User login
Thrombolysis is safe in stroke patients on oral anticoagulants
, a new observational study suggests, prompting researchers to ask whether guidelines that restrict its use should be updated.
Researchers found that DOAC users were significantly less likely to develop symptomatic intracerebral hemorrhage (sICH) after IVT, and there was no difference in functional independence at 3 months, compared with patients who received IVT but who did not receive DOAC.
“At the moment, the guidelines really pose a barrier and stop sign in front of the most important medical reperfusion therapy, which is thrombolysis,” said principal investigator Jan Purrucker, MD, professor of neurology at Heidelberg University Hospital.
“The main question we have to answer is, is IVT safe in patients with acute ischemic stroke who were pretreated with direct oral anticoagulants or not?”
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022, Lyon, France.
A ‘daily clinical problem’
As many as 20% of patients with atrial fibrillation experience ischemic stroke while receiving DOAC therapy. Reperfusion therapy with intravenous alteplase is considered standard of care for acute ischemic stroke, but current guidelines recommend against the use of IVT for patients who have recently received a DOAC, owing to safety concerns that researchers say are not backed by strong clinical evidence.
A recent study found no significant difference in sICH among patients who received IV alteplase for acute ischemic stroke within 7 days of receiving therapy with non–vitamin K antagonist oral anticoagulants.
“In our daily clinical practice, we face a lot of patients who have received oral anticoagulation, many with atrial fibrillation, but a lot of other indicators as well, and they suffer from ischemic stroke,” Dr. Purrucker said. “They usually are ineligible for medical reperfusion therapy because of quite strict guideline recommendations at the moment. This is a daily clinical problem.”
Dr. Purrucker and colleagues in New Zealand and Switzerland launched an international, observational, multicenter cohort study to examine the issue.
Researchers collected data on patients with ischemic stroke who had last received DOAC therapy 48 hours or less before the event or whose last intake was unknown and who had received IVT. They included 20,448 patients, 830 of whom were receiving DOAC therapy at the time of stroke onset.
Among the DOAC users, 30% received DOAC reversal prior to IVT, 27% had their DOAC level measured, and 42% received IVT without reversal treatment or knowledge of DOAC levels.
Overall, 4.5% of patients developed sICH. Compared with the control group, DOAC users were half as likely to develop sICH (adjusted odds ratio, 0.47; P = .003).
There was no significant difference between groups in independent outcome at 3 months, defined as a Modified Rankin Scale score of 1 to 3 (aOR, 1.21; 95% confidence interval, 0.99-1.49).
This finding held across patient subgroups, including patients for whom selection methods differed and patients with very recent intake of less than 12 hours.
“The question is whether we are so confident in these data that we would change our clinical practice now,” Dr. Purrucker said.
Infrastructure needed
While the findings are promising, more data are needed to strengthen the argument for revising current IVT guidelines, said Ho-Yan Yvonne Chun, PhD, honorary senior clinical lecturer with the Centre for Clinical Brain Sciences at the University of Edinburgh and a consultant in stroke medicine for NHS Lothian and Borders General Hospital, who commented on the findings.
“The study sample are a highly selected group of patients from selected centers that have the infrastructure to offer DOAC level checking and DOAC reversal,” Dr. Chun said. “The selected centers are not representative of the majority of hospitals that offer IVT to stroke patients with acute stroke.”
Most hospitals lack the equipment necessary to test DOAC levels and don’t have immediate access to DOAC reversal agents, Dr. Chun said. In those centers, she added, the administration of IVT could be delayed, which might affect clinical outcomes.
“Infrastructure needs to be in place to ensure timely delivery of IVT to these patients,” Dr. Chun added. “This means that in real-world practice, hospitals need to have right logistical pathway in place in order to provide timely DOAC level checking and DOAC reversal agents.”
Dr. Chun added that “large pragmatic clinical trials, preferably multicentered, are needed to provide the definitive evidence on the safety and effectiveness of using these approaches to select patients with prior DOAC use for IVT.”
But such a study may not be feasible, Dr. Purrucker said. Among the hurdles he noted are the large sample size needed for such a trial, uncertainty regarding funding, and patient selection bias, resulting from the fact that such studies would likely exclude patients eligible for mechanical thrombectomy or those eligible for reversal treatment.
In light of earlier studies, including preclinical data that support the safety of DOACs in IVT, and these new data, Dr. Purrucker said he hopes a change in guidelines might be taken up in the future.
“But it should be good academic practice to first let the results be externally evaluated, for example, during the manuscript submission process,” he said. “But once published, guideline working groups will have to evaluate the recent and new evidence and might reconsider previous recommendations.”
The study received no commercial funding. Dr. Purrucker and Dr. Chun reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new observational study suggests, prompting researchers to ask whether guidelines that restrict its use should be updated.
Researchers found that DOAC users were significantly less likely to develop symptomatic intracerebral hemorrhage (sICH) after IVT, and there was no difference in functional independence at 3 months, compared with patients who received IVT but who did not receive DOAC.
“At the moment, the guidelines really pose a barrier and stop sign in front of the most important medical reperfusion therapy, which is thrombolysis,” said principal investigator Jan Purrucker, MD, professor of neurology at Heidelberg University Hospital.
“The main question we have to answer is, is IVT safe in patients with acute ischemic stroke who were pretreated with direct oral anticoagulants or not?”
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022, Lyon, France.
A ‘daily clinical problem’
As many as 20% of patients with atrial fibrillation experience ischemic stroke while receiving DOAC therapy. Reperfusion therapy with intravenous alteplase is considered standard of care for acute ischemic stroke, but current guidelines recommend against the use of IVT for patients who have recently received a DOAC, owing to safety concerns that researchers say are not backed by strong clinical evidence.
A recent study found no significant difference in sICH among patients who received IV alteplase for acute ischemic stroke within 7 days of receiving therapy with non–vitamin K antagonist oral anticoagulants.
“In our daily clinical practice, we face a lot of patients who have received oral anticoagulation, many with atrial fibrillation, but a lot of other indicators as well, and they suffer from ischemic stroke,” Dr. Purrucker said. “They usually are ineligible for medical reperfusion therapy because of quite strict guideline recommendations at the moment. This is a daily clinical problem.”
Dr. Purrucker and colleagues in New Zealand and Switzerland launched an international, observational, multicenter cohort study to examine the issue.
Researchers collected data on patients with ischemic stroke who had last received DOAC therapy 48 hours or less before the event or whose last intake was unknown and who had received IVT. They included 20,448 patients, 830 of whom were receiving DOAC therapy at the time of stroke onset.
Among the DOAC users, 30% received DOAC reversal prior to IVT, 27% had their DOAC level measured, and 42% received IVT without reversal treatment or knowledge of DOAC levels.
Overall, 4.5% of patients developed sICH. Compared with the control group, DOAC users were half as likely to develop sICH (adjusted odds ratio, 0.47; P = .003).
There was no significant difference between groups in independent outcome at 3 months, defined as a Modified Rankin Scale score of 1 to 3 (aOR, 1.21; 95% confidence interval, 0.99-1.49).
This finding held across patient subgroups, including patients for whom selection methods differed and patients with very recent intake of less than 12 hours.
“The question is whether we are so confident in these data that we would change our clinical practice now,” Dr. Purrucker said.
Infrastructure needed
While the findings are promising, more data are needed to strengthen the argument for revising current IVT guidelines, said Ho-Yan Yvonne Chun, PhD, honorary senior clinical lecturer with the Centre for Clinical Brain Sciences at the University of Edinburgh and a consultant in stroke medicine for NHS Lothian and Borders General Hospital, who commented on the findings.
“The study sample are a highly selected group of patients from selected centers that have the infrastructure to offer DOAC level checking and DOAC reversal,” Dr. Chun said. “The selected centers are not representative of the majority of hospitals that offer IVT to stroke patients with acute stroke.”
Most hospitals lack the equipment necessary to test DOAC levels and don’t have immediate access to DOAC reversal agents, Dr. Chun said. In those centers, she added, the administration of IVT could be delayed, which might affect clinical outcomes.
“Infrastructure needs to be in place to ensure timely delivery of IVT to these patients,” Dr. Chun added. “This means that in real-world practice, hospitals need to have right logistical pathway in place in order to provide timely DOAC level checking and DOAC reversal agents.”
Dr. Chun added that “large pragmatic clinical trials, preferably multicentered, are needed to provide the definitive evidence on the safety and effectiveness of using these approaches to select patients with prior DOAC use for IVT.”
But such a study may not be feasible, Dr. Purrucker said. Among the hurdles he noted are the large sample size needed for such a trial, uncertainty regarding funding, and patient selection bias, resulting from the fact that such studies would likely exclude patients eligible for mechanical thrombectomy or those eligible for reversal treatment.
In light of earlier studies, including preclinical data that support the safety of DOACs in IVT, and these new data, Dr. Purrucker said he hopes a change in guidelines might be taken up in the future.
“But it should be good academic practice to first let the results be externally evaluated, for example, during the manuscript submission process,” he said. “But once published, guideline working groups will have to evaluate the recent and new evidence and might reconsider previous recommendations.”
The study received no commercial funding. Dr. Purrucker and Dr. Chun reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new observational study suggests, prompting researchers to ask whether guidelines that restrict its use should be updated.
Researchers found that DOAC users were significantly less likely to develop symptomatic intracerebral hemorrhage (sICH) after IVT, and there was no difference in functional independence at 3 months, compared with patients who received IVT but who did not receive DOAC.
“At the moment, the guidelines really pose a barrier and stop sign in front of the most important medical reperfusion therapy, which is thrombolysis,” said principal investigator Jan Purrucker, MD, professor of neurology at Heidelberg University Hospital.
“The main question we have to answer is, is IVT safe in patients with acute ischemic stroke who were pretreated with direct oral anticoagulants or not?”
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022, Lyon, France.
A ‘daily clinical problem’
As many as 20% of patients with atrial fibrillation experience ischemic stroke while receiving DOAC therapy. Reperfusion therapy with intravenous alteplase is considered standard of care for acute ischemic stroke, but current guidelines recommend against the use of IVT for patients who have recently received a DOAC, owing to safety concerns that researchers say are not backed by strong clinical evidence.
A recent study found no significant difference in sICH among patients who received IV alteplase for acute ischemic stroke within 7 days of receiving therapy with non–vitamin K antagonist oral anticoagulants.
“In our daily clinical practice, we face a lot of patients who have received oral anticoagulation, many with atrial fibrillation, but a lot of other indicators as well, and they suffer from ischemic stroke,” Dr. Purrucker said. “They usually are ineligible for medical reperfusion therapy because of quite strict guideline recommendations at the moment. This is a daily clinical problem.”
Dr. Purrucker and colleagues in New Zealand and Switzerland launched an international, observational, multicenter cohort study to examine the issue.
Researchers collected data on patients with ischemic stroke who had last received DOAC therapy 48 hours or less before the event or whose last intake was unknown and who had received IVT. They included 20,448 patients, 830 of whom were receiving DOAC therapy at the time of stroke onset.
Among the DOAC users, 30% received DOAC reversal prior to IVT, 27% had their DOAC level measured, and 42% received IVT without reversal treatment or knowledge of DOAC levels.
Overall, 4.5% of patients developed sICH. Compared with the control group, DOAC users were half as likely to develop sICH (adjusted odds ratio, 0.47; P = .003).
There was no significant difference between groups in independent outcome at 3 months, defined as a Modified Rankin Scale score of 1 to 3 (aOR, 1.21; 95% confidence interval, 0.99-1.49).
This finding held across patient subgroups, including patients for whom selection methods differed and patients with very recent intake of less than 12 hours.
“The question is whether we are so confident in these data that we would change our clinical practice now,” Dr. Purrucker said.
Infrastructure needed
While the findings are promising, more data are needed to strengthen the argument for revising current IVT guidelines, said Ho-Yan Yvonne Chun, PhD, honorary senior clinical lecturer with the Centre for Clinical Brain Sciences at the University of Edinburgh and a consultant in stroke medicine for NHS Lothian and Borders General Hospital, who commented on the findings.
“The study sample are a highly selected group of patients from selected centers that have the infrastructure to offer DOAC level checking and DOAC reversal,” Dr. Chun said. “The selected centers are not representative of the majority of hospitals that offer IVT to stroke patients with acute stroke.”
Most hospitals lack the equipment necessary to test DOAC levels and don’t have immediate access to DOAC reversal agents, Dr. Chun said. In those centers, she added, the administration of IVT could be delayed, which might affect clinical outcomes.
“Infrastructure needs to be in place to ensure timely delivery of IVT to these patients,” Dr. Chun added. “This means that in real-world practice, hospitals need to have right logistical pathway in place in order to provide timely DOAC level checking and DOAC reversal agents.”
Dr. Chun added that “large pragmatic clinical trials, preferably multicentered, are needed to provide the definitive evidence on the safety and effectiveness of using these approaches to select patients with prior DOAC use for IVT.”
But such a study may not be feasible, Dr. Purrucker said. Among the hurdles he noted are the large sample size needed for such a trial, uncertainty regarding funding, and patient selection bias, resulting from the fact that such studies would likely exclude patients eligible for mechanical thrombectomy or those eligible for reversal treatment.
In light of earlier studies, including preclinical data that support the safety of DOACs in IVT, and these new data, Dr. Purrucker said he hopes a change in guidelines might be taken up in the future.
“But it should be good academic practice to first let the results be externally evaluated, for example, during the manuscript submission process,” he said. “But once published, guideline working groups will have to evaluate the recent and new evidence and might reconsider previous recommendations.”
The study received no commercial funding. Dr. Purrucker and Dr. Chun reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESOC 2022
PA convicted of distributing more than 1.2 million opioid pills
A federal sting operation led to the recent conviction of a Texas physician assistant on charges of illegally prescribing a total of $3 million in drugs to patients at two “pill mill” clinics in Houston and helping others do the same.
The May 20 conviction of Charles Thompson, 76, of Houston, was based on charges of distributing more than 1.2 million opioid pills to thousands of individuals posing as patients at two pain management clinics, according to the U.S. Department of Justice.
Thompson’s conviction was the latest legal action in a string of cases involving the operation, including a doctor convicted in March who worked with Thompson at the West Parker Medical Clinic. Internist James Pierre, MD, 52, faces charges of unlawfully prescribing more than $1 million worth of opioid hydrocodone, according to federal officials.
Thompson also worked at Priority Wellness Clinic. Six people have pled guilty in connection with their conduct at West Parker or Priority Wellness, the justice department reported.
From June 2015 through July 2016, while Thompson was at West Parker, he helped Dr. Pierre unlawfully prescribe hydrocodone and the muscle relaxant carisoprodol, a combination of controlled substances for pain management known as the “Las Vegas cocktail,” to people in the sting operations pretending to be patients, authorities stated.
Thompson also distributed unlawful prescriptions for carisoprodol. So-called “runners” brought numerous people to pose as patients at West Parker and paid the clinic about $220 to $500 in cash for each visit that resulted in prescriptions for dangerous drugs. Throughout the scheme, West Parker pocketed about $1.75 million from prescriptions; Thompson was paid more than $208,000.
According to authorities, Thompson also helped others illegally prescribe controlled substances, including hydrocodone and oxycodone, from May to July 2017 at Priority Wellness, which opened in December 2016 after West Parker closed.
Priority Wellness reportedly operated as a pill mill similar to West Parker’s. Runners brought people posing as patients to Priority Wellness and paid the clinic between $300 and $600. The cost depended on whether the purported patient received a prescription for hydrocodone or oxycodone, almost always prescribed in combination with carisoprodol, authorities said. Throughout the scheme, Priority Wellness made about $1.1 million, and Thompson made between $700 and $900 a day.
He was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and seven counts of unlawfully distributing and dispensing controlled substances in connection with his conduct at West Parker. For his conduct at Priority Wellness, he was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and one count of unlawfully distributing and dispensing controlled substances.
He faces up to 20 years in prison for each count of conviction with sentencing scheduled for Oct. 3.
A version of this article first appeared on Medscape.com.
A federal sting operation led to the recent conviction of a Texas physician assistant on charges of illegally prescribing a total of $3 million in drugs to patients at two “pill mill” clinics in Houston and helping others do the same.
The May 20 conviction of Charles Thompson, 76, of Houston, was based on charges of distributing more than 1.2 million opioid pills to thousands of individuals posing as patients at two pain management clinics, according to the U.S. Department of Justice.
Thompson’s conviction was the latest legal action in a string of cases involving the operation, including a doctor convicted in March who worked with Thompson at the West Parker Medical Clinic. Internist James Pierre, MD, 52, faces charges of unlawfully prescribing more than $1 million worth of opioid hydrocodone, according to federal officials.
Thompson also worked at Priority Wellness Clinic. Six people have pled guilty in connection with their conduct at West Parker or Priority Wellness, the justice department reported.
From June 2015 through July 2016, while Thompson was at West Parker, he helped Dr. Pierre unlawfully prescribe hydrocodone and the muscle relaxant carisoprodol, a combination of controlled substances for pain management known as the “Las Vegas cocktail,” to people in the sting operations pretending to be patients, authorities stated.
Thompson also distributed unlawful prescriptions for carisoprodol. So-called “runners” brought numerous people to pose as patients at West Parker and paid the clinic about $220 to $500 in cash for each visit that resulted in prescriptions for dangerous drugs. Throughout the scheme, West Parker pocketed about $1.75 million from prescriptions; Thompson was paid more than $208,000.
According to authorities, Thompson also helped others illegally prescribe controlled substances, including hydrocodone and oxycodone, from May to July 2017 at Priority Wellness, which opened in December 2016 after West Parker closed.
Priority Wellness reportedly operated as a pill mill similar to West Parker’s. Runners brought people posing as patients to Priority Wellness and paid the clinic between $300 and $600. The cost depended on whether the purported patient received a prescription for hydrocodone or oxycodone, almost always prescribed in combination with carisoprodol, authorities said. Throughout the scheme, Priority Wellness made about $1.1 million, and Thompson made between $700 and $900 a day.
He was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and seven counts of unlawfully distributing and dispensing controlled substances in connection with his conduct at West Parker. For his conduct at Priority Wellness, he was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and one count of unlawfully distributing and dispensing controlled substances.
He faces up to 20 years in prison for each count of conviction with sentencing scheduled for Oct. 3.
A version of this article first appeared on Medscape.com.
A federal sting operation led to the recent conviction of a Texas physician assistant on charges of illegally prescribing a total of $3 million in drugs to patients at two “pill mill” clinics in Houston and helping others do the same.
The May 20 conviction of Charles Thompson, 76, of Houston, was based on charges of distributing more than 1.2 million opioid pills to thousands of individuals posing as patients at two pain management clinics, according to the U.S. Department of Justice.
Thompson’s conviction was the latest legal action in a string of cases involving the operation, including a doctor convicted in March who worked with Thompson at the West Parker Medical Clinic. Internist James Pierre, MD, 52, faces charges of unlawfully prescribing more than $1 million worth of opioid hydrocodone, according to federal officials.
Thompson also worked at Priority Wellness Clinic. Six people have pled guilty in connection with their conduct at West Parker or Priority Wellness, the justice department reported.
From June 2015 through July 2016, while Thompson was at West Parker, he helped Dr. Pierre unlawfully prescribe hydrocodone and the muscle relaxant carisoprodol, a combination of controlled substances for pain management known as the “Las Vegas cocktail,” to people in the sting operations pretending to be patients, authorities stated.
Thompson also distributed unlawful prescriptions for carisoprodol. So-called “runners” brought numerous people to pose as patients at West Parker and paid the clinic about $220 to $500 in cash for each visit that resulted in prescriptions for dangerous drugs. Throughout the scheme, West Parker pocketed about $1.75 million from prescriptions; Thompson was paid more than $208,000.
According to authorities, Thompson also helped others illegally prescribe controlled substances, including hydrocodone and oxycodone, from May to July 2017 at Priority Wellness, which opened in December 2016 after West Parker closed.
Priority Wellness reportedly operated as a pill mill similar to West Parker’s. Runners brought people posing as patients to Priority Wellness and paid the clinic between $300 and $600. The cost depended on whether the purported patient received a prescription for hydrocodone or oxycodone, almost always prescribed in combination with carisoprodol, authorities said. Throughout the scheme, Priority Wellness made about $1.1 million, and Thompson made between $700 and $900 a day.
He was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and seven counts of unlawfully distributing and dispensing controlled substances in connection with his conduct at West Parker. For his conduct at Priority Wellness, he was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and one count of unlawfully distributing and dispensing controlled substances.
He faces up to 20 years in prison for each count of conviction with sentencing scheduled for Oct. 3.
A version of this article first appeared on Medscape.com.
Phase-3 study: Leukemia patients live longer with ibrutinib
“This trial led to the first-line approval of ibrutinib for CLL patients,” lead author Paul M. Barr, MD, of the University of Rochester (N.Y.), said in an interview. “It is important to follow these patients long-term to understand the expected duration of response/disease control and to monitor for late toxicity,” he said “The data are useful in guiding clinicians who treat CLL and patients being treated with single agent BTK inhibitors,” he noted.
In the initial RESONATE-2, a phase 3, open-label study, 269 adults aged 65 years and older who were previously untreated for CLL or small lymphocytic leukemia were randomized to ibrutinib or the standard of care, chlorambucil. Patients received 420 mg of ibrutinib once daily until disease progression or unacceptable toxicity (136 patients) or up to 12 cycles of 0.5-0.8 mg/kg of chlorambucil (133 patients).
The long-term outcome data were published in Blood Advances.
Overall, at a median of 83 months’ follow-up, progression-free survival was significantly higher for ibrutinib patients than for chlorambucil patients (hazard ratio 0.154).
At 7 years, progression-free survival was 59% in the ibrutinib group vs. 9% in the chlorambucil group.
Notably, progression-free survival benefits with ibrutinib also were higher for patients with high-risk genomic features, identified as del(11q) and unmutated immunoglobulin heavy-chain variable region gene (IGHV).
Complete data were available for 54 patients with del(11q) and 118 with unmutated IGHV. In this subset of patients, progression-free survival rates at 7 years were significantly higher for those treated with ibrutinib vs. chlorambucil who had del(11q) or unmutated IGHV (52% vs. 0% and 58% vs. 2%, respectively).
Approximately 42% of patients with chronic lymphocytic leukemia treated with ibrutinib remained on the therapy at up to 8 years, with a median follow-up of 7.4 years. Overall survival at 7 years was 78% for ibrutinib; overall survival data were not collected for chlorambucil for patients with progressive disease after the median of 5 years, as these patients were eligible to switch to ibrutinib in a long-term extension study or exit the study.
Adverse events prompted reduction of ibrutinib in 30 patients and dose holding for at least 7 days in 79 patients. However, dose modification resolved or improved the adverse events in 85% of the patients with held doses and 90% of those with reduced doses.
The overall prevalence of adverse events was similar to previous follow-up data at 5 years. No new safety signals were observed during the longer study period. The rate of treatment discontinuation because of adverse events was highest in the first year.
“We have been surprised at how long the remissions have lasted with ibrutinib,” said Dr. Barr. “Even with up to 8 years of follow-up, we have yet to reach the median progression free-survival,” he noted.
“These data, in combination with other data sets, highlight the impact that ibrutinib and other BTK inhibitors have had in treating CLL,” said Dr. Barr. “Patients are living longer and avoiding the side effects of chemotherapy in the era of novel agent use,” he said.
However, research gaps remain, Dr. Barr noted. “We need to continue following these patients over time given the length of the remissions. Additionally, we need to continue investigating novel combinations,” he said. Such studies will help us understand the benefit of fixed durations regimens compared to single agent BTK inhibitors,” he emphasized.
Safety and efficacy remain promising
“Ibrutinib was approved for the treatment of CLL, but only in the relapsed setting,” Susan M. O’Brien, MD, of the University of California, Irvine, said in an interview. “This trial was important because it led to the approval of ibrutinib in the front-line setting, making it the first, and at the time, only, small molecule that could be used upfront,” said Dr. O’Brien, who was not involved with the study.
“The initial results were certainly not surprising, as given the efficacy of ibrutinib in the relapsed setting, it seemed likely that it would produce a longer PFS than chlorambucil,” said Dr. O’Brien. “What may not have been expected though, is the incredible durability of these responses with ibrutinib,” she noted.
The clinical implications of the long-term data are that ibrutinib is producing “very durable remissions with continuous therapy,” Dr. O’Brien said. “There are no late safety signals and most side effects diminish with time. However, hypertension and atrial fibrillation continue to occur, so continued monitoring of blood pressure in these patients is important,” she emphasized.
Minor, but annoying, side effects are not infrequent early on with ibrutinib and may present a barrier to use for some patients, Dr. O’Brien said. “Some side effects may be overcome with temporary pauses of drug or dose reduction,” she noted. However, “it is important for patients to be aware that most of these side effects will completely abate with time,” she added.
“The main limitation of this trial was that the comparison was to a rather weak chemotherapy agent, albeit it one frequently used in older patients, particularly in Europe,” said Dr. O’Brien. “Nevertheless, two subsequent trials comparing ibrutinib (with or without rituximab) with either BR [bendamustine/rituximab] or FCR [fludarabine/cyclophosphamide/rituximab] showed a longer PFS with ibrutinib, as compared to that seen with either chemoimmunotherapy regimen,” she said.
The study was supported by Pharmacyclics LLC, an AbbVie company. Dr. Barr collaborated with sponsor AbbVie on the study design, and disclosed relationships with companies including AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, MEI Pharma, Merck, Morphosys, Pharmacyclics LLC (an AbbVie company), Seattle Genetics, and TG Therapeutics. Dr. O’Brien had no relevant financial conflicts to disclose.
“This trial led to the first-line approval of ibrutinib for CLL patients,” lead author Paul M. Barr, MD, of the University of Rochester (N.Y.), said in an interview. “It is important to follow these patients long-term to understand the expected duration of response/disease control and to monitor for late toxicity,” he said “The data are useful in guiding clinicians who treat CLL and patients being treated with single agent BTK inhibitors,” he noted.
In the initial RESONATE-2, a phase 3, open-label study, 269 adults aged 65 years and older who were previously untreated for CLL or small lymphocytic leukemia were randomized to ibrutinib or the standard of care, chlorambucil. Patients received 420 mg of ibrutinib once daily until disease progression or unacceptable toxicity (136 patients) or up to 12 cycles of 0.5-0.8 mg/kg of chlorambucil (133 patients).
The long-term outcome data were published in Blood Advances.
Overall, at a median of 83 months’ follow-up, progression-free survival was significantly higher for ibrutinib patients than for chlorambucil patients (hazard ratio 0.154).
At 7 years, progression-free survival was 59% in the ibrutinib group vs. 9% in the chlorambucil group.
Notably, progression-free survival benefits with ibrutinib also were higher for patients with high-risk genomic features, identified as del(11q) and unmutated immunoglobulin heavy-chain variable region gene (IGHV).
Complete data were available for 54 patients with del(11q) and 118 with unmutated IGHV. In this subset of patients, progression-free survival rates at 7 years were significantly higher for those treated with ibrutinib vs. chlorambucil who had del(11q) or unmutated IGHV (52% vs. 0% and 58% vs. 2%, respectively).
Approximately 42% of patients with chronic lymphocytic leukemia treated with ibrutinib remained on the therapy at up to 8 years, with a median follow-up of 7.4 years. Overall survival at 7 years was 78% for ibrutinib; overall survival data were not collected for chlorambucil for patients with progressive disease after the median of 5 years, as these patients were eligible to switch to ibrutinib in a long-term extension study or exit the study.
Adverse events prompted reduction of ibrutinib in 30 patients and dose holding for at least 7 days in 79 patients. However, dose modification resolved or improved the adverse events in 85% of the patients with held doses and 90% of those with reduced doses.
The overall prevalence of adverse events was similar to previous follow-up data at 5 years. No new safety signals were observed during the longer study period. The rate of treatment discontinuation because of adverse events was highest in the first year.
“We have been surprised at how long the remissions have lasted with ibrutinib,” said Dr. Barr. “Even with up to 8 years of follow-up, we have yet to reach the median progression free-survival,” he noted.
“These data, in combination with other data sets, highlight the impact that ibrutinib and other BTK inhibitors have had in treating CLL,” said Dr. Barr. “Patients are living longer and avoiding the side effects of chemotherapy in the era of novel agent use,” he said.
However, research gaps remain, Dr. Barr noted. “We need to continue following these patients over time given the length of the remissions. Additionally, we need to continue investigating novel combinations,” he said. Such studies will help us understand the benefit of fixed durations regimens compared to single agent BTK inhibitors,” he emphasized.
Safety and efficacy remain promising
“Ibrutinib was approved for the treatment of CLL, but only in the relapsed setting,” Susan M. O’Brien, MD, of the University of California, Irvine, said in an interview. “This trial was important because it led to the approval of ibrutinib in the front-line setting, making it the first, and at the time, only, small molecule that could be used upfront,” said Dr. O’Brien, who was not involved with the study.
“The initial results were certainly not surprising, as given the efficacy of ibrutinib in the relapsed setting, it seemed likely that it would produce a longer PFS than chlorambucil,” said Dr. O’Brien. “What may not have been expected though, is the incredible durability of these responses with ibrutinib,” she noted.
The clinical implications of the long-term data are that ibrutinib is producing “very durable remissions with continuous therapy,” Dr. O’Brien said. “There are no late safety signals and most side effects diminish with time. However, hypertension and atrial fibrillation continue to occur, so continued monitoring of blood pressure in these patients is important,” she emphasized.
Minor, but annoying, side effects are not infrequent early on with ibrutinib and may present a barrier to use for some patients, Dr. O’Brien said. “Some side effects may be overcome with temporary pauses of drug or dose reduction,” she noted. However, “it is important for patients to be aware that most of these side effects will completely abate with time,” she added.
“The main limitation of this trial was that the comparison was to a rather weak chemotherapy agent, albeit it one frequently used in older patients, particularly in Europe,” said Dr. O’Brien. “Nevertheless, two subsequent trials comparing ibrutinib (with or without rituximab) with either BR [bendamustine/rituximab] or FCR [fludarabine/cyclophosphamide/rituximab] showed a longer PFS with ibrutinib, as compared to that seen with either chemoimmunotherapy regimen,” she said.
The study was supported by Pharmacyclics LLC, an AbbVie company. Dr. Barr collaborated with sponsor AbbVie on the study design, and disclosed relationships with companies including AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, MEI Pharma, Merck, Morphosys, Pharmacyclics LLC (an AbbVie company), Seattle Genetics, and TG Therapeutics. Dr. O’Brien had no relevant financial conflicts to disclose.
“This trial led to the first-line approval of ibrutinib for CLL patients,” lead author Paul M. Barr, MD, of the University of Rochester (N.Y.), said in an interview. “It is important to follow these patients long-term to understand the expected duration of response/disease control and to monitor for late toxicity,” he said “The data are useful in guiding clinicians who treat CLL and patients being treated with single agent BTK inhibitors,” he noted.
In the initial RESONATE-2, a phase 3, open-label study, 269 adults aged 65 years and older who were previously untreated for CLL or small lymphocytic leukemia were randomized to ibrutinib or the standard of care, chlorambucil. Patients received 420 mg of ibrutinib once daily until disease progression or unacceptable toxicity (136 patients) or up to 12 cycles of 0.5-0.8 mg/kg of chlorambucil (133 patients).
The long-term outcome data were published in Blood Advances.
Overall, at a median of 83 months’ follow-up, progression-free survival was significantly higher for ibrutinib patients than for chlorambucil patients (hazard ratio 0.154).
At 7 years, progression-free survival was 59% in the ibrutinib group vs. 9% in the chlorambucil group.
Notably, progression-free survival benefits with ibrutinib also were higher for patients with high-risk genomic features, identified as del(11q) and unmutated immunoglobulin heavy-chain variable region gene (IGHV).
Complete data were available for 54 patients with del(11q) and 118 with unmutated IGHV. In this subset of patients, progression-free survival rates at 7 years were significantly higher for those treated with ibrutinib vs. chlorambucil who had del(11q) or unmutated IGHV (52% vs. 0% and 58% vs. 2%, respectively).
Approximately 42% of patients with chronic lymphocytic leukemia treated with ibrutinib remained on the therapy at up to 8 years, with a median follow-up of 7.4 years. Overall survival at 7 years was 78% for ibrutinib; overall survival data were not collected for chlorambucil for patients with progressive disease after the median of 5 years, as these patients were eligible to switch to ibrutinib in a long-term extension study or exit the study.
Adverse events prompted reduction of ibrutinib in 30 patients and dose holding for at least 7 days in 79 patients. However, dose modification resolved or improved the adverse events in 85% of the patients with held doses and 90% of those with reduced doses.
The overall prevalence of adverse events was similar to previous follow-up data at 5 years. No new safety signals were observed during the longer study period. The rate of treatment discontinuation because of adverse events was highest in the first year.
“We have been surprised at how long the remissions have lasted with ibrutinib,” said Dr. Barr. “Even with up to 8 years of follow-up, we have yet to reach the median progression free-survival,” he noted.
“These data, in combination with other data sets, highlight the impact that ibrutinib and other BTK inhibitors have had in treating CLL,” said Dr. Barr. “Patients are living longer and avoiding the side effects of chemotherapy in the era of novel agent use,” he said.
However, research gaps remain, Dr. Barr noted. “We need to continue following these patients over time given the length of the remissions. Additionally, we need to continue investigating novel combinations,” he said. Such studies will help us understand the benefit of fixed durations regimens compared to single agent BTK inhibitors,” he emphasized.
Safety and efficacy remain promising
“Ibrutinib was approved for the treatment of CLL, but only in the relapsed setting,” Susan M. O’Brien, MD, of the University of California, Irvine, said in an interview. “This trial was important because it led to the approval of ibrutinib in the front-line setting, making it the first, and at the time, only, small molecule that could be used upfront,” said Dr. O’Brien, who was not involved with the study.
“The initial results were certainly not surprising, as given the efficacy of ibrutinib in the relapsed setting, it seemed likely that it would produce a longer PFS than chlorambucil,” said Dr. O’Brien. “What may not have been expected though, is the incredible durability of these responses with ibrutinib,” she noted.
The clinical implications of the long-term data are that ibrutinib is producing “very durable remissions with continuous therapy,” Dr. O’Brien said. “There are no late safety signals and most side effects diminish with time. However, hypertension and atrial fibrillation continue to occur, so continued monitoring of blood pressure in these patients is important,” she emphasized.
Minor, but annoying, side effects are not infrequent early on with ibrutinib and may present a barrier to use for some patients, Dr. O’Brien said. “Some side effects may be overcome with temporary pauses of drug or dose reduction,” she noted. However, “it is important for patients to be aware that most of these side effects will completely abate with time,” she added.
“The main limitation of this trial was that the comparison was to a rather weak chemotherapy agent, albeit it one frequently used in older patients, particularly in Europe,” said Dr. O’Brien. “Nevertheless, two subsequent trials comparing ibrutinib (with or without rituximab) with either BR [bendamustine/rituximab] or FCR [fludarabine/cyclophosphamide/rituximab] showed a longer PFS with ibrutinib, as compared to that seen with either chemoimmunotherapy regimen,” she said.
The study was supported by Pharmacyclics LLC, an AbbVie company. Dr. Barr collaborated with sponsor AbbVie on the study design, and disclosed relationships with companies including AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, MEI Pharma, Merck, Morphosys, Pharmacyclics LLC (an AbbVie company), Seattle Genetics, and TG Therapeutics. Dr. O’Brien had no relevant financial conflicts to disclose.
FROM BLOOD ADVANCES
Persistent Lip Swelling
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.
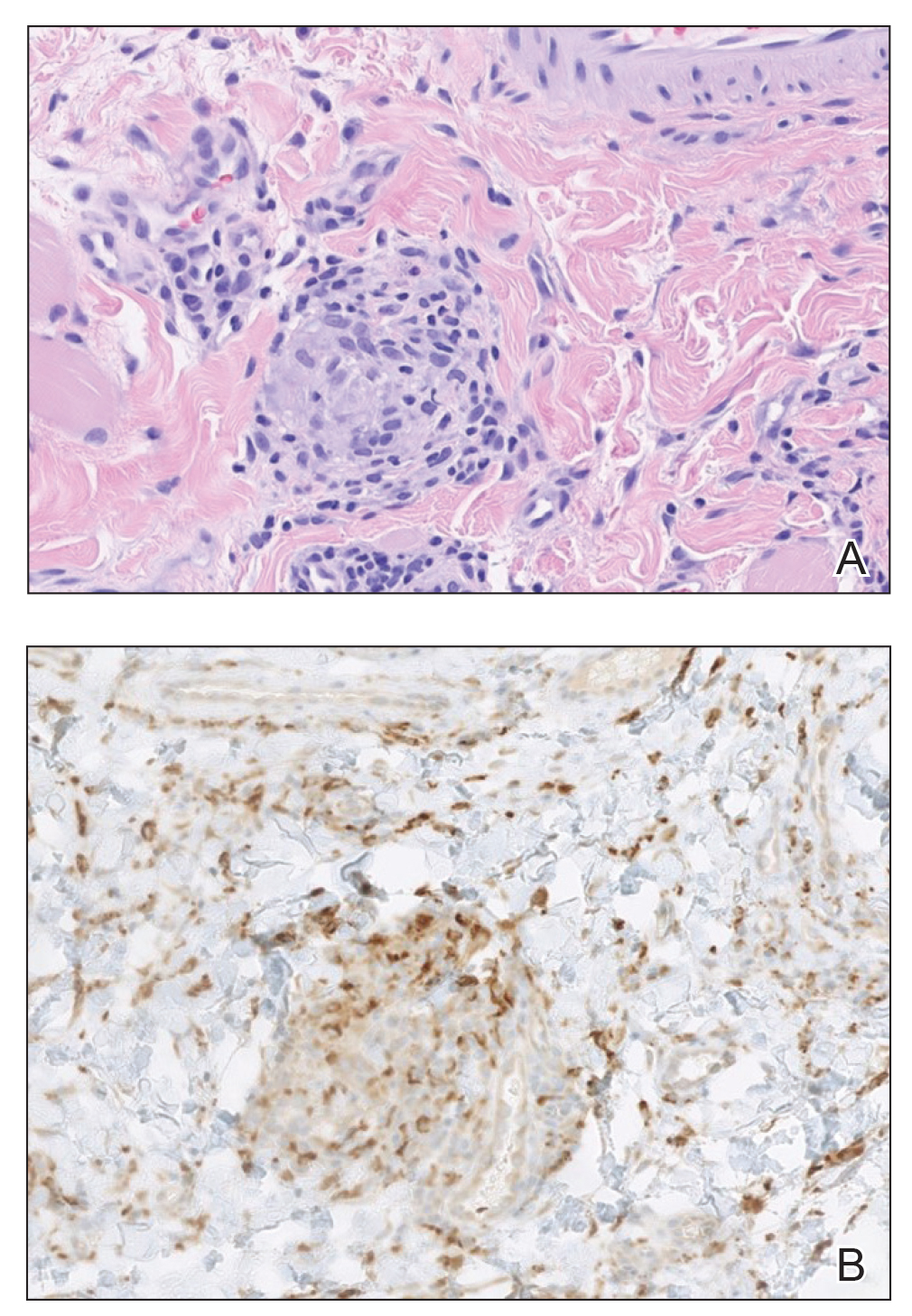
Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
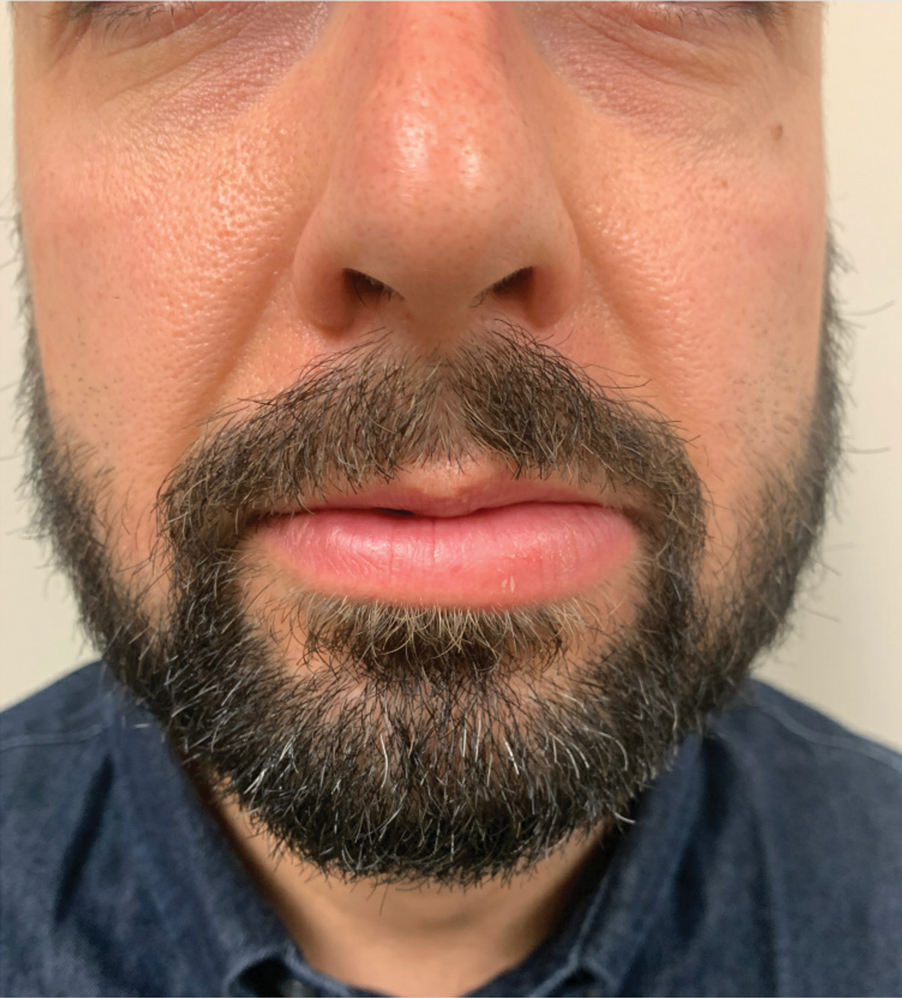
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
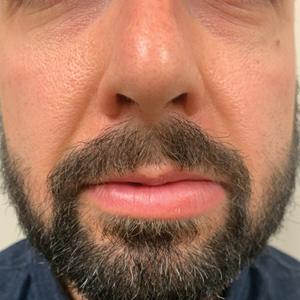
A 36-year-old man with allergic rhinitis presented with lower lip swelling of several months’ duration. The swelling was persistent and predominantly on the left side of the lower lip but occasionally spread to the entire lower lip. The episodes of increased swelling would last for several days and were not associated with any apparent triggers. He denied any pain, pruritus, or dryness. He noted more drooling from the affected side but denied any associated breathing difficulty or throat discomfort. Treatment with an oral antihistamine provided no relief. He denied any recent nonsteroidal anti-inflammatory drug or angiotensinconverting enzyme inhibitor use. His family history was notable for lupus in his maternal grandmother and maternal aunt. He denied any personal or family history of inflammatory bowel disease or recent gastrointestinal tract symptoms. Physical examination revealed nontender edema in the left side of the lower lip with no surface changes. No warmth or erythema were noted. The tongue and the rest of the oral cavity were unremarkable.
FDA approves topical tapinarof for plaque psoriasis
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
Videos may not increase vaccinations in IBD
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
AT DDW 2022
Sex hormones linked to breast cancer in men
The report follows other studies that generally failed to identify a statistically significant association, but the resources of Great Britain’s National Health Service made it possible to achieve greater power than previous efforts.
“It’s a very difficult disease to study because it’s about 100 times rarer than female breast cancer. To do the equivalent study, you either have to make your study 100 times bigger, or think laterally and approach it a different way. (That’s) why we did a case control study where you identify the cases and find controls, rather than wait for men to develop breast cancer,” said Michael Edwin Jones, PhD, the lead author of the study, which was published online in Breast Cancer Research.
The new study found that men who self-reported infertility had a doubled risk of breast cancer, while there was no difference if the fertility was linked to their female partner. Sex hormones are known to play a key role in female breast cancer, and they have a suspected role in male breast cancer as well, though it’s hard to pin down because there is no concentrated source of exposure like hormone therapy or activity from the ovary to cause spiked levels. “It’s more subtle in men, but there’s a reason to think it’s important,” Dr. Jones said.
