User login
PHM virtual conference promises practical pearls, plus Dr. Fauci
The Pediatric Hospital Medicine annual conference, though virtual in 2021, promises to retain its role as the premier educational event for pediatric hospitalists and other clinicians involved in treating pediatric patients.
The “can’t-miss” session, on August 5, at 6:30 p.m. ET, is a one-on-one discussion between Anthony S. Fauci, MD, and Lee Savio Beers, MD, president of the American Academic of Pediatrics, according to members of the meeting planning committee.
In addition to the conversation between Dr. Beers and Dr. Fauci, this year’s meeting offers a mix of workshops with pointers and pearls to improve practice, keynote and plenary sessions to inform and inspire, and abstract presentations of new research. Three members of the PHM Planning Committee shared their insights on the hot topics, advice for new clinicians, and tips for making the most of this year’s meeting.
Workshops worth watching
“The keynote plenary sessions by Julie Silver, MD, on ‘Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion,’ and by Ilan Alhadeff, MD, on ‘Leading through Adversity’ should inspire even the least enthusiastic among us,” Mirna Giordano, MD, FHM, of Columbia University Medical Center, New York, said in an interview. A talk by Nathan T. Chomilo, MD, “will likely prompt reflection on how George Floyd’s death changed us, and how we practice medicine forever.” In addition, “PHM Stories are not to be missed, they are voices that speak loud and move mountains.”
The PHM Stories are concise, narrative talks with minimal use of slides; each PHM Stories session includes three distinct talks and a 15-minute question and answer session. PHM Stories sessions are scheduled for each day of the conference, and topics include “Practicing Medicine While Human: The Secrets Physicians Keep,” by Uchenna Ewulonu, MD; “Finding the Power of the Imposter: How I Learned to Be Exactly the Color I Am, Everywhere I Go,” by Alexandra Coria, MD; and “Purple Butterflies: A Reflection on Why I’m a Pediatric Hospitalist,” by Joanne Mendoza, MD.
“The PHM community has been through a lot in the aftermath of the pandemic,” said Dr. Giordano. “The mini-plenary session on the mental health needs of our patients, and clinical quick-hit sessions on verbal deescalation of the agitated patients and cardiac effects of COVID-19 will likely be not only very popular, but also useful in clinical endeavors. The workshop on how to navigate the adult issues in hospitalized patients will provide the Med-Peds pearls we all wish we heard earlier.”
Although a 75-minute workshop session may seem long, “the workshop choices will offer something for everyone’s taste: education, research, clinical topics, diversity, and advocacy,” Dr. Giordano said. “I suggest that attendees check in advance which sessions will be available after the meeting, so that they prioritize highly interactive sessions like workshops, and that they experience, even if virtual, small group/room gatherings and networking.” There will be time for fun, too, she emphasized, with social sessions “that we hope will break the screen monotony and bring smiles to everyone’s faces.”
For younger clinicians relatively new to practice, Dr. Giordano recommended several workshops for a wealth of advice and guidance, including “New Kids on the Block: Thriving in your First Faculty Position,” “Channeling Your Inner Coach: Techniques to Enhance Clinical Teaching & Feedback,” “Palliative Care Pearls for the Pediatric Hospitalist,” “Perioperative Medicine for Medically Complex Children: Case Studies in Programmatic Approaches,” “The Bare Necessities: Social Determinant of Health Screening for the Hospitalist,” and “Mentorship, Autonomy, and Supervising a PHM Fellow.”
Classic topics and new concepts
“We are so excited to be able to offer a full spectrum of offerings at this year’s virtual meeting,” Yemisi Jones, MD, FHM, of Cincinnati Children’s Hospital, said in an interview. “We are covering some classic topics that we can’t do without at PHM, such as clinical updates in the management of sick and well newborns; workshops on best practices for educators; as well as the latest in PHM scholarship.” Sessions include “timely topics such as equity for women in medicine with one of our plenary speakers, Julie Silver, MD, and new febrile infant guidelines,” she added.
In particular, the COVID-19 and mental health session will help address clinicians’ evolving understanding of the COVID-19 pandemic and its effects on hospitalized children, said Dr. Jones. “Attendees can expect practical, timely updates on the current state of the science and ways to improve their practice to provide the best care for our patients.”
Attendees will be able to maximize the virtual conference format by accessing archived recordings, including clinical quick hits, mini-plenaries, and PHM Stories, which can be viewed during the scheduled meeting time or after, Dr. Jones said. “Workshops and abstract presentations will involve real-time interaction with presenters, so would be highest yield to attend during the live meeting. We also encourage all participants to take full advantage of the platform and the various networking opportunities to engage with others in our PHM community.”
For residents and new fellows, Dr. Jones advised making the workshop, “A Whole New World: Tips and Tools to Soar Into Your First Year of Fellowship,” a priority. “For early-career faculty, the ‘New Kids on the Block: Thriving in your First Faculty Position workshop will be a valuable resource.”
Make the meeting content a priority
This year’s conference has an exceptional slate of plenary speakers, Michelle Marks, DO, SFHM, of the Cleveland Clinic said in an interview. In addition to the much-anticipated session on vaccinations, school guidelines, and other topics with Dr. Fauci and Dr. Beers, the sessions on leading through adversity and workforce diversity and inclusion are “important topics to the PHM community and to our greater communities as a whole.”
Dr. Marks also highlighted the value of the COVID-19 and mental health session, as the long-term impact of COVID-19 on mental health of children and adults continues to grab headlines. “From this session specifically, I hope the attendees will gain awareness of the special mental health needs for child during a global disaster like a pandemic, which can be generalized to other situations and gain skills and resources to help meet and advocate for children’s mental health needs.”
For clinicians attending the virtual conference, “The most important strategy is to schedule time off of clinical work for the virtual meeting if you can so you can focus on the content,” said Dr. Marks. “For the longer sessions, it would be very important to block time in your day to fully attend the session, attend in a private space if possible since there will be breakouts with discussion, have your camera on, and engage with the workshop group as much as possible. The virtual format can be challenging because of all the external distractions, so intentional focus is necessary,” to get the most out of the experience.
The mini-plenary session on “The New AAP Clinical Practice Guideline on the Evaluation and Management of Febrile Infants 8-60 Days Old,” is an important session for all attendees, Dr. Marks said. She also recommended the Clinical Quick Hits sessions for anyone seeking “a diverse array of practical knowledge which can be easily applied to everyday practice.” The Clinical Quick Hits are designed as 35-minute, rapid-fire presentations focused on clinical knowledge. Each of these presentations will focus on the latest updates or evolutions in clinical practice in one area. Some key topics include counseling parents when a child has an abnormal exam finding, assessing pelvic pain in adolescent girls, and preventing venous thromboembolism in the inpatient setting.
“I would also recommend that younger clinicians take in at least one or two workshops or sessions on nonclinical topics to see the breath of content at the meeting and to develop a niche interest for themselves outside of clinical work,” Dr. Marks noted.
Nonclinical sessions at PHM 2021 include workshops on a pilot for a comprehensive LGBTQ+ curriculum, using media tools for public health messaging, and practicing health literacy.
To register for the Pediatric Hospital Medicine 2021 virtual conference, visit https://apaevents.regfox.com/phm21-virtual-conference.
Dr. Giordano, Dr. Jones, and Dr. Marks are members of the PHM conference planning committee and had no relevant financial conflicts to disclose.
The Pediatric Hospital Medicine annual conference, though virtual in 2021, promises to retain its role as the premier educational event for pediatric hospitalists and other clinicians involved in treating pediatric patients.
The “can’t-miss” session, on August 5, at 6:30 p.m. ET, is a one-on-one discussion between Anthony S. Fauci, MD, and Lee Savio Beers, MD, president of the American Academic of Pediatrics, according to members of the meeting planning committee.
In addition to the conversation between Dr. Beers and Dr. Fauci, this year’s meeting offers a mix of workshops with pointers and pearls to improve practice, keynote and plenary sessions to inform and inspire, and abstract presentations of new research. Three members of the PHM Planning Committee shared their insights on the hot topics, advice for new clinicians, and tips for making the most of this year’s meeting.
Workshops worth watching
“The keynote plenary sessions by Julie Silver, MD, on ‘Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion,’ and by Ilan Alhadeff, MD, on ‘Leading through Adversity’ should inspire even the least enthusiastic among us,” Mirna Giordano, MD, FHM, of Columbia University Medical Center, New York, said in an interview. A talk by Nathan T. Chomilo, MD, “will likely prompt reflection on how George Floyd’s death changed us, and how we practice medicine forever.” In addition, “PHM Stories are not to be missed, they are voices that speak loud and move mountains.”
The PHM Stories are concise, narrative talks with minimal use of slides; each PHM Stories session includes three distinct talks and a 15-minute question and answer session. PHM Stories sessions are scheduled for each day of the conference, and topics include “Practicing Medicine While Human: The Secrets Physicians Keep,” by Uchenna Ewulonu, MD; “Finding the Power of the Imposter: How I Learned to Be Exactly the Color I Am, Everywhere I Go,” by Alexandra Coria, MD; and “Purple Butterflies: A Reflection on Why I’m a Pediatric Hospitalist,” by Joanne Mendoza, MD.
“The PHM community has been through a lot in the aftermath of the pandemic,” said Dr. Giordano. “The mini-plenary session on the mental health needs of our patients, and clinical quick-hit sessions on verbal deescalation of the agitated patients and cardiac effects of COVID-19 will likely be not only very popular, but also useful in clinical endeavors. The workshop on how to navigate the adult issues in hospitalized patients will provide the Med-Peds pearls we all wish we heard earlier.”
Although a 75-minute workshop session may seem long, “the workshop choices will offer something for everyone’s taste: education, research, clinical topics, diversity, and advocacy,” Dr. Giordano said. “I suggest that attendees check in advance which sessions will be available after the meeting, so that they prioritize highly interactive sessions like workshops, and that they experience, even if virtual, small group/room gatherings and networking.” There will be time for fun, too, she emphasized, with social sessions “that we hope will break the screen monotony and bring smiles to everyone’s faces.”
For younger clinicians relatively new to practice, Dr. Giordano recommended several workshops for a wealth of advice and guidance, including “New Kids on the Block: Thriving in your First Faculty Position,” “Channeling Your Inner Coach: Techniques to Enhance Clinical Teaching & Feedback,” “Palliative Care Pearls for the Pediatric Hospitalist,” “Perioperative Medicine for Medically Complex Children: Case Studies in Programmatic Approaches,” “The Bare Necessities: Social Determinant of Health Screening for the Hospitalist,” and “Mentorship, Autonomy, and Supervising a PHM Fellow.”
Classic topics and new concepts
“We are so excited to be able to offer a full spectrum of offerings at this year’s virtual meeting,” Yemisi Jones, MD, FHM, of Cincinnati Children’s Hospital, said in an interview. “We are covering some classic topics that we can’t do without at PHM, such as clinical updates in the management of sick and well newborns; workshops on best practices for educators; as well as the latest in PHM scholarship.” Sessions include “timely topics such as equity for women in medicine with one of our plenary speakers, Julie Silver, MD, and new febrile infant guidelines,” she added.
In particular, the COVID-19 and mental health session will help address clinicians’ evolving understanding of the COVID-19 pandemic and its effects on hospitalized children, said Dr. Jones. “Attendees can expect practical, timely updates on the current state of the science and ways to improve their practice to provide the best care for our patients.”
Attendees will be able to maximize the virtual conference format by accessing archived recordings, including clinical quick hits, mini-plenaries, and PHM Stories, which can be viewed during the scheduled meeting time or after, Dr. Jones said. “Workshops and abstract presentations will involve real-time interaction with presenters, so would be highest yield to attend during the live meeting. We also encourage all participants to take full advantage of the platform and the various networking opportunities to engage with others in our PHM community.”
For residents and new fellows, Dr. Jones advised making the workshop, “A Whole New World: Tips and Tools to Soar Into Your First Year of Fellowship,” a priority. “For early-career faculty, the ‘New Kids on the Block: Thriving in your First Faculty Position workshop will be a valuable resource.”
Make the meeting content a priority
This year’s conference has an exceptional slate of plenary speakers, Michelle Marks, DO, SFHM, of the Cleveland Clinic said in an interview. In addition to the much-anticipated session on vaccinations, school guidelines, and other topics with Dr. Fauci and Dr. Beers, the sessions on leading through adversity and workforce diversity and inclusion are “important topics to the PHM community and to our greater communities as a whole.”
