User login
What is the diagnosis?
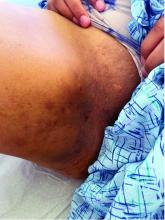
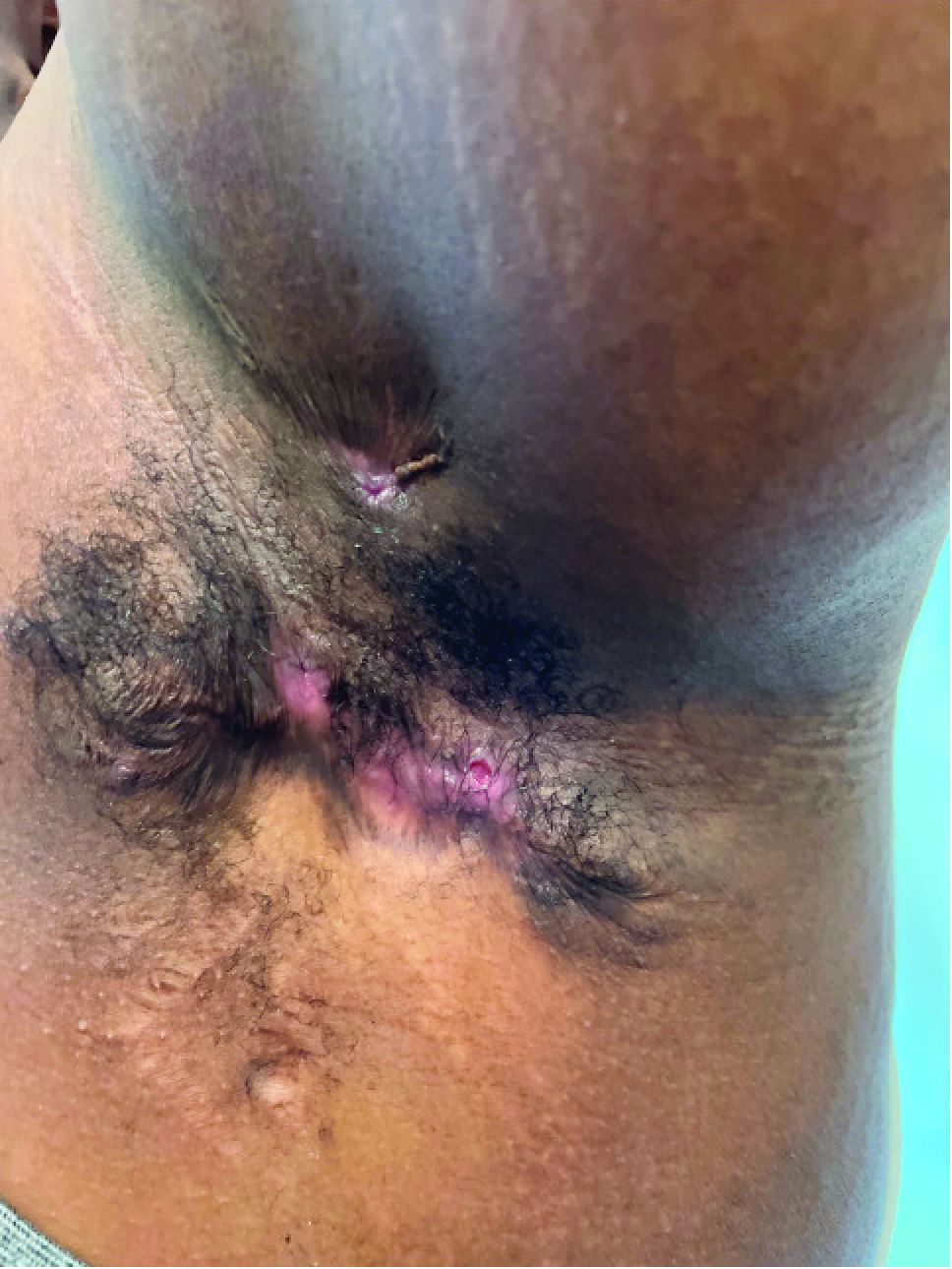
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that is becoming more recognized in children. It has a variable presentation, most commonly presenting as painful, recurrent cysts, abscesses, nodules, and/or pustules in classic locations with associated scarring and sinus tract formation.
The majority of patients present with bilateral lesions found most commonly in the axillae and inguinal folds.1 There are myriad other potential sites of involvement including the inframammary folds, inner thighs, buttocks, and groin.1 Diagnosis is made based on history and physical exam. There is a standard severity classification scheme called the Hurley score, which stratifies disease severity based on the presence of sinus tracts and extent of disease.1 HS is associated with comorbid conditions such as obesity, overweight, acne, and inflammatory bowel and joint disease.2 This painful, persistent condition is well documented to have a negative impact on quality of life in adult patients, and similar impairment has been found in pediatric patients.3,4
HS may be increasing in pediatric and adolescent patients, with recent studies showing onset coinciding most commonly with the onset of puberty.1,2 There is often a period of several years between symptom onset and diagnosis.1 A recent editorial highlighted the disparities that exist in HS, with disease more common in Black children and limited information about disease prevalence in Hispanic children.5
What’s the treatment plan?
HS is a difficult disease to treat, with few patients achieving remission and a significant proportion of patients with treatment-refractory disease.1 There are limited studies of HS treatment in pediatric patients. Topical and systemic antibiotic therapy are mainstays of HS treatment, with tetracyclines and a combination of clindamycin plus rifampin commonly used in adults and children alike. Topical therapies including topical antibiotics and antibacterial solutions are frequently used as adjunctive therapy.6 Adalimumab, a tumor necrosis factor receptor blocker, has been Food and Drug Administration approved for HS for ages 12 and up and is currently the only FDA-approved medication for HS in pediatric patients. Our patient was started on 100 mg doxycycline twice daily, with short-dose topical corticosteroids for symptom management of the most inflamed lesions.
What’s on the differential?
Acne conglobata
Acne conglobata is an uncommon, severe variant of acne vulgaris which arise in patients with a history of acne vulgaris and presents with comedones, cysts, abscesses, and scarring with possible drainage of pus. Lesions can present diffusely on the face, back, and body, including in the axillae, groin, and buttocks, and as such can be confused with HS.7
However, in contrast with HS, patients with acne conglobata will also develop disease in non–apocrine gland–bearing skin. This patient’s lack of preceding acne and restriction of lesions to the axillae, inguinal folds, and buttocks makes acne conglobata less likely.
Epidermal inclusion cyst
Epidermal inclusion cyst (EIC) is a common cutaneous cyst, presenting as a well-circumscribed nodule(s) with a central punctum. If not excised, lesions can sometimes become infected and painful.8 In contrast with HS, EIC presents only uncommonly as multiple lesions arising in different areas, and spontaneous drainage is uncommon. Our patient’s development of multiple draining lesions makes this diagnosis unlikely.
Furunculosis
Furunculosis is a common bacterial infection of the skin, presenting with inflammatory nodules or pustules centered around the hair follicle. Lesions may commonly present at sites of skin trauma and are found most frequently on the extremities.9 Though furunculosis lesions may drain pus and can coalesce to form larger “carbuncles,” our patient’s presence of significant scarring and lack of extremity involvement makes HS more likely.
Recurrent MRSA abscesses
Methicillin-resistant Staphylococcus aureus skin and soft-tissue infections are not uncommon in the pediatric population, with presentation of infection ranging from cellulitis to fluid-containing abscesses.10 Recurrent abscesses may be seen in MRSA infection, however in this patient the presence of draining, scarring lesions in multiple locations typical for HS over time is more consistent with a diagnosis of HS.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Appiah is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Appiah have no relevant financial disclosures.
References
1. Liy-Wong C et al. JAMA Dermatol. 2021;157(4):385-91.
2. Choi E et al. J Am Acad Dermatol. 2022;86(1):140-7.
3. Machado MO et al. JAMA Dermatol. 2019;155(8):939-45.
4. McAndrew R et al. J Am Acad Dermatol. 2021;84(3):829-30.
5. Kirby JS and Zaenglein AL. JAMA Dermatol. 2021;157(4):379-80.
6. Alikhan A et al. J Am Acad Dermatol. 2019;81(1):91-101.
7. Greydanus DE et al. Dis Mon. 2021;67(4):101103.
8. Weir CB, St. Hilaire NJ. Epidermal Inclusion Cyst, in “StatPearls.” Treasure Island, Fla: StatPearls Publishing, 2021.
9. Atanaskova N and Tomecki KJ. Dermatol Clin. 2010;28(3):479-87.
10. Papastefan ST et al. J Surg Res. 2019;242:70-7.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that is becoming more recognized in children. It has a variable presentation, most commonly presenting as painful, recurrent cysts, abscesses, nodules, and/or pustules in classic locations with associated scarring and sinus tract formation.
The majority of patients present with bilateral lesions found most commonly in the axillae and inguinal folds.1 There are myriad other potential sites of involvement including the inframammary folds, inner thighs, buttocks, and groin.1 Diagnosis is made based on history and physical exam. There is a standard severity classification scheme called the Hurley score, which stratifies disease severity based on the presence of sinus tracts and extent of disease.1 HS is associated with comorbid conditions such as obesity, overweight, acne, and inflammatory bowel and joint disease.2 This painful, persistent condition is well documented to have a negative impact on quality of life in adult patients, and similar impairment has been found in pediatric patients.3,4
HS may be increasing in pediatric and adolescent patients, with recent studies showing onset coinciding most commonly with the onset of puberty.1,2 There is often a period of several years between symptom onset and diagnosis.1 A recent editorial highlighted the disparities that exist in HS, with disease more common in Black children and limited information about disease prevalence in Hispanic children.5
What’s the treatment plan?
HS is a difficult disease to treat, with few patients achieving remission and a significant proportion of patients with treatment-refractory disease.1 There are limited studies of HS treatment in pediatric patients. Topical and systemic antibiotic therapy are mainstays of HS treatment, with tetracyclines and a combination of clindamycin plus rifampin commonly used in adults and children alike. Topical therapies including topical antibiotics and antibacterial solutions are frequently used as adjunctive therapy.6 Adalimumab, a tumor necrosis factor receptor blocker, has been Food and Drug Administration approved for HS for ages 12 and up and is currently the only FDA-approved medication for HS in pediatric patients. Our patient was started on 100 mg doxycycline twice daily, with short-dose topical corticosteroids for symptom management of the most inflamed lesions.
What’s on the differential?
Acne conglobata
Acne conglobata is an uncommon, severe variant of acne vulgaris which arise in patients with a history of acne vulgaris and presents with comedones, cysts, abscesses, and scarring with possible drainage of pus. Lesions can present diffusely on the face, back, and body, including in the axillae, groin, and buttocks, and as such can be confused with HS.7
However, in contrast with HS, patients with acne conglobata will also develop disease in non–apocrine gland–bearing skin. This patient’s lack of preceding acne and restriction of lesions to the axillae, inguinal folds, and buttocks makes acne conglobata less likely.
Epidermal inclusion cyst
Epidermal inclusion cyst (EIC) is a common cutaneous cyst, presenting as a well-circumscribed nodule(s) with a central punctum. If not excised, lesions can sometimes become infected and painful.8 In contrast with HS, EIC presents only uncommonly as multiple lesions arising in different areas, and spontaneous drainage is uncommon. Our patient’s development of multiple draining lesions makes this diagnosis unlikely.
Furunculosis
Furunculosis is a common bacterial infection of the skin, presenting with inflammatory nodules or pustules centered around the hair follicle. Lesions may commonly present at sites of skin trauma and are found most frequently on the extremities.9 Though furunculosis lesions may drain pus and can coalesce to form larger “carbuncles,” our patient’s presence of significant scarring and lack of extremity involvement makes HS more likely.
Recurrent MRSA abscesses
Methicillin-resistant Staphylococcus aureus skin and soft-tissue infections are not uncommon in the pediatric population, with presentation of infection ranging from cellulitis to fluid-containing abscesses.10 Recurrent abscesses may be seen in MRSA infection, however in this patient the presence of draining, scarring lesions in multiple locations typical for HS over time is more consistent with a diagnosis of HS.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Appiah is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Appiah have no relevant financial disclosures.
References
1. Liy-Wong C et al. JAMA Dermatol. 2021;157(4):385-91.
2. Choi E et al. J Am Acad Dermatol. 2022;86(1):140-7.
3. Machado MO et al. JAMA Dermatol. 2019;155(8):939-45.
4. McAndrew R et al. J Am Acad Dermatol. 2021;84(3):829-30.
5. Kirby JS and Zaenglein AL. JAMA Dermatol. 2021;157(4):379-80.
6. Alikhan A et al. J Am Acad Dermatol. 2019;81(1):91-101.
7. Greydanus DE et al. Dis Mon. 2021;67(4):101103.
8. Weir CB, St. Hilaire NJ. Epidermal Inclusion Cyst, in “StatPearls.” Treasure Island, Fla: StatPearls Publishing, 2021.
9. Atanaskova N and Tomecki KJ. Dermatol Clin. 2010;28(3):479-87.
10. Papastefan ST et al. J Surg Res. 2019;242:70-7.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that is becoming more recognized in children. It has a variable presentation, most commonly presenting as painful, recurrent cysts, abscesses, nodules, and/or pustules in classic locations with associated scarring and sinus tract formation.
The majority of patients present with bilateral lesions found most commonly in the axillae and inguinal folds.1 There are myriad other potential sites of involvement including the inframammary folds, inner thighs, buttocks, and groin.1 Diagnosis is made based on history and physical exam. There is a standard severity classification scheme called the Hurley score, which stratifies disease severity based on the presence of sinus tracts and extent of disease.1 HS is associated with comorbid conditions such as obesity, overweight, acne, and inflammatory bowel and joint disease.2 This painful, persistent condition is well documented to have a negative impact on quality of life in adult patients, and similar impairment has been found in pediatric patients.3,4
HS may be increasing in pediatric and adolescent patients, with recent studies showing onset coinciding most commonly with the onset of puberty.1,2 There is often a period of several years between symptom onset and diagnosis.1 A recent editorial highlighted the disparities that exist in HS, with disease more common in Black children and limited information about disease prevalence in Hispanic children.5
What’s the treatment plan?
HS is a difficult disease to treat, with few patients achieving remission and a significant proportion of patients with treatment-refractory disease.1 There are limited studies of HS treatment in pediatric patients. Topical and systemic antibiotic therapy are mainstays of HS treatment, with tetracyclines and a combination of clindamycin plus rifampin commonly used in adults and children alike. Topical therapies including topical antibiotics and antibacterial solutions are frequently used as adjunctive therapy.6 Adalimumab, a tumor necrosis factor receptor blocker, has been Food and Drug Administration approved for HS for ages 12 and up and is currently the only FDA-approved medication for HS in pediatric patients. Our patient was started on 100 mg doxycycline twice daily, with short-dose topical corticosteroids for symptom management of the most inflamed lesions.
What’s on the differential?
Acne conglobata
Acne conglobata is an uncommon, severe variant of acne vulgaris which arise in patients with a history of acne vulgaris and presents with comedones, cysts, abscesses, and scarring with possible drainage of pus. Lesions can present diffusely on the face, back, and body, including in the axillae, groin, and buttocks, and as such can be confused with HS.7
However, in contrast with HS, patients with acne conglobata will also develop disease in non–apocrine gland–bearing skin. This patient’s lack of preceding acne and restriction of lesions to the axillae, inguinal folds, and buttocks makes acne conglobata less likely.
