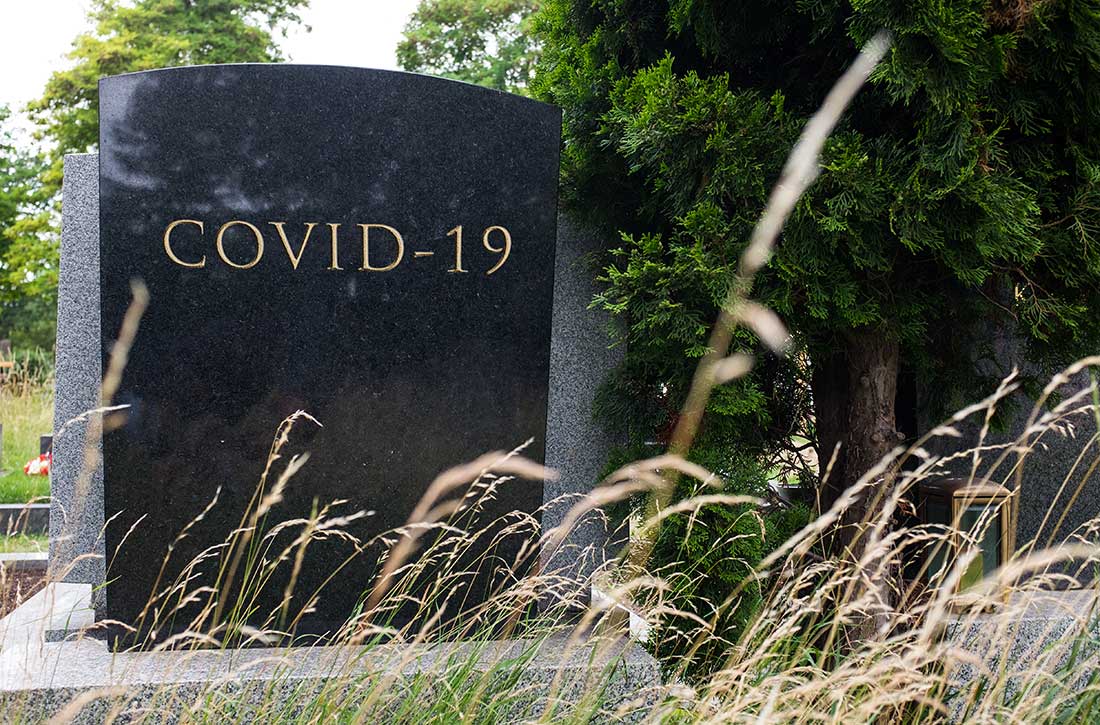User login
ACIP: Health workers, long-term care residents first tier for COVID-19 vaccine
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
Dilip V. Jeste, MD, on the state of psychiatry
Editor’s note: Psychiatry Leaders’ Perspectives is a new department in
In this first Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Dilip V. Jeste, MD. Dr. Jeste is Senior Associate Dean for Healthy Aging and Senior Care, Estelle and Edgar Levi Memorial Chair in Aging, Director of the Sam and Rose Stein Institute for Research on Aging, Distinguished Professor of Psychiatry and Neurosciences, University of California San Diego; and Co-Director of the UC San Diego-IBM Center on Artificial Intelligence for Healthy Living. His main areas of research include schizophrenia, neuropsychiatric interventions, and successful aging. He served as the 139th President of the American Psychiatric Association (APA) and also is a past president of the American Association for Geriatric Psychiatry, the West Coast College of Biological Psychiatry, and founding president of International College of Geriatric Psychoneuropharmacology.
Dr. Aftab: The focus of your term as president of the APA was on “positive psychiatry.” You are also one of the world’s foremost experts in this area. How successful have you been in your mission to promote positive psychiatry, and how has your message been received?
Dr. Jeste: Let me start with a little bit of background about why I got into positive psychiatry. As a geriatric psychiatrist, my research work has brought me face to face with the paradox of aging: although physical health declines with age, mental health and well-being improve on average. This is the case not just for individuals in the community but also for individuals with serious mental illnesses. That got me into thinking more and more about the ways in which we can bring positive change in the lives of patients. When I became the president of the APA, one of my main tasks was to finalize and publish the DSM-5, which rightly focuses on the disorders we treat, but it also provided me with an opportunity to highlight the side of psychiatry that focuses on the positive aspects of our own and our patients’ lives, such as wisdom, resilience, meaning, and social connectedness.
As is the case with any new idea, there is a lot of resistance in the beginning and this will always be the case. However, I would say that positive psychiatry has been received very well. We now have an APA Caucus and a World Psychiatric Association Section on positive psychiatry. Our book, Positive Psychiatry, turned out to be one of the best sellers for American Psychiatric Publishing! Every year, there are symposia on positive psychiatry and papers and books from other countries. Overall, the reception has been very promising.
Dr. Aftab: Thank you for this interesting background, Dr. Jeste. Now let me ask you about the current state of psychiatry. What do you see as some of the strengths of our profession?
Dr. Jeste: Psychiatry’s unique strength is our skill in promoting adaptive behavior change, with a focus on positive factors such as resilience, wisdom, optimism, social engagement, improved health, and longevity. If you look at the research literature, the effect sizes of factors such as optimism, resilience, and social engagement are equal to or greater than interventions such as statins, smoking cessation, and exercise. Cardiothoracic surgeons and radiologists can’t help people increase their resilience, optimism, and social engagement, but psychiatrists can. Behavior change is our expertise. When people are suicidal, we give them hope; we help depressed individuals become active, productive, and happy. We treat people with schizophrenia and bipolar disorder, reduce their psychopathological behaviors, and improve their everyday functioning.
Continue to: Dr. Aftab
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Jeste: Unfortunately, there are a lot of restrictions posed by the current reimbursement system. As a result, psychiatrists spend most of their time prescribing medications in clinical practice. I have nothing against psychopharmacology, but we also need to focus on important aspects of our lives, such as lifestyle, cognitive attitudes, self-care, and social engagement. We need to go beyond symptom reduction. A prominent example is loneliness, which is a major risk factor for morbidity and mortality; the treatment for loneliness is not increasing social network, it’s actually changing one’s perception of and ability to enhance appropriate socialization. Who can do that? Psychiatrists! But we don’t do that right now because the health insurance system doesn’t reimburse psychiatrists to do that.
Dr. Aftab: What is your perception of the threats that psychiatry faces? You had to fend off a variety of challenges during your year as APA president, such as issues surrounding revision of DSM-5. How has that experience shaped your assessment?
Dr. Jeste: I was honored to oversee the finalization and publication of DSM-5 as the president of the APA, even though I lost a lot of sleep working on it! What I found was that there was a lot of antagonism in the media, as well as among several advocacy groups, about the DSM. The misperception was that psychiatry and the APA were trying to expand diagnoses so that the drug companies could sell medications to more people, and psychiatry would benefit from this because of its relationship with the industry. That was actually not the case at all. What I tried to do was to understand where these groups were coming from, and to treat them as collaborators and partners, not as enemies. One thing I am particularly proud of is that we established the Summit Group for DSM-5, which brought together perspectives of the various stakeholders, and our communication both within and outside of the APA improved significantly. It’s gratifying to note that much of the controversy in the media died down after DSM-5 was published. The often-critical New York Times wrote that while DSM-5 is far from perfect, it is the best we have today clinically, and I’m very proud of the work we did on it.
Dr. Aftab: What sort of opportunities lie ahead for psychiatry? What do you envision for the future of the field?
Continue to: Dr. Jeste
Dr. Jeste: As a neuroscientist, I’m excited about the new developments in brain science. Our understanding of the neurobiologic basis of mental illnesses is slowly but surely increasing. I’m also very heartened by all the research going on with regard to the prevention of mental illnesses. I think we will be able to reduce the risk of many psychiatric disorders in the future. This is an exciting time for the field, and psychiatry is going to look very different 20 years from now!
Dr. Aftab: Some people think there’s a conflict between a neuroscientific and psychosocial understanding of psychiatry. How do you think the 2 relate to each other?
Dr. Jeste: The reality, I think, is that there is no conflict. Ultimately, the mind is a function of the brain, and the mind operates within a society. Neuroscientists are also realizing the importance of psychosocial aspects, and there is a growing social neuroscience, looking at the neurobiology of things such as loneliness, social isolation, and wisdom. The effects of psychosocial interventions such as meditation and long-term cognitive-behavioral therapy on the brain are now indisputable. I like to say that psychosocial interventions are often more biological in their effects than the drugs!
Dr. Aftab: Any words of wisdom for psychiatry trainees and early career psychiatrists?
Dr. Jeste: First of all, I congratulate them for going into psychiatry, which is rapidly advancing and is the field of the future. Looking at new developments, such as in artificial intelligence, I wish I could be a young person again just getting into psychiatry! The role of psychiatrists is also evolving, and psychiatrists will become leaders of multidisciplinary teams. I would advise trainees and early career psychiatrists not to get frustrated by issues such as insurance reimbursements; these obstacles will pass. Society is becoming far more conscious of the importance of mental health to our well-being. So I see a reason to be optimistic. I would also mention that the younger generation has a lot to teach the older generation while at the same time benefitting from the wisdom they have to offer. One of the best things we can promote is intergenerational activity, both within and outside of our profession.
Editor’s note: Psychiatry Leaders’ Perspectives is a new department in
In this first Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Dilip V. Jeste, MD. Dr. Jeste is Senior Associate Dean for Healthy Aging and Senior Care, Estelle and Edgar Levi Memorial Chair in Aging, Director of the Sam and Rose Stein Institute for Research on Aging, Distinguished Professor of Psychiatry and Neurosciences, University of California San Diego; and Co-Director of the UC San Diego-IBM Center on Artificial Intelligence for Healthy Living. His main areas of research include schizophrenia, neuropsychiatric interventions, and successful aging. He served as the 139th President of the American Psychiatric Association (APA) and also is a past president of the American Association for Geriatric Psychiatry, the West Coast College of Biological Psychiatry, and founding president of International College of Geriatric Psychoneuropharmacology.
Dr. Aftab: The focus of your term as president of the APA was on “positive psychiatry.” You are also one of the world’s foremost experts in this area. How successful have you been in your mission to promote positive psychiatry, and how has your message been received?
Dr. Jeste: Let me start with a little bit of background about why I got into positive psychiatry. As a geriatric psychiatrist, my research work has brought me face to face with the paradox of aging: although physical health declines with age, mental health and well-being improve on average. This is the case not just for individuals in the community but also for individuals with serious mental illnesses. That got me into thinking more and more about the ways in which we can bring positive change in the lives of patients. When I became the president of the APA, one of my main tasks was to finalize and publish the DSM-5, which rightly focuses on the disorders we treat, but it also provided me with an opportunity to highlight the side of psychiatry that focuses on the positive aspects of our own and our patients’ lives, such as wisdom, resilience, meaning, and social connectedness.
As is the case with any new idea, there is a lot of resistance in the beginning and this will always be the case. However, I would say that positive psychiatry has been received very well. We now have an APA Caucus and a World Psychiatric Association Section on positive psychiatry. Our book, Positive Psychiatry, turned out to be one of the best sellers for American Psychiatric Publishing! Every year, there are symposia on positive psychiatry and papers and books from other countries. Overall, the reception has been very promising.
Dr. Aftab: Thank you for this interesting background, Dr. Jeste. Now let me ask you about the current state of psychiatry. What do you see as some of the strengths of our profession?
Dr. Jeste: Psychiatry’s unique strength is our skill in promoting adaptive behavior change, with a focus on positive factors such as resilience, wisdom, optimism, social engagement, improved health, and longevity. If you look at the research literature, the effect sizes of factors such as optimism, resilience, and social engagement are equal to or greater than interventions such as statins, smoking cessation, and exercise. Cardiothoracic surgeons and radiologists can’t help people increase their resilience, optimism, and social engagement, but psychiatrists can. Behavior change is our expertise. When people are suicidal, we give them hope; we help depressed individuals become active, productive, and happy. We treat people with schizophrenia and bipolar disorder, reduce their psychopathological behaviors, and improve their everyday functioning.
Continue to: Dr. Aftab
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Jeste: Unfortunately, there are a lot of restrictions posed by the current reimbursement system. As a result, psychiatrists spend most of their time prescribing medications in clinical practice. I have nothing against psychopharmacology, but we also need to focus on important aspects of our lives, such as lifestyle, cognitive attitudes, self-care, and social engagement. We need to go beyond symptom reduction. A prominent example is loneliness, which is a major risk factor for morbidity and mortality; the treatment for loneliness is not increasing social network, it’s actually changing one’s perception of and ability to enhance appropriate socialization. Who can do that? Psychiatrists! But we don’t do that right now because the health insurance system doesn’t reimburse psychiatrists to do that.
Dr. Aftab: What is your perception of the threats that psychiatry faces? You had to fend off a variety of challenges during your year as APA president, such as issues surrounding revision of DSM-5. How has that experience shaped your assessment?
Dr. Jeste: I was honored to oversee the finalization and publication of DSM-5 as the president of the APA, even though I lost a lot of sleep working on it! What I found was that there was a lot of antagonism in the media, as well as among several advocacy groups, about the DSM. The misperception was that psychiatry and the APA were trying to expand diagnoses so that the drug companies could sell medications to more people, and psychiatry would benefit from this because of its relationship with the industry. That was actually not the case at all. What I tried to do was to understand where these groups were coming from, and to treat them as collaborators and partners, not as enemies. One thing I am particularly proud of is that we established the Summit Group for DSM-5, which brought together perspectives of the various stakeholders, and our communication both within and outside of the APA improved significantly. It’s gratifying to note that much of the controversy in the media died down after DSM-5 was published. The often-critical New York Times wrote that while DSM-5 is far from perfect, it is the best we have today clinically, and I’m very proud of the work we did on it.
Dr. Aftab: What sort of opportunities lie ahead for psychiatry? What do you envision for the future of the field?
Continue to: Dr. Jeste
Dr. Jeste: As a neuroscientist, I’m excited about the new developments in brain science. Our understanding of the neurobiologic basis of mental illnesses is slowly but surely increasing. I’m also very heartened by all the research going on with regard to the prevention of mental illnesses. I think we will be able to reduce the risk of many psychiatric disorders in the future. This is an exciting time for the field, and psychiatry is going to look very different 20 years from now!
Dr. Aftab: Some people think there’s a conflict between a neuroscientific and psychosocial understanding of psychiatry. How do you think the 2 relate to each other?
Dr. Jeste: The reality, I think, is that there is no conflict. Ultimately, the mind is a function of the brain, and the mind operates within a society. Neuroscientists are also realizing the importance of psychosocial aspects, and there is a growing social neuroscience, looking at the neurobiology of things such as loneliness, social isolation, and wisdom. The effects of psychosocial interventions such as meditation and long-term cognitive-behavioral therapy on the brain are now indisputable. I like to say that psychosocial interventions are often more biological in their effects than the drugs!
Dr. Aftab: Any words of wisdom for psychiatry trainees and early career psychiatrists?
Dr. Jeste: First of all, I congratulate them for going into psychiatry, which is rapidly advancing and is the field of the future. Looking at new developments, such as in artificial intelligence, I wish I could be a young person again just getting into psychiatry! The role of psychiatrists is also evolving, and psychiatrists will become leaders of multidisciplinary teams. I would advise trainees and early career psychiatrists not to get frustrated by issues such as insurance reimbursements; these obstacles will pass. Society is becoming far more conscious of the importance of mental health to our well-being. So I see a reason to be optimistic. I would also mention that the younger generation has a lot to teach the older generation while at the same time benefitting from the wisdom they have to offer. One of the best things we can promote is intergenerational activity, both within and outside of our profession.
Editor’s note: Psychiatry Leaders’ Perspectives is a new department in
In this first Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Dilip V. Jeste, MD. Dr. Jeste is Senior Associate Dean for Healthy Aging and Senior Care, Estelle and Edgar Levi Memorial Chair in Aging, Director of the Sam and Rose Stein Institute for Research on Aging, Distinguished Professor of Psychiatry and Neurosciences, University of California San Diego; and Co-Director of the UC San Diego-IBM Center on Artificial Intelligence for Healthy Living. His main areas of research include schizophrenia, neuropsychiatric interventions, and successful aging. He served as the 139th President of the American Psychiatric Association (APA) and also is a past president of the American Association for Geriatric Psychiatry, the West Coast College of Biological Psychiatry, and founding president of International College of Geriatric Psychoneuropharmacology.
Dr. Aftab: The focus of your term as president of the APA was on “positive psychiatry.” You are also one of the world’s foremost experts in this area. How successful have you been in your mission to promote positive psychiatry, and how has your message been received?
Dr. Jeste: Let me start with a little bit of background about why I got into positive psychiatry. As a geriatric psychiatrist, my research work has brought me face to face with the paradox of aging: although physical health declines with age, mental health and well-being improve on average. This is the case not just for individuals in the community but also for individuals with serious mental illnesses. That got me into thinking more and more about the ways in which we can bring positive change in the lives of patients. When I became the president of the APA, one of my main tasks was to finalize and publish the DSM-5, which rightly focuses on the disorders we treat, but it also provided me with an opportunity to highlight the side of psychiatry that focuses on the positive aspects of our own and our patients’ lives, such as wisdom, resilience, meaning, and social connectedness.
As is the case with any new idea, there is a lot of resistance in the beginning and this will always be the case. However, I would say that positive psychiatry has been received very well. We now have an APA Caucus and a World Psychiatric Association Section on positive psychiatry. Our book, Positive Psychiatry, turned out to be one of the best sellers for American Psychiatric Publishing! Every year, there are symposia on positive psychiatry and papers and books from other countries. Overall, the reception has been very promising.
Dr. Aftab: Thank you for this interesting background, Dr. Jeste. Now let me ask you about the current state of psychiatry. What do you see as some of the strengths of our profession?
Dr. Jeste: Psychiatry’s unique strength is our skill in promoting adaptive behavior change, with a focus on positive factors such as resilience, wisdom, optimism, social engagement, improved health, and longevity. If you look at the research literature, the effect sizes of factors such as optimism, resilience, and social engagement are equal to or greater than interventions such as statins, smoking cessation, and exercise. Cardiothoracic surgeons and radiologists can’t help people increase their resilience, optimism, and social engagement, but psychiatrists can. Behavior change is our expertise. When people are suicidal, we give them hope; we help depressed individuals become active, productive, and happy. We treat people with schizophrenia and bipolar disorder, reduce their psychopathological behaviors, and improve their everyday functioning.
Continue to: Dr. Aftab
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Jeste: Unfortunately, there are a lot of restrictions posed by the current reimbursement system. As a result, psychiatrists spend most of their time prescribing medications in clinical practice. I have nothing against psychopharmacology, but we also need to focus on important aspects of our lives, such as lifestyle, cognitive attitudes, self-care, and social engagement. We need to go beyond symptom reduction. A prominent example is loneliness, which is a major risk factor for morbidity and mortality; the treatment for loneliness is not increasing social network, it’s actually changing one’s perception of and ability to enhance appropriate socialization. Who can do that? Psychiatrists! But we don’t do that right now because the health insurance system doesn’t reimburse psychiatrists to do that.
Dr. Aftab: What is your perception of the threats that psychiatry faces? You had to fend off a variety of challenges during your year as APA president, such as issues surrounding revision of DSM-5. How has that experience shaped your assessment?
Dr. Jeste: I was honored to oversee the finalization and publication of DSM-5 as the president of the APA, even though I lost a lot of sleep working on it! What I found was that there was a lot of antagonism in the media, as well as among several advocacy groups, about the DSM. The misperception was that psychiatry and the APA were trying to expand diagnoses so that the drug companies could sell medications to more people, and psychiatry would benefit from this because of its relationship with the industry. That was actually not the case at all. What I tried to do was to understand where these groups were coming from, and to treat them as collaborators and partners, not as enemies. One thing I am particularly proud of is that we established the Summit Group for DSM-5, which brought together perspectives of the various stakeholders, and our communication both within and outside of the APA improved significantly. It’s gratifying to note that much of the controversy in the media died down after DSM-5 was published. The often-critical New York Times wrote that while DSM-5 is far from perfect, it is the best we have today clinically, and I’m very proud of the work we did on it.
Dr. Aftab: What sort of opportunities lie ahead for psychiatry? What do you envision for the future of the field?
Continue to: Dr. Jeste
Dr. Jeste: As a neuroscientist, I’m excited about the new developments in brain science. Our understanding of the neurobiologic basis of mental illnesses is slowly but surely increasing. I’m also very heartened by all the research going on with regard to the prevention of mental illnesses. I think we will be able to reduce the risk of many psychiatric disorders in the future. This is an exciting time for the field, and psychiatry is going to look very different 20 years from now!
Dr. Aftab: Some people think there’s a conflict between a neuroscientific and psychosocial understanding of psychiatry. How do you think the 2 relate to each other?
Dr. Jeste: The reality, I think, is that there is no conflict. Ultimately, the mind is a function of the brain, and the mind operates within a society. Neuroscientists are also realizing the importance of psychosocial aspects, and there is a growing social neuroscience, looking at the neurobiology of things such as loneliness, social isolation, and wisdom. The effects of psychosocial interventions such as meditation and long-term cognitive-behavioral therapy on the brain are now indisputable. I like to say that psychosocial interventions are often more biological in their effects than the drugs!
Dr. Aftab: Any words of wisdom for psychiatry trainees and early career psychiatrists?
Dr. Jeste: First of all, I congratulate them for going into psychiatry, which is rapidly advancing and is the field of the future. Looking at new developments, such as in artificial intelligence, I wish I could be a young person again just getting into psychiatry! The role of psychiatrists is also evolving, and psychiatrists will become leaders of multidisciplinary teams. I would advise trainees and early career psychiatrists not to get frustrated by issues such as insurance reimbursements; these obstacles will pass. Society is becoming far more conscious of the importance of mental health to our well-being. So I see a reason to be optimistic. I would also mention that the younger generation has a lot to teach the older generation while at the same time benefitting from the wisdom they have to offer. One of the best things we can promote is intergenerational activity, both within and outside of our profession.
First guidelines for keto diets in adults with epilepsy released
Just as in children with epilepsy, ketogenic diet therapies can be safe and effective in adults with epilepsy but should only be undertaken with the support of medical professionals trained in their use, the group said.
“Motivation is the key to successful ketogenic diet therapy adherence,” first author Mackenzie Cervenka, MD, director of the Adult Epilepsy Diet Center and associate professor of neurology at Johns Hopkins University, Baltimore, said in an interview.
“Patients who are autonomous require self-motivation and having a strong support structure is important as well. For those patients who are dependents, their caregivers need to be motivated to manage their diet,” said Dr. Cervenka.
The guidelines were published online Oct. 30 in Neurology Clinical Practice.
Novel in adult neurology
Ketogenic diet therapies are high-fat, low-carbohydrate, and adequate-protein diets that induce fat metabolism and ketone production. Despite its use as an effective antiseizure therapy since the 1920s, ketogenic diet therapies remain novel in adult neurology.
Furthermore, while there are established guidelines for ketogenic diet therapies to reduce seizures in children, there were no formal recommendations for adults, until now.
Drawing on the experience of experts at 20 centers using ketogenic diet therapies in more than 2,100 adults with epilepsy in 10 countries, Dr. Cervenka and an international team developed recommendations on use of ketogenic diet therapies in adults.
The panel noted, “with a relatively mild side effect profile and the potential to reduce seizures in nearly 60% of adults with drug-resistant epilepsy, ketogenic diet therapies should be part of the repertoire of available options.”
Ketogenic diet therapies are appropriate to offer to adults with seizure types and epilepsy syndromes for which these treatments are known to be effective in children, they said. These include tuberous sclerosis complex, Rett syndrome, Lennox-Gastaut syndrome, glucose transporter type 1 deficiency syndrome, genetic generalized epilepsies, and focal epilepsies caused by underlying migrational disorders and resistant to antiseizure medication.
However, adults with drug-resistant focal epilepsy should be offered surgical evaluation first, given the higher anticipated rate of seizure freedom via this route, the panel said.
A focus on compliance
Experts at nearly all of the centers report using two or more ketogenic diet therapies. Ninety percent use the modified Atkins diet, 84% use the classic ketogenic diet, and 63% use the modified ketogenic diet and/or low glycemic index treatment. More than half of the centers (58%) use medium-chain triglyceride oil in combination with another ketogenic diet therapy to boost ketone body production.
The most important factors influencing the choice of ketogenic diet therapy are ease of diet application for the patient (100%) and patient and/or caregiver preference, home setting, and mode of feeding (90% each).
The panel recommended that ketogenic diet therapies be tailored to fit the needs of the individual, taking into account his or her physical and mental characteristics, underlying medical conditions, food preferences, type and amount of support from family and others, level of self-sufficiency, feeding habits, and ease of following the diet.
“Most of the differences between the child and adult recommendations have to do with compliance. Often, it’s more of a challenge for adults than for children,” said Dr. Cervenka.
The panel recommended providing adult patients with recipe ideas, individualized training on the ketogenic diet lifestyle from a dietitian or nutritionist, and guidance for meal planning and preparation before starting the diet. This will provide the greatest likelihood of success, as patients often report difficulties coping with carbohydrate restriction.
“In pediatric practice, positive responders typically remain on a ketogenic diet therapy for 2 years before considering weaning. Ketogenic diet therapy in adults is not time-limited. However, a minimum of 3 months of ketogenic diet therapy is recommended before any judgment of response is made,” the panel advised.
The panel pointed out the absolute metabolic contraindications and cautions related to feeding difficulties, gastrointestinal dysfunction, and digestion remain the same for both children and adults. However, they added that a range of common adult conditions such as hyperlipidemia, heart disease, diabetes, low bone density, and pregnancy “bring additional consideration, caution, and monitoring to ketogenic diet therapy use.”
Beyond epilepsy
The guidelines also call for pre–ketogenic diet therapy biochemical studies to screen adults for preexisting abnormalities and establish a reference for comparing follow-up results after 3, 6, and 12 months, and then annually or as needed.
They also noted that metabolic studies such as urine organic acid and serum amino acid levels are generally not needed in adults unless there is a strong clinical suspicion for an underlying metabolic disorder.
Updated genetic evaluation may also be considered in adults with intellectual disability and epilepsy of unknown etiology. Serial bone mineral density scans may be obtained every 5 years.
The guidelines also call for ketone monitoring (blood beta-hydroxybutyrate or urine amino acids) during the early months of ketogenic diet therapy as an objective indication of compliance and biochemical response.
Dietary adjustments should focus on optimizing the treatment response, minimizing side effects, and maximizing sustainability.
Adults on a ketogenic diet therapy should also be advised to take multivitamin and mineral supplements and drink plenty of fluids.
The panel said emerging evidence also supports the use of ketogenic diet therapies in other adult neurologic disorders such as migraine, Parkinson’s disease, dementia, and multiple sclerosis.
However, the panel said further evidence is needed to guide recommendations on use of ketogenic diet therapies in other neurologic conditions.
The research had no targeted funding. Dr. Cervenka has reported receiving grants from Nutricia, Vitaflo, BrightFocus Foundation, and Army Research Laboratory; honoraria from the American Epilepsy Society, the Neurology Center, Epigenix, LivaNova, and Nutricia; royalties from Demos; and consulting for Nutricia, Glut1 Deficiency Foundation, and Sage Therapeutics. Disclosures for the other authors are listed in the article.
A version of this article originally appeared on Medscape.com.
Just as in children with epilepsy, ketogenic diet therapies can be safe and effective in adults with epilepsy but should only be undertaken with the support of medical professionals trained in their use, the group said.
“Motivation is the key to successful ketogenic diet therapy adherence,” first author Mackenzie Cervenka, MD, director of the Adult Epilepsy Diet Center and associate professor of neurology at Johns Hopkins University, Baltimore, said in an interview.
“Patients who are autonomous require self-motivation and having a strong support structure is important as well. For those patients who are dependents, their caregivers need to be motivated to manage their diet,” said Dr. Cervenka.
The guidelines were published online Oct. 30 in Neurology Clinical Practice.
Novel in adult neurology
Ketogenic diet therapies are high-fat, low-carbohydrate, and adequate-protein diets that induce fat metabolism and ketone production. Despite its use as an effective antiseizure therapy since the 1920s, ketogenic diet therapies remain novel in adult neurology.
Furthermore, while there are established guidelines for ketogenic diet therapies to reduce seizures in children, there were no formal recommendations for adults, until now.
Drawing on the experience of experts at 20 centers using ketogenic diet therapies in more than 2,100 adults with epilepsy in 10 countries, Dr. Cervenka and an international team developed recommendations on use of ketogenic diet therapies in adults.
The panel noted, “with a relatively mild side effect profile and the potential to reduce seizures in nearly 60% of adults with drug-resistant epilepsy, ketogenic diet therapies should be part of the repertoire of available options.”
Ketogenic diet therapies are appropriate to offer to adults with seizure types and epilepsy syndromes for which these treatments are known to be effective in children, they said. These include tuberous sclerosis complex, Rett syndrome, Lennox-Gastaut syndrome, glucose transporter type 1 deficiency syndrome, genetic generalized epilepsies, and focal epilepsies caused by underlying migrational disorders and resistant to antiseizure medication.
However, adults with drug-resistant focal epilepsy should be offered surgical evaluation first, given the higher anticipated rate of seizure freedom via this route, the panel said.
A focus on compliance
Experts at nearly all of the centers report using two or more ketogenic diet therapies. Ninety percent use the modified Atkins diet, 84% use the classic ketogenic diet, and 63% use the modified ketogenic diet and/or low glycemic index treatment. More than half of the centers (58%) use medium-chain triglyceride oil in combination with another ketogenic diet therapy to boost ketone body production.
The most important factors influencing the choice of ketogenic diet therapy are ease of diet application for the patient (100%) and patient and/or caregiver preference, home setting, and mode of feeding (90% each).
The panel recommended that ketogenic diet therapies be tailored to fit the needs of the individual, taking into account his or her physical and mental characteristics, underlying medical conditions, food preferences, type and amount of support from family and others, level of self-sufficiency, feeding habits, and ease of following the diet.
“Most of the differences between the child and adult recommendations have to do with compliance. Often, it’s more of a challenge for adults than for children,” said Dr. Cervenka.
The panel recommended providing adult patients with recipe ideas, individualized training on the ketogenic diet lifestyle from a dietitian or nutritionist, and guidance for meal planning and preparation before starting the diet. This will provide the greatest likelihood of success, as patients often report difficulties coping with carbohydrate restriction.
“In pediatric practice, positive responders typically remain on a ketogenic diet therapy for 2 years before considering weaning. Ketogenic diet therapy in adults is not time-limited. However, a minimum of 3 months of ketogenic diet therapy is recommended before any judgment of response is made,” the panel advised.
The panel pointed out the absolute metabolic contraindications and cautions related to feeding difficulties, gastrointestinal dysfunction, and digestion remain the same for both children and adults. However, they added that a range of common adult conditions such as hyperlipidemia, heart disease, diabetes, low bone density, and pregnancy “bring additional consideration, caution, and monitoring to ketogenic diet therapy use.”
Beyond epilepsy
The guidelines also call for pre–ketogenic diet therapy biochemical studies to screen adults for preexisting abnormalities and establish a reference for comparing follow-up results after 3, 6, and 12 months, and then annually or as needed.
They also noted that metabolic studies such as urine organic acid and serum amino acid levels are generally not needed in adults unless there is a strong clinical suspicion for an underlying metabolic disorder.
Updated genetic evaluation may also be considered in adults with intellectual disability and epilepsy of unknown etiology. Serial bone mineral density scans may be obtained every 5 years.
The guidelines also call for ketone monitoring (blood beta-hydroxybutyrate or urine amino acids) during the early months of ketogenic diet therapy as an objective indication of compliance and biochemical response.
Dietary adjustments should focus on optimizing the treatment response, minimizing side effects, and maximizing sustainability.
Adults on a ketogenic diet therapy should also be advised to take multivitamin and mineral supplements and drink plenty of fluids.
The panel said emerging evidence also supports the use of ketogenic diet therapies in other adult neurologic disorders such as migraine, Parkinson’s disease, dementia, and multiple sclerosis.
However, the panel said further evidence is needed to guide recommendations on use of ketogenic diet therapies in other neurologic conditions.
The research had no targeted funding. Dr. Cervenka has reported receiving grants from Nutricia, Vitaflo, BrightFocus Foundation, and Army Research Laboratory; honoraria from the American Epilepsy Society, the Neurology Center, Epigenix, LivaNova, and Nutricia; royalties from Demos; and consulting for Nutricia, Glut1 Deficiency Foundation, and Sage Therapeutics. Disclosures for the other authors are listed in the article.
A version of this article originally appeared on Medscape.com.
Just as in children with epilepsy, ketogenic diet therapies can be safe and effective in adults with epilepsy but should only be undertaken with the support of medical professionals trained in their use, the group said.
“Motivation is the key to successful ketogenic diet therapy adherence,” first author Mackenzie Cervenka, MD, director of the Adult Epilepsy Diet Center and associate professor of neurology at Johns Hopkins University, Baltimore, said in an interview.
“Patients who are autonomous require self-motivation and having a strong support structure is important as well. For those patients who are dependents, their caregivers need to be motivated to manage their diet,” said Dr. Cervenka.
The guidelines were published online Oct. 30 in Neurology Clinical Practice.
Novel in adult neurology
Ketogenic diet therapies are high-fat, low-carbohydrate, and adequate-protein diets that induce fat metabolism and ketone production. Despite its use as an effective antiseizure therapy since the 1920s, ketogenic diet therapies remain novel in adult neurology.
Furthermore, while there are established guidelines for ketogenic diet therapies to reduce seizures in children, there were no formal recommendations for adults, until now.
Drawing on the experience of experts at 20 centers using ketogenic diet therapies in more than 2,100 adults with epilepsy in 10 countries, Dr. Cervenka and an international team developed recommendations on use of ketogenic diet therapies in adults.
The panel noted, “with a relatively mild side effect profile and the potential to reduce seizures in nearly 60% of adults with drug-resistant epilepsy, ketogenic diet therapies should be part of the repertoire of available options.”
Ketogenic diet therapies are appropriate to offer to adults with seizure types and epilepsy syndromes for which these treatments are known to be effective in children, they said. These include tuberous sclerosis complex, Rett syndrome, Lennox-Gastaut syndrome, glucose transporter type 1 deficiency syndrome, genetic generalized epilepsies, and focal epilepsies caused by underlying migrational disorders and resistant to antiseizure medication.
However, adults with drug-resistant focal epilepsy should be offered surgical evaluation first, given the higher anticipated rate of seizure freedom via this route, the panel said.
A focus on compliance
Experts at nearly all of the centers report using two or more ketogenic diet therapies. Ninety percent use the modified Atkins diet, 84% use the classic ketogenic diet, and 63% use the modified ketogenic diet and/or low glycemic index treatment. More than half of the centers (58%) use medium-chain triglyceride oil in combination with another ketogenic diet therapy to boost ketone body production.
The most important factors influencing the choice of ketogenic diet therapy are ease of diet application for the patient (100%) and patient and/or caregiver preference, home setting, and mode of feeding (90% each).
The panel recommended that ketogenic diet therapies be tailored to fit the needs of the individual, taking into account his or her physical and mental characteristics, underlying medical conditions, food preferences, type and amount of support from family and others, level of self-sufficiency, feeding habits, and ease of following the diet.
“Most of the differences between the child and adult recommendations have to do with compliance. Often, it’s more of a challenge for adults than for children,” said Dr. Cervenka.
The panel recommended providing adult patients with recipe ideas, individualized training on the ketogenic diet lifestyle from a dietitian or nutritionist, and guidance for meal planning and preparation before starting the diet. This will provide the greatest likelihood of success, as patients often report difficulties coping with carbohydrate restriction.
“In pediatric practice, positive responders typically remain on a ketogenic diet therapy for 2 years before considering weaning. Ketogenic diet therapy in adults is not time-limited. However, a minimum of 3 months of ketogenic diet therapy is recommended before any judgment of response is made,” the panel advised.
The panel pointed out the absolute metabolic contraindications and cautions related to feeding difficulties, gastrointestinal dysfunction, and digestion remain the same for both children and adults. However, they added that a range of common adult conditions such as hyperlipidemia, heart disease, diabetes, low bone density, and pregnancy “bring additional consideration, caution, and monitoring to ketogenic diet therapy use.”
Beyond epilepsy
The guidelines also call for pre–ketogenic diet therapy biochemical studies to screen adults for preexisting abnormalities and establish a reference for comparing follow-up results after 3, 6, and 12 months, and then annually or as needed.
They also noted that metabolic studies such as urine organic acid and serum amino acid levels are generally not needed in adults unless there is a strong clinical suspicion for an underlying metabolic disorder.
Updated genetic evaluation may also be considered in adults with intellectual disability and epilepsy of unknown etiology. Serial bone mineral density scans may be obtained every 5 years.
The guidelines also call for ketone monitoring (blood beta-hydroxybutyrate or urine amino acids) during the early months of ketogenic diet therapy as an objective indication of compliance and biochemical response.
Dietary adjustments should focus on optimizing the treatment response, minimizing side effects, and maximizing sustainability.
Adults on a ketogenic diet therapy should also be advised to take multivitamin and mineral supplements and drink plenty of fluids.
The panel said emerging evidence also supports the use of ketogenic diet therapies in other adult neurologic disorders such as migraine, Parkinson’s disease, dementia, and multiple sclerosis.
However, the panel said further evidence is needed to guide recommendations on use of ketogenic diet therapies in other neurologic conditions.
The research had no targeted funding. Dr. Cervenka has reported receiving grants from Nutricia, Vitaflo, BrightFocus Foundation, and Army Research Laboratory; honoraria from the American Epilepsy Society, the Neurology Center, Epigenix, LivaNova, and Nutricia; royalties from Demos; and consulting for Nutricia, Glut1 Deficiency Foundation, and Sage Therapeutics. Disclosures for the other authors are listed in the article.
A version of this article originally appeared on Medscape.com.
2020: The year a viral asteroid collided with planet earth
Finally, 2020 is coming to an end, but the agony its viral pandemic inflicted on the entire world population will continue for a long time. And much as we would like to forget its damaging effects, it will surely be etched into our brains for the rest of our lives. The children who suffered the pain of the coronavirus disease 2019 (COVID-19) pandemic will endure its emotional scars for the rest of the 21st century.
Reading about the plagues of the past doesn’t come close to experiencing it and suffering through it. COVID-19 will continue to have ripple effects on every aspect of life on this planet, on individuals and on societies all over the world, especially on the biopsychosocial well-being of billions of humans around the globe.
Unprecedented disruptions
Think of the unprecedented disruptions inflicted by the trauma of the COVID-19 pandemic on our neural circuits. One of the wonders of the human brain is its continuous remodeling due to experiential neuroplasticity, and the formation of dendritic spines that immediately encode the memories of every experience. The turmoil of 2020 and its virulent pandemic will be forever etched into our collective brains, especially in our hippocampi and amygdalae. The impact on the developing brains of our children and grandchildren could be profound and may induce epigenetic changes that trigger psychopathology in the future.1,2
As with the dinosaurs, the 2020 pandemic is like a “viral asteroid” that left devastation on our social fabric and psychological well-being in its wake. We now have deep empathy with our 1918 ancestors and their tribulations, although so far, in the United States the proportion of people infected with COVID-19 (3% as of mid-November 20203) is dwarfed by the proportion infected with the influenza virus a century ago (30%). As of mid-November 2020, the number of global COVID-19 deaths (1.3 million3) was a tiny fraction of the 1918 influenza pandemic deaths (50 million worldwide and 675,000 in the United States4). Amazingly, researchers did not even know whether the killer germ was a virus or a bacterium until 1930, and it then took another 75 years to decode the genome of the influenza virus in 2005. In contrast, it took only a few short weeks to decode the genome of the virus that causes COVID-19 (severe acute respiratory syndrome-related coronavirus 2), and to begin developing multiple vaccines “at warp speed.” No vaccine or therapies were ever developed for victims of the 1918 pandemic.
An abundance of articles has been published about the pandemic since it ambushed us early in 2020, including many in
Most psychiatrists are familiar with the Holmes and Rahe Stress Scale,22 which contains 43 life events that cumulatively can progressively increase the odds of physical illness. It is likely that most of the world’s population will score very high on the Holmes and Rahe Stress Scale, which would predict an increased risk of medical illness, even after the pandemic subsides.
Exacerbating the situation is that hospitals and clinics had to shut down most of their operations to focus their resources on treating patients with COVID-19 in ICUs. This halted all routine screenings for cancer and heart, kidney, liver, lung, or brain diseases. In addition, diagnostic or therapeutic procedures such as endoscopies, colonoscopies, angiograms, or biopsies abruptly stopped, resulting in a surge of non–COVID-19 medical disorders and mortality as reported in several articles across many specialties.23 Going forward, in addition to COVID-19 morbidity and mortality, there is a significant likelihood of an increase in myriad medical disorders. The COVID-19 pandemic is obviously inflicting both direct and indirect casualties as it stretches into the next year and perhaps longer. The only hope for the community of nations is the rapid arrival of evidence-based treatments and vaccine(s).
Continue to: A progression of relentless stress
A progression of relentless stress
At the core of this pandemic is relentless stress. When it began in early 2020, the pandemic ignited an acute stress reaction due to the fear of death and the oddness of being isolated at home. Aggravating the acute stress was the realization that life as we knew it suddenly disappeared and all business or social activities had come to a screeching halt. It was almost surreal when streets usually bustling with human activity (such as Times Square in New York or Michigan Avenue in Chicago) became completely deserted and eerily silent. In addition, more stress was generated from watching television or scrolling through social media and being inundated with morbid and frightening news and updates about the number of individuals who became infected or died, and the official projections of tens of thousands or even hundreds of thousands of fatalities. Further intensifying the stress was hearing that there was a shortage of personal protective equipment (even masks), a lack of ventilators, and the absence of any medications to fight the overwhelming viral infection. Especially stressed were the front-line physicians and nurses, who heroically transcended their fears to save their patients’ lives. The sight of refrigerated trucks serving as temporary morgues outside hospital doors was chilling. The world became a macabre place where people died in hospitals without any relative to hold their hands or comfort them, and then were buried quickly without any formal funerals due to mandatory social distancing. The inability of families to grieve for their loved ones added another poignant layer of sadness and distress to the survivors who were unable to bid their loved ones goodbye. This was a jarring example of adding insult to injury.
With the protraction of the exceptional changes imposed by the pandemic, the acute stress reaction transmuted into posttraumatic stress disorder (PTSD) on a wide scale. Millions of previously healthy individuals began to succumb to the symptoms of PTSD (irritability, hypervigilance, intrusive thoughts, avoidance, insomnia, and bad dreams). The heaviest burden was inflicted on our patients, across all ages, with preexisting psychiatric conditions, who comprise approximately 25% of the population per the classic Epidemiological Catchment Area (ECA) study.24 These vulnerable patients, whom we see in our clinics and hospitals every day, had a significant exacerbation of their psychopathology, including anxiety, depression, psychosis, binge eating disorder, obsessive-compulsive disorder, alcohol and substance use disorders, child abuse, and intimate partner violence.25,26 The saving grace was the rapid adoption of telepsychiatry, which our psychiatric patients rapidly accepted. Many of them found it more convenient than dressing and driving and parking at the clinic. It also enabled psychiatrists to obtain useful collateral information from family members or partners.
If something good comes from this catastrophic social stress that emotionally hobbled the entire population, it would be the dilution of the stigma of mental illness because everyone has become more empathic due to their personal experience. Optimistically, this may also help expedite true health care parity for psychiatric brain disorders. And perhaps the government may see the need to train more psychiatrists and fund a higher number of residency stipends to all training programs.
Quo vadis COVID-19?
So, looking through the dense fog of the pandemic fatigue, what will 2021 bring us? Will waves of COVID-19 lead to pandemic exhaustion? Will the frayed public mental health care system be able to handle the surge of frayed nerves? Will social distancing intensify the widespread emotional disquietude? Will the children be able to manifest resilience and avoid disabling psychiatric disorders? Will the survivors of COVID-19 infections suffer from post–COVD-19 neuropsychiatric and other medical sequelae? Will efficacious therapies and vaccines emerge to blunt the spread of the virus? Will we all be able to gather in stadiums and arenas to enjoy sporting events, shows, and concerts? Will eating at our favorite restaurants become routine again? Will engaged couples be able to organize well-attended weddings and receptions? Will airplanes and hotels be fully booked again? Importantly, will all children and college students be able to resume their education in person and socialize ad lib? Will we be able to shed our masks and hug each other hello and goodbye? Will scientific journals and social media cover a wide array of topics again as before? Will the number of deaths dwindle to zero, and will we return to worrying mainly about the usual seasonal flu? Will everyone be able to leave home and go to work again?
I hope that the thick dust of this 2020 viral asteroid will settle in 2021, and that “normalcy” is eventually restored to our lives, allowing us to deal with other ongoing stresses such as social unrest and political hyperpartisanship.
1. Baumeister D, Akhtar R, Ciufolini S, et al. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 2016;21(5):642-649.
2. Zatti C, Rosa V, Barros A, et al. Childhood trauma and suicide attempt: a meta-analysis of longitudinal studies from the last decade. Psychiatry Res. 2017;256:353-358.
3. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/. Accessed November 11, 2020.
4. Centers for Disease Control and Prevention. 1918 Pandemic. https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html. Accessed November 4, 2020.
5. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
6. Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-33.
7. Joshi KG. Taking care of ourselves during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):46-47.
8. Frank B, Peterson T, Gupta S, et al. Telepsychiatry: what you need to know. Current Psychiatry. 2020;19(6):16-23.
9. Chahal K. Neuropsychiatric manifestations of COVID-19. Current Psychiatry. 2020;19(6):31-33.
10. Arbuck D. Changes in patient behavior during COVID-19: what I’ve observed. Current Psychiatry. 2020;19(6):46-47.
11. Joshi KG. Telepsychiatry during COVID-19: understanding the rules. Current Psychiatry. 2020;19(6):e12-e14.
12. Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
13. Brooks V. COVID-19’s effects on emergency psychiatry. Current Psychiatry. 2020;19(7):33-36,38-39.
14. Desarbo JR, DeSarbo L. Anorexia nervosa and COVID-19. Current Psychiatry. 2020;19(8):23-28.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-27,33-39.
16. Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
17. Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
18. Lodhi S, Marett C. Using seclusion to prevent COVID-19 transmission on inpatient psychiatry units. Current Psychiatry. 2020;19(11):37-41,53.
19. Nasrallah HA. COVID-19 and the precipitous dismantlement of societal norms. Current Psychiatry. 2020;19(7):12-14,16-17.
20. Nasrallah HA. The cataclysmic COVID-19 pandemic: THIS CHANGES EVERYTHING! Current Psychiatry. 2020;19(5):7-8,16.
21. Nasrallah HA. During a viral pandemic, anxiety is endemic: the psychiatric aspects of COVID-19. Current Psychiatry. 2020;19(4):e3-e5.
22. Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967;11(2):213-218.
23. Berkwits M, Flanagin A, Bauchner H, et al. The COVID-19 pandemic and the JAMA Network. JAMA. 2020;324(12):1159-1160.
24. Robins LN, Regier DA, eds. Psychiatric disorders in America. The Epidemiologic Catchment Area study. New York, NY: The Free Press; 1991.
25. Meninger KA. Psychosis associated with influenza. I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
26. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494.
Finally, 2020 is coming to an end, but the agony its viral pandemic inflicted on the entire world population will continue for a long time. And much as we would like to forget its damaging effects, it will surely be etched into our brains for the rest of our lives. The children who suffered the pain of the coronavirus disease 2019 (COVID-19) pandemic will endure its emotional scars for the rest of the 21st century.
Reading about the plagues of the past doesn’t come close to experiencing it and suffering through it. COVID-19 will continue to have ripple effects on every aspect of life on this planet, on individuals and on societies all over the world, especially on the biopsychosocial well-being of billions of humans around the globe.
Unprecedented disruptions
Think of the unprecedented disruptions inflicted by the trauma of the COVID-19 pandemic on our neural circuits. One of the wonders of the human brain is its continuous remodeling due to experiential neuroplasticity, and the formation of dendritic spines that immediately encode the memories of every experience. The turmoil of 2020 and its virulent pandemic will be forever etched into our collective brains, especially in our hippocampi and amygdalae. The impact on the developing brains of our children and grandchildren could be profound and may induce epigenetic changes that trigger psychopathology in the future.1,2
As with the dinosaurs, the 2020 pandemic is like a “viral asteroid” that left devastation on our social fabric and psychological well-being in its wake. We now have deep empathy with our 1918 ancestors and their tribulations, although so far, in the United States the proportion of people infected with COVID-19 (3% as of mid-November 20203) is dwarfed by the proportion infected with the influenza virus a century ago (30%). As of mid-November 2020, the number of global COVID-19 deaths (1.3 million3) was a tiny fraction of the 1918 influenza pandemic deaths (50 million worldwide and 675,000 in the United States4). Amazingly, researchers did not even know whether the killer germ was a virus or a bacterium until 1930, and it then took another 75 years to decode the genome of the influenza virus in 2005. In contrast, it took only a few short weeks to decode the genome of the virus that causes COVID-19 (severe acute respiratory syndrome-related coronavirus 2), and to begin developing multiple vaccines “at warp speed.” No vaccine or therapies were ever developed for victims of the 1918 pandemic.
An abundance of articles has been published about the pandemic since it ambushed us early in 2020, including many in

Most psychiatrists are familiar with the Holmes and Rahe Stress Scale,22 which contains 43 life events that cumulatively can progressively increase the odds of physical illness. It is likely that most of the world’s population will score very high on the Holmes and Rahe Stress Scale, which would predict an increased risk of medical illness, even after the pandemic subsides.
Exacerbating the situation is that hospitals and clinics had to shut down most of their operations to focus their resources on treating patients with COVID-19 in ICUs. This halted all routine screenings for cancer and heart, kidney, liver, lung, or brain diseases. In addition, diagnostic or therapeutic procedures such as endoscopies, colonoscopies, angiograms, or biopsies abruptly stopped, resulting in a surge of non–COVID-19 medical disorders and mortality as reported in several articles across many specialties.23 Going forward, in addition to COVID-19 morbidity and mortality, there is a significant likelihood of an increase in myriad medical disorders. The COVID-19 pandemic is obviously inflicting both direct and indirect casualties as it stretches into the next year and perhaps longer. The only hope for the community of nations is the rapid arrival of evidence-based treatments and vaccine(s).
Continue to: A progression of relentless stress
A progression of relentless stress
At the core of this pandemic is relentless stress. When it began in early 2020, the pandemic ignited an acute stress reaction due to the fear of death and the oddness of being isolated at home. Aggravating the acute stress was the realization that life as we knew it suddenly disappeared and all business or social activities had come to a screeching halt. It was almost surreal when streets usually bustling with human activity (such as Times Square in New York or Michigan Avenue in Chicago) became completely deserted and eerily silent. In addition, more stress was generated from watching television or scrolling through social media and being inundated with morbid and frightening news and updates about the number of individuals who became infected or died, and the official projections of tens of thousands or even hundreds of thousands of fatalities. Further intensifying the stress was hearing that there was a shortage of personal protective equipment (even masks), a lack of ventilators, and the absence of any medications to fight the overwhelming viral infection. Especially stressed were the front-line physicians and nurses, who heroically transcended their fears to save their patients’ lives. The sight of refrigerated trucks serving as temporary morgues outside hospital doors was chilling. The world became a macabre place where people died in hospitals without any relative to hold their hands or comfort them, and then were buried quickly without any formal funerals due to mandatory social distancing. The inability of families to grieve for their loved ones added another poignant layer of sadness and distress to the survivors who were unable to bid their loved ones goodbye. This was a jarring example of adding insult to injury.
With the protraction of the exceptional changes imposed by the pandemic, the acute stress reaction transmuted into posttraumatic stress disorder (PTSD) on a wide scale. Millions of previously healthy individuals began to succumb to the symptoms of PTSD (irritability, hypervigilance, intrusive thoughts, avoidance, insomnia, and bad dreams). The heaviest burden was inflicted on our patients, across all ages, with preexisting psychiatric conditions, who comprise approximately 25% of the population per the classic Epidemiological Catchment Area (ECA) study.24 These vulnerable patients, whom we see in our clinics and hospitals every day, had a significant exacerbation of their psychopathology, including anxiety, depression, psychosis, binge eating disorder, obsessive-compulsive disorder, alcohol and substance use disorders, child abuse, and intimate partner violence.25,26 The saving grace was the rapid adoption of telepsychiatry, which our psychiatric patients rapidly accepted. Many of them found it more convenient than dressing and driving and parking at the clinic. It also enabled psychiatrists to obtain useful collateral information from family members or partners.
If something good comes from this catastrophic social stress that emotionally hobbled the entire population, it would be the dilution of the stigma of mental illness because everyone has become more empathic due to their personal experience. Optimistically, this may also help expedite true health care parity for psychiatric brain disorders. And perhaps the government may see the need to train more psychiatrists and fund a higher number of residency stipends to all training programs.
Quo vadis COVID-19?
So, looking through the dense fog of the pandemic fatigue, what will 2021 bring us? Will waves of COVID-19 lead to pandemic exhaustion? Will the frayed public mental health care system be able to handle the surge of frayed nerves? Will social distancing intensify the widespread emotional disquietude? Will the children be able to manifest resilience and avoid disabling psychiatric disorders? Will the survivors of COVID-19 infections suffer from post–COVD-19 neuropsychiatric and other medical sequelae? Will efficacious therapies and vaccines emerge to blunt the spread of the virus? Will we all be able to gather in stadiums and arenas to enjoy sporting events, shows, and concerts? Will eating at our favorite restaurants become routine again? Will engaged couples be able to organize well-attended weddings and receptions? Will airplanes and hotels be fully booked again? Importantly, will all children and college students be able to resume their education in person and socialize ad lib? Will we be able to shed our masks and hug each other hello and goodbye? Will scientific journals and social media cover a wide array of topics again as before? Will the number of deaths dwindle to zero, and will we return to worrying mainly about the usual seasonal flu? Will everyone be able to leave home and go to work again?
I hope that the thick dust of this 2020 viral asteroid will settle in 2021, and that “normalcy” is eventually restored to our lives, allowing us to deal with other ongoing stresses such as social unrest and political hyperpartisanship.
Finally, 2020 is coming to an end, but the agony its viral pandemic inflicted on the entire world population will continue for a long time. And much as we would like to forget its damaging effects, it will surely be etched into our brains for the rest of our lives. The children who suffered the pain of the coronavirus disease 2019 (COVID-19) pandemic will endure its emotional scars for the rest of the 21st century.
Reading about the plagues of the past doesn’t come close to experiencing it and suffering through it. COVID-19 will continue to have ripple effects on every aspect of life on this planet, on individuals and on societies all over the world, especially on the biopsychosocial well-being of billions of humans around the globe.
Unprecedented disruptions
Think of the unprecedented disruptions inflicted by the trauma of the COVID-19 pandemic on our neural circuits. One of the wonders of the human brain is its continuous remodeling due to experiential neuroplasticity, and the formation of dendritic spines that immediately encode the memories of every experience. The turmoil of 2020 and its virulent pandemic will be forever etched into our collective brains, especially in our hippocampi and amygdalae. The impact on the developing brains of our children and grandchildren could be profound and may induce epigenetic changes that trigger psychopathology in the future.1,2
As with the dinosaurs, the 2020 pandemic is like a “viral asteroid” that left devastation on our social fabric and psychological well-being in its wake. We now have deep empathy with our 1918 ancestors and their tribulations, although so far, in the United States the proportion of people infected with COVID-19 (3% as of mid-November 20203) is dwarfed by the proportion infected with the influenza virus a century ago (30%). As of mid-November 2020, the number of global COVID-19 deaths (1.3 million3) was a tiny fraction of the 1918 influenza pandemic deaths (50 million worldwide and 675,000 in the United States4). Amazingly, researchers did not even know whether the killer germ was a virus or a bacterium until 1930, and it then took another 75 years to decode the genome of the influenza virus in 2005. In contrast, it took only a few short weeks to decode the genome of the virus that causes COVID-19 (severe acute respiratory syndrome-related coronavirus 2), and to begin developing multiple vaccines “at warp speed.” No vaccine or therapies were ever developed for victims of the 1918 pandemic.
An abundance of articles has been published about the pandemic since it ambushed us early in 2020, including many in

Most psychiatrists are familiar with the Holmes and Rahe Stress Scale,22 which contains 43 life events that cumulatively can progressively increase the odds of physical illness. It is likely that most of the world’s population will score very high on the Holmes and Rahe Stress Scale, which would predict an increased risk of medical illness, even after the pandemic subsides.
Exacerbating the situation is that hospitals and clinics had to shut down most of their operations to focus their resources on treating patients with COVID-19 in ICUs. This halted all routine screenings for cancer and heart, kidney, liver, lung, or brain diseases. In addition, diagnostic or therapeutic procedures such as endoscopies, colonoscopies, angiograms, or biopsies abruptly stopped, resulting in a surge of non–COVID-19 medical disorders and mortality as reported in several articles across many specialties.23 Going forward, in addition to COVID-19 morbidity and mortality, there is a significant likelihood of an increase in myriad medical disorders. The COVID-19 pandemic is obviously inflicting both direct and indirect casualties as it stretches into the next year and perhaps longer. The only hope for the community of nations is the rapid arrival of evidence-based treatments and vaccine(s).
Continue to: A progression of relentless stress
A progression of relentless stress
At the core of this pandemic is relentless stress. When it began in early 2020, the pandemic ignited an acute stress reaction due to the fear of death and the oddness of being isolated at home. Aggravating the acute stress was the realization that life as we knew it suddenly disappeared and all business or social activities had come to a screeching halt. It was almost surreal when streets usually bustling with human activity (such as Times Square in New York or Michigan Avenue in Chicago) became completely deserted and eerily silent. In addition, more stress was generated from watching television or scrolling through social media and being inundated with morbid and frightening news and updates about the number of individuals who became infected or died, and the official projections of tens of thousands or even hundreds of thousands of fatalities. Further intensifying the stress was hearing that there was a shortage of personal protective equipment (even masks), a lack of ventilators, and the absence of any medications to fight the overwhelming viral infection. Especially stressed were the front-line physicians and nurses, who heroically transcended their fears to save their patients’ lives. The sight of refrigerated trucks serving as temporary morgues outside hospital doors was chilling. The world became a macabre place where people died in hospitals without any relative to hold their hands or comfort them, and then were buried quickly without any formal funerals due to mandatory social distancing. The inability of families to grieve for their loved ones added another poignant layer of sadness and distress to the survivors who were unable to bid their loved ones goodbye. This was a jarring example of adding insult to injury.
With the protraction of the exceptional changes imposed by the pandemic, the acute stress reaction transmuted into posttraumatic stress disorder (PTSD) on a wide scale. Millions of previously healthy individuals began to succumb to the symptoms of PTSD (irritability, hypervigilance, intrusive thoughts, avoidance, insomnia, and bad dreams). The heaviest burden was inflicted on our patients, across all ages, with preexisting psychiatric conditions, who comprise approximately 25% of the population per the classic Epidemiological Catchment Area (ECA) study.24 These vulnerable patients, whom we see in our clinics and hospitals every day, had a significant exacerbation of their psychopathology, including anxiety, depression, psychosis, binge eating disorder, obsessive-compulsive disorder, alcohol and substance use disorders, child abuse, and intimate partner violence.25,26 The saving grace was the rapid adoption of telepsychiatry, which our psychiatric patients rapidly accepted. Many of them found it more convenient than dressing and driving and parking at the clinic. It also enabled psychiatrists to obtain useful collateral information from family members or partners.
If something good comes from this catastrophic social stress that emotionally hobbled the entire population, it would be the dilution of the stigma of mental illness because everyone has become more empathic due to their personal experience. Optimistically, this may also help expedite true health care parity for psychiatric brain disorders. And perhaps the government may see the need to train more psychiatrists and fund a higher number of residency stipends to all training programs.
Quo vadis COVID-19?
So, looking through the dense fog of the pandemic fatigue, what will 2021 bring us? Will waves of COVID-19 lead to pandemic exhaustion? Will the frayed public mental health care system be able to handle the surge of frayed nerves? Will social distancing intensify the widespread emotional disquietude? Will the children be able to manifest resilience and avoid disabling psychiatric disorders? Will the survivors of COVID-19 infections suffer from post–COVD-19 neuropsychiatric and other medical sequelae? Will efficacious therapies and vaccines emerge to blunt the spread of the virus? Will we all be able to gather in stadiums and arenas to enjoy sporting events, shows, and concerts? Will eating at our favorite restaurants become routine again? Will engaged couples be able to organize well-attended weddings and receptions? Will airplanes and hotels be fully booked again? Importantly, will all children and college students be able to resume their education in person and socialize ad lib? Will we be able to shed our masks and hug each other hello and goodbye? Will scientific journals and social media cover a wide array of topics again as before? Will the number of deaths dwindle to zero, and will we return to worrying mainly about the usual seasonal flu? Will everyone be able to leave home and go to work again?
I hope that the thick dust of this 2020 viral asteroid will settle in 2021, and that “normalcy” is eventually restored to our lives, allowing us to deal with other ongoing stresses such as social unrest and political hyperpartisanship.
1. Baumeister D, Akhtar R, Ciufolini S, et al. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 2016;21(5):642-649.
2. Zatti C, Rosa V, Barros A, et al. Childhood trauma and suicide attempt: a meta-analysis of longitudinal studies from the last decade. Psychiatry Res. 2017;256:353-358.
3. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/. Accessed November 11, 2020.
4. Centers for Disease Control and Prevention. 1918 Pandemic. https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html. Accessed November 4, 2020.
5. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
6. Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-33.
7. Joshi KG. Taking care of ourselves during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):46-47.
8. Frank B, Peterson T, Gupta S, et al. Telepsychiatry: what you need to know. Current Psychiatry. 2020;19(6):16-23.
9. Chahal K. Neuropsychiatric manifestations of COVID-19. Current Psychiatry. 2020;19(6):31-33.
10. Arbuck D. Changes in patient behavior during COVID-19: what I’ve observed. Current Psychiatry. 2020;19(6):46-47.
11. Joshi KG. Telepsychiatry during COVID-19: understanding the rules. Current Psychiatry. 2020;19(6):e12-e14.
12. Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
13. Brooks V. COVID-19’s effects on emergency psychiatry. Current Psychiatry. 2020;19(7):33-36,38-39.
14. Desarbo JR, DeSarbo L. Anorexia nervosa and COVID-19. Current Psychiatry. 2020;19(8):23-28.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-27,33-39.
16. Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
17. Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
18. Lodhi S, Marett C. Using seclusion to prevent COVID-19 transmission on inpatient psychiatry units. Current Psychiatry. 2020;19(11):37-41,53.
19. Nasrallah HA. COVID-19 and the precipitous dismantlement of societal norms. Current Psychiatry. 2020;19(7):12-14,16-17.
20. Nasrallah HA. The cataclysmic COVID-19 pandemic: THIS CHANGES EVERYTHING! Current Psychiatry. 2020;19(5):7-8,16.
21. Nasrallah HA. During a viral pandemic, anxiety is endemic: the psychiatric aspects of COVID-19. Current Psychiatry. 2020;19(4):e3-e5.
22. Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967;11(2):213-218.
23. Berkwits M, Flanagin A, Bauchner H, et al. The COVID-19 pandemic and the JAMA Network. JAMA. 2020;324(12):1159-1160.
24. Robins LN, Regier DA, eds. Psychiatric disorders in America. The Epidemiologic Catchment Area study. New York, NY: The Free Press; 1991.
25. Meninger KA. Psychosis associated with influenza. I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
26. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494.
1. Baumeister D, Akhtar R, Ciufolini S, et al. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 2016;21(5):642-649.
2. Zatti C, Rosa V, Barros A, et al. Childhood trauma and suicide attempt: a meta-analysis of longitudinal studies from the last decade. Psychiatry Res. 2017;256:353-358.
3. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/. Accessed November 11, 2020.
4. Centers for Disease Control and Prevention. 1918 Pandemic. https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html. Accessed November 4, 2020.
5. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
6. Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-33.
7. Joshi KG. Taking care of ourselves during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):46-47.
8. Frank B, Peterson T, Gupta S, et al. Telepsychiatry: what you need to know. Current Psychiatry. 2020;19(6):16-23.
9. Chahal K. Neuropsychiatric manifestations of COVID-19. Current Psychiatry. 2020;19(6):31-33.
10. Arbuck D. Changes in patient behavior during COVID-19: what I’ve observed. Current Psychiatry. 2020;19(6):46-47.
11. Joshi KG. Telepsychiatry during COVID-19: understanding the rules. Current Psychiatry. 2020;19(6):e12-e14.
12. Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
13. Brooks V. COVID-19’s effects on emergency psychiatry. Current Psychiatry. 2020;19(7):33-36,38-39.
14. Desarbo JR, DeSarbo L. Anorexia nervosa and COVID-19. Current Psychiatry. 2020;19(8):23-28.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-27,33-39.
16. Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
17. Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
18. Lodhi S, Marett C. Using seclusion to prevent COVID-19 transmission on inpatient psychiatry units. Current Psychiatry. 2020;19(11):37-41,53.
19. Nasrallah HA. COVID-19 and the precipitous dismantlement of societal norms. Current Psychiatry. 2020;19(7):12-14,16-17.
20. Nasrallah HA. The cataclysmic COVID-19 pandemic: THIS CHANGES EVERYTHING! Current Psychiatry. 2020;19(5):7-8,16.
21. Nasrallah HA. During a viral pandemic, anxiety is endemic: the psychiatric aspects of COVID-19. Current Psychiatry. 2020;19(4):e3-e5.
22. Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967;11(2):213-218.
23. Berkwits M, Flanagin A, Bauchner H, et al. The COVID-19 pandemic and the JAMA Network. JAMA. 2020;324(12):1159-1160.
24. Robins LN, Regier DA, eds. Psychiatric disorders in America. The Epidemiologic Catchment Area study. New York, NY: The Free Press; 1991.
25. Meninger KA. Psychosis associated with influenza. I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
26. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494.
Two-layer vaginal cuff closure may protect against laparoscopic hysterectomy complications
A two-layer vaginal cuff closure during total laparoscopic hysterectomy is associated with fewer postoperative complications, compared with a standard one-layer closure, according to a retrospective study of approximately 3,000 patients.
The difference is driven by fewer vaginal cuff complications among patients whose surgeons used the two-layer technique, said Ann Peters, MD, of Magee-Womens Hospital at the University of Pittsburgh Medical Center.
In light of these findings, Dr. Peters switched to using a two-layer closure. More surgeons may adopt this method, she said at the annual meeting sponsored by AAGL, held virtually this year.
Modifiable factors
Complications after total laparoscopic hysterectomy may be associated with modifiable surgical risk factors such as surgical volume, expertise, and suture material. The method of vaginal cuff closure also plays an important role, but few studies have compared multilayer and single-layer vaginal cuff closure, Dr. Peters said.
To investigate this question, Dr. Peters and colleagues analyzed data from 2,973 women who underwent total laparoscopic hysterectomy for benign indications during a 6-year period at their institution.
The analysis included 1,760 patients (59%) who underwent single-layer closure and 1,213 (41%) who underwent two-layer closure. The closure method was a matter of surgeon preference. Aside from the closure technique, other aspects of the surgeries were standardized.
The primary outcome was the rate of 30-day postoperative complications. Secondary outcomes included vaginal cuff complications during 6 months of follow-up.
The groups generally had similar baseline characteristics, although patients in the two-layer group had lower body mass index and were less likely to use tobacco.
Intraoperative complications and postoperative readmissions did not differ between the groups. The rate of postoperative complications, however, was lower in the two-layer group: 3.5% versus 5.6%. Likewise, the rate of vaginal cuff complications was lower in the two-layer group: 0.9% versus 2.5%.
No instances of vaginal cuff dehiscence or mucosal separation occurred in the two-layer group, whereas 12 cases of dehiscence and 4 cases of mucosal separation occurred in the one-layer group.
Although the study is limited by its retrospective design, the surgeons had similar training and many variables, including the sutures used, were equal or standardized, Dr. Peters noted.
Avoiding rare complications
Grace M. Janik, MD, of Reproductive Specialty Center in Milwaukee, has long theorized that two-layer closure may be beneficial. This study provides data to support that theory, Dr. Janik said in a discussion following the research presentation.
Given that hysterectomy is a common procedure, “any optimization ... has implications for a large number of women,” Dr. Janik said. Although rare outcomes such as dehiscence are difficult to study, the large number of patients in this analysis allowed the investigators to detect differences between the groups.
Studies of vaginal cuff closure have yielded mixed results. For example, various studies have suggested that laparoscopic closure may be inferior to, equal to, or superior to vaginal closure. Together, the findings indicate that “what we are doing is probably more important than the route,” said Dr. Janik.
Along with multilayer closure, the use of delayed absorbable sutures and adequate tissue bites are other factors that may lead to fewer complications, Dr. Janik noted.
Dr. Peters and Dr. Janik had no relevant financial disclosures. A study coauthor is a consultant for Medtronic and Olympus. The statistical analysis was supported by the National Institutes of Health.
SOURCE: Ali R et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.603.
A two-layer vaginal cuff closure during total laparoscopic hysterectomy is associated with fewer postoperative complications, compared with a standard one-layer closure, according to a retrospective study of approximately 3,000 patients.
The difference is driven by fewer vaginal cuff complications among patients whose surgeons used the two-layer technique, said Ann Peters, MD, of Magee-Womens Hospital at the University of Pittsburgh Medical Center.
In light of these findings, Dr. Peters switched to using a two-layer closure. More surgeons may adopt this method, she said at the annual meeting sponsored by AAGL, held virtually this year.
Modifiable factors
Complications after total laparoscopic hysterectomy may be associated with modifiable surgical risk factors such as surgical volume, expertise, and suture material. The method of vaginal cuff closure also plays an important role, but few studies have compared multilayer and single-layer vaginal cuff closure, Dr. Peters said.
To investigate this question, Dr. Peters and colleagues analyzed data from 2,973 women who underwent total laparoscopic hysterectomy for benign indications during a 6-year period at their institution.
The analysis included 1,760 patients (59%) who underwent single-layer closure and 1,213 (41%) who underwent two-layer closure. The closure method was a matter of surgeon preference. Aside from the closure technique, other aspects of the surgeries were standardized.
The primary outcome was the rate of 30-day postoperative complications. Secondary outcomes included vaginal cuff complications during 6 months of follow-up.
The groups generally had similar baseline characteristics, although patients in the two-layer group had lower body mass index and were less likely to use tobacco.
Intraoperative complications and postoperative readmissions did not differ between the groups. The rate of postoperative complications, however, was lower in the two-layer group: 3.5% versus 5.6%. Likewise, the rate of vaginal cuff complications was lower in the two-layer group: 0.9% versus 2.5%.
No instances of vaginal cuff dehiscence or mucosal separation occurred in the two-layer group, whereas 12 cases of dehiscence and 4 cases of mucosal separation occurred in the one-layer group.
Although the study is limited by its retrospective design, the surgeons had similar training and many variables, including the sutures used, were equal or standardized, Dr. Peters noted.
Avoiding rare complications
Grace M. Janik, MD, of Reproductive Specialty Center in Milwaukee, has long theorized that two-layer closure may be beneficial. This study provides data to support that theory, Dr. Janik said in a discussion following the research presentation.
Given that hysterectomy is a common procedure, “any optimization ... has implications for a large number of women,” Dr. Janik said. Although rare outcomes such as dehiscence are difficult to study, the large number of patients in this analysis allowed the investigators to detect differences between the groups.
Studies of vaginal cuff closure have yielded mixed results. For example, various studies have suggested that laparoscopic closure may be inferior to, equal to, or superior to vaginal closure. Together, the findings indicate that “what we are doing is probably more important than the route,” said Dr. Janik.
Along with multilayer closure, the use of delayed absorbable sutures and adequate tissue bites are other factors that may lead to fewer complications, Dr. Janik noted.
Dr. Peters and Dr. Janik had no relevant financial disclosures. A study coauthor is a consultant for Medtronic and Olympus. The statistical analysis was supported by the National Institutes of Health.
SOURCE: Ali R et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.603.
A two-layer vaginal cuff closure during total laparoscopic hysterectomy is associated with fewer postoperative complications, compared with a standard one-layer closure, according to a retrospective study of approximately 3,000 patients.
The difference is driven by fewer vaginal cuff complications among patients whose surgeons used the two-layer technique, said Ann Peters, MD, of Magee-Womens Hospital at the University of Pittsburgh Medical Center.
In light of these findings, Dr. Peters switched to using a two-layer closure. More surgeons may adopt this method, she said at the annual meeting sponsored by AAGL, held virtually this year.
Modifiable factors
Complications after total laparoscopic hysterectomy may be associated with modifiable surgical risk factors such as surgical volume, expertise, and suture material. The method of vaginal cuff closure also plays an important role, but few studies have compared multilayer and single-layer vaginal cuff closure, Dr. Peters said.
To investigate this question, Dr. Peters and colleagues analyzed data from 2,973 women who underwent total laparoscopic hysterectomy for benign indications during a 6-year period at their institution.
The analysis included 1,760 patients (59%) who underwent single-layer closure and 1,213 (41%) who underwent two-layer closure. The closure method was a matter of surgeon preference. Aside from the closure technique, other aspects of the surgeries were standardized.
The primary outcome was the rate of 30-day postoperative complications. Secondary outcomes included vaginal cuff complications during 6 months of follow-up.
The groups generally had similar baseline characteristics, although patients in the two-layer group had lower body mass index and were less likely to use tobacco.
Intraoperative complications and postoperative readmissions did not differ between the groups. The rate of postoperative complications, however, was lower in the two-layer group: 3.5% versus 5.6%. Likewise, the rate of vaginal cuff complications was lower in the two-layer group: 0.9% versus 2.5%.
No instances of vaginal cuff dehiscence or mucosal separation occurred in the two-layer group, whereas 12 cases of dehiscence and 4 cases of mucosal separation occurred in the one-layer group.
Although the study is limited by its retrospective design, the surgeons had similar training and many variables, including the sutures used, were equal or standardized, Dr. Peters noted.
Avoiding rare complications
Grace M. Janik, MD, of Reproductive Specialty Center in Milwaukee, has long theorized that two-layer closure may be beneficial. This study provides data to support that theory, Dr. Janik said in a discussion following the research presentation.
Given that hysterectomy is a common procedure, “any optimization ... has implications for a large number of women,” Dr. Janik said. Although rare outcomes such as dehiscence are difficult to study, the large number of patients in this analysis allowed the investigators to detect differences between the groups.
Studies of vaginal cuff closure have yielded mixed results. For example, various studies have suggested that laparoscopic closure may be inferior to, equal to, or superior to vaginal closure. Together, the findings indicate that “what we are doing is probably more important than the route,” said Dr. Janik.
Along with multilayer closure, the use of delayed absorbable sutures and adequate tissue bites are other factors that may lead to fewer complications, Dr. Janik noted.
Dr. Peters and Dr. Janik had no relevant financial disclosures. A study coauthor is a consultant for Medtronic and Olympus. The statistical analysis was supported by the National Institutes of Health.
SOURCE: Ali R et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.603.
FROM AAGL GLOBAL CONGRESS
My journey with mental illness
I am a retired advanced practice psychiatric nurse who has lived and worked on “both sides of the door.” This wording is paraphrased from psychologist and therapist Lauren Slater, PhD, who wrote about a time she went to McLean Hospital in Belmont, Massachusetts, as a therapist after staying there as a patient years earlier: “And now I am standing on the other—the wrong, I mean the right side of the door and I ring the buzzer.”1 Here I tell my story of the physical and emotional effects of my mental illness and treatment.
Onset of bipolar disorder. My bipolar illness started with a bout of depression in 1963 at age 13, which resulted in a low-key summer of often staying inside. I received no medication, and no one sent me for evaluation. In the fall, I went back to school and finished the year without incident. I continued as a quiet, shy kid through high school in the late 1960s. In my senior year, I decided to take an overload of difficult courses and run on the varsity cross-country team. The amount and intensity of these activities were too much. This resulted in my first manic episode, which started during a weekend visit to a college I hoped to attend. I became excitable, grandiose, and had delusions. A day later, I returned home, and my parents had me admitted to a psychiatric hospital, where I remained for 3 months.
At first, my diagnosis was unclear, and initially no one considered what at the time was called manic depression. At that point, I was unaware of my extensive family psychiatric history. My pharmacologic treatment consisted of chlorpromazine, trifluoperazine, and procyclidine. I returned home just before Christmas and barely finished my senior year of high school. A good college accepted me. But during the orientation, I was asked to leave because I experienced a second manic episode. After 4 more psychiatric hospitalizations, I finally stabilized.
During one of my hospitalizations, I had the good fortune to be interviewed by Dr. Thomas Detre. During this interview, I talked expansively about Don Quixote, Aldonza, and Sancho Panza. Dr. Detre diagnosed me with manic depression, and suggested that I see Dr. Christiaan van der Velde, who was researching lithium carbonate.2 In 1970, I was hospitalized at Norwich State Hospital in Preston, Connecticut and was started on lithium, even though it had not yet been FDA-approved. I responded well to lithium monotherapy.
An extensive family history. Having bipolar disorder was not something I would discuss with others because I felt ashamed. I commonly hid my medication during college, especially from my roommates or other friends. By then, I had learned a little about my family’s psychiatric history, but I knew few specifics. Over time, I became aware of a dense familial cluster of affective illness going back several generations. My maternal grandmother was hospitalized for depression in 1921 after her husband suddenly died during her fourth pregnancy. She became bereft and suicidal because she had no one to support her 4 children. During my grandmother’s hospitalization, her sister and sister’s husband took care of her children. My grandmother remained hospitalized until she died in 1943. At that time, no medications were available to treat her illness. Over the next 2 generations, 2 of her 4 children and 6 of her 12 grandchildren (including me) developed bipolar disorder.
A career and family. In 1970, I started to work as a nursing assistant, then as a nursing technician for 1.5 years in a specialty hospital in New England. In 1973, I began nursing school at a junior college. I received my RN in 1975, a BS in nursing in 1979, and an MS in psychiatric nursing in 1982. I worked steadily as a psychiatric nurse in both inpatient and outpatient settings from 1975 until I retired in 2019.
In the early 1980s, I married my first wife and had 2 wonderful children. During our courtship in 1981 and 1982, I became hypomanic, which perhaps made me more outgoing and sociable. In 1985, after my father required open heart surgery, I had a manic episode that lasted 1 week. Over the next 20 years, although I was not happy with my marriage, I remained euthymic and productive at work. My marriage ended in 2012.
Continue to: By the end of 2012...
By the end of 2012, I had been taking lithium continuously for 42 years. My laboratory tests showed peak lithium levels between 0.6 and 1.2 mmol/L. I remained otherwise healthy, as demonstrated by annual physical exams and laboratory test results. In 2015, I developed an increase in my blood pressure and my primary care physician (PCP) prescribed oral lisinopril, initially 10 mg/d, and later 10 mg twice daily. My blood pressure improved and ranged from 120/74 to 130/82 mm Hg.
Hyperparathyroidism. By 2016, my psychiatrist, PCP, and nephrologist all urged me to consider parathyroid surgery.3-5 Hypercalcemia and hyperparathyroidism caused the most worry. Laboratory tests indicated calcium 11.2 mg/dL, parathyroid hormone (PTH) 88 pg/mL, estimated glomerular filtration rate (eGFR) 59 mL/min, and thyroid-stimulating hormone (TSH) 0.78 mIU/L. Electrocardiographysometimes showed a slight QT elongation. A right bundle branch block, which was first noted in 2015, continued. Due to my elevated calcium levels, I eliminated most calcium from my diet. My psychiatrist began to speak more strongly of parathyroid surgery. I then consulted a senior endocrinologist and a senior nephrologist, who each recommended parathyroid surgery.
I remarried in July 2016, and we moved to a different area of the country. My second wife became a stabilizing force for me. My new PCP, however, found elevated high-density lipoproteins during a routine physical examination, and started me on simvastatin, 10 mg/d. My calcium and PTH levels continued to be elevated. My PCP, nephrologist, therapist, and wife urged me to proceed with the parathyroidectomy. After a short period of watchful waiting and a second consultation with a nephrologist, I agreed to schedule a subtotal parathyroidectomy.
Surgery. In spring 2017, I began preparation for parathyroidectomy. At the time, my lithium carbonate dose was 600 mg/d, alternating with 900 mg/d. My peak level of lithium was 0.6 mmol/L. Lisinopril is synergistic, which allowed me to take a smaller effective dose of lithium.
My parathyroid surgery occurred on June 28, 2017 at Norman Parathyroid Center in Tampa, Florida.6 The surgeon recorded my parathyroid glands as 136, 602, and 348 units using a measure developed at Norman Parathyroid Center. No reading was given for my fourth parathyroid gland, which they did not remove. Following the surgery, I resumed my previous functions, including employment as a visiting nurse. I initially took calcium supplements after surgery, and my lithium dose was reduced to 300 mg orally, twice daily, which I have continued. I have remained euthymic. On August 3, 2017 my laboratory workup showed an eGFR of 64 mL/min, calcium 10.0 mg/dL, and PTH 17 pg/mL. Vitamin D25 OH 33, glucose, BUN/Cr, electrolytes, complete blood count, and albumin were all within normal limits. Repeat bloodwork on September 19, 2017 showed Ca++ 10.1 mg/dL and PTH 18 pg/mL. Nine months after the surgery, I showed an incredibly positive physical and mental response, which has continued to this day.
Continue to: Clinical implications
Clinical implications. This is a single case study. However, it is important for clinicians treating patients with lithium carbonate to regularly order laboratory testing, including for lithium levels, PTH, and calcium, to detect early signs of complications from treatment, including hyperparathyroidism and hypercalcemia.7 These levels could be obtained every 6 months. If a patient’s PTH levels are >70 pg/mL and calcium levels are >11.0 mg/dL, it would be prudent to refer him/her for further medical evaluation. Additionally, it would be helpful to counsel the patient about considering alternative medication and adjunct mental health treatment. At some future point, it could be useful for the clinician and his/her patient to explore the idea of parathyroid surgery.
In addition to chronic lithium use, other causes of hyperparathyroidism include an adenoma on a gland, hyperplasia of ≥2 parathyroid glands, a malignant tumor, severe calcium deficiency, severe vitamin D deficiency, chronic renal failure, and (rarely) an inherited gene that causes hyperparathyroidism.
How I’m doing today. Currently, I am euthymic and in a happy marriage. My laboratory workup in May 2020 included glucose 107 mg/dL, Ca++ 9.5 mg/dL, eGFR 61 mL/min, PTH 32 pg/mL, lithium 0.3 mmol/L (300 mg twice daily), and TSH 1.79 mIU/L. A comprehensive metabolic panel, complete blood count, and lipid panel were all within normal limits.
I am fortunate to continue having excellent care provided by my PCP, nephrologist, urologist, and psychiatric APRN. Together with these wonderful professionals, I have been able to maintain my physical and mental health.
Acknowledgment: I gratefully acknowledge the help and skills of Robin Scharak and Gary Blake for providing some of the editing on this article.
Bill Greenberg MS, RN, APRN
Delray Beach, Florida
1. Slater L. Welcome to my country. New York, NY: Random House; 1996:187.
2. Van der Velde CD. Effectiveness of lithium in the treatment of manic-depressive illness. Am J Psychiatry. 1970;127(3):345-351.
3. Norman Parathyroid Center. Parathyroid glands, high calcium and hyperparathyroidism. www.parathyroid.com. Updated October 21, 2020. Accessed November 11, 2020.
4. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
5. Lally J, Lee B, McDonald C. Prevalence of hypercalcaemia in patients on maintenance lithium therapy monitored in primary care. Ir Med J. 2013;106(1):15-17.
6. Norman Parathyroid Center. Parathyroid surgery: minimally invasive 4-gland parathyroid surgery video. (4-Gland MIRP Parathyroid Operation). https://www.parathyroid.com/parathyroid-surgery.htm. Updated October 1, 2020. Accessed November 5, 2020.
7. MEDSAFE. Hyperparathyroidism and hypercalcaemia with lithium treatment. New Zealand Medicines and Medical Devices Safety Authority. 2014;35(3):37-38.
I am a retired advanced practice psychiatric nurse who has lived and worked on “both sides of the door.” This wording is paraphrased from psychologist and therapist Lauren Slater, PhD, who wrote about a time she went to McLean Hospital in Belmont, Massachusetts, as a therapist after staying there as a patient years earlier: “And now I am standing on the other—the wrong, I mean the right side of the door and I ring the buzzer.”1 Here I tell my story of the physical and emotional effects of my mental illness and treatment.
Onset of bipolar disorder. My bipolar illness started with a bout of depression in 1963 at age 13, which resulted in a low-key summer of often staying inside. I received no medication, and no one sent me for evaluation. In the fall, I went back to school and finished the year without incident. I continued as a quiet, shy kid through high school in the late 1960s. In my senior year, I decided to take an overload of difficult courses and run on the varsity cross-country team. The amount and intensity of these activities were too much. This resulted in my first manic episode, which started during a weekend visit to a college I hoped to attend. I became excitable, grandiose, and had delusions. A day later, I returned home, and my parents had me admitted to a psychiatric hospital, where I remained for 3 months.
At first, my diagnosis was unclear, and initially no one considered what at the time was called manic depression. At that point, I was unaware of my extensive family psychiatric history. My pharmacologic treatment consisted of chlorpromazine, trifluoperazine, and procyclidine. I returned home just before Christmas and barely finished my senior year of high school. A good college accepted me. But during the orientation, I was asked to leave because I experienced a second manic episode. After 4 more psychiatric hospitalizations, I finally stabilized.
During one of my hospitalizations, I had the good fortune to be interviewed by Dr. Thomas Detre. During this interview, I talked expansively about Don Quixote, Aldonza, and Sancho Panza. Dr. Detre diagnosed me with manic depression, and suggested that I see Dr. Christiaan van der Velde, who was researching lithium carbonate.2 In 1970, I was hospitalized at Norwich State Hospital in Preston, Connecticut and was started on lithium, even though it had not yet been FDA-approved. I responded well to lithium monotherapy.
An extensive family history. Having bipolar disorder was not something I would discuss with others because I felt ashamed. I commonly hid my medication during college, especially from my roommates or other friends. By then, I had learned a little about my family’s psychiatric history, but I knew few specifics. Over time, I became aware of a dense familial cluster of affective illness going back several generations. My maternal grandmother was hospitalized for depression in 1921 after her husband suddenly died during her fourth pregnancy. She became bereft and suicidal because she had no one to support her 4 children. During my grandmother’s hospitalization, her sister and sister’s husband took care of her children. My grandmother remained hospitalized until she died in 1943. At that time, no medications were available to treat her illness. Over the next 2 generations, 2 of her 4 children and 6 of her 12 grandchildren (including me) developed bipolar disorder.
A career and family. In 1970, I started to work as a nursing assistant, then as a nursing technician for 1.5 years in a specialty hospital in New England. In 1973, I began nursing school at a junior college. I received my RN in 1975, a BS in nursing in 1979, and an MS in psychiatric nursing in 1982. I worked steadily as a psychiatric nurse in both inpatient and outpatient settings from 1975 until I retired in 2019.
In the early 1980s, I married my first wife and had 2 wonderful children. During our courtship in 1981 and 1982, I became hypomanic, which perhaps made me more outgoing and sociable. In 1985, after my father required open heart surgery, I had a manic episode that lasted 1 week. Over the next 20 years, although I was not happy with my marriage, I remained euthymic and productive at work. My marriage ended in 2012.
Continue to: By the end of 2012...
By the end of 2012, I had been taking lithium continuously for 42 years. My laboratory tests showed peak lithium levels between 0.6 and 1.2 mmol/L. I remained otherwise healthy, as demonstrated by annual physical exams and laboratory test results. In 2015, I developed an increase in my blood pressure and my primary care physician (PCP) prescribed oral lisinopril, initially 10 mg/d, and later 10 mg twice daily. My blood pressure improved and ranged from 120/74 to 130/82 mm Hg.
Hyperparathyroidism. By 2016, my psychiatrist, PCP, and nephrologist all urged me to consider parathyroid surgery.3-5 Hypercalcemia and hyperparathyroidism caused the most worry. Laboratory tests indicated calcium 11.2 mg/dL, parathyroid hormone (PTH) 88 pg/mL, estimated glomerular filtration rate (eGFR) 59 mL/min, and thyroid-stimulating hormone (TSH) 0.78 mIU/L. Electrocardiographysometimes showed a slight QT elongation. A right bundle branch block, which was first noted in 2015, continued. Due to my elevated calcium levels, I eliminated most calcium from my diet. My psychiatrist began to speak more strongly of parathyroid surgery. I then consulted a senior endocrinologist and a senior nephrologist, who each recommended parathyroid surgery.
I remarried in July 2016, and we moved to a different area of the country. My second wife became a stabilizing force for me. My new PCP, however, found elevated high-density lipoproteins during a routine physical examination, and started me on simvastatin, 10 mg/d. My calcium and PTH levels continued to be elevated. My PCP, nephrologist, therapist, and wife urged me to proceed with the parathyroidectomy. After a short period of watchful waiting and a second consultation with a nephrologist, I agreed to schedule a subtotal parathyroidectomy.
Surgery. In spring 2017, I began preparation for parathyroidectomy. At the time, my lithium carbonate dose was 600 mg/d, alternating with 900 mg/d. My peak level of lithium was 0.6 mmol/L. Lisinopril is synergistic, which allowed me to take a smaller effective dose of lithium.
My parathyroid surgery occurred on June 28, 2017 at Norman Parathyroid Center in Tampa, Florida.6 The surgeon recorded my parathyroid glands as 136, 602, and 348 units using a measure developed at Norman Parathyroid Center. No reading was given for my fourth parathyroid gland, which they did not remove. Following the surgery, I resumed my previous functions, including employment as a visiting nurse. I initially took calcium supplements after surgery, and my lithium dose was reduced to 300 mg orally, twice daily, which I have continued. I have remained euthymic. On August 3, 2017 my laboratory workup showed an eGFR of 64 mL/min, calcium 10.0 mg/dL, and PTH 17 pg/mL. Vitamin D25 OH 33, glucose, BUN/Cr, electrolytes, complete blood count, and albumin were all within normal limits. Repeat bloodwork on September 19, 2017 showed Ca++ 10.1 mg/dL and PTH 18 pg/mL. Nine months after the surgery, I showed an incredibly positive physical and mental response, which has continued to this day.
Continue to: Clinical implications
Clinical implications. This is a single case study. However, it is important for clinicians treating patients with lithium carbonate to regularly order laboratory testing, including for lithium levels, PTH, and calcium, to detect early signs of complications from treatment, including hyperparathyroidism and hypercalcemia.7 These levels could be obtained every 6 months. If a patient’s PTH levels are >70 pg/mL and calcium levels are >11.0 mg/dL, it would be prudent to refer him/her for further medical evaluation. Additionally, it would be helpful to counsel the patient about considering alternative medication and adjunct mental health treatment. At some future point, it could be useful for the clinician and his/her patient to explore the idea of parathyroid surgery.
In addition to chronic lithium use, other causes of hyperparathyroidism include an adenoma on a gland, hyperplasia of ≥2 parathyroid glands, a malignant tumor, severe calcium deficiency, severe vitamin D deficiency, chronic renal failure, and (rarely) an inherited gene that causes hyperparathyroidism.
How I’m doing today. Currently, I am euthymic and in a happy marriage. My laboratory workup in May 2020 included glucose 107 mg/dL, Ca++ 9.5 mg/dL, eGFR 61 mL/min, PTH 32 pg/mL, lithium 0.3 mmol/L (300 mg twice daily), and TSH 1.79 mIU/L. A comprehensive metabolic panel, complete blood count, and lipid panel were all within normal limits.
I am fortunate to continue having excellent care provided by my PCP, nephrologist, urologist, and psychiatric APRN. Together with these wonderful professionals, I have been able to maintain my physical and mental health.
Acknowledgment: I gratefully acknowledge the help and skills of Robin Scharak and Gary Blake for providing some of the editing on this article.
Bill Greenberg MS, RN, APRN
Delray Beach, Florida
I am a retired advanced practice psychiatric nurse who has lived and worked on “both sides of the door.” This wording is paraphrased from psychologist and therapist Lauren Slater, PhD, who wrote about a time she went to McLean Hospital in Belmont, Massachusetts, as a therapist after staying there as a patient years earlier: “And now I am standing on the other—the wrong, I mean the right side of the door and I ring the buzzer.”1 Here I tell my story of the physical and emotional effects of my mental illness and treatment.
Onset of bipolar disorder. My bipolar illness started with a bout of depression in 1963 at age 13, which resulted in a low-key summer of often staying inside. I received no medication, and no one sent me for evaluation. In the fall, I went back to school and finished the year without incident. I continued as a quiet, shy kid through high school in the late 1960s. In my senior year, I decided to take an overload of difficult courses and run on the varsity cross-country team. The amount and intensity of these activities were too much. This resulted in my first manic episode, which started during a weekend visit to a college I hoped to attend. I became excitable, grandiose, and had delusions. A day later, I returned home, and my parents had me admitted to a psychiatric hospital, where I remained for 3 months.
At first, my diagnosis was unclear, and initially no one considered what at the time was called manic depression. At that point, I was unaware of my extensive family psychiatric history. My pharmacologic treatment consisted of chlorpromazine, trifluoperazine, and procyclidine. I returned home just before Christmas and barely finished my senior year of high school. A good college accepted me. But during the orientation, I was asked to leave because I experienced a second manic episode. After 4 more psychiatric hospitalizations, I finally stabilized.
During one of my hospitalizations, I had the good fortune to be interviewed by Dr. Thomas Detre. During this interview, I talked expansively about Don Quixote, Aldonza, and Sancho Panza. Dr. Detre diagnosed me with manic depression, and suggested that I see Dr. Christiaan van der Velde, who was researching lithium carbonate.2 In 1970, I was hospitalized at Norwich State Hospital in Preston, Connecticut and was started on lithium, even though it had not yet been FDA-approved. I responded well to lithium monotherapy.
An extensive family history. Having bipolar disorder was not something I would discuss with others because I felt ashamed. I commonly hid my medication during college, especially from my roommates or other friends. By then, I had learned a little about my family’s psychiatric history, but I knew few specifics. Over time, I became aware of a dense familial cluster of affective illness going back several generations. My maternal grandmother was hospitalized for depression in 1921 after her husband suddenly died during her fourth pregnancy. She became bereft and suicidal because she had no one to support her 4 children. During my grandmother’s hospitalization, her sister and sister’s husband took care of her children. My grandmother remained hospitalized until she died in 1943. At that time, no medications were available to treat her illness. Over the next 2 generations, 2 of her 4 children and 6 of her 12 grandchildren (including me) developed bipolar disorder.
A career and family. In 1970, I started to work as a nursing assistant, then as a nursing technician for 1.5 years in a specialty hospital in New England. In 1973, I began nursing school at a junior college. I received my RN in 1975, a BS in nursing in 1979, and an MS in psychiatric nursing in 1982. I worked steadily as a psychiatric nurse in both inpatient and outpatient settings from 1975 until I retired in 2019.
In the early 1980s, I married my first wife and had 2 wonderful children. During our courtship in 1981 and 1982, I became hypomanic, which perhaps made me more outgoing and sociable. In 1985, after my father required open heart surgery, I had a manic episode that lasted 1 week. Over the next 20 years, although I was not happy with my marriage, I remained euthymic and productive at work. My marriage ended in 2012.
Continue to: By the end of 2012...
By the end of 2012, I had been taking lithium continuously for 42 years. My laboratory tests showed peak lithium levels between 0.6 and 1.2 mmol/L. I remained otherwise healthy, as demonstrated by annual physical exams and laboratory test results. In 2015, I developed an increase in my blood pressure and my primary care physician (PCP) prescribed oral lisinopril, initially 10 mg/d, and later 10 mg twice daily. My blood pressure improved and ranged from 120/74 to 130/82 mm Hg.
Hyperparathyroidism. By 2016, my psychiatrist, PCP, and nephrologist all urged me to consider parathyroid surgery.3-5 Hypercalcemia and hyperparathyroidism caused the most worry. Laboratory tests indicated calcium 11.2 mg/dL, parathyroid hormone (PTH) 88 pg/mL, estimated glomerular filtration rate (eGFR) 59 mL/min, and thyroid-stimulating hormone (TSH) 0.78 mIU/L. Electrocardiographysometimes showed a slight QT elongation. A right bundle branch block, which was first noted in 2015, continued. Due to my elevated calcium levels, I eliminated most calcium from my diet. My psychiatrist began to speak more strongly of parathyroid surgery. I then consulted a senior endocrinologist and a senior nephrologist, who each recommended parathyroid surgery.
I remarried in July 2016, and we moved to a different area of the country. My second wife became a stabilizing force for me. My new PCP, however, found elevated high-density lipoproteins during a routine physical examination, and started me on simvastatin, 10 mg/d. My calcium and PTH levels continued to be elevated. My PCP, nephrologist, therapist, and wife urged me to proceed with the parathyroidectomy. After a short period of watchful waiting and a second consultation with a nephrologist, I agreed to schedule a subtotal parathyroidectomy.
Surgery. In spring 2017, I began preparation for parathyroidectomy. At the time, my lithium carbonate dose was 600 mg/d, alternating with 900 mg/d. My peak level of lithium was 0.6 mmol/L. Lisinopril is synergistic, which allowed me to take a smaller effective dose of lithium.
My parathyroid surgery occurred on June 28, 2017 at Norman Parathyroid Center in Tampa, Florida.6 The surgeon recorded my parathyroid glands as 136, 602, and 348 units using a measure developed at Norman Parathyroid Center. No reading was given for my fourth parathyroid gland, which they did not remove. Following the surgery, I resumed my previous functions, including employment as a visiting nurse. I initially took calcium supplements after surgery, and my lithium dose was reduced to 300 mg orally, twice daily, which I have continued. I have remained euthymic. On August 3, 2017 my laboratory workup showed an eGFR of 64 mL/min, calcium 10.0 mg/dL, and PTH 17 pg/mL. Vitamin D25 OH 33, glucose, BUN/Cr, electrolytes, complete blood count, and albumin were all within normal limits. Repeat bloodwork on September 19, 2017 showed Ca++ 10.1 mg/dL and PTH 18 pg/mL. Nine months after the surgery, I showed an incredibly positive physical and mental response, which has continued to this day.
Continue to: Clinical implications
Clinical implications. This is a single case study. However, it is important for clinicians treating patients with lithium carbonate to regularly order laboratory testing, including for lithium levels, PTH, and calcium, to detect early signs of complications from treatment, including hyperparathyroidism and hypercalcemia.7 These levels could be obtained every 6 months. If a patient’s PTH levels are >70 pg/mL and calcium levels are >11.0 mg/dL, it would be prudent to refer him/her for further medical evaluation. Additionally, it would be helpful to counsel the patient about considering alternative medication and adjunct mental health treatment. At some future point, it could be useful for the clinician and his/her patient to explore the idea of parathyroid surgery.
In addition to chronic lithium use, other causes of hyperparathyroidism include an adenoma on a gland, hyperplasia of ≥2 parathyroid glands, a malignant tumor, severe calcium deficiency, severe vitamin D deficiency, chronic renal failure, and (rarely) an inherited gene that causes hyperparathyroidism.
How I’m doing today. Currently, I am euthymic and in a happy marriage. My laboratory workup in May 2020 included glucose 107 mg/dL, Ca++ 9.5 mg/dL, eGFR 61 mL/min, PTH 32 pg/mL, lithium 0.3 mmol/L (300 mg twice daily), and TSH 1.79 mIU/L. A comprehensive metabolic panel, complete blood count, and lipid panel were all within normal limits.
I am fortunate to continue having excellent care provided by my PCP, nephrologist, urologist, and psychiatric APRN. Together with these wonderful professionals, I have been able to maintain my physical and mental health.
Acknowledgment: I gratefully acknowledge the help and skills of Robin Scharak and Gary Blake for providing some of the editing on this article.
Bill Greenberg MS, RN, APRN
Delray Beach, Florida
1. Slater L. Welcome to my country. New York, NY: Random House; 1996:187.
2. Van der Velde CD. Effectiveness of lithium in the treatment of manic-depressive illness. Am J Psychiatry. 1970;127(3):345-351.
3. Norman Parathyroid Center. Parathyroid glands, high calcium and hyperparathyroidism. www.parathyroid.com. Updated October 21, 2020. Accessed November 11, 2020.
4. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
5. Lally J, Lee B, McDonald C. Prevalence of hypercalcaemia in patients on maintenance lithium therapy monitored in primary care. Ir Med J. 2013;106(1):15-17.
6. Norman Parathyroid Center. Parathyroid surgery: minimally invasive 4-gland parathyroid surgery video. (4-Gland MIRP Parathyroid Operation). https://www.parathyroid.com/parathyroid-surgery.htm. Updated October 1, 2020. Accessed November 5, 2020.
7. MEDSAFE. Hyperparathyroidism and hypercalcaemia with lithium treatment. New Zealand Medicines and Medical Devices Safety Authority. 2014;35(3):37-38.
1. Slater L. Welcome to my country. New York, NY: Random House; 1996:187.
2. Van der Velde CD. Effectiveness of lithium in the treatment of manic-depressive illness. Am J Psychiatry. 1970;127(3):345-351.
3. Norman Parathyroid Center. Parathyroid glands, high calcium and hyperparathyroidism. www.parathyroid.com. Updated October 21, 2020. Accessed November 11, 2020.
4. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
5. Lally J, Lee B, McDonald C. Prevalence of hypercalcaemia in patients on maintenance lithium therapy monitored in primary care. Ir Med J. 2013;106(1):15-17.
6. Norman Parathyroid Center. Parathyroid surgery: minimally invasive 4-gland parathyroid surgery video. (4-Gland MIRP Parathyroid Operation). https://www.parathyroid.com/parathyroid-surgery.htm. Updated October 1, 2020. Accessed November 5, 2020.
7. MEDSAFE. Hyperparathyroidism and hypercalcaemia with lithium treatment. New Zealand Medicines and Medical Devices Safety Authority. 2014;35(3):37-38.
Researchers evaluate gynecology-specific laparoscopic simulator
Students have similar confidence levels during a simulated laparoscopic vaginal cuff suturing task whether they train with the current standard laparoscopic simulator or a newer gynecology-specific simulator, a randomized trial found.
Participants who trained on the gynecology-specific simulator, known as Essentials in Minimally Invasive Gynecology (EMIG), reported higher confidence scores, but differences between the groups were not statistically significant, a researcher reported at the annual meeting sponsored by AAGL, held virtually this year.
The study compared EMIG with Fundamentals of Laparoscopic Surgery (FLS), a laparoscopic simulator that general surgeons launched in 2004.
In 2018, the American Board of Obstetrics and Gynecology announced an FLS requirement for residents graduating after May 31, 2020. The same year, the AAGL began validating EMIG. AAGL developed the simulator in response to a growing trend for minimally invasive approaches and to provide a training tool geared toward gynecologists, said Emily G. Lin, MD, an obstetrics and gynecology resident at McGaw Medical Center at Northwestern University in Chicago.
A comparison of the two simulators
The simulators use different port placement and operator positioning. The operating fields within the box trainers also differ. In EMIG, laparoscopic tasks take place within a bowl that simulates a confined workspace similar to a pelvis, whereas FLS tasks take place in an open box trainer environment, Dr. Lin said.
To compare students’ self-reported confidence levels after performing a laparoscopic vaginal cuff suturing task after training with EMIG or FLS, Dr. Lin and colleagues conducted a randomized controlled trial.
The researchers recruited 45 participants who were preclinical medical students or premedical college students without prior training experience. Participants were randomized to EMIG or FLS training. After watching instructional videos about their simulator tasks and the vaginal cuff suturing task, they attempted the vaginal cuff suturing task as a pretest.
They then trained for about 2 hours on their assigned simulator. Training for both groups included practicing peg transfer and intracorporeal knot tying. In addition, the EMIG group trained on a running suture task, and the FLS group trained on a ligating loop task.
After training, participants retried the vaginal cuff suturing task. Participants subsequently rated their confidence during each simulation task on a 5-point Likert scale.
Confidence levels on the peg transfer (4.13 with EMIG vs. 4.10 with FLS), intracorporeal knot tying (3.0 with EMIG vs. 2.86 with FLS) and vaginal cuff suturing (2.46 with EMIG vs. 2.05 with FLS) were similar for both groups.
The study was small, included only one training session, and included only three of the five tasks for each simulator because of time and cost constraints, Dr. Lin noted.
Using simulation in residency training
The study was well designed and sheds light on inevitable comparisons between FLS and EMIG, Ido Sirota, MD, MHA, of New York-Presbyterian Queens, said in a discussion following the research presentation.
“The field of medical simulation has developed tremendously in the past decade,” Dr. Sirota said. “The paradigm that used to be common in our field – of see one, do one, teach one – belongs to the past. ... Current trainees need extensive practice on their surgical skills in a simulation setting before” entering the operating room.
A 2017 review found that simulation may be a useful adjunct to residency training.
And in a pilot study, EMIG’s laparoscopic and hysteroscopic simulation systems were considered to have good face validity, Dr. Sirota noted.
Using a gynecology-specific simulation may have advantages.
“In this day and age when we are trying to differentiate ourselves as a subspecialty, there is a great value to developing our own simulation-based curricula to validate our surgical skills during training, as well as for maintenance throughout our career,” Dr. Sirota said. “We as a subspecialty need specific tests tailored to our surgical procedures.”
Dr. Sirota disclosed consulting for Medtronic, Activ Surgical, Heracure, and HT, and he is on the speakers bureau for Medtronic. Dr. Lin had no relevant financial disclosures.
SOURCE: Lin E et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.593.
Students have similar confidence levels during a simulated laparoscopic vaginal cuff suturing task whether they train with the current standard laparoscopic simulator or a newer gynecology-specific simulator, a randomized trial found.
Participants who trained on the gynecology-specific simulator, known as Essentials in Minimally Invasive Gynecology (EMIG), reported higher confidence scores, but differences between the groups were not statistically significant, a researcher reported at the annual meeting sponsored by AAGL, held virtually this year.
The study compared EMIG with Fundamentals of Laparoscopic Surgery (FLS), a laparoscopic simulator that general surgeons launched in 2004.
In 2018, the American Board of Obstetrics and Gynecology announced an FLS requirement for residents graduating after May 31, 2020. The same year, the AAGL began validating EMIG. AAGL developed the simulator in response to a growing trend for minimally invasive approaches and to provide a training tool geared toward gynecologists, said Emily G. Lin, MD, an obstetrics and gynecology resident at McGaw Medical Center at Northwestern University in Chicago.
A comparison of the two simulators
The simulators use different port placement and operator positioning. The operating fields within the box trainers also differ. In EMIG, laparoscopic tasks take place within a bowl that simulates a confined workspace similar to a pelvis, whereas FLS tasks take place in an open box trainer environment, Dr. Lin said.
To compare students’ self-reported confidence levels after performing a laparoscopic vaginal cuff suturing task after training with EMIG or FLS, Dr. Lin and colleagues conducted a randomized controlled trial.
The researchers recruited 45 participants who were preclinical medical students or premedical college students without prior training experience. Participants were randomized to EMIG or FLS training. After watching instructional videos about their simulator tasks and the vaginal cuff suturing task, they attempted the vaginal cuff suturing task as a pretest.
They then trained for about 2 hours on their assigned simulator. Training for both groups included practicing peg transfer and intracorporeal knot tying. In addition, the EMIG group trained on a running suture task, and the FLS group trained on a ligating loop task.
After training, participants retried the vaginal cuff suturing task. Participants subsequently rated their confidence during each simulation task on a 5-point Likert scale.
Confidence levels on the peg transfer (4.13 with EMIG vs. 4.10 with FLS), intracorporeal knot tying (3.0 with EMIG vs. 2.86 with FLS) and vaginal cuff suturing (2.46 with EMIG vs. 2.05 with FLS) were similar for both groups.
The study was small, included only one training session, and included only three of the five tasks for each simulator because of time and cost constraints, Dr. Lin noted.
Using simulation in residency training
The study was well designed and sheds light on inevitable comparisons between FLS and EMIG, Ido Sirota, MD, MHA, of New York-Presbyterian Queens, said in a discussion following the research presentation.
“The field of medical simulation has developed tremendously in the past decade,” Dr. Sirota said. “The paradigm that used to be common in our field – of see one, do one, teach one – belongs to the past. ... Current trainees need extensive practice on their surgical skills in a simulation setting before” entering the operating room.
A 2017 review found that simulation may be a useful adjunct to residency training.
And in a pilot study, EMIG’s laparoscopic and hysteroscopic simulation systems were considered to have good face validity, Dr. Sirota noted.
Using a gynecology-specific simulation may have advantages.
“In this day and age when we are trying to differentiate ourselves as a subspecialty, there is a great value to developing our own simulation-based curricula to validate our surgical skills during training, as well as for maintenance throughout our career,” Dr. Sirota said. “We as a subspecialty need specific tests tailored to our surgical procedures.”
Dr. Sirota disclosed consulting for Medtronic, Activ Surgical, Heracure, and HT, and he is on the speakers bureau for Medtronic. Dr. Lin had no relevant financial disclosures.
SOURCE: Lin E et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.593.
Students have similar confidence levels during a simulated laparoscopic vaginal cuff suturing task whether they train with the current standard laparoscopic simulator or a newer gynecology-specific simulator, a randomized trial found.
Participants who trained on the gynecology-specific simulator, known as Essentials in Minimally Invasive Gynecology (EMIG), reported higher confidence scores, but differences between the groups were not statistically significant, a researcher reported at the annual meeting sponsored by AAGL, held virtually this year.
The study compared EMIG with Fundamentals of Laparoscopic Surgery (FLS), a laparoscopic simulator that general surgeons launched in 2004.
In 2018, the American Board of Obstetrics and Gynecology announced an FLS requirement for residents graduating after May 31, 2020. The same year, the AAGL began validating EMIG. AAGL developed the simulator in response to a growing trend for minimally invasive approaches and to provide a training tool geared toward gynecologists, said Emily G. Lin, MD, an obstetrics and gynecology resident at McGaw Medical Center at Northwestern University in Chicago.
A comparison of the two simulators
The simulators use different port placement and operator positioning. The operating fields within the box trainers also differ. In EMIG, laparoscopic tasks take place within a bowl that simulates a confined workspace similar to a pelvis, whereas FLS tasks take place in an open box trainer environment, Dr. Lin said.
To compare students’ self-reported confidence levels after performing a laparoscopic vaginal cuff suturing task after training with EMIG or FLS, Dr. Lin and colleagues conducted a randomized controlled trial.
The researchers recruited 45 participants who were preclinical medical students or premedical college students without prior training experience. Participants were randomized to EMIG or FLS training. After watching instructional videos about their simulator tasks and the vaginal cuff suturing task, they attempted the vaginal cuff suturing task as a pretest.
They then trained for about 2 hours on their assigned simulator. Training for both groups included practicing peg transfer and intracorporeal knot tying. In addition, the EMIG group trained on a running suture task, and the FLS group trained on a ligating loop task.
After training, participants retried the vaginal cuff suturing task. Participants subsequently rated their confidence during each simulation task on a 5-point Likert scale.
Confidence levels on the peg transfer (4.13 with EMIG vs. 4.10 with FLS), intracorporeal knot tying (3.0 with EMIG vs. 2.86 with FLS) and vaginal cuff suturing (2.46 with EMIG vs. 2.05 with FLS) were similar for both groups.
The study was small, included only one training session, and included only three of the five tasks for each simulator because of time and cost constraints, Dr. Lin noted.
Using simulation in residency training
The study was well designed and sheds light on inevitable comparisons between FLS and EMIG, Ido Sirota, MD, MHA, of New York-Presbyterian Queens, said in a discussion following the research presentation.
“The field of medical simulation has developed tremendously in the past decade,” Dr. Sirota said. “The paradigm that used to be common in our field – of see one, do one, teach one – belongs to the past. ... Current trainees need extensive practice on their surgical skills in a simulation setting before” entering the operating room.
A 2017 review found that simulation may be a useful adjunct to residency training.
And in a pilot study, EMIG’s laparoscopic and hysteroscopic simulation systems were considered to have good face validity, Dr. Sirota noted.
Using a gynecology-specific simulation may have advantages.
“In this day and age when we are trying to differentiate ourselves as a subspecialty, there is a great value to developing our own simulation-based curricula to validate our surgical skills during training, as well as for maintenance throughout our career,” Dr. Sirota said. “We as a subspecialty need specific tests tailored to our surgical procedures.”
Dr. Sirota disclosed consulting for Medtronic, Activ Surgical, Heracure, and HT, and he is on the speakers bureau for Medtronic. Dr. Lin had no relevant financial disclosures.
SOURCE: Lin E et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.593.
FROM AAGL GLOBAL CONGRESS
Reducing Inappropriate Laboratory Testing in the Hospital Setting: How Low Can We Go?
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12
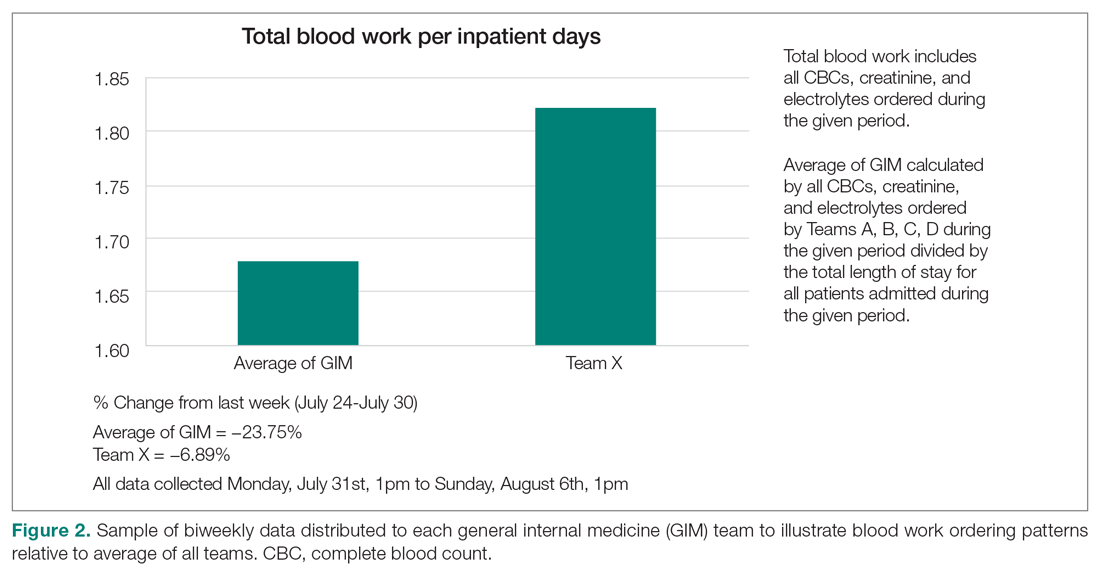
Data Collection and Analysis
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 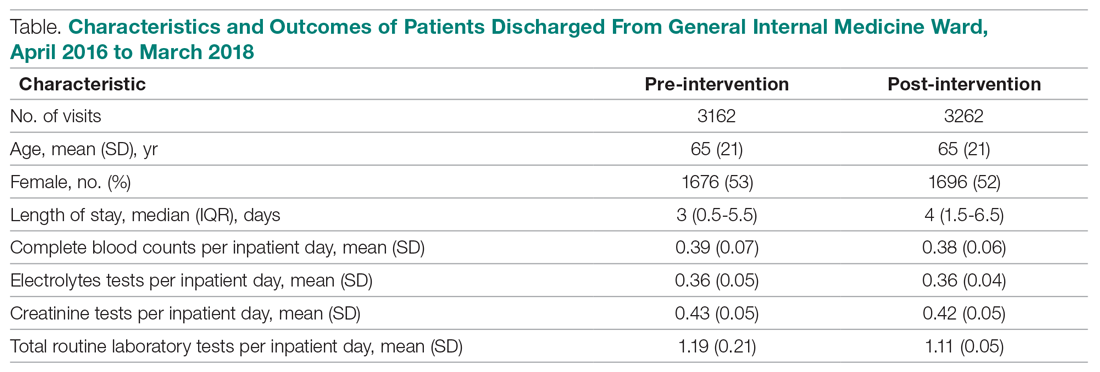
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.
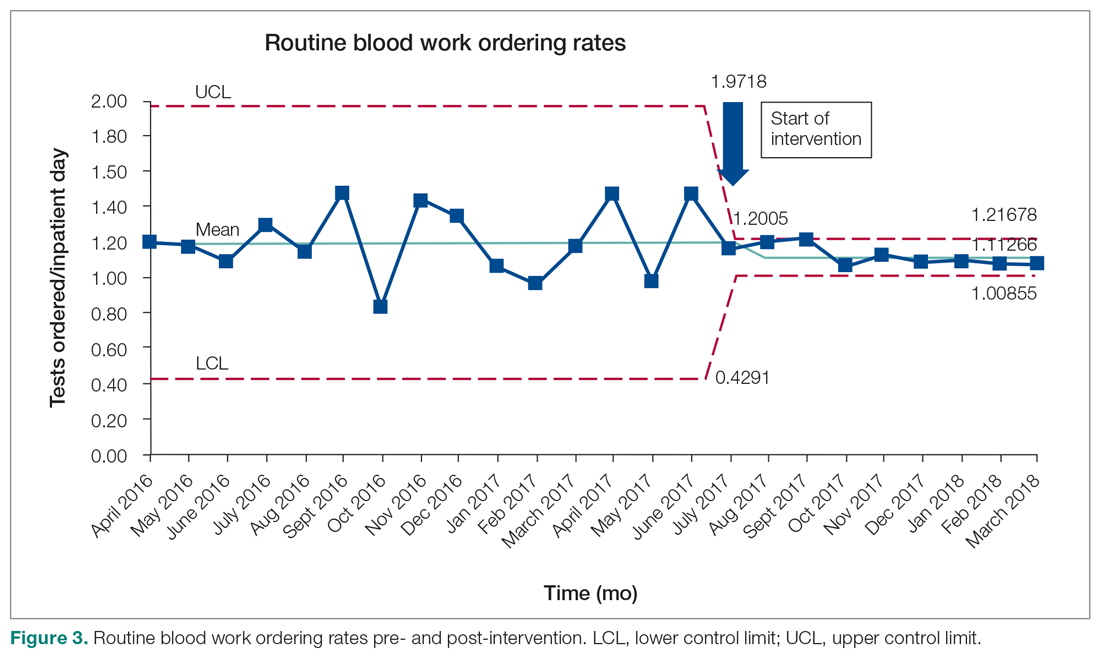
Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12

Data Collection and Analysis
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.

Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12

Data Collection and Analysis
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.

Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
Multiple Glomangiomas in a Patient With a History of Metastatic Melanoma
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
Practice Points
- The diagnosis of glomus tumor and glomangioma is easily suspected when the lesions are in the digital or subungual region.
- Multiple glomangiomas are rare and can clinically pose a diagnostic challenge to dermatologists.
- In patients with a recent history of malignancy, multiple glomangiomas may mimic cutaneous metastases. Therefore, multiple biopsies and histologic examination may be necessary.
Death anxiety among psychiatry trainees during COVID-19
The coronavirus disease 2019 (COVID-19) pandemic
The far-reaching effects of death anxiety
Postgraduate psychiatry training may expose one to stressful situations with adverse psychologic consequences.1 Furthermore, when caring for patients, psychiatry trainees frequently need to face issues of death and dying in the form of suicide risk assessments, grief and bereavement processes, near-death experiences, posttraumatic stress disorder, and psycho-oncology rotations. Because these interactions are incredibly personal, the emotions they provoke inevitably affect every interaction, theoretical discussion, diagnostic work-up, and treatment plan.
How each of us experiences death anxiety is unique. For some, it could be a fear of nonexistence, ultimate loss, disruption of the flow of life, worry about leaving loved ones behind, or fear of pain or loneliness in dying. Some might fear an untimely or violent death and subsequent judgment and retributions. The literature suggests that fear of death may be at the root of various mental health problems and, if left unaddressed, may adversely impact long-term treatment outcomes.2 Despite this, many standard treatment approaches typically do not target death anxiety, which potentially contributes to a “revolving door” of mental health problems.3
American existential psychiatrist Irvin Yalom, MD, cautioned psychiatrists not to “scratch where it does not itch.”4 Yet death, according to Dr. Yalom, does itch. Violent death is that caused by human intent or negligence, and is characterized by feeling helpless and terrorized at the time of dying. It may occur as an acute incident that denies the dying individual and his/her family members the time and space to prepare for the death.5 For survivors, accommodating the mental, emotional, psychological, and spiritual effects of violent death is a complex process that rarely has a conclusion. It often is accompanied by survivors’ guilt, which is replayed in the form of flashbacks and nightmares.6 With this understanding, I view COVID-19 deaths as violent deaths.
Pay close attention to countertransference
As much as we influence our patients and their families, we also are profoundly influenced by them. We need to pay attention to any feelings our clinical encounters generate within us, and to carefully use these feelings in our clinical judgment, and not just make causal inferences. For instance, if a clinician thinks that a patient with suicidal ideation would be better off dead, these feelings are a reliable indicator that the patient is, indeed, at a high risk of completing suicide.7 It is our ethical and moral responsibility towards our patients to listen to our countertransference responses. The aim is to identify countertransference and use it to inform us, not to rule us. By taking an active role in managing our emotional responses in the face of loss, we can harness the spirit of resilience. This is not always as easy as it seems. We need our peers, experienced clinicians, and supervisors to help us explore our feelings, resistances, and countertransference reactions.
Strategies to combat burnout
Psychiatric trainees must be encouraged to establish and maintain rigorous plans of self-care to prevent compassion fatigue and burnout. Most importantly, training programs must diversify residents’ clinical exposure by providing activities that promote mental health promotion activities, scholarly endeavors, and peer support groups. This will help trainees to restore meaning and purpose in life beyond.
1. Coverdale J, Balon R, Beresin EV, et al. What are some stressful adversities in psychiatry residency training, and how should they be managed professionally? Acad Psychiatry. 2019;43(2):145-150.
2. Russac RJ, Gatliff C, Reece M, et al. Death anxiety across the adult years: an examination of age and gender effects. Death Stud. 2007;31(6):549-561.
3. Lisa I, Menzies RG, Menzies RE. Death anxiety and its role in psychopathology: reviewing the status of a transdiagnostic construct. Clinical Psychology Review. 2014;34(7):580-593.
4. Yalom ID. Staring at the sun: being at peace with your own mortality. London, UK: Piatkus; 2011.
5. Rynearson EK, Johnson TA, Correa F. The horror and helplessness of violent death. In: Katz RS, Johnson TA (eds). When professionals weep: emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016:91-103.
6. Breggin PR. Guilt, shame, and anxiety: understanding and overcoming negative emotions. Buffalo, NY: Prometheus Books; 2014.
7. Katz RS, Johnson TA, (eds). When professionals weep: Emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016.
The coronavirus disease 2019 (COVID-19) pandemic
The far-reaching effects of death anxiety
Postgraduate psychiatry training may expose one to stressful situations with adverse psychologic consequences.1 Furthermore, when caring for patients, psychiatry trainees frequently need to face issues of death and dying in the form of suicide risk assessments, grief and bereavement processes, near-death experiences, posttraumatic stress disorder, and psycho-oncology rotations. Because these interactions are incredibly personal, the emotions they provoke inevitably affect every interaction, theoretical discussion, diagnostic work-up, and treatment plan.
How each of us experiences death anxiety is unique. For some, it could be a fear of nonexistence, ultimate loss, disruption of the flow of life, worry about leaving loved ones behind, or fear of pain or loneliness in dying. Some might fear an untimely or violent death and subsequent judgment and retributions. The literature suggests that fear of death may be at the root of various mental health problems and, if left unaddressed, may adversely impact long-term treatment outcomes.2 Despite this, many standard treatment approaches typically do not target death anxiety, which potentially contributes to a “revolving door” of mental health problems.3
American existential psychiatrist Irvin Yalom, MD, cautioned psychiatrists not to “scratch where it does not itch.”4 Yet death, according to Dr. Yalom, does itch. Violent death is that caused by human intent or negligence, and is characterized by feeling helpless and terrorized at the time of dying. It may occur as an acute incident that denies the dying individual and his/her family members the time and space to prepare for the death.5 For survivors, accommodating the mental, emotional, psychological, and spiritual effects of violent death is a complex process that rarely has a conclusion. It often is accompanied by survivors’ guilt, which is replayed in the form of flashbacks and nightmares.6 With this understanding, I view COVID-19 deaths as violent deaths.
Pay close attention to countertransference
As much as we influence our patients and their families, we also are profoundly influenced by them. We need to pay attention to any feelings our clinical encounters generate within us, and to carefully use these feelings in our clinical judgment, and not just make causal inferences. For instance, if a clinician thinks that a patient with suicidal ideation would be better off dead, these feelings are a reliable indicator that the patient is, indeed, at a high risk of completing suicide.7 It is our ethical and moral responsibility towards our patients to listen to our countertransference responses. The aim is to identify countertransference and use it to inform us, not to rule us. By taking an active role in managing our emotional responses in the face of loss, we can harness the spirit of resilience. This is not always as easy as it seems. We need our peers, experienced clinicians, and supervisors to help us explore our feelings, resistances, and countertransference reactions.
Strategies to combat burnout
Psychiatric trainees must be encouraged to establish and maintain rigorous plans of self-care to prevent compassion fatigue and burnout. Most importantly, training programs must diversify residents’ clinical exposure by providing activities that promote mental health promotion activities, scholarly endeavors, and peer support groups. This will help trainees to restore meaning and purpose in life beyond.
The coronavirus disease 2019 (COVID-19) pandemic
The far-reaching effects of death anxiety
Postgraduate psychiatry training may expose one to stressful situations with adverse psychologic consequences.1 Furthermore, when caring for patients, psychiatry trainees frequently need to face issues of death and dying in the form of suicide risk assessments, grief and bereavement processes, near-death experiences, posttraumatic stress disorder, and psycho-oncology rotations. Because these interactions are incredibly personal, the emotions they provoke inevitably affect every interaction, theoretical discussion, diagnostic work-up, and treatment plan.
How each of us experiences death anxiety is unique. For some, it could be a fear of nonexistence, ultimate loss, disruption of the flow of life, worry about leaving loved ones behind, or fear of pain or loneliness in dying. Some might fear an untimely or violent death and subsequent judgment and retributions. The literature suggests that fear of death may be at the root of various mental health problems and, if left unaddressed, may adversely impact long-term treatment outcomes.2 Despite this, many standard treatment approaches typically do not target death anxiety, which potentially contributes to a “revolving door” of mental health problems.3
American existential psychiatrist Irvin Yalom, MD, cautioned psychiatrists not to “scratch where it does not itch.”4 Yet death, according to Dr. Yalom, does itch. Violent death is that caused by human intent or negligence, and is characterized by feeling helpless and terrorized at the time of dying. It may occur as an acute incident that denies the dying individual and his/her family members the time and space to prepare for the death.5 For survivors, accommodating the mental, emotional, psychological, and spiritual effects of violent death is a complex process that rarely has a conclusion. It often is accompanied by survivors’ guilt, which is replayed in the form of flashbacks and nightmares.6 With this understanding, I view COVID-19 deaths as violent deaths.
Pay close attention to countertransference
As much as we influence our patients and their families, we also are profoundly influenced by them. We need to pay attention to any feelings our clinical encounters generate within us, and to carefully use these feelings in our clinical judgment, and not just make causal inferences. For instance, if a clinician thinks that a patient with suicidal ideation would be better off dead, these feelings are a reliable indicator that the patient is, indeed, at a high risk of completing suicide.7 It is our ethical and moral responsibility towards our patients to listen to our countertransference responses. The aim is to identify countertransference and use it to inform us, not to rule us. By taking an active role in managing our emotional responses in the face of loss, we can harness the spirit of resilience. This is not always as easy as it seems. We need our peers, experienced clinicians, and supervisors to help us explore our feelings, resistances, and countertransference reactions.
Strategies to combat burnout
Psychiatric trainees must be encouraged to establish and maintain rigorous plans of self-care to prevent compassion fatigue and burnout. Most importantly, training programs must diversify residents’ clinical exposure by providing activities that promote mental health promotion activities, scholarly endeavors, and peer support groups. This will help trainees to restore meaning and purpose in life beyond.
1. Coverdale J, Balon R, Beresin EV, et al. What are some stressful adversities in psychiatry residency training, and how should they be managed professionally? Acad Psychiatry. 2019;43(2):145-150.
2. Russac RJ, Gatliff C, Reece M, et al. Death anxiety across the adult years: an examination of age and gender effects. Death Stud. 2007;31(6):549-561.
3. Lisa I, Menzies RG, Menzies RE. Death anxiety and its role in psychopathology: reviewing the status of a transdiagnostic construct. Clinical Psychology Review. 2014;34(7):580-593.
4. Yalom ID. Staring at the sun: being at peace with your own mortality. London, UK: Piatkus; 2011.
5. Rynearson EK, Johnson TA, Correa F. The horror and helplessness of violent death. In: Katz RS, Johnson TA (eds). When professionals weep: emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016:91-103.
6. Breggin PR. Guilt, shame, and anxiety: understanding and overcoming negative emotions. Buffalo, NY: Prometheus Books; 2014.
7. Katz RS, Johnson TA, (eds). When professionals weep: Emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016.
1. Coverdale J, Balon R, Beresin EV, et al. What are some stressful adversities in psychiatry residency training, and how should they be managed professionally? Acad Psychiatry. 2019;43(2):145-150.
2. Russac RJ, Gatliff C, Reece M, et al. Death anxiety across the adult years: an examination of age and gender effects. Death Stud. 2007;31(6):549-561.
3. Lisa I, Menzies RG, Menzies RE. Death anxiety and its role in psychopathology: reviewing the status of a transdiagnostic construct. Clinical Psychology Review. 2014;34(7):580-593.
4. Yalom ID. Staring at the sun: being at peace with your own mortality. London, UK: Piatkus; 2011.
5. Rynearson EK, Johnson TA, Correa F. The horror and helplessness of violent death. In: Katz RS, Johnson TA (eds). When professionals weep: emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016:91-103.
6. Breggin PR. Guilt, shame, and anxiety: understanding and overcoming negative emotions. Buffalo, NY: Prometheus Books; 2014.
7. Katz RS, Johnson TA, (eds). When professionals weep: Emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016.