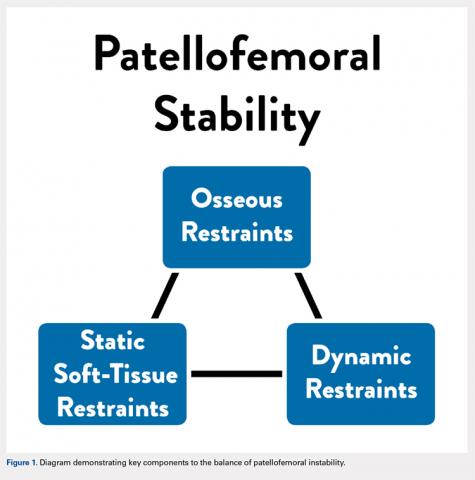
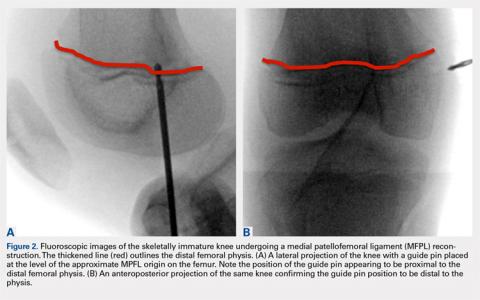
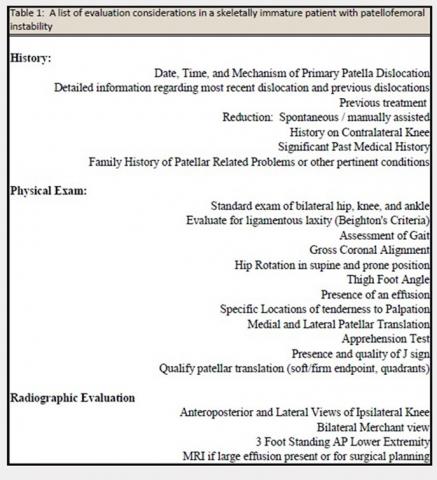
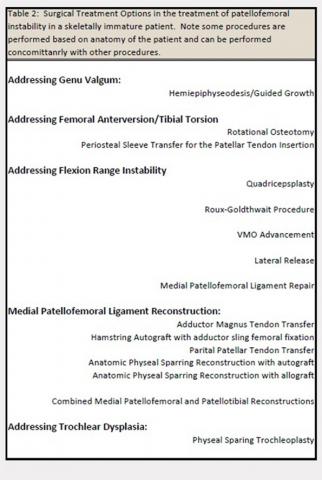
User login
Pregnant women commonly refuse the influenza vaccine
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Although almost all ob.gyns. recommend the influenza and Tdap vaccines for pregnant women, both commonly are refused.
Major finding: A total of 62% of ob.gyns. reported that 10% or greater of their pregnant patients refused the influenza vaccine; 32% reported this for Tdap vaccine.
Study details: An email and mail survey sent to a national network of ob.gyns. between March and June 2016.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
Source: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
QOL is poorer for young women after mastectomy than BCS
SAN ANTONIO – , according to investigators for a multicenter cross-sectional cohort study reported at the San Antonio Breast Cancer Symposium.
Women aged 40 or younger make up about 7% of all newly diagnosed cases of breast cancer in the United States, according to lead author, Laura S. Dominici, MD, of Dana-Farber/Brigham and Women’s Cancer Center and Harvard Medical School, Boston.
“Despite the fact that there is equivalent local-regional control with breast conservation and mastectomy, the rates of mastectomy and particularly bilateral mastectomy are increasing in young women, with a 10-fold increase seen from 1998 to 2011,” she noted in a press conference. “Young women are at particular risk for poorer psychosocial outcomes following a breast cancer diagnosis and in survivorship. However, little is known about the impact of surgery, particularly in the era of increasing bilateral mastectomy, on the quality of life of young survivors.”
Nearly three-fourths of the 560 young breast cancer survivors studied had undergone mastectomy, usually with some kind of reconstruction. Roughly 6 years later, compared with peers who had undergone breast-conserving surgery, women who had undergone unilateral or bilateral mastectomy had significantly poorer adjusted BREAST-Q scores for satisfaction with the appearance and feel of their breasts (beta, –8.7 and –9.3 points) and psychosocial well-being (–8.3 and –10.5 points). The latter also had poorer adjusted scores for sexual well-being (–8.1 points). Physical well-being, which captures aspects such as pain and range of motion, did not differ significantly by type of surgery.
“Local therapy decisions are associated with a persistent impact on quality of life in young breast cancer survivors,” Dr. Dominici concluded. “Knowledge of the potential long-term impact of surgery and quality of life is of critical importance for counseling young women about surgical decisions.”
Moving away from mastectomy
“The data are, to me anyway, more disconcerting when you consider the high mastectomy rate in this country relative to Europe, and this urge to have bilateral mastectomies, which, pardon the expression, is ridiculous in some cases because it doesn’t improve your outcome. And yet, it does have deleterious effects that last for years psychologically,” commented SABCS codirector and press conference moderator C. Kent Osborne, MD, who is director of the Dan L. Duncan Cancer Center at Baylor College of Medicine, Houston. “What can we do about that?” he asked.
“It’s a really challenging problem,” Dr. Dominici replied. “Part of what we are missing in the conversation that we have with our patients is this kind of information. We can certainly tell patients that the outcomes are equivalent, but if they don’t know that the long-term [quality of life] impact is potentially worse, then that may not affect their decision. The more prospective data that we generate to help us figure out which patients are going to have better or worse outcomes with these different types of surgery, the better we will be able to counsel patients with things that will be meaningful to them in the long run.”
The study was not designed to tease out the specific role of anxiety about a recurrence or a new breast cancer, which is a major driver of the decision to have mastectomy and also needs to be addressed during counseling, Dr. Dominici and Dr. Osborne agreed. “I think I spend more time talking patients out of bilateral mastectomy or mastectomy at all than anything,” he commented.
Study details
The women studied were participants in the prospective Young Women’s Breast Cancer Study (YWS) and had a mean age of 37 years at diagnosis. Most (86%) had stage 0-2 breast cancer. (Those with metastatic disease at diagnosis or a recurrence during follow-up were excluded.)
Overall, 52% of the women underwent bilateral mastectomy, 20% underwent unilateral mastectomy, and 28% underwent breast-conserving surgery, Dr. Dominici reported. Within the mastectomy group, most underwent implant-based reconstruction (69%) or flap reconstruction (12%), while some opted for no reconstruction (11%).
Multivariate analyses showed that, in addition to mastectomy, other significant predictors of poorer breast satisfaction were receipt of radiation therapy (beta, –7.5 points) and having a financially uncomfortable status as compared with a comfortable one (–5.4 points).
Additional significant predictors of poorer psychosocial well-being were receiving radiation (beta, –6.0 points), being financially uncomfortable (–7 points), and being overweight or obese (–4.2 points), and additional significant predictors of poorer sexual well-being were being financially uncomfortable (–6.8 points), being overweight or obese (–5.3 points), and having lymphedema a year after diagnosis (–3.8 points).
The only significant predictors of poorer physical health were financially uncomfortable status (beta, –4.8 points) and lymphedema (–6.4 points), whereas longer time since surgery (more than 5 years) predicted better physical health (+6.0 points), according to Dr. Dominici.
Age, race, marital status, work status, education level, disease stage, chemotherapy, and endocrine therapy did not significantly predict any of the outcomes studied.
“This was a one-time survey of women who were enrolled in an observational cohort study, and we know that preoperative quality of life likely drives surgical choices,” she commented, addressing the study’s limitations. “Our findings may have limited generalizability to a more diverse population in that the majority of our participants were white and of high socioeconomic status.”
Dr. Dominici disclosed that she had no conflicts of interest. The study was funded by the Agency for Healthcare Research and Quality, Susan G. Komen, the Breast Cancer Research Foundation, and The Pink Agenda.
SOURCE: Dominici LS et al. SABCS 2018, Abstract GS6-06,
SAN ANTONIO – , according to investigators for a multicenter cross-sectional cohort study reported at the San Antonio Breast Cancer Symposium.
Women aged 40 or younger make up about 7% of all newly diagnosed cases of breast cancer in the United States, according to lead author, Laura S. Dominici, MD, of Dana-Farber/Brigham and Women’s Cancer Center and Harvard Medical School, Boston.
“Despite the fact that there is equivalent local-regional control with breast conservation and mastectomy, the rates of mastectomy and particularly bilateral mastectomy are increasing in young women, with a 10-fold increase seen from 1998 to 2011,” she noted in a press conference. “Young women are at particular risk for poorer psychosocial outcomes following a breast cancer diagnosis and in survivorship. However, little is known about the impact of surgery, particularly in the era of increasing bilateral mastectomy, on the quality of life of young survivors.”
Nearly three-fourths of the 560 young breast cancer survivors studied had undergone mastectomy, usually with some kind of reconstruction. Roughly 6 years later, compared with peers who had undergone breast-conserving surgery, women who had undergone unilateral or bilateral mastectomy had significantly poorer adjusted BREAST-Q scores for satisfaction with the appearance and feel of their breasts (beta, –8.7 and –9.3 points) and psychosocial well-being (–8.3 and –10.5 points). The latter also had poorer adjusted scores for sexual well-being (–8.1 points). Physical well-being, which captures aspects such as pain and range of motion, did not differ significantly by type of surgery.
“Local therapy decisions are associated with a persistent impact on quality of life in young breast cancer survivors,” Dr. Dominici concluded. “Knowledge of the potential long-term impact of surgery and quality of life is of critical importance for counseling young women about surgical decisions.”
Moving away from mastectomy
“The data are, to me anyway, more disconcerting when you consider the high mastectomy rate in this country relative to Europe, and this urge to have bilateral mastectomies, which, pardon the expression, is ridiculous in some cases because it doesn’t improve your outcome. And yet, it does have deleterious effects that last for years psychologically,” commented SABCS codirector and press conference moderator C. Kent Osborne, MD, who is director of the Dan L. Duncan Cancer Center at Baylor College of Medicine, Houston. “What can we do about that?” he asked.
“It’s a really challenging problem,” Dr. Dominici replied. “Part of what we are missing in the conversation that we have with our patients is this kind of information. We can certainly tell patients that the outcomes are equivalent, but if they don’t know that the long-term [quality of life] impact is potentially worse, then that may not affect their decision. The more prospective data that we generate to help us figure out which patients are going to have better or worse outcomes with these different types of surgery, the better we will be able to counsel patients with things that will be meaningful to them in the long run.”
The study was not designed to tease out the specific role of anxiety about a recurrence or a new breast cancer, which is a major driver of the decision to have mastectomy and also needs to be addressed during counseling, Dr. Dominici and Dr. Osborne agreed. “I think I spend more time talking patients out of bilateral mastectomy or mastectomy at all than anything,” he commented.
Study details
The women studied were participants in the prospective Young Women’s Breast Cancer Study (YWS) and had a mean age of 37 years at diagnosis. Most (86%) had stage 0-2 breast cancer. (Those with metastatic disease at diagnosis or a recurrence during follow-up were excluded.)
Overall, 52% of the women underwent bilateral mastectomy, 20% underwent unilateral mastectomy, and 28% underwent breast-conserving surgery, Dr. Dominici reported. Within the mastectomy group, most underwent implant-based reconstruction (69%) or flap reconstruction (12%), while some opted for no reconstruction (11%).
Multivariate analyses showed that, in addition to mastectomy, other significant predictors of poorer breast satisfaction were receipt of radiation therapy (beta, –7.5 points) and having a financially uncomfortable status as compared with a comfortable one (–5.4 points).
Additional significant predictors of poorer psychosocial well-being were receiving radiation (beta, –6.0 points), being financially uncomfortable (–7 points), and being overweight or obese (–4.2 points), and additional significant predictors of poorer sexual well-being were being financially uncomfortable (–6.8 points), being overweight or obese (–5.3 points), and having lymphedema a year after diagnosis (–3.8 points).
The only significant predictors of poorer physical health were financially uncomfortable status (beta, –4.8 points) and lymphedema (–6.4 points), whereas longer time since surgery (more than 5 years) predicted better physical health (+6.0 points), according to Dr. Dominici.
Age, race, marital status, work status, education level, disease stage, chemotherapy, and endocrine therapy did not significantly predict any of the outcomes studied.