Epidermal inclusion cyst
Epidermal inclusion cyst (EIC) is a common cutaneous cyst, presenting as a well-circumscribed nodule(s) with a central punctum. If not excised, lesions can sometimes become infected and painful.8 In contrast with HS, EIC presents only uncommonly as multiple lesions arising in different areas, and spontaneous drainage is uncommon. Our patient’s development of multiple draining lesions makes this diagnosis unlikely.
Furunculosis
Furunculosis is a common bacterial infection of the skin, presenting with inflammatory nodules or pustules centered around the hair follicle. Lesions may commonly present at sites of skin trauma and are found most frequently on the extremities.9 Though furunculosis lesions may drain pus and can coalesce to form larger “carbuncles,” our patient’s presence of significant scarring and lack of extremity involvement makes HS more likely.
Recurrent MRSA abscesses
Methicillin-resistant Staphylococcus aureus skin and soft-tissue infections are not uncommon in the pediatric population, with presentation of infection ranging from cellulitis to fluid-containing abscesses.10 Recurrent abscesses may be seen in MRSA infection, however in this patient the presence of draining, scarring lesions in multiple locations typical for HS over time is more consistent with a diagnosis of HS.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Appiah is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Appiah have no relevant financial disclosures.
References
1. Liy-Wong C et al. JAMA Dermatol. 2021;157(4):385-91.
2. Choi E et al. J Am Acad Dermatol. 2022;86(1):140-7.
3. Machado MO et al. JAMA Dermatol. 2019;155(8):939-45.
4. McAndrew R et al. J Am Acad Dermatol. 2021;84(3):829-30.
5. Kirby JS and Zaenglein AL. JAMA Dermatol. 2021;157(4):379-80.
6. Alikhan A et al. J Am Acad Dermatol. 2019;81(1):91-101.
7. Greydanus DE et al. Dis Mon. 2021;67(4):101103.
8. Weir CB, St. Hilaire NJ. Epidermal Inclusion Cyst, in “StatPearls.” Treasure Island, Fla: StatPearls Publishing, 2021.
9. Atanaskova N and Tomecki KJ. Dermatol Clin. 2010;28(3):479-87.
10. Papastefan ST et al. J Surg Res. 2019;242:70-7.
Despite the stigma, ECT remains a gold standard
For Clayton Lively, electroconvulsive therapy, or ECT, has been a lifesaver.
“ECT was like a last resort to treat mania and psychosis for bipolar disorder,” said the 31-year-old financial firm associate who lives in Silver Spring, Md. “I had tried lots of different medications.”
The first course of treatments – three times per week for several weeks – was in 2005. They helped tremendously. “I came down from my mania,” Mr. Lively said. “The hallucinations stopped. The psychosis disappeared.”
He reached a point where medications and psychotherapy worked again. And for a decade, his condition was under control.
But in 2017, another episode of hallucinations and mania jolted him off course. Intrusive thoughts returned. For instance, while driving, he would visualize veering off the road. The thoughts were jarring, and yet, he couldn’t stop them from recurring.
“I wasn’t sleeping, and it just kind of wreaked havoc on my life,” Mr. Lively recalled. “I ended up being hospitalized again.”
Once again, ECT came to the rescue – and yet again, in 2018. Now, he’s on an effective maintenance regimen, receiving ECT once every 4 weeks, after tapering down from more frequent sessions.
When a combination of antidepressants and psychotherapy fails to control severe mental illness, there’s hope on the horizon. ECT can be a reliably safe and effective option.
For some patients, using it as maintenance therapy makes sense, said Vaughn McCall, MD, editor-in-chief of The Journal of ECT and professor and chairman of psychiatry at the Medical College of Georgia in Augusta. “I would think of it the same way as you have to treat any chronic illness,” such as blood pressure medicine to keep hypertension in check and dialysis to prevent kidney failure.
Despite a cacophony of contrarian voices – mainly from the Church of Scientology – “the number of psychiatrists who see controversy in ECT is vanishingly small,” Dr. McCall said.
In weighing the pros and cons of ECT, he noted that “when you’re trying to decide if it’s worth doing a treatment, you’re looking at the effectiveness on one hand and the side effects on the other hand.”
The answer to that emerges from several scales measuring patients’ quality of life by posing questions such as: “After receiving ECT, are you more able or less able to take care of yourself, to work, and enjoy the company of other people?”
In the end, Dr. McCall said, “we’ve applied these scales in probably half a dozen studies or more, and they always show that the patients’ qualify of life as a group is improved.”
A recent study published in The Lancet Psychiatry provides a significant degree of reassurance that ECT – also called “electroshock” or colloquially just “shock” therapy – does not increase the risk of serious medical side effects. In fact, the study suggests a potential benefit in reducing suicide risk.
First performed in 1938, the treatment has been well documented in the medical literature. But negative portrayals in books and movies, such as the 1975 film “One Flew Over the Cuckoo’s Nest,” have contributed to casting it in an unfavorable light.
“Unfortunately, over the past decades and years, there’s a lot of stigma and fear around the treatment,” said the study’s lead author, Tyler Kaster, MD, a psychiatrist and clinical fellow in brain stimulation at the University of Toronto.
For the study, Canadian investigators reviewed the admission records of 10,000 patients hospitalized for at least 3 days because of a severe depressive episode. Nearly two-thirds of the patients were women, and the average age for the entire group approached 57 years.
While half of the patients underwent ECT, the others received medication and psychotherapy. Researchers found that the group undergoing ECT did not have a heightened risk of death over the next 30 days and were not any more likely to be hospitalized for a medical problem.
Previous ECT comparative studies were at high risk of bias because of their inability to sufficiently account for confounding variables and differences between those who received the treatment and those who did not. The current study employed “rigorous methods with careful attention to bias and confounding to overcome limitations of previous work,” the authors wrote.
They used propensity score matching, which included more than 75 variables, such as measures of cognitive impairment, depression severity, medication use, other illnesses, and use of psychiatric and various medical services, capacity to consent to treatment, and sociodemographic factors.
“This is really a landmark study in terms of showing the medical safety of ECT,” said Mark S. George, MD, professor of psychiatry and neurology at the Medical University of South Carolina, Charleston, who was not involved in the study.
He added that “ECT is a life-saving treatment” for individuals with severe depression. “It’s good that we have this option for our patients.”
The authors highlight that depression is a major cause of illness and disability worldwide, with many individuals failing to achieve remission from initial therapies. Treatment-resistant depression is often described as being nonresponsive “to two or more medication trials of adequate dose and duration from different classes,” they wrote. In these instances, the authors point out, there is little evidence that psychotherapy would be helpful.
“The reason we consider ECT is someone has very severe depression that hasn’t responded to medications and talk therapy,” Dr. Kaster said. “The advantage of ECT is that it’s very effective in those circumstances.”
Of all therapies for treatment-resistant depression, ECT has the highest success rate, with 60% of patients attaining remission, according to the study, which cites prior research.
Compared with neurosurgery, the procedure is not invasive but requires general anesthesia. While the patient is asleep, Dr. Kaster said, the treating clinician places an electrical stimulus on the patient’s scalp, causing a generalized seizure inside the brain that lasts from 15 seconds to 2 minutes.
A course of ECT usually takes a total of 8-12 treatments, delivered two to three times per week over a month to a month and a half, Dr. George said.
Some patients need a new course of ECT if they relapse after several months. Others are unable to control their depression between courses and require repeated doses for maintenance. The time between these ECT sessions varies for each individual, Dr. George said, but is typically one session every 3-4 weeks.
To improve the odds of staying well, patients typically need to continue taking antidepressants and engaging in psychotherapy.
“It helps improve the efficacy of ECT and also down the road helps prevent relapse,” Dr. Kaster said, noting that “depression is, unfortunately, a chronic illness. We don’t have a cure.”
Murat Altinay, MD, associate professor of psychiatry at the Cleveland Clinic and a mood disorders specialist, said his patients generally need to demonstrate a lack of response to at least three or four antidepressants before he considers recommending ECT.
Confusion, short-term memory impairment, and muscle aches and pains may occur after the procedure, but they are relatively mild. Patients are monitored in a recovery room before discharge from the hospital, Dr. Altinay said.
The first few treatments will affect everyday function. After that initial period, however, people can resume most of their daily activities, he said.
“Maybe they won’t be able to work full-time right away, but anecdotally, we have had patients who were able to go back to the workforce relatively quickly or while they’re getting ECT,” Dr. Altinay said.
More significant adverse events are very rare, he noted, although heart rate and blood pressure can become elevated because of the electrical stimulus.
Dr. Altinay said he is pleased that the large-scale journal article has been published to help dispel myths surrounding ECT. While psychiatrists feel that ECT is generally safe and effective, the public maintains a negative view.
“It is an underutilized treatment,” he said. “In the media, it is almost depicted as a barbaric and archaic treatment in psychiatry.”
Patients are afraid of major side effects such as personality changes. Some fear they will forget someone’s birthday or other important factual information, “but that kind of stuff obviously does not happen,” Dr. Altinay said.
Sometimes it’s not only the patients who are hesitant to try ECT; it’s the family members who express concerns, said Irving Reti, MBBS, professor of psychiatry and neuroscience and director of the brain stimulation program at the Johns Hopkins University, Baltimore.
“It varies from one patient to another how agreeable or reluctant or cautious they are about their treatment if the doctor thinks it’s indicated for them,” Dr. Reti said. “Family members’ concerns may be very legitimate but may also be influenced by stigma and misunderstanding about the treatment. They may also not fully appreciate the severity of their loved one’s depression that warrants the administration of ECT.”
Hospitalized patients who are at risk of suicide have benefited from ECT. “It’s very effective,” he said. “I think it’s still the gold standard for severe treatment-resistant depression and also particularly helpful in people who are acutely suicidal.”
Dr. George cautioned that psychiatrists and the public should beware of questionable online sources that attempt to discredit ECT. “A quick Google search will find plenty of nonmedical doctors, many funded through Scientology, who will speak poorly of ECT. But they do not use evidence-based arguments and commonly do not treat patients,” he said.
“All good practicing psychiatrists that I know are in favor of ECT, as it clearly saves lives,” Dr. George added. “We all hope that the future will provide refinements of ECT, or even disruptive technologies that are more effective and with less hassle and will make ECT as we do it now obsolete. But we are not there yet.”
A version of this article first appeared on Medscape.com.
For Clayton Lively, electroconvulsive therapy, or ECT, has been a lifesaver.
“ECT was like a last resort to treat mania and psychosis for bipolar disorder,” said the 31-year-old financial firm associate who lives in Silver Spring, Md. “I had tried lots of different medications.”
The first course of treatments – three times per week for several weeks – was in 2005. They helped tremendously. “I came down from my mania,” Mr. Lively said. “The hallucinations stopped. The psychosis disappeared.”
He reached a point where medications and psychotherapy worked again. And for a decade, his condition was under control.
But in 2017, another episode of hallucinations and mania jolted him off course. Intrusive thoughts returned. For instance, while driving, he would visualize veering off the road. The thoughts were jarring, and yet, he couldn’t stop them from recurring.
“I wasn’t sleeping, and it just kind of wreaked havoc on my life,” Mr. Lively recalled. “I ended up being hospitalized again.”
Once again, ECT came to the rescue – and yet again, in 2018. Now, he’s on an effective maintenance regimen, receiving ECT once every 4 weeks, after tapering down from more frequent sessions.
When a combination of antidepressants and psychotherapy fails to control severe mental illness, there’s hope on the horizon. ECT can be a reliably safe and effective option.
For some patients, using it as maintenance therapy makes sense, said Vaughn McCall, MD, editor-in-chief of The Journal of ECT and professor and chairman of psychiatry at the Medical College of Georgia in Augusta. “I would think of it the same way as you have to treat any chronic illness,” such as blood pressure medicine to keep hypertension in check and dialysis to prevent kidney failure.
Despite a cacophony of contrarian voices – mainly from the Church of Scientology – “the number of psychiatrists who see controversy in ECT is vanishingly small,” Dr. McCall said.
In weighing the pros and cons of ECT, he noted that “when you’re trying to decide if it’s worth doing a treatment, you’re looking at the effectiveness on one hand and the side effects on the other hand.”
The answer to that emerges from several scales measuring patients’ quality of life by posing questions such as: “After receiving ECT, are you more able or less able to take care of yourself, to work, and enjoy the company of other people?”
In the end, Dr. McCall said, “we’ve applied these scales in probably half a dozen studies or more, and they always show that the patients’ qualify of life as a group is improved.”
A recent study published in The Lancet Psychiatry provides a significant degree of reassurance that ECT – also called “electroshock” or colloquially just “shock” therapy – does not increase the risk of serious medical side effects. In fact, the study suggests a potential benefit in reducing suicide risk.
First performed in 1938, the treatment has been well documented in the medical literature. But negative portrayals in books and movies, such as the 1975 film “One Flew Over the Cuckoo’s Nest,” have contributed to casting it in an unfavorable light.
“Unfortunately, over the past decades and years, there’s a lot of stigma and fear around the treatment,” said the study’s lead author, Tyler Kaster, MD, a psychiatrist and clinical fellow in brain stimulation at the University of Toronto.
For the study, Canadian investigators reviewed the admission records of 10,000 patients hospitalized for at least 3 days because of a severe depressive episode. Nearly two-thirds of the patients were women, and the average age for the entire group approached 57 years.
While half of the patients underwent ECT, the others received medication and psychotherapy. Researchers found that the group undergoing ECT did not have a heightened risk of death over the next 30 days and were not any more likely to be hospitalized for a medical problem.
Previous ECT comparative studies were at high risk of bias because of their inability to sufficiently account for confounding variables and differences between those who received the treatment and those who did not. The current study employed “rigorous methods with careful attention to bias and confounding to overcome limitations of previous work,” the authors wrote.
They used propensity score matching, which included more than 75 variables, such as measures of cognitive impairment, depression severity, medication use, other illnesses, and use of psychiatric and various medical services, capacity to consent to treatment, and sociodemographic factors.
“This is really a landmark study in terms of showing the medical safety of ECT,” said Mark S. George, MD, professor of psychiatry and neurology at the Medical University of South Carolina, Charleston, who was not involved in the study.
He added that “ECT is a life-saving treatment” for individuals with severe depression. “It’s good that we have this option for our patients.”
The authors highlight that depression is a major cause of illness and disability worldwide, with many individuals failing to achieve remission from initial therapies. Treatment-resistant depression is often described as being nonresponsive “to two or more medication trials of adequate dose and duration from different classes,” they wrote. In these instances, the authors point out, there is little evidence that psychotherapy would be helpful.
“The reason we consider ECT is someone has very severe depression that hasn’t responded to medications and talk therapy,” Dr. Kaster said. “The advantage of ECT is that it’s very effective in those circumstances.”
Of all therapies for treatment-resistant depression, ECT has the highest success rate, with 60% of patients attaining remission, according to the study, which cites prior research.
Compared with neurosurgery, the procedure is not invasive but requires general anesthesia. While the patient is asleep, Dr. Kaster said, the treating clinician places an electrical stimulus on the patient’s scalp, causing a generalized seizure inside the brain that lasts from 15 seconds to 2 minutes.
