User login
What makes a urinary tract infection complicated?
Consider anatomical and severity risk factors
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.
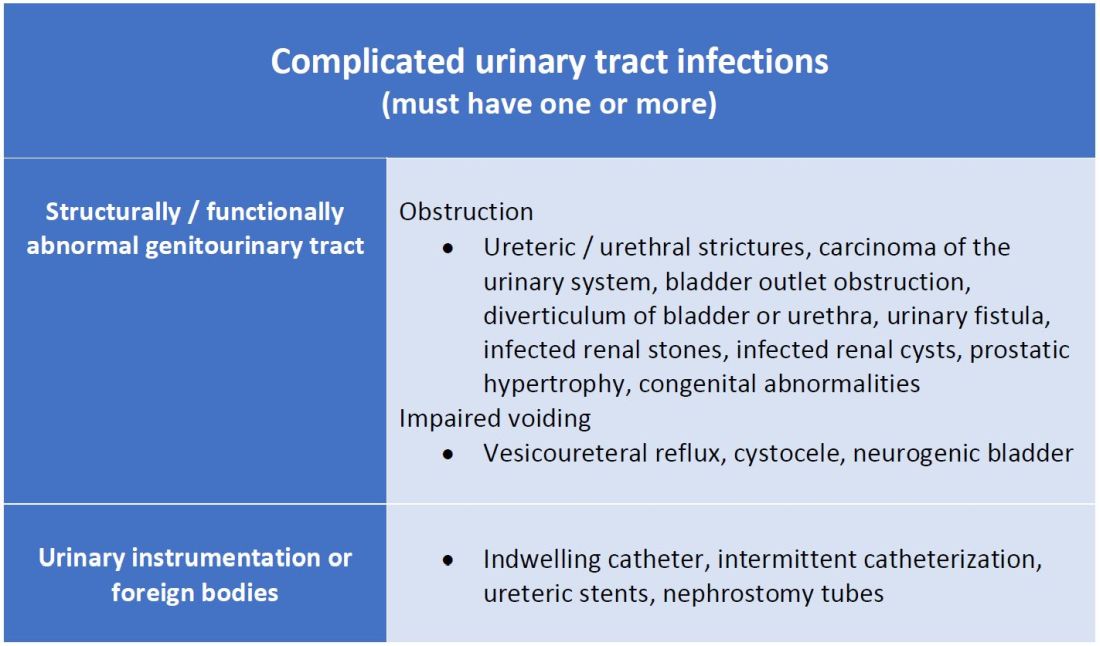
This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.
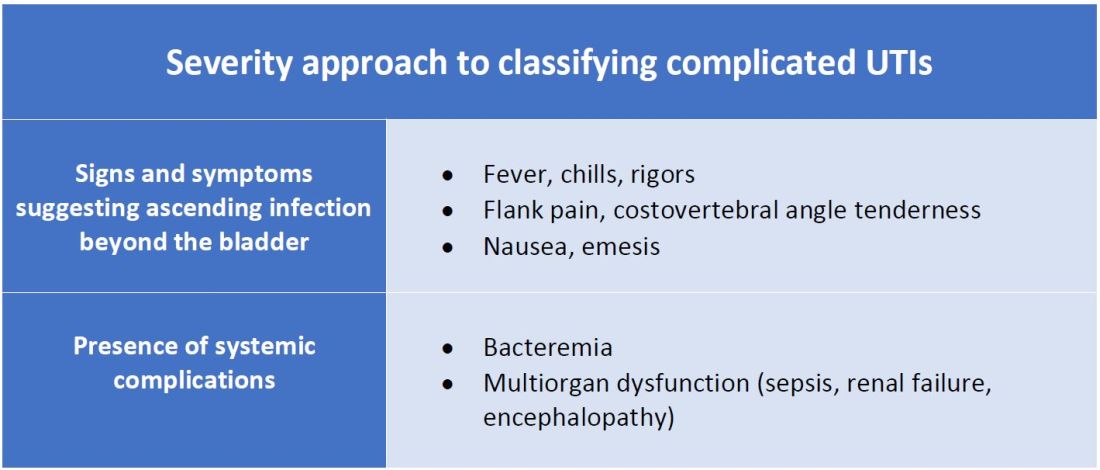
The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
Consider anatomical and severity risk factors
Consider anatomical and severity risk factors
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.

This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.

The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.

This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.

The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
Lithium’s antisuicidal effects questioned
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
FDA puts clozapine REMS requirements on temporary hold
U.S. regulators have put some of the new clozapine risk evaluation and mitigation strategy (REMS) program on temporary hold because of start-up difficulties, including long telephone wait times.
In a Nov. 19 statement, the Food and Drug Administration announced it is temporarily suspending certain aspects of the program because of challenges reported by medical professionals who were trying to meet the original Nov. 15 deadline.
In response, the FDA has conceded that pharmacists can dispense clozapine without a REMS dispense authorization (RDA). Wholesalers can continue to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS, the FDA also said.
“We encourage pharmacists and prescribers to continue working with the clozapine REMS to complete certification and patient enrollment,” the FDA said in a statement.
In July, the FDA approved modifications to the clozapine REMS strategy. Clozapine is used to treat schizophrenia that is not well controlled with standard antipsychotics. It is also prescribed to patients with recurrent suicidal behavior associated with schizophrenia or schizoaffective disorder.
Although it is highly effective in some patients, it also carries serious risks. Specifically, it can decrease the neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count (ANC) monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
HCP frustration
, including a high call volume and long call wait times for stakeholders.
“We understand that this has caused frustration and has led to patient access issues for clozapine,” the FDA said in a statement.
“Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA added. “We are working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.”
Abrupt discontinuation of clozapine can result in significant complications, the FDA said. The agency urged use of “clinical judgment” with respect to prescribing and dispensing clozapine to patients with an absolute neutrophil count within the acceptable range.
As previously reported by this news organization, the American Psychiatric Association and other national groups in a September letter asked the FDA to delay the implementation of a new REMS program until after Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
U.S. regulators have put some of the new clozapine risk evaluation and mitigation strategy (REMS) program on temporary hold because of start-up difficulties, including long telephone wait times.
In a Nov. 19 statement, the Food and Drug Administration announced it is temporarily suspending certain aspects of the program because of challenges reported by medical professionals who were trying to meet the original Nov. 15 deadline.
In response, the FDA has conceded that pharmacists can dispense clozapine without a REMS dispense authorization (RDA). Wholesalers can continue to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS, the FDA also said.
“We encourage pharmacists and prescribers to continue working with the clozapine REMS to complete certification and patient enrollment,” the FDA said in a statement.
In July, the FDA approved modifications to the clozapine REMS strategy. Clozapine is used to treat schizophrenia that is not well controlled with standard antipsychotics. It is also prescribed to patients with recurrent suicidal behavior associated with schizophrenia or schizoaffective disorder.
Although it is highly effective in some patients, it also carries serious risks. Specifically, it can decrease the neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count (ANC) monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
HCP frustration
, including a high call volume and long call wait times for stakeholders.
“We understand that this has caused frustration and has led to patient access issues for clozapine,” the FDA said in a statement.
“Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA added. “We are working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.”
Abrupt discontinuation of clozapine can result in significant complications, the FDA said. The agency urged use of “clinical judgment” with respect to prescribing and dispensing clozapine to patients with an absolute neutrophil count within the acceptable range.
As previously reported by this news organization, the American Psychiatric Association and other national groups in a September letter asked the FDA to delay the implementation of a new REMS program until after Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
U.S. regulators have put some of the new clozapine risk evaluation and mitigation strategy (REMS) program on temporary hold because of start-up difficulties, including long telephone wait times.
In a Nov. 19 statement, the Food and Drug Administration announced it is temporarily suspending certain aspects of the program because of challenges reported by medical professionals who were trying to meet the original Nov. 15 deadline.
In response, the FDA has conceded that pharmacists can dispense clozapine without a REMS dispense authorization (RDA). Wholesalers can continue to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS, the FDA also said.
“We encourage pharmacists and prescribers to continue working with the clozapine REMS to complete certification and patient enrollment,” the FDA said in a statement.
In July, the FDA approved modifications to the clozapine REMS strategy. Clozapine is used to treat schizophrenia that is not well controlled with standard antipsychotics. It is also prescribed to patients with recurrent suicidal behavior associated with schizophrenia or schizoaffective disorder.
Although it is highly effective in some patients, it also carries serious risks. Specifically, it can decrease the neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count (ANC) monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
HCP frustration
, including a high call volume and long call wait times for stakeholders.
“We understand that this has caused frustration and has led to patient access issues for clozapine,” the FDA said in a statement.
“Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA added. “We are working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.”
Abrupt discontinuation of clozapine can result in significant complications, the FDA said. The agency urged use of “clinical judgment” with respect to prescribing and dispensing clozapine to patients with an absolute neutrophil count within the acceptable range.
As previously reported by this news organization, the American Psychiatric Association and other national groups in a September letter asked the FDA to delay the implementation of a new REMS program until after Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
Schools, pediatricians look to make up lost ground on non–COVID-19 vaccinations
WESTMINSTER, COLO. – Melissa Blatzer was determined to get her three children caught up on their routine immunizations on a recent Saturday morning at a walk-in clinic in this Denver suburb. It had been about a year since the kids’ last shots, a delay Ms. Blatzer chalked up to the pandemic.
Two-year-old Lincoln Blatzer, in his fleece dinosaur pajamas, waited anxiously in line for his hepatitis A vaccine. His siblings, 14-year-old Nyla Kusumah and 11-year-old Nevan Kusumah, were there for their TDAP, HPV and meningococcal vaccines, plus a COVID-19 shot for Nyla.
“You don’t have to make an appointment and you can take all three at once,” said Ms. Blatzer, who lives several miles away in Commerce City. That convenience outweighed the difficulty of getting everyone up early on a weekend.
Child health experts hope community clinics like this – along with the return to in-person classes, more well-child visits, and the rollout of COVID shots for younger children – can help boost routine childhood immunizations, which dropped during the pandemic. Despite a rebound, immunization rates are still lower than in 2019, and disparities in rates between racial and economic groups, particularly for Black children, have been exacerbated.
“We’re still not back to where we need to be,” said Sean O’Leary, MD, a pediatric infectious disease doctor at Children’s Hospital Colorado and a professor of pediatrics at the University of Colorado at Denver, Aurora.
