User login
Prior exposure and response to triptans may not affect efficacy of ubrogepant
Key clinical point: Triptan historical responder status had no significant impact on the treatment effects of ubrogepant in patients with a history of migraine.
Major finding: Historical triptan responder status had no significant impact on the efficacy of ubrogepant for pain freedom (Pinteraction based on odds ratio [OR] = .290) and absence of migraine-associated symptom (Pinteraction based on OR = .705). The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs was not significantly different across historical triptan experience subgroups.
Study details: This was a post hoc analysis of pooled data from ACHIEVE I and II phase 3 trials involving 1,799 adults with a history of migraine with/without aura randomly allocated to either placebo or ubrogepant to treat a single attack. Based on previous experience with triptans, participants were categorized as responders, insufficient responders, and naïve.
Disclosures: The study was sponsored by Allergan. Some authors including the lead author declared receiving grants and personal fees; being consultant, speaker, or contributing author; or being on advisory boards for various sources including AbbVie. Some authors declared being current/former employees and/or holding stocks at AbbVie.
Source: Blumenfeld AM et al. Headache. 2021 Mar 22. doi: 10.1111/head.14089.
Key clinical point: Triptan historical responder status had no significant impact on the treatment effects of ubrogepant in patients with a history of migraine.
Major finding: Historical triptan responder status had no significant impact on the efficacy of ubrogepant for pain freedom (Pinteraction based on odds ratio [OR] = .290) and absence of migraine-associated symptom (Pinteraction based on OR = .705). The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs was not significantly different across historical triptan experience subgroups.
Study details: This was a post hoc analysis of pooled data from ACHIEVE I and II phase 3 trials involving 1,799 adults with a history of migraine with/without aura randomly allocated to either placebo or ubrogepant to treat a single attack. Based on previous experience with triptans, participants were categorized as responders, insufficient responders, and naïve.
Disclosures: The study was sponsored by Allergan. Some authors including the lead author declared receiving grants and personal fees; being consultant, speaker, or contributing author; or being on advisory boards for various sources including AbbVie. Some authors declared being current/former employees and/or holding stocks at AbbVie.
Source: Blumenfeld AM et al. Headache. 2021 Mar 22. doi: 10.1111/head.14089.
Key clinical point: Triptan historical responder status had no significant impact on the treatment effects of ubrogepant in patients with a history of migraine.
Major finding: Historical triptan responder status had no significant impact on the efficacy of ubrogepant for pain freedom (Pinteraction based on odds ratio [OR] = .290) and absence of migraine-associated symptom (Pinteraction based on OR = .705). The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs was not significantly different across historical triptan experience subgroups.
Study details: This was a post hoc analysis of pooled data from ACHIEVE I and II phase 3 trials involving 1,799 adults with a history of migraine with/without aura randomly allocated to either placebo or ubrogepant to treat a single attack. Based on previous experience with triptans, participants were categorized as responders, insufficient responders, and naïve.
Disclosures: The study was sponsored by Allergan. Some authors including the lead author declared receiving grants and personal fees; being consultant, speaker, or contributing author; or being on advisory boards for various sources including AbbVie. Some authors declared being current/former employees and/or holding stocks at AbbVie.
Source: Blumenfeld AM et al. Headache. 2021 Mar 22. doi: 10.1111/head.14089.
Migraine: Anti-CGRP mAbs beneficial in partial and nonresponders to onabotulinumtoxinA
Key clinical point: Patients with migraine respond to preventive treatment with monoclonal antibodies targeting calcitonin gene-related peptide (anti-CGRP mAbs) irrespective of their previous failure or partial response to onabotulinumtoxinA.
Major finding: A response of 50% or higher improvement in headache (P = .395) or migraine (P = .408) frequency was not significantly different in partial or complete nonresponders to onabotulinumtoxinA.
Study details: This was a real-world prospective observational study including 155 patients with migraine who initiated preventive treatment with anti-CGRP mAbs and were partial or nonresponders to onabotulinumtoxinA.
Disclosures: The authors declared receiving no financial support for the research, authorship, and/or publication of this article. The authors report no conflicts of interest in relation with this article.
Source: Alpuente A et al. Eur J Neurol. 2021 Mar 17. doi: 10.1111/ene.14828.
Key clinical point: Patients with migraine respond to preventive treatment with monoclonal antibodies targeting calcitonin gene-related peptide (anti-CGRP mAbs) irrespective of their previous failure or partial response to onabotulinumtoxinA.
Major finding: A response of 50% or higher improvement in headache (P = .395) or migraine (P = .408) frequency was not significantly different in partial or complete nonresponders to onabotulinumtoxinA.
Study details: This was a real-world prospective observational study including 155 patients with migraine who initiated preventive treatment with anti-CGRP mAbs and were partial or nonresponders to onabotulinumtoxinA.
Disclosures: The authors declared receiving no financial support for the research, authorship, and/or publication of this article. The authors report no conflicts of interest in relation with this article.
Source: Alpuente A et al. Eur J Neurol. 2021 Mar 17. doi: 10.1111/ene.14828.
Key clinical point: Patients with migraine respond to preventive treatment with monoclonal antibodies targeting calcitonin gene-related peptide (anti-CGRP mAbs) irrespective of their previous failure or partial response to onabotulinumtoxinA.
Major finding: A response of 50% or higher improvement in headache (P = .395) or migraine (P = .408) frequency was not significantly different in partial or complete nonresponders to onabotulinumtoxinA.
Study details: This was a real-world prospective observational study including 155 patients with migraine who initiated preventive treatment with anti-CGRP mAbs and were partial or nonresponders to onabotulinumtoxinA.
Disclosures: The authors declared receiving no financial support for the research, authorship, and/or publication of this article. The authors report no conflicts of interest in relation with this article.
Source: Alpuente A et al. Eur J Neurol. 2021 Mar 17. doi: 10.1111/ene.14828.
HHS proposes overturning Title X ‘gag’ rule
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
Phage-targeting PCR test picks up early Lyme disease
An investigational polymerase chain reaction (PCR) test that detects the presence of a viral gene in Lyme disease–causing bacteria can distinguish between early and late infection, according to the results of a study that the authors described as “systematic and comprehensive.”
“The current way of diagnosing Lyme disease is struggling to reflect the ‘true’ incidence of Lyme disease,” study investigator Jinyu Shan, PhD, said in an interview. Although there are tests for Lyme disease approved by the Food and Drug Administration, they are based on the development of antibodies in the blood, and the problem is that antibodies might not develop until several weeks after an infection.
Diagnosis therefore still relies heavily on the clinician’s experience. There are often telltale signs – such as a “bullseye” skin rash or having been to an area known to be infested with ticks that carry Lyme disease – but this might not always be the case.
For the new test, “we’re not targeting bacteria. We’re targeting bacteriophages,” said Dr. Shan, a research fellow in the department of genetics and genome biology at the University of Leicester (England).
Fortunately, there’s high correlation between the presence of the terL gene and the presence of Borrelia burgdorferi, the spirochete that causes Lyme disease. “If you find the bacteriophages, the bacteria are there,” said Dr. Shan.
“Importantly, there are 10 times more bacteriophages, compared with the bacteria, so you have a lot more targets,” he added.
In an evaluation of a total of 312 samples (156 whole blood and 156 serum samples), significantly fewer copies of the terL gene were found in samples from people with early Lyme disease than in those with late Lyme disease, whereas the fewest copies of terL were seen in healthy volunteers.
Most pathogenic bacteria carry viral DNA either as multiple complete or partial prophages, Dr. Shan explained. Knowing the prophage sequences means that quantitative PCR primers and probes can be designed and used to detect the presence of the associated bacteria.
Although the novel test still needs evaluation in a clinical trial, it could represent a “step-change” in the detection of Lyme disease, Dr. Shan and associates suggested in their report published in Frontiers in Microbiology.
Early treatment is key to the prevention of longer-term consequences of Lyme disease. Clinicians familiar with the treatment of Lyme disease might choose to initiate antibiotic treatment without a positive lab test. However, the lack of a test that can pick out people with Lyme disease in the first few weeks of infection means that many people are not diagnosed or treated early enough.
The new phage-based PCR test Dr. Shan and associates have developed could change all that. With only 0.3 mL of blood being needed, it can potentially be developed as a simple point-of-care test, but that’s a long way off.
At this stage, the research is very much a “proof of concept,” Dr. Shan said. One of the things he plans to try to work out next is whether the test can distinguish between active and dormant disease, which is a “big question” in the diagnosis of Lyme disease.
“Bacteriophages can only be sustained by actively growing bacteria,” explained Dr. Shan, so there is a chance that if they are present in a substantive amount the disease is active, and if they are not – or are in very low numbers – then the disease is dormant. The cutoff value, however, “is not trivial to establish, but we are working toward it,” added Dr. Shan.
Over the past 2 years, Dr. Shan and associates have been working with the Belgian-based diagnostics company, R.E.D Laboratories, to see how the test will fare in a real-world environment. This relationship is providing useful information to add to their bid to perform a clinical trial for which they are now seeking additional sponsorship.
“The lack of an early and effective diagnosis of Lyme disease remains a major cause of misdiagnosis and long-term patient suffering,” commented Rosie Milsom, charity manager for Caudwell LymeCo Charity in the United Kingdom.
It could be a game changer if the test passes the necessary clinical trial testing and validation stages, noted Ms. Milsom, who was not involved in the research.
“Not only would the test help to establish the level or length of infection,” she said, “but it could also act as a way to test after treatment to see if the infection levels are decreasing.” If levels are still high, “you would know more treatment is needed.
The research is being funded by the charity Phelix Research and Development with support from the University of Leicester and the Dutch-based Lyme Fund, Lymefonds. Dr. Shan is named as coinventor of the phage-targeting PCR test, alongside Martha R.J. Clokie, professor of microbiology at the University of Leicester and the senior author of the study. Dr. Shan is chief scientific officer for Phelix Research and Development. Ms. Clokie and other coauthors hold key positions within the medical research charity.
A version of this article first appeared on Medscape.com.
An investigational polymerase chain reaction (PCR) test that detects the presence of a viral gene in Lyme disease–causing bacteria can distinguish between early and late infection, according to the results of a study that the authors described as “systematic and comprehensive.”
“The current way of diagnosing Lyme disease is struggling to reflect the ‘true’ incidence of Lyme disease,” study investigator Jinyu Shan, PhD, said in an interview. Although there are tests for Lyme disease approved by the Food and Drug Administration, they are based on the development of antibodies in the blood, and the problem is that antibodies might not develop until several weeks after an infection.
Diagnosis therefore still relies heavily on the clinician’s experience. There are often telltale signs – such as a “bullseye” skin rash or having been to an area known to be infested with ticks that carry Lyme disease – but this might not always be the case.
For the new test, “we’re not targeting bacteria. We’re targeting bacteriophages,” said Dr. Shan, a research fellow in the department of genetics and genome biology at the University of Leicester (England).
Fortunately, there’s high correlation between the presence of the terL gene and the presence of Borrelia burgdorferi, the spirochete that causes Lyme disease. “If you find the bacteriophages, the bacteria are there,” said Dr. Shan.
“Importantly, there are 10 times more bacteriophages, compared with the bacteria, so you have a lot more targets,” he added.
In an evaluation of a total of 312 samples (156 whole blood and 156 serum samples), significantly fewer copies of the terL gene were found in samples from people with early Lyme disease than in those with late Lyme disease, whereas the fewest copies of terL were seen in healthy volunteers.
Most pathogenic bacteria carry viral DNA either as multiple complete or partial prophages, Dr. Shan explained. Knowing the prophage sequences means that quantitative PCR primers and probes can be designed and used to detect the presence of the associated bacteria.
Although the novel test still needs evaluation in a clinical trial, it could represent a “step-change” in the detection of Lyme disease, Dr. Shan and associates suggested in their report published in Frontiers in Microbiology.
Early treatment is key to the prevention of longer-term consequences of Lyme disease. Clinicians familiar with the treatment of Lyme disease might choose to initiate antibiotic treatment without a positive lab test. However, the lack of a test that can pick out people with Lyme disease in the first few weeks of infection means that many people are not diagnosed or treated early enough.
The new phage-based PCR test Dr. Shan and associates have developed could change all that. With only 0.3 mL of blood being needed, it can potentially be developed as a simple point-of-care test, but that’s a long way off.
At this stage, the research is very much a “proof of concept,” Dr. Shan said. One of the things he plans to try to work out next is whether the test can distinguish between active and dormant disease, which is a “big question” in the diagnosis of Lyme disease.
“Bacteriophages can only be sustained by actively growing bacteria,” explained Dr. Shan, so there is a chance that if they are present in a substantive amount the disease is active, and if they are not – or are in very low numbers – then the disease is dormant. The cutoff value, however, “is not trivial to establish, but we are working toward it,” added Dr. Shan.
Over the past 2 years, Dr. Shan and associates have been working with the Belgian-based diagnostics company, R.E.D Laboratories, to see how the test will fare in a real-world environment. This relationship is providing useful information to add to their bid to perform a clinical trial for which they are now seeking additional sponsorship.
“The lack of an early and effective diagnosis of Lyme disease remains a major cause of misdiagnosis and long-term patient suffering,” commented Rosie Milsom, charity manager for Caudwell LymeCo Charity in the United Kingdom.
It could be a game changer if the test passes the necessary clinical trial testing and validation stages, noted Ms. Milsom, who was not involved in the research.
“Not only would the test help to establish the level or length of infection,” she said, “but it could also act as a way to test after treatment to see if the infection levels are decreasing.” If levels are still high, “you would know more treatment is needed.
The research is being funded by the charity Phelix Research and Development with support from the University of Leicester and the Dutch-based Lyme Fund, Lymefonds. Dr. Shan is named as coinventor of the phage-targeting PCR test, alongside Martha R.J. Clokie, professor of microbiology at the University of Leicester and the senior author of the study. Dr. Shan is chief scientific officer for Phelix Research and Development. Ms. Clokie and other coauthors hold key positions within the medical research charity.
A version of this article first appeared on Medscape.com.
An investigational polymerase chain reaction (PCR) test that detects the presence of a viral gene in Lyme disease–causing bacteria can distinguish between early and late infection, according to the results of a study that the authors described as “systematic and comprehensive.”
“The current way of diagnosing Lyme disease is struggling to reflect the ‘true’ incidence of Lyme disease,” study investigator Jinyu Shan, PhD, said in an interview. Although there are tests for Lyme disease approved by the Food and Drug Administration, they are based on the development of antibodies in the blood, and the problem is that antibodies might not develop until several weeks after an infection.
Diagnosis therefore still relies heavily on the clinician’s experience. There are often telltale signs – such as a “bullseye” skin rash or having been to an area known to be infested with ticks that carry Lyme disease – but this might not always be the case.
For the new test, “we’re not targeting bacteria. We’re targeting bacteriophages,” said Dr. Shan, a research fellow in the department of genetics and genome biology at the University of Leicester (England).
Fortunately, there’s high correlation between the presence of the terL gene and the presence of Borrelia burgdorferi, the spirochete that causes Lyme disease. “If you find the bacteriophages, the bacteria are there,” said Dr. Shan.
“Importantly, there are 10 times more bacteriophages, compared with the bacteria, so you have a lot more targets,” he added.
In an evaluation of a total of 312 samples (156 whole blood and 156 serum samples), significantly fewer copies of the terL gene were found in samples from people with early Lyme disease than in those with late Lyme disease, whereas the fewest copies of terL were seen in healthy volunteers.
Most pathogenic bacteria carry viral DNA either as multiple complete or partial prophages, Dr. Shan explained. Knowing the prophage sequences means that quantitative PCR primers and probes can be designed and used to detect the presence of the associated bacteria.
Although the novel test still needs evaluation in a clinical trial, it could represent a “step-change” in the detection of Lyme disease, Dr. Shan and associates suggested in their report published in Frontiers in Microbiology.
Early treatment is key to the prevention of longer-term consequences of Lyme disease. Clinicians familiar with the treatment of Lyme disease might choose to initiate antibiotic treatment without a positive lab test. However, the lack of a test that can pick out people with Lyme disease in the first few weeks of infection means that many people are not diagnosed or treated early enough.
The new phage-based PCR test Dr. Shan and associates have developed could change all that. With only 0.3 mL of blood being needed, it can potentially be developed as a simple point-of-care test, but that’s a long way off.
At this stage, the research is very much a “proof of concept,” Dr. Shan said. One of the things he plans to try to work out next is whether the test can distinguish between active and dormant disease, which is a “big question” in the diagnosis of Lyme disease.
“Bacteriophages can only be sustained by actively growing bacteria,” explained Dr. Shan, so there is a chance that if they are present in a substantive amount the disease is active, and if they are not – or are in very low numbers – then the disease is dormant. The cutoff value, however, “is not trivial to establish, but we are working toward it,” added Dr. Shan.
Over the past 2 years, Dr. Shan and associates have been working with the Belgian-based diagnostics company, R.E.D Laboratories, to see how the test will fare in a real-world environment. This relationship is providing useful information to add to their bid to perform a clinical trial for which they are now seeking additional sponsorship.
“The lack of an early and effective diagnosis of Lyme disease remains a major cause of misdiagnosis and long-term patient suffering,” commented Rosie Milsom, charity manager for Caudwell LymeCo Charity in the United Kingdom.
It could be a game changer if the test passes the necessary clinical trial testing and validation stages, noted Ms. Milsom, who was not involved in the research.
“Not only would the test help to establish the level or length of infection,” she said, “but it could also act as a way to test after treatment to see if the infection levels are decreasing.” If levels are still high, “you would know more treatment is needed.
The research is being funded by the charity Phelix Research and Development with support from the University of Leicester and the Dutch-based Lyme Fund, Lymefonds. Dr. Shan is named as coinventor of the phage-targeting PCR test, alongside Martha R.J. Clokie, professor of microbiology at the University of Leicester and the senior author of the study. Dr. Shan is chief scientific officer for Phelix Research and Development. Ms. Clokie and other coauthors hold key positions within the medical research charity.
A version of this article first appeared on Medscape.com.
FDA panel supports islet cell treatment for type 1 diabetes
A Food and Drug Administration advisory panel has endorsed a pancreatic islet cell transplant therapy for the treatment of people with type 1 diabetes that can’t be managed with current therapies.
On April 15, the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee voted 12 to 4 in favor of approval of donislecel (Lantidra). There was one abstention. The panel regarded the drug as having “an overall favorable benefit-risk profile for some patients with type 1 diabetes.” The product consists of purified allogeneic pancreatic islets of Langerhans derived from cadaveric donors and is infused into the portal vein of the liver.
Benefits of the treatment include the potential for insulin independence and elimination of severe hypoglycemia. Risks are those associated with the surgical procedure and with long-term immunosuppression.
The therapy is manufactured by CellTrans. According to Jose Oberholzer, MD, the founder of CellTrans, the proposed indication is for adults with “brittle” type 1 diabetes who meet the American Diabetes Association’s (ADA) criteria for whole-organ pancreas-alone transplant (i.e., transplant of pancreas but not kidney).
The ADA criteria include the following: frequent, severe hypoglycemia, hyperglycemia, and/or ketoacidosis that requires medical attention; clinical or emotional problems regarding the use of exogenous insulin; and consistent failure of insulin-based management to prevent acute diabetes complications.
Success in two-thirds of patients in small studies
Dr. Oberholzer presented data from two single-arm open-label studies: a phase 1/2 trial initiated in 2004 with 10 patients, and a phase 3 study with 20 patients that began in 2007. The inclusion criteria differed somewhat between the two studies, but all 30 patients had hypoglycemic unawareness. Mean follow-up was 7.8 years for the phase 1/2 trial and 4.7 years for the phase 3 trial.
For all of the patients, C-peptide levels were positive after transplant. The composite endpoint for success – an A1c level of ≤ 6.5% and the absence of severe hypoglycemic episodes for 1 year – was met by 19 patients (63.3%). For five patients (16.7%), the target A1c level was not achieved, and seven patients (23.3%) experienced a severe episode of hypoglycemia.
Twenty of the 30 patients achieved insulin independence for at least 1 year.
Improvements were also seen at 1 year in mixed meal test outcomes, fasting blood glucose levels, and overall glycemic control. Graft survival 10 years post transplant was achieved by 60% of patients, Dr. Oberholzer said.
Adverse events not unexpected, but still of concern
Two patients died, one as a result of fulminant sepsis at 20 months post transplant, and the other as a result of severe dementia 9 years post transplant. Three patients experienced four serious procedure-related events, including one liver laceration and two hepatic hematomas. Elevations in portal pressure occurred in two patients.
Most adverse events were associated with immunosuppression. These included 178 infections in 26 of the 30 patients. The most common of these were herpes virus infections, Epstein-Barr virus infections, oral candidiasis, and cytomegalovirus infections. Twelve infections were severe. Renal function declined persistently in two patients (20%), and six (20%) experienced new-onset proteinuria at 1 year.
The adverse events related to the procedure and the problems associated with immunosuppression were not unexpected and were consistent with those described for patients receiving whole pancreas transplants, FDA reviewer Patricia Beaston, MD, said in her review of the CellTrans data.
Panel members support treatment for a small group of patients
During the discussion, several panel members pointed out that the target patient population for this treatment will likely be smaller today than it was when the two studies were initiated, given advances in diabetes care. Those advances include continuous glucose monitoring devices with alarms and closed-loop insulin delivery systems – the “artificial pancreas” that automatically suspends insulin delivery to prevent hypoglycemia.
Panel chair Lisa Butterfield, PhD, a surgeon and immunologist at the University of California, San Francisco, voted in favor of approval. But, she added, “I do support postapproval gathering of data to learn more about the product. ... I don’t know how many patients will really benefit, but I think it’s to be determined.”
Christopher K. Breuer, MD, a general and pediatric surgeon at the Center for Regenerative Medicine, Nationwide Children’s Hospital, Columbus, Ohio, said he supported approval for “two very small subpopulations where it would provide the only viable therapy”: those who are eligible for pancreas transplant but cannot tolerate a major operation, and those who already use the latest automated insulin delivery systems and still do not achieve acceptable glycemic control.
Temporary voting member David Harlan, MD, director of the University of Massachusetts Diabetes Center of Excellence, Worcester, Mass., voted no.
He noted that only about 100 whole pancreas-only transplants are performed annually in the United States and that such transplants are “very effective, so we’re talking about patients who aren’t pancreas transplant candidates who might get this.”
Moreover, Dr. Harlan said, “I’ve seen the awful things that can happen in posttransplant recipients. It’s really hard to get that informed consent from someone when you’re asking them to consider a future that they don’t know. When it works, it’s great. When it doesn’t work, it can be catastrophic. I just worry about opening Pandora’s box.”
The only other diabetes specialist on the panel, temporary voting member Ellen Leschek, MD, said she “reluctantly voted yes because a few people could benefit, but I think it’s a much smaller number than the company may believe.”
Dr. Leschek, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., said she’s concerned that “if it’s approved, too many people will get treated this way, when in fact, for a lot of those people, the risks will outweigh the benefits.”
Sandy Feng, MD, PhD, of the department of surgery at the University of California, San Francisco, pointed out that with regard to immunosuppressive therapy, “We’re concerned about the toxicity of what we currently use, but there are additional therapies being developed that might mitigate those toxicities that would be beneficial to this population.”
Dr. Feng, who voted yes, also said, “I do pancreas transplants. I can tell you that there is nothing that [patients with type 1 diabetes] like more than the freedom from dealing with the entire insulin issue. That has made a large impression on me over the last 20-plus years of clinical practice, so I do think this can help some people and will be incredibly meaningful to those people.”
FDA advisory panel members are vetted for conflicts of interest, and special waivers are granted if necessary. No such waivers were granted for this meeting.
A version of this article first appeared on Medscape.com.
A Food and Drug Administration advisory panel has endorsed a pancreatic islet cell transplant therapy for the treatment of people with type 1 diabetes that can’t be managed with current therapies.
On April 15, the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee voted 12 to 4 in favor of approval of donislecel (Lantidra). There was one abstention. The panel regarded the drug as having “an overall favorable benefit-risk profile for some patients with type 1 diabetes.” The product consists of purified allogeneic pancreatic islets of Langerhans derived from cadaveric donors and is infused into the portal vein of the liver.
Benefits of the treatment include the potential for insulin independence and elimination of severe hypoglycemia. Risks are those associated with the surgical procedure and with long-term immunosuppression.
The therapy is manufactured by CellTrans. According to Jose Oberholzer, MD, the founder of CellTrans, the proposed indication is for adults with “brittle” type 1 diabetes who meet the American Diabetes Association’s (ADA) criteria for whole-organ pancreas-alone transplant (i.e., transplant of pancreas but not kidney).
The ADA criteria include the following: frequent, severe hypoglycemia, hyperglycemia, and/or ketoacidosis that requires medical attention; clinical or emotional problems regarding the use of exogenous insulin; and consistent failure of insulin-based management to prevent acute diabetes complications.
Success in two-thirds of patients in small studies
Dr. Oberholzer presented data from two single-arm open-label studies: a phase 1/2 trial initiated in 2004 with 10 patients, and a phase 3 study with 20 patients that began in 2007. The inclusion criteria differed somewhat between the two studies, but all 30 patients had hypoglycemic unawareness. Mean follow-up was 7.8 years for the phase 1/2 trial and 4.7 years for the phase 3 trial.
For all of the patients, C-peptide levels were positive after transplant. The composite endpoint for success – an A1c level of ≤ 6.5% and the absence of severe hypoglycemic episodes for 1 year – was met by 19 patients (63.3%). For five patients (16.7%), the target A1c level was not achieved, and seven patients (23.3%) experienced a severe episode of hypoglycemia.
Twenty of the 30 patients achieved insulin independence for at least 1 year.
Improvements were also seen at 1 year in mixed meal test outcomes, fasting blood glucose levels, and overall glycemic control. Graft survival 10 years post transplant was achieved by 60% of patients, Dr. Oberholzer said.
Adverse events not unexpected, but still of concern
Two patients died, one as a result of fulminant sepsis at 20 months post transplant, and the other as a result of severe dementia 9 years post transplant. Three patients experienced four serious procedure-related events, including one liver laceration and two hepatic hematomas. Elevations in portal pressure occurred in two patients.
Most adverse events were associated with immunosuppression. These included 178 infections in 26 of the 30 patients. The most common of these were herpes virus infections, Epstein-Barr virus infections, oral candidiasis, and cytomegalovirus infections. Twelve infections were severe. Renal function declined persistently in two patients (20%), and six (20%) experienced new-onset proteinuria at 1 year.
The adverse events related to the procedure and the problems associated with immunosuppression were not unexpected and were consistent with those described for patients receiving whole pancreas transplants, FDA reviewer Patricia Beaston, MD, said in her review of the CellTrans data.
Panel members support treatment for a small group of patients
During the discussion, several panel members pointed out that the target patient population for this treatment will likely be smaller today than it was when the two studies were initiated, given advances in diabetes care. Those advances include continuous glucose monitoring devices with alarms and closed-loop insulin delivery systems – the “artificial pancreas” that automatically suspends insulin delivery to prevent hypoglycemia.
Panel chair Lisa Butterfield, PhD, a surgeon and immunologist at the University of California, San Francisco, voted in favor of approval. But, she added, “I do support postapproval gathering of data to learn more about the product. ... I don’t know how many patients will really benefit, but I think it’s to be determined.”
Christopher K. Breuer, MD, a general and pediatric surgeon at the Center for Regenerative Medicine, Nationwide Children’s Hospital, Columbus, Ohio, said he supported approval for “two very small subpopulations where it would provide the only viable therapy”: those who are eligible for pancreas transplant but cannot tolerate a major operation, and those who already use the latest automated insulin delivery systems and still do not achieve acceptable glycemic control.
Temporary voting member David Harlan, MD, director of the University of Massachusetts Diabetes Center of Excellence, Worcester, Mass., voted no.
He noted that only about 100 whole pancreas-only transplants are performed annually in the United States and that such transplants are “very effective, so we’re talking about patients who aren’t pancreas transplant candidates who might get this.”
Moreover, Dr. Harlan said, “I’ve seen the awful things that can happen in posttransplant recipients. It’s really hard to get that informed consent from someone when you’re asking them to consider a future that they don’t know. When it works, it’s great. When it doesn’t work, it can be catastrophic. I just worry about opening Pandora’s box.”
The only other diabetes specialist on the panel, temporary voting member Ellen Leschek, MD, said she “reluctantly voted yes because a few people could benefit, but I think it’s a much smaller number than the company may believe.”
Dr. Leschek, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., said she’s concerned that “if it’s approved, too many people will get treated this way, when in fact, for a lot of those people, the risks will outweigh the benefits.”
Sandy Feng, MD, PhD, of the department of surgery at the University of California, San Francisco, pointed out that with regard to immunosuppressive therapy, “We’re concerned about the toxicity of what we currently use, but there are additional therapies being developed that might mitigate those toxicities that would be beneficial to this population.”
Dr. Feng, who voted yes, also said, “I do pancreas transplants. I can tell you that there is nothing that [patients with type 1 diabetes] like more than the freedom from dealing with the entire insulin issue. That has made a large impression on me over the last 20-plus years of clinical practice, so I do think this can help some people and will be incredibly meaningful to those people.”
FDA advisory panel members are vetted for conflicts of interest, and special waivers are granted if necessary. No such waivers were granted for this meeting.
A version of this article first appeared on Medscape.com.
A Food and Drug Administration advisory panel has endorsed a pancreatic islet cell transplant therapy for the treatment of people with type 1 diabetes that can’t be managed with current therapies.
On April 15, the FDA’s Cellular, Tissue, and Gene Therapies Advisory Committee voted 12 to 4 in favor of approval of donislecel (Lantidra). There was one abstention. The panel regarded the drug as having “an overall favorable benefit-risk profile for some patients with type 1 diabetes.” The product consists of purified allogeneic pancreatic islets of Langerhans derived from cadaveric donors and is infused into the portal vein of the liver.
Benefits of the treatment include the potential for insulin independence and elimination of severe hypoglycemia. Risks are those associated with the surgical procedure and with long-term immunosuppression.
The therapy is manufactured by CellTrans. According to Jose Oberholzer, MD, the founder of CellTrans, the proposed indication is for adults with “brittle” type 1 diabetes who meet the American Diabetes Association’s (ADA) criteria for whole-organ pancreas-alone transplant (i.e., transplant of pancreas but not kidney).
The ADA criteria include the following: frequent, severe hypoglycemia, hyperglycemia, and/or ketoacidosis that requires medical attention; clinical or emotional problems regarding the use of exogenous insulin; and consistent failure of insulin-based management to prevent acute diabetes complications.
Success in two-thirds of patients in small studies
Dr. Oberholzer presented data from two single-arm open-label studies: a phase 1/2 trial initiated in 2004 with 10 patients, and a phase 3 study with 20 patients that began in 2007. The inclusion criteria differed somewhat between the two studies, but all 30 patients had hypoglycemic unawareness. Mean follow-up was 7.8 years for the phase 1/2 trial and 4.7 years for the phase 3 trial.
For all of the patients, C-peptide levels were positive after transplant. The composite endpoint for success – an A1c level of ≤ 6.5% and the absence of severe hypoglycemic episodes for 1 year – was met by 19 patients (63.3%). For five patients (16.7%), the target A1c level was not achieved, and seven patients (23.3%) experienced a severe episode of hypoglycemia.
Twenty of the 30 patients achieved insulin independence for at least 1 year.
Improvements were also seen at 1 year in mixed meal test outcomes, fasting blood glucose levels, and overall glycemic control. Graft survival 10 years post transplant was achieved by 60% of patients, Dr. Oberholzer said.
Adverse events not unexpected, but still of concern
Two patients died, one as a result of fulminant sepsis at 20 months post transplant, and the other as a result of severe dementia 9 years post transplant. Three patients experienced four serious procedure-related events, including one liver laceration and two hepatic hematomas. Elevations in portal pressure occurred in two patients.
Most adverse events were associated with immunosuppression. These included 178 infections in 26 of the 30 patients. The most common of these were herpes virus infections, Epstein-Barr virus infections, oral candidiasis, and cytomegalovirus infections. Twelve infections were severe. Renal function declined persistently in two patients (20%), and six (20%) experienced new-onset proteinuria at 1 year.
The adverse events related to the procedure and the problems associated with immunosuppression were not unexpected and were consistent with those described for patients receiving whole pancreas transplants, FDA reviewer Patricia Beaston, MD, said in her review of the CellTrans data.
Panel members support treatment for a small group of patients
During the discussion, several panel members pointed out that the target patient population for this treatment will likely be smaller today than it was when the two studies were initiated, given advances in diabetes care. Those advances include continuous glucose monitoring devices with alarms and closed-loop insulin delivery systems – the “artificial pancreas” that automatically suspends insulin delivery to prevent hypoglycemia.
Panel chair Lisa Butterfield, PhD, a surgeon and immunologist at the University of California, San Francisco, voted in favor of approval. But, she added, “I do support postapproval gathering of data to learn more about the product. ... I don’t know how many patients will really benefit, but I think it’s to be determined.”
Christopher K. Breuer, MD, a general and pediatric surgeon at the Center for Regenerative Medicine, Nationwide Children’s Hospital, Columbus, Ohio, said he supported approval for “two very small subpopulations where it would provide the only viable therapy”: those who are eligible for pancreas transplant but cannot tolerate a major operation, and those who already use the latest automated insulin delivery systems and still do not achieve acceptable glycemic control.
Temporary voting member David Harlan, MD, director of the University of Massachusetts Diabetes Center of Excellence, Worcester, Mass., voted no.
He noted that only about 100 whole pancreas-only transplants are performed annually in the United States and that such transplants are “very effective, so we’re talking about patients who aren’t pancreas transplant candidates who might get this.”
Moreover, Dr. Harlan said, “I’ve seen the awful things that can happen in posttransplant recipients. It’s really hard to get that informed consent from someone when you’re asking them to consider a future that they don’t know. When it works, it’s great. When it doesn’t work, it can be catastrophic. I just worry about opening Pandora’s box.”
The only other diabetes specialist on the panel, temporary voting member Ellen Leschek, MD, said she “reluctantly voted yes because a few people could benefit, but I think it’s a much smaller number than the company may believe.”
Dr. Leschek, of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., said she’s concerned that “if it’s approved, too many people will get treated this way, when in fact, for a lot of those people, the risks will outweigh the benefits.”
Sandy Feng, MD, PhD, of the department of surgery at the University of California, San Francisco, pointed out that with regard to immunosuppressive therapy, “We’re concerned about the toxicity of what we currently use, but there are additional therapies being developed that might mitigate those toxicities that would be beneficial to this population.”
Dr. Feng, who voted yes, also said, “I do pancreas transplants. I can tell you that there is nothing that [patients with type 1 diabetes] like more than the freedom from dealing with the entire insulin issue. That has made a large impression on me over the last 20-plus years of clinical practice, so I do think this can help some people and will be incredibly meaningful to those people.”
FDA advisory panel members are vetted for conflicts of interest, and special waivers are granted if necessary. No such waivers were granted for this meeting.
A version of this article first appeared on Medscape.com.
Leveraging the microbiome to enhance cancer treatment
Andrea Facciabene, PhD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a preclinical study in which vancomycin enhanced the efficacy of radiotherapy against melanoma and lung cancer. Now, researchers are conducting a clinical trial to determine if vancomycin can have the same effect in patients with non–small cell lung cancer.
Dr. Facciabene reviewed this research at the AACR Virtual Special Conference: Radiation Science and Medicine.
According to Dr. Facciabene, “gut microbiota” includes the more than 1,000 different strains of bacteria living in human intestines. He indicated that the average human has 10 times more bacteria than cells in the body and 150 times more genes in the gut microbiome than in the human genome.
In healthy individuals, the gut microbiota play a key role in intestinal function and digestive processes, modulation of hormones and vitamin secretion, energy extraction from food, and development and maintenance of a balanced immune system.
“Dysbiosis” is the term applied to a change in the composition, diversity, or metabolites of the microbiome from a healthy pattern to one associated with disease. Antibiotic therapy is a classic cause of dysbiosis, and dysbiosis has been implicated in a variety of inflammatory diseases.
The mechanisms by which the gut microbiome could influence systemic immunity is not known but is relevant to cancer therapy response. Augmenting the frequency and durability of response to immune-targeted treatments – potentially by manipulating the influence of gut microbiota on the immune system – could be highly impactful.
Gut microbiota and radiation-induced cell death
Immunogenic cell death – a process by which tumors die and release their intracellular molecular contents – is one of the mechanisms by which radiotherapy kills cancer cells.
Tumor cells succumbing to immunogenic cell death stimulate antigen presenting cells, such as dendritic cells, that engulf tumor antigens and cross-present them to CD8+ cytotoxic T lymphocytes. This process culminates in the generation of a specific immune response capable of killing the malignant cells in the irradiated area, but it also impacts distant nonirradiated tumors – an abscopal effect.
Dr. Facciabene and colleagues hypothesized that alterations of the gut microbiota could have an impact on the effect of radiotherapy. To investigate this, they studied mouse models of melanoma.
The team allowed B16-OVA tumors to grow for 9-12 days, then delivered a single dose of radiotherapy (21 Gy) to one – but not all – tumors. Simultaneously with the delivery of radiotherapy, the investigators started some animals on oral vancomycin. The team chose vancomycin because its effects are localized and impact the gut microbiota directly, without any known systemic effects.
Results showed that vancomycin significantly augmented the impact of radiotherapy in the irradiated area and was associated with regression of remote tumors.
The effects of the combination treatment on tumor volume were significantly greater than the effects of either treatment alone. Since manipulation of the gut microbiome potentiated radiotherapy effects both locally and distantly, the investigators concluded that immunogenic cell death may be involved in both the local and abscopal effects of radiotherapy.
When the experiment was repeated with a lung tumor model, similar findings were observed.
Involvement of cytotoxic T cells and interferon-gamma
Dr. Facciabene and colleagues found that the irradiated and unirradiated B16 OVA melanoma tumors treated with the radiotherapy-vancomycin combination were infiltrated by CD3+ and CD8+ T cells.
The investigators selectively depleted CD8+ T cells by pretreating the mice with an anti-CD8 monoclonal antibody. Depletion of CD8+ cells prior to administering radiotherapy plus vancomycin abrogated the antitumor effects of the combination treatment, demonstrating that the CD8+ T cells were required.
To characterize the antigen specificity of the tumor-infiltrating CD8+ T cells, Dr. Facciabene and colleagues used OVA MHC class 1 tetramer. Tumors from mice treated with vancomycin alone, radiotherapy alone, or the combination were dissected. Individual dendritic cells were assayed for OVA tetramer by flow cytometry.
The investigators found that tumors from mice treated with radiotherapy plus vancomycin had a significantly higher number of OVA-specific CD8+ T cells, in comparison with untreated tumors or tumors treated with either vancomycin alone or radiotherapy alone. Since antibody that impaired recognition of MHC class I peptides by T cells ablated the effect, it was clear that antigen recognition was vital.
Interferon-gamma (IFN-gamma) is known to play a critical role in both differentiation and effector functions of CD8+ cytolytic T cells in the antitumor immune response. To determine whether IFN-gamma is involved in the antitumor effects of the radiotherapy-vancomycin combination, the investigators measured intratumoral expression of IFN-gamma in the tumors 5 days after radiotherapy.
IFN-gamma messenger RNA expression levels were significantly elevated in the combination treatment group when compared with either treatment alone. In B16-OVA melanoma–challenged knockout mice, the enhancement of the radiotherapy effects by vancomycin was ablated.
The investigators concluded that vancomycin remodels the tumor microenvironment and increases the functionality of tumor-infiltrating, tumor-specific, CD8+ T cells. Furthermore, IFN-gamma is required to augment the radiotherapy-induced immune effect against the tumor.
Potential biochemical mediators of immune effects
The gut microbiota aid host digestion and generate a large repertoire of metabolites after defermentation of fiber. Short-chain fatty acids (SCFAs) constitute the major products of bacterial fermentation.
Acetic acid, propionic acid, and butyric acid represent 95% of total SCFAs present in the intestine. SCFAs are known to directly modulate cytokine production and dendritic cell function.
In their study, Dr. Facciabene and colleagues focused on butyric acid. Using mass spectroscopy, they demonstrated that vancomycin treatment reduces butyrate concentrations in tumor and tumor-draining lymph nodes by eradicating the major families of SCFA-producing Clostridia species.
To test whether supplementing butyrate could influence the synergy of the radiotherapy-vancomycin combination in vivo, the investigators added sodium butyrate to the mice’s drinking water when starting vancomycin treatment. The team then challenged the mice with B16-OVA tumors and treated them with radiotherapy.
In agreement with the group’s prior findings, vancomycin enhanced the tumor-inhibitory effects of radiotherapy, but dietary butyrate inhibited the benefit. The investigators found a significant decrease in the population of B16-OVA–presenting dendritic cells in the lymph nodes of mice receiving the supplemental butyrate.
Dr. Facciabene said these findings were supported by a recent publication. The authors observed that butyrate inhibited type I IFN expression in dendritic cells and radiotherapy-induced, tumor-specific cytotoxic T-cell immune responses without directly protecting tumor cells from the cytotoxic effects of radiotherapy.
Wide-ranging implications
Overall, Dr. Facciabene’s research has shown that:
- Vancomycin significantly enhances the tumor inhibitory effect of targeted radiation, including abscopal effects.
- The synergistic effects are dependent upon IFN-gamma and CD8+ cells.
- Depletion of some gut microbiome species increases antigen presentation by dendritic cells. This is mediated by SCFAs produced by certain bacterial families.
- There are promising new strategies to improve responses to radiotherapy, including targeting gut microbiota.
A clinical trial (NCT03546829) of vancomycin plus stereotactic body radiation in patients with locally advanced non–small cell lung cancer has been launched to investigate these findings further. Early data analysis has shown a significant impact of vancomycin on several species of gut microbiota, according to Dr. Facciabene.
Revolutionary results from immune-targeted therapy in the recent past have highlighted the important role the immune system can play in fighting cancer. Still, up to one-third of cancer patients fail to respond to overtly immune-targeted therapy.
The ability to inhibit cancer cells from evading immune surveillance by using new adjuvants – including those acting on non-traditional targets like gut microbiota – could herald the next major advances in cancer therapy. During his presentation, Dr. Facciabene gave participants an enticing hint of what could be coming for cancer patients in the years ahead.
Dr. Facciabene reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Andrea Facciabene, PhD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a preclinical study in which vancomycin enhanced the efficacy of radiotherapy against melanoma and lung cancer. Now, researchers are conducting a clinical trial to determine if vancomycin can have the same effect in patients with non–small cell lung cancer.
Dr. Facciabene reviewed this research at the AACR Virtual Special Conference: Radiation Science and Medicine.
According to Dr. Facciabene, “gut microbiota” includes the more than 1,000 different strains of bacteria living in human intestines. He indicated that the average human has 10 times more bacteria than cells in the body and 150 times more genes in the gut microbiome than in the human genome.
In healthy individuals, the gut microbiota play a key role in intestinal function and digestive processes, modulation of hormones and vitamin secretion, energy extraction from food, and development and maintenance of a balanced immune system.
“Dysbiosis” is the term applied to a change in the composition, diversity, or metabolites of the microbiome from a healthy pattern to one associated with disease. Antibiotic therapy is a classic cause of dysbiosis, and dysbiosis has been implicated in a variety of inflammatory diseases.
The mechanisms by which the gut microbiome could influence systemic immunity is not known but is relevant to cancer therapy response. Augmenting the frequency and durability of response to immune-targeted treatments – potentially by manipulating the influence of gut microbiota on the immune system – could be highly impactful.
Gut microbiota and radiation-induced cell death
Immunogenic cell death – a process by which tumors die and release their intracellular molecular contents – is one of the mechanisms by which radiotherapy kills cancer cells.
Tumor cells succumbing to immunogenic cell death stimulate antigen presenting cells, such as dendritic cells, that engulf tumor antigens and cross-present them to CD8+ cytotoxic T lymphocytes. This process culminates in the generation of a specific immune response capable of killing the malignant cells in the irradiated area, but it also impacts distant nonirradiated tumors – an abscopal effect.
Dr. Facciabene and colleagues hypothesized that alterations of the gut microbiota could have an impact on the effect of radiotherapy. To investigate this, they studied mouse models of melanoma.
The team allowed B16-OVA tumors to grow for 9-12 days, then delivered a single dose of radiotherapy (21 Gy) to one – but not all – tumors. Simultaneously with the delivery of radiotherapy, the investigators started some animals on oral vancomycin. The team chose vancomycin because its effects are localized and impact the gut microbiota directly, without any known systemic effects.
Results showed that vancomycin significantly augmented the impact of radiotherapy in the irradiated area and was associated with regression of remote tumors.
The effects of the combination treatment on tumor volume were significantly greater than the effects of either treatment alone. Since manipulation of the gut microbiome potentiated radiotherapy effects both locally and distantly, the investigators concluded that immunogenic cell death may be involved in both the local and abscopal effects of radiotherapy.
When the experiment was repeated with a lung tumor model, similar findings were observed.
Involvement of cytotoxic T cells and interferon-gamma
Dr. Facciabene and colleagues found that the irradiated and unirradiated B16 OVA melanoma tumors treated with the radiotherapy-vancomycin combination were infiltrated by CD3+ and CD8+ T cells.
The investigators selectively depleted CD8+ T cells by pretreating the mice with an anti-CD8 monoclonal antibody. Depletion of CD8+ cells prior to administering radiotherapy plus vancomycin abrogated the antitumor effects of the combination treatment, demonstrating that the CD8+ T cells were required.
To characterize the antigen specificity of the tumor-infiltrating CD8+ T cells, Dr. Facciabene and colleagues used OVA MHC class 1 tetramer. Tumors from mice treated with vancomycin alone, radiotherapy alone, or the combination were dissected. Individual dendritic cells were assayed for OVA tetramer by flow cytometry.
The investigators found that tumors from mice treated with radiotherapy plus vancomycin had a significantly higher number of OVA-specific CD8+ T cells, in comparison with untreated tumors or tumors treated with either vancomycin alone or radiotherapy alone. Since antibody that impaired recognition of MHC class I peptides by T cells ablated the effect, it was clear that antigen recognition was vital.
Interferon-gamma (IFN-gamma) is known to play a critical role in both differentiation and effector functions of CD8+ cytolytic T cells in the antitumor immune response. To determine whether IFN-gamma is involved in the antitumor effects of the radiotherapy-vancomycin combination, the investigators measured intratumoral expression of IFN-gamma in the tumors 5 days after radiotherapy.
IFN-gamma messenger RNA expression levels were significantly elevated in the combination treatment group when compared with either treatment alone. In B16-OVA melanoma–challenged knockout mice, the enhancement of the radiotherapy effects by vancomycin was ablated.
The investigators concluded that vancomycin remodels the tumor microenvironment and increases the functionality of tumor-infiltrating, tumor-specific, CD8+ T cells. Furthermore, IFN-gamma is required to augment the radiotherapy-induced immune effect against the tumor.
Potential biochemical mediators of immune effects
The gut microbiota aid host digestion and generate a large repertoire of metabolites after defermentation of fiber. Short-chain fatty acids (SCFAs) constitute the major products of bacterial fermentation.
Acetic acid, propionic acid, and butyric acid represent 95% of total SCFAs present in the intestine. SCFAs are known to directly modulate cytokine production and dendritic cell function.
In their study, Dr. Facciabene and colleagues focused on butyric acid. Using mass spectroscopy, they demonstrated that vancomycin treatment reduces butyrate concentrations in tumor and tumor-draining lymph nodes by eradicating the major families of SCFA-producing Clostridia species.
To test whether supplementing butyrate could influence the synergy of the radiotherapy-vancomycin combination in vivo, the investigators added sodium butyrate to the mice’s drinking water when starting vancomycin treatment. The team then challenged the mice with B16-OVA tumors and treated them with radiotherapy.
In agreement with the group’s prior findings, vancomycin enhanced the tumor-inhibitory effects of radiotherapy, but dietary butyrate inhibited the benefit. The investigators found a significant decrease in the population of B16-OVA–presenting dendritic cells in the lymph nodes of mice receiving the supplemental butyrate.
Dr. Facciabene said these findings were supported by a recent publication. The authors observed that butyrate inhibited type I IFN expression in dendritic cells and radiotherapy-induced, tumor-specific cytotoxic T-cell immune responses without directly protecting tumor cells from the cytotoxic effects of radiotherapy.
Wide-ranging implications
Overall, Dr. Facciabene’s research has shown that:
- Vancomycin significantly enhances the tumor inhibitory effect of targeted radiation, including abscopal effects.
- The synergistic effects are dependent upon IFN-gamma and CD8+ cells.
- Depletion of some gut microbiome species increases antigen presentation by dendritic cells. This is mediated by SCFAs produced by certain bacterial families.
- There are promising new strategies to improve responses to radiotherapy, including targeting gut microbiota.
A clinical trial (NCT03546829) of vancomycin plus stereotactic body radiation in patients with locally advanced non–small cell lung cancer has been launched to investigate these findings further. Early data analysis has shown a significant impact of vancomycin on several species of gut microbiota, according to Dr. Facciabene.
Revolutionary results from immune-targeted therapy in the recent past have highlighted the important role the immune system can play in fighting cancer. Still, up to one-third of cancer patients fail to respond to overtly immune-targeted therapy.
The ability to inhibit cancer cells from evading immune surveillance by using new adjuvants – including those acting on non-traditional targets like gut microbiota – could herald the next major advances in cancer therapy. During his presentation, Dr. Facciabene gave participants an enticing hint of what could be coming for cancer patients in the years ahead.
Dr. Facciabene reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Andrea Facciabene, PhD, of the University of Pennsylvania, Philadelphia, and colleagues conducted a preclinical study in which vancomycin enhanced the efficacy of radiotherapy against melanoma and lung cancer. Now, researchers are conducting a clinical trial to determine if vancomycin can have the same effect in patients with non–small cell lung cancer.
Dr. Facciabene reviewed this research at the AACR Virtual Special Conference: Radiation Science and Medicine.
According to Dr. Facciabene, “gut microbiota” includes the more than 1,000 different strains of bacteria living in human intestines. He indicated that the average human has 10 times more bacteria than cells in the body and 150 times more genes in the gut microbiome than in the human genome.
In healthy individuals, the gut microbiota play a key role in intestinal function and digestive processes, modulation of hormones and vitamin secretion, energy extraction from food, and development and maintenance of a balanced immune system.
“Dysbiosis” is the term applied to a change in the composition, diversity, or metabolites of the microbiome from a healthy pattern to one associated with disease. Antibiotic therapy is a classic cause of dysbiosis, and dysbiosis has been implicated in a variety of inflammatory diseases.
The mechanisms by which the gut microbiome could influence systemic immunity is not known but is relevant to cancer therapy response. Augmenting the frequency and durability of response to immune-targeted treatments – potentially by manipulating the influence of gut microbiota on the immune system – could be highly impactful.
Gut microbiota and radiation-induced cell death
Immunogenic cell death – a process by which tumors die and release their intracellular molecular contents – is one of the mechanisms by which radiotherapy kills cancer cells.
Tumor cells succumbing to immunogenic cell death stimulate antigen presenting cells, such as dendritic cells, that engulf tumor antigens and cross-present them to CD8+ cytotoxic T lymphocytes. This process culminates in the generation of a specific immune response capable of killing the malignant cells in the irradiated area, but it also impacts distant nonirradiated tumors – an abscopal effect.
Dr. Facciabene and colleagues hypothesized that alterations of the gut microbiota could have an impact on the effect of radiotherapy. To investigate this, they studied mouse models of melanoma.
The team allowed B16-OVA tumors to grow for 9-12 days, then delivered a single dose of radiotherapy (21 Gy) to one – but not all – tumors. Simultaneously with the delivery of radiotherapy, the investigators started some animals on oral vancomycin. The team chose vancomycin because its effects are localized and impact the gut microbiota directly, without any known systemic effects.
Results showed that vancomycin significantly augmented the impact of radiotherapy in the irradiated area and was associated with regression of remote tumors.
The effects of the combination treatment on tumor volume were significantly greater than the effects of either treatment alone. Since manipulation of the gut microbiome potentiated radiotherapy effects both locally and distantly, the investigators concluded that immunogenic cell death may be involved in both the local and abscopal effects of radiotherapy.
When the experiment was repeated with a lung tumor model, similar findings were observed.
Involvement of cytotoxic T cells and interferon-gamma
Dr. Facciabene and colleagues found that the irradiated and unirradiated B16 OVA melanoma tumors treated with the radiotherapy-vancomycin combination were infiltrated by CD3+ and CD8+ T cells.
The investigators selectively depleted CD8+ T cells by pretreating the mice with an anti-CD8 monoclonal antibody. Depletion of CD8+ cells prior to administering radiotherapy plus vancomycin abrogated the antitumor effects of the combination treatment, demonstrating that the CD8+ T cells were required.
To characterize the antigen specificity of the tumor-infiltrating CD8+ T cells, Dr. Facciabene and colleagues used OVA MHC class 1 tetramer. Tumors from mice treated with vancomycin alone, radiotherapy alone, or the combination were dissected. Individual dendritic cells were assayed for OVA tetramer by flow cytometry.
The investigators found that tumors from mice treated with radiotherapy plus vancomycin had a significantly higher number of OVA-specific CD8+ T cells, in comparison with untreated tumors or tumors treated with either vancomycin alone or radiotherapy alone. Since antibody that impaired recognition of MHC class I peptides by T cells ablated the effect, it was clear that antigen recognition was vital.
Interferon-gamma (IFN-gamma) is known to play a critical role in both differentiation and effector functions of CD8+ cytolytic T cells in the antitumor immune response. To determine whether IFN-gamma is involved in the antitumor effects of the radiotherapy-vancomycin combination, the investigators measured intratumoral expression of IFN-gamma in the tumors 5 days after radiotherapy.
IFN-gamma messenger RNA expression levels were significantly elevated in the combination treatment group when compared with either treatment alone. In B16-OVA melanoma–challenged knockout mice, the enhancement of the radiotherapy effects by vancomycin was ablated.
The investigators concluded that vancomycin remodels the tumor microenvironment and increases the functionality of tumor-infiltrating, tumor-specific, CD8+ T cells. Furthermore, IFN-gamma is required to augment the radiotherapy-induced immune effect against the tumor.
Potential biochemical mediators of immune effects
The gut microbiota aid host digestion and generate a large repertoire of metabolites after defermentation of fiber. Short-chain fatty acids (SCFAs) constitute the major products of bacterial fermentation.
Acetic acid, propionic acid, and butyric acid represent 95% of total SCFAs present in the intestine. SCFAs are known to directly modulate cytokine production and dendritic cell function.
In their study, Dr. Facciabene and colleagues focused on butyric acid. Using mass spectroscopy, they demonstrated that vancomycin treatment reduces butyrate concentrations in tumor and tumor-draining lymph nodes by eradicating the major families of SCFA-producing Clostridia species.
To test whether supplementing butyrate could influence the synergy of the radiotherapy-vancomycin combination in vivo, the investigators added sodium butyrate to the mice’s drinking water when starting vancomycin treatment. The team then challenged the mice with B16-OVA tumors and treated them with radiotherapy.
In agreement with the group’s prior findings, vancomycin enhanced the tumor-inhibitory effects of radiotherapy, but dietary butyrate inhibited the benefit. The investigators found a significant decrease in the population of B16-OVA–presenting dendritic cells in the lymph nodes of mice receiving the supplemental butyrate.
Dr. Facciabene said these findings were supported by a recent publication. The authors observed that butyrate inhibited type I IFN expression in dendritic cells and radiotherapy-induced, tumor-specific cytotoxic T-cell immune responses without directly protecting tumor cells from the cytotoxic effects of radiotherapy.
Wide-ranging implications
Overall, Dr. Facciabene’s research has shown that:
- Vancomycin significantly enhances the tumor inhibitory effect of targeted radiation, including abscopal effects.
- The synergistic effects are dependent upon IFN-gamma and CD8+ cells.
- Depletion of some gut microbiome species increases antigen presentation by dendritic cells. This is mediated by SCFAs produced by certain bacterial families.
- There are promising new strategies to improve responses to radiotherapy, including targeting gut microbiota.
A clinical trial (NCT03546829) of vancomycin plus stereotactic body radiation in patients with locally advanced non–small cell lung cancer has been launched to investigate these findings further. Early data analysis has shown a significant impact of vancomycin on several species of gut microbiota, according to Dr. Facciabene.
Revolutionary results from immune-targeted therapy in the recent past have highlighted the important role the immune system can play in fighting cancer. Still, up to one-third of cancer patients fail to respond to overtly immune-targeted therapy.
The ability to inhibit cancer cells from evading immune surveillance by using new adjuvants – including those acting on non-traditional targets like gut microbiota – could herald the next major advances in cancer therapy. During his presentation, Dr. Facciabene gave participants an enticing hint of what could be coming for cancer patients in the years ahead.
Dr. Facciabene reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR: RADIATION SCIENCE AND MEDICINE
AGA Clinical Practice Guidelines: Intragastric balloons in the management of obesity
For patients with obesity who want to lose weight but for whom conventional weight-loss strategies have failed, the combination of intragastric balloon placement and lifestyle modifications may be preferable to lifestyle modifications alone, according to new clinical practice guidelines from the American Gastroenterological Association.
In randomized clinical trials of patients with obesity (body mass index >30 kg/m2), placing an intragastric balloon (IGB) significantly improved key outcomes such as weight loss, metabolic parameters (such as fasting blood glucose, hemoglobin A1c), and rates of remission of diabetes, hypertension, and dyslipidemia, compared with standard noninvasive weight loss interventions, Thiruvengadam Muniraj, MD, MRCP, of Yale University in New Haven, Conn., and associates wrote in Gastroenterology. However, concomitant lifestyle modifications of “moderate to high intensity” are strongly recommended “to maintain and augment weight loss” after IGB placement, according to the guidelines published in Gastroenterology.
Obesity (BMI >30), affects approximately 40% of U.S. adults, but only about 1.1% of eligible patients receive bariatric weight-loss surgery, and few are aware that endoscopic treatment is an option, according to the guideline. Early IGB models were associated with “a number of devastating adverse events,” spurring their removal from the U.S. market in the 1980s and 1990s. Since then, however, several new models of IGBs have become available. The guidelines noted that, in seven randomized, controlled trials of these newer IGBs, there were no deaths and only a 5.6% overall rate of serious adverse events – most commonly injury to the gastrointestinal tract at 6-8 months’ follow-up. “More recently, postmarketing surveillance of IGB has reported additional rare adverse events of hyperinflation, acute pancreatitis, and death,” but overall, “IGBs appear to be associated with both a favorable adverse event and patient tolerability profile.”
Three models of fluid-filled balloons and two models of gas-filled balloons are currently available in the United States, the guidelines noted. The authors did not recommend one specific type or model over another. They cite limited data indicating that “fluid-filled balloons may be associated with more weight loss, lower tolerability, and less favorable safety profile, than gas filled balloons. Shared decision-making is suggested for determining device choice.”
Relatively few studies have evaluated lifestyle modifications after IGB placement. In one study of 80 patients, a very-low-calorie ketogenic diet led to significantly more weight loss (on average, 7.1 kg), compared with a conventional low-calorie diet. “Although diet does augment and sustain weight loss in patients receiving IGB therapy, it is unclear whether other lifestyle modifications (e.g., exercise) would have the same impact,” the guideline authors wrote.
They strongly recommended prophylactic proton pump inhibitor (PPI) therapy after IGB placement. The procedure can erode the gastrointestinal mucosa, and studies in which patients received prophylactic PPIs reported lower rates of serious adverse events, most notably upper GI bleeding. However, the numerous short- and long-term risks of these drugs make it “imperative that the lowest dose, frequency, and duration of PPIs be used in patients undergoing IGB therapy.”
Intragastric balloons can cause nausea and vomiting, leading to their premature removal. Therefore, when placing an IGB, concomitant antiemetic therapy is recommended along with an anesthetic that is unlikely to cause nausea. “Evidence is insufficient to recommend a specific antiemetic regimen” and “choice of regimen [should be] based on institutional policy, clinical context, and availability,” according to the guidelines.
Based on low-quality evidence, they included a conditional recommendation for daily vitamin supplementation with one to two adult-dose multivitamins after IGB placement. They suggest against perioperative laboratory screening for nutritional deficiencies, based on a lack of supporting evidence. However, since nutritional deficiencies with IGB placement have been reported, decisions about screening for nutritional deficiencies should be tailored based on clinical judgment.
To create the guideline, the authors reviewed databases for studies published through January 2020 in which patients with obesity had an IGB placed for at least 6 months. In all, 79 articles were cited, including more than 10 randomized clinical trials.
An update of the clinical practice guidelines is expected in 2024. The AGA Institute provided the only funding. Dr. Muniraj and five coauthors reported having no conflicts of interest. The other two coauthors disclosed relationships with Nestle Health Sciences, the American Society for Gastrointestinal Endoscopy, the American College of Gastroenterology, and the Association of American Indian Physicians.
For patients with obesity who want to lose weight but for whom conventional weight-loss strategies have failed, the combination of intragastric balloon placement and lifestyle modifications may be preferable to lifestyle modifications alone, according to new clinical practice guidelines from the American Gastroenterological Association.
In randomized clinical trials of patients with obesity (body mass index >30 kg/m2), placing an intragastric balloon (IGB) significantly improved key outcomes such as weight loss, metabolic parameters (such as fasting blood glucose, hemoglobin A1c), and rates of remission of diabetes, hypertension, and dyslipidemia, compared with standard noninvasive weight loss interventions, Thiruvengadam Muniraj, MD, MRCP, of Yale University in New Haven, Conn., and associates wrote in Gastroenterology. However, concomitant lifestyle modifications of “moderate to high intensity” are strongly recommended “to maintain and augment weight loss” after IGB placement, according to the guidelines published in Gastroenterology.
Obesity (BMI >30), affects approximately 40% of U.S. adults, but only about 1.1% of eligible patients receive bariatric weight-loss surgery, and few are aware that endoscopic treatment is an option, according to the guideline. Early IGB models were associated with “a number of devastating adverse events,” spurring their removal from the U.S. market in the 1980s and 1990s. Since then, however, several new models of IGBs have become available. The guidelines noted that, in seven randomized, controlled trials of these newer IGBs, there were no deaths and only a 5.6% overall rate of serious adverse events – most commonly injury to the gastrointestinal tract at 6-8 months’ follow-up. “More recently, postmarketing surveillance of IGB has reported additional rare adverse events of hyperinflation, acute pancreatitis, and death,” but overall, “IGBs appear to be associated with both a favorable adverse event and patient tolerability profile.”
Three models of fluid-filled balloons and two models of gas-filled balloons are currently available in the United States, the guidelines noted. The authors did not recommend one specific type or model over another. They cite limited data indicating that “fluid-filled balloons may be associated with more weight loss, lower tolerability, and less favorable safety profile, than gas filled balloons. Shared decision-making is suggested for determining device choice.”
Relatively few studies have evaluated lifestyle modifications after IGB placement. In one study of 80 patients, a very-low-calorie ketogenic diet led to significantly more weight loss (on average, 7.1 kg), compared with a conventional low-calorie diet. “Although diet does augment and sustain weight loss in patients receiving IGB therapy, it is unclear whether other lifestyle modifications (e.g., exercise) would have the same impact,” the guideline authors wrote.
They strongly recommended prophylactic proton pump inhibitor (PPI) therapy after IGB placement. The procedure can erode the gastrointestinal mucosa, and studies in which patients received prophylactic PPIs reported lower rates of serious adverse events, most notably upper GI bleeding. However, the numerous short- and long-term risks of these drugs make it “imperative that the lowest dose, frequency, and duration of PPIs be used in patients undergoing IGB therapy.”
Intragastric balloons can cause nausea and vomiting, leading to their premature removal. Therefore, when placing an IGB, concomitant antiemetic therapy is recommended along with an anesthetic that is unlikely to cause nausea. “Evidence is insufficient to recommend a specific antiemetic regimen” and “choice of regimen [should be] based on institutional policy, clinical context, and availability,” according to the guidelines.
Based on low-quality evidence, they included a conditional recommendation for daily vitamin supplementation with one to two adult-dose multivitamins after IGB placement. They suggest against perioperative laboratory screening for nutritional deficiencies, based on a lack of supporting evidence. However, since nutritional deficiencies with IGB placement have been reported, decisions about screening for nutritional deficiencies should be tailored based on clinical judgment.
To create the guideline, the authors reviewed databases for studies published through January 2020 in which patients with obesity had an IGB placed for at least 6 months. In all, 79 articles were cited, including more than 10 randomized clinical trials.
An update of the clinical practice guidelines is expected in 2024. The AGA Institute provided the only funding. Dr. Muniraj and five coauthors reported having no conflicts of interest. The other two coauthors disclosed relationships with Nestle Health Sciences, the American Society for Gastrointestinal Endoscopy, the American College of Gastroenterology, and the Association of American Indian Physicians.
For patients with obesity who want to lose weight but for whom conventional weight-loss strategies have failed, the combination of intragastric balloon placement and lifestyle modifications may be preferable to lifestyle modifications alone, according to new clinical practice guidelines from the American Gastroenterological Association.
In randomized clinical trials of patients with obesity (body mass index >30 kg/m2), placing an intragastric balloon (IGB) significantly improved key outcomes such as weight loss, metabolic parameters (such as fasting blood glucose, hemoglobin A1c), and rates of remission of diabetes, hypertension, and dyslipidemia, compared with standard noninvasive weight loss interventions, Thiruvengadam Muniraj, MD, MRCP, of Yale University in New Haven, Conn., and associates wrote in Gastroenterology. However, concomitant lifestyle modifications of “moderate to high intensity” are strongly recommended “to maintain and augment weight loss” after IGB placement, according to the guidelines published in Gastroenterology.
Obesity (BMI >30), affects approximately 40% of U.S. adults, but only about 1.1% of eligible patients receive bariatric weight-loss surgery, and few are aware that endoscopic treatment is an option, according to the guideline. Early IGB models were associated with “a number of devastating adverse events,” spurring their removal from the U.S. market in the 1980s and 1990s. Since then, however, several new models of IGBs have become available. The guidelines noted that, in seven randomized, controlled trials of these newer IGBs, there were no deaths and only a 5.6% overall rate of serious adverse events – most commonly injury to the gastrointestinal tract at 6-8 months’ follow-up. “More recently, postmarketing surveillance of IGB has reported additional rare adverse events of hyperinflation, acute pancreatitis, and death,” but overall, “IGBs appear to be associated with both a favorable adverse event and patient tolerability profile.”
Three models of fluid-filled balloons and two models of gas-filled balloons are currently available in the United States, the guidelines noted. The authors did not recommend one specific type or model over another. They cite limited data indicating that “fluid-filled balloons may be associated with more weight loss, lower tolerability, and less favorable safety profile, than gas filled balloons. Shared decision-making is suggested for determining device choice.”
Relatively few studies have evaluated lifestyle modifications after IGB placement. In one study of 80 patients, a very-low-calorie ketogenic diet led to significantly more weight loss (on average, 7.1 kg), compared with a conventional low-calorie diet. “Although diet does augment and sustain weight loss in patients receiving IGB therapy, it is unclear whether other lifestyle modifications (e.g., exercise) would have the same impact,” the guideline authors wrote.
They strongly recommended prophylactic proton pump inhibitor (PPI) therapy after IGB placement. The procedure can erode the gastrointestinal mucosa, and studies in which patients received prophylactic PPIs reported lower rates of serious adverse events, most notably upper GI bleeding. However, the numerous short- and long-term risks of these drugs make it “imperative that the lowest dose, frequency, and duration of PPIs be used in patients undergoing IGB therapy.”
Intragastric balloons can cause nausea and vomiting, leading to their premature removal. Therefore, when placing an IGB, concomitant antiemetic therapy is recommended along with an anesthetic that is unlikely to cause nausea. “Evidence is insufficient to recommend a specific antiemetic regimen” and “choice of regimen [should be] based on institutional policy, clinical context, and availability,” according to the guidelines.
Based on low-quality evidence, they included a conditional recommendation for daily vitamin supplementation with one to two adult-dose multivitamins after IGB placement. They suggest against perioperative laboratory screening for nutritional deficiencies, based on a lack of supporting evidence. However, since nutritional deficiencies with IGB placement have been reported, decisions about screening for nutritional deficiencies should be tailored based on clinical judgment.
To create the guideline, the authors reviewed databases for studies published through January 2020 in which patients with obesity had an IGB placed for at least 6 months. In all, 79 articles were cited, including more than 10 randomized clinical trials.
An update of the clinical practice guidelines is expected in 2024. The AGA Institute provided the only funding. Dr. Muniraj and five coauthors reported having no conflicts of interest. The other two coauthors disclosed relationships with Nestle Health Sciences, the American Society for Gastrointestinal Endoscopy, the American College of Gastroenterology, and the Association of American Indian Physicians.
FROM GASTROENTEROLOGY
Automating Measurement of Trainee Work Hours
Across the country, residents are bound to a set of rules from the Accreditation Council for Graduate Medical Education (ACGME) designed to mini mize fatigue, maintain quality of life, and reduce fatigue-related patient safety events. Adherence to work hours regulations is required to maintain accreditation. Among other guidelines, residents are required to work fewer than 80 hours per week on average over 4 consecutive weeks.1 When work hour violations occur, programs risk citation, penalties, and harm to the program’s reputation.
Residents self-report their adherence to program regulations in an annual survey conducted by the ACGME.2 To collect more frequent data, most training programs monitor resident work hours through self-report on an electronic tracking platform.3 These data generally are used internally to identify problems and opportunities for improvement. However, self-report approaches are subject to imperfect recall and incomplete reporting, and require time and effort to complete.4
The widespread adoption of electronic health records (EHRs) brings new opportunity to measure and promote adherence to work hours. EHR log data capture when users log in and out of the system, along with their location and specific actions. These data offer a compelling alternative to self-report because they are already being collected and can be analyzed almost immediately. Recent studies using EHR log data to approximate resident work hours in a pediatric hospital successfully approximated scheduled hours, but the approach was customized to their hospital’s workflows and might not generalize to other settings.5 Furthermore, earlier studies have not captured evening out-of-hospital work, which contributes to total work hours and is associated with physician burnout.6
We developed a computational method that sought to accurately capture work hours, including out-of-hospital work, which could be used as a screening tool to identify at-risk residents and rotations in near real-time. We estimated work hours, including EHR and non-EHR work, from these EHR data and compared these daily estimations to self-report. We then used a heuristic to estimate the frequency of exceeding the 80-hour workweek in a large internal medicine residency program.
METHODS
The population included 82 internal medicine interns (PGY-1) and 121 residents (PGY-2 = 60, PGY-3 = 61) who rotated through University of California, San Francisco Medical Center (UCSFMC) between July 1, 2018, and June 30, 2019, on inpatient rotations. In the UCSF internal medicine residency program, interns spend an average of 5 months per year and residents spend an average of 2 months per year on inpatient rotations at UCSFMC. Scheduled inpatient rotations generally are in 1-month blocks and include general medical wards, cardiology, liver transplant, night-float, and a procedures and jeopardy rotation where interns perform procedures at UCSFMC and serve as backup for their colleagues across sites. Although expected shift duration differs by rotation, types of shifts include regular length days, call days that are not overnight (but expected duration of work is into the late evening), 28-hour overnight call (PGY-2 and PGY-3), and night-float.
Data Source
This computational method was developed at UCSFMC. This study was approved by the University of California, San Francisco institutional review board. Using the UCSF Epic Clarity database, EHR access log data were obtained, including all Epic logins/logoffs, times, and access devices. Access devices identified included medical center computers, personal computers, and mobile devices.
Trainees self-report their work hours in MedHub, a widely used electronic tracking platform for self-report of resident work hours.7 Data were extracted from this database for interns and residents who matched the criteria above. The self-report data were considered the gold standard for comparison, because it is the best available despite its known limitations.
We used data collected from UCSF’s physician scheduling platform, AMiON, to identify interns and residents assigned to rotations at UCSF hospitals.8 AMiON also was used to capture half-days of off-site scheduled clinics and teaching, which count toward the workday but would not be associated with on-campus logins.
Developing a Computational Method to Measure Work Hours
We developed a heuristic to accomplish two goals: (1) infer the duration of continuous in-hospital work hours while providing clinical care and (2) measure “out-of-hospital” work. Logins from medical center computers were considered to be “on-campus” work. Logins from personal computers were considered to be “out-of-hospital.” “Out-of-hospital” login sessions were further subdivided into “out-of-hospital work” and “out-of-hospital study” based on activity during the session; if any work activities listed in Appendix Table 1 were performed, the session was attributed to work. If only chart review was performed, the session was attributed to study and did not count towards total hours worked. Logins from mobile devices also did not count towards total hours worked.
We inferred continuous in-hospital work by linking on-campus EHR sessions from the first on-campus login until the last on-campus logoff (Figure 1). 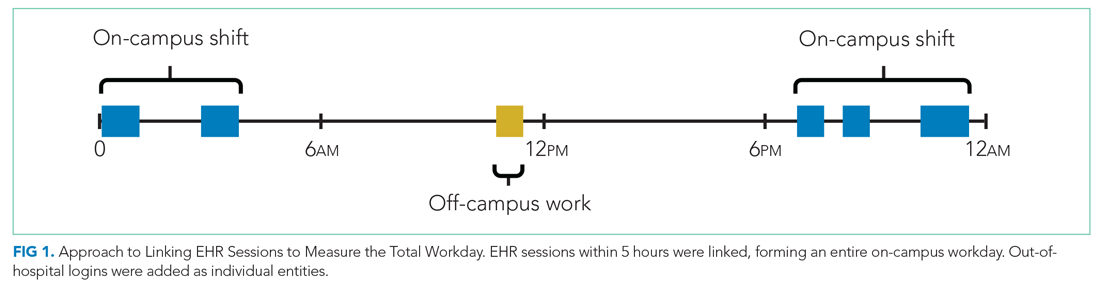
If there was overlapping time measurement between on-campus work and personal computer logins (for example, a resident was inferred to be doing on-campus work based on frequent medical center computer logins but there were also logins from personal computers), we inferred this to indicate that a personal device had been brought on-campus and the time was only attributed to on-campus work and was not double counted as out-of-hospital work. Out-of-hospital work that did not overlap with inferred on-campus work time contributed to the total hours worked in a week, consistent with ACGME guidelines.
Our internal medicine residents work at three hospitals: UCSFMC and two affiliated teaching hospitals. Although this study measured work hours while the residents were on an inpatient rotation at UCSFMC, trainees also might have occasional half-day clinics or teaching activities at other sites not captured by these EHR log data. The allocated time for that scheduled activity (extracted from AMiON) was counted as work hours. If the trainee was assigned to a morning half-day of off-site work (eg, didactics), this was counted the same as an 8
Comparison of EHR-Derived Work Hours Heuristic to Self-Report
Because resident adherence with daily self-report is imperfect, we compared EHR-derived work to self-report on days when both were available. We generated scatter plots of EHR-derived work hours compared with self-report and calculated the mean absolute error of estimation. We fit a linear mixed-effect model for each PGY, modeling self-reported hours as a linear function of estimated hours (fixed effect) with a random intercept (random effect) for each trainee to account for variations among individuals. StatsModels, version 0.11.1, was used for statistical analyses.9
We reviewed detailed data from outlier clusters to understand situations where the heuristic might not perform optimally. To assess whether EHR-derived work hours reasonably overlapped with expected shifts, 20 8-day blocks from separate interns and residents were randomly selected for qualitative detail review in comparison with AMiON schedule data.
Estimating Hours Worked and Work Hours Violations
After validating against self-report on a daily basis, we used our heuristic to infer the average rate at which the 80-hour workweek was exceeded across all inpatient rotations at UCSFMC. This was determined both including “out-of-hospital” work as derived from logins on personal computers and excluding it. Using the estimated daily hours worked, we built a near real-time dashboard to assist program leadership with identifying at-risk trainees and trends across the program.
RESULTS
Data from 82 interns (PGY-1) and 121 internal medicine residents (PGY-2 and PGY-3) who rotated at UCSFMC between July 1, 2018, and June 30, 2019, were included in the study. Table 1 shows the number of days and rotations worked at UCSFMC as well as the frequency of self-report of work hours according to program year. 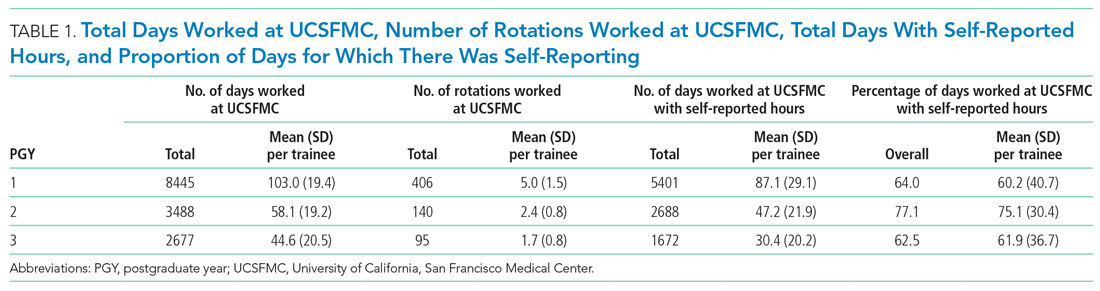
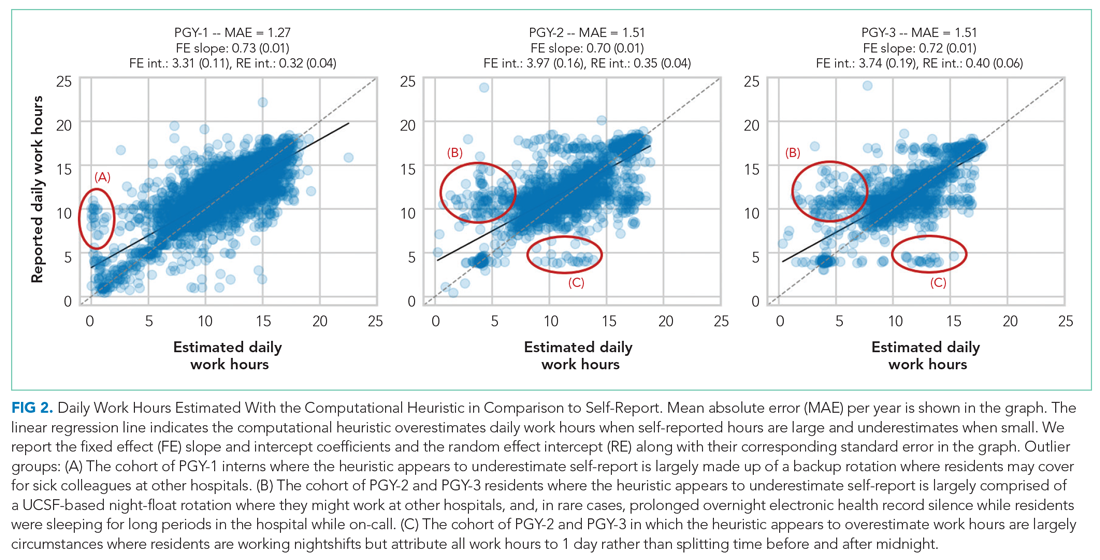
Qualitative review of EHR-derived data compared with schedule data showed that, although residents often reported homogenous daily work hours, EHR-derived work hours often varied as expected on a day-to-day basis according to the schedule (Appendix Table 2).
Because out-of-hospital EHR use does not count as work if done for educational purposes, we evaluated the proportion of out-of-hospital EHR use that is considered work and found that 67% of PGY-1, 50% of PGY-2, and 53% of PGY-3 out-of-hospital sessions included at least one work activity, as denoted in Appendix Table 1. Out-of-hospital work therefore represented 85% of PGY-1, 66% of PGY-2, and 73% of PGY-3 time spent in the EHR out-of-hospital. These sessions were counted towards work hours in accordance with ACGME rules and included 29% of PGY-1 workdays and 21% of PGY-2 and PGY-3 workdays. This amounted to a median of 1.0 hours per day (95% CI, 0.1-4.6 hours) of out-of-hospital work for PGY-1, 0.9 hours per day (95% CI, 0.1-4.1 hours) for PGY-2, and 0.8 hours per day (95% CI, 0.1-4.7 hours) for PGY-3 residents. Out-of-hospital logins that did not include work activities, as denoted in Appendix Table 1, were labeled out-of-hospital study and did not count towards work hours; this amounted to a median of 0.3 hours per day (95% CI, 0.02-1.6 hours) for PGY-1, 0.5 hours per day (95% CI, 0.04-0.25 hours) for PGY-2, and 0.3 hours per day (95% CI, 0.03-1.7 hours) for PGY-3. Mobile device logins also were not counted towards total work hours, with a median of 3 minutes per day for PGY-1, 6 minutes per day for PGY-2, and 5 minutes per day for PGY-3.
The percentage of rotation months where average hours worked exceeded 80 hours weekly is shown in Table 2. Inclusion of out-of-hospital work hours substantially increased the frequency at which the 80-hour workweek was exceeded. The frequency of individual residents working more than 80 hours weekly on average is shown in Appendix Figure 3. A narrow majority of PGY-1 and PGY-2 trainees and a larger majority of PGY-3 trainees never worked in excess of 80 hours per week when averaged over the course of a rotation, but several trainees did on several occasions.
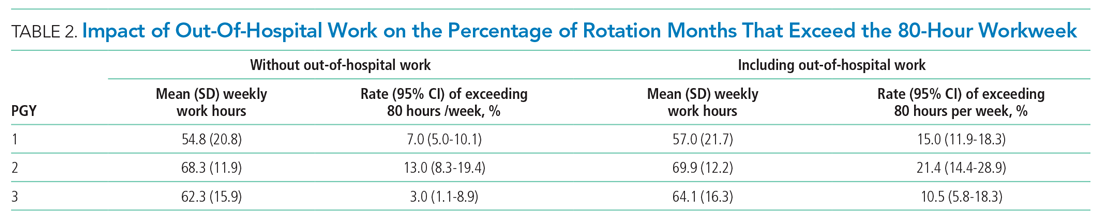
Estimations from the computational method were built into a dashboard for use as screening tool by residency program directors (Appendix Figure 4).
DISCUSSION
EHR log data can be used to automate measurement of trainee work hours, providing timely data to program directors for identifying residents at risk of exceeding work hours limits. We demonstrated this by developing a data-driven approach to link on-campus logins that can be replicated in other training programs. We further demonstrated that out-of-hospital work substantially contributed to resident work hours and the frequency with which they exceed the 80-hour workweek, making it a critical component of any work hour estimation approach. Inclusive of out-of-hospital work, our computational method found that residents exceeded the 80-hour workweek 10% to 21% of the time, depending on their year in residency, with a small majority of residents never exceeding the 80-hour workweek.
Historically, most ACGME residency programs have relied on resident self-report to determine work hours.3 The validity of this method has been extensively studied and results remain mixed; in some surveys, residents admit to underreporting their hours while other validation studies, including the use of clock-in and clock-out or time-stamped parking data, align with self-report relatively well.10-12 Regardless of the reliability of self-report, it is a cumbersome task that residents have difficulty adhering to, as shown in our study, where only slightly more than one-half of the days worked had associated self-report. By relying on resident self-report, we are adding to the burden of clerical work, which is associated with physician burnout.13 Furthermore, because self-report typically does not happen in real-time, it limits a program’s ability to intervene on recent or impending work-hour violations. Our computational method enabled us to build a dashboard that is updated daily and provides critical insight into resident work hours at any time, without waiting for retrospective self-report.
Our study builds on previous work by Dziorny et al using EHR log data to algorithmically measure in-hospital work.5 In their study, the authors isolated shifts with a login gap of 4 hours and then combined shifts according to a set of heuristics. However, their logic integrated an extensive workflow analysis of trainee shifts, which might limit generalizability.5 Our approach computationally derives the temporal threshold for linking EHR sessions, which in our data was 5 hours but might differ at other sites. Automated derivation of this threshold will support generalizability to other programs and sites, although programs will still need to manually account for off-site work such as didactics. In a subsequent study evaluating the 80-hour workweek, Dziorny et al evaluated shift duration and appropriate time-off between shifts and found systematic underreporting of work.14 In our study, we prioritized evaluation of the 80-hour workweek and found general alignment between self-report and EHR-derived work-hour estimates, with a tendency to underestimate at lower reported work hours and overestimate at higher reported work hours (potentially because of underreporting as illustrated by Dziorny et al). We included the important out-of-hospital logins as discrete work events because out-of-hospital work contributes to the total hours worked and to the number of workweeks that exceed the 80-hour workweek, and might contribute to burnout.15 The incidence of exceeding the 80-hour workweek increased by 7% to 8% across all residents when out-of-hospital work was included, demonstrating that tools such as ResQ (ResQ Medical) that rely primarily on geolocation data might not sufficiently capture the ways in which residents spend their time working.16
Our approach has limitations. We determined on-campus vs out-of-hospital locations based on whether the login device belonged to the medical center or was a personal computer. Consequently, if trainees exclusively used a personal computer while on-campus and never used a medical center computer, we would have captured this work done while logged into the EHR but would not have inferred on-campus work. Although nearly all trainees in our organization use medical center computers throughout the day, this might impact generalizability for programs where trainees use personal computers exclusively in the hospital. Our approach also assumes trainees will use the EHR at the beginning and end of their workdays, which could lead to underestimation of work hours in trainees who do not employ this practice. With regards to work done on personal computers, our heuristic required that at least one work activity (as denoted in Appendix Table 1) be included in the session in order for it to count as work. Although this approach allows us to exclude sessions where trainees might be reviewing charts exclusively for educational purposes, it is difficult to infer the true intent of chart review.
There might be periods of time where residents are doing in-hospital work but more than 5 hours elapsed between EHR user sessions. As we have started adapting this computational method for other residency programs, we have added logic that allows for long periods of time in the operating room to be considered part of a continuous workday. There also are limitations to assigning blocks of time to off-site clinics; clinics that are associated with after-hours work but use a different EHR would not be captured in total out-of-hospital work.
Although correlation with self-report was good, we identified clusters of inaccuracy. This likely resulted from our residency program covering three medical centers, two of which were not included in the data set. For example, if a resident had an off-site clinic that was not accounted for in AMiON, EHR-derived work hours might have been underestimated relative to self-report. Operationally leveraging an automated system for measuring work hours in the form of dashboards and other tools could provide the impetus to ensure accurate documentation of schedule anomalies.
CONCLUSION
Implementation of our EHR-derived work-hour model will allow ACGME residency programs to understand and act upon trainee work-hour violations closer to real time, as the data extraction is daily and automated. Automation will save busy residents a cumbersome task, provide more complete data than self-report, and empower residency programs to intervene quickly to support overworked trainees.
Acknowledgments
The authors thank Drs Bradley Monash, Larissa Thomas, and Rebecca Berman for providing residency program input.
1. Accreditation Council for Graduate Medical Education. Common program requirements. Accessed August 12, 2020. https://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements
2. Accreditation Council for Graduate Medical Education. Resident/fellow and faculty surveys. Accessed August 12, 2020. https://www.acgme.org/Data-Collection-Systems/Resident-Fellow-and-Faculty-Surveys
3. Petre M, Geana R, Cipparrone N, et al. Comparing electronic and manual tracking systems for monitoring resident duty hours. Ochsner J. 2016;16(1):16-21.
4. Gonzalo JD, Yang JJ, Ngo L, Clark A, Reynolds EE, Herzig SJ. Accuracy of residents’ retrospective perceptions of 16-hour call admitting shift compliance and characteristics. Grad Med Educ. 2013;5(4):630-633. https://doi.org/10.4300/jgme-d-12-00311.1
5. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Automatic detection of front-line clinician hospital shifts: a novel use of electronic health record timestamp data. Appl Clin Inform. 2019;10(1):28-37. https://doi.org/10.1055/s-0038-1676819
6. Gardner RL, Cooper E, Haskell J, et al. Physician stress and burnout: the impact of health information technology. J Am Med Inform Assoc. 2019;26(2):106-114. https://doi.org/10.1093/jamia/ocy145
7. MedHub. Accessed April 7, 2021. https://www.medhub.com
8. AMiON. Accessed April 7, 2021. https://www.amion.com
9. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference. https://conference.scipy.org/proceedings/scipy2010/pdfs/seabold.pdf
10. Todd SR, Fahy BN, Paukert JL, Mersinger D, Johnson ML, Bass BL. How accurate are self-reported resident duty hours? J Surg Educ. 2010;67(2):103-107. https://doi.org/10.1016/j.jsurg.2009.08.004
11. Chadaga SR, Keniston A, Casey D, Albert RK. Correlation between self-reported resident duty hours and time-stamped parking data. J Grad Med Educ. 2012;4(2):254-256. https://doi.org/10.4300/JGME-D-11-00142.1
12. Drolet BC, Schwede M, Bishop KD, Fischer SA. Compliance and falsification of duty hours: reports from residents and program directors. J Grad Med Educ. 2013;5(3):368-373. https://doi.org/10.4300/JGME-D-12-00375.1
13. Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317(9):901. https://doi.org/10.1001/jama.2017.0076
14. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Pediatric trainees systematically under-report duty hour violations compared to electronic health record defined shifts. PLOS ONE. 2019;14(12):e0226493. https://doi.org/10.1371/journal.pone.0226493
15. Saag HS, Shah K, Jones SA, Testa PA, Horwitz LI. Pajama time: working after work in the electronic health record. J Gen Intern Med. 2019;34(9):1695-1696. https://doi.org/10.1007/s11606-019-05055-x
16. ResQ Medical. Accessed April 7, 2021. https://resqmedical.com
Across the country, residents are bound to a set of rules from the Accreditation Council for Graduate Medical Education (ACGME) designed to mini mize fatigue, maintain quality of life, and reduce fatigue-related patient safety events. Adherence to work hours regulations is required to maintain accreditation. Among other guidelines, residents are required to work fewer than 80 hours per week on average over 4 consecutive weeks.1 When work hour violations occur, programs risk citation, penalties, and harm to the program’s reputation.
Residents self-report their adherence to program regulations in an annual survey conducted by the ACGME.2 To collect more frequent data, most training programs monitor resident work hours through self-report on an electronic tracking platform.3 These data generally are used internally to identify problems and opportunities for improvement. However, self-report approaches are subject to imperfect recall and incomplete reporting, and require time and effort to complete.4
The widespread adoption of electronic health records (EHRs) brings new opportunity to measure and promote adherence to work hours. EHR log data capture when users log in and out of the system, along with their location and specific actions. These data offer a compelling alternative to self-report because they are already being collected and can be analyzed almost immediately. Recent studies using EHR log data to approximate resident work hours in a pediatric hospital successfully approximated scheduled hours, but the approach was customized to their hospital’s workflows and might not generalize to other settings.5 Furthermore, earlier studies have not captured evening out-of-hospital work, which contributes to total work hours and is associated with physician burnout.6
We developed a computational method that sought to accurately capture work hours, including out-of-hospital work, which could be used as a screening tool to identify at-risk residents and rotations in near real-time. We estimated work hours, including EHR and non-EHR work, from these EHR data and compared these daily estimations to self-report. We then used a heuristic to estimate the frequency of exceeding the 80-hour workweek in a large internal medicine residency program.
METHODS
The population included 82 internal medicine interns (PGY-1) and 121 residents (PGY-2 = 60, PGY-3 = 61) who rotated through University of California, San Francisco Medical Center (UCSFMC) between July 1, 2018, and June 30, 2019, on inpatient rotations. In the UCSF internal medicine residency program, interns spend an average of 5 months per year and residents spend an average of 2 months per year on inpatient rotations at UCSFMC. Scheduled inpatient rotations generally are in 1-month blocks and include general medical wards, cardiology, liver transplant, night-float, and a procedures and jeopardy rotation where interns perform procedures at UCSFMC and serve as backup for their colleagues across sites. Although expected shift duration differs by rotation, types of shifts include regular length days, call days that are not overnight (but expected duration of work is into the late evening), 28-hour overnight call (PGY-2 and PGY-3), and night-float.
Data Source
This computational method was developed at UCSFMC. This study was approved by the University of California, San Francisco institutional review board. Using the UCSF Epic Clarity database, EHR access log data were obtained, including all Epic logins/logoffs, times, and access devices. Access devices identified included medical center computers, personal computers, and mobile devices.
Trainees self-report their work hours in MedHub, a widely used electronic tracking platform for self-report of resident work hours.7 Data were extracted from this database for interns and residents who matched the criteria above. The self-report data were considered the gold standard for comparison, because it is the best available despite its known limitations.
We used data collected from UCSF’s physician scheduling platform, AMiON, to identify interns and residents assigned to rotations at UCSF hospitals.8 AMiON also was used to capture half-days of off-site scheduled clinics and teaching, which count toward the workday but would not be associated with on-campus logins.
Developing a Computational Method to Measure Work Hours
We developed a heuristic to accomplish two goals: (1) infer the duration of continuous in-hospital work hours while providing clinical care and (2) measure “out-of-hospital” work. Logins from medical center computers were considered to be “on-campus” work. Logins from personal computers were considered to be “out-of-hospital.” “Out-of-hospital” login sessions were further subdivided into “out-of-hospital work” and “out-of-hospital study” based on activity during the session; if any work activities listed in Appendix Table 1 were performed, the session was attributed to work. If only chart review was performed, the session was attributed to study and did not count towards total hours worked. Logins from mobile devices also did not count towards total hours worked.
We inferred continuous in-hospital work by linking on-campus EHR sessions from the first on-campus login until the last on-campus logoff (Figure 1). 
If there was overlapping time measurement between on-campus work and personal computer logins (for example, a resident was inferred to be doing on-campus work based on frequent medical center computer logins but there were also logins from personal computers), we inferred this to indicate that a personal device had been brought on-campus and the time was only attributed to on-campus work and was not double counted as out-of-hospital work. Out-of-hospital work that did not overlap with inferred on-campus work time contributed to the total hours worked in a week, consistent with ACGME guidelines.
Our internal medicine residents work at three hospitals: UCSFMC and two affiliated teaching hospitals. Although this study measured work hours while the residents were on an inpatient rotation at UCSFMC, trainees also might have occasional half-day clinics or teaching activities at other sites not captured by these EHR log data. The allocated time for that scheduled activity (extracted from AMiON) was counted as work hours. If the trainee was assigned to a morning half-day of off-site work (eg, didactics), this was counted the same as an 8
Comparison of EHR-Derived Work Hours Heuristic to Self-Report
Because resident adherence with daily self-report is imperfect, we compared EHR-derived work to self-report on days when both were available. We generated scatter plots of EHR-derived work hours compared with self-report and calculated the mean absolute error of estimation. We fit a linear mixed-effect model for each PGY, modeling self-reported hours as a linear function of estimated hours (fixed effect) with a random intercept (random effect) for each trainee to account for variations among individuals. StatsModels, version 0.11.1, was used for statistical analyses.9
We reviewed detailed data from outlier clusters to understand situations where the heuristic might not perform optimally. To assess whether EHR-derived work hours reasonably overlapped with expected shifts, 20 8-day blocks from separate interns and residents were randomly selected for qualitative detail review in comparison with AMiON schedule data.
Estimating Hours Worked and Work Hours Violations
After validating against self-report on a daily basis, we used our heuristic to infer the average rate at which the 80-hour workweek was exceeded across all inpatient rotations at UCSFMC. This was determined both including “out-of-hospital” work as derived from logins on personal computers and excluding it. Using the estimated daily hours worked, we built a near real-time dashboard to assist program leadership with identifying at-risk trainees and trends across the program.
RESULTS
Data from 82 interns (PGY-1) and 121 internal medicine residents (PGY-2 and PGY-3) who rotated at UCSFMC between July 1, 2018, and June 30, 2019, were included in the study. Table 1 shows the number of days and rotations worked at UCSFMC as well as the frequency of self-report of work hours according to program year. 

Qualitative review of EHR-derived data compared with schedule data showed that, although residents often reported homogenous daily work hours, EHR-derived work hours often varied as expected on a day-to-day basis according to the schedule (Appendix Table 2).
Because out-of-hospital EHR use does not count as work if done for educational purposes, we evaluated the proportion of out-of-hospital EHR use that is considered work and found that 67% of PGY-1, 50% of PGY-2, and 53% of PGY-3 out-of-hospital sessions included at least one work activity, as denoted in Appendix Table 1. Out-of-hospital work therefore represented 85% of PGY-1, 66% of PGY-2, and 73% of PGY-3 time spent in the EHR out-of-hospital. These sessions were counted towards work hours in accordance with ACGME rules and included 29% of PGY-1 workdays and 21% of PGY-2 and PGY-3 workdays. This amounted to a median of 1.0 hours per day (95% CI, 0.1-4.6 hours) of out-of-hospital work for PGY-1, 0.9 hours per day (95% CI, 0.1-4.1 hours) for PGY-2, and 0.8 hours per day (95% CI, 0.1-4.7 hours) for PGY-3 residents. Out-of-hospital logins that did not include work activities, as denoted in Appendix Table 1, were labeled out-of-hospital study and did not count towards work hours; this amounted to a median of 0.3 hours per day (95% CI, 0.02-1.6 hours) for PGY-1, 0.5 hours per day (95% CI, 0.04-0.25 hours) for PGY-2, and 0.3 hours per day (95% CI, 0.03-1.7 hours) for PGY-3. Mobile device logins also were not counted towards total work hours, with a median of 3 minutes per day for PGY-1, 6 minutes per day for PGY-2, and 5 minutes per day for PGY-3.
The percentage of rotation months where average hours worked exceeded 80 hours weekly is shown in Table 2. Inclusion of out-of-hospital work hours substantially increased the frequency at which the 80-hour workweek was exceeded. The frequency of individual residents working more than 80 hours weekly on average is shown in Appendix Figure 3. A narrow majority of PGY-1 and PGY-2 trainees and a larger majority of PGY-3 trainees never worked in excess of 80 hours per week when averaged over the course of a rotation, but several trainees did on several occasions.

Estimations from the computational method were built into a dashboard for use as screening tool by residency program directors (Appendix Figure 4).
DISCUSSION
EHR log data can be used to automate measurement of trainee work hours, providing timely data to program directors for identifying residents at risk of exceeding work hours limits. We demonstrated this by developing a data-driven approach to link on-campus logins that can be replicated in other training programs. We further demonstrated that out-of-hospital work substantially contributed to resident work hours and the frequency with which they exceed the 80-hour workweek, making it a critical component of any work hour estimation approach. Inclusive of out-of-hospital work, our computational method found that residents exceeded the 80-hour workweek 10% to 21% of the time, depending on their year in residency, with a small majority of residents never exceeding the 80-hour workweek.
Historically, most ACGME residency programs have relied on resident self-report to determine work hours.3 The validity of this method has been extensively studied and results remain mixed; in some surveys, residents admit to underreporting their hours while other validation studies, including the use of clock-in and clock-out or time-stamped parking data, align with self-report relatively well.10-12 Regardless of the reliability of self-report, it is a cumbersome task that residents have difficulty adhering to, as shown in our study, where only slightly more than one-half of the days worked had associated self-report. By relying on resident self-report, we are adding to the burden of clerical work, which is associated with physician burnout.13 Furthermore, because self-report typically does not happen in real-time, it limits a program’s ability to intervene on recent or impending work-hour violations. Our computational method enabled us to build a dashboard that is updated daily and provides critical insight into resident work hours at any time, without waiting for retrospective self-report.
Our study builds on previous work by Dziorny et al using EHR log data to algorithmically measure in-hospital work.5 In their study, the authors isolated shifts with a login gap of 4 hours and then combined shifts according to a set of heuristics. However, their logic integrated an extensive workflow analysis of trainee shifts, which might limit generalizability.5 Our approach computationally derives the temporal threshold for linking EHR sessions, which in our data was 5 hours but might differ at other sites. Automated derivation of this threshold will support generalizability to other programs and sites, although programs will still need to manually account for off-site work such as didactics. In a subsequent study evaluating the 80-hour workweek, Dziorny et al evaluated shift duration and appropriate time-off between shifts and found systematic underreporting of work.14 In our study, we prioritized evaluation of the 80-hour workweek and found general alignment between self-report and EHR-derived work-hour estimates, with a tendency to underestimate at lower reported work hours and overestimate at higher reported work hours (potentially because of underreporting as illustrated by Dziorny et al). We included the important out-of-hospital logins as discrete work events because out-of-hospital work contributes to the total hours worked and to the number of workweeks that exceed the 80-hour workweek, and might contribute to burnout.15 The incidence of exceeding the 80-hour workweek increased by 7% to 8% across all residents when out-of-hospital work was included, demonstrating that tools such as ResQ (ResQ Medical) that rely primarily on geolocation data might not sufficiently capture the ways in which residents spend their time working.16
Our approach has limitations. We determined on-campus vs out-of-hospital locations based on whether the login device belonged to the medical center or was a personal computer. Consequently, if trainees exclusively used a personal computer while on-campus and never used a medical center computer, we would have captured this work done while logged into the EHR but would not have inferred on-campus work. Although nearly all trainees in our organization use medical center computers throughout the day, this might impact generalizability for programs where trainees use personal computers exclusively in the hospital. Our approach also assumes trainees will use the EHR at the beginning and end of their workdays, which could lead to underestimation of work hours in trainees who do not employ this practice. With regards to work done on personal computers, our heuristic required that at least one work activity (as denoted in Appendix Table 1) be included in the session in order for it to count as work. Although this approach allows us to exclude sessions where trainees might be reviewing charts exclusively for educational purposes, it is difficult to infer the true intent of chart review.
There might be periods of time where residents are doing in-hospital work but more than 5 hours elapsed between EHR user sessions. As we have started adapting this computational method for other residency programs, we have added logic that allows for long periods of time in the operating room to be considered part of a continuous workday. There also are limitations to assigning blocks of time to off-site clinics; clinics that are associated with after-hours work but use a different EHR would not be captured in total out-of-hospital work.
Although correlation with self-report was good, we identified clusters of inaccuracy. This likely resulted from our residency program covering three medical centers, two of which were not included in the data set. For example, if a resident had an off-site clinic that was not accounted for in AMiON, EHR-derived work hours might have been underestimated relative to self-report. Operationally leveraging an automated system for measuring work hours in the form of dashboards and other tools could provide the impetus to ensure accurate documentation of schedule anomalies.
CONCLUSION
Implementation of our EHR-derived work-hour model will allow ACGME residency programs to understand and act upon trainee work-hour violations closer to real time, as the data extraction is daily and automated. Automation will save busy residents a cumbersome task, provide more complete data than self-report, and empower residency programs to intervene quickly to support overworked trainees.
Acknowledgments
The authors thank Drs Bradley Monash, Larissa Thomas, and Rebecca Berman for providing residency program input.
Across the country, residents are bound to a set of rules from the Accreditation Council for Graduate Medical Education (ACGME) designed to mini mize fatigue, maintain quality of life, and reduce fatigue-related patient safety events. Adherence to work hours regulations is required to maintain accreditation. Among other guidelines, residents are required to work fewer than 80 hours per week on average over 4 consecutive weeks.1 When work hour violations occur, programs risk citation, penalties, and harm to the program’s reputation.
Residents self-report their adherence to program regulations in an annual survey conducted by the ACGME.2 To collect more frequent data, most training programs monitor resident work hours through self-report on an electronic tracking platform.3 These data generally are used internally to identify problems and opportunities for improvement. However, self-report approaches are subject to imperfect recall and incomplete reporting, and require time and effort to complete.4
The widespread adoption of electronic health records (EHRs) brings new opportunity to measure and promote adherence to work hours. EHR log data capture when users log in and out of the system, along with their location and specific actions. These data offer a compelling alternative to self-report because they are already being collected and can be analyzed almost immediately. Recent studies using EHR log data to approximate resident work hours in a pediatric hospital successfully approximated scheduled hours, but the approach was customized to their hospital’s workflows and might not generalize to other settings.5 Furthermore, earlier studies have not captured evening out-of-hospital work, which contributes to total work hours and is associated with physician burnout.6
We developed a computational method that sought to accurately capture work hours, including out-of-hospital work, which could be used as a screening tool to identify at-risk residents and rotations in near real-time. We estimated work hours, including EHR and non-EHR work, from these EHR data and compared these daily estimations to self-report. We then used a heuristic to estimate the frequency of exceeding the 80-hour workweek in a large internal medicine residency program.
METHODS
The population included 82 internal medicine interns (PGY-1) and 121 residents (PGY-2 = 60, PGY-3 = 61) who rotated through University of California, San Francisco Medical Center (UCSFMC) between July 1, 2018, and June 30, 2019, on inpatient rotations. In the UCSF internal medicine residency program, interns spend an average of 5 months per year and residents spend an average of 2 months per year on inpatient rotations at UCSFMC. Scheduled inpatient rotations generally are in 1-month blocks and include general medical wards, cardiology, liver transplant, night-float, and a procedures and jeopardy rotation where interns perform procedures at UCSFMC and serve as backup for their colleagues across sites. Although expected shift duration differs by rotation, types of shifts include regular length days, call days that are not overnight (but expected duration of work is into the late evening), 28-hour overnight call (PGY-2 and PGY-3), and night-float.
Data Source
This computational method was developed at UCSFMC. This study was approved by the University of California, San Francisco institutional review board. Using the UCSF Epic Clarity database, EHR access log data were obtained, including all Epic logins/logoffs, times, and access devices. Access devices identified included medical center computers, personal computers, and mobile devices.
Trainees self-report their work hours in MedHub, a widely used electronic tracking platform for self-report of resident work hours.7 Data were extracted from this database for interns and residents who matched the criteria above. The self-report data were considered the gold standard for comparison, because it is the best available despite its known limitations.
We used data collected from UCSF’s physician scheduling platform, AMiON, to identify interns and residents assigned to rotations at UCSF hospitals.8 AMiON also was used to capture half-days of off-site scheduled clinics and teaching, which count toward the workday but would not be associated with on-campus logins.
Developing a Computational Method to Measure Work Hours
We developed a heuristic to accomplish two goals: (1) infer the duration of continuous in-hospital work hours while providing clinical care and (2) measure “out-of-hospital” work. Logins from medical center computers were considered to be “on-campus” work. Logins from personal computers were considered to be “out-of-hospital.” “Out-of-hospital” login sessions were further subdivided into “out-of-hospital work” and “out-of-hospital study” based on activity during the session; if any work activities listed in Appendix Table 1 were performed, the session was attributed to work. If only chart review was performed, the session was attributed to study and did not count towards total hours worked. Logins from mobile devices also did not count towards total hours worked.
We inferred continuous in-hospital work by linking on-campus EHR sessions from the first on-campus login until the last on-campus logoff (Figure 1). 
If there was overlapping time measurement between on-campus work and personal computer logins (for example, a resident was inferred to be doing on-campus work based on frequent medical center computer logins but there were also logins from personal computers), we inferred this to indicate that a personal device had been brought on-campus and the time was only attributed to on-campus work and was not double counted as out-of-hospital work. Out-of-hospital work that did not overlap with inferred on-campus work time contributed to the total hours worked in a week, consistent with ACGME guidelines.
Our internal medicine residents work at three hospitals: UCSFMC and two affiliated teaching hospitals. Although this study measured work hours while the residents were on an inpatient rotation at UCSFMC, trainees also might have occasional half-day clinics or teaching activities at other sites not captured by these EHR log data. The allocated time for that scheduled activity (extracted from AMiON) was counted as work hours. If the trainee was assigned to a morning half-day of off-site work (eg, didactics), this was counted the same as an 8
Comparison of EHR-Derived Work Hours Heuristic to Self-Report
Because resident adherence with daily self-report is imperfect, we compared EHR-derived work to self-report on days when both were available. We generated scatter plots of EHR-derived work hours compared with self-report and calculated the mean absolute error of estimation. We fit a linear mixed-effect model for each PGY, modeling self-reported hours as a linear function of estimated hours (fixed effect) with a random intercept (random effect) for each trainee to account for variations among individuals. StatsModels, version 0.11.1, was used for statistical analyses.9
We reviewed detailed data from outlier clusters to understand situations where the heuristic might not perform optimally. To assess whether EHR-derived work hours reasonably overlapped with expected shifts, 20 8-day blocks from separate interns and residents were randomly selected for qualitative detail review in comparison with AMiON schedule data.
Estimating Hours Worked and Work Hours Violations
After validating against self-report on a daily basis, we used our heuristic to infer the average rate at which the 80-hour workweek was exceeded across all inpatient rotations at UCSFMC. This was determined both including “out-of-hospital” work as derived from logins on personal computers and excluding it. Using the estimated daily hours worked, we built a near real-time dashboard to assist program leadership with identifying at-risk trainees and trends across the program.
RESULTS
Data from 82 interns (PGY-1) and 121 internal medicine residents (PGY-2 and PGY-3) who rotated at UCSFMC between July 1, 2018, and June 30, 2019, were included in the study. Table 1 shows the number of days and rotations worked at UCSFMC as well as the frequency of self-report of work hours according to program year. 

Qualitative review of EHR-derived data compared with schedule data showed that, although residents often reported homogenous daily work hours, EHR-derived work hours often varied as expected on a day-to-day basis according to the schedule (Appendix Table 2).
Because out-of-hospital EHR use does not count as work if done for educational purposes, we evaluated the proportion of out-of-hospital EHR use that is considered work and found that 67% of PGY-1, 50% of PGY-2, and 53% of PGY-3 out-of-hospital sessions included at least one work activity, as denoted in Appendix Table 1. Out-of-hospital work therefore represented 85% of PGY-1, 66% of PGY-2, and 73% of PGY-3 time spent in the EHR out-of-hospital. These sessions were counted towards work hours in accordance with ACGME rules and included 29% of PGY-1 workdays and 21% of PGY-2 and PGY-3 workdays. This amounted to a median of 1.0 hours per day (95% CI, 0.1-4.6 hours) of out-of-hospital work for PGY-1, 0.9 hours per day (95% CI, 0.1-4.1 hours) for PGY-2, and 0.8 hours per day (95% CI, 0.1-4.7 hours) for PGY-3 residents. Out-of-hospital logins that did not include work activities, as denoted in Appendix Table 1, were labeled out-of-hospital study and did not count towards work hours; this amounted to a median of 0.3 hours per day (95% CI, 0.02-1.6 hours) for PGY-1, 0.5 hours per day (95% CI, 0.04-0.25 hours) for PGY-2, and 0.3 hours per day (95% CI, 0.03-1.7 hours) for PGY-3. Mobile device logins also were not counted towards total work hours, with a median of 3 minutes per day for PGY-1, 6 minutes per day for PGY-2, and 5 minutes per day for PGY-3.
The percentage of rotation months where average hours worked exceeded 80 hours weekly is shown in Table 2. Inclusion of out-of-hospital work hours substantially increased the frequency at which the 80-hour workweek was exceeded. The frequency of individual residents working more than 80 hours weekly on average is shown in Appendix Figure 3. A narrow majority of PGY-1 and PGY-2 trainees and a larger majority of PGY-3 trainees never worked in excess of 80 hours per week when averaged over the course of a rotation, but several trainees did on several occasions.

Estimations from the computational method were built into a dashboard for use as screening tool by residency program directors (Appendix Figure 4).
DISCUSSION
EHR log data can be used to automate measurement of trainee work hours, providing timely data to program directors for identifying residents at risk of exceeding work hours limits. We demonstrated this by developing a data-driven approach to link on-campus logins that can be replicated in other training programs. We further demonstrated that out-of-hospital work substantially contributed to resident work hours and the frequency with which they exceed the 80-hour workweek, making it a critical component of any work hour estimation approach. Inclusive of out-of-hospital work, our computational method found that residents exceeded the 80-hour workweek 10% to 21% of the time, depending on their year in residency, with a small majority of residents never exceeding the 80-hour workweek.
Historically, most ACGME residency programs have relied on resident self-report to determine work hours.3 The validity of this method has been extensively studied and results remain mixed; in some surveys, residents admit to underreporting their hours while other validation studies, including the use of clock-in and clock-out or time-stamped parking data, align with self-report relatively well.10-12 Regardless of the reliability of self-report, it is a cumbersome task that residents have difficulty adhering to, as shown in our study, where only slightly more than one-half of the days worked had associated self-report. By relying on resident self-report, we are adding to the burden of clerical work, which is associated with physician burnout.13 Furthermore, because self-report typically does not happen in real-time, it limits a program’s ability to intervene on recent or impending work-hour violations. Our computational method enabled us to build a dashboard that is updated daily and provides critical insight into resident work hours at any time, without waiting for retrospective self-report.
Our study builds on previous work by Dziorny et al using EHR log data to algorithmically measure in-hospital work.5 In their study, the authors isolated shifts with a login gap of 4 hours and then combined shifts according to a set of heuristics. However, their logic integrated an extensive workflow analysis of trainee shifts, which might limit generalizability.5 Our approach computationally derives the temporal threshold for linking EHR sessions, which in our data was 5 hours but might differ at other sites. Automated derivation of this threshold will support generalizability to other programs and sites, although programs will still need to manually account for off-site work such as didactics. In a subsequent study evaluating the 80-hour workweek, Dziorny et al evaluated shift duration and appropriate time-off between shifts and found systematic underreporting of work.14 In our study, we prioritized evaluation of the 80-hour workweek and found general alignment between self-report and EHR-derived work-hour estimates, with a tendency to underestimate at lower reported work hours and overestimate at higher reported work hours (potentially because of underreporting as illustrated by Dziorny et al). We included the important out-of-hospital logins as discrete work events because out-of-hospital work contributes to the total hours worked and to the number of workweeks that exceed the 80-hour workweek, and might contribute to burnout.15 The incidence of exceeding the 80-hour workweek increased by 7% to 8% across all residents when out-of-hospital work was included, demonstrating that tools such as ResQ (ResQ Medical) that rely primarily on geolocation data might not sufficiently capture the ways in which residents spend their time working.16
Our approach has limitations. We determined on-campus vs out-of-hospital locations based on whether the login device belonged to the medical center or was a personal computer. Consequently, if trainees exclusively used a personal computer while on-campus and never used a medical center computer, we would have captured this work done while logged into the EHR but would not have inferred on-campus work. Although nearly all trainees in our organization use medical center computers throughout the day, this might impact generalizability for programs where trainees use personal computers exclusively in the hospital. Our approach also assumes trainees will use the EHR at the beginning and end of their workdays, which could lead to underestimation of work hours in trainees who do not employ this practice. With regards to work done on personal computers, our heuristic required that at least one work activity (as denoted in Appendix Table 1) be included in the session in order for it to count as work. Although this approach allows us to exclude sessions where trainees might be reviewing charts exclusively for educational purposes, it is difficult to infer the true intent of chart review.
There might be periods of time where residents are doing in-hospital work but more than 5 hours elapsed between EHR user sessions. As we have started adapting this computational method for other residency programs, we have added logic that allows for long periods of time in the operating room to be considered part of a continuous workday. There also are limitations to assigning blocks of time to off-site clinics; clinics that are associated with after-hours work but use a different EHR would not be captured in total out-of-hospital work.
Although correlation with self-report was good, we identified clusters of inaccuracy. This likely resulted from our residency program covering three medical centers, two of which were not included in the data set. For example, if a resident had an off-site clinic that was not accounted for in AMiON, EHR-derived work hours might have been underestimated relative to self-report. Operationally leveraging an automated system for measuring work hours in the form of dashboards and other tools could provide the impetus to ensure accurate documentation of schedule anomalies.
CONCLUSION
Implementation of our EHR-derived work-hour model will allow ACGME residency programs to understand and act upon trainee work-hour violations closer to real time, as the data extraction is daily and automated. Automation will save busy residents a cumbersome task, provide more complete data than self-report, and empower residency programs to intervene quickly to support overworked trainees.
Acknowledgments
The authors thank Drs Bradley Monash, Larissa Thomas, and Rebecca Berman for providing residency program input.
1. Accreditation Council for Graduate Medical Education. Common program requirements. Accessed August 12, 2020. https://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements
2. Accreditation Council for Graduate Medical Education. Resident/fellow and faculty surveys. Accessed August 12, 2020. https://www.acgme.org/Data-Collection-Systems/Resident-Fellow-and-Faculty-Surveys
3. Petre M, Geana R, Cipparrone N, et al. Comparing electronic and manual tracking systems for monitoring resident duty hours. Ochsner J. 2016;16(1):16-21.
4. Gonzalo JD, Yang JJ, Ngo L, Clark A, Reynolds EE, Herzig SJ. Accuracy of residents’ retrospective perceptions of 16-hour call admitting shift compliance and characteristics. Grad Med Educ. 2013;5(4):630-633. https://doi.org/10.4300/jgme-d-12-00311.1
5. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Automatic detection of front-line clinician hospital shifts: a novel use of electronic health record timestamp data. Appl Clin Inform. 2019;10(1):28-37. https://doi.org/10.1055/s-0038-1676819
6. Gardner RL, Cooper E, Haskell J, et al. Physician stress and burnout: the impact of health information technology. J Am Med Inform Assoc. 2019;26(2):106-114. https://doi.org/10.1093/jamia/ocy145
7. MedHub. Accessed April 7, 2021. https://www.medhub.com
8. AMiON. Accessed April 7, 2021. https://www.amion.com
9. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference. https://conference.scipy.org/proceedings/scipy2010/pdfs/seabold.pdf
10. Todd SR, Fahy BN, Paukert JL, Mersinger D, Johnson ML, Bass BL. How accurate are self-reported resident duty hours? J Surg Educ. 2010;67(2):103-107. https://doi.org/10.1016/j.jsurg.2009.08.004
11. Chadaga SR, Keniston A, Casey D, Albert RK. Correlation between self-reported resident duty hours and time-stamped parking data. J Grad Med Educ. 2012;4(2):254-256. https://doi.org/10.4300/JGME-D-11-00142.1
12. Drolet BC, Schwede M, Bishop KD, Fischer SA. Compliance and falsification of duty hours: reports from residents and program directors. J Grad Med Educ. 2013;5(3):368-373. https://doi.org/10.4300/JGME-D-12-00375.1
13. Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317(9):901. https://doi.org/10.1001/jama.2017.0076
14. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Pediatric trainees systematically under-report duty hour violations compared to electronic health record defined shifts. PLOS ONE. 2019;14(12):e0226493. https://doi.org/10.1371/journal.pone.0226493
15. Saag HS, Shah K, Jones SA, Testa PA, Horwitz LI. Pajama time: working after work in the electronic health record. J Gen Intern Med. 2019;34(9):1695-1696. https://doi.org/10.1007/s11606-019-05055-x
16. ResQ Medical. Accessed April 7, 2021. https://resqmedical.com
1. Accreditation Council for Graduate Medical Education. Common program requirements. Accessed August 12, 2020. https://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements
2. Accreditation Council for Graduate Medical Education. Resident/fellow and faculty surveys. Accessed August 12, 2020. https://www.acgme.org/Data-Collection-Systems/Resident-Fellow-and-Faculty-Surveys
3. Petre M, Geana R, Cipparrone N, et al. Comparing electronic and manual tracking systems for monitoring resident duty hours. Ochsner J. 2016;16(1):16-21.
4. Gonzalo JD, Yang JJ, Ngo L, Clark A, Reynolds EE, Herzig SJ. Accuracy of residents’ retrospective perceptions of 16-hour call admitting shift compliance and characteristics. Grad Med Educ. 2013;5(4):630-633. https://doi.org/10.4300/jgme-d-12-00311.1
5. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Automatic detection of front-line clinician hospital shifts: a novel use of electronic health record timestamp data. Appl Clin Inform. 2019;10(1):28-37. https://doi.org/10.1055/s-0038-1676819
6. Gardner RL, Cooper E, Haskell J, et al. Physician stress and burnout: the impact of health information technology. J Am Med Inform Assoc. 2019;26(2):106-114. https://doi.org/10.1093/jamia/ocy145
7. MedHub. Accessed April 7, 2021. https://www.medhub.com
8. AMiON. Accessed April 7, 2021. https://www.amion.com
9. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference. https://conference.scipy.org/proceedings/scipy2010/pdfs/seabold.pdf
10. Todd SR, Fahy BN, Paukert JL, Mersinger D, Johnson ML, Bass BL. How accurate are self-reported resident duty hours? J Surg Educ. 2010;67(2):103-107. https://doi.org/10.1016/j.jsurg.2009.08.004
11. Chadaga SR, Keniston A, Casey D, Albert RK. Correlation between self-reported resident duty hours and time-stamped parking data. J Grad Med Educ. 2012;4(2):254-256. https://doi.org/10.4300/JGME-D-11-00142.1
12. Drolet BC, Schwede M, Bishop KD, Fischer SA. Compliance and falsification of duty hours: reports from residents and program directors. J Grad Med Educ. 2013;5(3):368-373. https://doi.org/10.4300/JGME-D-12-00375.1
13. Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317(9):901. https://doi.org/10.1001/jama.2017.0076
14. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Pediatric trainees systematically under-report duty hour violations compared to electronic health record defined shifts. PLOS ONE. 2019;14(12):e0226493. https://doi.org/10.1371/journal.pone.0226493
15. Saag HS, Shah K, Jones SA, Testa PA, Horwitz LI. Pajama time: working after work in the electronic health record. J Gen Intern Med. 2019;34(9):1695-1696. https://doi.org/10.1007/s11606-019-05055-x
16. ResQ Medical. Accessed April 7, 2021. https://resqmedical.com
© 2021 Society of Hospital Medicine
Female rheumatologists see fewer patients, earn less than males
A new study on the changing rheumatology workforce found that, although there has been a notable rise in female rheumatologists, they see fewer patients and have lower earnings than their male counterparts.
“In order for future health workforce policy and planning to be effective and equitable, it is essential to consider policies and other solutions to support the sustainability of rheumatology workforces in light of increasing feminization,” wrote Jessica Widdifield, PhD, of the Sunnybrook Research Institute in Toronto and her colleagues. The study was published in the Journal of Rheumatology.
To investigate potential workload and earnings disparities between male and female rheumatologists, the researchers launched a population-based study of rheumatologists practicing in Ontario, Canada, and their patient visits between April 1, 2000, and March 31, 2015. To quantify clinical activity, they calculated full-time equivalents (FTEs) using annual fee-for-service billing claims and defined rheumatologists practicing at least one clinical FTE as those at or above the 40th percentile of total billings each year. Any rheumatologists practicing less than one FTE were not included in the larger analysis.
Overall, they found that the total number of rheumatologists increased from 146 in 2000 to 194 in 2015, with 49% of the latter workforce being women. When assessing only rheumatologists practicing at greater than one FTE, the number increased from 89 in 2000 to 120 in 2015, with women making up 41.7% of the 2015 workforce. Although practice sizes decreased for both genders over the course of the study, in 2015 the median practice size was 1,948.5 patients (interquartile range, 1,433-2,562) for men, compared with 1,468.5 patients (IQR, 1,212-1,984) for women. In every year but 2001, men had larger median practice sizes than women.
Total patient visits remained relatively stable for men throughout the study period but declined for women, with the gap between genders widening over time. The peak gap in visits was 1,486 (95% confidence interval, 628-2,517) in 2008. And while median payments increased over time for all rheumatologists, median renumeration peaked in 2015 at $362,522 (IQR, $309,503-$437,127) for women, compared with $403,903 (IQR, $313,297-$544,703) for men. That said, the median difference that year – $45,556.10 (95% confidence interval, $951.60-$92,470.40; P = .04) – was the smallest for any in the study period. The largest difference was $102,176.10 (95% CI, $58,457.50-$152,821.20; P < .0001) in 2011.
An opportunity for female rheumatologists to reshape the specialty
Of course, gender gaps like these are not limited to rheumatology or even medicine, wrote Grace C. Wright, MD, PhD, president of the Association of Women in Rheumatology, in an accompanying editorial. “This issue exists across industries as well as across boundaries.”
“Particularly for women physicians, we do have additional demands on our time,” agreed April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School in Boston, in an interview. “For example, we know that women who work often have additional caregiving responsibilities at home, for kids and/or elderly relatives. I do think those are real reasons why certain providers, particularly women, might have a lower clinical volume.”
Despite the significant gender gaps that still exist, Dr. Jorge – who authored a previous study on the gaps in academic rheumatology – was heartened by the data that indicated more women finding their way into the specialty.
“I think it’s good news for rheumatology to be so balanced between men and women as providers,” she said. “For young women trainees, it’s really important to see role models in their field. For patients, it’s incredibly important for them to have a doctor who can relate and who can advocate for them. So many rheumatic conditions that we treat disproportionately affect women, often women of childbearing age. So it’s really important to have women involved in leading the specialty of rheumatology, including clinical practice but also research, education, and policy.”
Dr. Wright concurred in her editorial, stating that “this feminization of rheumatology provides an opportunity to assess the needs of working women, the generational shifts in attitudes toward work-life balance, and a change in clinical practice toward value over volume.”
The study’s authors shared its possible limitations, including the lack of a standard definition of a clinical FTE rheumatologist – thus their decision to define one – and a lack of context as to why certain rheumatologists were practicing less than others. In addition, they preemptively acknowledged Dr. Jorge’s concern by noting their inability to access gender-related details like marital status, family size, and childcare roles, all of which “could contribute to the relationship between physician gender and practice-level activity.”
The study was funded by an operating grant from the Canadian Initiative for Outcomes in Rheumatology Care and supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Two of the authors reported receiving support from the Arthritis Society Stars Career Development Award.
A new study on the changing rheumatology workforce found that, although there has been a notable rise in female rheumatologists, they see fewer patients and have lower earnings than their male counterparts.
“In order for future health workforce policy and planning to be effective and equitable, it is essential to consider policies and other solutions to support the sustainability of rheumatology workforces in light of increasing feminization,” wrote Jessica Widdifield, PhD, of the Sunnybrook Research Institute in Toronto and her colleagues. The study was published in the Journal of Rheumatology.
To investigate potential workload and earnings disparities between male and female rheumatologists, the researchers launched a population-based study of rheumatologists practicing in Ontario, Canada, and their patient visits between April 1, 2000, and March 31, 2015. To quantify clinical activity, they calculated full-time equivalents (FTEs) using annual fee-for-service billing claims and defined rheumatologists practicing at least one clinical FTE as those at or above the 40th percentile of total billings each year. Any rheumatologists practicing less than one FTE were not included in the larger analysis.
Overall, they found that the total number of rheumatologists increased from 146 in 2000 to 194 in 2015, with 49% of the latter workforce being women. When assessing only rheumatologists practicing at greater than one FTE, the number increased from 89 in 2000 to 120 in 2015, with women making up 41.7% of the 2015 workforce. Although practice sizes decreased for both genders over the course of the study, in 2015 the median practice size was 1,948.5 patients (interquartile range, 1,433-2,562) for men, compared with 1,468.5 patients (IQR, 1,212-1,984) for women. In every year but 2001, men had larger median practice sizes than women.
Total patient visits remained relatively stable for men throughout the study period but declined for women, with the gap between genders widening over time. The peak gap in visits was 1,486 (95% confidence interval, 628-2,517) in 2008. And while median payments increased over time for all rheumatologists, median renumeration peaked in 2015 at $362,522 (IQR, $309,503-$437,127) for women, compared with $403,903 (IQR, $313,297-$544,703) for men. That said, the median difference that year – $45,556.10 (95% confidence interval, $951.60-$92,470.40; P = .04) – was the smallest for any in the study period. The largest difference was $102,176.10 (95% CI, $58,457.50-$152,821.20; P < .0001) in 2011.
An opportunity for female rheumatologists to reshape the specialty
Of course, gender gaps like these are not limited to rheumatology or even medicine, wrote Grace C. Wright, MD, PhD, president of the Association of Women in Rheumatology, in an accompanying editorial. “This issue exists across industries as well as across boundaries.”
“Particularly for women physicians, we do have additional demands on our time,” agreed April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School in Boston, in an interview. “For example, we know that women who work often have additional caregiving responsibilities at home, for kids and/or elderly relatives. I do think those are real reasons why certain providers, particularly women, might have a lower clinical volume.”
Despite the significant gender gaps that still exist, Dr. Jorge – who authored a previous study on the gaps in academic rheumatology – was heartened by the data that indicated more women finding their way into the specialty.
“I think it’s good news for rheumatology to be so balanced between men and women as providers,” she said. “For young women trainees, it’s really important to see role models in their field. For patients, it’s incredibly important for them to have a doctor who can relate and who can advocate for them. So many rheumatic conditions that we treat disproportionately affect women, often women of childbearing age. So it’s really important to have women involved in leading the specialty of rheumatology, including clinical practice but also research, education, and policy.”
Dr. Wright concurred in her editorial, stating that “this feminization of rheumatology provides an opportunity to assess the needs of working women, the generational shifts in attitudes toward work-life balance, and a change in clinical practice toward value over volume.”
The study’s authors shared its possible limitations, including the lack of a standard definition of a clinical FTE rheumatologist – thus their decision to define one – and a lack of context as to why certain rheumatologists were practicing less than others. In addition, they preemptively acknowledged Dr. Jorge’s concern by noting their inability to access gender-related details like marital status, family size, and childcare roles, all of which “could contribute to the relationship between physician gender and practice-level activity.”
The study was funded by an operating grant from the Canadian Initiative for Outcomes in Rheumatology Care and supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Two of the authors reported receiving support from the Arthritis Society Stars Career Development Award.
A new study on the changing rheumatology workforce found that, although there has been a notable rise in female rheumatologists, they see fewer patients and have lower earnings than their male counterparts.
“In order for future health workforce policy and planning to be effective and equitable, it is essential to consider policies and other solutions to support the sustainability of rheumatology workforces in light of increasing feminization,” wrote Jessica Widdifield, PhD, of the Sunnybrook Research Institute in Toronto and her colleagues. The study was published in the Journal of Rheumatology.
To investigate potential workload and earnings disparities between male and female rheumatologists, the researchers launched a population-based study of rheumatologists practicing in Ontario, Canada, and their patient visits between April 1, 2000, and March 31, 2015. To quantify clinical activity, they calculated full-time equivalents (FTEs) using annual fee-for-service billing claims and defined rheumatologists practicing at least one clinical FTE as those at or above the 40th percentile of total billings each year. Any rheumatologists practicing less than one FTE were not included in the larger analysis.
Overall, they found that the total number of rheumatologists increased from 146 in 2000 to 194 in 2015, with 49% of the latter workforce being women. When assessing only rheumatologists practicing at greater than one FTE, the number increased from 89 in 2000 to 120 in 2015, with women making up 41.7% of the 2015 workforce. Although practice sizes decreased for both genders over the course of the study, in 2015 the median practice size was 1,948.5 patients (interquartile range, 1,433-2,562) for men, compared with 1,468.5 patients (IQR, 1,212-1,984) for women. In every year but 2001, men had larger median practice sizes than women.
Total patient visits remained relatively stable for men throughout the study period but declined for women, with the gap between genders widening over time. The peak gap in visits was 1,486 (95% confidence interval, 628-2,517) in 2008. And while median payments increased over time for all rheumatologists, median renumeration peaked in 2015 at $362,522 (IQR, $309,503-$437,127) for women, compared with $403,903 (IQR, $313,297-$544,703) for men. That said, the median difference that year – $45,556.10 (95% confidence interval, $951.60-$92,470.40; P = .04) – was the smallest for any in the study period. The largest difference was $102,176.10 (95% CI, $58,457.50-$152,821.20; P < .0001) in 2011.
An opportunity for female rheumatologists to reshape the specialty
Of course, gender gaps like these are not limited to rheumatology or even medicine, wrote Grace C. Wright, MD, PhD, president of the Association of Women in Rheumatology, in an accompanying editorial. “This issue exists across industries as well as across boundaries.”
“Particularly for women physicians, we do have additional demands on our time,” agreed April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School in Boston, in an interview. “For example, we know that women who work often have additional caregiving responsibilities at home, for kids and/or elderly relatives. I do think those are real reasons why certain providers, particularly women, might have a lower clinical volume.”
Despite the significant gender gaps that still exist, Dr. Jorge – who authored a previous study on the gaps in academic rheumatology – was heartened by the data that indicated more women finding their way into the specialty.
“I think it’s good news for rheumatology to be so balanced between men and women as providers,” she said. “For young women trainees, it’s really important to see role models in their field. For patients, it’s incredibly important for them to have a doctor who can relate and who can advocate for them. So many rheumatic conditions that we treat disproportionately affect women, often women of childbearing age. So it’s really important to have women involved in leading the specialty of rheumatology, including clinical practice but also research, education, and policy.”
Dr. Wright concurred in her editorial, stating that “this feminization of rheumatology provides an opportunity to assess the needs of working women, the generational shifts in attitudes toward work-life balance, and a change in clinical practice toward value over volume.”
The study’s authors shared its possible limitations, including the lack of a standard definition of a clinical FTE rheumatologist – thus their decision to define one – and a lack of context as to why certain rheumatologists were practicing less than others. In addition, they preemptively acknowledged Dr. Jorge’s concern by noting their inability to access gender-related details like marital status, family size, and childcare roles, all of which “could contribute to the relationship between physician gender and practice-level activity.”
The study was funded by an operating grant from the Canadian Initiative for Outcomes in Rheumatology Care and supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Two of the authors reported receiving support from the Arthritis Society Stars Career Development Award.
FROM THE JOURNAL OF RHEUMATOLOGY
COVID-19 vaccine response lower in kidney dialysis patients
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.