A course of ECT usually takes a total of 8-12 treatments, delivered two to three times per week over a month to a month and a half, Dr. George said.
Some patients need a new course of ECT if they relapse after several months. Others are unable to control their depression between courses and require repeated doses for maintenance. The time between these ECT sessions varies for each individual, Dr. George said, but is typically one session every 3-4 weeks.
To improve the odds of staying well, patients typically need to continue taking antidepressants and engaging in psychotherapy.
“It helps improve the efficacy of ECT and also down the road helps prevent relapse,” Dr. Kaster said, noting that “depression is, unfortunately, a chronic illness. We don’t have a cure.”
Murat Altinay, MD, associate professor of psychiatry at the Cleveland Clinic and a mood disorders specialist, said his patients generally need to demonstrate a lack of response to at least three or four antidepressants before he considers recommending ECT.
Confusion, short-term memory impairment, and muscle aches and pains may occur after the procedure, but they are relatively mild. Patients are monitored in a recovery room before discharge from the hospital, Dr. Altinay said.
The first few treatments will affect everyday function. After that initial period, however, people can resume most of their daily activities, he said.
“Maybe they won’t be able to work full-time right away, but anecdotally, we have had patients who were able to go back to the workforce relatively quickly or while they’re getting ECT,” Dr. Altinay said.
More significant adverse events are very rare, he noted, although heart rate and blood pressure can become elevated because of the electrical stimulus.
Dr. Altinay said he is pleased that the large-scale journal article has been published to help dispel myths surrounding ECT. While psychiatrists feel that ECT is generally safe and effective, the public maintains a negative view.
“It is an underutilized treatment,” he said. “In the media, it is almost depicted as a barbaric and archaic treatment in psychiatry.”
Patients are afraid of major side effects such as personality changes. Some fear they will forget someone’s birthday or other important factual information, “but that kind of stuff obviously does not happen,” Dr. Altinay said.
Sometimes it’s not only the patients who are hesitant to try ECT; it’s the family members who express concerns, said Irving Reti, MBBS, professor of psychiatry and neuroscience and director of the brain stimulation program at the Johns Hopkins University, Baltimore.
“It varies from one patient to another how agreeable or reluctant or cautious they are about their treatment if the doctor thinks it’s indicated for them,” Dr. Reti said. “Family members’ concerns may be very legitimate but may also be influenced by stigma and misunderstanding about the treatment. They may also not fully appreciate the severity of their loved one’s depression that warrants the administration of ECT.”
Hospitalized patients who are at risk of suicide have benefited from ECT. “It’s very effective,” he said. “I think it’s still the gold standard for severe treatment-resistant depression and also particularly helpful in people who are acutely suicidal.”
Dr. George cautioned that psychiatrists and the public should beware of questionable online sources that attempt to discredit ECT. “A quick Google search will find plenty of nonmedical doctors, many funded through Scientology, who will speak poorly of ECT. But they do not use evidence-based arguments and commonly do not treat patients,” he said.
“All good practicing psychiatrists that I know are in favor of ECT, as it clearly saves lives,” Dr. George added. “We all hope that the future will provide refinements of ECT, or even disruptive technologies that are more effective and with less hassle and will make ECT as we do it now obsolete. But we are not there yet.”
A version of this article first appeared on Medscape.com.
For Clayton Lively, electroconvulsive therapy, or ECT, has been a lifesaver.
“ECT was like a last resort to treat mania and psychosis for bipolar disorder,” said the 31-year-old financial firm associate who lives in Silver Spring, Md. “I had tried lots of different medications.”
The first course of treatments – three times per week for several weeks – was in 2005. They helped tremendously. “I came down from my mania,” Mr. Lively said. “The hallucinations stopped. The psychosis disappeared.”
He reached a point where medications and psychotherapy worked again. And for a decade, his condition was under control.
But in 2017, another episode of hallucinations and mania jolted him off course. Intrusive thoughts returned. For instance, while driving, he would visualize veering off the road. The thoughts were jarring, and yet, he couldn’t stop them from recurring.
“I wasn’t sleeping, and it just kind of wreaked havoc on my life,” Mr. Lively recalled. “I ended up being hospitalized again.”
Once again, ECT came to the rescue – and yet again, in 2018. Now, he’s on an effective maintenance regimen, receiving ECT once every 4 weeks, after tapering down from more frequent sessions.
When a combination of antidepressants and psychotherapy fails to control severe mental illness, there’s hope on the horizon. ECT can be a reliably safe and effective option.
For some patients, using it as maintenance therapy makes sense, said Vaughn McCall, MD, editor-in-chief of The Journal of ECT and professor and chairman of psychiatry at the Medical College of Georgia in Augusta. “I would think of it the same way as you have to treat any chronic illness,” such as blood pressure medicine to keep hypertension in check and dialysis to prevent kidney failure.
Despite a cacophony of contrarian voices – mainly from the Church of Scientology – “the number of psychiatrists who see controversy in ECT is vanishingly small,” Dr. McCall said.
In weighing the pros and cons of ECT, he noted that “when you’re trying to decide if it’s worth doing a treatment, you’re looking at the effectiveness on one hand and the side effects on the other hand.”
The answer to that emerges from several scales measuring patients’ quality of life by posing questions such as: “After receiving ECT, are you more able or less able to take care of yourself, to work, and enjoy the company of other people?”
In the end, Dr. McCall said, “we’ve applied these scales in probably half a dozen studies or more, and they always show that the patients’ qualify of life as a group is improved.”
A recent study published in The Lancet Psychiatry provides a significant degree of reassurance that ECT – also called “electroshock” or colloquially just “shock” therapy – does not increase the risk of serious medical side effects. In fact, the study suggests a potential benefit in reducing suicide risk.
First performed in 1938, the treatment has been well documented in the medical literature. But negative portrayals in books and movies, such as the 1975 film “One Flew Over the Cuckoo’s Nest,” have contributed to casting it in an unfavorable light.
“Unfortunately, over the past decades and years, there’s a lot of stigma and fear around the treatment,” said the study’s lead author, Tyler Kaster, MD, a psychiatrist and clinical fellow in brain stimulation at the University of Toronto.
For the study, Canadian investigators reviewed the admission records of 10,000 patients hospitalized for at least 3 days because of a severe depressive episode. Nearly two-thirds of the patients were women, and the average age for the entire group approached 57 years.
While half of the patients underwent ECT, the others received medication and psychotherapy. Researchers found that the group undergoing ECT did not have a heightened risk of death over the next 30 days and were not any more likely to be hospitalized for a medical problem.
Previous ECT comparative studies were at high risk of bias because of their inability to sufficiently account for confounding variables and differences between those who received the treatment and those who did not. The current study employed “rigorous methods with careful attention to bias and confounding to overcome limitations of previous work,” the authors wrote.
They used propensity score matching, which included more than 75 variables, such as measures of cognitive impairment, depression severity, medication use, other illnesses, and use of psychiatric and various medical services, capacity to consent to treatment, and sociodemographic factors.
“This is really a landmark study in terms of showing the medical safety of ECT,” said Mark S. George, MD, professor of psychiatry and neurology at the Medical University of South Carolina, Charleston, who was not involved in the study.
He added that “ECT is a life-saving treatment” for individuals with severe depression. “It’s good that we have this option for our patients.”
The authors highlight that depression is a major cause of illness and disability worldwide, with many individuals failing to achieve remission from initial therapies. Treatment-resistant depression is often described as being nonresponsive “to two or more medication trials of adequate dose and duration from different classes,” they wrote. In these instances, the authors point out, there is little evidence that psychotherapy would be helpful.
“The reason we consider ECT is someone has very severe depression that hasn’t responded to medications and talk therapy,” Dr. Kaster said. “The advantage of ECT is that it’s very effective in those circumstances.”
Of all therapies for treatment-resistant depression, ECT has the highest success rate, with 60% of patients attaining remission, according to the study, which cites prior research.
Compared with neurosurgery, the procedure is not invasive but requires general anesthesia. While the patient is asleep, Dr. Kaster said, the treating clinician places an electrical stimulus on the patient’s scalp, causing a generalized seizure inside the brain that lasts from 15 seconds to 2 minutes.
A course of ECT usually takes a total of 8-12 treatments, delivered two to three times per week over a month to a month and a half, Dr. George said.
Some patients need a new course of ECT if they relapse after several months. Others are unable to control their depression between courses and require repeated doses for maintenance. The time between these ECT sessions varies for each individual, Dr. George said, but is typically one session every 3-4 weeks.
To improve the odds of staying well, patients typically need to continue taking antidepressants and engaging in psychotherapy.
“It helps improve the efficacy of ECT and also down the road helps prevent relapse,” Dr. Kaster said, noting that “depression is, unfortunately, a chronic illness. We don’t have a cure.”
Murat Altinay, MD, associate professor of psychiatry at the Cleveland Clinic and a mood disorders specialist, said his patients generally need to demonstrate a lack of response to at least three or four antidepressants before he considers recommending ECT.
Confusion, short-term memory impairment, and muscle aches and pains may occur after the procedure, but they are relatively mild. Patients are monitored in a recovery room before discharge from the hospital, Dr. Altinay said.
The first few treatments will affect everyday function. After that initial period, however, people can resume most of their daily activities, he said.
“Maybe they won’t be able to work full-time right away, but anecdotally, we have had patients who were able to go back to the workforce relatively quickly or while they’re getting ECT,” Dr. Altinay said.
More significant adverse events are very rare, he noted, although heart rate and blood pressure can become elevated because of the electrical stimulus.
Dr. Altinay said he is pleased that the large-scale journal article has been published to help dispel myths surrounding ECT. While psychiatrists feel that ECT is generally safe and effective, the public maintains a negative view.
“It is an underutilized treatment,” he said. “In the media, it is almost depicted as a barbaric and archaic treatment in psychiatry.”
Patients are afraid of major side effects such as personality changes. Some fear they will forget someone’s birthday or other important factual information, “but that kind of stuff obviously does not happen,” Dr. Altinay said.
Sometimes it’s not only the patients who are hesitant to try ECT; it’s the family members who express concerns, said Irving Reti, MBBS, professor of psychiatry and neuroscience and director of the brain stimulation program at the Johns Hopkins University, Baltimore.
“It varies from one patient to another how agreeable or reluctant or cautious they are about their treatment if the doctor thinks it’s indicated for them,” Dr. Reti said. “Family members’ concerns may be very legitimate but may also be influenced by stigma and misunderstanding about the treatment. They may also not fully appreciate the severity of their loved one’s depression that warrants the administration of ECT.”
Hospitalized patients who are at risk of suicide have benefited from ECT. “It’s very effective,” he said. “I think it’s still the gold standard for severe treatment-resistant depression and also particularly helpful in people who are acutely suicidal.”
Dr. George cautioned that psychiatrists and the public should beware of questionable online sources that attempt to discredit ECT. “A quick Google search will find plenty of nonmedical doctors, many funded through Scientology, who will speak poorly of ECT. But they do not use evidence-based arguments and commonly do not treat patients,” he said.
“All good practicing psychiatrists that I know are in favor of ECT, as it clearly saves lives,” Dr. George added. “We all hope that the future will provide refinements of ECT, or even disruptive technologies that are more effective and with less hassle and will make ECT as we do it now obsolete. But we are not there yet.”
A version of this article first appeared on Medscape.com.
Increased access to LARC may improve birth outcomes
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA PEDIATRICS
Symptoms common in high-risk, early-stage ovarian cancer
A new study offers fresh insight into early indications of high-risk, early-stage, epithelial ovarian cancer: More than 70% have at least one symptom such as abdominal/pelvic pain or increased girth/fullness, and women with larger tumors have more symptoms.
“Even in early-stage disease, ovarian cancer is not necessarily a silent disease,” said lead author and gynecologic oncologist/surgeon John K. Chan, MD, of Palo Alto Medical Foundation/California Pacific/Sutter Research Institute.
The study appeared online Jan. 6, in the journal Obstetrics & Gynecology.*
According to Dr. Chan, most previous studies of symptoms in ovarian cancer have focused on those with advanced disease since that’s when it’s typically diagnosed. “Given these gaps in knowledge from prior reports, we performed this analysis to evaluate the presentation and characteristic symptoms of early-stage ovarian cancer and to attempt to identify the relationship between these symptoms with respect to clinicopathologic characteristics and prognosis in early-stage disease.”
Dr. Chan and colleagues retrospectively tracked 419 patients who were subjects in a clinical trial of chemotherapy doses. The patients all had high-risk, early-stage epithelial ovarian cancer (stage IA-IB and grade 3, any clear cell, stage IC or II).
Of the patients, 40% presented with one symptom, while 32% had multiple symptoms. The other 28% had no symptoms, and their masses were diagnosed upon discovery during physical examination. “Other investigators have found that nearly 95% of patients with ovarian cancer were symptomatic,” Dr. Chan said. “The lower percentage of symptomatic patients in our study may be because all 419 patients had early-stage disease as opposed to advanced-stage disease.”
The most common symptoms were abdominal or pelvic pain (31%; 95% confidence interval, 27%-36%), fullness or increased abdominal girth (27%; 95% CI, 22%-31%), abnormal vaginal bleeding (13%; 95% CI, 10%-17%), urinary problems (10%; 95% CI, 8%-14%), and gastrointestinal problems (6%; 95% CI, 4%-8%).
There was no statistically significant link between number of symptoms and age (younger than 60 or 60 or older), cancer stage, or histologic subtype. However, patients with the largest tumors (>15 cm) were more likely to have multiple symptoms than those with the smallest tumors (10 cm or smaller): 46% vs. 21% (P < .001).
Also, 79% of those with the largest tumors (>15 cm) had at least one symptom, compared with 65% of those with the smallest tumors (10 cm or smaller, P < .001)
Unlike other studies, this report didn’t find a link between the number of symptoms and mortality. This finding surprised the researchers, Dr. Chan said, as did the lack of connections between symptoms and age, stage, or histologic subtype. “We were expecting that the younger patients may have more symptoms given the association with endometriosis and clear cell cancers,” he said. “We also thought that those who are less symptomatic may have more stage I and low-grade indolent tumors with better survival, but we did not find that.”
The researchers noted limitations such as the lack of standardization in the patient data.
In the big picture, Dr. Chan said, “patients and health care professionals need to have a higher index of suspicion in symptomatic ovarian cancer patients to increase early detection and potentially improve cures. Ovarian cancer does not always kill. In fact, up to 80% of our early-stage disease patients are cured.”
He called for “additional research to evaluate symptom awareness in early-stage cancers and possibly incorporating novel serum biomarkers and wearable monitoring devices. Wearables may be able to assess for frequency or duration of symptoms, which may be an important factor in distinguishing symptoms that are more concerning for ovarian cancer.”
In an adjoining commentary, Barbara A. Goff, MD, chair of obstetrics and gynecology at the University of Washington, Seattle, noted that, while ovarian cancers diagnosed early have a high survival rate, prospective randomized trials of transvaginal ultrasonography and tumor marker screening strategies have failed to reduce mortality. There’s currently no recommended screening test for women at average risk.
There are other challenges, she wrote. For one, “many health care professionals are seemingly unaware of the symptoms typically associated with ovarian cancer, so misdiagnosis remains common.” And “one of the concerns about the symptoms of ovarian cancer is that they can be vague and commonly present in the general population.”
Dr. Goff praised the study, called for more education about the symptoms of ovarian cancer, and wrote that “symptom recognition with appropriate diagnostic testing remains very important in our efforts to improve outcomes.”
The National Institutes of Health funded the study. Several study authors, including Dr. Chan, reported various disclosures.
Correction, 1/31/22: An earlier version of this article misstated the date of publication.
A new study offers fresh insight into early indications of high-risk, early-stage, epithelial ovarian cancer: More than 70% have at least one symptom such as abdominal/pelvic pain or increased girth/fullness, and women with larger tumors have more symptoms.
“Even in early-stage disease, ovarian cancer is not necessarily a silent disease,” said lead author and gynecologic oncologist/surgeon John K. Chan, MD, of Palo Alto Medical Foundation/California Pacific/Sutter Research Institute.
The study appeared online Jan. 6, in the journal Obstetrics & Gynecology.*
According to Dr. Chan, most previous studies of symptoms in ovarian cancer have focused on those with advanced disease since that’s when it’s typically diagnosed. “Given these gaps in knowledge from prior reports, we performed this analysis to evaluate the presentation and characteristic symptoms of early-stage ovarian cancer and to attempt to identify the relationship between these symptoms with respect to clinicopathologic characteristics and prognosis in early-stage disease.”
Dr. Chan and colleagues retrospectively tracked 419 patients who were subjects in a clinical trial of chemotherapy doses. The patients all had high-risk, early-stage epithelial ovarian cancer (stage IA-IB and grade 3, any clear cell, stage IC or II).
Of the patients, 40% presented with one symptom, while 32% had multiple symptoms. The other 28% had no symptoms, and their masses were diagnosed upon discovery during physical examination. “Other investigators have found that nearly 95% of patients with ovarian cancer were symptomatic,” Dr. Chan said. “The lower percentage of symptomatic patients in our study may be because all 419 patients had early-stage disease as opposed to advanced-stage disease.”
The most common symptoms were abdominal or pelvic pain (31%; 95% confidence interval, 27%-36%), fullness or increased abdominal girth (27%; 95% CI, 22%-31%), abnormal vaginal bleeding (13%; 95% CI, 10%-17%), urinary problems (10%; 95% CI, 8%-14%), and gastrointestinal problems (6%; 95% CI, 4%-8%).
There was no statistically significant link between number of symptoms and age (younger than 60 or 60 or older), cancer stage, or histologic subtype. However, patients with the largest tumors (>15 cm) were more likely to have multiple symptoms than those with the smallest tumors (10 cm or smaller): 46% vs. 21% (P < .001).
Also, 79% of those with the largest tumors (>15 cm) had at least one symptom, compared with 65% of those with the smallest tumors (10 cm or smaller, P < .001)
Unlike other studies, this report didn’t find a link between the number of symptoms and mortality. This finding surprised the researchers, Dr. Chan said, as did the lack of connections between symptoms and age, stage, or histologic subtype. “We were expecting that the younger patients may have more symptoms given the association with endometriosis and clear cell cancers,” he said. “We also thought that those who are less symptomatic may have more stage I and low-grade indolent tumors with better survival, but we did not find that.”
The researchers noted limitations such as the lack of standardization in the patient data.
In the big picture, Dr. Chan said, “patients and health care professionals need to have a higher index of suspicion in symptomatic ovarian cancer patients to increase early detection and potentially improve cures. Ovarian cancer does not always kill. In fact, up to 80% of our early-stage disease patients are cured.”
He called for “additional research to evaluate symptom awareness in early-stage cancers and possibly incorporating novel serum biomarkers and wearable monitoring devices. Wearables may be able to assess for frequency or duration of symptoms, which may be an important factor in distinguishing symptoms that are more concerning for ovarian cancer.”
In an adjoining commentary, Barbara A. Goff, MD, chair of obstetrics and gynecology at the University of Washington, Seattle, noted that, while ovarian cancers diagnosed early have a high survival rate, prospective randomized trials of transvaginal ultrasonography and tumor marker screening strategies have failed to reduce mortality. There’s currently no recommended screening test for women at average risk.
There are other challenges, she wrote. For one, “many health care professionals are seemingly unaware of the symptoms typically associated with ovarian cancer, so misdiagnosis remains common.” And “one of the concerns about the symptoms of ovarian cancer is that they can be vague and commonly present in the general population.”
Dr. Goff praised the study, called for more education about the symptoms of ovarian cancer, and wrote that “symptom recognition with appropriate diagnostic testing remains very important in our efforts to improve outcomes.”
The National Institutes of Health funded the study. Several study authors, including Dr. Chan, reported various disclosures.
Correction, 1/31/22: An earlier version of this article misstated the date of publication.
A new study offers fresh insight into early indications of high-risk, early-stage, epithelial ovarian cancer: More than 70% have at least one symptom such as abdominal/pelvic pain or increased girth/fullness, and women with larger tumors have more symptoms.
“Even in early-stage disease, ovarian cancer is not necessarily a silent disease,” said lead author and gynecologic oncologist/surgeon John K. Chan, MD, of Palo Alto Medical Foundation/California Pacific/Sutter Research Institute.
The study appeared online Jan. 6, in the journal Obstetrics & Gynecology.*
According to Dr. Chan, most previous studies of symptoms in ovarian cancer have focused on those with advanced disease since that’s when it’s typically diagnosed. “Given these gaps in knowledge from prior reports, we performed this analysis to evaluate the presentation and characteristic symptoms of early-stage ovarian cancer and to attempt to identify the relationship between these symptoms with respect to clinicopathologic characteristics and prognosis in early-stage disease.”
Dr. Chan and colleagues retrospectively tracked 419 patients who were subjects in a clinical trial of chemotherapy doses. The patients all had high-risk, early-stage epithelial ovarian cancer (stage IA-IB and grade 3, any clear cell, stage IC or II).
Of the patients, 40% presented with one symptom, while 32% had multiple symptoms. The other 28% had no symptoms, and their masses were diagnosed upon discovery during physical examination. “Other investigators have found that nearly 95% of patients with ovarian cancer were symptomatic,” Dr. Chan said. “The lower percentage of symptomatic patients in our study may be because all 419 patients had early-stage disease as opposed to advanced-stage disease.”
The most common symptoms were abdominal or pelvic pain (31%; 95% confidence interval, 27%-36%), fullness or increased abdominal girth (27%; 95% CI, 22%-31%), abnormal vaginal bleeding (13%; 95% CI, 10%-17%), urinary problems (10%; 95% CI, 8%-14%), and gastrointestinal problems (6%; 95% CI, 4%-8%).
There was no statistically significant link between number of symptoms and age (younger than 60 or 60 or older), cancer stage, or histologic subtype. However, patients with the largest tumors (>15 cm) were more likely to have multiple symptoms than those with the smallest tumors (10 cm or smaller): 46% vs. 21% (P < .001).
Also, 79% of those with the largest tumors (>15 cm) had at least one symptom, compared with 65% of those with the smallest tumors (10 cm or smaller, P < .001)
Unlike other studies, this report didn’t find a link between the number of symptoms and mortality. This finding surprised the researchers, Dr. Chan said, as did the lack of connections between symptoms and age, stage, or histologic subtype. “We were expecting that the younger patients may have more symptoms given the association with endometriosis and clear cell cancers,” he said. “We also thought that those who are less symptomatic may have more stage I and low-grade indolent tumors with better survival, but we did not find that.”
The researchers noted limitations such as the lack of standardization in the patient data.
In the big picture, Dr. Chan said, “patients and health care professionals need to have a higher index of suspicion in symptomatic ovarian cancer patients to increase early detection and potentially improve cures. Ovarian cancer does not always kill. In fact, up to 80% of our early-stage disease patients are cured.”
He called for “additional research to evaluate symptom awareness in early-stage cancers and possibly incorporating novel serum biomarkers and wearable monitoring devices. Wearables may be able to assess for frequency or duration of symptoms, which may be an important factor in distinguishing symptoms that are more concerning for ovarian cancer.”
In an adjoining commentary, Barbara A. Goff, MD, chair of obstetrics and gynecology at the University of Washington, Seattle, noted that, while ovarian cancers diagnosed early have a high survival rate, prospective randomized trials of transvaginal ultrasonography and tumor marker screening strategies have failed to reduce mortality. There’s currently no recommended screening test for women at average risk.
There are other challenges, she wrote. For one, “many health care professionals are seemingly unaware of the symptoms typically associated with ovarian cancer, so misdiagnosis remains common.” And “one of the concerns about the symptoms of ovarian cancer is that they can be vague and commonly present in the general population.”
Dr. Goff praised the study, called for more education about the symptoms of ovarian cancer, and wrote that “symptom recognition with appropriate diagnostic testing remains very important in our efforts to improve outcomes.”
The National Institutes of Health funded the study. Several study authors, including Dr. Chan, reported various disclosures.
Correction, 1/31/22: An earlier version of this article misstated the date of publication.
FROM OBSTETRICS & GYNECOLOGY
Prior authorization abuse: It’s time for health insurance CEOs and their proxies to cease and desist the practice once and for all!
Before reading this editorial and concluding that the author (me) has lost his grip on reality, I would ask that you consider the facts I provide below and the ramifications incurred by your patients and practices, due to the misbehaviors adopted by the health insurance industry.
- Two of the most common issues discussed in today’s health care environment are revenue generation and provider/staff burnout.
While these issues are impacted by several factors, one of the most common denominators is increasing administrative workloads driven by non–revenue-generating activities. Consider this:
- A recent American Medical Association survey pointed out that during the course of the average workweek, a physician completes an average of 37 prior authorization requests. Physicians and their staff spend an average of 16.4 hours per week completing prior authorization requirements for patient medicines, procedures, and medical services that they may need.1
- While physicians report that about 65% of prior authorizations take only 1 day, they report that 26% take 3 or more days.2
The potential significance of the generated delays
While this may not seem like a long time (other than the impact it has on staff workload), consider the impact this can have on the patient if the medication being requested is: PrEP, the morning after pill, or other contraceptives? The consequences of the delay or denial could be a lifetime living with HIV, or an unintended pregnancy. This is to say nothing on the larger impact to family, partners, and the potential social stigma faced by all.
Beyond the personal costs and costs within your practice associated with the additional workload, consider the financial costs. The average cost to complete a prior authorization remains the single highest cost for the health care industry at $13.40 per manual transaction, and $7.19 per partially electronic web portal transaction,3 meaning that if I did only one prescription per week, I probably would not mind, but at $13.40 per prior authorization, this burden amounts to millions, actually $767 million by recent estimates.3 Additionally, if you factor in the number of denials and potential follow-ups, this creates a significant amount of waste and spending.
Ultimately, in my experience, I have found that most prior authorizations are simply unnecessary. Here, I’ve picked key examples from just my own recent experiences:
- My patient was denied access to a particular birth control pill she had been on successfully before, and my office was told she needed to try and fail on 5 different generic pills before she could be approved. However, the Affordable Care Act’s (ACA; aka Obamacare) Contraceptive Mandate requires coverage of all contraceptives determined to be most appropriate between a patient and their provider (see below).
- A menopausal patient was denied coverage twice (electronically) for generic micronized progesterone, and I was asked to write a letter of appeal because the insurance company wanted me to use medroxyprogesterone acetate instead. Polling my nearby retail independent pharmacy, the total cost difference per year was $19.96 savings/year ($47.01 ‒ $27.05 = $19.96). My pharmacist did note it could have been a different amount at a large chain pharmacy. Really? I had to write a letter, following two denials, to save less than $20, for a full year!
- A 78-year-old patient using Prolia for severe osteoporosis and preexisting fractures was delayed in getting her next Prolia injection due to a prior authorization snafu. She ended up with multiple additional fractures, a well-described effect of the increase in bone turnover when stopping or delaying this medication. She is now disabled.
- A 94-year-old patient was sent an email reminder to get the medical practice to authorize a refill of ileostomy bags. The email went to spam, and the patient ran out of bags prior to a holiday weekend. I got them in 2 days on Amazon Prime. But who emails a 94-year-old? And ileostomy bags! When does anyone stop needing ileostomy bags?
- I requested a prior authorization for Orilissa (clearly off label) because a severely progestogen-sensitive patient (augmented depression) with severe premenstrual dysphoric disorder requiring hospitalization was thought by her psychiatrist to be better off without menstrual periods. I completed the proper paperwork, two electronic appeals, and a letter of explanation including available references on the use of gonadotropin-releasing hormone analogues for such patients. I was then told I would need to have a peer-to-peer discussion, so I filled out that paperwork, which clearly noted that I am a board-certified reproductive endocrinologist. I got a phone call a few days later by a pleasant, young-sounding pediatric rheumatologist. Our interaction did not go well for him. This was not peer-to-peer!
Let us be clear, prior authorizations have nothing to do with patient care. In fact, they are solely about the money. We in ObGyn have mostly inexpensive and generic products, but even that fact has not lowered the excessive burden of the prior authorization process. In the case of contraception, whether you like the ACA or not it is the law, and it contains specific provisions regarding contraception. With the goals of providing broad access to patients and incentives to developers for new and novel contraceptive methods, these provisions require insurers to cover, without cost-sharing, women’s preventive services including the full range of FDA-approved contraceptives (currently 18 different method categories), and additional methods identified by the FDA as they become available. Further, providers must have an easily accessible, transparent, and sufficiently expedient exceptions process that is not unduly burdensome on the individual or a provider (or other individuals acting as a patient’s authorized representative).
And while I can regale you with chapter and verse and citations of the legal precedent and language, it boils down to this:
- The AMA reported that medical practices spend an average of 2 business days a week per physician to comply with health plans’ inefficient and overused prior-authorization protocols.4 To keep up with the administrative burden, 2 out of 5 physicians (40%) employ staff members who work exclusively on tasks associated with prior authorization.4
- About 86% of practices reported an increased burden of prior authorizations in the last 5 years.5
Continue to: What is to be done?
What is to be done?
I do have suggested solutions. Given the insurance industry’s complete lack of progress in voluntarily reducing the burdens of prior authorizations agreed to in their consensus statement with the AMA, American Hospital Association, America’s Health Insurance Plans, American Pharmacists Association, Blue Cross Blue Shield Association, and the Medical Group Management Association, I say, why not fine them? The AMA is calling on Congress to pass legislation that would codify much of the agreement, in which the above parties had already agreed that reforms were needed to reduce prior authorization burdens and enhance patient-centered care.6
A good model for enforcement via fines could be based on the old “incident to” rules of Medicare. These state that a physician needs to be “in the space” when advanced practice nurses or physician assistants see Medicare recipients. If they are not actually “in the space” they are subject to a fine. As a completely theoretical example, let’s say the claim was for $100. The practitioner would have to pay it back plus triple that amount in damages, or $400. They can also be fined up to $11,000 per claim and kick you out of Medicare and Medicaid. Take my example of Prolia from above…a single shot of Prolia is about $1,000. The insurer would theoretically have to pay $14,000/claim (the claim + triple damages + $11,000) if it was determined that the prior authorization was unnecessary. Seems about right to me. Or we could just sit the health insurance CEOs and their proxies in the corner on 2-foot-tall plastic Little Tikes® chairs for a “timeout” (dunce cap optional), like the outset of the article says.
