User login
Provocative Induction of PNES Doesn’t Require Placebos
Diagnosing psychogenic nonepileptic seizures (PNES) can prove challenging; using provocative induction is one way to detect the disorder. A recent experiment suggests that inducing seizures without the use of a placebo is just as effective as inducing them with one.
- Researchers compared 170 patients suspected of having PNES who underwent provocative induction plus placebo to 170 patients who underwent the same induction procedure without a saline solution placebo.
- Induction triggered a seizure in 79.4% of patients without the help of the placebo, compared to 73.5% with placebo, a non-significant difference.
- Investigators postulated that the greater success rate in the non-placebo group may have resulted from the greater cumulative induction experience of clinicians, which may have influenced the manner and presentation of how the induction was presented.
- The study concluded that experienced clinicians should opt for non-placebo based provocative induction.
Chen DK, Dave H, Gadelmola, K et al. Provocative induction of psychogenic nonepileptic seizures: Noninferiority of an induction technique without versus with placebo. Epilepsia. 2018; 59:e161-e165.
Diagnosing psychogenic nonepileptic seizures (PNES) can prove challenging; using provocative induction is one way to detect the disorder. A recent experiment suggests that inducing seizures without the use of a placebo is just as effective as inducing them with one.
- Researchers compared 170 patients suspected of having PNES who underwent provocative induction plus placebo to 170 patients who underwent the same induction procedure without a saline solution placebo.
- Induction triggered a seizure in 79.4% of patients without the help of the placebo, compared to 73.5% with placebo, a non-significant difference.
- Investigators postulated that the greater success rate in the non-placebo group may have resulted from the greater cumulative induction experience of clinicians, which may have influenced the manner and presentation of how the induction was presented.
- The study concluded that experienced clinicians should opt for non-placebo based provocative induction.
Chen DK, Dave H, Gadelmola, K et al. Provocative induction of psychogenic nonepileptic seizures: Noninferiority of an induction technique without versus with placebo. Epilepsia. 2018; 59:e161-e165.
Diagnosing psychogenic nonepileptic seizures (PNES) can prove challenging; using provocative induction is one way to detect the disorder. A recent experiment suggests that inducing seizures without the use of a placebo is just as effective as inducing them with one.
- Researchers compared 170 patients suspected of having PNES who underwent provocative induction plus placebo to 170 patients who underwent the same induction procedure without a saline solution placebo.
- Induction triggered a seizure in 79.4% of patients without the help of the placebo, compared to 73.5% with placebo, a non-significant difference.
- Investigators postulated that the greater success rate in the non-placebo group may have resulted from the greater cumulative induction experience of clinicians, which may have influenced the manner and presentation of how the induction was presented.
- The study concluded that experienced clinicians should opt for non-placebo based provocative induction.
Chen DK, Dave H, Gadelmola, K et al. Provocative induction of psychogenic nonepileptic seizures: Noninferiority of an induction technique without versus with placebo. Epilepsia. 2018; 59:e161-e165.
mRNA-based urine test performs well in bladder cancer surveillance
An mRNA-based assay for surveillance of patients with bladder cancer outperformed standard urine tests on certain measures in a validation study, investigators report.
The mRNA-based urine test (Xpert, Cepheid) had better sensitivity and negative predictive value compared to urine cytology and to UroVysion fluorescence in situ hybridization (FISH) testing, according to F. Johannes P. van Valenberg, of Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues.
The reported results suggest this mRNA test could partially replace white-light cystoscopy, helping urologists maintain the recommended follow-up schedules for patients with a history of bladder cancer, Dr. van Valenberg and his coauthors wrote. The report is in European Urology.
“If used in the follow-up of non–muscle invasive bladder cancer, a cystoscopy might be waived if the Xpert result is negative,” they wrote.
The negative predictive value for high-grade disease was 98%, suggesting the mRNA-based test could help urologists avoid invasive cystoscopies for intermediate- to high-risk patients, which could reduce costs and patient discomfort, they added.
The prospective, 19-center validation study enrolled 363 individuals with a history of non–muscle invasive bladder cancer who were scheduled for a standard cystoscopy. Voided urine specimens from a total of 259 patients were evaluated with all three methods: the mRNA test, FISH testing, and cytology.
In a comparison of the tests, the mRNA test identified more recurrent cancers correctly, and was more sensitive in detecting low-grade tumors than was FISH (P less than .001) or cytology (P = .021), they reported.
The mRNA test was more sensitive for detection of the most common recurrent tumors, independent of grade.
The sensitivity, specificity, and negative predictive values for mRNA testing were 74%, 80%, and 93%, respectively. By comparison the sensitivity, specificity, and negative predictive values were 51%, 80%, and 88% for FISH and 30%, 90%, and 86% for cytology.
Looking at high-grade disease only, the sensitivity, specificity, and negative predictive values were 83.3%, 75.8%, and 97.6% for mRNA testing, 75.0%, 79.5%, and 96.6% for FISH testing, and 50.0%, 90.7%, and 94.2% for cytology.
Bacillus Calmette-Guérin treatment in the past 90 days did not influence results of the mRNA test, one additional analysis showed.
The Xpert mRNA assay evaluates five mRNA targets: ABL1, ANXA10, UPK1B, CRH, and IGF2, results of which are combined to classify samples as either negative or positive. The test has a “hands-on time” of less than 2 minutes and provides results in 90 minutes, according to Dr. van Valenberg and his coinvestigators.
“Cytology requires a review by a pathologist, is not performed on the same day as a clinic visit, and has associated inter- and intraobserver variability,” the researchers wrote.
Dr. van Valenberg reported no financial disclosures related to the study. Several study coauthors reported employment or financial disclosures related to Cepheid, which provided funding and support for the conduct of the study. Study coauthors provided additional disclosures related to MDxHealth, PHotocure, Bristol-Myers Squibb, Johnson & Johnson, Roche, Bayer, and Astellas, among others.
SOURCE: van Valenberg FJP et al. Eur Urol. 2018 Dec 12. doi: 10.1016/j.eururo.2018.11.055.
An mRNA-based assay for surveillance of patients with bladder cancer outperformed standard urine tests on certain measures in a validation study, investigators report.
The mRNA-based urine test (Xpert, Cepheid) had better sensitivity and negative predictive value compared to urine cytology and to UroVysion fluorescence in situ hybridization (FISH) testing, according to F. Johannes P. van Valenberg, of Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues.
The reported results suggest this mRNA test could partially replace white-light cystoscopy, helping urologists maintain the recommended follow-up schedules for patients with a history of bladder cancer, Dr. van Valenberg and his coauthors wrote. The report is in European Urology.
“If used in the follow-up of non–muscle invasive bladder cancer, a cystoscopy might be waived if the Xpert result is negative,” they wrote.
The negative predictive value for high-grade disease was 98%, suggesting the mRNA-based test could help urologists avoid invasive cystoscopies for intermediate- to high-risk patients, which could reduce costs and patient discomfort, they added.
The prospective, 19-center validation study enrolled 363 individuals with a history of non–muscle invasive bladder cancer who were scheduled for a standard cystoscopy. Voided urine specimens from a total of 259 patients were evaluated with all three methods: the mRNA test, FISH testing, and cytology.
In a comparison of the tests, the mRNA test identified more recurrent cancers correctly, and was more sensitive in detecting low-grade tumors than was FISH (P less than .001) or cytology (P = .021), they reported.
The mRNA test was more sensitive for detection of the most common recurrent tumors, independent of grade.
The sensitivity, specificity, and negative predictive values for mRNA testing were 74%, 80%, and 93%, respectively. By comparison the sensitivity, specificity, and negative predictive values were 51%, 80%, and 88% for FISH and 30%, 90%, and 86% for cytology.
Looking at high-grade disease only, the sensitivity, specificity, and negative predictive values were 83.3%, 75.8%, and 97.6% for mRNA testing, 75.0%, 79.5%, and 96.6% for FISH testing, and 50.0%, 90.7%, and 94.2% for cytology.
Bacillus Calmette-Guérin treatment in the past 90 days did not influence results of the mRNA test, one additional analysis showed.
The Xpert mRNA assay evaluates five mRNA targets: ABL1, ANXA10, UPK1B, CRH, and IGF2, results of which are combined to classify samples as either negative or positive. The test has a “hands-on time” of less than 2 minutes and provides results in 90 minutes, according to Dr. van Valenberg and his coinvestigators.
“Cytology requires a review by a pathologist, is not performed on the same day as a clinic visit, and has associated inter- and intraobserver variability,” the researchers wrote.
Dr. van Valenberg reported no financial disclosures related to the study. Several study coauthors reported employment or financial disclosures related to Cepheid, which provided funding and support for the conduct of the study. Study coauthors provided additional disclosures related to MDxHealth, PHotocure, Bristol-Myers Squibb, Johnson & Johnson, Roche, Bayer, and Astellas, among others.
SOURCE: van Valenberg FJP et al. Eur Urol. 2018 Dec 12. doi: 10.1016/j.eururo.2018.11.055.
An mRNA-based assay for surveillance of patients with bladder cancer outperformed standard urine tests on certain measures in a validation study, investigators report.
The mRNA-based urine test (Xpert, Cepheid) had better sensitivity and negative predictive value compared to urine cytology and to UroVysion fluorescence in situ hybridization (FISH) testing, according to F. Johannes P. van Valenberg, of Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues.
The reported results suggest this mRNA test could partially replace white-light cystoscopy, helping urologists maintain the recommended follow-up schedules for patients with a history of bladder cancer, Dr. van Valenberg and his coauthors wrote. The report is in European Urology.
“If used in the follow-up of non–muscle invasive bladder cancer, a cystoscopy might be waived if the Xpert result is negative,” they wrote.
The negative predictive value for high-grade disease was 98%, suggesting the mRNA-based test could help urologists avoid invasive cystoscopies for intermediate- to high-risk patients, which could reduce costs and patient discomfort, they added.
The prospective, 19-center validation study enrolled 363 individuals with a history of non–muscle invasive bladder cancer who were scheduled for a standard cystoscopy. Voided urine specimens from a total of 259 patients were evaluated with all three methods: the mRNA test, FISH testing, and cytology.
In a comparison of the tests, the mRNA test identified more recurrent cancers correctly, and was more sensitive in detecting low-grade tumors than was FISH (P less than .001) or cytology (P = .021), they reported.
The mRNA test was more sensitive for detection of the most common recurrent tumors, independent of grade.
The sensitivity, specificity, and negative predictive values for mRNA testing were 74%, 80%, and 93%, respectively. By comparison the sensitivity, specificity, and negative predictive values were 51%, 80%, and 88% for FISH and 30%, 90%, and 86% for cytology.
Looking at high-grade disease only, the sensitivity, specificity, and negative predictive values were 83.3%, 75.8%, and 97.6% for mRNA testing, 75.0%, 79.5%, and 96.6% for FISH testing, and 50.0%, 90.7%, and 94.2% for cytology.
Bacillus Calmette-Guérin treatment in the past 90 days did not influence results of the mRNA test, one additional analysis showed.
The Xpert mRNA assay evaluates five mRNA targets: ABL1, ANXA10, UPK1B, CRH, and IGF2, results of which are combined to classify samples as either negative or positive. The test has a “hands-on time” of less than 2 minutes and provides results in 90 minutes, according to Dr. van Valenberg and his coinvestigators.
“Cytology requires a review by a pathologist, is not performed on the same day as a clinic visit, and has associated inter- and intraobserver variability,” the researchers wrote.
Dr. van Valenberg reported no financial disclosures related to the study. Several study coauthors reported employment or financial disclosures related to Cepheid, which provided funding and support for the conduct of the study. Study coauthors provided additional disclosures related to MDxHealth, PHotocure, Bristol-Myers Squibb, Johnson & Johnson, Roche, Bayer, and Astellas, among others.
SOURCE: van Valenberg FJP et al. Eur Urol. 2018 Dec 12. doi: 10.1016/j.eururo.2018.11.055.
FROM EUROPEAN UROLOGY
Key clinical point: A noninvasive mRNA-based urine test performed favorably compared with standard urine tests for surveillance of patients with bladder cancer.
Major finding: The mRNA test had a sensitivity of 74% and negative predictive value of 93%, superior to what was observed with fluorescence in situ hybridization (FISH) testing and cytology, according to investigators.
Study details: Prospective validation study including 363 patients with a history of non–muscle invasive bladder cancer.
Disclosures: Funding and trial support came from Cepheid. Several study coauthors reported employment or financial disclosures related to that company. Dr. van Valenberg reported no financial disclosures related to the study, while coauthors provided disclosures related to Bristol-Myers Squibb, Johnson & Johnson, Roche, Bayer, Astellas, and others.
Source: Van Valenberg FJP et al. Eur Urol. 2018 Dec 12. doi: 10.1016/j.eururo.2018.11.055.
FDA aims to boost safety of platelets for transfusion
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
Hematologists are outliers in care at the end of life
SAN DIEGO – When it comes to aggressive care at the end of life, hematologists stand alone.
Hematology patients are more likely than are other patients to undergo chemotherapy and visit emergency departments and intensive care units when they’re near death, and they’re less likely to be referred for palliative care, according to David Hui, MD, an oncologist and palliative care specialist at the MD Anderson Cancer Center in Houston.
An analysis at the center, for example, found that 43% of hematology cancer patients received chemotherapy within the last 30 days of life, compared with 14% of patients with solid tumors.
“That’s not a number we’re proud of,” Dr. Hui said at the annual meeting of the American Society of Hematology. “Ultimately, at the end of life, do we want our patients to be in this setting? There is room for improvement.”
The cancer center isn’t an outlier on this front, Dr. Hui said. Data from other institutions in the United States and internationally confirm that hematologic oncologists tend to provide more aggressive care at the end of a patient’s life, compared with other cancer specialists.
“If you’re one of those patients, this is a very big deal,” said Dr. Hui, especially in light of data that suggest hematology patients get fewer referrals to palliative care than do other cancer patients. “Oncologists are optimistic, and hematologic oncologists especially,” he said.
Dr. Hui led a 2014 study of 816 adult cancer patients who died while under care at MD Anderson Cancer Center during 6 months in 2009 and 2010 (Cancer. 2014 May 15; 120[10]:1572-8).
“We found that patients with hematological malignancies were more likely to have multiple emergency room visits, intensive care unit admissions and death, and cancer treatments in the last weeks of life compared to patients with solid tumors,” the study authors wrote. “We also identified a relative lack of palliative care involvement in hematologic patients.”
Specifically, hematology cancer patients were much more likely to get aggressive end-of-life care than were the other cancer patients (odds ratio, 6.63, P less than .001).
