User login
Novel topical acne combo hits marks in phase 3 trials
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
[email protected]
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
[email protected]
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
[email protected]
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Separating myth from reality: The role of cannabinoids in COVID-19
An intriguing pattern has emerged for cannabis enthusiasts as a result of lockdowns and statewide safety restrictions for COVID-19.
Consumers, as of late, have been shopping for larger marijuana baskets per trip to the dispensaries in various states, including California, Colorado, Nevada, and Washington, . However, they are also cutting down on the number of trips, perhaps, as a preventive measure to reduce the risk of exposure to coronavirus during this pandemic. Sales dipped considerably by the end of March only to experience a resurgence after the issuing of stimulus checks and unemployment benefits.
For the past few years, cannabis consumption remained steady while the industry continued to thrive with robust sales of the drug. It is a recession-proof phenomenon, therefore presenting a unique opportunity for clinicians with respect to patient education and individualized care.1
An unfortunate carryover of the governmental restrictions, self-isolation, and social estrangement is that consumers are now turning to the dark web as a source for continuous supply of cannabis. Prepandemic, according to the U.N. 2020 World Drug Report, there was already a 30% increase in sales of cannabis between 2009 and 2018. COVID-19 has fractured the drug’s supply chain and created an inescapable void that is being filled by drug traffickers.2 A clinical dilemma is posed when a user procures counterfeit cannabis or a drug batch with impurities.
Riding the cytokine storm
Cytokines are a host of proteins with designated regulatory and immune responses that play an instrumental role in cell signaling. The aptly named “cytokine storm” conjures up the image of an imperiled immune system spiraling out of control; it is, in fact, an extreme immune response that culminates into a massive influx of cytokines released into the bloodstream. Without the presence of an immunologic threat, cytokines are responsible for maintaining homeostasis and the functionality of immune cells. However, acute cytokine release (i.e., cytokine storm), as is the case with severe COVID-19, jeopardizes organ function (for example, interstitial lung disease) with clinical symptoms, such as fever, cough, dyspnea, and myalgia.
Benefits and drawbacks of immunosuppressive agents
To inhibit cytokine release (e.g., interleukin-6 cytokine levels), immunosuppressive agents such as tocilizumab have been leveraged to damper the body’s overactive inflammatory response to perceived immunologic stressors, in particular, COVID-19. While the aforementioned agent was remarkably effective with respect to lung consolidation clearance in most of the patients tested, a host of untoward effects prevent its general applicability and use. However, a team of researchers from the University of Nebraska, Omaha, with the Texas Biomedical Research Institute, San Antonio, might have stumbled upon a strategic workaround for mitigating the immune response.
They have proposed that cannabidiol (CBD) be used in lieu of other agents with potentially toxic effects. Animal and human trials have established that CBD confers a relatively high margin of safety coupled with favorable tolerance, providing a viable option for effectively targeting the inflammatory processes of SARS-CoV-2–based pulmonary disease. Furthermore, efficacy increased when CBD was combined with a terpene formulation, especially with respect to the more traditional steroid therapy.3
SARS-CoV-2 exhibits binding affinity for the ACE2 receptor, which is expressed in the lungs as well as other known predilection sites of infection. Ongoing studies attempt to modulate ACE2 expression, thereby eliminating its conspicuous role as “viral gateways,” perhaps even more so in patients with lung pathologies (e.g., people with chronic obstructive pulmonary disease [COPD] and smokers) as they already are prone to increased respiratory morbidity. CBD lacks tetrahydrocannabinol (THC), or the psychoactive component of cannabis sativa, rendering the agent to be particularly attractive from a therapeutic perspective. In addition to being devoid of abuse potential, CBD exhibits remarkable anti-inflammatory properties. It should be noted that considerable overlap exists between tobacco and cannabis users, and it is too early to determine the impact on COVID-19. As opposed to cannabis’s effect on ACE2 levels, smoking exhibits a proinflammatory role by up-regulating ACE2 expression.3 However, there are currently numerous conflicting reports in circulation about the positive effect of nicotine on COVID-19 outcome; confounding variables will need to be explored further in patients with a history of using nicotine and cannabis together.
From an immunologic perspective, the endocannabinoid system (ECS) plays an integral role in cell signaling by interacting with natural chemicals of the body, namely, cannabinoids with designated targets at the cannabinoid receptor 1 (CB1) and the CB2, respectively. The CB2 receptor is of particular interest as it is intimately involved in immune homeostasis; the primary goal of these COVID-19 studies is to modulate the endocannabinoid system via targeted CB2 therapies to produce an immunosuppressant effect.4 CB2 activation, be it by means of THC or CBD agonism, may prove to be beneficial by inhibiting the cytokine influx.
Unfortunately, there is a general dearth of data on COVID-19–exposed cannabis users, whether the drug is consumed for medication or recreational purposes. It has been suggested that cannabis intake might contribute toward the development of a cough, complicating the overall clinical outcome for those infected with the virus. The presence of a cough, even in an otherwise asymptomatic individual, facilitates viral spread. As for those cannabis users experiencing COVID-19 symptomatology, they can expect rapid clinical deterioration, including pronounced fatigue and a change in mental status.
According to pulmonary specialists and representatives of the American Lung Association, recreational cannabis use may be associated with a bronchitis-like inflammation (comparable with chronic bronchitis/COPD for chronic users) of the airways, along the lines of cigarette smoking.5 As far as cannabis smokers are concerned, the rationale for lung irritation is believed to stem from the relatively large portion of unburnt plant content that is inhaled in a given joint. If there is a superimposed infection, as is the case with COVID-19, the patient may experience further risk of adverse respiratory effects. This serves as a diagnostic dilemma for physicians, especially when they encounter patients who recently started dabbling with cannabis as a means of placating themselves or because they’ve heard rumors that it will somehow protect them from COVID-19. The entire assessment plan is slowed down as a result of the confounding variable (onset of a cough), which may arise independently of COVID-19 in cannabis users. Vulnerable populations include smokers and those with COPD or asthma, as they are more likely to require ventilator assistance during the course of COVID-19 therapy.5 Asthmatics and COPD patients are prone to bronchospasms because of sensitive airways.
COVID-19 safety protocols for cannabis users
Because of increased risk of respiratory morbidity, clinicians advise that consumption of recreational cannabinoids be scaled back during the course of the pandemic. In light of conflicting news from several media outlets regarding the efficacy of cannabis intake with respect to COVID-19, preexisting users might unwittingly increase their consumption as a preemptive measure against being exposed to the infection. To prevent transmission among users, clinicians should discourage patients from sharing joints. This recommendation is thematically consistent with general precautionary measures about the dangers of sharing utensils, drinking cups/glasses, and so on, amid the pandemic.
Despite promising preliminary research results, CBD cannot be wholeheartedly recommended at this time; patients already on medically administered cannabinoids are urged to discuss the risk-benefit ratio with their respective health care clinicians. Cannabinoid therapies present a massive opportunity from the perspective of immunomodulation, especially when considering the prevalence of drug use. However, to improve clinical guidelines with respect to COVID-19 outcomes, it would be prudent to increase the overall volume of preclinical knowledge by gathering retrospective data (from case-control designs) and randomized prospective trials.
A more comprehensive list of advice from physicians concerning casual or chronic cannabis users may also include: adopting a dedicated delivery or dispensing system for cannabis products, making considerations for decontamination (i.e., disinfecting mouthpieces), ensuring cleansing precautions are maintained (washing thoroughly before and after use or procurement), switching to inhalation alternates (e.g., tinctures, edibles, and/or oils) to decrease further irritation to the lungs. For bong users, it is recommended that they apply rubbing alcohol to clean their device followed with a minute of air-drying.6
Conclusion
The literature from preclinical studies appears to largely favor the use of CBD, but there remains an element of uncertainty with respect to implementing cannabinoids for the treatment of coronavirus.
COVID-19 cannabinoid intervention is a hot topic with renewed interest from the industry and the public at large, but viral-focused therapies remain a relatively underused area worth exploring with case-control designs and randomized prospective trials. As cannabis legalization is picking up momentum across five additional states, the time is ripe to systematically investigate the therapeutic applications of the drug beyond merely preclinical data. Aside from educational reform initiatives, clinicians might proactively launch a platform that integrates telemedicine as well as digital apps, directly linking the patient to the clinician and monitoring the efficacy of program initiatives in real time.
References
1. Long A. Consumers’ cannabis buying patterns change markedly in wake of COVID-19 pandemic. Marijuana Business Daily. 2020 Sep 22. https://mjbizdaily.com/consumers-cannabis-buying-patterns-change-markedly-in-wake-of-covid-pandemic/.
2. Bures B. How the coronavirus pandemic is increasing global demand for marijuana. Chicago Tribune. 2020 Jul 1. https://www.chicagotribune.com/marijuana/sns-tft-coronavirus-increases-global-marijuana-demand-20200701-oygaxryb7vhcjfeu44cgacicaa-story.html.
3. Walters J. Marijuana and COVID-19: Top studies. CannaMD. 2020 Aug 19. https://www.cannamd.com/marijuana-covid-19-top-studies/.
4. El Biali M et al. Med Cannabis Cannabinoids. 2020 Aug 19. doi: 10.1159/000510799.
5. LaMotte S. “Smoking weed and coronavirus: Even occasional use raises risk of COVID-19 complications.” CNN Health. 2020 Apr 10. https://www.cnn.com/2020/04/10/health/smoking-weed-coronavirus-wellness/index.html
6. Yafai S and Etengoff S. The case for cannabis: Advising cannabis users about COVID-19. Emergency Medicine News. 2020 May 20;42(5B).
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Mr. Choudhry is a research assistant at the IMCHF. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. Dr. Islam, Mr. Choudhry, and Dr. Choudhry reported no relevant disclosures.
An intriguing pattern has emerged for cannabis enthusiasts as a result of lockdowns and statewide safety restrictions for COVID-19.
Consumers, as of late, have been shopping for larger marijuana baskets per trip to the dispensaries in various states, including California, Colorado, Nevada, and Washington, . However, they are also cutting down on the number of trips, perhaps, as a preventive measure to reduce the risk of exposure to coronavirus during this pandemic. Sales dipped considerably by the end of March only to experience a resurgence after the issuing of stimulus checks and unemployment benefits.
For the past few years, cannabis consumption remained steady while the industry continued to thrive with robust sales of the drug. It is a recession-proof phenomenon, therefore presenting a unique opportunity for clinicians with respect to patient education and individualized care.1
An unfortunate carryover of the governmental restrictions, self-isolation, and social estrangement is that consumers are now turning to the dark web as a source for continuous supply of cannabis. Prepandemic, according to the U.N. 2020 World Drug Report, there was already a 30% increase in sales of cannabis between 2009 and 2018. COVID-19 has fractured the drug’s supply chain and created an inescapable void that is being filled by drug traffickers.2 A clinical dilemma is posed when a user procures counterfeit cannabis or a drug batch with impurities.
Riding the cytokine storm
Cytokines are a host of proteins with designated regulatory and immune responses that play an instrumental role in cell signaling. The aptly named “cytokine storm” conjures up the image of an imperiled immune system spiraling out of control; it is, in fact, an extreme immune response that culminates into a massive influx of cytokines released into the bloodstream. Without the presence of an immunologic threat, cytokines are responsible for maintaining homeostasis and the functionality of immune cells. However, acute cytokine release (i.e., cytokine storm), as is the case with severe COVID-19, jeopardizes organ function (for example, interstitial lung disease) with clinical symptoms, such as fever, cough, dyspnea, and myalgia.
Benefits and drawbacks of immunosuppressive agents
To inhibit cytokine release (e.g., interleukin-6 cytokine levels), immunosuppressive agents such as tocilizumab have been leveraged to damper the body’s overactive inflammatory response to perceived immunologic stressors, in particular, COVID-19. While the aforementioned agent was remarkably effective with respect to lung consolidation clearance in most of the patients tested, a host of untoward effects prevent its general applicability and use. However, a team of researchers from the University of Nebraska, Omaha, with the Texas Biomedical Research Institute, San Antonio, might have stumbled upon a strategic workaround for mitigating the immune response.
They have proposed that cannabidiol (CBD) be used in lieu of other agents with potentially toxic effects. Animal and human trials have established that CBD confers a relatively high margin of safety coupled with favorable tolerance, providing a viable option for effectively targeting the inflammatory processes of SARS-CoV-2–based pulmonary disease. Furthermore, efficacy increased when CBD was combined with a terpene formulation, especially with respect to the more traditional steroid therapy.3
SARS-CoV-2 exhibits binding affinity for the ACE2 receptor, which is expressed in the lungs as well as other known predilection sites of infection. Ongoing studies attempt to modulate ACE2 expression, thereby eliminating its conspicuous role as “viral gateways,” perhaps even more so in patients with lung pathologies (e.g., people with chronic obstructive pulmonary disease [COPD] and smokers) as they already are prone to increased respiratory morbidity. CBD lacks tetrahydrocannabinol (THC), or the psychoactive component of cannabis sativa, rendering the agent to be particularly attractive from a therapeutic perspective. In addition to being devoid of abuse potential, CBD exhibits remarkable anti-inflammatory properties. It should be noted that considerable overlap exists between tobacco and cannabis users, and it is too early to determine the impact on COVID-19. As opposed to cannabis’s effect on ACE2 levels, smoking exhibits a proinflammatory role by up-regulating ACE2 expression.3 However, there are currently numerous conflicting reports in circulation about the positive effect of nicotine on COVID-19 outcome; confounding variables will need to be explored further in patients with a history of using nicotine and cannabis together.
From an immunologic perspective, the endocannabinoid system (ECS) plays an integral role in cell signaling by interacting with natural chemicals of the body, namely, cannabinoids with designated targets at the cannabinoid receptor 1 (CB1) and the CB2, respectively. The CB2 receptor is of particular interest as it is intimately involved in immune homeostasis; the primary goal of these COVID-19 studies is to modulate the endocannabinoid system via targeted CB2 therapies to produce an immunosuppressant effect.4 CB2 activation, be it by means of THC or CBD agonism, may prove to be beneficial by inhibiting the cytokine influx.
Unfortunately, there is a general dearth of data on COVID-19–exposed cannabis users, whether the drug is consumed for medication or recreational purposes. It has been suggested that cannabis intake might contribute toward the development of a cough, complicating the overall clinical outcome for those infected with the virus. The presence of a cough, even in an otherwise asymptomatic individual, facilitates viral spread. As for those cannabis users experiencing COVID-19 symptomatology, they can expect rapid clinical deterioration, including pronounced fatigue and a change in mental status.
According to pulmonary specialists and representatives of the American Lung Association, recreational cannabis use may be associated with a bronchitis-like inflammation (comparable with chronic bronchitis/COPD for chronic users) of the airways, along the lines of cigarette smoking.5 As far as cannabis smokers are concerned, the rationale for lung irritation is believed to stem from the relatively large portion of unburnt plant content that is inhaled in a given joint. If there is a superimposed infection, as is the case with COVID-19, the patient may experience further risk of adverse respiratory effects. This serves as a diagnostic dilemma for physicians, especially when they encounter patients who recently started dabbling with cannabis as a means of placating themselves or because they’ve heard rumors that it will somehow protect them from COVID-19. The entire assessment plan is slowed down as a result of the confounding variable (onset of a cough), which may arise independently of COVID-19 in cannabis users. Vulnerable populations include smokers and those with COPD or asthma, as they are more likely to require ventilator assistance during the course of COVID-19 therapy.5 Asthmatics and COPD patients are prone to bronchospasms because of sensitive airways.
COVID-19 safety protocols for cannabis users
Because of increased risk of respiratory morbidity, clinicians advise that consumption of recreational cannabinoids be scaled back during the course of the pandemic. In light of conflicting news from several media outlets regarding the efficacy of cannabis intake with respect to COVID-19, preexisting users might unwittingly increase their consumption as a preemptive measure against being exposed to the infection. To prevent transmission among users, clinicians should discourage patients from sharing joints. This recommendation is thematically consistent with general precautionary measures about the dangers of sharing utensils, drinking cups/glasses, and so on, amid the pandemic.
Despite promising preliminary research results, CBD cannot be wholeheartedly recommended at this time; patients already on medically administered cannabinoids are urged to discuss the risk-benefit ratio with their respective health care clinicians. Cannabinoid therapies present a massive opportunity from the perspective of immunomodulation, especially when considering the prevalence of drug use. However, to improve clinical guidelines with respect to COVID-19 outcomes, it would be prudent to increase the overall volume of preclinical knowledge by gathering retrospective data (from case-control designs) and randomized prospective trials.
A more comprehensive list of advice from physicians concerning casual or chronic cannabis users may also include: adopting a dedicated delivery or dispensing system for cannabis products, making considerations for decontamination (i.e., disinfecting mouthpieces), ensuring cleansing precautions are maintained (washing thoroughly before and after use or procurement), switching to inhalation alternates (e.g., tinctures, edibles, and/or oils) to decrease further irritation to the lungs. For bong users, it is recommended that they apply rubbing alcohol to clean their device followed with a minute of air-drying.6
Conclusion
The literature from preclinical studies appears to largely favor the use of CBD, but there remains an element of uncertainty with respect to implementing cannabinoids for the treatment of coronavirus.
COVID-19 cannabinoid intervention is a hot topic with renewed interest from the industry and the public at large, but viral-focused therapies remain a relatively underused area worth exploring with case-control designs and randomized prospective trials. As cannabis legalization is picking up momentum across five additional states, the time is ripe to systematically investigate the therapeutic applications of the drug beyond merely preclinical data. Aside from educational reform initiatives, clinicians might proactively launch a platform that integrates telemedicine as well as digital apps, directly linking the patient to the clinician and monitoring the efficacy of program initiatives in real time.
References
1. Long A. Consumers’ cannabis buying patterns change markedly in wake of COVID-19 pandemic. Marijuana Business Daily. 2020 Sep 22. https://mjbizdaily.com/consumers-cannabis-buying-patterns-change-markedly-in-wake-of-covid-pandemic/.
2. Bures B. How the coronavirus pandemic is increasing global demand for marijuana. Chicago Tribune. 2020 Jul 1. https://www.chicagotribune.com/marijuana/sns-tft-coronavirus-increases-global-marijuana-demand-20200701-oygaxryb7vhcjfeu44cgacicaa-story.html.
3. Walters J. Marijuana and COVID-19: Top studies. CannaMD. 2020 Aug 19. https://www.cannamd.com/marijuana-covid-19-top-studies/.
4. El Biali M et al. Med Cannabis Cannabinoids. 2020 Aug 19. doi: 10.1159/000510799.
5. LaMotte S. “Smoking weed and coronavirus: Even occasional use raises risk of COVID-19 complications.” CNN Health. 2020 Apr 10. https://www.cnn.com/2020/04/10/health/smoking-weed-coronavirus-wellness/index.html
6. Yafai S and Etengoff S. The case for cannabis: Advising cannabis users about COVID-19. Emergency Medicine News. 2020 May 20;42(5B).
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Mr. Choudhry is a research assistant at the IMCHF. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. Dr. Islam, Mr. Choudhry, and Dr. Choudhry reported no relevant disclosures.
An intriguing pattern has emerged for cannabis enthusiasts as a result of lockdowns and statewide safety restrictions for COVID-19.
Consumers, as of late, have been shopping for larger marijuana baskets per trip to the dispensaries in various states, including California, Colorado, Nevada, and Washington, . However, they are also cutting down on the number of trips, perhaps, as a preventive measure to reduce the risk of exposure to coronavirus during this pandemic. Sales dipped considerably by the end of March only to experience a resurgence after the issuing of stimulus checks and unemployment benefits.
For the past few years, cannabis consumption remained steady while the industry continued to thrive with robust sales of the drug. It is a recession-proof phenomenon, therefore presenting a unique opportunity for clinicians with respect to patient education and individualized care.1
An unfortunate carryover of the governmental restrictions, self-isolation, and social estrangement is that consumers are now turning to the dark web as a source for continuous supply of cannabis. Prepandemic, according to the U.N. 2020 World Drug Report, there was already a 30% increase in sales of cannabis between 2009 and 2018. COVID-19 has fractured the drug’s supply chain and created an inescapable void that is being filled by drug traffickers.2 A clinical dilemma is posed when a user procures counterfeit cannabis or a drug batch with impurities.
Riding the cytokine storm
Cytokines are a host of proteins with designated regulatory and immune responses that play an instrumental role in cell signaling. The aptly named “cytokine storm” conjures up the image of an imperiled immune system spiraling out of control; it is, in fact, an extreme immune response that culminates into a massive influx of cytokines released into the bloodstream. Without the presence of an immunologic threat, cytokines are responsible for maintaining homeostasis and the functionality of immune cells. However, acute cytokine release (i.e., cytokine storm), as is the case with severe COVID-19, jeopardizes organ function (for example, interstitial lung disease) with clinical symptoms, such as fever, cough, dyspnea, and myalgia.
Benefits and drawbacks of immunosuppressive agents
To inhibit cytokine release (e.g., interleukin-6 cytokine levels), immunosuppressive agents such as tocilizumab have been leveraged to damper the body’s overactive inflammatory response to perceived immunologic stressors, in particular, COVID-19. While the aforementioned agent was remarkably effective with respect to lung consolidation clearance in most of the patients tested, a host of untoward effects prevent its general applicability and use. However, a team of researchers from the University of Nebraska, Omaha, with the Texas Biomedical Research Institute, San Antonio, might have stumbled upon a strategic workaround for mitigating the immune response.
They have proposed that cannabidiol (CBD) be used in lieu of other agents with potentially toxic effects. Animal and human trials have established that CBD confers a relatively high margin of safety coupled with favorable tolerance, providing a viable option for effectively targeting the inflammatory processes of SARS-CoV-2–based pulmonary disease. Furthermore, efficacy increased when CBD was combined with a terpene formulation, especially with respect to the more traditional steroid therapy.3
SARS-CoV-2 exhibits binding affinity for the ACE2 receptor, which is expressed in the lungs as well as other known predilection sites of infection. Ongoing studies attempt to modulate ACE2 expression, thereby eliminating its conspicuous role as “viral gateways,” perhaps even more so in patients with lung pathologies (e.g., people with chronic obstructive pulmonary disease [COPD] and smokers) as they already are prone to increased respiratory morbidity. CBD lacks tetrahydrocannabinol (THC), or the psychoactive component of cannabis sativa, rendering the agent to be particularly attractive from a therapeutic perspective. In addition to being devoid of abuse potential, CBD exhibits remarkable anti-inflammatory properties. It should be noted that considerable overlap exists between tobacco and cannabis users, and it is too early to determine the impact on COVID-19. As opposed to cannabis’s effect on ACE2 levels, smoking exhibits a proinflammatory role by up-regulating ACE2 expression.3 However, there are currently numerous conflicting reports in circulation about the positive effect of nicotine on COVID-19 outcome; confounding variables will need to be explored further in patients with a history of using nicotine and cannabis together.
From an immunologic perspective, the endocannabinoid system (ECS) plays an integral role in cell signaling by interacting with natural chemicals of the body, namely, cannabinoids with designated targets at the cannabinoid receptor 1 (CB1) and the CB2, respectively. The CB2 receptor is of particular interest as it is intimately involved in immune homeostasis; the primary goal of these COVID-19 studies is to modulate the endocannabinoid system via targeted CB2 therapies to produce an immunosuppressant effect.4 CB2 activation, be it by means of THC or CBD agonism, may prove to be beneficial by inhibiting the cytokine influx.
Unfortunately, there is a general dearth of data on COVID-19–exposed cannabis users, whether the drug is consumed for medication or recreational purposes. It has been suggested that cannabis intake might contribute toward the development of a cough, complicating the overall clinical outcome for those infected with the virus. The presence of a cough, even in an otherwise asymptomatic individual, facilitates viral spread. As for those cannabis users experiencing COVID-19 symptomatology, they can expect rapid clinical deterioration, including pronounced fatigue and a change in mental status.
According to pulmonary specialists and representatives of the American Lung Association, recreational cannabis use may be associated with a bronchitis-like inflammation (comparable with chronic bronchitis/COPD for chronic users) of the airways, along the lines of cigarette smoking.5 As far as cannabis smokers are concerned, the rationale for lung irritation is believed to stem from the relatively large portion of unburnt plant content that is inhaled in a given joint. If there is a superimposed infection, as is the case with COVID-19, the patient may experience further risk of adverse respiratory effects. This serves as a diagnostic dilemma for physicians, especially when they encounter patients who recently started dabbling with cannabis as a means of placating themselves or because they’ve heard rumors that it will somehow protect them from COVID-19. The entire assessment plan is slowed down as a result of the confounding variable (onset of a cough), which may arise independently of COVID-19 in cannabis users. Vulnerable populations include smokers and those with COPD or asthma, as they are more likely to require ventilator assistance during the course of COVID-19 therapy.5 Asthmatics and COPD patients are prone to bronchospasms because of sensitive airways.
COVID-19 safety protocols for cannabis users
Because of increased risk of respiratory morbidity, clinicians advise that consumption of recreational cannabinoids be scaled back during the course of the pandemic. In light of conflicting news from several media outlets regarding the efficacy of cannabis intake with respect to COVID-19, preexisting users might unwittingly increase their consumption as a preemptive measure against being exposed to the infection. To prevent transmission among users, clinicians should discourage patients from sharing joints. This recommendation is thematically consistent with general precautionary measures about the dangers of sharing utensils, drinking cups/glasses, and so on, amid the pandemic.
Despite promising preliminary research results, CBD cannot be wholeheartedly recommended at this time; patients already on medically administered cannabinoids are urged to discuss the risk-benefit ratio with their respective health care clinicians. Cannabinoid therapies present a massive opportunity from the perspective of immunomodulation, especially when considering the prevalence of drug use. However, to improve clinical guidelines with respect to COVID-19 outcomes, it would be prudent to increase the overall volume of preclinical knowledge by gathering retrospective data (from case-control designs) and randomized prospective trials.
A more comprehensive list of advice from physicians concerning casual or chronic cannabis users may also include: adopting a dedicated delivery or dispensing system for cannabis products, making considerations for decontamination (i.e., disinfecting mouthpieces), ensuring cleansing precautions are maintained (washing thoroughly before and after use or procurement), switching to inhalation alternates (e.g., tinctures, edibles, and/or oils) to decrease further irritation to the lungs. For bong users, it is recommended that they apply rubbing alcohol to clean their device followed with a minute of air-drying.6
Conclusion
The literature from preclinical studies appears to largely favor the use of CBD, but there remains an element of uncertainty with respect to implementing cannabinoids for the treatment of coronavirus.
COVID-19 cannabinoid intervention is a hot topic with renewed interest from the industry and the public at large, but viral-focused therapies remain a relatively underused area worth exploring with case-control designs and randomized prospective trials. As cannabis legalization is picking up momentum across five additional states, the time is ripe to systematically investigate the therapeutic applications of the drug beyond merely preclinical data. Aside from educational reform initiatives, clinicians might proactively launch a platform that integrates telemedicine as well as digital apps, directly linking the patient to the clinician and monitoring the efficacy of program initiatives in real time.
References
1. Long A. Consumers’ cannabis buying patterns change markedly in wake of COVID-19 pandemic. Marijuana Business Daily. 2020 Sep 22. https://mjbizdaily.com/consumers-cannabis-buying-patterns-change-markedly-in-wake-of-covid-pandemic/.
2. Bures B. How the coronavirus pandemic is increasing global demand for marijuana. Chicago Tribune. 2020 Jul 1. https://www.chicagotribune.com/marijuana/sns-tft-coronavirus-increases-global-marijuana-demand-20200701-oygaxryb7vhcjfeu44cgacicaa-story.html.
3. Walters J. Marijuana and COVID-19: Top studies. CannaMD. 2020 Aug 19. https://www.cannamd.com/marijuana-covid-19-top-studies/.
4. El Biali M et al. Med Cannabis Cannabinoids. 2020 Aug 19. doi: 10.1159/000510799.
5. LaMotte S. “Smoking weed and coronavirus: Even occasional use raises risk of COVID-19 complications.” CNN Health. 2020 Apr 10. https://www.cnn.com/2020/04/10/health/smoking-weed-coronavirus-wellness/index.html
6. Yafai S and Etengoff S. The case for cannabis: Advising cannabis users about COVID-19. Emergency Medicine News. 2020 May 20;42(5B).
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Mr. Choudhry is a research assistant at the IMCHF. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. Dr. Islam, Mr. Choudhry, and Dr. Choudhry reported no relevant disclosures.
Daily sunscreen use will prevent more melanoma deaths than early detection
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Lung cancer CT scan is chance for ‘opportunistic’ osteoporosis check
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
FDA approves first agent for PSMA-PET imaging in prostate cancer
A radioactive diagnostic agent has been approved by the U.S. Food and Drug Administration for use in patients with prostate cancer, but only for those treated at two institutions in California.
The product, Gallium 68 PSMA-11 (Ga 68 PSMA-11), is the first agent approved specifically for use in positron-emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA)–positive lesions in men with prostate cancer, the FDA noted.
This imaging approach can “detect whether or not the cancer has spread to other parts of the body,” commented Alex Gorovets, MD, acting deputy director of the Office of Specialty Medicine in the FDA’s Center for Drug Evaluation and Research.
Ga 68 PSMA-11 is indicated for use in patients with suspected prostate cancer metastasis whose conditions are potentially curable by surgery or radiotherapy and in patients with suspected prostate cancer recurrence, as determined on the basis of elevated serum prostate-specific antigen (PSA) levels.
Institutional use only
Ga 68 PSMA-11 has been approved for institutional use at the University of California, Los Angeles and the University of California, San Francisco under an academic new drug application (NDA).
The FDA approval was based partly on a clinical trial conducted by the UCSF and UCLA research teams on the effectiveness of PSMA-PET.
“It is rare for academic institutions to obtain FDA approval of a drug, and this unique collaboration has led to what is one of the first coapprovals of a drug at two institutions,” said Thomas Hope, MD, an associate professor at UCSF. “We hope that this first step will lead to a more widespread availability of this imaging test to men with prostate cancer throughout the country.”
Ga 68 PSMA-11 was developed outside the United States at the University of Heidelberg (Germany).
A commercial NDA from Telix Pharmaceuticals for TL591-CDx, a radiopharmaceutical cold kit for the preparation of Ga 68 PSMA-11 injection, is under consideration by the FDA.
The agency noted that two other PET diagnostic agents – fluciclovine F18 and choline C11 – are approved for prostate cancer imaging. However, they are only approved for use in patients with suspected cancer recurrence.
Trial results with PSMA-PET/CT
“PSMA-PET/CT is a novel molecular and functional imaging modality specific for prostate cancer cells that has good sensitivity and outstanding specificity in detecting metastasis,” commented T. Martin Ma, MD, PhD, of UCLA.
Dr. Ma presented a U.S. study on the technique at the recent annual meeting of the American Society for Radiation Oncology. That study showed that PSMA-PET/CT led to nodal upstaging in 19.7% of patients and metastasis upstaging in 9.4%.
He said these results were similar to those from the Australian proPSMA trial, which was published in The Lancet earlier this year. That trial found PSMA-PET/CT to be superior to conventional imaging with CT and bone scanning for primary staging of high-risk prostate cancer.
“These findings carry significant clinical implications and can affect treatment decision-making,” Dr. Ma commented.
“PSMA-PET has been a real game changer in high-risk prostate cancer and has implications in the various stages of prostate cancer management from diagnosis and staging to theranostics,” said Renu Eapen, MBBS, of Peter MacCallum Cancer Center, Melbourne, who was not involved in either study.
“PSMA-PET/CT has challenged conventional imaging in staging before curative-intent surgery or radiotherapy,” Dr. Eapen added.
The accuracy of PSMA-PET/CT was 27% higher than that of conventional imaging in the proPSMA trial, she noted in an interview last month. This superior accuracy can ultimately affect management. The imaging has additional benefits of lower radiation dose as well as reproducibility with high reporter agreement, potentially making it a “one-stop-shop” scan.
Trial results with Ga 68 PSMA-11
The safety and efficacy of Ga 68 PSMA-11 were evaluated in two prospective clinical trials with a total of 960 men with prostate cancer, each of whom received one injection of the product.
The first trial involved 325 patients with biopsy-proven prostate cancer who underwent PET/CT or PET/MRI scans performed with Ga 68 PSMA-11.
“These patients were candidates for surgical removal of the prostate gland and pelvic lymph nodes and were considered at higher risk for metastasis. Among the patients who proceeded to surgery, those with positive readings in the pelvic lymph nodes on Ga 68 PSMA-11 PET had a clinically important rate of metastatic cancer confirmed by surgical pathology,” the FDA noted.
“The availability of this information prior to treatment is expected to have important implications for patient care,” the FDA commented. “For example, it may spare certain patients from undergoing unnecessary surgery.”
The second trial enrolled 635 patients with rising serum PSA levels after initial prostate surgery or radiotherapy. All patients received a single Ga 68 PSMA-11 PET/CT scan or PET/MRI scan.
About three-quarters of patients (74%) had at least one positive lesion detected by Ga 68 PSMA-11 PET in at least one region – bone, prostate bed, pelvic lymph node, or extra-pelvic soft tissue.
“In patients with positive Ga 68 PSMA-11 PET readings who had correlative tissue pathology from biopsies, results from baseline or follow-up imaging by conventional methods, and serial PSA levels available for comparison, local recurrence or metastasis of prostate cancer was confirmed in an estimated 91% of cases,” the FDA noted.
“Thus, the second trial demonstrated that Ga 68 PSMA-11 PET can detect sites of disease in patients with biochemical evidence of recurrent prostate cancer, thereby providing important information that may impact the approach to therapy,” the agency added.
The FDA also noted that no serious adverse reactions were attributed to Ga 68 PSMA-11. The most common adverse reactions were nausea, diarrhea, and dizziness.
The FDA said there is a risk for misdiagnosis because Ga 68 PSMA-11 binding may occur in other types of cancer, and certain nonmalignant processes may lead to errors in interpreting images. In addition, there are radiation risks because Ga 68 PSMA-11 contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk for cancer.
A version of this article originally appeared on Medscape.com.
A radioactive diagnostic agent has been approved by the U.S. Food and Drug Administration for use in patients with prostate cancer, but only for those treated at two institutions in California.
The product, Gallium 68 PSMA-11 (Ga 68 PSMA-11), is the first agent approved specifically for use in positron-emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA)–positive lesions in men with prostate cancer, the FDA noted.
This imaging approach can “detect whether or not the cancer has spread to other parts of the body,” commented Alex Gorovets, MD, acting deputy director of the Office of Specialty Medicine in the FDA’s Center for Drug Evaluation and Research.
Ga 68 PSMA-11 is indicated for use in patients with suspected prostate cancer metastasis whose conditions are potentially curable by surgery or radiotherapy and in patients with suspected prostate cancer recurrence, as determined on the basis of elevated serum prostate-specific antigen (PSA) levels.
Institutional use only
Ga 68 PSMA-11 has been approved for institutional use at the University of California, Los Angeles and the University of California, San Francisco under an academic new drug application (NDA).
The FDA approval was based partly on a clinical trial conducted by the UCSF and UCLA research teams on the effectiveness of PSMA-PET.
“It is rare for academic institutions to obtain FDA approval of a drug, and this unique collaboration has led to what is one of the first coapprovals of a drug at two institutions,” said Thomas Hope, MD, an associate professor at UCSF. “We hope that this first step will lead to a more widespread availability of this imaging test to men with prostate cancer throughout the country.”
Ga 68 PSMA-11 was developed outside the United States at the University of Heidelberg (Germany).
A commercial NDA from Telix Pharmaceuticals for TL591-CDx, a radiopharmaceutical cold kit for the preparation of Ga 68 PSMA-11 injection, is under consideration by the FDA.
The agency noted that two other PET diagnostic agents – fluciclovine F18 and choline C11 – are approved for prostate cancer imaging. However, they are only approved for use in patients with suspected cancer recurrence.
Trial results with PSMA-PET/CT
“PSMA-PET/CT is a novel molecular and functional imaging modality specific for prostate cancer cells that has good sensitivity and outstanding specificity in detecting metastasis,” commented T. Martin Ma, MD, PhD, of UCLA.
Dr. Ma presented a U.S. study on the technique at the recent annual meeting of the American Society for Radiation Oncology. That study showed that PSMA-PET/CT led to nodal upstaging in 19.7% of patients and metastasis upstaging in 9.4%.
He said these results were similar to those from the Australian proPSMA trial, which was published in The Lancet earlier this year. That trial found PSMA-PET/CT to be superior to conventional imaging with CT and bone scanning for primary staging of high-risk prostate cancer.
“These findings carry significant clinical implications and can affect treatment decision-making,” Dr. Ma commented.
“PSMA-PET has been a real game changer in high-risk prostate cancer and has implications in the various stages of prostate cancer management from diagnosis and staging to theranostics,” said Renu Eapen, MBBS, of Peter MacCallum Cancer Center, Melbourne, who was not involved in either study.
“PSMA-PET/CT has challenged conventional imaging in staging before curative-intent surgery or radiotherapy,” Dr. Eapen added.
The accuracy of PSMA-PET/CT was 27% higher than that of conventional imaging in the proPSMA trial, she noted in an interview last month. This superior accuracy can ultimately affect management. The imaging has additional benefits of lower radiation dose as well as reproducibility with high reporter agreement, potentially making it a “one-stop-shop” scan.
Trial results with Ga 68 PSMA-11
The safety and efficacy of Ga 68 PSMA-11 were evaluated in two prospective clinical trials with a total of 960 men with prostate cancer, each of whom received one injection of the product.
The first trial involved 325 patients with biopsy-proven prostate cancer who underwent PET/CT or PET/MRI scans performed with Ga 68 PSMA-11.
“These patients were candidates for surgical removal of the prostate gland and pelvic lymph nodes and were considered at higher risk for metastasis. Among the patients who proceeded to surgery, those with positive readings in the pelvic lymph nodes on Ga 68 PSMA-11 PET had a clinically important rate of metastatic cancer confirmed by surgical pathology,” the FDA noted.
“The availability of this information prior to treatment is expected to have important implications for patient care,” the FDA commented. “For example, it may spare certain patients from undergoing unnecessary surgery.”
The second trial enrolled 635 patients with rising serum PSA levels after initial prostate surgery or radiotherapy. All patients received a single Ga 68 PSMA-11 PET/CT scan or PET/MRI scan.
About three-quarters of patients (74%) had at least one positive lesion detected by Ga 68 PSMA-11 PET in at least one region – bone, prostate bed, pelvic lymph node, or extra-pelvic soft tissue.
“In patients with positive Ga 68 PSMA-11 PET readings who had correlative tissue pathology from biopsies, results from baseline or follow-up imaging by conventional methods, and serial PSA levels available for comparison, local recurrence or metastasis of prostate cancer was confirmed in an estimated 91% of cases,” the FDA noted.
“Thus, the second trial demonstrated that Ga 68 PSMA-11 PET can detect sites of disease in patients with biochemical evidence of recurrent prostate cancer, thereby providing important information that may impact the approach to therapy,” the agency added.
The FDA also noted that no serious adverse reactions were attributed to Ga 68 PSMA-11. The most common adverse reactions were nausea, diarrhea, and dizziness.
The FDA said there is a risk for misdiagnosis because Ga 68 PSMA-11 binding may occur in other types of cancer, and certain nonmalignant processes may lead to errors in interpreting images. In addition, there are radiation risks because Ga 68 PSMA-11 contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk for cancer.
A version of this article originally appeared on Medscape.com.
A radioactive diagnostic agent has been approved by the U.S. Food and Drug Administration for use in patients with prostate cancer, but only for those treated at two institutions in California.
The product, Gallium 68 PSMA-11 (Ga 68 PSMA-11), is the first agent approved specifically for use in positron-emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA)–positive lesions in men with prostate cancer, the FDA noted.
This imaging approach can “detect whether or not the cancer has spread to other parts of the body,” commented Alex Gorovets, MD, acting deputy director of the Office of Specialty Medicine in the FDA’s Center for Drug Evaluation and Research.
Ga 68 PSMA-11 is indicated for use in patients with suspected prostate cancer metastasis whose conditions are potentially curable by surgery or radiotherapy and in patients with suspected prostate cancer recurrence, as determined on the basis of elevated serum prostate-specific antigen (PSA) levels.
Institutional use only
Ga 68 PSMA-11 has been approved for institutional use at the University of California, Los Angeles and the University of California, San Francisco under an academic new drug application (NDA).
The FDA approval was based partly on a clinical trial conducted by the UCSF and UCLA research teams on the effectiveness of PSMA-PET.
“It is rare for academic institutions to obtain FDA approval of a drug, and this unique collaboration has led to what is one of the first coapprovals of a drug at two institutions,” said Thomas Hope, MD, an associate professor at UCSF. “We hope that this first step will lead to a more widespread availability of this imaging test to men with prostate cancer throughout the country.”
Ga 68 PSMA-11 was developed outside the United States at the University of Heidelberg (Germany).
A commercial NDA from Telix Pharmaceuticals for TL591-CDx, a radiopharmaceutical cold kit for the preparation of Ga 68 PSMA-11 injection, is under consideration by the FDA.
The agency noted that two other PET diagnostic agents – fluciclovine F18 and choline C11 – are approved for prostate cancer imaging. However, they are only approved for use in patients with suspected cancer recurrence.
Trial results with PSMA-PET/CT
“PSMA-PET/CT is a novel molecular and functional imaging modality specific for prostate cancer cells that has good sensitivity and outstanding specificity in detecting metastasis,” commented T. Martin Ma, MD, PhD, of UCLA.
Dr. Ma presented a U.S. study on the technique at the recent annual meeting of the American Society for Radiation Oncology. That study showed that PSMA-PET/CT led to nodal upstaging in 19.7% of patients and metastasis upstaging in 9.4%.
He said these results were similar to those from the Australian proPSMA trial, which was published in The Lancet earlier this year. That trial found PSMA-PET/CT to be superior to conventional imaging with CT and bone scanning for primary staging of high-risk prostate cancer.
“These findings carry significant clinical implications and can affect treatment decision-making,” Dr. Ma commented.
“PSMA-PET has been a real game changer in high-risk prostate cancer and has implications in the various stages of prostate cancer management from diagnosis and staging to theranostics,” said Renu Eapen, MBBS, of Peter MacCallum Cancer Center, Melbourne, who was not involved in either study.
“PSMA-PET/CT has challenged conventional imaging in staging before curative-intent surgery or radiotherapy,” Dr. Eapen added.
The accuracy of PSMA-PET/CT was 27% higher than that of conventional imaging in the proPSMA trial, she noted in an interview last month. This superior accuracy can ultimately affect management. The imaging has additional benefits of lower radiation dose as well as reproducibility with high reporter agreement, potentially making it a “one-stop-shop” scan.
Trial results with Ga 68 PSMA-11
The safety and efficacy of Ga 68 PSMA-11 were evaluated in two prospective clinical trials with a total of 960 men with prostate cancer, each of whom received one injection of the product.
The first trial involved 325 patients with biopsy-proven prostate cancer who underwent PET/CT or PET/MRI scans performed with Ga 68 PSMA-11.
“These patients were candidates for surgical removal of the prostate gland and pelvic lymph nodes and were considered at higher risk for metastasis. Among the patients who proceeded to surgery, those with positive readings in the pelvic lymph nodes on Ga 68 PSMA-11 PET had a clinically important rate of metastatic cancer confirmed by surgical pathology,” the FDA noted.
“The availability of this information prior to treatment is expected to have important implications for patient care,” the FDA commented. “For example, it may spare certain patients from undergoing unnecessary surgery.”
The second trial enrolled 635 patients with rising serum PSA levels after initial prostate surgery or radiotherapy. All patients received a single Ga 68 PSMA-11 PET/CT scan or PET/MRI scan.
About three-quarters of patients (74%) had at least one positive lesion detected by Ga 68 PSMA-11 PET in at least one region – bone, prostate bed, pelvic lymph node, or extra-pelvic soft tissue.
“In patients with positive Ga 68 PSMA-11 PET readings who had correlative tissue pathology from biopsies, results from baseline or follow-up imaging by conventional methods, and serial PSA levels available for comparison, local recurrence or metastasis of prostate cancer was confirmed in an estimated 91% of cases,” the FDA noted.
“Thus, the second trial demonstrated that Ga 68 PSMA-11 PET can detect sites of disease in patients with biochemical evidence of recurrent prostate cancer, thereby providing important information that may impact the approach to therapy,” the agency added.
The FDA also noted that no serious adverse reactions were attributed to Ga 68 PSMA-11. The most common adverse reactions were nausea, diarrhea, and dizziness.
The FDA said there is a risk for misdiagnosis because Ga 68 PSMA-11 binding may occur in other types of cancer, and certain nonmalignant processes may lead to errors in interpreting images. In addition, there are radiation risks because Ga 68 PSMA-11 contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk for cancer.
A version of this article originally appeared on Medscape.com.
Pandemic increases need for home-based care with remote monitoring of patients
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
CRP testing in acute COPD exacerbations cuts antibiotic use without compromising outcomes
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
Three pillars of a successful coronavirus vaccine program in minorities
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
U.S. passes 1.3 million COVID-19 cases in children
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.
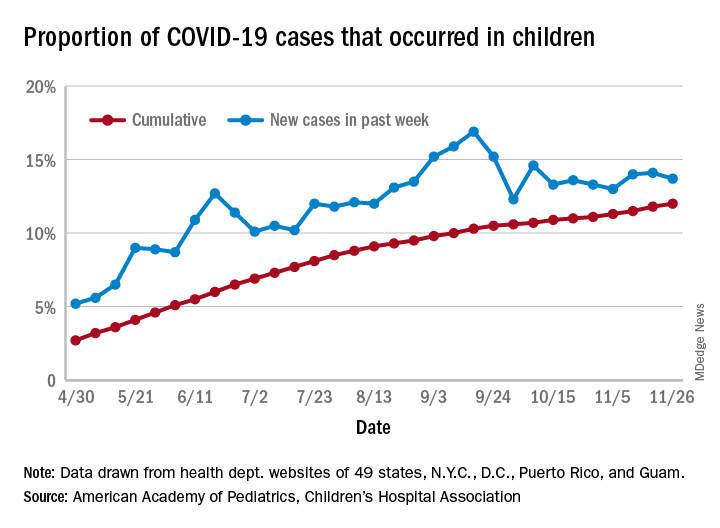
the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
Treating alcohol withdrawal syndrome: Going beyond ‘benzos’
Alcohol withdrawal syndrome (AWS) occurs when an individual who is alcohol-dependent suddenly stops or significantly reduces his/her alcohol intake.1 Symptoms of AWS, which can be fatal, include anxiety, restlessness, seizures, confusion, and delirium.1 Because benzodiazepines have been proven effective in improving symptoms of AWS, they are considered the first-line treatment, but they also carry the risk of abuse, psychomotor sedation, cognitive impairment, and interactions with alcohol.1
Non-benzodiazepine anticonvulsants (NBACs) such as valproic acid (VPA) and carbamazepine may offer benefit as alternatives or adjuncts to benzodiazepines.1 Many NBACs affect the functioning of glutamate and gamma-aminobutyric acid (GABA) neurotransmitters,2 which are particularly dysregulated in patients with AWS. Because NBACs help stabilize this imbalance, they may be useful for managing AWS and preventing relapse without the risks associated with benzodiazepines.2
Valproic acid: A better choice than carbamazepine
Compared with other NBACs, VPA and carbamazepine have been studied more extensively for treating patients with AWS, and their clinical effectiveness has been well documented.1 For mild-to-moderate AWS, VPA and carbamazepine may be as effective as benzodiazepines in reducing the severity of symptoms, and more potent for preventing withdrawal seizures.1
Increasing data suggests that compared with VPA, adjunctive treatment with carbamazepine for AWS may be more frequently associated with intolerable adverse effects such as ataxia, orthostatic hypotension, vertigo, nausea, and vomiting.1 The rapid onset of AWS requires rapid-acting pharmacotherapy.1 In attempting to rapidly achieve the desired plasma concentrations of carbamazepine, clinicians may risk inducing adverse effects. Compared with carbamazepine, VPA is associated with faster symptom resolution, a shorter duration of pharmacologic treatment, fewer transfers to the ICU, fewer withdrawal seizures, and a more favorable adverse effect profile.1 Likely due to its shorter half-life, VPA delivers its therapeutic effects without producing significant adverse effects.1
Early and aggressive treatment of AWS is needed to block kindling,3 which is characterized by the worsening of withdrawal symptoms each time an individual attempts to quit drinking alcohol. Compared with carbamazepine, VPA may be more helpful for blocking kindling.3
More data is needed
Due to ethical concerns, few studies have compared anticonvulsant medications with placebo for treating AWS.2 Most studies examining NBACs for AWS have either used the anticonvulsant as an adjunct to a benzodiazepine to examine improvement in withdrawal symptoms, or compared the anticonvulsant with placebo or another intervention to assess the amount of a benzodiazepine required for safe detoxification.2,4 Additionally, most studies examining NBACs have been short, and few followed patients after the active medication period, which limits our knowledge of the long-term effectiveness of NBACs.2 Before NBACs can replace benzodiazepines for managing AWS, further evidence from clinical trials is needed to assess their efficiency as a stand-alone treatment.
1. Maldonado JR. Novel algorithms for the prophylaxis and management of alcohol withdrawal syndromes–beyond benzodiazepines. Crit Care Clin. 2017;33(3):559-599.
2. Hammond CJ, Niciu MJ, Drew S, et al. Anticonvulsants for the treatment of alcohol withdrawal syndrome and alcohol use disorders. CNS Drugs. 2015;29(4):293-311.
3. Eyer F, Schreckenberg M, Hecht D, et al. Carbamazepine and valproate as adjuncts in the treatment of alcohol withdrawal syndrome: a retrospective cohort study. Alcohol Alcohol. 2011;46(2):177-184.
4. Guirguis E, Richardson J, Kuhn T, et al. Treatment of severe alcohol withdrawal: a focus on adjunctive agents. J Pharm Technol. 2017;33(5):204-212.
Alcohol withdrawal syndrome (AWS) occurs when an individual who is alcohol-dependent suddenly stops or significantly reduces his/her alcohol intake.1 Symptoms of AWS, which can be fatal, include anxiety, restlessness, seizures, confusion, and delirium.1 Because benzodiazepines have been proven effective in improving symptoms of AWS, they are considered the first-line treatment, but they also carry the risk of abuse, psychomotor sedation, cognitive impairment, and interactions with alcohol.1
Non-benzodiazepine anticonvulsants (NBACs) such as valproic acid (VPA) and carbamazepine may offer benefit as alternatives or adjuncts to benzodiazepines.1 Many NBACs affect the functioning of glutamate and gamma-aminobutyric acid (GABA) neurotransmitters,2 which are particularly dysregulated in patients with AWS. Because NBACs help stabilize this imbalance, they may be useful for managing AWS and preventing relapse without the risks associated with benzodiazepines.2
Valproic acid: A better choice than carbamazepine
Compared with other NBACs, VPA and carbamazepine have been studied more extensively for treating patients with AWS, and their clinical effectiveness has been well documented.1 For mild-to-moderate AWS, VPA and carbamazepine may be as effective as benzodiazepines in reducing the severity of symptoms, and more potent for preventing withdrawal seizures.1
Increasing data suggests that compared with VPA, adjunctive treatment with carbamazepine for AWS may be more frequently associated with intolerable adverse effects such as ataxia, orthostatic hypotension, vertigo, nausea, and vomiting.1 The rapid onset of AWS requires rapid-acting pharmacotherapy.1 In attempting to rapidly achieve the desired plasma concentrations of carbamazepine, clinicians may risk inducing adverse effects. Compared with carbamazepine, VPA is associated with faster symptom resolution, a shorter duration of pharmacologic treatment, fewer transfers to the ICU, fewer withdrawal seizures, and a more favorable adverse effect profile.1 Likely due to its shorter half-life, VPA delivers its therapeutic effects without producing significant adverse effects.1
Early and aggressive treatment of AWS is needed to block kindling,3 which is characterized by the worsening of withdrawal symptoms each time an individual attempts to quit drinking alcohol. Compared with carbamazepine, VPA may be more helpful for blocking kindling.3
More data is needed
Due to ethical concerns, few studies have compared anticonvulsant medications with placebo for treating AWS.2 Most studies examining NBACs for AWS have either used the anticonvulsant as an adjunct to a benzodiazepine to examine improvement in withdrawal symptoms, or compared the anticonvulsant with placebo or another intervention to assess the amount of a benzodiazepine required for safe detoxification.2,4 Additionally, most studies examining NBACs have been short, and few followed patients after the active medication period, which limits our knowledge of the long-term effectiveness of NBACs.2 Before NBACs can replace benzodiazepines for managing AWS, further evidence from clinical trials is needed to assess their efficiency as a stand-alone treatment.
Alcohol withdrawal syndrome (AWS) occurs when an individual who is alcohol-dependent suddenly stops or significantly reduces his/her alcohol intake.1 Symptoms of AWS, which can be fatal, include anxiety, restlessness, seizures, confusion, and delirium.1 Because benzodiazepines have been proven effective in improving symptoms of AWS, they are considered the first-line treatment, but they also carry the risk of abuse, psychomotor sedation, cognitive impairment, and interactions with alcohol.1
Non-benzodiazepine anticonvulsants (NBACs) such as valproic acid (VPA) and carbamazepine may offer benefit as alternatives or adjuncts to benzodiazepines.1 Many NBACs affect the functioning of glutamate and gamma-aminobutyric acid (GABA) neurotransmitters,2 which are particularly dysregulated in patients with AWS. Because NBACs help stabilize this imbalance, they may be useful for managing AWS and preventing relapse without the risks associated with benzodiazepines.2
Valproic acid: A better choice than carbamazepine
Compared with other NBACs, VPA and carbamazepine have been studied more extensively for treating patients with AWS, and their clinical effectiveness has been well documented.1 For mild-to-moderate AWS, VPA and carbamazepine may be as effective as benzodiazepines in reducing the severity of symptoms, and more potent for preventing withdrawal seizures.1
Increasing data suggests that compared with VPA, adjunctive treatment with carbamazepine for AWS may be more frequently associated with intolerable adverse effects such as ataxia, orthostatic hypotension, vertigo, nausea, and vomiting.1 The rapid onset of AWS requires rapid-acting pharmacotherapy.1 In attempting to rapidly achieve the desired plasma concentrations of carbamazepine, clinicians may risk inducing adverse effects. Compared with carbamazepine, VPA is associated with faster symptom resolution, a shorter duration of pharmacologic treatment, fewer transfers to the ICU, fewer withdrawal seizures, and a more favorable adverse effect profile.1 Likely due to its shorter half-life, VPA delivers its therapeutic effects without producing significant adverse effects.1
Early and aggressive treatment of AWS is needed to block kindling,3 which is characterized by the worsening of withdrawal symptoms each time an individual attempts to quit drinking alcohol. Compared with carbamazepine, VPA may be more helpful for blocking kindling.3
More data is needed
Due to ethical concerns, few studies have compared anticonvulsant medications with placebo for treating AWS.2 Most studies examining NBACs for AWS have either used the anticonvulsant as an adjunct to a benzodiazepine to examine improvement in withdrawal symptoms, or compared the anticonvulsant with placebo or another intervention to assess the amount of a benzodiazepine required for safe detoxification.2,4 Additionally, most studies examining NBACs have been short, and few followed patients after the active medication period, which limits our knowledge of the long-term effectiveness of NBACs.2 Before NBACs can replace benzodiazepines for managing AWS, further evidence from clinical trials is needed to assess their efficiency as a stand-alone treatment.
1. Maldonado JR. Novel algorithms for the prophylaxis and management of alcohol withdrawal syndromes–beyond benzodiazepines. Crit Care Clin. 2017;33(3):559-599.
2. Hammond CJ, Niciu MJ, Drew S, et al. Anticonvulsants for the treatment of alcohol withdrawal syndrome and alcohol use disorders. CNS Drugs. 2015;29(4):293-311.
3. Eyer F, Schreckenberg M, Hecht D, et al. Carbamazepine and valproate as adjuncts in the treatment of alcohol withdrawal syndrome: a retrospective cohort study. Alcohol Alcohol. 2011;46(2):177-184.
4. Guirguis E, Richardson J, Kuhn T, et al. Treatment of severe alcohol withdrawal: a focus on adjunctive agents. J Pharm Technol. 2017;33(5):204-212.
1. Maldonado JR. Novel algorithms for the prophylaxis and management of alcohol withdrawal syndromes–beyond benzodiazepines. Crit Care Clin. 2017;33(3):559-599.
2. Hammond CJ, Niciu MJ, Drew S, et al. Anticonvulsants for the treatment of alcohol withdrawal syndrome and alcohol use disorders. CNS Drugs. 2015;29(4):293-311.
3. Eyer F, Schreckenberg M, Hecht D, et al. Carbamazepine and valproate as adjuncts in the treatment of alcohol withdrawal syndrome: a retrospective cohort study. Alcohol Alcohol. 2011;46(2):177-184.
4. Guirguis E, Richardson J, Kuhn T, et al. Treatment of severe alcohol withdrawal: a focus on adjunctive agents. J Pharm Technol. 2017;33(5):204-212.