User login
Antibiotics may prolong PFS in HCC patients on immunotherapy
Response rates and overall survival (OS), on the other hand, did not seem to be affected by antibiotic administration, according to investigator Petros Fessas, MD, of Imperial College London.
Dr. Fessas and colleagues presented these findings in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract 485).
Dr. Fessas noted that, in other cancers, antibiotics have been shown to reduce both response and survival rates after ICI.
To assess the impact of early antibiotic exposure on ICI efficacy in HCC, Dr. Fessas and colleagues examined data from 449 patients treated at 12 centers in the United States, Europe, and Asia.
The patients’ median age was 65 years, and 79.1% were men. Nearly three-quarters (73.3%) were cirrhotic (60.4% because of viral hepatitis), 79.9% were Child-Pugh class A, 72.4% were Barcelona Clinic Liver Cancer stage C, and 79% had an Eastern Cooperative Oncology Group performance status of 0-1.
Response and survival results
The investigators compared outcomes between patients with and without antibiotic exposure in the early immunotherapy period (EIOP) 30 days before and after ICI initiation.
In all, 170 patients (37.9%) received antibiotics in the EIOP. There were no differences in response rates, disease control rates, or median OS between patients who received antibiotics and those who did not.
The objective response rate was 20.2% in patients who received antibiotics in the EIOP and 16.1% in patients who did not (P = .2808). Disease control rates were 63.1% and 55.4%, respectively (P = .1144). The median OS was 15.3 months and 15.4 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.72-1.21; P = .6275).
The median PFS, however, was significantly longer in patients who received antibiotics than in patients who did not – 6.1 months and 3.7 months, respectively (HR, 0.74; 95% CI, 0.60-0.93; P = .0135).
To overcome possible bias introduced by misclassification of patients who received antibiotics but discontinued immunotherapy within 30 days of initiation, the investigators conducted a landmark selection analysis among only those patients with a median follow-up for PFS of 30 days or longer (n = 402). This analysis confirmed the prior findings.
“Antibiotic exposure in the 30 days before or after immune checkpoint initiation in hepatocellular carcinoma is associated with prolonged progression-free survival,” Dr. Fessas concluded.
He added that a key question for future research is to discover the immune-microbiologic determinants of response to initiation of ICIs.
Positive effect surprising
“My group has shown that antibiotic therapy is normally detrimental in patients with cancer,” investigator David J. Pinato, MD, PhD, of Imperial College London, said in an interview. “So we were very surprised to see a positive effect on PFS.”
He added that the new findings should be interpreted with caution.
“My feeling is that, unlike with many other malignancies, the gut microbiome is heavily involved in the progression of the cirrhosis that pre-dates HCC onset,” he said.
That would suggest the relationship between antibiotics and perturbation of the gut microbiome is dictated by something more than changes in antitumor immune tolerance, he added.
“Overall, I think the interplay is more complex in HCC: cirrhosis/cancer/microbiome, not just microbiome/cancer as in many other tumors,” Dr. Pinato said. “So we are looking at microbial determinants of response in HCC patients undergoing ICI therapy, and we are hopeful to see some more mechanistic evidence behind this association.”
Dr. Pinato disclosed relationships with ViiV Healthcare, Bayer Healthcare, Bristol Myers Squibb, Mina Therapeutics, Eisai, Roche, and AstraZeneca. Dr. Fessas reported having no conflicts of interest. No funding source for the study was disclosed.
Response rates and overall survival (OS), on the other hand, did not seem to be affected by antibiotic administration, according to investigator Petros Fessas, MD, of Imperial College London.
Dr. Fessas and colleagues presented these findings in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract 485).
Dr. Fessas noted that, in other cancers, antibiotics have been shown to reduce both response and survival rates after ICI.
To assess the impact of early antibiotic exposure on ICI efficacy in HCC, Dr. Fessas and colleagues examined data from 449 patients treated at 12 centers in the United States, Europe, and Asia.
The patients’ median age was 65 years, and 79.1% were men. Nearly three-quarters (73.3%) were cirrhotic (60.4% because of viral hepatitis), 79.9% were Child-Pugh class A, 72.4% were Barcelona Clinic Liver Cancer stage C, and 79% had an Eastern Cooperative Oncology Group performance status of 0-1.
Response and survival results
The investigators compared outcomes between patients with and without antibiotic exposure in the early immunotherapy period (EIOP) 30 days before and after ICI initiation.
In all, 170 patients (37.9%) received antibiotics in the EIOP. There were no differences in response rates, disease control rates, or median OS between patients who received antibiotics and those who did not.
The objective response rate was 20.2% in patients who received antibiotics in the EIOP and 16.1% in patients who did not (P = .2808). Disease control rates were 63.1% and 55.4%, respectively (P = .1144). The median OS was 15.3 months and 15.4 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.72-1.21; P = .6275).
The median PFS, however, was significantly longer in patients who received antibiotics than in patients who did not – 6.1 months and 3.7 months, respectively (HR, 0.74; 95% CI, 0.60-0.93; P = .0135).
To overcome possible bias introduced by misclassification of patients who received antibiotics but discontinued immunotherapy within 30 days of initiation, the investigators conducted a landmark selection analysis among only those patients with a median follow-up for PFS of 30 days or longer (n = 402). This analysis confirmed the prior findings.
“Antibiotic exposure in the 30 days before or after immune checkpoint initiation in hepatocellular carcinoma is associated with prolonged progression-free survival,” Dr. Fessas concluded.
He added that a key question for future research is to discover the immune-microbiologic determinants of response to initiation of ICIs.
Positive effect surprising
“My group has shown that antibiotic therapy is normally detrimental in patients with cancer,” investigator David J. Pinato, MD, PhD, of Imperial College London, said in an interview. “So we were very surprised to see a positive effect on PFS.”
He added that the new findings should be interpreted with caution.
“My feeling is that, unlike with many other malignancies, the gut microbiome is heavily involved in the progression of the cirrhosis that pre-dates HCC onset,” he said.
That would suggest the relationship between antibiotics and perturbation of the gut microbiome is dictated by something more than changes in antitumor immune tolerance, he added.
“Overall, I think the interplay is more complex in HCC: cirrhosis/cancer/microbiome, not just microbiome/cancer as in many other tumors,” Dr. Pinato said. “So we are looking at microbial determinants of response in HCC patients undergoing ICI therapy, and we are hopeful to see some more mechanistic evidence behind this association.”
Dr. Pinato disclosed relationships with ViiV Healthcare, Bayer Healthcare, Bristol Myers Squibb, Mina Therapeutics, Eisai, Roche, and AstraZeneca. Dr. Fessas reported having no conflicts of interest. No funding source for the study was disclosed.
Response rates and overall survival (OS), on the other hand, did not seem to be affected by antibiotic administration, according to investigator Petros Fessas, MD, of Imperial College London.
Dr. Fessas and colleagues presented these findings in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract 485).
Dr. Fessas noted that, in other cancers, antibiotics have been shown to reduce both response and survival rates after ICI.
To assess the impact of early antibiotic exposure on ICI efficacy in HCC, Dr. Fessas and colleagues examined data from 449 patients treated at 12 centers in the United States, Europe, and Asia.
The patients’ median age was 65 years, and 79.1% were men. Nearly three-quarters (73.3%) were cirrhotic (60.4% because of viral hepatitis), 79.9% were Child-Pugh class A, 72.4% were Barcelona Clinic Liver Cancer stage C, and 79% had an Eastern Cooperative Oncology Group performance status of 0-1.
Response and survival results
The investigators compared outcomes between patients with and without antibiotic exposure in the early immunotherapy period (EIOP) 30 days before and after ICI initiation.
In all, 170 patients (37.9%) received antibiotics in the EIOP. There were no differences in response rates, disease control rates, or median OS between patients who received antibiotics and those who did not.
The objective response rate was 20.2% in patients who received antibiotics in the EIOP and 16.1% in patients who did not (P = .2808). Disease control rates were 63.1% and 55.4%, respectively (P = .1144). The median OS was 15.3 months and 15.4 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.72-1.21; P = .6275).
The median PFS, however, was significantly longer in patients who received antibiotics than in patients who did not – 6.1 months and 3.7 months, respectively (HR, 0.74; 95% CI, 0.60-0.93; P = .0135).
To overcome possible bias introduced by misclassification of patients who received antibiotics but discontinued immunotherapy within 30 days of initiation, the investigators conducted a landmark selection analysis among only those patients with a median follow-up for PFS of 30 days or longer (n = 402). This analysis confirmed the prior findings.
“Antibiotic exposure in the 30 days before or after immune checkpoint initiation in hepatocellular carcinoma is associated with prolonged progression-free survival,” Dr. Fessas concluded.
He added that a key question for future research is to discover the immune-microbiologic determinants of response to initiation of ICIs.
Positive effect surprising
“My group has shown that antibiotic therapy is normally detrimental in patients with cancer,” investigator David J. Pinato, MD, PhD, of Imperial College London, said in an interview. “So we were very surprised to see a positive effect on PFS.”
He added that the new findings should be interpreted with caution.
“My feeling is that, unlike with many other malignancies, the gut microbiome is heavily involved in the progression of the cirrhosis that pre-dates HCC onset,” he said.
That would suggest the relationship between antibiotics and perturbation of the gut microbiome is dictated by something more than changes in antitumor immune tolerance, he added.
“Overall, I think the interplay is more complex in HCC: cirrhosis/cancer/microbiome, not just microbiome/cancer as in many other tumors,” Dr. Pinato said. “So we are looking at microbial determinants of response in HCC patients undergoing ICI therapy, and we are hopeful to see some more mechanistic evidence behind this association.”
Dr. Pinato disclosed relationships with ViiV Healthcare, Bayer Healthcare, Bristol Myers Squibb, Mina Therapeutics, Eisai, Roche, and AstraZeneca. Dr. Fessas reported having no conflicts of interest. No funding source for the study was disclosed.
FROM AACR 2021
CLL patients: Diagnostic difficulties, treatment confusion with COVID-19
Chronic lymphocytic leukemia (CLL) patients present significant problems with regard to COVID-19 disease, according to a literature review by Yousef Roosta, MD, of Urmia (Iran) University of Medical Sciences, and colleagues.
Diagnostic interaction between CLL and COVID-19 provides a major challenge. CLL patients have a lower rate of anti–SARS-CoV-2 IgG development, and evidence shows worse therapeutic outcomes in these patients, according to study published in Leukemia Research Reports.
The researchers assessed 20 retrieved articles, 11 of which examined patients with CLL and with concomitant COVID-19; and 9 articles were designed as prospective or retrospective case series of such patients. The studies were assessed qualitatively by the QUADAS-2 tool.
Troubling results
Although the overall prevalence of CLL and COVID-19 concurrence was low, at 0.6% (95% confidence interval 0.5%-0.7%) according to the meta-analysis, the results showed some special challenges in the diagnosis and care of these patients.
Diagnostic difficulties are a unique problem. Lymphopenia is common in patients with COVID-19, while lymphocytosis may be considered a transient or even rare finding. The interplay between the two diseases is sometimes very misleading for specialists, and in patients with lymphocytosis, the diagnosis of CLL may be completely ignored, according to the researchers. They added that when performing a diagnostic approach for concurrent COVID-19 and CLL, due to differences in the amount and type of immune response, “relying on serological testing, and especially the evaluation of the anti–SARS-CoV-2 IgG levels may not be beneficial,” they indicated.
In addition, studies showed unacceptable therapeutic outcome in patients with concurrent CLL and COVID-19, with mortality ranging from 33% to 41.7%, showing a need to revise current treatment protocols, according to the authors. In one study, 85.7% of surviving patients showed a considerable decrease in functional class and significant fatigue, with such a poor prognosis occurring more commonly in the elderly.
With regard to treatment, “it is quite obvious that despite the use of current standard protocols, the prognosis of these patients will be much worse than the prognosis of CLL patients with no evidence of COVID-19. Even in the first-line treatment protocol for these patients, there is no agreement in combination therapy with selected CLL drugs along with management protocols of COVID-19 patients,” the researchers stated.
“[The] different hematological behaviors of two diseases might mimic the detection of COVID-19 in the CLL state and vise versa. Also, due to the low level of immune response against SARS-CoV-2 in CLL patients, both scheduled immunological-based diagnosis and treatment may fail,” the researchers added.
The authors reported that they had no disclosures.
Chronic lymphocytic leukemia (CLL) patients present significant problems with regard to COVID-19 disease, according to a literature review by Yousef Roosta, MD, of Urmia (Iran) University of Medical Sciences, and colleagues.
Diagnostic interaction between CLL and COVID-19 provides a major challenge. CLL patients have a lower rate of anti–SARS-CoV-2 IgG development, and evidence shows worse therapeutic outcomes in these patients, according to study published in Leukemia Research Reports.
The researchers assessed 20 retrieved articles, 11 of which examined patients with CLL and with concomitant COVID-19; and 9 articles were designed as prospective or retrospective case series of such patients. The studies were assessed qualitatively by the QUADAS-2 tool.
Troubling results
Although the overall prevalence of CLL and COVID-19 concurrence was low, at 0.6% (95% confidence interval 0.5%-0.7%) according to the meta-analysis, the results showed some special challenges in the diagnosis and care of these patients.
Diagnostic difficulties are a unique problem. Lymphopenia is common in patients with COVID-19, while lymphocytosis may be considered a transient or even rare finding. The interplay between the two diseases is sometimes very misleading for specialists, and in patients with lymphocytosis, the diagnosis of CLL may be completely ignored, according to the researchers. They added that when performing a diagnostic approach for concurrent COVID-19 and CLL, due to differences in the amount and type of immune response, “relying on serological testing, and especially the evaluation of the anti–SARS-CoV-2 IgG levels may not be beneficial,” they indicated.
In addition, studies showed unacceptable therapeutic outcome in patients with concurrent CLL and COVID-19, with mortality ranging from 33% to 41.7%, showing a need to revise current treatment protocols, according to the authors. In one study, 85.7% of surviving patients showed a considerable decrease in functional class and significant fatigue, with such a poor prognosis occurring more commonly in the elderly.
With regard to treatment, “it is quite obvious that despite the use of current standard protocols, the prognosis of these patients will be much worse than the prognosis of CLL patients with no evidence of COVID-19. Even in the first-line treatment protocol for these patients, there is no agreement in combination therapy with selected CLL drugs along with management protocols of COVID-19 patients,” the researchers stated.
“[The] different hematological behaviors of two diseases might mimic the detection of COVID-19 in the CLL state and vise versa. Also, due to the low level of immune response against SARS-CoV-2 in CLL patients, both scheduled immunological-based diagnosis and treatment may fail,” the researchers added.
The authors reported that they had no disclosures.
Chronic lymphocytic leukemia (CLL) patients present significant problems with regard to COVID-19 disease, according to a literature review by Yousef Roosta, MD, of Urmia (Iran) University of Medical Sciences, and colleagues.
Diagnostic interaction between CLL and COVID-19 provides a major challenge. CLL patients have a lower rate of anti–SARS-CoV-2 IgG development, and evidence shows worse therapeutic outcomes in these patients, according to study published in Leukemia Research Reports.
The researchers assessed 20 retrieved articles, 11 of which examined patients with CLL and with concomitant COVID-19; and 9 articles were designed as prospective or retrospective case series of such patients. The studies were assessed qualitatively by the QUADAS-2 tool.
Troubling results
Although the overall prevalence of CLL and COVID-19 concurrence was low, at 0.6% (95% confidence interval 0.5%-0.7%) according to the meta-analysis, the results showed some special challenges in the diagnosis and care of these patients.
Diagnostic difficulties are a unique problem. Lymphopenia is common in patients with COVID-19, while lymphocytosis may be considered a transient or even rare finding. The interplay between the two diseases is sometimes very misleading for specialists, and in patients with lymphocytosis, the diagnosis of CLL may be completely ignored, according to the researchers. They added that when performing a diagnostic approach for concurrent COVID-19 and CLL, due to differences in the amount and type of immune response, “relying on serological testing, and especially the evaluation of the anti–SARS-CoV-2 IgG levels may not be beneficial,” they indicated.
In addition, studies showed unacceptable therapeutic outcome in patients with concurrent CLL and COVID-19, with mortality ranging from 33% to 41.7%, showing a need to revise current treatment protocols, according to the authors. In one study, 85.7% of surviving patients showed a considerable decrease in functional class and significant fatigue, with such a poor prognosis occurring more commonly in the elderly.
With regard to treatment, “it is quite obvious that despite the use of current standard protocols, the prognosis of these patients will be much worse than the prognosis of CLL patients with no evidence of COVID-19. Even in the first-line treatment protocol for these patients, there is no agreement in combination therapy with selected CLL drugs along with management protocols of COVID-19 patients,” the researchers stated.
“[The] different hematological behaviors of two diseases might mimic the detection of COVID-19 in the CLL state and vise versa. Also, due to the low level of immune response against SARS-CoV-2 in CLL patients, both scheduled immunological-based diagnosis and treatment may fail,” the researchers added.
The authors reported that they had no disclosures.
FROM LEUKEMIA RESEARCH REPORTS
Eating more fat may boost borderline low testosterone
Low-fat diets appear to decrease testosterone levels in men, but further randomized, controlled trials are needed to confirm this effect, the authors of a meta-analysis of six small intervention studies concluded.
A total of 206 healthy men with normal testosterone received a high-fat diet followed by a low-fat diet (or vice versa), and their mean total testosterone levels were 10%-15% lower (but still in the normal range) during the low-fat diet.
The study by registered nutritionist Joseph Whittaker, MSc, University of Worcester (England), and statistician Kexin Wu, MSc, University of Warwick, Coventry, England, was published online in the Journal of Steroid Biochemistry and Molecular Biology.
“I think our results are consistent and fairly strong, but they are not strong enough to give blanket recommendations,” Mr. Whittaker said in an interview.
However, “if somebody has low testosterone, particularly borderline, they could try increasing their fat intake, maybe on a Mediterranean diet,” he said, and see if that works to increase their testosterone by 60 ng/dL, the weighted mean difference in total testosterone levels between the low-fat versus high-fat diet interventions in this meta-analysis.
“A Mediterranean diet is a good way to increase ‘healthy fats,’ mono- and polyunsaturated fatty acids, which will likely decrease cardiovascular disease risk, and boost testosterone at the same time,” Mr. Whittaker noted.
Olive oil has been shown to boost testosterone more than butter, and it also reduces CVD, he continued. Nuts are high in “healthy fats” and consistently decrease CVD and mortality and may boost testosterone. Other sources of “good fat” in a healthy diet include avocado, and red meat and poultry in moderation.
“It is controversial, but our results also indicate that foods with saturated fatty acids may boost testosterone,” he added, noting however that such foods are also associated with an increase in cholesterol.
Is waning testosterone explained by leaner diet?
Men need healthy testosterone levels for good physical performance, mental health, and sexual health, and low levels are associated with a higher risk of heart disease, diabetes, and Alzheimer’s disease, according to a statement about this research issued by the University of Worcester.
Although testosterone levels do decline with advancing age, there has also been an additional age-independent and persistent decline in testosterone levels that began roughly after nutrition guidelines began recommending a lower-fat diet in 1965.
Fat consumption dropped from 45% of the diet in 1965 to 35% of the diet in 1991, and stayed around that lower level through to 2011.
However, it is not clear if this decrease in dietary fat intake might explain part of the concurrent decline in men’s testosterone levels.
Mr. Whittaker and Mr. Wu conducted a systematic literature review and identified six crossover intervention studies that compared testosterone levels during low-fat versus high-fat diets – Dorgan 1996, Wang 2005, Hamalainen 1984, Hill 1980, Reed 1987, and Hill 1979 – and then they combined these studies in a meta-analysis.
Five studies each enrolled 6-43 healthy men from North America, the United Kingdom, and Scandinavia, and the sixth study (Hill 1980) enrolled 34 healthy men from North America and 39 farm laborers from South Africa.
Overall, on average, the men were aged 34-54 years and slightly overweight (a mean body mass index of roughly 27 kg/m2) with normal testosterone (i.e., >300 ng/dL, based on the 2018 American Urological Association guidelines criteria).
Most men received a high-fat diet (40% of calories from fat) first, followed by a low-fat diet (on average 20% of calories from fat; range, 7%-25%), but the subgroup of men from South Africa received the low-fat diet first.
To put this into context, U.K. guidelines recommend a fat intake of less than 35% of daily calories, and U.S. guidelines recommend a fat intake of 20%-35% of daily calories.
The low-and high-fat interventions ranged from 2 to 10 weeks.
Lowest testosterone levels with low-fat vegetarian diets
Overall, on average, the men’s total testosterone was 475 mg/dL when they were consuming a low-fat diet and 532 mg/dL when they were consuming a high-fat diet.
However, the South African men had higher testosterone levels when they consumed a low-fat diet. This suggests that “men with European ancestry may experience a greater decrease in testosterone in response to a low-fat diet,” the researchers wrote.
The decrease in total testosterone in the low-fat versus high-fat diet was largest (26%) in the two studies of men who consumed a vegetarian diet (Hill 1979 and Hill 1980). These diets may have been low in zinc, since a marginal zinc deficiency has been shown to decrease total testosterone, Mr. Whittaker and Mr. Wu speculated.
The meta-analysis also showed that levels of free testosterone, urinary testosterone, and dihydrotestosterone declined during the low-fat diet, whereas levels of luteinizing hormone or sex hormone binding globulin were similar with both diets.
Men with low testosterone and overweight, obesity
What nutritional advice should practitioners give to men who have low testosterone and overweight/obesity?
“If you are very overweight, losing weight is going to dramatically improve your testosterone,” Mr. Whittaker said.
However, proponents of various diets are often in stark disagreement about the merits of a low-fat versus low-carbohydrate diet to lose weight.
“In general,” he continued, “the literature shows low-carb (high-fat) diets are better for weight loss [although many will disagree with that statement].”
Although nutrition guidelines have stressed the importance of limiting fat intake, fat in the diet is also associated with lower triglyceride levels and blood pressure and higher HDL cholesterol levels, and now in this study, higher testosterone levels.
More research needed
The researchers acknowledge study limitations: The meta-analysis included just a few small studies with heterogeneous designs and findings, and there was possible bias from confounding variables.
“Ideally, we would like to see a few more studies to confirm our results,” Mr. Whittaker said in the statement. “However, these studies may never come; normally researchers want to find new results, not replicate old ones. In the meantime, men with low testosterone would be wise to avoid low-fat diets.”
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-fat diets appear to decrease testosterone levels in men, but further randomized, controlled trials are needed to confirm this effect, the authors of a meta-analysis of six small intervention studies concluded.
A total of 206 healthy men with normal testosterone received a high-fat diet followed by a low-fat diet (or vice versa), and their mean total testosterone levels were 10%-15% lower (but still in the normal range) during the low-fat diet.
The study by registered nutritionist Joseph Whittaker, MSc, University of Worcester (England), and statistician Kexin Wu, MSc, University of Warwick, Coventry, England, was published online in the Journal of Steroid Biochemistry and Molecular Biology.
“I think our results are consistent and fairly strong, but they are not strong enough to give blanket recommendations,” Mr. Whittaker said in an interview.
However, “if somebody has low testosterone, particularly borderline, they could try increasing their fat intake, maybe on a Mediterranean diet,” he said, and see if that works to increase their testosterone by 60 ng/dL, the weighted mean difference in total testosterone levels between the low-fat versus high-fat diet interventions in this meta-analysis.
“A Mediterranean diet is a good way to increase ‘healthy fats,’ mono- and polyunsaturated fatty acids, which will likely decrease cardiovascular disease risk, and boost testosterone at the same time,” Mr. Whittaker noted.
Olive oil has been shown to boost testosterone more than butter, and it also reduces CVD, he continued. Nuts are high in “healthy fats” and consistently decrease CVD and mortality and may boost testosterone. Other sources of “good fat” in a healthy diet include avocado, and red meat and poultry in moderation.
“It is controversial, but our results also indicate that foods with saturated fatty acids may boost testosterone,” he added, noting however that such foods are also associated with an increase in cholesterol.
Is waning testosterone explained by leaner diet?
Men need healthy testosterone levels for good physical performance, mental health, and sexual health, and low levels are associated with a higher risk of heart disease, diabetes, and Alzheimer’s disease, according to a statement about this research issued by the University of Worcester.
Although testosterone levels do decline with advancing age, there has also been an additional age-independent and persistent decline in testosterone levels that began roughly after nutrition guidelines began recommending a lower-fat diet in 1965.
Fat consumption dropped from 45% of the diet in 1965 to 35% of the diet in 1991, and stayed around that lower level through to 2011.
However, it is not clear if this decrease in dietary fat intake might explain part of the concurrent decline in men’s testosterone levels.
Mr. Whittaker and Mr. Wu conducted a systematic literature review and identified six crossover intervention studies that compared testosterone levels during low-fat versus high-fat diets – Dorgan 1996, Wang 2005, Hamalainen 1984, Hill 1980, Reed 1987, and Hill 1979 – and then they combined these studies in a meta-analysis.
Five studies each enrolled 6-43 healthy men from North America, the United Kingdom, and Scandinavia, and the sixth study (Hill 1980) enrolled 34 healthy men from North America and 39 farm laborers from South Africa.
Overall, on average, the men were aged 34-54 years and slightly overweight (a mean body mass index of roughly 27 kg/m2) with normal testosterone (i.e., >300 ng/dL, based on the 2018 American Urological Association guidelines criteria).
Most men received a high-fat diet (40% of calories from fat) first, followed by a low-fat diet (on average 20% of calories from fat; range, 7%-25%), but the subgroup of men from South Africa received the low-fat diet first.
To put this into context, U.K. guidelines recommend a fat intake of less than 35% of daily calories, and U.S. guidelines recommend a fat intake of 20%-35% of daily calories.
The low-and high-fat interventions ranged from 2 to 10 weeks.
Lowest testosterone levels with low-fat vegetarian diets
Overall, on average, the men’s total testosterone was 475 mg/dL when they were consuming a low-fat diet and 532 mg/dL when they were consuming a high-fat diet.
However, the South African men had higher testosterone levels when they consumed a low-fat diet. This suggests that “men with European ancestry may experience a greater decrease in testosterone in response to a low-fat diet,” the researchers wrote.
The decrease in total testosterone in the low-fat versus high-fat diet was largest (26%) in the two studies of men who consumed a vegetarian diet (Hill 1979 and Hill 1980). These diets may have been low in zinc, since a marginal zinc deficiency has been shown to decrease total testosterone, Mr. Whittaker and Mr. Wu speculated.
The meta-analysis also showed that levels of free testosterone, urinary testosterone, and dihydrotestosterone declined during the low-fat diet, whereas levels of luteinizing hormone or sex hormone binding globulin were similar with both diets.
Men with low testosterone and overweight, obesity
What nutritional advice should practitioners give to men who have low testosterone and overweight/obesity?
“If you are very overweight, losing weight is going to dramatically improve your testosterone,” Mr. Whittaker said.
However, proponents of various diets are often in stark disagreement about the merits of a low-fat versus low-carbohydrate diet to lose weight.
“In general,” he continued, “the literature shows low-carb (high-fat) diets are better for weight loss [although many will disagree with that statement].”
Although nutrition guidelines have stressed the importance of limiting fat intake, fat in the diet is also associated with lower triglyceride levels and blood pressure and higher HDL cholesterol levels, and now in this study, higher testosterone levels.
More research needed
The researchers acknowledge study limitations: The meta-analysis included just a few small studies with heterogeneous designs and findings, and there was possible bias from confounding variables.
“Ideally, we would like to see a few more studies to confirm our results,” Mr. Whittaker said in the statement. “However, these studies may never come; normally researchers want to find new results, not replicate old ones. In the meantime, men with low testosterone would be wise to avoid low-fat diets.”
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-fat diets appear to decrease testosterone levels in men, but further randomized, controlled trials are needed to confirm this effect, the authors of a meta-analysis of six small intervention studies concluded.
A total of 206 healthy men with normal testosterone received a high-fat diet followed by a low-fat diet (or vice versa), and their mean total testosterone levels were 10%-15% lower (but still in the normal range) during the low-fat diet.