Although the results hint at a possible role of sex hormones, the research can’t confirm that. Blood draws were taken from participants, but many were conducted after treatment had begun, leading to inconsistent results. Dr. Jones called for more research into biological mechanisms that might explain the increased risk, and suggested that such efforts could lead to a better understanding of breast cancer overall, since the disease in men is not effected by factors like pregnancy and menopause.
Historically, few clinical trials for breast cancer drugs included men, and this has resulted in few approved treatments. However, the impact of breast cancer on men is increasingly being recognized, and most such trials now accept male patients. The Food and Drug Administration has even produced a guidance document for inclusion of men in development of breast cancer drugs, which states that men should be excluded only if there is a clear scientific rationale. When there are too few male participants to draw direct conclusions, it may be possible to extrapolate findings in women to men for FDA approval, provided the mechanism of action suggests that there should be no difference in efficacy.
The Breast Cancer Now study included 1,998 cases and 1,597 controls, who were asked about infertility and whether they had children. Men with male-origin infertility had a higher risk of breast cancer (odds ratio, 2.03; 95% confidence interval [CI], 1.18-3.49), but not men who reported female-origin infertility (OR, 0.86; 95% CI, 0.51-1.45). There was also a heightened risk among men who had not fathered children versus those who had (OR, 1.50; 95% CI, 1.21-1.86).
The association was statistically significant for invasive tumors (OR, 1.96; P = .02), but only a trend was observed for in situ breast cancer (OR, 1.72; P = .39). A possible explanation is that diagnosis of in situ breast cancer is less common than invasive cancer in men, which could have led to the study being underpowered. “Unfortunately, there were too few in-situ breast cancers to allow us to say anything definitive,” said Dr. Jones.
Dr. Jones has no relevant financial disclosures. The study was funded by Breast Cancer Now.
The report follows other studies that generally failed to identify a statistically significant association, but the resources of Great Britain’s National Health Service made it possible to achieve greater power than previous efforts.
“It’s a very difficult disease to study because it’s about 100 times rarer than female breast cancer. To do the equivalent study, you either have to make your study 100 times bigger, or think laterally and approach it a different way. (That’s) why we did a case control study where you identify the cases and find controls, rather than wait for men to develop breast cancer,” said Michael Edwin Jones, PhD, the lead author of the study, which was published online in Breast Cancer Research.
The new study found that men who self-reported infertility had a doubled risk of breast cancer, while there was no difference if the fertility was linked to their female partner. Sex hormones are known to play a key role in female breast cancer, and they have a suspected role in male breast cancer as well, though it’s hard to pin down because there is no concentrated source of exposure like hormone therapy or activity from the ovary to cause spiked levels. “It’s more subtle in men, but there’s a reason to think it’s important,” Dr. Jones said.
Although the results hint at a possible role of sex hormones, the research can’t confirm that. Blood draws were taken from participants, but many were conducted after treatment had begun, leading to inconsistent results. Dr. Jones called for more research into biological mechanisms that might explain the increased risk, and suggested that such efforts could lead to a better understanding of breast cancer overall, since the disease in men is not effected by factors like pregnancy and menopause.
Historically, few clinical trials for breast cancer drugs included men, and this has resulted in few approved treatments. However, the impact of breast cancer on men is increasingly being recognized, and most such trials now accept male patients. The Food and Drug Administration has even produced a guidance document for inclusion of men in development of breast cancer drugs, which states that men should be excluded only if there is a clear scientific rationale. When there are too few male participants to draw direct conclusions, it may be possible to extrapolate findings in women to men for FDA approval, provided the mechanism of action suggests that there should be no difference in efficacy.
The Breast Cancer Now study included 1,998 cases and 1,597 controls, who were asked about infertility and whether they had children. Men with male-origin infertility had a higher risk of breast cancer (odds ratio, 2.03; 95% confidence interval [CI], 1.18-3.49), but not men who reported female-origin infertility (OR, 0.86; 95% CI, 0.51-1.45). There was also a heightened risk among men who had not fathered children versus those who had (OR, 1.50; 95% CI, 1.21-1.86).
The association was statistically significant for invasive tumors (OR, 1.96; P = .02), but only a trend was observed for in situ breast cancer (OR, 1.72; P = .39). A possible explanation is that diagnosis of in situ breast cancer is less common than invasive cancer in men, which could have led to the study being underpowered. “Unfortunately, there were too few in-situ breast cancers to allow us to say anything definitive,” said Dr. Jones.
Dr. Jones has no relevant financial disclosures. The study was funded by Breast Cancer Now.
The report follows other studies that generally failed to identify a statistically significant association, but the resources of Great Britain’s National Health Service made it possible to achieve greater power than previous efforts.
“It’s a very difficult disease to study because it’s about 100 times rarer than female breast cancer. To do the equivalent study, you either have to make your study 100 times bigger, or think laterally and approach it a different way. (That’s) why we did a case control study where you identify the cases and find controls, rather than wait for men to develop breast cancer,” said Michael Edwin Jones, PhD, the lead author of the study, which was published online in Breast Cancer Research.
The new study found that men who self-reported infertility had a doubled risk of breast cancer, while there was no difference if the fertility was linked to their female partner. Sex hormones are known to play a key role in female breast cancer, and they have a suspected role in male breast cancer as well, though it’s hard to pin down because there is no concentrated source of exposure like hormone therapy or activity from the ovary to cause spiked levels. “It’s more subtle in men, but there’s a reason to think it’s important,” Dr. Jones said.
Although the results hint at a possible role of sex hormones, the research can’t confirm that. Blood draws were taken from participants, but many were conducted after treatment had begun, leading to inconsistent results. Dr. Jones called for more research into biological mechanisms that might explain the increased risk, and suggested that such efforts could lead to a better understanding of breast cancer overall, since the disease in men is not effected by factors like pregnancy and menopause.
Historically, few clinical trials for breast cancer drugs included men, and this has resulted in few approved treatments. However, the impact of breast cancer on men is increasingly being recognized, and most such trials now accept male patients. The Food and Drug Administration has even produced a guidance document for inclusion of men in development of breast cancer drugs, which states that men should be excluded only if there is a clear scientific rationale. When there are too few male participants to draw direct conclusions, it may be possible to extrapolate findings in women to men for FDA approval, provided the mechanism of action suggests that there should be no difference in efficacy.
The Breast Cancer Now study included 1,998 cases and 1,597 controls, who were asked about infertility and whether they had children. Men with male-origin infertility had a higher risk of breast cancer (odds ratio, 2.03; 95% confidence interval [CI], 1.18-3.49), but not men who reported female-origin infertility (OR, 0.86; 95% CI, 0.51-1.45). There was also a heightened risk among men who had not fathered children versus those who had (OR, 1.50; 95% CI, 1.21-1.86).
The association was statistically significant for invasive tumors (OR, 1.96; P = .02), but only a trend was observed for in situ breast cancer (OR, 1.72; P = .39). A possible explanation is that diagnosis of in situ breast cancer is less common than invasive cancer in men, which could have led to the study being underpowered. “Unfortunately, there were too few in-situ breast cancers to allow us to say anything definitive,” said Dr. Jones.
Dr. Jones has no relevant financial disclosures. The study was funded by Breast Cancer Now.
FROM BREAST CANCER RESEARCH
Innovative med school curriculum could help curb the opioid epidemic
, new research suggests.
“Our study showed that implementing training for medical students about opioid use disorder and its treatment improves knowledge and understanding of clinical principles and may better prepare students to treat patients with this disorder,” study investigator Kimberly Hu, MD, psychiatry resident, Ohio State University, Columbus, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
The U.S. opioid epidemic claims thousands of lives every year, and there’s evidence it’s getting worse, said Dr. Hu. U.S. data from December 2020 to December 2021 show opioid-related deaths increased by almost 15%.
In 2019, about 70% of the nearly 71,000 drug overdose deaths in the United States involved opioids and now it exceeds 100,000 per year, said Dr. Hu. She noted 80% of heroin users report their addiction started with prescription opioids, data that she described as “pretty staggering.”
Although treatments such as buprenorphine are available for OUD, “insufficient access to medications for opioid use disorder remains a significant barrier for patients,” said Dr. Hu.
“Training the next generation of physicians across all specialties is one way that we can work to improve access to care and improve the health and well-being of our patients.”
The study, which is ongoing, included 405 3rd-year medical students at Ohio State. Researchers provided these students with in-person or virtual (during the pandemic) training in buprenorphine prescribing and in-person clinical experience.
Dr. Hu and her colleagues tested the students before and after the intervention and estimated improvement in knowledge (score 0-23) and approach to clinical management principles (1-5).
The investigators found a statistically significant increase in overall knowledge (from a mean total score of 18.34 to 19.32; P < .001). There was also a statistically significant increase in self-reported understanding of clinical management principles related to screening for and treating OUDs (from a mean of 3.12 to a mean of 4.02; P < .001).
An additional evaluation survey was completed by 162 students at the end of the program. About 83% of these students said they knew how to manage acute pain, 62% felt they knew how to manage chronic pain, and 77% agreed they knew how to screen a patient for OUD.
Dr. Hu noted 3rd-year medical students are a little over halfway through medical school, after which they will go into residency in various specialties. Providing them with this knowledge early on allows them to incorporate it as they continue their training, she said.
“If they are able to screen their patients in any specialty they eventually choose to go into, then they can help link these patients to resources early and make sure there aren’t patients who are slipping through the cracks.”
Worthwhile, important research
Howard Liu, MD, chair of the department of psychiatry at the University of Nebraska Medical Center, Omaha, and incoming chair of the APA’s Council on Communications, applauded the study.
The proposed curriculum, he said, instills confidence in students and teaches important lessons they can apply no matter what field they choose.
Dr. Liu, who moderated a press briefing highlighting the study, noted every state is affected differently by the opioid epidemic, but the shortage of appropriate treatments for OUD is nationwide.
Commenting on the study, addiction specialist Elie G. Aoun, MD, of the division of law, medicine, and psychiatry at Columbia University, New York, said this research is “very worthwhile and important.”
He noted that attitudes about addiction need to change. When he taught medical students about substance use disorders, he was struck by some of their negative beliefs about addiction. For example, considering addicts as “junkies” who are “taking resources away” from what they perceive as more deserving patients.
Addiction has been ignored in medicine for too long, added Dr. Aoun. He noted the requirement for addiction training for psychiatry residents is 2 months while they spend 4 months learning internal medicine. “That makes no sense,” he said.
“And now with the opioid epidemic, we’re faced with the consequences of dismissing addiction for such a long time.”
A lack of understanding about addiction, and the “very limited number” of experienced people treating addictions, has contributed to the “huge problem” experts now face in treating addictions, said Dr. Aoun.
“So you want to approach this problem from as many different angles as you can.”
He praised the study for presenting “a framework to ‘medicalize’ the addiction model” for students. This, he said, will help them build empathy and see those with a substance use disorder as no different from other patients with medical conditions.
A curriculum such as the one presented by Dr. Hu and colleagues may spur more medical students into the addiction field, he said. “It may make them more willing to treat patients with addiction using evidence-based medicine rather than dismissing them.”
The study was supported by the Substance Abuse and Mental Health Services Administration.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our study showed that implementing training for medical students about opioid use disorder and its treatment improves knowledge and understanding of clinical principles and may better prepare students to treat patients with this disorder,” study investigator Kimberly Hu, MD, psychiatry resident, Ohio State University, Columbus, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
The U.S. opioid epidemic claims thousands of lives every year, and there’s evidence it’s getting worse, said Dr. Hu. U.S. data from December 2020 to December 2021 show opioid-related deaths increased by almost 15%.
In 2019, about 70% of the nearly 71,000 drug overdose deaths in the United States involved opioids and now it exceeds 100,000 per year, said Dr. Hu. She noted 80% of heroin users report their addiction started with prescription opioids, data that she described as “pretty staggering.”
Although treatments such as buprenorphine are available for OUD, “insufficient access to medications for opioid use disorder remains a significant barrier for patients,” said Dr. Hu.
“Training the next generation of physicians across all specialties is one way that we can work to improve access to care and improve the health and well-being of our patients.”
The study, which is ongoing, included 405 3rd-year medical students at Ohio State. Researchers provided these students with in-person or virtual (during the pandemic) training in buprenorphine prescribing and in-person clinical experience.
Dr. Hu and her colleagues tested the students before and after the intervention and estimated improvement in knowledge (score 0-23) and approach to clinical management principles (1-5).
The investigators found a statistically significant increase in overall knowledge (from a mean total score of 18.34 to 19.32; P < .001). There was also a statistically significant increase in self-reported understanding of clinical management principles related to screening for and treating OUDs (from a mean of 3.12 to a mean of 4.02; P < .001).
An additional evaluation survey was completed by 162 students at the end of the program. About 83% of these students said they knew how to manage acute pain, 62% felt they knew how to manage chronic pain, and 77% agreed they knew how to screen a patient for OUD.
Dr. Hu noted 3rd-year medical students are a little over halfway through medical school, after which they will go into residency in various specialties. Providing them with this knowledge early on allows them to incorporate it as they continue their training, she said.
“If they are able to screen their patients in any specialty they eventually choose to go into, then they can help link these patients to resources early and make sure there aren’t patients who are slipping through the cracks.”
Worthwhile, important research
Howard Liu, MD, chair of the department of psychiatry at the University of Nebraska Medical Center, Omaha, and incoming chair of the APA’s Council on Communications, applauded the study.
The proposed curriculum, he said, instills confidence in students and teaches important lessons they can apply no matter what field they choose.
Dr. Liu, who moderated a press briefing highlighting the study, noted every state is affected differently by the opioid epidemic, but the shortage of appropriate treatments for OUD is nationwide.
Commenting on the study, addiction specialist Elie G. Aoun, MD, of the division of law, medicine, and psychiatry at Columbia University, New York, said this research is “very worthwhile and important.”
He noted that attitudes about addiction need to change. When he taught medical students about substance use disorders, he was struck by some of their negative beliefs about addiction. For example, considering addicts as “junkies” who are “taking resources away” from what they perceive as more deserving patients.
Addiction has been ignored in medicine for too long, added Dr. Aoun. He noted the requirement for addiction training for psychiatry residents is 2 months while they spend 4 months learning internal medicine. “That makes no sense,” he said.
“And now with the opioid epidemic, we’re faced with the consequences of dismissing addiction for such a long time.”
A lack of understanding about addiction, and the “very limited number” of experienced people treating addictions, has contributed to the “huge problem” experts now face in treating addictions, said Dr. Aoun.
“So you want to approach this problem from as many different angles as you can.”
He praised the study for presenting “a framework to ‘medicalize’ the addiction model” for students. This, he said, will help them build empathy and see those with a substance use disorder as no different from other patients with medical conditions.
A curriculum such as the one presented by Dr. Hu and colleagues may spur more medical students into the addiction field, he said. “It may make them more willing to treat patients with addiction using evidence-based medicine rather than dismissing them.”
The study was supported by the Substance Abuse and Mental Health Services Administration.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our study showed that implementing training for medical students about opioid use disorder and its treatment improves knowledge and understanding of clinical principles and may better prepare students to treat patients with this disorder,” study investigator Kimberly Hu, MD, psychiatry resident, Ohio State University, Columbus, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
The U.S. opioid epidemic claims thousands of lives every year, and there’s evidence it’s getting worse, said Dr. Hu. U.S. data from December 2020 to December 2021 show opioid-related deaths increased by almost 15%.
In 2019, about 70% of the nearly 71,000 drug overdose deaths in the United States involved opioids and now it exceeds 100,000 per year, said Dr. Hu. She noted 80% of heroin users report their addiction started with prescription opioids, data that she described as “pretty staggering.”
Although treatments such as buprenorphine are available for OUD, “insufficient access to medications for opioid use disorder remains a significant barrier for patients,” said Dr. Hu.
“Training the next generation of physicians across all specialties is one way that we can work to improve access to care and improve the health and well-being of our patients.”
The study, which is ongoing, included 405 3rd-year medical students at Ohio State. Researchers provided these students with in-person or virtual (during the pandemic) training in buprenorphine prescribing and in-person clinical experience.
Dr. Hu and her colleagues tested the students before and after the intervention and estimated improvement in knowledge (score 0-23) and approach to clinical management principles (1-5).
The investigators found a statistically significant increase in overall knowledge (from a mean total score of 18.34 to 19.32; P < .001). There was also a statistically significant increase in self-reported understanding of clinical management principles related to screening for and treating OUDs (from a mean of 3.12 to a mean of 4.02; P < .001).
An additional evaluation survey was completed by 162 students at the end of the program. About 83% of these students said they knew how to manage acute pain, 62% felt they knew how to manage chronic pain, and 77% agreed they knew how to screen a patient for OUD.
Dr. Hu noted 3rd-year medical students are a little over halfway through medical school, after which they will go into residency in various specialties. Providing them with this knowledge early on allows them to incorporate it as they continue their training, she said.
“If they are able to screen their patients in any specialty they eventually choose to go into, then they can help link these patients to resources early and make sure there aren’t patients who are slipping through the cracks.”
Worthwhile, important research
Howard Liu, MD, chair of the department of psychiatry at the University of Nebraska Medical Center, Omaha, and incoming chair of the APA’s Council on Communications, applauded the study.
The proposed curriculum, he said, instills confidence in students and teaches important lessons they can apply no matter what field they choose.
Dr. Liu, who moderated a press briefing highlighting the study, noted every state is affected differently by the opioid epidemic, but the shortage of appropriate treatments for OUD is nationwide.
Commenting on the study, addiction specialist Elie G. Aoun, MD, of the division of law, medicine, and psychiatry at Columbia University, New York, said this research is “very worthwhile and important.”
He noted that attitudes about addiction need to change. When he taught medical students about substance use disorders, he was struck by some of their negative beliefs about addiction. For example, considering addicts as “junkies” who are “taking resources away” from what they perceive as more deserving patients.
Addiction has been ignored in medicine for too long, added Dr. Aoun. He noted the requirement for addiction training for psychiatry residents is 2 months while they spend 4 months learning internal medicine. “That makes no sense,” he said.
“And now with the opioid epidemic, we’re faced with the consequences of dismissing addiction for such a long time.”
A lack of understanding about addiction, and the “very limited number” of experienced people treating addictions, has contributed to the “huge problem” experts now face in treating addictions, said Dr. Aoun.
“So you want to approach this problem from as many different angles as you can.”
He praised the study for presenting “a framework to ‘medicalize’ the addiction model” for students. This, he said, will help them build empathy and see those with a substance use disorder as no different from other patients with medical conditions.
A curriculum such as the one presented by Dr. Hu and colleagues may spur more medical students into the addiction field, he said. “It may make them more willing to treat patients with addiction using evidence-based medicine rather than dismissing them.”
The study was supported by the Substance Abuse and Mental Health Services Administration.
A version of this article first appeared on Medscape.com.
FROM APA 2022
New tool may identify pregnant women with eating disorders
A newly developed screening tool may help clinicians identify pregnant women with eating disorders.
The 12-question instrument is intended to be a quick way to help clinicians identify women who may need to be referred to a mental health expert for further evaluation, according to the researchers, who reported on the instrument in a study published in Archives of Women’s Mental Health.
“It would be most appropriate for clinical encounters so that women can get screened and referred,” said Elizabeth Claydon, MD, assistant professor in the department of social and behavioral sciences at West Virginia University’s School of Public Health, Morgantown, who led the study. “If you miss it, they may carry on their eating disorder throughout their pregnancy.”
Pregnant women who have an eating disorder are at increased risk for gestational diabetes, premature birth, labor complications, difficulties nursing, and postpartum depression, according to the National Eating Disorders Association. Their babies are at increased risk for premature birth, low birth weight, and poor development. However, clinicians have not had an accurate way of screening pregnant women who may have an eating disorder.
The American College of Obstetricians and Gynecologists offered its first clinical guidelines for managing anorexia in pregnancy in April 2022. The group’s recommendations include regular monitoring of cardiac and liver function, blood pressure, and heart rate, as well as tests to monitor iron, sodium, potassium, bone density, and blood sugar levels. Anorexia, bulimia, binge eating, and subthreshold disorders – also known as other specified feeding or eating disorders – are among the most common eating disorders among pregnant women.
There are no recent data on the incidence or prevalence of eating disorders among pregnant women, according to Lauren Smolar, vice president of mission and education at the National Eating Disorders Association.
“It’s hard to capture the number of pregnant women affected, since it so often goes undetected,” Ms. Smolar said.
Existing screening tools for eating disorders ask patients whether they’re currently pregnant; a questionnaire specifically tailored to pregnant women may help to better gather data on the prevalence within this group, Ms. Smolar said.
For the new study, Dr. Claydon and her colleagues tested the questionnaire among more than 400 mostly White women aged 25-34 years. They found that it could reliably identify women who may have an eating disorder. The questionnaire was validated for women to take during any trimester, according to the findings.
A score of 39 or above would serve as an indicator for follow-up. Women who score at least 39 were up to 16 times more likely to receive a diagnosis of an eating disorder, compared with women who scored less, the researchers found.
Eating disorders often escape the eye
Researchers developed the tool to screen all women, rather than just patients who present with recognizable symptoms, according to Dr. Claydon.
“Some people may relapse during pregnancy, some may develop [a disorder] while pregnant,” she said. “This makes sure there are no assumptions, because sometimes you can’t tell someone has an eating disorder just by looking at them.”
The researchers also worked to eliminate stigmatizing language to reduce the possibility of women withholding information about their symptoms.
The tool was developed following a qualitative study by Dr. Claydon and her colleagues that was published in 2018. In that study, the researchers analyzed self-perceptions and self-reported experiences of women going through pregnancy with an eating disorder.
“I heard a lot about how difficult it was to disclose eating disorders during pregnancy,” Dr. Claydon said. “It’s wonderful to do something applied to these findings. It’s very meaningful and personal work to me.”
Dr. Claydon said she and her colleagues now plan to test the tool by introducing it into clinics in West Virginia.
The Ophelia Fund/Rhode Island Foundation supported the creation of the tool and dissemination of the tool to clinicians. Research reported in the study was supported by the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
A newly developed screening tool may help clinicians identify pregnant women with eating disorders.
The 12-question instrument is intended to be a quick way to help clinicians identify women who may need to be referred to a mental health expert for further evaluation, according to the researchers, who reported on the instrument in a study published in Archives of Women’s Mental Health.
