User login
Where women’s voices still get heard less
“Our study provides the first analysis of gender and early-career faculty disparities in speakers at hematology and medical oncology board review meetings,” the authors reported in research published in Blood Advances.
“We covered six major board reviews over the last 5 years that are either conducted yearly or every other year, [and] the general trend across all meetings showed skewness toward men speakers,” the authors reported.
Recent data from 2021 suggests a closing of the gender gap in oncology, with women making up 44.6% of oncologists in training. However, they still only represented 35.2% of practicing oncologists and are underrepresented in leadership positions in academic oncology, the authors reported.
With speaking roles at academic meetings potentially marking a key step in career advancement and improved opportunities, the authors sought to investigate the balance of gender, as well as early-career faculty among speakers at prominent hematology and/or oncology board review lecture series taking place in the United States between 2017 and 2021.
The five institutions and one society presenting the board review lecture series included Baylor College of Medicine/MD Anderson Cancer Center, both in Houston; Dana-Farber Brigham Cancer Center, Boston; George Washington University, Washington; Memorial Sloan Kettering Cancer Center, New York; Seattle Cancer Care Alliance; and the hematology board review series from the American Society of Hematology.
During the period in question, among 1,224 board review lectures presented, women constituted only 37.7% of the speakers. In lectures presented by American Board of Internal Medicine–certified speakers (n = 1,016, 83%), women were found to have made up fewer than 50% of speakers in five of six courses.
Men were also more likely to be recurrent speakers; across all courses, 13 men but only 2 women conducted 10 or more lectures. And while 35 men gave six or more lectures across all courses, only 12 women did so.
The lecture topics with the lowest rates of women presenters included malignant hematology (24.8%), solid tumors (38.9%), and benign hematology lectures (44.1%).
“We suspected [the imbalance in malignant hematology] since multiple recurrent roles were concentrated in the malignant hematology,” senior author Samer Al Hadidi, MD, of the Myeloma Center, Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, Little Rock, AK, said in an interview.
He noted that “there are no regulations that such courses need to follow to ensure certain proportions of women and junior faculty are involved.”
Early-career faculty
In terms of early-career representation, more than 50% of lectures were given by faculty who had received their initial certifications more than 15 years earlier. The median time from initial certification was 12.5 years for hematology and 14 years for medical oncology.
The findings that more than half of the board review lectures were presented by faculty with more than 15 years’ experience since initial certification “reflects a lack of appropriate involvement of early-career faculty, who arguably may have more recent experience with board certification,” the authors wrote.
While being underrepresented in such roles is detrimental, there are no regulations that such courses follow to ensure certain proportions of women and junior faculty are involved, Dr. Al Hadidi noted.
Equal representation remains elusive
The study does suggest some notable gains. In a previous study of 181 academic conferences in the United States and Canada between 2007 and 2017, the rate of women speakers was only 15%, compared with 37.7% in the new study.
And an overall trend analysis in the study shows an approximately 10% increase in representation of women in all of the board reviews. However, only the ASH hematology board review achieved more than 50% women in their two courses.
“Overall, the proportion of women speakers is improving over the years, though it remains suboptimal,” Dr. Al Hadidi said.
The authors noted that oncology is clearly not the only specialty with gender disparities. They documented a lack of women speakers at conferences involving otolaryngology head and neck meetings, radiation oncology, emergency medicine, and research conferences.
They pointed to the work of ASH’s Women in Hematology Working Group as an important example of the needed effort to improve the balance of women hematologists.
Ariela Marshall, MD, director of women’s thrombosis and hemostasis at Penn Medicine in Philadelphia and a leader of ASH’s Women in Hematology Working Group, agreed that more efforts are needed to address both gender disparities as well as those of early career speakers. She asserted that the two disparities appear to be connected.
“If you broke down gender representation over time and the faculty/time since initial certification, the findings may mirror the percent of women in hematology-oncology at that given point in time,” Dr. Marshall said in an interview.
“If an institution is truly committed to taking action on gender equity, it needs to look at gender and experience equity of speakers,” she said. “Perhaps it’s the time to say ‘Dr. X has been doing this review course for 15 years. Let’s give someone else a chance.’
“This is not even just from a gender equity perspective but from a career development perspective overall,” she added. “Junior faculty need these speaking engagements a lot more than senior faculty.”
Meanwhile, the higher number of female trainees is a trend that ideally will be sustained as those trainees move into positions of leadership, Dr. Marshall noted.
“We do see that over time, we have achieved gender equity in the percent of women matriculating to medical school. And my hope is that, 20 years down the line, we will see the effects of this reflected in increased equity in leadership positions such as division/department chair, dean, and hospital CEO,” she said. “However, we have a lot of work to do because there are still huge inequities in the culture of medicine (institutional and more broadly), including gender-based discrimination, maternal discrimination, and high attrition rates for women physicians, compared to male physicians.
“It’s not enough to simply say ‘well, we have fixed the problem because our incoming medical student classes are now equitable in gender distribution,’ ”
The authors and Dr. Marshall had no disclosures to report.
“Our study provides the first analysis of gender and early-career faculty disparities in speakers at hematology and medical oncology board review meetings,” the authors reported in research published in Blood Advances.
“We covered six major board reviews over the last 5 years that are either conducted yearly or every other year, [and] the general trend across all meetings showed skewness toward men speakers,” the authors reported.
Recent data from 2021 suggests a closing of the gender gap in oncology, with women making up 44.6% of oncologists in training. However, they still only represented 35.2% of practicing oncologists and are underrepresented in leadership positions in academic oncology, the authors reported.
With speaking roles at academic meetings potentially marking a key step in career advancement and improved opportunities, the authors sought to investigate the balance of gender, as well as early-career faculty among speakers at prominent hematology and/or oncology board review lecture series taking place in the United States between 2017 and 2021.
The five institutions and one society presenting the board review lecture series included Baylor College of Medicine/MD Anderson Cancer Center, both in Houston; Dana-Farber Brigham Cancer Center, Boston; George Washington University, Washington; Memorial Sloan Kettering Cancer Center, New York; Seattle Cancer Care Alliance; and the hematology board review series from the American Society of Hematology.
During the period in question, among 1,224 board review lectures presented, women constituted only 37.7% of the speakers. In lectures presented by American Board of Internal Medicine–certified speakers (n = 1,016, 83%), women were found to have made up fewer than 50% of speakers in five of six courses.
Men were also more likely to be recurrent speakers; across all courses, 13 men but only 2 women conducted 10 or more lectures. And while 35 men gave six or more lectures across all courses, only 12 women did so.
The lecture topics with the lowest rates of women presenters included malignant hematology (24.8%), solid tumors (38.9%), and benign hematology lectures (44.1%).
“We suspected [the imbalance in malignant hematology] since multiple recurrent roles were concentrated in the malignant hematology,” senior author Samer Al Hadidi, MD, of the Myeloma Center, Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, Little Rock, AK, said in an interview.
He noted that “there are no regulations that such courses need to follow to ensure certain proportions of women and junior faculty are involved.”
Early-career faculty
In terms of early-career representation, more than 50% of lectures were given by faculty who had received their initial certifications more than 15 years earlier. The median time from initial certification was 12.5 years for hematology and 14 years for medical oncology.
The findings that more than half of the board review lectures were presented by faculty with more than 15 years’ experience since initial certification “reflects a lack of appropriate involvement of early-career faculty, who arguably may have more recent experience with board certification,” the authors wrote.
While being underrepresented in such roles is detrimental, there are no regulations that such courses follow to ensure certain proportions of women and junior faculty are involved, Dr. Al Hadidi noted.
Equal representation remains elusive
The study does suggest some notable gains. In a previous study of 181 academic conferences in the United States and Canada between 2007 and 2017, the rate of women speakers was only 15%, compared with 37.7% in the new study.
And an overall trend analysis in the study shows an approximately 10% increase in representation of women in all of the board reviews. However, only the ASH hematology board review achieved more than 50% women in their two courses.
“Overall, the proportion of women speakers is improving over the years, though it remains suboptimal,” Dr. Al Hadidi said.
The authors noted that oncology is clearly not the only specialty with gender disparities. They documented a lack of women speakers at conferences involving otolaryngology head and neck meetings, radiation oncology, emergency medicine, and research conferences.
They pointed to the work of ASH’s Women in Hematology Working Group as an important example of the needed effort to improve the balance of women hematologists.
Ariela Marshall, MD, director of women’s thrombosis and hemostasis at Penn Medicine in Philadelphia and a leader of ASH’s Women in Hematology Working Group, agreed that more efforts are needed to address both gender disparities as well as those of early career speakers. She asserted that the two disparities appear to be connected.
“If you broke down gender representation over time and the faculty/time since initial certification, the findings may mirror the percent of women in hematology-oncology at that given point in time,” Dr. Marshall said in an interview.
“If an institution is truly committed to taking action on gender equity, it needs to look at gender and experience equity of speakers,” she said. “Perhaps it’s the time to say ‘Dr. X has been doing this review course for 15 years. Let’s give someone else a chance.’
“This is not even just from a gender equity perspective but from a career development perspective overall,” she added. “Junior faculty need these speaking engagements a lot more than senior faculty.”
Meanwhile, the higher number of female trainees is a trend that ideally will be sustained as those trainees move into positions of leadership, Dr. Marshall noted.
“We do see that over time, we have achieved gender equity in the percent of women matriculating to medical school. And my hope is that, 20 years down the line, we will see the effects of this reflected in increased equity in leadership positions such as division/department chair, dean, and hospital CEO,” she said. “However, we have a lot of work to do because there are still huge inequities in the culture of medicine (institutional and more broadly), including gender-based discrimination, maternal discrimination, and high attrition rates for women physicians, compared to male physicians.
“It’s not enough to simply say ‘well, we have fixed the problem because our incoming medical student classes are now equitable in gender distribution,’ ”
The authors and Dr. Marshall had no disclosures to report.
“Our study provides the first analysis of gender and early-career faculty disparities in speakers at hematology and medical oncology board review meetings,” the authors reported in research published in Blood Advances.
“We covered six major board reviews over the last 5 years that are either conducted yearly or every other year, [and] the general trend across all meetings showed skewness toward men speakers,” the authors reported.
Recent data from 2021 suggests a closing of the gender gap in oncology, with women making up 44.6% of oncologists in training. However, they still only represented 35.2% of practicing oncologists and are underrepresented in leadership positions in academic oncology, the authors reported.
With speaking roles at academic meetings potentially marking a key step in career advancement and improved opportunities, the authors sought to investigate the balance of gender, as well as early-career faculty among speakers at prominent hematology and/or oncology board review lecture series taking place in the United States between 2017 and 2021.
