User login
Healthy lifestyle can reduce dementia risk despite family history
Individuals at increased risk for dementia because of family history can reduce that risk by adopting healthy lifestyle behaviors, data from more than 300,000 adults aged 50-73 years suggest.
Having a parent or sibling with dementia can increase a person’s risk of developing dementia themselves by nearly 75%, compared with someone with no first-degree family history of dementia, according to Angelique Brellenthin, PhD, of Iowa State University, Ames, and colleagues.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association, the researchers reviewed information for 302,239 men and women who were enrolled in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom, between 2006 and 2010.
The study participants had no evidence of dementia at baseline, and completed questionnaires about family history and lifestyle. The questions included details about six healthy lifestyle behaviors: eating a healthy diet, engaging in at least 150 minutes of moderate to vigorous physical activity per week, sleeping 6-9 hours each night, drinking alcohol in moderation, not smoking, and maintaining a body mass index below the obese level (less than 30 kg/m2).
The researchers identified 1,698 participants (0.6%) who developed dementia over an average follow-up period of 8 years. Those with a family history (first-degree relative) of dementia had a 70% increased risk of dementia, compared with those who had no such family history.
Overall, individuals who engaged in all six healthy behaviors reduced their risk of dementia by about half, compared with those who engaged in two or fewer healthy behaviors. Engaging in three healthy behaviors reduced the risk of dementia by 30%, compared with engaging in two or fewer healthy behaviors, and this association held after controlling not only for family history of dementia, but also for other dementia risk factors such as age, sex, race, and education level, as well as high blood pressure, high cholesterol, and the presence of type 2 diabetes.
Similarly, among participants with a family history of dementia, those who engaged in three healthy lifestyle behaviors showed a 25%-35% reduction in dementia risk, compared with those who engaged in two or fewer healthy behaviors.
The study findings were limited by several factors including the inability to prove that lifestyle can cause or prevent dementia, only to show an association, the researchers noted. Also, the findings were limited by the reliance on self-reports, rather than genetic data, to confirm familial dementia.
However, the findings were strengthened by the large sample size, and the results suggest that a healthy lifestyle can impact cognitive health, and support the value of encouraging healthy behaviors in general, and especially among individuals with a family history of dementia, they said.
Small changes may promote prevention
The study is important now because, as the population ages, many individuals have a family member who has had dementia, said lead author Dr. Brellenthin, in an interview. “It’s important to understand how lifestyle behaviors affect the risk of dementia when it runs in families,” she said.
Dr. Brellenthin said she was surprised by some of the findings. “It was surprising to see that the risk of dementia was reduced with just three healthy behaviors [but was further reduced as you added more behaviors] compared to two or fewer behaviors. However, it was not surprising to see that these same lifestyle behaviors that tend to be good for the heart and body are also good for the brain.”
The evidence that following just three healthy behaviors can reduce the risk of dementia by 25%-35% for individuals with a familial history of dementia has clinical implications, Dr. Brellenthin said. “Many people are already following some of these behaviors like not smoking, so it might be possible to focus on adding just one more behavior, like getting enough sleep, and going from there.”
Commenting on the study, AHA President Mitchell S. V. Elkind, MD, said that the study “tells us that, yes, family history is important [in determining the risk of dementia], and much of that may be driven by genetic factors, but some of that impact can be mitigated or decreased by engaging in those important behaviors that we know are good to maintain brain health.
“The tricky thing, of course, is getting people to engage in these behaviors. That’s where a lot of work in the future will be: changing people’s behavior to become more healthy, and figuring out exactly which behaviors may be the easiest to engage in and be most likely to have public health impact,” added Dr. Elkind, professor of neurology and epidemiology at Columbia University and attending neurologist at New York–Presbyterian/Columbia University Irving Medical Center, New York.
The study received no outside funding, but the was research was conducted using the U.K. Biobank resources. The researchers had no financial conflicts to disclose.
Individuals at increased risk for dementia because of family history can reduce that risk by adopting healthy lifestyle behaviors, data from more than 300,000 adults aged 50-73 years suggest.
Having a parent or sibling with dementia can increase a person’s risk of developing dementia themselves by nearly 75%, compared with someone with no first-degree family history of dementia, according to Angelique Brellenthin, PhD, of Iowa State University, Ames, and colleagues.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association, the researchers reviewed information for 302,239 men and women who were enrolled in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom, between 2006 and 2010.
The study participants had no evidence of dementia at baseline, and completed questionnaires about family history and lifestyle. The questions included details about six healthy lifestyle behaviors: eating a healthy diet, engaging in at least 150 minutes of moderate to vigorous physical activity per week, sleeping 6-9 hours each night, drinking alcohol in moderation, not smoking, and maintaining a body mass index below the obese level (less than 30 kg/m2).
The researchers identified 1,698 participants (0.6%) who developed dementia over an average follow-up period of 8 years. Those with a family history (first-degree relative) of dementia had a 70% increased risk of dementia, compared with those who had no such family history.
Overall, individuals who engaged in all six healthy behaviors reduced their risk of dementia by about half, compared with those who engaged in two or fewer healthy behaviors. Engaging in three healthy behaviors reduced the risk of dementia by 30%, compared with engaging in two or fewer healthy behaviors, and this association held after controlling not only for family history of dementia, but also for other dementia risk factors such as age, sex, race, and education level, as well as high blood pressure, high cholesterol, and the presence of type 2 diabetes.
Similarly, among participants with a family history of dementia, those who engaged in three healthy lifestyle behaviors showed a 25%-35% reduction in dementia risk, compared with those who engaged in two or fewer healthy behaviors.
The study findings were limited by several factors including the inability to prove that lifestyle can cause or prevent dementia, only to show an association, the researchers noted. Also, the findings were limited by the reliance on self-reports, rather than genetic data, to confirm familial dementia.
However, the findings were strengthened by the large sample size, and the results suggest that a healthy lifestyle can impact cognitive health, and support the value of encouraging healthy behaviors in general, and especially among individuals with a family history of dementia, they said.
Small changes may promote prevention
The study is important now because, as the population ages, many individuals have a family member who has had dementia, said lead author Dr. Brellenthin, in an interview. “It’s important to understand how lifestyle behaviors affect the risk of dementia when it runs in families,” she said.
Dr. Brellenthin said she was surprised by some of the findings. “It was surprising to see that the risk of dementia was reduced with just three healthy behaviors [but was further reduced as you added more behaviors] compared to two or fewer behaviors. However, it was not surprising to see that these same lifestyle behaviors that tend to be good for the heart and body are also good for the brain.”
The evidence that following just three healthy behaviors can reduce the risk of dementia by 25%-35% for individuals with a familial history of dementia has clinical implications, Dr. Brellenthin said. “Many people are already following some of these behaviors like not smoking, so it might be possible to focus on adding just one more behavior, like getting enough sleep, and going from there.”
Commenting on the study, AHA President Mitchell S. V. Elkind, MD, said that the study “tells us that, yes, family history is important [in determining the risk of dementia], and much of that may be driven by genetic factors, but some of that impact can be mitigated or decreased by engaging in those important behaviors that we know are good to maintain brain health.
“The tricky thing, of course, is getting people to engage in these behaviors. That’s where a lot of work in the future will be: changing people’s behavior to become more healthy, and figuring out exactly which behaviors may be the easiest to engage in and be most likely to have public health impact,” added Dr. Elkind, professor of neurology and epidemiology at Columbia University and attending neurologist at New York–Presbyterian/Columbia University Irving Medical Center, New York.
The study received no outside funding, but the was research was conducted using the U.K. Biobank resources. The researchers had no financial conflicts to disclose.
Individuals at increased risk for dementia because of family history can reduce that risk by adopting healthy lifestyle behaviors, data from more than 300,000 adults aged 50-73 years suggest.
Having a parent or sibling with dementia can increase a person’s risk of developing dementia themselves by nearly 75%, compared with someone with no first-degree family history of dementia, according to Angelique Brellenthin, PhD, of Iowa State University, Ames, and colleagues.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association, the researchers reviewed information for 302,239 men and women who were enrolled in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom, between 2006 and 2010.
The study participants had no evidence of dementia at baseline, and completed questionnaires about family history and lifestyle. The questions included details about six healthy lifestyle behaviors: eating a healthy diet, engaging in at least 150 minutes of moderate to vigorous physical activity per week, sleeping 6-9 hours each night, drinking alcohol in moderation, not smoking, and maintaining a body mass index below the obese level (less than 30 kg/m2).
The researchers identified 1,698 participants (0.6%) who developed dementia over an average follow-up period of 8 years. Those with a family history (first-degree relative) of dementia had a 70% increased risk of dementia, compared with those who had no such family history.
Overall, individuals who engaged in all six healthy behaviors reduced their risk of dementia by about half, compared with those who engaged in two or fewer healthy behaviors. Engaging in three healthy behaviors reduced the risk of dementia by 30%, compared with engaging in two or fewer healthy behaviors, and this association held after controlling not only for family history of dementia, but also for other dementia risk factors such as age, sex, race, and education level, as well as high blood pressure, high cholesterol, and the presence of type 2 diabetes.
Similarly, among participants with a family history of dementia, those who engaged in three healthy lifestyle behaviors showed a 25%-35% reduction in dementia risk, compared with those who engaged in two or fewer healthy behaviors.
The study findings were limited by several factors including the inability to prove that lifestyle can cause or prevent dementia, only to show an association, the researchers noted. Also, the findings were limited by the reliance on self-reports, rather than genetic data, to confirm familial dementia.
However, the findings were strengthened by the large sample size, and the results suggest that a healthy lifestyle can impact cognitive health, and support the value of encouraging healthy behaviors in general, and especially among individuals with a family history of dementia, they said.
Small changes may promote prevention
The study is important now because, as the population ages, many individuals have a family member who has had dementia, said lead author Dr. Brellenthin, in an interview. “It’s important to understand how lifestyle behaviors affect the risk of dementia when it runs in families,” she said.
Dr. Brellenthin said she was surprised by some of the findings. “It was surprising to see that the risk of dementia was reduced with just three healthy behaviors [but was further reduced as you added more behaviors] compared to two or fewer behaviors. However, it was not surprising to see that these same lifestyle behaviors that tend to be good for the heart and body are also good for the brain.”
The evidence that following just three healthy behaviors can reduce the risk of dementia by 25%-35% for individuals with a familial history of dementia has clinical implications, Dr. Brellenthin said. “Many people are already following some of these behaviors like not smoking, so it might be possible to focus on adding just one more behavior, like getting enough sleep, and going from there.”
Commenting on the study, AHA President Mitchell S. V. Elkind, MD, said that the study “tells us that, yes, family history is important [in determining the risk of dementia], and much of that may be driven by genetic factors, but some of that impact can be mitigated or decreased by engaging in those important behaviors that we know are good to maintain brain health.
“The tricky thing, of course, is getting people to engage in these behaviors. That’s where a lot of work in the future will be: changing people’s behavior to become more healthy, and figuring out exactly which behaviors may be the easiest to engage in and be most likely to have public health impact,” added Dr. Elkind, professor of neurology and epidemiology at Columbia University and attending neurologist at New York–Presbyterian/Columbia University Irving Medical Center, New York.
The study received no outside funding, but the was research was conducted using the U.K. Biobank resources. The researchers had no financial conflicts to disclose.
FROM EPI/LIFESTYLE 2021
Two key suicide risk factors identified in borderline personality disorder
Feelings of chronic emptiness and self-injury have been identified as two key risk factors for suicide attempts (SAs) in patients with borderline personality disorder (BPD), a new cross-sectional, nationally representative study suggests.
The findings also show lifetime and past-year SAs are common among patients with BPD, even when excluding self-injurious behaviors.
The results suggest that in addition to asking patients about self-harm during suicide risk screenings and assessments, clinicians should query them about “longstanding” feelings of emptiness, study investigator Carlos M. Grilo, PhD, professor of psychiatry and psychology, Yale University, New Haven, Conn., said in an interview.
Although related, chronic emptiness “is distinct and goes beyond feelings of sadness, loneliness, and hopelessness,” explained Dr. Grilo. he said.
The study was published online May 11 in JAMA Network Open.
Filling a research gap
While BPD and other psychiatric disorders are associated with suicide, the authors noted there is a “dearth of epidemiological research” examining the link between BPD and suicide.
Criteria for BPD diagnosis requires any five of the following criteria: relationships, affective instability, abandonment fear, anger, identity disturbance, emptiness, disassociation/paranoia, self-injurious behavior, and impulsivity, along with social-occupation dysfunction.
To determine SA risk with specific BPD diagnostic criteria, the investigators examined data on 36,309 individuals who participated in the third wave of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-III), conducted from 2012 to 2013.
During computer-assisted, face-to-face interviews, study participants answered questions based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5) of the National Institute on Alcohol Abuse and Alcoholism.
This structured interview assesses a range of DSM-5–defined psychiatric disorders and their criteria. In addition to BPD, the AUDADIS-5 generates diagnoses for mood disorders, anxiety disorders, posttraumatic stress disorder, substance use disorders, antisocial personality disorder, schizotypal disorder, and conduct disorder.
During the interviews, respondents were asked if they had ever attempted suicide. For those who had, interviewers recorded the total number of lifetime attempts.
Participants also answered questions about childhood maltreatment including physical neglect, emotional neglect, physical abuse, emotional abuse, and sexual abuse by parents or caregivers and other adverse events occurring before the age of 18.
Childhood trauma common
Patients with BPD frequently report a history of childhood trauma, noted Dr. Grilo, adding that such trauma is associated with self-harm and suicide attempts. Sociodemographic information, including age, sex, and ethnicity/race, education level, and income, was also gathered.
Investigators examined data on suicide attempts using relatively stringent coding that required serious dysfunction in at least five BPD criteria.
Using this definition, investigators found the lifetime SA prevalence in patients with BPD was 30.4%, and 3.2% for past-year SAs. This compared with a rate of 3.7% for lifetime SAs and 0.2% for past-year SAs in those without a BPD diagnosis.
The authors examined SA rates using diagnostic codes in the NESARC-III that required seriously impaired function in only 1 or 2 BPD criteria. Rates were higher using the 5-criteria definition.
When the researchers excluded the BPD criterion of self-injurious behavior, the prevalence was 28.1% for lifetime and 3.0% for past-year SAs among the BPD group, with corresponding rates of 3.8% and 0.2% in those without a BPD diagnosis.
It’s important to look at this, said Dr. Grilo, as some patients with BPD who engage in self-harm have suicidal intent while others don’t.
“We tested whether BPD had heightened risk for suicide attempts if we eliminated the self-injurious criterion and we found that heightened risk was still there,” he explained.
Looking at individual criteria for BPD, a model that adjusted for sociodemographic characteristics, other psychiatric disorders, age at BPD onset, and history of childhood adverse events uncovered two criteria that were significantly associated with increased odds of SAs.
