User login
April 2019 Highlights
Q&A on LGBT Youth: Affirmation in your practice
In recent years, pediatricians have learned more about the diversity of gender identities and gender expressions that exist, but many think they have gaps in their knowledge that need to be filled in order to provide better care to these patients.
On April 3, Pediatric News hosted a Twitter question-and-answer session for the purpose of trying to help pediatricians close some of these gaps, including ones related to the medical and mental health issues particular to LGBT patients. During this session Pediatric News’ LGBT Youth Consult columnists, Gerald T. Montano, DO, MS, and Gayathri Chelvakumar, MD, MPH, responded to four questions about working with children and teens who are lesbian, gay, bisexual, transgender, or questioning (LGBTQ). Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Dr. Chelvakumar, MD, MPH, is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
The following is an edited version of the Q&A session.
Question 1: How do people become aware they are lesbian, gay, bisexual, transgender?
Dr. Montano: For many adolescents, the teenage years are the time that these identities are forming and that questions around sexuality and gender identity may arise. Check out #genderunicorn! It illustrates the spectrum of normal gender and sexual identities that are part of the human experience. Many LGBT people recall how they felt when they were younger but couldn’t find the right words until they were older (usually during adolescence).
Dr. Chelvakumar: Every person’s experience is unique. I have had patients tell me they knew who they were attracted to in kindergarten, and other patients who are still figuring it out in college. It is important to note that every experience is valid.
Question 2: How can we address some of the specific health concerns of LGBT youth?
Dr. Chelvakumar: We need to educate ourselves. The American Academy of Pediatrics has a great resource for this, “Ensuring Comprehensive Care and Support for Transgender and Gender Diverse Children and Adolescents (Pediatrics. 2019 Oct. doi: 10.1542/peds.2018-2162).
Dr. Chelvakumar: Some LGBTQ youth are at increased risk of depression, anxiety, and suicidality related to stigma and internal/external transphobia/homophobia, NOT their identities.
Dr. Montano: A lot of LGBT youth report that health care providers fixate on sexual activity, even though that was not the reason why they came to the clinic.
Dr. Chelvakumar: A lot of these youth feel that providers won’t understand their needs or may discriminate against them because of their LGBTQ identity. This is why it is important to make sure our health care spaces are welcoming to everyone.
Dr. Montano: The role of the psychiatrist is not to determine the person’s gender identity or sexual orientation; rather, they should focus on making others feel comfortable with themselves.
Question 3: How can we make our clinics a safe space for LGBT youth?
Dr. Chelvakumar: Ensure that ALL clinic staff receive training on culturally affirming care of LGBTQ people. The National LGBT Health Education Center (@LGBTHealthEdCtr) offers some of these resources.
Dr. Montano: Train everyone in the clinic to be sensitive and aware of the needs of LGBT patients; it only takes one person to make a clinical experience horrible for an LGBT person.
Dr. Chelvakumar: To me, culturally affirming care means being aware of the spectrum of identities and experiences of all of our patients/families and being respectful of these identities. I also like the term cultural humility – we must continually learn about these diverse experiences.
Dr. Montano: Remember that if they get upset, these children and teens are not mad at you, they are mad at the situation.
Dr. Chelvakumar: An easy way to show that a clinic is a safe space is to clearly display a nondiscrimination policy, such as “We serve and respect all patients regardless of gender identity, race, sexual orientation, religion, socioeconomic status ... ”
Dr. Montano: Not every LGBT youth will disclose their sexual orientation or gender identity to the provider, even if the clinic appears welcoming. I suggest you begin a conversation with a patient by telling the patient your own pronouns, opening the door to additional conversations.
Dr. Chelvakumar: Many organizations are moving to universally asking questions about gender identity and pronouns. In the pediatric population, this can be difficult given privacy/confidentiality concerns. I usually ask these questions when obtaining my sensitive sexual history.
Question 4: What is gender dysphoria, and how is it treated?
Dr. Montano: Gender dysphoria is the distress related to gender identity that does not match sex/gender assigned at birth. Treat the distress, not the identity. There are many ways to treat gender dysphoria. You can provide social support, use pubertal blockers to prevent the development of secondary sex characteristics during adolescence, and/or use hormones or surgery to develop characteristics of the affirmed gender. And there is no “right” treatment for gender dysphoria. Each treatment is tailored to the needs of the youth.
Dr. Chelvakumar: It is important to recognize that each person’s journey/transition is different. Our job as providers is to help them on the path that will help them live their lives as their authentic selves. This may or may not involve taking medication and/or undergoing surgery.
Dr. Montano: Providers should respect the wishes of those who want to affirm their gender identity that makes sense to the patient and not try to impose their own idea of what transitioning should “look like.”
Dr. Chelvakumar: There are many guidelines that exist for patients with gender dysphoria. The World Professional Association for Transgender Health, the Endocrine Society, and University of California, San Francisco’s Center of Excellence for Transgender Health are all excellent resources.
In recent years, pediatricians have learned more about the diversity of gender identities and gender expressions that exist, but many think they have gaps in their knowledge that need to be filled in order to provide better care to these patients.
On April 3, Pediatric News hosted a Twitter question-and-answer session for the purpose of trying to help pediatricians close some of these gaps, including ones related to the medical and mental health issues particular to LGBT patients. During this session Pediatric News’ LGBT Youth Consult columnists, Gerald T. Montano, DO, MS, and Gayathri Chelvakumar, MD, MPH, responded to four questions about working with children and teens who are lesbian, gay, bisexual, transgender, or questioning (LGBTQ). Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Dr. Chelvakumar, MD, MPH, is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
The following is an edited version of the Q&A session.
Question 1: How do people become aware they are lesbian, gay, bisexual, transgender?
Dr. Montano: For many adolescents, the teenage years are the time that these identities are forming and that questions around sexuality and gender identity may arise. Check out #genderunicorn! It illustrates the spectrum of normal gender and sexual identities that are part of the human experience. Many LGBT people recall how they felt when they were younger but couldn’t find the right words until they were older (usually during adolescence).
Dr. Chelvakumar: Every person’s experience is unique. I have had patients tell me they knew who they were attracted to in kindergarten, and other patients who are still figuring it out in college. It is important to note that every experience is valid.
Question 2: How can we address some of the specific health concerns of LGBT youth?
Dr. Chelvakumar: We need to educate ourselves. The American Academy of Pediatrics has a great resource for this, “Ensuring Comprehensive Care and Support for Transgender and Gender Diverse Children and Adolescents (Pediatrics. 2019 Oct. doi: 10.1542/peds.2018-2162).
Dr. Chelvakumar: Some LGBTQ youth are at increased risk of depression, anxiety, and suicidality related to stigma and internal/external transphobia/homophobia, NOT their identities.
Dr. Montano: A lot of LGBT youth report that health care providers fixate on sexual activity, even though that was not the reason why they came to the clinic.
Dr. Chelvakumar: A lot of these youth feel that providers won’t understand their needs or may discriminate against them because of their LGBTQ identity. This is why it is important to make sure our health care spaces are welcoming to everyone.
Dr. Montano: The role of the psychiatrist is not to determine the person’s gender identity or sexual orientation; rather, they should focus on making others feel comfortable with themselves.
Question 3: How can we make our clinics a safe space for LGBT youth?
Dr. Chelvakumar: Ensure that ALL clinic staff receive training on culturally affirming care of LGBTQ people. The National LGBT Health Education Center (@LGBTHealthEdCtr) offers some of these resources.
Dr. Montano: Train everyone in the clinic to be sensitive and aware of the needs of LGBT patients; it only takes one person to make a clinical experience horrible for an LGBT person.
Dr. Chelvakumar: To me, culturally affirming care means being aware of the spectrum of identities and experiences of all of our patients/families and being respectful of these identities. I also like the term cultural humility – we must continually learn about these diverse experiences.
Dr. Montano: Remember that if they get upset, these children and teens are not mad at you, they are mad at the situation.
Dr. Chelvakumar: An easy way to show that a clinic is a safe space is to clearly display a nondiscrimination policy, such as “We serve and respect all patients regardless of gender identity, race, sexual orientation, religion, socioeconomic status ... ”
Dr. Montano: Not every LGBT youth will disclose their sexual orientation or gender identity to the provider, even if the clinic appears welcoming. I suggest you begin a conversation with a patient by telling the patient your own pronouns, opening the door to additional conversations.
Dr. Chelvakumar: Many organizations are moving to universally asking questions about gender identity and pronouns. In the pediatric population, this can be difficult given privacy/confidentiality concerns. I usually ask these questions when obtaining my sensitive sexual history.
Question 4: What is gender dysphoria, and how is it treated?
Dr. Montano: Gender dysphoria is the distress related to gender identity that does not match sex/gender assigned at birth. Treat the distress, not the identity. There are many ways to treat gender dysphoria. You can provide social support, use pubertal blockers to prevent the development of secondary sex characteristics during adolescence, and/or use hormones or surgery to develop characteristics of the affirmed gender. And there is no “right” treatment for gender dysphoria. Each treatment is tailored to the needs of the youth.
Dr. Chelvakumar: It is important to recognize that each person’s journey/transition is different. Our job as providers is to help them on the path that will help them live their lives as their authentic selves. This may or may not involve taking medication and/or undergoing surgery.
Dr. Montano: Providers should respect the wishes of those who want to affirm their gender identity that makes sense to the patient and not try to impose their own idea of what transitioning should “look like.”
Dr. Chelvakumar: There are many guidelines that exist for patients with gender dysphoria. The World Professional Association for Transgender Health, the Endocrine Society, and University of California, San Francisco’s Center of Excellence for Transgender Health are all excellent resources.
In recent years, pediatricians have learned more about the diversity of gender identities and gender expressions that exist, but many think they have gaps in their knowledge that need to be filled in order to provide better care to these patients.
On April 3, Pediatric News hosted a Twitter question-and-answer session for the purpose of trying to help pediatricians close some of these gaps, including ones related to the medical and mental health issues particular to LGBT patients. During this session Pediatric News’ LGBT Youth Consult columnists, Gerald T. Montano, DO, MS, and Gayathri Chelvakumar, MD, MPH, responded to four questions about working with children and teens who are lesbian, gay, bisexual, transgender, or questioning (LGBTQ). Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Dr. Chelvakumar, MD, MPH, is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
The following is an edited version of the Q&A session.
Question 1: How do people become aware they are lesbian, gay, bisexual, transgender?
Dr. Montano: For many adolescents, the teenage years are the time that these identities are forming and that questions around sexuality and gender identity may arise. Check out #genderunicorn! It illustrates the spectrum of normal gender and sexual identities that are part of the human experience. Many LGBT people recall how they felt when they were younger but couldn’t find the right words until they were older (usually during adolescence).
Dr. Chelvakumar: Every person’s experience is unique. I have had patients tell me they knew who they were attracted to in kindergarten, and other patients who are still figuring it out in college. It is important to note that every experience is valid.
Question 2: How can we address some of the specific health concerns of LGBT youth?
Dr. Chelvakumar: We need to educate ourselves. The American Academy of Pediatrics has a great resource for this, “Ensuring Comprehensive Care and Support for Transgender and Gender Diverse Children and Adolescents (Pediatrics. 2019 Oct. doi: 10.1542/peds.2018-2162).
Dr. Chelvakumar: Some LGBTQ youth are at increased risk of depression, anxiety, and suicidality related to stigma and internal/external transphobia/homophobia, NOT their identities.
Dr. Montano: A lot of LGBT youth report that health care providers fixate on sexual activity, even though that was not the reason why they came to the clinic.
Dr. Chelvakumar: A lot of these youth feel that providers won’t understand their needs or may discriminate against them because of their LGBTQ identity. This is why it is important to make sure our health care spaces are welcoming to everyone.
Dr. Montano: The role of the psychiatrist is not to determine the person’s gender identity or sexual orientation; rather, they should focus on making others feel comfortable with themselves.
Question 3: How can we make our clinics a safe space for LGBT youth?
Dr. Chelvakumar: Ensure that ALL clinic staff receive training on culturally affirming care of LGBTQ people. The National LGBT Health Education Center (@LGBTHealthEdCtr) offers some of these resources.
Dr. Montano: Train everyone in the clinic to be sensitive and aware of the needs of LGBT patients; it only takes one person to make a clinical experience horrible for an LGBT person.
Dr. Chelvakumar: To me, culturally affirming care means being aware of the spectrum of identities and experiences of all of our patients/families and being respectful of these identities. I also like the term cultural humility – we must continually learn about these diverse experiences.
Dr. Montano: Remember that if they get upset, these children and teens are not mad at you, they are mad at the situation.
Dr. Chelvakumar: An easy way to show that a clinic is a safe space is to clearly display a nondiscrimination policy, such as “We serve and respect all patients regardless of gender identity, race, sexual orientation, religion, socioeconomic status ... ”
Dr. Montano: Not every LGBT youth will disclose their sexual orientation or gender identity to the provider, even if the clinic appears welcoming. I suggest you begin a conversation with a patient by telling the patient your own pronouns, opening the door to additional conversations.
Dr. Chelvakumar: Many organizations are moving to universally asking questions about gender identity and pronouns. In the pediatric population, this can be difficult given privacy/confidentiality concerns. I usually ask these questions when obtaining my sensitive sexual history.
Question 4: What is gender dysphoria, and how is it treated?
Dr. Montano: Gender dysphoria is the distress related to gender identity that does not match sex/gender assigned at birth. Treat the distress, not the identity. There are many ways to treat gender dysphoria. You can provide social support, use pubertal blockers to prevent the development of secondary sex characteristics during adolescence, and/or use hormones or surgery to develop characteristics of the affirmed gender. And there is no “right” treatment for gender dysphoria. Each treatment is tailored to the needs of the youth.
Dr. Chelvakumar: It is important to recognize that each person’s journey/transition is different. Our job as providers is to help them on the path that will help them live their lives as their authentic selves. This may or may not involve taking medication and/or undergoing surgery.
Dr. Montano: Providers should respect the wishes of those who want to affirm their gender identity that makes sense to the patient and not try to impose their own idea of what transitioning should “look like.”
Dr. Chelvakumar: There are many guidelines that exist for patients with gender dysphoria. The World Professional Association for Transgender Health, the Endocrine Society, and University of California, San Francisco’s Center of Excellence for Transgender Health are all excellent resources.
ASCO, CCO issue multiple myeloma treatment guidelines
New clinical practice guidelines, jointly released by two leading cancer organizations, provide nearly 50 specific recommendations for the management of newly diagnosed and relapsed multiple myeloma patients.
The guidelines from the American Society of Clinical Oncology (ASCO) and Cancer Care Ontario (CCO) were authored by a panel of 21 experts in medical oncology, surgery, radiation oncology, and advocacy who reviewed 124 relevant studies published between 2005 and 2018.
“The treatment of multiple myeloma has changed significantly in the last 5 years. Since 2015, four new drugs have been approved, thus providing more options and adding to the complexity of treatment options,” the expert panel wrote in the Journal of Clinical Oncology.
The recommendations are intended to put in context recent randomized trials and drug advances, according to the experts, led by cochairs Joseph Mikhael, MD, of City of Hope Cancer Center, Phoenix, and the International Myeloma Foundation, North Hollywood, Calif., and Tom Martin, MD, of the University of California, San Francisco.
Specifically, the recently approved agents include the proteasome inhibitor ixazomib, the histone deacetylase inhibitor panobinostat, and the monoclonal antibodies daratumumab and elotuzumab, directed at CD38 and SLAMF7, respectively.
There are 20 specific recommendations for newly diagnosed, transplant-eligible patients with multiple myeloma; 10 recommendations for newly diagnosed, transplant-ineligible patients; and 16 recommendations related to relapsed disease in the ASCO/CCO guidelines.
All transplant-eligible patients should be offered up-front autologous stem cell transplant (ASCT), according to the guidelines. By contrast, allogeneic transplant is not routinely recommended but “may be considered” in select high-risk patients, and tandem transplant “should not be routinely recommended,” the expert panelists said in their report.
Lenalidomide maintenance therapy should be routinely offered to standard-risk, transplant-eligible patients, according to the panel, whereas bortezomib maintenance could be considered in those who are intolerant of lenalidomide or can’t receive that immunomodulatory drug.
“Evidence is emerging for the use of ixazomib as maintenance therapy and may also be considered,” the panel members said, citing the TOURMALINE-MM3 study results presented at the 2018 annual meeting of the American Society of Hematology and recently published in the Lancet.
Although minimal residual disease (MRD)–negative status is linked to improved outcomes, there is insufficient evidence that MRD can be used today to modify maintenance therapy based on depth of response in transplant-eligible patients, according to the guidelines. Likewise, in transplant-ineligible patients, MRD shouldn’t be used to guide treatment goals in clinical practice, the authors said.
Triplet therapies such as bortezomib, lenalidomide, and dexamethasone (VRd) can be considered for transplant ineligible patients, as can the combination of daratumumab and bortezomib plus melphalan and prednisone that was approved by the Food and Drug Administration in May 2018.
For patients with biochemically relapsed myeloma and high-risk disease – defined as early relapse and presence of high-risk cytogenetics – treatment should begin immediately, whereas close observation may be appropriate for patients with asymptomatic, slowly progressive relapse.
Triplets containing two novel therapies should be administered on first relapse, and should continue until disease progression, the expert panel advised.
If it was not already done after primary induction, ASCT should be offered to relapsed, transplant-eligible myeloma patients.
The expert panel reported numerous financial relationships with industry, including Celgene, Sanofi, AbbVie, TeneoBio, Roche, June Therapeutics, and others.
SOURCE: Mikhael J et al. J Clin Oncol. 2019 Apr 1. doi: 10.1200/JCO.18.02096.
New clinical practice guidelines, jointly released by two leading cancer organizations, provide nearly 50 specific recommendations for the management of newly diagnosed and relapsed multiple myeloma patients.
The guidelines from the American Society of Clinical Oncology (ASCO) and Cancer Care Ontario (CCO) were authored by a panel of 21 experts in medical oncology, surgery, radiation oncology, and advocacy who reviewed 124 relevant studies published between 2005 and 2018.
“The treatment of multiple myeloma has changed significantly in the last 5 years. Since 2015, four new drugs have been approved, thus providing more options and adding to the complexity of treatment options,” the expert panel wrote in the Journal of Clinical Oncology.
The recommendations are intended to put in context recent randomized trials and drug advances, according to the experts, led by cochairs Joseph Mikhael, MD, of City of Hope Cancer Center, Phoenix, and the International Myeloma Foundation, North Hollywood, Calif., and Tom Martin, MD, of the University of California, San Francisco.
Specifically, the recently approved agents include the proteasome inhibitor ixazomib, the histone deacetylase inhibitor panobinostat, and the monoclonal antibodies daratumumab and elotuzumab, directed at CD38 and SLAMF7, respectively.
There are 20 specific recommendations for newly diagnosed, transplant-eligible patients with multiple myeloma; 10 recommendations for newly diagnosed, transplant-ineligible patients; and 16 recommendations related to relapsed disease in the ASCO/CCO guidelines.
All transplant-eligible patients should be offered up-front autologous stem cell transplant (ASCT), according to the guidelines. By contrast, allogeneic transplant is not routinely recommended but “may be considered” in select high-risk patients, and tandem transplant “should not be routinely recommended,” the expert panelists said in their report.
Lenalidomide maintenance therapy should be routinely offered to standard-risk, transplant-eligible patients, according to the panel, whereas bortezomib maintenance could be considered in those who are intolerant of lenalidomide or can’t receive that immunomodulatory drug.
“Evidence is emerging for the use of ixazomib as maintenance therapy and may also be considered,” the panel members said, citing the TOURMALINE-MM3 study results presented at the 2018 annual meeting of the American Society of Hematology and recently published in the Lancet.
Although minimal residual disease (MRD)–negative status is linked to improved outcomes, there is insufficient evidence that MRD can be used today to modify maintenance therapy based on depth of response in transplant-eligible patients, according to the guidelines. Likewise, in transplant-ineligible patients, MRD shouldn’t be used to guide treatment goals in clinical practice, the authors said.
Triplet therapies such as bortezomib, lenalidomide, and dexamethasone (VRd) can be considered for transplant ineligible patients, as can the combination of daratumumab and bortezomib plus melphalan and prednisone that was approved by the Food and Drug Administration in May 2018.
For patients with biochemically relapsed myeloma and high-risk disease – defined as early relapse and presence of high-risk cytogenetics – treatment should begin immediately, whereas close observation may be appropriate for patients with asymptomatic, slowly progressive relapse.
Triplets containing two novel therapies should be administered on first relapse, and should continue until disease progression, the expert panel advised.
If it was not already done after primary induction, ASCT should be offered to relapsed, transplant-eligible myeloma patients.
The expert panel reported numerous financial relationships with industry, including Celgene, Sanofi, AbbVie, TeneoBio, Roche, June Therapeutics, and others.
SOURCE: Mikhael J et al. J Clin Oncol. 2019 Apr 1. doi: 10.1200/JCO.18.02096.
New clinical practice guidelines, jointly released by two leading cancer organizations, provide nearly 50 specific recommendations for the management of newly diagnosed and relapsed multiple myeloma patients.
The guidelines from the American Society of Clinical Oncology (ASCO) and Cancer Care Ontario (CCO) were authored by a panel of 21 experts in medical oncology, surgery, radiation oncology, and advocacy who reviewed 124 relevant studies published between 2005 and 2018.
“The treatment of multiple myeloma has changed significantly in the last 5 years. Since 2015, four new drugs have been approved, thus providing more options and adding to the complexity of treatment options,” the expert panel wrote in the Journal of Clinical Oncology.
The recommendations are intended to put in context recent randomized trials and drug advances, according to the experts, led by cochairs Joseph Mikhael, MD, of City of Hope Cancer Center, Phoenix, and the International Myeloma Foundation, North Hollywood, Calif., and Tom Martin, MD, of the University of California, San Francisco.
Specifically, the recently approved agents include the proteasome inhibitor ixazomib, the histone deacetylase inhibitor panobinostat, and the monoclonal antibodies daratumumab and elotuzumab, directed at CD38 and SLAMF7, respectively.
There are 20 specific recommendations for newly diagnosed, transplant-eligible patients with multiple myeloma; 10 recommendations for newly diagnosed, transplant-ineligible patients; and 16 recommendations related to relapsed disease in the ASCO/CCO guidelines.
All transplant-eligible patients should be offered up-front autologous stem cell transplant (ASCT), according to the guidelines. By contrast, allogeneic transplant is not routinely recommended but “may be considered” in select high-risk patients, and tandem transplant “should not be routinely recommended,” the expert panelists said in their report.
Lenalidomide maintenance therapy should be routinely offered to standard-risk, transplant-eligible patients, according to the panel, whereas bortezomib maintenance could be considered in those who are intolerant of lenalidomide or can’t receive that immunomodulatory drug.
“Evidence is emerging for the use of ixazomib as maintenance therapy and may also be considered,” the panel members said, citing the TOURMALINE-MM3 study results presented at the 2018 annual meeting of the American Society of Hematology and recently published in the Lancet.
Although minimal residual disease (MRD)–negative status is linked to improved outcomes, there is insufficient evidence that MRD can be used today to modify maintenance therapy based on depth of response in transplant-eligible patients, according to the guidelines. Likewise, in transplant-ineligible patients, MRD shouldn’t be used to guide treatment goals in clinical practice, the authors said.
Triplet therapies such as bortezomib, lenalidomide, and dexamethasone (VRd) can be considered for transplant ineligible patients, as can the combination of daratumumab and bortezomib plus melphalan and prednisone that was approved by the Food and Drug Administration in May 2018.
For patients with biochemically relapsed myeloma and high-risk disease – defined as early relapse and presence of high-risk cytogenetics – treatment should begin immediately, whereas close observation may be appropriate for patients with asymptomatic, slowly progressive relapse.
Triplets containing two novel therapies should be administered on first relapse, and should continue until disease progression, the expert panel advised.
If it was not already done after primary induction, ASCT should be offered to relapsed, transplant-eligible myeloma patients.
The expert panel reported numerous financial relationships with industry, including Celgene, Sanofi, AbbVie, TeneoBio, Roche, June Therapeutics, and others.
SOURCE: Mikhael J et al. J Clin Oncol. 2019 Apr 1. doi: 10.1200/JCO.18.02096.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
DDNA19: The role of the microbiome in liver disease

Stephen Brant, MD, and Nikolaos Pyrsopoulos, MD, discuss the latest news and the role of the microbiome in liver diseases at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.

Stephen Brant, MD, and Nikolaos Pyrsopoulos, MD, discuss the latest news and the role of the microbiome in liver diseases at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.

Stephen Brant, MD, and Nikolaos Pyrsopoulos, MD, discuss the latest news and the role of the microbiome in liver diseases at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.
REPORTING FROM DIGESTIVE DISEASES: NEW ADVANCES
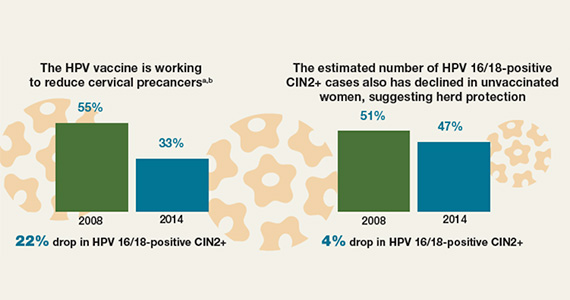
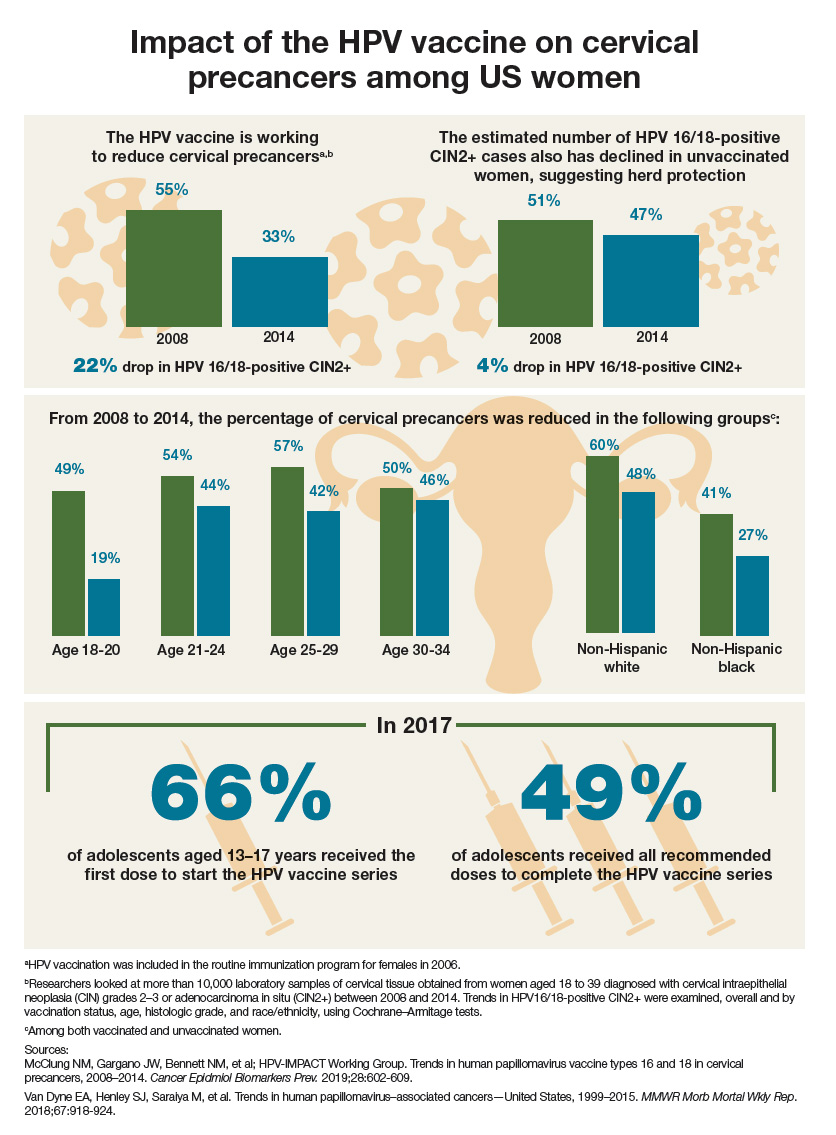
Impact of the HPV vaccine on cervical precancers among US women


Comorbidities, hematologic cancers drive high costs among elderly patients
High-cost hospital stays among elderly patients with cancer are associated with major procedures, more comorbid conditions, hematologic cancer, and metastatic cancer, according to a retrospective analysis of more than 570,000 inpatient visits.
Patients involved in the top 10% most expensive cancer-related hospital visits were more likely to have five or more comorbidities, or receive chemotherapy, reported lead author Jaqueline Avila, PhD candidate at the University of Texas Medical Branch in Galveston, and her colleagues.
“A small fraction (approximately 10%) of patients account for more than half of the overall health care costs incurred annually,” the investigators wrote. The report is in the Journal of Oncology Practice.
“[S]imilar to the general population, a small population of patients with cancer (the top 5% to 10% of patients with highest costs) accounts for more than 80% of the total cancer costs,” the investigators wrote. “However, we lack a detailed understanding of the characteristics of these high-cost and high-risk elderly patients with cancer.”
To gain insight, the investigators analyzed 574,367 cancer-related inpatient visits of patients aged 65 years or older, using data from the 2014 National Inpatient Sample. Visits were divided based on cost, with the top 10% most expensive visits in one group (n = 57,437) and the lower 90% of visits in another group (n = 516,930). The investigators then compared these groups based on a variety of patient factor covariates, including race, sex, age, type of cancer, comorbidities, treatments received, and hospital characteristics, such as private or public ownership and location.
The overall median cost of the top 10% of inpatient visits was $38,194 (interquartile range, $31,405-$51,802), compared with $8,257 in the lower-cost group (interquartile range, $5,032-$13,335). This was partly attributed to comorbidities. In the high-cost group, 38.4% of patients had five or more comorbidities, compared with 26.2% in the lower-cost group (P less than .001).
Procedures also factored into cost. The high-cost group had more procedures than did the lower-cost group (mean, 5.48 vs. 2.36; P less than .001), and expensive stays more often entailed major procedures (67.1% vs. 24.3%; P less than .001). Hematologic cancer and metastatic cancer were also more common in the high-cost group than in the lower-cost group, with rates of 23.5% versus 14.6% and 16.5% versus 11.8%, respectively. Among all cancer types, lymphoma, leukemia, and myeloma were the most expensive. The investigators noted that 97.9% of stem-cell or bone marrow transplants were received by patients with hematologic cancer.
Cost did not increase directly with age, as patients aged 65-84 years were more likely to have high-cost inpatient visits than were those who were aged 85 years or older.
The investigators suggested that complications of chronic diseases were likely at the root of this difference, particularly among patients in the 65- to 72-year range. “The difference in costs could also be because caregivers and clinicians may choose to provide only necessary and often less intensive procedures and care to the oldest patients,” the investigators wrote.
A variety of other factors were associated with high-cost visits, although to a lesser degree, including male sex, treatment at a metropolitan teaching hospital, higher median household income, radiation therapy, and large bed size.
The investigators stated that more research is needed to determine relationships between costs and medical necessity, and to develop strategies for reducing costs.
“Although we evaluated the drivers of hospital costs, we could not assess whether additional costs incurred by the high-cost group were medically necessary or could be prevented or whether these excess costs resulted in better outcomes,” the investigators wrote. “Additional research is needed to measure outcomes such as survival and quality of life in this high-cost group and also evaluate whether the excess cost is spent on medically necessary services.
“Future studies should address how implementation of models such as the integrative care model and hospice care may affect the distribution of high-cost and low-cost visits,” the investigators suggested.
Novartis funded the study. Dr. Chavez-MacGregor reported financial relationships with Pfizer and Genentech.
SOURCE: Avila et al. JOP. 2019 Apr 4. doi:10.1200/JOP.18.00706.
High-cost hospital stays among elderly patients with cancer are associated with major procedures, more comorbid conditions, hematologic cancer, and metastatic cancer, according to a retrospective analysis of more than 570,000 inpatient visits.
Patients involved in the top 10% most expensive cancer-related hospital visits were more likely to have five or more comorbidities, or receive chemotherapy, reported lead author Jaqueline Avila, PhD candidate at the University of Texas Medical Branch in Galveston, and her colleagues.
“A small fraction (approximately 10%) of patients account for more than half of the overall health care costs incurred annually,” the investigators wrote. The report is in the Journal of Oncology Practice.
“[S]imilar to the general population, a small population of patients with cancer (the top 5% to 10% of patients with highest costs) accounts for more than 80% of the total cancer costs,” the investigators wrote. “However, we lack a detailed understanding of the characteristics of these high-cost and high-risk elderly patients with cancer.”
To gain insight, the investigators analyzed 574,367 cancer-related inpatient visits of patients aged 65 years or older, using data from the 2014 National Inpatient Sample. Visits were divided based on cost, with the top 10% most expensive visits in one group (n = 57,437) and the lower 90% of visits in another group (n = 516,930). The investigators then compared these groups based on a variety of patient factor covariates, including race, sex, age, type of cancer, comorbidities, treatments received, and hospital characteristics, such as private or public ownership and location.
The overall median cost of the top 10% of inpatient visits was $38,194 (interquartile range, $31,405-$51,802), compared with $8,257 in the lower-cost group (interquartile range, $5,032-$13,335). This was partly attributed to comorbidities. In the high-cost group, 38.4% of patients had five or more comorbidities, compared with 26.2% in the lower-cost group (P less than .001).
Procedures also factored into cost. The high-cost group had more procedures than did the lower-cost group (mean, 5.48 vs. 2.36; P less than .001), and expensive stays more often entailed major procedures (67.1% vs. 24.3%; P less than .001). Hematologic cancer and metastatic cancer were also more common in the high-cost group than in the lower-cost group, with rates of 23.5% versus 14.6% and 16.5% versus 11.8%, respectively. Among all cancer types, lymphoma, leukemia, and myeloma were the most expensive. The investigators noted that 97.9% of stem-cell or bone marrow transplants were received by patients with hematologic cancer.
Cost did not increase directly with age, as patients aged 65-84 years were more likely to have high-cost inpatient visits than were those who were aged 85 years or older.
The investigators suggested that complications of chronic diseases were likely at the root of this difference, particularly among patients in the 65- to 72-year range. “The difference in costs could also be because caregivers and clinicians may choose to provide only necessary and often less intensive procedures and care to the oldest patients,” the investigators wrote.
A variety of other factors were associated with high-cost visits, although to a lesser degree, including male sex, treatment at a metropolitan teaching hospital, higher median household income, radiation therapy, and large bed size.
The investigators stated that more research is needed to determine relationships between costs and medical necessity, and to develop strategies for reducing costs.
“Although we evaluated the drivers of hospital costs, we could not assess whether additional costs incurred by the high-cost group were medically necessary or could be prevented or whether these excess costs resulted in better outcomes,” the investigators wrote. “Additional research is needed to measure outcomes such as survival and quality of life in this high-cost group and also evaluate whether the excess cost is spent on medically necessary services.
“Future studies should address how implementation of models such as the integrative care model and hospice care may affect the distribution of high-cost and low-cost visits,” the investigators suggested.
Novartis funded the study. Dr. Chavez-MacGregor reported financial relationships with Pfizer and Genentech.
SOURCE: Avila et al. JOP. 2019 Apr 4. doi:10.1200/JOP.18.00706.
High-cost hospital stays among elderly patients with cancer are associated with major procedures, more comorbid conditions, hematologic cancer, and metastatic cancer, according to a retrospective analysis of more than 570,000 inpatient visits.
Patients involved in the top 10% most expensive cancer-related hospital visits were more likely to have five or more comorbidities, or receive chemotherapy, reported lead author Jaqueline Avila, PhD candidate at the University of Texas Medical Branch in Galveston, and her colleagues.
“A small fraction (approximately 10%) of patients account for more than half of the overall health care costs incurred annually,” the investigators wrote. The report is in the Journal of Oncology Practice.
“[S]imilar to the general population, a small population of patients with cancer (the top 5% to 10% of patients with highest costs) accounts for more than 80% of the total cancer costs,” the investigators wrote. “However, we lack a detailed understanding of the characteristics of these high-cost and high-risk elderly patients with cancer.”
To gain insight, the investigators analyzed 574,367 cancer-related inpatient visits of patients aged 65 years or older, using data from the 2014 National Inpatient Sample. Visits were divided based on cost, with the top 10% most expensive visits in one group (n = 57,437) and the lower 90% of visits in another group (n = 516,930). The investigators then compared these groups based on a variety of patient factor covariates, including race, sex, age, type of cancer, comorbidities, treatments received, and hospital characteristics, such as private or public ownership and location.
The overall median cost of the top 10% of inpatient visits was $38,194 (interquartile range, $31,405-$51,802), compared with $8,257 in the lower-cost group (interquartile range, $5,032-$13,335). This was partly attributed to comorbidities. In the high-cost group, 38.4% of patients had five or more comorbidities, compared with 26.2% in the lower-cost group (P less than .001).
Procedures also factored into cost. The high-cost group had more procedures than did the lower-cost group (mean, 5.48 vs. 2.36; P less than .001), and expensive stays more often entailed major procedures (67.1% vs. 24.3%; P less than .001). Hematologic cancer and metastatic cancer were also more common in the high-cost group than in the lower-cost group, with rates of 23.5% versus 14.6% and 16.5% versus 11.8%, respectively. Among all cancer types, lymphoma, leukemia, and myeloma were the most expensive. The investigators noted that 97.9% of stem-cell or bone marrow transplants were received by patients with hematologic cancer.
Cost did not increase directly with age, as patients aged 65-84 years were more likely to have high-cost inpatient visits than were those who were aged 85 years or older.
The investigators suggested that complications of chronic diseases were likely at the root of this difference, particularly among patients in the 65- to 72-year range. “The difference in costs could also be because caregivers and clinicians may choose to provide only necessary and often less intensive procedures and care to the oldest patients,” the investigators wrote.
A variety of other factors were associated with high-cost visits, although to a lesser degree, including male sex, treatment at a metropolitan teaching hospital, higher median household income, radiation therapy, and large bed size.
The investigators stated that more research is needed to determine relationships between costs and medical necessity, and to develop strategies for reducing costs.
“Although we evaluated the drivers of hospital costs, we could not assess whether additional costs incurred by the high-cost group were medically necessary or could be prevented or whether these excess costs resulted in better outcomes,” the investigators wrote. “Additional research is needed to measure outcomes such as survival and quality of life in this high-cost group and also evaluate whether the excess cost is spent on medically necessary services.
“Future studies should address how implementation of models such as the integrative care model and hospice care may affect the distribution of high-cost and low-cost visits,” the investigators suggested.
Novartis funded the study. Dr. Chavez-MacGregor reported financial relationships with Pfizer and Genentech.
SOURCE: Avila et al. JOP. 2019 Apr 4. doi:10.1200/JOP.18.00706.
FROM JOURNAL OF ONCOLOGY PRACTICE
Key clinical point: High-cost hospital stays among elderly patients with cancer are associated with comorbid conditions, major procedures, hematologic cancer, and metastatic cancer.
Major finding: On a cost basis, the top 10% of hospital visits were almost five times more expensive than the bottom 90% of hospital stays (median, $38,194 vs. $8,257).
Study details: A retrospective cost analysis of cancer-related inpatient visits among patients aged 65 years or older, with data from the 2014 National Inpatient Sample.
Disclosures: Novartis funded the study. Dr. Chavez-MacGregor reported financial relationships with Pfizer and Genentech.
Source: Avila et al. J Oncol Pract. 2019 Apr 4. doi: 10.1200/JOP.18.00706.
FDA approves palbociclib for men with HR+/HER2- advanced breast cancer
The Food and Drug Administration has expanded the indication of palbociclib (Ibrance) in combination with specific endocrine therapies for hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative advanced or metastatic breast cancer in men.
Approval was based on postmarketing reports and electronic health records showing that the safety profile for men is consistent with that of women, the FDA said in a statement.
Less than 1% of all cases of breast cancer occur in men, but in the majority of those cases the tumors do express hormone receptors. Men are more likely to be diagnosed at a more advanced stage of disease. “According to the current clinical practice standards, male patients with breast cancer are treated similarly to women with breast cancer,” the FDA said.
The kinase inhibitor palbociclib was initially approved in 2015, in combination with an aromatase inhibitor, for postmenopausal women as first-line treatment of advanced disease.
The most common side effects in patients taking palbociclib are infections, leukopenia, fatigue, nausea, stomatitis, anemia, hair loss, diarrhea, and thrombocytopenia.
Because of the potential for genotoxicity, the FDA advised health care providers to tell male patients with female partners of reproductive potential to use effective contraception during treatment with palbociclib and for 3 months after the last dose.
The Food and Drug Administration has expanded the indication of palbociclib (Ibrance) in combination with specific endocrine therapies for hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative advanced or metastatic breast cancer in men.
Approval was based on postmarketing reports and electronic health records showing that the safety profile for men is consistent with that of women, the FDA said in a statement.
Less than 1% of all cases of breast cancer occur in men, but in the majority of those cases the tumors do express hormone receptors. Men are more likely to be diagnosed at a more advanced stage of disease. “According to the current clinical practice standards, male patients with breast cancer are treated similarly to women with breast cancer,” the FDA said.
The kinase inhibitor palbociclib was initially approved in 2015, in combination with an aromatase inhibitor, for postmenopausal women as first-line treatment of advanced disease.
The most common side effects in patients taking palbociclib are infections, leukopenia, fatigue, nausea, stomatitis, anemia, hair loss, diarrhea, and thrombocytopenia.
Because of the potential for genotoxicity, the FDA advised health care providers to tell male patients with female partners of reproductive potential to use effective contraception during treatment with palbociclib and for 3 months after the last dose.
The Food and Drug Administration has expanded the indication of palbociclib (Ibrance) in combination with specific endocrine therapies for hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative advanced or metastatic breast cancer in men.
Approval was based on postmarketing reports and electronic health records showing that the safety profile for men is consistent with that of women, the FDA said in a statement.
Less than 1% of all cases of breast cancer occur in men, but in the majority of those cases the tumors do express hormone receptors. Men are more likely to be diagnosed at a more advanced stage of disease. “According to the current clinical practice standards, male patients with breast cancer are treated similarly to women with breast cancer,” the FDA said.
The kinase inhibitor palbociclib was initially approved in 2015, in combination with an aromatase inhibitor, for postmenopausal women as first-line treatment of advanced disease.
The most common side effects in patients taking palbociclib are infections, leukopenia, fatigue, nausea, stomatitis, anemia, hair loss, diarrhea, and thrombocytopenia.
Because of the potential for genotoxicity, the FDA advised health care providers to tell male patients with female partners of reproductive potential to use effective contraception during treatment with palbociclib and for 3 months after the last dose.
Creating innovative discharge plans
‘Long Stay Committee’ may help
Hospitalists pay attention to length of stay as a measure of hospital efficiency and resource utilization; outliers on that measure – “long stay patients” – who present complex discharges are a barrier to length of stay reduction. To address this challenge, one institution formed a multidisciplinary Long Stay Committee and described the results in an abstract.
The Long Stay Committee is composed of medical directors, the chief quality officer, directors in nursing, directors of case management/social work, hospitalists, risk management, finance, ethics, psychiatry, and directors of rehabilitation. The most complex patient discharges, identified by case management and social work, are brought to the Long Stay Committee.
“Lack of guardianship is one of the most encountered barriers,” according to the authors. “The Long Stay Committee played an integral part in our institution partnering with the local county to form a guardian service board which facilitates guardianship appointments. Other solutions have included working with the patient and support persons to find appropriate discharge levels of care throughout the United States and other countries as well as guiding them through the process to gain the necessary financial resources.”
The authors conclude that the foundation of the committee’s success in coming up with innovative discharge solutions is the broad range of disciplines that attend this committee and the atmosphere of teamwork it creates.
Reference
Heacock A et al. Long Stay Committee finds innovative discharge plans for difficult discharges. Hospital Medicine 2018, Abstract 312. .
‘Long Stay Committee’ may help
‘Long Stay Committee’ may help
Hospitalists pay attention to length of stay as a measure of hospital efficiency and resource utilization; outliers on that measure – “long stay patients” – who present complex discharges are a barrier to length of stay reduction. To address this challenge, one institution formed a multidisciplinary Long Stay Committee and described the results in an abstract.
The Long Stay Committee is composed of medical directors, the chief quality officer, directors in nursing, directors of case management/social work, hospitalists, risk management, finance, ethics, psychiatry, and directors of rehabilitation. The most complex patient discharges, identified by case management and social work, are brought to the Long Stay Committee.
“Lack of guardianship is one of the most encountered barriers,” according to the authors. “The Long Stay Committee played an integral part in our institution partnering with the local county to form a guardian service board which facilitates guardianship appointments. Other solutions have included working with the patient and support persons to find appropriate discharge levels of care throughout the United States and other countries as well as guiding them through the process to gain the necessary financial resources.”
The authors conclude that the foundation of the committee’s success in coming up with innovative discharge solutions is the broad range of disciplines that attend this committee and the atmosphere of teamwork it creates.
Reference
Heacock A et al. Long Stay Committee finds innovative discharge plans for difficult discharges. Hospital Medicine 2018, Abstract 312. .
Hospitalists pay attention to length of stay as a measure of hospital efficiency and resource utilization; outliers on that measure – “long stay patients” – who present complex discharges are a barrier to length of stay reduction. To address this challenge, one institution formed a multidisciplinary Long Stay Committee and described the results in an abstract.
The Long Stay Committee is composed of medical directors, the chief quality officer, directors in nursing, directors of case management/social work, hospitalists, risk management, finance, ethics, psychiatry, and directors of rehabilitation. The most complex patient discharges, identified by case management and social work, are brought to the Long Stay Committee.
“Lack of guardianship is one of the most encountered barriers,” according to the authors. “The Long Stay Committee played an integral part in our institution partnering with the local county to form a guardian service board which facilitates guardianship appointments. Other solutions have included working with the patient and support persons to find appropriate discharge levels of care throughout the United States and other countries as well as guiding them through the process to gain the necessary financial resources.”
The authors conclude that the foundation of the committee’s success in coming up with innovative discharge solutions is the broad range of disciplines that attend this committee and the atmosphere of teamwork it creates.
Reference
Heacock A et al. Long Stay Committee finds innovative discharge plans for difficult discharges. Hospital Medicine 2018, Abstract 312. .
Polyglutamine diseases are rare, but not the mutations that cause them
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
FROM JAMA NEUROLOGY
Should the standard location for uterine aspiration be in the office?
[polldaddy:10286067]
[polldaddy:10286067]
[polldaddy:10286067]