User login
More first-degree relatives develop celiac than expected
Children who have first-degree relatives with celiac disease (CD) may be at higher risk of developing CD before age 10 than previously thought, according to results of an analysis of 10-year follow-up data. This analysis also yielded a web-based prediction model to help with screening for CD in children.
Screening is recommended for children who have a first-degree relative with celiac disease (CD) because they have a much higher risk for CD, but it has been unclear when to screen such children and how frequently. The researchers, led by Caroline Meijer, MD, in the department of pediatrics and medical statistics at Leiden (the Netherlands) University Medical Center, wrote in Gastroenterology that a prediction model they developed can help parents and providers with those questions.
The researchers analyzed prospective data from 10 years of follow-up from the PreventCD birth cohort with 944 children genetically predisposed to celiac disease with at least one first-degree relative affected. The children were enrolled at birth between 2007 and 2010 in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain.
“The data of the follow-up of the PreventCD cohort at the mean age of 10 years offers a unique opportunity to study the natural development of CD in children from high risk families,” the authors wrote.
Children were assessed regularly from birth for CD development at specific intervals, including seven times during the first 3 years of age and once a year after that or at least once between March 2016 and March 2019.
The researchers monitored parent-reported health status, recorded weight and height and gluten consumption and serum IgA against anti-transglutaminase. They found that risk for CD differs by gender, age, and human leukocyte antigen (HLA-DQ) genes. Variables that significantly affected the risk were combined to develop a risk score.
The noted that CD affects as many as 1%-3% of the general population. Until recently, the lifetime risk of CD when a child has a first-degree CD connection was considered to be 5%-10%, with one review indicating a pooled prevalence of 7.5%. However, the investigators found that the cumulative incidence of CD was 7.5%, 16.6%, and 17.5% at 3, 8, and 10 years of age, respectively.
“Our data show that, at the age of 8 years, this is as high as 17%, emphasizing the importance of a sound advice for early screening,” Dr. Meijer and coauthors wrote. “We also confirm that CD develops in children with affected FDR at a very young age, as the mean age of diagnosis in our cohort was 4 years of age.”
They found that CD developed more often in girls (P = .0005). Data show that, at the age of 10 years, girls in the cohort have a 7.7% higher cumulative incidence compared to boys (21.5% vs 13.8%). CD also developed more often in HLA-DQ2 homozygous participants than in other HLA-risk groups (8-year cumulative incidence of 35.4% vs. the maximum of the other groups of 18.2%; P < .001).
Benjamin Lebwohl, MD, MS, said the study provides data that can help parents of a child with a family history of celiac disease.
“The results of the study include an estimate of celiac disease risk over time, based on the child’s age, number of affected relatives, and genetic test results. Using this information, the web-based prediction model provides advice on how often to test the child for celiac disease, based on the child’s risk of developing the condition in the years ahead,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University Medical Center, New York, said in an interview.
“Overall,” he said, “the study advances our understanding of the natural history of celiac disease among children with a family history and gives clinicians and families practical advice on when to test.”
Validation of the prediction model was done with data from the independent NeoCel cohort. In that cohort, all children were assessed regularly from birth for CD development at predefined intervals, in a similar way as in the PreventCD cohort.
The investigators concluded that children should be screened early in life and that screening should include HLA-DQ2/8-typing. If children are genetically predisposed to CD, they should get further personalized screening advice using the web-based prediction “app” developed by the investigators.
“If the prediction for CD development [according to the app] is higher than 10% in the next 2 years, we advice to repeat the screening after 6 months. If the prediction is between 5%-10%, the advice is to repeat the screening after 1 year and if the prediction is lower than 5%, to repeat the screening after 2 years.”
The study authors reported no relevant financial relationships. The work is supported by funding from the European Commission; the Azrieli Foundation; Deutsche Zöliakie Gesellschaft; Eurospital; Fondazione Celiachia; Fria Bröd Sweden; Instituto de Salud Carlos III; Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Komitet Badań Naukowych; Fundacja Nutricia; Hungarian Scientific Research Funds; and TAMOP; Stichting Coeliakie Onderzoek Nederland; Thermo Fisher Scientific; and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Dr. Lebwohl reported having no relevant disclosures.
AGA offers guidance on celiac disease to help patients maintain a gluten free diet in the AGA GI Patient Center: www.gastro.org/celiac
Children who have first-degree relatives with celiac disease (CD) may be at higher risk of developing CD before age 10 than previously thought, according to results of an analysis of 10-year follow-up data. This analysis also yielded a web-based prediction model to help with screening for CD in children.
Screening is recommended for children who have a first-degree relative with celiac disease (CD) because they have a much higher risk for CD, but it has been unclear when to screen such children and how frequently. The researchers, led by Caroline Meijer, MD, in the department of pediatrics and medical statistics at Leiden (the Netherlands) University Medical Center, wrote in Gastroenterology that a prediction model they developed can help parents and providers with those questions.
The researchers analyzed prospective data from 10 years of follow-up from the PreventCD birth cohort with 944 children genetically predisposed to celiac disease with at least one first-degree relative affected. The children were enrolled at birth between 2007 and 2010 in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain.
“The data of the follow-up of the PreventCD cohort at the mean age of 10 years offers a unique opportunity to study the natural development of CD in children from high risk families,” the authors wrote.
Children were assessed regularly from birth for CD development at specific intervals, including seven times during the first 3 years of age and once a year after that or at least once between March 2016 and March 2019.
The researchers monitored parent-reported health status, recorded weight and height and gluten consumption and serum IgA against anti-transglutaminase. They found that risk for CD differs by gender, age, and human leukocyte antigen (HLA-DQ) genes. Variables that significantly affected the risk were combined to develop a risk score.
The noted that CD affects as many as 1%-3% of the general population. Until recently, the lifetime risk of CD when a child has a first-degree CD connection was considered to be 5%-10%, with one review indicating a pooled prevalence of 7.5%. However, the investigators found that the cumulative incidence of CD was 7.5%, 16.6%, and 17.5% at 3, 8, and 10 years of age, respectively.
“Our data show that, at the age of 8 years, this is as high as 17%, emphasizing the importance of a sound advice for early screening,” Dr. Meijer and coauthors wrote. “We also confirm that CD develops in children with affected FDR at a very young age, as the mean age of diagnosis in our cohort was 4 years of age.”
They found that CD developed more often in girls (P = .0005). Data show that, at the age of 10 years, girls in the cohort have a 7.7% higher cumulative incidence compared to boys (21.5% vs 13.8%). CD also developed more often in HLA-DQ2 homozygous participants than in other HLA-risk groups (8-year cumulative incidence of 35.4% vs. the maximum of the other groups of 18.2%; P < .001).
Benjamin Lebwohl, MD, MS, said the study provides data that can help parents of a child with a family history of celiac disease.
“The results of the study include an estimate of celiac disease risk over time, based on the child’s age, number of affected relatives, and genetic test results. Using this information, the web-based prediction model provides advice on how often to test the child for celiac disease, based on the child’s risk of developing the condition in the years ahead,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University Medical Center, New York, said in an interview.
“Overall,” he said, “the study advances our understanding of the natural history of celiac disease among children with a family history and gives clinicians and families practical advice on when to test.”
Validation of the prediction model was done with data from the independent NeoCel cohort. In that cohort, all children were assessed regularly from birth for CD development at predefined intervals, in a similar way as in the PreventCD cohort.
The investigators concluded that children should be screened early in life and that screening should include HLA-DQ2/8-typing. If children are genetically predisposed to CD, they should get further personalized screening advice using the web-based prediction “app” developed by the investigators.
“If the prediction for CD development [according to the app] is higher than 10% in the next 2 years, we advice to repeat the screening after 6 months. If the prediction is between 5%-10%, the advice is to repeat the screening after 1 year and if the prediction is lower than 5%, to repeat the screening after 2 years.”
