User login
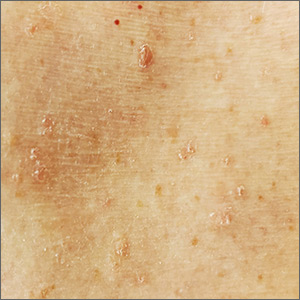
Itchy, scaly lesions with sweating

The patient’s history of a rash that worsened with sweating and clinical findings of erythematous papulosquamous lesions was consistent with Grover disease, also known as transient acantholytic dermatosis. Typically, this is a short-lived condition; however, when symptoms have manifested for years, it is deemed persistent acantholytic dermatosis. The gold standard for diagnostic confirmation is a skin biopsy, although it can also be diagnosed clinically. Since the patient had previously received this diagnosis from another clinician, and his clinical presentation was consistent, a confirmatory biopsy was not performed.
The specific pathophysiology of acantholytic dermatosis is unclear. A study to assess for an autoimmune component found that all participants had autoantibodies that are reactive to proteins involved in cell development, activation, growth, death, adhesion, and motility. Another hypothesis involves occlusion of eccrine sweat glands.
Typical triggers are sweating, heat, sunlight, and mechanical irritation, although it can also be triggered by end-stage renal disease and solid organ transplantation. It has also been linked to certain drugs (eg, ribavirin, anastrozole), other skin diseases (eg, atopic dermatitis, xerosis cutis), bacterial/viral infections, and malignancies.
As heat and perspiration are common triggers, avoidance of activities that expose patients to these conditions is recommended. Otherwise, topical corticosteroids and emollients are the recommended first-line therapy, along with antihistamines to control itching. Other therapies include systemic corticosteroids, topical vitamin D analogs (eg, calcipotriene), systemic retinoids (acitretin or isotretinoin), phototherapy and photochemotherapy (PUVA), red-light 5-aminolevulinic acid photodynamic therapy (ALA-PDT), and etanercept.
Although the patient could not remember the name of the previously prescribed medication, his description suggested that a systemic retinoid had already been tried, with no improvement. Treatment with emollients and oral antihistamines was also unsuccessful, as was topical antiperspirants to control perspiration on his affected skin. The patient agreed to try topical calcipotriene twice daily. He also agreed to switch to a topical emollient containing ceramides.
Image courtesy of Esther Walker, MD, and text courtesy of Esther Walker, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Phillips C, Kalantari-Dehaghi M, Marchenko S, et al. Is Grover's disease an autoimmune dermatosis? Exp Dermatol. 2013;22:781-784.

The patient’s history of a rash that worsened with sweating and clinical findings of erythematous papulosquamous lesions was consistent with Grover disease, also known as transient acantholytic dermatosis. Typically, this is a short-lived condition; however, when symptoms have manifested for years, it is deemed persistent acantholytic dermatosis. The gold standard for diagnostic confirmation is a skin biopsy, although it can also be diagnosed clinically. Since the patient had previously received this diagnosis from another clinician, and his clinical presentation was consistent, a confirmatory biopsy was not performed.
The specific pathophysiology of acantholytic dermatosis is unclear. A study to assess for an autoimmune component found that all participants had autoantibodies that are reactive to proteins involved in cell development, activation, growth, death, adhesion, and motility. Another hypothesis involves occlusion of eccrine sweat glands.
Typical triggers are sweating, heat, sunlight, and mechanical irritation, although it can also be triggered by end-stage renal disease and solid organ transplantation. It has also been linked to certain drugs (eg, ribavirin, anastrozole), other skin diseases (eg, atopic dermatitis, xerosis cutis), bacterial/viral infections, and malignancies.
As heat and perspiration are common triggers, avoidance of activities that expose patients to these conditions is recommended. Otherwise, topical corticosteroids and emollients are the recommended first-line therapy, along with antihistamines to control itching. Other therapies include systemic corticosteroids, topical vitamin D analogs (eg, calcipotriene), systemic retinoids (acitretin or isotretinoin), phototherapy and photochemotherapy (PUVA), red-light 5-aminolevulinic acid photodynamic therapy (ALA-PDT), and etanercept.
Although the patient could not remember the name of the previously prescribed medication, his description suggested that a systemic retinoid had already been tried, with no improvement. Treatment with emollients and oral antihistamines was also unsuccessful, as was topical antiperspirants to control perspiration on his affected skin. The patient agreed to try topical calcipotriene twice daily. He also agreed to switch to a topical emollient containing ceramides.
Image courtesy of Esther Walker, MD, and text courtesy of Esther Walker, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient’s history of a rash that worsened with sweating and clinical findings of erythematous papulosquamous lesions was consistent with Grover disease, also known as transient acantholytic dermatosis. Typically, this is a short-lived condition; however, when symptoms have manifested for years, it is deemed persistent acantholytic dermatosis. The gold standard for diagnostic confirmation is a skin biopsy, although it can also be diagnosed clinically. Since the patient had previously received this diagnosis from another clinician, and his clinical presentation was consistent, a confirmatory biopsy was not performed.
The specific pathophysiology of acantholytic dermatosis is unclear. A study to assess for an autoimmune component found that all participants had autoantibodies that are reactive to proteins involved in cell development, activation, growth, death, adhesion, and motility. Another hypothesis involves occlusion of eccrine sweat glands.
Typical triggers are sweating, heat, sunlight, and mechanical irritation, although it can also be triggered by end-stage renal disease and solid organ transplantation. It has also been linked to certain drugs (eg, ribavirin, anastrozole), other skin diseases (eg, atopic dermatitis, xerosis cutis), bacterial/viral infections, and malignancies.
As heat and perspiration are common triggers, avoidance of activities that expose patients to these conditions is recommended. Otherwise, topical corticosteroids and emollients are the recommended first-line therapy, along with antihistamines to control itching. Other therapies include systemic corticosteroids, topical vitamin D analogs (eg, calcipotriene), systemic retinoids (acitretin or isotretinoin), phototherapy and photochemotherapy (PUVA), red-light 5-aminolevulinic acid photodynamic therapy (ALA-PDT), and etanercept.
Although the patient could not remember the name of the previously prescribed medication, his description suggested that a systemic retinoid had already been tried, with no improvement. Treatment with emollients and oral antihistamines was also unsuccessful, as was topical antiperspirants to control perspiration on his affected skin. The patient agreed to try topical calcipotriene twice daily. He also agreed to switch to a topical emollient containing ceramides.
Image courtesy of Esther Walker, MD, and text courtesy of Esther Walker, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Phillips C, Kalantari-Dehaghi M, Marchenko S, et al. Is Grover's disease an autoimmune dermatosis? Exp Dermatol. 2013;22:781-784.
Phillips C, Kalantari-Dehaghi M, Marchenko S, et al. Is Grover's disease an autoimmune dermatosis? Exp Dermatol. 2013;22:781-784.
Matching Wits With a Viral Enemy: How the VA Has Responded to COVID-19
The numbers tell the story:
110,066 veterans diagnosed with COVID-19 as of November 30;
879,457 veterans and employees tested for COVID-19 as of November 6;
14,168 veterans admitted to a US Department of Veterans Affairs (VA) medical center for COVID-19 care;
1,525% increase in telehealth visits;
59,095 new staff hired to meet surge in demand for COVID-19 care;
75 completed Fourth Mission assignments; and
> 2,000 VA employees helping to support nonveteran patients and non-VA health care systems.
But those numbers are just some of the data in the COVID-19 Response Report, which the VA recently released. The report offers “an extensive look at VA’s complex COVID-19 response,” including how it prepared for the pandemic, the initial response, and key COVID-19 policies and directives.
The report was compiled from more than 90 interviews with health care leaders and stakeholders, along with documents and data pertaining to the Veterans Integrated Service Networks. The interviews were designed to “keep discussion at a strategic level.”
Meeting the crisis mandated that the Veterans Health Administration (VHA) act “with unity of effort and agility,” the authors note, across 18 networks with 170 medical centers. Not only is the VA called on to serve veterans, but its “Fourth Mission” explicitly calls on the VA to “improve the Nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters.” But the VHA possessed some major assets, they add, including a nationwide capacity for inpatient health care, “considerable experience” generating and managing response to regional and local public health emergencies, and strong clinical processes focused on evidence-based guidelines. However, “[w]ithout national analytics of data from outbreaks in other nations, and without a national plan addressing the VHA role, forecasting demand for VHA inpatient services under the Fourth Mission required assumptions with a high degree of uncertainty.”
VHA planners adapted the existing High Consequence Infections Base Plan to COVID-19 and then developed the COVID-19 Response Plan as an annex to that. They released their plan to the public in the interest of a coordinated national response—although not all states were aware of VHA’s important safety-net capabilities. Despite that, the report says, during the pandemic, the mission assignments under the VA’s Fourth Mission have grown to the greatest scale and scope in the VA’s history.
“[H]ealth care in the United States will never be the same,” said Richard Stone, MD, VHA Executive in Charge, in his foreword to the report. Much of what we now consider routine, he said, such as parking lot screenings, digital questionnaires and rapid testing “were revolutionary and challenging to implement” when the pandemic began. “While we are certainly not perfect, we are a learning organization and seek to always find ways to improve.”
Identifying root causes for complex process problems is essential to improvement, the report authors say, and require “new knowledge.” To that end, the VA also has played a critical role in COVID-19–related research, participating in more than 90 and leading 28 multiple-site COVID-19 research studies, including research on 3D-printed respirator masks and convalescent plasma treatment.
The VA’s pandemic response has been “robust and far-reaching,” said VA Secretary Robert Wilkie. The report, he adds, “reflects VA’s agility throughout the pandemic to adapt based on lessons learned.”
The numbers tell the story:
110,066 veterans diagnosed with COVID-19 as of November 30;
879,457 veterans and employees tested for COVID-19 as of November 6;
14,168 veterans admitted to a US Department of Veterans Affairs (VA) medical center for COVID-19 care;
1,525% increase in telehealth visits;
59,095 new staff hired to meet surge in demand for COVID-19 care;
75 completed Fourth Mission assignments; and
> 2,000 VA employees helping to support nonveteran patients and non-VA health care systems.
But those numbers are just some of the data in the COVID-19 Response Report, which the VA recently released. The report offers “an extensive look at VA’s complex COVID-19 response,” including how it prepared for the pandemic, the initial response, and key COVID-19 policies and directives.
The report was compiled from more than 90 interviews with health care leaders and stakeholders, along with documents and data pertaining to the Veterans Integrated Service Networks. The interviews were designed to “keep discussion at a strategic level.”
Meeting the crisis mandated that the Veterans Health Administration (VHA) act “with unity of effort and agility,” the authors note, across 18 networks with 170 medical centers. Not only is the VA called on to serve veterans, but its “Fourth Mission” explicitly calls on the VA to “improve the Nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters.” But the VHA possessed some major assets, they add, including a nationwide capacity for inpatient health care, “considerable experience” generating and managing response to regional and local public health emergencies, and strong clinical processes focused on evidence-based guidelines. However, “[w]ithout national analytics of data from outbreaks in other nations, and without a national plan addressing the VHA role, forecasting demand for VHA inpatient services under the Fourth Mission required assumptions with a high degree of uncertainty.”
VHA planners adapted the existing High Consequence Infections Base Plan to COVID-19 and then developed the COVID-19 Response Plan as an annex to that. They released their plan to the public in the interest of a coordinated national response—although not all states were aware of VHA’s important safety-net capabilities. Despite that, the report says, during the pandemic, the mission assignments under the VA’s Fourth Mission have grown to the greatest scale and scope in the VA’s history.
“[H]ealth care in the United States will never be the same,” said Richard Stone, MD, VHA Executive in Charge, in his foreword to the report. Much of what we now consider routine, he said, such as parking lot screenings, digital questionnaires and rapid testing “were revolutionary and challenging to implement” when the pandemic began. “While we are certainly not perfect, we are a learning organization and seek to always find ways to improve.”
Identifying root causes for complex process problems is essential to improvement, the report authors say, and require “new knowledge.” To that end, the VA also has played a critical role in COVID-19–related research, participating in more than 90 and leading 28 multiple-site COVID-19 research studies, including research on 3D-printed respirator masks and convalescent plasma treatment.
The VA’s pandemic response has been “robust and far-reaching,” said VA Secretary Robert Wilkie. The report, he adds, “reflects VA’s agility throughout the pandemic to adapt based on lessons learned.”
The numbers tell the story:
110,066 veterans diagnosed with COVID-19 as of November 30;
879,457 veterans and employees tested for COVID-19 as of November 6;
14,168 veterans admitted to a US Department of Veterans Affairs (VA) medical center for COVID-19 care;
1,525% increase in telehealth visits;
59,095 new staff hired to meet surge in demand for COVID-19 care;
75 completed Fourth Mission assignments; and
> 2,000 VA employees helping to support nonveteran patients and non-VA health care systems.
But those numbers are just some of the data in the COVID-19 Response Report, which the VA recently released. The report offers “an extensive look at VA’s complex COVID-19 response,” including how it prepared for the pandemic, the initial response, and key COVID-19 policies and directives.
The report was compiled from more than 90 interviews with health care leaders and stakeholders, along with documents and data pertaining to the Veterans Integrated Service Networks. The interviews were designed to “keep discussion at a strategic level.”
Meeting the crisis mandated that the Veterans Health Administration (VHA) act “with unity of effort and agility,” the authors note, across 18 networks with 170 medical centers. Not only is the VA called on to serve veterans, but its “Fourth Mission” explicitly calls on the VA to “improve the Nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters.” But the VHA possessed some major assets, they add, including a nationwide capacity for inpatient health care, “considerable experience” generating and managing response to regional and local public health emergencies, and strong clinical processes focused on evidence-based guidelines. However, “[w]ithout national analytics of data from outbreaks in other nations, and without a national plan addressing the VHA role, forecasting demand for VHA inpatient services under the Fourth Mission required assumptions with a high degree of uncertainty.”
VHA planners adapted the existing High Consequence Infections Base Plan to COVID-19 and then developed the COVID-19 Response Plan as an annex to that. They released their plan to the public in the interest of a coordinated national response—although not all states were aware of VHA’s important safety-net capabilities. Despite that, the report says, during the pandemic, the mission assignments under the VA’s Fourth Mission have grown to the greatest scale and scope in the VA’s history.
“[H]ealth care in the United States will never be the same,” said Richard Stone, MD, VHA Executive in Charge, in his foreword to the report. Much of what we now consider routine, he said, such as parking lot screenings, digital questionnaires and rapid testing “were revolutionary and challenging to implement” when the pandemic began. “While we are certainly not perfect, we are a learning organization and seek to always find ways to improve.”
Identifying root causes for complex process problems is essential to improvement, the report authors say, and require “new knowledge.” To that end, the VA also has played a critical role in COVID-19–related research, participating in more than 90 and leading 28 multiple-site COVID-19 research studies, including research on 3D-printed respirator masks and convalescent plasma treatment.
The VA’s pandemic response has been “robust and far-reaching,” said VA Secretary Robert Wilkie. The report, he adds, “reflects VA’s agility throughout the pandemic to adapt based on lessons learned.”
Caring for outpatients during COVID-19: 4 themes
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
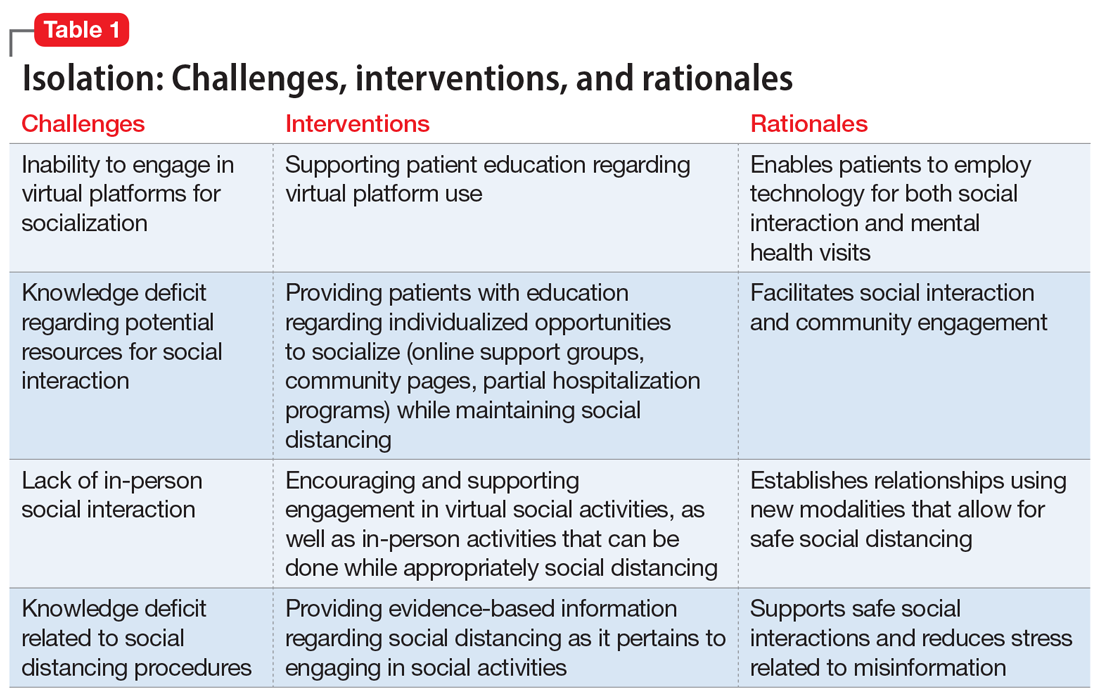
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.
Uncertainty
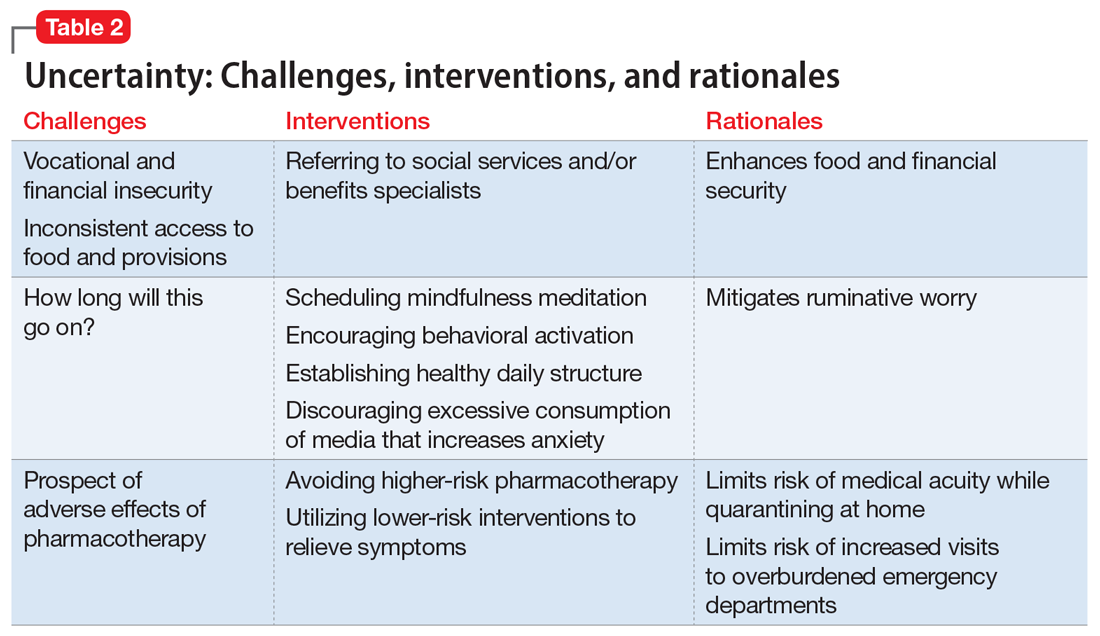
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11
Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

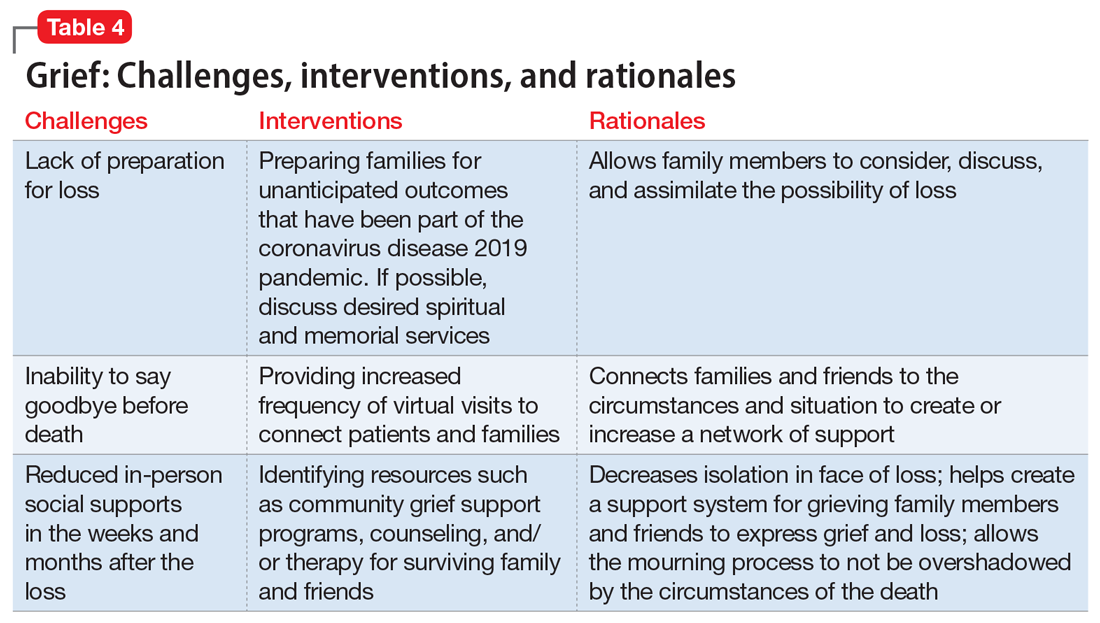
Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16
Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.

Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11

Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16

Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.

Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11

Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16

Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
Efforts underway to eradicate racism in photomedicine
For one thing, melanin’s extinction overlaps with common laser lines, which affects the safety and efficacy of laser treatments in dermatology, but also in imaging and wearable devices that use LEDs in the visible range. “Pheomelanin and eumelanin are chemically very similar and both have this property of having very high extinction coefficients in the visible range, meaning that melanins both absorb and scatter light which we commonly use for laser treatments and for wearable medical devices,” Dr. Marks, a research scientist in dermatology at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Melanins also shadow a number of other biological signals that we look for in the skin, such as those from hemoglobin.”
A number of different scales can be used to estimate the amount of eumelanin, or darkly pigmented melanin, in the skin, but the most famous is Fitzpatrick skin typing, the classification system that ranges from I to VI originally intended to quantify the skin’s response to UV light. “It’s so famous that it’s used in the emoji modifier of the Unicode Consortium lookup table,” said Dr. Marks, who spoke on behalf of the Wellman Anti-Racism Effort (WARE), a grassroots working group within the Wellman Center for Photomedicine at Massachusetts General Hospital. (The mission of the group is to eradicate racism in STEM, medicine, and academia starting with its own research and Center.)
Dr. Marks referred to a Northwestern University study published in 2013, which found that both patients and dermatologists failed to accurately determine Fitzpatrick skin type (FST) when compared with reflectance spectrophotometry used to measure melanin index objectively. “There is a need to classify skin type with reliable questions with responses suitable for all skin types,” the authors concluded.
Plenty more can go wrong when clinicians ignore or misunderstand the role of melanin as a background contrast agent, Dr. Marks continued. She cited the common misconception that melanomas do not occur in darker pigmented skin, a topic discussed in an article published online in January 2020 in Cancer Cytopathology.
“While they do occur at a lower rate, this misconception leads to an alarmingly low survival rate for black melanoma patients,” Dr. Marks said. “Acral lentiginous melanoma is one example of this. It is not related to sun exposure, yet it occurs in 30% to 70% of melanomas in black patients. This also exposes a mortality rate of 1 in 3 for Black melanoma patients, compared with 1 in 11 for White patients. In fact, Black patients face a lower survival for most cancers, often attributed to social and economic disparities rather than biological differences.”
Another significant contributing factor may be the lack of data and awareness of clinical research related to patients with skin of color. The Skin of Color Society’s “Find a Doctor” database is attempting to address this by improving patients’ access to board-certified dermatologists who specialize in skin of color. “Some of the discrepancies in dermatology education, screening, and treatment for Black, indigenous, and people of color is likely attributed to the fact that only 4.5% of images in general medical textbooks show darker skin, as they are only 5% of clinical trial participants despite making up 17% of the U.S. population,” Dr. Marks said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Mind The Gap, a handbook of clinical signs and symptoms in black and brown skin, was published in 2020 by students and staff at St. George’s University of London. It can be downloaded for free.
Some 40 years after Kodak was criticized for not acknowledging inherent biases in their film stocks based on its “Kodak Shirley” color correction card, Dr. Marks said that camera makers are still ignoring racial bias in their technologies. “This is likely a ‘garbage in, garbage out’ phenomenon,” she said. “Due to the lack of diverse images, these biases get ingrained into machine learning models themselves, either because patients were not served in the first place, resulting in missing data, or because of mislabeling due to a lack of knowledge in properly classifying these images. So, while machine learning has the potential to step in where dermatologists fall short, we must be very diligent about recognizing any bias we are ingraining into these algorithms,” she said.
“That said, no technology is ‘born racist,’ of course; it is up to us to prevent history from repeating itself and prevent these biases from being ingrained in our work,” she added. “We can start by holding ourselves and others accountable when designing studies that have exclusion criteria, by challenging our sponsors on the exclusion of Fitzpatrick V and VI if you feel it is not scientifically sound, and by ensuring inclusive algorithm development. If these things are not possible, please use a disclaimer to make these limitations clear.”
According to Dr. Marks and WARE, clinicians can increase diversity in clinical trials by widening eligibility criteria, tapping into community-based medical centers, connecting with patient advocacy groups, using point-of-care and telemedicine technologies, supporting diversity-focused public policy on a larger scale, and making diversity an internal mandate, “within your institution, and within yourselves.”
Some community efforts stemming from Wellman inventions so far include the Texas-based Removery INK-nitiative program, which removes racist and hateful tattoos for free via laser tattoo removal technology that was invented at Wellman. Dr. Marks and her WARE colleagues also work with the Dream Beam Foundation, which is a global initiative bringing laser-based technologies to children in Vietnam, Armenia, Israel, Brazil, and Lebanon.
Dr. Marks reported having no financial disclosures.
For one thing, melanin’s extinction overlaps with common laser lines, which affects the safety and efficacy of laser treatments in dermatology, but also in imaging and wearable devices that use LEDs in the visible range. “Pheomelanin and eumelanin are chemically very similar and both have this property of having very high extinction coefficients in the visible range, meaning that melanins both absorb and scatter light which we commonly use for laser treatments and for wearable medical devices,” Dr. Marks, a research scientist in dermatology at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Melanins also shadow a number of other biological signals that we look for in the skin, such as those from hemoglobin.”
A number of different scales can be used to estimate the amount of eumelanin, or darkly pigmented melanin, in the skin, but the most famous is Fitzpatrick skin typing, the classification system that ranges from I to VI originally intended to quantify the skin’s response to UV light. “It’s so famous that it’s used in the emoji modifier of the Unicode Consortium lookup table,” said Dr. Marks, who spoke on behalf of the Wellman Anti-Racism Effort (WARE), a grassroots working group within the Wellman Center for Photomedicine at Massachusetts General Hospital. (The mission of the group is to eradicate racism in STEM, medicine, and academia starting with its own research and Center.)