The five institutions and one society presenting the board review lecture series included Baylor College of Medicine/MD Anderson Cancer Center, both in Houston; Dana-Farber Brigham Cancer Center, Boston; George Washington University, Washington; Memorial Sloan Kettering Cancer Center, New York; Seattle Cancer Care Alliance; and the hematology board review series from the American Society of Hematology.
During the period in question, among 1,224 board review lectures presented, women constituted only 37.7% of the speakers. In lectures presented by American Board of Internal Medicine–certified speakers (n = 1,016, 83%), women were found to have made up fewer than 50% of speakers in five of six courses.
Men were also more likely to be recurrent speakers; across all courses, 13 men but only 2 women conducted 10 or more lectures. And while 35 men gave six or more lectures across all courses, only 12 women did so.
The lecture topics with the lowest rates of women presenters included malignant hematology (24.8%), solid tumors (38.9%), and benign hematology lectures (44.1%).
“We suspected [the imbalance in malignant hematology] since multiple recurrent roles were concentrated in the malignant hematology,” senior author Samer Al Hadidi, MD, of the Myeloma Center, Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, Little Rock, AK, said in an interview.
He noted that “there are no regulations that such courses need to follow to ensure certain proportions of women and junior faculty are involved.”
Early-career faculty
In terms of early-career representation, more than 50% of lectures were given by faculty who had received their initial certifications more than 15 years earlier. The median time from initial certification was 12.5 years for hematology and 14 years for medical oncology.
The findings that more than half of the board review lectures were presented by faculty with more than 15 years’ experience since initial certification “reflects a lack of appropriate involvement of early-career faculty, who arguably may have more recent experience with board certification,” the authors wrote.
While being underrepresented in such roles is detrimental, there are no regulations that such courses follow to ensure certain proportions of women and junior faculty are involved, Dr. Al Hadidi noted.
Equal representation remains elusive
The study does suggest some notable gains. In a previous study of 181 academic conferences in the United States and Canada between 2007 and 2017, the rate of women speakers was only 15%, compared with 37.7% in the new study.
And an overall trend analysis in the study shows an approximately 10% increase in representation of women in all of the board reviews. However, only the ASH hematology board review achieved more than 50% women in their two courses.
“Overall, the proportion of women speakers is improving over the years, though it remains suboptimal,” Dr. Al Hadidi said.
The authors noted that oncology is clearly not the only specialty with gender disparities. They documented a lack of women speakers at conferences involving otolaryngology head and neck meetings, radiation oncology, emergency medicine, and research conferences.
They pointed to the work of ASH’s Women in Hematology Working Group as an important example of the needed effort to improve the balance of women hematologists.
Ariela Marshall, MD, director of women’s thrombosis and hemostasis at Penn Medicine in Philadelphia and a leader of ASH’s Women in Hematology Working Group, agreed that more efforts are needed to address both gender disparities as well as those of early career speakers. She asserted that the two disparities appear to be connected.
“If you broke down gender representation over time and the faculty/time since initial certification, the findings may mirror the percent of women in hematology-oncology at that given point in time,” Dr. Marshall said in an interview.
“If an institution is truly committed to taking action on gender equity, it needs to look at gender and experience equity of speakers,” she said. “Perhaps it’s the time to say ‘Dr. X has been doing this review course for 15 years. Let’s give someone else a chance.’
“This is not even just from a gender equity perspective but from a career development perspective overall,” she added. “Junior faculty need these speaking engagements a lot more than senior faculty.”
Meanwhile, the higher number of female trainees is a trend that ideally will be sustained as those trainees move into positions of leadership, Dr. Marshall noted.
“We do see that over time, we have achieved gender equity in the percent of women matriculating to medical school. And my hope is that, 20 years down the line, we will see the effects of this reflected in increased equity in leadership positions such as division/department chair, dean, and hospital CEO,” she said. “However, we have a lot of work to do because there are still huge inequities in the culture of medicine (institutional and more broadly), including gender-based discrimination, maternal discrimination, and high attrition rates for women physicians, compared to male physicians.
“It’s not enough to simply say ‘well, we have fixed the problem because our incoming medical student classes are now equitable in gender distribution,’ ”
The authors and Dr. Marshall had no disclosures to report.
FROM BLOOD ADVANCES
Understanding the relationship between life satisfaction and cognitive decline
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
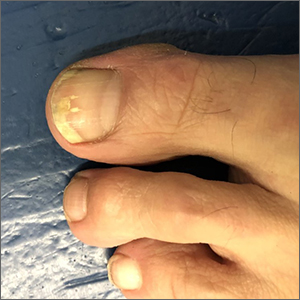
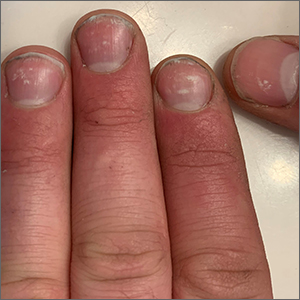
Ultrasound helps predict gout flares over the next year
Adding ultrasound (US) to the clinical exam helps predict the likelihood of future gout flares, results of a prospective, observational study conducted in Italy suggest.
“Baseline US findings indicative of MSU [monosodium urate] burden and US-detected inflammation are independent predictors of gout flares over 12 months,” lead author Edoardo Cipolletta, MD, of the rheumatology unit, department of clinical and molecular sciences at Marche Polytechnic University in Ancona, Italy, and colleagues wrote in Rheumatology.
“We demonstrated that US findings provided an additional value over clinical data in estimating the risk of flares. Moreover, we reported an association between US findings at a joint and the occurrence of gout flares at the same joint,” they added.
Predicting risk of flares and reducing their occurrence are two main challenges in managing gout, the authors wrote. US can be used to scan multiple joints and is widely used in Europe as a low-cost, radiation-free imaging tool that’s easily integrated into clinical practice.
To investigate whether US can predict gout flares, the researchers enrolled 81 consecutive adult patients with gout in the study between April 2019 and March 2021 at one academic rheumatology treatment site in Italy and followed them for 12 months. The authors compared cases (who developed at least one flare within 12 months of the baseline visit) with controls (who self-reported no gout flares over that period).
Patients diagnosed with other inflammatory arthritis and those with coexisting calcium pyrophosphate deposition disease were excluded from the study.
The 71 participants who completed the study were, on average, in their early 60s, and in both groups, all but one were male. At the baseline visit, all had been on stable urate-lowering therapy for at least 6 months and had not had any gout flares in 4 weeks. The mean gout duration was 7 years in the case group and 8 years in controls.
At baseline, all participants underwent physical examination and US of elbows, wrists, second metacarpophalangeal joints, knees, ankles, and first metatarsophalangeal joints by a member of the research team who was blinded to the clinical and laboratory data.
Clinical assessments were scheduled at baseline and at 6-month intervals, and all participants were evaluated by a second researcher who was blinded to US findings.
During follow-up visits, participants were asked to report any gout flare, considered to meet at least three of four criteria: patient-defined flare, pain at rest score higher than 3 on a 0-10 scale, at least one swollen joint, and at least one warm joint. Patients not reaching their target serum urate goal received escalated urate-lowering therapy dosage and anti-inflammatory prophylaxis.
The US indicators of MSU deposits – aggregates, double contour sign, and tophi – were recorded as present or absent. The power Doppler signal was scored from 0 through 4, and summated scores for each US finding were calculated.
Over 12 months, the researchers found:
- Thirty (42.3%) patients had at least one flare, with a median of 2.0 flares. Patients with flares had higher a US median total MSU score (5.0 vs. 2.0; P = .01) and power Doppler signal (3.0 vs. 0; P < .01) than controls.
- In multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the occurrence of flares. The adjusted odds ratio for total MSU score was 1.75 (95% confidence interval, 1.26-2.43) and for power Doppler score was 1.63 (95% CI, 1.12-2.40).
- Also in a multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the number of flares. The incidence risk ratio for total MSU score adjusted was 1.17 (95% CI, 1.08-1.26) and for power Doppler score was 1.29 (95% CI, 1.19-1.40).
Four rheumatologists welcome findings
Gout remains the most common cause of inflammatory arthritis and a significant reason for hospital visits, noted Narender Annapureddy, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn..
“The study adds to the growing utility of musculoskeletal ultrasound in rheumatology practices to treat various diseases,” he said. “Data that could provide risk prediction for gout flares would be associated with significant benefits in terms of reducing ED visits, hospital admission, and lost work productivity.”
One study limitation, Dr. Annapureddy mentioned, was the single experienced US reader, “which may limit generalizability of results at this time, at least in the United States.”
Yeohan Song, MD, an instructor at Ohio State University Wexner Medical Center, Columbus, integrates US into his practice.
“In gout management, musculoskeletal ultrasound is a useful adjunct to the clinical exam and laboratory markers, particularly [in patients] with recurrent flares despite guideline-directed target serum urate levels,” he said.
Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, pointed out that the US protocol in the study involved imaging knees, ankles, first metatarsophalangeal joints, elbows, wrists, and second metacarpophalangeal joints, and took around 30 minutes to complete.
“That would not be practical in the United States due to time constraints in most rheumatology clinics,” she said.
“The authors report that a ‘reduced scanning protocol’ of the bilateral knees, ankles, and first metatarsophalangeal joints demonstrated similar predictive ability as the full protocol,” she added, “although scanning six joints still might not be feasible during a typical return patient clinic visit in the United States.”
Philip Chu, MD, clinical associate at Duke University, Durham, N.C., uses diagnostic US to help differentiate borderline gout cases from other arthropathies.
“A baseline scan, a follow-up scan before deciding to stop prophylaxis, or a follow-up scan in the setting of recurrent gout flares despite reaching goal serum uric acid, may be cost-effective time points to perform diagnostic US,” he advised.
“Unfortunately,” he added, “reimbursement for diagnostic US has been decreasing over the years, which makes it challenging to increase diagnostic US to the [frequency of its use] in Europe.”
Asked how most gout care being provided by primary care doctors in the United States affects gout management, Dr. Chu said: “Depending on which guidelines one follows for treating gout – from the American College of Rheumatology or the American College of Physicians – one may be more or less likely to start urate-lowering therapy after the first gout flare.”
“Understanding MSU burden in each patient, or even seeing active inflammation at these sites by increased Doppler signal, may change the threshold for physicians to initiate therapy,” he added.
The study received no funding. Three study authors reported financial involvements with pharmaceutical companies. Dr. Cipolletta, Dr. Annapureddy, Dr. Song, Dr. Tedeschi, and Dr. Chu reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Adding ultrasound (US) to the clinical exam helps predict the likelihood of future gout flares, results of a prospective, observational study conducted in Italy suggest.
“Baseline US findings indicative of MSU [monosodium urate] burden and US-detected inflammation are independent predictors of gout flares over 12 months,” lead author Edoardo Cipolletta, MD, of the rheumatology unit, department of clinical and molecular sciences at Marche Polytechnic University in Ancona, Italy, and colleagues wrote in Rheumatology.
“We demonstrated that US findings provided an additional value over clinical data in estimating the risk of flares. Moreover, we reported an association between US findings at a joint and the occurrence of gout flares at the same joint,” they added.
Predicting risk of flares and reducing their occurrence are two main challenges in managing gout, the authors wrote. US can be used to scan multiple joints and is widely used in Europe as a low-cost, radiation-free imaging tool that’s easily integrated into clinical practice.
To investigate whether US can predict gout flares, the researchers enrolled 81 consecutive adult patients with gout in the study between April 2019 and March 2021 at one academic rheumatology treatment site in Italy and followed them for 12 months. The authors compared cases (who developed at least one flare within 12 months of the baseline visit) with controls (who self-reported no gout flares over that period).
Patients diagnosed with other inflammatory arthritis and those with coexisting calcium pyrophosphate deposition disease were excluded from the study.
The 71 participants who completed the study were, on average, in their early 60s, and in both groups, all but one were male. At the baseline visit, all had been on stable urate-lowering therapy for at least 6 months and had not had any gout flares in 4 weeks. The mean gout duration was 7 years in the case group and 8 years in controls.
At baseline, all participants underwent physical examination and US of elbows, wrists, second metacarpophalangeal joints, knees, ankles, and first metatarsophalangeal joints by a member of the research team who was blinded to the clinical and laboratory data.
Clinical assessments were scheduled at baseline and at 6-month intervals, and all participants were evaluated by a second researcher who was blinded to US findings.
During follow-up visits, participants were asked to report any gout flare, considered to meet at least three of four criteria: patient-defined flare, pain at rest score higher than 3 on a 0-10 scale, at least one swollen joint, and at least one warm joint. Patients not reaching their target serum urate goal received escalated urate-lowering therapy dosage and anti-inflammatory prophylaxis.
The US indicators of MSU deposits – aggregates, double contour sign, and tophi – were recorded as present or absent. The power Doppler signal was scored from 0 through 4, and summated scores for each US finding were calculated.
Over 12 months, the researchers found:
- Thirty (42.3%) patients had at least one flare, with a median of 2.0 flares. Patients with flares had higher a US median total MSU score (5.0 vs. 2.0; P = .01) and power Doppler signal (3.0 vs. 0; P < .01) than controls.
- In multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the occurrence of flares. The adjusted odds ratio for total MSU score was 1.75 (95% confidence interval, 1.26-2.43) and for power Doppler score was 1.63 (95% CI, 1.12-2.40).
- Also in a multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the number of flares. The incidence risk ratio for total MSU score adjusted was 1.17 (95% CI, 1.08-1.26) and for power Doppler score was 1.29 (95% CI, 1.19-1.40).
Four rheumatologists welcome findings
Gout remains the most common cause of inflammatory arthritis and a significant reason for hospital visits, noted Narender Annapureddy, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn..
“The study adds to the growing utility of musculoskeletal ultrasound in rheumatology practices to treat various diseases,” he said. “Data that could provide risk prediction for gout flares would be associated with significant benefits in terms of reducing ED visits, hospital admission, and lost work productivity.”
One study limitation, Dr. Annapureddy mentioned, was the single experienced US reader, “which may limit generalizability of results at this time, at least in the United States.”
Yeohan Song, MD, an instructor at Ohio State University Wexner Medical Center, Columbus, integrates US into his practice.
“In gout management, musculoskeletal ultrasound is a useful adjunct to the clinical exam and laboratory markers, particularly [in patients] with recurrent flares despite guideline-directed target serum urate levels,” he said.
Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, pointed out that the US protocol in the study involved imaging knees, ankles, first metatarsophalangeal joints, elbows, wrists, and second metacarpophalangeal joints, and took around 30 minutes to complete.
“That would not be practical in the United States due to time constraints in most rheumatology clinics,” she said.
“The authors report that a ‘reduced scanning protocol’ of the bilateral knees, ankles, and first metatarsophalangeal joints demonstrated similar predictive ability as the full protocol,” she added, “although scanning six joints still might not be feasible during a typical return patient clinic visit in the United States.”
Philip Chu, MD, clinical associate at Duke University, Durham, N.C., uses diagnostic US to help differentiate borderline gout cases from other arthropathies.
“A baseline scan, a follow-up scan before deciding to stop prophylaxis, or a follow-up scan in the setting of recurrent gout flares despite reaching goal serum uric acid, may be cost-effective time points to perform diagnostic US,” he advised.
“Unfortunately,” he added, “reimbursement for diagnostic US has been decreasing over the years, which makes it challenging to increase diagnostic US to the [frequency of its use] in Europe.”
Asked how most gout care being provided by primary care doctors in the United States affects gout management, Dr. Chu said: “Depending on which guidelines one follows for treating gout – from the American College of Rheumatology or the American College of Physicians – one may be more or less likely to start urate-lowering therapy after the first gout flare.”
“Understanding MSU burden in each patient, or even seeing active inflammation at these sites by increased Doppler signal, may change the threshold for physicians to initiate therapy,” he added.
The study received no funding. Three study authors reported financial involvements with pharmaceutical companies. Dr. Cipolletta, Dr. Annapureddy, Dr. Song, Dr. Tedeschi, and Dr. Chu reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Adding ultrasound (US) to the clinical exam helps predict the likelihood of future gout flares, results of a prospective, observational study conducted in Italy suggest.
“Baseline US findings indicative of MSU [monosodium urate] burden and US-detected inflammation are independent predictors of gout flares over 12 months,” lead author Edoardo Cipolletta, MD, of the rheumatology unit, department of clinical and molecular sciences at Marche Polytechnic University in Ancona, Italy, and colleagues wrote in Rheumatology.
“We demonstrated that US findings provided an additional value over clinical data in estimating the risk of flares. Moreover, we reported an association between US findings at a joint and the occurrence of gout flares at the same joint,” they added.
Predicting risk of flares and reducing their occurrence are two main challenges in managing gout, the authors wrote. US can be used to scan multiple joints and is widely used in Europe as a low-cost, radiation-free imaging tool that’s easily integrated into clinical practice.
To investigate whether US can predict gout flares, the researchers enrolled 81 consecutive adult patients with gout in the study between April 2019 and March 2021 at one academic rheumatology treatment site in Italy and followed them for 12 months. The authors compared cases (who developed at least one flare within 12 months of the baseline visit) with controls (who self-reported no gout flares over that period).
Patients diagnosed with other inflammatory arthritis and those with coexisting calcium pyrophosphate deposition disease were excluded from the study.
The 71 participants who completed the study were, on average, in their early 60s, and in both groups, all but one were male. At the baseline visit, all had been on stable urate-lowering therapy for at least 6 months and had not had any gout flares in 4 weeks. The mean gout duration was 7 years in the case group and 8 years in controls.
At baseline, all participants underwent physical examination and US of elbows, wrists, second metacarpophalangeal joints, knees, ankles, and first metatarsophalangeal joints by a member of the research team who was blinded to the clinical and laboratory data.
Clinical assessments were scheduled at baseline and at 6-month intervals, and all participants were evaluated by a second researcher who was blinded to US findings.
During follow-up visits, participants were asked to report any gout flare, considered to meet at least three of four criteria: patient-defined flare, pain at rest score higher than 3 on a 0-10 scale, at least one swollen joint, and at least one warm joint. Patients not reaching their target serum urate goal received escalated urate-lowering therapy dosage and anti-inflammatory prophylaxis.
The US indicators of MSU deposits – aggregates, double contour sign, and tophi – were recorded as present or absent. The power Doppler signal was scored from 0 through 4, and summated scores for each US finding were calculated.
Over 12 months, the researchers found:
- Thirty (42.3%) patients had at least one flare, with a median of 2.0 flares. Patients with flares had higher a US median total MSU score (5.0 vs. 2.0; P = .01) and power Doppler signal (3.0 vs. 0; P < .01) than controls.
- In multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the occurrence of flares. The adjusted odds ratio for total MSU score was 1.75 (95% confidence interval, 1.26-2.43) and for power Doppler score was 1.63 (95% CI, 1.12-2.40).
- Also in a multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the number of flares. The incidence risk ratio for total MSU score adjusted was 1.17 (95% CI, 1.08-1.26) and for power Doppler score was 1.29 (95% CI, 1.19-1.40).
Four rheumatologists welcome findings
Gout remains the most common cause of inflammatory arthritis and a significant reason for hospital visits, noted Narender Annapureddy, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn..
“The study adds to the growing utility of musculoskeletal ultrasound in rheumatology practices to treat various diseases,” he said. “Data that could provide risk prediction for gout flares would be associated with significant benefits in terms of reducing ED visits, hospital admission, and lost work productivity.”
One study limitation, Dr. Annapureddy mentioned, was the single experienced US reader, “which may limit generalizability of results at this time, at least in the United States.”
Yeohan Song, MD, an instructor at Ohio State University Wexner Medical Center, Columbus, integrates US into his practice.
“In gout management, musculoskeletal ultrasound is a useful adjunct to the clinical exam and laboratory markers, particularly [in patients] with recurrent flares despite guideline-directed target serum urate levels,” he said.
Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, pointed out that the US protocol in the study involved imaging knees, ankles, first metatarsophalangeal joints, elbows, wrists, and second metacarpophalangeal joints, and took around 30 minutes to complete.
“That would not be practical in the United States due to time constraints in most rheumatology clinics,” she said.
“The authors report that a ‘reduced scanning protocol’ of the bilateral knees, ankles, and first metatarsophalangeal joints demonstrated similar predictive ability as the full protocol,” she added, “although scanning six joints still might not be feasible during a typical return patient clinic visit in the United States.”
Philip Chu, MD, clinical associate at Duke University, Durham, N.C., uses diagnostic US to help differentiate borderline gout cases from other arthropathies.
“A baseline scan, a follow-up scan before deciding to stop prophylaxis, or a follow-up scan in the setting of recurrent gout flares despite reaching goal serum uric acid, may be cost-effective time points to perform diagnostic US,” he advised.
“Unfortunately,” he added, “reimbursement for diagnostic US has been decreasing over the years, which makes it challenging to increase diagnostic US to the [frequency of its use] in Europe.”
Asked how most gout care being provided by primary care doctors in the United States affects gout management, Dr. Chu said: “Depending on which guidelines one follows for treating gout – from the American College of Rheumatology or the American College of Physicians – one may be more or less likely to start urate-lowering therapy after the first gout flare.”
“Understanding MSU burden in each patient, or even seeing active inflammation at these sites by increased Doppler signal, may change the threshold for physicians to initiate therapy,” he added.