The study authors reported no relevant financial relationships. The work is supported by funding from the European Commission; the Azrieli Foundation; Deutsche Zöliakie Gesellschaft; Eurospital; Fondazione Celiachia; Fria Bröd Sweden; Instituto de Salud Carlos III; Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Komitet Badań Naukowych; Fundacja Nutricia; Hungarian Scientific Research Funds; and TAMOP; Stichting Coeliakie Onderzoek Nederland; Thermo Fisher Scientific; and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Dr. Lebwohl reported having no relevant disclosures.
AGA offers guidance on celiac disease to help patients maintain a gluten free diet in the AGA GI Patient Center: www.gastro.org/celiac
Children who have first-degree relatives with celiac disease (CD) may be at higher risk of developing CD before age 10 than previously thought, according to results of an analysis of 10-year follow-up data. This analysis also yielded a web-based prediction model to help with screening for CD in children.
Screening is recommended for children who have a first-degree relative with celiac disease (CD) because they have a much higher risk for CD, but it has been unclear when to screen such children and how frequently. The researchers, led by Caroline Meijer, MD, in the department of pediatrics and medical statistics at Leiden (the Netherlands) University Medical Center, wrote in Gastroenterology that a prediction model they developed can help parents and providers with those questions.
The researchers analyzed prospective data from 10 years of follow-up from the PreventCD birth cohort with 944 children genetically predisposed to celiac disease with at least one first-degree relative affected. The children were enrolled at birth between 2007 and 2010 in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain.
“The data of the follow-up of the PreventCD cohort at the mean age of 10 years offers a unique opportunity to study the natural development of CD in children from high risk families,” the authors wrote.
Children were assessed regularly from birth for CD development at specific intervals, including seven times during the first 3 years of age and once a year after that or at least once between March 2016 and March 2019.
The researchers monitored parent-reported health status, recorded weight and height and gluten consumption and serum IgA against anti-transglutaminase. They found that risk for CD differs by gender, age, and human leukocyte antigen (HLA-DQ) genes. Variables that significantly affected the risk were combined to develop a risk score.
The noted that CD affects as many as 1%-3% of the general population. Until recently, the lifetime risk of CD when a child has a first-degree CD connection was considered to be 5%-10%, with one review indicating a pooled prevalence of 7.5%. However, the investigators found that the cumulative incidence of CD was 7.5%, 16.6%, and 17.5% at 3, 8, and 10 years of age, respectively.
“Our data show that, at the age of 8 years, this is as high as 17%, emphasizing the importance of a sound advice for early screening,” Dr. Meijer and coauthors wrote. “We also confirm that CD develops in children with affected FDR at a very young age, as the mean age of diagnosis in our cohort was 4 years of age.”
They found that CD developed more often in girls (P = .0005). Data show that, at the age of 10 years, girls in the cohort have a 7.7% higher cumulative incidence compared to boys (21.5% vs 13.8%). CD also developed more often in HLA-DQ2 homozygous participants than in other HLA-risk groups (8-year cumulative incidence of 35.4% vs. the maximum of the other groups of 18.2%; P < .001).
Benjamin Lebwohl, MD, MS, said the study provides data that can help parents of a child with a family history of celiac disease.
“The results of the study include an estimate of celiac disease risk over time, based on the child’s age, number of affected relatives, and genetic test results. Using this information, the web-based prediction model provides advice on how often to test the child for celiac disease, based on the child’s risk of developing the condition in the years ahead,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University Medical Center, New York, said in an interview.
“Overall,” he said, “the study advances our understanding of the natural history of celiac disease among children with a family history and gives clinicians and families practical advice on when to test.”
Validation of the prediction model was done with data from the independent NeoCel cohort. In that cohort, all children were assessed regularly from birth for CD development at predefined intervals, in a similar way as in the PreventCD cohort.
The investigators concluded that children should be screened early in life and that screening should include HLA-DQ2/8-typing. If children are genetically predisposed to CD, they should get further personalized screening advice using the web-based prediction “app” developed by the investigators.
“If the prediction for CD development [according to the app] is higher than 10% in the next 2 years, we advice to repeat the screening after 6 months. If the prediction is between 5%-10%, the advice is to repeat the screening after 1 year and if the prediction is lower than 5%, to repeat the screening after 2 years.”
The study authors reported no relevant financial relationships. The work is supported by funding from the European Commission; the Azrieli Foundation; Deutsche Zöliakie Gesellschaft; Eurospital; Fondazione Celiachia; Fria Bröd Sweden; Instituto de Salud Carlos III; Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Komitet Badań Naukowych; Fundacja Nutricia; Hungarian Scientific Research Funds; and TAMOP; Stichting Coeliakie Onderzoek Nederland; Thermo Fisher Scientific; and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Dr. Lebwohl reported having no relevant disclosures.
AGA offers guidance on celiac disease to help patients maintain a gluten free diet in the AGA GI Patient Center: www.gastro.org/celiac
FROM GASTROENTEROLOGY
Monkeypox quarantines not needed in U.S., Biden says
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
Will ‘gold card’ legislation and others rein in prior authorizations?
I live in New Orleans and recently became aware of a piece of state legislation that would create a “gold card” system for prior authorizations in Louisiana. Before delving into what is a gold card and how it works, let’s take a look at the evolution of prior authorizations (PAs).
Commercial health insurance and Medicare/Medicaid had their beginnings in the 1950s and 1960s. Because the government would now be paying for medical services for seniors, there was a concern that there might be an “overutilization” of services. This concern resulted in the concepts of utilization review and “medical necessity.” These utilization reviews morphed into what are now known as utilization management tools (UMTs). The original intent of these tools was to link cost containment to quality assurance.
PAs are one of a number of UMTs, along with formulary step therapy and nonmedical switching, that are used by health insurance companies and pharmacy benefit managers to determine whether a prescribed product or service is medically necessary and cost effective. Originally, it also meant that the service/treatment would be reimbursed. That is not the case anymore.
Today, physicians face many frivolous PAs for generic medications, such as methotrexate and prednisone, and ironically sometimes higher-priced drugs are preferred over lower-priced ones.
A number of surveys, including a recent one of more than 1,000 specialty physicians by the Alliance of Specialty Medicine, show that PAs are not only a significant administrative burden on practices but also harm patients with significant delays in accessing needed treatments and diagnostic services.
The often-cited study by Zachary Wallace et al. clearly demonstrates significant harm to rheumatology patients whose treatments were delayed because of PAs. These delays caused a substantial increase in steroid dosages in patients whose PA was initially denied and even in those patients whose PAs were initially approved. These data and others support the urgent need to address the entire spectrum of PAs.
Over the last few years, we have seen many states passing laws, adding common-sense protections to mitigate the harmful consequences of UMTs. Such reforms are needed now to stop the indiscriminate use of PAs. Suggestions have included completely eliminating PAs for medications and services that are consistently approved, standardizing electronic forms across all health plans with real-time approval, and others, including “gold card” legislation. In addition to states’ efforts, Congress proposed H.R. 3173, the Improving Seniors’ Timely Access to Care Act of 2021, to protect seniors from the harm caused by PAs that are required by Medicare Advantage programs.
This brings us to the topic of gold card legislation, in which physicians would be given a gold card exempting them from PA for specific services (hopefully including prescription drugs). However, the criteria a physician needs to qualify for a gold card could vary from state to state. For example, it could be based on a physician’s PA approval rate during a specified review period, or it could be completely up to the insurance company to decide the criteria.
Texas is the only state that has passed gold card legislation thus far, although there is an active gold card bill in Louisiana (as of this writing). There are a few other states that have introduced gold card bills that have not yet passed, but there is definite interest throughout the country in this concept. In the Texas legislation, physicians would qualify for a gold card if they had a PA approval threshold of 90% for specific medications or services over a 6-month review period.
A few of the concerns about how this will be implemented and the potential unintended consequences of the legislation include:
- Would one gold card cover all drugs, a specific drug, or just a specific drug for a specific diagnosis?
- Will clinicians get bogged down appealing gold card denials/rescissions?
- Will health plans begin denying more requests up front to keep clinicians from qualifying for an exemption?
Unfortunately, the Louisiana gold card legislation has been amended from its original form to exclude “pharmacy services” and qualification for the gold card “shall be at the sole discretion of the health insurance issuer.”