Dr. Marks referred to a Northwestern University study published in 2013, which found that both patients and dermatologists failed to accurately determine Fitzpatrick skin type (FST) when compared with reflectance spectrophotometry used to measure melanin index objectively. “There is a need to classify skin type with reliable questions with responses suitable for all skin types,” the authors concluded.
Plenty more can go wrong when clinicians ignore or misunderstand the role of melanin as a background contrast agent, Dr. Marks continued. She cited the common misconception that melanomas do not occur in darker pigmented skin, a topic discussed in an article published online in January 2020 in Cancer Cytopathology.
“While they do occur at a lower rate, this misconception leads to an alarmingly low survival rate for black melanoma patients,” Dr. Marks said. “Acral lentiginous melanoma is one example of this. It is not related to sun exposure, yet it occurs in 30% to 70% of melanomas in black patients. This also exposes a mortality rate of 1 in 3 for Black melanoma patients, compared with 1 in 11 for White patients. In fact, Black patients face a lower survival for most cancers, often attributed to social and economic disparities rather than biological differences.”
Another significant contributing factor may be the lack of data and awareness of clinical research related to patients with skin of color. The Skin of Color Society’s “Find a Doctor” database is attempting to address this by improving patients’ access to board-certified dermatologists who specialize in skin of color. “Some of the discrepancies in dermatology education, screening, and treatment for Black, indigenous, and people of color is likely attributed to the fact that only 4.5% of images in general medical textbooks show darker skin, as they are only 5% of clinical trial participants despite making up 17% of the U.S. population,” Dr. Marks said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Mind The Gap, a handbook of clinical signs and symptoms in black and brown skin, was published in 2020 by students and staff at St. George’s University of London. It can be downloaded for free.
Some 40 years after Kodak was criticized for not acknowledging inherent biases in their film stocks based on its “Kodak Shirley” color correction card, Dr. Marks said that camera makers are still ignoring racial bias in their technologies. “This is likely a ‘garbage in, garbage out’ phenomenon,” she said. “Due to the lack of diverse images, these biases get ingrained into machine learning models themselves, either because patients were not served in the first place, resulting in missing data, or because of mislabeling due to a lack of knowledge in properly classifying these images. So, while machine learning has the potential to step in where dermatologists fall short, we must be very diligent about recognizing any bias we are ingraining into these algorithms,” she said.
“That said, no technology is ‘born racist,’ of course; it is up to us to prevent history from repeating itself and prevent these biases from being ingrained in our work,” she added. “We can start by holding ourselves and others accountable when designing studies that have exclusion criteria, by challenging our sponsors on the exclusion of Fitzpatrick V and VI if you feel it is not scientifically sound, and by ensuring inclusive algorithm development. If these things are not possible, please use a disclaimer to make these limitations clear.”
According to Dr. Marks and WARE, clinicians can increase diversity in clinical trials by widening eligibility criteria, tapping into community-based medical centers, connecting with patient advocacy groups, using point-of-care and telemedicine technologies, supporting diversity-focused public policy on a larger scale, and making diversity an internal mandate, “within your institution, and within yourselves.”
Some community efforts stemming from Wellman inventions so far include the Texas-based Removery INK-nitiative program, which removes racist and hateful tattoos for free via laser tattoo removal technology that was invented at Wellman. Dr. Marks and her WARE colleagues also work with the Dream Beam Foundation, which is a global initiative bringing laser-based technologies to children in Vietnam, Armenia, Israel, Brazil, and Lebanon.
Dr. Marks reported having no financial disclosures.
For one thing, melanin’s extinction overlaps with common laser lines, which affects the safety and efficacy of laser treatments in dermatology, but also in imaging and wearable devices that use LEDs in the visible range. “Pheomelanin and eumelanin are chemically very similar and both have this property of having very high extinction coefficients in the visible range, meaning that melanins both absorb and scatter light which we commonly use for laser treatments and for wearable medical devices,” Dr. Marks, a research scientist in dermatology at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Melanins also shadow a number of other biological signals that we look for in the skin, such as those from hemoglobin.”
A number of different scales can be used to estimate the amount of eumelanin, or darkly pigmented melanin, in the skin, but the most famous is Fitzpatrick skin typing, the classification system that ranges from I to VI originally intended to quantify the skin’s response to UV light. “It’s so famous that it’s used in the emoji modifier of the Unicode Consortium lookup table,” said Dr. Marks, who spoke on behalf of the Wellman Anti-Racism Effort (WARE), a grassroots working group within the Wellman Center for Photomedicine at Massachusetts General Hospital. (The mission of the group is to eradicate racism in STEM, medicine, and academia starting with its own research and Center.)
Dr. Marks referred to a Northwestern University study published in 2013, which found that both patients and dermatologists failed to accurately determine Fitzpatrick skin type (FST) when compared with reflectance spectrophotometry used to measure melanin index objectively. “There is a need to classify skin type with reliable questions with responses suitable for all skin types,” the authors concluded.
Plenty more can go wrong when clinicians ignore or misunderstand the role of melanin as a background contrast agent, Dr. Marks continued. She cited the common misconception that melanomas do not occur in darker pigmented skin, a topic discussed in an article published online in January 2020 in Cancer Cytopathology.
“While they do occur at a lower rate, this misconception leads to an alarmingly low survival rate for black melanoma patients,” Dr. Marks said. “Acral lentiginous melanoma is one example of this. It is not related to sun exposure, yet it occurs in 30% to 70% of melanomas in black patients. This also exposes a mortality rate of 1 in 3 for Black melanoma patients, compared with 1 in 11 for White patients. In fact, Black patients face a lower survival for most cancers, often attributed to social and economic disparities rather than biological differences.”
Another significant contributing factor may be the lack of data and awareness of clinical research related to patients with skin of color. The Skin of Color Society’s “Find a Doctor” database is attempting to address this by improving patients’ access to board-certified dermatologists who specialize in skin of color. “Some of the discrepancies in dermatology education, screening, and treatment for Black, indigenous, and people of color is likely attributed to the fact that only 4.5% of images in general medical textbooks show darker skin, as they are only 5% of clinical trial participants despite making up 17% of the U.S. population,” Dr. Marks said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Mind The Gap, a handbook of clinical signs and symptoms in black and brown skin, was published in 2020 by students and staff at St. George’s University of London. It can be downloaded for free.
Some 40 years after Kodak was criticized for not acknowledging inherent biases in their film stocks based on its “Kodak Shirley” color correction card, Dr. Marks said that camera makers are still ignoring racial bias in their technologies. “This is likely a ‘garbage in, garbage out’ phenomenon,” she said. “Due to the lack of diverse images, these biases get ingrained into machine learning models themselves, either because patients were not served in the first place, resulting in missing data, or because of mislabeling due to a lack of knowledge in properly classifying these images. So, while machine learning has the potential to step in where dermatologists fall short, we must be very diligent about recognizing any bias we are ingraining into these algorithms,” she said.
“That said, no technology is ‘born racist,’ of course; it is up to us to prevent history from repeating itself and prevent these biases from being ingrained in our work,” she added. “We can start by holding ourselves and others accountable when designing studies that have exclusion criteria, by challenging our sponsors on the exclusion of Fitzpatrick V and VI if you feel it is not scientifically sound, and by ensuring inclusive algorithm development. If these things are not possible, please use a disclaimer to make these limitations clear.”
According to Dr. Marks and WARE, clinicians can increase diversity in clinical trials by widening eligibility criteria, tapping into community-based medical centers, connecting with patient advocacy groups, using point-of-care and telemedicine technologies, supporting diversity-focused public policy on a larger scale, and making diversity an internal mandate, “within your institution, and within yourselves.”
Some community efforts stemming from Wellman inventions so far include the Texas-based Removery INK-nitiative program, which removes racist and hateful tattoos for free via laser tattoo removal technology that was invented at Wellman. Dr. Marks and her WARE colleagues also work with the Dream Beam Foundation, which is a global initiative bringing laser-based technologies to children in Vietnam, Armenia, Israel, Brazil, and Lebanon.
Dr. Marks reported having no financial disclosures.
EXPERT ANALYSIS FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Patient health suffers amid pandemic health care shortages
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
Managing metabolic syndrome in patients with schizophrenia
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.
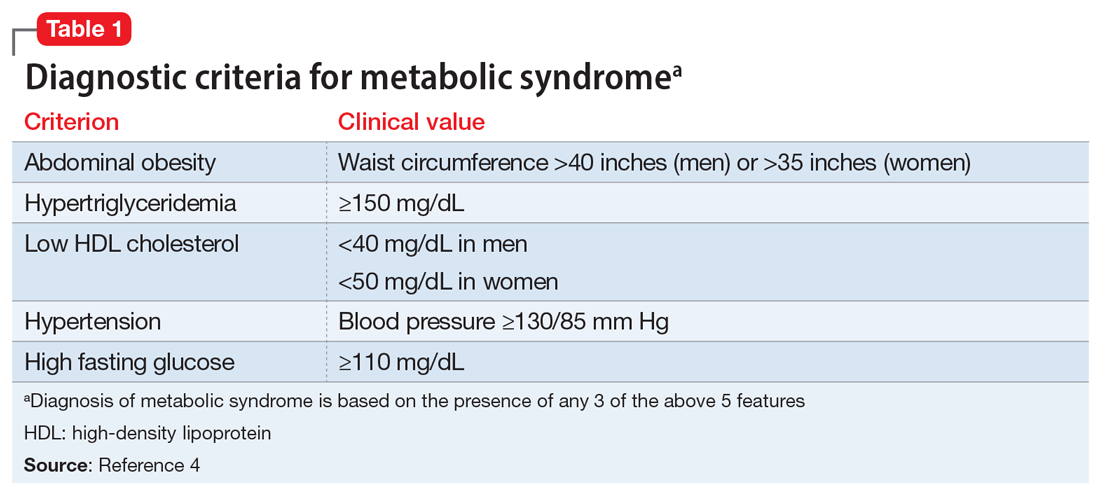
Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
Scientific power will save us
COVID-19 numbers again are increasing dramatically. Community infection rates have nearly doubled, and hospitals and health care workers are stretched beyond their limits. It is difficult not to feel anger about how poorly this pandemic was managed (mismanaged) by so many officials in charge and by a large segment of our population who still refuse protective actions to limit spread. While politics and ideology continue to cost American lives, scientific firepower will emerge as our saving grace.
My editorial board and I are entering our final year at the helm of GI & Hepatology News. AGA issued a search for the next Editor in Chief (EIC), who will take over October 2021. I urge anyone interested to apply (https://gastro.org/news/prestigious-aga-publications-seek-new-editors-in-chief/). As EIC, you will choose the next editorial board and forge professional friendships that are gratifying. You will assume responsibility for the content, where you must balance your own views with those of both the AGA and our readership.
As EIC, each month I am given space for 300 words to communicate interesting ideas and opinions. The AGA gives the newspaper great editorial freedom, and I hope we have supported AGA’s mission and values when we publish its official newspaper. I always have next month’s editorial in mind, and I look for useful phrases, quotes, ideas, and opinions. If you are interested in becoming EIC, please email [email protected] for more information.
I would be remiss not to acknowledge the contribution that Lora McGlade has made to GI & Hepatology News. She has been my partner, as the Frontline Medical Communications Editor in charge of GI & Hepatology News. Next month, she will move on to assume a new role. I cannot thank her enough for helping make this newspaper work. As the months go on, I will highlight the contributions of others from the AGA, our Board, and Frontline.
Please stay safe and do not let your guard down. COVID-19 is merciless and relentless. “If you think research is expensive, try disease.” – Mary Lasker.
John I. Allen, MD, MBA, AGA
Editor in Chief
COVID-19 numbers again are increasing dramatically. Community infection rates have nearly doubled, and hospitals and health care workers are stretched beyond their limits. It is difficult not to feel anger about how poorly this pandemic was managed (mismanaged) by so many officials in charge and by a large segment of our population who still refuse protective actions to limit spread. While politics and ideology continue to cost American lives, scientific firepower will emerge as our saving grace.
My editorial board and I are entering our final year at the helm of GI & Hepatology News. AGA issued a search for the next Editor in Chief (EIC), who will take over October 2021. I urge anyone interested to apply (https://gastro.org/news/prestigious-aga-publications-seek-new-editors-in-chief/). As EIC, you will choose the next editorial board and forge professional friendships that are gratifying. You will assume responsibility for the content, where you must balance your own views with those of both the AGA and our readership.
As EIC, each month I am given space for 300 words to communicate interesting ideas and opinions. The AGA gives the newspaper great editorial freedom, and I hope we have supported AGA’s mission and values when we publish its official newspaper. I always have next month’s editorial in mind, and I look for useful phrases, quotes, ideas, and opinions. If you are interested in becoming EIC, please email [email protected] for more information.
I would be remiss not to acknowledge the contribution that Lora McGlade has made to GI & Hepatology News. She has been my partner, as the Frontline Medical Communications Editor in charge of GI & Hepatology News. Next month, she will move on to assume a new role. I cannot thank her enough for helping make this newspaper work. As the months go on, I will highlight the contributions of others from the AGA, our Board, and Frontline.
Please stay safe and do not let your guard down. COVID-19 is merciless and relentless. “If you think research is expensive, try disease.” – Mary Lasker.
John I. Allen, MD, MBA, AGA
Editor in Chief
COVID-19 numbers again are increasing dramatically. Community infection rates have nearly doubled, and hospitals and health care workers are stretched beyond their limits. It is difficult not to feel anger about how poorly this pandemic was managed (mismanaged) by so many officials in charge and by a large segment of our population who still refuse protective actions to limit spread. While politics and ideology continue to cost American lives, scientific firepower will emerge as our saving grace.
My editorial board and I are entering our final year at the helm of GI & Hepatology News. AGA issued a search for the next Editor in Chief (EIC), who will take over October 2021. I urge anyone interested to apply (https://gastro.org/news/prestigious-aga-publications-seek-new-editors-in-chief/). As EIC, you will choose the next editorial board and forge professional friendships that are gratifying. You will assume responsibility for the content, where you must balance your own views with those of both the AGA and our readership.
As EIC, each month I am given space for 300 words to communicate interesting ideas and opinions. The AGA gives the newspaper great editorial freedom, and I hope we have supported AGA’s mission and values when we publish its official newspaper. I always have next month’s editorial in mind, and I look for useful phrases, quotes, ideas, and opinions. If you are interested in becoming EIC, please email [email protected] for more information.
I would be remiss not to acknowledge the contribution that Lora McGlade has made to GI & Hepatology News. She has been my partner, as the Frontline Medical Communications Editor in charge of GI & Hepatology News. Next month, she will move on to assume a new role. I cannot thank her enough for helping make this newspaper work. As the months go on, I will highlight the contributions of others from the AGA, our Board, and Frontline.
Please stay safe and do not let your guard down. COVID-19 is merciless and relentless. “If you think research is expensive, try disease.” – Mary Lasker.
John I. Allen, MD, MBA, AGA
Editor in Chief
How much longer?
SHM has changed direction as needed during the pandemic
“How much longer?” As a kid, I can remember the long holiday car ride with my parents from my home in Annapolis, Md., to Upstate New York where my grandparents lived. At the time, the ride felt like an eternity: endless miles of frozen landscape, limited food, and a brother who constantly crossed over the invisible line that was my side of the car.
We made our parents crazy asking, “how much longer?” every few minutes. This was the late 1970s, with no GPS or Google Maps to give you arrival times to the minute, traffic warnings, or reroutes when the inevitable delays occurred. We just plowed ahead, and my parents’ answer was always something vague like, “in a few hours” or “we’re about halfway through.” They did not know when we’d arrive with certainty either.
We at SHM have that same feeling about the pandemic. How much longer? No one can tell us when the COVID-19 threat will abate. The experts’ answers are understandably vague, and the tools for forecasting are non-existent. Months? That is the best we know for now.
At SHM, we believe we will make it through this journey by adapting to roadblocks, providing tools for success to our professional community, and identifying opportunities for us to connect with each other, even if that means virtually.
Like the rest of the planet, the spring of 2020 hit SHM with a shock. Hospital Medicine 2020 (HM20) in San Diego was shaping up to be the largest Annual Conference SHM ever had, the Pediatric Hospital Medicine 2020 (PHM20) conference was well planned and expected to be a huge success, regional SHM chapters were meeting (and growing), and membership was thriving. I was transitioning out of my roles at Johns Hopkins and looking forward to my new role as CEO of SHM. All in all, March 2020 began with a fantastic outlook.
Wow, what a difference a few weeks made. We watched as the pandemic spread across regions of the country, concerned for the wellbeing of our patients and our hospitalists. We saw how our members were at the forefront of patient care during this crisis and understood that SHM had to adapt rapidly to meet their needs in real time.
By May, SHM had canceled HM20, Chapter activity was halted, PHM20 was on its way to being canceled, SHM committee work was put on hold, and I was spending my last few months at Hopkins as the chief medical officer at the Baltimore Convention Center Field Hospital (which we got up and running in less than a month)! Whew.
But just like my dad could pivot our 1970s Chevy station wagon around a traffic jam in a flash, so too did SHM leadership start navigating around the COVID-19 landscape. As soon as HM20 was canceled, SHM immediately began planning for a virtual offering in August. We had hoped to attract at least 100 attendees and we were thrilled to have more than 1,000! PHM20 was switched from an in-person to a virtual meeting with 634 attendees. We launched numerous COVID-19 webinars and made our clinical and educational offerings open access. Our Public Policy Committee was active around both COVID-19 and hospitalist-related topics – immigration, telehealth, wellbeing, and financial impacts, to name a few. (And I even met with the POTUS & advocated for PPE.) The Journal of Hospital Medicine worked with authors to get important publications out at record speed. And of course, The Hospitalist connected all of us to our professional leaders and experts.
By the fall of 2020, SHM had actively adjusted to the “new normal” of this pandemic: SHM staff have settled into their new “work from home” environments, SHM Chapters are connecting members in the virtual world, SHM’s 2021 Annual Conference will be all virtual – rebranded as “SHM Converge” – and the State of Hospital Medicine Report (our every-other-year source for trends in hospital medicine) now has a COVID-19 supplement, which was developed at lightning speed. Even our SHM Board of Directors is meeting virtually! All this while advancing the routine work at SHM, which never faltered. Our work on resources for quality improvement, the opioid epidemic, wellbeing, diversity, equity and inclusion (DEI), leadership, professional development, advocacy, and so much more is as active as ever.
I don’t know how much longer we have on this very long pandemic journey, so I’ll use my father’s answer of “we’re about halfway through.” We have been immersed in it for months already, with months still ahead. But regardless of the upcoming twists and turns COVID-19 forces you, our patients, and our larger society to take, SHM is ready to change direction faster than a 1970s Chevy. The SHM staff, leadership, and members will be sure that hospitalists receive the tools to navigate these unprecedented times. Our patients need our skills to get through this as safely as possible. While we may not be able to tell them “how much longer,” we can certainly be prepared for the long road ahead as we begin 2021.
Dr. Howell is CEO of the Society of Hospital Medicine.
SHM has changed direction as needed during the pandemic
SHM has changed direction as needed during the pandemic
“How much longer?” As a kid, I can remember the long holiday car ride with my parents from my home in Annapolis, Md., to Upstate New York where my grandparents lived. At the time, the ride felt like an eternity: endless miles of frozen landscape, limited food, and a brother who constantly crossed over the invisible line that was my side of the car.
We made our parents crazy asking, “how much longer?” every few minutes. This was the late 1970s, with no GPS or Google Maps to give you arrival times to the minute, traffic warnings, or reroutes when the inevitable delays occurred. We just plowed ahead, and my parents’ answer was always something vague like, “in a few hours” or “we’re about halfway through.” They did not know when we’d arrive with certainty either.
We at SHM have that same feeling about the pandemic. How much longer? No one can tell us when the COVID-19 threat will abate. The experts’ answers are understandably vague, and the tools for forecasting are non-existent. Months? That is the best we know for now.
At SHM, we believe we will make it through this journey by adapting to roadblocks, providing tools for success to our professional community, and identifying opportunities for us to connect with each other, even if that means virtually.
Like the rest of the planet, the spring of 2020 hit SHM with a shock. Hospital Medicine 2020 (HM20) in San Diego was shaping up to be the largest Annual Conference SHM ever had, the Pediatric Hospital Medicine 2020 (PHM20) conference was well planned and expected to be a huge success, regional SHM chapters were meeting (and growing), and membership was thriving. I was transitioning out of my roles at Johns Hopkins and looking forward to my new role as CEO of SHM. All in all, March 2020 began with a fantastic outlook.
Wow, what a difference a few weeks made. We watched as the pandemic spread across regions of the country, concerned for the wellbeing of our patients and our hospitalists. We saw how our members were at the forefront of patient care during this crisis and understood that SHM had to adapt rapidly to meet their needs in real time.
By May, SHM had canceled HM20, Chapter activity was halted, PHM20 was on its way to being canceled, SHM committee work was put on hold, and I was spending my last few months at Hopkins as the chief medical officer at the Baltimore Convention Center Field Hospital (which we got up and running in less than a month)! Whew.
But just like my dad could pivot our 1970s Chevy station wagon around a traffic jam in a flash, so too did SHM leadership start navigating around the COVID-19 landscape. As soon as HM20 was canceled, SHM immediately began planning for a virtual offering in August. We had hoped to attract at least 100 attendees and we were thrilled to have more than 1,000! PHM20 was switched from an in-person to a virtual meeting with 634 attendees. We launched numerous COVID-19 webinars and made our clinical and educational offerings open access. Our Public Policy Committee was active around both COVID-19 and hospitalist-related topics – immigration, telehealth, wellbeing, and financial impacts, to name a few. (And I even met with the POTUS & advocated for PPE.) The Journal of Hospital Medicine worked with authors to get important publications out at record speed. And of course, The Hospitalist connected all of us to our professional leaders and experts.
By the fall of 2020, SHM had actively adjusted to the “new normal” of this pandemic: SHM staff have settled into their new “work from home” environments, SHM Chapters are connecting members in the virtual world, SHM’s 2021 Annual Conference will be all virtual – rebranded as “SHM Converge” – and the State of Hospital Medicine Report (our every-other-year source for trends in hospital medicine) now has a COVID-19 supplement, which was developed at lightning speed. Even our SHM Board of Directors is meeting virtually! All this while advancing the routine work at SHM, which never faltered. Our work on resources for quality improvement, the opioid epidemic, wellbeing, diversity, equity and inclusion (DEI), leadership, professional development, advocacy, and so much more is as active as ever.
I don’t know how much longer we have on this very long pandemic journey, so I’ll use my father’s answer of “we’re about halfway through.” We have been immersed in it for months already, with months still ahead. But regardless of the upcoming twists and turns COVID-19 forces you, our patients, and our larger society to take, SHM is ready to change direction faster than a 1970s Chevy. The SHM staff, leadership, and members will be sure that hospitalists receive the tools to navigate these unprecedented times. Our patients need our skills to get through this as safely as possible. While we may not be able to tell them “how much longer,” we can certainly be prepared for the long road ahead as we begin 2021.
Dr. Howell is CEO of the Society of Hospital Medicine.
“How much longer?” As a kid, I can remember the long holiday car ride with my parents from my home in Annapolis, Md., to Upstate New York where my grandparents lived. At the time, the ride felt like an eternity: endless miles of frozen landscape, limited food, and a brother who constantly crossed over the invisible line that was my side of the car.
We made our parents crazy asking, “how much longer?” every few minutes. This was the late 1970s, with no GPS or Google Maps to give you arrival times to the minute, traffic warnings, or reroutes when the inevitable delays occurred. We just plowed ahead, and my parents’ answer was always something vague like, “in a few hours” or “we’re about halfway through.” They did not know when we’d arrive with certainty either.
We at SHM have that same feeling about the pandemic. How much longer? No one can tell us when the COVID-19 threat will abate. The experts’ answers are understandably vague, and the tools for forecasting are non-existent. Months? That is the best we know for now.
At SHM, we believe we will make it through this journey by adapting to roadblocks, providing tools for success to our professional community, and identifying opportunities for us to connect with each other, even if that means virtually.
Like the rest of the planet, the spring of 2020 hit SHM with a shock. Hospital Medicine 2020 (HM20) in San Diego was shaping up to be the largest Annual Conference SHM ever had, the Pediatric Hospital Medicine 2020 (PHM20) conference was well planned and expected to be a huge success, regional SHM chapters were meeting (and growing), and membership was thriving. I was transitioning out of my roles at Johns Hopkins and looking forward to my new role as CEO of SHM. All in all, March 2020 began with a fantastic outlook.
Wow, what a difference a few weeks made. We watched as the pandemic spread across regions of the country, concerned for the wellbeing of our patients and our hospitalists. We saw how our members were at the forefront of patient care during this crisis and understood that SHM had to adapt rapidly to meet their needs in real time.
By May, SHM had canceled HM20, Chapter activity was halted, PHM20 was on its way to being canceled, SHM committee work was put on hold, and I was spending my last few months at Hopkins as the chief medical officer at the Baltimore Convention Center Field Hospital (which we got up and running in less than a month)! Whew.
But just like my dad could pivot our 1970s Chevy station wagon around a traffic jam in a flash, so too did SHM leadership start navigating around the COVID-19 landscape. As soon as HM20 was canceled, SHM immediately began planning for a virtual offering in August. We had hoped to attract at least 100 attendees and we were thrilled to have more than 1,000! PHM20 was switched from an in-person to a virtual meeting with 634 attendees. We launched numerous COVID-19 webinars and made our clinical and educational offerings open access. Our Public Policy Committee was active around both COVID-19 and hospitalist-related topics – immigration, telehealth, wellbeing, and financial impacts, to name a few. (And I even met with the POTUS & advocated for PPE.) The Journal of Hospital Medicine worked with authors to get important publications out at record speed. And of course, The Hospitalist connected all of us to our professional leaders and experts.
By the fall of 2020, SHM had actively adjusted to the “new normal” of this pandemic: SHM staff have settled into their new “work from home” environments, SHM Chapters are connecting members in the virtual world, SHM’s 2021 Annual Conference will be all virtual – rebranded as “SHM Converge” – and the State of Hospital Medicine Report (our every-other-year source for trends in hospital medicine) now has a COVID-19 supplement, which was developed at lightning speed. Even our SHM Board of Directors is meeting virtually! All this while advancing the routine work at SHM, which never faltered. Our work on resources for quality improvement, the opioid epidemic, wellbeing, diversity, equity and inclusion (DEI), leadership, professional development, advocacy, and so much more is as active as ever.
I don’t know how much longer we have on this very long pandemic journey, so I’ll use my father’s answer of “we’re about halfway through.” We have been immersed in it for months already, with months still ahead. But regardless of the upcoming twists and turns COVID-19 forces you, our patients, and our larger society to take, SHM is ready to change direction faster than a 1970s Chevy. The SHM staff, leadership, and members will be sure that hospitalists receive the tools to navigate these unprecedented times. Our patients need our skills to get through this as safely as possible. While we may not be able to tell them “how much longer,” we can certainly be prepared for the long road ahead as we begin 2021.
Dr. Howell is CEO of the Society of Hospital Medicine.
What the Biden-Harris COVID-19 Advisory Board is missing
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
Moderna filing for FDA emergency COVID-19 vaccine approval, reports 94.1% efficacy
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.