Dr. Hui had led an earlier study that looked at the same 816 cancer patients and found that 45% had received palliative care consultations. But the researchers also found that patients with hematologic malignancies had significantly fewer palliative care referrals, the longest time between an advanced cancer diagnosis and a palliative care consultation, and one of the largest numbers of medical team encounters – a median of 38 – before palliative care (Oncologist. 2012;17[12]:1574-80).
In light of these numbers, policies at MD Anderson Cancer Center “are evolving rapidly,” Dr. Hui said.
He urged colleagues to think about the wishes of their patients. “What do patients really want? Good symptom control, time with family, not being a burden, not a prolonging dying process, having a sense of control during the middle of the turmoil.”
Dr. Hui added that the attitudes of oncologists regarding palliative care can affect whether patients get timely referrals to consultations. He led a 2016 study that surveyed 182 oncologists about end-of-life care and found that “many oncologists have a favorable attitude toward EOL care; this, in turn, was associated with greater provision of primary palliative care and higher rates of referral to specialist palliative care.”
However, “we found that hematologic oncology specialists expressed lower comfort levels compared with their solid tumor counterparts,” a finding that reflects the results of other studies, the study authors wrote (Oncologist. 2016 Sep;21[9]:1149-55).
The stigma surrounding palliative care is a sticking point, Dr. Hui said, and has sparked a “rebranding” effort. Negative feelings about palliative decrease when it’s called “supportive care,” he said, and the new term is being adopted worldwide.
Dr. Hui reported having no financial disclosures.
SAN DIEGO – When it comes to aggressive care at the end of life, hematologists stand alone.
Hematology patients are more likely than are other patients to undergo chemotherapy and visit emergency departments and intensive care units when they’re near death, and they’re less likely to be referred for palliative care, according to David Hui, MD, an oncologist and palliative care specialist at the MD Anderson Cancer Center in Houston.
An analysis at the center, for example, found that 43% of hematology cancer patients received chemotherapy within the last 30 days of life, compared with 14% of patients with solid tumors.
“That’s not a number we’re proud of,” Dr. Hui said at the annual meeting of the American Society of Hematology. “Ultimately, at the end of life, do we want our patients to be in this setting? There is room for improvement.”
The cancer center isn’t an outlier on this front, Dr. Hui said. Data from other institutions in the United States and internationally confirm that hematologic oncologists tend to provide more aggressive care at the end of a patient’s life, compared with other cancer specialists.
“If you’re one of those patients, this is a very big deal,” said Dr. Hui, especially in light of data that suggest hematology patients get fewer referrals to palliative care than do other cancer patients. “Oncologists are optimistic, and hematologic oncologists especially,” he said.
Dr. Hui led a 2014 study of 816 adult cancer patients who died while under care at MD Anderson Cancer Center during 6 months in 2009 and 2010 (Cancer. 2014 May 15; 120[10]:1572-8).
“We found that patients with hematological malignancies were more likely to have multiple emergency room visits, intensive care unit admissions and death, and cancer treatments in the last weeks of life compared to patients with solid tumors,” the study authors wrote. “We also identified a relative lack of palliative care involvement in hematologic patients.”
Specifically, hematology cancer patients were much more likely to get aggressive end-of-life care than were the other cancer patients (odds ratio, 6.63, P less than .001).
Dr. Hui had led an earlier study that looked at the same 816 cancer patients and found that 45% had received palliative care consultations. But the researchers also found that patients with hematologic malignancies had significantly fewer palliative care referrals, the longest time between an advanced cancer diagnosis and a palliative care consultation, and one of the largest numbers of medical team encounters – a median of 38 – before palliative care (Oncologist. 2012;17[12]:1574-80).
In light of these numbers, policies at MD Anderson Cancer Center “are evolving rapidly,” Dr. Hui said.
He urged colleagues to think about the wishes of their patients. “What do patients really want? Good symptom control, time with family, not being a burden, not a prolonging dying process, having a sense of control during the middle of the turmoil.”
Dr. Hui added that the attitudes of oncologists regarding palliative care can affect whether patients get timely referrals to consultations. He led a 2016 study that surveyed 182 oncologists about end-of-life care and found that “many oncologists have a favorable attitude toward EOL care; this, in turn, was associated with greater provision of primary palliative care and higher rates of referral to specialist palliative care.”
However, “we found that hematologic oncology specialists expressed lower comfort levels compared with their solid tumor counterparts,” a finding that reflects the results of other studies, the study authors wrote (Oncologist. 2016 Sep;21[9]:1149-55).
The stigma surrounding palliative care is a sticking point, Dr. Hui said, and has sparked a “rebranding” effort. Negative feelings about palliative decrease when it’s called “supportive care,” he said, and the new term is being adopted worldwide.
Dr. Hui reported having no financial disclosures.
SAN DIEGO – When it comes to aggressive care at the end of life, hematologists stand alone.
Hematology patients are more likely than are other patients to undergo chemotherapy and visit emergency departments and intensive care units when they’re near death, and they’re less likely to be referred for palliative care, according to David Hui, MD, an oncologist and palliative care specialist at the MD Anderson Cancer Center in Houston.
An analysis at the center, for example, found that 43% of hematology cancer patients received chemotherapy within the last 30 days of life, compared with 14% of patients with solid tumors.
“That’s not a number we’re proud of,” Dr. Hui said at the annual meeting of the American Society of Hematology. “Ultimately, at the end of life, do we want our patients to be in this setting? There is room for improvement.”
The cancer center isn’t an outlier on this front, Dr. Hui said. Data from other institutions in the United States and internationally confirm that hematologic oncologists tend to provide more aggressive care at the end of a patient’s life, compared with other cancer specialists.
“If you’re one of those patients, this is a very big deal,” said Dr. Hui, especially in light of data that suggest hematology patients get fewer referrals to palliative care than do other cancer patients. “Oncologists are optimistic, and hematologic oncologists especially,” he said.
Dr. Hui led a 2014 study of 816 adult cancer patients who died while under care at MD Anderson Cancer Center during 6 months in 2009 and 2010 (Cancer. 2014 May 15; 120[10]:1572-8).
“We found that patients with hematological malignancies were more likely to have multiple emergency room visits, intensive care unit admissions and death, and cancer treatments in the last weeks of life compared to patients with solid tumors,” the study authors wrote. “We also identified a relative lack of palliative care involvement in hematologic patients.”
Specifically, hematology cancer patients were much more likely to get aggressive end-of-life care than were the other cancer patients (odds ratio, 6.63, P less than .001).
Dr. Hui had led an earlier study that looked at the same 816 cancer patients and found that 45% had received palliative care consultations. But the researchers also found that patients with hematologic malignancies had significantly fewer palliative care referrals, the longest time between an advanced cancer diagnosis and a palliative care consultation, and one of the largest numbers of medical team encounters – a median of 38 – before palliative care (Oncologist. 2012;17[12]:1574-80).
In light of these numbers, policies at MD Anderson Cancer Center “are evolving rapidly,” Dr. Hui said.
He urged colleagues to think about the wishes of their patients. “What do patients really want? Good symptom control, time with family, not being a burden, not a prolonging dying process, having a sense of control during the middle of the turmoil.”
Dr. Hui added that the attitudes of oncologists regarding palliative care can affect whether patients get timely referrals to consultations. He led a 2016 study that surveyed 182 oncologists about end-of-life care and found that “many oncologists have a favorable attitude toward EOL care; this, in turn, was associated with greater provision of primary palliative care and higher rates of referral to specialist palliative care.”
However, “we found that hematologic oncology specialists expressed lower comfort levels compared with their solid tumor counterparts,” a finding that reflects the results of other studies, the study authors wrote (Oncologist. 2016 Sep;21[9]:1149-55).
The stigma surrounding palliative care is a sticking point, Dr. Hui said, and has sparked a “rebranding” effort. Negative feelings about palliative decrease when it’s called “supportive care,” he said, and the new term is being adopted worldwide.
Dr. Hui reported having no financial disclosures.
EXPERT ANALYSIS FROM ASH 2018
MRI for Emergency Clinicians
The use of magnetic resonance imaging (MRI) by emergency physicians (EPs) is increasing steadily, as new MRI indications arise, technology evolves, and machines become faster and more widely available. It is therefore critically important that EPs understand the basics of this imaging modality, its uses, limitations, cautions, and contraindications.
A full explanation of the physics underpinning MRI is beyond this article’s scope. However, a comprehensive discussion of the topic is available in a 2013 review entitled, "Understanding MRI: basic MR physics for physicians."1 In short, three elements are necessary for an MRI machine to generate images: a strong magnetic field, radio waves, and a computer system. The body’s hydrogen nuclei with their single protons and north-south poles act as mini bar magnets with randomly aligned axes. However, when the body is subjected to the MRI machine magnetic field, these axes line up. When radio waves are applied to the magnetic field, the strength and direction of the magnetic field changes. Then, when the radio waves are turned off, the magnetic field strength and direction return to baseline and a signal is emitted. It is this signal that is interpreted by a computer system to generate images.2
Cautions and Limitations
Although limited availability is often cited as a reason for not obtaining MRI studies in the emergency department (ED), this limitation is institution specific and will likely improve over time. Recent statistics indicate that MRI availability in the United States is second only to that in Japan and climbing. MRI usage in the United States is the highest in the world.3
MRI cost (and the resultant patient bill) exceeds that of other commonly performed ED imaging roughly by a factor of 2:1 when compared to computed tomography (CT). This is unlikely to improve in the near term.
The time to complete an MRI study continues to fall for some indications, but significantly exceeds the time to obtain CT images. MRI scan times range from 20 to 60 minutes depending on test type.
Body habitus, particularly obesity, may limit the ability of certain patients to undergo MRI. Claustrophobia or the inability to lie still for the test’s entire duration may present a challenge for some patients. Be prepared to safely sedate patients with these issues. This is particularly relevant for pediatric patients. Consider a pre-MRI trial of sedation to assess which medication is best suited for individual patients.
Patients with certain medical devices may be unable to undergo MRI. Medical devices and implants from the U.S. and Europe manufactured within the past 30 years are non-ferromagnetic. This generally means they are MR-safe or MR-conditional. Realize, however, that certain non-ferromagnetic implants can heat during MR imaging.4 A free searchable database exists listing MRI-safe devices and implants along with limitations and cautions (http://www.mrisafety.com/TheList_search.asp).5
Pacemakers and defibrillators are worthy of special mention. Some are now considered MR-conditional in limited circumstances, and this situation will continue to evolve. Consult your radiologist and/or the physician who placed the medical device with any safety concerns.
Intraocular metallic foreign bodies are an MRI contraindication. If any concern exists for an intraocular metallic foreign body, perform an orbital CT before considering an MRI. Headphones and ear plugs are used during MRI examinations to prevent hearing damage due to machine noise or nerve and muscle stimulation.
A 2016 JAMA study of MRI in pregnancy involving more than 1.4 million deliveries concluded “exposure to MRI during the first trimester of pregnancy compared with non¬exposure was not associated with increased risk of harm to the fetus or in early childhood. Gadolinium MRI at any time during pregnancy was associated with an increased risk of a broad set of rheumatological, inflammatory, or infiltrative skin conditions and for stillbirth or neonatal death.”6
There is limited data on the use of MRI in pediatric patients, but a 2015 study noted, “to date, no studies have demonstrated any definite risk to the fetus, mother, or neonate when MR scanners are operated within the regulatory guidelines set forth by the FDA and other regulatory agencies.”7
A variety of gadolinium-based contrast agents (GBCAs) are currently used. GBCA administrations in renally impaired patients has been linked to nephrogenic systemic fibrosis (NSF), a rare, progressive, potentially fatal, incompletely understood, systemic disorder with a spectrum of manifestations. Its occurrence has prompted alerts, and a recent set of recommendations for at-risk patients (ie, those with acute kidney injury or an eGFR < 30 mL/min/1.73 m2 and those who are dialysis dependent) specifies that (1) a low-risk GBCA should be used; (2) GBCA dose should be as low as possible; and (3) dialysis should be performed as indicated immediately after GBCA-enhanced MRI.8,9 Additionally, the EP may wish to obtain informed consent from at-risk patients prior to the administration of GBCAs.
Common MRI indications in the ED
Central nervous system MRI
Spinal cord compression may occur due to a neoplastic process, either primary or metastatic, infection (epidural abscess is a particular concern), or hematoma. CT myelography is another diagnostic option, but MRI offers ease of performance, superior resolution, multiplanar imaging, lack of ionizing radiation, and the ability to detect multiple lesions with a single scan. For non-traumatic myelopathy evaluation (most commonly due to cancer), perform a non-contrast MRI of the entire spinal canal since multiple lesions may be present. Repeat the MRI with contrast if the cause of the myelopathy is not clear after the non-contrast study.10 Gadolinium does help detect and define inflammatory, infectious, and neoplastic lesions, but spinal cord compression can be diagnosed without it if the patient cannot receive gadolinium (see Cautions and Limitations section).11 Only a non-enhanced MRI, limited to the traumatized area, is required in the evaluation of trauma-induced myelopathy.10
Dural venous sinus thrombosis (DVST) is best assessed with a combination of MRI and MR venography.10 DVST is clot formation within any of five major dural venous sinuses. DVST risk factors include: dehydration; infections, both systemic and local; pregnancy and the puerperium; neoplastic incursion; trauma; and coagulopathies.10,12 MR venography is an essential part of DVST evaluation since it assesses patency of the involved dural venous sinus.10
Carotid artery dissection is a leading cause of stroke in those younger than 45 years of age.13 Carotid and vertebral artery dissection, due to trauma, hypertension, vascular disease, or local infections, can be diagnosed with endovascular angiography.10,14 However, MRI in combination with MRA can be diagnostic as well.10,13,14 MRI delineates the intramural clots while MRA shows the degree and extent of endovascular compromise.10,13
Meningoencephalitis and vasculitis are usually diagnosed with a combination of clinical findings, laboratory data, CT, and lumbar puncture results. However, MRI is highly sensitive for the CNS lesions associated with infection or vasculitis. Consider MRI as an alternative to the usual work up in selected patients if aggressive early therapy for viral infection (eg, herpes) or vasculitis is being contemplated.10
Acute subarachnoid hemorrhage (SAH) is usually best demonstrated on CT. However, MRI may have a role, especially in posterior fossa SAH.10
Cerebral Ischemia (TIA and Stroke) - The 2018 guidelines for early management of patients with acute ischemic stroke both recommended and considered equal (in patients selected for mechanical thrombectomy) CT, diffusion weighted MRI or MRI perfusion.15 This guideline was promulgated by the American Heart Association/American Stroke Association and endorsed by the Society for Academic Emergency Medicine, among other professional organizations.
In a joint statement published by the American Society of Neuroradiology, the American College of Radiology, and the Society of Neurointerventional Surgery, MRI was reported to be equivalent to a non-contrast brain CT. MRI was also found to have superior accuracy in detecting microhemorrhages.16
Spine MRI
Spine and spinal cord emergencies must be promptly and correctly diagnosed to avoid or minimize functional loss. Knowledge of the most appropriate imaging modalities is essential to facilitate diagnosis and treatment for patients presenting with spine-related emergencies.
Low back pain prompts many ED visits and is a major cause of disability in the United States.
MRI is unwarranted for those patients with acute (< 6 weeks duration) low back pain in whom serious pathology, such as cauda equina, malignancy, epidural hematoma, or infection is not suspected. Manage most low back pain patients conservatively and without imaging.17
Trauma is the most common reason for spine MRI. CT, and now increasingly MRI, have supplanted plain radiography in the evaluation of spinal trauma. Currently, CT alone is considered sufficient in the evaluation of thoracic and lumbar skeletal injuries. This is not true for cervical spine injuries.18
Initially, use either the NEXUS or Canadian C-Spine Rule criteria to determine if a trauma patient needs any imaging. Then, consider whether CT or MRI or both will be required, while realizing that the literature on this thorny issue continues to evolve. CT is the current standard for detecting bony injuries. MRI is usually reserved for patient in whom a soft-tissue, particularly ligamentous, injury is suspected. MRI is also required for the evaluation of any patient suspected of having sustained spinal cord injury.18 The downside of our increased MRI usage in the evaluation of potentially spine-injured patients has been the detection of many clinically insignificant findings.
Acute cauda equina syndrome is a neurosurgical emergency requiring prompt recognition, imaging, and immediate neurosurgical consultation. Common findings include: recent onset or worsening severe low back pain; bowel and/or bladder dysfunction; neurological deficits; and saddle anesthesia. Many processes can lead to the syndrome, but the most common is disc herniation with resultant cauda equina compression. The American College of Radiology appropriateness criteria cite MRI as the correct imaging modality for the diagnosis of acute cauda equina syndrome.19 In patients who’ve undergone previous herniated disc surgery, MRI with and without contrast must be obtained to differentiate between contrast-enhancing granulation tissue at the site of the surgery and nonenhancing herniated disc tissue.18
Infection is an important item in the differential diagnosis of back pain, with or without radiculopathy, and particularly important to consider if the patient has infectious disease risk factors. These risk factors include: spinal instrumentation via injections or surgery; intravenous drug use; prosthetic heart valves; systemic infections; other infectious sources in the body; and immunocompromising conditions.18 All spinal elements, including the spinal cord, meninges, joints, discs, and vertebrae can be affected. Realize that infection can occur by direct inoculation or contiguous or hematogenous spread. An MRI with and without contrast is essential to confirm the diagnosis.19 Your neurosurgical consultant will likely recommend imaging the entire spinal axis, since infectious lesions may be present at multiple levels.18
Pregnant patients with abdominal pain - concern for appendicitis (see the Cautions and Limitations section above on MRI in pregnancy)
Appendicitis occurs commonly in pregnancy. Missing the diagnosis can lead to fetal loss and other untoward outcomes. The 2018 American College of Radiology guidelines list MRI and ultrasound as imaging studies of choice in gravid patients in whom appendicitis is a concern.20 Ultrasound is more commonly available and less expensive but is limited by high rates of appendiceal non-visualization, likely due to appendix displacement by the uterus, patient habitus, bowel gas, and discomfort during the exam.21
MRI has high sensitivity and very high specificity for the diagnosis of appendicitis. Abnormal diagnostic findings include an appendiceal diameter > 7 mm and surrounding inflammatory changes.22 The low negative predictive value of MRI obviates the need for risky surgeries in pregnant patients in whom appendicitis is ruled out. MRI also allows for the diagnosis of other etiologies of abdominal pain in these patients.21
Pediatric patients with abdominal pain -concern for appendicitis (see the Cautions and Limitations section above on MRI in pediatric patients)
For pediatric patients with possible appendicitis, ultrasound is the first imaging modality of choice, followed by CT. However, ultrasound is operator dependent, with wide variability in its ability to correctly diagnose appendicitis, often leading to equivocal results. CT involves ionizing radiation exposure.20 Non-contrast MRI is the emerging imaging modality for these patients. A systematic review of almost 2000 pediatric patients found MRI sensitivity and specificity to be 97% and 97% with a low negative appendectomy rate.23
Cost and image acquisition time are limitations for MRI use for children. Pediatric patients may require sedation with long acquisition times in order to ensure that high-quality images are obtained, potentially introducing more associated costs and safety concerns. Shorter image-acquisition times would make MRI a more widely applicable test.23
Orthopedics
Various orthopedic conditions can be investigated by MRI, but this is not commonly done in the ED. Acute knee trauma with a concern for ligamentous, cartilaginous, or meniscal injury is one example. The patient with a concern for occult fracture or injury to the shoulder, elbow, or scaphoid represent others.
However, the special case of the patient with hip trauma with negative radiographs who will not weight bear or has significant pain is worth considering. MRI to either diagnose or exclude occult hip, pelvic, or acetabular fracture is traditionally considered to be the criterion standard. However, a 2016 study called this widely-held belief into question. It found that CT and MRI were similarly sensitive and concluded that starting with CT was a reasonable approach.24 MRI can be considered if the diagnosis remains in doubt.
Musculoskeletal infections
A wide variety of bone, joint, and soft-tissue infections can be diagnosed by MRI, which is often the imaging modality of choice. Some of these infections may be limb- or even life-threatening. One, epidural abscess, is both life-threatening and function-threatening and has been discussed briefly already.
If you are concerned about the possibility of a serious soft-tissue or bone infection, strongly consider giving gadolinium contrast, which is particularly useful for detecting abscesses, sinus tracts, and spine infections, and for providing other important anatomic details.25
Conclusion
MRI utilization by EPs will continue to increase as the factors governing its use evolves. These factors include: decreasing scan times; wider availability; possible cost reductions; new and changing indications; more research; and the always-present pressure on EPs to care for a broader spectrum of evermore challenging patients. It therefore benefits us to understand more about this dynamic part of our practice. Look to the scientific literature on stroke, neurosurgical emergencies, orthopedics, pediatrics, infectious disease and other fields that impact emergency medicine practice and MRI use as they continue to change.
SIDEBAR
Summary of Cautions and Limitations of MRI Use
Lack of availability
Cost
Exam completion time
Claustrophobia
Patient’s inability to lie still
Implanted medical devices
Metallic foreign bodies
Obesity
Hearing damage
Pregnancy
Pediatric patients (the developing brain)
Nephrogenic systemic fibrosis due to gadolinium-based contrast agents
SIDEBAR
Common ED MRI indications
Central Nervous System
- Spinal cord compression
- Dural venous sinus thrombosis
- Arterial dissections - carotid or vertebral
- Meningoencephalitis and vasculitis evaluation (possible)
- Subarachnoid hemorrhage (possible)
- Cerebral ischemia - TIA/Stroke
Spinal cord/surrounding structure disease or trauma - epidural abscess, cauda equina syndrome, cord/nerve trauma
Pregnant patients with abdominal pain (concern for appendicitis)
Children with abdominal pain (concern for appendicitis)
Musculoskeletal infections Orthopedic trauma
1. Currie S, Hoggard N, Craven IJ, Hadjivassiliou M, Wilkinson ID. Understanding MRI: Basic MR physics for physicians. Postgrad Med J. 2013;89:209-223.
2. Berger A. How does it work? Magnetic resonance imaging. BMJ. 2002;324:35.
3. Chung M, Dahabreh IJ, Hadar N, et al. Emerging MRI technologies for imaging musculoskeletal disorders under loading stress. Comparative Effectiveness Technical Briefs, No. 7. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. https://www.ncbi.nlm.nih.gov/books/NBK82287/
4. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451.
5. MRI Safety. http://www.mrisafety.com/TheList_search.asp.
6. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952-961.
7. Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A. MRI evaluation and safety in the developing brain. Semin Perinatol. 2015;39(2):73-104.
8. Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG. Revisiting the risks of MRI with Gadolinium based contrast agents—review of literature and guidelines. Insights Imaging. 2015;6(5):553-558.
9. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium-based contrast agents in kidney disease: A comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis. 2018;5:1-17.
10. Quint DJ. Indications for emergent MRI of the central nervous system. JAMA. 2000;283(7):853-855.
11. Broder J. Imaging the cervical, thoracic, and lumbar spine. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, PA: Elsevier; 2011:73-157.
12. Villringer A, Einhäupl KM. Dural sinus and cerebral venous thrombosis. New Horiz. 1997;5(4):332-341.
13. Ben Hassen W, Machet A, Edjlali-Goujon M, et al. Imaging of cervical artery dissection. Diagn Interv Imaging. 2014;95(12):1151-1161.
14. Jacobs A, Lanfermann H, Neveling M, Szelies B, Schröder R, Heiss W-D. MRI-and MRI-guided therapy of carotid and vertebral artery dissections. J Neurol Sci. 1997;147(1):27-34.
15. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
16. Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34(11):E117-127.
17. Lavi ES, Pal A, Bleicher D, Kang K, Sidani C. MR imaging of the spine: Urgent and emergent indications. Semin Ultrasound CT MR. (2018), doi: https://doi.org/10.1053/j.sult.2018.10.006
18. Kawakyu-O’Connor D, Bordia R, Nicola R. Magnetic resonance imaging of spinal emergencies. Magn Reson Imaging Clin N Am. 2016;24(2):325-344.
19. Patel ND, Broderick DF, Burns J, et al. ACR appropriateness criteria low back pain. J Am Coll of Radiol. 2016;13(9):1069-1078.
20. Garcia EM, Camacho MA, Karolyi DR, et al. ACR appropriateness criteria -right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15(11):S373-S387.
21. Duke E, Kalb B, Arif-Tiwari H, et al. A systematic review and meta-analysis of diagnostic performance of MRI for evaluation of acute appendicitis. AJR Am J Roentgenol. 2016;206(3):508-517.
22. Yu HS, Gupta A, Soto JA, et al. Emergency abdominal MRI: Current uses and trends. Br J Radiol. 2016;89(1061). doi:10.1259/bjr.20150804
23. Kim JR, Suh CH, Yoon HM, et al. Performance of MRI for suspected appendicitis in pediatric patients and negative appendectomy rate: A systematic review and meta-analysis. J Magn Reson Imaging. 2017;47(3):767-778.
24. Rehman H, Clement RG, Perks F, White TO. Imaging of occult hip fractures: CT or MRI? Injury. 2016;47(6):1297-1301.
25. Simpfendorfer CS. Radiologic approach to musculoskeletal infections. Infect Dis Clin N Am. 2017;31:299-324.
The use of magnetic resonance imaging (MRI) by emergency physicians (EPs) is increasing steadily, as new MRI indications arise, technology evolves, and machines become faster and more widely available. It is therefore critically important that EPs understand the basics of this imaging modality, its uses, limitations, cautions, and contraindications.
A full explanation of the physics underpinning MRI is beyond this article’s scope. However, a comprehensive discussion of the topic is available in a 2013 review entitled, "Understanding MRI: basic MR physics for physicians."1 In short, three elements are necessary for an MRI machine to generate images: a strong magnetic field, radio waves, and a computer system. The body’s hydrogen nuclei with their single protons and north-south poles act as mini bar magnets with randomly aligned axes. However, when the body is subjected to the MRI machine magnetic field, these axes line up. When radio waves are applied to the magnetic field, the strength and direction of the magnetic field changes. Then, when the radio waves are turned off, the magnetic field strength and direction return to baseline and a signal is emitted. It is this signal that is interpreted by a computer system to generate images.2
Cautions and Limitations
Although limited availability is often cited as a reason for not obtaining MRI studies in the emergency department (ED), this limitation is institution specific and will likely improve over time. Recent statistics indicate that MRI availability in the United States is second only to that in Japan and climbing. MRI usage in the United States is the highest in the world.3
MRI cost (and the resultant patient bill) exceeds that of other commonly performed ED imaging roughly by a factor of 2:1 when compared to computed tomography (CT). This is unlikely to improve in the near term.
The time to complete an MRI study continues to fall for some indications, but significantly exceeds the time to obtain CT images. MRI scan times range from 20 to 60 minutes depending on test type.
Body habitus, particularly obesity, may limit the ability of certain patients to undergo MRI. Claustrophobia or the inability to lie still for the test’s entire duration may present a challenge for some patients. Be prepared to safely sedate patients with these issues. This is particularly relevant for pediatric patients. Consider a pre-MRI trial of sedation to assess which medication is best suited for individual patients.
Patients with certain medical devices may be unable to undergo MRI. Medical devices and implants from the U.S. and Europe manufactured within the past 30 years are non-ferromagnetic. This generally means they are MR-safe or MR-conditional. Realize, however, that certain non-ferromagnetic implants can heat during MR imaging.4 A free searchable database exists listing MRI-safe devices and implants along with limitations and cautions (http://www.mrisafety.com/TheList_search.asp).5
Pacemakers and defibrillators are worthy of special mention. Some are now considered MR-conditional in limited circumstances, and this situation will continue to evolve. Consult your radiologist and/or the physician who placed the medical device with any safety concerns.
Intraocular metallic foreign bodies are an MRI contraindication. If any concern exists for an intraocular metallic foreign body, perform an orbital CT before considering an MRI. Headphones and ear plugs are used during MRI examinations to prevent hearing damage due to machine noise or nerve and muscle stimulation.
A 2016 JAMA study of MRI in pregnancy involving more than 1.4 million deliveries concluded “exposure to MRI during the first trimester of pregnancy compared with non¬exposure was not associated with increased risk of harm to the fetus or in early childhood. Gadolinium MRI at any time during pregnancy was associated with an increased risk of a broad set of rheumatological, inflammatory, or infiltrative skin conditions and for stillbirth or neonatal death.”6
There is limited data on the use of MRI in pediatric patients, but a 2015 study noted, “to date, no studies have demonstrated any definite risk to the fetus, mother, or neonate when MR scanners are operated within the regulatory guidelines set forth by the FDA and other regulatory agencies.”7
A variety of gadolinium-based contrast agents (GBCAs) are currently used. GBCA administrations in renally impaired patients has been linked to nephrogenic systemic fibrosis (NSF), a rare, progressive, potentially fatal, incompletely understood, systemic disorder with a spectrum of manifestations. Its occurrence has prompted alerts, and a recent set of recommendations for at-risk patients (ie, those with acute kidney injury or an eGFR < 30 mL/min/1.73 m2 and those who are dialysis dependent) specifies that (1) a low-risk GBCA should be used; (2) GBCA dose should be as low as possible; and (3) dialysis should be performed as indicated immediately after GBCA-enhanced MRI.8,9 Additionally, the EP may wish to obtain informed consent from at-risk patients prior to the administration of GBCAs.
Common MRI indications in the ED
Central nervous system MRI
Spinal cord compression may occur due to a neoplastic process, either primary or metastatic, infection (epidural abscess is a particular concern), or hematoma. CT myelography is another diagnostic option, but MRI offers ease of performance, superior resolution, multiplanar imaging, lack of ionizing radiation, and the ability to detect multiple lesions with a single scan. For non-traumatic myelopathy evaluation (most commonly due to cancer), perform a non-contrast MRI of the entire spinal canal since multiple lesions may be present. Repeat the MRI with contrast if the cause of the myelopathy is not clear after the non-contrast study.10 Gadolinium does help detect and define inflammatory, infectious, and neoplastic lesions, but spinal cord compression can be diagnosed without it if the patient cannot receive gadolinium (see Cautions and Limitations section).11 Only a non-enhanced MRI, limited to the traumatized area, is required in the evaluation of trauma-induced myelopathy.10
Dural venous sinus thrombosis (DVST) is best assessed with a combination of MRI and MR venography.10 DVST is clot formation within any of five major dural venous sinuses. DVST risk factors include: dehydration; infections, both systemic and local; pregnancy and the puerperium; neoplastic incursion; trauma; and coagulopathies.10,12 MR venography is an essential part of DVST evaluation since it assesses patency of the involved dural venous sinus.10
Carotid artery dissection is a leading cause of stroke in those younger than 45 years of age.13 Carotid and vertebral artery dissection, due to trauma, hypertension, vascular disease, or local infections, can be diagnosed with endovascular angiography.10,14 However, MRI in combination with MRA can be diagnostic as well.10,13,14 MRI delineates the intramural clots while MRA shows the degree and extent of endovascular compromise.10,13
Meningoencephalitis and vasculitis are usually diagnosed with a combination of clinical findings, laboratory data, CT, and lumbar puncture results. However, MRI is highly sensitive for the CNS lesions associated with infection or vasculitis. Consider MRI as an alternative to the usual work up in selected patients if aggressive early therapy for viral infection (eg, herpes) or vasculitis is being contemplated.10
Acute subarachnoid hemorrhage (SAH) is usually best demonstrated on CT. However, MRI may have a role, especially in posterior fossa SAH.10
Cerebral Ischemia (TIA and Stroke) - The 2018 guidelines for early management of patients with acute ischemic stroke both recommended and considered equal (in patients selected for mechanical thrombectomy) CT, diffusion weighted MRI or MRI perfusion.15 This guideline was promulgated by the American Heart Association/American Stroke Association and endorsed by the Society for Academic Emergency Medicine, among other professional organizations.
In a joint statement published by the American Society of Neuroradiology, the American College of Radiology, and the Society of Neurointerventional Surgery, MRI was reported to be equivalent to a non-contrast brain CT. MRI was also found to have superior accuracy in detecting microhemorrhages.16
Spine MRI
Spine and spinal cord emergencies must be promptly and correctly diagnosed to avoid or minimize functional loss. Knowledge of the most appropriate imaging modalities is essential to facilitate diagnosis and treatment for patients presenting with spine-related emergencies.
Low back pain prompts many ED visits and is a major cause of disability in the United States.
MRI is unwarranted for those patients with acute (< 6 weeks duration) low back pain in whom serious pathology, such as cauda equina, malignancy, epidural hematoma, or infection is not suspected. Manage most low back pain patients conservatively and without imaging.17
Trauma is the most common reason for spine MRI. CT, and now increasingly MRI, have supplanted plain radiography in the evaluation of spinal trauma. Currently, CT alone is considered sufficient in the evaluation of thoracic and lumbar skeletal injuries. This is not true for cervical spine injuries.18
Initially, use either the NEXUS or Canadian C-Spine Rule criteria to determine if a trauma patient needs any imaging. Then, consider whether CT or MRI or both will be required, while realizing that the literature on this thorny issue continues to evolve. CT is the current standard for detecting bony injuries. MRI is usually reserved for patient in whom a soft-tissue, particularly ligamentous, injury is suspected. MRI is also required for the evaluation of any patient suspected of having sustained spinal cord injury.18 The downside of our increased MRI usage in the evaluation of potentially spine-injured patients has been the detection of many clinically insignificant findings.
Acute cauda equina syndrome is a neurosurgical emergency requiring prompt recognition, imaging, and immediate neurosurgical consultation. Common findings include: recent onset or worsening severe low back pain; bowel and/or bladder dysfunction; neurological deficits; and saddle anesthesia. Many processes can lead to the syndrome, but the most common is disc herniation with resultant cauda equina compression. The American College of Radiology appropriateness criteria cite MRI as the correct imaging modality for the diagnosis of acute cauda equina syndrome.19 In patients who’ve undergone previous herniated disc surgery, MRI with and without contrast must be obtained to differentiate between contrast-enhancing granulation tissue at the site of the surgery and nonenhancing herniated disc tissue.18
Infection is an important item in the differential diagnosis of back pain, with or without radiculopathy, and particularly important to consider if the patient has infectious disease risk factors. These risk factors include: spinal instrumentation via injections or surgery; intravenous drug use; prosthetic heart valves; systemic infections; other infectious sources in the body; and immunocompromising conditions.18 All spinal elements, including the spinal cord, meninges, joints, discs, and vertebrae can be affected. Realize that infection can occur by direct inoculation or contiguous or hematogenous spread. An MRI with and without contrast is essential to confirm the diagnosis.19 Your neurosurgical consultant will likely recommend imaging the entire spinal axis, since infectious lesions may be present at multiple levels.18
Pregnant patients with abdominal pain - concern for appendicitis (see the Cautions and Limitations section above on MRI in pregnancy)
Appendicitis occurs commonly in pregnancy. Missing the diagnosis can lead to fetal loss and other untoward outcomes. The 2018 American College of Radiology guidelines list MRI and ultrasound as imaging studies of choice in gravid patients in whom appendicitis is a concern.20 Ultrasound is more commonly available and less expensive but is limited by high rates of appendiceal non-visualization, likely due to appendix displacement by the uterus, patient habitus, bowel gas, and discomfort during the exam.21
MRI has high sensitivity and very high specificity for the diagnosis of appendicitis. Abnormal diagnostic findings include an appendiceal diameter > 7 mm and surrounding inflammatory changes.22 The low negative predictive value of MRI obviates the need for risky surgeries in pregnant patients in whom appendicitis is ruled out. MRI also allows for the diagnosis of other etiologies of abdominal pain in these patients.21
Pediatric patients with abdominal pain -concern for appendicitis (see the Cautions and Limitations section above on MRI in pediatric patients)
For pediatric patients with possible appendicitis, ultrasound is the first imaging modality of choice, followed by CT. However, ultrasound is operator dependent, with wide variability in its ability to correctly diagnose appendicitis, often leading to equivocal results. CT involves ionizing radiation exposure.20 Non-contrast MRI is the emerging imaging modality for these patients. A systematic review of almost 2000 pediatric patients found MRI sensitivity and specificity to be 97% and 97% with a low negative appendectomy rate.23
Cost and image acquisition time are limitations for MRI use for children. Pediatric patients may require sedation with long acquisition times in order to ensure that high-quality images are obtained, potentially introducing more associated costs and safety concerns. Shorter image-acquisition times would make MRI a more widely applicable test.23
Orthopedics
Various orthopedic conditions can be investigated by MRI, but this is not commonly done in the ED. Acute knee trauma with a concern for ligamentous, cartilaginous, or meniscal injury is one example. The patient with a concern for occult fracture or injury to the shoulder, elbow, or scaphoid represent others.
However, the special case of the patient with hip trauma with negative radiographs who will not weight bear or has significant pain is worth considering. MRI to either diagnose or exclude occult hip, pelvic, or acetabular fracture is traditionally considered to be the criterion standard. However, a 2016 study called this widely-held belief into question. It found that CT and MRI were similarly sensitive and concluded that starting with CT was a reasonable approach.24 MRI can be considered if the diagnosis remains in doubt.
Musculoskeletal infections
A wide variety of bone, joint, and soft-tissue infections can be diagnosed by MRI, which is often the imaging modality of choice. Some of these infections may be limb- or even life-threatening. One, epidural abscess, is both life-threatening and function-threatening and has been discussed briefly already.
If you are concerned about the possibility of a serious soft-tissue or bone infection, strongly consider giving gadolinium contrast, which is particularly useful for detecting abscesses, sinus tracts, and spine infections, and for providing other important anatomic details.25
Conclusion
MRI utilization by EPs will continue to increase as the factors governing its use evolves. These factors include: decreasing scan times; wider availability; possible cost reductions; new and changing indications; more research; and the always-present pressure on EPs to care for a broader spectrum of evermore challenging patients. It therefore benefits us to understand more about this dynamic part of our practice. Look to the scientific literature on stroke, neurosurgical emergencies, orthopedics, pediatrics, infectious disease and other fields that impact emergency medicine practice and MRI use as they continue to change.
SIDEBAR
Summary of Cautions and Limitations of MRI Use
Lack of availability
Cost
Exam completion time
Claustrophobia
Patient’s inability to lie still
Implanted medical devices
Metallic foreign bodies
Obesity
Hearing damage
Pregnancy
Pediatric patients (the developing brain)
Nephrogenic systemic fibrosis due to gadolinium-based contrast agents
SIDEBAR
Common ED MRI indications
Central Nervous System
- Spinal cord compression
- Dural venous sinus thrombosis
- Arterial dissections - carotid or vertebral
- Meningoencephalitis and vasculitis evaluation (possible)
- Subarachnoid hemorrhage (possible)
- Cerebral ischemia - TIA/Stroke
Spinal cord/surrounding structure disease or trauma - epidural abscess, cauda equina syndrome, cord/nerve trauma
Pregnant patients with abdominal pain (concern for appendicitis)
Children with abdominal pain (concern for appendicitis)
Musculoskeletal infections Orthopedic trauma
The use of magnetic resonance imaging (MRI) by emergency physicians (EPs) is increasing steadily, as new MRI indications arise, technology evolves, and machines become faster and more widely available. It is therefore critically important that EPs understand the basics of this imaging modality, its uses, limitations, cautions, and contraindications.
A full explanation of the physics underpinning MRI is beyond this article’s scope. However, a comprehensive discussion of the topic is available in a 2013 review entitled, "Understanding MRI: basic MR physics for physicians."1 In short, three elements are necessary for an MRI machine to generate images: a strong magnetic field, radio waves, and a computer system. The body’s hydrogen nuclei with their single protons and north-south poles act as mini bar magnets with randomly aligned axes. However, when the body is subjected to the MRI machine magnetic field, these axes line up. When radio waves are applied to the magnetic field, the strength and direction of the magnetic field changes. Then, when the radio waves are turned off, the magnetic field strength and direction return to baseline and a signal is emitted. It is this signal that is interpreted by a computer system to generate images.2
Cautions and Limitations
Although limited availability is often cited as a reason for not obtaining MRI studies in the emergency department (ED), this limitation is institution specific and will likely improve over time. Recent statistics indicate that MRI availability in the United States is second only to that in Japan and climbing. MRI usage in the United States is the highest in the world.3
MRI cost (and the resultant patient bill) exceeds that of other commonly performed ED imaging roughly by a factor of 2:1 when compared to computed tomography (CT). This is unlikely to improve in the near term.
The time to complete an MRI study continues to fall for some indications, but significantly exceeds the time to obtain CT images. MRI scan times range from 20 to 60 minutes depending on test type.
Body habitus, particularly obesity, may limit the ability of certain patients to undergo MRI. Claustrophobia or the inability to lie still for the test’s entire duration may present a challenge for some patients. Be prepared to safely sedate patients with these issues. This is particularly relevant for pediatric patients. Consider a pre-MRI trial of sedation to assess which medication is best suited for individual patients.
Patients with certain medical devices may be unable to undergo MRI. Medical devices and implants from the U.S. and Europe manufactured within the past 30 years are non-ferromagnetic. This generally means they are MR-safe or MR-conditional. Realize, however, that certain non-ferromagnetic implants can heat during MR imaging.4 A free searchable database exists listing MRI-safe devices and implants along with limitations and cautions (http://www.mrisafety.com/TheList_search.asp).5
Pacemakers and defibrillators are worthy of special mention. Some are now considered MR-conditional in limited circumstances, and this situation will continue to evolve. Consult your radiologist and/or the physician who placed the medical device with any safety concerns.
Intraocular metallic foreign bodies are an MRI contraindication. If any concern exists for an intraocular metallic foreign body, perform an orbital CT before considering an MRI. Headphones and ear plugs are used during MRI examinations to prevent hearing damage due to machine noise or nerve and muscle stimulation.
A 2016 JAMA study of MRI in pregnancy involving more than 1.4 million deliveries concluded “exposure to MRI during the first trimester of pregnancy compared with non¬exposure was not associated with increased risk of harm to the fetus or in early childhood. Gadolinium MRI at any time during pregnancy was associated with an increased risk of a broad set of rheumatological, inflammatory, or infiltrative skin conditions and for stillbirth or neonatal death.”6
There is limited data on the use of MRI in pediatric patients, but a 2015 study noted, “to date, no studies have demonstrated any definite risk to the fetus, mother, or neonate when MR scanners are operated within the regulatory guidelines set forth by the FDA and other regulatory agencies.”7
A variety of gadolinium-based contrast agents (GBCAs) are currently used. GBCA administrations in renally impaired patients has been linked to nephrogenic systemic fibrosis (NSF), a rare, progressive, potentially fatal, incompletely understood, systemic disorder with a spectrum of manifestations. Its occurrence has prompted alerts, and a recent set of recommendations for at-risk patients (ie, those with acute kidney injury or an eGFR < 30 mL/min/1.73 m2 and those who are dialysis dependent) specifies that (1) a low-risk GBCA should be used; (2) GBCA dose should be as low as possible; and (3) dialysis should be performed as indicated immediately after GBCA-enhanced MRI.8,9 Additionally, the EP may wish to obtain informed consent from at-risk patients prior to the administration of GBCAs.
Common MRI indications in the ED
Central nervous system MRI
Spinal cord compression may occur due to a neoplastic process, either primary or metastatic, infection (epidural abscess is a particular concern), or hematoma. CT myelography is another diagnostic option, but MRI offers ease of performance, superior resolution, multiplanar imaging, lack of ionizing radiation, and the ability to detect multiple lesions with a single scan. For non-traumatic myelopathy evaluation (most commonly due to cancer), perform a non-contrast MRI of the entire spinal canal since multiple lesions may be present. Repeat the MRI with contrast if the cause of the myelopathy is not clear after the non-contrast study.10 Gadolinium does help detect and define inflammatory, infectious, and neoplastic lesions, but spinal cord compression can be diagnosed without it if the patient cannot receive gadolinium (see Cautions and Limitations section).11 Only a non-enhanced MRI, limited to the traumatized area, is required in the evaluation of trauma-induced myelopathy.10
Dural venous sinus thrombosis (DVST) is best assessed with a combination of MRI and MR venography.10 DVST is clot formation within any of five major dural venous sinuses. DVST risk factors include: dehydration; infections, both systemic and local; pregnancy and the puerperium; neoplastic incursion; trauma; and coagulopathies.10,12 MR venography is an essential part of DVST evaluation since it assesses patency of the involved dural venous sinus.10
Carotid artery dissection is a leading cause of stroke in those younger than 45 years of age.13 Carotid and vertebral artery dissection, due to trauma, hypertension, vascular disease, or local infections, can be diagnosed with endovascular angiography.10,14 However, MRI in combination with MRA can be diagnostic as well.10,13,14 MRI delineates the intramural clots while MRA shows the degree and extent of endovascular compromise.10,13
Meningoencephalitis and vasculitis are usually diagnosed with a combination of clinical findings, laboratory data, CT, and lumbar puncture results. However, MRI is highly sensitive for the CNS lesions associated with infection or vasculitis. Consider MRI as an alternative to the usual work up in selected patients if aggressive early therapy for viral infection (eg, herpes) or vasculitis is being contemplated.10
Acute subarachnoid hemorrhage (SAH) is usually best demonstrated on CT. However, MRI may have a role, especially in posterior fossa SAH.10
Cerebral Ischemia (TIA and Stroke) - The 2018 guidelines for early management of patients with acute ischemic stroke both recommended and considered equal (in patients selected for mechanical thrombectomy) CT, diffusion weighted MRI or MRI perfusion.15 This guideline was promulgated by the American Heart Association/American Stroke Association and endorsed by the Society for Academic Emergency Medicine, among other professional organizations.
In a joint statement published by the American Society of Neuroradiology, the American College of Radiology, and the Society of Neurointerventional Surgery, MRI was reported to be equivalent to a non-contrast brain CT. MRI was also found to have superior accuracy in detecting microhemorrhages.16
Spine MRI
Spine and spinal cord emergencies must be promptly and correctly diagnosed to avoid or minimize functional loss. Knowledge of the most appropriate imaging modalities is essential to facilitate diagnosis and treatment for patients presenting with spine-related emergencies.
Low back pain prompts many ED visits and is a major cause of disability in the United States.
MRI is unwarranted for those patients with acute (< 6 weeks duration) low back pain in whom serious pathology, such as cauda equina, malignancy, epidural hematoma, or infection is not suspected. Manage most low back pain patients conservatively and without imaging.17
Trauma is the most common reason for spine MRI. CT, and now increasingly MRI, have supplanted plain radiography in the evaluation of spinal trauma. Currently, CT alone is considered sufficient in the evaluation of thoracic and lumbar skeletal injuries. This is not true for cervical spine injuries.18
Initially, use either the NEXUS or Canadian C-Spine Rule criteria to determine if a trauma patient needs any imaging. Then, consider whether CT or MRI or both will be required, while realizing that the literature on this thorny issue continues to evolve. CT is the current standard for detecting bony injuries. MRI is usually reserved for patient in whom a soft-tissue, particularly ligamentous, injury is suspected. MRI is also required for the evaluation of any patient suspected of having sustained spinal cord injury.18 The downside of our increased MRI usage in the evaluation of potentially spine-injured patients has been the detection of many clinically insignificant findings.
Acute cauda equina syndrome is a neurosurgical emergency requiring prompt recognition, imaging, and immediate neurosurgical consultation. Common findings include: recent onset or worsening severe low back pain; bowel and/or bladder dysfunction; neurological deficits; and saddle anesthesia. Many processes can lead to the syndrome, but the most common is disc herniation with resultant cauda equina compression. The American College of Radiology appropriateness criteria cite MRI as the correct imaging modality for the diagnosis of acute cauda equina syndrome.19 In patients who’ve undergone previous herniated disc surgery, MRI with and without contrast must be obtained to differentiate between contrast-enhancing granulation tissue at the site of the surgery and nonenhancing herniated disc tissue.18
Infection is an important item in the differential diagnosis of back pain, with or without radiculopathy, and particularly important to consider if the patient has infectious disease risk factors. These risk factors include: spinal instrumentation via injections or surgery; intravenous drug use; prosthetic heart valves; systemic infections; other infectious sources in the body; and immunocompromising conditions.18 All spinal elements, including the spinal cord, meninges, joints, discs, and vertebrae can be affected. Realize that infection can occur by direct inoculation or contiguous or hematogenous spread. An MRI with and without contrast is essential to confirm the diagnosis.19 Your neurosurgical consultant will likely recommend imaging the entire spinal axis, since infectious lesions may be present at multiple levels.18
Pregnant patients with abdominal pain - concern for appendicitis (see the Cautions and Limitations section above on MRI in pregnancy)
Appendicitis occurs commonly in pregnancy. Missing the diagnosis can lead to fetal loss and other untoward outcomes. The 2018 American College of Radiology guidelines list MRI and ultrasound as imaging studies of choice in gravid patients in whom appendicitis is a concern.20 Ultrasound is more commonly available and less expensive but is limited by high rates of appendiceal non-visualization, likely due to appendix displacement by the uterus, patient habitus, bowel gas, and discomfort during the exam.21
MRI has high sensitivity and very high specificity for the diagnosis of appendicitis. Abnormal diagnostic findings include an appendiceal diameter > 7 mm and surrounding inflammatory changes.22 The low negative predictive value of MRI obviates the need for risky surgeries in pregnant patients in whom appendicitis is ruled out. MRI also allows for the diagnosis of other etiologies of abdominal pain in these patients.21
Pediatric patients with abdominal pain -concern for appendicitis (see the Cautions and Limitations section above on MRI in pediatric patients)
For pediatric patients with possible appendicitis, ultrasound is the first imaging modality of choice, followed by CT. However, ultrasound is operator dependent, with wide variability in its ability to correctly diagnose appendicitis, often leading to equivocal results. CT involves ionizing radiation exposure.20 Non-contrast MRI is the emerging imaging modality for these patients. A systematic review of almost 2000 pediatric patients found MRI sensitivity and specificity to be 97% and 97% with a low negative appendectomy rate.23
Cost and image acquisition time are limitations for MRI use for children. Pediatric patients may require sedation with long acquisition times in order to ensure that high-quality images are obtained, potentially introducing more associated costs and safety concerns. Shorter image-acquisition times would make MRI a more widely applicable test.23
Orthopedics
Various orthopedic conditions can be investigated by MRI, but this is not commonly done in the ED. Acute knee trauma with a concern for ligamentous, cartilaginous, or meniscal injury is one example. The patient with a concern for occult fracture or injury to the shoulder, elbow, or scaphoid represent others.
However, the special case of the patient with hip trauma with negative radiographs who will not weight bear or has significant pain is worth considering. MRI to either diagnose or exclude occult hip, pelvic, or acetabular fracture is traditionally considered to be the criterion standard. However, a 2016 study called this widely-held belief into question. It found that CT and MRI were similarly sensitive and concluded that starting with CT was a reasonable approach.24 MRI can be considered if the diagnosis remains in doubt.
Musculoskeletal infections
A wide variety of bone, joint, and soft-tissue infections can be diagnosed by MRI, which is often the imaging modality of choice. Some of these infections may be limb- or even life-threatening. One, epidural abscess, is both life-threatening and function-threatening and has been discussed briefly already.
If you are concerned about the possibility of a serious soft-tissue or bone infection, strongly consider giving gadolinium contrast, which is particularly useful for detecting abscesses, sinus tracts, and spine infections, and for providing other important anatomic details.25
Conclusion
MRI utilization by EPs will continue to increase as the factors governing its use evolves. These factors include: decreasing scan times; wider availability; possible cost reductions; new and changing indications; more research; and the always-present pressure on EPs to care for a broader spectrum of evermore challenging patients. It therefore benefits us to understand more about this dynamic part of our practice. Look to the scientific literature on stroke, neurosurgical emergencies, orthopedics, pediatrics, infectious disease and other fields that impact emergency medicine practice and MRI use as they continue to change.
SIDEBAR
Summary of Cautions and Limitations of MRI Use
Lack of availability
Cost
Exam completion time
Claustrophobia
Patient’s inability to lie still
Implanted medical devices
Metallic foreign bodies
Obesity
Hearing damage
Pregnancy
Pediatric patients (the developing brain)
Nephrogenic systemic fibrosis due to gadolinium-based contrast agents
SIDEBAR
Common ED MRI indications
Central Nervous System
- Spinal cord compression
- Dural venous sinus thrombosis
- Arterial dissections - carotid or vertebral
- Meningoencephalitis and vasculitis evaluation (possible)
- Subarachnoid hemorrhage (possible)
- Cerebral ischemia - TIA/Stroke
Spinal cord/surrounding structure disease or trauma - epidural abscess, cauda equina syndrome, cord/nerve trauma
Pregnant patients with abdominal pain (concern for appendicitis)
Children with abdominal pain (concern for appendicitis)
Musculoskeletal infections Orthopedic trauma
1. Currie S, Hoggard N, Craven IJ, Hadjivassiliou M, Wilkinson ID. Understanding MRI: Basic MR physics for physicians. Postgrad Med J. 2013;89:209-223.
2. Berger A. How does it work? Magnetic resonance imaging. BMJ. 2002;324:35.
3. Chung M, Dahabreh IJ, Hadar N, et al. Emerging MRI technologies for imaging musculoskeletal disorders under loading stress. Comparative Effectiveness Technical Briefs, No. 7. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. https://www.ncbi.nlm.nih.gov/books/NBK82287/
4. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451.
5. MRI Safety. http://www.mrisafety.com/TheList_search.asp.
6. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952-961.
7. Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A. MRI evaluation and safety in the developing brain. Semin Perinatol. 2015;39(2):73-104.
8. Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG. Revisiting the risks of MRI with Gadolinium based contrast agents—review of literature and guidelines. Insights Imaging. 2015;6(5):553-558.
9. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium-based contrast agents in kidney disease: A comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis. 2018;5:1-17.
10. Quint DJ. Indications for emergent MRI of the central nervous system. JAMA. 2000;283(7):853-855.
11. Broder J. Imaging the cervical, thoracic, and lumbar spine. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, PA: Elsevier; 2011:73-157.
12. Villringer A, Einhäupl KM. Dural sinus and cerebral venous thrombosis. New Horiz. 1997;5(4):332-341.
13. Ben Hassen W, Machet A, Edjlali-Goujon M, et al. Imaging of cervical artery dissection. Diagn Interv Imaging. 2014;95(12):1151-1161.
14. Jacobs A, Lanfermann H, Neveling M, Szelies B, Schröder R, Heiss W-D. MRI-and MRI-guided therapy of carotid and vertebral artery dissections. J Neurol Sci. 1997;147(1):27-34.
15. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
16. Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34(11):E117-127.
17. Lavi ES, Pal A, Bleicher D, Kang K, Sidani C. MR imaging of the spine: Urgent and emergent indications. Semin Ultrasound CT MR. (2018), doi: https://doi.org/10.1053/j.sult.2018.10.006
18. Kawakyu-O’Connor D, Bordia R, Nicola R. Magnetic resonance imaging of spinal emergencies. Magn Reson Imaging Clin N Am. 2016;24(2):325-344.
19. Patel ND, Broderick DF, Burns J, et al. ACR appropriateness criteria low back pain. J Am Coll of Radiol. 2016;13(9):1069-1078.
20. Garcia EM, Camacho MA, Karolyi DR, et al. ACR appropriateness criteria -right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15(11):S373-S387.
21. Duke E, Kalb B, Arif-Tiwari H, et al. A systematic review and meta-analysis of diagnostic performance of MRI for evaluation of acute appendicitis. AJR Am J Roentgenol. 2016;206(3):508-517.
22. Yu HS, Gupta A, Soto JA, et al. Emergency abdominal MRI: Current uses and trends. Br J Radiol. 2016;89(1061). doi:10.1259/bjr.20150804
23. Kim JR, Suh CH, Yoon HM, et al. Performance of MRI for suspected appendicitis in pediatric patients and negative appendectomy rate: A systematic review and meta-analysis. J Magn Reson Imaging. 2017;47(3):767-778.
24. Rehman H, Clement RG, Perks F, White TO. Imaging of occult hip fractures: CT or MRI? Injury. 2016;47(6):1297-1301.
25. Simpfendorfer CS. Radiologic approach to musculoskeletal infections. Infect Dis Clin N Am. 2017;31:299-324.
1. Currie S, Hoggard N, Craven IJ, Hadjivassiliou M, Wilkinson ID. Understanding MRI: Basic MR physics for physicians. Postgrad Med J. 2013;89:209-223.
2. Berger A. How does it work? Magnetic resonance imaging. BMJ. 2002;324:35.
3. Chung M, Dahabreh IJ, Hadar N, et al. Emerging MRI technologies for imaging musculoskeletal disorders under loading stress. Comparative Effectiveness Technical Briefs, No. 7. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. https://www.ncbi.nlm.nih.gov/books/NBK82287/
4. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451.
5. MRI Safety. http://www.mrisafety.com/TheList_search.asp.
6. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952-961.
7. Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A. MRI evaluation and safety in the developing brain. Semin Perinatol. 2015;39(2):73-104.
8. Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG. Revisiting the risks of MRI with Gadolinium based contrast agents—review of literature and guidelines. Insights Imaging. 2015;6(5):553-558.
9. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium-based contrast agents in kidney disease: A comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis. 2018;5:1-17.
10. Quint DJ. Indications for emergent MRI of the central nervous system. JAMA. 2000;283(7):853-855.
11. Broder J. Imaging the cervical, thoracic, and lumbar spine. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, PA: Elsevier; 2011:73-157.
12. Villringer A, Einhäupl KM. Dural sinus and cerebral venous thrombosis. New Horiz. 1997;5(4):332-341.
13. Ben Hassen W, Machet A, Edjlali-Goujon M, et al. Imaging of cervical artery dissection. Diagn Interv Imaging. 2014;95(12):1151-1161.
14. Jacobs A, Lanfermann H, Neveling M, Szelies B, Schröder R, Heiss W-D. MRI-and MRI-guided therapy of carotid and vertebral artery dissections. J Neurol Sci. 1997;147(1):27-34.
15. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
16. Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34(11):E117-127.
17. Lavi ES, Pal A, Bleicher D, Kang K, Sidani C. MR imaging of the spine: Urgent and emergent indications. Semin Ultrasound CT MR. (2018), doi: https://doi.org/10.1053/j.sult.2018.10.006
18. Kawakyu-O’Connor D, Bordia R, Nicola R. Magnetic resonance imaging of spinal emergencies. Magn Reson Imaging Clin N Am. 2016;24(2):325-344.