One was emptiness. For lifetime suicide attempts, the adjusted odds ratio (aOR) was 1.58 (95% confidence interval, 1.16-2.14) and for past-year attempts, the aOR was 1.99 (95% CI, 1.08-3.66).
The second was self-injurious behavior. For lifetime attempts, the aOR was 24.28 (95% CI, 16.83-32.03) and for past-year attempts, the aOR was 19.32 (95% CI, 5.22-71.58).
In a model in which all BPD-specific criteria were entered while excluding self-injurious behavior, the aORs for emptiness were 1.66 (95% CI, 1.23-2.24) for lifetime suicide attempts and 2.45 (95% CI, 1.18-5.08) for past year attempts.
Unlike another recent study that included more than 700 treatment-seeking patients with BPD who were followed for 10 years, the current study did not show significant associations with SAs for two other BPD criteria – identity disturbance and frantic attempts to avoid abandonment.
Dr. Grilo explained this might be because the earlier study included treatment-seeking patients instead of community cases, or because of differences in assessment interviews or other factors.
‘Compelling evidence’
“Our epidemiological sample has much broader generalizability and fewer potential confounds than the clinical treatment-seeking sample,” said Dr. Grilo.
However, he noted that the two studies “converge strongly and provide compelling evidence that BPD is associated with substantially heightened risk for suicide attempts over the lifetime.”
The two studies “also converge in finding that the presence of symptoms such as repeated self-harm and feelings of chronic emptiness are also associated with risk for suicide attempts.”
The new findings highlight the need to ask potentially at-risk patients about feelings of emptiness as well as self-injurious behaviors. Clinicians could, for example, ask: “Have you often felt like your life had no purpose or meaning?” or “Have you often felt empty inside?”
Limitations of the study include reliance on retrospective self-reports and use of lay interviewers, although these interviewers were trained and had an average of 5 years of experience conducting health-related surveys.
Although the study included a representative sample of U.S. adults, the sample did not include groups known to have high rates of suicide and self-harm behaviors, such as institutionalized, incarcerated, or homeless individuals.
In addition, the study did not evaluate severity and duration of BPD, although the authors noted they did adjust for age at BPD onset, this did not alter the findings.
Often misdiagnosed
Commenting on the study, John M. Oldham, MD, Distinguished Emeritus Professor, Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, and past-president, American Psychiatric Association, and an expert on BPD, had high praise for the research.
BPD is often misdiagnosed, Dr. Oldham said in an interview. Many patients seek help from primary care doctors who may label the symptoms as an anxiety disorder or a mood disorder, he said.
Although medications can help treat some BPD symptoms, “the primary, core evidence-based treatment for BPD is psychotherapy,” said Dr. Oldham, who some years ago helped develop evidence-based practice guidelines for BPD.
“It’s a clear and very well-designed study, and I don’t see any major limitations or problems with it,” he said. “The authors kept their focus rigorously on their goals and they used really careful methodology.”
He noted the “huge” numbers of patients included in the data and the relatively large percentage of men (43.7%).
“There’s a general belief that it’s mostly females who have BPD, but that’s not true; it’s females who come to treatment,” said Dr. Oldham.
Requiring that all five criteria lead to seriously impaired functioning “is a much more rigorous diagnostic methodology” than requiring only one or two criteria to lead to such impairment, said Dr. Oldham. “This is really important” and makes it “a much stronger study.”
The finding that self-harm behavior was linked to suicide attempts isn’t that surprising as this association has been well documented, but the finding that chronic emptiness is also predictive of future suicide attempts “is news,” said Dr. Oldham.
“We have not paid enough attention to this criterion in the clinical world or in the research world.”
Dr. Oldham said one patient with BPD gave him an ideal metaphor for emptiness. “She said it’s like there’s just nobody home. Think of it as an empty house that may look fine on the outside but you go inside and nobody lives there; there’s no furniture; no favorite things; no photos; no possessions.”
The authors have “important messages we need to pay attention to, and the main one is to explore this sense of chronic ‘nobody home’ emptiness,” said Dr. Oldham.
Dr. Grilo has reported receiving research grants from the National Institutes of Health; serving as a consultant for Sunovion and Weight Watchers; receiving honoraria for lectures, continuing medical education activities, and presentations at scientific conferences; and receiving royalties from Guilford Press and Taylor & Francis, all outside the submitted work.
A version of this article first appeared on Medscape.com.
Feelings of chronic emptiness and self-injury have been identified as two key risk factors for suicide attempts (SAs) in patients with borderline personality disorder (BPD), a new cross-sectional, nationally representative study suggests.
The findings also show lifetime and past-year SAs are common among patients with BPD, even when excluding self-injurious behaviors.
The results suggest that in addition to asking patients about self-harm during suicide risk screenings and assessments, clinicians should query them about “longstanding” feelings of emptiness, study investigator Carlos M. Grilo, PhD, professor of psychiatry and psychology, Yale University, New Haven, Conn., said in an interview.
Although related, chronic emptiness “is distinct and goes beyond feelings of sadness, loneliness, and hopelessness,” explained Dr. Grilo. he said.
The study was published online May 11 in JAMA Network Open.
Filling a research gap
While BPD and other psychiatric disorders are associated with suicide, the authors noted there is a “dearth of epidemiological research” examining the link between BPD and suicide.
Criteria for BPD diagnosis requires any five of the following criteria: relationships, affective instability, abandonment fear, anger, identity disturbance, emptiness, disassociation/paranoia, self-injurious behavior, and impulsivity, along with social-occupation dysfunction.
To determine SA risk with specific BPD diagnostic criteria, the investigators examined data on 36,309 individuals who participated in the third wave of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-III), conducted from 2012 to 2013.
During computer-assisted, face-to-face interviews, study participants answered questions based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5) of the National Institute on Alcohol Abuse and Alcoholism.
This structured interview assesses a range of DSM-5–defined psychiatric disorders and their criteria. In addition to BPD, the AUDADIS-5 generates diagnoses for mood disorders, anxiety disorders, posttraumatic stress disorder, substance use disorders, antisocial personality disorder, schizotypal disorder, and conduct disorder.
During the interviews, respondents were asked if they had ever attempted suicide. For those who had, interviewers recorded the total number of lifetime attempts.
Participants also answered questions about childhood maltreatment including physical neglect, emotional neglect, physical abuse, emotional abuse, and sexual abuse by parents or caregivers and other adverse events occurring before the age of 18.
Childhood trauma common
Patients with BPD frequently report a history of childhood trauma, noted Dr. Grilo, adding that such trauma is associated with self-harm and suicide attempts. Sociodemographic information, including age, sex, and ethnicity/race, education level, and income, was also gathered.
Investigators examined data on suicide attempts using relatively stringent coding that required serious dysfunction in at least five BPD criteria.
Using this definition, investigators found the lifetime SA prevalence in patients with BPD was 30.4%, and 3.2% for past-year SAs. This compared with a rate of 3.7% for lifetime SAs and 0.2% for past-year SAs in those without a BPD diagnosis.
The authors examined SA rates using diagnostic codes in the NESARC-III that required seriously impaired function in only 1 or 2 BPD criteria. Rates were higher using the 5-criteria definition.
When the researchers excluded the BPD criterion of self-injurious behavior, the prevalence was 28.1% for lifetime and 3.0% for past-year SAs among the BPD group, with corresponding rates of 3.8% and 0.2% in those without a BPD diagnosis.
It’s important to look at this, said Dr. Grilo, as some patients with BPD who engage in self-harm have suicidal intent while others don’t.
“We tested whether BPD had heightened risk for suicide attempts if we eliminated the self-injurious criterion and we found that heightened risk was still there,” he explained.
Looking at individual criteria for BPD, a model that adjusted for sociodemographic characteristics, other psychiatric disorders, age at BPD onset, and history of childhood adverse events uncovered two criteria that were significantly associated with increased odds of SAs.
One was emptiness. For lifetime suicide attempts, the adjusted odds ratio (aOR) was 1.58 (95% confidence interval, 1.16-2.14) and for past-year attempts, the aOR was 1.99 (95% CI, 1.08-3.66).
The second was self-injurious behavior. For lifetime attempts, the aOR was 24.28 (95% CI, 16.83-32.03) and for past-year attempts, the aOR was 19.32 (95% CI, 5.22-71.58).
In a model in which all BPD-specific criteria were entered while excluding self-injurious behavior, the aORs for emptiness were 1.66 (95% CI, 1.23-2.24) for lifetime suicide attempts and 2.45 (95% CI, 1.18-5.08) for past year attempts.
Unlike another recent study that included more than 700 treatment-seeking patients with BPD who were followed for 10 years, the current study did not show significant associations with SAs for two other BPD criteria – identity disturbance and frantic attempts to avoid abandonment.
Dr. Grilo explained this might be because the earlier study included treatment-seeking patients instead of community cases, or because of differences in assessment interviews or other factors.
‘Compelling evidence’
“Our epidemiological sample has much broader generalizability and fewer potential confounds than the clinical treatment-seeking sample,” said Dr. Grilo.
However, he noted that the two studies “converge strongly and provide compelling evidence that BPD is associated with substantially heightened risk for suicide attempts over the lifetime.”
The two studies “also converge in finding that the presence of symptoms such as repeated self-harm and feelings of chronic emptiness are also associated with risk for suicide attempts.”
The new findings highlight the need to ask potentially at-risk patients about feelings of emptiness as well as self-injurious behaviors. Clinicians could, for example, ask: “Have you often felt like your life had no purpose or meaning?” or “Have you often felt empty inside?”
Limitations of the study include reliance on retrospective self-reports and use of lay interviewers, although these interviewers were trained and had an average of 5 years of experience conducting health-related surveys.
Although the study included a representative sample of U.S. adults, the sample did not include groups known to have high rates of suicide and self-harm behaviors, such as institutionalized, incarcerated, or homeless individuals.
In addition, the study did not evaluate severity and duration of BPD, although the authors noted they did adjust for age at BPD onset, this did not alter the findings.
Often misdiagnosed
Commenting on the study, John M. Oldham, MD, Distinguished Emeritus Professor, Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, and past-president, American Psychiatric Association, and an expert on BPD, had high praise for the research.
BPD is often misdiagnosed, Dr. Oldham said in an interview. Many patients seek help from primary care doctors who may label the symptoms as an anxiety disorder or a mood disorder, he said.
Although medications can help treat some BPD symptoms, “the primary, core evidence-based treatment for BPD is psychotherapy,” said Dr. Oldham, who some years ago helped develop evidence-based practice guidelines for BPD.
“It’s a clear and very well-designed study, and I don’t see any major limitations or problems with it,” he said. “The authors kept their focus rigorously on their goals and they used really careful methodology.”
He noted the “huge” numbers of patients included in the data and the relatively large percentage of men (43.7%).
“There’s a general belief that it’s mostly females who have BPD, but that’s not true; it’s females who come to treatment,” said Dr. Oldham.
Requiring that all five criteria lead to seriously impaired functioning “is a much more rigorous diagnostic methodology” than requiring only one or two criteria to lead to such impairment, said Dr. Oldham. “This is really important” and makes it “a much stronger study.”
The finding that self-harm behavior was linked to suicide attempts isn’t that surprising as this association has been well documented, but the finding that chronic emptiness is also predictive of future suicide attempts “is news,” said Dr. Oldham.
“We have not paid enough attention to this criterion in the clinical world or in the research world.”
Dr. Oldham said one patient with BPD gave him an ideal metaphor for emptiness. “She said it’s like there’s just nobody home. Think of it as an empty house that may look fine on the outside but you go inside and nobody lives there; there’s no furniture; no favorite things; no photos; no possessions.”
The authors have “important messages we need to pay attention to, and the main one is to explore this sense of chronic ‘nobody home’ emptiness,” said Dr. Oldham.
Dr. Grilo has reported receiving research grants from the National Institutes of Health; serving as a consultant for Sunovion and Weight Watchers; receiving honoraria for lectures, continuing medical education activities, and presentations at scientific conferences; and receiving royalties from Guilford Press and Taylor & Francis, all outside the submitted work.
A version of this article first appeared on Medscape.com.
Feelings of chronic emptiness and self-injury have been identified as two key risk factors for suicide attempts (SAs) in patients with borderline personality disorder (BPD), a new cross-sectional, nationally representative study suggests.
The findings also show lifetime and past-year SAs are common among patients with BPD, even when excluding self-injurious behaviors.
The results suggest that in addition to asking patients about self-harm during suicide risk screenings and assessments, clinicians should query them about “longstanding” feelings of emptiness, study investigator Carlos M. Grilo, PhD, professor of psychiatry and psychology, Yale University, New Haven, Conn., said in an interview.
Although related, chronic emptiness “is distinct and goes beyond feelings of sadness, loneliness, and hopelessness,” explained Dr. Grilo. he said.
The study was published online May 11 in JAMA Network Open.
Filling a research gap
While BPD and other psychiatric disorders are associated with suicide, the authors noted there is a “dearth of epidemiological research” examining the link between BPD and suicide.
Criteria for BPD diagnosis requires any five of the following criteria: relationships, affective instability, abandonment fear, anger, identity disturbance, emptiness, disassociation/paranoia, self-injurious behavior, and impulsivity, along with social-occupation dysfunction.
To determine SA risk with specific BPD diagnostic criteria, the investigators examined data on 36,309 individuals who participated in the third wave of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-III), conducted from 2012 to 2013.
During computer-assisted, face-to-face interviews, study participants answered questions based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5) of the National Institute on Alcohol Abuse and Alcoholism.
This structured interview assesses a range of DSM-5–defined psychiatric disorders and their criteria. In addition to BPD, the AUDADIS-5 generates diagnoses for mood disorders, anxiety disorders, posttraumatic stress disorder, substance use disorders, antisocial personality disorder, schizotypal disorder, and conduct disorder.
During the interviews, respondents were asked if they had ever attempted suicide. For those who had, interviewers recorded the total number of lifetime attempts.
Participants also answered questions about childhood maltreatment including physical neglect, emotional neglect, physical abuse, emotional abuse, and sexual abuse by parents or caregivers and other adverse events occurring before the age of 18.
Childhood trauma common
Patients with BPD frequently report a history of childhood trauma, noted Dr. Grilo, adding that such trauma is associated with self-harm and suicide attempts. Sociodemographic information, including age, sex, and ethnicity/race, education level, and income, was also gathered.
Investigators examined data on suicide attempts using relatively stringent coding that required serious dysfunction in at least five BPD criteria.
Using this definition, investigators found the lifetime SA prevalence in patients with BPD was 30.4%, and 3.2% for past-year SAs. This compared with a rate of 3.7% for lifetime SAs and 0.2% for past-year SAs in those without a BPD diagnosis.
The authors examined SA rates using diagnostic codes in the NESARC-III that required seriously impaired function in only 1 or 2 BPD criteria. Rates were higher using the 5-criteria definition.
When the researchers excluded the BPD criterion of self-injurious behavior, the prevalence was 28.1% for lifetime and 3.0% for past-year SAs among the BPD group, with corresponding rates of 3.8% and 0.2% in those without a BPD diagnosis.
It’s important to look at this, said Dr. Grilo, as some patients with BPD who engage in self-harm have suicidal intent while others don’t.
“We tested whether BPD had heightened risk for suicide attempts if we eliminated the self-injurious criterion and we found that heightened risk was still there,” he explained.
Looking at individual criteria for BPD, a model that adjusted for sociodemographic characteristics, other psychiatric disorders, age at BPD onset, and history of childhood adverse events uncovered two criteria that were significantly associated with increased odds of SAs.
One was emptiness. For lifetime suicide attempts, the adjusted odds ratio (aOR) was 1.58 (95% confidence interval, 1.16-2.14) and for past-year attempts, the aOR was 1.99 (95% CI, 1.08-3.66).
The second was self-injurious behavior. For lifetime attempts, the aOR was 24.28 (95% CI, 16.83-32.03) and for past-year attempts, the aOR was 19.32 (95% CI, 5.22-71.58).
In a model in which all BPD-specific criteria were entered while excluding self-injurious behavior, the aORs for emptiness were 1.66 (95% CI, 1.23-2.24) for lifetime suicide attempts and 2.45 (95% CI, 1.18-5.08) for past year attempts.
Unlike another recent study that included more than 700 treatment-seeking patients with BPD who were followed for 10 years, the current study did not show significant associations with SAs for two other BPD criteria – identity disturbance and frantic attempts to avoid abandonment.
Dr. Grilo explained this might be because the earlier study included treatment-seeking patients instead of community cases, or because of differences in assessment interviews or other factors.
‘Compelling evidence’
“Our epidemiological sample has much broader generalizability and fewer potential confounds than the clinical treatment-seeking sample,” said Dr. Grilo.
However, he noted that the two studies “converge strongly and provide compelling evidence that BPD is associated with substantially heightened risk for suicide attempts over the lifetime.”
The two studies “also converge in finding that the presence of symptoms such as repeated self-harm and feelings of chronic emptiness are also associated with risk for suicide attempts.”
The new findings highlight the need to ask potentially at-risk patients about feelings of emptiness as well as self-injurious behaviors. Clinicians could, for example, ask: “Have you often felt like your life had no purpose or meaning?” or “Have you often felt empty inside?”
Limitations of the study include reliance on retrospective self-reports and use of lay interviewers, although these interviewers were trained and had an average of 5 years of experience conducting health-related surveys.
Although the study included a representative sample of U.S. adults, the sample did not include groups known to have high rates of suicide and self-harm behaviors, such as institutionalized, incarcerated, or homeless individuals.
In addition, the study did not evaluate severity and duration of BPD, although the authors noted they did adjust for age at BPD onset, this did not alter the findings.
Often misdiagnosed
Commenting on the study, John M. Oldham, MD, Distinguished Emeritus Professor, Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, and past-president, American Psychiatric Association, and an expert on BPD, had high praise for the research.
BPD is often misdiagnosed, Dr. Oldham said in an interview. Many patients seek help from primary care doctors who may label the symptoms as an anxiety disorder or a mood disorder, he said.
Although medications can help treat some BPD symptoms, “the primary, core evidence-based treatment for BPD is psychotherapy,” said Dr. Oldham, who some years ago helped develop evidence-based practice guidelines for BPD.
“It’s a clear and very well-designed study, and I don’t see any major limitations or problems with it,” he said. “The authors kept their focus rigorously on their goals and they used really careful methodology.”
He noted the “huge” numbers of patients included in the data and the relatively large percentage of men (43.7%).
“There’s a general belief that it’s mostly females who have BPD, but that’s not true; it’s females who come to treatment,” said Dr. Oldham.
Requiring that all five criteria lead to seriously impaired functioning “is a much more rigorous diagnostic methodology” than requiring only one or two criteria to lead to such impairment, said Dr. Oldham. “This is really important” and makes it “a much stronger study.”
The finding that self-harm behavior was linked to suicide attempts isn’t that surprising as this association has been well documented, but the finding that chronic emptiness is also predictive of future suicide attempts “is news,” said Dr. Oldham.
“We have not paid enough attention to this criterion in the clinical world or in the research world.”
Dr. Oldham said one patient with BPD gave him an ideal metaphor for emptiness. “She said it’s like there’s just nobody home. Think of it as an empty house that may look fine on the outside but you go inside and nobody lives there; there’s no furniture; no favorite things; no photos; no possessions.”
The authors have “important messages we need to pay attention to, and the main one is to explore this sense of chronic ‘nobody home’ emptiness,” said Dr. Oldham.
Dr. Grilo has reported receiving research grants from the National Institutes of Health; serving as a consultant for Sunovion and Weight Watchers; receiving honoraria for lectures, continuing medical education activities, and presentations at scientific conferences; and receiving royalties from Guilford Press and Taylor & Francis, all outside the submitted work.
A version of this article first appeared on Medscape.com.
45 researchers awarded millions in research funding
The Foundation introduced new awards in the 2021 awards cycle addressing diversity of GI investigators and the need for GI-specific COVID-19 research.
The American Gastroenterological Association is excited to announce the 45 researchers inducted into the 2021 class of AGA Research Foundation Awards Program recipients.
In the 2021 awards cycle, the AGA Research Foundation will provide more than $2.5 million in research funding to investigators working on projects that will further enhance our understanding of gastrointestinal and liver conditions and ultimately lead to the development of better treatment options for digestive diseases patients.
“This year, we made several enhancements to our awards portfolio to address current priorities for AGA and the field – we launched a new COVID-19 research award and established a summer undergraduate research fellowship to introduce talented underrepresented minority students into GI research,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We continue to change our funding program to meet the needs of GI research. What does not change is our long-standing commitment to support the research careers of talented early career investigators.”
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level.
View the full list of recipients online.
The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at http://foundation.gastro.org.
The Foundation introduced new awards in the 2021 awards cycle addressing diversity of GI investigators and the need for GI-specific COVID-19 research.
The American Gastroenterological Association is excited to announce the 45 researchers inducted into the 2021 class of AGA Research Foundation Awards Program recipients.
In the 2021 awards cycle, the AGA Research Foundation will provide more than $2.5 million in research funding to investigators working on projects that will further enhance our understanding of gastrointestinal and liver conditions and ultimately lead to the development of better treatment options for digestive diseases patients.
“This year, we made several enhancements to our awards portfolio to address current priorities for AGA and the field – we launched a new COVID-19 research award and established a summer undergraduate research fellowship to introduce talented underrepresented minority students into GI research,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We continue to change our funding program to meet the needs of GI research. What does not change is our long-standing commitment to support the research careers of talented early career investigators.”
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level.
View the full list of recipients online.
The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at http://foundation.gastro.org.
The Foundation introduced new awards in the 2021 awards cycle addressing diversity of GI investigators and the need for GI-specific COVID-19 research.
The American Gastroenterological Association is excited to announce the 45 researchers inducted into the 2021 class of AGA Research Foundation Awards Program recipients.
In the 2021 awards cycle, the AGA Research Foundation will provide more than $2.5 million in research funding to investigators working on projects that will further enhance our understanding of gastrointestinal and liver conditions and ultimately lead to the development of better treatment options for digestive diseases patients.
“This year, we made several enhancements to our awards portfolio to address current priorities for AGA and the field – we launched a new COVID-19 research award and established a summer undergraduate research fellowship to introduce talented underrepresented minority students into GI research,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We continue to change our funding program to meet the needs of GI research. What does not change is our long-standing commitment to support the research careers of talented early career investigators.”
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level.
View the full list of recipients online.
The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at http://foundation.gastro.org.
Digital GI Corner: Digital navigation to automate patient engagement and reduce procedure no-shows
Patient navigation as a best practice for GI procedures
Colonoscopy is the preferred method for colorectal cancer (CRC) screening. Among scheduled outpatient colonoscopies, key metrics like no-show rates and poor bowel preparation can be as high as 25% in some facilities. These missed appointments and repeated calls with patients have been an important source of wasted resources, poor patient outcomes, and revenue loss for endoscopy facilities (estimated to be up to $1 million dollars for 10-member GI practice).
Studies have shown that patient navigation (PN), a patient-centered approach, overcomes barriers in health care delivery, thus improving adherence to CRC screening. Typically, navigators are specialized health practitioners who fill a variety of functions, including providing updates and instructions to patients, as well as assisting with test-related fears. Despite the overall cost-effectiveness, PN programs require significant resources from hospitals or medical groups. The continued focus in the United States on value-based medicine has provided an urgent need for cost-effective treatments that are also readily available to most physicians.
Digital navigation to automate navigation for colonoscopy and other GI procedures
(see Figure below). Given the widespread use of mobile phones, DN has the ability to change the way doctors and health care providers work. This led to Mount Sinai Health System, New York, conducting a quality improvement program to automate and evaluate the effectiveness of an automated text messaging and web-based “digital navigation” platform for decreasing colonoscopy appointment no-show rates.
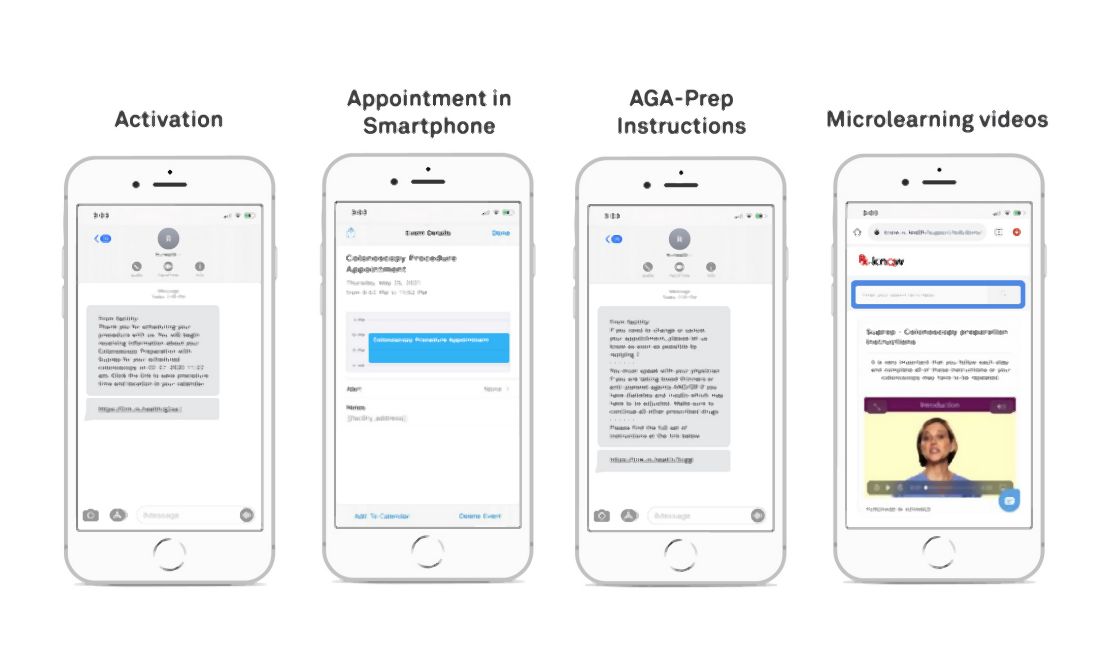
If a valid phone number was available in the patient’s electronic medical record chart and they did not opt out of receiving text message communications from the Mount Sinai Health System, patients over the age of 18 years who were scheduled for a colonoscopy at either of Mount Sinai Hospital, Mount Sinai Morningside, or Mount Sinai West were automatically sent DN SMS messages. The RxUniverse software platform (Rx.Health, New York) was used to send DN content through SMS to all eligible patients. The software platform interfaces with the EMR and endoscopy system (Provation) to automatically extract patient phone number and appointment details.
Impact of digital navigation and patient engagement
This study at Mount Sinai Health System demonstrated that patient engagement with SMS-based navigation is strongly predictive of colonoscopy completion. Patients with high engagement with digital navigation are about four times more likely to complete colonoscopy. Of all covariates included in the model, high DN engagement level had the largest effect size (odds ratio, 3.97), compared with no engagement. For health systems with patient navigators, targeting patients who are unlikely to engage DN or are low-engagers may be a more efficient use of person-to-person navigation.
Value-based reimbursement and cost-effectiveness have emerged as core principles in American health care reform, possibly requiring the creation of affordable, cost-effective approaches. Our research at Mount Sinai Health System suggested that SMS-based navigation can be a potential cost-effective strategy for reducing no-show rates. Beyond appointment no-shows, adequate bowel preparation is another important component of the preprocedure navigation process. Insufficient bowel preparation requires a repeat procedure, as poor visualization of the colon results in reduced therapeutic benefit from screening colonoscopy. We’ve shown in previous studies that our DN platform can increase bowel preparation efficiency, which results in lower rates of aborted procedures.
Missed colonoscopies not only cause longer wait times for patients, but they also cost the average facility $725 a day in lost revenue. It has been found through studies that traditional PN is cost-effective, with additional revenue generated from increased colonoscopy completion rates exceeding the costs of program implementation. While formal cost analyses have not been conducted on DN, estimates have shown around $1 million in annual savings for an average ambulatory surgery center or 10-member GI practice.
Looking ahead: AGA digital transformation network
After positive results for the Rx.Health’s platform were seen at Mount Sinai Health System, the American Gastroenterological Association partnered with Rx.Health to provide the GI community with a GI endoscopy transformation network. The core purpose of this endoscopy transformation network is to take an evidence-based approach and use digital medicine to positively affect key metrics and safety around periprocedural care and support “procedure bundles.” To illustrate the specific case of colonoscopy, these included the following: enhancing colorectal cancer surveillance rates though a comprehensive screening test strategy, decreasing no-show rates through shared decision-making and better preprocedure engagement, improving rates of adequate bowel preparation, benchmarking safety of procedures nationwide, and ensuring patient satisfaction and adequate recall for repeat procedures. These metrics represent key sources of revenue loss for provider organizations and, more importantly, have negative implications on patient care.
This collaboration is now supporting the implementation and expansion of the digital navigation program to all GI procedures at more than 15 different sites across the country.
Dr. Atreja is an adjunct associate professor at the Icahn School of Medicine at Mount Sinai, New York, and chief information officer and chief digital health officer at UC Davis Medical Center, Sacramento. The Icahn School of Medicine has licensed technology to Rx.Health. Dr. Atreja has no other conflicts to disclose
Patient navigation as a best practice for GI procedures
Colonoscopy is the preferred method for colorectal cancer (CRC) screening. Among scheduled outpatient colonoscopies, key metrics like no-show rates and poor bowel preparation can be as high as 25% in some facilities. These missed appointments and repeated calls with patients have been an important source of wasted resources, poor patient outcomes, and revenue loss for endoscopy facilities (estimated to be up to $1 million dollars for 10-member GI practice).
Studies have shown that patient navigation (PN), a patient-centered approach, overcomes barriers in health care delivery, thus improving adherence to CRC screening. Typically, navigators are specialized health practitioners who fill a variety of functions, including providing updates and instructions to patients, as well as assisting with test-related fears. Despite the overall cost-effectiveness, PN programs require significant resources from hospitals or medical groups. The continued focus in the United States on value-based medicine has provided an urgent need for cost-effective treatments that are also readily available to most physicians.
Digital navigation to automate navigation for colonoscopy and other GI procedures
(see Figure below). Given the widespread use of mobile phones, DN has the ability to change the way doctors and health care providers work. This led to Mount Sinai Health System, New York, conducting a quality improvement program to automate and evaluate the effectiveness of an automated text messaging and web-based “digital navigation” platform for decreasing colonoscopy appointment no-show rates.

If a valid phone number was available in the patient’s electronic medical record chart and they did not opt out of receiving text message communications from the Mount Sinai Health System, patients over the age of 18 years who were scheduled for a colonoscopy at either of Mount Sinai Hospital, Mount Sinai Morningside, or Mount Sinai West were automatically sent DN SMS messages. The RxUniverse software platform (Rx.Health, New York) was used to send DN content through SMS to all eligible patients. The software platform interfaces with the EMR and endoscopy system (Provation) to automatically extract patient phone number and appointment details.
Impact of digital navigation and patient engagement
This study at Mount Sinai Health System demonstrated that patient engagement with SMS-based navigation is strongly predictive of colonoscopy completion. Patients with high engagement with digital navigation are about four times more likely to complete colonoscopy. Of all covariates included in the model, high DN engagement level had the largest effect size (odds ratio, 3.97), compared with no engagement. For health systems with patient navigators, targeting patients who are unlikely to engage DN or are low-engagers may be a more efficient use of person-to-person navigation.
Value-based reimbursement and cost-effectiveness have emerged as core principles in American health care reform, possibly requiring the creation of affordable, cost-effective approaches. Our research at Mount Sinai Health System suggested that SMS-based navigation can be a potential cost-effective strategy for reducing no-show rates. Beyond appointment no-shows, adequate bowel preparation is another important component of the preprocedure navigation process. Insufficient bowel preparation requires a repeat procedure, as poor visualization of the colon results in reduced therapeutic benefit from screening colonoscopy. We’ve shown in previous studies that our DN platform can increase bowel preparation efficiency, which results in lower rates of aborted procedures.
Missed colonoscopies not only cause longer wait times for patients, but they also cost the average facility $725 a day in lost revenue. It has been found through studies that traditional PN is cost-effective, with additional revenue generated from increased colonoscopy completion rates exceeding the costs of program implementation. While formal cost analyses have not been conducted on DN, estimates have shown around $1 million in annual savings for an average ambulatory surgery center or 10-member GI practice.
Looking ahead: AGA digital transformation network
After positive results for the Rx.Health’s platform were seen at Mount Sinai Health System, the American Gastroenterological Association partnered with Rx.Health to provide the GI community with a GI endoscopy transformation network. The core purpose of this endoscopy transformation network is to take an evidence-based approach and use digital medicine to positively affect key metrics and safety around periprocedural care and support “procedure bundles.” To illustrate the specific case of colonoscopy, these included the following: enhancing colorectal cancer surveillance rates though a comprehensive screening test strategy, decreasing no-show rates through shared decision-making and better preprocedure engagement, improving rates of adequate bowel preparation, benchmarking safety of procedures nationwide, and ensuring patient satisfaction and adequate recall for repeat procedures. These metrics represent key sources of revenue loss for provider organizations and, more importantly, have negative implications on patient care.
This collaboration is now supporting the implementation and expansion of the digital navigation program to all GI procedures at more than 15 different sites across the country.
Dr. Atreja is an adjunct associate professor at the Icahn School of Medicine at Mount Sinai, New York, and chief information officer and chief digital health officer at UC Davis Medical Center, Sacramento. The Icahn School of Medicine has licensed technology to Rx.Health. Dr. Atreja has no other conflicts to disclose
Patient navigation as a best practice for GI procedures
Colonoscopy is the preferred method for colorectal cancer (CRC) screening. Among scheduled outpatient colonoscopies, key metrics like no-show rates and poor bowel preparation can be as high as 25% in some facilities. These missed appointments and repeated calls with patients have been an important source of wasted resources, poor patient outcomes, and revenue loss for endoscopy facilities (estimated to be up to $1 million dollars for 10-member GI practice).
Studies have shown that patient navigation (PN), a patient-centered approach, overcomes barriers in health care delivery, thus improving adherence to CRC screening. Typically, navigators are specialized health practitioners who fill a variety of functions, including providing updates and instructions to patients, as well as assisting with test-related fears. Despite the overall cost-effectiveness, PN programs require significant resources from hospitals or medical groups. The continued focus in the United States on value-based medicine has provided an urgent need for cost-effective treatments that are also readily available to most physicians.
Digital navigation to automate navigation for colonoscopy and other GI procedures
(see Figure below). Given the widespread use of mobile phones, DN has the ability to change the way doctors and health care providers work. This led to Mount Sinai Health System, New York, conducting a quality improvement program to automate and evaluate the effectiveness of an automated text messaging and web-based “digital navigation” platform for decreasing colonoscopy appointment no-show rates.

If a valid phone number was available in the patient’s electronic medical record chart and they did not opt out of receiving text message communications from the Mount Sinai Health System, patients over the age of 18 years who were scheduled for a colonoscopy at either of Mount Sinai Hospital, Mount Sinai Morningside, or Mount Sinai West were automatically sent DN SMS messages. The RxUniverse software platform (Rx.Health, New York) was used to send DN content through SMS to all eligible patients. The software platform interfaces with the EMR and endoscopy system (Provation) to automatically extract patient phone number and appointment details.
Impact of digital navigation and patient engagement
This study at Mount Sinai Health System demonstrated that patient engagement with SMS-based navigation is strongly predictive of colonoscopy completion. Patients with high engagement with digital navigation are about four times more likely to complete colonoscopy. Of all covariates included in the model, high DN engagement level had the largest effect size (odds ratio, 3.97), compared with no engagement. For health systems with patient navigators, targeting patients who are unlikely to engage DN or are low-engagers may be a more efficient use of person-to-person navigation.
Value-based reimbursement and cost-effectiveness have emerged as core principles in American health care reform, possibly requiring the creation of affordable, cost-effective approaches. Our research at Mount Sinai Health System suggested that SMS-based navigation can be a potential cost-effective strategy for reducing no-show rates. Beyond appointment no-shows, adequate bowel preparation is another important component of the preprocedure navigation process. Insufficient bowel preparation requires a repeat procedure, as poor visualization of the colon results in reduced therapeutic benefit from screening colonoscopy. We’ve shown in previous studies that our DN platform can increase bowel preparation efficiency, which results in lower rates of aborted procedures.
Missed colonoscopies not only cause longer wait times for patients, but they also cost the average facility $725 a day in lost revenue. It has been found through studies that traditional PN is cost-effective, with additional revenue generated from increased colonoscopy completion rates exceeding the costs of program implementation. While formal cost analyses have not been conducted on DN, estimates have shown around $1 million in annual savings for an average ambulatory surgery center or 10-member GI practice.
Looking ahead: AGA digital transformation network
After positive results for the Rx.Health’s platform were seen at Mount Sinai Health System, the American Gastroenterological Association partnered with Rx.Health to provide the GI community with a GI endoscopy transformation network. The core purpose of this endoscopy transformation network is to take an evidence-based approach and use digital medicine to positively affect key metrics and safety around periprocedural care and support “procedure bundles.” To illustrate the specific case of colonoscopy, these included the following: enhancing colorectal cancer surveillance rates though a comprehensive screening test strategy, decreasing no-show rates through shared decision-making and better preprocedure engagement, improving rates of adequate bowel preparation, benchmarking safety of procedures nationwide, and ensuring patient satisfaction and adequate recall for repeat procedures. These metrics represent key sources of revenue loss for provider organizations and, more importantly, have negative implications on patient care.
This collaboration is now supporting the implementation and expansion of the digital navigation program to all GI procedures at more than 15 different sites across the country.
Dr. Atreja is an adjunct associate professor at the Icahn School of Medicine at Mount Sinai, New York, and chief information officer and chief digital health officer at UC Davis Medical Center, Sacramento. The Icahn School of Medicine has licensed technology to Rx.Health. Dr. Atreja has no other conflicts to disclose
Novel immunotherapy relatlimab in advanced melanoma
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
Cervical cancer rates fall, but other HPV cancers increase
Cervical cancer incidence in the United States decreased by about 1% per year from 2001 to 2017, but at the same time there was an increase in the incidence of other human papillomavirus (HPV)–related cancers, a new study reveals.
Over the same period, there was an overall 1.3% annual increase in oropharyngeal, anal, rectal, and vulvar cancers in women, and a 2.3% annual increase in these cancers in men.
HPV is associated with more than 90% of cervical cancers and between 60% and 75% of oropharyngeal, vulvar, vaginal, and penile cancer in the United States, the researchers noted.
Oropharyngeal cancer incidence increased by 2.3% overall, with a 2.7% increase in men and a 0.77% increase in women. The incidence of this cancer was nearly fivefold greater in men at 8.89 per 100,000 population versus 1.68 per 100,000 population for women, the study found.
In addition, among women over age 50 years, anal and rectal cancer incidence increased by 3.5% per year; at the same time, cervical cancer incidence decreased 1.5% per year.
The increase in the incidence of oropharyngeal cancer and in anal and rectal cancers is expected to continue, the authors said.
The data showing these new trends come from an analysis of 657,317 individuals obtained from the U.S. Cancer Statistics program, conducted by Cheng-I Liao, MD, of Kaohsiung (Taiwan) Veterans Hospital and colleagues.
The study was highlighted at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented June 6.
These incidence trends may reflect the availability of clear guidelines for screening and vaccination for the prevention of HPV-related cervical cancer – and the dearth of guidelines and standardized screening and vaccination for the other HPV-related cancers, the authors said.
The team also found cervical cancer accounted for 52% of all HPV-related cancers during the study period. The decrease in the incidence of cervical cancer over time was greater among women aged 20-24 (4.6% per year), compared with those aged 25-29 years (1.6%) and 30-34 years (1.1%),
Dr. Liao speculated that this age-based difference suggests a potential effect of HPV vaccination, greater vaccine acceptance among younger women, and clear guidelines for screening and vaccination.
However, an expert approached for comment was not so sure. It is likely too soon to give HPV vaccination too much credit for lower cervical cancer rates, said Jennifer Young Pierce, MD, MPH, a gynecologic oncologist at the Mitchell Cancer Institute, University of South Alabama, Mobile.
The continued rise in HPV-related cancers other than cervical cancers supports the point that screening – rather than vaccination – accounts for much of the decline observed in cervical cancer incidence, Dr. Pierce said in an interview.
Vaccination in men lags behind that of women, and there is a lack of good screening methods for head and neck cancers, she explained.
“When we have both vaccination and screening in these other cancers at high rates, we’re going to see significant declines in those cancers also,” she said.
“I’m very excited by the data but I do not believe it is related to vaccination as a method of prevention,” said Dr. Pierce, a professor of interdisciplinary clinical oncology who has been involved in numerous HPV vaccine–related studies and initiatives to improve vaccine uptake since its approval in 2006.
HPV vaccination
The HPV vaccine was first approved for preventing HPV-related cervical cancer in 2006 with an indication for girls and women aged 9-26 years. The vaccine indication was expanded in 2011 to include boys aged 11-12 years and is now approved for those up to age 45 years.
However, neither standardized screening nor HPV vaccination is currently recommended for any HPV-related cancer other than cervical cancer, Dr. Liao said.
Vaccination during much of the current study time frame (2001-2017) didn’t apply to most of the people who got cancer, Dr. Pierce explained in an interview, noting that the vaccinated individuals “still aren’t old enough to be part of the group we’re talking about.”
Rather, the increased use of HPV screening along with Pap testing for cervical cancer was becoming much more widespread at the time and was likely picking up more precancerous lesions – and thereby helping to decrease cervical cancer incidence in women in their 40s, 50s, 60s, and 70s, she said.
Dr. Pierce does, however, credit the vaccine movement for improving awareness of HPV risk.
“It has done a great job of educating the population about the dangers of these cancers ... and that there’s more we can do to prevent them,” she said.
Like Dr. Liao, she stressed the need for research focused on finding more effective screening modalities and on vaccine efficacy.
Also commenting on the study, ASCO president Lori J. Pierce, MD, a radiation oncologist, professor, and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, said the findings underscore the need for ongoing exploration of potential strategies such as HPV screening for high-risk populations.
“We can pick out higher risk populations so it would make sense to do a screen,” she said.
“Clearly, this study shows that we still have a great deal of work to do in order to reverse the increasing incidence rates of other HPV-related cancers,” she added in a press statement.
In an interview prior to the press conference, Dr. Pierce said in an interview that the findings are important because the outcome “opens all of our eyes into the trends of HPV-related cancers in the United States.
“This is something that hasn’t been studied well over time,” she added, noting that, where guidelines do exist for HPV-related cancers other than cervical cancer, they are inconsistent.
Further, it is possible that the vaccine will “cover a significant portion of the etiologic viruses that cause these cancers,” thereby helping to prevent the other HPV-related cancers.
For that reason, additional research and strategies for overcoming vaccine hesitancy, increasing overall vaccination rates, and for developing consistent guidelines are needed.
“I think there needs to be further resources and research to address the lack of screening for these other HPV-related cancers and we need to have consistent vaccination guidelines, because these cancers are preventable,” she said
Dr. Liao and Dr. Pierce disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cervical cancer incidence in the United States decreased by about 1% per year from 2001 to 2017, but at the same time there was an increase in the incidence of other human papillomavirus (HPV)–related cancers, a new study reveals.
Over the same period, there was an overall 1.3% annual increase in oropharyngeal, anal, rectal, and vulvar cancers in women, and a 2.3% annual increase in these cancers in men.
HPV is associated with more than 90% of cervical cancers and between 60% and 75% of oropharyngeal, vulvar, vaginal, and penile cancer in the United States, the researchers noted.
Oropharyngeal cancer incidence increased by 2.3% overall, with a 2.7% increase in men and a 0.77% increase in women. The incidence of this cancer was nearly fivefold greater in men at 8.89 per 100,000 population versus 1.68 per 100,000 population for women, the study found.
In addition, among women over age 50 years, anal and rectal cancer incidence increased by 3.5% per year; at the same time, cervical cancer incidence decreased 1.5% per year.
The increase in the incidence of oropharyngeal cancer and in anal and rectal cancers is expected to continue, the authors said.
The data showing these new trends come from an analysis of 657,317 individuals obtained from the U.S. Cancer Statistics program, conducted by Cheng-I Liao, MD, of Kaohsiung (Taiwan) Veterans Hospital and colleagues.
The study was highlighted at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented June 6.
These incidence trends may reflect the availability of clear guidelines for screening and vaccination for the prevention of HPV-related cervical cancer – and the dearth of guidelines and standardized screening and vaccination for the other HPV-related cancers, the authors said.
The team also found cervical cancer accounted for 52% of all HPV-related cancers during the study period. The decrease in the incidence of cervical cancer over time was greater among women aged 20-24 (4.6% per year), compared with those aged 25-29 years (1.6%) and 30-34 years (1.1%),
Dr. Liao speculated that this age-based difference suggests a potential effect of HPV vaccination, greater vaccine acceptance among younger women, and clear guidelines for screening and vaccination.
However, an expert approached for comment was not so sure. It is likely too soon to give HPV vaccination too much credit for lower cervical cancer rates, said Jennifer Young Pierce, MD, MPH, a gynecologic oncologist at the Mitchell Cancer Institute, University of South Alabama, Mobile.
The continued rise in HPV-related cancers other than cervical cancers supports the point that screening – rather than vaccination – accounts for much of the decline observed in cervical cancer incidence, Dr. Pierce said in an interview.
Vaccination in men lags behind that of women, and there is a lack of good screening methods for head and neck cancers, she explained.
“When we have both vaccination and screening in these other cancers at high rates, we’re going to see significant declines in those cancers also,” she said.
“I’m very excited by the data but I do not believe it is related to vaccination as a method of prevention,” said Dr. Pierce, a professor of interdisciplinary clinical oncology who has been involved in numerous HPV vaccine–related studies and initiatives to improve vaccine uptake since its approval in 2006.
HPV vaccination
The HPV vaccine was first approved for preventing HPV-related cervical cancer in 2006 with an indication for girls and women aged 9-26 years. The vaccine indication was expanded in 2011 to include boys aged 11-12 years and is now approved for those up to age 45 years.
However, neither standardized screening nor HPV vaccination is currently recommended for any HPV-related cancer other than cervical cancer, Dr. Liao said.
Vaccination during much of the current study time frame (2001-2017) didn’t apply to most of the people who got cancer, Dr. Pierce explained in an interview, noting that the vaccinated individuals “still aren’t old enough to be part of the group we’re talking about.”
Rather, the increased use of HPV screening along with Pap testing for cervical cancer was becoming much more widespread at the time and was likely picking up more precancerous lesions – and thereby helping to decrease cervical cancer incidence in women in their 40s, 50s, 60s, and 70s, she said.
Dr. Pierce does, however, credit the vaccine movement for improving awareness of HPV risk.
“It has done a great job of educating the population about the dangers of these cancers ... and that there’s more we can do to prevent them,” she said.
Like Dr. Liao, she stressed the need for research focused on finding more effective screening modalities and on vaccine efficacy.
Also commenting on the study, ASCO president Lori J. Pierce, MD, a radiation oncologist, professor, and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, said the findings underscore the need for ongoing exploration of potential strategies such as HPV screening for high-risk populations.
“We can pick out higher risk populations so it would make sense to do a screen,” she said.
“Clearly, this study shows that we still have a great deal of work to do in order to reverse the increasing incidence rates of other HPV-related cancers,” she added in a press statement.
In an interview prior to the press conference, Dr. Pierce said in an interview that the findings are important because the outcome “opens all of our eyes into the trends of HPV-related cancers in the United States.
“This is something that hasn’t been studied well over time,” she added, noting that, where guidelines do exist for HPV-related cancers other than cervical cancer, they are inconsistent.
Further, it is possible that the vaccine will “cover a significant portion of the etiologic viruses that cause these cancers,” thereby helping to prevent the other HPV-related cancers.
For that reason, additional research and strategies for overcoming vaccine hesitancy, increasing overall vaccination rates, and for developing consistent guidelines are needed.
“I think there needs to be further resources and research to address the lack of screening for these other HPV-related cancers and we need to have consistent vaccination guidelines, because these cancers are preventable,” she said
Dr. Liao and Dr. Pierce disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cervical cancer incidence in the United States decreased by about 1% per year from 2001 to 2017, but at the same time there was an increase in the incidence of other human papillomavirus (HPV)–related cancers, a new study reveals.
Over the same period, there was an overall 1.3% annual increase in oropharyngeal, anal, rectal, and vulvar cancers in women, and a 2.3% annual increase in these cancers in men.
HPV is associated with more than 90% of cervical cancers and between 60% and 75% of oropharyngeal, vulvar, vaginal, and penile cancer in the United States, the researchers noted.
Oropharyngeal cancer incidence increased by 2.3% overall, with a 2.7% increase in men and a 0.77% increase in women. The incidence of this cancer was nearly fivefold greater in men at 8.89 per 100,000 population versus 1.68 per 100,000 population for women, the study found.
In addition, among women over age 50 years, anal and rectal cancer incidence increased by 3.5% per year; at the same time, cervical cancer incidence decreased 1.5% per year.
The increase in the incidence of oropharyngeal cancer and in anal and rectal cancers is expected to continue, the authors said.
The data showing these new trends come from an analysis of 657,317 individuals obtained from the U.S. Cancer Statistics program, conducted by Cheng-I Liao, MD, of Kaohsiung (Taiwan) Veterans Hospital and colleagues.
The study was highlighted at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology, where the study will be presented June 6.
These incidence trends may reflect the availability of clear guidelines for screening and vaccination for the prevention of HPV-related cervical cancer – and the dearth of guidelines and standardized screening and vaccination for the other HPV-related cancers, the authors said.
The team also found cervical cancer accounted for 52% of all HPV-related cancers during the study period. The decrease in the incidence of cervical cancer over time was greater among women aged 20-24 (4.6% per year), compared with those aged 25-29 years (1.6%) and 30-34 years (1.1%),
Dr. Liao speculated that this age-based difference suggests a potential effect of HPV vaccination, greater vaccine acceptance among younger women, and clear guidelines for screening and vaccination.
However, an expert approached for comment was not so sure. It is likely too soon to give HPV vaccination too much credit for lower cervical cancer rates, said Jennifer Young Pierce, MD, MPH, a gynecologic oncologist at the Mitchell Cancer Institute, University of South Alabama, Mobile.
The continued rise in HPV-related cancers other than cervical cancers supports the point that screening – rather than vaccination – accounts for much of the decline observed in cervical cancer incidence, Dr. Pierce said in an interview.
Vaccination in men lags behind that of women, and there is a lack of good screening methods for head and neck cancers, she explained.
“When we have both vaccination and screening in these other cancers at high rates, we’re going to see significant declines in those cancers also,” she said.
“I’m very excited by the data but I do not believe it is related to vaccination as a method of prevention,” said Dr. Pierce, a professor of interdisciplinary clinical oncology who has been involved in numerous HPV vaccine–related studies and initiatives to improve vaccine uptake since its approval in 2006.
HPV vaccination
The HPV vaccine was first approved for preventing HPV-related cervical cancer in 2006 with an indication for girls and women aged 9-26 years. The vaccine indication was expanded in 2011 to include boys aged 11-12 years and is now approved for those up to age 45 years.
However, neither standardized screening nor HPV vaccination is currently recommended for any HPV-related cancer other than cervical cancer, Dr. Liao said.
Vaccination during much of the current study time frame (2001-2017) didn’t apply to most of the people who got cancer, Dr. Pierce explained in an interview, noting that the vaccinated individuals “still aren’t old enough to be part of the group we’re talking about.”
Rather, the increased use of HPV screening along with Pap testing for cervical cancer was becoming much more widespread at the time and was likely picking up more precancerous lesions – and thereby helping to decrease cervical cancer incidence in women in their 40s, 50s, 60s, and 70s, she said.
Dr. Pierce does, however, credit the vaccine movement for improving awareness of HPV risk.
“It has done a great job of educating the population about the dangers of these cancers ... and that there’s more we can do to prevent them,” she said.
Like Dr. Liao, she stressed the need for research focused on finding more effective screening modalities and on vaccine efficacy.
Also commenting on the study, ASCO president Lori J. Pierce, MD, a radiation oncologist, professor, and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, said the findings underscore the need for ongoing exploration of potential strategies such as HPV screening for high-risk populations.
“We can pick out higher risk populations so it would make sense to do a screen,” she said.
“Clearly, this study shows that we still have a great deal of work to do in order to reverse the increasing incidence rates of other HPV-related cancers,” she added in a press statement.
In an interview prior to the press conference, Dr. Pierce said in an interview that the findings are important because the outcome “opens all of our eyes into the trends of HPV-related cancers in the United States.
“This is something that hasn’t been studied well over time,” she added, noting that, where guidelines do exist for HPV-related cancers other than cervical cancer, they are inconsistent.
Further, it is possible that the vaccine will “cover a significant portion of the etiologic viruses that cause these cancers,” thereby helping to prevent the other HPV-related cancers.
For that reason, additional research and strategies for overcoming vaccine hesitancy, increasing overall vaccination rates, and for developing consistent guidelines are needed.
“I think there needs to be further resources and research to address the lack of screening for these other HPV-related cancers and we need to have consistent vaccination guidelines, because these cancers are preventable,” she said
Dr. Liao and Dr. Pierce disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ID experts dole out practical advice to help with mask confusion
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
Sardines linked to reduced type 2 diabetes risk
Older people with prediabetes who followed a diet rich in sardines for 1 year show significant reductions in risk of developing type 2 diabetes compared with those placed on a similarly healthy diet but without the sardines, results from a new randomized trial show.
“A 1-year, sardine-enriched type 2 diabetes-preventive diet in an elderly population with prediabetes exerts a greater protective effect against developing type 2 diabetes and cardiovascular events, by improving anthropometric parameters, blood chemistry profile, lipid composition in erythrocytes membranes, and metabolomics data,” report the authors in research published in Clinical Nutrition by Diana Díaz-Rizzolo, PhD, of the Hospital Clinic of Barcelona, Spain, and colleagues.
While cardiovascular and other health benefits of unsaturated fats in oily fish are well-established and are a key component in diets such as the highly recommended Mediterranean diet, the authors note that the consumption of sardines for the prevention of type 2 diabetes has not previously been studied.
In addition to being rich in healthy omega-3 fatty acids, sardines have high concentrations of taurine – approximately 147 mg per 100 g serving – which, depending on the sardine species, is believed to have hypoglycemic, antioxidant, and anti-inflammatory benefits, the authors note.
Participants advised to consume the whole sardine, bones and all
To evaluate the effects, researchers enrolled 152 patients aged 65 and older who had been diagnosed with prediabetes (blood glucose levels between 100-124 mg/dL) and placed them all on a nutritional program to reduce the risk of diabetes for 1 year.
In addition, about half (n = 75) were also instructed to consume 200 g of canned sardines in olive oil per week, in 100 g servings consumed twice per week.
Those participants were recommended to consume the entire sardine, without removal of bones, due to their rich content of calcium and vitamin D. They were also provided with recipes that used canned sardines.
At 1 year, the percentage of participants classified as being at a very high risk of type 2 diabetes, assessed by the Finnish Diabetes Risk Score (FINDRISC), compared with baseline, had declined to a much greater degree in the sardine consumption group (37% at baseline vs. 8% at 1 year) compared with those in the control group, who only consumed the nutritional diet (27% vs. 22%) (P = .021).
In addition, those in the sardine group had greater increases in healthy HDL cholesterol and the glucose-regulating protein hormone adiponectin, with decreases in triglycerides compared with the nonsardine group (all P < .005).
Furthermore, the sardine consumption group had a greater decrease in insulin resistance, assessed by Homeostatic Model Assessment for Insulin Resistance (HOMA-IR; P = .032).
Sardines are cheap and reduce blood pressure too
“Not only are sardines reasonably priced and easy to find, but they are safe and help to prevent the onset of type 2 diabetes,” said Dr. Díaz-Rizzolo in a press statement.
Those in the sardine group also showed significant decreases in systolic blood pressure (P = .014) and diastolic blood pressure (P = .020) versus baseline, while no significant changes were observed in the control group. The authors suggest that sardines’ rich taurine concentrations could play a role in those effects.
“Previously, only lean fish consumption had demonstrated an improvement in blood pressure, not fatty fish consumption, perhaps because the species studied excluded those with a higher taurine content such as sardines,” they speculate.
In addition to showing improvements in levels of taurine, those in the sardine group also showed increases in nutrients that have been linked to health benefits, including omega-3 EPA and DHA, vitamin D, and fluorine (all P < .05).
The authors note that the increases could be attributed to sardines’ rich concentration of those nutrients, as well as to the olive oil that is present in the sardine can.
Some benefits seen in both groups
The patients in the study were a mean age of 71 and had been in a prediabetic state for an average of 4.8 years at the beginning of the study. They were 55% male and there were no other significant differences in characteristics between the groups.
While the conversion from being prediabetic to type 2 diabetes in the adult population has been reported to be about 10.6%, and the risk has been observed to be even higher in the 65 and older population, rates were lower than that in both groups.
“At the end of our 1-year study, we observed a [rate of] new-onset type 2 diabetes of 2.7% and 5.2% in the sardine group and control group, respectively,” the authors note. They add the differences were not statistically significant.
Both the sardine consumption and control groups showed significant reductions in A1c versus baseline (P = .011 and P = .010, respectively), as well as significant reductions in glucose fasting concentrations (P = .020 and P = .040, respectively).
And while the sardine group showed greater improvements in HDL versus the control group (P = .045), only the control group showed a significant decrease in total cholesterol versus baseline (P = .032).
Both groups showed improvements in the management of body weight, body mass index, and waist and hip circumference, in addition to improvement in body composition – despite no physical activity components in the programs, the authors note.
“This is probably because both groups followed the same base type 2 diabetes-preventive diet, with the one exception of sardine supplementation, and, although they did not modify their physical activity, both groups reduced their daily caloric intake through food,” the authors note.
The possibility of reducing diabetes risk through dietary changes as opposed to weight loss is especially important in the older population, the authors note, as some studies suggest a link between weight loss in the elderly and an increased risk of mortality.
In a second phase of the study, the researchers say they are evaluating the effect of sardines on the intestinal microbiota, “since it affects the regulation of many biological processes, and we need to understand if they have played a part in this protective effect against type 2 diabetes,” Dr. Díaz-Rizzolo concluded.
The study was funded by RecerCaixa 2013. The authors report that “no industry sponsorship was received for this work that could have influenced its outcome.”
A version of this article first appeared on Medscape.com.
Older people with prediabetes who followed a diet rich in sardines for 1 year show significant reductions in risk of developing type 2 diabetes compared with those placed on a similarly healthy diet but without the sardines, results from a new randomized trial show.
“A 1-year, sardine-enriched type 2 diabetes-preventive diet in an elderly population with prediabetes exerts a greater protective effect against developing type 2 diabetes and cardiovascular events, by improving anthropometric parameters, blood chemistry profile, lipid composition in erythrocytes membranes, and metabolomics data,” report the authors in research published in Clinical Nutrition by Diana Díaz-Rizzolo, PhD, of the Hospital Clinic of Barcelona, Spain, and colleagues.
While cardiovascular and other health benefits of unsaturated fats in oily fish are well-established and are a key component in diets such as the highly recommended Mediterranean diet, the authors note that the consumption of sardines for the prevention of type 2 diabetes has not previously been studied.
In addition to being rich in healthy omega-3 fatty acids, sardines have high concentrations of taurine – approximately 147 mg per 100 g serving – which, depending on the sardine species, is believed to have hypoglycemic, antioxidant, and anti-inflammatory benefits, the authors note.
Participants advised to consume the whole sardine, bones and all
To evaluate the effects, researchers enrolled 152 patients aged 65 and older who had been diagnosed with prediabetes (blood glucose levels between 100-124 mg/dL) and placed them all on a nutritional program to reduce the risk of diabetes for 1 year.
In addition, about half (n = 75) were also instructed to consume 200 g of canned sardines in olive oil per week, in 100 g servings consumed twice per week.
Those participants were recommended to consume the entire sardine, without removal of bones, due to their rich content of calcium and vitamin D. They were also provided with recipes that used canned sardines.
At 1 year, the percentage of participants classified as being at a very high risk of type 2 diabetes, assessed by the Finnish Diabetes Risk Score (FINDRISC), compared with baseline, had declined to a much greater degree in the sardine consumption group (37% at baseline vs. 8% at 1 year) compared with those in the control group, who only consumed the nutritional diet (27% vs. 22%) (P = .021).
In addition, those in the sardine group had greater increases in healthy HDL cholesterol and the glucose-regulating protein hormone adiponectin, with decreases in triglycerides compared with the nonsardine group (all P < .005).
Furthermore, the sardine consumption group had a greater decrease in insulin resistance, assessed by Homeostatic Model Assessment for Insulin Resistance (HOMA-IR; P = .032).
Sardines are cheap and reduce blood pressure too
“Not only are sardines reasonably priced and easy to find, but they are safe and help to prevent the onset of type 2 diabetes,” said Dr. Díaz-Rizzolo in a press statement.
Those in the sardine group also showed significant decreases in systolic blood pressure (P = .014) and diastolic blood pressure (P = .020) versus baseline, while no significant changes were observed in the control group. The authors suggest that sardines’ rich taurine concentrations could play a role in those effects.
“Previously, only lean fish consumption had demonstrated an improvement in blood pressure, not fatty fish consumption, perhaps because the species studied excluded those with a higher taurine content such as sardines,” they speculate.
In addition to showing improvements in levels of taurine, those in the sardine group also showed increases in nutrients that have been linked to health benefits, including omega-3 EPA and DHA, vitamin D, and fluorine (all P < .05).
The authors note that the increases could be attributed to sardines’ rich concentration of those nutrients, as well as to the olive oil that is present in the sardine can.
Some benefits seen in both groups
The patients in the study were a mean age of 71 and had been in a prediabetic state for an average of 4.8 years at the beginning of the study. They were 55% male and there were no other significant differences in characteristics between the groups.
While the conversion from being prediabetic to type 2 diabetes in the adult population has been reported to be about 10.6%, and the risk has been observed to be even higher in the 65 and older population, rates were lower than that in both groups.
“At the end of our 1-year study, we observed a [rate of] new-onset type 2 diabetes of 2.7% and 5.2% in the sardine group and control group, respectively,” the authors note. They add the differences were not statistically significant.
Both the sardine consumption and control groups showed significant reductions in A1c versus baseline (P = .011 and P = .010, respectively), as well as significant reductions in glucose fasting concentrations (P = .020 and P = .040, respectively).
And while the sardine group showed greater improvements in HDL versus the control group (P = .045), only the control group showed a significant decrease in total cholesterol versus baseline (P = .032).
Both groups showed improvements in the management of body weight, body mass index, and waist and hip circumference, in addition to improvement in body composition – despite no physical activity components in the programs, the authors note.
“This is probably because both groups followed the same base type 2 diabetes-preventive diet, with the one exception of sardine supplementation, and, although they did not modify their physical activity, both groups reduced their daily caloric intake through food,” the authors note.
The possibility of reducing diabetes risk through dietary changes as opposed to weight loss is especially important in the older population, the authors note, as some studies suggest a link between weight loss in the elderly and an increased risk of mortality.
In a second phase of the study, the researchers say they are evaluating the effect of sardines on the intestinal microbiota, “since it affects the regulation of many biological processes, and we need to understand if they have played a part in this protective effect against type 2 diabetes,” Dr. Díaz-Rizzolo concluded.
The study was funded by RecerCaixa 2013. The authors report that “no industry sponsorship was received for this work that could have influenced its outcome.”
A version of this article first appeared on Medscape.com.
Older people with prediabetes who followed a diet rich in sardines for 1 year show significant reductions in risk of developing type 2 diabetes compared with those placed on a similarly healthy diet but without the sardines, results from a new randomized trial show.
“A 1-year, sardine-enriched type 2 diabetes-preventive diet in an elderly population with prediabetes exerts a greater protective effect against developing type 2 diabetes and cardiovascular events, by improving anthropometric parameters, blood chemistry profile, lipid composition in erythrocytes membranes, and metabolomics data,” report the authors in research published in Clinical Nutrition by Diana Díaz-Rizzolo, PhD, of the Hospital Clinic of Barcelona, Spain, and colleagues.
While cardiovascular and other health benefits of unsaturated fats in oily fish are well-established and are a key component in diets such as the highly recommended Mediterranean diet, the authors note that the consumption of sardines for the prevention of type 2 diabetes has not previously been studied.
In addition to being rich in healthy omega-3 fatty acids, sardines have high concentrations of taurine – approximately 147 mg per 100 g serving – which, depending on the sardine species, is believed to have hypoglycemic, antioxidant, and anti-inflammatory benefits, the authors note.
Participants advised to consume the whole sardine, bones and all
To evaluate the effects, researchers enrolled 152 patients aged 65 and older who had been diagnosed with prediabetes (blood glucose levels between 100-124 mg/dL) and placed them all on a nutritional program to reduce the risk of diabetes for 1 year.
In addition, about half (n = 75) were also instructed to consume 200 g of canned sardines in olive oil per week, in 100 g servings consumed twice per week.
Those participants were recommended to consume the entire sardine, without removal of bones, due to their rich content of calcium and vitamin D. They were also provided with recipes that used canned sardines.
At 1 year, the percentage of participants classified as being at a very high risk of type 2 diabetes, assessed by the Finnish Diabetes Risk Score (FINDRISC), compared with baseline, had declined to a much greater degree in the sardine consumption group (37% at baseline vs. 8% at 1 year) compared with those in the control group, who only consumed the nutritional diet (27% vs. 22%) (P = .021).
In addition, those in the sardine group had greater increases in healthy HDL cholesterol and the glucose-regulating protein hormone adiponectin, with decreases in triglycerides compared with the nonsardine group (all P < .005).
Furthermore, the sardine consumption group had a greater decrease in insulin resistance, assessed by Homeostatic Model Assessment for Insulin Resistance (HOMA-IR; P = .032).
Sardines are cheap and reduce blood pressure too
“Not only are sardines reasonably priced and easy to find, but they are safe and help to prevent the onset of type 2 diabetes,” said Dr. Díaz-Rizzolo in a press statement.
Those in the sardine group also showed significant decreases in systolic blood pressure (P = .014) and diastolic blood pressure (P = .020) versus baseline, while no significant changes were observed in the control group. The authors suggest that sardines’ rich taurine concentrations could play a role in those effects.
“Previously, only lean fish consumption had demonstrated an improvement in blood pressure, not fatty fish consumption, perhaps because the species studied excluded those with a higher taurine content such as sardines,” they speculate.
In addition to showing improvements in levels of taurine, those in the sardine group also showed increases in nutrients that have been linked to health benefits, including omega-3 EPA and DHA, vitamin D, and fluorine (all P < .05).
The authors note that the increases could be attributed to sardines’ rich concentration of those nutrients, as well as to the olive oil that is present in the sardine can.
Some benefits seen in both groups
The patients in the study were a mean age of 71 and had been in a prediabetic state for an average of 4.8 years at the beginning of the study. They were 55% male and there were no other significant differences in characteristics between the groups.
While the conversion from being prediabetic to type 2 diabetes in the adult population has been reported to be about 10.6%, and the risk has been observed to be even higher in the 65 and older population, rates were lower than that in both groups.
“At the end of our 1-year study, we observed a [rate of] new-onset type 2 diabetes of 2.7% and 5.2% in the sardine group and control group, respectively,” the authors note. They add the differences were not statistically significant.
Both the sardine consumption and control groups showed significant reductions in A1c versus baseline (P = .011 and P = .010, respectively), as well as significant reductions in glucose fasting concentrations (P = .020 and P = .040, respectively).
And while the sardine group showed greater improvements in HDL versus the control group (P = .045), only the control group showed a significant decrease in total cholesterol versus baseline (P = .032).
Both groups showed improvements in the management of body weight, body mass index, and waist and hip circumference, in addition to improvement in body composition – despite no physical activity components in the programs, the authors note.
“This is probably because both groups followed the same base type 2 diabetes-preventive diet, with the one exception of sardine supplementation, and, although they did not modify their physical activity, both groups reduced their daily caloric intake through food,” the authors note.
The possibility of reducing diabetes risk through dietary changes as opposed to weight loss is especially important in the older population, the authors note, as some studies suggest a link between weight loss in the elderly and an increased risk of mortality.
In a second phase of the study, the researchers say they are evaluating the effect of sardines on the intestinal microbiota, “since it affects the regulation of many biological processes, and we need to understand if they have played a part in this protective effect against type 2 diabetes,” Dr. Díaz-Rizzolo concluded.
The study was funded by RecerCaixa 2013. The authors report that “no industry sponsorship was received for this work that could have influenced its outcome.”
A version of this article first appeared on Medscape.com.
Early aspirin withdrawal after PCI: More benefit for women?
A new analysis from the TWILIGHT study has shown that, in the high-risk population undergoing percutaneous coronary intervention (PCI) enrolled in the study, the benefits of early aspirin withdrawal and continuation on ticagrelor monotherapy were similar in women and men.
But there were some interesting observations in the analysis suggesting possible additional benefits of this strategy for women.
“These data support the use of ticagrelor monotherapy in women and men, and importantly show that the absolute risk reduction of bleeding was higher in women, as their bleeding rates were higher,” senior author Roxana Mehran, MD, the Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“These data also support the need for prospective dual antiplatelet therapy deescalation studies in women,” Dr. Mehran added.
The main results of the TWILIGHT study showed that after a short period of dual antiplatelet therapy, a strategy of ticagrelor monotherapy, compared with continued dual therapy led to reduced bleeding without an increase in ischemic events among patients at high risk for bleeding or ischemic events after PCI.
The new gender-based analysis was presented by Birgit Vogel, MD, on May 15 at the annual scientific sessions of the American College of Cardiology. It was also published online in JAMA Cardiology to coincide with the ACC presentation.
Dr. Vogel, also from Wiener Cardiovascular Institute, explained that the current analysis was undertaken to investigate whether the TWILIGHT results varied in relation to sex, given that women are believed to have an increased risk for bleeding after PCI, compared with men.
“The current analysis showed that, while women did have a higher bleeding risk, compared to men, this was no longer significant after adjustment for baseline characteristics; and ischemic events were similar between the two sexes,” she reported.
“Results showed that withdrawing aspirin while continuing ticagrelor after 3 months of dual antiplatelet therapy was associated with a reduction in bleeding and preserved ischemic benefits in both women and men,” she added.
The TWILIGHT trial randomized 7,119 patients at high risk of ischemic or bleeding events who had undergone successful PCI with at least one drug-eluting stent and had completed 3 months of dual antiplatelet therapy to aspirin or placebo for an additional 12 months plus open-label ticagrelor.
The main results showed that the primary endpoint of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding at 1 year was almost halved with ticagrelor monotherapy, occurring in 4% of these patients, compared with 7.1% of the ticagrelor/aspirin group (hazard ratio, 0.56). Ischemic events were similar in the two groups.
The current analysis focused on whether these effects varied in relation to sex.
Dr. Vogel noted that women made up 23.9% of the study population, were older than the men, and were more likely to have diabetes, chronic kidney disease, anemia and hypertension, while the men were more likely to be current smokers. Men had a higher incidence of coronary heart disease history, while women were more likely to have an ACS indication for PCI.
Unadjusted results showed a higher rate of BARC 2, 3, or 5 bleeding at 1 year in women (6.8%) versus men (5.2%), giving an HR of 1.32 (95% CI, 1.06-1.64).
But after adjustment for baseline characteristics, this became nonsignificant (HR, 1.20; 95% CI, 0.95-1.52).
Dr. Vogel pointed out that the most severe type of bleeding (BARC 3 and 5) was not attenuated as much by adjustment for baseline characteristics, with the HR reducing from 1.57 to 1.49.
The ischemic endpoint of death/stroke or MI was similar in men (4.0%) and women (3.5%), and this did not change after adjustment for baseline characteristics.
In terms of the two treatment groups, BARC 2, 3, or 5 bleeding was reduced to a similar extent with ticagrelor monotherapy in both men and women. This endpoint decreased from 8.6% in women on dual-antiplatelet therapy to 5.0% in women on ticagrelor alone (adjusted HR, 0.62) and from 6.6% to 3.7% in men (aHR, 0.57). But she noted that the absolute risk reduction in bleeding was greater in women (3.6%) versus men (2.9%).
“If we have a relative risk reduction in bleeding with early withdrawal of aspirin that is similar between the sexes but an overall higher risk of bleeding in women, that results in a greater absolute risk reduction,” Dr. Vogel commented.
The primary ischemic endpoint of death/MI/stroke was not increased in the ticagrelor group vs the dual antiplatelet group in either men (aHR, 1.06) or women (aHR, 1.04).
Greater reduction in mortality in women?
However, Dr. Vogel reported that there was a suggestion of a greater reduction in all-cause mortality with ticagrelor monotherapy in women versus men. “We found a significant interaction for treatment effect and sex for all-cause mortality, a prespecified endpoint, which was significantly lower in women treated with ticagrelor monotherapy, compared to dual antiplatelet therapy, but this was not the case in men.”
However, this observation was based on few events and should not be considered definitive, she added.
Dr. Vogel noted that the analysis had the limitations of the study not being powered to show differences in men versus women, and the results are only applicable to the population studied who were at high risk of bleeding post PCI.
Commenting on the study at the ACC session, Jacqueline Tamis-Holland, MD, associate professor of medicine at the Icahn School of Medicine at Mount Sinai, described the presentation as “very interesting.”
“We know that women notoriously have higher bleeding risk than men, but this particular study did not show a difference in bleeding risk after adjusting for other confounding variables,” she said.
“In fact, one would think that the relative benefit of a treatment designed to decrease bleeding would be more favorable to women, but this analysis didn’t show that,” she added.
Dr. Vogel replied that the HR of the most serious type of bleeding (BARC 3 and 5) in women versus men was only reduced minimally after adjustment for baseline characteristics, “which still makes us think that there are additional factors that might be important and contribute to an increased risk for bleeding and especially more serous types of bleeding in women.”
She pointed out that, while there was a similar risk reduction in bleeding in women and men, there was a potential mortality benefit in women. “The question is whether this mortality benefit is due to reduced bleeding that might be greater in women than men, and the reality is we don’t have a lot of data on that.”
Dr. Vogel added: “We know about the relationship between bleeding and mortality very well but the impact of sex on this is really not well investigated. It would be worth investigating this further to come up with bleeding reduction strategies for women because this is a really important issue.”
This work was supported by an investigator-initiated grant from AstraZeneca. Dr. Mehran reported grants and personal fees (paid to the institution) from Abbott, Abiomed, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, Chiesi, Concept Medical Research, Medtronic, Novartis and DSI Research; grants from Applied Therapeutics, AstraZeneca, Cerecor, CSL Behring, OrbusNeich, and Zoll; personal fees from Boston Scientific, California Institute for Regenerative Medicine, Cine-Med Research, Janssen Scientific Affairs, ACC, and WebMD; personal fees paid to the institution from CardiaWave, Duke University, and Idorsia Pharmaceuticals; serving as a consultant or committee or advisory board member for Society for Cardiovascular Angiography and Interventions, the American Medical Association, and Regeneron Pharmaceuticals; and owning stock in ControlRad, Elixir Medical, and STEL outside the submitted work. Dr. Vogel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis from the TWILIGHT study has shown that, in the high-risk population undergoing percutaneous coronary intervention (PCI) enrolled in the study, the benefits of early aspirin withdrawal and continuation on ticagrelor monotherapy were similar in women and men.
But there were some interesting observations in the analysis suggesting possible additional benefits of this strategy for women.
“These data support the use of ticagrelor monotherapy in women and men, and importantly show that the absolute risk reduction of bleeding was higher in women, as their bleeding rates were higher,” senior author Roxana Mehran, MD, the Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“These data also support the need for prospective dual antiplatelet therapy deescalation studies in women,” Dr. Mehran added.
The main results of the TWILIGHT study showed that after a short period of dual antiplatelet therapy, a strategy of ticagrelor monotherapy, compared with continued dual therapy led to reduced bleeding without an increase in ischemic events among patients at high risk for bleeding or ischemic events after PCI.
The new gender-based analysis was presented by Birgit Vogel, MD, on May 15 at the annual scientific sessions of the American College of Cardiology. It was also published online in JAMA Cardiology to coincide with the ACC presentation.
Dr. Vogel, also from Wiener Cardiovascular Institute, explained that the current analysis was undertaken to investigate whether the TWILIGHT results varied in relation to sex, given that women are believed to have an increased risk for bleeding after PCI, compared with men.
“The current analysis showed that, while women did have a higher bleeding risk, compared to men, this was no longer significant after adjustment for baseline characteristics; and ischemic events were similar between the two sexes,” she reported.
“Results showed that withdrawing aspirin while continuing ticagrelor after 3 months of dual antiplatelet therapy was associated with a reduction in bleeding and preserved ischemic benefits in both women and men,” she added.
The TWILIGHT trial randomized 7,119 patients at high risk of ischemic or bleeding events who had undergone successful PCI with at least one drug-eluting stent and had completed 3 months of dual antiplatelet therapy to aspirin or placebo for an additional 12 months plus open-label ticagrelor.
The main results showed that the primary endpoint of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding at 1 year was almost halved with ticagrelor monotherapy, occurring in 4% of these patients, compared with 7.1% of the ticagrelor/aspirin group (hazard ratio, 0.56). Ischemic events were similar in the two groups.
The current analysis focused on whether these effects varied in relation to sex.
Dr. Vogel noted that women made up 23.9% of the study population, were older than the men, and were more likely to have diabetes, chronic kidney disease, anemia and hypertension, while the men were more likely to be current smokers. Men had a higher incidence of coronary heart disease history, while women were more likely to have an ACS indication for PCI.
Unadjusted results showed a higher rate of BARC 2, 3, or 5 bleeding at 1 year in women (6.8%) versus men (5.2%), giving an HR of 1.32 (95% CI, 1.06-1.64).
But after adjustment for baseline characteristics, this became nonsignificant (HR, 1.20; 95% CI, 0.95-1.52).
Dr. Vogel pointed out that the most severe type of bleeding (BARC 3 and 5) was not attenuated as much by adjustment for baseline characteristics, with the HR reducing from 1.57 to 1.49.
The ischemic endpoint of death/stroke or MI was similar in men (4.0%) and women (3.5%), and this did not change after adjustment for baseline characteristics.
In terms of the two treatment groups, BARC 2, 3, or 5 bleeding was reduced to a similar extent with ticagrelor monotherapy in both men and women. This endpoint decreased from 8.6% in women on dual-antiplatelet therapy to 5.0% in women on ticagrelor alone (adjusted HR, 0.62) and from 6.6% to 3.7% in men (aHR, 0.57). But she noted that the absolute risk reduction in bleeding was greater in women (3.6%) versus men (2.9%).
“If we have a relative risk reduction in bleeding with early withdrawal of aspirin that is similar between the sexes but an overall higher risk of bleeding in women, that results in a greater absolute risk reduction,” Dr. Vogel commented.
The primary ischemic endpoint of death/MI/stroke was not increased in the ticagrelor group vs the dual antiplatelet group in either men (aHR, 1.06) or women (aHR, 1.04).
Greater reduction in mortality in women?
However, Dr. Vogel reported that there was a suggestion of a greater reduction in all-cause mortality with ticagrelor monotherapy in women versus men. “We found a significant interaction for treatment effect and sex for all-cause mortality, a prespecified endpoint, which was significantly lower in women treated with ticagrelor monotherapy, compared to dual antiplatelet therapy, but this was not the case in men.”
However, this observation was based on few events and should not be considered definitive, she added.
Dr. Vogel noted that the analysis had the limitations of the study not being powered to show differences in men versus women, and the results are only applicable to the population studied who were at high risk of bleeding post PCI.
Commenting on the study at the ACC session, Jacqueline Tamis-Holland, MD, associate professor of medicine at the Icahn School of Medicine at Mount Sinai, described the presentation as “very interesting.”
“We know that women notoriously have higher bleeding risk than men, but this particular study did not show a difference in bleeding risk after adjusting for other confounding variables,” she said.
“In fact, one would think that the relative benefit of a treatment designed to decrease bleeding would be more favorable to women, but this analysis didn’t show that,” she added.
Dr. Vogel replied that the HR of the most serious type of bleeding (BARC 3 and 5) in women versus men was only reduced minimally after adjustment for baseline characteristics, “which still makes us think that there are additional factors that might be important and contribute to an increased risk for bleeding and especially more serous types of bleeding in women.”
She pointed out that, while there was a similar risk reduction in bleeding in women and men, there was a potential mortality benefit in women. “The question is whether this mortality benefit is due to reduced bleeding that might be greater in women than men, and the reality is we don’t have a lot of data on that.”
Dr. Vogel added: “We know about the relationship between bleeding and mortality very well but the impact of sex on this is really not well investigated. It would be worth investigating this further to come up with bleeding reduction strategies for women because this is a really important issue.”
This work was supported by an investigator-initiated grant from AstraZeneca. Dr. Mehran reported grants and personal fees (paid to the institution) from Abbott, Abiomed, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, Chiesi, Concept Medical Research, Medtronic, Novartis and DSI Research; grants from Applied Therapeutics, AstraZeneca, Cerecor, CSL Behring, OrbusNeich, and Zoll; personal fees from Boston Scientific, California Institute for Regenerative Medicine, Cine-Med Research, Janssen Scientific Affairs, ACC, and WebMD; personal fees paid to the institution from CardiaWave, Duke University, and Idorsia Pharmaceuticals; serving as a consultant or committee or advisory board member for Society for Cardiovascular Angiography and Interventions, the American Medical Association, and Regeneron Pharmaceuticals; and owning stock in ControlRad, Elixir Medical, and STEL outside the submitted work. Dr. Vogel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis from the TWILIGHT study has shown that, in the high-risk population undergoing percutaneous coronary intervention (PCI) enrolled in the study, the benefits of early aspirin withdrawal and continuation on ticagrelor monotherapy were similar in women and men.
But there were some interesting observations in the analysis suggesting possible additional benefits of this strategy for women.
“These data support the use of ticagrelor monotherapy in women and men, and importantly show that the absolute risk reduction of bleeding was higher in women, as their bleeding rates were higher,” senior author Roxana Mehran, MD, the Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“These data also support the need for prospective dual antiplatelet therapy deescalation studies in women,” Dr. Mehran added.
The main results of the TWILIGHT study showed that after a short period of dual antiplatelet therapy, a strategy of ticagrelor monotherapy, compared with continued dual therapy led to reduced bleeding without an increase in ischemic events among patients at high risk for bleeding or ischemic events after PCI.
The new gender-based analysis was presented by Birgit Vogel, MD, on May 15 at the annual scientific sessions of the American College of Cardiology. It was also published online in JAMA Cardiology to coincide with the ACC presentation.
Dr. Vogel, also from Wiener Cardiovascular Institute, explained that the current analysis was undertaken to investigate whether the TWILIGHT results varied in relation to sex, given that women are believed to have an increased risk for bleeding after PCI, compared with men.
“The current analysis showed that, while women did have a higher bleeding risk, compared to men, this was no longer significant after adjustment for baseline characteristics; and ischemic events were similar between the two sexes,” she reported.
“Results showed that withdrawing aspirin while continuing ticagrelor after 3 months of dual antiplatelet therapy was associated with a reduction in bleeding and preserved ischemic benefits in both women and men,” she added.
The TWILIGHT trial randomized 7,119 patients at high risk of ischemic or bleeding events who had undergone successful PCI with at least one drug-eluting stent and had completed 3 months of dual antiplatelet therapy to aspirin or placebo for an additional 12 months plus open-label ticagrelor.
The main results showed that the primary endpoint of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding at 1 year was almost halved with ticagrelor monotherapy, occurring in 4% of these patients, compared with 7.1% of the ticagrelor/aspirin group (hazard ratio, 0.56). Ischemic events were similar in the two groups.
The current analysis focused on whether these effects varied in relation to sex.
Dr. Vogel noted that women made up 23.9% of the study population, were older than the men, and were more likely to have diabetes, chronic kidney disease, anemia and hypertension, while the men were more likely to be current smokers. Men had a higher incidence of coronary heart disease history, while women were more likely to have an ACS indication for PCI.
Unadjusted results showed a higher rate of BARC 2, 3, or 5 bleeding at 1 year in women (6.8%) versus men (5.2%), giving an HR of 1.32 (95% CI, 1.06-1.64).
But after adjustment for baseline characteristics, this became nonsignificant (HR, 1.20; 95% CI, 0.95-1.52).
Dr. Vogel pointed out that the most severe type of bleeding (BARC 3 and 5) was not attenuated as much by adjustment for baseline characteristics, with the HR reducing from 1.57 to 1.49.
The ischemic endpoint of death/stroke or MI was similar in men (4.0%) and women (3.5%), and this did not change after adjustment for baseline characteristics.
In terms of the two treatment groups, BARC 2, 3, or 5 bleeding was reduced to a similar extent with ticagrelor monotherapy in both men and women. This endpoint decreased from 8.6% in women on dual-antiplatelet therapy to 5.0% in women on ticagrelor alone (adjusted HR, 0.62) and from 6.6% to 3.7% in men (aHR, 0.57). But she noted that the absolute risk reduction in bleeding was greater in women (3.6%) versus men (2.9%).
“If we have a relative risk reduction in bleeding with early withdrawal of aspirin that is similar between the sexes but an overall higher risk of bleeding in women, that results in a greater absolute risk reduction,” Dr. Vogel commented.
The primary ischemic endpoint of death/MI/stroke was not increased in the ticagrelor group vs the dual antiplatelet group in either men (aHR, 1.06) or women (aHR, 1.04).
Greater reduction in mortality in women?
However, Dr. Vogel reported that there was a suggestion of a greater reduction in all-cause mortality with ticagrelor monotherapy in women versus men. “We found a significant interaction for treatment effect and sex for all-cause mortality, a prespecified endpoint, which was significantly lower in women treated with ticagrelor monotherapy, compared to dual antiplatelet therapy, but this was not the case in men.”
However, this observation was based on few events and should not be considered definitive, she added.
Dr. Vogel noted that the analysis had the limitations of the study not being powered to show differences in men versus women, and the results are only applicable to the population studied who were at high risk of bleeding post PCI.
Commenting on the study at the ACC session, Jacqueline Tamis-Holland, MD, associate professor of medicine at the Icahn School of Medicine at Mount Sinai, described the presentation as “very interesting.”
“We know that women notoriously have higher bleeding risk than men, but this particular study did not show a difference in bleeding risk after adjusting for other confounding variables,” she said.
“In fact, one would think that the relative benefit of a treatment designed to decrease bleeding would be more favorable to women, but this analysis didn’t show that,” she added.
Dr. Vogel replied that the HR of the most serious type of bleeding (BARC 3 and 5) in women versus men was only reduced minimally after adjustment for baseline characteristics, “which still makes us think that there are additional factors that might be important and contribute to an increased risk for bleeding and especially more serous types of bleeding in women.”
She pointed out that, while there was a similar risk reduction in bleeding in women and men, there was a potential mortality benefit in women. “The question is whether this mortality benefit is due to reduced bleeding that might be greater in women than men, and the reality is we don’t have a lot of data on that.”
Dr. Vogel added: “We know about the relationship between bleeding and mortality very well but the impact of sex on this is really not well investigated. It would be worth investigating this further to come up with bleeding reduction strategies for women because this is a really important issue.”
This work was supported by an investigator-initiated grant from AstraZeneca. Dr. Mehran reported grants and personal fees (paid to the institution) from Abbott, Abiomed, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, Chiesi, Concept Medical Research, Medtronic, Novartis and DSI Research; grants from Applied Therapeutics, AstraZeneca, Cerecor, CSL Behring, OrbusNeich, and Zoll; personal fees from Boston Scientific, California Institute for Regenerative Medicine, Cine-Med Research, Janssen Scientific Affairs, ACC, and WebMD; personal fees paid to the institution from CardiaWave, Duke University, and Idorsia Pharmaceuticals; serving as a consultant or committee or advisory board member for Society for Cardiovascular Angiography and Interventions, the American Medical Association, and Regeneron Pharmaceuticals; and owning stock in ControlRad, Elixir Medical, and STEL outside the submitted work. Dr. Vogel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Underlying heart rhythm, not ICD shocks, drives mortality
Combined data from five implantable cardioverter-defibrillator (ICD) trials suggest that it is the underlying arrhythmic disorder, rather than the ICD therapy itself, that affects mortality in these patients.
Analysis of the MADIT II, MADIT-RISK, MADIT-CRT, MADIT-RIT, and RAID trials showed that the major determinant of mortality in patients receiving a primary prevention ICD was the arrhythmic substrate that leads to occurrence of fast ventricular tachycardia (VT), defined as ≥ 200 bpm, or ventricular fibrillation (VF), not adverse effects of the ICD shock therapy itself.
Patients experiencing an episode of VT had more than a twofold increased risk for death during a follow-up of 2½ years; however, ICD therapies for VT less than 200 bpm and inappropriate ICD shocks were not associated with a higher risk for death.
The findings were published online in the Journal of the American College of Cardiology.
“We know that patients receiving an ICD shock have increased mortality during subsequent follow-up,” first author Mehmet K. Aktas, MD, MBA, University of Rochester (N.Y.), said in an interview.
“There are conflicting data on the impact of ICD shocks on subsequent mortality, and in this study, we aimed to determine whether shocks per se increase subsequent mortality risk or whether the arrhythmic substrate that leads to ICD therapy results in subsequent risk of death,” Dr. Aktas said.
He and his team evaluated the association of ICD therapy with subsequent mortality according to the type of ICD therapy (model I), type of arrhythmia for which ICD therapy was delivered (model II), combined assessment of all arrhythmia and therapy types during follow-up (model III), and incremental risk associated with repeated ICD shocks (model IV).
The study cohort included 5,516 patients. Of these, 1,001 patients (18%) received appropriate ICD therapy and 561 (10%) received inappropriate ICD therapy during an average of 2.4 years.
Patients receiving an appropriate ICD therapy were more likely to be male and to have prior atrial arrhythmia and nonsustained VT compared with those without ICD therapy.
Patients receiving an inappropriate shock were more likely to be younger, to be African American, and to be less likely to have prior nonsustained VT, compared with those without ICD therapy.
Most patients (90%) were receiving beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers regardless of device therapy during follow-up, and 10% of patients were treated with amiodarone.
In model I, at 3 years, the cumulative probability of death following an appropriate ICD shock was 38% compared with no appropriate ICD shock (P < .001). Inappropriate shock alone was not associated with mortality risk.
In model II, which looked at the type of arrhythmia for which ICD therapy was delivered, the cumulative death rate at 3 years following the first occurrence of ICD therapy for VT ≥ 200 beats/min or VF was 27%, compared with 10% in patients not experiencing VT ≥ 200 beats/min or VF (P < .001).
In model III, the highest risk for death was observed following shocks delivered after a failed antitachycardia pacing (ATP) for fast VT (hazard ratio [HR], 3.05), followed by ICD shock for VF (HR, 2.86), ICD shock for fast VT without a prior ATP (HR, 2.83), and ICD shock for slower VT (< 200 beats/min) without a prior ATP (HR, 2.39).
In contrast, other types of appropriate and inappropriate shock or ATP therapies were not associated with a significant risk increase.
In model IV, which assessed the association of shock therapy counts with the risk for death, two or more ICD appropriate shocks were not associated with increased risk after the first appropriate ICD shock.
“Our findings shed light on the mechanisms associated with increased mortality risk in primary prevention ICD recipients,” Dr. Aktas said.
“Studies that evaluate interventions focused on treating and stabilizing the myocardial substrate, which promotes ventricular tachyarrhythmias, such as catheter ablation, are needed to improve survival in heart failure patients,” he added.
Thoughtful study design
In an accompanying editorial, Rajat Deo, MD, and Naga Venkata K. Pothineni, MD, both from the University of Pennsylvania, Philadelphia, praised the researchers for their “thoughtful study design.”
“The take-home message that is most relevant to our clinical practice is clear: Sustained ventricular arrhythmias are a prognostic marker of death and heart failure hospitalization,” they wrote.
The editorialists also commented on the higher rate of inappropriate ICD therapies in African Americans.
“It is concerning to observe that Black patients had a markedly higher rate of inappropriate ICD therapies compared with White patients – and this was in the setting of some of the most respectable, established, and well-funded clinical trials,” they wrote.
Reasons for disparities in outcomes include access to appropriate and affordable medical therapies, access to specialty clinics and caregivers, remote ICD monitoring, and compliance issues.
“Future work will need to understand how the social determinants of health including race affect the treatment and outcomes of our primary prevention ICD population,” they wrote.
Identifying and characterizing the arrhythmic substrate will become a key component of sudden cardiac death risk stratification, the editorialists predicted.
“Concurrently, we must continue to partner with industry colleagues and work with our professional societies to ensure health equity across our patient population,” they concluded.
Dr. Aktas has received research grants from Boston Scientific and Medtronic. Dr. Deo and his coeditorialists report no relevant financial relationships. The MADIT trials were funded by an unrestricted research grant from Boston Scientific to the University of Rochester Medical Center. The RAID trial was funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Combined data from five implantable cardioverter-defibrillator (ICD) trials suggest that it is the underlying arrhythmic disorder, rather than the ICD therapy itself, that affects mortality in these patients.
Analysis of the MADIT II, MADIT-RISK, MADIT-CRT, MADIT-RIT, and RAID trials showed that the major determinant of mortality in patients receiving a primary prevention ICD was the arrhythmic substrate that leads to occurrence of fast ventricular tachycardia (VT), defined as ≥ 200 bpm, or ventricular fibrillation (VF), not adverse effects of the ICD shock therapy itself.
Patients experiencing an episode of VT had more than a twofold increased risk for death during a follow-up of 2½ years; however, ICD therapies for VT less than 200 bpm and inappropriate ICD shocks were not associated with a higher risk for death.
The findings were published online in the Journal of the American College of Cardiology.
“We know that patients receiving an ICD shock have increased mortality during subsequent follow-up,” first author Mehmet K. Aktas, MD, MBA, University of Rochester (N.Y.), said in an interview.
“There are conflicting data on the impact of ICD shocks on subsequent mortality, and in this study, we aimed to determine whether shocks per se increase subsequent mortality risk or whether the arrhythmic substrate that leads to ICD therapy results in subsequent risk of death,” Dr. Aktas said.
He and his team evaluated the association of ICD therapy with subsequent mortality according to the type of ICD therapy (model I), type of arrhythmia for which ICD therapy was delivered (model II), combined assessment of all arrhythmia and therapy types during follow-up (model III), and incremental risk associated with repeated ICD shocks (model IV).
The study cohort included 5,516 patients. Of these, 1,001 patients (18%) received appropriate ICD therapy and 561 (10%) received inappropriate ICD therapy during an average of 2.4 years.
Patients receiving an appropriate ICD therapy were more likely to be male and to have prior atrial arrhythmia and nonsustained VT compared with those without ICD therapy.
Patients receiving an inappropriate shock were more likely to be younger, to be African American, and to be less likely to have prior nonsustained VT, compared with those without ICD therapy.
Most patients (90%) were receiving beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers regardless of device therapy during follow-up, and 10% of patients were treated with amiodarone.
In model I, at 3 years, the cumulative probability of death following an appropriate ICD shock was 38% compared with no appropriate ICD shock (P < .001). Inappropriate shock alone was not associated with mortality risk.
In model II, which looked at the type of arrhythmia for which ICD therapy was delivered, the cumulative death rate at 3 years following the first occurrence of ICD therapy for VT ≥ 200 beats/min or VF was 27%, compared with 10% in patients not experiencing VT ≥ 200 beats/min or VF (P < .001).
In model III, the highest risk for death was observed following shocks delivered after a failed antitachycardia pacing (ATP) for fast VT (hazard ratio [HR], 3.05), followed by ICD shock for VF (HR, 2.86), ICD shock for fast VT without a prior ATP (HR, 2.83), and ICD shock for slower VT (< 200 beats/min) without a prior ATP (HR, 2.39).
In contrast, other types of appropriate and inappropriate shock or ATP therapies were not associated with a significant risk increase.
In model IV, which assessed the association of shock therapy counts with the risk for death, two or more ICD appropriate shocks were not associated with increased risk after the first appropriate ICD shock.
“Our findings shed light on the mechanisms associated with increased mortality risk in primary prevention ICD recipients,” Dr. Aktas said.
“Studies that evaluate interventions focused on treating and stabilizing the myocardial substrate, which promotes ventricular tachyarrhythmias, such as catheter ablation, are needed to improve survival in heart failure patients,” he added.
Thoughtful study design
In an accompanying editorial, Rajat Deo, MD, and Naga Venkata K. Pothineni, MD, both from the University of Pennsylvania, Philadelphia, praised the researchers for their “thoughtful study design.”
“The take-home message that is most relevant to our clinical practice is clear: Sustained ventricular arrhythmias are a prognostic marker of death and heart failure hospitalization,” they wrote.
The editorialists also commented on the higher rate of inappropriate ICD therapies in African Americans.
“It is concerning to observe that Black patients had a markedly higher rate of inappropriate ICD therapies compared with White patients – and this was in the setting of some of the most respectable, established, and well-funded clinical trials,” they wrote.
Reasons for disparities in outcomes include access to appropriate and affordable medical therapies, access to specialty clinics and caregivers, remote ICD monitoring, and compliance issues.
“Future work will need to understand how the social determinants of health including race affect the treatment and outcomes of our primary prevention ICD population,” they wrote.
Identifying and characterizing the arrhythmic substrate will become a key component of sudden cardiac death risk stratification, the editorialists predicted.
“Concurrently, we must continue to partner with industry colleagues and work with our professional societies to ensure health equity across our patient population,” they concluded.
Dr. Aktas has received research grants from Boston Scientific and Medtronic. Dr. Deo and his coeditorialists report no relevant financial relationships. The MADIT trials were funded by an unrestricted research grant from Boston Scientific to the University of Rochester Medical Center. The RAID trial was funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Combined data from five implantable cardioverter-defibrillator (ICD) trials suggest that it is the underlying arrhythmic disorder, rather than the ICD therapy itself, that affects mortality in these patients.
Analysis of the MADIT II, MADIT-RISK, MADIT-CRT, MADIT-RIT, and RAID trials showed that the major determinant of mortality in patients receiving a primary prevention ICD was the arrhythmic substrate that leads to occurrence of fast ventricular tachycardia (VT), defined as ≥ 200 bpm, or ventricular fibrillation (VF), not adverse effects of the ICD shock therapy itself.
Patients experiencing an episode of VT had more than a twofold increased risk for death during a follow-up of 2½ years; however, ICD therapies for VT less than 200 bpm and inappropriate ICD shocks were not associated with a higher risk for death.
The findings were published online in the Journal of the American College of Cardiology.
“We know that patients receiving an ICD shock have increased mortality during subsequent follow-up,” first author Mehmet K. Aktas, MD, MBA, University of Rochester (N.Y.), said in an interview.
“There are conflicting data on the impact of ICD shocks on subsequent mortality, and in this study, we aimed to determine whether shocks per se increase subsequent mortality risk or whether the arrhythmic substrate that leads to ICD therapy results in subsequent risk of death,” Dr. Aktas said.
He and his team evaluated the association of ICD therapy with subsequent mortality according to the type of ICD therapy (model I), type of arrhythmia for which ICD therapy was delivered (model II), combined assessment of all arrhythmia and therapy types during follow-up (model III), and incremental risk associated with repeated ICD shocks (model IV).
The study cohort included 5,516 patients. Of these, 1,001 patients (18%) received appropriate ICD therapy and 561 (10%) received inappropriate ICD therapy during an average of 2.4 years.
Patients receiving an appropriate ICD therapy were more likely to be male and to have prior atrial arrhythmia and nonsustained VT compared with those without ICD therapy.
Patients receiving an inappropriate shock were more likely to be younger, to be African American, and to be less likely to have prior nonsustained VT, compared with those without ICD therapy.
Most patients (90%) were receiving beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers regardless of device therapy during follow-up, and 10% of patients were treated with amiodarone.
In model I, at 3 years, the cumulative probability of death following an appropriate ICD shock was 38% compared with no appropriate ICD shock (P < .001). Inappropriate shock alone was not associated with mortality risk.
In model II, which looked at the type of arrhythmia for which ICD therapy was delivered, the cumulative death rate at 3 years following the first occurrence of ICD therapy for VT ≥ 200 beats/min or VF was 27%, compared with 10% in patients not experiencing VT ≥ 200 beats/min or VF (P < .001).
In model III, the highest risk for death was observed following shocks delivered after a failed antitachycardia pacing (ATP) for fast VT (hazard ratio [HR], 3.05), followed by ICD shock for VF (HR, 2.86), ICD shock for fast VT without a prior ATP (HR, 2.83), and ICD shock for slower VT (< 200 beats/min) without a prior ATP (HR, 2.39).
In contrast, other types of appropriate and inappropriate shock or ATP therapies were not associated with a significant risk increase.
In model IV, which assessed the association of shock therapy counts with the risk for death, two or more ICD appropriate shocks were not associated with increased risk after the first appropriate ICD shock.
“Our findings shed light on the mechanisms associated with increased mortality risk in primary prevention ICD recipients,” Dr. Aktas said.
“Studies that evaluate interventions focused on treating and stabilizing the myocardial substrate, which promotes ventricular tachyarrhythmias, such as catheter ablation, are needed to improve survival in heart failure patients,” he added.
Thoughtful study design
In an accompanying editorial, Rajat Deo, MD, and Naga Venkata K. Pothineni, MD, both from the University of Pennsylvania, Philadelphia, praised the researchers for their “thoughtful study design.”
“The take-home message that is most relevant to our clinical practice is clear: Sustained ventricular arrhythmias are a prognostic marker of death and heart failure hospitalization,” they wrote.
The editorialists also commented on the higher rate of inappropriate ICD therapies in African Americans.
“It is concerning to observe that Black patients had a markedly higher rate of inappropriate ICD therapies compared with White patients – and this was in the setting of some of the most respectable, established, and well-funded clinical trials,” they wrote.
Reasons for disparities in outcomes include access to appropriate and affordable medical therapies, access to specialty clinics and caregivers, remote ICD monitoring, and compliance issues.
“Future work will need to understand how the social determinants of health including race affect the treatment and outcomes of our primary prevention ICD population,” they wrote.
Identifying and characterizing the arrhythmic substrate will become a key component of sudden cardiac death risk stratification, the editorialists predicted.
“Concurrently, we must continue to partner with industry colleagues and work with our professional societies to ensure health equity across our patient population,” they concluded.
Dr. Aktas has received research grants from Boston Scientific and Medtronic. Dr. Deo and his coeditorialists report no relevant financial relationships. The MADIT trials were funded by an unrestricted research grant from Boston Scientific to the University of Rochester Medical Center. The RAID trial was funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.




