User login
This month in the journal CHEST®
Editor’s picks
Revisiting mild asthma: current knowledge and future needs. By Dr. A. Mohan, et al.
Treatment of Mycobacterium abscessus pulmonary disease. By Dr. D. Griffith, et al.
The utility of the rapid shallow breathing index in predicting successful extubation: A systematic review and meta-analysis. By Dr. K. Burns, et al.
National temporal trends in hospitalization and inpatient mortality in patients with pulmonary sarcoidosis in the United States between 2007 – 2018. By Dr. N. Obi Ogugua, et al.
How I Do It: Considering lung transplantation for patients with COVID-19. By Dr. S. Nathan.
Addressing race in pulmonary function testing by aligning intent and evidence with practice and perception. By Dr. N. Bhakta, et al.
Editor’s picks
Editor’s picks
Revisiting mild asthma: current knowledge and future needs. By Dr. A. Mohan, et al.
Treatment of Mycobacterium abscessus pulmonary disease. By Dr. D. Griffith, et al.
The utility of the rapid shallow breathing index in predicting successful extubation: A systematic review and meta-analysis. By Dr. K. Burns, et al.
National temporal trends in hospitalization and inpatient mortality in patients with pulmonary sarcoidosis in the United States between 2007 – 2018. By Dr. N. Obi Ogugua, et al.
How I Do It: Considering lung transplantation for patients with COVID-19. By Dr. S. Nathan.
Addressing race in pulmonary function testing by aligning intent and evidence with practice and perception. By Dr. N. Bhakta, et al.
Revisiting mild asthma: current knowledge and future needs. By Dr. A. Mohan, et al.
Treatment of Mycobacterium abscessus pulmonary disease. By Dr. D. Griffith, et al.
The utility of the rapid shallow breathing index in predicting successful extubation: A systematic review and meta-analysis. By Dr. K. Burns, et al.
National temporal trends in hospitalization and inpatient mortality in patients with pulmonary sarcoidosis in the United States between 2007 – 2018. By Dr. N. Obi Ogugua, et al.
How I Do It: Considering lung transplantation for patients with COVID-19. By Dr. S. Nathan.
Addressing race in pulmonary function testing by aligning intent and evidence with practice and perception. By Dr. N. Bhakta, et al.
The people’s paper
With this issue, we usher in a new era for CHEST Physician, as I hand over the reins of Editor-in-Chief to Angel Coz, MD, FCCP. I have had the pleasure of serving in this role over the last 4 years, and though I will still have the privilege of appearing within these pages with some frequency as I move into my new role as CHEST President, I would like to mark this milestone by passing along a few thoughts on how CHEST Physician has developed over the last few years, and reflecting on the goals I set for us way back in the January 2018 issue (on page 46 of that issue, for those of you holding on to our back issues).
I’ve always viewed CHEST Physician as “the People’s Paper” of CHEST. While we don’t feature first-run scientific manuscripts and authors aren’t likely to reference our articles in other publications, your editorial board and our partners at Frontline aim to give our readers a broad overview of recent publications and presentations in pulmonary, critical care, and sleep medicine, along with expert commentary about how those developments might affect the care we provide to our patients. I can’t thank our editorial board members enough for the hours they spend selecting a small number of items to feature among all of the new medical developments each month.
One of the main goals we had established over the last few years was to create more opportunities for CHEST Physician to serve as the voice of the members and leaders of the American College of Chest Physicians. We achieved the latter part of this goal, with leadership penning quarterly columns on actions of the Board of Regents, developments within the annual meeting, as well as ongoing columns from our NetWorks. And, we have also provided a more reliable voice for our members, with authors of our Sleep Strategies, Critical Care Commentary, and Pulmonary Perspectives columns providing a broader and more representative sample of our membership than ever before.
One of the areas where I would love to see more progress is with reader engagement. It has been a delight to receive feedback from CHEST members, even when the author is taking issue with something we have published. CHEST Physician will be a better publication than it already is with your ongoing input. Please, if you see something that we write that you particularly like (or don’t!) or if there’s something you’d like to see that we haven’t written, please reach out to us! You can always reach us at [email protected].
In closing, I want to thank all of the steadfast CHEST Physician readers for making my 4 years as Editor-in-Chief enjoyable and meaningful. While I am so pleased with the current state of this publication, I cannot wait to see its ongoing evolution under the leadership of Dr. Coz and his editorial board.
With this issue, we usher in a new era for CHEST Physician, as I hand over the reins of Editor-in-Chief to Angel Coz, MD, FCCP. I have had the pleasure of serving in this role over the last 4 years, and though I will still have the privilege of appearing within these pages with some frequency as I move into my new role as CHEST President, I would like to mark this milestone by passing along a few thoughts on how CHEST Physician has developed over the last few years, and reflecting on the goals I set for us way back in the January 2018 issue (on page 46 of that issue, for those of you holding on to our back issues).
I’ve always viewed CHEST Physician as “the People’s Paper” of CHEST. While we don’t feature first-run scientific manuscripts and authors aren’t likely to reference our articles in other publications, your editorial board and our partners at Frontline aim to give our readers a broad overview of recent publications and presentations in pulmonary, critical care, and sleep medicine, along with expert commentary about how those developments might affect the care we provide to our patients. I can’t thank our editorial board members enough for the hours they spend selecting a small number of items to feature among all of the new medical developments each month.
One of the main goals we had established over the last few years was to create more opportunities for CHEST Physician to serve as the voice of the members and leaders of the American College of Chest Physicians. We achieved the latter part of this goal, with leadership penning quarterly columns on actions of the Board of Regents, developments within the annual meeting, as well as ongoing columns from our NetWorks. And, we have also provided a more reliable voice for our members, with authors of our Sleep Strategies, Critical Care Commentary, and Pulmonary Perspectives columns providing a broader and more representative sample of our membership than ever before.
One of the areas where I would love to see more progress is with reader engagement. It has been a delight to receive feedback from CHEST members, even when the author is taking issue with something we have published. CHEST Physician will be a better publication than it already is with your ongoing input. Please, if you see something that we write that you particularly like (or don’t!) or if there’s something you’d like to see that we haven’t written, please reach out to us! You can always reach us at [email protected].
In closing, I want to thank all of the steadfast CHEST Physician readers for making my 4 years as Editor-in-Chief enjoyable and meaningful. While I am so pleased with the current state of this publication, I cannot wait to see its ongoing evolution under the leadership of Dr. Coz and his editorial board.
With this issue, we usher in a new era for CHEST Physician, as I hand over the reins of Editor-in-Chief to Angel Coz, MD, FCCP. I have had the pleasure of serving in this role over the last 4 years, and though I will still have the privilege of appearing within these pages with some frequency as I move into my new role as CHEST President, I would like to mark this milestone by passing along a few thoughts on how CHEST Physician has developed over the last few years, and reflecting on the goals I set for us way back in the January 2018 issue (on page 46 of that issue, for those of you holding on to our back issues).
I’ve always viewed CHEST Physician as “the People’s Paper” of CHEST. While we don’t feature first-run scientific manuscripts and authors aren’t likely to reference our articles in other publications, your editorial board and our partners at Frontline aim to give our readers a broad overview of recent publications and presentations in pulmonary, critical care, and sleep medicine, along with expert commentary about how those developments might affect the care we provide to our patients. I can’t thank our editorial board members enough for the hours they spend selecting a small number of items to feature among all of the new medical developments each month.
One of the main goals we had established over the last few years was to create more opportunities for CHEST Physician to serve as the voice of the members and leaders of the American College of Chest Physicians. We achieved the latter part of this goal, with leadership penning quarterly columns on actions of the Board of Regents, developments within the annual meeting, as well as ongoing columns from our NetWorks. And, we have also provided a more reliable voice for our members, with authors of our Sleep Strategies, Critical Care Commentary, and Pulmonary Perspectives columns providing a broader and more representative sample of our membership than ever before.
One of the areas where I would love to see more progress is with reader engagement. It has been a delight to receive feedback from CHEST members, even when the author is taking issue with something we have published. CHEST Physician will be a better publication than it already is with your ongoing input. Please, if you see something that we write that you particularly like (or don’t!) or if there’s something you’d like to see that we haven’t written, please reach out to us! You can always reach us at [email protected].
In closing, I want to thank all of the steadfast CHEST Physician readers for making my 4 years as Editor-in-Chief enjoyable and meaningful. While I am so pleased with the current state of this publication, I cannot wait to see its ongoing evolution under the leadership of Dr. Coz and his editorial board.
Clinical Edge Journal Scan Commentary: Migraine January 2022
Ferrari et al1 provided information on an open label extension to the “LIBERTY” study which investigated the use of erenumab in subjects with episodic migraine that have failed multiple prior preventive medications. The initial Calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) studies excluded more refractory patients. Most commercial insurances in the United States have a “step” policy that relates to use of these and other newer medications, meaning that the majority of patient in the US who receive these medications have previously tried other preventive medications. This raised the question whether migraine refractoriness is a negative predictive factor for erenumab.
This long-term open label study is more like the real-world use of erenumab, and as such the results are similar to what many practitioners are seeing in their clinical experience. Approximately 25% of subjects discontinued erenumab, mostly due to ineffectiveness. Adverse events were mild, and although erenumab has warnings for constipation and hypertension, this study did not show either as increasing over 2 years. Erenumab appeared to be tolerable over time. There were no newly noted safety signals in this study.
The efficacy of erenumab also appeared to be stable over time, without the development of tolerance to the medication. There is a slight decrease in the 50% responder rate at 2 years when these more refractory patients are compared to those that did not have multiple treatment failures. This study also looked at “functional parameters,” such as Migraine Disability Assessment (MIDAS) and Headache Impact Test (HIT-6), both of which were significantly improved over time.
Although there are some significant limitations in this study-primarily the fact that it is open label—this does give a more representative and real-world sample of patients who will be prescribed erenumab in the United States. Most practitioners will be glad to find that the long-term use of erenumab appears safe, and the efficacy remains stable, even in a more difficult-to-treat population.
A randomized controlled international study investigated the preventive use of occipital nerve blocks in migraine without aura.2 The majority of the literature for the use of occipital nerve blocks is for acute treatment, and arguably the most significant study prior to this was Friedman et al3 investigating the use of this procedure in the emergency ward. Prior occipital nerve studies have been inconclusive, and although occipital nerve blocks are considered standard of care for specific conditions in most headache centers, reimbursement is usually very limited. Insurance companies have quoted prior preventive occipital nerve studies to justify non-coverage of these procedures, making access to them for many patients very limited.
Occipital nerve blocks are not performed uniformly, both regarding the medications used—some practitioners use no steroids, some use lidocaine and bupivocaine—and regarding the placement of the injections. In this a small cohort study, 55 subjects were divided into four groups for intervention—one of which was a control group of saline—and all were given one 2.5 mL injection at a point in between the occipital protuberance and the mastoid process bilaterally. Due to adverse events (alopecia and cutaneous atrophy) in two of the triamcinolone groups, recruitment was halted for those two groups. Patients were assessed based on headache duration, frequency, and severity over a 4-week course.
Compared to baseline all interventional groups had significantly decreased headache severity, which did return closer to baseline during the final week. Headache duration was decreased in the first 2 weeks post-injection. Headache frequency was seen to return to baseline at week 4, but prior to that the groups injected with lidocaine had a significant decrease in migraine frequency, with an average decrease in headache days.
Occipital nerve blocks are performed frequently for migraine, occipital neuralgia, cervicogenic headache, and many other conditions with noted tenderness over the occiput. As noted above, they are not performed uniformly—sometimes they are given for acute headache pain or status migranosus, and other times they are used in regular intervals for prevention. This data does finally show a preventive benefit with occipital nerve blocks, and this may allow for modifications in how occipital nerve blocks are currently performed. Based on this study, if given preventively, occipital nerve blocks should only contain topical anesthetics, not steroids, and should be performed on an every 2-3 week basis.
The limitations of this study are significant as well. This is a very small cohort, and the injections were performed in only one manner (one bilateral injection), whereas many practitioners will target the greater and lesser branches of the occipital nerve individually. There were no exclusion criteria for subjects that already had occipital nerve blocks performed—those patients would be unblinded as there is a different sensation when injected with a topical anesthetic versus normal saline (normal saline does not cause burning subcutaneously).
These results should pave the way for further investigations in the use of occipital and other nerve blocks in the prevention of migraine. This should allow better access for our patients and the possibility of performing these procedures more uniformly in the future.
It can be challenging for many practitioners to determine which medication is ideal for individual situations. This is especially true when treating chronic migraine, where many potential complicating factors can influence positive to negative responses to treatment. The investigators here sought to determine which factors may potentially predict a positive response to galcanezumab.4
This is an observational study, where 156 subjects with a diagnosis of chronic migraine were enrolled. There was a 1-month run-in period where the following characteristics were collected: monthly headache days, monthly abortive medication intake, clinical features of migraine, and disability scores (MIDAS and HIT-6). These were tracked over a 3-month period after starting glacanezumab.
Approximately 40% of subjects experienced a 50% reduction in headache frequency. The better responders had a lower body mass index, fewer previously failed preventive medications, unilateral headache pain, and previous good response to triptan use. Surprisingly, the presence of medication overuse was associated with persistent improvement at 3 months as well, with over 60% of subjects with medication overuse no longer overusing acute medications at 3 months.
This study is helpful in identifying specific features that may allow a practitioner to better recommend CGRP mAb medications, such as galcanezumab. Chronic migraine can offer a challenge to even the best trained clinicians. Patients will often have multiple factors that have led to a conversion from episodic to chronic migraine, and a history of medication failures or intolerances. These patients are often referred specifically due to these challenges.
When deciding on a preventive medication for patients with chronic migraine, we often first consider which oral preventive medications may allow us to treat migraine in addition to another underlying issue—such as insomnia, depression, or hypertension. Although the oral class can improve other comorbidities, intolerance is significantly higher for most of these medications as well. The CGRP mAb class is somewhat more ideal for prevention of migraine; the focus when using this class is for migraine prevention alone, and the side effect profile is more tolerable for most patients. That said, if predictive factors were known a more individualized approach to migraine prevention would be possible.
The authors’ recognition of the factors associated with improvement in patients using glacanezumab allows this better individualization. Based on these results, patients with more unilateral pain, lower BMI, and good response to triptans could be recommended glacanuzumab with a great degree of confidence. This should be irrespective of even high frequency use of acute medications, as most of subjects in this study with medication overuse reverted after 3 months.
There is never a single ideal preventive or acute treatment for migraine in any population, however, recognizing factors that allow for an individualized approach improves the quality of life for our patients, and leaves them less disabled by migraine.
References
- Ferrari MD et al. Two-year efficacy and safety of erenumab in participants with episodic migraine and 2–4 prior preventive treatment failures: results from the LIBERTY study. J Neurol Neurosurg Psychiatry. 2021(Nov 29).
- Malekian N et al. Preventive effect of greater occipital nerve block on patients with episodic migraine: A randomized double‐blind placebo‐controlled clinical trial. Cephalalgia. 2021(Nov 17).
- Friedman BW et al. A Randomized, Sham-Controlled Trial of Bilateral Greater Occipital Nerve Blocks With Bupivacaine for Acute Migraine Patients Refractory to Standard Emergency Department Treatment With Metoclopramide. Headache. 2018(Oct);58(9):1427-34. https://doi.org/10.1111/head.13395.
- Vernieri F et al. Rapid response to galcanezumab and predictive factors in chronic migraine patients: A 3-month observational, longitudinal, cohort, multicenter, Italian real-life study. Eur J Neurol. 2021(Nov 26).
Ferrari et al1 provided information on an open label extension to the “LIBERTY” study which investigated the use of erenumab in subjects with episodic migraine that have failed multiple prior preventive medications. The initial Calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) studies excluded more refractory patients. Most commercial insurances in the United States have a “step” policy that relates to use of these and other newer medications, meaning that the majority of patient in the US who receive these medications have previously tried other preventive medications. This raised the question whether migraine refractoriness is a negative predictive factor for erenumab.
This long-term open label study is more like the real-world use of erenumab, and as such the results are similar to what many practitioners are seeing in their clinical experience. Approximately 25% of subjects discontinued erenumab, mostly due to ineffectiveness. Adverse events were mild, and although erenumab has warnings for constipation and hypertension, this study did not show either as increasing over 2 years. Erenumab appeared to be tolerable over time. There were no newly noted safety signals in this study.
The efficacy of erenumab also appeared to be stable over time, without the development of tolerance to the medication. There is a slight decrease in the 50% responder rate at 2 years when these more refractory patients are compared to those that did not have multiple treatment failures. This study also looked at “functional parameters,” such as Migraine Disability Assessment (MIDAS) and Headache Impact Test (HIT-6), both of which were significantly improved over time.
Although there are some significant limitations in this study-primarily the fact that it is open label—this does give a more representative and real-world sample of patients who will be prescribed erenumab in the United States. Most practitioners will be glad to find that the long-term use of erenumab appears safe, and the efficacy remains stable, even in a more difficult-to-treat population.
A randomized controlled international study investigated the preventive use of occipital nerve blocks in migraine without aura.2 The majority of the literature for the use of occipital nerve blocks is for acute treatment, and arguably the most significant study prior to this was Friedman et al3 investigating the use of this procedure in the emergency ward. Prior occipital nerve studies have been inconclusive, and although occipital nerve blocks are considered standard of care for specific conditions in most headache centers, reimbursement is usually very limited. Insurance companies have quoted prior preventive occipital nerve studies to justify non-coverage of these procedures, making access to them for many patients very limited.
Occipital nerve blocks are not performed uniformly, both regarding the medications used—some practitioners use no steroids, some use lidocaine and bupivocaine—and regarding the placement of the injections. In this a small cohort study, 55 subjects were divided into four groups for intervention—one of which was a control group of saline—and all were given one 2.5 mL injection at a point in between the occipital protuberance and the mastoid process bilaterally. Due to adverse events (alopecia and cutaneous atrophy) in two of the triamcinolone groups, recruitment was halted for those two groups. Patients were assessed based on headache duration, frequency, and severity over a 4-week course.
Compared to baseline all interventional groups had significantly decreased headache severity, which did return closer to baseline during the final week. Headache duration was decreased in the first 2 weeks post-injection. Headache frequency was seen to return to baseline at week 4, but prior to that the groups injected with lidocaine had a significant decrease in migraine frequency, with an average decrease in headache days.
Occipital nerve blocks are performed frequently for migraine, occipital neuralgia, cervicogenic headache, and many other conditions with noted tenderness over the occiput. As noted above, they are not performed uniformly—sometimes they are given for acute headache pain or status migranosus, and other times they are used in regular intervals for prevention. This data does finally show a preventive benefit with occipital nerve blocks, and this may allow for modifications in how occipital nerve blocks are currently performed. Based on this study, if given preventively, occipital nerve blocks should only contain topical anesthetics, not steroids, and should be performed on an every 2-3 week basis.
The limitations of this study are significant as well. This is a very small cohort, and the injections were performed in only one manner (one bilateral injection), whereas many practitioners will target the greater and lesser branches of the occipital nerve individually. There were no exclusion criteria for subjects that already had occipital nerve blocks performed—those patients would be unblinded as there is a different sensation when injected with a topical anesthetic versus normal saline (normal saline does not cause burning subcutaneously).
These results should pave the way for further investigations in the use of occipital and other nerve blocks in the prevention of migraine. This should allow better access for our patients and the possibility of performing these procedures more uniformly in the future.
It can be challenging for many practitioners to determine which medication is ideal for individual situations. This is especially true when treating chronic migraine, where many potential complicating factors can influence positive to negative responses to treatment. The investigators here sought to determine which factors may potentially predict a positive response to galcanezumab.4
This is an observational study, where 156 subjects with a diagnosis of chronic migraine were enrolled. There was a 1-month run-in period where the following characteristics were collected: monthly headache days, monthly abortive medication intake, clinical features of migraine, and disability scores (MIDAS and HIT-6). These were tracked over a 3-month period after starting glacanezumab.
Approximately 40% of subjects experienced a 50% reduction in headache frequency. The better responders had a lower body mass index, fewer previously failed preventive medications, unilateral headache pain, and previous good response to triptan use. Surprisingly, the presence of medication overuse was associated with persistent improvement at 3 months as well, with over 60% of subjects with medication overuse no longer overusing acute medications at 3 months.
This study is helpful in identifying specific features that may allow a practitioner to better recommend CGRP mAb medications, such as galcanezumab. Chronic migraine can offer a challenge to even the best trained clinicians. Patients will often have multiple factors that have led to a conversion from episodic to chronic migraine, and a history of medication failures or intolerances. These patients are often referred specifically due to these challenges.
When deciding on a preventive medication for patients with chronic migraine, we often first consider which oral preventive medications may allow us to treat migraine in addition to another underlying issue—such as insomnia, depression, or hypertension. Although the oral class can improve other comorbidities, intolerance is significantly higher for most of these medications as well. The CGRP mAb class is somewhat more ideal for prevention of migraine; the focus when using this class is for migraine prevention alone, and the side effect profile is more tolerable for most patients. That said, if predictive factors were known a more individualized approach to migraine prevention would be possible.
The authors’ recognition of the factors associated with improvement in patients using glacanezumab allows this better individualization. Based on these results, patients with more unilateral pain, lower BMI, and good response to triptans could be recommended glacanuzumab with a great degree of confidence. This should be irrespective of even high frequency use of acute medications, as most of subjects in this study with medication overuse reverted after 3 months.
There is never a single ideal preventive or acute treatment for migraine in any population, however, recognizing factors that allow for an individualized approach improves the quality of life for our patients, and leaves them less disabled by migraine.
References
- Ferrari MD et al. Two-year efficacy and safety of erenumab in participants with episodic migraine and 2–4 prior preventive treatment failures: results from the LIBERTY study. J Neurol Neurosurg Psychiatry. 2021(Nov 29).
- Malekian N et al. Preventive effect of greater occipital nerve block on patients with episodic migraine: A randomized double‐blind placebo‐controlled clinical trial. Cephalalgia. 2021(Nov 17).
- Friedman BW et al. A Randomized, Sham-Controlled Trial of Bilateral Greater Occipital Nerve Blocks With Bupivacaine for Acute Migraine Patients Refractory to Standard Emergency Department Treatment With Metoclopramide. Headache. 2018(Oct);58(9):1427-34. https://doi.org/10.1111/head.13395.
- Vernieri F et al. Rapid response to galcanezumab and predictive factors in chronic migraine patients: A 3-month observational, longitudinal, cohort, multicenter, Italian real-life study. Eur J Neurol. 2021(Nov 26).
Ferrari et al1 provided information on an open label extension to the “LIBERTY” study which investigated the use of erenumab in subjects with episodic migraine that have failed multiple prior preventive medications. The initial Calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) studies excluded more refractory patients. Most commercial insurances in the United States have a “step” policy that relates to use of these and other newer medications, meaning that the majority of patient in the US who receive these medications have previously tried other preventive medications. This raised the question whether migraine refractoriness is a negative predictive factor for erenumab.
This long-term open label study is more like the real-world use of erenumab, and as such the results are similar to what many practitioners are seeing in their clinical experience. Approximately 25% of subjects discontinued erenumab, mostly due to ineffectiveness. Adverse events were mild, and although erenumab has warnings for constipation and hypertension, this study did not show either as increasing over 2 years. Erenumab appeared to be tolerable over time. There were no newly noted safety signals in this study.
The efficacy of erenumab also appeared to be stable over time, without the development of tolerance to the medication. There is a slight decrease in the 50% responder rate at 2 years when these more refractory patients are compared to those that did not have multiple treatment failures. This study also looked at “functional parameters,” such as Migraine Disability Assessment (MIDAS) and Headache Impact Test (HIT-6), both of which were significantly improved over time.
Although there are some significant limitations in this study-primarily the fact that it is open label—this does give a more representative and real-world sample of patients who will be prescribed erenumab in the United States. Most practitioners will be glad to find that the long-term use of erenumab appears safe, and the efficacy remains stable, even in a more difficult-to-treat population.
A randomized controlled international study investigated the preventive use of occipital nerve blocks in migraine without aura.2 The majority of the literature for the use of occipital nerve blocks is for acute treatment, and arguably the most significant study prior to this was Friedman et al3 investigating the use of this procedure in the emergency ward. Prior occipital nerve studies have been inconclusive, and although occipital nerve blocks are considered standard of care for specific conditions in most headache centers, reimbursement is usually very limited. Insurance companies have quoted prior preventive occipital nerve studies to justify non-coverage of these procedures, making access to them for many patients very limited.
Occipital nerve blocks are not performed uniformly, both regarding the medications used—some practitioners use no steroids, some use lidocaine and bupivocaine—and regarding the placement of the injections. In this a small cohort study, 55 subjects were divided into four groups for intervention—one of which was a control group of saline—and all were given one 2.5 mL injection at a point in between the occipital protuberance and the mastoid process bilaterally. Due to adverse events (alopecia and cutaneous atrophy) in two of the triamcinolone groups, recruitment was halted for those two groups. Patients were assessed based on headache duration, frequency, and severity over a 4-week course.
Compared to baseline all interventional groups had significantly decreased headache severity, which did return closer to baseline during the final week. Headache duration was decreased in the first 2 weeks post-injection. Headache frequency was seen to return to baseline at week 4, but prior to that the groups injected with lidocaine had a significant decrease in migraine frequency, with an average decrease in headache days.
Occipital nerve blocks are performed frequently for migraine, occipital neuralgia, cervicogenic headache, and many other conditions with noted tenderness over the occiput. As noted above, they are not performed uniformly—sometimes they are given for acute headache pain or status migranosus, and other times they are used in regular intervals for prevention. This data does finally show a preventive benefit with occipital nerve blocks, and this may allow for modifications in how occipital nerve blocks are currently performed. Based on this study, if given preventively, occipital nerve blocks should only contain topical anesthetics, not steroids, and should be performed on an every 2-3 week basis.
The limitations of this study are significant as well. This is a very small cohort, and the injections were performed in only one manner (one bilateral injection), whereas many practitioners will target the greater and lesser branches of the occipital nerve individually. There were no exclusion criteria for subjects that already had occipital nerve blocks performed—those patients would be unblinded as there is a different sensation when injected with a topical anesthetic versus normal saline (normal saline does not cause burning subcutaneously).
These results should pave the way for further investigations in the use of occipital and other nerve blocks in the prevention of migraine. This should allow better access for our patients and the possibility of performing these procedures more uniformly in the future.
It can be challenging for many practitioners to determine which medication is ideal for individual situations. This is especially true when treating chronic migraine, where many potential complicating factors can influence positive to negative responses to treatment. The investigators here sought to determine which factors may potentially predict a positive response to galcanezumab.4
This is an observational study, where 156 subjects with a diagnosis of chronic migraine were enrolled. There was a 1-month run-in period where the following characteristics were collected: monthly headache days, monthly abortive medication intake, clinical features of migraine, and disability scores (MIDAS and HIT-6). These were tracked over a 3-month period after starting glacanezumab.
Approximately 40% of subjects experienced a 50% reduction in headache frequency. The better responders had a lower body mass index, fewer previously failed preventive medications, unilateral headache pain, and previous good response to triptan use. Surprisingly, the presence of medication overuse was associated with persistent improvement at 3 months as well, with over 60% of subjects with medication overuse no longer overusing acute medications at 3 months.
This study is helpful in identifying specific features that may allow a practitioner to better recommend CGRP mAb medications, such as galcanezumab. Chronic migraine can offer a challenge to even the best trained clinicians. Patients will often have multiple factors that have led to a conversion from episodic to chronic migraine, and a history of medication failures or intolerances. These patients are often referred specifically due to these challenges.
When deciding on a preventive medication for patients with chronic migraine, we often first consider which oral preventive medications may allow us to treat migraine in addition to another underlying issue—such as insomnia, depression, or hypertension. Although the oral class can improve other comorbidities, intolerance is significantly higher for most of these medications as well. The CGRP mAb class is somewhat more ideal for prevention of migraine; the focus when using this class is for migraine prevention alone, and the side effect profile is more tolerable for most patients. That said, if predictive factors were known a more individualized approach to migraine prevention would be possible.
The authors’ recognition of the factors associated with improvement in patients using glacanezumab allows this better individualization. Based on these results, patients with more unilateral pain, lower BMI, and good response to triptans could be recommended glacanuzumab with a great degree of confidence. This should be irrespective of even high frequency use of acute medications, as most of subjects in this study with medication overuse reverted after 3 months.
There is never a single ideal preventive or acute treatment for migraine in any population, however, recognizing factors that allow for an individualized approach improves the quality of life for our patients, and leaves them less disabled by migraine.
References
- Ferrari MD et al. Two-year efficacy and safety of erenumab in participants with episodic migraine and 2–4 prior preventive treatment failures: results from the LIBERTY study. J Neurol Neurosurg Psychiatry. 2021(Nov 29).
- Malekian N et al. Preventive effect of greater occipital nerve block on patients with episodic migraine: A randomized double‐blind placebo‐controlled clinical trial. Cephalalgia. 2021(Nov 17).
- Friedman BW et al. A Randomized, Sham-Controlled Trial of Bilateral Greater Occipital Nerve Blocks With Bupivacaine for Acute Migraine Patients Refractory to Standard Emergency Department Treatment With Metoclopramide. Headache. 2018(Oct);58(9):1427-34. https://doi.org/10.1111/head.13395.
