User login
New blood test may detect preclinical Alzheimer’s years in advance
, early research suggests.
Analysis of two studies showed the test (AlzoSure Predict), which uses less than 1 ml of blood, had numerous benefits compared with other blood tests that track AD pathology.
“We believe this has the potential to radically improve early stratification and identification of patients for trials 6 years in advance of a diagnosis, which can potentially enable more rapid and efficient approvals of therapies,” Paul Kinnon, CEO of Diadem, the test’s manufacturer, said in an interview.
The findings were presented at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Positive “discovery” results
P53, which is present in both the brain and elsewhere in the body, “is one of the most targeted proteins” for drug development in cancer and other conditions, said Mr. Kinnon.
The current blood test measures a derivative of P53 (U-p53AZ). Previous research suggests this derivative, which affects amyloid and oxidative stress, is also implicated in AD pathogenesis.
Researchers used blood samples from patients aged 60 years and older from the Australia Imaging, Biomarkers, and Lifestyles (AIBL) study who had various levels of cognitive function.
They analyzed samples at multiple timepoints over a 10-year period, “so we know when the marker is most accurate at predicting decline,” Mr. Kinnon said.
The first of two studies was considered a “discovery” study and included blood samples from 224 patients.
Results showed the test predicted decline from mild cognitive impairment (MCI) to AD at the end of 6 years, with an area under the curve (AUC) greater than 90%.
These results are “massive,” said Mr. Kinnon. “It’s the most accurate test I’ve seen anywhere for predicting decline of a patient.”
The test can also accurately classify a patient’s stage of cognition, he added. “Not only does it allow us to predict 6 years in advance, it also tells us if the patient has SMC [subjective memory complaints], MCI, or AD with a 95% certainty,” Mr. Kinnon said.
He noted that test sensitivity was higher than results found from traditional methods that are currently being used. The positive predictive value (PPV) and negative predictive value (NPV), which were at 90% or more, were “absolutely fantastic,” said Mr. Kinnon.
“Better than expected” results
In the second “validation” study, investigators examined samples from a completely different group of 482 patients. The “very compelling” results showed AUCs over 90%, PPVs over 90%, and “very high” NPVs, Mr. Kinnon said.
“These are great data, better than we expected,” he added.
However, he noted the test is “very specific” for decline to AD and not to other dementias.
In addition, Mr. Kinnon noted the test does not monitor levels of amyloid beta or tau, which accumulate at a later stage of AD. “Amyloid and tau tell you you’ve got it. We’re there way before those concentrations become detectable,” he said.
Identifying patients who will progress to AD years before they have symptoms gives them time to make medical decisions. These patients may also try treatments at an earlier stage of the disease, when these therapies are most likely to be helpful, said Mr. Kinnon.
In addition, using the test could speed up the approval of prospective drug treatments for AD. Currently, pharmaceutical companies enroll thousands of patients into a clinical study “and they don’t know which ones will have AD,” Mr. Kinnon noted.
“This test tells you these are the ones who are going to progress and should go into the study, and these are the ones that aren’t. So it makes the studies statistically relevant and accurate,” he said.
Investigators can also use the test to monitor patients during a study instead of relying on expensive PET scans and painful and costly spinal fluid taps, he added.
Previous surveys and market research have shown that neurologists and general practitioners “want a blood test to screen patients early, to help educate and inform patients,” said Mr. Kinnon.
Further results that will include biobank data on more than 1,000 patients in the United States and Europe are due for completion toward the end of this year.
The company is currently in negotiations to bring the product to North America, Europe, and elsewhere. “Our goal is to have it on the market by the middle of next year in multiple regions,” Mr. Kinnon said.
Encouraging, preliminary
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement at the Alzheimer’s Association, said “it’s exciting” to see development of novel ways for detecting or predicting AD.
“There is an urgent need for simple, inexpensive, noninvasive, and accessible early detection tools for Alzheimer’s, such as a blood test,” he said.
However, Dr. Griffin cautioned the test is still in the early stages of development and has not been tested extensively in large, diverse clinical trials.
In addition, although the test predicts whether a person will progress, it does not predict when the person will progress, he added.
“These preliminary results are encouraging, but further validation is needed before this test can be implemented widely,” he said.
Technologies that facilitate the early detection and intervention before significant loss of brain cells from AD “would be game-changing” for individuals, families, and the healthcare system, Dr. Griffin concluded.
A version of this article first appeared on Medscape.com.
, early research suggests.
Analysis of two studies showed the test (AlzoSure Predict), which uses less than 1 ml of blood, had numerous benefits compared with other blood tests that track AD pathology.
“We believe this has the potential to radically improve early stratification and identification of patients for trials 6 years in advance of a diagnosis, which can potentially enable more rapid and efficient approvals of therapies,” Paul Kinnon, CEO of Diadem, the test’s manufacturer, said in an interview.
The findings were presented at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Positive “discovery” results
P53, which is present in both the brain and elsewhere in the body, “is one of the most targeted proteins” for drug development in cancer and other conditions, said Mr. Kinnon.
The current blood test measures a derivative of P53 (U-p53AZ). Previous research suggests this derivative, which affects amyloid and oxidative stress, is also implicated in AD pathogenesis.
Researchers used blood samples from patients aged 60 years and older from the Australia Imaging, Biomarkers, and Lifestyles (AIBL) study who had various levels of cognitive function.
They analyzed samples at multiple timepoints over a 10-year period, “so we know when the marker is most accurate at predicting decline,” Mr. Kinnon said.
The first of two studies was considered a “discovery” study and included blood samples from 224 patients.
Results showed the test predicted decline from mild cognitive impairment (MCI) to AD at the end of 6 years, with an area under the curve (AUC) greater than 90%.
These results are “massive,” said Mr. Kinnon. “It’s the most accurate test I’ve seen anywhere for predicting decline of a patient.”
The test can also accurately classify a patient’s stage of cognition, he added. “Not only does it allow us to predict 6 years in advance, it also tells us if the patient has SMC [subjective memory complaints], MCI, or AD with a 95% certainty,” Mr. Kinnon said.
He noted that test sensitivity was higher than results found from traditional methods that are currently being used. The positive predictive value (PPV) and negative predictive value (NPV), which were at 90% or more, were “absolutely fantastic,” said Mr. Kinnon.
“Better than expected” results
In the second “validation” study, investigators examined samples from a completely different group of 482 patients. The “very compelling” results showed AUCs over 90%, PPVs over 90%, and “very high” NPVs, Mr. Kinnon said.
“These are great data, better than we expected,” he added.
However, he noted the test is “very specific” for decline to AD and not to other dementias.
In addition, Mr. Kinnon noted the test does not monitor levels of amyloid beta or tau, which accumulate at a later stage of AD. “Amyloid and tau tell you you’ve got it. We’re there way before those concentrations become detectable,” he said.
Identifying patients who will progress to AD years before they have symptoms gives them time to make medical decisions. These patients may also try treatments at an earlier stage of the disease, when these therapies are most likely to be helpful, said Mr. Kinnon.
In addition, using the test could speed up the approval of prospective drug treatments for AD. Currently, pharmaceutical companies enroll thousands of patients into a clinical study “and they don’t know which ones will have AD,” Mr. Kinnon noted.
“This test tells you these are the ones who are going to progress and should go into the study, and these are the ones that aren’t. So it makes the studies statistically relevant and accurate,” he said.
Investigators can also use the test to monitor patients during a study instead of relying on expensive PET scans and painful and costly spinal fluid taps, he added.
Previous surveys and market research have shown that neurologists and general practitioners “want a blood test to screen patients early, to help educate and inform patients,” said Mr. Kinnon.
Further results that will include biobank data on more than 1,000 patients in the United States and Europe are due for completion toward the end of this year.
The company is currently in negotiations to bring the product to North America, Europe, and elsewhere. “Our goal is to have it on the market by the middle of next year in multiple regions,” Mr. Kinnon said.
Encouraging, preliminary
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement at the Alzheimer’s Association, said “it’s exciting” to see development of novel ways for detecting or predicting AD.
“There is an urgent need for simple, inexpensive, noninvasive, and accessible early detection tools for Alzheimer’s, such as a blood test,” he said.
However, Dr. Griffin cautioned the test is still in the early stages of development and has not been tested extensively in large, diverse clinical trials.
In addition, although the test predicts whether a person will progress, it does not predict when the person will progress, he added.
“These preliminary results are encouraging, but further validation is needed before this test can be implemented widely,” he said.
Technologies that facilitate the early detection and intervention before significant loss of brain cells from AD “would be game-changing” for individuals, families, and the healthcare system, Dr. Griffin concluded.
A version of this article first appeared on Medscape.com.
, early research suggests.
Analysis of two studies showed the test (AlzoSure Predict), which uses less than 1 ml of blood, had numerous benefits compared with other blood tests that track AD pathology.
“We believe this has the potential to radically improve early stratification and identification of patients for trials 6 years in advance of a diagnosis, which can potentially enable more rapid and efficient approvals of therapies,” Paul Kinnon, CEO of Diadem, the test’s manufacturer, said in an interview.
The findings were presented at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Positive “discovery” results
P53, which is present in both the brain and elsewhere in the body, “is one of the most targeted proteins” for drug development in cancer and other conditions, said Mr. Kinnon.
The current blood test measures a derivative of P53 (U-p53AZ). Previous research suggests this derivative, which affects amyloid and oxidative stress, is also implicated in AD pathogenesis.
Researchers used blood samples from patients aged 60 years and older from the Australia Imaging, Biomarkers, and Lifestyles (AIBL) study who had various levels of cognitive function.
