User login
P3SONG: Evaluation for autism spectrum disorder
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
How to handle negative online reviews
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
2020 Peer Reviewers
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
Treating insomnia, anxiety in a pandemic
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The fourth trimester: Achieving improved postpartum care
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.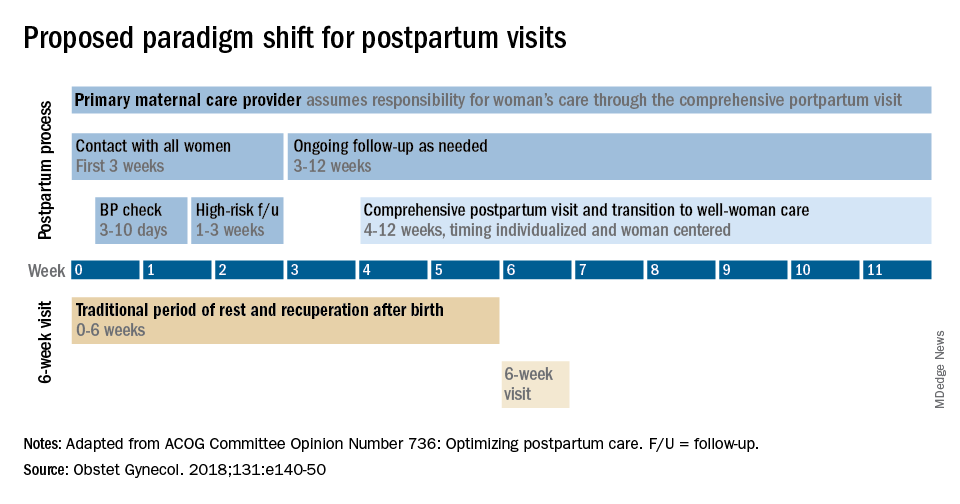
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
The fourth trimester
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
*This version has been updated to correct an erroneous byline, photo, and bio.
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
*This version has been updated to correct an erroneous byline, photo, and bio.
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
*This version has been updated to correct an erroneous byline, photo, and bio.
The COPD patient who couldn’t stop worrying
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Virtual residency/fellowship interviews: The good, the bad, and the future
As a psychiatry resident in the age of coronavirus disease 2019 (COVID-19), many of my educational experiences have undergone adjustments. Now, as I interview for a fellowship, I see firsthand that recruitment activities have not been spared from shifting paradigms levied by the pandemic. To adhere to social distancing guidelines and limit trainee interpersonal exposure, the American Association of Directors of Psychiatric Residency Training recommended that all psychiatry residency interviews be conducted virtually for 2020/2021.1 Trainees and programs alike are embarking on a new frontier of virtual interviews, and it is important that we evaluate the advantages and disadvantages of this approach. Because uncertainty abounds regarding when a sense of normalcy might eventually return to psychiatry residency and fellowship recruitment activities, I also provide recommendations to interviewers and interviewees who may navigate virtual recruitment in the future.
Advantages of virtual interviews
An immediately significant advantage of virtual interviews is the lack of travel, which for some applicants can be cost-prohibitive. The costs of airfare, rental vehicles, and lodging in multiple cities can add up, sometimes requiring students to budget interview travel into already-high student loans. In some cases, applicants may have limited days to interview, which makes the flexibility afforded by the lack of travel advantageous. Furthermore, navigating new locations can add to preexisting interview stress. Without travel, applicants can consider a broader set of programs and accept more interviews.
Another advantage is that virtual interviews allow interviewees latitude to shift the interview’s “frame.” Rather than sitting in an interviewer’s office, interviewees can choose a more comfortable environment for themselves, imparting a “home-field advantage” that may put them at ease. During my fellowship interviews, controlling the room temperature, using a familiar chair, and wearing comfortable shoes helped to reduce the anxiety inherent to interviewing.
Disadvantages of virtual interviews
Any new or unfamiliar experience can impart challenges. For example, applicants and interviewers must adjust to and observe different sets of etiquette during virtual interviews. These include muting microphones to avoid talking over each other, maintaining consistent eye contact, avoiding multitasking, and following up to avoid miscommunication.
Another potential problem is that virtual interviews can dampen an applicant’s ability to appreciate a program’s culture. Observing informal interactions between trainees and faculty is often as important as the formal interviews in ascertaining which programs have a supportive culture. Because my virtual fellowship interviews have generally been limited to formal one-on-one interviews, assessing program culture has become more challenging. Conversely, programs may find it difficult to grasp an applicant’s temperament and interaction style.
Virtual interviewing, while undeniably convenient in many regards, may fall prey to its own convenience. There can be disparities in the quantity, duration, and frequency of interviews. For me, the number of and time allotted for interviews has varied widely, ranging from 2.5 to 8 hours. The amount of allotted break time has also differed, with some programs providing longer breaks between interviews (30 to 60 minutes) and others offering shorter (5 to 10 minutes) or no breaks. Minimal breaks may fatigue applicants, while longer breaks may seem like wasted time. While virtual interviews may require no physical travel between offices, breaks are a necessity that should be implemented thoughtfully.
Finally, a troublesome challenge I encountered surprisingly often was unreliable internet service and other technical difficulties. Several times, my interviewers’ (or my) screen froze or shut off due to connectivity issues. This is an obstacle unique to virtual interviews that requires both a backup plan as well as patience and calm to navigate during an already taxing situation.
Continue to: What's next?
What’s next?
As applicants and programs adjust to the realities of virtual interviewing, this is likely an unfamiliar experience for all. While the benefits and shortcomings must be considered together, I, along with many of my peers,2 continue to prefer traditional in-person interviews. As the ongoing COVID-19 pandemic makes in-person interviews difficult, applicants and programs must embrace the experience of virtual interviews. However, a good understanding of the advantages and disadvantages are instrumental in preempting prospective challenges. Based on my recent experiences with virtual fellowship interviews, I have created some recommendations for applicants and psychiatry programs participating in virtual recruitment (Table).

After the COVID-19 pandemic subsides, it is conceivable that the advantages of virtual interviewing may justify its continued use. For example, applicants may be able to apply to geographically diverse programs without travel expenses. Currently, there is a paucity of evidence regarding virtual interviews specifically in psychiatry training programs, but the experiences of both applicants and psychiatry programs during this atypical time will allow us to improve the process going forward, and evaluate its utility well after COVID-19 recedes.
1. Arbuckle M, Kerlek A, Kovach J, et al. Consensus statement from the Association of Directors of Medical Student Education in Psychiatry (ADMSEP) and the American Association of Directors of Psychiatric Residency Training (AADPRT) on the 2020-21 Residency Application Cycle. https://www.aadprt.org/application/files/8816/0017/8240/admsep_aadprt_statement_9-14-20_Rev.pdf. Published May 18, 2020. Accessed November 20, 2020.
2. Seifi A, Mirahmadizadeh A, Eslami V. Perception of medical students and residents about virtual interviews for residency applications in the United States. PLoS ONE. 2020;15(8):e0238239. doi: 10.1371/journal.pone.0238239.
As a psychiatry resident in the age of coronavirus disease 2019 (COVID-19), many of my educational experiences have undergone adjustments. Now, as I interview for a fellowship, I see firsthand that recruitment activities have not been spared from shifting paradigms levied by the pandemic. To adhere to social distancing guidelines and limit trainee interpersonal exposure, the American Association of Directors of Psychiatric Residency Training recommended that all psychiatry residency interviews be conducted virtually for 2020/2021.1 Trainees and programs alike are embarking on a new frontier of virtual interviews, and it is important that we evaluate the advantages and disadvantages of this approach. Because uncertainty abounds regarding when a sense of normalcy might eventually return to psychiatry residency and fellowship recruitment activities, I also provide recommendations to interviewers and interviewees who may navigate virtual recruitment in the future.
Advantages of virtual interviews
An immediately significant advantage of virtual interviews is the lack of travel, which for some applicants can be cost-prohibitive. The costs of airfare, rental vehicles, and lodging in multiple cities can add up, sometimes requiring students to budget interview travel into already-high student loans. In some cases, applicants may have limited days to interview, which makes the flexibility afforded by the lack of travel advantageous. Furthermore, navigating new locations can add to preexisting interview stress. Without travel, applicants can consider a broader set of programs and accept more interviews.
Another advantage is that virtual interviews allow interviewees latitude to shift the interview’s “frame.” Rather than sitting in an interviewer’s office, interviewees can choose a more comfortable environment for themselves, imparting a “home-field advantage” that may put them at ease. During my fellowship interviews, controlling the room temperature, using a familiar chair, and wearing comfortable shoes helped to reduce the anxiety inherent to interviewing.
Disadvantages of virtual interviews
Any new or unfamiliar experience can impart challenges. For example, applicants and interviewers must adjust to and observe different sets of etiquette during virtual interviews. These include muting microphones to avoid talking over each other, maintaining consistent eye contact, avoiding multitasking, and following up to avoid miscommunication.
Another potential problem is that virtual interviews can dampen an applicant’s ability to appreciate a program’s culture. Observing informal interactions between trainees and faculty is often as important as the formal interviews in ascertaining which programs have a supportive culture. Because my virtual fellowship interviews have generally been limited to formal one-on-one interviews, assessing program culture has become more challenging. Conversely, programs may find it difficult to grasp an applicant’s temperament and interaction style.
Virtual interviewing, while undeniably convenient in many regards, may fall prey to its own convenience. There can be disparities in the quantity, duration, and frequency of interviews. For me, the number of and time allotted for interviews has varied widely, ranging from 2.5 to 8 hours. The amount of allotted break time has also differed, with some programs providing longer breaks between interviews (30 to 60 minutes) and others offering shorter (5 to 10 minutes) or no breaks. Minimal breaks may fatigue applicants, while longer breaks may seem like wasted time. While virtual interviews may require no physical travel between offices, breaks are a necessity that should be implemented thoughtfully.
Finally, a troublesome challenge I encountered surprisingly often was unreliable internet service and other technical difficulties. Several times, my interviewers’ (or my) screen froze or shut off due to connectivity issues. This is an obstacle unique to virtual interviews that requires both a backup plan as well as patience and calm to navigate during an already taxing situation.
Continue to: What's next?
What’s next?
As applicants and programs adjust to the realities of virtual interviewing, this is likely an unfamiliar experience for all. While the benefits and shortcomings must be considered together, I, along with many of my peers,2 continue to prefer traditional in-person interviews. As the ongoing COVID-19 pandemic makes in-person interviews difficult, applicants and programs must embrace the experience of virtual interviews. However, a good understanding of the advantages and disadvantages are instrumental in preempting prospective challenges. Based on my recent experiences with virtual fellowship interviews, I have created some recommendations for applicants and psychiatry programs participating in virtual recruitment (Table).

After the COVID-19 pandemic subsides, it is conceivable that the advantages of virtual interviewing may justify its continued use. For example, applicants may be able to apply to geographically diverse programs without travel expenses. Currently, there is a paucity of evidence regarding virtual interviews specifically in psychiatry training programs, but the experiences of both applicants and psychiatry programs during this atypical time will allow us to improve the process going forward, and evaluate its utility well after COVID-19 recedes.