“It would be most appropriate for clinical encounters so that women can get screened and referred,” said Elizabeth Claydon, MD, assistant professor in the department of social and behavioral sciences at West Virginia University’s School of Public Health, Morgantown, who led the study. “If you miss it, they may carry on their eating disorder throughout their pregnancy.”
Pregnant women who have an eating disorder are at increased risk for gestational diabetes, premature birth, labor complications, difficulties nursing, and postpartum depression, according to the National Eating Disorders Association. Their babies are at increased risk for premature birth, low birth weight, and poor development. However, clinicians have not had an accurate way of screening pregnant women who may have an eating disorder.
The American College of Obstetricians and Gynecologists offered its first clinical guidelines for managing anorexia in pregnancy in April 2022. The group’s recommendations include regular monitoring of cardiac and liver function, blood pressure, and heart rate, as well as tests to monitor iron, sodium, potassium, bone density, and blood sugar levels. Anorexia, bulimia, binge eating, and subthreshold disorders – also known as other specified feeding or eating disorders – are among the most common eating disorders among pregnant women.
There are no recent data on the incidence or prevalence of eating disorders among pregnant women, according to Lauren Smolar, vice president of mission and education at the National Eating Disorders Association.
“It’s hard to capture the number of pregnant women affected, since it so often goes undetected,” Ms. Smolar said.
Existing screening tools for eating disorders ask patients whether they’re currently pregnant; a questionnaire specifically tailored to pregnant women may help to better gather data on the prevalence within this group, Ms. Smolar said.
For the new study, Dr. Claydon and her colleagues tested the questionnaire among more than 400 mostly White women aged 25-34 years. They found that it could reliably identify women who may have an eating disorder. The questionnaire was validated for women to take during any trimester, according to the findings.
A score of 39 or above would serve as an indicator for follow-up. Women who score at least 39 were up to 16 times more likely to receive a diagnosis of an eating disorder, compared with women who scored less, the researchers found.
Eating disorders often escape the eye
Researchers developed the tool to screen all women, rather than just patients who present with recognizable symptoms, according to Dr. Claydon.
“Some people may relapse during pregnancy, some may develop [a disorder] while pregnant,” she said. “This makes sure there are no assumptions, because sometimes you can’t tell someone has an eating disorder just by looking at them.”
The researchers also worked to eliminate stigmatizing language to reduce the possibility of women withholding information about their symptoms.
The tool was developed following a qualitative study by Dr. Claydon and her colleagues that was published in 2018. In that study, the researchers analyzed self-perceptions and self-reported experiences of women going through pregnancy with an eating disorder.
“I heard a lot about how difficult it was to disclose eating disorders during pregnancy,” Dr. Claydon said. “It’s wonderful to do something applied to these findings. It’s very meaningful and personal work to me.”
Dr. Claydon said she and her colleagues now plan to test the tool by introducing it into clinics in West Virginia.
The Ophelia Fund/Rhode Island Foundation supported the creation of the tool and dissemination of the tool to clinicians. Research reported in the study was supported by the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
A newly developed screening tool may help clinicians identify pregnant women with eating disorders.
The 12-question instrument is intended to be a quick way to help clinicians identify women who may need to be referred to a mental health expert for further evaluation, according to the researchers, who reported on the instrument in a study published in Archives of Women’s Mental Health.
“It would be most appropriate for clinical encounters so that women can get screened and referred,” said Elizabeth Claydon, MD, assistant professor in the department of social and behavioral sciences at West Virginia University’s School of Public Health, Morgantown, who led the study. “If you miss it, they may carry on their eating disorder throughout their pregnancy.”
Pregnant women who have an eating disorder are at increased risk for gestational diabetes, premature birth, labor complications, difficulties nursing, and postpartum depression, according to the National Eating Disorders Association. Their babies are at increased risk for premature birth, low birth weight, and poor development. However, clinicians have not had an accurate way of screening pregnant women who may have an eating disorder.
The American College of Obstetricians and Gynecologists offered its first clinical guidelines for managing anorexia in pregnancy in April 2022. The group’s recommendations include regular monitoring of cardiac and liver function, blood pressure, and heart rate, as well as tests to monitor iron, sodium, potassium, bone density, and blood sugar levels. Anorexia, bulimia, binge eating, and subthreshold disorders – also known as other specified feeding or eating disorders – are among the most common eating disorders among pregnant women.
There are no recent data on the incidence or prevalence of eating disorders among pregnant women, according to Lauren Smolar, vice president of mission and education at the National Eating Disorders Association.
“It’s hard to capture the number of pregnant women affected, since it so often goes undetected,” Ms. Smolar said.
Existing screening tools for eating disorders ask patients whether they’re currently pregnant; a questionnaire specifically tailored to pregnant women may help to better gather data on the prevalence within this group, Ms. Smolar said.
For the new study, Dr. Claydon and her colleagues tested the questionnaire among more than 400 mostly White women aged 25-34 years. They found that it could reliably identify women who may have an eating disorder. The questionnaire was validated for women to take during any trimester, according to the findings.
A score of 39 or above would serve as an indicator for follow-up. Women who score at least 39 were up to 16 times more likely to receive a diagnosis of an eating disorder, compared with women who scored less, the researchers found.
Eating disorders often escape the eye
Researchers developed the tool to screen all women, rather than just patients who present with recognizable symptoms, according to Dr. Claydon.
“Some people may relapse during pregnancy, some may develop [a disorder] while pregnant,” she said. “This makes sure there are no assumptions, because sometimes you can’t tell someone has an eating disorder just by looking at them.”
The researchers also worked to eliminate stigmatizing language to reduce the possibility of women withholding information about their symptoms.
The tool was developed following a qualitative study by Dr. Claydon and her colleagues that was published in 2018. In that study, the researchers analyzed self-perceptions and self-reported experiences of women going through pregnancy with an eating disorder.
“I heard a lot about how difficult it was to disclose eating disorders during pregnancy,” Dr. Claydon said. “It’s wonderful to do something applied to these findings. It’s very meaningful and personal work to me.”
Dr. Claydon said she and her colleagues now plan to test the tool by introducing it into clinics in West Virginia.
The Ophelia Fund/Rhode Island Foundation supported the creation of the tool and dissemination of the tool to clinicians. Research reported in the study was supported by the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Urinary incontinence in transfeminine patients
Whether your patient is a cisgender female or a transfeminine patient, urinary incontinence is unfortunately common and can have a significant negative effect on a person’s quality of life. While the incidence of incontinence is relatively well established in the cisgender population, these statistics remain elusive among transfeminine individuals. Many studies today currently examine cosmetic results, sexual function, and major complications rates, and are now starting to explore the long-term effects of these surgeries on the urinary tract.1
As gender-affirming surgery increases in prevalence, our knowledge regarding long-term outcomes impacting quality of life needs to subsequently improve. A few small studies have examined the rates of incontinence and urinary dysfunction among transfeminine patients. In one study, changes in voiding were reported in 32% of patients, with 19% reporting worse voiding and 19% reporting some degree of incontinence.2 A small series of 52 transgender female patients found rates of urinary urgency to be 24.6% and stress incontinence 23%.1,3 Another study of only 18 patients demonstrated a significant rate of incontinence at 33%, which was due to stress urinary incontinence and overactive bladder.1,4 Other studies noted postvoid dribbling to be as high as 79%.1,2
Obtaining a thorough history is essential in evaluating patients with incontinence. Compared with cisgender females, risk factors for urinary incontinence in cisgender males are naturally different. For example, increasing age, parity, vaginal delivery, history of hysterectomy, and obesity are some risk factors for incontinence in cisgender women.1,6 However, in men, overall rates are lower and tend to be associated with factors such as a history of stroke, diabetes, and injury to the urethral sphincter – which can occur in radical prostatectomy.1
In addition to asking standard questions, such as caffeine use, beverage consumption, medication changes, physical activity, etc., the relationship of a patient’s symptoms to her vaginoplasty is crucial. Providers should elucidate whether patients experienced urinary symptoms prior to surgery, note the type of vaginoplasty performed, and determine if any temporal relationship exists with dilation or intercourse.
Communicating with the original surgeon and obtaining operative reports is often necessary to understand the flaps utilized and the current anatomic structures that were altered during surgery. Creation of the neovagina involves dissection through the levator ani, which can lead to neurologic injury and subsequently predispose patients to incontinence. The surgeon must be meticulous in their creation of the neovaginal space, particularly between the rectum and the prostatic urethra. As the dissection continues in a cephalad direction to the peritoneal reflection, the bladder can also suffer an iatrogenic injury.
In cases of the penile inversion vaginoplasty, a skin graft is typically used to line the neovaginal canal. If this graft fails to take appropriately it can prolapse and can contribute to urinary incontinence symptoms. Some surgeons will suspend the apical portion of the neovagina; however, the effect on rates of incontinence is mixed.
The physical exam of a transfeminine patient should consist of a general health assessment, neurological, abdominal, and genitourinary examinations. Palpation of the prostate is performed through the neovaginal canal if patent. During the urinary exam, the provider should make note of stenosis at the urethral meatus or urethral hypermobility. For patients reporting symptoms of stress incontinence, a cough stress test is useful. The neovagina should be carefully examined for fistula formation or any other structural abnormality.
Testing for urinary incontinence is similar to the evaluation in cisgender females in that every patient should undergo a urinalysis and a postvoid residual volume measurement, and should maintain a voiding diary. Indications for urodynamic testing are the same for transfeminine women and cisgender women – symptoms do not correlate with objective findings, failure to improve with treatment, prior incontinence from pelvic floor surgery, difficult diagnostic evaluation with unclear diagnosis.5,6 Cystoscopy is useful for patients experiencing hematuria, before anti-incontinence surgery, or prior to transurethral prostate intervention.1
Treatment is tailored to the type of incontinence diagnosed; however, there are no specific guidelines that are evidence-based for transfeminine patients after vaginoplasty. The therapies available are extrapolated from the general patient population. All patients can benefit from dietary modifications such as avoiding bladder irritants, monitoring fluid and caffeine intake, timed voiding, and pelvic floor exercises.1,6 If patients do not experience improvement from conservative measures, the mainstay treatment for overactive bladder is antimuscarinic agents. However, in cisgender male patients who have a prostate, these agents can lead to urinary retention related to bladder outlet obstruction, although the rates of urinary retention are low.1 Overall, these agents are relatively safe and effective in cisgender men with a prostate and by extension should be utilized in transfeminine patients when indicated.
In patients diagnosed with stress urinary incontinence, conservative options with weight loss, smoking cessation, and pelvic floor exercises should be attempted. If these measures fail in cisgender women, surgical treatment is often recommended. However, surgical treatment in transfeminine patients is significantly less straightforward and beyond the scope of this article.
Obstetrician/gynecologists are familiar with assessing and treating cisgender female patients reporting incontinence and should use this same knowledge for diagnosing and treating transfeminine patients. In addition, providers should be aware of complications of these procedures in evaluating patients presenting for symptoms of incontinence, as these complications directly contribute to incontinence in this patient population.1
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. Email her at [email protected] .
References
1. Ginzburg N. Care of transgender patients: Incontinence. In: Nikolavsky D, Blakley SA, eds. Urological Care for the Transgender Patient: A Comprehensive Guide. Syracuse, NY: Springer, 2021:203-17.
2. Hoebeke P et al. Eur Urol. 2005;47(3):398-402.
3. Kuhn A et al. Fertil Steril. 2011;95(7):2379-82.
4. Kuhn A et al. Eur J Obstet Gynecol Reprod Biol. 2007;131(2):226-30.
5. Winters JC et al. J Urol. 2012;188(6s):2464-72.
6. Practice Bulletin No. 155. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;126:e66-81.
Whether your patient is a cisgender female or a transfeminine patient, urinary incontinence is unfortunately common and can have a significant negative effect on a person’s quality of life. While the incidence of incontinence is relatively well established in the cisgender population, these statistics remain elusive among transfeminine individuals. Many studies today currently examine cosmetic results, sexual function, and major complications rates, and are now starting to explore the long-term effects of these surgeries on the urinary tract.1
As gender-affirming surgery increases in prevalence, our knowledge regarding long-term outcomes impacting quality of life needs to subsequently improve. A few small studies have examined the rates of incontinence and urinary dysfunction among transfeminine patients. In one study, changes in voiding were reported in 32% of patients, with 19% reporting worse voiding and 19% reporting some degree of incontinence.2 A small series of 52 transgender female patients found rates of urinary urgency to be 24.6% and stress incontinence 23%.1,3 Another study of only 18 patients demonstrated a significant rate of incontinence at 33%, which was due to stress urinary incontinence and overactive bladder.1,4 Other studies noted postvoid dribbling to be as high as 79%.1,2
Obtaining a thorough history is essential in evaluating patients with incontinence. Compared with cisgender females, risk factors for urinary incontinence in cisgender males are naturally different. For example, increasing age, parity, vaginal delivery, history of hysterectomy, and obesity are some risk factors for incontinence in cisgender women.1,6 However, in men, overall rates are lower and tend to be associated with factors such as a history of stroke, diabetes, and injury to the urethral sphincter – which can occur in radical prostatectomy.1
In addition to asking standard questions, such as caffeine use, beverage consumption, medication changes, physical activity, etc., the relationship of a patient’s symptoms to her vaginoplasty is crucial. Providers should elucidate whether patients experienced urinary symptoms prior to surgery, note the type of vaginoplasty performed, and determine if any temporal relationship exists with dilation or intercourse.
Communicating with the original surgeon and obtaining operative reports is often necessary to understand the flaps utilized and the current anatomic structures that were altered during surgery. Creation of the neovagina involves dissection through the levator ani, which can lead to neurologic injury and subsequently predispose patients to incontinence. The surgeon must be meticulous in their creation of the neovaginal space, particularly between the rectum and the prostatic urethra. As the dissection continues in a cephalad direction to the peritoneal reflection, the bladder can also suffer an iatrogenic injury.
In cases of the penile inversion vaginoplasty, a skin graft is typically used to line the neovaginal canal. If this graft fails to take appropriately it can prolapse and can contribute to urinary incontinence symptoms. Some surgeons will suspend the apical portion of the neovagina; however, the effect on rates of incontinence is mixed.
The physical exam of a transfeminine patient should consist of a general health assessment, neurological, abdominal, and genitourinary examinations. Palpation of the prostate is performed through the neovaginal canal if patent. During the urinary exam, the provider should make note of stenosis at the urethral meatus or urethral hypermobility. For patients reporting symptoms of stress incontinence, a cough stress test is useful. The neovagina should be carefully examined for fistula formation or any other structural abnormality.
Testing for urinary incontinence is similar to the evaluation in cisgender females in that every patient should undergo a urinalysis and a postvoid residual volume measurement, and should maintain a voiding diary. Indications for urodynamic testing are the same for transfeminine women and cisgender women – symptoms do not correlate with objective findings, failure to improve with treatment, prior incontinence from pelvic floor surgery, difficult diagnostic evaluation with unclear diagnosis.5,6 Cystoscopy is useful for patients experiencing hematuria, before anti-incontinence surgery, or prior to transurethral prostate intervention.1
Treatment is tailored to the type of incontinence diagnosed; however, there are no specific guidelines that are evidence-based for transfeminine patients after vaginoplasty. The therapies available are extrapolated from the general patient population. All patients can benefit from dietary modifications such as avoiding bladder irritants, monitoring fluid and caffeine intake, timed voiding, and pelvic floor exercises.1,6 If patients do not experience improvement from conservative measures, the mainstay treatment for overactive bladder is antimuscarinic agents. However, in cisgender male patients who have a prostate, these agents can lead to urinary retention related to bladder outlet obstruction, although the rates of urinary retention are low.1 Overall, these agents are relatively safe and effective in cisgender men with a prostate and by extension should be utilized in transfeminine patients when indicated.
In patients diagnosed with stress urinary incontinence, conservative options with weight loss, smoking cessation, and pelvic floor exercises should be attempted. If these measures fail in cisgender women, surgical treatment is often recommended. However, surgical treatment in transfeminine patients is significantly less straightforward and beyond the scope of this article.
Obstetrician/gynecologists are familiar with assessing and treating cisgender female patients reporting incontinence and should use this same knowledge for diagnosing and treating transfeminine patients. In addition, providers should be aware of complications of these procedures in evaluating patients presenting for symptoms of incontinence, as these complications directly contribute to incontinence in this patient population.1
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. Email her at [email protected] .
References
1. Ginzburg N. Care of transgender patients: Incontinence. In: Nikolavsky D, Blakley SA, eds. Urological Care for the Transgender Patient: A Comprehensive Guide. Syracuse, NY: Springer, 2021:203-17.
2. Hoebeke P et al. Eur Urol. 2005;47(3):398-402.
3. Kuhn A et al. Fertil Steril. 2011;95(7):2379-82.
4. Kuhn A et al. Eur J Obstet Gynecol Reprod Biol. 2007;131(2):226-30.
5. Winters JC et al. J Urol. 2012;188(6s):2464-72.
6. Practice Bulletin No. 155. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;126:e66-81.
Whether your patient is a cisgender female or a transfeminine patient, urinary incontinence is unfortunately common and can have a significant negative effect on a person’s quality of life. While the incidence of incontinence is relatively well established in the cisgender population, these statistics remain elusive among transfeminine individuals. Many studies today currently examine cosmetic results, sexual function, and major complications rates, and are now starting to explore the long-term effects of these surgeries on the urinary tract.1
As gender-affirming surgery increases in prevalence, our knowledge regarding long-term outcomes impacting quality of life needs to subsequently improve. A few small studies have examined the rates of incontinence and urinary dysfunction among transfeminine patients. In one study, changes in voiding were reported in 32% of patients, with 19% reporting worse voiding and 19% reporting some degree of incontinence.2 A small series of 52 transgender female patients found rates of urinary urgency to be 24.6% and stress incontinence 23%.1,3 Another study of only 18 patients demonstrated a significant rate of incontinence at 33%, which was due to stress urinary incontinence and overactive bladder.1,4 Other studies noted postvoid dribbling to be as high as 79%.1,2
Obtaining a thorough history is essential in evaluating patients with incontinence. Compared with cisgender females, risk factors for urinary incontinence in cisgender males are naturally different. For example, increasing age, parity, vaginal delivery, history of hysterectomy, and obesity are some risk factors for incontinence in cisgender women.1,6 However, in men, overall rates are lower and tend to be associated with factors such as a history of stroke, diabetes, and injury to the urethral sphincter – which can occur in radical prostatectomy.1
In addition to asking standard questions, such as caffeine use, beverage consumption, medication changes, physical activity, etc., the relationship of a patient’s symptoms to her vaginoplasty is crucial. Providers should elucidate whether patients experienced urinary symptoms prior to surgery, note the type of vaginoplasty performed, and determine if any temporal relationship exists with dilation or intercourse.
Communicating with the original surgeon and obtaining operative reports is often necessary to understand the flaps utilized and the current anatomic structures that were altered during surgery. Creation of the neovagina involves dissection through the levator ani, which can lead to neurologic injury and subsequently predispose patients to incontinence. The surgeon must be meticulous in their creation of the neovaginal space, particularly between the rectum and the prostatic urethra. As the dissection continues in a cephalad direction to the peritoneal reflection, the bladder can also suffer an iatrogenic injury.
In cases of the penile inversion vaginoplasty, a skin graft is typically used to line the neovaginal canal. If this graft fails to take appropriately it can prolapse and can contribute to urinary incontinence symptoms. Some surgeons will suspend the apical portion of the neovagina; however, the effect on rates of incontinence is mixed.
The physical exam of a transfeminine patient should consist of a general health assessment, neurological, abdominal, and genitourinary examinations. Palpation of the prostate is performed through the neovaginal canal if patent. During the urinary exam, the provider should make note of stenosis at the urethral meatus or urethral hypermobility. For patients reporting symptoms of stress incontinence, a cough stress test is useful. The neovagina should be carefully examined for fistula formation or any other structural abnormality.
Testing for urinary incontinence is similar to the evaluation in cisgender females in that every patient should undergo a urinalysis and a postvoid residual volume measurement, and should maintain a voiding diary. Indications for urodynamic testing are the same for transfeminine women and cisgender women – symptoms do not correlate with objective findings, failure to improve with treatment, prior incontinence from pelvic floor surgery, difficult diagnostic evaluation with unclear diagnosis.5,6 Cystoscopy is useful for patients experiencing hematuria, before anti-incontinence surgery, or prior to transurethral prostate intervention.1
Treatment is tailored to the type of incontinence diagnosed; however, there are no specific guidelines that are evidence-based for transfeminine patients after vaginoplasty. The therapies available are extrapolated from the general patient population. All patients can benefit from dietary modifications such as avoiding bladder irritants, monitoring fluid and caffeine intake, timed voiding, and pelvic floor exercises.1,6 If patients do not experience improvement from conservative measures, the mainstay treatment for overactive bladder is antimuscarinic agents. However, in cisgender male patients who have a prostate, these agents can lead to urinary retention related to bladder outlet obstruction, although the rates of urinary retention are low.1 Overall, these agents are relatively safe and effective in cisgender men with a prostate and by extension should be utilized in transfeminine patients when indicated.
In patients diagnosed with stress urinary incontinence, conservative options with weight loss, smoking cessation, and pelvic floor exercises should be attempted. If these measures fail in cisgender women, surgical treatment is often recommended. However, surgical treatment in transfeminine patients is significantly less straightforward and beyond the scope of this article.
Obstetrician/gynecologists are familiar with assessing and treating cisgender female patients reporting incontinence and should use this same knowledge for diagnosing and treating transfeminine patients. In addition, providers should be aware of complications of these procedures in evaluating patients presenting for symptoms of incontinence, as these complications directly contribute to incontinence in this patient population.1
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. Email her at [email protected] .
References
1. Ginzburg N. Care of transgender patients: Incontinence. In: Nikolavsky D, Blakley SA, eds. Urological Care for the Transgender Patient: A Comprehensive Guide. Syracuse, NY: Springer, 2021:203-17.
2. Hoebeke P et al. Eur Urol. 2005;47(3):398-402.
3. Kuhn A et al. Fertil Steril. 2011;95(7):2379-82.
4. Kuhn A et al. Eur J Obstet Gynecol Reprod Biol. 2007;131(2):226-30.
5. Winters JC et al. J Urol. 2012;188(6s):2464-72.
6. Practice Bulletin No. 155. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;126:e66-81.