User login
Site of infection linked to PsA onset regardless of pathogen
Key clinical point: Having sent a pharyngeal sample for culture was associated with an increased risk for psoriatic arthritis (PsA) onset, indicating site of infection being associated with the increased PsA risk rather than the pathogen.
Major finding: Among all samples sent for culture, a pharyngeal sample was associated with a higher risk for PsA onset within first 50 days compared with a urine (hazard ratio [HR] 8.78; 95% CI 3.23-23.91), nasopharyngeal (HR 8.26; 95% CI 2.23-30.63), or blood (HR 25.22; 95% CI 3.12-204.13) sample; however, streptococcal infections in the pharynx or at any other site were not associated with an increased risk for PsA.
Study details: Findings are from a population-based cohort study including 313,235 bacterial cultures from 128,982 individuals.
Disclosures: The study was supported by the Landspitali University Hospital, Iceland. A Ogdie and T Love declared serving as consultants and receiving grants and reimbursement from several sources.
Source: Thrastardottir T et al. Strong site-specific association of pharyngeal cultures with the onset of psoriatic arthritis and psoriasis, regardless of pathogen. Rheumatology (Oxford). 2022 (Apr 23). Doi: 10.1093/rheumatology/keac253
Key clinical point: Having sent a pharyngeal sample for culture was associated with an increased risk for psoriatic arthritis (PsA) onset, indicating site of infection being associated with the increased PsA risk rather than the pathogen.
Major finding: Among all samples sent for culture, a pharyngeal sample was associated with a higher risk for PsA onset within first 50 days compared with a urine (hazard ratio [HR] 8.78; 95% CI 3.23-23.91), nasopharyngeal (HR 8.26; 95% CI 2.23-30.63), or blood (HR 25.22; 95% CI 3.12-204.13) sample; however, streptococcal infections in the pharynx or at any other site were not associated with an increased risk for PsA.
Study details: Findings are from a population-based cohort study including 313,235 bacterial cultures from 128,982 individuals.
Disclosures: The study was supported by the Landspitali University Hospital, Iceland. A Ogdie and T Love declared serving as consultants and receiving grants and reimbursement from several sources.
Source: Thrastardottir T et al. Strong site-specific association of pharyngeal cultures with the onset of psoriatic arthritis and psoriasis, regardless of pathogen. Rheumatology (Oxford). 2022 (Apr 23). Doi: 10.1093/rheumatology/keac253
Key clinical point: Having sent a pharyngeal sample for culture was associated with an increased risk for psoriatic arthritis (PsA) onset, indicating site of infection being associated with the increased PsA risk rather than the pathogen.
Major finding: Among all samples sent for culture, a pharyngeal sample was associated with a higher risk for PsA onset within first 50 days compared with a urine (hazard ratio [HR] 8.78; 95% CI 3.23-23.91), nasopharyngeal (HR 8.26; 95% CI 2.23-30.63), or blood (HR 25.22; 95% CI 3.12-204.13) sample; however, streptococcal infections in the pharynx or at any other site were not associated with an increased risk for PsA.
Study details: Findings are from a population-based cohort study including 313,235 bacterial cultures from 128,982 individuals.
Disclosures: The study was supported by the Landspitali University Hospital, Iceland. A Ogdie and T Love declared serving as consultants and receiving grants and reimbursement from several sources.
Source: Thrastardottir T et al. Strong site-specific association of pharyngeal cultures with the onset of psoriatic arthritis and psoriasis, regardless of pathogen. Rheumatology (Oxford). 2022 (Apr 23). Doi: 10.1093/rheumatology/keac253
PsA: Real-world efficacy, safety, and retention rate of secukinumab
Key clinical point: Both first- and second-line secukinumab therapies demonstrated substantial improvement in disease activity, adequate retention rates, and a satisfactory safety profile in a real-world population of patients with psoriatic arthritis (PsA).
Major finding: Mean Disease Activity Score 28 using C-reactive protein decreased from 3.0 to 2.0 and 1.9 at the first and third years of follow-up, whereas the proportion of patients in remission/low disease activity increased from 52.1% and 71.4% to 100% after 2 years of the first- and second-line secukinumab treatments, respectively. The overall retention rates of secukinumab were 74.1%, 59.1%, and 54.2% in the first, second, and third years of treatment, respectively, with no new adverse events being reported.
Study details: This observational, retrospective analysis included 639 patients with PsA or axial spondyloarthritis from the BIOBADASER registry who had received secukinumab for >12 months. Of these patients, 350 had PsA.
Disclosures: This study was sponsored by Novartis Spain. C Sastré is an employee of Novartis. The other authors reported no conflicts of interest.
Source: Moreno-Ramos MJ et al. Real-world effectiveness and treatment retention of secukinumab in patients with psoriatic arthritis and axial spondyloarthritis: A descriptive observational analysis of the Spanish BIOBADASER Registry. Rheumatol Ther. 2022 (Apr 25). Doi: 10.1007/s40744-022-00446-9
Key clinical point: Both first- and second-line secukinumab therapies demonstrated substantial improvement in disease activity, adequate retention rates, and a satisfactory safety profile in a real-world population of patients with psoriatic arthritis (PsA).
Major finding: Mean Disease Activity Score 28 using C-reactive protein decreased from 3.0 to 2.0 and 1.9 at the first and third years of follow-up, whereas the proportion of patients in remission/low disease activity increased from 52.1% and 71.4% to 100% after 2 years of the first- and second-line secukinumab treatments, respectively. The overall retention rates of secukinumab were 74.1%, 59.1%, and 54.2% in the first, second, and third years of treatment, respectively, with no new adverse events being reported.
Study details: This observational, retrospective analysis included 639 patients with PsA or axial spondyloarthritis from the BIOBADASER registry who had received secukinumab for >12 months. Of these patients, 350 had PsA.
Disclosures: This study was sponsored by Novartis Spain. C Sastré is an employee of Novartis. The other authors reported no conflicts of interest.
Source: Moreno-Ramos MJ et al. Real-world effectiveness and treatment retention of secukinumab in patients with psoriatic arthritis and axial spondyloarthritis: A descriptive observational analysis of the Spanish BIOBADASER Registry. Rheumatol Ther. 2022 (Apr 25). Doi: 10.1007/s40744-022-00446-9
Key clinical point: Both first- and second-line secukinumab therapies demonstrated substantial improvement in disease activity, adequate retention rates, and a satisfactory safety profile in a real-world population of patients with psoriatic arthritis (PsA).
Major finding: Mean Disease Activity Score 28 using C-reactive protein decreased from 3.0 to 2.0 and 1.9 at the first and third years of follow-up, whereas the proportion of patients in remission/low disease activity increased from 52.1% and 71.4% to 100% after 2 years of the first- and second-line secukinumab treatments, respectively. The overall retention rates of secukinumab were 74.1%, 59.1%, and 54.2% in the first, second, and third years of treatment, respectively, with no new adverse events being reported.
Study details: This observational, retrospective analysis included 639 patients with PsA or axial spondyloarthritis from the BIOBADASER registry who had received secukinumab for >12 months. Of these patients, 350 had PsA.
Disclosures: This study was sponsored by Novartis Spain. C Sastré is an employee of Novartis. The other authors reported no conflicts of interest.
Source: Moreno-Ramos MJ et al. Real-world effectiveness and treatment retention of secukinumab in patients with psoriatic arthritis and axial spondyloarthritis: A descriptive observational analysis of the Spanish BIOBADASER Registry. Rheumatol Ther. 2022 (Apr 25). Doi: 10.1007/s40744-022-00446-9
Sex and BMI affect response to systemic PsA therapy
Key clinical point: Men vs women with psoriatic arthritis (PsA) experienced significantly improved outcomes with methotrexate+etanercept combination therapy, whereas those with lower body mass index (BMI) experienced better outcomes with no indication of any pattern with treatment received.
Major finding: At week 24, a higher proportion of men vs women receiving methotrexate+etanercept achieved American College of Rheumatology 20% (ACR20; 71.5% vs. 58.3%; P = .0194) and minimal disease activity (MDA; 45.8% vs 25.2%; P = .0003), whereas a higher proportion of patients with a BMI of ≤30 vs >30 kg/m2 in all treatment groups achieved MDA (all P < .05) and those in methotrexate+etanercept group achieved ACR20 (P = .0241).
Study details: This was a post hoc analysis of the phase 3 SEAM-PsA trial including 851 methotrexate/biologics naive patients with early PsA who were randomly assigned to receive methotrexate+placebo, etanercept+placebo, or methotrexate+etanercept.
Disclosures: This study was funded by Immunex, a subsidiary of Amgen. Two authors declared being employees of and owned stocks in Amgen. The other authors reported ties with various sources, including Amgen.
Source: Mease PJ et al. Potential impact of sex and body mass index on response to therapy in psoriatic arthritis: Post-hoc analysis of results from the SEAM-PsA trial. J Rheumatol. 2022 (Apr 15). Doi: 10.3899/jrheum.211037
Key clinical point: Men vs women with psoriatic arthritis (PsA) experienced significantly improved outcomes with methotrexate+etanercept combination therapy, whereas those with lower body mass index (BMI) experienced better outcomes with no indication of any pattern with treatment received.
Major finding: At week 24, a higher proportion of men vs women receiving methotrexate+etanercept achieved American College of Rheumatology 20% (ACR20; 71.5% vs. 58.3%; P = .0194) and minimal disease activity (MDA; 45.8% vs 25.2%; P = .0003), whereas a higher proportion of patients with a BMI of ≤30 vs >30 kg/m2 in all treatment groups achieved MDA (all P < .05) and those in methotrexate+etanercept group achieved ACR20 (P = .0241).