The study received no funding. Three study authors reported financial involvements with pharmaceutical companies. Dr. Cipolletta, Dr. Annapureddy, Dr. Song, Dr. Tedeschi, and Dr. Chu reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM RHEUMATOLOGY
Black Americans’ high gout rate stems from social causes
Gout prevalence is more common in Black Americans than White Americans, and the disparity in prevalence is attributable to social determinants of health, according to a recently published article in JAMA Network Open.
“There has been evidence from recent cohort studies in the U.S. that was suggesting that the prevalence and incidence [of gout] was growing among non-White populations,” said Natalie McCormick, PhD, the study’s lead author and postdoctoral research fellow in medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston. “We wanted to do this at the general population level to see how generalizable [that evidence] is.”
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Medical Group, Franklin, Wisc., noted the findings highlight inequities in care for patients with gout that could be improved with greater emphasis on educating patients about their condition.
“I think that what this shows is that in the U.S. ... there still are some disparities in treating gout,” said Dr. Wells, who was not involved with the study. “And that we have ways to mitigate that, with not only aggressive therapy, but also with other tools like counseling patients. At the end of the day, people all want to be educated about the disease.”
Greater prevalence disappears with adjustment for socioclinical factors
The cross-sectional analysis involved data from U.S. adult participants in the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2016 who self-reported Black or White race.
Investigators considered factors such as excess body mass index (BMI), chronic kidney disease (defined as estimated glomerular filtration rate less than 60 mL/min per 1.73 m2), poverty, poor-quality diet, lower educational level, alcohol consumption, and diuretic use in their analysis.
Dr. McCormick and coinvestigators included a total of 18,693 participants, consisting of 3,304 Black women, 6,195 White women, 3,085 Black men, and 6,109 White men.
They determined that the age-standardized prevalence of gout was 3.5% (95% confidence interval, 2.7%-4.3%) in Black women and 2.0% (95% CI, 1.5% - 2.5%) in White women (age-adjusted odds ratio, 1.81; 95% CI, 1.29-2.53). They calculated that the prevalence was 7.0% (95% CI, 6.2%-7.9%) in Black men and 5.4% (95% CI, 4.7%-6.2%) in White men (age-adjusted OR, 1.26; 95% CI, 1.02-1.55). They found similar differences in the prevalence of hyperuricemia between Black and White Americans.
The increased prevalence of gout in Black Americans, compared with White Americans, does not arise from genetics, according to McCormick. “Our conclusion was that it was due to social determinants of health,” she said. “When we adjusted for all socioclinical risk factors, the racial differences in gout and hyperuricemia prevalence disappeared. Importantly, stepwise regression analysis showed the two biggest drivers of the racial difference in gout prevalence among women were poverty itself, and excess BMI, which can be influenced by poverty.”
Dr. McCormick pointed out that in contrast to the current data, there was no racial difference in the prevalence of gout approximately 2 decades earlier, looking at data from the 1988-1994 NHANES III.
Given the findings, which included the fact that significantly more Black women and men were currently taking diuretics, compared with their White counterparts, Dr. McCormick pointed out clinicians should give more thought to medical therapies prescribed for conditions like high blood pressure to patients with gout or at risk for gout.
“One thing we found was that diuretic use was a driver” of gout, Dr. McCormick said. A prescriber “may want to consider different therapies that present a lower risk of gout if someone has hypertension. There could be greater consideration for prescribing alternatives to diuretics.”
More patient education and rheumatology referrals needed
An impediment to providing that education to patients with gout is unconscious bias on the part of the primary care provider, Dr. Wells said.
“It is about what your perspectives are and what you bring to the table,” he explained. “If you saw [a patient] who looked like someone in your family, that person will be treated differently [than someone who does not look like a family member]. That is where the whole concept [of unconscious bias] comes in.”
Primary care providers need to adopt a holistic approach to gout management that involves counseling about good nutrition, smoking cessation, regular exercise, and limiting alcohol consumption, in addition to medication adherence. Primary care providers may have a bias in treating their Black patients, failing to devote sufficient time and attention to assist them in getting their disease under control, he said.
“Gout should be just like any other chronic disease,” Dr. Wells said. “You need to have a target in mind, and you and your patient need to work together to get to that target. When [patients] end up in rheumatology offices, it is almost too late. I think the take-home message here is that in 2022 ... for any patient who has gout, that patient probably needs to be seen by a rheumatologist because, indeed, with aggressive therapy, preventive therapy, [and] education, and if they are on the right medications, they won’t end up with these crippling joints that we see all the time.”
Dr. McCormick and Dr. Wells disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout prevalence is more common in Black Americans than White Americans, and the disparity in prevalence is attributable to social determinants of health, according to a recently published article in JAMA Network Open.
“There has been evidence from recent cohort studies in the U.S. that was suggesting that the prevalence and incidence [of gout] was growing among non-White populations,” said Natalie McCormick, PhD, the study’s lead author and postdoctoral research fellow in medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston. “We wanted to do this at the general population level to see how generalizable [that evidence] is.”
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Medical Group, Franklin, Wisc., noted the findings highlight inequities in care for patients with gout that could be improved with greater emphasis on educating patients about their condition.
“I think that what this shows is that in the U.S. ... there still are some disparities in treating gout,” said Dr. Wells, who was not involved with the study. “And that we have ways to mitigate that, with not only aggressive therapy, but also with other tools like counseling patients. At the end of the day, people all want to be educated about the disease.”
Greater prevalence disappears with adjustment for socioclinical factors
The cross-sectional analysis involved data from U.S. adult participants in the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2016 who self-reported Black or White race.
Investigators considered factors such as excess body mass index (BMI), chronic kidney disease (defined as estimated glomerular filtration rate less than 60 mL/min per 1.73 m2), poverty, poor-quality diet, lower educational level, alcohol consumption, and diuretic use in their analysis.
Dr. McCormick and coinvestigators included a total of 18,693 participants, consisting of 3,304 Black women, 6,195 White women, 3,085 Black men, and 6,109 White men.
They determined that the age-standardized prevalence of gout was 3.5% (95% confidence interval, 2.7%-4.3%) in Black women and 2.0% (95% CI, 1.5% - 2.5%) in White women (age-adjusted odds ratio, 1.81; 95% CI, 1.29-2.53). They calculated that the prevalence was 7.0% (95% CI, 6.2%-7.9%) in Black men and 5.4% (95% CI, 4.7%-6.2%) in White men (age-adjusted OR, 1.26; 95% CI, 1.02-1.55). They found similar differences in the prevalence of hyperuricemia between Black and White Americans.
The increased prevalence of gout in Black Americans, compared with White Americans, does not arise from genetics, according to McCormick. “Our conclusion was that it was due to social determinants of health,” she said. “When we adjusted for all socioclinical risk factors, the racial differences in gout and hyperuricemia prevalence disappeared. Importantly, stepwise regression analysis showed the two biggest drivers of the racial difference in gout prevalence among women were poverty itself, and excess BMI, which can be influenced by poverty.”
Dr. McCormick pointed out that in contrast to the current data, there was no racial difference in the prevalence of gout approximately 2 decades earlier, looking at data from the 1988-1994 NHANES III.
Given the findings, which included the fact that significantly more Black women and men were currently taking diuretics, compared with their White counterparts, Dr. McCormick pointed out clinicians should give more thought to medical therapies prescribed for conditions like high blood pressure to patients with gout or at risk for gout.
“One thing we found was that diuretic use was a driver” of gout, Dr. McCormick said. A prescriber “may want to consider different therapies that present a lower risk of gout if someone has hypertension. There could be greater consideration for prescribing alternatives to diuretics.”
More patient education and rheumatology referrals needed
An impediment to providing that education to patients with gout is unconscious bias on the part of the primary care provider, Dr. Wells said.
“It is about what your perspectives are and what you bring to the table,” he explained. “If you saw [a patient] who looked like someone in your family, that person will be treated differently [than someone who does not look like a family member]. That is where the whole concept [of unconscious bias] comes in.”
Primary care providers need to adopt a holistic approach to gout management that involves counseling about good nutrition, smoking cessation, regular exercise, and limiting alcohol consumption, in addition to medication adherence. Primary care providers may have a bias in treating their Black patients, failing to devote sufficient time and attention to assist them in getting their disease under control, he said.
“Gout should be just like any other chronic disease,” Dr. Wells said. “You need to have a target in mind, and you and your patient need to work together to get to that target. When [patients] end up in rheumatology offices, it is almost too late. I think the take-home message here is that in 2022 ... for any patient who has gout, that patient probably needs to be seen by a rheumatologist because, indeed, with aggressive therapy, preventive therapy, [and] education, and if they are on the right medications, they won’t end up with these crippling joints that we see all the time.”
Dr. McCormick and Dr. Wells disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout prevalence is more common in Black Americans than White Americans, and the disparity in prevalence is attributable to social determinants of health, according to a recently published article in JAMA Network Open.
“There has been evidence from recent cohort studies in the U.S. that was suggesting that the prevalence and incidence [of gout] was growing among non-White populations,” said Natalie McCormick, PhD, the study’s lead author and postdoctoral research fellow in medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston. “We wanted to do this at the general population level to see how generalizable [that evidence] is.”
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Medical Group, Franklin, Wisc., noted the findings highlight inequities in care for patients with gout that could be improved with greater emphasis on educating patients about their condition.
“I think that what this shows is that in the U.S. ... there still are some disparities in treating gout,” said Dr. Wells, who was not involved with the study. “And that we have ways to mitigate that, with not only aggressive therapy, but also with other tools like counseling patients. At the end of the day, people all want to be educated about the disease.”
Greater prevalence disappears with adjustment for socioclinical factors
The cross-sectional analysis involved data from U.S. adult participants in the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2016 who self-reported Black or White race.
Investigators considered factors such as excess body mass index (BMI), chronic kidney disease (defined as estimated glomerular filtration rate less than 60 mL/min per 1.73 m2), poverty, poor-quality diet, lower educational level, alcohol consumption, and diuretic use in their analysis.
Dr. McCormick and coinvestigators included a total of 18,693 participants, consisting of 3,304 Black women, 6,195 White women, 3,085 Black men, and 6,109 White men.
They determined that the age-standardized prevalence of gout was 3.5% (95% confidence interval, 2.7%-4.3%) in Black women and 2.0% (95% CI, 1.5% - 2.5%) in White women (age-adjusted odds ratio, 1.81; 95% CI, 1.29-2.53). They calculated that the prevalence was 7.0% (95% CI, 6.2%-7.9%) in Black men and 5.4% (95% CI, 4.7%-6.2%) in White men (age-adjusted OR, 1.26; 95% CI, 1.02-1.55). They found similar differences in the prevalence of hyperuricemia between Black and White Americans.
The increased prevalence of gout in Black Americans, compared with White Americans, does not arise from genetics, according to McCormick. “Our conclusion was that it was due to social determinants of health,” she said. “When we adjusted for all socioclinical risk factors, the racial differences in gout and hyperuricemia prevalence disappeared. Importantly, stepwise regression analysis showed the two biggest drivers of the racial difference in gout prevalence among women were poverty itself, and excess BMI, which can be influenced by poverty.”