They analyzed samples at multiple timepoints over a 10-year period, “so we know when the marker is most accurate at predicting decline,” Mr. Kinnon said.
The first of two studies was considered a “discovery” study and included blood samples from 224 patients.
Results showed the test predicted decline from mild cognitive impairment (MCI) to AD at the end of 6 years, with an area under the curve (AUC) greater than 90%.
These results are “massive,” said Mr. Kinnon. “It’s the most accurate test I’ve seen anywhere for predicting decline of a patient.”
The test can also accurately classify a patient’s stage of cognition, he added. “Not only does it allow us to predict 6 years in advance, it also tells us if the patient has SMC [subjective memory complaints], MCI, or AD with a 95% certainty,” Mr. Kinnon said.
He noted that test sensitivity was higher than results found from traditional methods that are currently being used. The positive predictive value (PPV) and negative predictive value (NPV), which were at 90% or more, were “absolutely fantastic,” said Mr. Kinnon.
“Better than expected” results
In the second “validation” study, investigators examined samples from a completely different group of 482 patients. The “very compelling” results showed AUCs over 90%, PPVs over 90%, and “very high” NPVs, Mr. Kinnon said.
“These are great data, better than we expected,” he added.
However, he noted the test is “very specific” for decline to AD and not to other dementias.
In addition, Mr. Kinnon noted the test does not monitor levels of amyloid beta or tau, which accumulate at a later stage of AD. “Amyloid and tau tell you you’ve got it. We’re there way before those concentrations become detectable,” he said.
Identifying patients who will progress to AD years before they have symptoms gives them time to make medical decisions. These patients may also try treatments at an earlier stage of the disease, when these therapies are most likely to be helpful, said Mr. Kinnon.
In addition, using the test could speed up the approval of prospective drug treatments for AD. Currently, pharmaceutical companies enroll thousands of patients into a clinical study “and they don’t know which ones will have AD,” Mr. Kinnon noted.
“This test tells you these are the ones who are going to progress and should go into the study, and these are the ones that aren’t. So it makes the studies statistically relevant and accurate,” he said.
Investigators can also use the test to monitor patients during a study instead of relying on expensive PET scans and painful and costly spinal fluid taps, he added.
Previous surveys and market research have shown that neurologists and general practitioners “want a blood test to screen patients early, to help educate and inform patients,” said Mr. Kinnon.
Further results that will include biobank data on more than 1,000 patients in the United States and Europe are due for completion toward the end of this year.
The company is currently in negotiations to bring the product to North America, Europe, and elsewhere. “Our goal is to have it on the market by the middle of next year in multiple regions,” Mr. Kinnon said.
Encouraging, preliminary
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement at the Alzheimer’s Association, said “it’s exciting” to see development of novel ways for detecting or predicting AD.
“There is an urgent need for simple, inexpensive, noninvasive, and accessible early detection tools for Alzheimer’s, such as a blood test,” he said.
However, Dr. Griffin cautioned the test is still in the early stages of development and has not been tested extensively in large, diverse clinical trials.
In addition, although the test predicts whether a person will progress, it does not predict when the person will progress, he added.
“These preliminary results are encouraging, but further validation is needed before this test can be implemented widely,” he said.
Technologies that facilitate the early detection and intervention before significant loss of brain cells from AD “would be game-changing” for individuals, families, and the healthcare system, Dr. Griffin concluded.
A version of this article first appeared on Medscape.com.
FROM CTAD21
Headache is a common post–COVID-19 complaint
The Centers for Disease Control and Prevention has identified it as a sentinel symptom of COVID-19 disease. “A lot of the recommendations surrounding post-COVID headache is that if you identify a patient who has headaches associated with fever, and myalgia, and other systemic symptoms, the specificity of a COVID-19 diagnosis goes up. So [COVID-19] is a really important feature to look out for in patients with headache,” Deena Kuruvilla, MD, said during a presentation on post–COVID-19 headache at the 2021 Scottsdale Headache Symposium.
Estimates of the prevalence of headache in COVID-19 range widely, from 6.5% to 71%, but Dr. Kuruvilla has plenty of personal experience with it. “During my stint on the inpatient neurology service during the peak of COVID, I saw patients with headache being one of the most frequent complaints, [along with] dizziness, stroke, and seizure among many other neurological manifestations,” said Dr. Kuruvilla, director of the Westport (Conn.) Headache Institute.
One meta-analysis showed that 47% of patients with COVID-19 complain of headache within 30 days of diagnosis, and this drops to around 10% at 60-90 days, and around 8% at 180 days.
A survey of 3,458 patients, published in the Journal of Headache Pain, found that migraine is the most common type of post–COVID-19 headache phenotype, and patients reporting anosmia-ageusia were more likely to have post–COVID-19 headache (odds ratio [OR], 5.39; 95% confidence interval, 1.66-17.45).
A case-control study of post–COVID-19 headache patients with and without a history of migraine found that those with a history of migraine were more likely to have post–COVID-19 symptoms (OR, 1.70; P < .001) and fatigue (OR, 2.89; P = .008). “Interestingly, they found no difference in headache as post-COVID symptoms in people who had a history of migraine compared with people without a history of migraine,” said Dr. Kuruvilla.
Headache and COVID-19: What is the connection?
Several mechanisms have been proposed for direct invasion of the central nervous system, either via infection through the angiotensin-converting enzyme 2 (ACE-2) receptor, which is expressed in brain regions including the motor cortex, the posterior cingulate cortex, and the olfactory bulb, among other locations. Another potential mechanism is direct entry through the olfactory nerve and the associated olfactory epithelium. There are various potential mechanisms for spread among the peripheral nervous system, and the blood-brain barrier can be compromised by infection of vascular endothelial cells. According to the literature, neuronal damage seems to occur directly from viral damage rather than from the immune response, said Dr. Kuruvilla.
The virus may also gain entry to the CNS indirectly, as a result of hypoxia and metabolic disturbances, as well as dehydration and systematic inflammation. The cytokine storm associated with COVID-19 infection can activate C-reactive protein and calcitonin gene-related peptide (CGRP), which plays a key role in migraine pathology. The CGRP receptor antagonist vazegepant is being studied in a phase 2 clinical trial for the treatment of COVID-19–related lung inflammation.
Testing and treatment
“If I see patients with new headache, worsening headache from their baseline, or headache with systemic symptoms, I often consider screening them for COVID. If that screening is positive, I proceed with PCR testing. I also consider an MRI of the brain with and without gadolinium just to rule out any secondary causes for headache,” said Dr. Kuruvilla, noting that she has diagnosed patients with venous sinus thrombosis, ischemic stroke, and meningitis following COVID-19.
The existing literature suggests that lumbar puncture in patients with SARS-CoV-2 typically returns normal results, but Dr. Kuruvilla proceeds with it anyway with viral, bacterial, fungal, and autoimmune studies to rule out potential secondary causes for headache.
There are few studies on how to treat post–COVID-19 headache, and the general recommendation is that headache phenotype should drive treatment decisions.
In a case series, three patients with persistent headache following mild COVID-19 infection were treated with onabotulinumtoxinA and amitriptyline. They had daily headaches, along with post–COVID-19 symptoms including fatigue and insomnia. After treatment, each patient converted to episodic headaches.
One retrospective study of 37 patients found that a 5-day course of indomethacin 50 mg twice per day and pantoprazole 40 mg once per day was associated with a 50% or greater improvement in headache on the third day in 36 of the 37 patients. Five patients were free of pain by day 5.
A common problem
Neurologists have been involved in the treatment of COVID-19 since the beginning, and post–COVID-19 headache has added another layer. “It’s been a remarkably common clinical problem. And the fact that it’s actually reached the level of headache specialist actually shows that in some cases, it’s really quite a significant problem, in both its severity and persistence. So I think it’s a very, very significant issue,” said Andrew Charles, MD, professor of neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program.
Dr. Kuruvilla also discussed the question of whether neurological damage is due to direct damage from the virus, or indirect damage from an immune response. This was debated during the Q&A session following Dr. Kuruvilla’s talk, and it was pointed out that headache is a frequent side effect of the Pfizer and Moderna vaccines.
“It’s a huge open question about how much is direct invasion or damage or not even damage, but just change in function with the viral infection, as opposed to inflammation. The fact that very often the response to the vaccine is similar to what you see with COVID suggests that at least some component of it is inflammation. I wouldn’t commit to one mechanism or the other, but I’d say that it’s possible that it’s really both,” said Dr. Charles.
Dr. Kuruvilla has consulted for Cefaly, Neurolief, Theranica, Now What Media, and KX advisors. She has been on the speakers bureau for Abbvie/Allergan, Amgen/Novartis, and Lilly. She has been on advisory boards for Abbvie/Allergan, Lilly, Theranica, and Amgen/Novartis. Dr. Charles has no relevant financial disclosures.
The Centers for Disease Control and Prevention has identified it as a sentinel symptom of COVID-19 disease. “A lot of the recommendations surrounding post-COVID headache is that if you identify a patient who has headaches associated with fever, and myalgia, and other systemic symptoms, the specificity of a COVID-19 diagnosis goes up. So [COVID-19] is a really important feature to look out for in patients with headache,” Deena Kuruvilla, MD, said during a presentation on post–COVID-19 headache at the 2021 Scottsdale Headache Symposium.
Estimates of the prevalence of headache in COVID-19 range widely, from 6.5% to 71%, but Dr. Kuruvilla has plenty of personal experience with it. “During my stint on the inpatient neurology service during the peak of COVID, I saw patients with headache being one of the most frequent complaints, [along with] dizziness, stroke, and seizure among many other neurological manifestations,” said Dr. Kuruvilla, director of the Westport (Conn.) Headache Institute.
