User login
European survey finds wide variations in the use of phototherapy for atopic eczema
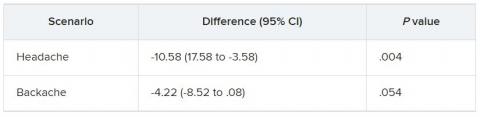
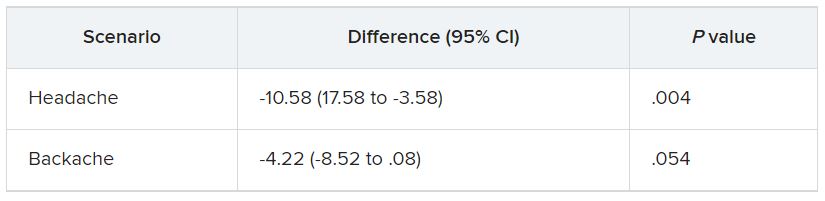
GLASGOW, Scotland – , which points to the need for management guidelines.
Over 140 phototherapy practitioners from 27 European countries responded to the survey. Of the practitioners surveyed, 96% used narrow-band ultraviolet B (NB-UVB), and about 50% prescribed psoralen and ultraviolet A (PUVA) for adults. Fewer than 10% did so for children.
There was considerable variation in prescribing practices, “especially when it comes to dosing and treatment duration,” said study presenter Mia Steyn, MD, dermatology registrar, St. John’s Institute of Dermatology, Guy’s and St. Thomas’s Hospital, London.
These results, she said, demonstrate that “an optimal treatment modality either is not known or agreed upon” and that studies are required to determine treatment efficacy, cost, and safety “in a range of skin types.”
Dr. Steyn said that what is needed first is a set of consensus treatment guidelines, “hopefully leading to a randomized controlled trial” that would compare the various treatment options.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 7.
Session co-chair Adam Fityan, MD, a consultant dermatologist at University Hospital Southampton NHS Foundation Trust, U.K., commented that the study was “fascinating” and “really helpful.”
Dr. Fityan, who was not involved with the survey, told this news organization that, “clearly, what we’ve seen is that there is a huge variation in the way everyone uses the different modalities of phototherapy.”
“Having that sort of knowledge will hopefully help us to think a bit more clearly about the regimens and protocols that we use and to maybe find the evidence that everyone needs to have the most effective protocol.”
The data from the study are also useful on an individual level, Dr. Fityan continued, as “you have no idea what anyone is doing” and whether “you are an outlier.”
Dr. Steyn said that phototherapy is commonly used for the treatment of atopic eczema, but the evidence for its efficacy, its impact on quality on life, its cost-effectiveness, and short- and long-term safety is “weak,” particularly in relation to real-life use.
Electronic survey
In lieu of a well-designed randomized controlled trial to answer these questions, the researchers set up a task force to assess how phototherapy is currently being used to treat atopic eczema across the United Kingdom and Europe so as to guide further research.
An electronic survey was devised, and 144 members of phototherapy groups from 27 European countries submitted their responses during 2020. Most responses came from the Netherlands (20), Italy (16), the United Kingdom (14), France (11), and Germany (10).
The results showed that NB-UVB was the most widely used modality of phototherapy, chosen by 96% of respondents. In addition, 17% of respondents said they also prescribed home-based NB-UVB, which was available in eight of the 27 countries.
When asked how they used NB-UVB, the majority (68%) of respondents said they had an age cutoff for use in children, which was set at an average age of 9 years and older, although the range was age 2 years to 16 years.
NBUVB was used as a second-line therapy instead of systemic treatments in up to 93% of adults and in 69% of children. It was used concomitantly with systemic treatment in up to 58% of adults and 11% of children, according to the survey responses.
For about 70% of respondents, the use of NB-UVB was determined by assessing the Fitzpatrick skin type, although almost 40% relied on clinical experience.
Frequency of treatment
NB-UVB was prescribed three times a week by 59% of respondents; 31% of respondents prescribed it twice a week; 7%, five times per week; and 2%, four times a week. The typical number of treatments was 21-30 for 53% of respondents, 0-20 treatments for 24%, and 31-40 treatments for 20%.
The dose was typically increased in 10% increments, although there were wide variations in how the treatment was stepped up. Dose was increased after each treatment by almost 50% of respondents, after every two treatments by almost 25%, and after every three treatments by approximately 15%.
For the majority (53%) of respondents, response to NBUVB was assessed after 7-15 treatments, while 43% waited until after 16-30 treatments. Success was defined as a 75% reduction in eczema from baseline by 56% of respondents, while 54% looked to patient satisfaction, and 47% relied on quality of life to determine success of treatment.
Maintenance NB-UVB was never used by 54% of respondents, but 44% said they used it occasionally, and 83% said they did not follow a weaning schedule at the end of treatment.
The most commonly reported adverse effects of NB-UVB were significant erythema, hyperpigmentation, and eczema flare, while the most commonly cited absolute contraindications included a history of melanoma, a history of squamous cell carcinoma, the use of photosensitizing medications, and claustrophobia.
Use of PUVA, UVA1
The next most commonly used phototherapy for atopic eczema was PUVA. Although it was available to 83% of respondents, only 52% of respondents had personally prescribed the treatment for adults, and only 7% prescribed it for children.
Of the respondents, 71% said they would switch from NB-UVB to PUVA if desired treatment outcomes were not achieved with the former, and 44% said they would “sometimes consider” PUVA as second-line therapy instead of systemic treatments. Only 13% said they would use it concomitantly with systemic treatment.
Ultraviolet A1 (UVA1) phototherapy was not widely available, with 66% of respondents declaring that they did not have access to this option and just 29% saying they prescribed it.
But when it was used, UVA1 was cited as being used often in adults by 24% of respondents, while 33% used it was used sometimes, and 43% said it was used rarely. It was used for children by 26% of respondents. In addition, 29% said they favored using UVA1 for chronic atopic eczema, and 33% favored using it for acute eczema while 38% had no preference over whether to use it for chronic versus acute atopic eczema.
Similarly to NB-UVB, there were wide variations in the use of PUVA and UVA1 by respondents in terms of dosing schedules, duration of treatment, and how response to treatment was measured.
No funding for the study has been reported. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – , which points to the need for management guidelines.
Over 140 phototherapy practitioners from 27 European countries responded to the survey. Of the practitioners surveyed, 96% used narrow-band ultraviolet B (NB-UVB), and about 50% prescribed psoralen and ultraviolet A (PUVA) for adults. Fewer than 10% did so for children.
There was considerable variation in prescribing practices, “especially when it comes to dosing and treatment duration,” said study presenter Mia Steyn, MD, dermatology registrar, St. John’s Institute of Dermatology, Guy’s and St. Thomas’s Hospital, London.
These results, she said, demonstrate that “an optimal treatment modality either is not known or agreed upon” and that studies are required to determine treatment efficacy, cost, and safety “in a range of skin types.”
Dr. Steyn said that what is needed first is a set of consensus treatment guidelines, “hopefully leading to a randomized controlled trial” that would compare the various treatment options.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 7.
Session co-chair Adam Fityan, MD, a consultant dermatologist at University Hospital Southampton NHS Foundation Trust, U.K., commented that the study was “fascinating” and “really helpful.”
Dr. Fityan, who was not involved with the survey, told this news organization that, “clearly, what we’ve seen is that there is a huge variation in the way everyone uses the different modalities of phototherapy.”
“Having that sort of knowledge will hopefully help us to think a bit more clearly about the regimens and protocols that we use and to maybe find the evidence that everyone needs to have the most effective protocol.”
The data from the study are also useful on an individual level, Dr. Fityan continued, as “you have no idea what anyone is doing” and whether “you are an outlier.”
Dr. Steyn said that phototherapy is commonly used for the treatment of atopic eczema, but the evidence for its efficacy, its impact on quality on life, its cost-effectiveness, and short- and long-term safety is “weak,” particularly in relation to real-life use.
Electronic survey
In lieu of a well-designed randomized controlled trial to answer these questions, the researchers set up a task force to assess how phototherapy is currently being used to treat atopic eczema across the United Kingdom and Europe so as to guide further research.
An electronic survey was devised, and 144 members of phototherapy groups from 27 European countries submitted their responses during 2020. Most responses came from the Netherlands (20), Italy (16), the United Kingdom (14), France (11), and Germany (10).
The results showed that NB-UVB was the most widely used modality of phototherapy, chosen by 96% of respondents. In addition, 17% of respondents said they also prescribed home-based NB-UVB, which was available in eight of the 27 countries.
When asked how they used NB-UVB, the majority (68%) of respondents said they had an age cutoff for use in children, which was set at an average age of 9 years and older, although the range was age 2 years to 16 years.
NBUVB was used as a second-line therapy instead of systemic treatments in up to 93% of adults and in 69% of children. It was used concomitantly with systemic treatment in up to 58% of adults and 11% of children, according to the survey responses.
For about 70% of respondents, the use of NB-UVB was determined by assessing the Fitzpatrick skin type, although almost 40% relied on clinical experience.
Frequency of treatment
NB-UVB was prescribed three times a week by 59% of respondents; 31% of respondents prescribed it twice a week; 7%, five times per week; and 2%, four times a week. The typical number of treatments was 21-30 for 53% of respondents, 0-20 treatments for 24%, and 31-40 treatments for 20%.
The dose was typically increased in 10% increments, although there were wide variations in how the treatment was stepped up. Dose was increased after each treatment by almost 50% of respondents, after every two treatments by almost 25%, and after every three treatments by approximately 15%.
For the majority (53%) of respondents, response to NBUVB was assessed after 7-15 treatments, while 43% waited until after 16-30 treatments. Success was defined as a 75% reduction in eczema from baseline by 56% of respondents, while 54% looked to patient satisfaction, and 47% relied on quality of life to determine success of treatment.
Maintenance NB-UVB was never used by 54% of respondents, but 44% said they used it occasionally, and 83% said they did not follow a weaning schedule at the end of treatment.
The most commonly reported adverse effects of NB-UVB were significant erythema, hyperpigmentation, and eczema flare, while the most commonly cited absolute contraindications included a history of melanoma, a history of squamous cell carcinoma, the use of photosensitizing medications, and claustrophobia.
Use of PUVA, UVA1
The next most commonly used phototherapy for atopic eczema was PUVA. Although it was available to 83% of respondents, only 52% of respondents had personally prescribed the treatment for adults, and only 7% prescribed it for children.
Of the respondents, 71% said they would switch from NB-UVB to PUVA if desired treatment outcomes were not achieved with the former, and 44% said they would “sometimes consider” PUVA as second-line therapy instead of systemic treatments. Only 13% said they would use it concomitantly with systemic treatment.
Ultraviolet A1 (UVA1) phototherapy was not widely available, with 66% of respondents declaring that they did not have access to this option and just 29% saying they prescribed it.
But when it was used, UVA1 was cited as being used often in adults by 24% of respondents, while 33% used it was used sometimes, and 43% said it was used rarely. It was used for children by 26% of respondents. In addition, 29% said they favored using UVA1 for chronic atopic eczema, and 33% favored using it for acute eczema while 38% had no preference over whether to use it for chronic versus acute atopic eczema.
Similarly to NB-UVB, there were wide variations in the use of PUVA and UVA1 by respondents in terms of dosing schedules, duration of treatment, and how response to treatment was measured.
No funding for the study has been reported. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – , which points to the need for management guidelines.
Over 140 phototherapy practitioners from 27 European countries responded to the survey. Of the practitioners surveyed, 96% used narrow-band ultraviolet B (NB-UVB), and about 50% prescribed psoralen and ultraviolet A (PUVA) for adults. Fewer than 10% did so for children.
There was considerable variation in prescribing practices, “especially when it comes to dosing and treatment duration,” said study presenter Mia Steyn, MD, dermatology registrar, St. John’s Institute of Dermatology, Guy’s and St. Thomas’s Hospital, London.
These results, she said, demonstrate that “an optimal treatment modality either is not known or agreed upon” and that studies are required to determine treatment efficacy, cost, and safety “in a range of skin types.”
