User login
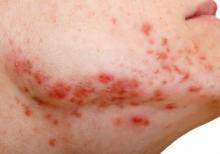
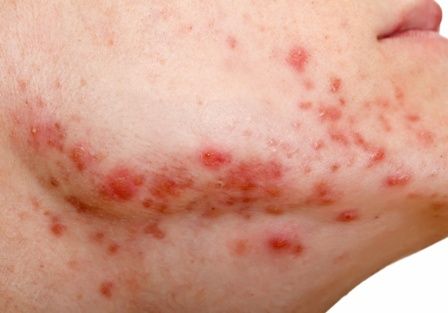
Review looks at effectiveness of isotretinoin for acne
There is when based on reductions in inflammatory lesion counts, according to a recent Cochrane Review.
“The current recommendation of clinical guidelines that oral isotretinoin should be the first-line treatment for moderate to severe acne unresponsive to previous therapies, being more effective than the use of oral antibiotics plus topical agents, underpins current dermatological practice,” Caroline S. Costa, MD, from the Emergency Medicine and Evidence-Based Medicine department at Universidade Federal de São Paulo and her colleagues wrote in their review. “The recommendation cannot be fully supported with certainty by the finding of this review; however, neither does this review challenge it.”
Dr. Costa and her colleagues identified 31 randomized, controlled trials in the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, and LILACS databases up to July 2017, which altogether included 3,836 participants with mild to severe acne who were aged 12-55 years. The patients received treatment with oral isotretinoin versus placebo or other treatments, such as antibiotics.
In 3 studies that altogether included 400 participants and compared isotretinoin with oral antibiotics plus topical treatments, there was no significant difference with isotretinoin in decreases in the investigator-assessed inflammatory lesion count in participants with moderate to severe acne after 20-24 weeks (risk ratio, 1.01; 95% confidence interval, 0.96-1.06). One serious side effect – Stevens-Johnson syndrome – was noted in the isotretinoin-treated group (RR, 3.00; 95% CI, 0.12-72.98). These results were based on “very-low-quality evidence,” the authors noted.
Two studies also comparing isotretinoin with oral antibiotics and topical treatments in a total of 351 participants showed a 15% improvement in acne severity (RR, 1.15; 95% CI, 1.00-1.32) as assessed by physician’s global evaluation but with a greater number of adverse events considered less severe (RR, 1.67; 95% CI, 1.42-1.98), such as vomiting, nausea, dry lips and skin, and cheilitis.
With regard to dosing, one study with 154 participants with severe acne showed 0.05 mg/kg per day of isotretinoin reduced inflammatory lesion count by 79% after 20 weeks, while 0.1 mg/kg per day and 0.2 mg/kg per day reduced inflammatory lesion counts by 80% and 84%, respectively, after 20 weeks. A different study of 150 participants with severe acne found a 95% decrease in inflammatory lesion counts for 58% of participants receiving 0.1 mg/kg per day, 80% of participants receiving 0.5 mg/kg per day, and 90% of participants receiving 1 mg/kg per day of oral isotretinoin after 20 weeks.
In a randomized, controlled trial with 40 participants with moderate acne comparing intermittent dosing (0.5-0.7 mg/kg per day for 1 week per month), continuous conventional dosing (0.5-0.7 mg/kg per day), and continuous low dosing (0.25-0.4 mg/kg per day) of oral isotretinoin found greater differences in mean decreased numbers of inflammatory lesions among participants in the continuous low dose (a decrease of 3.72 lesions; 95% CI, 2.13-5.31) and continuous conventional dose (a decrease of 3.87 lesions; 95% CI, 2.31-5.43) groups. The authors noted they were unable to perform a meta-analysis because of study heterogeneity in the three studies examining the primary outcome.
In 14 studies with a total of 906 participants who had moderate and severe acne, the authors noted there were no severe adverse events with different doses and regimens of isotretinoin treatment from 12 weeks to 32 weeks or at follow-up at the end of treatment at up to 48 weeks. There were some adverse events, such as skin dryness, hair loss, and itching in 13 studies with a total of 858 participants.
No birth defects were reported in the studies.
The authors took special note of the low-quality evidence in the review, and said that future studies should include larger sample sizes to observe more rare adverse events, as well as create subgroups with results based on acne severity, longer duration and follow-up, standardization of primary outcomes, and adherence to the 2010 CONSORT statement for reporting parallel-group randomized trials.
“With the aim of providing reliable physician guidelines and a robust evidence-based support for daily clinical practice in acne therapy, future randomized clinical trials on oral isotretinoin for acne should focus on treatment of acne when there is insufficient response to therapy with oral antibiotics plus topical agents,” Dr. Costa and her colleagues wrote.
Dr. Costa reports receiving a grant from the Brazilian government granting agency Coordenação de Aperfeicoamento de Pessoal de Nivel Superior. Another author reported a board membership with Bayer for studies and research and is a paid lecturer for a continuing medical education program on adult female acne. The remaining five authors reported no relevant conflicts of interest.
SOURCE: Costa CS et al. Cochrane Database Syst Rev. 2018. doi: 10.1002/14651858.CD009435.pub2.
I think dermatologists would agree with the assertions of the Cochrane Review that there is limited evidence according to today’s standards of quality evidence. The Cochrane Review process examines available data, ranking it by the quality of the data. The paper by Costa et al. shows where the Cochrane Review process is fatally flawed. It assumes that, where the highest quality data is lacking, the medication in question must be ineffective or that this at least casts doubt on its efficacy.
Isotretinoin was approved for use in acne in the 1980s. If you look at one initial report by Peck et al. examining the efficacy of isotretinoin versus placebo in patients with very severe nodulocystic acne, the isotretinoin group was so effective that half of the patients in the placebo arm broke code because of a 57% worsening of their acne and went to the treatment arm. Overall, there was a 95% improvement in the isotretinoin-treated group at the end of treatment (J Am Acad Dermatol. 1982 Apr;6[4 Pt 2 Suppl]:735-45). This study truly reflects how effective isotretinoin is in the treatment of severe acne, and any dermatologist who uses isotretinoin knows that for a fact. I suspect that, if someone applied the Cochrane Review process to insulin, they also would question its efficacy.
I agree there are questionable data on high versus standard versus low dosing. For most patients, standard dosing of 1.0 mg/kg per day over 20-24 weeks will result in complete and sustained clearance. There are always outliers, however, and alternative dosing may be needed – either higher cumulative dosing to achieve clearance or longer lower dosing to manage side effects.
The authors clearly have limited experience in treating acne, or the aim of the article was simply a review of available data with no consideration of actual clinical usage. However, if patients see this report, it could undermine dermatologists’ efforts to care for their patients with acne. It is rather irresponsible to publish these types of reviews, in which the efficacy of the medication in question was established long ago and is clearly without question, and it really discredits the integrity of the Cochrane Review.
Andrea L. Zaenglein, MD , is professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey, Penn., and is a cochair of the American Academy of Dermatology’s most recent acne management guidelines ( J Am Acad Dermatol. 2016 May;74[5]:945-73.e33 ). She reports being a consultant for Sun Pharmaceutical Industries.
I think dermatologists would agree with the assertions of the Cochrane Review that there is limited evidence according to today’s standards of quality evidence. The Cochrane Review process examines available data, ranking it by the quality of the data. The paper by Costa et al. shows where the Cochrane Review process is fatally flawed. It assumes that, where the highest quality data is lacking, the medication in question must be ineffective or that this at least casts doubt on its efficacy.
Isotretinoin was approved for use in acne in the 1980s. If you look at one initial report by Peck et al. examining the efficacy of isotretinoin versus placebo in patients with very severe nodulocystic acne, the isotretinoin group was so effective that half of the patients in the placebo arm broke code because of a 57% worsening of their acne and went to the treatment arm. Overall, there was a 95% improvement in the isotretinoin-treated group at the end of treatment (J Am Acad Dermatol. 1982 Apr;6[4 Pt 2 Suppl]:735-45). This study truly reflects how effective isotretinoin is in the treatment of severe acne, and any dermatologist who uses isotretinoin knows that for a fact. I suspect that, if someone applied the Cochrane Review process to insulin, they also would question its efficacy.
I agree there are questionable data on high versus standard versus low dosing. For most patients, standard dosing of 1.0 mg/kg per day over 20-24 weeks will result in complete and sustained clearance. There are always outliers, however, and alternative dosing may be needed – either higher cumulative dosing to achieve clearance or longer lower dosing to manage side effects.
The authors clearly have limited experience in treating acne, or the aim of the article was simply a review of available data with no consideration of actual clinical usage. However, if patients see this report, it could undermine dermatologists’ efforts to care for their patients with acne. It is rather irresponsible to publish these types of reviews, in which the efficacy of the medication in question was established long ago and is clearly without question, and it really discredits the integrity of the Cochrane Review.
Andrea L. Zaenglein, MD , is professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey, Penn., and is a cochair of the American Academy of Dermatology’s most recent acne management guidelines ( J Am Acad Dermatol. 2016 May;74[5]:945-73.e33 ). She reports being a consultant for Sun Pharmaceutical Industries.
I think dermatologists would agree with the assertions of the Cochrane Review that there is limited evidence according to today’s standards of quality evidence. The Cochrane Review process examines available data, ranking it by the quality of the data. The paper by Costa et al. shows where the Cochrane Review process is fatally flawed. It assumes that, where the highest quality data is lacking, the medication in question must be ineffective or that this at least casts doubt on its efficacy.
Isotretinoin was approved for use in acne in the 1980s. If you look at one initial report by Peck et al. examining the efficacy of isotretinoin versus placebo in patients with very severe nodulocystic acne, the isotretinoin group was so effective that half of the patients in the placebo arm broke code because of a 57% worsening of their acne and went to the treatment arm. Overall, there was a 95% improvement in the isotretinoin-treated group at the end of treatment (J Am Acad Dermatol. 1982 Apr;6[4 Pt 2 Suppl]:735-45). This study truly reflects how effective isotretinoin is in the treatment of severe acne, and any dermatologist who uses isotretinoin knows that for a fact. I suspect that, if someone applied the Cochrane Review process to insulin, they also would question its efficacy.
I agree there are questionable data on high versus standard versus low dosing. For most patients, standard dosing of 1.0 mg/kg per day over 20-24 weeks will result in complete and sustained clearance. There are always outliers, however, and alternative dosing may be needed – either higher cumulative dosing to achieve clearance or longer lower dosing to manage side effects.
The authors clearly have limited experience in treating acne, or the aim of the article was simply a review of available data with no consideration of actual clinical usage. However, if patients see this report, it could undermine dermatologists’ efforts to care for their patients with acne. It is rather irresponsible to publish these types of reviews, in which the efficacy of the medication in question was established long ago and is clearly without question, and it really discredits the integrity of the Cochrane Review.
Andrea L. Zaenglein, MD , is professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey, Penn., and is a cochair of the American Academy of Dermatology’s most recent acne management guidelines ( J Am Acad Dermatol. 2016 May;74[5]:945-73.e33 ). She reports being a consultant for Sun Pharmaceutical Industries.
There is when based on reductions in inflammatory lesion counts, according to a recent Cochrane Review.
“The current recommendation of clinical guidelines that oral isotretinoin should be the first-line treatment for moderate to severe acne unresponsive to previous therapies, being more effective than the use of oral antibiotics plus topical agents, underpins current dermatological practice,” Caroline S. Costa, MD, from the Emergency Medicine and Evidence-Based Medicine department at Universidade Federal de São Paulo and her colleagues wrote in their review. “The recommendation cannot be fully supported with certainty by the finding of this review; however, neither does this review challenge it.”
Dr. Costa and her colleagues identified 31 randomized, controlled trials in the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, and LILACS databases up to July 2017, which altogether included 3,836 participants with mild to severe acne who were aged 12-55 years. The patients received treatment with oral isotretinoin versus placebo or other treatments, such as antibiotics.
In 3 studies that altogether included 400 participants and compared isotretinoin with oral antibiotics plus topical treatments, there was no significant difference with isotretinoin in decreases in the investigator-assessed inflammatory lesion count in participants with moderate to severe acne after 20-24 weeks (risk ratio, 1.01; 95% confidence interval, 0.96-1.06). One serious side effect – Stevens-Johnson syndrome – was noted in the isotretinoin-treated group (RR, 3.00; 95% CI, 0.12-72.98). These results were based on “very-low-quality evidence,” the authors noted.
Two studies also comparing isotretinoin with oral antibiotics and topical treatments in a total of 351 participants showed a 15% improvement in acne severity (RR, 1.15; 95% CI, 1.00-1.32) as assessed by physician’s global evaluation but with a greater number of adverse events considered less severe (RR, 1.67; 95% CI, 1.42-1.98), such as vomiting, nausea, dry lips and skin, and cheilitis.
With regard to dosing, one study with 154 participants with severe acne showed 0.05 mg/kg per day of isotretinoin reduced inflammatory lesion count by 79% after 20 weeks, while 0.1 mg/kg per day and 0.2 mg/kg per day reduced inflammatory lesion counts by 80% and 84%, respectively, after 20 weeks. A different study of 150 participants with severe acne found a 95% decrease in inflammatory lesion counts for 58% of participants receiving 0.1 mg/kg per day, 80% of participants receiving 0.5 mg/kg per day, and 90% of participants receiving 1 mg/kg per day of oral isotretinoin after 20 weeks.
In a randomized, controlled trial with 40 participants with moderate acne comparing intermittent dosing (0.5-0.7 mg/kg per day for 1 week per month), continuous conventional dosing (0.5-0.7 mg/kg per day), and continuous low dosing (0.25-0.4 mg/kg per day) of oral isotretinoin found greater differences in mean decreased numbers of inflammatory lesions among participants in the continuous low dose (a decrease of 3.72 lesions; 95% CI, 2.13-5.31) and continuous conventional dose (a decrease of 3.87 lesions; 95% CI, 2.31-5.43) groups. The authors noted they were unable to perform a meta-analysis because of study heterogeneity in the three studies examining the primary outcome.
In 14 studies with a total of 906 participants who had moderate and severe acne, the authors noted there were no severe adverse events with different doses and regimens of isotretinoin treatment from 12 weeks to 32 weeks or at follow-up at the end of treatment at up to 48 weeks. There were some adverse events, such as skin dryness, hair loss, and itching in 13 studies with a total of 858 participants.
No birth defects were reported in the studies.
The authors took special note of the low-quality evidence in the review, and said that future studies should include larger sample sizes to observe more rare adverse events, as well as create subgroups with results based on acne severity, longer duration and follow-up, standardization of primary outcomes, and adherence to the 2010 CONSORT statement for reporting parallel-group randomized trials.
“With the aim of providing reliable physician guidelines and a robust evidence-based support for daily clinical practice in acne therapy, future randomized clinical trials on oral isotretinoin for acne should focus on treatment of acne when there is insufficient response to therapy with oral antibiotics plus topical agents,” Dr. Costa and her colleagues wrote.
Dr. Costa reports receiving a grant from the Brazilian government granting agency Coordenação de Aperfeicoamento de Pessoal de Nivel Superior. Another author reported a board membership with Bayer for studies and research and is a paid lecturer for a continuing medical education program on adult female acne. The remaining five authors reported no relevant conflicts of interest.
SOURCE: Costa CS et al. Cochrane Database Syst Rev. 2018. doi: 10.1002/14651858.CD009435.pub2.
There is when based on reductions in inflammatory lesion counts, according to a recent Cochrane Review.
“The current recommendation of clinical guidelines that oral isotretinoin should be the first-line treatment for moderate to severe acne unresponsive to previous therapies, being more effective than the use of oral antibiotics plus topical agents, underpins current dermatological practice,” Caroline S. Costa, MD, from the Emergency Medicine and Evidence-Based Medicine department at Universidade Federal de São Paulo and her colleagues wrote in their review. “The recommendation cannot be fully supported with certainty by the finding of this review; however, neither does this review challenge it.”
Dr. Costa and her colleagues identified 31 randomized, controlled trials in the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, and LILACS databases up to July 2017, which altogether included 3,836 participants with mild to severe acne who were aged 12-55 years. The patients received treatment with oral isotretinoin versus placebo or other treatments, such as antibiotics.
