User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Creating best practices for APPs
A holistic approach to integration
Hospital medicine groups (HMGs) nationally are confronted with a host of challenging issues: increased patient volume/complexities, resident duty-hour restrictions, and a rise in provider burnout. Many are turning to advanced practice providers (APPs) to help lighten these burdens.
But no practical guidelines exist around how to successfully incorporate APPs in a way that meets the needs of the patients, the providers, the HMG, and the health system, according to Kasey Bowden, MSN, FNP, AG-ACNP, lead author of a HM19 abstract on that subject.
“Much of the recent literature around APP utilization involves descriptive anecdotes on how individual HMGs have utilized APPs, and what metrics this helped them to achieve,” she said. “While these stories are often compelling, they provide no tangible value to HMGs looking to incorporate APPs into practice, as they do not address unique elements that limit successful APP integration, including diverse educational backgrounds of APPs and exceedingly high turnover rates (12.6% nationally).”
Ms. Bowden and coauthors created a conceptual framework, which recognizes that, without taking a holistic approach, many HMGs will fail to successfully integrate APPs. “Our hope is that by utilizing this framework to define APP-physician best practices, we will be able to create a useful tool for all HMGs that will promote successful APP-physician collaborative practice models.”
She thinks that hospitalists could also use this framework to examine their current practice models and to see where there may be opportunity for improvement. For example, a group may look at their own APP turnover rate. “If turnover rate is high in the first year, it may suggest inadequate onboarding/training, if it is high after 3 years, this may suggest minimal opportunities for professional growth and advancement,” Ms. Bowden said. “I would love to see a consensus group form within SHM of physician and APP leaders to utilize this framework to establish ‘APP-Physician best practices,’ and create a guideline available to all HMGs so that they can successfully incorporate APPs into their practice,” she said.
Reference
1. Bowden K et al. Creation of APP-physician best practices: A necessary tool for the growing APP workforce. Hospital Medicine 2019, Abstract 436.
A holistic approach to integration
A holistic approach to integration
Hospital medicine groups (HMGs) nationally are confronted with a host of challenging issues: increased patient volume/complexities, resident duty-hour restrictions, and a rise in provider burnout. Many are turning to advanced practice providers (APPs) to help lighten these burdens.
But no practical guidelines exist around how to successfully incorporate APPs in a way that meets the needs of the patients, the providers, the HMG, and the health system, according to Kasey Bowden, MSN, FNP, AG-ACNP, lead author of a HM19 abstract on that subject.
“Much of the recent literature around APP utilization involves descriptive anecdotes on how individual HMGs have utilized APPs, and what metrics this helped them to achieve,” she said. “While these stories are often compelling, they provide no tangible value to HMGs looking to incorporate APPs into practice, as they do not address unique elements that limit successful APP integration, including diverse educational backgrounds of APPs and exceedingly high turnover rates (12.6% nationally).”
Ms. Bowden and coauthors created a conceptual framework, which recognizes that, without taking a holistic approach, many HMGs will fail to successfully integrate APPs. “Our hope is that by utilizing this framework to define APP-physician best practices, we will be able to create a useful tool for all HMGs that will promote successful APP-physician collaborative practice models.”
She thinks that hospitalists could also use this framework to examine their current practice models and to see where there may be opportunity for improvement. For example, a group may look at their own APP turnover rate. “If turnover rate is high in the first year, it may suggest inadequate onboarding/training, if it is high after 3 years, this may suggest minimal opportunities for professional growth and advancement,” Ms. Bowden said. “I would love to see a consensus group form within SHM of physician and APP leaders to utilize this framework to establish ‘APP-Physician best practices,’ and create a guideline available to all HMGs so that they can successfully incorporate APPs into their practice,” she said.
Reference
1. Bowden K et al. Creation of APP-physician best practices: A necessary tool for the growing APP workforce. Hospital Medicine 2019, Abstract 436.
Hospital medicine groups (HMGs) nationally are confronted with a host of challenging issues: increased patient volume/complexities, resident duty-hour restrictions, and a rise in provider burnout. Many are turning to advanced practice providers (APPs) to help lighten these burdens.
But no practical guidelines exist around how to successfully incorporate APPs in a way that meets the needs of the patients, the providers, the HMG, and the health system, according to Kasey Bowden, MSN, FNP, AG-ACNP, lead author of a HM19 abstract on that subject.
“Much of the recent literature around APP utilization involves descriptive anecdotes on how individual HMGs have utilized APPs, and what metrics this helped them to achieve,” she said. “While these stories are often compelling, they provide no tangible value to HMGs looking to incorporate APPs into practice, as they do not address unique elements that limit successful APP integration, including diverse educational backgrounds of APPs and exceedingly high turnover rates (12.6% nationally).”
Ms. Bowden and coauthors created a conceptual framework, which recognizes that, without taking a holistic approach, many HMGs will fail to successfully integrate APPs. “Our hope is that by utilizing this framework to define APP-physician best practices, we will be able to create a useful tool for all HMGs that will promote successful APP-physician collaborative practice models.”
She thinks that hospitalists could also use this framework to examine their current practice models and to see where there may be opportunity for improvement. For example, a group may look at their own APP turnover rate. “If turnover rate is high in the first year, it may suggest inadequate onboarding/training, if it is high after 3 years, this may suggest minimal opportunities for professional growth and advancement,” Ms. Bowden said. “I would love to see a consensus group form within SHM of physician and APP leaders to utilize this framework to establish ‘APP-Physician best practices,’ and create a guideline available to all HMGs so that they can successfully incorporate APPs into their practice,” she said.
Reference
1. Bowden K et al. Creation of APP-physician best practices: A necessary tool for the growing APP workforce. Hospital Medicine 2019, Abstract 436.
DOACs for treatment of cancer-associated venous thromboembolism
Bleeding risk may determine best option
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3
In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4
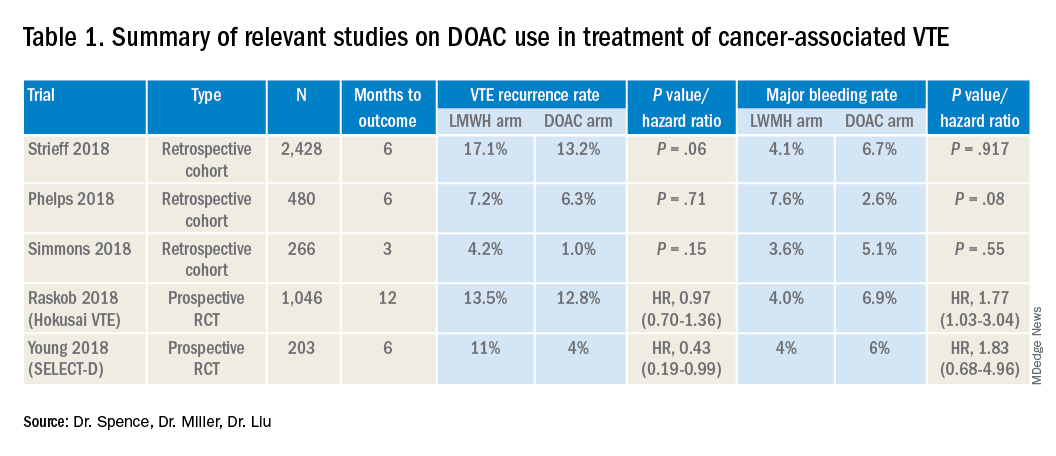
Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
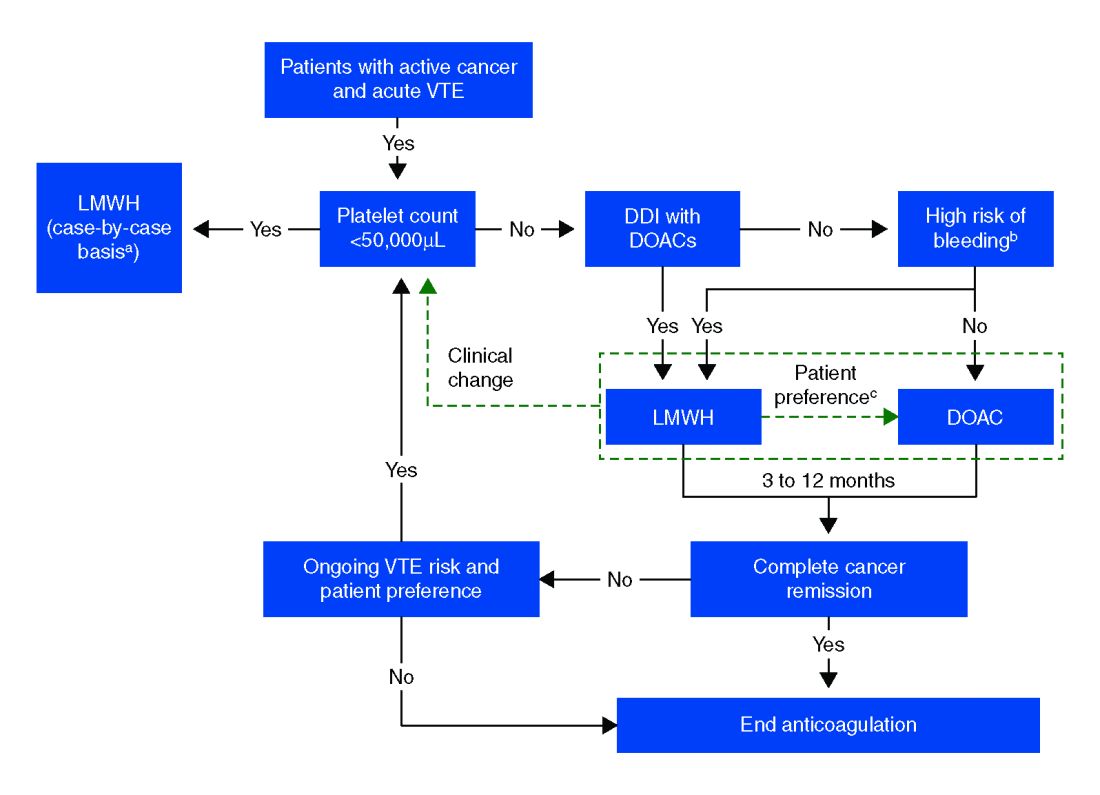
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
Bleeding risk may determine best option
Bleeding risk may determine best option
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3

In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4

Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3

In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4

Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
Cardiac biomarkers refine antihypertensive drug initiation decisions
PHILADELPHIA – Incorporation of cardiac biomarkers into current guideline-based decision-making regarding initiation of antihypertensive medication in patients with previously untreated mild or moderate high blood pressure leads to more appropriate and selective matching of intensive blood pressure control with true patient risk, Ambarish Pandey, MD, reported at the American Heart Association scientific sessions.
That’s because the 2017 American College of Cardiology/AHA blood pressure guidelines recommend incorporating the ACC/AHA 10-Year Atherosclerotic Cardiovascular Disease (ASCVD) Risk Calculator into decision making as to whether to start antihypertensive drug therapy in patients with stage 1 hypertension (130-139/80-89 mm Hg), but the risk calculator doesn’t account for the risk of heart failure.
Yet by far the greatest benefit of intensive BP lowering is in reducing the risk of developing heart failure, as demonstrated in the landmark SPRINT trial, which showed that intensive BP lowering achieved much greater risk reduction in new-onset heart failure than in atherosclerotic cardiovascular events.
Thus, there’s a need for better strategies to guide antihypertensive therapy. And therein lies the rationale for incorporating into the risk assessment an individual’s values for N-terminal pro–brain natriuretic peptide (NT-proBNP), which reflects chronic myocardial stress, and high-sensitivity cardiac troponin T (hs-cTnT), which when elevated signals myocardial injury.
“Cardiac biomarkers are intermediate phenotypes from hypertension to future cardiovascular events. They can identify individuals at increased risk for atherosclerotic events, and at even higher risk for heart failure events,” explained Dr. Pandey, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
He presented a study of 12,987 participants in three major U.S. cohort studies: the Atherosclerosis Risk In Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Dallas Heart Study. At baseline, none of the participants were on antihypertensive therapy or had known cardiovascular disease. During 10 years of prospective follow-up, 825 of them experienced a first cardiovascular disease event: 251 developed heart failure and 574 had an MI, stroke, or cardiovascular death. Dr. Pandey and his coworkers calculated the cardiovascular event incidence rate and number-needed-to-treat with intensive antihypertensive drug therapy to prevent a first cardiovascular disease event on the basis of whether patients in the various BP categories were positive or negative for one or more biomarkers.
The results
Fifty-four percent of subjects had normal BP, defined in the guidelines as less than 120/80 mm Hg. Another 3% had BP in excess of 160/100 mm Hg. No controversy exists regarding pharmacotherapy in either of these groups: It’s not warranted in the former, essential in the latter.
Another 3,000 individuals had what the ACC/AHA guidelines define as elevated BP, meaning 120-129/<80 mm Hg, or low-risk stage 1 hypertension of 130-139/80-89 mm Hg and a 10-year ASCVD risk score of less than 10%. Initiation of antihypertensive medication in these groups is not recommended in the guidelines. Yet 36% of these individuals had at least one positive cardiac biomarker. And here’s the eye-opening finding: Notably, the 10-year cardiovascular event incidence rate in this biomarker group not currently recommended for antihypertensive pharmacotherapy was 11%, more than double the 4.6% rate in the biomarker-negative group, which in turn was comparable to the 3.8% in the normal BP participants.
Antihypertensive therapy was recommended according to the guidelines in 20% of the total study population, comprising patients with stage 1 hypertension who had an ASCVD risk score of 10% or more as well as those with stage 2 hypertension, defined as BP greater than 140/90 mm Hg but less than 160/100 mm Hg. Forty-eight percent of these subjects were positive for at least one biomarker. Their cardiovascular incidence rate was 15.1%, compared to the 7.9% rate in biomarker-negative individuals.
The estimated number-needed-to-treat (NNT) with intensive blood pressure–lowering therapy to a target systolic BP of less than 120 mm Hg, as in SPRINT, to prevent one cardiovascular event in individuals not currently guideline-recommended for antihypertensive medications was 86 in those who were biomarker-negative. The NNT dropped to 36 in the biomarker-positive subgroup, a far more attractive figure that suggests a reasonable likelihood of benefit from intensive blood pressure control, in Dr. Pandey’s view.
Similarly, among individuals currently recommended for pharmacotherapy initiation, the NNTs were 49 if biomarker-negative, improving to 26 in those positive for one or both biomarkers, which was comparable to the NNT of 22 in the group with blood pressures greater than 160/100 mm Hg. The NNT of 49 in the biomarker-negative subgroup is in a borderline gray zone warranting individualized shared decision-making regarding pharmacotherapy, Dr. Pandey said.
In this study, an elevated hs-cTnT was defined as 6 ng/L or more, while an elevated NT-proBNP was considered to be at least 100 pg/mL.
“It’s noteworthy that the degree of elevation in hs-cTnT and NT-proBNP which were observed in our study were pretty subtle and much below the threshold used for diagnosis of ischemic events or heart failure. Thus, these elevations were largely representative of subtle chronic injury and not acute events,” according to the cardiologist.
One audience member asked if the elevated biomarkers could simply be a surrogate for longer duration of exposure of the heart to high BP. Sure, Dr. Pandey replied, pointing to the 6-year greater average age of the biomarker-positive participants.
“It is likely that biomarker-positive status is capturing the culmination of longstanding exposure. But the thing about hypertension is there are no symptoms that can signal to the patient or the doctor that they have this disease, so testing for the biomarkers can actually capture the high-risk group that may have had hypertension for a long duration but now needs to be treated in order to prevent the advance of downstream adverse events,” he said.
Dr. Pandey reported having no financial conflicts of interest regarding his study, conducted free of commercial support.
SOURCE: Pandey A. AHA 2019 Abstract EP.AOS.521.141
PHILADELPHIA – Incorporation of cardiac biomarkers into current guideline-based decision-making regarding initiation of antihypertensive medication in patients with previously untreated mild or moderate high blood pressure leads to more appropriate and selective matching of intensive blood pressure control with true patient risk, Ambarish Pandey, MD, reported at the American Heart Association scientific sessions.
That’s because the 2017 American College of Cardiology/AHA blood pressure guidelines recommend incorporating the ACC/AHA 10-Year Atherosclerotic Cardiovascular Disease (ASCVD) Risk Calculator into decision making as to whether to start antihypertensive drug therapy in patients with stage 1 hypertension (130-139/80-89 mm Hg), but the risk calculator doesn’t account for the risk of heart failure.
Yet by far the greatest benefit of intensive BP lowering is in reducing the risk of developing heart failure, as demonstrated in the landmark SPRINT trial, which showed that intensive BP lowering achieved much greater risk reduction in new-onset heart failure than in atherosclerotic cardiovascular events.
Thus, there’s a need for better strategies to guide antihypertensive therapy. And therein lies the rationale for incorporating into the risk assessment an individual’s values for N-terminal pro–brain natriuretic peptide (NT-proBNP), which reflects chronic myocardial stress, and high-sensitivity cardiac troponin T (hs-cTnT), which when elevated signals myocardial injury.
“Cardiac biomarkers are intermediate phenotypes from hypertension to future cardiovascular events. They can identify individuals at increased risk for atherosclerotic events, and at even higher risk for heart failure events,” explained Dr. Pandey, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
He presented a study of 12,987 participants in three major U.S. cohort studies: the Atherosclerosis Risk In Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Dallas Heart Study. At baseline, none of the participants were on antihypertensive therapy or had known cardiovascular disease. During 10 years of prospective follow-up, 825 of them experienced a first cardiovascular disease event: 251 developed heart failure and 574 had an MI, stroke, or cardiovascular death. Dr. Pandey and his coworkers calculated the cardiovascular event incidence rate and number-needed-to-treat with intensive antihypertensive drug therapy to prevent a first cardiovascular disease event on the basis of whether patients in the various BP categories were positive or negative for one or more biomarkers.
The results
Fifty-four percent of subjects had normal BP, defined in the guidelines as less than 120/80 mm Hg. Another 3% had BP in excess of 160/100 mm Hg. No controversy exists regarding pharmacotherapy in either of these groups: It’s not warranted in the former, essential in the latter.
Another 3,000 individuals had what the ACC/AHA guidelines define as elevated BP, meaning 120-129/<80 mm Hg, or low-risk stage 1 hypertension of 130-139/80-89 mm Hg and a 10-year ASCVD risk score of less than 10%. Initiation of antihypertensive medication in these groups is not recommended in the guidelines. Yet 36% of these individuals had at least one positive cardiac biomarker. And here’s the eye-opening finding: Notably, the 10-year cardiovascular event incidence rate in this biomarker group not currently recommended for antihypertensive pharmacotherapy was 11%, more than double the 4.6% rate in the biomarker-negative group, which in turn was comparable to the 3.8% in the normal BP participants.
Antihypertensive therapy was recommended according to the guidelines in 20% of the total study population, comprising patients with stage 1 hypertension who had an ASCVD risk score of 10% or more as well as those with stage 2 hypertension, defined as BP greater than 140/90 mm Hg but less than 160/100 mm Hg. Forty-eight percent of these subjects were positive for at least one biomarker. Their cardiovascular incidence rate was 15.1%, compared to the 7.9% rate in biomarker-negative individuals.
The estimated number-needed-to-treat (NNT) with intensive blood pressure–lowering therapy to a target systolic BP of less than 120 mm Hg, as in SPRINT, to prevent one cardiovascular event in individuals not currently guideline-recommended for antihypertensive medications was 86 in those who were biomarker-negative. The NNT dropped to 36 in the biomarker-positive subgroup, a far more attractive figure that suggests a reasonable likelihood of benefit from intensive blood pressure control, in Dr. Pandey’s view.
Similarly, among individuals currently recommended for pharmacotherapy initiation, the NNTs were 49 if biomarker-negative, improving to 26 in those positive for one or both biomarkers, which was comparable to the NNT of 22 in the group with blood pressures greater than 160/100 mm Hg. The NNT of 49 in the biomarker-negative subgroup is in a borderline gray zone warranting individualized shared decision-making regarding pharmacotherapy, Dr. Pandey said.
In this study, an elevated hs-cTnT was defined as 6 ng/L or more, while an elevated NT-proBNP was considered to be at least 100 pg/mL.
“It’s noteworthy that the degree of elevation in hs-cTnT and NT-proBNP which were observed in our study were pretty subtle and much below the threshold used for diagnosis of ischemic events or heart failure. Thus, these elevations were largely representative of subtle chronic injury and not acute events,” according to the cardiologist.
One audience member asked if the elevated biomarkers could simply be a surrogate for longer duration of exposure of the heart to high BP. Sure, Dr. Pandey replied, pointing to the 6-year greater average age of the biomarker-positive participants.
“It is likely that biomarker-positive status is capturing the culmination of longstanding exposure. But the thing about hypertension is there are no symptoms that can signal to the patient or the doctor that they have this disease, so testing for the biomarkers can actually capture the high-risk group that may have had hypertension for a long duration but now needs to be treated in order to prevent the advance of downstream adverse events,” he said.
Dr. Pandey reported having no financial conflicts of interest regarding his study, conducted free of commercial support.
SOURCE: Pandey A. AHA 2019 Abstract EP.AOS.521.141
PHILADELPHIA – Incorporation of cardiac biomarkers into current guideline-based decision-making regarding initiation of antihypertensive medication in patients with previously untreated mild or moderate high blood pressure leads to more appropriate and selective matching of intensive blood pressure control with true patient risk, Ambarish Pandey, MD, reported at the American Heart Association scientific sessions.
That’s because the 2017 American College of Cardiology/AHA blood pressure guidelines recommend incorporating the ACC/AHA 10-Year Atherosclerotic Cardiovascular Disease (ASCVD) Risk Calculator into decision making as to whether to start antihypertensive drug therapy in patients with stage 1 hypertension (130-139/80-89 mm Hg), but the risk calculator doesn’t account for the risk of heart failure.
Yet by far the greatest benefit of intensive BP lowering is in reducing the risk of developing heart failure, as demonstrated in the landmark SPRINT trial, which showed that intensive BP lowering achieved much greater risk reduction in new-onset heart failure than in atherosclerotic cardiovascular events.
Thus, there’s a need for better strategies to guide antihypertensive therapy. And therein lies the rationale for incorporating into the risk assessment an individual’s values for N-terminal pro–brain natriuretic peptide (NT-proBNP), which reflects chronic myocardial stress, and high-sensitivity cardiac troponin T (hs-cTnT), which when elevated signals myocardial injury.
“Cardiac biomarkers are intermediate phenotypes from hypertension to future cardiovascular events. They can identify individuals at increased risk for atherosclerotic events, and at even higher risk for heart failure events,” explained Dr. Pandey, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
He presented a study of 12,987 participants in three major U.S. cohort studies: the Atherosclerosis Risk In Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Dallas Heart Study. At baseline, none of the participants were on antihypertensive therapy or had known cardiovascular disease. During 10 years of prospective follow-up, 825 of them experienced a first cardiovascular disease event: 251 developed heart failure and 574 had an MI, stroke, or cardiovascular death. Dr. Pandey and his coworkers calculated the cardiovascular event incidence rate and number-needed-to-treat with intensive antihypertensive drug therapy to prevent a first cardiovascular disease event on the basis of whether patients in the various BP categories were positive or negative for one or more biomarkers.
The results
Fifty-four percent of subjects had normal BP, defined in the guidelines as less than 120/80 mm Hg. Another 3% had BP in excess of 160/100 mm Hg. No controversy exists regarding pharmacotherapy in either of these groups: It’s not warranted in the former, essential in the latter.
Another 3,000 individuals had what the ACC/AHA guidelines define as elevated BP, meaning 120-129/<80 mm Hg, or low-risk stage 1 hypertension of 130-139/80-89 mm Hg and a 10-year ASCVD risk score of less than 10%. Initiation of antihypertensive medication in these groups is not recommended in the guidelines. Yet 36% of these individuals had at least one positive cardiac biomarker. And here’s the eye-opening finding: Notably, the 10-year cardiovascular event incidence rate in this biomarker group not currently recommended for antihypertensive pharmacotherapy was 11%, more than double the 4.6% rate in the biomarker-negative group, which in turn was comparable to the 3.8% in the normal BP participants.
Antihypertensive therapy was recommended according to the guidelines in 20% of the total study population, comprising patients with stage 1 hypertension who had an ASCVD risk score of 10% or more as well as those with stage 2 hypertension, defined as BP greater than 140/90 mm Hg but less than 160/100 mm Hg. Forty-eight percent of these subjects were positive for at least one biomarker. Their cardiovascular incidence rate was 15.1%, compared to the 7.9% rate in biomarker-negative individuals.
The estimated number-needed-to-treat (NNT) with intensive blood pressure–lowering therapy to a target systolic BP of less than 120 mm Hg, as in SPRINT, to prevent one cardiovascular event in individuals not currently guideline-recommended for antihypertensive medications was 86 in those who were biomarker-negative. The NNT dropped to 36 in the biomarker-positive subgroup, a far more attractive figure that suggests a reasonable likelihood of benefit from intensive blood pressure control, in Dr. Pandey’s view.
Similarly, among individuals currently recommended for pharmacotherapy initiation, the NNTs were 49 if biomarker-negative, improving to 26 in those positive for one or both biomarkers, which was comparable to the NNT of 22 in the group with blood pressures greater than 160/100 mm Hg. The NNT of 49 in the biomarker-negative subgroup is in a borderline gray zone warranting individualized shared decision-making regarding pharmacotherapy, Dr. Pandey said.
In this study, an elevated hs-cTnT was defined as 6 ng/L or more, while an elevated NT-proBNP was considered to be at least 100 pg/mL.
“It’s noteworthy that the degree of elevation in hs-cTnT and NT-proBNP which were observed in our study were pretty subtle and much below the threshold used for diagnosis of ischemic events or heart failure. Thus, these elevations were largely representative of subtle chronic injury and not acute events,” according to the cardiologist.
One audience member asked if the elevated biomarkers could simply be a surrogate for longer duration of exposure of the heart to high BP. Sure, Dr. Pandey replied, pointing to the 6-year greater average age of the biomarker-positive participants.
“It is likely that biomarker-positive status is capturing the culmination of longstanding exposure. But the thing about hypertension is there are no symptoms that can signal to the patient or the doctor that they have this disease, so testing for the biomarkers can actually capture the high-risk group that may have had hypertension for a long duration but now needs to be treated in order to prevent the advance of downstream adverse events,” he said.
Dr. Pandey reported having no financial conflicts of interest regarding his study, conducted free of commercial support.
SOURCE: Pandey A. AHA 2019 Abstract EP.AOS.521.141
REPORTING FROM AHA 2019
FDA advisers set high bar for new opioids
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
Medscape survey points to generational differences in physician burnout
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”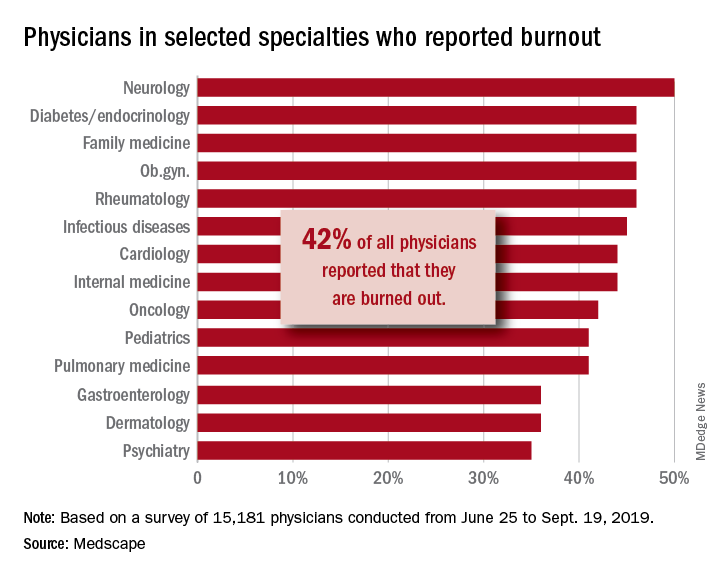
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Nontuberculous mycobacterial lung disease cases on the rise across U.S.
To assess the NTM lung disease burden on a national level, Kevin L. Winthrop, MD, of Oregon Health & Science University, Portland, and associates analyzed patient data from a U.S. managed care claims database between 2008 and 2015. Their findings were published in the Annals of the American Thoracic Society.
A case of NTM lung disease was defined as a patient with at least two medical claims with the disease’s diagnostic codes – 031.0 and A31.0 – that were at least 30 days apart. Of the 74,984,596 beneficiaries in the database, 9,476 met the case definition for NTM lung disease; 69% (n = 6,530) were women.
From 2008 to 2015, the annual incidence of NTM lung disease increased from 3.13 (95% confidence interval, 2.88-3.40) to 4.73 (95% CI, 4.43-5.05) per 100,000 person-years, with the average rate of yearly change being +5.2% (95% CI, 4.0%-6.4%; P less than .01).The annual prevalence increased from 6.78 (95% CI, 6.45-7.14) to 11.70 (95% CI, 11.26-12.16) per 100,000 persons, with the average rate of yearly change being +7.5% (95% CI, 6.7-8.2%; P less than .01).
The majority of NTM lung disease in the United States is caused by Mycobacterium avium complex (17), although other species such as M. abscessus, M. kansasii, M. xenopi, and others contribute to this disease burden.
“It’s a classic chicken-or-egg scenario,” said Sachin Gupta, MD, a pulmonologist in San Francisco, in regard to the rising numbers. “Increased awareness of NTM lung disease is, in part, why we’re seeing prevalence and incidence go up. And yet the disease itself may also be growing in clusters and pockets, as the data show, in various places across the nation.
“The worrisome aspect here,” he added, “is that future studies will likely show that, as incidence is increasing, mortality is increasing as well. That speaks to the challenges with these bugs: Very hard to diagnose, very hard to treat.”
The authors acknowledged their study’s limitations, including the lack of microbiologic or radiographic confirmation of the NTM infection and the inherent shortcomings of claims data–based studies overall. They did note a previous report, however, that “claims-based case identification has a high positive predictive value of approximately 82% for NTM lung disease.”
The study was funded by Insmed; the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases; and the National Heart, Lung, and Blood Institute. The authors reported no conflicts of interest.
SOURCE: Winthrop KL et al. Ann Am Thorac Soc. 2019 Dec 13. doi: 10.1513/AnnalsATS.201804-236OC.
To assess the NTM lung disease burden on a national level, Kevin L. Winthrop, MD, of Oregon Health & Science University, Portland, and associates analyzed patient data from a U.S. managed care claims database between 2008 and 2015. Their findings were published in the Annals of the American Thoracic Society.
A case of NTM lung disease was defined as a patient with at least two medical claims with the disease’s diagnostic codes – 031.0 and A31.0 – that were at least 30 days apart. Of the 74,984,596 beneficiaries in the database, 9,476 met the case definition for NTM lung disease; 69% (n = 6,530) were women.
From 2008 to 2015, the annual incidence of NTM lung disease increased from 3.13 (95% confidence interval, 2.88-3.40) to 4.73 (95% CI, 4.43-5.05) per 100,000 person-years, with the average rate of yearly change being +5.2% (95% CI, 4.0%-6.4%; P less than .01).The annual prevalence increased from 6.78 (95% CI, 6.45-7.14) to 11.70 (95% CI, 11.26-12.16) per 100,000 persons, with the average rate of yearly change being +7.5% (95% CI, 6.7-8.2%; P less than .01).
The majority of NTM lung disease in the United States is caused by Mycobacterium avium complex (17), although other species such as M. abscessus, M. kansasii, M. xenopi, and others contribute to this disease burden.
“It’s a classic chicken-or-egg scenario,” said Sachin Gupta, MD, a pulmonologist in San Francisco, in regard to the rising numbers. “Increased awareness of NTM lung disease is, in part, why we’re seeing prevalence and incidence go up. And yet the disease itself may also be growing in clusters and pockets, as the data show, in various places across the nation.
“The worrisome aspect here,” he added, “is that future studies will likely show that, as incidence is increasing, mortality is increasing as well. That speaks to the challenges with these bugs: Very hard to diagnose, very hard to treat.”
The authors acknowledged their study’s limitations, including the lack of microbiologic or radiographic confirmation of the NTM infection and the inherent shortcomings of claims data–based studies overall. They did note a previous report, however, that “claims-based case identification has a high positive predictive value of approximately 82% for NTM lung disease.”
The study was funded by Insmed; the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases; and the National Heart, Lung, and Blood Institute. The authors reported no conflicts of interest.
SOURCE: Winthrop KL et al. Ann Am Thorac Soc. 2019 Dec 13. doi: 10.1513/AnnalsATS.201804-236OC.
To assess the NTM lung disease burden on a national level, Kevin L. Winthrop, MD, of Oregon Health & Science University, Portland, and associates analyzed patient data from a U.S. managed care claims database between 2008 and 2015. Their findings were published in the Annals of the American Thoracic Society.
A case of NTM lung disease was defined as a patient with at least two medical claims with the disease’s diagnostic codes – 031.0 and A31.0 – that were at least 30 days apart. Of the 74,984,596 beneficiaries in the database, 9,476 met the case definition for NTM lung disease; 69% (n = 6,530) were women.
From 2008 to 2015, the annual incidence of NTM lung disease increased from 3.13 (95% confidence interval, 2.88-3.40) to 4.73 (95% CI, 4.43-5.05) per 100,000 person-years, with the average rate of yearly change being +5.2% (95% CI, 4.0%-6.4%; P less than .01).The annual prevalence increased from 6.78 (95% CI, 6.45-7.14) to 11.70 (95% CI, 11.26-12.16) per 100,000 persons, with the average rate of yearly change being +7.5% (95% CI, 6.7-8.2%; P less than .01).
The majority of NTM lung disease in the United States is caused by Mycobacterium avium complex (17), although other species such as M. abscessus, M. kansasii, M. xenopi, and others contribute to this disease burden.
“It’s a classic chicken-or-egg scenario,” said Sachin Gupta, MD, a pulmonologist in San Francisco, in regard to the rising numbers. “Increased awareness of NTM lung disease is, in part, why we’re seeing prevalence and incidence go up. And yet the disease itself may also be growing in clusters and pockets, as the data show, in various places across the nation.
“The worrisome aspect here,” he added, “is that future studies will likely show that, as incidence is increasing, mortality is increasing as well. That speaks to the challenges with these bugs: Very hard to diagnose, very hard to treat.”
The authors acknowledged their study’s limitations, including the lack of microbiologic or radiographic confirmation of the NTM infection and the inherent shortcomings of claims data–based studies overall. They did note a previous report, however, that “claims-based case identification has a high positive predictive value of approximately 82% for NTM lung disease.”
The study was funded by Insmed; the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases; and the National Heart, Lung, and Blood Institute. The authors reported no conflicts of interest.
SOURCE: Winthrop KL et al. Ann Am Thorac Soc. 2019 Dec 13. doi: 10.1513/AnnalsATS.201804-236OC.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Cognitive screening of older physicians: What’s fair?
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
FROM JAMA
Reducing alarm fatigue in the hospital
Noise increases patient anxiety
Researchers are exploring ways to make alarms and monitors less irritating and more informative.
“Hospitals today can be sonic hellscapes, which studies have shown regularly exceed levels set by the World Health Organization: droning IV pumps, ding-donging nurse call buttons, voices crackling on loudspeakers, ringing telephones, beeping elevators, buzzing ID scanners, clattering carts, coughing, screaming, vomiting,” according to a recent article in the New York Times.
And that’s not to mention all the alarms that blare regularly, day and night. “A single patient might trigger hundreds each day, challenging caregivers to figure out which machine is beeping, and what is wrong with the patient, if anything,” according to the article.
All this noise contributes to patient anxiety and delirium and to staff burnout too. Alarm fatigue is a serious problem, related to the high rate of false alarms, the lack of alarm standardization, and the number of medical devices that emit an alarm. Its effect is to make caregivers less responsive.
A group of researchers is developing new sounds that could replace current alarms. These new signals might mimic electronic dance music or the sounds of a heartbeat; they may combine audible alarms with visual cues such as interactive screens; they will certainly be quieter. Testing remains to be done around how quickly clinicians will be able to learn the sounds and how loud they need to be. The researchers say a new standard is likely to go into effect in 2020.
Reference
1. Rueb ES. To Reduce Hospital Noise, Researchers Create Alarms That Whistle and Sing. New York Times. July 9, 2019.
Noise increases patient anxiety
Noise increases patient anxiety
Researchers are exploring ways to make alarms and monitors less irritating and more informative.
“Hospitals today can be sonic hellscapes, which studies have shown regularly exceed levels set by the World Health Organization: droning IV pumps, ding-donging nurse call buttons, voices crackling on loudspeakers, ringing telephones, beeping elevators, buzzing ID scanners, clattering carts, coughing, screaming, vomiting,” according to a recent article in the New York Times.
And that’s not to mention all the alarms that blare regularly, day and night. “A single patient might trigger hundreds each day, challenging caregivers to figure out which machine is beeping, and what is wrong with the patient, if anything,” according to the article.
All this noise contributes to patient anxiety and delirium and to staff burnout too. Alarm fatigue is a serious problem, related to the high rate of false alarms, the lack of alarm standardization, and the number of medical devices that emit an alarm. Its effect is to make caregivers less responsive.
A group of researchers is developing new sounds that could replace current alarms. These new signals might mimic electronic dance music or the sounds of a heartbeat; they may combine audible alarms with visual cues such as interactive screens; they will certainly be quieter. Testing remains to be done around how quickly clinicians will be able to learn the sounds and how loud they need to be. The researchers say a new standard is likely to go into effect in 2020.
Reference
1. Rueb ES. To Reduce Hospital Noise, Researchers Create Alarms That Whistle and Sing. New York Times. July 9, 2019.
Researchers are exploring ways to make alarms and monitors less irritating and more informative.
“Hospitals today can be sonic hellscapes, which studies have shown regularly exceed levels set by the World Health Organization: droning IV pumps, ding-donging nurse call buttons, voices crackling on loudspeakers, ringing telephones, beeping elevators, buzzing ID scanners, clattering carts, coughing, screaming, vomiting,” according to a recent article in the New York Times.
And that’s not to mention all the alarms that blare regularly, day and night. “A single patient might trigger hundreds each day, challenging caregivers to figure out which machine is beeping, and what is wrong with the patient, if anything,” according to the article.
All this noise contributes to patient anxiety and delirium and to staff burnout too. Alarm fatigue is a serious problem, related to the high rate of false alarms, the lack of alarm standardization, and the number of medical devices that emit an alarm. Its effect is to make caregivers less responsive.
A group of researchers is developing new sounds that could replace current alarms. These new signals might mimic electronic dance music or the sounds of a heartbeat; they may combine audible alarms with visual cues such as interactive screens; they will certainly be quieter. Testing remains to be done around how quickly clinicians will be able to learn the sounds and how loud they need to be. The researchers say a new standard is likely to go into effect in 2020.
Reference
1. Rueb ES. To Reduce Hospital Noise, Researchers Create Alarms That Whistle and Sing. New York Times. July 9, 2019.
Children with resistant UTIs unexpectedly may respond to discordant antibiotics
Children with urinary tract infections (UTIs) may improve clinically, and pyuria may resolve, during empiric treatment with an antibiotic that turns out to be discordant, according a retrospective study in Pediatrics.
“The low rate of care escalation and high rate of clinical improvement while on discordant antibiotics suggests that, for most patients, it would be reasonable to continue current empiric antibiotic practices until urine culture sensitivities return,” said first author Marie E. Wang, MD, a pediatric hospitalist at Stanford (Calif.) University, and colleagues.
The researchers examined the initial clinical response and escalation of care for 316 children with UTIs who received therapy to which the infecting isolate was not susceptible. The study included patients who had infections that were resistant to third-generation cephalosporins – that is, urinalysis found that the infections were not susceptible to ceftriaxone or cefotaxime in vitro. Before the resistant organisms were identified, however, the patients were started on discordant antibiotics.
Escalation of care was uncommon
The patients had a median age of 2.4 years, and 78% were girls. Approximately 90% were started on a cephalosporin, and about 65% received a first-generation cephalosporin. Patients presented during 2012-2017 to one of five children’s hospitals or to a large managed care organization with 10 hospitals in the United States. The investigators defined care escalation as a visit to the emergency department, hospitalization, or transfer to the ICU.
In all, seven patients (2%) had escalation of care on discordant antibiotics. Four children visited an emergency department without hospitalization, and three children were hospitalized because of persistent symptoms.
Among 230 cases for which the researchers had data about clinical response at a median follow-up of 3 days, 84% “had overall clinical improvement while on discordant antibiotics,” the authors said.
For 22 children who had repeat urine testing while on discordant antibiotics, 53% had resolution of pyuria, and 32% had improvement of pyuria, whereas 16% did not have improvement. Of the three patients without improvement, one had no change, and two had worsening.
Of 17 patients who had a repeat urine culture on discordant therapy, 65% had a negative repeat culture, and 18% grew the same pathogen with a decreased colony count. Two patients had a colony count that remained unchanged, and one patient had an increased colony count.
Small studies outside the United States have reported similar results, the researchers noted. Spontaneous resolution of UTIs or antibiotics reaching a sufficient concentration in the urine and renal parenchyma to achieve a clinical response are possible explanations for the findings, they wrote.
“Few children required escalation of care and most experienced initial clinical improvement,” noted Dr. Wang and colleagues. “Furthermore, in the small group of children that underwent repeat urine testing while on discordant therapy, most had resolution or improvement in pyuria and sterilization of their urine cultures. Our findings suggest that Additionally, given that these patients initially received what would generally be considered inadequate treatment, our findings may provide some insight into the natural history of UTIs and/or trigger further investigation into the relationship between in vitro urine culture susceptibilities and in vivo clinical response to treatment.”
‘Caution is needed’
The study “highlights an intriguing observation about children with UTIs unexpectedly responding to discordant antibiotic therapy,” Tej K. Mattoo, MD, and Basim I. Asmar, MD, wrote in an accompanying commentary.(doi: 10.1542/peds.2019-3512). Dr. Mattoo and Dr. Asmar, a pediatric nephrologist and a specialist in pediatric infectious diseases, respectively, at Wayne State University and affiliated with Children’s Hospital of Michigan, both in Detroit.
In an inpatient setting, it may be easy for physicians to reassess patients “once urine culture results reveal resistance to the treating antibiotic,” they noted. In an ambulatory setting, however, “it is likely that some patients will receive a full course of an antibiotic that does not have in vitro activity against the urinary pathogen.”
Physicians have a responsibility to use antibiotics judiciously, they said. Widely accepted principles include avoiding repeated courses of antibiotics, diagnosing UTIs appropriately, and not treating asymptomatic bacteriuria.
The study had no external funding. The authors had no relevant financial disclosures.
SOURCE: Wang ME et al. Pediatrics. 2020 Jan 17. doi: 10.1542/peds.2019-1608.
This article was updated 2/4/2020.
Children with urinary tract infections (UTIs) may improve clinically, and pyuria may resolve, during empiric treatment with an antibiotic that turns out to be discordant, according a retrospective study in Pediatrics.
“The low rate of care escalation and high rate of clinical improvement while on discordant antibiotics suggests that, for most patients, it would be reasonable to continue current empiric antibiotic practices until urine culture sensitivities return,” said first author Marie E. Wang, MD, a pediatric hospitalist at Stanford (Calif.) University, and colleagues.
The researchers examined the initial clinical response and escalation of care for 316 children with UTIs who received therapy to which the infecting isolate was not susceptible. The study included patients who had infections that were resistant to third-generation cephalosporins – that is, urinalysis found that the infections were not susceptible to ceftriaxone or cefotaxime in vitro. Before the resistant organisms were identified, however, the patients were started on discordant antibiotics.
Escalation of care was uncommon
The patients had a median age of 2.4 years, and 78% were girls. Approximately 90% were started on a cephalosporin, and about 65% received a first-generation cephalosporin. Patients presented during 2012-2017 to one of five children’s hospitals or to a large managed care organization with 10 hospitals in the United States. The investigators defined care escalation as a visit to the emergency department, hospitalization, or transfer to the ICU.
In all, seven patients (2%) had escalation of care on discordant antibiotics. Four children visited an emergency department without hospitalization, and three children were hospitalized because of persistent symptoms.
Among 230 cases for which the researchers had data about clinical response at a median follow-up of 3 days, 84% “had overall clinical improvement while on discordant antibiotics,” the authors said.
For 22 children who had repeat urine testing while on discordant antibiotics, 53% had resolution of pyuria, and 32% had improvement of pyuria, whereas 16% did not have improvement. Of the three patients without improvement, one had no change, and two had worsening.
Of 17 patients who had a repeat urine culture on discordant therapy, 65% had a negative repeat culture, and 18% grew the same pathogen with a decreased colony count. Two patients had a colony count that remained unchanged, and one patient had an increased colony count.
Small studies outside the United States have reported similar results, the researchers noted. Spontaneous resolution of UTIs or antibiotics reaching a sufficient concentration in the urine and renal parenchyma to achieve a clinical response are possible explanations for the findings, they wrote.
“Few children required escalation of care and most experienced initial clinical improvement,” noted Dr. Wang and colleagues. “Furthermore, in the small group of children that underwent repeat urine testing while on discordant therapy, most had resolution or improvement in pyuria and sterilization of their urine cultures. Our findings suggest that Additionally, given that these patients initially received what would generally be considered inadequate treatment, our findings may provide some insight into the natural history of UTIs and/or trigger further investigation into the relationship between in vitro urine culture susceptibilities and in vivo clinical response to treatment.”
‘Caution is needed’
The study “highlights an intriguing observation about children with UTIs unexpectedly responding to discordant antibiotic therapy,” Tej K. Mattoo, MD, and Basim I. Asmar, MD, wrote in an accompanying commentary.(doi: 10.1542/peds.2019-3512). Dr. Mattoo and Dr. Asmar, a pediatric nephrologist and a specialist in pediatric infectious diseases, respectively, at Wayne State University and affiliated with Children’s Hospital of Michigan, both in Detroit.
In an inpatient setting, it may be easy for physicians to reassess patients “once urine culture results reveal resistance to the treating antibiotic,” they noted. In an ambulatory setting, however, “it is likely that some patients will receive a full course of an antibiotic that does not have in vitro activity against the urinary pathogen.”
Physicians have a responsibility to use antibiotics judiciously, they said. Widely accepted principles include avoiding repeated courses of antibiotics, diagnosing UTIs appropriately, and not treating asymptomatic bacteriuria.
The study had no external funding. The authors had no relevant financial disclosures.
SOURCE: Wang ME et al. Pediatrics. 2020 Jan 17. doi: 10.1542/peds.2019-1608.
This article was updated 2/4/2020.
Children with urinary tract infections (UTIs) may improve clinically, and pyuria may resolve, during empiric treatment with an antibiotic that turns out to be discordant, according a retrospective study in Pediatrics.
“The low rate of care escalation and high rate of clinical improvement while on discordant antibiotics suggests that, for most patients, it would be reasonable to continue current empiric antibiotic practices until urine culture sensitivities return,” said first author Marie E. Wang, MD, a pediatric hospitalist at Stanford (Calif.) University, and colleagues.
The researchers examined the initial clinical response and escalation of care for 316 children with UTIs who received therapy to which the infecting isolate was not susceptible. The study included patients who had infections that were resistant to third-generation cephalosporins – that is, urinalysis found that the infections were not susceptible to ceftriaxone or cefotaxime in vitro. Before the resistant organisms were identified, however, the patients were started on discordant antibiotics.
Escalation of care was uncommon
The patients had a median age of 2.4 years, and 78% were girls. Approximately 90% were started on a cephalosporin, and about 65% received a first-generation cephalosporin. Patients presented during 2012-2017 to one of five children’s hospitals or to a large managed care organization with 10 hospitals in the United States. The investigators defined care escalation as a visit to the emergency department, hospitalization, or transfer to the ICU.
In all, seven patients (2%) had escalation of care on discordant antibiotics. Four children visited an emergency department without hospitalization, and three children were hospitalized because of persistent symptoms.
Among 230 cases for which the researchers had data about clinical response at a median follow-up of 3 days, 84% “had overall clinical improvement while on discordant antibiotics,” the authors said.
For 22 children who had repeat urine testing while on discordant antibiotics, 53% had resolution of pyuria, and 32% had improvement of pyuria, whereas 16% did not have improvement. Of the three patients without improvement, one had no change, and two had worsening.
Of 17 patients who had a repeat urine culture on discordant therapy, 65% had a negative repeat culture, and 18% grew the same pathogen with a decreased colony count. Two patients had a colony count that remained unchanged, and one patient had an increased colony count.
Small studies outside the United States have reported similar results, the researchers noted. Spontaneous resolution of UTIs or antibiotics reaching a sufficient concentration in the urine and renal parenchyma to achieve a clinical response are possible explanations for the findings, they wrote.
“Few children required escalation of care and most experienced initial clinical improvement,” noted Dr. Wang and colleagues. “Furthermore, in the small group of children that underwent repeat urine testing while on discordant therapy, most had resolution or improvement in pyuria and sterilization of their urine cultures. Our findings suggest that Additionally, given that these patients initially received what would generally be considered inadequate treatment, our findings may provide some insight into the natural history of UTIs and/or trigger further investigation into the relationship between in vitro urine culture susceptibilities and in vivo clinical response to treatment.”
‘Caution is needed’
The study “highlights an intriguing observation about children with UTIs unexpectedly responding to discordant antibiotic therapy,” Tej K. Mattoo, MD, and Basim I. Asmar, MD, wrote in an accompanying commentary.(doi: 10.1542/peds.2019-3512). Dr. Mattoo and Dr. Asmar, a pediatric nephrologist and a specialist in pediatric infectious diseases, respectively, at Wayne State University and affiliated with Children’s Hospital of Michigan, both in Detroit.
In an inpatient setting, it may be easy for physicians to reassess patients “once urine culture results reveal resistance to the treating antibiotic,” they noted. In an ambulatory setting, however, “it is likely that some patients will receive a full course of an antibiotic that does not have in vitro activity against the urinary pathogen.”
Physicians have a responsibility to use antibiotics judiciously, they said. Widely accepted principles include avoiding repeated courses of antibiotics, diagnosing UTIs appropriately, and not treating asymptomatic bacteriuria.
The study had no external funding. The authors had no relevant financial disclosures.
SOURCE: Wang ME et al. Pediatrics. 2020 Jan 17. doi: 10.1542/peds.2019-1608.
This article was updated 2/4/2020.
FROM PEDIATRICS
SHM Pediatric Core Competencies get fresh update
New core competencies reflect a decade of change
Over the past 10 years, much has changed in the world of pediatric hospital medicine. The annual national PHM conference sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA) is robust; textbooks and journal articles in the field abound; and networks and training in research, quality improvement, and education are successful and ongoing.
Much of this did not exist or was in its infancy back in 2010. Since then, it has grown and greatly evolved. In parallel, medicine and society have changed. These influences on health care, along with the growth of the field over time, prompted a review and revision of the 2010 PHM Core Competencies published by SHM. With support from the society, the Pediatric Hospital Medicine Special Interest Group launched the plan for revision of the PHM Core Competencies.
The selected editors included Sandra Gage, MD, PhD, SFHM, of Phoenix Children’s Hospital; Erin Stucky Fisher, MD, MHM, of UCSD/Rady Children’s Hospital in San Diego; Jennifer Maniscalco, MD, MPH, of Johns Hopkins All Children’s Hospital in St. Petersburg, Fla.; and Sofia Teferi, MD, SFHM, a pediatric hospitalist based at Bon Secours St. Mary’s Hospital in Richmond, Va. They began their work in 2017 along with six associate editors, meeting every 2 weeks via conference call, dividing the work accordingly.
Dr. Teferi served in a new and critical role as contributing editor. She described her role as a “sweeper” of sorts, bringing her unique perspective to the process. “The other three members are from academic settings, and I’m from a community setting, which is very different,” Dr. Teferi said. “I went through all the chapters to ensure they were inclusive of the community setting.”
According to Dr. Gage, “the purpose of the original PHM Core Competencies was to define the roles and responsibilities of a PHM practitioner. In the intervening 10 years, the field has changed and matured, and we have solidified our role since then.”
Today’s pediatric hospitalists, for instance, may coordinate care in EDs, provide inpatient consultations, engage or lead quality improvement programs, and teach. The demands for pediatric hospital care today go beyond the training provided in a standard pediatric residency. The core competencies need to provide the information necessary, therefore, to ensure pediatric hospital medicine is practiced at its most informed level.
A profession transformed
At the time of the first set of core competencies, there were over 2,500 members in three core societies in which pediatric hospitalists were members: the AAP, the APA, and SHM. As of 2017, those numbers have swelled as the care for children in the hospital setting has shifted away from these patients’ primary care providers.
The original core competencies included 54 chapters, designed to be used independent of the others. They provided a foundation for the creation of pediatric hospital medicine and served to standardize and improve inpatient training practices.
For the new core competencies, every single chapter was reviewed line by line, Dr. Gage said. Many chapters had content modified, and new chapters were added to reflect the evolution of the field and of medicine. “We added about 14 new chapters, adjusted the titles of others, and significantly changed the content of over half,” Dr. Gage explained. “They are fairly broad changes, related to the breadth of the practice today.”
Dr. Teferi noted that practitioners can use the updated competencies with additions to the service lines that have arisen since the last version. “These include areas like step down and newborn nursery, things that weren’t part of our portfolio 10 years ago,” she said. “This reflects the fact that often you’ll see a hospital leader who might want to add to a hospitalist’s portfolio of services because there is no one else to do it. Or maybe community pediatrics no longer want to treat babies, so we add that. The settings vary widely today and we need the competencies to address that.”
Practices within these settings can also vary widely. Teaching, palliative care, airway management, critical care, and anesthesia may all come into play, among other factors. Research opportunities throughout the field also continue to expand.
Dr. Fisher said that the editors and associate editors kept in mind the fact that not every hospital would have all the resources necessary at its fingertips. “The competencies must reflect the realities of the variety of community settings,” she said. “Also, on a national level, the majority of pediatric patients are not seen in a children’s hospital. Community sites are where pediatric hospitalists are not only advocates for care, but can be working with limited resources – the ‘lone soldiers.’ We wanted to make sure the competencies reflect that reality and environment community site or not; academic site or not; tertiary care site or not; rural or not – these are overlapping but independent considerations for all who practice pediatric hospital medicine – a Venn diagram, and the PHM core competencies try to attend to all of those.”
This made Dr. Teferi’s perspective all the more important. “While many, including other editors and associate editors, work in community sites, Dr. Teferi has this as her unique and sole focus. She brought a unique viewpoint to the table,” Dr. Fisher said.
A goal of the core competencies is to make it possible for a pediatric hospitalist to move to a different practice environment and still provide the same level of high-quality care. “It’s difficult but important to grasp the concepts and competencies of various settings,” Dr. Fisher said. “In this way, our competencies are a parallel model to the adult hospitalist competencies.”
The editors surveyed practitioners across the country to gather their input on content, and brought on topic experts to write the new chapters. “If we didn’t have an author for a specific chapter or area from the last set of competencies, we came to a consensus on who the new one should be,” Dr. Gage explained. “We looked for known and accepted experts in the field by reviewing the literature and conference lecturers at all major PHM meetings.”
Once the editors and associate editors worked with authors to refine their chapter(s), the chapters were sent to multiple external reviewers including subgroups of SHM, AAP, and APA, as well as a variety of other associations. They provided input that the editors and associate editors collated, reviewed, and incorporated according to consensus and discussion with the authors.
A preview
As far as the actual changes go, some of new chapters include four common clinical, two core skills, three specialized services, and five health care systems, with many others undergoing content changes, according to Dr. Gage.
Major considerations in developing the new competencies include the national trend of rising mental health issues among young patients. According to the AAP, over the last decade the number of young people aged 6-17 years requiring mental health care has risen from 9% to more than 14%. In outpatient settings, many pediatricians report that half or more of their visits are dedicated to these issues, a number that may spill out into the hospital setting as well.
According to Dr. Fisher, pediatric hospitalists today see increasing numbers of chronic and acute diseases accompanied by mental and behavioral health issues. “We wanted to underscore this complexity in the competencies,” she explained. “We needed to focus new attention on how to identify and treat children with behavioral or psychiatric diagnoses or needs.”
Other new areas of focus include infection care and antimicrobial stewardship. “We see kids on antibiotics in hospital settings and we need to focus on narrowing choices, decreasing use, and shortening duration,” Dr. Gage said.
Dr. Maniscalco said that, overall, the changes represent the evolution of the field. “Pediatric hospitalists are taking on far more patients with acute and complex issues,” she explained. “Our skill set is coming into focus.”
Dr. Gage added that there is an increased need for pediatric hospitalists to be adept at “managing acute psychiatric care and navigating the mental health care arena.”
There’s also the growing need for an understanding of neonatal abstinence and opioid withdrawal syndrome. “This is definitely a hot topic and one that most hospitalists must address today,” Dr. Gage said. “That wasn’t the case a decade ago.”
Hospital care for pediatrics today often means a team effort, including pediatric hospitalists, surgeons, mental health professionals, and others. Often missing from the picture today are primary care physicians, who instead refer a growing percentage of their patients to hospitalists. The pediatric hospitalist’s role has evolved and grown from what it was 10 years ago, as reflected in the competencies.
“We are very much coordinating care and collaborating today in ways we weren’t 10 years ago,” said Dr. Gage. “There’s a lot more attention on creating partnerships. While we may not always be the ones performing procedures, we will most likely take part in patient care, especially as surgeons step farther away from care outside of the OR.”
The field has also become more family centered, said Dr. Gage. “All of health care today is more astute about the participation of families in care,” she said. “We kept that in mind in developing the competencies.”
Also important in this set of competencies was the concept of high-value care using evidence-based medicine.
Into the field
How exactly the core competencies will be utilized from one hospital or setting to the next will vary, said Dr. Fisher. “For some sites, they can aid existing teaching programs, and they will most likely adapt their curriculum to address the new competencies, informing how they teach.”
Even in centers where there isn’t a formal academic role, teaching still occurs. “Pediatric hospitalists have roles on committees and projects, and giving a talk to respiratory therapists, having group meetings – these all involve teaching in some form,” Dr. Fisher said. “Most physicians will determine how they wish to insert the competencies into their own education, as well as use them to educate others.”
Regardless of how they may be used locally, Dr. Fisher anticipates that the entire pediatric hospitalist community will appreciate the updates. “The competencies address our rapidly changing health care environment,” she said. “We believe the field will benefit from the additions and changes.”
Indeed, the core competencies will help standardize and improve consistency of care across the board. Improved efficiencies, economics, and practices are all desired and expected outcomes from the release of the revised competencies.
To ensure that the changes to the competencies are highlighted in settings nationwide, the editors and associate editors hope to present about them at upcoming conferences, including at the SHM 2020 Annual Conference, the Pediatric Hospital Medicine conference, the Pediatric Academic Societies conference, and the American Pediatric Association.
“We want to present to as many venues as possible to bring people up to speed and ensure they are aware of the changes,” Dr. Teferi said. “We’ll be including workshops with visual aids, along with our presentations.”
While this update represents a 10-year evolution, the editors and the SHM Pediatric Special Interest Group do not have an exact time frame for when the core competencies will need another revision. As quickly as the profession is developing, it may be as few as 5 years, but may also be another full decade.
“Like most fields, we will continue to evolve as our roles become better defined and we gain more knowledge,” Dr. Maniscalco said. “The core competencies represent the field whether a senior pediatric hospitalist, a fellow, or an educator. They bring the field together and provide education for everyone. That’s their role.”
New core competencies reflect a decade of change
New core competencies reflect a decade of change
Over the past 10 years, much has changed in the world of pediatric hospital medicine. The annual national PHM conference sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA) is robust; textbooks and journal articles in the field abound; and networks and training in research, quality improvement, and education are successful and ongoing.
Much of this did not exist or was in its infancy back in 2010. Since then, it has grown and greatly evolved. In parallel, medicine and society have changed. These influences on health care, along with the growth of the field over time, prompted a review and revision of the 2010 PHM Core Competencies published by SHM. With support from the society, the Pediatric Hospital Medicine Special Interest Group launched the plan for revision of the PHM Core Competencies.
The selected editors included Sandra Gage, MD, PhD, SFHM, of Phoenix Children’s Hospital; Erin Stucky Fisher, MD, MHM, of UCSD/Rady Children’s Hospital in San Diego; Jennifer Maniscalco, MD, MPH, of Johns Hopkins All Children’s Hospital in St. Petersburg, Fla.; and Sofia Teferi, MD, SFHM, a pediatric hospitalist based at Bon Secours St. Mary’s Hospital in Richmond, Va. They began their work in 2017 along with six associate editors, meeting every 2 weeks via conference call, dividing the work accordingly.
Dr. Teferi served in a new and critical role as contributing editor. She described her role as a “sweeper” of sorts, bringing her unique perspective to the process. “The other three members are from academic settings, and I’m from a community setting, which is very different,” Dr. Teferi said. “I went through all the chapters to ensure they were inclusive of the community setting.”
According to Dr. Gage, “the purpose of the original PHM Core Competencies was to define the roles and responsibilities of a PHM practitioner. In the intervening 10 years, the field has changed and matured, and we have solidified our role since then.”
Today’s pediatric hospitalists, for instance, may coordinate care in EDs, provide inpatient consultations, engage or lead quality improvement programs, and teach. The demands for pediatric hospital care today go beyond the training provided in a standard pediatric residency. The core competencies need to provide the information necessary, therefore, to ensure pediatric hospital medicine is practiced at its most informed level.
A profession transformed
At the time of the first set of core competencies, there were over 2,500 members in three core societies in which pediatric hospitalists were members: the AAP, the APA, and SHM. As of 2017, those numbers have swelled as the care for children in the hospital setting has shifted away from these patients’ primary care providers.
The original core competencies included 54 chapters, designed to be used independent of the others. They provided a foundation for the creation of pediatric hospital medicine and served to standardize and improve inpatient training practices.
For the new core competencies, every single chapter was reviewed line by line, Dr. Gage said. Many chapters had content modified, and new chapters were added to reflect the evolution of the field and of medicine. “We added about 14 new chapters, adjusted the titles of others, and significantly changed the content of over half,” Dr. Gage explained. “They are fairly broad changes, related to the breadth of the practice today.”
Dr. Teferi noted that practitioners can use the updated competencies with additions to the service lines that have arisen since the last version. “These include areas like step down and newborn nursery, things that weren’t part of our portfolio 10 years ago,” she said. “This reflects the fact that often you’ll see a hospital leader who might want to add to a hospitalist’s portfolio of services because there is no one else to do it. Or maybe community pediatrics no longer want to treat babies, so we add that. The settings vary widely today and we need the competencies to address that.”
Practices within these settings can also vary widely. Teaching, palliative care, airway management, critical care, and anesthesia may all come into play, among other factors. Research opportunities throughout the field also continue to expand.
Dr. Fisher said that the editors and associate editors kept in mind the fact that not every hospital would have all the resources necessary at its fingertips. “The competencies must reflect the realities of the variety of community settings,” she said. “Also, on a national level, the majority of pediatric patients are not seen in a children’s hospital. Community sites are where pediatric hospitalists are not only advocates for care, but can be working with limited resources – the ‘lone soldiers.’ We wanted to make sure the competencies reflect that reality and environment community site or not; academic site or not; tertiary care site or not; rural or not – these are overlapping but independent considerations for all who practice pediatric hospital medicine – a Venn diagram, and the PHM core competencies try to attend to all of those.”
This made Dr. Teferi’s perspective all the more important. “While many, including other editors and associate editors, work in community sites, Dr. Teferi has this as her unique and sole focus. She brought a unique viewpoint to the table,” Dr. Fisher said.
A goal of the core competencies is to make it possible for a pediatric hospitalist to move to a different practice environment and still provide the same level of high-quality care. “It’s difficult but important to grasp the concepts and competencies of various settings,” Dr. Fisher said. “In this way, our competencies are a parallel model to the adult hospitalist competencies.”
The editors surveyed practitioners across the country to gather their input on content, and brought on topic experts to write the new chapters. “If we didn’t have an author for a specific chapter or area from the last set of competencies, we came to a consensus on who the new one should be,” Dr. Gage explained. “We looked for known and accepted experts in the field by reviewing the literature and conference lecturers at all major PHM meetings.”
Once the editors and associate editors worked with authors to refine their chapter(s), the chapters were sent to multiple external reviewers including subgroups of SHM, AAP, and APA, as well as a variety of other associations. They provided input that the editors and associate editors collated, reviewed, and incorporated according to consensus and discussion with the authors.
A preview
As far as the actual changes go, some of new chapters include four common clinical, two core skills, three specialized services, and five health care systems, with many others undergoing content changes, according to Dr. Gage.
Major considerations in developing the new competencies include the national trend of rising mental health issues among young patients. According to the AAP, over the last decade the number of young people aged 6-17 years requiring mental health care has risen from 9% to more than 14%. In outpatient settings, many pediatricians report that half or more of their visits are dedicated to these issues, a number that may spill out into the hospital setting as well.
According to Dr. Fisher, pediatric hospitalists today see increasing numbers of chronic and acute diseases accompanied by mental and behavioral health issues. “We wanted to underscore this complexity in the competencies,” she explained. “We needed to focus new attention on how to identify and treat children with behavioral or psychiatric diagnoses or needs.”
Other new areas of focus include infection care and antimicrobial stewardship. “We see kids on antibiotics in hospital settings and we need to focus on narrowing choices, decreasing use, and shortening duration,” Dr. Gage said.
Dr. Maniscalco said that, overall, the changes represent the evolution of the field. “Pediatric hospitalists are taking on far more patients with acute and complex issues,” she explained. “Our skill set is coming into focus.”
Dr. Gage added that there is an increased need for pediatric hospitalists to be adept at “managing acute psychiatric care and navigating the mental health care arena.”
There’s also the growing need for an understanding of neonatal abstinence and opioid withdrawal syndrome. “This is definitely a hot topic and one that most hospitalists must address today,” Dr. Gage said. “That wasn’t the case a decade ago.”
Hospital care for pediatrics today often means a team effort, including pediatric hospitalists, surgeons, mental health professionals, and others. Often missing from the picture today are primary care physicians, who instead refer a growing percentage of their patients to hospitalists. The pediatric hospitalist’s role has evolved and grown from what it was 10 years ago, as reflected in the competencies.
“We are very much coordinating care and collaborating today in ways we weren’t 10 years ago,” said Dr. Gage. “There’s a lot more attention on creating partnerships. While we may not always be the ones performing procedures, we will most likely take part in patient care, especially as surgeons step farther away from care outside of the OR.”
The field has also become more family centered, said Dr. Gage. “All of health care today is more astute about the participation of families in care,” she said. “We kept that in mind in developing the competencies.”
Also important in this set of competencies was the concept of high-value care using evidence-based medicine.
Into the field
How exactly the core competencies will be utilized from one hospital or setting to the next will vary, said Dr. Fisher. “For some sites, they can aid existing teaching programs, and they will most likely adapt their curriculum to address the new competencies, informing how they teach.”
Even in centers where there isn’t a formal academic role, teaching still occurs. “Pediatric hospitalists have roles on committees and projects, and giving a talk to respiratory therapists, having group meetings – these all involve teaching in some form,” Dr. Fisher said. “Most physicians will determine how they wish to insert the competencies into their own education, as well as use them to educate others.”
Regardless of how they may be used locally, Dr. Fisher anticipates that the entire pediatric hospitalist community will appreciate the updates. “The competencies address our rapidly changing health care environment,” she said. “We believe the field will benefit from the additions and changes.”
Indeed, the core competencies will help standardize and improve consistency of care across the board. Improved efficiencies, economics, and practices are all desired and expected outcomes from the release of the revised competencies.
To ensure that the changes to the competencies are highlighted in settings nationwide, the editors and associate editors hope to present about them at upcoming conferences, including at the SHM 2020 Annual Conference, the Pediatric Hospital Medicine conference, the Pediatric Academic Societies conference, and the American Pediatric Association.
