User login
Intravascular lithotripsy hailed as ‘game changer’ for coronary calcification
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
FROM TCT 2020
Key clinical point: Intravascular lithotripsy was safe and effective for treatment of severely calcified coronary stenoses in a pivotal trial.
Major finding: The 30-day rate of freedom from major adverse cardiovascular events was 92.2%, well above the prespecified performance goal of 84.4%.
Study details: Disrupt CAD III study is a multicenter, single-arm, prospective study of intravascular lithotripsy in 384 patients with severe coronary calcification.
Disclosures: The presenter reported serving as a consultant to Shockwave Medical Inc., the study sponsor, as well as several other medical device companies.
Source: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
Paraneoplastic Pemphigus With Cicatricial Nail Involvement
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.
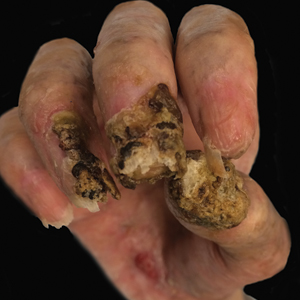
Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.
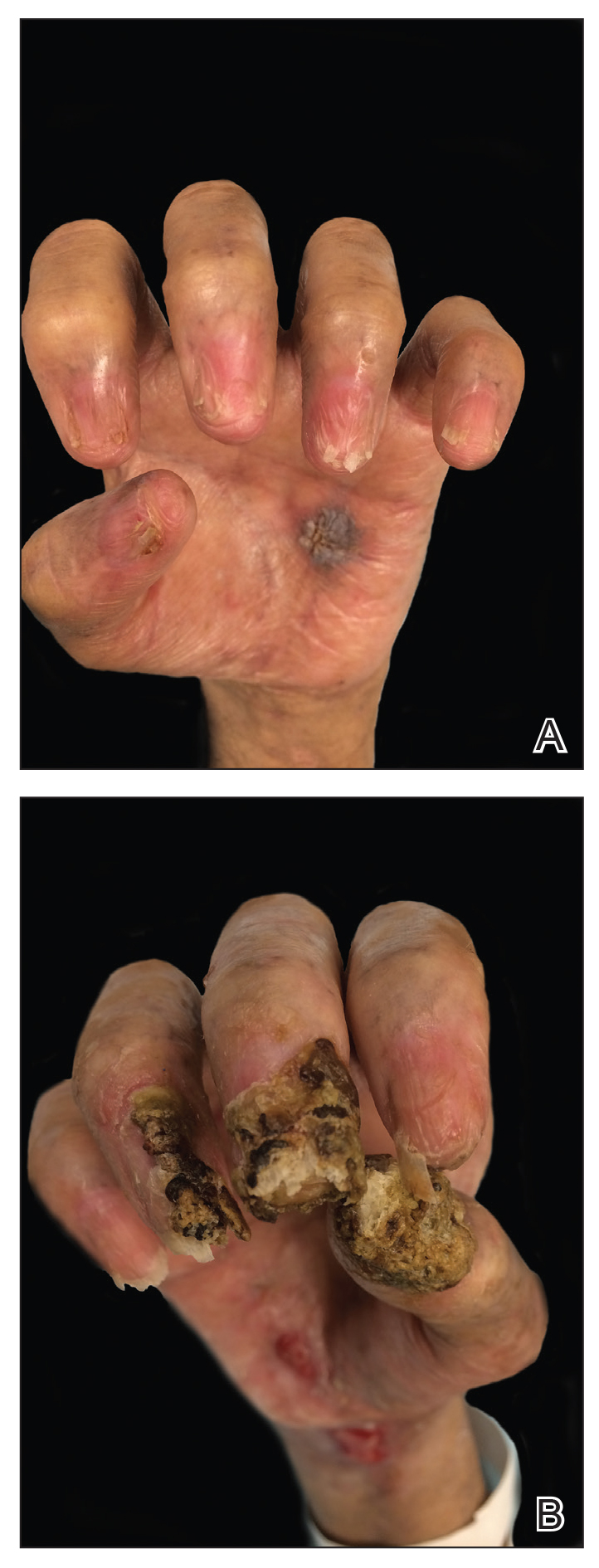
Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.

Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.

Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.

Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.

Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
Practice Points
- Paraneoplastic pemphigus (PNP) is a rare blistering skin eruption commonly associated with an underlying malignancy.
- Paraneoplastic pemphigus generally presents with erosive stomatitis with involvement of the vermillion lip but also can involve the skin and nails.
- Nail involvement can lead to scarring of the nails and can mimic lichen planus, bullous pemphigoid, or epidermolysis bullosa of the nails. These nail changes likely are due to the pronounced lichenoid interphase dermatitis seen in PNP.
Lichen Sclerosus of the Eyelid
To the Editor:
Lichen sclerosus is a chronic inflammatory skin disease of unknown cause that predominantly affects the anogenital region, but isolated extragenital lesions occur in 6% to 15% of patients. The buttocks, thighs, neck, shoulder, upper torso, and wrists most commonly are involved; the face rarely is affected.1,2 Although the etiology of lichen sclerosus remains undetermined, there is growing evidence that autoimmunity may play a role.1 Lichen sclerosus more commonly is seen in women, and the disease can present at any age, with a bimodal onset in prepubertal children and in postmenopausal women and men in the fourth decade of life.1-3 A PubMed search of articles indexed for MEDLINE using the terms lichen and eyelid and manually screened revealed 6 cases of lichen sclerosus involving the eyelid.2-4 We describe a case of lichen sclerosus involving the eyelid and its histopathology.
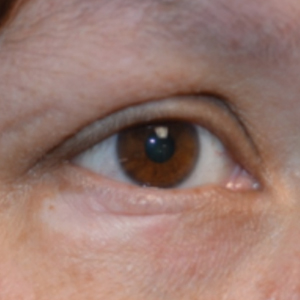
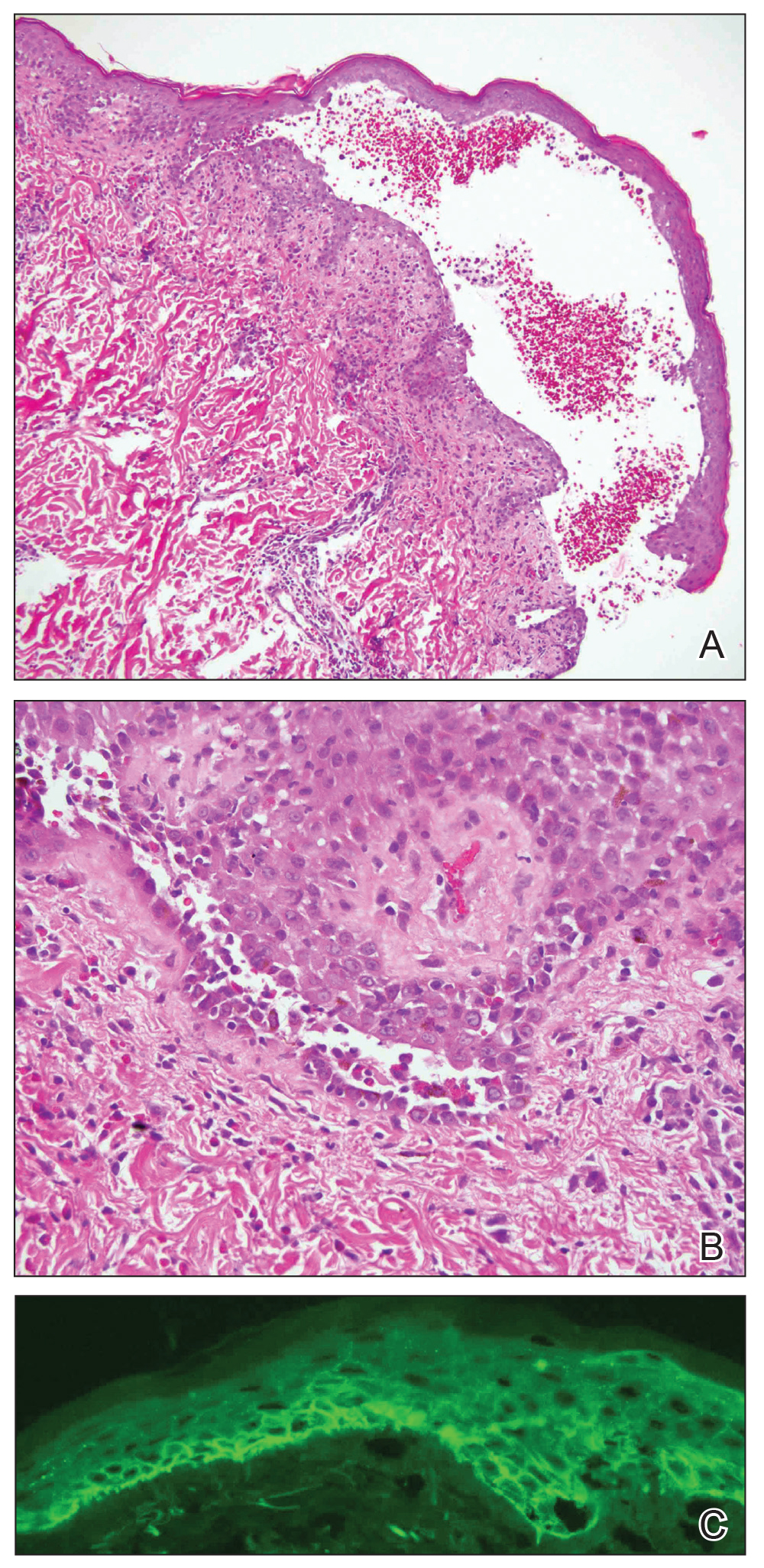
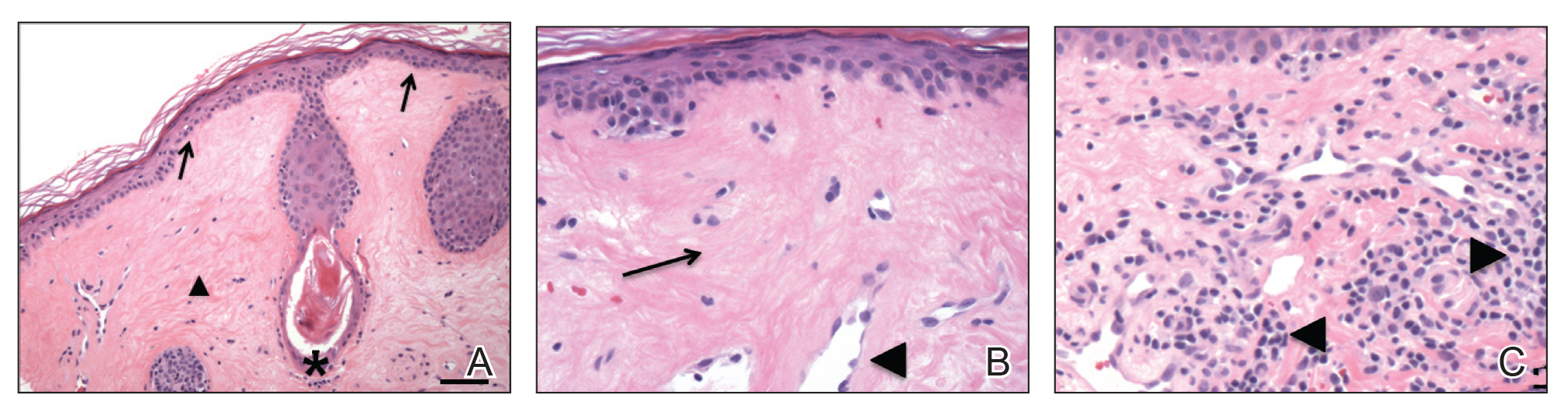
A 45-year-old woman was referred to dermatology for evaluation of a right lower eyelid lesion of 3 months’ duration. She first noted a small white patch under the eyelid that had doubled in size and felt firm without bleeding or ulceration. Her medical history was unremarkable, and there was no history of ophthalmic conditions, autoimmune disease, trauma, or cancer. An ophthalmic examination was normal, except for a 20×8-mm, flat, depigmented, firm papule with scalloped borders involving the right lower eyelid margin and extending inferiorly without evidence of madarosis or ulceration (Figure 1). She underwent an incisional biopsy that revealed the diagnosis of lichen sclerosus et atrophicus (Figure 2). A full dermatologic evaluation included a genital examination and did not reveal any additional lesions. Tacrolimus ointment was started to avoid the need for long-term use of periocular steroids and their complications.
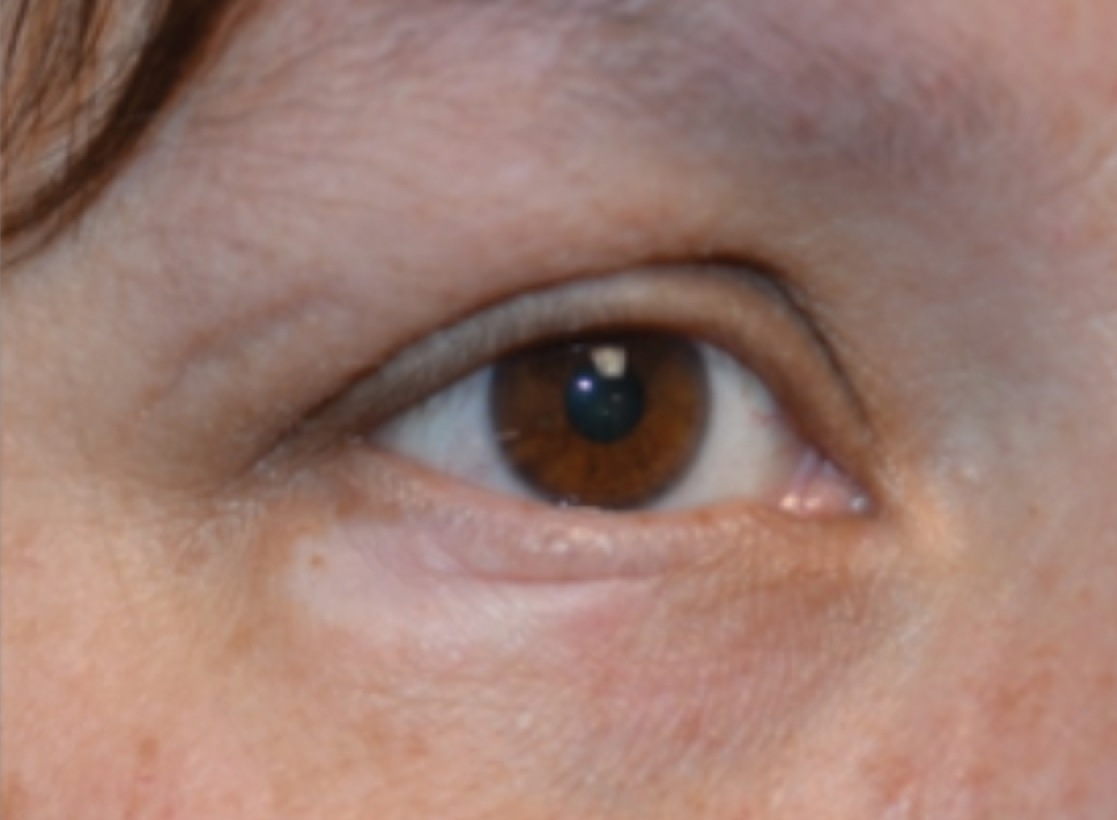
Extragenital lichen sclerosus typically is asymptomatic and only rarely presents with pruritus, in contrast to genital lichen sclerosus, which characteristically involves pruritus and dyspareunia. Although eyelid involvement is rare, ophthalmic manifestations of lichen sclerosus have included lid notching, ectropion, acquired Brown syndrome, and associated keratoconjunctivitis sicca.3-5 It characteristically appears as a well-demarcated hypopigmented papule. The differential diagnosis for a hypopigmented papule also includes amelanotic melanoma, basal cell carcinoma, vitiligo, tinea versicolor, lichen simplex chronicus, lichen planus, morphea (localized scleroderma), and systemic scleroderma with eyelid involvement.1,5
Differentiating lichen sclerosus from these conditions is of importance, as some of them can have notable morbidity and/or mortality. Of all the autoimmune connective tissue disorders, systemic sclerosus has the highest disease-specific mortality.6 Morphea, on the other hand, can have considerable morbidity. Morphea involving the head and neck notably increases the risk for neurologic complications such as seizures or central nervous system vasculitis as well as ocular complications such as anterior uveitis.6 Of note, genital lichen sclerosus carries an increased risk for squamous cell carcinoma and verrucous carcinoma; however, there have been no reported cases of malignant transformation with extragenital lesions.2
Histopathology is useful to distinguish among these entities. Although there are no specific features separating lichen sclerosus from a morphea overlap and both entities often are classified by clinical presentation, lichen sclerosus demonstrates epidermal atrophy, follicular plugging, homogenized collagen in the upper dermis with dermal edema, and lichenoid lymphocytic infiltrate (Figure 2).1 Extragenital lesions in particular also have been noted to have more epidermal atrophy and decreased rete ridges.2
First-line treatment of lichen sclerosus includes topical corticosteroids with emollients for supportive therapy. A topical calcineurin inhibitor such as tacrolimus should be considered for patients who do not respond to corticosteroid therapy or in cases in which corticosteroid therapy is contraindicated to avoid steroid-induced glaucoma or undesirable skin atrophy and hypopigmentation.2 A collaborative approach including dermatology and internal medicine can help identify a systemic or multisystem process.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Rosenthal IM, Taube JM, Nelson DL, et al. A case of infraorbital lichen sclerosus. Dermatol Online J. 2013;19:20021.
- Rabinowitz R, Rosenthal G, Yerushalmy J, et al. Keratoconjunctivitis sicca associated with lichen sclerosus et atrophicus. Eye. 2000;14:103-104.
- Olver J, Laidler P. Acquired Brown’s syndrome in a patient with combined lichen sclerosus et atrophicus and morphea. Br J Ophthalmol. 1988;72:552-557.
- El-Baba F, Frangieh GT, Iliff WJ, et al. Morphea of the eyelids. Ophthalmology. 1982;89:125-128.
- Fett N. Scleroderma: nomenclatures, etiology, pathogenesis, prognosis, and treatment: facts and controversies. Clin Dermatol. 2013;31:432-437.
To the Editor:
Lichen sclerosus is a chronic inflammatory skin disease of unknown cause that predominantly affects the anogenital region, but isolated extragenital lesions occur in 6% to 15% of patients. The buttocks, thighs, neck, shoulder, upper torso, and wrists most commonly are involved; the face rarely is affected.1,2 Although the etiology of lichen sclerosus remains undetermined, there is growing evidence that autoimmunity may play a role.1 Lichen sclerosus more commonly is seen in women, and the disease can present at any age, with a bimodal onset in prepubertal children and in postmenopausal women and men in the fourth decade of life.1-3 A PubMed search of articles indexed for MEDLINE using the terms lichen and eyelid and manually screened revealed 6 cases of lichen sclerosus involving the eyelid.2-4 We describe a case of lichen sclerosus involving the eyelid and its histopathology.
A 45-year-old woman was referred to dermatology for evaluation of a right lower eyelid lesion of 3 months’ duration. She first noted a small white patch under the eyelid that had doubled in size and felt firm without bleeding or ulceration. Her medical history was unremarkable, and there was no history of ophthalmic conditions, autoimmune disease, trauma, or cancer. An ophthalmic examination was normal, except for a 20×8-mm, flat, depigmented, firm papule with scalloped borders involving the right lower eyelid margin and extending inferiorly without evidence of madarosis or ulceration (Figure 1). She underwent an incisional biopsy that revealed the diagnosis of lichen sclerosus et atrophicus (Figure 2). A full dermatologic evaluation included a genital examination and did not reveal any additional lesions. Tacrolimus ointment was started to avoid the need for long-term use of periocular steroids and their complications.

Extragenital lichen sclerosus typically is asymptomatic and only rarely presents with pruritus, in contrast to genital lichen sclerosus, which characteristically involves pruritus and dyspareunia. Although eyelid involvement is rare, ophthalmic manifestations of lichen sclerosus have included lid notching, ectropion, acquired Brown syndrome, and associated keratoconjunctivitis sicca.3-5 It characteristically appears as a well-demarcated hypopigmented papule. The differential diagnosis for a hypopigmented papule also includes amelanotic melanoma, basal cell carcinoma, vitiligo, tinea versicolor, lichen simplex chronicus, lichen planus, morphea (localized scleroderma), and systemic scleroderma with eyelid involvement.1,5
Differentiating lichen sclerosus from these conditions is of importance, as some of them can have notable morbidity and/or mortality. Of all the autoimmune connective tissue disorders, systemic sclerosus has the highest disease-specific mortality.6 Morphea, on the other hand, can have considerable morbidity. Morphea involving the head and neck notably increases the risk for neurologic complications such as seizures or central nervous system vasculitis as well as ocular complications such as anterior uveitis.6 Of note, genital lichen sclerosus carries an increased risk for squamous cell carcinoma and verrucous carcinoma; however, there have been no reported cases of malignant transformation with extragenital lesions.2
Histopathology is useful to distinguish among these entities. Although there are no specific features separating lichen sclerosus from a morphea overlap and both entities often are classified by clinical presentation, lichen sclerosus demonstrates epidermal atrophy, follicular plugging, homogenized collagen in the upper dermis with dermal edema, and lichenoid lymphocytic infiltrate (Figure 2).1 Extragenital lesions in particular also have been noted to have more epidermal atrophy and decreased rete ridges.2

First-line treatment of lichen sclerosus includes topical corticosteroids with emollients for supportive therapy. A topical calcineurin inhibitor such as tacrolimus should be considered for patients who do not respond to corticosteroid therapy or in cases in which corticosteroid therapy is contraindicated to avoid steroid-induced glaucoma or undesirable skin atrophy and hypopigmentation.2 A collaborative approach including dermatology and internal medicine can help identify a systemic or multisystem process.
To the Editor:
Lichen sclerosus is a chronic inflammatory skin disease of unknown cause that predominantly affects the anogenital region, but isolated extragenital lesions occur in 6% to 15% of patients. The buttocks, thighs, neck, shoulder, upper torso, and wrists most commonly are involved; the face rarely is affected.1,2 Although the etiology of lichen sclerosus remains undetermined, there is growing evidence that autoimmunity may play a role.1 Lichen sclerosus more commonly is seen in women, and the disease can present at any age, with a bimodal onset in prepubertal children and in postmenopausal women and men in the fourth decade of life.1-3 A PubMed search of articles indexed for MEDLINE using the terms lichen and eyelid and manually screened revealed 6 cases of lichen sclerosus involving the eyelid.2-4 We describe a case of lichen sclerosus involving the eyelid and its histopathology.
A 45-year-old woman was referred to dermatology for evaluation of a right lower eyelid lesion of 3 months’ duration. She first noted a small white patch under the eyelid that had doubled in size and felt firm without bleeding or ulceration. Her medical history was unremarkable, and there was no history of ophthalmic conditions, autoimmune disease, trauma, or cancer. An ophthalmic examination was normal, except for a 20×8-mm, flat, depigmented, firm papule with scalloped borders involving the right lower eyelid margin and extending inferiorly without evidence of madarosis or ulceration (Figure 1). She underwent an incisional biopsy that revealed the diagnosis of lichen sclerosus et atrophicus (Figure 2). A full dermatologic evaluation included a genital examination and did not reveal any additional lesions. Tacrolimus ointment was started to avoid the need for long-term use of periocular steroids and their complications.

Extragenital lichen sclerosus typically is asymptomatic and only rarely presents with pruritus, in contrast to genital lichen sclerosus, which characteristically involves pruritus and dyspareunia. Although eyelid involvement is rare, ophthalmic manifestations of lichen sclerosus have included lid notching, ectropion, acquired Brown syndrome, and associated keratoconjunctivitis sicca.3-5 It characteristically appears as a well-demarcated hypopigmented papule. The differential diagnosis for a hypopigmented papule also includes amelanotic melanoma, basal cell carcinoma, vitiligo, tinea versicolor, lichen simplex chronicus, lichen planus, morphea (localized scleroderma), and systemic scleroderma with eyelid involvement.1,5
Differentiating lichen sclerosus from these conditions is of importance, as some of them can have notable morbidity and/or mortality. Of all the autoimmune connective tissue disorders, systemic sclerosus has the highest disease-specific mortality.6 Morphea, on the other hand, can have considerable morbidity. Morphea involving the head and neck notably increases the risk for neurologic complications such as seizures or central nervous system vasculitis as well as ocular complications such as anterior uveitis.6 Of note, genital lichen sclerosus carries an increased risk for squamous cell carcinoma and verrucous carcinoma; however, there have been no reported cases of malignant transformation with extragenital lesions.2
Histopathology is useful to distinguish among these entities. Although there are no specific features separating lichen sclerosus from a morphea overlap and both entities often are classified by clinical presentation, lichen sclerosus demonstrates epidermal atrophy, follicular plugging, homogenized collagen in the upper dermis with dermal edema, and lichenoid lymphocytic infiltrate (Figure 2).1 Extragenital lesions in particular also have been noted to have more epidermal atrophy and decreased rete ridges.2

First-line treatment of lichen sclerosus includes topical corticosteroids with emollients for supportive therapy. A topical calcineurin inhibitor such as tacrolimus should be considered for patients who do not respond to corticosteroid therapy or in cases in which corticosteroid therapy is contraindicated to avoid steroid-induced glaucoma or undesirable skin atrophy and hypopigmentation.2 A collaborative approach including dermatology and internal medicine can help identify a systemic or multisystem process.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Rosenthal IM, Taube JM, Nelson DL, et al. A case of infraorbital lichen sclerosus. Dermatol Online J. 2013;19:20021.
- Rabinowitz R, Rosenthal G, Yerushalmy J, et al. Keratoconjunctivitis sicca associated with lichen sclerosus et atrophicus. Eye. 2000;14:103-104.
- Olver J, Laidler P. Acquired Brown’s syndrome in a patient with combined lichen sclerosus et atrophicus and morphea. Br J Ophthalmol. 1988;72:552-557.
- El-Baba F, Frangieh GT, Iliff WJ, et al. Morphea of the eyelids. Ophthalmology. 1982;89:125-128.
- Fett N. Scleroderma: nomenclatures, etiology, pathogenesis, prognosis, and treatment: facts and controversies. Clin Dermatol. 2013;31:432-437.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Rosenthal IM, Taube JM, Nelson DL, et al. A case of infraorbital lichen sclerosus. Dermatol Online J. 2013;19:20021.
- Rabinowitz R, Rosenthal G, Yerushalmy J, et al. Keratoconjunctivitis sicca associated with lichen sclerosus et atrophicus. Eye. 2000;14:103-104.
- Olver J, Laidler P. Acquired Brown’s syndrome in a patient with combined lichen sclerosus et atrophicus and morphea. Br J Ophthalmol. 1988;72:552-557.
- El-Baba F, Frangieh GT, Iliff WJ, et al. Morphea of the eyelids. Ophthalmology. 1982;89:125-128.
- Fett N. Scleroderma: nomenclatures, etiology, pathogenesis, prognosis, and treatment: facts and controversies. Clin Dermatol. 2013;31:432-437.
Practice Points
- Lichen sclerosus is not confined to only the anogenital area and can affect the face in rare cases.
Dermatology Resident Education for Skin of Color
An article recently was published in The New York Times with a headline that read, “Dermatology Has a Problem With Skin Color.” 1 The article featured interviews with many well-known dermatologists who are experts in skin of color (SOC), and their points followed a similar pattern—skin disease often looks different in patients with darker skin, and diagnoses often are delayed or missed altogether as a consequence of clinical uncertainty. The article included an interview with Jenna Lester, MD, who leads the SOC clinic at the University of California, San Francisco. In the article, she discussed how dermatologists are trained to recognize findings through pattern recognition. However, if we are only trained to diagnose dermatologic diseases on white skin, we will be unable to recognize diseases in patients with darker skin, leading to suboptimal patient care. 1
Dermatology is a visual specialty, and residents go through thousands of photographs during residency training to distinguish different presentations and unique findings of a variety of skin diseases. Nevertheless, to Dr. Lester’s point, our learning is limited by the photographs and patients that we see.
Additionally, residents training in locations without diverse patient populations rely even more on images in educational resources to recognize clinical presentations in patients with darker skin. A study was published in Cutis earlier this year that surveyed dermatology residents about multiethnic training in residency.2 It showed that residents training in less ethnically diverse areas such as the Midwest and Northwest were more likely to agree that dedicated multiethnic clinics and rotations are important to gain competence compared to residents training in more ethnically diverse regions such as the Southeast, Northeast, and Southwest. Most residents believed 1 to 5 hours per month of lectures covering conditions affecting SOC and/or multiethnic skin are needed to become competent.2
Limitations of Educational Resources
The images in dermatology educational resources do not reflect the diversity of our country’s population. A research letter recently was published in the Journal of the American Academy of Dermatology (JAAD) in which the authors assessed the number of images of dark skin—Fitzpatrick skin types V and VI—in dermatology educational resources.3 The authors analyzed images from 8 resources commonly used to study dermatology, including 6 printed texts and 2 online resources. Of the printed texts, Andrews’ Diseases of the Skin had the highest percentage of images of dark skin at 19.9%. Overall, VisualDx had the highest percentage of photographs of dark skin at 28.5%, while DermNet NZ had the lowest of all resources at only 2.8%.3
Similarly, a research letter published in the British Journal of Dermatology reviewed images in 2 standard dermatology textbooks.4 Although images of SOC made up 22% to 32% of the overall content, the number of images of sexually transmitted infections in SOC was disproportionate (47%–58%) compared to images of non–sexually transmitted infections (28%). The authors also stated that communities of color often have legacies of mistrust with the health care system, and diagnostic uncertainty can further impair the physician-patient relationship.4
The lack of diversity in clinical images and research was further exemplified by recent publications regarding the perniolike eruption associated with coronavirus disease 2019 (COVID-19), commonly referred to as COVID toes. A research letter was published in the British Journal of Dermatology earlier this year about the lack of images of SOC in publications about the cutaneous manifestations of COVID-19.5 At that time, there were zero published images of cutaneous COVID-19 manifestations in Fitzpatrick skin types V and VI, yet COVID-19 disproportionately affects Black individuals and other people of color.5,6 A case series recently was published in JAAD Case Reports that included images of cutaneous COVID-19 findings in patients with Fitzpatrick skin types III through V.7 The authors noted that the findings were more subtle on darker skin as the erythema was harder to discern. The inability to identify the perniolike eruption ultimately can delay diagnosis.7
Resident Education
Over the past few months, I have reflected on my role as a dermatology resident and my dedication to antiracism in my personal and professional life. It is not a valid response or excuse to say that certain diagnoses are harder to make because of darker skin tone. It is our responsibility to do better for all patients. To that end, our educational resources should reflect our entire patient population.
I have been working with my coresident Annika Weinhammer, MD, on a quality improvement project to strengthen our educational curriculum at the University of Wisconsin regarding SOC. This project aims to enhance our skills as dermatologists in diagnosing and treating diseases in SOC. Moving forward, we have set an expectation that all didactic lectures must include images of SOC. Below, I have listed some of our initiatives along with recommendations for educational resources. There are multiple dermatology textbooks focused on SOC, including the following:
- Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders 8
- Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology 9
- Dermatology Atlas for Skin of Color 10
- Fundamentals of Ethnic Hair: The Dermatologist’s Perspective 11
- Light-Based Therapies for Skin of Color 12
- Pediatric Skin of Color 13
- Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment 14
- Taylor and Kelly’s Dermatology for Skin of Color 15
- Treatments for Skin of Color 16
Our program has provided residents with Taylor and Kelly’s Dermatology for Skin of Color15 and Treatments for Skin of Color.16 Residents and medical students should search their institution’s electronic library for e-books and other resources including VisualDx, which includes many photographs of SOC that can be used and cited in resident didactics.
There also are a variety of online resources. Mind the Gap is a handbook written by Malone Mukwende, a medical student in London.17,18 The handbook focuses on common clinical signs and how they present in black and brown skin. Another online resource with clinical images is Skin Deep (https://dftbskindeep.com/), a project aimed at improving the diversity of pediatric skin images. An additional online resource is Brown Skin Matters on Instagram (@brownskinmatters) that shows photographs of dermatologic conditions in SOC; however, these photographs are submitted by users and not independently verified.
I also encourage residents to join the Skin of Color Society, which promotes awareness and excellence within the special interest area of SOC. Some of the society's initiatives include educational series, networking events, diversity town halls, and a scientific symposium. Patient information for common dermatologic diagnoses exists on the society's website (https://skinofcolorsociety.org/). The society waives membership fees for resident applicants who provide a letter of good standing from their residency program. The society hosted the Skin of Color Update virtually this year (September 12–13, 2020). It costs $49 to attend, and the recorded lectures are available to stream through the end of 2020. Our department sponsored residents to attend virtually.
Finally, our department has been taking steps to implement antiracism measures in how we work, learn, conduct research, and treat patients. We are leading a resident book club discussing How to Be an Antiracist19 by Ibram X. Kendi. Residents are involved in the local chapter of White Coats for Black Lives (https://whitecoats4blacklives.org/). We also have compiled a list of antiracism resources that was shared with the department, including books, documentaries, podcasts, local and online Black-owned businesses to support, and local Black-led nonprofits.
Final Thoughts
Dermatology residents must be comfortable diagnosing and treating diseases in darker skin tones to provide the best possible care for patients with SOC. Although some common dermatology educational resources have a paucity of clinical images of SOC, there are a variety of additional educational resources through textbooks and websites.
- Rabin RC. Dermatology has a problem with skin color. New York Times. August 30, 2020. https://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed October 5, 2020.
- Cline A, Winter R, Kouroush S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595.
- Golden SH. Coronavirus in African Americans and other people of color. Johns Hopkins Medicine website. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid19-racial-disparities. Published April 20, 2020. Accessed October 5, 2020.
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders. Switzerland: Springer; 2016.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology. Switzerland: Springer; 2016.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. New York, NY: Springer; 2014.
- Aguh C, Okoye GA, eds. Fundamentals of Ethnic Hair: The Dermatologist’s Perspective. Switzerland: Springer; 2017.
- Baron E, ed. Light-Based Therapies for Skin of Color. London: Springer; 2009.
- Silverberg NB, Durán-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. New York, NY: Springer; 2015.
- Alexis AF, Barbosa VH, eds. Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment. New York, NY: Springer; 2013.
- Taylor SC, Kelly AP, Lim H, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw Hill Professional; 2016.
- Taylor SC, Badreshia-Bansal S, Calendar VD, et al. Treatments for Skin of Color. China: Saunders Elsevier; 2011.
- Page S. A medical student couldn’t find how symptoms look on darker skin. he decided to publish a book about it. Washington Post. July 22, 2020. https://www.washingtonpost.com/lifestyle/2020/07/22/malone-mukwende-medical-handbook/. Accessed October 5, 2020.
- Mukwende M, Tamony P, Turner M. Mind the Gap: A Handbook of Clinical Signs in Black and Brown Skin. London, England: St. George’s University of London; 2020. https://www.blackandbrownskin.co.uk/mindthegap. Accessed October 5, 2020.
- Kendi IX. How to Be an Antiracist. New York, NY: Random House; 2019.
An article recently was published in The New York Times with a headline that read, “Dermatology Has a Problem With Skin Color.” 1 The article featured interviews with many well-known dermatologists who are experts in skin of color (SOC), and their points followed a similar pattern—skin disease often looks different in patients with darker skin, and diagnoses often are delayed or missed altogether as a consequence of clinical uncertainty. The article included an interview with Jenna Lester, MD, who leads the SOC clinic at the University of California, San Francisco. In the article, she discussed how dermatologists are trained to recognize findings through pattern recognition. However, if we are only trained to diagnose dermatologic diseases on white skin, we will be unable to recognize diseases in patients with darker skin, leading to suboptimal patient care. 1
Dermatology is a visual specialty, and residents go through thousands of photographs during residency training to distinguish different presentations and unique findings of a variety of skin diseases. Nevertheless, to Dr. Lester’s point, our learning is limited by the photographs and patients that we see.
Additionally, residents training in locations without diverse patient populations rely even more on images in educational resources to recognize clinical presentations in patients with darker skin. A study was published in Cutis earlier this year that surveyed dermatology residents about multiethnic training in residency.2 It showed that residents training in less ethnically diverse areas such as the Midwest and Northwest were more likely to agree that dedicated multiethnic clinics and rotations are important to gain competence compared to residents training in more ethnically diverse regions such as the Southeast, Northeast, and Southwest. Most residents believed 1 to 5 hours per month of lectures covering conditions affecting SOC and/or multiethnic skin are needed to become competent.2
Limitations of Educational Resources
The images in dermatology educational resources do not reflect the diversity of our country’s population. A research letter recently was published in the Journal of the American Academy of Dermatology (JAAD) in which the authors assessed the number of images of dark skin—Fitzpatrick skin types V and VI—in dermatology educational resources.3 The authors analyzed images from 8 resources commonly used to study dermatology, including 6 printed texts and 2 online resources. Of the printed texts, Andrews’ Diseases of the Skin had the highest percentage of images of dark skin at 19.9%. Overall, VisualDx had the highest percentage of photographs of dark skin at 28.5%, while DermNet NZ had the lowest of all resources at only 2.8%.3
Similarly, a research letter published in the British Journal of Dermatology reviewed images in 2 standard dermatology textbooks.4 Although images of SOC made up 22% to 32% of the overall content, the number of images of sexually transmitted infections in SOC was disproportionate (47%–58%) compared to images of non–sexually transmitted infections (28%). The authors also stated that communities of color often have legacies of mistrust with the health care system, and diagnostic uncertainty can further impair the physician-patient relationship.4
The lack of diversity in clinical images and research was further exemplified by recent publications regarding the perniolike eruption associated with coronavirus disease 2019 (COVID-19), commonly referred to as COVID toes. A research letter was published in the British Journal of Dermatology earlier this year about the lack of images of SOC in publications about the cutaneous manifestations of COVID-19.5 At that time, there were zero published images of cutaneous COVID-19 manifestations in Fitzpatrick skin types V and VI, yet COVID-19 disproportionately affects Black individuals and other people of color.5,6 A case series recently was published in JAAD Case Reports that included images of cutaneous COVID-19 findings in patients with Fitzpatrick skin types III through V.7 The authors noted that the findings were more subtle on darker skin as the erythema was harder to discern. The inability to identify the perniolike eruption ultimately can delay diagnosis.7
Resident Education
Over the past few months, I have reflected on my role as a dermatology resident and my dedication to antiracism in my personal and professional life. It is not a valid response or excuse to say that certain diagnoses are harder to make because of darker skin tone. It is our responsibility to do better for all patients. To that end, our educational resources should reflect our entire patient population.
I have been working with my coresident Annika Weinhammer, MD, on a quality improvement project to strengthen our educational curriculum at the University of Wisconsin regarding SOC. This project aims to enhance our skills as dermatologists in diagnosing and treating diseases in SOC. Moving forward, we have set an expectation that all didactic lectures must include images of SOC. Below, I have listed some of our initiatives along with recommendations for educational resources. There are multiple dermatology textbooks focused on SOC, including the following:
- Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders 8
- Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology 9
- Dermatology Atlas for Skin of Color 10
- Fundamentals of Ethnic Hair: The Dermatologist’s Perspective 11
- Light-Based Therapies for Skin of Color 12
- Pediatric Skin of Color 13
- Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment 14
- Taylor and Kelly’s Dermatology for Skin of Color 15
- Treatments for Skin of Color 16
Our program has provided residents with Taylor and Kelly’s Dermatology for Skin of Color15 and Treatments for Skin of Color.16 Residents and medical students should search their institution’s electronic library for e-books and other resources including VisualDx, which includes many photographs of SOC that can be used and cited in resident didactics.
There also are a variety of online resources. Mind the Gap is a handbook written by Malone Mukwende, a medical student in London.17,18 The handbook focuses on common clinical signs and how they present in black and brown skin. Another online resource with clinical images is Skin Deep (https://dftbskindeep.com/), a project aimed at improving the diversity of pediatric skin images. An additional online resource is Brown Skin Matters on Instagram (@brownskinmatters) that shows photographs of dermatologic conditions in SOC; however, these photographs are submitted by users and not independently verified.
I also encourage residents to join the Skin of Color Society, which promotes awareness and excellence within the special interest area of SOC. Some of the society's initiatives include educational series, networking events, diversity town halls, and a scientific symposium. Patient information for common dermatologic diagnoses exists on the society's website (https://skinofcolorsociety.org/). The society waives membership fees for resident applicants who provide a letter of good standing from their residency program. The society hosted the Skin of Color Update virtually this year (September 12–13, 2020). It costs $49 to attend, and the recorded lectures are available to stream through the end of 2020. Our department sponsored residents to attend virtually.
Finally, our department has been taking steps to implement antiracism measures in how we work, learn, conduct research, and treat patients. We are leading a resident book club discussing How to Be an Antiracist19 by Ibram X. Kendi. Residents are involved in the local chapter of White Coats for Black Lives (https://whitecoats4blacklives.org/). We also have compiled a list of antiracism resources that was shared with the department, including books, documentaries, podcasts, local and online Black-owned businesses to support, and local Black-led nonprofits.
Final Thoughts
Dermatology residents must be comfortable diagnosing and treating diseases in darker skin tones to provide the best possible care for patients with SOC. Although some common dermatology educational resources have a paucity of clinical images of SOC, there are a variety of additional educational resources through textbooks and websites.
An article recently was published in The New York Times with a headline that read, “Dermatology Has a Problem With Skin Color.” 1 The article featured interviews with many well-known dermatologists who are experts in skin of color (SOC), and their points followed a similar pattern—skin disease often looks different in patients with darker skin, and diagnoses often are delayed or missed altogether as a consequence of clinical uncertainty. The article included an interview with Jenna Lester, MD, who leads the SOC clinic at the University of California, San Francisco. In the article, she discussed how dermatologists are trained to recognize findings through pattern recognition. However, if we are only trained to diagnose dermatologic diseases on white skin, we will be unable to recognize diseases in patients with darker skin, leading to suboptimal patient care. 1
Dermatology is a visual specialty, and residents go through thousands of photographs during residency training to distinguish different presentations and unique findings of a variety of skin diseases. Nevertheless, to Dr. Lester’s point, our learning is limited by the photographs and patients that we see.
Additionally, residents training in locations without diverse patient populations rely even more on images in educational resources to recognize clinical presentations in patients with darker skin. A study was published in Cutis earlier this year that surveyed dermatology residents about multiethnic training in residency.2 It showed that residents training in less ethnically diverse areas such as the Midwest and Northwest were more likely to agree that dedicated multiethnic clinics and rotations are important to gain competence compared to residents training in more ethnically diverse regions such as the Southeast, Northeast, and Southwest. Most residents believed 1 to 5 hours per month of lectures covering conditions affecting SOC and/or multiethnic skin are needed to become competent.2
Limitations of Educational Resources
The images in dermatology educational resources do not reflect the diversity of our country’s population. A research letter recently was published in the Journal of the American Academy of Dermatology (JAAD) in which the authors assessed the number of images of dark skin—Fitzpatrick skin types V and VI—in dermatology educational resources.3 The authors analyzed images from 8 resources commonly used to study dermatology, including 6 printed texts and 2 online resources. Of the printed texts, Andrews’ Diseases of the Skin had the highest percentage of images of dark skin at 19.9%. Overall, VisualDx had the highest percentage of photographs of dark skin at 28.5%, while DermNet NZ had the lowest of all resources at only 2.8%.3
Similarly, a research letter published in the British Journal of Dermatology reviewed images in 2 standard dermatology textbooks.4 Although images of SOC made up 22% to 32% of the overall content, the number of images of sexually transmitted infections in SOC was disproportionate (47%–58%) compared to images of non–sexually transmitted infections (28%). The authors also stated that communities of color often have legacies of mistrust with the health care system, and diagnostic uncertainty can further impair the physician-patient relationship.4
The lack of diversity in clinical images and research was further exemplified by recent publications regarding the perniolike eruption associated with coronavirus disease 2019 (COVID-19), commonly referred to as COVID toes. A research letter was published in the British Journal of Dermatology earlier this year about the lack of images of SOC in publications about the cutaneous manifestations of COVID-19.5 At that time, there were zero published images of cutaneous COVID-19 manifestations in Fitzpatrick skin types V and VI, yet COVID-19 disproportionately affects Black individuals and other people of color.5,6 A case series recently was published in JAAD Case Reports that included images of cutaneous COVID-19 findings in patients with Fitzpatrick skin types III through V.7 The authors noted that the findings were more subtle on darker skin as the erythema was harder to discern. The inability to identify the perniolike eruption ultimately can delay diagnosis.7
Resident Education
Over the past few months, I have reflected on my role as a dermatology resident and my dedication to antiracism in my personal and professional life. It is not a valid response or excuse to say that certain diagnoses are harder to make because of darker skin tone. It is our responsibility to do better for all patients. To that end, our educational resources should reflect our entire patient population.
I have been working with my coresident Annika Weinhammer, MD, on a quality improvement project to strengthen our educational curriculum at the University of Wisconsin regarding SOC. This project aims to enhance our skills as dermatologists in diagnosing and treating diseases in SOC. Moving forward, we have set an expectation that all didactic lectures must include images of SOC. Below, I have listed some of our initiatives along with recommendations for educational resources. There are multiple dermatology textbooks focused on SOC, including the following:
- Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders 8
- Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology 9
- Dermatology Atlas for Skin of Color 10
- Fundamentals of Ethnic Hair: The Dermatologist’s Perspective 11
- Light-Based Therapies for Skin of Color 12
- Pediatric Skin of Color 13
- Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment 14
- Taylor and Kelly’s Dermatology for Skin of Color 15
- Treatments for Skin of Color 16
Our program has provided residents with Taylor and Kelly’s Dermatology for Skin of Color15 and Treatments for Skin of Color.16 Residents and medical students should search their institution’s electronic library for e-books and other resources including VisualDx, which includes many photographs of SOC that can be used and cited in resident didactics.
There also are a variety of online resources. Mind the Gap is a handbook written by Malone Mukwende, a medical student in London.17,18 The handbook focuses on common clinical signs and how they present in black and brown skin. Another online resource with clinical images is Skin Deep (https://dftbskindeep.com/), a project aimed at improving the diversity of pediatric skin images. An additional online resource is Brown Skin Matters on Instagram (@brownskinmatters) that shows photographs of dermatologic conditions in SOC; however, these photographs are submitted by users and not independently verified.
I also encourage residents to join the Skin of Color Society, which promotes awareness and excellence within the special interest area of SOC. Some of the society's initiatives include educational series, networking events, diversity town halls, and a scientific symposium. Patient information for common dermatologic diagnoses exists on the society's website (https://skinofcolorsociety.org/). The society waives membership fees for resident applicants who provide a letter of good standing from their residency program. The society hosted the Skin of Color Update virtually this year (September 12–13, 2020). It costs $49 to attend, and the recorded lectures are available to stream through the end of 2020. Our department sponsored residents to attend virtually.
Finally, our department has been taking steps to implement antiracism measures in how we work, learn, conduct research, and treat patients. We are leading a resident book club discussing How to Be an Antiracist19 by Ibram X. Kendi. Residents are involved in the local chapter of White Coats for Black Lives (https://whitecoats4blacklives.org/). We also have compiled a list of antiracism resources that was shared with the department, including books, documentaries, podcasts, local and online Black-owned businesses to support, and local Black-led nonprofits.
Final Thoughts
Dermatology residents must be comfortable diagnosing and treating diseases in darker skin tones to provide the best possible care for patients with SOC. Although some common dermatology educational resources have a paucity of clinical images of SOC, there are a variety of additional educational resources through textbooks and websites.
- Rabin RC. Dermatology has a problem with skin color. New York Times. August 30, 2020. https://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed October 5, 2020.
- Cline A, Winter R, Kouroush S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595.
- Golden SH. Coronavirus in African Americans and other people of color. Johns Hopkins Medicine website. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid19-racial-disparities. Published April 20, 2020. Accessed October 5, 2020.
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders. Switzerland: Springer; 2016.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology. Switzerland: Springer; 2016.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. New York, NY: Springer; 2014.
- Aguh C, Okoye GA, eds. Fundamentals of Ethnic Hair: The Dermatologist’s Perspective. Switzerland: Springer; 2017.
- Baron E, ed. Light-Based Therapies for Skin of Color. London: Springer; 2009.
- Silverberg NB, Durán-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. New York, NY: Springer; 2015.
- Alexis AF, Barbosa VH, eds. Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment. New York, NY: Springer; 2013.
- Taylor SC, Kelly AP, Lim H, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw Hill Professional; 2016.
- Taylor SC, Badreshia-Bansal S, Calendar VD, et al. Treatments for Skin of Color. China: Saunders Elsevier; 2011.
- Page S. A medical student couldn’t find how symptoms look on darker skin. he decided to publish a book about it. Washington Post. July 22, 2020. https://www.washingtonpost.com/lifestyle/2020/07/22/malone-mukwende-medical-handbook/. Accessed October 5, 2020.
- Mukwende M, Tamony P, Turner M. Mind the Gap: A Handbook of Clinical Signs in Black and Brown Skin. London, England: St. George’s University of London; 2020. https://www.blackandbrownskin.co.uk/mindthegap. Accessed October 5, 2020.
- Kendi IX. How to Be an Antiracist. New York, NY: Random House; 2019.
- Rabin RC. Dermatology has a problem with skin color. New York Times. August 30, 2020. https://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed October 5, 2020.
- Cline A, Winter R, Kouroush S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595.
- Golden SH. Coronavirus in African Americans and other people of color. Johns Hopkins Medicine website. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid19-racial-disparities. Published April 20, 2020. Accessed October 5, 2020.
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Adnexal, Inflammation, Infections, and Pigmentary Disorders. Switzerland: Springer; 2016.
- Love PB, Kundu RV, eds. Clinical Cases in Skin of Color: Medical, Oncological and Hair Disorders, and Cosmetic Dermatology. Switzerland: Springer; 2016.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. New York, NY: Springer; 2014.
- Aguh C, Okoye GA, eds. Fundamentals of Ethnic Hair: The Dermatologist’s Perspective. Switzerland: Springer; 2017.
- Baron E, ed. Light-Based Therapies for Skin of Color. London: Springer; 2009.
- Silverberg NB, Durán-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. New York, NY: Springer; 2015.
- Alexis AF, Barbosa VH, eds. Skin of Color: A Practical Guide to Dermatologic Diagnosis and Treatment. New York, NY: Springer; 2013.
- Taylor SC, Kelly AP, Lim H, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw Hill Professional; 2016.
- Taylor SC, Badreshia-Bansal S, Calendar VD, et al. Treatments for Skin of Color. China: Saunders Elsevier; 2011.
- Page S. A medical student couldn’t find how symptoms look on darker skin. he decided to publish a book about it. Washington Post. July 22, 2020. https://www.washingtonpost.com/lifestyle/2020/07/22/malone-mukwende-medical-handbook/. Accessed October 5, 2020.
- Mukwende M, Tamony P, Turner M. Mind the Gap: A Handbook of Clinical Signs in Black and Brown Skin. London, England: St. George’s University of London; 2020. https://www.blackandbrownskin.co.uk/mindthegap. Accessed October 5, 2020.
- Kendi IX. How to Be an Antiracist. New York, NY: Random House; 2019.
Resident Pearls
- Images of skin of color (SOC) are greatly underrepresented in dermatology educational resources.
- Inadequate training in recognizing skin disease in patients with darker skin can lead to delayed or missed diagnoses.
- There are various educational resources and opportunities available to improve and diversify dermatology education, ensuring the best possible care for patients with SOC.
Diabetic neuropathic pain linked to brain bioenergic anomalies
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
FROM EASD 2020
Pathologic CR in HER2+ breast cancer predicts long-term survival
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
In fact, for the majority of women, pCR appears to be a marker of cure.
The trial was conducted among 455 women with HER2-positive breast cancer tumors measuring at least 2 cm who were randomized to neoadjuvant trastuzumab, lapatinib, or both drugs in combination, each together with paclitaxel, followed by more chemotherapy and more of the same targeted therapy after surgery.
Relative to trastuzumab alone, trastuzumab plus lapatinib improved rates of pCR, as shown by data published in The Lancet in 2012. However, the dual therapy did not significantly prolong event-free or overall survival, according to data published in The Lancet Oncology in 2014. Findings were similar in an update at a median follow-up of 6.7 years, published in the European Journal of Cancer in 2019.
Study investigator Paolo Nuciforo, MD, PhD, of the Vall d’Hebron Institute of Oncology in Barcelona, reported the trial’s final results, now at a median follow-up of 9.7 years, at the 12th European Breast Cancer Conference.
There were no significant differences in 9-year outcomes by specific HER2-targeted therapy. However, in a landmark analysis among women who were event free and still on follow-up 30 weeks after randomization, those achieving pCR with any of the therapies were 52% less likely to experience events and 63% less likely to die. Benefit was greatest in the subset of patients with hormone receptor–negative disease.
“The long-term follow-up confirms that, independent of the treatment regimen that we use – in this case, the dual blockade was with lapatinib, but similar results can be expected with other dual blockade – the pCR is a very robust surrogate biomarker of long-term survival,” Dr. Nuciforo commented in a press conference, noting that dual trastuzumab and pertuzumab has emerged as the standard of care.
“If we really pay attention to the curve, it’s maybe interesting to see that, after year 6, we actually don’t see any events in the pCR population. So this means that these patients are almost cured. We cannot say the word ‘cure’ in cancer, but it’s very reassuring to see the long-term survival analysis support the use of pCR as an endpoint,” he elaborated.
“Our results support the design of future trial concepts in HER2-positive early breast cancer which use pCR as an early efficacy readout of long-term benefit to escalate or deescalate therapy, particularly for hormone receptor–negative tumors,” Dr. Nuciforo concluded.
Support for current practice
“The study lends support for the current practice of risk-stratifying by pCR as well as making treatment decisions regarding T-DM1 [trastuzumab emtansine], and there hasn’t been a big change between 5-year and 9-year outcomes,” Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, commented in an interview.
The lack of late events in the group with pCR technically meets the definition of cure, Dr. Carey said. “I think it speaks to the relatively early relapse risk in HER2-positive breast cancer and the impact of anti-HER2 therapy that carries forward. In general, these are findings similar to long-term findings of other trials and I suspect will be the same for any regimen.”
Although the analysis of dual lapatinib-trastuzumab therapy was underpowered, the trends seen align with favorable results in the adjuvant APHINITY trial (which combined trastuzumab with pertuzumab) and the neoadjuvant CALGB 40601 trial (which combined trastuzumab with lapatinib), according to Dr. Carey. “There has been a trend in every other study [of dual therapy] performed, so this is consistent.”
Study details
NeoALTTO is noteworthy for having the longest follow-up among all neoadjuvant studies of dual HER2 blockade in early breast cancer, Dr. Nuciforo said.
He reported no significant difference in survival between the treatment arms at 9 years.
The 9-year rate of event-free survival was 69% with lapatinib-trastuzumab, 63% with lapatinib alone, and 65% with trastuzumab alone. The corresponding 9-year rates of overall survival were 80%, 77%, and 76%, respectively.
However, there were significant differences in event-free and overall survival among women who achieved pCR and those who did not.
“pCR was achieved for almost twice as many patients treated with dual HER2 blockade, compared with patients in the single-agent arms,” Dr. Nuciforo pointed out. The pCR rate was 51.3% with lapatinib-trastuzumab, 24.7% with lapatinib alone, and 29.5% with trastuzumab alone.
Relative to peers who did not achieve pCR, women who did had better 9-year event-free survival (77% vs. 61%; adjusted hazard ratio, 0.48; P = .0008). The benefit was stronger in hormone receptor–negative disease (HR, 0.43; P = .002) than in hormone receptor–positive disease (HR, 0.60; P = .15).
The pattern was similar for overall survival at 9 years – 88% in those who achieved a pCR and 72% in those who did not (adjusted HR, 0.37; P = .0004). Again, greater benefit was seen in hormone receptor–negative disease (HR, 0.33; P = .002) than in hormone receptor–positive disease (HR, 0.44; P = .09).
“Biomarker-driven approaches may improve selection of those patients who are more likely to respond to anti-HER2 therapies,” Dr. Nuciforo proposed.
From 6 years onward, there were no additional fatal adverse events or nonfatal serious adverse events recorded, and no additional primary cardiac endpoints were recorded.
The study was funded by Novartis. Dr. Nuciforo and Dr. Carey disclosed no conflicts of interest.
SOURCE: Nuciforo P et al. EBCC-12 Virtual Conference, Abstract 23.
FROM EBCC-12 VIRTUAL CONFERENCE
Was This Tattoo a Rash Choice?
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.
ANSWER
The correct answer is koebnerization of pre-existing psoriasis (choice “c”).
DISCUSSION
Tattoos have been known to cause bacterial infection (choice “a”), but this was unlikely given the diffuse nature of the rash and the lack of pain or adenopathy. Allergic reactions to tattoo dyes (choice “b”) are certainly common, but usually red or yellow dyes—which were not used for this tattoo—provoke the worst reactions. Furthermore, itching would have been a more prominent feature of the patient's complaint. Had it been fungal infection (choice “d”), the steroid cream would have made it worse.
One possibility remained: the so-called isomorphic phenomenon (otherwise known as koebnerization). First described by Heinrich Koebner in the mid-19th century, koebnerization is characterized by the appearance of psoriasis in traumatized skin such as surgical wounds, abrasions, burns, or even tattoos. Several other conditions also exhibit this same linear response to trauma, including warts, molluscum, and lichen planus.
To test for this diagnosis, corroborative findings of psoriasis were sought and found in the patient’s nails. His history of rashes on the knees and elbows also contributed to establishing the diagnosis. Moreover, his complaint of arthritis was quite suggestive of psoriatic arthropathy, which afflicts about 25% of patients with psoriasis and has little to do with the severity of the skin disease itself. Once the diagnosis became more apparent, the patient recalled a family history of psoriasis. Had any question remained, a biopsy could remove doubt.
TREATMENT
For the patient, twice-daily application of a stronger steroid cream (augmented betamethasone) was prescribed. Though this quickly cleared the koebnerizing psoriasis, it is likely we haven’t seen the last of this disease.

Weeks ago, a 43-year-old man received a birthday tattoo of his choice: a geometric pattern etched in blue ink on his wrist. Unfortunately, a rash began to develop within the lines of the tattoo. The rash itches, but its appearance is of greater concern to the patient. He’s gotten some relief from topical creams, although the rash quickly returns with cessation of treatment.
Past medical history is notable for arthritis affecting his left elbow and right heel. He also has intermittent rashes that manifest on his elbows and knees, but these are partially relieved by a steroid cream (triamcinolone 0.1%).
His tattoo is located on the extensor right wrist. The affected areas show a brisk, red, inflammatory response, which—in several locations—is also scaly. There is no tenderness or induration on palpation of the rash and no palpable adenopathy in local nodal locations (epitrochlear and axillary). Elsewhere, 5 of his 10 fingernails demonstrate pitting; 2 show onycholysis and oil spotting. His scalp, knees, and elbows are free of any notable changes.
Combined features of benign breast disease tied to breast cancer risk
“Benign breast disease is a key risk factor for breast cancer risk prediction,” commented presenting investigator Marta Román, PhD, of the Hospital del Mar Medical Research Institute in Barcelona. “Those women who have had a benign breast disease diagnosis have an increased risk that lasts for at least 20 years.”
To assess the combined influence of various attributes of benign breast disease, the investigators studied 629,087 women, aged 50-69 years, in Spain who underwent population-based mammographic breast cancer screening during 1994-2015 and did not have breast cancer at their prevalent (first) screen. The mean follow-up was 7.8 years.
Results showed that breast cancer risk was about three times higher for women with benign breast disease that was proliferative or that was detected on an incident screen, relative to peers with no benign breast disease. When combinations of factors were considered, breast cancer risk was most elevated – more than four times higher – for women with proliferative benign breast disease with atypia detected on an incident screen.
“We believe that these findings should be considered when discussing risk-based personalized screening strategies because these differences between prevalent and incident screens might be important if we want to personalize the screening, whether it’s the first time a woman comes to the screening program or a subsequent screen,” Dr. Román said.
Practice changing?
The study’s large size and population-based design, likely permitting capture of most biopsy results, are strengths, Mark David Pearlman, MD, of the University of Michigan, Ann Arbor, commented in an interview.
But its observational, retrospective nature opens the study up to biases, such as uncertainty as to how many women were symptomatic at the time of their mammogram and the likelihood of heightened monitoring after a biopsy showing hyperplasia, Dr. Pearlman cautioned.
“Moreover, the relative risk in this study for proliferative benign breast disease without atypia is substantially higher than prior observations of this group. This discrepancy was not discussed by the authors,” Dr. Pearlman said.
At present, women’s risk of breast cancer is predicted using well-validated models that include the question of prior breast biopsies, such as the Gail Model, the Tyrer-Cuzick model (IBIS tool), and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm, Dr. Pearlman noted.
“This study, without further validation within a model, would not change risk assessment,” he said, disagreeing with the investigators’ conclusions. “What I would say is that further study to determine how to use this observation to decide if any change in screening or management should occur would be more appropriate.”
Study details
The 629,087 women studied underwent 2,327,384 screens, Dr. Román reported. In total, screening detected 9,184 cases of benign breast disease and 9,431 breast cancers.
Breast cancer was diagnosed in 2.4% and 3.0% of women with benign breast disease detected on prevalent and incident screens, respectively, compared with 1.5% of women without any benign breast disease detected.
Elevation of breast cancer risk varied across benign breast disease subtype. Relative to peers without any benign disease, risk was significantly elevated for women with nonproliferative disease (adjusted hazard ratio, 1.95), proliferative disease without atypia (aHR, 3.19), and proliferative disease with atypia (aHR, 3.82).
Similarly, elevation of risk varied depending on the screening at which the benign disease was detected. Risk was significantly elevated when the disease was found at prevalent screens (aHR, 1.87) and more so when it was found at incident screens (aHR, 2.67).
There was no significant interaction of these two factors (P = .83). However, when combinations were considered, risk was highest for women with proliferative benign breast disease with atypia detected on incident screens (aHR, 4.35) or prevalent screens (aHR, 3.35), and women with proliferative benign breast disease without atypia detected on incident screens (aHR, 3.83).
This study was supported by grants from Instituto de Salud Carlos III FEDER and by the Research Network on Health Services in Chronic Diseases. Dr. Román and Dr. Pearlman disclosed no conflicts of interest.
SOURCE: Román M et al. EBCC-12 Virtual Conference, Abstract 15.
“Benign breast disease is a key risk factor for breast cancer risk prediction,” commented presenting investigator Marta Román, PhD, of the Hospital del Mar Medical Research Institute in Barcelona. “Those women who have had a benign breast disease diagnosis have an increased risk that lasts for at least 20 years.”
To assess the combined influence of various attributes of benign breast disease, the investigators studied 629,087 women, aged 50-69 years, in Spain who underwent population-based mammographic breast cancer screening during 1994-2015 and did not have breast cancer at their prevalent (first) screen. The mean follow-up was 7.8 years.
Results showed that breast cancer risk was about three times higher for women with benign breast disease that was proliferative or that was detected on an incident screen, relative to peers with no benign breast disease. When combinations of factors were considered, breast cancer risk was most elevated – more than four times higher – for women with proliferative benign breast disease with atypia detected on an incident screen.
“We believe that these findings should be considered when discussing risk-based personalized screening strategies because these differences between prevalent and incident screens might be important if we want to personalize the screening, whether it’s the first time a woman comes to the screening program or a subsequent screen,” Dr. Román said.
Practice changing?
The study’s large size and population-based design, likely permitting capture of most biopsy results, are strengths, Mark David Pearlman, MD, of the University of Michigan, Ann Arbor, commented in an interview.
But its observational, retrospective nature opens the study up to biases, such as uncertainty as to how many women were symptomatic at the time of their mammogram and the likelihood of heightened monitoring after a biopsy showing hyperplasia, Dr. Pearlman cautioned.
“Moreover, the relative risk in this study for proliferative benign breast disease without atypia is substantially higher than prior observations of this group. This discrepancy was not discussed by the authors,” Dr. Pearlman said.
At present, women’s risk of breast cancer is predicted using well-validated models that include the question of prior breast biopsies, such as the Gail Model, the Tyrer-Cuzick model (IBIS tool), and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm, Dr. Pearlman noted.
“This study, without further validation within a model, would not change risk assessment,” he said, disagreeing with the investigators’ conclusions. “What I would say is that further study to determine how to use this observation to decide if any change in screening or management should occur would be more appropriate.”
Study details
The 629,087 women studied underwent 2,327,384 screens, Dr. Román reported. In total, screening detected 9,184 cases of benign breast disease and 9,431 breast cancers.
Breast cancer was diagnosed in 2.4% and 3.0% of women with benign breast disease detected on prevalent and incident screens, respectively, compared with 1.5% of women without any benign breast disease detected.
Elevation of breast cancer risk varied across benign breast disease subtype. Relative to peers without any benign disease, risk was significantly elevated for women with nonproliferative disease (adjusted hazard ratio, 1.95), proliferative disease without atypia (aHR, 3.19), and proliferative disease with atypia (aHR, 3.82).
Similarly, elevation of risk varied depending on the screening at which the benign disease was detected. Risk was significantly elevated when the disease was found at prevalent screens (aHR, 1.87) and more so when it was found at incident screens (aHR, 2.67).
There was no significant interaction of these two factors (P = .83). However, when combinations were considered, risk was highest for women with proliferative benign breast disease with atypia detected on incident screens (aHR, 4.35) or prevalent screens (aHR, 3.35), and women with proliferative benign breast disease without atypia detected on incident screens (aHR, 3.83).
This study was supported by grants from Instituto de Salud Carlos III FEDER and by the Research Network on Health Services in Chronic Diseases. Dr. Román and Dr. Pearlman disclosed no conflicts of interest.
SOURCE: Román M et al. EBCC-12 Virtual Conference, Abstract 15.
“Benign breast disease is a key risk factor for breast cancer risk prediction,” commented presenting investigator Marta Román, PhD, of the Hospital del Mar Medical Research Institute in Barcelona. “Those women who have had a benign breast disease diagnosis have an increased risk that lasts for at least 20 years.”
To assess the combined influence of various attributes of benign breast disease, the investigators studied 629,087 women, aged 50-69 years, in Spain who underwent population-based mammographic breast cancer screening during 1994-2015 and did not have breast cancer at their prevalent (first) screen. The mean follow-up was 7.8 years.
Results showed that breast cancer risk was about three times higher for women with benign breast disease that was proliferative or that was detected on an incident screen, relative to peers with no benign breast disease. When combinations of factors were considered, breast cancer risk was most elevated – more than four times higher – for women with proliferative benign breast disease with atypia detected on an incident screen.
“We believe that these findings should be considered when discussing risk-based personalized screening strategies because these differences between prevalent and incident screens might be important if we want to personalize the screening, whether it’s the first time a woman comes to the screening program or a subsequent screen,” Dr. Román said.
Practice changing?
The study’s large size and population-based design, likely permitting capture of most biopsy results, are strengths, Mark David Pearlman, MD, of the University of Michigan, Ann Arbor, commented in an interview.
But its observational, retrospective nature opens the study up to biases, such as uncertainty as to how many women were symptomatic at the time of their mammogram and the likelihood of heightened monitoring after a biopsy showing hyperplasia, Dr. Pearlman cautioned.
“Moreover, the relative risk in this study for proliferative benign breast disease without atypia is substantially higher than prior observations of this group. This discrepancy was not discussed by the authors,” Dr. Pearlman said.
At present, women’s risk of breast cancer is predicted using well-validated models that include the question of prior breast biopsies, such as the Gail Model, the Tyrer-Cuzick model (IBIS tool), and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm, Dr. Pearlman noted.
“This study, without further validation within a model, would not change risk assessment,” he said, disagreeing with the investigators’ conclusions. “What I would say is that further study to determine how to use this observation to decide if any change in screening or management should occur would be more appropriate.”
Study details
The 629,087 women studied underwent 2,327,384 screens, Dr. Román reported. In total, screening detected 9,184 cases of benign breast disease and 9,431 breast cancers.
Breast cancer was diagnosed in 2.4% and 3.0% of women with benign breast disease detected on prevalent and incident screens, respectively, compared with 1.5% of women without any benign breast disease detected.
Elevation of breast cancer risk varied across benign breast disease subtype. Relative to peers without any benign disease, risk was significantly elevated for women with nonproliferative disease (adjusted hazard ratio, 1.95), proliferative disease without atypia (aHR, 3.19), and proliferative disease with atypia (aHR, 3.82).
Similarly, elevation of risk varied depending on the screening at which the benign disease was detected. Risk was significantly elevated when the disease was found at prevalent screens (aHR, 1.87) and more so when it was found at incident screens (aHR, 2.67).
There was no significant interaction of these two factors (P = .83). However, when combinations were considered, risk was highest for women with proliferative benign breast disease with atypia detected on incident screens (aHR, 4.35) or prevalent screens (aHR, 3.35), and women with proliferative benign breast disease without atypia detected on incident screens (aHR, 3.83).
This study was supported by grants from Instituto de Salud Carlos III FEDER and by the Research Network on Health Services in Chronic Diseases. Dr. Román and Dr. Pearlman disclosed no conflicts of interest.
SOURCE: Román M et al. EBCC-12 Virtual Conference, Abstract 15.
FROM EBCC-12 VIRTUAL CONFERENCE
Fauci: Cautious optimism for COVID-19 vaccine by end of 2020
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
FROM CHEST 2020
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021