User login
Web-based interviews, financial planning in a pandemic, and more
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Role of gastroenterologists in the U.S. in the management of gastric cancer
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.
These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
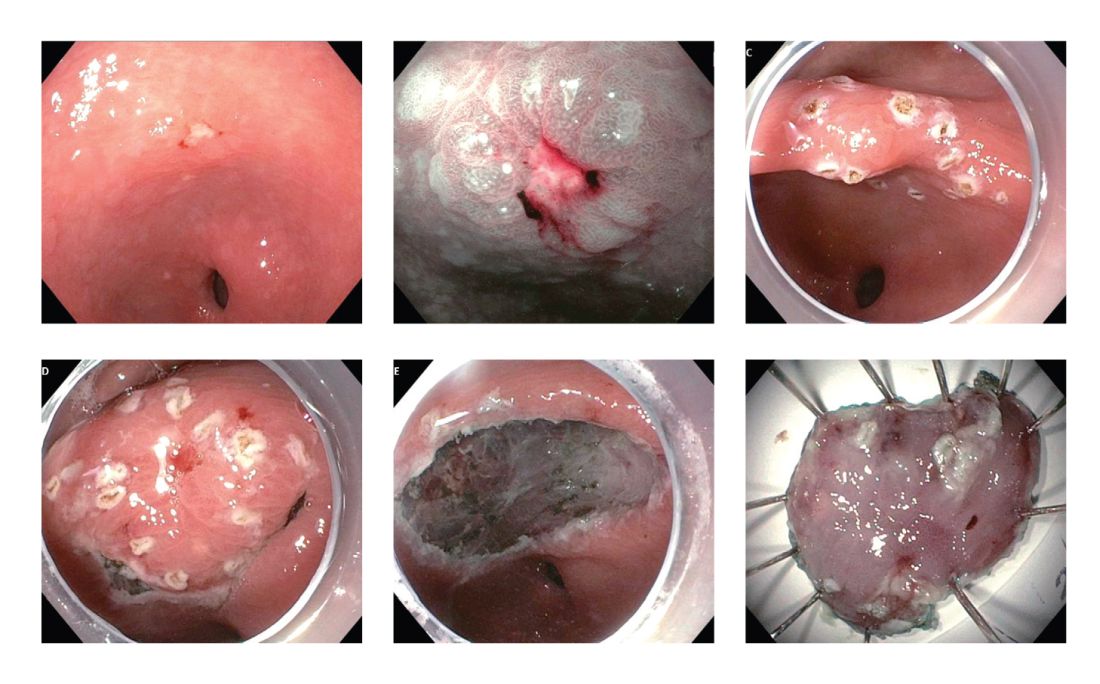
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.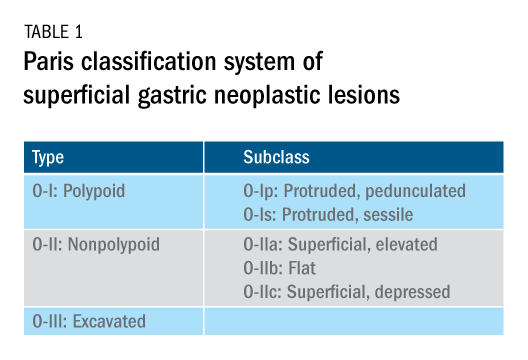
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.

These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.

These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Disruption of postpandemic world will precipitate innovation
When this editorial is published, we will know the results of the national election (hopefully) and whether there will be a smooth transition of power. We should know whether the Affordable Care Act will remain intact, and we will have indications about the impact of a COVID/flu combination. Health care will never be the same.
According to a recent Medscape survey, 62% of U.S. physicians saw a reduction of monthly income (12% saw a reduction of over 70%) in the first 6 months of this year. Almost a third of the physician workforce is contemplating retirement earlier than anticipated. As worrisome, according to a JAMA article (Aug 4, 2020;324:510-3) the United States saw a 35% increase in excess deaths because of non-COVID etiologies, an indication of health care deferral and avoidance. We all are scrambling to catch up and accommodate an enormous demand.
We are witnessing a “K” shaped recovery for both individuals and GI practices. If your health care is covered by Medicare, you own a mortgage-free home and your wealth is based on a balanced equity/bond portfolio, then all of your assets increased in value compared to last year’s peak valuations. For the other 90% of Americans, the recovery is modest, neutral, or more often nonexistent. Gastroenterologists who work in academic centers or large health systems did not lose income this year and were protected by billion-dollar credit lines and cash-on-hand accounts from robust days available to these entities. Independent practices (critically dependent on monthly cash flow) were decimated, furthering the trend towards consolidation, retirement, and acquisitions. With the new CMS E/M valuations we will see further reduction in procedural reimbursement.
However, disruption always precipitates innovation. Challenges are great but opportunities are clearly evident for those willing to risk.
John I. Allen, MD, MBA, AGAF
Editor in Chief
When this editorial is published, we will know the results of the national election (hopefully) and whether there will be a smooth transition of power. We should know whether the Affordable Care Act will remain intact, and we will have indications about the impact of a COVID/flu combination. Health care will never be the same.
According to a recent Medscape survey, 62% of U.S. physicians saw a reduction of monthly income (12% saw a reduction of over 70%) in the first 6 months of this year. Almost a third of the physician workforce is contemplating retirement earlier than anticipated. As worrisome, according to a JAMA article (Aug 4, 2020;324:510-3) the United States saw a 35% increase in excess deaths because of non-COVID etiologies, an indication of health care deferral and avoidance. We all are scrambling to catch up and accommodate an enormous demand.
We are witnessing a “K” shaped recovery for both individuals and GI practices. If your health care is covered by Medicare, you own a mortgage-free home and your wealth is based on a balanced equity/bond portfolio, then all of your assets increased in value compared to last year’s peak valuations. For the other 90% of Americans, the recovery is modest, neutral, or more often nonexistent. Gastroenterologists who work in academic centers or large health systems did not lose income this year and were protected by billion-dollar credit lines and cash-on-hand accounts from robust days available to these entities. Independent practices (critically dependent on monthly cash flow) were decimated, furthering the trend towards consolidation, retirement, and acquisitions. With the new CMS E/M valuations we will see further reduction in procedural reimbursement.
However, disruption always precipitates innovation. Challenges are great but opportunities are clearly evident for those willing to risk.
John I. Allen, MD, MBA, AGAF
Editor in Chief
When this editorial is published, we will know the results of the national election (hopefully) and whether there will be a smooth transition of power. We should know whether the Affordable Care Act will remain intact, and we will have indications about the impact of a COVID/flu combination. Health care will never be the same.
According to a recent Medscape survey, 62% of U.S. physicians saw a reduction of monthly income (12% saw a reduction of over 70%) in the first 6 months of this year. Almost a third of the physician workforce is contemplating retirement earlier than anticipated. As worrisome, according to a JAMA article (Aug 4, 2020;324:510-3) the United States saw a 35% increase in excess deaths because of non-COVID etiologies, an indication of health care deferral and avoidance. We all are scrambling to catch up and accommodate an enormous demand.
We are witnessing a “K” shaped recovery for both individuals and GI practices. If your health care is covered by Medicare, you own a mortgage-free home and your wealth is based on a balanced equity/bond portfolio, then all of your assets increased in value compared to last year’s peak valuations. For the other 90% of Americans, the recovery is modest, neutral, or more often nonexistent. Gastroenterologists who work in academic centers or large health systems did not lose income this year and were protected by billion-dollar credit lines and cash-on-hand accounts from robust days available to these entities. Independent practices (critically dependent on monthly cash flow) were decimated, furthering the trend towards consolidation, retirement, and acquisitions. With the new CMS E/M valuations we will see further reduction in procedural reimbursement.
However, disruption always precipitates innovation. Challenges are great but opportunities are clearly evident for those willing to risk.
John I. Allen, MD, MBA, AGAF
Editor in Chief
No lab monitoring needed in adolescents on dupilumab
, Michael J. Cork, MBBS, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These reassuring results from the ongoing LIBERTY AD PED-OLE study confirm that, as previously established in adults, no blood monitoring is required in adolescents on the monoclonal antibody, which inhibits signaling of interleukins-4 and -13, said Dr. Cork, professor of dermatology and head of Sheffield Dermatology Research at the University of Sheffield (England).
“The practical importance of this finding is that there are no other systemic drugs available that don’t require blood samples. Cyclosporine, methotrexate, and the others used for atopic dermatitis require a lot of blood monitoring, and they’re off-license anyway for use in children and adolescents,” he said in an interview.
Many pediatric patients are afraid of needles and have an intense dislike of blood draws. And in a pandemic, no one wants to come into the office for blood draws if they don’t need to.
“Blood draws are very different from the injection for dupilumab. Taking a blood sample is much more painful for children. The needle in the autoinjector is really, really tiny; you can hardly feel it, and with the autoinjector you can’t even see it,” noted Dr. Cork, who is both a pediatric and adult dermatologist.
This report from the ongoing LIBERTY AD PED-OLE study included 105 patients aged 12-17 years who completed 52 weeks on dupilumab (Dupixent) with assessments of hematologic and serum chemistry parameters at baseline and weeks 16 and 52.
“The results were anticipated, but we want to know the drug is safe in every age group. The immune system is different in different age groups, so we have to be really careful,” Dr. Cork said.
The clinical side-effect profile was the same as in adults, consisting mainly of mild conjunctivitis and injection-site reactions. It’s a much less problematic side effect picture than with the older drugs.
“We’re finding the conjunctivitis to be slightly less severe than in adults, maybe because we’ve learned from the first trials in adults and from clinical experience to use prophylactic therapy. There would be no child going on dupilumab now – and no adult – that I wouldn’t put on prophylactic eye drops with replacement tears. I start them 2 weeks before I start dupilumab,” the dermatologist explained.
He and others with extensive experience using the biologic agent also work closely with an ophthalmologist.
“If we see an eye problem before going on dupilumab we get an assessment and then ophthalmologic monitoring during treatment,” Dr. Cork said.
As a dermatologist specializing in atopic dermatitis, he confessed to feeling deprived over the years as he watched the multitude of targeted biologic agents being developed for psoriasis. When he became involved in the first pediatric clinical trials of dupilumab, he had a realization: “It’s a miraculous treatment.”
“The first child I put on dupilumab spent 70 days in the hospital for IV antibiotics in the prior year. Seventy days! He almost died from MRSA septicemia. His serum IgE was 155,000 kU/L. And his IgE just went down and down and down as the dupilumab took effect. It was just incredible,” he recalled.
Dr. Cork reported receiving research funding from and serving as a consultant to Sanofi and Regeneron, which fund the LIBERTY AD PED-OLE study, as well as numerous other pharmaceutical companies.
SOURCE: Cork MJ. EADV 2020, Abstract 1772.
, Michael J. Cork, MBBS, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These reassuring results from the ongoing LIBERTY AD PED-OLE study confirm that, as previously established in adults, no blood monitoring is required in adolescents on the monoclonal antibody, which inhibits signaling of interleukins-4 and -13, said Dr. Cork, professor of dermatology and head of Sheffield Dermatology Research at the University of Sheffield (England).
“The practical importance of this finding is that there are no other systemic drugs available that don’t require blood samples. Cyclosporine, methotrexate, and the others used for atopic dermatitis require a lot of blood monitoring, and they’re off-license anyway for use in children and adolescents,” he said in an interview.
Many pediatric patients are afraid of needles and have an intense dislike of blood draws. And in a pandemic, no one wants to come into the office for blood draws if they don’t need to.
“Blood draws are very different from the injection for dupilumab. Taking a blood sample is much more painful for children. The needle in the autoinjector is really, really tiny; you can hardly feel it, and with the autoinjector you can’t even see it,” noted Dr. Cork, who is both a pediatric and adult dermatologist.
This report from the ongoing LIBERTY AD PED-OLE study included 105 patients aged 12-17 years who completed 52 weeks on dupilumab (Dupixent) with assessments of hematologic and serum chemistry parameters at baseline and weeks 16 and 52.
“The results were anticipated, but we want to know the drug is safe in every age group. The immune system is different in different age groups, so we have to be really careful,” Dr. Cork said.
The clinical side-effect profile was the same as in adults, consisting mainly of mild conjunctivitis and injection-site reactions. It’s a much less problematic side effect picture than with the older drugs.
“We’re finding the conjunctivitis to be slightly less severe than in adults, maybe because we’ve learned from the first trials in adults and from clinical experience to use prophylactic therapy. There would be no child going on dupilumab now – and no adult – that I wouldn’t put on prophylactic eye drops with replacement tears. I start them 2 weeks before I start dupilumab,” the dermatologist explained.
He and others with extensive experience using the biologic agent also work closely with an ophthalmologist.
“If we see an eye problem before going on dupilumab we get an assessment and then ophthalmologic monitoring during treatment,” Dr. Cork said.
As a dermatologist specializing in atopic dermatitis, he confessed to feeling deprived over the years as he watched the multitude of targeted biologic agents being developed for psoriasis. When he became involved in the first pediatric clinical trials of dupilumab, he had a realization: “It’s a miraculous treatment.”
“The first child I put on dupilumab spent 70 days in the hospital for IV antibiotics in the prior year. Seventy days! He almost died from MRSA septicemia. His serum IgE was 155,000 kU/L. And his IgE just went down and down and down as the dupilumab took effect. It was just incredible,” he recalled.
Dr. Cork reported receiving research funding from and serving as a consultant to Sanofi and Regeneron, which fund the LIBERTY AD PED-OLE study, as well as numerous other pharmaceutical companies.
SOURCE: Cork MJ. EADV 2020, Abstract 1772.
, Michael J. Cork, MBBS, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These reassuring results from the ongoing LIBERTY AD PED-OLE study confirm that, as previously established in adults, no blood monitoring is required in adolescents on the monoclonal antibody, which inhibits signaling of interleukins-4 and -13, said Dr. Cork, professor of dermatology and head of Sheffield Dermatology Research at the University of Sheffield (England).
“The practical importance of this finding is that there are no other systemic drugs available that don’t require blood samples. Cyclosporine, methotrexate, and the others used for atopic dermatitis require a lot of blood monitoring, and they’re off-license anyway for use in children and adolescents,” he said in an interview.
Many pediatric patients are afraid of needles and have an intense dislike of blood draws. And in a pandemic, no one wants to come into the office for blood draws if they don’t need to.
“Blood draws are very different from the injection for dupilumab. Taking a blood sample is much more painful for children. The needle in the autoinjector is really, really tiny; you can hardly feel it, and with the autoinjector you can’t even see it,” noted Dr. Cork, who is both a pediatric and adult dermatologist.
This report from the ongoing LIBERTY AD PED-OLE study included 105 patients aged 12-17 years who completed 52 weeks on dupilumab (Dupixent) with assessments of hematologic and serum chemistry parameters at baseline and weeks 16 and 52.
“The results were anticipated, but we want to know the drug is safe in every age group. The immune system is different in different age groups, so we have to be really careful,” Dr. Cork said.
The clinical side-effect profile was the same as in adults, consisting mainly of mild conjunctivitis and injection-site reactions. It’s a much less problematic side effect picture than with the older drugs.
“We’re finding the conjunctivitis to be slightly less severe than in adults, maybe because we’ve learned from the first trials in adults and from clinical experience to use prophylactic therapy. There would be no child going on dupilumab now – and no adult – that I wouldn’t put on prophylactic eye drops with replacement tears. I start them 2 weeks before I start dupilumab,” the dermatologist explained.
He and others with extensive experience using the biologic agent also work closely with an ophthalmologist.
“If we see an eye problem before going on dupilumab we get an assessment and then ophthalmologic monitoring during treatment,” Dr. Cork said.
As a dermatologist specializing in atopic dermatitis, he confessed to feeling deprived over the years as he watched the multitude of targeted biologic agents being developed for psoriasis. When he became involved in the first pediatric clinical trials of dupilumab, he had a realization: “It’s a miraculous treatment.”
“The first child I put on dupilumab spent 70 days in the hospital for IV antibiotics in the prior year. Seventy days! He almost died from MRSA septicemia. His serum IgE was 155,000 kU/L. And his IgE just went down and down and down as the dupilumab took effect. It was just incredible,” he recalled.
Dr. Cork reported receiving research funding from and serving as a consultant to Sanofi and Regeneron, which fund the LIBERTY AD PED-OLE study, as well as numerous other pharmaceutical companies.
SOURCE: Cork MJ. EADV 2020, Abstract 1772.
FROM THE EADV CONGRESS
Add-on atypicals for depression carry ‘substantial’ death risk
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
OTC topical ivermectin lotion earns FDA approval for head lice
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.
Hospitalists are natural leaders in the COVID-19 battle
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Echocardiography in AMI not associated with improved outcomes
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Fulminant C. diff debate: Fecal transplants or antibiotics?
Two experts at IDWeek 2020 debated the best treatment for patients with the most severe type of Clostridioides difficile infection – fulminant C. diff. The discussion pitted fecal microbiota transplants (FMT) from the stool of healthy donors against traditional antibiotics.
Fulminant C. diff infection (CDI) represents about 8% of all CDI cases and is often fatal. Patients frequently don’t respond to maximum antibiotic therapy.
Should these patients be treated with FMT before surgery is considered?
“Unequivocally, yes,” said Jessica R. Allegretti, MD, MPH, associate director of the Crohn’s and Colitis Center at Brigham and Women’s Hospital in Boston.
Patients face full colectomy
Fulminant infection, she says, typically requires a total abdominal colectomy with end ileostomy.
“Patients have a quite high perioperative and intraoperative mortality because this is typically an older population with significant comorbidities,” she said.
Often the patients are poor candidates for surgery, she added.
She pointed to the efficacy of FMT in studies such as one published in Gut Microbes in 2017. The study, by Monika Fischer, MD, of Indiana University, Indianapolis, and colleagues showed a 91% cure rate at 1 month in severe patients with an average of 1.5 fecal transplants, noting that was “quite remarkable” in this very sick population.
Though FMT is not approved by the US Food and Drug Administration for fulminant CDI, Dr. Allegretti said, the FDA does allow treatment under “enforcement discretion,” which means no investigational new drug license is needed specifically if treating CDI patients who haven’t responded to standard therapy, as long as proper consent has been obtained.
“This is a patient population that is likely going to die,” she said. “If you were the one in the ICU with fulminant C. diff and you’ve been on maximum therapy for 3-5 days and you’re not getting better, wouldn’t you want somebody to offer you a fecal transplant and give you the chance to recover and leave the hospital with your colon intact? The data suggest that is possible, with a high likelihood and a good safety profile.”
She said the most recent guidelines have supported FMT, and emerging guidelines coming within months “will support this as well.”
Unknowns with FMT
Taking the other side of the debate, Kevin Garey, PharmD, chair of the department of pharmacy practice and translational research at University of Houston College of Pharmacy, warned against trading traditional antibiotics, such as vancomycin and fidaxomicin, for the novelty of FMT.
“With the science of the microbiome and the novelty of fecal microbiota transplantation in expanding use, I think people have somewhat forgotten pharmacotherapy,” he said.
He pointed out safety concerns with FMT reported in June 2019, after which the FDA issued an alert. Two immunocompromised patients who received FMT, both from the same donor, developed invasive infections caused by extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli. One died.
The FDA explained that the donated FMT samples the patients received were not tested for ESBL-producing gram-negative organisms before use.
Dr. Allegretti agreed antibiotics play a role in treatment with FMT, but she argued that the safety profile of FMT remains strong and that the safety issues came from isolated incidents at a single center.
Dr. Garey countered that there are just too many unknowns with FMT.
“We will never know what the next superbug that’s going to land in an FMT is until we’ve identified that superbug in somebody – the next Candida auris, the next CRE [carbapenem-resistant Enterobacteriaceae], the next thing that’s going to show up in FMT – until we get rid of the ‘F,’ “ Dr. Garey said.
“[Until] we get microbial therapy that’s generated without the need for healthy donors, I think we’re always going to be in this problem.”
He said although FMT “has an amazing ability to alter a microbiome” it “pales in comparison” to vancomycin’s ability to do so.
Disruption of the microbiome is, without a doubt, a hallmark of C. diff, but we don’t have to run to FMT,” Dr. Garey said. “We can think about prophylaxis strategies, we can think about new drug development that spares the microbiota. The need for FMT might be a consequence of poor pharmacotherapy management, not a part of pharmacotherapy management.”
Moderator Sam Aitken, PharmD, MPH, a clinical pharmacy specialist in infectious disease at MD Anderson Cancer Center in Houston, said in an interview the speakers found some common ground.
“I think there was a general consensus between both Dr. Allegretti and Dr. Garey that both traditional therapeutics and fecal microbiota transplantation have a role to play in these patients, although there is still quite a bit of discussion around where those might be best positioned,” Dr. Aitken said.
He added, “There’s also a general consensus that there is not likely to be one right answer for all patients with multiple recurrent CDI.”
Dr. Allegretti, Dr. Garey, and Dr. Aitken have disclosed no relevant financial relationships.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more and register to participate at www.gastro.org/fmtregistry.
A version of this article originally appeared on Medscape.com.
Two experts at IDWeek 2020 debated the best treatment for patients with the most severe type of Clostridioides difficile infection – fulminant C. diff. The discussion pitted fecal microbiota transplants (FMT) from the stool of healthy donors against traditional antibiotics.
Fulminant C. diff infection (CDI) represents about 8% of all CDI cases and is often fatal. Patients frequently don’t respond to maximum antibiotic therapy.
Should these patients be treated with FMT before surgery is considered?
“Unequivocally, yes,” said Jessica R. Allegretti, MD, MPH, associate director of the Crohn’s and Colitis Center at Brigham and Women’s Hospital in Boston.
Patients face full colectomy
Fulminant infection, she says, typically requires a total abdominal colectomy with end ileostomy.
“Patients have a quite high perioperative and intraoperative mortality because this is typically an older population with significant comorbidities,” she said.
Often the patients are poor candidates for surgery, she added.
She pointed to the efficacy of FMT in studies such as one published in Gut Microbes in 2017. The study, by Monika Fischer, MD, of Indiana University, Indianapolis, and colleagues showed a 91% cure rate at 1 month in severe patients with an average of 1.5 fecal transplants, noting that was “quite remarkable” in this very sick population.
Though FMT is not approved by the US Food and Drug Administration for fulminant CDI, Dr. Allegretti said, the FDA does allow treatment under “enforcement discretion,” which means no investigational new drug license is needed specifically if treating CDI patients who haven’t responded to standard therapy, as long as proper consent has been obtained.
“This is a patient population that is likely going to die,” she said. “If you were the one in the ICU with fulminant C. diff and you’ve been on maximum therapy for 3-5 days and you’re not getting better, wouldn’t you want somebody to offer you a fecal transplant and give you the chance to recover and leave the hospital with your colon intact? The data suggest that is possible, with a high likelihood and a good safety profile.”
She said the most recent guidelines have supported FMT, and emerging guidelines coming within months “will support this as well.”
Unknowns with FMT
Taking the other side of the debate, Kevin Garey, PharmD, chair of the department of pharmacy practice and translational research at University of Houston College of Pharmacy, warned against trading traditional antibiotics, such as vancomycin and fidaxomicin, for the novelty of FMT.
“With the science of the microbiome and the novelty of fecal microbiota transplantation in expanding use, I think people have somewhat forgotten pharmacotherapy,” he said.
He pointed out safety concerns with FMT reported in June 2019, after which the FDA issued an alert. Two immunocompromised patients who received FMT, both from the same donor, developed invasive infections caused by extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli. One died.
The FDA explained that the donated FMT samples the patients received were not tested for ESBL-producing gram-negative organisms before use.
Dr. Allegretti agreed antibiotics play a role in treatment with FMT, but she argued that the safety profile of FMT remains strong and that the safety issues came from isolated incidents at a single center.
Dr. Garey countered that there are just too many unknowns with FMT.
“We will never know what the next superbug that’s going to land in an FMT is until we’ve identified that superbug in somebody – the next Candida auris, the next CRE [carbapenem-resistant Enterobacteriaceae], the next thing that’s going to show up in FMT – until we get rid of the ‘F,’ “ Dr. Garey said.
“[Until] we get microbial therapy that’s generated without the need for healthy donors, I think we’re always going to be in this problem.”
He said although FMT “has an amazing ability to alter a microbiome” it “pales in comparison” to vancomycin’s ability to do so.
Disruption of the microbiome is, without a doubt, a hallmark of C. diff, but we don’t have to run to FMT,” Dr. Garey said. “We can think about prophylaxis strategies, we can think about new drug development that spares the microbiota. The need for FMT might be a consequence of poor pharmacotherapy management, not a part of pharmacotherapy management.”
Moderator Sam Aitken, PharmD, MPH, a clinical pharmacy specialist in infectious disease at MD Anderson Cancer Center in Houston, said in an interview the speakers found some common ground.
“I think there was a general consensus between both Dr. Allegretti and Dr. Garey that both traditional therapeutics and fecal microbiota transplantation have a role to play in these patients, although there is still quite a bit of discussion around where those might be best positioned,” Dr. Aitken said.
He added, “There’s also a general consensus that there is not likely to be one right answer for all patients with multiple recurrent CDI.”
Dr. Allegretti, Dr. Garey, and Dr. Aitken have disclosed no relevant financial relationships.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more and register to participate at www.gastro.org/fmtregistry.
A version of this article originally appeared on Medscape.com.
Two experts at IDWeek 2020 debated the best treatment for patients with the most severe type of Clostridioides difficile infection – fulminant C. diff. The discussion pitted fecal microbiota transplants (FMT) from the stool of healthy donors against traditional antibiotics.
Fulminant C. diff infection (CDI) represents about 8% of all CDI cases and is often fatal. Patients frequently don’t respond to maximum antibiotic therapy.
Should these patients be treated with FMT before surgery is considered?
“Unequivocally, yes,” said Jessica R. Allegretti, MD, MPH, associate director of the Crohn’s and Colitis Center at Brigham and Women’s Hospital in Boston.
Patients face full colectomy
Fulminant infection, she says, typically requires a total abdominal colectomy with end ileostomy.
“Patients have a quite high perioperative and intraoperative mortality because this is typically an older population with significant comorbidities,” she said.
Often the patients are poor candidates for surgery, she added.
She pointed to the efficacy of FMT in studies such as one published in Gut Microbes in 2017. The study, by Monika Fischer, MD, of Indiana University, Indianapolis, and colleagues showed a 91% cure rate at 1 month in severe patients with an average of 1.5 fecal transplants, noting that was “quite remarkable” in this very sick population.
Though FMT is not approved by the US Food and Drug Administration for fulminant CDI, Dr. Allegretti said, the FDA does allow treatment under “enforcement discretion,” which means no investigational new drug license is needed specifically if treating CDI patients who haven’t responded to standard therapy, as long as proper consent has been obtained.
“This is a patient population that is likely going to die,” she said. “If you were the one in the ICU with fulminant C. diff and you’ve been on maximum therapy for 3-5 days and you’re not getting better, wouldn’t you want somebody to offer you a fecal transplant and give you the chance to recover and leave the hospital with your colon intact? The data suggest that is possible, with a high likelihood and a good safety profile.”
She said the most recent guidelines have supported FMT, and emerging guidelines coming within months “will support this as well.”
Unknowns with FMT
Taking the other side of the debate, Kevin Garey, PharmD, chair of the department of pharmacy practice and translational research at University of Houston College of Pharmacy, warned against trading traditional antibiotics, such as vancomycin and fidaxomicin, for the novelty of FMT.
“With the science of the microbiome and the novelty of fecal microbiota transplantation in expanding use, I think people have somewhat forgotten pharmacotherapy,” he said.
He pointed out safety concerns with FMT reported in June 2019, after which the FDA issued an alert. Two immunocompromised patients who received FMT, both from the same donor, developed invasive infections caused by extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli. One died.
The FDA explained that the donated FMT samples the patients received were not tested for ESBL-producing gram-negative organisms before use.
Dr. Allegretti agreed antibiotics play a role in treatment with FMT, but she argued that the safety profile of FMT remains strong and that the safety issues came from isolated incidents at a single center.
Dr. Garey countered that there are just too many unknowns with FMT.
“We will never know what the next superbug that’s going to land in an FMT is until we’ve identified that superbug in somebody – the next Candida auris, the next CRE [carbapenem-resistant Enterobacteriaceae], the next thing that’s going to show up in FMT – until we get rid of the ‘F,’ “ Dr. Garey said.
“[Until] we get microbial therapy that’s generated without the need for healthy donors, I think we’re always going to be in this problem.”
He said although FMT “has an amazing ability to alter a microbiome” it “pales in comparison” to vancomycin’s ability to do so.
Disruption of the microbiome is, without a doubt, a hallmark of C. diff, but we don’t have to run to FMT,” Dr. Garey said. “We can think about prophylaxis strategies, we can think about new drug development that spares the microbiota. The need for FMT might be a consequence of poor pharmacotherapy management, not a part of pharmacotherapy management.”
Moderator Sam Aitken, PharmD, MPH, a clinical pharmacy specialist in infectious disease at MD Anderson Cancer Center in Houston, said in an interview the speakers found some common ground.
“I think there was a general consensus between both Dr. Allegretti and Dr. Garey that both traditional therapeutics and fecal microbiota transplantation have a role to play in these patients, although there is still quite a bit of discussion around where those might be best positioned,” Dr. Aitken said.
He added, “There’s also a general consensus that there is not likely to be one right answer for all patients with multiple recurrent CDI.”
Dr. Allegretti, Dr. Garey, and Dr. Aitken have disclosed no relevant financial relationships.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more and register to participate at www.gastro.org/fmtregistry.
A version of this article originally appeared on Medscape.com.
COVID and med ed cost: Are future docs paying more for less?
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.