Consequently, my initial excitement surrounding the Louisiana gold card legislation, for our specialty, has for the most part disappeared. Nonetheless, there is clear excitement behind the gold card concept throughout the country.
What is clear is that health insurance companies and pharmacy benefit managers have lost sight of the original purpose of UMTs, which is to ensure that patients have access to cost-effective quality care. Over the years, the aggressive use of PAs and other UMTs has led to a significant increase in administrative burden for our offices, and more importantly, a loss of disease control in many of our patients, resulting in an increase in overall health care costs.
While it is extremely disturbing that we need legislation to force health plans to keep our patients safe and ensure quality of care, it certainly proves that now, more than ever, we must make our voices be heard.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
I live in New Orleans and recently became aware of a piece of state legislation that would create a “gold card” system for prior authorizations in Louisiana. Before delving into what is a gold card and how it works, let’s take a look at the evolution of prior authorizations (PAs).
Commercial health insurance and Medicare/Medicaid had their beginnings in the 1950s and 1960s. Because the government would now be paying for medical services for seniors, there was a concern that there might be an “overutilization” of services. This concern resulted in the concepts of utilization review and “medical necessity.” These utilization reviews morphed into what are now known as utilization management tools (UMTs). The original intent of these tools was to link cost containment to quality assurance.
PAs are one of a number of UMTs, along with formulary step therapy and nonmedical switching, that are used by health insurance companies and pharmacy benefit managers to determine whether a prescribed product or service is medically necessary and cost effective. Originally, it also meant that the service/treatment would be reimbursed. That is not the case anymore.
Today, physicians face many frivolous PAs for generic medications, such as methotrexate and prednisone, and ironically sometimes higher-priced drugs are preferred over lower-priced ones.
A number of surveys, including a recent one of more than 1,000 specialty physicians by the Alliance of Specialty Medicine, show that PAs are not only a significant administrative burden on practices but also harm patients with significant delays in accessing needed treatments and diagnostic services.
The often-cited study by Zachary Wallace et al. clearly demonstrates significant harm to rheumatology patients whose treatments were delayed because of PAs. These delays caused a substantial increase in steroid dosages in patients whose PA was initially denied and even in those patients whose PAs were initially approved. These data and others support the urgent need to address the entire spectrum of PAs.
Over the last few years, we have seen many states passing laws, adding common-sense protections to mitigate the harmful consequences of UMTs. Such reforms are needed now to stop the indiscriminate use of PAs. Suggestions have included completely eliminating PAs for medications and services that are consistently approved, standardizing electronic forms across all health plans with real-time approval, and others, including “gold card” legislation. In addition to states’ efforts, Congress proposed H.R. 3173, the Improving Seniors’ Timely Access to Care Act of 2021, to protect seniors from the harm caused by PAs that are required by Medicare Advantage programs.
This brings us to the topic of gold card legislation, in which physicians would be given a gold card exempting them from PA for specific services (hopefully including prescription drugs). However, the criteria a physician needs to qualify for a gold card could vary from state to state. For example, it could be based on a physician’s PA approval rate during a specified review period, or it could be completely up to the insurance company to decide the criteria.
Texas is the only state that has passed gold card legislation thus far, although there is an active gold card bill in Louisiana (as of this writing). There are a few other states that have introduced gold card bills that have not yet passed, but there is definite interest throughout the country in this concept. In the Texas legislation, physicians would qualify for a gold card if they had a PA approval threshold of 90% for specific medications or services over a 6-month review period.
A few of the concerns about how this will be implemented and the potential unintended consequences of the legislation include:
- Would one gold card cover all drugs, a specific drug, or just a specific drug for a specific diagnosis?
- Will clinicians get bogged down appealing gold card denials/rescissions?
- Will health plans begin denying more requests up front to keep clinicians from qualifying for an exemption?
Unfortunately, the Louisiana gold card legislation has been amended from its original form to exclude “pharmacy services” and qualification for the gold card “shall be at the sole discretion of the health insurance issuer.”
Consequently, my initial excitement surrounding the Louisiana gold card legislation, for our specialty, has for the most part disappeared. Nonetheless, there is clear excitement behind the gold card concept throughout the country.
What is clear is that health insurance companies and pharmacy benefit managers have lost sight of the original purpose of UMTs, which is to ensure that patients have access to cost-effective quality care. Over the years, the aggressive use of PAs and other UMTs has led to a significant increase in administrative burden for our offices, and more importantly, a loss of disease control in many of our patients, resulting in an increase in overall health care costs.
While it is extremely disturbing that we need legislation to force health plans to keep our patients safe and ensure quality of care, it certainly proves that now, more than ever, we must make our voices be heard.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
I live in New Orleans and recently became aware of a piece of state legislation that would create a “gold card” system for prior authorizations in Louisiana. Before delving into what is a gold card and how it works, let’s take a look at the evolution of prior authorizations (PAs).
Commercial health insurance and Medicare/Medicaid had their beginnings in the 1950s and 1960s. Because the government would now be paying for medical services for seniors, there was a concern that there might be an “overutilization” of services. This concern resulted in the concepts of utilization review and “medical necessity.” These utilization reviews morphed into what are now known as utilization management tools (UMTs). The original intent of these tools was to link cost containment to quality assurance.
PAs are one of a number of UMTs, along with formulary step therapy and nonmedical switching, that are used by health insurance companies and pharmacy benefit managers to determine whether a prescribed product or service is medically necessary and cost effective. Originally, it also meant that the service/treatment would be reimbursed. That is not the case anymore.
Today, physicians face many frivolous PAs for generic medications, such as methotrexate and prednisone, and ironically sometimes higher-priced drugs are preferred over lower-priced ones.
A number of surveys, including a recent one of more than 1,000 specialty physicians by the Alliance of Specialty Medicine, show that PAs are not only a significant administrative burden on practices but also harm patients with significant delays in accessing needed treatments and diagnostic services.
The often-cited study by Zachary Wallace et al. clearly demonstrates significant harm to rheumatology patients whose treatments were delayed because of PAs. These delays caused a substantial increase in steroid dosages in patients whose PA was initially denied and even in those patients whose PAs were initially approved. These data and others support the urgent need to address the entire spectrum of PAs.
Over the last few years, we have seen many states passing laws, adding common-sense protections to mitigate the harmful consequences of UMTs. Such reforms are needed now to stop the indiscriminate use of PAs. Suggestions have included completely eliminating PAs for medications and services that are consistently approved, standardizing electronic forms across all health plans with real-time approval, and others, including “gold card” legislation. In addition to states’ efforts, Congress proposed H.R. 3173, the Improving Seniors’ Timely Access to Care Act of 2021, to protect seniors from the harm caused by PAs that are required by Medicare Advantage programs.
This brings us to the topic of gold card legislation, in which physicians would be given a gold card exempting them from PA for specific services (hopefully including prescription drugs). However, the criteria a physician needs to qualify for a gold card could vary from state to state. For example, it could be based on a physician’s PA approval rate during a specified review period, or it could be completely up to the insurance company to decide the criteria.
Texas is the only state that has passed gold card legislation thus far, although there is an active gold card bill in Louisiana (as of this writing). There are a few other states that have introduced gold card bills that have not yet passed, but there is definite interest throughout the country in this concept. In the Texas legislation, physicians would qualify for a gold card if they had a PA approval threshold of 90% for specific medications or services over a 6-month review period.
A few of the concerns about how this will be implemented and the potential unintended consequences of the legislation include:
- Would one gold card cover all drugs, a specific drug, or just a specific drug for a specific diagnosis?
- Will clinicians get bogged down appealing gold card denials/rescissions?
- Will health plans begin denying more requests up front to keep clinicians from qualifying for an exemption?
Unfortunately, the Louisiana gold card legislation has been amended from its original form to exclude “pharmacy services” and qualification for the gold card “shall be at the sole discretion of the health insurance issuer.”
Consequently, my initial excitement surrounding the Louisiana gold card legislation, for our specialty, has for the most part disappeared. Nonetheless, there is clear excitement behind the gold card concept throughout the country.