- Vernieri F et al. Rapid response to galcanezumab and predictive factors in chronic migraine patients: A 3-month observational, longitudinal, cohort, multicenter, Italian real-life study. Eur J Neurol. 2021(Nov 26).
Much lower risk of false-positive breast screen in Norway versus U.S.
Nearly 1 in 5 women who receive the recommended 10 biennial screening rounds for breast cancer in Norway will get a false positive result, and 1 in 20 women will receive a false positive result that leads to an invasive procedure, a new analysis shows.
While the risk may seem high, it is actually much lower than what researchers have reported in the U.S., the study authors note in their paper, published online Dec. 21 in Cancer.
“I am proud about the low rate of recalls we have in Norway and Europe – and hope we can keep it that low for the future,” said senior author Solveig Hofvind, PhD, head of BreastScreen Norway, a nationwide screening program that invites women aged 50 to 69 to mammographic screening every other year.
“The double reading in Europe is probably the main reason for the lower rate in Europe compared to the U.S., where single reading is used,” she said in an interview.
Until now, Dr. Hofvind and her colleagues say, no studies have been performed using exclusively empirical data to describe the cumulative risk of experiencing a false positive screening result in Europe because of the need for long-term follow-up and complete data registration.
For their study, the researchers turned to the Cancer Registry of Norway, which administers BreastScreen Norway. They focused on data from 1995 to 2019 on women aged 50 to 69 years who had attended one or more screening rounds and could potentially attend all 10 screening examinations over the 20-year period.
Women were excluded if they were diagnosed with breast cancer before attending screening, participated in interventional research, self-referred for screening, were recalled due to self-reported symptoms or technically inadequate mammograms, or declined follow-up after a positive screen.
Among more than 421,000 women who underwent nearly 1.9 million screening examinations, 11.3% experienced at least one false positive result and 3.3% experienced at least one false positive involving an invasive procedure, such as fine-needle aspiration cytology, core-needle biopsy, or open biopsy.
The cumulative risk of experiencing a first false positive screen was 18.0% and that of experiencing a false positive that involved an invasive procedure was 5.01%. Adjusting for irregular attendance, age at screening, or the number of screens attended had little effect on the estimates.
The results closely match earlier findings from Norway that have been based on assumptions rather than exclusively empirical data. However, these findings differ from results reported in U.S. studies, which have relied largely on data from the Breast Cancer Surveillance Consortium, the researchers say.
“The latter have indicated that, for women who initiate biennial screening at the age of 50 years, the cumulative risk after 10 years is 42% for experiencing at least one false-positive screening result and 6.4% for experiencing at least one false-positive screening result involving an invasive procedure,” Dr. Hofvind and her colleagues write.
Several principal investigators with the Breast Cancer Surveillance Consortium did not respond or were unavailable for comment when contacted by this news organization.
However, the study authors highlighted several factors that could help explain the discrepancy between the U.S. and European results.
In addition to double mammogram reading, “European guidelines recommend that breast radiologists read 3,500 to 11,000 mammograms annually, whereas 960 every 2 years are required by the U.S. Mammography Quality Standards Act,” the researchers note. They also point out that previous screening mammograms are readily available in Norway, whereas this is not always the case in the U.S.
“False-positive screening results are a part of the screening for breast cancer – and the women need to be informed about the risk,” Dr. Hofvind concluded. “The screening programs should aim to keep the rate as low as possible for the women [given] the costs.”
The study was supported by the Dam Foundation via the Norwegian Breast Cancer Society.
A version of this article first appeared on Medscape.com.
Nearly 1 in 5 women who receive the recommended 10 biennial screening rounds for breast cancer in Norway will get a false positive result, and 1 in 20 women will receive a false positive result that leads to an invasive procedure, a new analysis shows.
While the risk may seem high, it is actually much lower than what researchers have reported in the U.S., the study authors note in their paper, published online Dec. 21 in Cancer.
“I am proud about the low rate of recalls we have in Norway and Europe – and hope we can keep it that low for the future,” said senior author Solveig Hofvind, PhD, head of BreastScreen Norway, a nationwide screening program that invites women aged 50 to 69 to mammographic screening every other year.
“The double reading in Europe is probably the main reason for the lower rate in Europe compared to the U.S., where single reading is used,” she said in an interview.
Until now, Dr. Hofvind and her colleagues say, no studies have been performed using exclusively empirical data to describe the cumulative risk of experiencing a false positive screening result in Europe because of the need for long-term follow-up and complete data registration.
For their study, the researchers turned to the Cancer Registry of Norway, which administers BreastScreen Norway. They focused on data from 1995 to 2019 on women aged 50 to 69 years who had attended one or more screening rounds and could potentially attend all 10 screening examinations over the 20-year period.
Women were excluded if they were diagnosed with breast cancer before attending screening, participated in interventional research, self-referred for screening, were recalled due to self-reported symptoms or technically inadequate mammograms, or declined follow-up after a positive screen.
Among more than 421,000 women who underwent nearly 1.9 million screening examinations, 11.3% experienced at least one false positive result and 3.3% experienced at least one false positive involving an invasive procedure, such as fine-needle aspiration cytology, core-needle biopsy, or open biopsy.
The cumulative risk of experiencing a first false positive screen was 18.0% and that of experiencing a false positive that involved an invasive procedure was 5.01%. Adjusting for irregular attendance, age at screening, or the number of screens attended had little effect on the estimates.
The results closely match earlier findings from Norway that have been based on assumptions rather than exclusively empirical data. However, these findings differ from results reported in U.S. studies, which have relied largely on data from the Breast Cancer Surveillance Consortium, the researchers say.
“The latter have indicated that, for women who initiate biennial screening at the age of 50 years, the cumulative risk after 10 years is 42% for experiencing at least one false-positive screening result and 6.4% for experiencing at least one false-positive screening result involving an invasive procedure,” Dr. Hofvind and her colleagues write.
Several principal investigators with the Breast Cancer Surveillance Consortium did not respond or were unavailable for comment when contacted by this news organization.
However, the study authors highlighted several factors that could help explain the discrepancy between the U.S. and European results.
In addition to double mammogram reading, “European guidelines recommend that breast radiologists read 3,500 to 11,000 mammograms annually, whereas 960 every 2 years are required by the U.S. Mammography Quality Standards Act,” the researchers note. They also point out that previous screening mammograms are readily available in Norway, whereas this is not always the case in the U.S.
“False-positive screening results are a part of the screening for breast cancer – and the women need to be informed about the risk,” Dr. Hofvind concluded. “The screening programs should aim to keep the rate as low as possible for the women [given] the costs.”
The study was supported by the Dam Foundation via the Norwegian Breast Cancer Society.
A version of this article first appeared on Medscape.com.
Nearly 1 in 5 women who receive the recommended 10 biennial screening rounds for breast cancer in Norway will get a false positive result, and 1 in 20 women will receive a false positive result that leads to an invasive procedure, a new analysis shows.
While the risk may seem high, it is actually much lower than what researchers have reported in the U.S., the study authors note in their paper, published online Dec. 21 in Cancer.
“I am proud about the low rate of recalls we have in Norway and Europe – and hope we can keep it that low for the future,” said senior author Solveig Hofvind, PhD, head of BreastScreen Norway, a nationwide screening program that invites women aged 50 to 69 to mammographic screening every other year.
“The double reading in Europe is probably the main reason for the lower rate in Europe compared to the U.S., where single reading is used,” she said in an interview.
Until now, Dr. Hofvind and her colleagues say, no studies have been performed using exclusively empirical data to describe the cumulative risk of experiencing a false positive screening result in Europe because of the need for long-term follow-up and complete data registration.
For their study, the researchers turned to the Cancer Registry of Norway, which administers BreastScreen Norway. They focused on data from 1995 to 2019 on women aged 50 to 69 years who had attended one or more screening rounds and could potentially attend all 10 screening examinations over the 20-year period.
Women were excluded if they were diagnosed with breast cancer before attending screening, participated in interventional research, self-referred for screening, were recalled due to self-reported symptoms or technically inadequate mammograms, or declined follow-up after a positive screen.
Among more than 421,000 women who underwent nearly 1.9 million screening examinations, 11.3% experienced at least one false positive result and 3.3% experienced at least one false positive involving an invasive procedure, such as fine-needle aspiration cytology, core-needle biopsy, or open biopsy.
The cumulative risk of experiencing a first false positive screen was 18.0% and that of experiencing a false positive that involved an invasive procedure was 5.01%. Adjusting for irregular attendance, age at screening, or the number of screens attended had little effect on the estimates.
The results closely match earlier findings from Norway that have been based on assumptions rather than exclusively empirical data. However, these findings differ from results reported in U.S. studies, which have relied largely on data from the Breast Cancer Surveillance Consortium, the researchers say.
“The latter have indicated that, for women who initiate biennial screening at the age of 50 years, the cumulative risk after 10 years is 42% for experiencing at least one false-positive screening result and 6.4% for experiencing at least one false-positive screening result involving an invasive procedure,” Dr. Hofvind and her colleagues write.
Several principal investigators with the Breast Cancer Surveillance Consortium did not respond or were unavailable for comment when contacted by this news organization.
However, the study authors highlighted several factors that could help explain the discrepancy between the U.S. and European results.
In addition to double mammogram reading, “European guidelines recommend that breast radiologists read 3,500 to 11,000 mammograms annually, whereas 960 every 2 years are required by the U.S. Mammography Quality Standards Act,” the researchers note. They also point out that previous screening mammograms are readily available in Norway, whereas this is not always the case in the U.S.
“False-positive screening results are a part of the screening for breast cancer – and the women need to be informed about the risk,” Dr. Hofvind concluded. “The screening programs should aim to keep the rate as low as possible for the women [given] the costs.”
The study was supported by the Dam Foundation via the Norwegian Breast Cancer Society.
A version of this article first appeared on Medscape.com.
Progress still needed for pregnant and postpartum gastroenterologists
Despite increasing numbers joining the field, women remain a minority group in gastroenterology, where they constitute only 18% of these physicians.1 Additionally, women continue to be underrepresented among senior faculty and in leadership roles in both academic and private practice settings.2 While women now make up a majority of medical school matriculants3,4 women trainees are frequently dissuaded from pursuing specialty fellowships following residency, particularly in procedurally based fields like gastroenterology, because of perceived incompatibility with childbearing and child-rearing.5-8 For many who choose to enter the field despite these challenges, gastroenterology training and early practice often coincide with childbearing years.910 These structural impediments may contribute to the “leaky pipeline” and female physician attrition during the first decade of independent practice after fellowship.11-13 Urgent changes are needed in order to retain and support clinicians and physician-scientists through this period so that they, their offspring, their patients, and the field are able to thrive.
Fertility and pregnancy
The decision to have a child is a major milestone for many physicians and often occurs during gastroenterology training or early practice.10 Medical-training and early-career environments are not yet optimized to support women who become pregnant. At baseline, the formative years of a career are challenging ones, punctuated by long hours and both intellectually and emotionally demanding work. They are also often physically grueling, particularly while one is learning and becoming efficient in endoscopy. The ergonomics in the endoscopy suite (as in other areas of medicine) are not optimized for physicians of shorter stature, smaller hand sizes, and those who may have difficulty pushing a several-hundred-pound endoscopy cart bedside, all of which contribute to increased injury risk for female proceduralists.7,14-16 Methods to reduce endoscopic injuries in pregnant endoscopists have not yet been studied. Additionally, the existence of maternity and gender bias has been well-documented, in our field and beyond.17-20 Not surprisingly, women in gastroenterology commonly report delayed childbearing, with expected consequences, including increased infertility rates, compared with nonphysician peers.21 After 5 and 10 years as attendings, female gastroenterologists continue to report fewer children than male colleagues.22,23 Once pregnant, there are a number of field-specific challenges to navigate. These include decisions about the safety of performing procedures involving fluoroscopy or high infectious risk, particularly early in pregnancy when organogenesis occurs.7,24 Additionally, engaging in appropriate obstetric care can be challenging given the need for regular physician and ultrasound appointments.
Simple, cost-efficient interventions may be effective in decreasing infertility rates, pregnancy loss, and poor physician experiences during pregnancy. For one, all gastroenterology divisions could craft written policies that include a no-tolerance approach to expressions of maternity bias against pregnant or postpartum trainees and faculty.12,25 Additionally, ergonomic improvements, such as standing pads, dial extenders, and adjusted screen heights may decrease injury rates and increase comfort for female endoscopists.26,27 There should also be a no-penalty, no-questions-asked approach for any female endoscopist who defers performance of an obstetrically high-risk procedure to a nonpregnant colleague. Additionally, pregnant gastroenterologists should be supported in obtaining high-quality obstetric care. At an individual level, nonpregnant gastroenterologists, and particularly male allies, can support pregnant colleagues by agreeing to perform higher-risk procedures, stepping in if a fellow is unable to perform endoscopy because of pregnancy, and by offering to push the endoscopy cart on behalf of a pregnant colleague to bedside, if necessary.10,28
Parental leave
Following delivery, parental leave presents an additional challenge for the physician parent. Paid maternal leave has been associated with improved child and maternal outcomes and is widely available to physicians outside the United States.29,30 At present, duration of leave varies significantly by career stage (fellows versus attending), practice setting (academic center versus private practice), and geographic location. The American Academy of Pediatrics recommends a minimum of 12 weeks of leave.31 This length has been associated with lower rates of postpartum depression and higher rates of sustained breastfeeding, with subsequent improved health outcomes for mother and child.32-34 An increasing number of states have passed laws mandating minimum paid and unpaid parental leave time (for example, in Massachusetts, gastroenterology trainees and faculty are afforded 12 weeks of leave, in accordance with state law).35 Recent changes to board eligibility and training requirements via the American Board of Medical Specialties and the American Council for Graduate Medical Education now provide 6 weeks for parental leave. This is an improvement over prior policies which rendered many physician-parents board-ineligible if they took more than 4 weeks of leave, although it must be noted that even the revised policies allow for less time than either that of Obstetricians and Gynecologists or than the American Academy of Pediatrics recommends.
Our data, presented at the 2021 ACG conference, suggest that many trainees report receiving 4 weeks or less of parental leave, despite the ACGME and ABMS policies described above. We also found that physicians were frequently not aware of their institution or division leave policies.10 Ideally, all gastroenterology divisions in the United States would follow the recommended leave duration set forth by the medical societies of specialties that care for pregnant and postpartum mothers and their infants. Additionally, the impact of leave time on graduation and board eligibility, as well as academic and practice promotion, should be made clear at the time of leave and should minimize adverse consequences for the careers of pregnant and postpartum gastroenterologists. Gastroenterology trainees and faculty should be educated in the existence and details of their institution or practice policies, and these policies should be made readily available to all physicians and administrators.