Study details: This was a post hoc analysis of the phase 3 SEAM-PsA trial including 851 methotrexate/biologics naive patients with early PsA who were randomly assigned to receive methotrexate+placebo, etanercept+placebo, or methotrexate+etanercept.
Disclosures: This study was funded by Immunex, a subsidiary of Amgen. Two authors declared being employees of and owned stocks in Amgen. The other authors reported ties with various sources, including Amgen.
Source: Mease PJ et al. Potential impact of sex and body mass index on response to therapy in psoriatic arthritis: Post-hoc analysis of results from the SEAM-PsA trial. J Rheumatol. 2022 (Apr 15). Doi: 10.3899/jrheum.211037
Key clinical point: Men vs women with psoriatic arthritis (PsA) experienced significantly improved outcomes with methotrexate+etanercept combination therapy, whereas those with lower body mass index (BMI) experienced better outcomes with no indication of any pattern with treatment received.
Major finding: At week 24, a higher proportion of men vs women receiving methotrexate+etanercept achieved American College of Rheumatology 20% (ACR20; 71.5% vs. 58.3%; P = .0194) and minimal disease activity (MDA; 45.8% vs 25.2%; P = .0003), whereas a higher proportion of patients with a BMI of ≤30 vs >30 kg/m2 in all treatment groups achieved MDA (all P < .05) and those in methotrexate+etanercept group achieved ACR20 (P = .0241).
Study details: This was a post hoc analysis of the phase 3 SEAM-PsA trial including 851 methotrexate/biologics naive patients with early PsA who were randomly assigned to receive methotrexate+placebo, etanercept+placebo, or methotrexate+etanercept.
Disclosures: This study was funded by Immunex, a subsidiary of Amgen. Two authors declared being employees of and owned stocks in Amgen. The other authors reported ties with various sources, including Amgen.
Source: Mease PJ et al. Potential impact of sex and body mass index on response to therapy in psoriatic arthritis: Post-hoc analysis of results from the SEAM-PsA trial. J Rheumatol. 2022 (Apr 15). Doi: 10.3899/jrheum.211037
Improved outcomes with 6-month secukinumab use in PsA
Key clinical point: More than one-third of real-world patients with psoriatic arthritis (PsA) who were not in minimal disease activity (MDA) at secukinumab initiation achieved MDA after 6 months of initiating secukinumab along with improvement in other patient-reported outcomes.
Major finding: At 6 months, 36.6% of patients not in MDA at secukinumab initiation achieved MDA and 41.2%, 44.4%, 60.7%, and 75.0% of patients with ≥1 tender joint, ≥1 swollen joint, enthesitis, and dactylitis, respectively, at secukinumab initiation achieved symptom resolution along with improvement in pain, fatigue, and other scores.
Study details: Findings are from an analysis of 100 patients with PsA from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry who initiated secukinumab and maintained the treatment at 6-month follow-up visit.
Disclosures: This study was sponsored by CorEvitas, LLC. Three authors declared being employees of CorEvitas. The other authors reported ties with several sources.
Source: Mease PJ et al. Effectiveness of 6-month use of secukinumab in patients with psoriatic arthritis in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol. 2022 (Apr 15). Doi: 10.3899/jrheum.211033
Key clinical point: More than one-third of real-world patients with psoriatic arthritis (PsA) who were not in minimal disease activity (MDA) at secukinumab initiation achieved MDA after 6 months of initiating secukinumab along with improvement in other patient-reported outcomes.
Major finding: At 6 months, 36.6% of patients not in MDA at secukinumab initiation achieved MDA and 41.2%, 44.4%, 60.7%, and 75.0% of patients with ≥1 tender joint, ≥1 swollen joint, enthesitis, and dactylitis, respectively, at secukinumab initiation achieved symptom resolution along with improvement in pain, fatigue, and other scores.
Study details: Findings are from an analysis of 100 patients with PsA from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry who initiated secukinumab and maintained the treatment at 6-month follow-up visit.
Disclosures: This study was sponsored by CorEvitas, LLC. Three authors declared being employees of CorEvitas. The other authors reported ties with several sources.
Source: Mease PJ et al. Effectiveness of 6-month use of secukinumab in patients with psoriatic arthritis in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol. 2022 (Apr 15). Doi: 10.3899/jrheum.211033
Key clinical point: More than one-third of real-world patients with psoriatic arthritis (PsA) who were not in minimal disease activity (MDA) at secukinumab initiation achieved MDA after 6 months of initiating secukinumab along with improvement in other patient-reported outcomes.
Major finding: At 6 months, 36.6% of patients not in MDA at secukinumab initiation achieved MDA and 41.2%, 44.4%, 60.7%, and 75.0% of patients with ≥1 tender joint, ≥1 swollen joint, enthesitis, and dactylitis, respectively, at secukinumab initiation achieved symptom resolution along with improvement in pain, fatigue, and other scores.
Study details: Findings are from an analysis of 100 patients with PsA from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry who initiated secukinumab and maintained the treatment at 6-month follow-up visit.
Disclosures: This study was sponsored by CorEvitas, LLC. Three authors declared being employees of CorEvitas. The other authors reported ties with several sources.
Source: Mease PJ et al. Effectiveness of 6-month use of secukinumab in patients with psoriatic arthritis in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol. 2022 (Apr 15). Doi: 10.3899/jrheum.211033
Disasters abroad a major trigger for mental illness in expats
The 2020 explosion that rocked Beirut, killing more than 200, injuring more than 7,000 and causing millions of dollars in damage had a significant impact on the mental health of Lebanese expatriates, leaving many grappling with anxiety, depression, and posttraumatic stress disorder, results of a new survey show.
The findings highlight the importance of considering the well-being of expatriates dealing with adverse events in their home countries, the investigators say.
“Everyone, including doctors, should be more sensitive to expatriates around them; we should look out for them especially when their home country is going through a traumatic event,” study investigator Gaëlle Rached, MD, MSc, research postdoctoral fellow, Northwestern University, Chicago, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
A historic explosion
It is estimated that approximately 14 million Lebanese citizens live outside their home country, which is more than double the population of Lebanon. However, the trauma-related mental health of these and other expatriate communities is understudied, said Dr. Rached.
“If you look at the literature, next to no one has examined expatriates’ mental health, and more so in the context of trauma.”
Dr. Rached has personal experience with the event. She was in Beirut on Aug. 4, 2020, when the Lebanese capital was rocked by an explosion attributed to ammonium nitrate stored at the city’s port. It was one of the biggest nonnuclear explosions in history and left hundreds homeless, killed, or injured. Dr. Rached watched as her father was injured and her house destroyed.
She heard anecdotes of Lebanese expatriates, experiencing trauma as a result of the blast. Many were unable to contact friends and loved ones in the wake of the tragedy.
“That prompted us to look at expatriate mental health following this traumatic incident,” she said.
She and her colleagues used various social media platforms to advertise the survey. They also reached out to the International Lebanese Medical Association, which has “a strong base” in the United States, said Dr. Rached.
She was “shocked” at how many expatriates responded. “People really wanted to speak up and express themselves” – whether because of survivor’s guilt or for some other reason, she said.
The survey included 670 adults with Lebanese nationality or who were first generation Lebanese living abroad. The study population had a median age 31 years and 62.2% female, most living in North America or Europe. Over one-third of respondents (270) had been living abroad from 1-5 years but many had been away for more than 20 years.
Study participants completed the Hopkins Symptoms Checklist (HSCL), which screens for anxiety and depression. On this checklist, a score of 1.75 is a typical cutoff value for symptomatic cases.
The investigators found 41.2% of participants scored higher than this threshold. Being younger, female and visiting Lebanon at the time of the blast, were factors associated with higher HSCL scores.
No tincture of time
Interestingly, the amount of time since emigrating from Lebanon was unrelated to the score. “Our results show that, no matter how long you’ve been away, you’re prone to the same negative outcome,” said Dr. Rached.
Of the total study population, 268 personally experienced the explosion and/or had close friends or family physically affected by it. These expatriates completed the Post-traumatic Checklist for DSM-5 (PCL-5).
Here, the analysis showed that many of these respondents (57.5%) scored above 33, which is higher than the threshold for probable PTSD. Being female was linked to higher PCL-5 scores.
The results may be especially timely as many countries are taking in a flood of refugees fleeing war in Ukraine. However, Dr. Rached said, the findings from her research may not apply to Ukrainians.
“I don’t think the results can be extrapolated, given that the nature of the trauma is a little bit different,” she said, adding that the Beirut blast was “monumental” but it was over quickly. In contrast, there’s no end in sight for the Russian invasion of Ukraine.
Dr. Rached noted the study data are preliminary and limited because there’s no way to determine whether respondents had mental health issues before the blast.
Global psychiatrist shortage
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry in Omaha, and incoming chair of the APA’s Council on Communications, said he found the presentation “fascinating on several levels.”
It’s increasingly important for psychiatrists to be “trauma informed,” Dr. Liu told a press briefing highlighting the study. “It’s not just about looking at the biological correlates of illness,” meaning looking at genetic markers etc, “but also looking at the environment in which people live, work, and/or are in therapy or in treatment.”
In a later interview, Dr. Liu said he was impressed by the fact that Dr. Rached, who has “a very deep personal connection to this community,” is using her own personal trauma to help identify others are at risk who may need future care.
Dr. Liu, whose own family sponsors Afghan refugees, said the research underlines the need to ensure training for psychiatrists everywhere to help manage the expatriate population. As it stands, there’s “a huge shortage of psychiatrists around the world,” particularly in countries that have been affected by trauma, said Dr. Liu.
The researchers and Dr. Liu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 2020 explosion that rocked Beirut, killing more than 200, injuring more than 7,000 and causing millions of dollars in damage had a significant impact on the mental health of Lebanese expatriates, leaving many grappling with anxiety, depression, and posttraumatic stress disorder, results of a new survey show.
The findings highlight the importance of considering the well-being of expatriates dealing with adverse events in their home countries, the investigators say.
“Everyone, including doctors, should be more sensitive to expatriates around them; we should look out for them especially when their home country is going through a traumatic event,” study investigator Gaëlle Rached, MD, MSc, research postdoctoral fellow, Northwestern University, Chicago, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
A historic explosion
It is estimated that approximately 14 million Lebanese citizens live outside their home country, which is more than double the population of Lebanon. However, the trauma-related mental health of these and other expatriate communities is understudied, said Dr. Rached.
“If you look at the literature, next to no one has examined expatriates’ mental health, and more so in the context of trauma.”
Dr. Rached has personal experience with the event. She was in Beirut on Aug. 4, 2020, when the Lebanese capital was rocked by an explosion attributed to ammonium nitrate stored at the city’s port. It was one of the biggest nonnuclear explosions in history and left hundreds homeless, killed, or injured. Dr. Rached watched as her father was injured and her house destroyed.
She heard anecdotes of Lebanese expatriates, experiencing trauma as a result of the blast. Many were unable to contact friends and loved ones in the wake of the tragedy.
“That prompted us to look at expatriate mental health following this traumatic incident,” she said.
She and her colleagues used various social media platforms to advertise the survey. They also reached out to the International Lebanese Medical Association, which has “a strong base” in the United States, said Dr. Rached.
She was “shocked” at how many expatriates responded. “People really wanted to speak up and express themselves” – whether because of survivor’s guilt or for some other reason, she said.
The survey included 670 adults with Lebanese nationality or who were first generation Lebanese living abroad. The study population had a median age 31 years and 62.2% female, most living in North America or Europe. Over one-third of respondents (270) had been living abroad from 1-5 years but many had been away for more than 20 years.
Study participants completed the Hopkins Symptoms Checklist (HSCL), which screens for anxiety and depression. On this checklist, a score of 1.75 is a typical cutoff value for symptomatic cases.
The investigators found 41.2% of participants scored higher than this threshold. Being younger, female and visiting Lebanon at the time of the blast, were factors associated with higher HSCL scores.
No tincture of time
Interestingly, the amount of time since emigrating from Lebanon was unrelated to the score. “Our results show that, no matter how long you’ve been away, you’re prone to the same negative outcome,” said Dr. Rached.
Of the total study population, 268 personally experienced the explosion and/or had close friends or family physically affected by it. These expatriates completed the Post-traumatic Checklist for DSM-5 (PCL-5).
Here, the analysis showed that many of these respondents (57.5%) scored above 33, which is higher than the threshold for probable PTSD. Being female was linked to higher PCL-5 scores.
The results may be especially timely as many countries are taking in a flood of refugees fleeing war in Ukraine. However, Dr. Rached said, the findings from her research may not apply to Ukrainians.
“I don’t think the results can be extrapolated, given that the nature of the trauma is a little bit different,” she said, adding that the Beirut blast was “monumental” but it was over quickly. In contrast, there’s no end in sight for the Russian invasion of Ukraine.
Dr. Rached noted the study data are preliminary and limited because there’s no way to determine whether respondents had mental health issues before the blast.
Global psychiatrist shortage
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry in Omaha, and incoming chair of the APA’s Council on Communications, said he found the presentation “fascinating on several levels.”
It’s increasingly important for psychiatrists to be “trauma informed,” Dr. Liu told a press briefing highlighting the study. “It’s not just about looking at the biological correlates of illness,” meaning looking at genetic markers etc, “but also looking at the environment in which people live, work, and/or are in therapy or in treatment.”
In a later interview, Dr. Liu said he was impressed by the fact that Dr. Rached, who has “a very deep personal connection to this community,” is using her own personal trauma to help identify others are at risk who may need future care.
Dr. Liu, whose own family sponsors Afghan refugees, said the research underlines the need to ensure training for psychiatrists everywhere to help manage the expatriate population. As it stands, there’s “a huge shortage of psychiatrists around the world,” particularly in countries that have been affected by trauma, said Dr. Liu.
The researchers and Dr. Liu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 2020 explosion that rocked Beirut, killing more than 200, injuring more than 7,000 and causing millions of dollars in damage had a significant impact on the mental health of Lebanese expatriates, leaving many grappling with anxiety, depression, and posttraumatic stress disorder, results of a new survey show.
The findings highlight the importance of considering the well-being of expatriates dealing with adverse events in their home countries, the investigators say.