Routine immunizations protect children against 16 infectious diseases, including measles, diphtheria and chickenpox, and inhibit transmission to the community.
The rollout of COVID shots for younger kids is an opportunity to catch up on routine vaccinations, said Dr. O’Leary, adding that children can receive these vaccines together. Primary care practices, where many children are likely to receive the COVID shots, usually have other childhood vaccines on hand.
“It’s really important that parents and health care providers work together so that all children are up to date on these recommended vaccines,” said Malini DeSilva, MD, an internist and pediatrician at HealthPartners in the Minneapolis–St. Paul area. “Not only for the child’s health but for our community’s health.”
People were reluctant to come out for routine immunizations at the height of the pandemic, said Karen Miller, an immunization nurse manager for the Denver area’s Tri-County Health Department, which ran the Westminster clinic. National and global data confirm what Ms. Miller saw on the ground.
Global vaccine coverage in children fell from 2019 to 2020, according to a recent study by scientists at the Centers for Disease Control and Prevention, the World Health Organization, and UNICEF. Reasons included reduced access, lack of transportation, worries about COVID exposure and supply chain interruptions, the study said.
Third doses of the DTP vaccine and of the polio vaccine decreased from 86% of all eligible children in 2019 to 83% in 2020, according to the study. Worldwide, 22.7 million children had not had their third dose of DTP in 2020, compared with 19 million in 2019. Three doses are far more effective than one or two at protecting children and communities.
In the United States, researchers who studied 2019 and 2020 data on routine vaccinations in California, Colorado, Minnesota, Oregon, Washington, and Wisconsin found substantial disruptions in vaccination rates during the pandemic that continued into September 2020. For example, the percentage of 7-month-old babies who were up to date on vaccinations decreased from 81% in September 2019 to 74% a year later.
The proportion of Black children up to date on immunizations in almost all age groups was lower than that of children in other racial and ethnic groups. This was most pronounced in those turning 18 months old: Only 41% of Black children that age were caught up on vaccinations in September 2020, compared with 57% of all children at 18 months, said Dr. DeSilva, who led that study.
A CDC study of data from the National Immunization Surveys found that race and ethnicity, poverty, and lack of insurance created the greatest disparities in vaccination rates, and the authors noted that extra efforts are needed to counter the pandemic’s disruptions.
In addition to the problems caused by COVID, Ms. Miller said, competing life priorities like work and school impede families from keeping up with shots. Weekend vaccination clinics can help working parents get their children caught up on routine immunizations while they get a flu or COVID shot. Ms. Miller and O’Leary also said reminders via phone, text or email can boost immunizations.
“Vaccines are so effective that I think it’s easy for families to put immunizations on the back burner because we don’t often hear about these diseases,” she said.
It’s a long and nasty list that includes hepatitis A and B, measles, mumps, whooping cough, polio, rubella, rotavirus, pneumococcus, tetanus, diphtheria, human papillomavirus, and meningococcal disease, among others. Even small drops in vaccination coverage can lead to outbreaks. And measles is the perfect example that worries experts, particularly as international travel opens up.
“Measles is among the most contagious diseases known to humankind, meaning that we have to keep very high vaccination coverage to keep it from spreading,” said Dr. O’Leary.
In 2019, 22 measles outbreaks occurred in 17 states in mostly unvaccinated children and adults. Dr. O’Leary said outbreaks in New York City were contained because surrounding areas had high vaccination coverage. But an outbreak in an undervaccinated community still could spread beyond its borders.
In some states a significant number of parents were opposed to routine childhood vaccines even before the pandemic for religious or personal reasons, posing another challenge for health professionals. For example, 87% of Colorado kindergartners were vaccinated against measles, mumps, and rubella during the 2018-19 school year, one of the nation’s lowest rates.
Those rates bumped up to 91% in 2019-20 but are still below the CDC’s target of 95%.
Dr. O’Leary said he does not see the same level of hesitancy for routine immunizations as for COVID. “There has always been vaccine hesitancy and vaccine refusers. But we’ve maintained vaccination rates north of 90% for all routine childhood vaccines for a long time now,” he said.
Dr. DeSilva said the “ripple effects” of missed vaccinations earlier in the pandemic continued into 2021. As children returned to in-person learning this fall, schools may have been the first place families heard about missed vaccinations. Individual states set vaccination requirements, and allowable exemptions, for entry at schools and child care facilities. In 2020, Colorado passed a school entry immunization law that tightened allowable exemptions.
“Schools, where vaccination requirements are generally enforced, are stretched thin for a variety of reasons, including COVID,” said Dr. O’Leary, adding that managing vaccine requirements may be more difficult for some, but not all, schools.
Anayeli Dominguez, 13, was at the Westminster clinic for a Tdap vaccine because her middle school had noticed she was not up to date.
“School nurses play an important role in helping identify students in need of immunizations, and also by connecting families to resources both within the district and in the larger community,” said Denver Public Schools spokesperson Will Jones.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
WESTMINSTER, COLO. – Melissa Blatzer was determined to get her three children caught up on their routine immunizations on a recent Saturday morning at a walk-in clinic in this Denver suburb. It had been about a year since the kids’ last shots, a delay Ms. Blatzer chalked up to the pandemic.
Two-year-old Lincoln Blatzer, in his fleece dinosaur pajamas, waited anxiously in line for his hepatitis A vaccine. His siblings, 14-year-old Nyla Kusumah and 11-year-old Nevan Kusumah, were there for their TDAP, HPV and meningococcal vaccines, plus a COVID-19 shot for Nyla.
“You don’t have to make an appointment and you can take all three at once,” said Ms. Blatzer, who lives several miles away in Commerce City. That convenience outweighed the difficulty of getting everyone up early on a weekend.
Child health experts hope community clinics like this – along with the return to in-person classes, more well-child visits, and the rollout of COVID shots for younger children – can help boost routine childhood immunizations, which dropped during the pandemic. Despite a rebound, immunization rates are still lower than in 2019, and disparities in rates between racial and economic groups, particularly for Black children, have been exacerbated.
“We’re still not back to where we need to be,” said Sean O’Leary, MD, a pediatric infectious disease doctor at Children’s Hospital Colorado and a professor of pediatrics at the University of Colorado at Denver, Aurora.
Routine immunizations protect children against 16 infectious diseases, including measles, diphtheria and chickenpox, and inhibit transmission to the community.
The rollout of COVID shots for younger kids is an opportunity to catch up on routine vaccinations, said Dr. O’Leary, adding that children can receive these vaccines together. Primary care practices, where many children are likely to receive the COVID shots, usually have other childhood vaccines on hand.
“It’s really important that parents and health care providers work together so that all children are up to date on these recommended vaccines,” said Malini DeSilva, MD, an internist and pediatrician at HealthPartners in the Minneapolis–St. Paul area. “Not only for the child’s health but for our community’s health.”
People were reluctant to come out for routine immunizations at the height of the pandemic, said Karen Miller, an immunization nurse manager for the Denver area’s Tri-County Health Department, which ran the Westminster clinic. National and global data confirm what Ms. Miller saw on the ground.
Global vaccine coverage in children fell from 2019 to 2020, according to a recent study by scientists at the Centers for Disease Control and Prevention, the World Health Organization, and UNICEF. Reasons included reduced access, lack of transportation, worries about COVID exposure and supply chain interruptions, the study said.
Third doses of the DTP vaccine and of the polio vaccine decreased from 86% of all eligible children in 2019 to 83% in 2020, according to the study. Worldwide, 22.7 million children had not had their third dose of DTP in 2020, compared with 19 million in 2019. Three doses are far more effective than one or two at protecting children and communities.
In the United States, researchers who studied 2019 and 2020 data on routine vaccinations in California, Colorado, Minnesota, Oregon, Washington, and Wisconsin found substantial disruptions in vaccination rates during the pandemic that continued into September 2020. For example, the percentage of 7-month-old babies who were up to date on vaccinations decreased from 81% in September 2019 to 74% a year later.
The proportion of Black children up to date on immunizations in almost all age groups was lower than that of children in other racial and ethnic groups. This was most pronounced in those turning 18 months old: Only 41% of Black children that age were caught up on vaccinations in September 2020, compared with 57% of all children at 18 months, said Dr. DeSilva, who led that study.
A CDC study of data from the National Immunization Surveys found that race and ethnicity, poverty, and lack of insurance created the greatest disparities in vaccination rates, and the authors noted that extra efforts are needed to counter the pandemic’s disruptions.
In addition to the problems caused by COVID, Ms. Miller said, competing life priorities like work and school impede families from keeping up with shots. Weekend vaccination clinics can help working parents get their children caught up on routine immunizations while they get a flu or COVID shot. Ms. Miller and O’Leary also said reminders via phone, text or email can boost immunizations.
“Vaccines are so effective that I think it’s easy for families to put immunizations on the back burner because we don’t often hear about these diseases,” she said.
It’s a long and nasty list that includes hepatitis A and B, measles, mumps, whooping cough, polio, rubella, rotavirus, pneumococcus, tetanus, diphtheria, human papillomavirus, and meningococcal disease, among others. Even small drops in vaccination coverage can lead to outbreaks. And measles is the perfect example that worries experts, particularly as international travel opens up.
“Measles is among the most contagious diseases known to humankind, meaning that we have to keep very high vaccination coverage to keep it from spreading,” said Dr. O’Leary.
In 2019, 22 measles outbreaks occurred in 17 states in mostly unvaccinated children and adults. Dr. O’Leary said outbreaks in New York City were contained because surrounding areas had high vaccination coverage. But an outbreak in an undervaccinated community still could spread beyond its borders.
In some states a significant number of parents were opposed to routine childhood vaccines even before the pandemic for religious or personal reasons, posing another challenge for health professionals. For example, 87% of Colorado kindergartners were vaccinated against measles, mumps, and rubella during the 2018-19 school year, one of the nation’s lowest rates.
Those rates bumped up to 91% in 2019-20 but are still below the CDC’s target of 95%.
Dr. O’Leary said he does not see the same level of hesitancy for routine immunizations as for COVID. “There has always been vaccine hesitancy and vaccine refusers. But we’ve maintained vaccination rates north of 90% for all routine childhood vaccines for a long time now,” he said.
Dr. DeSilva said the “ripple effects” of missed vaccinations earlier in the pandemic continued into 2021. As children returned to in-person learning this fall, schools may have been the first place families heard about missed vaccinations. Individual states set vaccination requirements, and allowable exemptions, for entry at schools and child care facilities. In 2020, Colorado passed a school entry immunization law that tightened allowable exemptions.