Postpartum period
The transition back to work is a challenging one for mothers in all fields of medicine, particularly for those returning to procedurally based subspecialties such as gastroenterology. This is especially true for trainees and faculty who have returned to work sooner than the recommended 12 weeks and for those who are post cesarean section, for whom physical healing may not be complete. Long days performing endoscopy may be physically challenging or impossible for some women during the postpartum period. Additionally, expressing breast milk, a metabolically intensive activity, also necessitates time, space, and privacy to perform and is frequently made more difficult by insufficient lactation accommodations. The COVID-19 pandemic has increased logistic challenges for lactating mothers, because of the need for well-ventilated lactation spaces to minimize infectious risk.19 Our colleagues have reported pumping in their vehicles, in supply closets, and in spaces that require so much travel time (in addition to time required to express milk, store milk, and clean pump equipment) that the practice was unsustainable, and the physician stopped breastfeeding prematurely.36
The benefits of breastfeeding for mother and infant are well-established, and exclusive breastfeeding for the first 6 months of life is supported by the American College of Obstetricians and Gynecologists, whose position statement reads as follows: “Policies that protect the right of a woman and her child to breastfeed ... and that accommodate milk expression, such as ... paid maternity leave, on-site childcare, break time for expressing milk, and a clean, private location for expressing milk, are essential to sustaining breastfeeding.”37 We would add to these recommendations provision of dedicated milk storage space and establishment of clear, supportive policies that allow lactating physicians to breastfeed and express breast milk if they choose without career penalty. Several institutions offer scheduled protected clinical time and modified work relative value units (RVU) for lactating physicians, such that returning parents can have protected time for expressing breast milk and still meet RVU targets.38 Additionally, many academic institutions offer productivity adjustments for tenure-track faculty who have recently had children.
Creating a more supportive environment for women gastroenterologists who desire children allows the field to be more representative of our patient population and has been shown to positively impact outcomes from improved colorectal cancer screening rates to more guideline-directed informed consent conversations.39-41 Gastroenterology should comprise a physician workforce predicated on clinical and research excellence alone and should not require its practitioners to delay or abstain from pregnancy and child rearing. Robust, clear, and generous parental leave and postpartum accommodations will allow the field to retain and promote talented physicians, who will then contribute to the betterment of patients and the field over decades.
Dr. Rabinowitz is a faculty member in the department of medicine and division of gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Feld is a transplant hepatology fellow, division of gastroenterology, department of medicine, University of Washington, Seattle. Dr. Rabinowitz and Dr. Feld have no conflicts of interest to disclose.
References
1. AAMC. Diversity in Medicine: Facts and Figures 2019. 2018.
2. Colleges AoAM. The State of Women in Academic Medicine: The Pipeline and Pathways to Leadership, 2015-2016. 2016. www.aamc.org/download/481206/data/2015table11.pdf.
3. AAMC. Table B-3: Total U.S. Medical School Enrollment by Race/Ethnicity and Sex, 2014-2015 through 2018-2019, 2019.
4. Rabinowitz LG. Recognizing blind spots – a remedy for gender bias in medicine? (N Engl. J Med. 2018; 378[24]: 2253-5).
5. Douglas PS et al. Career preferences and perceptions of cardiology among US internal medicine trainees: Factors influencing cardiology career choice. JAMA Cardiol 2018; 3(8):682-91.
6. Stack SW et al. Childbearing decisions in residency: A multicenter survey of female residents. Acad Med 2020;95(10):1550-7.
7. David YN et al. Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges. Gastroenterology 2021;161(3):756-60.
8. Rembacken BJ et al. Barriers and bias standing in the way of female trainees wanting to learn advanced endoscopy. United European Gastroenterol J. 2019;7(8):1141-5.
9. Arlow FL et al. Gastroenterology training and career choices: A prospective longitudinal study of the impact of gender and of managed care. Am J Gastroenterol. 2002;97(2):459-69.
10. Feld L et al. Parental leave for gastroenterology fellows: A national survey of current fellows. Am J Gastroenterol. 2021;116:S611-2.
11. Rabinowitz LG et al. Addressing gender in gastroenterology: opportunities for change. Gastrointest Endosc. 2020;91(1):155-61.
12. Feld LD. Baby steps in the right direction: Toward a parental leave policy for gastroenterology fellows. Am J Gastroenterol. 2021;116(3):505-8.
13. Feld LD. Interviewing for two. Am J Gastroenterol. 2020;116(3):445-6
14. Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93(5):1047-56.e5.
15. Harvin G. Review of musculoskeletal injuries and prevention in the endoscopy practitioner. J Clin Gastroenterol. 2014;48(7):590-4.
16. LabX Oecs. www.labx.com/product/endoscopy-cart (accessed 2021 Nov 19.
17. Heilman ME and Okimoto TG. Motherhood: A potential source of bias in employment decisions. J Appl Psychol. 2008;93(1):189-98.
18. Robinson K et al. Racism, bias, and discrimination as modifiable barriers to breastfeeding for African American women: A scoping review of the literature. J Midwifery Womens Health. 2019;64(6):734-42.
19. Rabinowitz LG and Rabinowitz DG. Women on the Frontline: A Changed Workforce and the Fight Against COVID-19. Acad Med. 2021 Jun 1;96(6):808-12.
20. Rabinowitz LG et al. Gender in the endoscopy suite. Lancet Gastroenterol Hepatol. 2020 Dec;5(12):1032-4.
21. Stentz NC et al. Fertility and childbearing among American female physicians. J Womens Health. 2016; 25(10):1059-65.
22. Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol. 2005;100(2):259-64.
23. Singh A et al. Women in gastroenterology committee of American College of G. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103(7):1589-95.
24. Krueger KJ and Hoffman BJ. Radiation exposure during gastroenterologic fluoroscopy: Risk assessment for pregnant workers. Am J Gastroenterol. 1992;87(4):429-31.
25. Krause ML et al. Impact of pregnancy and gender on internal medicine resident evaluations: A retrospective cohort study. J Gen Intern Med. 2017;32(6):648-53.
26. Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
27. David YN et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Am J Gastroenterol. 2021;116(3):539-50.
28. Bilal M et al. The need for allyship in achieving gender equity in gastroenterology. Am J Gastroenterol. 2021 Oct 19. doi: 10.14309/ajg.0000000000001508. Online ahead of print.
29. Jou J et al. Paid maternity leave in the United States: Associations with maternal and infant health. Matern Child Health J. 2018;22(2):216-25.
30. Aitken Z et al. The maternal health outcomes of paid maternity leave: A systematic review. Soc Sci Med. 2015;130:32-41.
31. Dodson NA and Talib HJ. Paid parental leave for mothers and fathers can improve physician wellness. AAP News. 2020 Jul 1. https://publications.aap.org/aapnews/news/12432.
32. Kornfeind KR and Sipsma HL. Exploring the link between maternity leave and postpartum depression. Womens Health Issues 2018;28(4):321-6.
33. Navarro-Rosenblatt D and Garmendia ML. Maternity leave and its impact on breastfeeding: A review of the literature. Breastfeed Med 2018;13(9):589-97.
34. Stack SW et al. Maternity leave in residency: A multicenter study of determinants and wellness outcomes. Acad Med. 2019;94(11):1738-45.
35. Mass.gov. Paid Family and Medical Leave Information for Massachusetts Employers. 2020.
36. Ares Segura S et al. en representacion del Comite de Lactancia Materna de la Asociacion Espanola de P. [The importance of maternal nutrition during breastfeeding: Do breastfeeding mothers need nutritional supplements?]. An Pediatr. (Barc) 2016;84(6):347 e1-7.
37. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 658: Optimizing Support for Breastfeeding as Part of Obstetric Practice. Obstet Gynecol. 2016;127(2):e86-92.
38. Porter KK et al. A lactation credit model to support breastfeeding in radiology: The new gold standard to support “liquid gold.” Clin Imaging 2021;80:16-8.
39. Davis J et al. Clinical practice patterns suggest female patients prefer female endoscopists. Dig Dis Sci. 2015;60(10):3149-50.
40. Menees SB et al. Women patients’ preference for women physicians is a barrier to colon cancer screening. Gastrointest Endosc. 2005;62(2):219-23.
41. Feld LD et al. Management of code status in the periendoscopic period: A national survey of current practices and beliefs of U.S. gastroenterologists. Gastrointest Endosc. 2021;94(1):172-7.e2.
Despite increasing numbers joining the field, women remain a minority group in gastroenterology, where they constitute only 18% of these physicians.1 Additionally, women continue to be underrepresented among senior faculty and in leadership roles in both academic and private practice settings.2 While women now make up a majority of medical school matriculants3,4 women trainees are frequently dissuaded from pursuing specialty fellowships following residency, particularly in procedurally based fields like gastroenterology, because of perceived incompatibility with childbearing and child-rearing.5-8 For many who choose to enter the field despite these challenges, gastroenterology training and early practice often coincide with childbearing years.910 These structural impediments may contribute to the “leaky pipeline” and female physician attrition during the first decade of independent practice after fellowship.11-13 Urgent changes are needed in order to retain and support clinicians and physician-scientists through this period so that they, their offspring, their patients, and the field are able to thrive.
Fertility and pregnancy
The decision to have a child is a major milestone for many physicians and often occurs during gastroenterology training or early practice.10 Medical-training and early-career environments are not yet optimized to support women who become pregnant. At baseline, the formative years of a career are challenging ones, punctuated by long hours and both intellectually and emotionally demanding work. They are also often physically grueling, particularly while one is learning and becoming efficient in endoscopy. The ergonomics in the endoscopy suite (as in other areas of medicine) are not optimized for physicians of shorter stature, smaller hand sizes, and those who may have difficulty pushing a several-hundred-pound endoscopy cart bedside, all of which contribute to increased injury risk for female proceduralists.7,14-16 Methods to reduce endoscopic injuries in pregnant endoscopists have not yet been studied. Additionally, the existence of maternity and gender bias has been well-documented, in our field and beyond.17-20 Not surprisingly, women in gastroenterology commonly report delayed childbearing, with expected consequences, including increased infertility rates, compared with nonphysician peers.21 After 5 and 10 years as attendings, female gastroenterologists continue to report fewer children than male colleagues.22,23 Once pregnant, there are a number of field-specific challenges to navigate. These include decisions about the safety of performing procedures involving fluoroscopy or high infectious risk, particularly early in pregnancy when organogenesis occurs.7,24 Additionally, engaging in appropriate obstetric care can be challenging given the need for regular physician and ultrasound appointments.
Simple, cost-efficient interventions may be effective in decreasing infertility rates, pregnancy loss, and poor physician experiences during pregnancy. For one, all gastroenterology divisions could craft written policies that include a no-tolerance approach to expressions of maternity bias against pregnant or postpartum trainees and faculty.12,25 Additionally, ergonomic improvements, such as standing pads, dial extenders, and adjusted screen heights may decrease injury rates and increase comfort for female endoscopists.26,27 There should also be a no-penalty, no-questions-asked approach for any female endoscopist who defers performance of an obstetrically high-risk procedure to a nonpregnant colleague. Additionally, pregnant gastroenterologists should be supported in obtaining high-quality obstetric care. At an individual level, nonpregnant gastroenterologists, and particularly male allies, can support pregnant colleagues by agreeing to perform higher-risk procedures, stepping in if a fellow is unable to perform endoscopy because of pregnancy, and by offering to push the endoscopy cart on behalf of a pregnant colleague to bedside, if necessary.10,28
Parental leave
Following delivery, parental leave presents an additional challenge for the physician parent. Paid maternal leave has been associated with improved child and maternal outcomes and is widely available to physicians outside the United States.29,30 At present, duration of leave varies significantly by career stage (fellows versus attending), practice setting (academic center versus private practice), and geographic location. The American Academy of Pediatrics recommends a minimum of 12 weeks of leave.31 This length has been associated with lower rates of postpartum depression and higher rates of sustained breastfeeding, with subsequent improved health outcomes for mother and child.32-34 An increasing number of states have passed laws mandating minimum paid and unpaid parental leave time (for example, in Massachusetts, gastroenterology trainees and faculty are afforded 12 weeks of leave, in accordance with state law).35 Recent changes to board eligibility and training requirements via the American Board of Medical Specialties and the American Council for Graduate Medical Education now provide 6 weeks for parental leave. This is an improvement over prior policies which rendered many physician-parents board-ineligible if they took more than 4 weeks of leave, although it must be noted that even the revised policies allow for less time than either that of Obstetricians and Gynecologists or than the American Academy of Pediatrics recommends.
Our data, presented at the 2021 ACG conference, suggest that many trainees report receiving 4 weeks or less of parental leave, despite the ACGME and ABMS policies described above. We also found that physicians were frequently not aware of their institution or division leave policies.10 Ideally, all gastroenterology divisions in the United States would follow the recommended leave duration set forth by the medical societies of specialties that care for pregnant and postpartum mothers and their infants. Additionally, the impact of leave time on graduation and board eligibility, as well as academic and practice promotion, should be made clear at the time of leave and should minimize adverse consequences for the careers of pregnant and postpartum gastroenterologists. Gastroenterology trainees and faculty should be educated in the existence and details of their institution or practice policies, and these policies should be made readily available to all physicians and administrators.
Postpartum period
The transition back to work is a challenging one for mothers in all fields of medicine, particularly for those returning to procedurally based subspecialties such as gastroenterology. This is especially true for trainees and faculty who have returned to work sooner than the recommended 12 weeks and for those who are post cesarean section, for whom physical healing may not be complete. Long days performing endoscopy may be physically challenging or impossible for some women during the postpartum period. Additionally, expressing breast milk, a metabolically intensive activity, also necessitates time, space, and privacy to perform and is frequently made more difficult by insufficient lactation accommodations. The COVID-19 pandemic has increased logistic challenges for lactating mothers, because of the need for well-ventilated lactation spaces to minimize infectious risk.19 Our colleagues have reported pumping in their vehicles, in supply closets, and in spaces that require so much travel time (in addition to time required to express milk, store milk, and clean pump equipment) that the practice was unsustainable, and the physician stopped breastfeeding prematurely.36
The benefits of breastfeeding for mother and infant are well-established, and exclusive breastfeeding for the first 6 months of life is supported by the American College of Obstetricians and Gynecologists, whose position statement reads as follows: “Policies that protect the right of a woman and her child to breastfeed ... and that accommodate milk expression, such as ... paid maternity leave, on-site childcare, break time for expressing milk, and a clean, private location for expressing milk, are essential to sustaining breastfeeding.”37 We would add to these recommendations provision of dedicated milk storage space and establishment of clear, supportive policies that allow lactating physicians to breastfeed and express breast milk if they choose without career penalty. Several institutions offer scheduled protected clinical time and modified work relative value units (RVU) for lactating physicians, such that returning parents can have protected time for expressing breast milk and still meet RVU targets.38 Additionally, many academic institutions offer productivity adjustments for tenure-track faculty who have recently had children.
