User login
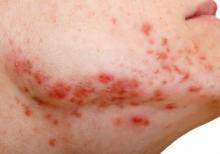
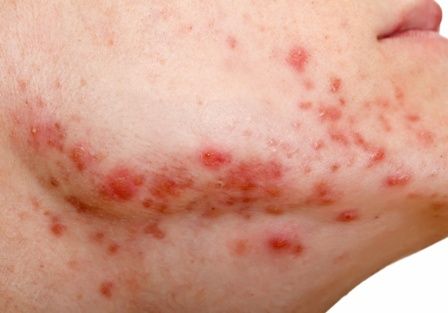
Large cohort study finds isotretinoin not associated with IBD
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Almonds may be a good diet option
according to researchers at the University of South Australia’s Alliance for Research in Exercise, Nutrition and Activity.
What to know
People who consume as few as 30-50 g of almonds, as opposed to an energy-equivalent carbohydrate snack, can lower their energy intake significantly at the subsequent meal.
People who eat almonds can experience changes in their appetite-regulating hormones that may contribute to less food intake.
Almond consumption can lower C-peptide responses, which can improve insulin sensitivity and reduce the risk of developing diabetes and cardiovascular disease.
Eating almonds can raise levels of glucose-dependent insulinotropic polypeptide glucagon, which can send satiety signals to the brain, and pancreatic polypeptide, which slows digestion, which may reduce food intake, supporting weight loss.
Almonds are high in protein, fiber, and unsaturated fatty acids, which may contribute to their satiating properties and help explain why fewer calories are consumed.
A version of this article originally appeared on Medscape.com.
This is a summary of the article “Acute Feeding With Almonds Compared to a Carbohydrate-Based Snack Improves Appetite-Regulating Hormones With No Effect on Self-reported Appetite Sensations: A Randomised Controlled Trial,” published in the European Journal of Nutrition on Oct. 11, 2022. The full article can be found on link.springer.com.
according to researchers at the University of South Australia’s Alliance for Research in Exercise, Nutrition and Activity.
What to know
People who consume as few as 30-50 g of almonds, as opposed to an energy-equivalent carbohydrate snack, can lower their energy intake significantly at the subsequent meal.
People who eat almonds can experience changes in their appetite-regulating hormones that may contribute to less food intake.
Almond consumption can lower C-peptide responses, which can improve insulin sensitivity and reduce the risk of developing diabetes and cardiovascular disease.
Eating almonds can raise levels of glucose-dependent insulinotropic polypeptide glucagon, which can send satiety signals to the brain, and pancreatic polypeptide, which slows digestion, which may reduce food intake, supporting weight loss.
Almonds are high in protein, fiber, and unsaturated fatty acids, which may contribute to their satiating properties and help explain why fewer calories are consumed.
A version of this article originally appeared on Medscape.com.
This is a summary of the article “Acute Feeding With Almonds Compared to a Carbohydrate-Based Snack Improves Appetite-Regulating Hormones With No Effect on Self-reported Appetite Sensations: A Randomised Controlled Trial,” published in the European Journal of Nutrition on Oct. 11, 2022. The full article can be found on link.springer.com.
according to researchers at the University of South Australia’s Alliance for Research in Exercise, Nutrition and Activity.
What to know
People who consume as few as 30-50 g of almonds, as opposed to an energy-equivalent carbohydrate snack, can lower their energy intake significantly at the subsequent meal.
People who eat almonds can experience changes in their appetite-regulating hormones that may contribute to less food intake.
Almond consumption can lower C-peptide responses, which can improve insulin sensitivity and reduce the risk of developing diabetes and cardiovascular disease.
Eating almonds can raise levels of glucose-dependent insulinotropic polypeptide glucagon, which can send satiety signals to the brain, and pancreatic polypeptide, which slows digestion, which may reduce food intake, supporting weight loss.
Almonds are high in protein, fiber, and unsaturated fatty acids, which may contribute to their satiating properties and help explain why fewer calories are consumed.
A version of this article originally appeared on Medscape.com.
This is a summary of the article “Acute Feeding With Almonds Compared to a Carbohydrate-Based Snack Improves Appetite-Regulating Hormones With No Effect on Self-reported Appetite Sensations: A Randomised Controlled Trial,” published in the European Journal of Nutrition on Oct. 11, 2022. The full article can be found on link.springer.com.
FROM THE EUROPEAN JOURNAL OF NUTRITION
Colorectal cancer treatment outcomes in older adults
A phase 2, multi-institutional feasibility study found a completion rate of 67.3%, while a prospective study found that completion was associated with improved disease-free survival.
Both studies were presented in January at the ASCO Gastrointestinal Cancers Symposium 2023.
In HiSCO-04, Japanese researchers found that of 64 older patients with stage 3A colorectal cancer who underwent adjuvant chemotherapy, 53% completed the treatment with an improvement in disease-free survival. Patients who completed adjuvant chemotherapy had better disease-free survival (P = .03), while the survival was lower among those who did not receive adjuvant chemotherapy, and lowest among those who discontinued adjuvant chemotherapy.
“The results showed that adjuvant chemotherapy is not always recommended for elderly patients, and that patients who are able to complete treatment may have a better prognosis for survival. However, the results do not indicate which patients are unable to complete chemotherapy, and it will be necessary to identify patients who are intolerant of chemotherapy,” said the study’s lead author Manabu Shimomura, MD, PhD, an assistant professor of gastroenterological and transplant surgery at the Hiroshima University Graduate School of Biomedical and Health Sciences in Japan.
The study, which was conducted between 2013 and 2021, enrolled 214 patients (99 men, 115 women, 80-101 years old) who were in stage 3 cancer (27 cases 3A, 158 cases 3B, and 29 cases 3C). A total of 41 patients were ineligible for chemotherapy. Of the remaining patients, 65 received adjuvant chemotherapy and 108 did not receive adjuvant chemotherapy.
The 3-year disease-free survival was 63.6%, the 3-year overall survival was 76.9%, and the 3-year relapse-free survival was 63.1%. Thirty-six patients died because of colorectal cancer, and 30 patients died of other causes. There was recurrence in 58 cases and secondary cancers were observed in 17 cases during the 42.5 months–long follow-up period.
There were few reports of serious adverse events, but some cases of treatment discontinuation were because of adverse events.
In a second study presented by Dr. Shimomura’s group, called HiSCO-03, 65 patients (33 female) underwent curative resection and received five courses of uracil-tegafur and leucovorin (UFT/LV).
The completion rate of 67.3% had a 95% lower bound of 54.9%, which were lower than the predefined thresholds of 75% completion and a lower bound of 60%. “Based on the results of a previous (ACTS-CC phase III) study, we set the expected value of UFT/LV therapy in patients over 80 years of age at 75% and the threshold at 60%. Since the target age group of previous study was 75 years or younger, we concluded from the results of the current study that UFT/LV therapy is less well tolerated in patients 80 years of age and older than in patients 75 years of age and younger,” Dr. Shimomura said.
The treatment completion rate trended higher in males than females (77.6% versus 57.2%; P = .06) and performance status of 0 versus 1 or 2 (74.3% versus 58.9%; P = .10). The most common adverse events were anorexia (33.8%), diarrhea (30.8%), and anemia (24.6%). The median relative dose intensity was 84% for UFT and 100% for LV.
The challenges of treating older patients
If and how older patients with colorectal cancer should be treated is not clear cut. While 20% of patients in the United States who have colorectal cancer are over 80 years old, each case should be evaluated individually, experts say.
Writing in a 2015 review of colorectal cancer treatment in older adults, Monica Millan, MD, PhD, of Joan XXIII University Hospital, Tarragona, Spain, and colleagues, wrote that physiological heterogeneity and coexisting medical conditions make treating older patients with colorectal cancer challenging.
“Age in itself should not be an exclusion criterion for radical treatment, but there will be many elderly patients that will not tolerate or respond well to standard therapies. These patients need to be properly assessed before proposing treatment, and a tailored, individualized approach should be offered in a multidisciplinary setting,” wrote Dr. Millan, who is a colorectal surgeon.
The authors suggest that older patients who are fit could be treated similarly to younger patients, but there remain uncertainties about how to proceed in frail older adults with comorbidities.
“Most elderly patients with cancer will have priorities besides simply prolonging their lives. Surveys have found that their top concerns include avoiding suffering, strengthening relationships with family and friends, being mentally aware, not being a burden on others, and achieving a sense that their life is complete. The treatment plan should be comprehensive: cancer-specific treatment, symptom-specific treatment, supportive treatment modalities, and end-of-life care,” they wrote.
The U.S. Preventive Services Task Force recommends colorectal cancer screening for men and women who are between 45 and 75 years old; however, screening for patients between 76 and 85 years old should be done on a case-by-case basis based on a patient’s overall health, screening history, and the patient’s preferences.
Colorectal cancer incidence rates have been declining since the mid-1980s because of an increase in screening among adults 50 years and older, according to the American Cancer Society. Likewise, mortality rates have dropped from 29.2% in 1970 to 12.6% in 2020 – mostly because of screening.
Dr. Shimomura has no relevant financial disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A phase 2, multi-institutional feasibility study found a completion rate of 67.3%, while a prospective study found that completion was associated with improved disease-free survival.
Both studies were presented in January at the ASCO Gastrointestinal Cancers Symposium 2023.
In HiSCO-04, Japanese researchers found that of 64 older patients with stage 3A colorectal cancer who underwent adjuvant chemotherapy, 53% completed the treatment with an improvement in disease-free survival. Patients who completed adjuvant chemotherapy had better disease-free survival (P = .03), while the survival was lower among those who did not receive adjuvant chemotherapy, and lowest among those who discontinued adjuvant chemotherapy.
“The results showed that adjuvant chemotherapy is not always recommended for elderly patients, and that patients who are able to complete treatment may have a better prognosis for survival. However, the results do not indicate which patients are unable to complete chemotherapy, and it will be necessary to identify patients who are intolerant of chemotherapy,” said the study’s lead author Manabu Shimomura, MD, PhD, an assistant professor of gastroenterological and transplant surgery at the Hiroshima University Graduate School of Biomedical and Health Sciences in Japan.
The study, which was conducted between 2013 and 2021, enrolled 214 patients (99 men, 115 women, 80-101 years old) who were in stage 3 cancer (27 cases 3A, 158 cases 3B, and 29 cases 3C). A total of 41 patients were ineligible for chemotherapy. Of the remaining patients, 65 received adjuvant chemotherapy and 108 did not receive adjuvant chemotherapy.
The 3-year disease-free survival was 63.6%, the 3-year overall survival was 76.9%, and the 3-year relapse-free survival was 63.1%. Thirty-six patients died because of colorectal cancer, and 30 patients died of other causes. There was recurrence in 58 cases and secondary cancers were observed in 17 cases during the 42.5 months–long follow-up period.
There were few reports of serious adverse events, but some cases of treatment discontinuation were because of adverse events.
In a second study presented by Dr. Shimomura’s group, called HiSCO-03, 65 patients (33 female) underwent curative resection and received five courses of uracil-tegafur and leucovorin (UFT/LV).
The completion rate of 67.3% had a 95% lower bound of 54.9%, which were lower than the predefined thresholds of 75% completion and a lower bound of 60%. “Based on the results of a previous (ACTS-CC phase III) study, we set the expected value of UFT/LV therapy in patients over 80 years of age at 75% and the threshold at 60%. Since the target age group of previous study was 75 years or younger, we concluded from the results of the current study that UFT/LV therapy is less well tolerated in patients 80 years of age and older than in patients 75 years of age and younger,” Dr. Shimomura said.
The treatment completion rate trended higher in males than females (77.6% versus 57.2%; P = .06) and performance status of 0 versus 1 or 2 (74.3% versus 58.9%; P = .10). The most common adverse events were anorexia (33.8%), diarrhea (30.8%), and anemia (24.6%). The median relative dose intensity was 84% for UFT and 100% for LV.
The challenges of treating older patients
If and how older patients with colorectal cancer should be treated is not clear cut. While 20% of patients in the United States who have colorectal cancer are over 80 years old, each case should be evaluated individually, experts say.
Writing in a 2015 review of colorectal cancer treatment in older adults, Monica Millan, MD, PhD, of Joan XXIII University Hospital, Tarragona, Spain, and colleagues, wrote that physiological heterogeneity and coexisting medical conditions make treating older patients with colorectal cancer challenging.
“Age in itself should not be an exclusion criterion for radical treatment, but there will be many elderly patients that will not tolerate or respond well to standard therapies. These patients need to be properly assessed before proposing treatment, and a tailored, individualized approach should be offered in a multidisciplinary setting,” wrote Dr. Millan, who is a colorectal surgeon.
The authors suggest that older patients who are fit could be treated similarly to younger patients, but there remain uncertainties about how to proceed in frail older adults with comorbidities.
“Most elderly patients with cancer will have priorities besides simply prolonging their lives. Surveys have found that their top concerns include avoiding suffering, strengthening relationships with family and friends, being mentally aware, not being a burden on others, and achieving a sense that their life is complete. The treatment plan should be comprehensive: cancer-specific treatment, symptom-specific treatment, supportive treatment modalities, and end-of-life care,” they wrote.
The U.S. Preventive Services Task Force recommends colorectal cancer screening for men and women who are between 45 and 75 years old; however, screening for patients between 76 and 85 years old should be done on a case-by-case basis based on a patient’s overall health, screening history, and the patient’s preferences.
Colorectal cancer incidence rates have been declining since the mid-1980s because of an increase in screening among adults 50 years and older, according to the American Cancer Society. Likewise, mortality rates have dropped from 29.2% in 1970 to 12.6% in 2020 – mostly because of screening.
Dr. Shimomura has no relevant financial disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A phase 2, multi-institutional feasibility study found a completion rate of 67.3%, while a prospective study found that completion was associated with improved disease-free survival.
Both studies were presented in January at the ASCO Gastrointestinal Cancers Symposium 2023.
In HiSCO-04, Japanese researchers found that of 64 older patients with stage 3A colorectal cancer who underwent adjuvant chemotherapy, 53% completed the treatment with an improvement in disease-free survival. Patients who completed adjuvant chemotherapy had better disease-free survival (P = .03), while the survival was lower among those who did not receive adjuvant chemotherapy, and lowest among those who discontinued adjuvant chemotherapy.
“The results showed that adjuvant chemotherapy is not always recommended for elderly patients, and that patients who are able to complete treatment may have a better prognosis for survival. However, the results do not indicate which patients are unable to complete chemotherapy, and it will be necessary to identify patients who are intolerant of chemotherapy,” said the study’s lead author Manabu Shimomura, MD, PhD, an assistant professor of gastroenterological and transplant surgery at the Hiroshima University Graduate School of Biomedical and Health Sciences in Japan.
The study, which was conducted between 2013 and 2021, enrolled 214 patients (99 men, 115 women, 80-101 years old) who were in stage 3 cancer (27 cases 3A, 158 cases 3B, and 29 cases 3C). A total of 41 patients were ineligible for chemotherapy. Of the remaining patients, 65 received adjuvant chemotherapy and 108 did not receive adjuvant chemotherapy.
The 3-year disease-free survival was 63.6%, the 3-year overall survival was 76.9%, and the 3-year relapse-free survival was 63.1%. Thirty-six patients died because of colorectal cancer, and 30 patients died of other causes. There was recurrence in 58 cases and secondary cancers were observed in 17 cases during the 42.5 months–long follow-up period.
There were few reports of serious adverse events, but some cases of treatment discontinuation were because of adverse events.
In a second study presented by Dr. Shimomura’s group, called HiSCO-03, 65 patients (33 female) underwent curative resection and received five courses of uracil-tegafur and leucovorin (UFT/LV).
The completion rate of 67.3% had a 95% lower bound of 54.9%, which were lower than the predefined thresholds of 75% completion and a lower bound of 60%. “Based on the results of a previous (ACTS-CC phase III) study, we set the expected value of UFT/LV therapy in patients over 80 years of age at 75% and the threshold at 60%. Since the target age group of previous study was 75 years or younger, we concluded from the results of the current study that UFT/LV therapy is less well tolerated in patients 80 years of age and older than in patients 75 years of age and younger,” Dr. Shimomura said.
The treatment completion rate trended higher in males than females (77.6% versus 57.2%; P = .06) and performance status of 0 versus 1 or 2 (74.3% versus 58.9%; P = .10). The most common adverse events were anorexia (33.8%), diarrhea (30.8%), and anemia (24.6%). The median relative dose intensity was 84% for UFT and 100% for LV.
The challenges of treating older patients
If and how older patients with colorectal cancer should be treated is not clear cut. While 20% of patients in the United States who have colorectal cancer are over 80 years old, each case should be evaluated individually, experts say.
Writing in a 2015 review of colorectal cancer treatment in older adults, Monica Millan, MD, PhD, of Joan XXIII University Hospital, Tarragona, Spain, and colleagues, wrote that physiological heterogeneity and coexisting medical conditions make treating older patients with colorectal cancer challenging.
“Age in itself should not be an exclusion criterion for radical treatment, but there will be many elderly patients that will not tolerate or respond well to standard therapies. These patients need to be properly assessed before proposing treatment, and a tailored, individualized approach should be offered in a multidisciplinary setting,” wrote Dr. Millan, who is a colorectal surgeon.
The authors suggest that older patients who are fit could be treated similarly to younger patients, but there remain uncertainties about how to proceed in frail older adults with comorbidities.
“Most elderly patients with cancer will have priorities besides simply prolonging their lives. Surveys have found that their top concerns include avoiding suffering, strengthening relationships with family and friends, being mentally aware, not being a burden on others, and achieving a sense that their life is complete. The treatment plan should be comprehensive: cancer-specific treatment, symptom-specific treatment, supportive treatment modalities, and end-of-life care,” they wrote.
The U.S. Preventive Services Task Force recommends colorectal cancer screening for men and women who are between 45 and 75 years old; however, screening for patients between 76 and 85 years old should be done on a case-by-case basis based on a patient’s overall health, screening history, and the patient’s preferences.
Colorectal cancer incidence rates have been declining since the mid-1980s because of an increase in screening among adults 50 years and older, according to the American Cancer Society. Likewise, mortality rates have dropped from 29.2% in 1970 to 12.6% in 2020 – mostly because of screening.
Dr. Shimomura has no relevant financial disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
FROM ASCO GI 2023
FDA OKs sacituzumab govitecan for HR+ metastatic breast cancer
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
Immunotherapy with antibiotics doesn’t worsen biliary tract cancer outcomes
according to a new analysis of the landmark TOPAZ-1 clinical trial.
The findings, released at the ASCO Gastrointestinal Cancers Symposium 2023, suggest that “people with advanced biliary tract cancer can safely be treated with antibiotics while still benefiting from treatment with durvalumab plus chemotherapy,” said lead author Aiwu Ruth He, MD, PhD, a gastrointestinal oncologist with MedStar Georgetown University Hospital, Washington.
Antibiotic use during immune checkpoint inhibitor therapy has been associated with poorer outcomes. A review of 12 studies published in Frontiers in Oncology found that antibiotic use was associated with worse progression-free and overall survival.
“Patients with biliary tract cancer have the increased risk of biliary tract infection as the result of biliary tract obstruction, and they often receive antibiotics,” Dr. He said.
A 2020 report in eCancer suggested that antibiotics may disrupt gut bacteria and, as a result, interfere with the immune system’s responsiveness. “It has been a consensus that the use of broad-spectrum antibiotics should be avoided during the use of immunotherapy whenever possible,” the report authors wrote. “In addition, antibiotics should be prescribed only when properly indicated.”
However, cutting down on antibiotic use may be especially difficult in cancer patients since they frequently suffer from infections. “An antibiotic-resistant bacterial infection may cause serious issues for a cancer patient, who likely already has a suppressed immune system,” according to a 2017 information sheet posted by the Cancer Treatment Centers of America. “Chemotherapy may cause neutropenia, a reduction of white blood cells that help fight infections and viruses. Radiation therapy may damage the skin and cause irritation and wounds. Immunotherapy or targeted therapy drugs may trigger side effects that may lead to infections. Incisions from surgery or to insert ports or catheters may be vulnerable to infections.”
The new study
For the new subgroup analysis, researchers analyzed data from the phase 3 TOPAZ-1 clinical trial, which was a double-blinded analysis of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. The previously reported main findings from the study were positive with a median overall survival of 12.8 months in the durvalumab arm versus 11.5 months in the placebo arm (hazard ratio, 0.80; P = .021). These findings contributed to the Food and Drug Administration’s decision in 2022 to approve the treatment for use in locally advanced or metastatic biliary tract cancer.
Of 341 patients who received durvalumab treatment, 167 also took antibiotics. The median overall survival in the antibiotic and nonantibiotic groups were similar at 12.6 months (95% confidence interval, 9.7-14.8 months) and 13 months (95% CI, 10.8-14.7 months), respectively. Median progression-free survival was 7.3 months (95% CI, 6.5-7.7 months) and 7.2 months (95% CI, 5.9-7.4 months), respectively.
“The results support that advanced patients’ risk of death, and the risk that their cancer would grow, spread, or get worse, was not meaningfully different between patients who used antibiotics and those who did not use antibiotics at the same time as they were receiving durvalumab-based treatment,” Dr. He said. “The result is not surprising to me since it is not clear to me how and why antibiotics may affect the effectiveness of immunotherapy.”
Moving forward, she said, “additional studies are needed to further investigator the relationship between antibiotics use and effectiveness of immunotherapy. We need to understand why use of antibiotics during treatment with immunotherapy is correlated with poor outcomes in some circumstances but not in other circumstances.”
The study was funded by AstraZeneca. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
according to a new analysis of the landmark TOPAZ-1 clinical trial.
The findings, released at the ASCO Gastrointestinal Cancers Symposium 2023, suggest that “people with advanced biliary tract cancer can safely be treated with antibiotics while still benefiting from treatment with durvalumab plus chemotherapy,” said lead author Aiwu Ruth He, MD, PhD, a gastrointestinal oncologist with MedStar Georgetown University Hospital, Washington.
Antibiotic use during immune checkpoint inhibitor therapy has been associated with poorer outcomes. A review of 12 studies published in Frontiers in Oncology found that antibiotic use was associated with worse progression-free and overall survival.
“Patients with biliary tract cancer have the increased risk of biliary tract infection as the result of biliary tract obstruction, and they often receive antibiotics,” Dr. He said.
A 2020 report in eCancer suggested that antibiotics may disrupt gut bacteria and, as a result, interfere with the immune system’s responsiveness. “It has been a consensus that the use of broad-spectrum antibiotics should be avoided during the use of immunotherapy whenever possible,” the report authors wrote. “In addition, antibiotics should be prescribed only when properly indicated.”
However, cutting down on antibiotic use may be especially difficult in cancer patients since they frequently suffer from infections. “An antibiotic-resistant bacterial infection may cause serious issues for a cancer patient, who likely already has a suppressed immune system,” according to a 2017 information sheet posted by the Cancer Treatment Centers of America. “Chemotherapy may cause neutropenia, a reduction of white blood cells that help fight infections and viruses. Radiation therapy may damage the skin and cause irritation and wounds. Immunotherapy or targeted therapy drugs may trigger side effects that may lead to infections. Incisions from surgery or to insert ports or catheters may be vulnerable to infections.”
The new study
For the new subgroup analysis, researchers analyzed data from the phase 3 TOPAZ-1 clinical trial, which was a double-blinded analysis of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. The previously reported main findings from the study were positive with a median overall survival of 12.8 months in the durvalumab arm versus 11.5 months in the placebo arm (hazard ratio, 0.80; P = .021). These findings contributed to the Food and Drug Administration’s decision in 2022 to approve the treatment for use in locally advanced or metastatic biliary tract cancer.
Of 341 patients who received durvalumab treatment, 167 also took antibiotics. The median overall survival in the antibiotic and nonantibiotic groups were similar at 12.6 months (95% confidence interval, 9.7-14.8 months) and 13 months (95% CI, 10.8-14.7 months), respectively. Median progression-free survival was 7.3 months (95% CI, 6.5-7.7 months) and 7.2 months (95% CI, 5.9-7.4 months), respectively.
“The results support that advanced patients’ risk of death, and the risk that their cancer would grow, spread, or get worse, was not meaningfully different between patients who used antibiotics and those who did not use antibiotics at the same time as they were receiving durvalumab-based treatment,” Dr. He said. “The result is not surprising to me since it is not clear to me how and why antibiotics may affect the effectiveness of immunotherapy.”
Moving forward, she said, “additional studies are needed to further investigator the relationship between antibiotics use and effectiveness of immunotherapy. We need to understand why use of antibiotics during treatment with immunotherapy is correlated with poor outcomes in some circumstances but not in other circumstances.”
The study was funded by AstraZeneca. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
according to a new analysis of the landmark TOPAZ-1 clinical trial.
The findings, released at the ASCO Gastrointestinal Cancers Symposium 2023, suggest that “people with advanced biliary tract cancer can safely be treated with antibiotics while still benefiting from treatment with durvalumab plus chemotherapy,” said lead author Aiwu Ruth He, MD, PhD, a gastrointestinal oncologist with MedStar Georgetown University Hospital, Washington.
Antibiotic use during immune checkpoint inhibitor therapy has been associated with poorer outcomes. A review of 12 studies published in Frontiers in Oncology found that antibiotic use was associated with worse progression-free and overall survival.
“Patients with biliary tract cancer have the increased risk of biliary tract infection as the result of biliary tract obstruction, and they often receive antibiotics,” Dr. He said.
A 2020 report in eCancer suggested that antibiotics may disrupt gut bacteria and, as a result, interfere with the immune system’s responsiveness. “It has been a consensus that the use of broad-spectrum antibiotics should be avoided during the use of immunotherapy whenever possible,” the report authors wrote. “In addition, antibiotics should be prescribed only when properly indicated.”
However, cutting down on antibiotic use may be especially difficult in cancer patients since they frequently suffer from infections. “An antibiotic-resistant bacterial infection may cause serious issues for a cancer patient, who likely already has a suppressed immune system,” according to a 2017 information sheet posted by the Cancer Treatment Centers of America. “Chemotherapy may cause neutropenia, a reduction of white blood cells that help fight infections and viruses. Radiation therapy may damage the skin and cause irritation and wounds. Immunotherapy or targeted therapy drugs may trigger side effects that may lead to infections. Incisions from surgery or to insert ports or catheters may be vulnerable to infections.”
The new study
For the new subgroup analysis, researchers analyzed data from the phase 3 TOPAZ-1 clinical trial, which was a double-blinded analysis of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. The previously reported main findings from the study were positive with a median overall survival of 12.8 months in the durvalumab arm versus 11.5 months in the placebo arm (hazard ratio, 0.80; P = .021). These findings contributed to the Food and Drug Administration’s decision in 2022 to approve the treatment for use in locally advanced or metastatic biliary tract cancer.
Of 341 patients who received durvalumab treatment, 167 also took antibiotics. The median overall survival in the antibiotic and nonantibiotic groups were similar at 12.6 months (95% confidence interval, 9.7-14.8 months) and 13 months (95% CI, 10.8-14.7 months), respectively. Median progression-free survival was 7.3 months (95% CI, 6.5-7.7 months) and 7.2 months (95% CI, 5.9-7.4 months), respectively.
“The results support that advanced patients’ risk of death, and the risk that their cancer would grow, spread, or get worse, was not meaningfully different between patients who used antibiotics and those who did not use antibiotics at the same time as they were receiving durvalumab-based treatment,” Dr. He said. “The result is not surprising to me since it is not clear to me how and why antibiotics may affect the effectiveness of immunotherapy.”
Moving forward, she said, “additional studies are needed to further investigator the relationship between antibiotics use and effectiveness of immunotherapy. We need to understand why use of antibiotics during treatment with immunotherapy is correlated with poor outcomes in some circumstances but not in other circumstances.”
The study was funded by AstraZeneca. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
FROM ASCO GI 2023
Race and geography tied to breast cancer care delays
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER
Periorbital Orange Spots
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
A 63-year-old White man with a history of melanoma presented to our dermatology clinic for evaluation of gradually worsening yellow discoloration around the eyes of 2 years’ duration. Physical examination revealed periorbital yellow-orange patches (top). The discolorations were nonelevated and nonpalpable. Dermoscopy revealed yellow blotches with sparing of the hair follicles (bottom). The remainder of the skin examination was unremarkable.
A White male presented with a 1½-year history of a progressive hypoesthetic annular, hyperpigmented plaque on the upper arm
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
A 44-year-old White male presented with a 1½-year history of a progressive hypoesthetic annular, mildly hyperpigmented plaque on the left posterior upper arm.
He denied pruritus, pain, or systemic symptoms including weight loss, visual changes, cough, dyspnea, and abdominal pain. He also denied any paresthesia or weakness. On physical examination, there is a subtle, solitary 4-cm annular skin-colored thin plaque on the patient's left posterior upper arm (Figure 1).
Punch biopsy of the lesion was performed, and the histopathological findings are illustrated in Figure 2.
Advice on antibiotics for kids during shortages
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Despite limits, COVID vaccines protect CLL patients
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
FROM BLOOD ADVANCES