Until the detrimental prior authorization process is challenged at all levels, we will continue to see and feel the effects of the harm it causes. Being able to drive change through advocacy and education is the best way we as clinicians can impact not just the future of health care but provide for the daily care of our patients who depend on and trust us to provide for their medical needs. We must be the impactors of change for ourselves, colleagues, staff, and profession if we are to really make advancements into the future.
Oh…and health insurance CEOs and their proxies, to get out of their “time-out” would still be entitled to one phone call to beg forgiveness from their mommies/daddies, priest/ rabbi/pastor, psychologist/psychiatrist/mystic healer, etc., but alas, the average wait time is an hour, and if anyone answers the phone, they have a grade school education used in following an irrelevant algorithm. ●
- Corder JC. Streamlining the insurance prior authorization debacle. Mo Med. 2018;115:312-314.
- Prior authorization hurdles have led to serious adverse events. American Medical Association website. February 5, 2019. https://www.ama-assn .org/press-center/press-releases/prior-author ization-hurdles-have-led-serious-adverse -events. Accessed November 29, 2021.
- Council for Affordable Quality Healthcare. 2020 CAQH INDEX. https://www.caqh.org/sites /default/files/explorations/index/2020-caqh -index.pdf. Accessed November 22, 2021.
- Most physicians had little relief from prior authorization as COVID cases soared. American Medical Association website. April 7, 2021. https:// www.ama-assn.org/press-center/press-releases /most-physicians-had-little-relief-prior-author ization-covid-cases. Accessed November 29, 2021.
- Robeznieks A. 1 in 4 doctors say prior authorization has led to a serious adverse event. American Medical Association website. February 5, 2019. https://www .ama-assn.org/practice-management/sustainability /1-4-doctors-say-prior-authorization-has-led-serious -adverse. Accessed November 29, 2021.
- Physicians call on Congress to address prior authorization reform. American Medical Association website. May 14, 2021. https://www .ama-assn.org/press-center/press-releases /physicians-call-congress-address-prior-author ization-reform. Accessed November 29, 2021.
Before reading this editorial and concluding that the author (me) has lost his grip on reality, I would ask that you consider the facts I provide below and the ramifications incurred by your patients and practices, due to the misbehaviors adopted by the health insurance industry.
- Two of the most common issues discussed in today’s health care environment are revenue generation and provider/staff burnout.
While these issues are impacted by several factors, one of the most common denominators is increasing administrative workloads driven by non–revenue-generating activities. Consider this:
- A recent American Medical Association survey pointed out that during the course of the average workweek, a physician completes an average of 37 prior authorization requests. Physicians and their staff spend an average of 16.4 hours per week completing prior authorization requirements for patient medicines, procedures, and medical services that they may need.1
- While physicians report that about 65% of prior authorizations take only 1 day, they report that 26% take 3 or more days.2
The potential significance of the generated delays
While this may not seem like a long time (other than the impact it has on staff workload), consider the impact this can have on the patient if the medication being requested is: PrEP, the morning after pill, or other contraceptives? The consequences of the delay or denial could be a lifetime living with HIV, or an unintended pregnancy. This is to say nothing on the larger impact to family, partners, and the potential social stigma faced by all.
Beyond the personal costs and costs within your practice associated with the additional workload, consider the financial costs. The average cost to complete a prior authorization remains the single highest cost for the health care industry at $13.40 per manual transaction, and $7.19 per partially electronic web portal transaction,3 meaning that if I did only one prescription per week, I probably would not mind, but at $13.40 per prior authorization, this burden amounts to millions, actually $767 million by recent estimates.3 Additionally, if you factor in the number of denials and potential follow-ups, this creates a significant amount of waste and spending.
Ultimately, in my experience, I have found that most prior authorizations are simply unnecessary. Here, I’ve picked key examples from just my own recent experiences:
- My patient was denied access to a particular birth control pill she had been on successfully before, and my office was told she needed to try and fail on 5 different generic pills before she could be approved. However, the Affordable Care Act’s (ACA; aka Obamacare) Contraceptive Mandate requires coverage of all contraceptives determined to be most appropriate between a patient and their provider (see below).
- A menopausal patient was denied coverage twice (electronically) for generic micronized progesterone, and I was asked to write a letter of appeal because the insurance company wanted me to use medroxyprogesterone acetate instead. Polling my nearby retail independent pharmacy, the total cost difference per year was $19.96 savings/year ($47.01 ‒ $27.05 = $19.96). My pharmacist did note it could have been a different amount at a large chain pharmacy. Really? I had to write a letter, following two denials, to save less than $20, for a full year!
- A 78-year-old patient using Prolia for severe osteoporosis and preexisting fractures was delayed in getting her next Prolia injection due to a prior authorization snafu. She ended up with multiple additional fractures, a well-described effect of the increase in bone turnover when stopping or delaying this medication. She is now disabled.
- A 94-year-old patient was sent an email reminder to get the medical practice to authorize a refill of ileostomy bags. The email went to spam, and the patient ran out of bags prior to a holiday weekend. I got them in 2 days on Amazon Prime. But who emails a 94-year-old? And ileostomy bags! When does anyone stop needing ileostomy bags?
- I requested a prior authorization for Orilissa (clearly off label) because a severely progestogen-sensitive patient (augmented depression) with severe premenstrual dysphoric disorder requiring hospitalization was thought by her psychiatrist to be better off without menstrual periods. I completed the proper paperwork, two electronic appeals, and a letter of explanation including available references on the use of gonadotropin-releasing hormone analogues for such patients. I was then told I would need to have a peer-to-peer discussion, so I filled out that paperwork, which clearly noted that I am a board-certified reproductive endocrinologist. I got a phone call a few days later by a pleasant, young-sounding pediatric rheumatologist. Our interaction did not go well for him. This was not peer-to-peer!
Let us be clear, prior authorizations have nothing to do with patient care. In fact, they are solely about the money. We in ObGyn have mostly inexpensive and generic products, but even that fact has not lowered the excessive burden of the prior authorization process. In the case of contraception, whether you like the ACA or not it is the law, and it contains specific provisions regarding contraception. With the goals of providing broad access to patients and incentives to developers for new and novel contraceptive methods, these provisions require insurers to cover, without cost-sharing, women’s preventive services including the full range of FDA-approved contraceptives (currently 18 different method categories), and additional methods identified by the FDA as they become available. Further, providers must have an easily accessible, transparent, and sufficiently expedient exceptions process that is not unduly burdensome on the individual or a provider (or other individuals acting as a patient’s authorized representative).
And while I can regale you with chapter and verse and citations of the legal precedent and language, it boils down to this:
- The AMA reported that medical practices spend an average of 2 business days a week per physician to comply with health plans’ inefficient and overused prior-authorization protocols.4 To keep up with the administrative burden, 2 out of 5 physicians (40%) employ staff members who work exclusively on tasks associated with prior authorization.4
- About 86% of practices reported an increased burden of prior authorizations in the last 5 years.5
Continue to: What is to be done?
What is to be done?
I do have suggested solutions. Given the insurance industry’s complete lack of progress in voluntarily reducing the burdens of prior authorizations agreed to in their consensus statement with the AMA, American Hospital Association, America’s Health Insurance Plans, American Pharmacists Association, Blue Cross Blue Shield Association, and the Medical Group Management Association, I say, why not fine them? The AMA is calling on Congress to pass legislation that would codify much of the agreement, in which the above parties had already agreed that reforms were needed to reduce prior authorization burdens and enhance patient-centered care.6
A good model for enforcement via fines could be based on the old “incident to” rules of Medicare. These state that a physician needs to be “in the space” when advanced practice nurses or physician assistants see Medicare recipients. If they are not actually “in the space” they are subject to a fine. As a completely theoretical example, let’s say the claim was for $100. The practitioner would have to pay it back plus triple that amount in damages, or $400. They can also be fined up to $11,000 per claim and kick you out of Medicare and Medicaid. Take my example of Prolia from above…a single shot of Prolia is about $1,000. The insurer would theoretically have to pay $14,000/claim (the claim + triple damages + $11,000) if it was determined that the prior authorization was unnecessary. Seems about right to me. Or we could just sit the health insurance CEOs and their proxies in the corner on 2-foot-tall plastic Little Tikes® chairs for a “timeout” (dunce cap optional), like the outset of the article says.
Until the detrimental prior authorization process is challenged at all levels, we will continue to see and feel the effects of the harm it causes. Being able to drive change through advocacy and education is the best way we as clinicians can impact not just the future of health care but provide for the daily care of our patients who depend on and trust us to provide for their medical needs. We must be the impactors of change for ourselves, colleagues, staff, and profession if we are to really make advancements into the future.
Oh…and health insurance CEOs and their proxies, to get out of their “time-out” would still be entitled to one phone call to beg forgiveness from their mommies/daddies, priest/ rabbi/pastor, psychologist/psychiatrist/mystic healer, etc., but alas, the average wait time is an hour, and if anyone answers the phone, they have a grade school education used in following an irrelevant algorithm. ●
Before reading this editorial and concluding that the author (me) has lost his grip on reality, I would ask that you consider the facts I provide below and the ramifications incurred by your patients and practices, due to the misbehaviors adopted by the health insurance industry.
- Two of the most common issues discussed in today’s health care environment are revenue generation and provider/staff burnout.
While these issues are impacted by several factors, one of the most common denominators is increasing administrative workloads driven by non–revenue-generating activities. Consider this:
- A recent American Medical Association survey pointed out that during the course of the average workweek, a physician completes an average of 37 prior authorization requests. Physicians and their staff spend an average of 16.4 hours per week completing prior authorization requirements for patient medicines, procedures, and medical services that they may need.1
- While physicians report that about 65% of prior authorizations take only 1 day, they report that 26% take 3 or more days.2
The potential significance of the generated delays
While this may not seem like a long time (other than the impact it has on staff workload), consider the impact this can have on the patient if the medication being requested is: PrEP, the morning after pill, or other contraceptives? The consequences of the delay or denial could be a lifetime living with HIV, or an unintended pregnancy. This is to say nothing on the larger impact to family, partners, and the potential social stigma faced by all.
Beyond the personal costs and costs within your practice associated with the additional workload, consider the financial costs. The average cost to complete a prior authorization remains the single highest cost for the health care industry at $13.40 per manual transaction, and $7.19 per partially electronic web portal transaction,3 meaning that if I did only one prescription per week, I probably would not mind, but at $13.40 per prior authorization, this burden amounts to millions, actually $767 million by recent estimates.3 Additionally, if you factor in the number of denials and potential follow-ups, this creates a significant amount of waste and spending.
Ultimately, in my experience, I have found that most prior authorizations are simply unnecessary. Here, I’ve picked key examples from just my own recent experiences:
- My patient was denied access to a particular birth control pill she had been on successfully before, and my office was told she needed to try and fail on 5 different generic pills before she could be approved. However, the Affordable Care Act’s (ACA; aka Obamacare) Contraceptive Mandate requires coverage of all contraceptives determined to be most appropriate between a patient and their provider (see below).
- A menopausal patient was denied coverage twice (electronically) for generic micronized progesterone, and I was asked to write a letter of appeal because the insurance company wanted me to use medroxyprogesterone acetate instead. Polling my nearby retail independent pharmacy, the total cost difference per year was $19.96 savings/year ($47.01 ‒ $27.05 = $19.96). My pharmacist did note it could have been a different amount at a large chain pharmacy. Really? I had to write a letter, following two denials, to save less than $20, for a full year!
- A 78-year-old patient using Prolia for severe osteoporosis and preexisting fractures was delayed in getting her next Prolia injection due to a prior authorization snafu. She ended up with multiple additional fractures, a well-described effect of the increase in bone turnover when stopping or delaying this medication. She is now disabled.
- A 94-year-old patient was sent an email reminder to get the medical practice to authorize a refill of ileostomy bags. The email went to spam, and the patient ran out of bags prior to a holiday weekend. I got them in 2 days on Amazon Prime. But who emails a 94-year-old? And ileostomy bags! When does anyone stop needing ileostomy bags?
- I requested a prior authorization for Orilissa (clearly off label) because a severely progestogen-sensitive patient (augmented depression) with severe premenstrual dysphoric disorder requiring hospitalization was thought by her psychiatrist to be better off without menstrual periods. I completed the proper paperwork, two electronic appeals, and a letter of explanation including available references on the use of gonadotropin-releasing hormone analogues for such patients. I was then told I would need to have a peer-to-peer discussion, so I filled out that paperwork, which clearly noted that I am a board-certified reproductive endocrinologist. I got a phone call a few days later by a pleasant, young-sounding pediatric rheumatologist. Our interaction did not go well for him. This was not peer-to-peer!
Let us be clear, prior authorizations have nothing to do with patient care. In fact, they are solely about the money. We in ObGyn have mostly inexpensive and generic products, but even that fact has not lowered the excessive burden of the prior authorization process. In the case of contraception, whether you like the ACA or not it is the law, and it contains specific provisions regarding contraception. With the goals of providing broad access to patients and incentives to developers for new and novel contraceptive methods, these provisions require insurers to cover, without cost-sharing, women’s preventive services including the full range of FDA-approved contraceptives (currently 18 different method categories), and additional methods identified by the FDA as they become available. Further, providers must have an easily accessible, transparent, and sufficiently expedient exceptions process that is not unduly burdensome on the individual or a provider (or other individuals acting as a patient’s authorized representative).
And while I can regale you with chapter and verse and citations of the legal precedent and language, it boils down to this:
- The AMA reported that medical practices spend an average of 2 business days a week per physician to comply with health plans’ inefficient and overused prior-authorization protocols.4 To keep up with the administrative burden, 2 out of 5 physicians (40%) employ staff members who work exclusively on tasks associated with prior authorization.4
- About 86% of practices reported an increased burden of prior authorizations in the last 5 years.5
Continue to: What is to be done?
What is to be done?
I do have suggested solutions. Given the insurance industry’s complete lack of progress in voluntarily reducing the burdens of prior authorizations agreed to in their consensus statement with the AMA, American Hospital Association, America’s Health Insurance Plans, American Pharmacists Association, Blue Cross Blue Shield Association, and the Medical Group Management Association, I say, why not fine them? The AMA is calling on Congress to pass legislation that would codify much of the agreement, in which the above parties had already agreed that reforms were needed to reduce prior authorization burdens and enhance patient-centered care.6
A good model for enforcement via fines could be based on the old “incident to” rules of Medicare. These state that a physician needs to be “in the space” when advanced practice nurses or physician assistants see Medicare recipients. If they are not actually “in the space” they are subject to a fine. As a completely theoretical example, let’s say the claim was for $100. The practitioner would have to pay it back plus triple that amount in damages, or $400. They can also be fined up to $11,000 per claim and kick you out of Medicare and Medicaid. Take my example of Prolia from above…a single shot of Prolia is about $1,000. The insurer would theoretically have to pay $14,000/claim (the claim + triple damages + $11,000) if it was determined that the prior authorization was unnecessary. Seems about right to me. Or we could just sit the health insurance CEOs and their proxies in the corner on 2-foot-tall plastic Little Tikes® chairs for a “timeout” (dunce cap optional), like the outset of the article says.
Until the detrimental prior authorization process is challenged at all levels, we will continue to see and feel the effects of the harm it causes. Being able to drive change through advocacy and education is the best way we as clinicians can impact not just the future of health care but provide for the daily care of our patients who depend on and trust us to provide for their medical needs. We must be the impactors of change for ourselves, colleagues, staff, and profession if we are to really make advancements into the future.
Oh…and health insurance CEOs and their proxies, to get out of their “time-out” would still be entitled to one phone call to beg forgiveness from their mommies/daddies, priest/ rabbi/pastor, psychologist/psychiatrist/mystic healer, etc., but alas, the average wait time is an hour, and if anyone answers the phone, they have a grade school education used in following an irrelevant algorithm. ●
- Corder JC. Streamlining the insurance prior authorization debacle. Mo Med. 2018;115:312-314.
- Prior authorization hurdles have led to serious adverse events. American Medical Association website. February 5, 2019. https://www.ama-assn .org/press-center/press-releases/prior-author ization-hurdles-have-led-serious-adverse -events. Accessed November 29, 2021.
- Council for Affordable Quality Healthcare. 2020 CAQH INDEX. https://www.caqh.org/sites /default/files/explorations/index/2020-caqh -index.pdf. Accessed November 22, 2021.
- Most physicians had little relief from prior authorization as COVID cases soared. American Medical Association website. April 7, 2021. https:// www.ama-assn.org/press-center/press-releases /most-physicians-had-little-relief-prior-author ization-covid-cases. Accessed November 29, 2021.
- Robeznieks A. 1 in 4 doctors say prior authorization has led to a serious adverse event. American Medical Association website. February 5, 2019. https://www .ama-assn.org/practice-management/sustainability /1-4-doctors-say-prior-authorization-has-led-serious -adverse. Accessed November 29, 2021.
- Physicians call on Congress to address prior authorization reform. American Medical Association website. May 14, 2021. https://www .ama-assn.org/press-center/press-releases /physicians-call-congress-address-prior-author ization-reform. Accessed November 29, 2021.
- Corder JC. Streamlining the insurance prior authorization debacle. Mo Med. 2018;115:312-314.
- Prior authorization hurdles have led to serious adverse events. American Medical Association website. February 5, 2019. https://www.ama-assn .org/press-center/press-releases/prior-author ization-hurdles-have-led-serious-adverse -events. Accessed November 29, 2021.
- Council for Affordable Quality Healthcare. 2020 CAQH INDEX. https://www.caqh.org/sites /default/files/explorations/index/2020-caqh -index.pdf. Accessed November 22, 2021.
- Most physicians had little relief from prior authorization as COVID cases soared. American Medical Association website. April 7, 2021. https:// www.ama-assn.org/press-center/press-releases /most-physicians-had-little-relief-prior-author ization-covid-cases. Accessed November 29, 2021.
- Robeznieks A. 1 in 4 doctors say prior authorization has led to a serious adverse event. American Medical Association website. February 5, 2019. https://www .ama-assn.org/practice-management/sustainability /1-4-doctors-say-prior-authorization-has-led-serious -adverse. Accessed November 29, 2021.
- Physicians call on Congress to address prior authorization reform. American Medical Association website. May 14, 2021. https://www .ama-assn.org/press-center/press-releases /physicians-call-congress-address-prior-author ization-reform. Accessed November 29, 2021.
Common cold could protect against COVID-19, study says
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com
Ranking seven COVID-19 antigen tests by ease of use: Report
Some COVID-19 rapid antigen home test kits are much easier to use than others, according to an analysis by ECRI, an independent, nonprofit patient safety organization.
None of the tests were rated as “excellent” in terms of usability and some had “noteworthy” usability concerns, the company said.
If a test is hard to use, “chances are that you may miss a step or not follow the right order, or contaminate the testing area and that can definitely influence the accuracy of the test and lead to a wrong test result,” Marcus Schabacker, MD, PhD, president and CEO of ECRI, told this news organization.
To gauge usability, ECRI used the “industry-standard” system usability scale (SUS), which rates products on a scale of 0 to 100 with 100 being the easiest to use.
More than 30 points separated the top and bottom tests analyzed. The top performer was On/Go, followed by CareStart and Flowflex.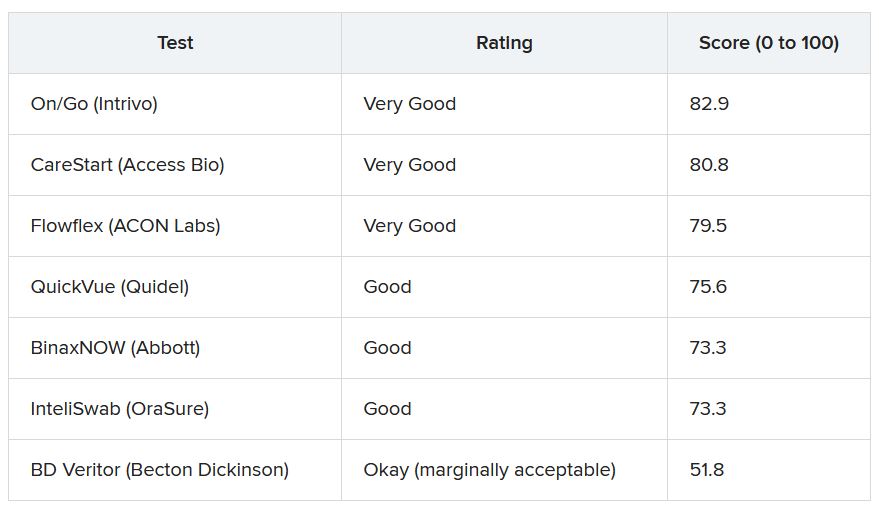
ECRI analysts found that some tests require particularly fine motor skills or have instructions with extremely small font size that may make it hard for older adults or people with complex health conditions to use the tests correctly.
“If you have a tremor from Parkinson’s, for example, or anything which won’t allow you to handle small items, you will have difficulties to do that test by yourself. That is the No. 1 concern we have,” Dr. Schabacker said.
“The second concern is readability, as all of these tests have relatively small instructions. One of them actually has doesn’t even have instructions – you have to download an app,” he noted.
Given demand and supply issues, Dr. Schabacker acknowledged that consumers might not have a choice in which test to use and may have to rely on whatever is available.
These tests are a “hot commodity right now,” he said. “If you have a choice, people should use the ones which are easiest to use, which is the On/Go, the CareStart, or the Flowflex.”
A version of this article first appeared on Medscape.com.
Some COVID-19 rapid antigen home test kits are much easier to use than others, according to an analysis by ECRI, an independent, nonprofit patient safety organization.
None of the tests were rated as “excellent” in terms of usability and some had “noteworthy” usability concerns, the company said.
If a test is hard to use, “chances are that you may miss a step or not follow the right order, or contaminate the testing area and that can definitely influence the accuracy of the test and lead to a wrong test result,” Marcus Schabacker, MD, PhD, president and CEO of ECRI, told this news organization.
To gauge usability, ECRI used the “industry-standard” system usability scale (SUS), which rates products on a scale of 0 to 100 with 100 being the easiest to use.
More than 30 points separated the top and bottom tests analyzed. The top performer was On/Go, followed by CareStart and Flowflex.
ECRI analysts found that some tests require particularly fine motor skills or have instructions with extremely small font size that may make it hard for older adults or people with complex health conditions to use the tests correctly.
“If you have a tremor from Parkinson’s, for example, or anything which won’t allow you to handle small items, you will have difficulties to do that test by yourself. That is the No. 1 concern we have,” Dr. Schabacker said.
“The second concern is readability, as all of these tests have relatively small instructions. One of them actually has doesn’t even have instructions – you have to download an app,” he noted.
Given demand and supply issues, Dr. Schabacker acknowledged that consumers might not have a choice in which test to use and may have to rely on whatever is available.
These tests are a “hot commodity right now,” he said. “If you have a choice, people should use the ones which are easiest to use, which is the On/Go, the CareStart, or the Flowflex.”
A version of this article first appeared on Medscape.com.
Some COVID-19 rapid antigen home test kits are much easier to use than others, according to an analysis by ECRI, an independent, nonprofit patient safety organization.
None of the tests were rated as “excellent” in terms of usability and some had “noteworthy” usability concerns, the company said.
If a test is hard to use, “chances are that you may miss a step or not follow the right order, or contaminate the testing area and that can definitely influence the accuracy of the test and lead to a wrong test result,” Marcus Schabacker, MD, PhD, president and CEO of ECRI, told this news organization.
To gauge usability, ECRI used the “industry-standard” system usability scale (SUS), which rates products on a scale of 0 to 100 with 100 being the easiest to use.
More than 30 points separated the top and bottom tests analyzed. The top performer was On/Go, followed by CareStart and Flowflex.
ECRI analysts found that some tests require particularly fine motor skills or have instructions with extremely small font size that may make it hard for older adults or people with complex health conditions to use the tests correctly.
“If you have a tremor from Parkinson’s, for example, or anything which won’t allow you to handle small items, you will have difficulties to do that test by yourself. That is the No. 1 concern we have,” Dr. Schabacker said.
“The second concern is readability, as all of these tests have relatively small instructions. One of them actually has doesn’t even have instructions – you have to download an app,” he noted.
Given demand and supply issues, Dr. Schabacker acknowledged that consumers might not have a choice in which test to use and may have to rely on whatever is available.
These tests are a “hot commodity right now,” he said. “If you have a choice, people should use the ones which are easiest to use, which is the On/Go, the CareStart, or the Flowflex.”
A version of this article first appeared on Medscape.com.
Physicians react: Should docs lose their licenses for spreading false COVID information?
Doctors providing “fraudulent” COVID-19 information became a hot-button issue for physicians responding to Medscape’s recent article, "Shouldn’t Doctors Who Spread False COVID-19 Information Lose Their Licenses?”
COVID-19 safety recommendations are set by mainstream medical organizations as new information becomes available, but some doctors consistently oppose advice from the Centers for Disease Control and Prevention and other medical authorities. These physicians often promote off-label, unapproved use of medications for COVID-19 and/or contradict mainstream safety guidelines such as vaccines, masks, and social distancing.
Some medical organizations are concerned that these doctors are hampering efforts to control the highly contagious coronavirus and are, at worst, placing lives in danger with their contrarian views that can spread like wildfire on social media sites. Their words are often used by those who refuse to be vaccinated or wear masks.
State licensing boards have mostly refused to discipline these doctors for making false and/or misleading claims, but as the virus spreads, there are calls to take action against them. However, others worry that such actions would violate free speech and critical thought.
Yes, those doctors are doing wrong
Several physicians took a strong stand against their fellow doctors who are spreading misinformation about COVID-19.
One doctor endorsed the idea of removing licenses for spreading misinformation and called for criminal prosecution: “It should certainly be grounds for cancellation of all licensing (after appropriate examination to rule out acute psychotic episodes, dementia, tumor, etc.) and very likely [include] a charge of manslaughter.”
Another health care provider said, “A person who does not accept science should not, of course, be allowed to practice medicine. One who argues publicly that vaccines and masks don’t work should be prosecuted for crimes ranging from reckless endangerment to attempted murder.”
One reader framed COVID-19 misinformers in stark terms: “These men and women are medical prostitutes. Their medical and surgical colleges [should] have a panel to track in-court testimony and the disinformation they spread ...”
“This is malpractice of the worst kind,” said a clinician. “Public health officials and science are quite clear on [the] best practices for safety during a pandemic, which is killing millions. This is a standard of care.”
“Medical Boards should suspend licenses and give the physician a chance to testify [about] the scientific basis for his comments,” added a health care provider. “Boards involve themselves in all kinds of perceived disciplinary infractions. We are in the midst of a lethal pandemic. I would think that would take precedence over many other issues?”
“I do believe that physicians have the responsibility to speak the truth and have scientifically displayed minds,” said a reader. “Not [to] promulgate misleading, false, and/or unverified information.”
“Any physician, who holds a license, should abide [by] government and state regulation,” asserted a doctor. “He should be disciplined by the board for spreading medical/public misinformation since he is creating potential harm to the population.”
One specialist insisted that “state boards do not do enough to restrict/limit the practice of physicians touting questionable therapies.”
“Any doctor who spreads false information about Covid is hurting our country, our individuals, and our economy and leading to needless deaths,” asserted a physician. “However, there are uncertainties, and where those exist, physicians [should] simply say ‘it is unknown.’”
No, those physicians have a right to speak their beliefs
However, many physicians worried that science and controversial thought were being muzzled.
“Absolutely no,” a doctor stated. “Who judges what is misinformation in this age where debate is canceled? Science advances with challenge, and it’s not about an authority dictating the allowable opinion.”
Another clinician claimed the “truth is very difficult to discern from less-than-truth in a country running on a profit-oriented economic ideology.”
One specialist warned that if disinformation doctors are held responsible, then “that means a lot of doctors” will be “gone” because “almost anything that is written or said about COVID can be contested.”
Another physician warned his colleagues about suppressing new ideas: “To condemn what we didn’t try, or purposefully ignore a different approach because [it] doesn’t agree with our opinion is suppression of information.”
Some doctors insisted the issue extended beyond medicine and into Constitutional freedoms. They also expressed their mistrust in the government to regulate physicians.
“There is a First Amendment in this country,” said one reader. “What you think is false may not be so. The people can listen to whoever they want to and make their own medical decisions. We do not need one iota more of politicizing medicine. Having an MD or DO does not mean you relinquish your First Amendment rights.”
“One of the fundamental problems with a system that allows government to ‘license’ physicians, or any other profession, is that politics inevitably turn to cronyism, and big businesses and wealthy people start controlling the government,” argued a doctor.
One clinician suggested enforcement against health food, drug company commercials, and talk shows: “What about all the [misinformation] at the health food stores and the like. Doctors of natural-whatever? Those info-commercials on tv. How many faxes do I get to ‘approve’ because ‘patients request’ braces and pain-treating expensive compounds advertised on TV? We tolerate those ... What about Dr. Oz and the docs on talk shows claiming BS?”
And the debate goes even further
Some physicians questioned the very notion of claiming “truth.”
“Nobody should be certain that they have the ‘absolute truth,’” said one reader. “In fact, the best clinical insights exceed so-called knowledge by at least one step.”
“Who can determine exactly what is truth?” asked another clinician. “For sure, the ‘Federal Government,’ who ‘is here to help you,’ is not qualified to make such determinations, and who are you to make such a suggestion as to remove someone’s license because they disagree with you? Give me a break!”
Another physician echoed that sentiment: “What’s true and false is often and certainly currently debatable. There are well-qualified physicians (with credentials such as the development of mRNA technology), virologists, and biostatisticians that have valid thoughts on this but do not necessarily agree with the drug company-sponsored journals and news channels (most of them). Their voices should be heard, and they should not lose their licenses. They are doing their work in good conscience.”
One reader commented that he wanted his “freedom of speech,” and offered this defiant advice: “You can take this license and shove it.”
Finally, a physician noted that the political climate has influenced medical directives: “If someone in a leadership role knowingly, and with intent, spread false information, that is wrong. However, during this global pandemic the active and the politics have combined. Red state no mandate, blue state mandate – what does that tell you about American leadership?”
A version of this article first appeared on Medscape.com.