What is clear is that health insurance companies and pharmacy benefit managers have lost sight of the original purpose of UMTs, which is to ensure that patients have access to cost-effective quality care. Over the years, the aggressive use of PAs and other UMTs has led to a significant increase in administrative burden for our offices, and more importantly, a loss of disease control in many of our patients, resulting in an increase in overall health care costs.
While it is extremely disturbing that we need legislation to force health plans to keep our patients safe and ensure quality of care, it certainly proves that now, more than ever, we must make our voices be heard.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Downtime? Enjoy it
Everything in medicine, and pretty much the universe, is based on averages. Average reduction of seizures, average blood levels, average response to treatment, average insurance reimbursement, average time spent with a new consult.
Statistics are helpful in working through large amounts of data, but on a smaller scale, like my practice, statistics aren’t quite as helpful.
I see, on average, maybe 10 patients per day, consisting of new ones, follow-ups, and electromyography and nerve conduction velocity (EMG/NCV) studies. That is, by far, a smaller number of patients than my colleagues in primary care see, and probably other neurology practices as well. But it works for me.
But that’s on averages and not always. Sometimes we all hit slumps. Who knows why? Everyone is on vacation, or the holidays are coming, or they’ve been abducted by aliens. Whatever the reason, I get the occasional week where I’m pretty bored. Maybe one or two patients in a day. I start to feel like the lonely Maytag repairman behind my desk. I check to see if any drug samples have expired. I wonder if people are actually reading my online reviews and going elsewhere.
Years ago weeks like that terrified me. I was worried my little practice might fail (granted, it still could). But as years – and cycles that make up the averages – go by, they don’t bother me as much.
After 23 years I’ve learned that it’s just part of the normal fluctuations that make up an average. One morning I’ll roll the phones and the lines will explode (figuratively, I hope) with calls. At times like these my secretary seems to grow another pair of arms as she frantically schedules callers, puts others on hold, copies insurance cards, and gives the evil eye to drug reps who step in and ask her if she’s busy.
Then my schedule gets packed. My secretary crams patients in my emergency slots of 7:00, 8:00, and 12:00. MRI results come in that require me to see people sooner rather than later. My “average” of 10 patients per day suddenly doesn’t exist. I go home with a pile of dictations to do and work away into the night to catch up.
With experience we learn to take this in stride. Now, when I hit a slow patch, I remind myself that it’s not the average, and to enjoy it while I can. Read a book, take a long lunch, go home early and nap.
Worrying about where the patients are isn’t productive, or good for your mental health. They know where I am, and will find me when they need me.
. Enjoy the slow times while you can.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Everything in medicine, and pretty much the universe, is based on averages. Average reduction of seizures, average blood levels, average response to treatment, average insurance reimbursement, average time spent with a new consult.
Statistics are helpful in working through large amounts of data, but on a smaller scale, like my practice, statistics aren’t quite as helpful.
I see, on average, maybe 10 patients per day, consisting of new ones, follow-ups, and electromyography and nerve conduction velocity (EMG/NCV) studies. That is, by far, a smaller number of patients than my colleagues in primary care see, and probably other neurology practices as well. But it works for me.
But that’s on averages and not always. Sometimes we all hit slumps. Who knows why? Everyone is on vacation, or the holidays are coming, or they’ve been abducted by aliens. Whatever the reason, I get the occasional week where I’m pretty bored. Maybe one or two patients in a day. I start to feel like the lonely Maytag repairman behind my desk. I check to see if any drug samples have expired. I wonder if people are actually reading my online reviews and going elsewhere.
Years ago weeks like that terrified me. I was worried my little practice might fail (granted, it still could). But as years – and cycles that make up the averages – go by, they don’t bother me as much.
After 23 years I’ve learned that it’s just part of the normal fluctuations that make up an average. One morning I’ll roll the phones and the lines will explode (figuratively, I hope) with calls. At times like these my secretary seems to grow another pair of arms as she frantically schedules callers, puts others on hold, copies insurance cards, and gives the evil eye to drug reps who step in and ask her if she’s busy.
Then my schedule gets packed. My secretary crams patients in my emergency slots of 7:00, 8:00, and 12:00. MRI results come in that require me to see people sooner rather than later. My “average” of 10 patients per day suddenly doesn’t exist. I go home with a pile of dictations to do and work away into the night to catch up.
With experience we learn to take this in stride. Now, when I hit a slow patch, I remind myself that it’s not the average, and to enjoy it while I can. Read a book, take a long lunch, go home early and nap.
Worrying about where the patients are isn’t productive, or good for your mental health. They know where I am, and will find me when they need me.
. Enjoy the slow times while you can.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Everything in medicine, and pretty much the universe, is based on averages. Average reduction of seizures, average blood levels, average response to treatment, average insurance reimbursement, average time spent with a new consult.
Statistics are helpful in working through large amounts of data, but on a smaller scale, like my practice, statistics aren’t quite as helpful.
I see, on average, maybe 10 patients per day, consisting of new ones, follow-ups, and electromyography and nerve conduction velocity (EMG/NCV) studies. That is, by far, a smaller number of patients than my colleagues in primary care see, and probably other neurology practices as well. But it works for me.
But that’s on averages and not always. Sometimes we all hit slumps. Who knows why? Everyone is on vacation, or the holidays are coming, or they’ve been abducted by aliens. Whatever the reason, I get the occasional week where I’m pretty bored. Maybe one or two patients in a day. I start to feel like the lonely Maytag repairman behind my desk. I check to see if any drug samples have expired. I wonder if people are actually reading my online reviews and going elsewhere.
Years ago weeks like that terrified me. I was worried my little practice might fail (granted, it still could). But as years – and cycles that make up the averages – go by, they don’t bother me as much.
After 23 years I’ve learned that it’s just part of the normal fluctuations that make up an average. One morning I’ll roll the phones and the lines will explode (figuratively, I hope) with calls. At times like these my secretary seems to grow another pair of arms as she frantically schedules callers, puts others on hold, copies insurance cards, and gives the evil eye to drug reps who step in and ask her if she’s busy.
Then my schedule gets packed. My secretary crams patients in my emergency slots of 7:00, 8:00, and 12:00. MRI results come in that require me to see people sooner rather than later. My “average” of 10 patients per day suddenly doesn’t exist. I go home with a pile of dictations to do and work away into the night to catch up.
With experience we learn to take this in stride. Now, when I hit a slow patch, I remind myself that it’s not the average, and to enjoy it while I can. Read a book, take a long lunch, go home early and nap.
Worrying about where the patients are isn’t productive, or good for your mental health. They know where I am, and will find me when they need me.
. Enjoy the slow times while you can.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Alarming increase in esophageal cancer in middle-aged adults
An alarming increase in both esophageal cancer (EC) and the primary precursor lesion for esophageal adenocarcinoma known as Barrett’s esophagus (BE) has been observed among middle-aged adults over the past 5 years, and it’s not because of better or more frequent screening, warn the authors of a new study from Florida.
“We found that the [prevalence of] esophageal cancer and Barrett’s esophagus may have in fact plateaued in the elderly, but there is a concerning increase in their prevalence in middle-aged adults despite the fact that there has been no increase in the use of endoscopy in this population,” Bashar Qumseya, MD, MPH, associate professor of medicine and chief of endoscopy, University of Florida, Gainesville, told a press briefing.
“This should be of great concern to physicians and to patients, and it is our suggestion that maybe we should consider screening middle-aged patients or even those at younger ages for both conditions,” he added.
The study was highlighted during a press briefing in advance of the annual Digestive Disease Week® (DDW).
Research network
The analysis was carried out using electronic health records from the OneFlorida Clinical Data Research Network, a database that covers over 40% of residents living in Florida. The researchers identified patients who had been diagnosed with EC or BE between 2012 and 2019. “The primary outcome of interest was the adjusted prevalence of EC and BE in the population,” they stated.
The cohort was categorized by age: those aged 18-44 years (young); those aged 45-64 years (middle-aged), and those older than 65 (elderly). The number of patients included in the database varied by year and ranged from 4,238,884 to 5,411,838 patients per year, the investigators noted. Interestingly, in the most recent year, 2019, more women, at over 57%, were diagnosed with EC than were men.