One meta-analysis showed that 47% of patients with COVID-19 complain of headache within 30 days of diagnosis, and this drops to around 10% at 60-90 days, and around 8% at 180 days.
A survey of 3,458 patients, published in the Journal of Headache Pain, found that migraine is the most common type of post–COVID-19 headache phenotype, and patients reporting anosmia-ageusia were more likely to have post–COVID-19 headache (odds ratio [OR], 5.39; 95% confidence interval, 1.66-17.45).
A case-control study of post–COVID-19 headache patients with and without a history of migraine found that those with a history of migraine were more likely to have post–COVID-19 symptoms (OR, 1.70; P < .001) and fatigue (OR, 2.89; P = .008). “Interestingly, they found no difference in headache as post-COVID symptoms in people who had a history of migraine compared with people without a history of migraine,” said Dr. Kuruvilla.
Headache and COVID-19: What is the connection?
Several mechanisms have been proposed for direct invasion of the central nervous system, either via infection through the angiotensin-converting enzyme 2 (ACE-2) receptor, which is expressed in brain regions including the motor cortex, the posterior cingulate cortex, and the olfactory bulb, among other locations. Another potential mechanism is direct entry through the olfactory nerve and the associated olfactory epithelium. There are various potential mechanisms for spread among the peripheral nervous system, and the blood-brain barrier can be compromised by infection of vascular endothelial cells. According to the literature, neuronal damage seems to occur directly from viral damage rather than from the immune response, said Dr. Kuruvilla.
The virus may also gain entry to the CNS indirectly, as a result of hypoxia and metabolic disturbances, as well as dehydration and systematic inflammation. The cytokine storm associated with COVID-19 infection can activate C-reactive protein and calcitonin gene-related peptide (CGRP), which plays a key role in migraine pathology. The CGRP receptor antagonist vazegepant is being studied in a phase 2 clinical trial for the treatment of COVID-19–related lung inflammation.
Testing and treatment
“If I see patients with new headache, worsening headache from their baseline, or headache with systemic symptoms, I often consider screening them for COVID. If that screening is positive, I proceed with PCR testing. I also consider an MRI of the brain with and without gadolinium just to rule out any secondary causes for headache,” said Dr. Kuruvilla, noting that she has diagnosed patients with venous sinus thrombosis, ischemic stroke, and meningitis following COVID-19.
The existing literature suggests that lumbar puncture in patients with SARS-CoV-2 typically returns normal results, but Dr. Kuruvilla proceeds with it anyway with viral, bacterial, fungal, and autoimmune studies to rule out potential secondary causes for headache.
There are few studies on how to treat post–COVID-19 headache, and the general recommendation is that headache phenotype should drive treatment decisions.
In a case series, three patients with persistent headache following mild COVID-19 infection were treated with onabotulinumtoxinA and amitriptyline. They had daily headaches, along with post–COVID-19 symptoms including fatigue and insomnia. After treatment, each patient converted to episodic headaches.
One retrospective study of 37 patients found that a 5-day course of indomethacin 50 mg twice per day and pantoprazole 40 mg once per day was associated with a 50% or greater improvement in headache on the third day in 36 of the 37 patients. Five patients were free of pain by day 5.
A common problem
Neurologists have been involved in the treatment of COVID-19 since the beginning, and post–COVID-19 headache has added another layer. “It’s been a remarkably common clinical problem. And the fact that it’s actually reached the level of headache specialist actually shows that in some cases, it’s really quite a significant problem, in both its severity and persistence. So I think it’s a very, very significant issue,” said Andrew Charles, MD, professor of neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program.
Dr. Kuruvilla also discussed the question of whether neurological damage is due to direct damage from the virus, or indirect damage from an immune response. This was debated during the Q&A session following Dr. Kuruvilla’s talk, and it was pointed out that headache is a frequent side effect of the Pfizer and Moderna vaccines.
“It’s a huge open question about how much is direct invasion or damage or not even damage, but just change in function with the viral infection, as opposed to inflammation. The fact that very often the response to the vaccine is similar to what you see with COVID suggests that at least some component of it is inflammation. I wouldn’t commit to one mechanism or the other, but I’d say that it’s possible that it’s really both,” said Dr. Charles.
Dr. Kuruvilla has consulted for Cefaly, Neurolief, Theranica, Now What Media, and KX advisors. She has been on the speakers bureau for Abbvie/Allergan, Amgen/Novartis, and Lilly. She has been on advisory boards for Abbvie/Allergan, Lilly, Theranica, and Amgen/Novartis. Dr. Charles has no relevant financial disclosures.
The Centers for Disease Control and Prevention has identified it as a sentinel symptom of COVID-19 disease. “A lot of the recommendations surrounding post-COVID headache is that if you identify a patient who has headaches associated with fever, and myalgia, and other systemic symptoms, the specificity of a COVID-19 diagnosis goes up. So [COVID-19] is a really important feature to look out for in patients with headache,” Deena Kuruvilla, MD, said during a presentation on post–COVID-19 headache at the 2021 Scottsdale Headache Symposium.
Estimates of the prevalence of headache in COVID-19 range widely, from 6.5% to 71%, but Dr. Kuruvilla has plenty of personal experience with it. “During my stint on the inpatient neurology service during the peak of COVID, I saw patients with headache being one of the most frequent complaints, [along with] dizziness, stroke, and seizure among many other neurological manifestations,” said Dr. Kuruvilla, director of the Westport (Conn.) Headache Institute.
One meta-analysis showed that 47% of patients with COVID-19 complain of headache within 30 days of diagnosis, and this drops to around 10% at 60-90 days, and around 8% at 180 days.
A survey of 3,458 patients, published in the Journal of Headache Pain, found that migraine is the most common type of post–COVID-19 headache phenotype, and patients reporting anosmia-ageusia were more likely to have post–COVID-19 headache (odds ratio [OR], 5.39; 95% confidence interval, 1.66-17.45).
A case-control study of post–COVID-19 headache patients with and without a history of migraine found that those with a history of migraine were more likely to have post–COVID-19 symptoms (OR, 1.70; P < .001) and fatigue (OR, 2.89; P = .008). “Interestingly, they found no difference in headache as post-COVID symptoms in people who had a history of migraine compared with people without a history of migraine,” said Dr. Kuruvilla.
Headache and COVID-19: What is the connection?
Several mechanisms have been proposed for direct invasion of the central nervous system, either via infection through the angiotensin-converting enzyme 2 (ACE-2) receptor, which is expressed in brain regions including the motor cortex, the posterior cingulate cortex, and the olfactory bulb, among other locations. Another potential mechanism is direct entry through the olfactory nerve and the associated olfactory epithelium. There are various potential mechanisms for spread among the peripheral nervous system, and the blood-brain barrier can be compromised by infection of vascular endothelial cells. According to the literature, neuronal damage seems to occur directly from viral damage rather than from the immune response, said Dr. Kuruvilla.
The virus may also gain entry to the CNS indirectly, as a result of hypoxia and metabolic disturbances, as well as dehydration and systematic inflammation. The cytokine storm associated with COVID-19 infection can activate C-reactive protein and calcitonin gene-related peptide (CGRP), which plays a key role in migraine pathology. The CGRP receptor antagonist vazegepant is being studied in a phase 2 clinical trial for the treatment of COVID-19–related lung inflammation.
Testing and treatment
“If I see patients with new headache, worsening headache from their baseline, or headache with systemic symptoms, I often consider screening them for COVID. If that screening is positive, I proceed with PCR testing. I also consider an MRI of the brain with and without gadolinium just to rule out any secondary causes for headache,” said Dr. Kuruvilla, noting that she has diagnosed patients with venous sinus thrombosis, ischemic stroke, and meningitis following COVID-19.
The existing literature suggests that lumbar puncture in patients with SARS-CoV-2 typically returns normal results, but Dr. Kuruvilla proceeds with it anyway with viral, bacterial, fungal, and autoimmune studies to rule out potential secondary causes for headache.
There are few studies on how to treat post–COVID-19 headache, and the general recommendation is that headache phenotype should drive treatment decisions.
In a case series, three patients with persistent headache following mild COVID-19 infection were treated with onabotulinumtoxinA and amitriptyline. They had daily headaches, along with post–COVID-19 symptoms including fatigue and insomnia. After treatment, each patient converted to episodic headaches.
One retrospective study of 37 patients found that a 5-day course of indomethacin 50 mg twice per day and pantoprazole 40 mg once per day was associated with a 50% or greater improvement in headache on the third day in 36 of the 37 patients. Five patients were free of pain by day 5.
A common problem
Neurologists have been involved in the treatment of COVID-19 since the beginning, and post–COVID-19 headache has added another layer. “It’s been a remarkably common clinical problem. And the fact that it’s actually reached the level of headache specialist actually shows that in some cases, it’s really quite a significant problem, in both its severity and persistence. So I think it’s a very, very significant issue,” said Andrew Charles, MD, professor of neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program.
Dr. Kuruvilla also discussed the question of whether neurological damage is due to direct damage from the virus, or indirect damage from an immune response. This was debated during the Q&A session following Dr. Kuruvilla’s talk, and it was pointed out that headache is a frequent side effect of the Pfizer and Moderna vaccines.
“It’s a huge open question about how much is direct invasion or damage or not even damage, but just change in function with the viral infection, as opposed to inflammation. The fact that very often the response to the vaccine is similar to what you see with COVID suggests that at least some component of it is inflammation. I wouldn’t commit to one mechanism or the other, but I’d say that it’s possible that it’s really both,” said Dr. Charles.
