User login
Trust in a Time of Uncertainty: A Call for Articles
A functioning healthcare system requires trust on many levels. In its simplest form, this is the trust between an individual patient and their physician that allows for candor, autonomy, informed decisions, and compassionate care. Trust is a central component of medical education, as trainees gradually earn the trust of their supervisors to achieve autonomy. And, on a much larger scale, societal trust in science, the facts, and the medical system influences individual and group decisions that can have far-reaching consequences.
Defining trust is challenging. Trust is relational, an often subconscious decision “by one individual to depend on another,” but it can also be as broad as trust in an institution or a national system.1 Trust also requires vulnerability—trusting another person or system means ceding some level of personal control and accepting risk. Thus, to ask patients and society to trust in physicians, the healthcare system, or public health institutions, though essential, is no small request.
Physicians and the medical system at large have not always behaved in ways that warrant trust. Medical research on vulnerable populations (historically marginalized communities, prisoners, residents of institutions) has occurred within living memory. Systemic racism within medicine has led to marked disparities in access and outcomes between White and minoritized communities.2 These disparities have been accentuated by the pandemic. Black and Brown patients have higher infection rates and higher mortality rates but less access to healthcare.3 Vaccine distribution, which has been complicated by historic earned distrust from Black and Brown communities, revealed systemic racism. For example, many early mass vaccination sites, such as Dodger Stadium in Los Angeles, could only be easily reached by car. Online appointment scheduling platforms were opaque and required access to technology.4
Public trust in institutions has been eroding over the past several decades, but healthcare has unfortunately seen the largest decline.5 Individual healthcare decisions have also been increasingly politicized; the net result is the creation of laws, such as those limiting discussions of firearm safety or banning gender-affirming treatments for transgender children, that influence patient-physician interactions. This combination of erosion of trust and politicization of medical decisions has been harshly highlighted by the global pandemic, complicating public health policy and doctor-patient discussions. Public health measures such as masking and vaccination have become polarized.6 Further, there is diminishing trust in medical recommendations, brought about by the current media landscape and by frequent modifications to public health recommendations. Science and medicine are constantly changing, and knowledge in these fields is ultimately provisional. Unfortunately, when new data are published that contradict prior information or report new or dramatic findings, it can appear that the medical system was somehow obscuring the truth in the past, rather than simply advancing its knowledge in the present.
How do we build trust? How do we function in a healthcare system where trust has been eroded? Trust is ultimately a fragile thing. The process of earning it is not swift or straightforward, but it can be lost in a moment.
In partnership with the ABIM Foundation, the Journal of Hospital Medicine will explore the concept of trust in all facets of healthcare and medical education, including understanding the drivers of trust in a multitude of settings and in different relationships (patient-clinician, clinician-trainee, clinician- or trainee-organization, health system-community), interventions to build trust, and the enablers of those interventions. To this end, we are seeking articles that explore or evaluate trust. These include original research, brief reports, perspectives, and Leadership & Professional Development articles. Articles focusing on trust should be submitted by December 31, 2021.
1. Hendren EM, Kumagai AK. A matter of trust. Acad Med. 2019;94(9):1270-1272. https://doi.org/10.1097/ACM.0000000000002846
2. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(7):572-573. https://doi.org/10.12788/jhm.3492
3. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
4. Dembosky A. It’s not Tuskegee. Current medical racism fuels Black Americans’ vaccine hesitancy. Los Angeles Times. March 25, 2021.
5. Lynch TJ, Wolfson DB, Baron RJ. A trust initiative in health care: why and why now? Acad Med. 2019;94(4):463-465. https://doi.org/10.1097/ACM.0000000000002599
6. Sherling DH, Bell M. Masks, seat belts, and the politicization of public health. J Hosp Med. 2020;15(11):692-693. https://doi.org/10.12788/jhm.3524
A functioning healthcare system requires trust on many levels. In its simplest form, this is the trust between an individual patient and their physician that allows for candor, autonomy, informed decisions, and compassionate care. Trust is a central component of medical education, as trainees gradually earn the trust of their supervisors to achieve autonomy. And, on a much larger scale, societal trust in science, the facts, and the medical system influences individual and group decisions that can have far-reaching consequences.
Defining trust is challenging. Trust is relational, an often subconscious decision “by one individual to depend on another,” but it can also be as broad as trust in an institution or a national system.1 Trust also requires vulnerability—trusting another person or system means ceding some level of personal control and accepting risk. Thus, to ask patients and society to trust in physicians, the healthcare system, or public health institutions, though essential, is no small request.
Physicians and the medical system at large have not always behaved in ways that warrant trust. Medical research on vulnerable populations (historically marginalized communities, prisoners, residents of institutions) has occurred within living memory. Systemic racism within medicine has led to marked disparities in access and outcomes between White and minoritized communities.2 These disparities have been accentuated by the pandemic. Black and Brown patients have higher infection rates and higher mortality rates but less access to healthcare.3 Vaccine distribution, which has been complicated by historic earned distrust from Black and Brown communities, revealed systemic racism. For example, many early mass vaccination sites, such as Dodger Stadium in Los Angeles, could only be easily reached by car. Online appointment scheduling platforms were opaque and required access to technology.4
Public trust in institutions has been eroding over the past several decades, but healthcare has unfortunately seen the largest decline.5 Individual healthcare decisions have also been increasingly politicized; the net result is the creation of laws, such as those limiting discussions of firearm safety or banning gender-affirming treatments for transgender children, that influence patient-physician interactions. This combination of erosion of trust and politicization of medical decisions has been harshly highlighted by the global pandemic, complicating public health policy and doctor-patient discussions. Public health measures such as masking and vaccination have become polarized.6 Further, there is diminishing trust in medical recommendations, brought about by the current media landscape and by frequent modifications to public health recommendations. Science and medicine are constantly changing, and knowledge in these fields is ultimately provisional. Unfortunately, when new data are published that contradict prior information or report new or dramatic findings, it can appear that the medical system was somehow obscuring the truth in the past, rather than simply advancing its knowledge in the present.
How do we build trust? How do we function in a healthcare system where trust has been eroded? Trust is ultimately a fragile thing. The process of earning it is not swift or straightforward, but it can be lost in a moment.
In partnership with the ABIM Foundation, the Journal of Hospital Medicine will explore the concept of trust in all facets of healthcare and medical education, including understanding the drivers of trust in a multitude of settings and in different relationships (patient-clinician, clinician-trainee, clinician- or trainee-organization, health system-community), interventions to build trust, and the enablers of those interventions. To this end, we are seeking articles that explore or evaluate trust. These include original research, brief reports, perspectives, and Leadership & Professional Development articles. Articles focusing on trust should be submitted by December 31, 2021.
A functioning healthcare system requires trust on many levels. In its simplest form, this is the trust between an individual patient and their physician that allows for candor, autonomy, informed decisions, and compassionate care. Trust is a central component of medical education, as trainees gradually earn the trust of their supervisors to achieve autonomy. And, on a much larger scale, societal trust in science, the facts, and the medical system influences individual and group decisions that can have far-reaching consequences.
Defining trust is challenging. Trust is relational, an often subconscious decision “by one individual to depend on another,” but it can also be as broad as trust in an institution or a national system.1 Trust also requires vulnerability—trusting another person or system means ceding some level of personal control and accepting risk. Thus, to ask patients and society to trust in physicians, the healthcare system, or public health institutions, though essential, is no small request.
Physicians and the medical system at large have not always behaved in ways that warrant trust. Medical research on vulnerable populations (historically marginalized communities, prisoners, residents of institutions) has occurred within living memory. Systemic racism within medicine has led to marked disparities in access and outcomes between White and minoritized communities.2 These disparities have been accentuated by the pandemic. Black and Brown patients have higher infection rates and higher mortality rates but less access to healthcare.3 Vaccine distribution, which has been complicated by historic earned distrust from Black and Brown communities, revealed systemic racism. For example, many early mass vaccination sites, such as Dodger Stadium in Los Angeles, could only be easily reached by car. Online appointment scheduling platforms were opaque and required access to technology.4
Public trust in institutions has been eroding over the past several decades, but healthcare has unfortunately seen the largest decline.5 Individual healthcare decisions have also been increasingly politicized; the net result is the creation of laws, such as those limiting discussions of firearm safety or banning gender-affirming treatments for transgender children, that influence patient-physician interactions. This combination of erosion of trust and politicization of medical decisions has been harshly highlighted by the global pandemic, complicating public health policy and doctor-patient discussions. Public health measures such as masking and vaccination have become polarized.6 Further, there is diminishing trust in medical recommendations, brought about by the current media landscape and by frequent modifications to public health recommendations. Science and medicine are constantly changing, and knowledge in these fields is ultimately provisional. Unfortunately, when new data are published that contradict prior information or report new or dramatic findings, it can appear that the medical system was somehow obscuring the truth in the past, rather than simply advancing its knowledge in the present.
How do we build trust? How do we function in a healthcare system where trust has been eroded? Trust is ultimately a fragile thing. The process of earning it is not swift or straightforward, but it can be lost in a moment.
In partnership with the ABIM Foundation, the Journal of Hospital Medicine will explore the concept of trust in all facets of healthcare and medical education, including understanding the drivers of trust in a multitude of settings and in different relationships (patient-clinician, clinician-trainee, clinician- or trainee-organization, health system-community), interventions to build trust, and the enablers of those interventions. To this end, we are seeking articles that explore or evaluate trust. These include original research, brief reports, perspectives, and Leadership & Professional Development articles. Articles focusing on trust should be submitted by December 31, 2021.
1. Hendren EM, Kumagai AK. A matter of trust. Acad Med. 2019;94(9):1270-1272. https://doi.org/10.1097/ACM.0000000000002846
2. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(7):572-573. https://doi.org/10.12788/jhm.3492
3. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
4. Dembosky A. It’s not Tuskegee. Current medical racism fuels Black Americans’ vaccine hesitancy. Los Angeles Times. March 25, 2021.
5. Lynch TJ, Wolfson DB, Baron RJ. A trust initiative in health care: why and why now? Acad Med. 2019;94(4):463-465. https://doi.org/10.1097/ACM.0000000000002599
6. Sherling DH, Bell M. Masks, seat belts, and the politicization of public health. J Hosp Med. 2020;15(11):692-693. https://doi.org/10.12788/jhm.3524
1. Hendren EM, Kumagai AK. A matter of trust. Acad Med. 2019;94(9):1270-1272. https://doi.org/10.1097/ACM.0000000000002846
2. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(7):572-573. https://doi.org/10.12788/jhm.3492
3. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
4. Dembosky A. It’s not Tuskegee. Current medical racism fuels Black Americans’ vaccine hesitancy. Los Angeles Times. March 25, 2021.
5. Lynch TJ, Wolfson DB, Baron RJ. A trust initiative in health care: why and why now? Acad Med. 2019;94(4):463-465. https://doi.org/10.1097/ACM.0000000000002599
6. Sherling DH, Bell M. Masks, seat belts, and the politicization of public health. J Hosp Med. 2020;15(11):692-693. https://doi.org/10.12788/jhm.3524
© 2021 Society of Hospital Medicine
GALACTIC-HF: Novel drug most effective in sickest HFrEF patients
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
FROM ACC 2021
Ulcerative Heliotrope Rash in Antimelanoma Differentiation–Associated Gene 5 Dermatomyositis
Dermatomyositis (DM) is an autoimmune condition characterized by skin and muscle inflammation with an estimated incidence of 9 cases per 1 million people. The incidence of amyopathic DM, which includes antimelanoma differentiation–associated gene 5 (anti-MDA5) DM, is approximately 2 cases per 1 million people.1 Classic cutaneous manifestations of DM include a heliotrope rash, Gottron papules, and the shawl sign.
Case Reports
Patient 1
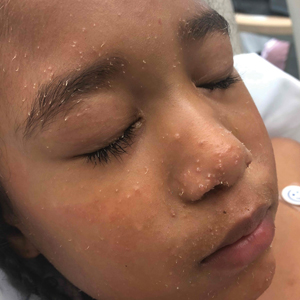
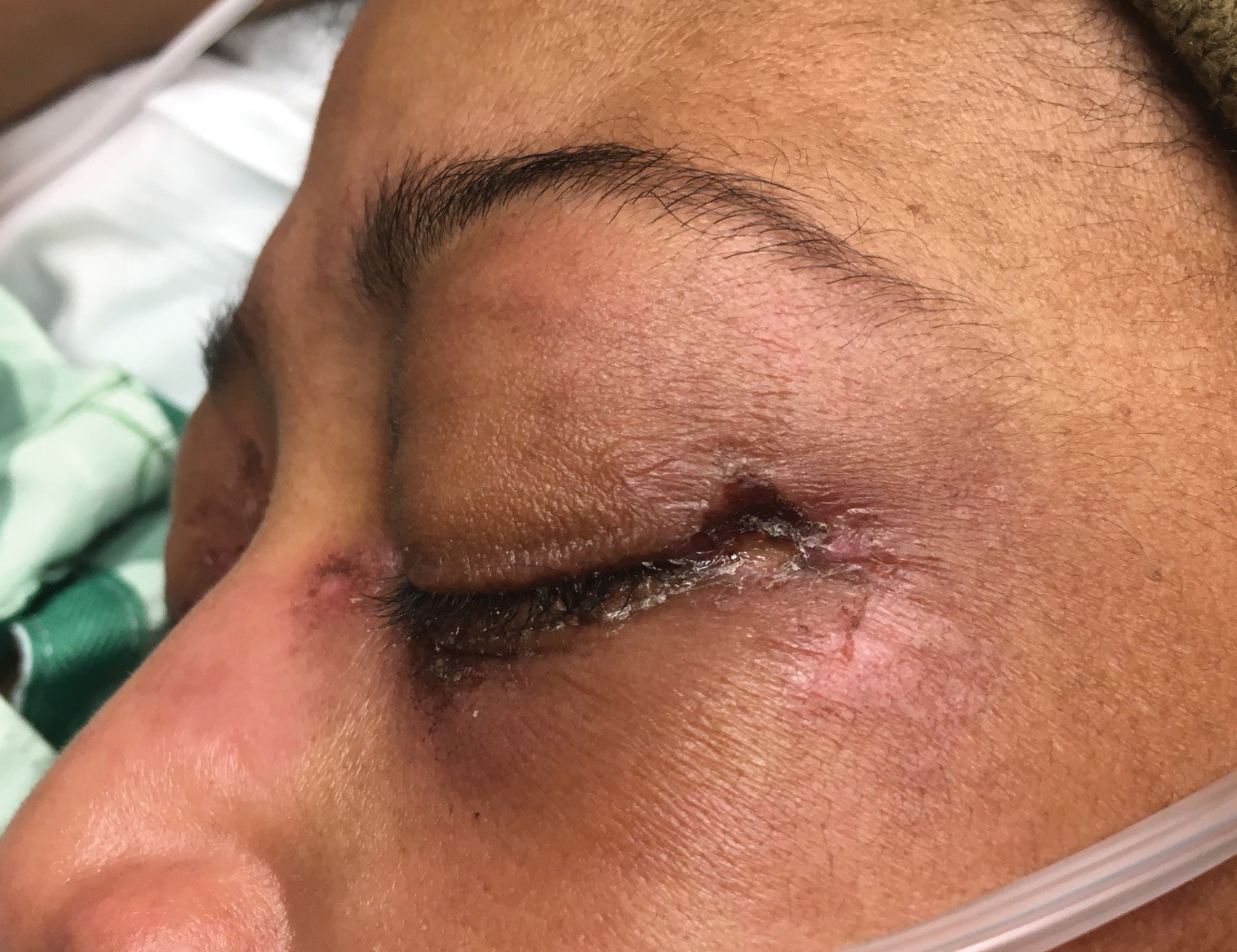
A woman in her 30s presented with diffuse arthralgias, bilateral eyelid edema, fatigue, and a progressive diffuse exanthem of 3 months’ duration. A review of systems was notable for the absence of myalgias. Physical examination revealed periorbital poikilodermatous patches with erythematous-to-violaceous plaques along the eyelid margins, violaceous papules on the dorsal knuckles, and edematous eroded plaques on the palmar fingertips. The patient was found to have a positive antinuclear antibody titer of 1:320 (reference range, <1:80) with a speckled pattern. A computed tomography (CT) scan of the chest showed patchy bilateral ground-glass opacities that were concerning for ILD. The cutaneous erosions, absence of myalgias, considerable proximal weakness, radiographic evidence of ILD, and positive antinuclear antibody test were clinically suggestive of anti-MDA5 DM. Further workup confirmed this diagnosis with positive reactivity to MDA5 by line immunoassay. The patient was treated with intravenous corticosteroids and was discharged after a 17-day hospitalization; however, she presented 2 months later to outpatient dermatology for progression of the cutaneous ulcerations, at which time an ulcerative heliotrope rash (Figure 1) was identified. Despite compliance with oral corticosteroids (1 mg/kg/d), she was hospitalized 1 month later for progressive respiratory insufficiency. A chest CT showed ground-glass linear opacities centrally located in all lobes of both lungs, consistent with rapidly progressive ILD. Over the course of her 5-day hospitalization, she was treated with corticosteroids, intravenous immunoglobulin (IVIG), and mycophenolate mofetil. The patient responded well to these therapies, leading to resolution of the respiratory symptoms, and she was discharged with plans to continue this regimen as an outpatient.
Patient 2
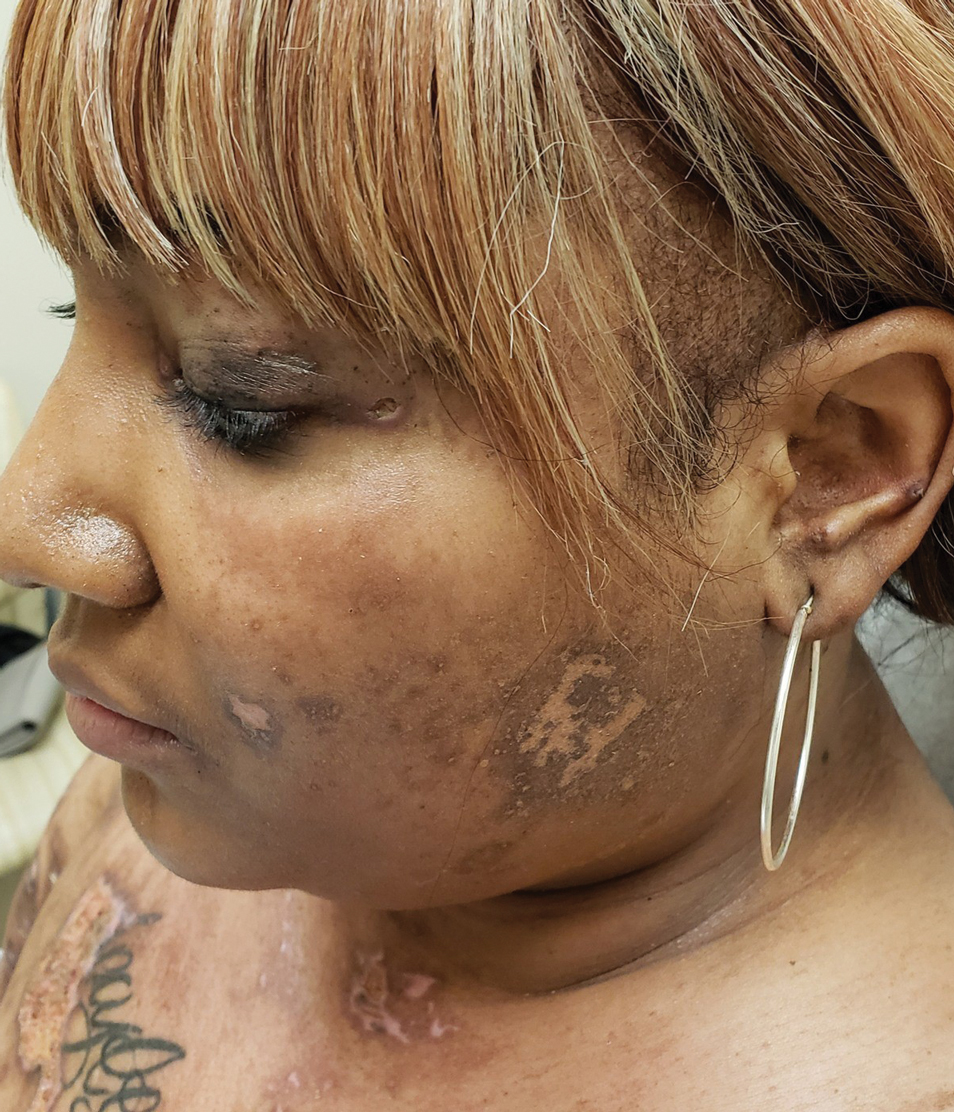
A woman in her late 30s with a history of known anti-MDA5 DM confirmed by line immunoassay 1 year prior presented to the emergency department with shortness of breath due to progressive ILD and a worsening exanthem. Dermatology was consulted to provide treatment recommendations. The treatment team was concerned for infection or anti-MDA5 DM disease progression. Physical examination revealed an ulcerative heliotrope rash (Figure 2) in addition to cutaneous findings classic for anti-MDA5 DM. Despite interventions, including high-dose corticosteroids, rituximab, IVIG, and plasma exchange, the ILD continued to progress, and the patient and her family elected to de-escalate aggressive medical care and pursue comfort care. The patient later died in in patient hospice.
Comment
Clinical Presentation of Anti-MDA5 DM
Dermatomyositis classically presents with cutaneous manifestations including a heliotropic erythematous rash and Gottron papules as well as accompanying muscle weakness.2 However, a subtype known as amyopathic DM, which includes anti-MDA5 DM, usually presents without muscle involvement.3 Clinical muscle weakness has been reported in cases of anti-MDA5 DM, though it is less likely in these patients.4 The characteristic cutaneous phenotype of
While a heliotrope rash is classic for DM, and ulcerations are a hallmark of the anti-MDA5 DM subtype, overlap of these cutaneous manifestations is not commonly reported. In both cases presented here, ulcerations of the lateral canthi were associated with progression of ILD.
Diagnosis of Anti-MDA5 DM
Anti-MDA5 DM is defined by the presence of the anti-MDA5 antibody in the serum, named for its reactivity against the RNA helicase encoded by MDA5, within the clinical context of cutaneous signs of DM as described above.12
As described by Rider et al,13 a thorough laboratory analysis, including complete blood cell count, serum electrolytes, calcium, magnesium, phosphorus, and thyroid-stimulating hormone, is necessary to rule out conditions with similar presentations. Additionally, serum analysis for elevated muscle enzymes (creatinine phosphokinase, aldolase, lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase) is necessary to assess for subclinical muscle involvement. Serologic evidence of myositis usually denotes an alternative diagnosis.13 Antinuclear antibodies and myositis-specific antibody positivity are much less frequent in the anti-MDA5 DM subtype than in other forms of DM.6
Anti-MDA5 antibody titer, ferritin, and IL-18 can be trended and may be useful in the evaluation of the response to treatment and ILD status in patients with anti-MDA5 DM.14,15 Elevated alveolar-arterial gradient, serum ferritin, serum chitotriosidase, and serum chitinase-3-like protein 1 (YKL-40) have each been associated with poorer prognosis of anti-MDA5 DM. The aforementioned serologies therefore may be helpful in determination of risk stratification and treatment aggressiveness.16-19
Because of its strong association with RP-ILD, screening for pulmonary disease is necessary in all patients with confirmed or strongly suspected anti-MDA5 DM. Screening can be performed with pulmonary function testing; however, high-resolution chest CT is the gold standard for diagnosis of ILD.20
Finally, all patients with a new diagnosis of DM should be evaluated for underlying malignancy through cancer screenings, given the propensity for DM to present as a paraneoplastic process.21 However, reports have indicated that the anti-MDA5 DM subtype may have a reduced risk for or an inverse relationship with underlying malignancy.5
Treatment Options for Anti-MDA5 DM
Early and aggressive therapy should be considered in the treatment of anti-MDA5 DM because of its association with RP-ILD. No treatment protocol is well established; thus, an individualized therapeutic approach may be guided by symptom severity and the clinical, radiographic, or functional evidence of ILD.6 High-dose systemic corticosteroids are first line, either in combination with or as a bridge to corticosteroid-sparing agents for immunosuppression. Many steroid-sparing medications have been employed with varying success. Mycophenolate mofetil is a reasonable first-line corticosteroid-sparing immunosuppressant agent, given its added benefit of attenuating ILD progression.6 A combination of high-dose corticosteroids, cyclosporine, and cyclophosphamide is utilized by some initially in the treatment of anti-MDA5 with ILD.22,23 While others have used combinations of these immunomodulatory agents with mycophenolate mofetil, IVIG, rituximab, azathioprine, tofacitinib, and polymyxin B, direct hemoperfusion has been added, leading to successful remission.23-28
Conclusion
We present 2 patients with anti-MDA5 DM who demonstrated a rare cutaneous manifestation of an ulcerative heliotrope rash. In both cases, this cutaneous finding was associated with the development of RP-ILD. Because of the strong association with and rapid progression of ILD seen in anti-MDA5 DM, early identification and aggressive treatment of this subtype are imperative. The clinician should recognize nonacral locations of cutaneous ulcerations, including an ulcerated heliotrope rash, to optimize diagnosis and management.
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30. doi:10.1001/archdermatol.2009.328
- Bogdanov I, Kazandjieva J, Darlenski R, et al. Dermatomyositis: current concepts. Clin Dermatol. 2018;36:450-458. doi:10.1016/j.clindermatol.2018.04.003
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27. doi:10.1001/archderm.138.1.23
- Li J, Liu Y, Li Y, et al. Associations between anti-melanoma differentiation-associated gene 5 antibody and demographics, clinical characteristics and laboratory results of patients with dermatomyositis: a systematic meta-analysis. J Dermatol. 2018;45:46-52. doi:10.1111/1346-8138.14092
- Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25-34. doi:10.1016/j.jaad.2010.09.016
- Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation–associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78:776-785. doi:10.1016/j.jaad.2017.12.010
- Narang NS, Casciola-Rosen L, Li S, et al. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease: analysis of skin ulcers in dermatomyositis. Arthritis Care Res. 2015;67:667-672. doi:10.1002/acr.22498
- Charrow A, Vleugels RA. Cutaneous ulcerations in anti-MDA5 dermatomyositis. N Engl J Med. 2019;381:465. doi:10.1056/NEJMicm1816147
- Cao H, Xia Q, Pan M, et al. Gottron papules and Gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43:1735-1742. doi:10.3899/jrheum.160024
- Moghadam-Kia S, Oddis CV, Sato S, et al. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol. 2017;44:319-325. doi:10.3899/jrheum.160682
- Li L, Wang Q, Wen X, et al. Assessment of anti-MDA5 antibody as a diagnostic biomarker in patients with dermatomyositis-associated interstitial lung disease or rapidly progressive interstitial lung disease. Oncotarget. 2017;876129-76140. doi:10.18632/oncotarget.19050
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193-2200. doi:10.1002/art.24621
- Rider LG, Miller FW. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA. 2011;305:183-190. doi:10.1001/jama.2010.1977
- Nishioka A, Tsunoda S, Abe T, et al. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod Rheumatol. 2019;29:814-820. doi:10.1080/14397595.2018.1548918
- Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology. 2012;51:1563-1570. doi:10.1093/rheumatology/kes102
- Jiang L, Wang Y, Peng Q, et al. Serum YKL-40 level is associated with severity of interstitial lung disease and poor prognosis in dermatomyositis with anti-MDA5 antibody. Clin Rheumatol. 2019;38:1655-1663. doi:10.1007/s10067-019-04457-w
- Fujisawa T, Hozumi H, Yasui H, et al. Clinical significance of serum chitotriosidase level in anti-MDA5 antibody–positive dermatomyositis-associated interstitial lung disease. J Rheumatol. 2019;46:935-942. doi:10.3899/jrheum.180825
- Enomoto N, Oyama Y, Enomoto Y, et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Resp J. 2018;12:2378-2389. doi:10.1111/crj.12918
- Fujiki Y, Kotani T, Isoda K, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. 2018;28:133-140. doi:10.1080/14397595.2017.1318468
- Raghu G, Remy-Jardin M, Myers JL, et al; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:E44-E68. doi:10.1164/rccm.201807-1255ST
- Yang Z, Lin F, Qin B, et al. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42:282-291. doi:10.3899/jrheum.140566
- Hisanaga J, Kotani T, Fujiki Y, et al. Successful multi-target therapy including rituximab and mycophenolate mofetil in anti-melanoma differentiation-associated gene 5 antibody-positive rapidly progressive interstitial lung disease with clinically amyopathic dermatomyositis. Int J Rheumatic Dis. 2017;20:2182-2185. doi:10.1111/1756-185X.13136
- Kameda H, Nagasawa H, Ogawa H, et al. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol. 2005;32:1719-1726.
- Endo Y, Koga T, Suzuki T, et al. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti–MDA5 antibody–positive dermatomyositis: a case report. Medicine. 2018;97:e0436. doi:10.1097/MD.0000000000010436
- So H, Wong VTL, Lao VWN, et al. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. 2018;37:1983-1989. doi:10.1007/s10067-018-4122-2
- Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology. 2018;57:2114-2119. doi:10.1093/rheumatology/key188
- Nawata T, Kubo M, Okuda S, et al. Successful treatment with intravenous cyclophosphamide for anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis associated with myelodysplastic syndrome. Scand J Rheumatol. 2017;46:496-498. doi:10.1080/03009742.2016.1253770
- Griger Z, Nagy-Vincze M, Dankó K. Pharmacological management of dermatomyositis. Exp Rev Clin Pharmacol. 2017;10:1109-1118. doi:10.1080/17512433.2017.1353910
Dermatomyositis (DM) is an autoimmune condition characterized by skin and muscle inflammation with an estimated incidence of 9 cases per 1 million people. The incidence of amyopathic DM, which includes antimelanoma differentiation–associated gene 5 (anti-MDA5) DM, is approximately 2 cases per 1 million people.1 Classic cutaneous manifestations of DM include a heliotrope rash, Gottron papules, and the shawl sign.
Case Reports
Patient 1
A woman in her 30s presented with diffuse arthralgias, bilateral eyelid edema, fatigue, and a progressive diffuse exanthem of 3 months’ duration. A review of systems was notable for the absence of myalgias. Physical examination revealed periorbital poikilodermatous patches with erythematous-to-violaceous plaques along the eyelid margins, violaceous papules on the dorsal knuckles, and edematous eroded plaques on the palmar fingertips. The patient was found to have a positive antinuclear antibody titer of 1:320 (reference range, <1:80) with a speckled pattern. A computed tomography (CT) scan of the chest showed patchy bilateral ground-glass opacities that were concerning for ILD. The cutaneous erosions, absence of myalgias, considerable proximal weakness, radiographic evidence of ILD, and positive antinuclear antibody test were clinically suggestive of anti-MDA5 DM. Further workup confirmed this diagnosis with positive reactivity to MDA5 by line immunoassay. The patient was treated with intravenous corticosteroids and was discharged after a 17-day hospitalization; however, she presented 2 months later to outpatient dermatology for progression of the cutaneous ulcerations, at which time an ulcerative heliotrope rash (Figure 1) was identified. Despite compliance with oral corticosteroids (1 mg/kg/d), she was hospitalized 1 month later for progressive respiratory insufficiency. A chest CT showed ground-glass linear opacities centrally located in all lobes of both lungs, consistent with rapidly progressive ILD. Over the course of her 5-day hospitalization, she was treated with corticosteroids, intravenous immunoglobulin (IVIG), and mycophenolate mofetil. The patient responded well to these therapies, leading to resolution of the respiratory symptoms, and she was discharged with plans to continue this regimen as an outpatient.

Patient 2
A woman in her late 30s with a history of known anti-MDA5 DM confirmed by line immunoassay 1 year prior presented to the emergency department with shortness of breath due to progressive ILD and a worsening exanthem. Dermatology was consulted to provide treatment recommendations. The treatment team was concerned for infection or anti-MDA5 DM disease progression. Physical examination revealed an ulcerative heliotrope rash (Figure 2) in addition to cutaneous findings classic for anti-MDA5 DM. Despite interventions, including high-dose corticosteroids, rituximab, IVIG, and plasma exchange, the ILD continued to progress, and the patient and her family elected to de-escalate aggressive medical care and pursue comfort care. The patient later died in in patient hospice.

Comment
Clinical Presentation of Anti-MDA5 DM
Dermatomyositis classically presents with cutaneous manifestations including a heliotropic erythematous rash and Gottron papules as well as accompanying muscle weakness.2 However, a subtype known as amyopathic DM, which includes anti-MDA5 DM, usually presents without muscle involvement.3 Clinical muscle weakness has been reported in cases of anti-MDA5 DM, though it is less likely in these patients.4 The characteristic cutaneous phenotype of
While a heliotrope rash is classic for DM, and ulcerations are a hallmark of the anti-MDA5 DM subtype, overlap of these cutaneous manifestations is not commonly reported. In both cases presented here, ulcerations of the lateral canthi were associated with progression of ILD.
Diagnosis of Anti-MDA5 DM
Anti-MDA5 DM is defined by the presence of the anti-MDA5 antibody in the serum, named for its reactivity against the RNA helicase encoded by MDA5, within the clinical context of cutaneous signs of DM as described above.12
As described by Rider et al,13 a thorough laboratory analysis, including complete blood cell count, serum electrolytes, calcium, magnesium, phosphorus, and thyroid-stimulating hormone, is necessary to rule out conditions with similar presentations. Additionally, serum analysis for elevated muscle enzymes (creatinine phosphokinase, aldolase, lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase) is necessary to assess for subclinical muscle involvement. Serologic evidence of myositis usually denotes an alternative diagnosis.13 Antinuclear antibodies and myositis-specific antibody positivity are much less frequent in the anti-MDA5 DM subtype than in other forms of DM.6
Anti-MDA5 antibody titer, ferritin, and IL-18 can be trended and may be useful in the evaluation of the response to treatment and ILD status in patients with anti-MDA5 DM.14,15 Elevated alveolar-arterial gradient, serum ferritin, serum chitotriosidase, and serum chitinase-3-like protein 1 (YKL-40) have each been associated with poorer prognosis of anti-MDA5 DM. The aforementioned serologies therefore may be helpful in determination of risk stratification and treatment aggressiveness.16-19
Because of its strong association with RP-ILD, screening for pulmonary disease is necessary in all patients with confirmed or strongly suspected anti-MDA5 DM. Screening can be performed with pulmonary function testing; however, high-resolution chest CT is the gold standard for diagnosis of ILD.20
Finally, all patients with a new diagnosis of DM should be evaluated for underlying malignancy through cancer screenings, given the propensity for DM to present as a paraneoplastic process.21 However, reports have indicated that the anti-MDA5 DM subtype may have a reduced risk for or an inverse relationship with underlying malignancy.5
Treatment Options for Anti-MDA5 DM
Early and aggressive therapy should be considered in the treatment of anti-MDA5 DM because of its association with RP-ILD. No treatment protocol is well established; thus, an individualized therapeutic approach may be guided by symptom severity and the clinical, radiographic, or functional evidence of ILD.6 High-dose systemic corticosteroids are first line, either in combination with or as a bridge to corticosteroid-sparing agents for immunosuppression. Many steroid-sparing medications have been employed with varying success. Mycophenolate mofetil is a reasonable first-line corticosteroid-sparing immunosuppressant agent, given its added benefit of attenuating ILD progression.6 A combination of high-dose corticosteroids, cyclosporine, and cyclophosphamide is utilized by some initially in the treatment of anti-MDA5 with ILD.22,23 While others have used combinations of these immunomodulatory agents with mycophenolate mofetil, IVIG, rituximab, azathioprine, tofacitinib, and polymyxin B, direct hemoperfusion has been added, leading to successful remission.23-28
Conclusion
We present 2 patients with anti-MDA5 DM who demonstrated a rare cutaneous manifestation of an ulcerative heliotrope rash. In both cases, this cutaneous finding was associated with the development of RP-ILD. Because of the strong association with and rapid progression of ILD seen in anti-MDA5 DM, early identification and aggressive treatment of this subtype are imperative. The clinician should recognize nonacral locations of cutaneous ulcerations, including an ulcerated heliotrope rash, to optimize diagnosis and management.
Dermatomyositis (DM) is an autoimmune condition characterized by skin and muscle inflammation with an estimated incidence of 9 cases per 1 million people. The incidence of amyopathic DM, which includes antimelanoma differentiation–associated gene 5 (anti-MDA5) DM, is approximately 2 cases per 1 million people.1 Classic cutaneous manifestations of DM include a heliotrope rash, Gottron papules, and the shawl sign.
Case Reports
Patient 1
A woman in her 30s presented with diffuse arthralgias, bilateral eyelid edema, fatigue, and a progressive diffuse exanthem of 3 months’ duration. A review of systems was notable for the absence of myalgias. Physical examination revealed periorbital poikilodermatous patches with erythematous-to-violaceous plaques along the eyelid margins, violaceous papules on the dorsal knuckles, and edematous eroded plaques on the palmar fingertips. The patient was found to have a positive antinuclear antibody titer of 1:320 (reference range, <1:80) with a speckled pattern. A computed tomography (CT) scan of the chest showed patchy bilateral ground-glass opacities that were concerning for ILD. The cutaneous erosions, absence of myalgias, considerable proximal weakness, radiographic evidence of ILD, and positive antinuclear antibody test were clinically suggestive of anti-MDA5 DM. Further workup confirmed this diagnosis with positive reactivity to MDA5 by line immunoassay. The patient was treated with intravenous corticosteroids and was discharged after a 17-day hospitalization; however, she presented 2 months later to outpatient dermatology for progression of the cutaneous ulcerations, at which time an ulcerative heliotrope rash (Figure 1) was identified. Despite compliance with oral corticosteroids (1 mg/kg/d), she was hospitalized 1 month later for progressive respiratory insufficiency. A chest CT showed ground-glass linear opacities centrally located in all lobes of both lungs, consistent with rapidly progressive ILD. Over the course of her 5-day hospitalization, she was treated with corticosteroids, intravenous immunoglobulin (IVIG), and mycophenolate mofetil. The patient responded well to these therapies, leading to resolution of the respiratory symptoms, and she was discharged with plans to continue this regimen as an outpatient.

Patient 2
A woman in her late 30s with a history of known anti-MDA5 DM confirmed by line immunoassay 1 year prior presented to the emergency department with shortness of breath due to progressive ILD and a worsening exanthem. Dermatology was consulted to provide treatment recommendations. The treatment team was concerned for infection or anti-MDA5 DM disease progression. Physical examination revealed an ulcerative heliotrope rash (Figure 2) in addition to cutaneous findings classic for anti-MDA5 DM. Despite interventions, including high-dose corticosteroids, rituximab, IVIG, and plasma exchange, the ILD continued to progress, and the patient and her family elected to de-escalate aggressive medical care and pursue comfort care. The patient later died in in patient hospice.

Comment
Clinical Presentation of Anti-MDA5 DM
Dermatomyositis classically presents with cutaneous manifestations including a heliotropic erythematous rash and Gottron papules as well as accompanying muscle weakness.2 However, a subtype known as amyopathic DM, which includes anti-MDA5 DM, usually presents without muscle involvement.3 Clinical muscle weakness has been reported in cases of anti-MDA5 DM, though it is less likely in these patients.4 The characteristic cutaneous phenotype of
While a heliotrope rash is classic for DM, and ulcerations are a hallmark of the anti-MDA5 DM subtype, overlap of these cutaneous manifestations is not commonly reported. In both cases presented here, ulcerations of the lateral canthi were associated with progression of ILD.
Diagnosis of Anti-MDA5 DM
Anti-MDA5 DM is defined by the presence of the anti-MDA5 antibody in the serum, named for its reactivity against the RNA helicase encoded by MDA5, within the clinical context of cutaneous signs of DM as described above.12
As described by Rider et al,13 a thorough laboratory analysis, including complete blood cell count, serum electrolytes, calcium, magnesium, phosphorus, and thyroid-stimulating hormone, is necessary to rule out conditions with similar presentations. Additionally, serum analysis for elevated muscle enzymes (creatinine phosphokinase, aldolase, lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase) is necessary to assess for subclinical muscle involvement. Serologic evidence of myositis usually denotes an alternative diagnosis.13 Antinuclear antibodies and myositis-specific antibody positivity are much less frequent in the anti-MDA5 DM subtype than in other forms of DM.6
Anti-MDA5 antibody titer, ferritin, and IL-18 can be trended and may be useful in the evaluation of the response to treatment and ILD status in patients with anti-MDA5 DM.14,15 Elevated alveolar-arterial gradient, serum ferritin, serum chitotriosidase, and serum chitinase-3-like protein 1 (YKL-40) have each been associated with poorer prognosis of anti-MDA5 DM. The aforementioned serologies therefore may be helpful in determination of risk stratification and treatment aggressiveness.16-19
Because of its strong association with RP-ILD, screening for pulmonary disease is necessary in all patients with confirmed or strongly suspected anti-MDA5 DM. Screening can be performed with pulmonary function testing; however, high-resolution chest CT is the gold standard for diagnosis of ILD.20
Finally, all patients with a new diagnosis of DM should be evaluated for underlying malignancy through cancer screenings, given the propensity for DM to present as a paraneoplastic process.21 However, reports have indicated that the anti-MDA5 DM subtype may have a reduced risk for or an inverse relationship with underlying malignancy.5
Treatment Options for Anti-MDA5 DM
Early and aggressive therapy should be considered in the treatment of anti-MDA5 DM because of its association with RP-ILD. No treatment protocol is well established; thus, an individualized therapeutic approach may be guided by symptom severity and the clinical, radiographic, or functional evidence of ILD.6 High-dose systemic corticosteroids are first line, either in combination with or as a bridge to corticosteroid-sparing agents for immunosuppression. Many steroid-sparing medications have been employed with varying success. Mycophenolate mofetil is a reasonable first-line corticosteroid-sparing immunosuppressant agent, given its added benefit of attenuating ILD progression.6 A combination of high-dose corticosteroids, cyclosporine, and cyclophosphamide is utilized by some initially in the treatment of anti-MDA5 with ILD.22,23 While others have used combinations of these immunomodulatory agents with mycophenolate mofetil, IVIG, rituximab, azathioprine, tofacitinib, and polymyxin B, direct hemoperfusion has been added, leading to successful remission.23-28
Conclusion
We present 2 patients with anti-MDA5 DM who demonstrated a rare cutaneous manifestation of an ulcerative heliotrope rash. In both cases, this cutaneous finding was associated with the development of RP-ILD. Because of the strong association with and rapid progression of ILD seen in anti-MDA5 DM, early identification and aggressive treatment of this subtype are imperative. The clinician should recognize nonacral locations of cutaneous ulcerations, including an ulcerated heliotrope rash, to optimize diagnosis and management.
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30. doi:10.1001/archdermatol.2009.328
- Bogdanov I, Kazandjieva J, Darlenski R, et al. Dermatomyositis: current concepts. Clin Dermatol. 2018;36:450-458. doi:10.1016/j.clindermatol.2018.04.003
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27. doi:10.1001/archderm.138.1.23
- Li J, Liu Y, Li Y, et al. Associations between anti-melanoma differentiation-associated gene 5 antibody and demographics, clinical characteristics and laboratory results of patients with dermatomyositis: a systematic meta-analysis. J Dermatol. 2018;45:46-52. doi:10.1111/1346-8138.14092
- Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25-34. doi:10.1016/j.jaad.2010.09.016
- Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation–associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78:776-785. doi:10.1016/j.jaad.2017.12.010
- Narang NS, Casciola-Rosen L, Li S, et al. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease: analysis of skin ulcers in dermatomyositis. Arthritis Care Res. 2015;67:667-672. doi:10.1002/acr.22498
- Charrow A, Vleugels RA. Cutaneous ulcerations in anti-MDA5 dermatomyositis. N Engl J Med. 2019;381:465. doi:10.1056/NEJMicm1816147
- Cao H, Xia Q, Pan M, et al. Gottron papules and Gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43:1735-1742. doi:10.3899/jrheum.160024
- Moghadam-Kia S, Oddis CV, Sato S, et al. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol. 2017;44:319-325. doi:10.3899/jrheum.160682
- Li L, Wang Q, Wen X, et al. Assessment of anti-MDA5 antibody as a diagnostic biomarker in patients with dermatomyositis-associated interstitial lung disease or rapidly progressive interstitial lung disease. Oncotarget. 2017;876129-76140. doi:10.18632/oncotarget.19050
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193-2200. doi:10.1002/art.24621
- Rider LG, Miller FW. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA. 2011;305:183-190. doi:10.1001/jama.2010.1977
- Nishioka A, Tsunoda S, Abe T, et al. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod Rheumatol. 2019;29:814-820. doi:10.1080/14397595.2018.1548918
- Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology. 2012;51:1563-1570. doi:10.1093/rheumatology/kes102
- Jiang L, Wang Y, Peng Q, et al. Serum YKL-40 level is associated with severity of interstitial lung disease and poor prognosis in dermatomyositis with anti-MDA5 antibody. Clin Rheumatol. 2019;38:1655-1663. doi:10.1007/s10067-019-04457-w
- Fujisawa T, Hozumi H, Yasui H, et al. Clinical significance of serum chitotriosidase level in anti-MDA5 antibody–positive dermatomyositis-associated interstitial lung disease. J Rheumatol. 2019;46:935-942. doi:10.3899/jrheum.180825
- Enomoto N, Oyama Y, Enomoto Y, et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Resp J. 2018;12:2378-2389. doi:10.1111/crj.12918
- Fujiki Y, Kotani T, Isoda K, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. 2018;28:133-140. doi:10.1080/14397595.2017.1318468
- Raghu G, Remy-Jardin M, Myers JL, et al; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:E44-E68. doi:10.1164/rccm.201807-1255ST
- Yang Z, Lin F, Qin B, et al. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42:282-291. doi:10.3899/jrheum.140566
- Hisanaga J, Kotani T, Fujiki Y, et al. Successful multi-target therapy including rituximab and mycophenolate mofetil in anti-melanoma differentiation-associated gene 5 antibody-positive rapidly progressive interstitial lung disease with clinically amyopathic dermatomyositis. Int J Rheumatic Dis. 2017;20:2182-2185. doi:10.1111/1756-185X.13136
- Kameda H, Nagasawa H, Ogawa H, et al. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol. 2005;32:1719-1726.
- Endo Y, Koga T, Suzuki T, et al. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti–MDA5 antibody–positive dermatomyositis: a case report. Medicine. 2018;97:e0436. doi:10.1097/MD.0000000000010436
- So H, Wong VTL, Lao VWN, et al. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. 2018;37:1983-1989. doi:10.1007/s10067-018-4122-2
- Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology. 2018;57:2114-2119. doi:10.1093/rheumatology/key188
- Nawata T, Kubo M, Okuda S, et al. Successful treatment with intravenous cyclophosphamide for anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis associated with myelodysplastic syndrome. Scand J Rheumatol. 2017;46:496-498. doi:10.1080/03009742.2016.1253770
- Griger Z, Nagy-Vincze M, Dankó K. Pharmacological management of dermatomyositis. Exp Rev Clin Pharmacol. 2017;10:1109-1118. doi:10.1080/17512433.2017.1353910
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30. doi:10.1001/archdermatol.2009.328
- Bogdanov I, Kazandjieva J, Darlenski R, et al. Dermatomyositis: current concepts. Clin Dermatol. 2018;36:450-458. doi:10.1016/j.clindermatol.2018.04.003
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27. doi:10.1001/archderm.138.1.23
- Li J, Liu Y, Li Y, et al. Associations between anti-melanoma differentiation-associated gene 5 antibody and demographics, clinical characteristics and laboratory results of patients with dermatomyositis: a systematic meta-analysis. J Dermatol. 2018;45:46-52. doi:10.1111/1346-8138.14092
- Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25-34. doi:10.1016/j.jaad.2010.09.016
- Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation–associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78:776-785. doi:10.1016/j.jaad.2017.12.010
- Narang NS, Casciola-Rosen L, Li S, et al. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease: analysis of skin ulcers in dermatomyositis. Arthritis Care Res. 2015;67:667-672. doi:10.1002/acr.22498
- Charrow A, Vleugels RA. Cutaneous ulcerations in anti-MDA5 dermatomyositis. N Engl J Med. 2019;381:465. doi:10.1056/NEJMicm1816147
- Cao H, Xia Q, Pan M, et al. Gottron papules and Gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43:1735-1742. doi:10.3899/jrheum.160024
- Moghadam-Kia S, Oddis CV, Sato S, et al. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol. 2017;44:319-325. doi:10.3899/jrheum.160682
- Li L, Wang Q, Wen X, et al. Assessment of anti-MDA5 antibody as a diagnostic biomarker in patients with dermatomyositis-associated interstitial lung disease or rapidly progressive interstitial lung disease. Oncotarget. 2017;876129-76140. doi:10.18632/oncotarget.19050
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193-2200. doi:10.1002/art.24621
- Rider LG, Miller FW. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA. 2011;305:183-190. doi:10.1001/jama.2010.1977
- Nishioka A, Tsunoda S, Abe T, et al. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod Rheumatol. 2019;29:814-820. doi:10.1080/14397595.2018.1548918
- Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology. 2012;51:1563-1570. doi:10.1093/rheumatology/kes102
- Jiang L, Wang Y, Peng Q, et al. Serum YKL-40 level is associated with severity of interstitial lung disease and poor prognosis in dermatomyositis with anti-MDA5 antibody. Clin Rheumatol. 2019;38:1655-1663. doi:10.1007/s10067-019-04457-w
- Fujisawa T, Hozumi H, Yasui H, et al. Clinical significance of serum chitotriosidase level in anti-MDA5 antibody–positive dermatomyositis-associated interstitial lung disease. J Rheumatol. 2019;46:935-942. doi:10.3899/jrheum.180825
- Enomoto N, Oyama Y, Enomoto Y, et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Resp J. 2018;12:2378-2389. doi:10.1111/crj.12918
- Fujiki Y, Kotani T, Isoda K, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. 2018;28:133-140. doi:10.1080/14397595.2017.1318468
- Raghu G, Remy-Jardin M, Myers JL, et al; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:E44-E68. doi:10.1164/rccm.201807-1255ST
- Yang Z, Lin F, Qin B, et al. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42:282-291. doi:10.3899/jrheum.140566
- Hisanaga J, Kotani T, Fujiki Y, et al. Successful multi-target therapy including rituximab and mycophenolate mofetil in anti-melanoma differentiation-associated gene 5 antibody-positive rapidly progressive interstitial lung disease with clinically amyopathic dermatomyositis. Int J Rheumatic Dis. 2017;20:2182-2185. doi:10.1111/1756-185X.13136
- Kameda H, Nagasawa H, Ogawa H, et al. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol. 2005;32:1719-1726.
- Endo Y, Koga T, Suzuki T, et al. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti–MDA5 antibody–positive dermatomyositis: a case report. Medicine. 2018;97:e0436. doi:10.1097/MD.0000000000010436
- So H, Wong VTL, Lao VWN, et al. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. 2018;37:1983-1989. doi:10.1007/s10067-018-4122-2
- Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology. 2018;57:2114-2119. doi:10.1093/rheumatology/key188
- Nawata T, Kubo M, Okuda S, et al. Successful treatment with intravenous cyclophosphamide for anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis associated with myelodysplastic syndrome. Scand J Rheumatol. 2017;46:496-498. doi:10.1080/03009742.2016.1253770
- Griger Z, Nagy-Vincze M, Dankó K. Pharmacological management of dermatomyositis. Exp Rev Clin Pharmacol. 2017;10:1109-1118. doi:10.1080/17512433.2017.1353910
Practice Points
- Antimelanoma differentiation–associated gene 5 dermatomyositis (anti-MDA5 DM) can present with an ulcerative heliotrope rash.
- Ulceration of the heliotrope rash in anti-MDA5 DM may indicate disease progression.
- Rapidly progressive interstitial lung disease is highly associated with anti-MDA5 DM.
Cultivating emotional awareness
A path to resilience and joy in the hospital
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
A path to resilience and joy in the hospital
A path to resilience and joy in the hospital
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Approaching the nursing unit, I heard the anxiety in my masked colleagues’ voices. I was starting another rotation on our COVID unit; this week I was trying to develop my emotional awareness in an effort to help with the stress of the job and, just as importantly, take in the moments of positive emotions when they arose. I was making a conscious effort to take in all I saw and felt in the same way I approached my patient examinations: my mind quiet, receptive, and curious.
Seeing my nursing teammates covered with personal protective equipment, I felt a little reverence at the purpose they bring to work. Thinking of our patients, isolated and scared in noisy, ventilated rooms, there was compassion welling up in my chest. Thinking about my role on the team, I felt humbled by the challenges of treating this new disease and meeting the needs of staff and patient.
A few years ago, a period of frustration and disaffectedness had led me to apply my diagnostic eye to myself: I was burning out. Developing a mindfulness practice has transformed my experience at work. Now, the pandemic pushed me to go beyond a few minutes of quieting the mind before work. I was developing my emotional awareness. A growing body of research suggests that emotional awareness helps temper the negative experiences and savor the good. This week on the COVID unit was an opportunity to put this idea to the test.
Across the hall from the desk was Ms. A, 85-year-old woman who always clutched her rosary. My Spanish is not great, but I understood her prayer when I entered the room. She had tested positive for COVID about 7 days before – so had all the people in her multigenerational home. Over the din of the negative-pressure machine, with damp eyes she kept saying she wanted to go home. I felt my body soften and, in my chest, it felt as if my heart moved towards her which is the manifestation of compassion. “I will do my best to get you there soon,” I said in an effort to comfort her.
We often resist strong emotions, especially at work, because they can increase stress in situations where we need to be in control. In high-emotion situations, our brain’s warning centers alert both body and brain. This has helped our ancestors to action over the millennia, but in the hospital, these responses hurt more than help. Our bodies amplifying the emotion, our mind races for solutions and we can feel overwhelmed.
Simply recognizing the emotion and naming it puts the brakes on this process. fMRI data demonstrate that naming the emotions moves the brain activity away from the emotion centers to the appraisal centers in the frontal lobe. Just the perspective to see the emotional process calms it down.
Name it to tame it – this is what those in the field call this act. “This is sadness,” I said to myself as I left Ms. A’s room.
Down the hall was Mr. D; he was an 81-year-old former Vietnamese refugee. He had come in 3 days prior to my coming on service. While he didn’t talk, even with an interpreter, he ate well and had looked comfortable for days on 50% O2.
Ms. A’s O2 needs crept up each day as did her anxiety, the plaintive tenor of her prayers and inquiries about going home. I got a priest to visit, not for last rites but just for some support. Over the phone, I updated the family on the prognosis.
A couple of days later, she needed 95% O2 and with PO2 was only 70. I told her family it seemed she was losing her battle with the virus. I said we could see how she did on 60% – that’s the max she could get at home with hospice. I called them after 2 hours on 60% to tell them she was up eating and despite slight increased resp rate, she looked okay. “Can you guarantee that she would not make it if she stayed in the hospital? “
My body vibrating with uncertainty – an emotional mix of fear and sadness – I said, “I am sorry, but this is such a new disease, I can’t say that for certain.” On the call, family members voiced different opinions, but in the end, they were unable to give up hope, so we agreed to keep her in hospital.
Down the hall, Mr. D had stopped eating and his sats dropped as did his blood pressure. A nurse exited his room; despite the mask and steamed-up glasses, I could read her body language. “That poor man is dying,” she said. I told her I agreed and called the family with the news and to offer them a chance to visit and to talk about home hospice.
“He has not seen any of us in 10 months,” said his daughter over the phone, “We would love to visit and talk about bringing him home on hospice.” The next morning four of his nine kids showed up with a quart of jook, an Asian rice porridge, for him and pastries for the staff.
They left the room smiling an hour later. “He ate all the jook and he smiled! Yes, let’s work on home with hospice.” That night his blood pressure was better, and we were able to move him to 8 liters oxymizer; the staff was excited by his improvement, too.
The next day Ms. A was less responsive with sats in the 80’s on 100% FiO2, but she still had this great sense of warmth and dignity about her. When I entered the room, Spanish Catholic hymns were playing, two of her kids stood leaning over the bed and on an iPod, there was a chorus of tears. 20 family members were all crying on a Zoom call. Together this made the most beautiful soundtrack to an end of life I have ever heard. I tried hard not to join the chorus as we talked about turning off the oxygen to help limit her suffering.
We added a bolus of morphine to her drip and removed the oxygen. She looked more beautiful and peaceful without it. Briefly, she closed her eyes then opened them, her breathing calmer. And with the hymns and the chorus of family crying she lived another 20 minutes in the loving presence of her big family.
Leaving the room, I was flooded with “woulda, coulda, shouldas” that accompany work with so much uncertainty and high stakes. “Maybe I should have tried convalescent plasma. Maybe I should have told them she must go home,” and so on my mind went on looking for solutions when there were none. I turned to my body – my chest ached, and I whispered to myself: “This is how sadness feels.”
By thinking about how the emotion feels in the body, we move the mind away from problem solving that can end up leading to unhealthy ruminations. Such thoughts in times of high emotions lead to that pressurized, tightness feeling we get when overwhelmed. Taking in the universal sensations of the emotions is calming and connects us with these deep human experiences in healthy ways. At the same time, the racing and ruminations stop.
Meanwhile, down the hall, Mr. D’s family arrived in great spirits armed with more food for patient and staff. He was to go home later that day with hospice. When they saw him up in the chair without the oxygen, they said: “It is a miracle, Dr. Hass! He is going home on hospice but having beat COVID! We can’t thank you enough!”
“Don’t thank me! He was cured by love and jook! What a lesson for us all. Sometimes there is no better medicine than food from home and love!” With the explosive expansiveness of joy, we shared some “elbow bumps” and took some pictures before he was wheeled home.
Back at the nurse’s station, there were tears. Sometimes life is so full of emotion that it is hard to give it a name – joy? grief? Our bodies almost pulsing, our minds searching for words, it is as if an ancient process is marking a time and place in our souls. “This is what it is to be a human being living with love and creating meaning,” the experience seems to be telling us.
This is awesome work. In fact, awe was what we were feeling then – that sense of wonder we have in the presence of something beautiful or vast that we cannot easily comprehend. Taking in these moments of awe at the power and depth of the human experience is critical to keep us humble, engaged, and emotionally involved.
Cultivating emotional awareness is a simple technique to maintain equanimity as we do the emotionally turbulent work of caring for vulnerable and seriously ill members of our community. It uses the same techniques of attention and diagnosis we use on those we care for. It is a practice that can be seamlessly incorporated into our workday with no time added. Recognizing it, naming it, and feeling it will give us the resilience to handle the challenges this amazing work inevitably brings.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley–UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Microaggressions in Medicine
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10
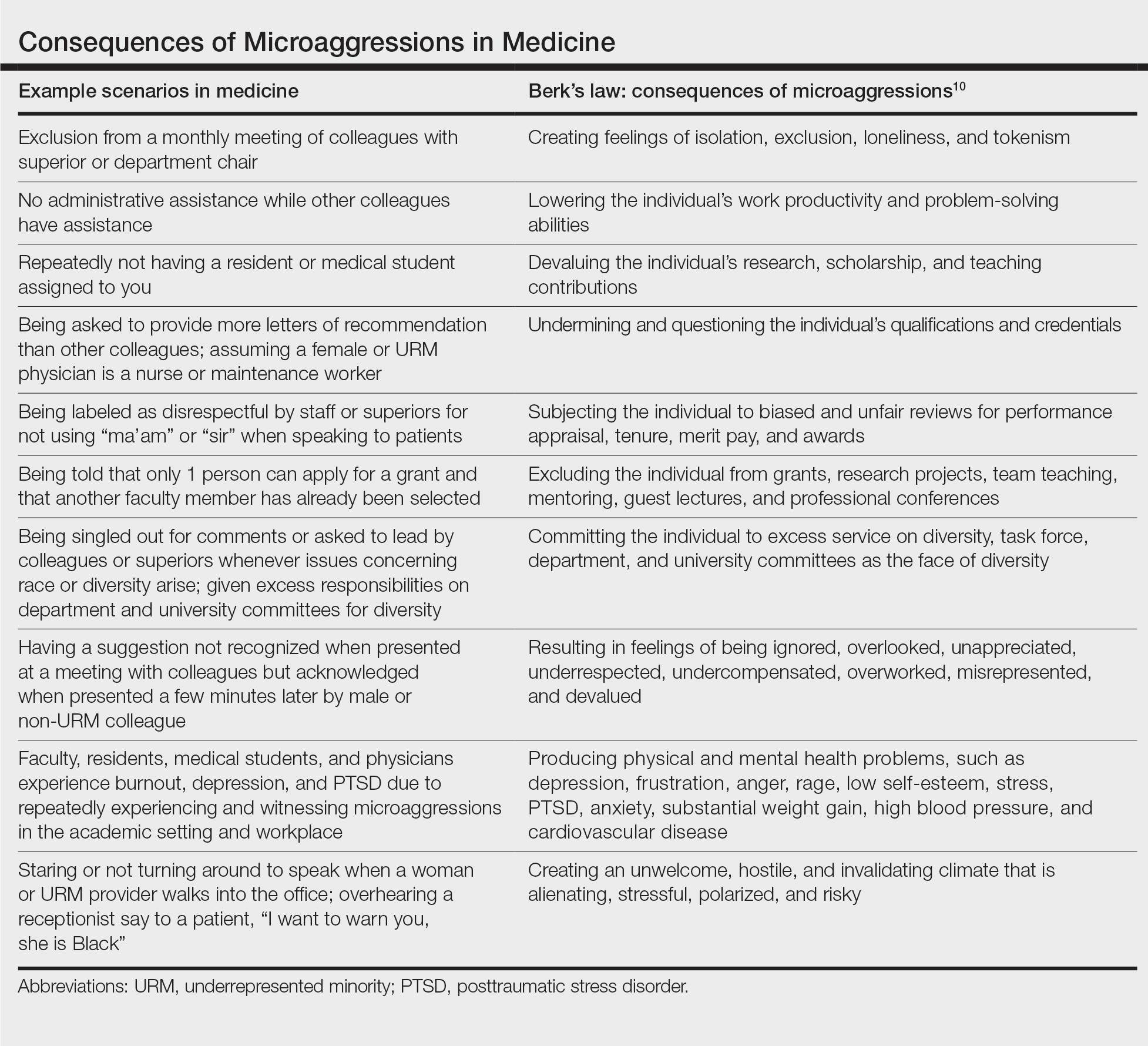
Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10

Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
As manifestations of overt racism and macroaggressions have gained increased visibility, there is a need for discussion of another expression of racism: microaggressions. Although racism classically is viewed as blatant structural, attitudinal, and behavioral prejudice, experts pose that the face of racism has evolved into a more covert insidious form. This form of racism was originally coined racial microaggressions by psychiatrist Chester M. Pierce, MD, 50 years ago.1,2 Since that time, microaggressions have further expanded to describe “brief and commonplace daily verbal, behavioral, and environmental indignities, whether intentional or unintentional, that communicate hostile, derogatory, or negative racial, gender, sexual-orientation, and religious slights and insults to the target person or group.” 3 This article aims to define and depict examples of microaggressions in medicine, discuss the resulting harmful effects, and offer strategies to minimize and counter these negative ramifications.
What are microaggressions?
Microaggressions are behaviors that stem from implicit bias and occur at an interpersonal level. Implicit bias refers to unconscious stereotypes, assumptions, and beliefs held about an individual’s identity. One of the earliest microaggressions—invisibility—was characterized by Ralph Ellison in his novel Invisible Man. Ellison states, “I am invisible, understand, simply because people refuse to see me . . . When they approach me they see only my surroundings, themselves, or figments of their imagination—indeed, everything and anything except me.”4 This concept of invisibility is a primary microaggression faced by people of color.
In medicine, microaggressions and implicit bias may be encountered throughout medical training and clinical practice in interactions with colleagues, superiors, patients, and patients’ families.5,6 Examples of microaggressions in medicine include demeaning comments, nonverbal disrespect, generalizations of social identity, assumption of nonphysician status, role- or credential-questioning behavior, explicit epithets, rejection of care, questioning or inquiries of ethnic/racial origin, and sexual harassment.7
An example of microaggressions in medicine was fully displayed when physician Tamika Cross described her experience of being turned away from helping an unresponsive passenger during a flight emergency.
[T]he flight attendant yells “call overhead for a physician on board.” I raised my hand to grab her attention. She said to me “oh no sweetie put [your] hand down, we are looking for actual physicians or nurses or some type of medical personnel, we don’t have time to talk to you” . . . Another “seasoned” white male approaches the row and says he is a physician as well. She says to me “thanks for your help but he can help us, and he has his credentials.”8
What are the effects of microaggressions?
Although microaggressions may be unconscious and unintentional by the offender, the negative ramifications are notable. Recent studies report that women and underrepresented minority (URM) medical students, residents, and physicians experience microaggressions and implicit bias at a higher prevalence and frequency compared with their male and non-URM counterparts.7,9 Repetitive microaggressions are harmful to the health and safety of women and URM medical students, residents, physicians, other providers, and patients. The Table provides example scenarios of microaggressions in medicine categorized according to Berk.10

Microaggressions negatively impact physical, mental, and emotional well-being. Current data support that medical students and residents who experience microaggressions are more likely to report associated symptoms of burnout, depression, and suicidal thoughts.11,12 Subjection to persistent bias can lead to minority status stress and racial battle fatigue, creating feelings of invisibility, isolation, exclusion, and loneliness for those impacted.13,14
In the book Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine, Damon Tweedy, MD, reflects on race in medicine. Tweedy notes his experience as a medical student when a professor mistakenly assumed he was a maintenance worker in the classroom. Tweedy describes how he internalized the exchange and, despite his success throughout the course of his medical training, combatted feelings of anxiety, self-doubt, and implied inferiority.15
Although microaggressions are harmful to one’s health, they also undermine the learning and teaching experience for students, residents, and faculty, and they detract from the larger goal of providing care for patients.11 Frequent devaluing and questioning of an individual’s contributions, qualifications, and credentials based on identity can lower productivity and problem-solving abilities. These behaviors cultivate an unwelcome and hostile work/learning environment that is stressful and polarizing for the recipient.
Despite the heavy burden of microaggressions, most students, residents, and faculty physicians do not report incidents to their institutions and feel that training, resources, and policies to respond to bias adequately are lacking.7 As a result of implicit bias and microaggressions, women and URM medical students and providers are unable to focus solely on the practice of medicine. They are tasked with the additional burden of shouldering the emotional and cognitive complexities that microaggressions produce.16
What are strategies to reduce microaggressions in medicine?
To minimize the harmful effects of microaggressions, intervention strategies must be implemented that reduce the likelihood of the occurrence of microaggressions and challenge the stereotypes that undergird implicit bias. These strategies include cultivating allies, followed by demanding structural accountability. Allies are members of the majority group who collectively collaborate with members of the nonmajority group to effect change through the promotion of diversity, equity, and inclusion efforts.17 Cultivating allies involves building a network of collaboration among these groups and emphasizes education. Education is critical for allies to address microaggressions at the interpersonal level. This process of education involves personal reflection and self-awareness in exploring one’s biases, fears, and assumptions. Integral to this step is broadening one’s acceptance of different cultures, racial/ethnic groups, and identities. There must be a willingness to engage in difficult or uncomfortable conversations and a readiness to actively listen to concerns rather than perpetuating further harm through avoidance and dismissive or defensive behavior.18
Demanding structural accountability facilitates deconstruction of bias and microaggression at the larger systemic level. This strategy involves implicit bias and antiracism training, development of retention plans, and identification of mentors for women and URM providers and students. Implicit bias and microaggression training and policies should be incorporated into medical education and resident curriculums. Similarly, educational resources and training must be made available to practicing physicians, faculty, and other providers through their institutions and places of employment. Equipping students and providers with the tools needed when microaggressions are witnessed or experienced demonstrates systemic-level accountability and communicates the importance of the issue. Furthermore, the development of retention plans and identification of mentors provide a support system and foster a culture of inclusion where recipients of microaggressions feel protected and valued. Increased feelings of inclusivity and belonging help bridge the gap created through microaggressions and implicit bias.
Final Thoughts
Despite an often covert nature, the detrimental effects of microaggressions are tangible and far reaching. As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice. It is our obligation to undertake the challenge of “making the ‘invisible’ visible” as we confront microaggressions and implicit bias to promote a safer and more inclusive medical community and workforce.19
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
- Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154:868-872. doi:10.1001/jamasurg.2019.1648
- Williams MT. Microaggressions: clarification, evidence, and impact. Perspect Psychol Sci. 2020;15:3-26. doi:10.1177/1745691619827499
- Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Wiley; 2010.
- Ellison R. Invisible Man. Random House; 1952.
- Molina MF, Landry AI, Chary AN, et al. Addressing the elephant in the room: microaggressions in medicine. Ann Emerg Med. 2020;76:387-391. doi:10.1016/j.annemergmed.2020.04.009
- Overland MK, Zumsteg JM, Lindo EG, et al. Microaggressions in clinical training and practice. PM R. 2019;11:1004-1012. doi:10.1002/pmrj.12229
- de Bourmont SS, Burra A, Nouri SS, et al. Resident physician experiences with and responses to biased patients. JAMA Netw Open. 2020;3:e2021769. doi:10.1001/jamanetworkopen.2020.21769
- TK Cross Facebook page. October 9, 2016. Accessed April 19, 2021. https://www.facebook.com/tamika.cross.52/posts/658443077654049
- Periyakoil VS, Chaudron L, Hill EV, et al. Common types of gender-based microaggressions in medicine. Acad Med. 2020;95:450-457. doi:10.1097/ACM.0000000000003057
- Berk RA. Microaggressions trilogy: part 1. why do microaggressions matter? J Fac Dev. 2017;31:63-73.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2020:S0027-9684(20)30428-4. doi:10.1016/j.jnma.2020.11.009
- Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381:1741-1752. doi:10.1056/NEJMsa1903759
- Acholonu RG, Oyeku SO. Addressing microaggressions in the health care workforce-a path toward achieving equity and inclusion. JAMA Netw Open. 2020;3:E2021770. doi:10.1001/jamanetworkopen.2020.21770
- O’Keefe VM, Wingate LR, Cole AB, et al. Seemingly harmless racial communications are not so harmless: racial microaggressions lead to suicidal ideation by way of depression symptoms. Suicide Life Threat Behav. 2015;45:567-576. doi:10.1111/sltb.12150
- Tweedy D. Black Man in a White Coat: A Doctor’s Reflections on Race and Medicine. Picador; 2016.
- Osseo-Asare A, Balasuriya L, Huot SJ, et al. Minority resident physicians’ views on the role of race/ethnicity in their training experiences in the workplace. JAMA Netw Open. 2018;1:E182723. doi: 10.1001/jamanetworkopen.2018.2723
- Melaku TM, Beeman A, Smith DG, et al. Be a better ally. Harvard Business Review. Published November-December 2020. Accessed April 23, 2021. https://hbr.org/2020/11/be-a-better-ally
- Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62:271-286. doi:10.1037/0003-066X.62.4.271
- Sue DW. Whiteness and ethnocentric monoculturalism: making the “invisible” visible. Am Psychol. 2004;59:761-769. doi:10.1037/0003-066X.59.8.761
Practice Points
- As providers, we must strive to understand all categories of racism and expose the many ways prejudice manifests within medical training and clinical practice.
- Intervention strategies must be implemented to reduce the likelihood of the occurrence of microaggressions in medicine and challenge the stereotypes that undergird implicit bias.
- It is important to promote collaboration in diversity, equity, and inclusion efforts to demonstrate support for women and underrepresented minority medical students, residents, physicians, providers, and patients.
Psychosis, depression tied to neurodegeneration in Parkinson’s
Depression and psychosis are significantly associated with neuronal loss and gliosis – but not with Lewy body scores – in Parkinson’s disease, data from analyses of the brains of 175 patients suggest.
Previous research has suggested a link between neuronal loss and depression in Parkinson’s disease (PD) but the impact of Lewy bodies has not been well studied, Nicole Mercado Fischer, MPH, of Johns Hopkins University, Baltimore, and colleagues wrote.
Evaluating Lewy body scores and neuronal loss/gliosis in the substantia nigra pars compacta (SN) and locus coeruleus (LC) could increase understanding of pathophysiology in PD, they said.
In a study published in the American Journal of Geriatric Psychiatry, the researchers analyzed the brains of 175 individuals with a primary diagnosis of PD.
A total of 98 participants had diagnoses of psychosis, 88 had depression, and 55 had anxiety. The average age of onset for PD was 62.4 years; 67.4% of the subjects were male, and 97.8% were White. The mean duration of illness was 16 years, and the average age at death was 78 years.
Psychosis was significantly associated with severe neuronal loss and gliosis in both the LC and SN (P = .048 and P = .042, respectively). Depression was significantly associated with severe neuronal loss in the SN (P = .042) but not in the LC. Anxiety was not associated with severe neuronal loss in either brain region. These results remained significant after a multivariate analysis, the researchers noted. However, Lewy body scores were not associated with any neuropsychiatric symptom, and severity of neuronal loss and gliosis was not correlated with Lewy body scores.
The study findings were limited by several factors, including the retrospective design and inability to collect pathology data for all patients, the researchers noted. Also, in some cases, the collection of clinical data and observation of brain tissue pathology took place years apart, and the researchers did not assess medication records.
However, the results were strengthened by the large sample size and “further support the notion that in vivo clinical symptoms of PD are either not caused by Lewy body pathology or that the relationship is confounded by the time of autopsy,” they said. and eventually by using new functional imaging techniques in vivo.”
The researchers had no financial conflicts to disclose. Two coauthors were supported in part by the National Institutes of Health.
Depression and psychosis are significantly associated with neuronal loss and gliosis – but not with Lewy body scores – in Parkinson’s disease, data from analyses of the brains of 175 patients suggest.
Previous research has suggested a link between neuronal loss and depression in Parkinson’s disease (PD) but the impact of Lewy bodies has not been well studied, Nicole Mercado Fischer, MPH, of Johns Hopkins University, Baltimore, and colleagues wrote.
Evaluating Lewy body scores and neuronal loss/gliosis in the substantia nigra pars compacta (SN) and locus coeruleus (LC) could increase understanding of pathophysiology in PD, they said.
In a study published in the American Journal of Geriatric Psychiatry, the researchers analyzed the brains of 175 individuals with a primary diagnosis of PD.
A total of 98 participants had diagnoses of psychosis, 88 had depression, and 55 had anxiety. The average age of onset for PD was 62.4 years; 67.4% of the subjects were male, and 97.8% were White. The mean duration of illness was 16 years, and the average age at death was 78 years.
Psychosis was significantly associated with severe neuronal loss and gliosis in both the LC and SN (P = .048 and P = .042, respectively). Depression was significantly associated with severe neuronal loss in the SN (P = .042) but not in the LC. Anxiety was not associated with severe neuronal loss in either brain region. These results remained significant after a multivariate analysis, the researchers noted. However, Lewy body scores were not associated with any neuropsychiatric symptom, and severity of neuronal loss and gliosis was not correlated with Lewy body scores.
The study findings were limited by several factors, including the retrospective design and inability to collect pathology data for all patients, the researchers noted. Also, in some cases, the collection of clinical data and observation of brain tissue pathology took place years apart, and the researchers did not assess medication records.
However, the results were strengthened by the large sample size and “further support the notion that in vivo clinical symptoms of PD are either not caused by Lewy body pathology or that the relationship is confounded by the time of autopsy,” they said. and eventually by using new functional imaging techniques in vivo.”
The researchers had no financial conflicts to disclose. Two coauthors were supported in part by the National Institutes of Health.
Depression and psychosis are significantly associated with neuronal loss and gliosis – but not with Lewy body scores – in Parkinson’s disease, data from analyses of the brains of 175 patients suggest.
Previous research has suggested a link between neuronal loss and depression in Parkinson’s disease (PD) but the impact of Lewy bodies has not been well studied, Nicole Mercado Fischer, MPH, of Johns Hopkins University, Baltimore, and colleagues wrote.
Evaluating Lewy body scores and neuronal loss/gliosis in the substantia nigra pars compacta (SN) and locus coeruleus (LC) could increase understanding of pathophysiology in PD, they said.
In a study published in the American Journal of Geriatric Psychiatry, the researchers analyzed the brains of 175 individuals with a primary diagnosis of PD.
A total of 98 participants had diagnoses of psychosis, 88 had depression, and 55 had anxiety. The average age of onset for PD was 62.4 years; 67.4% of the subjects were male, and 97.8% were White. The mean duration of illness was 16 years, and the average age at death was 78 years.
Psychosis was significantly associated with severe neuronal loss and gliosis in both the LC and SN (P = .048 and P = .042, respectively). Depression was significantly associated with severe neuronal loss in the SN (P = .042) but not in the LC. Anxiety was not associated with severe neuronal loss in either brain region. These results remained significant after a multivariate analysis, the researchers noted. However, Lewy body scores were not associated with any neuropsychiatric symptom, and severity of neuronal loss and gliosis was not correlated with Lewy body scores.
The study findings were limited by several factors, including the retrospective design and inability to collect pathology data for all patients, the researchers noted. Also, in some cases, the collection of clinical data and observation of brain tissue pathology took place years apart, and the researchers did not assess medication records.
However, the results were strengthened by the large sample size and “further support the notion that in vivo clinical symptoms of PD are either not caused by Lewy body pathology or that the relationship is confounded by the time of autopsy,” they said. and eventually by using new functional imaging techniques in vivo.”
The researchers had no financial conflicts to disclose. Two coauthors were supported in part by the National Institutes of Health.
FROM THE AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Prevalence of psychiatric disorders higher in adult cerebral palsy patients
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
Adults with cerebral palsy, especially those with intellectual disabilities, are significantly more likely to be diagnosed with a psychiatric disorder, compared with the general population, a review of seven datasets shows.
The body of literature on psychiatric issues in children with cerebral palsy (CP) is increasing, but population-based studies of psychiatric issues in adults with CP have been limited in number and in scope. Most of those studies focus mainly on anxiety and depression, rather than on other issues such as psychosis or schizophrenia, Carly A. McMorris, PhD, of the University of Calgary (Alta.) and colleagues wrote.
In a retrospective, cross-sectional study published in Research in Developmental Disabilities, the researchers reviewed information from five health data sets, one registry, and census data for adults aged 18-64 years with a CP diagnosis living in Ontario, including those with and without diagnosed intellectual disabilities (ID) and a comparison group of individuals in the general population. The researchers examined the proportion of individuals with a psychiatric disorder in each of four groups: total CP, CP without ID, CP with ID, and the general population.
The study participants included 9,388 individuals with CP, 4,767 individuals with CP and ID, and a general population of 2,757,744 individuals. About half of the participants were male, and at least 85% lived in urban areas.
Overall, compared with the general population group, over a 2-year period (33.7 % vs. 24.7%). Also, the CP group was more than twice as likely to be diagnosed with a psychotic disorder, schizophrenia, personality disorder, or bipolar disorder, compared with the general population. Individuals with CP were significantly more likely to suffer from mood or affective disorders, and depression and anxiety disorders, compared with the general population, but less likely to suffer from substance use disorders.
When the data were assessed by ID status, disorders such as psychotic disorders, bipolar disorders, and schizophrenia were six times more common among individuals with CP and ID, compared with the general population (adjusted prevalence ratios, 6.26 and 6.46, respectively).
Individuals with CP and ID also had a notably higher prevalence of bipolar disorder (confidence interval, 2.06-2.89) and personality disorder, compared with the general population (aPR, 2.44 and 4.22, respectively), but this subgroup also was less likely than the general population to engage in substance use (aPR, 0.44).
The study findings were limited by several factors, including the absence of universal definitions for some of the conditions studied, potential misclassification of ID, the inclusion of data on specific psychiatric diagnoses but not elevated symptoms, and by the challenges of diagnosing psychiatric disorders in individuals with ID, the researchers noted.
However, “the present study contributes important information to the existing literature, highlighting that psychiatric issues are common in adults with CP, similar to what has been reported in children and youth,” they said. “Further research is needed to determine the validity and reliability of mental health assessment measures for this population, the efficacy of evidence-based psychotherapeutic approaches ... and the underlying causes or mechanisms of psychiatric issues in individuals with CP.”
The findings also highlight the need for health care clinicians to screen for psychiatric issues in CP patients, they said.
The study was supported in part by the Province of Ontario research grants and the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The researchers had no disclosures.
FROM RESEARCH IN DEVELOPMENTAL DISABILITIES
Sotagliflozin’s HFpEF benefit confirmed by new analyses
It’s now official: The investigational sodium-glucose cotransporter (SGLT) 1/2 inhibitor sotagliflozin is the first agent clearly shown in a prespecified analysis of randomized trials to improve clinical outcomes in patients with heart failure with reduced ejection fraction (HFpEF).
Researchers who ran the SCORED and SOLOIST-WHF pivotal trials for sotagliflozin first made that claim in November 2020 when reporting top-line results from a prespecified meta-analysis of the two trials during the American Heart Association annual scientific sessions. A follow-up report during the annual scientific sessions of the American College of Cardiology fleshed out the evidence and firmed up their landmark conclusion.
The meta-analysis (Abstract 410-08) included 4,500 patients with type 2 diabetes and diagnosed heart failure at entry; its primary endpoint, which was the same in both trials, was the combined incidence of cardiovascular death and the total number of either hospitalization for heart failure or urgent outpatient visits for heart failure.
Compared with placebo, treatment with sotagliflozin for a median of about 15 months dropped this composite endpoint by a relative 33% among the 1,931 who began the study with a left ventricular ejection fraction (LVEF) of at least 50% (HFpEF), by a relative 22% in the 1,758 patients who entered with an LVEF of less than 40% (patients with heart failure with reduced ejection fraction), and by a relative 43% among the 811 patients who began with an LVEF of 40%-49% (patients with heart failure with mid-range ejection fraction). The relative risk reductions were significant for all three subgroups, Deepak L. Bhatt, MD, reported at the meeting.
Equally effective ‘across the full range of LVEFs.’
Perhaps as notable and unprecedented was the further finding that the clinical benefits seen with treatment of patients with type 2 diabetes with sotagliflozin was consistent regardless of the ejection fraction they had at entry. Enrolled patients with baseline LVEFs in the range of 25% received a relative benefit from sotagliflozin treatment that was statistically no different from the benefit seen in patients who entered with an LVEF in the neighborhood of 45%, 65%, or at any other level across the LVEF spectrum, a finding that Dr. Bhatt called “remarkable” during a press briefing. “The results show the benefit of sotagliflozin across the full range of LVEFs.”
“We are very excited in the heart failure world by the SGLT2 inhibitors; we’ve been impressed by their reduction in heart failure hospitalizations, but we wonder about the patients with HFpEF, where we haven’t had a blockbuster drug to give,” said Ileana L. Piña, MD, a heart failure specialist and medical officer with the Food and Drug Administration.
The new findings “look like they could pose a regulatory indication [for sotagliflozin] for patients with type 2 diabetes and heart failure across the entire spectrum of heart failure,” said Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart & Vascular institute in Falls Church, Va., and designated discussant for Dr. Bhatt’s report.
SCORED randomized 10,584 patients with type 2 diabetes and chronic kidney disease to treatment with sotagliflozin or placebo on top of guideline-directed medical therapy. During a median 16 months of treatment, the combined primary endpoint occurred at a rate of 5.6 events/100 patient years on sotagliflozin and 7.5 events/100 patient years in the controls, a significant 26% relative reduction with sotagliflozin (N Engl J Med. 2021 Jan 14;384[2]:129-39). Nearly a third of the enrolled patients had heart failure, with representation across the range of LVEF.
SOLOIST-WHF randomized 1,222 patients with type 2 diabetes who were recently hospitalized for worsening heart failure. During a median 9 months of follow-up, the primary endpoint occurred at a rate of 51 events/100 patient years in the sotagliflozin-treated patients and a rate of 76 events/100 patient years in the controls, a significant 33% relative reduction with sotagliflozin (N Engl J Med. 2021 Jan 14;384[2]:117-28). Both trials stopped prematurely because of sponsorship issues.
In addition to the 4,500 patients with heart failure at entry in both trials, SCORED included a total of more than 6,700 without diagnosed heart failure at baseline, and in this subgroup treatment with sotagliflozin cut the incidence of the primary endpoint by a significant 27% compared with control patients.
A significant on-treatment reduction in CV death
Other new, notable findings from the meta-analysis included the observation that while treatment with sotagliflozin failed to produce a significant reduction in cardiovascular death, compared with placebo, in the intent-to-treat analysis of all patients and of those with heart failure at baseline (it produced nonsignificant point-estimate reductions of 11% compared with placebo for all patients, and of 23% for patients who began the study with heart failure), it did result in a significant 23% relative risk reduction when the researchers focused on patients while they remained adherent to their sotagliflozin regimen (the on-treatment analysis). This 23% relative reduction appeared among all enrolled patients, as well as in the subgroup that started with diagnosed heart failure.
“Given the totality of data from the SGLT2 inhibitors, I think this is a real finding,” Dr. Bhatt said.
Additional analyses also showed that, among women, treatment with sotagliflozin was linked with significant relative reductions in the primary endpoint of roughly 30% compared with placebo among all patients, and also among those with heart failure at baseline. “HFpEF is a problem particularly in older women, and we showed that the benefit was consistent in men and women,” Dr. Bhatt said.
He acknowledged that results are expected soon from two pivotal trials that are examining two different SGLT2 inhibitors, dapagliflozin and empagliflozin, in patients with HFpEF. “I think there will be a class effect for both SGLT2 inhibitors and sotagliflozin for reducing heart failure events in patients with HFpEF, and I predict that the dapagliflozin and empagliflozin trials will have positive results,” Dr. Bhatt said.
Sotagliflozin differs from the SGLT2 inhibitors by also inhibiting SGLT1, an enzyme found in the gastrointestinal system that, when inhibited, results in increased glucose excretion from the gut and a cut in bloodstream levels of postprandial glucose levels. The Food and Drug Administration accepted data from SCORED and SOLOIST-WHF as part of the evidence the agency is now considering for granting a new drug approval to sotagliflozin.
SCORED and SOLOIST-WHF were initially sponsored by Sanofi, and later by Lexicon Pharmaceuticals. Dr. Bhatt’s institution, Brigham and Women’s Hospital, has received funding from Sanofi and Lexicon Pharmaceuticals. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an adviser to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies. Dr. Piña has no relevant disclosures. Dr. O’Connor has been a consultant to Arena, Bayer, Bristol-Myers Squibb, Merck, and Windtree, and he has an ownership interest in Biscardia.
It’s now official: The investigational sodium-glucose cotransporter (SGLT) 1/2 inhibitor sotagliflozin is the first agent clearly shown in a prespecified analysis of randomized trials to improve clinical outcomes in patients with heart failure with reduced ejection fraction (HFpEF).
Researchers who ran the SCORED and SOLOIST-WHF pivotal trials for sotagliflozin first made that claim in November 2020 when reporting top-line results from a prespecified meta-analysis of the two trials during the American Heart Association annual scientific sessions. A follow-up report during the annual scientific sessions of the American College of Cardiology fleshed out the evidence and firmed up their landmark conclusion.
The meta-analysis (Abstract 410-08) included 4,500 patients with type 2 diabetes and diagnosed heart failure at entry; its primary endpoint, which was the same in both trials, was the combined incidence of cardiovascular death and the total number of either hospitalization for heart failure or urgent outpatient visits for heart failure.
Compared with placebo, treatment with sotagliflozin for a median of about 15 months dropped this composite endpoint by a relative 33% among the 1,931 who began the study with a left ventricular ejection fraction (LVEF) of at least 50% (HFpEF), by a relative 22% in the 1,758 patients who entered with an LVEF of less than 40% (patients with heart failure with reduced ejection fraction), and by a relative 43% among the 811 patients who began with an LVEF of 40%-49% (patients with heart failure with mid-range ejection fraction). The relative risk reductions were significant for all three subgroups, Deepak L. Bhatt, MD, reported at the meeting.
Equally effective ‘across the full range of LVEFs.’
Perhaps as notable and unprecedented was the further finding that the clinical benefits seen with treatment of patients with type 2 diabetes with sotagliflozin was consistent regardless of the ejection fraction they had at entry. Enrolled patients with baseline LVEFs in the range of 25% received a relative benefit from sotagliflozin treatment that was statistically no different from the benefit seen in patients who entered with an LVEF in the neighborhood of 45%, 65%, or at any other level across the LVEF spectrum, a finding that Dr. Bhatt called “remarkable” during a press briefing. “The results show the benefit of sotagliflozin across the full range of LVEFs.”
“We are very excited in the heart failure world by the SGLT2 inhibitors; we’ve been impressed by their reduction in heart failure hospitalizations, but we wonder about the patients with HFpEF, where we haven’t had a blockbuster drug to give,” said Ileana L. Piña, MD, a heart failure specialist and medical officer with the Food and Drug Administration.
The new findings “look like they could pose a regulatory indication [for sotagliflozin] for patients with type 2 diabetes and heart failure across the entire spectrum of heart failure,” said Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart & Vascular institute in Falls Church, Va., and designated discussant for Dr. Bhatt’s report.
SCORED randomized 10,584 patients with type 2 diabetes and chronic kidney disease to treatment with sotagliflozin or placebo on top of guideline-directed medical therapy. During a median 16 months of treatment, the combined primary endpoint occurred at a rate of 5.6 events/100 patient years on sotagliflozin and 7.5 events/100 patient years in the controls, a significant 26% relative reduction with sotagliflozin (N Engl J Med. 2021 Jan 14;384[2]:129-39). Nearly a third of the enrolled patients had heart failure, with representation across the range of LVEF.
SOLOIST-WHF randomized 1,222 patients with type 2 diabetes who were recently hospitalized for worsening heart failure. During a median 9 months of follow-up, the primary endpoint occurred at a rate of 51 events/100 patient years in the sotagliflozin-treated patients and a rate of 76 events/100 patient years in the controls, a significant 33% relative reduction with sotagliflozin (N Engl J Med. 2021 Jan 14;384[2]:117-28). Both trials stopped prematurely because of sponsorship issues.
In addition to the 4,500 patients with heart failure at entry in both trials, SCORED included a total of more than 6,700 without diagnosed heart failure at baseline, and in this subgroup treatment with sotagliflozin cut the incidence of the primary endpoint by a significant 27% compared with control patients.
A significant on-treatment reduction in CV death
Other new, notable findings from the meta-analysis included the observation that while treatment with sotagliflozin failed to produce a significant reduction in cardiovascular death, compared with placebo, in the intent-to-treat analysis of all patients and of those with heart failure at baseline (it produced nonsignificant point-estimate reductions of 11% compared with placebo for all patients, and of 23% for patients who began the study with heart failure), it did result in a significant 23% relative risk reduction when the researchers focused on patients while they remained adherent to their sotagliflozin regimen (the on-treatment analysis). This 23% relative reduction appeared among all enrolled patients, as well as in the subgroup that started with diagnosed heart failure.
“Given the totality of data from the SGLT2 inhibitors, I think this is a real finding,” Dr. Bhatt said.
Additional analyses also showed that, among women, treatment with sotagliflozin was linked with significant relative reductions in the primary endpoint of roughly 30% compared with placebo among all patients, and also among those with heart failure at baseline. “HFpEF is a problem particularly in older women, and we showed that the benefit was consistent in men and women,” Dr. Bhatt said.
He acknowledged that results are expected soon from two pivotal trials that are examining two different SGLT2 inhibitors, dapagliflozin and empagliflozin, in patients with HFpEF. “I think there will be a class effect for both SGLT2 inhibitors and sotagliflozin for reducing heart failure events in patients with HFpEF, and I predict that the dapagliflozin and empagliflozin trials will have positive results,” Dr. Bhatt said.
Sotagliflozin differs from the SGLT2 inhibitors by also inhibiting SGLT1, an enzyme found in the gastrointestinal system that, when inhibited, results in increased glucose excretion from the gut and a cut in bloodstream levels of postprandial glucose levels. The Food and Drug Administration accepted data from SCORED and SOLOIST-WHF as part of the evidence the agency is now considering for granting a new drug approval to sotagliflozin.
SCORED and SOLOIST-WHF were initially sponsored by Sanofi, and later by Lexicon Pharmaceuticals. Dr. Bhatt’s institution, Brigham and Women’s Hospital, has received funding from Sanofi and Lexicon Pharmaceuticals. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an adviser to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies. Dr. Piña has no relevant disclosures. Dr. O’Connor has been a consultant to Arena, Bayer, Bristol-Myers Squibb, Merck, and Windtree, and he has an ownership interest in Biscardia.
It’s now official: The investigational sodium-glucose cotransporter (SGLT) 1/2 inhibitor sotagliflozin is the first agent clearly shown in a prespecified analysis of randomized trials to improve clinical outcomes in patients with heart failure with reduced ejection fraction (HFpEF).
Researchers who ran the SCORED and SOLOIST-WHF pivotal trials for sotagliflozin first made that claim in November 2020 when reporting top-line results from a prespecified meta-analysis of the two trials during the American Heart Association annual scientific sessions. A follow-up report during the annual scientific sessions of the American College of Cardiology fleshed out the evidence and firmed up their landmark conclusion.
The meta-analysis (Abstract 410-08) included 4,500 patients with type 2 diabetes and diagnosed heart failure at entry; its primary endpoint, which was the same in both trials, was the combined incidence of cardiovascular death and the total number of either hospitalization for heart failure or urgent outpatient visits for heart failure.
Compared with placebo, treatment with sotagliflozin for a median of about 15 months dropped this composite endpoint by a relative 33% among the 1,931 who began the study with a left ventricular ejection fraction (LVEF) of at least 50% (HFpEF), by a relative 22% in the 1,758 patients who entered with an LVEF of less than 40% (patients with heart failure with reduced ejection fraction), and by a relative 43% among the 811 patients who began with an LVEF of 40%-49% (patients with heart failure with mid-range ejection fraction). The relative risk reductions were significant for all three subgroups, Deepak L. Bhatt, MD, reported at the meeting.
Equally effective ‘across the full range of LVEFs.’
Perhaps as notable and unprecedented was the further finding that the clinical benefits seen with treatment of patients with type 2 diabetes with sotagliflozin was consistent regardless of the ejection fraction they had at entry. Enrolled patients with baseline LVEFs in the range of 25% received a relative benefit from sotagliflozin treatment that was statistically no different from the benefit seen in patients who entered with an LVEF in the neighborhood of 45%, 65%, or at any other level across the LVEF spectrum, a finding that Dr. Bhatt called “remarkable” during a press briefing. “The results show the benefit of sotagliflozin across the full range of LVEFs.”
“We are very excited in the heart failure world by the SGLT2 inhibitors; we’ve been impressed by their reduction in heart failure hospitalizations, but we wonder about the patients with HFpEF, where we haven’t had a blockbuster drug to give,” said Ileana L. Piña, MD, a heart failure specialist and medical officer with the Food and Drug Administration.
The new findings “look like they could pose a regulatory indication [for sotagliflozin] for patients with type 2 diabetes and heart failure across the entire spectrum of heart failure,” said Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart & Vascular institute in Falls Church, Va., and designated discussant for Dr. Bhatt’s report.
SCORED randomized 10,584 patients with type 2 diabetes and chronic kidney disease to treatment with sotagliflozin or placebo on top of guideline-directed medical therapy. During a median 16 months of treatment, the combined primary endpoint occurred at a rate of 5.6 events/100 patient years on sotagliflozin and 7.5 events/100 patient years in the controls, a significant 26% relative reduction with sotagliflozin (N Engl J Med. 2021 Jan 14;384[2]:129-39). Nearly a third of the enrolled patients had heart failure, with representation across the range of LVEF.
SOLOIST-WHF randomized 1,222 patients with type 2 diabetes who were recently hospitalized for worsening heart failure. During a median 9 months of follow-up, the primary endpoint occurred at a rate of 51 events/100 patient years in the sotagliflozin-treated patients and a rate of 76 events/100 patient years in the controls, a significant 33% relative reduction with sotagliflozin (N Engl J Med. 2021 Jan 14;384[2]:117-28). Both trials stopped prematurely because of sponsorship issues.
In addition to the 4,500 patients with heart failure at entry in both trials, SCORED included a total of more than 6,700 without diagnosed heart failure at baseline, and in this subgroup treatment with sotagliflozin cut the incidence of the primary endpoint by a significant 27% compared with control patients.
A significant on-treatment reduction in CV death
Other new, notable findings from the meta-analysis included the observation that while treatment with sotagliflozin failed to produce a significant reduction in cardiovascular death, compared with placebo, in the intent-to-treat analysis of all patients and of those with heart failure at baseline (it produced nonsignificant point-estimate reductions of 11% compared with placebo for all patients, and of 23% for patients who began the study with heart failure), it did result in a significant 23% relative risk reduction when the researchers focused on patients while they remained adherent to their sotagliflozin regimen (the on-treatment analysis). This 23% relative reduction appeared among all enrolled patients, as well as in the subgroup that started with diagnosed heart failure.
“Given the totality of data from the SGLT2 inhibitors, I think this is a real finding,” Dr. Bhatt said.
Additional analyses also showed that, among women, treatment with sotagliflozin was linked with significant relative reductions in the primary endpoint of roughly 30% compared with placebo among all patients, and also among those with heart failure at baseline. “HFpEF is a problem particularly in older women, and we showed that the benefit was consistent in men and women,” Dr. Bhatt said.
He acknowledged that results are expected soon from two pivotal trials that are examining two different SGLT2 inhibitors, dapagliflozin and empagliflozin, in patients with HFpEF. “I think there will be a class effect for both SGLT2 inhibitors and sotagliflozin for reducing heart failure events in patients with HFpEF, and I predict that the dapagliflozin and empagliflozin trials will have positive results,” Dr. Bhatt said.
Sotagliflozin differs from the SGLT2 inhibitors by also inhibiting SGLT1, an enzyme found in the gastrointestinal system that, when inhibited, results in increased glucose excretion from the gut and a cut in bloodstream levels of postprandial glucose levels. The Food and Drug Administration accepted data from SCORED and SOLOIST-WHF as part of the evidence the agency is now considering for granting a new drug approval to sotagliflozin.
SCORED and SOLOIST-WHF were initially sponsored by Sanofi, and later by Lexicon Pharmaceuticals. Dr. Bhatt’s institution, Brigham and Women’s Hospital, has received funding from Sanofi and Lexicon Pharmaceuticals. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an adviser to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies. Dr. Piña has no relevant disclosures. Dr. O’Connor has been a consultant to Arena, Bayer, Bristol-Myers Squibb, Merck, and Windtree, and he has an ownership interest in Biscardia.
FROM ACC 2021
How advances in genomics have informed obstetrics practice
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Genetic screening and diagnosis: Key advancements and the role of genetic counseling
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)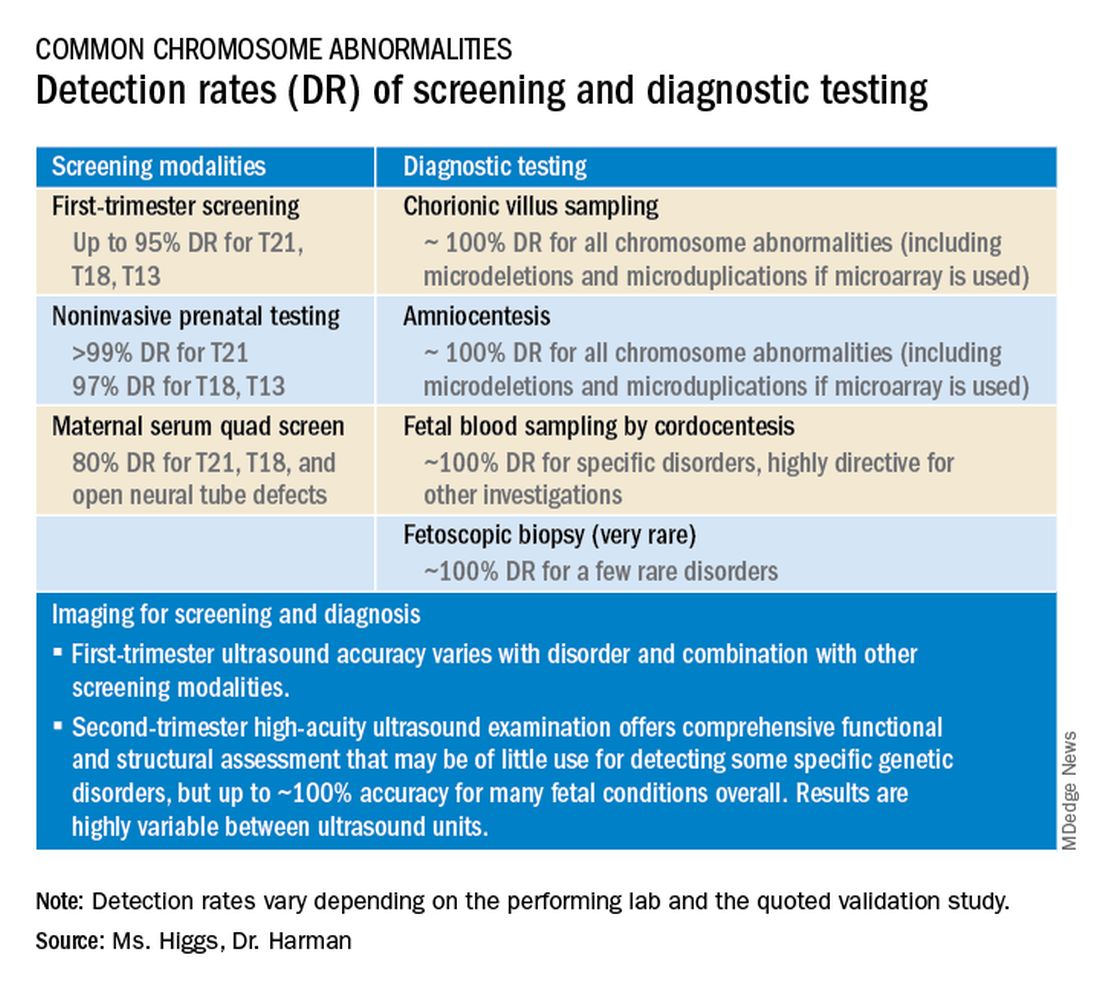
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.
Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
[email protected]
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.

Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
[email protected]
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.

Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
[email protected]