The study by registered nutritionist Joseph Whittaker, MSc, University of Worcester (England), and statistician Kexin Wu, MSc, University of Warwick, Coventry, England, was published online in the Journal of Steroid Biochemistry and Molecular Biology.
“I think our results are consistent and fairly strong, but they are not strong enough to give blanket recommendations,” Mr. Whittaker said in an interview.
However, “if somebody has low testosterone, particularly borderline, they could try increasing their fat intake, maybe on a Mediterranean diet,” he said, and see if that works to increase their testosterone by 60 ng/dL, the weighted mean difference in total testosterone levels between the low-fat versus high-fat diet interventions in this meta-analysis.
“A Mediterranean diet is a good way to increase ‘healthy fats,’ mono- and polyunsaturated fatty acids, which will likely decrease cardiovascular disease risk, and boost testosterone at the same time,” Mr. Whittaker noted.
Olive oil has been shown to boost testosterone more than butter, and it also reduces CVD, he continued. Nuts are high in “healthy fats” and consistently decrease CVD and mortality and may boost testosterone. Other sources of “good fat” in a healthy diet include avocado, and red meat and poultry in moderation.
“It is controversial, but our results also indicate that foods with saturated fatty acids may boost testosterone,” he added, noting however that such foods are also associated with an increase in cholesterol.
Is waning testosterone explained by leaner diet?
Men need healthy testosterone levels for good physical performance, mental health, and sexual health, and low levels are associated with a higher risk of heart disease, diabetes, and Alzheimer’s disease, according to a statement about this research issued by the University of Worcester.
Although testosterone levels do decline with advancing age, there has also been an additional age-independent and persistent decline in testosterone levels that began roughly after nutrition guidelines began recommending a lower-fat diet in 1965.
Fat consumption dropped from 45% of the diet in 1965 to 35% of the diet in 1991, and stayed around that lower level through to 2011.
However, it is not clear if this decrease in dietary fat intake might explain part of the concurrent decline in men’s testosterone levels.
Mr. Whittaker and Mr. Wu conducted a systematic literature review and identified six crossover intervention studies that compared testosterone levels during low-fat versus high-fat diets – Dorgan 1996, Wang 2005, Hamalainen 1984, Hill 1980, Reed 1987, and Hill 1979 – and then they combined these studies in a meta-analysis.
Five studies each enrolled 6-43 healthy men from North America, the United Kingdom, and Scandinavia, and the sixth study (Hill 1980) enrolled 34 healthy men from North America and 39 farm laborers from South Africa.
Overall, on average, the men were aged 34-54 years and slightly overweight (a mean body mass index of roughly 27 kg/m2) with normal testosterone (i.e., >300 ng/dL, based on the 2018 American Urological Association guidelines criteria).
Most men received a high-fat diet (40% of calories from fat) first, followed by a low-fat diet (on average 20% of calories from fat; range, 7%-25%), but the subgroup of men from South Africa received the low-fat diet first.
To put this into context, U.K. guidelines recommend a fat intake of less than 35% of daily calories, and U.S. guidelines recommend a fat intake of 20%-35% of daily calories.
The low-and high-fat interventions ranged from 2 to 10 weeks.
Lowest testosterone levels with low-fat vegetarian diets
Overall, on average, the men’s total testosterone was 475 mg/dL when they were consuming a low-fat diet and 532 mg/dL when they were consuming a high-fat diet.
However, the South African men had higher testosterone levels when they consumed a low-fat diet. This suggests that “men with European ancestry may experience a greater decrease in testosterone in response to a low-fat diet,” the researchers wrote.
The decrease in total testosterone in the low-fat versus high-fat diet was largest (26%) in the two studies of men who consumed a vegetarian diet (Hill 1979 and Hill 1980). These diets may have been low in zinc, since a marginal zinc deficiency has been shown to decrease total testosterone, Mr. Whittaker and Mr. Wu speculated.
The meta-analysis also showed that levels of free testosterone, urinary testosterone, and dihydrotestosterone declined during the low-fat diet, whereas levels of luteinizing hormone or sex hormone binding globulin were similar with both diets.
Men with low testosterone and overweight, obesity
What nutritional advice should practitioners give to men who have low testosterone and overweight/obesity?
“If you are very overweight, losing weight is going to dramatically improve your testosterone,” Mr. Whittaker said.
However, proponents of various diets are often in stark disagreement about the merits of a low-fat versus low-carbohydrate diet to lose weight.
“In general,” he continued, “the literature shows low-carb (high-fat) diets are better for weight loss [although many will disagree with that statement].”
Although nutrition guidelines have stressed the importance of limiting fat intake, fat in the diet is also associated with lower triglyceride levels and blood pressure and higher HDL cholesterol levels, and now in this study, higher testosterone levels.
More research needed
The researchers acknowledge study limitations: The meta-analysis included just a few small studies with heterogeneous designs and findings, and there was possible bias from confounding variables.
“Ideally, we would like to see a few more studies to confirm our results,” Mr. Whittaker said in the statement. “However, these studies may never come; normally researchers want to find new results, not replicate old ones. In the meantime, men with low testosterone would be wise to avoid low-fat diets.”
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How often should we check EKGs in patients starting antipsychotic medications?
Determining relative risk with available data
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 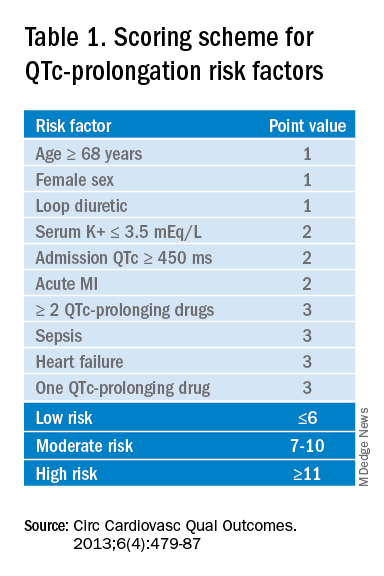
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.
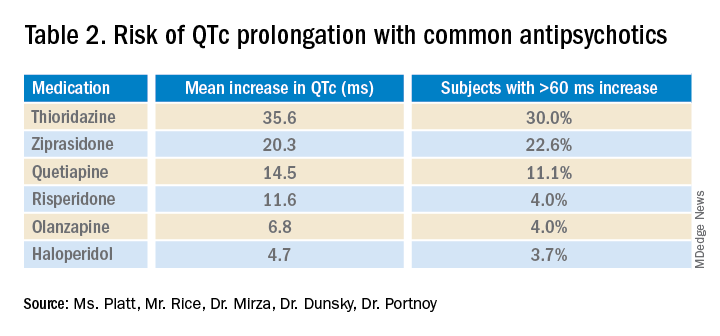
Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.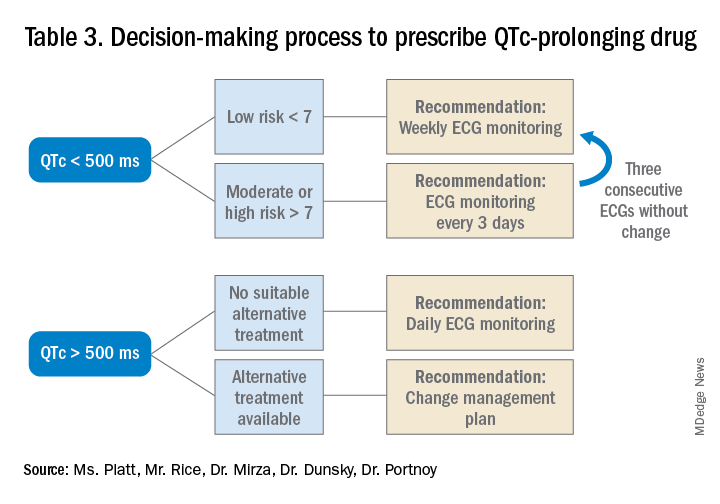
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Determining relative risk with available data
Determining relative risk with available data
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.

Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.

Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Risk of hypogammaglobulinemia, infections with rituximab increased in pediatric patients
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
FROM CARRA 2021
Postpartum health care needs exceed those of nonpostpartum patients
Postpartum women with commercial health insurance use substantially more inpatient and outpatient care than nonpostpartum women, particularly in the first 2 months after giving birth, according to a retrospective cohort study published in the May 2021 issue of Obstetrics & Gynecology.
“These findings are consistent with previous studies that have documented elevated postpartum ED and hospitalization rates, and we contribute new evidence that indicates postpartum health care utilization rates are significantly higher than rates of health care utilization in the general population of reproductive aged women when they are not pregnant or postpartum,” Maria W. Steenland, ScD, of Brown University, Providence, R.I., and colleagues wrote. “Most notably, postpartum women were more than three times more likely to have an ED visit and eight times more likely to be hospitalized than nonpostpartum women in the early postpartum period.”
Approximately one-third of maternal deaths occur between 1 week and 1 year post partum, the authors noted in their background information. The overall U.S. maternal mortality rate was 17.4 per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention.
“This study underscores the importance of access to health care for women, in particular in the postpartum period,” Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, said in an interview. “Maternal mortality is a public health crisis. The majority of maternal deaths are preventable and occur up to 1 year postpartum. Studies such as these are needed as the postpartum period is a critical time in the care of reproductive age women that is often overlooked.”
Using data from a large national commercial claims database, the researchers analyzed data from 149,563 women aged 18-44 years who gave birth in 2016 and from 2,048,831 women who were neither pregnant nor post partum during the same study period. In postpartum women, the researchers specifically looked at hospitalization, preventive visits, problem visits, and ED visits during three periods for postpartum women: early postpartum, defined as within 21 days of giving birth; postpartum, defined as 21-60 days after birth; and extended postpartum, defined as 61 days to 1 year after birth. These data were then compared with equivalent periods in nonpostpartum women.
“For the comparison group, we created a random start date of follow-up by generating a random variable between 0 and 365 and adding this integer to Jan. 1, 2016,” the authors explained. “These start dates correspond to the range of possible follow-up start dates among postpartum women who gave birth in 2016.”
The groups differed in age composition: 62% of the postpartum women were between 25 and 34 years old while the nonpostpartum women were more evenly distributed across the age range. A higher proportion of nonpostpartum women had a chronic disease, but median income and geographic region were similar between the groups. Just over a third of the postpartum women (36%) had a cesarean delivery, higher than the 2016 national average of 32%.
Nearly a quarter (23.7%) of postpartum women had a problem visit in the early postpartum period, compared with one in five (19.7%) nonpostpartum women in the equivalent period. This 4-point difference increased to an 4.8-point difference after adjustment for age group, chronic disease, geographic region, income, and month when follow-up began.
Postpartum women were also three times more likely to have an ED visit (3.2%) in the early postpartum period than nonpostpartum women (1%). Postpartum women’s problem visits and ED visits were most prevalent in the first 2 weeks after childbirth: 12.4% in the first week and 10.4% in the second. Complaints for these visits primarily included urinary tract infections and other genitourinary issues, hypertension, breast or breastfeeding issues, and respiratory issues, such as shortness of breath or chest pain. Hospitalization rates were also higher in the early postpartum period for postpartum women (0.8%) than nonpostpartum women (0.1%).
Problem visits showed a similar pattern during the postpartum period from 21 to 60 days after birth: 39.4% of postpartum women, compared with 30.2% of nonpostpartum women. Rate differences were narrower for ED visits (2% post partum vs. 1.9% non–post partum) and hospitalization (0.3% post partum vs. 0.2% non–post partum), but the differences remained significant, and ED visits were still 0.3 points higher after adjustment.
The biggest differences between the groups in the first 2 months occurred with preventive care. In the early postpartum period, 15% of postpartum women had a preventive visit, compared with 3.3% of nonpostpartum women. Similarly, the rates were 28.2% in postpartum women and 6.5% in nonpostpartum women in the postpartum period.
Differences between the groups evened out in the extended postpartum period, when postpartum women had slightly fewer preventive visits (42.5%) than nonpostpartum women (42.7%). ED rates (11.2% postpartum vs 11.1%) and hospitalization rates (1.4% postpartum vs. 1.6%) were similar, but postpartum women were significantly more likely to have problem visits (79.2%) in the year after childbirth than nonpostpartum women (72.8%).
“Compared with postpartum women overall, postpartum women with chronic disease, pregnancy complications, and cesarean births were more likely to receive health care of all types in the early postpartum period,” the researchers reported. “Of the three subgroups, health care use was highest among postpartum women with chronic disease, among whom 35% had at least one outpatient problem visit, 5% had an ED visit, and 1.3% were hospitalized within the first 20 days of childbirth.”
These findings as a whole point to an increased need for health care not only in the first 3 weeks after women give birth but “beyond the traditional 6-week postpartum period, which adds to the argument that the way we care for women in the postpartum period should be revised,” Dr. Krishna said.
The American College of Obstetricians and Gynecologists updated their postpartum recommendations in 2018 to advise that all postpartum women have a follow-up visit in the first 3 weeks after birth.
Dr. Krishna reiterated that postpartum patients should ideally have an initial follow-up within 3 weeks of giving birth, which the rapid expansion of telehealth has made more viable for both clinicians and mothers.
The authors similarly noted that telehealth and home visits “are promising options to promote early and consistent health care contact and reduce known barriers to postpartum care seeking such as fatigue, lack of transportation, and child care.” Predischarge guidance may also help meet postpartum patients’ health care needs.
“Health care professionals also may be able to reduce the escalation of common early postpartum problems identified in this study (e.g., respiratory problems, pain, urinary tract infections) with anticipatory postpartum counseling and care before hospital discharge such as ensuring that women have inhalers at home, developing a pain management plan, and screening for signs of urinary tract infection,” the authors wrote.
Dr. Krishna also pointed out the need to address racial inequalities in health care and material mortality.
“Black women have a maternal mortality rate two times the rate of non-Hispanic White women,” Dr. Krishna said. “One way to address health disparities between commercially insured and uninsured women is improving access to health care through Medicaid expansion for at least 1 year post partum. States that participated in the Affordable Care Acts Medicaid expansion have noted improvement in maternal mortality and a decrease in racial/ethnic inequities.”
The research was funded by the Robert Wood Johnson Foundation and the National Institutes of Health. Data was provided by Aetna, Humana, Kaiser Permanente and UnitedHealthcare. The authors and Dr. Krishna reported no disclosures.
Postpartum women with commercial health insurance use substantially more inpatient and outpatient care than nonpostpartum women, particularly in the first 2 months after giving birth, according to a retrospective cohort study published in the May 2021 issue of Obstetrics & Gynecology.
“These findings are consistent with previous studies that have documented elevated postpartum ED and hospitalization rates, and we contribute new evidence that indicates postpartum health care utilization rates are significantly higher than rates of health care utilization in the general population of reproductive aged women when they are not pregnant or postpartum,” Maria W. Steenland, ScD, of Brown University, Providence, R.I., and colleagues wrote. “Most notably, postpartum women were more than three times more likely to have an ED visit and eight times more likely to be hospitalized than nonpostpartum women in the early postpartum period.”
Approximately one-third of maternal deaths occur between 1 week and 1 year post partum, the authors noted in their background information. The overall U.S. maternal mortality rate was 17.4 per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention.
“This study underscores the importance of access to health care for women, in particular in the postpartum period,” Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, said in an interview. “Maternal mortality is a public health crisis. The majority of maternal deaths are preventable and occur up to 1 year postpartum. Studies such as these are needed as the postpartum period is a critical time in the care of reproductive age women that is often overlooked.”
Using data from a large national commercial claims database, the researchers analyzed data from 149,563 women aged 18-44 years who gave birth in 2016 and from 2,048,831 women who were neither pregnant nor post partum during the same study period. In postpartum women, the researchers specifically looked at hospitalization, preventive visits, problem visits, and ED visits during three periods for postpartum women: early postpartum, defined as within 21 days of giving birth; postpartum, defined as 21-60 days after birth; and extended postpartum, defined as 61 days to 1 year after birth. These data were then compared with equivalent periods in nonpostpartum women.
“For the comparison group, we created a random start date of follow-up by generating a random variable between 0 and 365 and adding this integer to Jan. 1, 2016,” the authors explained. “These start dates correspond to the range of possible follow-up start dates among postpartum women who gave birth in 2016.”
The groups differed in age composition: 62% of the postpartum women were between 25 and 34 years old while the nonpostpartum women were more evenly distributed across the age range. A higher proportion of nonpostpartum women had a chronic disease, but median income and geographic region were similar between the groups. Just over a third of the postpartum women (36%) had a cesarean delivery, higher than the 2016 national average of 32%.
Nearly a quarter (23.7%) of postpartum women had a problem visit in the early postpartum period, compared with one in five (19.7%) nonpostpartum women in the equivalent period. This 4-point difference increased to an 4.8-point difference after adjustment for age group, chronic disease, geographic region, income, and month when follow-up began.
Postpartum women were also three times more likely to have an ED visit (3.2%) in the early postpartum period than nonpostpartum women (1%). Postpartum women’s problem visits and ED visits were most prevalent in the first 2 weeks after childbirth: 12.4% in the first week and 10.4% in the second. Complaints for these visits primarily included urinary tract infections and other genitourinary issues, hypertension, breast or breastfeeding issues, and respiratory issues, such as shortness of breath or chest pain. Hospitalization rates were also higher in the early postpartum period for postpartum women (0.8%) than nonpostpartum women (0.1%).
Problem visits showed a similar pattern during the postpartum period from 21 to 60 days after birth: 39.4% of postpartum women, compared with 30.2% of nonpostpartum women. Rate differences were narrower for ED visits (2% post partum vs. 1.9% non–post partum) and hospitalization (0.3% post partum vs. 0.2% non–post partum), but the differences remained significant, and ED visits were still 0.3 points higher after adjustment.
The biggest differences between the groups in the first 2 months occurred with preventive care. In the early postpartum period, 15% of postpartum women had a preventive visit, compared with 3.3% of nonpostpartum women. Similarly, the rates were 28.2% in postpartum women and 6.5% in nonpostpartum women in the postpartum period.
Differences between the groups evened out in the extended postpartum period, when postpartum women had slightly fewer preventive visits (42.5%) than nonpostpartum women (42.7%). ED rates (11.2% postpartum vs 11.1%) and hospitalization rates (1.4% postpartum vs. 1.6%) were similar, but postpartum women were significantly more likely to have problem visits (79.2%) in the year after childbirth than nonpostpartum women (72.8%).
“Compared with postpartum women overall, postpartum women with chronic disease, pregnancy complications, and cesarean births were more likely to receive health care of all types in the early postpartum period,” the researchers reported. “Of the three subgroups, health care use was highest among postpartum women with chronic disease, among whom 35% had at least one outpatient problem visit, 5% had an ED visit, and 1.3% were hospitalized within the first 20 days of childbirth.”
These findings as a whole point to an increased need for health care not only in the first 3 weeks after women give birth but “beyond the traditional 6-week postpartum period, which adds to the argument that the way we care for women in the postpartum period should be revised,” Dr. Krishna said.
The American College of Obstetricians and Gynecologists updated their postpartum recommendations in 2018 to advise that all postpartum women have a follow-up visit in the first 3 weeks after birth.
Dr. Krishna reiterated that postpartum patients should ideally have an initial follow-up within 3 weeks of giving birth, which the rapid expansion of telehealth has made more viable for both clinicians and mothers.
The authors similarly noted that telehealth and home visits “are promising options to promote early and consistent health care contact and reduce known barriers to postpartum care seeking such as fatigue, lack of transportation, and child care.” Predischarge guidance may also help meet postpartum patients’ health care needs.
“Health care professionals also may be able to reduce the escalation of common early postpartum problems identified in this study (e.g., respiratory problems, pain, urinary tract infections) with anticipatory postpartum counseling and care before hospital discharge such as ensuring that women have inhalers at home, developing a pain management plan, and screening for signs of urinary tract infection,” the authors wrote.
Dr. Krishna also pointed out the need to address racial inequalities in health care and material mortality.
“Black women have a maternal mortality rate two times the rate of non-Hispanic White women,” Dr. Krishna said. “One way to address health disparities between commercially insured and uninsured women is improving access to health care through Medicaid expansion for at least 1 year post partum. States that participated in the Affordable Care Acts Medicaid expansion have noted improvement in maternal mortality and a decrease in racial/ethnic inequities.”
The research was funded by the Robert Wood Johnson Foundation and the National Institutes of Health. Data was provided by Aetna, Humana, Kaiser Permanente and UnitedHealthcare. The authors and Dr. Krishna reported no disclosures.
Postpartum women with commercial health insurance use substantially more inpatient and outpatient care than nonpostpartum women, particularly in the first 2 months after giving birth, according to a retrospective cohort study published in the May 2021 issue of Obstetrics & Gynecology.
“These findings are consistent with previous studies that have documented elevated postpartum ED and hospitalization rates, and we contribute new evidence that indicates postpartum health care utilization rates are significantly higher than rates of health care utilization in the general population of reproductive aged women when they are not pregnant or postpartum,” Maria W. Steenland, ScD, of Brown University, Providence, R.I., and colleagues wrote. “Most notably, postpartum women were more than three times more likely to have an ED visit and eight times more likely to be hospitalized than nonpostpartum women in the early postpartum period.”
Approximately one-third of maternal deaths occur between 1 week and 1 year post partum, the authors noted in their background information. The overall U.S. maternal mortality rate was 17.4 per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention.
“This study underscores the importance of access to health care for women, in particular in the postpartum period,” Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, said in an interview. “Maternal mortality is a public health crisis. The majority of maternal deaths are preventable and occur up to 1 year postpartum. Studies such as these are needed as the postpartum period is a critical time in the care of reproductive age women that is often overlooked.”
Using data from a large national commercial claims database, the researchers analyzed data from 149,563 women aged 18-44 years who gave birth in 2016 and from 2,048,831 women who were neither pregnant nor post partum during the same study period. In postpartum women, the researchers specifically looked at hospitalization, preventive visits, problem visits, and ED visits during three periods for postpartum women: early postpartum, defined as within 21 days of giving birth; postpartum, defined as 21-60 days after birth; and extended postpartum, defined as 61 days to 1 year after birth. These data were then compared with equivalent periods in nonpostpartum women.
“For the comparison group, we created a random start date of follow-up by generating a random variable between 0 and 365 and adding this integer to Jan. 1, 2016,” the authors explained. “These start dates correspond to the range of possible follow-up start dates among postpartum women who gave birth in 2016.”
The groups differed in age composition: 62% of the postpartum women were between 25 and 34 years old while the nonpostpartum women were more evenly distributed across the age range. A higher proportion of nonpostpartum women had a chronic disease, but median income and geographic region were similar between the groups. Just over a third of the postpartum women (36%) had a cesarean delivery, higher than the 2016 national average of 32%.
Nearly a quarter (23.7%) of postpartum women had a problem visit in the early postpartum period, compared with one in five (19.7%) nonpostpartum women in the equivalent period. This 4-point difference increased to an 4.8-point difference after adjustment for age group, chronic disease, geographic region, income, and month when follow-up began.
Postpartum women were also three times more likely to have an ED visit (3.2%) in the early postpartum period than nonpostpartum women (1%). Postpartum women’s problem visits and ED visits were most prevalent in the first 2 weeks after childbirth: 12.4% in the first week and 10.4% in the second. Complaints for these visits primarily included urinary tract infections and other genitourinary issues, hypertension, breast or breastfeeding issues, and respiratory issues, such as shortness of breath or chest pain. Hospitalization rates were also higher in the early postpartum period for postpartum women (0.8%) than nonpostpartum women (0.1%).
Problem visits showed a similar pattern during the postpartum period from 21 to 60 days after birth: 39.4% of postpartum women, compared with 30.2% of nonpostpartum women. Rate differences were narrower for ED visits (2% post partum vs. 1.9% non–post partum) and hospitalization (0.3% post partum vs. 0.2% non–post partum), but the differences remained significant, and ED visits were still 0.3 points higher after adjustment.
The biggest differences between the groups in the first 2 months occurred with preventive care. In the early postpartum period, 15% of postpartum women had a preventive visit, compared with 3.3% of nonpostpartum women. Similarly, the rates were 28.2% in postpartum women and 6.5% in nonpostpartum women in the postpartum period.
Differences between the groups evened out in the extended postpartum period, when postpartum women had slightly fewer preventive visits (42.5%) than nonpostpartum women (42.7%). ED rates (11.2% postpartum vs 11.1%) and hospitalization rates (1.4% postpartum vs. 1.6%) were similar, but postpartum women were significantly more likely to have problem visits (79.2%) in the year after childbirth than nonpostpartum women (72.8%).
“Compared with postpartum women overall, postpartum women with chronic disease, pregnancy complications, and cesarean births were more likely to receive health care of all types in the early postpartum period,” the researchers reported. “Of the three subgroups, health care use was highest among postpartum women with chronic disease, among whom 35% had at least one outpatient problem visit, 5% had an ED visit, and 1.3% were hospitalized within the first 20 days of childbirth.”
These findings as a whole point to an increased need for health care not only in the first 3 weeks after women give birth but “beyond the traditional 6-week postpartum period, which adds to the argument that the way we care for women in the postpartum period should be revised,” Dr. Krishna said.
The American College of Obstetricians and Gynecologists updated their postpartum recommendations in 2018 to advise that all postpartum women have a follow-up visit in the first 3 weeks after birth.
Dr. Krishna reiterated that postpartum patients should ideally have an initial follow-up within 3 weeks of giving birth, which the rapid expansion of telehealth has made more viable for both clinicians and mothers.
The authors similarly noted that telehealth and home visits “are promising options to promote early and consistent health care contact and reduce known barriers to postpartum care seeking such as fatigue, lack of transportation, and child care.” Predischarge guidance may also help meet postpartum patients’ health care needs.
“Health care professionals also may be able to reduce the escalation of common early postpartum problems identified in this study (e.g., respiratory problems, pain, urinary tract infections) with anticipatory postpartum counseling and care before hospital discharge such as ensuring that women have inhalers at home, developing a pain management plan, and screening for signs of urinary tract infection,” the authors wrote.
Dr. Krishna also pointed out the need to address racial inequalities in health care and material mortality.
“Black women have a maternal mortality rate two times the rate of non-Hispanic White women,” Dr. Krishna said. “One way to address health disparities between commercially insured and uninsured women is improving access to health care through Medicaid expansion for at least 1 year post partum. States that participated in the Affordable Care Acts Medicaid expansion have noted improvement in maternal mortality and a decrease in racial/ethnic inequities.”
The research was funded by the Robert Wood Johnson Foundation and the National Institutes of Health. Data was provided by Aetna, Humana, Kaiser Permanente and UnitedHealthcare. The authors and Dr. Krishna reported no disclosures.
FROM OBSTETRICS & GYNECOLOGY
Quicker fertility rebound in young women with breast cancer
Researchers found that omitting cyclophosphamide from a regimen of epirubicin and paclitaxel increased the likelihood of an early return of menses, and there was a trend toward improved disease-free survival.
The phase 3 SPECTRUM trial involved 521 women with estrogen receptor–positive, HER2-negative breast cancer who had undergone definitive surgery at one of eight institutions in China. The average age of the patients was 34 years.
Cyclophosphamide is a standard component of adjuvant chemotherapy, but it’s strongly associated with premature ovarian failure and infertility.
“For the first time, we demonstrate that a cyclophosphamide-free regimen [can] increase the rate of menses recovery without compromising survival,” said the researchers, led by Ke-Da Yu, MD, PhD, of the Fudan University Shanghai (China) Cancer Center.
They also reported that, among the women who tried to conceive at a later date, there was a higher pregnancy success rate among those who did not take cyclophosphamide.
“Our findings can be extrapolated to patients with other subtypes of breast cancer, such as triple-negative or HER2-enriched, because the effect of paclitaxel and cyclophosphamide on menstrual resumption is not subtype specific,” the investigators commented.
The results were published online in the Journal of the National Cancer Institute.
This is the first prospective trial specifically designed to find an adjuvant breast cancer regimen less toxic to the ovaries. The “investigators ... should be applauded,” wrote Matteo Lambertini, MD, PhD, of the University of Genova (Italy), and Ann Partridge, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
Although promising, there are a few caveats, the editorialists wrote. In a past trial of doxorubicin and docetaxel in lieu of a cyclophosphamide regimen, disease outcomes were inferior. There is also a question as to whether the SPECTRUM results apply to non-Asian women.
The editorialists also noted that enrollment in this trial ended in 2016, before it was recommended that ovarian suppression be used in conjunction with adjuvant chemotherapy to prevent premature menopause.
“[It’s] notable that the absolute benefit in reducing [premature ovarian insufficiency] rates with the use of a cyclophosphamide-free regimen is similar to the effect demonstrated with the administration of a gonadotropin-releasing hormone agonist during cyclophosphamide-based chemotherapy,” they commented. It’s possible that combining the two approaches might have an additive effect, but for now the possibility remains unknown.
In addition, the SPECTRUM trial predates the widespread use of genetic testing to guide treatment, the editorialists pointed out.
“Therefore, caution should be taken in adopting wholesale such regimens,” Dr. Lambertini and Dr. Partridge said.
Switch to paclitaxel
The research team was inspired by previous reports that swapping out cyclophosphamide for paclitaxel did not reduce adjuvant efficacy in the general breast cancer population.
The SPECTRUM trial randomly assigned 260 women to receive a cyclophosphamide-free regimen of epirubicin (75 mg/m2) and paclitaxel (175 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks.
Another 261 women were randomly assigned to receive cyclophosphamide (600 mg/m2) and epirubicin (75 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks. These patients constituted the control group.
A year after completing chemotherapy, 63.1% of the cyclophosphamide-free arm versus 48.3% of the control group, had resumed menses, defined as having two consecutive menstrual cycles or one cycle but with premenopausal levels of estradiol and follicle-stimulating hormone (P < .001).
Another caveat of the study is that assessments of women who resumed menses were conducted at the 1-year point; rates may have been higher in the cyclophosphamide arm had the investigators conducted the assessments at 2 years, the editorialists said.
The 5-year disease-free survival was 84.7% in the cyclophosphamide-free arm versus 78.3% in the control group, an absolute difference of 14.8% (P = .07).
Patients with node-positive disease appeared to benefit the most from cyclophosphamide sparing.
There were no statistically significant differences in overall or distant disease-free survival.
Higher pregnancy rates
Almost 18% of women in the experimental arm reported trying to conceive, and 9.6% of them did so. About 10% of women in the cyclophosphamide arm tried to conceive, and 2.7% did so (P = .03).
The median interval between randomization and pregnancy was 42 months.
For all of the women who became pregnant, endocrine therapy was interrupted. “Women who temporarily interrupt endocrine therapy due to pregnancy should be reminded to resume endocrine therapy following attempted or successful pregnancy,” the investigators wrote.
The patients were taking tamoxifen at least 5 years after receiving chemotherapy, most often as monotherapy. About 5% of the patients underwent up-front ovarian suppression with an aromatase inhibitor, which is a current standard option.
The study was supported by the National Natural Science Foundation of China and other organizations. The investigators and Dr. Partridge disclosed no relevant financial relationships. Dr. Lambertini has consulted for and/or has received speakers fees from Roche, AstraZeneca, Lilly, Novartis, and other companies.
A version of this article first appeared on Medscape.com.
Researchers found that omitting cyclophosphamide from a regimen of epirubicin and paclitaxel increased the likelihood of an early return of menses, and there was a trend toward improved disease-free survival.
The phase 3 SPECTRUM trial involved 521 women with estrogen receptor–positive, HER2-negative breast cancer who had undergone definitive surgery at one of eight institutions in China. The average age of the patients was 34 years.
Cyclophosphamide is a standard component of adjuvant chemotherapy, but it’s strongly associated with premature ovarian failure and infertility.
“For the first time, we demonstrate that a cyclophosphamide-free regimen [can] increase the rate of menses recovery without compromising survival,” said the researchers, led by Ke-Da Yu, MD, PhD, of the Fudan University Shanghai (China) Cancer Center.
They also reported that, among the women who tried to conceive at a later date, there was a higher pregnancy success rate among those who did not take cyclophosphamide.
“Our findings can be extrapolated to patients with other subtypes of breast cancer, such as triple-negative or HER2-enriched, because the effect of paclitaxel and cyclophosphamide on menstrual resumption is not subtype specific,” the investigators commented.
The results were published online in the Journal of the National Cancer Institute.
This is the first prospective trial specifically designed to find an adjuvant breast cancer regimen less toxic to the ovaries. The “investigators ... should be applauded,” wrote Matteo Lambertini, MD, PhD, of the University of Genova (Italy), and Ann Partridge, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
Although promising, there are a few caveats, the editorialists wrote. In a past trial of doxorubicin and docetaxel in lieu of a cyclophosphamide regimen, disease outcomes were inferior. There is also a question as to whether the SPECTRUM results apply to non-Asian women.
The editorialists also noted that enrollment in this trial ended in 2016, before it was recommended that ovarian suppression be used in conjunction with adjuvant chemotherapy to prevent premature menopause.
“[It’s] notable that the absolute benefit in reducing [premature ovarian insufficiency] rates with the use of a cyclophosphamide-free regimen is similar to the effect demonstrated with the administration of a gonadotropin-releasing hormone agonist during cyclophosphamide-based chemotherapy,” they commented. It’s possible that combining the two approaches might have an additive effect, but for now the possibility remains unknown.
In addition, the SPECTRUM trial predates the widespread use of genetic testing to guide treatment, the editorialists pointed out.
“Therefore, caution should be taken in adopting wholesale such regimens,” Dr. Lambertini and Dr. Partridge said.
Switch to paclitaxel
The research team was inspired by previous reports that swapping out cyclophosphamide for paclitaxel did not reduce adjuvant efficacy in the general breast cancer population.
The SPECTRUM trial randomly assigned 260 women to receive a cyclophosphamide-free regimen of epirubicin (75 mg/m2) and paclitaxel (175 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks.
Another 261 women were randomly assigned to receive cyclophosphamide (600 mg/m2) and epirubicin (75 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks. These patients constituted the control group.
A year after completing chemotherapy, 63.1% of the cyclophosphamide-free arm versus 48.3% of the control group, had resumed menses, defined as having two consecutive menstrual cycles or one cycle but with premenopausal levels of estradiol and follicle-stimulating hormone (P < .001).
Another caveat of the study is that assessments of women who resumed menses were conducted at the 1-year point; rates may have been higher in the cyclophosphamide arm had the investigators conducted the assessments at 2 years, the editorialists said.
The 5-year disease-free survival was 84.7% in the cyclophosphamide-free arm versus 78.3% in the control group, an absolute difference of 14.8% (P = .07).
Patients with node-positive disease appeared to benefit the most from cyclophosphamide sparing.
There were no statistically significant differences in overall or distant disease-free survival.
Higher pregnancy rates
Almost 18% of women in the experimental arm reported trying to conceive, and 9.6% of them did so. About 10% of women in the cyclophosphamide arm tried to conceive, and 2.7% did so (P = .03).
The median interval between randomization and pregnancy was 42 months.
For all of the women who became pregnant, endocrine therapy was interrupted. “Women who temporarily interrupt endocrine therapy due to pregnancy should be reminded to resume endocrine therapy following attempted or successful pregnancy,” the investigators wrote.
The patients were taking tamoxifen at least 5 years after receiving chemotherapy, most often as monotherapy. About 5% of the patients underwent up-front ovarian suppression with an aromatase inhibitor, which is a current standard option.
The study was supported by the National Natural Science Foundation of China and other organizations. The investigators and Dr. Partridge disclosed no relevant financial relationships. Dr. Lambertini has consulted for and/or has received speakers fees from Roche, AstraZeneca, Lilly, Novartis, and other companies.
A version of this article first appeared on Medscape.com.
Researchers found that omitting cyclophosphamide from a regimen of epirubicin and paclitaxel increased the likelihood of an early return of menses, and there was a trend toward improved disease-free survival.
The phase 3 SPECTRUM trial involved 521 women with estrogen receptor–positive, HER2-negative breast cancer who had undergone definitive surgery at one of eight institutions in China. The average age of the patients was 34 years.
Cyclophosphamide is a standard component of adjuvant chemotherapy, but it’s strongly associated with premature ovarian failure and infertility.
“For the first time, we demonstrate that a cyclophosphamide-free regimen [can] increase the rate of menses recovery without compromising survival,” said the researchers, led by Ke-Da Yu, MD, PhD, of the Fudan University Shanghai (China) Cancer Center.
They also reported that, among the women who tried to conceive at a later date, there was a higher pregnancy success rate among those who did not take cyclophosphamide.
“Our findings can be extrapolated to patients with other subtypes of breast cancer, such as triple-negative or HER2-enriched, because the effect of paclitaxel and cyclophosphamide on menstrual resumption is not subtype specific,” the investigators commented.
The results were published online in the Journal of the National Cancer Institute.
This is the first prospective trial specifically designed to find an adjuvant breast cancer regimen less toxic to the ovaries. The “investigators ... should be applauded,” wrote Matteo Lambertini, MD, PhD, of the University of Genova (Italy), and Ann Partridge, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
Although promising, there are a few caveats, the editorialists wrote. In a past trial of doxorubicin and docetaxel in lieu of a cyclophosphamide regimen, disease outcomes were inferior. There is also a question as to whether the SPECTRUM results apply to non-Asian women.
The editorialists also noted that enrollment in this trial ended in 2016, before it was recommended that ovarian suppression be used in conjunction with adjuvant chemotherapy to prevent premature menopause.
“[It’s] notable that the absolute benefit in reducing [premature ovarian insufficiency] rates with the use of a cyclophosphamide-free regimen is similar to the effect demonstrated with the administration of a gonadotropin-releasing hormone agonist during cyclophosphamide-based chemotherapy,” they commented. It’s possible that combining the two approaches might have an additive effect, but for now the possibility remains unknown.
In addition, the SPECTRUM trial predates the widespread use of genetic testing to guide treatment, the editorialists pointed out.
“Therefore, caution should be taken in adopting wholesale such regimens,” Dr. Lambertini and Dr. Partridge said.
Switch to paclitaxel
The research team was inspired by previous reports that swapping out cyclophosphamide for paclitaxel did not reduce adjuvant efficacy in the general breast cancer population.
The SPECTRUM trial randomly assigned 260 women to receive a cyclophosphamide-free regimen of epirubicin (75 mg/m2) and paclitaxel (175 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks.
Another 261 women were randomly assigned to receive cyclophosphamide (600 mg/m2) and epirubicin (75 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks. These patients constituted the control group.
A year after completing chemotherapy, 63.1% of the cyclophosphamide-free arm versus 48.3% of the control group, had resumed menses, defined as having two consecutive menstrual cycles or one cycle but with premenopausal levels of estradiol and follicle-stimulating hormone (P < .001).
Another caveat of the study is that assessments of women who resumed menses were conducted at the 1-year point; rates may have been higher in the cyclophosphamide arm had the investigators conducted the assessments at 2 years, the editorialists said.
The 5-year disease-free survival was 84.7% in the cyclophosphamide-free arm versus 78.3% in the control group, an absolute difference of 14.8% (P = .07).
Patients with node-positive disease appeared to benefit the most from cyclophosphamide sparing.
There were no statistically significant differences in overall or distant disease-free survival.
Higher pregnancy rates
Almost 18% of women in the experimental arm reported trying to conceive, and 9.6% of them did so. About 10% of women in the cyclophosphamide arm tried to conceive, and 2.7% did so (P = .03).
The median interval between randomization and pregnancy was 42 months.
For all of the women who became pregnant, endocrine therapy was interrupted. “Women who temporarily interrupt endocrine therapy due to pregnancy should be reminded to resume endocrine therapy following attempted or successful pregnancy,” the investigators wrote.
The patients were taking tamoxifen at least 5 years after receiving chemotherapy, most often as monotherapy. About 5% of the patients underwent up-front ovarian suppression with an aromatase inhibitor, which is a current standard option.
The study was supported by the National Natural Science Foundation of China and other organizations. The investigators and Dr. Partridge disclosed no relevant financial relationships. Dr. Lambertini has consulted for and/or has received speakers fees from Roche, AstraZeneca, Lilly, Novartis, and other companies.
A version of this article first appeared on Medscape.com.
Made-to-order TILs effective against metastatic melanoma
In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents, objective clinical responses occurred with a customized cell therapy based on T cells extracted directly from tumor tissue.
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies that utilize T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” said Jason Alan Chesney, MD, PhD, of the James Graham Brown Cancer Center at the University of Louisville (Ky.)
He presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT008).
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the clinical research division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, where the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2.
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The had received a mean of 3.3 prior lines of therapy. All patients had received prior anti–PD-1/PD-L1 agents; 53 had received a CTLA4 inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
In all, 24 patients (36.4%) had an objective response, 3 patients had a complete response, and 21 had a partial response. There were 29 patients who had stable disease and 9 who progressed. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to more than 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade. All but two patients experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, hypophosphatemia, and lymphopenia.
“The adverse-event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloablative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg said one of the study’s limitations is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stem-like T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents, objective clinical responses occurred with a customized cell therapy based on T cells extracted directly from tumor tissue.
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies that utilize T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” said Jason Alan Chesney, MD, PhD, of the James Graham Brown Cancer Center at the University of Louisville (Ky.)
He presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT008).
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the clinical research division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, where the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2.
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The had received a mean of 3.3 prior lines of therapy. All patients had received prior anti–PD-1/PD-L1 agents; 53 had received a CTLA4 inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
In all, 24 patients (36.4%) had an objective response, 3 patients had a complete response, and 21 had a partial response. There were 29 patients who had stable disease and 9 who progressed. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to more than 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade. All but two patients experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, hypophosphatemia, and lymphopenia.
“The adverse-event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloablative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg said one of the study’s limitations is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stem-like T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents, objective clinical responses occurred with a customized cell therapy based on T cells extracted directly from tumor tissue.
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies that utilize T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” said Jason Alan Chesney, MD, PhD, of the James Graham Brown Cancer Center at the University of Louisville (Ky.)
He presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT008).
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the clinical research division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, where the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2.
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The had received a mean of 3.3 prior lines of therapy. All patients had received prior anti–PD-1/PD-L1 agents; 53 had received a CTLA4 inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
In all, 24 patients (36.4%) had an objective response, 3 patients had a complete response, and 21 had a partial response. There were 29 patients who had stable disease and 9 who progressed. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to more than 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade. All but two patients experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, hypophosphatemia, and lymphopenia.
“The adverse-event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloablative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg said one of the study’s limitations is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stem-like T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
FDA approves frontline immunotherapy for gastric cancers
This is the first immunotherapy approved for the frontline treatment of gastric cancers, the agency says in a press release.
The approval comes after nivolumab received Priority Review and Orphan Drug designations for this indication. There are approximately 28,000 new diagnoses of gastric cancer annually in the United States, and overall survival is generally poor with currently available therapy, points out the FDA.
“Today’s approval is the first treatment in more than a decade to show a survival benefit for patients with advanced or metastatic gastric cancer who are being treated for the first time,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, states in an FDA press release.
Efficacy in the gastric cancer setting was demonstrated in the randomized, phase 3, open-label CheckMate 649 study of 1,518 untreated patients. Median survival was 13.8 months among those treated with nivolumab, compared with 11.6 months with chemotherapy alone (hazard ratio, 0.80; P = .0002).
Common side effects experienced by patients in the nivolumab group included peripheral neuropathy, nausea, fatigue, diarrhea, vomiting, decreased appetite, abdominal pain, constipation, and musculoskeletal pain.
Nivolumab is also approved for numerous other cancers. Other known adverse effects include immune-mediated inflammation of the lungs, colon, liver, endocrine glands, and kidneys.
“Patients should tell their health care providers if they have immune system problems, lung or breathing problems, liver problems, have had an organ transplant, or are pregnant or plan to become pregnant before starting treatment,” the FDA states.
A version of this article first appeared on Medscape.com.
This is the first immunotherapy approved for the frontline treatment of gastric cancers, the agency says in a press release.
The approval comes after nivolumab received Priority Review and Orphan Drug designations for this indication. There are approximately 28,000 new diagnoses of gastric cancer annually in the United States, and overall survival is generally poor with currently available therapy, points out the FDA.
“Today’s approval is the first treatment in more than a decade to show a survival benefit for patients with advanced or metastatic gastric cancer who are being treated for the first time,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, states in an FDA press release.
Efficacy in the gastric cancer setting was demonstrated in the randomized, phase 3, open-label CheckMate 649 study of 1,518 untreated patients. Median survival was 13.8 months among those treated with nivolumab, compared with 11.6 months with chemotherapy alone (hazard ratio, 0.80; P = .0002).
Common side effects experienced by patients in the nivolumab group included peripheral neuropathy, nausea, fatigue, diarrhea, vomiting, decreased appetite, abdominal pain, constipation, and musculoskeletal pain.
Nivolumab is also approved for numerous other cancers. Other known adverse effects include immune-mediated inflammation of the lungs, colon, liver, endocrine glands, and kidneys.
“Patients should tell their health care providers if they have immune system problems, lung or breathing problems, liver problems, have had an organ transplant, or are pregnant or plan to become pregnant before starting treatment,” the FDA states.
A version of this article first appeared on Medscape.com.
This is the first immunotherapy approved for the frontline treatment of gastric cancers, the agency says in a press release.
The approval comes after nivolumab received Priority Review and Orphan Drug designations for this indication. There are approximately 28,000 new diagnoses of gastric cancer annually in the United States, and overall survival is generally poor with currently available therapy, points out the FDA.
“Today’s approval is the first treatment in more than a decade to show a survival benefit for patients with advanced or metastatic gastric cancer who are being treated for the first time,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, states in an FDA press release.
Efficacy in the gastric cancer setting was demonstrated in the randomized, phase 3, open-label CheckMate 649 study of 1,518 untreated patients. Median survival was 13.8 months among those treated with nivolumab, compared with 11.6 months with chemotherapy alone (hazard ratio, 0.80; P = .0002).
Common side effects experienced by patients in the nivolumab group included peripheral neuropathy, nausea, fatigue, diarrhea, vomiting, decreased appetite, abdominal pain, constipation, and musculoskeletal pain.
Nivolumab is also approved for numerous other cancers. Other known adverse effects include immune-mediated inflammation of the lungs, colon, liver, endocrine glands, and kidneys.
“Patients should tell their health care providers if they have immune system problems, lung or breathing problems, liver problems, have had an organ transplant, or are pregnant or plan to become pregnant before starting treatment,” the FDA states.
A version of this article first appeared on Medscape.com.
Study aims to enhance understanding of ‘tremendously understudied’ prurigo nodularis
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY