User login
A Case Series of Catheter-Directed Thrombolysis With Mechanical Thrombectomy for Treating Severe Deep Vein Thrombosis
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
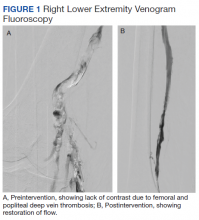
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
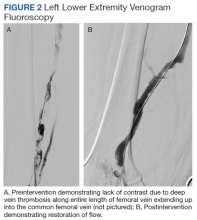
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
Management of Do Not Resuscitate Orders Before Invasive Procedures
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
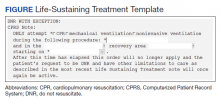
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
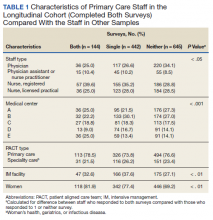
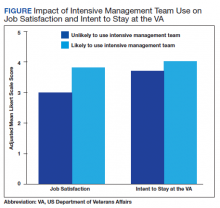
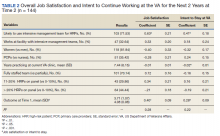
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
Managing cancer outpatients during the pandemic: Tips from MSKCC
“We’ve tried a lot of new things to ensure optimal care for our patients,” said Tiffany A. Traina, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “We need to effectively utilize all resources at our disposal to keep in touch with our patients during this time.”
Dr. Traina described the approach to outpatient management used at MSKCC during a presentation at the AACR Virtual Meeting: COVID-19 and Cancer.
Four guiding principles
MSKCC has established four guiding principles on how to manage cancer patients during the pandemic: openness, safety, technology, and staffing.
Openness ensures that decisions are guided by clinical priorities to provide optimal patient care and allow for prioritization of clinical research and education, Dr. Traina said.
The safety of patients and staff is of the utmost importance, she added. To ensure safety in the context of outpatient care, several operational levers were developed, including COVID surge planning, universal masking and personal protective equipment guidelines, remote work, clinical levers, and new dashboards and communications.
Dr. Traina said data analytics and dashboards have been key technological tools used to support evidence-based decision-making and deliver care remotely for patients during the pandemic.
Staffing resources have also shifted to support demand at different health system locations.
Screening, cohorting, and telemedicine
One measure MSKCC adopted is the MSK Engage Questionnaire, a COVID-19 screening questionnaire assigned to every patient with a scheduled outpatient visit. After completing the questionnaire, patients receive a response denoting whether they need to come into the outpatient setting.
On the staffing side, clinic coordinators prepare appointments accordingly, based on the risk level for each patient.
“We also try to cohort COVID-positive patients into particular areas within the outpatient setting,” Dr. Traina explained. “In addition, we control flow through ambulatory care locations by having separate patient entrances and use other tools to make flow as efficient as possible.”
On the technology side, interactive dashboards are being used to model traffic through different buildings.
“These data and analytics are useful for operational engineering, answering questions such as (1) Are there backups in chemotherapy? and (2) Are patients seeing one particular physician?” Dr. Traina explained. “One important key takeaway is the importance of frequently communicating simple messages through multiple mechanisms, including signage, websites, and dedicated resources.”
Other key technological measures are leveraging telemedicine to convert inpatient appointments to a virtual setting, as well as developing and deploying a system for centralized outpatient follow-up of COVID-19-positive patients.
“We saw a 3,000% increase in telemedicine utilization from February 2020 to June 2020,” Dr. Traina reported. “In a given month, we have approximately 230,000 outpatient visits, and a substantial proportion of these are now done via telemedicine.”
Dr. Traina also noted that multiple organizations have released guidelines addressing when to resume anticancer therapy in patients who have been COVID-19 positive. Adherence is important, as unnecessary COVID-19 testing may delay cancer therapy and is not recommended.
During a live discussion, Louis P. Voigt, MD, of MSKCC, said Dr. Traina’s presentation provided “a lot of good ideas for other institutions who may be facing similar challenges.”
Dr. Traina and Dr. Voigt disclosed no conflicts of interest. No funding sources were reported.
“We’ve tried a lot of new things to ensure optimal care for our patients,” said Tiffany A. Traina, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “We need to effectively utilize all resources at our disposal to keep in touch with our patients during this time.”
Dr. Traina described the approach to outpatient management used at MSKCC during a presentation at the AACR Virtual Meeting: COVID-19 and Cancer.
Four guiding principles
MSKCC has established four guiding principles on how to manage cancer patients during the pandemic: openness, safety, technology, and staffing.
Openness ensures that decisions are guided by clinical priorities to provide optimal patient care and allow for prioritization of clinical research and education, Dr. Traina said.
The safety of patients and staff is of the utmost importance, she added. To ensure safety in the context of outpatient care, several operational levers were developed, including COVID surge planning, universal masking and personal protective equipment guidelines, remote work, clinical levers, and new dashboards and communications.
Dr. Traina said data analytics and dashboards have been key technological tools used to support evidence-based decision-making and deliver care remotely for patients during the pandemic.
Staffing resources have also shifted to support demand at different health system locations.
Screening, cohorting, and telemedicine
One measure MSKCC adopted is the MSK Engage Questionnaire, a COVID-19 screening questionnaire assigned to every patient with a scheduled outpatient visit. After completing the questionnaire, patients receive a response denoting whether they need to come into the outpatient setting.
On the staffing side, clinic coordinators prepare appointments accordingly, based on the risk level for each patient.
“We also try to cohort COVID-positive patients into particular areas within the outpatient setting,” Dr. Traina explained. “In addition, we control flow through ambulatory care locations by having separate patient entrances and use other tools to make flow as efficient as possible.”
On the technology side, interactive dashboards are being used to model traffic through different buildings.
“These data and analytics are useful for operational engineering, answering questions such as (1) Are there backups in chemotherapy? and (2) Are patients seeing one particular physician?” Dr. Traina explained. “One important key takeaway is the importance of frequently communicating simple messages through multiple mechanisms, including signage, websites, and dedicated resources.”
Other key technological measures are leveraging telemedicine to convert inpatient appointments to a virtual setting, as well as developing and deploying a system for centralized outpatient follow-up of COVID-19-positive patients.
“We saw a 3,000% increase in telemedicine utilization from February 2020 to June 2020,” Dr. Traina reported. “In a given month, we have approximately 230,000 outpatient visits, and a substantial proportion of these are now done via telemedicine.”
Dr. Traina also noted that multiple organizations have released guidelines addressing when to resume anticancer therapy in patients who have been COVID-19 positive. Adherence is important, as unnecessary COVID-19 testing may delay cancer therapy and is not recommended.
During a live discussion, Louis P. Voigt, MD, of MSKCC, said Dr. Traina’s presentation provided “a lot of good ideas for other institutions who may be facing similar challenges.”
Dr. Traina and Dr. Voigt disclosed no conflicts of interest. No funding sources were reported.
“We’ve tried a lot of new things to ensure optimal care for our patients,” said Tiffany A. Traina, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York. “We need to effectively utilize all resources at our disposal to keep in touch with our patients during this time.”
Dr. Traina described the approach to outpatient management used at MSKCC during a presentation at the AACR Virtual Meeting: COVID-19 and Cancer.
Four guiding principles
MSKCC has established four guiding principles on how to manage cancer patients during the pandemic: openness, safety, technology, and staffing.
Openness ensures that decisions are guided by clinical priorities to provide optimal patient care and allow for prioritization of clinical research and education, Dr. Traina said.
The safety of patients and staff is of the utmost importance, she added. To ensure safety in the context of outpatient care, several operational levers were developed, including COVID surge planning, universal masking and personal protective equipment guidelines, remote work, clinical levers, and new dashboards and communications.
Dr. Traina said data analytics and dashboards have been key technological tools used to support evidence-based decision-making and deliver care remotely for patients during the pandemic.
Staffing resources have also shifted to support demand at different health system locations.
Screening, cohorting, and telemedicine
One measure MSKCC adopted is the MSK Engage Questionnaire, a COVID-19 screening questionnaire assigned to every patient with a scheduled outpatient visit. After completing the questionnaire, patients receive a response denoting whether they need to come into the outpatient setting.
On the staffing side, clinic coordinators prepare appointments accordingly, based on the risk level for each patient.
“We also try to cohort COVID-positive patients into particular areas within the outpatient setting,” Dr. Traina explained. “In addition, we control flow through ambulatory care locations by having separate patient entrances and use other tools to make flow as efficient as possible.”
On the technology side, interactive dashboards are being used to model traffic through different buildings.
“These data and analytics are useful for operational engineering, answering questions such as (1) Are there backups in chemotherapy? and (2) Are patients seeing one particular physician?” Dr. Traina explained. “One important key takeaway is the importance of frequently communicating simple messages through multiple mechanisms, including signage, websites, and dedicated resources.”
Other key technological measures are leveraging telemedicine to convert inpatient appointments to a virtual setting, as well as developing and deploying a system for centralized outpatient follow-up of COVID-19-positive patients.
“We saw a 3,000% increase in telemedicine utilization from February 2020 to June 2020,” Dr. Traina reported. “In a given month, we have approximately 230,000 outpatient visits, and a substantial proportion of these are now done via telemedicine.”
Dr. Traina also noted that multiple organizations have released guidelines addressing when to resume anticancer therapy in patients who have been COVID-19 positive. Adherence is important, as unnecessary COVID-19 testing may delay cancer therapy and is not recommended.
During a live discussion, Louis P. Voigt, MD, of MSKCC, said Dr. Traina’s presentation provided “a lot of good ideas for other institutions who may be facing similar challenges.”
Dr. Traina and Dr. Voigt disclosed no conflicts of interest. No funding sources were reported.
FROM AACR: COVID-19 AND CANCER 2021
Can Using an Intensive Management Program Improve Primary Care Staff Experiences With Caring for High-Risk Patients?
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
Patients with complex medical and psychosocial needs are at the highest risk for fragmented care and adverse health outcomes.1,2 Although these high-risk patients make up only about 5% of the US patient population, they can account for as much as half of total health care costs.1 High-risk patients are complicated to treat because most have multiple chronic medical conditions, and many have a wide variety of psychological and social needs. Thus, physician, physician assistant, and nurse practitioner primary care providers (PCPs), and nurses (registered nurses, licensed vocational nurses, and licensed practical nurses) must address the complexity of the human condition in conjunction with health problems.2
Background
Caring for high-risk patients within a tight clinic schedule geared to the provision of comprehensive care to large panels of less complex patients can be a source of stress for PCPs and nurses.3-5 These conditions may lead to reduced well-being among primary care team members and to potential turnover.6 Furthermore, primary care staff may feel uncomfortable or lack the ability to address nonmedical concerns because of “person-specific factors that interfere with the delivery of usual care and decision making for whatever condition the patient has.”7,8 Having additional support for complex patients, such as intensive outpatient management teams, may be protective both by reducing health care provider (HCP) stress and improving patient outcomes.3,4
Caring for high-risk patients is challenging.9-11 High-risk patient care may require additional, often unpaid, work hoursand may be discouraging because these patients can be difficult to engage in care.7,12 Furthermore, high-risk patient care is challenging for primary care teams, since these complex patients may fall through the cracks and experience potentially preventable hospitalization or even death. Avoiding these negative consequences typically requires substantial time for the primary care team to engage and counsel the patient, family, and caregiver, through more frequent visits and additional communication. Furthermore, the primary care team typically must coordinate with other HCPs and resources—as many as 16 in a single year and as much as 12 for a single patient over an 80-day period.13,14 Not surprisingly, primary care teams identify help with care coordination as a critical need that may be addressed with intensive management support.
Primary care at the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care for a large proportion of high-risk patients.15 Accordingly, VHA provides a variety of intensive management options for equipping primary care teams with expanded resources for caring for high-risk patients, including those offered in a few sites by a pilot intensive management program.16 As part of the pilot’s evaluation, we studied the work experiences of PCPs and nurses, some of whom had experienced the pilot program and some of whom only had access to typical VHA intensive management resources, such as telehealth and specialty medical homes (referred to in the VA as patient aligned care teams, or PACT), eg, for women patients, for patients who are homeless, or for older adults.17 Surveys assessed whether HCPs who indicated they were likely to seek help from PACT intensive management (PIM) teams to care for high-risk patients had higher job satisfaction and intention to stay at VHA compared with those who were not likely to seek help.
While substantial research on high-risk patients’ intensive management needs has focused on patient-level outcomes of interventions for meeting those needs,little research has examined links between primary care team access to intensive management resources and experiences, such as job satisfaction and job retention.18 In the work presented here, our objectives were to (1) assess the likelihood that a PCP or nurse intent to manage high-risk patients by seeking care coordination help from or transferring care to an intensive management team; and (2) evaluate the relationship between PCP or nurse intentions regarding using intensive management help for high-risk patients and their job satisfaction and likelihood of leaving VA primary care. We hypothesized that the accessibility of intensive management resources and PCP and nurse receptivity to accessing those resources may affect job-related experiences.
Methods
This study was conducted as part of the evaluation of a VA pilot project to provide general primary care teams with intensive management support from interdisciplinary teams for high-risk patients in 5 VHA systems in 5 states (Ohio, Georgia, North Carolina, Wisconsin, and California).6 We sampled primary care staff at 39 primary care clinics within those systems, all of whom had access to VA intensive management resources. These included telehealth, health coaches, integrated mental health providers, and specialty PACTs for specific populations (eg, those who are women, elderly, homeless, HIV-positive, or who have serious mental illness). Of the 39 primary care clinics that participated in the survey, 8 also participated in the pilot program offering an intensive management team to support general primary care in their care of high-risk patients.
Data are from PCPs and nurses who completed 2 cross-sectional surveys (online or hard copy). We invited 1,000 PCPs and nurses to complete the first survey (fielded December 2014 to May 2015) and 863 to complete the second survey (fielded October 2016 to January 2017). A total of 436 completed the first survey for a response rate of 44%, and 313 completed the second survey for a response rate of 36%. We constructed a longitudinal cohort of 144 PCPs and nurses who completed both surveys and had data at 2 timepoints. This longitudinal cohort represents 33% of the 442 unique respondents who completed either the first or second survey. Overlap across surveys was low because of high staff turnover between survey waves.
Measures
Outcomes. We examined 2 single-item outcome measures to assess job satisfaction and retention (ie, intent to stay in primary care at the VA) measured in both surveys. These items were worded “Overall, I am satisfied with my job.” and “I intend to continue working in primary care at the VA for the next two years.” Both items were rated on a 5-point Likert scale.
Independent Variable. We assessed proclivity to seek assistance in caring for high-risk patients based on PCPs or nurses indicating that they are likely to either “manage these patients with ongoing care coordination assistance from an intensive management team” and/or “transfer these patients from primary care to another intensive management team or program specializing in high-risk patients.” These 2 items were rated on a 5-point Likert scale; we dichotomized the scale with likely or very likely indicating high proclivity (likelihood) for ease of interpretation of the combined items.
Covariates. We also controlled for indicators of staff demographic and practice characteristics in multivariate analyses. These included gender, staff type (PCP vs nurse), years practicing at a VA clinic, team staffing level (full vs partial), proportion of the panel consisting of high-risk patients (using binary indicators: 11 to 20% or > 20% compared with 0 to 10% as the reference group), and whether or not the site participated in the pilot program offering an intensive management team to support primary care for high-risk patients to distinguish the 8 pilot sites from nonpilot sites.
Statistical Analysis
We used ordinary least squares regression analysis to examine associations between the independent variable measured at time 1 and outcomes measured at time 2, controlling for time 1 outcomes among staff who completed both surveys (eg, the longitudinal cohort). We adjusted for time 1 covariates and clustering of staff within clinics, assuming a random effect with robust standard errors, and conducted multiple imputations for item-level missing data. Poststratification weights were used to adjust for survey nonresponse by staff type, gender, facilities participating in the innovations, and type of specialty PACT. We calculated weights based on the sampling frame of all PCPs and nurses for each survey.
Results
Table 1 shows the proportion of primary care staff responding to the surveys. For the longitudinal cohort, the response by staff type was similar to the sample of staff that responded only to a single survey, but the sample that did not respond to either survey included more physicians. There was also some variation by medical center. For example, a smaller proportion of the cohort was from site D and more was from site E compared with the other samples. The proportion of primary care staff in facilities that participated in the intensive management pilot was higher than the proportion in other facilities. More women (81.9%) were in the longitudinal cohort compared with 77.4% in the single-survey sample and 69.2% in the sample that responded to neither survey.
Both surveys were completed by 144 respondents while 442 completed 1 survey and 645 did not respond to either survey. The cohort was predominantly nurses (64.6%); Of the PCPs, 25% were physicians. Most staff were women (81.9%) and aged > 45 years (72.2%). Staff had practiced at their current VA clinics for a mean of 7.4 years, and most reported being on a fully-staffed primary care team (70%).
Multivariate Analyses
In the multivariable regression analyses, we found that the primary care staff, which reported being more likely to use intensive management teams to help care for high-risk patients at time 1, reported significantly higher satisfaction (0.63 points higher on a 5-point scale) and intention to stay (0.41 points higher) at VA primary care (both P < .05) at time 2, 18 months later (Table 2). These effect sizes are equivalent to nearly two-thirds and half of a standard deviation, respectively. Among our control variables, years practicing in the VA was significantly associated with a lower likelihood of intent to stay at the VA. Models account for 28% of the variation in satisfaction and 22% of the variation in retention. The Figure shows the adjusted means based on parameters from the regression models for job satisfaction and intent to stay at the VA as well as likelihood of using an intensive management team for high-risk patients. Job satisfaction is nearly 1 point higher among those who report being likely to draw on support from an intensive management team to care for high-risk patients compared with those who reported that they were unlikely to use such a team. The pattern for intent to stay at the VA, while less pronounced, is similar to that for satisfaction.
Discussion
Our findings are consistent with our hypothesis that augmenting primary care with high-risk patient intensive management assistance would improve primary care staff job satisfaction and retention. Findings also mirror recent qualitative studies, which have found that systemic approaches to augment primary care of high-risk patients are likely needed to maintain well-being.7,19 We found a positive relationship between the likelihood of using intensive management teams to help care for their high-risk patients and reported job satisfaction and intent to continue to work within VA primary care 18 months later. To our knowledge, this study is the first to examine the potential impact on PCPs and nurses of using intensive management teams to help care for high-risk patients.
Our study suggests that this approach has the potential to alleviate PCP and nurse stress by incorporating intensive management teams as an extension of the medical home. Even high-functioning medical homes may find it challenging to meet the needs of their high-risk patients.3,7,8 Time constraints and a structured clinic schedule may limit the ability of medical homes to balance the needs of the general panel vs the individual needs of high-risk patients who might benefit from intensive services. Limited knowledge and lack of training to address the broad array of problems faced by high-risk patients also makes care challenging.2
Intensive management services often include interdisciplinary and comprehensive assessments, care coordination, health care system navigation, and linkages to social and home care services.20 Medical homes may benefit from these services, especially resources to support care coordination and communication with specialists and social services in large medical neighborhoods.21 For example, including a social worker on the intensive patient care team can help primary care staff by focusing specialized resources on nonmedical issues, such as chronic homelessness, substance use disorders, food insecurity, access to transportation, and poverty.18
Limitations
This study is subject to some limitations, including those typical of surveys, such as reliance on self-reported data. The longitudinal sample we studied had response rates that varied by site, participation in the pilot program, and gender relative to those who did not respond to both surveys; selection bias is possible. While we use a longitudinal cohort, we cannot attribute causality; it is possible that more satisfied staff are more likely to use intensive management teams rather than the use of intensive management teams contributing to higher satisfaction. Although each study site includes at least 1 type of intensive management resource, we cannot ascertain which intensive management resource primary care staff accessed, if any. While our sample size for the longitudinal cohort responders was limited, focusing on our longitudinal cohort provides more valid and reliable estimates than does using 2 cross-sectional samples with all responders. In addition, our models do not completely explain variation in the outcomes (R2= 0.28 and 0.22), although we included major explanatory factors, such as team staffing and professional type; other unmeasured factors may explain our outcomes. Finally, our provider sample may not generalize to HCPs in non-VA settings.
Conclusions
Our study expands on the limited data regarding the primary care staff experience of caring for high-risk patients and the potential impact of using interdisciplinary assistance to help care for this population. A strength of this study is the longitudinal cohort design that allowed us to understand staff receptivity to having access to intensive management resources to help care for high-risk patients over time among the same group of primary care staff. Given that an economic analysis has determined that the addition of the pilot intensive management program has been cost neutral to the VA, the possibility of its benefit, as suggested by our study findings, would support further implementation and evaluation of intensive management teams as a resource for PCPs caring for high-risk patients.22
Understanding the mechanisms by which primary care staff benefit most from high-risk patient assistance, and how to optimize communication and coordination between primary care staff and intensive management teams for high-risk patients might further increase primary care satisfaction and retention.
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
1. Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016;26:1-14.
2. Bowman MA. The complexity of family medicine care. J Am Board Fam Med. 2011;24(1):4-5. doi:10.3122/jabfm.2011.01.100268
3. Grant RW, Adams AS, Bayliss EA, Heisler M. Establishing visit priorities for complex patients: a summary of the literature and conceptual model to guide innovative interventions. Healthc (Amst). 2013;1(3-4):117-122. doi:10.1016/j.hjdsi.2013.07.008
4. Okunogbe A, Meredith LS, Chang ET, Simon A, Stockdale SE, Rubenstein LV. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018;33(1):65-71. doi:10.1007/s11606-017-4186-8
5. Weiner JZ, McCloskey JK, Uratsu CS, Grant RW. Primary care physician stress driven by social and financial needs of complex patients. J Gen Intern Med. 2019;34(6):818-819. doi:10.1007/s11606-018-4815-x
6. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114(6):513-519. doi:10.1016/s0002-9343(03)00117-7
7. Loeb DF, Bayliss EA, Candrian C, deGruy FV, Binswanger IA. Primary care providers’ experiences caring for complex patients in primary care: a qualitative study. BMC Fam Pract. 2016;17:34. Published 2016 Mar 22. doi:10.1186/s12875-016-0433-z
8. Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health. 2009;27(4):287-302. doi:10.1037/a0018048
9. Powers BW, Chaguturu SK, Ferris TG. Optimizing high-risk care management. JAMA. 2015;313(8):795-796. doi:10.1001/jama.2014.18171
10. Skinner HG, Coffey R, Jones J, Heslin KC, Moy E. The effects of multiple chronic conditions on hospitalization costs and utilization for ambulatory care sensitive conditions in the United States: a nationally representative cross-sectional study. BMC Health Serv Res. 2016;16:77. Published 2016 Mar 1. doi:10.1186/s12913-016-1304-y
11. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. Published 2015 Apr 16. doi:10.1136/bmjopen-2015-007771
12. Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthc (Amst). 2016;4(1):22-29. doi:10.1016/j.hjdsi.2015.12.005
13. Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. doi:10.1056/NEJMhpr0706165
14. Press MJ. Instant replay--a quarterback’s view of care coordination. N Engl J Med. 2014;371(6):489-491. doi:10.1056/NEJMp1406033
15. Chang ET, Piegari RI, Zulman DM, et al. High-risk patients in VHA: where do they get their primary care? Abstract presented at the 2017 Society of General Internal Medicine Annual Meeting. J Gen Intern Med. 2017;32(suppl 2):83-808. doi:10.1007/s11606-017-4028-8
16. Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): Study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018;69:65-75. doi:10.1016/j.cct.2018.04.008
17. Olmos-Ochoa TT, Bharath P, Ganz DA, et al. Staff perspectives on primary care teams as de facto “hubs” for care coordination in VA: a qualitative study. J Gen Intern Med. 2019;34(suppl 1):82-89. doi:10.1007/s11606-019-04967-y
18. Iovan S, Lantz PM, Allan K, Abir M. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99-111. doi:10.1177/1077558719845722
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). J Gen Intern Med. 2014;29 Suppl 4(Suppl 4):861-869. doi:10.1007/s11606-014-3022-7
20. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018;6(4):231-237. doi:10.1016/j.hjdsi.2017.10.001
21. Rich E, Lipson D, Libersky J, Parchman M; Mathematica Policy Research. Coordinating care for adults with complex care needs in the patient-centered medical home: challenges and solutions. Published January 2012. Accessed January 12, 2021. https://pcmh.ahrq.gov/page/coordinating-care-adults-complex-care-needs-patient-centered-medical-home-challenges-and-0
22. Yoon J, Chang E, Rubenstein LV, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization: a randomized quality improvement trial [published correction appears in Ann Intern Med. 2018 Oct 2;169(7):516]. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
FDA alert confirms heart and cancer risks with tofacitinib (Xeljanz)
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
Sotorasib in NSCLC: ‘Historic milestone’ reached
“This is a historic milestone in lung cancer therapy. After 4 decades of scientific efforts in targeting KRAS, sotorasib has potential to be the first targeted treatment option for this patient population with a high unmet need,” said Bob T. Li, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Li reported results with sotorasib in NSCLC, from the phase 2 part of the CodeBreaK 100 trial, at the 2020 World Conference on Lung Cancer (Abstract PS01.07), which was rescheduled for January 2021.
“It’s an absolutely remarkable study,” said Dean A. Fennell, MBBS, PhD, of the University of Leicester and University Hospitals of Leicester NHS Trust in the United Kingdom.
“The ‘un-druggability’ of KRAS has been something of a challenge for decades. To see results like this from Dr. Li is absolutely fabulous and will lead to a new stratification option.”
Rationale and study details
Dr. Li noted that the KRAS p.G12C mutation is a key oncogenic driver, occurring in about 13% of lung adenocarcinomas.
Sotorasib is a first-in-class, highly selective, irreversible KRASG12C inhibitor. It showed durable clinical benefit in 59 NSCLC patients enrolled in the phase 1 part of the CodeBreaK 100 trial (N Engl J Med 2020;383:1207-17). One-third of the patients had an objective response across all doses tested. The median duration of response was 10.9 months, and the median progression-free survival was 6.3 months.
The phase 2 part of CodeBreaK 100 included 126 patients from 11 countries in North America, Europe, and Asia-Pacific. Their median age was 63.5 years (range, 37-80 years), and 92.9% were current or former smokers.
Patients had locally advanced or metastatic NSCLC and a centrally confirmed KRAS p.G12C mutation. They had progressed after three or fewer prior lines of therapy.
Patients received oral sotorasib at 960 mg daily until disease progression. They were followed for a median of 12.2 months. An independent blinded central review found that 124 patients had at least one measurable lesion at baseline and were therefore evaluable for efficacy.
Phase 2 results
Sotorasib “demonstrated early, deep, and durable responses,” Dr. Li said.
In all, 46 patients had a confirmed response – 3 complete responses and 43 partial responses – for an objective response rate of 37.1%.
The median time to objective response was 1.4 months, the median duration of response was 10 months, and 43% of responders were still on treatment without progression at the data cutoff.
“Tumor response to sotorasib was observed across a range of biomarker subgroups, including patients with negative or low PD-L1 expression level and those with mutant STK11,” Dr. Li said.
The disease control rate was 80.6%, and tumors shrank by an average of about 60%. The median progression-free survival was 6.8 months.
Treatment-related adverse events (TRAEs) were acceptable, with no surprises compared to phase 1 results, Dr. Li said.
TRAEs of any grade occurred in 69.8% of patients and led to discontinuation in 7.1%. TRAEs led to dose modification in 22.2% of patients.
Grade 3 TRAEs were reported in 19.8% of patients, including alanine aminotransferase increase (6.3%), aspartate aminotransferase increase (5.6%), diarrhea (4.0%), and blood alkaline phosphatase increase (0.8%).
“Sotorasib was well tolerated, with no deaths attributed to treatment and low incidence of grade 3 or 4 TRAEs, treatment discontinuation, and dose modification,” Dr. Li said.
A phase 3 trial of sotorasib compared with second-line docetaxel is now enrolling patients.
The phase 1/2 CodeBreaK 100 trial was funded by Amgen. Dr. Li disclosed relationships with Amgen and many other companies. Dr. Fennell disclosed relationships with AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Eli Lilly, Merck, Roche, Astex Therapeutics, Bayer, Lab21, Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
“This is a historic milestone in lung cancer therapy. After 4 decades of scientific efforts in targeting KRAS, sotorasib has potential to be the first targeted treatment option for this patient population with a high unmet need,” said Bob T. Li, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Li reported results with sotorasib in NSCLC, from the phase 2 part of the CodeBreaK 100 trial, at the 2020 World Conference on Lung Cancer (Abstract PS01.07), which was rescheduled for January 2021.
“It’s an absolutely remarkable study,” said Dean A. Fennell, MBBS, PhD, of the University of Leicester and University Hospitals of Leicester NHS Trust in the United Kingdom.
“The ‘un-druggability’ of KRAS has been something of a challenge for decades. To see results like this from Dr. Li is absolutely fabulous and will lead to a new stratification option.”
Rationale and study details
Dr. Li noted that the KRAS p.G12C mutation is a key oncogenic driver, occurring in about 13% of lung adenocarcinomas.
Sotorasib is a first-in-class, highly selective, irreversible KRASG12C inhibitor. It showed durable clinical benefit in 59 NSCLC patients enrolled in the phase 1 part of the CodeBreaK 100 trial (N Engl J Med 2020;383:1207-17). One-third of the patients had an objective response across all doses tested. The median duration of response was 10.9 months, and the median progression-free survival was 6.3 months.
The phase 2 part of CodeBreaK 100 included 126 patients from 11 countries in North America, Europe, and Asia-Pacific. Their median age was 63.5 years (range, 37-80 years), and 92.9% were current or former smokers.
Patients had locally advanced or metastatic NSCLC and a centrally confirmed KRAS p.G12C mutation. They had progressed after three or fewer prior lines of therapy.
Patients received oral sotorasib at 960 mg daily until disease progression. They were followed for a median of 12.2 months. An independent blinded central review found that 124 patients had at least one measurable lesion at baseline and were therefore evaluable for efficacy.
Phase 2 results
Sotorasib “demonstrated early, deep, and durable responses,” Dr. Li said.
In all, 46 patients had a confirmed response – 3 complete responses and 43 partial responses – for an objective response rate of 37.1%.
The median time to objective response was 1.4 months, the median duration of response was 10 months, and 43% of responders were still on treatment without progression at the data cutoff.
“Tumor response to sotorasib was observed across a range of biomarker subgroups, including patients with negative or low PD-L1 expression level and those with mutant STK11,” Dr. Li said.
The disease control rate was 80.6%, and tumors shrank by an average of about 60%. The median progression-free survival was 6.8 months.
Treatment-related adverse events (TRAEs) were acceptable, with no surprises compared to phase 1 results, Dr. Li said.
TRAEs of any grade occurred in 69.8% of patients and led to discontinuation in 7.1%. TRAEs led to dose modification in 22.2% of patients.
Grade 3 TRAEs were reported in 19.8% of patients, including alanine aminotransferase increase (6.3%), aspartate aminotransferase increase (5.6%), diarrhea (4.0%), and blood alkaline phosphatase increase (0.8%).
“Sotorasib was well tolerated, with no deaths attributed to treatment and low incidence of grade 3 or 4 TRAEs, treatment discontinuation, and dose modification,” Dr. Li said.
A phase 3 trial of sotorasib compared with second-line docetaxel is now enrolling patients.
The phase 1/2 CodeBreaK 100 trial was funded by Amgen. Dr. Li disclosed relationships with Amgen and many other companies. Dr. Fennell disclosed relationships with AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Eli Lilly, Merck, Roche, Astex Therapeutics, Bayer, Lab21, Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
“This is a historic milestone in lung cancer therapy. After 4 decades of scientific efforts in targeting KRAS, sotorasib has potential to be the first targeted treatment option for this patient population with a high unmet need,” said Bob T. Li, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Li reported results with sotorasib in NSCLC, from the phase 2 part of the CodeBreaK 100 trial, at the 2020 World Conference on Lung Cancer (Abstract PS01.07), which was rescheduled for January 2021.
“It’s an absolutely remarkable study,” said Dean A. Fennell, MBBS, PhD, of the University of Leicester and University Hospitals of Leicester NHS Trust in the United Kingdom.
“The ‘un-druggability’ of KRAS has been something of a challenge for decades. To see results like this from Dr. Li is absolutely fabulous and will lead to a new stratification option.”
Rationale and study details
Dr. Li noted that the KRAS p.G12C mutation is a key oncogenic driver, occurring in about 13% of lung adenocarcinomas.
Sotorasib is a first-in-class, highly selective, irreversible KRASG12C inhibitor. It showed durable clinical benefit in 59 NSCLC patients enrolled in the phase 1 part of the CodeBreaK 100 trial (N Engl J Med 2020;383:1207-17). One-third of the patients had an objective response across all doses tested. The median duration of response was 10.9 months, and the median progression-free survival was 6.3 months.
The phase 2 part of CodeBreaK 100 included 126 patients from 11 countries in North America, Europe, and Asia-Pacific. Their median age was 63.5 years (range, 37-80 years), and 92.9% were current or former smokers.
Patients had locally advanced or metastatic NSCLC and a centrally confirmed KRAS p.G12C mutation. They had progressed after three or fewer prior lines of therapy.
Patients received oral sotorasib at 960 mg daily until disease progression. They were followed for a median of 12.2 months. An independent blinded central review found that 124 patients had at least one measurable lesion at baseline and were therefore evaluable for efficacy.
Phase 2 results
Sotorasib “demonstrated early, deep, and durable responses,” Dr. Li said.
In all, 46 patients had a confirmed response – 3 complete responses and 43 partial responses – for an objective response rate of 37.1%.
The median time to objective response was 1.4 months, the median duration of response was 10 months, and 43% of responders were still on treatment without progression at the data cutoff.
“Tumor response to sotorasib was observed across a range of biomarker subgroups, including patients with negative or low PD-L1 expression level and those with mutant STK11,” Dr. Li said.
The disease control rate was 80.6%, and tumors shrank by an average of about 60%. The median progression-free survival was 6.8 months.
Treatment-related adverse events (TRAEs) were acceptable, with no surprises compared to phase 1 results, Dr. Li said.
TRAEs of any grade occurred in 69.8% of patients and led to discontinuation in 7.1%. TRAEs led to dose modification in 22.2% of patients.
Grade 3 TRAEs were reported in 19.8% of patients, including alanine aminotransferase increase (6.3%), aspartate aminotransferase increase (5.6%), diarrhea (4.0%), and blood alkaline phosphatase increase (0.8%).
“Sotorasib was well tolerated, with no deaths attributed to treatment and low incidence of grade 3 or 4 TRAEs, treatment discontinuation, and dose modification,” Dr. Li said.
A phase 3 trial of sotorasib compared with second-line docetaxel is now enrolling patients.
The phase 1/2 CodeBreaK 100 trial was funded by Amgen. Dr. Li disclosed relationships with Amgen and many other companies. Dr. Fennell disclosed relationships with AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Eli Lilly, Merck, Roche, Astex Therapeutics, Bayer, Lab21, Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
FROM WCLC 2020
A third discontinuing levothyroxine have normal thyroid levels
Approximately a third of patients treated for hypothyroidism continue to maintain normal thyroid levels after discontinuing thyroid hormone replacement therapy.
Those who were treated for overt hypothyroidism were less likely to maintain normal hormone levels than those with subclinical disease, the new meta-analysis shows.
“This analysis is the first to summarize the limited evidence regarding successful thyroid hormone discontinuation, but unfortunately more research is needed to develop an evidenced-based strategy for deprescribing thyroid hormone replacement,” Nydia Burgos, MD, and colleagues write in their article published online in Thyroid.
Nevertheless, the main findings were somewhat surprising, Dr. Burgos of the division of endocrinology, diabetes and metabolism, University of Puerto Rico, told this news organization.
“I expected that a considerable portion of patients would remain euthyroid, but up to a third of patients was an impressive number,” she said.
The finding could be an indicator of people who may not have had much benefit from the treatment in the first place, she noted.
“The truth of the matter is that levothyroxine (LT4) is among the top-prescribed drugs in the United States, and every day in clinics we encounter patients that were started on thyroid hormone replacement therapy for unclear reasons, as a therapeutic trial that was never reassessed, or as treatment for subclinical hypothyroidism without having convincing criteria for treatment,” she observed.
Meta-analysis of 17 studies examining LT4 discontinuation
Known to be highly effective in the treatment of overt hypothyroidism, LT4 is often prescribed long term; however, it is also commonly prescribed for patients with subclinical hypothyroidism, despite research suggesting no benefits in these patients.
With a guideline panel underscoring the lack of evidence and issuing a “strong recommendation” in May 2019 against treatment with thyroid hormones in adults with subclinical hypothyroidism (elevated thyroid-stimulating hormone [TSH] levels and normal free T4 levels), clinicians may increasingly be considering discontinuation strategies.
To examine the evidence to date on the clinical outcomes of discontinuing LT4, Dr. Burgos and colleagues conducted a meta-analysis in which they identified 17 observational studies that met the inclusion criteria. Of a total of 1,103 patients in the studies, 86% were women. Most studies included only adults.
With a median follow-up of 5 years, the pooled estimate of patients maintaining euthyroidism after treatment discontinuation was 37.2%.
The estimated rate of remaining euthyroid was significantly lower among those with overt hypothyroidism (11.8%) compared with those with subclinical hypothyroidism (35.6%).
Meanwhile, as many as 65.8% of patients ended up restarting thyroid hormone treatment during the follow-up period, according to pooled estimates, and the rate was as high as 87.2% in patients with overt hypothyroidism. The mean increase in TSH from time of LT4 discontinuation to follow-up was 9.4 mIU/L.
Among specific factors shown to be linked to a lower likelihood of euthyroidism at follow-up were inconsistent echogenicity on thyroid ultrasound, elevated TSH (8-9 mIU/L), and the presence of thyroid antibodies.
Only a few of the studies evaluated thyroid hormones other than synthetic LT4 (such as the commonly used desiccated thyroid), and so the analysis did not compare differences between therapies, Dr. Burgos noted.
Despite the lack of evidence of benefits of LT4 treatment for subclinical hypothyroidism, the finding that, even among those patients, approximately two-thirds were not euthyroid at follow-up was not unexpected, she added.
“I am not surprised that, even in the subclinical hypothyroidism group about two-thirds of participants were not euthyroid, because when looking at the natural history of subclinical hypothyroidism in other studies, only a fifth had normalized thyroid hormone tests, while the majority continue with mild subclinical hypothyroidism and a fifth progress to overt hypothyroidism,” she explained.
More work needed to determine best way to taper down LT4
The specific regimens for discontinuing LT4 were detailed in only three studies and reflected varying approaches, ranging from tapering down the dose over 2 weeks to reducing the dose over several more weeks, or even months, Dr. Burgos noted
“We need more studies to figure out which tapering regimen will promote a more favorable outcome,” she said.
“The ideal regimen will be one in which patients can comply with follow-up visits and have thyroid function testing done before symptoms of hypothyroidism develop.”
In addition to likely offering no benefit to people with subclinical hypothyroidism, other reasons for discontinuing LT4 in patients who are considered appropriate candidates include concerns about side effects in older patients.
The authors say there is evidence indicating that as many as 50% of patients older than 65 who take thyroid hormones develop iatrogenic hyperthyroidism, which can have detrimental effects including an increased risk for cardiac arrhythmias, angina pectoris, bone loss, and fractures.
Collaborative approach to ‘deprescribing’ suggested
To get patients off LT4, the authors suggest a collaborative approach of “deprescribing,” whereby the health care professional supervises with a goal of managing polypharmacy and improving outcomes.
“This systematic process starts with an accurate evaluation of the medication list, followed by identification of potentially inappropriate medications, collaboration between patients and clinicians to decide whether deprescribing would be appropriate, and establishing a supportive plan to safely deprescribe the medication,” they write.
When decision-making is shared, patients are more likely to consider discontinuation if they understand why the medication is inappropriate, have their concerns related to the discontinuation addressed, understand the process, and feel that they have the support of the clinical team, the authors conclude.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately a third of patients treated for hypothyroidism continue to maintain normal thyroid levels after discontinuing thyroid hormone replacement therapy.
Those who were treated for overt hypothyroidism were less likely to maintain normal hormone levels than those with subclinical disease, the new meta-analysis shows.
“This analysis is the first to summarize the limited evidence regarding successful thyroid hormone discontinuation, but unfortunately more research is needed to develop an evidenced-based strategy for deprescribing thyroid hormone replacement,” Nydia Burgos, MD, and colleagues write in their article published online in Thyroid.
Nevertheless, the main findings were somewhat surprising, Dr. Burgos of the division of endocrinology, diabetes and metabolism, University of Puerto Rico, told this news organization.
“I expected that a considerable portion of patients would remain euthyroid, but up to a third of patients was an impressive number,” she said.
The finding could be an indicator of people who may not have had much benefit from the treatment in the first place, she noted.
“The truth of the matter is that levothyroxine (LT4) is among the top-prescribed drugs in the United States, and every day in clinics we encounter patients that were started on thyroid hormone replacement therapy for unclear reasons, as a therapeutic trial that was never reassessed, or as treatment for subclinical hypothyroidism without having convincing criteria for treatment,” she observed.
Meta-analysis of 17 studies examining LT4 discontinuation
Known to be highly effective in the treatment of overt hypothyroidism, LT4 is often prescribed long term; however, it is also commonly prescribed for patients with subclinical hypothyroidism, despite research suggesting no benefits in these patients.
With a guideline panel underscoring the lack of evidence and issuing a “strong recommendation” in May 2019 against treatment with thyroid hormones in adults with subclinical hypothyroidism (elevated thyroid-stimulating hormone [TSH] levels and normal free T4 levels), clinicians may increasingly be considering discontinuation strategies.
To examine the evidence to date on the clinical outcomes of discontinuing LT4, Dr. Burgos and colleagues conducted a meta-analysis in which they identified 17 observational studies that met the inclusion criteria. Of a total of 1,103 patients in the studies, 86% were women. Most studies included only adults.
With a median follow-up of 5 years, the pooled estimate of patients maintaining euthyroidism after treatment discontinuation was 37.2%.
The estimated rate of remaining euthyroid was significantly lower among those with overt hypothyroidism (11.8%) compared with those with subclinical hypothyroidism (35.6%).
Meanwhile, as many as 65.8% of patients ended up restarting thyroid hormone treatment during the follow-up period, according to pooled estimates, and the rate was as high as 87.2% in patients with overt hypothyroidism. The mean increase in TSH from time of LT4 discontinuation to follow-up was 9.4 mIU/L.
Among specific factors shown to be linked to a lower likelihood of euthyroidism at follow-up were inconsistent echogenicity on thyroid ultrasound, elevated TSH (8-9 mIU/L), and the presence of thyroid antibodies.
Only a few of the studies evaluated thyroid hormones other than synthetic LT4 (such as the commonly used desiccated thyroid), and so the analysis did not compare differences between therapies, Dr. Burgos noted.
Despite the lack of evidence of benefits of LT4 treatment for subclinical hypothyroidism, the finding that, even among those patients, approximately two-thirds were not euthyroid at follow-up was not unexpected, she added.
“I am not surprised that, even in the subclinical hypothyroidism group about two-thirds of participants were not euthyroid, because when looking at the natural history of subclinical hypothyroidism in other studies, only a fifth had normalized thyroid hormone tests, while the majority continue with mild subclinical hypothyroidism and a fifth progress to overt hypothyroidism,” she explained.
More work needed to determine best way to taper down LT4
The specific regimens for discontinuing LT4 were detailed in only three studies and reflected varying approaches, ranging from tapering down the dose over 2 weeks to reducing the dose over several more weeks, or even months, Dr. Burgos noted
“We need more studies to figure out which tapering regimen will promote a more favorable outcome,” she said.
“The ideal regimen will be one in which patients can comply with follow-up visits and have thyroid function testing done before symptoms of hypothyroidism develop.”
In addition to likely offering no benefit to people with subclinical hypothyroidism, other reasons for discontinuing LT4 in patients who are considered appropriate candidates include concerns about side effects in older patients.
The authors say there is evidence indicating that as many as 50% of patients older than 65 who take thyroid hormones develop iatrogenic hyperthyroidism, which can have detrimental effects including an increased risk for cardiac arrhythmias, angina pectoris, bone loss, and fractures.
Collaborative approach to ‘deprescribing’ suggested
To get patients off LT4, the authors suggest a collaborative approach of “deprescribing,” whereby the health care professional supervises with a goal of managing polypharmacy and improving outcomes.
“This systematic process starts with an accurate evaluation of the medication list, followed by identification of potentially inappropriate medications, collaboration between patients and clinicians to decide whether deprescribing would be appropriate, and establishing a supportive plan to safely deprescribe the medication,” they write.
When decision-making is shared, patients are more likely to consider discontinuation if they understand why the medication is inappropriate, have their concerns related to the discontinuation addressed, understand the process, and feel that they have the support of the clinical team, the authors conclude.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately a third of patients treated for hypothyroidism continue to maintain normal thyroid levels after discontinuing thyroid hormone replacement therapy.
Those who were treated for overt hypothyroidism were less likely to maintain normal hormone levels than those with subclinical disease, the new meta-analysis shows.
“This analysis is the first to summarize the limited evidence regarding successful thyroid hormone discontinuation, but unfortunately more research is needed to develop an evidenced-based strategy for deprescribing thyroid hormone replacement,” Nydia Burgos, MD, and colleagues write in their article published online in Thyroid.
Nevertheless, the main findings were somewhat surprising, Dr. Burgos of the division of endocrinology, diabetes and metabolism, University of Puerto Rico, told this news organization.
“I expected that a considerable portion of patients would remain euthyroid, but up to a third of patients was an impressive number,” she said.
The finding could be an indicator of people who may not have had much benefit from the treatment in the first place, she noted.
“The truth of the matter is that levothyroxine (LT4) is among the top-prescribed drugs in the United States, and every day in clinics we encounter patients that were started on thyroid hormone replacement therapy for unclear reasons, as a therapeutic trial that was never reassessed, or as treatment for subclinical hypothyroidism without having convincing criteria for treatment,” she observed.
Meta-analysis of 17 studies examining LT4 discontinuation
Known to be highly effective in the treatment of overt hypothyroidism, LT4 is often prescribed long term; however, it is also commonly prescribed for patients with subclinical hypothyroidism, despite research suggesting no benefits in these patients.
With a guideline panel underscoring the lack of evidence and issuing a “strong recommendation” in May 2019 against treatment with thyroid hormones in adults with subclinical hypothyroidism (elevated thyroid-stimulating hormone [TSH] levels and normal free T4 levels), clinicians may increasingly be considering discontinuation strategies.
To examine the evidence to date on the clinical outcomes of discontinuing LT4, Dr. Burgos and colleagues conducted a meta-analysis in which they identified 17 observational studies that met the inclusion criteria. Of a total of 1,103 patients in the studies, 86% were women. Most studies included only adults.
With a median follow-up of 5 years, the pooled estimate of patients maintaining euthyroidism after treatment discontinuation was 37.2%.
The estimated rate of remaining euthyroid was significantly lower among those with overt hypothyroidism (11.8%) compared with those with subclinical hypothyroidism (35.6%).
Meanwhile, as many as 65.8% of patients ended up restarting thyroid hormone treatment during the follow-up period, according to pooled estimates, and the rate was as high as 87.2% in patients with overt hypothyroidism. The mean increase in TSH from time of LT4 discontinuation to follow-up was 9.4 mIU/L.
Among specific factors shown to be linked to a lower likelihood of euthyroidism at follow-up were inconsistent echogenicity on thyroid ultrasound, elevated TSH (8-9 mIU/L), and the presence of thyroid antibodies.
Only a few of the studies evaluated thyroid hormones other than synthetic LT4 (such as the commonly used desiccated thyroid), and so the analysis did not compare differences between therapies, Dr. Burgos noted.
Despite the lack of evidence of benefits of LT4 treatment for subclinical hypothyroidism, the finding that, even among those patients, approximately two-thirds were not euthyroid at follow-up was not unexpected, she added.
“I am not surprised that, even in the subclinical hypothyroidism group about two-thirds of participants were not euthyroid, because when looking at the natural history of subclinical hypothyroidism in other studies, only a fifth had normalized thyroid hormone tests, while the majority continue with mild subclinical hypothyroidism and a fifth progress to overt hypothyroidism,” she explained.
More work needed to determine best way to taper down LT4
The specific regimens for discontinuing LT4 were detailed in only three studies and reflected varying approaches, ranging from tapering down the dose over 2 weeks to reducing the dose over several more weeks, or even months, Dr. Burgos noted
“We need more studies to figure out which tapering regimen will promote a more favorable outcome,” she said.
“The ideal regimen will be one in which patients can comply with follow-up visits and have thyroid function testing done before symptoms of hypothyroidism develop.”
In addition to likely offering no benefit to people with subclinical hypothyroidism, other reasons for discontinuing LT4 in patients who are considered appropriate candidates include concerns about side effects in older patients.
The authors say there is evidence indicating that as many as 50% of patients older than 65 who take thyroid hormones develop iatrogenic hyperthyroidism, which can have detrimental effects including an increased risk for cardiac arrhythmias, angina pectoris, bone loss, and fractures.
Collaborative approach to ‘deprescribing’ suggested
To get patients off LT4, the authors suggest a collaborative approach of “deprescribing,” whereby the health care professional supervises with a goal of managing polypharmacy and improving outcomes.
“This systematic process starts with an accurate evaluation of the medication list, followed by identification of potentially inappropriate medications, collaboration between patients and clinicians to decide whether deprescribing would be appropriate, and establishing a supportive plan to safely deprescribe the medication,” they write.
When decision-making is shared, patients are more likely to consider discontinuation if they understand why the medication is inappropriate, have their concerns related to the discontinuation addressed, understand the process, and feel that they have the support of the clinical team, the authors conclude.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA okays new CAR T-cell treatment for large B-cell lymphomas
The Food and Drug Administration has approved lisocabtagene maraleucel (Breyanzi), a chimeric antigen receptor (CAR) T-cell product for the treatment of adults with certain types of relapsed or refractory large B-cell lymphoma who relapse or fail to respond to at least two systemic treatments.
The new approval comes with a risk evaluation and mitigation strategy (REMS) because of the risk for serious adverse events, including cytokine release syndrome (CRS).
The product, from Juno Therapeutics, a Bristol Myers Squibb company, is the third gene therapy to receive FDA approval for non-Hodgkin lymphoma, including diffuse large B-cell lymphoma (DLBCL). DLBCL is the most common type of non-Hodgkin lymphoma in adults, accounting for about a third of the approximately 77,000 cases diagnosed each year in the United States.
The FDA previously granted Breyanzi orphan drug, regenerative medicine advanced therapy (RMAT), and breakthrough therapy designations. The product is the first therapy with an RMAT designation to be licensed by the agency.
The new approval is based on efficacy and safety demonstrated in a pivotal phase 1 trial of more than 250 adults with relapsed or refractory large B-cell lymphoma. The complete remission rate after treatment with Breyanzi was 54%.
“Treatment with Breyanzi has the potential to cause severe side effects. The labeling carries a boxed warning for cytokine release syndrome (CRS), which is a systemic response to the activation and proliferation of CAR T cells, causing high fever and flu-like symptoms and neurologic toxicities,” the FDA explained. “Both CRS and neurological events can be life-threatening.”
Other side effects, which typically present within 1-2 weeks after treatment, include hypersensitivity reactions, serious infections, low blood cell counts, and a weakened immune system, but some side effects may occur later.
The REMS requires special certification for facilities that dispense the product and “specifies that patients be informed of the signs and symptoms of CRS and neurological toxicities following infusion – and of the importance of promptly returning to the treatment site if they develop fever or other adverse reactions after receiving treatment with Breyanzi,” the FDA noted.
Breyanzi is not indicated for patients with primary central nervous system lymphoma, the FDA noted.
Facility certification involves training to recognize and manage the risks of CRS and neurologic toxicities.
A postmarketing study to further evaluate the long-term safety will also be required.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved lisocabtagene maraleucel (Breyanzi), a chimeric antigen receptor (CAR) T-cell product for the treatment of adults with certain types of relapsed or refractory large B-cell lymphoma who relapse or fail to respond to at least two systemic treatments.
The new approval comes with a risk evaluation and mitigation strategy (REMS) because of the risk for serious adverse events, including cytokine release syndrome (CRS).
The product, from Juno Therapeutics, a Bristol Myers Squibb company, is the third gene therapy to receive FDA approval for non-Hodgkin lymphoma, including diffuse large B-cell lymphoma (DLBCL). DLBCL is the most common type of non-Hodgkin lymphoma in adults, accounting for about a third of the approximately 77,000 cases diagnosed each year in the United States.
The FDA previously granted Breyanzi orphan drug, regenerative medicine advanced therapy (RMAT), and breakthrough therapy designations. The product is the first therapy with an RMAT designation to be licensed by the agency.
The new approval is based on efficacy and safety demonstrated in a pivotal phase 1 trial of more than 250 adults with relapsed or refractory large B-cell lymphoma. The complete remission rate after treatment with Breyanzi was 54%.
“Treatment with Breyanzi has the potential to cause severe side effects. The labeling carries a boxed warning for cytokine release syndrome (CRS), which is a systemic response to the activation and proliferation of CAR T cells, causing high fever and flu-like symptoms and neurologic toxicities,” the FDA explained. “Both CRS and neurological events can be life-threatening.”
Other side effects, which typically present within 1-2 weeks after treatment, include hypersensitivity reactions, serious infections, low blood cell counts, and a weakened immune system, but some side effects may occur later.
The REMS requires special certification for facilities that dispense the product and “specifies that patients be informed of the signs and symptoms of CRS and neurological toxicities following infusion – and of the importance of promptly returning to the treatment site if they develop fever or other adverse reactions after receiving treatment with Breyanzi,” the FDA noted.
Breyanzi is not indicated for patients with primary central nervous system lymphoma, the FDA noted.
Facility certification involves training to recognize and manage the risks of CRS and neurologic toxicities.
A postmarketing study to further evaluate the long-term safety will also be required.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved lisocabtagene maraleucel (Breyanzi), a chimeric antigen receptor (CAR) T-cell product for the treatment of adults with certain types of relapsed or refractory large B-cell lymphoma who relapse or fail to respond to at least two systemic treatments.
The new approval comes with a risk evaluation and mitigation strategy (REMS) because of the risk for serious adverse events, including cytokine release syndrome (CRS).
The product, from Juno Therapeutics, a Bristol Myers Squibb company, is the third gene therapy to receive FDA approval for non-Hodgkin lymphoma, including diffuse large B-cell lymphoma (DLBCL). DLBCL is the most common type of non-Hodgkin lymphoma in adults, accounting for about a third of the approximately 77,000 cases diagnosed each year in the United States.
The FDA previously granted Breyanzi orphan drug, regenerative medicine advanced therapy (RMAT), and breakthrough therapy designations. The product is the first therapy with an RMAT designation to be licensed by the agency.
The new approval is based on efficacy and safety demonstrated in a pivotal phase 1 trial of more than 250 adults with relapsed or refractory large B-cell lymphoma. The complete remission rate after treatment with Breyanzi was 54%.
“Treatment with Breyanzi has the potential to cause severe side effects. The labeling carries a boxed warning for cytokine release syndrome (CRS), which is a systemic response to the activation and proliferation of CAR T cells, causing high fever and flu-like symptoms and neurologic toxicities,” the FDA explained. “Both CRS and neurological events can be life-threatening.”
Other side effects, which typically present within 1-2 weeks after treatment, include hypersensitivity reactions, serious infections, low blood cell counts, and a weakened immune system, but some side effects may occur later.
The REMS requires special certification for facilities that dispense the product and “specifies that patients be informed of the signs and symptoms of CRS and neurological toxicities following infusion – and of the importance of promptly returning to the treatment site if they develop fever or other adverse reactions after receiving treatment with Breyanzi,” the FDA noted.
Breyanzi is not indicated for patients with primary central nervous system lymphoma, the FDA noted.
Facility certification involves training to recognize and manage the risks of CRS and neurologic toxicities.
A postmarketing study to further evaluate the long-term safety will also be required.
A version of this article first appeared on Medscape.com.
Minimizing Opioids After Joint Operation: Protocol to Decrease Postoperative Opioid Use After Primary Total Knee Arthroplasty
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
The Veterans Health Administration Approach to COVID-19 Vaccine Allocation—Balancing Utility and Equity
The Veterans Health Administration (VHA) COVID-19 vaccine allocation plan showcases several lessons for government and health care leaders in planning for future pandemics.1 Many state governments—underresourced and overwhelmed with other COVID-19 demands—have struggled to get COVID-19 vaccines into the arms of their residents.2 In contrast, the VHA was able to mobilize early to identify vaccine allocation guidelines and proactively prepare facilities to vaccinate VHA staff and veterans as soon as vaccines were approved under Emergency Use Authorization by the US Food and Drug Administration.3,4
In August 2020, VHA formed a COVID-19 Vaccine Integrated Project Team, composed of 6 subgroups: communications, distribution, education, measurement, policy, prioritization, and vaccine safety. The National Center for Ethics in Health Care weighed in on the ethical justification for the developed vaccination risk stratification framework, which was informed by, but not identical to, that recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.5
Prioritizing who gets early access to a potentially life-saving vaccine weighs heavily on those leaders charged with making such decisions. The ethics of scarce resource allocation and triage protocols that may be necessary in a pandemic are often in tension with the patient-centered clinical ethics that health care practitioners (HCPs) encounter. HCPs require assistance in appreciating the ethical rationale for this shift in focus from the preference of the individual to the common good. The same is true for the risk stratification criteria required when there is not sufficient vaccine for all those who could benefit from immunization. Decisions must be transparent to ensure widespread acceptance and trust in the vaccination process. The ethical reasoning and values that are the basis for allocation criteria must be clearly, compassionately, and consistently communicated to the public, as outlined below. Ethical questions or concerns involve a conflict between core values: one of the central tasks of ethical analysis is to identify the available ethical options to resolve value conflicts. Several ethical frameworks for vaccine allocation are available—each balances and weighs the primary values of equity, dignity, beneficence, and utility slightly differently.6
For example, utilitarian ethics looks to produce the most good and avoid the most harm for the greatest number of people. Within this framework, there can be different notions of “good,” for example, saving the most lives, the most life years, the most quality life years, or the lives of those who have more life “innings” ahead. The approach of the US Department of Veterans Affairs (VA) focuses on saving the most lives in combination with avoiding suffering from serious illness, minimizing contagion, and preserving the essential workforce. Frameworks that give primacy to 1 notion of the good (ie, saving the most lives) may deprioritize other beneficial outcomes, such as allowing earlier return to work, school, and leisure activities that many find integral to human flourishing. Other ethical theories and principles may be used to support various allocation frameworks. For example, a pragmatic ethics approach might emphasize the importance of adapting the approach based on the evolving science and innovation surrounding COVID-19. Having more than 1 ethically defensible approach is common; the goal in ethics work is to be open to diversity of thought and reflect on the strength of one’s reasoning in resolving a core values conflict. We identify 2 central tenets of pandemic ethics that inform vaccine allocation.
1. Pandemic Ethics Requires Proactive Planning and Reevaluation of Continually Evolving Facts
There is an oft quoted saying among bioethicists: “Good ethics begins with good facts.” One obvious challenge during the COVID-19 pandemic has been the difficulty accessing up-to-date facts to inform decision making. If a main goal of a vaccination plan is to minimize the incidence of serious or fatal COVID-19 disease and contagion, myriad data points are needed to identify the best way to do this. For example, if 2 doses of the same vaccine are needed, this impacts the logistics of identifying, inviting, and scheduling eligible individuals and staffing vaccine clinics as well as ensuring that sufficient personal protective equipment and rescue equipment/medication are available to treat allergic reactions. If the adverse effects of vaccines lead to staff absenteeism or vaccine hesitancy, this needs to be factored into logistics.7 Tailored messaging is important to reduce appointment no-shows and vaccine nonadopters.8 Transportation to vaccination sites is a relevant factor: how a vaccine is stored, thawed, and reconstituted and its shelf life impacts whether it can be transported after thawing and what must be provided on site.
Consideration of the multifaceted factors influencing a successful vaccination campaign requires proactive planning and the readiness to pivot when new information is revealed. For example, vaccine appointment no-shows should be anticipated along with a fair process for allocating unused vaccine that would otherwise be wasted. This is an example of responsible stewardship of a scarce and life-saving resource. A higher than anticipated no-show rate would require revisiting a facility’s approach to ensuring that waste is avoided while the process is perceived to be fair and transparent. Ethical theories and principles cannot do all the work here; mindful attention to detail and proactive, informed planning are critical. Fortunately, the VA is well resourced in this domain, whereas many state health departments floundered in their response, causing unnecessary vaccination delays.9
2. Utility: Necessary But Insufficient
Most ethical approaches recognize to some extent that seeking good and minimizing harm is of value. However, a strictly utilitarian approach is insufficient to address the core values in conflict surrounding how best to allocate limited doses of COVID-19 vaccine. For example, some may argue that prioritizing the elderly or those in long-term care facilities like VA’s community living centers because they have the highest COVID-19 mortality rate produces less net benefit than prioritizing younger veterans with comorbidities or certain higher risk essential workers. There are 2 important points to make here.
First, the VHA vaccination plan balances utility with other ethical principles, namely, treating people with equal concern, and addressing health inequities, including a focus on justice and valuing the worth and dignity of each person. Rather than giving everyone an equal chance via lottery, the prioritization plan recognizes that some people have greater need or would stand to better mitigate viral contagion and preserve the essential workforce if they were vaccinated earlier. However, the principle of justice requires that efforts are made to treat like cases the same to avoid perceptions of bias, and to demonstrate respect for the dignity of each individual by way of promoting a fair vaccination process.
This requires transparency, consistency, and delivery of respectful and accurate communication. For example, the VA recognizes that lifetime exposure to social injustice produces health inequities that make Black, Hispanic, and Native American persons more susceptible to contracting COVID-19 and suffering serious or fatal illness. The approach to addressing this inequity is by giving priority to those with higher risk factors. Again, this is an example of blending and balancing ethical principles of utility and justice—that is, recognizing and remedying social injustice is of value both because it will help achieve better outcomes for persons of color and because it is inherently worthwhile to oppose injustice.
However, contrary to some news reports, the VHA approach does not allocate by race/ethnicity alone, as it does by age.10,11 Doing so would present logistical challenges—for example, race/ethnicity is not an objective classification as is age, and reconciling individuals’ self-reports could create confusion or chaos that is antithetical to a fair, streamlined vaccination program. Putting veterans of color at the front of the vaccination line could backfire by amplifying worries that they are being exposed to vaccine that is not fully tested (a common contributor to vaccine hesitancy, particularly among communities of color familiar with prior exploitation and abuse in the name of science).
Discriminating based on race/ethnicity alone in the spirit of achieving equity would be precedent setting for the VA and would require a strong ethical justification. The decision to prioritize for vaccine based on risk factors strives to achieve this balance of equity and utility, as it encompasses VA staff and veterans of color by way of their status as essential workers or those with comorbidities. However, it is important to address race-based access barriers and vaccine hesitancy to satisfy the equity demands. This effort is underway (eg, engaging community champions and developing tailored educational resources to reach diverse communities).
In addition, pragmatic ethics recognizes that an overly granular, complicated allocation plan would be inefficient to implement. While it might be true that some veterans who are aged < 65 years may be at higher risk from COVID-19 than some elderly veterans, achieving the goals of fairness and transparency requires establishing a vaccine prioritization plan that is both ethically defensible and feasibly implementable (ie, achieves its goal of getting “needles into arms”). For example, veterans aged ≥ 65 years may be invited to schedule their vaccination before younger veterans, but any veteran may be accepted “on-call” for vaccine appointment no-shows via first-come, first-served or by lottery. Flexibility of response is crucial. This played out in adding flexibility around the decision to vaccinate veterans aged ≥ 75 years before those aged 65 to 74 years, after revisiting how this prioritization might affect feasibility and throughput and opting to allow the opportunity to include those aged ≥ 65 years.
There will no doubt be additional modifications to the vaccine allocation plan as more data become available. Since the danger of fueling suspicion and distrust is high (ie, that certain privileged people are jumping the line, as we heard reports of in some non-VA facilities).12 There is an obvious ethical duty to explain why the chosen approach is ethically defensible. VA facility leaders should be able to answer how their approach achieves the goals of avoiding serious or fatal illness, reducing contagion, and preserving the essential workforce while ensuring a fair, respectful, evidence-based, and transparent process.
1. US Department of Veterans Affairs. COVID-19 vaccination plan for the Veterans Health Administration. Version 2.0, Published December 14, 2020. Accessed February 3, 2021. https://www.publichealth.va.gov/docs/n-coronavirus/VHA-COVID-Vaccine-Plan-14Dec2020.pdf
2. Hennigann WJ, Park A, Ducharme J. The U.S. fumbled its early vaccine rollout. Will the Biden Administration put America back on track? TIME. January 21, 2021. Accessed February 3, 2021. https://time.com/5932028/vaccine-rollout-joe-biden/
3. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
4. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
5. McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine-United States, 2020. Am J Transplant. 2021;21(1):420-425. doi:10.1111/ajt.16437
6. National Academies of Sciences, Engineering, and Medicine. 2020. Framework for equitable allocation of COVID-19 vaccine. The National Academies Press; 2020. doi:10.17226/25917
7 . Wood S, Schulman K. Beyond Politics - Promoting Covid-19 vaccination in the United States [published online ahead of print, 2021 Jan 6]. N Engl J Med. 2021;10.1056/NEJMms2033790. doi:10.1056/NEJMms2033790
8 . Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID-19, who to vaccinate first? medRxiv . 2020 Aug 16. doi:10.1101/2020.08.14.20175257
9 . Makary M. Hospitals: stop playing vaccine games and show leadership. Published January 12, 2021. Accessed February 3, 2021. https://www.medpagetoday.com/blogs/marty-makary/90649
10 . Wentling N. Minority veterans to receive priority for coronavirus vaccines. Stars and Stripes. December 10, 2020. Accessed February 3, 2021. https://www.stripes.com/news/us/minority-veterans-to-receive-priority-for-coronavirus-vaccines-1.654624
11 . Kime, P. Minority veterans on VA’s priority list for COVID-19 vaccine distribution. Published December 8, 2020. Accessed February 3, 2021. https://www.military.com/daily-news/2020/12/08/minority-veterans-vas-priority-list-covid-19-vaccine-distribution.html
12 . Rosenthal, E. Yes, it matters that people are jumping the vaccine line. The New York Times . Published January 28, 2021. Accessed February 3, 2021. https://www.nytimes.com/2021/01/28/opinion/covid-vaccine-line.html
The Veterans Health Administration (VHA) COVID-19 vaccine allocation plan showcases several lessons for government and health care leaders in planning for future pandemics.1 Many state governments—underresourced and overwhelmed with other COVID-19 demands—have struggled to get COVID-19 vaccines into the arms of their residents.2 In contrast, the VHA was able to mobilize early to identify vaccine allocation guidelines and proactively prepare facilities to vaccinate VHA staff and veterans as soon as vaccines were approved under Emergency Use Authorization by the US Food and Drug Administration.3,4
In August 2020, VHA formed a COVID-19 Vaccine Integrated Project Team, composed of 6 subgroups: communications, distribution, education, measurement, policy, prioritization, and vaccine safety. The National Center for Ethics in Health Care weighed in on the ethical justification for the developed vaccination risk stratification framework, which was informed by, but not identical to, that recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.5
Prioritizing who gets early access to a potentially life-saving vaccine weighs heavily on those leaders charged with making such decisions. The ethics of scarce resource allocation and triage protocols that may be necessary in a pandemic are often in tension with the patient-centered clinical ethics that health care practitioners (HCPs) encounter. HCPs require assistance in appreciating the ethical rationale for this shift in focus from the preference of the individual to the common good. The same is true for the risk stratification criteria required when there is not sufficient vaccine for all those who could benefit from immunization. Decisions must be transparent to ensure widespread acceptance and trust in the vaccination process. The ethical reasoning and values that are the basis for allocation criteria must be clearly, compassionately, and consistently communicated to the public, as outlined below. Ethical questions or concerns involve a conflict between core values: one of the central tasks of ethical analysis is to identify the available ethical options to resolve value conflicts. Several ethical frameworks for vaccine allocation are available—each balances and weighs the primary values of equity, dignity, beneficence, and utility slightly differently.6
For example, utilitarian ethics looks to produce the most good and avoid the most harm for the greatest number of people. Within this framework, there can be different notions of “good,” for example, saving the most lives, the most life years, the most quality life years, or the lives of those who have more life “innings” ahead. The approach of the US Department of Veterans Affairs (VA) focuses on saving the most lives in combination with avoiding suffering from serious illness, minimizing contagion, and preserving the essential workforce. Frameworks that give primacy to 1 notion of the good (ie, saving the most lives) may deprioritize other beneficial outcomes, such as allowing earlier return to work, school, and leisure activities that many find integral to human flourishing. Other ethical theories and principles may be used to support various allocation frameworks. For example, a pragmatic ethics approach might emphasize the importance of adapting the approach based on the evolving science and innovation surrounding COVID-19. Having more than 1 ethically defensible approach is common; the goal in ethics work is to be open to diversity of thought and reflect on the strength of one’s reasoning in resolving a core values conflict. We identify 2 central tenets of pandemic ethics that inform vaccine allocation.
1. Pandemic Ethics Requires Proactive Planning and Reevaluation of Continually Evolving Facts
There is an oft quoted saying among bioethicists: “Good ethics begins with good facts.” One obvious challenge during the COVID-19 pandemic has been the difficulty accessing up-to-date facts to inform decision making. If a main goal of a vaccination plan is to minimize the incidence of serious or fatal COVID-19 disease and contagion, myriad data points are needed to identify the best way to do this. For example, if 2 doses of the same vaccine are needed, this impacts the logistics of identifying, inviting, and scheduling eligible individuals and staffing vaccine clinics as well as ensuring that sufficient personal protective equipment and rescue equipment/medication are available to treat allergic reactions. If the adverse effects of vaccines lead to staff absenteeism or vaccine hesitancy, this needs to be factored into logistics.7 Tailored messaging is important to reduce appointment no-shows and vaccine nonadopters.8 Transportation to vaccination sites is a relevant factor: how a vaccine is stored, thawed, and reconstituted and its shelf life impacts whether it can be transported after thawing and what must be provided on site.
Consideration of the multifaceted factors influencing a successful vaccination campaign requires proactive planning and the readiness to pivot when new information is revealed. For example, vaccine appointment no-shows should be anticipated along with a fair process for allocating unused vaccine that would otherwise be wasted. This is an example of responsible stewardship of a scarce and life-saving resource. A higher than anticipated no-show rate would require revisiting a facility’s approach to ensuring that waste is avoided while the process is perceived to be fair and transparent. Ethical theories and principles cannot do all the work here; mindful attention to detail and proactive, informed planning are critical. Fortunately, the VA is well resourced in this domain, whereas many state health departments floundered in their response, causing unnecessary vaccination delays.9
2. Utility: Necessary But Insufficient
Most ethical approaches recognize to some extent that seeking good and minimizing harm is of value. However, a strictly utilitarian approach is insufficient to address the core values in conflict surrounding how best to allocate limited doses of COVID-19 vaccine. For example, some may argue that prioritizing the elderly or those in long-term care facilities like VA’s community living centers because they have the highest COVID-19 mortality rate produces less net benefit than prioritizing younger veterans with comorbidities or certain higher risk essential workers. There are 2 important points to make here.
First, the VHA vaccination plan balances utility with other ethical principles, namely, treating people with equal concern, and addressing health inequities, including a focus on justice and valuing the worth and dignity of each person. Rather than giving everyone an equal chance via lottery, the prioritization plan recognizes that some people have greater need or would stand to better mitigate viral contagion and preserve the essential workforce if they were vaccinated earlier. However, the principle of justice requires that efforts are made to treat like cases the same to avoid perceptions of bias, and to demonstrate respect for the dignity of each individual by way of promoting a fair vaccination process.
This requires transparency, consistency, and delivery of respectful and accurate communication. For example, the VA recognizes that lifetime exposure to social injustice produces health inequities that make Black, Hispanic, and Native American persons more susceptible to contracting COVID-19 and suffering serious or fatal illness. The approach to addressing this inequity is by giving priority to those with higher risk factors. Again, this is an example of blending and balancing ethical principles of utility and justice—that is, recognizing and remedying social injustice is of value both because it will help achieve better outcomes for persons of color and because it is inherently worthwhile to oppose injustice.
However, contrary to some news reports, the VHA approach does not allocate by race/ethnicity alone, as it does by age.10,11 Doing so would present logistical challenges—for example, race/ethnicity is not an objective classification as is age, and reconciling individuals’ self-reports could create confusion or chaos that is antithetical to a fair, streamlined vaccination program. Putting veterans of color at the front of the vaccination line could backfire by amplifying worries that they are being exposed to vaccine that is not fully tested (a common contributor to vaccine hesitancy, particularly among communities of color familiar with prior exploitation and abuse in the name of science).
Discriminating based on race/ethnicity alone in the spirit of achieving equity would be precedent setting for the VA and would require a strong ethical justification. The decision to prioritize for vaccine based on risk factors strives to achieve this balance of equity and utility, as it encompasses VA staff and veterans of color by way of their status as essential workers or those with comorbidities. However, it is important to address race-based access barriers and vaccine hesitancy to satisfy the equity demands. This effort is underway (eg, engaging community champions and developing tailored educational resources to reach diverse communities).
In addition, pragmatic ethics recognizes that an overly granular, complicated allocation plan would be inefficient to implement. While it might be true that some veterans who are aged < 65 years may be at higher risk from COVID-19 than some elderly veterans, achieving the goals of fairness and transparency requires establishing a vaccine prioritization plan that is both ethically defensible and feasibly implementable (ie, achieves its goal of getting “needles into arms”). For example, veterans aged ≥ 65 years may be invited to schedule their vaccination before younger veterans, but any veteran may be accepted “on-call” for vaccine appointment no-shows via first-come, first-served or by lottery. Flexibility of response is crucial. This played out in adding flexibility around the decision to vaccinate veterans aged ≥ 75 years before those aged 65 to 74 years, after revisiting how this prioritization might affect feasibility and throughput and opting to allow the opportunity to include those aged ≥ 65 years.
There will no doubt be additional modifications to the vaccine allocation plan as more data become available. Since the danger of fueling suspicion and distrust is high (ie, that certain privileged people are jumping the line, as we heard reports of in some non-VA facilities).12 There is an obvious ethical duty to explain why the chosen approach is ethically defensible. VA facility leaders should be able to answer how their approach achieves the goals of avoiding serious or fatal illness, reducing contagion, and preserving the essential workforce while ensuring a fair, respectful, evidence-based, and transparent process.
The Veterans Health Administration (VHA) COVID-19 vaccine allocation plan showcases several lessons for government and health care leaders in planning for future pandemics.1 Many state governments—underresourced and overwhelmed with other COVID-19 demands—have struggled to get COVID-19 vaccines into the arms of their residents.2 In contrast, the VHA was able to mobilize early to identify vaccine allocation guidelines and proactively prepare facilities to vaccinate VHA staff and veterans as soon as vaccines were approved under Emergency Use Authorization by the US Food and Drug Administration.3,4
In August 2020, VHA formed a COVID-19 Vaccine Integrated Project Team, composed of 6 subgroups: communications, distribution, education, measurement, policy, prioritization, and vaccine safety. The National Center for Ethics in Health Care weighed in on the ethical justification for the developed vaccination risk stratification framework, which was informed by, but not identical to, that recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.5
Prioritizing who gets early access to a potentially life-saving vaccine weighs heavily on those leaders charged with making such decisions. The ethics of scarce resource allocation and triage protocols that may be necessary in a pandemic are often in tension with the patient-centered clinical ethics that health care practitioners (HCPs) encounter. HCPs require assistance in appreciating the ethical rationale for this shift in focus from the preference of the individual to the common good. The same is true for the risk stratification criteria required when there is not sufficient vaccine for all those who could benefit from immunization. Decisions must be transparent to ensure widespread acceptance and trust in the vaccination process. The ethical reasoning and values that are the basis for allocation criteria must be clearly, compassionately, and consistently communicated to the public, as outlined below. Ethical questions or concerns involve a conflict between core values: one of the central tasks of ethical analysis is to identify the available ethical options to resolve value conflicts. Several ethical frameworks for vaccine allocation are available—each balances and weighs the primary values of equity, dignity, beneficence, and utility slightly differently.6
For example, utilitarian ethics looks to produce the most good and avoid the most harm for the greatest number of people. Within this framework, there can be different notions of “good,” for example, saving the most lives, the most life years, the most quality life years, or the lives of those who have more life “innings” ahead. The approach of the US Department of Veterans Affairs (VA) focuses on saving the most lives in combination with avoiding suffering from serious illness, minimizing contagion, and preserving the essential workforce. Frameworks that give primacy to 1 notion of the good (ie, saving the most lives) may deprioritize other beneficial outcomes, such as allowing earlier return to work, school, and leisure activities that many find integral to human flourishing. Other ethical theories and principles may be used to support various allocation frameworks. For example, a pragmatic ethics approach might emphasize the importance of adapting the approach based on the evolving science and innovation surrounding COVID-19. Having more than 1 ethically defensible approach is common; the goal in ethics work is to be open to diversity of thought and reflect on the strength of one’s reasoning in resolving a core values conflict. We identify 2 central tenets of pandemic ethics that inform vaccine allocation.
1. Pandemic Ethics Requires Proactive Planning and Reevaluation of Continually Evolving Facts
There is an oft quoted saying among bioethicists: “Good ethics begins with good facts.” One obvious challenge during the COVID-19 pandemic has been the difficulty accessing up-to-date facts to inform decision making. If a main goal of a vaccination plan is to minimize the incidence of serious or fatal COVID-19 disease and contagion, myriad data points are needed to identify the best way to do this. For example, if 2 doses of the same vaccine are needed, this impacts the logistics of identifying, inviting, and scheduling eligible individuals and staffing vaccine clinics as well as ensuring that sufficient personal protective equipment and rescue equipment/medication are available to treat allergic reactions. If the adverse effects of vaccines lead to staff absenteeism or vaccine hesitancy, this needs to be factored into logistics.7 Tailored messaging is important to reduce appointment no-shows and vaccine nonadopters.8 Transportation to vaccination sites is a relevant factor: how a vaccine is stored, thawed, and reconstituted and its shelf life impacts whether it can be transported after thawing and what must be provided on site.
Consideration of the multifaceted factors influencing a successful vaccination campaign requires proactive planning and the readiness to pivot when new information is revealed. For example, vaccine appointment no-shows should be anticipated along with a fair process for allocating unused vaccine that would otherwise be wasted. This is an example of responsible stewardship of a scarce and life-saving resource. A higher than anticipated no-show rate would require revisiting a facility’s approach to ensuring that waste is avoided while the process is perceived to be fair and transparent. Ethical theories and principles cannot do all the work here; mindful attention to detail and proactive, informed planning are critical. Fortunately, the VA is well resourced in this domain, whereas many state health departments floundered in their response, causing unnecessary vaccination delays.9
2. Utility: Necessary But Insufficient
Most ethical approaches recognize to some extent that seeking good and minimizing harm is of value. However, a strictly utilitarian approach is insufficient to address the core values in conflict surrounding how best to allocate limited doses of COVID-19 vaccine. For example, some may argue that prioritizing the elderly or those in long-term care facilities like VA’s community living centers because they have the highest COVID-19 mortality rate produces less net benefit than prioritizing younger veterans with comorbidities or certain higher risk essential workers. There are 2 important points to make here.
First, the VHA vaccination plan balances utility with other ethical principles, namely, treating people with equal concern, and addressing health inequities, including a focus on justice and valuing the worth and dignity of each person. Rather than giving everyone an equal chance via lottery, the prioritization plan recognizes that some people have greater need or would stand to better mitigate viral contagion and preserve the essential workforce if they were vaccinated earlier. However, the principle of justice requires that efforts are made to treat like cases the same to avoid perceptions of bias, and to demonstrate respect for the dignity of each individual by way of promoting a fair vaccination process.
This requires transparency, consistency, and delivery of respectful and accurate communication. For example, the VA recognizes that lifetime exposure to social injustice produces health inequities that make Black, Hispanic, and Native American persons more susceptible to contracting COVID-19 and suffering serious or fatal illness. The approach to addressing this inequity is by giving priority to those with higher risk factors. Again, this is an example of blending and balancing ethical principles of utility and justice—that is, recognizing and remedying social injustice is of value both because it will help achieve better outcomes for persons of color and because it is inherently worthwhile to oppose injustice.
However, contrary to some news reports, the VHA approach does not allocate by race/ethnicity alone, as it does by age.10,11 Doing so would present logistical challenges—for example, race/ethnicity is not an objective classification as is age, and reconciling individuals’ self-reports could create confusion or chaos that is antithetical to a fair, streamlined vaccination program. Putting veterans of color at the front of the vaccination line could backfire by amplifying worries that they are being exposed to vaccine that is not fully tested (a common contributor to vaccine hesitancy, particularly among communities of color familiar with prior exploitation and abuse in the name of science).
Discriminating based on race/ethnicity alone in the spirit of achieving equity would be precedent setting for the VA and would require a strong ethical justification. The decision to prioritize for vaccine based on risk factors strives to achieve this balance of equity and utility, as it encompasses VA staff and veterans of color by way of their status as essential workers or those with comorbidities. However, it is important to address race-based access barriers and vaccine hesitancy to satisfy the equity demands. This effort is underway (eg, engaging community champions and developing tailored educational resources to reach diverse communities).
In addition, pragmatic ethics recognizes that an overly granular, complicated allocation plan would be inefficient to implement. While it might be true that some veterans who are aged < 65 years may be at higher risk from COVID-19 than some elderly veterans, achieving the goals of fairness and transparency requires establishing a vaccine prioritization plan that is both ethically defensible and feasibly implementable (ie, achieves its goal of getting “needles into arms”). For example, veterans aged ≥ 65 years may be invited to schedule their vaccination before younger veterans, but any veteran may be accepted “on-call” for vaccine appointment no-shows via first-come, first-served or by lottery. Flexibility of response is crucial. This played out in adding flexibility around the decision to vaccinate veterans aged ≥ 75 years before those aged 65 to 74 years, after revisiting how this prioritization might affect feasibility and throughput and opting to allow the opportunity to include those aged ≥ 65 years.
There will no doubt be additional modifications to the vaccine allocation plan as more data become available. Since the danger of fueling suspicion and distrust is high (ie, that certain privileged people are jumping the line, as we heard reports of in some non-VA facilities).12 There is an obvious ethical duty to explain why the chosen approach is ethically defensible. VA facility leaders should be able to answer how their approach achieves the goals of avoiding serious or fatal illness, reducing contagion, and preserving the essential workforce while ensuring a fair, respectful, evidence-based, and transparent process.
1. US Department of Veterans Affairs. COVID-19 vaccination plan for the Veterans Health Administration. Version 2.0, Published December 14, 2020. Accessed February 3, 2021. https://www.publichealth.va.gov/docs/n-coronavirus/VHA-COVID-Vaccine-Plan-14Dec2020.pdf
2. Hennigann WJ, Park A, Ducharme J. The U.S. fumbled its early vaccine rollout. Will the Biden Administration put America back on track? TIME. January 21, 2021. Accessed February 3, 2021. https://time.com/5932028/vaccine-rollout-joe-biden/
3. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
4. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
5. McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine-United States, 2020. Am J Transplant. 2021;21(1):420-425. doi:10.1111/ajt.16437
6. National Academies of Sciences, Engineering, and Medicine. 2020. Framework for equitable allocation of COVID-19 vaccine. The National Academies Press; 2020. doi:10.17226/25917
7 . Wood S, Schulman K. Beyond Politics - Promoting Covid-19 vaccination in the United States [published online ahead of print, 2021 Jan 6]. N Engl J Med. 2021;10.1056/NEJMms2033790. doi:10.1056/NEJMms2033790
8 . Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID-19, who to vaccinate first? medRxiv . 2020 Aug 16. doi:10.1101/2020.08.14.20175257
9 . Makary M. Hospitals: stop playing vaccine games and show leadership. Published January 12, 2021. Accessed February 3, 2021. https://www.medpagetoday.com/blogs/marty-makary/90649
10 . Wentling N. Minority veterans to receive priority for coronavirus vaccines. Stars and Stripes. December 10, 2020. Accessed February 3, 2021. https://www.stripes.com/news/us/minority-veterans-to-receive-priority-for-coronavirus-vaccines-1.654624
11 . Kime, P. Minority veterans on VA’s priority list for COVID-19 vaccine distribution. Published December 8, 2020. Accessed February 3, 2021. https://www.military.com/daily-news/2020/12/08/minority-veterans-vas-priority-list-covid-19-vaccine-distribution.html
12 . Rosenthal, E. Yes, it matters that people are jumping the vaccine line. The New York Times . Published January 28, 2021. Accessed February 3, 2021. https://www.nytimes.com/2021/01/28/opinion/covid-vaccine-line.html
1. US Department of Veterans Affairs. COVID-19 vaccination plan for the Veterans Health Administration. Version 2.0, Published December 14, 2020. Accessed February 3, 2021. https://www.publichealth.va.gov/docs/n-coronavirus/VHA-COVID-Vaccine-Plan-14Dec2020.pdf
2. Hennigann WJ, Park A, Ducharme J. The U.S. fumbled its early vaccine rollout. Will the Biden Administration put America back on track? TIME. January 21, 2021. Accessed February 3, 2021. https://time.com/5932028/vaccine-rollout-joe-biden/
3. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
4. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
5. McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine-United States, 2020. Am J Transplant. 2021;21(1):420-425. doi:10.1111/ajt.16437
6. National Academies of Sciences, Engineering, and Medicine. 2020. Framework for equitable allocation of COVID-19 vaccine. The National Academies Press; 2020. doi:10.17226/25917
7 . Wood S, Schulman K. Beyond Politics - Promoting Covid-19 vaccination in the United States [published online ahead of print, 2021 Jan 6]. N Engl J Med. 2021;10.1056/NEJMms2033790. doi:10.1056/NEJMms2033790
8 . Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID-19, who to vaccinate first? medRxiv . 2020 Aug 16. doi:10.1101/2020.08.14.20175257
9 . Makary M. Hospitals: stop playing vaccine games and show leadership. Published January 12, 2021. Accessed February 3, 2021. https://www.medpagetoday.com/blogs/marty-makary/90649
10 . Wentling N. Minority veterans to receive priority for coronavirus vaccines. Stars and Stripes. December 10, 2020. Accessed February 3, 2021. https://www.stripes.com/news/us/minority-veterans-to-receive-priority-for-coronavirus-vaccines-1.654624
11 . Kime, P. Minority veterans on VA’s priority list for COVID-19 vaccine distribution. Published December 8, 2020. Accessed February 3, 2021. https://www.military.com/daily-news/2020/12/08/minority-veterans-vas-priority-list-covid-19-vaccine-distribution.html
12 . Rosenthal, E. Yes, it matters that people are jumping the vaccine line. The New York Times . Published January 28, 2021. Accessed February 3, 2021. https://www.nytimes.com/2021/01/28/opinion/covid-vaccine-line.html