19. Patel ND, Broderick DF, Burns J, et al. ACR appropriateness criteria low back pain. J Am Coll of Radiol. 2016;13(9):1069-1078.
20. Garcia EM, Camacho MA, Karolyi DR, et al. ACR appropriateness criteria -right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15(11):S373-S387.
21. Duke E, Kalb B, Arif-Tiwari H, et al. A systematic review and meta-analysis of diagnostic performance of MRI for evaluation of acute appendicitis. AJR Am J Roentgenol. 2016;206(3):508-517.
22. Yu HS, Gupta A, Soto JA, et al. Emergency abdominal MRI: Current uses and trends. Br J Radiol. 2016;89(1061). doi:10.1259/bjr.20150804
23. Kim JR, Suh CH, Yoon HM, et al. Performance of MRI for suspected appendicitis in pediatric patients and negative appendectomy rate: A systematic review and meta-analysis. J Magn Reson Imaging. 2017;47(3):767-778.
24. Rehman H, Clement RG, Perks F, White TO. Imaging of occult hip fractures: CT or MRI? Injury. 2016;47(6):1297-1301.
25. Simpfendorfer CS. Radiologic approach to musculoskeletal infections. Infect Dis Clin N Am. 2017;31:299-324.
Dileep Nair, MD
Moderate approach best for NET liver mets
BOSTON – Even partial reduction of liver metastases from malignant small bowel neuroendocrine tumors will help symptoms and improve survival, according to James Howe, MD, director of surgical oncology and endocrine surgery at the University of Iowa, Iowa City.
“Hepatic cytoreduction is not just to cut down on hormone production” to relieve carcinoid syndrome symptoms, but also “there seems to be a survival benefit,” he said at the annual clinical congress of the American College of Surgeons.
There was a time when it was thought that at least 90% of liver metastases needed to be removed for patients benefit, but it’s become clear that even clearing 70% will help.
It’s important, however, to use parenchymal sparing techniques such as wedge excision, enucleation, and ablation. “We don’t do a lot of right and left hepatectomies anymore,” because patients often live a long time with the disease, many past 10 years, so the goal is management without undo side effects from aggressive treatment. “The key thing with all patients is to avoid morbidity. These patients are going to live a long time if you do not do very much,” Dr. Howe said.
The general surgical approach is to remove the primary neuroendocrine tumor (NET) plus regional lymph nodes; debulk peritoneal disease; cytoreduce liver metastases, and remove the gallbladder, because many patients go on to somatostatin analogs; in 2 years or so, they’ll develop gallstones.
CT is overall the most useful imaging tool for workup. The terminal ileum is the most common location of small bowel NETs, but often “the primary will not be seen,” so “the main thing I look for is mesenteric lymphadenopathy. Liver metastases are even more suspicious.” PET imaging with gallium-labeled somatostatin analogues is also “very good for determining if a lesion is indeed a NET and to determine sites of distant metastases,” Dr. Howe said.
Suspicious findings are followed by surgical exploration, which he usually does by midline incision, the size of which depends on if hepatic cytoreduction is planned. “It’s critically important to palpate the entire small bowel, or you are going to miss lesions. You can feel them quite easily even if they are just 1 or 2 mm,” he said.
Laparoscopy is an option for quicker recovery and other benefits, but the downside is “you are going to miss multifocal disease if you are using metal graspers to feel the small bowel,” and challenging node resection is impossible. One compromise is to do a small incision of a few inches; “you can actually palpate the small bowel through a small incision, and resect a section of bowel through it,” he said.
He saves systemic therapy for patients who progress despite surgery and somatostatin analogues. Everolimus is an option, but the most promising option is lutetium Lu 177 dotatate (Lutathera), approved by the Food and Drug Administration in early 2018 for pancreatic and gastrointestinal NETs.
BOSTON – Even partial reduction of liver metastases from malignant small bowel neuroendocrine tumors will help symptoms and improve survival, according to James Howe, MD, director of surgical oncology and endocrine surgery at the University of Iowa, Iowa City.
“Hepatic cytoreduction is not just to cut down on hormone production” to relieve carcinoid syndrome symptoms, but also “there seems to be a survival benefit,” he said at the annual clinical congress of the American College of Surgeons.
There was a time when it was thought that at least 90% of liver metastases needed to be removed for patients benefit, but it’s become clear that even clearing 70% will help.
It’s important, however, to use parenchymal sparing techniques such as wedge excision, enucleation, and ablation. “We don’t do a lot of right and left hepatectomies anymore,” because patients often live a long time with the disease, many past 10 years, so the goal is management without undo side effects from aggressive treatment. “The key thing with all patients is to avoid morbidity. These patients are going to live a long time if you do not do very much,” Dr. Howe said.
The general surgical approach is to remove the primary neuroendocrine tumor (NET) plus regional lymph nodes; debulk peritoneal disease; cytoreduce liver metastases, and remove the gallbladder, because many patients go on to somatostatin analogs; in 2 years or so, they’ll develop gallstones.
CT is overall the most useful imaging tool for workup. The terminal ileum is the most common location of small bowel NETs, but often “the primary will not be seen,” so “the main thing I look for is mesenteric lymphadenopathy. Liver metastases are even more suspicious.” PET imaging with gallium-labeled somatostatin analogues is also “very good for determining if a lesion is indeed a NET and to determine sites of distant metastases,” Dr. Howe said.
Suspicious findings are followed by surgical exploration, which he usually does by midline incision, the size of which depends on if hepatic cytoreduction is planned. “It’s critically important to palpate the entire small bowel, or you are going to miss lesions. You can feel them quite easily even if they are just 1 or 2 mm,” he said.
Laparoscopy is an option for quicker recovery and other benefits, but the downside is “you are going to miss multifocal disease if you are using metal graspers to feel the small bowel,” and challenging node resection is impossible. One compromise is to do a small incision of a few inches; “you can actually palpate the small bowel through a small incision, and resect a section of bowel through it,” he said.
He saves systemic therapy for patients who progress despite surgery and somatostatin analogues. Everolimus is an option, but the most promising option is lutetium Lu 177 dotatate (Lutathera), approved by the Food and Drug Administration in early 2018 for pancreatic and gastrointestinal NETs.
BOSTON – Even partial reduction of liver metastases from malignant small bowel neuroendocrine tumors will help symptoms and improve survival, according to James Howe, MD, director of surgical oncology and endocrine surgery at the University of Iowa, Iowa City.
“Hepatic cytoreduction is not just to cut down on hormone production” to relieve carcinoid syndrome symptoms, but also “there seems to be a survival benefit,” he said at the annual clinical congress of the American College of Surgeons.
There was a time when it was thought that at least 90% of liver metastases needed to be removed for patients benefit, but it’s become clear that even clearing 70% will help.
It’s important, however, to use parenchymal sparing techniques such as wedge excision, enucleation, and ablation. “We don’t do a lot of right and left hepatectomies anymore,” because patients often live a long time with the disease, many past 10 years, so the goal is management without undo side effects from aggressive treatment. “The key thing with all patients is to avoid morbidity. These patients are going to live a long time if you do not do very much,” Dr. Howe said.
The general surgical approach is to remove the primary neuroendocrine tumor (NET) plus regional lymph nodes; debulk peritoneal disease; cytoreduce liver metastases, and remove the gallbladder, because many patients go on to somatostatin analogs; in 2 years or so, they’ll develop gallstones.
CT is overall the most useful imaging tool for workup. The terminal ileum is the most common location of small bowel NETs, but often “the primary will not be seen,” so “the main thing I look for is mesenteric lymphadenopathy. Liver metastases are even more suspicious.” PET imaging with gallium-labeled somatostatin analogues is also “very good for determining if a lesion is indeed a NET and to determine sites of distant metastases,” Dr. Howe said.
Suspicious findings are followed by surgical exploration, which he usually does by midline incision, the size of which depends on if hepatic cytoreduction is planned. “It’s critically important to palpate the entire small bowel, or you are going to miss lesions. You can feel them quite easily even if they are just 1 or 2 mm,” he said.
Laparoscopy is an option for quicker recovery and other benefits, but the downside is “you are going to miss multifocal disease if you are using metal graspers to feel the small bowel,” and challenging node resection is impossible. One compromise is to do a small incision of a few inches; “you can actually palpate the small bowel through a small incision, and resect a section of bowel through it,” he said.
He saves systemic therapy for patients who progress despite surgery and somatostatin analogues. Everolimus is an option, but the most promising option is lutetium Lu 177 dotatate (Lutathera), approved by the Food and Drug Administration in early 2018 for pancreatic and gastrointestinal NETs.
EXPERT ANALYSIS FROM THE ACS CLINICAL CONGRESS
Lipoblastoma of the Scalp in a Child
To the Editor:
A 2-year-old boy was referred to our pediatric dermatology clinic by his pediatrician for an enlarging mass on the mid frontal scalp. The lesion had been present since birth and slowly enlarged. His parents thought the lesion was mostly asymptomatic; however, if it was irritated, the child would cry. He was otherwise healthy and had no history of skin conditions. There was no family history of skin conditions, birthmarks, or vascular malformations. On physical examination, we observed an isolated, approximately 3-cm, well-circumscribed, mobile, flesh-colored and violaceous nodule on the mid frontal scalp (Figure 1). At that time our differential diagnosis included a complex hemangioma or other vascular proliferation, nevus lipomatosis, or even a soft-tissue malignancy such as a sarcoma. Prior to biopsy, we ordered magnetic resonance imaging (MRI) to evaluate for intracranial extension of the lesion. The MRI revealed a 3.2-cm frontal midline scalp mass with complex imaging characteristics, and the radiologist gave a differential diagnosis of hemangioma, teratoma, or less likely liposarcoma (Figure 2). Fortunately, there was no evidence of central nervous system or intracranial invasion. We then proceeded with excisional biopsy, which grossly revealed a nodular, well-circumscribed, yellow mass (Figure 3). The wound was closed with primary closure. Histologically, there was a lobulated tumor with thin, well-vascularized connective tissue septa within a myxoid stroma (Figure 4A). The tumor was composed of lipocytes in varying stages of maturity without obvious nuclear atypia (Figures 4B and 4C), leading to a diagnosis of lipoblastoma.
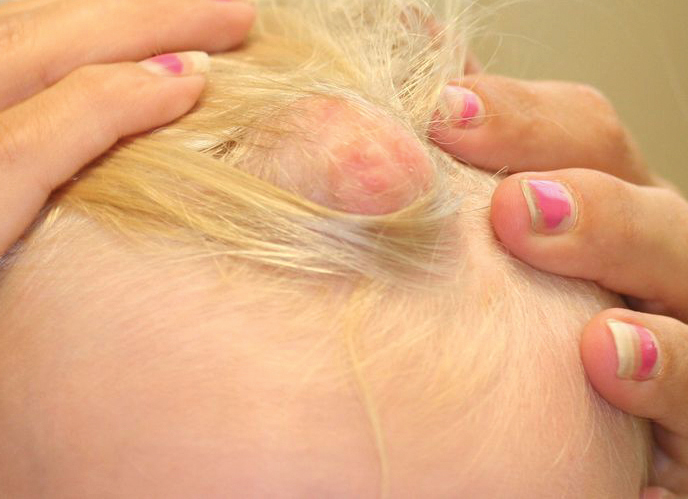
Lipoblastoma (also known as an embryonic lipoma) is a rare variant of lipoma. It is a benign neoplasm of immature white fat cells primarily seen in children younger than 3 years. It is reportedly twice as common in boys versus girls. Lipoblastomas present as enlarging, soft, mobile, painless nodules, usually 3 to 5 cm in diameter. The extremities are the favored location, but they also have been described on the head, neck, and trunk. Additionally, mediastinal and retroperitoneal lipoblastomas have been documented.1 The tumors may be symptomatic, particularly when involving the neck or mediastinum. In rare instances, they may present with respiratory distress.2 Multiple cases of head and neck lipoblastomas have been published in the English-language literature.3 Growing evidence supports that a chromosomal breakpoint abnormality at 8q11-q13 may be implicated in the pathogenesis.4
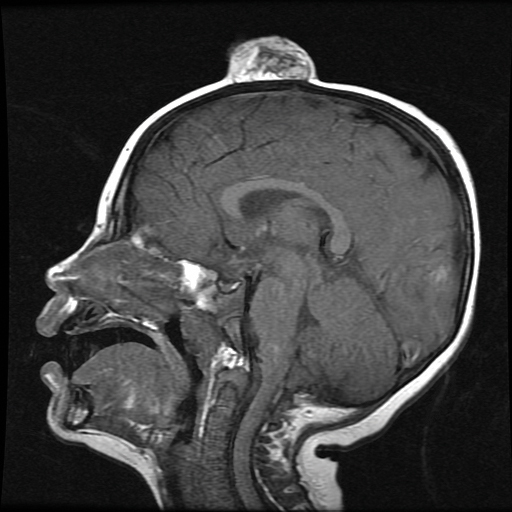
mass without intracranial extension.
Most lipoblastomas are well circumscribed, encapsulated, and limited to the subcutis. However, lipoblastomatosis is the diffuse counterpart to lipoblastoma, affecting deeper soft tissue and often infiltrating adjacent skeletal muscle.1

Diagnosis is made by histologic evaluation. Lipoblastoma appears as immature fat cells in varying stages of maturity with septa separating them into lobules. There should not be nuclear atypia.5 The histologic differential diagnosis includes other adipose tumors, most chiefly myxoid liposarcoma. These tumors have a lobular pattern without prominent septae and contain nuclear atypia with atypical mitotic figures. Myxoid liposarcoma has an infiltrating pattern similar to lipoblastomatosis and has a metastatic rate up to 60%.6 Imaging studies such as MRI are helpful in diagnosis, particularly in head and neck or visceral cases.3 The treatment of choice of lipoblastoma is wide excision. With complete removal, tumors rarely recur. Recurrences are more common in lipoblastomatosis or with incompletely excised primary lesions.3 A 14% to 24% recurrence rate has been recognized. Cytogenic analysis of lipomatous tumors has begun to reveal translocations in chromosome 8q11-13 region breakpoint abnormalities and translocations, specifically involving the pleomorphic adenoma gene 1, PLAG1, as the oncogenic target in lipoblastoma.6 Identification of these molecular mutations may provide aid in differentiating histologically similar-appearing tumors in the future.
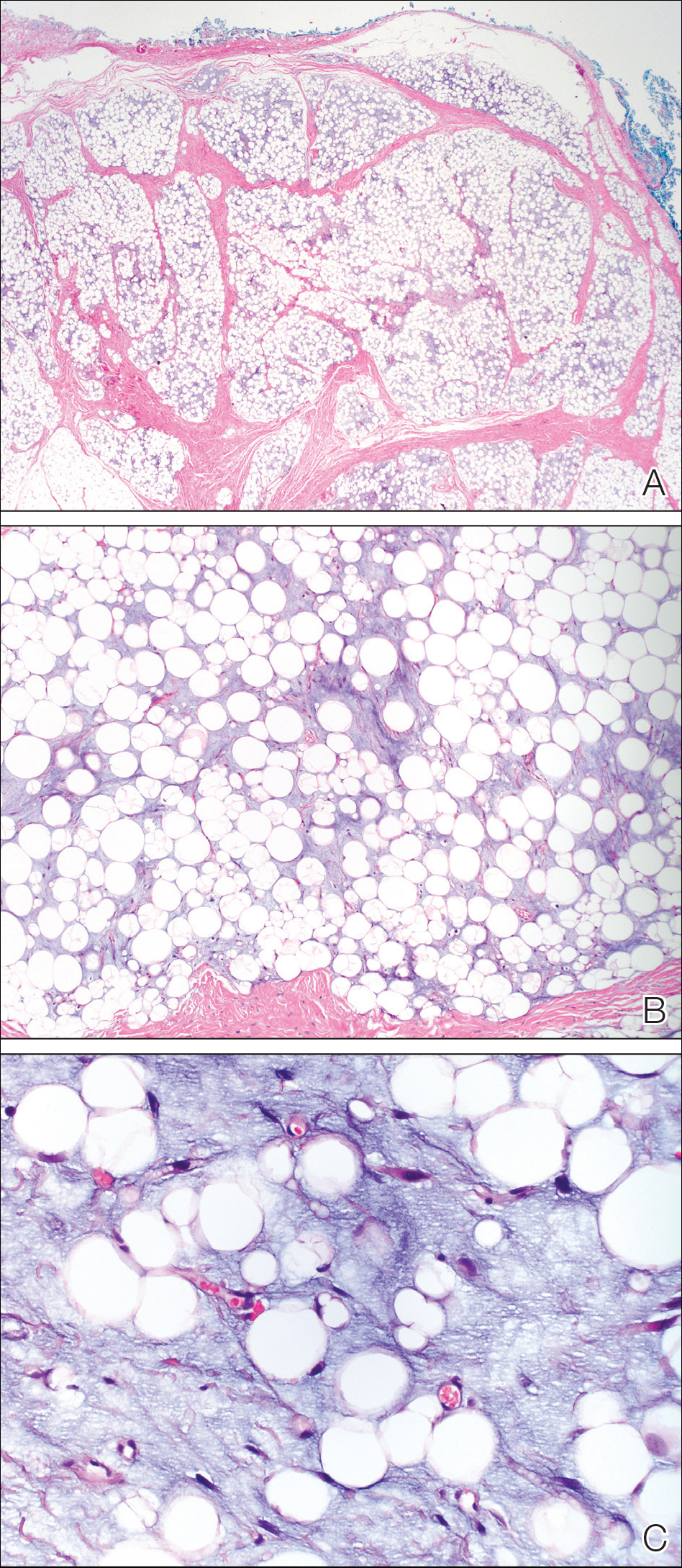
This case illustrates a rare benign childhood tumor that can be difficult to diagnose prior to histologic examination. Our patient did not fit the typical description of a lipoblastoma, as his tumor was axially located as opposed to the more common peripheral presentation. We aim to raise awareness of this diagnosis as more cases are being recognized.
- Kaddu S, Kohler S. Muscle, adipose, and cartilage neoplasms. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. St. Louis, MO: Mosby; 2003:1988-1989.
- Benato C, Falezza G, Lonardoni A, et al. Acute respiratory distress caused by a giant mediastinal lipoblastoma in a 16-month-old boy. Ann Thorac Surg. 2011;92:119-120.
- Pham NS, Poirier B, Fuller SC, et al. Pediatric lipoblastoma in the head and neck: a systematic review of 48 reported cases. Int J Pediatr Otorhinolaryngol. 2010;74:723-728.
- Chen Z, Coffin CM, Scott S, et al. Evidence by spectral karyotyping that 8q11.2 is nonrandomly involved in lipoblastoma. J Mol Diagn. 2000;2:73-77.
- Weedon, D. Weedon’s Skin Pathology. 3rd ed. China: Churchill Livingstone; 2010.
- Hicks J, Dilley A, Patal D. Lipoblastoma and lipoblastomatosis in infancy and childhood: histopathologic, ultrastructural, and cytogenetic features. Ultrastruct Pathol. 2001;25:321-333.
To the Editor:
A 2-year-old boy was referred to our pediatric dermatology clinic by his pediatrician for an enlarging mass on the mid frontal scalp. The lesion had been present since birth and slowly enlarged. His parents thought the lesion was mostly asymptomatic; however, if it was irritated, the child would cry. He was otherwise healthy and had no history of skin conditions. There was no family history of skin conditions, birthmarks, or vascular malformations. On physical examination, we observed an isolated, approximately 3-cm, well-circumscribed, mobile, flesh-colored and violaceous nodule on the mid frontal scalp (Figure 1). At that time our differential diagnosis included a complex hemangioma or other vascular proliferation, nevus lipomatosis, or even a soft-tissue malignancy such as a sarcoma. Prior to biopsy, we ordered magnetic resonance imaging (MRI) to evaluate for intracranial extension of the lesion. The MRI revealed a 3.2-cm frontal midline scalp mass with complex imaging characteristics, and the radiologist gave a differential diagnosis of hemangioma, teratoma, or less likely liposarcoma (Figure 2). Fortunately, there was no evidence of central nervous system or intracranial invasion. We then proceeded with excisional biopsy, which grossly revealed a nodular, well-circumscribed, yellow mass (Figure 3). The wound was closed with primary closure. Histologically, there was a lobulated tumor with thin, well-vascularized connective tissue septa within a myxoid stroma (Figure 4A). The tumor was composed of lipocytes in varying stages of maturity without obvious nuclear atypia (Figures 4B and 4C), leading to a diagnosis of lipoblastoma.

Lipoblastoma (also known as an embryonic lipoma) is a rare variant of lipoma. It is a benign neoplasm of immature white fat cells primarily seen in children younger than 3 years. It is reportedly twice as common in boys versus girls. Lipoblastomas present as enlarging, soft, mobile, painless nodules, usually 3 to 5 cm in diameter. The extremities are the favored location, but they also have been described on the head, neck, and trunk. Additionally, mediastinal and retroperitoneal lipoblastomas have been documented.1 The tumors may be symptomatic, particularly when involving the neck or mediastinum. In rare instances, they may present with respiratory distress.2 Multiple cases of head and neck lipoblastomas have been published in the English-language literature.3 Growing evidence supports that a chromosomal breakpoint abnormality at 8q11-q13 may be implicated in the pathogenesis.4

mass without intracranial extension.
Most lipoblastomas are well circumscribed, encapsulated, and limited to the subcutis. However, lipoblastomatosis is the diffuse counterpart to lipoblastoma, affecting deeper soft tissue and often infiltrating adjacent skeletal muscle.1

Diagnosis is made by histologic evaluation. Lipoblastoma appears as immature fat cells in varying stages of maturity with septa separating them into lobules. There should not be nuclear atypia.5 The histologic differential diagnosis includes other adipose tumors, most chiefly myxoid liposarcoma. These tumors have a lobular pattern without prominent septae and contain nuclear atypia with atypical mitotic figures. Myxoid liposarcoma has an infiltrating pattern similar to lipoblastomatosis and has a metastatic rate up to 60%.6 Imaging studies such as MRI are helpful in diagnosis, particularly in head and neck or visceral cases.3 The treatment of choice of lipoblastoma is wide excision. With complete removal, tumors rarely recur. Recurrences are more common in lipoblastomatosis or with incompletely excised primary lesions.3 A 14% to 24% recurrence rate has been recognized. Cytogenic analysis of lipomatous tumors has begun to reveal translocations in chromosome 8q11-13 region breakpoint abnormalities and translocations, specifically involving the pleomorphic adenoma gene 1, PLAG1, as the oncogenic target in lipoblastoma.6 Identification of these molecular mutations may provide aid in differentiating histologically similar-appearing tumors in the future.

This case illustrates a rare benign childhood tumor that can be difficult to diagnose prior to histologic examination. Our patient did not fit the typical description of a lipoblastoma, as his tumor was axially located as opposed to the more common peripheral presentation. We aim to raise awareness of this diagnosis as more cases are being recognized.
To the Editor:
A 2-year-old boy was referred to our pediatric dermatology clinic by his pediatrician for an enlarging mass on the mid frontal scalp. The lesion had been present since birth and slowly enlarged. His parents thought the lesion was mostly asymptomatic; however, if it was irritated, the child would cry. He was otherwise healthy and had no history of skin conditions. There was no family history of skin conditions, birthmarks, or vascular malformations. On physical examination, we observed an isolated, approximately 3-cm, well-circumscribed, mobile, flesh-colored and violaceous nodule on the mid frontal scalp (Figure 1). At that time our differential diagnosis included a complex hemangioma or other vascular proliferation, nevus lipomatosis, or even a soft-tissue malignancy such as a sarcoma. Prior to biopsy, we ordered magnetic resonance imaging (MRI) to evaluate for intracranial extension of the lesion. The MRI revealed a 3.2-cm frontal midline scalp mass with complex imaging characteristics, and the radiologist gave a differential diagnosis of hemangioma, teratoma, or less likely liposarcoma (Figure 2). Fortunately, there was no evidence of central nervous system or intracranial invasion. We then proceeded with excisional biopsy, which grossly revealed a nodular, well-circumscribed, yellow mass (Figure 3). The wound was closed with primary closure. Histologically, there was a lobulated tumor with thin, well-vascularized connective tissue septa within a myxoid stroma (Figure 4A). The tumor was composed of lipocytes in varying stages of maturity without obvious nuclear atypia (Figures 4B and 4C), leading to a diagnosis of lipoblastoma.

Lipoblastoma (also known as an embryonic lipoma) is a rare variant of lipoma. It is a benign neoplasm of immature white fat cells primarily seen in children younger than 3 years. It is reportedly twice as common in boys versus girls. Lipoblastomas present as enlarging, soft, mobile, painless nodules, usually 3 to 5 cm in diameter. The extremities are the favored location, but they also have been described on the head, neck, and trunk. Additionally, mediastinal and retroperitoneal lipoblastomas have been documented.1 The tumors may be symptomatic, particularly when involving the neck or mediastinum. In rare instances, they may present with respiratory distress.2 Multiple cases of head and neck lipoblastomas have been published in the English-language literature.3 Growing evidence supports that a chromosomal breakpoint abnormality at 8q11-q13 may be implicated in the pathogenesis.4

mass without intracranial extension.
Most lipoblastomas are well circumscribed, encapsulated, and limited to the subcutis. However, lipoblastomatosis is the diffuse counterpart to lipoblastoma, affecting deeper soft tissue and often infiltrating adjacent skeletal muscle.1

Diagnosis is made by histologic evaluation. Lipoblastoma appears as immature fat cells in varying stages of maturity with septa separating them into lobules. There should not be nuclear atypia.5 The histologic differential diagnosis includes other adipose tumors, most chiefly myxoid liposarcoma. These tumors have a lobular pattern without prominent septae and contain nuclear atypia with atypical mitotic figures. Myxoid liposarcoma has an infiltrating pattern similar to lipoblastomatosis and has a metastatic rate up to 60%.6 Imaging studies such as MRI are helpful in diagnosis, particularly in head and neck or visceral cases.3 The treatment of choice of lipoblastoma is wide excision. With complete removal, tumors rarely recur. Recurrences are more common in lipoblastomatosis or with incompletely excised primary lesions.3 A 14% to 24% recurrence rate has been recognized. Cytogenic analysis of lipomatous tumors has begun to reveal translocations in chromosome 8q11-13 region breakpoint abnormalities and translocations, specifically involving the pleomorphic adenoma gene 1, PLAG1, as the oncogenic target in lipoblastoma.6 Identification of these molecular mutations may provide aid in differentiating histologically similar-appearing tumors in the future.

This case illustrates a rare benign childhood tumor that can be difficult to diagnose prior to histologic examination. Our patient did not fit the typical description of a lipoblastoma, as his tumor was axially located as opposed to the more common peripheral presentation. We aim to raise awareness of this diagnosis as more cases are being recognized.
- Kaddu S, Kohler S. Muscle, adipose, and cartilage neoplasms. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. St. Louis, MO: Mosby; 2003:1988-1989.
- Benato C, Falezza G, Lonardoni A, et al. Acute respiratory distress caused by a giant mediastinal lipoblastoma in a 16-month-old boy. Ann Thorac Surg. 2011;92:119-120.
- Pham NS, Poirier B, Fuller SC, et al. Pediatric lipoblastoma in the head and neck: a systematic review of 48 reported cases. Int J Pediatr Otorhinolaryngol. 2010;74:723-728.
- Chen Z, Coffin CM, Scott S, et al. Evidence by spectral karyotyping that 8q11.2 is nonrandomly involved in lipoblastoma. J Mol Diagn. 2000;2:73-77.
- Weedon, D. Weedon’s Skin Pathology. 3rd ed. China: Churchill Livingstone; 2010.
- Hicks J, Dilley A, Patal D. Lipoblastoma and lipoblastomatosis in infancy and childhood: histopathologic, ultrastructural, and cytogenetic features. Ultrastruct Pathol. 2001;25:321-333.
- Kaddu S, Kohler S. Muscle, adipose, and cartilage neoplasms. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. St. Louis, MO: Mosby; 2003:1988-1989.
- Benato C, Falezza G, Lonardoni A, et al. Acute respiratory distress caused by a giant mediastinal lipoblastoma in a 16-month-old boy. Ann Thorac Surg. 2011;92:119-120.
- Pham NS, Poirier B, Fuller SC, et al. Pediatric lipoblastoma in the head and neck: a systematic review of 48 reported cases. Int J Pediatr Otorhinolaryngol. 2010;74:723-728.
- Chen Z, Coffin CM, Scott S, et al. Evidence by spectral karyotyping that 8q11.2 is nonrandomly involved in lipoblastoma. J Mol Diagn. 2000;2:73-77.
- Weedon, D. Weedon’s Skin Pathology. 3rd ed. China: Churchill Livingstone; 2010.
- Hicks J, Dilley A, Patal D. Lipoblastoma and lipoblastomatosis in infancy and childhood: histopathologic, ultrastructural, and cytogenetic features. Ultrastruct Pathol. 2001;25:321-333.
Practice Points
- Lipoblastoma is a benign neoplasm of immature white fat cells primarily seen in children younger than 3 years.
- Lipoblastomas often present as painless nodules located on the extremities.
- Histologically, lipoblastoma reveals immature adipose cells in varying stages of maturity arranged into lobules separated by septae.
- Consider magnetic resonance imaging if visceral extension is a concern; otherwise, surgical excision is curative in most cases.
TOO GOOD TO LAST?
When I first started in my training, I could not wait until the next issue of Emergency Medicine arrived in my mailbox (not yet inbox!). Emergency Medicine provided a service to our community and specialty at a time when no other publication was willing or able to do so. For no cost, no membership requirements, and no strings attached, we all received a monthly treasure trove of contemporary and practical information. The articles within were written by credible authors, in an approachable style, with professional illustrations that focused on key clinical issues that we saw in our everyday clinical practices.
As my career matured, I was asked to write and subsequently oversee a recurring feature on practical aspects of managing poisoning. Unclear if this was going to be well read, I agreed with trepidation. I quickly learned just how widely appreciated this journal was. People around the country wrote to let me know their thoughts on our thoughts (which meant they were reading it at least!). And people around the country offered to submit interesting toxicology cases for publication. For many of these authors, and many of my med tox trainees, this journal represented the first time they saw their name in print.
Clearly, to me at least, despite all the available blogs, podcasts, reddit and subreddit streams, and continuing medical education programs out there, people still loved getting this small but effective educational tool sent to them. And it is certainly sad to me, and likely many, that this wonderful benefactor of high-quality EM knowledge is losing its hard-fought battle against the modern reality of medical publishing.
I rest assured that there are other credible sources of education that we all can access. I know that our authors and readers will miss the journal dearly. But to paraphrase an unknown author: We should not be sad that it’s over, but glad that it happened.
Lewis S. Nelson, MD
Rutgers New Jersey Medical School
When I first started in my training, I could not wait until the next issue of Emergency Medicine arrived in my mailbox (not yet inbox!). Emergency Medicine provided a service to our community and specialty at a time when no other publication was willing or able to do so. For no cost, no membership requirements, and no strings attached, we all received a monthly treasure trove of contemporary and practical information. The articles within were written by credible authors, in an approachable style, with professional illustrations that focused on key clinical issues that we saw in our everyday clinical practices.
As my career matured, I was asked to write and subsequently oversee a recurring feature on practical aspects of managing poisoning. Unclear if this was going to be well read, I agreed with trepidation. I quickly learned just how widely appreciated this journal was. People around the country wrote to let me know their thoughts on our thoughts (which meant they were reading it at least!). And people around the country offered to submit interesting toxicology cases for publication. For many of these authors, and many of my med tox trainees, this journal represented the first time they saw their name in print.
Clearly, to me at least, despite all the available blogs, podcasts, reddit and subreddit streams, and continuing medical education programs out there, people still loved getting this small but effective educational tool sent to them. And it is certainly sad to me, and likely many, that this wonderful benefactor of high-quality EM knowledge is losing its hard-fought battle against the modern reality of medical publishing.
I rest assured that there are other credible sources of education that we all can access. I know that our authors and readers will miss the journal dearly. But to paraphrase an unknown author: We should not be sad that it’s over, but glad that it happened.
Lewis S. Nelson, MD
Rutgers New Jersey Medical School
When I first started in my training, I could not wait until the next issue of Emergency Medicine arrived in my mailbox (not yet inbox!). Emergency Medicine provided a service to our community and specialty at a time when no other publication was willing or able to do so. For no cost, no membership requirements, and no strings attached, we all received a monthly treasure trove of contemporary and practical information. The articles within were written by credible authors, in an approachable style, with professional illustrations that focused on key clinical issues that we saw in our everyday clinical practices.
As my career matured, I was asked to write and subsequently oversee a recurring feature on practical aspects of managing poisoning. Unclear if this was going to be well read, I agreed with trepidation. I quickly learned just how widely appreciated this journal was. People around the country wrote to let me know their thoughts on our thoughts (which meant they were reading it at least!). And people around the country offered to submit interesting toxicology cases for publication. For many of these authors, and many of my med tox trainees, this journal represented the first time they saw their name in print.
Clearly, to me at least, despite all the available blogs, podcasts, reddit and subreddit streams, and continuing medical education programs out there, people still loved getting this small but effective educational tool sent to them. And it is certainly sad to me, and likely many, that this wonderful benefactor of high-quality EM knowledge is losing its hard-fought battle against the modern reality of medical publishing.
I rest assured that there are other credible sources of education that we all can access. I know that our authors and readers will miss the journal dearly. But to paraphrase an unknown author: We should not be sad that it’s over, but glad that it happened.
Lewis S. Nelson, MD
Rutgers New Jersey Medical School
Extended-release naltrexone competes with buprenorphine-naloxone combo
Improvements in comorbid symptoms result in both treatments
Patients with opioid dependence and anxiety, depression, and insomnia symptoms who receive extended-release naltrexone injections experience similar results to those treated with daily oral buprenorphine-naloxone, research shows. The results suggest that those comorbid symptoms should not prevent a switch from treating patients with an opioid agonist to treating them with extended-release naltrexone, reported Zill-e-Huma Latif, MD, and colleagues.
“Our results showed improvements in anxiety, depression, and insomnia within only a few weeks of beginning either study treatment,” Dr. Latif and colleagues wrote in JAMA Psychiatry. “Our study also showed reductions in the use of opioids and other illegal substances in both treatment groups.”
Dr. Latif and colleagues conducted a prospective, randomized trial of 159 men and women with opioid dependence to receive a 380-mg injectable dose of extended-release naltrexone every 4 weeks or flexible doses (4-24 mg) of daily combined buprenorphine-naloxone for 12 weeks and then a 9-month, open-label period with one of the treatments as chosen by the participant. Of the participants, 105 reached the end of the trial (66%).
The participants were aged 18-60 years and had similar ages, gender distributions, and heroin use duration between treatment groups. The researchers used the 25-item Hopkins Symptom Checklist (HSCL-25) and the Insomnia Severity Index to assess anxiety, depression, and insomnia symptoms every 4 weeks until 48 weeks, reported Dr. Latif, of the department of research and development in mental health at Akershus University Hospital in Norway, and colleagues.
Over 12 weeks of the trial, However, the Insomnia Severity Index score was significantly lower among participants taking extended-release naltrexone (ES, −0.32; 95% CI, −0.65 to 0.02; P = .008). In the 9-month, open-label follow-up period, no significant differences were found in anxiety (ES, 0.04; 95% CI, −0.34 to 0.42), depression (ES, −0.04; 95% CI, −0.42 to 0.33), or insomnia (ES, 0.04; 95% CI, −0.33 to 0.42) among participants who switched to extended-release naltrexone or continued using extended-release naltrexone.
“Because participants receiving treatment with [extended-release naltrexone] or [buprenorphine-naloxone] showed equal improvements in anxiety, depression, and insomnia as assessed by the HSCL-25 and the Insomnia Severity Index, such symptoms should not preclude the choice to leave opioid agonist treatment and to start treatment with [extended-release naltrexone],” Dr. Latif and colleagues wrote.
With regard to study limitations, the researchers noted that they used self-reported questionnaires for anxiety, depression, and insomnia symptoms, did not confirm reported drug use through urine samples, and used the HSCL-25 when it is not a diagnostic tool.
The study was funded by grants from the Research Council of Norway and the Western Norway Regional Health Authority; financial support was provided by the Norwegian Center for Addiction Research, University of Oslo, and Akershus University Hospital. The authors report no relevant conflicts of interest.
SOURCE: Latif Z-H et al. JAMA Psychiatry. 2018 Dec 19. doi: 10.1001/jamapsychiatry.2018.3537.
Improvements in comorbid symptoms result in both treatments
Improvements in comorbid symptoms result in both treatments
Patients with opioid dependence and anxiety, depression, and insomnia symptoms who receive extended-release naltrexone injections experience similar results to those treated with daily oral buprenorphine-naloxone, research shows. The results suggest that those comorbid symptoms should not prevent a switch from treating patients with an opioid agonist to treating them with extended-release naltrexone, reported Zill-e-Huma Latif, MD, and colleagues.
“Our results showed improvements in anxiety, depression, and insomnia within only a few weeks of beginning either study treatment,” Dr. Latif and colleagues wrote in JAMA Psychiatry. “Our study also showed reductions in the use of opioids and other illegal substances in both treatment groups.”
Dr. Latif and colleagues conducted a prospective, randomized trial of 159 men and women with opioid dependence to receive a 380-mg injectable dose of extended-release naltrexone every 4 weeks or flexible doses (4-24 mg) of daily combined buprenorphine-naloxone for 12 weeks and then a 9-month, open-label period with one of the treatments as chosen by the participant. Of the participants, 105 reached the end of the trial (66%).
The participants were aged 18-60 years and had similar ages, gender distributions, and heroin use duration between treatment groups. The researchers used the 25-item Hopkins Symptom Checklist (HSCL-25) and the Insomnia Severity Index to assess anxiety, depression, and insomnia symptoms every 4 weeks until 48 weeks, reported Dr. Latif, of the department of research and development in mental health at Akershus University Hospital in Norway, and colleagues.
Over 12 weeks of the trial, However, the Insomnia Severity Index score was significantly lower among participants taking extended-release naltrexone (ES, −0.32; 95% CI, −0.65 to 0.02; P = .008). In the 9-month, open-label follow-up period, no significant differences were found in anxiety (ES, 0.04; 95% CI, −0.34 to 0.42), depression (ES, −0.04; 95% CI, −0.42 to 0.33), or insomnia (ES, 0.04; 95% CI, −0.33 to 0.42) among participants who switched to extended-release naltrexone or continued using extended-release naltrexone.
“Because participants receiving treatment with [extended-release naltrexone] or [buprenorphine-naloxone] showed equal improvements in anxiety, depression, and insomnia as assessed by the HSCL-25 and the Insomnia Severity Index, such symptoms should not preclude the choice to leave opioid agonist treatment and to start treatment with [extended-release naltrexone],” Dr. Latif and colleagues wrote.
With regard to study limitations, the researchers noted that they used self-reported questionnaires for anxiety, depression, and insomnia symptoms, did not confirm reported drug use through urine samples, and used the HSCL-25 when it is not a diagnostic tool.
The study was funded by grants from the Research Council of Norway and the Western Norway Regional Health Authority; financial support was provided by the Norwegian Center for Addiction Research, University of Oslo, and Akershus University Hospital. The authors report no relevant conflicts of interest.
SOURCE: Latif Z-H et al. JAMA Psychiatry. 2018 Dec 19. doi: 10.1001/jamapsychiatry.2018.3537.
Patients with opioid dependence and anxiety, depression, and insomnia symptoms who receive extended-release naltrexone injections experience similar results to those treated with daily oral buprenorphine-naloxone, research shows. The results suggest that those comorbid symptoms should not prevent a switch from treating patients with an opioid agonist to treating them with extended-release naltrexone, reported Zill-e-Huma Latif, MD, and colleagues.
“Our results showed improvements in anxiety, depression, and insomnia within only a few weeks of beginning either study treatment,” Dr. Latif and colleagues wrote in JAMA Psychiatry. “Our study also showed reductions in the use of opioids and other illegal substances in both treatment groups.”
Dr. Latif and colleagues conducted a prospective, randomized trial of 159 men and women with opioid dependence to receive a 380-mg injectable dose of extended-release naltrexone every 4 weeks or flexible doses (4-24 mg) of daily combined buprenorphine-naloxone for 12 weeks and then a 9-month, open-label period with one of the treatments as chosen by the participant. Of the participants, 105 reached the end of the trial (66%).
The participants were aged 18-60 years and had similar ages, gender distributions, and heroin use duration between treatment groups. The researchers used the 25-item Hopkins Symptom Checklist (HSCL-25) and the Insomnia Severity Index to assess anxiety, depression, and insomnia symptoms every 4 weeks until 48 weeks, reported Dr. Latif, of the department of research and development in mental health at Akershus University Hospital in Norway, and colleagues.
Over 12 weeks of the trial, However, the Insomnia Severity Index score was significantly lower among participants taking extended-release naltrexone (ES, −0.32; 95% CI, −0.65 to 0.02; P = .008). In the 9-month, open-label follow-up period, no significant differences were found in anxiety (ES, 0.04; 95% CI, −0.34 to 0.42), depression (ES, −0.04; 95% CI, −0.42 to 0.33), or insomnia (ES, 0.04; 95% CI, −0.33 to 0.42) among participants who switched to extended-release naltrexone or continued using extended-release naltrexone.
“Because participants receiving treatment with [extended-release naltrexone] or [buprenorphine-naloxone] showed equal improvements in anxiety, depression, and insomnia as assessed by the HSCL-25 and the Insomnia Severity Index, such symptoms should not preclude the choice to leave opioid agonist treatment and to start treatment with [extended-release naltrexone],” Dr. Latif and colleagues wrote.
With regard to study limitations, the researchers noted that they used self-reported questionnaires for anxiety, depression, and insomnia symptoms, did not confirm reported drug use through urine samples, and used the HSCL-25 when it is not a diagnostic tool.
The study was funded by grants from the Research Council of Norway and the Western Norway Regional Health Authority; financial support was provided by the Norwegian Center for Addiction Research, University of Oslo, and Akershus University Hospital. The authors report no relevant conflicts of interest.
SOURCE: Latif Z-H et al. JAMA Psychiatry. 2018 Dec 19. doi: 10.1001/jamapsychiatry.2018.3537.
FROM JAMA PSYCHIATRY
Key clinical point: No significant differences were found in the improvement of anxiety, depression, and insomnia symptoms among individuals with opioid dependence when receiving extended-release naltrexone, compared with combined buprenorphine-naloxone.
Major finding: In the 9-month, open-label follow-up period, there were no significant differences in anxiety (effect size, 0.04; 95% confidence interval, −0.34 to 0.42), depression (ES, −0.04; 95% CI, −0.42 to 0.33), or insomnia (ES, 0.04; 95% CI, −0.33 to 0.42) among participants who switched to extended-release naltrexone or continued using buprenorphine-naloxone.
Study details: A prospective, randomized trial of 159 men and women with opioid dependence who received extended-release naltrexone or combined buprenorphine-naloxone between November 2012 and October 2015.
Disclosures: This study was funded by grants from the Research Council of Norway and the Western Norway Regional Health Authority, and financial support was provided by the Norwegian Center for Addiction Research, University of Oslo, and Akershus University Hospital. The authors reported no relevant conflicts of interest.
Source: Latif Z-H et al. JAMA Psychiatry. 2018 Dec 19. doi: 10.1001/jamapsychiatry.2018.3537.