“Schools, where vaccination requirements are generally enforced, are stretched thin for a variety of reasons, including COVID,” said Dr. O’Leary, adding that managing vaccine requirements may be more difficult for some, but not all, schools.
Anayeli Dominguez, 13, was at the Westminster clinic for a Tdap vaccine because her middle school had noticed she was not up to date.
“School nurses play an important role in helping identify students in need of immunizations, and also by connecting families to resources both within the district and in the larger community,” said Denver Public Schools spokesperson Will Jones.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
WESTMINSTER, COLO. – Melissa Blatzer was determined to get her three children caught up on their routine immunizations on a recent Saturday morning at a walk-in clinic in this Denver suburb. It had been about a year since the kids’ last shots, a delay Ms. Blatzer chalked up to the pandemic.
Two-year-old Lincoln Blatzer, in his fleece dinosaur pajamas, waited anxiously in line for his hepatitis A vaccine. His siblings, 14-year-old Nyla Kusumah and 11-year-old Nevan Kusumah, were there for their TDAP, HPV and meningococcal vaccines, plus a COVID-19 shot for Nyla.
“You don’t have to make an appointment and you can take all three at once,” said Ms. Blatzer, who lives several miles away in Commerce City. That convenience outweighed the difficulty of getting everyone up early on a weekend.
Child health experts hope community clinics like this – along with the return to in-person classes, more well-child visits, and the rollout of COVID shots for younger children – can help boost routine childhood immunizations, which dropped during the pandemic. Despite a rebound, immunization rates are still lower than in 2019, and disparities in rates between racial and economic groups, particularly for Black children, have been exacerbated.
“We’re still not back to where we need to be,” said Sean O’Leary, MD, a pediatric infectious disease doctor at Children’s Hospital Colorado and a professor of pediatrics at the University of Colorado at Denver, Aurora.
Routine immunizations protect children against 16 infectious diseases, including measles, diphtheria and chickenpox, and inhibit transmission to the community.
The rollout of COVID shots for younger kids is an opportunity to catch up on routine vaccinations, said Dr. O’Leary, adding that children can receive these vaccines together. Primary care practices, where many children are likely to receive the COVID shots, usually have other childhood vaccines on hand.
“It’s really important that parents and health care providers work together so that all children are up to date on these recommended vaccines,” said Malini DeSilva, MD, an internist and pediatrician at HealthPartners in the Minneapolis–St. Paul area. “Not only for the child’s health but for our community’s health.”
People were reluctant to come out for routine immunizations at the height of the pandemic, said Karen Miller, an immunization nurse manager for the Denver area’s Tri-County Health Department, which ran the Westminster clinic. National and global data confirm what Ms. Miller saw on the ground.
Global vaccine coverage in children fell from 2019 to 2020, according to a recent study by scientists at the Centers for Disease Control and Prevention, the World Health Organization, and UNICEF. Reasons included reduced access, lack of transportation, worries about COVID exposure and supply chain interruptions, the study said.
Third doses of the DTP vaccine and of the polio vaccine decreased from 86% of all eligible children in 2019 to 83% in 2020, according to the study. Worldwide, 22.7 million children had not had their third dose of DTP in 2020, compared with 19 million in 2019. Three doses are far more effective than one or two at protecting children and communities.
In the United States, researchers who studied 2019 and 2020 data on routine vaccinations in California, Colorado, Minnesota, Oregon, Washington, and Wisconsin found substantial disruptions in vaccination rates during the pandemic that continued into September 2020. For example, the percentage of 7-month-old babies who were up to date on vaccinations decreased from 81% in September 2019 to 74% a year later.
The proportion of Black children up to date on immunizations in almost all age groups was lower than that of children in other racial and ethnic groups. This was most pronounced in those turning 18 months old: Only 41% of Black children that age were caught up on vaccinations in September 2020, compared with 57% of all children at 18 months, said Dr. DeSilva, who led that study.
A CDC study of data from the National Immunization Surveys found that race and ethnicity, poverty, and lack of insurance created the greatest disparities in vaccination rates, and the authors noted that extra efforts are needed to counter the pandemic’s disruptions.
In addition to the problems caused by COVID, Ms. Miller said, competing life priorities like work and school impede families from keeping up with shots. Weekend vaccination clinics can help working parents get their children caught up on routine immunizations while they get a flu or COVID shot. Ms. Miller and O’Leary also said reminders via phone, text or email can boost immunizations.
“Vaccines are so effective that I think it’s easy for families to put immunizations on the back burner because we don’t often hear about these diseases,” she said.
It’s a long and nasty list that includes hepatitis A and B, measles, mumps, whooping cough, polio, rubella, rotavirus, pneumococcus, tetanus, diphtheria, human papillomavirus, and meningococcal disease, among others. Even small drops in vaccination coverage can lead to outbreaks. And measles is the perfect example that worries experts, particularly as international travel opens up.
“Measles is among the most contagious diseases known to humankind, meaning that we have to keep very high vaccination coverage to keep it from spreading,” said Dr. O’Leary.
In 2019, 22 measles outbreaks occurred in 17 states in mostly unvaccinated children and adults. Dr. O’Leary said outbreaks in New York City were contained because surrounding areas had high vaccination coverage. But an outbreak in an undervaccinated community still could spread beyond its borders.
In some states a significant number of parents were opposed to routine childhood vaccines even before the pandemic for religious or personal reasons, posing another challenge for health professionals. For example, 87% of Colorado kindergartners were vaccinated against measles, mumps, and rubella during the 2018-19 school year, one of the nation’s lowest rates.
Those rates bumped up to 91% in 2019-20 but are still below the CDC’s target of 95%.
Dr. O’Leary said he does not see the same level of hesitancy for routine immunizations as for COVID. “There has always been vaccine hesitancy and vaccine refusers. But we’ve maintained vaccination rates north of 90% for all routine childhood vaccines for a long time now,” he said.
Dr. DeSilva said the “ripple effects” of missed vaccinations earlier in the pandemic continued into 2021. As children returned to in-person learning this fall, schools may have been the first place families heard about missed vaccinations. Individual states set vaccination requirements, and allowable exemptions, for entry at schools and child care facilities. In 2020, Colorado passed a school entry immunization law that tightened allowable exemptions.
“Schools, where vaccination requirements are generally enforced, are stretched thin for a variety of reasons, including COVID,” said Dr. O’Leary, adding that managing vaccine requirements may be more difficult for some, but not all, schools.
Anayeli Dominguez, 13, was at the Westminster clinic for a Tdap vaccine because her middle school had noticed she was not up to date.
“School nurses play an important role in helping identify students in need of immunizations, and also by connecting families to resources both within the district and in the larger community,” said Denver Public Schools spokesperson Will Jones.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Leadership & Professional Development: Relational Leadership—It’s Not About You
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
“Lead me, follow me, or get the hell out of my way.”
—George Patton
The concept of leadership is often viewed through the lens of the individual. Terms such as “born leader” are canon in our lexicon, and motivational images are common, frequently paired with a singular majestic animal on a mountain peak, meant to inspire awe in the value of the individual leader. This mindset can be problematically reductive, suggesting that leadership is binary and mutually exclusive: we are either leaders or followers. The terminology can also be pejorative, as few are likely to populate a curriculum vitae with examples of being a great follower.
Leadership can instead be regarded as a role rather than a personality trait or superpower. Many of us inhabit multiple leadership roles in our professional lives. Whether participating on a committee, designing an educational curriculum, overseeing a clinical service line, or supervising learners as ward teaching attending, we function as leaders in the context of our relationships with other members of the numerous cohorts within which we work. As leaders, we must consider our relationships to others in a group as opposed to our intrinsic personalities.1
The following pearls can help operationalize relational leadership concepts2,3:
We are not alone. In any given leadership role, we must understand with whom we work (and often depend upon) and what we need to do to allow others to help us succeed. When entering a leadership role with a new group, it is important to assess the interests and skill sets of the rest of the team by either formal or informal means. Many are used to doing so on the first day of attending on a new ward service, but this concept also applies to other roles, such as chairing a new committee.
Work with individuals and groups whose knowledge, experience, skills, and/or attitudes are complementary to our own. This is not as easy as it sounds; when hiring individuals or assembling groups, we tend to gravitate to those like ourselves. Seeking different opinions and styles can be valuable, and promoting diversity, inclusion, and equity is paramount. To do so, we must make efforts to understand our own personal strengths and limitations, ideally supplemented with observation and feedback from a trusted mentor or coach. Taking an honest look at our preconceptions and assumptions is crucial. Consider how we view other silos with which we interact and the presuppositions we make, such as the “typical” surgeon or emergency department practitioner.
Recognize and publicly share shortcomings. Transparency about our limitations allows us to build relationships that are more effective and impactful. A leader who meaningfully reveals a weakness for which they need other group members to contribute specific expertise can allow team members to feel more connected or engaged with that leader or group by shifting from interpreting an ask as “Do this task” to the more empowering “I need your help.”
Leadership can be effectively conceptualized as a relational skill, fulfilled by various roles in our professional lives. Collaboration, introspection, and transparency are essential to becoming a successful leader.
Acknowledgments
The author gratefully acknowledges Dr David Berg for his invaluable mentorship as well as the core faculty of the SHM-SGIM Academic Hospitalist Academy 2.0 for their support and encouragement.
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8
1. Wood M, Dibben M. Leadership as a relational process. Process Studies. 2015;44(1): 24-47. https://doi.org/10.5840/process20154412
2. Berg DN. Resurrecting the muse: followership in organizations. In: Klein EB, Gabelnick E, Herr R, eds. Psychodynamics of Leadership. Psychosocial Press; 1998.
3. Berg DN, Bradley EH. Leadership: Rhetoric vs. Reality. 2015. Accessed September 22, 2021. https://www.youtube.com/watch?v=77IwJ8wXaM8
Simulation-Based Training in Medical Education: Immediate Growth or Cautious Optimism?
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
For years, professional athletes have used simulation-based training (SBT), a combination of virtual and experiential learning that aims to optimize technical skills, teamwork, and communication.1 In SBT, critical plays and skills are first watched on video or reviewed on a chalkboard, and then run in the presence of a coach who offers immediate feedback to the player. The hope is that the individual will then be able to perfectly execute that play or scenario when it is game time. While SBT is a developing tool in medical education—allowing learners to practice important clinical skills prior to practicing in the higher-stakes clinical environment—an important question remains: what training can go virtual and what needs to stay in person?
In this issue, Carter et al2 present a single-site, telesimulation curriculum that addresses consult request and handoff communication using SBT. Due to the COVID-19 pandemic, the authors converted an in-person intern bootcamp into a virtual, Zoom®-based workshop and compared assessments and evaluations to the previous year’s (2019) in-person bootcamp. Compared to the in-person class, the telesimulation-based cohort were equally or better trained in the consult request portion of the workshop. However, participants were significantly less likely to perform the assessed handoff skills optimally, with only a quarter (26%) appropriately prioritizing patients and less than half (49%) providing an appropriate amount of information in the patient summary. Additionally, postworkshop surveys found that SBT participants were more satisfied with their performance in both the consult request and handoff scenarios and felt more prepared (99% vs 91%) to perform handoffs in clinical practice compared to the previous year’s in-person cohort.
We focus on this work as it explores the role that SBT or virtual training could have in hospital communication and patient safety training. While previous work has highlighted that technical and procedural skills often lend themselves to in-person adaptation (eg, point-of-care ultrasound), this work suggests that nontechnical skills training could be adapted to the virtual environment. Hospitalists and internal medicine trainees perform a myriad of nontechnical activities, such as end-of-life discussions, obtaining informed consent, providing peer-to-peer feedback, and leading multidisciplinary teams. Activities like these, which require no hands-on interactions, may be well-suited for simulation or virtual-based training.3
However, we make this suggestion with some caution. In Carter et al’s study,2 while we assumed that telesimulation would work for the handoff portion of the workshop, interestingly, the telesimulation-based cohort performed worse than the interns who participated in the previous year’s in-person training while simultaneously and paradoxically reporting that they felt more prepared. The authors offer several possible explanations, including alterations in the assessment checklist and a shift in the facilitators from peer observers to faculty hospitalists. We suspect that differences in the participants’ experiences prior to the bootcamp may also be at play. Given the onset of the pandemic during their final year in undergraduate training, many in this intern cohort were likely removed from their fourth-year clinical clerkships,4 taking from them pivotal opportunities to hone and refine this skill set prior to starting their graduate medical education.
As telesimulation and other virtual care educational opportunities continue to evolve, we must ensure that such training does not sacrifice quality for ease and satisfaction. As the authors’ findings show, simply replicating an in-person curriculum in a virtual environment does not ensure equivalence for all skill sets. We remain cautiously optimistic that as we adjust to a postpandemic world, more SBT and virtual-based educational interventions will allow medical trainees to be ready to perform come game time.
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
1. McCaskill S. Sports tech comes of age with VR training, coaching apps and smart gear. Forbes. March 31, 2020. https://www.forbes.com/sites/stevemccaskill/2020/03/31/sports-tech-comes-of-age-with-vr-training-coaching-apps-and-smart-gear/?sh=309a8fa219c9
2. Carter K, Podczerwinski J, Love L, et al. Utilizing telesimulation for advanced skills training in consultation and handoff communication: a post-COVID-19 GME bootcamp experience. J Hosp Med. 2021;16(12)730-734. https://doi.org/10.12788/jhm.3733
3. Paige JT, Sonesh SC, Garbee DD, Bonanno LS. Comprensive Healthcare Simulation: Interprofessional Team Training and Simulation. 1st ed. Springer International Publishing; 2020. https://doi.org/10.1007/978-3-030-28845-7
4. Goldenberg MN, Hersh DC, Wilkins KM, Schwartz ML. Suspending medical student clerkships due to COVID-19. Med Sci Educ. 2020;30(3):1-4. https://doi.org/10.1007/s40670-020-00994-1
Firefighters’ blood pressure surges when they are called to action
In response to a 911 alert or page, firefighters’ systolic and diastolic blood pressure surges and their heart rate accelerates, with a similar response whether the call is for a fire or medical emergency, a small study suggests.
On average, the 41 firefighters monitored in the study, who were middle-aged and overweight, had a 9% increase in systolic blood pressure when called to a fire, a 9% increase in diastolic blood pressure when called to a medical emergency, and a 16% increase in heart rate for both types of calls.
Senior study author Deborah Feairheller, PhD, presented these results at the virtual American Heart Association scientific sessions.
Firefighters have a higher prevalence of cardiovascular disease (CVD) than that of the general population, explained Dr. Feairheller, director of the Hypertension and Endothelial Function with Aerobic and Resistance Training (HEART) Lab and clinical associate professor of kinesiology at the University of New Hampshire, Durham.
More than 50% of firefighter deaths in the line of duty are from CVD, she noted. Moreover, almost 75% of firefighters have hypertension and fewer than 25% have it under control.
The study findings show that all emergency and first responders “should know what their typical blood pressure level is and be aware of how it fluctuates,” Dr. Feairheller said in a press release from the AHA. “Most important, if they have high blood pressure, they should make sure it is well controlled,” she said.
“I really hope that fire departments everywhere see these data, rise to the occasion, and advocate for BP awareness in their crews,” Dr. Feairheller, a volunteer firefighter, said in an interview.
“I do think this has value to any occupation that wears a pager,” she added. “Clinicians, physicians, other emergency responders, all of those occupations are stressful and could place people at risk if they have undiagnosed or uncontrolled hypertension.”
Invited to comment, Comilla Sasson, MD, PhD, an emergency department physician who was not involved with this research, said in an interview that she saw parallels between stress experienced by firefighters and, for example, emergency department physicians.
The transient increases in BP, both systolic and diastolic, along with the heart rate are likely due to the body’s natural fight or flight response to an emergency call, including increases in epinephrine and cortisol, said Dr. Sasson, vice president of science and innovation for emergency cardiovascular care at the American Heart Association.
“The thing that is most interesting to me,” said Dr. Sasson, who can be subject to a series of high-stress situations on a shift, such as multiple trauma victims, a stroke victim, or a person in cardiac arrest, is “what is the cumulative impact of this over time?”
She said she wonders if “having to be ‘ready to go’ at any time, along with disruptions in sleep/wake schedules, and poorer eating and working-out habits when you are on shift, has long-term sequelae on the body.”
Stress-related surges in blood pressure “could be a reason for worse health outcomes in this group,” Dr. Sasson said, adding that this needs to be investigated further.
Firefighters with high normal BP, high BMI
Dr. Feairheller and colleagues recruited 41 volunteer and employee firefighters from suburban Philadelphia and Dover, N.H.
On average, the 37 men and 4 women had a mean age of 41 years, had been working as firefighters for 16.9 years, and had a mean body mass index of 30.3 kg/m2.
They wore ambulatory blood pressure monitors during an on-call work shift for at least 12 consecutive hours.
In addition to the automatic readings, the participants were instructed to prompt the machine to take a reading whenever a pager or emergency call sounded or when they felt they were entering a stressful situation.
Over the 12-hour shift, on average, participants had a blood pressure of 131/79.3 mm Hg and a heart rate of 75.7 bpm.
When they were alerted go to a fire, their blood pressure surged by 19.2/10.5 mm Hg, and their heart rate rose to 85.5 bpm.
Similarly, when they were alerted to go to a medical emergency, their blood pressure jumped up by 18.7/16.5 mm Hg and their heart rate climbed to 90.5 bpm.
The surges in blood pressure and heart rate were similar when participants were riding in the fire truck to a call or when the call turned out to be a false alarm.
What can be done?
“If we can increase awareness and identify specific risk factors in firefighters,” Dr. Feairheller said, this could “save a life of someone who spends their day saving lives and property.”
To start, “regular, in-station or home BP monitoring should be encouraged,” she said. “Firefighters should start to track their BP levels in the morning, at night, at work. Being a volunteer firefighter myself, I know the stress and anxiety and sadness and heavy work that comes with the job,” she said. “I want to be able to do what I can to help make the crews healthier.”
Dr. Sasson suggested that ways to increase awareness and improve the health of firefighters might include “counseling, appropriate breaks, possibly food service/delivery to provide better nutritional options, built-in time for exercise (gym or cardio equipment on site), and discussions about how stress can impact the body over time.”
It is important to advocate for better mental health care, because people may have PTSD, depression, substance abuse, or other mental health conditions brought on by their stressful jobs, she said.
“Also, it would be interesting to know what is the current state of health monitoring (both physical, mental, and emotional) that occurs for firefighters,” she said.
The American Heart Association funded the study. The authors and Dr. Sasson report no disclosures.
A version of this article first appeared on Medscape.com.
In response to a 911 alert or page, firefighters’ systolic and diastolic blood pressure surges and their heart rate accelerates, with a similar response whether the call is for a fire or medical emergency, a small study suggests.
On average, the 41 firefighters monitored in the study, who were middle-aged and overweight, had a 9% increase in systolic blood pressure when called to a fire, a 9% increase in diastolic blood pressure when called to a medical emergency, and a 16% increase in heart rate for both types of calls.
Senior study author Deborah Feairheller, PhD, presented these results at the virtual American Heart Association scientific sessions.
Firefighters have a higher prevalence of cardiovascular disease (CVD) than that of the general population, explained Dr. Feairheller, director of the Hypertension and Endothelial Function with Aerobic and Resistance Training (HEART) Lab and clinical associate professor of kinesiology at the University of New Hampshire, Durham.
More than 50% of firefighter deaths in the line of duty are from CVD, she noted. Moreover, almost 75% of firefighters have hypertension and fewer than 25% have it under control.
The study findings show that all emergency and first responders “should know what their typical blood pressure level is and be aware of how it fluctuates,” Dr. Feairheller said in a press release from the AHA. “Most important, if they have high blood pressure, they should make sure it is well controlled,” she said.
“I really hope that fire departments everywhere see these data, rise to the occasion, and advocate for BP awareness in their crews,” Dr. Feairheller, a volunteer firefighter, said in an interview.
“I do think this has value to any occupation that wears a pager,” she added. “Clinicians, physicians, other emergency responders, all of those occupations are stressful and could place people at risk if they have undiagnosed or uncontrolled hypertension.”
Invited to comment, Comilla Sasson, MD, PhD, an emergency department physician who was not involved with this research, said in an interview that she saw parallels between stress experienced by firefighters and, for example, emergency department physicians.
The transient increases in BP, both systolic and diastolic, along with the heart rate are likely due to the body’s natural fight or flight response to an emergency call, including increases in epinephrine and cortisol, said Dr. Sasson, vice president of science and innovation for emergency cardiovascular care at the American Heart Association.
“The thing that is most interesting to me,” said Dr. Sasson, who can be subject to a series of high-stress situations on a shift, such as multiple trauma victims, a stroke victim, or a person in cardiac arrest, is “what is the cumulative impact of this over time?”
She said she wonders if “having to be ‘ready to go’ at any time, along with disruptions in sleep/wake schedules, and poorer eating and working-out habits when you are on shift, has long-term sequelae on the body.”
Stress-related surges in blood pressure “could be a reason for worse health outcomes in this group,” Dr. Sasson said, adding that this needs to be investigated further.
Firefighters with high normal BP, high BMI
Dr. Feairheller and colleagues recruited 41 volunteer and employee firefighters from suburban Philadelphia and Dover, N.H.
On average, the 37 men and 4 women had a mean age of 41 years, had been working as firefighters for 16.9 years, and had a mean body mass index of 30.3 kg/m2.
They wore ambulatory blood pressure monitors during an on-call work shift for at least 12 consecutive hours.
In addition to the automatic readings, the participants were instructed to prompt the machine to take a reading whenever a pager or emergency call sounded or when they felt they were entering a stressful situation.
Over the 12-hour shift, on average, participants had a blood pressure of 131/79.3 mm Hg and a heart rate of 75.7 bpm.
When they were alerted go to a fire, their blood pressure surged by 19.2/10.5 mm Hg, and their heart rate rose to 85.5 bpm.
Similarly, when they were alerted to go to a medical emergency, their blood pressure jumped up by 18.7/16.5 mm Hg and their heart rate climbed to 90.5 bpm.
The surges in blood pressure and heart rate were similar when participants were riding in the fire truck to a call or when the call turned out to be a false alarm.
What can be done?
“If we can increase awareness and identify specific risk factors in firefighters,” Dr. Feairheller said, this could “save a life of someone who spends their day saving lives and property.”
To start, “regular, in-station or home BP monitoring should be encouraged,” she said. “Firefighters should start to track their BP levels in the morning, at night, at work. Being a volunteer firefighter myself, I know the stress and anxiety and sadness and heavy work that comes with the job,” she said. “I want to be able to do what I can to help make the crews healthier.”
Dr. Sasson suggested that ways to increase awareness and improve the health of firefighters might include “counseling, appropriate breaks, possibly food service/delivery to provide better nutritional options, built-in time for exercise (gym or cardio equipment on site), and discussions about how stress can impact the body over time.”
It is important to advocate for better mental health care, because people may have PTSD, depression, substance abuse, or other mental health conditions brought on by their stressful jobs, she said.
“Also, it would be interesting to know what is the current state of health monitoring (both physical, mental, and emotional) that occurs for firefighters,” she said.
The American Heart Association funded the study. The authors and Dr. Sasson report no disclosures.
A version of this article first appeared on Medscape.com.
In response to a 911 alert or page, firefighters’ systolic and diastolic blood pressure surges and their heart rate accelerates, with a similar response whether the call is for a fire or medical emergency, a small study suggests.
On average, the 41 firefighters monitored in the study, who were middle-aged and overweight, had a 9% increase in systolic blood pressure when called to a fire, a 9% increase in diastolic blood pressure when called to a medical emergency, and a 16% increase in heart rate for both types of calls.
Senior study author Deborah Feairheller, PhD, presented these results at the virtual American Heart Association scientific sessions.
Firefighters have a higher prevalence of cardiovascular disease (CVD) than that of the general population, explained Dr. Feairheller, director of the Hypertension and Endothelial Function with Aerobic and Resistance Training (HEART) Lab and clinical associate professor of kinesiology at the University of New Hampshire, Durham.
More than 50% of firefighter deaths in the line of duty are from CVD, she noted. Moreover, almost 75% of firefighters have hypertension and fewer than 25% have it under control.
The study findings show that all emergency and first responders “should know what their typical blood pressure level is and be aware of how it fluctuates,” Dr. Feairheller said in a press release from the AHA. “Most important, if they have high blood pressure, they should make sure it is well controlled,” she said.
“I really hope that fire departments everywhere see these data, rise to the occasion, and advocate for BP awareness in their crews,” Dr. Feairheller, a volunteer firefighter, said in an interview.
“I do think this has value to any occupation that wears a pager,” she added. “Clinicians, physicians, other emergency responders, all of those occupations are stressful and could place people at risk if they have undiagnosed or uncontrolled hypertension.”
Invited to comment, Comilla Sasson, MD, PhD, an emergency department physician who was not involved with this research, said in an interview that she saw parallels between stress experienced by firefighters and, for example, emergency department physicians.
The transient increases in BP, both systolic and diastolic, along with the heart rate are likely due to the body’s natural fight or flight response to an emergency call, including increases in epinephrine and cortisol, said Dr. Sasson, vice president of science and innovation for emergency cardiovascular care at the American Heart Association.
“The thing that is most interesting to me,” said Dr. Sasson, who can be subject to a series of high-stress situations on a shift, such as multiple trauma victims, a stroke victim, or a person in cardiac arrest, is “what is the cumulative impact of this over time?”
She said she wonders if “having to be ‘ready to go’ at any time, along with disruptions in sleep/wake schedules, and poorer eating and working-out habits when you are on shift, has long-term sequelae on the body.”
Stress-related surges in blood pressure “could be a reason for worse health outcomes in this group,” Dr. Sasson said, adding that this needs to be investigated further.
Firefighters with high normal BP, high BMI
Dr. Feairheller and colleagues recruited 41 volunteer and employee firefighters from suburban Philadelphia and Dover, N.H.
On average, the 37 men and 4 women had a mean age of 41 years, had been working as firefighters for 16.9 years, and had a mean body mass index of 30.3 kg/m2.
They wore ambulatory blood pressure monitors during an on-call work shift for at least 12 consecutive hours.
In addition to the automatic readings, the participants were instructed to prompt the machine to take a reading whenever a pager or emergency call sounded or when they felt they were entering a stressful situation.
Over the 12-hour shift, on average, participants had a blood pressure of 131/79.3 mm Hg and a heart rate of 75.7 bpm.
When they were alerted go to a fire, their blood pressure surged by 19.2/10.5 mm Hg, and their heart rate rose to 85.5 bpm.
Similarly, when they were alerted to go to a medical emergency, their blood pressure jumped up by 18.7/16.5 mm Hg and their heart rate climbed to 90.5 bpm.
The surges in blood pressure and heart rate were similar when participants were riding in the fire truck to a call or when the call turned out to be a false alarm.
What can be done?
“If we can increase awareness and identify specific risk factors in firefighters,” Dr. Feairheller said, this could “save a life of someone who spends their day saving lives and property.”
To start, “regular, in-station or home BP monitoring should be encouraged,” she said. “Firefighters should start to track their BP levels in the morning, at night, at work. Being a volunteer firefighter myself, I know the stress and anxiety and sadness and heavy work that comes with the job,” she said. “I want to be able to do what I can to help make the crews healthier.”
Dr. Sasson suggested that ways to increase awareness and improve the health of firefighters might include “counseling, appropriate breaks, possibly food service/delivery to provide better nutritional options, built-in time for exercise (gym or cardio equipment on site), and discussions about how stress can impact the body over time.”
It is important to advocate for better mental health care, because people may have PTSD, depression, substance abuse, or other mental health conditions brought on by their stressful jobs, she said.
“Also, it would be interesting to know what is the current state of health monitoring (both physical, mental, and emotional) that occurs for firefighters,” she said.
The American Heart Association funded the study. The authors and Dr. Sasson report no disclosures.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Large Leg Ulcers After Swimming in the Ocean
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3 Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4 Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3 Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4 Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
The Diagnosis: Vibrio vulnificus Infection
At the initial presentation, the differential diagnosis included infectious processes such as bacterial or angioinvasive fungal infections or an inflammatory process such as pyoderma gangrenosum. Blood cultures were found to be positive for pansensitive Vibrio vulnificus. He initially was treated with piperacillin-tazobactam and received surgical debridement of the affected tissues. Pathologic interpretation of the wound tissues revealed a diagnosis of necrotizing softtissue infection and positive Candida albicans growth. He received topical bacitracin on discharge as well as a 7-day course of amoxicillin-clavulanate and fluconazole. He continued to receive debridement procedures and skin grafts, followed by topical mupirocin treatment and silver sulfadiazine. He was seen 6 weeks after discharge with healing wounds and healthy-appearing granulation tissue at the base.
Our patient’s presentation of retiform purpura with stellate necrosis was consistent with a wide range of serious pathologies ranging from medium-vessel vasculitis to thromboembolic phenomena and angioinvasive fungal infections.1 Although Vibrio infection rarely is the first explanation that comes to mind when observing necrotic retiform purpura, the chronic nonhealing injury on the leg combined with the recent history of ocean swimming made V vulnificus stand out as a likely culprit. Although V vulnificus infection traditionally presents with cellulitis, edema, and hemorrhagic bulla,2 necrosis also has been observed.3 Vibrio vulnificus produces multiple virulence factors, and it is believed that these severe cutaneous symptoms are attributable to the production of a specific metalloprotease that enhances vascular permeability, thereby inducing hemorrhage within the vascular basement membrane zone.2
Vibrio vulnificus is an opportunistic bacterial pathogen associated with consumption of contaminated seafood or swimming in ocean waters with open wounds. Infections are rare, with only approximately 100 cases reported annually in the United States.4 However, V vulnificus infections have demonstrated increasing incidence in recent years, especially infections of pre-existing wounds.4,5 Risk factors for their development include age over 40 years and underlying conditions including liver disease, diabetes mellitus, and immune dysfunction.4 Vibrio vulnificus infections also demonstrate a strong male predilection, with almost 90% of infections occurring in males.4 Although the precise etiology of this sex discrepancy remains unknown, estrogen has been suggested to be a protective factor.6 Alternatively, behavioral differences also have been proposed as possible explanations for this discrepancy, with women less likely to consume seafood or go swimming. However, epidemiologic data reveal strong correlations between male sex and liver cirrhosis, a primary risk factor for V vulnificus infections, suggesting that male sex may simply be a confounding variable.7
Infections with V vulnificus are notable for their short incubation periods, with onset of symptoms occurring within 24 hours of exposure, making prompt diagnosis and treatment of high importance.8 Although rare, V vulnificus infections are associated with high mortality rates. From 1988 to 2010, nearly 600 deaths were reported secondary to V vulnificus infections.4 Wound infections carry a 17.6% fatality rate,4 while bloodborne V vulnificus infections exceed 50% fatality.8 Although sepsis secondary to V vulnificus usually is caused by ingestion of raw or undercooked shellfish, primarily oysters,4 our case highlights a rarer instance of both sepsis and localized infection stemming from ocean water exposure.