“Everyone, including doctors, should be more sensitive to expatriates around them; we should look out for them especially when their home country is going through a traumatic event,” study investigator Gaëlle Rached, MD, MSc, research postdoctoral fellow, Northwestern University, Chicago, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
A historic explosion
It is estimated that approximately 14 million Lebanese citizens live outside their home country, which is more than double the population of Lebanon. However, the trauma-related mental health of these and other expatriate communities is understudied, said Dr. Rached.
“If you look at the literature, next to no one has examined expatriates’ mental health, and more so in the context of trauma.”
Dr. Rached has personal experience with the event. She was in Beirut on Aug. 4, 2020, when the Lebanese capital was rocked by an explosion attributed to ammonium nitrate stored at the city’s port. It was one of the biggest nonnuclear explosions in history and left hundreds homeless, killed, or injured. Dr. Rached watched as her father was injured and her house destroyed.
She heard anecdotes of Lebanese expatriates, experiencing trauma as a result of the blast. Many were unable to contact friends and loved ones in the wake of the tragedy.
“That prompted us to look at expatriate mental health following this traumatic incident,” she said.
She and her colleagues used various social media platforms to advertise the survey. They also reached out to the International Lebanese Medical Association, which has “a strong base” in the United States, said Dr. Rached.
She was “shocked” at how many expatriates responded. “People really wanted to speak up and express themselves” – whether because of survivor’s guilt or for some other reason, she said.
The survey included 670 adults with Lebanese nationality or who were first generation Lebanese living abroad. The study population had a median age 31 years and 62.2% female, most living in North America or Europe. Over one-third of respondents (270) had been living abroad from 1-5 years but many had been away for more than 20 years.
Study participants completed the Hopkins Symptoms Checklist (HSCL), which screens for anxiety and depression. On this checklist, a score of 1.75 is a typical cutoff value for symptomatic cases.
The investigators found 41.2% of participants scored higher than this threshold. Being younger, female and visiting Lebanon at the time of the blast, were factors associated with higher HSCL scores.
No tincture of time
Interestingly, the amount of time since emigrating from Lebanon was unrelated to the score. “Our results show that, no matter how long you’ve been away, you’re prone to the same negative outcome,” said Dr. Rached.
Of the total study population, 268 personally experienced the explosion and/or had close friends or family physically affected by it. These expatriates completed the Post-traumatic Checklist for DSM-5 (PCL-5).
Here, the analysis showed that many of these respondents (57.5%) scored above 33, which is higher than the threshold for probable PTSD. Being female was linked to higher PCL-5 scores.
The results may be especially timely as many countries are taking in a flood of refugees fleeing war in Ukraine. However, Dr. Rached said, the findings from her research may not apply to Ukrainians.
“I don’t think the results can be extrapolated, given that the nature of the trauma is a little bit different,” she said, adding that the Beirut blast was “monumental” but it was over quickly. In contrast, there’s no end in sight for the Russian invasion of Ukraine.
Dr. Rached noted the study data are preliminary and limited because there’s no way to determine whether respondents had mental health issues before the blast.
Global psychiatrist shortage
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry in Omaha, and incoming chair of the APA’s Council on Communications, said he found the presentation “fascinating on several levels.”
It’s increasingly important for psychiatrists to be “trauma informed,” Dr. Liu told a press briefing highlighting the study. “It’s not just about looking at the biological correlates of illness,” meaning looking at genetic markers etc, “but also looking at the environment in which people live, work, and/or are in therapy or in treatment.”
In a later interview, Dr. Liu said he was impressed by the fact that Dr. Rached, who has “a very deep personal connection to this community,” is using her own personal trauma to help identify others are at risk who may need future care.
Dr. Liu, whose own family sponsors Afghan refugees, said the research underlines the need to ensure training for psychiatrists everywhere to help manage the expatriate population. As it stands, there’s “a huge shortage of psychiatrists around the world,” particularly in countries that have been affected by trauma, said Dr. Liu.