Doctors providing “fraudulent” COVID-19 information became a hot-button issue for physicians responding to Medscape’s recent article, "Shouldn’t Doctors Who Spread False COVID-19 Information Lose Their Licenses?”
COVID-19 safety recommendations are set by mainstream medical organizations as new information becomes available, but some doctors consistently oppose advice from the Centers for Disease Control and Prevention and other medical authorities. These physicians often promote off-label, unapproved use of medications for COVID-19 and/or contradict mainstream safety guidelines such as vaccines, masks, and social distancing.
Some medical organizations are concerned that these doctors are hampering efforts to control the highly contagious coronavirus and are, at worst, placing lives in danger with their contrarian views that can spread like wildfire on social media sites. Their words are often used by those who refuse to be vaccinated or wear masks.
State licensing boards have mostly refused to discipline these doctors for making false and/or misleading claims, but as the virus spreads, there are calls to take action against them. However, others worry that such actions would violate free speech and critical thought.
Yes, those doctors are doing wrong
Several physicians took a strong stand against their fellow doctors who are spreading misinformation about COVID-19.
One doctor endorsed the idea of removing licenses for spreading misinformation and called for criminal prosecution: “It should certainly be grounds for cancellation of all licensing (after appropriate examination to rule out acute psychotic episodes, dementia, tumor, etc.) and very likely [include] a charge of manslaughter.”
Another health care provider said, “A person who does not accept science should not, of course, be allowed to practice medicine. One who argues publicly that vaccines and masks don’t work should be prosecuted for crimes ranging from reckless endangerment to attempted murder.”
One reader framed COVID-19 misinformers in stark terms: “These men and women are medical prostitutes. Their medical and surgical colleges [should] have a panel to track in-court testimony and the disinformation they spread ...”
“This is malpractice of the worst kind,” said a clinician. “Public health officials and science are quite clear on [the] best practices for safety during a pandemic, which is killing millions. This is a standard of care.”
“Medical Boards should suspend licenses and give the physician a chance to testify [about] the scientific basis for his comments,” added a health care provider. “Boards involve themselves in all kinds of perceived disciplinary infractions. We are in the midst of a lethal pandemic. I would think that would take precedence over many other issues?”
“I do believe that physicians have the responsibility to speak the truth and have scientifically displayed minds,” said a reader. “Not [to] promulgate misleading, false, and/or unverified information.”
“Any physician, who holds a license, should abide [by] government and state regulation,” asserted a doctor. “He should be disciplined by the board for spreading medical/public misinformation since he is creating potential harm to the population.”
One specialist insisted that “state boards do not do enough to restrict/limit the practice of physicians touting questionable therapies.”
“Any doctor who spreads false information about Covid is hurting our country, our individuals, and our economy and leading to needless deaths,” asserted a physician. “However, there are uncertainties, and where those exist, physicians [should] simply say ‘it is unknown.’”
No, those physicians have a right to speak their beliefs
However, many physicians worried that science and controversial thought were being muzzled.
“Absolutely no,” a doctor stated. “Who judges what is misinformation in this age where debate is canceled? Science advances with challenge, and it’s not about an authority dictating the allowable opinion.”
Another clinician claimed the “truth is very difficult to discern from less-than-truth in a country running on a profit-oriented economic ideology.”
One specialist warned that if disinformation doctors are held responsible, then “that means a lot of doctors” will be “gone” because “almost anything that is written or said about COVID can be contested.”
Another physician warned his colleagues about suppressing new ideas: “To condemn what we didn’t try, or purposefully ignore a different approach because [it] doesn’t agree with our opinion is suppression of information.”
Some doctors insisted the issue extended beyond medicine and into Constitutional freedoms. They also expressed their mistrust in the government to regulate physicians.
“There is a First Amendment in this country,” said one reader. “What you think is false may not be so. The people can listen to whoever they want to and make their own medical decisions. We do not need one iota more of politicizing medicine. Having an MD or DO does not mean you relinquish your First Amendment rights.”
“One of the fundamental problems with a system that allows government to ‘license’ physicians, or any other profession, is that politics inevitably turn to cronyism, and big businesses and wealthy people start controlling the government,” argued a doctor.
One clinician suggested enforcement against health food, drug company commercials, and talk shows: “What about all the [misinformation] at the health food stores and the like. Doctors of natural-whatever? Those info-commercials on tv. How many faxes do I get to ‘approve’ because ‘patients request’ braces and pain-treating expensive compounds advertised on TV? We tolerate those ... What about Dr. Oz and the docs on talk shows claiming BS?”
And the debate goes even further
Some physicians questioned the very notion of claiming “truth.”
“Nobody should be certain that they have the ‘absolute truth,’” said one reader. “In fact, the best clinical insights exceed so-called knowledge by at least one step.”
“Who can determine exactly what is truth?” asked another clinician. “For sure, the ‘Federal Government,’ who ‘is here to help you,’ is not qualified to make such determinations, and who are you to make such a suggestion as to remove someone’s license because they disagree with you? Give me a break!”
Another physician echoed that sentiment: “What’s true and false is often and certainly currently debatable. There are well-qualified physicians (with credentials such as the development of mRNA technology), virologists, and biostatisticians that have valid thoughts on this but do not necessarily agree with the drug company-sponsored journals and news channels (most of them). Their voices should be heard, and they should not lose their licenses. They are doing their work in good conscience.”
One reader commented that he wanted his “freedom of speech,” and offered this defiant advice: “You can take this license and shove it.”
Finally, a physician noted that the political climate has influenced medical directives: “If someone in a leadership role knowingly, and with intent, spread false information, that is wrong. However, during this global pandemic the active and the politics have combined. Red state no mandate, blue state mandate – what does that tell you about American leadership?”
A version of this article first appeared on Medscape.com.
Doctors providing “fraudulent” COVID-19 information became a hot-button issue for physicians responding to Medscape’s recent article, "Shouldn’t Doctors Who Spread False COVID-19 Information Lose Their Licenses?”
COVID-19 safety recommendations are set by mainstream medical organizations as new information becomes available, but some doctors consistently oppose advice from the Centers for Disease Control and Prevention and other medical authorities. These physicians often promote off-label, unapproved use of medications for COVID-19 and/or contradict mainstream safety guidelines such as vaccines, masks, and social distancing.
Some medical organizations are concerned that these doctors are hampering efforts to control the highly contagious coronavirus and are, at worst, placing lives in danger with their contrarian views that can spread like wildfire on social media sites. Their words are often used by those who refuse to be vaccinated or wear masks.
State licensing boards have mostly refused to discipline these doctors for making false and/or misleading claims, but as the virus spreads, there are calls to take action against them. However, others worry that such actions would violate free speech and critical thought.
Yes, those doctors are doing wrong
Several physicians took a strong stand against their fellow doctors who are spreading misinformation about COVID-19.
One doctor endorsed the idea of removing licenses for spreading misinformation and called for criminal prosecution: “It should certainly be grounds for cancellation of all licensing (after appropriate examination to rule out acute psychotic episodes, dementia, tumor, etc.) and very likely [include] a charge of manslaughter.”
Another health care provider said, “A person who does not accept science should not, of course, be allowed to practice medicine. One who argues publicly that vaccines and masks don’t work should be prosecuted for crimes ranging from reckless endangerment to attempted murder.”
One reader framed COVID-19 misinformers in stark terms: “These men and women are medical prostitutes. Their medical and surgical colleges [should] have a panel to track in-court testimony and the disinformation they spread ...”
“This is malpractice of the worst kind,” said a clinician. “Public health officials and science are quite clear on [the] best practices for safety during a pandemic, which is killing millions. This is a standard of care.”
“Medical Boards should suspend licenses and give the physician a chance to testify [about] the scientific basis for his comments,” added a health care provider. “Boards involve themselves in all kinds of perceived disciplinary infractions. We are in the midst of a lethal pandemic. I would think that would take precedence over many other issues?”
“I do believe that physicians have the responsibility to speak the truth and have scientifically displayed minds,” said a reader. “Not [to] promulgate misleading, false, and/or unverified information.”
“Any physician, who holds a license, should abide [by] government and state regulation,” asserted a doctor. “He should be disciplined by the board for spreading medical/public misinformation since he is creating potential harm to the population.”
One specialist insisted that “state boards do not do enough to restrict/limit the practice of physicians touting questionable therapies.”
“Any doctor who spreads false information about Covid is hurting our country, our individuals, and our economy and leading to needless deaths,” asserted a physician. “However, there are uncertainties, and where those exist, physicians [should] simply say ‘it is unknown.’”
No, those physicians have a right to speak their beliefs
However, many physicians worried that science and controversial thought were being muzzled.
“Absolutely no,” a doctor stated. “Who judges what is misinformation in this age where debate is canceled? Science advances with challenge, and it’s not about an authority dictating the allowable opinion.”
Another clinician claimed the “truth is very difficult to discern from less-than-truth in a country running on a profit-oriented economic ideology.”
One specialist warned that if disinformation doctors are held responsible, then “that means a lot of doctors” will be “gone” because “almost anything that is written or said about COVID can be contested.”
Another physician warned his colleagues about suppressing new ideas: “To condemn what we didn’t try, or purposefully ignore a different approach because [it] doesn’t agree with our opinion is suppression of information.”
Some doctors insisted the issue extended beyond medicine and into Constitutional freedoms. They also expressed their mistrust in the government to regulate physicians.
“There is a First Amendment in this country,” said one reader. “What you think is false may not be so. The people can listen to whoever they want to and make their own medical decisions. We do not need one iota more of politicizing medicine. Having an MD or DO does not mean you relinquish your First Amendment rights.”
“One of the fundamental problems with a system that allows government to ‘license’ physicians, or any other profession, is that politics inevitably turn to cronyism, and big businesses and wealthy people start controlling the government,” argued a doctor.
One clinician suggested enforcement against health food, drug company commercials, and talk shows: “What about all the [misinformation] at the health food stores and the like. Doctors of natural-whatever? Those info-commercials on tv. How many faxes do I get to ‘approve’ because ‘patients request’ braces and pain-treating expensive compounds advertised on TV? We tolerate those ... What about Dr. Oz and the docs on talk shows claiming BS?”
And the debate goes even further
Some physicians questioned the very notion of claiming “truth.”
“Nobody should be certain that they have the ‘absolute truth,’” said one reader. “In fact, the best clinical insights exceed so-called knowledge by at least one step.”
“Who can determine exactly what is truth?” asked another clinician. “For sure, the ‘Federal Government,’ who ‘is here to help you,’ is not qualified to make such determinations, and who are you to make such a suggestion as to remove someone’s license because they disagree with you? Give me a break!”
Another physician echoed that sentiment: “What’s true and false is often and certainly currently debatable. There are well-qualified physicians (with credentials such as the development of mRNA technology), virologists, and biostatisticians that have valid thoughts on this but do not necessarily agree with the drug company-sponsored journals and news channels (most of them). Their voices should be heard, and they should not lose their licenses. They are doing their work in good conscience.”
One reader commented that he wanted his “freedom of speech,” and offered this defiant advice: “You can take this license and shove it.”
Finally, a physician noted that the political climate has influenced medical directives: “If someone in a leadership role knowingly, and with intent, spread false information, that is wrong. However, during this global pandemic the active and the politics have combined. Red state no mandate, blue state mandate – what does that tell you about American leadership?”
A version of this article first appeared on Medscape.com.
Single-use duodenoscope is cost effective in ERCP
, according to a new analysis.
The study compared the EXALT Model-D, HLD, culture-and-quarantine (CQ), and ethylene oxide sterilization (ETO). The results came from a simulated cohort of patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) to treat choledocholithiasis.
Although EXALT was the costliest option and HLD the cheapest, EXALT produced the most quality-adjusted life years (QALYs) and allowed the hospital to decrease net costs, and sensitivity analysis showed that it was a better option than HLD over a range of willingness-to-pay values.
“When evaluating technologies based on cost-effectiveness and additionally in the context of TPT [transitional passthrough] or NTAP [new technology add-on payment], the EXALT approach meets typically used cost-effectiveness thresholds compared to all other evaluated strategies and should be considered for standard practice,” wrote the authors, who were led by Ananya Das, MD, of the Arizona Centers for Digestive Health, Gilbert. The study was published in Techniques and Innovations in Gastrointestinal Endoscopy.
Duodenoscope contamination has resulted in outbreaks of various multidrug-resistant organisms in hospital settings, which has led to the publication of various reprocessing guidelines. Although many hospitals have adopted HLD protocols, others use additional or alternative reprocessing methods such as CQ or ETO. Despite these efforts, a recent Food and Drug Administration study found that 1.9%-22% of samples taken from duodenoscopes tested positive for bacteria of concern, such as pathogens. Those and other findings have led some to suggest that it would be best to move away from HLD, and instead employ sterilizable or disposable endoscopes.
In another study, The EXALT Model-D (Boston Scientific) had been shown to be a good alternative to standard reusable duodenoscopes.
The researchers used a Markov-model to determine the cost-effectiveness of EXALT Model-D against other approaches in a simulated cohort. They found that EXALT Model-D created the most QALYs (21.9265) at the highest cost ($3,000), and HLD the fewest QALYs (21.8938) at the lowest cost ($962). Compared with HLD, the incremental cost-effectiveness ratio (ICER) of EXALT was $62,185, and $38,461 for ETO gas sterilization. CQ was dominated, indicating that it had a higher cost but was not more effective than HLD.
The researchers conducted a subanalysis of ERCP and Medicare patients to consider the recently approved TPT payment and the NTAP, in both hospital outpatient and inpatient settings. With TPT, EXALT had no cost after reimbursement, with a net saving of $962 per patient when compared with HLD, plus an increase in 0.033 QALYs (0.15%). The other procedures cost more and were less effective. With NTAP, EXALT had a net cost of $323 versus HLD, with a similar QALY benefit.
A Monte Carlo analysis of EXALT versus HLD found reductions in duodenoscope infection-related ICU admission (relative risk reduction, 0.996; 95% confidence interval, 0.936-1.0; number needed to treat, 79; 95% CI, 67-95) and death (RRR, 0.973; 95% CI, 0.552-0.998; number needed to treat, 556; 95% CI, 350-997).
In willingness-to-pay estimates from $50,000 to $100,000, EXALT was cost effective in 67.28% of trials with ICER under $100,000 per QALY.
The study did not consider medicolegal costs, which could lead to an underestimation of EXALT’s cost-effectiveness. The study also relied on available published information to determine cost per patient of hospital outbreaks in the United States and Europe since 2012, but the authors did not include costs of administrative sanctions, litigation, and poor publicity due to inconsistencies in the literature.
“While more research is needed to understand and quantify the determinants of the natural history after exposure to contaminated duodenoscopes, such as the risk of transmission and the subsequent development of serious clinical infections, this economic analysis demonstrates an approach using EXALT Model-D is cost effective in the U.S. health care system when compared to the currently utilized strategies of duodenoscope reprocessing,” the researchers concluded.
The study did not receive any funding. One of the authors is an employee and stockholder of Boston Scientific, which manufactures and markets EXALT. The other two authors have consulted for Boston Scientific.
