User login
Pencil-core Granuloma Forming 62 Years After Initial Injury
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
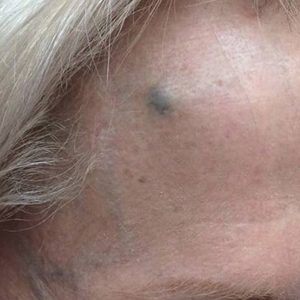
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.
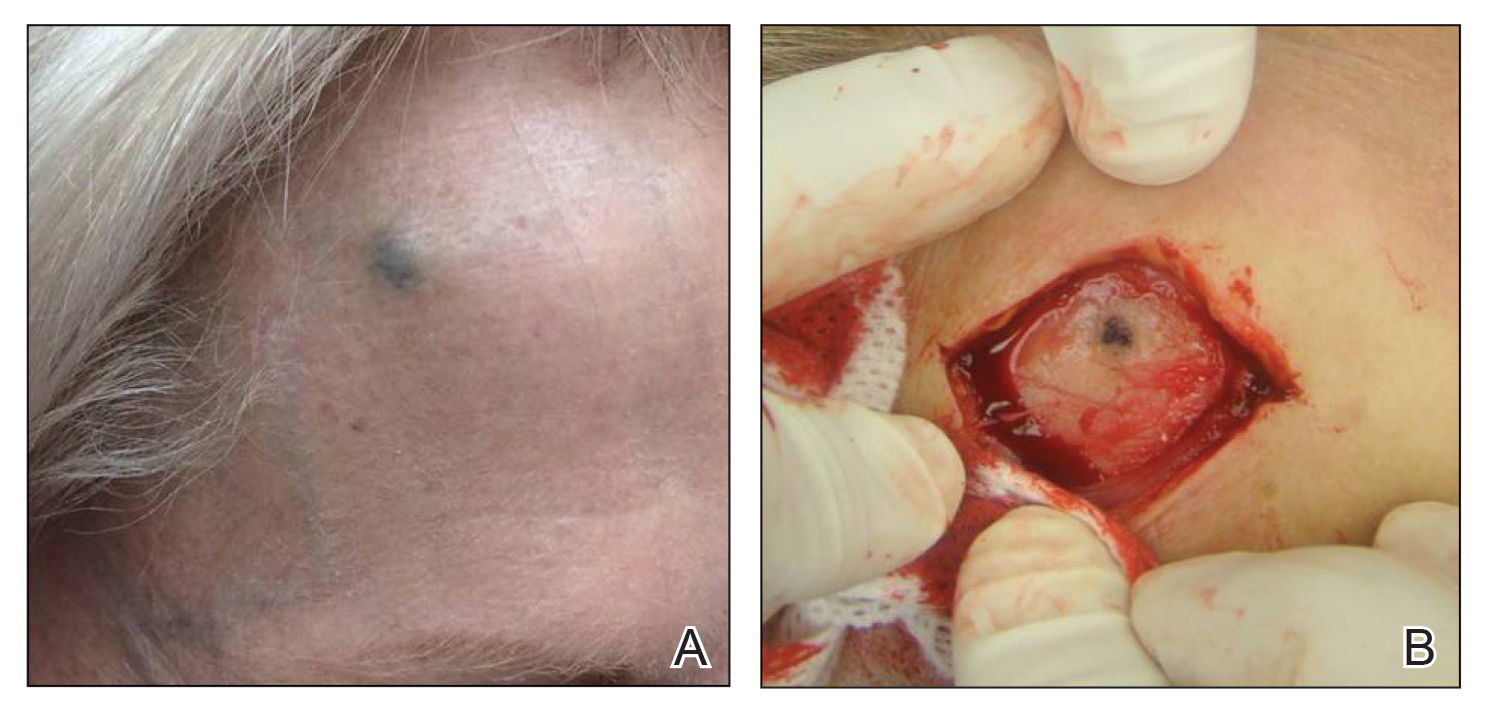
An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.
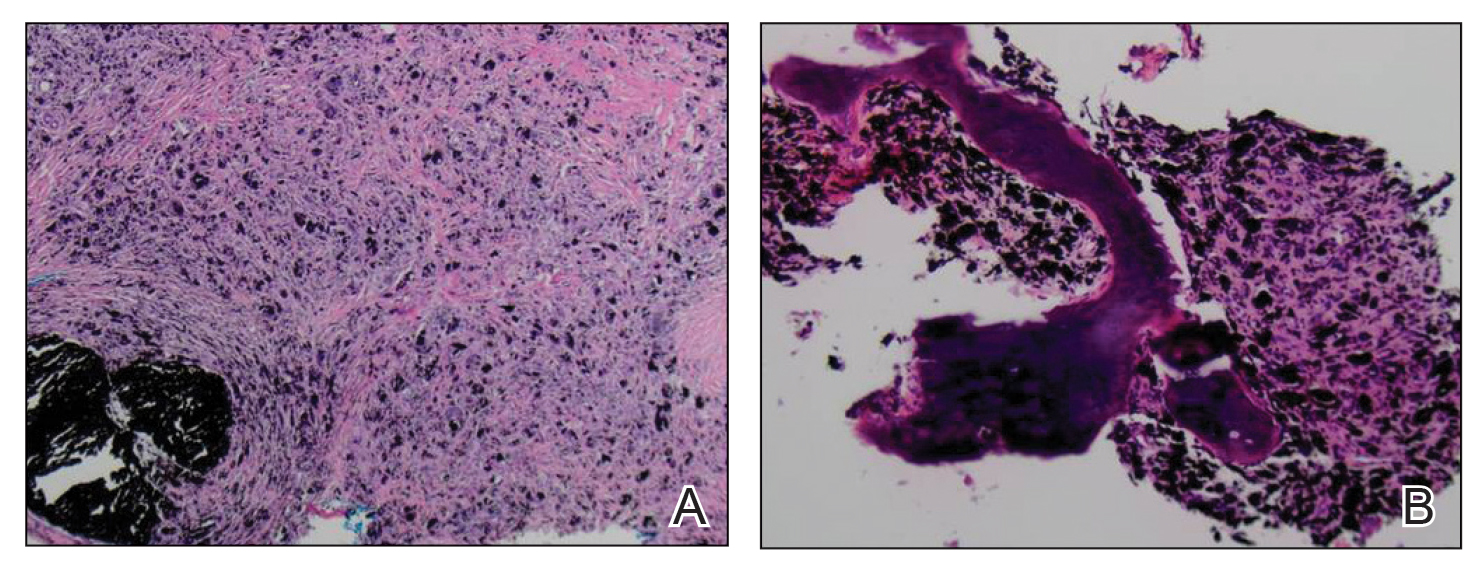
Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.

An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.

Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.

An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.

Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
Practice Points
- Pencil-core granulomas can arise even decades after the lead is embedded in the skin.
- It is important to biopsy to confirm the diagnosis, as pencil-core granulomas can very closely mimic melanomas.
ARFID or reasonable food restriction? The jury is out
Problems with eating and nutrition are common among patients with inflammatory bowel disease (IBD) and other gastrointestinal disorders, but clinicians who treat them should be careful not to automatically assume that patients have eating disorders, according to a psychologist who specializes in the psychological and social aspects of chronic digestive diseases.
On the other hand, clinicians must also be aware of the possibility that patients could have a recently identified syndrome cluster called avoidant restrictive food intake disorder (ARFID), said Tiffany Taft, PsyD, a research associate professor of medicine (gastroenterology and hepatology), medical social sciences, and psychiatry and behavioral sciences at Northwestern University, Chicago. In a recent study, she and her colleagues defined ARFID as “failure to meet one’s nutritional needs owing to sensory hypersensitivity, lack of interest in eating, or fear of aversive consequences from eating, and is associated with negative medical and psychosocial outcomes.”
ARFID “is a hot topic that we really don’t understand,” she said in an online presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nutritional deficiencies
Nutritional deficiencies are common among patients with IBD, “and nutritional deficiencies themselves can lead to symptoms or side effects that can cause people to eat less,” she said.
“As our vitamin B12 goes down, our cognitive functioning starts to decline, and we might not be making clear decisions in how we’re deciding what to eat, when to eat, if we should be eating at all – just something to think about in your patients who have nutritional deficiencies,” she told the audience.
Other common nutritional deficiencies that can affect eating and food choice among patients with IBD include low folate (B9) levels associated with sore tongue and weight loss, low iron levels leading to nausea and loss of appetite, and zinc deficiency leading to loss of appetite and alterations in taste and/or smell, she said.
Newly recognized in GI
She noted that “ARFID actually originates in the pediatric psychiatric literature, mostly in children with sensory issues [such as] autism spectrum disorder, so this is not a construct that started in digestive disease, but has been adapted and applied to patients with digestive disease, including IBD.”
The DSM-5 lists four criteria for ARFID: significant weight loss, significant nutritional deficiency, dependence on enteral nutrition or oral supplements, and marked interference with psychosocial functions.
Helen Burton Murray, PhD, director of the gastrointestinal behavioral health program in the Center for Neurointestinal Health at Massachusetts General Hospital, Boston, who is familiar with Dr. Taft’s work, said in an interview that inclusion of ARFID in DSM-5 has put a name to a syndrome or symptom cluster that in all likelihood already existed.
However, “the jury is still out about whether, if we do diagnose patients who have digestive diseases with ARFID, that then helps them get to a treatment that improves their relationship with food and improves nutritional issues that may have occurred as a result of a restricted food intake,” she said.
“We don’t know yet if the diagnosis will actually improve things. In our clinical practice, anecdotally, it has, both for patients with IBDs and for patients with other GI conditions, particularly GI functional motility disorders. We’re a little bit more confident about making the diagnosis of ARFID in GI functional motility disorders than we are in IBD of course,” she said.
Screening measures
To get a better sense of the prevalence of ARFID, compared with reasonable responses to digestive diseases, Dr. Taft and colleagues conducted their cross-sectional study in 289 adults with achalasia, celiac, eosinophilic esophagitis, or IBD.
They found that 51.3% of the total sample met the diagnostic criteria for ARFID based on the Nine-Item ARFID Screen (NIAS), including 75.7 % of patients with achalasia. But Dr. Taft had cautions
“I can tell you, working with achalasia patients, 75% do not have ARFID,” Dr. Taft said.
She noted that the 51.3% of patients with IBD identified by NIAS or the 53% identified by the ARFID+ scale as having ARFID was also highly doubtful.
Dr. Taft and colleagues determined that nearly half of the variance in the NIAS could be accounted for by GI symptoms rather than psychosocial factors, making it less than ideal for use in the clinic or by researchers.
She also noted, however, that she received an email from one of the creators of NIAS, Hana F. Zickgraf, PhD, from the University of South Alabama, Mobile. Dr. Zickgraf agreed that the scale had drawbacks when applied to patients with GI disease, and pointed instead to the Fear of Food Questionnaire, a newly developed 18-item GI disease-specific instrument. Dr. Taft recommended the new questionnaire for research purposes, and expressed hope that a shorter version could be made available for screening patients in clinic.
Dr. Burton Murray said that while the Fear of Food Questionnaire, perhaps in combination with NIAS, has the potential to be a useful screening tool, cutoffs for it have yet to be established.
“At the end of the day, the diagnosis would be made by a clinician who is able to determine whether the life impairment or if the nutritional impairment or restricted food intake are reasonable in the realm of their digestive disease, or could a treatment for ARFID be warranted to help them to make changes to improve their quality of life and nutrition,” she said.
Check biases at the door
Before arriving at a diagnosis of ARFID, clinicians should also consider biases, Dr. Taft said.
“Eating disorders are highly stigmatized and stereotyped diagnoses,” more often attributed to young White women than to either men or to people of racial or ethnic minorities, she said.
Cultural background may contribute to food restrictions, and the risk may increase with age, with 68% of patients with later-onset IBD restricting diets to control the disease. It’s also possible that beliefs about food and “clean and healthy” eating may influence food and eating choices after a patient receives an IBD diagnosis.
Dr. Taft also pointed out that clinicians and patients may have different ideas about what constitutes significant food avoidance. Clinicians may expect patients with IBD to eat despite feeling nauseated, having abdominal pains, or diarrhea, for example, when the same food avoidance might be deemed reasonable in patients with short-term GI infections.
“Severe IBD symptoms are a significant predictor of posttraumatic stress disorder symptoms, and PTSD is hallmarked by avoidance behaviors,” she added.
She emphasized the need for clinicians to ask the right questions of patients to get at the roots of their nutritional deficiency or eating behavior, and to refer patients to mental health professionals with expertise in disordered eating or GI psychology.
Dr. Taft and Dr. Burton Murray reported having no conflicts of interest to disclose.
This article was updated on Feb. 4, 2022.
Problems with eating and nutrition are common among patients with inflammatory bowel disease (IBD) and other gastrointestinal disorders, but clinicians who treat them should be careful not to automatically assume that patients have eating disorders, according to a psychologist who specializes in the psychological and social aspects of chronic digestive diseases.
On the other hand, clinicians must also be aware of the possibility that patients could have a recently identified syndrome cluster called avoidant restrictive food intake disorder (ARFID), said Tiffany Taft, PsyD, a research associate professor of medicine (gastroenterology and hepatology), medical social sciences, and psychiatry and behavioral sciences at Northwestern University, Chicago. In a recent study, she and her colleagues defined ARFID as “failure to meet one’s nutritional needs owing to sensory hypersensitivity, lack of interest in eating, or fear of aversive consequences from eating, and is associated with negative medical and psychosocial outcomes.”
ARFID “is a hot topic that we really don’t understand,” she said in an online presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nutritional deficiencies
Nutritional deficiencies are common among patients with IBD, “and nutritional deficiencies themselves can lead to symptoms or side effects that can cause people to eat less,” she said.
“As our vitamin B12 goes down, our cognitive functioning starts to decline, and we might not be making clear decisions in how we’re deciding what to eat, when to eat, if we should be eating at all – just something to think about in your patients who have nutritional deficiencies,” she told the audience.
Other common nutritional deficiencies that can affect eating and food choice among patients with IBD include low folate (B9) levels associated with sore tongue and weight loss, low iron levels leading to nausea and loss of appetite, and zinc deficiency leading to loss of appetite and alterations in taste and/or smell, she said.
Newly recognized in GI
She noted that “ARFID actually originates in the pediatric psychiatric literature, mostly in children with sensory issues [such as] autism spectrum disorder, so this is not a construct that started in digestive disease, but has been adapted and applied to patients with digestive disease, including IBD.”
The DSM-5 lists four criteria for ARFID: significant weight loss, significant nutritional deficiency, dependence on enteral nutrition or oral supplements, and marked interference with psychosocial functions.
Helen Burton Murray, PhD, director of the gastrointestinal behavioral health program in the Center for Neurointestinal Health at Massachusetts General Hospital, Boston, who is familiar with Dr. Taft’s work, said in an interview that inclusion of ARFID in DSM-5 has put a name to a syndrome or symptom cluster that in all likelihood already existed.
However, “the jury is still out about whether, if we do diagnose patients who have digestive diseases with ARFID, that then helps them get to a treatment that improves their relationship with food and improves nutritional issues that may have occurred as a result of a restricted food intake,” she said.
“We don’t know yet if the diagnosis will actually improve things. In our clinical practice, anecdotally, it has, both for patients with IBDs and for patients with other GI conditions, particularly GI functional motility disorders. We’re a little bit more confident about making the diagnosis of ARFID in GI functional motility disorders than we are in IBD of course,” she said.
Screening measures
To get a better sense of the prevalence of ARFID, compared with reasonable responses to digestive diseases, Dr. Taft and colleagues conducted their cross-sectional study in 289 adults with achalasia, celiac, eosinophilic esophagitis, or IBD.
They found that 51.3% of the total sample met the diagnostic criteria for ARFID based on the Nine-Item ARFID Screen (NIAS), including 75.7 % of patients with achalasia. But Dr. Taft had cautions
“I can tell you, working with achalasia patients, 75% do not have ARFID,” Dr. Taft said.
She noted that the 51.3% of patients with IBD identified by NIAS or the 53% identified by the ARFID+ scale as having ARFID was also highly doubtful.
Dr. Taft and colleagues determined that nearly half of the variance in the NIAS could be accounted for by GI symptoms rather than psychosocial factors, making it less than ideal for use in the clinic or by researchers.
She also noted, however, that she received an email from one of the creators of NIAS, Hana F. Zickgraf, PhD, from the University of South Alabama, Mobile. Dr. Zickgraf agreed that the scale had drawbacks when applied to patients with GI disease, and pointed instead to the Fear of Food Questionnaire, a newly developed 18-item GI disease-specific instrument. Dr. Taft recommended the new questionnaire for research purposes, and expressed hope that a shorter version could be made available for screening patients in clinic.
Dr. Burton Murray said that while the Fear of Food Questionnaire, perhaps in combination with NIAS, has the potential to be a useful screening tool, cutoffs for it have yet to be established.
“At the end of the day, the diagnosis would be made by a clinician who is able to determine whether the life impairment or if the nutritional impairment or restricted food intake are reasonable in the realm of their digestive disease, or could a treatment for ARFID be warranted to help them to make changes to improve their quality of life and nutrition,” she said.
Check biases at the door
Before arriving at a diagnosis of ARFID, clinicians should also consider biases, Dr. Taft said.
“Eating disorders are highly stigmatized and stereotyped diagnoses,” more often attributed to young White women than to either men or to people of racial or ethnic minorities, she said.
Cultural background may contribute to food restrictions, and the risk may increase with age, with 68% of patients with later-onset IBD restricting diets to control the disease. It’s also possible that beliefs about food and “clean and healthy” eating may influence food and eating choices after a patient receives an IBD diagnosis.
Dr. Taft also pointed out that clinicians and patients may have different ideas about what constitutes significant food avoidance. Clinicians may expect patients with IBD to eat despite feeling nauseated, having abdominal pains, or diarrhea, for example, when the same food avoidance might be deemed reasonable in patients with short-term GI infections.
“Severe IBD symptoms are a significant predictor of posttraumatic stress disorder symptoms, and PTSD is hallmarked by avoidance behaviors,” she added.
She emphasized the need for clinicians to ask the right questions of patients to get at the roots of their nutritional deficiency or eating behavior, and to refer patients to mental health professionals with expertise in disordered eating or GI psychology.
Dr. Taft and Dr. Burton Murray reported having no conflicts of interest to disclose.
This article was updated on Feb. 4, 2022.
Problems with eating and nutrition are common among patients with inflammatory bowel disease (IBD) and other gastrointestinal disorders, but clinicians who treat them should be careful not to automatically assume that patients have eating disorders, according to a psychologist who specializes in the psychological and social aspects of chronic digestive diseases.
On the other hand, clinicians must also be aware of the possibility that patients could have a recently identified syndrome cluster called avoidant restrictive food intake disorder (ARFID), said Tiffany Taft, PsyD, a research associate professor of medicine (gastroenterology and hepatology), medical social sciences, and psychiatry and behavioral sciences at Northwestern University, Chicago. In a recent study, she and her colleagues defined ARFID as “failure to meet one’s nutritional needs owing to sensory hypersensitivity, lack of interest in eating, or fear of aversive consequences from eating, and is associated with negative medical and psychosocial outcomes.”
ARFID “is a hot topic that we really don’t understand,” she said in an online presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nutritional deficiencies
Nutritional deficiencies are common among patients with IBD, “and nutritional deficiencies themselves can lead to symptoms or side effects that can cause people to eat less,” she said.
“As our vitamin B12 goes down, our cognitive functioning starts to decline, and we might not be making clear decisions in how we’re deciding what to eat, when to eat, if we should be eating at all – just something to think about in your patients who have nutritional deficiencies,” she told the audience.
Other common nutritional deficiencies that can affect eating and food choice among patients with IBD include low folate (B9) levels associated with sore tongue and weight loss, low iron levels leading to nausea and loss of appetite, and zinc deficiency leading to loss of appetite and alterations in taste and/or smell, she said.
Newly recognized in GI
She noted that “ARFID actually originates in the pediatric psychiatric literature, mostly in children with sensory issues [such as] autism spectrum disorder, so this is not a construct that started in digestive disease, but has been adapted and applied to patients with digestive disease, including IBD.”
The DSM-5 lists four criteria for ARFID: significant weight loss, significant nutritional deficiency, dependence on enteral nutrition or oral supplements, and marked interference with psychosocial functions.
Helen Burton Murray, PhD, director of the gastrointestinal behavioral health program in the Center for Neurointestinal Health at Massachusetts General Hospital, Boston, who is familiar with Dr. Taft’s work, said in an interview that inclusion of ARFID in DSM-5 has put a name to a syndrome or symptom cluster that in all likelihood already existed.
However, “the jury is still out about whether, if we do diagnose patients who have digestive diseases with ARFID, that then helps them get to a treatment that improves their relationship with food and improves nutritional issues that may have occurred as a result of a restricted food intake,” she said.
“We don’t know yet if the diagnosis will actually improve things. In our clinical practice, anecdotally, it has, both for patients with IBDs and for patients with other GI conditions, particularly GI functional motility disorders. We’re a little bit more confident about making the diagnosis of ARFID in GI functional motility disorders than we are in IBD of course,” she said.
Screening measures
To get a better sense of the prevalence of ARFID, compared with reasonable responses to digestive diseases, Dr. Taft and colleagues conducted their cross-sectional study in 289 adults with achalasia, celiac, eosinophilic esophagitis, or IBD.
They found that 51.3% of the total sample met the diagnostic criteria for ARFID based on the Nine-Item ARFID Screen (NIAS), including 75.7 % of patients with achalasia. But Dr. Taft had cautions
“I can tell you, working with achalasia patients, 75% do not have ARFID,” Dr. Taft said.
She noted that the 51.3% of patients with IBD identified by NIAS or the 53% identified by the ARFID+ scale as having ARFID was also highly doubtful.
Dr. Taft and colleagues determined that nearly half of the variance in the NIAS could be accounted for by GI symptoms rather than psychosocial factors, making it less than ideal for use in the clinic or by researchers.
She also noted, however, that she received an email from one of the creators of NIAS, Hana F. Zickgraf, PhD, from the University of South Alabama, Mobile. Dr. Zickgraf agreed that the scale had drawbacks when applied to patients with GI disease, and pointed instead to the Fear of Food Questionnaire, a newly developed 18-item GI disease-specific instrument. Dr. Taft recommended the new questionnaire for research purposes, and expressed hope that a shorter version could be made available for screening patients in clinic.
Dr. Burton Murray said that while the Fear of Food Questionnaire, perhaps in combination with NIAS, has the potential to be a useful screening tool, cutoffs for it have yet to be established.
“At the end of the day, the diagnosis would be made by a clinician who is able to determine whether the life impairment or if the nutritional impairment or restricted food intake are reasonable in the realm of their digestive disease, or could a treatment for ARFID be warranted to help them to make changes to improve their quality of life and nutrition,” she said.
Check biases at the door
Before arriving at a diagnosis of ARFID, clinicians should also consider biases, Dr. Taft said.
“Eating disorders are highly stigmatized and stereotyped diagnoses,” more often attributed to young White women than to either men or to people of racial or ethnic minorities, she said.
Cultural background may contribute to food restrictions, and the risk may increase with age, with 68% of patients with later-onset IBD restricting diets to control the disease. It’s also possible that beliefs about food and “clean and healthy” eating may influence food and eating choices after a patient receives an IBD diagnosis.
Dr. Taft also pointed out that clinicians and patients may have different ideas about what constitutes significant food avoidance. Clinicians may expect patients with IBD to eat despite feeling nauseated, having abdominal pains, or diarrhea, for example, when the same food avoidance might be deemed reasonable in patients with short-term GI infections.
“Severe IBD symptoms are a significant predictor of posttraumatic stress disorder symptoms, and PTSD is hallmarked by avoidance behaviors,” she added.
She emphasized the need for clinicians to ask the right questions of patients to get at the roots of their nutritional deficiency or eating behavior, and to refer patients to mental health professionals with expertise in disordered eating or GI psychology.
Dr. Taft and Dr. Burton Murray reported having no conflicts of interest to disclose.
This article was updated on Feb. 4, 2022.
FROM CROHN’S & COLITIS CONGRESS
Lower Leg Hyperpigmentation in MYH9-Related Disorder
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
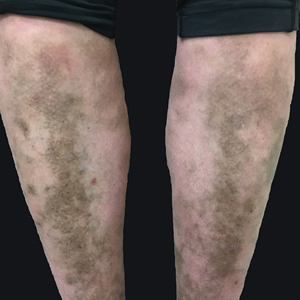
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.
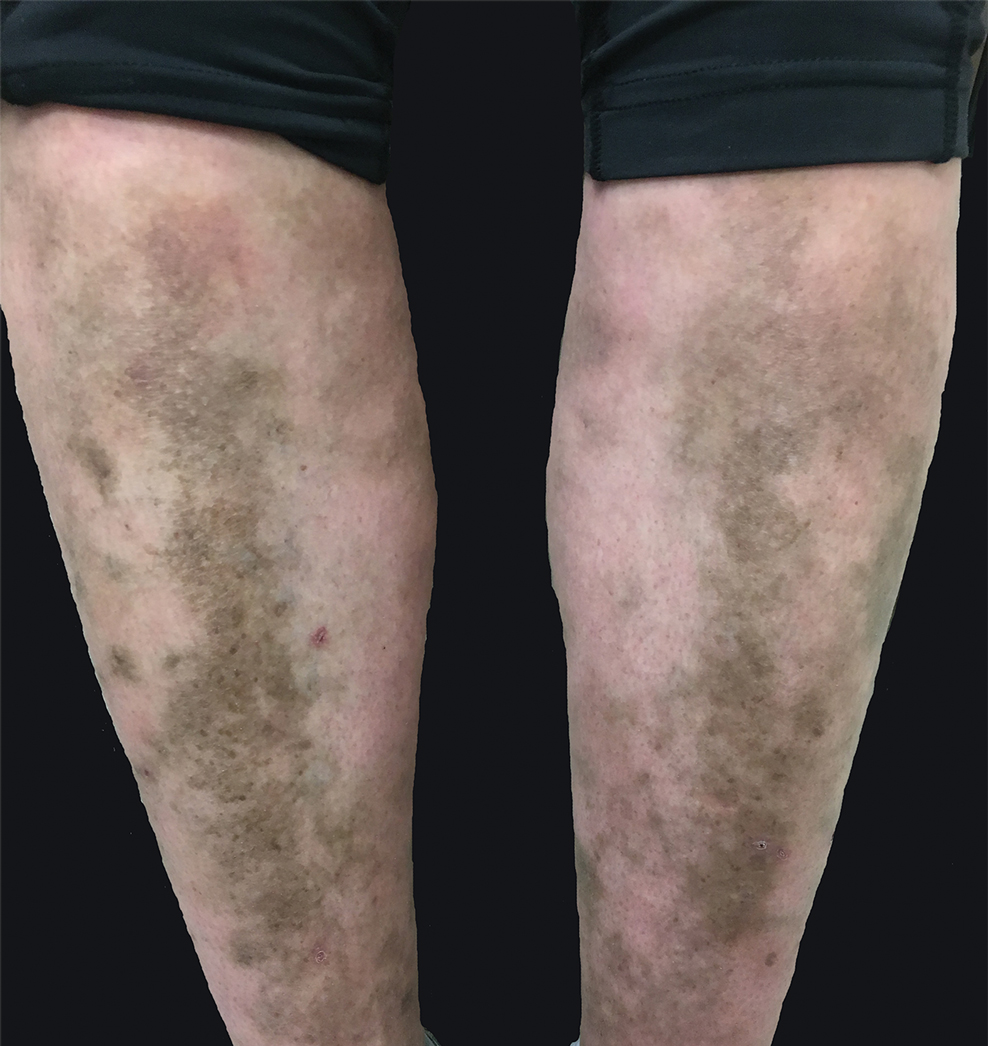
Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.

Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.

Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
Practice Points
- MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.
- Leg hyperpigmentation can occur secondary to hemosiderin deposition from MYH9-related disorder.
- The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder.
- Lasers and intense pulsed light therapy are modalities to consider for the hyperpigmentation of MYH9- related disorder.
Topline data for aficamten positive in obstructive HCM
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.
If you give a mouse a genetically engineered bitcoin wallet
The world’s most valuable mouse
You’ve heard of Mighty Mouse. Now say hello to the world’s newest mouse superhero, Crypto-Mouse! After being bitten by a radioactive cryptocurrency investor, Crypto-Mouse can tap directly into the power of the blockchain itself, allowing it to perform incredible, death-defying feats of strength!
We’re going to stop right there before Crypto-Mouse gains entry into the Marvel cinematic universe. Let’s rewind to the beginning, because that’s precisely where this crazy scheme is at. In late January, a new decentralized autonomous organization, BitMouseDAO, launched to enormous … -ly little fanfare, according to Vice. Two investors as of Jan. 31. But what they lack in money they make up for in sheer ambition.
BitMouseDAO’s $100 million dollar idea is to genetically engineer mice to carry bitcoin, the first cryptocurrency and one of the most valuable. This isn’t as crazy an idea as it sounds since DNA can be modified to store information, potentially even bitcoin information. Their plan is to create a private bitcoin wallet, which will be stored in the mouse DNA, and purchase online bitcoin to store in this wallet.
BitMouseDAO, being a “collection of artists,” plans to partner with a lab to translate its private key into a specific DNA sequence to be encoded into the mice during fertilization; or, if that doesn’t work, inject them with a harmless virus that carries the key.
Since these are artists, their ultimate plan is to use their bitcoin mice to make NFTs (scratch that off your cryptocurrency bingo card) and auction them off to people. Or, as Vice put it, BitMouseDAO essentially plans to send preserved dead mice to people. Artistic dead mice! Artistic dead mice worth millions! Maybe. Even BitMouseDAO admits bitcoin could be worthless by the time the project gets off the ground.
If this all sounds completely insane, that’s because it is. But it also sounds crazy enough to work. Now, if you’ll excuse us, we’re off to write a screenplay about a scrappy group of high-tech thieves who steal a group of genetically altered bitcoin mice to sell for millions, only to keep them as their adorable pets. Trust us Hollywood, it’ll make millions!
Alcoholic monkeys vs. the future of feces
Which is more important, the journey or the destination? Science is all about the destination, yes? Solving the problem, saving a life, expanding horizons. That’s science. Or is it? The scientific method is a process, so does that make it a journey?
For us, today’s journey begins at the University of Iowa, where investigators are trying to reduce alcohol consumption. A worthy goal, and they seem to have made some progress by targeting a liver hormone called fibroblast growth factor 21 (FGF21). But we’re more interested in the process right now, so bring on the alcoholic monkeys. And no, that’s not a death metal/reggae fusion band. Should be, though.
“The vervet monkey population is [composed] of alcohol avoiders, moderate alcohol drinkers, and a group of heavy drinkers,” Matthew Potthoff, PhD, and associates wrote in Cell Metabolism. When this particular bunch of heavy-drinking vervets were given FGF21, they consumed 50% less alcohol than did vehicle-treated controls, so mission accomplished.
Maybe it could be a breakfast cereal. Who wouldn’t enjoy a bowl of alcoholic monkeys in the morning?
And after breakfast, you might be ready for a digitized bowel movement, courtesy of researchers at University of California, San Diego. They’re studying ulcerative colitis (UC) by examining the gut microbiome, and their “most useful biological sample is patient stool,” according to a written statement from the university.
“Once we had all the technology to digitize the stool, the question was, is this going to tell us what’s happening in these patients? The answer turned out to be yes,” co-senior author Rob Knight, PhD, said in the statement. “Digitizing fecal material is the future.” The road to UC treatment, in other words, is paved with digital stool.
About 40% of the UC patients had elevated protease levels, and their high-protease feces were then transplanted into germ-free mice, which subsequently developed colitis and were successfully treated with protease inhibitors. And that is our final destination.
As our revered founder and mentor, Josephine Lotmevich, used to say, an alcoholic monkey in the hand is worth a number 2 in the bush.
Raise a glass to delinquency
You wouldn’t think that a glass of water could lead to a life of crime, but a recent study suggests just that.
Children exposed to lead in their drinking water during their early years had a 21% higher risk of delinquency after the age of 14 years and a 38% higher risk of having a record for a serious complaint, Jackie MacDonald Gibson and associates said in a statement on Eurekalert.
Data for the study came from Wake County, N.C., which includes rural areas, wealthy exurban developments, and predominantly Black communities. The investigators compared the blood lead levels for children tested between 1998 and 2011 with juvenile delinquency reports of the same children from the N.C. Department of Public Safety.
The main culprit, they found, was well water. Blood lead levels were 11% higher in the children whose water came from private wells, compared with children using community water. About 13% of U.S. households rely on private wells, which are not regulated under the Safe Drinking Water Act, for their water supply.
The researchers said there is an urgent need for better drinking-water solutions in communities that rely on well water, whether it be through subsidized home filtration or infrastructure redevelopment.
An earlier study had estimated that preventing just one child from entering the adult criminal justice system would save $1.3 to $1.5 million in 1997 dollars. That’s about $2.2 to $2.5 million dollars today!
If you do the math, it’s not hard to see what’s cheaper (and healthier) in the long run.
A ‘dirty’ scam
Another one? This is just getting sad. You’ve probably heard of muds and clays being good for the skin and maybe you’ve gone to a spa and sat in a mud bath, but would you believe it if someone told you that mud can cure all your ailments? No? Neither would we. Senatorial candidate Beto O’Rourke was definitely someone who brought this strange treatment to light, but it seems like this is something that has been going on for years, even before the pandemic.
A company called Black Oxygen Organics (BOO) was selling “magic dirt” for $110 per 4-ounce package. It claimed the dirt was high in fulvic acid and humic acid, which are good for many things. They were, however, literally getting this mud from bogs with landfills nearby, Mel magazine reported.
That doesn’t sound appealing at all, but wait, there’s more. People were eating, drinking, bathing, and feeding their families this sludge in hopes that they would be cured of their ailments. A lot of people jumped aboard the magic dirt train when the pandemic arose, but it quickly became clear that this mud was not as helpful as BOO claimed it to be.
“We began to receive inquiries and calls on our website with people having problems and issues. Ultimately, we sent the products out for independent testing, and then when that came back and showed that there were toxic heavy metals [lead, arsenic, and cadmium among them] at an unsafe level, that’s when we knew we had to act,” Atlanta-based attorney Matt Wetherington, who filed a federal lawsuit against BOO, told Mel.
After a very complicated series of events involving an expose by NBC, product recalls, extortion claims, and grassroots activism, BOO was shut down by both the Canadian and U.S. governments.
As always, please listen only to health care professionals when you wish to use natural remedies for illnesses and ailments.
The world’s most valuable mouse
You’ve heard of Mighty Mouse. Now say hello to the world’s newest mouse superhero, Crypto-Mouse! After being bitten by a radioactive cryptocurrency investor, Crypto-Mouse can tap directly into the power of the blockchain itself, allowing it to perform incredible, death-defying feats of strength!
We’re going to stop right there before Crypto-Mouse gains entry into the Marvel cinematic universe. Let’s rewind to the beginning, because that’s precisely where this crazy scheme is at. In late January, a new decentralized autonomous organization, BitMouseDAO, launched to enormous … -ly little fanfare, according to Vice. Two investors as of Jan. 31. But what they lack in money they make up for in sheer ambition.
BitMouseDAO’s $100 million dollar idea is to genetically engineer mice to carry bitcoin, the first cryptocurrency and one of the most valuable. This isn’t as crazy an idea as it sounds since DNA can be modified to store information, potentially even bitcoin information. Their plan is to create a private bitcoin wallet, which will be stored in the mouse DNA, and purchase online bitcoin to store in this wallet.
BitMouseDAO, being a “collection of artists,” plans to partner with a lab to translate its private key into a specific DNA sequence to be encoded into the mice during fertilization; or, if that doesn’t work, inject them with a harmless virus that carries the key.
Since these are artists, their ultimate plan is to use their bitcoin mice to make NFTs (scratch that off your cryptocurrency bingo card) and auction them off to people. Or, as Vice put it, BitMouseDAO essentially plans to send preserved dead mice to people. Artistic dead mice! Artistic dead mice worth millions! Maybe. Even BitMouseDAO admits bitcoin could be worthless by the time the project gets off the ground.
If this all sounds completely insane, that’s because it is. But it also sounds crazy enough to work. Now, if you’ll excuse us, we’re off to write a screenplay about a scrappy group of high-tech thieves who steal a group of genetically altered bitcoin mice to sell for millions, only to keep them as their adorable pets. Trust us Hollywood, it’ll make millions!
Alcoholic monkeys vs. the future of feces
Which is more important, the journey or the destination? Science is all about the destination, yes? Solving the problem, saving a life, expanding horizons. That’s science. Or is it? The scientific method is a process, so does that make it a journey?
For us, today’s journey begins at the University of Iowa, where investigators are trying to reduce alcohol consumption. A worthy goal, and they seem to have made some progress by targeting a liver hormone called fibroblast growth factor 21 (FGF21). But we’re more interested in the process right now, so bring on the alcoholic monkeys. And no, that’s not a death metal/reggae fusion band. Should be, though.
“The vervet monkey population is [composed] of alcohol avoiders, moderate alcohol drinkers, and a group of heavy drinkers,” Matthew Potthoff, PhD, and associates wrote in Cell Metabolism. When this particular bunch of heavy-drinking vervets were given FGF21, they consumed 50% less alcohol than did vehicle-treated controls, so mission accomplished.
Maybe it could be a breakfast cereal. Who wouldn’t enjoy a bowl of alcoholic monkeys in the morning?
And after breakfast, you might be ready for a digitized bowel movement, courtesy of researchers at University of California, San Diego. They’re studying ulcerative colitis (UC) by examining the gut microbiome, and their “most useful biological sample is patient stool,” according to a written statement from the university.
“Once we had all the technology to digitize the stool, the question was, is this going to tell us what’s happening in these patients? The answer turned out to be yes,” co-senior author Rob Knight, PhD, said in the statement. “Digitizing fecal material is the future.” The road to UC treatment, in other words, is paved with digital stool.
About 40% of the UC patients had elevated protease levels, and their high-protease feces were then transplanted into germ-free mice, which subsequently developed colitis and were successfully treated with protease inhibitors. And that is our final destination.
As our revered founder and mentor, Josephine Lotmevich, used to say, an alcoholic monkey in the hand is worth a number 2 in the bush.
Raise a glass to delinquency
You wouldn’t think that a glass of water could lead to a life of crime, but a recent study suggests just that.
Children exposed to lead in their drinking water during their early years had a 21% higher risk of delinquency after the age of 14 years and a 38% higher risk of having a record for a serious complaint, Jackie MacDonald Gibson and associates said in a statement on Eurekalert.
Data for the study came from Wake County, N.C., which includes rural areas, wealthy exurban developments, and predominantly Black communities. The investigators compared the blood lead levels for children tested between 1998 and 2011 with juvenile delinquency reports of the same children from the N.C. Department of Public Safety.
The main culprit, they found, was well water. Blood lead levels were 11% higher in the children whose water came from private wells, compared with children using community water. About 13% of U.S. households rely on private wells, which are not regulated under the Safe Drinking Water Act, for their water supply.
The researchers said there is an urgent need for better drinking-water solutions in communities that rely on well water, whether it be through subsidized home filtration or infrastructure redevelopment.
An earlier study had estimated that preventing just one child from entering the adult criminal justice system would save $1.3 to $1.5 million in 1997 dollars. That’s about $2.2 to $2.5 million dollars today!
If you do the math, it’s not hard to see what’s cheaper (and healthier) in the long run.
A ‘dirty’ scam
Another one? This is just getting sad. You’ve probably heard of muds and clays being good for the skin and maybe you’ve gone to a spa and sat in a mud bath, but would you believe it if someone told you that mud can cure all your ailments? No? Neither would we. Senatorial candidate Beto O’Rourke was definitely someone who brought this strange treatment to light, but it seems like this is something that has been going on for years, even before the pandemic.
A company called Black Oxygen Organics (BOO) was selling “magic dirt” for $110 per 4-ounce package. It claimed the dirt was high in fulvic acid and humic acid, which are good for many things. They were, however, literally getting this mud from bogs with landfills nearby, Mel magazine reported.
That doesn’t sound appealing at all, but wait, there’s more. People were eating, drinking, bathing, and feeding their families this sludge in hopes that they would be cured of their ailments. A lot of people jumped aboard the magic dirt train when the pandemic arose, but it quickly became clear that this mud was not as helpful as BOO claimed it to be.
“We began to receive inquiries and calls on our website with people having problems and issues. Ultimately, we sent the products out for independent testing, and then when that came back and showed that there were toxic heavy metals [lead, arsenic, and cadmium among them] at an unsafe level, that’s when we knew we had to act,” Atlanta-based attorney Matt Wetherington, who filed a federal lawsuit against BOO, told Mel.
After a very complicated series of events involving an expose by NBC, product recalls, extortion claims, and grassroots activism, BOO was shut down by both the Canadian and U.S. governments.
As always, please listen only to health care professionals when you wish to use natural remedies for illnesses and ailments.
The world’s most valuable mouse
You’ve heard of Mighty Mouse. Now say hello to the world’s newest mouse superhero, Crypto-Mouse! After being bitten by a radioactive cryptocurrency investor, Crypto-Mouse can tap directly into the power of the blockchain itself, allowing it to perform incredible, death-defying feats of strength!
We’re going to stop right there before Crypto-Mouse gains entry into the Marvel cinematic universe. Let’s rewind to the beginning, because that’s precisely where this crazy scheme is at. In late January, a new decentralized autonomous organization, BitMouseDAO, launched to enormous … -ly little fanfare, according to Vice. Two investors as of Jan. 31. But what they lack in money they make up for in sheer ambition.
BitMouseDAO’s $100 million dollar idea is to genetically engineer mice to carry bitcoin, the first cryptocurrency and one of the most valuable. This isn’t as crazy an idea as it sounds since DNA can be modified to store information, potentially even bitcoin information. Their plan is to create a private bitcoin wallet, which will be stored in the mouse DNA, and purchase online bitcoin to store in this wallet.
BitMouseDAO, being a “collection of artists,” plans to partner with a lab to translate its private key into a specific DNA sequence to be encoded into the mice during fertilization; or, if that doesn’t work, inject them with a harmless virus that carries the key.
Since these are artists, their ultimate plan is to use their bitcoin mice to make NFTs (scratch that off your cryptocurrency bingo card) and auction them off to people. Or, as Vice put it, BitMouseDAO essentially plans to send preserved dead mice to people. Artistic dead mice! Artistic dead mice worth millions! Maybe. Even BitMouseDAO admits bitcoin could be worthless by the time the project gets off the ground.
If this all sounds completely insane, that’s because it is. But it also sounds crazy enough to work. Now, if you’ll excuse us, we’re off to write a screenplay about a scrappy group of high-tech thieves who steal a group of genetically altered bitcoin mice to sell for millions, only to keep them as their adorable pets. Trust us Hollywood, it’ll make millions!
Alcoholic monkeys vs. the future of feces
Which is more important, the journey or the destination? Science is all about the destination, yes? Solving the problem, saving a life, expanding horizons. That’s science. Or is it? The scientific method is a process, so does that make it a journey?
For us, today’s journey begins at the University of Iowa, where investigators are trying to reduce alcohol consumption. A worthy goal, and they seem to have made some progress by targeting a liver hormone called fibroblast growth factor 21 (FGF21). But we’re more interested in the process right now, so bring on the alcoholic monkeys. And no, that’s not a death metal/reggae fusion band. Should be, though.
“The vervet monkey population is [composed] of alcohol avoiders, moderate alcohol drinkers, and a group of heavy drinkers,” Matthew Potthoff, PhD, and associates wrote in Cell Metabolism. When this particular bunch of heavy-drinking vervets were given FGF21, they consumed 50% less alcohol than did vehicle-treated controls, so mission accomplished.
Maybe it could be a breakfast cereal. Who wouldn’t enjoy a bowl of alcoholic monkeys in the morning?
And after breakfast, you might be ready for a digitized bowel movement, courtesy of researchers at University of California, San Diego. They’re studying ulcerative colitis (UC) by examining the gut microbiome, and their “most useful biological sample is patient stool,” according to a written statement from the university.
“Once we had all the technology to digitize the stool, the question was, is this going to tell us what’s happening in these patients? The answer turned out to be yes,” co-senior author Rob Knight, PhD, said in the statement. “Digitizing fecal material is the future.” The road to UC treatment, in other words, is paved with digital stool.
About 40% of the UC patients had elevated protease levels, and their high-protease feces were then transplanted into germ-free mice, which subsequently developed colitis and were successfully treated with protease inhibitors. And that is our final destination.
As our revered founder and mentor, Josephine Lotmevich, used to say, an alcoholic monkey in the hand is worth a number 2 in the bush.
Raise a glass to delinquency
You wouldn’t think that a glass of water could lead to a life of crime, but a recent study suggests just that.
Children exposed to lead in their drinking water during their early years had a 21% higher risk of delinquency after the age of 14 years and a 38% higher risk of having a record for a serious complaint, Jackie MacDonald Gibson and associates said in a statement on Eurekalert.
Data for the study came from Wake County, N.C., which includes rural areas, wealthy exurban developments, and predominantly Black communities. The investigators compared the blood lead levels for children tested between 1998 and 2011 with juvenile delinquency reports of the same children from the N.C. Department of Public Safety.
The main culprit, they found, was well water. Blood lead levels were 11% higher in the children whose water came from private wells, compared with children using community water. About 13% of U.S. households rely on private wells, which are not regulated under the Safe Drinking Water Act, for their water supply.
The researchers said there is an urgent need for better drinking-water solutions in communities that rely on well water, whether it be through subsidized home filtration or infrastructure redevelopment.
An earlier study had estimated that preventing just one child from entering the adult criminal justice system would save $1.3 to $1.5 million in 1997 dollars. That’s about $2.2 to $2.5 million dollars today!
If you do the math, it’s not hard to see what’s cheaper (and healthier) in the long run.
A ‘dirty’ scam
Another one? This is just getting sad. You’ve probably heard of muds and clays being good for the skin and maybe you’ve gone to a spa and sat in a mud bath, but would you believe it if someone told you that mud can cure all your ailments? No? Neither would we. Senatorial candidate Beto O’Rourke was definitely someone who brought this strange treatment to light, but it seems like this is something that has been going on for years, even before the pandemic.
A company called Black Oxygen Organics (BOO) was selling “magic dirt” for $110 per 4-ounce package. It claimed the dirt was high in fulvic acid and humic acid, which are good for many things. They were, however, literally getting this mud from bogs with landfills nearby, Mel magazine reported.
That doesn’t sound appealing at all, but wait, there’s more. People were eating, drinking, bathing, and feeding their families this sludge in hopes that they would be cured of their ailments. A lot of people jumped aboard the magic dirt train when the pandemic arose, but it quickly became clear that this mud was not as helpful as BOO claimed it to be.
“We began to receive inquiries and calls on our website with people having problems and issues. Ultimately, we sent the products out for independent testing, and then when that came back and showed that there were toxic heavy metals [lead, arsenic, and cadmium among them] at an unsafe level, that’s when we knew we had to act,” Atlanta-based attorney Matt Wetherington, who filed a federal lawsuit against BOO, told Mel.
After a very complicated series of events involving an expose by NBC, product recalls, extortion claims, and grassroots activism, BOO was shut down by both the Canadian and U.S. governments.
As always, please listen only to health care professionals when you wish to use natural remedies for illnesses and ailments.
Chronic respiratory conditions occur more often in RSV vs. flu
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
FROM CHEST
CLL patients ‘cured’: 10 years post infusion, CAR T cells persist
“We can now conclude that CAR T cells can actually cure patients with leukemia based on these results,” said senior author Carl H. June, MD, in a press briefing on the study published in Nature.
“The major finding from this paper is that, 10 years down the road, you can find these [CAR T] cells,” Dr. June, director of the Center for Cellular Immunotherapies, University of Pennsylvania, Philadelphia, added. “The cells have evolved, and that was a big surprise ... but they are still able to kill leukemia cells 10 years after infusion.”
CAR T-cell therapy, in which patients’ own T cells are removed, reprogrammed in a lab to recognize and attack cancer cells, and then infused back into the patients, has transformed treatment of various blood cancers and shows often-remarkable results in achieving remissions.
While the treatment has become a routine therapy for certain leukemias, long-term results on the fate and function of the cells over time has been highly anticipated.
In the first published observations of a 10-year follow-up of patients treated with CAR T cells, Dr. June and colleagues described the findings for two patients, both with CLL, who back in 2010 were among the first to be treated with this groundbreaking therapy at the University of Pennsylvania.
A decade later, the CAR T cells are found to have remained detectable in both patients, who achieved complete remission in their first year of treatment, and both have sustained that remission.
Notably, the cells have evolved over the years – from initially being dominated by killer T cells to being dominated primarily by proliferative CD4-positive CAR T cells – with one of the patients exclusively having CD4-positive cells at year 9.3.
“The killer T cells did the initial heavy lifting of eliminating the tumor, “ first author J. Joseph Melenhorst, PhD, said in an interview.
“Once their job was done, those cells went down to very low levels, but the CD4-positive population persisted,” said Dr. Melenhorst, who established the lab at the University of Pennsylvania to follow patients treated with CAR T-cell therapy. “[This] delayed phase of immune response against cancer is a novel insight, and we were surprised to see it.”
Dr. Melenhorst noted that the clonal makeup of the CD4-positive cells importantly stabilized and became dominated by a small number of clones, suggesting further sustainability.
When one of the two patients, Doug Olson, who participated in the press conference, donated his cells back to the center after 9.3 years, the researchers found that his cells were still capable of destroying leukemia cells in the lab.
“Ten years [post infusion], we can’t find any of the leukemia cells and we still have the CAR T cells that are on patrol and on surveillance for residual leukemia,” Dr. June said.
One challenge of the otherwise desirable elimination of leukemia cells is that some aspects of sustaining CAR T-cell activity become problematic.
“The aspect of how the remission is maintained [is] very hard to study in a patient when there is no leukemia at all,” Dr. June explained. “It could be the last cell was gone within 3 weeks [of treatment], or it could be that the [cancer cells] are coming up like whack-a-moles, and they are killed because these CAR T cells are on patrol.”
Sadly, the other CLL patient, Bill Ludwig, who was first to receive the CAR T-cell treatment, died in 2021 from COVID-19.
Effects in other blood diseases similar?
CAR T-cell therapy is currently approved in the United States for several blood cancers, and whether similar long-term patterns of the cells may be observed in other patient and cancer types remains to be seen, Dr. Melenhorst said.
“I think in CLL we will see something similar, but in other diseases, we have yet to learn,” he said. “It may depend on issues including which domain has been engineered into the CAR.”
While the prospect of some patients being “cured” is exciting, responses to the therapy have generally been mixed. In CLL, for instance, full remissions have been observed to be maintained in about a quarter of patients, with higher rates observed in some lymphomas and pediatric ALL patients, Dr. Melenhorst explained.
The effects of CAR T-cell therapy in solid cancers have so far been more disappointing, with no research centers reproducing the kinds of results that have been seen with blood cancers.
“There appear to be a number of reasons, including that the [solid] tumor is more complex, and these solid cancers have ways to evade the immune system that need to be overcome,” Dr. June explained.
And despite the more encouraging findings in blood cancers, even with those, “the biggest disappointment is that CAR T-cell therapy doesn’t work all the time. It doesn’t work in every patient,” coauthor David Porter, MD, the University of Pennsylvania oncologist who treated the two patients, said in the press briefing.
“I think the importance of the Nature study is that we are starting to learn the mechanisms of why and how this works, so that we can start to get at how to make it work for more people,” Dr. Porter added. “But what we do see is that, when it works, it really is beyond what we expected 10 or 11 years ago.”
Speaking in the press briefing, Mr. Olson described how several weeks after his treatment in 2010, he became very ill with what has become known as the common, short-term side effect of cytokine release syndrome.
However, after Mr. Olson recovered a few days later, Dr. Porter gave him the remarkable news that “we cannot find a single cancer cell. You appear completely free of CLL.”
Mr. Olson reported that he has since lived a “full life,” kept working, and has even run some half-marathons.
Dr. June confided that the current 10-year results far exceed the team’s early expectations for CAR T-cell therapy. “After Doug [initially] signed his informed consent document for this, we thought that the cells would all be gone within a month or 2. The fact that they have survived for 10 years was a major surprise – and a happy one at that.”
Dr. June, Dr. Melenhorst, and Dr. Porter reported holding patents related to CAR T-cell manufacturing and biomarker discovery.
“We can now conclude that CAR T cells can actually cure patients with leukemia based on these results,” said senior author Carl H. June, MD, in a press briefing on the study published in Nature.
“The major finding from this paper is that, 10 years down the road, you can find these [CAR T] cells,” Dr. June, director of the Center for Cellular Immunotherapies, University of Pennsylvania, Philadelphia, added. “The cells have evolved, and that was a big surprise ... but they are still able to kill leukemia cells 10 years after infusion.”
CAR T-cell therapy, in which patients’ own T cells are removed, reprogrammed in a lab to recognize and attack cancer cells, and then infused back into the patients, has transformed treatment of various blood cancers and shows often-remarkable results in achieving remissions.
While the treatment has become a routine therapy for certain leukemias, long-term results on the fate and function of the cells over time has been highly anticipated.
In the first published observations of a 10-year follow-up of patients treated with CAR T cells, Dr. June and colleagues described the findings for two patients, both with CLL, who back in 2010 were among the first to be treated with this groundbreaking therapy at the University of Pennsylvania.
A decade later, the CAR T cells are found to have remained detectable in both patients, who achieved complete remission in their first year of treatment, and both have sustained that remission.
Notably, the cells have evolved over the years – from initially being dominated by killer T cells to being dominated primarily by proliferative CD4-positive CAR T cells – with one of the patients exclusively having CD4-positive cells at year 9.3.
“The killer T cells did the initial heavy lifting of eliminating the tumor, “ first author J. Joseph Melenhorst, PhD, said in an interview.
“Once their job was done, those cells went down to very low levels, but the CD4-positive population persisted,” said Dr. Melenhorst, who established the lab at the University of Pennsylvania to follow patients treated with CAR T-cell therapy. “[This] delayed phase of immune response against cancer is a novel insight, and we were surprised to see it.”
Dr. Melenhorst noted that the clonal makeup of the CD4-positive cells importantly stabilized and became dominated by a small number of clones, suggesting further sustainability.
When one of the two patients, Doug Olson, who participated in the press conference, donated his cells back to the center after 9.3 years, the researchers found that his cells were still capable of destroying leukemia cells in the lab.
“Ten years [post infusion], we can’t find any of the leukemia cells and we still have the CAR T cells that are on patrol and on surveillance for residual leukemia,” Dr. June said.
One challenge of the otherwise desirable elimination of leukemia cells is that some aspects of sustaining CAR T-cell activity become problematic.
“The aspect of how the remission is maintained [is] very hard to study in a patient when there is no leukemia at all,” Dr. June explained. “It could be the last cell was gone within 3 weeks [of treatment], or it could be that the [cancer cells] are coming up like whack-a-moles, and they are killed because these CAR T cells are on patrol.”
Sadly, the other CLL patient, Bill Ludwig, who was first to receive the CAR T-cell treatment, died in 2021 from COVID-19.
Effects in other blood diseases similar?
CAR T-cell therapy is currently approved in the United States for several blood cancers, and whether similar long-term patterns of the cells may be observed in other patient and cancer types remains to be seen, Dr. Melenhorst said.
“I think in CLL we will see something similar, but in other diseases, we have yet to learn,” he said. “It may depend on issues including which domain has been engineered into the CAR.”
While the prospect of some patients being “cured” is exciting, responses to the therapy have generally been mixed. In CLL, for instance, full remissions have been observed to be maintained in about a quarter of patients, with higher rates observed in some lymphomas and pediatric ALL patients, Dr. Melenhorst explained.
The effects of CAR T-cell therapy in solid cancers have so far been more disappointing, with no research centers reproducing the kinds of results that have been seen with blood cancers.
“There appear to be a number of reasons, including that the [solid] tumor is more complex, and these solid cancers have ways to evade the immune system that need to be overcome,” Dr. June explained.
And despite the more encouraging findings in blood cancers, even with those, “the biggest disappointment is that CAR T-cell therapy doesn’t work all the time. It doesn’t work in every patient,” coauthor David Porter, MD, the University of Pennsylvania oncologist who treated the two patients, said in the press briefing.
“I think the importance of the Nature study is that we are starting to learn the mechanisms of why and how this works, so that we can start to get at how to make it work for more people,” Dr. Porter added. “But what we do see is that, when it works, it really is beyond what we expected 10 or 11 years ago.”
Speaking in the press briefing, Mr. Olson described how several weeks after his treatment in 2010, he became very ill with what has become known as the common, short-term side effect of cytokine release syndrome.
However, after Mr. Olson recovered a few days later, Dr. Porter gave him the remarkable news that “we cannot find a single cancer cell. You appear completely free of CLL.”
Mr. Olson reported that he has since lived a “full life,” kept working, and has even run some half-marathons.
Dr. June confided that the current 10-year results far exceed the team’s early expectations for CAR T-cell therapy. “After Doug [initially] signed his informed consent document for this, we thought that the cells would all be gone within a month or 2. The fact that they have survived for 10 years was a major surprise – and a happy one at that.”
Dr. June, Dr. Melenhorst, and Dr. Porter reported holding patents related to CAR T-cell manufacturing and biomarker discovery.
“We can now conclude that CAR T cells can actually cure patients with leukemia based on these results,” said senior author Carl H. June, MD, in a press briefing on the study published in Nature.
“The major finding from this paper is that, 10 years down the road, you can find these [CAR T] cells,” Dr. June, director of the Center for Cellular Immunotherapies, University of Pennsylvania, Philadelphia, added. “The cells have evolved, and that was a big surprise ... but they are still able to kill leukemia cells 10 years after infusion.”
CAR T-cell therapy, in which patients’ own T cells are removed, reprogrammed in a lab to recognize and attack cancer cells, and then infused back into the patients, has transformed treatment of various blood cancers and shows often-remarkable results in achieving remissions.
While the treatment has become a routine therapy for certain leukemias, long-term results on the fate and function of the cells over time has been highly anticipated.
In the first published observations of a 10-year follow-up of patients treated with CAR T cells, Dr. June and colleagues described the findings for two patients, both with CLL, who back in 2010 were among the first to be treated with this groundbreaking therapy at the University of Pennsylvania.
A decade later, the CAR T cells are found to have remained detectable in both patients, who achieved complete remission in their first year of treatment, and both have sustained that remission.
Notably, the cells have evolved over the years – from initially being dominated by killer T cells to being dominated primarily by proliferative CD4-positive CAR T cells – with one of the patients exclusively having CD4-positive cells at year 9.3.
“The killer T cells did the initial heavy lifting of eliminating the tumor, “ first author J. Joseph Melenhorst, PhD, said in an interview.
“Once their job was done, those cells went down to very low levels, but the CD4-positive population persisted,” said Dr. Melenhorst, who established the lab at the University of Pennsylvania to follow patients treated with CAR T-cell therapy. “[This] delayed phase of immune response against cancer is a novel insight, and we were surprised to see it.”
Dr. Melenhorst noted that the clonal makeup of the CD4-positive cells importantly stabilized and became dominated by a small number of clones, suggesting further sustainability.
When one of the two patients, Doug Olson, who participated in the press conference, donated his cells back to the center after 9.3 years, the researchers found that his cells were still capable of destroying leukemia cells in the lab.
“Ten years [post infusion], we can’t find any of the leukemia cells and we still have the CAR T cells that are on patrol and on surveillance for residual leukemia,” Dr. June said.
One challenge of the otherwise desirable elimination of leukemia cells is that some aspects of sustaining CAR T-cell activity become problematic.
“The aspect of how the remission is maintained [is] very hard to study in a patient when there is no leukemia at all,” Dr. June explained. “It could be the last cell was gone within 3 weeks [of treatment], or it could be that the [cancer cells] are coming up like whack-a-moles, and they are killed because these CAR T cells are on patrol.”
Sadly, the other CLL patient, Bill Ludwig, who was first to receive the CAR T-cell treatment, died in 2021 from COVID-19.
Effects in other blood diseases similar?
CAR T-cell therapy is currently approved in the United States for several blood cancers, and whether similar long-term patterns of the cells may be observed in other patient and cancer types remains to be seen, Dr. Melenhorst said.
“I think in CLL we will see something similar, but in other diseases, we have yet to learn,” he said. “It may depend on issues including which domain has been engineered into the CAR.”
While the prospect of some patients being “cured” is exciting, responses to the therapy have generally been mixed. In CLL, for instance, full remissions have been observed to be maintained in about a quarter of patients, with higher rates observed in some lymphomas and pediatric ALL patients, Dr. Melenhorst explained.
The effects of CAR T-cell therapy in solid cancers have so far been more disappointing, with no research centers reproducing the kinds of results that have been seen with blood cancers.
“There appear to be a number of reasons, including that the [solid] tumor is more complex, and these solid cancers have ways to evade the immune system that need to be overcome,” Dr. June explained.
And despite the more encouraging findings in blood cancers, even with those, “the biggest disappointment is that CAR T-cell therapy doesn’t work all the time. It doesn’t work in every patient,” coauthor David Porter, MD, the University of Pennsylvania oncologist who treated the two patients, said in the press briefing.
“I think the importance of the Nature study is that we are starting to learn the mechanisms of why and how this works, so that we can start to get at how to make it work for more people,” Dr. Porter added. “But what we do see is that, when it works, it really is beyond what we expected 10 or 11 years ago.”
Speaking in the press briefing, Mr. Olson described how several weeks after his treatment in 2010, he became very ill with what has become known as the common, short-term side effect of cytokine release syndrome.
However, after Mr. Olson recovered a few days later, Dr. Porter gave him the remarkable news that “we cannot find a single cancer cell. You appear completely free of CLL.”
Mr. Olson reported that he has since lived a “full life,” kept working, and has even run some half-marathons.
Dr. June confided that the current 10-year results far exceed the team’s early expectations for CAR T-cell therapy. “After Doug [initially] signed his informed consent document for this, we thought that the cells would all be gone within a month or 2. The fact that they have survived for 10 years was a major surprise – and a happy one at that.”
Dr. June, Dr. Melenhorst, and Dr. Porter reported holding patents related to CAR T-cell manufacturing and biomarker discovery.
FROM NATURE
‘Lucky genes’ may protect against some obesity-related diseases
in a large new genetics study.
That is, people with unfavorable adiposity gene variants had fat stored under the skin throughout the body, but they also had more ectopic fat (fat in the “wrong place”) surrounding the pancreas and liver, which is associated with a higher risk of metabolic diseases such as heart disease and type 2 diabetes.
In contrast, people with favorable adiposity gene variants had more subcutaneous fat (such as a paunch or a double chin).
The study by Susan Martin, PhD, a postdoctoral research associate at the University of Exeter (England) and colleagues, was recently published in eLife.
“Some people have ‘unlucky fat genes,’ meaning they store higher levels of fat everywhere, including under the skin [and around the] liver and pancreas. That’s associated with a higher risk of diseases such as type 2 diabetes,” senior author Hanieh Yaghootkar, MD, PhD, summarized in a press release from the University of Exeter.
“Others are luckier and have genes that mean higher fat under the skin but lower liver fat and a lower risk of diseases like type 2 diabetes,” added Dr. Yaghootkar, from Brunel University London.
Among 37 chronic diseases that are associated with obesity, the researchers found the metabolic effects of adiposity are likely the main cause of the following 11: type 2 diabetes, polycystic ovary syndrome, coronary artery disease, peripheral artery disease, hypertension, stroke, heart failure, atrial fibrillation, chronic kidney disease, renal cancer, and gout.
On the other hand, excess weight itself (such as a heavy load on the joints) rather than a metabolic effect is associated with nine other obesity-related diseases: osteoarthritis, rheumatoid arthritis, osteoporosis, gastro-esophageal reflux disease, gallstones, adult-onset asthma, psoriasis, deep vein thrombosis, and venous thromboembolism.
Good genes no substitute for a healthy lifestyle
“People with more favorable adiposity gene variants are still at risk of the nine diseases” that are not caused by metabolic effects – such as osteoarthritis – but are caused by the effect of excess weight on the joints, another author, Timothy M. Frayling, PhD, stressed.
“People with obesity and unfavorable adiposity gene variants are at higher risk of all 20 diseases because they have the double hit of the excess mechanical effects and the adverse metabolic effects,” Dr. Frayling of the University of Exeter, told this news organization in an email.
The main clinical message, he said, is that “this research helps inform which conditions may respond better to therapies that lower the adverse effects” of risk factors such as high cholesterol and blood glucose levels, “and high blood pressure, even with no weight loss.”
“In contrast, other conditions really require the weight loss.”
“These results emphasize that many people in the community who are of higher body mass index are at risk of multiple chronic conditions that can severely impair their quality of life or cause morbidity or mortality, even if their metabolic parameters appear relatively normal,” the researchers conclude.
“Whilst it’s important that we identify the causes of obesity-related disease, good genes [are] still no substitute for a healthy lifestyle,” Dr. Martin stressed.
“A favorable adiposity will only go so far. If you’re obese, the advice is to still try and shift the excess weight where you can,” she said.
“The authors have conducted a robust and very comprehensive study using Mendelian randomization to disentangle metabolic and nonmetabolic effects of overweight on a long list of disease outcomes,” reviewing editor Edward D. Janus, MD, PhD, of the University of Melbourne summarized.
“This is an important topic and can help us better understand how overweight influences risk of several important outcomes.”
Metabolic and nonmetabolic diseases caused by obesity
The researchers aimed to investigate the effects of adiposity on metabolic and nonmetabolic diseases caused by obesity.
They used data from 176,899 individuals in the FinnGen project in Finland and from over 500,000 individuals in the UK Biobank database.
They performed Mendelian randomization studies to investigate the causal association between BMI, body fat percentage, favorable adiposity alleles, and unfavorable adiposity alleles with 37 disease outcomes.
Of these 37 chronic diseases associated with obesity, 11 diseases were directly related to the metabolic effect of adiposity (where favorable adiposity or unfavorable adiposity gene variants had opposite effects). Nine other diseases were unrelated to the metabolic effects of adiposity.
For most of the remaining diseases – for example, Alzheimer’s disease and different cancers – it was difficult to draw firm conclusions about the respective roles of favorable adiposity and unfavorable adiposity gene variants.
The study was funded by Diabetes UK, the UK Medical Research Council, the World Cancer Research Fund, and the National Cancer Institute. Author disclosures are listed with the article.
A version of this article first appeared on Medscape.com.
in a large new genetics study.
That is, people with unfavorable adiposity gene variants had fat stored under the skin throughout the body, but they also had more ectopic fat (fat in the “wrong place”) surrounding the pancreas and liver, which is associated with a higher risk of metabolic diseases such as heart disease and type 2 diabetes.
In contrast, people with favorable adiposity gene variants had more subcutaneous fat (such as a paunch or a double chin).
The study by Susan Martin, PhD, a postdoctoral research associate at the University of Exeter (England) and colleagues, was recently published in eLife.
“Some people have ‘unlucky fat genes,’ meaning they store higher levels of fat everywhere, including under the skin [and around the] liver and pancreas. That’s associated with a higher risk of diseases such as type 2 diabetes,” senior author Hanieh Yaghootkar, MD, PhD, summarized in a press release from the University of Exeter.
“Others are luckier and have genes that mean higher fat under the skin but lower liver fat and a lower risk of diseases like type 2 diabetes,” added Dr. Yaghootkar, from Brunel University London.
Among 37 chronic diseases that are associated with obesity, the researchers found the metabolic effects of adiposity are likely the main cause of the following 11: type 2 diabetes, polycystic ovary syndrome, coronary artery disease, peripheral artery disease, hypertension, stroke, heart failure, atrial fibrillation, chronic kidney disease, renal cancer, and gout.
On the other hand, excess weight itself (such as a heavy load on the joints) rather than a metabolic effect is associated with nine other obesity-related diseases: osteoarthritis, rheumatoid arthritis, osteoporosis, gastro-esophageal reflux disease, gallstones, adult-onset asthma, psoriasis, deep vein thrombosis, and venous thromboembolism.
Good genes no substitute for a healthy lifestyle
“People with more favorable adiposity gene variants are still at risk of the nine diseases” that are not caused by metabolic effects – such as osteoarthritis – but are caused by the effect of excess weight on the joints, another author, Timothy M. Frayling, PhD, stressed.
“People with obesity and unfavorable adiposity gene variants are at higher risk of all 20 diseases because they have the double hit of the excess mechanical effects and the adverse metabolic effects,” Dr. Frayling of the University of Exeter, told this news organization in an email.
The main clinical message, he said, is that “this research helps inform which conditions may respond better to therapies that lower the adverse effects” of risk factors such as high cholesterol and blood glucose levels, “and high blood pressure, even with no weight loss.”
“In contrast, other conditions really require the weight loss.”
“These results emphasize that many people in the community who are of higher body mass index are at risk of multiple chronic conditions that can severely impair their quality of life or cause morbidity or mortality, even if their metabolic parameters appear relatively normal,” the researchers conclude.
“Whilst it’s important that we identify the causes of obesity-related disease, good genes [are] still no substitute for a healthy lifestyle,” Dr. Martin stressed.
“A favorable adiposity will only go so far. If you’re obese, the advice is to still try and shift the excess weight where you can,” she said.
“The authors have conducted a robust and very comprehensive study using Mendelian randomization to disentangle metabolic and nonmetabolic effects of overweight on a long list of disease outcomes,” reviewing editor Edward D. Janus, MD, PhD, of the University of Melbourne summarized.
“This is an important topic and can help us better understand how overweight influences risk of several important outcomes.”
Metabolic and nonmetabolic diseases caused by obesity
The researchers aimed to investigate the effects of adiposity on metabolic and nonmetabolic diseases caused by obesity.
They used data from 176,899 individuals in the FinnGen project in Finland and from over 500,000 individuals in the UK Biobank database.
They performed Mendelian randomization studies to investigate the causal association between BMI, body fat percentage, favorable adiposity alleles, and unfavorable adiposity alleles with 37 disease outcomes.
Of these 37 chronic diseases associated with obesity, 11 diseases were directly related to the metabolic effect of adiposity (where favorable adiposity or unfavorable adiposity gene variants had opposite effects). Nine other diseases were unrelated to the metabolic effects of adiposity.
For most of the remaining diseases – for example, Alzheimer’s disease and different cancers – it was difficult to draw firm conclusions about the respective roles of favorable adiposity and unfavorable adiposity gene variants.
The study was funded by Diabetes UK, the UK Medical Research Council, the World Cancer Research Fund, and the National Cancer Institute. Author disclosures are listed with the article.
A version of this article first appeared on Medscape.com.
in a large new genetics study.
That is, people with unfavorable adiposity gene variants had fat stored under the skin throughout the body, but they also had more ectopic fat (fat in the “wrong place”) surrounding the pancreas and liver, which is associated with a higher risk of metabolic diseases such as heart disease and type 2 diabetes.
In contrast, people with favorable adiposity gene variants had more subcutaneous fat (such as a paunch or a double chin).
The study by Susan Martin, PhD, a postdoctoral research associate at the University of Exeter (England) and colleagues, was recently published in eLife.
“Some people have ‘unlucky fat genes,’ meaning they store higher levels of fat everywhere, including under the skin [and around the] liver and pancreas. That’s associated with a higher risk of diseases such as type 2 diabetes,” senior author Hanieh Yaghootkar, MD, PhD, summarized in a press release from the University of Exeter.
“Others are luckier and have genes that mean higher fat under the skin but lower liver fat and a lower risk of diseases like type 2 diabetes,” added Dr. Yaghootkar, from Brunel University London.
Among 37 chronic diseases that are associated with obesity, the researchers found the metabolic effects of adiposity are likely the main cause of the following 11: type 2 diabetes, polycystic ovary syndrome, coronary artery disease, peripheral artery disease, hypertension, stroke, heart failure, atrial fibrillation, chronic kidney disease, renal cancer, and gout.
On the other hand, excess weight itself (such as a heavy load on the joints) rather than a metabolic effect is associated with nine other obesity-related diseases: osteoarthritis, rheumatoid arthritis, osteoporosis, gastro-esophageal reflux disease, gallstones, adult-onset asthma, psoriasis, deep vein thrombosis, and venous thromboembolism.
Good genes no substitute for a healthy lifestyle
“People with more favorable adiposity gene variants are still at risk of the nine diseases” that are not caused by metabolic effects – such as osteoarthritis – but are caused by the effect of excess weight on the joints, another author, Timothy M. Frayling, PhD, stressed.
“People with obesity and unfavorable adiposity gene variants are at higher risk of all 20 diseases because they have the double hit of the excess mechanical effects and the adverse metabolic effects,” Dr. Frayling of the University of Exeter, told this news organization in an email.
The main clinical message, he said, is that “this research helps inform which conditions may respond better to therapies that lower the adverse effects” of risk factors such as high cholesterol and blood glucose levels, “and high blood pressure, even with no weight loss.”
“In contrast, other conditions really require the weight loss.”
“These results emphasize that many people in the community who are of higher body mass index are at risk of multiple chronic conditions that can severely impair their quality of life or cause morbidity or mortality, even if their metabolic parameters appear relatively normal,” the researchers conclude.
“Whilst it’s important that we identify the causes of obesity-related disease, good genes [are] still no substitute for a healthy lifestyle,” Dr. Martin stressed.
“A favorable adiposity will only go so far. If you’re obese, the advice is to still try and shift the excess weight where you can,” she said.
“The authors have conducted a robust and very comprehensive study using Mendelian randomization to disentangle metabolic and nonmetabolic effects of overweight on a long list of disease outcomes,” reviewing editor Edward D. Janus, MD, PhD, of the University of Melbourne summarized.
“This is an important topic and can help us better understand how overweight influences risk of several important outcomes.”
Metabolic and nonmetabolic diseases caused by obesity
The researchers aimed to investigate the effects of adiposity on metabolic and nonmetabolic diseases caused by obesity.
They used data from 176,899 individuals in the FinnGen project in Finland and from over 500,000 individuals in the UK Biobank database.
They performed Mendelian randomization studies to investigate the causal association between BMI, body fat percentage, favorable adiposity alleles, and unfavorable adiposity alleles with 37 disease outcomes.
Of these 37 chronic diseases associated with obesity, 11 diseases were directly related to the metabolic effect of adiposity (where favorable adiposity or unfavorable adiposity gene variants had opposite effects). Nine other diseases were unrelated to the metabolic effects of adiposity.
For most of the remaining diseases – for example, Alzheimer’s disease and different cancers – it was difficult to draw firm conclusions about the respective roles of favorable adiposity and unfavorable adiposity gene variants.
The study was funded by Diabetes UK, the UK Medical Research Council, the World Cancer Research Fund, and the National Cancer Institute. Author disclosures are listed with the article.
A version of this article first appeared on Medscape.com.
10 things not to do in a medical board hearing
A Florida doctor told his patient her test result would be available in 3-4 days. When the patient didn’t hear back, she called the practice several times, but she didn’t receive a return call. So she filed a complaint against the doctor with the medical board.
When the board investigator interviewed the doctor, the physician said he wasn’t aware the patient had called. But his staff said otherwise. Because the doctor had not been truthful, the board sent him a letter of guidance and required him to attend a training program in ethics.
Miami attorney William J. Spratt Jr., who supplied this anecdote about a former client, said that
The following are some common mistakes that physicians make when dealing with a board complaint.
1. Not responding to the complaint
The complaint you get from the board – which often comes with a subpoena and a response deadline – usually asks for medical records pertinent to the case.
You can’t disregard the board’s letter, said Doug Brocker, an attorney handling board actions in Raleigh, N.C. “It’s amazing to me that some people just ignore a board complaint. Sometimes it’s because the doctor is just burnt out, which may have gotten the doctor into trouble in the first place.”
If you do not respond to a subpoena, “the board can file a court order holding you in contempt and start taking action on your license,” said Jeff Segal, MD, a neurosurgeon and attorney in Greensboro, N.C. Dr. Segal is CEO of Medical Justice Services, which protects physicians’ reputations associated with malpractice suits and board actions. “Not responding is not much different from agreeing to all of the charges.”
2. Not recognizing the seriousness of the complaint
“The biggest mistake is not taking a complaint seriously,” said Linda Stimmel, an attorney at Wilson Elser in Dallas. “Physicians who get a complaint often fire off a brief response stating that the complaint has no merit, without offering any evidence.”
According to Ms. Stimmel, “it’s really important to back up your assertions, such as using excerpts from the medical record, citations of peer-reviewed articles, or a letter of support from a colleague.”
“Weigh your answers carefully, because lack of accuracy will complicate your case,” Mr. Brocker said. “Consult the medical record rather than rely on your memory.”
“Present your version of events, in your own words, because that’s almost always better than the board’s version,” said Dr. Segal.
Even if there was a bad clinical outcome, Dr. Segal said you might point out that the patient was at high risk, or you could show that your clinical outcomes are better than the national average.
3. Thinking the board is on your side
You may be lulled into a false sense of security because the physicians on the medical board are your peers, but they can be as tough as any medical malpractice judge, said William P. Sullivan, DO, an emergency physician and attorney in Frankfort, Ill.
As per the National Practitioner Data Bank, physicians are three to four times more likely to incur an adverse board action than make a malpractice payout, Dr. Sullivan said.
Also, although a malpractice lawsuit rarely involves more than a monetary payment, a board action, like a monitoring plan, can restrict your ability to practice medicine. In fact, any kind of board action against you can make it harder to find employment.
4. Not being honest or forthcoming
“Lying to the board is the fastest way to turn what would have been a minor infraction into putting your license at risk,” Mr. Brocker said. This can happen when doctors update a medical record to support their version of events.
As per Dr. Sullivan, another way to put your license at risk is to withhold adverse information, which the board can detect by obtaining your application for hospital privileges or for licensure to another state, in which you revealed the adverse information.
Dr. Sullivan also advised against claiming you “always” take a certain precautionary measure. “In reality, we doctors don’t always do what we would like to have done. By saying you always do it when you didn’t, you appear less than truthful to the board, and boards have a hard time with that.”
Similarly, “when doctors don’t want to recognize that they could have handled things better, they tend to dance around the issue,” Mr. Brocker said. “This does not sit well with the board.” Insisting that you did everything right when it’s obvious that you didn’t can lead to harsher sanctions. “The board wants to make sure doctors recognize their mistakes and are willing to learn from them.”
5. Providing too much information
You may think that providing a great deal of information strengthens your case, but it can actually weaken it, Mr. Brocker said. Irrelevant information makes your response hard to follow, and it may contain evidence that could prompt another line of inquiry.
“Less is more,” Dr. Segal advised. “Present a coherent argument and keep to the most salient points.” Being concise is also good advice if your complaint proceeds to the board and you have to present your case.
Dr. Segal said the board will stop paying attention to long-winded presentations. He tells his clients to imagine the board is watching a movie. “If your presentation is tedious or hard to follow, you will lose them.”
6. Trying to contact the complainant
Complaints are kept anonymous, but in many cases, the doctor has an idea who the complainant was and may try to contact that person. “It’s natural to wonder why a patient would file a complaint against you,” Mr. Brocker said, but if you reach out to the patient to ask why, “it could look like you’re trying to persuade the patient to drop the complaint.”
Doctors who are involved in a practice breakup or a divorce can be victims of false and malicious complaints, but Beth Y. Collis, a partner at the law firm of Dinsmore & Shohl in Columbus, said boards are onto this tactic and usually reject these complaints.
The doctor may be tempted to sue the complainant, but Mr. Brocker said this won’t stop the complaint and could strengthen it. “Most statements to the medical board are protected from defamation lawsuits, and any lawsuit could appear to be intimidation.”
7. Simply signing a consent agreement
A small minority of complaints may result in the board taking action against the doctor. Typically, this involves getting the doctor to sign a consent agreement stating that he or she agrees with the board’s decision and its remedy, such as continuing education, a fine, or being placed under another doctor’s supervision.
“When the board sends you a consent agreement, it’s usually about something fairly minor,” Ms. Collis said. “You can make a counteroffer and see if they accept that. But once you enter into the agreement, you waive any right to appeal the board’s decision.”
8. Not hiring an attorney
Although some doctors manage to deal with a board complaint on their own, many will need to get an attorney, Mr. Brocker said. “An experienced attorney can help you navigate the board’s process.”
Clients often look for attorneys at the end of the process, when formal charges have already been filed, Mr. Brocker said. At that point, “it’s harder to get things moving in the right direction. You can’t unring the bell.”
Even if you don’t think you need an attorney throughout the case, “it helps to get advice from an attorney at the beginning,” Dr. Segal said. Doctors may think they can’t afford an attorney, but many malpractice carriers pay attorneys’ fees in medical board investigations.
Mr. Brocker advised finding an attorney who is familiar with licensing boards. “Malpractice attorneys may think they can deal with medical boards, but boards are quite different.” For example, “malpractice cases involve an adversarial approach, but licensing boards normally require working collaboratively.”
9. Not requesting a hearing
When the board takes action against you, it can be tempting to just accept the allegations and move on with your life, but it may be possible to undo the action, Dr. Sullivan said. “The board still has to prove its allegations, and it may not have a strong case against you.”
In some states, the medical board has to meet a very high standard of proof, Dr. Sullivan said. In Illinois, for example, the board must show “clear and convincing evidence,” while a malpractice plaintiff must only prove that it’s “more likely than not” that a physician violated the standard of care.
A hearing can especially help doctors facing harsh sanctions for minor offenses. For example, in a case handled by the law firm of Ray & Bishop in Newport Beach, Calif., a doctor who was stopped by police while driving home after having wine at a family gathering was found to have a blood alcohol level of 0.11%. Noting that the physician was on call at the time, the Medical Board of California decided to give him 5 years of probation.
Ray & Bishop asked for a judicial hearing to contest the decision. At the hearing, the physician noted that other physicians were also available to take call that night, and an expert stated that the doctor was not an alcohol abuser. The judge ruled that the board’s action was unduly harsh, and the physician received a public reprimand with no further penalties.
10. Getting upset with board officials
A board investigator may show up at your office uninvited and ask you to answer some questions, but you aren’t required to answer then and there, said Ms. Collis.
In fact, she noted, it’s never a good idea to let investigators into your office. “They can walk around, look through your records, and find more things to investigate.” For this reason, Ms. Collis makes it a point to schedule meetings with investigators at her office.
When you have to interact with board officials, such as during hearings, expressing anger is a mistake. “Some board members may raise their voices and make untrue assertions about your medical care,” Dr. Sullivan said. “You may wish you could respond in kind, but that will not help you.” Instead, calmly provide studies or guidelines supporting the care you provided.
Taking board investigators to task is also a mistake, Mr. Brocker pointed out. In his words, “investigators have to follow the rules. Getting mad at them will only make your case more difficult. Even if you believe the complaint against you is totally without merit, the process needs to run its course.”
A version of this article first appeared on Medscape.com.
A Florida doctor told his patient her test result would be available in 3-4 days. When the patient didn’t hear back, she called the practice several times, but she didn’t receive a return call. So she filed a complaint against the doctor with the medical board.
When the board investigator interviewed the doctor, the physician said he wasn’t aware the patient had called. But his staff said otherwise. Because the doctor had not been truthful, the board sent him a letter of guidance and required him to attend a training program in ethics.
Miami attorney William J. Spratt Jr., who supplied this anecdote about a former client, said that
The following are some common mistakes that physicians make when dealing with a board complaint.
1. Not responding to the complaint
The complaint you get from the board – which often comes with a subpoena and a response deadline – usually asks for medical records pertinent to the case.
You can’t disregard the board’s letter, said Doug Brocker, an attorney handling board actions in Raleigh, N.C. “It’s amazing to me that some people just ignore a board complaint. Sometimes it’s because the doctor is just burnt out, which may have gotten the doctor into trouble in the first place.”
If you do not respond to a subpoena, “the board can file a court order holding you in contempt and start taking action on your license,” said Jeff Segal, MD, a neurosurgeon and attorney in Greensboro, N.C. Dr. Segal is CEO of Medical Justice Services, which protects physicians’ reputations associated with malpractice suits and board actions. “Not responding is not much different from agreeing to all of the charges.”
2. Not recognizing the seriousness of the complaint
“The biggest mistake is not taking a complaint seriously,” said Linda Stimmel, an attorney at Wilson Elser in Dallas. “Physicians who get a complaint often fire off a brief response stating that the complaint has no merit, without offering any evidence.”
According to Ms. Stimmel, “it’s really important to back up your assertions, such as using excerpts from the medical record, citations of peer-reviewed articles, or a letter of support from a colleague.”
“Weigh your answers carefully, because lack of accuracy will complicate your case,” Mr. Brocker said. “Consult the medical record rather than rely on your memory.”
“Present your version of events, in your own words, because that’s almost always better than the board’s version,” said Dr. Segal.
Even if there was a bad clinical outcome, Dr. Segal said you might point out that the patient was at high risk, or you could show that your clinical outcomes are better than the national average.
3. Thinking the board is on your side
You may be lulled into a false sense of security because the physicians on the medical board are your peers, but they can be as tough as any medical malpractice judge, said William P. Sullivan, DO, an emergency physician and attorney in Frankfort, Ill.
As per the National Practitioner Data Bank, physicians are three to four times more likely to incur an adverse board action than make a malpractice payout, Dr. Sullivan said.
Also, although a malpractice lawsuit rarely involves more than a monetary payment, a board action, like a monitoring plan, can restrict your ability to practice medicine. In fact, any kind of board action against you can make it harder to find employment.
4. Not being honest or forthcoming
“Lying to the board is the fastest way to turn what would have been a minor infraction into putting your license at risk,” Mr. Brocker said. This can happen when doctors update a medical record to support their version of events.
As per Dr. Sullivan, another way to put your license at risk is to withhold adverse information, which the board can detect by obtaining your application for hospital privileges or for licensure to another state, in which you revealed the adverse information.
Dr. Sullivan also advised against claiming you “always” take a certain precautionary measure. “In reality, we doctors don’t always do what we would like to have done. By saying you always do it when you didn’t, you appear less than truthful to the board, and boards have a hard time with that.”
Similarly, “when doctors don’t want to recognize that they could have handled things better, they tend to dance around the issue,” Mr. Brocker said. “This does not sit well with the board.” Insisting that you did everything right when it’s obvious that you didn’t can lead to harsher sanctions. “The board wants to make sure doctors recognize their mistakes and are willing to learn from them.”
5. Providing too much information
You may think that providing a great deal of information strengthens your case, but it can actually weaken it, Mr. Brocker said. Irrelevant information makes your response hard to follow, and it may contain evidence that could prompt another line of inquiry.
“Less is more,” Dr. Segal advised. “Present a coherent argument and keep to the most salient points.” Being concise is also good advice if your complaint proceeds to the board and you have to present your case.
Dr. Segal said the board will stop paying attention to long-winded presentations. He tells his clients to imagine the board is watching a movie. “If your presentation is tedious or hard to follow, you will lose them.”
6. Trying to contact the complainant
Complaints are kept anonymous, but in many cases, the doctor has an idea who the complainant was and may try to contact that person. “It’s natural to wonder why a patient would file a complaint against you,” Mr. Brocker said, but if you reach out to the patient to ask why, “it could look like you’re trying to persuade the patient to drop the complaint.”
Doctors who are involved in a practice breakup or a divorce can be victims of false and malicious complaints, but Beth Y. Collis, a partner at the law firm of Dinsmore & Shohl in Columbus, said boards are onto this tactic and usually reject these complaints.
The doctor may be tempted to sue the complainant, but Mr. Brocker said this won’t stop the complaint and could strengthen it. “Most statements to the medical board are protected from defamation lawsuits, and any lawsuit could appear to be intimidation.”
7. Simply signing a consent agreement
A small minority of complaints may result in the board taking action against the doctor. Typically, this involves getting the doctor to sign a consent agreement stating that he or she agrees with the board’s decision and its remedy, such as continuing education, a fine, or being placed under another doctor’s supervision.
“When the board sends you a consent agreement, it’s usually about something fairly minor,” Ms. Collis said. “You can make a counteroffer and see if they accept that. But once you enter into the agreement, you waive any right to appeal the board’s decision.”
8. Not hiring an attorney
Although some doctors manage to deal with a board complaint on their own, many will need to get an attorney, Mr. Brocker said. “An experienced attorney can help you navigate the board’s process.”
Clients often look for attorneys at the end of the process, when formal charges have already been filed, Mr. Brocker said. At that point, “it’s harder to get things moving in the right direction. You can’t unring the bell.”
Even if you don’t think you need an attorney throughout the case, “it helps to get advice from an attorney at the beginning,” Dr. Segal said. Doctors may think they can’t afford an attorney, but many malpractice carriers pay attorneys’ fees in medical board investigations.
Mr. Brocker advised finding an attorney who is familiar with licensing boards. “Malpractice attorneys may think they can deal with medical boards, but boards are quite different.” For example, “malpractice cases involve an adversarial approach, but licensing boards normally require working collaboratively.”
9. Not requesting a hearing
When the board takes action against you, it can be tempting to just accept the allegations and move on with your life, but it may be possible to undo the action, Dr. Sullivan said. “The board still has to prove its allegations, and it may not have a strong case against you.”
In some states, the medical board has to meet a very high standard of proof, Dr. Sullivan said. In Illinois, for example, the board must show “clear and convincing evidence,” while a malpractice plaintiff must only prove that it’s “more likely than not” that a physician violated the standard of care.
A hearing can especially help doctors facing harsh sanctions for minor offenses. For example, in a case handled by the law firm of Ray & Bishop in Newport Beach, Calif., a doctor who was stopped by police while driving home after having wine at a family gathering was found to have a blood alcohol level of 0.11%. Noting that the physician was on call at the time, the Medical Board of California decided to give him 5 years of probation.
Ray & Bishop asked for a judicial hearing to contest the decision. At the hearing, the physician noted that other physicians were also available to take call that night, and an expert stated that the doctor was not an alcohol abuser. The judge ruled that the board’s action was unduly harsh, and the physician received a public reprimand with no further penalties.
10. Getting upset with board officials
A board investigator may show up at your office uninvited and ask you to answer some questions, but you aren’t required to answer then and there, said Ms. Collis.
In fact, she noted, it’s never a good idea to let investigators into your office. “They can walk around, look through your records, and find more things to investigate.” For this reason, Ms. Collis makes it a point to schedule meetings with investigators at her office.
When you have to interact with board officials, such as during hearings, expressing anger is a mistake. “Some board members may raise their voices and make untrue assertions about your medical care,” Dr. Sullivan said. “You may wish you could respond in kind, but that will not help you.” Instead, calmly provide studies or guidelines supporting the care you provided.
Taking board investigators to task is also a mistake, Mr. Brocker pointed out. In his words, “investigators have to follow the rules. Getting mad at them will only make your case more difficult. Even if you believe the complaint against you is totally without merit, the process needs to run its course.”
A version of this article first appeared on Medscape.com.
A Florida doctor told his patient her test result would be available in 3-4 days. When the patient didn’t hear back, she called the practice several times, but she didn’t receive a return call. So she filed a complaint against the doctor with the medical board.
When the board investigator interviewed the doctor, the physician said he wasn’t aware the patient had called. But his staff said otherwise. Because the doctor had not been truthful, the board sent him a letter of guidance and required him to attend a training program in ethics.
Miami attorney William J. Spratt Jr., who supplied this anecdote about a former client, said that
The following are some common mistakes that physicians make when dealing with a board complaint.
1. Not responding to the complaint
The complaint you get from the board – which often comes with a subpoena and a response deadline – usually asks for medical records pertinent to the case.
You can’t disregard the board’s letter, said Doug Brocker, an attorney handling board actions in Raleigh, N.C. “It’s amazing to me that some people just ignore a board complaint. Sometimes it’s because the doctor is just burnt out, which may have gotten the doctor into trouble in the first place.”
If you do not respond to a subpoena, “the board can file a court order holding you in contempt and start taking action on your license,” said Jeff Segal, MD, a neurosurgeon and attorney in Greensboro, N.C. Dr. Segal is CEO of Medical Justice Services, which protects physicians’ reputations associated with malpractice suits and board actions. “Not responding is not much different from agreeing to all of the charges.”
2. Not recognizing the seriousness of the complaint
“The biggest mistake is not taking a complaint seriously,” said Linda Stimmel, an attorney at Wilson Elser in Dallas. “Physicians who get a complaint often fire off a brief response stating that the complaint has no merit, without offering any evidence.”
According to Ms. Stimmel, “it’s really important to back up your assertions, such as using excerpts from the medical record, citations of peer-reviewed articles, or a letter of support from a colleague.”
“Weigh your answers carefully, because lack of accuracy will complicate your case,” Mr. Brocker said. “Consult the medical record rather than rely on your memory.”
“Present your version of events, in your own words, because that’s almost always better than the board’s version,” said Dr. Segal.
Even if there was a bad clinical outcome, Dr. Segal said you might point out that the patient was at high risk, or you could show that your clinical outcomes are better than the national average.
3. Thinking the board is on your side
You may be lulled into a false sense of security because the physicians on the medical board are your peers, but they can be as tough as any medical malpractice judge, said William P. Sullivan, DO, an emergency physician and attorney in Frankfort, Ill.
As per the National Practitioner Data Bank, physicians are three to four times more likely to incur an adverse board action than make a malpractice payout, Dr. Sullivan said.
Also, although a malpractice lawsuit rarely involves more than a monetary payment, a board action, like a monitoring plan, can restrict your ability to practice medicine. In fact, any kind of board action against you can make it harder to find employment.
4. Not being honest or forthcoming
“Lying to the board is the fastest way to turn what would have been a minor infraction into putting your license at risk,” Mr. Brocker said. This can happen when doctors update a medical record to support their version of events.
As per Dr. Sullivan, another way to put your license at risk is to withhold adverse information, which the board can detect by obtaining your application for hospital privileges or for licensure to another state, in which you revealed the adverse information.
Dr. Sullivan also advised against claiming you “always” take a certain precautionary measure. “In reality, we doctors don’t always do what we would like to have done. By saying you always do it when you didn’t, you appear less than truthful to the board, and boards have a hard time with that.”
Similarly, “when doctors don’t want to recognize that they could have handled things better, they tend to dance around the issue,” Mr. Brocker said. “This does not sit well with the board.” Insisting that you did everything right when it’s obvious that you didn’t can lead to harsher sanctions. “The board wants to make sure doctors recognize their mistakes and are willing to learn from them.”
5. Providing too much information
You may think that providing a great deal of information strengthens your case, but it can actually weaken it, Mr. Brocker said. Irrelevant information makes your response hard to follow, and it may contain evidence that could prompt another line of inquiry.
“Less is more,” Dr. Segal advised. “Present a coherent argument and keep to the most salient points.” Being concise is also good advice if your complaint proceeds to the board and you have to present your case.
Dr. Segal said the board will stop paying attention to long-winded presentations. He tells his clients to imagine the board is watching a movie. “If your presentation is tedious or hard to follow, you will lose them.”
6. Trying to contact the complainant
Complaints are kept anonymous, but in many cases, the doctor has an idea who the complainant was and may try to contact that person. “It’s natural to wonder why a patient would file a complaint against you,” Mr. Brocker said, but if you reach out to the patient to ask why, “it could look like you’re trying to persuade the patient to drop the complaint.”
Doctors who are involved in a practice breakup or a divorce can be victims of false and malicious complaints, but Beth Y. Collis, a partner at the law firm of Dinsmore & Shohl in Columbus, said boards are onto this tactic and usually reject these complaints.
The doctor may be tempted to sue the complainant, but Mr. Brocker said this won’t stop the complaint and could strengthen it. “Most statements to the medical board are protected from defamation lawsuits, and any lawsuit could appear to be intimidation.”
7. Simply signing a consent agreement
A small minority of complaints may result in the board taking action against the doctor. Typically, this involves getting the doctor to sign a consent agreement stating that he or she agrees with the board’s decision and its remedy, such as continuing education, a fine, or being placed under another doctor’s supervision.
“When the board sends you a consent agreement, it’s usually about something fairly minor,” Ms. Collis said. “You can make a counteroffer and see if they accept that. But once you enter into the agreement, you waive any right to appeal the board’s decision.”
8. Not hiring an attorney
Although some doctors manage to deal with a board complaint on their own, many will need to get an attorney, Mr. Brocker said. “An experienced attorney can help you navigate the board’s process.”
Clients often look for attorneys at the end of the process, when formal charges have already been filed, Mr. Brocker said. At that point, “it’s harder to get things moving in the right direction. You can’t unring the bell.”
Even if you don’t think you need an attorney throughout the case, “it helps to get advice from an attorney at the beginning,” Dr. Segal said. Doctors may think they can’t afford an attorney, but many malpractice carriers pay attorneys’ fees in medical board investigations.
Mr. Brocker advised finding an attorney who is familiar with licensing boards. “Malpractice attorneys may think they can deal with medical boards, but boards are quite different.” For example, “malpractice cases involve an adversarial approach, but licensing boards normally require working collaboratively.”
9. Not requesting a hearing
When the board takes action against you, it can be tempting to just accept the allegations and move on with your life, but it may be possible to undo the action, Dr. Sullivan said. “The board still has to prove its allegations, and it may not have a strong case against you.”
In some states, the medical board has to meet a very high standard of proof, Dr. Sullivan said. In Illinois, for example, the board must show “clear and convincing evidence,” while a malpractice plaintiff must only prove that it’s “more likely than not” that a physician violated the standard of care.
A hearing can especially help doctors facing harsh sanctions for minor offenses. For example, in a case handled by the law firm of Ray & Bishop in Newport Beach, Calif., a doctor who was stopped by police while driving home after having wine at a family gathering was found to have a blood alcohol level of 0.11%. Noting that the physician was on call at the time, the Medical Board of California decided to give him 5 years of probation.
Ray & Bishop asked for a judicial hearing to contest the decision. At the hearing, the physician noted that other physicians were also available to take call that night, and an expert stated that the doctor was not an alcohol abuser. The judge ruled that the board’s action was unduly harsh, and the physician received a public reprimand with no further penalties.
10. Getting upset with board officials
A board investigator may show up at your office uninvited and ask you to answer some questions, but you aren’t required to answer then and there, said Ms. Collis.
In fact, she noted, it’s never a good idea to let investigators into your office. “They can walk around, look through your records, and find more things to investigate.” For this reason, Ms. Collis makes it a point to schedule meetings with investigators at her office.
When you have to interact with board officials, such as during hearings, expressing anger is a mistake. “Some board members may raise their voices and make untrue assertions about your medical care,” Dr. Sullivan said. “You may wish you could respond in kind, but that will not help you.” Instead, calmly provide studies or guidelines supporting the care you provided.
Taking board investigators to task is also a mistake, Mr. Brocker pointed out. In his words, “investigators have to follow the rules. Getting mad at them will only make your case more difficult. Even if you believe the complaint against you is totally without merit, the process needs to run its course.”
A version of this article first appeared on Medscape.com.
Omalizumab curbs airway inflammation in severe asthma
Patients with severe asthma who were new to omalizumab showed significant clinical improvement after 2 weeks of treatment, according to data from a pilot study of 26 adults.
Although omalizumab is approved for severe allergic asthma, not all patients respond well, and are considered nonresponders in the absence of clinical benefits within 16 weeks of starting treatment, wrote Todor A. Popov, MD, of the University Hospital St. Ivan Rilski, Sofia, Bulgaria, and colleagues.
“Since airway inflammation is a cardinal feature of asthma, we reasoned that early changes in its level may determine the subsequent course of the disease,” they said.
In a study published in Annals of Allergy, Asthma & Immunology, the researchers recruited 26 adults with severe asthma who were new to biologic therapy and eligible for omalizumab. The patients ranged in age from 22 to 70 years, and 13 were men. Patients received omalizumab doses between 150 mg and 375 mg every 2-4 weeks based on body weight and pretreatment serum IgE levels, and they were assessed at baseline and followed for a total of 18 weeks (2-week run-in and 16 weeks of treatment).
Patients rated their overall discomfort from asthma on a 100-mm visual analogue scale (VAS). Asthma control was assessed via the asthma control questionnaire (ACQ), and disease-related quality of life was assessed via the Asthma Quality of Life Questionnaires (AQLQ). All patients reported significant improvement across all three measures after 2 weeks and through the study period after the first administration of omalizumab at week 0 (P < .001).
Clinical response was based on quantitative indicators of airway and systemic eosinophilic inflammation: fractional exhaled nitric oxide (FeNO), eosinophil cationic peptide (ECP), and the temperature of the exhaled air (EBT, exhaled breath temperature). The researchers also measured fractional EBT (FrEBT) by measuring the EBT of central and peripheral airways at the beginning and end of the expiration.
Overall, EBT decreased significantly after 2 weeks, and the decrease lasted until week 16. FrEBT decreased significantly after 4 weeks. ECP reached statistical significance at week 16 (P = .029). FeNO showed a downward trend, but the decrease did not reach statistical significance, the researchers wrote.
These results might suggest that “after blocking IgE, the eosinophilic inflammation is not suppressed well and fast enough,” the researchers noted. “Consequently, indicators of eosinophilic inflammation may not be suited for early predictors of success of omalizumab treatment,” they added. The drop in EBT after the first dose of omalizumab may predict effectiveness for a particular patient, while the FrEBT results “may mean that it takes longer to suppress the inflammatory process in the vast basin of the small airways,” they noted.
A key limitation of the findings was the small sample size, although the study was designed as a proof-of-concept on which to base sample size calculation for larger trials with EBT as a predictive marker, the researchers said.
However, the EBT and FrEBT signals reached statistical significance, and the results warrant confirmation in larger trials; such confirmation may spare patients from expensive and ineffective treatments, they concluded.
The study was funded by Novartis. The researchers had no financial conflicts to disclose.
Patients with severe asthma who were new to omalizumab showed significant clinical improvement after 2 weeks of treatment, according to data from a pilot study of 26 adults.
Although omalizumab is approved for severe allergic asthma, not all patients respond well, and are considered nonresponders in the absence of clinical benefits within 16 weeks of starting treatment, wrote Todor A. Popov, MD, of the University Hospital St. Ivan Rilski, Sofia, Bulgaria, and colleagues.
“Since airway inflammation is a cardinal feature of asthma, we reasoned that early changes in its level may determine the subsequent course of the disease,” they said.
In a study published in Annals of Allergy, Asthma & Immunology, the researchers recruited 26 adults with severe asthma who were new to biologic therapy and eligible for omalizumab. The patients ranged in age from 22 to 70 years, and 13 were men. Patients received omalizumab doses between 150 mg and 375 mg every 2-4 weeks based on body weight and pretreatment serum IgE levels, and they were assessed at baseline and followed for a total of 18 weeks (2-week run-in and 16 weeks of treatment).
Patients rated their overall discomfort from asthma on a 100-mm visual analogue scale (VAS). Asthma control was assessed via the asthma control questionnaire (ACQ), and disease-related quality of life was assessed via the Asthma Quality of Life Questionnaires (AQLQ). All patients reported significant improvement across all three measures after 2 weeks and through the study period after the first administration of omalizumab at week 0 (P < .001).
Clinical response was based on quantitative indicators of airway and systemic eosinophilic inflammation: fractional exhaled nitric oxide (FeNO), eosinophil cationic peptide (ECP), and the temperature of the exhaled air (EBT, exhaled breath temperature). The researchers also measured fractional EBT (FrEBT) by measuring the EBT of central and peripheral airways at the beginning and end of the expiration.
Overall, EBT decreased significantly after 2 weeks, and the decrease lasted until week 16. FrEBT decreased significantly after 4 weeks. ECP reached statistical significance at week 16 (P = .029). FeNO showed a downward trend, but the decrease did not reach statistical significance, the researchers wrote.
These results might suggest that “after blocking IgE, the eosinophilic inflammation is not suppressed well and fast enough,” the researchers noted. “Consequently, indicators of eosinophilic inflammation may not be suited for early predictors of success of omalizumab treatment,” they added. The drop in EBT after the first dose of omalizumab may predict effectiveness for a particular patient, while the FrEBT results “may mean that it takes longer to suppress the inflammatory process in the vast basin of the small airways,” they noted.
A key limitation of the findings was the small sample size, although the study was designed as a proof-of-concept on which to base sample size calculation for larger trials with EBT as a predictive marker, the researchers said.
However, the EBT and FrEBT signals reached statistical significance, and the results warrant confirmation in larger trials; such confirmation may spare patients from expensive and ineffective treatments, they concluded.
The study was funded by Novartis. The researchers had no financial conflicts to disclose.
Patients with severe asthma who were new to omalizumab showed significant clinical improvement after 2 weeks of treatment, according to data from a pilot study of 26 adults.
Although omalizumab is approved for severe allergic asthma, not all patients respond well, and are considered nonresponders in the absence of clinical benefits within 16 weeks of starting treatment, wrote Todor A. Popov, MD, of the University Hospital St. Ivan Rilski, Sofia, Bulgaria, and colleagues.
“Since airway inflammation is a cardinal feature of asthma, we reasoned that early changes in its level may determine the subsequent course of the disease,” they said.
In a study published in Annals of Allergy, Asthma & Immunology, the researchers recruited 26 adults with severe asthma who were new to biologic therapy and eligible for omalizumab. The patients ranged in age from 22 to 70 years, and 13 were men. Patients received omalizumab doses between 150 mg and 375 mg every 2-4 weeks based on body weight and pretreatment serum IgE levels, and they were assessed at baseline and followed for a total of 18 weeks (2-week run-in and 16 weeks of treatment).
Patients rated their overall discomfort from asthma on a 100-mm visual analogue scale (VAS). Asthma control was assessed via the asthma control questionnaire (ACQ), and disease-related quality of life was assessed via the Asthma Quality of Life Questionnaires (AQLQ). All patients reported significant improvement across all three measures after 2 weeks and through the study period after the first administration of omalizumab at week 0 (P < .001).
Clinical response was based on quantitative indicators of airway and systemic eosinophilic inflammation: fractional exhaled nitric oxide (FeNO), eosinophil cationic peptide (ECP), and the temperature of the exhaled air (EBT, exhaled breath temperature). The researchers also measured fractional EBT (FrEBT) by measuring the EBT of central and peripheral airways at the beginning and end of the expiration.
Overall, EBT decreased significantly after 2 weeks, and the decrease lasted until week 16. FrEBT decreased significantly after 4 weeks. ECP reached statistical significance at week 16 (P = .029). FeNO showed a downward trend, but the decrease did not reach statistical significance, the researchers wrote.
These results might suggest that “after blocking IgE, the eosinophilic inflammation is not suppressed well and fast enough,” the researchers noted. “Consequently, indicators of eosinophilic inflammation may not be suited for early predictors of success of omalizumab treatment,” they added. The drop in EBT after the first dose of omalizumab may predict effectiveness for a particular patient, while the FrEBT results “may mean that it takes longer to suppress the inflammatory process in the vast basin of the small airways,” they noted.
A key limitation of the findings was the small sample size, although the study was designed as a proof-of-concept on which to base sample size calculation for larger trials with EBT as a predictive marker, the researchers said.
However, the EBT and FrEBT signals reached statistical significance, and the results warrant confirmation in larger trials; such confirmation may spare patients from expensive and ineffective treatments, they concluded.
The study was funded by Novartis. The researchers had no financial conflicts to disclose.
FROM ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY