User login
Adjunctive pimavanserin looks promising for anxious depression
Adjunctive pimavanserin brought clinically meaningful improvement in patients with anxious major depressive disorder inadequately responsive to standard antidepressants alone in a post hoc analysis of the CLARITY trial, Bryan Dirks, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
This is an intriguing observation, because it’s estimated that roughly 50% of individuals with major depressive disorder (MDD) have comorbid anxiety disorders or a high level of anxiety symptoms. Moreover, anxious depression has been associated with increased risk of suicidality, high unemployment, and impaired functioning.
CLARITY was a phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial whose positive results for the primary outcome have been published (J Clin Psychiatry. 2019 Sep 24;80[6]:19m12928. doi: 10.4088/JCP.19m12928). Because the encouraging findings regarding pimavanserin’s impact on anxious depression came from a post hoc analysis, the results need replication. That’s ongoing in a phase 3 trial of adjunctive pimavanserin versus placebo in patients with MDD, according to Dr. Dirks, director of clinical research at Acadia Pharmaceuticals, San Diego.
The CLARITY post hoc analysis included 104 patients with baseline MDD inadequately responsive to an SSRI or a serotonin norepinephrine reuptake inhibitor and anxious depression as defined by a Hamilton Depression Rating Scale (HAMD-17) anxiety/somatization factor subscale score of 7 or more. Twenty-nine of the patients were randomized to 34 mg of adjunctive oral pimavanserin once daily, and 75 to placebo. At 5 weeks, the HAMD-17 anxiety/somatization factor score in the pimavanserin group had dropped by a mean of 5 points from a baseline of 8.8, a significantly greater effect than the 2.8-point drop in placebo-treated controls.
By week 5, the treatment response rate as defined by at least a 50% reduction in HAMD-17 total score from baseline was 55% with pimavanserin and 22% with placebo. The remission rate as indicated by a HAMD-17 total score below 7 was 24% in the pimavanserin group, compared with 5% with placebo. These results translated into an effect size of 0.78, considered by statisticians to be on the border between medium and large. Those response and remission rates in patients with anxious depression were higher with pimavanserin and lower with placebo than in the overall CLARITY trial.
as defined by a HAMD-17 total score of 24 or more plus an anxiety/somatization factor score of 7 or greater. Seventeen such patients were randomized to adjunctive pimavanserin, 36 to placebo. At 5 weeks, the mean HAMD total score had dropped by 17.4 points from a baseline of 27.6 in the pimavanserin group, compared with a 9.3-point reduction in controls.
“Of note, significant differences from placebo were observed as early as week 2 with pimavanserin,” Dr. Dirks said.
Pimavanserin is a novel selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors. At present pimavanserin is Food Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, but because of the drug’s unique mechanism of action it is under study for a variety of other mental disorders. Indeed, pimavanserin is now under FDA review for a possible expanded indication for treatment of dementia-related psychosis. The drug is also under study for schizophrenia as well as for MDD.
The CLARITY trial and this post hoc analysis were sponsored by Acadia Pharmaceuticals.
SOURCE: Dirks B. ECNP 2020. Abstract P 094.
Adjunctive pimavanserin brought clinically meaningful improvement in patients with anxious major depressive disorder inadequately responsive to standard antidepressants alone in a post hoc analysis of the CLARITY trial, Bryan Dirks, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
This is an intriguing observation, because it’s estimated that roughly 50% of individuals with major depressive disorder (MDD) have comorbid anxiety disorders or a high level of anxiety symptoms. Moreover, anxious depression has been associated with increased risk of suicidality, high unemployment, and impaired functioning.
CLARITY was a phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial whose positive results for the primary outcome have been published (J Clin Psychiatry. 2019 Sep 24;80[6]:19m12928. doi: 10.4088/JCP.19m12928). Because the encouraging findings regarding pimavanserin’s impact on anxious depression came from a post hoc analysis, the results need replication. That’s ongoing in a phase 3 trial of adjunctive pimavanserin versus placebo in patients with MDD, according to Dr. Dirks, director of clinical research at Acadia Pharmaceuticals, San Diego.
The CLARITY post hoc analysis included 104 patients with baseline MDD inadequately responsive to an SSRI or a serotonin norepinephrine reuptake inhibitor and anxious depression as defined by a Hamilton Depression Rating Scale (HAMD-17) anxiety/somatization factor subscale score of 7 or more. Twenty-nine of the patients were randomized to 34 mg of adjunctive oral pimavanserin once daily, and 75 to placebo. At 5 weeks, the HAMD-17 anxiety/somatization factor score in the pimavanserin group had dropped by a mean of 5 points from a baseline of 8.8, a significantly greater effect than the 2.8-point drop in placebo-treated controls.
By week 5, the treatment response rate as defined by at least a 50% reduction in HAMD-17 total score from baseline was 55% with pimavanserin and 22% with placebo. The remission rate as indicated by a HAMD-17 total score below 7 was 24% in the pimavanserin group, compared with 5% with placebo. These results translated into an effect size of 0.78, considered by statisticians to be on the border between medium and large. Those response and remission rates in patients with anxious depression were higher with pimavanserin and lower with placebo than in the overall CLARITY trial.
as defined by a HAMD-17 total score of 24 or more plus an anxiety/somatization factor score of 7 or greater. Seventeen such patients were randomized to adjunctive pimavanserin, 36 to placebo. At 5 weeks, the mean HAMD total score had dropped by 17.4 points from a baseline of 27.6 in the pimavanserin group, compared with a 9.3-point reduction in controls.
“Of note, significant differences from placebo were observed as early as week 2 with pimavanserin,” Dr. Dirks said.
Pimavanserin is a novel selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors. At present pimavanserin is Food Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, but because of the drug’s unique mechanism of action it is under study for a variety of other mental disorders. Indeed, pimavanserin is now under FDA review for a possible expanded indication for treatment of dementia-related psychosis. The drug is also under study for schizophrenia as well as for MDD.
The CLARITY trial and this post hoc analysis were sponsored by Acadia Pharmaceuticals.
SOURCE: Dirks B. ECNP 2020. Abstract P 094.
Adjunctive pimavanserin brought clinically meaningful improvement in patients with anxious major depressive disorder inadequately responsive to standard antidepressants alone in a post hoc analysis of the CLARITY trial, Bryan Dirks, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
This is an intriguing observation, because it’s estimated that roughly 50% of individuals with major depressive disorder (MDD) have comorbid anxiety disorders or a high level of anxiety symptoms. Moreover, anxious depression has been associated with increased risk of suicidality, high unemployment, and impaired functioning.
CLARITY was a phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial whose positive results for the primary outcome have been published (J Clin Psychiatry. 2019 Sep 24;80[6]:19m12928. doi: 10.4088/JCP.19m12928). Because the encouraging findings regarding pimavanserin’s impact on anxious depression came from a post hoc analysis, the results need replication. That’s ongoing in a phase 3 trial of adjunctive pimavanserin versus placebo in patients with MDD, according to Dr. Dirks, director of clinical research at Acadia Pharmaceuticals, San Diego.
The CLARITY post hoc analysis included 104 patients with baseline MDD inadequately responsive to an SSRI or a serotonin norepinephrine reuptake inhibitor and anxious depression as defined by a Hamilton Depression Rating Scale (HAMD-17) anxiety/somatization factor subscale score of 7 or more. Twenty-nine of the patients were randomized to 34 mg of adjunctive oral pimavanserin once daily, and 75 to placebo. At 5 weeks, the HAMD-17 anxiety/somatization factor score in the pimavanserin group had dropped by a mean of 5 points from a baseline of 8.8, a significantly greater effect than the 2.8-point drop in placebo-treated controls.
By week 5, the treatment response rate as defined by at least a 50% reduction in HAMD-17 total score from baseline was 55% with pimavanserin and 22% with placebo. The remission rate as indicated by a HAMD-17 total score below 7 was 24% in the pimavanserin group, compared with 5% with placebo. These results translated into an effect size of 0.78, considered by statisticians to be on the border between medium and large. Those response and remission rates in patients with anxious depression were higher with pimavanserin and lower with placebo than in the overall CLARITY trial.
as defined by a HAMD-17 total score of 24 or more plus an anxiety/somatization factor score of 7 or greater. Seventeen such patients were randomized to adjunctive pimavanserin, 36 to placebo. At 5 weeks, the mean HAMD total score had dropped by 17.4 points from a baseline of 27.6 in the pimavanserin group, compared with a 9.3-point reduction in controls.
“Of note, significant differences from placebo were observed as early as week 2 with pimavanserin,” Dr. Dirks said.
Pimavanserin is a novel selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors. At present pimavanserin is Food Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis, but because of the drug’s unique mechanism of action it is under study for a variety of other mental disorders. Indeed, pimavanserin is now under FDA review for a possible expanded indication for treatment of dementia-related psychosis. The drug is also under study for schizophrenia as well as for MDD.
The CLARITY trial and this post hoc analysis were sponsored by Acadia Pharmaceuticals.
SOURCE: Dirks B. ECNP 2020. Abstract P 094.
FROM ECNP 2020
Key clinical point: Pimavanserin may have a future as a novel treatment for anxious depression.
Major finding: Twenty-four percent of patients with anxious major depressive disorder inadequately responsive to standard antidepressant therapy achieved remission with 5 weeks of adjunctive pimavanserin, compared with 5% with placebo.
Study details: This was a post hoc analysis of the phase 2, multicenter, randomized, double-blind CLARITY trial.
Disclosures: The study was sponsored by Acadia Pharmaceuticals and presented by a company employee.
Source: Dirks B. ECNP 2020. Abstract P 094.
MitraClip effective for post-MI acute mitral regurgitation with cardiogenic shock
Percutaneous mitral valve repair with the MitraClip appears to be a safe, effective, and life-saving new treatment for severe acute mitral regurgitation (MR) secondary to MI in surgical noncandidates, even when accompanied by cardiogenic shock, according to data from the international IREMMI registry.
“Cardiogenic shock, when adequately supported, does not seem to influence short- and mid-term outcomes, so the development of cardiogenic shock should not preclude percutaneous mitral valve repair in this scenario,” Rodrigo Estevez-Loureiro, MD, PhD, said in presenting the IREMMI (International Registry of MitraClip in Acute Myocardial Infarction) findings reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
Commentators hailed the prospective IREMMI data as potentially practice changing in light of the dire prognosis of such patients when surgery is deemed unacceptably high risk because medical management, the traditionally the only alternative, has a 30-day mortality of up to 50%.
Severe acute MR occurs in an estimated 3% of acute MIs, and in roughly 10% of patients who present with acute MI complicated by cardiogenic shock (CS). The impact of intervening with the MitraClip in an effort to correct the acute MR arising from MI with CS has previously been addressed only in sparse case reports. The new IREMMI study is easily the largest dataset to date detailing clinical and echocardiographic outcomes, Dr. Estevez-Loureiro of Alvaro Cunqueiro Hospital in Vigo, Spain, said at the meeting, sponsored by the Cardiovascular Research Foundation.
He reported on 93 consecutive patients who underwent MitraClip implantation for acute MR arising in the setting of MI, including 50 patients in CS at the time of the procedure. All 93 patients had been turned down by their surgical team because of extreme surgical risk. Three-quarters of the MIs showed ST-segment elevation. Only six patients had a papillary muscle rupture; in the rest, the mechanism of acute MR involved left ventricular global remodeling associated with mitral valve leaflet tethering. Percutaneous valve repair was performed at 18 expert valvular heart centers in the United States, Canada, Israel, and five European countries.
Procedural success
Time from MI to MitraClip implantation averaged 24 days in the CS patients and 33 days in the comparator arm without CS.
“These patients had been turned down for surgery, so the attending physicians generally followed a strategy of trying to cool them down with mechanical circulatory support and vasopressors. MitraClip wasn’t an option at the beginning, but after two or three failed weanings from all the possible therapies, then MitraClip becomes an option. This is one of the reasons why the time lapse between MI and the clip is so large,” the cardiologist explained.
Procedural success rates were similar in the two groups: 90% in those with CS and 93% in those without. However, average procedure time was significantly longer in the CS patients: 143 minutes versus 83 minutes in the patients without CS.
At baseline, 86% of the CS group had grade 4+ MR, similar to the 79% rate in the non-CS patients. Postprocedurally, 60% of the CS group were MR grade 0/1 and 34% were grade 2, comparable to the rates of 65% and 23% in the non-CS group.
At 3 months’ follow-up, 83.4% of the CS group had MR grade 2 or less, again not significantly different from the 90.5% rate in non-CS patients. Systolic pulmonary artery pressure was also similar: 39.6 mm Hg in the CS patients, 44 mm Hg in those without. While everyone was New York Heart Association functional class IV preprocedurally, 79.5% of the CS group were NYHA class I or II at 3 months, not significantly different from the 86.5% prevalence in the comparator arm.
Longer-term clinical outcomes
At a median follow-up of 7 months, the composite primary clinical outcome composed of all-cause mortality or heart failure rehospitalization did not differ between the two groups: a 28% rate in the CS group and 25.6% in non-CS patients. All-cause mortality occurred in 16% with CS and 9.3% without, again not a significant difference.
In a Cox regression analysis, neither surgical risk score, patient age, left ventricular geometry, nor CS was independently associated with the primary composite endpoint. Indeed, the only independent predictor of freedom from mortality or heart failure readmission at follow-up was procedural success, which is very much a function of the experience of the heart team, Dr. Estevez-Loureiro continued.
Michael A. Borger, MD, PhD, who comoderated the late-breaking clinical science session, was wowed by the IREMMI results.
“The mortality rates, I can tell you, compared to traditional surgical series of acute MR in the face of ACS [acute cardiogenic shock] are very, very respectable,” commented Dr. Borger, director of the cardiac surgery clinic at the Leipzig (Ger.) University Heart Center.
“Extremely impressive,” agreed discussant Vinayak N. Bapat, MD, a cardiothoracic surgeon and valve scientist at the Minneapolis Heart Institute Foundation. He posed a practical question: “Should we take from this presentation that patients should be stabilized with something like ECMO [extracorporeal membrane oxygenation] or Impella [left ventricular assist device], then transferred to an expert center for the procedure?”
“I think that the stabilization is essential in the patients with cardiogenic shock,” Dr. Estevez-Loureiro replied. “Unlike with surgery, it’s very difficult to establish a MitraClip procedure in a couple of hours in the middle of the night. You have to stabilize them and then treat for shock with ECMO, Impella, or both. I think they should be transferred to a center than can deliver the best treatment. In centers with less experience, patients can be put on mechanical support and transferred to an expert valve center, not only for MitraClip implantation, but for discussion of all the treatment possibilities, including surgery.”
At a press conference in which Dr. Estevez-Loureiro presented highlights of the IREMMI study, discussant Dee Dee Wang, MD, said the international coinvestigators “need to be applauded” for this study.
“Having these outcomes is incredible,” declared Dr. Wang, a structural heart disease specialist at the Henry Ford Health System, Detroit.
While this is an observational study, it’s a high-quality dataset with excellent methodology. And conducting a randomized trial in patients with such high surgical risk scores – the CS group had an average EuroSCORE II of 21 – would be extremely difficult, according to the cardiologist.
Dr. Estevez-Loureiro reported receiving research grants from Abbott and serving as a consultant to that company as well as Boston Scientific.
SOURCE: Estevez-Loureiro, R. TCT 2020, LBCS session IV.
Percutaneous mitral valve repair with the MitraClip appears to be a safe, effective, and life-saving new treatment for severe acute mitral regurgitation (MR) secondary to MI in surgical noncandidates, even when accompanied by cardiogenic shock, according to data from the international IREMMI registry.
“Cardiogenic shock, when adequately supported, does not seem to influence short- and mid-term outcomes, so the development of cardiogenic shock should not preclude percutaneous mitral valve repair in this scenario,” Rodrigo Estevez-Loureiro, MD, PhD, said in presenting the IREMMI (International Registry of MitraClip in Acute Myocardial Infarction) findings reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
Commentators hailed the prospective IREMMI data as potentially practice changing in light of the dire prognosis of such patients when surgery is deemed unacceptably high risk because medical management, the traditionally the only alternative, has a 30-day mortality of up to 50%.
Severe acute MR occurs in an estimated 3% of acute MIs, and in roughly 10% of patients who present with acute MI complicated by cardiogenic shock (CS). The impact of intervening with the MitraClip in an effort to correct the acute MR arising from MI with CS has previously been addressed only in sparse case reports. The new IREMMI study is easily the largest dataset to date detailing clinical and echocardiographic outcomes, Dr. Estevez-Loureiro of Alvaro Cunqueiro Hospital in Vigo, Spain, said at the meeting, sponsored by the Cardiovascular Research Foundation.
He reported on 93 consecutive patients who underwent MitraClip implantation for acute MR arising in the setting of MI, including 50 patients in CS at the time of the procedure. All 93 patients had been turned down by their surgical team because of extreme surgical risk. Three-quarters of the MIs showed ST-segment elevation. Only six patients had a papillary muscle rupture; in the rest, the mechanism of acute MR involved left ventricular global remodeling associated with mitral valve leaflet tethering. Percutaneous valve repair was performed at 18 expert valvular heart centers in the United States, Canada, Israel, and five European countries.
Procedural success
Time from MI to MitraClip implantation averaged 24 days in the CS patients and 33 days in the comparator arm without CS.
“These patients had been turned down for surgery, so the attending physicians generally followed a strategy of trying to cool them down with mechanical circulatory support and vasopressors. MitraClip wasn’t an option at the beginning, but after two or three failed weanings from all the possible therapies, then MitraClip becomes an option. This is one of the reasons why the time lapse between MI and the clip is so large,” the cardiologist explained.
Procedural success rates were similar in the two groups: 90% in those with CS and 93% in those without. However, average procedure time was significantly longer in the CS patients: 143 minutes versus 83 minutes in the patients without CS.
At baseline, 86% of the CS group had grade 4+ MR, similar to the 79% rate in the non-CS patients. Postprocedurally, 60% of the CS group were MR grade 0/1 and 34% were grade 2, comparable to the rates of 65% and 23% in the non-CS group.
At 3 months’ follow-up, 83.4% of the CS group had MR grade 2 or less, again not significantly different from the 90.5% rate in non-CS patients. Systolic pulmonary artery pressure was also similar: 39.6 mm Hg in the CS patients, 44 mm Hg in those without. While everyone was New York Heart Association functional class IV preprocedurally, 79.5% of the CS group were NYHA class I or II at 3 months, not significantly different from the 86.5% prevalence in the comparator arm.
Longer-term clinical outcomes
At a median follow-up of 7 months, the composite primary clinical outcome composed of all-cause mortality or heart failure rehospitalization did not differ between the two groups: a 28% rate in the CS group and 25.6% in non-CS patients. All-cause mortality occurred in 16% with CS and 9.3% without, again not a significant difference.
In a Cox regression analysis, neither surgical risk score, patient age, left ventricular geometry, nor CS was independently associated with the primary composite endpoint. Indeed, the only independent predictor of freedom from mortality or heart failure readmission at follow-up was procedural success, which is very much a function of the experience of the heart team, Dr. Estevez-Loureiro continued.
Michael A. Borger, MD, PhD, who comoderated the late-breaking clinical science session, was wowed by the IREMMI results.
“The mortality rates, I can tell you, compared to traditional surgical series of acute MR in the face of ACS [acute cardiogenic shock] are very, very respectable,” commented Dr. Borger, director of the cardiac surgery clinic at the Leipzig (Ger.) University Heart Center.
“Extremely impressive,” agreed discussant Vinayak N. Bapat, MD, a cardiothoracic surgeon and valve scientist at the Minneapolis Heart Institute Foundation. He posed a practical question: “Should we take from this presentation that patients should be stabilized with something like ECMO [extracorporeal membrane oxygenation] or Impella [left ventricular assist device], then transferred to an expert center for the procedure?”
“I think that the stabilization is essential in the patients with cardiogenic shock,” Dr. Estevez-Loureiro replied. “Unlike with surgery, it’s very difficult to establish a MitraClip procedure in a couple of hours in the middle of the night. You have to stabilize them and then treat for shock with ECMO, Impella, or both. I think they should be transferred to a center than can deliver the best treatment. In centers with less experience, patients can be put on mechanical support and transferred to an expert valve center, not only for MitraClip implantation, but for discussion of all the treatment possibilities, including surgery.”
At a press conference in which Dr. Estevez-Loureiro presented highlights of the IREMMI study, discussant Dee Dee Wang, MD, said the international coinvestigators “need to be applauded” for this study.
“Having these outcomes is incredible,” declared Dr. Wang, a structural heart disease specialist at the Henry Ford Health System, Detroit.
While this is an observational study, it’s a high-quality dataset with excellent methodology. And conducting a randomized trial in patients with such high surgical risk scores – the CS group had an average EuroSCORE II of 21 – would be extremely difficult, according to the cardiologist.
Dr. Estevez-Loureiro reported receiving research grants from Abbott and serving as a consultant to that company as well as Boston Scientific.
SOURCE: Estevez-Loureiro, R. TCT 2020, LBCS session IV.
Percutaneous mitral valve repair with the MitraClip appears to be a safe, effective, and life-saving new treatment for severe acute mitral regurgitation (MR) secondary to MI in surgical noncandidates, even when accompanied by cardiogenic shock, according to data from the international IREMMI registry.
“Cardiogenic shock, when adequately supported, does not seem to influence short- and mid-term outcomes, so the development of cardiogenic shock should not preclude percutaneous mitral valve repair in this scenario,” Rodrigo Estevez-Loureiro, MD, PhD, said in presenting the IREMMI (International Registry of MitraClip in Acute Myocardial Infarction) findings reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
Commentators hailed the prospective IREMMI data as potentially practice changing in light of the dire prognosis of such patients when surgery is deemed unacceptably high risk because medical management, the traditionally the only alternative, has a 30-day mortality of up to 50%.
Severe acute MR occurs in an estimated 3% of acute MIs, and in roughly 10% of patients who present with acute MI complicated by cardiogenic shock (CS). The impact of intervening with the MitraClip in an effort to correct the acute MR arising from MI with CS has previously been addressed only in sparse case reports. The new IREMMI study is easily the largest dataset to date detailing clinical and echocardiographic outcomes, Dr. Estevez-Loureiro of Alvaro Cunqueiro Hospital in Vigo, Spain, said at the meeting, sponsored by the Cardiovascular Research Foundation.
He reported on 93 consecutive patients who underwent MitraClip implantation for acute MR arising in the setting of MI, including 50 patients in CS at the time of the procedure. All 93 patients had been turned down by their surgical team because of extreme surgical risk. Three-quarters of the MIs showed ST-segment elevation. Only six patients had a papillary muscle rupture; in the rest, the mechanism of acute MR involved left ventricular global remodeling associated with mitral valve leaflet tethering. Percutaneous valve repair was performed at 18 expert valvular heart centers in the United States, Canada, Israel, and five European countries.
Procedural success
Time from MI to MitraClip implantation averaged 24 days in the CS patients and 33 days in the comparator arm without CS.
“These patients had been turned down for surgery, so the attending physicians generally followed a strategy of trying to cool them down with mechanical circulatory support and vasopressors. MitraClip wasn’t an option at the beginning, but after two or three failed weanings from all the possible therapies, then MitraClip becomes an option. This is one of the reasons why the time lapse between MI and the clip is so large,” the cardiologist explained.
Procedural success rates were similar in the two groups: 90% in those with CS and 93% in those without. However, average procedure time was significantly longer in the CS patients: 143 minutes versus 83 minutes in the patients without CS.
At baseline, 86% of the CS group had grade 4+ MR, similar to the 79% rate in the non-CS patients. Postprocedurally, 60% of the CS group were MR grade 0/1 and 34% were grade 2, comparable to the rates of 65% and 23% in the non-CS group.
At 3 months’ follow-up, 83.4% of the CS group had MR grade 2 or less, again not significantly different from the 90.5% rate in non-CS patients. Systolic pulmonary artery pressure was also similar: 39.6 mm Hg in the CS patients, 44 mm Hg in those without. While everyone was New York Heart Association functional class IV preprocedurally, 79.5% of the CS group were NYHA class I or II at 3 months, not significantly different from the 86.5% prevalence in the comparator arm.
Longer-term clinical outcomes
At a median follow-up of 7 months, the composite primary clinical outcome composed of all-cause mortality or heart failure rehospitalization did not differ between the two groups: a 28% rate in the CS group and 25.6% in non-CS patients. All-cause mortality occurred in 16% with CS and 9.3% without, again not a significant difference.
In a Cox regression analysis, neither surgical risk score, patient age, left ventricular geometry, nor CS was independently associated with the primary composite endpoint. Indeed, the only independent predictor of freedom from mortality or heart failure readmission at follow-up was procedural success, which is very much a function of the experience of the heart team, Dr. Estevez-Loureiro continued.
Michael A. Borger, MD, PhD, who comoderated the late-breaking clinical science session, was wowed by the IREMMI results.
“The mortality rates, I can tell you, compared to traditional surgical series of acute MR in the face of ACS [acute cardiogenic shock] are very, very respectable,” commented Dr. Borger, director of the cardiac surgery clinic at the Leipzig (Ger.) University Heart Center.
“Extremely impressive,” agreed discussant Vinayak N. Bapat, MD, a cardiothoracic surgeon and valve scientist at the Minneapolis Heart Institute Foundation. He posed a practical question: “Should we take from this presentation that patients should be stabilized with something like ECMO [extracorporeal membrane oxygenation] or Impella [left ventricular assist device], then transferred to an expert center for the procedure?”
“I think that the stabilization is essential in the patients with cardiogenic shock,” Dr. Estevez-Loureiro replied. “Unlike with surgery, it’s very difficult to establish a MitraClip procedure in a couple of hours in the middle of the night. You have to stabilize them and then treat for shock with ECMO, Impella, or both. I think they should be transferred to a center than can deliver the best treatment. In centers with less experience, patients can be put on mechanical support and transferred to an expert valve center, not only for MitraClip implantation, but for discussion of all the treatment possibilities, including surgery.”
At a press conference in which Dr. Estevez-Loureiro presented highlights of the IREMMI study, discussant Dee Dee Wang, MD, said the international coinvestigators “need to be applauded” for this study.
“Having these outcomes is incredible,” declared Dr. Wang, a structural heart disease specialist at the Henry Ford Health System, Detroit.
While this is an observational study, it’s a high-quality dataset with excellent methodology. And conducting a randomized trial in patients with such high surgical risk scores – the CS group had an average EuroSCORE II of 21 – would be extremely difficult, according to the cardiologist.
Dr. Estevez-Loureiro reported receiving research grants from Abbott and serving as a consultant to that company as well as Boston Scientific.
SOURCE: Estevez-Loureiro, R. TCT 2020, LBCS session IV.
FROM TCT 2020
IL-23 plays key roles in antimicrobial macrophage activity
Interleukin-23 optimizes antimicrobial macrophage activity, which is reduced among persons harboring an IL-23 receptor variant that helps protect against inflammatory bowel disease (IBD), recent research has found.
“These [findings] highlight that the susceptibility to infections with therapeutic blockade of the IL-23/IL-12 pathways may be owing in part to the essential role for IL-23 in mediating antimicrobial functions in macrophages. They further highlight that carriers of the IL-23R–Q381 variant, who are relatively protected from IBD and other immune-mediated diseases, may be at increased risk for bacterial infection,” Rui Sun and Clara Abraham, MD, of Yale University, New Haven, Conn., wrote in Cellular and Molecular Gastroenterology and Hepatology.
IL-23 is key to the pathogenesis of IBD and is being studied as a therapeutic target, both alone and in combination with IL-12 blocking. Although human macrophages express low levels of IL-23 receptor, recent research reveals that IL-23R is up-regulated “within minutes of exposure to IL-23,” which promotes signaling and cytokine secretion, the investigators wrote. However, the extent to which IL-23 supports macrophage antimicrobial activity was unknown. To characterize protein expression, signaling, and bacterial uptake and clearance of bacteria by human macrophages derived from monocytes, the investigators tested these cells with Western blot, flow cytometry, and gentamicin protection, which involved coculturing human macrophages with bacteria, adding gentamicin solution, and then lysing and plating the cells onto agar to assess the extent to which the macrophages had taken up the bacteria.
After 48 hours of exposure to IL-23 or IL-12, macrophages increased their intracellular clearance of clinically relevant bacteria, including Enterococcus faecalis, adherent invasive Escherichia coli, and Salmonella typhimurium. Notably, this did not occur when the investigators reduced (“knocked down”) macrophage expression of either IL-23R or IL-12 receptor alpha 2. Additional investigations showed that in macrophages, IL-23 promotes bacterial uptake, clearance, and autophagy by inducing a pyruvate dehydrogenase kinase 1 (PDK1)–dependent pathway mediated by Janus kinase 2/tyrosine kinase 2 and by inducing reactive oxygen species (ROS) and reactive nitrogen species (RNS) pathways. IL-23 also activates two key proteins involved in autophagy (ATG5 and ATG16L1), the researchers reported. “ROS, RNS, and autophagy cooperate to mediate IL-23-induced bacterial clearance. Reduction of each ROS, RNS, and autophagy pathway partially reversed the enhanced bacterial clearance observed with chronic IL-23 treatment.”
Further tests found that IL-23 mediates antimicrobial pathways through the Janus kinase 2, tyrosine kinase 2, and STAT3 pathways, which “cooperate to mediate optimal IL-23-induced intracellular bacterial clearance in human macrophages.” Importantly, human macrophages showed less antimicrobial activity when transfected with the IL-23R–Q381 variant than with IL-23R–R381. The IL-23R-Q381 variant, which reduces susceptibility to IBD, “decreased IL-23-induced and NOD2-induced antimicrobial pathways and intracellular bacterial clearance in monocyte-derived macrophages,” the researchers explained. Evaluating actual carriers of these variants showed the same results – macrophages harboring IBD-protective IL-23R–R381/Q381 exhibited lower antimicrobial activity and less intracellular bacterial clearance compared with macrophages from carriers of IL-23R–R381/R381.
“Taken together, IL-23 promotes increased bacterial uptake and then induces a more rapid and effective clearance of these intracellular bacteria in human monocyte-derived macrophages,” the researchers wrote. “The reduced inflammatory responses observed in IL-23R Q381 carriers are associated with protection from multiple immune-mediated diseases. This would imply that loss-of-function observed with the common IL-23R–R381Q variant may lead to a disadvantage in select infectious diseases, including through [this variant’s] now identified role in promoting antimicrobial pathways in macrophages.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SOURCE: Sun R, Abraham C. Cell Molec Gastro Hepatol. 2020 May 28. doi.: 10.1016/j.jcmgh.2020.05.007.
Both genetic studies in humans and functional studies in mice have pinpointed interleukin-23 and its receptor as a key pathway in the pathogenesis of inflammatory bowel disease (IBD). IL-23 is released from myeloid cells in response to sensing of invading pathogens or danger-associated molecular patterns, where it drives induction of Th17, innate lymphoid cell responses, and inflammation.
Alison Simmons, FRCP, PhD, is professor of gastroenterology, honorary consultant gastroenterologist, MRC human immunology unit, Weatherall Institute of Molecular Medicine, University of Oxford (England), and translational gastroenterology unit, Oxford University Hospitals NHS Trust. She has consultancies from AbbVie, Bristol-Myers Squibb, and Janssen, and is a cofounder and equity holder in TRexBio.
Both genetic studies in humans and functional studies in mice have pinpointed interleukin-23 and its receptor as a key pathway in the pathogenesis of inflammatory bowel disease (IBD). IL-23 is released from myeloid cells in response to sensing of invading pathogens or danger-associated molecular patterns, where it drives induction of Th17, innate lymphoid cell responses, and inflammation.
Alison Simmons, FRCP, PhD, is professor of gastroenterology, honorary consultant gastroenterologist, MRC human immunology unit, Weatherall Institute of Molecular Medicine, University of Oxford (England), and translational gastroenterology unit, Oxford University Hospitals NHS Trust. She has consultancies from AbbVie, Bristol-Myers Squibb, and Janssen, and is a cofounder and equity holder in TRexBio.
Both genetic studies in humans and functional studies in mice have pinpointed interleukin-23 and its receptor as a key pathway in the pathogenesis of inflammatory bowel disease (IBD). IL-23 is released from myeloid cells in response to sensing of invading pathogens or danger-associated molecular patterns, where it drives induction of Th17, innate lymphoid cell responses, and inflammation.
Alison Simmons, FRCP, PhD, is professor of gastroenterology, honorary consultant gastroenterologist, MRC human immunology unit, Weatherall Institute of Molecular Medicine, University of Oxford (England), and translational gastroenterology unit, Oxford University Hospitals NHS Trust. She has consultancies from AbbVie, Bristol-Myers Squibb, and Janssen, and is a cofounder and equity holder in TRexBio.
Interleukin-23 optimizes antimicrobial macrophage activity, which is reduced among persons harboring an IL-23 receptor variant that helps protect against inflammatory bowel disease (IBD), recent research has found.
“These [findings] highlight that the susceptibility to infections with therapeutic blockade of the IL-23/IL-12 pathways may be owing in part to the essential role for IL-23 in mediating antimicrobial functions in macrophages. They further highlight that carriers of the IL-23R–Q381 variant, who are relatively protected from IBD and other immune-mediated diseases, may be at increased risk for bacterial infection,” Rui Sun and Clara Abraham, MD, of Yale University, New Haven, Conn., wrote in Cellular and Molecular Gastroenterology and Hepatology.
IL-23 is key to the pathogenesis of IBD and is being studied as a therapeutic target, both alone and in combination with IL-12 blocking. Although human macrophages express low levels of IL-23 receptor, recent research reveals that IL-23R is up-regulated “within minutes of exposure to IL-23,” which promotes signaling and cytokine secretion, the investigators wrote. However, the extent to which IL-23 supports macrophage antimicrobial activity was unknown. To characterize protein expression, signaling, and bacterial uptake and clearance of bacteria by human macrophages derived from monocytes, the investigators tested these cells with Western blot, flow cytometry, and gentamicin protection, which involved coculturing human macrophages with bacteria, adding gentamicin solution, and then lysing and plating the cells onto agar to assess the extent to which the macrophages had taken up the bacteria.
After 48 hours of exposure to IL-23 or IL-12, macrophages increased their intracellular clearance of clinically relevant bacteria, including Enterococcus faecalis, adherent invasive Escherichia coli, and Salmonella typhimurium. Notably, this did not occur when the investigators reduced (“knocked down”) macrophage expression of either IL-23R or IL-12 receptor alpha 2. Additional investigations showed that in macrophages, IL-23 promotes bacterial uptake, clearance, and autophagy by inducing a pyruvate dehydrogenase kinase 1 (PDK1)–dependent pathway mediated by Janus kinase 2/tyrosine kinase 2 and by inducing reactive oxygen species (ROS) and reactive nitrogen species (RNS) pathways. IL-23 also activates two key proteins involved in autophagy (ATG5 and ATG16L1), the researchers reported. “ROS, RNS, and autophagy cooperate to mediate IL-23-induced bacterial clearance. Reduction of each ROS, RNS, and autophagy pathway partially reversed the enhanced bacterial clearance observed with chronic IL-23 treatment.”
Further tests found that IL-23 mediates antimicrobial pathways through the Janus kinase 2, tyrosine kinase 2, and STAT3 pathways, which “cooperate to mediate optimal IL-23-induced intracellular bacterial clearance in human macrophages.” Importantly, human macrophages showed less antimicrobial activity when transfected with the IL-23R–Q381 variant than with IL-23R–R381. The IL-23R-Q381 variant, which reduces susceptibility to IBD, “decreased IL-23-induced and NOD2-induced antimicrobial pathways and intracellular bacterial clearance in monocyte-derived macrophages,” the researchers explained. Evaluating actual carriers of these variants showed the same results – macrophages harboring IBD-protective IL-23R–R381/Q381 exhibited lower antimicrobial activity and less intracellular bacterial clearance compared with macrophages from carriers of IL-23R–R381/R381.
“Taken together, IL-23 promotes increased bacterial uptake and then induces a more rapid and effective clearance of these intracellular bacteria in human monocyte-derived macrophages,” the researchers wrote. “The reduced inflammatory responses observed in IL-23R Q381 carriers are associated with protection from multiple immune-mediated diseases. This would imply that loss-of-function observed with the common IL-23R–R381Q variant may lead to a disadvantage in select infectious diseases, including through [this variant’s] now identified role in promoting antimicrobial pathways in macrophages.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SOURCE: Sun R, Abraham C. Cell Molec Gastro Hepatol. 2020 May 28. doi.: 10.1016/j.jcmgh.2020.05.007.
Interleukin-23 optimizes antimicrobial macrophage activity, which is reduced among persons harboring an IL-23 receptor variant that helps protect against inflammatory bowel disease (IBD), recent research has found.
“These [findings] highlight that the susceptibility to infections with therapeutic blockade of the IL-23/IL-12 pathways may be owing in part to the essential role for IL-23 in mediating antimicrobial functions in macrophages. They further highlight that carriers of the IL-23R–Q381 variant, who are relatively protected from IBD and other immune-mediated diseases, may be at increased risk for bacterial infection,” Rui Sun and Clara Abraham, MD, of Yale University, New Haven, Conn., wrote in Cellular and Molecular Gastroenterology and Hepatology.
IL-23 is key to the pathogenesis of IBD and is being studied as a therapeutic target, both alone and in combination with IL-12 blocking. Although human macrophages express low levels of IL-23 receptor, recent research reveals that IL-23R is up-regulated “within minutes of exposure to IL-23,” which promotes signaling and cytokine secretion, the investigators wrote. However, the extent to which IL-23 supports macrophage antimicrobial activity was unknown. To characterize protein expression, signaling, and bacterial uptake and clearance of bacteria by human macrophages derived from monocytes, the investigators tested these cells with Western blot, flow cytometry, and gentamicin protection, which involved coculturing human macrophages with bacteria, adding gentamicin solution, and then lysing and plating the cells onto agar to assess the extent to which the macrophages had taken up the bacteria.
After 48 hours of exposure to IL-23 or IL-12, macrophages increased their intracellular clearance of clinically relevant bacteria, including Enterococcus faecalis, adherent invasive Escherichia coli, and Salmonella typhimurium. Notably, this did not occur when the investigators reduced (“knocked down”) macrophage expression of either IL-23R or IL-12 receptor alpha 2. Additional investigations showed that in macrophages, IL-23 promotes bacterial uptake, clearance, and autophagy by inducing a pyruvate dehydrogenase kinase 1 (PDK1)–dependent pathway mediated by Janus kinase 2/tyrosine kinase 2 and by inducing reactive oxygen species (ROS) and reactive nitrogen species (RNS) pathways. IL-23 also activates two key proteins involved in autophagy (ATG5 and ATG16L1), the researchers reported. “ROS, RNS, and autophagy cooperate to mediate IL-23-induced bacterial clearance. Reduction of each ROS, RNS, and autophagy pathway partially reversed the enhanced bacterial clearance observed with chronic IL-23 treatment.”
Further tests found that IL-23 mediates antimicrobial pathways through the Janus kinase 2, tyrosine kinase 2, and STAT3 pathways, which “cooperate to mediate optimal IL-23-induced intracellular bacterial clearance in human macrophages.” Importantly, human macrophages showed less antimicrobial activity when transfected with the IL-23R–Q381 variant than with IL-23R–R381. The IL-23R-Q381 variant, which reduces susceptibility to IBD, “decreased IL-23-induced and NOD2-induced antimicrobial pathways and intracellular bacterial clearance in monocyte-derived macrophages,” the researchers explained. Evaluating actual carriers of these variants showed the same results – macrophages harboring IBD-protective IL-23R–R381/Q381 exhibited lower antimicrobial activity and less intracellular bacterial clearance compared with macrophages from carriers of IL-23R–R381/R381.
“Taken together, IL-23 promotes increased bacterial uptake and then induces a more rapid and effective clearance of these intracellular bacteria in human monocyte-derived macrophages,” the researchers wrote. “The reduced inflammatory responses observed in IL-23R Q381 carriers are associated with protection from multiple immune-mediated diseases. This would imply that loss-of-function observed with the common IL-23R–R381Q variant may lead to a disadvantage in select infectious diseases, including through [this variant’s] now identified role in promoting antimicrobial pathways in macrophages.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SOURCE: Sun R, Abraham C. Cell Molec Gastro Hepatol. 2020 May 28. doi.: 10.1016/j.jcmgh.2020.05.007.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Intravascular lithotripsy hailed as ‘game changer’ for coronary calcification
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
FROM TCT 2020
Key clinical point: Intravascular lithotripsy was safe and effective for treatment of severely calcified coronary stenoses in a pivotal trial.
Major finding: The 30-day rate of freedom from major adverse cardiovascular events was 92.2%, well above the prespecified performance goal of 84.4%.
Study details: Disrupt CAD III study is a multicenter, single-arm, prospective study of intravascular lithotripsy in 384 patients with severe coronary calcification.
Disclosures: The presenter reported serving as a consultant to Shockwave Medical Inc., the study sponsor, as well as several other medical device companies.
Source: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
Paraneoplastic Pemphigus With Cicatricial Nail Involvement
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
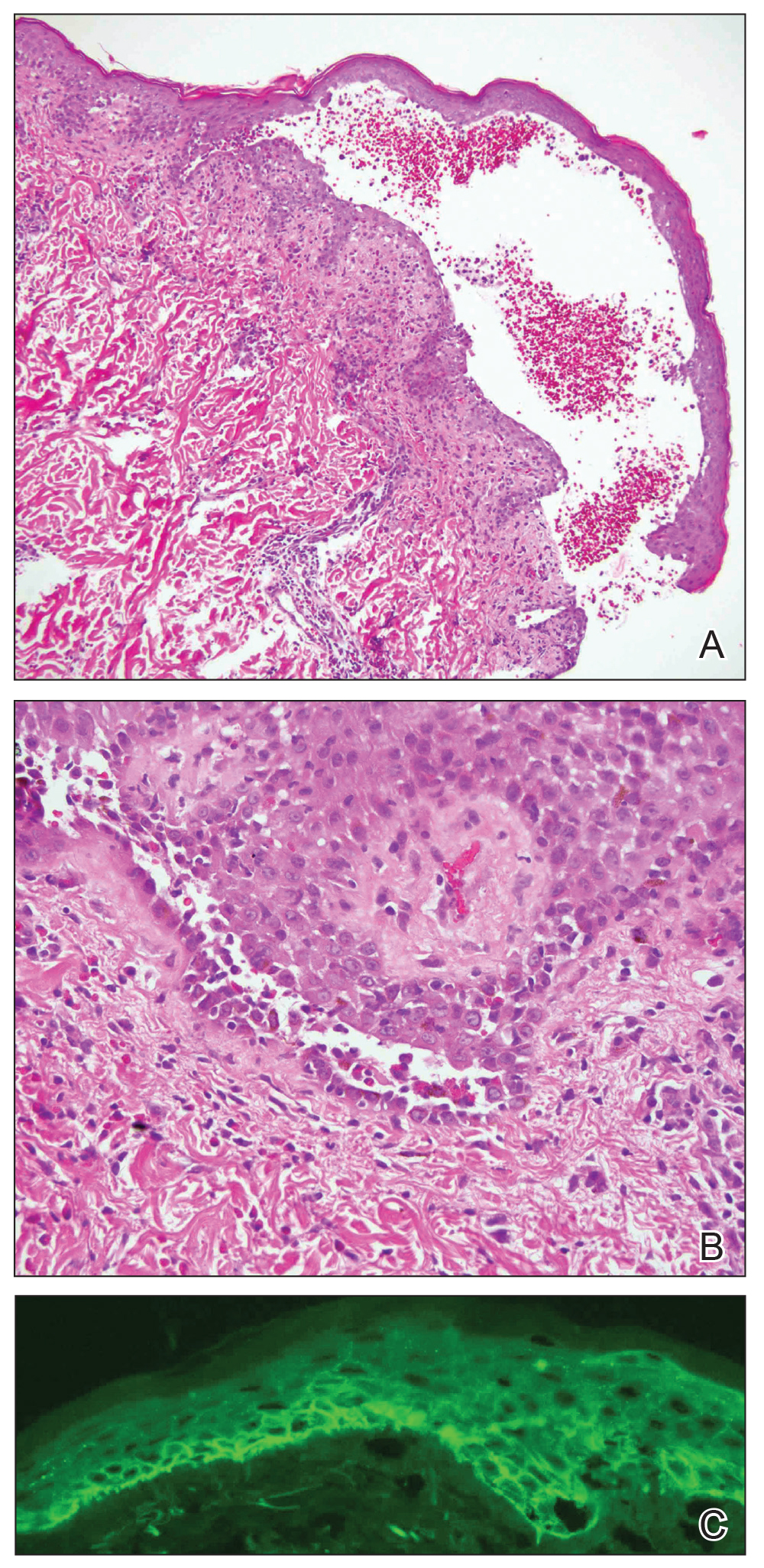
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.
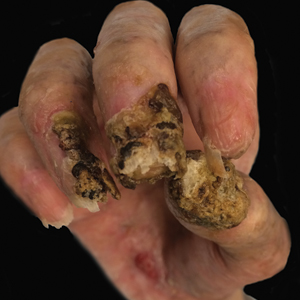
Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.
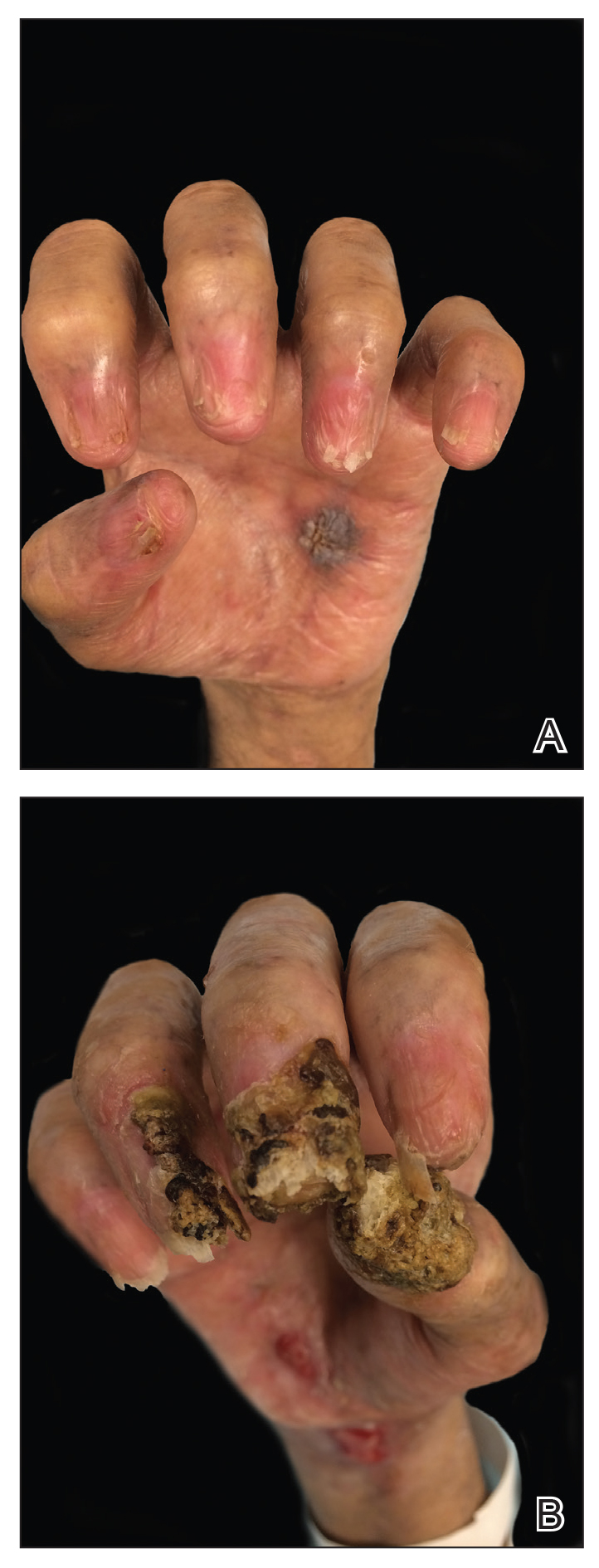
Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.

Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.

Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.

Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.

Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
Practice Points
- Paraneoplastic pemphigus (PNP) is a rare blistering skin eruption commonly associated with an underlying malignancy.
- Paraneoplastic pemphigus generally presents with erosive stomatitis with involvement of the vermillion lip but also can involve the skin and nails.
- Nail involvement can lead to scarring of the nails and can mimic lichen planus, bullous pemphigoid, or epidermolysis bullosa of the nails. These nail changes likely are due to the pronounced lichenoid interphase dermatitis seen in PNP.
Lichen Sclerosus of the Eyelid
To the Editor:
Lichen sclerosus is a chronic inflammatory skin disease of unknown cause that predominantly affects the anogenital region, but isolated extragenital lesions occur in 6% to 15% of patients. The buttocks, thighs, neck, shoulder, upper torso, and wrists most commonly are involved; the face rarely is affected.1,2 Although the etiology of lichen sclerosus remains undetermined, there is growing evidence that autoimmunity may play a role.1 Lichen sclerosus more commonly is seen in women, and the disease can present at any age, with a bimodal onset in prepubertal children and in postmenopausal women and men in the fourth decade of life.1-3 A PubMed search of articles indexed for MEDLINE using the terms lichen and eyelid and manually screened revealed 6 cases of lichen sclerosus involving the eyelid.2-4 We describe a case of lichen sclerosus involving the eyelid and its histopathology.
A 45-year-old woman was referred to dermatology for evaluation of a right lower eyelid lesion of 3 months’ duration. She first noted a small white patch under the eyelid that had doubled in size and felt firm without bleeding or ulceration. Her medical history was unremarkable, and there was no history of ophthalmic conditions, autoimmune disease, trauma, or cancer. An ophthalmic examination was normal, except for a 20×8-mm, flat, depigmented, firm papule with scalloped borders involving the right lower eyelid margin and extending inferiorly without evidence of madarosis or ulceration (Figure 1). She underwent an incisional biopsy that revealed the diagnosis of lichen sclerosus et atrophicus (Figure 2). A full dermatologic evaluation included a genital examination and did not reveal any additional lesions. Tacrolimus ointment was started to avoid the need for long-term use of periocular steroids and their complications.
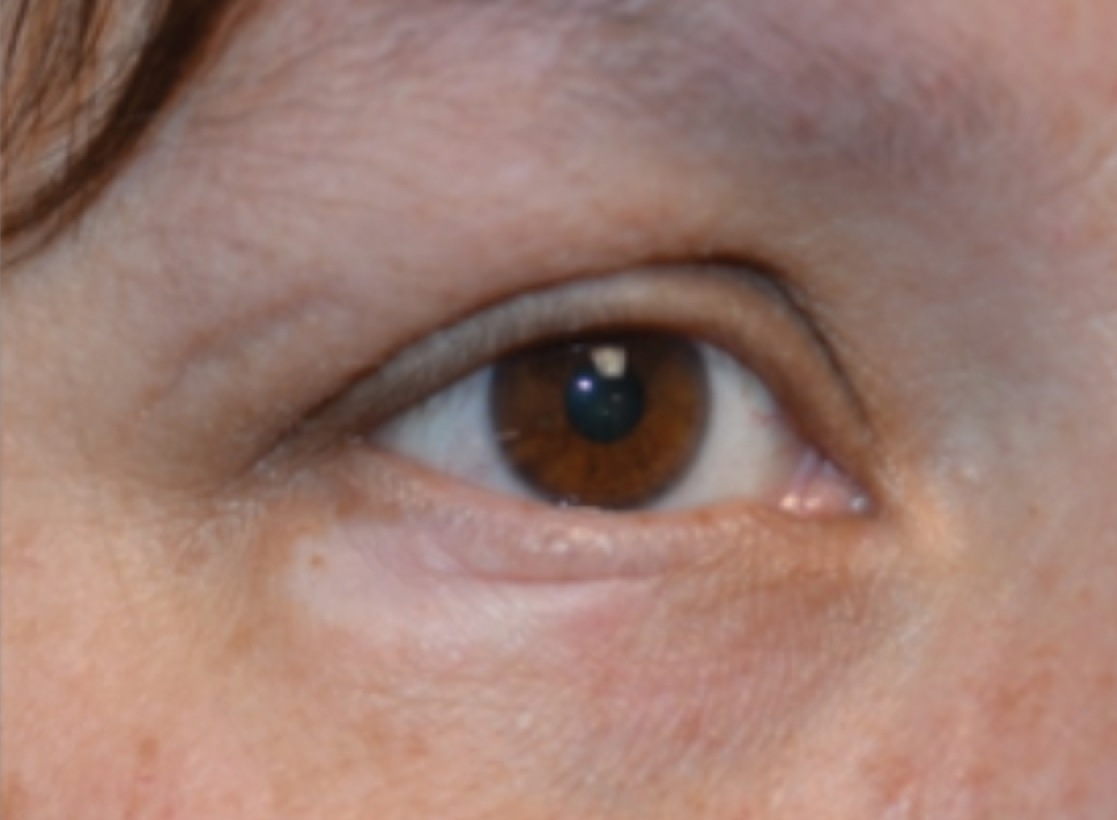
Extragenital lichen sclerosus typically is asymptomatic and only rarely presents with pruritus, in contrast to genital lichen sclerosus, which characteristically involves pruritus and dyspareunia. Although eyelid involvement is rare, ophthalmic manifestations of lichen sclerosus have included lid notching, ectropion, acquired Brown syndrome, and associated keratoconjunctivitis sicca.3-5 It characteristically appears as a well-demarcated hypopigmented papule. The differential diagnosis for a hypopigmented papule also includes amelanotic melanoma, basal cell carcinoma, vitiligo, tinea versicolor, lichen simplex chronicus, lichen planus, morphea (localized scleroderma), and systemic scleroderma with eyelid involvement.1,5
Differentiating lichen sclerosus from these conditions is of importance, as some of them can have notable morbidity and/or mortality. Of all the autoimmune connective tissue disorders, systemic sclerosus has the highest disease-specific mortality.6 Morphea, on the other hand, can have considerable morbidity. Morphea involving the head and neck notably increases the risk for neurologic complications such as seizures or central nervous system vasculitis as well as ocular complications such as anterior uveitis.6 Of note, genital lichen sclerosus carries an increased risk for squamous cell carcinoma and verrucous carcinoma; however, there have been no reported cases of malignant transformation with extragenital lesions.2
Histopathology is useful to distinguish among these entities. Although there are no specific features separating lichen sclerosus from a morphea overlap and both entities often are classified by clinical presentation, lichen sclerosus demonstrates epidermal atrophy, follicular plugging, homogenized collagen in the upper dermis with dermal edema, and lichenoid lymphocytic infiltrate (Figure 2).1 Extragenital lesions in particular also have been noted to have more epidermal atrophy and decreased rete ridges.2
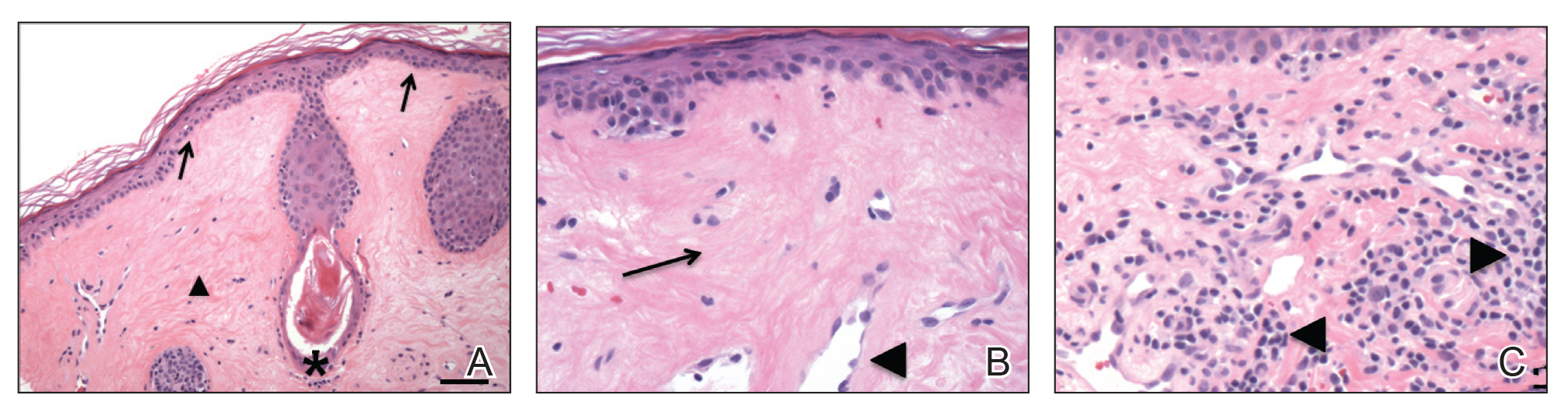
First-line treatment of lichen sclerosus includes topical corticosteroids with emollients for supportive therapy. A topical calcineurin inhibitor such as tacrolimus should be considered for patients who do not respond to corticosteroid therapy or in cases in which corticosteroid therapy is contraindicated to avoid steroid-induced glaucoma or undesirable skin atrophy and hypopigmentation.2 A collaborative approach including dermatology and internal medicine can help identify a systemic or multisystem process.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Rosenthal IM, Taube JM, Nelson DL, et al. A case of infraorbital lichen sclerosus. Dermatol Online J. 2013;19:20021.
- Rabinowitz R, Rosenthal G, Yerushalmy J, et al. Keratoconjunctivitis sicca associated with lichen sclerosus et atrophicus. Eye. 2000;14:103-104.
- Olver J, Laidler P. Acquired Brown’s syndrome in a patient with combined lichen sclerosus et atrophicus and morphea. Br J Ophthalmol. 1988;72:552-557.
- El-Baba F, Frangieh GT, Iliff WJ, et al. Morphea of the eyelids. Ophthalmology. 1982;89:125-128.
- Fett N. Scleroderma: nomenclatures, etiology, pathogenesis, prognosis, and treatment: facts and controversies. Clin Dermatol. 2013;31:432-437.
To the Editor:
Lichen sclerosus is a chronic inflammatory skin disease of unknown cause that predominantly affects the anogenital region, but isolated extragenital lesions occur in 6% to 15% of patients. The buttocks, thighs, neck, shoulder, upper torso, and wrists most commonly are involved; the face rarely is affected.1,2 Although the etiology of lichen sclerosus remains undetermined, there is growing evidence that autoimmunity may play a role.1 Lichen sclerosus more commonly is seen in women, and the disease can present at any age, with a bimodal onset in prepubertal children and in postmenopausal women and men in the fourth decade of life.1-3 A PubMed search of articles indexed for MEDLINE using the terms lichen and eyelid and manually screened revealed 6 cases of lichen sclerosus involving the eyelid.2-4 We describe a case of lichen sclerosus involving the eyelid and its histopathology.
A 45-year-old woman was referred to dermatology for evaluation of a right lower eyelid lesion of 3 months’ duration. She first noted a small white patch under the eyelid that had doubled in size and felt firm without bleeding or ulceration. Her medical history was unremarkable, and there was no history of ophthalmic conditions, autoimmune disease, trauma, or cancer. An ophthalmic examination was normal, except for a 20×8-mm, flat, depigmented, firm papule with scalloped borders involving the right lower eyelid margin and extending inferiorly without evidence of madarosis or ulceration (Figure 1). She underwent an incisional biopsy that revealed the diagnosis of lichen sclerosus et atrophicus (Figure 2). A full dermatologic evaluation included a genital examination and did not reveal any additional lesions. Tacrolimus ointment was started to avoid the need for long-term use of periocular steroids and their complications.

Extragenital lichen sclerosus typically is asymptomatic and only rarely presents with pruritus, in contrast to genital lichen sclerosus, which characteristically involves pruritus and dyspareunia. Although eyelid involvement is rare, ophthalmic manifestations of lichen sclerosus have included lid notching, ectropion, acquired Brown syndrome, and associated keratoconjunctivitis sicca.3-5 It characteristically appears as a well-demarcated hypopigmented papule. The differential diagnosis for a hypopigmented papule also includes amelanotic melanoma, basal cell carcinoma, vitiligo, tinea versicolor, lichen simplex chronicus, lichen planus, morphea (localized scleroderma), and systemic scleroderma with eyelid involvement.1,5
Differentiating lichen sclerosus from these conditions is of importance, as some of them can have notable morbidity and/or mortality. Of all the autoimmune connective tissue disorders, systemic sclerosus has the highest disease-specific mortality.6 Morphea, on the other hand, can have considerable morbidity. Morphea involving the head and neck notably increases the risk for neurologic complications such as seizures or central nervous system vasculitis as well as ocular complications such as anterior uveitis.6 Of note, genital lichen sclerosus carries an increased risk for squamous cell carcinoma and verrucous carcinoma; however, there have been no reported cases of malignant transformation with extragenital lesions.2
Histopathology is useful to distinguish among these entities. Although there are no specific features separating lichen sclerosus from a morphea overlap and both entities often are classified by clinical presentation, lichen sclerosus demonstrates epidermal atrophy, follicular plugging, homogenized collagen in the upper dermis with dermal edema, and lichenoid lymphocytic infiltrate (Figure 2).1 Extragenital lesions in particular also have been noted to have more epidermal atrophy and decreased rete ridges.2

First-line treatment of lichen sclerosus includes topical corticosteroids with emollients for supportive therapy. A topical calcineurin inhibitor such as tacrolimus should be considered for patients who do not respond to corticosteroid therapy or in cases in which corticosteroid therapy is contraindicated to avoid steroid-induced glaucoma or undesirable skin atrophy and hypopigmentation.2 A collaborative approach including dermatology and internal medicine can help identify a systemic or multisystem process.
To the Editor:
Lichen sclerosus is a chronic inflammatory skin disease of unknown cause that predominantly affects the anogenital region, but isolated extragenital lesions occur in 6% to 15% of patients. The buttocks, thighs, neck, shoulder, upper torso, and wrists most commonly are involved; the face rarely is affected.1,2 Although the etiology of lichen sclerosus remains undetermined, there is growing evidence that autoimmunity may play a role.1 Lichen sclerosus more commonly is seen in women, and the disease can present at any age, with a bimodal onset in prepubertal children and in postmenopausal women and men in the fourth decade of life.1-3 A PubMed search of articles indexed for MEDLINE using the terms lichen and eyelid and manually screened revealed 6 cases of lichen sclerosus involving the eyelid.2-4 We describe a case of lichen sclerosus involving the eyelid and its histopathology.
A 45-year-old woman was referred to dermatology for evaluation of a right lower eyelid lesion of 3 months’ duration. She first noted a small white patch under the eyelid that had doubled in size and felt firm without bleeding or ulceration. Her medical history was unremarkable, and there was no history of ophthalmic conditions, autoimmune disease, trauma, or cancer. An ophthalmic examination was normal, except for a 20×8-mm, flat, depigmented, firm papule with scalloped borders involving the right lower eyelid margin and extending inferiorly without evidence of madarosis or ulceration (Figure 1). She underwent an incisional biopsy that revealed the diagnosis of lichen sclerosus et atrophicus (Figure 2). A full dermatologic evaluation included a genital examination and did not reveal any additional lesions. Tacrolimus ointment was started to avoid the need for long-term use of periocular steroids and their complications.

Extragenital lichen sclerosus typically is asymptomatic and only rarely presents with pruritus, in contrast to genital lichen sclerosus, which characteristically involves pruritus and dyspareunia. Although eyelid involvement is rare, ophthalmic manifestations of lichen sclerosus have included lid notching, ectropion, acquired Brown syndrome, and associated keratoconjunctivitis sicca.3-5 It characteristically appears as a well-demarcated hypopigmented papule. The differential diagnosis for a hypopigmented papule also includes amelanotic melanoma, basal cell carcinoma, vitiligo, tinea versicolor, lichen simplex chronicus, lichen planus, morphea (localized scleroderma), and systemic scleroderma with eyelid involvement.1,5
Differentiating lichen sclerosus from these conditions is of importance, as some of them can have notable morbidity and/or mortality. Of all the autoimmune connective tissue disorders, systemic sclerosus has the highest disease-specific mortality.6 Morphea, on the other hand, can have considerable morbidity. Morphea involving the head and neck notably increases the risk for neurologic complications such as seizures or central nervous system vasculitis as well as ocular complications such as anterior uveitis.6 Of note, genital lichen sclerosus carries an increased risk for squamous cell carcinoma and verrucous carcinoma; however, there have been no reported cases of malignant transformation with extragenital lesions.2
Histopathology is useful to distinguish among these entities. Although there are no specific features separating lichen sclerosus from a morphea overlap and both entities often are classified by clinical presentation, lichen sclerosus demonstrates epidermal atrophy, follicular plugging, homogenized collagen in the upper dermis with dermal edema, and lichenoid lymphocytic infiltrate (Figure 2).1 Extragenital lesions in particular also have been noted to have more epidermal atrophy and decreased rete ridges.2

First-line treatment of lichen sclerosus includes topical corticosteroids with emollients for supportive therapy. A topical calcineurin inhibitor such as tacrolimus should be considered for patients who do not respond to corticosteroid therapy or in cases in which corticosteroid therapy is contraindicated to avoid steroid-induced glaucoma or undesirable skin atrophy and hypopigmentation.2 A collaborative approach including dermatology and internal medicine can help identify a systemic or multisystem process.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Rosenthal IM, Taube JM, Nelson DL, et al. A case of infraorbital lichen sclerosus. Dermatol Online J. 2013;19:20021.
- Rabinowitz R, Rosenthal G, Yerushalmy J, et al. Keratoconjunctivitis sicca associated with lichen sclerosus et atrophicus. Eye. 2000;14:103-104.
- Olver J, Laidler P. Acquired Brown’s syndrome in a patient with combined lichen sclerosus et atrophicus and morphea. Br J Ophthalmol. 1988;72:552-557.
- El-Baba F, Frangieh GT, Iliff WJ, et al. Morphea of the eyelids. Ophthalmology. 1982;89:125-128.
- Fett N. Scleroderma: nomenclatures, etiology, pathogenesis, prognosis, and treatment: facts and controversies. Clin Dermatol. 2013;31:432-437.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Rosenthal IM, Taube JM, Nelson DL, et al. A case of infraorbital lichen sclerosus. Dermatol Online J. 2013;19:20021.
- Rabinowitz R, Rosenthal G, Yerushalmy J, et al. Keratoconjunctivitis sicca associated with lichen sclerosus et atrophicus. Eye. 2000;14:103-104.
- Olver J, Laidler P. Acquired Brown’s syndrome in a patient with combined lichen sclerosus et atrophicus and morphea. Br J Ophthalmol. 1988;72:552-557.
- El-Baba F, Frangieh GT, Iliff WJ, et al. Morphea of the eyelids. Ophthalmology. 1982;89:125-128.
- Fett N. Scleroderma: nomenclatures, etiology, pathogenesis, prognosis, and treatment: facts and controversies. Clin Dermatol. 2013;31:432-437.
Practice Points
- Lichen sclerosus is not confined to only the anogenital area and can affect the face in rare cases.
Dermatology Resident Education for Skin of Color
An article recently was published in The New York Times with a headline that read, “Dermatology Has a Problem With Skin Color.” 1 The article featured interviews with many well-known dermatologists who are experts in skin of color (SOC), and their points followed a similar pattern—skin disease often looks different in patients with darker skin, and diagnoses often are delayed or missed altogether as a consequence of clinical uncertainty. The article included an interview with Jenna Lester, MD, who leads the SOC clinic at the University of California, San Francisco. In the article, she discussed how dermatologists are trained to recognize findings through pattern recognition. However, if we are only trained to diagnose dermatologic diseases on white skin, we will be unable to recognize diseases in patients with darker skin, leading to suboptimal patient care. 1
Dermatology is a visual specialty, and residents go through thousands of photographs during residency training to distinguish different presentations and unique findings of a variety of skin diseases. Nevertheless, to Dr. Lester’s point, our learning is limited by the photographs and patients that we see.
Additionally, residents training in locations without diverse patient populations rely even more on images in educational resources to recognize clinical presentations in patients with darker skin. A study was published in Cutis earlier this year that surveyed dermatology residents about multiethnic training in residency.2 It showed that residents training in less ethnically diverse areas such as the Midwest and Northwest were more likely to agree that dedicated multiethnic clinics and rotations are important to gain competence compared to residents training in more ethnically diverse regions such as the Southeast, Northeast, and Southwest. Most residents believed 1 to 5 hours per month of lectures covering conditions affecting SOC and/or multiethnic skin are needed to become competent.2
Limitations of Educational Resources
The images in dermatology educational resources do not reflect the diversity of our country’s population. A research letter recently was published in the Journal of the American Academy of Dermatology (JAAD) in which the authors assessed the number of images of dark skin—Fitzpatrick skin types V and VI—in dermatology educational resources.3 The authors analyzed images from 8 resources commonly used to study dermatology, including 6 printed texts and 2 online resources. Of the printed texts, Andrews’ Diseases of the Skin had the highest percentage of images of dark skin at 19.9%. Overall, VisualDx had the highest percentage of photographs of dark skin at 28.5%, while DermNet NZ had the lowest of all resources at only 2.8%.3
Similarly, a research letter published in the British Journal of Dermatology reviewed images in 2 standard dermatology textbooks.4 Although images of SOC made up 22% to 32% of the overall content, the number of images of sexually transmitted infections in SOC was disproportionate (47%–58%) compared to images of non–sexually transmitted infections (28%). The authors also stated that communities of color often have legacies of mistrust with the health care system, and diagnostic uncertainty can further impair the physician-patient relationship.4
The lack of diversity in clinical images and research was further exemplified by recent publications regarding the perniolike eruption associated with coronavirus disease 2019 (COVID-19), commonly referred to as COVID toes. A research letter was published in the British Journal of Dermatology earlier this year about the lack of images of SOC in publications about the cutaneous manifestations of COVID-19.5 At that time, there were zero published images of cutaneous COVID-19 manifestations in Fitzpatrick skin types V and VI, yet COVID-19 disproportionately affects Black individuals and other people of color.5,6 A case series recently was published in JAAD Case Reports that included images of cutaneous COVID-19 findings in patients with Fitzpatrick skin types III through V.7 The authors noted that the findings were more subtle on darker skin as the erythema was harder to discern. The inability to identify the perniolike eruption ultimately can delay diagnosis.7
Resident Education
Over the past few months, I have reflected on my role as a dermatology resident and my dedication to antiracism in my personal and professional life. It is not a valid response or excuse to say that certain diagnoses are harder to make because of darker skin tone. It is our responsibility to do better for all patients. To that end, our educational resources should reflect our entire patient population.
I have been working with my coresident Annika Weinhammer, MD, on a quality improvement project to strengthen our educational curriculum at the University of Wisconsin regarding SOC. This project aims to enhance our skills as dermatologists in diagnosing and treating diseases in SOC. Moving forward, we have set an expectation that all didactic lectures must include images of SOC. Below, I have listed some of our initiatives along with recommendations for educational resources. There are multiple dermatology textbooks focused on SOC, including the following:
- Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders 8
- Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology 9
- Dermatology Atlas for Skin of Color 10
- Fundamentals of Ethnic Hair: The Dermatologist’s Perspective 11
- Light-Based Therapies for Skin of Color 12
- Pediatric Skin of Color 13
- Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment 14
- Taylor and Kelly’s Dermatology for Skin of Color 15
- Treatments for Skin of Color 16
Our program has provided residents with Taylor and Kelly’s Dermatology for Skin of Color15 and Treatments for Skin of Color.16 Residents and medical students should search their institution’s electronic library for e-books and other resources including VisualDx, which includes many photographs of SOC that can be used and cited in resident didactics.
There also are a variety of online resources. Mind the Gap is a handbook written by Malone Mukwende, a medical student in London.17,18 The handbook focuses on common clinical signs and how they present in black and brown skin. Another online resource with clinical images is Skin Deep (https://dftbskindeep.com/), a project aimed at improving the diversity of pediatric skin images. An additional online resource is Brown Skin Matters on Instagram (@brownskinmatters) that shows photographs of dermatologic conditions in SOC; however, these photographs are submitted by users and not independently verified.
I also encourage residents to join the Skin of Color Society, which promotes awareness and excellence within the special interest area of SOC. Some of the society's initiatives include educational series, networking events, diversity town halls, and a scientific symposium. Patient information for common dermatologic diagnoses exists on the society's website (https://skinofcolorsociety.org/). The society waives membership fees for resident applicants who provide a letter of good standing from their residency program. The society hosted the Skin of Color Update virtually this year (September 12–13, 2020). It costs $49 to attend, and the recorded lectures are available to stream through the end of 2020. Our department sponsored residents to attend virtually.
Finally, our department has been taking steps to implement antiracism measures in how we work, learn, conduct research, and treat patients. We are leading a resident book club discussing How to Be an Antiracist19 by Ibram X. Kendi. Residents are involved in the local chapter of White Coats for Black Lives (https://whitecoats4blacklives.org/). We also have compiled a list of antiracism resources that was shared with the department, including books, documentaries, podcasts, local and online Black-owned businesses to support, and local Black-led nonprofits.
Final Thoughts
Dermatology residents must be comfortable diagnosing and treating diseases in darker skin tones to provide the best possible care for patients with SOC. Although some common dermatology educational resources have a paucity of clinical images of SOC, there are a variety of additional educational resources through textbooks and websites.
- Rabin RC. Dermatology has a problem with skin color. New York Times. August 30, 2020. https://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed October 5, 2020.
- Cline A, Winter R, Kouroush S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595.
- Golden SH. Coronavirus in African Americans and other people of color. Johns Hopkins Medicine website. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid19-racial-disparities. Published April 20, 2020. Accessed October 5, 2020.
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders. Switzerland: Springer; 2016.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology. Switzerland: Springer; 2016.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. New York, NY: Springer; 2014.
- Aguh C, Okoye GA, eds. Fundamentals of Ethnic Hair: The Dermatologist’s Perspective. Switzerland: Springer; 2017.
- Baron E, ed. Light-Based Therapies for Skin of Color. London: Springer; 2009.
- Silverberg NB, Durán-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. New York, NY: Springer; 2015.
- Alexis AF, Barbosa VH, eds. Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment. New York, NY: Springer; 2013.
- Taylor SC, Kelly AP, Lim H, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw Hill Professional; 2016.
- Taylor SC, Badreshia-Bansal S, Calendar VD, et al. Treatments for Skin of Color. China: Saunders Elsevier; 2011.
- Page S. A medical student couldn’t find how symptoms look on darker skin. he decided to publish a book about it. Washington Post. July 22, 2020. https://www.washingtonpost.com/lifestyle/2020/07/22/malone-mukwende-medical-handbook/. Accessed October 5, 2020.
- Mukwende M, Tamony P, Turner M. Mind the Gap: A Handbook of Clinical Signs in Black and Brown Skin. London, England: St. George’s University of London; 2020. https://www.blackandbrownskin.co.uk/mindthegap. Accessed October 5, 2020.
- Kendi IX. How to Be an Antiracist. New York, NY: Random House; 2019.
An article recently was published in The New York Times with a headline that read, “Dermatology Has a Problem With Skin Color.” 1 The article featured interviews with many well-known dermatologists who are experts in skin of color (SOC), and their points followed a similar pattern—skin disease often looks different in patients with darker skin, and diagnoses often are delayed or missed altogether as a consequence of clinical uncertainty. The article included an interview with Jenna Lester, MD, who leads the SOC clinic at the University of California, San Francisco. In the article, she discussed how dermatologists are trained to recognize findings through pattern recognition. However, if we are only trained to diagnose dermatologic diseases on white skin, we will be unable to recognize diseases in patients with darker skin, leading to suboptimal patient care. 1
Dermatology is a visual specialty, and residents go through thousands of photographs during residency training to distinguish different presentations and unique findings of a variety of skin diseases. Nevertheless, to Dr. Lester’s point, our learning is limited by the photographs and patients that we see.
Additionally, residents training in locations without diverse patient populations rely even more on images in educational resources to recognize clinical presentations in patients with darker skin. A study was published in Cutis earlier this year that surveyed dermatology residents about multiethnic training in residency.2 It showed that residents training in less ethnically diverse areas such as the Midwest and Northwest were more likely to agree that dedicated multiethnic clinics and rotations are important to gain competence compared to residents training in more ethnically diverse regions such as the Southeast, Northeast, and Southwest. Most residents believed 1 to 5 hours per month of lectures covering conditions affecting SOC and/or multiethnic skin are needed to become competent.2
Limitations of Educational Resources
The images in dermatology educational resources do not reflect the diversity of our country’s population. A research letter recently was published in the Journal of the American Academy of Dermatology (JAAD) in which the authors assessed the number of images of dark skin—Fitzpatrick skin types V and VI—in dermatology educational resources.3 The authors analyzed images from 8 resources commonly used to study dermatology, including 6 printed texts and 2 online resources. Of the printed texts, Andrews’ Diseases of the Skin had the highest percentage of images of dark skin at 19.9%. Overall, VisualDx had the highest percentage of photographs of dark skin at 28.5%, while DermNet NZ had the lowest of all resources at only 2.8%.3
Similarly, a research letter published in the British Journal of Dermatology reviewed images in 2 standard dermatology textbooks.4 Although images of SOC made up 22% to 32% of the overall content, the number of images of sexually transmitted infections in SOC was disproportionate (47%–58%) compared to images of non–sexually transmitted infections (28%). The authors also stated that communities of color often have legacies of mistrust with the health care system, and diagnostic uncertainty can further impair the physician-patient relationship.4
The lack of diversity in clinical images and research was further exemplified by recent publications regarding the perniolike eruption associated with coronavirus disease 2019 (COVID-19), commonly referred to as COVID toes. A research letter was published in the British Journal of Dermatology earlier this year about the lack of images of SOC in publications about the cutaneous manifestations of COVID-19.5 At that time, there were zero published images of cutaneous COVID-19 manifestations in Fitzpatrick skin types V and VI, yet COVID-19 disproportionately affects Black individuals and other people of color.5,6 A case series recently was published in JAAD Case Reports that included images of cutaneous COVID-19 findings in patients with Fitzpatrick skin types III through V.7 The authors noted that the findings were more subtle on darker skin as the erythema was harder to discern. The inability to identify the perniolike eruption ultimately can delay diagnosis.7
Resident Education
Over the past few months, I have reflected on my role as a dermatology resident and my dedication to antiracism in my personal and professional life. It is not a valid response or excuse to say that certain diagnoses are harder to make because of darker skin tone. It is our responsibility to do better for all patients. To that end, our educational resources should reflect our entire patient population.
I have been working with my coresident Annika Weinhammer, MD, on a quality improvement project to strengthen our educational curriculum at the University of Wisconsin regarding SOC. This project aims to enhance our skills as dermatologists in diagnosing and treating diseases in SOC. Moving forward, we have set an expectation that all didactic lectures must include images of SOC. Below, I have listed some of our initiatives along with recommendations for educational resources. There are multiple dermatology textbooks focused on SOC, including the following:
- Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders 8
- Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology 9
- Dermatology Atlas for Skin of Color 10
- Fundamentals of Ethnic Hair: The Dermatologist’s Perspective 11
- Light-Based Therapies for Skin of Color 12
- Pediatric Skin of Color 13
- Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment 14
- Taylor and Kelly’s Dermatology for Skin of Color 15
- Treatments for Skin of Color 16
Our program has provided residents with Taylor and Kelly’s Dermatology for Skin of Color15 and Treatments for Skin of Color.16 Residents and medical students should search their institution’s electronic library for e-books and other resources including VisualDx, which includes many photographs of SOC that can be used and cited in resident didactics.
There also are a variety of online resources. Mind the Gap is a handbook written by Malone Mukwende, a medical student in London.17,18 The handbook focuses on common clinical signs and how they present in black and brown skin. Another online resource with clinical images is Skin Deep (https://dftbskindeep.com/), a project aimed at improving the diversity of pediatric skin images. An additional online resource is Brown Skin Matters on Instagram (@brownskinmatters) that shows photographs of dermatologic conditions in SOC; however, these photographs are submitted by users and not independently verified.
I also encourage residents to join the Skin of Color Society, which promotes awareness and excellence within the special interest area of SOC. Some of the society's initiatives include educational series, networking events, diversity town halls, and a scientific symposium. Patient information for common dermatologic diagnoses exists on the society's website (https://skinofcolorsociety.org/). The society waives membership fees for resident applicants who provide a letter of good standing from their residency program. The society hosted the Skin of Color Update virtually this year (September 12–13, 2020). It costs $49 to attend, and the recorded lectures are available to stream through the end of 2020. Our department sponsored residents to attend virtually.
Finally, our department has been taking steps to implement antiracism measures in how we work, learn, conduct research, and treat patients. We are leading a resident book club discussing How to Be an Antiracist19 by Ibram X. Kendi. Residents are involved in the local chapter of White Coats for Black Lives (https://whitecoats4blacklives.org/). We also have compiled a list of antiracism resources that was shared with the department, including books, documentaries, podcasts, local and online Black-owned businesses to support, and local Black-led nonprofits.
Final Thoughts
Dermatology residents must be comfortable diagnosing and treating diseases in darker skin tones to provide the best possible care for patients with SOC. Although some common dermatology educational resources have a paucity of clinical images of SOC, there are a variety of additional educational resources through textbooks and websites.
An article recently was published in The New York Times with a headline that read, “Dermatology Has a Problem With Skin Color.” 1 The article featured interviews with many well-known dermatologists who are experts in skin of color (SOC), and their points followed a similar pattern—skin disease often looks different in patients with darker skin, and diagnoses often are delayed or missed altogether as a consequence of clinical uncertainty. The article included an interview with Jenna Lester, MD, who leads the SOC clinic at the University of California, San Francisco. In the article, she discussed how dermatologists are trained to recognize findings through pattern recognition. However, if we are only trained to diagnose dermatologic diseases on white skin, we will be unable to recognize diseases in patients with darker skin, leading to suboptimal patient care. 1
Dermatology is a visual specialty, and residents go through thousands of photographs during residency training to distinguish different presentations and unique findings of a variety of skin diseases. Nevertheless, to Dr. Lester’s point, our learning is limited by the photographs and patients that we see.
Additionally, residents training in locations without diverse patient populations rely even more on images in educational resources to recognize clinical presentations in patients with darker skin. A study was published in Cutis earlier this year that surveyed dermatology residents about multiethnic training in residency.2 It showed that residents training in less ethnically diverse areas such as the Midwest and Northwest were more likely to agree that dedicated multiethnic clinics and rotations are important to gain competence compared to residents training in more ethnically diverse regions such as the Southeast, Northeast, and Southwest. Most residents believed 1 to 5 hours per month of lectures covering conditions affecting SOC and/or multiethnic skin are needed to become competent.2
Limitations of Educational Resources
The images in dermatology educational resources do not reflect the diversity of our country’s population. A research letter recently was published in the Journal of the American Academy of Dermatology (JAAD) in which the authors assessed the number of images of dark skin—Fitzpatrick skin types V and VI—in dermatology educational resources.3 The authors analyzed images from 8 resources commonly used to study dermatology, including 6 printed texts and 2 online resources. Of the printed texts, Andrews’ Diseases of the Skin had the highest percentage of images of dark skin at 19.9%. Overall, VisualDx had the highest percentage of photographs of dark skin at 28.5%, while DermNet NZ had the lowest of all resources at only 2.8%.3
Similarly, a research letter published in the British Journal of Dermatology reviewed images in 2 standard dermatology textbooks.4 Although images of SOC made up 22% to 32% of the overall content, the number of images of sexually transmitted infections in SOC was disproportionate (47%–58%) compared to images of non–sexually transmitted infections (28%). The authors also stated that communities of color often have legacies of mistrust with the health care system, and diagnostic uncertainty can further impair the physician-patient relationship.4
The lack of diversity in clinical images and research was further exemplified by recent publications regarding the perniolike eruption associated with coronavirus disease 2019 (COVID-19), commonly referred to as COVID toes. A research letter was published in the British Journal of Dermatology earlier this year about the lack of images of SOC in publications about the cutaneous manifestations of COVID-19.5 At that time, there were zero published images of cutaneous COVID-19 manifestations in Fitzpatrick skin types V and VI, yet COVID-19 disproportionately affects Black individuals and other people of color.5,6 A case series recently was published in JAAD Case Reports that included images of cutaneous COVID-19 findings in patients with Fitzpatrick skin types III through V.7 The authors noted that the findings were more subtle on darker skin as the erythema was harder to discern. The inability to identify the perniolike eruption ultimately can delay diagnosis.7
Resident Education
Over the past few months, I have reflected on my role as a dermatology resident and my dedication to antiracism in my personal and professional life. It is not a valid response or excuse to say that certain diagnoses are harder to make because of darker skin tone. It is our responsibility to do better for all patients. To that end, our educational resources should reflect our entire patient population.
I have been working with my coresident Annika Weinhammer, MD, on a quality improvement project to strengthen our educational curriculum at the University of Wisconsin regarding SOC. This project aims to enhance our skills as dermatologists in diagnosing and treating diseases in SOC. Moving forward, we have set an expectation that all didactic lectures must include images of SOC. Below, I have listed some of our initiatives along with recommendations for educational resources. There are multiple dermatology textbooks focused on SOC, including the following:
- Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders 8
- Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology 9
- Dermatology Atlas for Skin of Color 10
- Fundamentals of Ethnic Hair: The Dermatologist’s Perspective 11
- Light-Based Therapies for Skin of Color 12
- Pediatric Skin of Color 13
- Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment 14
- Taylor and Kelly’s Dermatology for Skin of Color 15
- Treatments for Skin of Color 16
Our program has provided residents with Taylor and Kelly’s Dermatology for Skin of Color15 and Treatments for Skin of Color.16 Residents and medical students should search their institution’s electronic library for e-books and other resources including VisualDx, which includes many photographs of SOC that can be used and cited in resident didactics.
There also are a variety of online resources. Mind the Gap is a handbook written by Malone Mukwende, a medical student in London.17,18 The handbook focuses on common clinical signs and how they present in black and brown skin. Another online resource with clinical images is Skin Deep (https://dftbskindeep.com/), a project aimed at improving the diversity of pediatric skin images. An additional online resource is Brown Skin Matters on Instagram (@brownskinmatters) that shows photographs of dermatologic conditions in SOC; however, these photographs are submitted by users and not independently verified.
I also encourage residents to join the Skin of Color Society, which promotes awareness and excellence within the special interest area of SOC. Some of the society's initiatives include educational series, networking events, diversity town halls, and a scientific symposium. Patient information for common dermatologic diagnoses exists on the society's website (https://skinofcolorsociety.org/). The society waives membership fees for resident applicants who provide a letter of good standing from their residency program. The society hosted the Skin of Color Update virtually this year (September 12–13, 2020). It costs $49 to attend, and the recorded lectures are available to stream through the end of 2020. Our department sponsored residents to attend virtually.
Finally, our department has been taking steps to implement antiracism measures in how we work, learn, conduct research, and treat patients. We are leading a resident book club discussing How to Be an Antiracist19 by Ibram X. Kendi. Residents are involved in the local chapter of White Coats for Black Lives (https://whitecoats4blacklives.org/). We also have compiled a list of antiracism resources that was shared with the department, including books, documentaries, podcasts, local and online Black-owned businesses to support, and local Black-led nonprofits.
Final Thoughts
Dermatology residents must be comfortable diagnosing and treating diseases in darker skin tones to provide the best possible care for patients with SOC. Although some common dermatology educational resources have a paucity of clinical images of SOC, there are a variety of additional educational resources through textbooks and websites.
- Rabin RC. Dermatology has a problem with skin color. New York Times. August 30, 2020. https://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed October 5, 2020.
- Cline A, Winter R, Kouroush S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595.
- Golden SH. Coronavirus in African Americans and other people of color. Johns Hopkins Medicine website. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid19-racial-disparities. Published April 20, 2020. Accessed October 5, 2020.
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders. Switzerland: Springer; 2016.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology. Switzerland: Springer; 2016.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. New York, NY: Springer; 2014.
- Aguh C, Okoye GA, eds. Fundamentals of Ethnic Hair: The Dermatologist’s Perspective. Switzerland: Springer; 2017.
- Baron E, ed. Light-Based Therapies for Skin of Color. London: Springer; 2009.
- Silverberg NB, Durán-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. New York, NY: Springer; 2015.
- Alexis AF, Barbosa VH, eds. Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment. New York, NY: Springer; 2013.
- Taylor SC, Kelly AP, Lim H, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw Hill Professional; 2016.
- Taylor SC, Badreshia-Bansal S, Calendar VD, et al. Treatments for Skin of Color. China: Saunders Elsevier; 2011.
- Page S. A medical student couldn’t find how symptoms look on darker skin. he decided to publish a book about it. Washington Post. July 22, 2020. https://www.washingtonpost.com/lifestyle/2020/07/22/malone-mukwende-medical-handbook/. Accessed October 5, 2020.
- Mukwende M, Tamony P, Turner M. Mind the Gap: A Handbook of Clinical Signs in Black and Brown Skin. London, England: St. George’s University of London; 2020. https://www.blackandbrownskin.co.uk/mindthegap. Accessed October 5, 2020.
- Kendi IX. How to Be an Antiracist. New York, NY: Random House; 2019.
- Rabin RC. Dermatology has a problem with skin color. New York Times. August 30, 2020. https://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed October 5, 2020.
- Cline A, Winter R, Kouroush S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595.
- Golden SH. Coronavirus in African Americans and other people of color. Johns Hopkins Medicine website. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid19-racial-disparities. Published April 20, 2020. Accessed October 5, 2020.
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders. Switzerland: Springer; 2016.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology. Switzerland: Springer; 2016.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. New York, NY: Springer; 2014.
- Aguh C, Okoye GA, eds. Fundamentals of Ethnic Hair: The Dermatologist’s Perspective. Switzerland: Springer; 2017.
- Baron E, ed. Light-Based Therapies for Skin of Color. London: Springer; 2009.
- Silverberg NB, Durán-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. New York, NY: Springer; 2015.
- Alexis AF, Barbosa VH, eds. Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment. New York, NY: Springer; 2013.
- Taylor SC, Kelly AP, Lim H, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw Hill Professional; 2016.
- Taylor SC, Badreshia-Bansal S, Calendar VD, et al. Treatments for Skin of Color. China: Saunders Elsevier; 2011.
- Page S. A medical student couldn’t find how symptoms look on darker skin. he decided to publish a book about it. Washington Post. July 22, 2020. https://www.washingtonpost.com/lifestyle/2020/07/22/malone-mukwende-medical-handbook/. Accessed October 5, 2020.
- Mukwende M, Tamony P, Turner M. Mind the Gap: A Handbook of Clinical Signs in Black and Brown Skin. London, England: St. George’s University of London; 2020. https://www.blackandbrownskin.co.uk/mindthegap. Accessed October 5, 2020.
- Kendi IX. How to Be an Antiracist. New York, NY: Random House; 2019.
Resident Pearls
- Images of skin of color (SOC) are greatly underrepresented in dermatology educational resources.
- Inadequate training in recognizing skin disease in patients with darker skin can lead to delayed or missed diagnoses.
- There are various educational resources and opportunities available to improve and diversify dermatology education, ensuring the best possible care for patients with SOC.
Diabetic neuropathic pain linked to brain bioenergic anomalies
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
FROM EASD 2020
Pathologic CR in HER2+ breast cancer predicts long-term survival
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
FROM EBCC-12 VIRTUAL CONFERENCE
Was This Tattoo a Rash Choice?
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.

Weeks ago, a 43-year-old man received a birthday tattoo of his choice: a geometric pattern etched in blue ink on his wrist. Unfortunately, a rash began to develop within the lines of the tattoo. The rash itches, but its appearance is of greater concern to the patient. He’s gotten some relief from topical creams, although the rash quickly returns with cessation of treatment.
Past medical history is notable for arthritis affecting his left elbow and right heel. He also has intermittent rashes that manifest on his elbows and knees, but these are partially relieved by a steroid cream (triamcinolone 0.1%).
His tattoo is located on the extensor right wrist. The affected areas show a brisk, red, inflammatory response, which—in several locations—is also scaly. There is no tenderness or induration on palpation of the rash and no palpable adenopathy in local nodal locations (epitrochlear and axillary). Elsewhere, 5 of his 10 fingernails demonstrate pitting; 2 show onycholysis and oil spotting. His scalp, knees, and elbows are free of any notable changes.