Over the study interval, the prevalence of EC remained stable among the elderly but nearly doubled among middle-aged patients, from a rate of 49 per 100,000 in 2012 to a rate of 94 per 100,000 in 2019.
Similarly, there was a 50% increase in BE over the same study interval, from 304 per 100,000 in 2012 to 466 per 100,000 in 2019, again in the middle-aged group. The increase in the prevalence of BE was highest in those aged 51-60 years, followed by those aged 61-70 years and then by those aged 41-50.
Data from the same cohort also indicated that the great majority of patients with multiple risk factors for EC or BE – obesity, diet, and gastroesophageal reflux disease – had never undergone endoscopy, “so we can definitely do better,” Dr. Qumseya said. One simple way to “do better” is to offer patients an endoscopy when they undergo their first colonoscopy at the recommended age of 45 years.
“I am not in a position to make the guidelines,”Dr. Qumseya commented. “But we do [already] have guidelines that suggest that patients with multiple risk factors [for EC and BE] be screened, and since we know from our data that this is not happening, I believe that if a patient has multiple risk factors, they should have at least one screening endoscopy at the time of colonoscopy. Otherwise, we are missing a good opportunity to do so, and personally, I think this is something that we should be considering.”
A version of this article first appeared on Medscape.com.
An alarming increase in both esophageal cancer (EC) and the primary precursor lesion for esophageal adenocarcinoma known as Barrett’s esophagus (BE) has been observed among middle-aged adults over the past 5 years, and it’s not because of better or more frequent screening, warn the authors of a new study from Florida.
“We found that the [prevalence of] esophageal cancer and Barrett’s esophagus may have in fact plateaued in the elderly, but there is a concerning increase in their prevalence in middle-aged adults despite the fact that there has been no increase in the use of endoscopy in this population,” Bashar Qumseya, MD, MPH, associate professor of medicine and chief of endoscopy, University of Florida, Gainesville, told a press briefing.
“This should be of great concern to physicians and to patients, and it is our suggestion that maybe we should consider screening middle-aged patients or even those at younger ages for both conditions,” he added.
The study was highlighted during a press briefing in advance of the annual Digestive Disease Week® (DDW).
Research network
The analysis was carried out using electronic health records from the OneFlorida Clinical Data Research Network, a database that covers over 40% of residents living in Florida. The researchers identified patients who had been diagnosed with EC or BE between 2012 and 2019. “The primary outcome of interest was the adjusted prevalence of EC and BE in the population,” they stated.
The cohort was categorized by age: those aged 18-44 years (young); those aged 45-64 years (middle-aged), and those older than 65 (elderly). The number of patients included in the database varied by year and ranged from 4,238,884 to 5,411,838 patients per year, the investigators noted. Interestingly, in the most recent year, 2019, more women, at over 57%, were diagnosed with EC than were men.
Over the study interval, the prevalence of EC remained stable among the elderly but nearly doubled among middle-aged patients, from a rate of 49 per 100,000 in 2012 to a rate of 94 per 100,000 in 2019.
Similarly, there was a 50% increase in BE over the same study interval, from 304 per 100,000 in 2012 to 466 per 100,000 in 2019, again in the middle-aged group. The increase in the prevalence of BE was highest in those aged 51-60 years, followed by those aged 61-70 years and then by those aged 41-50.
Data from the same cohort also indicated that the great majority of patients with multiple risk factors for EC or BE – obesity, diet, and gastroesophageal reflux disease – had never undergone endoscopy, “so we can definitely do better,” Dr. Qumseya said. One simple way to “do better” is to offer patients an endoscopy when they undergo their first colonoscopy at the recommended age of 45 years.
“I am not in a position to make the guidelines,”Dr. Qumseya commented. “But we do [already] have guidelines that suggest that patients with multiple risk factors [for EC and BE] be screened, and since we know from our data that this is not happening, I believe that if a patient has multiple risk factors, they should have at least one screening endoscopy at the time of colonoscopy. Otherwise, we are missing a good opportunity to do so, and personally, I think this is something that we should be considering.”
A version of this article first appeared on Medscape.com.
An alarming increase in both esophageal cancer (EC) and the primary precursor lesion for esophageal adenocarcinoma known as Barrett’s esophagus (BE) has been observed among middle-aged adults over the past 5 years, and it’s not because of better or more frequent screening, warn the authors of a new study from Florida.
“We found that the [prevalence of] esophageal cancer and Barrett’s esophagus may have in fact plateaued in the elderly, but there is a concerning increase in their prevalence in middle-aged adults despite the fact that there has been no increase in the use of endoscopy in this population,” Bashar Qumseya, MD, MPH, associate professor of medicine and chief of endoscopy, University of Florida, Gainesville, told a press briefing.
“This should be of great concern to physicians and to patients, and it is our suggestion that maybe we should consider screening middle-aged patients or even those at younger ages for both conditions,” he added.
The study was highlighted during a press briefing in advance of the annual Digestive Disease Week® (DDW).
Research network
The analysis was carried out using electronic health records from the OneFlorida Clinical Data Research Network, a database that covers over 40% of residents living in Florida. The researchers identified patients who had been diagnosed with EC or BE between 2012 and 2019. “The primary outcome of interest was the adjusted prevalence of EC and BE in the population,” they stated.
The cohort was categorized by age: those aged 18-44 years (young); those aged 45-64 years (middle-aged), and those older than 65 (elderly). The number of patients included in the database varied by year and ranged from 4,238,884 to 5,411,838 patients per year, the investigators noted. Interestingly, in the most recent year, 2019, more women, at over 57%, were diagnosed with EC than were men.
Over the study interval, the prevalence of EC remained stable among the elderly but nearly doubled among middle-aged patients, from a rate of 49 per 100,000 in 2012 to a rate of 94 per 100,000 in 2019.
Similarly, there was a 50% increase in BE over the same study interval, from 304 per 100,000 in 2012 to 466 per 100,000 in 2019, again in the middle-aged group. The increase in the prevalence of BE was highest in those aged 51-60 years, followed by those aged 61-70 years and then by those aged 41-50.
Data from the same cohort also indicated that the great majority of patients with multiple risk factors for EC or BE – obesity, diet, and gastroesophageal reflux disease – had never undergone endoscopy, “so we can definitely do better,” Dr. Qumseya said. One simple way to “do better” is to offer patients an endoscopy when they undergo their first colonoscopy at the recommended age of 45 years.
“I am not in a position to make the guidelines,”Dr. Qumseya commented. “But we do [already] have guidelines that suggest that patients with multiple risk factors [for EC and BE] be screened, and since we know from our data that this is not happening, I believe that if a patient has multiple risk factors, they should have at least one screening endoscopy at the time of colonoscopy. Otherwise, we are missing a good opportunity to do so, and personally, I think this is something that we should be considering.”
A version of this article first appeared on Medscape.com.
New insight into how brain stimulation eases major depression
For the first time, researchers understand what happens to the brain when patients with treatment-resistant depression receive repetitive transcranial magnetic stimulation (rTMS).
Using functional magnetic resonance imaging (fMRI), they showed that rTMS induces widespread alterations in functional connectivity in brain regions involved in emotion and motor control.
“‘How does rTMS work?’ is one of the most frequent questions I get in clinic. Providing an accurate explanation and narrative to patients is critical,” senior investigator Fidel Vila-Rodriguez, MD, PhD, director of the Non-Invasive Neurostimulation Therapies Laboratory, University of British Columbia, Vancouver, told this news organization.
“Our findings suggest that rTMS might rely on the brain’s capacity for change (neuroplasticity) to exert its effects and that rTMS effects on the brain are widespread beyond the focal area stimulated (functional network effects),” Dr. Vila-Rodriguez added.
The study was published online in the American Journal of Psychiatry.
Mechanistic insights
Although rTMS has proven efficacy for treatment-resistant depression, the mechanisms behind how it affects the brain are not well understood.
In the current study, researchers used fMRI to assess changes in functional connectivity induced by a single rTMS session in 26 women and 12 men with treatment-resistant depression.
