User login
Commenting on weight’s not rude. It’s dangerous.
It was the start of the fall semester of my sophomore year of college.
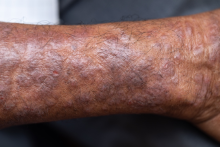
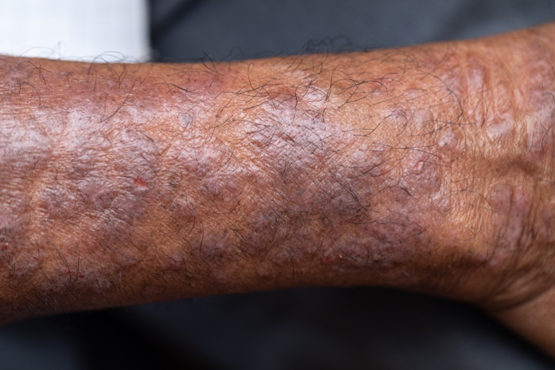
At my small women’s college, the previous semester’s gossip had been about our classmate, S*. She had gone from being very thin to noticeably gaining a lot of weight in a few months. The rumors were that S was pregnant and gave birth over summer break. As a busy biology premed major, this was my first time hearing the news. So when I saw her standing in the hallway, back to her previous weight, I was excited for her.
In true extravert fashion, I commented on the baby and her new size. But no sooner had the words left my mouth than I regretted them.
The hall grew awkwardly silent as S’s face flushed and she asked, “Excuse me?!” Instantly I knew that the rumors weren’t true.
Thankfully, at that moment, the classroom opened and we walked in. Whew! After class, S asked if we could talk. She explained that she had a thyroid tumor and struggled to adjust to the treatments, which caused her weight fluctuations. She had never been pregnant.
My awkward statement had been the first time anyone on campus had directly mentioned her weight, though she suspected that people were talking about her. We became fast friends after this rocky beginning. Although we lost touch after college, S taught me an invaluable lesson about making assumptions about people’s weight: Ask before you assume.
Now, years later, as an internist and obesity specialist, this lesson continues to be reinforced daily.
In daily life, comments about weight can be perceived as rude. In the clinical setting, however, assumptions about weight are a form of weight bias. Weight bias can lead to weight stigma and even be dangerous to health care.
Let’s discuss the insidious influence of weight bias in health care through two commonly used phrases and then look at a few solutions to address weight bias in health care individually and systematically.
Common weight bias assumptions
“Great job, you lost weight!” In checking your patient’s vital signs, you notice that this patient with obesity has a significant weight change. You congratulate them upon entering the room. Unfortunately, their weight loss was a result of minimal eating after losing a loved one. This isn’t healthy weight loss. One of the adverse effects of weight bias is that it infers that weight loss is always a good thing, especially in people with larger bodies. This is a dangerous presumption. Let’s remember that the body favors fat storage, hence why “unintentional weight loss” is a recognized medical condition prompting evaluation. We have to be careful not to celebrate weight loss “at all costs,” such as fad diets that haven’t been shown to improve health outcomes.
Furthermore, patients who lose weight quickly (more than 4-8 lb/month) require closer follow-up and evaluation for secondary causes of weight loss. Patients may lose weight at a faster rate with the new antiobesity medications, but clinicians still should ensure that age-appropriate health maintenance screening is done and be vigilant for secondary causes of weight changes.
“Have you tried losing weight yet?” Three times. That’s how many times Chanté Burkett went to her doctor about her painful, enlarging firm stomach. She was advised to continue working on weight loss, which she did diligently. But Ms. Burkett’s abdomen kept growing and her concerns were dismissed. A visit to urgent care and a CT scan revealed that Ms. Burkett’s excess abdominal “fat” was a 13-lb mucinous cystadenoma. Sadly, cases like hers aren’t rare, isolated events. Weight bias can cause anchoring on one diagnosis, preventing consideration of other diagnostic possibilities. Even worse, anchoring will lead to the wrong intervention, such as prescribing weight loss for presumed increased adiposity instead of ordering the appropriate testing.
It’s also essential to recognize that, even if someone does have the disease of obesity, weight loss isn’t the solution to every medical concern. Even if weight loss is helpful, other, more pressing treatments may still be necessary. Telling a person with obesity who has an acute complaint to “just lose weight” is comparable to telling a patient with coronary artery disease who presents with an 80% vessel occlusion and chest pain to follow a low-fat diet. In both cases, you need to address the acute concern appropriately, then focus on the chronic treatment.
Ways to reduce clinical weight bias
How do you reduce clinical weight bias?
Ask, don’t assume. The information from the scale is simply data. Instead of judging it positively or negatively and creating a story, ask the patient. An unbiased way to approach the conversation is to say, “Great to see you. You seem [positive adjective of choice]. How have you been?” Wait until the vitals section to objectively discuss weight unless the patient offers the discussion earlier or their chief complaint lists a weight-related concern.
Order necessary tests to evaluate weight. Weight is the vital sign that people wear externally, so we feel that we can readily interpret it without any further assessment. However, resist the urge to interpret scale data without context. Keeping an open mind helps prevent anchoring and missing critical clues in the clinical history.
Address weight changes effectively. Sometimes there is an indication to prescribe weight loss as part of the treatment plan. However, remember that weight loss isn’t simply “calories in vs. calories out.” Obesity is a complex medical disease that requires a multimodal treatment approach. As clinicians, we have access to the most powerful tools for weight loss. Unfortunately, weight bias contributes to limited prescribing of metabolic medications (“antiobesity medications” or AOMs). In addition, systemic weight bias prevents insurance coverage of AOMs. The Treat and Reduce Obesity Act has been introduced into Congress to help improve life-transforming access to AOMs.
Acknowledge your bias. Our experiences make us all susceptible to bias. The Harvard Weight Implicit Association Test is free and a helpful way to assess your level of weight bias. I take it annually to ensure that I remain objective in my practice.
Addressing weight bias needs to extend beyond the individual level.
Systemically, health care needs to address the following:
Language. Use people-centered language. For example, “People aren’t obese. They have obesity.”
Accessibility. Health care settings must be comfortable and accessible for people of all sizes. Furthermore, improvements to access the services that comprehensive obesity care requires, such as AOMs, bariatric procedures and bariatric surgery, mental health care, nutrition, fitness specialists, health coaches, and more, are needed.
Education. Medical students and trainees have to learn the newest obesity science and know how to treat obesity effectively. Acknowledge and address biased tools. Recent data have shown that some of our screening tools, such as body mass index, have inherent bias. It’s time to focus on using improved diagnostic tools and personalized treatments.
We are at a pivotal time in our scientific understanding of body weight regulation and the disease of obesity. Clinical weight bias is primarily rooted in flawed science influenced by biased cultural norms and other forms of discrimination, such as racial and gender bias. We must move past assumptions to give our patients the optimal individualized care they need. So next time you observe a weight change, instead of commenting on their weight, say, “Great to see you! How have you been?”
S*: Initial has been changed to protect privacy.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness”, was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
It was the start of the fall semester of my sophomore year of college.
At my small women’s college, the previous semester’s gossip had been about our classmate, S*. She had gone from being very thin to noticeably gaining a lot of weight in a few months. The rumors were that S was pregnant and gave birth over summer break. As a busy biology premed major, this was my first time hearing the news. So when I saw her standing in the hallway, back to her previous weight, I was excited for her.
In true extravert fashion, I commented on the baby and her new size. But no sooner had the words left my mouth than I regretted them.
The hall grew awkwardly silent as S’s face flushed and she asked, “Excuse me?!” Instantly I knew that the rumors weren’t true.
Thankfully, at that moment, the classroom opened and we walked in. Whew! After class, S asked if we could talk. She explained that she had a thyroid tumor and struggled to adjust to the treatments, which caused her weight fluctuations. She had never been pregnant.
My awkward statement had been the first time anyone on campus had directly mentioned her weight, though she suspected that people were talking about her. We became fast friends after this rocky beginning. Although we lost touch after college, S taught me an invaluable lesson about making assumptions about people’s weight: Ask before you assume.
Now, years later, as an internist and obesity specialist, this lesson continues to be reinforced daily.
In daily life, comments about weight can be perceived as rude. In the clinical setting, however, assumptions about weight are a form of weight bias. Weight bias can lead to weight stigma and even be dangerous to health care.
Let’s discuss the insidious influence of weight bias in health care through two commonly used phrases and then look at a few solutions to address weight bias in health care individually and systematically.
Common weight bias assumptions
“Great job, you lost weight!” In checking your patient’s vital signs, you notice that this patient with obesity has a significant weight change. You congratulate them upon entering the room. Unfortunately, their weight loss was a result of minimal eating after losing a loved one. This isn’t healthy weight loss. One of the adverse effects of weight bias is that it infers that weight loss is always a good thing, especially in people with larger bodies. This is a dangerous presumption. Let’s remember that the body favors fat storage, hence why “unintentional weight loss” is a recognized medical condition prompting evaluation. We have to be careful not to celebrate weight loss “at all costs,” such as fad diets that haven’t been shown to improve health outcomes.
Furthermore, patients who lose weight quickly (more than 4-8 lb/month) require closer follow-up and evaluation for secondary causes of weight loss. Patients may lose weight at a faster rate with the new antiobesity medications, but clinicians still should ensure that age-appropriate health maintenance screening is done and be vigilant for secondary causes of weight changes.
“Have you tried losing weight yet?” Three times. That’s how many times Chanté Burkett went to her doctor about her painful, enlarging firm stomach. She was advised to continue working on weight loss, which she did diligently. But Ms. Burkett’s abdomen kept growing and her concerns were dismissed. A visit to urgent care and a CT scan revealed that Ms. Burkett’s excess abdominal “fat” was a 13-lb mucinous cystadenoma. Sadly, cases like hers aren’t rare, isolated events. Weight bias can cause anchoring on one diagnosis, preventing consideration of other diagnostic possibilities. Even worse, anchoring will lead to the wrong intervention, such as prescribing weight loss for presumed increased adiposity instead of ordering the appropriate testing.
It’s also essential to recognize that, even if someone does have the disease of obesity, weight loss isn’t the solution to every medical concern. Even if weight loss is helpful, other, more pressing treatments may still be necessary. Telling a person with obesity who has an acute complaint to “just lose weight” is comparable to telling a patient with coronary artery disease who presents with an 80% vessel occlusion and chest pain to follow a low-fat diet. In both cases, you need to address the acute concern appropriately, then focus on the chronic treatment.
Ways to reduce clinical weight bias
How do you reduce clinical weight bias?
Ask, don’t assume. The information from the scale is simply data. Instead of judging it positively or negatively and creating a story, ask the patient. An unbiased way to approach the conversation is to say, “Great to see you. You seem [positive adjective of choice]. How have you been?” Wait until the vitals section to objectively discuss weight unless the patient offers the discussion earlier or their chief complaint lists a weight-related concern.
Order necessary tests to evaluate weight. Weight is the vital sign that people wear externally, so we feel that we can readily interpret it without any further assessment. However, resist the urge to interpret scale data without context. Keeping an open mind helps prevent anchoring and missing critical clues in the clinical history.
Address weight changes effectively. Sometimes there is an indication to prescribe weight loss as part of the treatment plan. However, remember that weight loss isn’t simply “calories in vs. calories out.” Obesity is a complex medical disease that requires a multimodal treatment approach. As clinicians, we have access to the most powerful tools for weight loss. Unfortunately, weight bias contributes to limited prescribing of metabolic medications (“antiobesity medications” or AOMs). In addition, systemic weight bias prevents insurance coverage of AOMs. The Treat and Reduce Obesity Act has been introduced into Congress to help improve life-transforming access to AOMs.
Acknowledge your bias. Our experiences make us all susceptible to bias. The Harvard Weight Implicit Association Test is free and a helpful way to assess your level of weight bias. I take it annually to ensure that I remain objective in my practice.
Addressing weight bias needs to extend beyond the individual level.
Systemically, health care needs to address the following:
Language. Use people-centered language. For example, “People aren’t obese. They have obesity.”
Accessibility. Health care settings must be comfortable and accessible for people of all sizes. Furthermore, improvements to access the services that comprehensive obesity care requires, such as AOMs, bariatric procedures and bariatric surgery, mental health care, nutrition, fitness specialists, health coaches, and more, are needed.
Education. Medical students and trainees have to learn the newest obesity science and know how to treat obesity effectively. Acknowledge and address biased tools. Recent data have shown that some of our screening tools, such as body mass index, have inherent bias. It’s time to focus on using improved diagnostic tools and personalized treatments.
We are at a pivotal time in our scientific understanding of body weight regulation and the disease of obesity. Clinical weight bias is primarily rooted in flawed science influenced by biased cultural norms and other forms of discrimination, such as racial and gender bias. We must move past assumptions to give our patients the optimal individualized care they need. So next time you observe a weight change, instead of commenting on their weight, say, “Great to see you! How have you been?”
S*: Initial has been changed to protect privacy.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness”, was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
It was the start of the fall semester of my sophomore year of college.
At my small women’s college, the previous semester’s gossip had been about our classmate, S*. She had gone from being very thin to noticeably gaining a lot of weight in a few months. The rumors were that S was pregnant and gave birth over summer break. As a busy biology premed major, this was my first time hearing the news. So when I saw her standing in the hallway, back to her previous weight, I was excited for her.
In true extravert fashion, I commented on the baby and her new size. But no sooner had the words left my mouth than I regretted them.
The hall grew awkwardly silent as S’s face flushed and she asked, “Excuse me?!” Instantly I knew that the rumors weren’t true.
Thankfully, at that moment, the classroom opened and we walked in. Whew! After class, S asked if we could talk. She explained that she had a thyroid tumor and struggled to adjust to the treatments, which caused her weight fluctuations. She had never been pregnant.
My awkward statement had been the first time anyone on campus had directly mentioned her weight, though she suspected that people were talking about her. We became fast friends after this rocky beginning. Although we lost touch after college, S taught me an invaluable lesson about making assumptions about people’s weight: Ask before you assume.
Now, years later, as an internist and obesity specialist, this lesson continues to be reinforced daily.
In daily life, comments about weight can be perceived as rude. In the clinical setting, however, assumptions about weight are a form of weight bias. Weight bias can lead to weight stigma and even be dangerous to health care.
Let’s discuss the insidious influence of weight bias in health care through two commonly used phrases and then look at a few solutions to address weight bias in health care individually and systematically.
Common weight bias assumptions
“Great job, you lost weight!” In checking your patient’s vital signs, you notice that this patient with obesity has a significant weight change. You congratulate them upon entering the room. Unfortunately, their weight loss was a result of minimal eating after losing a loved one. This isn’t healthy weight loss. One of the adverse effects of weight bias is that it infers that weight loss is always a good thing, especially in people with larger bodies. This is a dangerous presumption. Let’s remember that the body favors fat storage, hence why “unintentional weight loss” is a recognized medical condition prompting evaluation. We have to be careful not to celebrate weight loss “at all costs,” such as fad diets that haven’t been shown to improve health outcomes.
Furthermore, patients who lose weight quickly (more than 4-8 lb/month) require closer follow-up and evaluation for secondary causes of weight loss. Patients may lose weight at a faster rate with the new antiobesity medications, but clinicians still should ensure that age-appropriate health maintenance screening is done and be vigilant for secondary causes of weight changes.
“Have you tried losing weight yet?” Three times. That’s how many times Chanté Burkett went to her doctor about her painful, enlarging firm stomach. She was advised to continue working on weight loss, which she did diligently. But Ms. Burkett’s abdomen kept growing and her concerns were dismissed. A visit to urgent care and a CT scan revealed that Ms. Burkett’s excess abdominal “fat” was a 13-lb mucinous cystadenoma. Sadly, cases like hers aren’t rare, isolated events. Weight bias can cause anchoring on one diagnosis, preventing consideration of other diagnostic possibilities. Even worse, anchoring will lead to the wrong intervention, such as prescribing weight loss for presumed increased adiposity instead of ordering the appropriate testing.
It’s also essential to recognize that, even if someone does have the disease of obesity, weight loss isn’t the solution to every medical concern. Even if weight loss is helpful, other, more pressing treatments may still be necessary. Telling a person with obesity who has an acute complaint to “just lose weight” is comparable to telling a patient with coronary artery disease who presents with an 80% vessel occlusion and chest pain to follow a low-fat diet. In both cases, you need to address the acute concern appropriately, then focus on the chronic treatment.
Ways to reduce clinical weight bias
How do you reduce clinical weight bias?
Ask, don’t assume. The information from the scale is simply data. Instead of judging it positively or negatively and creating a story, ask the patient. An unbiased way to approach the conversation is to say, “Great to see you. You seem [positive adjective of choice]. How have you been?” Wait until the vitals section to objectively discuss weight unless the patient offers the discussion earlier or their chief complaint lists a weight-related concern.
Order necessary tests to evaluate weight. Weight is the vital sign that people wear externally, so we feel that we can readily interpret it without any further assessment. However, resist the urge to interpret scale data without context. Keeping an open mind helps prevent anchoring and missing critical clues in the clinical history.
Address weight changes effectively. Sometimes there is an indication to prescribe weight loss as part of the treatment plan. However, remember that weight loss isn’t simply “calories in vs. calories out.” Obesity is a complex medical disease that requires a multimodal treatment approach. As clinicians, we have access to the most powerful tools for weight loss. Unfortunately, weight bias contributes to limited prescribing of metabolic medications (“antiobesity medications” or AOMs). In addition, systemic weight bias prevents insurance coverage of AOMs. The Treat and Reduce Obesity Act has been introduced into Congress to help improve life-transforming access to AOMs.
Acknowledge your bias. Our experiences make us all susceptible to bias. The Harvard Weight Implicit Association Test is free and a helpful way to assess your level of weight bias. I take it annually to ensure that I remain objective in my practice.
Addressing weight bias needs to extend beyond the individual level.
Systemically, health care needs to address the following:
Language. Use people-centered language. For example, “People aren’t obese. They have obesity.”
Accessibility. Health care settings must be comfortable and accessible for people of all sizes. Furthermore, improvements to access the services that comprehensive obesity care requires, such as AOMs, bariatric procedures and bariatric surgery, mental health care, nutrition, fitness specialists, health coaches, and more, are needed.
Education. Medical students and trainees have to learn the newest obesity science and know how to treat obesity effectively. Acknowledge and address biased tools. Recent data have shown that some of our screening tools, such as body mass index, have inherent bias. It’s time to focus on using improved diagnostic tools and personalized treatments.
We are at a pivotal time in our scientific understanding of body weight regulation and the disease of obesity. Clinical weight bias is primarily rooted in flawed science influenced by biased cultural norms and other forms of discrimination, such as racial and gender bias. We must move past assumptions to give our patients the optimal individualized care they need. So next time you observe a weight change, instead of commenting on their weight, say, “Great to see you! How have you been?”
S*: Initial has been changed to protect privacy.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness”, was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Bacterial vaginosis linked with persistent HPV infections
Montrouge, France – Four in five women will be infected by one or more human papillomavirus (HPV) strains during their lifetimes. For most of these women, the HPV will be cleared from the body, but 5% of them will develop precancerous lesions in the cervix.
At a press conference ahead of the 46th meeting of the French Colposcopy and Cervical and Vaginal Diseases Society, Julia Maruani, MD, a medical gynecologist in Marseille, France, took the opportunity to discuss the importance of vaginal flora and the need to treat cases of bacterial vaginosis.
Striking a balance
Essential for reducing the risk of sexually transmitted infections, a healthy vaginal flora is made up of millions of microorganisms, mainly lactobacilli, as well as other bacteria (Gardnerella vaginalis, Atopobium vaginae, Prevotella, streptococcus, gonococcus), HPV, and fungi.
Lactobacilli produce lactic acid, which reduces the vagina’s pH, as well as hydrogen peroxide, which is toxic to the other bacteria.
Different factors, such as alcohol, a diet rich in polyunsaturated fatty acids and sugar, and especially smoking, can lead to an imbalance of the bacteria in the vaginal flora and thus result in vaginosis. What occurs is an abnormal multiplication of different types of anaerobic bacteria that are normally present in much lower numbers. There is a relative reduction in lactobacilli, which results in an increased vaginal pH, a greater risk of contracting an STI, and reduced clearance of the HPV infection. “Women who smoke probably experience persistent HPV infections due to an imbalance in vaginal flora,” said Dr. Maruani.
Vaginosis and HPV
When there are fewer lactobacilli than there should be, these bacteria can no longer protect the vaginal mucosa, which is disrupted by other bacteria. “HPV then has access to the basal cells,” said Dr. Maruani, acknowledging that the relationship between bacterial vaginosis and persistent HPV infections has been the subject of numerous research studies over the past decade or so. “For years, I would see this same link in my patients. Those with persistent vaginosis were also the ones with persistent HPV. And I’m not the only one to notice this. Studies have also been carried out investigating this exact correlation,” she added.
These studies have shown that HPV infections persist in cases of vaginosis, resulting in the appearance of epithelial lesions. Additionally, the lesions are more severe when dysbiosis is more severe.
What about probiotics? Can they treat dysbiosis and an HPV infection at the same time? “Probiotics work very well for vaginosis, provided they are used for a long time. We know that they lessen HPV infections and low-grade lesions,” said Dr. Maruani, although no randomized studies support this conclusion. “It’s not a one size fits all. We aren’t about to treat patients with precancerous lesions with probiotics.” There are currently no data concerning the efficacy of probiotics on high-grade lesions. These days, Dr. Maruani has been thinking about a new issue: the benefit of diagnosing cases of asymptomatic vaginosis – because treating them would reduce the risk of persistent HPV infection.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Montrouge, France – Four in five women will be infected by one or more human papillomavirus (HPV) strains during their lifetimes. For most of these women, the HPV will be cleared from the body, but 5% of them will develop precancerous lesions in the cervix.
At a press conference ahead of the 46th meeting of the French Colposcopy and Cervical and Vaginal Diseases Society, Julia Maruani, MD, a medical gynecologist in Marseille, France, took the opportunity to discuss the importance of vaginal flora and the need to treat cases of bacterial vaginosis.
Striking a balance
Essential for reducing the risk of sexually transmitted infections, a healthy vaginal flora is made up of millions of microorganisms, mainly lactobacilli, as well as other bacteria (Gardnerella vaginalis, Atopobium vaginae, Prevotella, streptococcus, gonococcus), HPV, and fungi.
Lactobacilli produce lactic acid, which reduces the vagina’s pH, as well as hydrogen peroxide, which is toxic to the other bacteria.
Different factors, such as alcohol, a diet rich in polyunsaturated fatty acids and sugar, and especially smoking, can lead to an imbalance of the bacteria in the vaginal flora and thus result in vaginosis. What occurs is an abnormal multiplication of different types of anaerobic bacteria that are normally present in much lower numbers. There is a relative reduction in lactobacilli, which results in an increased vaginal pH, a greater risk of contracting an STI, and reduced clearance of the HPV infection. “Women who smoke probably experience persistent HPV infections due to an imbalance in vaginal flora,” said Dr. Maruani.
Vaginosis and HPV
When there are fewer lactobacilli than there should be, these bacteria can no longer protect the vaginal mucosa, which is disrupted by other bacteria. “HPV then has access to the basal cells,” said Dr. Maruani, acknowledging that the relationship between bacterial vaginosis and persistent HPV infections has been the subject of numerous research studies over the past decade or so. “For years, I would see this same link in my patients. Those with persistent vaginosis were also the ones with persistent HPV. And I’m not the only one to notice this. Studies have also been carried out investigating this exact correlation,” she added.
These studies have shown that HPV infections persist in cases of vaginosis, resulting in the appearance of epithelial lesions. Additionally, the lesions are more severe when dysbiosis is more severe.
What about probiotics? Can they treat dysbiosis and an HPV infection at the same time? “Probiotics work very well for vaginosis, provided they are used for a long time. We know that they lessen HPV infections and low-grade lesions,” said Dr. Maruani, although no randomized studies support this conclusion. “It’s not a one size fits all. We aren’t about to treat patients with precancerous lesions with probiotics.” There are currently no data concerning the efficacy of probiotics on high-grade lesions. These days, Dr. Maruani has been thinking about a new issue: the benefit of diagnosing cases of asymptomatic vaginosis – because treating them would reduce the risk of persistent HPV infection.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Montrouge, France – Four in five women will be infected by one or more human papillomavirus (HPV) strains during their lifetimes. For most of these women, the HPV will be cleared from the body, but 5% of them will develop precancerous lesions in the cervix.
At a press conference ahead of the 46th meeting of the French Colposcopy and Cervical and Vaginal Diseases Society, Julia Maruani, MD, a medical gynecologist in Marseille, France, took the opportunity to discuss the importance of vaginal flora and the need to treat cases of bacterial vaginosis.
Striking a balance
Essential for reducing the risk of sexually transmitted infections, a healthy vaginal flora is made up of millions of microorganisms, mainly lactobacilli, as well as other bacteria (Gardnerella vaginalis, Atopobium vaginae, Prevotella, streptococcus, gonococcus), HPV, and fungi.
Lactobacilli produce lactic acid, which reduces the vagina’s pH, as well as hydrogen peroxide, which is toxic to the other bacteria.
Different factors, such as alcohol, a diet rich in polyunsaturated fatty acids and sugar, and especially smoking, can lead to an imbalance of the bacteria in the vaginal flora and thus result in vaginosis. What occurs is an abnormal multiplication of different types of anaerobic bacteria that are normally present in much lower numbers. There is a relative reduction in lactobacilli, which results in an increased vaginal pH, a greater risk of contracting an STI, and reduced clearance of the HPV infection. “Women who smoke probably experience persistent HPV infections due to an imbalance in vaginal flora,” said Dr. Maruani.
Vaginosis and HPV
When there are fewer lactobacilli than there should be, these bacteria can no longer protect the vaginal mucosa, which is disrupted by other bacteria. “HPV then has access to the basal cells,” said Dr. Maruani, acknowledging that the relationship between bacterial vaginosis and persistent HPV infections has been the subject of numerous research studies over the past decade or so. “For years, I would see this same link in my patients. Those with persistent vaginosis were also the ones with persistent HPV. And I’m not the only one to notice this. Studies have also been carried out investigating this exact correlation,” she added.
These studies have shown that HPV infections persist in cases of vaginosis, resulting in the appearance of epithelial lesions. Additionally, the lesions are more severe when dysbiosis is more severe.
What about probiotics? Can they treat dysbiosis and an HPV infection at the same time? “Probiotics work very well for vaginosis, provided they are used for a long time. We know that they lessen HPV infections and low-grade lesions,” said Dr. Maruani, although no randomized studies support this conclusion. “It’s not a one size fits all. We aren’t about to treat patients with precancerous lesions with probiotics.” There are currently no data concerning the efficacy of probiotics on high-grade lesions. These days, Dr. Maruani has been thinking about a new issue: the benefit of diagnosing cases of asymptomatic vaginosis – because treating them would reduce the risk of persistent HPV infection.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
The challenge of incidentally detected interstitial lung abnormalities
Clinicians working within the U.S. health care system order CTs; it’s just what we do, and we do it a lot. This isn’t necessarily bad, but an inevitable byproduct is the pandemic of incidental findings. One underrecognized but frequent “incidentaloma” on CT is an interstitial lung abnormality (ILA). The Fleischner Society defines an ILA as honeycombing, traction bronchiectasis, parenchymal distortions, and reticular abnormalities that take up more than 5% of a particular lung zone in a patient without a clinical diagnosis of interstitial lung disease (ILD). In essence, ILAs are both a radiographic and a clinical diagnosis.
ILAs are common. With the advent of lung cancer screening and advances in CT technology, we’re now inundated with detailed images of lung parenchyma in older smokers who are at high risk for respiratory disease. The resulting opportunity for early identification of disease is as exciting as the risk for overdiagnosis, excessive testing, and unnecessary treatment is frightening. Early diagnosis remains critical for preventing irreversible respiratory disease. But as with any disease process, when we attempt to detect pathology before it has become apparent, the line between benign change and true abnormality is blurred.
Such is the challenge with ILAs. Past studies have shown an association between ILAs and morbidity and mortality, but considerable uncertainty persists over what the ILAs represent and how they should be managed. A recent study published in the American Journal of Respiratory and Critical Care Medicine provides some clarity. The authors used data from the COPDGene cohort to correlate ILAs with lung testing, and functional and respiratory outcomes. As with other studies, they found that approximately 10% of the COPDGene patients that they examined had ILAs on CT and half of those met their criteria for “suspected ILD.” Suspected ILD was defined radiographically (definite fibrosis) and on lung function testing (abnormal forced vital capacity [FVC] or diffusing capacity of the lungs for carbon monoxide [DLCO]). The patients with suspected ILD had worse clinical outcomes; being a Black individual, pack-years of smoking, and GOLD stage on spirometry were independently associated with suspected ILD.
This type of study is urgently needed. Given their high prevalence, we’re in dire need of a valid model for risk stratifying ILAs. The authors of this study have moved us closer, but we’ve still got a long way to go. The study has significant limitations. First, although patients with previous documentation of ILD were excluded from COPDGene, no formal, multidisciplinary assessment was performed; therefore, some of the patients labeled as having ILA probably had diagnosable ILD. Their possible inclusion would falsely increase the prevalence of clinically important ILAs and exaggerate the relationship between ILAs and clinical outcomes.
The rhetorical gymnastics performed throughout the paper are necessary yet problematic. “Suspected ILD” is not a recognized diagnosis and the definition is therefore arbitrary. To the extent that “suspected ILD” requires an abnormality on spirometry or DLCO, one could argue it’s the lung function changes and not the radiographic findings that are driving the differences. In fact, “suspected ILD” was defined by lung function more often than radiographic criteria (16% had definite fibrosis on CT, 57% had an abnormal FVC, and 67% had an abnormal DLCO). Patients with ILAs without suspected ILD had outcomes that weren’t statistically different from those with no ILAs at all, implying that the lung testing and not the ILA is the better discriminator. Regardless, this leads us back to where we started before this paper was published: ILAs require lung function testing and referral to a pulmonologist for proper risk stratification. An accompanying editorial highlights these and other limitations.
One particular problem that isn’t addressed by the authors or the editorial is their findings on race. The authors concluded that Black persons with ILAs are more likely to have “suspected ILD.” However, their definition suffers from an insidious form of incorporation bias generated by the way they handled their DLCO reference values. The Global Lung Function Initiative equations they used were derived exclusively from White persons. In accordance with the recent American Thoracic Society/European Respiratory Society (ATS/ERS) statement on lung testing, the authors did not apply a fixed correction factor to adjust for race. Without such an adjustment, Black persons would be biased toward having lower percent predicted values for DLCO. In short, self-identified Black individuals would be more likely to have a predicted DLCO of less than 70% and to therefore meet criteria for “suspected ILD.” The resulting effects on biologic plausibility, causal inference, and the strength of the relationship between “suspected ILD” and clinical outcomes will vary by whether the association between race and lung function is considered a product of inherent biologic variability or a result of external (socioeconomic and environmental) effects.
In summary, ILAs remain a challenge for radiologists, primary care providers, pulmonologists, and anyone else who orders a CT of the lungs. Despite its limitations, I believe the recently published paper pushes us forward conceptually. Perhaps its most important contribution is showing that 50% of ILAs are clinically insignificant by definition. This offers further reassurance that a subset of ILAs can be dismissed. Now, all we need is an easy, cost-effective, and efficient way to identify this subset.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He covers a wide range of topics in pulmonary, critical care, and sleep medicine. He disclosed ties to Metapharm Inc., CHEST College, and WebMD. A version of this article originally appeared on Medscape.com.
Clinicians working within the U.S. health care system order CTs; it’s just what we do, and we do it a lot. This isn’t necessarily bad, but an inevitable byproduct is the pandemic of incidental findings. One underrecognized but frequent “incidentaloma” on CT is an interstitial lung abnormality (ILA). The Fleischner Society defines an ILA as honeycombing, traction bronchiectasis, parenchymal distortions, and reticular abnormalities that take up more than 5% of a particular lung zone in a patient without a clinical diagnosis of interstitial lung disease (ILD). In essence, ILAs are both a radiographic and a clinical diagnosis.
ILAs are common. With the advent of lung cancer screening and advances in CT technology, we’re now inundated with detailed images of lung parenchyma in older smokers who are at high risk for respiratory disease. The resulting opportunity for early identification of disease is as exciting as the risk for overdiagnosis, excessive testing, and unnecessary treatment is frightening. Early diagnosis remains critical for preventing irreversible respiratory disease. But as with any disease process, when we attempt to detect pathology before it has become apparent, the line between benign change and true abnormality is blurred.
Such is the challenge with ILAs. Past studies have shown an association between ILAs and morbidity and mortality, but considerable uncertainty persists over what the ILAs represent and how they should be managed. A recent study published in the American Journal of Respiratory and Critical Care Medicine provides some clarity. The authors used data from the COPDGene cohort to correlate ILAs with lung testing, and functional and respiratory outcomes. As with other studies, they found that approximately 10% of the COPDGene patients that they examined had ILAs on CT and half of those met their criteria for “suspected ILD.” Suspected ILD was defined radiographically (definite fibrosis) and on lung function testing (abnormal forced vital capacity [FVC] or diffusing capacity of the lungs for carbon monoxide [DLCO]). The patients with suspected ILD had worse clinical outcomes; being a Black individual, pack-years of smoking, and GOLD stage on spirometry were independently associated with suspected ILD.
This type of study is urgently needed. Given their high prevalence, we’re in dire need of a valid model for risk stratifying ILAs. The authors of this study have moved us closer, but we’ve still got a long way to go. The study has significant limitations. First, although patients with previous documentation of ILD were excluded from COPDGene, no formal, multidisciplinary assessment was performed; therefore, some of the patients labeled as having ILA probably had diagnosable ILD. Their possible inclusion would falsely increase the prevalence of clinically important ILAs and exaggerate the relationship between ILAs and clinical outcomes.
The rhetorical gymnastics performed throughout the paper are necessary yet problematic. “Suspected ILD” is not a recognized diagnosis and the definition is therefore arbitrary. To the extent that “suspected ILD” requires an abnormality on spirometry or DLCO, one could argue it’s the lung function changes and not the radiographic findings that are driving the differences. In fact, “suspected ILD” was defined by lung function more often than radiographic criteria (16% had definite fibrosis on CT, 57% had an abnormal FVC, and 67% had an abnormal DLCO). Patients with ILAs without suspected ILD had outcomes that weren’t statistically different from those with no ILAs at all, implying that the lung testing and not the ILA is the better discriminator. Regardless, this leads us back to where we started before this paper was published: ILAs require lung function testing and referral to a pulmonologist for proper risk stratification. An accompanying editorial highlights these and other limitations.
One particular problem that isn’t addressed by the authors or the editorial is their findings on race. The authors concluded that Black persons with ILAs are more likely to have “suspected ILD.” However, their definition suffers from an insidious form of incorporation bias generated by the way they handled their DLCO reference values. The Global Lung Function Initiative equations they used were derived exclusively from White persons. In accordance with the recent American Thoracic Society/European Respiratory Society (ATS/ERS) statement on lung testing, the authors did not apply a fixed correction factor to adjust for race. Without such an adjustment, Black persons would be biased toward having lower percent predicted values for DLCO. In short, self-identified Black individuals would be more likely to have a predicted DLCO of less than 70% and to therefore meet criteria for “suspected ILD.” The resulting effects on biologic plausibility, causal inference, and the strength of the relationship between “suspected ILD” and clinical outcomes will vary by whether the association between race and lung function is considered a product of inherent biologic variability or a result of external (socioeconomic and environmental) effects.
In summary, ILAs remain a challenge for radiologists, primary care providers, pulmonologists, and anyone else who orders a CT of the lungs. Despite its limitations, I believe the recently published paper pushes us forward conceptually. Perhaps its most important contribution is showing that 50% of ILAs are clinically insignificant by definition. This offers further reassurance that a subset of ILAs can be dismissed. Now, all we need is an easy, cost-effective, and efficient way to identify this subset.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He covers a wide range of topics in pulmonary, critical care, and sleep medicine. He disclosed ties to Metapharm Inc., CHEST College, and WebMD. A version of this article originally appeared on Medscape.com.
Clinicians working within the U.S. health care system order CTs; it’s just what we do, and we do it a lot. This isn’t necessarily bad, but an inevitable byproduct is the pandemic of incidental findings. One underrecognized but frequent “incidentaloma” on CT is an interstitial lung abnormality (ILA). The Fleischner Society defines an ILA as honeycombing, traction bronchiectasis, parenchymal distortions, and reticular abnormalities that take up more than 5% of a particular lung zone in a patient without a clinical diagnosis of interstitial lung disease (ILD). In essence, ILAs are both a radiographic and a clinical diagnosis.
ILAs are common. With the advent of lung cancer screening and advances in CT technology, we’re now inundated with detailed images of lung parenchyma in older smokers who are at high risk for respiratory disease. The resulting opportunity for early identification of disease is as exciting as the risk for overdiagnosis, excessive testing, and unnecessary treatment is frightening. Early diagnosis remains critical for preventing irreversible respiratory disease. But as with any disease process, when we attempt to detect pathology before it has become apparent, the line between benign change and true abnormality is blurred.
Such is the challenge with ILAs. Past studies have shown an association between ILAs and morbidity and mortality, but considerable uncertainty persists over what the ILAs represent and how they should be managed. A recent study published in the American Journal of Respiratory and Critical Care Medicine provides some clarity. The authors used data from the COPDGene cohort to correlate ILAs with lung testing, and functional and respiratory outcomes. As with other studies, they found that approximately 10% of the COPDGene patients that they examined had ILAs on CT and half of those met their criteria for “suspected ILD.” Suspected ILD was defined radiographically (definite fibrosis) and on lung function testing (abnormal forced vital capacity [FVC] or diffusing capacity of the lungs for carbon monoxide [DLCO]). The patients with suspected ILD had worse clinical outcomes; being a Black individual, pack-years of smoking, and GOLD stage on spirometry were independently associated with suspected ILD.
This type of study is urgently needed. Given their high prevalence, we’re in dire need of a valid model for risk stratifying ILAs. The authors of this study have moved us closer, but we’ve still got a long way to go. The study has significant limitations. First, although patients with previous documentation of ILD were excluded from COPDGene, no formal, multidisciplinary assessment was performed; therefore, some of the patients labeled as having ILA probably had diagnosable ILD. Their possible inclusion would falsely increase the prevalence of clinically important ILAs and exaggerate the relationship between ILAs and clinical outcomes.
The rhetorical gymnastics performed throughout the paper are necessary yet problematic. “Suspected ILD” is not a recognized diagnosis and the definition is therefore arbitrary. To the extent that “suspected ILD” requires an abnormality on spirometry or DLCO, one could argue it’s the lung function changes and not the radiographic findings that are driving the differences. In fact, “suspected ILD” was defined by lung function more often than radiographic criteria (16% had definite fibrosis on CT, 57% had an abnormal FVC, and 67% had an abnormal DLCO). Patients with ILAs without suspected ILD had outcomes that weren’t statistically different from those with no ILAs at all, implying that the lung testing and not the ILA is the better discriminator. Regardless, this leads us back to where we started before this paper was published: ILAs require lung function testing and referral to a pulmonologist for proper risk stratification. An accompanying editorial highlights these and other limitations.
One particular problem that isn’t addressed by the authors or the editorial is their findings on race. The authors concluded that Black persons with ILAs are more likely to have “suspected ILD.” However, their definition suffers from an insidious form of incorporation bias generated by the way they handled their DLCO reference values. The Global Lung Function Initiative equations they used were derived exclusively from White persons. In accordance with the recent American Thoracic Society/European Respiratory Society (ATS/ERS) statement on lung testing, the authors did not apply a fixed correction factor to adjust for race. Without such an adjustment, Black persons would be biased toward having lower percent predicted values for DLCO. In short, self-identified Black individuals would be more likely to have a predicted DLCO of less than 70% and to therefore meet criteria for “suspected ILD.” The resulting effects on biologic plausibility, causal inference, and the strength of the relationship between “suspected ILD” and clinical outcomes will vary by whether the association between race and lung function is considered a product of inherent biologic variability or a result of external (socioeconomic and environmental) effects.
In summary, ILAs remain a challenge for radiologists, primary care providers, pulmonologists, and anyone else who orders a CT of the lungs. Despite its limitations, I believe the recently published paper pushes us forward conceptually. Perhaps its most important contribution is showing that 50% of ILAs are clinically insignificant by definition. This offers further reassurance that a subset of ILAs can be dismissed. Now, all we need is an easy, cost-effective, and efficient way to identify this subset.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He covers a wide range of topics in pulmonary, critical care, and sleep medicine. He disclosed ties to Metapharm Inc., CHEST College, and WebMD. A version of this article originally appeared on Medscape.com.
‘Ozempic face’: Accepting wrinkles for improved health
This transcript has been edited for clarity.
Last week, a number of patients emailed me regarding their concerns about this phenomenon known as Ozempic face. I went on to read about what this meant. I live in Los Angeles, where most people appear to be on semaglutide (Ozempic). It’s the phenomenon where people lose weight relatively rapidly, making their faces thin out. Then what happens, apparently, is they look older because their face is more wrinkled and baggier. They might have to have further plastic surgery. I say that with slight sarcasm because of where I live.
I want to talk about what I think about this, living here where there’s a great pressure to prescribe semaglutide off label, and what I think about it for my patients with diabetes.
Historically, we haven’t had much in terms of effective medication for treating obesity, and frankly, now we do. We now have agents that are effective, that have relatively few side effects, and that have become part of what’s out there. People now want to use these agents, semaglutide, and there’s been a great need for these agents.
The problem, however, is twofold. One, as we all know, is that it has basically caused a shortage of medication for treating our patients who actually have type 2 diabetes and really need these medications to manage their disease. Then we have people who want these medications who can’t pay for them. Insurance doesn’t cover obesity medications, which is problematic and actually quite frustrating for people who, I think, really would benefit from using these medications.
What I tell people, frankly, is that until I have enough supply for my patients with type 2 diabetes, who need these agents to control their blood sugars, I want to keep this class of drugs available to them. I also hope we’re able to expand it more and more with improving insurance coverage – and that’s a big if, if you ask me – both for people who have prediabetes and for patients who are overweight and obese, because I think it’s really hard for people to lose weight.
It’s frustrating, and for many people, being overweight and obese causes all sorts of other health issues, not only diabetes. I believe that these drugs are both safe and effective and should be more available. I do think we need to be careful in terms of who we prescribe them to, at least at the moment. Hopefully, we’ll be able to expand their use.
Anything that can encourage our population to lose weight and maintain that weight loss is very important. We need to couple weight loss medications with lifestyle interventions. I think people can out-eat any medication; therefore, it’s very important to encourage our patients to eat better, to exercise more, and to do all the other things they need to do to reduce their risks for other comorbidities.
I am incredibly happy to have these newer agents on the market. I tell my patients – at least those who have diabetes – that they have to accept looking a little bit too thin for the benefits that we can see in using these medications.
Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She has ties with Abbott Diabetes Care, AstraZeneca Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Last week, a number of patients emailed me regarding their concerns about this phenomenon known as Ozempic face. I went on to read about what this meant. I live in Los Angeles, where most people appear to be on semaglutide (Ozempic). It’s the phenomenon where people lose weight relatively rapidly, making their faces thin out. Then what happens, apparently, is they look older because their face is more wrinkled and baggier. They might have to have further plastic surgery. I say that with slight sarcasm because of where I live.
I want to talk about what I think about this, living here where there’s a great pressure to prescribe semaglutide off label, and what I think about it for my patients with diabetes.
Historically, we haven’t had much in terms of effective medication for treating obesity, and frankly, now we do. We now have agents that are effective, that have relatively few side effects, and that have become part of what’s out there. People now want to use these agents, semaglutide, and there’s been a great need for these agents.
The problem, however, is twofold. One, as we all know, is that it has basically caused a shortage of medication for treating our patients who actually have type 2 diabetes and really need these medications to manage their disease. Then we have people who want these medications who can’t pay for them. Insurance doesn’t cover obesity medications, which is problematic and actually quite frustrating for people who, I think, really would benefit from using these medications.
What I tell people, frankly, is that until I have enough supply for my patients with type 2 diabetes, who need these agents to control their blood sugars, I want to keep this class of drugs available to them. I also hope we’re able to expand it more and more with improving insurance coverage – and that’s a big if, if you ask me – both for people who have prediabetes and for patients who are overweight and obese, because I think it’s really hard for people to lose weight.
It’s frustrating, and for many people, being overweight and obese causes all sorts of other health issues, not only diabetes. I believe that these drugs are both safe and effective and should be more available. I do think we need to be careful in terms of who we prescribe them to, at least at the moment. Hopefully, we’ll be able to expand their use.
Anything that can encourage our population to lose weight and maintain that weight loss is very important. We need to couple weight loss medications with lifestyle interventions. I think people can out-eat any medication; therefore, it’s very important to encourage our patients to eat better, to exercise more, and to do all the other things they need to do to reduce their risks for other comorbidities.
I am incredibly happy to have these newer agents on the market. I tell my patients – at least those who have diabetes – that they have to accept looking a little bit too thin for the benefits that we can see in using these medications.
Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She has ties with Abbott Diabetes Care, AstraZeneca Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Last week, a number of patients emailed me regarding their concerns about this phenomenon known as Ozempic face. I went on to read about what this meant. I live in Los Angeles, where most people appear to be on semaglutide (Ozempic). It’s the phenomenon where people lose weight relatively rapidly, making their faces thin out. Then what happens, apparently, is they look older because their face is more wrinkled and baggier. They might have to have further plastic surgery. I say that with slight sarcasm because of where I live.
I want to talk about what I think about this, living here where there’s a great pressure to prescribe semaglutide off label, and what I think about it for my patients with diabetes.
Historically, we haven’t had much in terms of effective medication for treating obesity, and frankly, now we do. We now have agents that are effective, that have relatively few side effects, and that have become part of what’s out there. People now want to use these agents, semaglutide, and there’s been a great need for these agents.
The problem, however, is twofold. One, as we all know, is that it has basically caused a shortage of medication for treating our patients who actually have type 2 diabetes and really need these medications to manage their disease. Then we have people who want these medications who can’t pay for them. Insurance doesn’t cover obesity medications, which is problematic and actually quite frustrating for people who, I think, really would benefit from using these medications.
What I tell people, frankly, is that until I have enough supply for my patients with type 2 diabetes, who need these agents to control their blood sugars, I want to keep this class of drugs available to them. I also hope we’re able to expand it more and more with improving insurance coverage – and that’s a big if, if you ask me – both for people who have prediabetes and for patients who are overweight and obese, because I think it’s really hard for people to lose weight.
It’s frustrating, and for many people, being overweight and obese causes all sorts of other health issues, not only diabetes. I believe that these drugs are both safe and effective and should be more available. I do think we need to be careful in terms of who we prescribe them to, at least at the moment. Hopefully, we’ll be able to expand their use.
Anything that can encourage our population to lose weight and maintain that weight loss is very important. We need to couple weight loss medications with lifestyle interventions. I think people can out-eat any medication; therefore, it’s very important to encourage our patients to eat better, to exercise more, and to do all the other things they need to do to reduce their risks for other comorbidities.
I am incredibly happy to have these newer agents on the market. I tell my patients – at least those who have diabetes – that they have to accept looking a little bit too thin for the benefits that we can see in using these medications.
Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She has ties with Abbott Diabetes Care, AstraZeneca Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article originally appeared on Medscape.com.
Accelerated pacing a possible strategy for HFpEF?
Evidence supporting medications that slow the heart rate (HR), notably beta-blockers, is overwhelming in heart failure (HF) with reduced ejection fraction. Underwhelming, however, is clinical trial support for such agents in patients with HF with preserved ejection fraction (HFpEF).
Indeed, at least for some such patients, a treatment that modestly accelerates resting HR may be a more promising strategy, suggests an early line of research that challenges prevalent thinking about HFpEF therapy.
In a small, randomized test of the idea, patients with HFpEF and standard pacemakers set to a backup resting HR a bit higher than a standard of care 60 bpm, usually to about 75 bpm, reaped important quality of life benefits.
More strikingly, their natriuretic peptide levels and burden of atrial fibrillation (AFib) fell significantly, the latter by 27% over 1 year.
The trial enrolled only HFpEF patients with pacemakers previously implanted for sick sinus syndrome or atrioventricular block. But researchers say their 107-patient study called myPACE – if confirmed in larger, multicenter trials – lays the groundwork for a device therapy that is broadly useful, potentially, in patients with “preclinical or overt” HFpEF.
Indeed, some of the intervention’s “quite substantial” benefits rivaled or surpassed what his group has observed with available HFpEF drug therapies, including the sodium-glucose cotransporter 2 inhibitors, observed Markus Meyer, MD, PhD, University of Minnesota, Minneapolis.
Moreover, the study may be “the first to show that, with this approach, we can actually also reduce atrial fibrillation,” he said in an interview.
Dr. Meyer said his group is “confident” that the HR-modulation strategy will be successful in appropriate clinical trials and that “pacemakers, in the end, will become a treatment modality for HFpEF.”
Meyer is senior author on the trial’s publication in JAMA Cardiology in JAMA Cardiology (2023 Feb 1. doi: 10.1001/jamacardio.2022.5320), with lead author Margaret Infeld, MD, University of Vermont, Burlington.
The trial entered pacemaker patients with HFpEF of stage B or C – that is, either asymptomatic with structural disease or fully symptomatic. But, Dr. Meyer said, “we saw that the treatment effect was much more pronounced in the patients who had overt heart failure with preserved ejection fraction.”
Challenging beta-blocker dogma
The study, the report states, “contradicts canonical thinking” by suggesting HFpEF patients may benefit from a higher resting heart rate, which would presumably shorten diastolic filling time. It also “may help reduce the overprescription of beta-blockers to allow higher heart rates in this population.”
Indeed, Dr. Meyer observed, no one really knows whether beta-blockers work in HFpEF, “because they really have never been studied in a sufficiently powered randomized controlled trial.”
The current study “basically rewrites what we know about the pathophysiology of this form of clinical heart failure,” said Michael R. Zile, MD, Medical University of South Carolina and Veterans Affairs Medical Center, both in Charleston, who was not part of the trial or report.
Previously in HFpEF, Dr. Zile said in an interview, “everybody thought you needed to make diastole longer to give the ventricle a longer time to fill. And none of that really made any sense. It was just sort of accepted as dogma.”
The idea led to widespread use of beta-blockers in HFpEF but “turned out just not to be true.” Indeed, European and North American guidelines, Dr. Zile observed, “have all taken beta-blockers out of the equation for HFpEF” except for treating comorbidities that can be associated with HFpEF, like hypertension or AFib.
Many patients with HFpEF and chronotropic incompetence could be provided with standard pacemakers with primarily conduction-system pacing but are not getting them, he observed.
The current study might help change that. No one is suggesting, based on the current study, “that we start putting pacemakers in every single patient with HFpEF,” Dr. Zile said. Still, for HFpEF patients already with a pacemaker, the study provides “reasonable assurance” that its criteria for elevated resting HR may well improve symptoms.
Moreover, it suggests such pacemakers, programmed as in the study, might potentially give a boost to HFpEF patients without chronotropic incompetence but with persisting symptoms despite guideline-directed drug therapy. That’s certainly worth exploring in further trials, Dr. Zile said.
How the study worked
The single-center trial entered 107 participants with HFpEF and pacemakers set, at baseline, to a backup resting HR of 60 bpm; their age averaged 75 and 48% were women. Only patients with devices for atrial pacing, conduction-system pacing, or biventricular pacing – which are unlikely to promote ventricular dyssynchrony – were included.
They were randomly assigned, double-blind, to have their devices set to an accelerated backup rate or to be continued at 60 bpm. The backup resting rate set for the intervention group’s 50 patients was individualized based on height and other factors; the median was 75 bpm.
Scores on the Minnesota Living with Heart Failure Questionnaire, the primary endpoint, improved in the intervention group, compared with baseline, by about 11 points after 1 month and by 15 points after 1 year (P < .001).
The scores in the usual-care group deteriorated by half a point and by 3.5 points at 1 month and 1 year (P = .03), respectively.
Consistent advantages for the accelerated-HR strategy were evident throughout the major secondary endpoints. For example, levels of N-terminal pro-B-type natriuretic peptide fell an average 109 pg/dL after 1 month in the accelerated-HR group and rose a mean of 128 pg/dL in the usual-care group (P = .02).
Mean daily pacemaker-monitored activity level rose by 47 minutes by 1 year in the accelerated-HR group, compared with a drop of 22 minutes for those assigned to the standard-care rate (P < .001).
AFib was detected in 18% of intervention patients at the 1-year follow-up, down from 31% at baseline. Their risk ratio for AFib at 1 year was 0.73 (95% confidence interval, 0.55-0.99, P = .04), compared with the control group.
In other patients with HFpEF “we have done pacing studies where we just ramped up the pacing rate, and we see that these pressures in the left atrium actually drop immediately,” Dr. Meyer said. It’s that “unburdening of the atria,” he added, that probably leads to the reduction in AFib.
Dr. Meyer reported holding a patent for pacemakers for HFpEF licensed to Medtronic. Dr. Zile said he consults for Medtronic and has no other relevant financial relationships.
A version of this article first appeared on Medscape.com.
Evidence supporting medications that slow the heart rate (HR), notably beta-blockers, is overwhelming in heart failure (HF) with reduced ejection fraction. Underwhelming, however, is clinical trial support for such agents in patients with HF with preserved ejection fraction (HFpEF).
Indeed, at least for some such patients, a treatment that modestly accelerates resting HR may be a more promising strategy, suggests an early line of research that challenges prevalent thinking about HFpEF therapy.
In a small, randomized test of the idea, patients with HFpEF and standard pacemakers set to a backup resting HR a bit higher than a standard of care 60 bpm, usually to about 75 bpm, reaped important quality of life benefits.
More strikingly, their natriuretic peptide levels and burden of atrial fibrillation (AFib) fell significantly, the latter by 27% over 1 year.
The trial enrolled only HFpEF patients with pacemakers previously implanted for sick sinus syndrome or atrioventricular block. But researchers say their 107-patient study called myPACE – if confirmed in larger, multicenter trials – lays the groundwork for a device therapy that is broadly useful, potentially, in patients with “preclinical or overt” HFpEF.
Indeed, some of the intervention’s “quite substantial” benefits rivaled or surpassed what his group has observed with available HFpEF drug therapies, including the sodium-glucose cotransporter 2 inhibitors, observed Markus Meyer, MD, PhD, University of Minnesota, Minneapolis.
Moreover, the study may be “the first to show that, with this approach, we can actually also reduce atrial fibrillation,” he said in an interview.
Dr. Meyer said his group is “confident” that the HR-modulation strategy will be successful in appropriate clinical trials and that “pacemakers, in the end, will become a treatment modality for HFpEF.”
Meyer is senior author on the trial’s publication in JAMA Cardiology in JAMA Cardiology (2023 Feb 1. doi: 10.1001/jamacardio.2022.5320), with lead author Margaret Infeld, MD, University of Vermont, Burlington.
The trial entered pacemaker patients with HFpEF of stage B or C – that is, either asymptomatic with structural disease or fully symptomatic. But, Dr. Meyer said, “we saw that the treatment effect was much more pronounced in the patients who had overt heart failure with preserved ejection fraction.”
Challenging beta-blocker dogma
The study, the report states, “contradicts canonical thinking” by suggesting HFpEF patients may benefit from a higher resting heart rate, which would presumably shorten diastolic filling time. It also “may help reduce the overprescription of beta-blockers to allow higher heart rates in this population.”
Indeed, Dr. Meyer observed, no one really knows whether beta-blockers work in HFpEF, “because they really have never been studied in a sufficiently powered randomized controlled trial.”
The current study “basically rewrites what we know about the pathophysiology of this form of clinical heart failure,” said Michael R. Zile, MD, Medical University of South Carolina and Veterans Affairs Medical Center, both in Charleston, who was not part of the trial or report.
Previously in HFpEF, Dr. Zile said in an interview, “everybody thought you needed to make diastole longer to give the ventricle a longer time to fill. And none of that really made any sense. It was just sort of accepted as dogma.”
The idea led to widespread use of beta-blockers in HFpEF but “turned out just not to be true.” Indeed, European and North American guidelines, Dr. Zile observed, “have all taken beta-blockers out of the equation for HFpEF” except for treating comorbidities that can be associated with HFpEF, like hypertension or AFib.
Many patients with HFpEF and chronotropic incompetence could be provided with standard pacemakers with primarily conduction-system pacing but are not getting them, he observed.
The current study might help change that. No one is suggesting, based on the current study, “that we start putting pacemakers in every single patient with HFpEF,” Dr. Zile said. Still, for HFpEF patients already with a pacemaker, the study provides “reasonable assurance” that its criteria for elevated resting HR may well improve symptoms.
Moreover, it suggests such pacemakers, programmed as in the study, might potentially give a boost to HFpEF patients without chronotropic incompetence but with persisting symptoms despite guideline-directed drug therapy. That’s certainly worth exploring in further trials, Dr. Zile said.
How the study worked
The single-center trial entered 107 participants with HFpEF and pacemakers set, at baseline, to a backup resting HR of 60 bpm; their age averaged 75 and 48% were women. Only patients with devices for atrial pacing, conduction-system pacing, or biventricular pacing – which are unlikely to promote ventricular dyssynchrony – were included.
They were randomly assigned, double-blind, to have their devices set to an accelerated backup rate or to be continued at 60 bpm. The backup resting rate set for the intervention group’s 50 patients was individualized based on height and other factors; the median was 75 bpm.
Scores on the Minnesota Living with Heart Failure Questionnaire, the primary endpoint, improved in the intervention group, compared with baseline, by about 11 points after 1 month and by 15 points after 1 year (P < .001).
The scores in the usual-care group deteriorated by half a point and by 3.5 points at 1 month and 1 year (P = .03), respectively.
Consistent advantages for the accelerated-HR strategy were evident throughout the major secondary endpoints. For example, levels of N-terminal pro-B-type natriuretic peptide fell an average 109 pg/dL after 1 month in the accelerated-HR group and rose a mean of 128 pg/dL in the usual-care group (P = .02).
Mean daily pacemaker-monitored activity level rose by 47 minutes by 1 year in the accelerated-HR group, compared with a drop of 22 minutes for those assigned to the standard-care rate (P < .001).
AFib was detected in 18% of intervention patients at the 1-year follow-up, down from 31% at baseline. Their risk ratio for AFib at 1 year was 0.73 (95% confidence interval, 0.55-0.99, P = .04), compared with the control group.
In other patients with HFpEF “we have done pacing studies where we just ramped up the pacing rate, and we see that these pressures in the left atrium actually drop immediately,” Dr. Meyer said. It’s that “unburdening of the atria,” he added, that probably leads to the reduction in AFib.
Dr. Meyer reported holding a patent for pacemakers for HFpEF licensed to Medtronic. Dr. Zile said he consults for Medtronic and has no other relevant financial relationships.
A version of this article first appeared on Medscape.com.
Evidence supporting medications that slow the heart rate (HR), notably beta-blockers, is overwhelming in heart failure (HF) with reduced ejection fraction. Underwhelming, however, is clinical trial support for such agents in patients with HF with preserved ejection fraction (HFpEF).
Indeed, at least for some such patients, a treatment that modestly accelerates resting HR may be a more promising strategy, suggests an early line of research that challenges prevalent thinking about HFpEF therapy.
In a small, randomized test of the idea, patients with HFpEF and standard pacemakers set to a backup resting HR a bit higher than a standard of care 60 bpm, usually to about 75 bpm, reaped important quality of life benefits.
More strikingly, their natriuretic peptide levels and burden of atrial fibrillation (AFib) fell significantly, the latter by 27% over 1 year.
The trial enrolled only HFpEF patients with pacemakers previously implanted for sick sinus syndrome or atrioventricular block. But researchers say their 107-patient study called myPACE – if confirmed in larger, multicenter trials – lays the groundwork for a device therapy that is broadly useful, potentially, in patients with “preclinical or overt” HFpEF.
Indeed, some of the intervention’s “quite substantial” benefits rivaled or surpassed what his group has observed with available HFpEF drug therapies, including the sodium-glucose cotransporter 2 inhibitors, observed Markus Meyer, MD, PhD, University of Minnesota, Minneapolis.
Moreover, the study may be “the first to show that, with this approach, we can actually also reduce atrial fibrillation,” he said in an interview.
Dr. Meyer said his group is “confident” that the HR-modulation strategy will be successful in appropriate clinical trials and that “pacemakers, in the end, will become a treatment modality for HFpEF.”
Meyer is senior author on the trial’s publication in JAMA Cardiology in JAMA Cardiology (2023 Feb 1. doi: 10.1001/jamacardio.2022.5320), with lead author Margaret Infeld, MD, University of Vermont, Burlington.
The trial entered pacemaker patients with HFpEF of stage B or C – that is, either asymptomatic with structural disease or fully symptomatic. But, Dr. Meyer said, “we saw that the treatment effect was much more pronounced in the patients who had overt heart failure with preserved ejection fraction.”
Challenging beta-blocker dogma
The study, the report states, “contradicts canonical thinking” by suggesting HFpEF patients may benefit from a higher resting heart rate, which would presumably shorten diastolic filling time. It also “may help reduce the overprescription of beta-blockers to allow higher heart rates in this population.”
Indeed, Dr. Meyer observed, no one really knows whether beta-blockers work in HFpEF, “because they really have never been studied in a sufficiently powered randomized controlled trial.”
The current study “basically rewrites what we know about the pathophysiology of this form of clinical heart failure,” said Michael R. Zile, MD, Medical University of South Carolina and Veterans Affairs Medical Center, both in Charleston, who was not part of the trial or report.
Previously in HFpEF, Dr. Zile said in an interview, “everybody thought you needed to make diastole longer to give the ventricle a longer time to fill. And none of that really made any sense. It was just sort of accepted as dogma.”
The idea led to widespread use of beta-blockers in HFpEF but “turned out just not to be true.” Indeed, European and North American guidelines, Dr. Zile observed, “have all taken beta-blockers out of the equation for HFpEF” except for treating comorbidities that can be associated with HFpEF, like hypertension or AFib.
Many patients with HFpEF and chronotropic incompetence could be provided with standard pacemakers with primarily conduction-system pacing but are not getting them, he observed.
The current study might help change that. No one is suggesting, based on the current study, “that we start putting pacemakers in every single patient with HFpEF,” Dr. Zile said. Still, for HFpEF patients already with a pacemaker, the study provides “reasonable assurance” that its criteria for elevated resting HR may well improve symptoms.
Moreover, it suggests such pacemakers, programmed as in the study, might potentially give a boost to HFpEF patients without chronotropic incompetence but with persisting symptoms despite guideline-directed drug therapy. That’s certainly worth exploring in further trials, Dr. Zile said.
How the study worked
The single-center trial entered 107 participants with HFpEF and pacemakers set, at baseline, to a backup resting HR of 60 bpm; their age averaged 75 and 48% were women. Only patients with devices for atrial pacing, conduction-system pacing, or biventricular pacing – which are unlikely to promote ventricular dyssynchrony – were included.
They were randomly assigned, double-blind, to have their devices set to an accelerated backup rate or to be continued at 60 bpm. The backup resting rate set for the intervention group’s 50 patients was individualized based on height and other factors; the median was 75 bpm.
Scores on the Minnesota Living with Heart Failure Questionnaire, the primary endpoint, improved in the intervention group, compared with baseline, by about 11 points after 1 month and by 15 points after 1 year (P < .001).
The scores in the usual-care group deteriorated by half a point and by 3.5 points at 1 month and 1 year (P = .03), respectively.
Consistent advantages for the accelerated-HR strategy were evident throughout the major secondary endpoints. For example, levels of N-terminal pro-B-type natriuretic peptide fell an average 109 pg/dL after 1 month in the accelerated-HR group and rose a mean of 128 pg/dL in the usual-care group (P = .02).
Mean daily pacemaker-monitored activity level rose by 47 minutes by 1 year in the accelerated-HR group, compared with a drop of 22 minutes for those assigned to the standard-care rate (P < .001).
AFib was detected in 18% of intervention patients at the 1-year follow-up, down from 31% at baseline. Their risk ratio for AFib at 1 year was 0.73 (95% confidence interval, 0.55-0.99, P = .04), compared with the control group.
In other patients with HFpEF “we have done pacing studies where we just ramped up the pacing rate, and we see that these pressures in the left atrium actually drop immediately,” Dr. Meyer said. It’s that “unburdening of the atria,” he added, that probably leads to the reduction in AFib.
Dr. Meyer reported holding a patent for pacemakers for HFpEF licensed to Medtronic. Dr. Zile said he consults for Medtronic and has no other relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Longer diabetes duration links with increased heart failure
The longer people had diabetes, the greater their rate of incident heart failure, suggests a recently published review of prospectively collected observational data from nearly 24,000 people with diabetes in the UK Biobank.
The findings “add to the growing body of evidence suggesting that duration of diabetes is an important and independent determinant of heart failure among patients with diabetes,” comments Justin B. Echouffo-Tcheugui, MD, PhD, in an accompanying editorial.
Collectively, the new UK Biobank results and prior findings, “provide additional persuasive evidence that the link between duration of diabetes and heart failure is real,” although the physiological mechanisms behind the relationship remain incompletely understood, wrote Dr. Echouffo-Tcheugui, an endocrinologist at Johns Hopkins Medicine, Baltimore.
“The duration of diabetes may reflect cumulative effects of various adverse processes in the setting of diabetes” that result in “intrinsic myocardial lesions,” he suggested. These adverse processes might include not only hyperglycemia, but also glucotoxicity, lipotoxicity, hyperinsulinemia, advanced glycosylation end products, oxidative stress, mitochondrial dysfunction, cardiac autonomic neuropathy, and coronary microvascular dysfunction. Long-duration diabetes may also contribute to declining kidney function, which can further worsen heart failure risk.
The upshot is that clinicians may need to consider more systematically the duration of diabetes when assessing people with diabetes for heart failure.
Existing risk-assessment tools for predicting heart failure in people with diabetes “have not always accounted for diabetes duration,” Dr. Echouffo-Tcheugui noted.
Intensify heart failure detection with longer diabetes duration
“Active heart failure detection should perhaps be intensified with increased diabetes duration,” Dr. Echouffo-Tcheugui suggested in his editorial. He noted that a 2022 consensus report by the American Diabetes Association recommends clinicians measure natriuretic peptide or high-sensitivity cardiac troponin in all people with diabetes “on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure.”
The UK Biobank study was run by investigators primarily based in China and included data from 23,754 people with type 1 or type 2 diabetes and no heart failure at baseline. The prospectively collected data allowed for a median follow-up of 11.7 years, during which time 2,081 people developed incident heart failure.
In an analysis that divided participants into four categories of diabetes duration (< 5 years, 5-9 years, 10-14 years, and ≥ 15 years) and adjusted for potential confounders, heart failure incidence showed a significant 32% increased incidence among those with diabetes for at least 15 years, compared with those with diabetes for less than 5 years. People with a diabetes duration of 5-14 years showed a trend toward having more incident heart failure, compared with those with diabetes for less than 5 years, but the difference was not significant.
An adjusted analysis also showed poor glycemic control at baseline (hemoglobin A1c ≥ 8.0%) significantly linked with a 46% increased incidence of heart failure, compared with those with baseline A1c less than 7.0%.
Additive effect?
When the authors analyzed the effect of both these variables, they saw a roughly additive effect.
Patients with diabetes for at least 15 years and a baseline A1c of at least 8.0% had a 98% increased incidence of heart failure, compared with those who had diabetes for less than 5 years and a baseline A1c less than 7.0%, after adjustment. This association was independent of age, sex, and race.
These findings “highlight the paramount role of the duration of diabetes and its interaction with glycemic control in the development of heart failure,” the authors concluded. “Long duration of diabetes and poor glycemic control may result in structural and functional changes in the myocardium, which is likely to underlie the pathogenesis of heart failure among individuals with diabetes.”
In his editorial, Dr. Echouffo-Tcheugui lauded the report for its “robust” analyses that included a large sample and accounted for key confounders, such as glycemic control. However, he also cited eight “shortcomings” of the study, including its sole reliance on A1c levels to identify diabetes, a likely underestimation of diabetes duration, the lumping together of people with type 1 and type 2 diabetes, and lack of a subanalysis of incident heart failure in those with preserved or reduced left ventricular ejection fraction.
Among prior reports of evidence also suggesting an effect of diabetes duration on incident heart failure, Dr. Echouffo-Tcheugui cited a study he led, published in 2021, that analyzed prospective, longitudinal, observational data from 9,734 adults enrolled in the Atherosclerosis Risk in Communities study. The results showed that, compared with those without diabetes, the incidence of heart failure rose with longer diabetes duration, with the highest risk among those with diabetes for at least 15 years, who had a 2.8-fold increase in heart failure versus the reference group. Each 5-year increase in diabetes duration was associated with a significant 17% relative increase in heart failure incidence.
The study received no commercial funding. The authors and editorialist reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The longer people had diabetes, the greater their rate of incident heart failure, suggests a recently published review of prospectively collected observational data from nearly 24,000 people with diabetes in the UK Biobank.
The findings “add to the growing body of evidence suggesting that duration of diabetes is an important and independent determinant of heart failure among patients with diabetes,” comments Justin B. Echouffo-Tcheugui, MD, PhD, in an accompanying editorial.
Collectively, the new UK Biobank results and prior findings, “provide additional persuasive evidence that the link between duration of diabetes and heart failure is real,” although the physiological mechanisms behind the relationship remain incompletely understood, wrote Dr. Echouffo-Tcheugui, an endocrinologist at Johns Hopkins Medicine, Baltimore.
“The duration of diabetes may reflect cumulative effects of various adverse processes in the setting of diabetes” that result in “intrinsic myocardial lesions,” he suggested. These adverse processes might include not only hyperglycemia, but also glucotoxicity, lipotoxicity, hyperinsulinemia, advanced glycosylation end products, oxidative stress, mitochondrial dysfunction, cardiac autonomic neuropathy, and coronary microvascular dysfunction. Long-duration diabetes may also contribute to declining kidney function, which can further worsen heart failure risk.
The upshot is that clinicians may need to consider more systematically the duration of diabetes when assessing people with diabetes for heart failure.
Existing risk-assessment tools for predicting heart failure in people with diabetes “have not always accounted for diabetes duration,” Dr. Echouffo-Tcheugui noted.
Intensify heart failure detection with longer diabetes duration
“Active heart failure detection should perhaps be intensified with increased diabetes duration,” Dr. Echouffo-Tcheugui suggested in his editorial. He noted that a 2022 consensus report by the American Diabetes Association recommends clinicians measure natriuretic peptide or high-sensitivity cardiac troponin in all people with diabetes “on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure.”
The UK Biobank study was run by investigators primarily based in China and included data from 23,754 people with type 1 or type 2 diabetes and no heart failure at baseline. The prospectively collected data allowed for a median follow-up of 11.7 years, during which time 2,081 people developed incident heart failure.
In an analysis that divided participants into four categories of diabetes duration (< 5 years, 5-9 years, 10-14 years, and ≥ 15 years) and adjusted for potential confounders, heart failure incidence showed a significant 32% increased incidence among those with diabetes for at least 15 years, compared with those with diabetes for less than 5 years. People with a diabetes duration of 5-14 years showed a trend toward having more incident heart failure, compared with those with diabetes for less than 5 years, but the difference was not significant.
An adjusted analysis also showed poor glycemic control at baseline (hemoglobin A1c ≥ 8.0%) significantly linked with a 46% increased incidence of heart failure, compared with those with baseline A1c less than 7.0%.
Additive effect?
When the authors analyzed the effect of both these variables, they saw a roughly additive effect.
Patients with diabetes for at least 15 years and a baseline A1c of at least 8.0% had a 98% increased incidence of heart failure, compared with those who had diabetes for less than 5 years and a baseline A1c less than 7.0%, after adjustment. This association was independent of age, sex, and race.
These findings “highlight the paramount role of the duration of diabetes and its interaction with glycemic control in the development of heart failure,” the authors concluded. “Long duration of diabetes and poor glycemic control may result in structural and functional changes in the myocardium, which is likely to underlie the pathogenesis of heart failure among individuals with diabetes.”
In his editorial, Dr. Echouffo-Tcheugui lauded the report for its “robust” analyses that included a large sample and accounted for key confounders, such as glycemic control. However, he also cited eight “shortcomings” of the study, including its sole reliance on A1c levels to identify diabetes, a likely underestimation of diabetes duration, the lumping together of people with type 1 and type 2 diabetes, and lack of a subanalysis of incident heart failure in those with preserved or reduced left ventricular ejection fraction.
Among prior reports of evidence also suggesting an effect of diabetes duration on incident heart failure, Dr. Echouffo-Tcheugui cited a study he led, published in 2021, that analyzed prospective, longitudinal, observational data from 9,734 adults enrolled in the Atherosclerosis Risk in Communities study. The results showed that, compared with those without diabetes, the incidence of heart failure rose with longer diabetes duration, with the highest risk among those with diabetes for at least 15 years, who had a 2.8-fold increase in heart failure versus the reference group. Each 5-year increase in diabetes duration was associated with a significant 17% relative increase in heart failure incidence.
The study received no commercial funding. The authors and editorialist reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The longer people had diabetes, the greater their rate of incident heart failure, suggests a recently published review of prospectively collected observational data from nearly 24,000 people with diabetes in the UK Biobank.
The findings “add to the growing body of evidence suggesting that duration of diabetes is an important and independent determinant of heart failure among patients with diabetes,” comments Justin B. Echouffo-Tcheugui, MD, PhD, in an accompanying editorial.
Collectively, the new UK Biobank results and prior findings, “provide additional persuasive evidence that the link between duration of diabetes and heart failure is real,” although the physiological mechanisms behind the relationship remain incompletely understood, wrote Dr. Echouffo-Tcheugui, an endocrinologist at Johns Hopkins Medicine, Baltimore.
“The duration of diabetes may reflect cumulative effects of various adverse processes in the setting of diabetes” that result in “intrinsic myocardial lesions,” he suggested. These adverse processes might include not only hyperglycemia, but also glucotoxicity, lipotoxicity, hyperinsulinemia, advanced glycosylation end products, oxidative stress, mitochondrial dysfunction, cardiac autonomic neuropathy, and coronary microvascular dysfunction. Long-duration diabetes may also contribute to declining kidney function, which can further worsen heart failure risk.
The upshot is that clinicians may need to consider more systematically the duration of diabetes when assessing people with diabetes for heart failure.
Existing risk-assessment tools for predicting heart failure in people with diabetes “have not always accounted for diabetes duration,” Dr. Echouffo-Tcheugui noted.
Intensify heart failure detection with longer diabetes duration
“Active heart failure detection should perhaps be intensified with increased diabetes duration,” Dr. Echouffo-Tcheugui suggested in his editorial. He noted that a 2022 consensus report by the American Diabetes Association recommends clinicians measure natriuretic peptide or high-sensitivity cardiac troponin in all people with diabetes “on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure.”
The UK Biobank study was run by investigators primarily based in China and included data from 23,754 people with type 1 or type 2 diabetes and no heart failure at baseline. The prospectively collected data allowed for a median follow-up of 11.7 years, during which time 2,081 people developed incident heart failure.
In an analysis that divided participants into four categories of diabetes duration (< 5 years, 5-9 years, 10-14 years, and ≥ 15 years) and adjusted for potential confounders, heart failure incidence showed a significant 32% increased incidence among those with diabetes for at least 15 years, compared with those with diabetes for less than 5 years. People with a diabetes duration of 5-14 years showed a trend toward having more incident heart failure, compared with those with diabetes for less than 5 years, but the difference was not significant.
An adjusted analysis also showed poor glycemic control at baseline (hemoglobin A1c ≥ 8.0%) significantly linked with a 46% increased incidence of heart failure, compared with those with baseline A1c less than 7.0%.
Additive effect?
When the authors analyzed the effect of both these variables, they saw a roughly additive effect.
Patients with diabetes for at least 15 years and a baseline A1c of at least 8.0% had a 98% increased incidence of heart failure, compared with those who had diabetes for less than 5 years and a baseline A1c less than 7.0%, after adjustment. This association was independent of age, sex, and race.
These findings “highlight the paramount role of the duration of diabetes and its interaction with glycemic control in the development of heart failure,” the authors concluded. “Long duration of diabetes and poor glycemic control may result in structural and functional changes in the myocardium, which is likely to underlie the pathogenesis of heart failure among individuals with diabetes.”
In his editorial, Dr. Echouffo-Tcheugui lauded the report for its “robust” analyses that included a large sample and accounted for key confounders, such as glycemic control. However, he also cited eight “shortcomings” of the study, including its sole reliance on A1c levels to identify diabetes, a likely underestimation of diabetes duration, the lumping together of people with type 1 and type 2 diabetes, and lack of a subanalysis of incident heart failure in those with preserved or reduced left ventricular ejection fraction.
Among prior reports of evidence also suggesting an effect of diabetes duration on incident heart failure, Dr. Echouffo-Tcheugui cited a study he led, published in 2021, that analyzed prospective, longitudinal, observational data from 9,734 adults enrolled in the Atherosclerosis Risk in Communities study. The results showed that, compared with those without diabetes, the incidence of heart failure rose with longer diabetes duration, with the highest risk among those with diabetes for at least 15 years, who had a 2.8-fold increase in heart failure versus the reference group. Each 5-year increase in diabetes duration was associated with a significant 17% relative increase in heart failure incidence.
The study received no commercial funding. The authors and editorialist reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Nearly 12% of PsA patients need musculoskeletal surgery
Among adults with psoriatic arthritis (PsA), 11.8% required at least one musculoskeletal surgery related to their disease, based on data from more than 1,500 individuals at the University of Toronto’s Psoriatic Arthritis Clinic.
“Despite optimal medical therapy to control systemic inflammation and preserve joint function, a subset of patients with PsA still require musculoskeletal (MSK) surgery for disease-related morbidity,” but data on the prevalence of MSK surgeries and the associated risk factors are lacking, wrote Timothy S.H. Kwok, MD, of the University of Toronto, and colleagues.
In a study published in The Journal of Rheumatology, the researchers reviewed data from a longitudinal cohort of 1,574 adults aged 18 years and older with PsA established at the Toronto clinic during 1978-2019.
Overall, 185 patients had 379 MSK surgeries related to PsA during the study period for a prevalence of 11.8%.
The most common procedures were arthrodesis and arthroplasty (27% for both). More than half (59%) of the surgeries were joint sacrificing, and 41% were joint retaining, and 57 procedures were revisions related to the primary surgery.
Among 1,018 patients with data complete enough for a multivariate analysis, including 71 PsA surgeries, factors significantly associated with an increased risk for surgery were a higher number of damaged joints (hazard ratio [HR], 1.03; P < .001), tender or swollen joints (HR, 1.04; P = .01), and the presence of nail lesions (HR, 2.08; P < .01). Other predictors of surgery were higher scores on the Health Assessment Questionnaire (HR, 2.01; P < .001), elevated erythrocyte sedimentation rate (HR, 2.37; P = .02), and HLA-B27 positivity (HR, 2.22; P = .048).
However, a higher score on the Psoriasis Area Severity Index was significantly associated with lower risk of surgery (HR, 0.88; P < .002) The use of biologics had no significant impact on MSK surgery, the researchers noted.
The high percentage of joint sacrificing surgeries suggests a high burden of MSK surgery in patients with PsA, the researchers wrote in their discussion. The current study supports findings from previous studies and highlights the potential limitations and need for improvement in the current medical treatment paradigm for PsA, they said.
The findings were limited by several factors, including the potential for referral bias of complex cases to the center, which might have caused overestimation of the number of surgeries. The similarity in surgeries specifically related to PsA and degenerative arthritis also makes overestimation of surgeries possible, the researchers noted.
However, the study is one of the largest known to evaluate the prevalence of risk factors for MSK surgery in PsA patients over a long period of time, and identified surgeries directly attributable to PsA, they said. The study ended prior to the onset of the COVID-19 pandemic, which increased the external validity of the findings, they added.
The study was supported by the Krembil Foundation. The researchers had no financial conflicts to disclose.
Among adults with psoriatic arthritis (PsA), 11.8% required at least one musculoskeletal surgery related to their disease, based on data from more than 1,500 individuals at the University of Toronto’s Psoriatic Arthritis Clinic.
“Despite optimal medical therapy to control systemic inflammation and preserve joint function, a subset of patients with PsA still require musculoskeletal (MSK) surgery for disease-related morbidity,” but data on the prevalence of MSK surgeries and the associated risk factors are lacking, wrote Timothy S.H. Kwok, MD, of the University of Toronto, and colleagues.
In a study published in The Journal of Rheumatology, the researchers reviewed data from a longitudinal cohort of 1,574 adults aged 18 years and older with PsA established at the Toronto clinic during 1978-2019.
Overall, 185 patients had 379 MSK surgeries related to PsA during the study period for a prevalence of 11.8%.
The most common procedures were arthrodesis and arthroplasty (27% for both). More than half (59%) of the surgeries were joint sacrificing, and 41% were joint retaining, and 57 procedures were revisions related to the primary surgery.
Among 1,018 patients with data complete enough for a multivariate analysis, including 71 PsA surgeries, factors significantly associated with an increased risk for surgery were a higher number of damaged joints (hazard ratio [HR], 1.03; P < .001), tender or swollen joints (HR, 1.04; P = .01), and the presence of nail lesions (HR, 2.08; P < .01). Other predictors of surgery were higher scores on the Health Assessment Questionnaire (HR, 2.01; P < .001), elevated erythrocyte sedimentation rate (HR, 2.37; P = .02), and HLA-B27 positivity (HR, 2.22; P = .048).
However, a higher score on the Psoriasis Area Severity Index was significantly associated with lower risk of surgery (HR, 0.88; P < .002) The use of biologics had no significant impact on MSK surgery, the researchers noted.
The high percentage of joint sacrificing surgeries suggests a high burden of MSK surgery in patients with PsA, the researchers wrote in their discussion. The current study supports findings from previous studies and highlights the potential limitations and need for improvement in the current medical treatment paradigm for PsA, they said.
The findings were limited by several factors, including the potential for referral bias of complex cases to the center, which might have caused overestimation of the number of surgeries. The similarity in surgeries specifically related to PsA and degenerative arthritis also makes overestimation of surgeries possible, the researchers noted.
However, the study is one of the largest known to evaluate the prevalence of risk factors for MSK surgery in PsA patients over a long period of time, and identified surgeries directly attributable to PsA, they said. The study ended prior to the onset of the COVID-19 pandemic, which increased the external validity of the findings, they added.
The study was supported by the Krembil Foundation. The researchers had no financial conflicts to disclose.
Among adults with psoriatic arthritis (PsA), 11.8% required at least one musculoskeletal surgery related to their disease, based on data from more than 1,500 individuals at the University of Toronto’s Psoriatic Arthritis Clinic.
“Despite optimal medical therapy to control systemic inflammation and preserve joint function, a subset of patients with PsA still require musculoskeletal (MSK) surgery for disease-related morbidity,” but data on the prevalence of MSK surgeries and the associated risk factors are lacking, wrote Timothy S.H. Kwok, MD, of the University of Toronto, and colleagues.
In a study published in The Journal of Rheumatology, the researchers reviewed data from a longitudinal cohort of 1,574 adults aged 18 years and older with PsA established at the Toronto clinic during 1978-2019.
Overall, 185 patients had 379 MSK surgeries related to PsA during the study period for a prevalence of 11.8%.
The most common procedures were arthrodesis and arthroplasty (27% for both). More than half (59%) of the surgeries were joint sacrificing, and 41% were joint retaining, and 57 procedures were revisions related to the primary surgery.
Among 1,018 patients with data complete enough for a multivariate analysis, including 71 PsA surgeries, factors significantly associated with an increased risk for surgery were a higher number of damaged joints (hazard ratio [HR], 1.03; P < .001), tender or swollen joints (HR, 1.04; P = .01), and the presence of nail lesions (HR, 2.08; P < .01). Other predictors of surgery were higher scores on the Health Assessment Questionnaire (HR, 2.01; P < .001), elevated erythrocyte sedimentation rate (HR, 2.37; P = .02), and HLA-B27 positivity (HR, 2.22; P = .048).
However, a higher score on the Psoriasis Area Severity Index was significantly associated with lower risk of surgery (HR, 0.88; P < .002) The use of biologics had no significant impact on MSK surgery, the researchers noted.
The high percentage of joint sacrificing surgeries suggests a high burden of MSK surgery in patients with PsA, the researchers wrote in their discussion. The current study supports findings from previous studies and highlights the potential limitations and need for improvement in the current medical treatment paradigm for PsA, they said.
The findings were limited by several factors, including the potential for referral bias of complex cases to the center, which might have caused overestimation of the number of surgeries. The similarity in surgeries specifically related to PsA and degenerative arthritis also makes overestimation of surgeries possible, the researchers noted.
However, the study is one of the largest known to evaluate the prevalence of risk factors for MSK surgery in PsA patients over a long period of time, and identified surgeries directly attributable to PsA, they said. The study ended prior to the onset of the COVID-19 pandemic, which increased the external validity of the findings, they added.
The study was supported by the Krembil Foundation. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF RHEUMATOLOGY
Could boosting fat taste receptors help cut calories?
Desire for dietary lipids has been traced to the taste receptors CD36 and GPR120, and these have been found to be malfunctioning in both obese animals and humans, leading to low perception of fat levels in food.
The study was published online in Cellular and Molecular Gastroenterology and Hepatology. “Ours is the first study on targeting fat taste receptors, leading to [the] activation of tongue-gut loop as a therapeutic approach, and it opens new vistas to synthesize more potent chemical compounds to decrease progressive weight gain under [high-fat diet] consumption,” the authors wrote.
The perception of fat has recently been identified as a potential sixth basic taste quality, joining sweet, sour, bitter, salt, and umami. CD36 is expressed by taste cells, where it senses dietary long-chain fatty acids (LCFAs), and its deletion led mice to ignore LCFAs and oily solutions that they would otherwise prefer. GPR120 has also been proposed as a lipid sensor.
Previous researchers had suggested that CD36 may play a role in the preference for eating fats, while GPR120 could have a role in lipid satiation following consumption. “Our team also supported these conclusions and proposed that CD36 might be involved in immediate early detection [of fat in foods], whereas GPR120 will be responsible for post-ingestive regulation of lipid food intake,” the authors wrote.
The researchers showed that lipids bind to CD36 when they are present in low concentrations, but at high concentrations they bind to GPR120, suggesting that the two receptors are nonoverlapping but nevertheless complement one another during fatty acid–mediated signaling with taste bud cells (TBC). They are also coexpressed within the same type of TBC.
Experiments in rodents suggest that obese animals have reduced capacity to sense dietary fatty acids, which drives consumption of greater amounts. Fat-rich diets can also reduce fat taste perception, and this has been shown cross-sectionally in obese human subjects, and a single nucleotide polymorphism in CD36 that leads to a reduction in expression is linked to reduced perception of dietary fatty acids.
To test the idea that altering the receptors could change behavior, the researchers synthesized two novel fat taste receptor agonists (FTAs) that are derived from the LCFA linoleic acid, which is abundant in Western diets.
Using nerve recordings, the researchers confirmed that a message from TBCs is sent to the brain via the chorda tympani nerve, and the two FTAs increased the nerve signal. The signals from LCFAs alone were boosted with the addition of the FTAs, suggesting that these molecules can be effective even in the presence of dietary lipids. They also confirmed that FTAs activate the tongue-brain-gut loop by increasing pancreato-bile secretion more than linoleic acid alone.
Given the choice between two bottles, mice preferred the one containing FTAs, and the experiments indicated that FTAs are 95-142 times more potent than natural LCFA as food attractants.
It is well known that diet and lifestyle interventions rarely result in long-term weight loss, and products designed to mimic ‘fat-like’ texture – such as maltodextrin, inulin, and plant fibers – have had limited success because they do not have a fat-like taste and can lead to gastrointestinal side effects. Agonists of CD36 and GPR120 added to low- or noncaloric foods could boost their appeal and lead to earlier satiation.
Importantly, in obese mice, both TFAs led to decreased food intake as well as reduced weight gain and fat mass, without affecting lean mass. One of the agents also promoted a higher metabolic rate through increased energy expenditure.
The researchers also examined the agents’ effects on the microbiota of the obese mice, which contain high concentrations of bacteria belonging to the Lachnospiraceae family. Both inhibitors reduced the numbers of Lachnospiraceae bacteria, and promoted other bacterial families that may contribute to an anti-inflammatory effect. Obese animals exposed to TFAs also showed improvements in dyslipidemia, and there was evidence that they could reduce liver lipid concentrations.
There was no evidence of any mutagenicity, genotoxicity, or endocrine disruption. In sum, these new agonists might enable the development of novel treatments of obesity, which would have a major impact on human health.
The authors stated that they have no financial conflicts of interest. The study received financial support from institutions including the Société d'Accélération du Transfert de Technologies and the University of Burgundy.
This article was updated 2/15/23.
The obesity epidemic represents a significant public health crisis that has spread to most countries on the planet. In addition to being a major risk factor for diabetes and cardiovascular disease, obesity also impacts the incidence of gastrointestinal cancers. Despite major efforts of health professionals and public health messaging, it remains very difficult for patients to achieve sustained weight loss by changing diet and increasing physical activity alone. Novel approaches to regulate food intake and thus obesity are urgently needed.
While these are preclinical studies, it will now be fascinating to determine if these or similar compounds can be developed into drugs or food additives to impact human food intake and thus become an additional tool in the fight against the obesity epidemic.
Klaus H. Kaestner, PhD, MS, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, University of Pennsylvania, Philadelphia. He has no financial conflicts of interest.
The obesity epidemic represents a significant public health crisis that has spread to most countries on the planet. In addition to being a major risk factor for diabetes and cardiovascular disease, obesity also impacts the incidence of gastrointestinal cancers. Despite major efforts of health professionals and public health messaging, it remains very difficult for patients to achieve sustained weight loss by changing diet and increasing physical activity alone. Novel approaches to regulate food intake and thus obesity are urgently needed.
While these are preclinical studies, it will now be fascinating to determine if these or similar compounds can be developed into drugs or food additives to impact human food intake and thus become an additional tool in the fight against the obesity epidemic.
Klaus H. Kaestner, PhD, MS, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, University of Pennsylvania, Philadelphia. He has no financial conflicts of interest.
The obesity epidemic represents a significant public health crisis that has spread to most countries on the planet. In addition to being a major risk factor for diabetes and cardiovascular disease, obesity also impacts the incidence of gastrointestinal cancers. Despite major efforts of health professionals and public health messaging, it remains very difficult for patients to achieve sustained weight loss by changing diet and increasing physical activity alone. Novel approaches to regulate food intake and thus obesity are urgently needed.
While these are preclinical studies, it will now be fascinating to determine if these or similar compounds can be developed into drugs or food additives to impact human food intake and thus become an additional tool in the fight against the obesity epidemic.
Klaus H. Kaestner, PhD, MS, is with the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases, University of Pennsylvania, Philadelphia. He has no financial conflicts of interest.
Desire for dietary lipids has been traced to the taste receptors CD36 and GPR120, and these have been found to be malfunctioning in both obese animals and humans, leading to low perception of fat levels in food.
The study was published online in Cellular and Molecular Gastroenterology and Hepatology. “Ours is the first study on targeting fat taste receptors, leading to [the] activation of tongue-gut loop as a therapeutic approach, and it opens new vistas to synthesize more potent chemical compounds to decrease progressive weight gain under [high-fat diet] consumption,” the authors wrote.
The perception of fat has recently been identified as a potential sixth basic taste quality, joining sweet, sour, bitter, salt, and umami. CD36 is expressed by taste cells, where it senses dietary long-chain fatty acids (LCFAs), and its deletion led mice to ignore LCFAs and oily solutions that they would otherwise prefer. GPR120 has also been proposed as a lipid sensor.
Previous researchers had suggested that CD36 may play a role in the preference for eating fats, while GPR120 could have a role in lipid satiation following consumption. “Our team also supported these conclusions and proposed that CD36 might be involved in immediate early detection [of fat in foods], whereas GPR120 will be responsible for post-ingestive regulation of lipid food intake,” the authors wrote.
The researchers showed that lipids bind to CD36 when they are present in low concentrations, but at high concentrations they bind to GPR120, suggesting that the two receptors are nonoverlapping but nevertheless complement one another during fatty acid–mediated signaling with taste bud cells (TBC). They are also coexpressed within the same type of TBC.
Experiments in rodents suggest that obese animals have reduced capacity to sense dietary fatty acids, which drives consumption of greater amounts. Fat-rich diets can also reduce fat taste perception, and this has been shown cross-sectionally in obese human subjects, and a single nucleotide polymorphism in CD36 that leads to a reduction in expression is linked to reduced perception of dietary fatty acids.
To test the idea that altering the receptors could change behavior, the researchers synthesized two novel fat taste receptor agonists (FTAs) that are derived from the LCFA linoleic acid, which is abundant in Western diets.
Using nerve recordings, the researchers confirmed that a message from TBCs is sent to the brain via the chorda tympani nerve, and the two FTAs increased the nerve signal. The signals from LCFAs alone were boosted with the addition of the FTAs, suggesting that these molecules can be effective even in the presence of dietary lipids. They also confirmed that FTAs activate the tongue-brain-gut loop by increasing pancreato-bile secretion more than linoleic acid alone.
Given the choice between two bottles, mice preferred the one containing FTAs, and the experiments indicated that FTAs are 95-142 times more potent than natural LCFA as food attractants.
It is well known that diet and lifestyle interventions rarely result in long-term weight loss, and products designed to mimic ‘fat-like’ texture – such as maltodextrin, inulin, and plant fibers – have had limited success because they do not have a fat-like taste and can lead to gastrointestinal side effects. Agonists of CD36 and GPR120 added to low- or noncaloric foods could boost their appeal and lead to earlier satiation.
Importantly, in obese mice, both TFAs led to decreased food intake as well as reduced weight gain and fat mass, without affecting lean mass. One of the agents also promoted a higher metabolic rate through increased energy expenditure.
The researchers also examined the agents’ effects on the microbiota of the obese mice, which contain high concentrations of bacteria belonging to the Lachnospiraceae family. Both inhibitors reduced the numbers of Lachnospiraceae bacteria, and promoted other bacterial families that may contribute to an anti-inflammatory effect. Obese animals exposed to TFAs also showed improvements in dyslipidemia, and there was evidence that they could reduce liver lipid concentrations.
There was no evidence of any mutagenicity, genotoxicity, or endocrine disruption. In sum, these new agonists might enable the development of novel treatments of obesity, which would have a major impact on human health.
The authors stated that they have no financial conflicts of interest. The study received financial support from institutions including the Société d'Accélération du Transfert de Technologies and the University of Burgundy.
This article was updated 2/15/23.
Desire for dietary lipids has been traced to the taste receptors CD36 and GPR120, and these have been found to be malfunctioning in both obese animals and humans, leading to low perception of fat levels in food.
The study was published online in Cellular and Molecular Gastroenterology and Hepatology. “Ours is the first study on targeting fat taste receptors, leading to [the] activation of tongue-gut loop as a therapeutic approach, and it opens new vistas to synthesize more potent chemical compounds to decrease progressive weight gain under [high-fat diet] consumption,” the authors wrote.
The perception of fat has recently been identified as a potential sixth basic taste quality, joining sweet, sour, bitter, salt, and umami. CD36 is expressed by taste cells, where it senses dietary long-chain fatty acids (LCFAs), and its deletion led mice to ignore LCFAs and oily solutions that they would otherwise prefer. GPR120 has also been proposed as a lipid sensor.
Previous researchers had suggested that CD36 may play a role in the preference for eating fats, while GPR120 could have a role in lipid satiation following consumption. “Our team also supported these conclusions and proposed that CD36 might be involved in immediate early detection [of fat in foods], whereas GPR120 will be responsible for post-ingestive regulation of lipid food intake,” the authors wrote.
The researchers showed that lipids bind to CD36 when they are present in low concentrations, but at high concentrations they bind to GPR120, suggesting that the two receptors are nonoverlapping but nevertheless complement one another during fatty acid–mediated signaling with taste bud cells (TBC). They are also coexpressed within the same type of TBC.
Experiments in rodents suggest that obese animals have reduced capacity to sense dietary fatty acids, which drives consumption of greater amounts. Fat-rich diets can also reduce fat taste perception, and this has been shown cross-sectionally in obese human subjects, and a single nucleotide polymorphism in CD36 that leads to a reduction in expression is linked to reduced perception of dietary fatty acids.
To test the idea that altering the receptors could change behavior, the researchers synthesized two novel fat taste receptor agonists (FTAs) that are derived from the LCFA linoleic acid, which is abundant in Western diets.
Using nerve recordings, the researchers confirmed that a message from TBCs is sent to the brain via the chorda tympani nerve, and the two FTAs increased the nerve signal. The signals from LCFAs alone were boosted with the addition of the FTAs, suggesting that these molecules can be effective even in the presence of dietary lipids. They also confirmed that FTAs activate the tongue-brain-gut loop by increasing pancreato-bile secretion more than linoleic acid alone.
Given the choice between two bottles, mice preferred the one containing FTAs, and the experiments indicated that FTAs are 95-142 times more potent than natural LCFA as food attractants.
It is well known that diet and lifestyle interventions rarely result in long-term weight loss, and products designed to mimic ‘fat-like’ texture – such as maltodextrin, inulin, and plant fibers – have had limited success because they do not have a fat-like taste and can lead to gastrointestinal side effects. Agonists of CD36 and GPR120 added to low- or noncaloric foods could boost their appeal and lead to earlier satiation.
Importantly, in obese mice, both TFAs led to decreased food intake as well as reduced weight gain and fat mass, without affecting lean mass. One of the agents also promoted a higher metabolic rate through increased energy expenditure.
The researchers also examined the agents’ effects on the microbiota of the obese mice, which contain high concentrations of bacteria belonging to the Lachnospiraceae family. Both inhibitors reduced the numbers of Lachnospiraceae bacteria, and promoted other bacterial families that may contribute to an anti-inflammatory effect. Obese animals exposed to TFAs also showed improvements in dyslipidemia, and there was evidence that they could reduce liver lipid concentrations.
There was no evidence of any mutagenicity, genotoxicity, or endocrine disruption. In sum, these new agonists might enable the development of novel treatments of obesity, which would have a major impact on human health.
The authors stated that they have no financial conflicts of interest. The study received financial support from institutions including the Société d'Accélération du Transfert de Technologies and the University of Burgundy.
This article was updated 2/15/23.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Pruritic rash on arms and legs
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 27-year-old student presents with a pruritic rash on his hands and in the bends of his arms and legs. He recently started clinical rotations in a nursing facility and has been using hand sanitizer multiple times per day, which has exacerbated the rash on his hands, causing them to ooze and sting. He describes the rash as itchy, especially at night. At times he reports that the itching causes difficulty sleeping. In addition, his skin has little cracks that frequently bleed. He notes that he has experienced similar symptoms in the past, which resolved with moisturizers and topical cream from the drugstore. He has tried over-the-counter hydrocortisone during this episode, with minimal improvement in symptoms. He denies any change in laundry detergents or use of new household products.
Physical examination reveals large erythematous plaques on the hands and flexure surfaces of his neck, antecubital fossa, and behind the knees with scattered excoriations. Erythematous, slightly lichenified coalescing papules are noted on the proximal arms. His face is clear. General skin pigmentation is brown and free of masses and lumps.
Product updates and reviews
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.