“We want to present to as many venues as possible to bring people up to speed and ensure they are aware of the changes,” Dr. Teferi said. “We’ll be including workshops with visual aids, along with our presentations.”
While this update represents a 10-year evolution, the editors and the SHM Pediatric Special Interest Group do not have an exact time frame for when the core competencies will need another revision. As quickly as the profession is developing, it may be as few as 5 years, but may also be another full decade.
“Like most fields, we will continue to evolve as our roles become better defined and we gain more knowledge,” Dr. Maniscalco said. “The core competencies represent the field whether a senior pediatric hospitalist, a fellow, or an educator. They bring the field together and provide education for everyone. That’s their role.”
Over the past 10 years, much has changed in the world of pediatric hospital medicine. The annual national PHM conference sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA) is robust; textbooks and journal articles in the field abound; and networks and training in research, quality improvement, and education are successful and ongoing.
Much of this did not exist or was in its infancy back in 2010. Since then, it has grown and greatly evolved. In parallel, medicine and society have changed. These influences on health care, along with the growth of the field over time, prompted a review and revision of the 2010 PHM Core Competencies published by SHM. With support from the society, the Pediatric Hospital Medicine Special Interest Group launched the plan for revision of the PHM Core Competencies.
The selected editors included Sandra Gage, MD, PhD, SFHM, of Phoenix Children’s Hospital; Erin Stucky Fisher, MD, MHM, of UCSD/Rady Children’s Hospital in San Diego; Jennifer Maniscalco, MD, MPH, of Johns Hopkins All Children’s Hospital in St. Petersburg, Fla.; and Sofia Teferi, MD, SFHM, a pediatric hospitalist based at Bon Secours St. Mary’s Hospital in Richmond, Va. They began their work in 2017 along with six associate editors, meeting every 2 weeks via conference call, dividing the work accordingly.
Dr. Teferi served in a new and critical role as contributing editor. She described her role as a “sweeper” of sorts, bringing her unique perspective to the process. “The other three members are from academic settings, and I’m from a community setting, which is very different,” Dr. Teferi said. “I went through all the chapters to ensure they were inclusive of the community setting.”
According to Dr. Gage, “the purpose of the original PHM Core Competencies was to define the roles and responsibilities of a PHM practitioner. In the intervening 10 years, the field has changed and matured, and we have solidified our role since then.”
Today’s pediatric hospitalists, for instance, may coordinate care in EDs, provide inpatient consultations, engage or lead quality improvement programs, and teach. The demands for pediatric hospital care today go beyond the training provided in a standard pediatric residency. The core competencies need to provide the information necessary, therefore, to ensure pediatric hospital medicine is practiced at its most informed level.
A profession transformed
At the time of the first set of core competencies, there were over 2,500 members in three core societies in which pediatric hospitalists were members: the AAP, the APA, and SHM. As of 2017, those numbers have swelled as the care for children in the hospital setting has shifted away from these patients’ primary care providers.
The original core competencies included 54 chapters, designed to be used independent of the others. They provided a foundation for the creation of pediatric hospital medicine and served to standardize and improve inpatient training practices.
For the new core competencies, every single chapter was reviewed line by line, Dr. Gage said. Many chapters had content modified, and new chapters were added to reflect the evolution of the field and of medicine. “We added about 14 new chapters, adjusted the titles of others, and significantly changed the content of over half,” Dr. Gage explained. “They are fairly broad changes, related to the breadth of the practice today.”
Dr. Teferi noted that practitioners can use the updated competencies with additions to the service lines that have arisen since the last version. “These include areas like step down and newborn nursery, things that weren’t part of our portfolio 10 years ago,” she said. “This reflects the fact that often you’ll see a hospital leader who might want to add to a hospitalist’s portfolio of services because there is no one else to do it. Or maybe community pediatrics no longer want to treat babies, so we add that. The settings vary widely today and we need the competencies to address that.”
Practices within these settings can also vary widely. Teaching, palliative care, airway management, critical care, and anesthesia may all come into play, among other factors. Research opportunities throughout the field also continue to expand.
Dr. Fisher said that the editors and associate editors kept in mind the fact that not every hospital would have all the resources necessary at its fingertips. “The competencies must reflect the realities of the variety of community settings,” she said. “Also, on a national level, the majority of pediatric patients are not seen in a children’s hospital. Community sites are where pediatric hospitalists are not only advocates for care, but can be working with limited resources – the ‘lone soldiers.’ We wanted to make sure the competencies reflect that reality and environment community site or not; academic site or not; tertiary care site or not; rural or not – these are overlapping but independent considerations for all who practice pediatric hospital medicine – a Venn diagram, and the PHM core competencies try to attend to all of those.”
This made Dr. Teferi’s perspective all the more important. “While many, including other editors and associate editors, work in community sites, Dr. Teferi has this as her unique and sole focus. She brought a unique viewpoint to the table,” Dr. Fisher said.
A goal of the core competencies is to make it possible for a pediatric hospitalist to move to a different practice environment and still provide the same level of high-quality care. “It’s difficult but important to grasp the concepts and competencies of various settings,” Dr. Fisher said. “In this way, our competencies are a parallel model to the adult hospitalist competencies.”
The editors surveyed practitioners across the country to gather their input on content, and brought on topic experts to write the new chapters. “If we didn’t have an author for a specific chapter or area from the last set of competencies, we came to a consensus on who the new one should be,” Dr. Gage explained. “We looked for known and accepted experts in the field by reviewing the literature and conference lecturers at all major PHM meetings.”
Once the editors and associate editors worked with authors to refine their chapter(s), the chapters were sent to multiple external reviewers including subgroups of SHM, AAP, and APA, as well as a variety of other associations. They provided input that the editors and associate editors collated, reviewed, and incorporated according to consensus and discussion with the authors.
A preview
As far as the actual changes go, some of new chapters include four common clinical, two core skills, three specialized services, and five health care systems, with many others undergoing content changes, according to Dr. Gage.
Major considerations in developing the new competencies include the national trend of rising mental health issues among young patients. According to the AAP, over the last decade the number of young people aged 6-17 years requiring mental health care has risen from 9% to more than 14%. In outpatient settings, many pediatricians report that half or more of their visits are dedicated to these issues, a number that may spill out into the hospital setting as well.
According to Dr. Fisher, pediatric hospitalists today see increasing numbers of chronic and acute diseases accompanied by mental and behavioral health issues. “We wanted to underscore this complexity in the competencies,” she explained. “We needed to focus new attention on how to identify and treat children with behavioral or psychiatric diagnoses or needs.”
Other new areas of focus include infection care and antimicrobial stewardship. “We see kids on antibiotics in hospital settings and we need to focus on narrowing choices, decreasing use, and shortening duration,” Dr. Gage said.
Dr. Maniscalco said that, overall, the changes represent the evolution of the field. “Pediatric hospitalists are taking on far more patients with acute and complex issues,” she explained. “Our skill set is coming into focus.”
Dr. Gage added that there is an increased need for pediatric hospitalists to be adept at “managing acute psychiatric care and navigating the mental health care arena.”
There’s also the growing need for an understanding of neonatal abstinence and opioid withdrawal syndrome. “This is definitely a hot topic and one that most hospitalists must address today,” Dr. Gage said. “That wasn’t the case a decade ago.”
Hospital care for pediatrics today often means a team effort, including pediatric hospitalists, surgeons, mental health professionals, and others. Often missing from the picture today are primary care physicians, who instead refer a growing percentage of their patients to hospitalists. The pediatric hospitalist’s role has evolved and grown from what it was 10 years ago, as reflected in the competencies.
“We are very much coordinating care and collaborating today in ways we weren’t 10 years ago,” said Dr. Gage. “There’s a lot more attention on creating partnerships. While we may not always be the ones performing procedures, we will most likely take part in patient care, especially as surgeons step farther away from care outside of the OR.”
The field has also become more family centered, said Dr. Gage. “All of health care today is more astute about the participation of families in care,” she said. “We kept that in mind in developing the competencies.”
Also important in this set of competencies was the concept of high-value care using evidence-based medicine.
Into the field
How exactly the core competencies will be utilized from one hospital or setting to the next will vary, said Dr. Fisher. “For some sites, they can aid existing teaching programs, and they will most likely adapt their curriculum to address the new competencies, informing how they teach.”
Even in centers where there isn’t a formal academic role, teaching still occurs. “Pediatric hospitalists have roles on committees and projects, and giving a talk to respiratory therapists, having group meetings – these all involve teaching in some form,” Dr. Fisher said. “Most physicians will determine how they wish to insert the competencies into their own education, as well as use them to educate others.”
Regardless of how they may be used locally, Dr. Fisher anticipates that the entire pediatric hospitalist community will appreciate the updates. “The competencies address our rapidly changing health care environment,” she said. “We believe the field will benefit from the additions and changes.”
Indeed, the core competencies will help standardize and improve consistency of care across the board. Improved efficiencies, economics, and practices are all desired and expected outcomes from the release of the revised competencies.
To ensure that the changes to the competencies are highlighted in settings nationwide, the editors and associate editors hope to present about them at upcoming conferences, including at the SHM 2020 Annual Conference, the Pediatric Hospital Medicine conference, the Pediatric Academic Societies conference, and the American Pediatric Association.
“We want to present to as many venues as possible to bring people up to speed and ensure they are aware of the changes,” Dr. Teferi said. “We’ll be including workshops with visual aids, along with our presentations.”
While this update represents a 10-year evolution, the editors and the SHM Pediatric Special Interest Group do not have an exact time frame for when the core competencies will need another revision. As quickly as the profession is developing, it may be as few as 5 years, but may also be another full decade.
“Like most fields, we will continue to evolve as our roles become better defined and we gain more knowledge,” Dr. Maniscalco said. “The core competencies represent the field whether a senior pediatric hospitalist, a fellow, or an educator. They bring the field together and provide education for everyone. That’s their role.”