In 3 studies that altogether included 400 participants and compared isotretinoin with oral antibiotics plus topical treatments, there was no significant difference with isotretinoin in decreases in the investigator-assessed inflammatory lesion count in participants with moderate to severe acne after 20-24 weeks (risk ratio, 1.01; 95% confidence interval, 0.96-1.06). One serious side effect – Stevens-Johnson syndrome – was noted in the isotretinoin-treated group (RR, 3.00; 95% CI, 0.12-72.98). These results were based on “very-low-quality evidence,” the authors noted.
Two studies also comparing isotretinoin with oral antibiotics and topical treatments in a total of 351 participants showed a 15% improvement in acne severity (RR, 1.15; 95% CI, 1.00-1.32) as assessed by physician’s global evaluation but with a greater number of adverse events considered less severe (RR, 1.67; 95% CI, 1.42-1.98), such as vomiting, nausea, dry lips and skin, and cheilitis.
With regard to dosing, one study with 154 participants with severe acne showed 0.05 mg/kg per day of isotretinoin reduced inflammatory lesion count by 79% after 20 weeks, while 0.1 mg/kg per day and 0.2 mg/kg per day reduced inflammatory lesion counts by 80% and 84%, respectively, after 20 weeks. A different study of 150 participants with severe acne found a 95% decrease in inflammatory lesion counts for 58% of participants receiving 0.1 mg/kg per day, 80% of participants receiving 0.5 mg/kg per day, and 90% of participants receiving 1 mg/kg per day of oral isotretinoin after 20 weeks.
In a randomized, controlled trial with 40 participants with moderate acne comparing intermittent dosing (0.5-0.7 mg/kg per day for 1 week per month), continuous conventional dosing (0.5-0.7 mg/kg per day), and continuous low dosing (0.25-0.4 mg/kg per day) of oral isotretinoin found greater differences in mean decreased numbers of inflammatory lesions among participants in the continuous low dose (a decrease of 3.72 lesions; 95% CI, 2.13-5.31) and continuous conventional dose (a decrease of 3.87 lesions; 95% CI, 2.31-5.43) groups. The authors noted they were unable to perform a meta-analysis because of study heterogeneity in the three studies examining the primary outcome.
In 14 studies with a total of 906 participants who had moderate and severe acne, the authors noted there were no severe adverse events with different doses and regimens of isotretinoin treatment from 12 weeks to 32 weeks or at follow-up at the end of treatment at up to 48 weeks. There were some adverse events, such as skin dryness, hair loss, and itching in 13 studies with a total of 858 participants.
No birth defects were reported in the studies.
The authors took special note of the low-quality evidence in the review, and said that future studies should include larger sample sizes to observe more rare adverse events, as well as create subgroups with results based on acne severity, longer duration and follow-up, standardization of primary outcomes, and adherence to the 2010 CONSORT statement for reporting parallel-group randomized trials.
“With the aim of providing reliable physician guidelines and a robust evidence-based support for daily clinical practice in acne therapy, future randomized clinical trials on oral isotretinoin for acne should focus on treatment of acne when there is insufficient response to therapy with oral antibiotics plus topical agents,” Dr. Costa and her colleagues wrote.
Dr. Costa reports receiving a grant from the Brazilian government granting agency Coordenação de Aperfeicoamento de Pessoal de Nivel Superior. Another author reported a board membership with Bayer for studies and research and is a paid lecturer for a continuing medical education program on adult female acne. The remaining five authors reported no relevant conflicts of interest.
SOURCE: Costa CS et al. Cochrane Database Syst Rev. 2018. doi: 10.1002/14651858.CD009435.pub2.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point: A Cochrane Review found low quality of evidence surrounding oral isotretinoin’s effectiveness for acne.
Major finding: Isotretinoin did not decrease inflammatory lesion count, compared with (risk ratio, 1.01; 95% confidence interval, 0.96-1.06), in 3 studies, and there was low evidence to suggest daily treatment was more effective than treatment for 1 week per month.
Study details: A Cochrane Review of 31 studies that altogether included 3,836 patients with mild to severe acne who received oral isotretinoin, placebo, or other treatments such as antibiotics.
Disclosures: Dr. Costa reported receiving a grant from the Brazilian government granting agency Coordenação de Aperfeicoamento de Pessoal de Nivel Superior. Dr. Bagatin reported a board membership with Bayer for studies and research and is a paid lecturer for a continuing medical education program on adult female acne. The other authors report no relevant conflicts of interest.
Source: Costa CS et al. Cochrane Database Syst Rev. 2018. doi: 10.1002/14651858.CD009435.pub2.
Atopic dermatitis associated with increased suicidality
Patients with atopic dermatitis might face up to a 44% increased risk of suicidal ideation and are 36% more likely to attempt suicide than those without the disorder, a large meta-analysis has determined.
The analysis, which included data from studies published as far back as 1945, also found some correlation of increased suicide risk and increasing disease severity, although the numbers were small, Jeena K. Sandhu and her colleagues reported in JAMA Dermatology.
Both physical and psychological factors could be involved in the link, wrote Ms. Sandhu, a medical student at the University of Missouri–Kansas City, and her coauthors.
“Atopic dermatitis is associated with multiple physical comorbidities, such as asthma, allergic rhinitis, metabolic syndrome, and sleep disturbances, which all contribute to the overall physical burden of the disease. Many patients also have a profound psychosocial burden. Because of the visibility of the disease, patients may experience shame, embarrassment, and stigmatization,” they wrote.
But the disease also is associated with high levels of proinflammatory cytokines, and those proteins have been isolated in the cerebrospinal fluid of patients who have attempted suicide, the investigators noted. “Treatments targeting cytokines, such as interleukin-4 and interleukin-13, have been shown to decrease symptoms of depression and anxiety in patients with atopic dermatitis.”
The investigators plumbed several databases of medical literature, searching for studies that mentioned both atopic dermatitis (AD) and suicide, suicidal ideation, or suicidal behavior. They found 15 studies, published from 1945 to May 2018. Most (13) were cross sectional; the remainder were cohort studies. Together, they comprised a total of 4.7 million subjects, 310,681 of whom had AD. The analysis looked at risks in three areas: suicidal ideation, suicide attempts, and completed suicides.
Of the studies, 11 investigated suicidal ideation. Pooled data determined that patients with AD were a significant 44% more likely to experience suicidal ideation than those without the disease.
Three studies mentioned suicide attempts and had complete data for pooling. Taken together, they showed a significant 36% increased risk of attempted suicide among patients with AD, compared with those without the disorder.
Two studies investigated the prevalence of completed suicides among patients. One did report a significantly increased risk of 40%, compared with the control group, but it failed to report the number of suicides in the control group. The other study found no increased risk of completed suicides in patients with either mild or moderate to severe disease, compared with controls.
Two studies involved only pediatric patients. One, conducted in Korea, found a significant 23% increased risk of suicidal ideation and a 31% increased risk of attempted suicide. The other failed to find any increased risks in the overall analysis, but did find small increases in the risks of ideation and attempt in girls with AD, compared with healthy controls.
the team concluded. “Dermatology providers may use several tools to screen patients for suicidality. Asking patients about suicidal ideation with a question may be integrated into a patient visit. If a patient screens positive for suicidality, the dermatology provider should send a referral to the patient’s primary care or mental health provider for follow-up care. If the patient reports an orchestrated plan to commit suicide, this patient should be urgently referred to the emergency department for further assessment.”
Ms. Sandhu reported no financial disclosures.
SOURCE: Sandhu JK et al. JAMA Dermatol. 2018 Dec 12. doi: 10.1001/jamadermatol.2018.4566.
Patients with atopic dermatitis might face up to a 44% increased risk of suicidal ideation and are 36% more likely to attempt suicide than those without the disorder, a large meta-analysis has determined.
The analysis, which included data from studies published as far back as 1945, also found some correlation of increased suicide risk and increasing disease severity, although the numbers were small, Jeena K. Sandhu and her colleagues reported in JAMA Dermatology.
Both physical and psychological factors could be involved in the link, wrote Ms. Sandhu, a medical student at the University of Missouri–Kansas City, and her coauthors.
“Atopic dermatitis is associated with multiple physical comorbidities, such as asthma, allergic rhinitis, metabolic syndrome, and sleep disturbances, which all contribute to the overall physical burden of the disease. Many patients also have a profound psychosocial burden. Because of the visibility of the disease, patients may experience shame, embarrassment, and stigmatization,” they wrote.
But the disease also is associated with high levels of proinflammatory cytokines, and those proteins have been isolated in the cerebrospinal fluid of patients who have attempted suicide, the investigators noted. “Treatments targeting cytokines, such as interleukin-4 and interleukin-13, have been shown to decrease symptoms of depression and anxiety in patients with atopic dermatitis.”
The investigators plumbed several databases of medical literature, searching for studies that mentioned both atopic dermatitis (AD) and suicide, suicidal ideation, or suicidal behavior. They found 15 studies, published from 1945 to May 2018. Most (13) were cross sectional; the remainder were cohort studies. Together, they comprised a total of 4.7 million subjects, 310,681 of whom had AD. The analysis looked at risks in three areas: suicidal ideation, suicide attempts, and completed suicides.
Of the studies, 11 investigated suicidal ideation. Pooled data determined that patients with AD were a significant 44% more likely to experience suicidal ideation than those without the disease.
Three studies mentioned suicide attempts and had complete data for pooling. Taken together, they showed a significant 36% increased risk of attempted suicide among patients with AD, compared with those without the disorder.
Two studies investigated the prevalence of completed suicides among patients. One did report a significantly increased risk of 40%, compared with the control group, but it failed to report the number of suicides in the control group. The other study found no increased risk of completed suicides in patients with either mild or moderate to severe disease, compared with controls.
Two studies involved only pediatric patients. One, conducted in Korea, found a significant 23% increased risk of suicidal ideation and a 31% increased risk of attempted suicide. The other failed to find any increased risks in the overall analysis, but did find small increases in the risks of ideation and attempt in girls with AD, compared with healthy controls.
the team concluded. “Dermatology providers may use several tools to screen patients for suicidality. Asking patients about suicidal ideation with a question may be integrated into a patient visit. If a patient screens positive for suicidality, the dermatology provider should send a referral to the patient’s primary care or mental health provider for follow-up care. If the patient reports an orchestrated plan to commit suicide, this patient should be urgently referred to the emergency department for further assessment.”
Ms. Sandhu reported no financial disclosures.
SOURCE: Sandhu JK et al. JAMA Dermatol. 2018 Dec 12. doi: 10.1001/jamadermatol.2018.4566.
Patients with atopic dermatitis might face up to a 44% increased risk of suicidal ideation and are 36% more likely to attempt suicide than those without the disorder, a large meta-analysis has determined.
The analysis, which included data from studies published as far back as 1945, also found some correlation of increased suicide risk and increasing disease severity, although the numbers were small, Jeena K. Sandhu and her colleagues reported in JAMA Dermatology.
Both physical and psychological factors could be involved in the link, wrote Ms. Sandhu, a medical student at the University of Missouri–Kansas City, and her coauthors.
“Atopic dermatitis is associated with multiple physical comorbidities, such as asthma, allergic rhinitis, metabolic syndrome, and sleep disturbances, which all contribute to the overall physical burden of the disease. Many patients also have a profound psychosocial burden. Because of the visibility of the disease, patients may experience shame, embarrassment, and stigmatization,” they wrote.
But the disease also is associated with high levels of proinflammatory cytokines, and those proteins have been isolated in the cerebrospinal fluid of patients who have attempted suicide, the investigators noted. “Treatments targeting cytokines, such as interleukin-4 and interleukin-13, have been shown to decrease symptoms of depression and anxiety in patients with atopic dermatitis.”
The investigators plumbed several databases of medical literature, searching for studies that mentioned both atopic dermatitis (AD) and suicide, suicidal ideation, or suicidal behavior. They found 15 studies, published from 1945 to May 2018. Most (13) were cross sectional; the remainder were cohort studies. Together, they comprised a total of 4.7 million subjects, 310,681 of whom had AD. The analysis looked at risks in three areas: suicidal ideation, suicide attempts, and completed suicides.
Of the studies, 11 investigated suicidal ideation. Pooled data determined that patients with AD were a significant 44% more likely to experience suicidal ideation than those without the disease.
Three studies mentioned suicide attempts and had complete data for pooling. Taken together, they showed a significant 36% increased risk of attempted suicide among patients with AD, compared with those without the disorder.
Two studies investigated the prevalence of completed suicides among patients. One did report a significantly increased risk of 40%, compared with the control group, but it failed to report the number of suicides in the control group. The other study found no increased risk of completed suicides in patients with either mild or moderate to severe disease, compared with controls.
Two studies involved only pediatric patients. One, conducted in Korea, found a significant 23% increased risk of suicidal ideation and a 31% increased risk of attempted suicide. The other failed to find any increased risks in the overall analysis, but did find small increases in the risks of ideation and attempt in girls with AD, compared with healthy controls.
the team concluded. “Dermatology providers may use several tools to screen patients for suicidality. Asking patients about suicidal ideation with a question may be integrated into a patient visit. If a patient screens positive for suicidality, the dermatology provider should send a referral to the patient’s primary care or mental health provider for follow-up care. If the patient reports an orchestrated plan to commit suicide, this patient should be urgently referred to the emergency department for further assessment.”
Ms. Sandhu reported no financial disclosures.
SOURCE: Sandhu JK et al. JAMA Dermatol. 2018 Dec 12. doi: 10.1001/jamadermatol.2018.4566.
FROM JAMA DERMATOLOGY
Key clinical point: Suicidal ideation and suicide attempts seem to be more common among people with atopic dermatitis than those without the disease.
Major finding: Patients were 44% more likely to have suicidal ideation and 36% more likely to attempt suicide.
Study details: The meta-analysis comprised 15 studies with a total of 4.7 million participants, 310,681 of whom had the disease.
Disclosures: Ms. Sandhu reported no financial disclosures.
Source: Sandhu JK et al. JAMA Dermatol. 2018 Dec 12. doi: 10.1001/jamadermatol.2018.4566.
FDA reclassifies ECT devices for resistant depression, other conditions
The Food and Drug Administration issued a final order Dec. 21 reclassifying electroconvulsive therapy (ECT) devices from class III, indicating higher risk, to class II, indicating moderate risk, in certain cases.
Conditions included in the new order are catatonia or a severe major depressive episode associated with major depressive disorder or bipolar disorder in patients over the age of 13 years who are resistant to treatment or who require a rapid response because of the severity of their psychiatric or medical condition, according to an FDA press release.
In addition, the final order requires the filing of premarket approval application for class III devices used for all conditions not reclassified as class II.
“The FDA is issuing this final order to regulate ECT devices in a way that appropriately reflects the known benefits and risks of these devices for their indications for use, provides patients with additional protections, and gives physicians more information on the safe and effective use of these devices,” the agency said in the press release.
The Food and Drug Administration issued a final order Dec. 21 reclassifying electroconvulsive therapy (ECT) devices from class III, indicating higher risk, to class II, indicating moderate risk, in certain cases.
Conditions included in the new order are catatonia or a severe major depressive episode associated with major depressive disorder or bipolar disorder in patients over the age of 13 years who are resistant to treatment or who require a rapid response because of the severity of their psychiatric or medical condition, according to an FDA press release.
In addition, the final order requires the filing of premarket approval application for class III devices used for all conditions not reclassified as class II.
“The FDA is issuing this final order to regulate ECT devices in a way that appropriately reflects the known benefits and risks of these devices for their indications for use, provides patients with additional protections, and gives physicians more information on the safe and effective use of these devices,” the agency said in the press release.
The Food and Drug Administration issued a final order Dec. 21 reclassifying electroconvulsive therapy (ECT) devices from class III, indicating higher risk, to class II, indicating moderate risk, in certain cases.
Conditions included in the new order are catatonia or a severe major depressive episode associated with major depressive disorder or bipolar disorder in patients over the age of 13 years who are resistant to treatment or who require a rapid response because of the severity of their psychiatric or medical condition, according to an FDA press release.
In addition, the final order requires the filing of premarket approval application for class III devices used for all conditions not reclassified as class II.
“The FDA is issuing this final order to regulate ECT devices in a way that appropriately reflects the known benefits and risks of these devices for their indications for use, provides patients with additional protections, and gives physicians more information on the safe and effective use of these devices,” the agency said in the press release.
Blanchable Erythematous Patches on the Fingers
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
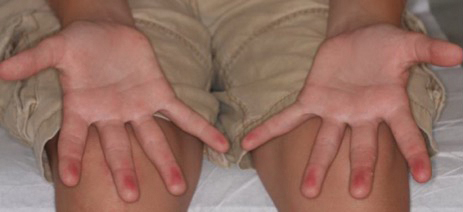
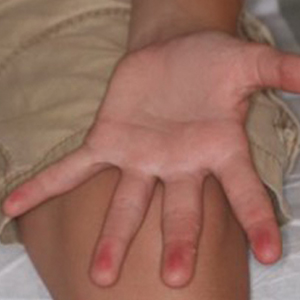
A 12-year-old boy presented with well-defined, blanchable, erythematous patches on the distal bilateral palmar aspects of the second to fifth fingers of 2 years’ duration. The patient stated that he skateboarded daily throughout the year and swam once weekly in the summer months. Furthermore, the patient cited frequent contact with the rough undersurface of the skateboard and concrete road surfaces while skateboarding. He stated that the lesions were always present and worsened in the summer months. The lesions had an occasional burning sensation when they were more prominently erythematous, and the patient denied any pattern of exacerbation, numbness, bleeding, or itching. There was no notable family history or evidence of systemic disease.
FDA approves Elzonris for blastic plasmacytoid dendritic cell neoplasm
The Food and Drug Administration has approved tagraxofusp-erzs (Elzonris) infusion for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and pediatric patients, 2 years of age and older.
Approval was based on efficacy in two cohorts of patients in a single-arm clinical trial. Seven patients (54%) out of 13 with untreated BPDCN achieved complete remission (CR) or CR with a skin abnormality not indicative of active disease (CRc) in the first cohort. In the second cohort of 15 patients, one patient achieved CR and one patient achieved CRc.
Common side effects for patients receiving tagraxofusp-erzs infusion were capillary leak syndrome, nausea, fatigue, peripheral edema, pyrexia, chills, and weight increase. Most common laboratory abnormalities were decreases in lymphocytes, albumin, platelets, hemoglobin, and calcium, and increases in glucose and liver enzymes (ALT and AST), the FDA said in a press statement.
The FDA placed a Boxed Warning on the drug to alert health care professionals and patients about the increased risk of capillary leak syndrome and recommends that health care providers monitor liver enzyme levels for signs of intolerance to the infusion.
BPDCN is an aggressive and rare disease of the bone marrow and blood that can affect multiple organs, including the lymph nodes and the skin. It often presents as leukemia or evolves into acute leukemia, the FDA said.
“Prior to today’s approval, there had been no FDA approved therapies for BPDCN. The standard of care has been intensive chemotherapy followed by bone marrow transplantation. Many patients with BPDCN are unable to tolerate this intensive therapy, so there is an urgent need for alternative treatment options,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
The Food and Drug Administration has approved tagraxofusp-erzs (Elzonris) infusion for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and pediatric patients, 2 years of age and older.
Approval was based on efficacy in two cohorts of patients in a single-arm clinical trial. Seven patients (54%) out of 13 with untreated BPDCN achieved complete remission (CR) or CR with a skin abnormality not indicative of active disease (CRc) in the first cohort. In the second cohort of 15 patients, one patient achieved CR and one patient achieved CRc.
Common side effects for patients receiving tagraxofusp-erzs infusion were capillary leak syndrome, nausea, fatigue, peripheral edema, pyrexia, chills, and weight increase. Most common laboratory abnormalities were decreases in lymphocytes, albumin, platelets, hemoglobin, and calcium, and increases in glucose and liver enzymes (ALT and AST), the FDA said in a press statement.
The FDA placed a Boxed Warning on the drug to alert health care professionals and patients about the increased risk of capillary leak syndrome and recommends that health care providers monitor liver enzyme levels for signs of intolerance to the infusion.
BPDCN is an aggressive and rare disease of the bone marrow and blood that can affect multiple organs, including the lymph nodes and the skin. It often presents as leukemia or evolves into acute leukemia, the FDA said.
“Prior to today’s approval, there had been no FDA approved therapies for BPDCN. The standard of care has been intensive chemotherapy followed by bone marrow transplantation. Many patients with BPDCN are unable to tolerate this intensive therapy, so there is an urgent need for alternative treatment options,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
The Food and Drug Administration has approved tagraxofusp-erzs (Elzonris) infusion for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and pediatric patients, 2 years of age and older.
Approval was based on efficacy in two cohorts of patients in a single-arm clinical trial. Seven patients (54%) out of 13 with untreated BPDCN achieved complete remission (CR) or CR with a skin abnormality not indicative of active disease (CRc) in the first cohort. In the second cohort of 15 patients, one patient achieved CR and one patient achieved CRc.
Common side effects for patients receiving tagraxofusp-erzs infusion were capillary leak syndrome, nausea, fatigue, peripheral edema, pyrexia, chills, and weight increase. Most common laboratory abnormalities were decreases in lymphocytes, albumin, platelets, hemoglobin, and calcium, and increases in glucose and liver enzymes (ALT and AST), the FDA said in a press statement.
The FDA placed a Boxed Warning on the drug to alert health care professionals and patients about the increased risk of capillary leak syndrome and recommends that health care providers monitor liver enzyme levels for signs of intolerance to the infusion.
BPDCN is an aggressive and rare disease of the bone marrow and blood that can affect multiple organs, including the lymph nodes and the skin. It often presents as leukemia or evolves into acute leukemia, the FDA said.
“Prior to today’s approval, there had been no FDA approved therapies for BPDCN. The standard of care has been intensive chemotherapy followed by bone marrow transplantation. Many patients with BPDCN are unable to tolerate this intensive therapy, so there is an urgent need for alternative treatment options,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
FDA approves olaparib for maintenance treatment of BRCA-mutated advanced ovarian cancer
The Food and Drug Administration has approved olaparib for the maintenance treatment of adult patients with deleterious or suspected deleterious germline or somatic BRCA-mutated (gBRCAm or sBRCAm) advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to first-line, platinum-based chemotherapy.
The FDA also approved the BRACAnalysis CDx test (Myriad Genetic Laboratories) to identify patients who are eligible for olaparib.
Approval of olaparib was based on improvement in progression-free survival (PFS) in the phase 3 SOLO-1 trial of 391 women with BRCA-mutated advanced ovarian, fallopian tube, or primary peritoneal cancer following first-line, platinum-based chemotherapy. Patients were randomized (2:1) to receive olaparib tablets 300 mg orally twice daily or placebo.
Estimated median investigator-assessed PFS was not reached in the olaparib arm and was 13.8 months in the placebo arm (hazard ratio, 0.30; 95% confidence interval, 0.23-0.41; P less than .0001). Overall survival data are not yet mature.
The most common adverse reactions in women who received olaparib in SOLO-1 were nausea, fatigue, abdominal pain, vomiting, anemia, diarrhea, upper respiratory tract infection/influenza/nasopharyngitis/bronchitis, constipation, dysgeusia, decreased appetite, dizziness, neutropenia, dyspepsia, dyspnea, urinary tract infection, leukopenia, thrombocytopenia, and stomatitis.
The recommended olaparib dose is 300 mg (two 150 mg tablets) taken orally twice daily, with or without food, for a total daily dose of 600 mg, the FDA said in a press statement.
Olaparib is marketed as Lynparza by AstraZeneca Pharmaceuticals.
The Food and Drug Administration has approved olaparib for the maintenance treatment of adult patients with deleterious or suspected deleterious germline or somatic BRCA-mutated (gBRCAm or sBRCAm) advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to first-line, platinum-based chemotherapy.
The FDA also approved the BRACAnalysis CDx test (Myriad Genetic Laboratories) to identify patients who are eligible for olaparib.
Approval of olaparib was based on improvement in progression-free survival (PFS) in the phase 3 SOLO-1 trial of 391 women with BRCA-mutated advanced ovarian, fallopian tube, or primary peritoneal cancer following first-line, platinum-based chemotherapy. Patients were randomized (2:1) to receive olaparib tablets 300 mg orally twice daily or placebo.
Estimated median investigator-assessed PFS was not reached in the olaparib arm and was 13.8 months in the placebo arm (hazard ratio, 0.30; 95% confidence interval, 0.23-0.41; P less than .0001). Overall survival data are not yet mature.
The most common adverse reactions in women who received olaparib in SOLO-1 were nausea, fatigue, abdominal pain, vomiting, anemia, diarrhea, upper respiratory tract infection/influenza/nasopharyngitis/bronchitis, constipation, dysgeusia, decreased appetite, dizziness, neutropenia, dyspepsia, dyspnea, urinary tract infection, leukopenia, thrombocytopenia, and stomatitis.
The recommended olaparib dose is 300 mg (two 150 mg tablets) taken orally twice daily, with or without food, for a total daily dose of 600 mg, the FDA said in a press statement.
Olaparib is marketed as Lynparza by AstraZeneca Pharmaceuticals.
The Food and Drug Administration has approved olaparib for the maintenance treatment of adult patients with deleterious or suspected deleterious germline or somatic BRCA-mutated (gBRCAm or sBRCAm) advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to first-line, platinum-based chemotherapy.
The FDA also approved the BRACAnalysis CDx test (Myriad Genetic Laboratories) to identify patients who are eligible for olaparib.
Approval of olaparib was based on improvement in progression-free survival (PFS) in the phase 3 SOLO-1 trial of 391 women with BRCA-mutated advanced ovarian, fallopian tube, or primary peritoneal cancer following first-line, platinum-based chemotherapy. Patients were randomized (2:1) to receive olaparib tablets 300 mg orally twice daily or placebo.
Estimated median investigator-assessed PFS was not reached in the olaparib arm and was 13.8 months in the placebo arm (hazard ratio, 0.30; 95% confidence interval, 0.23-0.41; P less than .0001). Overall survival data are not yet mature.
The most common adverse reactions in women who received olaparib in SOLO-1 were nausea, fatigue, abdominal pain, vomiting, anemia, diarrhea, upper respiratory tract infection/influenza/nasopharyngitis/bronchitis, constipation, dysgeusia, decreased appetite, dizziness, neutropenia, dyspepsia, dyspnea, urinary tract infection, leukopenia, thrombocytopenia, and stomatitis.
The recommended olaparib dose is 300 mg (two 150 mg tablets) taken orally twice daily, with or without food, for a total daily dose of 600 mg, the FDA said in a press statement.
Olaparib is marketed as Lynparza by AstraZeneca Pharmaceuticals.
Frailty-adjusted treatment strategy emerges in myeloma
SAN DIEGO – Switching to lenalidomide maintenance after nine cycles of lenalidomide/dexamethasone (Rd) may avoid toxicity without sacrificing survival benefit in elderly multiple myeloma patients of intermediate fitness, results from a randomized trial showed.
The Rd-R strategy yielded a “slight improvement” in event-free survival due largely to fewer adverse events, and no significant differences in progression-free or overall survival versus continuous Rd, reported Alessandra Larocca, MD, of GIMEMA/European Myeloma Network in Italy.
That finding suggests the promise of adapting myeloma treatment to a patient’s level of frailty or fitness, as determined by a myeloma frailty score, Dr. Larocca said at the annual meeting of the American Society of Hematology.
“A frailty-adjusted treatment approach is important in intermediate-fit patients to balance efficacy and safety,” she said.
The frailty score, developed by the International Myeloma Working Group (IMWG), classifies individuals as fit, intermediate, or frail based on age, comorbidities, cognitive status, and functional status. In a 2015 report in Blood, the IMWG frailty score was shown to predict mortality and treatment-related toxicity in elderly myeloma patients.
Dr. Larocca described results of the RV-MM-PI-0752 phase 3 study, which enrolled 199 newly diagnosed myeloma patients of intermediate fitness and randomized them to continuous Rd or nine cycles of Rd induction followed by lenalidomide maintenance (Rd-R).
The goal was to see if Rd could be “further optimized” for elderly, intermediate-fit patients, Dr. Larocca said.
The primary endpoint of RV-MM-PI-0752 was event-free survival, which included grade 4 hematologic and grade 3-4 nonhematologic adverse events, lenalidomide discontinuation, disease progression, or death.
Median event-free survival was 9.3 months for the Rd-R strategy, compared with 6.6 months for continuous Rd (hazard ratio, 0.72; 95% confidence interval, 0.52-0.99; P = .044), Dr. Larocca reported.
No difference was seen in survival outcomes, she added. The 20-month progression-free survival was 43% and 42% for Rd-R and continuous Rd, respectively. The 20-month overall survival was 84% vs. 79%, with P values that were not significant for either comparison.
Patients in the Rd-R group had a somewhat higher incidence of grade 3 or greater neutropenia, but the continuous Rd group had a somewhat higher rate of nonhematologic adverse events, leading to slightly higher rates of lenalidomide discontinuation and dose reduction, Dr. Larocca said.
Overall, 9% of patients dropped out of the RV-MM-PI-0752 trial within the first 60 days, due mainly to toxicity, she added.
“We have to evaluate how to better prevent toxicity, potentially enabling patients to stay on therapy longer,” Dr. Larocca said. “Probably we have to evaluate, in prospective clinical trials, the role of up-front dose adjustment or dose reduction, and subsequent dose increase in a subgroup of patients.”
Dr. Larocca reported disclosures related to Celgene, Bristol-Myers Squibb, Janssen-Cilag, Takeda, and Amgen.
SOURCE: Larocca A, et al. ASH 2018, Abstract 305.
SAN DIEGO – Switching to lenalidomide maintenance after nine cycles of lenalidomide/dexamethasone (Rd) may avoid toxicity without sacrificing survival benefit in elderly multiple myeloma patients of intermediate fitness, results from a randomized trial showed.
The Rd-R strategy yielded a “slight improvement” in event-free survival due largely to fewer adverse events, and no significant differences in progression-free or overall survival versus continuous Rd, reported Alessandra Larocca, MD, of GIMEMA/European Myeloma Network in Italy.
That finding suggests the promise of adapting myeloma treatment to a patient’s level of frailty or fitness, as determined by a myeloma frailty score, Dr. Larocca said at the annual meeting of the American Society of Hematology.
“A frailty-adjusted treatment approach is important in intermediate-fit patients to balance efficacy and safety,” she said.
The frailty score, developed by the International Myeloma Working Group (IMWG), classifies individuals as fit, intermediate, or frail based on age, comorbidities, cognitive status, and functional status. In a 2015 report in Blood, the IMWG frailty score was shown to predict mortality and treatment-related toxicity in elderly myeloma patients.
Dr. Larocca described results of the RV-MM-PI-0752 phase 3 study, which enrolled 199 newly diagnosed myeloma patients of intermediate fitness and randomized them to continuous Rd or nine cycles of Rd induction followed by lenalidomide maintenance (Rd-R).
The goal was to see if Rd could be “further optimized” for elderly, intermediate-fit patients, Dr. Larocca said.
The primary endpoint of RV-MM-PI-0752 was event-free survival, which included grade 4 hematologic and grade 3-4 nonhematologic adverse events, lenalidomide discontinuation, disease progression, or death.
Median event-free survival was 9.3 months for the Rd-R strategy, compared with 6.6 months for continuous Rd (hazard ratio, 0.72; 95% confidence interval, 0.52-0.99; P = .044), Dr. Larocca reported.
No difference was seen in survival outcomes, she added. The 20-month progression-free survival was 43% and 42% for Rd-R and continuous Rd, respectively. The 20-month overall survival was 84% vs. 79%, with P values that were not significant for either comparison.
Patients in the Rd-R group had a somewhat higher incidence of grade 3 or greater neutropenia, but the continuous Rd group had a somewhat higher rate of nonhematologic adverse events, leading to slightly higher rates of lenalidomide discontinuation and dose reduction, Dr. Larocca said.
Overall, 9% of patients dropped out of the RV-MM-PI-0752 trial within the first 60 days, due mainly to toxicity, she added.
“We have to evaluate how to better prevent toxicity, potentially enabling patients to stay on therapy longer,” Dr. Larocca said. “Probably we have to evaluate, in prospective clinical trials, the role of up-front dose adjustment or dose reduction, and subsequent dose increase in a subgroup of patients.”
Dr. Larocca reported disclosures related to Celgene, Bristol-Myers Squibb, Janssen-Cilag, Takeda, and Amgen.
SOURCE: Larocca A, et al. ASH 2018, Abstract 305.
SAN DIEGO – Switching to lenalidomide maintenance after nine cycles of lenalidomide/dexamethasone (Rd) may avoid toxicity without sacrificing survival benefit in elderly multiple myeloma patients of intermediate fitness, results from a randomized trial showed.
The Rd-R strategy yielded a “slight improvement” in event-free survival due largely to fewer adverse events, and no significant differences in progression-free or overall survival versus continuous Rd, reported Alessandra Larocca, MD, of GIMEMA/European Myeloma Network in Italy.
That finding suggests the promise of adapting myeloma treatment to a patient’s level of frailty or fitness, as determined by a myeloma frailty score, Dr. Larocca said at the annual meeting of the American Society of Hematology.
“A frailty-adjusted treatment approach is important in intermediate-fit patients to balance efficacy and safety,” she said.
The frailty score, developed by the International Myeloma Working Group (IMWG), classifies individuals as fit, intermediate, or frail based on age, comorbidities, cognitive status, and functional status. In a 2015 report in Blood, the IMWG frailty score was shown to predict mortality and treatment-related toxicity in elderly myeloma patients.
Dr. Larocca described results of the RV-MM-PI-0752 phase 3 study, which enrolled 199 newly diagnosed myeloma patients of intermediate fitness and randomized them to continuous Rd or nine cycles of Rd induction followed by lenalidomide maintenance (Rd-R).
The goal was to see if Rd could be “further optimized” for elderly, intermediate-fit patients, Dr. Larocca said.
The primary endpoint of RV-MM-PI-0752 was event-free survival, which included grade 4 hematologic and grade 3-4 nonhematologic adverse events, lenalidomide discontinuation, disease progression, or death.
Median event-free survival was 9.3 months for the Rd-R strategy, compared with 6.6 months for continuous Rd (hazard ratio, 0.72; 95% confidence interval, 0.52-0.99; P = .044), Dr. Larocca reported.
No difference was seen in survival outcomes, she added. The 20-month progression-free survival was 43% and 42% for Rd-R and continuous Rd, respectively. The 20-month overall survival was 84% vs. 79%, with P values that were not significant for either comparison.
Patients in the Rd-R group had a somewhat higher incidence of grade 3 or greater neutropenia, but the continuous Rd group had a somewhat higher rate of nonhematologic adverse events, leading to slightly higher rates of lenalidomide discontinuation and dose reduction, Dr. Larocca said.
Overall, 9% of patients dropped out of the RV-MM-PI-0752 trial within the first 60 days, due mainly to toxicity, she added.
“We have to evaluate how to better prevent toxicity, potentially enabling patients to stay on therapy longer,” Dr. Larocca said. “Probably we have to evaluate, in prospective clinical trials, the role of up-front dose adjustment or dose reduction, and subsequent dose increase in a subgroup of patients.”
Dr. Larocca reported disclosures related to Celgene, Bristol-Myers Squibb, Janssen-Cilag, Takeda, and Amgen.
SOURCE: Larocca A, et al. ASH 2018, Abstract 305.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Median event-free survival was 9.3 months for the Rd induction followed by lenalidomide maintenance, compared with 6.6 months for continuous Rd (P = .044).
Study details: Results of the RV-MM-PI-0752 phase 3 study, which enrolled 199 newly diagnosed multiple myeloma patients of intermediate fitness.
Disclosures: Dr. Larocca reported disclosures related to Celgene, Bristol-Myers Squibb, Janssen-Cilag, Takeda, and Amgen.
Source: Larocca A et al. ASH 2018, Abstract 305.
Checkmate 436: Two-drug combo is ‘promising’ for PMBCL
SAN DIEGO – Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising ... to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president of Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the annual meeting of the American Society of Hematology.
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
The interim analysis of this trial included 30 patients with relapsed/refractory PMBCL. Their median age at enrollment was 35.5 and 57% of patients were female. More than half of the patients (60%) had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was two and 13% of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit, disease progression, AEs unrelated to treatment, patient request, and other concerns.
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment related. There were three other deaths in the trial, but they were considered unrelated to treatment.
The complete response rate was 27% (n = 8), and the partial response rate was 43% (n = 13), for an overall response rate of 70% (n = 21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n = 3) had stable disease, 13% (n = 4) progressed, and investigators were unable to determine the status for 7% of patients (n = 2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics. Investigators reported relationships with Bristol-Myers Squibb, Seattle Genetics, and a range of other companies.
SOURCE: Moskowitz AJ et al. ASH 2018. Abstract 1691.
SAN DIEGO – Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising ... to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president of Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the annual meeting of the American Society of Hematology.
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
The interim analysis of this trial included 30 patients with relapsed/refractory PMBCL. Their median age at enrollment was 35.5 and 57% of patients were female. More than half of the patients (60%) had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was two and 13% of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit, disease progression, AEs unrelated to treatment, patient request, and other concerns.
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment related. There were three other deaths in the trial, but they were considered unrelated to treatment.
The complete response rate was 27% (n = 8), and the partial response rate was 43% (n = 13), for an overall response rate of 70% (n = 21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n = 3) had stable disease, 13% (n = 4) progressed, and investigators were unable to determine the status for 7% of patients (n = 2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics. Investigators reported relationships with Bristol-Myers Squibb, Seattle Genetics, and a range of other companies.
SOURCE: Moskowitz AJ et al. ASH 2018. Abstract 1691.
SAN DIEGO – Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising ... to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president of Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the annual meeting of the American Society of Hematology.
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
The interim analysis of this trial included 30 patients with relapsed/refractory PMBCL. Their median age at enrollment was 35.5 and 57% of patients were female. More than half of the patients (60%) had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was two and 13% of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit, disease progression, AEs unrelated to treatment, patient request, and other concerns.
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment related. There were three other deaths in the trial, but they were considered unrelated to treatment.
The complete response rate was 27% (n = 8), and the partial response rate was 43% (n = 13), for an overall response rate of 70% (n = 21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n = 3) had stable disease, 13% (n = 4) progressed, and investigators were unable to determine the status for 7% of patients (n = 2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics. Investigators reported relationships with Bristol-Myers Squibb, Seattle Genetics, and a range of other companies.
SOURCE: Moskowitz AJ et al. ASH 2018. Abstract 1691.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The overall response rate was 70%, including a complete response rate of 27%.
Study details: A phase 1/2 study of 30 patients.
Disclosures: This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics, and investigators reported relationships with a range of other companies.
Source: Moskowitz AJ et al. ASH 2018, Abstract 1691.
Lack of gut diversity hurts survival after HCT
SAN DIEGO – The success or failure of hematopoietic cell transplants may be related to the diversity of species in the gut microbiome, investigators contend.
A study of fecal samples from patients scheduled to undergo hematopoietic cell transplant (HCT) in the United States, Europe, and Japan showed that intestinal microbial diversity was significantly associated with overall survival, reported Jonathan U. Peled, MD, PhD, from the bone marrow transplantation service at Memorial Sloan Kettering Cancer Center in New York City.
“Patients with low diversity pretransplant have a poorer overall survival than patients with a higher diversity after transplantation,” he said at the annual meeting of the American Society of Hematology. “The implication is, if we could come up with a way to remediate microbiome injury, there might be time to implement it before the transplant.”
There are about 100 trillion symbiotic microbes in and on the human body, and 95% of them live in the gastrointestinal tract, which has a surface area that if stretched out would equal the size of two tennis courts, Dr. Peled said.
Previous studies by his group and others have shown that the composition of intestinal microbiota is associated with survival, relapses, graft-versus-host disease, and infections in patients in the early weeks after allogeneic HCT.
The international team previously reported that intestinal diversity measured around the time of neutrophil engraftment was predictive of overall survival, and they hypothesized that the same might be true of the pretransplant microbiota composition.
To test this idea, they collected 1,922 stools samples approximately once weekly for 3 weeks pretransplant from 991 adult patients scheduled for allo-HCT for various diagnoses in two U.S. cohorts and one cohort each in Europe and Japan.
They found that on average patients in all four cohorts had microbiota diversity values ranging from 70% to 150% lower than those of healthy volunteers whose samples were sequenced by the investigators, as well as those in a publicly available database.
The investigators also asked whether one or more bacterial species accounted for 30% or more of a microbiome, a phenomenon known as “monodomination.”
“A third, or in some cases 95+%, of bacteria in an individual’s intestine are all the exact same strain,” Dr. Peled said.
They found that the incidence and prevalence of the monodomination phenotype was “strikingly similar” at all four transplant sites.
The patterns of microbiota injury were similar despite differences in antibiotic strategies among the four centers and different dietary patterns of patients in the regions studied.
Studies in animal models suggest that T cells responsible for graft-versus-host disease migrate to the gut as early as 2 or 3 days post transplant, and by that time more than half of transplant patients have a monodomination event in their guts, Dr. Peled said.
Some strategies to remediate or prevent microbiome damage – and potentially improve HCT outcomes – may include the use of over-the-counter or custom-made probiotics, fecal transplants, a “prebiotic” approach in which the patient is asked to follow a specific diet that promotes the growth of beneficial bacteria, or a “postbiotic” approach in which patients receive metabolites of agents or foods thought to have a beneficial effect.
“Finally, we can think about different antibiotic strategies, to use or not use different types of antibiotics at different times in a rational way,” Dr. Peled said.
Dr. Peled reported current or prior financial relationships with Seres Therapeutics, the Parker Institute for Cancer Immunotherapy, and Merck/Society for Immunotherapy of Cancer.
SOURCE: Peled JU et al. ASH 2018, Abstract 811.
SAN DIEGO – The success or failure of hematopoietic cell transplants may be related to the diversity of species in the gut microbiome, investigators contend.
A study of fecal samples from patients scheduled to undergo hematopoietic cell transplant (HCT) in the United States, Europe, and Japan showed that intestinal microbial diversity was significantly associated with overall survival, reported Jonathan U. Peled, MD, PhD, from the bone marrow transplantation service at Memorial Sloan Kettering Cancer Center in New York City.
“Patients with low diversity pretransplant have a poorer overall survival than patients with a higher diversity after transplantation,” he said at the annual meeting of the American Society of Hematology. “The implication is, if we could come up with a way to remediate microbiome injury, there might be time to implement it before the transplant.”
There are about 100 trillion symbiotic microbes in and on the human body, and 95% of them live in the gastrointestinal tract, which has a surface area that if stretched out would equal the size of two tennis courts, Dr. Peled said.
Previous studies by his group and others have shown that the composition of intestinal microbiota is associated with survival, relapses, graft-versus-host disease, and infections in patients in the early weeks after allogeneic HCT.
The international team previously reported that intestinal diversity measured around the time of neutrophil engraftment was predictive of overall survival, and they hypothesized that the same might be true of the pretransplant microbiota composition.
To test this idea, they collected 1,922 stools samples approximately once weekly for 3 weeks pretransplant from 991 adult patients scheduled for allo-HCT for various diagnoses in two U.S. cohorts and one cohort each in Europe and Japan.
They found that on average patients in all four cohorts had microbiota diversity values ranging from 70% to 150% lower than those of healthy volunteers whose samples were sequenced by the investigators, as well as those in a publicly available database.
The investigators also asked whether one or more bacterial species accounted for 30% or more of a microbiome, a phenomenon known as “monodomination.”
“A third, or in some cases 95+%, of bacteria in an individual’s intestine are all the exact same strain,” Dr. Peled said.
They found that the incidence and prevalence of the monodomination phenotype was “strikingly similar” at all four transplant sites.
The patterns of microbiota injury were similar despite differences in antibiotic strategies among the four centers and different dietary patterns of patients in the regions studied.
Studies in animal models suggest that T cells responsible for graft-versus-host disease migrate to the gut as early as 2 or 3 days post transplant, and by that time more than half of transplant patients have a monodomination event in their guts, Dr. Peled said.
Some strategies to remediate or prevent microbiome damage – and potentially improve HCT outcomes – may include the use of over-the-counter or custom-made probiotics, fecal transplants, a “prebiotic” approach in which the patient is asked to follow a specific diet that promotes the growth of beneficial bacteria, or a “postbiotic” approach in which patients receive metabolites of agents or foods thought to have a beneficial effect.
“Finally, we can think about different antibiotic strategies, to use or not use different types of antibiotics at different times in a rational way,” Dr. Peled said.
Dr. Peled reported current or prior financial relationships with Seres Therapeutics, the Parker Institute for Cancer Immunotherapy, and Merck/Society for Immunotherapy of Cancer.
SOURCE: Peled JU et al. ASH 2018, Abstract 811.
SAN DIEGO – The success or failure of hematopoietic cell transplants may be related to the diversity of species in the gut microbiome, investigators contend.
A study of fecal samples from patients scheduled to undergo hematopoietic cell transplant (HCT) in the United States, Europe, and Japan showed that intestinal microbial diversity was significantly associated with overall survival, reported Jonathan U. Peled, MD, PhD, from the bone marrow transplantation service at Memorial Sloan Kettering Cancer Center in New York City.
“Patients with low diversity pretransplant have a poorer overall survival than patients with a higher diversity after transplantation,” he said at the annual meeting of the American Society of Hematology. “The implication is, if we could come up with a way to remediate microbiome injury, there might be time to implement it before the transplant.”
There are about 100 trillion symbiotic microbes in and on the human body, and 95% of them live in the gastrointestinal tract, which has a surface area that if stretched out would equal the size of two tennis courts, Dr. Peled said.
Previous studies by his group and others have shown that the composition of intestinal microbiota is associated with survival, relapses, graft-versus-host disease, and infections in patients in the early weeks after allogeneic HCT.
The international team previously reported that intestinal diversity measured around the time of neutrophil engraftment was predictive of overall survival, and they hypothesized that the same might be true of the pretransplant microbiota composition.
To test this idea, they collected 1,922 stools samples approximately once weekly for 3 weeks pretransplant from 991 adult patients scheduled for allo-HCT for various diagnoses in two U.S. cohorts and one cohort each in Europe and Japan.
They found that on average patients in all four cohorts had microbiota diversity values ranging from 70% to 150% lower than those of healthy volunteers whose samples were sequenced by the investigators, as well as those in a publicly available database.
The investigators also asked whether one or more bacterial species accounted for 30% or more of a microbiome, a phenomenon known as “monodomination.”
“A third, or in some cases 95+%, of bacteria in an individual’s intestine are all the exact same strain,” Dr. Peled said.
They found that the incidence and prevalence of the monodomination phenotype was “strikingly similar” at all four transplant sites.
The patterns of microbiota injury were similar despite differences in antibiotic strategies among the four centers and different dietary patterns of patients in the regions studied.
Studies in animal models suggest that T cells responsible for graft-versus-host disease migrate to the gut as early as 2 or 3 days post transplant, and by that time more than half of transplant patients have a monodomination event in their guts, Dr. Peled said.
Some strategies to remediate or prevent microbiome damage – and potentially improve HCT outcomes – may include the use of over-the-counter or custom-made probiotics, fecal transplants, a “prebiotic” approach in which the patient is asked to follow a specific diet that promotes the growth of beneficial bacteria, or a “postbiotic” approach in which patients receive metabolites of agents or foods thought to have a beneficial effect.
“Finally, we can think about different antibiotic strategies, to use or not use different types of antibiotics at different times in a rational way,” Dr. Peled said.
Dr. Peled reported current or prior financial relationships with Seres Therapeutics, the Parker Institute for Cancer Immunotherapy, and Merck/Society for Immunotherapy of Cancer.
SOURCE: Peled JU et al. ASH 2018, Abstract 811.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Hematopoietic cell transplant candidates in the United States, Europe, and Japan all had similar patterns of low intestinal microbiota diversity and predomination of a single taxonomic species.
Study details: An analysis of stool samples from 991 adults scheduled for hematopoietic cell transplant at four transplant centers in the United States, Germany, and Japan.
Disclosures: Dr. Peled reported current or prior financial relationships with Seres Therapeutics, the Parker Institute for Cancer Immunotherapy, and Merck/Society for Immunotherapy of Cancer.
Source: Peled JU et al. ASH 2018, Abstract 811.
Best of Psychopharmacology: Stimulants, ketamine, benzodiazapines
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify