User login
HIV vaccine trial makes pivotal leap toward making ‘super antibodies’
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
FROM SCIENCE
The new obesity breakthrough drugs
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.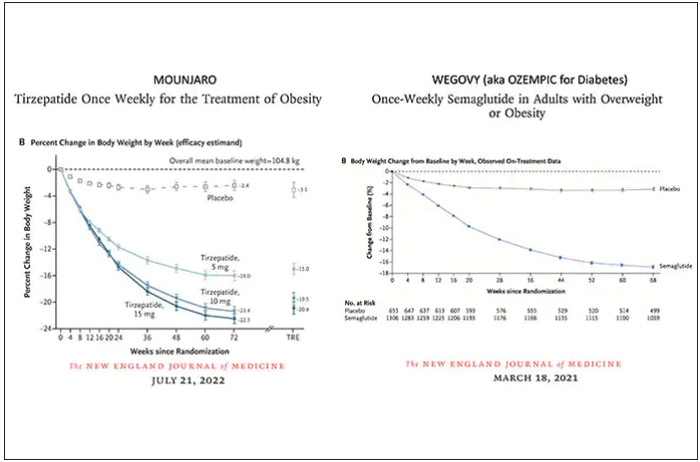
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction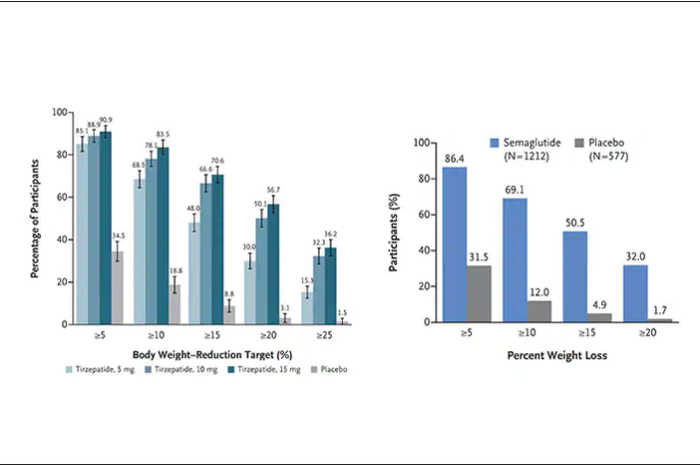
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.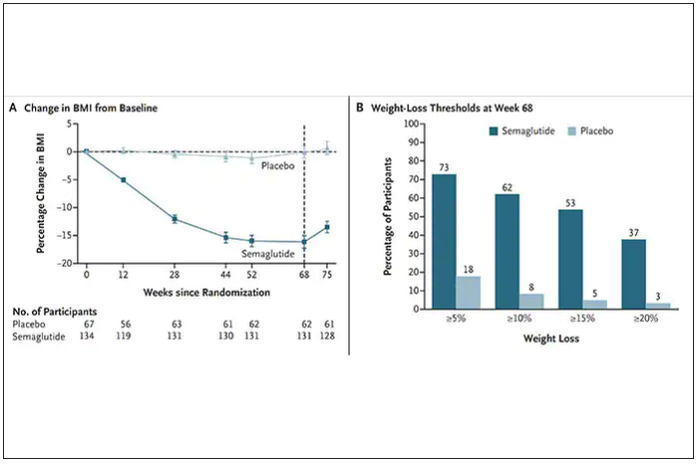
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.
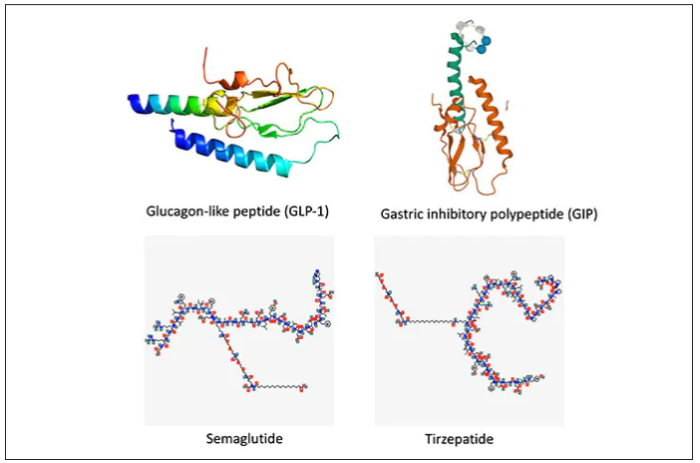
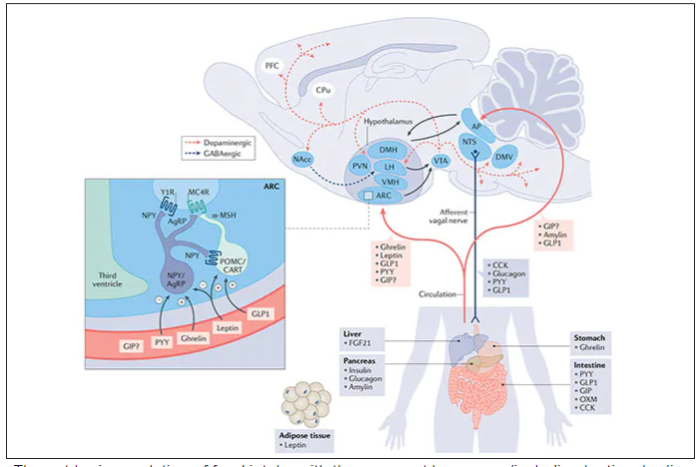
These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
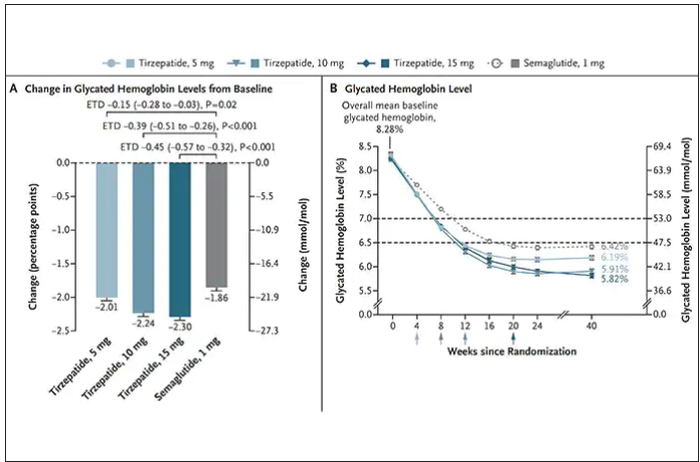
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.
Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.
While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
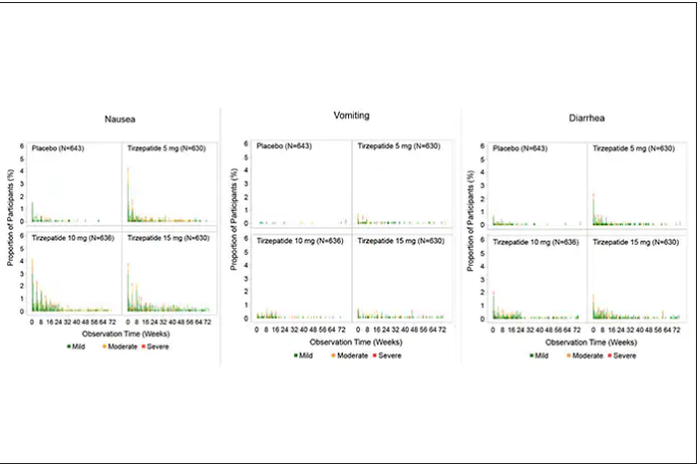
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.
Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.

Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.

While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.

Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.

While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
Can a Mediterranean diet ease depression in young men?
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
Noninvasive laser therapy tied to improved short-term memory
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Terminally ill cancer patients struggle to access psilocybin
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
Should you quit employment to open a practice? These docs share how they did it
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
CAB-LA’s full potential for HIV prevention hits snags
, say authors of a new review article.
CAB-LA “represents the most important breakthrough in HIV prevention in recent years,” write Geoffroy Liegeon, MD, and Jade Ghosn, MD, PhD, with Université Paris Cité, in this month’s HIV Medicine.
It has been found to be safe, and more effective in phase 3 trials than oral PrEP, and is well-accepted in men who have sex with men, and transgender and cisgender women.
Reductions in stigma
Surveys show patients at high risk for HIV – especially those who see PrEP as burdensome – are highly interested in long-acting injectable drugs. Reduced stigma with the injections also appears to steer the choice toward a long-acting agent and may attract more people to HIV prevention programs.
The first two injections are given 4 weeks apart, followed by an injection every 8 weeks.
Models designed to increase uptake, adherence, and persistence when on and after discontinuing CAB-LA will be important for wider rollout, as will better patient education and demonstrated efficacy and safety in populations not included in clinical trials, Dr. Liegeon and Dr. Ghosn note.
Still, they point out that its broader integration into clinical routine is held back by factors including breakthrough infections despite timely injections, complexity of follow-up, logistical considerations, and its cost-effectiveness compared with oral PrEP.
A hefty price tag
“[T]he cost effectiveness compared with TDF-FTC [tenofovir/emtricitabine] generics may not support its use at the current price in many settings,” the authors write.
For low- and middle-income countries, the TDF/FTC price is about $55, according to the World Health Organization’s Global Price Reporting, while the current price of CAB-LA in the United States is about $22,000, according to Dr. Ghosn. He said in an interview that because the cost of generics can reach $400-$500 per year in the United States, depending on the pharmaceutical companies, the price for CAB-LA is almost 60 times higher than TDF/FTC in the Untied States.
The biggest hope for the price reduction, at least in lower-income countries, he said, is a new licensing agreement.
ViiV Healthcare signed a new voluntary licensing agreement with the Medicines Patent Pool in July to help access in low-income, lower-middle-income, and sub-Saharan African countries, he explained.
The authors summarize: “[E]stablishing the effectiveness of CAB-LA does not guarantee its uptake into clinical routine.”
Because of the combined issues, the WHO recommended CAB-LA as an additional prevention choice for PrEP in its recent guidelines, pending further studies.
Barriers frustrate providers
Lauren Fontana, DO, assistant professor at the University of Minnesota, Minneapolis, and infectious disease physician at M Health Fairview, said in an interview that “as a health care provider, cost and insurance barriers can be frustrating, especially when CAB-LA is identified as the best option for a patient.”
Lack of nonphysician-led initiatives, such as nurse- or pharmacy-led services for CAB-LA, may limit availability to marginalized and at-risk populations, she said.
“If a clinic can acquire CAB-LA, clinic protocols need to be developed and considerations of missed visits and doses must be thought about when implementing a program,” Dr. Fontana said.
Clinics need resources to engage with patients to promote retention in the program with case management and pharmacy support, she added.
“Simplification processes need to be developed to make CAB-LA an option for more clinics and patients,” she continued. “We are still learning about the incidence of breakthrough HIV infections, patterns of HIV seroconversion, and how to optimize testing so that HIV infections are detected early.”
Dr. Liegeon, Dr. Ghosn, and Dr. Fontana report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, say authors of a new review article.
CAB-LA “represents the most important breakthrough in HIV prevention in recent years,” write Geoffroy Liegeon, MD, and Jade Ghosn, MD, PhD, with Université Paris Cité, in this month’s HIV Medicine.
It has been found to be safe, and more effective in phase 3 trials than oral PrEP, and is well-accepted in men who have sex with men, and transgender and cisgender women.
Reductions in stigma
Surveys show patients at high risk for HIV – especially those who see PrEP as burdensome – are highly interested in long-acting injectable drugs. Reduced stigma with the injections also appears to steer the choice toward a long-acting agent and may attract more people to HIV prevention programs.
The first two injections are given 4 weeks apart, followed by an injection every 8 weeks.
Models designed to increase uptake, adherence, and persistence when on and after discontinuing CAB-LA will be important for wider rollout, as will better patient education and demonstrated efficacy and safety in populations not included in clinical trials, Dr. Liegeon and Dr. Ghosn note.
Still, they point out that its broader integration into clinical routine is held back by factors including breakthrough infections despite timely injections, complexity of follow-up, logistical considerations, and its cost-effectiveness compared with oral PrEP.
A hefty price tag
“[T]he cost effectiveness compared with TDF-FTC [tenofovir/emtricitabine] generics may not support its use at the current price in many settings,” the authors write.
For low- and middle-income countries, the TDF/FTC price is about $55, according to the World Health Organization’s Global Price Reporting, while the current price of CAB-LA in the United States is about $22,000, according to Dr. Ghosn. He said in an interview that because the cost of generics can reach $400-$500 per year in the United States, depending on the pharmaceutical companies, the price for CAB-LA is almost 60 times higher than TDF/FTC in the Untied States.
The biggest hope for the price reduction, at least in lower-income countries, he said, is a new licensing agreement.
ViiV Healthcare signed a new voluntary licensing agreement with the Medicines Patent Pool in July to help access in low-income, lower-middle-income, and sub-Saharan African countries, he explained.
The authors summarize: “[E]stablishing the effectiveness of CAB-LA does not guarantee its uptake into clinical routine.”
Because of the combined issues, the WHO recommended CAB-LA as an additional prevention choice for PrEP in its recent guidelines, pending further studies.
Barriers frustrate providers
Lauren Fontana, DO, assistant professor at the University of Minnesota, Minneapolis, and infectious disease physician at M Health Fairview, said in an interview that “as a health care provider, cost and insurance barriers can be frustrating, especially when CAB-LA is identified as the best option for a patient.”
Lack of nonphysician-led initiatives, such as nurse- or pharmacy-led services for CAB-LA, may limit availability to marginalized and at-risk populations, she said.
“If a clinic can acquire CAB-LA, clinic protocols need to be developed and considerations of missed visits and doses must be thought about when implementing a program,” Dr. Fontana said.
Clinics need resources to engage with patients to promote retention in the program with case management and pharmacy support, she added.
“Simplification processes need to be developed to make CAB-LA an option for more clinics and patients,” she continued. “We are still learning about the incidence of breakthrough HIV infections, patterns of HIV seroconversion, and how to optimize testing so that HIV infections are detected early.”
Dr. Liegeon, Dr. Ghosn, and Dr. Fontana report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, say authors of a new review article.
CAB-LA “represents the most important breakthrough in HIV prevention in recent years,” write Geoffroy Liegeon, MD, and Jade Ghosn, MD, PhD, with Université Paris Cité, in this month’s HIV Medicine.
It has been found to be safe, and more effective in phase 3 trials than oral PrEP, and is well-accepted in men who have sex with men, and transgender and cisgender women.
Reductions in stigma
Surveys show patients at high risk for HIV – especially those who see PrEP as burdensome – are highly interested in long-acting injectable drugs. Reduced stigma with the injections also appears to steer the choice toward a long-acting agent and may attract more people to HIV prevention programs.
The first two injections are given 4 weeks apart, followed by an injection every 8 weeks.
Models designed to increase uptake, adherence, and persistence when on and after discontinuing CAB-LA will be important for wider rollout, as will better patient education and demonstrated efficacy and safety in populations not included in clinical trials, Dr. Liegeon and Dr. Ghosn note.
Still, they point out that its broader integration into clinical routine is held back by factors including breakthrough infections despite timely injections, complexity of follow-up, logistical considerations, and its cost-effectiveness compared with oral PrEP.
A hefty price tag
“[T]he cost effectiveness compared with TDF-FTC [tenofovir/emtricitabine] generics may not support its use at the current price in many settings,” the authors write.
For low- and middle-income countries, the TDF/FTC price is about $55, according to the World Health Organization’s Global Price Reporting, while the current price of CAB-LA in the United States is about $22,000, according to Dr. Ghosn. He said in an interview that because the cost of generics can reach $400-$500 per year in the United States, depending on the pharmaceutical companies, the price for CAB-LA is almost 60 times higher than TDF/FTC in the Untied States.
The biggest hope for the price reduction, at least in lower-income countries, he said, is a new licensing agreement.
ViiV Healthcare signed a new voluntary licensing agreement with the Medicines Patent Pool in July to help access in low-income, lower-middle-income, and sub-Saharan African countries, he explained.
The authors summarize: “[E]stablishing the effectiveness of CAB-LA does not guarantee its uptake into clinical routine.”
Because of the combined issues, the WHO recommended CAB-LA as an additional prevention choice for PrEP in its recent guidelines, pending further studies.
Barriers frustrate providers
Lauren Fontana, DO, assistant professor at the University of Minnesota, Minneapolis, and infectious disease physician at M Health Fairview, said in an interview that “as a health care provider, cost and insurance barriers can be frustrating, especially when CAB-LA is identified as the best option for a patient.”
Lack of nonphysician-led initiatives, such as nurse- or pharmacy-led services for CAB-LA, may limit availability to marginalized and at-risk populations, she said.
“If a clinic can acquire CAB-LA, clinic protocols need to be developed and considerations of missed visits and doses must be thought about when implementing a program,” Dr. Fontana said.
Clinics need resources to engage with patients to promote retention in the program with case management and pharmacy support, she added.
“Simplification processes need to be developed to make CAB-LA an option for more clinics and patients,” she continued. “We are still learning about the incidence of breakthrough HIV infections, patterns of HIV seroconversion, and how to optimize testing so that HIV infections are detected early.”
Dr. Liegeon, Dr. Ghosn, and Dr. Fontana report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HIV MEDICINE
A Transdisciplinary Program for Care of Veterans With Neurocognitive Disorders
Dementia is a devastating condition resulting in major functional, emotional, and financial impact on patients, their caregivers, and families. Approximately 6.5 million Americans are living with Alzheimer disease (AD), the most common of many causes of dementia.1 The prevalence of AD could increase to 12.7 million Americans by 2050 as the population ages.1 Studies suggest that dementia, also known as major neurocognitive disorder, is common and underdiagnosed among US veterans, a population with a mean age of 65 years.2 During cognitive screening, memory impairment is present in approximately 20% of veterans aged ≥ 75 years who have not been diagnosed with a neurocognitive disorder.3 In addition, veterans might be particularly vulnerable to dementia at an earlier age than the general population because of vascular risk factors and traumatic brain injuries.4 These concerns highlight the need for effective dementia care programs at US Department of Veterans Affairs (VA) facilities.
The US health care system often does not adequately address the needs of patients with dementia and their caregivers.5 Dementia care requires specialized medical care among collaborating professionals and caregiver and psychosocial interventions and services. However, the US health care system is fragmented with different clinicians and services siloed into separate practices and most dementia care occurring in primary care settings.6 Primary care professionals (PCPs) often are uncomfortable diagnosing and managing dementia because of time constraints, lack of expertise and training, and inability to deal with the range of care needs.7 PCPs do not identify approximately 42% of their patients with dementia and, when recognized, do not adhere to dementia care guidelines and address caregiver needs.8-10 Research indicates that caregiver support improves dementia care by teaching behavioral management skills and caregiver coping strategies, allowing patients to stay at home and delay institutionalization.6,11,12 Clinicians underuse available resources and do not incorporate them in their patient care.10 These community services benefit patients and caregivers and significantly improve the overall quality of care.6
Memory clinics have emerged to address these deficiencies when managing dementia.13 The most effective memory clinics maximize the use of specialists with different expertise in dementia care, particularly integrated programs where disciplines function together rather than independently.1,5,14 Systematic reviews and meta-analyses have documented the effectiveness of collaborative care management programs.11,12,15 Integration of dementia care management is associated with earlier diagnosis and interventions, decreased functional and cognitive symptom severity, decreased or delayed institutionalization, improved quality of life for patients and caregivers, enhanced overall quality of care and cost-effectiveness, and better integration of community services.11,12,14-19 In these programs, designating a dementia care manager (DCM) as the patient’s advocate facilitates the integrated structure, increases the quality of care, helps caregivers, facilitates adherence to dementia practice guidelines, and prevents behavioral and psychological symptoms of dementia (BPSD).1,6,11,12,20,21
The best interprofessional model for dementia care might be the transdisciplinary model that includes a DCM. To meet the specific demands of dementia care, there must be a high level of interprofessional collaboration rather than multiple health care professionals (HCPs) delivering care in isolation—an approach that is time consuming and often difficult to implement.22 Whereas multidisciplinary care refers to delivery of parallel services and interdisciplinary care implies a joint formulation, transdisciplinary care aims to maximize integration of HCPs and their specific expertise and contributions through interactions and discussions that deliver focused input to the lead physician. The transdisciplinary model addresses needs that often are missed and can minimize disparities in the quality of dementia care.23 A DCM is an integral part of our program, facilitating understanding and implementation of the final care plan and providing long-term follow-up and care. We outline a conference-centered transdisciplinary dementia care model with a social worker as DCM (SW-DCM) at our VA medical center.
Program Description
In 2020, the VA Greater Los Angeles Healthcare System (VAGLAHS) in California established a multispecialty clinic dedicated to evaluation and treatment of veterans with memory and neurocognitive disorders and to provide support for their caregivers and families. With the agreement of leadership in mental health, neurology, and geriatrics services on the importance of collaboration for dementia care, the psychiatry and neurology services created a joint Memory and Neurobehavior Clinic, which completed its first 2 years of operation as a full-day program. In recent months, the clinic has scheduled 24 veterans per day, approximately 50% new evaluations and 50% follow-up patients, with wait times of < 2 months. There is a mean of 12 intake or lead physicians who could attend sessions in the morning, afternoon, or both. The general clinic flow consists of a 2-hour intake evaluation of new referrals by the lead physician followed by a clinic conference with transdisciplinary discussion. The DCM then follows up with the veteran/caregiver presenting a final care plan individualized to the veterans, caregivers, and families.
The Memory and Neurobehavior team includes behavioral neurologists, geriatric psychiatrists, neuropsychologists, geriatric fellows, advanced clinical nurses, and social workers who function as the DCM (Table 1).
Procedures
Before the office visit, the coordinating geriatric psychiatrist triages veterans to neurology, psychiatry, or geriatric physicians based on the clinical presentation, history of neurologic signs or symptoms, BPSD or psychiatric history, functional decline, or comorbid medical illnesses. Although veterans often have overlapping concerns, the triage process aims to coordinate the intake evaluations with the most indicated and available specialist with the intention to notify the other specialists during the transdisciplinary conference.
Referrals to the program occur from many sources, notably from primary care (70.8%), mental health (16.7%), and specialty clinics (12.5%). The clinic also receives referrals from the affiliated Veterans Cognitive Assessment and Management Program, which provides dementia evaluation and support via telehealth screening. This VAGLAHS program services a diverse population of veterans: 87% male; 43% aged > 65 years (75% in our clinic); 51% non-Hispanic White; 19% non-Hispanic African American; 16% Hispanic; 4% Asian; and 1% Native American. This population receives care at regional VA medical centers and community-based outpatient clinics over a wide geographic service area.
The initial standardized assessments by intake or lead physicians includes mental status screening with the Montreal Cognitive Assessment (with certified clinicians), the Neurobehavioral Status Examination for a more detailed assessment of cognitive domains, the Columbia-Suicide Severity Rating Scale, the Patient Health Questionnaire for depression screening, and assessment for impairments in instrumental or basic activities of daily living. This initial evaluation aims to apply clinical guidelines and diagnostic criteria for the differential diagnosis of neurocognitive disorders, determine eligibility for cognitive-enhancing medications and techniques, assess for BPSD and the need for nonpharmacologic or pharmacologic interventions, determine functional status, and evaluate the need for supervision, safety concerns, and evidence of neglect or abuse.
As part of its mission, the clinic is charged with implementing the VA Dementia System of Care (DSOC). The stated goals of the DSOC are to provide individualized person-centered dementia care to help veterans experiencing dementia and their caregivers maintain a positive and optimal quality of life and create an environment where VA medical center staff understand the health care needs of veterans with dementia and their caregivers’ role. As part of this initiative, the clinic includes (1) coordination of care through a SW-DCM; (2)
Transdisciplinary Conference
Clinic conferences are held after the veterans are seen. Staff gather to discuss the patient and review management. All team members are present, as well as the head of the clinical clerical staff who can facilitate appointments, make lobby and wait times more bearable for our patients and caregivers, and help manage emergencies. Although this is an in-person conference, the COVID-19 pandemic has allowed us to include staff who screen at remote sites via videoconferencing, similar to other VA programs.24 The Memory and Neurobehavior Clinic has two ≤ 90-minute conferences daily. The lead physicians and their senior attendings present the new intake evaluations (4-6 at each conference session) with a preliminary formulation and questions for discussion. The moderator solicits contributions from the different disciplines, going from one to the next and recording their responses for each veteran. Further specialists are available for consultation through the conference mechanism if necessary. The final assessment is reviewed, a diagnosis is established, and a tailored, individualized care plan for adjusting or optimizing the veteran’s care is presented to the lead physician who makes the final determination. At the close of the conference, the team’s discussion is recorded along with the lead physician’s original detailed intake evaluation. Currently, the records go into the Computerized Patient Record System, but we are making plans to transition to Cerner as it is implemented.
During the discussion, team members review several areas of consideration. If there is neuroimaging, neurologists review the images projected on a large computer screen. Team members also will assess for the need to obtain biomarker studies, such as blood, cerebrospinal fluid, or positron emission tomography. Psychiatrists could review management of BPSD and use of psychotropic agents, and neuropsychologists might consider the need for more precise cognitive testing and whether a capacity assessment is indicated. Social work might bring up the need for a durable power of attorney as well as applicable caregiver and community resources. Geriatric medicine and nursing could provide input into medical management and care and the ability of veterans and caregivers to follow the prescribed regimen. Further areas of discussion include driving safety and restrictions on driving (as required in California) and the presence of guns in the home. Finally, brief education is provided in short 10-to-15-minute lectures covering pertinent topics so staff remain up-to-date in this changing field.