“This was a one-time survey of women who were enrolled in an observational cohort study, and we know that preoperative quality of life likely drives surgical choices,” she commented, addressing the study’s limitations. “Our findings may have limited generalizability to a more diverse population in that the majority of our participants were white and of high socioeconomic status.”
Dr. Dominici disclosed that she had no conflicts of interest. The study was funded by the Agency for Healthcare Research and Quality, Susan G. Komen, the Breast Cancer Research Foundation, and The Pink Agenda.
SOURCE: Dominici LS et al. SABCS 2018, Abstract GS6-06,
SAN ANTONIO – , according to investigators for a multicenter cross-sectional cohort study reported at the San Antonio Breast Cancer Symposium.
Women aged 40 or younger make up about 7% of all newly diagnosed cases of breast cancer in the United States, according to lead author, Laura S. Dominici, MD, of Dana-Farber/Brigham and Women’s Cancer Center and Harvard Medical School, Boston.
“Despite the fact that there is equivalent local-regional control with breast conservation and mastectomy, the rates of mastectomy and particularly bilateral mastectomy are increasing in young women, with a 10-fold increase seen from 1998 to 2011,” she noted in a press conference. “Young women are at particular risk for poorer psychosocial outcomes following a breast cancer diagnosis and in survivorship. However, little is known about the impact of surgery, particularly in the era of increasing bilateral mastectomy, on the quality of life of young survivors.”
Nearly three-fourths of the 560 young breast cancer survivors studied had undergone mastectomy, usually with some kind of reconstruction. Roughly 6 years later, compared with peers who had undergone breast-conserving surgery, women who had undergone unilateral or bilateral mastectomy had significantly poorer adjusted BREAST-Q scores for satisfaction with the appearance and feel of their breasts (beta, –8.7 and –9.3 points) and psychosocial well-being (–8.3 and –10.5 points). The latter also had poorer adjusted scores for sexual well-being (–8.1 points). Physical well-being, which captures aspects such as pain and range of motion, did not differ significantly by type of surgery.
“Local therapy decisions are associated with a persistent impact on quality of life in young breast cancer survivors,” Dr. Dominici concluded. “Knowledge of the potential long-term impact of surgery and quality of life is of critical importance for counseling young women about surgical decisions.”
Moving away from mastectomy
“The data are, to me anyway, more disconcerting when you consider the high mastectomy rate in this country relative to Europe, and this urge to have bilateral mastectomies, which, pardon the expression, is ridiculous in some cases because it doesn’t improve your outcome. And yet, it does have deleterious effects that last for years psychologically,” commented SABCS codirector and press conference moderator C. Kent Osborne, MD, who is director of the Dan L. Duncan Cancer Center at Baylor College of Medicine, Houston. “What can we do about that?” he asked.
“It’s a really challenging problem,” Dr. Dominici replied. “Part of what we are missing in the conversation that we have with our patients is this kind of information. We can certainly tell patients that the outcomes are equivalent, but if they don’t know that the long-term [quality of life] impact is potentially worse, then that may not affect their decision. The more prospective data that we generate to help us figure out which patients are going to have better or worse outcomes with these different types of surgery, the better we will be able to counsel patients with things that will be meaningful to them in the long run.”
The study was not designed to tease out the specific role of anxiety about a recurrence or a new breast cancer, which is a major driver of the decision to have mastectomy and also needs to be addressed during counseling, Dr. Dominici and Dr. Osborne agreed. “I think I spend more time talking patients out of bilateral mastectomy or mastectomy at all than anything,” he commented.
Study details
The women studied were participants in the prospective Young Women’s Breast Cancer Study (YWS) and had a mean age of 37 years at diagnosis. Most (86%) had stage 0-2 breast cancer. (Those with metastatic disease at diagnosis or a recurrence during follow-up were excluded.)
Overall, 52% of the women underwent bilateral mastectomy, 20% underwent unilateral mastectomy, and 28% underwent breast-conserving surgery, Dr. Dominici reported. Within the mastectomy group, most underwent implant-based reconstruction (69%) or flap reconstruction (12%), while some opted for no reconstruction (11%).
Multivariate analyses showed that, in addition to mastectomy, other significant predictors of poorer breast satisfaction were receipt of radiation therapy (beta, –7.5 points) and having a financially uncomfortable status as compared with a comfortable one (–5.4 points).
Additional significant predictors of poorer psychosocial well-being were receiving radiation (beta, –6.0 points), being financially uncomfortable (–7 points), and being overweight or obese (–4.2 points), and additional significant predictors of poorer sexual well-being were being financially uncomfortable (–6.8 points), being overweight or obese (–5.3 points), and having lymphedema a year after diagnosis (–3.8 points).
The only significant predictors of poorer physical health were financially uncomfortable status (beta, –4.8 points) and lymphedema (–6.4 points), whereas longer time since surgery (more than 5 years) predicted better physical health (+6.0 points), according to Dr. Dominici.
Age, race, marital status, work status, education level, disease stage, chemotherapy, and endocrine therapy did not significantly predict any of the outcomes studied.
“This was a one-time survey of women who were enrolled in an observational cohort study, and we know that preoperative quality of life likely drives surgical choices,” she commented, addressing the study’s limitations. “Our findings may have limited generalizability to a more diverse population in that the majority of our participants were white and of high socioeconomic status.”
Dr. Dominici disclosed that she had no conflicts of interest. The study was funded by the Agency for Healthcare Research and Quality, Susan G. Komen, the Breast Cancer Research Foundation, and The Pink Agenda.
SOURCE: Dominici LS et al. SABCS 2018, Abstract GS6-06,
REPORTING FROM SABCS 2018
Key clinical point: More extensive breast surgery has a long-term negative impact on QOL for young breast cancer survivors.
Major finding: Compared with peers who underwent breast-conserving surgery, young women who underwent unilateral or bilateral mastectomy had significantly poorer adjusted scores for breast satisfaction (beta, –8.7 and –9.3 points) and psychosocial well-being (beta, –8.3 and –10.5 points).
Study details: A multicenter cross-sectional cohort study of 560 women with a mean age of 37 years at breast cancer diagnosis who completed the BREAST-Q questionnaire a median of 5.8 years later.
Disclosures: Dr. Dominici disclosed that she had no conflicts of interest. The study was funded by the Agency for Healthcare Research and Quality, Susan G. Komen, the Breast Cancer Research Foundation, and The Pink Agenda.
Source: Dominici LS et al. SABCS 2018, Abstract GS6-06.
Untreated OSA linked to resistant hypertension in black patients
according to findings published in Circulation.
In an analysis of 664 patients with hypertension, those with moderate to severe OSA had twofold higher odds of resistant hypertension, compared with those with no or mild OSA (odds ratio, 2.04; 95% confidence interval, 1.14-3.67), reported Dayna A. Johnson, PhD, of the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital, Boston, and coauthors.
Participants were enrolled in the JHSS, an ancillary trial conducted during December 2012 – May 2016 as part of the Jackson Heart Study, a longitudinal study of 5,306 black adults aged 21-95 years in Jackson, Miss. Patients included in the analysis had hypertension (defined as high blood pressure, use of antihypertensive medication, or self-reported diagnosis). Those without a valid in-home sleep apnea test and with missing data on hypertension, measured blood pressure, or use of antihypertensive medications and diuretics were excluded from analysis.
Sleep apnea was assessed using measures of nasal pressure, thoracic and abdominal inductance plethysmography, finger pulse oximetry, body position, and electrocardiography with a validated Type 3 home sleep apnea device. Obstructive apneas were identified as a flat or nearly flat amplitude of the nasal pressure signal for greater than 10 seconds, accompanied by respiratory effort on the abdominal or thoracic inductance plethysmography bands. Severity was defined by the standard Respiratory Event Index (REI) categories: fewer than 5 events (unaffected), greater than or equal to 5 events to fewer than 15 events (mild), greater than or equal to 15 events to fewer than 30 events (moderate), and greater than or equal to 30 events (severe), the authors reported.
High blood pressure (BP) was defined as systolic BP greater than or equal to 130 mm Hg or diastolic BP greater than or equal to 80 mm Hg. Controlled hypertension was defined as systolic BP less than 130 mmHg and diastolic BP less than 80 mm Hg.
Uncontrolled BP was defined as high BP with use of one or two classes of antihypertensive medications; resistant hypertension was defined as having high BP while on greater than or equal to three classes of antihypertensive medications with one being a diuretic or as using of greater than four classes of antihypertensive medications regardless of BP control, Dr. Johnson and colleagues reported.
A total of 25.7% of hypertension patients had moderate or severe OSA, though only 6% of these patients had an OSA diagnosis from a physician. In addition, 48.2% of patients had uncontrolled hypertension, and 14.5% had resistant hypertension.
Moderate or severe OSA was associated with nearly twofold higher unadjusted odds of resistant hypertension (OR, 1.92; 95% CI, 1.15-3.20). In adjusted models, moderate or severe OSA and nocturnal hypoxemia were not associated with uncontrolled hypertension but were associated with resistant hypertension (OR, 2.04; 95% CI, 1.14-3.67; OR, 1.25; 95% CI, 1.01-1.55, respectively).
Compared with no OSA, severe OSA was associated with more than three times higher odds of resistant hypertension (OR, 3.50; 95% CI, 1.54-7.91). This association was even higher after adjustment for covariates (OR, 3.58; 95% CI, 1.39-9.19).
“These data suggest that untreated OSA may contribute to the high burden of resistant hypertension in blacks,” Dr. Johnson and coauthors wrote. “Future studies should test whether diagnosis and treatment of OSA may be interventions for improving BP control” and reducing this burden, they added.
“These findings are particularly important given that most adults with OSA are undiagnosed and untreated.”
The study was funded by grants from the National Heart, Lung, and Blood Institute. One of the authors reported receiving funding from Amgen. No other disclosures were reported.
SOURCE: Johnson D et al. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.118.036675.
according to findings published in Circulation.
In an analysis of 664 patients with hypertension, those with moderate to severe OSA had twofold higher odds of resistant hypertension, compared with those with no or mild OSA (odds ratio, 2.04; 95% confidence interval, 1.14-3.67), reported Dayna A. Johnson, PhD, of the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital, Boston, and coauthors.
Participants were enrolled in the JHSS, an ancillary trial conducted during December 2012 – May 2016 as part of the Jackson Heart Study, a longitudinal study of 5,306 black adults aged 21-95 years in Jackson, Miss. Patients included in the analysis had hypertension (defined as high blood pressure, use of antihypertensive medication, or self-reported diagnosis). Those without a valid in-home sleep apnea test and with missing data on hypertension, measured blood pressure, or use of antihypertensive medications and diuretics were excluded from analysis.
Sleep apnea was assessed using measures of nasal pressure, thoracic and abdominal inductance plethysmography, finger pulse oximetry, body position, and electrocardiography with a validated Type 3 home sleep apnea device. Obstructive apneas were identified as a flat or nearly flat amplitude of the nasal pressure signal for greater than 10 seconds, accompanied by respiratory effort on the abdominal or thoracic inductance plethysmography bands. Severity was defined by the standard Respiratory Event Index (REI) categories: fewer than 5 events (unaffected), greater than or equal to 5 events to fewer than 15 events (mild), greater than or equal to 15 events to fewer than 30 events (moderate), and greater than or equal to 30 events (severe), the authors reported.
High blood pressure (BP) was defined as systolic BP greater than or equal to 130 mm Hg or diastolic BP greater than or equal to 80 mm Hg. Controlled hypertension was defined as systolic BP less than 130 mmHg and diastolic BP less than 80 mm Hg.
Uncontrolled BP was defined as high BP with use of one or two classes of antihypertensive medications; resistant hypertension was defined as having high BP while on greater than or equal to three classes of antihypertensive medications with one being a diuretic or as using of greater than four classes of antihypertensive medications regardless of BP control, Dr. Johnson and colleagues reported.
A total of 25.7% of hypertension patients had moderate or severe OSA, though only 6% of these patients had an OSA diagnosis from a physician. In addition, 48.2% of patients had uncontrolled hypertension, and 14.5% had resistant hypertension.
Moderate or severe OSA was associated with nearly twofold higher unadjusted odds of resistant hypertension (OR, 1.92; 95% CI, 1.15-3.20). In adjusted models, moderate or severe OSA and nocturnal hypoxemia were not associated with uncontrolled hypertension but were associated with resistant hypertension (OR, 2.04; 95% CI, 1.14-3.67; OR, 1.25; 95% CI, 1.01-1.55, respectively).
