User login
Two-stage surgery to reduce ovarian cancer risk piques interest
“Many physicians would assume that prevention of cancer, especially cancer as serious as ovarian cancer, trumps all other decision making, but when we really listen to high-risk women, they want to have options,” Karen Lu, MD, chair of gynecologic oncology and reproductive medicine at MD Anderson Cancer Center, Houston, Texas, told Medscape Medical News.
She was commenting on the findings from a UK survey conducted among women at an increased risk for ovarian cancer (OC), some of whom had already undergone salpingo-oophorectomy (RRSO), a standard risk-reducing surgery that involves removal of fallopian tubes and ovaries.
The survey found that these women were just as likely to consider an alternative two-stage surgical approach in which the fallopian tubes are removed but removal of the ovaries is delayed ― risk-reducing early salpingectomy with delayed oophorectomy (RRESDO).
In the survey, women were asked which option they would theorectically prefer. At present, the two-step surgery is recommended only within the context of a research trial (several of which are ongoing).
The UK survey was published online August 16 in the British Journal of Obstetrics and Gynaecology.
It found that premenopausal women concerned about the sexual dysfunction that can occur after RRSO were most likely to embrace the two-step surgery option.
The likelihood of finding this option acceptable was nearly three times higher among this subgroup of patients (odds ratio [RR], 2.9). It was more than five times higher among patients who had already undergone RRSO and had experienced sexual dysfunction after the surgery (OR, 5.3), the authors report.
These findings largely mirror those from a 2014 survey of US women, which set the stage for the Women Choosing Surgical Prevention (WISP) study.
The WISP investigators, led by Lu, are assessing quality-of-life outcomes related to sexual function with RRESDO vs RRSO.
Final results from the WISP study and from a similar Dutch study, TUBA, which is evaluating RRESDO’s effects on menopause-related quality of life, are anticipated in late 2020 or early 2021.
The investigators from both the WISP and the TUBA trials are planning a joint trial to evaluate the safety and efficacy of RRESDO, Lu told Medscape Medical News.
The PROTECTOR study, in the United Kingdom, is currently enrolling patients. Like WISP, its primary endpoint will be quality-of-life measures related to sexual function. The PROTECTOR trial will offer the option of RRESDO to the “large proportion of eligible women” who are interested in this two-stage approach, as evidenced by the UK survey, said Faiza Gaba, MBB, first author on the survey results. Gaba is affiliated with the Wolfson Institute of Preventive Medicine at the Queen Mary University of London and the Department of Gynaecological Oncology at St Bartholomew’s Hospital, London, United Kingdom.
Survey findings
The 39-item survey was offered from October 2017 to June 2019 at multiple clinics in the United Kingdom and to members of a support group for BRCA gene carriers. Of the 683 respondents, 346 had undergone RRSO and 337 had not. Those who had not were significantly younger (38.3 years vs 51.5 years); 262 were premenopausal.
Overall, 88.8% of the premenopausal and 95.2% of the postmenopausal women who had undergone RRSO were satisfied with their decision, but, respectively, 9.4% and 1.2% of these women regretted their decision.
More than half (55.3%) said they would consider participating in a study offering RRESDO, 20.2% said they wouldn’t consider it, and 24% weren’t sure.
Among the premenopausal respondents who had not undergone RRSO, 69.1% said they would consider it, and 30.9% said they would not.
Those wanting to delay hot flashes were five times more likely to find RRESDO acceptable (OR, 5.0).
Willingness to undergo RRESDO in a trial setting was also higher among those who considered it acceptable to undergo two surgeries (OR, 444.1), to undergo interval monitoring between surgeries (OR, 59.0), to have uncertainty about the level of OC risk reduction with RRESDO (OR, 14.6), and to potentially experience interval OC between the two surgeries (OR, 9.6).
Notably, 74.1% of the premenopausal RRSO patients used hormone replacement therapy (HRT), and most said it reduced symptoms of vaginal dryness. HRT use was not significantly associated with satisfaction or regret regarding decisions to undergo RRSO, the authors found.
Rather, the high regret rates among premenopausal women who underwent RRSO were driven largely by certain symptoms. Regret was highest among those who experienced night sweats (OR, 13.8), sleep disturbance (OR, 18.8), sexual dysfunction (OR, 5.3), or urinary incontinence (OR 17.2). More of those women than those who did not experience these symptoms said they regretted their decision (OR, 6.4) and that RRSO did them a lot of harm (OR, 3.9). These women were also significantly more likely to say they would have opted for RRESDO instead of RRSO had they been given the option, whereas those with hot flashes, osteoporosis, or fatigue after RRSO were less likely, retrospectively, to choose RRESDO.
The findings suggest “there is a range of tolerability and acceptability of various symptoms among women which affects surgical decision making,” the authors comment.
RRSO remains the gold standard for OC risk reduction, but about 10% of premenopausal women regret having undergone RRSO, mainly because of the menopausal sequelae, they note.
RRESDO could offer an alternative for relatively young women who wish to delay the onset of menopause, they suggest.
The approach is supported by evidence that most high-grade, serous OC originates in the fallopian tubes, meaning delayed oophorectomy with RRESDO may have a favorable risk-benefit profile for those wishing to avoid surgical menopause.
Preliminary reports from WISP and TUBA were presented at the annual meeting of the Society of Gynecologic Oncology in 2019. These initial results showed, as expected, that menopausal symptoms were worse with RRSO. This was true even among those who used HRT, WISP lead investigator Lu told Medscape Medical News.
She applauded the work by Gaba and colleagues, saying that the survey shows that women appreciate having options.
“It’s quite a daunting dilemma” for a woman at high risk but who is without cancer ― a “previvor” ― to be told that the standard-of-care recommendation is to undergo surgical menopause years earlier than would occur naturally, Lu added.
However, it is most important to know whether a given approach is safe and effective, and that’s where the joint international study planned by her team and the TUBA study investigators comes in.
“Acceptability is important; showing the impact on menopausal symptoms and sexual function is important,” she said. “But ultimately, we really need to know that [RRESDO] protects women from ovarian cancer.”
The UK survey was funded by a grant from Rosetrees Trust. Gaba and Lu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“Many physicians would assume that prevention of cancer, especially cancer as serious as ovarian cancer, trumps all other decision making, but when we really listen to high-risk women, they want to have options,” Karen Lu, MD, chair of gynecologic oncology and reproductive medicine at MD Anderson Cancer Center, Houston, Texas, told Medscape Medical News.
She was commenting on the findings from a UK survey conducted among women at an increased risk for ovarian cancer (OC), some of whom had already undergone salpingo-oophorectomy (RRSO), a standard risk-reducing surgery that involves removal of fallopian tubes and ovaries.
The survey found that these women were just as likely to consider an alternative two-stage surgical approach in which the fallopian tubes are removed but removal of the ovaries is delayed ― risk-reducing early salpingectomy with delayed oophorectomy (RRESDO).
In the survey, women were asked which option they would theorectically prefer. At present, the two-step surgery is recommended only within the context of a research trial (several of which are ongoing).
The UK survey was published online August 16 in the British Journal of Obstetrics and Gynaecology.
It found that premenopausal women concerned about the sexual dysfunction that can occur after RRSO were most likely to embrace the two-step surgery option.
The likelihood of finding this option acceptable was nearly three times higher among this subgroup of patients (odds ratio [RR], 2.9). It was more than five times higher among patients who had already undergone RRSO and had experienced sexual dysfunction after the surgery (OR, 5.3), the authors report.
These findings largely mirror those from a 2014 survey of US women, which set the stage for the Women Choosing Surgical Prevention (WISP) study.
The WISP investigators, led by Lu, are assessing quality-of-life outcomes related to sexual function with RRESDO vs RRSO.
Final results from the WISP study and from a similar Dutch study, TUBA, which is evaluating RRESDO’s effects on menopause-related quality of life, are anticipated in late 2020 or early 2021.
The investigators from both the WISP and the TUBA trials are planning a joint trial to evaluate the safety and efficacy of RRESDO, Lu told Medscape Medical News.
The PROTECTOR study, in the United Kingdom, is currently enrolling patients. Like WISP, its primary endpoint will be quality-of-life measures related to sexual function. The PROTECTOR trial will offer the option of RRESDO to the “large proportion of eligible women” who are interested in this two-stage approach, as evidenced by the UK survey, said Faiza Gaba, MBB, first author on the survey results. Gaba is affiliated with the Wolfson Institute of Preventive Medicine at the Queen Mary University of London and the Department of Gynaecological Oncology at St Bartholomew’s Hospital, London, United Kingdom.
Survey findings
The 39-item survey was offered from October 2017 to June 2019 at multiple clinics in the United Kingdom and to members of a support group for BRCA gene carriers. Of the 683 respondents, 346 had undergone RRSO and 337 had not. Those who had not were significantly younger (38.3 years vs 51.5 years); 262 were premenopausal.
Overall, 88.8% of the premenopausal and 95.2% of the postmenopausal women who had undergone RRSO were satisfied with their decision, but, respectively, 9.4% and 1.2% of these women regretted their decision.
More than half (55.3%) said they would consider participating in a study offering RRESDO, 20.2% said they wouldn’t consider it, and 24% weren’t sure.
Among the premenopausal respondents who had not undergone RRSO, 69.1% said they would consider it, and 30.9% said they would not.
Those wanting to delay hot flashes were five times more likely to find RRESDO acceptable (OR, 5.0).
Willingness to undergo RRESDO in a trial setting was also higher among those who considered it acceptable to undergo two surgeries (OR, 444.1), to undergo interval monitoring between surgeries (OR, 59.0), to have uncertainty about the level of OC risk reduction with RRESDO (OR, 14.6), and to potentially experience interval OC between the two surgeries (OR, 9.6).
Notably, 74.1% of the premenopausal RRSO patients used hormone replacement therapy (HRT), and most said it reduced symptoms of vaginal dryness. HRT use was not significantly associated with satisfaction or regret regarding decisions to undergo RRSO, the authors found.
Rather, the high regret rates among premenopausal women who underwent RRSO were driven largely by certain symptoms. Regret was highest among those who experienced night sweats (OR, 13.8), sleep disturbance (OR, 18.8), sexual dysfunction (OR, 5.3), or urinary incontinence (OR 17.2). More of those women than those who did not experience these symptoms said they regretted their decision (OR, 6.4) and that RRSO did them a lot of harm (OR, 3.9). These women were also significantly more likely to say they would have opted for RRESDO instead of RRSO had they been given the option, whereas those with hot flashes, osteoporosis, or fatigue after RRSO were less likely, retrospectively, to choose RRESDO.
The findings suggest “there is a range of tolerability and acceptability of various symptoms among women which affects surgical decision making,” the authors comment.
RRSO remains the gold standard for OC risk reduction, but about 10% of premenopausal women regret having undergone RRSO, mainly because of the menopausal sequelae, they note.
RRESDO could offer an alternative for relatively young women who wish to delay the onset of menopause, they suggest.
The approach is supported by evidence that most high-grade, serous OC originates in the fallopian tubes, meaning delayed oophorectomy with RRESDO may have a favorable risk-benefit profile for those wishing to avoid surgical menopause.
Preliminary reports from WISP and TUBA were presented at the annual meeting of the Society of Gynecologic Oncology in 2019. These initial results showed, as expected, that menopausal symptoms were worse with RRSO. This was true even among those who used HRT, WISP lead investigator Lu told Medscape Medical News.
She applauded the work by Gaba and colleagues, saying that the survey shows that women appreciate having options.
“It’s quite a daunting dilemma” for a woman at high risk but who is without cancer ― a “previvor” ― to be told that the standard-of-care recommendation is to undergo surgical menopause years earlier than would occur naturally, Lu added.
However, it is most important to know whether a given approach is safe and effective, and that’s where the joint international study planned by her team and the TUBA study investigators comes in.
“Acceptability is important; showing the impact on menopausal symptoms and sexual function is important,” she said. “But ultimately, we really need to know that [RRESDO] protects women from ovarian cancer.”
The UK survey was funded by a grant from Rosetrees Trust. Gaba and Lu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“Many physicians would assume that prevention of cancer, especially cancer as serious as ovarian cancer, trumps all other decision making, but when we really listen to high-risk women, they want to have options,” Karen Lu, MD, chair of gynecologic oncology and reproductive medicine at MD Anderson Cancer Center, Houston, Texas, told Medscape Medical News.
She was commenting on the findings from a UK survey conducted among women at an increased risk for ovarian cancer (OC), some of whom had already undergone salpingo-oophorectomy (RRSO), a standard risk-reducing surgery that involves removal of fallopian tubes and ovaries.
The survey found that these women were just as likely to consider an alternative two-stage surgical approach in which the fallopian tubes are removed but removal of the ovaries is delayed ― risk-reducing early salpingectomy with delayed oophorectomy (RRESDO).
In the survey, women were asked which option they would theorectically prefer. At present, the two-step surgery is recommended only within the context of a research trial (several of which are ongoing).
The UK survey was published online August 16 in the British Journal of Obstetrics and Gynaecology.
It found that premenopausal women concerned about the sexual dysfunction that can occur after RRSO were most likely to embrace the two-step surgery option.
The likelihood of finding this option acceptable was nearly three times higher among this subgroup of patients (odds ratio [RR], 2.9). It was more than five times higher among patients who had already undergone RRSO and had experienced sexual dysfunction after the surgery (OR, 5.3), the authors report.
These findings largely mirror those from a 2014 survey of US women, which set the stage for the Women Choosing Surgical Prevention (WISP) study.
The WISP investigators, led by Lu, are assessing quality-of-life outcomes related to sexual function with RRESDO vs RRSO.
Final results from the WISP study and from a similar Dutch study, TUBA, which is evaluating RRESDO’s effects on menopause-related quality of life, are anticipated in late 2020 or early 2021.
The investigators from both the WISP and the TUBA trials are planning a joint trial to evaluate the safety and efficacy of RRESDO, Lu told Medscape Medical News.
The PROTECTOR study, in the United Kingdom, is currently enrolling patients. Like WISP, its primary endpoint will be quality-of-life measures related to sexual function. The PROTECTOR trial will offer the option of RRESDO to the “large proportion of eligible women” who are interested in this two-stage approach, as evidenced by the UK survey, said Faiza Gaba, MBB, first author on the survey results. Gaba is affiliated with the Wolfson Institute of Preventive Medicine at the Queen Mary University of London and the Department of Gynaecological Oncology at St Bartholomew’s Hospital, London, United Kingdom.
Survey findings
The 39-item survey was offered from October 2017 to June 2019 at multiple clinics in the United Kingdom and to members of a support group for BRCA gene carriers. Of the 683 respondents, 346 had undergone RRSO and 337 had not. Those who had not were significantly younger (38.3 years vs 51.5 years); 262 were premenopausal.
Overall, 88.8% of the premenopausal and 95.2% of the postmenopausal women who had undergone RRSO were satisfied with their decision, but, respectively, 9.4% and 1.2% of these women regretted their decision.
More than half (55.3%) said they would consider participating in a study offering RRESDO, 20.2% said they wouldn’t consider it, and 24% weren’t sure.
Among the premenopausal respondents who had not undergone RRSO, 69.1% said they would consider it, and 30.9% said they would not.
Those wanting to delay hot flashes were five times more likely to find RRESDO acceptable (OR, 5.0).
Willingness to undergo RRESDO in a trial setting was also higher among those who considered it acceptable to undergo two surgeries (OR, 444.1), to undergo interval monitoring between surgeries (OR, 59.0), to have uncertainty about the level of OC risk reduction with RRESDO (OR, 14.6), and to potentially experience interval OC between the two surgeries (OR, 9.6).
Notably, 74.1% of the premenopausal RRSO patients used hormone replacement therapy (HRT), and most said it reduced symptoms of vaginal dryness. HRT use was not significantly associated with satisfaction or regret regarding decisions to undergo RRSO, the authors found.
Rather, the high regret rates among premenopausal women who underwent RRSO were driven largely by certain symptoms. Regret was highest among those who experienced night sweats (OR, 13.8), sleep disturbance (OR, 18.8), sexual dysfunction (OR, 5.3), or urinary incontinence (OR 17.2). More of those women than those who did not experience these symptoms said they regretted their decision (OR, 6.4) and that RRSO did them a lot of harm (OR, 3.9). These women were also significantly more likely to say they would have opted for RRESDO instead of RRSO had they been given the option, whereas those with hot flashes, osteoporosis, or fatigue after RRSO were less likely, retrospectively, to choose RRESDO.
The findings suggest “there is a range of tolerability and acceptability of various symptoms among women which affects surgical decision making,” the authors comment.
RRSO remains the gold standard for OC risk reduction, but about 10% of premenopausal women regret having undergone RRSO, mainly because of the menopausal sequelae, they note.
RRESDO could offer an alternative for relatively young women who wish to delay the onset of menopause, they suggest.
The approach is supported by evidence that most high-grade, serous OC originates in the fallopian tubes, meaning delayed oophorectomy with RRESDO may have a favorable risk-benefit profile for those wishing to avoid surgical menopause.
Preliminary reports from WISP and TUBA were presented at the annual meeting of the Society of Gynecologic Oncology in 2019. These initial results showed, as expected, that menopausal symptoms were worse with RRSO. This was true even among those who used HRT, WISP lead investigator Lu told Medscape Medical News.
She applauded the work by Gaba and colleagues, saying that the survey shows that women appreciate having options.
“It’s quite a daunting dilemma” for a woman at high risk but who is without cancer ― a “previvor” ― to be told that the standard-of-care recommendation is to undergo surgical menopause years earlier than would occur naturally, Lu added.
However, it is most important to know whether a given approach is safe and effective, and that’s where the joint international study planned by her team and the TUBA study investigators comes in.
“Acceptability is important; showing the impact on menopausal symptoms and sexual function is important,” she said. “But ultimately, we really need to know that [RRESDO] protects women from ovarian cancer.”
The UK survey was funded by a grant from Rosetrees Trust. Gaba and Lu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Scrubs ad that insulted women and DOs pulled after outcry
A video that advertised scrubs but denigrated women and DOs has been removed from the company’s website after fierce backlash.
On Tuesday Kevin Klauer, DO, EJD, directed this tweet to the medical uniform company Figs: “@wearfigs REMOVE YOUR DO offensive web ad immediately or the @AOAforDOs will proceed promptly with a defamation lawsuit on behalf of our members and profession.”
Also on Tuesday, the American Association of Colleges of Osteopathic Medicine demanded a public apology.
The video ad featured a woman carrying a “Medical Terminology for Dummies” book upside down while modeling the pink scrubs from all angles and dancing. At one point in the ad, the camera zooms in on the badge clipped to her waistband that read “DO.”
Agnieszka Solberg, MD, a vascular and interventional radiologist and assistant clinical professor at the University of North Dakota in Grand Forks, was among those voicing pointed criticism on social media.
“This was another hit for our DO colleagues,” she said in an interview, emphasizing that MDs and DOs provide the same level of care.
AACOM tweeted: “We are outraged women physicians & doctors of osteopathic medicine are still attacked in ignorant marketing campaigns. A company like @wearfigs should be ashamed for promoting these stereotypes. We demand the respect we’ve earned AND a public apology.”
Dr. Solberg says this is not the first offense by the company. She said she had stopped buying the company’s scrubs a year ago because the ads “have been portraying female providers as dumb and silly. This was the final straw.”
She said the timing of the ad is suspect as DOs had been swept into a storm of negativity earlier this month, as Medscape Medical News reported, when some questioned the qualifications of President Donald Trump’s physician, Sean Conley, who is a DO.
The scrubs ad ignited criticism across specialties, provider levels, and genders.
Jessica K. Willett, MD, tweeted: “As women physicians in 2020, we still struggle to be taken seriously compared to our male counterparts, as we battle stereotypes like THIS EXACT ONE. We expect the brands we support to reflect the badasses we are.”
The company responded to her tweet: “Thank you so much for the feedback! Totally not our intent – we’re taking down both the men’s and women’s versions of this ASAP! I really appreciate you taking the time to share this.”
The company did not respond to a request for comment but issued an apology on social media: “A lot of you guys have pointed out an insensitive video we had on our site – we are incredibly sorry for any hurt this has caused you, especially our female DOs (who are amazing!) FIGS is a female founded company whose only mission is to make you guys feel awesome.”
The Los Angeles–based company, which Forbes estimated will make $250 million in sales this year, was founded by co-CEOs Heather Hasson and Trina Spear.
A med student wrote on Twitter: “As a female and a DO student, how would I ever “feel awesome” about myself knowing that this is how you view me??? And how you want others to view me??? Women and DO’s have fought stereotypes way too long for you to go ahead and put this out there. Do better.”
Even the company’s apology was tinged with disrespect, some noted, with the use of “you guys” and for what it didn’t include.
As Liesl Young, MD, tweeted: “We are not “guys”, we are women. MD = DO. We stand together.”
Dr. Solberg said the apology came across as an apology that feelings were hurt. It should have detailed the changes the company would make to prevent another incident and address the processes that led to the video.
Dr. Solberg said she is seeing something positive come from the whole incident in that, “women are taking up the torch of feminism in such a volatile and divisive time.”
Dr. Solberg reported no relevant financial relationships.
This article first appeared on Medscape.com.
A video that advertised scrubs but denigrated women and DOs has been removed from the company’s website after fierce backlash.
On Tuesday Kevin Klauer, DO, EJD, directed this tweet to the medical uniform company Figs: “@wearfigs REMOVE YOUR DO offensive web ad immediately or the @AOAforDOs will proceed promptly with a defamation lawsuit on behalf of our members and profession.”
Also on Tuesday, the American Association of Colleges of Osteopathic Medicine demanded a public apology.
The video ad featured a woman carrying a “Medical Terminology for Dummies” book upside down while modeling the pink scrubs from all angles and dancing. At one point in the ad, the camera zooms in on the badge clipped to her waistband that read “DO.”
Agnieszka Solberg, MD, a vascular and interventional radiologist and assistant clinical professor at the University of North Dakota in Grand Forks, was among those voicing pointed criticism on social media.
“This was another hit for our DO colleagues,” she said in an interview, emphasizing that MDs and DOs provide the same level of care.
AACOM tweeted: “We are outraged women physicians & doctors of osteopathic medicine are still attacked in ignorant marketing campaigns. A company like @wearfigs should be ashamed for promoting these stereotypes. We demand the respect we’ve earned AND a public apology.”
Dr. Solberg says this is not the first offense by the company. She said she had stopped buying the company’s scrubs a year ago because the ads “have been portraying female providers as dumb and silly. This was the final straw.”
She said the timing of the ad is suspect as DOs had been swept into a storm of negativity earlier this month, as Medscape Medical News reported, when some questioned the qualifications of President Donald Trump’s physician, Sean Conley, who is a DO.
The scrubs ad ignited criticism across specialties, provider levels, and genders.
Jessica K. Willett, MD, tweeted: “As women physicians in 2020, we still struggle to be taken seriously compared to our male counterparts, as we battle stereotypes like THIS EXACT ONE. We expect the brands we support to reflect the badasses we are.”
The company responded to her tweet: “Thank you so much for the feedback! Totally not our intent – we’re taking down both the men’s and women’s versions of this ASAP! I really appreciate you taking the time to share this.”
The company did not respond to a request for comment but issued an apology on social media: “A lot of you guys have pointed out an insensitive video we had on our site – we are incredibly sorry for any hurt this has caused you, especially our female DOs (who are amazing!) FIGS is a female founded company whose only mission is to make you guys feel awesome.”
The Los Angeles–based company, which Forbes estimated will make $250 million in sales this year, was founded by co-CEOs Heather Hasson and Trina Spear.
A med student wrote on Twitter: “As a female and a DO student, how would I ever “feel awesome” about myself knowing that this is how you view me??? And how you want others to view me??? Women and DO’s have fought stereotypes way too long for you to go ahead and put this out there. Do better.”
Even the company’s apology was tinged with disrespect, some noted, with the use of “you guys” and for what it didn’t include.
As Liesl Young, MD, tweeted: “We are not “guys”, we are women. MD = DO. We stand together.”
Dr. Solberg said the apology came across as an apology that feelings were hurt. It should have detailed the changes the company would make to prevent another incident and address the processes that led to the video.
Dr. Solberg said she is seeing something positive come from the whole incident in that, “women are taking up the torch of feminism in such a volatile and divisive time.”
Dr. Solberg reported no relevant financial relationships.
This article first appeared on Medscape.com.
A video that advertised scrubs but denigrated women and DOs has been removed from the company’s website after fierce backlash.
On Tuesday Kevin Klauer, DO, EJD, directed this tweet to the medical uniform company Figs: “@wearfigs REMOVE YOUR DO offensive web ad immediately or the @AOAforDOs will proceed promptly with a defamation lawsuit on behalf of our members and profession.”
Also on Tuesday, the American Association of Colleges of Osteopathic Medicine demanded a public apology.
The video ad featured a woman carrying a “Medical Terminology for Dummies” book upside down while modeling the pink scrubs from all angles and dancing. At one point in the ad, the camera zooms in on the badge clipped to her waistband that read “DO.”
Agnieszka Solberg, MD, a vascular and interventional radiologist and assistant clinical professor at the University of North Dakota in Grand Forks, was among those voicing pointed criticism on social media.
“This was another hit for our DO colleagues,” she said in an interview, emphasizing that MDs and DOs provide the same level of care.
AACOM tweeted: “We are outraged women physicians & doctors of osteopathic medicine are still attacked in ignorant marketing campaigns. A company like @wearfigs should be ashamed for promoting these stereotypes. We demand the respect we’ve earned AND a public apology.”
Dr. Solberg says this is not the first offense by the company. She said she had stopped buying the company’s scrubs a year ago because the ads “have been portraying female providers as dumb and silly. This was the final straw.”
She said the timing of the ad is suspect as DOs had been swept into a storm of negativity earlier this month, as Medscape Medical News reported, when some questioned the qualifications of President Donald Trump’s physician, Sean Conley, who is a DO.
The scrubs ad ignited criticism across specialties, provider levels, and genders.
Jessica K. Willett, MD, tweeted: “As women physicians in 2020, we still struggle to be taken seriously compared to our male counterparts, as we battle stereotypes like THIS EXACT ONE. We expect the brands we support to reflect the badasses we are.”
The company responded to her tweet: “Thank you so much for the feedback! Totally not our intent – we’re taking down both the men’s and women’s versions of this ASAP! I really appreciate you taking the time to share this.”
The company did not respond to a request for comment but issued an apology on social media: “A lot of you guys have pointed out an insensitive video we had on our site – we are incredibly sorry for any hurt this has caused you, especially our female DOs (who are amazing!) FIGS is a female founded company whose only mission is to make you guys feel awesome.”
The Los Angeles–based company, which Forbes estimated will make $250 million in sales this year, was founded by co-CEOs Heather Hasson and Trina Spear.
A med student wrote on Twitter: “As a female and a DO student, how would I ever “feel awesome” about myself knowing that this is how you view me??? And how you want others to view me??? Women and DO’s have fought stereotypes way too long for you to go ahead and put this out there. Do better.”
Even the company’s apology was tinged with disrespect, some noted, with the use of “you guys” and for what it didn’t include.
As Liesl Young, MD, tweeted: “We are not “guys”, we are women. MD = DO. We stand together.”
Dr. Solberg said the apology came across as an apology that feelings were hurt. It should have detailed the changes the company would make to prevent another incident and address the processes that led to the video.
Dr. Solberg said she is seeing something positive come from the whole incident in that, “women are taking up the torch of feminism in such a volatile and divisive time.”
Dr. Solberg reported no relevant financial relationships.
This article first appeared on Medscape.com.
Lower TNF inhibitor efficacy observed in women with nonradiographic axSpA
according to results from a prospective cohort study.
Despite these similarities between the sexes, first author Regula Neuenschwander of the department of rheumatology at Zurich University Hospital and colleagues reported in Arthritis Research & Therapy that women treated with a TNF inhibitor were 81% less likely than men to have a 40% or greater improvement on Assessment of Spondyloarthritis International Society (ASAS) response criteria by 1 year. Statistically significant differences at baseline included women’s longer time to nr-axSpA diagnosis, slightly lower HLA-B27 positivity rate, higher mean baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, higher rate of current enthesitis, and lower mean body mass index (BMI).
With radiographic disease, women have been reported to more often “present with higher self-reported disease activity and functional impairment, a lower quality of life, less severe spinal radiographic changes, and more peripheral disease (arthritis and enthesitis),” whereas men more often have “objective markers of inflammation, such as elevated C-reactive protein (CRP) levels and magnetic resonance imaging (MRI) inflammation of the axial skeleton,” the researchers wrote. Radiographic disease also tends to occur more often in men, and some studies have reported men to have a greater response to TNF inhibitors. However, the current study sought to understand whether these differences between sexes exist in patients with nonradiographic disease.
The researchers included 495 patients (231 men and 264 women) with a clinical diagnosis of nr-axSpA in the Swiss Clinical Quality Management cohort during 2005-2018 who fulfilled ASAS classification criteria for axSpA and lacked definite radiographic sacroiliac joint changes according to the modified New York criteria. The radiographs were centrally digitized and independently scored in a blinded manner by a rotating group of two readers (out of six total).
Both women and men had a mean age of around 28 years at symptom onset, but women had a significantly longer diagnostic delay of 6.0 years vs. 4.7 years. Also, women were significantly less likely to be HLA-B27 positive (67.0% vs. 76.5%) and had a significantly higher mean BASDAI score at baseline (5.3 vs. 4.6). More women than men also showed signs of current enthesitis (79.6% vs. 64.0%), and women had a lower mean BMI (24.3 vs. 25.7 kg/m2). Concomitant clinically diagnosed fibromyalgia was higher in women than in men (13.1% vs. 2.7%), and when patients with fibromylagia (n = 25) were excluded the remaining differences in BASDAI were mainly because of fatigue and enthesitis, both of which occurred more often in women than in men.
A total of 163 patients without fibromyalgia started a first TNF inhibitor, and 120 had a follow-up visit at 1 year. An ASAS40 response is defined as 40% improvement in at least three of four domains on the ASAS response criteria: patient global assessment of disease activity for the past week, patient assessment of back pain over the past week, function (as assessed on the Bath Ankylosing Spondylitis Functional Index [BASFI]), and inflammation (mean of BASDAI questions 5 and 6). An ASAS40 response was achieved by 17% of women and 38% of men (odds ratio, 0.34; 95% confidence interval, 0.12-0.93), and this difference became more pronounced after adjustment for baseline differences in BASDAI, Maastricht Ankylosing Spondylitis Enthesitis Score, BMI, and diagnostic delay (OR, 0.19; 95% CI, 0.05-0.61). ASAS40 response rates were lower for patients with higher BMI but better for those with higher BASDAI levels. The researchers found comparable results when they excluded patients who stopped a TNF inhibitor because of other reasons for discontinuation and also when they counted patients who discontinued the TNF inhibitor because of remission as responders.
The sex difference in nr-axSpA patients’ treatment response to TNF inhibitors was even larger than the 56% lower odds the same group of researchers reported finding between women and men with radiographic disease in an earlier report, according to the new paper.
Given that this study and others in nr-axSpA patients have found higher remission rates to TNF inhibitor therapy in men versus women, the “current study therefore adds to available data to support the claim for future randomized controlled trials in axSpA to be sufficiently powered to detect potential sex differences,” the researchers said.
The authors acknowledged that a lack of MRI scans available for central scoring made it impossible to evaluate potential imaging misinterpretation, such as possible abnormalities mimicking mild sacroiliitis that have been reported to be more prevalent in women. It is also possible that some patients with fibromyalgia were missed because of screening for the condition by expert opinion of the treating rheumatologist “on a comorbidity questionnaire and not through fulfillment of classification criteria for fibromyalgia or via the use of a standardized fibromyalgia questionnaire,” they said.
The study was funded by the Stiftung für Rheumaforschung in Zurich. The Swiss Clinical Quality Management Foundation is supported by the Swiss Society of Rheumatology and by 11 pharmaceutical companies. Two study authors reported receiving consulting and/or speaking fees from some of those same companies.
SOURCE: Neuenschwander R et al. Arthritis Res Ther. 2020;22(1):233.
according to results from a prospective cohort study.
Despite these similarities between the sexes, first author Regula Neuenschwander of the department of rheumatology at Zurich University Hospital and colleagues reported in Arthritis Research & Therapy that women treated with a TNF inhibitor were 81% less likely than men to have a 40% or greater improvement on Assessment of Spondyloarthritis International Society (ASAS) response criteria by 1 year. Statistically significant differences at baseline included women’s longer time to nr-axSpA diagnosis, slightly lower HLA-B27 positivity rate, higher mean baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, higher rate of current enthesitis, and lower mean body mass index (BMI).
With radiographic disease, women have been reported to more often “present with higher self-reported disease activity and functional impairment, a lower quality of life, less severe spinal radiographic changes, and more peripheral disease (arthritis and enthesitis),” whereas men more often have “objective markers of inflammation, such as elevated C-reactive protein (CRP) levels and magnetic resonance imaging (MRI) inflammation of the axial skeleton,” the researchers wrote. Radiographic disease also tends to occur more often in men, and some studies have reported men to have a greater response to TNF inhibitors. However, the current study sought to understand whether these differences between sexes exist in patients with nonradiographic disease.
The researchers included 495 patients (231 men and 264 women) with a clinical diagnosis of nr-axSpA in the Swiss Clinical Quality Management cohort during 2005-2018 who fulfilled ASAS classification criteria for axSpA and lacked definite radiographic sacroiliac joint changes according to the modified New York criteria. The radiographs were centrally digitized and independently scored in a blinded manner by a rotating group of two readers (out of six total).
Both women and men had a mean age of around 28 years at symptom onset, but women had a significantly longer diagnostic delay of 6.0 years vs. 4.7 years. Also, women were significantly less likely to be HLA-B27 positive (67.0% vs. 76.5%) and had a significantly higher mean BASDAI score at baseline (5.3 vs. 4.6). More women than men also showed signs of current enthesitis (79.6% vs. 64.0%), and women had a lower mean BMI (24.3 vs. 25.7 kg/m2). Concomitant clinically diagnosed fibromyalgia was higher in women than in men (13.1% vs. 2.7%), and when patients with fibromylagia (n = 25) were excluded the remaining differences in BASDAI were mainly because of fatigue and enthesitis, both of which occurred more often in women than in men.
A total of 163 patients without fibromyalgia started a first TNF inhibitor, and 120 had a follow-up visit at 1 year. An ASAS40 response is defined as 40% improvement in at least three of four domains on the ASAS response criteria: patient global assessment of disease activity for the past week, patient assessment of back pain over the past week, function (as assessed on the Bath Ankylosing Spondylitis Functional Index [BASFI]), and inflammation (mean of BASDAI questions 5 and 6). An ASAS40 response was achieved by 17% of women and 38% of men (odds ratio, 0.34; 95% confidence interval, 0.12-0.93), and this difference became more pronounced after adjustment for baseline differences in BASDAI, Maastricht Ankylosing Spondylitis Enthesitis Score, BMI, and diagnostic delay (OR, 0.19; 95% CI, 0.05-0.61). ASAS40 response rates were lower for patients with higher BMI but better for those with higher BASDAI levels. The researchers found comparable results when they excluded patients who stopped a TNF inhibitor because of other reasons for discontinuation and also when they counted patients who discontinued the TNF inhibitor because of remission as responders.
The sex difference in nr-axSpA patients’ treatment response to TNF inhibitors was even larger than the 56% lower odds the same group of researchers reported finding between women and men with radiographic disease in an earlier report, according to the new paper.
Given that this study and others in nr-axSpA patients have found higher remission rates to TNF inhibitor therapy in men versus women, the “current study therefore adds to available data to support the claim for future randomized controlled trials in axSpA to be sufficiently powered to detect potential sex differences,” the researchers said.
The authors acknowledged that a lack of MRI scans available for central scoring made it impossible to evaluate potential imaging misinterpretation, such as possible abnormalities mimicking mild sacroiliitis that have been reported to be more prevalent in women. It is also possible that some patients with fibromyalgia were missed because of screening for the condition by expert opinion of the treating rheumatologist “on a comorbidity questionnaire and not through fulfillment of classification criteria for fibromyalgia or via the use of a standardized fibromyalgia questionnaire,” they said.
The study was funded by the Stiftung für Rheumaforschung in Zurich. The Swiss Clinical Quality Management Foundation is supported by the Swiss Society of Rheumatology and by 11 pharmaceutical companies. Two study authors reported receiving consulting and/or speaking fees from some of those same companies.
SOURCE: Neuenschwander R et al. Arthritis Res Ther. 2020;22(1):233.
according to results from a prospective cohort study.
Despite these similarities between the sexes, first author Regula Neuenschwander of the department of rheumatology at Zurich University Hospital and colleagues reported in Arthritis Research & Therapy that women treated with a TNF inhibitor were 81% less likely than men to have a 40% or greater improvement on Assessment of Spondyloarthritis International Society (ASAS) response criteria by 1 year. Statistically significant differences at baseline included women’s longer time to nr-axSpA diagnosis, slightly lower HLA-B27 positivity rate, higher mean baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, higher rate of current enthesitis, and lower mean body mass index (BMI).
With radiographic disease, women have been reported to more often “present with higher self-reported disease activity and functional impairment, a lower quality of life, less severe spinal radiographic changes, and more peripheral disease (arthritis and enthesitis),” whereas men more often have “objective markers of inflammation, such as elevated C-reactive protein (CRP) levels and magnetic resonance imaging (MRI) inflammation of the axial skeleton,” the researchers wrote. Radiographic disease also tends to occur more often in men, and some studies have reported men to have a greater response to TNF inhibitors. However, the current study sought to understand whether these differences between sexes exist in patients with nonradiographic disease.
The researchers included 495 patients (231 men and 264 women) with a clinical diagnosis of nr-axSpA in the Swiss Clinical Quality Management cohort during 2005-2018 who fulfilled ASAS classification criteria for axSpA and lacked definite radiographic sacroiliac joint changes according to the modified New York criteria. The radiographs were centrally digitized and independently scored in a blinded manner by a rotating group of two readers (out of six total).
Both women and men had a mean age of around 28 years at symptom onset, but women had a significantly longer diagnostic delay of 6.0 years vs. 4.7 years. Also, women were significantly less likely to be HLA-B27 positive (67.0% vs. 76.5%) and had a significantly higher mean BASDAI score at baseline (5.3 vs. 4.6). More women than men also showed signs of current enthesitis (79.6% vs. 64.0%), and women had a lower mean BMI (24.3 vs. 25.7 kg/m2). Concomitant clinically diagnosed fibromyalgia was higher in women than in men (13.1% vs. 2.7%), and when patients with fibromylagia (n = 25) were excluded the remaining differences in BASDAI were mainly because of fatigue and enthesitis, both of which occurred more often in women than in men.
A total of 163 patients without fibromyalgia started a first TNF inhibitor, and 120 had a follow-up visit at 1 year. An ASAS40 response is defined as 40% improvement in at least three of four domains on the ASAS response criteria: patient global assessment of disease activity for the past week, patient assessment of back pain over the past week, function (as assessed on the Bath Ankylosing Spondylitis Functional Index [BASFI]), and inflammation (mean of BASDAI questions 5 and 6). An ASAS40 response was achieved by 17% of women and 38% of men (odds ratio, 0.34; 95% confidence interval, 0.12-0.93), and this difference became more pronounced after adjustment for baseline differences in BASDAI, Maastricht Ankylosing Spondylitis Enthesitis Score, BMI, and diagnostic delay (OR, 0.19; 95% CI, 0.05-0.61). ASAS40 response rates were lower for patients with higher BMI but better for those with higher BASDAI levels. The researchers found comparable results when they excluded patients who stopped a TNF inhibitor because of other reasons for discontinuation and also when they counted patients who discontinued the TNF inhibitor because of remission as responders.
The sex difference in nr-axSpA patients’ treatment response to TNF inhibitors was even larger than the 56% lower odds the same group of researchers reported finding between women and men with radiographic disease in an earlier report, according to the new paper.
Given that this study and others in nr-axSpA patients have found higher remission rates to TNF inhibitor therapy in men versus women, the “current study therefore adds to available data to support the claim for future randomized controlled trials in axSpA to be sufficiently powered to detect potential sex differences,” the researchers said.
The authors acknowledged that a lack of MRI scans available for central scoring made it impossible to evaluate potential imaging misinterpretation, such as possible abnormalities mimicking mild sacroiliitis that have been reported to be more prevalent in women. It is also possible that some patients with fibromyalgia were missed because of screening for the condition by expert opinion of the treating rheumatologist “on a comorbidity questionnaire and not through fulfillment of classification criteria for fibromyalgia or via the use of a standardized fibromyalgia questionnaire,” they said.
The study was funded by the Stiftung für Rheumaforschung in Zurich. The Swiss Clinical Quality Management Foundation is supported by the Swiss Society of Rheumatology and by 11 pharmaceutical companies. Two study authors reported receiving consulting and/or speaking fees from some of those same companies.
SOURCE: Neuenschwander R et al. Arthritis Res Ther. 2020;22(1):233.
FROM ARTHRITIS RESEARCH & THERAPY
NMOSD challenges in children
, but have led to some uncertainty and confusion as well.
At the2020 CNS-ICNA Conjoint Meeting, held virtually this year, presenters discussed some of the challenges of differential diagnosis and treatment choice in pediatric NMOSD, which is easily confused with multiple sclerosis.
NMOSD used to be considered a monophasic disease restricted to the optic nerve and spinal cord, but is now known to affect other regions of the central nervous system and to relapse in some patients.
Diagnosis
The disease is often mediated by antibodies to the aquaporin-4 (AQP-4) water channel, but about 30% of adult patients lack the antibody, and AQP-4 seronegativity is more common in the pediatric population. Another common antibody found in 40%–50% of children with NMOSD targets myelin oligodendrocyte glycoprotein (MOG).
It is important to be aware that false negatives can occur in serology assays, and false positives are common, particularly in ELISA assays, Silvia N. Tenembaum, MD, said during her presentation. For those reasons, serology is not enough for a diagnosis. “Patients should also have compatible symptoms and MRI findings,” said Dr. Tenembaum, director of the pediatric neuroimmunology program at National Pediatric Hospital in Buenos Aires.
According to international consensus criteria, to be diagnosed with NMOSD, AQP-4 seropositive patients should also have at least one core clinical symptom: optic neuritis, acute myelitis, area postrema syndrome, other acute brainstem syndrome, symptomatic narcolepsy or acute diencephalic clinical syndrome, or symptomatic cerebral syndrome. AQP-4 seronegative patients or with unknown status should have at least two core symptoms, one of which must be optic neuritis, acute myelitis, or area postrema syndrome. Both conventional MRI and advanced new techniques are important for achieving differential diagnosis.
The most common symptom in children is optic neuritis, which occurs in 50%-70% of patients. Cerebral syndromes with or without encephalopathy and large tumefactive white matter lesions are also common, according to Dr. Tenembaum.
There are many conditions that mimic the spinal cord and optic nerve symptoms of NMOSD, which must be ruled out. One example is optic myelopathy and vision loss from late-onset biotinylase deficiency. It is critical to rule that out because it is treatable with supplements. Optic neuropathy, papillitis, and papilledema can also resemble NMOSD.
It is critical to achieve an early diagnosis of NMOSD in children, because some MS drugs can worsen NMOSD, according to Thaís Armangue, MD, PhD, head of neuroimmunology at SJD Barcelona Children’s Hospital, who also presented at the session. She pointed out that the MOG antibody, while common in children, is also associated with many demyelinating diseases. Some 50%-60% of children with acute disseminated encephalomyelitis (ADEM) have high titers of MOG antibodies. Although early studies suggested that persistent anti-MOG antibodies were associated with risk of developing MS, more recent studies show it predicts a non-MS disease course, particularly at titers greater than 1:1280, according to Dr. Tenembaum. Persistent anti-MOG antibodies are also associated with relapsing disease, but it is associated with other syndromes besides NMOSD. “The probability is that [MOG antibodies are] useful, but they cannot guide chronic immunotherapy, because even monophasic patients can last maybe 12 months before they become MOG negative, and we cannot wait so many months” to determine treatment course, said Dr. Tenembaum.
For monophasic ADEM or NMOSD, there is no need for chronic treatment. But children with MS and recurrent NMOSD require early chronic immunotherapy because specific therapies have been shown to improve prognosis.
Acute treatment
When it comes to acute treatment of NMOSD, the goal is to suppress the inflammatory attack but also to minimize long-term damage and optimize long-term neurological function. “The potential for irreversible injury with an attack is very high, and cumulative disabilities in NMOSD can result directly from attacks,” E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children at the University of Toronto, said during her talk.
IV steroids are generally the first choice, with a preference for methylprednisolone. Pediatric patients that are MOG antibody positive usually respond better and more quickly than do adults, with rapid daily improvements in mobility, vomiting, and eyesight. Dr. Yeh recommends weaning good responders off steroids because AQP-4 positive patients are likely to relapse without a steroid wean, and antibody testing may be unavailable or results may be delayed. The wean can range from 4 weeks to 4-6 months, depending on antibody status, likelihood of AQP-4 positivity, and clinical parameters.
Inadequate responses are usually pretty evident. If there is only light perception by day 4 or 5, or paralyzed patients are nonambulatory and achieve only twitchy movements by that time, second-line therapies should be considered, including therapeutic plasma exchange (TPE) with 5-7 exchanges or intravenous immunoglobulins (IVIg).
Dr. Yeh called for quick treatment. Whatever you do, “please do it sooner rather than later if you think there’s no response [to steroids],” Dr. Yeh said.
TPE is the first choice, according to Dr. Yeh. “There seems to be a fair amount of information that suggests that if you’re having difficulty getting a response to steroids, TPE can make a difference in these patients,” she said. But in some cases TPE may not be available, and IVIg can be attempted first. If it achieves no or only marginal improvement, TPE can be attempted later, but it must be kept in mind that TPE conducted too soon could wash out IVIg. Patients who get much better on IVIg can undergo a steroid wean, and then be evaluated for prophylactic therapy, said Dr. Yeh.
The evidence for IVIg is limited, reflecting the difficulty of studying treatments in rare populations. Still, when TPE is not available and the patient is quite impaired, IVIg makes sense to try. “Absence of evidence does not mean that the therapy doesn’t work, and I don’t think we should throw out the baby with the bath water,” said Dr. Yeh.
Although IVIg treatment is generally well tolerated, there have been a few serious adverse events, such as anaphylactic shock and aseptic meningitis, according to Andrea Savransky, MD, a pediatrician at National Pediatric Hospital in Buenos Aires, who also spoke at the session. “I think it is important to weigh the benefits against the risk,” Dr. Savransky said. She noted that TPE should not be taken lightly. One study showed more complications in pediatric patients than in adult patients, and it must be performed in specialized centers.
Emerging treaments
Tanuja Chitnis, MD, director of the Partners Pediatric MS Center at Massachusetts General Hospital, Boston, discussed some of the emerging treatments for pediatric NMOSD. Rituximab has been associated with success in some retrospective studies, but dosing should be personalized. Dr. Chitnis reported that B cells can return before 6 months, so she monitors B cells beginning 2 months after induction, redosing after 4 or 5 months rather than 6 if B cells return.
Nevertheless, relapses can still occur after rituximab therapy. “There is room for additional therapies to address this gap,” said Dr. Chitnis. Three new antibodies have received approval for treatment of NMOSD in adults. These include the complement inhibitor eculizumab, the IL-6 receptor antibody satralizumab, and the anti-CD19 antibody inebilizumab. Phase 3 clinical trials in children have been conducted for eculizumab and are in the planning stage for inebilizumab, and pediatric patients were included in pivotal trials for satralizumab.
Eculizumab treatment resulted in a 94.2% reduction in relapse risk in AQP4-positive adults. Satralizumab showed a 79% reduction in relapse risk among AQP-4 positive subjects with NMOSD or neuromyelitis optica and a 34% reduction in those who were AQP-4 negative. The pediatric subgroup had similar levels of response to adults, though the numbers were too small for a subgroup analysis.
In AQP-4 positive patients, inebilizumab treatment yielded a 77% reduction in relapse rate. In all patients, there was a 73% reduction.
For MOG antibody-positive patients with AQP-4 negative disease, novel therapies are at earlier stages of development. Typical MS therapies such as interferon beta and glatiramer acetate don’t seem to be effective. Some that have shown signs of efficacy include azathioprine, mycophenylate mofetil, rituximab, and IVIg infusion, but the state of the field is not encouraging. “This is an observation now being studied in larger cohorts, but in general I have not found that there’s a very strong response to any of these therapies, possibly with the exception of IVIg,” said Dr. Chitnis.
Dr. Tenembaum has no relevant financial disclosures. Dr. Armangue has received speaking honoraria from Novartis and travel expenses for scientific meetings from Merck, Biogen, and Roche. Dr. Yeh is on the scientific advisory board of Juno Therapeutics and has received research support from Biogen. Dr. Chitnis advises Biogen-Idec, Novartis, and Alexion, serves on clinical trial advisory boards for Novartis and Sanofi Aventis, and has received research support from Verily, EMD Serono, and Novartis. Dr. Savransky has received honoraria from Genzyme de Argentina SA.
, but have led to some uncertainty and confusion as well.
At the2020 CNS-ICNA Conjoint Meeting, held virtually this year, presenters discussed some of the challenges of differential diagnosis and treatment choice in pediatric NMOSD, which is easily confused with multiple sclerosis.
NMOSD used to be considered a monophasic disease restricted to the optic nerve and spinal cord, but is now known to affect other regions of the central nervous system and to relapse in some patients.
Diagnosis
The disease is often mediated by antibodies to the aquaporin-4 (AQP-4) water channel, but about 30% of adult patients lack the antibody, and AQP-4 seronegativity is more common in the pediatric population. Another common antibody found in 40%–50% of children with NMOSD targets myelin oligodendrocyte glycoprotein (MOG).
It is important to be aware that false negatives can occur in serology assays, and false positives are common, particularly in ELISA assays, Silvia N. Tenembaum, MD, said during her presentation. For those reasons, serology is not enough for a diagnosis. “Patients should also have compatible symptoms and MRI findings,” said Dr. Tenembaum, director of the pediatric neuroimmunology program at National Pediatric Hospital in Buenos Aires.
According to international consensus criteria, to be diagnosed with NMOSD, AQP-4 seropositive patients should also have at least one core clinical symptom: optic neuritis, acute myelitis, area postrema syndrome, other acute brainstem syndrome, symptomatic narcolepsy or acute diencephalic clinical syndrome, or symptomatic cerebral syndrome. AQP-4 seronegative patients or with unknown status should have at least two core symptoms, one of which must be optic neuritis, acute myelitis, or area postrema syndrome. Both conventional MRI and advanced new techniques are important for achieving differential diagnosis.
The most common symptom in children is optic neuritis, which occurs in 50%-70% of patients. Cerebral syndromes with or without encephalopathy and large tumefactive white matter lesions are also common, according to Dr. Tenembaum.
There are many conditions that mimic the spinal cord and optic nerve symptoms of NMOSD, which must be ruled out. One example is optic myelopathy and vision loss from late-onset biotinylase deficiency. It is critical to rule that out because it is treatable with supplements. Optic neuropathy, papillitis, and papilledema can also resemble NMOSD.
It is critical to achieve an early diagnosis of NMOSD in children, because some MS drugs can worsen NMOSD, according to Thaís Armangue, MD, PhD, head of neuroimmunology at SJD Barcelona Children’s Hospital, who also presented at the session. She pointed out that the MOG antibody, while common in children, is also associated with many demyelinating diseases. Some 50%-60% of children with acute disseminated encephalomyelitis (ADEM) have high titers of MOG antibodies. Although early studies suggested that persistent anti-MOG antibodies were associated with risk of developing MS, more recent studies show it predicts a non-MS disease course, particularly at titers greater than 1:1280, according to Dr. Tenembaum. Persistent anti-MOG antibodies are also associated with relapsing disease, but it is associated with other syndromes besides NMOSD. “The probability is that [MOG antibodies are] useful, but they cannot guide chronic immunotherapy, because even monophasic patients can last maybe 12 months before they become MOG negative, and we cannot wait so many months” to determine treatment course, said Dr. Tenembaum.
For monophasic ADEM or NMOSD, there is no need for chronic treatment. But children with MS and recurrent NMOSD require early chronic immunotherapy because specific therapies have been shown to improve prognosis.
Acute treatment
When it comes to acute treatment of NMOSD, the goal is to suppress the inflammatory attack but also to minimize long-term damage and optimize long-term neurological function. “The potential for irreversible injury with an attack is very high, and cumulative disabilities in NMOSD can result directly from attacks,” E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children at the University of Toronto, said during her talk.
IV steroids are generally the first choice, with a preference for methylprednisolone. Pediatric patients that are MOG antibody positive usually respond better and more quickly than do adults, with rapid daily improvements in mobility, vomiting, and eyesight. Dr. Yeh recommends weaning good responders off steroids because AQP-4 positive patients are likely to relapse without a steroid wean, and antibody testing may be unavailable or results may be delayed. The wean can range from 4 weeks to 4-6 months, depending on antibody status, likelihood of AQP-4 positivity, and clinical parameters.
Inadequate responses are usually pretty evident. If there is only light perception by day 4 or 5, or paralyzed patients are nonambulatory and achieve only twitchy movements by that time, second-line therapies should be considered, including therapeutic plasma exchange (TPE) with 5-7 exchanges or intravenous immunoglobulins (IVIg).
Dr. Yeh called for quick treatment. Whatever you do, “please do it sooner rather than later if you think there’s no response [to steroids],” Dr. Yeh said.
TPE is the first choice, according to Dr. Yeh. “There seems to be a fair amount of information that suggests that if you’re having difficulty getting a response to steroids, TPE can make a difference in these patients,” she said. But in some cases TPE may not be available, and IVIg can be attempted first. If it achieves no or only marginal improvement, TPE can be attempted later, but it must be kept in mind that TPE conducted too soon could wash out IVIg. Patients who get much better on IVIg can undergo a steroid wean, and then be evaluated for prophylactic therapy, said Dr. Yeh.
The evidence for IVIg is limited, reflecting the difficulty of studying treatments in rare populations. Still, when TPE is not available and the patient is quite impaired, IVIg makes sense to try. “Absence of evidence does not mean that the therapy doesn’t work, and I don’t think we should throw out the baby with the bath water,” said Dr. Yeh.
Although IVIg treatment is generally well tolerated, there have been a few serious adverse events, such as anaphylactic shock and aseptic meningitis, according to Andrea Savransky, MD, a pediatrician at National Pediatric Hospital in Buenos Aires, who also spoke at the session. “I think it is important to weigh the benefits against the risk,” Dr. Savransky said. She noted that TPE should not be taken lightly. One study showed more complications in pediatric patients than in adult patients, and it must be performed in specialized centers.
Emerging treaments
Tanuja Chitnis, MD, director of the Partners Pediatric MS Center at Massachusetts General Hospital, Boston, discussed some of the emerging treatments for pediatric NMOSD. Rituximab has been associated with success in some retrospective studies, but dosing should be personalized. Dr. Chitnis reported that B cells can return before 6 months, so she monitors B cells beginning 2 months after induction, redosing after 4 or 5 months rather than 6 if B cells return.
Nevertheless, relapses can still occur after rituximab therapy. “There is room for additional therapies to address this gap,” said Dr. Chitnis. Three new antibodies have received approval for treatment of NMOSD in adults. These include the complement inhibitor eculizumab, the IL-6 receptor antibody satralizumab, and the anti-CD19 antibody inebilizumab. Phase 3 clinical trials in children have been conducted for eculizumab and are in the planning stage for inebilizumab, and pediatric patients were included in pivotal trials for satralizumab.
Eculizumab treatment resulted in a 94.2% reduction in relapse risk in AQP4-positive adults. Satralizumab showed a 79% reduction in relapse risk among AQP-4 positive subjects with NMOSD or neuromyelitis optica and a 34% reduction in those who were AQP-4 negative. The pediatric subgroup had similar levels of response to adults, though the numbers were too small for a subgroup analysis.
In AQP-4 positive patients, inebilizumab treatment yielded a 77% reduction in relapse rate. In all patients, there was a 73% reduction.
For MOG antibody-positive patients with AQP-4 negative disease, novel therapies are at earlier stages of development. Typical MS therapies such as interferon beta and glatiramer acetate don’t seem to be effective. Some that have shown signs of efficacy include azathioprine, mycophenylate mofetil, rituximab, and IVIg infusion, but the state of the field is not encouraging. “This is an observation now being studied in larger cohorts, but in general I have not found that there’s a very strong response to any of these therapies, possibly with the exception of IVIg,” said Dr. Chitnis.
Dr. Tenembaum has no relevant financial disclosures. Dr. Armangue has received speaking honoraria from Novartis and travel expenses for scientific meetings from Merck, Biogen, and Roche. Dr. Yeh is on the scientific advisory board of Juno Therapeutics and has received research support from Biogen. Dr. Chitnis advises Biogen-Idec, Novartis, and Alexion, serves on clinical trial advisory boards for Novartis and Sanofi Aventis, and has received research support from Verily, EMD Serono, and Novartis. Dr. Savransky has received honoraria from Genzyme de Argentina SA.
, but have led to some uncertainty and confusion as well.
At the2020 CNS-ICNA Conjoint Meeting, held virtually this year, presenters discussed some of the challenges of differential diagnosis and treatment choice in pediatric NMOSD, which is easily confused with multiple sclerosis.
NMOSD used to be considered a monophasic disease restricted to the optic nerve and spinal cord, but is now known to affect other regions of the central nervous system and to relapse in some patients.
Diagnosis
The disease is often mediated by antibodies to the aquaporin-4 (AQP-4) water channel, but about 30% of adult patients lack the antibody, and AQP-4 seronegativity is more common in the pediatric population. Another common antibody found in 40%–50% of children with NMOSD targets myelin oligodendrocyte glycoprotein (MOG).
It is important to be aware that false negatives can occur in serology assays, and false positives are common, particularly in ELISA assays, Silvia N. Tenembaum, MD, said during her presentation. For those reasons, serology is not enough for a diagnosis. “Patients should also have compatible symptoms and MRI findings,” said Dr. Tenembaum, director of the pediatric neuroimmunology program at National Pediatric Hospital in Buenos Aires.
According to international consensus criteria, to be diagnosed with NMOSD, AQP-4 seropositive patients should also have at least one core clinical symptom: optic neuritis, acute myelitis, area postrema syndrome, other acute brainstem syndrome, symptomatic narcolepsy or acute diencephalic clinical syndrome, or symptomatic cerebral syndrome. AQP-4 seronegative patients or with unknown status should have at least two core symptoms, one of which must be optic neuritis, acute myelitis, or area postrema syndrome. Both conventional MRI and advanced new techniques are important for achieving differential diagnosis.
The most common symptom in children is optic neuritis, which occurs in 50%-70% of patients. Cerebral syndromes with or without encephalopathy and large tumefactive white matter lesions are also common, according to Dr. Tenembaum.
There are many conditions that mimic the spinal cord and optic nerve symptoms of NMOSD, which must be ruled out. One example is optic myelopathy and vision loss from late-onset biotinylase deficiency. It is critical to rule that out because it is treatable with supplements. Optic neuropathy, papillitis, and papilledema can also resemble NMOSD.
It is critical to achieve an early diagnosis of NMOSD in children, because some MS drugs can worsen NMOSD, according to Thaís Armangue, MD, PhD, head of neuroimmunology at SJD Barcelona Children’s Hospital, who also presented at the session. She pointed out that the MOG antibody, while common in children, is also associated with many demyelinating diseases. Some 50%-60% of children with acute disseminated encephalomyelitis (ADEM) have high titers of MOG antibodies. Although early studies suggested that persistent anti-MOG antibodies were associated with risk of developing MS, more recent studies show it predicts a non-MS disease course, particularly at titers greater than 1:1280, according to Dr. Tenembaum. Persistent anti-MOG antibodies are also associated with relapsing disease, but it is associated with other syndromes besides NMOSD. “The probability is that [MOG antibodies are] useful, but they cannot guide chronic immunotherapy, because even monophasic patients can last maybe 12 months before they become MOG negative, and we cannot wait so many months” to determine treatment course, said Dr. Tenembaum.
For monophasic ADEM or NMOSD, there is no need for chronic treatment. But children with MS and recurrent NMOSD require early chronic immunotherapy because specific therapies have been shown to improve prognosis.
Acute treatment
When it comes to acute treatment of NMOSD, the goal is to suppress the inflammatory attack but also to minimize long-term damage and optimize long-term neurological function. “The potential for irreversible injury with an attack is very high, and cumulative disabilities in NMOSD can result directly from attacks,” E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children at the University of Toronto, said during her talk.
IV steroids are generally the first choice, with a preference for methylprednisolone. Pediatric patients that are MOG antibody positive usually respond better and more quickly than do adults, with rapid daily improvements in mobility, vomiting, and eyesight. Dr. Yeh recommends weaning good responders off steroids because AQP-4 positive patients are likely to relapse without a steroid wean, and antibody testing may be unavailable or results may be delayed. The wean can range from 4 weeks to 4-6 months, depending on antibody status, likelihood of AQP-4 positivity, and clinical parameters.
Inadequate responses are usually pretty evident. If there is only light perception by day 4 or 5, or paralyzed patients are nonambulatory and achieve only twitchy movements by that time, second-line therapies should be considered, including therapeutic plasma exchange (TPE) with 5-7 exchanges or intravenous immunoglobulins (IVIg).
Dr. Yeh called for quick treatment. Whatever you do, “please do it sooner rather than later if you think there’s no response [to steroids],” Dr. Yeh said.
TPE is the first choice, according to Dr. Yeh. “There seems to be a fair amount of information that suggests that if you’re having difficulty getting a response to steroids, TPE can make a difference in these patients,” she said. But in some cases TPE may not be available, and IVIg can be attempted first. If it achieves no or only marginal improvement, TPE can be attempted later, but it must be kept in mind that TPE conducted too soon could wash out IVIg. Patients who get much better on IVIg can undergo a steroid wean, and then be evaluated for prophylactic therapy, said Dr. Yeh.
The evidence for IVIg is limited, reflecting the difficulty of studying treatments in rare populations. Still, when TPE is not available and the patient is quite impaired, IVIg makes sense to try. “Absence of evidence does not mean that the therapy doesn’t work, and I don’t think we should throw out the baby with the bath water,” said Dr. Yeh.
Although IVIg treatment is generally well tolerated, there have been a few serious adverse events, such as anaphylactic shock and aseptic meningitis, according to Andrea Savransky, MD, a pediatrician at National Pediatric Hospital in Buenos Aires, who also spoke at the session. “I think it is important to weigh the benefits against the risk,” Dr. Savransky said. She noted that TPE should not be taken lightly. One study showed more complications in pediatric patients than in adult patients, and it must be performed in specialized centers.
Emerging treaments
Tanuja Chitnis, MD, director of the Partners Pediatric MS Center at Massachusetts General Hospital, Boston, discussed some of the emerging treatments for pediatric NMOSD. Rituximab has been associated with success in some retrospective studies, but dosing should be personalized. Dr. Chitnis reported that B cells can return before 6 months, so she monitors B cells beginning 2 months after induction, redosing after 4 or 5 months rather than 6 if B cells return.
Nevertheless, relapses can still occur after rituximab therapy. “There is room for additional therapies to address this gap,” said Dr. Chitnis. Three new antibodies have received approval for treatment of NMOSD in adults. These include the complement inhibitor eculizumab, the IL-6 receptor antibody satralizumab, and the anti-CD19 antibody inebilizumab. Phase 3 clinical trials in children have been conducted for eculizumab and are in the planning stage for inebilizumab, and pediatric patients were included in pivotal trials for satralizumab.
Eculizumab treatment resulted in a 94.2% reduction in relapse risk in AQP4-positive adults. Satralizumab showed a 79% reduction in relapse risk among AQP-4 positive subjects with NMOSD or neuromyelitis optica and a 34% reduction in those who were AQP-4 negative. The pediatric subgroup had similar levels of response to adults, though the numbers were too small for a subgroup analysis.
In AQP-4 positive patients, inebilizumab treatment yielded a 77% reduction in relapse rate. In all patients, there was a 73% reduction.
For MOG antibody-positive patients with AQP-4 negative disease, novel therapies are at earlier stages of development. Typical MS therapies such as interferon beta and glatiramer acetate don’t seem to be effective. Some that have shown signs of efficacy include azathioprine, mycophenylate mofetil, rituximab, and IVIg infusion, but the state of the field is not encouraging. “This is an observation now being studied in larger cohorts, but in general I have not found that there’s a very strong response to any of these therapies, possibly with the exception of IVIg,” said Dr. Chitnis.
Dr. Tenembaum has no relevant financial disclosures. Dr. Armangue has received speaking honoraria from Novartis and travel expenses for scientific meetings from Merck, Biogen, and Roche. Dr. Yeh is on the scientific advisory board of Juno Therapeutics and has received research support from Biogen. Dr. Chitnis advises Biogen-Idec, Novartis, and Alexion, serves on clinical trial advisory boards for Novartis and Sanofi Aventis, and has received research support from Verily, EMD Serono, and Novartis. Dr. Savransky has received honoraria from Genzyme de Argentina SA.
FROM CNS-ICNA 2020
Worldwide measles vaccination is flagging
After almost 2 decades of progress, the global state of measles vaccination and measles mortality is deteriorating.
One of the most serious concerns of measles infection is its long-term neurological complications, including the fatal subacute sclerosing panencephalitis (SSPE) and measles inclusion-body encephalitis (MIBE), which is usually seen in immune deficient children. Although some efforts are being made to determine which patients might be most vulnerable to these outcomes, and to treat them, the best approach is still prevention and vaccination, according to Banu Anlar, MD, of Hacettepe University, Ankara, Turkey, who spoke during a session at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
Worldwide vaccination strategies have slipped in recent years, leading to upticks in measles cases and vaccination rates. As a result, in 2018 the World Health Organization postponed its goal of eliminating measles by 2020. Future eradication goals will likely need to be modified, according to Anaita Udwadia Hegde MD, a pediatric neurologist in Mumbai, India, who also presented at the session.
After measles deaths dropped 74% between 2000 and 2010, coinciding with widespread increases in vaccination, the WHO felt emboldened to deal the disease a knockout blow. In 2010, it held a Global Technical Consultation to determine the feasibility of an eradication campaign, which concluded it should be possible by 2020. Several characteristics of measles made that a reasonable goal: It is passed only among humans, with no known animal reservoir; natural infection grants lifelong immunity; there is only one serotype; the virus is genetically stable; the vaccine is safe and leads to 95%-97% seroconversion after two doses, which provides long-term protection against known genotypes; the disease is easily recognized and tested for; and it had been successfully eliminated already in some regions of the world.
As of 2017, analyses showed that the vaccination program saved the lives of about 1.5 million children. That was a cause for celebration, but the goal of eradication has remained elusive. Vaccination rates have trailed targets. In 2018, UNICEF and WHO estimated that 86% of children globally received the first measles vaccine, unchanged from 2010 and below the goal of 95%. Only 69% of children received the second dose, below the goal of 80%. Four countries in Europe lost their measles elimination status in 2018.
Other attempts to eradicate diseases have met with mixed results. The only full success was smallpox, eliminated in 1977. Similar efforts with polio, malaria, guinea worm, and now measles have all come up short. Those failures could complicate future efforts because global agencies and donors may be leery of past failures because of potential harm to their reputations, according to Dr. Hegde.
Such programs require sustained financial commitment and political support as well as local trust. Nevertheless, they must continue for ethical reasons, said Dr. Hegde, but also for economic ones: Every $1 spent on vaccination programs saves $58 in future costs in low- and middle-income countries. Missed childhood vaccination also results in future vulnerable teenagers and young adults, and these populations are much harder to reach and can drive large outbreaks.
Several factors are contributing to the global regression in vaccine coverage, according to Kristen Feemster, MD, MPH, a pediatric infectious disease physician and the global director of medical affairs at Merck. Globalization has enabled the spread of the disease. Most cases in the United States are imported by travelers to countries where the disease is endemic. “Measles can happen anywhere in the world, and when it does it can travel and spread. If you have an unvaccinated traveler who is exposed to measles abroad, they can return home and spread it to anyone else who is unvaccinated or not otherwise immune. When we see cases they’ve been sporadic, but if you return to a community where immunization rates are low, you have the potential for more sustained spread,” Dr. Feemster said during her presentation.
Why are so many travelers unvaccinated? A key reason is that vaccine hesitance is growing. Most affected individuals involved in outbreaks are unvaccinated, usually by choice rather than for medical reasons. Concerns continue over the measles vaccine and autism, growing out of the debunked studies of Andrew Wakefield. In one example, a Somali community in Minnesota experienced a higher than usual number of autism cases and parents sought reasons to explain it. They discovered the supposed connection between vaccination and autism, and Wakefield himself met with a group of them. The result was a drop in vaccination and, in 2011 and 2017, sizable measles outbreaks.
2020 has of course brought a fresh challenge to measles vaccine with the COVID-19 pandemic, which has reduced access to health care and shifted scientific and health care interest away from measles and other vaccine-preventable diseases. On the positive side, social distancing, mask wearing, and restricted movement are likely reducing exposure to measles, but reduced vaccination rates are likely to result in future outbreaks. “There’s been a significant decrease in rates for routine immunizations globally, so there’s a potential for yet another resurgence of measles and other vaccine-preventable diseases,” said Dr. Feemster.
Dr. Feemster is an employee of Merck. Dr. Anlar and Dr. Hegde did not disclose any relevant financial relationships.
After almost 2 decades of progress, the global state of measles vaccination and measles mortality is deteriorating.
One of the most serious concerns of measles infection is its long-term neurological complications, including the fatal subacute sclerosing panencephalitis (SSPE) and measles inclusion-body encephalitis (MIBE), which is usually seen in immune deficient children. Although some efforts are being made to determine which patients might be most vulnerable to these outcomes, and to treat them, the best approach is still prevention and vaccination, according to Banu Anlar, MD, of Hacettepe University, Ankara, Turkey, who spoke during a session at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
Worldwide vaccination strategies have slipped in recent years, leading to upticks in measles cases and vaccination rates. As a result, in 2018 the World Health Organization postponed its goal of eliminating measles by 2020. Future eradication goals will likely need to be modified, according to Anaita Udwadia Hegde MD, a pediatric neurologist in Mumbai, India, who also presented at the session.
After measles deaths dropped 74% between 2000 and 2010, coinciding with widespread increases in vaccination, the WHO felt emboldened to deal the disease a knockout blow. In 2010, it held a Global Technical Consultation to determine the feasibility of an eradication campaign, which concluded it should be possible by 2020. Several characteristics of measles made that a reasonable goal: It is passed only among humans, with no known animal reservoir; natural infection grants lifelong immunity; there is only one serotype; the virus is genetically stable; the vaccine is safe and leads to 95%-97% seroconversion after two doses, which provides long-term protection against known genotypes; the disease is easily recognized and tested for; and it had been successfully eliminated already in some regions of the world.
As of 2017, analyses showed that the vaccination program saved the lives of about 1.5 million children. That was a cause for celebration, but the goal of eradication has remained elusive. Vaccination rates have trailed targets. In 2018, UNICEF and WHO estimated that 86% of children globally received the first measles vaccine, unchanged from 2010 and below the goal of 95%. Only 69% of children received the second dose, below the goal of 80%. Four countries in Europe lost their measles elimination status in 2018.
Other attempts to eradicate diseases have met with mixed results. The only full success was smallpox, eliminated in 1977. Similar efforts with polio, malaria, guinea worm, and now measles have all come up short. Those failures could complicate future efforts because global agencies and donors may be leery of past failures because of potential harm to their reputations, according to Dr. Hegde.
Such programs require sustained financial commitment and political support as well as local trust. Nevertheless, they must continue for ethical reasons, said Dr. Hegde, but also for economic ones: Every $1 spent on vaccination programs saves $58 in future costs in low- and middle-income countries. Missed childhood vaccination also results in future vulnerable teenagers and young adults, and these populations are much harder to reach and can drive large outbreaks.
Several factors are contributing to the global regression in vaccine coverage, according to Kristen Feemster, MD, MPH, a pediatric infectious disease physician and the global director of medical affairs at Merck. Globalization has enabled the spread of the disease. Most cases in the United States are imported by travelers to countries where the disease is endemic. “Measles can happen anywhere in the world, and when it does it can travel and spread. If you have an unvaccinated traveler who is exposed to measles abroad, they can return home and spread it to anyone else who is unvaccinated or not otherwise immune. When we see cases they’ve been sporadic, but if you return to a community where immunization rates are low, you have the potential for more sustained spread,” Dr. Feemster said during her presentation.
Why are so many travelers unvaccinated? A key reason is that vaccine hesitance is growing. Most affected individuals involved in outbreaks are unvaccinated, usually by choice rather than for medical reasons. Concerns continue over the measles vaccine and autism, growing out of the debunked studies of Andrew Wakefield. In one example, a Somali community in Minnesota experienced a higher than usual number of autism cases and parents sought reasons to explain it. They discovered the supposed connection between vaccination and autism, and Wakefield himself met with a group of them. The result was a drop in vaccination and, in 2011 and 2017, sizable measles outbreaks.
2020 has of course brought a fresh challenge to measles vaccine with the COVID-19 pandemic, which has reduced access to health care and shifted scientific and health care interest away from measles and other vaccine-preventable diseases. On the positive side, social distancing, mask wearing, and restricted movement are likely reducing exposure to measles, but reduced vaccination rates are likely to result in future outbreaks. “There’s been a significant decrease in rates for routine immunizations globally, so there’s a potential for yet another resurgence of measles and other vaccine-preventable diseases,” said Dr. Feemster.
Dr. Feemster is an employee of Merck. Dr. Anlar and Dr. Hegde did not disclose any relevant financial relationships.
After almost 2 decades of progress, the global state of measles vaccination and measles mortality is deteriorating.
One of the most serious concerns of measles infection is its long-term neurological complications, including the fatal subacute sclerosing panencephalitis (SSPE) and measles inclusion-body encephalitis (MIBE), which is usually seen in immune deficient children. Although some efforts are being made to determine which patients might be most vulnerable to these outcomes, and to treat them, the best approach is still prevention and vaccination, according to Banu Anlar, MD, of Hacettepe University, Ankara, Turkey, who spoke during a session at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
Worldwide vaccination strategies have slipped in recent years, leading to upticks in measles cases and vaccination rates. As a result, in 2018 the World Health Organization postponed its goal of eliminating measles by 2020. Future eradication goals will likely need to be modified, according to Anaita Udwadia Hegde MD, a pediatric neurologist in Mumbai, India, who also presented at the session.
After measles deaths dropped 74% between 2000 and 2010, coinciding with widespread increases in vaccination, the WHO felt emboldened to deal the disease a knockout blow. In 2010, it held a Global Technical Consultation to determine the feasibility of an eradication campaign, which concluded it should be possible by 2020. Several characteristics of measles made that a reasonable goal: It is passed only among humans, with no known animal reservoir; natural infection grants lifelong immunity; there is only one serotype; the virus is genetically stable; the vaccine is safe and leads to 95%-97% seroconversion after two doses, which provides long-term protection against known genotypes; the disease is easily recognized and tested for; and it had been successfully eliminated already in some regions of the world.
As of 2017, analyses showed that the vaccination program saved the lives of about 1.5 million children. That was a cause for celebration, but the goal of eradication has remained elusive. Vaccination rates have trailed targets. In 2018, UNICEF and WHO estimated that 86% of children globally received the first measles vaccine, unchanged from 2010 and below the goal of 95%. Only 69% of children received the second dose, below the goal of 80%. Four countries in Europe lost their measles elimination status in 2018.
Other attempts to eradicate diseases have met with mixed results. The only full success was smallpox, eliminated in 1977. Similar efforts with polio, malaria, guinea worm, and now measles have all come up short. Those failures could complicate future efforts because global agencies and donors may be leery of past failures because of potential harm to their reputations, according to Dr. Hegde.
Such programs require sustained financial commitment and political support as well as local trust. Nevertheless, they must continue for ethical reasons, said Dr. Hegde, but also for economic ones: Every $1 spent on vaccination programs saves $58 in future costs in low- and middle-income countries. Missed childhood vaccination also results in future vulnerable teenagers and young adults, and these populations are much harder to reach and can drive large outbreaks.
Several factors are contributing to the global regression in vaccine coverage, according to Kristen Feemster, MD, MPH, a pediatric infectious disease physician and the global director of medical affairs at Merck. Globalization has enabled the spread of the disease. Most cases in the United States are imported by travelers to countries where the disease is endemic. “Measles can happen anywhere in the world, and when it does it can travel and spread. If you have an unvaccinated traveler who is exposed to measles abroad, they can return home and spread it to anyone else who is unvaccinated or not otherwise immune. When we see cases they’ve been sporadic, but if you return to a community where immunization rates are low, you have the potential for more sustained spread,” Dr. Feemster said during her presentation.
Why are so many travelers unvaccinated? A key reason is that vaccine hesitance is growing. Most affected individuals involved in outbreaks are unvaccinated, usually by choice rather than for medical reasons. Concerns continue over the measles vaccine and autism, growing out of the debunked studies of Andrew Wakefield. In one example, a Somali community in Minnesota experienced a higher than usual number of autism cases and parents sought reasons to explain it. They discovered the supposed connection between vaccination and autism, and Wakefield himself met with a group of them. The result was a drop in vaccination and, in 2011 and 2017, sizable measles outbreaks.
2020 has of course brought a fresh challenge to measles vaccine with the COVID-19 pandemic, which has reduced access to health care and shifted scientific and health care interest away from measles and other vaccine-preventable diseases. On the positive side, social distancing, mask wearing, and restricted movement are likely reducing exposure to measles, but reduced vaccination rates are likely to result in future outbreaks. “There’s been a significant decrease in rates for routine immunizations globally, so there’s a potential for yet another resurgence of measles and other vaccine-preventable diseases,” said Dr. Feemster.
Dr. Feemster is an employee of Merck. Dr. Anlar and Dr. Hegde did not disclose any relevant financial relationships.
FROM CNS-ICNA 2020
Latest week brings 44,000 more children with COVID-19
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
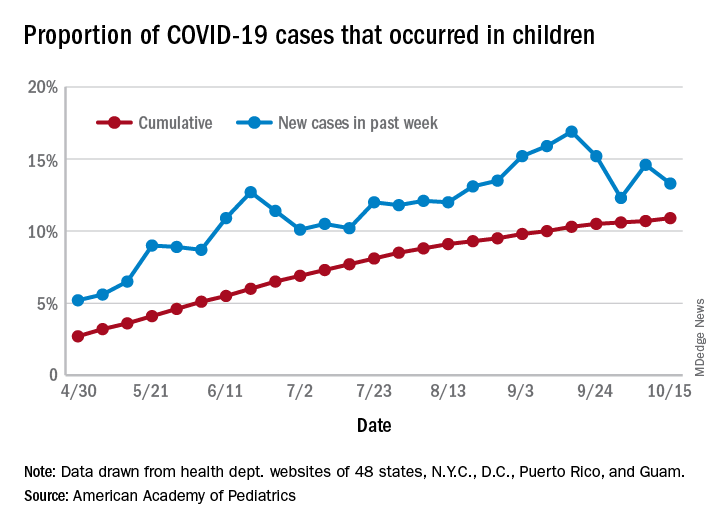
The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
Systemic sclerosis patients share their perspectives and needs in treatment trials
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
Patients with systemic sclerosis have variable disease progression but often experience debilitating fatigue, pain, and digestive issues – and they’re extremely concerned about progressive organ damage, according to those who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs, and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Patients rate their most impactful symptoms
Dinesh Khanna, MBBS, MSc, a rheumatologist who directs a scleroderma research program at the University of Michigan, Ann Arbor, attended the meeting after giving an opening presentation on the disease to FDA officials, patients, and other participants. In a later interview, he said that patients’ ratings of their most impactful symptoms was especially striking.
Raynaud’s phenomenon, digestive symptoms, and fatigue were the top three answers to a poll question that asked patients what symptom had the most significant impact on daily life, he noted, “and none of these are being [strongly] addressed right now [in clinical trials] apart from Raynaud’s phenomenon, for which there are some trials ongoing.”
He and other researchers are “struggling with what outcomes measures to use [in their studies],” said Dr. Khanna, the Frederick G.L. Huetwell Professor of Rheumatology at the University. “My takeaway from the meeting as a clinical trialist is that we should be paying close attention to the symptoms that patients tell us are the most important. We should be including these in our trial designs as secondary endpoints, if not primary endpoints. We have not done that [thus far], really.”
Approximately 200,000 patients in the United States have scleroderma, and approximately 75,000-80,000 of these patients have systemic scleroderma, or systemic sclerosis, Dr. Khanna said in his opening presentation. Each year, he estimates, about 6,000 new diagnoses of systemic sclerosis are made.
More than 200 people – patients, FDA officials, and others – participated in the PFDD meeting. Patients participated in one of two panels – one focused on health effects and daily impacts, and the other on treatments – or submitted input electronically. All were invited to answer poll questions.
Raj Nair, MD, one of eight FDA leaders attending the meeting, noted in closing remarks that the pain experienced by patients with systemic sclerosis includes severe pain from Raynaud’s phenomenon and pain caused by digital ulcers and by calcinosis. “We heard about how paralyzing the pain from calcinosis is, and that there are very few options for alleviating this pain,” said Dr. Nair, of the division of rheumatology and transplant medicine.
Another takeaway, he said, is that the “fatigue can be severe and debilitating, leading to days where it is impossible to get out of bed,” and that digestive symptoms can also be severe. “Reflux,” he noted, “requires significant medical intervention.”
Patients describe their experiences
Rosemary Lyons, diagnosed with scleroderma 35 years ago, explained that while her skin is no longer hardened, she is overly sensitive to fabrics and skin care products and has difficulty with sleeping and eating. She moved away from family in the Northeast to live in the South where the climate is warmer, but even on a 90-degree night she needs a blanket and two comforters to curb the cold and attempt to sleep.
Impaired gastrointestinal motility has made food her “biggest problem” for the past 10 years, and because of GI symptoms, she can eat only one meal a day. She also experiences fainting, brain fog, and severe fatigue. On a good day, Ms. Lyons noted, she sometimes opts to do some house chores “knowing that I’ll have 1-3 days of recovery.”
Another patient, Amy Harding, said that 22 years after her scleroderma diagnosis, “the calcinosis I get in my fingers, elbows, toes, and ears tops all the prior symptoms.” The skin tightening and digital ulcers that she experienced in the first 10 years have tapered off, and while Raynaud’s symptoms and heartburn have worsened, they are at least partly manageable with medications, unlike the pain from calcinosis.
Treating symptoms vs. disease may be key in risk-benefit analysis
In questions after patient presentations, FDA officials probed for more perspective on issues such as how fatigue should be assessed, the differences between fatigue and brain fog, the impact of calcinosis on functioning, and how much risk patients would be willing to assume from treatments that have side effects and that may or may not modulate the disease and slow disease progression.
Most patients said in response to an FDA poll question that they definitely (almost 40%) or possibly (almost 50%) would be willing to try a hypothetical new self-injectable medication if it were shown to reduce their most impactful symptoms but had side effects.
“I think what [we’ve been hearing] today is that whether we’re working on the symptoms or the disease itself is [the key]” to patients’ risk-benefit analysis, said meeting moderator Capt. Robyn Bent, RN, MS, of the U.S. Public Health Service, and director of the PFDD.
Anita Devine, diagnosed 13 years ago with systemic sclerosis, was one of several panel members who said she would accept more bothersome treatment side effects and risks “if the gain was control of disease progression and overall quality of life ... and organ preservation.” Ms. Devine, who has needed kidney dialysis and multiple hand surgeries, noted that she previously took anti-neoplastic and anti-inflammatory agents “to try to stem the course of my disease, but unfortunately the disease did not abate.”
Treatments for systemic sclerosis include vasodilators, immunosuppressive medications, antifibrotic therapies, and stem cell transplants, Dr. Khanna said in his opening remarks.
Trials of drugs for scleroderma have focused on early disease that may be amenable to treatment, with the exception of trials for pulmonary arterial hypertension, which affects some patients with systemic sclerosis. There are multiple FDA-approved drugs for pulmonary arterial hypertension and more trials are underway.
Outcomes such as pain and fatigue are included in many of the trials currently underway, but they tend to be lower-level secondary outcomes measures that cannot be incorporated into drug labeling or are more “exploratory in nature,” Dr. Khanna said in the interview.
Dr. Khanna disclosed that he is the chief medical officer (an equity position) for CiVi Biopharma/Eicos Sciences Inc., which is developing a drug for Raynaud’s, and serves as a consultant and grant recipient for numerous companies that make or are developing drugs for systemic sclerosis.
The FDA will accept patient comments until Dec. 15, 2020, at which time comments will be compiled into a summary report, Ms. Bent said.
FROM AN FDA PATIENT-FOCUSED DRUG DEVELOPMENT MEETING
Bariatric surgery tied to lower aortic dissection risk
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.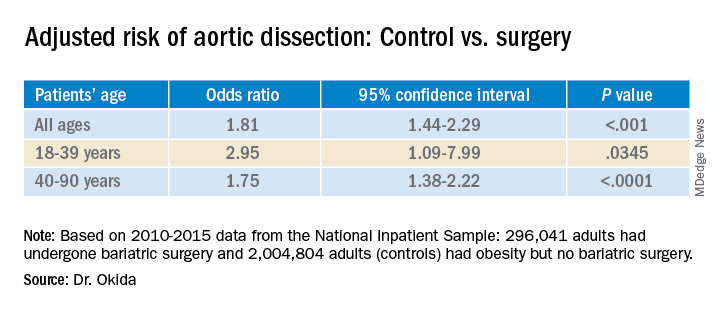
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ESMO offers new clinical practice guideline for CLL
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
FROM ANNALS OF ONCOLOGY
Increasing racial diversity in hospital medicine’s leadership ranks
Have you ever done something where you’re not quite sure why you did it at the time, but later on you realize it was part of some larger cosmic purpose, and you go, “Ahhh, now I understand…that’s why!”? Call it a fortuitous coincidence. Or a subconscious act of anticipation. Maybe a little push from God.
Last summer, as SHM’s Practice Analysis Committee was planning the State of Hospital Medicine survey for 2020, we received a request from SHM’s Diversity, Equity & Inclusion (DEI) Special Interest Group (SIG) to include a series of questions related to hospitalist gender, race and ethnic distribution in the new survey. We’ve generally resisted doing things like this because the SoHM is designed to capture data at the group level, not the individual level – and honestly, it’s as much as a lot of groups can do to tell us reliably how many FTEs they have, much less provide details about individual providers. In addition, the survey is already really long, and we are always looking for ways to make it shorter and easier for participants while still collecting the information report users care most about.
But we wanted to take the asks from the DEI SIG seriously, and as we considered their request, we realized that though it wasn’t practical to collect this information for individual hospital medicine group (HMG) members, we could collect it for group leaders. Little did we know last summer that issues of gender and racial diversity and equity would be so front-and-center right now, as we prepare to release the 2020 SoHM Report in early September. Ahhh, now I understand…that’s why – with the prompting of the DEI SIG – we so fortuitously chose to include those questions this year!
Here’s a sneak preview of what we learned. Among SoHM respondents, 57.1% reported that the highest-ranking leader in their HMG is White, and 23.5% of highest-ranking leaders are Asian. Only 5.5% of HMG leaders were Black/African American. Ethnicity was a separate question, and only 2.2% of HMG leaders were reported as Hispanic/Latino.
I have been profoundly moved by the wretched deaths of George Floyd and other people of color at the hands of police in recent months, and by the subsequent protests and our growing national reckoning over issues of racial equity and justice. In my efforts to understand more about race in America, I have been challenged by my friend Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., and others to go beyond just learning about these issues. I want to use my voice to advocate for change, and my actions to participate in effecting change, within the context of my sphere of influence.
So, what does that have to do with the SoHM data on HMG leader demographics? Well, it’s clear that Black and brown people are woefully underrepresented in the ranks of hospital medicine leadership.
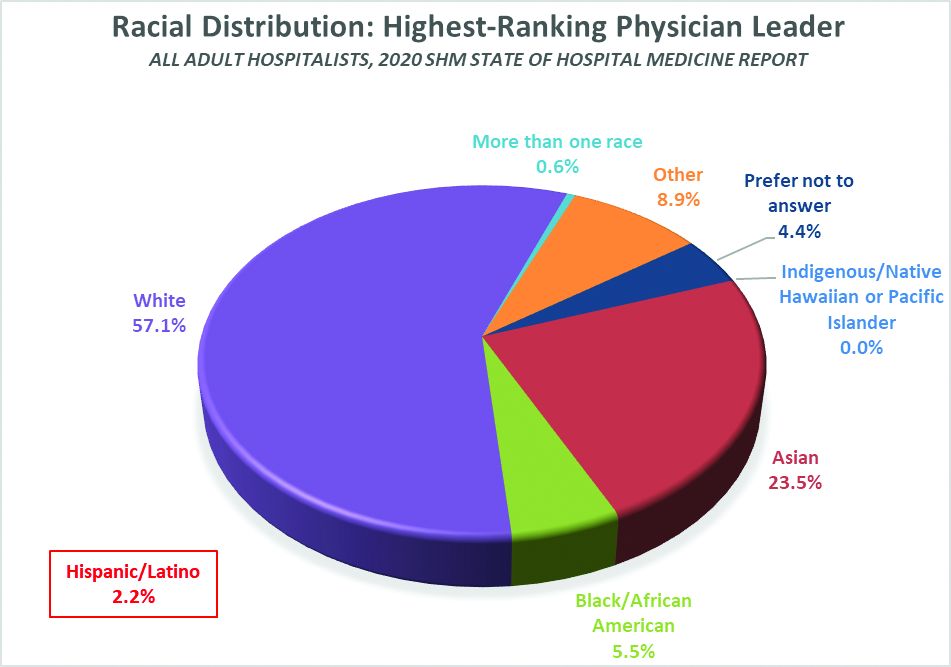
Unfortunately, we don’t have good information on racial diversity for hospitalists as a specialty, though I understand that SHM is working on plans to update membership profiles to begin collecting this information. In searching the Internet, I found a 2018 paper from the Journal of Health Care for the Poor and Underserved that studied racial and ethnic distribution of U.S. primary care physicians (doi: 10.1353/hpu.2018.0036). It reported that, in 2012, 7.8% of general internists were Black, along with 5.8% of family medicine/general practice physicians and 6.8% of pediatricians. A separate data set issued by the Association of American Medical Colleges reported that, in 2019, 6.4% of all actively practicing general internal medicine doctors were Black (5.5% of male IM physicians and 7.9% of female IM physicians). While this doesn’t mean hospitalists have the same racial and ethnic distribution, this is probably the best proxy we can come up with.
At first glance, having 5.5% of HMG leaders who are Black doesn’t seem terribly out of line with the reported range of 6.4 to 7.8% in the general population of internal medicine physicians (apologies to the family medicine and pediatric hospitalists reading this, but I’ll confine my discussion to internists for ease and brevity, since they represent the vast majority of the nation’s hospitalists). But do the math. It means Black hospitalists are likely underrepresented in HMG leadership ranks by something like 14% to 29% compared to their likely presence among hospitalists in general.
The real problem, of course, is that according the U.S. Census Bureau, 13.4% of the U.S. population is Black. So even if the racial distribution of HMG leaders catches up to the general hospitalist population, hospital medicine is still woefully underrepresenting the racial and ethnic distribution of our patient population.
The disconnect between the ethnic distribution of HMG leaders vs. hospitalists (based on general internal medicine distribution) is even more pronounced for Latinos. The JHCPU paper reported that, in 2012, 5.6% of general internists were Hispanic. The AAMC data set reported 5.8% of IM doctors were Hispanic/Latino. But only 2.2% of SoHM respondent HMGs reported a Hispanic/Latino leader, which means Latinos are underrepresented by somewhere around 61% or so relative to the likely hospitalist population, and by a whole lot more considering the fact that Latinos make up about 18.5% of the U.S. population.
I’m not saying that a White or Asian doctor can’t provide skilled, compassionate care to a Black or Latino patient, or vice-versa. It happens every day. I guess what I am saying is that we as a country and in the medical profession need to do a better job of creating pathways and promoting careers in medicine for people of color. A JAMA paper from 2019 reported that while the numbers and proportions of minority medical school matriculants has slowly been increasing from 2002 to 2017, the rate of increase was “slower than their age-matched counterparts in the U.S. population, resulting in increased underrepresentation” (doi:10.1001/jamanetworkopen.2019.10490). This means we’re falling behind, not catching up.
We need to make sure that people like Dr. Ryan Brown aren’t discouraged from pursuing medicine by teachers or school counselors because of their skin color or accent, or their gender or sexual orientation. And among those who become doctors, we need to promote hospital medicine as a desirable specialty for people of color and actively invite them in.
In my view, much of this starts with creating more and better paths to leadership within hospital medicine for people of color. Hospital medicine group leaders wield enormous – and increasing – influence, not only within their HMGs and within SHM, but within their institutions and health care systems. We need their voices and their influence to promote diversity within their groups, their institutions, within hospital medicine, and within medicine and the U.S. health care system more broadly.
The Society of Hospital Medicine is already taking steps to promote diversity, equity and inclusion. These include issuing a formal Diversity and Inclusion Statement, creating the DEI SIG, and the recent formation of a Board-designated DEI task force charged with making recommendations to promote DEI within SHM and in hospital medicine more broadly. But I want to challenge SHM to do more, particularly with regard to promoting diversity in leadership. Here are a few ideas to consider:
- Create and sponsor a mentoring program in which hospitalists volunteer to mentor minority junior high and high school students and help them prepare to pursue a career in medicine.
- Develop a formal, structured advocacy or collaboration effort with organizations like AAMC and the Accreditation Council for Graduate Medical Education designed to promote meaningful increases in the proportion of medical school students and residents who are people of color, and in the proportion who choose primary care – and ultimately, hospital medicine.
- Work hard to collect reliable racial, ethnic and gender information about SHM members and consider collaborating with MGMA to incorporate demographic questions into its survey tool for individual hospitalist compensation and productivity data. Challenge us on the Practice Analysis Committee who are responsible for the SoHM survey to continue surveying leadership demographics, and to consider how we can expand our collection of DEI information in 2022.
- Undertake a public relations campaign to highlight to health systems and other employers the under-representation of Black and Latino hospitalists in leadership positions, and to promote conscious efforts to increase those ranks.
- Create scholarships for hospitalists from underrepresented racial and ethnic groups to attend SHM-sponsored leadership development programs such as Leadership Academy, Academic Hospitalist Academy, and Quality and Safety Educators Academy, with the goal of increasing their ranks in positions of influence throughout healthcare. A scholarship program might even include raising funds to help minority hospitalists pursue Master’s-level programs such as an MBA, MHA, or MMM.
- Develop an educational track, mentoring program, or other support initiative for early-career hospitalist leaders and those interested in developing leadership skills, and ensure it gives specific attention to strategies for increasing the proportion of hospitalists of color in leadership positions.
- Review and revise existing SHM documents such as The Key Principles and Characteristics of an Effective Hospital Medicine Group, the Core Competencies in Hospital Medicine, and various white papers and position statements to ensure they address diversity, equity and inclusion – both with regard to the hospital medicine workforce and leadership, and with regard to patient care and eliminating health disparities.
I’m sure there are plenty of other similar actions we can take that I haven’t thought of. But we need to start the conversation about concrete steps our Society, and the medical specialty we represent, can take to foster real change. And then, we need to follow our words up with actions.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
Have you ever done something where you’re not quite sure why you did it at the time, but later on you realize it was part of some larger cosmic purpose, and you go, “Ahhh, now I understand…that’s why!”? Call it a fortuitous coincidence. Or a subconscious act of anticipation. Maybe a little push from God.
Last summer, as SHM’s Practice Analysis Committee was planning the State of Hospital Medicine survey for 2020, we received a request from SHM’s Diversity, Equity & Inclusion (DEI) Special Interest Group (SIG) to include a series of questions related to hospitalist gender, race and ethnic distribution in the new survey. We’ve generally resisted doing things like this because the SoHM is designed to capture data at the group level, not the individual level – and honestly, it’s as much as a lot of groups can do to tell us reliably how many FTEs they have, much less provide details about individual providers. In addition, the survey is already really long, and we are always looking for ways to make it shorter and easier for participants while still collecting the information report users care most about.
But we wanted to take the asks from the DEI SIG seriously, and as we considered their request, we realized that though it wasn’t practical to collect this information for individual hospital medicine group (HMG) members, we could collect it for group leaders. Little did we know last summer that issues of gender and racial diversity and equity would be so front-and-center right now, as we prepare to release the 2020 SoHM Report in early September. Ahhh, now I understand…that’s why – with the prompting of the DEI SIG – we so fortuitously chose to include those questions this year!
Here’s a sneak preview of what we learned. Among SoHM respondents, 57.1% reported that the highest-ranking leader in their HMG is White, and 23.5% of highest-ranking leaders are Asian. Only 5.5% of HMG leaders were Black/African American. Ethnicity was a separate question, and only 2.2% of HMG leaders were reported as Hispanic/Latino.
I have been profoundly moved by the wretched deaths of George Floyd and other people of color at the hands of police in recent months, and by the subsequent protests and our growing national reckoning over issues of racial equity and justice. In my efforts to understand more about race in America, I have been challenged by my friend Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., and others to go beyond just learning about these issues. I want to use my voice to advocate for change, and my actions to participate in effecting change, within the context of my sphere of influence.
So, what does that have to do with the SoHM data on HMG leader demographics? Well, it’s clear that Black and brown people are woefully underrepresented in the ranks of hospital medicine leadership.

Unfortunately, we don’t have good information on racial diversity for hospitalists as a specialty, though I understand that SHM is working on plans to update membership profiles to begin collecting this information. In searching the Internet, I found a 2018 paper from the Journal of Health Care for the Poor and Underserved that studied racial and ethnic distribution of U.S. primary care physicians (doi: 10.1353/hpu.2018.0036). It reported that, in 2012, 7.8% of general internists were Black, along with 5.8% of family medicine/general practice physicians and 6.8% of pediatricians. A separate data set issued by the Association of American Medical Colleges reported that, in 2019, 6.4% of all actively practicing general internal medicine doctors were Black (5.5% of male IM physicians and 7.9% of female IM physicians). While this doesn’t mean hospitalists have the same racial and ethnic distribution, this is probably the best proxy we can come up with.
At first glance, having 5.5% of HMG leaders who are Black doesn’t seem terribly out of line with the reported range of 6.4 to 7.8% in the general population of internal medicine physicians (apologies to the family medicine and pediatric hospitalists reading this, but I’ll confine my discussion to internists for ease and brevity, since they represent the vast majority of the nation’s hospitalists). But do the math. It means Black hospitalists are likely underrepresented in HMG leadership ranks by something like 14% to 29% compared to their likely presence among hospitalists in general.
The real problem, of course, is that according the U.S. Census Bureau, 13.4% of the U.S. population is Black. So even if the racial distribution of HMG leaders catches up to the general hospitalist population, hospital medicine is still woefully underrepresenting the racial and ethnic distribution of our patient population.
The disconnect between the ethnic distribution of HMG leaders vs. hospitalists (based on general internal medicine distribution) is even more pronounced for Latinos. The JHCPU paper reported that, in 2012, 5.6% of general internists were Hispanic. The AAMC data set reported 5.8% of IM doctors were Hispanic/Latino. But only 2.2% of SoHM respondent HMGs reported a Hispanic/Latino leader, which means Latinos are underrepresented by somewhere around 61% or so relative to the likely hospitalist population, and by a whole lot more considering the fact that Latinos make up about 18.5% of the U.S. population.
I’m not saying that a White or Asian doctor can’t provide skilled, compassionate care to a Black or Latino patient, or vice-versa. It happens every day. I guess what I am saying is that we as a country and in the medical profession need to do a better job of creating pathways and promoting careers in medicine for people of color. A JAMA paper from 2019 reported that while the numbers and proportions of minority medical school matriculants has slowly been increasing from 2002 to 2017, the rate of increase was “slower than their age-matched counterparts in the U.S. population, resulting in increased underrepresentation” (doi:10.1001/jamanetworkopen.2019.10490). This means we’re falling behind, not catching up.
We need to make sure that people like Dr. Ryan Brown aren’t discouraged from pursuing medicine by teachers or school counselors because of their skin color or accent, or their gender or sexual orientation. And among those who become doctors, we need to promote hospital medicine as a desirable specialty for people of color and actively invite them in.
In my view, much of this starts with creating more and better paths to leadership within hospital medicine for people of color. Hospital medicine group leaders wield enormous – and increasing – influence, not only within their HMGs and within SHM, but within their institutions and health care systems. We need their voices and their influence to promote diversity within their groups, their institutions, within hospital medicine, and within medicine and the U.S. health care system more broadly.
The Society of Hospital Medicine is already taking steps to promote diversity, equity and inclusion. These include issuing a formal Diversity and Inclusion Statement, creating the DEI SIG, and the recent formation of a Board-designated DEI task force charged with making recommendations to promote DEI within SHM and in hospital medicine more broadly. But I want to challenge SHM to do more, particularly with regard to promoting diversity in leadership. Here are a few ideas to consider:
- Create and sponsor a mentoring program in which hospitalists volunteer to mentor minority junior high and high school students and help them prepare to pursue a career in medicine.
- Develop a formal, structured advocacy or collaboration effort with organizations like AAMC and the Accreditation Council for Graduate Medical Education designed to promote meaningful increases in the proportion of medical school students and residents who are people of color, and in the proportion who choose primary care – and ultimately, hospital medicine.
- Work hard to collect reliable racial, ethnic and gender information about SHM members and consider collaborating with MGMA to incorporate demographic questions into its survey tool for individual hospitalist compensation and productivity data. Challenge us on the Practice Analysis Committee who are responsible for the SoHM survey to continue surveying leadership demographics, and to consider how we can expand our collection of DEI information in 2022.
- Undertake a public relations campaign to highlight to health systems and other employers the under-representation of Black and Latino hospitalists in leadership positions, and to promote conscious efforts to increase those ranks.
- Create scholarships for hospitalists from underrepresented racial and ethnic groups to attend SHM-sponsored leadership development programs such as Leadership Academy, Academic Hospitalist Academy, and Quality and Safety Educators Academy, with the goal of increasing their ranks in positions of influence throughout healthcare. A scholarship program might even include raising funds to help minority hospitalists pursue Master’s-level programs such as an MBA, MHA, or MMM.
- Develop an educational track, mentoring program, or other support initiative for early-career hospitalist leaders and those interested in developing leadership skills, and ensure it gives specific attention to strategies for increasing the proportion of hospitalists of color in leadership positions.
- Review and revise existing SHM documents such as The Key Principles and Characteristics of an Effective Hospital Medicine Group, the Core Competencies in Hospital Medicine, and various white papers and position statements to ensure they address diversity, equity and inclusion – both with regard to the hospital medicine workforce and leadership, and with regard to patient care and eliminating health disparities.
I’m sure there are plenty of other similar actions we can take that I haven’t thought of. But we need to start the conversation about concrete steps our Society, and the medical specialty we represent, can take to foster real change. And then, we need to follow our words up with actions.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
Have you ever done something where you’re not quite sure why you did it at the time, but later on you realize it was part of some larger cosmic purpose, and you go, “Ahhh, now I understand…that’s why!”? Call it a fortuitous coincidence. Or a subconscious act of anticipation. Maybe a little push from God.
Last summer, as SHM’s Practice Analysis Committee was planning the State of Hospital Medicine survey for 2020, we received a request from SHM’s Diversity, Equity & Inclusion (DEI) Special Interest Group (SIG) to include a series of questions related to hospitalist gender, race and ethnic distribution in the new survey. We’ve generally resisted doing things like this because the SoHM is designed to capture data at the group level, not the individual level – and honestly, it’s as much as a lot of groups can do to tell us reliably how many FTEs they have, much less provide details about individual providers. In addition, the survey is already really long, and we are always looking for ways to make it shorter and easier for participants while still collecting the information report users care most about.
But we wanted to take the asks from the DEI SIG seriously, and as we considered their request, we realized that though it wasn’t practical to collect this information for individual hospital medicine group (HMG) members, we could collect it for group leaders. Little did we know last summer that issues of gender and racial diversity and equity would be so front-and-center right now, as we prepare to release the 2020 SoHM Report in early September. Ahhh, now I understand…that’s why – with the prompting of the DEI SIG – we so fortuitously chose to include those questions this year!
Here’s a sneak preview of what we learned. Among SoHM respondents, 57.1% reported that the highest-ranking leader in their HMG is White, and 23.5% of highest-ranking leaders are Asian. Only 5.5% of HMG leaders were Black/African American. Ethnicity was a separate question, and only 2.2% of HMG leaders were reported as Hispanic/Latino.
I have been profoundly moved by the wretched deaths of George Floyd and other people of color at the hands of police in recent months, and by the subsequent protests and our growing national reckoning over issues of racial equity and justice. In my efforts to understand more about race in America, I have been challenged by my friend Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., and others to go beyond just learning about these issues. I want to use my voice to advocate for change, and my actions to participate in effecting change, within the context of my sphere of influence.
So, what does that have to do with the SoHM data on HMG leader demographics? Well, it’s clear that Black and brown people are woefully underrepresented in the ranks of hospital medicine leadership.

Unfortunately, we don’t have good information on racial diversity for hospitalists as a specialty, though I understand that SHM is working on plans to update membership profiles to begin collecting this information. In searching the Internet, I found a 2018 paper from the Journal of Health Care for the Poor and Underserved that studied racial and ethnic distribution of U.S. primary care physicians (doi: 10.1353/hpu.2018.0036). It reported that, in 2012, 7.8% of general internists were Black, along with 5.8% of family medicine/general practice physicians and 6.8% of pediatricians. A separate data set issued by the Association of American Medical Colleges reported that, in 2019, 6.4% of all actively practicing general internal medicine doctors were Black (5.5% of male IM physicians and 7.9% of female IM physicians). While this doesn’t mean hospitalists have the same racial and ethnic distribution, this is probably the best proxy we can come up with.
At first glance, having 5.5% of HMG leaders who are Black doesn’t seem terribly out of line with the reported range of 6.4 to 7.8% in the general population of internal medicine physicians (apologies to the family medicine and pediatric hospitalists reading this, but I’ll confine my discussion to internists for ease and brevity, since they represent the vast majority of the nation’s hospitalists). But do the math. It means Black hospitalists are likely underrepresented in HMG leadership ranks by something like 14% to 29% compared to their likely presence among hospitalists in general.
The real problem, of course, is that according the U.S. Census Bureau, 13.4% of the U.S. population is Black. So even if the racial distribution of HMG leaders catches up to the general hospitalist population, hospital medicine is still woefully underrepresenting the racial and ethnic distribution of our patient population.
The disconnect between the ethnic distribution of HMG leaders vs. hospitalists (based on general internal medicine distribution) is even more pronounced for Latinos. The JHCPU paper reported that, in 2012, 5.6% of general internists were Hispanic. The AAMC data set reported 5.8% of IM doctors were Hispanic/Latino. But only 2.2% of SoHM respondent HMGs reported a Hispanic/Latino leader, which means Latinos are underrepresented by somewhere around 61% or so relative to the likely hospitalist population, and by a whole lot more considering the fact that Latinos make up about 18.5% of the U.S. population.
I’m not saying that a White or Asian doctor can’t provide skilled, compassionate care to a Black or Latino patient, or vice-versa. It happens every day. I guess what I am saying is that we as a country and in the medical profession need to do a better job of creating pathways and promoting careers in medicine for people of color. A JAMA paper from 2019 reported that while the numbers and proportions of minority medical school matriculants has slowly been increasing from 2002 to 2017, the rate of increase was “slower than their age-matched counterparts in the U.S. population, resulting in increased underrepresentation” (doi:10.1001/jamanetworkopen.2019.10490). This means we’re falling behind, not catching up.
We need to make sure that people like Dr. Ryan Brown aren’t discouraged from pursuing medicine by teachers or school counselors because of their skin color or accent, or their gender or sexual orientation. And among those who become doctors, we need to promote hospital medicine as a desirable specialty for people of color and actively invite them in.
In my view, much of this starts with creating more and better paths to leadership within hospital medicine for people of color. Hospital medicine group leaders wield enormous – and increasing – influence, not only within their HMGs and within SHM, but within their institutions and health care systems. We need their voices and their influence to promote diversity within their groups, their institutions, within hospital medicine, and within medicine and the U.S. health care system more broadly.
The Society of Hospital Medicine is already taking steps to promote diversity, equity and inclusion. These include issuing a formal Diversity and Inclusion Statement, creating the DEI SIG, and the recent formation of a Board-designated DEI task force charged with making recommendations to promote DEI within SHM and in hospital medicine more broadly. But I want to challenge SHM to do more, particularly with regard to promoting diversity in leadership. Here are a few ideas to consider:
- Create and sponsor a mentoring program in which hospitalists volunteer to mentor minority junior high and high school students and help them prepare to pursue a career in medicine.
- Develop a formal, structured advocacy or collaboration effort with organizations like AAMC and the Accreditation Council for Graduate Medical Education designed to promote meaningful increases in the proportion of medical school students and residents who are people of color, and in the proportion who choose primary care – and ultimately, hospital medicine.
- Work hard to collect reliable racial, ethnic and gender information about SHM members and consider collaborating with MGMA to incorporate demographic questions into its survey tool for individual hospitalist compensation and productivity data. Challenge us on the Practice Analysis Committee who are responsible for the SoHM survey to continue surveying leadership demographics, and to consider how we can expand our collection of DEI information in 2022.
- Undertake a public relations campaign to highlight to health systems and other employers the under-representation of Black and Latino hospitalists in leadership positions, and to promote conscious efforts to increase those ranks.
- Create scholarships for hospitalists from underrepresented racial and ethnic groups to attend SHM-sponsored leadership development programs such as Leadership Academy, Academic Hospitalist Academy, and Quality and Safety Educators Academy, with the goal of increasing their ranks in positions of influence throughout healthcare. A scholarship program might even include raising funds to help minority hospitalists pursue Master’s-level programs such as an MBA, MHA, or MMM.
- Develop an educational track, mentoring program, or other support initiative for early-career hospitalist leaders and those interested in developing leadership skills, and ensure it gives specific attention to strategies for increasing the proportion of hospitalists of color in leadership positions.
- Review and revise existing SHM documents such as The Key Principles and Characteristics of an Effective Hospital Medicine Group, the Core Competencies in Hospital Medicine, and various white papers and position statements to ensure they address diversity, equity and inclusion – both with regard to the hospital medicine workforce and leadership, and with regard to patient care and eliminating health disparities.
I’m sure there are plenty of other similar actions we can take that I haven’t thought of. But we need to start the conversation about concrete steps our Society, and the medical specialty we represent, can take to foster real change. And then, we need to follow our words up with actions.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.