Creating a more supportive environment for women gastroenterologists who desire children allows the field to be more representative of our patient population and has been shown to positively impact outcomes from improved colorectal cancer screening rates to more guideline-directed informed consent conversations.39-41 Gastroenterology should comprise a physician workforce predicated on clinical and research excellence alone and should not require its practitioners to delay or abstain from pregnancy and child rearing. Robust, clear, and generous parental leave and postpartum accommodations will allow the field to retain and promote talented physicians, who will then contribute to the betterment of patients and the field over decades.
Dr. Rabinowitz is a faculty member in the department of medicine and division of gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Feld is a transplant hepatology fellow, division of gastroenterology, department of medicine, University of Washington, Seattle. Dr. Rabinowitz and Dr. Feld have no conflicts of interest to disclose.
References
1. AAMC. Diversity in Medicine: Facts and Figures 2019. 2018.
2. Colleges AoAM. The State of Women in Academic Medicine: The Pipeline and Pathways to Leadership, 2015-2016. 2016. www.aamc.org/download/481206/data/2015table11.pdf.
3. AAMC. Table B-3: Total U.S. Medical School Enrollment by Race/Ethnicity and Sex, 2014-2015 through 2018-2019, 2019.
4. Rabinowitz LG. Recognizing blind spots – a remedy for gender bias in medicine? (N Engl. J Med. 2018; 378[24]: 2253-5).
5. Douglas PS et al. Career preferences and perceptions of cardiology among US internal medicine trainees: Factors influencing cardiology career choice. JAMA Cardiol 2018; 3(8):682-91.
6. Stack SW et al. Childbearing decisions in residency: A multicenter survey of female residents. Acad Med 2020;95(10):1550-7.
7. David YN et al. Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges. Gastroenterology 2021;161(3):756-60.
8. Rembacken BJ et al. Barriers and bias standing in the way of female trainees wanting to learn advanced endoscopy. United European Gastroenterol J. 2019;7(8):1141-5.
9. Arlow FL et al. Gastroenterology training and career choices: A prospective longitudinal study of the impact of gender and of managed care. Am J Gastroenterol. 2002;97(2):459-69.
10. Feld L et al. Parental leave for gastroenterology fellows: A national survey of current fellows. Am J Gastroenterol. 2021;116:S611-2.
11. Rabinowitz LG et al. Addressing gender in gastroenterology: opportunities for change. Gastrointest Endosc. 2020;91(1):155-61.
12. Feld LD. Baby steps in the right direction: Toward a parental leave policy for gastroenterology fellows. Am J Gastroenterol. 2021;116(3):505-8.
13. Feld LD. Interviewing for two. Am J Gastroenterol. 2020;116(3):445-6
14. Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93(5):1047-56.e5.
15. Harvin G. Review of musculoskeletal injuries and prevention in the endoscopy practitioner. J Clin Gastroenterol. 2014;48(7):590-4.
16. LabX Oecs. www.labx.com/product/endoscopy-cart (accessed 2021 Nov 19.
17. Heilman ME and Okimoto TG. Motherhood: A potential source of bias in employment decisions. J Appl Psychol. 2008;93(1):189-98.
18. Robinson K et al. Racism, bias, and discrimination as modifiable barriers to breastfeeding for African American women: A scoping review of the literature. J Midwifery Womens Health. 2019;64(6):734-42.
19. Rabinowitz LG and Rabinowitz DG. Women on the Frontline: A Changed Workforce and the Fight Against COVID-19. Acad Med. 2021 Jun 1;96(6):808-12.
20. Rabinowitz LG et al. Gender in the endoscopy suite. Lancet Gastroenterol Hepatol. 2020 Dec;5(12):1032-4.
21. Stentz NC et al. Fertility and childbearing among American female physicians. J Womens Health. 2016; 25(10):1059-65.
22. Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol. 2005;100(2):259-64.
23. Singh A et al. Women in gastroenterology committee of American College of G. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103(7):1589-95.
24. Krueger KJ and Hoffman BJ. Radiation exposure during gastroenterologic fluoroscopy: Risk assessment for pregnant workers. Am J Gastroenterol. 1992;87(4):429-31.
25. Krause ML et al. Impact of pregnancy and gender on internal medicine resident evaluations: A retrospective cohort study. J Gen Intern Med. 2017;32(6):648-53.
26. Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
27. David YN et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Am J Gastroenterol. 2021;116(3):539-50.
28. Bilal M et al. The need for allyship in achieving gender equity in gastroenterology. Am J Gastroenterol. 2021 Oct 19. doi: 10.14309/ajg.0000000000001508. Online ahead of print.
29. Jou J et al. Paid maternity leave in the United States: Associations with maternal and infant health. Matern Child Health J. 2018;22(2):216-25.
30. Aitken Z et al. The maternal health outcomes of paid maternity leave: A systematic review. Soc Sci Med. 2015;130:32-41.
31. Dodson NA and Talib HJ. Paid parental leave for mothers and fathers can improve physician wellness. AAP News. 2020 Jul 1. https://publications.aap.org/aapnews/news/12432.
32. Kornfeind KR and Sipsma HL. Exploring the link between maternity leave and postpartum depression. Womens Health Issues 2018;28(4):321-6.
33. Navarro-Rosenblatt D and Garmendia ML. Maternity leave and its impact on breastfeeding: A review of the literature. Breastfeed Med 2018;13(9):589-97.
34. Stack SW et al. Maternity leave in residency: A multicenter study of determinants and wellness outcomes. Acad Med. 2019;94(11):1738-45.
35. Mass.gov. Paid Family and Medical Leave Information for Massachusetts Employers. 2020.
36. Ares Segura S et al. en representacion del Comite de Lactancia Materna de la Asociacion Espanola de P. [The importance of maternal nutrition during breastfeeding: Do breastfeeding mothers need nutritional supplements?]. An Pediatr. (Barc) 2016;84(6):347 e1-7.
37. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 658: Optimizing Support for Breastfeeding as Part of Obstetric Practice. Obstet Gynecol. 2016;127(2):e86-92.
38. Porter KK et al. A lactation credit model to support breastfeeding in radiology: The new gold standard to support “liquid gold.” Clin Imaging 2021;80:16-8.
39. Davis J et al. Clinical practice patterns suggest female patients prefer female endoscopists. Dig Dis Sci. 2015;60(10):3149-50.
40. Menees SB et al. Women patients’ preference for women physicians is a barrier to colon cancer screening. Gastrointest Endosc. 2005;62(2):219-23.
41. Feld LD et al. Management of code status in the periendoscopic period: A national survey of current practices and beliefs of U.S. gastroenterologists. Gastrointest Endosc. 2021;94(1):172-7.e2.
Despite increasing numbers joining the field, women remain a minority group in gastroenterology, where they constitute only 18% of these physicians.1 Additionally, women continue to be underrepresented among senior faculty and in leadership roles in both academic and private practice settings.2 While women now make up a majority of medical school matriculants3,4 women trainees are frequently dissuaded from pursuing specialty fellowships following residency, particularly in procedurally based fields like gastroenterology, because of perceived incompatibility with childbearing and child-rearing.5-8 For many who choose to enter the field despite these challenges, gastroenterology training and early practice often coincide with childbearing years.910 These structural impediments may contribute to the “leaky pipeline” and female physician attrition during the first decade of independent practice after fellowship.11-13 Urgent changes are needed in order to retain and support clinicians and physician-scientists through this period so that they, their offspring, their patients, and the field are able to thrive.
Fertility and pregnancy
The decision to have a child is a major milestone for many physicians and often occurs during gastroenterology training or early practice.10 Medical-training and early-career environments are not yet optimized to support women who become pregnant. At baseline, the formative years of a career are challenging ones, punctuated by long hours and both intellectually and emotionally demanding work. They are also often physically grueling, particularly while one is learning and becoming efficient in endoscopy. The ergonomics in the endoscopy suite (as in other areas of medicine) are not optimized for physicians of shorter stature, smaller hand sizes, and those who may have difficulty pushing a several-hundred-pound endoscopy cart bedside, all of which contribute to increased injury risk for female proceduralists.7,14-16 Methods to reduce endoscopic injuries in pregnant endoscopists have not yet been studied. Additionally, the existence of maternity and gender bias has been well-documented, in our field and beyond.17-20 Not surprisingly, women in gastroenterology commonly report delayed childbearing, with expected consequences, including increased infertility rates, compared with nonphysician peers.21 After 5 and 10 years as attendings, female gastroenterologists continue to report fewer children than male colleagues.22,23 Once pregnant, there are a number of field-specific challenges to navigate. These include decisions about the safety of performing procedures involving fluoroscopy or high infectious risk, particularly early in pregnancy when organogenesis occurs.7,24 Additionally, engaging in appropriate obstetric care can be challenging given the need for regular physician and ultrasound appointments.
Simple, cost-efficient interventions may be effective in decreasing infertility rates, pregnancy loss, and poor physician experiences during pregnancy. For one, all gastroenterology divisions could craft written policies that include a no-tolerance approach to expressions of maternity bias against pregnant or postpartum trainees and faculty.12,25 Additionally, ergonomic improvements, such as standing pads, dial extenders, and adjusted screen heights may decrease injury rates and increase comfort for female endoscopists.26,27 There should also be a no-penalty, no-questions-asked approach for any female endoscopist who defers performance of an obstetrically high-risk procedure to a nonpregnant colleague. Additionally, pregnant gastroenterologists should be supported in obtaining high-quality obstetric care. At an individual level, nonpregnant gastroenterologists, and particularly male allies, can support pregnant colleagues by agreeing to perform higher-risk procedures, stepping in if a fellow is unable to perform endoscopy because of pregnancy, and by offering to push the endoscopy cart on behalf of a pregnant colleague to bedside, if necessary.10,28
Parental leave
Following delivery, parental leave presents an additional challenge for the physician parent. Paid maternal leave has been associated with improved child and maternal outcomes and is widely available to physicians outside the United States.29,30 At present, duration of leave varies significantly by career stage (fellows versus attending), practice setting (academic center versus private practice), and geographic location. The American Academy of Pediatrics recommends a minimum of 12 weeks of leave.31 This length has been associated with lower rates of postpartum depression and higher rates of sustained breastfeeding, with subsequent improved health outcomes for mother and child.32-34 An increasing number of states have passed laws mandating minimum paid and unpaid parental leave time (for example, in Massachusetts, gastroenterology trainees and faculty are afforded 12 weeks of leave, in accordance with state law).35 Recent changes to board eligibility and training requirements via the American Board of Medical Specialties and the American Council for Graduate Medical Education now provide 6 weeks for parental leave. This is an improvement over prior policies which rendered many physician-parents board-ineligible if they took more than 4 weeks of leave, although it must be noted that even the revised policies allow for less time than either that of Obstetricians and Gynecologists or than the American Academy of Pediatrics recommends.
Our data, presented at the 2021 ACG conference, suggest that many trainees report receiving 4 weeks or less of parental leave, despite the ACGME and ABMS policies described above. We also found that physicians were frequently not aware of their institution or division leave policies.10 Ideally, all gastroenterology divisions in the United States would follow the recommended leave duration set forth by the medical societies of specialties that care for pregnant and postpartum mothers and their infants. Additionally, the impact of leave time on graduation and board eligibility, as well as academic and practice promotion, should be made clear at the time of leave and should minimize adverse consequences for the careers of pregnant and postpartum gastroenterologists. Gastroenterology trainees and faculty should be educated in the existence and details of their institution or practice policies, and these policies should be made readily available to all physicians and administrators.
Postpartum period
The transition back to work is a challenging one for mothers in all fields of medicine, particularly for those returning to procedurally based subspecialties such as gastroenterology. This is especially true for trainees and faculty who have returned to work sooner than the recommended 12 weeks and for those who are post cesarean section, for whom physical healing may not be complete. Long days performing endoscopy may be physically challenging or impossible for some women during the postpartum period. Additionally, expressing breast milk, a metabolically intensive activity, also necessitates time, space, and privacy to perform and is frequently made more difficult by insufficient lactation accommodations. The COVID-19 pandemic has increased logistic challenges for lactating mothers, because of the need for well-ventilated lactation spaces to minimize infectious risk.19 Our colleagues have reported pumping in their vehicles, in supply closets, and in spaces that require so much travel time (in addition to time required to express milk, store milk, and clean pump equipment) that the practice was unsustainable, and the physician stopped breastfeeding prematurely.36
The benefits of breastfeeding for mother and infant are well-established, and exclusive breastfeeding for the first 6 months of life is supported by the American College of Obstetricians and Gynecologists, whose position statement reads as follows: “Policies that protect the right of a woman and her child to breastfeed ... and that accommodate milk expression, such as ... paid maternity leave, on-site childcare, break time for expressing milk, and a clean, private location for expressing milk, are essential to sustaining breastfeeding.”37 We would add to these recommendations provision of dedicated milk storage space and establishment of clear, supportive policies that allow lactating physicians to breastfeed and express breast milk if they choose without career penalty. Several institutions offer scheduled protected clinical time and modified work relative value units (RVU) for lactating physicians, such that returning parents can have protected time for expressing breast milk and still meet RVU targets.38 Additionally, many academic institutions offer productivity adjustments for tenure-track faculty who have recently had children.
Creating a more supportive environment for women gastroenterologists who desire children allows the field to be more representative of our patient population and has been shown to positively impact outcomes from improved colorectal cancer screening rates to more guideline-directed informed consent conversations.39-41 Gastroenterology should comprise a physician workforce predicated on clinical and research excellence alone and should not require its practitioners to delay or abstain from pregnancy and child rearing. Robust, clear, and generous parental leave and postpartum accommodations will allow the field to retain and promote talented physicians, who will then contribute to the betterment of patients and the field over decades.
Dr. Rabinowitz is a faculty member in the department of medicine and division of gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Feld is a transplant hepatology fellow, division of gastroenterology, department of medicine, University of Washington, Seattle. Dr. Rabinowitz and Dr. Feld have no conflicts of interest to disclose.
References
1. AAMC. Diversity in Medicine: Facts and Figures 2019. 2018.
2. Colleges AoAM. The State of Women in Academic Medicine: The Pipeline and Pathways to Leadership, 2015-2016. 2016. www.aamc.org/download/481206/data/2015table11.pdf.
3. AAMC. Table B-3: Total U.S. Medical School Enrollment by Race/Ethnicity and Sex, 2014-2015 through 2018-2019, 2019.
4. Rabinowitz LG. Recognizing blind spots – a remedy for gender bias in medicine? (N Engl. J Med. 2018; 378[24]: 2253-5).
5. Douglas PS et al. Career preferences and perceptions of cardiology among US internal medicine trainees: Factors influencing cardiology career choice. JAMA Cardiol 2018; 3(8):682-91.
6. Stack SW et al. Childbearing decisions in residency: A multicenter survey of female residents. Acad Med 2020;95(10):1550-7.
7. David YN et al. Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges. Gastroenterology 2021;161(3):756-60.
8. Rembacken BJ et al. Barriers and bias standing in the way of female trainees wanting to learn advanced endoscopy. United European Gastroenterol J. 2019;7(8):1141-5.
9. Arlow FL et al. Gastroenterology training and career choices: A prospective longitudinal study of the impact of gender and of managed care. Am J Gastroenterol. 2002;97(2):459-69.
10. Feld L et al. Parental leave for gastroenterology fellows: A national survey of current fellows. Am J Gastroenterol. 2021;116:S611-2.
11. Rabinowitz LG et al. Addressing gender in gastroenterology: opportunities for change. Gastrointest Endosc. 2020;91(1):155-61.
12. Feld LD. Baby steps in the right direction: Toward a parental leave policy for gastroenterology fellows. Am J Gastroenterol. 2021;116(3):505-8.
13. Feld LD. Interviewing for two. Am J Gastroenterol. 2020;116(3):445-6
14. Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93(5):1047-56.e5.
15. Harvin G. Review of musculoskeletal injuries and prevention in the endoscopy practitioner. J Clin Gastroenterol. 2014;48(7):590-4.
16. LabX Oecs. www.labx.com/product/endoscopy-cart (accessed 2021 Nov 19.
17. Heilman ME and Okimoto TG. Motherhood: A potential source of bias in employment decisions. J Appl Psychol. 2008;93(1):189-98.
18. Robinson K et al. Racism, bias, and discrimination as modifiable barriers to breastfeeding for African American women: A scoping review of the literature. J Midwifery Womens Health. 2019;64(6):734-42.
19. Rabinowitz LG and Rabinowitz DG. Women on the Frontline: A Changed Workforce and the Fight Against COVID-19. Acad Med. 2021 Jun 1;96(6):808-12.
20. Rabinowitz LG et al. Gender in the endoscopy suite. Lancet Gastroenterol Hepatol. 2020 Dec;5(12):1032-4.
21. Stentz NC et al. Fertility and childbearing among American female physicians. J Womens Health. 2016; 25(10):1059-65.
22. Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol. 2005;100(2):259-64.
23. Singh A et al. Women in gastroenterology committee of American College of G. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103(7):1589-95.
24. Krueger KJ and Hoffman BJ. Radiation exposure during gastroenterologic fluoroscopy: Risk assessment for pregnant workers. Am J Gastroenterol. 1992;87(4):429-31.
25. Krause ML et al. Impact of pregnancy and gender on internal medicine resident evaluations: A retrospective cohort study. J Gen Intern Med. 2017;32(6):648-53.
26. Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
27. David YN et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Am J Gastroenterol. 2021;116(3):539-50.
28. Bilal M et al. The need for allyship in achieving gender equity in gastroenterology. Am J Gastroenterol. 2021 Oct 19. doi: 10.14309/ajg.0000000000001508. Online ahead of print.
29. Jou J et al. Paid maternity leave in the United States: Associations with maternal and infant health. Matern Child Health J. 2018;22(2):216-25.
30. Aitken Z et al. The maternal health outcomes of paid maternity leave: A systematic review. Soc Sci Med. 2015;130:32-41.
31. Dodson NA and Talib HJ. Paid parental leave for mothers and fathers can improve physician wellness. AAP News. 2020 Jul 1. https://publications.aap.org/aapnews/news/12432.
32. Kornfeind KR and Sipsma HL. Exploring the link between maternity leave and postpartum depression. Womens Health Issues 2018;28(4):321-6.
33. Navarro-Rosenblatt D and Garmendia ML. Maternity leave and its impact on breastfeeding: A review of the literature. Breastfeed Med 2018;13(9):589-97.
34. Stack SW et al. Maternity leave in residency: A multicenter study of determinants and wellness outcomes. Acad Med. 2019;94(11):1738-45.
35. Mass.gov. Paid Family and Medical Leave Information for Massachusetts Employers. 2020.
36. Ares Segura S et al. en representacion del Comite de Lactancia Materna de la Asociacion Espanola de P. [The importance of maternal nutrition during breastfeeding: Do breastfeeding mothers need nutritional supplements?]. An Pediatr. (Barc) 2016;84(6):347 e1-7.
37. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 658: Optimizing Support for Breastfeeding as Part of Obstetric Practice. Obstet Gynecol. 2016;127(2):e86-92.
38. Porter KK et al. A lactation credit model to support breastfeeding in radiology: The new gold standard to support “liquid gold.” Clin Imaging 2021;80:16-8.
39. Davis J et al. Clinical practice patterns suggest female patients prefer female endoscopists. Dig Dis Sci. 2015;60(10):3149-50.
40. Menees SB et al. Women patients’ preference for women physicians is a barrier to colon cancer screening. Gastrointest Endosc. 2005;62(2):219-23.
41. Feld LD et al. Management of code status in the periendoscopic period: A national survey of current practices and beliefs of U.S. gastroenterologists. Gastrointest Endosc. 2021;94(1):172-7.e2.
The etiology of acute otitis media in young children in recent years
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.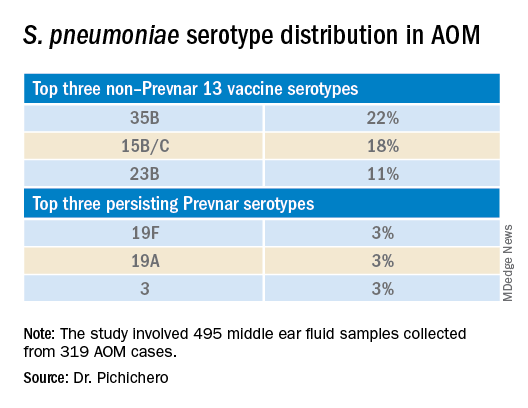
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
Responding to the Pandemic: How is the VA doing?
The VA’s Coronavirus Disease 2019 Response Report is now in its third iteration as the pandemic continues. On the bright side, as Steven Lieberman, MD, deputy under secretary for health at the US Department of Veterans Affairs (VA), writes in the report’s introduction, “we have learned a great deal about mounting a national response to a public health crisis.”
“Annex B” covers January 1, 2021 to July 31, 2021, building on the 2 previous reports. All 3 have sought to capture and share lessons learned, with updated information on vaccination, elder care, health equity, mental health, health care ethics, preparedness, and other topics.
As the pandemic evolved, so did the VA efforts to cope with it. This iteration, for instance, deals with details of the campaign that vaccinated more than 2.5 million people “while sustaining all other aspects of the pandemic response and veteran health services,” and how the VA implemented a vaccine mandate for all VA employees in health care roles—the first federal agency to do so. In addition to vaccinating veterans, the Strengthening and Amplifying Vaccination Efforts to Locally Immunize All Veterans and Every Spouse (SAVE LIVES) Act led to nearly 80,000 other vaccinations among families, caregivers, and veterans who do not use VHA services.
The VA also conducted extensive COVID-19 testing, processing as many as 70,000 to 90,000 tests per week. It enhanced telehealth services to reach home-based and rural veterans, for an almost 2,500% increase in home-based primary care. Recognizing the added stress the pandemic put on people at risk for suicide, the VHA used predictive analytic tools specific to veterans with COVID-19 and monitored “high-risk flags,” using them to identify veterans for tailored outreach.
The response also included carrying out 158 Federal Emergency Management Agency Fourth Mission assignments. The report highlights the contributions of the more than 1,600 Veterans Health Administration (VHA) employees who volunteered to deploy across the country, often multiple times.
In addition to active response, more than 300 studies on COVID-19 have been published by VA researchers.
The current status report discusses how to expand what worked and to improve what did not. For instance, one unsurprising finding was that “the sustained pandemic response has imposed stress on the workforce, most evident in the nursing workforce.” The recommendation: Develop a comprehensive strategy with metrics and actions to monitor and mitigate stress on the health care workforce, facilitate wellness, and enhance retention.
The finding that VHA has demonstrated that telehealth usage for care to elderly veterans is “beneficial and feasible with the right technical support” led to recommendations for expanded research to identify effective COVID-19 prevention and intervention measures for elderly veterans residing at home or in long-term care facilities.
The research found that VHA processes for protecting community living center (CLC) residents during the pandemic “have succeeded in keeping rates of CLC-onset COVID-19 at the same rate as for the population of enrolled veterans over 65,” the report says. The recommendation based on that finding is to develop an information system to facilitate monitoring of state-run veterans homes for indicators of infectious disease risk, combining periodic assessment results with epidemiologic community data.
However, the report also acknowledges unexpected detours or blocks. “Planning for the mass vaccination campaign was highly effective, but did not anticipate the complexity of interagency support.” And “[t[he inability to access state vaccination data left VHA with an incomplete picture of the vaccination status of enrolled veterans.” In response, the VA recommends incorporating interagency support into planning templates and pursuing legislative action to enable the VA to obtain vaccination data from states.
Overall, the report gives the VA high marks for managing a “well-coordinated response” to an overwhelming crisis. But the lessons are not over.
“As we continue to address the pandemic and as new variants arise,” Dr. Lieberman said in comments, “it is clear that continuous learning and improvement are essential to a successful COVID-19 response. We will continue to update this report to document our efforts so veterans, doctors, and the public can understand and learn from what we’ve discovered to better serve our veterans and communities.” Stay tuned for Annex C.
The VA’s Coronavirus Disease 2019 Response Report is now in its third iteration as the pandemic continues. On the bright side, as Steven Lieberman, MD, deputy under secretary for health at the US Department of Veterans Affairs (VA), writes in the report’s introduction, “we have learned a great deal about mounting a national response to a public health crisis.”
“Annex B” covers January 1, 2021 to July 31, 2021, building on the 2 previous reports. All 3 have sought to capture and share lessons learned, with updated information on vaccination, elder care, health equity, mental health, health care ethics, preparedness, and other topics.
As the pandemic evolved, so did the VA efforts to cope with it. This iteration, for instance, deals with details of the campaign that vaccinated more than 2.5 million people “while sustaining all other aspects of the pandemic response and veteran health services,” and how the VA implemented a vaccine mandate for all VA employees in health care roles—the first federal agency to do so. In addition to vaccinating veterans, the Strengthening and Amplifying Vaccination Efforts to Locally Immunize All Veterans and Every Spouse (SAVE LIVES) Act led to nearly 80,000 other vaccinations among families, caregivers, and veterans who do not use VHA services.
The VA also conducted extensive COVID-19 testing, processing as many as 70,000 to 90,000 tests per week. It enhanced telehealth services to reach home-based and rural veterans, for an almost 2,500% increase in home-based primary care. Recognizing the added stress the pandemic put on people at risk for suicide, the VHA used predictive analytic tools specific to veterans with COVID-19 and monitored “high-risk flags,” using them to identify veterans for tailored outreach.
The response also included carrying out 158 Federal Emergency Management Agency Fourth Mission assignments. The report highlights the contributions of the more than 1,600 Veterans Health Administration (VHA) employees who volunteered to deploy across the country, often multiple times.
In addition to active response, more than 300 studies on COVID-19 have been published by VA researchers.
The current status report discusses how to expand what worked and to improve what did not. For instance, one unsurprising finding was that “the sustained pandemic response has imposed stress on the workforce, most evident in the nursing workforce.” The recommendation: Develop a comprehensive strategy with metrics and actions to monitor and mitigate stress on the health care workforce, facilitate wellness, and enhance retention.
The finding that VHA has demonstrated that telehealth usage for care to elderly veterans is “beneficial and feasible with the right technical support” led to recommendations for expanded research to identify effective COVID-19 prevention and intervention measures for elderly veterans residing at home or in long-term care facilities.
The research found that VHA processes for protecting community living center (CLC) residents during the pandemic “have succeeded in keeping rates of CLC-onset COVID-19 at the same rate as for the population of enrolled veterans over 65,” the report says. The recommendation based on that finding is to develop an information system to facilitate monitoring of state-run veterans homes for indicators of infectious disease risk, combining periodic assessment results with epidemiologic community data.
However, the report also acknowledges unexpected detours or blocks. “Planning for the mass vaccination campaign was highly effective, but did not anticipate the complexity of interagency support.” And “[t[he inability to access state vaccination data left VHA with an incomplete picture of the vaccination status of enrolled veterans.” In response, the VA recommends incorporating interagency support into planning templates and pursuing legislative action to enable the VA to obtain vaccination data from states.
Overall, the report gives the VA high marks for managing a “well-coordinated response” to an overwhelming crisis. But the lessons are not over.
“As we continue to address the pandemic and as new variants arise,” Dr. Lieberman said in comments, “it is clear that continuous learning and improvement are essential to a successful COVID-19 response. We will continue to update this report to document our efforts so veterans, doctors, and the public can understand and learn from what we’ve discovered to better serve our veterans and communities.” Stay tuned for Annex C.
The VA’s Coronavirus Disease 2019 Response Report is now in its third iteration as the pandemic continues. On the bright side, as Steven Lieberman, MD, deputy under secretary for health at the US Department of Veterans Affairs (VA), writes in the report’s introduction, “we have learned a great deal about mounting a national response to a public health crisis.”
“Annex B” covers January 1, 2021 to July 31, 2021, building on the 2 previous reports. All 3 have sought to capture and share lessons learned, with updated information on vaccination, elder care, health equity, mental health, health care ethics, preparedness, and other topics.
As the pandemic evolved, so did the VA efforts to cope with it. This iteration, for instance, deals with details of the campaign that vaccinated more than 2.5 million people “while sustaining all other aspects of the pandemic response and veteran health services,” and how the VA implemented a vaccine mandate for all VA employees in health care roles—the first federal agency to do so. In addition to vaccinating veterans, the Strengthening and Amplifying Vaccination Efforts to Locally Immunize All Veterans and Every Spouse (SAVE LIVES) Act led to nearly 80,000 other vaccinations among families, caregivers, and veterans who do not use VHA services.
The VA also conducted extensive COVID-19 testing, processing as many as 70,000 to 90,000 tests per week. It enhanced telehealth services to reach home-based and rural veterans, for an almost 2,500% increase in home-based primary care. Recognizing the added stress the pandemic put on people at risk for suicide, the VHA used predictive analytic tools specific to veterans with COVID-19 and monitored “high-risk flags,” using them to identify veterans for tailored outreach.
The response also included carrying out 158 Federal Emergency Management Agency Fourth Mission assignments. The report highlights the contributions of the more than 1,600 Veterans Health Administration (VHA) employees who volunteered to deploy across the country, often multiple times.
In addition to active response, more than 300 studies on COVID-19 have been published by VA researchers.
The current status report discusses how to expand what worked and to improve what did not. For instance, one unsurprising finding was that “the sustained pandemic response has imposed stress on the workforce, most evident in the nursing workforce.” The recommendation: Develop a comprehensive strategy with metrics and actions to monitor and mitigate stress on the health care workforce, facilitate wellness, and enhance retention.
The finding that VHA has demonstrated that telehealth usage for care to elderly veterans is “beneficial and feasible with the right technical support” led to recommendations for expanded research to identify effective COVID-19 prevention and intervention measures for elderly veterans residing at home or in long-term care facilities.
The research found that VHA processes for protecting community living center (CLC) residents during the pandemic “have succeeded in keeping rates of CLC-onset COVID-19 at the same rate as for the population of enrolled veterans over 65,” the report says. The recommendation based on that finding is to develop an information system to facilitate monitoring of state-run veterans homes for indicators of infectious disease risk, combining periodic assessment results with epidemiologic community data.
However, the report also acknowledges unexpected detours or blocks. “Planning for the mass vaccination campaign was highly effective, but did not anticipate the complexity of interagency support.” And “[t[he inability to access state vaccination data left VHA with an incomplete picture of the vaccination status of enrolled veterans.” In response, the VA recommends incorporating interagency support into planning templates and pursuing legislative action to enable the VA to obtain vaccination data from states.
Overall, the report gives the VA high marks for managing a “well-coordinated response” to an overwhelming crisis. But the lessons are not over.
“As we continue to address the pandemic and as new variants arise,” Dr. Lieberman said in comments, “it is clear that continuous learning and improvement are essential to a successful COVID-19 response. We will continue to update this report to document our efforts so veterans, doctors, and the public can understand and learn from what we’ve discovered to better serve our veterans and communities.” Stay tuned for Annex C.
Children and COVID: New cases and hospital admissions skyrocket
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.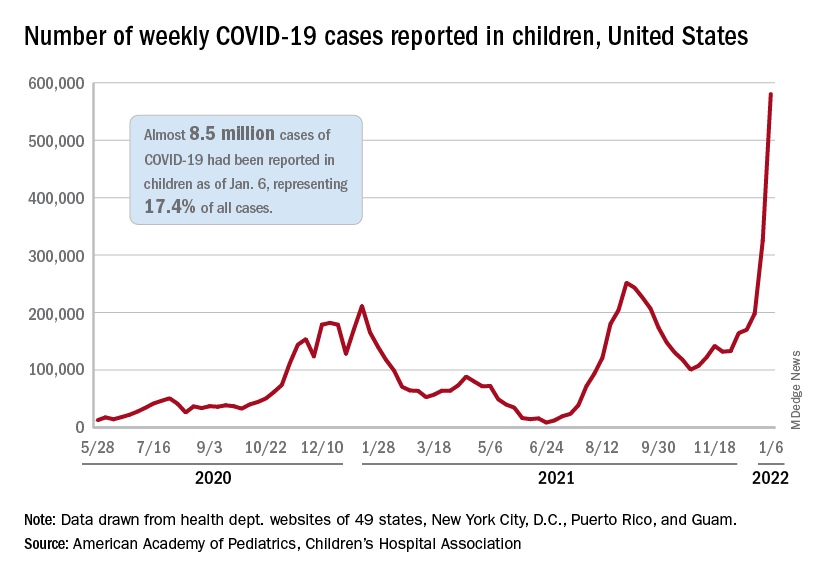
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
Olive oil intake tied to reduced mortality
Compared with men and women who rarely or never consumed olive oil (the lowest intake), those who consumed greater than 0.5 tablespoon/day or more than 7 g/day (the highest intake) had a 19% lower mortality risk over a 28-year follow-up, starting from an average age of 56 years.
Moreover, compared with those with the lowest olive oil intake, those with the highest intake had a 19% lower cardiovascular disease (CVD) mortality, a 17% lower risk of dying from cancer, a 29% lower risk of dying from neurodegenerative disease, and an 18% lower risk of dying from respiratory disease during follow-up.
The researchers estimate that replacing 10 g/day of margarine, butter, mayonnaise, or dairy fat with the same amount of olive oil is associated with an 8%-34% lower risk of death from various causes.
The study by Marta Guasch-Ferré, PhD, and colleagues was published online Jan. 10 in the Journal of the American College of Cardiology.
Results support plant-based dietary fat recommendations
“Our results support current dietary recommendations to increase the intake of olive oil and other unsaturated vegetable oils in place of other fats to improve overall health and longevity,” the researchers summarize.
However, “I wouldn’t say that olive oil is the only way to help you live longer,” Dr. Guasch-Ferré, a senior research scientist in the department of nutrition, Harvard T.H. Chan School of Public Health, Boston, cautioned in an interview with this news organization.
“Other things are very important, such as not smoking, doing physical activity, etc., but one recommendation could be to try to eat more plant-based food including olive oil and healthy fat,” she added, and to use it for cooking, salad dressing, and baking, and substitute it for saturated fat or animal fat, especially for cooking.
The study suggests that people should “consume a more plant-based diet and prioritize fatty acids such as olive oil because they have a better nutritional composition (high in phenols and antioxidants), instead of using butter or margarines or other animal fats that have been shown to have detrimental effects for health,” she added, which is consistent with recommendations in the Dietary Guidelines for Americans.
“That said,” Dr. Guasch-Ferré summarized, “replication is needed in other cohorts and populations to see if the results are similar.”
In an accompanying editorial, Susanna C. Larsson, PhD, writes that “this was a well-designed study, with long-term follow-up and repeated measurements of dietary intake and other risk factors for diseases.”
“However, the difference in olive oil consumption between those with the highest and those with the lowest/no olive oil consumption was very low (0.5 tablespoon) and a [12%] reduced mortality risk was observed already at a much lower intake (0.5 teaspoon, about 1.5 g/day) of olive oil,” she noted in an email to this news organization.
“It’s a bit hard to believe that such a small amount could have an independent effect on mortality risk,” Dr. Larsson, associate professor of epidemiology at the Karolinska Institutet, Stockholm, cautioned.
Like Dr. Guasch-Ferré, she noted that “just adding one or two teaspoons of olive oil to the diet each day will likely not change the risk of mortality.”
Rather, “people may need to make larger changes in the whole diet, not focus on fat only. An overall healthier diet, rich in nonrefined plant-based foods (vegetables, whole grains, nuts), low/no intake of processed foods, and a switch to healthier fat (eg, olive oil) is needed.”
Importantly, “this study cannot say anything about causality, that is, whether it’s olive oil specifically that reduces mortality risk or if there are many other beneficial factors that act together to reduce mortality rate among those with high olive oil consumption.”
The researchers acknowledge this observational study limitation and that the findings may not be generalizable to other populations.
Novel findings regarding Alzheimer’s and respiratory disease
Dr. Larsson highlights two novel findings of this study.
First, it showed a 27% reduction in risk of dementia-related mortality for those in the highest versus lowest category of olive oil consumption. “Considering the lack of preventive strategies for Alzheimer’s disease and the high morbidity and mortality related to this disease, this finding, if confirmed, is of great public health importance,” she said.
Second, the study reported an inverse association of olive oil consumption with risk of respiratory disease mortality. “Because residual confounding from smoking cannot be ruled out,” Dr. Larsson said, “this finding is tentative and requires confirmation in a study that is less susceptible to confounding, such as a randomized trial.”
And although the current study and previous studies have found that consumption of olive oil may have health benefits, she identified several remaining questions.
“Are the associations causal or spurious?” she noted. Is olive oil consumption protective for certain cardiovascular diseases like stroke or atrial fibrillation only, as has been shown in other studies, or also for other major diseases and causes of death, she added. What is the amount of olive oil required for a protective effect?
Further, is the potential effect related to monounsaturated fatty acids (MUFAs) or phenolic compounds; that is, “is the protective effect confined to polyphenol-rich extra-virgin olive oil or are refined olive oil and other vegetable oils as beneficial? More research is needed to address these questions,” she concludes.
“Further studies are needed,” the researchers agree, “to confirm the association of olive oil consumption with reduced mortality, clarify the mechanisms responsible, and quantify the dose/volume boundaries around this effect.”
Virgin olive oil has more polyphenols
Olive oil, a key component of the Mediterranean diet, is high in MUFAs, especially oleic acid, as well as vitamin E and polyphenols, which contribute to its anti-inflammatory and antioxidant properties, the researchers explain.
Virgin olive oil, produced by mechanically pressing ripe olives, contains multiple bioactive and antioxidant components and has an acidity of less than 1.5%. And extra-virgin olive oil is produced the same way but has a higher quality, more intense taste, and lower acidity (less than 1%).
Refined or processed olive oil contains less phytochemicals, as some are lost during processing; it usually contains more than 80% refined oil, plus virgin oil added back to enhance flavor, and may also be labeled “pure” or “light.” However, refined olive oil “still has a good amount of healthy fatty acids but less bioactive compounds,” Dr. Guasch-Ferré noted.
Until now, no large prospective study has examined the link between olive oil intake and all-cause and cause-specific mortality in a U.S. population, where olive oil consumption is limited, compared with Mediterranean countries.
The researchers identified 60,582 women in the Nurses’ Health Study and 31,801 men in the Health Professionals Follow-up Study who were free of CVD or cancer in 1990, the first year that food frequency questionnaires in these studies asked about olive oil.
Participants replied to questionnaires every 4 years that asked about use of olive oil (for salad dressing, baking, frying, sautéing, and spreading on bread), other vegetable oils (for example, corn, safflower, soybean, canola oil), margarine, butter, and dairy fat. The researchers averaged the consumption of these fats during the follow-up years.
From 1990 to 2019, the average consumption of olive oil increased from 1.6 g/day to 4 g/day. Margarine in the 1990s contained saturated fat and trans fats, whereas more recently margarine contains beneficial olive oil or vegetable fat, Dr. Guasch-Ferré noted.
Baseline olive oil consumption in this U.S. population “differed remarkably” from that in the Spanish population in the PREDIMED (Prevención con Dieta Mediterránea) trial, which was, on average, 20-22 g/day of extra-virgin olive oil and 16-18 g/day of refined/mixed olive oil, Larsson pointed out.
Because olive oil consumption was so low in this U.S. study, the researchers did not distinguish between virgin/extra-virgin olive oil and refined/processed olive oil.
The participants were almost all White (99%) and were generally healthier than the average U.S. population; on average, they had a body mass index of 25.3-25.8 kg/m2 and ate 4.8-7.2 fruits and vegetables/day.
Those with the highest olive oil consumption were more physically active, had a healthier diet, were more likely to have Southern European or Mediterranean ancestry, and were less likely to smoke.
During 28 years of follow-up, 36,856 participants died. The researchers classified the deaths into five categories: CVD, cancer, neurodegenerative disease (including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis), respiratory disease (such as chronic obstructive pulmonary disease), and all other causes (including suicide, injury, infections, diabetes, and kidney disease).
After adjusting for multiple confounders, compared with participants who rarely or never consumed olive oil, those in the highest quartile for olive oil consumption had a decreased risk of death from all causes (hazard ratio, 0.81; 95% confidence interval, 0.78 - 0.84) and from CVD (HR, 0.81; 95% CI, 0.75-0.87), cancer (HR, 0.83; 95% CI, 0.78-0.89), neurodegenerative disease (HR, 0.71; 95% CI, 0.64-0.78), and respiratory disease (HR, 0.82; 95% CI, 0.72-0.93).
There was no decrease in mortality in models where the researchers substituted olive oil for vegetable oil, suggesting that vegetable oils may provide similar health benefits as olive oil.
The research was supported by grants from the National Institutes of Health. Dr. Guasch-Ferré was supported by the American Diabetes Association. Coauthor Salas-Salvadó is partially supported by the Catalan Institution for Research and Advanced Studies and received the virgin olive oil that was used in the PREDIMED and PREDIMED-Plus studies from the Patrimonio Communal Olivalero and Hojiblanca (Málaga, Spain). The other study authors and Dr. Larsson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Compared with men and women who rarely or never consumed olive oil (the lowest intake), those who consumed greater than 0.5 tablespoon/day or more than 7 g/day (the highest intake) had a 19% lower mortality risk over a 28-year follow-up, starting from an average age of 56 years.
Moreover, compared with those with the lowest olive oil intake, those with the highest intake had a 19% lower cardiovascular disease (CVD) mortality, a 17% lower risk of dying from cancer, a 29% lower risk of dying from neurodegenerative disease, and an 18% lower risk of dying from respiratory disease during follow-up.
The researchers estimate that replacing 10 g/day of margarine, butter, mayonnaise, or dairy fat with the same amount of olive oil is associated with an 8%-34% lower risk of death from various causes.
The study by Marta Guasch-Ferré, PhD, and colleagues was published online Jan. 10 in the Journal of the American College of Cardiology.
Results support plant-based dietary fat recommendations
“Our results support current dietary recommendations to increase the intake of olive oil and other unsaturated vegetable oils in place of other fats to improve overall health and longevity,” the researchers summarize.
However, “I wouldn’t say that olive oil is the only way to help you live longer,” Dr. Guasch-Ferré, a senior research scientist in the department of nutrition, Harvard T.H. Chan School of Public Health, Boston, cautioned in an interview with this news organization.
“Other things are very important, such as not smoking, doing physical activity, etc., but one recommendation could be to try to eat more plant-based food including olive oil and healthy fat,” she added, and to use it for cooking, salad dressing, and baking, and substitute it for saturated fat or animal fat, especially for cooking.
The study suggests that people should “consume a more plant-based diet and prioritize fatty acids such as olive oil because they have a better nutritional composition (high in phenols and antioxidants), instead of using butter or margarines or other animal fats that have been shown to have detrimental effects for health,” she added, which is consistent with recommendations in the Dietary Guidelines for Americans.
“That said,” Dr. Guasch-Ferré summarized, “replication is needed in other cohorts and populations to see if the results are similar.”
In an accompanying editorial, Susanna C. Larsson, PhD, writes that “this was a well-designed study, with long-term follow-up and repeated measurements of dietary intake and other risk factors for diseases.”
“However, the difference in olive oil consumption between those with the highest and those with the lowest/no olive oil consumption was very low (0.5 tablespoon) and a [12%] reduced mortality risk was observed already at a much lower intake (0.5 teaspoon, about 1.5 g/day) of olive oil,” she noted in an email to this news organization.
“It’s a bit hard to believe that such a small amount could have an independent effect on mortality risk,” Dr. Larsson, associate professor of epidemiology at the Karolinska Institutet, Stockholm, cautioned.
Like Dr. Guasch-Ferré, she noted that “just adding one or two teaspoons of olive oil to the diet each day will likely not change the risk of mortality.”
Rather, “people may need to make larger changes in the whole diet, not focus on fat only. An overall healthier diet, rich in nonrefined plant-based foods (vegetables, whole grains, nuts), low/no intake of processed foods, and a switch to healthier fat (eg, olive oil) is needed.”
Importantly, “this study cannot say anything about causality, that is, whether it’s olive oil specifically that reduces mortality risk or if there are many other beneficial factors that act together to reduce mortality rate among those with high olive oil consumption.”
The researchers acknowledge this observational study limitation and that the findings may not be generalizable to other populations.
Novel findings regarding Alzheimer’s and respiratory disease
Dr. Larsson highlights two novel findings of this study.
First, it showed a 27% reduction in risk of dementia-related mortality for those in the highest versus lowest category of olive oil consumption. “Considering the lack of preventive strategies for Alzheimer’s disease and the high morbidity and mortality related to this disease, this finding, if confirmed, is of great public health importance,” she said.
Second, the study reported an inverse association of olive oil consumption with risk of respiratory disease mortality. “Because residual confounding from smoking cannot be ruled out,” Dr. Larsson said, “this finding is tentative and requires confirmation in a study that is less susceptible to confounding, such as a randomized trial.”
And although the current study and previous studies have found that consumption of olive oil may have health benefits, she identified several remaining questions.
“Are the associations causal or spurious?” she noted. Is olive oil consumption protective for certain cardiovascular diseases like stroke or atrial fibrillation only, as has been shown in other studies, or also for other major diseases and causes of death, she added. What is the amount of olive oil required for a protective effect?
Further, is the potential effect related to monounsaturated fatty acids (MUFAs) or phenolic compounds; that is, “is the protective effect confined to polyphenol-rich extra-virgin olive oil or are refined olive oil and other vegetable oils as beneficial? More research is needed to address these questions,” she concludes.
“Further studies are needed,” the researchers agree, “to confirm the association of olive oil consumption with reduced mortality, clarify the mechanisms responsible, and quantify the dose/volume boundaries around this effect.”
Virgin olive oil has more polyphenols
Olive oil, a key component of the Mediterranean diet, is high in MUFAs, especially oleic acid, as well as vitamin E and polyphenols, which contribute to its anti-inflammatory and antioxidant properties, the researchers explain.
Virgin olive oil, produced by mechanically pressing ripe olives, contains multiple bioactive and antioxidant components and has an acidity of less than 1.5%. And extra-virgin olive oil is produced the same way but has a higher quality, more intense taste, and lower acidity (less than 1%).
Refined or processed olive oil contains less phytochemicals, as some are lost during processing; it usually contains more than 80% refined oil, plus virgin oil added back to enhance flavor, and may also be labeled “pure” or “light.” However, refined olive oil “still has a good amount of healthy fatty acids but less bioactive compounds,” Dr. Guasch-Ferré noted.
Until now, no large prospective study has examined the link between olive oil intake and all-cause and cause-specific mortality in a U.S. population, where olive oil consumption is limited, compared with Mediterranean countries.
The researchers identified 60,582 women in the Nurses’ Health Study and 31,801 men in the Health Professionals Follow-up Study who were free of CVD or cancer in 1990, the first year that food frequency questionnaires in these studies asked about olive oil.
Participants replied to questionnaires every 4 years that asked about use of olive oil (for salad dressing, baking, frying, sautéing, and spreading on bread), other vegetable oils (for example, corn, safflower, soybean, canola oil), margarine, butter, and dairy fat. The researchers averaged the consumption of these fats during the follow-up years.
From 1990 to 2019, the average consumption of olive oil increased from 1.6 g/day to 4 g/day. Margarine in the 1990s contained saturated fat and trans fats, whereas more recently margarine contains beneficial olive oil or vegetable fat, Dr. Guasch-Ferré noted.
Baseline olive oil consumption in this U.S. population “differed remarkably” from that in the Spanish population in the PREDIMED (Prevención con Dieta Mediterránea) trial, which was, on average, 20-22 g/day of extra-virgin olive oil and 16-18 g/day of refined/mixed olive oil, Larsson pointed out.
Because olive oil consumption was so low in this U.S. study, the researchers did not distinguish between virgin/extra-virgin olive oil and refined/processed olive oil.
The participants were almost all White (99%) and were generally healthier than the average U.S. population; on average, they had a body mass index of 25.3-25.8 kg/m2 and ate 4.8-7.2 fruits and vegetables/day.
Those with the highest olive oil consumption were more physically active, had a healthier diet, were more likely to have Southern European or Mediterranean ancestry, and were less likely to smoke.
During 28 years of follow-up, 36,856 participants died. The researchers classified the deaths into five categories: CVD, cancer, neurodegenerative disease (including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis), respiratory disease (such as chronic obstructive pulmonary disease), and all other causes (including suicide, injury, infections, diabetes, and kidney disease).
After adjusting for multiple confounders, compared with participants who rarely or never consumed olive oil, those in the highest quartile for olive oil consumption had a decreased risk of death from all causes (hazard ratio, 0.81; 95% confidence interval, 0.78 - 0.84) and from CVD (HR, 0.81; 95% CI, 0.75-0.87), cancer (HR, 0.83; 95% CI, 0.78-0.89), neurodegenerative disease (HR, 0.71; 95% CI, 0.64-0.78), and respiratory disease (HR, 0.82; 95% CI, 0.72-0.93).
There was no decrease in mortality in models where the researchers substituted olive oil for vegetable oil, suggesting that vegetable oils may provide similar health benefits as olive oil.
The research was supported by grants from the National Institutes of Health. Dr. Guasch-Ferré was supported by the American Diabetes Association. Coauthor Salas-Salvadó is partially supported by the Catalan Institution for Research and Advanced Studies and received the virgin olive oil that was used in the PREDIMED and PREDIMED-Plus studies from the Patrimonio Communal Olivalero and Hojiblanca (Málaga, Spain). The other study authors and Dr. Larsson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Compared with men and women who rarely or never consumed olive oil (the lowest intake), those who consumed greater than 0.5 tablespoon/day or more than 7 g/day (the highest intake) had a 19% lower mortality risk over a 28-year follow-up, starting from an average age of 56 years.
Moreover, compared with those with the lowest olive oil intake, those with the highest intake had a 19% lower cardiovascular disease (CVD) mortality, a 17% lower risk of dying from cancer, a 29% lower risk of dying from neurodegenerative disease, and an 18% lower risk of dying from respiratory disease during follow-up.
The researchers estimate that replacing 10 g/day of margarine, butter, mayonnaise, or dairy fat with the same amount of olive oil is associated with an 8%-34% lower risk of death from various causes.
The study by Marta Guasch-Ferré, PhD, and colleagues was published online Jan. 10 in the Journal of the American College of Cardiology.
Results support plant-based dietary fat recommendations
“Our results support current dietary recommendations to increase the intake of olive oil and other unsaturated vegetable oils in place of other fats to improve overall health and longevity,” the researchers summarize.
However, “I wouldn’t say that olive oil is the only way to help you live longer,” Dr. Guasch-Ferré, a senior research scientist in the department of nutrition, Harvard T.H. Chan School of Public Health, Boston, cautioned in an interview with this news organization.
“Other things are very important, such as not smoking, doing physical activity, etc., but one recommendation could be to try to eat more plant-based food including olive oil and healthy fat,” she added, and to use it for cooking, salad dressing, and baking, and substitute it for saturated fat or animal fat, especially for cooking.
The study suggests that people should “consume a more plant-based diet and prioritize fatty acids such as olive oil because they have a better nutritional composition (high in phenols and antioxidants), instead of using butter or margarines or other animal fats that have been shown to have detrimental effects for health,” she added, which is consistent with recommendations in the Dietary Guidelines for Americans.
“That said,” Dr. Guasch-Ferré summarized, “replication is needed in other cohorts and populations to see if the results are similar.”
In an accompanying editorial, Susanna C. Larsson, PhD, writes that “this was a well-designed study, with long-term follow-up and repeated measurements of dietary intake and other risk factors for diseases.”
“However, the difference in olive oil consumption between those with the highest and those with the lowest/no olive oil consumption was very low (0.5 tablespoon) and a [12%] reduced mortality risk was observed already at a much lower intake (0.5 teaspoon, about 1.5 g/day) of olive oil,” she noted in an email to this news organization.
“It’s a bit hard to believe that such a small amount could have an independent effect on mortality risk,” Dr. Larsson, associate professor of epidemiology at the Karolinska Institutet, Stockholm, cautioned.
Like Dr. Guasch-Ferré, she noted that “just adding one or two teaspoons of olive oil to the diet each day will likely not change the risk of mortality.”
Rather, “people may need to make larger changes in the whole diet, not focus on fat only. An overall healthier diet, rich in nonrefined plant-based foods (vegetables, whole grains, nuts), low/no intake of processed foods, and a switch to healthier fat (eg, olive oil) is needed.”
Importantly, “this study cannot say anything about causality, that is, whether it’s olive oil specifically that reduces mortality risk or if there are many other beneficial factors that act together to reduce mortality rate among those with high olive oil consumption.”
The researchers acknowledge this observational study limitation and that the findings may not be generalizable to other populations.
Novel findings regarding Alzheimer’s and respiratory disease
Dr. Larsson highlights two novel findings of this study.
First, it showed a 27% reduction in risk of dementia-related mortality for those in the highest versus lowest category of olive oil consumption. “Considering the lack of preventive strategies for Alzheimer’s disease and the high morbidity and mortality related to this disease, this finding, if confirmed, is of great public health importance,” she said.
Second, the study reported an inverse association of olive oil consumption with risk of respiratory disease mortality. “Because residual confounding from smoking cannot be ruled out,” Dr. Larsson said, “this finding is tentative and requires confirmation in a study that is less susceptible to confounding, such as a randomized trial.”
And although the current study and previous studies have found that consumption of olive oil may have health benefits, she identified several remaining questions.
“Are the associations causal or spurious?” she noted. Is olive oil consumption protective for certain cardiovascular diseases like stroke or atrial fibrillation only, as has been shown in other studies, or also for other major diseases and causes of death, she added. What is the amount of olive oil required for a protective effect?
Further, is the potential effect related to monounsaturated fatty acids (MUFAs) or phenolic compounds; that is, “is the protective effect confined to polyphenol-rich extra-virgin olive oil or are refined olive oil and other vegetable oils as beneficial? More research is needed to address these questions,” she concludes.
“Further studies are needed,” the researchers agree, “to confirm the association of olive oil consumption with reduced mortality, clarify the mechanisms responsible, and quantify the dose/volume boundaries around this effect.”
Virgin olive oil has more polyphenols
Olive oil, a key component of the Mediterranean diet, is high in MUFAs, especially oleic acid, as well as vitamin E and polyphenols, which contribute to its anti-inflammatory and antioxidant properties, the researchers explain.
Virgin olive oil, produced by mechanically pressing ripe olives, contains multiple bioactive and antioxidant components and has an acidity of less than 1.5%. And extra-virgin olive oil is produced the same way but has a higher quality, more intense taste, and lower acidity (less than 1%).
Refined or processed olive oil contains less phytochemicals, as some are lost during processing; it usually contains more than 80% refined oil, plus virgin oil added back to enhance flavor, and may also be labeled “pure” or “light.” However, refined olive oil “still has a good amount of healthy fatty acids but less bioactive compounds,” Dr. Guasch-Ferré noted.
Until now, no large prospective study has examined the link between olive oil intake and all-cause and cause-specific mortality in a U.S. population, where olive oil consumption is limited, compared with Mediterranean countries.
The researchers identified 60,582 women in the Nurses’ Health Study and 31,801 men in the Health Professionals Follow-up Study who were free of CVD or cancer in 1990, the first year that food frequency questionnaires in these studies asked about olive oil.
Participants replied to questionnaires every 4 years that asked about use of olive oil (for salad dressing, baking, frying, sautéing, and spreading on bread), other vegetable oils (for example, corn, safflower, soybean, canola oil), margarine, butter, and dairy fat. The researchers averaged the consumption of these fats during the follow-up years.
From 1990 to 2019, the average consumption of olive oil increased from 1.6 g/day to 4 g/day. Margarine in the 1990s contained saturated fat and trans fats, whereas more recently margarine contains beneficial olive oil or vegetable fat, Dr. Guasch-Ferré noted.
Baseline olive oil consumption in this U.S. population “differed remarkably” from that in the Spanish population in the PREDIMED (Prevención con Dieta Mediterránea) trial, which was, on average, 20-22 g/day of extra-virgin olive oil and 16-18 g/day of refined/mixed olive oil, Larsson pointed out.
Because olive oil consumption was so low in this U.S. study, the researchers did not distinguish between virgin/extra-virgin olive oil and refined/processed olive oil.
The participants were almost all White (99%) and were generally healthier than the average U.S. population; on average, they had a body mass index of 25.3-25.8 kg/m2 and ate 4.8-7.2 fruits and vegetables/day.
Those with the highest olive oil consumption were more physically active, had a healthier diet, were more likely to have Southern European or Mediterranean ancestry, and were less likely to smoke.
During 28 years of follow-up, 36,856 participants died. The researchers classified the deaths into five categories: CVD, cancer, neurodegenerative disease (including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis), respiratory disease (such as chronic obstructive pulmonary disease), and all other causes (including suicide, injury, infections, diabetes, and kidney disease).
After adjusting for multiple confounders, compared with participants who rarely or never consumed olive oil, those in the highest quartile for olive oil consumption had a decreased risk of death from all causes (hazard ratio, 0.81; 95% confidence interval, 0.78 - 0.84) and from CVD (HR, 0.81; 95% CI, 0.75-0.87), cancer (HR, 0.83; 95% CI, 0.78-0.89), neurodegenerative disease (HR, 0.71; 95% CI, 0.64-0.78), and respiratory disease (HR, 0.82; 95% CI, 0.72-0.93).
There was no decrease in mortality in models where the researchers substituted olive oil for vegetable oil, suggesting that vegetable oils may provide similar health benefits as olive oil.
The research was supported by grants from the National Institutes of Health. Dr. Guasch-Ferré was supported by the American Diabetes Association. Coauthor Salas-Salvadó is partially supported by the Catalan Institution for Research and Advanced Studies and received the virgin olive oil that was used in the PREDIMED and PREDIMED-Plus studies from the Patrimonio Communal Olivalero and Hojiblanca (Málaga, Spain). The other study authors and Dr. Larsson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S. reports record-breaking 1.35 million new COVID cases in a day
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.