They found that – from managing emotional responses to memory and motor control.
Following a 4-week course of rTMS, these connectivity changes predicted about 30% of the variance of improvement in scores on the Montgomery-Åsberg Depression Rating Scale after rTMS treatment.
The most robust predictive associations involved connections between prefrontal regions and motor, parietal, and insular cortices and between bilateral regions of the thalamus.
“By demonstrating this principle and identifying regions of the brain that are activated by rTMS, we can now try to understand whether this pattern can be used as a biomarker,” Dr. Vila-Rodriguez said in a news release.
“This work provides a mechanistic explanation of what rTMS does to treat depression and supports the notion that for rTMS to treat depressive symptoms a distributed change in brain activity (network or circuit base) is necessary,” he told this news organization.
With funding from the Canadian Institutes of Health Research (CIHR), the team will next see if they can use fMRI to guide rTMS at the individual level, with the ultimate goal of “personalizing” rTMS using individualized functional targets, Dr. Vila-Rodriguez said.
New generation of tms researchers
Reached for comment, Jonathan Downar, MD, PhD, department of psychiatry, University of Toronto, noted that TMS can be “very effective” for treatment-resistant depression, and it has a “very clean side effect profile compared to medications.”
What the field is trying to figure out now is “who it works for and how we can predict more effectively who’s going to benefit from it,” Dr. Downar said in an interview.
He noted that the study’s investigators are part of a “new generation of TMS researchers who are bringing new ideas into the fold and figuring out how to use brain imaging to personalize the treatment.” This study represents “a step” in that direction.
“A challenge for the field is that it’s often pretty easy to demonstrate a change at the group level, but the question is whether we can use that at the individual level. That’s a higher bar to meet, and we’re still not there yet,” Dr. Downar added.
Support for the study was provided by Brain Canada, the Michael Smith Foundation for Health Research and the Vancouver Coastal Health Research Institute. Dr. Vila-Rodriguez has received research support from CIHR, Brain Canada, the Michael Smith Foundation for Health Research, the Vancouver Coastal Health Research Institute, and the Weston Brain Institute for investigator-initiated research and philanthropic support from the Seedlings Foundation; he received in-kind equipment support from MagVenture for this investigator-initiated trial; and he has received honoraria for participation on an advisory board for Janssen. Dr. Downar has served as an adviser for BrainCheck, NeuroStim TMS, and Salience Neuro Health; received research grant from CIHR, National Institute for Mental Health, Brain Canada, Canadian Biomarker Integration Network in Depression, Ontario Brain Institute, Klarman Family Foundation, Arrell Family Foundation and the Edgestone Foundation; received travel stipends from Lundbeck and ANT Neuro; and received in-kind equipment support for investigator-initiated trials from MagVenture.
A version of this article first appeared on Medscape.com.
For the first time, researchers understand what happens to the brain when patients with treatment-resistant depression receive repetitive transcranial magnetic stimulation (rTMS).
Using functional magnetic resonance imaging (fMRI), they showed that rTMS induces widespread alterations in functional connectivity in brain regions involved in emotion and motor control.
“‘How does rTMS work?’ is one of the most frequent questions I get in clinic. Providing an accurate explanation and narrative to patients is critical,” senior investigator Fidel Vila-Rodriguez, MD, PhD, director of the Non-Invasive Neurostimulation Therapies Laboratory, University of British Columbia, Vancouver, told this news organization.
“Our findings suggest that rTMS might rely on the brain’s capacity for change (neuroplasticity) to exert its effects and that rTMS effects on the brain are widespread beyond the focal area stimulated (functional network effects),” Dr. Vila-Rodriguez added.
The study was published online in the American Journal of Psychiatry.
Mechanistic insights
Although rTMS has proven efficacy for treatment-resistant depression, the mechanisms behind how it affects the brain are not well understood.
In the current study, researchers used fMRI to assess changes in functional connectivity induced by a single rTMS session in 26 women and 12 men with treatment-resistant depression.
They found that – from managing emotional responses to memory and motor control.
Following a 4-week course of rTMS, these connectivity changes predicted about 30% of the variance of improvement in scores on the Montgomery-Åsberg Depression Rating Scale after rTMS treatment.
The most robust predictive associations involved connections between prefrontal regions and motor, parietal, and insular cortices and between bilateral regions of the thalamus.
“By demonstrating this principle and identifying regions of the brain that are activated by rTMS, we can now try to understand whether this pattern can be used as a biomarker,” Dr. Vila-Rodriguez said in a news release.
“This work provides a mechanistic explanation of what rTMS does to treat depression and supports the notion that for rTMS to treat depressive symptoms a distributed change in brain activity (network or circuit base) is necessary,” he told this news organization.
With funding from the Canadian Institutes of Health Research (CIHR), the team will next see if they can use fMRI to guide rTMS at the individual level, with the ultimate goal of “personalizing” rTMS using individualized functional targets, Dr. Vila-Rodriguez said.
New generation of tms researchers
Reached for comment, Jonathan Downar, MD, PhD, department of psychiatry, University of Toronto, noted that TMS can be “very effective” for treatment-resistant depression, and it has a “very clean side effect profile compared to medications.”
What the field is trying to figure out now is “who it works for and how we can predict more effectively who’s going to benefit from it,” Dr. Downar said in an interview.
He noted that the study’s investigators are part of a “new generation of TMS researchers who are bringing new ideas into the fold and figuring out how to use brain imaging to personalize the treatment.” This study represents “a step” in that direction.
“A challenge for the field is that it’s often pretty easy to demonstrate a change at the group level, but the question is whether we can use that at the individual level. That’s a higher bar to meet, and we’re still not there yet,” Dr. Downar added.
Support for the study was provided by Brain Canada, the Michael Smith Foundation for Health Research and the Vancouver Coastal Health Research Institute. Dr. Vila-Rodriguez has received research support from CIHR, Brain Canada, the Michael Smith Foundation for Health Research, the Vancouver Coastal Health Research Institute, and the Weston Brain Institute for investigator-initiated research and philanthropic support from the Seedlings Foundation; he received in-kind equipment support from MagVenture for this investigator-initiated trial; and he has received honoraria for participation on an advisory board for Janssen. Dr. Downar has served as an adviser for BrainCheck, NeuroStim TMS, and Salience Neuro Health; received research grant from CIHR, National Institute for Mental Health, Brain Canada, Canadian Biomarker Integration Network in Depression, Ontario Brain Institute, Klarman Family Foundation, Arrell Family Foundation and the Edgestone Foundation; received travel stipends from Lundbeck and ANT Neuro; and received in-kind equipment support for investigator-initiated trials from MagVenture.
A version of this article first appeared on Medscape.com.
For the first time, researchers understand what happens to the brain when patients with treatment-resistant depression receive repetitive transcranial magnetic stimulation (rTMS).
Using functional magnetic resonance imaging (fMRI), they showed that rTMS induces widespread alterations in functional connectivity in brain regions involved in emotion and motor control.
“‘How does rTMS work?’ is one of the most frequent questions I get in clinic. Providing an accurate explanation and narrative to patients is critical,” senior investigator Fidel Vila-Rodriguez, MD, PhD, director of the Non-Invasive Neurostimulation Therapies Laboratory, University of British Columbia, Vancouver, told this news organization.
“Our findings suggest that rTMS might rely on the brain’s capacity for change (neuroplasticity) to exert its effects and that rTMS effects on the brain are widespread beyond the focal area stimulated (functional network effects),” Dr. Vila-Rodriguez added.
The study was published online in the American Journal of Psychiatry.
Mechanistic insights
Although rTMS has proven efficacy for treatment-resistant depression, the mechanisms behind how it affects the brain are not well understood.
In the current study, researchers used fMRI to assess changes in functional connectivity induced by a single rTMS session in 26 women and 12 men with treatment-resistant depression.
They found that – from managing emotional responses to memory and motor control.
Following a 4-week course of rTMS, these connectivity changes predicted about 30% of the variance of improvement in scores on the Montgomery-Åsberg Depression Rating Scale after rTMS treatment.