Compared with no OSA, severe OSA was associated with more than three times higher odds of resistant hypertension (OR, 3.50; 95% CI, 1.54-7.91). This association was even higher after adjustment for covariates (OR, 3.58; 95% CI, 1.39-9.19).
“These data suggest that untreated OSA may contribute to the high burden of resistant hypertension in blacks,” Dr. Johnson and coauthors wrote. “Future studies should test whether diagnosis and treatment of OSA may be interventions for improving BP control” and reducing this burden, they added.
“These findings are particularly important given that most adults with OSA are undiagnosed and untreated.”
The study was funded by grants from the National Heart, Lung, and Blood Institute. One of the authors reported receiving funding from Amgen. No other disclosures were reported.
SOURCE: Johnson D et al. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.118.036675.
according to findings published in Circulation.
In an analysis of 664 patients with hypertension, those with moderate to severe OSA had twofold higher odds of resistant hypertension, compared with those with no or mild OSA (odds ratio, 2.04; 95% confidence interval, 1.14-3.67), reported Dayna A. Johnson, PhD, of the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital, Boston, and coauthors.
Participants were enrolled in the JHSS, an ancillary trial conducted during December 2012 – May 2016 as part of the Jackson Heart Study, a longitudinal study of 5,306 black adults aged 21-95 years in Jackson, Miss. Patients included in the analysis had hypertension (defined as high blood pressure, use of antihypertensive medication, or self-reported diagnosis). Those without a valid in-home sleep apnea test and with missing data on hypertension, measured blood pressure, or use of antihypertensive medications and diuretics were excluded from analysis.
Sleep apnea was assessed using measures of nasal pressure, thoracic and abdominal inductance plethysmography, finger pulse oximetry, body position, and electrocardiography with a validated Type 3 home sleep apnea device. Obstructive apneas were identified as a flat or nearly flat amplitude of the nasal pressure signal for greater than 10 seconds, accompanied by respiratory effort on the abdominal or thoracic inductance plethysmography bands. Severity was defined by the standard Respiratory Event Index (REI) categories: fewer than 5 events (unaffected), greater than or equal to 5 events to fewer than 15 events (mild), greater than or equal to 15 events to fewer than 30 events (moderate), and greater than or equal to 30 events (severe), the authors reported.
High blood pressure (BP) was defined as systolic BP greater than or equal to 130 mm Hg or diastolic BP greater than or equal to 80 mm Hg. Controlled hypertension was defined as systolic BP less than 130 mmHg and diastolic BP less than 80 mm Hg.
Uncontrolled BP was defined as high BP with use of one or two classes of antihypertensive medications; resistant hypertension was defined as having high BP while on greater than or equal to three classes of antihypertensive medications with one being a diuretic or as using of greater than four classes of antihypertensive medications regardless of BP control, Dr. Johnson and colleagues reported.
A total of 25.7% of hypertension patients had moderate or severe OSA, though only 6% of these patients had an OSA diagnosis from a physician. In addition, 48.2% of patients had uncontrolled hypertension, and 14.5% had resistant hypertension.
Moderate or severe OSA was associated with nearly twofold higher unadjusted odds of resistant hypertension (OR, 1.92; 95% CI, 1.15-3.20). In adjusted models, moderate or severe OSA and nocturnal hypoxemia were not associated with uncontrolled hypertension but were associated with resistant hypertension (OR, 2.04; 95% CI, 1.14-3.67; OR, 1.25; 95% CI, 1.01-1.55, respectively).
Compared with no OSA, severe OSA was associated with more than three times higher odds of resistant hypertension (OR, 3.50; 95% CI, 1.54-7.91). This association was even higher after adjustment for covariates (OR, 3.58; 95% CI, 1.39-9.19).
“These data suggest that untreated OSA may contribute to the high burden of resistant hypertension in blacks,” Dr. Johnson and coauthors wrote. “Future studies should test whether diagnosis and treatment of OSA may be interventions for improving BP control” and reducing this burden, they added.
“These findings are particularly important given that most adults with OSA are undiagnosed and untreated.”
The study was funded by grants from the National Heart, Lung, and Blood Institute. One of the authors reported receiving funding from Amgen. No other disclosures were reported.
SOURCE: Johnson D et al. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.118.036675.
FROM CIRCULATION
Key clinical point: Untreated moderate or severe obstructive sleep apnea was associated with greater odds of resistant hypertension.
Major finding: In patients with hypertension, those with moderate to severe OSA had twofold higher odds of resistant hypertension, compared with those with no or mild OSA.
Study details: A total of 664 participants were enrolled in the JHSS, an ancillary trial as part of the Jackson Heart Study, a longitudinal study of 5,306 black adults.
Disclosures: The study was funded by grants from the National Heart, Lung, and Blood Institute. One of the authors reported receiving funding from Amgen. No other disclosures were reported.
Source: Johnson D et al. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.118.036675.
New risk-prediction model for diabetes under development
LOS ANGELES – Clinicians treating patients with diabetes rely heavily on the U.K. Prospective Diabetes Study (UKPDS) Risk Engine and the Framingham Risk Score to predict outcomes, but the populations used for developing these tools differ significantly from the current U.S. diabetes population.
“All these risk engines have various degrees of accuracy along with several limitations, including that they are derived from data from various populations,” Vivian A. Fonseca, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “Sometimes the results may not be generalizable. That’s one of the big problems with the risk engines we’re using.”
To address these shortcomings, Dr. Fonseca, Hui Shao, PhD, and Lizheng Shi, PhD, have developed the Building, Relating, Assessing, Validating Outcomes (BRAVO) of Diabetes Model, a patient-level microsimulation model based on data from the ACCORD trial. The model predicts both primary and secondary CVD events, microvascular events, the progress of hemoglobin A1c and other key biomarkers over time, quality-adjusted life-year (QALY) function decrements associated with complications, and an ability to predict outcomes in patients from other regions in the world. The risk engine contains three modules for 17 equations in total, including angina, blindness, and hypoglycemia (Pharmacoeconomics. 2018;36[9]:1125-34). “There are lots of data now showing that if you get hypoglycemia, your risk of a cardiovascular event goes up greatly over the subsequent 2 years,” said Dr. Fonseca, who is chief of the section of endocrinology at Tulane University Health Science Center, New Orleans. “No other risk engine has that.”
When he and his associates applied the UKPDS Risk Engine to the ACCORD cohort, they found that the UPKDS Risk Engine overpredicted the risk of stroke (2.3% vs. 1.4% observed), MI (6.5% vs. 4.9% observed), and all-cause mortality (10.3% vs. 4% observed); yet it underpredicted congestive heart failure (2.2% vs. 4% observed), end-stage renal disease (0.5% vs. 3% observed), and blindness (1.35% vs. 8.1% observed). In the ACCORD cohort, baseline duration varied from 0 to 35 years. “Using left truncated regression, we can piece together the segmented follow-up times for 10,251 patients to a complete diabetes progression track from 0 years to 40 years after diabetes onset,” he said.
Dr. Fonseca said that Internal validations studies found that BRAVO predicted outcomes from the ACCORD trial, including congestive heart failure, MI, stroke, angina, blindness, end-stage renal disease, and neuropathy. Data from the ASPEN, CARDS, and ADVANCE trials were used to conduct external validation, and the incidence rates of 28 endpoints correlated with that of BRAVO “extremely well.” In addition, BRAVO has been calibrated against 18 large randomized, controlled trials conducted after the year 2000. “Regional variation in CVD [cardiovascular disease] outcomes were included as an important risk factor in the simulation,” said Dr. Fonseca, who is also assistant dean for clinical research at Tulane. Results to date show a high prediction accuracy (R-squared value = .91).
He and his associates are currently examining ways to apply BRAVO in clinical practice, including for risk stratification. “Let’s say you have a large health system, and you want to separate out your patients who have high, medium, or low risk for diabetes and make sure they get they get the right care according to their stratification,” he explained. “A couple of large health systems are trying this out right now.”
BRAVO can also be used as a tool for cost-effectiveness analysis and program evaluation. In fact, he and his colleagues at five medical centers are working with the American Diabetes Association “to see what effect a certain intervention will have on outcomes in people with diabetes over a number of years, and how cost effective it might be.”
Finally, BRAVO can be used for diabetes management in clinical practice. “Based on an individual’s characteristics, the BRAVO model potentially simulates future outcomes such as complications and mortality, providing a transparent platform for shared decision making,” he said.
Dr. Fonseca disclosed that he has an ownership interest in the development of BRAVO.
LOS ANGELES – Clinicians treating patients with diabetes rely heavily on the U.K. Prospective Diabetes Study (UKPDS) Risk Engine and the Framingham Risk Score to predict outcomes, but the populations used for developing these tools differ significantly from the current U.S. diabetes population.
“All these risk engines have various degrees of accuracy along with several limitations, including that they are derived from data from various populations,” Vivian A. Fonseca, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “Sometimes the results may not be generalizable. That’s one of the big problems with the risk engines we’re using.”
To address these shortcomings, Dr. Fonseca, Hui Shao, PhD, and Lizheng Shi, PhD, have developed the Building, Relating, Assessing, Validating Outcomes (BRAVO) of Diabetes Model, a patient-level microsimulation model based on data from the ACCORD trial. The model predicts both primary and secondary CVD events, microvascular events, the progress of hemoglobin A1c and other key biomarkers over time, quality-adjusted life-year (QALY) function decrements associated with complications, and an ability to predict outcomes in patients from other regions in the world. The risk engine contains three modules for 17 equations in total, including angina, blindness, and hypoglycemia (Pharmacoeconomics. 2018;36[9]:1125-34). “There are lots of data now showing that if you get hypoglycemia, your risk of a cardiovascular event goes up greatly over the subsequent 2 years,” said Dr. Fonseca, who is chief of the section of endocrinology at Tulane University Health Science Center, New Orleans. “No other risk engine has that.”
When he and his associates applied the UKPDS Risk Engine to the ACCORD cohort, they found that the UPKDS Risk Engine overpredicted the risk of stroke (2.3% vs. 1.4% observed), MI (6.5% vs. 4.9% observed), and all-cause mortality (10.3% vs. 4% observed); yet it underpredicted congestive heart failure (2.2% vs. 4% observed), end-stage renal disease (0.5% vs. 3% observed), and blindness (1.35% vs. 8.1% observed). In the ACCORD cohort, baseline duration varied from 0 to 35 years. “Using left truncated regression, we can piece together the segmented follow-up times for 10,251 patients to a complete diabetes progression track from 0 years to 40 years after diabetes onset,” he said.
Dr. Fonseca said that Internal validations studies found that BRAVO predicted outcomes from the ACCORD trial, including congestive heart failure, MI, stroke, angina, blindness, end-stage renal disease, and neuropathy. Data from the ASPEN, CARDS, and ADVANCE trials were used to conduct external validation, and the incidence rates of 28 endpoints correlated with that of BRAVO “extremely well.” In addition, BRAVO has been calibrated against 18 large randomized, controlled trials conducted after the year 2000. “Regional variation in CVD [cardiovascular disease] outcomes were included as an important risk factor in the simulation,” said Dr. Fonseca, who is also assistant dean for clinical research at Tulane. Results to date show a high prediction accuracy (R-squared value = .91).
He and his associates are currently examining ways to apply BRAVO in clinical practice, including for risk stratification. “Let’s say you have a large health system, and you want to separate out your patients who have high, medium, or low risk for diabetes and make sure they get they get the right care according to their stratification,” he explained. “A couple of large health systems are trying this out right now.”
BRAVO can also be used as a tool for cost-effectiveness analysis and program evaluation. In fact, he and his colleagues at five medical centers are working with the American Diabetes Association “to see what effect a certain intervention will have on outcomes in people with diabetes over a number of years, and how cost effective it might be.”
Finally, BRAVO can be used for diabetes management in clinical practice. “Based on an individual’s characteristics, the BRAVO model potentially simulates future outcomes such as complications and mortality, providing a transparent platform for shared decision making,” he said.
Dr. Fonseca disclosed that he has an ownership interest in the development of BRAVO.
LOS ANGELES – Clinicians treating patients with diabetes rely heavily on the U.K. Prospective Diabetes Study (UKPDS) Risk Engine and the Framingham Risk Score to predict outcomes, but the populations used for developing these tools differ significantly from the current U.S. diabetes population.
“All these risk engines have various degrees of accuracy along with several limitations, including that they are derived from data from various populations,” Vivian A. Fonseca, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “Sometimes the results may not be generalizable. That’s one of the big problems with the risk engines we’re using.”
To address these shortcomings, Dr. Fonseca, Hui Shao, PhD, and Lizheng Shi, PhD, have developed the Building, Relating, Assessing, Validating Outcomes (BRAVO) of Diabetes Model, a patient-level microsimulation model based on data from the ACCORD trial. The model predicts both primary and secondary CVD events, microvascular events, the progress of hemoglobin A1c and other key biomarkers over time, quality-adjusted life-year (QALY) function decrements associated with complications, and an ability to predict outcomes in patients from other regions in the world. The risk engine contains three modules for 17 equations in total, including angina, blindness, and hypoglycemia (Pharmacoeconomics. 2018;36[9]:1125-34). “There are lots of data now showing that if you get hypoglycemia, your risk of a cardiovascular event goes up greatly over the subsequent 2 years,” said Dr. Fonseca, who is chief of the section of endocrinology at Tulane University Health Science Center, New Orleans. “No other risk engine has that.”
When he and his associates applied the UKPDS Risk Engine to the ACCORD cohort, they found that the UPKDS Risk Engine overpredicted the risk of stroke (2.3% vs. 1.4% observed), MI (6.5% vs. 4.9% observed), and all-cause mortality (10.3% vs. 4% observed); yet it underpredicted congestive heart failure (2.2% vs. 4% observed), end-stage renal disease (0.5% vs. 3% observed), and blindness (1.35% vs. 8.1% observed). In the ACCORD cohort, baseline duration varied from 0 to 35 years. “Using left truncated regression, we can piece together the segmented follow-up times for 10,251 patients to a complete diabetes progression track from 0 years to 40 years after diabetes onset,” he said.
Dr. Fonseca said that Internal validations studies found that BRAVO predicted outcomes from the ACCORD trial, including congestive heart failure, MI, stroke, angina, blindness, end-stage renal disease, and neuropathy. Data from the ASPEN, CARDS, and ADVANCE trials were used to conduct external validation, and the incidence rates of 28 endpoints correlated with that of BRAVO “extremely well.” In addition, BRAVO has been calibrated against 18 large randomized, controlled trials conducted after the year 2000. “Regional variation in CVD [cardiovascular disease] outcomes were included as an important risk factor in the simulation,” said Dr. Fonseca, who is also assistant dean for clinical research at Tulane. Results to date show a high prediction accuracy (R-squared value = .91).
He and his associates are currently examining ways to apply BRAVO in clinical practice, including for risk stratification. “Let’s say you have a large health system, and you want to separate out your patients who have high, medium, or low risk for diabetes and make sure they get they get the right care according to their stratification,” he explained. “A couple of large health systems are trying this out right now.”
BRAVO can also be used as a tool for cost-effectiveness analysis and program evaluation. In fact, he and his colleagues at five medical centers are working with the American Diabetes Association “to see what effect a certain intervention will have on outcomes in people with diabetes over a number of years, and how cost effective it might be.”
Finally, BRAVO can be used for diabetes management in clinical practice. “Based on an individual’s characteristics, the BRAVO model potentially simulates future outcomes such as complications and mortality, providing a transparent platform for shared decision making,” he said.
Dr. Fonseca disclosed that he has an ownership interest in the development of BRAVO.
EXPERT ANALYSIS FROM THE WCIRCD 2018
Risk-based testing missed 35% of HCV-positive prison inmates
Routine testing for hepatitis C virus at inmate entry should be considered by U.S. state prisons, according to Sabrina A. Assoumou, MD, of the Boston Medical Center and her colleagues.
The researchers performed a retrospective analysis of individuals entering the Washington state prison system, which routinely offers hepatitis C virus (HCV) testing, in order to compare routine opt-out testing with current risk-based and one-time testing for individuals born between 1945 and 1965. Additionally, liver fibrosis stage was characterized in blood samples from HCV-positive individuals, the investigators wrote in the American Journal of Preventative Medicine.
Between 2012 and 2016, 24,567 (83%) individuals were tested for HCV antibody, and of these, 4,921 (20%) tested positive. A total of 2,403 (49%) of those testing positive had subsequent hepatitis HCV RNA testing, with 1,727 (72%) of these showing chronic infection.
As expected, Dr. Assoumou and her colleagues found that reactive antibodies was more prevalent in individuals born between 1945 and 1965, compared with other years (44% vs. 17%). However, in actual case numbers, most (72%) were outside of this age bracket. Overall, they calculated that up to 35% of positive HCV tests would be missed using testing targeted by birth cohort and risk behavior alone. Among the chronically infected individuals, 23% had showed at least moderate liver fibrosis.
“Routine opt-out testing identified a substantial number of HCV cases that would have been missed by targeted testing. Almost one-quarter of individuals with chronic HCV had significant liver fibrosis and thus a more urgent need for treatment to prevent complications,” Dr Assoumou and her colleagues concluded.
The researchers reported that they had no conflicts of interest.
SOURCE: Assoumou SA et al, Am J Prev Med. 2019;56:8-16.
Routine testing for hepatitis C virus at inmate entry should be considered by U.S. state prisons, according to Sabrina A. Assoumou, MD, of the Boston Medical Center and her colleagues.
The researchers performed a retrospective analysis of individuals entering the Washington state prison system, which routinely offers hepatitis C virus (HCV) testing, in order to compare routine opt-out testing with current risk-based and one-time testing for individuals born between 1945 and 1965. Additionally, liver fibrosis stage was characterized in blood samples from HCV-positive individuals, the investigators wrote in the American Journal of Preventative Medicine.
Between 2012 and 2016, 24,567 (83%) individuals were tested for HCV antibody, and of these, 4,921 (20%) tested positive. A total of 2,403 (49%) of those testing positive had subsequent hepatitis HCV RNA testing, with 1,727 (72%) of these showing chronic infection.
As expected, Dr. Assoumou and her colleagues found that reactive antibodies was more prevalent in individuals born between 1945 and 1965, compared with other years (44% vs. 17%). However, in actual case numbers, most (72%) were outside of this age bracket. Overall, they calculated that up to 35% of positive HCV tests would be missed using testing targeted by birth cohort and risk behavior alone. Among the chronically infected individuals, 23% had showed at least moderate liver fibrosis.
“Routine opt-out testing identified a substantial number of HCV cases that would have been missed by targeted testing. Almost one-quarter of individuals with chronic HCV had significant liver fibrosis and thus a more urgent need for treatment to prevent complications,” Dr Assoumou and her colleagues concluded.
The researchers reported that they had no conflicts of interest.
SOURCE: Assoumou SA et al, Am J Prev Med. 2019;56:8-16.
Routine testing for hepatitis C virus at inmate entry should be considered by U.S. state prisons, according to Sabrina A. Assoumou, MD, of the Boston Medical Center and her colleagues.
The researchers performed a retrospective analysis of individuals entering the Washington state prison system, which routinely offers hepatitis C virus (HCV) testing, in order to compare routine opt-out testing with current risk-based and one-time testing for individuals born between 1945 and 1965. Additionally, liver fibrosis stage was characterized in blood samples from HCV-positive individuals, the investigators wrote in the American Journal of Preventative Medicine.
Between 2012 and 2016, 24,567 (83%) individuals were tested for HCV antibody, and of these, 4,921 (20%) tested positive. A total of 2,403 (49%) of those testing positive had subsequent hepatitis HCV RNA testing, with 1,727 (72%) of these showing chronic infection.
As expected, Dr. Assoumou and her colleagues found that reactive antibodies was more prevalent in individuals born between 1945 and 1965, compared with other years (44% vs. 17%). However, in actual case numbers, most (72%) were outside of this age bracket. Overall, they calculated that up to 35% of positive HCV tests would be missed using testing targeted by birth cohort and risk behavior alone. Among the chronically infected individuals, 23% had showed at least moderate liver fibrosis.
“Routine opt-out testing identified a substantial number of HCV cases that would have been missed by targeted testing. Almost one-quarter of individuals with chronic HCV had significant liver fibrosis and thus a more urgent need for treatment to prevent complications,” Dr Assoumou and her colleagues concluded.
The researchers reported that they had no conflicts of interest.
SOURCE: Assoumou SA et al, Am J Prev Med. 2019;56:8-16.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Canakinumab reduces arthroplasty rates
CHICAGO – Canakinumab, a human monoclonal antibody targeting interleukin-1 beta, was associated with an eye-popping 45% relative risk reduction in the rate of total knee or hip replacement in a prespecified secondary analysis of the landmark CANTOS trial, Matthias Schieker, MD, reported at the annual meeting of the American College of Rheumatology.
For the broader composite endpoint of all osteoarthritis-related adverse events, including new-onset OA or worsening of symptoms in those with OA at baseline, the relative risk reduction was 23% in patients randomized to canakinumab rather than placebo. For CANTOS participants who already had OA at baseline, the relative risk reduction was 31%, according to Dr. Schieker, who is head of the joint, bone, and tendon disease group at the Novartis Institute for Biomedical Research in Basel, Switzerland, and professor of regenerative medicine at the University of Munich.
CANTOS (the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) was designed as a massive phase 3 secondary cardiovascular prevention trial. It included 10,061 patients with a history of acute MI and an elevated high-sensitivity C-reactive protein (hsCRP) level of 2 mg/L or more who were randomized double blind to subcutaneous canakinumab at 50, 150, or 300 mg or placebo given once every 3 months. During a median 3.7 years of prospective follow-up, patients in the 150-mg group had a highly significant 17% reduction relative to placebo in the risk of the composite efficacy endpoint comprising cardiovascular death, MI, stroke, or hospitalization for unstable angina resulting in urgent coronary revascularization (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Since this result was achieved with a 39% reduction in CRP, compared with placebo, and involved no lipid-lowering effect, it was hailed in the cardiology world as the long-awaited proof of the inflammatory hypothesis of atherosclerotic cardiovascular disease.
CANTOS has proved to be the gift that keeps on giving. Secondary analyses of the study data have found statistically significant reductions in the incidence of and mortality caused by lung cancer in the coronary disease patients on canakinumab, as well as a decreased risk of developing gout. Moreover, the CANTOS investigators, well aware that there are no approved therapies to prevent disease progression in OA, had the foresight to prospectively collect data on OA-related symptoms and outcomes.
At baseline, 15.6% of CANTOS participants had a history of OA. During follow-up, patients in that subgroup had a 3.4% incidence of total knee replacement or total hip replacement if they had been assigned to canakinumab, compared with a 6.3% incidence if they got placebo. In the full 10,000-plus CANTOS cohort, the arthroplasty rates were 0.8% and 1.4%, respectively.
The combined rate of OA-related adverse events in the full CANTOS cohort was 5.4% with canakinumab and 7.0% with placebo. In the subgroup with baseline OA, the rates were 14.5% and 20.8%.
Canakinumab is marketed by Novartis as Ilaris and is already approved for cryopyrin-associated periodic syndromes, familial Mediterranean fever, juvenile idiopathic arthritis, and other rare autoimmune inflammatory diseases. Based upon the positive primary outcomes of the CANTOS trial, Novartis applied to the Food and Drug Administration for a major expanded indication of the IL-1B inhibitor for cardiovascular risk reduction. However, the regulatory agency has turned down that bid.
Although the CANTOS OA-related outcomes data caused quite a stir at the meeting, Dr. Schieker said in an interview that the impressive findings didn’t really come as a surprise to him.
“I think everyone in the field has assumed that IL-1 plays a role in OA. That idea has been around for quite a long time, but until now no effects could be shown in OA. We were lucky to have an enriched population with elevated hsCRP that was so large and followed for so long that we could finally show these relative risk reductions,” he explained.
SOURCE: Schieker M et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 445.
CHICAGO – Canakinumab, a human monoclonal antibody targeting interleukin-1 beta, was associated with an eye-popping 45% relative risk reduction in the rate of total knee or hip replacement in a prespecified secondary analysis of the landmark CANTOS trial, Matthias Schieker, MD, reported at the annual meeting of the American College of Rheumatology.
For the broader composite endpoint of all osteoarthritis-related adverse events, including new-onset OA or worsening of symptoms in those with OA at baseline, the relative risk reduction was 23% in patients randomized to canakinumab rather than placebo. For CANTOS participants who already had OA at baseline, the relative risk reduction was 31%, according to Dr. Schieker, who is head of the joint, bone, and tendon disease group at the Novartis Institute for Biomedical Research in Basel, Switzerland, and professor of regenerative medicine at the University of Munich.
CANTOS (the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) was designed as a massive phase 3 secondary cardiovascular prevention trial. It included 10,061 patients with a history of acute MI and an elevated high-sensitivity C-reactive protein (hsCRP) level of 2 mg/L or more who were randomized double blind to subcutaneous canakinumab at 50, 150, or 300 mg or placebo given once every 3 months. During a median 3.7 years of prospective follow-up, patients in the 150-mg group had a highly significant 17% reduction relative to placebo in the risk of the composite efficacy endpoint comprising cardiovascular death, MI, stroke, or hospitalization for unstable angina resulting in urgent coronary revascularization (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Since this result was achieved with a 39% reduction in CRP, compared with placebo, and involved no lipid-lowering effect, it was hailed in the cardiology world as the long-awaited proof of the inflammatory hypothesis of atherosclerotic cardiovascular disease.
CANTOS has proved to be the gift that keeps on giving. Secondary analyses of the study data have found statistically significant reductions in the incidence of and mortality caused by lung cancer in the coronary disease patients on canakinumab, as well as a decreased risk of developing gout. Moreover, the CANTOS investigators, well aware that there are no approved therapies to prevent disease progression in OA, had the foresight to prospectively collect data on OA-related symptoms and outcomes.
At baseline, 15.6% of CANTOS participants had a history of OA. During follow-up, patients in that subgroup had a 3.4% incidence of total knee replacement or total hip replacement if they had been assigned to canakinumab, compared with a 6.3% incidence if they got placebo. In the full 10,000-plus CANTOS cohort, the arthroplasty rates were 0.8% and 1.4%, respectively.
The combined rate of OA-related adverse events in the full CANTOS cohort was 5.4% with canakinumab and 7.0% with placebo. In the subgroup with baseline OA, the rates were 14.5% and 20.8%.
Canakinumab is marketed by Novartis as Ilaris and is already approved for cryopyrin-associated periodic syndromes, familial Mediterranean fever, juvenile idiopathic arthritis, and other rare autoimmune inflammatory diseases. Based upon the positive primary outcomes of the CANTOS trial, Novartis applied to the Food and Drug Administration for a major expanded indication of the IL-1B inhibitor for cardiovascular risk reduction. However, the regulatory agency has turned down that bid.
Although the CANTOS OA-related outcomes data caused quite a stir at the meeting, Dr. Schieker said in an interview that the impressive findings didn’t really come as a surprise to him.
“I think everyone in the field has assumed that IL-1 plays a role in OA. That idea has been around for quite a long time, but until now no effects could be shown in OA. We were lucky to have an enriched population with elevated hsCRP that was so large and followed for so long that we could finally show these relative risk reductions,” he explained.
SOURCE: Schieker M et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 445.
CHICAGO – Canakinumab, a human monoclonal antibody targeting interleukin-1 beta, was associated with an eye-popping 45% relative risk reduction in the rate of total knee or hip replacement in a prespecified secondary analysis of the landmark CANTOS trial, Matthias Schieker, MD, reported at the annual meeting of the American College of Rheumatology.
For the broader composite endpoint of all osteoarthritis-related adverse events, including new-onset OA or worsening of symptoms in those with OA at baseline, the relative risk reduction was 23% in patients randomized to canakinumab rather than placebo. For CANTOS participants who already had OA at baseline, the relative risk reduction was 31%, according to Dr. Schieker, who is head of the joint, bone, and tendon disease group at the Novartis Institute for Biomedical Research in Basel, Switzerland, and professor of regenerative medicine at the University of Munich.
CANTOS (the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) was designed as a massive phase 3 secondary cardiovascular prevention trial. It included 10,061 patients with a history of acute MI and an elevated high-sensitivity C-reactive protein (hsCRP) level of 2 mg/L or more who were randomized double blind to subcutaneous canakinumab at 50, 150, or 300 mg or placebo given once every 3 months. During a median 3.7 years of prospective follow-up, patients in the 150-mg group had a highly significant 17% reduction relative to placebo in the risk of the composite efficacy endpoint comprising cardiovascular death, MI, stroke, or hospitalization for unstable angina resulting in urgent coronary revascularization (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Since this result was achieved with a 39% reduction in CRP, compared with placebo, and involved no lipid-lowering effect, it was hailed in the cardiology world as the long-awaited proof of the inflammatory hypothesis of atherosclerotic cardiovascular disease.
CANTOS has proved to be the gift that keeps on giving. Secondary analyses of the study data have found statistically significant reductions in the incidence of and mortality caused by lung cancer in the coronary disease patients on canakinumab, as well as a decreased risk of developing gout. Moreover, the CANTOS investigators, well aware that there are no approved therapies to prevent disease progression in OA, had the foresight to prospectively collect data on OA-related symptoms and outcomes.
At baseline, 15.6% of CANTOS participants had a history of OA. During follow-up, patients in that subgroup had a 3.4% incidence of total knee replacement or total hip replacement if they had been assigned to canakinumab, compared with a 6.3% incidence if they got placebo. In the full 10,000-plus CANTOS cohort, the arthroplasty rates were 0.8% and 1.4%, respectively.
The combined rate of OA-related adverse events in the full CANTOS cohort was 5.4% with canakinumab and 7.0% with placebo. In the subgroup with baseline OA, the rates were 14.5% and 20.8%.
Canakinumab is marketed by Novartis as Ilaris and is already approved for cryopyrin-associated periodic syndromes, familial Mediterranean fever, juvenile idiopathic arthritis, and other rare autoimmune inflammatory diseases. Based upon the positive primary outcomes of the CANTOS trial, Novartis applied to the Food and Drug Administration for a major expanded indication of the IL-1B inhibitor for cardiovascular risk reduction. However, the regulatory agency has turned down that bid.
Although the CANTOS OA-related outcomes data caused quite a stir at the meeting, Dr. Schieker said in an interview that the impressive findings didn’t really come as a surprise to him.
“I think everyone in the field has assumed that IL-1 plays a role in OA. That idea has been around for quite a long time, but until now no effects could be shown in OA. We were lucky to have an enriched population with elevated hsCRP that was so large and followed for so long that we could finally show these relative risk reductions,” he explained.
SOURCE: Schieker M et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 445.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Patients on the IL-1B inhibitor canakinumab for secondary cardiovascular prevention also experienced a 45% risk reduction in total knee or total hip replacement, compared with placebo.
Study details: This was a prespecified secondary analysis of OA-related outcomes in the 10,061 participants in the randomized, double-blind CANTOS trial.
Disclosures: The presenter is an employee of Novartis, which markets canakinumab and sponsored CANTOS.
Source: Schieker M et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 445.
Hospice liability
Question: Hospice liability may exist in which of the following?
A. False claims in violation of Medicare rules regarding eligible beneficiaries.
B. False claims for continuous home care services.
C. Negligent billing practices.
D. Only A and B are correct.
E. A, B, and C are correct.
Answer: D. With an aging population and better end-of-life care, the United States has in the last decade witnessed about a 50% increase in the number of hospices. Hospice care is a Medicare-covered benefit, and most hospices operate on a for-profit basis. Although occasionally institution based, services are more often offered as an outpatient or home-care option. In 2016, hospice care reached 1.4 million beneficiaries, with total Medicare expenditure of $16.7 billion.1
There are two broad categories of legal jeopardy that hospices face: Medicare fraud and malpractice lawsuits. This article will address these two issues. In addition, hospices, like all health care institutions, face numerous other liabilities, such as negligent hiring, breach of confidentiality, premise liability, HIPAA violations, sexual harassment, vicarious liability, and many others.
Medicare fraud
The False Claims Act (FCA) is an old law enacted by Congress way back in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false.2
Intent to defraud is not a required element. But knowing or reckless disregard of the truth or material misrepresentation are required, although negligence is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim – as well as possible imprisonment. The FCA is the most prominent health care antifraud statute.3 Two others are the federal Anti-Kickback Statute and the Stark Law.
A recent example of hospice fraud involved Ohio’s Chemed and Vitas Hospice Services, which were accused of knowingly billing for hospice-ineligible patients and inflated levels of care.4
The government alleged that the defendants rewarded employees with bonuses based on the number of patients receiving hospice services, irrespective of whether they were actually terminally ill or needed continuous home care services (CHCS). CHCS commands the highest Medicare daily rate and is meant only for the temporary treatment of acute symptoms constituting a medical crisis.
According to the complaint, the defendants set aggressive billing goals for CHCS without regard to whether the patients actually required such a level of service. The defendants agreed to pay $75 million to settle the lawsuit, the largest in the history of hospice false-claim settlements.
Can an alleged wrong prognosis regarding life expectancy amount to a false claim? Under Medicare rules, a physician certifying that a patient is eligible for hospice care must attest that the condition is terminal, with death expected within 6 months.
AseraCare, a hospice company, was accused of knowingly submitting false claims to Medicare by certifying patients as eligible for hospice. The government claimed that the medical records of the 123 patients at issue did not contain clinical information and other documentation that supported the medical prognosis, and thus, AseraCare’s claims for those patients were false.
AseraCare won a summary judgment defending against the $200 million lawsuit in a federal district court in Alabama. The court opined that, when hospice-certifying physicians and government medical experts look at the very same medical records and disagree about eligibility, the opinion of one medical expert alone cannot prove falsity without further evidence of an objective falsehood.5 The government, however, has appealed the decision to the Court of Appeals for the Eleventh Circuit.
Malpractice
Hospices have their share of malpractice litigation, and judgments may be substantial because of noneconomic losses such as pain and suffering, not to mention punitive damages.
For example, in 2013a Maryland jury awarded more than $950,000 to a family that alleged that the decedent’s death was caused by the excessive use of morphine and oxycodone in treating her infected ulcers. Such treatment was deemed suitable for a hospice-type situation, but in fact, the patient was not expected to die within 6 months.
Her husband and two children argued successfully that the hospital committed malpractice by misdiagnosing her need for hospice care and by performing unnecessary surgery. The bulk of the judgment was for pain and suffering and other noneconomic damages.
In another negligence suit, a 66-year-old woman died in a hospice after receiving an overdose of Dilaudid for pancreatic cancer, which an autopsy revealed she did not have. In that case, the plaintiffs were awarded $4.5 million in a wrongful death lawsuit filed against Hospice Ministries and its medical director. The jury awarded the family $4 million in monetary compensation and $500,000 in punitive damages.
The case of McGregor v. Hospice Care of Louisiana is illustrative of a malpractice action with a focus on expert testimony.6 The issue in this case was whether the testimony of the plaintiffs’ expert, Bruce Samuels, MD, was admissible and whether it correctly addressed the requisite standard of care.
The decedent had terminal metastatic prostate cancer and was under the care of an oncologist. He eventually enrolled as a patient of Hospice of Baton Rouge, whose nurses visited him in his home several times a week. They reported their findings to the attending oncologist, who prescribed a total of 40 morphine suppositories to be administered 1-2 per hour as needed for pain. However, the prescription noted that only half – that is, 20 suppositories – were to be filled, and stipulated when the remaining 20 suppositories could be released.
Believing that his father was in pain, the patient’s son demanded the early release of the remaining 20 morphine suppositories; he also refused to allow the nurse to assess the patient and exhibited threatening behavior toward her. After conferring with the oncologist on call, the hospice discharged the patient from its care. An ambulance later took the patient to a hospital, where he died that evening.
The family filed a lawsuit against the hospice, alleging negligence in failing to release the remaining 20 morphine suppositories and in abandoning the patient by discharging him. At trial, the jury rendered a verdict in favor of the hospice, after the court excluded the testimony of the plaintiffs’ expert as being outside his expertise. However, the Louisiana Supreme Court found that the trial court erred in excluding his testimony.
On remand, the appellate court affirmed the trial court’s judgment that the plaintiffs had failed to meet the burden of proof showing negligence. It found that the expert, Dr. Samuels, admitted he had never written a partial-fill prescription before and that he did not know who had the authority to authorize the pharmacist to release the remainder of the partial fill prescription in this case.
In addition, Dr. Samuels acknowledged that a nurse has the obligation to assess a patient and report her findings to the physician and follow any orders of the physician. Further, the nurse indicated that the doctor had instructed her to discharge the patient, not from the doctor’s care, but for treatment to be continued at the hospital.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from earlier columns in Internal Medicine News. For additional information, readers may contact the author at [email protected].
References
1. “Medicare’s most indefensible fraud hotspot: Hospice care.” CNBC, Modern Medicine, Aug. 3, 2018.
2. 31 U.S. Code, Section 3729(a)(1)(A).
3. Tan SY. “Update on the False Claims Act.” Internal Medicine News, April 5, 2017.
4. U.S. Department of Justice, Office of Public Affairs, Oct. 30, 2017.
5. U.S. ex rel. Paradies et al. v. AseraCare Inc. et al., case number 2:12-CV-245-KOB, in the U.S. District Court for the Northern District of Alabama, March 31, 2016.
6. McGregor v. Hospice Care of Louisiana in Baton Rouge, LLC, No. 2013 CA 1979R, consolidated with No. 2013 CA 1980R. Court of Appeals of Louisiana, First Circuit, judgment rendered Sept. 21, 2015.
Question: Hospice liability may exist in which of the following?
A. False claims in violation of Medicare rules regarding eligible beneficiaries.
B. False claims for continuous home care services.
C. Negligent billing practices.
D. Only A and B are correct.
E. A, B, and C are correct.
Answer: D. With an aging population and better end-of-life care, the United States has in the last decade witnessed about a 50% increase in the number of hospices. Hospice care is a Medicare-covered benefit, and most hospices operate on a for-profit basis. Although occasionally institution based, services are more often offered as an outpatient or home-care option. In 2016, hospice care reached 1.4 million beneficiaries, with total Medicare expenditure of $16.7 billion.1
There are two broad categories of legal jeopardy that hospices face: Medicare fraud and malpractice lawsuits. This article will address these two issues. In addition, hospices, like all health care institutions, face numerous other liabilities, such as negligent hiring, breach of confidentiality, premise liability, HIPAA violations, sexual harassment, vicarious liability, and many others.
Medicare fraud
The False Claims Act (FCA) is an old law enacted by Congress way back in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false.2
Intent to defraud is not a required element. But knowing or reckless disregard of the truth or material misrepresentation are required, although negligence is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim – as well as possible imprisonment. The FCA is the most prominent health care antifraud statute.3 Two others are the federal Anti-Kickback Statute and the Stark Law.
A recent example of hospice fraud involved Ohio’s Chemed and Vitas Hospice Services, which were accused of knowingly billing for hospice-ineligible patients and inflated levels of care.4
The government alleged that the defendants rewarded employees with bonuses based on the number of patients receiving hospice services, irrespective of whether they were actually terminally ill or needed continuous home care services (CHCS). CHCS commands the highest Medicare daily rate and is meant only for the temporary treatment of acute symptoms constituting a medical crisis.
According to the complaint, the defendants set aggressive billing goals for CHCS without regard to whether the patients actually required such a level of service. The defendants agreed to pay $75 million to settle the lawsuit, the largest in the history of hospice false-claim settlements.
Can an alleged wrong prognosis regarding life expectancy amount to a false claim? Under Medicare rules, a physician certifying that a patient is eligible for hospice care must attest that the condition is terminal, with death expected within 6 months.
AseraCare, a hospice company, was accused of knowingly submitting false claims to Medicare by certifying patients as eligible for hospice. The government claimed that the medical records of the 123 patients at issue did not contain clinical information and other documentation that supported the medical prognosis, and thus, AseraCare’s claims for those patients were false.
AseraCare won a summary judgment defending against the $200 million lawsuit in a federal district court in Alabama. The court opined that, when hospice-certifying physicians and government medical experts look at the very same medical records and disagree about eligibility, the opinion of one medical expert alone cannot prove falsity without further evidence of an objective falsehood.5 The government, however, has appealed the decision to the Court of Appeals for the Eleventh Circuit.
Malpractice
Hospices have their share of malpractice litigation, and judgments may be substantial because of noneconomic losses such as pain and suffering, not to mention punitive damages.
For example, in 2013a Maryland jury awarded more than $950,000 to a family that alleged that the decedent’s death was caused by the excessive use of morphine and oxycodone in treating her infected ulcers. Such treatment was deemed suitable for a hospice-type situation, but in fact, the patient was not expected to die within 6 months.
Her husband and two children argued successfully that the hospital committed malpractice by misdiagnosing her need for hospice care and by performing unnecessary surgery. The bulk of the judgment was for pain and suffering and other noneconomic damages.
In another negligence suit, a 66-year-old woman died in a hospice after receiving an overdose of Dilaudid for pancreatic cancer, which an autopsy revealed she did not have. In that case, the plaintiffs were awarded $4.5 million in a wrongful death lawsuit filed against Hospice Ministries and its medical director. The jury awarded the family $4 million in monetary compensation and $500,000 in punitive damages.
The case of McGregor v. Hospice Care of Louisiana is illustrative of a malpractice action with a focus on expert testimony.6 The issue in this case was whether the testimony of the plaintiffs’ expert, Bruce Samuels, MD, was admissible and whether it correctly addressed the requisite standard of care.
The decedent had terminal metastatic prostate cancer and was under the care of an oncologist. He eventually enrolled as a patient of Hospice of Baton Rouge, whose nurses visited him in his home several times a week. They reported their findings to the attending oncologist, who prescribed a total of 40 morphine suppositories to be administered 1-2 per hour as needed for pain. However, the prescription noted that only half – that is, 20 suppositories – were to be filled, and stipulated when the remaining 20 suppositories could be released.
Believing that his father was in pain, the patient’s son demanded the early release of the remaining 20 morphine suppositories; he also refused to allow the nurse to assess the patient and exhibited threatening behavior toward her. After conferring with the oncologist on call, the hospice discharged the patient from its care. An ambulance later took the patient to a hospital, where he died that evening.
The family filed a lawsuit against the hospice, alleging negligence in failing to release the remaining 20 morphine suppositories and in abandoning the patient by discharging him. At trial, the jury rendered a verdict in favor of the hospice, after the court excluded the testimony of the plaintiffs’ expert as being outside his expertise. However, the Louisiana Supreme Court found that the trial court erred in excluding his testimony.
On remand, the appellate court affirmed the trial court’s judgment that the plaintiffs had failed to meet the burden of proof showing negligence. It found that the expert, Dr. Samuels, admitted he had never written a partial-fill prescription before and that he did not know who had the authority to authorize the pharmacist to release the remainder of the partial fill prescription in this case.
In addition, Dr. Samuels acknowledged that a nurse has the obligation to assess a patient and report her findings to the physician and follow any orders of the physician. Further, the nurse indicated that the doctor had instructed her to discharge the patient, not from the doctor’s care, but for treatment to be continued at the hospital.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from earlier columns in Internal Medicine News. For additional information, readers may contact the author at [email protected].
References
1. “Medicare’s most indefensible fraud hotspot: Hospice care.” CNBC, Modern Medicine, Aug. 3, 2018.
2. 31 U.S. Code, Section 3729(a)(1)(A).
3. Tan SY. “Update on the False Claims Act.” Internal Medicine News, April 5, 2017.
4. U.S. Department of Justice, Office of Public Affairs, Oct. 30, 2017.
5. U.S. ex rel. Paradies et al. v. AseraCare Inc. et al., case number 2:12-CV-245-KOB, in the U.S. District Court for the Northern District of Alabama, March 31, 2016.
6. McGregor v. Hospice Care of Louisiana in Baton Rouge, LLC, No. 2013 CA 1979R, consolidated with No. 2013 CA 1980R. Court of Appeals of Louisiana, First Circuit, judgment rendered Sept. 21, 2015.
Question: Hospice liability may exist in which of the following?
A. False claims in violation of Medicare rules regarding eligible beneficiaries.
B. False claims for continuous home care services.
C. Negligent billing practices.
D. Only A and B are correct.
E. A, B, and C are correct.
Answer: D. With an aging population and better end-of-life care, the United States has in the last decade witnessed about a 50% increase in the number of hospices. Hospice care is a Medicare-covered benefit, and most hospices operate on a for-profit basis. Although occasionally institution based, services are more often offered as an outpatient or home-care option. In 2016, hospice care reached 1.4 million beneficiaries, with total Medicare expenditure of $16.7 billion.1
There are two broad categories of legal jeopardy that hospices face: Medicare fraud and malpractice lawsuits. This article will address these two issues. In addition, hospices, like all health care institutions, face numerous other liabilities, such as negligent hiring, breach of confidentiality, premise liability, HIPAA violations, sexual harassment, vicarious liability, and many others.
Medicare fraud
The False Claims Act (FCA) is an old law enacted by Congress way back in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false.2
Intent to defraud is not a required element. But knowing or reckless disregard of the truth or material misrepresentation are required, although negligence is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim – as well as possible imprisonment. The FCA is the most prominent health care antifraud statute.3 Two others are the federal Anti-Kickback Statute and the Stark Law.
A recent example of hospice fraud involved Ohio’s Chemed and Vitas Hospice Services, which were accused of knowingly billing for hospice-ineligible patients and inflated levels of care.4
The government alleged that the defendants rewarded employees with bonuses based on the number of patients receiving hospice services, irrespective of whether they were actually terminally ill or needed continuous home care services (CHCS). CHCS commands the highest Medicare daily rate and is meant only for the temporary treatment of acute symptoms constituting a medical crisis.
According to the complaint, the defendants set aggressive billing goals for CHCS without regard to whether the patients actually required such a level of service. The defendants agreed to pay $75 million to settle the lawsuit, the largest in the history of hospice false-claim settlements.
Can an alleged wrong prognosis regarding life expectancy amount to a false claim? Under Medicare rules, a physician certifying that a patient is eligible for hospice care must attest that the condition is terminal, with death expected within 6 months.
AseraCare, a hospice company, was accused of knowingly submitting false claims to Medicare by certifying patients as eligible for hospice. The government claimed that the medical records of the 123 patients at issue did not contain clinical information and other documentation that supported the medical prognosis, and thus, AseraCare’s claims for those patients were false.
AseraCare won a summary judgment defending against the $200 million lawsuit in a federal district court in Alabama. The court opined that, when hospice-certifying physicians and government medical experts look at the very same medical records and disagree about eligibility, the opinion of one medical expert alone cannot prove falsity without further evidence of an objective falsehood.5 The government, however, has appealed the decision to the Court of Appeals for the Eleventh Circuit.
Malpractice
Hospices have their share of malpractice litigation, and judgments may be substantial because of noneconomic losses such as pain and suffering, not to mention punitive damages.
For example, in 2013a Maryland jury awarded more than $950,000 to a family that alleged that the decedent’s death was caused by the excessive use of morphine and oxycodone in treating her infected ulcers. Such treatment was deemed suitable for a hospice-type situation, but in fact, the patient was not expected to die within 6 months.
Her husband and two children argued successfully that the hospital committed malpractice by misdiagnosing her need for hospice care and by performing unnecessary surgery. The bulk of the judgment was for pain and suffering and other noneconomic damages.
In another negligence suit, a 66-year-old woman died in a hospice after receiving an overdose of Dilaudid for pancreatic cancer, which an autopsy revealed she did not have. In that case, the plaintiffs were awarded $4.5 million in a wrongful death lawsuit filed against Hospice Ministries and its medical director. The jury awarded the family $4 million in monetary compensation and $500,000 in punitive damages.
The case of McGregor v. Hospice Care of Louisiana is illustrative of a malpractice action with a focus on expert testimony.6 The issue in this case was whether the testimony of the plaintiffs’ expert, Bruce Samuels, MD, was admissible and whether it correctly addressed the requisite standard of care.
The decedent had terminal metastatic prostate cancer and was under the care of an oncologist. He eventually enrolled as a patient of Hospice of Baton Rouge, whose nurses visited him in his home several times a week. They reported their findings to the attending oncologist, who prescribed a total of 40 morphine suppositories to be administered 1-2 per hour as needed for pain. However, the prescription noted that only half – that is, 20 suppositories – were to be filled, and stipulated when the remaining 20 suppositories could be released.
Believing that his father was in pain, the patient’s son demanded the early release of the remaining 20 morphine suppositories; he also refused to allow the nurse to assess the patient and exhibited threatening behavior toward her. After conferring with the oncologist on call, the hospice discharged the patient from its care. An ambulance later took the patient to a hospital, where he died that evening.
The family filed a lawsuit against the hospice, alleging negligence in failing to release the remaining 20 morphine suppositories and in abandoning the patient by discharging him. At trial, the jury rendered a verdict in favor of the hospice, after the court excluded the testimony of the plaintiffs’ expert as being outside his expertise. However, the Louisiana Supreme Court found that the trial court erred in excluding his testimony.
On remand, the appellate court affirmed the trial court’s judgment that the plaintiffs had failed to meet the burden of proof showing negligence. It found that the expert, Dr. Samuels, admitted he had never written a partial-fill prescription before and that he did not know who had the authority to authorize the pharmacist to release the remainder of the partial fill prescription in this case.
In addition, Dr. Samuels acknowledged that a nurse has the obligation to assess a patient and report her findings to the physician and follow any orders of the physician. Further, the nurse indicated that the doctor had instructed her to discharge the patient, not from the doctor’s care, but for treatment to be continued at the hospital.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from earlier columns in Internal Medicine News. For additional information, readers may contact the author at [email protected].
References
1. “Medicare’s most indefensible fraud hotspot: Hospice care.” CNBC, Modern Medicine, Aug. 3, 2018.
2. 31 U.S. Code, Section 3729(a)(1)(A).
3. Tan SY. “Update on the False Claims Act.” Internal Medicine News, April 5, 2017.
4. U.S. Department of Justice, Office of Public Affairs, Oct. 30, 2017.
5. U.S. ex rel. Paradies et al. v. AseraCare Inc. et al., case number 2:12-CV-245-KOB, in the U.S. District Court for the Northern District of Alabama, March 31, 2016.
6. McGregor v. Hospice Care of Louisiana in Baton Rouge, LLC, No. 2013 CA 1979R, consolidated with No. 2013 CA 1980R. Court of Appeals of Louisiana, First Circuit, judgment rendered Sept. 21, 2015.
In-hospital blood saving strategy appears safe with anemia
A blood management initiative that reduced RBC transfusions in the hospital did not adversely impact long-term outcomes after discharge, a retrospective analysis of an extensive patient database suggested.
Tolerating moderate in-hospital anemia did not increase subsequent RBC use, readmission, or mortality over the next 6 months, according to results of the study, which drew on nearly half a million patient records.
In fact, modest mortality decreases were seen over time for patients with moderate anemia, perhaps because of concomitant initiatives that targeted infectious and circulatory conditions, reported Nareg H. Roubinian, MD, of Kaiser Permanente Northern California in Oakland and the University of California, San Francisco, and coinvestigators.
“These data support the efficacy and safety of practice recommendations to limit red blood cell transfusion in patients with anemia during and after hospitalization,” Dr. Roubinian and colleagues wrote in their report, which appears in the Annals of Internal Medicine.
However, additional studies are needed to guide anemia management, they wrote, particularly since persistent anemia has impacts on quality of life that are “likely substantial” and linked to the severity of that anemia.
Dr. Roubinian and colleagues sought to evaluate the impact of blood management programs – initiated starting in 2010 – that included blood-sparing surgical and medical techniques, increased use of hemostatic and cell salvage agents, and treatment of suboptimal iron stores before surgery.
In previous retrospective cohort studies, the researchers had found that the blood conservation strategies did not impact in-hospital or 30-day mortality rates, which was consistent with short-term safety data from clinical trials and other observational studies.
Their latest report on longer-term outcomes was based on data from Kaiser Permanente Northern California for 445,371 adults who had 801,261 hospitalizations with discharges between 2010 and 2014. In this cohort, moderate anemia (hemoglobin between 7 g/dL and 10 g/dL) at discharge occurred in 119,489 patients (27%) and 187,440 hospitalizations overall (23%).
Over the 2010-2014 period, RBC transfusions decreased by more than 25% in the inpatient and outpatient settings; and in parallel, the prevalence of moderate anemia at hospital discharge increased from 20% to 25%.
However, the risks of subsequent RBC transfusions and rehospitalization after discharge with anemia decreased during the study period, and mortality rates stayed steady or decreased slightly.
Among patients with moderate anemia, the proportion with subsequent RBC transfusions within 6 months decreased from 18.9% in 2010 to 16.8% in 2014 (P less than .001), while the rate of rehospitalization within 6 months decreased from 36.5% to 32.8% over that same time period (P less than .001).
The adjusted 6-month mortality rate likewise decreased from 16.1% to 15.6% (P = .004) over that time period among patients with moderate anemia.
The study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Roubinian and several coauthors reported grants during the conduct of the study from the National Institutes of Health.
SOURCE: Roubinian NH et al. Ann Intern Med. 2018 Dec 18. doi: 10.7326/M17-3253.
Some scrutiny is warranted of the observation of Roubinian et al. that long-term transfusion, readmission, and mortality outcomes were apparently unaffected by decreased in-hospital RBC transfusions, according to the authors of an accompanying editorial.
“Missing here is a wide spectrum of morbidity outcomes and issues related to diminished quality of life that do not reach the level of severity that would necessitate admission but nonetheless detract from patients’ health and well-being,” wrote Aryeh Shander, MD, and Lawrence Tim Goodnough, MD.
Moreover, transfusion rate is not a clinical outcome, they noted, adding that readmission and mortality are important outcomes but that they do not accurately or fully reflect patient well-being.
While blood management initiatives may be a safe practice, as Roubinian et al. found, proper management of anemia after discharge may actually improve outcomes, given the many consequences of anemia.
Instead of again testing whether restricting transfusions is acceptable because of lack of impact on outcomes, future studies could evaluate a “more sensible” hypothesis that proper anemia management – especially post discharge – could improve outcomes.
“Let’s increase efforts to prevent and treat anemia properly, rather than requiring patients to tolerate it,” they wrote.
Dr. Shander is with Englewood (N.J.) Hospital and Medical Center; Dr. Goodnough is with Stanford (Calif.) University. Dr. Shander reported consulting fees from Vifor and AMAG. Dr. Goodnough reported having no relevant financial disclosures. Their comments are taken from an accompanying editorial (Ann Intern Med. 2018 Dec 18. doi: 10.7326/M18-3145).
Some scrutiny is warranted of the observation of Roubinian et al. that long-term transfusion, readmission, and mortality outcomes were apparently unaffected by decreased in-hospital RBC transfusions, according to the authors of an accompanying editorial.
“Missing here is a wide spectrum of morbidity outcomes and issues related to diminished quality of life that do not reach the level of severity that would necessitate admission but nonetheless detract from patients’ health and well-being,” wrote Aryeh Shander, MD, and Lawrence Tim Goodnough, MD.
Moreover, transfusion rate is not a clinical outcome, they noted, adding that readmission and mortality are important outcomes but that they do not accurately or fully reflect patient well-being.
While blood management initiatives may be a safe practice, as Roubinian et al. found, proper management of anemia after discharge may actually improve outcomes, given the many consequences of anemia.
Instead of again testing whether restricting transfusions is acceptable because of lack of impact on outcomes, future studies could evaluate a “more sensible” hypothesis that proper anemia management – especially post discharge – could improve outcomes.
“Let’s increase efforts to prevent and treat anemia properly, rather than requiring patients to tolerate it,” they wrote.
Dr. Shander is with Englewood (N.J.) Hospital and Medical Center; Dr. Goodnough is with Stanford (Calif.) University. Dr. Shander reported consulting fees from Vifor and AMAG. Dr. Goodnough reported having no relevant financial disclosures. Their comments are taken from an accompanying editorial (Ann Intern Med. 2018 Dec 18. doi: 10.7326/M18-3145).
Some scrutiny is warranted of the observation of Roubinian et al. that long-term transfusion, readmission, and mortality outcomes were apparently unaffected by decreased in-hospital RBC transfusions, according to the authors of an accompanying editorial.
“Missing here is a wide spectrum of morbidity outcomes and issues related to diminished quality of life that do not reach the level of severity that would necessitate admission but nonetheless detract from patients’ health and well-being,” wrote Aryeh Shander, MD, and Lawrence Tim Goodnough, MD.
Moreover, transfusion rate is not a clinical outcome, they noted, adding that readmission and mortality are important outcomes but that they do not accurately or fully reflect patient well-being.
While blood management initiatives may be a safe practice, as Roubinian et al. found, proper management of anemia after discharge may actually improve outcomes, given the many consequences of anemia.
Instead of again testing whether restricting transfusions is acceptable because of lack of impact on outcomes, future studies could evaluate a “more sensible” hypothesis that proper anemia management – especially post discharge – could improve outcomes.
“Let’s increase efforts to prevent and treat anemia properly, rather than requiring patients to tolerate it,” they wrote.
Dr. Shander is with Englewood (N.J.) Hospital and Medical Center; Dr. Goodnough is with Stanford (Calif.) University. Dr. Shander reported consulting fees from Vifor and AMAG. Dr. Goodnough reported having no relevant financial disclosures. Their comments are taken from an accompanying editorial (Ann Intern Med. 2018 Dec 18. doi: 10.7326/M18-3145).
A blood management initiative that reduced RBC transfusions in the hospital did not adversely impact long-term outcomes after discharge, a retrospective analysis of an extensive patient database suggested.
Tolerating moderate in-hospital anemia did not increase subsequent RBC use, readmission, or mortality over the next 6 months, according to results of the study, which drew on nearly half a million patient records.
In fact, modest mortality decreases were seen over time for patients with moderate anemia, perhaps because of concomitant initiatives that targeted infectious and circulatory conditions, reported Nareg H. Roubinian, MD, of Kaiser Permanente Northern California in Oakland and the University of California, San Francisco, and coinvestigators.
“These data support the efficacy and safety of practice recommendations to limit red blood cell transfusion in patients with anemia during and after hospitalization,” Dr. Roubinian and colleagues wrote in their report, which appears in the Annals of Internal Medicine.
However, additional studies are needed to guide anemia management, they wrote, particularly since persistent anemia has impacts on quality of life that are “likely substantial” and linked to the severity of that anemia.
Dr. Roubinian and colleagues sought to evaluate the impact of blood management programs – initiated starting in 2010 – that included blood-sparing surgical and medical techniques, increased use of hemostatic and cell salvage agents, and treatment of suboptimal iron stores before surgery.
In previous retrospective cohort studies, the researchers had found that the blood conservation strategies did not impact in-hospital or 30-day mortality rates, which was consistent with short-term safety data from clinical trials and other observational studies.
Their latest report on longer-term outcomes was based on data from Kaiser Permanente Northern California for 445,371 adults who had 801,261 hospitalizations with discharges between 2010 and 2014. In this cohort, moderate anemia (hemoglobin between 7 g/dL and 10 g/dL) at discharge occurred in 119,489 patients (27%) and 187,440 hospitalizations overall (23%).
Over the 2010-2014 period, RBC transfusions decreased by more than 25% in the inpatient and outpatient settings; and in parallel, the prevalence of moderate anemia at hospital discharge increased from 20% to 25%.
However, the risks of subsequent RBC transfusions and rehospitalization after discharge with anemia decreased during the study period, and mortality rates stayed steady or decreased slightly.
Among patients with moderate anemia, the proportion with subsequent RBC transfusions within 6 months decreased from 18.9% in 2010 to 16.8% in 2014 (P less than .001), while the rate of rehospitalization within 6 months decreased from 36.5% to 32.8% over that same time period (P less than .001).
The adjusted 6-month mortality rate likewise decreased from 16.1% to 15.6% (P = .004) over that time period among patients with moderate anemia.
The study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Roubinian and several coauthors reported grants during the conduct of the study from the National Institutes of Health.
SOURCE: Roubinian NH et al. Ann Intern Med. 2018 Dec 18. doi: 10.7326/M17-3253.
A blood management initiative that reduced RBC transfusions in the hospital did not adversely impact long-term outcomes after discharge, a retrospective analysis of an extensive patient database suggested.
Tolerating moderate in-hospital anemia did not increase subsequent RBC use, readmission, or mortality over the next 6 months, according to results of the study, which drew on nearly half a million patient records.
In fact, modest mortality decreases were seen over time for patients with moderate anemia, perhaps because of concomitant initiatives that targeted infectious and circulatory conditions, reported Nareg H. Roubinian, MD, of Kaiser Permanente Northern California in Oakland and the University of California, San Francisco, and coinvestigators.
“These data support the efficacy and safety of practice recommendations to limit red blood cell transfusion in patients with anemia during and after hospitalization,” Dr. Roubinian and colleagues wrote in their report, which appears in the Annals of Internal Medicine.
However, additional studies are needed to guide anemia management, they wrote, particularly since persistent anemia has impacts on quality of life that are “likely substantial” and linked to the severity of that anemia.
Dr. Roubinian and colleagues sought to evaluate the impact of blood management programs – initiated starting in 2010 – that included blood-sparing surgical and medical techniques, increased use of hemostatic and cell salvage agents, and treatment of suboptimal iron stores before surgery.
In previous retrospective cohort studies, the researchers had found that the blood conservation strategies did not impact in-hospital or 30-day mortality rates, which was consistent with short-term safety data from clinical trials and other observational studies.
Their latest report on longer-term outcomes was based on data from Kaiser Permanente Northern California for 445,371 adults who had 801,261 hospitalizations with discharges between 2010 and 2014. In this cohort, moderate anemia (hemoglobin between 7 g/dL and 10 g/dL) at discharge occurred in 119,489 patients (27%) and 187,440 hospitalizations overall (23%).
Over the 2010-2014 period, RBC transfusions decreased by more than 25% in the inpatient and outpatient settings; and in parallel, the prevalence of moderate anemia at hospital discharge increased from 20% to 25%.
However, the risks of subsequent RBC transfusions and rehospitalization after discharge with anemia decreased during the study period, and mortality rates stayed steady or decreased slightly.
Among patients with moderate anemia, the proportion with subsequent RBC transfusions within 6 months decreased from 18.9% in 2010 to 16.8% in 2014 (P less than .001), while the rate of rehospitalization within 6 months decreased from 36.5% to 32.8% over that same time period (P less than .001).
The adjusted 6-month mortality rate likewise decreased from 16.1% to 15.6% (P = .004) over that time period among patients with moderate anemia.
The study was supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Roubinian and several coauthors reported grants during the conduct of the study from the National Institutes of Health.
SOURCE: Roubinian NH et al. Ann Intern Med. 2018 Dec 18. doi: 10.7326/M17-3253.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: The adjusted 6-month mortality rate decreased from 16.1% to 15.6% (P = .004) in the 4-year period following implementation of blood conservation strategies.
Study details: A retrospective cohort study including 445,371 adults hospitalized and discharged between 2010 and 2014.
Disclosures: The study was supported by a grant from the National Heart, Lung, and Blood Institute. Several authors reported grants during the conduct of the study from the National Institutes of Health.
Source: Roubinian NH et al. Ann Intern Med. 2018 Dec 18. doi: 10.7326/M17-3253.
Low BMP-10 levels correlate with poor ovarian cancer survival
Loss of expression of bone morphogenetic protein-10 (BMP-10) in ovarian cancer cells appears to be a marker for poor prognosis, investigators claim.
Low expression of BMP-10 in ovarian cancer tissues and cell lines significantly correlated with higher-stage disease, high-risk features, and poor prognosis. Conversely, over-expression of BMP-10 was significantly associated with inhibition of ovarian cancer cell proliferation, migration, and invasion, reported Yufeng Jin, PhD, and colleagues from Nantong (China) University.
“Loss of BMP-10 expression might represent a poor prognosis in ovarian cancer patients. Meanwhile, loss of BMP-10 could promote malignant behaviors in ovarian cell lines, suggesting it might be a promising tumor suppressor,” they wrote in Pathology – Research and Practice.
Other members of the BMP family of cytokines and metabolites have recently been implicated in development of esophageal squamous cell cancer, breast cancer, and colorectal cancer, and BMP-10 has been linked to ovarian tumor progression, the investigators noted.
To get a better sense of BMP-10’s potential role as an ovarian cancer suppressor, the investigators first examined eight paired samples of ovarian cancers and adjacent normal tissues, and observed that in most pairs expression of the protein was significantly lower in the cancerous tissues (P less than .01).
Next, they looked at BMP-10 expression in ovarian tissues with varying histological grades; they found that it was overexpressed in normal tissues, but underexpressed in ovarian cancer tissues, especially those from patients with advanced grade disease.
The investigators then looked at the association between BMP-10 expression and clinicopathologic features, using a cutoff of 30% as a mean value for BMP-10 expression in tissues. They found the expression of the protein trended toward correlation with FIGO (International Federation of Gynecology and Obstetrics) stage (P = .08) and correlated significantly with histologic grade (P = .018), lymph node metastasis (P = .004), and peritoneal fluid (P = .032). There were no significant correlations with patient age, histologic subtype, residual tumor size or tumor cells in peritoneal fluid, however.
The authors also found that patients whose tissues had low levels of BMP-10 expression had significantly shorter overall survival (P less than .01), and in multivariate analysis, they saw that expression of the protein was an independent prognostic factor (hazard ratio, 4.834; P = .002).
Turning to in vitro studies, they observed that overexpression of the protein inhibited proliferation of an ovarian cancer cell line (Ovca3), while introduction of an antibody that neutralized BMP-10 allowed proliferation to occur unimpeded.
Finally, they showed that BMP-10 overexpression impaired the wound-healing and invasive properties of Ovca3 cells, while knockdown of the protein’s expression promoted migration and invasion of a different ovarian carcinoma cell line (Skov3).
The investigators acknowledged that their study was limited by small sample sizes, and they noted that they did not investigate potential mechanisms for BMP-10’s effect on ovarian cells.
Nonetheless, they concluded that “BMP-10 should be considered as a promising prognostic marker and a crucial regulator for progression of ovarian cancer.”
The study was supported by the Nantong Health and Family Planning Commission. All authors declared having no conflicts of interest.
SOURCE: Jin Y et al. Pathol Res Pract. 2019 Oct 28. doi: 10.1016/j.prp.2018.10.025.
Loss of expression of bone morphogenetic protein-10 (BMP-10) in ovarian cancer cells appears to be a marker for poor prognosis, investigators claim.
Low expression of BMP-10 in ovarian cancer tissues and cell lines significantly correlated with higher-stage disease, high-risk features, and poor prognosis. Conversely, over-expression of BMP-10 was significantly associated with inhibition of ovarian cancer cell proliferation, migration, and invasion, reported Yufeng Jin, PhD, and colleagues from Nantong (China) University.
“Loss of BMP-10 expression might represent a poor prognosis in ovarian cancer patients. Meanwhile, loss of BMP-10 could promote malignant behaviors in ovarian cell lines, suggesting it might be a promising tumor suppressor,” they wrote in Pathology – Research and Practice.
Other members of the BMP family of cytokines and metabolites have recently been implicated in development of esophageal squamous cell cancer, breast cancer, and colorectal cancer, and BMP-10 has been linked to ovarian tumor progression, the investigators noted.
To get a better sense of BMP-10’s potential role as an ovarian cancer suppressor, the investigators first examined eight paired samples of ovarian cancers and adjacent normal tissues, and observed that in most pairs expression of the protein was significantly lower in the cancerous tissues (P less than .01).
Next, they looked at BMP-10 expression in ovarian tissues with varying histological grades; they found that it was overexpressed in normal tissues, but underexpressed in ovarian cancer tissues, especially those from patients with advanced grade disease.
The investigators then looked at the association between BMP-10 expression and clinicopathologic features, using a cutoff of 30% as a mean value for BMP-10 expression in tissues. They found the expression of the protein trended toward correlation with FIGO (International Federation of Gynecology and Obstetrics) stage (P = .08) and correlated significantly with histologic grade (P = .018), lymph node metastasis (P = .004), and peritoneal fluid (P = .032). There were no significant correlations with patient age, histologic subtype, residual tumor size or tumor cells in peritoneal fluid, however.
The authors also found that patients whose tissues had low levels of BMP-10 expression had significantly shorter overall survival (P less than .01), and in multivariate analysis, they saw that expression of the protein was an independent prognostic factor (hazard ratio, 4.834; P = .002).
Turning to in vitro studies, they observed that overexpression of the protein inhibited proliferation of an ovarian cancer cell line (Ovca3), while introduction of an antibody that neutralized BMP-10 allowed proliferation to occur unimpeded.
Finally, they showed that BMP-10 overexpression impaired the wound-healing and invasive properties of Ovca3 cells, while knockdown of the protein’s expression promoted migration and invasion of a different ovarian carcinoma cell line (Skov3).
The investigators acknowledged that their study was limited by small sample sizes, and they noted that they did not investigate potential mechanisms for BMP-10’s effect on ovarian cells.
Nonetheless, they concluded that “BMP-10 should be considered as a promising prognostic marker and a crucial regulator for progression of ovarian cancer.”
The study was supported by the Nantong Health and Family Planning Commission. All authors declared having no conflicts of interest.
SOURCE: Jin Y et al. Pathol Res Pract. 2019 Oct 28. doi: 10.1016/j.prp.2018.10.025.
Loss of expression of bone morphogenetic protein-10 (BMP-10) in ovarian cancer cells appears to be a marker for poor prognosis, investigators claim.
Low expression of BMP-10 in ovarian cancer tissues and cell lines significantly correlated with higher-stage disease, high-risk features, and poor prognosis. Conversely, over-expression of BMP-10 was significantly associated with inhibition of ovarian cancer cell proliferation, migration, and invasion, reported Yufeng Jin, PhD, and colleagues from Nantong (China) University.
“Loss of BMP-10 expression might represent a poor prognosis in ovarian cancer patients. Meanwhile, loss of BMP-10 could promote malignant behaviors in ovarian cell lines, suggesting it might be a promising tumor suppressor,” they wrote in Pathology – Research and Practice.
Other members of the BMP family of cytokines and metabolites have recently been implicated in development of esophageal squamous cell cancer, breast cancer, and colorectal cancer, and BMP-10 has been linked to ovarian tumor progression, the investigators noted.
To get a better sense of BMP-10’s potential role as an ovarian cancer suppressor, the investigators first examined eight paired samples of ovarian cancers and adjacent normal tissues, and observed that in most pairs expression of the protein was significantly lower in the cancerous tissues (P less than .01).
Next, they looked at BMP-10 expression in ovarian tissues with varying histological grades; they found that it was overexpressed in normal tissues, but underexpressed in ovarian cancer tissues, especially those from patients with advanced grade disease.
The investigators then looked at the association between BMP-10 expression and clinicopathologic features, using a cutoff of 30% as a mean value for BMP-10 expression in tissues. They found the expression of the protein trended toward correlation with FIGO (International Federation of Gynecology and Obstetrics) stage (P = .08) and correlated significantly with histologic grade (P = .018), lymph node metastasis (P = .004), and peritoneal fluid (P = .032). There were no significant correlations with patient age, histologic subtype, residual tumor size or tumor cells in peritoneal fluid, however.
The authors also found that patients whose tissues had low levels of BMP-10 expression had significantly shorter overall survival (P less than .01), and in multivariate analysis, they saw that expression of the protein was an independent prognostic factor (hazard ratio, 4.834; P = .002).
Turning to in vitro studies, they observed that overexpression of the protein inhibited proliferation of an ovarian cancer cell line (Ovca3), while introduction of an antibody that neutralized BMP-10 allowed proliferation to occur unimpeded.
Finally, they showed that BMP-10 overexpression impaired the wound-healing and invasive properties of Ovca3 cells, while knockdown of the protein’s expression promoted migration and invasion of a different ovarian carcinoma cell line (Skov3).
The investigators acknowledged that their study was limited by small sample sizes, and they noted that they did not investigate potential mechanisms for BMP-10’s effect on ovarian cells.
Nonetheless, they concluded that “BMP-10 should be considered as a promising prognostic marker and a crucial regulator for progression of ovarian cancer.”
The study was supported by the Nantong Health and Family Planning Commission. All authors declared having no conflicts of interest.
SOURCE: Jin Y et al. Pathol Res Pract. 2019 Oct 28. doi: 10.1016/j.prp.2018.10.025.
Key clinical point: Expression levels of BMP-10 significantly correlated with ovarian cancer features and patient prognosis.
Major finding: Patients whose tissues had low levels of BMP-10 expression had significantly shorter overall survival (P less than .01),
Study details: Analysis of BMP-10 expression in ovarian cancers, normal tissues, and ovarian cancer cell lines.
Disclosures: The study was supported by the Nantong Health and Family Planning Commission. All authors declared having no conflicts of interest.
Source: Jin Y et al. Pathol Res Pract. 2018 Oct 28. doi: 10.1016/j.prp.2018.10.025.
Patellofemoral Instability in the Skeletally Immature Patient: A Review and Technical Description of Medial Patellofemoral Ligament Reconstruction in Patients with Open Physes
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
TAKE-HOME POINTS
- Patellofemoral instability is common in the skeletally immature age group.
- Many treatment options exist for patellofemoral instability; however, a medial patellofemoral ligament (MPFL) reconstruction is an option in a skeletally immature patient.
- Allograft or autografts may be utilized for the MPFL reconstruction.
- Three options exist to confirm location of the MPFL origin on the patella, but only one drill tunnel is recommended in pediatrics.
- During identification of MPFL origin, special views in the coronal plane should be considered to avoid injury or damage to the growth plate.