Dr. Steyn said that what is needed first is a set of consensus treatment guidelines, “hopefully leading to a randomized controlled trial” that would compare the various treatment options.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 7.
Session co-chair Adam Fityan, MD, a consultant dermatologist at University Hospital Southampton NHS Foundation Trust, U.K., commented that the study was “fascinating” and “really helpful.”
Dr. Fityan, who was not involved with the survey, told this news organization that, “clearly, what we’ve seen is that there is a huge variation in the way everyone uses the different modalities of phototherapy.”
“Having that sort of knowledge will hopefully help us to think a bit more clearly about the regimens and protocols that we use and to maybe find the evidence that everyone needs to have the most effective protocol.”
The data from the study are also useful on an individual level, Dr. Fityan continued, as “you have no idea what anyone is doing” and whether “you are an outlier.”
Dr. Steyn said that phototherapy is commonly used for the treatment of atopic eczema, but the evidence for its efficacy, its impact on quality on life, its cost-effectiveness, and short- and long-term safety is “weak,” particularly in relation to real-life use.
Electronic survey
In lieu of a well-designed randomized controlled trial to answer these questions, the researchers set up a task force to assess how phototherapy is currently being used to treat atopic eczema across the United Kingdom and Europe so as to guide further research.
An electronic survey was devised, and 144 members of phototherapy groups from 27 European countries submitted their responses during 2020. Most responses came from the Netherlands (20), Italy (16), the United Kingdom (14), France (11), and Germany (10).
The results showed that NB-UVB was the most widely used modality of phototherapy, chosen by 96% of respondents. In addition, 17% of respondents said they also prescribed home-based NB-UVB, which was available in eight of the 27 countries.
When asked how they used NB-UVB, the majority (68%) of respondents said they had an age cutoff for use in children, which was set at an average age of 9 years and older, although the range was age 2 years to 16 years.
NBUVB was used as a second-line therapy instead of systemic treatments in up to 93% of adults and in 69% of children. It was used concomitantly with systemic treatment in up to 58% of adults and 11% of children, according to the survey responses.
For about 70% of respondents, the use of NB-UVB was determined by assessing the Fitzpatrick skin type, although almost 40% relied on clinical experience.
Frequency of treatment
NB-UVB was prescribed three times a week by 59% of respondents; 31% of respondents prescribed it twice a week; 7%, five times per week; and 2%, four times a week. The typical number of treatments was 21-30 for 53% of respondents, 0-20 treatments for 24%, and 31-40 treatments for 20%.
The dose was typically increased in 10% increments, although there were wide variations in how the treatment was stepped up. Dose was increased after each treatment by almost 50% of respondents, after every two treatments by almost 25%, and after every three treatments by approximately 15%.
For the majority (53%) of respondents, response to NBUVB was assessed after 7-15 treatments, while 43% waited until after 16-30 treatments. Success was defined as a 75% reduction in eczema from baseline by 56% of respondents, while 54% looked to patient satisfaction, and 47% relied on quality of life to determine success of treatment.
Maintenance NB-UVB was never used by 54% of respondents, but 44% said they used it occasionally, and 83% said they did not follow a weaning schedule at the end of treatment.
The most commonly reported adverse effects of NB-UVB were significant erythema, hyperpigmentation, and eczema flare, while the most commonly cited absolute contraindications included a history of melanoma, a history of squamous cell carcinoma, the use of photosensitizing medications, and claustrophobia.
Use of PUVA, UVA1
The next most commonly used phototherapy for atopic eczema was PUVA. Although it was available to 83% of respondents, only 52% of respondents had personally prescribed the treatment for adults, and only 7% prescribed it for children.
Of the respondents, 71% said they would switch from NB-UVB to PUVA if desired treatment outcomes were not achieved with the former, and 44% said they would “sometimes consider” PUVA as second-line therapy instead of systemic treatments. Only 13% said they would use it concomitantly with systemic treatment.
Ultraviolet A1 (UVA1) phototherapy was not widely available, with 66% of respondents declaring that they did not have access to this option and just 29% saying they prescribed it.
But when it was used, UVA1 was cited as being used often in adults by 24% of respondents, while 33% used it was used sometimes, and 43% said it was used rarely. It was used for children by 26% of respondents. In addition, 29% said they favored using UVA1 for chronic atopic eczema, and 33% favored using it for acute eczema while 38% had no preference over whether to use it for chronic versus acute atopic eczema.
Similarly to NB-UVB, there were wide variations in the use of PUVA and UVA1 by respondents in terms of dosing schedules, duration of treatment, and how response to treatment was measured.
No funding for the study has been reported. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Depression screens do not reduce suicidal acts in teens: Study
Screening adolescents for signs of depression does not reduce their emergency department visits, hospitalizations, or treatment for suicidal behaviors, according to research published in Preventive Medicine. Adolescents who underwent a depression screening were just as likely to need these services as those who did not.
In 2016, the U.S. Preventive Services Task Force recommended that adolescents aged 12-18 years be screened for major depressive disorder, provided that effective treatment options and follow-up strategies are in place.
“The main goal of depression screening is really to reduce adverse psychiatric outcomes. But I think a collateral hope is that, in reducing these adverse psychiatric outcomes, you would also reduce avoidable health services use,” such as ED visits or hospitalizations, said Kira Riehm, PhD, a postdoctoral fellow in epidemiology at Columbia University, New York, who led the research. Dr. Riehm designed the new study, which was part of her doctoral work at Johns Hopkins University, Baltimore, to test this premise.
Dr. Riehm and colleagues compared 14,433 adolescents aged 12-18 years who were screened for depression at least once during a wellness visit from 2014 to 2017 to 43,299 adolescents who were not screened for depression during such visits. Depression screenings were interspersed among a total of 281,463 adolescent wellness visits from 2014 to 2017, which represented 5% of all visits.
The researchers used diagnostic codes from a database of insurance claims to determine who had undergone depression screening. They then compared use of ED services, inpatient hospitalizations, and the number of treatments for suicidal behaviors between the two groups for the 2 years following the wellness visit.
The average age of the adolescents who underwent screening was 13-14 years, as was the average age of adolescents who were not screened. Both groups were evenly matched with respect to being male or female.
The researchers estimated that a high majority of adolescents in the sample were White (83%). Black persons represented 7% of the sample; Hispanic/Latino, 5%; and Asian, 3%. Insurance claims don’t always include racial and ethnicity data, Dr. Riehm said, so her group statistically imputed these proportions. The claims data also do not include details about which type of screening tool was used or the results of the screening, such as whether a teen exhibited mild or severe depression.
Adolescents in both groups were just as likely to go to the ED for any reason, be admitted to the hospital for any reason, or undergo treatment for suicidal behaviors. The researchers observed a slight association between being screened for depression and going to the ED specifically for a mental health reason (relative risk, 1.16; 95% confidence interval, 1.00-1.33). The sex of the adolescents had no bearing on whether they used these services.
“I think people think of [depression screening] as one event. But in reality, screening is a series of different events that all have to be happening in order for a screening program to work,” Dr. Riehm told this news organization.
These events could include ensuring that adolescents who exhibit signs of depression receive a proper assessment, receive medications if needed, and have access to psychotherapists who can help them. Without these supports in place, she said, a one-off depression screening may have limited benefit.
“There’s a lot of places where people could drop out of that care continuum,” Dr. Riehm said.
“One-time screening may not be enough,” said Trân Đoàn, PhD, MPH, a postdoctoral researcher in the University of Pittsburgh department of pediatrics.
Dr. Đoàn, who was not involved in the research, noted that the American Academy of Pediatrics recommends annual screening of all adolescents for depressive symptoms. Given that only 5% of the visits in this sample included any kind of depression screening, Dr. Đoàn said, some pediatric practices may not have felt they had the resources to adequately address positive screenings for depression.
Both Dr. Riehm and Dr. Đoàn are focusing on the link between depression screening and health outcomes. In her own doctoral work at the University of Michigan, Dr. Đoàn modeled the effects of universal annual depression screening in primary care settings on the health status of people aged 12-22 years. She is currently preparing this work for publication.
“I did find that, over the long term, there is improvement in health outcomes if we were to screen on an annual basis,” provided improved screening is coupled with comprehensive treatment plans, Dr. Đoàn said. The model’s health outcomes measures included an increase in life expectancy as well as a greater proportion of depression-free days among adolescents who receive appropriate treatment.
Dr. Riehm and Dr. Đoàn disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Screening adolescents for signs of depression does not reduce their emergency department visits, hospitalizations, or treatment for suicidal behaviors, according to research published in Preventive Medicine. Adolescents who underwent a depression screening were just as likely to need these services as those who did not.
In 2016, the U.S. Preventive Services Task Force recommended that adolescents aged 12-18 years be screened for major depressive disorder, provided that effective treatment options and follow-up strategies are in place.
“The main goal of depression screening is really to reduce adverse psychiatric outcomes. But I think a collateral hope is that, in reducing these adverse psychiatric outcomes, you would also reduce avoidable health services use,” such as ED visits or hospitalizations, said Kira Riehm, PhD, a postdoctoral fellow in epidemiology at Columbia University, New York, who led the research. Dr. Riehm designed the new study, which was part of her doctoral work at Johns Hopkins University, Baltimore, to test this premise.
Dr. Riehm and colleagues compared 14,433 adolescents aged 12-18 years who were screened for depression at least once during a wellness visit from 2014 to 2017 to 43,299 adolescents who were not screened for depression during such visits. Depression screenings were interspersed among a total of 281,463 adolescent wellness visits from 2014 to 2017, which represented 5% of all visits.
The researchers used diagnostic codes from a database of insurance claims to determine who had undergone depression screening. They then compared use of ED services, inpatient hospitalizations, and the number of treatments for suicidal behaviors between the two groups for the 2 years following the wellness visit.
The average age of the adolescents who underwent screening was 13-14 years, as was the average age of adolescents who were not screened. Both groups were evenly matched with respect to being male or female.
The researchers estimated that a high majority of adolescents in the sample were White (83%). Black persons represented 7% of the sample; Hispanic/Latino, 5%; and Asian, 3%. Insurance claims don’t always include racial and ethnicity data, Dr. Riehm said, so her group statistically imputed these proportions. The claims data also do not include details about which type of screening tool was used or the results of the screening, such as whether a teen exhibited mild or severe depression.
Adolescents in both groups were just as likely to go to the ED for any reason, be admitted to the hospital for any reason, or undergo treatment for suicidal behaviors. The researchers observed a slight association between being screened for depression and going to the ED specifically for a mental health reason (relative risk, 1.16; 95% confidence interval, 1.00-1.33). The sex of the adolescents had no bearing on whether they used these services.
“I think people think of [depression screening] as one event. But in reality, screening is a series of different events that all have to be happening in order for a screening program to work,” Dr. Riehm told this news organization.
These events could include ensuring that adolescents who exhibit signs of depression receive a proper assessment, receive medications if needed, and have access to psychotherapists who can help them. Without these supports in place, she said, a one-off depression screening may have limited benefit.
“There’s a lot of places where people could drop out of that care continuum,” Dr. Riehm said.
“One-time screening may not be enough,” said Trân Đoàn, PhD, MPH, a postdoctoral researcher in the University of Pittsburgh department of pediatrics.
Dr. Đoàn, who was not involved in the research, noted that the American Academy of Pediatrics recommends annual screening of all adolescents for depressive symptoms. Given that only 5% of the visits in this sample included any kind of depression screening, Dr. Đoàn said, some pediatric practices may not have felt they had the resources to adequately address positive screenings for depression.
Both Dr. Riehm and Dr. Đoàn are focusing on the link between depression screening and health outcomes. In her own doctoral work at the University of Michigan, Dr. Đoàn modeled the effects of universal annual depression screening in primary care settings on the health status of people aged 12-22 years. She is currently preparing this work for publication.
“I did find that, over the long term, there is improvement in health outcomes if we were to screen on an annual basis,” provided improved screening is coupled with comprehensive treatment plans, Dr. Đoàn said. The model’s health outcomes measures included an increase in life expectancy as well as a greater proportion of depression-free days among adolescents who receive appropriate treatment.