The researchers and Dr. Liu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Conjunctival Melanoma of the Left Lower Eyelid
To the Editor:
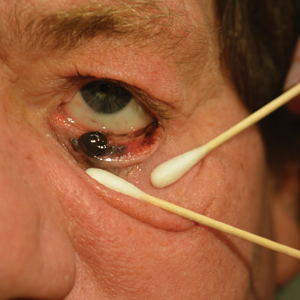
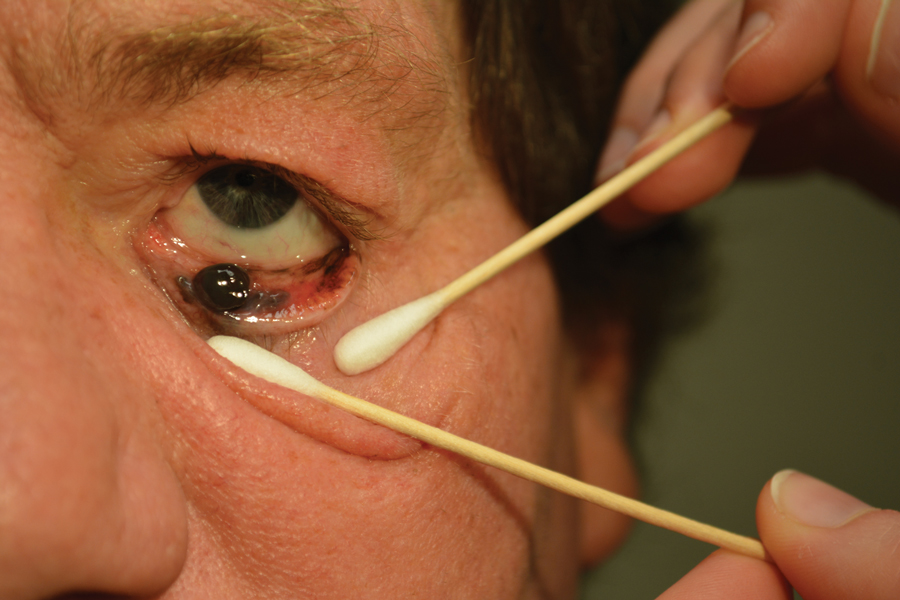
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.
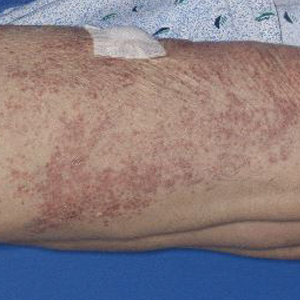
A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.

A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.

A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
Practice Points
- Ophthalmologists should carefully examine palpebral and bulbar conjunctiva at each annual visit paying careful attention to pigmented nevi.
- Conjunctival abnormalities should be thoroughly documented via color photography to accurately follow for suspicious change.
Müllerian anomalies – old problem, new approach and classification
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at [email protected].
Vibrating pill counters constipation
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
FROM DDW 2022
Cutaneous Lupus Erythematosus–like Isotopic Response to Herpes Zoster Infection
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.
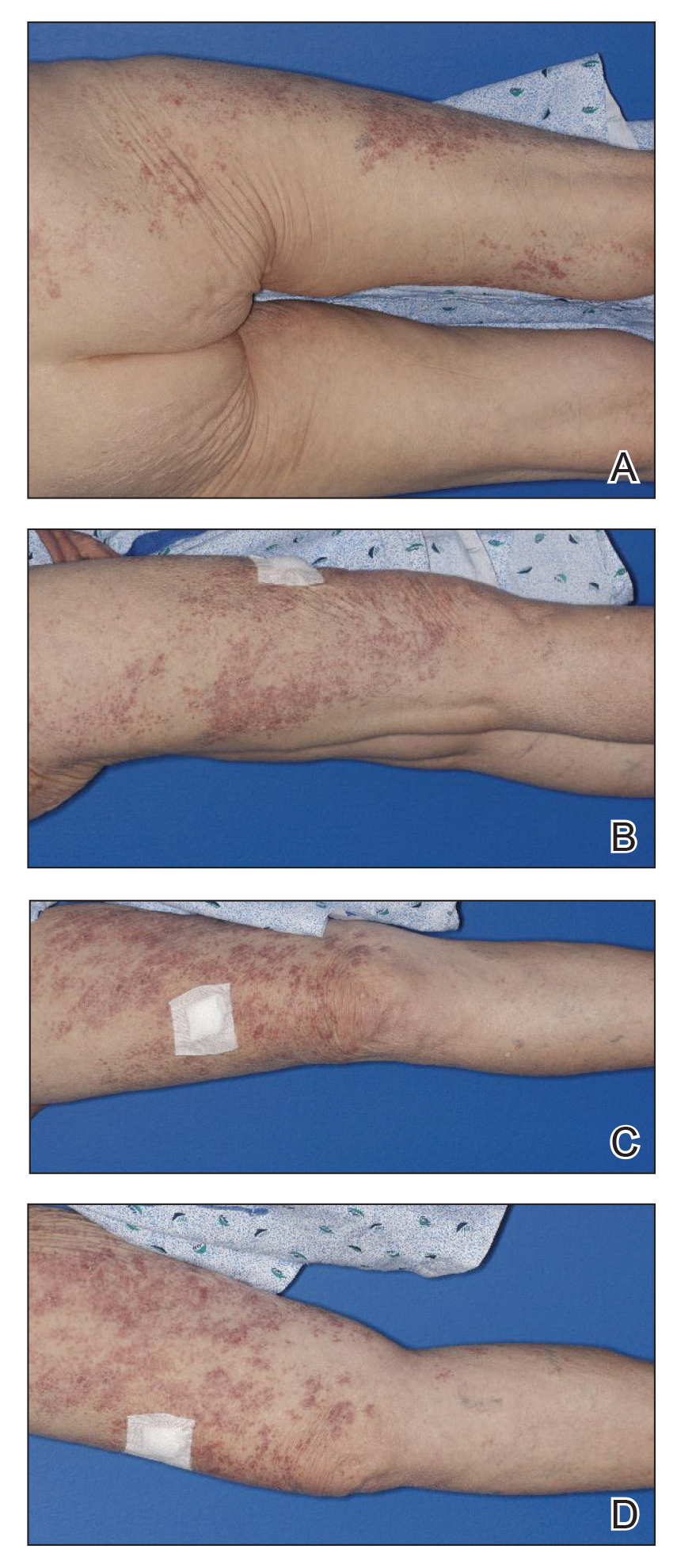
Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.
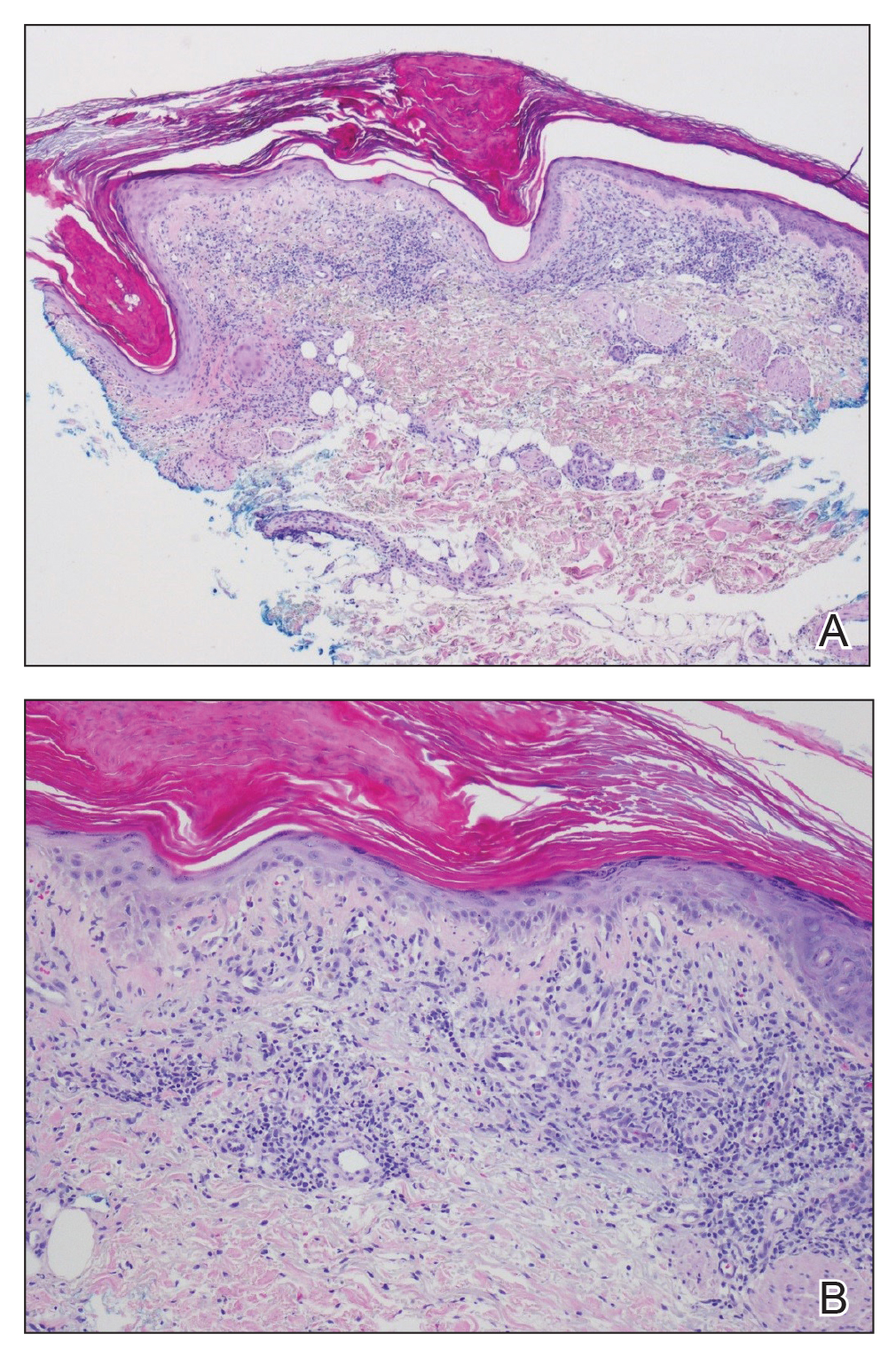
Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.

Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.

Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.

Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.

Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
Practice Points
- Wolf isotopic response describes the occurrence of a new skin condition at the site of a previously healed and unrelated skin disorder; a granulomatous reaction is a commonly reported isotopic response.
- Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses.
FDA, AMA prepare for potential COVID-19 shots for children younger than 6
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.