The most robust predictive associations involved connections between prefrontal regions and motor, parietal, and insular cortices and between bilateral regions of the thalamus.
“By demonstrating this principle and identifying regions of the brain that are activated by rTMS, we can now try to understand whether this pattern can be used as a biomarker,” Dr. Vila-Rodriguez said in a news release.
“This work provides a mechanistic explanation of what rTMS does to treat depression and supports the notion that for rTMS to treat depressive symptoms a distributed change in brain activity (network or circuit base) is necessary,” he told this news organization.
With funding from the Canadian Institutes of Health Research (CIHR), the team will next see if they can use fMRI to guide rTMS at the individual level, with the ultimate goal of “personalizing” rTMS using individualized functional targets, Dr. Vila-Rodriguez said.
New generation of tms researchers
Reached for comment, Jonathan Downar, MD, PhD, department of psychiatry, University of Toronto, noted that TMS can be “very effective” for treatment-resistant depression, and it has a “very clean side effect profile compared to medications.”
What the field is trying to figure out now is “who it works for and how we can predict more effectively who’s going to benefit from it,” Dr. Downar said in an interview.
He noted that the study’s investigators are part of a “new generation of TMS researchers who are bringing new ideas into the fold and figuring out how to use brain imaging to personalize the treatment.” This study represents “a step” in that direction.
“A challenge for the field is that it’s often pretty easy to demonstrate a change at the group level, but the question is whether we can use that at the individual level. That’s a higher bar to meet, and we’re still not there yet,” Dr. Downar added.
Support for the study was provided by Brain Canada, the Michael Smith Foundation for Health Research and the Vancouver Coastal Health Research Institute. Dr. Vila-Rodriguez has received research support from CIHR, Brain Canada, the Michael Smith Foundation for Health Research, the Vancouver Coastal Health Research Institute, and the Weston Brain Institute for investigator-initiated research and philanthropic support from the Seedlings Foundation; he received in-kind equipment support from MagVenture for this investigator-initiated trial; and he has received honoraria for participation on an advisory board for Janssen. Dr. Downar has served as an adviser for BrainCheck, NeuroStim TMS, and Salience Neuro Health; received research grant from CIHR, National Institute for Mental Health, Brain Canada, Canadian Biomarker Integration Network in Depression, Ontario Brain Institute, Klarman Family Foundation, Arrell Family Foundation and the Edgestone Foundation; received travel stipends from Lundbeck and ANT Neuro; and received in-kind equipment support for investigator-initiated trials from MagVenture.
A version of this article first appeared on Medscape.com.
Type 2 Diabetes: Pathophysiology
SAFE-PAD shows long-term safety of paclitaxel devices
Patients who have paclitaxel-coated stents and balloons have survival and outcomes comparable to those who have a bare-metal stent or percutaneous transluminal angioplasty, according to updated results from a large study of almost 170,000 Medicare beneficiaries.
The SAFE-PAD study analyzed Medicare claims data of 168,533 patients, including 70,584 who were treated with drug-coated devices (DCD), from April 2015 through 2018.
Notably, Eric A. Secemsky, MD, MSc, said in an interview, that included more than 32,000 patients with more than 5 years of follow-up. He presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“What we’re seeing now with this study is that paclitaxel-coated devices [PCDs] have the same long-term survival compared to those treated with non–drug-coated devices (NDCDs),” said Dr. Secemsky, director of vascular intervention at Beth Israel Deaconess Medical Center in Boston. “I think this is another important piece and some of the longest-term data in this size population to demonstrate the long-term safety of PCD, and hopefully it will help us get back to normal practice that has been halted now for over 3 years.”
That was a reference to the 2018 meta-analysis by Konstantinos Katsanos, MD, PhD, of Patras University in Greece, and colleagues, which showed an increased risk of death after PCD placements. That study threw a wet blanket of sorts on PCD use, Dr. Secemsky said.
The median follow-up for SAFE-PAD (formally called the Safety Assessment of Femoropopliteal Endovascular treatment with Paclitaxel-coated Devices) was 3.5 years, with the longest follow-up, 6.3 years. The weighted cumulative incidence of mortality at 6.3 years was 63.6% with NDCDs and 62.5% with DCDs (hazard ratio, 0.98; 95% confidence interval, 0.96-0.99; P < .0001). A subgroup analysis found no link between DCDs and increased death in low-risk patients, low-comorbid patients, inpatient or outpatient treatment, patients without critical limb ischemia, or patients treated with stents or balloon angioplasty alone.
“This report and the length of follow-up is one more piece that has continued to demonstrate safety with PCDs,” Dr. Secemsky said. He added that these results fall in line with smaller studies that failed to show a link between DCDs and long-term mortality, notably the SWEDEPAD randomized study of 2,289 patients evaluated through 4 years, and a subanalysis of 4,000 patients in VOYAGER-PAD through 42 months of follow-up.
“So we’ve really shown through these data sets and others that we can’t replicate any harms that we’ve seen in that Katsanos meta-analysis, and it suggests that there was some bias in that meta-analysis.”
Strengths of the study are its size and the way it followed the patients longitudinally, Sahil A. Parikh, MD, director of endovascular services at Columbia University Vagelos College of Physicians and Surgeons in New York, said in an interview.
With regard to its limitations, Dr. Parikh said, “On the other hand, it’s a claims database which doesn’t have the granularity about the patients’ specific procedural factors,” he said. “There are gaps that might further inform the value of lack thereof of the drug-coated device, but certainly at the topline, which is the hard endpoint of mortality, you can read quite a lot and you can assume that with such large numbers, the signal-to-noise ratio would be sufficiently sensitive that you get a real signal.”
With these updated SAFE-PAD results along with other studies, Dr. Parikh said, “If one weighs the risk benefit of cardiac lesion revascularization regarding requiring a repeat procedure vs. the risk of mortality from paclitaxel, if there is such a thing, I think most physicians have come back and the pendulum has swung back considering it reasonable to use paclitaxel products.”
That’s a message that will resonate with patients reluctant to return to the hospital since the COVID-19 outbreak, he said. “If you can tell them we can avoid a repeat trip to the hospital, they’re all for it,” Dr. Parikh said.
The study results were published simultaneously with Dr. Secemsky’s presentation. Funding for SAFE-PAD came from a multi-industry consortium consisting of BD, Boston Scientific, Cook Medical, Medtronic and Philips, which wasn’t involved in the study design or analysis.
Dr. Secemsky disclosed relationships with Abbott, BD, Bayer, Boston Scientific, Cook Medical, CSI, Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and Venture Med. Dr. Parikh disclosed relationships with TriReme Medical, Boston Scientific, Heartflow, Cordis, Janssen, Terumo, Canon, Shockwave, Abiomed, Abbott, Cardiovascular Systems, Inari and Surmodics.
Patients who have paclitaxel-coated stents and balloons have survival and outcomes comparable to those who have a bare-metal stent or percutaneous transluminal angioplasty, according to updated results from a large study of almost 170,000 Medicare beneficiaries.
The SAFE-PAD study analyzed Medicare claims data of 168,533 patients, including 70,584 who were treated with drug-coated devices (DCD), from April 2015 through 2018.
Notably, Eric A. Secemsky, MD, MSc, said in an interview, that included more than 32,000 patients with more than 5 years of follow-up. He presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“What we’re seeing now with this study is that paclitaxel-coated devices [PCDs] have the same long-term survival compared to those treated with non–drug-coated devices (NDCDs),” said Dr. Secemsky, director of vascular intervention at Beth Israel Deaconess Medical Center in Boston. “I think this is another important piece and some of the longest-term data in this size population to demonstrate the long-term safety of PCD, and hopefully it will help us get back to normal practice that has been halted now for over 3 years.”
That was a reference to the 2018 meta-analysis by Konstantinos Katsanos, MD, PhD, of Patras University in Greece, and colleagues, which showed an increased risk of death after PCD placements. That study threw a wet blanket of sorts on PCD use, Dr. Secemsky said.
The median follow-up for SAFE-PAD (formally called the Safety Assessment of Femoropopliteal Endovascular treatment with Paclitaxel-coated Devices) was 3.5 years, with the longest follow-up, 6.3 years. The weighted cumulative incidence of mortality at 6.3 years was 63.6% with NDCDs and 62.5% with DCDs (hazard ratio, 0.98; 95% confidence interval, 0.96-0.99; P < .0001). A subgroup analysis found no link between DCDs and increased death in low-risk patients, low-comorbid patients, inpatient or outpatient treatment, patients without critical limb ischemia, or patients treated with stents or balloon angioplasty alone.
“This report and the length of follow-up is one more piece that has continued to demonstrate safety with PCDs,” Dr. Secemsky said. He added that these results fall in line with smaller studies that failed to show a link between DCDs and long-term mortality, notably the SWEDEPAD randomized study of 2,289 patients evaluated through 4 years, and a subanalysis of 4,000 patients in VOYAGER-PAD through 42 months of follow-up.
“So we’ve really shown through these data sets and others that we can’t replicate any harms that we’ve seen in that Katsanos meta-analysis, and it suggests that there was some bias in that meta-analysis.”
Strengths of the study are its size and the way it followed the patients longitudinally, Sahil A. Parikh, MD, director of endovascular services at Columbia University Vagelos College of Physicians and Surgeons in New York, said in an interview.
With regard to its limitations, Dr. Parikh said, “On the other hand, it’s a claims database which doesn’t have the granularity about the patients’ specific procedural factors,” he said. “There are gaps that might further inform the value of lack thereof of the drug-coated device, but certainly at the topline, which is the hard endpoint of mortality, you can read quite a lot and you can assume that with such large numbers, the signal-to-noise ratio would be sufficiently sensitive that you get a real signal.”
With these updated SAFE-PAD results along with other studies, Dr. Parikh said, “If one weighs the risk benefit of cardiac lesion revascularization regarding requiring a repeat procedure vs. the risk of mortality from paclitaxel, if there is such a thing, I think most physicians have come back and the pendulum has swung back considering it reasonable to use paclitaxel products.”
That’s a message that will resonate with patients reluctant to return to the hospital since the COVID-19 outbreak, he said. “If you can tell them we can avoid a repeat trip to the hospital, they’re all for it,” Dr. Parikh said.
The study results were published simultaneously with Dr. Secemsky’s presentation. Funding for SAFE-PAD came from a multi-industry consortium consisting of BD, Boston Scientific, Cook Medical, Medtronic and Philips, which wasn’t involved in the study design or analysis.
Dr. Secemsky disclosed relationships with Abbott, BD, Bayer, Boston Scientific, Cook Medical, CSI, Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and Venture Med. Dr. Parikh disclosed relationships with TriReme Medical, Boston Scientific, Heartflow, Cordis, Janssen, Terumo, Canon, Shockwave, Abiomed, Abbott, Cardiovascular Systems, Inari and Surmodics.
Patients who have paclitaxel-coated stents and balloons have survival and outcomes comparable to those who have a bare-metal stent or percutaneous transluminal angioplasty, according to updated results from a large study of almost 170,000 Medicare beneficiaries.
The SAFE-PAD study analyzed Medicare claims data of 168,533 patients, including 70,584 who were treated with drug-coated devices (DCD), from April 2015 through 2018.
Notably, Eric A. Secemsky, MD, MSc, said in an interview, that included more than 32,000 patients with more than 5 years of follow-up. He presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“What we’re seeing now with this study is that paclitaxel-coated devices [PCDs] have the same long-term survival compared to those treated with non–drug-coated devices (NDCDs),” said Dr. Secemsky, director of vascular intervention at Beth Israel Deaconess Medical Center in Boston. “I think this is another important piece and some of the longest-term data in this size population to demonstrate the long-term safety of PCD, and hopefully it will help us get back to normal practice that has been halted now for over 3 years.”
That was a reference to the 2018 meta-analysis by Konstantinos Katsanos, MD, PhD, of Patras University in Greece, and colleagues, which showed an increased risk of death after PCD placements. That study threw a wet blanket of sorts on PCD use, Dr. Secemsky said.
The median follow-up for SAFE-PAD (formally called the Safety Assessment of Femoropopliteal Endovascular treatment with Paclitaxel-coated Devices) was 3.5 years, with the longest follow-up, 6.3 years. The weighted cumulative incidence of mortality at 6.3 years was 63.6% with NDCDs and 62.5% with DCDs (hazard ratio, 0.98; 95% confidence interval, 0.96-0.99; P < .0001). A subgroup analysis found no link between DCDs and increased death in low-risk patients, low-comorbid patients, inpatient or outpatient treatment, patients without critical limb ischemia, or patients treated with stents or balloon angioplasty alone.
“This report and the length of follow-up is one more piece that has continued to demonstrate safety with PCDs,” Dr. Secemsky said. He added that these results fall in line with smaller studies that failed to show a link between DCDs and long-term mortality, notably the SWEDEPAD randomized study of 2,289 patients evaluated through 4 years, and a subanalysis of 4,000 patients in VOYAGER-PAD through 42 months of follow-up.
“So we’ve really shown through these data sets and others that we can’t replicate any harms that we’ve seen in that Katsanos meta-analysis, and it suggests that there was some bias in that meta-analysis.”
Strengths of the study are its size and the way it followed the patients longitudinally, Sahil A. Parikh, MD, director of endovascular services at Columbia University Vagelos College of Physicians and Surgeons in New York, said in an interview.
With regard to its limitations, Dr. Parikh said, “On the other hand, it’s a claims database which doesn’t have the granularity about the patients’ specific procedural factors,” he said. “There are gaps that might further inform the value of lack thereof of the drug-coated device, but certainly at the topline, which is the hard endpoint of mortality, you can read quite a lot and you can assume that with such large numbers, the signal-to-noise ratio would be sufficiently sensitive that you get a real signal.”
With these updated SAFE-PAD results along with other studies, Dr. Parikh said, “If one weighs the risk benefit of cardiac lesion revascularization regarding requiring a repeat procedure vs. the risk of mortality from paclitaxel, if there is such a thing, I think most physicians have come back and the pendulum has swung back considering it reasonable to use paclitaxel products.”
That’s a message that will resonate with patients reluctant to return to the hospital since the COVID-19 outbreak, he said. “If you can tell them we can avoid a repeat trip to the hospital, they’re all for it,” Dr. Parikh said.
The study results were published simultaneously with Dr. Secemsky’s presentation. Funding for SAFE-PAD came from a multi-industry consortium consisting of BD, Boston Scientific, Cook Medical, Medtronic and Philips, which wasn’t involved in the study design or analysis.
Dr. Secemsky disclosed relationships with Abbott, BD, Bayer, Boston Scientific, Cook Medical, CSI, Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and Venture Med. Dr. Parikh disclosed relationships with TriReme Medical, Boston Scientific, Heartflow, Cordis, Janssen, Terumo, Canon, Shockwave, Abiomed, Abbott, Cardiovascular Systems, Inari and Surmodics.
FROM SCAI 2022
A psychiatric patient confesses to murder: Now what?
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
AT APA 2022
Fever after a tropical trip: A guide to differential diagnosis
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.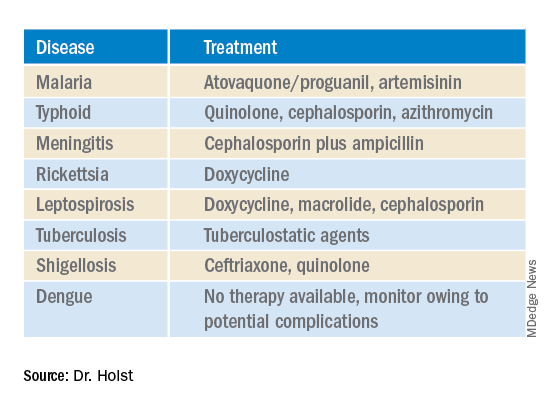
This article was translated from Coliquio. A version of this article appeared on Medscape.com.
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.
This article was translated from Coliquio. A version of this article appeared on Medscape.com.
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.
This article was translated from Coliquio. A version of this article appeared on Medscape.com.