Dr. Riehm and Dr. Đoàn disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Screening adolescents for signs of depression does not reduce their emergency department visits, hospitalizations, or treatment for suicidal behaviors, according to research published in Preventive Medicine. Adolescents who underwent a depression screening were just as likely to need these services as those who did not.
In 2016, the U.S. Preventive Services Task Force recommended that adolescents aged 12-18 years be screened for major depressive disorder, provided that effective treatment options and follow-up strategies are in place.
“The main goal of depression screening is really to reduce adverse psychiatric outcomes. But I think a collateral hope is that, in reducing these adverse psychiatric outcomes, you would also reduce avoidable health services use,” such as ED visits or hospitalizations, said Kira Riehm, PhD, a postdoctoral fellow in epidemiology at Columbia University, New York, who led the research. Dr. Riehm designed the new study, which was part of her doctoral work at Johns Hopkins University, Baltimore, to test this premise.
Dr. Riehm and colleagues compared 14,433 adolescents aged 12-18 years who were screened for depression at least once during a wellness visit from 2014 to 2017 to 43,299 adolescents who were not screened for depression during such visits. Depression screenings were interspersed among a total of 281,463 adolescent wellness visits from 2014 to 2017, which represented 5% of all visits.
The researchers used diagnostic codes from a database of insurance claims to determine who had undergone depression screening. They then compared use of ED services, inpatient hospitalizations, and the number of treatments for suicidal behaviors between the two groups for the 2 years following the wellness visit.
The average age of the adolescents who underwent screening was 13-14 years, as was the average age of adolescents who were not screened. Both groups were evenly matched with respect to being male or female.
The researchers estimated that a high majority of adolescents in the sample were White (83%). Black persons represented 7% of the sample; Hispanic/Latino, 5%; and Asian, 3%. Insurance claims don’t always include racial and ethnicity data, Dr. Riehm said, so her group statistically imputed these proportions. The claims data also do not include details about which type of screening tool was used or the results of the screening, such as whether a teen exhibited mild or severe depression.
Adolescents in both groups were just as likely to go to the ED for any reason, be admitted to the hospital for any reason, or undergo treatment for suicidal behaviors. The researchers observed a slight association between being screened for depression and going to the ED specifically for a mental health reason (relative risk, 1.16; 95% confidence interval, 1.00-1.33). The sex of the adolescents had no bearing on whether they used these services.
“I think people think of [depression screening] as one event. But in reality, screening is a series of different events that all have to be happening in order for a screening program to work,” Dr. Riehm told this news organization.
These events could include ensuring that adolescents who exhibit signs of depression receive a proper assessment, receive medications if needed, and have access to psychotherapists who can help them. Without these supports in place, she said, a one-off depression screening may have limited benefit.
“There’s a lot of places where people could drop out of that care continuum,” Dr. Riehm said.
“One-time screening may not be enough,” said Trân Đoàn, PhD, MPH, a postdoctoral researcher in the University of Pittsburgh department of pediatrics.
Dr. Đoàn, who was not involved in the research, noted that the American Academy of Pediatrics recommends annual screening of all adolescents for depressive symptoms. Given that only 5% of the visits in this sample included any kind of depression screening, Dr. Đoàn said, some pediatric practices may not have felt they had the resources to adequately address positive screenings for depression.
Both Dr. Riehm and Dr. Đoàn are focusing on the link between depression screening and health outcomes. In her own doctoral work at the University of Michigan, Dr. Đoàn modeled the effects of universal annual depression screening in primary care settings on the health status of people aged 12-22 years. She is currently preparing this work for publication.
“I did find that, over the long term, there is improvement in health outcomes if we were to screen on an annual basis,” provided improved screening is coupled with comprehensive treatment plans, Dr. Đoàn said. The model’s health outcomes measures included an increase in life expectancy as well as a greater proportion of depression-free days among adolescents who receive appropriate treatment.
Dr. Riehm and Dr. Đoàn disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PREVENTIVE MEDICINE
PCOS ups risk of heart complications during delivery period
Pregnant women with polycystic ovary syndrome (PCOS) appear to be at significantly increased risk of experiencing cardiac complications while hospitalized during and after delivery.
An estimated 5 million women of childbearing age in the United States have PCOS, a hormone disorder linked to infertility. PCOS is also known to contribute to the development of cardiometabolic abnormalities like high cholesterol and high blood pressure, which are associated with acute cardiovascular complications during delivery.
But a study, published online in the Journal of the American Heart Association, found that even after accounting for pre-eclampsia, age, comorbidities, and race, PCOS was linked to a 76% increased risk for heart failure, a 79% higher risk of a weakened heart, and an 82% increased risk of having blood clots in the hours and days around giving birth in hospital settings, compared with women without PCOS.
“Perhaps women need a closer follow-up during their pregnancy,” said Erin Michos, MD, MHS, associate director of preventive cardiology at Johns Hopkins Medicine, Baltimore, and a co-author of the study. “They’re counseled about the difficulties of getting pregnant, but what about when they get pregnant?”
Hospitalizations of women with PCOS were also associated with longer stays (3 vs. 2 days) and higher costs ($4,901 vs. $3616; P < .01), compared with women without PCOS.
Over the 17-year analysis period, the number of women with PCOS rose from 569 per 100,000 deliveries to 15,349 per 100,000 deliveries. The researchers attributed the increase in part to greater awareness and diagnosis of the disorder. Dr. Michos and her colleagues used the National Inpatient Sample, managed by the Agency for Healthcare Research and Quality, to pull claims data for women who gave birth in hospitals between 2002 and 2019.
Solutions?
Dr. Michos said there may be more prevention work from og.gyns. to both educate patients about their heart risks during the delivery process and also to refer them to relevant cardiac specialists.
“These women may seek out a gynecologist because of the symptoms, perhaps irregular menses, but along with that should come counseling of the long-term cardiovascular complication,” Dr. Michos said. “And after a pregnancy there should be a good handoff to a primary care provider, so they get a cardiovascular assessment.”
Lifestyle management before, during, and after pregnancy can help prevent the onset of the long-term consequences of cardiac complications during delivery, according to Valerie Baker, MD, director of the division of reproductive endocrinology and infertility at Hopkins Medicine, and her colleagues in a viewpoint published in the journal Fertility and Sterility.
“Once women with PCOS are identified by screening to be at higher risk for [cardiovascular disease], the foundational approach should be lifestyle management followed by statin therapy,” Dr. Baker’s group wrote. “These interventions should include dietary management and physical activity, especially for those who are prediabetic.”
The current study came on the heels of a June 14 meta-analysis by Dr. Michos’ group that found that women with PCOS may be twice as likely as those without PCOS to have coronary artery calcification, a precursor to atherosclerosis and a sign of the early onset of cardiovascular disease.
“We shouldn’t assume that all women of reproductive age are low risk,” Dr. Michos said. “This is the window of time that we can reshape the trajectory early in life.”
The study was supported by the Amato Fund for Women’s Cardiovascular Health research at Johns Hopkins University and through grant support from the American Heart Association (940166). Dr. Michos reported advisory board participation for AstraZeneca, Amarin, Novartis, Novo Nordisk, Bayer, Boehringer Ingelheim, Esperion, and Pfizer. Study coauthor Michael Honigberg, MD, reported consulting fees from CRISPR Therapeutics, unrelated to the present work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women with polycystic ovary syndrome (PCOS) appear to be at significantly increased risk of experiencing cardiac complications while hospitalized during and after delivery.
An estimated 5 million women of childbearing age in the United States have PCOS, a hormone disorder linked to infertility. PCOS is also known to contribute to the development of cardiometabolic abnormalities like high cholesterol and high blood pressure, which are associated with acute cardiovascular complications during delivery.
But a study, published online in the Journal of the American Heart Association, found that even after accounting for pre-eclampsia, age, comorbidities, and race, PCOS was linked to a 76% increased risk for heart failure, a 79% higher risk of a weakened heart, and an 82% increased risk of having blood clots in the hours and days around giving birth in hospital settings, compared with women without PCOS.
“Perhaps women need a closer follow-up during their pregnancy,” said Erin Michos, MD, MHS, associate director of preventive cardiology at Johns Hopkins Medicine, Baltimore, and a co-author of the study. “They’re counseled about the difficulties of getting pregnant, but what about when they get pregnant?”
Hospitalizations of women with PCOS were also associated with longer stays (3 vs. 2 days) and higher costs ($4,901 vs. $3616; P < .01), compared with women without PCOS.
Over the 17-year analysis period, the number of women with PCOS rose from 569 per 100,000 deliveries to 15,349 per 100,000 deliveries. The researchers attributed the increase in part to greater awareness and diagnosis of the disorder. Dr. Michos and her colleagues used the National Inpatient Sample, managed by the Agency for Healthcare Research and Quality, to pull claims data for women who gave birth in hospitals between 2002 and 2019.
Solutions?
Dr. Michos said there may be more prevention work from og.gyns. to both educate patients about their heart risks during the delivery process and also to refer them to relevant cardiac specialists.
“These women may seek out a gynecologist because of the symptoms, perhaps irregular menses, but along with that should come counseling of the long-term cardiovascular complication,” Dr. Michos said. “And after a pregnancy there should be a good handoff to a primary care provider, so they get a cardiovascular assessment.”
Lifestyle management before, during, and after pregnancy can help prevent the onset of the long-term consequences of cardiac complications during delivery, according to Valerie Baker, MD, director of the division of reproductive endocrinology and infertility at Hopkins Medicine, and her colleagues in a viewpoint published in the journal Fertility and Sterility.
“Once women with PCOS are identified by screening to be at higher risk for [cardiovascular disease], the foundational approach should be lifestyle management followed by statin therapy,” Dr. Baker’s group wrote. “These interventions should include dietary management and physical activity, especially for those who are prediabetic.”
The current study came on the heels of a June 14 meta-analysis by Dr. Michos’ group that found that women with PCOS may be twice as likely as those without PCOS to have coronary artery calcification, a precursor to atherosclerosis and a sign of the early onset of cardiovascular disease.
“We shouldn’t assume that all women of reproductive age are low risk,” Dr. Michos said. “This is the window of time that we can reshape the trajectory early in life.”
The study was supported by the Amato Fund for Women’s Cardiovascular Health research at Johns Hopkins University and through grant support from the American Heart Association (940166). Dr. Michos reported advisory board participation for AstraZeneca, Amarin, Novartis, Novo Nordisk, Bayer, Boehringer Ingelheim, Esperion, and Pfizer. Study coauthor Michael Honigberg, MD, reported consulting fees from CRISPR Therapeutics, unrelated to the present work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women with polycystic ovary syndrome (PCOS) appear to be at significantly increased risk of experiencing cardiac complications while hospitalized during and after delivery.
An estimated 5 million women of childbearing age in the United States have PCOS, a hormone disorder linked to infertility. PCOS is also known to contribute to the development of cardiometabolic abnormalities like high cholesterol and high blood pressure, which are associated with acute cardiovascular complications during delivery.
But a study, published online in the Journal of the American Heart Association, found that even after accounting for pre-eclampsia, age, comorbidities, and race, PCOS was linked to a 76% increased risk for heart failure, a 79% higher risk of a weakened heart, and an 82% increased risk of having blood clots in the hours and days around giving birth in hospital settings, compared with women without PCOS.
“Perhaps women need a closer follow-up during their pregnancy,” said Erin Michos, MD, MHS, associate director of preventive cardiology at Johns Hopkins Medicine, Baltimore, and a co-author of the study. “They’re counseled about the difficulties of getting pregnant, but what about when they get pregnant?”
Hospitalizations of women with PCOS were also associated with longer stays (3 vs. 2 days) and higher costs ($4,901 vs. $3616; P < .01), compared with women without PCOS.
Over the 17-year analysis period, the number of women with PCOS rose from 569 per 100,000 deliveries to 15,349 per 100,000 deliveries. The researchers attributed the increase in part to greater awareness and diagnosis of the disorder. Dr. Michos and her colleagues used the National Inpatient Sample, managed by the Agency for Healthcare Research and Quality, to pull claims data for women who gave birth in hospitals between 2002 and 2019.
Solutions?
Dr. Michos said there may be more prevention work from og.gyns. to both educate patients about their heart risks during the delivery process and also to refer them to relevant cardiac specialists.
“These women may seek out a gynecologist because of the symptoms, perhaps irregular menses, but along with that should come counseling of the long-term cardiovascular complication,” Dr. Michos said. “And after a pregnancy there should be a good handoff to a primary care provider, so they get a cardiovascular assessment.”
Lifestyle management before, during, and after pregnancy can help prevent the onset of the long-term consequences of cardiac complications during delivery, according to Valerie Baker, MD, director of the division of reproductive endocrinology and infertility at Hopkins Medicine, and her colleagues in a viewpoint published in the journal Fertility and Sterility.
“Once women with PCOS are identified by screening to be at higher risk for [cardiovascular disease], the foundational approach should be lifestyle management followed by statin therapy,” Dr. Baker’s group wrote. “These interventions should include dietary management and physical activity, especially for those who are prediabetic.”
The current study came on the heels of a June 14 meta-analysis by Dr. Michos’ group that found that women with PCOS may be twice as likely as those without PCOS to have coronary artery calcification, a precursor to atherosclerosis and a sign of the early onset of cardiovascular disease.
“We shouldn’t assume that all women of reproductive age are low risk,” Dr. Michos said. “This is the window of time that we can reshape the trajectory early in life.”
The study was supported by the Amato Fund for Women’s Cardiovascular Health research at Johns Hopkins University and through grant support from the American Heart Association (940166). Dr. Michos reported advisory board participation for AstraZeneca, Amarin, Novartis, Novo Nordisk, Bayer, Boehringer Ingelheim, Esperion, and Pfizer. Study coauthor Michael Honigberg, MD, reported consulting fees from CRISPR Therapeutics, unrelated to the present work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
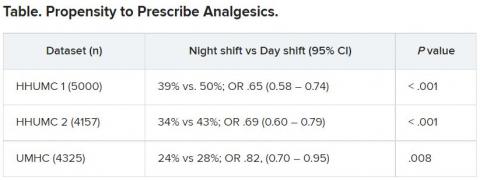
Biologics reduce exacerbations in severe asthma
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
FROM THE ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Adding salt to food linked to higher risk of premature death
in a new study.
In the study of more than 500,000 people, compared with those who never or rarely added salt, those who always added salt to their food had a 28% increased risk of dying prematurely (defined as death before the age of 75 years).
Results also showed that adding salt to food was linked to a lower life expectancy. At the age of 50 years, life expectancy was reduced by 1.5 years in women and by 2.28 years in men who always added salt to their food, compared with those who never or rarely did.
However, these increased risks appeared to be attenuated with increasing intakes of high-potassium foods (vegetables and fruits).
The study was published online in the European Heart Journal.
“As far as we are aware, this is the first study to analyze adding salt to meals as a unique measurement for dietary sodium intake. Such a measure is less likely affected by other dietary components, especially potassium intake,” senior author Lu Qi, MD, Tulane University, New Orleans, told this news organization.
“Our study provides supportive evidence from a novel perspective to show the adverse effects of high sodium intake on human health, which is still a controversial topic. Our findings support the advice that reduction of salt intake by reducing the salt added to meals may benefit health and improve life expectancy. Our results also suggest that high intakes of fruits and vegetables are beneficial regarding lowering the adverse effects of salt,” he added.
Link between dietary salt and health is subject of longstanding debate
The researchers explained that the relationship between dietary salt intake and health remains a subject of longstanding debate, with previous studies on the association between sodium intake and mortality having shown conflicting results.
They attributed the inconsistent results to the low accuracy of sodium measurement, noting that sodium intake varies widely from day to day, but the majority of previous studies have largely relied on a single day’s urine collection or dietary survey for estimating the sodium intake, which is inadequate to assess an individual’s usual consumption levels.
They also pointed out that it is difficult to separate the contributions of intakes of sodium and potassium to health based on current methods for measuring dietary sodium and potassium, and this may confound the association between sodium intake and health outcomes.
They noted that the hypothesis that a high-potassium intake may attenuate the adverse association of high-sodium intake with health outcomes has been proposed for many years, but studies assessing the interaction between sodium intake and potassium intake on the risk of mortality are scarce.
Adding salt to food at the table is a common eating behavior directly related to an individual’s long-term preference for salty tasting foods and habitual salt intake, the authors said, adding that commonly used table salt contains 97%-99% sodium chloride, minimizing the potential confounding effects of other dietary factors including potassium. “Therefore, adding salt to foods provides a unique assessment to evaluate the association between habitual sodium intake and mortality.”
UK Biobank study
For the current study Dr. Qi and colleagues analyzed data from 501,379 people taking part in the UK Biobank study. When joining the study between 2006 and 2010, the participants were asked whether they added salt to their foods never/rarely, sometimes, usually or always. Participants were then followed for a median of 9 years.
After adjustment for sex, age, race, smoking, moderate drinking, body mass index, physical activity, Townsend deprivation index, high cholesterol, chronic kidney disease, diabetes, cardiovascular disease, and cancer, results showed an increasing risk of all-cause premature mortality rose with increasing frequency of adding salt to foods.
The adjusted hazard ratios, compared with those who never or rarely added salt, were 1.02 (95% CI, 0.99-1.06) for those who added salt sometimes, 1.07 (95% CI, 1.02-1.11) for those who usually added salt, and 1.28 (95% CI, 1.20-1.35) for those who always added salt.
The researchers also estimated the lower survival time caused by the high frequency of adding salt to foods. At age 50, women who always added salt to foods had an average 1.50 fewer years of life expectancy, and men who always added salt had an average 2.28 fewer years of life expectancy, as compared with their counterparts who never/rarely added salt to foods.
For cause-specific premature mortality, results showed that higher frequency of adding salt to foods was significantly associated with a higher risk of cardiovascular mortality and cancer mortality, but not for dementia mortality or respiratory mortality. For the subtypes of cardiovascular mortality, adding salt to foods was significantly associated with higher risk of stroke mortality but not coronary heart disease mortality.
Other analyses suggested that the association of adding salt to foods with an increased risk of premature mortality appeared to be attenuated with increasing intake of food high in potassium (fruits and vegetables).
The authors point out that the amounts of discretionary sodium intake (the salt used at the table or in home cooking) have been largely overlooked in previous studies, even though adding salt to foods accounts for a considerable proportion of total sodium intake (6%-20%) in Western diets.
“Our findings also support the notion that even a modest reduction in sodium intake is likely to result in substantial health benefits, especially when it is achieved in the general population,” they conclude.
Conflicting information from different studies
But the current findings seem to directly contradict those from another recent study by Messerli and colleagues showing higher sodium intake correlates with improved life expectancy.
Addressing these contradictory results, Dr. Qi commented: “The study of Messerli et al. is based on an ecological design, in which the analysis is performed on country average sodium intake, rather than at the individual level. This type of ecological study has several major limitations, such as the lack of individuals’ sodium intake, uncontrolled confounding, and the cross-sectional nature. Typically, ecological studies are not considered useful for testing hypothesis in epidemiological studies.”
Dr. Qi noted that, in contrast, his current study analyzes individuals’ exposure, and has a prospective design. “Our findings are supported by previous large-scale observational studies and clinical trials which show the high intake of sodium may adversely affect chronic diseases such as cardiovascular disease and hypertension.” =
Lead author of the ecological study, Franz Messerli, MD, Bern (Switzerland) University Hospital, however, was not convinced by the findings from Dr. Qi’s study.
“The difference in 24-hour sodium intake between those who never/rarely added salt and those who always did is a minuscule 0.17 g. It is highly unlikely that such negligible quantity has any impact on blood pressure, not to mention cardiovascular mortality or life expectancy,” he commented in an interview.
He also pointed out that, in Dr. Qi’s study, people who added salt more frequently also consumed more red meat and processed meat, as well as less fish and less fruit and vegetables. “I would suggest that the bad habit of adding salt at the table is simply a powerful marker for an unhealthy diet.”
“There is no question that an excessive salt intake is harmful in hypertensive patients and increases the risk of stroke. But 0.17 g is not going to make any difference,” Dr. Messerli added.
What is the optimum level?
In an editorial accompanying the study by Dr. Qi and colleagues in the European Heart Journal, Annika Rosengren, MD, PhD, Sahlgrenska University Hospital, Gothenburg, Sweden, noted that guidelines recommend a salt intake below 5 g, or about a teaspoon, per day. But few individuals meet this recommendation.
Because several recent studies show a U- or J-shaped association between salt and atherosclerotic cardiovascular disease, reducing salt intake across the whole population may not be universally beneficial, Dr. Rosengren said.
“So far, what the collective evidence about salt seems to indicate is that healthy people consuming what constitutes normal levels of ordinary salt need not worry too much about their salt intake,” she wrote.
Instead, she advised a diet rich in fruit and vegetables should be a priority to counterbalance potentially harmful effects of salt, and for many other reasons.
And she added that people at high risk, such as those with hypertension who have a high salt intake, are probably well advised to cut down, and not adding extra salt to already prepared foods is one way of achieving this. However, at the individual level, the optimal salt consumption range, or the “sweet spot” remains to be determined.
“Not adding extra salt to food is unlikely to be harmful and could contribute to strategies to lower population blood pressure levels,” Dr. Rosengren concluded.
A version of this article first appeared on Medscape.com.
in a new study.
In the study of more than 500,000 people, compared with those who never or rarely added salt, those who always added salt to their food had a 28% increased risk of dying prematurely (defined as death before the age of 75 years).
Results also showed that adding salt to food was linked to a lower life expectancy. At the age of 50 years, life expectancy was reduced by 1.5 years in women and by 2.28 years in men who always added salt to their food, compared with those who never or rarely did.
However, these increased risks appeared to be attenuated with increasing intakes of high-potassium foods (vegetables and fruits).
The study was published online in the European Heart Journal.
“As far as we are aware, this is the first study to analyze adding salt to meals as a unique measurement for dietary sodium intake. Such a measure is less likely affected by other dietary components, especially potassium intake,” senior author Lu Qi, MD, Tulane University, New Orleans, told this news organization.
“Our study provides supportive evidence from a novel perspective to show the adverse effects of high sodium intake on human health, which is still a controversial topic. Our findings support the advice that reduction of salt intake by reducing the salt added to meals may benefit health and improve life expectancy. Our results also suggest that high intakes of fruits and vegetables are beneficial regarding lowering the adverse effects of salt,” he added.
Link between dietary salt and health is subject of longstanding debate
The researchers explained that the relationship between dietary salt intake and health remains a subject of longstanding debate, with previous studies on the association between sodium intake and mortality having shown conflicting results.
They attributed the inconsistent results to the low accuracy of sodium measurement, noting that sodium intake varies widely from day to day, but the majority of previous studies have largely relied on a single day’s urine collection or dietary survey for estimating the sodium intake, which is inadequate to assess an individual’s usual consumption levels.
They also pointed out that it is difficult to separate the contributions of intakes of sodium and potassium to health based on current methods for measuring dietary sodium and potassium, and this may confound the association between sodium intake and health outcomes.
They noted that the hypothesis that a high-potassium intake may attenuate the adverse association of high-sodium intake with health outcomes has been proposed for many years, but studies assessing the interaction between sodium intake and potassium intake on the risk of mortality are scarce.
Adding salt to food at the table is a common eating behavior directly related to an individual’s long-term preference for salty tasting foods and habitual salt intake, the authors said, adding that commonly used table salt contains 97%-99% sodium chloride, minimizing the potential confounding effects of other dietary factors including potassium. “Therefore, adding salt to foods provides a unique assessment to evaluate the association between habitual sodium intake and mortality.”
UK Biobank study
For the current study Dr. Qi and colleagues analyzed data from 501,379 people taking part in the UK Biobank study. When joining the study between 2006 and 2010, the participants were asked whether they added salt to their foods never/rarely, sometimes, usually or always. Participants were then followed for a median of 9 years.
After adjustment for sex, age, race, smoking, moderate drinking, body mass index, physical activity, Townsend deprivation index, high cholesterol, chronic kidney disease, diabetes, cardiovascular disease, and cancer, results showed an increasing risk of all-cause premature mortality rose with increasing frequency of adding salt to foods.
The adjusted hazard ratios, compared with those who never or rarely added salt, were 1.02 (95% CI, 0.99-1.06) for those who added salt sometimes, 1.07 (95% CI, 1.02-1.11) for those who usually added salt, and 1.28 (95% CI, 1.20-1.35) for those who always added salt.
The researchers also estimated the lower survival time caused by the high frequency of adding salt to foods. At age 50, women who always added salt to foods had an average 1.50 fewer years of life expectancy, and men who always added salt had an average 2.28 fewer years of life expectancy, as compared with their counterparts who never/rarely added salt to foods.
For cause-specific premature mortality, results showed that higher frequency of adding salt to foods was significantly associated with a higher risk of cardiovascular mortality and cancer mortality, but not for dementia mortality or respiratory mortality. For the subtypes of cardiovascular mortality, adding salt to foods was significantly associated with higher risk of stroke mortality but not coronary heart disease mortality.
Other analyses suggested that the association of adding salt to foods with an increased risk of premature mortality appeared to be attenuated with increasing intake of food high in potassium (fruits and vegetables).
The authors point out that the amounts of discretionary sodium intake (the salt used at the table or in home cooking) have been largely overlooked in previous studies, even though adding salt to foods accounts for a considerable proportion of total sodium intake (6%-20%) in Western diets.
“Our findings also support the notion that even a modest reduction in sodium intake is likely to result in substantial health benefits, especially when it is achieved in the general population,” they conclude.
Conflicting information from different studies
But the current findings seem to directly contradict those from another recent study by Messerli and colleagues showing higher sodium intake correlates with improved life expectancy.
Addressing these contradictory results, Dr. Qi commented: “The study of Messerli et al. is based on an ecological design, in which the analysis is performed on country average sodium intake, rather than at the individual level. This type of ecological study has several major limitations, such as the lack of individuals’ sodium intake, uncontrolled confounding, and the cross-sectional nature. Typically, ecological studies are not considered useful for testing hypothesis in epidemiological studies.”
Dr. Qi noted that, in contrast, his current study analyzes individuals’ exposure, and has a prospective design. “Our findings are supported by previous large-scale observational studies and clinical trials which show the high intake of sodium may adversely affect chronic diseases such as cardiovascular disease and hypertension.” =
Lead author of the ecological study, Franz Messerli, MD, Bern (Switzerland) University Hospital, however, was not convinced by the findings from Dr. Qi’s study.
“The difference in 24-hour sodium intake between those who never/rarely added salt and those who always did is a minuscule 0.17 g. It is highly unlikely that such negligible quantity has any impact on blood pressure, not to mention cardiovascular mortality or life expectancy,” he commented in an interview.
He also pointed out that, in Dr. Qi’s study, people who added salt more frequently also consumed more red meat and processed meat, as well as less fish and less fruit and vegetables. “I would suggest that the bad habit of adding salt at the table is simply a powerful marker for an unhealthy diet.”
“There is no question that an excessive salt intake is harmful in hypertensive patients and increases the risk of stroke. But 0.17 g is not going to make any difference,” Dr. Messerli added.
What is the optimum level?
In an editorial accompanying the study by Dr. Qi and colleagues in the European Heart Journal, Annika Rosengren, MD, PhD, Sahlgrenska University Hospital, Gothenburg, Sweden, noted that guidelines recommend a salt intake below 5 g, or about a teaspoon, per day. But few individuals meet this recommendation.
Because several recent studies show a U- or J-shaped association between salt and atherosclerotic cardiovascular disease, reducing salt intake across the whole population may not be universally beneficial, Dr. Rosengren said.
“So far, what the collective evidence about salt seems to indicate is that healthy people consuming what constitutes normal levels of ordinary salt need not worry too much about their salt intake,” she wrote.
Instead, she advised a diet rich in fruit and vegetables should be a priority to counterbalance potentially harmful effects of salt, and for many other reasons.
And she added that people at high risk, such as those with hypertension who have a high salt intake, are probably well advised to cut down, and not adding extra salt to already prepared foods is one way of achieving this. However, at the individual level, the optimal salt consumption range, or the “sweet spot” remains to be determined.
“Not adding extra salt to food is unlikely to be harmful and could contribute to strategies to lower population blood pressure levels,” Dr. Rosengren concluded.
A version of this article first appeared on Medscape.com.
in a new study.
In the study of more than 500,000 people, compared with those who never or rarely added salt, those who always added salt to their food had a 28% increased risk of dying prematurely (defined as death before the age of 75 years).
Results also showed that adding salt to food was linked to a lower life expectancy. At the age of 50 years, life expectancy was reduced by 1.5 years in women and by 2.28 years in men who always added salt to their food, compared with those who never or rarely did.
However, these increased risks appeared to be attenuated with increasing intakes of high-potassium foods (vegetables and fruits).
The study was published online in the European Heart Journal.
“As far as we are aware, this is the first study to analyze adding salt to meals as a unique measurement for dietary sodium intake. Such a measure is less likely affected by other dietary components, especially potassium intake,” senior author Lu Qi, MD, Tulane University, New Orleans, told this news organization.
“Our study provides supportive evidence from a novel perspective to show the adverse effects of high sodium intake on human health, which is still a controversial topic. Our findings support the advice that reduction of salt intake by reducing the salt added to meals may benefit health and improve life expectancy. Our results also suggest that high intakes of fruits and vegetables are beneficial regarding lowering the adverse effects of salt,” he added.
Link between dietary salt and health is subject of longstanding debate
The researchers explained that the relationship between dietary salt intake and health remains a subject of longstanding debate, with previous studies on the association between sodium intake and mortality having shown conflicting results.
They attributed the inconsistent results to the low accuracy of sodium measurement, noting that sodium intake varies widely from day to day, but the majority of previous studies have largely relied on a single day’s urine collection or dietary survey for estimating the sodium intake, which is inadequate to assess an individual’s usual consumption levels.
They also pointed out that it is difficult to separate the contributions of intakes of sodium and potassium to health based on current methods for measuring dietary sodium and potassium, and this may confound the association between sodium intake and health outcomes.
They noted that the hypothesis that a high-potassium intake may attenuate the adverse association of high-sodium intake with health outcomes has been proposed for many years, but studies assessing the interaction between sodium intake and potassium intake on the risk of mortality are scarce.
Adding salt to food at the table is a common eating behavior directly related to an individual’s long-term preference for salty tasting foods and habitual salt intake, the authors said, adding that commonly used table salt contains 97%-99% sodium chloride, minimizing the potential confounding effects of other dietary factors including potassium. “Therefore, adding salt to foods provides a unique assessment to evaluate the association between habitual sodium intake and mortality.”
UK Biobank study
For the current study Dr. Qi and colleagues analyzed data from 501,379 people taking part in the UK Biobank study. When joining the study between 2006 and 2010, the participants were asked whether they added salt to their foods never/rarely, sometimes, usually or always. Participants were then followed for a median of 9 years.
After adjustment for sex, age, race, smoking, moderate drinking, body mass index, physical activity, Townsend deprivation index, high cholesterol, chronic kidney disease, diabetes, cardiovascular disease, and cancer, results showed an increasing risk of all-cause premature mortality rose with increasing frequency of adding salt to foods.
The adjusted hazard ratios, compared with those who never or rarely added salt, were 1.02 (95% CI, 0.99-1.06) for those who added salt sometimes, 1.07 (95% CI, 1.02-1.11) for those who usually added salt, and 1.28 (95% CI, 1.20-1.35) for those who always added salt.
The researchers also estimated the lower survival time caused by the high frequency of adding salt to foods. At age 50, women who always added salt to foods had an average 1.50 fewer years of life expectancy, and men who always added salt had an average 2.28 fewer years of life expectancy, as compared with their counterparts who never/rarely added salt to foods.
For cause-specific premature mortality, results showed that higher frequency of adding salt to foods was significantly associated with a higher risk of cardiovascular mortality and cancer mortality, but not for dementia mortality or respiratory mortality. For the subtypes of cardiovascular mortality, adding salt to foods was significantly associated with higher risk of stroke mortality but not coronary heart disease mortality.
Other analyses suggested that the association of adding salt to foods with an increased risk of premature mortality appeared to be attenuated with increasing intake of food high in potassium (fruits and vegetables).
The authors point out that the amounts of discretionary sodium intake (the salt used at the table or in home cooking) have been largely overlooked in previous studies, even though adding salt to foods accounts for a considerable proportion of total sodium intake (6%-20%) in Western diets.
“Our findings also support the notion that even a modest reduction in sodium intake is likely to result in substantial health benefits, especially when it is achieved in the general population,” they conclude.
Conflicting information from different studies
But the current findings seem to directly contradict those from another recent study by Messerli and colleagues showing higher sodium intake correlates with improved life expectancy.
Addressing these contradictory results, Dr. Qi commented: “The study of Messerli et al. is based on an ecological design, in which the analysis is performed on country average sodium intake, rather than at the individual level. This type of ecological study has several major limitations, such as the lack of individuals’ sodium intake, uncontrolled confounding, and the cross-sectional nature. Typically, ecological studies are not considered useful for testing hypothesis in epidemiological studies.”
Dr. Qi noted that, in contrast, his current study analyzes individuals’ exposure, and has a prospective design. “Our findings are supported by previous large-scale observational studies and clinical trials which show the high intake of sodium may adversely affect chronic diseases such as cardiovascular disease and hypertension.” =
Lead author of the ecological study, Franz Messerli, MD, Bern (Switzerland) University Hospital, however, was not convinced by the findings from Dr. Qi’s study.
“The difference in 24-hour sodium intake between those who never/rarely added salt and those who always did is a minuscule 0.17 g. It is highly unlikely that such negligible quantity has any impact on blood pressure, not to mention cardiovascular mortality or life expectancy,” he commented in an interview.
He also pointed out that, in Dr. Qi’s study, people who added salt more frequently also consumed more red meat and processed meat, as well as less fish and less fruit and vegetables. “I would suggest that the bad habit of adding salt at the table is simply a powerful marker for an unhealthy diet.”
“There is no question that an excessive salt intake is harmful in hypertensive patients and increases the risk of stroke. But 0.17 g is not going to make any difference,” Dr. Messerli added.
What is the optimum level?
In an editorial accompanying the study by Dr. Qi and colleagues in the European Heart Journal, Annika Rosengren, MD, PhD, Sahlgrenska University Hospital, Gothenburg, Sweden, noted that guidelines recommend a salt intake below 5 g, or about a teaspoon, per day. But few individuals meet this recommendation.
Because several recent studies show a U- or J-shaped association between salt and atherosclerotic cardiovascular disease, reducing salt intake across the whole population may not be universally beneficial, Dr. Rosengren said.
“So far, what the collective evidence about salt seems to indicate is that healthy people consuming what constitutes normal levels of ordinary salt need not worry too much about their salt intake,” she wrote.
Instead, she advised a diet rich in fruit and vegetables should be a priority to counterbalance potentially harmful effects of salt, and for many other reasons.
And she added that people at high risk, such as those with hypertension who have a high salt intake, are probably well advised to cut down, and not adding extra salt to already prepared foods is one way of achieving this. However, at the individual level, the optimal salt consumption range, or the “sweet spot” remains to be determined.
“Not adding extra salt to food is unlikely to be harmful and could contribute to strategies to lower population blood pressure levels,” Dr. Rosengren concluded.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN HEART JOURNAL
Zoster vaccination does not appear to increase flare risk in patients with immune-mediated inflammatory disease
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
Sleep-deprived physicians less empathetic to patient pain?
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Algorithm method versus spidey sense
One to two times a week I go through my junk mail folder. Usually it’s a collection of, well, junk: ads for CME, office software, car warranties, gift cards, dating sites, eyeglass or razor sellers, etc.
But there are usually a few items I’m glad I found, ones that I’m not sure how they ended up there. Bank notifications, package-tracking updates, a few other things. By the same token, every day a few pieces of junk land in my inbox.
This is, however, what we do for a living in this job.
Some patients are straightforward. The story is clear, the plan obvious.
Some require a bit more thinking.
And some are all over the place. Histories that wander everywhere, a million symptoms and clues. Most are likely red herrings, but which ones isn’t immediately obvious. And it’s up to the doctor to work this out.
With my junk folder, though, it’s usually immediately obvious what the useless things are compared with those of value. In medicine it’s often not so simple. You have to be careful what you discard, and you always need to be ready to change your mind and backtrack.
Artificial intelligence gets better every year but still makes plenty of mistakes. In sorting email my computer has to work out the signal-to-noise ratio of incoming items and decide which ones mean something. If my junk folder is any indication, it still has a ways to go.
This isn’t to say I’m infallible. I’m not. Unlike the algorithms my email program uses, there are no definite rules in medical cases. Picking through the clues is something that comes with training, experience, and a bit of luck. When I realize I’m going in the wrong direction I have to step back and rethink it all.
A lot of chart systems try to incorporate algorithms into medical decision-making. Sometimes they’re helpful, such as pointing out a drug interaction I wasn’t aware of. At other times they’re not, telling me I shouldn’t be ordering a test because such-and-such criteria haven’t been met. The trouble is these algorithms are written to apply to all cases, even though every patient is different. Sometimes the best we can go on is what I call “spidey sense” – realizing that there’s more than meets the eye here. In 24 years it’s served me well, far better than any computer algorithm has.
People talk about a natural fear of being replaced by computers. I agree that there are some things they’re very good at, and they keep getting better. But medicine isn’t a one-size-fits-all field. And the consequences are a lot higher than those from my bank statement being overlooked for a few days.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
One to two times a week I go through my junk mail folder. Usually it’s a collection of, well, junk: ads for CME, office software, car warranties, gift cards, dating sites, eyeglass or razor sellers, etc.
But there are usually a few items I’m glad I found, ones that I’m not sure how they ended up there. Bank notifications, package-tracking updates, a few other things. By the same token, every day a few pieces of junk land in my inbox.
This is, however, what we do for a living in this job.
Some patients are straightforward. The story is clear, the plan obvious.
Some require a bit more thinking.
And some are all over the place. Histories that wander everywhere, a million symptoms and clues. Most are likely red herrings, but which ones isn’t immediately obvious. And it’s up to the doctor to work this out.
With my junk folder, though, it’s usually immediately obvious what the useless things are compared with those of value. In medicine it’s often not so simple. You have to be careful what you discard, and you always need to be ready to change your mind and backtrack.
Artificial intelligence gets better every year but still makes plenty of mistakes. In sorting email my computer has to work out the signal-to-noise ratio of incoming items and decide which ones mean something. If my junk folder is any indication, it still has a ways to go.
This isn’t to say I’m infallible. I’m not. Unlike the algorithms my email program uses, there are no definite rules in medical cases. Picking through the clues is something that comes with training, experience, and a bit of luck. When I realize I’m going in the wrong direction I have to step back and rethink it all.
A lot of chart systems try to incorporate algorithms into medical decision-making. Sometimes they’re helpful, such as pointing out a drug interaction I wasn’t aware of. At other times they’re not, telling me I shouldn’t be ordering a test because such-and-such criteria haven’t been met. The trouble is these algorithms are written to apply to all cases, even though every patient is different. Sometimes the best we can go on is what I call “spidey sense” – realizing that there’s more than meets the eye here. In 24 years it’s served me well, far better than any computer algorithm has.
People talk about a natural fear of being replaced by computers. I agree that there are some things they’re very good at, and they keep getting better. But medicine isn’t a one-size-fits-all field. And the consequences are a lot higher than those from my bank statement being overlooked for a few days.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
One to two times a week I go through my junk mail folder. Usually it’s a collection of, well, junk: ads for CME, office software, car warranties, gift cards, dating sites, eyeglass or razor sellers, etc.
But there are usually a few items I’m glad I found, ones that I’m not sure how they ended up there. Bank notifications, package-tracking updates, a few other things. By the same token, every day a few pieces of junk land in my inbox.
This is, however, what we do for a living in this job.
Some patients are straightforward. The story is clear, the plan obvious.
Some require a bit more thinking.
And some are all over the place. Histories that wander everywhere, a million symptoms and clues. Most are likely red herrings, but which ones isn’t immediately obvious. And it’s up to the doctor to work this out.
With my junk folder, though, it’s usually immediately obvious what the useless things are compared with those of value. In medicine it’s often not so simple. You have to be careful what you discard, and you always need to be ready to change your mind and backtrack.
Artificial intelligence gets better every year but still makes plenty of mistakes. In sorting email my computer has to work out the signal-to-noise ratio of incoming items and decide which ones mean something. If my junk folder is any indication, it still has a ways to go.
This isn’t to say I’m infallible. I’m not. Unlike the algorithms my email program uses, there are no definite rules in medical cases. Picking through the clues is something that comes with training, experience, and a bit of luck. When I realize I’m going in the wrong direction I have to step back and rethink it all.
A lot of chart systems try to incorporate algorithms into medical decision-making. Sometimes they’re helpful, such as pointing out a drug interaction I wasn’t aware of. At other times they’re not, telling me I shouldn’t be ordering a test because such-and-such criteria haven’t been met. The trouble is these algorithms are written to apply to all cases, even though every patient is different. Sometimes the best we can go on is what I call “spidey sense” – realizing that there’s more than meets the eye here. In 24 years it’s served me well, far better than any computer algorithm has.
People talk about a natural fear of being replaced by computers. I agree that there are some things they’re very good at, and they keep getting better. But medicine isn’t a one-size-fits-all field. And the consequences are a lot higher than those from my bank statement being overlooked for a few days.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
New European guidelines ‘drastically’ reduce statin eligibility
New risk thresholds used to guide statin therapy for primary prevention of atherosclerotic cardiovascular disease in the latest European guidelines dramatically reduce eligibility for statin use in low-risk countries, a new study has found.
The authors reported that and essentially eliminate a statin indication in women.
“We have guidelines in place to try to prevent cardiovascular disease but the risk threshold in this new guideline means that almost nobody qualifies for treatment in many countries, which will lead to almost no prevention of future cardiovascular disease in those countries,” lead author Martin Bødtker Mortensen, MD, PhD, Aarhus (Denmark) University Hospital, commented in an interview.
“We argue that the risk thresholds need to be lowered to get the statin eligibility in European countries to be in line with thresholds in the U.K. and U.S., which are based on randomized, controlled trials,” he added.
The study was published online in JAMA Cardiology.
An accompanying editorial describes the results of the study as “alarming,” and, if confirmed, said the guidelines should be revisited to “prevent a step backwards in the use of statins in primary prevention.”
For the study, Dr. Mortensen and colleagues set out to compare the clinical performance of the new European prevention guidelines with American College of Cardiology/American Heart Association, United Kingdom–National Institute for Health and Care Excellence, and the 2019 European guidelines in a contemporary European cohort of 66,909 apparently healthy individuals from the Copenhagen General Population Study.
During the 9-year follow-up, a range of 2,962-4,277 nonfatal and fatal cardiovascular events was observed, as defined by the models in the various guidelines.
Results showed that although the new 2021 European guidelines introduced a new and improved risk model, known as SCORE2, the updated age-specific recommendations dramatically reduced eligibility for a class I recommendation for statin therapy to only 4% of individuals, aged 40-69 years, and less than 1% of women.
This is in sharp contrast to the previous 2019 European guidelines as well as current UK-NICE and US-ACC/AHA guidelines that provide class I/strong recommendations to 20%, 26%, and 34% of individuals, respectively, with better clinical performance in both men and women, the authors report.
The researchers also reported other analyses in which the sensitivity of the new European guidelines was improved considerably by lowering the treatment thresholds.
Dr. Mortensen explained to this news organization that the original SCORE risk model used in ESC guidelines was problematic as it only predicts the 10-year risk of fatal atherosclerotic cardiovascular events, whereas those from the United States and United Kingdom used both fatal and nonfatal cardiovascular events.
“Now the ESC has updated its model and the new model is much better in that it predicts both fatal and nonfatal events, and the predicted risk correlates well with the actual risk. So that’s a big step forward. However, the new thresholds for statin treatment are far too high for low-risk European countries because very few individuals will now qualify for statin therapy,” he said.
“The problem is that, if we use these guidelines, the vast majority of those individuals who will develop cardiovascular disease within 10 years will not be assigned statin therapy that can reduce this risk. There will be lots of individuals who are at high risk of cardiovascular disease, but these guidelines will not identify them as needing to take a statin,” Dr. Mortensen commented.
“If we use the U.K. or U.S. guidelines, far more people in these low-risk European countries would be eligible for statin therapy and we would prevent far more events than if we use the new ESC guidelines,” he added.
Dr. Mortensen explained that the problem arises from having four different risk score models in Europe for areas at different risk, but they all use the same risk thresholds for statin treatment.
“In general, Eastern European countries have higher risk than Western European countries, so these guidelines may work quite well in Eastern European countries but in low-risk Western European countries, where the low-risk score model is used, very few people will qualify for statin therapy,” he said.
While Dr. Mortensen is not against the idea of different risk models in areas that have different risks, he says this needs to be accompanied by different risk thresholds in the different risk areas.
Asked whether there is an argument that most individuals in low-risk countries may not need to take a statin, Dr. Mortensen countered: “One of the reasons the risk is low in many of these European countries is the high use of preventative medication. So, if a threshold that is too high is used most people will not take a statin anymore and the risk in these countries will increase again.”
Authors of the accompanying editorial, Ann Marie Navar, MD, PhD, University of Texas Southwestern Medical Center, Dallas; Gregg C. Fonarow, MD, University of California, Los Angeles; and Michael J. Pencina, PhD, Duke University Medical Center, Durham, N.C., agreed with Dr. Mortensen that the problems appear to arise from use of a risk score that is highly influenced by regional cardiovascular burden.
They point out that under the current guidelines, a 55-year-old woman (smoker; systolic blood pressure, 130 mm Hg; non–HDL cholesterol, 4.0 mmol/L) would have a 10-year predicted risk of having a cardiovascular event of 5% in Denmark but a predicted risk of 18% in Romania.
“While there may be regional differences in environmental risk factors, location alone should not cause a fourfold difference in an individual’s predicted cardiovascular risk,” they wrote.
The editorialists also elaborated on Dr. Mortensen’s point that the new guideline creates a system that eventually becomes a victim of its own success.
“As countries are successful in implementing statin therapy to lower CVD, CVD rates drop, and progressively fewer individuals are then eligible for the very therapy that contributed to the decline in CVD in the first place,” they noted.
The editorialists called for the analysis to be replicated in other low-risk countries and extended to higher-risk regions, with a focus on potential overtreatment of men and older adults.
“If confirmed, the present findings should be a catalyst for the ESC to revisit or augment their current guidelines to prevent a step backward in the use of statins in primary prevention,” they concluded.
This news organization asked the ESC for a response to the findings, but did not comment by press time.
This work was supported by the Lundbeck Foundation, Herlev and Gentofte Hospital, Copenhagen University Hospital, the Copenhagen County Foundation, and Aarhus University, Denmark. Dr. Mortensen reported no disclosures.
A version of this article first appeared on Medscape.com.
New risk thresholds used to guide statin therapy for primary prevention of atherosclerotic cardiovascular disease in the latest European guidelines dramatically reduce eligibility for statin use in low-risk countries, a new study has found.
The authors reported that and essentially eliminate a statin indication in women.
“We have guidelines in place to try to prevent cardiovascular disease but the risk threshold in this new guideline means that almost nobody qualifies for treatment in many countries, which will lead to almost no prevention of future cardiovascular disease in those countries,” lead author Martin Bødtker Mortensen, MD, PhD, Aarhus (Denmark) University Hospital, commented in an interview.
“We argue that the risk thresholds need to be lowered to get the statin eligibility in European countries to be in line with thresholds in the U.K. and U.S., which are based on randomized, controlled trials,” he added.
The study was published online in JAMA Cardiology.
An accompanying editorial describes the results of the study as “alarming,” and, if confirmed, said the guidelines should be revisited to “prevent a step backwards in the use of statins in primary prevention.”
For the study, Dr. Mortensen and colleagues set out to compare the clinical performance of the new European prevention guidelines with American College of Cardiology/American Heart Association, United Kingdom–National Institute for Health and Care Excellence, and the 2019 European guidelines in a contemporary European cohort of 66,909 apparently healthy individuals from the Copenhagen General Population Study.
During the 9-year follow-up, a range of 2,962-4,277 nonfatal and fatal cardiovascular events was observed, as defined by the models in the various guidelines.
Results showed that although the new 2021 European guidelines introduced a new and improved risk model, known as SCORE2, the updated age-specific recommendations dramatically reduced eligibility for a class I recommendation for statin therapy to only 4% of individuals, aged 40-69 years, and less than 1% of women.
This is in sharp contrast to the previous 2019 European guidelines as well as current UK-NICE and US-ACC/AHA guidelines that provide class I/strong recommendations to 20%, 26%, and 34% of individuals, respectively, with better clinical performance in both men and women, the authors report.
The researchers also reported other analyses in which the sensitivity of the new European guidelines was improved considerably by lowering the treatment thresholds.
Dr. Mortensen explained to this news organization that the original SCORE risk model used in ESC guidelines was problematic as it only predicts the 10-year risk of fatal atherosclerotic cardiovascular events, whereas those from the United States and United Kingdom used both fatal and nonfatal cardiovascular events.
“Now the ESC has updated its model and the new model is much better in that it predicts both fatal and nonfatal events, and the predicted risk correlates well with the actual risk. So that’s a big step forward. However, the new thresholds for statin treatment are far too high for low-risk European countries because very few individuals will now qualify for statin therapy,” he said.
“The problem is that, if we use these guidelines, the vast majority of those individuals who will develop cardiovascular disease within 10 years will not be assigned statin therapy that can reduce this risk. There will be lots of individuals who are at high risk of cardiovascular disease, but these guidelines will not identify them as needing to take a statin,” Dr. Mortensen commented.
“If we use the U.K. or U.S. guidelines, far more people in these low-risk European countries would be eligible for statin therapy and we would prevent far more events than if we use the new ESC guidelines,” he added.
Dr. Mortensen explained that the problem arises from having four different risk score models in Europe for areas at different risk, but they all use the same risk thresholds for statin treatment.
“In general, Eastern European countries have higher risk than Western European countries, so these guidelines may work quite well in Eastern European countries but in low-risk Western European countries, where the low-risk score model is used, very few people will qualify for statin therapy,” he said.
While Dr. Mortensen is not against the idea of different risk models in areas that have different risks, he says this needs to be accompanied by different risk thresholds in the different risk areas.
Asked whether there is an argument that most individuals in low-risk countries may not need to take a statin, Dr. Mortensen countered: “One of the reasons the risk is low in many of these European countries is the high use of preventative medication. So, if a threshold that is too high is used most people will not take a statin anymore and the risk in these countries will increase again.”
Authors of the accompanying editorial, Ann Marie Navar, MD, PhD, University of Texas Southwestern Medical Center, Dallas; Gregg C. Fonarow, MD, University of California, Los Angeles; and Michael J. Pencina, PhD, Duke University Medical Center, Durham, N.C., agreed with Dr. Mortensen that the problems appear to arise from use of a risk score that is highly influenced by regional cardiovascular burden.
They point out that under the current guidelines, a 55-year-old woman (smoker; systolic blood pressure, 130 mm Hg; non–HDL cholesterol, 4.0 mmol/L) would have a 10-year predicted risk of having a cardiovascular event of 5% in Denmark but a predicted risk of 18% in Romania.
“While there may be regional differences in environmental risk factors, location alone should not cause a fourfold difference in an individual’s predicted cardiovascular risk,” they wrote.
The editorialists also elaborated on Dr. Mortensen’s point that the new guideline creates a system that eventually becomes a victim of its own success.
“As countries are successful in implementing statin therapy to lower CVD, CVD rates drop, and progressively fewer individuals are then eligible for the very therapy that contributed to the decline in CVD in the first place,” they noted.
The editorialists called for the analysis to be replicated in other low-risk countries and extended to higher-risk regions, with a focus on potential overtreatment of men and older adults.
“If confirmed, the present findings should be a catalyst for the ESC to revisit or augment their current guidelines to prevent a step backward in the use of statins in primary prevention,” they concluded.
This news organization asked the ESC for a response to the findings, but did not comment by press time.
This work was supported by the Lundbeck Foundation, Herlev and Gentofte Hospital, Copenhagen University Hospital, the Copenhagen County Foundation, and Aarhus University, Denmark. Dr. Mortensen reported no disclosures.
A version of this article first appeared on Medscape.com.
New risk thresholds used to guide statin therapy for primary prevention of atherosclerotic cardiovascular disease in the latest European guidelines dramatically reduce eligibility for statin use in low-risk countries, a new study has found.
The authors reported that and essentially eliminate a statin indication in women.
“We have guidelines in place to try to prevent cardiovascular disease but the risk threshold in this new guideline means that almost nobody qualifies for treatment in many countries, which will lead to almost no prevention of future cardiovascular disease in those countries,” lead author Martin Bødtker Mortensen, MD, PhD, Aarhus (Denmark) University Hospital, commented in an interview.
“We argue that the risk thresholds need to be lowered to get the statin eligibility in European countries to be in line with thresholds in the U.K. and U.S., which are based on randomized, controlled trials,” he added.
The study was published online in JAMA Cardiology.
An accompanying editorial describes the results of the study as “alarming,” and, if confirmed, said the guidelines should be revisited to “prevent a step backwards in the use of statins in primary prevention.”
For the study, Dr. Mortensen and colleagues set out to compare the clinical performance of the new European prevention guidelines with American College of Cardiology/American Heart Association, United Kingdom–National Institute for Health and Care Excellence, and the 2019 European guidelines in a contemporary European cohort of 66,909 apparently healthy individuals from the Copenhagen General Population Study.
During the 9-year follow-up, a range of 2,962-4,277 nonfatal and fatal cardiovascular events was observed, as defined by the models in the various guidelines.
Results showed that although the new 2021 European guidelines introduced a new and improved risk model, known as SCORE2, the updated age-specific recommendations dramatically reduced eligibility for a class I recommendation for statin therapy to only 4% of individuals, aged 40-69 years, and less than 1% of women.
This is in sharp contrast to the previous 2019 European guidelines as well as current UK-NICE and US-ACC/AHA guidelines that provide class I/strong recommendations to 20%, 26%, and 34% of individuals, respectively, with better clinical performance in both men and women, the authors report.
The researchers also reported other analyses in which the sensitivity of the new European guidelines was improved considerably by lowering the treatment thresholds.
Dr. Mortensen explained to this news organization that the original SCORE risk model used in ESC guidelines was problematic as it only predicts the 10-year risk of fatal atherosclerotic cardiovascular events, whereas those from the United States and United Kingdom used both fatal and nonfatal cardiovascular events.
“Now the ESC has updated its model and the new model is much better in that it predicts both fatal and nonfatal events, and the predicted risk correlates well with the actual risk. So that’s a big step forward. However, the new thresholds for statin treatment are far too high for low-risk European countries because very few individuals will now qualify for statin therapy,” he said.
“The problem is that, if we use these guidelines, the vast majority of those individuals who will develop cardiovascular disease within 10 years will not be assigned statin therapy that can reduce this risk. There will be lots of individuals who are at high risk of cardiovascular disease, but these guidelines will not identify them as needing to take a statin,” Dr. Mortensen commented.
“If we use the U.K. or U.S. guidelines, far more people in these low-risk European countries would be eligible for statin therapy and we would prevent far more events than if we use the new ESC guidelines,” he added.
Dr. Mortensen explained that the problem arises from having four different risk score models in Europe for areas at different risk, but they all use the same risk thresholds for statin treatment.
“In general, Eastern European countries have higher risk than Western European countries, so these guidelines may work quite well in Eastern European countries but in low-risk Western European countries, where the low-risk score model is used, very few people will qualify for statin therapy,” he said.
While Dr. Mortensen is not against the idea of different risk models in areas that have different risks, he says this needs to be accompanied by different risk thresholds in the different risk areas.
Asked whether there is an argument that most individuals in low-risk countries may not need to take a statin, Dr. Mortensen countered: “One of the reasons the risk is low in many of these European countries is the high use of preventative medication. So, if a threshold that is too high is used most people will not take a statin anymore and the risk in these countries will increase again.”
Authors of the accompanying editorial, Ann Marie Navar, MD, PhD, University of Texas Southwestern Medical Center, Dallas; Gregg C. Fonarow, MD, University of California, Los Angeles; and Michael J. Pencina, PhD, Duke University Medical Center, Durham, N.C., agreed with Dr. Mortensen that the problems appear to arise from use of a risk score that is highly influenced by regional cardiovascular burden.
They point out that under the current guidelines, a 55-year-old woman (smoker; systolic blood pressure, 130 mm Hg; non–HDL cholesterol, 4.0 mmol/L) would have a 10-year predicted risk of having a cardiovascular event of 5% in Denmark but a predicted risk of 18% in Romania.
“While there may be regional differences in environmental risk factors, location alone should not cause a fourfold difference in an individual’s predicted cardiovascular risk,” they wrote.
The editorialists also elaborated on Dr. Mortensen’s point that the new guideline creates a system that eventually becomes a victim of its own success.
“As countries are successful in implementing statin therapy to lower CVD, CVD rates drop, and progressively fewer individuals are then eligible for the very therapy that contributed to the decline in CVD in the first place,” they noted.
The editorialists called for the analysis to be replicated in other low-risk countries and extended to higher-risk regions, with a focus on potential overtreatment of men and older adults.
“If confirmed, the present findings should be a catalyst for the ESC to revisit or augment their current guidelines to prevent a step backward in the use of statins in primary prevention,” they concluded.
This news organization asked the ESC for a response to the findings, but did not comment by press time.
This work was supported by the Lundbeck Foundation, Herlev and Gentofte Hospital, Copenhagen University Hospital, the Copenhagen County Foundation, and Aarhus University, Denmark. Dr. Mortensen reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Nocturnal sleep key to successful kindergarten adjustment
Children who regularly slept 10-plus hours per night, particularly just before starting kindergarten, transitioned more successfully to kindergarten than those with less regular sleeping patterns, an observational study found. The effect held across the kindergarten year regardless of socioeconomic and health covariates, according to a new study by Douglas M. Teti, PhD, a developmental scientist and a professor of pediatrics at Penn State University, University Park, and colleagues.
“These effects were ubiquitous, extending to socioemotional learning engagement and academic domains,” they wrote online in Pediatrics
Furthermore, it was the regularity of sufficient nocturnal sleep that appeared to be more important for school adjustment than overall amounts of sleep accumulated across the day or the proportion of 24-hour periods in which children got 10 or more hours of sleep.
The American Academy of Sleep Medicine has recommended that 3- to 5-year-olds get 10-13 hours of sleep per day, including naps.
The findings by Dr. Teti’s group suggest that family-based interventions to establish consistent patterns of sufficient nighttime sleep should begin 5 or 6 months before the start of kindergarten.
“The importance of sleep as a predictor of school functioning in children is well-established, but relatively less is known about how sleep impacts children as they make their first transition into formal schooling,” Dr. Teti told this news organization. “School readiness and adjustment can be impacted by many factors, including socioeconomic status, child health, and missed days of school, but few studies have isolated the role of sleep in the transition to kindergarten net of these other influences, and few studies have examined the role that sleep plays on children’s school functioning throughout the full kindergarten year.”
The study
During 2016-2019, the researcher recruited 230 families from three Pennsylvania school districts, of which 221 completed the study. At several time points, the study examined three different measures of child sleep duration in 7-day bursts: at pre-kindergarten (July to August), early kindergarten (late September), mid-kindergarten (late November), and late kindergarten (mid-to-late April), using wrist actigraphy. These measures included:
- mean amounts of child sleep per 24-hour period across the full week
- proportion of 24-hour periods per week that children slept 10 or more hours
- proportion of nighttime sleep periods per week that children slept 10 or more hours
Outcomes at the designated school year time points were provided by 64 teachers blinded to the pupils’ sleep histories and by assessments administered by project staff.
Among the sleep measures examined, regularity of nighttime sleep involving 10 or more hours of sleep over the nocturnal period, especially at the pre-kindergarten stage, consistently predicted more favorable outcomes in socioemotional, learning engagement, and academic domains. These findings were controlled for income-to-poverty threshold ratios, child health status, and number of missed school days.
The study results generally align with those of previous studies, showing the importance of sleep for children’s school functioning, Dr. Teti told this news organization. “But they differed significantly in terms of finding that it was the regularity of 10-plus hours concentrated during the nighttime sleep period that was most important for predicting school adjustment, in particular, regular or sufficient sleep that occurred prior to the start of kindergarten.”
Calling the study “thought provoking,” Michael B. Grosso, MD, chair of pediatrics at Huntington (N.Y.) Hospital, said it confirms a robust correlation between total sleep duration and outcomes important to successful adjustment to kindergarten. “And we find out that uninterrupted sleep time of 10 hours or more seems to matter as well.”
In his view, the biggest limitation to the analysis is the one inherent to any observational study, “which is that association cannot prove causality. The authors did attempt to control for other health factors, but that can be hard to do,” he said. “The point is that if a child faces any of several health challenges, from sleep apnea to uncontrolled asthma, to ADHD or an autistic spectrum disorder, those issues will cause disrupted, abnormal sleep and also interfere with the outcomes the study addresses. In other words, it’s hard to know if sleep is affecting kindergarten adjustment or whether some X factor is affecting sleep and also affecting kindergarten performance.”
Getting children into bed earlier in long bright evenings of spring and summer before onset of kindergarten may not be easy, Dr. Teti acknowledged. “Arranging children’s sleep schedule as they approach kindergarten so that most, if not all, of their sleep takes place during the night – and as a corollary, reducing the frequency of naps during the day – should help children shift into sleeping nighttime primarily if not exclusively,” he said.
If necessary, he added, parents can work with sleep professionals to gradually concentrate children’s sleep during the night. They should normalize earlier bedtimes by reducing access to electronic screens before bedtime and removing televisions from their bedrooms. “A consistent bedtime routine should be a central feature of parental attempts to shape better, more regular sleep in their children.”
Dr. Grosso added that pediatricians need to talk about the importance of consistent routines and especially adequate sleep when counseling parents during pre-school health supervision visits. “And as the authors mention, it’s hard to ensure good sleep hygiene for children if parents aren’t also getting a good night sleep. It all goes together.”
This study was supported by the National Institutes of Health. The authors had no competing interests to declare. Dr. Grosso disclosed no relevant conflicts of interest.
Children who regularly slept 10-plus hours per night, particularly just before starting kindergarten, transitioned more successfully to kindergarten than those with less regular sleeping patterns, an observational study found. The effect held across the kindergarten year regardless of socioeconomic and health covariates, according to a new study by Douglas M. Teti, PhD, a developmental scientist and a professor of pediatrics at Penn State University, University Park, and colleagues.
“These effects were ubiquitous, extending to socioemotional learning engagement and academic domains,” they wrote online in Pediatrics
Furthermore, it was the regularity of sufficient nocturnal sleep that appeared to be more important for school adjustment than overall amounts of sleep accumulated across the day or the proportion of 24-hour periods in which children got 10 or more hours of sleep.
The American Academy of Sleep Medicine has recommended that 3- to 5-year-olds get 10-13 hours of sleep per day, including naps.
The findings by Dr. Teti’s group suggest that family-based interventions to establish consistent patterns of sufficient nighttime sleep should begin 5 or 6 months before the start of kindergarten.
“The importance of sleep as a predictor of school functioning in children is well-established, but relatively less is known about how sleep impacts children as they make their first transition into formal schooling,” Dr. Teti told this news organization. “School readiness and adjustment can be impacted by many factors, including socioeconomic status, child health, and missed days of school, but few studies have isolated the role of sleep in the transition to kindergarten net of these other influences, and few studies have examined the role that sleep plays on children’s school functioning throughout the full kindergarten year.”
The study
During 2016-2019, the researcher recruited 230 families from three Pennsylvania school districts, of which 221 completed the study. At several time points, the study examined three different measures of child sleep duration in 7-day bursts: at pre-kindergarten (July to August), early kindergarten (late September), mid-kindergarten (late November), and late kindergarten (mid-to-late April), using wrist actigraphy. These measures included:
- mean amounts of child sleep per 24-hour period across the full week
- proportion of 24-hour periods per week that children slept 10 or more hours
- proportion of nighttime sleep periods per week that children slept 10 or more hours
Outcomes at the designated school year time points were provided by 64 teachers blinded to the pupils’ sleep histories and by assessments administered by project staff.
Among the sleep measures examined, regularity of nighttime sleep involving 10 or more hours of sleep over the nocturnal period, especially at the pre-kindergarten stage, consistently predicted more favorable outcomes in socioemotional, learning engagement, and academic domains. These findings were controlled for income-to-poverty threshold ratios, child health status, and number of missed school days.
The study results generally align with those of previous studies, showing the importance of sleep for children’s school functioning, Dr. Teti told this news organization. “But they differed significantly in terms of finding that it was the regularity of 10-plus hours concentrated during the nighttime sleep period that was most important for predicting school adjustment, in particular, regular or sufficient sleep that occurred prior to the start of kindergarten.”
Calling the study “thought provoking,” Michael B. Grosso, MD, chair of pediatrics at Huntington (N.Y.) Hospital, said it confirms a robust correlation between total sleep duration and outcomes important to successful adjustment to kindergarten. “And we find out that uninterrupted sleep time of 10 hours or more seems to matter as well.”
In his view, the biggest limitation to the analysis is the one inherent to any observational study, “which is that association cannot prove causality. The authors did attempt to control for other health factors, but that can be hard to do,” he said. “The point is that if a child faces any of several health challenges, from sleep apnea to uncontrolled asthma, to ADHD or an autistic spectrum disorder, those issues will cause disrupted, abnormal sleep and also interfere with the outcomes the study addresses. In other words, it’s hard to know if sleep is affecting kindergarten adjustment or whether some X factor is affecting sleep and also affecting kindergarten performance.”
Getting children into bed earlier in long bright evenings of spring and summer before onset of kindergarten may not be easy, Dr. Teti acknowledged. “Arranging children’s sleep schedule as they approach kindergarten so that most, if not all, of their sleep takes place during the night – and as a corollary, reducing the frequency of naps during the day – should help children shift into sleeping nighttime primarily if not exclusively,” he said.
If necessary, he added, parents can work with sleep professionals to gradually concentrate children’s sleep during the night. They should normalize earlier bedtimes by reducing access to electronic screens before bedtime and removing televisions from their bedrooms. “A consistent bedtime routine should be a central feature of parental attempts to shape better, more regular sleep in their children.”
Dr. Grosso added that pediatricians need to talk about the importance of consistent routines and especially adequate sleep when counseling parents during pre-school health supervision visits. “And as the authors mention, it’s hard to ensure good sleep hygiene for children if parents aren’t also getting a good night sleep. It all goes together.”
This study was supported by the National Institutes of Health. The authors had no competing interests to declare. Dr. Grosso disclosed no relevant conflicts of interest.
Children who regularly slept 10-plus hours per night, particularly just before starting kindergarten, transitioned more successfully to kindergarten than those with less regular sleeping patterns, an observational study found. The effect held across the kindergarten year regardless of socioeconomic and health covariates, according to a new study by Douglas M. Teti, PhD, a developmental scientist and a professor of pediatrics at Penn State University, University Park, and colleagues.
“These effects were ubiquitous, extending to socioemotional learning engagement and academic domains,” they wrote online in Pediatrics
Furthermore, it was the regularity of sufficient nocturnal sleep that appeared to be more important for school adjustment than overall amounts of sleep accumulated across the day or the proportion of 24-hour periods in which children got 10 or more hours of sleep.
The American Academy of Sleep Medicine has recommended that 3- to 5-year-olds get 10-13 hours of sleep per day, including naps.
The findings by Dr. Teti’s group suggest that family-based interventions to establish consistent patterns of sufficient nighttime sleep should begin 5 or 6 months before the start of kindergarten.
“The importance of sleep as a predictor of school functioning in children is well-established, but relatively less is known about how sleep impacts children as they make their first transition into formal schooling,” Dr. Teti told this news organization. “School readiness and adjustment can be impacted by many factors, including socioeconomic status, child health, and missed days of school, but few studies have isolated the role of sleep in the transition to kindergarten net of these other influences, and few studies have examined the role that sleep plays on children’s school functioning throughout the full kindergarten year.”
The study
During 2016-2019, the researcher recruited 230 families from three Pennsylvania school districts, of which 221 completed the study. At several time points, the study examined three different measures of child sleep duration in 7-day bursts: at pre-kindergarten (July to August), early kindergarten (late September), mid-kindergarten (late November), and late kindergarten (mid-to-late April), using wrist actigraphy. These measures included:
- mean amounts of child sleep per 24-hour period across the full week
- proportion of 24-hour periods per week that children slept 10 or more hours
- proportion of nighttime sleep periods per week that children slept 10 or more hours
Outcomes at the designated school year time points were provided by 64 teachers blinded to the pupils’ sleep histories and by assessments administered by project staff.
Among the sleep measures examined, regularity of nighttime sleep involving 10 or more hours of sleep over the nocturnal period, especially at the pre-kindergarten stage, consistently predicted more favorable outcomes in socioemotional, learning engagement, and academic domains. These findings were controlled for income-to-poverty threshold ratios, child health status, and number of missed school days.
The study results generally align with those of previous studies, showing the importance of sleep for children’s school functioning, Dr. Teti told this news organization. “But they differed significantly in terms of finding that it was the regularity of 10-plus hours concentrated during the nighttime sleep period that was most important for predicting school adjustment, in particular, regular or sufficient sleep that occurred prior to the start of kindergarten.”
Calling the study “thought provoking,” Michael B. Grosso, MD, chair of pediatrics at Huntington (N.Y.) Hospital, said it confirms a robust correlation between total sleep duration and outcomes important to successful adjustment to kindergarten. “And we find out that uninterrupted sleep time of 10 hours or more seems to matter as well.”
In his view, the biggest limitation to the analysis is the one inherent to any observational study, “which is that association cannot prove causality. The authors did attempt to control for other health factors, but that can be hard to do,” he said. “The point is that if a child faces any of several health challenges, from sleep apnea to uncontrolled asthma, to ADHD or an autistic spectrum disorder, those issues will cause disrupted, abnormal sleep and also interfere with the outcomes the study addresses. In other words, it’s hard to know if sleep is affecting kindergarten adjustment or whether some X factor is affecting sleep and also affecting kindergarten performance.”
Getting children into bed earlier in long bright evenings of spring and summer before onset of kindergarten may not be easy, Dr. Teti acknowledged. “Arranging children’s sleep schedule as they approach kindergarten so that most, if not all, of their sleep takes place during the night – and as a corollary, reducing the frequency of naps during the day – should help children shift into sleeping nighttime primarily if not exclusively,” he said.
If necessary, he added, parents can work with sleep professionals to gradually concentrate children’s sleep during the night. They should normalize earlier bedtimes by reducing access to electronic screens before bedtime and removing televisions from their bedrooms. “A consistent bedtime routine should be a central feature of parental attempts to shape better, more regular sleep in their children.”
Dr. Grosso added that pediatricians need to talk about the importance of consistent routines and especially adequate sleep when counseling parents during pre-school health supervision visits. “And as the authors mention, it’s hard to ensure good sleep hygiene for children if parents aren’t also getting a good night sleep. It all goes together.”
This study was supported by the National Institutes of Health. The authors had no competing interests to declare. Dr. Grosso disclosed no relevant conflicts of interest.
FROM PEDIATRICS