User login
Frivolous lawsuits: Still a big threat to doctors?
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.
The psychiatric consequences of COVID-19: 8 Studies
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
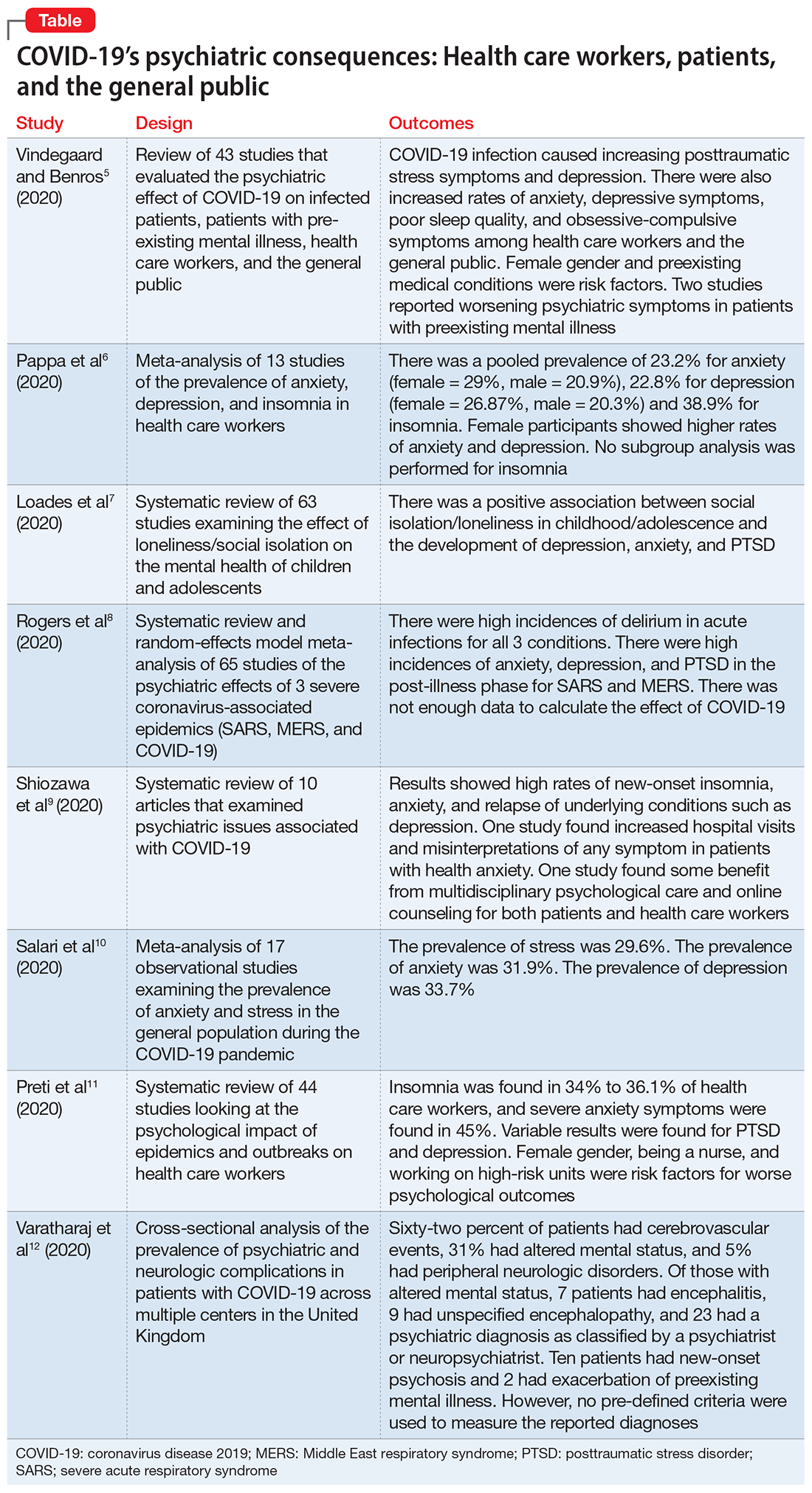
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.

1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.

1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Using seclusion to prevent COVID-19 transmission on inpatient psychiatry units
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.
Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.

Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
Mr. T, age 26, presents to the psychiatric emergency department with acutely worsening symptoms of schizophrenia. The treating team decides to admit him to the inpatient psychiatry unit. The patient agrees to admission bloodwork, but adamantly refuses a coronavirus disease 2019 (COVID-19) nasal swab, stating that he does not consent to “having COVID-19 injected into his nose.” His nurse pages the psychiatry resident on call, asking her for seclusion orders to be placed for the patient in order to quarantine him.
This case illustrates a quandary that has arisen during the COVID-19 era. Traditionally, the use of seclusion in inpatient psychiatry wards has been restricted to the management of violent or self-destructive behavior. Most guidelines advise that seclusion should be used only to ensure the immediate physical safety of a patient, staff members, or other patients.1 Using seclusion for other purposes, such as to quarantine patients suspected of having an infectious disease, raises ethical questions.
What is seclusion?
To best understand the questions that arise from the above scenario, a thorough understanding of the terminology used is needed. Although the terms “isolation,” “quarantine,” and “seclusion” are often used interchangeably, each has a distinct definition and unique history.
Isolation in a medical context refers to the practice of isolating people confirmed to have a disease from the general population. The earliest description of medical isolation dates back to the 7th century BC in the Book of Leviticus, which mentions a protocol for separating individuals infected with leprosy from those who are healthy.2
Quarantine hearkens back to the most fatal pandemic recorded in human history, the Black Death. In 1377, on the advice of the city’s chief physician, the Mediterranean seaport of Ragusa passed a law establishing an isolation period for all visitors from plague-endemic lands.2 Initially a 30-day isolation period (a trentino), this was extended to 40 days (a quarantino). Distinct from isolation, quarantine is the practice of limiting movements of apparently healthy individuals who may have been exposed to a disease but do not have a confirmed diagnosis.
Seclusion, a term used most often in psychiatry, is defined as “the involuntary confinement of a patient alone in a room or area from which the patient is physically prevented from leaving.”3 The use of seclusion rooms in psychiatric facilities was originally championed by the 19th century British psychiatrist John Conolly.4 In The Treatment of the Insane without Mechanical Restraints, Conolly argued that a padded seclusion room was far more humane and effective in calming a violent patient than mechanical restraints. After exhausting less restrictive measures, seclusion is one of the most common means of restraining violent patients in inpatient psychiatric facilities.
Why consider seclusion?
The discussion of using seclusion as a means of quarantine has arisen recently due to the COVID-19 pandemic. This infectious disease was first identified in December 2019 in Wuhan, China.5 Since then, it has spread rapidly across the world. As of mid-October 2020, >39 million cases across 189 countries had been reported.6 The primary means by which the virus is spread is through respiratory droplets released from infected individuals through coughing, sneezing, or talking.7 These droplets can remain airborne or fall onto surfaces that become fomites. Transmission is possible before symptoms appear in an infected individual or even from individuals who are asymptomatic.8
Continue to: The typical layout and requirements...
The typical layout and requirements of an inpatient psychiatric ward intensify the risk of COVID-19 transmission.9 Unlike most medical specialty wards, psychiatric wards are set up with a therapeutic milieu where patients have the opportunity to mingle and interact with each other and staff members. Patients are allowed to walk around the unit, spend time in group therapy, eat meals with each other, and have visitation hours. The therapeutic benefit of such a milieu, however, must be weighed against the risks that patients pose to staff members and other patients. While many facilities have restricted some of these activities to limit COVID-19 exposure, the overall risk of transmission is still elevated. Early in course of the pandemic, the virus spread to an inpatient psychiatric ward in South Korea. Although health officials put the ward on lockdown, given the heightened risk of transmission, the virus quickly spread from patient to patient. Out of 103 inpatients, 101 contracted COVID-19.10
To mitigate this risk, many inpatient psychiatric facilities have mandated that all newly admitted patients be tested for COVID-19. By obtaining COVID-19 testing, facilities are better able to risk stratify their patient population and appropriately protect all patients. A dilemma arises, however, when a patient refuses to consent to COVID-19 testing. In such cases, the infectious risk of the patient remains unknown. Given the potentially disastrous consequences of an unchecked COVID-19 infection running rampant in an inpatient ward, some facilities have elected to use seclusion as a means of quarantining the patient.
Is seclusion justifiable?
There are legitimate objections to using seclusion as a means of quarantine. Most guidelines state that the only time seclusion is ethical is when it is used to prevent immediate physical danger, either to the patient or others.11 Involuntary confinement entails considerable restriction of a patient’s rights and thus should be used only after all other options have been exhausted. People opposed to the use of seclusion point out that outside of the hospital, people are not forcibly restrained in order to enforce social distancing,12 so by extension, those who are inside the hospital should not be forced to seclude.
Seclusion also comes with potentially harmful effects. For the 14 days that a patient is in quarantine, they are cut off from most social contact, which is the opposite of the intended purpose of the therapeutic milieu in inpatient psychiatric wards. Several quantitative studies have shown that individuals who are quarantined tend to report a high prevalence of symptoms of psychological distress, including low mood, irritability, depression, stress, anger, and posttraumatic stress disorder.13
Furthermore, there is considerable evidence that a negative test does not definitively rule out a COVID-19 infection. Nasal swabs for COVID-19 have a false-negative rate of 27%.14 In other words, patients on an inpatient psychiatry ward who are free to walk around the unit and interact with others are only probably COVID-19 free, not definitively. This fact throws into question the original justification for seclusion—to protect other patients from COVID-19.
Continue to: Support for using seclusion as quarantine
Support for using seclusion as quarantine
Despite these objections, there are clear arguments in favor of using seclusion as a means of quarantine. First, the danger posed by an unidentified COVID-19 infection to the inpatient psychiatric population is not small. As of mid-October 2020, >217,000 Americans had died of COVID-19.6 Psychiatric patients, especially those who are acutely decompensated and hospitalized, have a heightened risk.15 Those with underlying medical issues are more likely to be seriously affected by an infection. Patients with serious mental illness have higher rates of medical comorbidities16 and premature death.17 The risk of a patient contracting and then dying from COVID-19 is elevated in an inpatient psychiatric ward. Even if a test is not 100% sensitive or specific, the balance of probability it provides is sufficient to make an informed decision about transmission risk.
In choosing to seclude a patient who refuses COVID-19 testing, the treating team must weigh one person’s autonomy against the safety of every other individual on the ward. From a purely utilitarian perspective, the lives of the many outweigh the discomfort of one. Addressing this balance, the American Medical Association (AMA) Code of Ethics states “Although physicians’ primary ethical obligation is to their individual patients, they also have a long-recognized public health responsibility. In the context of infectious disease, this may include the use of quarantine and isolation to reduce the transmission of disease and protect the health of the public. In such situations, physicians have a further responsibility to protect their own health to ensure that they remain able to provide care. These responsibilities potentially conflict with patients’ rights of self-determination and with physicians’ duty to advocate for the best interests of individual patients and to provide care in emergencies.”18
The AMA Code of Ethics further mentions that physicians should “support mandatory quarantine and isolation when a patient fails to adhere voluntarily.” Medical evidence supports both quarantine19 and enacting isolation measures for COVID-19–positive hospitalized patients.20 Table 121-24 summarizes the recommendations of major medical societies regarding isolation on hospital units.

Further, public health officials and law enforcement officials do in fact have the authority25 to enforce quarantine and restrict a citizen’s movement outside a hospital setting. Recent cases have illustrated how this has been enforced, particularly with the use of electronic monitoring units and even criminal sanctions.26,27
It is also important to consider that when used as quarantine, seclusion is not an indefinite action. Current recommendations suggest the longest period of time a patient would need to be in seclusion is 14 days. A patient could potentially reduce this period by agreeing to COVID-19 testing and obtaining a negative test result.
Continue to: Enacting inpatient quarantine
Enacting inpatient quarantine
In Mr. T’s case, the resident physician was asked to make a decision regarding seclusion on the spot. Prudent facilities will set policies and educate clinicians before they need to face this conundrum. The following practical considerations may guide implementation of seclusion as a measure of quarantine on an inpatient psychiatric unit:
- given the risk of asymptomatic carriers, all admitted patients should be tested for COVID-19
- patients who refuse a test should be evaluated by the psychiatrist on duty to determine if the patient has the capacity to make this decision
- if a patient demonstrates capacity to refuse and continues to refuse testing, seclusion orders should then be placed
- the facility should create a protocol to ensure consistent application of seclusion orders.
So that they can make an informed decision, patients should be educated about the risks of not undergoing testing. It is important to correctly frame a seclusion decision to the patient. Explain that seclusion is not a punitive measure, but rather a means of respecting the patient’s right to refuse testing while ensuring other patients’ right to be protected from COVID-19 transmission.
It is crucial to not allow psychiatric care to be diminished because a patient is isolated due to COVID-19. Psychiatrists have legal duties to provide care when a patient is admitted to their unit,28-30 and state laws generally outline patients’ rights while they are hospitalized.31 The use of technology can ensure these duties are fulfilled. Patient rounds and group treatment can be conducted through telehealth.10,32 When in-person interaction is required, caretakers should don proper personal protective equipment and interact with the patient as often as they would if the patient were not in seclusion. Table 233-36 summarizes further ethical considerations when implementing quarantine measures on a psychiatry unit.
The contemporary inpatient unit
The ideal design to optimize care and safety is to create designated COVID-19 psychiatric units. Indeed, the US Substance Abuse and Mental Health Services Administration recommends segregating floors based on infection status where possible.37 This minimizes the risk of transmission to other patients while maintaining the same standards of psychiatric treatment, including milieu and group therapy (which may also require adjustments). Such a unit already has precedent.38 Although designated COVID-19 psychiatric units present clinical and administrative hurdles,39 they may become more commonplace as the number of COVID-19–positive inpatients continues to rise.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has created challenges for inpatient psychiatric facilities. Although seclusion is a serious decision and should not be undertaken lightly, there are clear ethical and practical justifications for using it as a means of quarantine for patients who are COVID-19–positive or refuse testing.
Related Resources
- Askew L, Fisher P, Beazley P. What are adult psychiatric inpatients’ experience of seclusion: a systematic review of qualitative studies. J Psychiatr Ment Health Nurs. 2019; 26(7-8):274-285.
- Komrad MS. Medical ethics in the time of COVID-19. Current Psychiatry. 2020;19(7):29-32,46.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
1. Knox DK, Holloman GH Jr. Use and avoidance of seclusion and restraint: consensus statement of the American Association for Emergency Psychiatry Project BETA Seclusion and Restraint Workgroup. West J Emerg Med. 2012;13(1):35-40.
2. Sehdev PS. The origin of quarantine. Clin Infect Dis. 2002;35(9):1071-1072.
3. 42 CFR § 482.13. Condition of participation: patient’s rights.
4. Colaizzi J. Seclusion & restraint: a historical perspective. J Psychosoc Nurs Ment Health Serv. 2005;43(2):31-37.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
6. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed October 16, 2020.
7. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11.
8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407.
9. Li L. Challenges and priorities in responding to COVID-19 in inpatient psychiatry. Psychiatr Serv. 2020;71(6):624-626.
10. Kim MJ. ‘It was a medical disaster’: the psychiatric ward that saw 100 patients diagnosed with new coronavirus. Independent. https://www.independent.co.uk/news/world/asia/coronavirus-south-korea-outbreak-hospital-patients-lockdown-a9367486.html. Published March 1, 2020. Accessed July 12, 2020.
11. Petrini C. Ethical considerations for evaluating the issue of physical restraint in psychiatry. Ann Ist Super Sanita. 2013;49(3):281-285.
12. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed July 12, 2020.
13. Brooks SK, Webster RK, Smith, LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
14. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897.
15. Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiatry. 2020;77(9):891-892.
16. Rao S, Raney L, Xiong GL. Reducing medical comorbidity and mortality in severe mental illness. Current Psychiatry. 2015;14(7):14-20.
17. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827-1835.
18. American Medical Association. Ethical use of quarantine and isolation. Code of Ethics Opinion 8.4. https://www.ama-assn.org/delivering-care/ethics/ethical-use-quarantine-isolation. Published November 14, 2016. Accessed July 12, 2020.
19. Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4(4):CD013574.
20. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Duration of isolation & precautions for adults. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Updated August 16, 2020. Accessed August 21, 2020.
21. American College of Gynecologists. Novel coronavirus 2019 (COVID-19). https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Updated August 12, 2020. Accessed August 26, 2020.
22. American College of Physicians. COVID-19: an ACP physician’s guide + resources. Chapter 14 of 31. Infection control: advice for physicians. https://assets.acponline.org/coronavirus/scormcontent/#/. Updated September 3, 2020. Accessed September 9, 2020.
23. Infectious Disease Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients with Suspected or Known COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/#toc-9-9. Updated April 20, 2020. Accessed August 26, 2020.
24. American College of Emergency Physicians. Joint Statement for Care of Patients with Behavioral Health Emergencies and Suspected or Confirmed COVID-19. https://www.acep.org/corona/covid-19-field-guide/special-populations/behavioral-health-patients/. Updated June 17, 2020. Accessed August 26, 2020.
25. Centers for Disease Control and Prevention. Quarantine and isolation. Legal authorities. https://www.cdc.gov/quarantine/aboutlawsregulationsquarantineisolation.html. Updated February 24, 2020. Accessed August 31, 2020.
26. Roberts A. Kentucky couple under house arrest after refusing to sign self-quarantine agreement. https://abcnews.go.com/US/kentucky-couple-house-arrest-refusing-sign-quarantine-agreement/story?id=71886479. Published July 20, 2020. Accessed July 24, 2020.
27. Satter R. To keep COVID-19 patients home, some U.S. states weigh house arrest tech. https://www.reuters.com/article/us-health-coronavirus-quarantine-tech/to-keep-covid-19-patients-home-some-us-states-weigh-house-arrest-tech-idUSKBN22J1U8. Published May 7, 2020. Accessed July 24, 2020.
28. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
29. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
30. Donaldson v O’Connor, 519, F2d 59 (5th Cir 1975).
31. Ohio Revised Code § 5122.290.
32. Shore JH. Telepsychiatry: videoconferencing in the delivery of psychiatric care. Am J Psychiatry. 2013;170(3):256-262.
33. Bostick NA, Levine MA, Sade RM. Ethical obligations of physicians participating in public health quarantine and isolation measures. Public Health Rep. 2008;123(1):3-8.
34. Upshur RE. Principles for the justification of public health intervention. Can J Public Health. 2002;93(2):101-103.
35. Barbera J, Macintyre A, Gostin L, et al. Large-scale quarantine following biological terrorism in the United States: scientific examination, logistic and legal limits, and possible consequences. JAMA. 2001;286(21):2711-2717.
36. Stanford Encyclopedia of Philosophy. Doctrine of double effect. https://plato.stanford.edu/entries/double-effect/. Revised December 24, 2018. Accessed July 12, 2020.
37. Substance Abuse and Mental Health Services Administration. Covid19: interim considerations for state psychiatric hospitals. https://www.samhsa.gov/sites/default/files/covid19-interim-considerations-for-state-psychiatric-hospitals.pdf. Updated May 8, 2020. Accessed July 24, 2020.
38. Augenstein TM, Pigeon WR, DiGiovanni SK, et al. Creating a novel inpatient psychiatric unit with integrated medical support for patients with COVID-19. N Engl J Med Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0249. Published June 22, 2020. Accessed July 12, 2020.
39. Bojdani E, Rajagopalan A, Chen A, et al. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289:113069. doi: 10.1016/j.psychres.2020.113069.
Issues with the Maintenance of Certification program; Overcoming a ‘quadruple threat’
Issues with the MOC
In Dr. Nasrallah’s editorial “Revamp the MOC” (From the Editor,
I was not so fortunate to have been grandfathered with lifetime certification, so I have been forced to recertify twice now. I will be 70 years old when I will need to decide whether to recertify once again. It is my belief that the MOC process is cumbersome and nonsensical, having little, if any, relevance in assessing one’s competency. Again, the ABPN’s purpose is not to “protect the public” and ensure safe and competent care, but to generate tremendous revenue for the Board. How can any rational individual believe that this exam is a legitimate test of one’s knowledge and competency when the pass rates are so stratospherically high year after year? I do not know of a single individual who has failed the recertification exam, so it would appear that if you pay the fees and sit for the exam, you will pass. It saddens me that the Board can perpetrate such a hoax on the public, leading them to believe that the MOC actually means something.
The cost to recertify is not inexpensive. Apparently, in a desire to add to its coffers, the ABPN has recently implemented the Physician Folios portal, whereby psychiatrists are forced to pay an annual fee. Its purpose, according to the Board, is to provide“a dynamic conduit for important data exchange such as making updates to personal contact information, updating medical license information, and applying and paying for an examination.”1 Give me a break!
It is my hope that a better, less expensive, more appropriate system is developed, allowing the psychiatrist to focus his/her efforts on treating patients.
Terrence Boyadjis, MD
Private psychiatric practice
West Chester, Pennsylvania
Reference
1. American Board of Psychiatry and Neurology. ABPN Physician Folios. https://application.abpn.com/webclient/landing_page.asp. Accessed October 20, 2020.
Dr. Nasrallah’s editorial about the MOC process is another addition to his collection of many of the best editorials I’ve ever read. I related fondly to his experiences taking the oral exam, which I took in 1972. I also became an examiner during the mid-1970s. Dr. Nasrallah continues to be a source of down-to-earth wisdom for our beloved profession.
Richard W. Worst, MD
Twin Falls, Idaho
Continue to: Overcoming a ‘quadruple threat’
Overcoming a ‘quadruple threat’
Dr. Nasrallah’s editorial “Enduring the ordeal of a quadruple threat is especially arduous for psychiatric patients” (From the Editor,
Robert W. Pollack, MD
Founder/COO
Psychiatric Associates of Southwest Florida
Fort Myers, Florida
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Issues with the MOC
In Dr. Nasrallah’s editorial “Revamp the MOC” (From the Editor,
I was not so fortunate to have been grandfathered with lifetime certification, so I have been forced to recertify twice now. I will be 70 years old when I will need to decide whether to recertify once again. It is my belief that the MOC process is cumbersome and nonsensical, having little, if any, relevance in assessing one’s competency. Again, the ABPN’s purpose is not to “protect the public” and ensure safe and competent care, but to generate tremendous revenue for the Board. How can any rational individual believe that this exam is a legitimate test of one’s knowledge and competency when the pass rates are so stratospherically high year after year? I do not know of a single individual who has failed the recertification exam, so it would appear that if you pay the fees and sit for the exam, you will pass. It saddens me that the Board can perpetrate such a hoax on the public, leading them to believe that the MOC actually means something.
The cost to recertify is not inexpensive. Apparently, in a desire to add to its coffers, the ABPN has recently implemented the Physician Folios portal, whereby psychiatrists are forced to pay an annual fee. Its purpose, according to the Board, is to provide“a dynamic conduit for important data exchange such as making updates to personal contact information, updating medical license information, and applying and paying for an examination.”1 Give me a break!
It is my hope that a better, less expensive, more appropriate system is developed, allowing the psychiatrist to focus his/her efforts on treating patients.
Terrence Boyadjis, MD
Private psychiatric practice
West Chester, Pennsylvania
Reference
1. American Board of Psychiatry and Neurology. ABPN Physician Folios. https://application.abpn.com/webclient/landing_page.asp. Accessed October 20, 2020.
Dr. Nasrallah’s editorial about the MOC process is another addition to his collection of many of the best editorials I’ve ever read. I related fondly to his experiences taking the oral exam, which I took in 1972. I also became an examiner during the mid-1970s. Dr. Nasrallah continues to be a source of down-to-earth wisdom for our beloved profession.
Richard W. Worst, MD
Twin Falls, Idaho
Continue to: Overcoming a ‘quadruple threat’
Overcoming a ‘quadruple threat’
Dr. Nasrallah’s editorial “Enduring the ordeal of a quadruple threat is especially arduous for psychiatric patients” (From the Editor,
Robert W. Pollack, MD
Founder/COO
Psychiatric Associates of Southwest Florida
Fort Myers, Florida
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Issues with the MOC
In Dr. Nasrallah’s editorial “Revamp the MOC” (From the Editor,
I was not so fortunate to have been grandfathered with lifetime certification, so I have been forced to recertify twice now. I will be 70 years old when I will need to decide whether to recertify once again. It is my belief that the MOC process is cumbersome and nonsensical, having little, if any, relevance in assessing one’s competency. Again, the ABPN’s purpose is not to “protect the public” and ensure safe and competent care, but to generate tremendous revenue for the Board. How can any rational individual believe that this exam is a legitimate test of one’s knowledge and competency when the pass rates are so stratospherically high year after year? I do not know of a single individual who has failed the recertification exam, so it would appear that if you pay the fees and sit for the exam, you will pass. It saddens me that the Board can perpetrate such a hoax on the public, leading them to believe that the MOC actually means something.
The cost to recertify is not inexpensive. Apparently, in a desire to add to its coffers, the ABPN has recently implemented the Physician Folios portal, whereby psychiatrists are forced to pay an annual fee. Its purpose, according to the Board, is to provide“a dynamic conduit for important data exchange such as making updates to personal contact information, updating medical license information, and applying and paying for an examination.”1 Give me a break!
It is my hope that a better, less expensive, more appropriate system is developed, allowing the psychiatrist to focus his/her efforts on treating patients.
Terrence Boyadjis, MD
Private psychiatric practice
West Chester, Pennsylvania
Reference
1. American Board of Psychiatry and Neurology. ABPN Physician Folios. https://application.abpn.com/webclient/landing_page.asp. Accessed October 20, 2020.
Dr. Nasrallah’s editorial about the MOC process is another addition to his collection of many of the best editorials I’ve ever read. I related fondly to his experiences taking the oral exam, which I took in 1972. I also became an examiner during the mid-1970s. Dr. Nasrallah continues to be a source of down-to-earth wisdom for our beloved profession.