Dr. Kuruvilla has consulted for Cefaly, Neurolief, Theranica, Now What Media, and KX advisors. She has been on the speakers bureau for Abbvie/Allergan, Amgen/Novartis, and Lilly. She has been on advisory boards for Abbvie/Allergan, Lilly, Theranica, and Amgen/Novartis. Dr. Charles has no relevant financial disclosures.
FROM 2021 SCOTTSDALE HEADACHE SYMPOSIUM
Psychiatrist’s killer gets life in prison
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
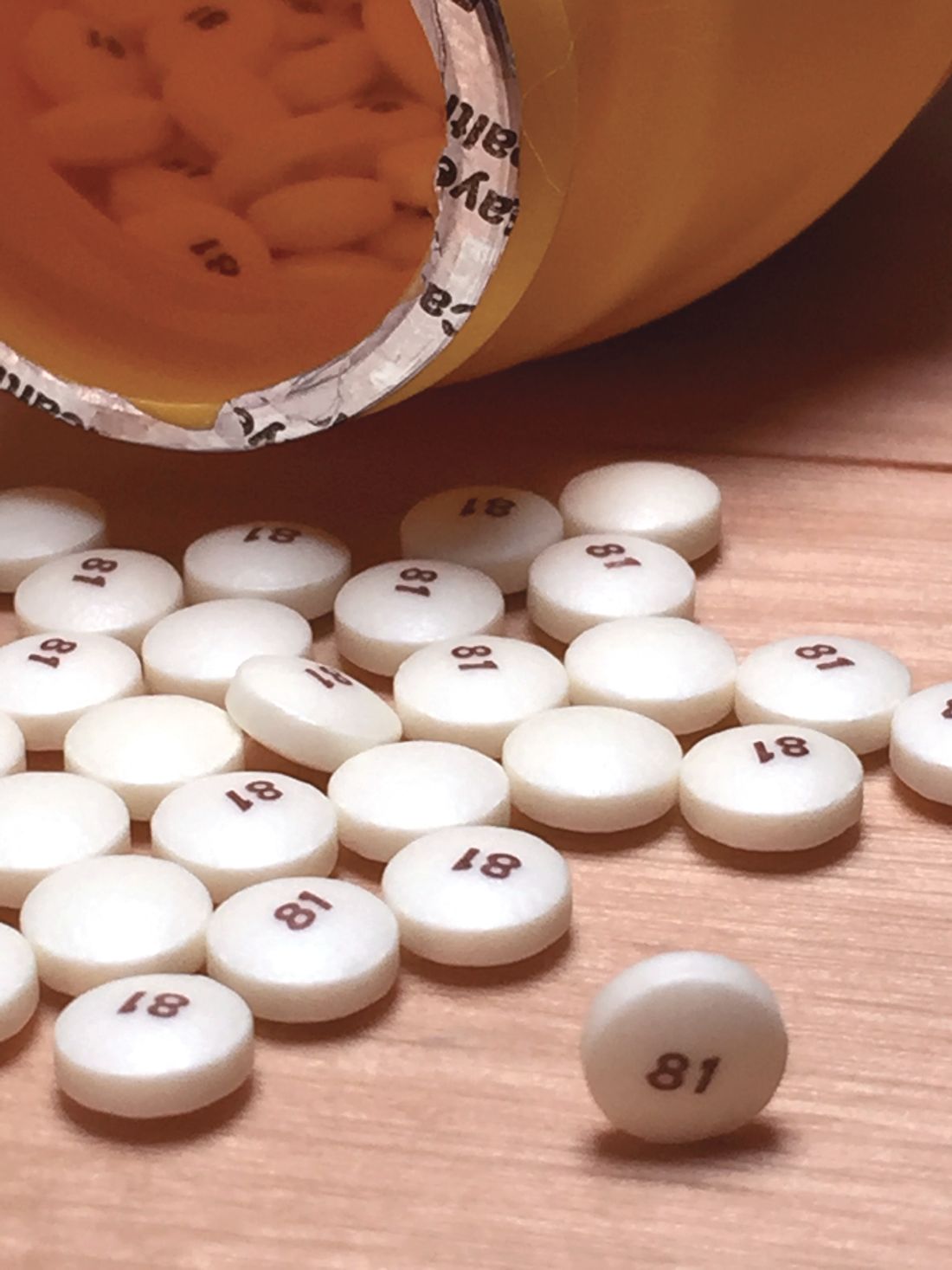
Daily aspirin linked to increased risk of heart failure
Daily aspirin is associated with new onset heart failure independent of other risk factors, according to data derived from a database with follow-up from more than 30,000 patients who did not have HF when they were enrolled.
These data are not relevant to primary or secondary prevention of cardiovascular events but “refer only to starting aspirin for secondary prevention of HF in patients at high risk of HF or with symptomatic HF,” according to the senior investigator, Jan A. Staessen, MD, PhD, professor emeritus at the University of Leuven (Belgium).
In data from 30,827 patients at risk for HF enrolled in six observational studies, the hazard ratio (HR) for developing HF among those taking daily aspirin at baseline relative to those who were not was 1.26 (P ≤ .001) over 5.3 years of follow-up. In the 22,690 patients without a prior history cardiovascular disease (CVD), the HF risk increase for exposure to daily aspirin was about the same (HR 1.27; P = .001).
This study was launched because multiple conflicting studies have made the relationship between aspirin and HF risk unclear, according to the multinational team of authors, whose finding were published in ESC Heart Failure.
In principle, HF is recognized as a prothrombotic condition for which an antithrombotic therapy such as aspirin would be expected to have a protective role, but the investigators pointed out that the evidence is mixed. In a population-based Danish study of 12,277 patients with new-onset HF, for example, there was no relationship seen between aspirin use and a reduction in the composite outcome of all-cause mortality, myocardial infarction, or stroke.
Aspirin use linked to HF admissions
“Interestingly, this study reported that aspirin use was associated with an increased risk of readmissions for HF,” wrote the authors of the newly published data. “Uncertainty on aspirin use has been reflected in current guideline recommendations,” they added.
The population studied was drawn from the HOMAGE database, which has collated data on 46,437 participants in 21 studies. After the exclusion of studies with patients who already had HF as well as studies without information on HF incidence over time, six studies with 30,827 participants provided the basis for this analysis.
One study, ASCOT, which was randomized and blinded, served as the derivation data set. The remaining five studies, FLEMENGHO, HEALTH ABC, HULL LIFE LAB, PREDICTOR, and PROSPER, served as the validation data set.
In addition to identifying participants as aspirin users or nonusers at baseline, all of the studies had detailed baseline data on a wide variety of patient characteristics and risk factors, such as body mass index, blood cholesterol levels, blood glucose concentrations, blood pressure, and creatinine.
No patient in any trial was on an antithrombotic therapy other than aspirin at baseline.
Of the minority of patients with CVD at baseline, more than 80% had coronary heart disease. Only 2.8% of the total population had a prior myocardial infarction. In the study population overall, most (86%) had hypertension, and there was a sizeable proportion with diabetes (22%). The average age was 67 years, and 34% were women.
HF incidence on aspirin: 14.5/1000 person-years
Overall, the incidence rate of HF per 1,000 person-years for the entire population before adjustment was 14.5 in the group on daily aspirin versus 5.9 in the non-aspirin group. These absolute rates were lower in the discovery data set than in the validation set, but the relative differences in HF incidence rates for those who were versus those who were not on aspirin at baseline were similar.
Numerous sensitivity analyses supported the basic conclusions. This not only included one omitting patients with a history of CVD, but another that excluded patients who developed HF within the first 2 years. Stratified analyses looking for overall consistency across variables showed increased risk of new onset heart failure among those taking daily aspirin regardless of relative age, body weight, or blood pressure levels.
The most important limitation of this study was that it evaluated data taken from studies not originally designed to test the study hypothesis. In addition, only baseline data were available, so the drugs that patients took over the course of follow-up are unknown. However, the authors believe these data have a clinical message.
Given the consistency of these results, “our observations suggest that aspirin should be prescribed with caution in patients at risk of HF or having HF,” the investigators concluded.
“If such treatment is initiated in these patients, use low-dose aspirin,” Dr. Staessen told this news organization.
Aspirin for CVD versus HF risk
Many patients take low-dose aspirin to prevent the types of cardiovascular events, such as MI, that lead to heart failure. In attempting to address a controversy regarding aspirin and risk of new onset heart failure, it appears to create another regarding CVD risk reduction.
Deepak L. Bhatt, MD, executive director of Interventional Cardiovascular Programs at Brigham and Women’s Health, Boston, expressed some reluctance in applying these data to routine practice.
“It is important to emphasize that this pooled analysis draws upon six observational studies, not randomized trials of aspirin,” Dr. Bhatt said.
He called these findings “provocative,” but he said they “would need to be confirmed in databases of already completed randomized trials of aspirin versus a control before being actionable.” For Dr. Bhatt, one obstacle to a change in practice based on these data is that, “to my knowledge, no such signal [of a relationship between aspirin and incident heart failure] exists in the cumulative randomized data.”
Dr. Staessen reports no potential conflicts of interest for this study. Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PLx Pharma, for which he performs aspirin-related research.
Daily aspirin is associated with new onset heart failure independent of other risk factors, according to data derived from a database with follow-up from more than 30,000 patients who did not have HF when they were enrolled.
These data are not relevant to primary or secondary prevention of cardiovascular events but “refer only to starting aspirin for secondary prevention of HF in patients at high risk of HF or with symptomatic HF,” according to the senior investigator, Jan A. Staessen, MD, PhD, professor emeritus at the University of Leuven (Belgium).
In data from 30,827 patients at risk for HF enrolled in six observational studies, the hazard ratio (HR) for developing HF among those taking daily aspirin at baseline relative to those who were not was 1.26 (P ≤ .001) over 5.3 years of follow-up. In the 22,690 patients without a prior history cardiovascular disease (CVD), the HF risk increase for exposure to daily aspirin was about the same (HR 1.27; P = .001).
This study was launched because multiple conflicting studies have made the relationship between aspirin and HF risk unclear, according to the multinational team of authors, whose finding were published in ESC Heart Failure.
In principle, HF is recognized as a prothrombotic condition for which an antithrombotic therapy such as aspirin would be expected to have a protective role, but the investigators pointed out that the evidence is mixed. In a population-based Danish study of 12,277 patients with new-onset HF, for example, there was no relationship seen between aspirin use and a reduction in the composite outcome of all-cause mortality, myocardial infarction, or stroke.
Aspirin use linked to HF admissions
“Interestingly, this study reported that aspirin use was associated with an increased risk of readmissions for HF,” wrote the authors of the newly published data. “Uncertainty on aspirin use has been reflected in current guideline recommendations,” they added.
The population studied was drawn from the HOMAGE database, which has collated data on 46,437 participants in 21 studies. After the exclusion of studies with patients who already had HF as well as studies without information on HF incidence over time, six studies with 30,827 participants provided the basis for this analysis.
One study, ASCOT, which was randomized and blinded, served as the derivation data set. The remaining five studies, FLEMENGHO, HEALTH ABC, HULL LIFE LAB, PREDICTOR, and PROSPER, served as the validation data set.
In addition to identifying participants as aspirin users or nonusers at baseline, all of the studies had detailed baseline data on a wide variety of patient characteristics and risk factors, such as body mass index, blood cholesterol levels, blood glucose concentrations, blood pressure, and creatinine.
No patient in any trial was on an antithrombotic therapy other than aspirin at baseline.
Of the minority of patients with CVD at baseline, more than 80% had coronary heart disease. Only 2.8% of the total population had a prior myocardial infarction. In the study population overall, most (86%) had hypertension, and there was a sizeable proportion with diabetes (22%). The average age was 67 years, and 34% were women.
HF incidence on aspirin: 14.5/1000 person-years
Overall, the incidence rate of HF per 1,000 person-years for the entire population before adjustment was 14.5 in the group on daily aspirin versus 5.9 in the non-aspirin group. These absolute rates were lower in the discovery data set than in the validation set, but the relative differences in HF incidence rates for those who were versus those who were not on aspirin at baseline were similar.
Numerous sensitivity analyses supported the basic conclusions. This not only included one omitting patients with a history of CVD, but another that excluded patients who developed HF within the first 2 years. Stratified analyses looking for overall consistency across variables showed increased risk of new onset heart failure among those taking daily aspirin regardless of relative age, body weight, or blood pressure levels.
The most important limitation of this study was that it evaluated data taken from studies not originally designed to test the study hypothesis. In addition, only baseline data were available, so the drugs that patients took over the course of follow-up are unknown. However, the authors believe these data have a clinical message.
Given the consistency of these results, “our observations suggest that aspirin should be prescribed with caution in patients at risk of HF or having HF,” the investigators concluded.
“If such treatment is initiated in these patients, use low-dose aspirin,” Dr. Staessen told this news organization.
Aspirin for CVD versus HF risk
Many patients take low-dose aspirin to prevent the types of cardiovascular events, such as MI, that lead to heart failure. In attempting to address a controversy regarding aspirin and risk of new onset heart failure, it appears to create another regarding CVD risk reduction.
Deepak L. Bhatt, MD, executive director of Interventional Cardiovascular Programs at Brigham and Women’s Health, Boston, expressed some reluctance in applying these data to routine practice.
“It is important to emphasize that this pooled analysis draws upon six observational studies, not randomized trials of aspirin,” Dr. Bhatt said.
He called these findings “provocative,” but he said they “would need to be confirmed in databases of already completed randomized trials of aspirin versus a control before being actionable.” For Dr. Bhatt, one obstacle to a change in practice based on these data is that, “to my knowledge, no such signal [of a relationship between aspirin and incident heart failure] exists in the cumulative randomized data.”
Dr. Staessen reports no potential conflicts of interest for this study. Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PLx Pharma, for which he performs aspirin-related research.
Daily aspirin is associated with new onset heart failure independent of other risk factors, according to data derived from a database with follow-up from more than 30,000 patients who did not have HF when they were enrolled.
These data are not relevant to primary or secondary prevention of cardiovascular events but “refer only to starting aspirin for secondary prevention of HF in patients at high risk of HF or with symptomatic HF,” according to the senior investigator, Jan A. Staessen, MD, PhD, professor emeritus at the University of Leuven (Belgium).
In data from 30,827 patients at risk for HF enrolled in six observational studies, the hazard ratio (HR) for developing HF among those taking daily aspirin at baseline relative to those who were not was 1.26 (P ≤ .001) over 5.3 years of follow-up. In the 22,690 patients without a prior history cardiovascular disease (CVD), the HF risk increase for exposure to daily aspirin was about the same (HR 1.27; P = .001).
This study was launched because multiple conflicting studies have made the relationship between aspirin and HF risk unclear, according to the multinational team of authors, whose finding were published in ESC Heart Failure.
In principle, HF is recognized as a prothrombotic condition for which an antithrombotic therapy such as aspirin would be expected to have a protective role, but the investigators pointed out that the evidence is mixed. In a population-based Danish study of 12,277 patients with new-onset HF, for example, there was no relationship seen between aspirin use and a reduction in the composite outcome of all-cause mortality, myocardial infarction, or stroke.
Aspirin use linked to HF admissions
“Interestingly, this study reported that aspirin use was associated with an increased risk of readmissions for HF,” wrote the authors of the newly published data. “Uncertainty on aspirin use has been reflected in current guideline recommendations,” they added.
The population studied was drawn from the HOMAGE database, which has collated data on 46,437 participants in 21 studies. After the exclusion of studies with patients who already had HF as well as studies without information on HF incidence over time, six studies with 30,827 participants provided the basis for this analysis.
One study, ASCOT, which was randomized and blinded, served as the derivation data set. The remaining five studies, FLEMENGHO, HEALTH ABC, HULL LIFE LAB, PREDICTOR, and PROSPER, served as the validation data set.
In addition to identifying participants as aspirin users or nonusers at baseline, all of the studies had detailed baseline data on a wide variety of patient characteristics and risk factors, such as body mass index, blood cholesterol levels, blood glucose concentrations, blood pressure, and creatinine.
No patient in any trial was on an antithrombotic therapy other than aspirin at baseline.
Of the minority of patients with CVD at baseline, more than 80% had coronary heart disease. Only 2.8% of the total population had a prior myocardial infarction. In the study population overall, most (86%) had hypertension, and there was a sizeable proportion with diabetes (22%). The average age was 67 years, and 34% were women.
HF incidence on aspirin: 14.5/1000 person-years
Overall, the incidence rate of HF per 1,000 person-years for the entire population before adjustment was 14.5 in the group on daily aspirin versus 5.9 in the non-aspirin group. These absolute rates were lower in the discovery data set than in the validation set, but the relative differences in HF incidence rates for those who were versus those who were not on aspirin at baseline were similar.
Numerous sensitivity analyses supported the basic conclusions. This not only included one omitting patients with a history of CVD, but another that excluded patients who developed HF within the first 2 years. Stratified analyses looking for overall consistency across variables showed increased risk of new onset heart failure among those taking daily aspirin regardless of relative age, body weight, or blood pressure levels.
The most important limitation of this study was that it evaluated data taken from studies not originally designed to test the study hypothesis. In addition, only baseline data were available, so the drugs that patients took over the course of follow-up are unknown. However, the authors believe these data have a clinical message.
Given the consistency of these results, “our observations suggest that aspirin should be prescribed with caution in patients at risk of HF or having HF,” the investigators concluded.
“If such treatment is initiated in these patients, use low-dose aspirin,” Dr. Staessen told this news organization.
Aspirin for CVD versus HF risk
Many patients take low-dose aspirin to prevent the types of cardiovascular events, such as MI, that lead to heart failure. In attempting to address a controversy regarding aspirin and risk of new onset heart failure, it appears to create another regarding CVD risk reduction.
Deepak L. Bhatt, MD, executive director of Interventional Cardiovascular Programs at Brigham and Women’s Health, Boston, expressed some reluctance in applying these data to routine practice.
“It is important to emphasize that this pooled analysis draws upon six observational studies, not randomized trials of aspirin,” Dr. Bhatt said.
He called these findings “provocative,” but he said they “would need to be confirmed in databases of already completed randomized trials of aspirin versus a control before being actionable.” For Dr. Bhatt, one obstacle to a change in practice based on these data is that, “to my knowledge, no such signal [of a relationship between aspirin and incident heart failure] exists in the cumulative randomized data.”
Dr. Staessen reports no potential conflicts of interest for this study. Dr. Bhatt has a financial relationship with a large number of pharmaceutical companies, including PLx Pharma, for which he performs aspirin-related research.
FROM JACC HEART FAILURE
MRI before tongue carcinoma biopsy may be more reliable than other radiologic methods
Tongue squamous cell carcinomas are one of the most common oral cancers yet one of the most difficult to successfully treat. Getting an accurate measure of the depth of the tumor is critical to determining a patient’s prognosis and currently CT, MRI, and ultrasound are used to determine the depth and location of the tumor.
“Several studies have reported a significant relationship between radiological depth of invasion measured on MRI and pathological depth of invasion in the tongue squamous cell carcinoma. To the best of our knowledge, this is the first study on MRI divided into ‘before biopsy’ and ‘after incision biopsy,’ ” Hiroyuki Harada, DDS, PhD, of Tokyo Medical and Dental University, and colleagues wrote.
The issue of depth of invasion is so critical to determining metastasis to neck lymph nodes that in 2017 it was added to the AJCC Cancer Staging Manual.
This retrospective Japanese study included 128 patients with tongue carcinoma who underwent glossectomy between 2006 and 2019. The researchers evaluated which radiologic depth of invasion measurement – MRI before or after biopsy and ultrasound – was the most agreeable to clinical depth of invasion.
All the participants had undergone ultrasound, while 18 had undergone MRI before biopsy and 110 had undergone MRI after biopsy. The coefficients of determination were 0.664, 0.891, and 0.422 respectively, suggesting that calculating radiologic depth of invasion using MRI before biopsy was the most reliable radiologic method. MRI before biopsy slightly overestimated clinical depth of tumor invasion, MRI after biopsy severely overestimated the clinical measurement, and ultrasound slightly underestimated the clinical depth of tumor invasion.
Ultrasound is often preferred because of its easy use, yet measuring ulcerous lesions is difficult with ultrasound. But with MRI, there is no upper limit of measured value, but tongue movement during the procedure can make getting accurate images during the procedure difficult.
“The advantage of the MRI is that the image is acquired in the tongue’s resting position and that there is no upper limit of the measured value. The disadvantages include difficult detection of superficial tumors, influence of metal artifacts, and movement of the tongue,” the authors wrote. “Biopsy may lead to edema or hemorrhage and subsequent overestimation of tumor size and invasion depth in MRI.”
A small Canadian study published in 2016 in the Journal of Otolaryngology–Head & Neck Surgery, found a strong correlation between clinical, pathological, and MRI measurements of depth of invasion in oral tongue SCC. Meanwhile, a 2017 U.K. study published in the British Journal of Oral and Maxillofacial Surgery found that the accuracy of clinical staging in oral SCC did not differ whether the biopsy was taken before or after MRI. While a good radiologic-pathological tumor thickness correlation has been reported in 2018 in the American Journal of Neuroradiology, tongue carcinoma often shows exophytic or ulcerous lesions, which are difficult to measure with MRI, CT, or ultrasound.
The authors suggested that further studies are needed to determine the effects on MRI of postbiopsy tongue inflammation. Limitations of the study included the small number of cases with MRI before biopsy.
The authors declared no conflicts of interest.
Tongue squamous cell carcinomas are one of the most common oral cancers yet one of the most difficult to successfully treat. Getting an accurate measure of the depth of the tumor is critical to determining a patient’s prognosis and currently CT, MRI, and ultrasound are used to determine the depth and location of the tumor.
“Several studies have reported a significant relationship between radiological depth of invasion measured on MRI and pathological depth of invasion in the tongue squamous cell carcinoma. To the best of our knowledge, this is the first study on MRI divided into ‘before biopsy’ and ‘after incision biopsy,’ ” Hiroyuki Harada, DDS, PhD, of Tokyo Medical and Dental University, and colleagues wrote.
The issue of depth of invasion is so critical to determining metastasis to neck lymph nodes that in 2017 it was added to the AJCC Cancer Staging Manual.
This retrospective Japanese study included 128 patients with tongue carcinoma who underwent glossectomy between 2006 and 2019. The researchers evaluated which radiologic depth of invasion measurement – MRI before or after biopsy and ultrasound – was the most agreeable to clinical depth of invasion.
All the participants had undergone ultrasound, while 18 had undergone MRI before biopsy and 110 had undergone MRI after biopsy. The coefficients of determination were 0.664, 0.891, and 0.422 respectively, suggesting that calculating radiologic depth of invasion using MRI before biopsy was the most reliable radiologic method. MRI before biopsy slightly overestimated clinical depth of tumor invasion, MRI after biopsy severely overestimated the clinical measurement, and ultrasound slightly underestimated the clinical depth of tumor invasion.
Ultrasound is often preferred because of its easy use, yet measuring ulcerous lesions is difficult with ultrasound. But with MRI, there is no upper limit of measured value, but tongue movement during the procedure can make getting accurate images during the procedure difficult.
“The advantage of the MRI is that the image is acquired in the tongue’s resting position and that there is no upper limit of the measured value. The disadvantages include difficult detection of superficial tumors, influence of metal artifacts, and movement of the tongue,” the authors wrote. “Biopsy may lead to edema or hemorrhage and subsequent overestimation of tumor size and invasion depth in MRI.”
A small Canadian study published in 2016 in the Journal of Otolaryngology–Head & Neck Surgery, found a strong correlation between clinical, pathological, and MRI measurements of depth of invasion in oral tongue SCC. Meanwhile, a 2017 U.K. study published in the British Journal of Oral and Maxillofacial Surgery found that the accuracy of clinical staging in oral SCC did not differ whether the biopsy was taken before or after MRI. While a good radiologic-pathological tumor thickness correlation has been reported in 2018 in the American Journal of Neuroradiology, tongue carcinoma often shows exophytic or ulcerous lesions, which are difficult to measure with MRI, CT, or ultrasound.
The authors suggested that further studies are needed to determine the effects on MRI of postbiopsy tongue inflammation. Limitations of the study included the small number of cases with MRI before biopsy.
The authors declared no conflicts of interest.
Tongue squamous cell carcinomas are one of the most common oral cancers yet one of the most difficult to successfully treat. Getting an accurate measure of the depth of the tumor is critical to determining a patient’s prognosis and currently CT, MRI, and ultrasound are used to determine the depth and location of the tumor.
“Several studies have reported a significant relationship between radiological depth of invasion measured on MRI and pathological depth of invasion in the tongue squamous cell carcinoma. To the best of our knowledge, this is the first study on MRI divided into ‘before biopsy’ and ‘after incision biopsy,’ ” Hiroyuki Harada, DDS, PhD, of Tokyo Medical and Dental University, and colleagues wrote.
The issue of depth of invasion is so critical to determining metastasis to neck lymph nodes that in 2017 it was added to the AJCC Cancer Staging Manual.
This retrospective Japanese study included 128 patients with tongue carcinoma who underwent glossectomy between 2006 and 2019. The researchers evaluated which radiologic depth of invasion measurement – MRI before or after biopsy and ultrasound – was the most agreeable to clinical depth of invasion.
All the participants had undergone ultrasound, while 18 had undergone MRI before biopsy and 110 had undergone MRI after biopsy. The coefficients of determination were 0.664, 0.891, and 0.422 respectively, suggesting that calculating radiologic depth of invasion using MRI before biopsy was the most reliable radiologic method. MRI before biopsy slightly overestimated clinical depth of tumor invasion, MRI after biopsy severely overestimated the clinical measurement, and ultrasound slightly underestimated the clinical depth of tumor invasion.
Ultrasound is often preferred because of its easy use, yet measuring ulcerous lesions is difficult with ultrasound. But with MRI, there is no upper limit of measured value, but tongue movement during the procedure can make getting accurate images during the procedure difficult.
“The advantage of the MRI is that the image is acquired in the tongue’s resting position and that there is no upper limit of the measured value. The disadvantages include difficult detection of superficial tumors, influence of metal artifacts, and movement of the tongue,” the authors wrote. “Biopsy may lead to edema or hemorrhage and subsequent overestimation of tumor size and invasion depth in MRI.”
A small Canadian study published in 2016 in the Journal of Otolaryngology–Head & Neck Surgery, found a strong correlation between clinical, pathological, and MRI measurements of depth of invasion in oral tongue SCC. Meanwhile, a 2017 U.K. study published in the British Journal of Oral and Maxillofacial Surgery found that the accuracy of clinical staging in oral SCC did not differ whether the biopsy was taken before or after MRI. While a good radiologic-pathological tumor thickness correlation has been reported in 2018 in the American Journal of Neuroradiology, tongue carcinoma often shows exophytic or ulcerous lesions, which are difficult to measure with MRI, CT, or ultrasound.
The authors suggested that further studies are needed to determine the effects on MRI of postbiopsy tongue inflammation. Limitations of the study included the small number of cases with MRI before biopsy.
The authors declared no conflicts of interest.
FROM SCIENTIFIC REPORTS
AAN issues ethical guidance on controversial Alzheimer’s drug
The statement includes ethical considerations and recommendations for informed consent, and the AAN notes that neurologists should ensure that patients understand all of the issues and uncertainties surrounding the use of aducanumab.
“Neurologists and other clinicians want to provide the best care to patients and families, particularly for a disease that is as challenging as Alzheimer’s. We hope that this statement can be a guide for clinicians in communicating with patients and families in order to carefully consider decisions about the use of aducanumab,” said lead author Winston Chiong, MD, PhD, University of California San Francisco Memory and Aging Center, and a member of the AAN’s Ethics, Law, and Humanities Committee.
The statement was published online Nov. 17 in Neurology.
Open, honest communication
The Food and Drug Administration approved the antiamyloid agent aducanumab based on two studies that were both stopped prematurely for futility. In subsequent post hoc analyses of the available data, one of those studies indicated a statistically significant, albeit small, benefit with high-dose aducanumab, while the other study continued to show no benefit.
The clinical importance of the small statistical benefit in the single trial for daily function is unclear, and aducanumab was also associated with brain inflammation and brain bleeds in more than one-third of patients who received the FDA-approved dose, which requires regular brain MRI monitoring.
All of this should be communicated to patients, the AAN advises.
Patients should know that while aducanumab reduces beta-amyloid plaques in the brain that are markers of Alzheimer’s disease, it remains unclear whether this provides any meaningful benefit.
The AAN adds that it is equally important to tell patients and families that aducanumab does not restore cognitive function and that there is insufficient data to offer it to people with moderate or advanced dementia or to those without evidence of beta-amyloid plaques.
It’s important to note that very few participants in the aducanumab trials were Hispanic, Black, or Indigenous.
“Informed consent conversations with patients of populations underrepresented in clinical trials should include disclosure about the absence of safety and efficacy data in these groups,” the authors noted.
‘New territory’ for neurologists
“There are two aspects of aducanumab that are relatively new territory for us as neurologists,” Dr. Chiong said. One is the controversy about the evidence for the drug. “In the statement, we’ve tried to help clinicians communicate the uncertainty over aducanumab’s risks and potential benefits,” Dr. Chiong said. The other is the high cost of the drug and how it will be covered.
Aducanumab has a price tag of $56,000 per year, which does not include the cost of infusing the drug, required repeat imaging, and medical management.
The AAN estimates annual costs of prescribing aducanumab may top $100,000 per year. With Medicare generally covering 80%, patients and families must be told that the full costs of treatment may not be covered.
“Regarding cost, we probably don’t think often enough about what prescribing a drug means for an individual patient’s finances and for the health system,” said Dr. Chiong. “In particular, when patients are in Medicare we might assume their health care costs will be sufficiently covered, but because aducanumab is so expensive its use is likely to impose very significant costs on individual patients as well as to the Medicare program,” Dr. Chiong said.
“It is understandable why a new drug for Alzheimer’s disease generates so much interest, because while its approval has been controversial, it still offers a glimmer of hope to patients and their families,” AAN President Orly Avitzur, MD, said in a news release. “By using ethical principles to create this position statement, the American Academy of Neurology aims to help neurologists and other physicians transparently counsel patients and their families with a goal of providing the highest quality patient-centered care,” Dr. Avitzur said.
This statement was approved by the Ethics, Law, and Humanities Committee, a joint committee of the AAN, American Neurological Association, and Child Neurology Society.
This research had no targeted funding. Dr. Chiong has received personal compensation for serving on the Neuroethics Working Group of the National Institutes of Health BRAIN Initiative, and his institution has received research support from the National Institutes of Health. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
The statement includes ethical considerations and recommendations for informed consent, and the AAN notes that neurologists should ensure that patients understand all of the issues and uncertainties surrounding the use of aducanumab.
“Neurologists and other clinicians want to provide the best care to patients and families, particularly for a disease that is as challenging as Alzheimer’s. We hope that this statement can be a guide for clinicians in communicating with patients and families in order to carefully consider decisions about the use of aducanumab,” said lead author Winston Chiong, MD, PhD, University of California San Francisco Memory and Aging Center, and a member of the AAN’s Ethics, Law, and Humanities Committee.
The statement was published online Nov. 17 in Neurology.
Open, honest communication
The Food and Drug Administration approved the antiamyloid agent aducanumab based on two studies that were both stopped prematurely for futility. In subsequent post hoc analyses of the available data, one of those studies indicated a statistically significant, albeit small, benefit with high-dose aducanumab, while the other study continued to show no benefit.
The clinical importance of the small statistical benefit in the single trial for daily function is unclear, and aducanumab was also associated with brain inflammation and brain bleeds in more than one-third of patients who received the FDA-approved dose, which requires regular brain MRI monitoring.
All of this should be communicated to patients, the AAN advises.
Patients should know that while aducanumab reduces beta-amyloid plaques in the brain that are markers of Alzheimer’s disease, it remains unclear whether this provides any meaningful benefit.
The AAN adds that it is equally important to tell patients and families that aducanumab does not restore cognitive function and that there is insufficient data to offer it to people with moderate or advanced dementia or to those without evidence of beta-amyloid plaques.
It’s important to note that very few participants in the aducanumab trials were Hispanic, Black, or Indigenous.
“Informed consent conversations with patients of populations underrepresented in clinical trials should include disclosure about the absence of safety and efficacy data in these groups,” the authors noted.
‘New territory’ for neurologists
“There are two aspects of aducanumab that are relatively new territory for us as neurologists,” Dr. Chiong said. One is the controversy about the evidence for the drug. “In the statement, we’ve tried to help clinicians communicate the uncertainty over aducanumab’s risks and potential benefits,” Dr. Chiong said. The other is the high cost of the drug and how it will be covered.
Aducanumab has a price tag of $56,000 per year, which does not include the cost of infusing the drug, required repeat imaging, and medical management.
The AAN estimates annual costs of prescribing aducanumab may top $100,000 per year. With Medicare generally covering 80%, patients and families must be told that the full costs of treatment may not be covered.
“Regarding cost, we probably don’t think often enough about what prescribing a drug means for an individual patient’s finances and for the health system,” said Dr. Chiong. “In particular, when patients are in Medicare we might assume their health care costs will be sufficiently covered, but because aducanumab is so expensive its use is likely to impose very significant costs on individual patients as well as to the Medicare program,” Dr. Chiong said.
“It is understandable why a new drug for Alzheimer’s disease generates so much interest, because while its approval has been controversial, it still offers a glimmer of hope to patients and their families,” AAN President Orly Avitzur, MD, said in a news release. “By using ethical principles to create this position statement, the American Academy of Neurology aims to help neurologists and other physicians transparently counsel patients and their families with a goal of providing the highest quality patient-centered care,” Dr. Avitzur said.
This statement was approved by the Ethics, Law, and Humanities Committee, a joint committee of the AAN, American Neurological Association, and Child Neurology Society.
This research had no targeted funding. Dr. Chiong has received personal compensation for serving on the Neuroethics Working Group of the National Institutes of Health BRAIN Initiative, and his institution has received research support from the National Institutes of Health. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
The statement includes ethical considerations and recommendations for informed consent, and the AAN notes that neurologists should ensure that patients understand all of the issues and uncertainties surrounding the use of aducanumab.
“Neurologists and other clinicians want to provide the best care to patients and families, particularly for a disease that is as challenging as Alzheimer’s. We hope that this statement can be a guide for clinicians in communicating with patients and families in order to carefully consider decisions about the use of aducanumab,” said lead author Winston Chiong, MD, PhD, University of California San Francisco Memory and Aging Center, and a member of the AAN’s Ethics, Law, and Humanities Committee.
The statement was published online Nov. 17 in Neurology.
Open, honest communication
The Food and Drug Administration approved the antiamyloid agent aducanumab based on two studies that were both stopped prematurely for futility. In subsequent post hoc analyses of the available data, one of those studies indicated a statistically significant, albeit small, benefit with high-dose aducanumab, while the other study continued to show no benefit.
The clinical importance of the small statistical benefit in the single trial for daily function is unclear, and aducanumab was also associated with brain inflammation and brain bleeds in more than one-third of patients who received the FDA-approved dose, which requires regular brain MRI monitoring.
All of this should be communicated to patients, the AAN advises.
Patients should know that while aducanumab reduces beta-amyloid plaques in the brain that are markers of Alzheimer’s disease, it remains unclear whether this provides any meaningful benefit.
The AAN adds that it is equally important to tell patients and families that aducanumab does not restore cognitive function and that there is insufficient data to offer it to people with moderate or advanced dementia or to those without evidence of beta-amyloid plaques.
It’s important to note that very few participants in the aducanumab trials were Hispanic, Black, or Indigenous.
“Informed consent conversations with patients of populations underrepresented in clinical trials should include disclosure about the absence of safety and efficacy data in these groups,” the authors noted.
‘New territory’ for neurologists
“There are two aspects of aducanumab that are relatively new territory for us as neurologists,” Dr. Chiong said. One is the controversy about the evidence for the drug. “In the statement, we’ve tried to help clinicians communicate the uncertainty over aducanumab’s risks and potential benefits,” Dr. Chiong said. The other is the high cost of the drug and how it will be covered.
Aducanumab has a price tag of $56,000 per year, which does not include the cost of infusing the drug, required repeat imaging, and medical management.
The AAN estimates annual costs of prescribing aducanumab may top $100,000 per year. With Medicare generally covering 80%, patients and families must be told that the full costs of treatment may not be covered.
“Regarding cost, we probably don’t think often enough about what prescribing a drug means for an individual patient’s finances and for the health system,” said Dr. Chiong. “In particular, when patients are in Medicare we might assume their health care costs will be sufficiently covered, but because aducanumab is so expensive its use is likely to impose very significant costs on individual patients as well as to the Medicare program,” Dr. Chiong said.
“It is understandable why a new drug for Alzheimer’s disease generates so much interest, because while its approval has been controversial, it still offers a glimmer of hope to patients and their families,” AAN President Orly Avitzur, MD, said in a news release. “By using ethical principles to create this position statement, the American Academy of Neurology aims to help neurologists and other physicians transparently counsel patients and their families with a goal of providing the highest quality patient-centered care,” Dr. Avitzur said.
This statement was approved by the Ethics, Law, and Humanities Committee, a joint committee of the AAN, American Neurological Association, and Child Neurology Society.
This research had no targeted funding. Dr. Chiong has received personal compensation for serving on the Neuroethics Working Group of the National Institutes of Health BRAIN Initiative, and his institution has received research support from the National Institutes of Health. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Validity of commercial serologic tests for dermatomyositis still questionable
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Axial spondyloarthritis survey raises importance of discussing treatment changes
More than half of patients with axial spondyloarthritis in a survey of ArthritisPower Registry participants said they discussed a change in treatment with their doctor at their most recent visit, and these discussions were about changing medication or increasing the dose in more than two-thirds of instances.
The cross-sectional survey, published in ACR Open Rheumatology, is believed to be the first “to look at treatment decision-making from the patient perspective, meaning this is our first quantitative analysis to examine how patients think about important disease management decisions and communicate with their doctor about their care,” W. Benjamin Nowell, PhD, director of patient-centered research at CreakyJoints and principal investigator of the ArthritisPower registry, said in a news release.
“This study makes it clear that there are unmet treatment needs in the axial spondyloarthritis [axSpA] patient community,” senior author Jessica A. Walsh, MD, rheumatologist and associate professor at the University of Utah, Salt Lake City, said in the release. “In the future, we need to identify the tools that this specific arthritis community needs to ensure that shared decision-making about disease management and treatment escalation is working effectively between the patient and the provider.”
Survey results
Of the survey’s 274 participants with physician-diagnosed axSpA, 57% said they discussed treatment change at their last physician visit, and nearly half of the time it was brought up by the patient. About 80% of patients in the survey said they researched treatment changes before the visit.
The most common discussion points were about changing medicines or increasing dose (69%), compared with reducing dose (28%) or switching treatments (39%). Another 12% of respondents entered free-text responses to an “other” option with things such as exercise, physical therapy, surgery, waiting on results, insurance, and pregnancy.
Close to half (47%) of the patients were taking biologic disease-modifying antirheumatic drugs (bDMARDs), followed by prescription NSAIDs (44%), steroids (16%), or conventional synthetic DMARDs (11%). Half of all patients said they also took prescription muscle relaxers, nerve pain medications or antidepressants, and opioids.
More than half (55%) of patients taking a bDMARD were at least somewhat satisfied with their treatment for axSpA, and about half were satisfied with their control of axSpA-related pain.
Of the 12% of patients in the survey who reported being very satisfied overall with their treatment, 77% were taking a bDMARD, and these bDMARD users said that they prioritized the prevention of long-term consequences and their physician’s advice in their decision-making process.
A large percentage – 43% – said they were somewhat or very dissatisfied with treatment, and nearly two-thirds of these patients had discussed treatment change at their last physician visit.
A large majority of patients who discussed a treatment change agreed to it (85%), most often because their disease was not controlled by their previous treatment or because they thought it could be better controlled by a change in treatment.
The survey respondents were about 50 years old on average, and most were women (87%) and White (85%). They experienced a delay in diagnosis averaging more than 10 years from first onset of axSpA symptoms to initial axSpA diagnosis by a physician.
The study was sponsored by Eli Lilly. The study was also indirectly partially supported by a grant from the Patient-Centered Outcomes Research Institute for ArthritisPower. Dr. Nowell reported receiving grants/contracts from AbbVie, Eli Lilly, and PCORI and is an employee of the Global Healthy Living Foundation. The GHLF receives grants, sponsorships, and contracts from pharmaceutical manufacturers and private foundations. Five authors are employees and shareholders of Eli Lilly. Two authors reported financial relationships with multiple pharmaceutical companies.
More than half of patients with axial spondyloarthritis in a survey of ArthritisPower Registry participants said they discussed a change in treatment with their doctor at their most recent visit, and these discussions were about changing medication or increasing the dose in more than two-thirds of instances.
The cross-sectional survey, published in ACR Open Rheumatology, is believed to be the first “to look at treatment decision-making from the patient perspective, meaning this is our first quantitative analysis to examine how patients think about important disease management decisions and communicate with their doctor about their care,” W. Benjamin Nowell, PhD, director of patient-centered research at CreakyJoints and principal investigator of the ArthritisPower registry, said in a news release.
“This study makes it clear that there are unmet treatment needs in the axial spondyloarthritis [axSpA] patient community,” senior author Jessica A. Walsh, MD, rheumatologist and associate professor at the University of Utah, Salt Lake City, said in the release. “In the future, we need to identify the tools that this specific arthritis community needs to ensure that shared decision-making about disease management and treatment escalation is working effectively between the patient and the provider.”
Survey results
Of the survey’s 274 participants with physician-diagnosed axSpA, 57% said they discussed treatment change at their last physician visit, and nearly half of the time it was brought up by the patient. About 80% of patients in the survey said they researched treatment changes before the visit.
The most common discussion points were about changing medicines or increasing dose (69%), compared with reducing dose (28%) or switching treatments (39%). Another 12% of respondents entered free-text responses to an “other” option with things such as exercise, physical therapy, surgery, waiting on results, insurance, and pregnancy.
Close to half (47%) of the patients were taking biologic disease-modifying antirheumatic drugs (bDMARDs), followed by prescription NSAIDs (44%), steroids (16%), or conventional synthetic DMARDs (11%). Half of all patients said they also took prescription muscle relaxers, nerve pain medications or antidepressants, and opioids.
More than half (55%) of patients taking a bDMARD were at least somewhat satisfied with their treatment for axSpA, and about half were satisfied with their control of axSpA-related pain.
Of the 12% of patients in the survey who reported being very satisfied overall with their treatment, 77% were taking a bDMARD, and these bDMARD users said that they prioritized the prevention of long-term consequences and their physician’s advice in their decision-making process.
A large percentage – 43% – said they were somewhat or very dissatisfied with treatment, and nearly two-thirds of these patients had discussed treatment change at their last physician visit.
A large majority of patients who discussed a treatment change agreed to it (85%), most often because their disease was not controlled by their previous treatment or because they thought it could be better controlled by a change in treatment.
The survey respondents were about 50 years old on average, and most were women (87%) and White (85%). They experienced a delay in diagnosis averaging more than 10 years from first onset of axSpA symptoms to initial axSpA diagnosis by a physician.
The study was sponsored by Eli Lilly. The study was also indirectly partially supported by a grant from the Patient-Centered Outcomes Research Institute for ArthritisPower. Dr. Nowell reported receiving grants/contracts from AbbVie, Eli Lilly, and PCORI and is an employee of the Global Healthy Living Foundation. The GHLF receives grants, sponsorships, and contracts from pharmaceutical manufacturers and private foundations. Five authors are employees and shareholders of Eli Lilly. Two authors reported financial relationships with multiple pharmaceutical companies.
More than half of patients with axial spondyloarthritis in a survey of ArthritisPower Registry participants said they discussed a change in treatment with their doctor at their most recent visit, and these discussions were about changing medication or increasing the dose in more than two-thirds of instances.
The cross-sectional survey, published in ACR Open Rheumatology, is believed to be the first “to look at treatment decision-making from the patient perspective, meaning this is our first quantitative analysis to examine how patients think about important disease management decisions and communicate with their doctor about their care,” W. Benjamin Nowell, PhD, director of patient-centered research at CreakyJoints and principal investigator of the ArthritisPower registry, said in a news release.
“This study makes it clear that there are unmet treatment needs in the axial spondyloarthritis [axSpA] patient community,” senior author Jessica A. Walsh, MD, rheumatologist and associate professor at the University of Utah, Salt Lake City, said in the release. “In the future, we need to identify the tools that this specific arthritis community needs to ensure that shared decision-making about disease management and treatment escalation is working effectively between the patient and the provider.”
Survey results
Of the survey’s 274 participants with physician-diagnosed axSpA, 57% said they discussed treatment change at their last physician visit, and nearly half of the time it was brought up by the patient. About 80% of patients in the survey said they researched treatment changes before the visit.
The most common discussion points were about changing medicines or increasing dose (69%), compared with reducing dose (28%) or switching treatments (39%). Another 12% of respondents entered free-text responses to an “other” option with things such as exercise, physical therapy, surgery, waiting on results, insurance, and pregnancy.
Close to half (47%) of the patients were taking biologic disease-modifying antirheumatic drugs (bDMARDs), followed by prescription NSAIDs (44%), steroids (16%), or conventional synthetic DMARDs (11%). Half of all patients said they also took prescription muscle relaxers, nerve pain medications or antidepressants, and opioids.
More than half (55%) of patients taking a bDMARD were at least somewhat satisfied with their treatment for axSpA, and about half were satisfied with their control of axSpA-related pain.
Of the 12% of patients in the survey who reported being very satisfied overall with their treatment, 77% were taking a bDMARD, and these bDMARD users said that they prioritized the prevention of long-term consequences and their physician’s advice in their decision-making process.
A large percentage – 43% – said they were somewhat or very dissatisfied with treatment, and nearly two-thirds of these patients had discussed treatment change at their last physician visit.
A large majority of patients who discussed a treatment change agreed to it (85%), most often because their disease was not controlled by their previous treatment or because they thought it could be better controlled by a change in treatment.
The survey respondents were about 50 years old on average, and most were women (87%) and White (85%). They experienced a delay in diagnosis averaging more than 10 years from first onset of axSpA symptoms to initial axSpA diagnosis by a physician.
The study was sponsored by Eli Lilly. The study was also indirectly partially supported by a grant from the Patient-Centered Outcomes Research Institute for ArthritisPower. Dr. Nowell reported receiving grants/contracts from AbbVie, Eli Lilly, and PCORI and is an employee of the Global Healthy Living Foundation. The GHLF receives grants, sponsorships, and contracts from pharmaceutical manufacturers and private foundations. Five authors are employees and shareholders of Eli Lilly. Two authors reported financial relationships with multiple pharmaceutical companies.
FROM ACR OPEN RHEUMATOLOGY
Evaluation of Intermittent Energy Restriction and Continuous Energy Restriction on Weight Loss and Blood Pressure Control in Overweight and Obese Patients With Hypertension
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
Preoperative Code Status Discussion in Older Adults: Are We Doing Enough?
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521