Consider for a moment: The single-use duodenoscope (SUD) represents a revolutionary approach to duodenoscope infection control. Who, even 10 years ago, would have imagined that a disposable duodenoscope would even be technically achievable, much less economically feasible? Notwithstanding, determining how to incorporate such a revolutionary new technology and its associated capital and recurring costs can be every bit as complex and challenging as conceiving and developing the SUD. The authors provide insights into answering these questions through Markov modeling, comparing cost-effectiveness of SUDs to traditional duodenoscopes (TD) using available data on TD and SUD performance, and extrapolating from nonendoscopic infection management data.
This analysis is helpful because it demonstrates that, despite SUD cost approaching $3,000, Centers for Medicare & Medicaid Services inpatient and outpatient cost-defrayment payments may result in SUDs being cost-effective within limits and assumptions the study incorporates. This information is also timely, because these CMS subsidies are guaranteed only through mid-2022 for Medicare inpatients and 2023 for Medicare outpatients.
Though useful and timely, this study does make assumptions that narrow its applicability to real-world endoscopic retrograde cholangiopancreatography (ERCP). Clinically, it considers only patients with uncomplicated common bile duct stones. While choledocholithiasis is the indication for ERCP in the majority of patients, over 40% of ERCPs in the United States are performed for other, often more complex applications. While most procedures in the referenced studies were performed by high-volume ERCP experts, a substantial proportion of ERCPs are performed by lower-volume ERCP proceduralists, who actually perform a substantial proportion of straight-forward ERCPs addressing uncomplicated choledocholithiasis.
This study focuses on cost implications on CMS Transitional Pass-through (TPT) and New Technology Add-On Payment (NTAP) subsidies available only for Medicare inpatients and outpatients, respectively. These reimbursements are set to expire in 2022 (inpatients) and 2023 (outpatients). What will happen after that? Also, the amount of TPT and NTAP cost defrayments are institution-dependent, because cost-to-charge ratio (CCR), an important factor in calculating these subsidies, varies substantially between institutions and regions. Looking to the future, how will the cost of SUDs be incorporated into the hospital business model when TPT and NTAP are over?
SUDs are a technological marvel and a remarkable advance in endoscopic infection control. But innovations in medical technology are expectedly accompanied by new operational challenges: How to incorporate them into day-to-day practice and develop a business model that avails valuable new resources to patients. Such operational challenges require as much heavy lifting as the technological innovation needed to produce innovative devices like SUDs. The authors’ vision and effort in ideating and executing this study give us a head-start on this path by helping us to imagine what is possible.
John A. Martin, MD, is associate professor and consultant at the Mayo Clinic, Rochester, Minn. He is a former member of the editorial board for GI & Hepatology News, but has no relevant conflicts to disclose.
Consider for a moment: The single-use duodenoscope (SUD) represents a revolutionary approach to duodenoscope infection control. Who, even 10 years ago, would have imagined that a disposable duodenoscope would even be technically achievable, much less economically feasible? Notwithstanding, determining how to incorporate such a revolutionary new technology and its associated capital and recurring costs can be every bit as complex and challenging as conceiving and developing the SUD. The authors provide insights into answering these questions through Markov modeling, comparing cost-effectiveness of SUDs to traditional duodenoscopes (TD) using available data on TD and SUD performance, and extrapolating from nonendoscopic infection management data.
This analysis is helpful because it demonstrates that, despite SUD cost approaching $3,000, Centers for Medicare & Medicaid Services inpatient and outpatient cost-defrayment payments may result in SUDs being cost-effective within limits and assumptions the study incorporates. This information is also timely, because these CMS subsidies are guaranteed only through mid-2022 for Medicare inpatients and 2023 for Medicare outpatients.
Though useful and timely, this study does make assumptions that narrow its applicability to real-world endoscopic retrograde cholangiopancreatography (ERCP). Clinically, it considers only patients with uncomplicated common bile duct stones. While choledocholithiasis is the indication for ERCP in the majority of patients, over 40% of ERCPs in the United States are performed for other, often more complex applications. While most procedures in the referenced studies were performed by high-volume ERCP experts, a substantial proportion of ERCPs are performed by lower-volume ERCP proceduralists, who actually perform a substantial proportion of straight-forward ERCPs addressing uncomplicated choledocholithiasis.
This study focuses on cost implications on CMS Transitional Pass-through (TPT) and New Technology Add-On Payment (NTAP) subsidies available only for Medicare inpatients and outpatients, respectively. These reimbursements are set to expire in 2022 (inpatients) and 2023 (outpatients). What will happen after that? Also, the amount of TPT and NTAP cost defrayments are institution-dependent, because cost-to-charge ratio (CCR), an important factor in calculating these subsidies, varies substantially between institutions and regions. Looking to the future, how will the cost of SUDs be incorporated into the hospital business model when TPT and NTAP are over?
SUDs are a technological marvel and a remarkable advance in endoscopic infection control. But innovations in medical technology are expectedly accompanied by new operational challenges: How to incorporate them into day-to-day practice and develop a business model that avails valuable new resources to patients. Such operational challenges require as much heavy lifting as the technological innovation needed to produce innovative devices like SUDs. The authors’ vision and effort in ideating and executing this study give us a head-start on this path by helping us to imagine what is possible.
John A. Martin, MD, is associate professor and consultant at the Mayo Clinic, Rochester, Minn. He is a former member of the editorial board for GI & Hepatology News, but has no relevant conflicts to disclose.
Consider for a moment: The single-use duodenoscope (SUD) represents a revolutionary approach to duodenoscope infection control. Who, even 10 years ago, would have imagined that a disposable duodenoscope would even be technically achievable, much less economically feasible? Notwithstanding, determining how to incorporate such a revolutionary new technology and its associated capital and recurring costs can be every bit as complex and challenging as conceiving and developing the SUD. The authors provide insights into answering these questions through Markov modeling, comparing cost-effectiveness of SUDs to traditional duodenoscopes (TD) using available data on TD and SUD performance, and extrapolating from nonendoscopic infection management data.
This analysis is helpful because it demonstrates that, despite SUD cost approaching $3,000, Centers for Medicare & Medicaid Services inpatient and outpatient cost-defrayment payments may result in SUDs being cost-effective within limits and assumptions the study incorporates. This information is also timely, because these CMS subsidies are guaranteed only through mid-2022 for Medicare inpatients and 2023 for Medicare outpatients.
Though useful and timely, this study does make assumptions that narrow its applicability to real-world endoscopic retrograde cholangiopancreatography (ERCP). Clinically, it considers only patients with uncomplicated common bile duct stones. While choledocholithiasis is the indication for ERCP in the majority of patients, over 40% of ERCPs in the United States are performed for other, often more complex applications. While most procedures in the referenced studies were performed by high-volume ERCP experts, a substantial proportion of ERCPs are performed by lower-volume ERCP proceduralists, who actually perform a substantial proportion of straight-forward ERCPs addressing uncomplicated choledocholithiasis.
This study focuses on cost implications on CMS Transitional Pass-through (TPT) and New Technology Add-On Payment (NTAP) subsidies available only for Medicare inpatients and outpatients, respectively. These reimbursements are set to expire in 2022 (inpatients) and 2023 (outpatients). What will happen after that? Also, the amount of TPT and NTAP cost defrayments are institution-dependent, because cost-to-charge ratio (CCR), an important factor in calculating these subsidies, varies substantially between institutions and regions. Looking to the future, how will the cost of SUDs be incorporated into the hospital business model when TPT and NTAP are over?
SUDs are a technological marvel and a remarkable advance in endoscopic infection control. But innovations in medical technology are expectedly accompanied by new operational challenges: How to incorporate them into day-to-day practice and develop a business model that avails valuable new resources to patients. Such operational challenges require as much heavy lifting as the technological innovation needed to produce innovative devices like SUDs. The authors’ vision and effort in ideating and executing this study give us a head-start on this path by helping us to imagine what is possible.
John A. Martin, MD, is associate professor and consultant at the Mayo Clinic, Rochester, Minn. He is a former member of the editorial board for GI & Hepatology News, but has no relevant conflicts to disclose.
, according to a new analysis.
The study compared the EXALT Model-D, HLD, culture-and-quarantine (CQ), and ethylene oxide sterilization (ETO). The results came from a simulated cohort of patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) to treat choledocholithiasis.
Although EXALT was the costliest option and HLD the cheapest, EXALT produced the most quality-adjusted life years (QALYs) and allowed the hospital to decrease net costs, and sensitivity analysis showed that it was a better option than HLD over a range of willingness-to-pay values.
“When evaluating technologies based on cost-effectiveness and additionally in the context of TPT [transitional passthrough] or NTAP [new technology add-on payment], the EXALT approach meets typically used cost-effectiveness thresholds compared to all other evaluated strategies and should be considered for standard practice,” wrote the authors, who were led by Ananya Das, MD, of the Arizona Centers for Digestive Health, Gilbert. The study was published in Techniques and Innovations in Gastrointestinal Endoscopy.
Duodenoscope contamination has resulted in outbreaks of various multidrug-resistant organisms in hospital settings, which has led to the publication of various reprocessing guidelines. Although many hospitals have adopted HLD protocols, others use additional or alternative reprocessing methods such as CQ or ETO. Despite these efforts, a recent Food and Drug Administration study found that 1.9%-22% of samples taken from duodenoscopes tested positive for bacteria of concern, such as pathogens. Those and other findings have led some to suggest that it would be best to move away from HLD, and instead employ sterilizable or disposable endoscopes.
In another study, The EXALT Model-D (Boston Scientific) had been shown to be a good alternative to standard reusable duodenoscopes.
The researchers used a Markov-model to determine the cost-effectiveness of EXALT Model-D against other approaches in a simulated cohort. They found that EXALT Model-D created the most QALYs (21.9265) at the highest cost ($3,000), and HLD the fewest QALYs (21.8938) at the lowest cost ($962). Compared with HLD, the incremental cost-effectiveness ratio (ICER) of EXALT was $62,185, and $38,461 for ETO gas sterilization. CQ was dominated, indicating that it had a higher cost but was not more effective than HLD.
The researchers conducted a subanalysis of ERCP and Medicare patients to consider the recently approved TPT payment and the NTAP, in both hospital outpatient and inpatient settings. With TPT, EXALT had no cost after reimbursement, with a net saving of $962 per patient when compared with HLD, plus an increase in 0.033 QALYs (0.15%). The other procedures cost more and were less effective. With NTAP, EXALT had a net cost of $323 versus HLD, with a similar QALY benefit.
A Monte Carlo analysis of EXALT versus HLD found reductions in duodenoscope infection-related ICU admission (relative risk reduction, 0.996; 95% confidence interval, 0.936-1.0; number needed to treat, 79; 95% CI, 67-95) and death (RRR, 0.973; 95% CI, 0.552-0.998; number needed to treat, 556; 95% CI, 350-997).
In willingness-to-pay estimates from $50,000 to $100,000, EXALT was cost effective in 67.28% of trials with ICER under $100,000 per QALY.
The study did not consider medicolegal costs, which could lead to an underestimation of EXALT’s cost-effectiveness. The study also relied on available published information to determine cost per patient of hospital outbreaks in the United States and Europe since 2012, but the authors did not include costs of administrative sanctions, litigation, and poor publicity due to inconsistencies in the literature.
“While more research is needed to understand and quantify the determinants of the natural history after exposure to contaminated duodenoscopes, such as the risk of transmission and the subsequent development of serious clinical infections, this economic analysis demonstrates an approach using EXALT Model-D is cost effective in the U.S. health care system when compared to the currently utilized strategies of duodenoscope reprocessing,” the researchers concluded.
The study did not receive any funding. One of the authors is an employee and stockholder of Boston Scientific, which manufactures and markets EXALT. The other two authors have consulted for Boston Scientific.
, according to a new analysis.
The study compared the EXALT Model-D, HLD, culture-and-quarantine (CQ), and ethylene oxide sterilization (ETO). The results came from a simulated cohort of patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) to treat choledocholithiasis.
Although EXALT was the costliest option and HLD the cheapest, EXALT produced the most quality-adjusted life years (QALYs) and allowed the hospital to decrease net costs, and sensitivity analysis showed that it was a better option than HLD over a range of willingness-to-pay values.
“When evaluating technologies based on cost-effectiveness and additionally in the context of TPT [transitional passthrough] or NTAP [new technology add-on payment], the EXALT approach meets typically used cost-effectiveness thresholds compared to all other evaluated strategies and should be considered for standard practice,” wrote the authors, who were led by Ananya Das, MD, of the Arizona Centers for Digestive Health, Gilbert. The study was published in Techniques and Innovations in Gastrointestinal Endoscopy.
Duodenoscope contamination has resulted in outbreaks of various multidrug-resistant organisms in hospital settings, which has led to the publication of various reprocessing guidelines. Although many hospitals have adopted HLD protocols, others use additional or alternative reprocessing methods such as CQ or ETO. Despite these efforts, a recent Food and Drug Administration study found that 1.9%-22% of samples taken from duodenoscopes tested positive for bacteria of concern, such as pathogens. Those and other findings have led some to suggest that it would be best to move away from HLD, and instead employ sterilizable or disposable endoscopes.
In another study, The EXALT Model-D (Boston Scientific) had been shown to be a good alternative to standard reusable duodenoscopes.
The researchers used a Markov-model to determine the cost-effectiveness of EXALT Model-D against other approaches in a simulated cohort. They found that EXALT Model-D created the most QALYs (21.9265) at the highest cost ($3,000), and HLD the fewest QALYs (21.8938) at the lowest cost ($962). Compared with HLD, the incremental cost-effectiveness ratio (ICER) of EXALT was $62,185, and $38,461 for ETO gas sterilization. CQ was dominated, indicating that it had a higher cost but was not more effective than HLD.
The researchers conducted a subanalysis of ERCP and Medicare patients to consider the recently approved TPT payment and the NTAP, in both hospital outpatient and inpatient settings. With TPT, EXALT had no cost after reimbursement, with a net saving of $962 per patient when compared with HLD, plus an increase in 0.033 QALYs (0.15%). The other procedures cost more and were less effective. With NTAP, EXALT had a net cost of $323 versus HLD, with a similar QALY benefit.
A Monte Carlo analysis of EXALT versus HLD found reductions in duodenoscope infection-related ICU admission (relative risk reduction, 0.996; 95% confidence interval, 0.936-1.0; number needed to treat, 79; 95% CI, 67-95) and death (RRR, 0.973; 95% CI, 0.552-0.998; number needed to treat, 556; 95% CI, 350-997).
In willingness-to-pay estimates from $50,000 to $100,000, EXALT was cost effective in 67.28% of trials with ICER under $100,000 per QALY.
The study did not consider medicolegal costs, which could lead to an underestimation of EXALT’s cost-effectiveness. The study also relied on available published information to determine cost per patient of hospital outbreaks in the United States and Europe since 2012, but the authors did not include costs of administrative sanctions, litigation, and poor publicity due to inconsistencies in the literature.
“While more research is needed to understand and quantify the determinants of the natural history after exposure to contaminated duodenoscopes, such as the risk of transmission and the subsequent development of serious clinical infections, this economic analysis demonstrates an approach using EXALT Model-D is cost effective in the U.S. health care system when compared to the currently utilized strategies of duodenoscope reprocessing,” the researchers concluded.
The study did not receive any funding. One of the authors is an employee and stockholder of Boston Scientific, which manufactures and markets EXALT. The other two authors have consulted for Boston Scientific.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY