User login
Real world responses mirror TOURMALINE-MM1 data
Glasgow – Patients with relapsed or refractory multiple myeloma (RRMM) who were treated with a combination of the oral protease inhibitor ixazomib with lenalidomide and dexamethasone (IRd) in routine clinical practice had similar responses to clinical trial patients, according to a global observational study.
Real-world progression-free survival (PFS) and overall response (OR) rates closely approximated data from the TOURMALINE‑MM1 trial, reported lead author Gordon Cook, MB ChB, PhD, clinical director of hematology at the University of Leeds (England).
Tolerability appeared slightly higher in routine clinical practice, and in agreement with previous real-world studies for RRMM, patients who received IRd in earlier lines of therapy had better outcomes than did those who received IRd in later lines of therapy. “The translation of clinical trial data into the real world is really important because we practice in the real world,” Dr. Cook said at the annual meeting of the British Society for Haematology. “We know that trials are really important for establishing efficacy and safety of drugs so they can get licensed and market access, but [clinical trials] often don’t tell us about the true effectiveness of the drugs and tolerability because the populations in trials are often different from [patients in] the real world.”
This situation leads to an evidence gap, which the present trial, dubbed INSIGHT MM aims to fill. INSIGHT is the largest global, prospective, observational trial for multiple myeloma conducted to date, with ongoing enrollment of about 4,200 patients from 15 countries with newly diagnosed or refractory/relapsed multiple myeloma. Dr. Cook estimated that recruitment would be complete by June of 2019.
“The aim of [INSIGHT MM] is to evaluate real-world treatment and outcomes [in multiple myeloma] over 5 years and beyond,” Dr. Cook said.
In combination with interim data from INSIGHT MM (n = 50), Dr. Cook reported patient outcomes from the Czech Registry of Monoclonal Gammopathies (n = 113), a similar database. Unlike INSIGHT MM, which includes patients treated with between one and three prior lines of therapy, the Czech registry does not cap the number of prior therapies. Overall, in the data presented by Dr. Cook, nine countries were represented; about 90% of which were European, although approximately 10% of patients were treated in the United States and about 1% were treated in Taiwan.
The median age of diagnosis was 67 years, with about 14% of patients over the age of 75 years. Median time from diagnosis to initiation of IRd was about 3.5 years (42.6 months), at which point 71% of patients had an Eastern Cooperative Oncology Group performance status of at least 1.
About two-thirds of the patients (65%) had IgG multiple myeloma, and 14% had extramedullary disease. The most common prior therapy was bortezomib (89%), followed by transplant (61%), thalidomide (42%), lenalidomide (21%), carfilzomib (11%), daratumumab (3%), and pomalidomide (2%).
Half of the patients received IRd as second-line therapy, while the other half received the treatment third-line (30%), or fourth-line or later (20%). Median duration of therapy was just over 1 year (14 months), with 62% of patients still receiving therapy at data cutoff.
Dr. Cook cautioned that with a median follow-up of 9.3 months, data are still immature. However, the results so far suggest strong similarities in tolerability and efficacy when comparing real-world and clinical trial administration of IRd.
Routine clinical use was associated with an overall response rate of 74%, compared with 78% in the TOURMALINE‑MM1 trial. Again, showing high similarity, median PFS rates were 20.9 months and 20.6 months for the present data set and the TOURMALINE‑MM1 trial, respectively.
Just 4% of patients permanently discontinued ixazomib in the real-world study, compared with 17% in the clinical trial, suggesting that IRd may be better tolerated in routine clinical practice than the trial data indicated.
“IRd is effective in this setting,” Dr. Cook said. “Bear in mind that patients in the real-world database were further down the line in terms of the treatment pathway, they had prior heavier exposure to bortezomib and lenalidomide, and their performance status was slightly less impressive than it was in [TOURMALINE‑MM1]; therefore, to see this level of response in the real world is very pleasing.”
When asked by an attendee if clinical trials should push for inclusion of patients more representative of real-world populations, Dr. Cook said no. “I think the way we conduct phase 3 clinical trials, in particular, has to be the way it is in order for us to ensure that we can actually get the absolute efficacy and the safety, and that has to be done by a refined population, I’m afraid,” he said.
However, Dr. Cook supported efforts to improve reliability of data for clinicians at the time of drug licensing.
“We should be running real-world exposure in parallel with phase 3 studies, which is harder to do but just requires a bit of imagination,” Dr. Cook said.
The study was funded by Takeda. The investigators reported financial relationships with Takeda and other companies.
SOURCE: Cook G et al. BSH 2019, Abstract OR-018.
Glasgow – Patients with relapsed or refractory multiple myeloma (RRMM) who were treated with a combination of the oral protease inhibitor ixazomib with lenalidomide and dexamethasone (IRd) in routine clinical practice had similar responses to clinical trial patients, according to a global observational study.
Real-world progression-free survival (PFS) and overall response (OR) rates closely approximated data from the TOURMALINE‑MM1 trial, reported lead author Gordon Cook, MB ChB, PhD, clinical director of hematology at the University of Leeds (England).
Tolerability appeared slightly higher in routine clinical practice, and in agreement with previous real-world studies for RRMM, patients who received IRd in earlier lines of therapy had better outcomes than did those who received IRd in later lines of therapy. “The translation of clinical trial data into the real world is really important because we practice in the real world,” Dr. Cook said at the annual meeting of the British Society for Haematology. “We know that trials are really important for establishing efficacy and safety of drugs so they can get licensed and market access, but [clinical trials] often don’t tell us about the true effectiveness of the drugs and tolerability because the populations in trials are often different from [patients in] the real world.”
This situation leads to an evidence gap, which the present trial, dubbed INSIGHT MM aims to fill. INSIGHT is the largest global, prospective, observational trial for multiple myeloma conducted to date, with ongoing enrollment of about 4,200 patients from 15 countries with newly diagnosed or refractory/relapsed multiple myeloma. Dr. Cook estimated that recruitment would be complete by June of 2019.
“The aim of [INSIGHT MM] is to evaluate real-world treatment and outcomes [in multiple myeloma] over 5 years and beyond,” Dr. Cook said.
In combination with interim data from INSIGHT MM (n = 50), Dr. Cook reported patient outcomes from the Czech Registry of Monoclonal Gammopathies (n = 113), a similar database. Unlike INSIGHT MM, which includes patients treated with between one and three prior lines of therapy, the Czech registry does not cap the number of prior therapies. Overall, in the data presented by Dr. Cook, nine countries were represented; about 90% of which were European, although approximately 10% of patients were treated in the United States and about 1% were treated in Taiwan.
The median age of diagnosis was 67 years, with about 14% of patients over the age of 75 years. Median time from diagnosis to initiation of IRd was about 3.5 years (42.6 months), at which point 71% of patients had an Eastern Cooperative Oncology Group performance status of at least 1.
About two-thirds of the patients (65%) had IgG multiple myeloma, and 14% had extramedullary disease. The most common prior therapy was bortezomib (89%), followed by transplant (61%), thalidomide (42%), lenalidomide (21%), carfilzomib (11%), daratumumab (3%), and pomalidomide (2%).
Half of the patients received IRd as second-line therapy, while the other half received the treatment third-line (30%), or fourth-line or later (20%). Median duration of therapy was just over 1 year (14 months), with 62% of patients still receiving therapy at data cutoff.
Dr. Cook cautioned that with a median follow-up of 9.3 months, data are still immature. However, the results so far suggest strong similarities in tolerability and efficacy when comparing real-world and clinical trial administration of IRd.
Routine clinical use was associated with an overall response rate of 74%, compared with 78% in the TOURMALINE‑MM1 trial. Again, showing high similarity, median PFS rates were 20.9 months and 20.6 months for the present data set and the TOURMALINE‑MM1 trial, respectively.
Just 4% of patients permanently discontinued ixazomib in the real-world study, compared with 17% in the clinical trial, suggesting that IRd may be better tolerated in routine clinical practice than the trial data indicated.
“IRd is effective in this setting,” Dr. Cook said. “Bear in mind that patients in the real-world database were further down the line in terms of the treatment pathway, they had prior heavier exposure to bortezomib and lenalidomide, and their performance status was slightly less impressive than it was in [TOURMALINE‑MM1]; therefore, to see this level of response in the real world is very pleasing.”
When asked by an attendee if clinical trials should push for inclusion of patients more representative of real-world populations, Dr. Cook said no. “I think the way we conduct phase 3 clinical trials, in particular, has to be the way it is in order for us to ensure that we can actually get the absolute efficacy and the safety, and that has to be done by a refined population, I’m afraid,” he said.
However, Dr. Cook supported efforts to improve reliability of data for clinicians at the time of drug licensing.
“We should be running real-world exposure in parallel with phase 3 studies, which is harder to do but just requires a bit of imagination,” Dr. Cook said.
The study was funded by Takeda. The investigators reported financial relationships with Takeda and other companies.
SOURCE: Cook G et al. BSH 2019, Abstract OR-018.
Glasgow – Patients with relapsed or refractory multiple myeloma (RRMM) who were treated with a combination of the oral protease inhibitor ixazomib with lenalidomide and dexamethasone (IRd) in routine clinical practice had similar responses to clinical trial patients, according to a global observational study.
Real-world progression-free survival (PFS) and overall response (OR) rates closely approximated data from the TOURMALINE‑MM1 trial, reported lead author Gordon Cook, MB ChB, PhD, clinical director of hematology at the University of Leeds (England).
Tolerability appeared slightly higher in routine clinical practice, and in agreement with previous real-world studies for RRMM, patients who received IRd in earlier lines of therapy had better outcomes than did those who received IRd in later lines of therapy. “The translation of clinical trial data into the real world is really important because we practice in the real world,” Dr. Cook said at the annual meeting of the British Society for Haematology. “We know that trials are really important for establishing efficacy and safety of drugs so they can get licensed and market access, but [clinical trials] often don’t tell us about the true effectiveness of the drugs and tolerability because the populations in trials are often different from [patients in] the real world.”
This situation leads to an evidence gap, which the present trial, dubbed INSIGHT MM aims to fill. INSIGHT is the largest global, prospective, observational trial for multiple myeloma conducted to date, with ongoing enrollment of about 4,200 patients from 15 countries with newly diagnosed or refractory/relapsed multiple myeloma. Dr. Cook estimated that recruitment would be complete by June of 2019.
“The aim of [INSIGHT MM] is to evaluate real-world treatment and outcomes [in multiple myeloma] over 5 years and beyond,” Dr. Cook said.
In combination with interim data from INSIGHT MM (n = 50), Dr. Cook reported patient outcomes from the Czech Registry of Monoclonal Gammopathies (n = 113), a similar database. Unlike INSIGHT MM, which includes patients treated with between one and three prior lines of therapy, the Czech registry does not cap the number of prior therapies. Overall, in the data presented by Dr. Cook, nine countries were represented; about 90% of which were European, although approximately 10% of patients were treated in the United States and about 1% were treated in Taiwan.
The median age of diagnosis was 67 years, with about 14% of patients over the age of 75 years. Median time from diagnosis to initiation of IRd was about 3.5 years (42.6 months), at which point 71% of patients had an Eastern Cooperative Oncology Group performance status of at least 1.
About two-thirds of the patients (65%) had IgG multiple myeloma, and 14% had extramedullary disease. The most common prior therapy was bortezomib (89%), followed by transplant (61%), thalidomide (42%), lenalidomide (21%), carfilzomib (11%), daratumumab (3%), and pomalidomide (2%).
Half of the patients received IRd as second-line therapy, while the other half received the treatment third-line (30%), or fourth-line or later (20%). Median duration of therapy was just over 1 year (14 months), with 62% of patients still receiving therapy at data cutoff.
Dr. Cook cautioned that with a median follow-up of 9.3 months, data are still immature. However, the results so far suggest strong similarities in tolerability and efficacy when comparing real-world and clinical trial administration of IRd.
Routine clinical use was associated with an overall response rate of 74%, compared with 78% in the TOURMALINE‑MM1 trial. Again, showing high similarity, median PFS rates were 20.9 months and 20.6 months for the present data set and the TOURMALINE‑MM1 trial, respectively.
Just 4% of patients permanently discontinued ixazomib in the real-world study, compared with 17% in the clinical trial, suggesting that IRd may be better tolerated in routine clinical practice than the trial data indicated.
“IRd is effective in this setting,” Dr. Cook said. “Bear in mind that patients in the real-world database were further down the line in terms of the treatment pathway, they had prior heavier exposure to bortezomib and lenalidomide, and their performance status was slightly less impressive than it was in [TOURMALINE‑MM1]; therefore, to see this level of response in the real world is very pleasing.”
When asked by an attendee if clinical trials should push for inclusion of patients more representative of real-world populations, Dr. Cook said no. “I think the way we conduct phase 3 clinical trials, in particular, has to be the way it is in order for us to ensure that we can actually get the absolute efficacy and the safety, and that has to be done by a refined population, I’m afraid,” he said.
However, Dr. Cook supported efforts to improve reliability of data for clinicians at the time of drug licensing.
“We should be running real-world exposure in parallel with phase 3 studies, which is harder to do but just requires a bit of imagination,” Dr. Cook said.
The study was funded by Takeda. The investigators reported financial relationships with Takeda and other companies.
SOURCE: Cook G et al. BSH 2019, Abstract OR-018.
Reporting from BSH 2019
Powerful breast-implant testimony constrained by limited evidence
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
Scientific Abstracts; Skin Disease Education Foundation’s 43rd Annual Hawaii Dermatology Seminar
Despite failed primary endpoint, MI alert device has predictive value
WASHINGTON –Although an implantable device for detecting myocardial infarction missed the primary composite outcome endpoint in a controlled trial, a newly completed extended analysis associated the device with a higher positive predictive value and a lower false positive rate when compared to sham control, according to data presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
“Among high risk patients, this system may be beneficial in the identification of both symptomatic and asymptomatic coronary events,” reported C. Michael Gibson, MD, chief of clinical research in the cardiology division at Beth Israel Deaconess Hospital, Boston.
The implantable device (AngelMed Guardian System), which received Food and Drug Administration approval in April 2018, is designed to identify MI through detection of ST-segment elevations in the absence of an elevated heart rate. When the system detects an event during continuous monitoring, it sends a signal (internal vibration and auditory signal to an external monitor) designed to tell the patient to seek medical care.
The previously published multicenter and randomized ALERTS (AngelMed for Early Recognition and Treatment of STEMI) trial that tested this device was negative for primary composite endpoint of cardiac or unexplained death, new Q-wave MI, or presentation at the emergency department (ED) more than 2 hours after symptom onset (J Am Coll Cardiol. 2019 Feb 25. pii: S0735-1097[19]30237-2). In that trial 907 patients were fitted with the device and then randomized to having the device switched on or left off.
At 7 days, a primary endpoint was reached by 3.8% of those in the device-on group versus 4.9% of those in the device-off group, which was not significantly different.
Although the primary endpoint was not met, there were promising results. For example, in those who did have an occlusive event, patients in the device-on group had better preserved left ventricular function when evaluated after the event, a result consistent with earlier presentation in the ED and earlier treatment. In fact, 85% of patients with an MI in the device-on group presented to a hospital within 2 hours, compared with just 5% of those in the device-off control group during the initial study period.
More evidence of a potential clinical role for the device has now been generated in a new extended analysis. This analysis was made possible because patients in both of the randomized groups continue to wear the device, including those in the device-off group who had their devices activated after 6 months. There are now 3 more years of data of follow-up from those initially in the device-on group and those switched from the device-off group.
“So we started the clock over with a new statistical analysis plan and new endpoints,” Dr. Gibson explained. The FDA was consulted in selecting endpoints, particularly regarding evidence that the device did not increase false-positive ED visits.
There were numerous encouraging findings. One was that 42 silent MIs, which would otherwise have been missed, were detected over the extended follow-up. Another was that the annualized false-positive rate was lower in those with an activated device (0.164/year) when compared to the original device-off group (0.678/year; P less than .001). Lastly, the positive predictive value of an alarm during the extended follow-up was higher than that of symptoms alone among the original device-off group (25.8% vs. 18.2%).
The device was found safe. The rate of system-related complications was under 4%, which Dr. Gibson said is noninferior to that associated with pacemakers.
One of the potential explanations for the failure of the device to achieve the primary endpoint in the original trial was an unexpectedly low event rate, according to Dr. Gibson.
Even before this extended analysis, the FDA had accepted the potential benefits of this device as demonstrated in the approval last year. In the labeling, the device is called “a more accurate predictor of acute coronary syndrome events when compared to patient recognized symptoms alone and demonstrates a reduced rate over time of patient presentations without ACS events.”
“About 50% of patients wait more than 3 hours after the onset of symptoms before reaching an emergency room,” observed Dr. Gibson. Emphasizing the evidence that delay is an important predictor of adverse outcomes, he suggested the alarm device might be useful in accelerating care in some high risk groups.
WASHINGTON –Although an implantable device for detecting myocardial infarction missed the primary composite outcome endpoint in a controlled trial, a newly completed extended analysis associated the device with a higher positive predictive value and a lower false positive rate when compared to sham control, according to data presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
“Among high risk patients, this system may be beneficial in the identification of both symptomatic and asymptomatic coronary events,” reported C. Michael Gibson, MD, chief of clinical research in the cardiology division at Beth Israel Deaconess Hospital, Boston.
The implantable device (AngelMed Guardian System), which received Food and Drug Administration approval in April 2018, is designed to identify MI through detection of ST-segment elevations in the absence of an elevated heart rate. When the system detects an event during continuous monitoring, it sends a signal (internal vibration and auditory signal to an external monitor) designed to tell the patient to seek medical care.
The previously published multicenter and randomized ALERTS (AngelMed for Early Recognition and Treatment of STEMI) trial that tested this device was negative for primary composite endpoint of cardiac or unexplained death, new Q-wave MI, or presentation at the emergency department (ED) more than 2 hours after symptom onset (J Am Coll Cardiol. 2019 Feb 25. pii: S0735-1097[19]30237-2). In that trial 907 patients were fitted with the device and then randomized to having the device switched on or left off.
At 7 days, a primary endpoint was reached by 3.8% of those in the device-on group versus 4.9% of those in the device-off group, which was not significantly different.
Although the primary endpoint was not met, there were promising results. For example, in those who did have an occlusive event, patients in the device-on group had better preserved left ventricular function when evaluated after the event, a result consistent with earlier presentation in the ED and earlier treatment. In fact, 85% of patients with an MI in the device-on group presented to a hospital within 2 hours, compared with just 5% of those in the device-off control group during the initial study period.
More evidence of a potential clinical role for the device has now been generated in a new extended analysis. This analysis was made possible because patients in both of the randomized groups continue to wear the device, including those in the device-off group who had their devices activated after 6 months. There are now 3 more years of data of follow-up from those initially in the device-on group and those switched from the device-off group.
“So we started the clock over with a new statistical analysis plan and new endpoints,” Dr. Gibson explained. The FDA was consulted in selecting endpoints, particularly regarding evidence that the device did not increase false-positive ED visits.
There were numerous encouraging findings. One was that 42 silent MIs, which would otherwise have been missed, were detected over the extended follow-up. Another was that the annualized false-positive rate was lower in those with an activated device (0.164/year) when compared to the original device-off group (0.678/year; P less than .001). Lastly, the positive predictive value of an alarm during the extended follow-up was higher than that of symptoms alone among the original device-off group (25.8% vs. 18.2%).
The device was found safe. The rate of system-related complications was under 4%, which Dr. Gibson said is noninferior to that associated with pacemakers.
One of the potential explanations for the failure of the device to achieve the primary endpoint in the original trial was an unexpectedly low event rate, according to Dr. Gibson.
Even before this extended analysis, the FDA had accepted the potential benefits of this device as demonstrated in the approval last year. In the labeling, the device is called “a more accurate predictor of acute coronary syndrome events when compared to patient recognized symptoms alone and demonstrates a reduced rate over time of patient presentations without ACS events.”
“About 50% of patients wait more than 3 hours after the onset of symptoms before reaching an emergency room,” observed Dr. Gibson. Emphasizing the evidence that delay is an important predictor of adverse outcomes, he suggested the alarm device might be useful in accelerating care in some high risk groups.
WASHINGTON –Although an implantable device for detecting myocardial infarction missed the primary composite outcome endpoint in a controlled trial, a newly completed extended analysis associated the device with a higher positive predictive value and a lower false positive rate when compared to sham control, according to data presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
“Among high risk patients, this system may be beneficial in the identification of both symptomatic and asymptomatic coronary events,” reported C. Michael Gibson, MD, chief of clinical research in the cardiology division at Beth Israel Deaconess Hospital, Boston.
The implantable device (AngelMed Guardian System), which received Food and Drug Administration approval in April 2018, is designed to identify MI through detection of ST-segment elevations in the absence of an elevated heart rate. When the system detects an event during continuous monitoring, it sends a signal (internal vibration and auditory signal to an external monitor) designed to tell the patient to seek medical care.
The previously published multicenter and randomized ALERTS (AngelMed for Early Recognition and Treatment of STEMI) trial that tested this device was negative for primary composite endpoint of cardiac or unexplained death, new Q-wave MI, or presentation at the emergency department (ED) more than 2 hours after symptom onset (J Am Coll Cardiol. 2019 Feb 25. pii: S0735-1097[19]30237-2). In that trial 907 patients were fitted with the device and then randomized to having the device switched on or left off.
At 7 days, a primary endpoint was reached by 3.8% of those in the device-on group versus 4.9% of those in the device-off group, which was not significantly different.
Although the primary endpoint was not met, there were promising results. For example, in those who did have an occlusive event, patients in the device-on group had better preserved left ventricular function when evaluated after the event, a result consistent with earlier presentation in the ED and earlier treatment. In fact, 85% of patients with an MI in the device-on group presented to a hospital within 2 hours, compared with just 5% of those in the device-off control group during the initial study period.
More evidence of a potential clinical role for the device has now been generated in a new extended analysis. This analysis was made possible because patients in both of the randomized groups continue to wear the device, including those in the device-off group who had their devices activated after 6 months. There are now 3 more years of data of follow-up from those initially in the device-on group and those switched from the device-off group.
“So we started the clock over with a new statistical analysis plan and new endpoints,” Dr. Gibson explained. The FDA was consulted in selecting endpoints, particularly regarding evidence that the device did not increase false-positive ED visits.
There were numerous encouraging findings. One was that 42 silent MIs, which would otherwise have been missed, were detected over the extended follow-up. Another was that the annualized false-positive rate was lower in those with an activated device (0.164/year) when compared to the original device-off group (0.678/year; P less than .001). Lastly, the positive predictive value of an alarm during the extended follow-up was higher than that of symptoms alone among the original device-off group (25.8% vs. 18.2%).
The device was found safe. The rate of system-related complications was under 4%, which Dr. Gibson said is noninferior to that associated with pacemakers.
One of the potential explanations for the failure of the device to achieve the primary endpoint in the original trial was an unexpectedly low event rate, according to Dr. Gibson.
Even before this extended analysis, the FDA had accepted the potential benefits of this device as demonstrated in the approval last year. In the labeling, the device is called “a more accurate predictor of acute coronary syndrome events when compared to patient recognized symptoms alone and demonstrates a reduced rate over time of patient presentations without ACS events.”
“About 50% of patients wait more than 3 hours after the onset of symptoms before reaching an emergency room,” observed Dr. Gibson. Emphasizing the evidence that delay is an important predictor of adverse outcomes, he suggested the alarm device might be useful in accelerating care in some high risk groups.
REPORTING FROM CRT 2019
Whole-genome sequencing demonstrates clinical relevance
GLASGOW – Whole genome sequencing (WGS) appears capable of replacing cytogenetic testing and next generation sequencing (NGS) for the detection of clinically relevant molecular abnormalities in hematological malignancies, according to investigators.
A comparison of WGS with fluorescence in situ hybridization (FISH) showed that WGS caught all the same significant structural variants, plus some abnormalities that FISH had not detected, reported lead author Shirley Henderson, PhD, lead for cancer molecular diagnostics at Genomics England in Oxford.
Although further validation is needed, these findings, reported at the annual meeting of the British Society for Haematology, support an ongoing effort to validate the clinical reliability of WGS, which is currently reserved for research purposes.
“It’s vitally important that the clinical community engage with this and understand both the power and the limitations of this technique and how this work is going to be interpreted for the benefit of patients,” said Adele Fielding, PhD, session chair from University College London’s Cancer Institute.
The investigators compared WGS with FISH for detection of clinically significant structural variants (SVs) and copy number variants (CNVs) in tumor samples from 34 patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
The 252 standard of care FISH tests – conducted at three separate clinical diagnostic centers in the United Kingdom – included 138 SVs and 114 CNVs. WGS relied on a combination of bioinformatics and visual inspection of Circos plots. WGS confirmed all of the SVs detected by FISH with high confidence; WGS detected four additional SVs, also with high confidence, including an ETV6-RUNX1 fusion not detected by FISH because of probe limitations.
Results for CNVs were similar, with WGS detecting 78 out of 85 positive CNVs. Six of the missed positives were associated with low quality samples or low level mutations in the FISH test, suggesting that at least some positives may have been detected with better samples. Only one negative CNV from FISH was missed by WGS.
Overall, WGS had a false positive rate of less than 5% and a positive percentage agreement with FISH that exceeded 90%.
“Further work is required to fully validate all aspects of the WGS analysis pipeline,” Dr. Henderson said. “But these results indicate that WGS has the potential to reliably detect SVs and CNVs in these conditions while offering the advantage of detecting all SVs and CNVs present without the need for additional interrogation of the sample by multiple tests or probes.”
Dr. Henderson noted that there is really no “perfect method” for identifying structural and copy number variants at the present time.
Small variants are relatively easy to detect with techniques such as karyotyping and gene banding, but these tests have shortcomings, namely, that they require live cells and have “fairly high failure rates for various reasons,” Dr. Henderson said.
“FISH is an incredibly useful test and it has higher resolution than gene banding, but the problem with FISH is that you only find what you’re looking at,” Dr. Henderson said. “It’s not genome wide; it’s very targeted.”
Similarly, polymerase chain reaction (PCR), including next generation sequencing (NGS), can detect molecular abnormalities, but only those that are targeted, which may necessitate multiple tests, she said.
“If you start looking for all of the structural variants [with existing techniques], then you’re going to be doing an awful lot of tests,” Dr. Henderson said.
Another potential benefit of WGS is that it is “future resistant,” Dr. Henderson said. “As new biomarkers are discovered, you don’t have to redesign a new targeted test. It will also detect emerging biomarkers, such as mutational signatures and burden.”
The study was sponsored by NHS England. The investigators reported having no conflicts of interest.
SOURCE: Henderson S et al. BSH 2019, Abstract OR-002.
GLASGOW – Whole genome sequencing (WGS) appears capable of replacing cytogenetic testing and next generation sequencing (NGS) for the detection of clinically relevant molecular abnormalities in hematological malignancies, according to investigators.
A comparison of WGS with fluorescence in situ hybridization (FISH) showed that WGS caught all the same significant structural variants, plus some abnormalities that FISH had not detected, reported lead author Shirley Henderson, PhD, lead for cancer molecular diagnostics at Genomics England in Oxford.
Although further validation is needed, these findings, reported at the annual meeting of the British Society for Haematology, support an ongoing effort to validate the clinical reliability of WGS, which is currently reserved for research purposes.
“It’s vitally important that the clinical community engage with this and understand both the power and the limitations of this technique and how this work is going to be interpreted for the benefit of patients,” said Adele Fielding, PhD, session chair from University College London’s Cancer Institute.
The investigators compared WGS with FISH for detection of clinically significant structural variants (SVs) and copy number variants (CNVs) in tumor samples from 34 patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
The 252 standard of care FISH tests – conducted at three separate clinical diagnostic centers in the United Kingdom – included 138 SVs and 114 CNVs. WGS relied on a combination of bioinformatics and visual inspection of Circos plots. WGS confirmed all of the SVs detected by FISH with high confidence; WGS detected four additional SVs, also with high confidence, including an ETV6-RUNX1 fusion not detected by FISH because of probe limitations.
Results for CNVs were similar, with WGS detecting 78 out of 85 positive CNVs. Six of the missed positives were associated with low quality samples or low level mutations in the FISH test, suggesting that at least some positives may have been detected with better samples. Only one negative CNV from FISH was missed by WGS.
Overall, WGS had a false positive rate of less than 5% and a positive percentage agreement with FISH that exceeded 90%.
“Further work is required to fully validate all aspects of the WGS analysis pipeline,” Dr. Henderson said. “But these results indicate that WGS has the potential to reliably detect SVs and CNVs in these conditions while offering the advantage of detecting all SVs and CNVs present without the need for additional interrogation of the sample by multiple tests or probes.”
Dr. Henderson noted that there is really no “perfect method” for identifying structural and copy number variants at the present time.
Small variants are relatively easy to detect with techniques such as karyotyping and gene banding, but these tests have shortcomings, namely, that they require live cells and have “fairly high failure rates for various reasons,” Dr. Henderson said.
“FISH is an incredibly useful test and it has higher resolution than gene banding, but the problem with FISH is that you only find what you’re looking at,” Dr. Henderson said. “It’s not genome wide; it’s very targeted.”
Similarly, polymerase chain reaction (PCR), including next generation sequencing (NGS), can detect molecular abnormalities, but only those that are targeted, which may necessitate multiple tests, she said.
“If you start looking for all of the structural variants [with existing techniques], then you’re going to be doing an awful lot of tests,” Dr. Henderson said.
Another potential benefit of WGS is that it is “future resistant,” Dr. Henderson said. “As new biomarkers are discovered, you don’t have to redesign a new targeted test. It will also detect emerging biomarkers, such as mutational signatures and burden.”
The study was sponsored by NHS England. The investigators reported having no conflicts of interest.
SOURCE: Henderson S et al. BSH 2019, Abstract OR-002.
GLASGOW – Whole genome sequencing (WGS) appears capable of replacing cytogenetic testing and next generation sequencing (NGS) for the detection of clinically relevant molecular abnormalities in hematological malignancies, according to investigators.
A comparison of WGS with fluorescence in situ hybridization (FISH) showed that WGS caught all the same significant structural variants, plus some abnormalities that FISH had not detected, reported lead author Shirley Henderson, PhD, lead for cancer molecular diagnostics at Genomics England in Oxford.
Although further validation is needed, these findings, reported at the annual meeting of the British Society for Haematology, support an ongoing effort to validate the clinical reliability of WGS, which is currently reserved for research purposes.
“It’s vitally important that the clinical community engage with this and understand both the power and the limitations of this technique and how this work is going to be interpreted for the benefit of patients,” said Adele Fielding, PhD, session chair from University College London’s Cancer Institute.
The investigators compared WGS with FISH for detection of clinically significant structural variants (SVs) and copy number variants (CNVs) in tumor samples from 34 patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
The 252 standard of care FISH tests – conducted at three separate clinical diagnostic centers in the United Kingdom – included 138 SVs and 114 CNVs. WGS relied on a combination of bioinformatics and visual inspection of Circos plots. WGS confirmed all of the SVs detected by FISH with high confidence; WGS detected four additional SVs, also with high confidence, including an ETV6-RUNX1 fusion not detected by FISH because of probe limitations.
Results for CNVs were similar, with WGS detecting 78 out of 85 positive CNVs. Six of the missed positives were associated with low quality samples or low level mutations in the FISH test, suggesting that at least some positives may have been detected with better samples. Only one negative CNV from FISH was missed by WGS.
Overall, WGS had a false positive rate of less than 5% and a positive percentage agreement with FISH that exceeded 90%.
“Further work is required to fully validate all aspects of the WGS analysis pipeline,” Dr. Henderson said. “But these results indicate that WGS has the potential to reliably detect SVs and CNVs in these conditions while offering the advantage of detecting all SVs and CNVs present without the need for additional interrogation of the sample by multiple tests or probes.”
Dr. Henderson noted that there is really no “perfect method” for identifying structural and copy number variants at the present time.
Small variants are relatively easy to detect with techniques such as karyotyping and gene banding, but these tests have shortcomings, namely, that they require live cells and have “fairly high failure rates for various reasons,” Dr. Henderson said.
“FISH is an incredibly useful test and it has higher resolution than gene banding, but the problem with FISH is that you only find what you’re looking at,” Dr. Henderson said. “It’s not genome wide; it’s very targeted.”
Similarly, polymerase chain reaction (PCR), including next generation sequencing (NGS), can detect molecular abnormalities, but only those that are targeted, which may necessitate multiple tests, she said.
“If you start looking for all of the structural variants [with existing techniques], then you’re going to be doing an awful lot of tests,” Dr. Henderson said.
Another potential benefit of WGS is that it is “future resistant,” Dr. Henderson said. “As new biomarkers are discovered, you don’t have to redesign a new targeted test. It will also detect emerging biomarkers, such as mutational signatures and burden.”
The study was sponsored by NHS England. The investigators reported having no conflicts of interest.
SOURCE: Henderson S et al. BSH 2019, Abstract OR-002.
REPORTING FROM BSH 2019
Bothersome Blisters: Localized Epidermolysis Bullosa Simplex
To the Editor:
Epidermolysis bullosa (EB) was first described in 1886, with the first classification scheme proposed in 1962 utilizing transmission electron microscopy (TEM) findings to delineate categories: epidermolytic (EB simplex [EBS]), lucidolytic (junctional EB), and dermolytic (dystrophic EB).1 Localized EBS (EBS-loc) is an autosomal-dominant disorder caused by negative mutations in keratin-5 and keratin-14, proteins expressed in the intermediate filaments of basal keratinocytes, which result in fragility of the skin in response to minor trauma.2 The incidence of EBS-loc is approximately 10 to 30 cases per million live births, with the age of presentation typically between the first and third decades of life.3,4 Because EBS-loc is the most common and often mildest form of EB, not all patients present for medical evaluation and true prevalence may be underestimated.4 We report a case of EBS-loc.
A 26-year-old woman with no notable medical history presented to the dermatology clinic for evaluation of skin blisters that had been intermittently present since infancy. The blisters primarily occurred on the feet, but she did occasionally develop blisters on the hands, knees, and elbows and at sites of friction or trauma (eg, bra line, medial thighs) following exercise. The blisters were worsened by heat and tight-fitting shoes. Because of the painful nature of the blisters, she would lance them with a needle. On the medial thighs, she utilized nonstick and gauze bandage roll dressings to minimize friction. A review of systems was positive for hyperhidrosis. Her family history revealed multiple family members with blisters involving the feet and areas of friction or trauma for 4 generations with no known diagnosis.
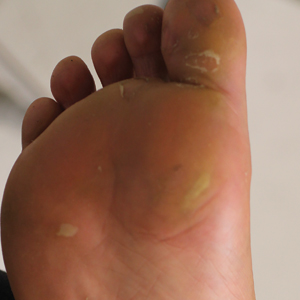
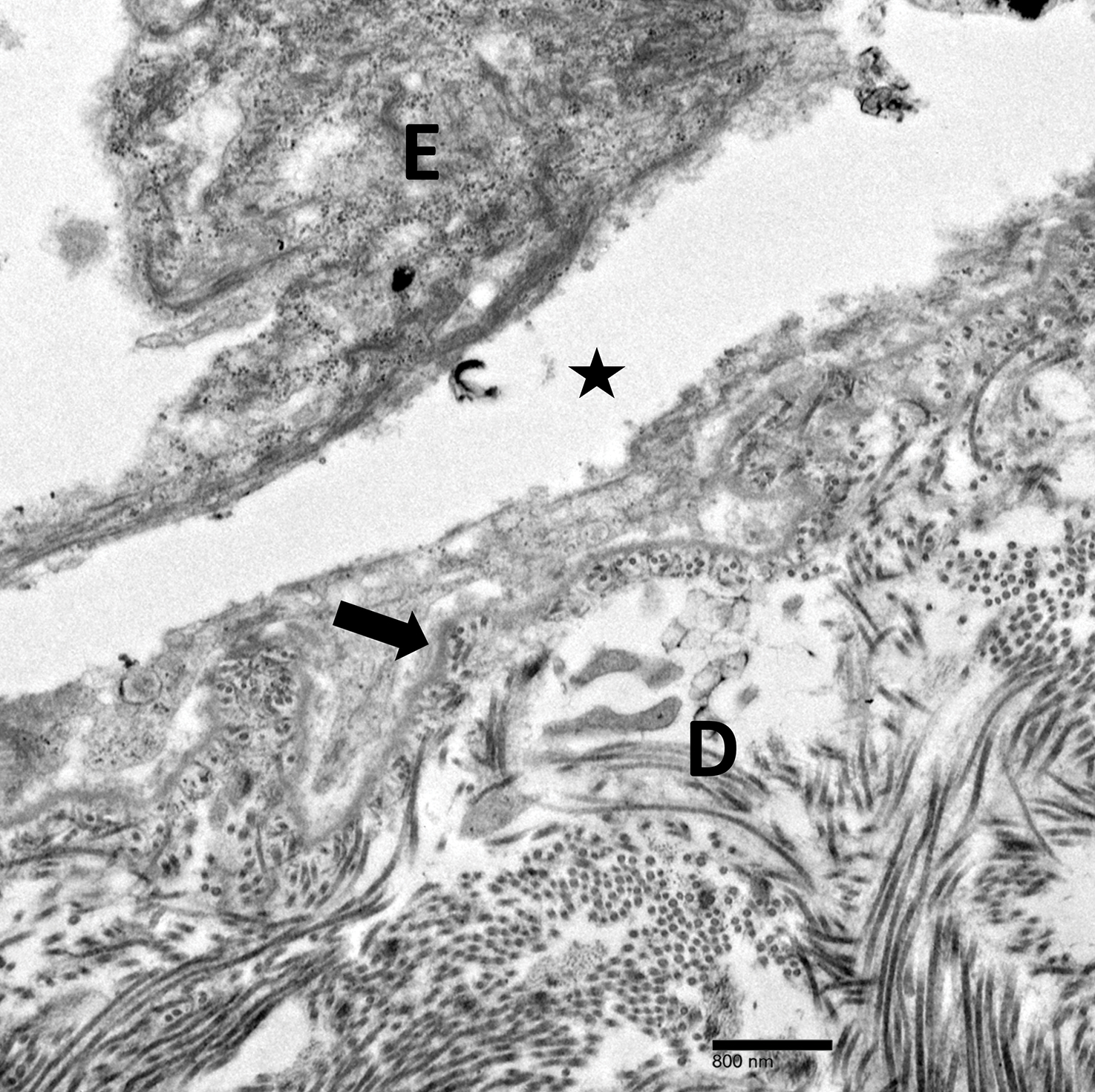
Physical examination revealed multiple tense bullae and calluses scattered over the bilateral plantar and distal dorsal feet with a few healing, superficially eroded, erythematous papules and plaques on the bilateral medial thighs (Figure 1). A biopsy from an induced blister on the right dorsal second toe was performed and sent in glutaraldehyde to the Epidermolysis Bullosa Clinic at Stanford University (Redwood City, California) for electron microscopy, which revealed lysis within the basal keratinocytes through the tonofilaments with continuous and intact lamina densa and lamina lucida (Figure 2). In this clinical context with the relevant family history, the findings were consistent with the diagnosis of EBS-loc (formerly Weber-Cockayne syndrome).2

Skin manifestations of EBS-loc typically consist of friction-induced blisters, erosions, and calluses primarily on the palms and soles, often associated with hyperhidrosis and worsening of symptoms in summer months and hot temperatures.3 Milia, atrophic scarring, and dystrophic nails are uncommon.1 Extracutaneous involvement is rare with the exception of oral cavity erosions, which typically are asymptomatic and usually are only seen during infancy.1
Light microscopy does not have a notable role in diagnosis of classic forms of inherited EB unless another autoimmune blistering disorder is suspected.2,5 Both TEM and immunofluorescence mapping are used to diagnose EB.1 DNA mutational analysis is not considered a first-line diagnostic test for EB given it is a costly labor-intensive technique with limited access at present, but it may be considered in settings of prenatal diagnosis or in vitro fertilization.1 Biopsy of a freshly induced blister should be performed, as early reepithelialization of an existing blister makes it difficult to establish the level of cleavage.5 Applying firm pressure using a pencil eraser and rotating it on intact skin induces a subclinical blister. Two punch biopsies (4 mm) at the edge of the blister with one-third lesional and two-thirds perilesional skin should be obtained, with one biopsy sent for immunofluorescence mapping in Michel fixative and the other for TEM in glutaraldehyde.3,5 Transmission electron microscopy of an induced blister in EBS-loc shows cleavage within the most inferior portion of the basilar keratinocyte.2 Immunofluorescence mapping with anti–epidermal basement membrane monoclonal antibodies can distinguish between EB subtypes and assess expression of specific skin-associated proteins on both a qualitative or semiquantitative basis, providing insight on which structural protein is mutated.1,5
No specific treatments are available for EBS-loc. Mainstays of treatment include prevention of mechanical trauma and secondary infection. Hyperhidrosis of thepalms and soles may be treated with topical aluminum chloride hexahydrate or injections of botulinum toxin type A.2,6 Patients have normal life expectancy, though some cases may have complications with substantial morbidity.1 Awareness of this disease, its clinical course, and therapeutic options will allow physicians to more appropriately counsel patients on the disease process.
Localized EBS may be more common than previously thought, as not all patients seek medical care. Given its impact on patient quality of life, it is important for clinicians to recognize EBS-loc. Although no specific treatments are available, wound care counseling and explanation of the genetics of the disease should be provided to patients.
- Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931-950.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Limited; 2012.
- Eichenfield LF, Frieden IJ, Mathes EF, et al, eds. Neonatal and Infant Dermatology. 3rd ed. New York, NY: Elsevier Health Sciences; 2015.
- Spitz JL. Genodermatoses: A Clinical Guide to Genetic Skin Disorders. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Epidermolysis bullosa. Stanford Medicine website. http://med.stanford.edu/dermatopathology/dermpath-services/epiderm.html. Accessed April 3, 2019.
- Abitbol RJ, Zhou LH. Treatment of epidermolysis bullosa simplex, Weber-Cockayne type, with botulinum toxin type A. Arch Dermatol. 2009;145:13-15.
To the Editor:
Epidermolysis bullosa (EB) was first described in 1886, with the first classification scheme proposed in 1962 utilizing transmission electron microscopy (TEM) findings to delineate categories: epidermolytic (EB simplex [EBS]), lucidolytic (junctional EB), and dermolytic (dystrophic EB).1 Localized EBS (EBS-loc) is an autosomal-dominant disorder caused by negative mutations in keratin-5 and keratin-14, proteins expressed in the intermediate filaments of basal keratinocytes, which result in fragility of the skin in response to minor trauma.2 The incidence of EBS-loc is approximately 10 to 30 cases per million live births, with the age of presentation typically between the first and third decades of life.3,4 Because EBS-loc is the most common and often mildest form of EB, not all patients present for medical evaluation and true prevalence may be underestimated.4 We report a case of EBS-loc.
A 26-year-old woman with no notable medical history presented to the dermatology clinic for evaluation of skin blisters that had been intermittently present since infancy. The blisters primarily occurred on the feet, but she did occasionally develop blisters on the hands, knees, and elbows and at sites of friction or trauma (eg, bra line, medial thighs) following exercise. The blisters were worsened by heat and tight-fitting shoes. Because of the painful nature of the blisters, she would lance them with a needle. On the medial thighs, she utilized nonstick and gauze bandage roll dressings to minimize friction. A review of systems was positive for hyperhidrosis. Her family history revealed multiple family members with blisters involving the feet and areas of friction or trauma for 4 generations with no known diagnosis.
Physical examination revealed multiple tense bullae and calluses scattered over the bilateral plantar and distal dorsal feet with a few healing, superficially eroded, erythematous papules and plaques on the bilateral medial thighs (Figure 1). A biopsy from an induced blister on the right dorsal second toe was performed and sent in glutaraldehyde to the Epidermolysis Bullosa Clinic at Stanford University (Redwood City, California) for electron microscopy, which revealed lysis within the basal keratinocytes through the tonofilaments with continuous and intact lamina densa and lamina lucida (Figure 2). In this clinical context with the relevant family history, the findings were consistent with the diagnosis of EBS-loc (formerly Weber-Cockayne syndrome).2


Skin manifestations of EBS-loc typically consist of friction-induced blisters, erosions, and calluses primarily on the palms and soles, often associated with hyperhidrosis and worsening of symptoms in summer months and hot temperatures.3 Milia, atrophic scarring, and dystrophic nails are uncommon.1 Extracutaneous involvement is rare with the exception of oral cavity erosions, which typically are asymptomatic and usually are only seen during infancy.1
Light microscopy does not have a notable role in diagnosis of classic forms of inherited EB unless another autoimmune blistering disorder is suspected.2,5 Both TEM and immunofluorescence mapping are used to diagnose EB.1 DNA mutational analysis is not considered a first-line diagnostic test for EB given it is a costly labor-intensive technique with limited access at present, but it may be considered in settings of prenatal diagnosis or in vitro fertilization.1 Biopsy of a freshly induced blister should be performed, as early reepithelialization of an existing blister makes it difficult to establish the level of cleavage.5 Applying firm pressure using a pencil eraser and rotating it on intact skin induces a subclinical blister. Two punch biopsies (4 mm) at the edge of the blister with one-third lesional and two-thirds perilesional skin should be obtained, with one biopsy sent for immunofluorescence mapping in Michel fixative and the other for TEM in glutaraldehyde.3,5 Transmission electron microscopy of an induced blister in EBS-loc shows cleavage within the most inferior portion of the basilar keratinocyte.2 Immunofluorescence mapping with anti–epidermal basement membrane monoclonal antibodies can distinguish between EB subtypes and assess expression of specific skin-associated proteins on both a qualitative or semiquantitative basis, providing insight on which structural protein is mutated.1,5
No specific treatments are available for EBS-loc. Mainstays of treatment include prevention of mechanical trauma and secondary infection. Hyperhidrosis of thepalms and soles may be treated with topical aluminum chloride hexahydrate or injections of botulinum toxin type A.2,6 Patients have normal life expectancy, though some cases may have complications with substantial morbidity.1 Awareness of this disease, its clinical course, and therapeutic options will allow physicians to more appropriately counsel patients on the disease process.
Localized EBS may be more common than previously thought, as not all patients seek medical care. Given its impact on patient quality of life, it is important for clinicians to recognize EBS-loc. Although no specific treatments are available, wound care counseling and explanation of the genetics of the disease should be provided to patients.
To the Editor:
Epidermolysis bullosa (EB) was first described in 1886, with the first classification scheme proposed in 1962 utilizing transmission electron microscopy (TEM) findings to delineate categories: epidermolytic (EB simplex [EBS]), lucidolytic (junctional EB), and dermolytic (dystrophic EB).1 Localized EBS (EBS-loc) is an autosomal-dominant disorder caused by negative mutations in keratin-5 and keratin-14, proteins expressed in the intermediate filaments of basal keratinocytes, which result in fragility of the skin in response to minor trauma.2 The incidence of EBS-loc is approximately 10 to 30 cases per million live births, with the age of presentation typically between the first and third decades of life.3,4 Because EBS-loc is the most common and often mildest form of EB, not all patients present for medical evaluation and true prevalence may be underestimated.4 We report a case of EBS-loc.
A 26-year-old woman with no notable medical history presented to the dermatology clinic for evaluation of skin blisters that had been intermittently present since infancy. The blisters primarily occurred on the feet, but she did occasionally develop blisters on the hands, knees, and elbows and at sites of friction or trauma (eg, bra line, medial thighs) following exercise. The blisters were worsened by heat and tight-fitting shoes. Because of the painful nature of the blisters, she would lance them with a needle. On the medial thighs, she utilized nonstick and gauze bandage roll dressings to minimize friction. A review of systems was positive for hyperhidrosis. Her family history revealed multiple family members with blisters involving the feet and areas of friction or trauma for 4 generations with no known diagnosis.
Physical examination revealed multiple tense bullae and calluses scattered over the bilateral plantar and distal dorsal feet with a few healing, superficially eroded, erythematous papules and plaques on the bilateral medial thighs (Figure 1). A biopsy from an induced blister on the right dorsal second toe was performed and sent in glutaraldehyde to the Epidermolysis Bullosa Clinic at Stanford University (Redwood City, California) for electron microscopy, which revealed lysis within the basal keratinocytes through the tonofilaments with continuous and intact lamina densa and lamina lucida (Figure 2). In this clinical context with the relevant family history, the findings were consistent with the diagnosis of EBS-loc (formerly Weber-Cockayne syndrome).2


Skin manifestations of EBS-loc typically consist of friction-induced blisters, erosions, and calluses primarily on the palms and soles, often associated with hyperhidrosis and worsening of symptoms in summer months and hot temperatures.3 Milia, atrophic scarring, and dystrophic nails are uncommon.1 Extracutaneous involvement is rare with the exception of oral cavity erosions, which typically are asymptomatic and usually are only seen during infancy.1
Light microscopy does not have a notable role in diagnosis of classic forms of inherited EB unless another autoimmune blistering disorder is suspected.2,5 Both TEM and immunofluorescence mapping are used to diagnose EB.1 DNA mutational analysis is not considered a first-line diagnostic test for EB given it is a costly labor-intensive technique with limited access at present, but it may be considered in settings of prenatal diagnosis or in vitro fertilization.1 Biopsy of a freshly induced blister should be performed, as early reepithelialization of an existing blister makes it difficult to establish the level of cleavage.5 Applying firm pressure using a pencil eraser and rotating it on intact skin induces a subclinical blister. Two punch biopsies (4 mm) at the edge of the blister with one-third lesional and two-thirds perilesional skin should be obtained, with one biopsy sent for immunofluorescence mapping in Michel fixative and the other for TEM in glutaraldehyde.3,5 Transmission electron microscopy of an induced blister in EBS-loc shows cleavage within the most inferior portion of the basilar keratinocyte.2 Immunofluorescence mapping with anti–epidermal basement membrane monoclonal antibodies can distinguish between EB subtypes and assess expression of specific skin-associated proteins on both a qualitative or semiquantitative basis, providing insight on which structural protein is mutated.1,5
No specific treatments are available for EBS-loc. Mainstays of treatment include prevention of mechanical trauma and secondary infection. Hyperhidrosis of thepalms and soles may be treated with topical aluminum chloride hexahydrate or injections of botulinum toxin type A.2,6 Patients have normal life expectancy, though some cases may have complications with substantial morbidity.1 Awareness of this disease, its clinical course, and therapeutic options will allow physicians to more appropriately counsel patients on the disease process.
Localized EBS may be more common than previously thought, as not all patients seek medical care. Given its impact on patient quality of life, it is important for clinicians to recognize EBS-loc. Although no specific treatments are available, wound care counseling and explanation of the genetics of the disease should be provided to patients.
- Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931-950.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Limited; 2012.
- Eichenfield LF, Frieden IJ, Mathes EF, et al, eds. Neonatal and Infant Dermatology. 3rd ed. New York, NY: Elsevier Health Sciences; 2015.
- Spitz JL. Genodermatoses: A Clinical Guide to Genetic Skin Disorders. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Epidermolysis bullosa. Stanford Medicine website. http://med.stanford.edu/dermatopathology/dermpath-services/epiderm.html. Accessed April 3, 2019.
- Abitbol RJ, Zhou LH. Treatment of epidermolysis bullosa simplex, Weber-Cockayne type, with botulinum toxin type A. Arch Dermatol. 2009;145:13-15.
- Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931-950.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Limited; 2012.
- Eichenfield LF, Frieden IJ, Mathes EF, et al, eds. Neonatal and Infant Dermatology. 3rd ed. New York, NY: Elsevier Health Sciences; 2015.
- Spitz JL. Genodermatoses: A Clinical Guide to Genetic Skin Disorders. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Epidermolysis bullosa. Stanford Medicine website. http://med.stanford.edu/dermatopathology/dermpath-services/epiderm.html. Accessed April 3, 2019.
- Abitbol RJ, Zhou LH. Treatment of epidermolysis bullosa simplex, Weber-Cockayne type, with botulinum toxin type A. Arch Dermatol. 2009;145:13-15.
Practice Points
- Localized epidermolysis bullosa simplex (formerly Weber-Cockayne syndrome) presents with flaccid bullae and erosions predominantly on the hands and feet, most commonly related to mechanical friction and heat.
- It is inherited in an autosomal-dominant fashion with defects in keratin-5 and keratin-14.
- Biopsy of a freshly induced blister should be examined by transmission electron microscopy or immunofluorescence mapping.
- Treatment is focused on wound management and infection control of the blisters.
Adjunctive devices for transcatheter valve replacement improve outcomes
WASHINGTON – One transcatheter device designed to prevent left ventricular outflow tract (LVOT) obstruction relevant to transcatheter mitral valve replacement (TMVR) and another designed to prevent coronary obstruction relevant to transcatheter aortic valve replacement (TAVR) performed well in feasibility studies, according to data presented in two separate late breaking clinical trial sessions at the CRT 2019, sponsored by MedStar Heart & Vascular Institute.
LVOT obstruction prevention
“The 30-day survival in subjects with an increased risk of LVOT obstruction was significantly better than that previously reported in registries,” said Jaffar M. Khan, BM BCh, of the National Heart, Lung, and Blood Institute, who addressing results with the LAMPOON device prior to TMVR,.
LAMPOON is an acronym for intentional Laceration of the Anterior Mitral valve leaflet to Prevent LVOT ObstructioN. Introduced percutaneously and guided to the valve with wires, it was designed to tear existing mitral valve leaflets to prevent them from causing life-threatening LVOT obstruction. It is used immediately prior to TMVR in patients at risk for this complication.
In a feasibility study, delivery, deployment, and retrieval of the device was achieved in all 30 patients. On the basis of the primary endpoint of LVOT gradients of less than 50 mm Hg and no emergency surgery, the procedural success was 73%. The 30-day survival was 93%.
Citing data from registries, Dr. Khan said that the expected survival in TMVR patients with LVOT obstruction caused by a native mitral valve leaflet has been less than 40%. With few existing options to prevent this complication, none of which are reliable, LAMPOON is poised to permit patients who are poor candidates or are contraindicated for TMVR to undergo this treatment, according to Dr. Khan.
“LAMPOON is feasible in all anatomies and calcium patterns,” said Dr. Khan, who noted that gradients of less than 30 mm Hg was achieved in 29 of the 30 patients. Although Dr. Khan acknowledged that this study was small and uncontrolled, and he further cautioned that current strategies for predicting mitral valve leaflet LVOT obstruction are “imprecise,” he believes larger studies of LAMPOON are warranted based on these results.
Coronary artery obstruction prevention
Dr. Khan also presented data on the BASILICA device from a second feasibility study. The device is employed immediately prior to TAVR in order to prevent large aortic valve leaflets, whether native or from an existing bioprosthetic valve, from producing coronary obstruction. BASILICA is an acronym for Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction.
This device is also introduced percutaneously and uses radiofrequency ablation to split leaflets that are considered to pose a risk for coronary obstruction. Even though Dr. Khan acknowledged that there is also a lack of precision for predicting which TAVR candidates require an intervention to prevent coronary obstruction, he cited mortality rates exceeding 40% when this complication occurs.
In the feasibility study, 30 patients, of whom 80% were female, were enrolled. In half of the cases, the target for BASILICA was a native valve. The remainder was treated for risk of coronary obstruction posed by a bioprosthetic valve. Multiple comorbidities, including a high proportion with prior stroke, made those selected for enrollment poor candidates for surgery.
The BASILICA intervention was successful in 28 of the 30 participants and in 35 of the 37 leaflets treated. At 30 days, there was one death and one disabling stroke. The overall success rate of the procedure was 93%, according to Dr. Khan.
“One hundred percent of patients were discharged from the cath lab without coronary obstruction despite the high baseline risk,” Dr. Khan said. Again, larger studies are needed to validate the safety and efficacy of this approach, but Dr. Khan believes the outcomes in this study warrant expanded clinical studies.
WASHINGTON – One transcatheter device designed to prevent left ventricular outflow tract (LVOT) obstruction relevant to transcatheter mitral valve replacement (TMVR) and another designed to prevent coronary obstruction relevant to transcatheter aortic valve replacement (TAVR) performed well in feasibility studies, according to data presented in two separate late breaking clinical trial sessions at the CRT 2019, sponsored by MedStar Heart & Vascular Institute.
LVOT obstruction prevention
“The 30-day survival in subjects with an increased risk of LVOT obstruction was significantly better than that previously reported in registries,” said Jaffar M. Khan, BM BCh, of the National Heart, Lung, and Blood Institute, who addressing results with the LAMPOON device prior to TMVR,.
LAMPOON is an acronym for intentional Laceration of the Anterior Mitral valve leaflet to Prevent LVOT ObstructioN. Introduced percutaneously and guided to the valve with wires, it was designed to tear existing mitral valve leaflets to prevent them from causing life-threatening LVOT obstruction. It is used immediately prior to TMVR in patients at risk for this complication.
In a feasibility study, delivery, deployment, and retrieval of the device was achieved in all 30 patients. On the basis of the primary endpoint of LVOT gradients of less than 50 mm Hg and no emergency surgery, the procedural success was 73%. The 30-day survival was 93%.
Citing data from registries, Dr. Khan said that the expected survival in TMVR patients with LVOT obstruction caused by a native mitral valve leaflet has been less than 40%. With few existing options to prevent this complication, none of which are reliable, LAMPOON is poised to permit patients who are poor candidates or are contraindicated for TMVR to undergo this treatment, according to Dr. Khan.
“LAMPOON is feasible in all anatomies and calcium patterns,” said Dr. Khan, who noted that gradients of less than 30 mm Hg was achieved in 29 of the 30 patients. Although Dr. Khan acknowledged that this study was small and uncontrolled, and he further cautioned that current strategies for predicting mitral valve leaflet LVOT obstruction are “imprecise,” he believes larger studies of LAMPOON are warranted based on these results.
Coronary artery obstruction prevention
Dr. Khan also presented data on the BASILICA device from a second feasibility study. The device is employed immediately prior to TAVR in order to prevent large aortic valve leaflets, whether native or from an existing bioprosthetic valve, from producing coronary obstruction. BASILICA is an acronym for Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction.
This device is also introduced percutaneously and uses radiofrequency ablation to split leaflets that are considered to pose a risk for coronary obstruction. Even though Dr. Khan acknowledged that there is also a lack of precision for predicting which TAVR candidates require an intervention to prevent coronary obstruction, he cited mortality rates exceeding 40% when this complication occurs.
In the feasibility study, 30 patients, of whom 80% were female, were enrolled. In half of the cases, the target for BASILICA was a native valve. The remainder was treated for risk of coronary obstruction posed by a bioprosthetic valve. Multiple comorbidities, including a high proportion with prior stroke, made those selected for enrollment poor candidates for surgery.
The BASILICA intervention was successful in 28 of the 30 participants and in 35 of the 37 leaflets treated. At 30 days, there was one death and one disabling stroke. The overall success rate of the procedure was 93%, according to Dr. Khan.
“One hundred percent of patients were discharged from the cath lab without coronary obstruction despite the high baseline risk,” Dr. Khan said. Again, larger studies are needed to validate the safety and efficacy of this approach, but Dr. Khan believes the outcomes in this study warrant expanded clinical studies.
WASHINGTON – One transcatheter device designed to prevent left ventricular outflow tract (LVOT) obstruction relevant to transcatheter mitral valve replacement (TMVR) and another designed to prevent coronary obstruction relevant to transcatheter aortic valve replacement (TAVR) performed well in feasibility studies, according to data presented in two separate late breaking clinical trial sessions at the CRT 2019, sponsored by MedStar Heart & Vascular Institute.
LVOT obstruction prevention
“The 30-day survival in subjects with an increased risk of LVOT obstruction was significantly better than that previously reported in registries,” said Jaffar M. Khan, BM BCh, of the National Heart, Lung, and Blood Institute, who addressing results with the LAMPOON device prior to TMVR,.
LAMPOON is an acronym for intentional Laceration of the Anterior Mitral valve leaflet to Prevent LVOT ObstructioN. Introduced percutaneously and guided to the valve with wires, it was designed to tear existing mitral valve leaflets to prevent them from causing life-threatening LVOT obstruction. It is used immediately prior to TMVR in patients at risk for this complication.
In a feasibility study, delivery, deployment, and retrieval of the device was achieved in all 30 patients. On the basis of the primary endpoint of LVOT gradients of less than 50 mm Hg and no emergency surgery, the procedural success was 73%. The 30-day survival was 93%.
Citing data from registries, Dr. Khan said that the expected survival in TMVR patients with LVOT obstruction caused by a native mitral valve leaflet has been less than 40%. With few existing options to prevent this complication, none of which are reliable, LAMPOON is poised to permit patients who are poor candidates or are contraindicated for TMVR to undergo this treatment, according to Dr. Khan.
“LAMPOON is feasible in all anatomies and calcium patterns,” said Dr. Khan, who noted that gradients of less than 30 mm Hg was achieved in 29 of the 30 patients. Although Dr. Khan acknowledged that this study was small and uncontrolled, and he further cautioned that current strategies for predicting mitral valve leaflet LVOT obstruction are “imprecise,” he believes larger studies of LAMPOON are warranted based on these results.
Coronary artery obstruction prevention
Dr. Khan also presented data on the BASILICA device from a second feasibility study. The device is employed immediately prior to TAVR in order to prevent large aortic valve leaflets, whether native or from an existing bioprosthetic valve, from producing coronary obstruction. BASILICA is an acronym for Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction.
This device is also introduced percutaneously and uses radiofrequency ablation to split leaflets that are considered to pose a risk for coronary obstruction. Even though Dr. Khan acknowledged that there is also a lack of precision for predicting which TAVR candidates require an intervention to prevent coronary obstruction, he cited mortality rates exceeding 40% when this complication occurs.
In the feasibility study, 30 patients, of whom 80% were female, were enrolled. In half of the cases, the target for BASILICA was a native valve. The remainder was treated for risk of coronary obstruction posed by a bioprosthetic valve. Multiple comorbidities, including a high proportion with prior stroke, made those selected for enrollment poor candidates for surgery.
The BASILICA intervention was successful in 28 of the 30 participants and in 35 of the 37 leaflets treated. At 30 days, there was one death and one disabling stroke. The overall success rate of the procedure was 93%, according to Dr. Khan.
“One hundred percent of patients were discharged from the cath lab without coronary obstruction despite the high baseline risk,” Dr. Khan said. Again, larger studies are needed to validate the safety and efficacy of this approach, but Dr. Khan believes the outcomes in this study warrant expanded clinical studies.
REPORTING FROM CRT 2019
Large mass on shoulder
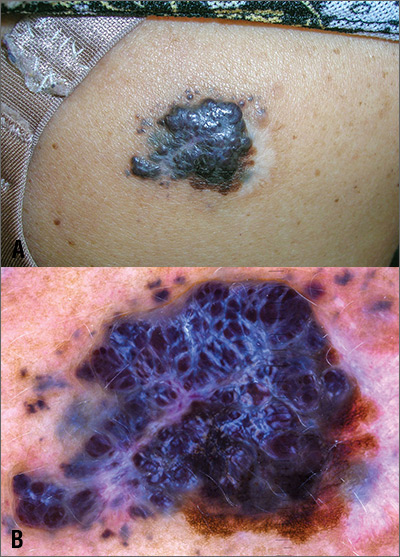
The FP was extremely concerned that this was a large melanoma due the lesion’s chaotic appearance, with multiple colors and an irregular border. Going through the ABCDE criteria, he noted that the lesion was Asymmetric, the Border was irregular, the Colors were varied, the Diameter was > 6 mm, and it was Enlarging by history.
With his dermatoscope attached to his smart phone, the FP looked at the lesion and took a photograph (Figure B). The image revealed a pigment network of the original nevus at 5:00 to 6:00 o’clock and extensions of the tumor due to in-transit metastases. Satellites were visible, especially in the top right and left corners. The FP noted the shiny white lines caused by collagen deposition found in growing tumors. (See “Dermoscopy in family medicine: A primer.”)
The FP knew that the mass needed to be biopsied, but because of its size, the best he could do would be to perform a partial biopsy. So on the day of presentation, the FP performed a 6-mm punch biopsy in the most raised area of the lesion to try and get sufficient depth and breadth for diagnosis and prognosis. (See the Watch & Learn video on “Punch biopsy.”)
The pathology report indicated that the lesion was a nodular melanoma with a Breslow depth of 5.5 mm. This melanoma arose in a nevus, which occurs in about 30% of melanomas. Most melanomas arise de novo.
The FP referred the patient to a surgical oncologist for an excision with 2 cm margins and a sentinel lymph node biopsy. The sentinel node was in the right axilla and was remarkably negative despite the local in-transit metastases/satellites. One year after the original diagnosis was made, there was no evidence of metastatic disease and no new melanomas. The patient follows up with the dermatologist for skin and lymph node surveillance every 3 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP was extremely concerned that this was a large melanoma due the lesion’s chaotic appearance, with multiple colors and an irregular border. Going through the ABCDE criteria, he noted that the lesion was Asymmetric, the Border was irregular, the Colors were varied, the Diameter was > 6 mm, and it was Enlarging by history.
With his dermatoscope attached to his smart phone, the FP looked at the lesion and took a photograph (Figure B). The image revealed a pigment network of the original nevus at 5:00 to 6:00 o’clock and extensions of the tumor due to in-transit metastases. Satellites were visible, especially in the top right and left corners. The FP noted the shiny white lines caused by collagen deposition found in growing tumors. (See “Dermoscopy in family medicine: A primer.”)
The FP knew that the mass needed to be biopsied, but because of its size, the best he could do would be to perform a partial biopsy. So on the day of presentation, the FP performed a 6-mm punch biopsy in the most raised area of the lesion to try and get sufficient depth and breadth for diagnosis and prognosis. (See the Watch & Learn video on “Punch biopsy.”)
The pathology report indicated that the lesion was a nodular melanoma with a Breslow depth of 5.5 mm. This melanoma arose in a nevus, which occurs in about 30% of melanomas. Most melanomas arise de novo.
The FP referred the patient to a surgical oncologist for an excision with 2 cm margins and a sentinel lymph node biopsy. The sentinel node was in the right axilla and was remarkably negative despite the local in-transit metastases/satellites. One year after the original diagnosis was made, there was no evidence of metastatic disease and no new melanomas. The patient follows up with the dermatologist for skin and lymph node surveillance every 3 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP was extremely concerned that this was a large melanoma due the lesion’s chaotic appearance, with multiple colors and an irregular border. Going through the ABCDE criteria, he noted that the lesion was Asymmetric, the Border was irregular, the Colors were varied, the Diameter was > 6 mm, and it was Enlarging by history.
With his dermatoscope attached to his smart phone, the FP looked at the lesion and took a photograph (Figure B). The image revealed a pigment network of the original nevus at 5:00 to 6:00 o’clock and extensions of the tumor due to in-transit metastases. Satellites were visible, especially in the top right and left corners. The FP noted the shiny white lines caused by collagen deposition found in growing tumors. (See “Dermoscopy in family medicine: A primer.”)
The FP knew that the mass needed to be biopsied, but because of its size, the best he could do would be to perform a partial biopsy. So on the day of presentation, the FP performed a 6-mm punch biopsy in the most raised area of the lesion to try and get sufficient depth and breadth for diagnosis and prognosis. (See the Watch & Learn video on “Punch biopsy.”)
The pathology report indicated that the lesion was a nodular melanoma with a Breslow depth of 5.5 mm. This melanoma arose in a nevus, which occurs in about 30% of melanomas. Most melanomas arise de novo.
The FP referred the patient to a surgical oncologist for an excision with 2 cm margins and a sentinel lymph node biopsy. The sentinel node was in the right axilla and was remarkably negative despite the local in-transit metastases/satellites. One year after the original diagnosis was made, there was no evidence of metastatic disease and no new melanomas. The patient follows up with the dermatologist for skin and lymph node surveillance every 3 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
What are the risks of long-term PPI use for GERD symptoms in patients > 65 years?
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE-BASED ANSWER:
The use of proton pump inhibitors (PPIs) to control gastroesophageal reflux disease (GERD) is significantly associated with an increased risk of cardiovascular events such as acute myocardial infarction and myocardial ischemia, especially with treatment longer than 8 weeks (strength of recommendation [SOR]: A, systematic review of randomized, controlled trials [RCTs]). This summary is based on data extrapolated from studies on all adults because there is limited evidence that specifically addresses patients older than 65 years.
Adults taking PPIs also appear to be at increased risk of Clostridium difficile infection, community-acquired pneumonia (CAP; with use for < 30 days), and fracture (SOR: B, systematic reviews of heterogeneous prospective and retrospective observational studies).
Daily headaches • associated nausea • obesity • Dx?
THE CASE
A 22-year-old woman presented to our office complaining of headaches that started 6 weeks earlier. Initially the headache was throbbing, nonpositional, infrequent, and intermittent, lasting 15 to 45 minutes, often starting in the neck and migrating towards the right frontotemporal region. During the week prior to presentation, the headaches became daily and constant, with brief periods of relief after the patient took ibuprofen 400 mg 4 times a day as needed. The patient reported associated nausea, a sensation of pressure changes in the ears, and intermittent dimming of vision in the right eye (sometimes independent of headache). The patient denied photophobia and phonophobia. Her only medication was an oral contraceptive pill (OCP). She had no prior history of headaches.
Physical examination showed a blood pressure of 148/66 mm Hg, body mass index of 44.38, muscle tenderness in the neck and upper back, and no focal neurological findings. Funduscopic examination was unsuccessful. A working diagnosis of atypical migraine was made, but because of unilateral visual disturbance the patient was referred to Ophthalmology for further evaluation. The following day, ophthalmological consultation found bilateral papilledema and the patient was admitted to our hospitalist service via the Emergency Department. She subsequently was referred to inpatient Neurology.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of the brain and orbits with and without contrast was unremarkable. Magnetic resonance venography (MRV) with contrast of the brain showed possible stenosis at the junction of the transverse and sigmoid sinuses but no mass lesion nor venous sinus thrombosis. Lumbar puncture (LP) revealed an opening pressure of 650 mm H20 (reference range, 60–250 mm H2O).1 A diagnosis of idiopathic intracranial hypertension (IIH) was made.
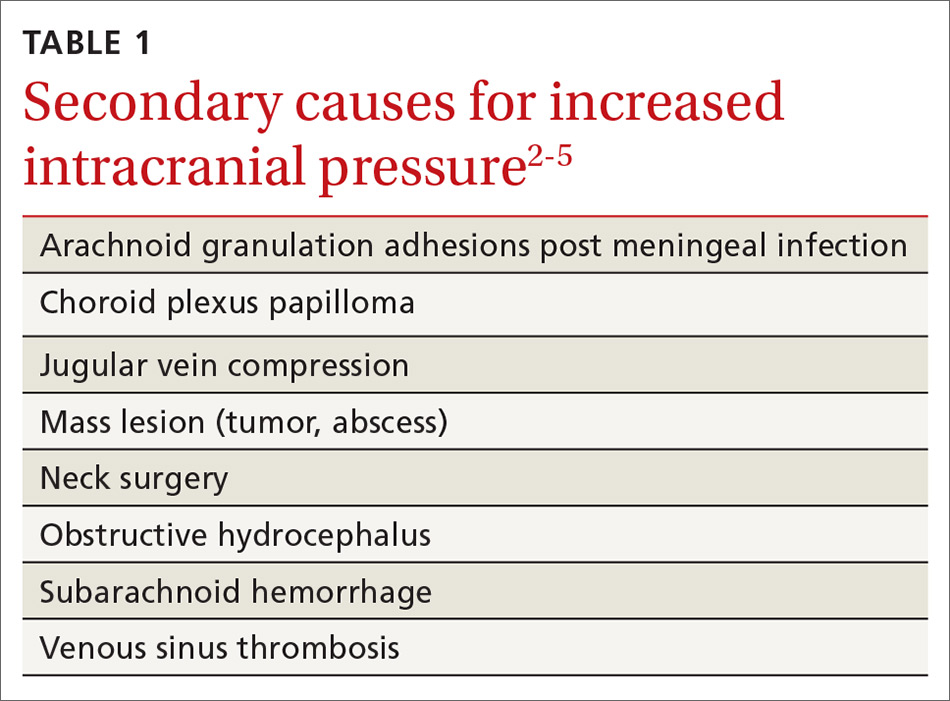
DISCUSSION
IIH, previously known as pseudotumor cerebri and benign intracranial hypertension, is defined by signs and symptoms of elevated intracranial pressure (ICP) without obvious cause on neuroimaging (TABLE 12-5). It is well documented that IIH is consequential and can result in vision loss and intractable chronic headaches.5,6 Older terms such as pseudotumor cerebri and benign intracranial hypertension are therefore no longer recommended because they are considered misleading and not reflective of the severity of potential injury caused by the condition3,4,6 IIH is considered a diagnosis of exclusion requiring certain criteria to be met (TABLE 22). Although the etiology of IIH is unclear, associations have been made between IIH and various medications and conditions2-5,7 (TABLE 33,5).
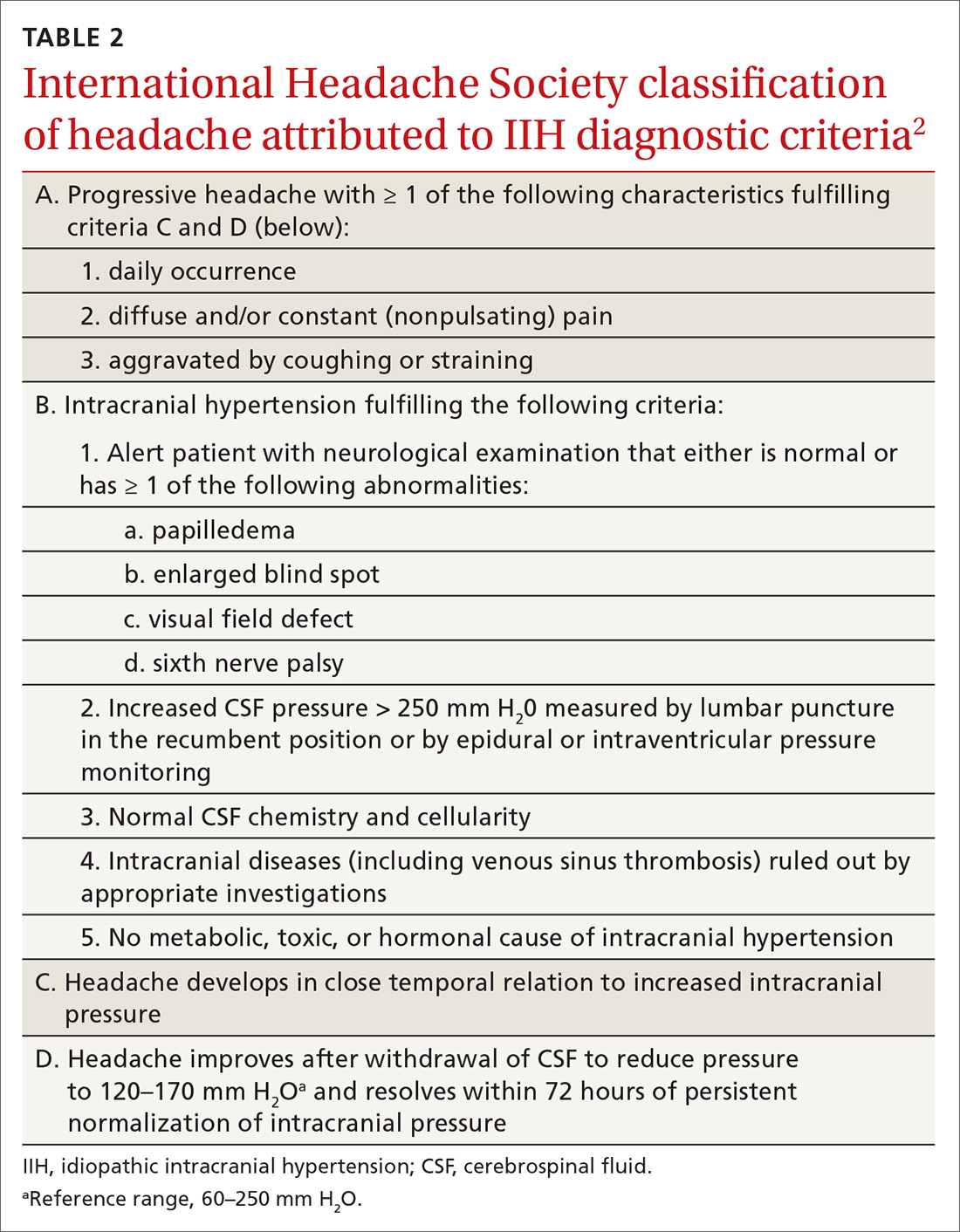
Classically, IIH affects women who are obese and of childbearing age, but studies have shown that this condition also can affect men and children—albeit less frequently.3,5-7 The incidence of IIH in the general population is between 0.03 to 2.36/100,000 people per year, but in women, the incidence is 0.65 to 4.65/100,000 per year.6 Furthermore, females who are obese have an incidence of 2.7 to 19.3/100,000 per year.6
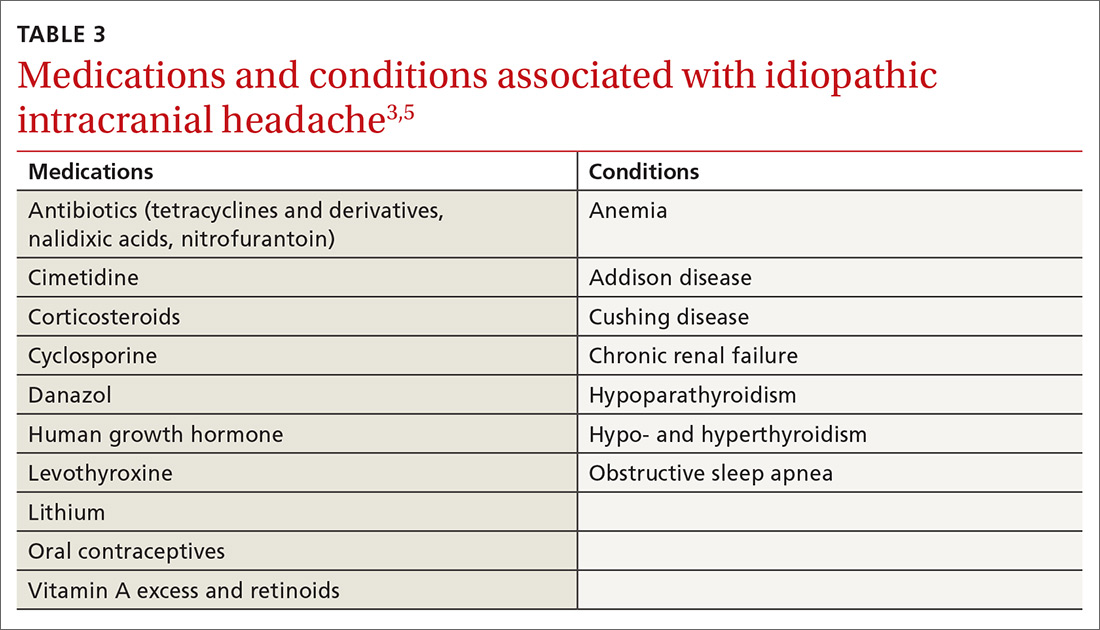
Headache is the most common symptom of IIH. Unfortunately, the differential diagnosis of headache is vast; thus, a careful history is needed to narrow the field3,5-7 (TABLE 42). Associated symptoms of transient visual changes, pulsatile tinnitus, neck and back pain, nausea, vomiting, photo/phonophobia, and findings of abducens nerve palsy or papilledema—while nonspecific— should raise suspicion for elevated ICP and IIH, especially in women who are obese.2-8 Once IIH is suspected, an urgent diagnosis and treatment is necessary to prevent permanent vision loss.3,4,6
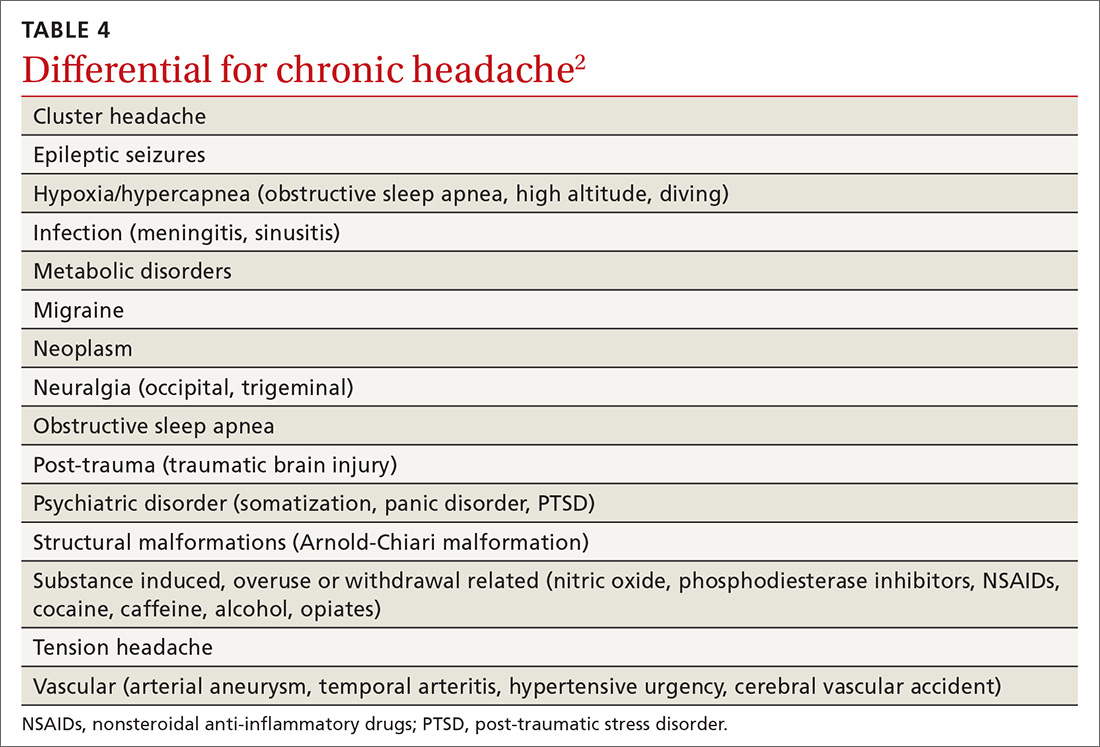
Headache with findings of papilledema warrants neuroimaging, preferably with MRI, to rule out intracranial mass and hydrocephalus.1,2,5 MRV also is recommended to assess for intracranial venous thrombosis, an alternate cause for papilledema and increased ICP.1,2,4,5
Continue to: Recently, a classification of IIH...
Recently, a classification of IIH without papilledema has been acknowledged by the International Headache Society.2,8 Specific MRI findings have been suggested to help make this diagnosis5,9 (TABLE 55).
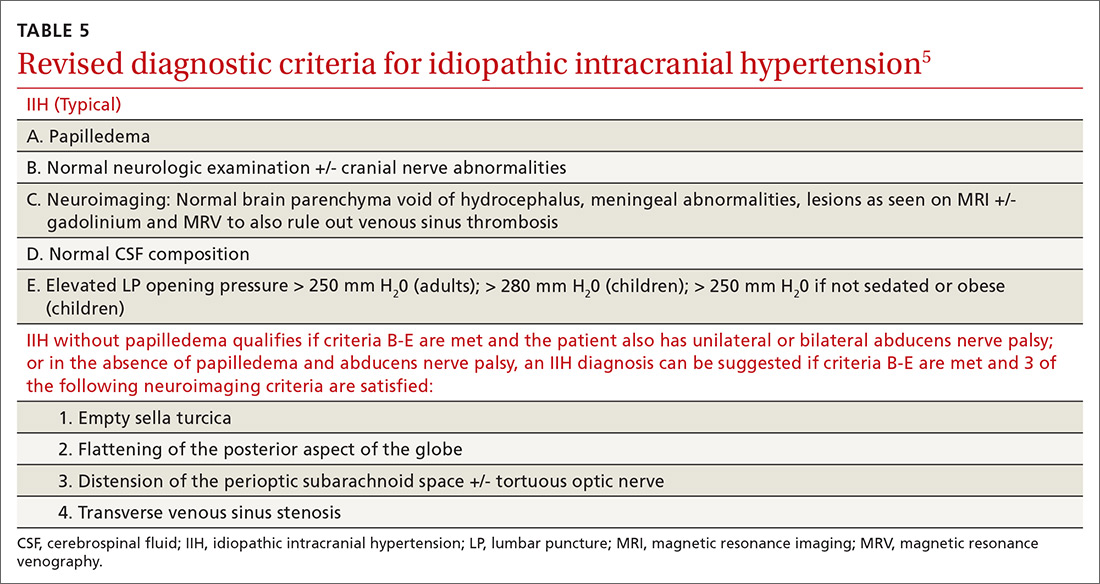
TREATMENT FOR IIH CAN BE MEDICAL OR SURGICAL
Medications associated with IIH should be discontinued.7 The first-line medication for IIH is acetazolamide, a carbonic anhydrase inhibitor that works in the choroid plexus to decrease cerebrospinal fluid (CSF) production and thus, lower ICP.3,6 An adult dose of 1 to 2 g/day3,4,6 is tolerated well, but can be increased to 4 g/day,10 if necessary. Weight loss via diet and exercise or bariatric surgery has been shown to be effective in patients who are obese and have been given a diagnosis of IIH.3,4
Topiramate also has been suggested as a treatment option, based on its usefulness in weight loss and because of its action as a weak carbonic anhydrase inhibitor.3,6 Also, LP has therapeutic merit—although relief is only short-term.3,6 Patients who fail medical therapy and have intractable headache or progressive visual loss appear to benefit from optic nerve sheath fenestration.3,7,8
Our patient experienced notable improvement in her headache after LP. Her OCP was discontinued, a diuretic regimen started, and weight loss counseling was provided. Prior to discharge, the patient was seen by a neuro-ophthalmologist for perimetry, a visual field test that assesses for acute vision loss and establishes a baseline for follow-up monitoring of vision.7
THE TAKEAWAY
Headache is a common condition that may be challenging to correctly diagnose. A thorough history and neurological examination, including fundoscopy, are essential in the evaluation of headache and suspected IIH. In the primary care setting, limited time, lack of mydriatic agents, suboptimal lighting, and practitioner inexperience may pose challenges for funduscopic examination. Ophthalmoscopes incorporating new technology to expand and magnify the examiner’s field of view may facilitate this exam.11 A global rise in the prevalence of obesity underscores a need for primary care providers to be compulsive about their clinical evaluation when symptoms suspicious of IIH are present. Lastly, if IIH cannot be ruled out confidently, recommend a prompt evaluation by an ophthalmologist.
CORRESPONDENCE
Aarti Paltoo, MD, MSc, CCFP, Peel Village Medical Center, 28 Rambler Drive, Brampton, Ontario L6W 1E2 Canada; [email protected]
1. Lee SC, Lueck CJ. Cerebrospinal fluid pressure in adults. J Neuroophthalmol. 2014;34:278-283.
2. International Headache Society. Idiopathic intracranial hypertension. The International Classification of Headache Disorders. 2nd ed. Oxford, UK: Blackwell Publishing; 2003:1-232.
3. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83:488-494.
4. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14:380-390.
5. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159-1165.
6. Julayanont P, Karukote A, Ruthirago D, et al. Idiopathic intracranial hypertension: ongoing clinical challenges and future prospects. J Pain Res. 2016;9:87-99.
7. Friedman DI, Digre KB. Headache medicine meets neuro-ophthalmology: exam techniques and challenging cases. Headache. 2013;53:703-716.
8. Digre KB, Nakamoto BK, Warner JE, et al. A comparison of idiopathic intracranaial hypertension with and without papilledema. Headache. 2009;49:185-193.
9. Digre KB. Imaging characteristics of IIH: are they reliable? Cephalagia. 2013;33:1067-1069.
10. Horton J. Acetazolamide for pseudotumor cerebri--evidence from the NORDIC trial. JAMA. 2014;311:1618-1619.
11. Petrushkin H, Barsam A, Mavrakakis M, et al. Optic disc assessment in the emergency department: a comparative study between the PanOptic and direct ophthalmoscopes. Emerg Med J. 2012;29:1007-1008.
THE CASE
A 22-year-old woman presented to our office complaining of headaches that started 6 weeks earlier. Initially the headache was throbbing, nonpositional, infrequent, and intermittent, lasting 15 to 45 minutes, often starting in the neck and migrating towards the right frontotemporal region. During the week prior to presentation, the headaches became daily and constant, with brief periods of relief after the patient took ibuprofen 400 mg 4 times a day as needed. The patient reported associated nausea, a sensation of pressure changes in the ears, and intermittent dimming of vision in the right eye (sometimes independent of headache). The patient denied photophobia and phonophobia. Her only medication was an oral contraceptive pill (OCP). She had no prior history of headaches.
Physical examination showed a blood pressure of 148/66 mm Hg, body mass index of 44.38, muscle tenderness in the neck and upper back, and no focal neurological findings. Funduscopic examination was unsuccessful. A working diagnosis of atypical migraine was made, but because of unilateral visual disturbance the patient was referred to Ophthalmology for further evaluation. The following day, ophthalmological consultation found bilateral papilledema and the patient was admitted to our hospitalist service via the Emergency Department. She subsequently was referred to inpatient Neurology.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of the brain and orbits with and without contrast was unremarkable. Magnetic resonance venography (MRV) with contrast of the brain showed possible stenosis at the junction of the transverse and sigmoid sinuses but no mass lesion nor venous sinus thrombosis. Lumbar puncture (LP) revealed an opening pressure of 650 mm H20 (reference range, 60–250 mm H2O).1 A diagnosis of idiopathic intracranial hypertension (IIH) was made.

DISCUSSION
IIH, previously known as pseudotumor cerebri and benign intracranial hypertension, is defined by signs and symptoms of elevated intracranial pressure (ICP) without obvious cause on neuroimaging (TABLE 12-5). It is well documented that IIH is consequential and can result in vision loss and intractable chronic headaches.5,6 Older terms such as pseudotumor cerebri and benign intracranial hypertension are therefore no longer recommended because they are considered misleading and not reflective of the severity of potential injury caused by the condition3,4,6 IIH is considered a diagnosis of exclusion requiring certain criteria to be met (TABLE 22). Although the etiology of IIH is unclear, associations have been made between IIH and various medications and conditions2-5,7 (TABLE 33,5).

Classically, IIH affects women who are obese and of childbearing age, but studies have shown that this condition also can affect men and children—albeit less frequently.3,5-7 The incidence of IIH in the general population is between 0.03 to 2.36/100,000 people per year, but in women, the incidence is 0.65 to 4.65/100,000 per year.6 Furthermore, females who are obese have an incidence of 2.7 to 19.3/100,000 per year.6

Headache is the most common symptom of IIH. Unfortunately, the differential diagnosis of headache is vast; thus, a careful history is needed to narrow the field3,5-7 (TABLE 42). Associated symptoms of transient visual changes, pulsatile tinnitus, neck and back pain, nausea, vomiting, photo/phonophobia, and findings of abducens nerve palsy or papilledema—while nonspecific— should raise suspicion for elevated ICP and IIH, especially in women who are obese.2-8 Once IIH is suspected, an urgent diagnosis and treatment is necessary to prevent permanent vision loss.3,4,6

Headache with findings of papilledema warrants neuroimaging, preferably with MRI, to rule out intracranial mass and hydrocephalus.1,2,5 MRV also is recommended to assess for intracranial venous thrombosis, an alternate cause for papilledema and increased ICP.1,2,4,5
Continue to: Recently, a classification of IIH...
Recently, a classification of IIH without papilledema has been acknowledged by the International Headache Society.2,8 Specific MRI findings have been suggested to help make this diagnosis5,9 (TABLE 55).

TREATMENT FOR IIH CAN BE MEDICAL OR SURGICAL
Medications associated with IIH should be discontinued.7 The first-line medication for IIH is acetazolamide, a carbonic anhydrase inhibitor that works in the choroid plexus to decrease cerebrospinal fluid (CSF) production and thus, lower ICP.3,6 An adult dose of 1 to 2 g/day3,4,6 is tolerated well, but can be increased to 4 g/day,10 if necessary. Weight loss via diet and exercise or bariatric surgery has been shown to be effective in patients who are obese and have been given a diagnosis of IIH.3,4
Topiramate also has been suggested as a treatment option, based on its usefulness in weight loss and because of its action as a weak carbonic anhydrase inhibitor.3,6 Also, LP has therapeutic merit—although relief is only short-term.3,6 Patients who fail medical therapy and have intractable headache or progressive visual loss appear to benefit from optic nerve sheath fenestration.3,7,8
Our patient experienced notable improvement in her headache after LP. Her OCP was discontinued, a diuretic regimen started, and weight loss counseling was provided. Prior to discharge, the patient was seen by a neuro-ophthalmologist for perimetry, a visual field test that assesses for acute vision loss and establishes a baseline for follow-up monitoring of vision.7
THE TAKEAWAY
Headache is a common condition that may be challenging to correctly diagnose. A thorough history and neurological examination, including fundoscopy, are essential in the evaluation of headache and suspected IIH. In the primary care setting, limited time, lack of mydriatic agents, suboptimal lighting, and practitioner inexperience may pose challenges for funduscopic examination. Ophthalmoscopes incorporating new technology to expand and magnify the examiner’s field of view may facilitate this exam.11 A global rise in the prevalence of obesity underscores a need for primary care providers to be compulsive about their clinical evaluation when symptoms suspicious of IIH are present. Lastly, if IIH cannot be ruled out confidently, recommend a prompt evaluation by an ophthalmologist.
CORRESPONDENCE
Aarti Paltoo, MD, MSc, CCFP, Peel Village Medical Center, 28 Rambler Drive, Brampton, Ontario L6W 1E2 Canada; [email protected]
THE CASE
A 22-year-old woman presented to our office complaining of headaches that started 6 weeks earlier. Initially the headache was throbbing, nonpositional, infrequent, and intermittent, lasting 15 to 45 minutes, often starting in the neck and migrating towards the right frontotemporal region. During the week prior to presentation, the headaches became daily and constant, with brief periods of relief after the patient took ibuprofen 400 mg 4 times a day as needed. The patient reported associated nausea, a sensation of pressure changes in the ears, and intermittent dimming of vision in the right eye (sometimes independent of headache). The patient denied photophobia and phonophobia. Her only medication was an oral contraceptive pill (OCP). She had no prior history of headaches.
Physical examination showed a blood pressure of 148/66 mm Hg, body mass index of 44.38, muscle tenderness in the neck and upper back, and no focal neurological findings. Funduscopic examination was unsuccessful. A working diagnosis of atypical migraine was made, but because of unilateral visual disturbance the patient was referred to Ophthalmology for further evaluation. The following day, ophthalmological consultation found bilateral papilledema and the patient was admitted to our hospitalist service via the Emergency Department. She subsequently was referred to inpatient Neurology.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of the brain and orbits with and without contrast was unremarkable. Magnetic resonance venography (MRV) with contrast of the brain showed possible stenosis at the junction of the transverse and sigmoid sinuses but no mass lesion nor venous sinus thrombosis. Lumbar puncture (LP) revealed an opening pressure of 650 mm H20 (reference range, 60–250 mm H2O).1 A diagnosis of idiopathic intracranial hypertension (IIH) was made.

DISCUSSION
IIH, previously known as pseudotumor cerebri and benign intracranial hypertension, is defined by signs and symptoms of elevated intracranial pressure (ICP) without obvious cause on neuroimaging (TABLE 12-5). It is well documented that IIH is consequential and can result in vision loss and intractable chronic headaches.5,6 Older terms such as pseudotumor cerebri and benign intracranial hypertension are therefore no longer recommended because they are considered misleading and not reflective of the severity of potential injury caused by the condition3,4,6 IIH is considered a diagnosis of exclusion requiring certain criteria to be met (TABLE 22). Although the etiology of IIH is unclear, associations have been made between IIH and various medications and conditions2-5,7 (TABLE 33,5).

Classically, IIH affects women who are obese and of childbearing age, but studies have shown that this condition also can affect men and children—albeit less frequently.3,5-7 The incidence of IIH in the general population is between 0.03 to 2.36/100,000 people per year, but in women, the incidence is 0.65 to 4.65/100,000 per year.6 Furthermore, females who are obese have an incidence of 2.7 to 19.3/100,000 per year.6

Headache is the most common symptom of IIH. Unfortunately, the differential diagnosis of headache is vast; thus, a careful history is needed to narrow the field3,5-7 (TABLE 42). Associated symptoms of transient visual changes, pulsatile tinnitus, neck and back pain, nausea, vomiting, photo/phonophobia, and findings of abducens nerve palsy or papilledema—while nonspecific— should raise suspicion for elevated ICP and IIH, especially in women who are obese.2-8 Once IIH is suspected, an urgent diagnosis and treatment is necessary to prevent permanent vision loss.3,4,6

Headache with findings of papilledema warrants neuroimaging, preferably with MRI, to rule out intracranial mass and hydrocephalus.1,2,5 MRV also is recommended to assess for intracranial venous thrombosis, an alternate cause for papilledema and increased ICP.1,2,4,5
Continue to: Recently, a classification of IIH...
Recently, a classification of IIH without papilledema has been acknowledged by the International Headache Society.2,8 Specific MRI findings have been suggested to help make this diagnosis5,9 (TABLE 55).

TREATMENT FOR IIH CAN BE MEDICAL OR SURGICAL
Medications associated with IIH should be discontinued.7 The first-line medication for IIH is acetazolamide, a carbonic anhydrase inhibitor that works in the choroid plexus to decrease cerebrospinal fluid (CSF) production and thus, lower ICP.3,6 An adult dose of 1 to 2 g/day3,4,6 is tolerated well, but can be increased to 4 g/day,10 if necessary. Weight loss via diet and exercise or bariatric surgery has been shown to be effective in patients who are obese and have been given a diagnosis of IIH.3,4
Topiramate also has been suggested as a treatment option, based on its usefulness in weight loss and because of its action as a weak carbonic anhydrase inhibitor.3,6 Also, LP has therapeutic merit—although relief is only short-term.3,6 Patients who fail medical therapy and have intractable headache or progressive visual loss appear to benefit from optic nerve sheath fenestration.3,7,8
Our patient experienced notable improvement in her headache after LP. Her OCP was discontinued, a diuretic regimen started, and weight loss counseling was provided. Prior to discharge, the patient was seen by a neuro-ophthalmologist for perimetry, a visual field test that assesses for acute vision loss and establishes a baseline for follow-up monitoring of vision.7
THE TAKEAWAY
Headache is a common condition that may be challenging to correctly diagnose. A thorough history and neurological examination, including fundoscopy, are essential in the evaluation of headache and suspected IIH. In the primary care setting, limited time, lack of mydriatic agents, suboptimal lighting, and practitioner inexperience may pose challenges for funduscopic examination. Ophthalmoscopes incorporating new technology to expand and magnify the examiner’s field of view may facilitate this exam.11 A global rise in the prevalence of obesity underscores a need for primary care providers to be compulsive about their clinical evaluation when symptoms suspicious of IIH are present. Lastly, if IIH cannot be ruled out confidently, recommend a prompt evaluation by an ophthalmologist.
CORRESPONDENCE
Aarti Paltoo, MD, MSc, CCFP, Peel Village Medical Center, 28 Rambler Drive, Brampton, Ontario L6W 1E2 Canada; [email protected]
1. Lee SC, Lueck CJ. Cerebrospinal fluid pressure in adults. J Neuroophthalmol. 2014;34:278-283.
2. International Headache Society. Idiopathic intracranial hypertension. The International Classification of Headache Disorders. 2nd ed. Oxford, UK: Blackwell Publishing; 2003:1-232.
3. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83:488-494.
4. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14:380-390.
5. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159-1165.
6. Julayanont P, Karukote A, Ruthirago D, et al. Idiopathic intracranial hypertension: ongoing clinical challenges and future prospects. J Pain Res. 2016;9:87-99.
7. Friedman DI, Digre KB. Headache medicine meets neuro-ophthalmology: exam techniques and challenging cases. Headache. 2013;53:703-716.
8. Digre KB, Nakamoto BK, Warner JE, et al. A comparison of idiopathic intracranaial hypertension with and without papilledema. Headache. 2009;49:185-193.
9. Digre KB. Imaging characteristics of IIH: are they reliable? Cephalagia. 2013;33:1067-1069.
10. Horton J. Acetazolamide for pseudotumor cerebri--evidence from the NORDIC trial. JAMA. 2014;311:1618-1619.
11. Petrushkin H, Barsam A, Mavrakakis M, et al. Optic disc assessment in the emergency department: a comparative study between the PanOptic and direct ophthalmoscopes. Emerg Med J. 2012;29:1007-1008.
1. Lee SC, Lueck CJ. Cerebrospinal fluid pressure in adults. J Neuroophthalmol. 2014;34:278-283.
2. International Headache Society. Idiopathic intracranial hypertension. The International Classification of Headache Disorders. 2nd ed. Oxford, UK: Blackwell Publishing; 2003:1-232.
3. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83:488-494.
4. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14:380-390.
5. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159-1165.
6. Julayanont P, Karukote A, Ruthirago D, et al. Idiopathic intracranial hypertension: ongoing clinical challenges and future prospects. J Pain Res. 2016;9:87-99.
7. Friedman DI, Digre KB. Headache medicine meets neuro-ophthalmology: exam techniques and challenging cases. Headache. 2013;53:703-716.
8. Digre KB, Nakamoto BK, Warner JE, et al. A comparison of idiopathic intracranaial hypertension with and without papilledema. Headache. 2009;49:185-193.
9. Digre KB. Imaging characteristics of IIH: are they reliable? Cephalagia. 2013;33:1067-1069.
10. Horton J. Acetazolamide for pseudotumor cerebri--evidence from the NORDIC trial. JAMA. 2014;311:1618-1619.
11. Petrushkin H, Barsam A, Mavrakakis M, et al. Optic disc assessment in the emergency department: a comparative study between the PanOptic and direct ophthalmoscopes. Emerg Med J. 2012;29:1007-1008.