Dr. McCormick pointed out that in contrast to the current data, there was no racial difference in the prevalence of gout approximately 2 decades earlier, looking at data from the 1988-1994 NHANES III.
Given the findings, which included the fact that significantly more Black women and men were currently taking diuretics, compared with their White counterparts, Dr. McCormick pointed out clinicians should give more thought to medical therapies prescribed for conditions like high blood pressure to patients with gout or at risk for gout.
“One thing we found was that diuretic use was a driver” of gout, Dr. McCormick said. A prescriber “may want to consider different therapies that present a lower risk of gout if someone has hypertension. There could be greater consideration for prescribing alternatives to diuretics.”
More patient education and rheumatology referrals needed
An impediment to providing that education to patients with gout is unconscious bias on the part of the primary care provider, Dr. Wells said.
“It is about what your perspectives are and what you bring to the table,” he explained. “If you saw [a patient] who looked like someone in your family, that person will be treated differently [than someone who does not look like a family member]. That is where the whole concept [of unconscious bias] comes in.”
Primary care providers need to adopt a holistic approach to gout management that involves counseling about good nutrition, smoking cessation, regular exercise, and limiting alcohol consumption, in addition to medication adherence. Primary care providers may have a bias in treating their Black patients, failing to devote sufficient time and attention to assist them in getting their disease under control, he said.
“Gout should be just like any other chronic disease,” Dr. Wells said. “You need to have a target in mind, and you and your patient need to work together to get to that target. When [patients] end up in rheumatology offices, it is almost too late. I think the take-home message here is that in 2022 ... for any patient who has gout, that patient probably needs to be seen by a rheumatologist because, indeed, with aggressive therapy, preventive therapy, [and] education, and if they are on the right medications, they won’t end up with these crippling joints that we see all the time.”
Dr. McCormick and Dr. Wells disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Toenail trauma
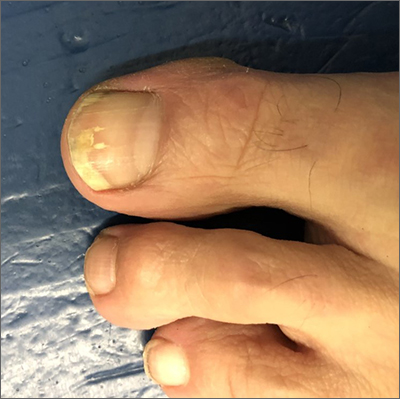
The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111

The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111
Spotted white fingernails
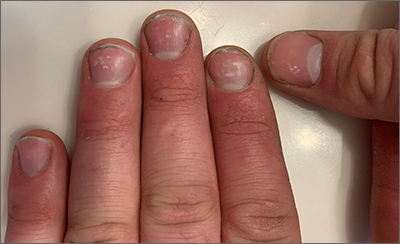
White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6

White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

White nail changes are broadly called leukonychia: “leuko” meaning white and “nychia” referring to the nail. Scattered or single asymptomatic cloudy white nail lesions occurring without other associated skin or nail disorders are more specifically called punctate leukonychia.
Punctate leukonychia is theorized to be caused by trauma at the proximal nail matrix, affecting the developing nail.1 The trauma may result from aggressive nail care practices or damage to the cuticle. In many cases, there is no history of known trauma. For this patient with multiple lesions, who performed manual work, multiple small traumas may have induced the punctate leukonychia.
Other causes of leukonychia include superficial onychomycosis (in which discoloration may be whiter than the usual yellow-brown), renal disease, and arsenic toxicity.1 Arsenic toxicity causes transverse leukonychia in a band-like fashion, since it is a systemic insult to the growing nails. Longitudinal leukonychia is due to a more localized insult to the nail matrix, causing the white lines to grow out with the nail along the axis of the digit. Other than avoiding trauma, there is no treatment needed or recommended for punctate leukonychia.
The patient was counseled on the benign nature of his punctate leukonychia and assured that no treatment was necessary.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6
1. Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193. doi: 10.1007/s40257-022-00671-6
Primary care now offering physicians the 26.7-hour day
Taking ‘not enough hours in the day’ to new heights
It’s no secret that there’s a big doctor shortage in the United States. Going through medical school is long, expensive, and stressful, and it’s not like those long, stressful hours stop once you finally do get that degree. There is, however, an excellent reason to take that dive into doctorhood: You’ll gain mastery over time itself.
A study from the University of Chicago, Johns Hopkins University, and Imperial College London has revealed the truth. By using data pulled from the National Health and Nutrition Examination Survey, the researchers found that primary care physicians who see an average number of patients and follow all the current national guidelines for preventive care, chronic disease care, and acute care – plus administrative tasks – must work 26.7 hours a day. That works out to 14.1 hours of preventive care, 7.2 hours of chronic disease care, 2.2 hours of acute care, and 3.2 hours of documentation and inbox management.
Astute readers may note that this is a bit more than the traditional 8-hour workday. It is, in fact, more hours than there actually are in a day. As it turns out, Doctor Strange is more of a documentary than …
Hang on, we’re receiving word that doctors are not in fact wizards who can bend time and space to their will, nor are they sitting on a stash of Time-Turners they saved from the Ministry of Magic before Voldemort destroyed them all. They are, according to the study, overworked and overburdened with too many things and too little time. This is why outcomes haven’t improved despite technological advances and why burnout is so common. We’d be burned out too, having to work temporally impossible hours.
The study authors suggested a team-based approach to medicine that would spread the workload out to nurses, physician assistants, dietitians, etc., estimating that about two-thirds of what a primary care physician does can be handled by someone else. A team-based approach would reduce the physician’s required hours down to 9.3 hours a day, which is at least physically possible. It’s either that or we make the day longer, which sounds like the plot of an episode of Futurama. Swap overwork for global warming and a longer day for a longer year and it is actually the plot of an episode of Futurama.
After a hard day of thinking, brains need their rest
Do you ever feel like you have no more capacity to think or make any more decisions after a long day at work? Do you need a few extra cups of coffee to even make it through the day, even though you’re mostly just sitting around talking and typing? Have we got the research for you: Mental exhaustion is an actual thing. Imagine that double whammy of having a job that’s physically and mentally demanding.
A recent study in Current Biology explained why we feel so exhausted after doing something mentally demanding for several hours. Over that time, glutamate builds up in synapses of the prefrontal cortex, which affects our decision making and leads to cognitive lethargy. Your brain eventually becomes more interested in tasks that are less mentally fatiguing, and that’s probably why you’re reading this LOTME right now instead of getting back to work.
“Our findings show that cognitive work results in a true functional alteration – accumulation of noxious substances – so fatigue would indeed be a signal that makes us stop working but for a different purpose: to preserve the integrity of brain functioning,” senior author Mathias Pessiglione of Pitié-Salpêtrière University, Paris, said in a written statement.
The group of researchers conducted studies by using magnetic resonance spectroscopy to look at two groups of people over the course of a workday: One group had mentally tasking jobs and one didn’t. Those who had to think harder for their jobs had more signs of fatigue, such as reduced pupil dilation and glutamate in synapses of the prefrontal cortex. They also looked for more rewards that required less thinking.
For those whose mentally exhausting jobs probably won’t get better or change, the researchers suggest getting as much rest as possible. Those who don’t have that option will have to continue drinking those 7 cups of coffee a day. ... and reading LOTME.
Hmm, might be a new tagline for us in there somewhere. LOTME: Tired brains love us? When you’re too tired to think, think of LOTME? You can’t spell mental exhaustion without L-O-T-M-E?
Testosterone shows its warm and fuzzy side
Stereotypically, men are loud, knuckle-dragging Neanderthals. The hair coming out of our faces is kind of a dead giveaway, right? We grunt, we scratch, we start wars, we watch sports on TV. But why? It’s the testosterone. Everyone knows that. Testosterone makes men aggressive … or does it?
Since this sort of research generally isn’t done with actual men, investigators at Emory University used Mongolian gerbils. The advantage being that males exhibit cuddling behavior after females become pregnant and they don’t watch a lot of sports on TV. They introduced a male and female gerbil, who then formed a pair bond and the female became pregnant. When the male started displaying cuddling behaviors, the researchers injected him with testosterone, expecting to see his antisocial side.
“Instead, we were surprised that a male gerbil became even more cuddly and prosocial with his partner. He became like ‘super partner,’ ” lead author Aubrey Kelly, PhD, said in a written statement from the university.
For the next experiment, the female was removed and another male was introduced to a male who had already received a testosterone injection. That male was surprisingly unaggressive toward the intruder, at least initially. Then he received a second injection of testosterone. “It was like they suddenly woke up and realized they weren’t supposed to be friendly in that context,” Dr. Kelly said.
The testosterone seemed to influence the activity of oxytocin, the so-called “love hormone,” the investigators suggested. “It’s surprising because normally we think of testosterone as increasing sexual behaviors and aggression. But we’ve shown that it can have more nuanced effects, depending on the social context.”
The researchers were not as surprised when their use of the phrase “super partner” led to a bidding war between DC and Marvel. Then came the contact from the Department of Defense, wondering about weaponized testosterone: Would it be possible for some sort of bomb to turn Vlad “the Impaler” Putin into Vlad “the Cuddler” Putin?
Are instruments spreading the sounds of COVID?
COVID restrictions are practically a thing of the past now. With more people laxed on being in close proximity to each other and the CDC not even recommending social distancing anymore, live concerts and events are back in full swing. But with new variants on the rise and people being a little more cautious, should we be worried about musical instruments spreading COVID?
Yes and no.
A study published in Physics of Fluids looked at wind instruments specifically and how much aerosol is produced and dispersed when playing them. For the study, the investigators measured fog particles with a laser and aerosol concentration with a particle counter to see how fast these particles decay in the air from the distance of the instrument.
Musicians in an orchestra typically would sit close together to produce the best sound, but with COVID that became an issue, senior author Paulo Arratia of the University of Pennsylvania, Philadelphia, noted in a separate written statement. By looking at the distance traveled by the particles coming from a single instrument and how quickly they decayed, they could determine if sitting in close proximity is an actual threat.
Well, the threat was no greater than talking to someone face to face. Particle exit speeds were lower than for a cough or a sneeze, and the maximum decay length was 2 meters from the instrument’s opening.
But that’s just one instrument: What kind of impact does a whole orchestra have on a space? The researchers are looking into that too, but for now they suggest that musicians continue to stay 6 feet away from each other.
So, yeah, there is a threat, but it’s probably safer for you to see that orchestra than have someone sneeze on you.
Music to our ears.
Taking ‘not enough hours in the day’ to new heights
It’s no secret that there’s a big doctor shortage in the United States. Going through medical school is long, expensive, and stressful, and it’s not like those long, stressful hours stop once you finally do get that degree. There is, however, an excellent reason to take that dive into doctorhood: You’ll gain mastery over time itself.
A study from the University of Chicago, Johns Hopkins University, and Imperial College London has revealed the truth. By using data pulled from the National Health and Nutrition Examination Survey, the researchers found that primary care physicians who see an average number of patients and follow all the current national guidelines for preventive care, chronic disease care, and acute care – plus administrative tasks – must work 26.7 hours a day. That works out to 14.1 hours of preventive care, 7.2 hours of chronic disease care, 2.2 hours of acute care, and 3.2 hours of documentation and inbox management.
Astute readers may note that this is a bit more than the traditional 8-hour workday. It is, in fact, more hours than there actually are in a day. As it turns out, Doctor Strange is more of a documentary than …
Hang on, we’re receiving word that doctors are not in fact wizards who can bend time and space to their will, nor are they sitting on a stash of Time-Turners they saved from the Ministry of Magic before Voldemort destroyed them all. They are, according to the study, overworked and overburdened with too many things and too little time. This is why outcomes haven’t improved despite technological advances and why burnout is so common. We’d be burned out too, having to work temporally impossible hours.
The study authors suggested a team-based approach to medicine that would spread the workload out to nurses, physician assistants, dietitians, etc., estimating that about two-thirds of what a primary care physician does can be handled by someone else. A team-based approach would reduce the physician’s required hours down to 9.3 hours a day, which is at least physically possible. It’s either that or we make the day longer, which sounds like the plot of an episode of Futurama. Swap overwork for global warming and a longer day for a longer year and it is actually the plot of an episode of Futurama.
After a hard day of thinking, brains need their rest
Do you ever feel like you have no more capacity to think or make any more decisions after a long day at work? Do you need a few extra cups of coffee to even make it through the day, even though you’re mostly just sitting around talking and typing? Have we got the research for you: Mental exhaustion is an actual thing. Imagine that double whammy of having a job that’s physically and mentally demanding.
A recent study in Current Biology explained why we feel so exhausted after doing something mentally demanding for several hours. Over that time, glutamate builds up in synapses of the prefrontal cortex, which affects our decision making and leads to cognitive lethargy. Your brain eventually becomes more interested in tasks that are less mentally fatiguing, and that’s probably why you’re reading this LOTME right now instead of getting back to work.
“Our findings show that cognitive work results in a true functional alteration – accumulation of noxious substances – so fatigue would indeed be a signal that makes us stop working but for a different purpose: to preserve the integrity of brain functioning,” senior author Mathias Pessiglione of Pitié-Salpêtrière University, Paris, said in a written statement.
The group of researchers conducted studies by using magnetic resonance spectroscopy to look at two groups of people over the course of a workday: One group had mentally tasking jobs and one didn’t. Those who had to think harder for their jobs had more signs of fatigue, such as reduced pupil dilation and glutamate in synapses of the prefrontal cortex. They also looked for more rewards that required less thinking.
For those whose mentally exhausting jobs probably won’t get better or change, the researchers suggest getting as much rest as possible. Those who don’t have that option will have to continue drinking those 7 cups of coffee a day. ... and reading LOTME.
Hmm, might be a new tagline for us in there somewhere. LOTME: Tired brains love us? When you’re too tired to think, think of LOTME? You can’t spell mental exhaustion without L-O-T-M-E?
Testosterone shows its warm and fuzzy side
Stereotypically, men are loud, knuckle-dragging Neanderthals. The hair coming out of our faces is kind of a dead giveaway, right? We grunt, we scratch, we start wars, we watch sports on TV. But why? It’s the testosterone. Everyone knows that. Testosterone makes men aggressive … or does it?
Since this sort of research generally isn’t done with actual men, investigators at Emory University used Mongolian gerbils. The advantage being that males exhibit cuddling behavior after females become pregnant and they don’t watch a lot of sports on TV. They introduced a male and female gerbil, who then formed a pair bond and the female became pregnant. When the male started displaying cuddling behaviors, the researchers injected him with testosterone, expecting to see his antisocial side.
“Instead, we were surprised that a male gerbil became even more cuddly and prosocial with his partner. He became like ‘super partner,’ ” lead author Aubrey Kelly, PhD, said in a written statement from the university.
For the next experiment, the female was removed and another male was introduced to a male who had already received a testosterone injection. That male was surprisingly unaggressive toward the intruder, at least initially. Then he received a second injection of testosterone. “It was like they suddenly woke up and realized they weren’t supposed to be friendly in that context,” Dr. Kelly said.
The testosterone seemed to influence the activity of oxytocin, the so-called “love hormone,” the investigators suggested. “It’s surprising because normally we think of testosterone as increasing sexual behaviors and aggression. But we’ve shown that it can have more nuanced effects, depending on the social context.”
The researchers were not as surprised when their use of the phrase “super partner” led to a bidding war between DC and Marvel. Then came the contact from the Department of Defense, wondering about weaponized testosterone: Would it be possible for some sort of bomb to turn Vlad “the Impaler” Putin into Vlad “the Cuddler” Putin?
Are instruments spreading the sounds of COVID?
COVID restrictions are practically a thing of the past now. With more people laxed on being in close proximity to each other and the CDC not even recommending social distancing anymore, live concerts and events are back in full swing. But with new variants on the rise and people being a little more cautious, should we be worried about musical instruments spreading COVID?
Yes and no.
A study published in Physics of Fluids looked at wind instruments specifically and how much aerosol is produced and dispersed when playing them. For the study, the investigators measured fog particles with a laser and aerosol concentration with a particle counter to see how fast these particles decay in the air from the distance of the instrument.
Musicians in an orchestra typically would sit close together to produce the best sound, but with COVID that became an issue, senior author Paulo Arratia of the University of Pennsylvania, Philadelphia, noted in a separate written statement. By looking at the distance traveled by the particles coming from a single instrument and how quickly they decayed, they could determine if sitting in close proximity is an actual threat.
Well, the threat was no greater than talking to someone face to face. Particle exit speeds were lower than for a cough or a sneeze, and the maximum decay length was 2 meters from the instrument’s opening.
But that’s just one instrument: What kind of impact does a whole orchestra have on a space? The researchers are looking into that too, but for now they suggest that musicians continue to stay 6 feet away from each other.
So, yeah, there is a threat, but it’s probably safer for you to see that orchestra than have someone sneeze on you.
Music to our ears.
Taking ‘not enough hours in the day’ to new heights
It’s no secret that there’s a big doctor shortage in the United States. Going through medical school is long, expensive, and stressful, and it’s not like those long, stressful hours stop once you finally do get that degree. There is, however, an excellent reason to take that dive into doctorhood: You’ll gain mastery over time itself.
A study from the University of Chicago, Johns Hopkins University, and Imperial College London has revealed the truth. By using data pulled from the National Health and Nutrition Examination Survey, the researchers found that primary care physicians who see an average number of patients and follow all the current national guidelines for preventive care, chronic disease care, and acute care – plus administrative tasks – must work 26.7 hours a day. That works out to 14.1 hours of preventive care, 7.2 hours of chronic disease care, 2.2 hours of acute care, and 3.2 hours of documentation and inbox management.
Astute readers may note that this is a bit more than the traditional 8-hour workday. It is, in fact, more hours than there actually are in a day. As it turns out, Doctor Strange is more of a documentary than …
Hang on, we’re receiving word that doctors are not in fact wizards who can bend time and space to their will, nor are they sitting on a stash of Time-Turners they saved from the Ministry of Magic before Voldemort destroyed them all. They are, according to the study, overworked and overburdened with too many things and too little time. This is why outcomes haven’t improved despite technological advances and why burnout is so common. We’d be burned out too, having to work temporally impossible hours.
The study authors suggested a team-based approach to medicine that would spread the workload out to nurses, physician assistants, dietitians, etc., estimating that about two-thirds of what a primary care physician does can be handled by someone else. A team-based approach would reduce the physician’s required hours down to 9.3 hours a day, which is at least physically possible. It’s either that or we make the day longer, which sounds like the plot of an episode of Futurama. Swap overwork for global warming and a longer day for a longer year and it is actually the plot of an episode of Futurama.
After a hard day of thinking, brains need their rest
Do you ever feel like you have no more capacity to think or make any more decisions after a long day at work? Do you need a few extra cups of coffee to even make it through the day, even though you’re mostly just sitting around talking and typing? Have we got the research for you: Mental exhaustion is an actual thing. Imagine that double whammy of having a job that’s physically and mentally demanding.
A recent study in Current Biology explained why we feel so exhausted after doing something mentally demanding for several hours. Over that time, glutamate builds up in synapses of the prefrontal cortex, which affects our decision making and leads to cognitive lethargy. Your brain eventually becomes more interested in tasks that are less mentally fatiguing, and that’s probably why you’re reading this LOTME right now instead of getting back to work.
“Our findings show that cognitive work results in a true functional alteration – accumulation of noxious substances – so fatigue would indeed be a signal that makes us stop working but for a different purpose: to preserve the integrity of brain functioning,” senior author Mathias Pessiglione of Pitié-Salpêtrière University, Paris, said in a written statement.
The group of researchers conducted studies by using magnetic resonance spectroscopy to look at two groups of people over the course of a workday: One group had mentally tasking jobs and one didn’t. Those who had to think harder for their jobs had more signs of fatigue, such as reduced pupil dilation and glutamate in synapses of the prefrontal cortex. They also looked for more rewards that required less thinking.
For those whose mentally exhausting jobs probably won’t get better or change, the researchers suggest getting as much rest as possible. Those who don’t have that option will have to continue drinking those 7 cups of coffee a day. ... and reading LOTME.
Hmm, might be a new tagline for us in there somewhere. LOTME: Tired brains love us? When you’re too tired to think, think of LOTME? You can’t spell mental exhaustion without L-O-T-M-E?
Testosterone shows its warm and fuzzy side
Stereotypically, men are loud, knuckle-dragging Neanderthals. The hair coming out of our faces is kind of a dead giveaway, right? We grunt, we scratch, we start wars, we watch sports on TV. But why? It’s the testosterone. Everyone knows that. Testosterone makes men aggressive … or does it?
Since this sort of research generally isn’t done with actual men, investigators at Emory University used Mongolian gerbils. The advantage being that males exhibit cuddling behavior after females become pregnant and they don’t watch a lot of sports on TV. They introduced a male and female gerbil, who then formed a pair bond and the female became pregnant. When the male started displaying cuddling behaviors, the researchers injected him with testosterone, expecting to see his antisocial side.
“Instead, we were surprised that a male gerbil became even more cuddly and prosocial with his partner. He became like ‘super partner,’ ” lead author Aubrey Kelly, PhD, said in a written statement from the university.
For the next experiment, the female was removed and another male was introduced to a male who had already received a testosterone injection. That male was surprisingly unaggressive toward the intruder, at least initially. Then he received a second injection of testosterone. “It was like they suddenly woke up and realized they weren’t supposed to be friendly in that context,” Dr. Kelly said.
The testosterone seemed to influence the activity of oxytocin, the so-called “love hormone,” the investigators suggested. “It’s surprising because normally we think of testosterone as increasing sexual behaviors and aggression. But we’ve shown that it can have more nuanced effects, depending on the social context.”
The researchers were not as surprised when their use of the phrase “super partner” led to a bidding war between DC and Marvel. Then came the contact from the Department of Defense, wondering about weaponized testosterone: Would it be possible for some sort of bomb to turn Vlad “the Impaler” Putin into Vlad “the Cuddler” Putin?
Are instruments spreading the sounds of COVID?
COVID restrictions are practically a thing of the past now. With more people laxed on being in close proximity to each other and the CDC not even recommending social distancing anymore, live concerts and events are back in full swing. But with new variants on the rise and people being a little more cautious, should we be worried about musical instruments spreading COVID?
Yes and no.
A study published in Physics of Fluids looked at wind instruments specifically and how much aerosol is produced and dispersed when playing them. For the study, the investigators measured fog particles with a laser and aerosol concentration with a particle counter to see how fast these particles decay in the air from the distance of the instrument.
Musicians in an orchestra typically would sit close together to produce the best sound, but with COVID that became an issue, senior author Paulo Arratia of the University of Pennsylvania, Philadelphia, noted in a separate written statement. By looking at the distance traveled by the particles coming from a single instrument and how quickly they decayed, they could determine if sitting in close proximity is an actual threat.
Well, the threat was no greater than talking to someone face to face. Particle exit speeds were lower than for a cough or a sneeze, and the maximum decay length was 2 meters from the instrument’s opening.
But that’s just one instrument: What kind of impact does a whole orchestra have on a space? The researchers are looking into that too, but for now they suggest that musicians continue to stay 6 feet away from each other.
So, yeah, there is a threat, but it’s probably safer for you to see that orchestra than have someone sneeze on you.
Music to our ears.
Reliably solving complex problems
The James Webb Space Telescope (JWST) is an engineering marvel. Costing over $10 billion, it should be. The project cost overrun was 900%. The launch was delayed by more than a decade. The Human Genome Project from 1990 to 2003 was completed slightly ahead of schedule and for less than the $4-$5 billion original estimates. This HGP success story is partly because of private entrepreneurial involvement. The Superconducting Super Collider in Texas spent $2 billion but never got off the ground. Successfully shepherding huge public projects like these involves the art of politics and management as well as science.
Whatever the earlier missteps, the JWST project is now performing above expectations. It has launched, taken up residence a million miles from Earth, deployed its mirrors (a process that had more than 300 possible single points of failure, any one of which would reduce the thing to scrap metal), and been calibrated. The JWST has even been dented by a micrometeoroid – sort of like a parking lot ding on the door of your brand new car. The first images are visually amazing and producing new scientific insights. This is a pinnacle of scientific achievement.
What characteristics enable such an achievement? How do we foster those same characteristics in the practice of medicine and medical research? Will the success of the JWST increase and restore the public’s trust in science and scientists?
After all the bickering over vaccines and masks for the past 2+ years, medical science could use a boost. The gravitas of scientists, and indeed all experts, has diminished over the 5 decades since humans walked on the moon. It has been harmed by mercenary scientists who sought to sow doubt about whether smoking caused cancer and whether fossil fuels created climate change. No proof was needed, just doubt.
The trust in science has also been harmed by the vast amount of published medical research that is wrong. An effort was made 20 years ago to rid research of the bias of taking money from drug companies. To my observation, that change produced only a small benefit that has been overwhelmed by the unintended harms. The large, well-funded academic labs of full-time researchers have been replaced with unfunded, undertrained, and inadequately supported part-time junior faculty trying to publish enough articles to be promoted. In my opinion, this change is worse than funding from Big Pharma. (Disclosure – I worked in industry prior to graduate school.)
The pressure to publish reduces skepticism, so more incorrect data are published. The small size of these amateur studies produces unconvincing conclusions that feed an industry of meta-analysis that tries to overcome the deficiencies of the individual studies. This fragmented, biased approach is not how you build, launch, deploy, and operate the JWST, which requires very high reliability.
This approach is not working well for pediatrics either. I look at the history of the recommended workup of the febrile young infant from the 1980s until today. I see constant changes to the guidelines but no real progress toward a validated, evidence-based approach. A similar history is behind treatment of neonatal hyperbilirubinemia. In the 1994 publication, there was a movement toward being less aggressive. The 2004 and 2009 editions increased the frequency of screening and phototherapy. Now, the 2022 guidelines have moved in the direction we were headed in the 1990s. The workup of infants and children with possible urinary tract infections has undergone a similar trajectory. So has the screening for neonatal herpes infections. The practice changes are more like Brownian motion than real progress. This inconsistency has led me to be skeptical of the process the American Academy of Pediatrics uses to create guidelines.
Part of solving complex problems is allowing all stakeholders’ voices to be heard. On Jan. 28, 1986, seconds after liftoff, the space shuttle Challenger exploded. In the aftermath, it was determined that some engineers had expressed concern about the very cold weather that morning. The rubber in the O-ring would not be as flexible as designed. Their objection was not listened to. The O-ring failed, the fuel tank exploded, and the ship and crew were lost. It is a lesson many engineers of my generation took to heart. Do not suppress voices.
For example, 1 year ago (September 2021), the Royal Australian and New Zealand College of Psychiatrists published a position statement, “Recognising and addressing the mental health needs of people experiencing gender dysphoria/gender incongruence.” The statement expressed concern about the marked increase in incidence of rapid-onset gender dysphoria and therefore urged more thorough assessment by psychiatry before embarking on puberty-blocking therapies. The RANZCP position is at variance with recent trends in the United States. The topic was censored at the 2021 AAP national conference. Lately, I have heard the words disinformation and homophobic used to describe my RANZCP colleagues. I have been comparing AAP, Britain’s National Institute for Health and Care Excellence, and Royal Children’s Hospital Melbourne guidelines for 20 years. The variation is enlightening. I do not know the correct answer to treating gender dysphoria, but I know suppressing viewpoints and debate leads to exploding spaceships.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
The James Webb Space Telescope (JWST) is an engineering marvel. Costing over $10 billion, it should be. The project cost overrun was 900%. The launch was delayed by more than a decade. The Human Genome Project from 1990 to 2003 was completed slightly ahead of schedule and for less than the $4-$5 billion original estimates. This HGP success story is partly because of private entrepreneurial involvement. The Superconducting Super Collider in Texas spent $2 billion but never got off the ground. Successfully shepherding huge public projects like these involves the art of politics and management as well as science.
Whatever the earlier missteps, the JWST project is now performing above expectations. It has launched, taken up residence a million miles from Earth, deployed its mirrors (a process that had more than 300 possible single points of failure, any one of which would reduce the thing to scrap metal), and been calibrated. The JWST has even been dented by a micrometeoroid – sort of like a parking lot ding on the door of your brand new car. The first images are visually amazing and producing new scientific insights. This is a pinnacle of scientific achievement.
What characteristics enable such an achievement? How do we foster those same characteristics in the practice of medicine and medical research? Will the success of the JWST increase and restore the public’s trust in science and scientists?
After all the bickering over vaccines and masks for the past 2+ years, medical science could use a boost. The gravitas of scientists, and indeed all experts, has diminished over the 5 decades since humans walked on the moon. It has been harmed by mercenary scientists who sought to sow doubt about whether smoking caused cancer and whether fossil fuels created climate change. No proof was needed, just doubt.
The trust in science has also been harmed by the vast amount of published medical research that is wrong. An effort was made 20 years ago to rid research of the bias of taking money from drug companies. To my observation, that change produced only a small benefit that has been overwhelmed by the unintended harms. The large, well-funded academic labs of full-time researchers have been replaced with unfunded, undertrained, and inadequately supported part-time junior faculty trying to publish enough articles to be promoted. In my opinion, this change is worse than funding from Big Pharma. (Disclosure – I worked in industry prior to graduate school.)
The pressure to publish reduces skepticism, so more incorrect data are published. The small size of these amateur studies produces unconvincing conclusions that feed an industry of meta-analysis that tries to overcome the deficiencies of the individual studies. This fragmented, biased approach is not how you build, launch, deploy, and operate the JWST, which requires very high reliability.
This approach is not working well for pediatrics either. I look at the history of the recommended workup of the febrile young infant from the 1980s until today. I see constant changes to the guidelines but no real progress toward a validated, evidence-based approach. A similar history is behind treatment of neonatal hyperbilirubinemia. In the 1994 publication, there was a movement toward being less aggressive. The 2004 and 2009 editions increased the frequency of screening and phototherapy. Now, the 2022 guidelines have moved in the direction we were headed in the 1990s. The workup of infants and children with possible urinary tract infections has undergone a similar trajectory. So has the screening for neonatal herpes infections. The practice changes are more like Brownian motion than real progress. This inconsistency has led me to be skeptical of the process the American Academy of Pediatrics uses to create guidelines.
Part of solving complex problems is allowing all stakeholders’ voices to be heard. On Jan. 28, 1986, seconds after liftoff, the space shuttle Challenger exploded. In the aftermath, it was determined that some engineers had expressed concern about the very cold weather that morning. The rubber in the O-ring would not be as flexible as designed. Their objection was not listened to. The O-ring failed, the fuel tank exploded, and the ship and crew were lost. It is a lesson many engineers of my generation took to heart. Do not suppress voices.
For example, 1 year ago (September 2021), the Royal Australian and New Zealand College of Psychiatrists published a position statement, “Recognising and addressing the mental health needs of people experiencing gender dysphoria/gender incongruence.” The statement expressed concern about the marked increase in incidence of rapid-onset gender dysphoria and therefore urged more thorough assessment by psychiatry before embarking on puberty-blocking therapies. The RANZCP position is at variance with recent trends in the United States. The topic was censored at the 2021 AAP national conference. Lately, I have heard the words disinformation and homophobic used to describe my RANZCP colleagues. I have been comparing AAP, Britain’s National Institute for Health and Care Excellence, and Royal Children’s Hospital Melbourne guidelines for 20 years. The variation is enlightening. I do not know the correct answer to treating gender dysphoria, but I know suppressing viewpoints and debate leads to exploding spaceships.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
The James Webb Space Telescope (JWST) is an engineering marvel. Costing over $10 billion, it should be. The project cost overrun was 900%. The launch was delayed by more than a decade. The Human Genome Project from 1990 to 2003 was completed slightly ahead of schedule and for less than the $4-$5 billion original estimates. This HGP success story is partly because of private entrepreneurial involvement. The Superconducting Super Collider in Texas spent $2 billion but never got off the ground. Successfully shepherding huge public projects like these involves the art of politics and management as well as science.