Vibrio vulnificus is an obligate halophile and therefore is found in marine environments rather than freshwater bodies. However, it rarely is isolated from bodies of water with salinities over 25 parts per thousand, such as the Mediterranean Sea; it usually is found in warmer waters, making it more common in the summer months from May to October.4 Given this proclivity for warmer environments, climate change has contributed to both a greater incidence and global distribution of V vulnificus. 9,10
Treatment of V vulnificus infections centers on antibiotic treatment, with Vibrio species generally demonstrating susceptibility to most antibiotics of human significance.11 However, some Vibrio isolates within the United States have demonstrated antibiotic resistance; 45% of a variety of clinical and environmental samples from South Carolina and Georgia demonstrated resistance to at least 3 antibiotic classes, and 17.3% resisted 8 or more classes of antibiotics.12 These included medications such as doxycycline, tetracycline, aminoglycosides, and cephalosporins—agents that normally are prescribed for V vulnificus infections. Although tetracyclines have long been touted as the preferred treatment of V vulnificus infections, the spread of antibiotic resistance may require greater reliance on alternative regimens such as combinations of cephalosporins and doxycycline or a single fluoroquinolone.13 Although rare, Vibrio infections can have rapidly fatal consequences and should be given serious consideration when evaluating patients with relevant risk factors.
The differential diagnosis included angioinvasive mucormycosis, calciphylaxis, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Mucormycosis is a fungal infection caused by Mucorales fungi that most commonly is seen in patients with diabetes mellitus, hematologic malignancies, neutropenia, and immunocompromise.14 Calciphylaxis is a condition involving microvascular occlusion due to diffuse calcium deposition in cutaneous blood vessels. It typically presents as violaceous retiform patches and plaques commonly seen on areas such as the thighs, buttocks, or abdomen and usually is associated with chronic renal failure, hemodialysis, and/or secondary hyperparathyroidism.15 Pyoderma gangrenosum is an inflammatory condition involving neutrophilic ulceration of the skin that typically presents as ulceration with a classically undermined border. It frequently is considered a diagnosis of exclusion and therefore requires that providers rule out other causes of ulceration prior to diagnosis.16 Stevens-Johnson syndrome/toxic epidermal necrolysis is a rare drug reaction involving mucosal erosions and cutaneous detachment.17 This diagnosis is less likely given that our patient lacked mucosal involvement and did not have any notable medication exposures prior to symptom onset.
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
- Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172. doi:10.1111/j .1529-8019.2011.01392.x
- Miyoshi S-I. Vibrio vulnificus infection and metalloprotease. J Dermatol. 2006;33:589-595. doi:10.1111/j.1346-8138.2006.00139.x
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145. doi:10.1067 /mjd.2002.107778
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430. doi:10.1111/1462-2920.13955
- Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food —10 states, 2009. CDC website. Published April 16, 2010. Accessed November 3, 2021. https://www.cdc .gov/mmwr/preview/mmwrhtml/mm5914a2.htm
- Merkel SM, Alexander S, Zufall E, et al. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. doi:10.1128/IAI.69.10.6119 -6122.2001
- Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690-696. doi:10.1097/MCG.0000000000000208
- Jones M, Oliver J. Vibrio vulnificus: disease and pathogenesis [published online December 20, 2020]. Infect Immun. https://doi.org/10.1128 /IAI.01046-08
- Paz S, Bisharat N, Paz E, et al. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res. 2007;103:390-396. doi:10.1016/j.envres.2006.07.002
- Martinez-Urtaza J, Bowers JC, Trinanes J, et al. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780-1790. doi:10.1016/j. foodres.2010.04.001
- Oliver JD. Vibrio vulnificus. In: Thompson FL, Austin B, Swings J, eds. The Biology of Vibrios. ASM Press; 2006:349-366.
- Baker-Austin C, McArthur JV, Lindell AH, et al. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol. 2009;57:151-159. doi:10.1007 /s00248-008-9413-8
- Elmahdi S, DaSilva LV, Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128-134. doi:10.1016/j.fm.2016.02.008
- Prasad P, Wong V, Burgin S, et al. Mucormycosis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/mucormycosis?diagnosisId=51981 &moduleId=101
- Blum A, Song P, Tan B, et al. Calciphylaxis. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/calciphylaxis?diagnosisId=51241&moduleId=101
- Cohen J, Wong V, Burgin S. Pyoderma gangrenosum. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/pyoderma+gangrenosum?diagnosis Id=52242&moduleId=101
- Walls A, Burgin S. Stevens-Johnson syndrome. VisualDx website. Accessed November 13, 2021. https://www-visualdx-com.proxy.lib.ohio-state.edu/visualdx/diagnosis/stevens-johnson+syndrome?diagnosisId=52342&moduleId=101
A 48-year-old man presented to the emergency department with pain in both legs after swimming in the ocean surrounding Florida 1 month prior to presentation. His medical history included skin graft treatment of burns during childhood and a chronic lower extremity ulcer that developed after trauma. He received hemodialysis for acute renal failure approximately 1 month prior to the current presentation. At the current presentation he was found to be septic and quickly developed rapidly expanding regions of retiform purpura with stellate necrosis on the legs.
Neurologist guilty of overprescribing thousands of doses of painkillers
Ohio doctor convicted of prescribing unnecessary controlled substances, fraud
A federal jury found William R. Bauer, 84, of Port Clinton, Ohio, guilty of prescribing powerful controlled substances, including opioids, to patients without medical necessity and outside the usual course of medical practice.
Dr. Bauer, a neurologist with over 50 years of experience, was convicted of 76 counts of distribution of controlled substances and 25 counts of healthcare fraud. According to television station WTOL, a federal indictment from 2019 listed 270 charges against the physician.
Federal officials claim that through his practice in Bellevue, Ohio, Dr. Bauer repeatedly prescribed controlled substances, including oxycodone, fentanyl, morphine, and tramadol, outside the usual course of professional practice and without legitimate medical purpose. The charges focused on 14 of his patients, to whom he prescribed high doses of opioids and other controlled substances without medical necessity. He also prescribed dangerous drug combinations. He ignored patients’ signs of addiction and abuse, such as early requests for refills, claims that medications had been lost, and claims that family members were stealing pills.
Dr. Bauer was also convicted of healthcare fraud for regularly administering epidural injections and trigger-point injections without medical necessity. Because these injections failed to meet the procedural requirements, they were rendered ineffective and were fraudulently billed to insurers. Dr. Bauer’s illegal prescriptions resulted in insurers paying for these medically unnecessary controlled substances.
Evidence at trial indicated that between January 2007 and August 16, 2019, Dr. Bauer prescribed controlled substances outside the usual course of medical practice and for illegitimate medical purposes. Insurers paid for these medically unnecessary controlled substances as well.
He will be sentenced at a later date.
Lab pays $1.2 million to resolve allegations of false claims for drug testing
Bluewater Toxicology, LLC, a clinical laboratory in Mount Washington, Ky., has agreed to pay $1.2 million to resolve civil allegations that it violated the False Claims Act.
The U.S. Department of Justice alleged three issues relating to claims for urine drug testing services that Bluewater submitted to Medicare, Kentucky Medicaid, Indiana Medicaid, TRICARE, and CHAMPVA. First, Bluewater submitted claims in which it misrepresented the number of drug classes it tested. Bluewater claimed it conducted definitive urine drug tests of 22 or more drug classes. In truth, Bluewater tested for fewer than 22 drug classes and secured reimbursement for drug tests that it did not conduct.
Second, Bluewater submitted certain claims without sufficient documentation to support the physician’s intent to order the test that was billed. In this way Bluewater obtained further unwarranted reimbursements.
Finally, Bluewater billed Medicare for specimen validity testing, a quality control process used to analyze a urine specimen to ensure that it has not been diluted or adulterated. Since January 2014, Medicare’s guidance has stated that specimen validity testing should not be separately billed to Medicare, but Bluewater did so anyway.
Home care company owner pays $1 million in Medicare fraud restitution
Richard Wennerberg, 72, of Grantham, N.H., pleaded guilty and was sentenced to two counts of class B felony Medicaid fraud, according to the New Hampshire Department of Justice.
Mr. Wennerberg is the owner of Alternative Care @ Home, LLC, a company licensed to provide in-home personal care services to Medicaid beneficiaries. He also pleaded guilty to a third charge of Medicaid fraud, through which Alternative Care @ Home, LLC, will be excluded from future participation in federal healthcare programs.
According to New Hampshire officials, Mr. Wennerberg submitted claims for reimbursement for in-home, personal care services that were never provided. Wennerberg billed Medicaid up to the maximum hours allowed under certain clients’ service authorizations, knowing that his employees did not provide care for all of those hours. He would use the difference to reimburse some caregivers for mileage.
Mr. Wennerberg will serve 1 year in state prison and will pay $1 million in restitution.
North Carolina wins two “Operation Root Canal” settlements
North Carolina Attorney General Josh Stein announced two separate civil settlements with ProHealth Dental Inc and Henry W. Davis, Jr, DDS, as part of Operation Root Canal, an ongoing effort to find and stop healthcare fraud among dental practitioners. The settlements, totaling $75,000, resolve allegations of the submission of false claims to the North Carolina Medicaid program.
In Operation Root Canal, the state Medicaid investigations department reviews billing practices for a wide variety of dental services, including dental cleanings, use of nitrous oxide, repetitive restorations on the same tooth, palliative care, and upcoding of patient examinations. In total, the operation has netted more than $7 million for the state.
The recent settlement relates to a prior criminal plea the attorney general’s Medicaid Investigations Division obtained involving Mr. Christian Ekberg, of Maryland, who was sentenced to 18 months in prison for healthcare fraud and was ordered to pay $173,870.12 to the North Carolina Medicaid Fund in restitution. Ekberg was an officer and minority shareholder of ProHealth Dental, a company that entered into a practice management agreement with Henry W. Davis, Jr, DDS., a North Carolina dentist and Medicaid practitioner who provided dental services to patients living in skilled nursing facilities throughout North Carolina. ProHealth Dental would provide professional management services to Dr. Davis, including submitting Medicaid claims. The company submitted claims for dental services that Dr. Davis did not perform on Medicaid recipients.
A version of this article first appeared on Medscape.com.
Ohio doctor convicted of prescribing unnecessary controlled substances, fraud
Ohio doctor convicted of prescribing unnecessary controlled substances, fraud
A federal jury found William R. Bauer, 84, of Port Clinton, Ohio, guilty of prescribing powerful controlled substances, including opioids, to patients without medical necessity and outside the usual course of medical practice.
Dr. Bauer, a neurologist with over 50 years of experience, was convicted of 76 counts of distribution of controlled substances and 25 counts of healthcare fraud. According to television station WTOL, a federal indictment from 2019 listed 270 charges against the physician.
Federal officials claim that through his practice in Bellevue, Ohio, Dr. Bauer repeatedly prescribed controlled substances, including oxycodone, fentanyl, morphine, and tramadol, outside the usual course of professional practice and without legitimate medical purpose. The charges focused on 14 of his patients, to whom he prescribed high doses of opioids and other controlled substances without medical necessity. He also prescribed dangerous drug combinations. He ignored patients’ signs of addiction and abuse, such as early requests for refills, claims that medications had been lost, and claims that family members were stealing pills.
Dr. Bauer was also convicted of healthcare fraud for regularly administering epidural injections and trigger-point injections without medical necessity. Because these injections failed to meet the procedural requirements, they were rendered ineffective and were fraudulently billed to insurers. Dr. Bauer’s illegal prescriptions resulted in insurers paying for these medically unnecessary controlled substances.
Evidence at trial indicated that between January 2007 and August 16, 2019, Dr. Bauer prescribed controlled substances outside the usual course of medical practice and for illegitimate medical purposes. Insurers paid for these medically unnecessary controlled substances as well.
He will be sentenced at a later date.
Lab pays $1.2 million to resolve allegations of false claims for drug testing
Bluewater Toxicology, LLC, a clinical laboratory in Mount Washington, Ky., has agreed to pay $1.2 million to resolve civil allegations that it violated the False Claims Act.
The U.S. Department of Justice alleged three issues relating to claims for urine drug testing services that Bluewater submitted to Medicare, Kentucky Medicaid, Indiana Medicaid, TRICARE, and CHAMPVA. First, Bluewater submitted claims in which it misrepresented the number of drug classes it tested. Bluewater claimed it conducted definitive urine drug tests of 22 or more drug classes. In truth, Bluewater tested for fewer than 22 drug classes and secured reimbursement for drug tests that it did not conduct.
Second, Bluewater submitted certain claims without sufficient documentation to support the physician’s intent to order the test that was billed. In this way Bluewater obtained further unwarranted reimbursements.
Finally, Bluewater billed Medicare for specimen validity testing, a quality control process used to analyze a urine specimen to ensure that it has not been diluted or adulterated. Since January 2014, Medicare’s guidance has stated that specimen validity testing should not be separately billed to Medicare, but Bluewater did so anyway.
Home care company owner pays $1 million in Medicare fraud restitution
Richard Wennerberg, 72, of Grantham, N.H., pleaded guilty and was sentenced to two counts of class B felony Medicaid fraud, according to the New Hampshire Department of Justice.
Mr. Wennerberg is the owner of Alternative Care @ Home, LLC, a company licensed to provide in-home personal care services to Medicaid beneficiaries. He also pleaded guilty to a third charge of Medicaid fraud, through which Alternative Care @ Home, LLC, will be excluded from future participation in federal healthcare programs.
According to New Hampshire officials, Mr. Wennerberg submitted claims for reimbursement for in-home, personal care services that were never provided. Wennerberg billed Medicaid up to the maximum hours allowed under certain clients’ service authorizations, knowing that his employees did not provide care for all of those hours. He would use the difference to reimburse some caregivers for mileage.
Mr. Wennerberg will serve 1 year in state prison and will pay $1 million in restitution.
North Carolina wins two “Operation Root Canal” settlements
North Carolina Attorney General Josh Stein announced two separate civil settlements with ProHealth Dental Inc and Henry W. Davis, Jr, DDS, as part of Operation Root Canal, an ongoing effort to find and stop healthcare fraud among dental practitioners. The settlements, totaling $75,000, resolve allegations of the submission of false claims to the North Carolina Medicaid program.
In Operation Root Canal, the state Medicaid investigations department reviews billing practices for a wide variety of dental services, including dental cleanings, use of nitrous oxide, repetitive restorations on the same tooth, palliative care, and upcoding of patient examinations. In total, the operation has netted more than $7 million for the state.
The recent settlement relates to a prior criminal plea the attorney general’s Medicaid Investigations Division obtained involving Mr. Christian Ekberg, of Maryland, who was sentenced to 18 months in prison for healthcare fraud and was ordered to pay $173,870.12 to the North Carolina Medicaid Fund in restitution. Ekberg was an officer and minority shareholder of ProHealth Dental, a company that entered into a practice management agreement with Henry W. Davis, Jr, DDS., a North Carolina dentist and Medicaid practitioner who provided dental services to patients living in skilled nursing facilities throughout North Carolina. ProHealth Dental would provide professional management services to Dr. Davis, including submitting Medicaid claims. The company submitted claims for dental services that Dr. Davis did not perform on Medicaid recipients.
A version of this article first appeared on Medscape.com.
A federal jury found William R. Bauer, 84, of Port Clinton, Ohio, guilty of prescribing powerful controlled substances, including opioids, to patients without medical necessity and outside the usual course of medical practice.
Dr. Bauer, a neurologist with over 50 years of experience, was convicted of 76 counts of distribution of controlled substances and 25 counts of healthcare fraud. According to television station WTOL, a federal indictment from 2019 listed 270 charges against the physician.
Federal officials claim that through his practice in Bellevue, Ohio, Dr. Bauer repeatedly prescribed controlled substances, including oxycodone, fentanyl, morphine, and tramadol, outside the usual course of professional practice and without legitimate medical purpose. The charges focused on 14 of his patients, to whom he prescribed high doses of opioids and other controlled substances without medical necessity. He also prescribed dangerous drug combinations. He ignored patients’ signs of addiction and abuse, such as early requests for refills, claims that medications had been lost, and claims that family members were stealing pills.
Dr. Bauer was also convicted of healthcare fraud for regularly administering epidural injections and trigger-point injections without medical necessity. Because these injections failed to meet the procedural requirements, they were rendered ineffective and were fraudulently billed to insurers. Dr. Bauer’s illegal prescriptions resulted in insurers paying for these medically unnecessary controlled substances.
Evidence at trial indicated that between January 2007 and August 16, 2019, Dr. Bauer prescribed controlled substances outside the usual course of medical practice and for illegitimate medical purposes. Insurers paid for these medically unnecessary controlled substances as well.
He will be sentenced at a later date.
Lab pays $1.2 million to resolve allegations of false claims for drug testing
Bluewater Toxicology, LLC, a clinical laboratory in Mount Washington, Ky., has agreed to pay $1.2 million to resolve civil allegations that it violated the False Claims Act.
The U.S. Department of Justice alleged three issues relating to claims for urine drug testing services that Bluewater submitted to Medicare, Kentucky Medicaid, Indiana Medicaid, TRICARE, and CHAMPVA. First, Bluewater submitted claims in which it misrepresented the number of drug classes it tested. Bluewater claimed it conducted definitive urine drug tests of 22 or more drug classes. In truth, Bluewater tested for fewer than 22 drug classes and secured reimbursement for drug tests that it did not conduct.
Second, Bluewater submitted certain claims without sufficient documentation to support the physician’s intent to order the test that was billed. In this way Bluewater obtained further unwarranted reimbursements.
Finally, Bluewater billed Medicare for specimen validity testing, a quality control process used to analyze a urine specimen to ensure that it has not been diluted or adulterated. Since January 2014, Medicare’s guidance has stated that specimen validity testing should not be separately billed to Medicare, but Bluewater did so anyway.
Home care company owner pays $1 million in Medicare fraud restitution
Richard Wennerberg, 72, of Grantham, N.H., pleaded guilty and was sentenced to two counts of class B felony Medicaid fraud, according to the New Hampshire Department of Justice.
Mr. Wennerberg is the owner of Alternative Care @ Home, LLC, a company licensed to provide in-home personal care services to Medicaid beneficiaries. He also pleaded guilty to a third charge of Medicaid fraud, through which Alternative Care @ Home, LLC, will be excluded from future participation in federal healthcare programs.
According to New Hampshire officials, Mr. Wennerberg submitted claims for reimbursement for in-home, personal care services that were never provided. Wennerberg billed Medicaid up to the maximum hours allowed under certain clients’ service authorizations, knowing that his employees did not provide care for all of those hours. He would use the difference to reimburse some caregivers for mileage.
Mr. Wennerberg will serve 1 year in state prison and will pay $1 million in restitution.
North Carolina wins two “Operation Root Canal” settlements
North Carolina Attorney General Josh Stein announced two separate civil settlements with ProHealth Dental Inc and Henry W. Davis, Jr, DDS, as part of Operation Root Canal, an ongoing effort to find and stop healthcare fraud among dental practitioners. The settlements, totaling $75,000, resolve allegations of the submission of false claims to the North Carolina Medicaid program.
In Operation Root Canal, the state Medicaid investigations department reviews billing practices for a wide variety of dental services, including dental cleanings, use of nitrous oxide, repetitive restorations on the same tooth, palliative care, and upcoding of patient examinations. In total, the operation has netted more than $7 million for the state.
The recent settlement relates to a prior criminal plea the attorney general’s Medicaid Investigations Division obtained involving Mr. Christian Ekberg, of Maryland, who was sentenced to 18 months in prison for healthcare fraud and was ordered to pay $173,870.12 to the North Carolina Medicaid Fund in restitution. Ekberg was an officer and minority shareholder of ProHealth Dental, a company that entered into a practice management agreement with Henry W. Davis, Jr, DDS., a North Carolina dentist and Medicaid practitioner who provided dental services to patients living in skilled nursing facilities throughout North Carolina. ProHealth Dental would provide professional management services to Dr. Davis, including submitting Medicaid claims. The company submitted claims for dental services that Dr. Davis did not perform on Medicaid recipients.
A version of this article first appeared on Medscape.com.
Transdermal patches ease extrapyramidal symptoms in schizophrenia
Use of a transdermal blonanserin patch significantly improved extrapyramidal symptoms (EPS), compared with oral blonanserin tablets in patients with schizophrenia, according to results of an open-label study of 155 adults.
Blonanserin, a second-generation antipsychotic, has been shown to reduce extrapyramidal symptoms when used to treat schizophrenia, but the impact of switching to a patch on extrapyramidal symptoms and on the use of antiparkinson drugs has not been well studied, Kazutaka Ohi, MD, of Gifu University Graduate School of Medicine, Seki, Japan, and colleagues wrote. Advantages of the patch include the ability to provide stable blood concentrations and the ability to be concealed under clothing to avoid patients’ embarrassment at taking oral medications.
In a study published in Progress in Neuropsychopharmacology & Biological Psychiatry, the researchers identified 155 adults aged 18 years and older diagnosed with schizophrenia who were treated at 37 medical institutions in Japan between February 2015 and May 2017.
The first cohort of 97 patients received blonanserin tablets (8-16 mg/day) for 6 weeks, followed by blonanserin transdermal patches (40-80 mg/day) once daily for 1 year. The second cohort of 58 patients received continuous blonanserin patch therapy. Extrapyramidal symptoms were assessed using the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS); individual scores ranged from a 0 for normal to a 4 for severe.
Overall, DIEPSS scores decreased significantly in both cohorts after switching from blonanserin tablets or powders to transdermal patches. The average DIEPSS change from baseline at 3, 6, and 12 months was –0.44, –0.07, and –0.14, respectively, in cohort 1, and –0.16, –0.74, and –0.81, respectively, in cohort 2.
The researchers also assessed the impact of transition to transdermal patches on the use of antiparkinsonism drugs using the biperiden equivalents of total antiparkinsonian drugs (BPD-eq) measure. At baseline, about 22% of patients used concomitant antiparkinsonism drugs, compared with 25.8% at 1 year after starting patch treatment. The dose of antiparkinson drugs was not significantly decreased after switching to transdermal patches, in part because of psychiatrists’ prescribing behaviors, Dr. Ohi and colleagues noted.
As a secondary outcome, the researchers examined psychotic symptoms and found that Positive and Negative Syndrome Scale (PANSS) negative symptom scores decreased significantly in patients in cohort 1 who switched from tablets or powders to patches. Changes in scores from baseline to 3, 6, and 12 months were –0.7, –1.0, and –1.3, respectively. Positive PANSS scores did not change significantly in cohort 1. In cohort 2, both positive and negative PANSS scores decreased significantly over 12 months after switching from blonanserin tablets/powders to patches. The mean changes in scores from baseline to 3, 6, and 12 months were –1.6, –2.3, and –2.4, respectively, for PANSS positive symptom scores, and –1.4, –2.7, and –2.8, respectively, for negative symptom scores.
A total of 41.2% of cohort 1 patients and 44.8% of cohort 2 patients discontinued patch treatments by 1 year. Four patients discontinued the patch because of EPS during the treatment period in cohort 1; no patients in cohort 2 discontinued because of EPS.
The study findings were limited by several factors, including the open-label design and lack of controls; also, the study did not examine crossover changes in patients who switched from tablets or powders to patches, the researchers noted.
However, the results indicate that direct switching from blonanserin tablets or powders to transdermal patches reduced EPS and psychotic symptoms in schizophrenia and may be more acceptable to patients, compared with oral medications, as well as more effective, they concluded.
The study received no outside funding, and Dr. Ohi and colleagues had no disclosures.
Use of a transdermal blonanserin patch significantly improved extrapyramidal symptoms (EPS), compared with oral blonanserin tablets in patients with schizophrenia, according to results of an open-label study of 155 adults.
Blonanserin, a second-generation antipsychotic, has been shown to reduce extrapyramidal symptoms when used to treat schizophrenia, but the impact of switching to a patch on extrapyramidal symptoms and on the use of antiparkinson drugs has not been well studied, Kazutaka Ohi, MD, of Gifu University Graduate School of Medicine, Seki, Japan, and colleagues wrote. Advantages of the patch include the ability to provide stable blood concentrations and the ability to be concealed under clothing to avoid patients’ embarrassment at taking oral medications.
In a study published in Progress in Neuropsychopharmacology & Biological Psychiatry, the researchers identified 155 adults aged 18 years and older diagnosed with schizophrenia who were treated at 37 medical institutions in Japan between February 2015 and May 2017.
The first cohort of 97 patients received blonanserin tablets (8-16 mg/day) for 6 weeks, followed by blonanserin transdermal patches (40-80 mg/day) once daily for 1 year. The second cohort of 58 patients received continuous blonanserin patch therapy. Extrapyramidal symptoms were assessed using the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS); individual scores ranged from a 0 for normal to a 4 for severe.
Overall, DIEPSS scores decreased significantly in both cohorts after switching from blonanserin tablets or powders to transdermal patches. The average DIEPSS change from baseline at 3, 6, and 12 months was –0.44, –0.07, and –0.14, respectively, in cohort 1, and –0.16, –0.74, and –0.81, respectively, in cohort 2.
The researchers also assessed the impact of transition to transdermal patches on the use of antiparkinsonism drugs using the biperiden equivalents of total antiparkinsonian drugs (BPD-eq) measure. At baseline, about 22% of patients used concomitant antiparkinsonism drugs, compared with 25.8% at 1 year after starting patch treatment. The dose of antiparkinson drugs was not significantly decreased after switching to transdermal patches, in part because of psychiatrists’ prescribing behaviors, Dr. Ohi and colleagues noted.
As a secondary outcome, the researchers examined psychotic symptoms and found that Positive and Negative Syndrome Scale (PANSS) negative symptom scores decreased significantly in patients in cohort 1 who switched from tablets or powders to patches. Changes in scores from baseline to 3, 6, and 12 months were –0.7, –1.0, and –1.3, respectively. Positive PANSS scores did not change significantly in cohort 1. In cohort 2, both positive and negative PANSS scores decreased significantly over 12 months after switching from blonanserin tablets/powders to patches. The mean changes in scores from baseline to 3, 6, and 12 months were –1.6, –2.3, and –2.4, respectively, for PANSS positive symptom scores, and –1.4, –2.7, and –2.8, respectively, for negative symptom scores.
A total of 41.2% of cohort 1 patients and 44.8% of cohort 2 patients discontinued patch treatments by 1 year. Four patients discontinued the patch because of EPS during the treatment period in cohort 1; no patients in cohort 2 discontinued because of EPS.
The study findings were limited by several factors, including the open-label design and lack of controls; also, the study did not examine crossover changes in patients who switched from tablets or powders to patches, the researchers noted.
However, the results indicate that direct switching from blonanserin tablets or powders to transdermal patches reduced EPS and psychotic symptoms in schizophrenia and may be more acceptable to patients, compared with oral medications, as well as more effective, they concluded.
The study received no outside funding, and Dr. Ohi and colleagues had no disclosures.
Use of a transdermal blonanserin patch significantly improved extrapyramidal symptoms (EPS), compared with oral blonanserin tablets in patients with schizophrenia, according to results of an open-label study of 155 adults.
Blonanserin, a second-generation antipsychotic, has been shown to reduce extrapyramidal symptoms when used to treat schizophrenia, but the impact of switching to a patch on extrapyramidal symptoms and on the use of antiparkinson drugs has not been well studied, Kazutaka Ohi, MD, of Gifu University Graduate School of Medicine, Seki, Japan, and colleagues wrote. Advantages of the patch include the ability to provide stable blood concentrations and the ability to be concealed under clothing to avoid patients’ embarrassment at taking oral medications.
In a study published in Progress in Neuropsychopharmacology & Biological Psychiatry, the researchers identified 155 adults aged 18 years and older diagnosed with schizophrenia who were treated at 37 medical institutions in Japan between February 2015 and May 2017.
The first cohort of 97 patients received blonanserin tablets (8-16 mg/day) for 6 weeks, followed by blonanserin transdermal patches (40-80 mg/day) once daily for 1 year. The second cohort of 58 patients received continuous blonanserin patch therapy. Extrapyramidal symptoms were assessed using the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS); individual scores ranged from a 0 for normal to a 4 for severe.
Overall, DIEPSS scores decreased significantly in both cohorts after switching from blonanserin tablets or powders to transdermal patches. The average DIEPSS change from baseline at 3, 6, and 12 months was –0.44, –0.07, and –0.14, respectively, in cohort 1, and –0.16, –0.74, and –0.81, respectively, in cohort 2.
The researchers also assessed the impact of transition to transdermal patches on the use of antiparkinsonism drugs using the biperiden equivalents of total antiparkinsonian drugs (BPD-eq) measure. At baseline, about 22% of patients used concomitant antiparkinsonism drugs, compared with 25.8% at 1 year after starting patch treatment. The dose of antiparkinson drugs was not significantly decreased after switching to transdermal patches, in part because of psychiatrists’ prescribing behaviors, Dr. Ohi and colleagues noted.
As a secondary outcome, the researchers examined psychotic symptoms and found that Positive and Negative Syndrome Scale (PANSS) negative symptom scores decreased significantly in patients in cohort 1 who switched from tablets or powders to patches. Changes in scores from baseline to 3, 6, and 12 months were –0.7, –1.0, and –1.3, respectively. Positive PANSS scores did not change significantly in cohort 1. In cohort 2, both positive and negative PANSS scores decreased significantly over 12 months after switching from blonanserin tablets/powders to patches. The mean changes in scores from baseline to 3, 6, and 12 months were –1.6, –2.3, and –2.4, respectively, for PANSS positive symptom scores, and –1.4, –2.7, and –2.8, respectively, for negative symptom scores.
A total of 41.2% of cohort 1 patients and 44.8% of cohort 2 patients discontinued patch treatments by 1 year. Four patients discontinued the patch because of EPS during the treatment period in cohort 1; no patients in cohort 2 discontinued because of EPS.
The study findings were limited by several factors, including the open-label design and lack of controls; also, the study did not examine crossover changes in patients who switched from tablets or powders to patches, the researchers noted.
However, the results indicate that direct switching from blonanserin tablets or powders to transdermal patches reduced EPS and psychotic symptoms in schizophrenia and may be more acceptable to patients, compared with oral medications, as well as more effective, they concluded.
The study received no outside funding, and Dr. Ohi and colleagues had no disclosures.
FROM PROGRESS IN NEUROPSYCHOPHARMACOLOGY & BIOLOGICAL PSYCHIATRY