Postconference Continuity
After the conference, the SW-DCM continues to provide support throughout the disease course, helping veterans and their caregivers understand and follow through on the team’s recommendations. The SW-DCM, who is experienced and trained in case management, forms an ongoing relationship with the veterans and their caregivers and remains an advocate for their care. The SW-DCM communicates the final plan by phone and, when necessary, requests the lead physician to call to clarify any poorly understood or technical aspects of the care plan. About 50% of our veterans—primarily those who do not have a neurocognitive disorder or have mild cognitive impairment—return to their PCPs with our care plan consultation; about 25% are already enrolled in geriatric and other programs with long-term follow-up. The assigned SW-DCM follows up with the remaining veterans and caregivers regularly by phone, facilitates communication with other team members, and endeavors to assure postvisit continuity of care and support during advancing stages of the disease. In addition, the SW-DCM can provide supportive counseling and psychotherapy for stressed caregivers, refer to support groups and cognitive rehabilitation programs, and help develop long-term goals and consideration for supervised living environments. The nurse specialist participates with follow-up calls regarding medications and scheduled tests and appointments, clearing up confusion about instructions, avoiding medication errors, and providing education in dementia care. Both social worker and nurse are present throughout the week, reachable by phone, and, in turn, able to contact the clinic physicians for veterans’ needs.
Discussion
Because of the heterogenous medical and psychosocial needs of veterans with dementia and their caregivers, a transdisciplinary team with a dedicated DCM might offer the most effective and efficient model for dementia care. We present a transdisciplinary program that incorporates dementia specialists in a single evaluation by maximizing their time through a conference-centered program. Our program involves neurologists, psychiatrists, geriatricians, psychologists, nurses, and social workers collaborating and communicating to enact effective dementia care. It further meets the goals of the VA-DSOC in implementing individualized patient and caregiver care.
This transdisciplinary model addresses a number of issues, starting with the differential diagnosis of underlying neurologic conditions. Within the transdisciplinary team, the neurologist can provide specific insights into any neurologic findings and illnesses, such as Alzheimer disease and other neurodegenerative dementias, vascular dementia syndromes, normal pressure hydrocephalus, Creutzfeldt-Jakob disease, neurosyphilis, and others. Most veterans with dementia experience BPSD at some point during of their illness. The psychiatrists on the transdisciplinary team can maximize management of BPSD with nonpharmacologic interventions and the fewest and least aversive psychoactive medications. Our program also addresses the need for more precise cognitive evaluation. Neuropsychologists are present and available for administrating neuropsychologic tests and interpreting cognitive performance and any earlier neuropsychologic testing. This model also cares for the caregivers and assesses their needs. The social worker—as well as other members of the team—can provide caregivers with strategies for coping with disruptive and other behaviors related to dementia, counsel them on how to manage the veteran’s functional decline, and aid in establishing a safe living space. Because the social worker serves as a DCM, these coping and adjustment questions occupy significant clinical attention between appointments. This transdisciplinary model places the patient’s illness in the context of their functional status, diagnoses, and medications. The team geriatrician and the nurse specialist are indispensable resources. The clinic conference provides a teaching venue for staff and trainees and a mechanism to discuss new developments in dementia care, such as the increasing need to assess individuals with mild cognitive impairment.25 This model depends on the DCM’s invaluable role in ensuring implementation of the dementia care plan and continuity of care.
Conclusions
We describe effective dementia care with a transdisciplinary team in a conference setting and with the participation of a dedicated DCM.5 To date, this program appears to be an efficient, sustainable application of the limited resources allocated to dementia care. Nevertheless, we are collecting data to compare with performance measures, track use, and assess the programs effects on continuity of care. We look forward to presenting metrics from our program that show improvement in the health care for veterans experiencing a devastating and increasingly common disorder.
1. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789. doi:10.1002/alz.12638
2. National Center for Veterans Analysis and Statistics. Profile of veterans: 2016. Accessed October 12, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf
3. Chodosh J, Sultzer DL, Lee ML, et al. Memory impairment among primary care veterans. Aging Ment Health. 2007;11(4):444-450. doi:10.1080/13607860601086272
4. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627. doi:10.1080/02699052.2022.20338465. Heintz H, Monette P, Epstein-Lubow G, Smith L, Rowlett S, Forester BP. Emerging collaborative care models for dementia care in the primary care setting: a narrative review. Am J Geriatr Psychiatry. 2020;28(3):320-330. doi:10.1016/j.jagp.2019.07.015
6. Reuben DB, Evertson LC, Wenger NS, et al. The University of California at Los Angeles Alzheimer’s and Dementia Care program for comprehensive, coordinated, patient-centered care: preliminary data. J Am Geriatr Soc. 2013;61(12):2214-2218. doi:10.1111/jgs.12562
7. Apesoa-Varano EC, Barker JC, Hinton L. Curing and caring: the work of primary care physicians with dementia patients. Qual Health Res. 2011;21(11):1469-1483. doi:10.1177/1049732311412788
8. Creavin ST, Noel-Storr AH, Langdon RJ, et al. Clinical judgement by primary care physicians for the diagnosis of all-cause dementia or cognitive impairment in symptomatic people. Cochrane Database Syst Rev. 2022;6:CD012558. doi:10.1002/14651858.CD012558.pub2
9. Sivananthan SN, Puyat JH, McGrail KM. Variations in self-reported practice of physicians providing clinical care to individuals with dementia: a systematic review. J Am Geriatr Soc. 2013;61(8):1277-1285. doi:10.1111/jgs.12368
10. Rosen CS, Chow HC, Greenbaum MA, et al. How well are clinicians following dementia practice guidelines? Alzheimer Dis Assoc Disord. 2002;16(1):15-23. doi:10.1097/00002093-200201000-00003
11. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345. doi:10.1002/14651858.CD008345.pub2
12. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2013;28(9):889-902. doi:10.1002/gps.3906
13. Jolley D, Benbow SM, Grizzell M. Memory clinics. Postgrad Med J. 2006;82(965):199-206. doi:10.1136/pgmj.2005.040592
14. Muhlichen F, Michalowsky B, Radke A, et al. Tasks and activities of an effective collaborative dementia care management program in German primary care. J Alzheimers Dis. 2022;87(4):1615-1625. doi:10.3233/JAD-215656
15. Somme D, Trouve H, Drame M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement. 2012;8(5):426-436. doi:10.1016/j.jalz.2011.06.004
16. Ramakers IH, Verhey FR. Development of memory clinics in the Netherlands: 1998 to 2009. Aging Ment Health. 2011;15(1):34-39. doi:10.1080/13607863.2010.519321
17. LaMantia MA, Alder CA, Callahan CM, et al. The aging brain care medical home: preliminary data. J Am Geriatr Soc. 2015;63(6):1209-1213. doi:10.1111/jgs.13447
18. Rubinsztein JS, van Rensburg MJ, Al-Salihy Z, et al. A memory clinic v. traditional community mental health team service: comparison of costs and quality. BJPsych Bull. 2015;39(1):6-11. doi:10.1192/pb.bp.113.044263
19. Lee L, Hillier LM, Harvey D. Integrating community services into primary care: improving the quality of dementia care. Neurodegener Dis Manag. 2014;4(1):11-21. doi:10.2217/nmt.13.72
20. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386. doi:10.1111/jgs.12362
21. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157. doi:10.1001/jama.295.18.2148
22. Leggett A, Connell C, Dubin L, et al. Dementia care across a tertiary care health system: what exists now and what needs to change. J Am Med Dir Assoc. 2019;20(10):1307-12 e1. doi:10.1016/j.jamda.2019.04.006
23. Brown AF, Vassar SD, Connor KI, Vickrey BG. Collaborative care management reduces disparities in dementia care quality for caregivers with less education. J Am Geriatr Soc. 2013;61(2):243-251. doi:10.1111/jgs.12079
24. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
25. Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s Disease: evolving the care team for optimal patient management. Front Neurol. 2020;11:592302. doi:10.3389/fneur.2020.592302
Dementia is a devastating condition resulting in major functional, emotional, and financial impact on patients, their caregivers, and families. Approximately 6.5 million Americans are living with Alzheimer disease (AD), the most common of many causes of dementia.1 The prevalence of AD could increase to 12.7 million Americans by 2050 as the population ages.1 Studies suggest that dementia, also known as major neurocognitive disorder, is common and underdiagnosed among US veterans, a population with a mean age of 65 years.2 During cognitive screening, memory impairment is present in approximately 20% of veterans aged ≥ 75 years who have not been diagnosed with a neurocognitive disorder.3 In addition, veterans might be particularly vulnerable to dementia at an earlier age than the general population because of vascular risk factors and traumatic brain injuries.4 These concerns highlight the need for effective dementia care programs at US Department of Veterans Affairs (VA) facilities.
The US health care system often does not adequately address the needs of patients with dementia and their caregivers.5 Dementia care requires specialized medical care among collaborating professionals and caregiver and psychosocial interventions and services. However, the US health care system is fragmented with different clinicians and services siloed into separate practices and most dementia care occurring in primary care settings.6 Primary care professionals (PCPs) often are uncomfortable diagnosing and managing dementia because of time constraints, lack of expertise and training, and inability to deal with the range of care needs.7 PCPs do not identify approximately 42% of their patients with dementia and, when recognized, do not adhere to dementia care guidelines and address caregiver needs.8-10 Research indicates that caregiver support improves dementia care by teaching behavioral management skills and caregiver coping strategies, allowing patients to stay at home and delay institutionalization.6,11,12 Clinicians underuse available resources and do not incorporate them in their patient care.10 These community services benefit patients and caregivers and significantly improve the overall quality of care.6
Memory clinics have emerged to address these deficiencies when managing dementia.13 The most effective memory clinics maximize the use of specialists with different expertise in dementia care, particularly integrated programs where disciplines function together rather than independently.1,5,14 Systematic reviews and meta-analyses have documented the effectiveness of collaborative care management programs.11,12,15 Integration of dementia care management is associated with earlier diagnosis and interventions, decreased functional and cognitive symptom severity, decreased or delayed institutionalization, improved quality of life for patients and caregivers, enhanced overall quality of care and cost-effectiveness, and better integration of community services.11,12,14-19 In these programs, designating a dementia care manager (DCM) as the patient’s advocate facilitates the integrated structure, increases the quality of care, helps caregivers, facilitates adherence to dementia practice guidelines, and prevents behavioral and psychological symptoms of dementia (BPSD).1,6,11,12,20,21
The best interprofessional model for dementia care might be the transdisciplinary model that includes a DCM. To meet the specific demands of dementia care, there must be a high level of interprofessional collaboration rather than multiple health care professionals (HCPs) delivering care in isolation—an approach that is time consuming and often difficult to implement.22 Whereas multidisciplinary care refers to delivery of parallel services and interdisciplinary care implies a joint formulation, transdisciplinary care aims to maximize integration of HCPs and their specific expertise and contributions through interactions and discussions that deliver focused input to the lead physician. The transdisciplinary model addresses needs that often are missed and can minimize disparities in the quality of dementia care.23 A DCM is an integral part of our program, facilitating understanding and implementation of the final care plan and providing long-term follow-up and care. We outline a conference-centered transdisciplinary dementia care model with a social worker as DCM (SW-DCM) at our VA medical center.
Program Description
In 2020, the VA Greater Los Angeles Healthcare System (VAGLAHS) in California established a multispecialty clinic dedicated to evaluation and treatment of veterans with memory and neurocognitive disorders and to provide support for their caregivers and families. With the agreement of leadership in mental health, neurology, and geriatrics services on the importance of collaboration for dementia care, the psychiatry and neurology services created a joint Memory and Neurobehavior Clinic, which completed its first 2 years of operation as a full-day program. In recent months, the clinic has scheduled 24 veterans per day, approximately 50% new evaluations and 50% follow-up patients, with wait times of < 2 months. There is a mean of 12 intake or lead physicians who could attend sessions in the morning, afternoon, or both. The general clinic flow consists of a 2-hour intake evaluation of new referrals by the lead physician followed by a clinic conference with transdisciplinary discussion. The DCM then follows up with the veteran/caregiver presenting a final care plan individualized to the veterans, caregivers, and families.
The Memory and Neurobehavior team includes behavioral neurologists, geriatric psychiatrists, neuropsychologists, geriatric fellows, advanced clinical nurses, and social workers who function as the DCM (Table 1).
Procedures
Before the office visit, the coordinating geriatric psychiatrist triages veterans to neurology, psychiatry, or geriatric physicians based on the clinical presentation, history of neurologic signs or symptoms, BPSD or psychiatric history, functional decline, or comorbid medical illnesses. Although veterans often have overlapping concerns, the triage process aims to coordinate the intake evaluations with the most indicated and available specialist with the intention to notify the other specialists during the transdisciplinary conference.
Referrals to the program occur from many sources, notably from primary care (70.8%), mental health (16.7%), and specialty clinics (12.5%). The clinic also receives referrals from the affiliated Veterans Cognitive Assessment and Management Program, which provides dementia evaluation and support via telehealth screening. This VAGLAHS program services a diverse population of veterans: 87% male; 43% aged > 65 years (75% in our clinic); 51% non-Hispanic White; 19% non-Hispanic African American; 16% Hispanic; 4% Asian; and 1% Native American. This population receives care at regional VA medical centers and community-based outpatient clinics over a wide geographic service area.
The initial standardized assessments by intake or lead physicians includes mental status screening with the Montreal Cognitive Assessment (with certified clinicians), the Neurobehavioral Status Examination for a more detailed assessment of cognitive domains, the Columbia-Suicide Severity Rating Scale, the Patient Health Questionnaire for depression screening, and assessment for impairments in instrumental or basic activities of daily living. This initial evaluation aims to apply clinical guidelines and diagnostic criteria for the differential diagnosis of neurocognitive disorders, determine eligibility for cognitive-enhancing medications and techniques, assess for BPSD and the need for nonpharmacologic or pharmacologic interventions, determine functional status, and evaluate the need for supervision, safety concerns, and evidence of neglect or abuse.
As part of its mission, the clinic is charged with implementing the VA Dementia System of Care (DSOC). The stated goals of the DSOC are to provide individualized person-centered dementia care to help veterans experiencing dementia and their caregivers maintain a positive and optimal quality of life and create an environment where VA medical center staff understand the health care needs of veterans with dementia and their caregivers’ role. As part of this initiative, the clinic includes (1) coordination of care through a SW-DCM; (2)
Transdisciplinary Conference
Clinic conferences are held after the veterans are seen. Staff gather to discuss the patient and review management. All team members are present, as well as the head of the clinical clerical staff who can facilitate appointments, make lobby and wait times more bearable for our patients and caregivers, and help manage emergencies. Although this is an in-person conference, the COVID-19 pandemic has allowed us to include staff who screen at remote sites via videoconferencing, similar to other VA programs.24 The Memory and Neurobehavior Clinic has two ≤ 90-minute conferences daily. The lead physicians and their senior attendings present the new intake evaluations (4-6 at each conference session) with a preliminary formulation and questions for discussion. The moderator solicits contributions from the different disciplines, going from one to the next and recording their responses for each veteran. Further specialists are available for consultation through the conference mechanism if necessary. The final assessment is reviewed, a diagnosis is established, and a tailored, individualized care plan for adjusting or optimizing the veteran’s care is presented to the lead physician who makes the final determination. At the close of the conference, the team’s discussion is recorded along with the lead physician’s original detailed intake evaluation. Currently, the records go into the Computerized Patient Record System, but we are making plans to transition to Cerner as it is implemented.
During the discussion, team members review several areas of consideration. If there is neuroimaging, neurologists review the images projected on a large computer screen. Team members also will assess for the need to obtain biomarker studies, such as blood, cerebrospinal fluid, or positron emission tomography. Psychiatrists could review management of BPSD and use of psychotropic agents, and neuropsychologists might consider the need for more precise cognitive testing and whether a capacity assessment is indicated. Social work might bring up the need for a durable power of attorney as well as applicable caregiver and community resources. Geriatric medicine and nursing could provide input into medical management and care and the ability of veterans and caregivers to follow the prescribed regimen. Further areas of discussion include driving safety and restrictions on driving (as required in California) and the presence of guns in the home. Finally, brief education is provided in short 10-to-15-minute lectures covering pertinent topics so staff remain up-to-date in this changing field.
Postconference Continuity
After the conference, the SW-DCM continues to provide support throughout the disease course, helping veterans and their caregivers understand and follow through on the team’s recommendations. The SW-DCM, who is experienced and trained in case management, forms an ongoing relationship with the veterans and their caregivers and remains an advocate for their care. The SW-DCM communicates the final plan by phone and, when necessary, requests the lead physician to call to clarify any poorly understood or technical aspects of the care plan. About 50% of our veterans—primarily those who do not have a neurocognitive disorder or have mild cognitive impairment—return to their PCPs with our care plan consultation; about 25% are already enrolled in geriatric and other programs with long-term follow-up. The assigned SW-DCM follows up with the remaining veterans and caregivers regularly by phone, facilitates communication with other team members, and endeavors to assure postvisit continuity of care and support during advancing stages of the disease. In addition, the SW-DCM can provide supportive counseling and psychotherapy for stressed caregivers, refer to support groups and cognitive rehabilitation programs, and help develop long-term goals and consideration for supervised living environments. The nurse specialist participates with follow-up calls regarding medications and scheduled tests and appointments, clearing up confusion about instructions, avoiding medication errors, and providing education in dementia care. Both social worker and nurse are present throughout the week, reachable by phone, and, in turn, able to contact the clinic physicians for veterans’ needs.
Discussion
Because of the heterogenous medical and psychosocial needs of veterans with dementia and their caregivers, a transdisciplinary team with a dedicated DCM might offer the most effective and efficient model for dementia care. We present a transdisciplinary program that incorporates dementia specialists in a single evaluation by maximizing their time through a conference-centered program. Our program involves neurologists, psychiatrists, geriatricians, psychologists, nurses, and social workers collaborating and communicating to enact effective dementia care. It further meets the goals of the VA-DSOC in implementing individualized patient and caregiver care.
This transdisciplinary model addresses a number of issues, starting with the differential diagnosis of underlying neurologic conditions. Within the transdisciplinary team, the neurologist can provide specific insights into any neurologic findings and illnesses, such as Alzheimer disease and other neurodegenerative dementias, vascular dementia syndromes, normal pressure hydrocephalus, Creutzfeldt-Jakob disease, neurosyphilis, and others. Most veterans with dementia experience BPSD at some point during of their illness. The psychiatrists on the transdisciplinary team can maximize management of BPSD with nonpharmacologic interventions and the fewest and least aversive psychoactive medications. Our program also addresses the need for more precise cognitive evaluation. Neuropsychologists are present and available for administrating neuropsychologic tests and interpreting cognitive performance and any earlier neuropsychologic testing. This model also cares for the caregivers and assesses their needs. The social worker—as well as other members of the team—can provide caregivers with strategies for coping with disruptive and other behaviors related to dementia, counsel them on how to manage the veteran’s functional decline, and aid in establishing a safe living space. Because the social worker serves as a DCM, these coping and adjustment questions occupy significant clinical attention between appointments. This transdisciplinary model places the patient’s illness in the context of their functional status, diagnoses, and medications. The team geriatrician and the nurse specialist are indispensable resources. The clinic conference provides a teaching venue for staff and trainees and a mechanism to discuss new developments in dementia care, such as the increasing need to assess individuals with mild cognitive impairment.25 This model depends on the DCM’s invaluable role in ensuring implementation of the dementia care plan and continuity of care.
Conclusions
We describe effective dementia care with a transdisciplinary team in a conference setting and with the participation of a dedicated DCM.5 To date, this program appears to be an efficient, sustainable application of the limited resources allocated to dementia care. Nevertheless, we are collecting data to compare with performance measures, track use, and assess the programs effects on continuity of care. We look forward to presenting metrics from our program that show improvement in the health care for veterans experiencing a devastating and increasingly common disorder.
Dementia is a devastating condition resulting in major functional, emotional, and financial impact on patients, their caregivers, and families. Approximately 6.5 million Americans are living with Alzheimer disease (AD), the most common of many causes of dementia.1 The prevalence of AD could increase to 12.7 million Americans by 2050 as the population ages.1 Studies suggest that dementia, also known as major neurocognitive disorder, is common and underdiagnosed among US veterans, a population with a mean age of 65 years.2 During cognitive screening, memory impairment is present in approximately 20% of veterans aged ≥ 75 years who have not been diagnosed with a neurocognitive disorder.3 In addition, veterans might be particularly vulnerable to dementia at an earlier age than the general population because of vascular risk factors and traumatic brain injuries.4 These concerns highlight the need for effective dementia care programs at US Department of Veterans Affairs (VA) facilities.
The US health care system often does not adequately address the needs of patients with dementia and their caregivers.5 Dementia care requires specialized medical care among collaborating professionals and caregiver and psychosocial interventions and services. However, the US health care system is fragmented with different clinicians and services siloed into separate practices and most dementia care occurring in primary care settings.6 Primary care professionals (PCPs) often are uncomfortable diagnosing and managing dementia because of time constraints, lack of expertise and training, and inability to deal with the range of care needs.7 PCPs do not identify approximately 42% of their patients with dementia and, when recognized, do not adhere to dementia care guidelines and address caregiver needs.8-10 Research indicates that caregiver support improves dementia care by teaching behavioral management skills and caregiver coping strategies, allowing patients to stay at home and delay institutionalization.6,11,12 Clinicians underuse available resources and do not incorporate them in their patient care.10 These community services benefit patients and caregivers and significantly improve the overall quality of care.6
Memory clinics have emerged to address these deficiencies when managing dementia.13 The most effective memory clinics maximize the use of specialists with different expertise in dementia care, particularly integrated programs where disciplines function together rather than independently.1,5,14 Systematic reviews and meta-analyses have documented the effectiveness of collaborative care management programs.11,12,15 Integration of dementia care management is associated with earlier diagnosis and interventions, decreased functional and cognitive symptom severity, decreased or delayed institutionalization, improved quality of life for patients and caregivers, enhanced overall quality of care and cost-effectiveness, and better integration of community services.11,12,14-19 In these programs, designating a dementia care manager (DCM) as the patient’s advocate facilitates the integrated structure, increases the quality of care, helps caregivers, facilitates adherence to dementia practice guidelines, and prevents behavioral and psychological symptoms of dementia (BPSD).1,6,11,12,20,21
The best interprofessional model for dementia care might be the transdisciplinary model that includes a DCM. To meet the specific demands of dementia care, there must be a high level of interprofessional collaboration rather than multiple health care professionals (HCPs) delivering care in isolation—an approach that is time consuming and often difficult to implement.22 Whereas multidisciplinary care refers to delivery of parallel services and interdisciplinary care implies a joint formulation, transdisciplinary care aims to maximize integration of HCPs and their specific expertise and contributions through interactions and discussions that deliver focused input to the lead physician. The transdisciplinary model addresses needs that often are missed and can minimize disparities in the quality of dementia care.23 A DCM is an integral part of our program, facilitating understanding and implementation of the final care plan and providing long-term follow-up and care. We outline a conference-centered transdisciplinary dementia care model with a social worker as DCM (SW-DCM) at our VA medical center.
Program Description
In 2020, the VA Greater Los Angeles Healthcare System (VAGLAHS) in California established a multispecialty clinic dedicated to evaluation and treatment of veterans with memory and neurocognitive disorders and to provide support for their caregivers and families. With the agreement of leadership in mental health, neurology, and geriatrics services on the importance of collaboration for dementia care, the psychiatry and neurology services created a joint Memory and Neurobehavior Clinic, which completed its first 2 years of operation as a full-day program. In recent months, the clinic has scheduled 24 veterans per day, approximately 50% new evaluations and 50% follow-up patients, with wait times of < 2 months. There is a mean of 12 intake or lead physicians who could attend sessions in the morning, afternoon, or both. The general clinic flow consists of a 2-hour intake evaluation of new referrals by the lead physician followed by a clinic conference with transdisciplinary discussion. The DCM then follows up with the veteran/caregiver presenting a final care plan individualized to the veterans, caregivers, and families.
The Memory and Neurobehavior team includes behavioral neurologists, geriatric psychiatrists, neuropsychologists, geriatric fellows, advanced clinical nurses, and social workers who function as the DCM (Table 1).
Procedures
Before the office visit, the coordinating geriatric psychiatrist triages veterans to neurology, psychiatry, or geriatric physicians based on the clinical presentation, history of neurologic signs or symptoms, BPSD or psychiatric history, functional decline, or comorbid medical illnesses. Although veterans often have overlapping concerns, the triage process aims to coordinate the intake evaluations with the most indicated and available specialist with the intention to notify the other specialists during the transdisciplinary conference.
Referrals to the program occur from many sources, notably from primary care (70.8%), mental health (16.7%), and specialty clinics (12.5%). The clinic also receives referrals from the affiliated Veterans Cognitive Assessment and Management Program, which provides dementia evaluation and support via telehealth screening. This VAGLAHS program services a diverse population of veterans: 87% male; 43% aged > 65 years (75% in our clinic); 51% non-Hispanic White; 19% non-Hispanic African American; 16% Hispanic; 4% Asian; and 1% Native American. This population receives care at regional VA medical centers and community-based outpatient clinics over a wide geographic service area.
The initial standardized assessments by intake or lead physicians includes mental status screening with the Montreal Cognitive Assessment (with certified clinicians), the Neurobehavioral Status Examination for a more detailed assessment of cognitive domains, the Columbia-Suicide Severity Rating Scale, the Patient Health Questionnaire for depression screening, and assessment for impairments in instrumental or basic activities of daily living. This initial evaluation aims to apply clinical guidelines and diagnostic criteria for the differential diagnosis of neurocognitive disorders, determine eligibility for cognitive-enhancing medications and techniques, assess for BPSD and the need for nonpharmacologic or pharmacologic interventions, determine functional status, and evaluate the need for supervision, safety concerns, and evidence of neglect or abuse.
As part of its mission, the clinic is charged with implementing the VA Dementia System of Care (DSOC). The stated goals of the DSOC are to provide individualized person-centered dementia care to help veterans experiencing dementia and their caregivers maintain a positive and optimal quality of life and create an environment where VA medical center staff understand the health care needs of veterans with dementia and their caregivers’ role. As part of this initiative, the clinic includes (1) coordination of care through a SW-DCM; (2)
Transdisciplinary Conference
Clinic conferences are held after the veterans are seen. Staff gather to discuss the patient and review management. All team members are present, as well as the head of the clinical clerical staff who can facilitate appointments, make lobby and wait times more bearable for our patients and caregivers, and help manage emergencies. Although this is an in-person conference, the COVID-19 pandemic has allowed us to include staff who screen at remote sites via videoconferencing, similar to other VA programs.24 The Memory and Neurobehavior Clinic has two ≤ 90-minute conferences daily. The lead physicians and their senior attendings present the new intake evaluations (4-6 at each conference session) with a preliminary formulation and questions for discussion. The moderator solicits contributions from the different disciplines, going from one to the next and recording their responses for each veteran. Further specialists are available for consultation through the conference mechanism if necessary. The final assessment is reviewed, a diagnosis is established, and a tailored, individualized care plan for adjusting or optimizing the veteran’s care is presented to the lead physician who makes the final determination. At the close of the conference, the team’s discussion is recorded along with the lead physician’s original detailed intake evaluation. Currently, the records go into the Computerized Patient Record System, but we are making plans to transition to Cerner as it is implemented.
During the discussion, team members review several areas of consideration. If there is neuroimaging, neurologists review the images projected on a large computer screen. Team members also will assess for the need to obtain biomarker studies, such as blood, cerebrospinal fluid, or positron emission tomography. Psychiatrists could review management of BPSD and use of psychotropic agents, and neuropsychologists might consider the need for more precise cognitive testing and whether a capacity assessment is indicated. Social work might bring up the need for a durable power of attorney as well as applicable caregiver and community resources. Geriatric medicine and nursing could provide input into medical management and care and the ability of veterans and caregivers to follow the prescribed regimen. Further areas of discussion include driving safety and restrictions on driving (as required in California) and the presence of guns in the home. Finally, brief education is provided in short 10-to-15-minute lectures covering pertinent topics so staff remain up-to-date in this changing field.
Postconference Continuity
After the conference, the SW-DCM continues to provide support throughout the disease course, helping veterans and their caregivers understand and follow through on the team’s recommendations. The SW-DCM, who is experienced and trained in case management, forms an ongoing relationship with the veterans and their caregivers and remains an advocate for their care. The SW-DCM communicates the final plan by phone and, when necessary, requests the lead physician to call to clarify any poorly understood or technical aspects of the care plan. About 50% of our veterans—primarily those who do not have a neurocognitive disorder or have mild cognitive impairment—return to their PCPs with our care plan consultation; about 25% are already enrolled in geriatric and other programs with long-term follow-up. The assigned SW-DCM follows up with the remaining veterans and caregivers regularly by phone, facilitates communication with other team members, and endeavors to assure postvisit continuity of care and support during advancing stages of the disease. In addition, the SW-DCM can provide supportive counseling and psychotherapy for stressed caregivers, refer to support groups and cognitive rehabilitation programs, and help develop long-term goals and consideration for supervised living environments. The nurse specialist participates with follow-up calls regarding medications and scheduled tests and appointments, clearing up confusion about instructions, avoiding medication errors, and providing education in dementia care. Both social worker and nurse are present throughout the week, reachable by phone, and, in turn, able to contact the clinic physicians for veterans’ needs.
Discussion
Because of the heterogenous medical and psychosocial needs of veterans with dementia and their caregivers, a transdisciplinary team with a dedicated DCM might offer the most effective and efficient model for dementia care. We present a transdisciplinary program that incorporates dementia specialists in a single evaluation by maximizing their time through a conference-centered program. Our program involves neurologists, psychiatrists, geriatricians, psychologists, nurses, and social workers collaborating and communicating to enact effective dementia care. It further meets the goals of the VA-DSOC in implementing individualized patient and caregiver care.
This transdisciplinary model addresses a number of issues, starting with the differential diagnosis of underlying neurologic conditions. Within the transdisciplinary team, the neurologist can provide specific insights into any neurologic findings and illnesses, such as Alzheimer disease and other neurodegenerative dementias, vascular dementia syndromes, normal pressure hydrocephalus, Creutzfeldt-Jakob disease, neurosyphilis, and others. Most veterans with dementia experience BPSD at some point during of their illness. The psychiatrists on the transdisciplinary team can maximize management of BPSD with nonpharmacologic interventions and the fewest and least aversive psychoactive medications. Our program also addresses the need for more precise cognitive evaluation. Neuropsychologists are present and available for administrating neuropsychologic tests and interpreting cognitive performance and any earlier neuropsychologic testing. This model also cares for the caregivers and assesses their needs. The social worker—as well as other members of the team—can provide caregivers with strategies for coping with disruptive and other behaviors related to dementia, counsel them on how to manage the veteran’s functional decline, and aid in establishing a safe living space. Because the social worker serves as a DCM, these coping and adjustment questions occupy significant clinical attention between appointments. This transdisciplinary model places the patient’s illness in the context of their functional status, diagnoses, and medications. The team geriatrician and the nurse specialist are indispensable resources. The clinic conference provides a teaching venue for staff and trainees and a mechanism to discuss new developments in dementia care, such as the increasing need to assess individuals with mild cognitive impairment.25 This model depends on the DCM’s invaluable role in ensuring implementation of the dementia care plan and continuity of care.
Conclusions
We describe effective dementia care with a transdisciplinary team in a conference setting and with the participation of a dedicated DCM.5 To date, this program appears to be an efficient, sustainable application of the limited resources allocated to dementia care. Nevertheless, we are collecting data to compare with performance measures, track use, and assess the programs effects on continuity of care. We look forward to presenting metrics from our program that show improvement in the health care for veterans experiencing a devastating and increasingly common disorder.
1. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789. doi:10.1002/alz.12638
2. National Center for Veterans Analysis and Statistics. Profile of veterans: 2016. Accessed October 12, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf
3. Chodosh J, Sultzer DL, Lee ML, et al. Memory impairment among primary care veterans. Aging Ment Health. 2007;11(4):444-450. doi:10.1080/13607860601086272
4. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627. doi:10.1080/02699052.2022.20338465. Heintz H, Monette P, Epstein-Lubow G, Smith L, Rowlett S, Forester BP. Emerging collaborative care models for dementia care in the primary care setting: a narrative review. Am J Geriatr Psychiatry. 2020;28(3):320-330. doi:10.1016/j.jagp.2019.07.015
6. Reuben DB, Evertson LC, Wenger NS, et al. The University of California at Los Angeles Alzheimer’s and Dementia Care program for comprehensive, coordinated, patient-centered care: preliminary data. J Am Geriatr Soc. 2013;61(12):2214-2218. doi:10.1111/jgs.12562
7. Apesoa-Varano EC, Barker JC, Hinton L. Curing and caring: the work of primary care physicians with dementia patients. Qual Health Res. 2011;21(11):1469-1483. doi:10.1177/1049732311412788
8. Creavin ST, Noel-Storr AH, Langdon RJ, et al. Clinical judgement by primary care physicians for the diagnosis of all-cause dementia or cognitive impairment in symptomatic people. Cochrane Database Syst Rev. 2022;6:CD012558. doi:10.1002/14651858.CD012558.pub2
9. Sivananthan SN, Puyat JH, McGrail KM. Variations in self-reported practice of physicians providing clinical care to individuals with dementia: a systematic review. J Am Geriatr Soc. 2013;61(8):1277-1285. doi:10.1111/jgs.12368
10. Rosen CS, Chow HC, Greenbaum MA, et al. How well are clinicians following dementia practice guidelines? Alzheimer Dis Assoc Disord. 2002;16(1):15-23. doi:10.1097/00002093-200201000-00003
11. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345. doi:10.1002/14651858.CD008345.pub2
12. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2013;28(9):889-902. doi:10.1002/gps.3906
13. Jolley D, Benbow SM, Grizzell M. Memory clinics. Postgrad Med J. 2006;82(965):199-206. doi:10.1136/pgmj.2005.040592
14. Muhlichen F, Michalowsky B, Radke A, et al. Tasks and activities of an effective collaborative dementia care management program in German primary care. J Alzheimers Dis. 2022;87(4):1615-1625. doi:10.3233/JAD-215656
15. Somme D, Trouve H, Drame M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement. 2012;8(5):426-436. doi:10.1016/j.jalz.2011.06.004
16. Ramakers IH, Verhey FR. Development of memory clinics in the Netherlands: 1998 to 2009. Aging Ment Health. 2011;15(1):34-39. doi:10.1080/13607863.2010.519321
17. LaMantia MA, Alder CA, Callahan CM, et al. The aging brain care medical home: preliminary data. J Am Geriatr Soc. 2015;63(6):1209-1213. doi:10.1111/jgs.13447
18. Rubinsztein JS, van Rensburg MJ, Al-Salihy Z, et al. A memory clinic v. traditional community mental health team service: comparison of costs and quality. BJPsych Bull. 2015;39(1):6-11. doi:10.1192/pb.bp.113.044263
19. Lee L, Hillier LM, Harvey D. Integrating community services into primary care: improving the quality of dementia care. Neurodegener Dis Manag. 2014;4(1):11-21. doi:10.2217/nmt.13.72
20. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386. doi:10.1111/jgs.12362
21. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157. doi:10.1001/jama.295.18.2148
22. Leggett A, Connell C, Dubin L, et al. Dementia care across a tertiary care health system: what exists now and what needs to change. J Am Med Dir Assoc. 2019;20(10):1307-12 e1. doi:10.1016/j.jamda.2019.04.006
23. Brown AF, Vassar SD, Connor KI, Vickrey BG. Collaborative care management reduces disparities in dementia care quality for caregivers with less education. J Am Geriatr Soc. 2013;61(2):243-251. doi:10.1111/jgs.12079
24. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
25. Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s Disease: evolving the care team for optimal patient management. Front Neurol. 2020;11:592302. doi:10.3389/fneur.2020.592302
1. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789. doi:10.1002/alz.12638
2. National Center for Veterans Analysis and Statistics. Profile of veterans: 2016. Accessed October 12, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf
3. Chodosh J, Sultzer DL, Lee ML, et al. Memory impairment among primary care veterans. Aging Ment Health. 2007;11(4):444-450. doi:10.1080/13607860601086272
4. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627. doi:10.1080/02699052.2022.20338465. Heintz H, Monette P, Epstein-Lubow G, Smith L, Rowlett S, Forester BP. Emerging collaborative care models for dementia care in the primary care setting: a narrative review. Am J Geriatr Psychiatry. 2020;28(3):320-330. doi:10.1016/j.jagp.2019.07.015
6. Reuben DB, Evertson LC, Wenger NS, et al. The University of California at Los Angeles Alzheimer’s and Dementia Care program for comprehensive, coordinated, patient-centered care: preliminary data. J Am Geriatr Soc. 2013;61(12):2214-2218. doi:10.1111/jgs.12562
7. Apesoa-Varano EC, Barker JC, Hinton L. Curing and caring: the work of primary care physicians with dementia patients. Qual Health Res. 2011;21(11):1469-1483. doi:10.1177/1049732311412788
8. Creavin ST, Noel-Storr AH, Langdon RJ, et al. Clinical judgement by primary care physicians for the diagnosis of all-cause dementia or cognitive impairment in symptomatic people. Cochrane Database Syst Rev. 2022;6:CD012558. doi:10.1002/14651858.CD012558.pub2
9. Sivananthan SN, Puyat JH, McGrail KM. Variations in self-reported practice of physicians providing clinical care to individuals with dementia: a systematic review. J Am Geriatr Soc. 2013;61(8):1277-1285. doi:10.1111/jgs.12368
10. Rosen CS, Chow HC, Greenbaum MA, et al. How well are clinicians following dementia practice guidelines? Alzheimer Dis Assoc Disord. 2002;16(1):15-23. doi:10.1097/00002093-200201000-00003
11. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345. doi:10.1002/14651858.CD008345.pub2
12. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2013;28(9):889-902. doi:10.1002/gps.3906
13. Jolley D, Benbow SM, Grizzell M. Memory clinics. Postgrad Med J. 2006;82(965):199-206. doi:10.1136/pgmj.2005.040592
14. Muhlichen F, Michalowsky B, Radke A, et al. Tasks and activities of an effective collaborative dementia care management program in German primary care. J Alzheimers Dis. 2022;87(4):1615-1625. doi:10.3233/JAD-215656
15. Somme D, Trouve H, Drame M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement. 2012;8(5):426-436. doi:10.1016/j.jalz.2011.06.004
16. Ramakers IH, Verhey FR. Development of memory clinics in the Netherlands: 1998 to 2009. Aging Ment Health. 2011;15(1):34-39. doi:10.1080/13607863.2010.519321
17. LaMantia MA, Alder CA, Callahan CM, et al. The aging brain care medical home: preliminary data. J Am Geriatr Soc. 2015;63(6):1209-1213. doi:10.1111/jgs.13447
18. Rubinsztein JS, van Rensburg MJ, Al-Salihy Z, et al. A memory clinic v. traditional community mental health team service: comparison of costs and quality. BJPsych Bull. 2015;39(1):6-11. doi:10.1192/pb.bp.113.044263
19. Lee L, Hillier LM, Harvey D. Integrating community services into primary care: improving the quality of dementia care. Neurodegener Dis Manag. 2014;4(1):11-21. doi:10.2217/nmt.13.72
20. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386. doi:10.1111/jgs.12362
21. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157. doi:10.1001/jama.295.18.2148
22. Leggett A, Connell C, Dubin L, et al. Dementia care across a tertiary care health system: what exists now and what needs to change. J Am Med Dir Assoc. 2019;20(10):1307-12 e1. doi:10.1016/j.jamda.2019.04.006
23. Brown AF, Vassar SD, Connor KI, Vickrey BG. Collaborative care management reduces disparities in dementia care quality for caregivers with less education. J Am Geriatr Soc. 2013;61(2):243-251. doi:10.1111/jgs.12079
24. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
25. Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s Disease: evolving the care team for optimal patient management. Front Neurol. 2020;11:592302. doi:10.3389/fneur.2020.592302
A Novel Text Message Protocol to Improve Bowel Preparation for Outpatient Colonoscopies in Veterans
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
Contralateral Constrictor Dose Predicts Swallowing Function After Radiation for Head and Neck Cancer
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck (H&N) cancer.1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia.4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was.5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures.6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume for the lowest dose target volume. NRG H&N trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for H&N squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as H&N cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the H&N.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for H&N cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy (IMRT). Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 69.96 Gy in 33 fractions, with elective volumes treated to 54.45 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased 1-year dysphagia.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year, compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for H&N cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant.
An advantage of contralateral constrictor is that it is independent of planning target volume (PTV) size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with H&N cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after IMRT for H&N cancer.16 Radiation has been shown to decrease pharyngeal function in patients with H&N cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with H&N cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with H&N cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck (H&N) cancer.1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia.4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was.5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures.6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume for the lowest dose target volume. NRG H&N trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for H&N squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as H&N cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the H&N.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for H&N cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy (IMRT). Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 69.96 Gy in 33 fractions, with elective volumes treated to 54.45 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased 1-year dysphagia.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year, compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for H&N cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant.
An advantage of contralateral constrictor is that it is independent of planning target volume (PTV) size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with H&N cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after IMRT for H&N cancer.16 Radiation has been shown to decrease pharyngeal function in patients with H&N cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with H&N cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with H&N cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck (H&N) cancer.1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia.4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was.5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures.6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume for the lowest dose target volume. NRG H&N trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for H&N squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as H&N cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the H&N.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for H&N cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy (IMRT). Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 69.96 Gy in 33 fractions, with elective volumes treated to 54.45 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased 1-year dysphagia.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year, compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for H&N cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant.
An advantage of contralateral constrictor is that it is independent of planning target volume (PTV) size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with H&N cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after IMRT for H&N cancer.16 Radiation has been shown to decrease pharyngeal function in patients with H&N cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with H&N cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with H&N cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938