Dr. Marks also highlighted the value of the COVID-19 and mental health session, as the long-term impact of COVID-19 on mental health of children and adults continues to grab headlines. “From this session specifically, I hope the attendees will gain awareness of the special mental health needs for child during a global disaster like a pandemic, which can be generalized to other situations and gain skills and resources to help meet and advocate for children’s mental health needs.”
For clinicians attending the virtual conference, “The most important strategy is to schedule time off of clinical work for the virtual meeting if you can so you can focus on the content,” said Dr. Marks. “For the longer sessions, it would be very important to block time in your day to fully attend the session, attend in a private space if possible since there will be breakouts with discussion, have your camera on, and engage with the workshop group as much as possible. The virtual format can be challenging because of all the external distractions, so intentional focus is necessary,” to get the most out of the experience.
The mini-plenary session on “The New AAP Clinical Practice Guideline on the Evaluation and Management of Febrile Infants 8-60 Days Old,” is an important session for all attendees, Dr. Marks said. She also recommended the Clinical Quick Hits sessions for anyone seeking “a diverse array of practical knowledge which can be easily applied to everyday practice.” The Clinical Quick Hits are designed as 35-minute, rapid-fire presentations focused on clinical knowledge. Each of these presentations will focus on the latest updates or evolutions in clinical practice in one area. Some key topics include counseling parents when a child has an abnormal exam finding, assessing pelvic pain in adolescent girls, and preventing venous thromboembolism in the inpatient setting.
“I would also recommend that younger clinicians take in at least one or two workshops or sessions on nonclinical topics to see the breath of content at the meeting and to develop a niche interest for themselves outside of clinical work,” Dr. Marks noted.
Nonclinical sessions at PHM 2021 include workshops on a pilot for a comprehensive LGBTQ+ curriculum, using media tools for public health messaging, and practicing health literacy.
To register for the Pediatric Hospital Medicine 2021 virtual conference, visit https://apaevents.regfox.com/phm21-virtual-conference.
Dr. Giordano, Dr. Jones, and Dr. Marks are members of the PHM conference planning committee and had no relevant financial conflicts to disclose.
The Pediatric Hospital Medicine annual conference, though virtual in 2021, promises to retain its role as the premier educational event for pediatric hospitalists and other clinicians involved in treating pediatric patients.
The “can’t-miss” session, on August 5, at 6:30 p.m. ET, is a one-on-one discussion between Anthony S. Fauci, MD, and Lee Savio Beers, MD, president of the American Academic of Pediatrics, according to members of the meeting planning committee.
In addition to the conversation between Dr. Beers and Dr. Fauci, this year’s meeting offers a mix of workshops with pointers and pearls to improve practice, keynote and plenary sessions to inform and inspire, and abstract presentations of new research. Three members of the PHM Planning Committee shared their insights on the hot topics, advice for new clinicians, and tips for making the most of this year’s meeting.
Workshops worth watching
“The keynote plenary sessions by Julie Silver, MD, on ‘Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion,’ and by Ilan Alhadeff, MD, on ‘Leading through Adversity’ should inspire even the least enthusiastic among us,” Mirna Giordano, MD, FHM, of Columbia University Medical Center, New York, said in an interview. A talk by Nathan T. Chomilo, MD, “will likely prompt reflection on how George Floyd’s death changed us, and how we practice medicine forever.” In addition, “PHM Stories are not to be missed, they are voices that speak loud and move mountains.”
The PHM Stories are concise, narrative talks with minimal use of slides; each PHM Stories session includes three distinct talks and a 15-minute question and answer session. PHM Stories sessions are scheduled for each day of the conference, and topics include “Practicing Medicine While Human: The Secrets Physicians Keep,” by Uchenna Ewulonu, MD; “Finding the Power of the Imposter: How I Learned to Be Exactly the Color I Am, Everywhere I Go,” by Alexandra Coria, MD; and “Purple Butterflies: A Reflection on Why I’m a Pediatric Hospitalist,” by Joanne Mendoza, MD.
“The PHM community has been through a lot in the aftermath of the pandemic,” said Dr. Giordano. “The mini-plenary session on the mental health needs of our patients, and clinical quick-hit sessions on verbal deescalation of the agitated patients and cardiac effects of COVID-19 will likely be not only very popular, but also useful in clinical endeavors. The workshop on how to navigate the adult issues in hospitalized patients will provide the Med-Peds pearls we all wish we heard earlier.”
Although a 75-minute workshop session may seem long, “the workshop choices will offer something for everyone’s taste: education, research, clinical topics, diversity, and advocacy,” Dr. Giordano said. “I suggest that attendees check in advance which sessions will be available after the meeting, so that they prioritize highly interactive sessions like workshops, and that they experience, even if virtual, small group/room gatherings and networking.” There will be time for fun, too, she emphasized, with social sessions “that we hope will break the screen monotony and bring smiles to everyone’s faces.”
For younger clinicians relatively new to practice, Dr. Giordano recommended several workshops for a wealth of advice and guidance, including “New Kids on the Block: Thriving in your First Faculty Position,” “Channeling Your Inner Coach: Techniques to Enhance Clinical Teaching & Feedback,” “Palliative Care Pearls for the Pediatric Hospitalist,” “Perioperative Medicine for Medically Complex Children: Case Studies in Programmatic Approaches,” “The Bare Necessities: Social Determinant of Health Screening for the Hospitalist,” and “Mentorship, Autonomy, and Supervising a PHM Fellow.”
Classic topics and new concepts
“We are so excited to be able to offer a full spectrum of offerings at this year’s virtual meeting,” Yemisi Jones, MD, FHM, of Cincinnati Children’s Hospital, said in an interview. “We are covering some classic topics that we can’t do without at PHM, such as clinical updates in the management of sick and well newborns; workshops on best practices for educators; as well as the latest in PHM scholarship.” Sessions include “timely topics such as equity for women in medicine with one of our plenary speakers, Julie Silver, MD, and new febrile infant guidelines,” she added.
In particular, the COVID-19 and mental health session will help address clinicians’ evolving understanding of the COVID-19 pandemic and its effects on hospitalized children, said Dr. Jones. “Attendees can expect practical, timely updates on the current state of the science and ways to improve their practice to provide the best care for our patients.”
Attendees will be able to maximize the virtual conference format by accessing archived recordings, including clinical quick hits, mini-plenaries, and PHM Stories, which can be viewed during the scheduled meeting time or after, Dr. Jones said. “Workshops and abstract presentations will involve real-time interaction with presenters, so would be highest yield to attend during the live meeting. We also encourage all participants to take full advantage of the platform and the various networking opportunities to engage with others in our PHM community.”
For residents and new fellows, Dr. Jones advised making the workshop, “A Whole New World: Tips and Tools to Soar Into Your First Year of Fellowship,” a priority. “For early-career faculty, the ‘New Kids on the Block: Thriving in your First Faculty Position workshop will be a valuable resource.”
Make the meeting content a priority
This year’s conference has an exceptional slate of plenary speakers, Michelle Marks, DO, SFHM, of the Cleveland Clinic said in an interview. In addition to the much-anticipated session on vaccinations, school guidelines, and other topics with Dr. Fauci and Dr. Beers, the sessions on leading through adversity and workforce diversity and inclusion are “important topics to the PHM community and to our greater communities as a whole.”
Dr. Marks also highlighted the value of the COVID-19 and mental health session, as the long-term impact of COVID-19 on mental health of children and adults continues to grab headlines. “From this session specifically, I hope the attendees will gain awareness of the special mental health needs for child during a global disaster like a pandemic, which can be generalized to other situations and gain skills and resources to help meet and advocate for children’s mental health needs.”
For clinicians attending the virtual conference, “The most important strategy is to schedule time off of clinical work for the virtual meeting if you can so you can focus on the content,” said Dr. Marks. “For the longer sessions, it would be very important to block time in your day to fully attend the session, attend in a private space if possible since there will be breakouts with discussion, have your camera on, and engage with the workshop group as much as possible. The virtual format can be challenging because of all the external distractions, so intentional focus is necessary,” to get the most out of the experience.
The mini-plenary session on “The New AAP Clinical Practice Guideline on the Evaluation and Management of Febrile Infants 8-60 Days Old,” is an important session for all attendees, Dr. Marks said. She also recommended the Clinical Quick Hits sessions for anyone seeking “a diverse array of practical knowledge which can be easily applied to everyday practice.” The Clinical Quick Hits are designed as 35-minute, rapid-fire presentations focused on clinical knowledge. Each of these presentations will focus on the latest updates or evolutions in clinical practice in one area. Some key topics include counseling parents when a child has an abnormal exam finding, assessing pelvic pain in adolescent girls, and preventing venous thromboembolism in the inpatient setting.
“I would also recommend that younger clinicians take in at least one or two workshops or sessions on nonclinical topics to see the breath of content at the meeting and to develop a niche interest for themselves outside of clinical work,” Dr. Marks noted.
Nonclinical sessions at PHM 2021 include workshops on a pilot for a comprehensive LGBTQ+ curriculum, using media tools for public health messaging, and practicing health literacy.
To register for the Pediatric Hospital Medicine 2021 virtual conference, visit https://apaevents.regfox.com/phm21-virtual-conference.
Dr. Giordano, Dr. Jones, and Dr. Marks are members of the PHM conference planning committee and had no relevant financial conflicts to disclose.
Advice on biopsies, workups, and referrals
Over the next 2 months we will dedicate this column to some general tips and pearls from the perspective of a gynecologic oncologist to guide general obstetrician gynecologists in the workup and management of preinvasive or invasive gynecologic diseases. The goal of these recommendations is to minimize misdiagnosis or delayed diagnosis and avoid unnecessary or untimely referrals.
Perform biopsy, not Pap smears, on visible cervical and vaginal lesions
The purpose of the Pap smear is to screen asymptomatic patients for cervical dysplasia or microscopic invasive disease. Cytology is an unreliable diagnostic tool for visible, symptomatic lesions in large part because of sampling errors, and the lack of architectural information in cytologic versus histopathologic specimens. Invasive lesions can be mischaracterized as preinvasive on a Pap smear. This can result in delayed diagnosis and unnecessary diagnostic procedures. For example, if a visible, abnormal-appearing, cervical lesion is seen during a routine visit and a Pap smear is performed (rather than a biopsy of the mass), the patient may receive an incorrect preliminary diagnosis of “high-grade dysplasia, carcinoma in situ” as it can be difficult to distinguish invasive carcinoma from carcinoma in situ on cytology. If the patient and provider do not understand the limitations of Pap smears in diagnosing invasive cancers, they may be falsely reassured and possibly delay or abstain from follow-up for an excisional procedure. If she does return for the loop electrosurgical excision procedure (LEEP), there might still be unnecessary delays in making referrals and definitive treatment while waiting for results. Radical hysterectomy may not promptly follow because, if performed within 6 weeks of an excisional procedure, it is associated with a significantly higher risk for perioperative complication, and therefore, if the excisional procedure was unnecessary to begin with, there may be additional time lost that need not be.1
Some clinicians avoid biopsy of visible lesions because they are concerned about bleeding complications that might arise in the office. Straightforward strategies to control bleeding are readily available in most gynecology offices, especially those already equipped for procedures such as LEEP and colposcopy. Prior to performing the biopsy, clinicians should ensure that they have supplies such as gauze sponges and ring forceps or packing forceps, silver nitrate, and ferric subsulfate solution (“Monsel’s solution”) close at hand. In the vast majority of cases, direct pressure for 5 minutes with gauze sponges and ferric subsulfate is highly effective at resolving most bleeding from a cervical or vaginal biopsy site. If this does not bring hemostasis, cautery devices or suture can be employed. If all else fails, be prepared to place vaginal packing (always with the insertion of a urinary Foley catheter to prevent urinary retention). In my experience, this is rarely needed.
Wherever possible, visible cervical or vaginal (or vulvar, see below) lesions should be biopsied for histopathology, sampling representative areas of the most concerning portion, in order to minimize misdiagnosis and expedite referral and definitive treatment. For necrotic-appearing lesions I recommend taking multiple samples of the tumor, as necrotic, nonviable tissue can prevent accurate diagnosis of a cancer. In general, Pap smears should be reserved as screening tests for asymptomatic women without visible pathology.
Don’t treat or refer low-grade dysplasia, even if persistent
Increasingly we are understanding that low-grade dysplasia of the lower genital tract (CIN I, VAIN I, VIN I) is less a precursor for cancer, and more a phenomenon of benign HPV-associated changes.2 This HPV change may be chronically persistent, may require years of observation and serial Pap smears, and may be a general nuisance for the patient. However, current guidelines do not recommend intervention for low-grade dysplasia of the lower genital tract.2 Interventions to resect these lesions can result in morbidity, including perineal pain, vaginal scarring, and cervical stenosis or insufficiency. Given the extremely low risk for progression to cancer, these morbidities do not outweigh any small potential benefit.
When I am conferring with patients who have chronic low-grade dysplasia I spend a great deal of time exploring their understanding of the diagnosis and its pathophysiology, their fears, and their expectation regarding “success” of treatment. I spend the time educating them that this is a sequela of chronic viral infection that will not be eradicated with local surgical excisions, that their cancer risk and need for surveillance would persist even if surgical intervention were offered, and that the side effects of treatment would outweigh any benefit from the small risk of cancer or high-grade dysplasia.
In summary, the treatment of choice for persistent low-grade dysplasia of the lower genital tract is comprehensive patient education, not surgical resection or referral to gynecologic oncology.
Repeat sampling if there’s a discordance between imaging and biopsy results
Delay in cancer diagnosis is one of the greatest concerns for front-line gynecology providers. One of the more modifiable strategies to avoid missed or delayed diagnosis is to ensure that there is concordance between clinical findings and testing results. Otherwise said: The results and findings should make sense in aggregate. An example was cited above in which a visible cervical mass demonstrated CIN III on cytologic testing. Another common example is a biopsy result of “scant benign endometrium” in a patient with postmenopausal bleeding and thickened endometrial stripe on ultrasound. In both of these cases there is clear discordance between physical findings and the results of pathology sampling. A pathology report, in all of its black and white certitude, seems like the most reliable source of information. However, always trust your clinical judgment. If the clinical picture is suggesting something far worse than these limited, often random or blind samplings, I recommend repeated or more extensive sampling (for example, dilation and curettage). At the very least, schedule close follow-up with repeated sampling if the symptom or finding persists. The emphasis here is on scheduled follow-up, rather than “p.r.n.,” because a patient who was given a “normal” pathology result to explain her abnormal symptoms may not volunteer that those symptoms are persistent as she may feel that anything sinister was already ruled out. Make certain that you explain the potential for misdiagnosis as the reason for why you would like to see her back shortly to ensure the issue has resolved.
Biopsy vulvar lesions, minimize empiric treatment
Vulvar cancer is notoriously associated with delayed diagnosis. Unfortunately, it is commonplace for gynecologic oncologists to see women who have vulvar cancers that have been empirically treated, sometimes for months or years, with steroids or other topical agents. If a lesion on the vulva is characteristically benign in appearance (such as condyloma or lichen sclerosis), it may be reasonable to start empiric treatment. However, all patients who are treated without biopsy should be rescheduled for a planned follow-up appointment in 2-3 months. If the lesion/area remains unchanged, or worse, the lesion should be biopsied before proceeding with a change in therapy or continued therapy. Once again, don’t rely on patients to return for evaluation if the lesion doesn’t improve. Many patients assume that our first empiric diagnosis is “gospel,” and therefore may not return if the treatment doesn’t work. Meanwhile, providers may assume that patients will know that there is uncertainty in our interpretation and that they will know to report if the initial treatment didn’t work. These assumptions are the recipe for delayed diagnosis. If there is too great a burden on the patient to schedule a return visit because of social or financial reasons then the patient should have a biopsy prior to initiation of treatment. As a rule, empiric treatment is not a good strategy for patients without good access to follow-up.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Sullivan S. et al Gynecol Oncol. 2017 Feb;144(2):294-8.
2. Perkins R .et al J Low Genit Tract Dis. 2020 Apr;24(2):102-31.
Over the next 2 months we will dedicate this column to some general tips and pearls from the perspective of a gynecologic oncologist to guide general obstetrician gynecologists in the workup and management of preinvasive or invasive gynecologic diseases. The goal of these recommendations is to minimize misdiagnosis or delayed diagnosis and avoid unnecessary or untimely referrals.
Perform biopsy, not Pap smears, on visible cervical and vaginal lesions
The purpose of the Pap smear is to screen asymptomatic patients for cervical dysplasia or microscopic invasive disease. Cytology is an unreliable diagnostic tool for visible, symptomatic lesions in large part because of sampling errors, and the lack of architectural information in cytologic versus histopathologic specimens. Invasive lesions can be mischaracterized as preinvasive on a Pap smear. This can result in delayed diagnosis and unnecessary diagnostic procedures. For example, if a visible, abnormal-appearing, cervical lesion is seen during a routine visit and a Pap smear is performed (rather than a biopsy of the mass), the patient may receive an incorrect preliminary diagnosis of “high-grade dysplasia, carcinoma in situ” as it can be difficult to distinguish invasive carcinoma from carcinoma in situ on cytology. If the patient and provider do not understand the limitations of Pap smears in diagnosing invasive cancers, they may be falsely reassured and possibly delay or abstain from follow-up for an excisional procedure. If she does return for the loop electrosurgical excision procedure (LEEP), there might still be unnecessary delays in making referrals and definitive treatment while waiting for results. Radical hysterectomy may not promptly follow because, if performed within 6 weeks of an excisional procedure, it is associated with a significantly higher risk for perioperative complication, and therefore, if the excisional procedure was unnecessary to begin with, there may be additional time lost that need not be.1
Some clinicians avoid biopsy of visible lesions because they are concerned about bleeding complications that might arise in the office. Straightforward strategies to control bleeding are readily available in most gynecology offices, especially those already equipped for procedures such as LEEP and colposcopy. Prior to performing the biopsy, clinicians should ensure that they have supplies such as gauze sponges and ring forceps or packing forceps, silver nitrate, and ferric subsulfate solution (“Monsel’s solution”) close at hand. In the vast majority of cases, direct pressure for 5 minutes with gauze sponges and ferric subsulfate is highly effective at resolving most bleeding from a cervical or vaginal biopsy site. If this does not bring hemostasis, cautery devices or suture can be employed. If all else fails, be prepared to place vaginal packing (always with the insertion of a urinary Foley catheter to prevent urinary retention). In my experience, this is rarely needed.
Wherever possible, visible cervical or vaginal (or vulvar, see below) lesions should be biopsied for histopathology, sampling representative areas of the most concerning portion, in order to minimize misdiagnosis and expedite referral and definitive treatment. For necrotic-appearing lesions I recommend taking multiple samples of the tumor, as necrotic, nonviable tissue can prevent accurate diagnosis of a cancer. In general, Pap smears should be reserved as screening tests for asymptomatic women without visible pathology.
Don’t treat or refer low-grade dysplasia, even if persistent
Increasingly we are understanding that low-grade dysplasia of the lower genital tract (CIN I, VAIN I, VIN I) is less a precursor for cancer, and more a phenomenon of benign HPV-associated changes.2 This HPV change may be chronically persistent, may require years of observation and serial Pap smears, and may be a general nuisance for the patient. However, current guidelines do not recommend intervention for low-grade dysplasia of the lower genital tract.2 Interventions to resect these lesions can result in morbidity, including perineal pain, vaginal scarring, and cervical stenosis or insufficiency. Given the extremely low risk for progression to cancer, these morbidities do not outweigh any small potential benefit.
When I am conferring with patients who have chronic low-grade dysplasia I spend a great deal of time exploring their understanding of the diagnosis and its pathophysiology, their fears, and their expectation regarding “success” of treatment. I spend the time educating them that this is a sequela of chronic viral infection that will not be eradicated with local surgical excisions, that their cancer risk and need for surveillance would persist even if surgical intervention were offered, and that the side effects of treatment would outweigh any benefit from the small risk of cancer or high-grade dysplasia.
In summary, the treatment of choice for persistent low-grade dysplasia of the lower genital tract is comprehensive patient education, not surgical resection or referral to gynecologic oncology.
Repeat sampling if there’s a discordance between imaging and biopsy results
Delay in cancer diagnosis is one of the greatest concerns for front-line gynecology providers. One of the more modifiable strategies to avoid missed or delayed diagnosis is to ensure that there is concordance between clinical findings and testing results. Otherwise said: The results and findings should make sense in aggregate. An example was cited above in which a visible cervical mass demonstrated CIN III on cytologic testing. Another common example is a biopsy result of “scant benign endometrium” in a patient with postmenopausal bleeding and thickened endometrial stripe on ultrasound. In both of these cases there is clear discordance between physical findings and the results of pathology sampling. A pathology report, in all of its black and white certitude, seems like the most reliable source of information. However, always trust your clinical judgment. If the clinical picture is suggesting something far worse than these limited, often random or blind samplings, I recommend repeated or more extensive sampling (for example, dilation and curettage). At the very least, schedule close follow-up with repeated sampling if the symptom or finding persists. The emphasis here is on scheduled follow-up, rather than “p.r.n.,” because a patient who was given a “normal” pathology result to explain her abnormal symptoms may not volunteer that those symptoms are persistent as she may feel that anything sinister was already ruled out. Make certain that you explain the potential for misdiagnosis as the reason for why you would like to see her back shortly to ensure the issue has resolved.
Biopsy vulvar lesions, minimize empiric treatment
Vulvar cancer is notoriously associated with delayed diagnosis. Unfortunately, it is commonplace for gynecologic oncologists to see women who have vulvar cancers that have been empirically treated, sometimes for months or years, with steroids or other topical agents. If a lesion on the vulva is characteristically benign in appearance (such as condyloma or lichen sclerosis), it may be reasonable to start empiric treatment. However, all patients who are treated without biopsy should be rescheduled for a planned follow-up appointment in 2-3 months. If the lesion/area remains unchanged, or worse, the lesion should be biopsied before proceeding with a change in therapy or continued therapy. Once again, don’t rely on patients to return for evaluation if the lesion doesn’t improve. Many patients assume that our first empiric diagnosis is “gospel,” and therefore may not return if the treatment doesn’t work. Meanwhile, providers may assume that patients will know that there is uncertainty in our interpretation and that they will know to report if the initial treatment didn’t work. These assumptions are the recipe for delayed diagnosis. If there is too great a burden on the patient to schedule a return visit because of social or financial reasons then the patient should have a biopsy prior to initiation of treatment. As a rule, empiric treatment is not a good strategy for patients without good access to follow-up.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Sullivan S. et al Gynecol Oncol. 2017 Feb;144(2):294-8.
2. Perkins R .et al J Low Genit Tract Dis. 2020 Apr;24(2):102-31.
Over the next 2 months we will dedicate this column to some general tips and pearls from the perspective of a gynecologic oncologist to guide general obstetrician gynecologists in the workup and management of preinvasive or invasive gynecologic diseases. The goal of these recommendations is to minimize misdiagnosis or delayed diagnosis and avoid unnecessary or untimely referrals.
Perform biopsy, not Pap smears, on visible cervical and vaginal lesions
The purpose of the Pap smear is to screen asymptomatic patients for cervical dysplasia or microscopic invasive disease. Cytology is an unreliable diagnostic tool for visible, symptomatic lesions in large part because of sampling errors, and the lack of architectural information in cytologic versus histopathologic specimens. Invasive lesions can be mischaracterized as preinvasive on a Pap smear. This can result in delayed diagnosis and unnecessary diagnostic procedures. For example, if a visible, abnormal-appearing, cervical lesion is seen during a routine visit and a Pap smear is performed (rather than a biopsy of the mass), the patient may receive an incorrect preliminary diagnosis of “high-grade dysplasia, carcinoma in situ” as it can be difficult to distinguish invasive carcinoma from carcinoma in situ on cytology. If the patient and provider do not understand the limitations of Pap smears in diagnosing invasive cancers, they may be falsely reassured and possibly delay or abstain from follow-up for an excisional procedure. If she does return for the loop electrosurgical excision procedure (LEEP), there might still be unnecessary delays in making referrals and definitive treatment while waiting for results. Radical hysterectomy may not promptly follow because, if performed within 6 weeks of an excisional procedure, it is associated with a significantly higher risk for perioperative complication, and therefore, if the excisional procedure was unnecessary to begin with, there may be additional time lost that need not be.1
Some clinicians avoid biopsy of visible lesions because they are concerned about bleeding complications that might arise in the office. Straightforward strategies to control bleeding are readily available in most gynecology offices, especially those already equipped for procedures such as LEEP and colposcopy. Prior to performing the biopsy, clinicians should ensure that they have supplies such as gauze sponges and ring forceps or packing forceps, silver nitrate, and ferric subsulfate solution (“Monsel’s solution”) close at hand. In the vast majority of cases, direct pressure for 5 minutes with gauze sponges and ferric subsulfate is highly effective at resolving most bleeding from a cervical or vaginal biopsy site. If this does not bring hemostasis, cautery devices or suture can be employed. If all else fails, be prepared to place vaginal packing (always with the insertion of a urinary Foley catheter to prevent urinary retention). In my experience, this is rarely needed.
Wherever possible, visible cervical or vaginal (or vulvar, see below) lesions should be biopsied for histopathology, sampling representative areas of the most concerning portion, in order to minimize misdiagnosis and expedite referral and definitive treatment. For necrotic-appearing lesions I recommend taking multiple samples of the tumor, as necrotic, nonviable tissue can prevent accurate diagnosis of a cancer. In general, Pap smears should be reserved as screening tests for asymptomatic women without visible pathology.
Don’t treat or refer low-grade dysplasia, even if persistent
Increasingly we are understanding that low-grade dysplasia of the lower genital tract (CIN I, VAIN I, VIN I) is less a precursor for cancer, and more a phenomenon of benign HPV-associated changes.2 This HPV change may be chronically persistent, may require years of observation and serial Pap smears, and may be a general nuisance for the patient. However, current guidelines do not recommend intervention for low-grade dysplasia of the lower genital tract.2 Interventions to resect these lesions can result in morbidity, including perineal pain, vaginal scarring, and cervical stenosis or insufficiency. Given the extremely low risk for progression to cancer, these morbidities do not outweigh any small potential benefit.
When I am conferring with patients who have chronic low-grade dysplasia I spend a great deal of time exploring their understanding of the diagnosis and its pathophysiology, their fears, and their expectation regarding “success” of treatment. I spend the time educating them that this is a sequela of chronic viral infection that will not be eradicated with local surgical excisions, that their cancer risk and need for surveillance would persist even if surgical intervention were offered, and that the side effects of treatment would outweigh any benefit from the small risk of cancer or high-grade dysplasia.
In summary, the treatment of choice for persistent low-grade dysplasia of the lower genital tract is comprehensive patient education, not surgical resection or referral to gynecologic oncology.
Repeat sampling if there’s a discordance between imaging and biopsy results
Delay in cancer diagnosis is one of the greatest concerns for front-line gynecology providers. One of the more modifiable strategies to avoid missed or delayed diagnosis is to ensure that there is concordance between clinical findings and testing results. Otherwise said: The results and findings should make sense in aggregate. An example was cited above in which a visible cervical mass demonstrated CIN III on cytologic testing. Another common example is a biopsy result of “scant benign endometrium” in a patient with postmenopausal bleeding and thickened endometrial stripe on ultrasound. In both of these cases there is clear discordance between physical findings and the results of pathology sampling. A pathology report, in all of its black and white certitude, seems like the most reliable source of information. However, always trust your clinical judgment. If the clinical picture is suggesting something far worse than these limited, often random or blind samplings, I recommend repeated or more extensive sampling (for example, dilation and curettage). At the very least, schedule close follow-up with repeated sampling if the symptom or finding persists. The emphasis here is on scheduled follow-up, rather than “p.r.n.,” because a patient who was given a “normal” pathology result to explain her abnormal symptoms may not volunteer that those symptoms are persistent as she may feel that anything sinister was already ruled out. Make certain that you explain the potential for misdiagnosis as the reason for why you would like to see her back shortly to ensure the issue has resolved.
Biopsy vulvar lesions, minimize empiric treatment
Vulvar cancer is notoriously associated with delayed diagnosis. Unfortunately, it is commonplace for gynecologic oncologists to see women who have vulvar cancers that have been empirically treated, sometimes for months or years, with steroids or other topical agents. If a lesion on the vulva is characteristically benign in appearance (such as condyloma or lichen sclerosis), it may be reasonable to start empiric treatment. However, all patients who are treated without biopsy should be rescheduled for a planned follow-up appointment in 2-3 months. If the lesion/area remains unchanged, or worse, the lesion should be biopsied before proceeding with a change in therapy or continued therapy. Once again, don’t rely on patients to return for evaluation if the lesion doesn’t improve. Many patients assume that our first empiric diagnosis is “gospel,” and therefore may not return if the treatment doesn’t work. Meanwhile, providers may assume that patients will know that there is uncertainty in our interpretation and that they will know to report if the initial treatment didn’t work. These assumptions are the recipe for delayed diagnosis. If there is too great a burden on the patient to schedule a return visit because of social or financial reasons then the patient should have a biopsy prior to initiation of treatment. As a rule, empiric treatment is not a good strategy for patients without good access to follow-up.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Sullivan S. et al Gynecol Oncol. 2017 Feb;144(2):294-8.
2. Perkins R .et al J Low Genit Tract Dis. 2020 Apr;24(2):102-31.
Combination atezolizumab and bevacizumab succeeds in real-world setting
Key clinical point: In the real-world setting, patients with unresectable HCC who did not meet clinical trial criteria had similar responses to the treatment combination as those who met clinical trial criteria.
Major finding: The objective response rates and disease control rates of 5.2% and 82.8% at 6 weeks and 10.0% and 84.0% at 12 weeks, respectively, were similar between patients who met and did not meet the IMbrave150 clinical trial criteria, as were safety profiles.
Study details: The data come from a multicenter study of 64 adults with unresectable hepatocellular carcinoma, including 46 who did not meet the study criteria. All patients were treated with a combination of atezolizumab plus bevacizumab. Treatment response and safety issues were assessed at 6 weeks and 12 weeks.
Disclosures: The study was supported by the Japan Agency for Medical Research and Development. The researchers had no financial conflicts to disclose.
Source: Sho T et al. Hepatol Res. 2021 Jul 10. doi: 10.1111/hepr.13693.
Key clinical point: In the real-world setting, patients with unresectable HCC who did not meet clinical trial criteria had similar responses to the treatment combination as those who met clinical trial criteria.
Major finding: The objective response rates and disease control rates of 5.2% and 82.8% at 6 weeks and 10.0% and 84.0% at 12 weeks, respectively, were similar between patients who met and did not meet the IMbrave150 clinical trial criteria, as were safety profiles.
Study details: The data come from a multicenter study of 64 adults with unresectable hepatocellular carcinoma, including 46 who did not meet the study criteria. All patients were treated with a combination of atezolizumab plus bevacizumab. Treatment response and safety issues were assessed at 6 weeks and 12 weeks.
Disclosures: The study was supported by the Japan Agency for Medical Research and Development. The researchers had no financial conflicts to disclose.
Source: Sho T et al. Hepatol Res. 2021 Jul 10. doi: 10.1111/hepr.13693.
Key clinical point: In the real-world setting, patients with unresectable HCC who did not meet clinical trial criteria had similar responses to the treatment combination as those who met clinical trial criteria.
Major finding: The objective response rates and disease control rates of 5.2% and 82.8% at 6 weeks and 10.0% and 84.0% at 12 weeks, respectively, were similar between patients who met and did not meet the IMbrave150 clinical trial criteria, as were safety profiles.
Study details: The data come from a multicenter study of 64 adults with unresectable hepatocellular carcinoma, including 46 who did not meet the study criteria. All patients were treated with a combination of atezolizumab plus bevacizumab. Treatment response and safety issues were assessed at 6 weeks and 12 weeks.
Disclosures: The study was supported by the Japan Agency for Medical Research and Development. The researchers had no financial conflicts to disclose.
Source: Sho T et al. Hepatol Res. 2021 Jul 10. doi: 10.1111/hepr.13693.
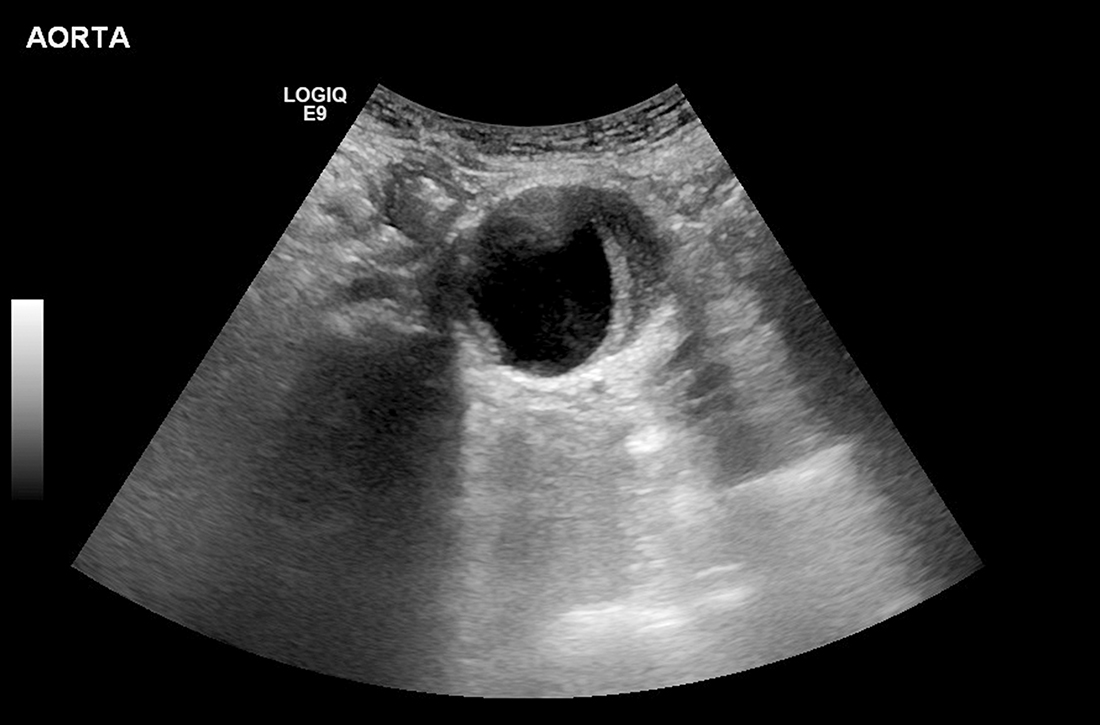
Can family physicians accurately screen for AAA with point-of-care ultrasound?
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
EVIDENCE-BASED ANSWER:
Likely yes. Point-of-care ultrasound (POCUS) screening for abdominal aortic aneurysm (AAA) by nonradiologist physicians is 98% sensitive and 99% specific, compared with imaging performed by radiologists (strength of recommendation [SOR]: B, meta-analysis of diagnostic accuracy studies mostly involving emergency medicine physicians). European family physicians demonstrated 100% concordance with radiologist readings (SOR: C, very small subsequent diagnostic accuracy studies).
‘Gold cards’ allow Texas docs to skip prior authorizations
In what could be a model for other states, Texas has become the first state to exempt physicians from prior authorizations for meeting insurer benchmarks.
The law was passed in June and will take effect in September. It excuses physicians from having to obtain prior authorization if, during the previous 6 months, 90% of their treatments met medical necessity criteria by the health insurer. Through this law, doctors in the state will spend less time getting approvals for treatments for their patients.
Automatic approval of authorizations for treatments – or what the Texas Medical Association calls a “gold card” – “allows patients to get the care they need in a more timely fashion,” says Debra Patt, MD, an Austin, Tex.–based oncologist and former chair of the council on legislation for the TMA.
About 87% of Texas physicians reported a “drastic increase over the past 5 years in the burden of prior authorization on their patients and their practices,” per a 2020 survey by the TMA. Nearly half (48%) of Texas physicians have hired staff whose work focuses on processing requests for prior authorization, according to the survey.
Jack Resneck Jr., MD, a San Francisco–based dermatologist and president-elect of the American Medical Association, said other states have investigated ways to ease the impact of prior authorizations on physicians, but no other state has passed such a law.
Administrative burdens plague physicians around the country. The Medscape Physician Compensation Report 2021 found that physicians spend on average 15.6 hours per week on paperwork and administrative duties.
Better outcomes, less anxiety for patients
Dr. Patt, who testified in support of the law’s passage in the Texas legislature, says automatic approval of authorizations “is better for patients because it reduces their anxiety about whether they’re able to get the treatments they need now, and they will have better outcomes if they’re able to receive more timely care.”
Recently, a chemotherapy treatment Dr. Patt prescribed for one of her patients was not authorized by an insurer. The result is “a lot of anxiety and potentially health problems” for the patient.
She expects that automatic approval for treatments will be based on prescribing patterns during the preceding 6 months. “It means that when I order a test today, the [health insurer] looks back at my record 6 months previously,” she said. Still, Dr. Patt awaits guidance from the Texas Department of Insurance, which regulates health insurers in the state, regarding the law.
Dr. Resneck said the pharmacy counter is where most patients encounter prior authorization delays. “That’s when the pharmacist looks at them and says: ‘Actually, this isn’t covered by your health insurer’s formulary,’ or it isn’t covered fully on their formulary.”
One of Dr. Resneck’s patients had a life-altering case of eczema that lasted many years. Because of the condition, the patient couldn’t work or maintain meaningful bonds with family members. A biologic treatment transformed his patient’s life. The patient was able to return to work and to reengage with family, said Dr. Resneck. But a year after his patient started the treatment, the health insurer wouldn’t authorize the treatment because the patient wasn’t experiencing the same symptoms.
The patient didn’t have the same symptoms because the biologic treatment worked, said Dr. Resneck.
Kristine Grow, a spokesperson for America’s Health Insurance Plans, a national association for health insurers, said: “The use of prior authorization is relatively small – typically, less than 15% – and can help ensure safer opioid prescribing, help prevent dangerous drug interactions, and help protect patients from unnecessary exposure to potentially harmful radiation for inappropriate diagnostic imaging. Numerous studies show that Americans frequently receive inappropriate care, and 25% of unnecessary treatments are associated with complications or adverse events.”
Medical management tools, such as prior authorization, are “an important way” to deliver “safe, high-quality care” to patients, she added.
Potential for harm?
Sadeea Abbasi, MD, a practicing physician at Cedars-Sinai in the gastroenterology clinical office in Santa Monica, Calif., can attest that these practices are harmful for her patients.
“Prior authorization requirements have been on the rise across various medical specialties. For GI, we have seen an increase of required approvals for procedures like upper endoscopy, colonoscopy, and wireless capsule endoscopy and in medications prescribed, including biologic infusions for inflammatory bowel disease.”
Dr. Abbasi added: “One of the largest concerns I have with this growing ‘cost-savings’ trend is the impact it has on clinical outcomes. I have seen patients suffer with symptoms while waiting for a decision on a prior authorization for a medication. My patients have endured confusion and chaos when arriving for imaging appointments, only to learn the insurance has not reached a decision on whether the study is approved. When patients learn their procedure has been delayed, they have to reschedule the appointment, take another day off work, coordinate transportation and most importantly, postpone subsequent treatments to alleviate symptoms.”
According to an AMA survey, almost all physicians (94%) said prior authorization delays care and 79% percent have had patients abandon their recommended treatment because of issues related to prior authorization. This delay causes potentially irreversible damage to patients’ digestive system and increases the likelihood of hospitalization. This is a huge issue for America’s seniors: Medicare Advantage (MA) plans, which represent 24.1 million of the 62 million Medicare beneficiaries, the increase in prior authorization requests has been substantial.
State and federal efforts to curb prior authorization
In addition to efforts to curb prior authorization in other states, the AMA and nearly 300 other stakeholders, including the American Gastroenterological Association, support the Improving Seniors’ Timely Access to Care Act (H.R. 3173). The legislation includes a provision related to “gold carding,” said Robert Mills, an AMA spokesperson.
The bill aims to establish transparency requirements and standards for prior authorization processes related to MA plans. The requirements and standards for MA plans include the following:
- Establishing an electronic prior authorization program that meets specific standards, such as the ability to provide real-time decisions in response to requests for items and services that are routinely approved.
- Publishing on an annual basis specific prior authorization information, including the percentage of requests approved and the average response time.
- Ensuring prior authorization requests are reviewed by qualified medical personnel.
- Meeting standards set by the Centers for Medicare & Medicaid Services related to the quality and timeliness of prior authorization determinations.
This legislation was introduced in the U.S. House of Representatives in May by representatives Suzan DelBene (D-Wash.); Mike Kelly (R-Pa.); Ami Bera, MD (D-Calif.); and Larry Bucshon (R-Ind.), after which it was referred to the House Committee on Energy and Commerce and the House Committee on Ways and Means for consideration.
Gaining support for this legislation is a priority for AGA and as such the legislation will be featured as a top policy request at AGA’s upcoming fall Advocacy Day on Sept. 23. The AGA encourages all physicians to contact their lawmakers, urging for support of the bill in the 117th Congress.
In addition to AGA’s advocacy efforts on prior authorization reform, the Regulatory Relief Coalition, a group of national physician specialty organizations, advocates for regulatory burden reduction in Medicare so that physicians can spend more time treating patients. The physician community has banded together to address prior authorization burdens in our field and improve delivery of patient care. Learn more about prior authorization burdens and the various advocacy efforts being pursued.
With additional reporting by staff from this news organization.
In what could be a model for other states, Texas has become the first state to exempt physicians from prior authorizations for meeting insurer benchmarks.
The law was passed in June and will take effect in September. It excuses physicians from having to obtain prior authorization if, during the previous 6 months, 90% of their treatments met medical necessity criteria by the health insurer. Through this law, doctors in the state will spend less time getting approvals for treatments for their patients.
Automatic approval of authorizations for treatments – or what the Texas Medical Association calls a “gold card” – “allows patients to get the care they need in a more timely fashion,” says Debra Patt, MD, an Austin, Tex.–based oncologist and former chair of the council on legislation for the TMA.
About 87% of Texas physicians reported a “drastic increase over the past 5 years in the burden of prior authorization on their patients and their practices,” per a 2020 survey by the TMA. Nearly half (48%) of Texas physicians have hired staff whose work focuses on processing requests for prior authorization, according to the survey.
Jack Resneck Jr., MD, a San Francisco–based dermatologist and president-elect of the American Medical Association, said other states have investigated ways to ease the impact of prior authorizations on physicians, but no other state has passed such a law.
Administrative burdens plague physicians around the country. The Medscape Physician Compensation Report 2021 found that physicians spend on average 15.6 hours per week on paperwork and administrative duties.
Better outcomes, less anxiety for patients
Dr. Patt, who testified in support of the law’s passage in the Texas legislature, says automatic approval of authorizations “is better for patients because it reduces their anxiety about whether they’re able to get the treatments they need now, and they will have better outcomes if they’re able to receive more timely care.”
Recently, a chemotherapy treatment Dr. Patt prescribed for one of her patients was not authorized by an insurer. The result is “a lot of anxiety and potentially health problems” for the patient.
She expects that automatic approval for treatments will be based on prescribing patterns during the preceding 6 months. “It means that when I order a test today, the [health insurer] looks back at my record 6 months previously,” she said. Still, Dr. Patt awaits guidance from the Texas Department of Insurance, which regulates health insurers in the state, regarding the law.
Dr. Resneck said the pharmacy counter is where most patients encounter prior authorization delays. “That’s when the pharmacist looks at them and says: ‘Actually, this isn’t covered by your health insurer’s formulary,’ or it isn’t covered fully on their formulary.”
One of Dr. Resneck’s patients had a life-altering case of eczema that lasted many years. Because of the condition, the patient couldn’t work or maintain meaningful bonds with family members. A biologic treatment transformed his patient’s life. The patient was able to return to work and to reengage with family, said Dr. Resneck. But a year after his patient started the treatment, the health insurer wouldn’t authorize the treatment because the patient wasn’t experiencing the same symptoms.
The patient didn’t have the same symptoms because the biologic treatment worked, said Dr. Resneck.
Kristine Grow, a spokesperson for America’s Health Insurance Plans, a national association for health insurers, said: “The use of prior authorization is relatively small – typically, less than 15% – and can help ensure safer opioid prescribing, help prevent dangerous drug interactions, and help protect patients from unnecessary exposure to potentially harmful radiation for inappropriate diagnostic imaging. Numerous studies show that Americans frequently receive inappropriate care, and 25% of unnecessary treatments are associated with complications or adverse events.”
Medical management tools, such as prior authorization, are “an important way” to deliver “safe, high-quality care” to patients, she added.
Potential for harm?
Sadeea Abbasi, MD, a practicing physician at Cedars-Sinai in the gastroenterology clinical office in Santa Monica, Calif., can attest that these practices are harmful for her patients.
“Prior authorization requirements have been on the rise across various medical specialties. For GI, we have seen an increase of required approvals for procedures like upper endoscopy, colonoscopy, and wireless capsule endoscopy and in medications prescribed, including biologic infusions for inflammatory bowel disease.”
Dr. Abbasi added: “One of the largest concerns I have with this growing ‘cost-savings’ trend is the impact it has on clinical outcomes. I have seen patients suffer with symptoms while waiting for a decision on a prior authorization for a medication. My patients have endured confusion and chaos when arriving for imaging appointments, only to learn the insurance has not reached a decision on whether the study is approved. When patients learn their procedure has been delayed, they have to reschedule the appointment, take another day off work, coordinate transportation and most importantly, postpone subsequent treatments to alleviate symptoms.”
According to an AMA survey, almost all physicians (94%) said prior authorization delays care and 79% percent have had patients abandon their recommended treatment because of issues related to prior authorization. This delay causes potentially irreversible damage to patients’ digestive system and increases the likelihood of hospitalization. This is a huge issue for America’s seniors: Medicare Advantage (MA) plans, which represent 24.1 million of the 62 million Medicare beneficiaries, the increase in prior authorization requests has been substantial.
State and federal efforts to curb prior authorization
In addition to efforts to curb prior authorization in other states, the AMA and nearly 300 other stakeholders, including the American Gastroenterological Association, support the Improving Seniors’ Timely Access to Care Act (H.R. 3173). The legislation includes a provision related to “gold carding,” said Robert Mills, an AMA spokesperson.
The bill aims to establish transparency requirements and standards for prior authorization processes related to MA plans. The requirements and standards for MA plans include the following:
- Establishing an electronic prior authorization program that meets specific standards, such as the ability to provide real-time decisions in response to requests for items and services that are routinely approved.
- Publishing on an annual basis specific prior authorization information, including the percentage of requests approved and the average response time.
- Ensuring prior authorization requests are reviewed by qualified medical personnel.
- Meeting standards set by the Centers for Medicare & Medicaid Services related to the quality and timeliness of prior authorization determinations.
This legislation was introduced in the U.S. House of Representatives in May by representatives Suzan DelBene (D-Wash.); Mike Kelly (R-Pa.); Ami Bera, MD (D-Calif.); and Larry Bucshon (R-Ind.), after which it was referred to the House Committee on Energy and Commerce and the House Committee on Ways and Means for consideration.
Gaining support for this legislation is a priority for AGA and as such the legislation will be featured as a top policy request at AGA’s upcoming fall Advocacy Day on Sept. 23. The AGA encourages all physicians to contact their lawmakers, urging for support of the bill in the 117th Congress.
In addition to AGA’s advocacy efforts on prior authorization reform, the Regulatory Relief Coalition, a group of national physician specialty organizations, advocates for regulatory burden reduction in Medicare so that physicians can spend more time treating patients. The physician community has banded together to address prior authorization burdens in our field and improve delivery of patient care. Learn more about prior authorization burdens and the various advocacy efforts being pursued.
With additional reporting by staff from this news organization.
In what could be a model for other states, Texas has become the first state to exempt physicians from prior authorizations for meeting insurer benchmarks.
The law was passed in June and will take effect in September. It excuses physicians from having to obtain prior authorization if, during the previous 6 months, 90% of their treatments met medical necessity criteria by the health insurer. Through this law, doctors in the state will spend less time getting approvals for treatments for their patients.
Automatic approval of authorizations for treatments – or what the Texas Medical Association calls a “gold card” – “allows patients to get the care they need in a more timely fashion,” says Debra Patt, MD, an Austin, Tex.–based oncologist and former chair of the council on legislation for the TMA.
About 87% of Texas physicians reported a “drastic increase over the past 5 years in the burden of prior authorization on their patients and their practices,” per a 2020 survey by the TMA. Nearly half (48%) of Texas physicians have hired staff whose work focuses on processing requests for prior authorization, according to the survey.
Jack Resneck Jr., MD, a San Francisco–based dermatologist and president-elect of the American Medical Association, said other states have investigated ways to ease the impact of prior authorizations on physicians, but no other state has passed such a law.
Administrative burdens plague physicians around the country. The Medscape Physician Compensation Report 2021 found that physicians spend on average 15.6 hours per week on paperwork and administrative duties.
Better outcomes, less anxiety for patients
Dr. Patt, who testified in support of the law’s passage in the Texas legislature, says automatic approval of authorizations “is better for patients because it reduces their anxiety about whether they’re able to get the treatments they need now, and they will have better outcomes if they’re able to receive more timely care.”
Recently, a chemotherapy treatment Dr. Patt prescribed for one of her patients was not authorized by an insurer. The result is “a lot of anxiety and potentially health problems” for the patient.
She expects that automatic approval for treatments will be based on prescribing patterns during the preceding 6 months. “It means that when I order a test today, the [health insurer] looks back at my record 6 months previously,” she said. Still, Dr. Patt awaits guidance from the Texas Department of Insurance, which regulates health insurers in the state, regarding the law.
Dr. Resneck said the pharmacy counter is where most patients encounter prior authorization delays. “That’s when the pharmacist looks at them and says: ‘Actually, this isn’t covered by your health insurer’s formulary,’ or it isn’t covered fully on their formulary.”
One of Dr. Resneck’s patients had a life-altering case of eczema that lasted many years. Because of the condition, the patient couldn’t work or maintain meaningful bonds with family members. A biologic treatment transformed his patient’s life. The patient was able to return to work and to reengage with family, said Dr. Resneck. But a year after his patient started the treatment, the health insurer wouldn’t authorize the treatment because the patient wasn’t experiencing the same symptoms.
The patient didn’t have the same symptoms because the biologic treatment worked, said Dr. Resneck.
Kristine Grow, a spokesperson for America’s Health Insurance Plans, a national association for health insurers, said: “The use of prior authorization is relatively small – typically, less than 15% – and can help ensure safer opioid prescribing, help prevent dangerous drug interactions, and help protect patients from unnecessary exposure to potentially harmful radiation for inappropriate diagnostic imaging. Numerous studies show that Americans frequently receive inappropriate care, and 25% of unnecessary treatments are associated with complications or adverse events.”
Medical management tools, such as prior authorization, are “an important way” to deliver “safe, high-quality care” to patients, she added.
Potential for harm?
Sadeea Abbasi, MD, a practicing physician at Cedars-Sinai in the gastroenterology clinical office in Santa Monica, Calif., can attest that these practices are harmful for her patients.
“Prior authorization requirements have been on the rise across various medical specialties. For GI, we have seen an increase of required approvals for procedures like upper endoscopy, colonoscopy, and wireless capsule endoscopy and in medications prescribed, including biologic infusions for inflammatory bowel disease.”
Dr. Abbasi added: “One of the largest concerns I have with this growing ‘cost-savings’ trend is the impact it has on clinical outcomes. I have seen patients suffer with symptoms while waiting for a decision on a prior authorization for a medication. My patients have endured confusion and chaos when arriving for imaging appointments, only to learn the insurance has not reached a decision on whether the study is approved. When patients learn their procedure has been delayed, they have to reschedule the appointment, take another day off work, coordinate transportation and most importantly, postpone subsequent treatments to alleviate symptoms.”
According to an AMA survey, almost all physicians (94%) said prior authorization delays care and 79% percent have had patients abandon their recommended treatment because of issues related to prior authorization. This delay causes potentially irreversible damage to patients’ digestive system and increases the likelihood of hospitalization. This is a huge issue for America’s seniors: Medicare Advantage (MA) plans, which represent 24.1 million of the 62 million Medicare beneficiaries, the increase in prior authorization requests has been substantial.
State and federal efforts to curb prior authorization
In addition to efforts to curb prior authorization in other states, the AMA and nearly 300 other stakeholders, including the American Gastroenterological Association, support the Improving Seniors’ Timely Access to Care Act (H.R. 3173). The legislation includes a provision related to “gold carding,” said Robert Mills, an AMA spokesperson.
The bill aims to establish transparency requirements and standards for prior authorization processes related to MA plans. The requirements and standards for MA plans include the following:
- Establishing an electronic prior authorization program that meets specific standards, such as the ability to provide real-time decisions in response to requests for items and services that are routinely approved.
- Publishing on an annual basis specific prior authorization information, including the percentage of requests approved and the average response time.
- Ensuring prior authorization requests are reviewed by qualified medical personnel.
- Meeting standards set by the Centers for Medicare & Medicaid Services related to the quality and timeliness of prior authorization determinations.
This legislation was introduced in the U.S. House of Representatives in May by representatives Suzan DelBene (D-Wash.); Mike Kelly (R-Pa.); Ami Bera, MD (D-Calif.); and Larry Bucshon (R-Ind.), after which it was referred to the House Committee on Energy and Commerce and the House Committee on Ways and Means for consideration.
Gaining support for this legislation is a priority for AGA and as such the legislation will be featured as a top policy request at AGA’s upcoming fall Advocacy Day on Sept. 23. The AGA encourages all physicians to contact their lawmakers, urging for support of the bill in the 117th Congress.
In addition to AGA’s advocacy efforts on prior authorization reform, the Regulatory Relief Coalition, a group of national physician specialty organizations, advocates for regulatory burden reduction in Medicare so that physicians can spend more time treating patients. The physician community has banded together to address prior authorization burdens in our field and improve delivery of patient care. Learn more about prior authorization burdens and the various advocacy efforts being pursued.
With additional reporting by staff from this news organization.
FDA okays extended-release exenatide for children with T2D
the agency announced July 22.
Previously approved in adults, the injectable is now the second glucagonlike peptide-1 receptor agonist approved for use in pediatric type 2 diabetes, after liraglutide (Victoza, Novo Nordisk) in 2019, and the first with once-weekly administration.
The two extended-release Bydureon products – which differ in delivery device and mixing procedure – are now indicated for use in addition to diet and exercise to improve glycemic control in pediatric patients 10 years of age or older with type 2 diabetes.
Exenatide extended release is not recommended as first-line treatment following diet and exercise.
The approval was based on a 24-week, double-blind, placebo-controlled study in 82 children with type 2 diabetes aged 10 and older. They were randomized to 2 mg once-weekly exenatide extended release or placebo. At week 24, hemoglobin A1c in those randomized to the drug had dropped by 0.25 percentage points, compared with a 0.45 percentage point increase in the placebo group.
Side effects were similar to those seen in adults, including injection site reactions, headaches, and gastrointestinal discomfort.
Currently, metformin is the only oral medication approved for treating pediatric type 2 diabetes, while the injectables also include insulin in addition to the two GLP-1 receptor agonists. During a symposium held in June 2021 at the annual scientific sessions of the American Diabetes Association, speakers expressed alarm about the rise in youth developing type 2 diabetes, noting that the condition typically progresses more rapidly and is less likely to respond well to metformin, compared with adults.
But, the panelists were also optimistic about extended-release exenatide as well as several other therapies for pediatric patients with type 2 diabetes in ongoing phase 3 trials, including the sodium-glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin, and the dipeptidyl peptidase–4 inhibitors alogliptin and linagliptin. Results are expected in the next 1-2 years.
the agency announced July 22.
Previously approved in adults, the injectable is now the second glucagonlike peptide-1 receptor agonist approved for use in pediatric type 2 diabetes, after liraglutide (Victoza, Novo Nordisk) in 2019, and the first with once-weekly administration.
The two extended-release Bydureon products – which differ in delivery device and mixing procedure – are now indicated for use in addition to diet and exercise to improve glycemic control in pediatric patients 10 years of age or older with type 2 diabetes.
Exenatide extended release is not recommended as first-line treatment following diet and exercise.
The approval was based on a 24-week, double-blind, placebo-controlled study in 82 children with type 2 diabetes aged 10 and older. They were randomized to 2 mg once-weekly exenatide extended release or placebo. At week 24, hemoglobin A1c in those randomized to the drug had dropped by 0.25 percentage points, compared with a 0.45 percentage point increase in the placebo group.
Side effects were similar to those seen in adults, including injection site reactions, headaches, and gastrointestinal discomfort.
Currently, metformin is the only oral medication approved for treating pediatric type 2 diabetes, while the injectables also include insulin in addition to the two GLP-1 receptor agonists. During a symposium held in June 2021 at the annual scientific sessions of the American Diabetes Association, speakers expressed alarm about the rise in youth developing type 2 diabetes, noting that the condition typically progresses more rapidly and is less likely to respond well to metformin, compared with adults.
But, the panelists were also optimistic about extended-release exenatide as well as several other therapies for pediatric patients with type 2 diabetes in ongoing phase 3 trials, including the sodium-glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin, and the dipeptidyl peptidase–4 inhibitors alogliptin and linagliptin. Results are expected in the next 1-2 years.
the agency announced July 22.
Previously approved in adults, the injectable is now the second glucagonlike peptide-1 receptor agonist approved for use in pediatric type 2 diabetes, after liraglutide (Victoza, Novo Nordisk) in 2019, and the first with once-weekly administration.
The two extended-release Bydureon products – which differ in delivery device and mixing procedure – are now indicated for use in addition to diet and exercise to improve glycemic control in pediatric patients 10 years of age or older with type 2 diabetes.
Exenatide extended release is not recommended as first-line treatment following diet and exercise.
The approval was based on a 24-week, double-blind, placebo-controlled study in 82 children with type 2 diabetes aged 10 and older. They were randomized to 2 mg once-weekly exenatide extended release or placebo. At week 24, hemoglobin A1c in those randomized to the drug had dropped by 0.25 percentage points, compared with a 0.45 percentage point increase in the placebo group.
Side effects were similar to those seen in adults, including injection site reactions, headaches, and gastrointestinal discomfort.
Currently, metformin is the only oral medication approved for treating pediatric type 2 diabetes, while the injectables also include insulin in addition to the two GLP-1 receptor agonists. During a symposium held in June 2021 at the annual scientific sessions of the American Diabetes Association, speakers expressed alarm about the rise in youth developing type 2 diabetes, noting that the condition typically progresses more rapidly and is less likely to respond well to metformin, compared with adults.
But, the panelists were also optimistic about extended-release exenatide as well as several other therapies for pediatric patients with type 2 diabetes in ongoing phase 3 trials, including the sodium-glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin, and the dipeptidyl peptidase–4 inhibitors alogliptin and linagliptin. Results are expected in the next 1-2 years.
Twofold increased risk for death from COVID-19 in psych patients
compared with those without a psychiatric diagnosis, according to the results of the largest study of its kind to date.
These findings, the investigators noted, highlight the need to prioritize vaccination in patients with preexisting mental health disorders.
“We have proven beyond a shadow of a doubt that there are increased risks” among psychiatric patients who get COVID-19, study investigator Livia De Picker, MD, PhD, psychiatrist and postdoctoral researcher, University Psychiatric Hospital Campus Duffel and University of Antwerp (Belgium), told this news organization.
“Doctors need to look at these patients the same way they would other high-risk people, for example those with diabetes or chronic obstructive pulmonary disease,” all of whom should be protected against COVID-19, Dr. De Picker added.
The study was published online July 15, 2021, in Lancet Psychiatry.
Risk by mental illness type
The systematic review included 33 studies from 22 countries that reported risk estimates for mortality, hospitalization, and ICU admission in patients with confirmed SARS-CoV-2 infection. The meta-analysis included 23 of these studies with a total of 1.47 million participants. Of these, 43,938 had a psychiatric disorder.
The primary outcome was mortality after COVID-19. Secondary outcomes included hospitalization and ICU admission after COVID-19. Researchers adjusted for age, sex, and other covariates.
Results showed the presence of any comorbid mental illness was associated with an increased risk for death after SARS-CoV-2 infection (odds ratio, 2.00; 95% confidence interval, 1.58-2.54; P < .0001).
When researchers stratified mortality risk by psychiatric disorder type, the most robust associations were for psychotic and mood disorders. Substance use disorders, intellectual disabilities, and developmental disorders were associated with higher mortality only in crude estimates. There was no increased death risk associated with anxiety disorders.
“That there are differences between the various types of disorders was an interesting finding,” said Dr. De Picker, adding that previous research “just lumped together all diagnostic categories.”
Potential mechanisms
The study did not explore why psychiatric illness raise the risk for death in the setting of COVID-19, so potential mechanisms are purely speculative. However, the investigators believe it may reflect biological processes such as immune-inflammatory alterations.
Psychotic disorders and mood disorders in particular, are associated with immune changes, including immunogenetic abnormalities, raised cytokine concentrations, autoantibodies, acute-phase proteins, and aberrant counts of leukocyte cell types, said Dr. De Picker.
She likened this to elderly people being at increased risk following COVID-19 because their immune system is compromised and less able to fight infection.
There are likely other factors at play, said Dr. De Picker. These could include social isolation and lifestyle factors like poor diet, physical inactivity, high alcohol and tobacco use, and sleep disturbances.
In addition, psychiatric patients have a higher prevalence of comorbidities including diabetes, cardiovascular disease, and respiratory disease, which could also play a role.
The increased mortality might also reflect reduced access to care. “Some of these patients may be living in difficult socioeconomic conditions,” said Dr. De Picker.
She noted that, while the in-hospital mortality was not increased, the risk was significantly increased in samples that were outside of the hospital. This reinforces the need for providing close monitoring and early referral to hospital for psychiatric patients with COVID-19.
Mortality varied significantly among countries, with the lowest risk in Europe and the United States. This difference might be attributable to differences in health care systems and access to care, said Dr. De Picker.
Overall, the risk for hospitalization was about double for COVID patients with a mental illness, but when stratified by disorder, there was only a significantly increased risk for substance use and mood disorders. “But mood disorders were not even significant any more after adjusting for age, sex, and comorbid conditions, and we don’t see an increased risk for psychotic disorders whereas they had the highest mortality risks,” said Dr. De Picker.
Psych meds a risk factor?
The studies were primarily based on electronic medical records, so investigators were unable to carry out “a fine grain analysis” into clinical factors affecting outcomes, she noted.
Antipsychotics were consistently associated with an increased risk for mortality (adjusted OR, 2.43; 95% CI, 1.81-3.25), as were anxiolytics (aOR, 1.47; 95% CI, 1.15-1.88).
“There are some theoretical reasons why we believe there could be a risk associated with these drugs,” said Dr. De Picker. For example, antipsychotics can increase the risk for cardiac arrhythmias and thromboembolic events, and cause interactions with drugs used to treat COVID-19.
As for anxiolytics, especially benzodiazepines, these drugs are associated with respiratory risk and with all-cause mortality. “So you could imagine that someone who is infected with a respiratory virus and [is] then using these drugs on top of that would have a worse outcome,” said Dr. De Picker.
In contrast to antipsychotics and anxiolytics, antidepressants did not increase mortality risk.
Dr. De Picker noted a new study by French researchers showing a protective effect of certain serotonergic antidepressants on COVID outcomes, including mortality.
There was no robust evidence of an increased risk for ICU admission for patients with mental disorders. However, the authors noted some studies included small samples of patients with psychiatric disorders, “contributing to a low certainty of evidence for ICU admission.”
Dr. De Picker criticized COVID vaccine policies that don’t prioritize patients with psychiatric disorders. In many countries, groups that were initially green-lighted for the vaccine included health care workers, the elderly, and those with underlying conditions such as diabetes, obesity and even mild hypertension – but not mental illness, which is also an underlying risk.
‘Outstanding’ research
Commenting on the study for this news organization, Jonathan E. Alpert, MD, PhD, department of psychiatry and behavioral sciences, Montefiore Medical Center, New York, and chair of the American Psychiatric Association Council on Research, called it “outstanding” and the largest of its kind.
“There have been a number of studies that have come to similar conclusions, that people with psychiatric illness are at greater risk for poorer outcomes, but because any given study had a relatively limited sample, perhaps from one health system or one country, there were some inconsistencies,” said Dr. Alpert.
“This is the strongest report so far that has made the point that people with psychiatric illness are a vulnerable population for a negative outcome from COVID, including the most worrisome – mortality.”
The study helps drive home a “very important public health lesson” that applies to COVID-19 but goes “beyond,” said Dr. Alpert.
“As a society, we need to keep in mind that people with serious mental disorders are a vulnerable population for poorer outcomes in most general medical conditions,” he stressed, “whether it’s cancer or heart disease or diabetes, and special efforts need to be made to reach out to those populations.”
Dr. Alpert agreed that, at the start of the pandemic, psychiatric patients in the United States were not prioritized for vaccination, and although psychiatric patients may initially have found it difficult to navigate the health care system to learn where and how to get a COVID shot, today that barrier has mostly been removed.
“Our patients are at least as willing as any other subgroup to get the vaccine, and that includes people with psychotic disorders,” he said.
The study was supported by the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry network and Fondazione Centro San Raffaele (Milan). Dr. De Picker reported receiving grants from Boehringer Ingelheim and Janssen outside the submitted work. She is a member of the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry Thematic Working Group.
A version of this article first appeared on Medscape.com.
compared with those without a psychiatric diagnosis, according to the results of the largest study of its kind to date.
These findings, the investigators noted, highlight the need to prioritize vaccination in patients with preexisting mental health disorders.
“We have proven beyond a shadow of a doubt that there are increased risks” among psychiatric patients who get COVID-19, study investigator Livia De Picker, MD, PhD, psychiatrist and postdoctoral researcher, University Psychiatric Hospital Campus Duffel and University of Antwerp (Belgium), told this news organization.
“Doctors need to look at these patients the same way they would other high-risk people, for example those with diabetes or chronic obstructive pulmonary disease,” all of whom should be protected against COVID-19, Dr. De Picker added.
The study was published online July 15, 2021, in Lancet Psychiatry.
Risk by mental illness type
The systematic review included 33 studies from 22 countries that reported risk estimates for mortality, hospitalization, and ICU admission in patients with confirmed SARS-CoV-2 infection. The meta-analysis included 23 of these studies with a total of 1.47 million participants. Of these, 43,938 had a psychiatric disorder.
The primary outcome was mortality after COVID-19. Secondary outcomes included hospitalization and ICU admission after COVID-19. Researchers adjusted for age, sex, and other covariates.
Results showed the presence of any comorbid mental illness was associated with an increased risk for death after SARS-CoV-2 infection (odds ratio, 2.00; 95% confidence interval, 1.58-2.54; P < .0001).
When researchers stratified mortality risk by psychiatric disorder type, the most robust associations were for psychotic and mood disorders. Substance use disorders, intellectual disabilities, and developmental disorders were associated with higher mortality only in crude estimates. There was no increased death risk associated with anxiety disorders.
“That there are differences between the various types of disorders was an interesting finding,” said Dr. De Picker, adding that previous research “just lumped together all diagnostic categories.”
Potential mechanisms
The study did not explore why psychiatric illness raise the risk for death in the setting of COVID-19, so potential mechanisms are purely speculative. However, the investigators believe it may reflect biological processes such as immune-inflammatory alterations.
Psychotic disorders and mood disorders in particular, are associated with immune changes, including immunogenetic abnormalities, raised cytokine concentrations, autoantibodies, acute-phase proteins, and aberrant counts of leukocyte cell types, said Dr. De Picker.
She likened this to elderly people being at increased risk following COVID-19 because their immune system is compromised and less able to fight infection.
There are likely other factors at play, said Dr. De Picker. These could include social isolation and lifestyle factors like poor diet, physical inactivity, high alcohol and tobacco use, and sleep disturbances.
In addition, psychiatric patients have a higher prevalence of comorbidities including diabetes, cardiovascular disease, and respiratory disease, which could also play a role.
The increased mortality might also reflect reduced access to care. “Some of these patients may be living in difficult socioeconomic conditions,” said Dr. De Picker.
She noted that, while the in-hospital mortality was not increased, the risk was significantly increased in samples that were outside of the hospital. This reinforces the need for providing close monitoring and early referral to hospital for psychiatric patients with COVID-19.
Mortality varied significantly among countries, with the lowest risk in Europe and the United States. This difference might be attributable to differences in health care systems and access to care, said Dr. De Picker.
Overall, the risk for hospitalization was about double for COVID patients with a mental illness, but when stratified by disorder, there was only a significantly increased risk for substance use and mood disorders. “But mood disorders were not even significant any more after adjusting for age, sex, and comorbid conditions, and we don’t see an increased risk for psychotic disorders whereas they had the highest mortality risks,” said Dr. De Picker.
Psych meds a risk factor?
The studies were primarily based on electronic medical records, so investigators were unable to carry out “a fine grain analysis” into clinical factors affecting outcomes, she noted.
Antipsychotics were consistently associated with an increased risk for mortality (adjusted OR, 2.43; 95% CI, 1.81-3.25), as were anxiolytics (aOR, 1.47; 95% CI, 1.15-1.88).
“There are some theoretical reasons why we believe there could be a risk associated with these drugs,” said Dr. De Picker. For example, antipsychotics can increase the risk for cardiac arrhythmias and thromboembolic events, and cause interactions with drugs used to treat COVID-19.
As for anxiolytics, especially benzodiazepines, these drugs are associated with respiratory risk and with all-cause mortality. “So you could imagine that someone who is infected with a respiratory virus and [is] then using these drugs on top of that would have a worse outcome,” said Dr. De Picker.
In contrast to antipsychotics and anxiolytics, antidepressants did not increase mortality risk.
Dr. De Picker noted a new study by French researchers showing a protective effect of certain serotonergic antidepressants on COVID outcomes, including mortality.
There was no robust evidence of an increased risk for ICU admission for patients with mental disorders. However, the authors noted some studies included small samples of patients with psychiatric disorders, “contributing to a low certainty of evidence for ICU admission.”
Dr. De Picker criticized COVID vaccine policies that don’t prioritize patients with psychiatric disorders. In many countries, groups that were initially green-lighted for the vaccine included health care workers, the elderly, and those with underlying conditions such as diabetes, obesity and even mild hypertension – but not mental illness, which is also an underlying risk.
‘Outstanding’ research
Commenting on the study for this news organization, Jonathan E. Alpert, MD, PhD, department of psychiatry and behavioral sciences, Montefiore Medical Center, New York, and chair of the American Psychiatric Association Council on Research, called it “outstanding” and the largest of its kind.
“There have been a number of studies that have come to similar conclusions, that people with psychiatric illness are at greater risk for poorer outcomes, but because any given study had a relatively limited sample, perhaps from one health system or one country, there were some inconsistencies,” said Dr. Alpert.
“This is the strongest report so far that has made the point that people with psychiatric illness are a vulnerable population for a negative outcome from COVID, including the most worrisome – mortality.”
The study helps drive home a “very important public health lesson” that applies to COVID-19 but goes “beyond,” said Dr. Alpert.
“As a society, we need to keep in mind that people with serious mental disorders are a vulnerable population for poorer outcomes in most general medical conditions,” he stressed, “whether it’s cancer or heart disease or diabetes, and special efforts need to be made to reach out to those populations.”
Dr. Alpert agreed that, at the start of the pandemic, psychiatric patients in the United States were not prioritized for vaccination, and although psychiatric patients may initially have found it difficult to navigate the health care system to learn where and how to get a COVID shot, today that barrier has mostly been removed.
“Our patients are at least as willing as any other subgroup to get the vaccine, and that includes people with psychotic disorders,” he said.
The study was supported by the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry network and Fondazione Centro San Raffaele (Milan). Dr. De Picker reported receiving grants from Boehringer Ingelheim and Janssen outside the submitted work. She is a member of the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry Thematic Working Group.
A version of this article first appeared on Medscape.com.
compared with those without a psychiatric diagnosis, according to the results of the largest study of its kind to date.
These findings, the investigators noted, highlight the need to prioritize vaccination in patients with preexisting mental health disorders.
“We have proven beyond a shadow of a doubt that there are increased risks” among psychiatric patients who get COVID-19, study investigator Livia De Picker, MD, PhD, psychiatrist and postdoctoral researcher, University Psychiatric Hospital Campus Duffel and University of Antwerp (Belgium), told this news organization.
“Doctors need to look at these patients the same way they would other high-risk people, for example those with diabetes or chronic obstructive pulmonary disease,” all of whom should be protected against COVID-19, Dr. De Picker added.
The study was published online July 15, 2021, in Lancet Psychiatry.
Risk by mental illness type
The systematic review included 33 studies from 22 countries that reported risk estimates for mortality, hospitalization, and ICU admission in patients with confirmed SARS-CoV-2 infection. The meta-analysis included 23 of these studies with a total of 1.47 million participants. Of these, 43,938 had a psychiatric disorder.
The primary outcome was mortality after COVID-19. Secondary outcomes included hospitalization and ICU admission after COVID-19. Researchers adjusted for age, sex, and other covariates.
Results showed the presence of any comorbid mental illness was associated with an increased risk for death after SARS-CoV-2 infection (odds ratio, 2.00; 95% confidence interval, 1.58-2.54; P < .0001).
When researchers stratified mortality risk by psychiatric disorder type, the most robust associations were for psychotic and mood disorders. Substance use disorders, intellectual disabilities, and developmental disorders were associated with higher mortality only in crude estimates. There was no increased death risk associated with anxiety disorders.
“That there are differences between the various types of disorders was an interesting finding,” said Dr. De Picker, adding that previous research “just lumped together all diagnostic categories.”
Potential mechanisms
The study did not explore why psychiatric illness raise the risk for death in the setting of COVID-19, so potential mechanisms are purely speculative. However, the investigators believe it may reflect biological processes such as immune-inflammatory alterations.
Psychotic disorders and mood disorders in particular, are associated with immune changes, including immunogenetic abnormalities, raised cytokine concentrations, autoantibodies, acute-phase proteins, and aberrant counts of leukocyte cell types, said Dr. De Picker.
She likened this to elderly people being at increased risk following COVID-19 because their immune system is compromised and less able to fight infection.
There are likely other factors at play, said Dr. De Picker. These could include social isolation and lifestyle factors like poor diet, physical inactivity, high alcohol and tobacco use, and sleep disturbances.
In addition, psychiatric patients have a higher prevalence of comorbidities including diabetes, cardiovascular disease, and respiratory disease, which could also play a role.
The increased mortality might also reflect reduced access to care. “Some of these patients may be living in difficult socioeconomic conditions,” said Dr. De Picker.
She noted that, while the in-hospital mortality was not increased, the risk was significantly increased in samples that were outside of the hospital. This reinforces the need for providing close monitoring and early referral to hospital for psychiatric patients with COVID-19.
Mortality varied significantly among countries, with the lowest risk in Europe and the United States. This difference might be attributable to differences in health care systems and access to care, said Dr. De Picker.
Overall, the risk for hospitalization was about double for COVID patients with a mental illness, but when stratified by disorder, there was only a significantly increased risk for substance use and mood disorders. “But mood disorders were not even significant any more after adjusting for age, sex, and comorbid conditions, and we don’t see an increased risk for psychotic disorders whereas they had the highest mortality risks,” said Dr. De Picker.
Psych meds a risk factor?
The studies were primarily based on electronic medical records, so investigators were unable to carry out “a fine grain analysis” into clinical factors affecting outcomes, she noted.
Antipsychotics were consistently associated with an increased risk for mortality (adjusted OR, 2.43; 95% CI, 1.81-3.25), as were anxiolytics (aOR, 1.47; 95% CI, 1.15-1.88).
“There are some theoretical reasons why we believe there could be a risk associated with these drugs,” said Dr. De Picker. For example, antipsychotics can increase the risk for cardiac arrhythmias and thromboembolic events, and cause interactions with drugs used to treat COVID-19.
As for anxiolytics, especially benzodiazepines, these drugs are associated with respiratory risk and with all-cause mortality. “So you could imagine that someone who is infected with a respiratory virus and [is] then using these drugs on top of that would have a worse outcome,” said Dr. De Picker.
In contrast to antipsychotics and anxiolytics, antidepressants did not increase mortality risk.
Dr. De Picker noted a new study by French researchers showing a protective effect of certain serotonergic antidepressants on COVID outcomes, including mortality.
There was no robust evidence of an increased risk for ICU admission for patients with mental disorders. However, the authors noted some studies included small samples of patients with psychiatric disorders, “contributing to a low certainty of evidence for ICU admission.”
Dr. De Picker criticized COVID vaccine policies that don’t prioritize patients with psychiatric disorders. In many countries, groups that were initially green-lighted for the vaccine included health care workers, the elderly, and those with underlying conditions such as diabetes, obesity and even mild hypertension – but not mental illness, which is also an underlying risk.
‘Outstanding’ research
Commenting on the study for this news organization, Jonathan E. Alpert, MD, PhD, department of psychiatry and behavioral sciences, Montefiore Medical Center, New York, and chair of the American Psychiatric Association Council on Research, called it “outstanding” and the largest of its kind.
“There have been a number of studies that have come to similar conclusions, that people with psychiatric illness are at greater risk for poorer outcomes, but because any given study had a relatively limited sample, perhaps from one health system or one country, there were some inconsistencies,” said Dr. Alpert.
“This is the strongest report so far that has made the point that people with psychiatric illness are a vulnerable population for a negative outcome from COVID, including the most worrisome – mortality.”
The study helps drive home a “very important public health lesson” that applies to COVID-19 but goes “beyond,” said Dr. Alpert.
“As a society, we need to keep in mind that people with serious mental disorders are a vulnerable population for poorer outcomes in most general medical conditions,” he stressed, “whether it’s cancer or heart disease or diabetes, and special efforts need to be made to reach out to those populations.”
Dr. Alpert agreed that, at the start of the pandemic, psychiatric patients in the United States were not prioritized for vaccination, and although psychiatric patients may initially have found it difficult to navigate the health care system to learn where and how to get a COVID shot, today that barrier has mostly been removed.
“Our patients are at least as willing as any other subgroup to get the vaccine, and that includes people with psychotic disorders,” he said.
The study was supported by the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry network and Fondazione Centro San Raffaele (Milan). Dr. De Picker reported receiving grants from Boehringer Ingelheim and Janssen outside the submitted work. She is a member of the European College of Neuropsychopharmacology Immuno-NeuroPsychiatry Thematic Working Group.
A version of this article first appeared on Medscape.com.
CDC revamps STI treatment guidelines
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.
Abdominal pain and urinary frequency
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
A 58-year-old Black man presents with abdominal pain, urinary frequency and urgency, dysuria, incomplete voiding, and postmicturition dribble. The patient's medical history is unremarkable apart from stage 1 hypertension, for which he receives losartan plus amlodipine. Physical examination findings reveal an overdistended bladder with associated tenderness and a mildly enlarged prostate with a large, firm nodule on digital rectal exam. Urinalysis shows hematuria. Complete blood count and chemistry panel are normal. The total prostate-specific antigen level is 22 ng/mL.
Autoinflammatory diseases ‘not so rare after all,’ expert says
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
Not long ago, after all.
“Patients with autoinflammatory diseases are all around us, but many go several years without a diagnosis,” Dr. Dissanayake, a rheumatologist at the Autoinflammatory Disease Clinic at the Hospital for Sick Children, Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “The median time to diagnosis has been estimated to be between 2.5 and 5 years. You can imagine that this type of delay can lead to significant issues, not only with quality of life but also morbidity due to unchecked inflammation that can cause organ damage, and in the most severe cases, can result in an early death.”
Effective treatment options such as biologic medications, however, can prevent these negative sequelae if the disease is recognized early. “Dermatologists are in a unique position because they will often be the first specialist to see these patients and therefore make the diagnosis early on and really alter the lives of these patients,” he said.
While it’s common to classify autoinflammatory diseases by presenting features, such as age of onset, associated symptoms, family history/ethnicity, and triggers/alleviating factors for episodes, Dr. Dissanayake prefers to classify them into one of three groups based on pathophysiology, the first being inflammasomopathies. “When activated, an inflammasome is responsible for processing cytokines from the [interleukin]-1 family from the pro form to the active form,” he explained. As a result, if there is dysregulation and overactivity of the inflammasome, there is excessive production of cytokines like IL-1 beta and IL-18 driving the disease.
Clinical characteristics include fevers and organ involvement, notably abdominal pain, nonvasculitic rashes, uveitis, arthritis, elevated white blood cell count/neutrophils, and highly elevated inflammatory markers. Potential treatments include IL-1 blockers.
The second category of autoinflammatory diseases are the interferonopathies, which are caused by overactivity of the antiviral side of the innate immune system. “For example, if you have overactivity of a sensor for a nucleic acid in your cytosol, the cell misinterprets this as a viral infection and will turn on type 1 interferon production,” said Dr. Dissanayake, who is also an assistant professor of pediatrics at the University of Toronto. “As a result, if you have dysregulation of these pathways, you will get excessive type 1 interferon that contributes to your disease manifestations.” Clinical characteristics include fevers and organ involvement, notably vasculitic rashes, interstitial lung disease, and intracranial calcifications. Inflammatory markers may not be as elevated, and autoantibodies may be present. Janus kinase inhibitors are a potential treatment, he said.
The third category of autoinflammatory diseases are the NF-kappaBopathies, which are caused by overactivity of the NF-kappaB signaling pathway. Clinical characteristics can include fevers with organ involvement that can be highly variable but may include mucocutaneous lesions or granulomatous disease as potential clues. Treatment options depend on the pathway that is involved but tumor necrosis factor blockers often play a role because of the importance of NF-KB in this signaling pathway.
From a skin perspective, most of the rashes Dr. Dissanayake and colleagues see in the rheumatology clinic consist of nonspecific dermohypodermatitis: macules, papules, patches, or plaques. The most common monogenic autoinflammatory disease is Familial Mediterranean Fever syndrome, which “commonly presents as an erysipelas-like rash of the lower extremities, typically below the knee, often over the malleolus,” he said.
Other monogenic autoinflammatory diseases with similar rashes include TNF receptor–associated periodic syndrome, Hyper-IgD syndrome, and systemic juvenile idiopathic arthritis.
Other patients present with urticarial rashes, most commonly cryopyrin-associated periodic syndrome (CAPS). “This is a neutrophilic urticaria, so it tends not to be pruritic and can actually sometimes be tender,” he said. “It also tends not to be as transient as your typical urticaria.” Urticarial rashes can also appear with NLRP12-associated autoinflammatory syndrome (familial cold autoinflammatory syndrome–2), PLCgamma2-associated antibody deficiency and immune dysregulation, and Schnitzler syndrome (monoclonal IgM gammopathy).
Patients can also present with pyogenic or pustular lesions, which can appear with pyoderma gangrenosum–related diseases, such as pyogenic arthritis, pyoderma gangrenosum, arthritis (PAPA) syndrome; pyrin-associated inflammation with neutrophilic dermatosis; deficiency of the IL-1 receptor antagonist; deficiency of IL-36 receptor antagonist; and Majeed syndrome, a mutation in the LPIN2 gene.
The mucocutaneous system can also be affected in autoinflammatory diseases, often presenting with symptoms such as periodic fever, aphthous stomatitis, and pharyngitis. Cervical adenitis syndrome is the most common autoinflammatory disease in childhood and can present with aphthous stomatitis, he said, while Behcet’s disease typically presents with oral and genital ulcers. “More recently, monogenic forms of Behcet’s disease have been described, with haploinsufficiency of A20 and RelA, which are both part of the NF-KB pathway,” he said.
Finally, the presence of vasculitic lesions often suggest interferonopathies such as STING-associated vasculopathy in infancy, proteasome-associated autoinflammatory syndrome and deficiency of adenosine deaminase 2.
Dr. Dissanayake noted that dermatologists should suspect an autoimmune disease if a patient has recurrent fevers, evidence of systemic inflammation on blood work, and if multiple organ systems are involved, especially the lungs, gut, joints, CNS system, and eyes. “Many of these patients have episodic and stereotypical attacks,” he said.
“One of the tools we use in the autoinflammatory clinic is to have patients and families keep a symptom diary where they track the dates of the various symptoms. We can review this during their appointment and try to come up with a diagnosis based on the pattern,” he said.
Since many of these diseases are due to a single gene defect, if there’s any evidence to suggest a monogenic cause, consider an autoinflammatory disease, he added. “If there’s a family history, if there’s consanguinity, or if there’s early age of onset – these may all lead you to think about monogenic autoinflammatory disease.”
During a question-and-answer session, a meeting attendee asked what type of workup he recommends when an autoinflammatory syndrome is suspected. “It partially depends on what organ systems you suspect to be involved,” Dr. Dissanayake said. “As a routine baseline, typically what we would check is CBC and differential, [erythrocyte sedimentation rate] and [C-reactive protein], and we screen for liver transaminases and creatinine to check for liver and kidney issues. A serum albumin will also tell you if the patient is hypoalbuminemic, that there’s been some chronic inflammation and they’re starting to leak the protein out. It’s good to check blood work during the flare and off the flare, to get a sense of the persistence of that inflammation.”
Dr. Dissanayake disclosed that he has received research finding from Gilead Sciences and speaker fees from Novartis.
*This story was updated on 9/20/2021.
FROM SPD 2021