Whatever the earlier missteps, the JWST project is now performing above expectations. It has launched, taken up residence a million miles from Earth, deployed its mirrors (a process that had more than 300 possible single points of failure, any one of which would reduce the thing to scrap metal), and been calibrated. The JWST has even been dented by a micrometeoroid – sort of like a parking lot ding on the door of your brand new car. The first images are visually amazing and producing new scientific insights. This is a pinnacle of scientific achievement.
What characteristics enable such an achievement? How do we foster those same characteristics in the practice of medicine and medical research? Will the success of the JWST increase and restore the public’s trust in science and scientists?
After all the bickering over vaccines and masks for the past 2+ years, medical science could use a boost. The gravitas of scientists, and indeed all experts, has diminished over the 5 decades since humans walked on the moon. It has been harmed by mercenary scientists who sought to sow doubt about whether smoking caused cancer and whether fossil fuels created climate change. No proof was needed, just doubt.
The trust in science has also been harmed by the vast amount of published medical research that is wrong. An effort was made 20 years ago to rid research of the bias of taking money from drug companies. To my observation, that change produced only a small benefit that has been overwhelmed by the unintended harms. The large, well-funded academic labs of full-time researchers have been replaced with unfunded, undertrained, and inadequately supported part-time junior faculty trying to publish enough articles to be promoted. In my opinion, this change is worse than funding from Big Pharma. (Disclosure – I worked in industry prior to graduate school.)
The pressure to publish reduces skepticism, so more incorrect data are published. The small size of these amateur studies produces unconvincing conclusions that feed an industry of meta-analysis that tries to overcome the deficiencies of the individual studies. This fragmented, biased approach is not how you build, launch, deploy, and operate the JWST, which requires very high reliability.
This approach is not working well for pediatrics either. I look at the history of the recommended workup of the febrile young infant from the 1980s until today. I see constant changes to the guidelines but no real progress toward a validated, evidence-based approach. A similar history is behind treatment of neonatal hyperbilirubinemia. In the 1994 publication, there was a movement toward being less aggressive. The 2004 and 2009 editions increased the frequency of screening and phototherapy. Now, the 2022 guidelines have moved in the direction we were headed in the 1990s. The workup of infants and children with possible urinary tract infections has undergone a similar trajectory. So has the screening for neonatal herpes infections. The practice changes are more like Brownian motion than real progress. This inconsistency has led me to be skeptical of the process the American Academy of Pediatrics uses to create guidelines.
Part of solving complex problems is allowing all stakeholders’ voices to be heard. On Jan. 28, 1986, seconds after liftoff, the space shuttle Challenger exploded. In the aftermath, it was determined that some engineers had expressed concern about the very cold weather that morning. The rubber in the O-ring would not be as flexible as designed. Their objection was not listened to. The O-ring failed, the fuel tank exploded, and the ship and crew were lost. It is a lesson many engineers of my generation took to heart. Do not suppress voices.
For example, 1 year ago (September 2021), the Royal Australian and New Zealand College of Psychiatrists published a position statement, “Recognising and addressing the mental health needs of people experiencing gender dysphoria/gender incongruence.” The statement expressed concern about the marked increase in incidence of rapid-onset gender dysphoria and therefore urged more thorough assessment by psychiatry before embarking on puberty-blocking therapies. The RANZCP position is at variance with recent trends in the United States. The topic was censored at the 2021 AAP national conference. Lately, I have heard the words disinformation and homophobic used to describe my RANZCP colleagues. I have been comparing AAP, Britain’s National Institute for Health and Care Excellence, and Royal Children’s Hospital Melbourne guidelines for 20 years. The variation is enlightening. I do not know the correct answer to treating gender dysphoria, but I know suppressing viewpoints and debate leads to exploding spaceships.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
FDA approves first gene therapy, betibeglogene autotemcel (Zynteglo), for beta-thalassemia
Betibeglogene autotemcel, a one-time gene therapy, represents a potential cure in which functional copies of the mutated gene are inserted into patients’ hematopoietic stem cells via a replication-defective lentivirus.
“Today’s approval is an important advance in the treatment of beta-thalassemia, particularly in individuals who require ongoing red blood cell transfusions,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an FDA press release. “Given the potential health complications associated with this serious disease, this action highlights the FDA’s continued commitment to supporting development of innovative therapies for patients who have limited treatment options.”
The approval was based on phase 3 trials, in which 89% of 41 patients aged 4-34 years who received the therapy maintained normal or near-normal hemoglobin levels and didn’t need transfusions for at least a year. The patients were as young as age 4, maker Bluebird Bio said in a press release.
FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee unanimously recommended approval in June. The gene therapy had been approved in Europe, where it carried a price tag of about $1.8 million, but Bluebird pulled it from the market in 2021 because of problems with reimbursement.
“The decision to discontinue operations in Europe resulted from prolonged negotiations with European payers and challenges to achieving appropriate value recognition and market access,” the company said in a Securities and Exchange Commission filing.
The projected price in the United States is even higher: $2.1 million.
But the Institute for Clinical and Economic Review, an influential Boston-based nonprofit organization that specializes in medical cost-effectiveness analyses, concluded in June that, “given the high annual costs of standard care ... this new treatment meets commonly accepted value thresholds at an anticipated price of $2.1 million,” particularly with Bluebird’s proposal to pay back 80% of the cost if patients need a transfusion within 5 years.
The company is planning an October 2022 launch and estimates the U.S. market for betibeglogene autotemcel to be about 1,500 patients.
Adverse events in studies were “infrequent and consisted primarily of nonserious infusion-related reactions,” such as abdominal pain, hot flush, dyspnea, tachycardia, noncardiac chest pain, and cytopenias, including thrombocytopenia, leukopenia, and neutropenia. One case of thrombocytopenia was considered serious but resolved, according to the company.
Most of the serious adverse events were related to hematopoietic stem cell collection and the busulfan conditioning regimen. Insertional oncogenesis and/or cancer have been reported with Bluebird’s other gene therapy products, but no cases have been associated with betibeglogene autotemcel.
A version of this article first appeared on Medscape.com.
Betibeglogene autotemcel, a one-time gene therapy, represents a potential cure in which functional copies of the mutated gene are inserted into patients’ hematopoietic stem cells via a replication-defective lentivirus.
“Today’s approval is an important advance in the treatment of beta-thalassemia, particularly in individuals who require ongoing red blood cell transfusions,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an FDA press release. “Given the potential health complications associated with this serious disease, this action highlights the FDA’s continued commitment to supporting development of innovative therapies for patients who have limited treatment options.”
The approval was based on phase 3 trials, in which 89% of 41 patients aged 4-34 years who received the therapy maintained normal or near-normal hemoglobin levels and didn’t need transfusions for at least a year. The patients were as young as age 4, maker Bluebird Bio said in a press release.
FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee unanimously recommended approval in June. The gene therapy had been approved in Europe, where it carried a price tag of about $1.8 million, but Bluebird pulled it from the market in 2021 because of problems with reimbursement.
“The decision to discontinue operations in Europe resulted from prolonged negotiations with European payers and challenges to achieving appropriate value recognition and market access,” the company said in a Securities and Exchange Commission filing.
The projected price in the United States is even higher: $2.1 million.
But the Institute for Clinical and Economic Review, an influential Boston-based nonprofit organization that specializes in medical cost-effectiveness analyses, concluded in June that, “given the high annual costs of standard care ... this new treatment meets commonly accepted value thresholds at an anticipated price of $2.1 million,” particularly with Bluebird’s proposal to pay back 80% of the cost if patients need a transfusion within 5 years.
The company is planning an October 2022 launch and estimates the U.S. market for betibeglogene autotemcel to be about 1,500 patients.
Adverse events in studies were “infrequent and consisted primarily of nonserious infusion-related reactions,” such as abdominal pain, hot flush, dyspnea, tachycardia, noncardiac chest pain, and cytopenias, including thrombocytopenia, leukopenia, and neutropenia. One case of thrombocytopenia was considered serious but resolved, according to the company.
Most of the serious adverse events were related to hematopoietic stem cell collection and the busulfan conditioning regimen. Insertional oncogenesis and/or cancer have been reported with Bluebird’s other gene therapy products, but no cases have been associated with betibeglogene autotemcel.
A version of this article first appeared on Medscape.com.
Betibeglogene autotemcel, a one-time gene therapy, represents a potential cure in which functional copies of the mutated gene are inserted into patients’ hematopoietic stem cells via a replication-defective lentivirus.
“Today’s approval is an important advance in the treatment of beta-thalassemia, particularly in individuals who require ongoing red blood cell transfusions,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in an FDA press release. “Given the potential health complications associated with this serious disease, this action highlights the FDA’s continued commitment to supporting development of innovative therapies for patients who have limited treatment options.”
The approval was based on phase 3 trials, in which 89% of 41 patients aged 4-34 years who received the therapy maintained normal or near-normal hemoglobin levels and didn’t need transfusions for at least a year. The patients were as young as age 4, maker Bluebird Bio said in a press release.
FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee unanimously recommended approval in June. The gene therapy had been approved in Europe, where it carried a price tag of about $1.8 million, but Bluebird pulled it from the market in 2021 because of problems with reimbursement.
“The decision to discontinue operations in Europe resulted from prolonged negotiations with European payers and challenges to achieving appropriate value recognition and market access,” the company said in a Securities and Exchange Commission filing.
The projected price in the United States is even higher: $2.1 million.
But the Institute for Clinical and Economic Review, an influential Boston-based nonprofit organization that specializes in medical cost-effectiveness analyses, concluded in June that, “given the high annual costs of standard care ... this new treatment meets commonly accepted value thresholds at an anticipated price of $2.1 million,” particularly with Bluebird’s proposal to pay back 80% of the cost if patients need a transfusion within 5 years.
The company is planning an October 2022 launch and estimates the U.S. market for betibeglogene autotemcel to be about 1,500 patients.
Adverse events in studies were “infrequent and consisted primarily of nonserious infusion-related reactions,” such as abdominal pain, hot flush, dyspnea, tachycardia, noncardiac chest pain, and cytopenias, including thrombocytopenia, leukopenia, and neutropenia. One case of thrombocytopenia was considered serious but resolved, according to the company.
Most of the serious adverse events were related to hematopoietic stem cell collection and the busulfan conditioning regimen. Insertional oncogenesis and/or cancer have been reported with Bluebird’s other gene therapy products, but no cases have been associated with betibeglogene autotemcel.
A version of this article first appeared on Medscape.com.
FDA approves adalimumab-bwwd biosimilar (Hadlima) in high-concentration form
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.