User login
RA Flare Risk Rises Following DMARD Taper to Discontinuation With Conventional Synthetics or TNF Inhibitors
Patients with rheumatoid arthritis (RA) in remission who tapered and then fully stopped either conventional synthetic disease-modifying antirheumatic drug (csDMARD) or tumor necrosis factor (TNF)–inhibitor therapy experienced more disease flares than those who received stable dose treatment in an open-label, randomized trial.
In the 3-year trial, called ARCTIC REWIND, 80% of patients taking stable doses of only csMARDs remained flare-free compared with 38% in another treatment arm taking only csDMARDs who tapered to a half dose and then discontinued all after 1 year. In patients who continued to receive half-dose csDMARDs for the entire study period, 57% remained flare-free.
A separate two treatment arms of the study that assessed the effect of tapering TNF-inhibitor treatment to withdrawal showed that only 25% of patients who tapered TNF inhibitor to withdrawal remained flare-free over 3 years compared with 85% who remained on a stable TNF-inhibitor dose.
Though the risk for flare was higher in both the half-dose csDMARD and drug-free groups, the results also suggested that tapering medication “could be a realistic option for some patients with rheumatoid arthritis in sustained remission on csDMARDs,” wrote Kaja Kjørholt, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and colleagues.
The 3-year results for the csDMARD-only arms of the trial were published in The Lancet Rheumatology. The 3-year results of the TNF-inhibitor arms of the study were presented as an abstract at the annual meeting of the American College of Rheumatology (ACR).
Don’t Avoid Tapering But Take an Individualized Approach
Many rheumatologists will taper patients with RA in remission to lower doses of medication, but the protocols for this study do not reflect clinical practice, noted James R. O’Dell, MD, chief of the Division of Rheumatology at the University of Nebraska Medical Center in Omaha, Nebraska. He was not involved with the research.
“I don’t know of any rheumatologist who would ever think that it was a good idea to taper somebody completely off of all DMARDs,” he told this news organization. “The only surprise is that more of them didn’t flare,” he continued, though he suspected that more patients would flare if they were followed for more time. Rheumatologists also would take a much more individualized approach when tapering to lower doses, he added, and do so at a much slower rate than what was observed in this study.
Both the ACR and the European Alliance of Associations for Rheumatology recommendations for the management of RA stated that tapering DMARDs can be considered for patients who have sustained remission, but they do not mention discontinuing medication entirely.
In the TNF-inhibitor arms of the trial, the tapering group received a half dose of a TNF inhibitor for 4 months before stopping therapy entirely, which Dr. O’Dell noted was a large dip in too short a period.
“Nobody should be surprised that these people flared a lot,” he said. However, tapering to lower doses of a TNF inhibitor can be successful, he noted, adding that more than half of his patients taking a TNF inhibitor are on less than their original dose. Completely tapering off a TNF inhibitor is less common and depends on what other DMARDs a patient is taking, he said, and complete drug-free remission in this population is highly unlikely.
Dr. O’Dell emphasized that the takeaway from these results should not be to avoid tapering medication because of flare risk but instead a tailored approach — something that is not possible with a study protocol — is needed.
“We want our patients to have all the medicine they need and no more,” he said. “That sweet spot is different for each individual patient for how much TNF inhibition or how much conventional therapy they need. If we’re thoughtful about that in the clinic, we can find that sweet spot,” he said.
Details of ARCTIC REWIND
The open-label ARCTIC REWIND trial enrolled patients with RA in sustained remission, determined via Disease Activity Score (DAS), from 10 different hospitals in Norway. Researchers enrolled 160 patients in the csDMARD-only arms and randomized them to receive stable dose csDMARDs for 3 years or half-dose csDMARDs for 1 year, followed by complete withdrawal for the next 2 years; withdrawal of csDMARDs was only done in patients who had not had a flare during the first year. Participants had scheduled clinic visits every 4 months, and full-dose csDMARD therapy was resumed in patients who experienced disease flares.
There was a total of 99 patients randomized in the TNF-inhibitor arms to continue stable TNF-inhibitor therapy or to taper to a half dose for 4 months before discontinuing therapy. Like the csDMARD study, clinic visits occurred every 4 months, and full-dose therapy was resumed if a flare occurred. Patients taking a TNF inhibitor could also take a csDMARD as needed.
Last year, 1-year results for the csDMARD arms were published in JAMA, and 1-year results for the TNF-inhibitor arms were reported in Annals of the Rheumatic Diseases.
At baseline, most patients across the three csDMARD groups (81%) had received methotrexate monotherapy. Triple therapy (methotrexate, sulfasalazine, and hydroxychloroquine) was used in 13% of the stable-dose group, 7% of the half-dose group, and 8% of the half-dose tapering to withdrawal group. Seven individuals in the stable-dose group, three individuals in the half-dose group, and three individuals the half-dose tapering to withdrawal group used other mono/duo therapies.
A total of 139 participants in the csDMARD-only arms completed 3 years of follow-up, with 68 in the stable-dose group, 36 in the half-dose group, and 35 in the half-dose tapering to withdrawal group.
Compared with the stable-dose group, the risk for flare was more than four times higher in the half-dose tapering to withdrawal group (hazard ratio [HR], 4.2; 95% CI, 2.2-8.2) and about three times higher in the half-dose group (HR, 2.9; 95% CI, 1.5-5.9). The flare risk between the half dose and half-dose tapering to withdrawal group was not statistically significant.
Most patients regained DAS remission status in the next clinic visit following a flare, the authors reported. Comparing the last visit to baseline, 10 patients in the taper-to-withdrawal group (27%) had increased treatment — either by adding a biologic or increasing csDMARD dose — compared with one patient (3%) in the half-dose group and 11 patients (14%) in the stable-dose group. Adverse events were common across all three groups, though were highest in the tapering to withdrawal group.
In the TNF-inhibitor arms, a total of 80 patients completed the 3-year follow-up. By the end of 3 years, 75% of the tapering group experienced a disease flare compared with 15% of the stable TNF-inhibitor group. Most patients regained DAS remission status in the next clinic visit following a flare, the authors reported. During the study, 23% of the tapering group and 13% of the stable TNF-inhibitor group used systemic glucocorticoids. Four patients in the tapering group and two patients in the stable TNF-inhibitor group switched to another TNF inhibitor during the study. An additional two patients in the stable TNF-inhibitor group switched to a Janus kinase inhibitor during the 3-year study.
Adverse events were similar in both treatment groups, but serious adverse events were more common in the tapering group (21%) than in the stable-dose group (11%).
The authors concluded that the findings did not support tapering a TNF inhibitor to withdrawal for patients in sustained remission, but they noted that additional research is needed to identify which patients would fare better or worse tapering csDMARDs.
ARCTIC REWIND was funded by grants from The Research Council of Norway and The South-Eastern Norway Regional Health Authorities. Many of the authors disclosed financial ties to pharmaceutical companies. Dr. O’Dell disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with rheumatoid arthritis (RA) in remission who tapered and then fully stopped either conventional synthetic disease-modifying antirheumatic drug (csDMARD) or tumor necrosis factor (TNF)–inhibitor therapy experienced more disease flares than those who received stable dose treatment in an open-label, randomized trial.
In the 3-year trial, called ARCTIC REWIND, 80% of patients taking stable doses of only csMARDs remained flare-free compared with 38% in another treatment arm taking only csDMARDs who tapered to a half dose and then discontinued all after 1 year. In patients who continued to receive half-dose csDMARDs for the entire study period, 57% remained flare-free.
A separate two treatment arms of the study that assessed the effect of tapering TNF-inhibitor treatment to withdrawal showed that only 25% of patients who tapered TNF inhibitor to withdrawal remained flare-free over 3 years compared with 85% who remained on a stable TNF-inhibitor dose.
Though the risk for flare was higher in both the half-dose csDMARD and drug-free groups, the results also suggested that tapering medication “could be a realistic option for some patients with rheumatoid arthritis in sustained remission on csDMARDs,” wrote Kaja Kjørholt, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and colleagues.
The 3-year results for the csDMARD-only arms of the trial were published in The Lancet Rheumatology. The 3-year results of the TNF-inhibitor arms of the study were presented as an abstract at the annual meeting of the American College of Rheumatology (ACR).
Don’t Avoid Tapering But Take an Individualized Approach
Many rheumatologists will taper patients with RA in remission to lower doses of medication, but the protocols for this study do not reflect clinical practice, noted James R. O’Dell, MD, chief of the Division of Rheumatology at the University of Nebraska Medical Center in Omaha, Nebraska. He was not involved with the research.
“I don’t know of any rheumatologist who would ever think that it was a good idea to taper somebody completely off of all DMARDs,” he told this news organization. “The only surprise is that more of them didn’t flare,” he continued, though he suspected that more patients would flare if they were followed for more time. Rheumatologists also would take a much more individualized approach when tapering to lower doses, he added, and do so at a much slower rate than what was observed in this study.
Both the ACR and the European Alliance of Associations for Rheumatology recommendations for the management of RA stated that tapering DMARDs can be considered for patients who have sustained remission, but they do not mention discontinuing medication entirely.
In the TNF-inhibitor arms of the trial, the tapering group received a half dose of a TNF inhibitor for 4 months before stopping therapy entirely, which Dr. O’Dell noted was a large dip in too short a period.
“Nobody should be surprised that these people flared a lot,” he said. However, tapering to lower doses of a TNF inhibitor can be successful, he noted, adding that more than half of his patients taking a TNF inhibitor are on less than their original dose. Completely tapering off a TNF inhibitor is less common and depends on what other DMARDs a patient is taking, he said, and complete drug-free remission in this population is highly unlikely.
Dr. O’Dell emphasized that the takeaway from these results should not be to avoid tapering medication because of flare risk but instead a tailored approach — something that is not possible with a study protocol — is needed.
“We want our patients to have all the medicine they need and no more,” he said. “That sweet spot is different for each individual patient for how much TNF inhibition or how much conventional therapy they need. If we’re thoughtful about that in the clinic, we can find that sweet spot,” he said.
Details of ARCTIC REWIND
The open-label ARCTIC REWIND trial enrolled patients with RA in sustained remission, determined via Disease Activity Score (DAS), from 10 different hospitals in Norway. Researchers enrolled 160 patients in the csDMARD-only arms and randomized them to receive stable dose csDMARDs for 3 years or half-dose csDMARDs for 1 year, followed by complete withdrawal for the next 2 years; withdrawal of csDMARDs was only done in patients who had not had a flare during the first year. Participants had scheduled clinic visits every 4 months, and full-dose csDMARD therapy was resumed in patients who experienced disease flares.
There was a total of 99 patients randomized in the TNF-inhibitor arms to continue stable TNF-inhibitor therapy or to taper to a half dose for 4 months before discontinuing therapy. Like the csDMARD study, clinic visits occurred every 4 months, and full-dose therapy was resumed if a flare occurred. Patients taking a TNF inhibitor could also take a csDMARD as needed.
Last year, 1-year results for the csDMARD arms were published in JAMA, and 1-year results for the TNF-inhibitor arms were reported in Annals of the Rheumatic Diseases.
At baseline, most patients across the three csDMARD groups (81%) had received methotrexate monotherapy. Triple therapy (methotrexate, sulfasalazine, and hydroxychloroquine) was used in 13% of the stable-dose group, 7% of the half-dose group, and 8% of the half-dose tapering to withdrawal group. Seven individuals in the stable-dose group, three individuals in the half-dose group, and three individuals the half-dose tapering to withdrawal group used other mono/duo therapies.
A total of 139 participants in the csDMARD-only arms completed 3 years of follow-up, with 68 in the stable-dose group, 36 in the half-dose group, and 35 in the half-dose tapering to withdrawal group.
Compared with the stable-dose group, the risk for flare was more than four times higher in the half-dose tapering to withdrawal group (hazard ratio [HR], 4.2; 95% CI, 2.2-8.2) and about three times higher in the half-dose group (HR, 2.9; 95% CI, 1.5-5.9). The flare risk between the half dose and half-dose tapering to withdrawal group was not statistically significant.
Most patients regained DAS remission status in the next clinic visit following a flare, the authors reported. Comparing the last visit to baseline, 10 patients in the taper-to-withdrawal group (27%) had increased treatment — either by adding a biologic or increasing csDMARD dose — compared with one patient (3%) in the half-dose group and 11 patients (14%) in the stable-dose group. Adverse events were common across all three groups, though were highest in the tapering to withdrawal group.
In the TNF-inhibitor arms, a total of 80 patients completed the 3-year follow-up. By the end of 3 years, 75% of the tapering group experienced a disease flare compared with 15% of the stable TNF-inhibitor group. Most patients regained DAS remission status in the next clinic visit following a flare, the authors reported. During the study, 23% of the tapering group and 13% of the stable TNF-inhibitor group used systemic glucocorticoids. Four patients in the tapering group and two patients in the stable TNF-inhibitor group switched to another TNF inhibitor during the study. An additional two patients in the stable TNF-inhibitor group switched to a Janus kinase inhibitor during the 3-year study.
Adverse events were similar in both treatment groups, but serious adverse events were more common in the tapering group (21%) than in the stable-dose group (11%).
The authors concluded that the findings did not support tapering a TNF inhibitor to withdrawal for patients in sustained remission, but they noted that additional research is needed to identify which patients would fare better or worse tapering csDMARDs.
ARCTIC REWIND was funded by grants from The Research Council of Norway and The South-Eastern Norway Regional Health Authorities. Many of the authors disclosed financial ties to pharmaceutical companies. Dr. O’Dell disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with rheumatoid arthritis (RA) in remission who tapered and then fully stopped either conventional synthetic disease-modifying antirheumatic drug (csDMARD) or tumor necrosis factor (TNF)–inhibitor therapy experienced more disease flares than those who received stable dose treatment in an open-label, randomized trial.
In the 3-year trial, called ARCTIC REWIND, 80% of patients taking stable doses of only csMARDs remained flare-free compared with 38% in another treatment arm taking only csDMARDs who tapered to a half dose and then discontinued all after 1 year. In patients who continued to receive half-dose csDMARDs for the entire study period, 57% remained flare-free.
A separate two treatment arms of the study that assessed the effect of tapering TNF-inhibitor treatment to withdrawal showed that only 25% of patients who tapered TNF inhibitor to withdrawal remained flare-free over 3 years compared with 85% who remained on a stable TNF-inhibitor dose.
Though the risk for flare was higher in both the half-dose csDMARD and drug-free groups, the results also suggested that tapering medication “could be a realistic option for some patients with rheumatoid arthritis in sustained remission on csDMARDs,” wrote Kaja Kjørholt, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and colleagues.
The 3-year results for the csDMARD-only arms of the trial were published in The Lancet Rheumatology. The 3-year results of the TNF-inhibitor arms of the study were presented as an abstract at the annual meeting of the American College of Rheumatology (ACR).
Don’t Avoid Tapering But Take an Individualized Approach
Many rheumatologists will taper patients with RA in remission to lower doses of medication, but the protocols for this study do not reflect clinical practice, noted James R. O’Dell, MD, chief of the Division of Rheumatology at the University of Nebraska Medical Center in Omaha, Nebraska. He was not involved with the research.
“I don’t know of any rheumatologist who would ever think that it was a good idea to taper somebody completely off of all DMARDs,” he told this news organization. “The only surprise is that more of them didn’t flare,” he continued, though he suspected that more patients would flare if they were followed for more time. Rheumatologists also would take a much more individualized approach when tapering to lower doses, he added, and do so at a much slower rate than what was observed in this study.
Both the ACR and the European Alliance of Associations for Rheumatology recommendations for the management of RA stated that tapering DMARDs can be considered for patients who have sustained remission, but they do not mention discontinuing medication entirely.
In the TNF-inhibitor arms of the trial, the tapering group received a half dose of a TNF inhibitor for 4 months before stopping therapy entirely, which Dr. O’Dell noted was a large dip in too short a period.
“Nobody should be surprised that these people flared a lot,” he said. However, tapering to lower doses of a TNF inhibitor can be successful, he noted, adding that more than half of his patients taking a TNF inhibitor are on less than their original dose. Completely tapering off a TNF inhibitor is less common and depends on what other DMARDs a patient is taking, he said, and complete drug-free remission in this population is highly unlikely.
Dr. O’Dell emphasized that the takeaway from these results should not be to avoid tapering medication because of flare risk but instead a tailored approach — something that is not possible with a study protocol — is needed.
“We want our patients to have all the medicine they need and no more,” he said. “That sweet spot is different for each individual patient for how much TNF inhibition or how much conventional therapy they need. If we’re thoughtful about that in the clinic, we can find that sweet spot,” he said.
Details of ARCTIC REWIND
The open-label ARCTIC REWIND trial enrolled patients with RA in sustained remission, determined via Disease Activity Score (DAS), from 10 different hospitals in Norway. Researchers enrolled 160 patients in the csDMARD-only arms and randomized them to receive stable dose csDMARDs for 3 years or half-dose csDMARDs for 1 year, followed by complete withdrawal for the next 2 years; withdrawal of csDMARDs was only done in patients who had not had a flare during the first year. Participants had scheduled clinic visits every 4 months, and full-dose csDMARD therapy was resumed in patients who experienced disease flares.
There was a total of 99 patients randomized in the TNF-inhibitor arms to continue stable TNF-inhibitor therapy or to taper to a half dose for 4 months before discontinuing therapy. Like the csDMARD study, clinic visits occurred every 4 months, and full-dose therapy was resumed if a flare occurred. Patients taking a TNF inhibitor could also take a csDMARD as needed.
Last year, 1-year results for the csDMARD arms were published in JAMA, and 1-year results for the TNF-inhibitor arms were reported in Annals of the Rheumatic Diseases.
At baseline, most patients across the three csDMARD groups (81%) had received methotrexate monotherapy. Triple therapy (methotrexate, sulfasalazine, and hydroxychloroquine) was used in 13% of the stable-dose group, 7% of the half-dose group, and 8% of the half-dose tapering to withdrawal group. Seven individuals in the stable-dose group, three individuals in the half-dose group, and three individuals the half-dose tapering to withdrawal group used other mono/duo therapies.
A total of 139 participants in the csDMARD-only arms completed 3 years of follow-up, with 68 in the stable-dose group, 36 in the half-dose group, and 35 in the half-dose tapering to withdrawal group.
Compared with the stable-dose group, the risk for flare was more than four times higher in the half-dose tapering to withdrawal group (hazard ratio [HR], 4.2; 95% CI, 2.2-8.2) and about three times higher in the half-dose group (HR, 2.9; 95% CI, 1.5-5.9). The flare risk between the half dose and half-dose tapering to withdrawal group was not statistically significant.
Most patients regained DAS remission status in the next clinic visit following a flare, the authors reported. Comparing the last visit to baseline, 10 patients in the taper-to-withdrawal group (27%) had increased treatment — either by adding a biologic or increasing csDMARD dose — compared with one patient (3%) in the half-dose group and 11 patients (14%) in the stable-dose group. Adverse events were common across all three groups, though were highest in the tapering to withdrawal group.
In the TNF-inhibitor arms, a total of 80 patients completed the 3-year follow-up. By the end of 3 years, 75% of the tapering group experienced a disease flare compared with 15% of the stable TNF-inhibitor group. Most patients regained DAS remission status in the next clinic visit following a flare, the authors reported. During the study, 23% of the tapering group and 13% of the stable TNF-inhibitor group used systemic glucocorticoids. Four patients in the tapering group and two patients in the stable TNF-inhibitor group switched to another TNF inhibitor during the study. An additional two patients in the stable TNF-inhibitor group switched to a Janus kinase inhibitor during the 3-year study.
Adverse events were similar in both treatment groups, but serious adverse events were more common in the tapering group (21%) than in the stable-dose group (11%).
The authors concluded that the findings did not support tapering a TNF inhibitor to withdrawal for patients in sustained remission, but they noted that additional research is needed to identify which patients would fare better or worse tapering csDMARDs.
ARCTIC REWIND was funded by grants from The Research Council of Norway and The South-Eastern Norway Regional Health Authorities. Many of the authors disclosed financial ties to pharmaceutical companies. Dr. O’Dell disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Worldwide Uptick in Invasive Group A Streptococcus Disease Post Pandemic — What Should We Know?
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.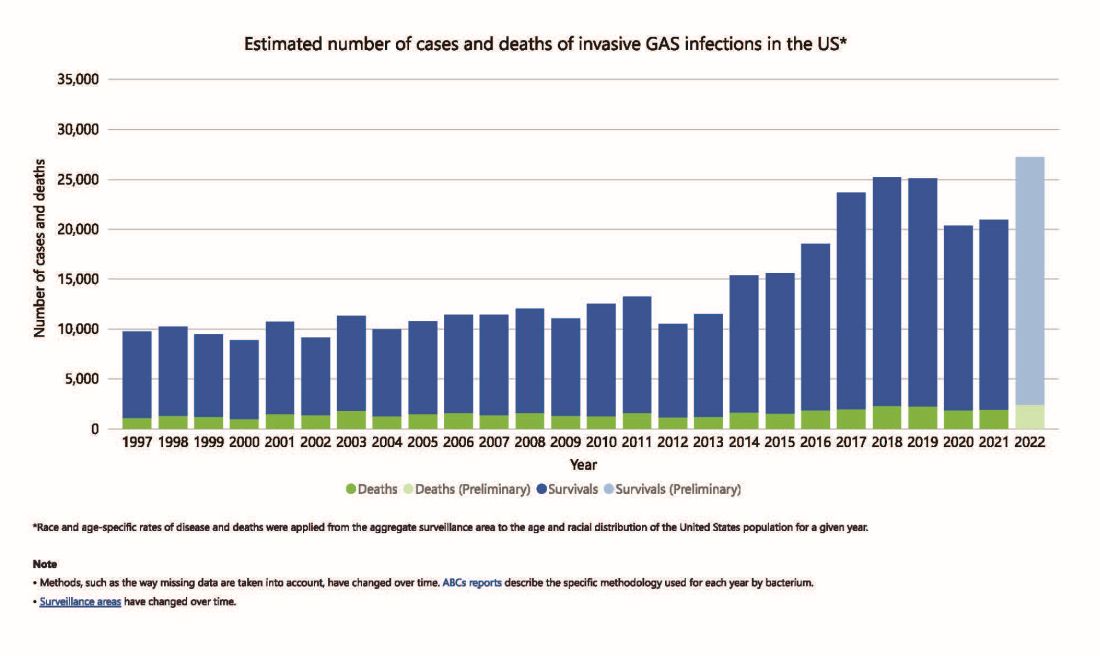
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 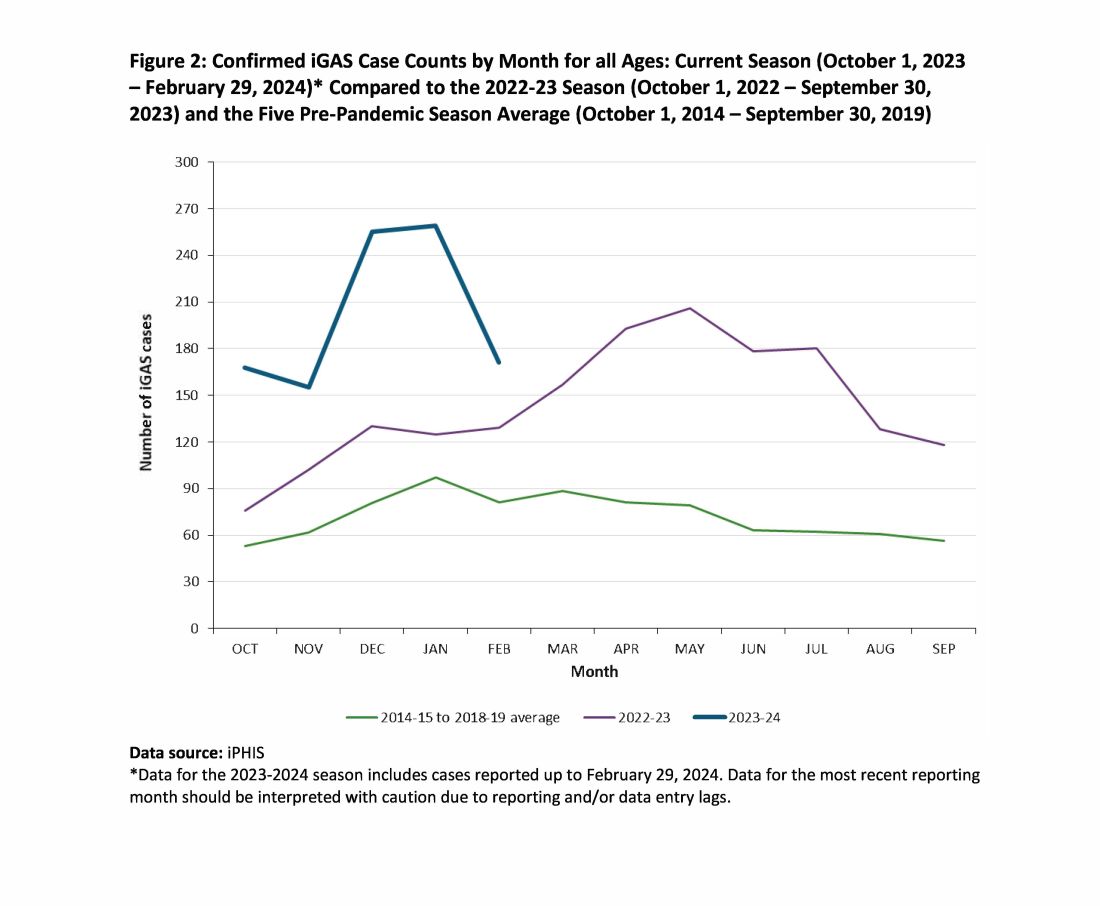
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
One-Minute Speech Test Could Help Assess Dementia Risk
BUDAPEST, HUNGARY — , suggests research.
János Kálmán, MD, PhD, and colleagues at the University of Szeged in Hungary have developed an automated speech analysis approach called the Speech-Gap Test (S-GAP Test) that is unique because it focuses on the temporal changes made when someone talks. This means it does not overcomplicate matters by also assessing the phonetics and semantics of speech, Dr. Kálmán said.
Dr. Kálmán presented his findings at the 32nd European Congress of Psychiatry.
Temporal Speech Parameters
The test analyzes parameters such as how quickly someone speaks, whether they hesitate when they talk, how long the hesitation lasts, and how many silent pauses they make. This can be done with a mere 60-second sample of speech, Dr. Kálmán said, noting that other automated speech and language tools currently in development need much longer audio samples.
“We tried different approaches and we finally ended up with the temporal speech parameters because these are not culture-dependent, not education-dependent, and could be more reliable than the semantic parts of [speech] analysis,” he explained.
The analysis of temporal speech parameters is also not language-dependent. Although the S-GAP Test was developed using audio samples from native Hungarian speakers, Dr. Kálmán and his collaborators have shown that it works just as well with samples from native English and German speakers. They now plan to validate the test further using samples from native Spanish speakers.
For Screening, Not Diagnosis
Currently, “the only purpose of this tool would be initial screening,” Dr. Kálmán said at the congress. It is not for diagnosis, and there is no intention to get it registered as a medical device.
A national survey of primary care physicians conducted by Dr. Kálmán and collaborators showed that there was little time for performing standard cognitive tests during the average consultation. Thus, the original idea was that the S-GAP Test would be an aid to help primary care physicians quickly flag whether a patient might have cognitive problems that needed further assessment at a memory clinic or by more specialist neurology services.
The goalposts have since been moved, from developing a pure telemedicine solution to a more widespread application that perhaps anyone could buy and download from the internet or using a smartphone.
Dr. Kálmán doesn’t discount developing a more sophisticated version of the S-GAP Test in the future that combines temporal speech parameters with biomarkers for mild cognitive impairment or Alzheimer’s disease and could be used in memory clinics and by neurologists for the purpose of diagnosis.
Detect to Prevent?
The big question is: What happens to all the people that could be flagged as needing further assessment using tools such as the S-GAP Test?
Tackling risk factors for dementia will probably be key, said Robert Perneckzy, MD, MBA, professor of translational dementia research at Ludwig Maximilian University of Munich, Imperial College London, and the University of Sheffield.
According to the Lancet Commission on dementia, there are 12 potentially modifiable risk factors for dementia. Their influence varies throughout the life course, but certain early events, such as the level of education attained, can’t be modified in an older patient.
That said, there are many risk factors that might still be influenced later in life, such as adequately treating comorbid conditions such as diabetes, and addressing alcohol consumption and smoking practices.
“We can do things in terms of personal risk, risk mitigation, which have a huge effect on dementia risk much later in life,” said Dr. Perneckzy.
“The speech-based assessments are another opportunity to save our time as doctors to do assessments before someone comes to the memory clinic,” he said.
The S-GAP Test is under development by the University of Szeged. Dr. Kálmán is a co-inventor. Dr. Perneckzy had no relevant conflicts of interest but has helped validate the S-GAP Test in the German language.
A version of this article appeared on Medscape.com.
BUDAPEST, HUNGARY — , suggests research.
János Kálmán, MD, PhD, and colleagues at the University of Szeged in Hungary have developed an automated speech analysis approach called the Speech-Gap Test (S-GAP Test) that is unique because it focuses on the temporal changes made when someone talks. This means it does not overcomplicate matters by also assessing the phonetics and semantics of speech, Dr. Kálmán said.
Dr. Kálmán presented his findings at the 32nd European Congress of Psychiatry.
Temporal Speech Parameters
The test analyzes parameters such as how quickly someone speaks, whether they hesitate when they talk, how long the hesitation lasts, and how many silent pauses they make. This can be done with a mere 60-second sample of speech, Dr. Kálmán said, noting that other automated speech and language tools currently in development need much longer audio samples.
“We tried different approaches and we finally ended up with the temporal speech parameters because these are not culture-dependent, not education-dependent, and could be more reliable than the semantic parts of [speech] analysis,” he explained.
The analysis of temporal speech parameters is also not language-dependent. Although the S-GAP Test was developed using audio samples from native Hungarian speakers, Dr. Kálmán and his collaborators have shown that it works just as well with samples from native English and German speakers. They now plan to validate the test further using samples from native Spanish speakers.
For Screening, Not Diagnosis
Currently, “the only purpose of this tool would be initial screening,” Dr. Kálmán said at the congress. It is not for diagnosis, and there is no intention to get it registered as a medical device.
A national survey of primary care physicians conducted by Dr. Kálmán and collaborators showed that there was little time for performing standard cognitive tests during the average consultation. Thus, the original idea was that the S-GAP Test would be an aid to help primary care physicians quickly flag whether a patient might have cognitive problems that needed further assessment at a memory clinic or by more specialist neurology services.
The goalposts have since been moved, from developing a pure telemedicine solution to a more widespread application that perhaps anyone could buy and download from the internet or using a smartphone.
Dr. Kálmán doesn’t discount developing a more sophisticated version of the S-GAP Test in the future that combines temporal speech parameters with biomarkers for mild cognitive impairment or Alzheimer’s disease and could be used in memory clinics and by neurologists for the purpose of diagnosis.
Detect to Prevent?
The big question is: What happens to all the people that could be flagged as needing further assessment using tools such as the S-GAP Test?
Tackling risk factors for dementia will probably be key, said Robert Perneckzy, MD, MBA, professor of translational dementia research at Ludwig Maximilian University of Munich, Imperial College London, and the University of Sheffield.
According to the Lancet Commission on dementia, there are 12 potentially modifiable risk factors for dementia. Their influence varies throughout the life course, but certain early events, such as the level of education attained, can’t be modified in an older patient.
That said, there are many risk factors that might still be influenced later in life, such as adequately treating comorbid conditions such as diabetes, and addressing alcohol consumption and smoking practices.
“We can do things in terms of personal risk, risk mitigation, which have a huge effect on dementia risk much later in life,” said Dr. Perneckzy.
“The speech-based assessments are another opportunity to save our time as doctors to do assessments before someone comes to the memory clinic,” he said.
The S-GAP Test is under development by the University of Szeged. Dr. Kálmán is a co-inventor. Dr. Perneckzy had no relevant conflicts of interest but has helped validate the S-GAP Test in the German language.
A version of this article appeared on Medscape.com.
BUDAPEST, HUNGARY — , suggests research.
János Kálmán, MD, PhD, and colleagues at the University of Szeged in Hungary have developed an automated speech analysis approach called the Speech-Gap Test (S-GAP Test) that is unique because it focuses on the temporal changes made when someone talks. This means it does not overcomplicate matters by also assessing the phonetics and semantics of speech, Dr. Kálmán said.
Dr. Kálmán presented his findings at the 32nd European Congress of Psychiatry.
Temporal Speech Parameters
The test analyzes parameters such as how quickly someone speaks, whether they hesitate when they talk, how long the hesitation lasts, and how many silent pauses they make. This can be done with a mere 60-second sample of speech, Dr. Kálmán said, noting that other automated speech and language tools currently in development need much longer audio samples.
“We tried different approaches and we finally ended up with the temporal speech parameters because these are not culture-dependent, not education-dependent, and could be more reliable than the semantic parts of [speech] analysis,” he explained.
The analysis of temporal speech parameters is also not language-dependent. Although the S-GAP Test was developed using audio samples from native Hungarian speakers, Dr. Kálmán and his collaborators have shown that it works just as well with samples from native English and German speakers. They now plan to validate the test further using samples from native Spanish speakers.
For Screening, Not Diagnosis
Currently, “the only purpose of this tool would be initial screening,” Dr. Kálmán said at the congress. It is not for diagnosis, and there is no intention to get it registered as a medical device.
A national survey of primary care physicians conducted by Dr. Kálmán and collaborators showed that there was little time for performing standard cognitive tests during the average consultation. Thus, the original idea was that the S-GAP Test would be an aid to help primary care physicians quickly flag whether a patient might have cognitive problems that needed further assessment at a memory clinic or by more specialist neurology services.
The goalposts have since been moved, from developing a pure telemedicine solution to a more widespread application that perhaps anyone could buy and download from the internet or using a smartphone.
Dr. Kálmán doesn’t discount developing a more sophisticated version of the S-GAP Test in the future that combines temporal speech parameters with biomarkers for mild cognitive impairment or Alzheimer’s disease and could be used in memory clinics and by neurologists for the purpose of diagnosis.
Detect to Prevent?
The big question is: What happens to all the people that could be flagged as needing further assessment using tools such as the S-GAP Test?
Tackling risk factors for dementia will probably be key, said Robert Perneckzy, MD, MBA, professor of translational dementia research at Ludwig Maximilian University of Munich, Imperial College London, and the University of Sheffield.
According to the Lancet Commission on dementia, there are 12 potentially modifiable risk factors for dementia. Their influence varies throughout the life course, but certain early events, such as the level of education attained, can’t be modified in an older patient.
That said, there are many risk factors that might still be influenced later in life, such as adequately treating comorbid conditions such as diabetes, and addressing alcohol consumption and smoking practices.
“We can do things in terms of personal risk, risk mitigation, which have a huge effect on dementia risk much later in life,” said Dr. Perneckzy.
“The speech-based assessments are another opportunity to save our time as doctors to do assessments before someone comes to the memory clinic,” he said.
The S-GAP Test is under development by the University of Szeged. Dr. Kálmán is a co-inventor. Dr. Perneckzy had no relevant conflicts of interest but has helped validate the S-GAP Test in the German language.
A version of this article appeared on Medscape.com.
FROM THE EUROPEAN CONGRESS OF PSYCHIATRY
CDC Investigating Adverse Events Related to Counterfeit, Mishandled Botulinum Toxin
, such as homes and spas, according to an announcement of an investigation into these reports from the Centers for Disease Control and Prevention posted online April 15.
Reactions have included blurry vision, double vision, drooping eyelids, difficult swallowing or breathing, and other symptoms of botulism.
Of the 19 individuals — all of whom identified as female and had a mean age of 39 years — 9 (60%) were hospitalized and 4 (21%) were treated with botulism antitoxin because of concerns that the botulinum toxin could have spread beyond the injection site. Also, five were tested for botulism and their results were negative.
The CDC, several state and local health departments, and the US Food and Drug Administration (FDA) are investigating these reports, according to the announcement.
States reporting these cases include Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee, and Washington. According to the CDC summary, some of the individuals “received injections with counterfeit products or products with unverified sources. Investigation into the sources of these products is ongoing.” All but one report involved receiving botulinum toxin injections for cosmetic purposes.
Recent cases of botulism-like illnesses possibly related to counterfeit botulinum toxin reported in Illinois and Tennessee, prompted the American Society for Dermatologic Surgery Association (ASDSA) to call on states to increase oversight of medical care in all settings, including medical spas, the ASDSA announced on April 12.
The CDC summary advises clinicians to consider the possibility of adverse effects from botulinum toxin injection, including for cosmetic reasons, when patients present with signs and symptoms consistent with botulism near the injection site. Symptoms of botulism include blurry or double vision, drooping eyelids, difficulty swallowing, difficulty breathing, and muscle weakness.
For people who are considering botulinum toxin for cosmetic or medical reasons, recommendations from the CDC include asking the provider and setting, such as a clinic or spa, if they are licensed and trained to provide these injections, and to ask if the product is approved by the FDA and from a reliable source, and, “if in doubt, don’t get the injection.”
This ‘Should Never Happen’
“The report of people getting botulism from botulinum toxin injections is frightening, and should never happen,” Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, told this news organization.
These reports show “how important it is to receive botulinum toxin injections only in a medical office, and from or under the direction of a qualified, trained, and licensed individual, like a board certified dermatologist,” added Dr. Green, who practices in Rockville, Maryland. “Other types of practitioners may not adhere to the same standards of professionalism, especially not always putting patient safety first.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
, such as homes and spas, according to an announcement of an investigation into these reports from the Centers for Disease Control and Prevention posted online April 15.
Reactions have included blurry vision, double vision, drooping eyelids, difficult swallowing or breathing, and other symptoms of botulism.
Of the 19 individuals — all of whom identified as female and had a mean age of 39 years — 9 (60%) were hospitalized and 4 (21%) were treated with botulism antitoxin because of concerns that the botulinum toxin could have spread beyond the injection site. Also, five were tested for botulism and their results were negative.
The CDC, several state and local health departments, and the US Food and Drug Administration (FDA) are investigating these reports, according to the announcement.
States reporting these cases include Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee, and Washington. According to the CDC summary, some of the individuals “received injections with counterfeit products or products with unverified sources. Investigation into the sources of these products is ongoing.” All but one report involved receiving botulinum toxin injections for cosmetic purposes.
Recent cases of botulism-like illnesses possibly related to counterfeit botulinum toxin reported in Illinois and Tennessee, prompted the American Society for Dermatologic Surgery Association (ASDSA) to call on states to increase oversight of medical care in all settings, including medical spas, the ASDSA announced on April 12.
The CDC summary advises clinicians to consider the possibility of adverse effects from botulinum toxin injection, including for cosmetic reasons, when patients present with signs and symptoms consistent with botulism near the injection site. Symptoms of botulism include blurry or double vision, drooping eyelids, difficulty swallowing, difficulty breathing, and muscle weakness.
For people who are considering botulinum toxin for cosmetic or medical reasons, recommendations from the CDC include asking the provider and setting, such as a clinic or spa, if they are licensed and trained to provide these injections, and to ask if the product is approved by the FDA and from a reliable source, and, “if in doubt, don’t get the injection.”
This ‘Should Never Happen’
“The report of people getting botulism from botulinum toxin injections is frightening, and should never happen,” Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, told this news organization.
These reports show “how important it is to receive botulinum toxin injections only in a medical office, and from or under the direction of a qualified, trained, and licensed individual, like a board certified dermatologist,” added Dr. Green, who practices in Rockville, Maryland. “Other types of practitioners may not adhere to the same standards of professionalism, especially not always putting patient safety first.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
, such as homes and spas, according to an announcement of an investigation into these reports from the Centers for Disease Control and Prevention posted online April 15.
Reactions have included blurry vision, double vision, drooping eyelids, difficult swallowing or breathing, and other symptoms of botulism.
Of the 19 individuals — all of whom identified as female and had a mean age of 39 years — 9 (60%) were hospitalized and 4 (21%) were treated with botulism antitoxin because of concerns that the botulinum toxin could have spread beyond the injection site. Also, five were tested for botulism and their results were negative.
The CDC, several state and local health departments, and the US Food and Drug Administration (FDA) are investigating these reports, according to the announcement.
States reporting these cases include Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee, and Washington. According to the CDC summary, some of the individuals “received injections with counterfeit products or products with unverified sources. Investigation into the sources of these products is ongoing.” All but one report involved receiving botulinum toxin injections for cosmetic purposes.
Recent cases of botulism-like illnesses possibly related to counterfeit botulinum toxin reported in Illinois and Tennessee, prompted the American Society for Dermatologic Surgery Association (ASDSA) to call on states to increase oversight of medical care in all settings, including medical spas, the ASDSA announced on April 12.
The CDC summary advises clinicians to consider the possibility of adverse effects from botulinum toxin injection, including for cosmetic reasons, when patients present with signs and symptoms consistent with botulism near the injection site. Symptoms of botulism include blurry or double vision, drooping eyelids, difficulty swallowing, difficulty breathing, and muscle weakness.
For people who are considering botulinum toxin for cosmetic or medical reasons, recommendations from the CDC include asking the provider and setting, such as a clinic or spa, if they are licensed and trained to provide these injections, and to ask if the product is approved by the FDA and from a reliable source, and, “if in doubt, don’t get the injection.”
This ‘Should Never Happen’
“The report of people getting botulism from botulinum toxin injections is frightening, and should never happen,” Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, told this news organization.
These reports show “how important it is to receive botulinum toxin injections only in a medical office, and from or under the direction of a qualified, trained, and licensed individual, like a board certified dermatologist,” added Dr. Green, who practices in Rockville, Maryland. “Other types of practitioners may not adhere to the same standards of professionalism, especially not always putting patient safety first.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
Working From Home: Doctors’ Options Are Not Limited to Classic Telemedicine
The appeal of working from home is undeniable. It comes with no daily commute, casual dress, and the ability to manage work-life balance more effectively.
Telemedicine is often the first thing that comes to mind when physicians think about remote medical practice. In its traditional sense, telemedicine entails live video consults, replicating the in-person experience as closely as possible, minus the hands-on component. However, this format is just one of many types of virtual care presenting opportunities to practice medicine from home.
The scope and volume of such opportunities are expanding due to technology, regulatory shifts at the state and federal levels favoring remote healthcare, and a wider move toward remote work. Virtual practice options for physicians range from full-time employment to flexible part-time positions that can be used to earn supplementary income.
Just a few of those virtual options are:
Remote Patient Monitoring
Remote patient monitoring uses technology for tracking patient health data, applicable in real-time or asynchronously, through devices ranging from specialized monitors to consumer wearables. Data are securely transmitted to healthcare providers, enabling them to guide or make treatment choices remotely. This method has proven particularly valuable in managing chronic diseases where continuous monitoring can significantly affect outcomes.
Like standard telemedicine, remote patient monitoring offers flexibility, autonomy, and the ability to work from home. It is picking up steam across the healthcare industry, especially in critical care, surgery, post-acute care, and primary care, so there are opportunities for physicians across a variety of specialties.
Online Medication Management and Text-Based Consults
Gathering necessary information for patient care decisions often doesn’t require a direct, face-to-face visit in person or by telemedicine. Clinical data can be efficiently collected through online forms, HIPAA-compliant messaging, medical record reviews, and information gathered by staff.
An approach that uses all these sources enables effective medication management for stable chronic conditions (such as hypertension), as well as straightforward but simple acute issues (such as urinary tract infections). It also is useful for quick follow-ups with patients after starting new treatments, to address questions between visits, and to give them educational material.
Some medical practices and virtual healthcare corporations have made online medication management and text-based consults the center of their business model. Part-time positions with platforms that offer this type of care let physicians fit consultations into their schedule as time permits, without committing to scheduled appointments.
eConsults
Electronic consultations, or eConsults, facilitate collaboration among healthcare professionals about complex cases without direct patient interaction.
These services operate via online platforms that support asynchronous communication and often bypass the need for a traditional referral. Typically, a primary care provider submits a query that is then assigned to a specialist. Next, the specialist reviews the information and offers recommendations for the patient’s care plan.
Major eConsult platforms such as AristaMD and RubiconMD contract with healthcare systems and medical practices. Physicians can easily join the specialist panels of these companies and complete assigned consultations from their homes or offices, paid on a per-consult basis. They should check their employment contracts to make sure such independent contract work is allowed.
Phone-Only On-Call Positions
On-call rotations for after-hour care bring with them challenges in staffing and scheduling vacations. These challenges have helped trigger as-needed or per diem on-call roles, in which a physician provides recommendations and orders over the phone without needing to visit an office or a hospital.
Examples of workplaces that employ phone-only on-call physicians include smaller jails, mental health facilities, dialysis centers, long-term care facilities, and sporting groups or events needing back-up for on-site nurses or emergency medical technicians.
While these positions can sometimes be challenging for a physician to find, they are out there. They can be a fantastic option to earn additional income through low-stress clinical work performed from home.
Supervision of Nurse Practitioners (NPs) and Physician Assistants (PAs)
In states that mandate such physician oversight, it often be conducted remotely — depending on that state’s rules, the practice type, and the scope of services being provided. This remote option introduces part-time opportunities for physicians to oversee NPs and PAs without being in the medical office. Essentially, the doctor needs to be available for phone or email consultations, complete chart reviews, and meet regularly with the provider.
Remote supervision roles are available across various types of healthcare organizations and medical practices. There also are opportunities with insurers, many of which have established NP-run, in-home member assessment programs that require remote supervision by a doctor.
Remote Medical Directorships
Medical directors are a key part of the clinician team in a wide variety of healthcare settings requiring clinical protocol oversight, regulatory compliance, and guidance for other clinicians making treatment decisions. Many directorships do not require direct patient contact and therefore are conducive to remote work, given technologies such as electronic health record and secure messaging systems.
Organizations such as emergency medical service agencies, hospice services, med spas, blood and plasma donation centers, home health agencies, and substance use disorder treatment programs increasingly rely on remote medical directorships to meet legal requirements and accreditation standards.
Although these positions are often viewed as “nonclinical,” they carry significant clinical responsibilities. Examples are developing and reviewing treatment protocols, ensuring adherence to healthcare regulations, and sometimes intervening in complex patient cases or when adverse outcomes occur.
Keeping a Role in Patient Welfare
Clearly, working from home as a physician doesn’t have to mean taking on a nonclinical job. Beyond the options already mentioned, there are numerous others — for example, working as a medical monitor for clinical trials, in utilization management for insurance companies, or in conducting independent medical exams for insurance claims. While these roles don’t involve direct patient treatment, they require similar skills and affect the quality of care.
If such remote opportunities aren’t currently available in your workplace, consider approaching your management about trying them.
Physicians who are experiencing burnout, seeking a career change, or interested in earning extra income should consider exploring more of the unconventional ways that they can practice medicine.
A version of this article appeared on Medscape.com.
The appeal of working from home is undeniable. It comes with no daily commute, casual dress, and the ability to manage work-life balance more effectively.
Telemedicine is often the first thing that comes to mind when physicians think about remote medical practice. In its traditional sense, telemedicine entails live video consults, replicating the in-person experience as closely as possible, minus the hands-on component. However, this format is just one of many types of virtual care presenting opportunities to practice medicine from home.
The scope and volume of such opportunities are expanding due to technology, regulatory shifts at the state and federal levels favoring remote healthcare, and a wider move toward remote work. Virtual practice options for physicians range from full-time employment to flexible part-time positions that can be used to earn supplementary income.
Just a few of those virtual options are:
Remote Patient Monitoring
Remote patient monitoring uses technology for tracking patient health data, applicable in real-time or asynchronously, through devices ranging from specialized monitors to consumer wearables. Data are securely transmitted to healthcare providers, enabling them to guide or make treatment choices remotely. This method has proven particularly valuable in managing chronic diseases where continuous monitoring can significantly affect outcomes.
Like standard telemedicine, remote patient monitoring offers flexibility, autonomy, and the ability to work from home. It is picking up steam across the healthcare industry, especially in critical care, surgery, post-acute care, and primary care, so there are opportunities for physicians across a variety of specialties.
Online Medication Management and Text-Based Consults
Gathering necessary information for patient care decisions often doesn’t require a direct, face-to-face visit in person or by telemedicine. Clinical data can be efficiently collected through online forms, HIPAA-compliant messaging, medical record reviews, and information gathered by staff.
An approach that uses all these sources enables effective medication management for stable chronic conditions (such as hypertension), as well as straightforward but simple acute issues (such as urinary tract infections). It also is useful for quick follow-ups with patients after starting new treatments, to address questions between visits, and to give them educational material.
Some medical practices and virtual healthcare corporations have made online medication management and text-based consults the center of their business model. Part-time positions with platforms that offer this type of care let physicians fit consultations into their schedule as time permits, without committing to scheduled appointments.
eConsults
Electronic consultations, or eConsults, facilitate collaboration among healthcare professionals about complex cases without direct patient interaction.
These services operate via online platforms that support asynchronous communication and often bypass the need for a traditional referral. Typically, a primary care provider submits a query that is then assigned to a specialist. Next, the specialist reviews the information and offers recommendations for the patient’s care plan.
Major eConsult platforms such as AristaMD and RubiconMD contract with healthcare systems and medical practices. Physicians can easily join the specialist panels of these companies and complete assigned consultations from their homes or offices, paid on a per-consult basis. They should check their employment contracts to make sure such independent contract work is allowed.
Phone-Only On-Call Positions
On-call rotations for after-hour care bring with them challenges in staffing and scheduling vacations. These challenges have helped trigger as-needed or per diem on-call roles, in which a physician provides recommendations and orders over the phone without needing to visit an office or a hospital.
Examples of workplaces that employ phone-only on-call physicians include smaller jails, mental health facilities, dialysis centers, long-term care facilities, and sporting groups or events needing back-up for on-site nurses or emergency medical technicians.
While these positions can sometimes be challenging for a physician to find, they are out there. They can be a fantastic option to earn additional income through low-stress clinical work performed from home.
Supervision of Nurse Practitioners (NPs) and Physician Assistants (PAs)
In states that mandate such physician oversight, it often be conducted remotely — depending on that state’s rules, the practice type, and the scope of services being provided. This remote option introduces part-time opportunities for physicians to oversee NPs and PAs without being in the medical office. Essentially, the doctor needs to be available for phone or email consultations, complete chart reviews, and meet regularly with the provider.
Remote supervision roles are available across various types of healthcare organizations and medical practices. There also are opportunities with insurers, many of which have established NP-run, in-home member assessment programs that require remote supervision by a doctor.
Remote Medical Directorships
Medical directors are a key part of the clinician team in a wide variety of healthcare settings requiring clinical protocol oversight, regulatory compliance, and guidance for other clinicians making treatment decisions. Many directorships do not require direct patient contact and therefore are conducive to remote work, given technologies such as electronic health record and secure messaging systems.
Organizations such as emergency medical service agencies, hospice services, med spas, blood and plasma donation centers, home health agencies, and substance use disorder treatment programs increasingly rely on remote medical directorships to meet legal requirements and accreditation standards.
Although these positions are often viewed as “nonclinical,” they carry significant clinical responsibilities. Examples are developing and reviewing treatment protocols, ensuring adherence to healthcare regulations, and sometimes intervening in complex patient cases or when adverse outcomes occur.
Keeping a Role in Patient Welfare
Clearly, working from home as a physician doesn’t have to mean taking on a nonclinical job. Beyond the options already mentioned, there are numerous others — for example, working as a medical monitor for clinical trials, in utilization management for insurance companies, or in conducting independent medical exams for insurance claims. While these roles don’t involve direct patient treatment, they require similar skills and affect the quality of care.
If such remote opportunities aren’t currently available in your workplace, consider approaching your management about trying them.
Physicians who are experiencing burnout, seeking a career change, or interested in earning extra income should consider exploring more of the unconventional ways that they can practice medicine.
A version of this article appeared on Medscape.com.
The appeal of working from home is undeniable. It comes with no daily commute, casual dress, and the ability to manage work-life balance more effectively.
Telemedicine is often the first thing that comes to mind when physicians think about remote medical practice. In its traditional sense, telemedicine entails live video consults, replicating the in-person experience as closely as possible, minus the hands-on component. However, this format is just one of many types of virtual care presenting opportunities to practice medicine from home.
The scope and volume of such opportunities are expanding due to technology, regulatory shifts at the state and federal levels favoring remote healthcare, and a wider move toward remote work. Virtual practice options for physicians range from full-time employment to flexible part-time positions that can be used to earn supplementary income.
Just a few of those virtual options are:
Remote Patient Monitoring
Remote patient monitoring uses technology for tracking patient health data, applicable in real-time or asynchronously, through devices ranging from specialized monitors to consumer wearables. Data are securely transmitted to healthcare providers, enabling them to guide or make treatment choices remotely. This method has proven particularly valuable in managing chronic diseases where continuous monitoring can significantly affect outcomes.
Like standard telemedicine, remote patient monitoring offers flexibility, autonomy, and the ability to work from home. It is picking up steam across the healthcare industry, especially in critical care, surgery, post-acute care, and primary care, so there are opportunities for physicians across a variety of specialties.
Online Medication Management and Text-Based Consults
Gathering necessary information for patient care decisions often doesn’t require a direct, face-to-face visit in person or by telemedicine. Clinical data can be efficiently collected through online forms, HIPAA-compliant messaging, medical record reviews, and information gathered by staff.
An approach that uses all these sources enables effective medication management for stable chronic conditions (such as hypertension), as well as straightforward but simple acute issues (such as urinary tract infections). It also is useful for quick follow-ups with patients after starting new treatments, to address questions between visits, and to give them educational material.
Some medical practices and virtual healthcare corporations have made online medication management and text-based consults the center of their business model. Part-time positions with platforms that offer this type of care let physicians fit consultations into their schedule as time permits, without committing to scheduled appointments.
eConsults
Electronic consultations, or eConsults, facilitate collaboration among healthcare professionals about complex cases without direct patient interaction.
These services operate via online platforms that support asynchronous communication and often bypass the need for a traditional referral. Typically, a primary care provider submits a query that is then assigned to a specialist. Next, the specialist reviews the information and offers recommendations for the patient’s care plan.
Major eConsult platforms such as AristaMD and RubiconMD contract with healthcare systems and medical practices. Physicians can easily join the specialist panels of these companies and complete assigned consultations from their homes or offices, paid on a per-consult basis. They should check their employment contracts to make sure such independent contract work is allowed.
Phone-Only On-Call Positions
On-call rotations for after-hour care bring with them challenges in staffing and scheduling vacations. These challenges have helped trigger as-needed or per diem on-call roles, in which a physician provides recommendations and orders over the phone without needing to visit an office or a hospital.
Examples of workplaces that employ phone-only on-call physicians include smaller jails, mental health facilities, dialysis centers, long-term care facilities, and sporting groups or events needing back-up for on-site nurses or emergency medical technicians.
While these positions can sometimes be challenging for a physician to find, they are out there. They can be a fantastic option to earn additional income through low-stress clinical work performed from home.
Supervision of Nurse Practitioners (NPs) and Physician Assistants (PAs)
In states that mandate such physician oversight, it often be conducted remotely — depending on that state’s rules, the practice type, and the scope of services being provided. This remote option introduces part-time opportunities for physicians to oversee NPs and PAs without being in the medical office. Essentially, the doctor needs to be available for phone or email consultations, complete chart reviews, and meet regularly with the provider.
Remote supervision roles are available across various types of healthcare organizations and medical practices. There also are opportunities with insurers, many of which have established NP-run, in-home member assessment programs that require remote supervision by a doctor.
Remote Medical Directorships
Medical directors are a key part of the clinician team in a wide variety of healthcare settings requiring clinical protocol oversight, regulatory compliance, and guidance for other clinicians making treatment decisions. Many directorships do not require direct patient contact and therefore are conducive to remote work, given technologies such as electronic health record and secure messaging systems.
Organizations such as emergency medical service agencies, hospice services, med spas, blood and plasma donation centers, home health agencies, and substance use disorder treatment programs increasingly rely on remote medical directorships to meet legal requirements and accreditation standards.
Although these positions are often viewed as “nonclinical,” they carry significant clinical responsibilities. Examples are developing and reviewing treatment protocols, ensuring adherence to healthcare regulations, and sometimes intervening in complex patient cases or when adverse outcomes occur.
Keeping a Role in Patient Welfare
Clearly, working from home as a physician doesn’t have to mean taking on a nonclinical job. Beyond the options already mentioned, there are numerous others — for example, working as a medical monitor for clinical trials, in utilization management for insurance companies, or in conducting independent medical exams for insurance claims. While these roles don’t involve direct patient treatment, they require similar skills and affect the quality of care.
If such remote opportunities aren’t currently available in your workplace, consider approaching your management about trying them.
Physicians who are experiencing burnout, seeking a career change, or interested in earning extra income should consider exploring more of the unconventional ways that they can practice medicine.
A version of this article appeared on Medscape.com.
Meat Linked to Higher Erectile Dysfunction Risk
Rachel S. Rubin, MD: Welcome to another episode of Sex Matters. I’m Dr. Rachel Rubin. I’m a urologist and sexual medicine specialist based in the Washington, DC area, and I interview amazingly cool people doing research in sexual medicine.
I heard an incredible lecture while I was at the Mayo Clinic urology conference by Dr. Stacy Loeb, who is a wonderful researcher of all things prostate cancer and men’s health, who is now talking more plant-based diets. Her lecture was so good, I begged her to join me for this discussion.
Dr. Loeb, I would love for you to introduce yourself.
Stacy Loeb, MD: I’m Dr. Loeb. I’m a urologist at New York University in the Manhattan VA, and I recently became board certified in lifestyle medicine because it’s so important for sexual health and, really, everything that we do.
Dr. Rubin: You recently became very interested in studying plant-based diets. How did that start, and how has the research evolved over time?
Dr. Loeb: It’s really amazing. For one thing, more of our patients with prostate cancer die of heart disease than of prostate cancer. And erectile dysfunction is really an early warning sign of cardiovascular disease. We felt like it was incumbent upon us, even within urology and sexual medicine, to better understand the basis for lifestyle modification that can help with these issues.
Dr. Rubin: Tell us more about what you found for erectile dysfunction. How much benefit do people get by switching to a plant-based diet?
Dr. Loeb: First we looked at erectile function in men without prostate cancer in the health professionals follow-up study, a very large cohort study out of Harvard University. We found that among omnivorous people, those who ate more plant-based and less animal-based food were less likely to have incident erectile dysfunction. Then, we published a new paper looking at patients with prostate cancer. These men have extra challenges for sexual function because in addition to the standard cardiovascular changes with aging, prostate cancer treatment can affect the nerves that are involved in erections. But amazingly, even in that population, we found that the men who ate more plant-based and less animal-based food had better scores for erectile function.
That was really good news, and it’s a win-win. There is no reason not to counsel our patients to eat more plant-based foods. Meat is not masculine. Meat is associated with a higher risk for erectile dysfunction and is considered carcinogenic. It’s just something that we should try to stay away from.
Dr. Rubin: How do you counsel patients who might not be ready to go fully plant-based? Is a little better than nothing? How do you even start these conversations with people? Do you have any tips for primary care doctors?
Dr. Loeb: Great question. A little bit is very much better than nothing. In fact, in the health professionals follow-up study, we actually looked at quintiles of people who ate the most meat and animal-based foods and the least plant-based foods all the way up to the most plant-based and the least animal-based diets. Along that spectrum, it really does make a big difference. Anywhere that patients can start from is definitely better than nothing.
Simple things such as Meatless Monday or choosing a few days that they will give up animal-based foods will help. For some people, trying new things is easier than cutting things out, for example, trying a milk substitute such as oat, almond, or soy milk instead of dairy milk. That could be a great first step, or trying some dishes that don’t include meat — maybe a tofu stir fry or a taco or burrito without the meat.
There are many great options out there. In terms of resources for doctors, the Physicians Committee for Responsible Medicine has a great website. They have fact sheets for a lot of the common questions that people ask such as how can I get enough protein or calcium on a plant-based diet? This isn’t a problem at all. In fact, Novak Djokovic and many other elite athletes eat plant-based diets, and they get enough protein with a much higher requirement than most of us who are not elite athletes. These fact sheets explain which plant foods are the best
I also like Nutritionfacts.org. They also have all kinds of great videos and resources. Both of these websites have recipes that were created by doctors and nutritionists.
We can suggest that our patients work with a nutritionist or join a virtual program. For example, Plant Powered here in New York has virtual plant-based jumpstart programs. People around the country can get in on programs that have nutritionists and health coaches — for people who want a boost.
Dr. Rubin: The data are really compelling. When you were speaking, not a person in the room was interested in having a steak that night for dinner, even with a steakhouse in the hotel.
What do you say to men who have prostate cancer or suffer from erectile dysfunction? Do any data show that by going plant-based you may show improvements? We have recent studies that show that regular exercise might be as good as Viagra.
Dr. Loeb: It’s definitely not too late, even if you’ve already been diagnosed with these conditions. In my own practice, I have seen changes in patients. In fact, one of the case scenarios that I submitted for the lifestyle medicine boards was a patient who adopted a whole food, plant-based diet and no longer uses Viagra. This is definitely something that’s possible to do with intensive lifestyle modification.
Dr. Rubin: Maybe vegetables are the new sexual health aide. How can people find out more? I know you have a Sirius XM radio show.
Dr. Loeb: It’s the Men’s Health Show on Sirius XM channel 110. It’s on Wednesdays from 6:00 to 8:00 PM ET, or you can listen to it on demand anytime through the Sirius XM app.
Dr. Rubin: You have done an enormous amount of research in prostate cancer and sexual medicine. You are an all-star in the field. Thank you for sharing all of your knowledge about plant-based diets. You’ve given us all a lot to think about today.
Dr. Rubin has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
Rachel S. Rubin, MD: Welcome to another episode of Sex Matters. I’m Dr. Rachel Rubin. I’m a urologist and sexual medicine specialist based in the Washington, DC area, and I interview amazingly cool people doing research in sexual medicine.
I heard an incredible lecture while I was at the Mayo Clinic urology conference by Dr. Stacy Loeb, who is a wonderful researcher of all things prostate cancer and men’s health, who is now talking more plant-based diets. Her lecture was so good, I begged her to join me for this discussion.
Dr. Loeb, I would love for you to introduce yourself.
Stacy Loeb, MD: I’m Dr. Loeb. I’m a urologist at New York University in the Manhattan VA, and I recently became board certified in lifestyle medicine because it’s so important for sexual health and, really, everything that we do.
Dr. Rubin: You recently became very interested in studying plant-based diets. How did that start, and how has the research evolved over time?
Dr. Loeb: It’s really amazing. For one thing, more of our patients with prostate cancer die of heart disease than of prostate cancer. And erectile dysfunction is really an early warning sign of cardiovascular disease. We felt like it was incumbent upon us, even within urology and sexual medicine, to better understand the basis for lifestyle modification that can help with these issues.
Dr. Rubin: Tell us more about what you found for erectile dysfunction. How much benefit do people get by switching to a plant-based diet?
Dr. Loeb: First we looked at erectile function in men without prostate cancer in the health professionals follow-up study, a very large cohort study out of Harvard University. We found that among omnivorous people, those who ate more plant-based and less animal-based food were less likely to have incident erectile dysfunction. Then, we published a new paper looking at patients with prostate cancer. These men have extra challenges for sexual function because in addition to the standard cardiovascular changes with aging, prostate cancer treatment can affect the nerves that are involved in erections. But amazingly, even in that population, we found that the men who ate more plant-based and less animal-based food had better scores for erectile function.
That was really good news, and it’s a win-win. There is no reason not to counsel our patients to eat more plant-based foods. Meat is not masculine. Meat is associated with a higher risk for erectile dysfunction and is considered carcinogenic. It’s just something that we should try to stay away from.
Dr. Rubin: How do you counsel patients who might not be ready to go fully plant-based? Is a little better than nothing? How do you even start these conversations with people? Do you have any tips for primary care doctors?
Dr. Loeb: Great question. A little bit is very much better than nothing. In fact, in the health professionals follow-up study, we actually looked at quintiles of people who ate the most meat and animal-based foods and the least plant-based foods all the way up to the most plant-based and the least animal-based diets. Along that spectrum, it really does make a big difference. Anywhere that patients can start from is definitely better than nothing.
Simple things such as Meatless Monday or choosing a few days that they will give up animal-based foods will help. For some people, trying new things is easier than cutting things out, for example, trying a milk substitute such as oat, almond, or soy milk instead of dairy milk. That could be a great first step, or trying some dishes that don’t include meat — maybe a tofu stir fry or a taco or burrito without the meat.
There are many great options out there. In terms of resources for doctors, the Physicians Committee for Responsible Medicine has a great website. They have fact sheets for a lot of the common questions that people ask such as how can I get enough protein or calcium on a plant-based diet? This isn’t a problem at all. In fact, Novak Djokovic and many other elite athletes eat plant-based diets, and they get enough protein with a much higher requirement than most of us who are not elite athletes. These fact sheets explain which plant foods are the best
I also like Nutritionfacts.org. They also have all kinds of great videos and resources. Both of these websites have recipes that were created by doctors and nutritionists.
We can suggest that our patients work with a nutritionist or join a virtual program. For example, Plant Powered here in New York has virtual plant-based jumpstart programs. People around the country can get in on programs that have nutritionists and health coaches — for people who want a boost.
Dr. Rubin: The data are really compelling. When you were speaking, not a person in the room was interested in having a steak that night for dinner, even with a steakhouse in the hotel.
What do you say to men who have prostate cancer or suffer from erectile dysfunction? Do any data show that by going plant-based you may show improvements? We have recent studies that show that regular exercise might be as good as Viagra.
Dr. Loeb: It’s definitely not too late, even if you’ve already been diagnosed with these conditions. In my own practice, I have seen changes in patients. In fact, one of the case scenarios that I submitted for the lifestyle medicine boards was a patient who adopted a whole food, plant-based diet and no longer uses Viagra. This is definitely something that’s possible to do with intensive lifestyle modification.
Dr. Rubin: Maybe vegetables are the new sexual health aide. How can people find out more? I know you have a Sirius XM radio show.
Dr. Loeb: It’s the Men’s Health Show on Sirius XM channel 110. It’s on Wednesdays from 6:00 to 8:00 PM ET, or you can listen to it on demand anytime through the Sirius XM app.
Dr. Rubin: You have done an enormous amount of research in prostate cancer and sexual medicine. You are an all-star in the field. Thank you for sharing all of your knowledge about plant-based diets. You’ve given us all a lot to think about today.
Dr. Rubin has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
Rachel S. Rubin, MD: Welcome to another episode of Sex Matters. I’m Dr. Rachel Rubin. I’m a urologist and sexual medicine specialist based in the Washington, DC area, and I interview amazingly cool people doing research in sexual medicine.
I heard an incredible lecture while I was at the Mayo Clinic urology conference by Dr. Stacy Loeb, who is a wonderful researcher of all things prostate cancer and men’s health, who is now talking more plant-based diets. Her lecture was so good, I begged her to join me for this discussion.
Dr. Loeb, I would love for you to introduce yourself.
Stacy Loeb, MD: I’m Dr. Loeb. I’m a urologist at New York University in the Manhattan VA, and I recently became board certified in lifestyle medicine because it’s so important for sexual health and, really, everything that we do.
Dr. Rubin: You recently became very interested in studying plant-based diets. How did that start, and how has the research evolved over time?
Dr. Loeb: It’s really amazing. For one thing, more of our patients with prostate cancer die of heart disease than of prostate cancer. And erectile dysfunction is really an early warning sign of cardiovascular disease. We felt like it was incumbent upon us, even within urology and sexual medicine, to better understand the basis for lifestyle modification that can help with these issues.
Dr. Rubin: Tell us more about what you found for erectile dysfunction. How much benefit do people get by switching to a plant-based diet?
Dr. Loeb: First we looked at erectile function in men without prostate cancer in the health professionals follow-up study, a very large cohort study out of Harvard University. We found that among omnivorous people, those who ate more plant-based and less animal-based food were less likely to have incident erectile dysfunction. Then, we published a new paper looking at patients with prostate cancer. These men have extra challenges for sexual function because in addition to the standard cardiovascular changes with aging, prostate cancer treatment can affect the nerves that are involved in erections. But amazingly, even in that population, we found that the men who ate more plant-based and less animal-based food had better scores for erectile function.
That was really good news, and it’s a win-win. There is no reason not to counsel our patients to eat more plant-based foods. Meat is not masculine. Meat is associated with a higher risk for erectile dysfunction and is considered carcinogenic. It’s just something that we should try to stay away from.
Dr. Rubin: How do you counsel patients who might not be ready to go fully plant-based? Is a little better than nothing? How do you even start these conversations with people? Do you have any tips for primary care doctors?
Dr. Loeb: Great question. A little bit is very much better than nothing. In fact, in the health professionals follow-up study, we actually looked at quintiles of people who ate the most meat and animal-based foods and the least plant-based foods all the way up to the most plant-based and the least animal-based diets. Along that spectrum, it really does make a big difference. Anywhere that patients can start from is definitely better than nothing.
Simple things such as Meatless Monday or choosing a few days that they will give up animal-based foods will help. For some people, trying new things is easier than cutting things out, for example, trying a milk substitute such as oat, almond, or soy milk instead of dairy milk. That could be a great first step, or trying some dishes that don’t include meat — maybe a tofu stir fry or a taco or burrito without the meat.
There are many great options out there. In terms of resources for doctors, the Physicians Committee for Responsible Medicine has a great website. They have fact sheets for a lot of the common questions that people ask such as how can I get enough protein or calcium on a plant-based diet? This isn’t a problem at all. In fact, Novak Djokovic and many other elite athletes eat plant-based diets, and they get enough protein with a much higher requirement than most of us who are not elite athletes. These fact sheets explain which plant foods are the best
I also like Nutritionfacts.org. They also have all kinds of great videos and resources. Both of these websites have recipes that were created by doctors and nutritionists.
We can suggest that our patients work with a nutritionist or join a virtual program. For example, Plant Powered here in New York has virtual plant-based jumpstart programs. People around the country can get in on programs that have nutritionists and health coaches — for people who want a boost.
Dr. Rubin: The data are really compelling. When you were speaking, not a person in the room was interested in having a steak that night for dinner, even with a steakhouse in the hotel.
What do you say to men who have prostate cancer or suffer from erectile dysfunction? Do any data show that by going plant-based you may show improvements? We have recent studies that show that regular exercise might be as good as Viagra.
Dr. Loeb: It’s definitely not too late, even if you’ve already been diagnosed with these conditions. In my own practice, I have seen changes in patients. In fact, one of the case scenarios that I submitted for the lifestyle medicine boards was a patient who adopted a whole food, plant-based diet and no longer uses Viagra. This is definitely something that’s possible to do with intensive lifestyle modification.
Dr. Rubin: Maybe vegetables are the new sexual health aide. How can people find out more? I know you have a Sirius XM radio show.
Dr. Loeb: It’s the Men’s Health Show on Sirius XM channel 110. It’s on Wednesdays from 6:00 to 8:00 PM ET, or you can listen to it on demand anytime through the Sirius XM app.
Dr. Rubin: You have done an enormous amount of research in prostate cancer and sexual medicine. You are an all-star in the field. Thank you for sharing all of your knowledge about plant-based diets. You’ve given us all a lot to think about today.
Dr. Rubin has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
Blood Test Shows Promise for Improving CRC Screening
say the authors of new research.
Rachel B. Issaka, MD, MAS, of the Fred Hutchinson Cancer Center, Seattle, presented the clinical data, which was published in The New England Journal of Medicine, at the American Association for Cancer Research annual meeting.
The authors of the study evaluated the performance of a cfDNA blood-based test in a population eligible for colorectal cancer screening. The researchers found that the test had high sensitivity for the detection of colorectal cancer and high specificity for advanced precancerous lesions.
This novel blood test could improve screening adherence and, ultimately, reduce colorectal cancer-related mortality, Dr. Issaka said during her presentation.
“This test has the potential to help us reach the 80% screening target in colorectal cancer. However, this will depend on many factors, including access, implementation, follow-up colonoscopy, and characteristics of the test,” Dr. Issaka said in an interview.
She added that, when approved for broader use, anyone who wants to use this blood test for colorectal cancer screening should have a frank conversation with their healthcare provider.
“Considering the person’s age, medical history, family history, and any potential symptoms, and how the test performs will dictate if it’s the right test for that person versus another screening strategy,” Dr. Issaka explained.
The Blood Test Detects Colorectal Cancer With High Accuracy
The investigators of the observational ECLIPSE trial evaluated the performance of the cfDNA-based blood test in 7861 individuals who were eligible for colorectal cancer screening. The study population included people from more than 200 rural and urban sites across 34 states, including community hospitals, private practices, gastroenterology clinics, and academic centers. “The study enrolled a diverse cohort that is reflective of the demographics of the intended use population in the US,” Dr. Issaka said during her talk.
The co-primary outcomes of the study were the test’s sensitivity for detecting colorectal cancer and its specificity for identifying advanced neoplasia.
In her presentation, Dr. Issaka highlighted that the test had 83.1% (95% confidence interval [CI], 72.2%-90.3%) sensitivity for the detection of colorectal cancer, meaning that it was able to correctly identify most participants with the disease. The test’s sensitivity was even higher (87.5%; 95% CI, 75.3%-94.1%) for stage I, II, or III colorectal cancer. “These are the stages at which early intervention can have the greatest impact on patient prognosis,” Dr. Issaka said.
Moreover, the blood test showed 89.6% (95% CI, 88.8%-90.3%) specificity for advanced neoplasia, including colorectal cancer and advanced precancerous lesions. The specificity of the test for negative colonoscopy results (no colorectal cancer, advanced precancerous lesions, or nonadvanced precancerous lesions) was 89.9% (95% CI, 89.0%-90.7%).
Dr. Issaka highlighted that this cfDNA assay is the first blood-based test with performance comparable to current guideline-recommended noninvasive options for CRC.
The Blood Test Shows Limited Ability To Detect Advanced Precancerous Lesions
During her presentation, Dr. Issaka acknowledged that the cfDNA-based blood test had a lower sensitivity (13.2%; 95% CI, 11.3%-15.3%) for the detection of advanced precancerous lesions, suggesting that it may be more effective at identifying established cancers than early-stage precancerous changes. Low sensitivity was also observed for high-grade dysplasia (22.6%; 95% CI, 11.4%-39.8%). However, she emphasized that the test could still play a valuable role in a comprehensive screening approach, potentially serving as a first-line tool to identify individuals who would then undergo follow-up colonoscopy.
“Although blood-based tests perform well at finding cancers, they do not do so well at finding precancerous polyps. This is relevant because colorectal cancer is one of the few cancers that we can prevent by finding and removing precancerous polyps,” Folasade P. May, MD, PhD, MPhil, said in an interview.
“Users must also understand that if the test result is abnormal, a colonoscopy is required to look for cancers and polyps that might have caused the abnormal result,” added Dr. May, associate professor at UCLA. She was not involved in the study.
Clinical Implications and Future Steps
According to the study published in the NEJM, colorectal cancer is the third most commonly diagnosed cancer in the United States, and early detection is crucial for effective treatment. However, over a third of eligible individuals are not up to date with recommended screening.
During her talk, Dr. Issaka noted that colonoscopy is the most commonly used screening method for colorectal cancer. What contributes to the low adherence to getting a colonoscopy among the eligible population is that some find it inconvenient, and the test is invasive, she added.
According to Dr. May, the key advantage of cfDNA-based screening is that many people will find it easier to complete a blood test than the currently available screening tests.
“This option may allow us to screen individuals that we have previously struggled to convince to get screened for colorectal cancer,” she said.
In an interview, Dr. Issaka acknowledged that the potential public health impact of any noninvasive screening test depends on how many people with abnormal results complete a follow-up colonoscopy. “This is an important quality metric to track,” she said.
In an interview, Dr. Issaka emphasized that comparing this cfDNA blood test with emerging blood tests and other noninvasive screening strategies will empower patients and clinicians to select the right test at the right time for the right patient.
She added that the study was conducted in an average-risk screening population and that further research is needed to evaluate the test’s performance in higher-risk groups and to assess its real-world impact on screening adherence and colorectal cancer-related outcomes.
Commenting on potential challenges with implementing this cfDNA blood test in clinical practice, Dr. May said, “As we consider incorporating blood-based tests into clinical practice, some challenges include cost, equitable access to tests and follow-up, performance in young adults who are newly eligible for screening, and follow-up after abnormal results.”
She added that, if there is uptake of these tests, it will be important to track how that impacts colorectal cancer screening rates, stage at diagnosis, and whether there is stage migration, incidence, and mortality.
“At this time, I feel that these tests are appropriate for individuals who will not or cannot participate in one of the currently recommended screening tests. These include colonoscopy and stool-based tests, like FIT and FIT-DNA,” Dr. May concluded.
Dr. Issaka reported financial relationships with the National Institutes of Health/National Cancer Institute, American College of Gastroenterology, and Guardant Health Inc. Dr. May reported financial relationships with Takeda, Medtronic, Johnson & Johnson, Saint Supply, Exact Sciences, Freenome, Geneoscopy, Guardant Health, InterVenn, Natura, National Institutes of Health/National Cancer Institute, Veterans Affairs HSR&D, Broad Institute, Stand up to Cancer, and NRG Oncology.
say the authors of new research.
Rachel B. Issaka, MD, MAS, of the Fred Hutchinson Cancer Center, Seattle, presented the clinical data, which was published in The New England Journal of Medicine, at the American Association for Cancer Research annual meeting.
The authors of the study evaluated the performance of a cfDNA blood-based test in a population eligible for colorectal cancer screening. The researchers found that the test had high sensitivity for the detection of colorectal cancer and high specificity for advanced precancerous lesions.
This novel blood test could improve screening adherence and, ultimately, reduce colorectal cancer-related mortality, Dr. Issaka said during her presentation.
“This test has the potential to help us reach the 80% screening target in colorectal cancer. However, this will depend on many factors, including access, implementation, follow-up colonoscopy, and characteristics of the test,” Dr. Issaka said in an interview.
She added that, when approved for broader use, anyone who wants to use this blood test for colorectal cancer screening should have a frank conversation with their healthcare provider.
“Considering the person’s age, medical history, family history, and any potential symptoms, and how the test performs will dictate if it’s the right test for that person versus another screening strategy,” Dr. Issaka explained.
The Blood Test Detects Colorectal Cancer With High Accuracy
The investigators of the observational ECLIPSE trial evaluated the performance of the cfDNA-based blood test in 7861 individuals who were eligible for colorectal cancer screening. The study population included people from more than 200 rural and urban sites across 34 states, including community hospitals, private practices, gastroenterology clinics, and academic centers. “The study enrolled a diverse cohort that is reflective of the demographics of the intended use population in the US,” Dr. Issaka said during her talk.
The co-primary outcomes of the study were the test’s sensitivity for detecting colorectal cancer and its specificity for identifying advanced neoplasia.
In her presentation, Dr. Issaka highlighted that the test had 83.1% (95% confidence interval [CI], 72.2%-90.3%) sensitivity for the detection of colorectal cancer, meaning that it was able to correctly identify most participants with the disease. The test’s sensitivity was even higher (87.5%; 95% CI, 75.3%-94.1%) for stage I, II, or III colorectal cancer. “These are the stages at which early intervention can have the greatest impact on patient prognosis,” Dr. Issaka said.
Moreover, the blood test showed 89.6% (95% CI, 88.8%-90.3%) specificity for advanced neoplasia, including colorectal cancer and advanced precancerous lesions. The specificity of the test for negative colonoscopy results (no colorectal cancer, advanced precancerous lesions, or nonadvanced precancerous lesions) was 89.9% (95% CI, 89.0%-90.7%).
Dr. Issaka highlighted that this cfDNA assay is the first blood-based test with performance comparable to current guideline-recommended noninvasive options for CRC.
The Blood Test Shows Limited Ability To Detect Advanced Precancerous Lesions
During her presentation, Dr. Issaka acknowledged that the cfDNA-based blood test had a lower sensitivity (13.2%; 95% CI, 11.3%-15.3%) for the detection of advanced precancerous lesions, suggesting that it may be more effective at identifying established cancers than early-stage precancerous changes. Low sensitivity was also observed for high-grade dysplasia (22.6%; 95% CI, 11.4%-39.8%). However, she emphasized that the test could still play a valuable role in a comprehensive screening approach, potentially serving as a first-line tool to identify individuals who would then undergo follow-up colonoscopy.
“Although blood-based tests perform well at finding cancers, they do not do so well at finding precancerous polyps. This is relevant because colorectal cancer is one of the few cancers that we can prevent by finding and removing precancerous polyps,” Folasade P. May, MD, PhD, MPhil, said in an interview.
“Users must also understand that if the test result is abnormal, a colonoscopy is required to look for cancers and polyps that might have caused the abnormal result,” added Dr. May, associate professor at UCLA. She was not involved in the study.
Clinical Implications and Future Steps
According to the study published in the NEJM, colorectal cancer is the third most commonly diagnosed cancer in the United States, and early detection is crucial for effective treatment. However, over a third of eligible individuals are not up to date with recommended screening.
During her talk, Dr. Issaka noted that colonoscopy is the most commonly used screening method for colorectal cancer. What contributes to the low adherence to getting a colonoscopy among the eligible population is that some find it inconvenient, and the test is invasive, she added.
According to Dr. May, the key advantage of cfDNA-based screening is that many people will find it easier to complete a blood test than the currently available screening tests.
“This option may allow us to screen individuals that we have previously struggled to convince to get screened for colorectal cancer,” she said.
In an interview, Dr. Issaka acknowledged that the potential public health impact of any noninvasive screening test depends on how many people with abnormal results complete a follow-up colonoscopy. “This is an important quality metric to track,” she said.
In an interview, Dr. Issaka emphasized that comparing this cfDNA blood test with emerging blood tests and other noninvasive screening strategies will empower patients and clinicians to select the right test at the right time for the right patient.
She added that the study was conducted in an average-risk screening population and that further research is needed to evaluate the test’s performance in higher-risk groups and to assess its real-world impact on screening adherence and colorectal cancer-related outcomes.
Commenting on potential challenges with implementing this cfDNA blood test in clinical practice, Dr. May said, “As we consider incorporating blood-based tests into clinical practice, some challenges include cost, equitable access to tests and follow-up, performance in young adults who are newly eligible for screening, and follow-up after abnormal results.”
She added that, if there is uptake of these tests, it will be important to track how that impacts colorectal cancer screening rates, stage at diagnosis, and whether there is stage migration, incidence, and mortality.
“At this time, I feel that these tests are appropriate for individuals who will not or cannot participate in one of the currently recommended screening tests. These include colonoscopy and stool-based tests, like FIT and FIT-DNA,” Dr. May concluded.
Dr. Issaka reported financial relationships with the National Institutes of Health/National Cancer Institute, American College of Gastroenterology, and Guardant Health Inc. Dr. May reported financial relationships with Takeda, Medtronic, Johnson & Johnson, Saint Supply, Exact Sciences, Freenome, Geneoscopy, Guardant Health, InterVenn, Natura, National Institutes of Health/National Cancer Institute, Veterans Affairs HSR&D, Broad Institute, Stand up to Cancer, and NRG Oncology.
say the authors of new research.
Rachel B. Issaka, MD, MAS, of the Fred Hutchinson Cancer Center, Seattle, presented the clinical data, which was published in The New England Journal of Medicine, at the American Association for Cancer Research annual meeting.
The authors of the study evaluated the performance of a cfDNA blood-based test in a population eligible for colorectal cancer screening. The researchers found that the test had high sensitivity for the detection of colorectal cancer and high specificity for advanced precancerous lesions.
This novel blood test could improve screening adherence and, ultimately, reduce colorectal cancer-related mortality, Dr. Issaka said during her presentation.
“This test has the potential to help us reach the 80% screening target in colorectal cancer. However, this will depend on many factors, including access, implementation, follow-up colonoscopy, and characteristics of the test,” Dr. Issaka said in an interview.
She added that, when approved for broader use, anyone who wants to use this blood test for colorectal cancer screening should have a frank conversation with their healthcare provider.
“Considering the person’s age, medical history, family history, and any potential symptoms, and how the test performs will dictate if it’s the right test for that person versus another screening strategy,” Dr. Issaka explained.
The Blood Test Detects Colorectal Cancer With High Accuracy
The investigators of the observational ECLIPSE trial evaluated the performance of the cfDNA-based blood test in 7861 individuals who were eligible for colorectal cancer screening. The study population included people from more than 200 rural and urban sites across 34 states, including community hospitals, private practices, gastroenterology clinics, and academic centers. “The study enrolled a diverse cohort that is reflective of the demographics of the intended use population in the US,” Dr. Issaka said during her talk.
The co-primary outcomes of the study were the test’s sensitivity for detecting colorectal cancer and its specificity for identifying advanced neoplasia.
In her presentation, Dr. Issaka highlighted that the test had 83.1% (95% confidence interval [CI], 72.2%-90.3%) sensitivity for the detection of colorectal cancer, meaning that it was able to correctly identify most participants with the disease. The test’s sensitivity was even higher (87.5%; 95% CI, 75.3%-94.1%) for stage I, II, or III colorectal cancer. “These are the stages at which early intervention can have the greatest impact on patient prognosis,” Dr. Issaka said.
Moreover, the blood test showed 89.6% (95% CI, 88.8%-90.3%) specificity for advanced neoplasia, including colorectal cancer and advanced precancerous lesions. The specificity of the test for negative colonoscopy results (no colorectal cancer, advanced precancerous lesions, or nonadvanced precancerous lesions) was 89.9% (95% CI, 89.0%-90.7%).
Dr. Issaka highlighted that this cfDNA assay is the first blood-based test with performance comparable to current guideline-recommended noninvasive options for CRC.
The Blood Test Shows Limited Ability To Detect Advanced Precancerous Lesions
During her presentation, Dr. Issaka acknowledged that the cfDNA-based blood test had a lower sensitivity (13.2%; 95% CI, 11.3%-15.3%) for the detection of advanced precancerous lesions, suggesting that it may be more effective at identifying established cancers than early-stage precancerous changes. Low sensitivity was also observed for high-grade dysplasia (22.6%; 95% CI, 11.4%-39.8%). However, she emphasized that the test could still play a valuable role in a comprehensive screening approach, potentially serving as a first-line tool to identify individuals who would then undergo follow-up colonoscopy.
“Although blood-based tests perform well at finding cancers, they do not do so well at finding precancerous polyps. This is relevant because colorectal cancer is one of the few cancers that we can prevent by finding and removing precancerous polyps,” Folasade P. May, MD, PhD, MPhil, said in an interview.
“Users must also understand that if the test result is abnormal, a colonoscopy is required to look for cancers and polyps that might have caused the abnormal result,” added Dr. May, associate professor at UCLA. She was not involved in the study.
Clinical Implications and Future Steps
According to the study published in the NEJM, colorectal cancer is the third most commonly diagnosed cancer in the United States, and early detection is crucial for effective treatment. However, over a third of eligible individuals are not up to date with recommended screening.
During her talk, Dr. Issaka noted that colonoscopy is the most commonly used screening method for colorectal cancer. What contributes to the low adherence to getting a colonoscopy among the eligible population is that some find it inconvenient, and the test is invasive, she added.
According to Dr. May, the key advantage of cfDNA-based screening is that many people will find it easier to complete a blood test than the currently available screening tests.
“This option may allow us to screen individuals that we have previously struggled to convince to get screened for colorectal cancer,” she said.
In an interview, Dr. Issaka acknowledged that the potential public health impact of any noninvasive screening test depends on how many people with abnormal results complete a follow-up colonoscopy. “This is an important quality metric to track,” she said.
In an interview, Dr. Issaka emphasized that comparing this cfDNA blood test with emerging blood tests and other noninvasive screening strategies will empower patients and clinicians to select the right test at the right time for the right patient.
She added that the study was conducted in an average-risk screening population and that further research is needed to evaluate the test’s performance in higher-risk groups and to assess its real-world impact on screening adherence and colorectal cancer-related outcomes.
Commenting on potential challenges with implementing this cfDNA blood test in clinical practice, Dr. May said, “As we consider incorporating blood-based tests into clinical practice, some challenges include cost, equitable access to tests and follow-up, performance in young adults who are newly eligible for screening, and follow-up after abnormal results.”
She added that, if there is uptake of these tests, it will be important to track how that impacts colorectal cancer screening rates, stage at diagnosis, and whether there is stage migration, incidence, and mortality.
“At this time, I feel that these tests are appropriate for individuals who will not or cannot participate in one of the currently recommended screening tests. These include colonoscopy and stool-based tests, like FIT and FIT-DNA,” Dr. May concluded.
Dr. Issaka reported financial relationships with the National Institutes of Health/National Cancer Institute, American College of Gastroenterology, and Guardant Health Inc. Dr. May reported financial relationships with Takeda, Medtronic, Johnson & Johnson, Saint Supply, Exact Sciences, Freenome, Geneoscopy, Guardant Health, InterVenn, Natura, National Institutes of Health/National Cancer Institute, Veterans Affairs HSR&D, Broad Institute, Stand up to Cancer, and NRG Oncology.
Positive Results for Intranasal Oxytocin in Adults With Autism
BUDAPEST, HUNGARY — Twice daily intranasal oxytocin has been associated with improved social functioning, quality of life, and overall symptoms in adults with autism spectrum disorder (ASD), results of a small randomized control trial showed.
“One of the challenges for adults with autism is experiencing poor social interactions and difficulties in making friends. Insufficient social support from peers, friends, and family members can contribute to loneliness in adolescents with ASD, which in turn leads to anxiety, sadness, and social isolation,” said study investigator Saba Faraji Niri, MD, assistant professor of psychiatry, Tehran University of Medical Sciences in Iran.
Recent US data show it is relatively common. In addition, previous research suggests intranasal oxytocin significantly increases activity in brain regions that play a role in establishing social interactions.
To evaluate the therapeutic effects and safety of intranasal oxytocin the researchers randomly assigned 39 adult patients with ASD to receive intranasal oxytocin or placebo with 24 units administered every 12 hours for 8 weeks.
Dr. Faraji Niri said study participants were required to stop all psychotropic medications for at least 8 weeks prior to study entry.
Participants were assessed at baseline and weeks 4 and 8 using the Autism Quotient, Ritvo Autism Asperger Diagnostic Scale — Revised (RAADS-R), Social Responsiveness Scale (SRS), Clinical Global Impression (CGI) scale, and the World Health Organization Quality of Life-BREF (WHOQL-BREF) questionnaire. Adverse events were also evaluated.
Dr. Faraji Niri said that those receiving intranasal oxytocin showed clinical improvement on RAADS-R scores (P = .010), as well as on the social communication subscale of the SRS (P = .002), the CGI scale (P = .000), and the physical (P = .004), psychological (P = .006), and social relationships (P = .046) domains of the WHOQL-BREF.
However, although the findings were positive, she said at this point it’s not possible to draw any definitive conclusions. She noted the study had several potential confounders. These included differences in baseline levels of endogenous oxytocin among study participants individuals, as well as difference in required treatment doses, which were adjusted by age and sex. The presence of comorbidities and interactions with other treatments could also affect the results.
Commenting on the findings for this news organization, session chair Szabolcs Kéri, PhD, Professor, Sztárai Institute, University of Tokaj, Sárospatak, Hungary, said the use of oxytocin for ASD is controversial. He said that, while the research contributes to the scientific debate, the clinical significance of the findings is unclear.
The investigators and Dr Keri reported no relevant financial disclosures.
A version of this article appeared on Medscape.com .
BUDAPEST, HUNGARY — Twice daily intranasal oxytocin has been associated with improved social functioning, quality of life, and overall symptoms in adults with autism spectrum disorder (ASD), results of a small randomized control trial showed.
“One of the challenges for adults with autism is experiencing poor social interactions and difficulties in making friends. Insufficient social support from peers, friends, and family members can contribute to loneliness in adolescents with ASD, which in turn leads to anxiety, sadness, and social isolation,” said study investigator Saba Faraji Niri, MD, assistant professor of psychiatry, Tehran University of Medical Sciences in Iran.
Recent US data show it is relatively common. In addition, previous research suggests intranasal oxytocin significantly increases activity in brain regions that play a role in establishing social interactions.
To evaluate the therapeutic effects and safety of intranasal oxytocin the researchers randomly assigned 39 adult patients with ASD to receive intranasal oxytocin or placebo with 24 units administered every 12 hours for 8 weeks.
Dr. Faraji Niri said study participants were required to stop all psychotropic medications for at least 8 weeks prior to study entry.
Participants were assessed at baseline and weeks 4 and 8 using the Autism Quotient, Ritvo Autism Asperger Diagnostic Scale — Revised (RAADS-R), Social Responsiveness Scale (SRS), Clinical Global Impression (CGI) scale, and the World Health Organization Quality of Life-BREF (WHOQL-BREF) questionnaire. Adverse events were also evaluated.
Dr. Faraji Niri said that those receiving intranasal oxytocin showed clinical improvement on RAADS-R scores (P = .010), as well as on the social communication subscale of the SRS (P = .002), the CGI scale (P = .000), and the physical (P = .004), psychological (P = .006), and social relationships (P = .046) domains of the WHOQL-BREF.
However, although the findings were positive, she said at this point it’s not possible to draw any definitive conclusions. She noted the study had several potential confounders. These included differences in baseline levels of endogenous oxytocin among study participants individuals, as well as difference in required treatment doses, which were adjusted by age and sex. The presence of comorbidities and interactions with other treatments could also affect the results.
Commenting on the findings for this news organization, session chair Szabolcs Kéri, PhD, Professor, Sztárai Institute, University of Tokaj, Sárospatak, Hungary, said the use of oxytocin for ASD is controversial. He said that, while the research contributes to the scientific debate, the clinical significance of the findings is unclear.
The investigators and Dr Keri reported no relevant financial disclosures.
A version of this article appeared on Medscape.com .
BUDAPEST, HUNGARY — Twice daily intranasal oxytocin has been associated with improved social functioning, quality of life, and overall symptoms in adults with autism spectrum disorder (ASD), results of a small randomized control trial showed.
“One of the challenges for adults with autism is experiencing poor social interactions and difficulties in making friends. Insufficient social support from peers, friends, and family members can contribute to loneliness in adolescents with ASD, which in turn leads to anxiety, sadness, and social isolation,” said study investigator Saba Faraji Niri, MD, assistant professor of psychiatry, Tehran University of Medical Sciences in Iran.
Recent US data show it is relatively common. In addition, previous research suggests intranasal oxytocin significantly increases activity in brain regions that play a role in establishing social interactions.
To evaluate the therapeutic effects and safety of intranasal oxytocin the researchers randomly assigned 39 adult patients with ASD to receive intranasal oxytocin or placebo with 24 units administered every 12 hours for 8 weeks.
Dr. Faraji Niri said study participants were required to stop all psychotropic medications for at least 8 weeks prior to study entry.
Participants were assessed at baseline and weeks 4 and 8 using the Autism Quotient, Ritvo Autism Asperger Diagnostic Scale — Revised (RAADS-R), Social Responsiveness Scale (SRS), Clinical Global Impression (CGI) scale, and the World Health Organization Quality of Life-BREF (WHOQL-BREF) questionnaire. Adverse events were also evaluated.
Dr. Faraji Niri said that those receiving intranasal oxytocin showed clinical improvement on RAADS-R scores (P = .010), as well as on the social communication subscale of the SRS (P = .002), the CGI scale (P = .000), and the physical (P = .004), psychological (P = .006), and social relationships (P = .046) domains of the WHOQL-BREF.
However, although the findings were positive, she said at this point it’s not possible to draw any definitive conclusions. She noted the study had several potential confounders. These included differences in baseline levels of endogenous oxytocin among study participants individuals, as well as difference in required treatment doses, which were adjusted by age and sex. The presence of comorbidities and interactions with other treatments could also affect the results.
Commenting on the findings for this news organization, session chair Szabolcs Kéri, PhD, Professor, Sztárai Institute, University of Tokaj, Sárospatak, Hungary, said the use of oxytocin for ASD is controversial. He said that, while the research contributes to the scientific debate, the clinical significance of the findings is unclear.
The investigators and Dr Keri reported no relevant financial disclosures.
A version of this article appeared on Medscape.com .
Is Axillary Surgery in Early Breast Cancer on Its Way Out?
TOPLINE:
METHODOLOGY:
- A growing body of evidence has indicated that patients with one or two positive sentinel nodes undergoing breast-conserving surgery and radiation therapy can skip axillary lymph node dissection and achieve similar outcomes compared with patients receiving axillary dissection.
- However, these earlier studies had notable limitations, such as limited statistical power, uncertain nodal radiotherapy target volumes, and minimal data on relevant clinical subgroups.
- To fill the gaps in the literature, the researchers conducted a trial with a large, inclusive cohort of patients with node-negative stage T1-T3 breast cancer who had one or two sentinel-node macrometastases and had undergone a mastectomy or breast-conserving surgery.
- The trial randomized 2540 patients to either completion axillary lymph node dissection (n = 1205) or sentinel-node biopsy only (n = 1335). Nearly 90% of patients received adjuvant radiation therapy, and the majority also received systematic therapy.
- Earlier recurrence-free survival findings and patient-reported outcomes were reported last December. The researchers now reported overall survival findings as well as secondary endpoints of breast cancer-specific survival.
TAKEAWAY:
- The researchers reported 191 recurrences or deaths over a median follow-up of 46.8 months; 62 patients (4.6%) in the sentinel-node biopsy–only group died, and 69 patients (5.7%) in the dissection group died.
- The biopsy-only group had an estimated 5-year overall survival of 92.9% compared with 92.0% in the dissection group and an estimated 5-year breast cancer-specific survival of 97.1% vs 96.6% in the dissection group.
- The estimated 5-year recurrence-free survival was 89.7% in the biopsy-only group vs 88.7% in the dissection group (hazard ratio [HR], 0.89; 95% CI, 0.66-1.19).
- This non-inferior difference held across all prespecified patient subgroups, except in patients with estrogen receptor-positive, human epidermal growth factor receptor 2–positive disease, in which sentinel biopsy alone appeared to be better (HR, 0.26).
IN PRACTICE:
“This trial provides robust evidence that the omission of completion axillary-lymph-node dissection was safe in patients with clinically node-negative T1, T2, or T3 breast cancer and one or two sentinel-node macrometastases who received adjuvant systemic treatment and radiation therapy according to national guidelines,” the authors concluded.
“It is clear that the role of axillary dissection is rapidly disappearing,” Kandace P. McGuire, MD, of Virginia Commonwealth University, Richmond, wrote in an accompanying editorial. “However, axillary staging continues to be vital with regard to decisions about appropriate breast cancer therapy.”
SOURCE:
This work, led by Jana de Boniface, MD, PhD, from Karolinska Institute, Stockholm, was published online in The New England Journal of Medicine, alongside the accompanying editorial by Dr. McGuire.
LIMITATIONS:
The study limitations include unavailable radiation therapy details for comparison, low male recruitment hindering sex-based analysis, short follow-up for luminal subtype breast cancer, unmet enrollment targets, and higher withdrawal rates in the dissection group.
DISCLOSURES:
This study was supported by the Swedish Research Council, Swedish Cancer Society, Nordic Cancer Union, and Swedish Breast Cancer Association. One coauthor reported receiving consultancy fees from various pharmaceutical companies outside this work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A growing body of evidence has indicated that patients with one or two positive sentinel nodes undergoing breast-conserving surgery and radiation therapy can skip axillary lymph node dissection and achieve similar outcomes compared with patients receiving axillary dissection.
- However, these earlier studies had notable limitations, such as limited statistical power, uncertain nodal radiotherapy target volumes, and minimal data on relevant clinical subgroups.
- To fill the gaps in the literature, the researchers conducted a trial with a large, inclusive cohort of patients with node-negative stage T1-T3 breast cancer who had one or two sentinel-node macrometastases and had undergone a mastectomy or breast-conserving surgery.
- The trial randomized 2540 patients to either completion axillary lymph node dissection (n = 1205) or sentinel-node biopsy only (n = 1335). Nearly 90% of patients received adjuvant radiation therapy, and the majority also received systematic therapy.
- Earlier recurrence-free survival findings and patient-reported outcomes were reported last December. The researchers now reported overall survival findings as well as secondary endpoints of breast cancer-specific survival.
TAKEAWAY:
- The researchers reported 191 recurrences or deaths over a median follow-up of 46.8 months; 62 patients (4.6%) in the sentinel-node biopsy–only group died, and 69 patients (5.7%) in the dissection group died.
- The biopsy-only group had an estimated 5-year overall survival of 92.9% compared with 92.0% in the dissection group and an estimated 5-year breast cancer-specific survival of 97.1% vs 96.6% in the dissection group.
- The estimated 5-year recurrence-free survival was 89.7% in the biopsy-only group vs 88.7% in the dissection group (hazard ratio [HR], 0.89; 95% CI, 0.66-1.19).
- This non-inferior difference held across all prespecified patient subgroups, except in patients with estrogen receptor-positive, human epidermal growth factor receptor 2–positive disease, in which sentinel biopsy alone appeared to be better (HR, 0.26).
IN PRACTICE:
“This trial provides robust evidence that the omission of completion axillary-lymph-node dissection was safe in patients with clinically node-negative T1, T2, or T3 breast cancer and one or two sentinel-node macrometastases who received adjuvant systemic treatment and radiation therapy according to national guidelines,” the authors concluded.
“It is clear that the role of axillary dissection is rapidly disappearing,” Kandace P. McGuire, MD, of Virginia Commonwealth University, Richmond, wrote in an accompanying editorial. “However, axillary staging continues to be vital with regard to decisions about appropriate breast cancer therapy.”
SOURCE:
This work, led by Jana de Boniface, MD, PhD, from Karolinska Institute, Stockholm, was published online in The New England Journal of Medicine, alongside the accompanying editorial by Dr. McGuire.
LIMITATIONS:
The study limitations include unavailable radiation therapy details for comparison, low male recruitment hindering sex-based analysis, short follow-up for luminal subtype breast cancer, unmet enrollment targets, and higher withdrawal rates in the dissection group.
DISCLOSURES:
This study was supported by the Swedish Research Council, Swedish Cancer Society, Nordic Cancer Union, and Swedish Breast Cancer Association. One coauthor reported receiving consultancy fees from various pharmaceutical companies outside this work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A growing body of evidence has indicated that patients with one or two positive sentinel nodes undergoing breast-conserving surgery and radiation therapy can skip axillary lymph node dissection and achieve similar outcomes compared with patients receiving axillary dissection.
- However, these earlier studies had notable limitations, such as limited statistical power, uncertain nodal radiotherapy target volumes, and minimal data on relevant clinical subgroups.
- To fill the gaps in the literature, the researchers conducted a trial with a large, inclusive cohort of patients with node-negative stage T1-T3 breast cancer who had one or two sentinel-node macrometastases and had undergone a mastectomy or breast-conserving surgery.
- The trial randomized 2540 patients to either completion axillary lymph node dissection (n = 1205) or sentinel-node biopsy only (n = 1335). Nearly 90% of patients received adjuvant radiation therapy, and the majority also received systematic therapy.
- Earlier recurrence-free survival findings and patient-reported outcomes were reported last December. The researchers now reported overall survival findings as well as secondary endpoints of breast cancer-specific survival.
TAKEAWAY:
- The researchers reported 191 recurrences or deaths over a median follow-up of 46.8 months; 62 patients (4.6%) in the sentinel-node biopsy–only group died, and 69 patients (5.7%) in the dissection group died.
- The biopsy-only group had an estimated 5-year overall survival of 92.9% compared with 92.0% in the dissection group and an estimated 5-year breast cancer-specific survival of 97.1% vs 96.6% in the dissection group.
- The estimated 5-year recurrence-free survival was 89.7% in the biopsy-only group vs 88.7% in the dissection group (hazard ratio [HR], 0.89; 95% CI, 0.66-1.19).
- This non-inferior difference held across all prespecified patient subgroups, except in patients with estrogen receptor-positive, human epidermal growth factor receptor 2–positive disease, in which sentinel biopsy alone appeared to be better (HR, 0.26).
IN PRACTICE:
“This trial provides robust evidence that the omission of completion axillary-lymph-node dissection was safe in patients with clinically node-negative T1, T2, or T3 breast cancer and one or two sentinel-node macrometastases who received adjuvant systemic treatment and radiation therapy according to national guidelines,” the authors concluded.
“It is clear that the role of axillary dissection is rapidly disappearing,” Kandace P. McGuire, MD, of Virginia Commonwealth University, Richmond, wrote in an accompanying editorial. “However, axillary staging continues to be vital with regard to decisions about appropriate breast cancer therapy.”
SOURCE:
This work, led by Jana de Boniface, MD, PhD, from Karolinska Institute, Stockholm, was published online in The New England Journal of Medicine, alongside the accompanying editorial by Dr. McGuire.
LIMITATIONS:
The study limitations include unavailable radiation therapy details for comparison, low male recruitment hindering sex-based analysis, short follow-up for luminal subtype breast cancer, unmet enrollment targets, and higher withdrawal rates in the dissection group.
DISCLOSURES:
This study was supported by the Swedish Research Council, Swedish Cancer Society, Nordic Cancer Union, and Swedish Breast Cancer Association. One coauthor reported receiving consultancy fees from various pharmaceutical companies outside this work.
A version of this article appeared on Medscape.com.
Low-Fat Vegan Diet May Improve Cardiometabolic Health in T1D
TOPLINE:
A low-fat vegan diet — high in fiber and carbohydrates and moderate in protein — reduces insulin requirement, increases insulin sensitivity, and improves glycemic control in individuals with type 1 diabetes (T1D) compared with a conventional portion-controlled diet.
METHODOLOGY:
- The effects of a low-fat vegan diet (without carbohydrate or portion restriction) were compared with those of a conventional portion-controlled, carbohydrate-controlled diet in 58 patients with T1D (age, ≥ 18 years) who had been receiving stable insulin treatment for the past 3 months.
- Participants were randomly assigned to receive either the vegan diet (n = 29), comprising vegetables, grains, legumes, and fruits, or the portion-controlled diet (n = 29), which reduced daily energy intake by 500-1000 kcal/d in participants with overweight while maintaining a stable carbohydrate intake.
- The primary clinical outcomes were insulin requirement (total daily dose of insulin), insulin sensitivity, and glycemic control (A1c).
- Other assessments included the blood, lipid profile, blood urea nitrogen, blood urea nitrogen-to-creatinine ratio, and body weight.
TAKEAWAY:
- The study was completed by 18 participants in the vegan-diet group and 17 in the portion-controlled group.
- In the vegan group, the total daily dose of insulin decreased by 12.1 units/d (P = .007) and insulin sensitivity increased by 6.6 g of carbohydrate per unit of insulin on average (P = .002), with no significant changes in the portion-controlled diet group.
- Participants on the vegan diet had lower levels of total and low-density lipoprotein cholesterol and blood urea nitrogen and a lower blood urea nitrogen-to-creatinine ratio (P for all < .001), whereas both vegan and portion-controlled groups had lower A1c levels.
- Body weight decreased by 5.2 kg (P < .001) in the vegan group; there were no significant changes in the portion-controlled group.
- For every 1-kg weight loss, there was a 2.16-unit decrease in the insulin total daily dose and a 0.9-unit increase in insulin sensitivity.
IN PRACTICE:
“This study provides substantial support for a low-fat vegan diet that is high in fiber and carbohydrates, low in fat, and moderate in protein” and suggests the potential therapeutic use of this diet in type 1 diabetes management, the authors wrote.
SOURCE:
The study led by Hana Kahleova, MD, PhD, Physicians Committee for Responsible Medicine, Washington, was published in Clinical Diabetes.
LIMITATIONS:
Dietary intake was recorded on the basis of self-reported data. A higher attrition rate was observed due to meal and blood glucose monitoring. The findings may have limited generalizability as the study participants comprised those seeking help for T1D.
DISCLOSURES:
The study was supported by the Physicians Committee for Responsible Medicine and a grant from the Institute for Technology in Healthcare. Some authors reported receiving compensation, being cofounders of a coaching program, writing books, providing nutrition coaching, giving lectures, or receiving royalties and honoraria from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A low-fat vegan diet — high in fiber and carbohydrates and moderate in protein — reduces insulin requirement, increases insulin sensitivity, and improves glycemic control in individuals with type 1 diabetes (T1D) compared with a conventional portion-controlled diet.
METHODOLOGY:
- The effects of a low-fat vegan diet (without carbohydrate or portion restriction) were compared with those of a conventional portion-controlled, carbohydrate-controlled diet in 58 patients with T1D (age, ≥ 18 years) who had been receiving stable insulin treatment for the past 3 months.
- Participants were randomly assigned to receive either the vegan diet (n = 29), comprising vegetables, grains, legumes, and fruits, or the portion-controlled diet (n = 29), which reduced daily energy intake by 500-1000 kcal/d in participants with overweight while maintaining a stable carbohydrate intake.
- The primary clinical outcomes were insulin requirement (total daily dose of insulin), insulin sensitivity, and glycemic control (A1c).
- Other assessments included the blood, lipid profile, blood urea nitrogen, blood urea nitrogen-to-creatinine ratio, and body weight.
TAKEAWAY:
- The study was completed by 18 participants in the vegan-diet group and 17 in the portion-controlled group.
- In the vegan group, the total daily dose of insulin decreased by 12.1 units/d (P = .007) and insulin sensitivity increased by 6.6 g of carbohydrate per unit of insulin on average (P = .002), with no significant changes in the portion-controlled diet group.
- Participants on the vegan diet had lower levels of total and low-density lipoprotein cholesterol and blood urea nitrogen and a lower blood urea nitrogen-to-creatinine ratio (P for all < .001), whereas both vegan and portion-controlled groups had lower A1c levels.
- Body weight decreased by 5.2 kg (P < .001) in the vegan group; there were no significant changes in the portion-controlled group.
- For every 1-kg weight loss, there was a 2.16-unit decrease in the insulin total daily dose and a 0.9-unit increase in insulin sensitivity.
IN PRACTICE:
“This study provides substantial support for a low-fat vegan diet that is high in fiber and carbohydrates, low in fat, and moderate in protein” and suggests the potential therapeutic use of this diet in type 1 diabetes management, the authors wrote.
SOURCE:
The study led by Hana Kahleova, MD, PhD, Physicians Committee for Responsible Medicine, Washington, was published in Clinical Diabetes.
LIMITATIONS:
Dietary intake was recorded on the basis of self-reported data. A higher attrition rate was observed due to meal and blood glucose monitoring. The findings may have limited generalizability as the study participants comprised those seeking help for T1D.
DISCLOSURES:
The study was supported by the Physicians Committee for Responsible Medicine and a grant from the Institute for Technology in Healthcare. Some authors reported receiving compensation, being cofounders of a coaching program, writing books, providing nutrition coaching, giving lectures, or receiving royalties and honoraria from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A low-fat vegan diet — high in fiber and carbohydrates and moderate in protein — reduces insulin requirement, increases insulin sensitivity, and improves glycemic control in individuals with type 1 diabetes (T1D) compared with a conventional portion-controlled diet.
METHODOLOGY:
- The effects of a low-fat vegan diet (without carbohydrate or portion restriction) were compared with those of a conventional portion-controlled, carbohydrate-controlled diet in 58 patients with T1D (age, ≥ 18 years) who had been receiving stable insulin treatment for the past 3 months.
- Participants were randomly assigned to receive either the vegan diet (n = 29), comprising vegetables, grains, legumes, and fruits, or the portion-controlled diet (n = 29), which reduced daily energy intake by 500-1000 kcal/d in participants with overweight while maintaining a stable carbohydrate intake.
- The primary clinical outcomes were insulin requirement (total daily dose of insulin), insulin sensitivity, and glycemic control (A1c).
- Other assessments included the blood, lipid profile, blood urea nitrogen, blood urea nitrogen-to-creatinine ratio, and body weight.
TAKEAWAY:
- The study was completed by 18 participants in the vegan-diet group and 17 in the portion-controlled group.
- In the vegan group, the total daily dose of insulin decreased by 12.1 units/d (P = .007) and insulin sensitivity increased by 6.6 g of carbohydrate per unit of insulin on average (P = .002), with no significant changes in the portion-controlled diet group.
- Participants on the vegan diet had lower levels of total and low-density lipoprotein cholesterol and blood urea nitrogen and a lower blood urea nitrogen-to-creatinine ratio (P for all < .001), whereas both vegan and portion-controlled groups had lower A1c levels.
- Body weight decreased by 5.2 kg (P < .001) in the vegan group; there were no significant changes in the portion-controlled group.
- For every 1-kg weight loss, there was a 2.16-unit decrease in the insulin total daily dose and a 0.9-unit increase in insulin sensitivity.
IN PRACTICE:
“This study provides substantial support for a low-fat vegan diet that is high in fiber and carbohydrates, low in fat, and moderate in protein” and suggests the potential therapeutic use of this diet in type 1 diabetes management, the authors wrote.
SOURCE:
The study led by Hana Kahleova, MD, PhD, Physicians Committee for Responsible Medicine, Washington, was published in Clinical Diabetes.
LIMITATIONS:
Dietary intake was recorded on the basis of self-reported data. A higher attrition rate was observed due to meal and blood glucose monitoring. The findings may have limited generalizability as the study participants comprised those seeking help for T1D.
DISCLOSURES:
The study was supported by the Physicians Committee for Responsible Medicine and a grant from the Institute for Technology in Healthcare. Some authors reported receiving compensation, being cofounders of a coaching program, writing books, providing nutrition coaching, giving lectures, or receiving royalties and honoraria from various sources.
A version of this article appeared on Medscape.com.