Richard W. Worst, MD
Twin Falls, Idaho
Continue to: Overcoming a ‘quadruple threat’
Overcoming a ‘quadruple threat’
Dr. Nasrallah’s editorial “Enduring the ordeal of a quadruple threat is especially arduous for psychiatric patients” (From the Editor,
Robert W. Pollack, MD
Founder/COO
Psychiatric Associates of Southwest Florida
Fort Myers, Florida
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Unmet needs in the pharmacotherapy of psychiatric brain syndromes
Let’s face it: The greatest unmet need in psychiatry is discovering a treatment for the infamous syndrome of toxic political extremism. Its ugly symptoms include blind hatred, visceral malice, bigotry, vandalism, hypocrisy, racism, hubris, intransigence, narcissism, demagoguery, mutual contempt, and intense schadenfreude.
This corrosive affliction has engulfed and polluted our society, and compromised our well-being and quality of life. Treating this malignant syndrome is beyond the reach of psychopharmacology!
Thus, we psychiatrists should focus on the mood, psychotic, anxiety, and addiction syndromes that we encounter daily in our hospitals, clinics, and private offices. They affect tens of millions of patients. We currently have many psychotropic medications for these conditions. When combined with psychotherapy, the resulting synergy can be magical and immensely gratifying. However, some of those agents have limited efficacy due to the extensive heterogeneity of syndromes such as schizophrenia or depression, which are often confounded with comorbidities. A perfect balance between efficacy, tolerability, and safety are often hard to come by in pharmacotherapy.
The most glaring psychopharmacologic unmet need is that 80% of DSM disorders still do not have a single FDA-approved (evidence-based) medication.1 It will take decades, hundreds of billions of dollars, and the motivation of the often-maligned pharmaceutical industry (indispensable, because they are the only entity with the large R&D infrastructure to develop medications for psychiatry). Both academic and clinical psychiatrists must advise pharmaceutical companies about the unmet needs in our field and urge them to develop novel pharmacotherapies to address the gaps in the clinical care of psychiatric patients.
An inventory of unmet needs
With that in mind, here is a list of unmet needs I have been thinking about lately, and hoping that they will be resolved to help our patients achieve better clinical and functional outcomes.
Rapid-onset antipsychotics. The discovery that ketamine can rapidly convert refractory patients who are chronically depressed or suicidal to normal mood within a few hours shattered the dogma that weeks and months are needed for severe depression to improve, let alone achieve full remission. There is a similar dogma about psychosis requiring a protracted duration of antipsychotic treatment to attain significant impact. A rapid-acting antipsychotic agent would represent a major advance in psychiatry and its pharmaco-economic benefits would be substantial, given the high cost of inpatient hospitalization. Just as neurobiologic research guided the discovery of ketamine as a dramatic paradigm shift in treating depression, targeted research, especially focusing on glutamate pathways, may help identify a rapid-onset agent, whether oral, intranasal, IV, or even (why not) intrathecal. Research is known to enhance serendipity, which has been kind to psychiatry and has led to the discovery of several pharmacologic therapies in psychiatry, such as chlorpromazine, monoamine oxidase inhibitor antidepressants, and lithium.
Long-acting antidepressants and anxiolytics. This can be regarded as low-hanging fruit. Several technologies have been developed for long-acting formulations, yet they have been exploited mainly for antipsychotic medications. Some of these technologies can be employed to convert commonly used antidepressants (such as selective serotonin reuptake inhibitors) into long-acting antidepressants that can also reduce anxiety. Nonadherence among patients with depression is quite common, and relapses may lead to suicide attempts. The use of injectable, long-acting antidepressants can also reduce the incidence of overdoses because the patient will not have possession of potentially fatal pills.
Continue to: Long-acting mood stabilizers
Long-acting mood stabilizers. The rationale for long-acting mood stabilizers is the same as for long-acting antidepressants. Patients with bipolar disorder are known to stop taking their medications because they miss their “highs.” Some long-acting antipsychotics are approved for bipolar disorder, but these are often associated with adverse effects, such as metabolic dysregulation, extrapyramidal symptoms, and tardive dyskinesia. Mood stabilizers are essential for the bipolar spectrum.
A “real” treatment for alcohol use disorders that eliminates craving for alcohol. Alcoholism is associated with more than 100 medical complications and is one of the leading causes of disability in the world. It is frustrating that very few drug companies have focused on this widely prevalent brain disorder, which is also a common comorbid condition in many psychiatric syndromes.
Treatment-resistance pharmacotherapy solutions. All psychiatric syndromes are heterogeneous and contain ≥1 subgroups (biotypes) that fail to respond to what is considered the “standard” psychopharmacologic treatment (such as antipsychotics, antidepressants, mood stabilizers, or anti-obsessive medications). Technically, those so-called treatment-resistant subtypes need medications with a different mechanism of action. For example, clozapine for treatment-resistant schizophrenia and ketamine for treatment-resistant depression provide proof that treatment resistance is treatable but by a mechanism of action that is completely different from that of standard therapies, such as N-methyl-
Negative symptoms of schizophrenia cause significant functional disability and are well known to be a major unmet need. Some promising data are emerging on agents such as pimavanserin, cariprazine, and roluperidone, which is encouraging, but nothing is approved yet.
Cognitive deficits of schizophrenia, both neurocognition and social cognition, are another major unmet need that impair function in many patients. Many attempts to develop a pharmacologic treatment for these serious cognitive impairments have been made, but several candidates that initially appeared promising have bitten the dust. A focus on modulating the glutamate NMDA receptor may eventually lead to a breakthrough, and that may also help patients with bipolar disorder and major depressive disorder, both of whom also have cognitive deficits in several domains, albeit less severe than those experienced by patients with schizophrenia.
Continue to: Personality disorders
Personality disorders, especially borderline personality disorder, are very challenging to treat pharmacologically despite their prevalence and serious disruption to people’s lives. Hardly any FDA clinical trials have been conducted on any personality disorder. It is an unmet need that all psychiatrists would love to see addressed. But the mythical notion that personality disorders are untreatable may be an impediment in the pursuit of novel pharmacotherapy for borderline, narcissistic, antisocial, or schizotypal personality disorders, and other disorders. Heart attacks and religious conversion often change the baseline personality dramatically.
Childhood disorders. Apart from attention-deficit/hyperactivity disorder (ADHD), very few childhood psychiatric disorders have an FDA-approved medication. Why do drug companies avoid conducting controlled clinical trials in children age <10 who have autism, spectrum disorders, conduct disorder, oppositional defiant disorder, and other disorders? Effective pharmacotherapy for these children can be regarded as a desirable early intervention that may short-circuit their progression to serious adult psychopathology.
Parsimonious psychopharmacology for the treatment of trans-diagnostic psychiatric disorders. Recent research strongly suggests there is a strong overlap among psychiatric conditions, genetically, clinically, and biologically.2,3 For example, bipolar disorder is frequently accompanied by anxiety or substance use, patients with schizophrenia often experience anxiety, depression, or substance use, and ADHD has been found to share genes with autism.4,5
Eating disorders. There are no truly efficacious pharmacologic treatments for anorexia or bulimia nervosa. Research in this area is thin, and needs to be beefed up.
Sexual disorders. A huge unmet need exists for the pharmacotherapy of many sexual disorders that can have serious legal consequences (paraphilias) or quality-of-life repercussions (low sexual desire and orgasm disorders).
Continue to: A coordinated effort
A coordinated effort
It will take a massive collaboration among multiple stakeholders to launch the herculean process of addressing the unmet needs of all the above psychiatric disorders. This includes:
- the pharmaceutical industry (to provide the massive financial investment and R&D expertise)
- the federal government (to provide incentives)
- the FDA (to allow novel clinical trial designs)
- academic psychiatrists (to conduct research to discover the pathophysiology of psychiatric diseases)
- clinical psychiatrists (to provide consultations and advise about the clinical gaps in current psychopharmacological treatments)
- psychiatric patients (who are needed to volunteer for large-scale clinical trials).
This will be a veritable “psychiatric Manhattan Project” to advance the treatment of numerous psychiatric illnesses. The greatest benefit of discovering cures for disabling mental disorders is the evaporation of the virulent stigma that continues to plague our patients.
As for the political extremism that has corroded our society, it may be beyond pharmacologic redemption. An antidote to the “kool aid” has not yet been invented…
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nasrallah HA. Is there only 1 neurobiologic disorder, with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
3. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
4. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
5. Marshall M. Roots of mental illness. Nature. 2020;581:19-21.
Let’s face it: The greatest unmet need in psychiatry is discovering a treatment for the infamous syndrome of toxic political extremism. Its ugly symptoms include blind hatred, visceral malice, bigotry, vandalism, hypocrisy, racism, hubris, intransigence, narcissism, demagoguery, mutual contempt, and intense schadenfreude.
This corrosive affliction has engulfed and polluted our society, and compromised our well-being and quality of life. Treating this malignant syndrome is beyond the reach of psychopharmacology!
Thus, we psychiatrists should focus on the mood, psychotic, anxiety, and addiction syndromes that we encounter daily in our hospitals, clinics, and private offices. They affect tens of millions of patients. We currently have many psychotropic medications for these conditions. When combined with psychotherapy, the resulting synergy can be magical and immensely gratifying. However, some of those agents have limited efficacy due to the extensive heterogeneity of syndromes such as schizophrenia or depression, which are often confounded with comorbidities. A perfect balance between efficacy, tolerability, and safety are often hard to come by in pharmacotherapy.
The most glaring psychopharmacologic unmet need is that 80% of DSM disorders still do not have a single FDA-approved (evidence-based) medication.1 It will take decades, hundreds of billions of dollars, and the motivation of the often-maligned pharmaceutical industry (indispensable, because they are the only entity with the large R&D infrastructure to develop medications for psychiatry). Both academic and clinical psychiatrists must advise pharmaceutical companies about the unmet needs in our field and urge them to develop novel pharmacotherapies to address the gaps in the clinical care of psychiatric patients.
An inventory of unmet needs
With that in mind, here is a list of unmet needs I have been thinking about lately, and hoping that they will be resolved to help our patients achieve better clinical and functional outcomes.
Rapid-onset antipsychotics. The discovery that ketamine can rapidly convert refractory patients who are chronically depressed or suicidal to normal mood within a few hours shattered the dogma that weeks and months are needed for severe depression to improve, let alone achieve full remission. There is a similar dogma about psychosis requiring a protracted duration of antipsychotic treatment to attain significant impact. A rapid-acting antipsychotic agent would represent a major advance in psychiatry and its pharmaco-economic benefits would be substantial, given the high cost of inpatient hospitalization. Just as neurobiologic research guided the discovery of ketamine as a dramatic paradigm shift in treating depression, targeted research, especially focusing on glutamate pathways, may help identify a rapid-onset agent, whether oral, intranasal, IV, or even (why not) intrathecal. Research is known to enhance serendipity, which has been kind to psychiatry and has led to the discovery of several pharmacologic therapies in psychiatry, such as chlorpromazine, monoamine oxidase inhibitor antidepressants, and lithium.
Long-acting antidepressants and anxiolytics. This can be regarded as low-hanging fruit. Several technologies have been developed for long-acting formulations, yet they have been exploited mainly for antipsychotic medications. Some of these technologies can be employed to convert commonly used antidepressants (such as selective serotonin reuptake inhibitors) into long-acting antidepressants that can also reduce anxiety. Nonadherence among patients with depression is quite common, and relapses may lead to suicide attempts. The use of injectable, long-acting antidepressants can also reduce the incidence of overdoses because the patient will not have possession of potentially fatal pills.
Continue to: Long-acting mood stabilizers
Long-acting mood stabilizers. The rationale for long-acting mood stabilizers is the same as for long-acting antidepressants. Patients with bipolar disorder are known to stop taking their medications because they miss their “highs.” Some long-acting antipsychotics are approved for bipolar disorder, but these are often associated with adverse effects, such as metabolic dysregulation, extrapyramidal symptoms, and tardive dyskinesia. Mood stabilizers are essential for the bipolar spectrum.
A “real” treatment for alcohol use disorders that eliminates craving for alcohol. Alcoholism is associated with more than 100 medical complications and is one of the leading causes of disability in the world. It is frustrating that very few drug companies have focused on this widely prevalent brain disorder, which is also a common comorbid condition in many psychiatric syndromes.
Treatment-resistance pharmacotherapy solutions. All psychiatric syndromes are heterogeneous and contain ≥1 subgroups (biotypes) that fail to respond to what is considered the “standard” psychopharmacologic treatment (such as antipsychotics, antidepressants, mood stabilizers, or anti-obsessive medications). Technically, those so-called treatment-resistant subtypes need medications with a different mechanism of action. For example, clozapine for treatment-resistant schizophrenia and ketamine for treatment-resistant depression provide proof that treatment resistance is treatable but by a mechanism of action that is completely different from that of standard therapies, such as N-methyl-
Negative symptoms of schizophrenia cause significant functional disability and are well known to be a major unmet need. Some promising data are emerging on agents such as pimavanserin, cariprazine, and roluperidone, which is encouraging, but nothing is approved yet.
Cognitive deficits of schizophrenia, both neurocognition and social cognition, are another major unmet need that impair function in many patients. Many attempts to develop a pharmacologic treatment for these serious cognitive impairments have been made, but several candidates that initially appeared promising have bitten the dust. A focus on modulating the glutamate NMDA receptor may eventually lead to a breakthrough, and that may also help patients with bipolar disorder and major depressive disorder, both of whom also have cognitive deficits in several domains, albeit less severe than those experienced by patients with schizophrenia.
Continue to: Personality disorders
Personality disorders, especially borderline personality disorder, are very challenging to treat pharmacologically despite their prevalence and serious disruption to people’s lives. Hardly any FDA clinical trials have been conducted on any personality disorder. It is an unmet need that all psychiatrists would love to see addressed. But the mythical notion that personality disorders are untreatable may be an impediment in the pursuit of novel pharmacotherapy for borderline, narcissistic, antisocial, or schizotypal personality disorders, and other disorders. Heart attacks and religious conversion often change the baseline personality dramatically.
Childhood disorders. Apart from attention-deficit/hyperactivity disorder (ADHD), very few childhood psychiatric disorders have an FDA-approved medication. Why do drug companies avoid conducting controlled clinical trials in children age <10 who have autism, spectrum disorders, conduct disorder, oppositional defiant disorder, and other disorders? Effective pharmacotherapy for these children can be regarded as a desirable early intervention that may short-circuit their progression to serious adult psychopathology.
Parsimonious psychopharmacology for the treatment of trans-diagnostic psychiatric disorders. Recent research strongly suggests there is a strong overlap among psychiatric conditions, genetically, clinically, and biologically.2,3 For example, bipolar disorder is frequently accompanied by anxiety or substance use, patients with schizophrenia often experience anxiety, depression, or substance use, and ADHD has been found to share genes with autism.4,5
Eating disorders. There are no truly efficacious pharmacologic treatments for anorexia or bulimia nervosa. Research in this area is thin, and needs to be beefed up.
Sexual disorders. A huge unmet need exists for the pharmacotherapy of many sexual disorders that can have serious legal consequences (paraphilias) or quality-of-life repercussions (low sexual desire and orgasm disorders).
Continue to: A coordinated effort
A coordinated effort
It will take a massive collaboration among multiple stakeholders to launch the herculean process of addressing the unmet needs of all the above psychiatric disorders. This includes:
- the pharmaceutical industry (to provide the massive financial investment and R&D expertise)
- the federal government (to provide incentives)
- the FDA (to allow novel clinical trial designs)
- academic psychiatrists (to conduct research to discover the pathophysiology of psychiatric diseases)
- clinical psychiatrists (to provide consultations and advise about the clinical gaps in current psychopharmacological treatments)
- psychiatric patients (who are needed to volunteer for large-scale clinical trials).
This will be a veritable “psychiatric Manhattan Project” to advance the treatment of numerous psychiatric illnesses. The greatest benefit of discovering cures for disabling mental disorders is the evaporation of the virulent stigma that continues to plague our patients.
As for the political extremism that has corroded our society, it may be beyond pharmacologic redemption. An antidote to the “kool aid” has not yet been invented…
Let’s face it: The greatest unmet need in psychiatry is discovering a treatment for the infamous syndrome of toxic political extremism. Its ugly symptoms include blind hatred, visceral malice, bigotry, vandalism, hypocrisy, racism, hubris, intransigence, narcissism, demagoguery, mutual contempt, and intense schadenfreude.
This corrosive affliction has engulfed and polluted our society, and compromised our well-being and quality of life. Treating this malignant syndrome is beyond the reach of psychopharmacology!
Thus, we psychiatrists should focus on the mood, psychotic, anxiety, and addiction syndromes that we encounter daily in our hospitals, clinics, and private offices. They affect tens of millions of patients. We currently have many psychotropic medications for these conditions. When combined with psychotherapy, the resulting synergy can be magical and immensely gratifying. However, some of those agents have limited efficacy due to the extensive heterogeneity of syndromes such as schizophrenia or depression, which are often confounded with comorbidities. A perfect balance between efficacy, tolerability, and safety are often hard to come by in pharmacotherapy.
The most glaring psychopharmacologic unmet need is that 80% of DSM disorders still do not have a single FDA-approved (evidence-based) medication.1 It will take decades, hundreds of billions of dollars, and the motivation of the often-maligned pharmaceutical industry (indispensable, because they are the only entity with the large R&D infrastructure to develop medications for psychiatry). Both academic and clinical psychiatrists must advise pharmaceutical companies about the unmet needs in our field and urge them to develop novel pharmacotherapies to address the gaps in the clinical care of psychiatric patients.
An inventory of unmet needs
With that in mind, here is a list of unmet needs I have been thinking about lately, and hoping that they will be resolved to help our patients achieve better clinical and functional outcomes.
Rapid-onset antipsychotics. The discovery that ketamine can rapidly convert refractory patients who are chronically depressed or suicidal to normal mood within a few hours shattered the dogma that weeks and months are needed for severe depression to improve, let alone achieve full remission. There is a similar dogma about psychosis requiring a protracted duration of antipsychotic treatment to attain significant impact. A rapid-acting antipsychotic agent would represent a major advance in psychiatry and its pharmaco-economic benefits would be substantial, given the high cost of inpatient hospitalization. Just as neurobiologic research guided the discovery of ketamine as a dramatic paradigm shift in treating depression, targeted research, especially focusing on glutamate pathways, may help identify a rapid-onset agent, whether oral, intranasal, IV, or even (why not) intrathecal. Research is known to enhance serendipity, which has been kind to psychiatry and has led to the discovery of several pharmacologic therapies in psychiatry, such as chlorpromazine, monoamine oxidase inhibitor antidepressants, and lithium.
Long-acting antidepressants and anxiolytics. This can be regarded as low-hanging fruit. Several technologies have been developed for long-acting formulations, yet they have been exploited mainly for antipsychotic medications. Some of these technologies can be employed to convert commonly used antidepressants (such as selective serotonin reuptake inhibitors) into long-acting antidepressants that can also reduce anxiety. Nonadherence among patients with depression is quite common, and relapses may lead to suicide attempts. The use of injectable, long-acting antidepressants can also reduce the incidence of overdoses because the patient will not have possession of potentially fatal pills.
Continue to: Long-acting mood stabilizers
Long-acting mood stabilizers. The rationale for long-acting mood stabilizers is the same as for long-acting antidepressants. Patients with bipolar disorder are known to stop taking their medications because they miss their “highs.” Some long-acting antipsychotics are approved for bipolar disorder, but these are often associated with adverse effects, such as metabolic dysregulation, extrapyramidal symptoms, and tardive dyskinesia. Mood stabilizers are essential for the bipolar spectrum.
A “real” treatment for alcohol use disorders that eliminates craving for alcohol. Alcoholism is associated with more than 100 medical complications and is one of the leading causes of disability in the world. It is frustrating that very few drug companies have focused on this widely prevalent brain disorder, which is also a common comorbid condition in many psychiatric syndromes.
Treatment-resistance pharmacotherapy solutions. All psychiatric syndromes are heterogeneous and contain ≥1 subgroups (biotypes) that fail to respond to what is considered the “standard” psychopharmacologic treatment (such as antipsychotics, antidepressants, mood stabilizers, or anti-obsessive medications). Technically, those so-called treatment-resistant subtypes need medications with a different mechanism of action. For example, clozapine for treatment-resistant schizophrenia and ketamine for treatment-resistant depression provide proof that treatment resistance is treatable but by a mechanism of action that is completely different from that of standard therapies, such as N-methyl-
Negative symptoms of schizophrenia cause significant functional disability and are well known to be a major unmet need. Some promising data are emerging on agents such as pimavanserin, cariprazine, and roluperidone, which is encouraging, but nothing is approved yet.
Cognitive deficits of schizophrenia, both neurocognition and social cognition, are another major unmet need that impair function in many patients. Many attempts to develop a pharmacologic treatment for these serious cognitive impairments have been made, but several candidates that initially appeared promising have bitten the dust. A focus on modulating the glutamate NMDA receptor may eventually lead to a breakthrough, and that may also help patients with bipolar disorder and major depressive disorder, both of whom also have cognitive deficits in several domains, albeit less severe than those experienced by patients with schizophrenia.
Continue to: Personality disorders
Personality disorders, especially borderline personality disorder, are very challenging to treat pharmacologically despite their prevalence and serious disruption to people’s lives. Hardly any FDA clinical trials have been conducted on any personality disorder. It is an unmet need that all psychiatrists would love to see addressed. But the mythical notion that personality disorders are untreatable may be an impediment in the pursuit of novel pharmacotherapy for borderline, narcissistic, antisocial, or schizotypal personality disorders, and other disorders. Heart attacks and religious conversion often change the baseline personality dramatically.
Childhood disorders. Apart from attention-deficit/hyperactivity disorder (ADHD), very few childhood psychiatric disorders have an FDA-approved medication. Why do drug companies avoid conducting controlled clinical trials in children age <10 who have autism, spectrum disorders, conduct disorder, oppositional defiant disorder, and other disorders? Effective pharmacotherapy for these children can be regarded as a desirable early intervention that may short-circuit their progression to serious adult psychopathology.
Parsimonious psychopharmacology for the treatment of trans-diagnostic psychiatric disorders. Recent research strongly suggests there is a strong overlap among psychiatric conditions, genetically, clinically, and biologically.2,3 For example, bipolar disorder is frequently accompanied by anxiety or substance use, patients with schizophrenia often experience anxiety, depression, or substance use, and ADHD has been found to share genes with autism.4,5
Eating disorders. There are no truly efficacious pharmacologic treatments for anorexia or bulimia nervosa. Research in this area is thin, and needs to be beefed up.
Sexual disorders. A huge unmet need exists for the pharmacotherapy of many sexual disorders that can have serious legal consequences (paraphilias) or quality-of-life repercussions (low sexual desire and orgasm disorders).
Continue to: A coordinated effort
A coordinated effort
It will take a massive collaboration among multiple stakeholders to launch the herculean process of addressing the unmet needs of all the above psychiatric disorders. This includes:
- the pharmaceutical industry (to provide the massive financial investment and R&D expertise)
- the federal government (to provide incentives)
- the FDA (to allow novel clinical trial designs)
- academic psychiatrists (to conduct research to discover the pathophysiology of psychiatric diseases)
- clinical psychiatrists (to provide consultations and advise about the clinical gaps in current psychopharmacological treatments)
- psychiatric patients (who are needed to volunteer for large-scale clinical trials).
This will be a veritable “psychiatric Manhattan Project” to advance the treatment of numerous psychiatric illnesses. The greatest benefit of discovering cures for disabling mental disorders is the evaporation of the virulent stigma that continues to plague our patients.
As for the political extremism that has corroded our society, it may be beyond pharmacologic redemption. An antidote to the “kool aid” has not yet been invented…
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nasrallah HA. Is there only 1 neurobiologic disorder, with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
3. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
4. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
5. Marshall M. Roots of mental illness. Nature. 2020;581:19-21.
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nasrallah HA. Is there only 1 neurobiologic disorder, with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
3. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
4. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
5. Marshall M. Roots of mental illness. Nature. 2020;581:19-21.
Infected with COVID-19: One psychiatrist’s story
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Pharmacogenetic testing: 5 Questions
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
When selecting a psychotropic medication for a patient with a challenging illness, you may want to consider ordering pharmacogenetic testing. By characterizing how a patient’s genetic profile affects their medication metabolism, pharmacogenetic testing can potentially help improve medication adherence, reduce “trial-and-error” prescribing, and target an effective treatment. Here we address 5 important questions about using pharmacogenetic testing.
1. What can pharmacogenetic testing tell you? Pharmacogenetic testing looks for variants in genes that can affect how a patient metabolizes specific medications. While the results will not inform you about a specific medication’s effectiveness, they can describe the patient’s tolerability of that medication based on his/her metabolism. Most psychotropic medications are biotransformed in the liver by cytochrome P450 (CYP) through pathway enzymes such as 2D6, 2C19, and 3A4. For example, fluoxetine and paroxetine exert their inhibition on CYP2D6, while other psychotropic medications, such as lurasidone, are metabolized at CYP3A4 and are contraindicated with potent CYP3A4 inhibitors (eg, grapefruit juice).1
In addition to CYP450 enzymes, pharmacogenetic testing can assess for the serotonin transporter gene, SLC6A4, and its sequence promoter variant, 5-HTTLPR. This sequence variation influences response to selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tertiary amine tricyclic antidepressants.2 Pharmacogenetic testing also looks for genes related to Stevens-Johnson syndrome, such as HLA-B*1502, which is associated with adverse effects of carbamazepine and lamotrigine.
2. When should you order pharmacogenetic testing? Not all patients require pharmacogenetic testing. Anxious patients who have had multiple unsuccessful medication trials may be good candidates for testing. Consider testing for patients with a history of sensitivity to medications, or whose family members have experienced unusual drug responses.2
3. What steps should you take before ordering pharmacogenetic testing? First, obtain your patient’s informed consent, because clinical testing reveals personal genetic information. Make sure your patient understands that such testing is voluntary and that he/she can opt out. Also, explain that the information obtained from pharmacogenetic testing is confidential and will become part of the patient’s medical record.
Second, choose the best test for your patient’s needs. Pharmacogenetic tests can assess for single genes encoded for selected CYP450 enzymes based on pharmacokinetics (metabolism), or for multiple genes based on pharmacodynamic (mechanism of action) factors.5 In a recent randomized controlled trial, Bradley et al6 found that testing for multiple genes improved response and remission rates in patients with depression and/or anxiety.
Third, confirm that your patient’s insurance covers pharmacogenetic testing, because this testing can be expensive, although some genetic testing companies may offer patients financial assistance.
Continue to: How are samples taken?
4. How are samples taken? Several methods are used for obtaining samples, including saliva, buccal swab, and peripheral blood. Your patient should not smoke, eat, or drink for at least 30 minutes before providing a saliva sample. For a buccal swab, a cotton swab is rubbed in a circular motion along the oral lining inside each of the patient’s cheeks. The most invasive method is peripheral blood obtained via venipuncture. The sample is sent through expedited mail to an accredited genetic processing laboratory for analysis.
5. How do you interpret the results? Pharmacogenetic testing results are provided in a confidential report. A single gene report allows you to choose psychotropic agents based on pharmacokinetics.5 Some laboratories assess multiple genes in a single report, and create categories of medications (such as “use as directed” or “use with caution”) based on the pharmacodynamic factors of each agent.5,6 Certain laboratories offer dosing guidelines for types of medications that you should use with caution.1,5,6
When interpreting such testing results, it is critical to use your clinical judgment, because pharmacogenetic testing alone does not assess whether a medication will help improve the patient’s symptoms. It is of utmost importance that you have an understanding of pharmacodynamics, knowledge of the patient’s diet and age, and a caring doctor–patient relationship.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
1. Madhusoodanan S, Velama U, Parmar J, et al. A current review of cytochrome P450 interactions of psychotropic drugs. Ann Clin Psychiatry. 2014;26(2):120-138.
2. Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69-76.
3. Drozda K, Müller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166-184.
4. Shelton RC, Sloan Manning J, Barrentine LW, et al. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):PCC.13m01520. doi: 10.4088/PCC.13m01520.
5. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded randomized, controlled study. J Psychiatr Res. 2019;111:59-67.
6. Bradley P, Shiekh M, Mehra V, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psych Res. 2018;96:100-107.
Lemborexant for insomnia
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
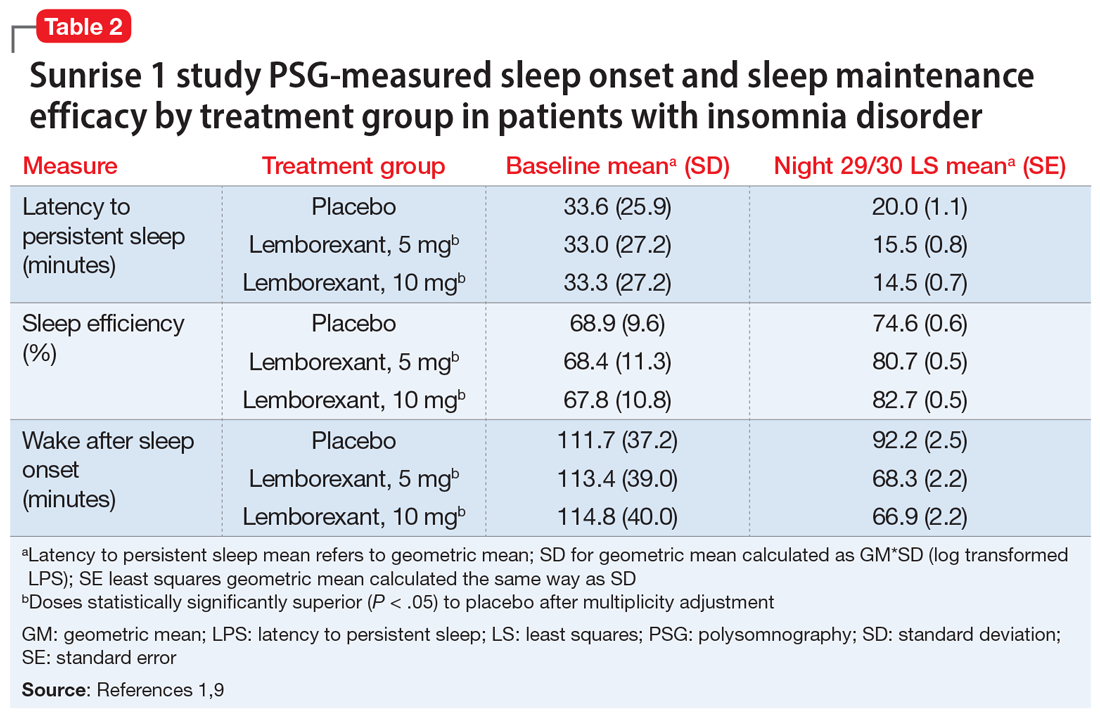
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
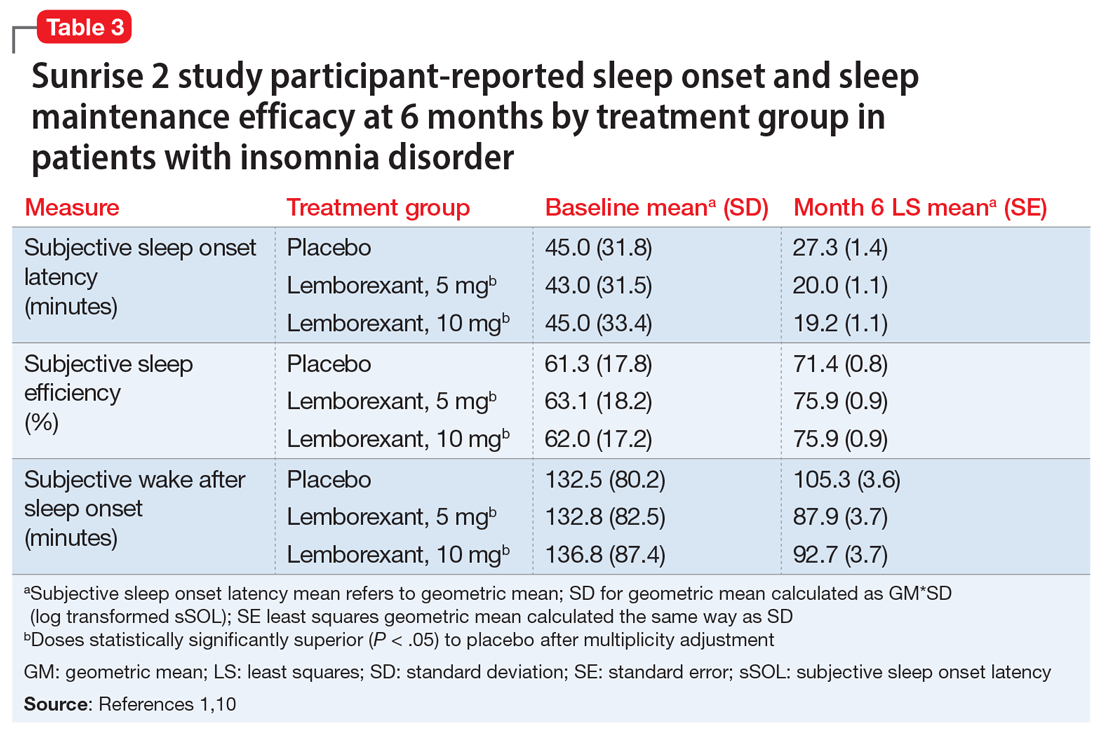
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.

In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.

Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.

In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.

Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Painful erections while being treated for OCD
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
Leadership & Professional Development: Fighting Reputational Inertia
“Becoming is better than being.”
—Carol Dweck
The words spoken about her in the staff meeting were flattering. She’d just been acknowledged with a departmental teaching award for the second year in a row. With only 3 years under her belt since completing training, the former chief resident was living up to all they’d anticipated.
Eager students requested to be on her team and colleagues delighted in sharing patients with her. “Great, as always,” her peers and learners said in hallways and evaluations. This would come to define her identity.
Things were going well. She was succeeding. But she began to wonder if this reciprocating engine of accolades represented who she truly was. Was she really that good? Was she an imposter? In her performance meetings, the feedback never wavered: “Great, as always.”
The following year she would leave for a different job.
THE THREAT OF REPUTATIONAL INERTIA
While specific plans for growth and improvement often get laid out for struggling colleagues and learners, far less effort is devoted to coaching high performers. Feedback that consists of nonspecific compliments may hinder potential, growth, and job satisfaction. We outline strategies for preventing this professional plateau in those you lead.
ENCOURAGE A GROWTH MINDSET
In Mindset: The New Psychology of Success, psychologist Carol Dweck describes how emphasis on qualities such as “being smart” or, in this example, “great,” underscores this “fixed mindset” that certain attributes are set in stone.1 Conversely, she defines the “growth mindset” as a belief that potential can be cultivated through efforts. Even when there aren’t obvious issues with performance, the failure, fine-tuning, and feedback necessary for resilience and, ultimately, sustained growth require intention.
Emphasize Effort
Instead of lauding an individual for being “great, as always,” consider focusing on the effort it required to get there. For example, regarding the aforementioned junior colleague who’d just won awards, a typical compliment might be: “Wow, you’re on fire!” An option, to promote a growth mindset, might be: “You work very hard at bedside teaching and innovative curriculum development. I’m happy to see that our learners and department have recognized your commitment and effort.” This language also affirms others and makes achievements seem attainable to all.
Provide Active Coaching
Identifying specific opportunities for development can challenge individuals to expand their skills. Even those who are doing well have room to become even better. Coproduction of new milestones that push beyond current comfort zones can acknowledge current achievements while encouraging continued growth—and make things personal. For example, encouraging an individual to apply to a national faculty development program, such as the Society of Hospital Medicine’s Academic Hospitalist Academy, could help them expand their skills and social network.
Offer Meaningful Feedback
Prioritizing feedback is essential for growth and peak performance. This can be particularly powerful when the observer moves beyond basic expectations to incorporate personal goals. Concrete feedback measured against individual potential then takes the place of nondescript compliments. For example, you could say: “Your teaching on systolic ejection murmurs was on target for the students. Next time I want to challenge you to broaden your teaching script to include points appropriate for more seasoned learners.” This feedback leaves them with a set of tailored “marching orders” to guide practice and improvement.
CONCLUSION
No matter where a person stands on the spectrum of performance, growth in medicine relies on deliberate practice, active coaching, meaningful feedback, and graduated opportunities. Even the most proficient among us can stagnate without these things. If we aren’t careful, this reputational inertia could amplify imposter syndrome, prevent individuals from achieving their full potential, and threaten faculty retention. Intentional work toward a growth mindset allows everyone to grow—and be seen.
Disclosures
The authors have nothing to disclose.
1. Dweck CS. Mindset: The New Psychology of Success. New York: Ballantine Books; 2008.
“Becoming is better than being.”
—Carol Dweck
The words spoken about her in the staff meeting were flattering. She’d just been acknowledged with a departmental teaching award for the second year in a row. With only 3 years under her belt since completing training, the former chief resident was living up to all they’d anticipated.
Eager students requested to be on her team and colleagues delighted in sharing patients with her. “Great, as always,” her peers and learners said in hallways and evaluations. This would come to define her identity.
Things were going well. She was succeeding. But she began to wonder if this reciprocating engine of accolades represented who she truly was. Was she really that good? Was she an imposter? In her performance meetings, the feedback never wavered: “Great, as always.”
The following year she would leave for a different job.
THE THREAT OF REPUTATIONAL INERTIA
While specific plans for growth and improvement often get laid out for struggling colleagues and learners, far less effort is devoted to coaching high performers. Feedback that consists of nonspecific compliments may hinder potential, growth, and job satisfaction. We outline strategies for preventing this professional plateau in those you lead.
ENCOURAGE A GROWTH MINDSET
In Mindset: The New Psychology of Success, psychologist Carol Dweck describes how emphasis on qualities such as “being smart” or, in this example, “great,” underscores this “fixed mindset” that certain attributes are set in stone.1 Conversely, she defines the “growth mindset” as a belief that potential can be cultivated through efforts. Even when there aren’t obvious issues with performance, the failure, fine-tuning, and feedback necessary for resilience and, ultimately, sustained growth require intention.
Emphasize Effort
Instead of lauding an individual for being “great, as always,” consider focusing on the effort it required to get there. For example, regarding the aforementioned junior colleague who’d just won awards, a typical compliment might be: “Wow, you’re on fire!” An option, to promote a growth mindset, might be: “You work very hard at bedside teaching and innovative curriculum development. I’m happy to see that our learners and department have recognized your commitment and effort.” This language also affirms others and makes achievements seem attainable to all.
Provide Active Coaching
Identifying specific opportunities for development can challenge individuals to expand their skills. Even those who are doing well have room to become even better. Coproduction of new milestones that push beyond current comfort zones can acknowledge current achievements while encouraging continued growth—and make things personal. For example, encouraging an individual to apply to a national faculty development program, such as the Society of Hospital Medicine’s Academic Hospitalist Academy, could help them expand their skills and social network.
Offer Meaningful Feedback
Prioritizing feedback is essential for growth and peak performance. This can be particularly powerful when the observer moves beyond basic expectations to incorporate personal goals. Concrete feedback measured against individual potential then takes the place of nondescript compliments. For example, you could say: “Your teaching on systolic ejection murmurs was on target for the students. Next time I want to challenge you to broaden your teaching script to include points appropriate for more seasoned learners.” This feedback leaves them with a set of tailored “marching orders” to guide practice and improvement.
CONCLUSION
No matter where a person stands on the spectrum of performance, growth in medicine relies on deliberate practice, active coaching, meaningful feedback, and graduated opportunities. Even the most proficient among us can stagnate without these things. If we aren’t careful, this reputational inertia could amplify imposter syndrome, prevent individuals from achieving their full potential, and threaten faculty retention. Intentional work toward a growth mindset allows everyone to grow—and be seen.
Disclosures
The authors have nothing to disclose.
“Becoming is better than being.”
—Carol Dweck
The words spoken about her in the staff meeting were flattering. She’d just been acknowledged with a departmental teaching award for the second year in a row. With only 3 years under her belt since completing training, the former chief resident was living up to all they’d anticipated.
Eager students requested to be on her team and colleagues delighted in sharing patients with her. “Great, as always,” her peers and learners said in hallways and evaluations. This would come to define her identity.
Things were going well. She was succeeding. But she began to wonder if this reciprocating engine of accolades represented who she truly was. Was she really that good? Was she an imposter? In her performance meetings, the feedback never wavered: “Great, as always.”
The following year she would leave for a different job.
THE THREAT OF REPUTATIONAL INERTIA
While specific plans for growth and improvement often get laid out for struggling colleagues and learners, far less effort is devoted to coaching high performers. Feedback that consists of nonspecific compliments may hinder potential, growth, and job satisfaction. We outline strategies for preventing this professional plateau in those you lead.
ENCOURAGE A GROWTH MINDSET
In Mindset: The New Psychology of Success, psychologist Carol Dweck describes how emphasis on qualities such as “being smart” or, in this example, “great,” underscores this “fixed mindset” that certain attributes are set in stone.1 Conversely, she defines the “growth mindset” as a belief that potential can be cultivated through efforts. Even when there aren’t obvious issues with performance, the failure, fine-tuning, and feedback necessary for resilience and, ultimately, sustained growth require intention.
Emphasize Effort
Instead of lauding an individual for being “great, as always,” consider focusing on the effort it required to get there. For example, regarding the aforementioned junior colleague who’d just won awards, a typical compliment might be: “Wow, you’re on fire!” An option, to promote a growth mindset, might be: “You work very hard at bedside teaching and innovative curriculum development. I’m happy to see that our learners and department have recognized your commitment and effort.” This language also affirms others and makes achievements seem attainable to all.
Provide Active Coaching
Identifying specific opportunities for development can challenge individuals to expand their skills. Even those who are doing well have room to become even better. Coproduction of new milestones that push beyond current comfort zones can acknowledge current achievements while encouraging continued growth—and make things personal. For example, encouraging an individual to apply to a national faculty development program, such as the Society of Hospital Medicine’s Academic Hospitalist Academy, could help them expand their skills and social network.
Offer Meaningful Feedback
Prioritizing feedback is essential for growth and peak performance. This can be particularly powerful when the observer moves beyond basic expectations to incorporate personal goals. Concrete feedback measured against individual potential then takes the place of nondescript compliments. For example, you could say: “Your teaching on systolic ejection murmurs was on target for the students. Next time I want to challenge you to broaden your teaching script to include points appropriate for more seasoned learners.” This feedback leaves them with a set of tailored “marching orders” to guide practice and improvement.
CONCLUSION
No matter where a person stands on the spectrum of performance, growth in medicine relies on deliberate practice, active coaching, meaningful feedback, and graduated opportunities. Even the most proficient among us can stagnate without these things. If we aren’t careful, this reputational inertia could amplify imposter syndrome, prevent individuals from achieving their full potential, and threaten faculty retention. Intentional work toward a growth mindset allows everyone to grow—and be seen.
Disclosures
The authors have nothing to disclose.
1. Dweck CS. Mindset: The New Psychology of Success. New York: Ballantine Books; 2008.
1. Dweck CS. Mindset: The New Psychology of Success. New York: Ballantine Books; 2008.
© 2020 Society of Hospital Medicine