As a psychiatry resident in the age of coronavirus disease 2019 (COVID-19), many of my educational experiences have undergone adjustments. Now, as I interview for a fellowship, I see firsthand that recruitment activities have not been spared from shifting paradigms levied by the pandemic. To adhere to social distancing guidelines and limit trainee interpersonal exposure, the American Association of Directors of Psychiatric Residency Training recommended that all psychiatry residency interviews be conducted virtually for 2020/2021.1 Trainees and programs alike are embarking on a new frontier of virtual interviews, and it is important that we evaluate the advantages and disadvantages of this approach. Because uncertainty abounds regarding when a sense of normalcy might eventually return to psychiatry residency and fellowship recruitment activities, I also provide recommendations to interviewers and interviewees who may navigate virtual recruitment in the future.
Advantages of virtual interviews
An immediately significant advantage of virtual interviews is the lack of travel, which for some applicants can be cost-prohibitive. The costs of airfare, rental vehicles, and lodging in multiple cities can add up, sometimes requiring students to budget interview travel into already-high student loans. In some cases, applicants may have limited days to interview, which makes the flexibility afforded by the lack of travel advantageous. Furthermore, navigating new locations can add to preexisting interview stress. Without travel, applicants can consider a broader set of programs and accept more interviews.
Another advantage is that virtual interviews allow interviewees latitude to shift the interview’s “frame.” Rather than sitting in an interviewer’s office, interviewees can choose a more comfortable environment for themselves, imparting a “home-field advantage” that may put them at ease. During my fellowship interviews, controlling the room temperature, using a familiar chair, and wearing comfortable shoes helped to reduce the anxiety inherent to interviewing.
Disadvantages of virtual interviews
Any new or unfamiliar experience can impart challenges. For example, applicants and interviewers must adjust to and observe different sets of etiquette during virtual interviews. These include muting microphones to avoid talking over each other, maintaining consistent eye contact, avoiding multitasking, and following up to avoid miscommunication.
Another potential problem is that virtual interviews can dampen an applicant’s ability to appreciate a program’s culture. Observing informal interactions between trainees and faculty is often as important as the formal interviews in ascertaining which programs have a supportive culture. Because my virtual fellowship interviews have generally been limited to formal one-on-one interviews, assessing program culture has become more challenging. Conversely, programs may find it difficult to grasp an applicant’s temperament and interaction style.
Virtual interviewing, while undeniably convenient in many regards, may fall prey to its own convenience. There can be disparities in the quantity, duration, and frequency of interviews. For me, the number of and time allotted for interviews has varied widely, ranging from 2.5 to 8 hours. The amount of allotted break time has also differed, with some programs providing longer breaks between interviews (30 to 60 minutes) and others offering shorter (5 to 10 minutes) or no breaks. Minimal breaks may fatigue applicants, while longer breaks may seem like wasted time. While virtual interviews may require no physical travel between offices, breaks are a necessity that should be implemented thoughtfully.
Finally, a troublesome challenge I encountered surprisingly often was unreliable internet service and other technical difficulties. Several times, my interviewers’ (or my) screen froze or shut off due to connectivity issues. This is an obstacle unique to virtual interviews that requires both a backup plan as well as patience and calm to navigate during an already taxing situation.
Continue to: What's next?
What’s next?
As applicants and programs adjust to the realities of virtual interviewing, this is likely an unfamiliar experience for all. While the benefits and shortcomings must be considered together, I, along with many of my peers,2 continue to prefer traditional in-person interviews. As the ongoing COVID-19 pandemic makes in-person interviews difficult, applicants and programs must embrace the experience of virtual interviews. However, a good understanding of the advantages and disadvantages are instrumental in preempting prospective challenges. Based on my recent experiences with virtual fellowship interviews, I have created some recommendations for applicants and psychiatry programs participating in virtual recruitment (Table).

After the COVID-19 pandemic subsides, it is conceivable that the advantages of virtual interviewing may justify its continued use. For example, applicants may be able to apply to geographically diverse programs without travel expenses. Currently, there is a paucity of evidence regarding virtual interviews specifically in psychiatry training programs, but the experiences of both applicants and psychiatry programs during this atypical time will allow us to improve the process going forward, and evaluate its utility well after COVID-19 recedes.
1. Arbuckle M, Kerlek A, Kovach J, et al. Consensus statement from the Association of Directors of Medical Student Education in Psychiatry (ADMSEP) and the American Association of Directors of Psychiatric Residency Training (AADPRT) on the 2020-21 Residency Application Cycle. https://www.aadprt.org/application/files/8816/0017/8240/admsep_aadprt_statement_9-14-20_Rev.pdf. Published May 18, 2020. Accessed November 20, 2020.
2. Seifi A, Mirahmadizadeh A, Eslami V. Perception of medical students and residents about virtual interviews for residency applications in the United States. PLoS ONE. 2020;15(8):e0238239. doi: 10.1371/journal.pone.0238239.
1. Arbuckle M, Kerlek A, Kovach J, et al. Consensus statement from the Association of Directors of Medical Student Education in Psychiatry (ADMSEP) and the American Association of Directors of Psychiatric Residency Training (AADPRT) on the 2020-21 Residency Application Cycle. https://www.aadprt.org/application/files/8816/0017/8240/admsep_aadprt_statement_9-14-20_Rev.pdf. Published May 18, 2020. Accessed November 20, 2020.
2. Seifi A, Mirahmadizadeh A, Eslami V. Perception of medical students and residents about virtual interviews for residency applications in the United States. PLoS ONE. 2020;15(8):e0238239. doi: 10.1371/journal.pone.0238239.
COVID-19’s religious strain: Differentiating spirituality from pathology
As the world grapples with the coronavirus disease 2019 (COVID-19) pandemic, the search for answers, comfort, how to cope, and how to make sense of it all has become paramount. People commonly turn to their faith in times of crisis, but this recent global public health emergency is unlike many have ever seen or could have imagined.1 What happens when the well-intentioned journey for spiritual insight intersects with psychiatric symptomatology? Where does the line between these phenomena get crossed? As a psychiatric resident and person who was raised in the Pentecostal faith, I have observed faith and psychopathology come to a head in the last 6 months. COVID-19 has dealt a religious strain of undocumented cases; I hope to shed light on the topic by sharing my experience of navigating the assessment and treatment plan of patients with psychiatric symptoms whose spiritual beliefs are a cornerstone of life.
Piety, or pathology?
The following approaches have helped me to identify what is driven by faith vs what is psychopathology:
While taking the patient history. Obtaining a history from a patient who professes to have strong spiritual beliefs and presents with psychiatric symptoms is similar to a standard patient interview, but pay special attention to how the patient came to the emergency department. Was there a family member, friend, or emergency medical services present at that time? During the interview, patients often appear “normal,” which may lead a clinician to question the reason for the consult, yet considering the recent events preceding the presentation will be a good place to start gathering the appropriate information for investigation.
Next, compare the patient’s recent daily functioning with his/her baseline. If this information comes solely from the patient, it may be skewed, so try to retrieve information from a collateral source. If the patient was accompanied by someone, request permission from the patient to speak with him/her. It may also be best in some instances to speak with the collateral source out of earshot of the patient. Be aware that collateral information that comes from just one source also could be biased, so search for additional contacts to help acquire a comprehensive representation of the circumstances.
Information about a patient could come from a faith leader because people often rely on their faith leaders when they are ill, in need of support, or in crisis.2 Faith leaders may have valuable information and insight into the patient and the history of the patient’s illness. In addition, diverse sources of collateral reports may be helpful because specific spiritual views and practices can vary even within one family or congregation. What may be an abnormal practice to some followers may be normal for others.3 When approaching these situations with parishioners, it is essential to maintain confidentiality.
While performing the clinical examination. As with any psychiatric diagnosis, other causative factors (metabolic and organic) need to be ruled out. Also, assess for the use of mood-altering substances. The patient may express offense or resistance to such questions, but maintain a matter-of-fact approach and explain that assessment for substance use is a routine part of the clinical examination. Approximately 18% of people in the United States with psychiatric disorders have a comorbid substance use disorder.4 However, keep in mind that a patient who refuses substance use screening is not necessarily hiding something. The road to being thorough may lead to strained rapport with the patient, and this risk must be balanced with providing the best care. As in any other clinical situation, seek evidence to both verify and clarify information without being deterred by a patient’s vocalization of spiritual tenants.
Learn about your patients’ beliefs
Do not feel defeated if you find these interviews difficult. Religion and symptomatology can overlap and fluctuate within the same faith group, which can make these types of assessments complex.5 In an effort to understand the patient more clearly, be sensitive to their spiritual practices and receptive to learning about unfamiliar spiritual beliefs. Be transparent about not knowing a specific belief or practice, and exhibit humility. Most patients are open to sharing their religious/spiritual views with a clinician who is sincere about wanting insight. Understanding the value of spiritual care is an important skill that many medical practitioners often lack.6 This understanding is especially critical when patients express worries related to the COVID-19 pandemic and how they are coping.
Continue to: Integrate your patient's spiritual requests
Integrate your patient’s spiritual requests
If you are comfortable with certain practices that do not compromise your values or beliefs or put a patient at risk, try to integrate your patients’ spiritual request(s) in their care. For a patient who serves a higher power, admitting to a problem (eg, fears related to COVID-19) or seeking professional help for symptoms (eg, anxiety, depression) may imply spiritual doubt. Patients may believe that seeking professional assistance means they are questioning the omnipotence of their deity to prevent or heal a condition. While spiritual distress can stimulate changes in behavior, it may not be pathological.
To avoid misdiagnosis, refer to the description “V62.89 (Z65.8) Religious or Spiritual Problem” in the DSM-5.7 If you find that it is a discord in faith that is affecting the patient’s presentation, and that this has not caused a psychiatric disorder, document this appropriately and provide the necessary resources to continue supporting the patient holistically.
1. Dein S, Loewenthal K, Lewis CA, et al. COVID-19, mental health and religion: an agenda for future research. Mental Health, Religion & Culture. 2020;23(1):1-9.
2. American Psychiatric Association Foundation. Mental health: a guide for faith leaders. Arlington, VA: American Psychiatric Association Foundation; 2018.
3. Johnson CV, Friedman HL. Enlightened or delusional? Differentiating religious, spiritual, and transpersonal experiences from psychopathology. Journal of Humanistic Psychology. 2008;48(4):505-527.
4. Han B, Compton WM, Blanco C, et al. Prevalence, treatment, and unmet treatment needs of US adults with mental health and substance use disorders. Health Aff (Millwood). 2017;36(10):1739-1747.
5. Menezes Jr A, Moreira-Almeida A. Differential diagnosis between spiritual experiences and mental disorders of religious content. Rev Psiq Clín. 2009;36(2):75-82.
6. Best M, Butow P, Olver I. Doctors discussing religion and spirituality: a systematic literature review. Palliat Med. 2016;30(4):327-337.
7. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:725.
As the world grapples with the coronavirus disease 2019 (COVID-19) pandemic, the search for answers, comfort, how to cope, and how to make sense of it all has become paramount. People commonly turn to their faith in times of crisis, but this recent global public health emergency is unlike many have ever seen or could have imagined.1 What happens when the well-intentioned journey for spiritual insight intersects with psychiatric symptomatology? Where does the line between these phenomena get crossed? As a psychiatric resident and person who was raised in the Pentecostal faith, I have observed faith and psychopathology come to a head in the last 6 months. COVID-19 has dealt a religious strain of undocumented cases; I hope to shed light on the topic by sharing my experience of navigating the assessment and treatment plan of patients with psychiatric symptoms whose spiritual beliefs are a cornerstone of life.
Piety, or pathology?
The following approaches have helped me to identify what is driven by faith vs what is psychopathology:
While taking the patient history. Obtaining a history from a patient who professes to have strong spiritual beliefs and presents with psychiatric symptoms is similar to a standard patient interview, but pay special attention to how the patient came to the emergency department. Was there a family member, friend, or emergency medical services present at that time? During the interview, patients often appear “normal,” which may lead a clinician to question the reason for the consult, yet considering the recent events preceding the presentation will be a good place to start gathering the appropriate information for investigation.
Next, compare the patient’s recent daily functioning with his/her baseline. If this information comes solely from the patient, it may be skewed, so try to retrieve information from a collateral source. If the patient was accompanied by someone, request permission from the patient to speak with him/her. It may also be best in some instances to speak with the collateral source out of earshot of the patient. Be aware that collateral information that comes from just one source also could be biased, so search for additional contacts to help acquire a comprehensive representation of the circumstances.
Information about a patient could come from a faith leader because people often rely on their faith leaders when they are ill, in need of support, or in crisis.2 Faith leaders may have valuable information and insight into the patient and the history of the patient’s illness. In addition, diverse sources of collateral reports may be helpful because specific spiritual views and practices can vary even within one family or congregation. What may be an abnormal practice to some followers may be normal for others.3 When approaching these situations with parishioners, it is essential to maintain confidentiality.
While performing the clinical examination. As with any psychiatric diagnosis, other causative factors (metabolic and organic) need to be ruled out. Also, assess for the use of mood-altering substances. The patient may express offense or resistance to such questions, but maintain a matter-of-fact approach and explain that assessment for substance use is a routine part of the clinical examination. Approximately 18% of people in the United States with psychiatric disorders have a comorbid substance use disorder.4 However, keep in mind that a patient who refuses substance use screening is not necessarily hiding something. The road to being thorough may lead to strained rapport with the patient, and this risk must be balanced with providing the best care. As in any other clinical situation, seek evidence to both verify and clarify information without being deterred by a patient’s vocalization of spiritual tenants.
Learn about your patients’ beliefs
Do not feel defeated if you find these interviews difficult. Religion and symptomatology can overlap and fluctuate within the same faith group, which can make these types of assessments complex.5 In an effort to understand the patient more clearly, be sensitive to their spiritual practices and receptive to learning about unfamiliar spiritual beliefs. Be transparent about not knowing a specific belief or practice, and exhibit humility. Most patients are open to sharing their religious/spiritual views with a clinician who is sincere about wanting insight. Understanding the value of spiritual care is an important skill that many medical practitioners often lack.6 This understanding is especially critical when patients express worries related to the COVID-19 pandemic and how they are coping.
Continue to: Integrate your patient's spiritual requests
Integrate your patient’s spiritual requests
If you are comfortable with certain practices that do not compromise your values or beliefs or put a patient at risk, try to integrate your patients’ spiritual request(s) in their care. For a patient who serves a higher power, admitting to a problem (eg, fears related to COVID-19) or seeking professional help for symptoms (eg, anxiety, depression) may imply spiritual doubt. Patients may believe that seeking professional assistance means they are questioning the omnipotence of their deity to prevent or heal a condition. While spiritual distress can stimulate changes in behavior, it may not be pathological.
To avoid misdiagnosis, refer to the description “V62.89 (Z65.8) Religious or Spiritual Problem” in the DSM-5.7 If you find that it is a discord in faith that is affecting the patient’s presentation, and that this has not caused a psychiatric disorder, document this appropriately and provide the necessary resources to continue supporting the patient holistically.
As the world grapples with the coronavirus disease 2019 (COVID-19) pandemic, the search for answers, comfort, how to cope, and how to make sense of it all has become paramount. People commonly turn to their faith in times of crisis, but this recent global public health emergency is unlike many have ever seen or could have imagined.1 What happens when the well-intentioned journey for spiritual insight intersects with psychiatric symptomatology? Where does the line between these phenomena get crossed? As a psychiatric resident and person who was raised in the Pentecostal faith, I have observed faith and psychopathology come to a head in the last 6 months. COVID-19 has dealt a religious strain of undocumented cases; I hope to shed light on the topic by sharing my experience of navigating the assessment and treatment plan of patients with psychiatric symptoms whose spiritual beliefs are a cornerstone of life.
Piety, or pathology?
The following approaches have helped me to identify what is driven by faith vs what is psychopathology:
While taking the patient history. Obtaining a history from a patient who professes to have strong spiritual beliefs and presents with psychiatric symptoms is similar to a standard patient interview, but pay special attention to how the patient came to the emergency department. Was there a family member, friend, or emergency medical services present at that time? During the interview, patients often appear “normal,” which may lead a clinician to question the reason for the consult, yet considering the recent events preceding the presentation will be a good place to start gathering the appropriate information for investigation.
Next, compare the patient’s recent daily functioning with his/her baseline. If this information comes solely from the patient, it may be skewed, so try to retrieve information from a collateral source. If the patient was accompanied by someone, request permission from the patient to speak with him/her. It may also be best in some instances to speak with the collateral source out of earshot of the patient. Be aware that collateral information that comes from just one source also could be biased, so search for additional contacts to help acquire a comprehensive representation of the circumstances.
Information about a patient could come from a faith leader because people often rely on their faith leaders when they are ill, in need of support, or in crisis.2 Faith leaders may have valuable information and insight into the patient and the history of the patient’s illness. In addition, diverse sources of collateral reports may be helpful because specific spiritual views and practices can vary even within one family or congregation. What may be an abnormal practice to some followers may be normal for others.3 When approaching these situations with parishioners, it is essential to maintain confidentiality.
While performing the clinical examination. As with any psychiatric diagnosis, other causative factors (metabolic and organic) need to be ruled out. Also, assess for the use of mood-altering substances. The patient may express offense or resistance to such questions, but maintain a matter-of-fact approach and explain that assessment for substance use is a routine part of the clinical examination. Approximately 18% of people in the United States with psychiatric disorders have a comorbid substance use disorder.4 However, keep in mind that a patient who refuses substance use screening is not necessarily hiding something. The road to being thorough may lead to strained rapport with the patient, and this risk must be balanced with providing the best care. As in any other clinical situation, seek evidence to both verify and clarify information without being deterred by a patient’s vocalization of spiritual tenants.
Learn about your patients’ beliefs
Do not feel defeated if you find these interviews difficult. Religion and symptomatology can overlap and fluctuate within the same faith group, which can make these types of assessments complex.5 In an effort to understand the patient more clearly, be sensitive to their spiritual practices and receptive to learning about unfamiliar spiritual beliefs. Be transparent about not knowing a specific belief or practice, and exhibit humility. Most patients are open to sharing their religious/spiritual views with a clinician who is sincere about wanting insight. Understanding the value of spiritual care is an important skill that many medical practitioners often lack.6 This understanding is especially critical when patients express worries related to the COVID-19 pandemic and how they are coping.
Continue to: Integrate your patient's spiritual requests
Integrate your patient’s spiritual requests
If you are comfortable with certain practices that do not compromise your values or beliefs or put a patient at risk, try to integrate your patients’ spiritual request(s) in their care. For a patient who serves a higher power, admitting to a problem (eg, fears related to COVID-19) or seeking professional help for symptoms (eg, anxiety, depression) may imply spiritual doubt. Patients may believe that seeking professional assistance means they are questioning the omnipotence of their deity to prevent or heal a condition. While spiritual distress can stimulate changes in behavior, it may not be pathological.
To avoid misdiagnosis, refer to the description “V62.89 (Z65.8) Religious or Spiritual Problem” in the DSM-5.7 If you find that it is a discord in faith that is affecting the patient’s presentation, and that this has not caused a psychiatric disorder, document this appropriately and provide the necessary resources to continue supporting the patient holistically.
1. Dein S, Loewenthal K, Lewis CA, et al. COVID-19, mental health and religion: an agenda for future research. Mental Health, Religion & Culture. 2020;23(1):1-9.
2. American Psychiatric Association Foundation. Mental health: a guide for faith leaders. Arlington, VA: American Psychiatric Association Foundation; 2018.
3. Johnson CV, Friedman HL. Enlightened or delusional? Differentiating religious, spiritual, and transpersonal experiences from psychopathology. Journal of Humanistic Psychology. 2008;48(4):505-527.
4. Han B, Compton WM, Blanco C, et al. Prevalence, treatment, and unmet treatment needs of US adults with mental health and substance use disorders. Health Aff (Millwood). 2017;36(10):1739-1747.
5. Menezes Jr A, Moreira-Almeida A. Differential diagnosis between spiritual experiences and mental disorders of religious content. Rev Psiq Clín. 2009;36(2):75-82.
6. Best M, Butow P, Olver I. Doctors discussing religion and spirituality: a systematic literature review. Palliat Med. 2016;30(4):327-337.
7. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:725.
1. Dein S, Loewenthal K, Lewis CA, et al. COVID-19, mental health and religion: an agenda for future research. Mental Health, Religion & Culture. 2020;23(1):1-9.
2. American Psychiatric Association Foundation. Mental health: a guide for faith leaders. Arlington, VA: American Psychiatric Association Foundation; 2018.
3. Johnson CV, Friedman HL. Enlightened or delusional? Differentiating religious, spiritual, and transpersonal experiences from psychopathology. Journal of Humanistic Psychology. 2008;48(4):505-527.
4. Han B, Compton WM, Blanco C, et al. Prevalence, treatment, and unmet treatment needs of US adults with mental health and substance use disorders. Health Aff (Millwood). 2017;36(10):1739-1747.
5. Menezes Jr A, Moreira-Almeida A. Differential diagnosis between spiritual experiences and mental disorders of religious content. Rev Psiq Clín. 2009;36(2):75-82.
6. Best M, Butow P, Olver I. Doctors discussing religion and spirituality: a systematic literature review. Palliat Med. 2016;30(4):327-337.
7. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:725.
Maintaining credibility when evaluating new dermatologic technologies
A healthy dose of skepticism is reasonable when evaluating new technologies to potentially use in your practice, but being overly critical can backfire, according to E. Victor Ross, MD.
During a virtual course on laser and aesthetic skin therapy, he noted that dermatologists may be evaluating new technologies in roles as an investigator or provider, or as a provider who buys the equipment outright, without any direct compensation from industry. “If you’re doing investigative work, you’re already in a conflicted situation because you’re trying to serve two masters,” said Dr. Ross, who directs the Scripps Clinic Laser and Cosmetic Dermatology Center in San Diego. “You’re trying to advance science and to make sure your reputation is intact and maybe even enhanced, but you’re also kind of at the whim of industry, because they have a goal. Sometimes the goals are similar. Your goal is to advance the technology and advance patient care, but they have a goal of selling equipment. Those goals should be compatible, and they should be in the same pathway; they should be parallel.”
Being too critical as an investigator/researcher of new technologies can hinder further interactions with industry. “Sometimes your criticism can be premature, and small changes in the technology and/or waiting for results can validate the technology,” he said. “Maybe it’s a skin-tightening technology, or maybe there’s something you don’t like about it; you have a prototype and you say: ‘This is not so great,’ but these are studies that take a long time to evaluate, like hair removal. Generally, if you’re a big critic, after a while, nobody wants to hear it. So, you can’t be overly critical. I think you can be skeptical, but not overly critical.”
On the other hand, Dr. Ross continued, if you cheerlead for the device industry, your reputation may be sold to the highest bidder. “You may compromise your ability to be trusted in future work or presentations. I’ve regretted some things I said many years ago, not because I was being dishonest but because I really wasn’t as skeptical as I should have been about the types of results I was getting. You do tend to get on a bandwagon; you want everything to be positive,” he said, adding: “The other thing that can happen is, if things don’t work out with other buyers, they’re going to say, ‘I can’t get the same results.’ If somebody can’t replicate what you’re doing, it’s going to put your reputation on the line to some degree. So, you have to be very careful.”
Before agreeing to evaluate a new technology as an investigator or in your own practice, Dr. Ross recommended asking yourself if the intervention makes sense on a gross or microanatomic level. “There should be a physical basis for how it works, and ideally there should be some histology that backs it up,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Where is the data to support the outcomes? If it is your own data, how skeptical have you been in its acquisition and assessment?”
When evaluating a new technology, Dr. Ross recommended starting with test spots and low settings. “It behooves you not to be too aggressive because there are some untoward things you may not see right away.” He advised evaluating short- and long-term outcomes and being wary of devices that heat very deeply and rely on long-term outcomes, such as hair removal, acne improvement, scar improvement, and skin tightening – all of which require long intervals for assessment. As one of his “Ross Rules” states: “The deeper the heating and the less focused the heating, the less we can expect results.” Another Ross Rule calls for being skeptical about technologies without an immediate finding that can be “seen” on routine histology. “To me, the deeper the procedure, particularly if it’s not fractional, the confidence of my outcome is diminished,” he said. “The exception is any intervention that relies on selective photothermolysis.”
Using new technologies in practice
Dr. Ross also offered tips on how to properly incorporate newly approved technologies into your practice safely, including the use of visual endpoints. “That’s tough for technologies like laser liposuction or fractional technologies, where we rely more on ‘guidelines’ than endpoints,” he said. In addition, he recommended gradually increasing settings and using test areas to stall and/or hone techniques. “It’s exciting, but you’re like a test pilot. You want to be careful that you are doing things that are not likely to risk the patient. Try some off-the-face applications first. When you’re using a new technology, push a little harder with each patient so you can find a safe zone for that technology. You don’t have to get it all in one treatment session. Be conservative. Anticipate that you may be underassessing the immediate response.”
Above all, be careful. “Use your judgment more than laser company-prescribed settings,” he said. “Most companies have the go-by settings on the low side for patient protection, but sometimes efficacy suffers. Use cautiously on friends and family. If you treat a spouse or a friend and things don’t go perfectly, that’s always a recipe for a problem.”
Dr. Ross reported having received financial grants and research grants from Candela, Cutera, Lumenis, and Lutronic; consulting fees from Palomar; and honoraria from Cynosure, Cutera, and Lumenis. He has also received research funding from Venous Concepts, Pulsed Biosciences, and Cynosure.
A healthy dose of skepticism is reasonable when evaluating new technologies to potentially use in your practice, but being overly critical can backfire, according to E. Victor Ross, MD.
During a virtual course on laser and aesthetic skin therapy, he noted that dermatologists may be evaluating new technologies in roles as an investigator or provider, or as a provider who buys the equipment outright, without any direct compensation from industry. “If you’re doing investigative work, you’re already in a conflicted situation because you’re trying to serve two masters,” said Dr. Ross, who directs the Scripps Clinic Laser and Cosmetic Dermatology Center in San Diego. “You’re trying to advance science and to make sure your reputation is intact and maybe even enhanced, but you’re also kind of at the whim of industry, because they have a goal. Sometimes the goals are similar. Your goal is to advance the technology and advance patient care, but they have a goal of selling equipment. Those goals should be compatible, and they should be in the same pathway; they should be parallel.”
Being too critical as an investigator/researcher of new technologies can hinder further interactions with industry. “Sometimes your criticism can be premature, and small changes in the technology and/or waiting for results can validate the technology,” he said. “Maybe it’s a skin-tightening technology, or maybe there’s something you don’t like about it; you have a prototype and you say: ‘This is not so great,’ but these are studies that take a long time to evaluate, like hair removal. Generally, if you’re a big critic, after a while, nobody wants to hear it. So, you can’t be overly critical. I think you can be skeptical, but not overly critical.”
On the other hand, Dr. Ross continued, if you cheerlead for the device industry, your reputation may be sold to the highest bidder. “You may compromise your ability to be trusted in future work or presentations. I’ve regretted some things I said many years ago, not because I was being dishonest but because I really wasn’t as skeptical as I should have been about the types of results I was getting. You do tend to get on a bandwagon; you want everything to be positive,” he said, adding: “The other thing that can happen is, if things don’t work out with other buyers, they’re going to say, ‘I can’t get the same results.’ If somebody can’t replicate what you’re doing, it’s going to put your reputation on the line to some degree. So, you have to be very careful.”
Before agreeing to evaluate a new technology as an investigator or in your own practice, Dr. Ross recommended asking yourself if the intervention makes sense on a gross or microanatomic level. “There should be a physical basis for how it works, and ideally there should be some histology that backs it up,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Where is the data to support the outcomes? If it is your own data, how skeptical have you been in its acquisition and assessment?”
When evaluating a new technology, Dr. Ross recommended starting with test spots and low settings. “It behooves you not to be too aggressive because there are some untoward things you may not see right away.” He advised evaluating short- and long-term outcomes and being wary of devices that heat very deeply and rely on long-term outcomes, such as hair removal, acne improvement, scar improvement, and skin tightening – all of which require long intervals for assessment. As one of his “Ross Rules” states: “The deeper the heating and the less focused the heating, the less we can expect results.” Another Ross Rule calls for being skeptical about technologies without an immediate finding that can be “seen” on routine histology. “To me, the deeper the procedure, particularly if it’s not fractional, the confidence of my outcome is diminished,” he said. “The exception is any intervention that relies on selective photothermolysis.”
Using new technologies in practice
Dr. Ross also offered tips on how to properly incorporate newly approved technologies into your practice safely, including the use of visual endpoints. “That’s tough for technologies like laser liposuction or fractional technologies, where we rely more on ‘guidelines’ than endpoints,” he said. In addition, he recommended gradually increasing settings and using test areas to stall and/or hone techniques. “It’s exciting, but you’re like a test pilot. You want to be careful that you are doing things that are not likely to risk the patient. Try some off-the-face applications first. When you’re using a new technology, push a little harder with each patient so you can find a safe zone for that technology. You don’t have to get it all in one treatment session. Be conservative. Anticipate that you may be underassessing the immediate response.”
Above all, be careful. “Use your judgment more than laser company-prescribed settings,” he said. “Most companies have the go-by settings on the low side for patient protection, but sometimes efficacy suffers. Use cautiously on friends and family. If you treat a spouse or a friend and things don’t go perfectly, that’s always a recipe for a problem.”
Dr. Ross reported having received financial grants and research grants from Candela, Cutera, Lumenis, and Lutronic; consulting fees from Palomar; and honoraria from Cynosure, Cutera, and Lumenis. He has also received research funding from Venous Concepts, Pulsed Biosciences, and Cynosure.
A healthy dose of skepticism is reasonable when evaluating new technologies to potentially use in your practice, but being overly critical can backfire, according to E. Victor Ross, MD.
During a virtual course on laser and aesthetic skin therapy, he noted that dermatologists may be evaluating new technologies in roles as an investigator or provider, or as a provider who buys the equipment outright, without any direct compensation from industry. “If you’re doing investigative work, you’re already in a conflicted situation because you’re trying to serve two masters,” said Dr. Ross, who directs the Scripps Clinic Laser and Cosmetic Dermatology Center in San Diego. “You’re trying to advance science and to make sure your reputation is intact and maybe even enhanced, but you’re also kind of at the whim of industry, because they have a goal. Sometimes the goals are similar. Your goal is to advance the technology and advance patient care, but they have a goal of selling equipment. Those goals should be compatible, and they should be in the same pathway; they should be parallel.”
Being too critical as an investigator/researcher of new technologies can hinder further interactions with industry. “Sometimes your criticism can be premature, and small changes in the technology and/or waiting for results can validate the technology,” he said. “Maybe it’s a skin-tightening technology, or maybe there’s something you don’t like about it; you have a prototype and you say: ‘This is not so great,’ but these are studies that take a long time to evaluate, like hair removal. Generally, if you’re a big critic, after a while, nobody wants to hear it. So, you can’t be overly critical. I think you can be skeptical, but not overly critical.”
On the other hand, Dr. Ross continued, if you cheerlead for the device industry, your reputation may be sold to the highest bidder. “You may compromise your ability to be trusted in future work or presentations. I’ve regretted some things I said many years ago, not because I was being dishonest but because I really wasn’t as skeptical as I should have been about the types of results I was getting. You do tend to get on a bandwagon; you want everything to be positive,” he said, adding: “The other thing that can happen is, if things don’t work out with other buyers, they’re going to say, ‘I can’t get the same results.’ If somebody can’t replicate what you’re doing, it’s going to put your reputation on the line to some degree. So, you have to be very careful.”
Before agreeing to evaluate a new technology as an investigator or in your own practice, Dr. Ross recommended asking yourself if the intervention makes sense on a gross or microanatomic level. “There should be a physical basis for how it works, and ideally there should be some histology that backs it up,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Where is the data to support the outcomes? If it is your own data, how skeptical have you been in its acquisition and assessment?”
When evaluating a new technology, Dr. Ross recommended starting with test spots and low settings. “It behooves you not to be too aggressive because there are some untoward things you may not see right away.” He advised evaluating short- and long-term outcomes and being wary of devices that heat very deeply and rely on long-term outcomes, such as hair removal, acne improvement, scar improvement, and skin tightening – all of which require long intervals for assessment. As one of his “Ross Rules” states: “The deeper the heating and the less focused the heating, the less we can expect results.” Another Ross Rule calls for being skeptical about technologies without an immediate finding that can be “seen” on routine histology. “To me, the deeper the procedure, particularly if it’s not fractional, the confidence of my outcome is diminished,” he said. “The exception is any intervention that relies on selective photothermolysis.”
Using new technologies in practice
Dr. Ross also offered tips on how to properly incorporate newly approved technologies into your practice safely, including the use of visual endpoints. “That’s tough for technologies like laser liposuction or fractional technologies, where we rely more on ‘guidelines’ than endpoints,” he said. In addition, he recommended gradually increasing settings and using test areas to stall and/or hone techniques. “It’s exciting, but you’re like a test pilot. You want to be careful that you are doing things that are not likely to risk the patient. Try some off-the-face applications first. When you’re using a new technology, push a little harder with each patient so you can find a safe zone for that technology. You don’t have to get it all in one treatment session. Be conservative. Anticipate that you may be underassessing the immediate response.”
Above all, be careful. “Use your judgment more than laser company-prescribed settings,” he said. “Most companies have the go-by settings on the low side for patient protection, but sometimes efficacy suffers. Use cautiously on friends and family. If you treat a spouse or a friend and things don’t go perfectly, that’s always a recipe for a problem.”
Dr. Ross reported having received financial grants and research grants from Candela, Cutera, Lumenis, and Lutronic; consulting fees from Palomar; and honoraria from Cynosure, Cutera, and Lumenis. He has also received research funding from Venous Concepts, Pulsed Biosciences, and Cynosure.
REPORTING FROM A LASER & AESTHETIC SKIN THERAPY COURSE










