User login
CLL at ASH: A ‘mountain of data’ for targeted therapies
SAN DIEGO – There was a mountain of data presented at the annual meeting of the American Society of Hematology on the use of novel agents – both as frontline therapy and in combination – for the treatment of chronic lymphocytic leukemia (CLL).
In a video interview at the meeting, Brian T. Hill, MD, PhD, of the Cleveland Clinic and Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center, New York, summed up the key studies and what they mean in practice. They also looked ahead at what data are still missing that could aid in making important treatment decisions.
Dr. Hill highlighted the late-breaking abstract on the ECOG-ACRIN Cancer Research Group E1912 trial comparing ibrutinib-rituximab to a chemotherapy regimen of fludarabine, cyclophosphamide, and rituximab (FCR) in previously untreated patients under age 70 years (Abstract LBA-4). Not only was there a progression-free survival benefit with the use of the ibrutinib regimen, but there was an overall survival benefit as well, he noted.
Dr. Mato pointed to notable results from the Alliance A041202 trial of older patients with previously untreated disease that compared ibrutinib alone or in combination with rituximab, with bendamustine plus rituximab (Abstract #6). The ibrutinib-containing regimens resulted in superior progression-free survival.
The two trials taken together show a movement away from chemotherapy in the frontline setting and toward targeted agents for CLL, Dr. Mato said. “What that agent or combination of agents will be, remains to be seen,” he said. “We have now a real message about the fact that we’re ending, potentially, the era of chemotherapy for patients with CLL, which is a very welcome change.”
Dr. Mato and Dr. Hill will be discussing these trials and more CLL data during a Twitter chat on Jan. 31, 2019, from 7 p.m. to 8 p.m. EST. Join in the conversation by using and following #MDedgeChats.
SAN DIEGO – There was a mountain of data presented at the annual meeting of the American Society of Hematology on the use of novel agents – both as frontline therapy and in combination – for the treatment of chronic lymphocytic leukemia (CLL).
In a video interview at the meeting, Brian T. Hill, MD, PhD, of the Cleveland Clinic and Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center, New York, summed up the key studies and what they mean in practice. They also looked ahead at what data are still missing that could aid in making important treatment decisions.
Dr. Hill highlighted the late-breaking abstract on the ECOG-ACRIN Cancer Research Group E1912 trial comparing ibrutinib-rituximab to a chemotherapy regimen of fludarabine, cyclophosphamide, and rituximab (FCR) in previously untreated patients under age 70 years (Abstract LBA-4). Not only was there a progression-free survival benefit with the use of the ibrutinib regimen, but there was an overall survival benefit as well, he noted.
Dr. Mato pointed to notable results from the Alliance A041202 trial of older patients with previously untreated disease that compared ibrutinib alone or in combination with rituximab, with bendamustine plus rituximab (Abstract #6). The ibrutinib-containing regimens resulted in superior progression-free survival.
The two trials taken together show a movement away from chemotherapy in the frontline setting and toward targeted agents for CLL, Dr. Mato said. “What that agent or combination of agents will be, remains to be seen,” he said. “We have now a real message about the fact that we’re ending, potentially, the era of chemotherapy for patients with CLL, which is a very welcome change.”
Dr. Mato and Dr. Hill will be discussing these trials and more CLL data during a Twitter chat on Jan. 31, 2019, from 7 p.m. to 8 p.m. EST. Join in the conversation by using and following #MDedgeChats.
SAN DIEGO – There was a mountain of data presented at the annual meeting of the American Society of Hematology on the use of novel agents – both as frontline therapy and in combination – for the treatment of chronic lymphocytic leukemia (CLL).
In a video interview at the meeting, Brian T. Hill, MD, PhD, of the Cleveland Clinic and Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center, New York, summed up the key studies and what they mean in practice. They also looked ahead at what data are still missing that could aid in making important treatment decisions.
Dr. Hill highlighted the late-breaking abstract on the ECOG-ACRIN Cancer Research Group E1912 trial comparing ibrutinib-rituximab to a chemotherapy regimen of fludarabine, cyclophosphamide, and rituximab (FCR) in previously untreated patients under age 70 years (Abstract LBA-4). Not only was there a progression-free survival benefit with the use of the ibrutinib regimen, but there was an overall survival benefit as well, he noted.
Dr. Mato pointed to notable results from the Alliance A041202 trial of older patients with previously untreated disease that compared ibrutinib alone or in combination with rituximab, with bendamustine plus rituximab (Abstract #6). The ibrutinib-containing regimens resulted in superior progression-free survival.
The two trials taken together show a movement away from chemotherapy in the frontline setting and toward targeted agents for CLL, Dr. Mato said. “What that agent or combination of agents will be, remains to be seen,” he said. “We have now a real message about the fact that we’re ending, potentially, the era of chemotherapy for patients with CLL, which is a very welcome change.”
Dr. Mato and Dr. Hill will be discussing these trials and more CLL data during a Twitter chat on Jan. 31, 2019, from 7 p.m. to 8 p.m. EST. Join in the conversation by using and following #MDedgeChats.
REPORTING FROM ASH 2018
For weight-loss apps, the evidence base is still small
NASHVILLE –
Beginning with a pool of 1,380 publications, Christina Hopkins and her colleagues at Duke University, Durham, N.C., eventually identified just nine trials of all-digital interventions for weight loss that met their inclusion criteria.
Presenting their findings at a late-breaking poster session during Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery, Ms. Hopkins, a clinical psychology graduate student at Duke, and her colleagues found that three of the nine studies showed statistically significant weight loss, compared with a control state. Absolute weight loss in these three trials ranged from 3 kg to about 7 kg (between-group differences, P less than .001 for all).
Participants in another trial didn’t lose a statistically significant amount of weight, compared with the control arm of the study. However, the mean 5 kg lost by those in the intervention arm was enough to be clinically significant, so Ms. Hopkins and her colleagues included this study in a subanalysis that looked for effective modalities and interventions among the studies with significant results.
The duration of the studies ranged from 6 to 24 months, though five of the trials were less than 1 year long. Women made up the majority of participants in all but one trial.
“There is limited evidence that standalone digital weight-loss interventions produce clinically meaningful outcomes,” wrote Ms. Hopkins and her coauthors. “Absolute magnitude of weight loss was low, and the short intervention lengths call into question the sustainability of these weight losses.”
The systematic review cast a broad net to include digital modalities such as wireless scales, text messaging, email, and web-based interventions, as well as the use of smartphone apps and tracking devices. All interventions used multiple digital modalities.
The most frequently used technologies were the use of a website, used in six (67%) of the trials, followed by text messaging and smartphone apps, each used in five (56%) of the trials. Tracking devices, email, message boards, and gamification of some sort were all used in three (33%) of the trials.
In terms of the specific interventions used in the trials, weight, diet, and activity were all tracked in eight trials (89%). Similarly, all but one trial gave feedback and weight and health education to participants. Behavior change education, as well as calorie goals, were each used in six trials (67%).
Ms. Hopkins and her colleagues looked at which trials incorporated which modalities and interventions, finding that “trials that integrated components unique to digital interventions, such as gamification, podcasts, or interactive features, yielded significantly greater and more clinically meaningful weight losses.”
To be included in the systematic review, trials had to include adult participants with a body mass index of at least 25 kg/m2 and use a standalone digital intervention of at least 6 months’ duration. The primary outcome of interest in the review was the change in participant weight from baseline to the end of the minimum 6-month follow-up period. Randomized, controlled trials and feasibility trials were included, so long as participants were allocated randomly.
Of the 126 trials reviewed at the full text level, the most frequent reason for exclusion was the inclusion of human coaching. Also, 30 of the trials didn’t report weight change as an outcome, the investigators said.
Future directions should include comparing digital interventions that “utilize features unique to digital delivery” with those that more closely resemble in-person weight-loss management interventions, suggested Ms. Hopkins and her collaborators.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Hopkins C et al. Obesity Week 2018, Abstract T-P-LB-3640.
NASHVILLE –
Beginning with a pool of 1,380 publications, Christina Hopkins and her colleagues at Duke University, Durham, N.C., eventually identified just nine trials of all-digital interventions for weight loss that met their inclusion criteria.
Presenting their findings at a late-breaking poster session during Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery, Ms. Hopkins, a clinical psychology graduate student at Duke, and her colleagues found that three of the nine studies showed statistically significant weight loss, compared with a control state. Absolute weight loss in these three trials ranged from 3 kg to about 7 kg (between-group differences, P less than .001 for all).
Participants in another trial didn’t lose a statistically significant amount of weight, compared with the control arm of the study. However, the mean 5 kg lost by those in the intervention arm was enough to be clinically significant, so Ms. Hopkins and her colleagues included this study in a subanalysis that looked for effective modalities and interventions among the studies with significant results.
The duration of the studies ranged from 6 to 24 months, though five of the trials were less than 1 year long. Women made up the majority of participants in all but one trial.
“There is limited evidence that standalone digital weight-loss interventions produce clinically meaningful outcomes,” wrote Ms. Hopkins and her coauthors. “Absolute magnitude of weight loss was low, and the short intervention lengths call into question the sustainability of these weight losses.”
The systematic review cast a broad net to include digital modalities such as wireless scales, text messaging, email, and web-based interventions, as well as the use of smartphone apps and tracking devices. All interventions used multiple digital modalities.
The most frequently used technologies were the use of a website, used in six (67%) of the trials, followed by text messaging and smartphone apps, each used in five (56%) of the trials. Tracking devices, email, message boards, and gamification of some sort were all used in three (33%) of the trials.
In terms of the specific interventions used in the trials, weight, diet, and activity were all tracked in eight trials (89%). Similarly, all but one trial gave feedback and weight and health education to participants. Behavior change education, as well as calorie goals, were each used in six trials (67%).
Ms. Hopkins and her colleagues looked at which trials incorporated which modalities and interventions, finding that “trials that integrated components unique to digital interventions, such as gamification, podcasts, or interactive features, yielded significantly greater and more clinically meaningful weight losses.”
To be included in the systematic review, trials had to include adult participants with a body mass index of at least 25 kg/m2 and use a standalone digital intervention of at least 6 months’ duration. The primary outcome of interest in the review was the change in participant weight from baseline to the end of the minimum 6-month follow-up period. Randomized, controlled trials and feasibility trials were included, so long as participants were allocated randomly.
Of the 126 trials reviewed at the full text level, the most frequent reason for exclusion was the inclusion of human coaching. Also, 30 of the trials didn’t report weight change as an outcome, the investigators said.
Future directions should include comparing digital interventions that “utilize features unique to digital delivery” with those that more closely resemble in-person weight-loss management interventions, suggested Ms. Hopkins and her collaborators.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Hopkins C et al. Obesity Week 2018, Abstract T-P-LB-3640.
NASHVILLE –
Beginning with a pool of 1,380 publications, Christina Hopkins and her colleagues at Duke University, Durham, N.C., eventually identified just nine trials of all-digital interventions for weight loss that met their inclusion criteria.
Presenting their findings at a late-breaking poster session during Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery, Ms. Hopkins, a clinical psychology graduate student at Duke, and her colleagues found that three of the nine studies showed statistically significant weight loss, compared with a control state. Absolute weight loss in these three trials ranged from 3 kg to about 7 kg (between-group differences, P less than .001 for all).
Participants in another trial didn’t lose a statistically significant amount of weight, compared with the control arm of the study. However, the mean 5 kg lost by those in the intervention arm was enough to be clinically significant, so Ms. Hopkins and her colleagues included this study in a subanalysis that looked for effective modalities and interventions among the studies with significant results.
The duration of the studies ranged from 6 to 24 months, though five of the trials were less than 1 year long. Women made up the majority of participants in all but one trial.
“There is limited evidence that standalone digital weight-loss interventions produce clinically meaningful outcomes,” wrote Ms. Hopkins and her coauthors. “Absolute magnitude of weight loss was low, and the short intervention lengths call into question the sustainability of these weight losses.”
The systematic review cast a broad net to include digital modalities such as wireless scales, text messaging, email, and web-based interventions, as well as the use of smartphone apps and tracking devices. All interventions used multiple digital modalities.
The most frequently used technologies were the use of a website, used in six (67%) of the trials, followed by text messaging and smartphone apps, each used in five (56%) of the trials. Tracking devices, email, message boards, and gamification of some sort were all used in three (33%) of the trials.
In terms of the specific interventions used in the trials, weight, diet, and activity were all tracked in eight trials (89%). Similarly, all but one trial gave feedback and weight and health education to participants. Behavior change education, as well as calorie goals, were each used in six trials (67%).
Ms. Hopkins and her colleagues looked at which trials incorporated which modalities and interventions, finding that “trials that integrated components unique to digital interventions, such as gamification, podcasts, or interactive features, yielded significantly greater and more clinically meaningful weight losses.”
To be included in the systematic review, trials had to include adult participants with a body mass index of at least 25 kg/m2 and use a standalone digital intervention of at least 6 months’ duration. The primary outcome of interest in the review was the change in participant weight from baseline to the end of the minimum 6-month follow-up period. Randomized, controlled trials and feasibility trials were included, so long as participants were allocated randomly.
Of the 126 trials reviewed at the full text level, the most frequent reason for exclusion was the inclusion of human coaching. Also, 30 of the trials didn’t report weight change as an outcome, the investigators said.
Future directions should include comparing digital interventions that “utilize features unique to digital delivery” with those that more closely resemble in-person weight-loss management interventions, suggested Ms. Hopkins and her collaborators.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Hopkins C et al. Obesity Week 2018, Abstract T-P-LB-3640.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Three of nine studies found statistically significant weight loss with digital interventions.
Major finding: The largest effect was seen in one study showing 7 kg of long-term weight loss (P less than .001).
Study details: A systematic review of nine studies of digital-only interventions for weight loss.
Disclosures: The authors reported no outside sources of funding and no conflicts of interest.
Source: Hopkins C et al. Obesity Week 2018, Abstract T-P-PB-3640.
How I Became a Derm Guru (And How You Can, Too)
Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).

Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
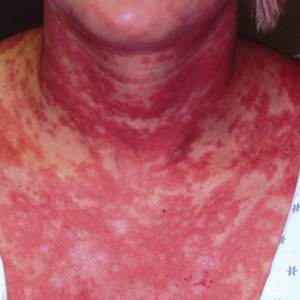
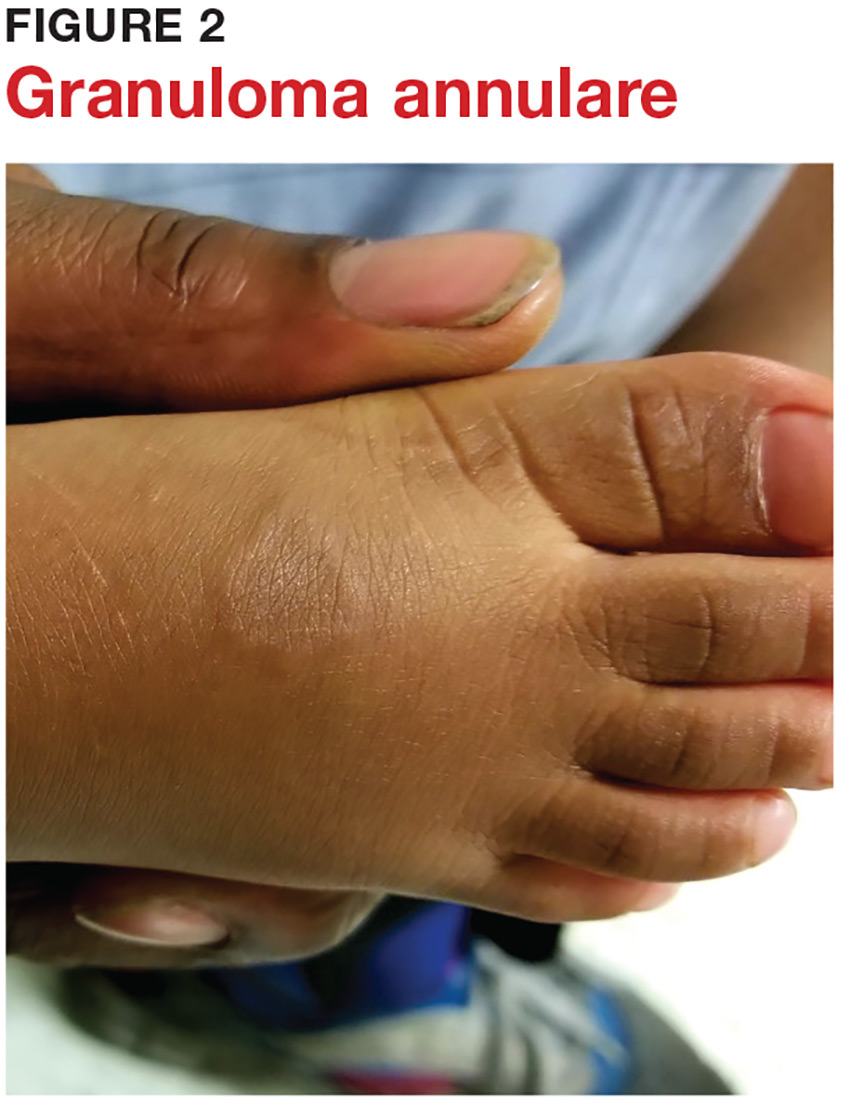
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).
4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
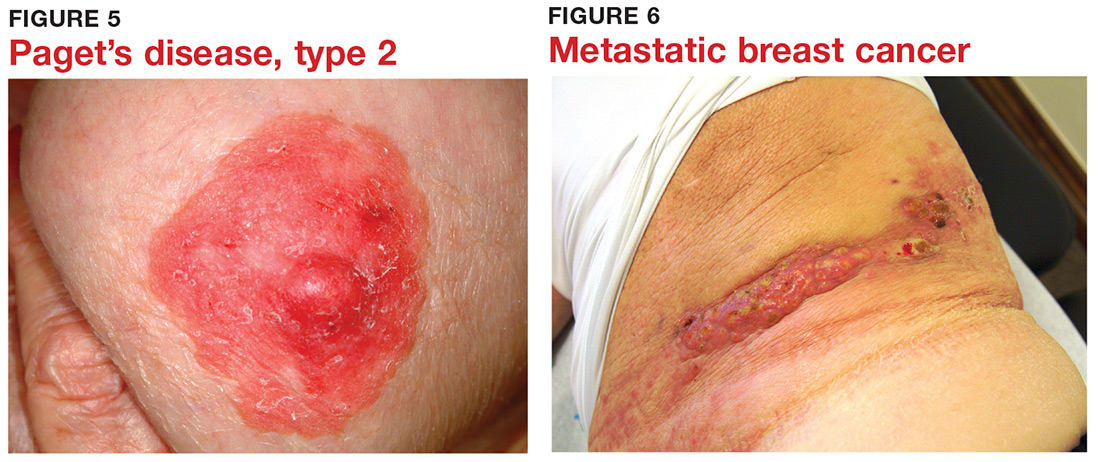
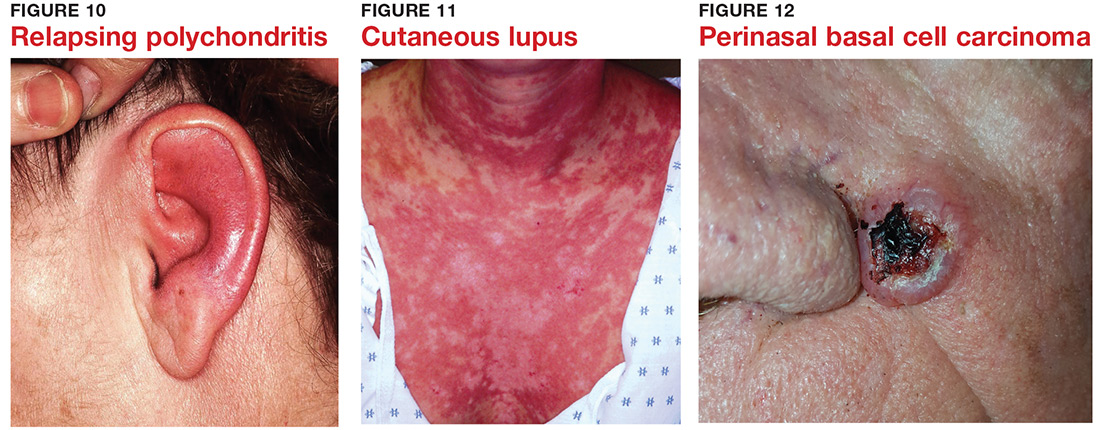
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.
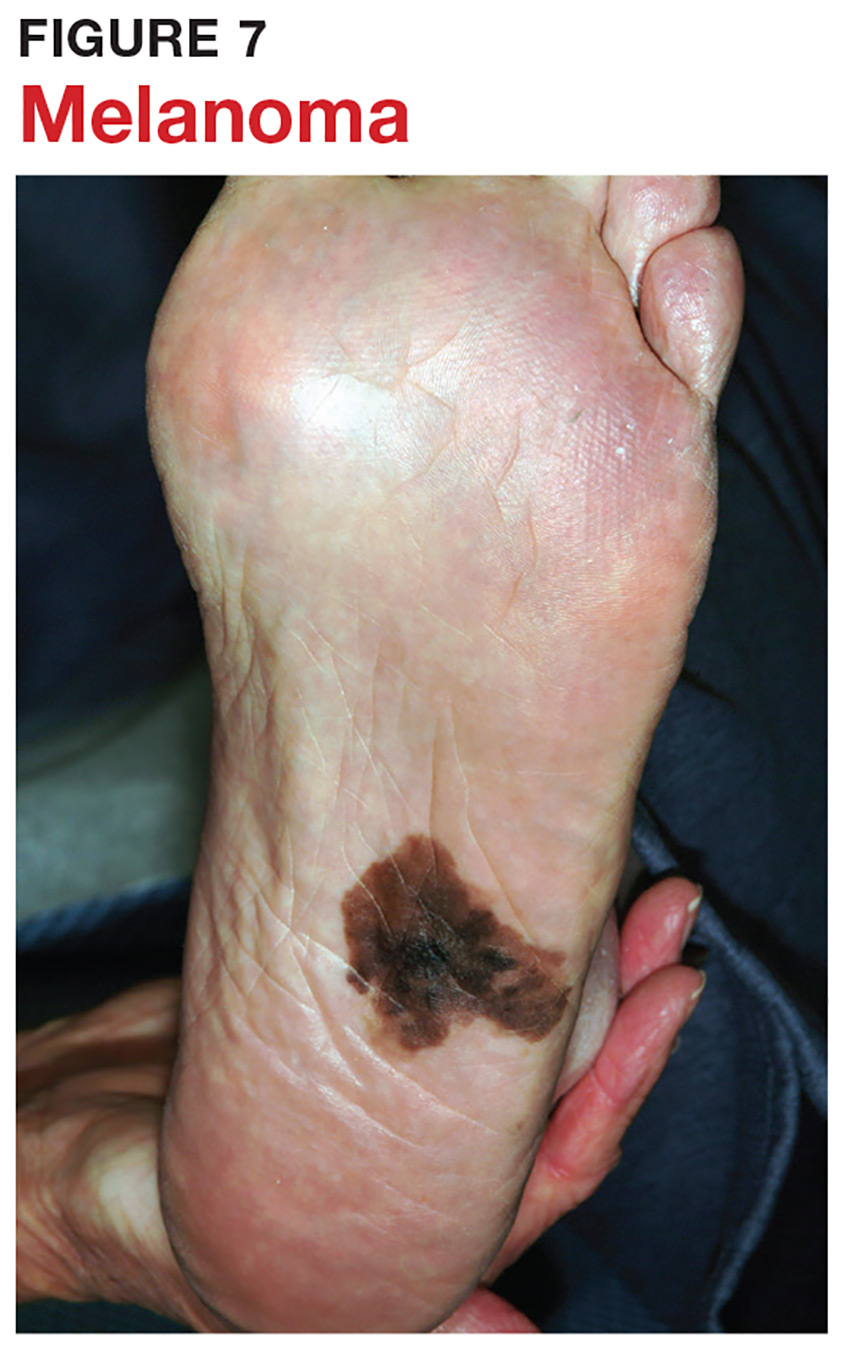
6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).
Continue to: #7...
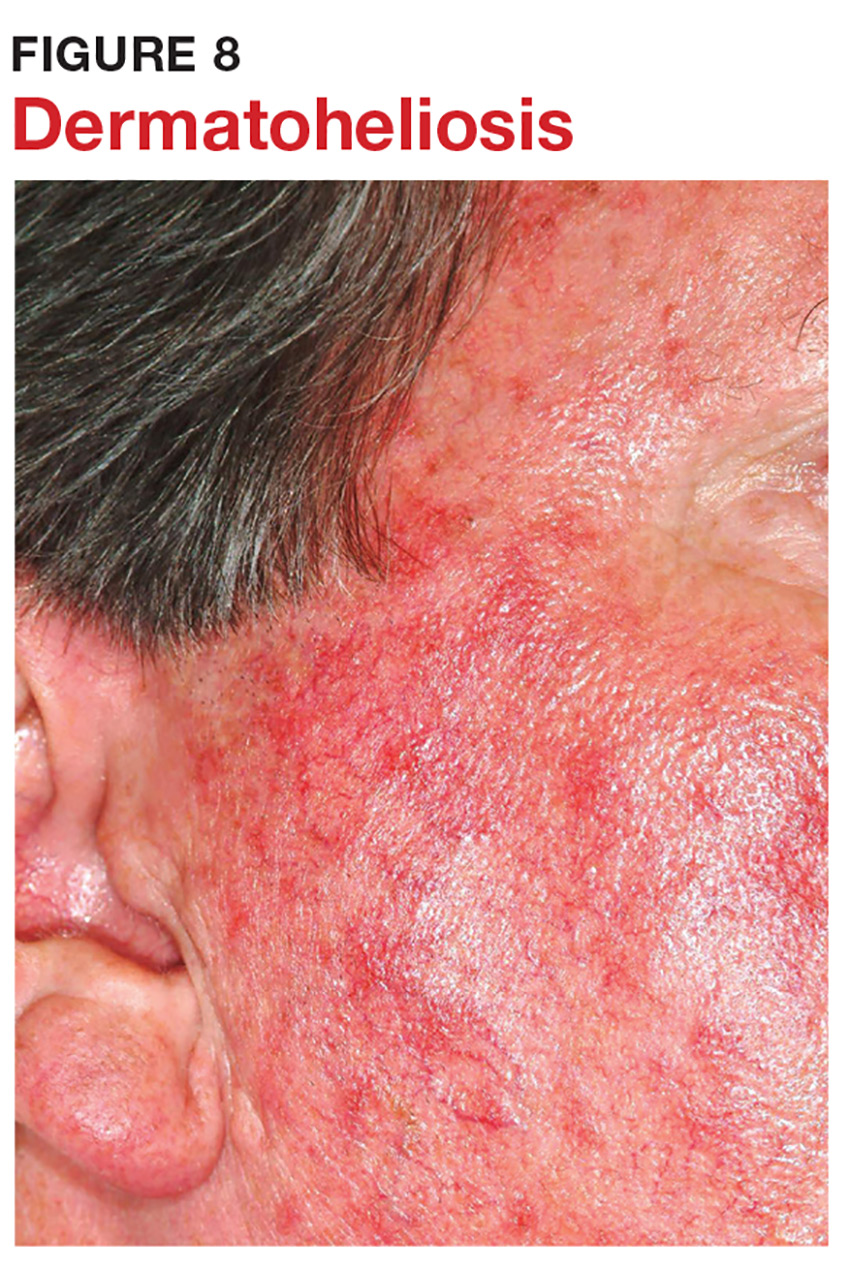
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).
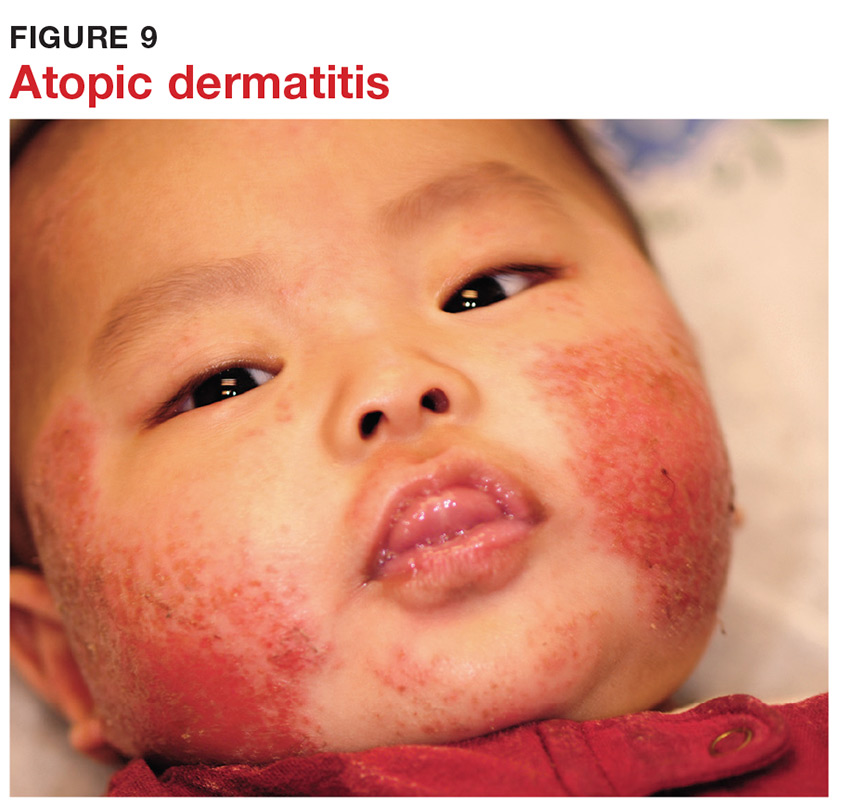
8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.
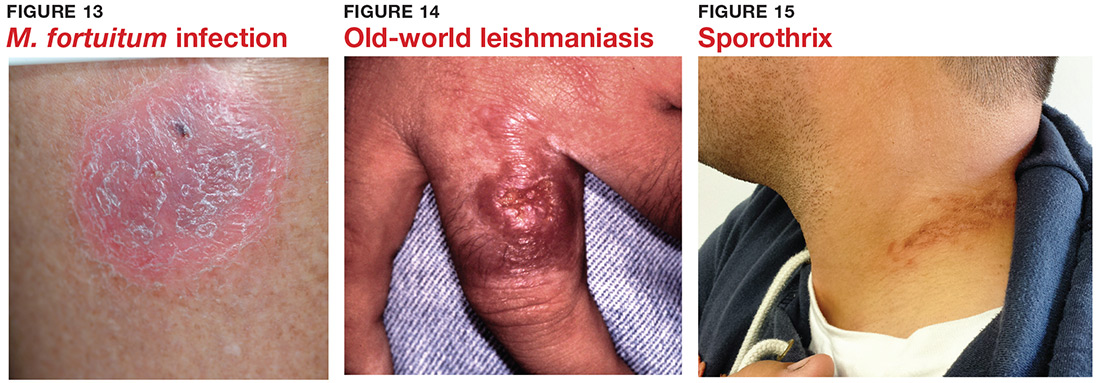
9 “Infections” are not always what they seem
10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).
Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).

Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).

4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.

6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).

Continue to: #7...
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).

8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.

9 “Infections” are not always what they seem

10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).

Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).

Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).

4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.

6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).

Continue to: #7...
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).

8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.

9 “Infections” are not always what they seem

10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).

Guideline-concordant treatment still unlikely in nonchildren’s hospitals for pediatric CAP
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
FROM JAMA PEDIATRICS
Medical marijuana for autism facing good prospects in Colorado
Five years after the legalization of marijuana in Colorado, anticipated legislation in 2019 could see home delivery of cannabis and cannabis-related products, and expanded medical availability.
Governor-elect Jared Polis, who takes office in the new year, probably will take a different approach from outgoing Gov. John Hickenlooper, according to a recent article in the Denver Post. Mr. Hickenlooper vetoed previous legislation intended to increase drug’s accessibility.
“ says Albert Gutierrez, CEO of MedPharm Holdings, a cannabis research and cultivation company.
“You’re going to probably have more variety from these companies, whether they’re offering drinks or chocolate bars. But these companies are going to be the household names that people are going to come to know over the next 30, 50, 100 years,” he says.
Not everyone is on board. “We should all be able to agree that Colorado’s increasingly potent marijuana products are harmful to youth and that we have a collective responsibility to protect Colorado kids,” writes Henny Lasley, the cofounder of Smart Colorado, which was formed in opposition to the legalization of marijuana in the state.
The availability of medical marijuana for people diagnosed with autism spectrum disorders is among the vetoed initiatives that are likely to reemerge in 2019. That bill reportedly was opposed by the Colorado Child and Adolescent Psychiatric Society, the Colorado Psychiatric Society, and by Larry Wolk, MD, who recently stepped down as chief medical officer of the state’s department of public health and environment.
Adjusting to life after fires
The latest wildfires have been vanquished in California. For those affected recently and in the past several years has come the reality that the draw of living on the edge of nature means living surrounded by tinder-dry terrain. It’s a great location – until it ignites.
A year ago, the Thomas Fire devastated Ventura and Santa Barbara counties, burning more than 440 square miles. Few people died, but more than 1,000 buildings were destroyed – and hundreds of people were left homeless. A year later, in the Clearpoint neighborhood of Ventura, residential lots sit empty, their owners having abandoned the effort to rebuild. Others, like Sandra and Ed Fuller, are choosing to begin again. The beauty of the area that pulled them there years ago remains strong.
They have come to terms with losing their home to the fire. “I think it was a sort of a breaking point where there was just a flood of peace that kind of went through. It’s like there is nothing we can do about this. We know what we have to do now. We’ll just get on with it,” Ed Fuller says in an interview with NPR.
Having the Christmas season looming has been a boost to their spirits and planning. “My wife is absolutely obsessed that she’s ready for Christmas. Last Christmas we sort of lost.”
The invisibility of asexuality
It can be hard for some to fathom that sex just isn’t important for some. “They are the friends and family members who don’t express any desire to pursue sexual intimacy, who don’t often or ever seem interested in conventional dating, and who get pushed to the sidelines in any conversation about sexual health,” Kate Sloan writes in a recent article in the Walrus.
“Much like same-sex attraction decades ago, this nonattraction was initially (and is sometimes still) conflated with a sexual-desire disorder, worthy of pathologization and medical treatment with pharmaceuticals or therapy. But scientists have confirmed asexuality isn’t a medical issue; it is a sexual orientation on the same plane as heterosexuality, homosexuality, and bisexuality,” Ms. Sloan writes.
“If someone is gay, as an example, it’s pretty easy to say, ‘Okay, well, I experience the same type of attraction that everyone else does, it’s just pointed at a different gender,’ ” says Brian Langevin, executive director of the nonprofit organization Asexual Outreach. “For asexual people ... they might not even know that sexual attraction exists, and to them, the whole world could seem very confusing.”
Meanwhile, a 2013 study in British Columbia showed that asexual individuals are more likely to be socially isolated, depressed, and anxious.
“True emotional intimacy is created, according to psychology, by honesty, empathy, and listening,” Ms. Sloan writes. “When we oversimplify relationships by insisting, on a sociocultural level, that sex is the ultimate key to and only sign of a profound connection, we deprive ourselves of the more holistic affinities available to us if we look for more.”
Fundamental churches face allegations
Joy Evans Ryder was 15 when she reportedly was raped by Dave Hyles, youth director at her Baptist church in Hammond, Ind. She was not the youth director’s only alleged victim. He never faced charges; in a scenario strikingly similar to that of hundreds of Roman Catholic priests, he escaped local prosecution by being moved on to other assignments.
An investigation by the Fort Worth (Tex.) Star-Telegram has unearthed a decades-old cover-up of more than 400 cases of sexual abuse at independent fundamental Baptist churches across the United States.
Former members of congregations point to the cultlike power of many independent fundamental Baptist churches and the constant pressure to never question pastors or leave the church.
“We didn’t have a compound ... but it may as well have been. Our mind was the compound,” says a former member. Some of the abused believed that if they disobeyed the pastor or left the church, God would kill them or their family.
Some independent fundamental Baptist churches preach separation from the world, nonbelievers, and Christians with other religious views. A natural outcome, according to Josh Elliott, a former member of Vineyard’s Oklahoma City church, is that for any issues, “even legal issues, you go to the pastor first, not the police. ... You don’t report to police because the pastor is the ultimate authority, not the government.”
“I see a culture where pastoral authority is taken to a level that’s beyond what the Scripture teaches,” says Tim Heck, who was a deacon at Faith Baptist Church in Wildomar, Calif., and whose daughter said she had been abused by the youth pastor there. “I think the independent fundamental Baptists have lost their way.”
Adam Lanza’s ‘separateness’ exposed
Written musings and other documents by Adam Lanza – who slaughtered 20 first-graders and six teachers at Sandy Hook Elementary School in Newtown, Conn., on Dec. 14, 2012 – have been reported by the Hartford Courant.
Adam Lanza was challenged by speech and sensory issues as a child but had a keen intellect. That potential was eclipsed in his teenage years by paranoia, disdain for relationships, and contempt for others, the documents show. Family, teachers, and counselors were aware of his isolation. And, with time, his obsessions and mental/physical deterioration grew. But the documents make clear that no one really had a full grasp of the person he was becoming.
“As a teenager, his sensory condition made him exceedingly sensitive to textures, sound, light, and movement. He shunned his classmates, bothered by their choice of clothes and the noises they made. He cultivated a set of ground rules that fed his separateness,” write reporters Josh Kovner and Dave Altimari. The critical addition to this toxic brew was an absence of empathy and social compassion, according to Harold I. Schwartz, MD, a psychiatrist and former member of the Sandy Hook Advisory Commission, which studied the shootings.
“In this mental state, known as solipsism, only the solipsist is real. Everyone else in the world is a cardboard cutout, placed there for your benefit and otherwise devoid of meaning or value. It is the most extreme end of one form of malignant narcissism. If the victims have no value, then there is nothing to constrain you from shooting them,” Dr. Schwartz says.
In a note accompanying the article, the editors write: “Understanding what a mass killer was thinking not only paints a clearer picture of the individual, it helps us identify and understand red flags that could be part of a prevention formula for future mass shootings.”
Five years after the legalization of marijuana in Colorado, anticipated legislation in 2019 could see home delivery of cannabis and cannabis-related products, and expanded medical availability.
Governor-elect Jared Polis, who takes office in the new year, probably will take a different approach from outgoing Gov. John Hickenlooper, according to a recent article in the Denver Post. Mr. Hickenlooper vetoed previous legislation intended to increase drug’s accessibility.
“ says Albert Gutierrez, CEO of MedPharm Holdings, a cannabis research and cultivation company.
“You’re going to probably have more variety from these companies, whether they’re offering drinks or chocolate bars. But these companies are going to be the household names that people are going to come to know over the next 30, 50, 100 years,” he says.
Not everyone is on board. “We should all be able to agree that Colorado’s increasingly potent marijuana products are harmful to youth and that we have a collective responsibility to protect Colorado kids,” writes Henny Lasley, the cofounder of Smart Colorado, which was formed in opposition to the legalization of marijuana in the state.
The availability of medical marijuana for people diagnosed with autism spectrum disorders is among the vetoed initiatives that are likely to reemerge in 2019. That bill reportedly was opposed by the Colorado Child and Adolescent Psychiatric Society, the Colorado Psychiatric Society, and by Larry Wolk, MD, who recently stepped down as chief medical officer of the state’s department of public health and environment.
Adjusting to life after fires
The latest wildfires have been vanquished in California. For those affected recently and in the past several years has come the reality that the draw of living on the edge of nature means living surrounded by tinder-dry terrain. It’s a great location – until it ignites.
A year ago, the Thomas Fire devastated Ventura and Santa Barbara counties, burning more than 440 square miles. Few people died, but more than 1,000 buildings were destroyed – and hundreds of people were left homeless. A year later, in the Clearpoint neighborhood of Ventura, residential lots sit empty, their owners having abandoned the effort to rebuild. Others, like Sandra and Ed Fuller, are choosing to begin again. The beauty of the area that pulled them there years ago remains strong.
They have come to terms with losing their home to the fire. “I think it was a sort of a breaking point where there was just a flood of peace that kind of went through. It’s like there is nothing we can do about this. We know what we have to do now. We’ll just get on with it,” Ed Fuller says in an interview with NPR.
Having the Christmas season looming has been a boost to their spirits and planning. “My wife is absolutely obsessed that she’s ready for Christmas. Last Christmas we sort of lost.”
The invisibility of asexuality
It can be hard for some to fathom that sex just isn’t important for some. “They are the friends and family members who don’t express any desire to pursue sexual intimacy, who don’t often or ever seem interested in conventional dating, and who get pushed to the sidelines in any conversation about sexual health,” Kate Sloan writes in a recent article in the Walrus.
“Much like same-sex attraction decades ago, this nonattraction was initially (and is sometimes still) conflated with a sexual-desire disorder, worthy of pathologization and medical treatment with pharmaceuticals or therapy. But scientists have confirmed asexuality isn’t a medical issue; it is a sexual orientation on the same plane as heterosexuality, homosexuality, and bisexuality,” Ms. Sloan writes.
“If someone is gay, as an example, it’s pretty easy to say, ‘Okay, well, I experience the same type of attraction that everyone else does, it’s just pointed at a different gender,’ ” says Brian Langevin, executive director of the nonprofit organization Asexual Outreach. “For asexual people ... they might not even know that sexual attraction exists, and to them, the whole world could seem very confusing.”
Meanwhile, a 2013 study in British Columbia showed that asexual individuals are more likely to be socially isolated, depressed, and anxious.
“True emotional intimacy is created, according to psychology, by honesty, empathy, and listening,” Ms. Sloan writes. “When we oversimplify relationships by insisting, on a sociocultural level, that sex is the ultimate key to and only sign of a profound connection, we deprive ourselves of the more holistic affinities available to us if we look for more.”
Fundamental churches face allegations
Joy Evans Ryder was 15 when she reportedly was raped by Dave Hyles, youth director at her Baptist church in Hammond, Ind. She was not the youth director’s only alleged victim. He never faced charges; in a scenario strikingly similar to that of hundreds of Roman Catholic priests, he escaped local prosecution by being moved on to other assignments.
An investigation by the Fort Worth (Tex.) Star-Telegram has unearthed a decades-old cover-up of more than 400 cases of sexual abuse at independent fundamental Baptist churches across the United States.
Former members of congregations point to the cultlike power of many independent fundamental Baptist churches and the constant pressure to never question pastors or leave the church.
“We didn’t have a compound ... but it may as well have been. Our mind was the compound,” says a former member. Some of the abused believed that if they disobeyed the pastor or left the church, God would kill them or their family.
Some independent fundamental Baptist churches preach separation from the world, nonbelievers, and Christians with other religious views. A natural outcome, according to Josh Elliott, a former member of Vineyard’s Oklahoma City church, is that for any issues, “even legal issues, you go to the pastor first, not the police. ... You don’t report to police because the pastor is the ultimate authority, not the government.”
“I see a culture where pastoral authority is taken to a level that’s beyond what the Scripture teaches,” says Tim Heck, who was a deacon at Faith Baptist Church in Wildomar, Calif., and whose daughter said she had been abused by the youth pastor there. “I think the independent fundamental Baptists have lost their way.”
Adam Lanza’s ‘separateness’ exposed
Written musings and other documents by Adam Lanza – who slaughtered 20 first-graders and six teachers at Sandy Hook Elementary School in Newtown, Conn., on Dec. 14, 2012 – have been reported by the Hartford Courant.
Adam Lanza was challenged by speech and sensory issues as a child but had a keen intellect. That potential was eclipsed in his teenage years by paranoia, disdain for relationships, and contempt for others, the documents show. Family, teachers, and counselors were aware of his isolation. And, with time, his obsessions and mental/physical deterioration grew. But the documents make clear that no one really had a full grasp of the person he was becoming.
“As a teenager, his sensory condition made him exceedingly sensitive to textures, sound, light, and movement. He shunned his classmates, bothered by their choice of clothes and the noises they made. He cultivated a set of ground rules that fed his separateness,” write reporters Josh Kovner and Dave Altimari. The critical addition to this toxic brew was an absence of empathy and social compassion, according to Harold I. Schwartz, MD, a psychiatrist and former member of the Sandy Hook Advisory Commission, which studied the shootings.
“In this mental state, known as solipsism, only the solipsist is real. Everyone else in the world is a cardboard cutout, placed there for your benefit and otherwise devoid of meaning or value. It is the most extreme end of one form of malignant narcissism. If the victims have no value, then there is nothing to constrain you from shooting them,” Dr. Schwartz says.
In a note accompanying the article, the editors write: “Understanding what a mass killer was thinking not only paints a clearer picture of the individual, it helps us identify and understand red flags that could be part of a prevention formula for future mass shootings.”
Five years after the legalization of marijuana in Colorado, anticipated legislation in 2019 could see home delivery of cannabis and cannabis-related products, and expanded medical availability.
Governor-elect Jared Polis, who takes office in the new year, probably will take a different approach from outgoing Gov. John Hickenlooper, according to a recent article in the Denver Post. Mr. Hickenlooper vetoed previous legislation intended to increase drug’s accessibility.
“ says Albert Gutierrez, CEO of MedPharm Holdings, a cannabis research and cultivation company.
“You’re going to probably have more variety from these companies, whether they’re offering drinks or chocolate bars. But these companies are going to be the household names that people are going to come to know over the next 30, 50, 100 years,” he says.
Not everyone is on board. “We should all be able to agree that Colorado’s increasingly potent marijuana products are harmful to youth and that we have a collective responsibility to protect Colorado kids,” writes Henny Lasley, the cofounder of Smart Colorado, which was formed in opposition to the legalization of marijuana in the state.
The availability of medical marijuana for people diagnosed with autism spectrum disorders is among the vetoed initiatives that are likely to reemerge in 2019. That bill reportedly was opposed by the Colorado Child and Adolescent Psychiatric Society, the Colorado Psychiatric Society, and by Larry Wolk, MD, who recently stepped down as chief medical officer of the state’s department of public health and environment.
Adjusting to life after fires
The latest wildfires have been vanquished in California. For those affected recently and in the past several years has come the reality that the draw of living on the edge of nature means living surrounded by tinder-dry terrain. It’s a great location – until it ignites.
A year ago, the Thomas Fire devastated Ventura and Santa Barbara counties, burning more than 440 square miles. Few people died, but more than 1,000 buildings were destroyed – and hundreds of people were left homeless. A year later, in the Clearpoint neighborhood of Ventura, residential lots sit empty, their owners having abandoned the effort to rebuild. Others, like Sandra and Ed Fuller, are choosing to begin again. The beauty of the area that pulled them there years ago remains strong.
They have come to terms with losing their home to the fire. “I think it was a sort of a breaking point where there was just a flood of peace that kind of went through. It’s like there is nothing we can do about this. We know what we have to do now. We’ll just get on with it,” Ed Fuller says in an interview with NPR.
Having the Christmas season looming has been a boost to their spirits and planning. “My wife is absolutely obsessed that she’s ready for Christmas. Last Christmas we sort of lost.”
The invisibility of asexuality
It can be hard for some to fathom that sex just isn’t important for some. “They are the friends and family members who don’t express any desire to pursue sexual intimacy, who don’t often or ever seem interested in conventional dating, and who get pushed to the sidelines in any conversation about sexual health,” Kate Sloan writes in a recent article in the Walrus.
“Much like same-sex attraction decades ago, this nonattraction was initially (and is sometimes still) conflated with a sexual-desire disorder, worthy of pathologization and medical treatment with pharmaceuticals or therapy. But scientists have confirmed asexuality isn’t a medical issue; it is a sexual orientation on the same plane as heterosexuality, homosexuality, and bisexuality,” Ms. Sloan writes.
“If someone is gay, as an example, it’s pretty easy to say, ‘Okay, well, I experience the same type of attraction that everyone else does, it’s just pointed at a different gender,’ ” says Brian Langevin, executive director of the nonprofit organization Asexual Outreach. “For asexual people ... they might not even know that sexual attraction exists, and to them, the whole world could seem very confusing.”
Meanwhile, a 2013 study in British Columbia showed that asexual individuals are more likely to be socially isolated, depressed, and anxious.
“True emotional intimacy is created, according to psychology, by honesty, empathy, and listening,” Ms. Sloan writes. “When we oversimplify relationships by insisting, on a sociocultural level, that sex is the ultimate key to and only sign of a profound connection, we deprive ourselves of the more holistic affinities available to us if we look for more.”
Fundamental churches face allegations
Joy Evans Ryder was 15 when she reportedly was raped by Dave Hyles, youth director at her Baptist church in Hammond, Ind. She was not the youth director’s only alleged victim. He never faced charges; in a scenario strikingly similar to that of hundreds of Roman Catholic priests, he escaped local prosecution by being moved on to other assignments.
An investigation by the Fort Worth (Tex.) Star-Telegram has unearthed a decades-old cover-up of more than 400 cases of sexual abuse at independent fundamental Baptist churches across the United States.
Former members of congregations point to the cultlike power of many independent fundamental Baptist churches and the constant pressure to never question pastors or leave the church.
“We didn’t have a compound ... but it may as well have been. Our mind was the compound,” says a former member. Some of the abused believed that if they disobeyed the pastor or left the church, God would kill them or their family.
Some independent fundamental Baptist churches preach separation from the world, nonbelievers, and Christians with other religious views. A natural outcome, according to Josh Elliott, a former member of Vineyard’s Oklahoma City church, is that for any issues, “even legal issues, you go to the pastor first, not the police. ... You don’t report to police because the pastor is the ultimate authority, not the government.”
“I see a culture where pastoral authority is taken to a level that’s beyond what the Scripture teaches,” says Tim Heck, who was a deacon at Faith Baptist Church in Wildomar, Calif., and whose daughter said she had been abused by the youth pastor there. “I think the independent fundamental Baptists have lost their way.”
Adam Lanza’s ‘separateness’ exposed
Written musings and other documents by Adam Lanza – who slaughtered 20 first-graders and six teachers at Sandy Hook Elementary School in Newtown, Conn., on Dec. 14, 2012 – have been reported by the Hartford Courant.
Adam Lanza was challenged by speech and sensory issues as a child but had a keen intellect. That potential was eclipsed in his teenage years by paranoia, disdain for relationships, and contempt for others, the documents show. Family, teachers, and counselors were aware of his isolation. And, with time, his obsessions and mental/physical deterioration grew. But the documents make clear that no one really had a full grasp of the person he was becoming.
“As a teenager, his sensory condition made him exceedingly sensitive to textures, sound, light, and movement. He shunned his classmates, bothered by their choice of clothes and the noises they made. He cultivated a set of ground rules that fed his separateness,” write reporters Josh Kovner and Dave Altimari. The critical addition to this toxic brew was an absence of empathy and social compassion, according to Harold I. Schwartz, MD, a psychiatrist and former member of the Sandy Hook Advisory Commission, which studied the shootings.
“In this mental state, known as solipsism, only the solipsist is real. Everyone else in the world is a cardboard cutout, placed there for your benefit and otherwise devoid of meaning or value. It is the most extreme end of one form of malignant narcissism. If the victims have no value, then there is nothing to constrain you from shooting them,” Dr. Schwartz says.
In a note accompanying the article, the editors write: “Understanding what a mass killer was thinking not only paints a clearer picture of the individual, it helps us identify and understand red flags that could be part of a prevention formula for future mass shootings.”
Fragility Fractures: Diagnosis and Treatment
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
TAKE-HOME POINTS
- 3 million people sustain fragility fractures annually, and nearly 30% die within a year of the fracture.
- The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.
- The World Health Organization has developed a set of T-core criteria to diagnose osteoporosis in postmenopausal women: a score >–1 is normal; <–1 but >–2.5 signifies osteopenia; <–2.5 denotes osteoporosis; and <–2.5 with fragility fracture indicates severe osteoporosis.
- The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children. The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender. Z scores <–2.0 indicate low BMD for chronological age. A Z score > –2.0 is considered within the expected range for age.
- After an initial fragility fracture, the risk for additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture.26
The Characteristics of Surgeons Performing Total Shoulder Arthroplasty: Volume Consistency, Training, and Specialization
ABSTRACT
Total shoulder arthroplasty (TSA) has proved a cost-effective, reproducible procedure for multiple shoulder pathologies. As utilization of TSA continues to grow, it is important to investigate procedure diversity, training, and other characteristics of surgeons performing TSA. To identify surgeons performing TSA in the Medicare population, the Medicare Provider Utilization and Payment Databases from 2012 through 2014 were used. This dataset includes any provider who bills Medicare >10 times with a single billing code. A web-based search was performed for each physician performing >10 TSA in all years of the study to identify their surgical training characteristics. Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year (71,973 TSA). Only 44.3% (609/1374) of surgeons met this threshold for all 3 years (55,538 TSA). Of these 609 surgeons, 191 (31.3%) were shoulder and elbow fellowship trained (21,444 TSA). Shoulder and elbow fellowship-trained surgeons were at earlier points in their careers and practiced in large referral-based centers with other surgeons performing TSA. In addition to TSA, surgeons performed other non-arthroplasty shoulder procedures (80.2% of surgeons), total knee arthroplasty (46.3%), repairs of traumatic injuries (29.8%), total hip arthroplasty (27.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%) during the study period. With less than one-third of TSA performed by shoulder and elbow fellowship-trained surgeons with consistent moderate-volume practices, the impact of consistent high-volume practices and targeted fellowship training on quality must be determined.
Continue to: With the adoption of reverse shoulder arthroplasty...
With the adoption of reverse shoulder arthroplasty, utilization of total shoulder arthroplasty (TSA) has increased substantially over the last decade.1–3 Such increases are likely secondary to an aging population, increased comfort with the procedure, and the adoption of broadened indications for reverse shoulder arthroplasty, especially in the setting of proximal humerus fractures in the elderly.4–7 Between 2012 and 2014 alone, the number of surgeons performing >10 TSA in Medicare patients annually increased by 28.6% (824 to 1060 surgeons) providing a 26.6% (20,824-26,365 procedures) increase in national volume in the Medicare population.2 With this boom in utilization, scrutiny of this now routine procedure and those performing it is necessary.
Prior reviews have demonstrated a strong link between surgeon and hospital TSA volume and outcomes of the procedure.8–10 Somerson and colleagues11 investigated fellowship training among surgeons performing TSA in 2012 and found that only 28% had completed a shoulder and elbow fellowship. In addition to prior analyses2, 12, Somerson and colleagues confirmed a persistent geographic variation in utilization of TSA.11 In conjunction with the evolution of shoulder arthroplasty, dedicated shoulder and elbow fellowship training has expanded. With a shift toward specialization in care, nearly 90% of orthopedic surgery residents plan to pursue shoulder and elbow fellowships, comprising 4.6% of (42/897) of available positions.13
What remains unknown is the specialization of surgeons performing TSA, the regularity of their arthroplasty volume, and trends in TSA specialization over time. Therefore, this study aims to (a) identify surgeons performing shoulder arthroplasty and cohort changes over time, (b) determine the case profile of surgeons consistently performing shoulder arthroplasty, and (c) establish the characteristics of shoulder arthroplasty surgeons with a specific focus on fellowship training.
METHODS
Prior to collecting surgeon-specific data, we identified surgeons performing TSA through the Centers for Medicare and Medicaid Services’ public release of “Medicare Provider Utilization and Payment Data: Physician and Other Supplier.”14 Datasets from 2012, 2013, and 2014 were used to identify all surgeons performing >10 TSAs (Current Procedural Terminology [CPT] Code 23472) during at least 1 of those years. This dataset provides the name, identification number, address, and all billing (by volume) for each unique CPT code submitted ≥10 times in a calendar year.
Once the cohort of surgeons had been generated, the number of surgeons consistently performing TSA year-over-year was determined. This allowed for an analysis of the consistency with which surgeons are performing moderate- to high-volume TSA. To form a case profile of surgeons performing TSA and observe how this shifted over time, a count and a description of each CPT code submitted by each surgeon was identified. To maintain patient privacy, only those claims made >10 times are reported for a provider (both physicians and physician-extenders are included in this dataset). First, all CPT codes were reviewed and tagged as surgical or non-surgical events. Then, every procedural CPT code identified was reviewed and categorized based upon anatomic location and procedure (eg, total knee arthroplasty [TKA]). It is important to note that all claims in this dataset are limited to those patients participating in Medicare’s fee-for-service program.
Specialization was defined as the number of categorized procedures as a percentage of all procedures performed on Medicare patients. The trends for national, regional, and individual specialization of TSA, arthroplasty (major joint), and shoulder procedures were determined.
Continue to: To investigate the characteristics of surgeons...
To investigate the characteristics of surgeons consistently performing TSA, all surgeons performing a minimum of 11 TSA in Medicare fee-for-service beneficiaries in all years between 2012 and 2014 were identified. Such surgeons were defined as consistent TSA surgeons. Investigation of this cohort included a web-based search of their self-reported post-graduate fellowship training and year of graduation from medical school. Using these data, the percentage of surgeons performing TSA who underwent formal shoulder and elbow training was determined. In addition, the impact of fellowship training on shoulder specialization and practice location was determined. Surgeons who had completed multiple fellowships were categorized under all of them. As such, there may be some duplication of surgeons in the comparisons. In addition, other potential characteristics of shoulder and elbow fellowship-trained surgeons were investigated: number of regional shoulder surgeons, urban area, total number of Medicare beneficiaries, average reimbursement for TSA, ethnicity of Medicare beneficiaries, and percentage of Medicare patients eligible for Medicaid. Geographic regions were defined by the Dartmouth Atlas and assigned by hospital referral region.15 These defined regions were used to assess the beneficiaries (number and characteristics) that individual surgeons were likely serving. The United States Census Bureau characterization of zip code-based regions as urban areas (population >50,000), urban clusters (2500 to 50,000), and rural region (<2500) was used to categorize practice location.16
Descriptive statistics were used initially to report these findings. To analyze predictors of utilization and specialization, comparative statistics were undertaken. For comparison of binomial variables between groups, a χ2analysis was utilized. For continuous variables, data normality was assessed. A skewness and kurtosis <2 and 12, respectively, was considered to represent parametric data. For parametric data, the mean was reported; conversely, the median is reported for non-parametric data. To assess continuous variables between groups, a t test or a Wilcoxon rank-sum test was used for parametric and non-parametric distributions, respectively.
RESULTS
Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year, for a combined total of 71,973 TSAs (Table 1). In 2012, only 834 surgeons (13 females [1.6%]) performed a minimum of 10 TSA in Medicare patients (21,137 arthroplasties; 25.3 per surgeon). This increased to 1078 surgeons (33 females [3.1%]; P = .04) performing 26,865 TSA (24.92 per surgeon) in 2014. Utilization of non-physician assistants in TSA also increased significantly over this period, with 307 assisting in 6885 TSAs (22.4 per provider) in 2012 and 465 assisting 10,433 TSA (22.4 per provider) in 2014. When all procedures were considered, including those performed at outpatient visits, 1319 physicians (95.9% of cohort) were active in 2012—providing either surgical procedures or outpatient consults to the Medicare population. Yet, only 63.2% performed >10 TSA in Medicare patients. The number of active surgeons performing TSA increased to 79.6% (1078/1353) in 2014 (P < .001).
Table 1. Trends in the Number of Providers Performing TSA between 2012 and 2014*
2012 | 2013 | 2014 | Total | |
| Providers (no.) | 1141 | 1373 | 1543 | 1994 |
Physicians | 834 | 984 | 1,078 | 1,374 |
Non-physicians | 307 | 389 | 465 | 620 |
| TSA (no.) | 28,022 | 32,641 | 37,298 | 97,961 |
| Physicians | 21,137 | 23,971 | 26,865 | 71,973 |
| Non-physicians | 6,885 | 8,670 | 10,433 | 25,988 |
| TSA per provider | 24.5 | 23.8 | 24.2 | 49.2 |
| Physicians | 25.3 | 24.3 | 24.9 | 52.4 |
| Non-physicians | 22.4 | 22.3 | 22.4 | 41.2 |
| Procedures (no.) | 210,845 | 224,123 | 227,305 | 662,273 |
| Physicians | 152,862 | 160,114 | 160,851 | 473,827 |
| Non-physicians | 57,983 | 64,009 | 66,454 | 188,446 |
| Procedure per provider | 114.4 | 116.8 | 116.5 | 332.13 |
| Physicians | 115.9 | 118.9 | 118.9 | 344.9 |
| Non-physicians | 110.7 | 111.9 | 111.1 | 303.9 |
| Active providers (no.) | 1843 | 1919 | 1951 | 1994 |
| Physicians | 1319 | 1347 | 1353 | 1374 |
| Non-physicians | 524 | 572 | 598 | 620 |
* Included are the number of arthroplasties and total procedures over time among this cohort. The number of active providers, determined by billing Medicare for office or surgical procedures within that year, is reported.
Abbreviation: TSA, total shoulder arthroplasty.
In addition to TSA, this cohort of surgeons submitted 240 unique CPT codes with case volumes >10 annually over the 3-year study period. Of these, 80.2% (1102/1374) of surgeons performed non-arthroplasty shoulder procedures on Medicare patients, for a combined total of 202,335 procedures over the 3-year study period (Table 2). A significant proportion of these procedures were arthroscopic debridement (60,014 procedures performed by 908 surgeons) and arthroscopic rotator cuff repair (47,089 procedures performed by 809 surgeons). Just under half (49.1%; 674/1374) of surgeons performing TSA also performed TKA during this period (77,873 arthroplasties). Fewer surgeons (27.8%; 382/1374) performed total hip arthroplasty during this period (27,322 arthroplasties). Other procedure types that this group of surgeons routinely performed on Medicare patients were repairs of traumatic injuries (29.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%). By case load, non-arthroplasty shoulder procedures consisted of 43% of Medicare volume over the study period (Figure 1). Between 2012 and 2014, the average proportion of Medicare cases that were shoulder arthroplasties increased from 13.8% (21,137/152,862) to 16.7% (26,865/160,851; P = .001). Shoulder arthroplasty constituted 100% of the Medicare surgical case volume for 67 (4.9%; 67/1374) of the surgeons.
Table 2. Case Volumes over Time with All Procedures Categorized by Anatomic Region and Arthroplasty vs Non-arthroplasty*
2012 | 2013 | 2014 | Total | |
| Shoulder arthroplasty | 21,351 (n=837) | 24,128 (n=984) | 26,902 (n=1,078) | 72,381 (n=1,374) |
23472: total shoulder arthroplasty | 21,137 (n=834) | 23,971 (n=984) | 26,865 (n=1,078) | 71,973 (n=1,374) |
23470: Hemiarthroplasty | 214 (n=14) | 84 (n=6) | 37 (n=2) | 335 (n=15) |
| Shoulder (non-arthroplasty) | 65,947 (n=887) | 68,746 (n=942) | 67,642 (n=932) | 202,335 (n=1,102) |
29826: arthroscopic acromioplasty | 19,152 (n=724) | 20,367 (n=760) | 20,495 (n=754) | 60,014 (n=908) |
29827: arthroscopic rotator cuff repair | 14,700 (n=613) | 15,963 (n=664) | 16,426 (n=658) | 47,089 (n=809) |
23412: open rotator cuff repair | 1957 (n=88) | 2046 (n=90) | 2112 (n=2,112) | 6115 (n=143) |
23430: Open biceps tenodesis | 4063 (n=178) | 3998 (n=167) | 4601 (n=185) | 12,662 (n=288) |
29823: arthroscopic major debridement | 7428 (n=301) | 7745 (n=309) | 5202 (n=210) | 20,375 (n=417) |
Total knee arthroplasty | 25,640 (n=565) | 26,558 (n=587) | 25,675 (n=580) | 77,873 (n=637) |
Total hip arthroplasty | 8729 (n=316) | 9226 (n=318) | 9367 (n=330) | 27,322 (n=382) |
Trauma | 6454 (n=260) | 6396 (n=254) | 6364 (n=261) | 19,214 (n=410) |
27245: surgical treatment of broken thigh bone (intertrochanteric) | 2602 (n=162) | 2654 (n=164) | 2537 (n=160) | 7793 (n=274) |
27236: surgical treatment of broken thigh bone (hemiarthroplasty) | 1961 (n=123) | 1703 (n=111) | 1702 (n=112) | 5366 (n=205) |
Hand | 6343 (n=139) | 7321 (n=154) | 8006 (n=172) | 21,670 (n=211) |
Elbow | 6113 (n=198) | 6139 (n=204) | 6131 (n=198) | 18,383 (n=270) |
Knee (non-arthroplasty) | 8514 (n=275) | 8140 (n=275) | 7689 (n=230) | 24,343 (n=374) |
Outpatient visits | 879,740 (n=1,282) | 907,124 (n=1,320) | 921,291 (n=1,327) | 2,708,155 (n=1,342) |
New patient | 195,898 (n=1,276) | 192,937 (n=1,305) | 191,427 (n=1,315) | 571,203 (n=1,332) |
Existing patient | 740,307 (n=1,279) | 714,187 (n=1,316) | 729,864 (n=1,324) | 2,29,976 (n=1,338) |
* Procedures of interest with high case volumes are reported individually.
Only 44.3% (609/1374) of surgeons performed TSA in a minimum of 11 Medicare patients in all 3 years of the study period (consistent providers of TSA), providing a total of 55,538 TSA (77.2%; 55,538/71,973). When fellowship training was evaluated, 191 (31.4%; 191/609) of these surgeons were shoulder and elbow fellowship trained (21,444 TSA; 38.6%; Table 3). More than one-third (36.6%; 223/609) had completed a sports surgery fellowship (18,899 TSA; 34.0%). Surgeons trained in hand surgery (12.5%; 76/609) and adult reconstruction (5.3%; 32/610) also made contributions to meeting the TSA demand with 6971 (12.6%) and 2485 (4.5%) TSA, respectively. One-fifth of this cohort (18.1%; 110/609) had unknown fellowship training: they either reported no fellowship (13.6%; 83/609) or did not specify the type of training (4.4%; 27/609). Shoulder and elbow fellowship-trained surgeons performed more TSA (median: 89.0 TSA per surgeon between 2012 and 2014) than surgeons without shoulder and elbow fellowship training (median: 67.0 TSA per surgeon; P < 0.001). More than one-third (37%) of shoulder and elbow fellowship-trained surgeons’ surgical case volume was comprised of TSA, with an additional 35% from non-arthroplasty shoulder procedures (Figure 2). In order for the current supply of shoulder and elbow fellowship-trained surgeons to meet the Medicare TSA demand, each fellowship graduate would have to perform 140.6 TSA in Medicare patients annually. Shoulder and elbow fellowship-trained surgeons were more likely to practice in referral regions with an increased Medicare population (P < .001), an increased number of surgeons performing TSA (P < .001), and a higher proportion of Medicaid-eligible patients (P = .01; Table 4). Shoulder and elbow fellowship-trained surgeons (18.7 years post-medical school graduation) were also earlier in their careers than other consistent TSA surgeons (23.1 years post-graduation; P < .001).
Table 3. A Representation of Fellowships Among TSA Surgeons and Their Shoulder Arthroplasty Case Load*
| Fellowship | Surgeons (%) | 2012-2014 (no, %) | SA Medicare Cases (%) | Average Surgeon Annual SA Volume |
| Shoulder and elbow | 191 (31.4%) | 21,444 (38.6%) | 29.3% | 37.4 |
| Hand surgery | 76 (12.5%) | 6971 (12.6%) | 17.1% | 30.6 |
| Sports | 223 (36.6%) | 18,899 (34.0%) | 19.4% | 28.3 |
| Trauma | 14 (2.3%) | 1270 (2.3%) | 10.9% | 30.2 |
| Adult reconstruction | 32 (5.3%) | 2485 (4.5%) | 10.2% | 25.9 |
| Unknown/none | 110 (18.1%) | 8489 (15.3%) | 16.3% | 25.7 |
|
|
|
| |
| 1 Fellowship | 459 (75.3%) | 42,065 (75.7%) | 20.7% | 30.5 |
| ≥2 Fellowships | 67 (11.0%) | 7122 (12.8%) | 22.5% | 35.4 |
* Not all fellowships (eg, oncology) included due to small numbers. Also, many surgeons performed multiple fellowships.
Abbreviations: SA, shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 4. Breakdown of Geographic Characteristics of Orthopedic Surgeons Consistently
Performing TSA between 2012 and 2014 Stratified by Fellowship Training
Abbreviations: HRR, hospital referral region; TSA, total shoulder arthroplasty.
| Fellowship | Percentage in Non-Urban Area | Average No. of Other TSA Surgeons within HRR | Median Proportion of Patients Eligible for Medicaid within HRR | Average Proportion of Caucasian Patients within HRR | Average Population in Practicing Zip Code | Average No. of Medicare Beneficiaries in HRR | Average No. Years from Medical School Graduation |
| Shoulder and elbow | 7.3% | 10.5 | 12.6 | 84.7 | 26,620.1 | 224,868.3 | 18.7 |
| Other fellowships | 10.3% | 8.6 | 11.1 | 85.6 | 27,619.7 | 177,939.7 | 23.1 |
P value | 0.29 | <0.001 | 0.01 | 0.30 | 0.41 | <0.001 | <0.001 |
Hand surgery | 7.9% | 8.1 | 12.8 | 83.7 | 24,022.8 | 179,370.8 | 23.9 |
Sports | 11.2% | 8.9 | 11.9 | 85.6 | 28,588.9 | 185,902.4 | 21.2 |
Trauma | 21.4% | 7.7 | 13.8 | 85.5 | 20,065.9 | 170,807.6 | 25.6 |
Adult reconstruction | 6.3% | 8.7 | 12.8 | 86.9 | 26,601.5 | 173,280.1 | 22.4 |
None/unknown | 10.9% | 8.5 | 12.0 | 86.4 | 28,173.6 | 166,522.5 | 27.0 |
Continue to: DISCUSSION...
DISCUSSION
Utilization of TSA has continued to rise; however, access to this cost-effective procedure was recently demonstrated to be limited.11 In a separate analysis, we established the continued rise in use of TSA in the Medicare population, coupled with an increase in the number of surgeons routinely performing TSA.2 Multiple analyses have demonstrated the importance of high-volume surgeons and hospitals familiar with the intricacies of shoulder arthroplasty concepts in minimizing complications, improving the quality and decreasing the cost of TSA.6,10,17 Specifically, Singh and colleagues18 demonstrated from a multi-center registry that surgeons and hospitals with greater shoulder arthroplasty volumes had decreased intra-operative blood loss, operative time, and hospital length of stay. As the demand for TSA, both anatomic and reverse, continues to rise, it is imperative that the healthcare delivery system is optimized to provide the best possible care. Before we can determine whether specialized training in shoulder arthroplasty influences surgical outcomes, characteristics and training of surgeons performing TSA should be described.
The number of surgeons performing >10 TSA in the Medicare population rose significantly between 2012 and 2014 (29.3%). However, the number of TSAs per surgeon over this time period remained consistent (approximately 25 per surgeon). Furthermore, the increase in the number of surgeons performing a reportable volume of TSA by 2014 was from the addition of already active surgeons (ie, the growth in TSA was not from the addition of newly trained arthroplasty surgeons but originated from the existing orthopedic surgeon workforce). In a recently published analysis, Somerson and colleagues, 11 using this same dataset, demonstrated persistent limitations in access to high-volume TSA surgeons. In a more recent analysis, we showed that while still lacking for some patients, access to a high-volume TSA surgeon has improved significantly over the past 3 years, with 96.9% of the United States population residing within 200 kilometers of a high-volume TSA surgeon (>20 Medicare cases).2 This analysis validates those findings, with the caveat that the average annual volume per surgeon is not increasing. What remains unknown, due to limitations of this dataset, is how many surgeons are not identified because they are performing ≤10 TSA each year or are performing TSA in non-Medicare patients.
With the specialization of healthcare delivery, specifically in orthopedics, it is imperative that mechanisms for providing specialty-focused care be established. However, the proportion of their practice that surgeons dedicate to TSA was unknown. This study demonstrates that this proportion is increasing. Including non-arthroplasty procedures, more than half (58%) of the procedures performed by this surgeon cohort were shoulder-specific. Furthermore, this analysis demonstrates that surgeons performing TSA have significant case diversity, including nearly half of the cohort performing TKA. Repeated evidence has demonstrated the effect of case volume on improved outcomes following orthopedic procedures.8,19–21 The pre-existing location-based model for delivering orthopedic care supports case diversity; however, this model continues to be challenged with high-volume centers of excellence and patient travel.22–24 Hip and knee arthroplasty experienced a similar surge in demand, with a subsequent shift in care to high-volume surgeons and centers.25 Shoulder and elbow fellowship-trained surgeons would need to nearly quadruple their current Medicare TSA volume to meet the entire current demand for TSA in the Medicare population (and this does not account for TSA performed by very low-volume surgeons not included in this cohort). With increased utilization of TSA, policymakers and the orthopedic community must determine the structure of delivery (centers of excellence or medium-volume disseminated throughout the country) that is optimal.
For those surgeons consistently performing TSA over the study period, fellowship training was diverse. While the current focus in orthopedics is on case volume, research in other specialties, namely general surgery, has provided repeated evidence that surgical specialization (more so than high case volume) provides improved outcomes.26–29Furthermore, Leopold and colleagues30 demonstrated an inverse relationship between competency in performing a procedure and confidence in one’s ability to do so. In their study, educational intervention provided improved competency in the procedure. Less than one-third (29.8%) of TSA in this cohort were performed by a shoulder and elbow fellowship-trained surgeon consistently performing this procedure. Approximately another quarter (26.2%) were performed by consistent TSA surgeons trained in sports surgery. Meanwhile, 34.6% of TSA in this study cohort were performed by a surgeon who did not consistently meet the minimum threshold in all study years (16,435 TSA; 22.8%) or by a surgeon performing TSA without fellowship training (8,489 TSA; 11.8%). There has been a trend toward orthopedic subspecialty training with an increased demand for fellowship-trained surgeons.31 Despite this and the complexities of TSA, many continue to be performed by surgeons with an inconsistent volume and those without arthroplasty-specific fellowship training. The available evidence supports a push toward the fellowship-trained, high-volume TSA surgeon in providing reproducible high-quality shoulder arthroplasty care. For now, that surgeon is more likely to be earlier in his/her career and reside in large, referral-based centers surrounded by other surgeons performing TSA.
These findings must be considered in the light of the study limitations. First, this is a large publicly available database. While this type of database provides a unique opportunity to assess the geographic distributions and characteristics of orthopedic surgeons, specifically those performing TSA, it completely prevents any assessment of the relationship between these findings and quality. As such, while the reader may generate hypotheses regarding the implications of our findings on the quality of TSA delivery, the true effects cannot be determined. In the same vein, for the purpose of privacy, surgeons performing ≤10 TSA were not included in this dataset. This limitation prevents the identification of low-volume TSA surgeons. Also, it is likely that the observed increase in surgeons over time is likely a reflection of small increases in volume for surgeons already performing TSA. Lastly, a web-based search was undertaken to identify surgeons’ self-reported fellowship training. The results of this web-based search could not be validated, and it is possible that fellowship training, or the lack thereof, was mischaracterized and simply not obtainable through a web-based search. Furthermore, it is not possible to fully assess the extent of high-quality TSA training in these various fellowships.
CONCLUSION
In just the past decade, the utilization of TSA in the Medicare population has increased significantly. However, this increase was not achieved by the addition of highly specialized, high-volume surgeons but by the addition of many surgeons performing lower numbers of TSA surgeries. Furthermore, for those performing this cost-effective procedure, TSA constitutes a relatively small proportion of the surgeries they perform. Shoulder and elbow fellowship-trained surgeons currently account for a low percentage of the overall number of surgeons performing TSA. The implications of these findings must be considered and investigated.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
2. Zmistowski B, Padegimas EM, Howley M, Abboud J, Williams G, Namdari S. Trends and Variability in the Use of Total Shoulder Arthroplasty for Medicare Patients. J Am Acad Orthop Surg. 2018;26(4):133-141. doi:10.5435/JAAOS-D-16-00720
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: http://www.jshoulderelbow.org/article/S1058-2746(10)00110-2/abstract.
4. Day JS, Paxton ES, Lau E, Gordon VA, Abboud JA, Williams GR. Use of reverse total shoulder arthroplasty in the Medicare population. J Shoulder Elbow Surg. 2015;24(5):766-772. doi:10.1016/j.jse.2014.12.023.
5. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
6. Westermann RW, Pugely AJ, Martin CT, Gao Y, Wolf BR, Hettrich CM. Reverse shoulder arthroplasty in the United States: A comparison of national volume, patient demographics, complications, and surgical indications. Iowa Orthop J. 2015;35:1-7.
7. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367. doi: http://www.jshoulderelbow.org/article/S1058-2746(14)00036-6/abstract.
8. Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2003;85-A(12):2318-2324.
9. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop Relat Res. 2012;471(2):655-664. doi:10.1007/s11999-012-2481-6.
10. Lyman S, Jones EC, Bach PB, Peterson MGE, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;(432):132-137. doi:10.1097/01.blo.0000150571.51381.9a.
11. Somerson JS, Stein BA, Wirth MA. Distribution of high-volume shoulder arthroplasty surgeons in the United States: Data from the 2014 Medicare provider utilization and payment data release. J Bone Joint Surg Am. 2016;98(18):e77. doi:10.2106/JBJS.15.00776.
12. Fisher ES, Bell J-E, Tomek IM, Esty AR, Goodman DC. Trends and regional variation in hip, knee, and shoulder Replacement. Atlases and Reports. Dartmouth Atlas of Health Care. https://www.dartmouthatlas.org/atlases-and-reports/. Accessed December 14, 2018.
13. Daniels AH, DiGiovanni CW. Is subspecialty fellowship training emerging as a necessary component of contemporary orthopaedic surgery education? J Grad Med Educ. 2014;6(2):218-221. doi:10.4300/JGME-D-14-00120.1.
14. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Physician and other supplier Data 2012 CY 2012. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Tren.... Published October 5, 2015. Accessed July 25, 2016.
15. The Dartmouth Institute for Health Policy and Clinical Practice. Dartmouth Atlas of Health Care. Understanding the Efficiency and Effectiveness of the Health Care System. http://www.dartmouthatlas.org/. Accessed January 31, 2014.
16. United States Department of Commerce. United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed September 30, 2016.
17. Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469(9):2496-2504. doi:10.1007/s11999-011-1811-4.
18. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Bone Joint Surg Am. 2014;23(8):1187-1194. doi:10.1016/j.jse.2013.11.017.
19. Jain N, Pietrobon R, Hocker S, Guller U, Shankar A, Higgins LD. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(3):496-505.
20. Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital patient volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12(3):235-242. doi:10.1016/S0883-5403(97)90018-8.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Bone Joint Surg Am. 2012;21(11):1470-1477. doi:10.1016/j.jse.2011.11.010.
22. FitzGerald JD, Soohoo NF, Losina E, Katz JN. Potential impact on patient residence to hospital travel distance and access to care under a policy of preferential referral to high-volume knee replacement hospitals. Arthritis Care Res. 2012;64(6):890-897. doi:10.1002/acr.21611.
23. Maradit Kremers H, Salduz A, Schleck CD, Larson DR, Berry DJ, Lewallen DG. Referral bias in primary total knee arthroplasty: retrospective analysis of 22,614 surgeries in a tertiary referral center. J Arthroplasty. doi:10.1016/j.arth.2016.08.014.
24. Robinson JC, MacPherson K. Payers test reference pricing and centers of excellence to steer patients to low-price and high-quality providers. Health Affairs. 2012;31(9):2028-2036. doi: 10.1377/hlthaff.2011.1313
25. Laucis NC, Chowdhury M, Dasgupta A, Bhattacharyya T. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am. 2016;98(9):707-712. doi:10.2106/JBJS.15.00399.
26. Anwar S, Fraser S, Hill J. Surgical specialization and training–its relation to clinical outcome for colorectal cancer surgery. J Eval Clin Pract. 2012;18(1):5-11. doi:10.1111/j.1365-2753.2010.01525.x.
27. Snow BW, Catwright PC, Young MD. Does surgical subspecialization in pediatrics provide high-quality, cost-effective patient care? Pediatrics. 1996;97(1):14-17.
28. Smith J a. E, King PM, Lane RHS, Thompson MR. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90(5):583-592. doi:10.1002/bjs.4085.
29. Hall BL, Hsaio EY, Majercik S, Hirbe M, Hamilton BH. The impact of surgeon specialization on patient mortality: Examination of a continuous Herfindahl-Hirschman Index. Ann Surg. 2009;249(5):708-716. doi: 10.1097/SLA.0b013e3181a335f8.
30. Leopold SS, Morgan HD, Kadel NJ, Gardner GC, Schaad DC, Wolf FM. Impact of educational intervention on confidence and competence in the performance of a simple surgical task. J Bone Joint Surg Am. 2005;87(5):1031-1037. doi:10.2106/JBJS.D.02434.
31. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560. doi:10.3928/01477447-20120327-13.
ABSTRACT
Total shoulder arthroplasty (TSA) has proved a cost-effective, reproducible procedure for multiple shoulder pathologies. As utilization of TSA continues to grow, it is important to investigate procedure diversity, training, and other characteristics of surgeons performing TSA. To identify surgeons performing TSA in the Medicare population, the Medicare Provider Utilization and Payment Databases from 2012 through 2014 were used. This dataset includes any provider who bills Medicare >10 times with a single billing code. A web-based search was performed for each physician performing >10 TSA in all years of the study to identify their surgical training characteristics. Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year (71,973 TSA). Only 44.3% (609/1374) of surgeons met this threshold for all 3 years (55,538 TSA). Of these 609 surgeons, 191 (31.3%) were shoulder and elbow fellowship trained (21,444 TSA). Shoulder and elbow fellowship-trained surgeons were at earlier points in their careers and practiced in large referral-based centers with other surgeons performing TSA. In addition to TSA, surgeons performed other non-arthroplasty shoulder procedures (80.2% of surgeons), total knee arthroplasty (46.3%), repairs of traumatic injuries (29.8%), total hip arthroplasty (27.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%) during the study period. With less than one-third of TSA performed by shoulder and elbow fellowship-trained surgeons with consistent moderate-volume practices, the impact of consistent high-volume practices and targeted fellowship training on quality must be determined.
Continue to: With the adoption of reverse shoulder arthroplasty...
With the adoption of reverse shoulder arthroplasty, utilization of total shoulder arthroplasty (TSA) has increased substantially over the last decade.1–3 Such increases are likely secondary to an aging population, increased comfort with the procedure, and the adoption of broadened indications for reverse shoulder arthroplasty, especially in the setting of proximal humerus fractures in the elderly.4–7 Between 2012 and 2014 alone, the number of surgeons performing >10 TSA in Medicare patients annually increased by 28.6% (824 to 1060 surgeons) providing a 26.6% (20,824-26,365 procedures) increase in national volume in the Medicare population.2 With this boom in utilization, scrutiny of this now routine procedure and those performing it is necessary.
Prior reviews have demonstrated a strong link between surgeon and hospital TSA volume and outcomes of the procedure.8–10 Somerson and colleagues11 investigated fellowship training among surgeons performing TSA in 2012 and found that only 28% had completed a shoulder and elbow fellowship. In addition to prior analyses2, 12, Somerson and colleagues confirmed a persistent geographic variation in utilization of TSA.11 In conjunction with the evolution of shoulder arthroplasty, dedicated shoulder and elbow fellowship training has expanded. With a shift toward specialization in care, nearly 90% of orthopedic surgery residents plan to pursue shoulder and elbow fellowships, comprising 4.6% of (42/897) of available positions.13
What remains unknown is the specialization of surgeons performing TSA, the regularity of their arthroplasty volume, and trends in TSA specialization over time. Therefore, this study aims to (a) identify surgeons performing shoulder arthroplasty and cohort changes over time, (b) determine the case profile of surgeons consistently performing shoulder arthroplasty, and (c) establish the characteristics of shoulder arthroplasty surgeons with a specific focus on fellowship training.
METHODS
Prior to collecting surgeon-specific data, we identified surgeons performing TSA through the Centers for Medicare and Medicaid Services’ public release of “Medicare Provider Utilization and Payment Data: Physician and Other Supplier.”14 Datasets from 2012, 2013, and 2014 were used to identify all surgeons performing >10 TSAs (Current Procedural Terminology [CPT] Code 23472) during at least 1 of those years. This dataset provides the name, identification number, address, and all billing (by volume) for each unique CPT code submitted ≥10 times in a calendar year.
Once the cohort of surgeons had been generated, the number of surgeons consistently performing TSA year-over-year was determined. This allowed for an analysis of the consistency with which surgeons are performing moderate- to high-volume TSA. To form a case profile of surgeons performing TSA and observe how this shifted over time, a count and a description of each CPT code submitted by each surgeon was identified. To maintain patient privacy, only those claims made >10 times are reported for a provider (both physicians and physician-extenders are included in this dataset). First, all CPT codes were reviewed and tagged as surgical or non-surgical events. Then, every procedural CPT code identified was reviewed and categorized based upon anatomic location and procedure (eg, total knee arthroplasty [TKA]). It is important to note that all claims in this dataset are limited to those patients participating in Medicare’s fee-for-service program.
Specialization was defined as the number of categorized procedures as a percentage of all procedures performed on Medicare patients. The trends for national, regional, and individual specialization of TSA, arthroplasty (major joint), and shoulder procedures were determined.
Continue to: To investigate the characteristics of surgeons...
To investigate the characteristics of surgeons consistently performing TSA, all surgeons performing a minimum of 11 TSA in Medicare fee-for-service beneficiaries in all years between 2012 and 2014 were identified. Such surgeons were defined as consistent TSA surgeons. Investigation of this cohort included a web-based search of their self-reported post-graduate fellowship training and year of graduation from medical school. Using these data, the percentage of surgeons performing TSA who underwent formal shoulder and elbow training was determined. In addition, the impact of fellowship training on shoulder specialization and practice location was determined. Surgeons who had completed multiple fellowships were categorized under all of them. As such, there may be some duplication of surgeons in the comparisons. In addition, other potential characteristics of shoulder and elbow fellowship-trained surgeons were investigated: number of regional shoulder surgeons, urban area, total number of Medicare beneficiaries, average reimbursement for TSA, ethnicity of Medicare beneficiaries, and percentage of Medicare patients eligible for Medicaid. Geographic regions were defined by the Dartmouth Atlas and assigned by hospital referral region.15 These defined regions were used to assess the beneficiaries (number and characteristics) that individual surgeons were likely serving. The United States Census Bureau characterization of zip code-based regions as urban areas (population >50,000), urban clusters (2500 to 50,000), and rural region (<2500) was used to categorize practice location.16
Descriptive statistics were used initially to report these findings. To analyze predictors of utilization and specialization, comparative statistics were undertaken. For comparison of binomial variables between groups, a χ2analysis was utilized. For continuous variables, data normality was assessed. A skewness and kurtosis <2 and 12, respectively, was considered to represent parametric data. For parametric data, the mean was reported; conversely, the median is reported for non-parametric data. To assess continuous variables between groups, a t test or a Wilcoxon rank-sum test was used for parametric and non-parametric distributions, respectively.
RESULTS
Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year, for a combined total of 71,973 TSAs (Table 1). In 2012, only 834 surgeons (13 females [1.6%]) performed a minimum of 10 TSA in Medicare patients (21,137 arthroplasties; 25.3 per surgeon). This increased to 1078 surgeons (33 females [3.1%]; P = .04) performing 26,865 TSA (24.92 per surgeon) in 2014. Utilization of non-physician assistants in TSA also increased significantly over this period, with 307 assisting in 6885 TSAs (22.4 per provider) in 2012 and 465 assisting 10,433 TSA (22.4 per provider) in 2014. When all procedures were considered, including those performed at outpatient visits, 1319 physicians (95.9% of cohort) were active in 2012—providing either surgical procedures or outpatient consults to the Medicare population. Yet, only 63.2% performed >10 TSA in Medicare patients. The number of active surgeons performing TSA increased to 79.6% (1078/1353) in 2014 (P < .001).
Table 1. Trends in the Number of Providers Performing TSA between 2012 and 2014*
2012 | 2013 | 2014 | Total | |
| Providers (no.) | 1141 | 1373 | 1543 | 1994 |
Physicians | 834 | 984 | 1,078 | 1,374 |
Non-physicians | 307 | 389 | 465 | 620 |
| TSA (no.) | 28,022 | 32,641 | 37,298 | 97,961 |
| Physicians | 21,137 | 23,971 | 26,865 | 71,973 |
| Non-physicians | 6,885 | 8,670 | 10,433 | 25,988 |
| TSA per provider | 24.5 | 23.8 | 24.2 | 49.2 |
| Physicians | 25.3 | 24.3 | 24.9 | 52.4 |
| Non-physicians | 22.4 | 22.3 | 22.4 | 41.2 |
| Procedures (no.) | 210,845 | 224,123 | 227,305 | 662,273 |
| Physicians | 152,862 | 160,114 | 160,851 | 473,827 |
| Non-physicians | 57,983 | 64,009 | 66,454 | 188,446 |
| Procedure per provider | 114.4 | 116.8 | 116.5 | 332.13 |
| Physicians | 115.9 | 118.9 | 118.9 | 344.9 |
| Non-physicians | 110.7 | 111.9 | 111.1 | 303.9 |
| Active providers (no.) | 1843 | 1919 | 1951 | 1994 |
| Physicians | 1319 | 1347 | 1353 | 1374 |
| Non-physicians | 524 | 572 | 598 | 620 |
* Included are the number of arthroplasties and total procedures over time among this cohort. The number of active providers, determined by billing Medicare for office or surgical procedures within that year, is reported.
Abbreviation: TSA, total shoulder arthroplasty.
In addition to TSA, this cohort of surgeons submitted 240 unique CPT codes with case volumes >10 annually over the 3-year study period. Of these, 80.2% (1102/1374) of surgeons performed non-arthroplasty shoulder procedures on Medicare patients, for a combined total of 202,335 procedures over the 3-year study period (Table 2). A significant proportion of these procedures were arthroscopic debridement (60,014 procedures performed by 908 surgeons) and arthroscopic rotator cuff repair (47,089 procedures performed by 809 surgeons). Just under half (49.1%; 674/1374) of surgeons performing TSA also performed TKA during this period (77,873 arthroplasties). Fewer surgeons (27.8%; 382/1374) performed total hip arthroplasty during this period (27,322 arthroplasties). Other procedure types that this group of surgeons routinely performed on Medicare patients were repairs of traumatic injuries (29.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%). By case load, non-arthroplasty shoulder procedures consisted of 43% of Medicare volume over the study period (Figure 1). Between 2012 and 2014, the average proportion of Medicare cases that were shoulder arthroplasties increased from 13.8% (21,137/152,862) to 16.7% (26,865/160,851; P = .001). Shoulder arthroplasty constituted 100% of the Medicare surgical case volume for 67 (4.9%; 67/1374) of the surgeons.

Table 2. Case Volumes over Time with All Procedures Categorized by Anatomic Region and Arthroplasty vs Non-arthroplasty*
2012 | 2013 | 2014 | Total | |
| Shoulder arthroplasty | 21,351 (n=837) | 24,128 (n=984) | 26,902 (n=1,078) | 72,381 (n=1,374) |
23472: total shoulder arthroplasty | 21,137 (n=834) | 23,971 (n=984) | 26,865 (n=1,078) | 71,973 (n=1,374) |
23470: Hemiarthroplasty | 214 (n=14) | 84 (n=6) | 37 (n=2) | 335 (n=15) |
| Shoulder (non-arthroplasty) | 65,947 (n=887) | 68,746 (n=942) | 67,642 (n=932) | 202,335 (n=1,102) |
29826: arthroscopic acromioplasty | 19,152 (n=724) | 20,367 (n=760) | 20,495 (n=754) | 60,014 (n=908) |
29827: arthroscopic rotator cuff repair | 14,700 (n=613) | 15,963 (n=664) | 16,426 (n=658) | 47,089 (n=809) |
23412: open rotator cuff repair | 1957 (n=88) | 2046 (n=90) | 2112 (n=2,112) | 6115 (n=143) |
23430: Open biceps tenodesis | 4063 (n=178) | 3998 (n=167) | 4601 (n=185) | 12,662 (n=288) |
29823: arthroscopic major debridement | 7428 (n=301) | 7745 (n=309) | 5202 (n=210) | 20,375 (n=417) |
Total knee arthroplasty | 25,640 (n=565) | 26,558 (n=587) | 25,675 (n=580) | 77,873 (n=637) |
Total hip arthroplasty | 8729 (n=316) | 9226 (n=318) | 9367 (n=330) | 27,322 (n=382) |
Trauma | 6454 (n=260) | 6396 (n=254) | 6364 (n=261) | 19,214 (n=410) |
27245: surgical treatment of broken thigh bone (intertrochanteric) | 2602 (n=162) | 2654 (n=164) | 2537 (n=160) | 7793 (n=274) |
27236: surgical treatment of broken thigh bone (hemiarthroplasty) | 1961 (n=123) | 1703 (n=111) | 1702 (n=112) | 5366 (n=205) |
Hand | 6343 (n=139) | 7321 (n=154) | 8006 (n=172) | 21,670 (n=211) |
Elbow | 6113 (n=198) | 6139 (n=204) | 6131 (n=198) | 18,383 (n=270) |
Knee (non-arthroplasty) | 8514 (n=275) | 8140 (n=275) | 7689 (n=230) | 24,343 (n=374) |
Outpatient visits | 879,740 (n=1,282) | 907,124 (n=1,320) | 921,291 (n=1,327) | 2,708,155 (n=1,342) |
New patient | 195,898 (n=1,276) | 192,937 (n=1,305) | 191,427 (n=1,315) | 571,203 (n=1,332) |
Existing patient | 740,307 (n=1,279) | 714,187 (n=1,316) | 729,864 (n=1,324) | 2,29,976 (n=1,338) |
* Procedures of interest with high case volumes are reported individually.

Only 44.3% (609/1374) of surgeons performed TSA in a minimum of 11 Medicare patients in all 3 years of the study period (consistent providers of TSA), providing a total of 55,538 TSA (77.2%; 55,538/71,973). When fellowship training was evaluated, 191 (31.4%; 191/609) of these surgeons were shoulder and elbow fellowship trained (21,444 TSA; 38.6%; Table 3). More than one-third (36.6%; 223/609) had completed a sports surgery fellowship (18,899 TSA; 34.0%). Surgeons trained in hand surgery (12.5%; 76/609) and adult reconstruction (5.3%; 32/610) also made contributions to meeting the TSA demand with 6971 (12.6%) and 2485 (4.5%) TSA, respectively. One-fifth of this cohort (18.1%; 110/609) had unknown fellowship training: they either reported no fellowship (13.6%; 83/609) or did not specify the type of training (4.4%; 27/609). Shoulder and elbow fellowship-trained surgeons performed more TSA (median: 89.0 TSA per surgeon between 2012 and 2014) than surgeons without shoulder and elbow fellowship training (median: 67.0 TSA per surgeon; P < 0.001). More than one-third (37%) of shoulder and elbow fellowship-trained surgeons’ surgical case volume was comprised of TSA, with an additional 35% from non-arthroplasty shoulder procedures (Figure 2). In order for the current supply of shoulder and elbow fellowship-trained surgeons to meet the Medicare TSA demand, each fellowship graduate would have to perform 140.6 TSA in Medicare patients annually. Shoulder and elbow fellowship-trained surgeons were more likely to practice in referral regions with an increased Medicare population (P < .001), an increased number of surgeons performing TSA (P < .001), and a higher proportion of Medicaid-eligible patients (P = .01; Table 4). Shoulder and elbow fellowship-trained surgeons (18.7 years post-medical school graduation) were also earlier in their careers than other consistent TSA surgeons (23.1 years post-graduation; P < .001).
Table 3. A Representation of Fellowships Among TSA Surgeons and Their Shoulder Arthroplasty Case Load*
| Fellowship | Surgeons (%) | 2012-2014 (no, %) | SA Medicare Cases (%) | Average Surgeon Annual SA Volume |
| Shoulder and elbow | 191 (31.4%) | 21,444 (38.6%) | 29.3% | 37.4 |
| Hand surgery | 76 (12.5%) | 6971 (12.6%) | 17.1% | 30.6 |
| Sports | 223 (36.6%) | 18,899 (34.0%) | 19.4% | 28.3 |
| Trauma | 14 (2.3%) | 1270 (2.3%) | 10.9% | 30.2 |
| Adult reconstruction | 32 (5.3%) | 2485 (4.5%) | 10.2% | 25.9 |
| Unknown/none | 110 (18.1%) | 8489 (15.3%) | 16.3% | 25.7 |
|
|
|
| |
| 1 Fellowship | 459 (75.3%) | 42,065 (75.7%) | 20.7% | 30.5 |
| ≥2 Fellowships | 67 (11.0%) | 7122 (12.8%) | 22.5% | 35.4 |
* Not all fellowships (eg, oncology) included due to small numbers. Also, many surgeons performed multiple fellowships.
Abbreviations: SA, shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 4. Breakdown of Geographic Characteristics of Orthopedic Surgeons Consistently
Performing TSA between 2012 and 2014 Stratified by Fellowship Training
Abbreviations: HRR, hospital referral region; TSA, total shoulder arthroplasty.
| Fellowship | Percentage in Non-Urban Area | Average No. of Other TSA Surgeons within HRR | Median Proportion of Patients Eligible for Medicaid within HRR | Average Proportion of Caucasian Patients within HRR | Average Population in Practicing Zip Code | Average No. of Medicare Beneficiaries in HRR | Average No. Years from Medical School Graduation |
| Shoulder and elbow | 7.3% | 10.5 | 12.6 | 84.7 | 26,620.1 | 224,868.3 | 18.7 |
| Other fellowships | 10.3% | 8.6 | 11.1 | 85.6 | 27,619.7 | 177,939.7 | 23.1 |
P value | 0.29 | <0.001 | 0.01 | 0.30 | 0.41 | <0.001 | <0.001 |
Hand surgery | 7.9% | 8.1 | 12.8 | 83.7 | 24,022.8 | 179,370.8 | 23.9 |
Sports | 11.2% | 8.9 | 11.9 | 85.6 | 28,588.9 | 185,902.4 | 21.2 |
Trauma | 21.4% | 7.7 | 13.8 | 85.5 | 20,065.9 | 170,807.6 | 25.6 |
Adult reconstruction | 6.3% | 8.7 | 12.8 | 86.9 | 26,601.5 | 173,280.1 | 22.4 |
None/unknown | 10.9% | 8.5 | 12.0 | 86.4 | 28,173.6 | 166,522.5 | 27.0 |
Continue to: DISCUSSION...
DISCUSSION
Utilization of TSA has continued to rise; however, access to this cost-effective procedure was recently demonstrated to be limited.11 In a separate analysis, we established the continued rise in use of TSA in the Medicare population, coupled with an increase in the number of surgeons routinely performing TSA.2 Multiple analyses have demonstrated the importance of high-volume surgeons and hospitals familiar with the intricacies of shoulder arthroplasty concepts in minimizing complications, improving the quality and decreasing the cost of TSA.6,10,17 Specifically, Singh and colleagues18 demonstrated from a multi-center registry that surgeons and hospitals with greater shoulder arthroplasty volumes had decreased intra-operative blood loss, operative time, and hospital length of stay. As the demand for TSA, both anatomic and reverse, continues to rise, it is imperative that the healthcare delivery system is optimized to provide the best possible care. Before we can determine whether specialized training in shoulder arthroplasty influences surgical outcomes, characteristics and training of surgeons performing TSA should be described.
The number of surgeons performing >10 TSA in the Medicare population rose significantly between 2012 and 2014 (29.3%). However, the number of TSAs per surgeon over this time period remained consistent (approximately 25 per surgeon). Furthermore, the increase in the number of surgeons performing a reportable volume of TSA by 2014 was from the addition of already active surgeons (ie, the growth in TSA was not from the addition of newly trained arthroplasty surgeons but originated from the existing orthopedic surgeon workforce). In a recently published analysis, Somerson and colleagues, 11 using this same dataset, demonstrated persistent limitations in access to high-volume TSA surgeons. In a more recent analysis, we showed that while still lacking for some patients, access to a high-volume TSA surgeon has improved significantly over the past 3 years, with 96.9% of the United States population residing within 200 kilometers of a high-volume TSA surgeon (>20 Medicare cases).2 This analysis validates those findings, with the caveat that the average annual volume per surgeon is not increasing. What remains unknown, due to limitations of this dataset, is how many surgeons are not identified because they are performing ≤10 TSA each year or are performing TSA in non-Medicare patients.
With the specialization of healthcare delivery, specifically in orthopedics, it is imperative that mechanisms for providing specialty-focused care be established. However, the proportion of their practice that surgeons dedicate to TSA was unknown. This study demonstrates that this proportion is increasing. Including non-arthroplasty procedures, more than half (58%) of the procedures performed by this surgeon cohort were shoulder-specific. Furthermore, this analysis demonstrates that surgeons performing TSA have significant case diversity, including nearly half of the cohort performing TKA. Repeated evidence has demonstrated the effect of case volume on improved outcomes following orthopedic procedures.8,19–21 The pre-existing location-based model for delivering orthopedic care supports case diversity; however, this model continues to be challenged with high-volume centers of excellence and patient travel.22–24 Hip and knee arthroplasty experienced a similar surge in demand, with a subsequent shift in care to high-volume surgeons and centers.25 Shoulder and elbow fellowship-trained surgeons would need to nearly quadruple their current Medicare TSA volume to meet the entire current demand for TSA in the Medicare population (and this does not account for TSA performed by very low-volume surgeons not included in this cohort). With increased utilization of TSA, policymakers and the orthopedic community must determine the structure of delivery (centers of excellence or medium-volume disseminated throughout the country) that is optimal.
For those surgeons consistently performing TSA over the study period, fellowship training was diverse. While the current focus in orthopedics is on case volume, research in other specialties, namely general surgery, has provided repeated evidence that surgical specialization (more so than high case volume) provides improved outcomes.26–29Furthermore, Leopold and colleagues30 demonstrated an inverse relationship between competency in performing a procedure and confidence in one’s ability to do so. In their study, educational intervention provided improved competency in the procedure. Less than one-third (29.8%) of TSA in this cohort were performed by a shoulder and elbow fellowship-trained surgeon consistently performing this procedure. Approximately another quarter (26.2%) were performed by consistent TSA surgeons trained in sports surgery. Meanwhile, 34.6% of TSA in this study cohort were performed by a surgeon who did not consistently meet the minimum threshold in all study years (16,435 TSA; 22.8%) or by a surgeon performing TSA without fellowship training (8,489 TSA; 11.8%). There has been a trend toward orthopedic subspecialty training with an increased demand for fellowship-trained surgeons.31 Despite this and the complexities of TSA, many continue to be performed by surgeons with an inconsistent volume and those without arthroplasty-specific fellowship training. The available evidence supports a push toward the fellowship-trained, high-volume TSA surgeon in providing reproducible high-quality shoulder arthroplasty care. For now, that surgeon is more likely to be earlier in his/her career and reside in large, referral-based centers surrounded by other surgeons performing TSA.
These findings must be considered in the light of the study limitations. First, this is a large publicly available database. While this type of database provides a unique opportunity to assess the geographic distributions and characteristics of orthopedic surgeons, specifically those performing TSA, it completely prevents any assessment of the relationship between these findings and quality. As such, while the reader may generate hypotheses regarding the implications of our findings on the quality of TSA delivery, the true effects cannot be determined. In the same vein, for the purpose of privacy, surgeons performing ≤10 TSA were not included in this dataset. This limitation prevents the identification of low-volume TSA surgeons. Also, it is likely that the observed increase in surgeons over time is likely a reflection of small increases in volume for surgeons already performing TSA. Lastly, a web-based search was undertaken to identify surgeons’ self-reported fellowship training. The results of this web-based search could not be validated, and it is possible that fellowship training, or the lack thereof, was mischaracterized and simply not obtainable through a web-based search. Furthermore, it is not possible to fully assess the extent of high-quality TSA training in these various fellowships.
CONCLUSION
In just the past decade, the utilization of TSA in the Medicare population has increased significantly. However, this increase was not achieved by the addition of highly specialized, high-volume surgeons but by the addition of many surgeons performing lower numbers of TSA surgeries. Furthermore, for those performing this cost-effective procedure, TSA constitutes a relatively small proportion of the surgeries they perform. Shoulder and elbow fellowship-trained surgeons currently account for a low percentage of the overall number of surgeons performing TSA. The implications of these findings must be considered and investigated.
ABSTRACT
Total shoulder arthroplasty (TSA) has proved a cost-effective, reproducible procedure for multiple shoulder pathologies. As utilization of TSA continues to grow, it is important to investigate procedure diversity, training, and other characteristics of surgeons performing TSA. To identify surgeons performing TSA in the Medicare population, the Medicare Provider Utilization and Payment Databases from 2012 through 2014 were used. This dataset includes any provider who bills Medicare >10 times with a single billing code. A web-based search was performed for each physician performing >10 TSA in all years of the study to identify their surgical training characteristics. Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year (71,973 TSA). Only 44.3% (609/1374) of surgeons met this threshold for all 3 years (55,538 TSA). Of these 609 surgeons, 191 (31.3%) were shoulder and elbow fellowship trained (21,444 TSA). Shoulder and elbow fellowship-trained surgeons were at earlier points in their careers and practiced in large referral-based centers with other surgeons performing TSA. In addition to TSA, surgeons performed other non-arthroplasty shoulder procedures (80.2% of surgeons), total knee arthroplasty (46.3%), repairs of traumatic injuries (29.8%), total hip arthroplasty (27.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%) during the study period. With less than one-third of TSA performed by shoulder and elbow fellowship-trained surgeons with consistent moderate-volume practices, the impact of consistent high-volume practices and targeted fellowship training on quality must be determined.
Continue to: With the adoption of reverse shoulder arthroplasty...
With the adoption of reverse shoulder arthroplasty, utilization of total shoulder arthroplasty (TSA) has increased substantially over the last decade.1–3 Such increases are likely secondary to an aging population, increased comfort with the procedure, and the adoption of broadened indications for reverse shoulder arthroplasty, especially in the setting of proximal humerus fractures in the elderly.4–7 Between 2012 and 2014 alone, the number of surgeons performing >10 TSA in Medicare patients annually increased by 28.6% (824 to 1060 surgeons) providing a 26.6% (20,824-26,365 procedures) increase in national volume in the Medicare population.2 With this boom in utilization, scrutiny of this now routine procedure and those performing it is necessary.
Prior reviews have demonstrated a strong link between surgeon and hospital TSA volume and outcomes of the procedure.8–10 Somerson and colleagues11 investigated fellowship training among surgeons performing TSA in 2012 and found that only 28% had completed a shoulder and elbow fellowship. In addition to prior analyses2, 12, Somerson and colleagues confirmed a persistent geographic variation in utilization of TSA.11 In conjunction with the evolution of shoulder arthroplasty, dedicated shoulder and elbow fellowship training has expanded. With a shift toward specialization in care, nearly 90% of orthopedic surgery residents plan to pursue shoulder and elbow fellowships, comprising 4.6% of (42/897) of available positions.13
What remains unknown is the specialization of surgeons performing TSA, the regularity of their arthroplasty volume, and trends in TSA specialization over time. Therefore, this study aims to (a) identify surgeons performing shoulder arthroplasty and cohort changes over time, (b) determine the case profile of surgeons consistently performing shoulder arthroplasty, and (c) establish the characteristics of shoulder arthroplasty surgeons with a specific focus on fellowship training.
METHODS
Prior to collecting surgeon-specific data, we identified surgeons performing TSA through the Centers for Medicare and Medicaid Services’ public release of “Medicare Provider Utilization and Payment Data: Physician and Other Supplier.”14 Datasets from 2012, 2013, and 2014 were used to identify all surgeons performing >10 TSAs (Current Procedural Terminology [CPT] Code 23472) during at least 1 of those years. This dataset provides the name, identification number, address, and all billing (by volume) for each unique CPT code submitted ≥10 times in a calendar year.
Once the cohort of surgeons had been generated, the number of surgeons consistently performing TSA year-over-year was determined. This allowed for an analysis of the consistency with which surgeons are performing moderate- to high-volume TSA. To form a case profile of surgeons performing TSA and observe how this shifted over time, a count and a description of each CPT code submitted by each surgeon was identified. To maintain patient privacy, only those claims made >10 times are reported for a provider (both physicians and physician-extenders are included in this dataset). First, all CPT codes were reviewed and tagged as surgical or non-surgical events. Then, every procedural CPT code identified was reviewed and categorized based upon anatomic location and procedure (eg, total knee arthroplasty [TKA]). It is important to note that all claims in this dataset are limited to those patients participating in Medicare’s fee-for-service program.
Specialization was defined as the number of categorized procedures as a percentage of all procedures performed on Medicare patients. The trends for national, regional, and individual specialization of TSA, arthroplasty (major joint), and shoulder procedures were determined.
Continue to: To investigate the characteristics of surgeons...
To investigate the characteristics of surgeons consistently performing TSA, all surgeons performing a minimum of 11 TSA in Medicare fee-for-service beneficiaries in all years between 2012 and 2014 were identified. Such surgeons were defined as consistent TSA surgeons. Investigation of this cohort included a web-based search of their self-reported post-graduate fellowship training and year of graduation from medical school. Using these data, the percentage of surgeons performing TSA who underwent formal shoulder and elbow training was determined. In addition, the impact of fellowship training on shoulder specialization and practice location was determined. Surgeons who had completed multiple fellowships were categorized under all of them. As such, there may be some duplication of surgeons in the comparisons. In addition, other potential characteristics of shoulder and elbow fellowship-trained surgeons were investigated: number of regional shoulder surgeons, urban area, total number of Medicare beneficiaries, average reimbursement for TSA, ethnicity of Medicare beneficiaries, and percentage of Medicare patients eligible for Medicaid. Geographic regions were defined by the Dartmouth Atlas and assigned by hospital referral region.15 These defined regions were used to assess the beneficiaries (number and characteristics) that individual surgeons were likely serving. The United States Census Bureau characterization of zip code-based regions as urban areas (population >50,000), urban clusters (2500 to 50,000), and rural region (<2500) was used to categorize practice location.16
Descriptive statistics were used initially to report these findings. To analyze predictors of utilization and specialization, comparative statistics were undertaken. For comparison of binomial variables between groups, a χ2analysis was utilized. For continuous variables, data normality was assessed. A skewness and kurtosis <2 and 12, respectively, was considered to represent parametric data. For parametric data, the mean was reported; conversely, the median is reported for non-parametric data. To assess continuous variables between groups, a t test or a Wilcoxon rank-sum test was used for parametric and non-parametric distributions, respectively.
RESULTS
Between 2012 and 2014, 1374 surgeons (39 females [2.8%]) performed >10 TSA in Medicare patients in at least 1 year, for a combined total of 71,973 TSAs (Table 1). In 2012, only 834 surgeons (13 females [1.6%]) performed a minimum of 10 TSA in Medicare patients (21,137 arthroplasties; 25.3 per surgeon). This increased to 1078 surgeons (33 females [3.1%]; P = .04) performing 26,865 TSA (24.92 per surgeon) in 2014. Utilization of non-physician assistants in TSA also increased significantly over this period, with 307 assisting in 6885 TSAs (22.4 per provider) in 2012 and 465 assisting 10,433 TSA (22.4 per provider) in 2014. When all procedures were considered, including those performed at outpatient visits, 1319 physicians (95.9% of cohort) were active in 2012—providing either surgical procedures or outpatient consults to the Medicare population. Yet, only 63.2% performed >10 TSA in Medicare patients. The number of active surgeons performing TSA increased to 79.6% (1078/1353) in 2014 (P < .001).
Table 1. Trends in the Number of Providers Performing TSA between 2012 and 2014*
2012 | 2013 | 2014 | Total | |
| Providers (no.) | 1141 | 1373 | 1543 | 1994 |
Physicians | 834 | 984 | 1,078 | 1,374 |
Non-physicians | 307 | 389 | 465 | 620 |
| TSA (no.) | 28,022 | 32,641 | 37,298 | 97,961 |
| Physicians | 21,137 | 23,971 | 26,865 | 71,973 |
| Non-physicians | 6,885 | 8,670 | 10,433 | 25,988 |
| TSA per provider | 24.5 | 23.8 | 24.2 | 49.2 |
| Physicians | 25.3 | 24.3 | 24.9 | 52.4 |
| Non-physicians | 22.4 | 22.3 | 22.4 | 41.2 |
| Procedures (no.) | 210,845 | 224,123 | 227,305 | 662,273 |
| Physicians | 152,862 | 160,114 | 160,851 | 473,827 |
| Non-physicians | 57,983 | 64,009 | 66,454 | 188,446 |
| Procedure per provider | 114.4 | 116.8 | 116.5 | 332.13 |
| Physicians | 115.9 | 118.9 | 118.9 | 344.9 |
| Non-physicians | 110.7 | 111.9 | 111.1 | 303.9 |
| Active providers (no.) | 1843 | 1919 | 1951 | 1994 |
| Physicians | 1319 | 1347 | 1353 | 1374 |
| Non-physicians | 524 | 572 | 598 | 620 |
* Included are the number of arthroplasties and total procedures over time among this cohort. The number of active providers, determined by billing Medicare for office or surgical procedures within that year, is reported.
Abbreviation: TSA, total shoulder arthroplasty.
In addition to TSA, this cohort of surgeons submitted 240 unique CPT codes with case volumes >10 annually over the 3-year study period. Of these, 80.2% (1102/1374) of surgeons performed non-arthroplasty shoulder procedures on Medicare patients, for a combined total of 202,335 procedures over the 3-year study period (Table 2). A significant proportion of these procedures were arthroscopic debridement (60,014 procedures performed by 908 surgeons) and arthroscopic rotator cuff repair (47,089 procedures performed by 809 surgeons). Just under half (49.1%; 674/1374) of surgeons performing TSA also performed TKA during this period (77,873 arthroplasties). Fewer surgeons (27.8%; 382/1374) performed total hip arthroplasty during this period (27,322 arthroplasties). Other procedure types that this group of surgeons routinely performed on Medicare patients were repairs of traumatic injuries (29.8%), non-arthroplasty knee surgeries (27.2%), elbow procedures (19.6%), and hand surgery (15.4%). By case load, non-arthroplasty shoulder procedures consisted of 43% of Medicare volume over the study period (Figure 1). Between 2012 and 2014, the average proportion of Medicare cases that were shoulder arthroplasties increased from 13.8% (21,137/152,862) to 16.7% (26,865/160,851; P = .001). Shoulder arthroplasty constituted 100% of the Medicare surgical case volume for 67 (4.9%; 67/1374) of the surgeons.

Table 2. Case Volumes over Time with All Procedures Categorized by Anatomic Region and Arthroplasty vs Non-arthroplasty*
2012 | 2013 | 2014 | Total | |
| Shoulder arthroplasty | 21,351 (n=837) | 24,128 (n=984) | 26,902 (n=1,078) | 72,381 (n=1,374) |
23472: total shoulder arthroplasty | 21,137 (n=834) | 23,971 (n=984) | 26,865 (n=1,078) | 71,973 (n=1,374) |
23470: Hemiarthroplasty | 214 (n=14) | 84 (n=6) | 37 (n=2) | 335 (n=15) |
| Shoulder (non-arthroplasty) | 65,947 (n=887) | 68,746 (n=942) | 67,642 (n=932) | 202,335 (n=1,102) |
29826: arthroscopic acromioplasty | 19,152 (n=724) | 20,367 (n=760) | 20,495 (n=754) | 60,014 (n=908) |
29827: arthroscopic rotator cuff repair | 14,700 (n=613) | 15,963 (n=664) | 16,426 (n=658) | 47,089 (n=809) |
23412: open rotator cuff repair | 1957 (n=88) | 2046 (n=90) | 2112 (n=2,112) | 6115 (n=143) |
23430: Open biceps tenodesis | 4063 (n=178) | 3998 (n=167) | 4601 (n=185) | 12,662 (n=288) |
29823: arthroscopic major debridement | 7428 (n=301) | 7745 (n=309) | 5202 (n=210) | 20,375 (n=417) |
Total knee arthroplasty | 25,640 (n=565) | 26,558 (n=587) | 25,675 (n=580) | 77,873 (n=637) |
Total hip arthroplasty | 8729 (n=316) | 9226 (n=318) | 9367 (n=330) | 27,322 (n=382) |
Trauma | 6454 (n=260) | 6396 (n=254) | 6364 (n=261) | 19,214 (n=410) |
27245: surgical treatment of broken thigh bone (intertrochanteric) | 2602 (n=162) | 2654 (n=164) | 2537 (n=160) | 7793 (n=274) |
27236: surgical treatment of broken thigh bone (hemiarthroplasty) | 1961 (n=123) | 1703 (n=111) | 1702 (n=112) | 5366 (n=205) |
Hand | 6343 (n=139) | 7321 (n=154) | 8006 (n=172) | 21,670 (n=211) |
Elbow | 6113 (n=198) | 6139 (n=204) | 6131 (n=198) | 18,383 (n=270) |
Knee (non-arthroplasty) | 8514 (n=275) | 8140 (n=275) | 7689 (n=230) | 24,343 (n=374) |
Outpatient visits | 879,740 (n=1,282) | 907,124 (n=1,320) | 921,291 (n=1,327) | 2,708,155 (n=1,342) |
New patient | 195,898 (n=1,276) | 192,937 (n=1,305) | 191,427 (n=1,315) | 571,203 (n=1,332) |
Existing patient | 740,307 (n=1,279) | 714,187 (n=1,316) | 729,864 (n=1,324) | 2,29,976 (n=1,338) |
* Procedures of interest with high case volumes are reported individually.

Only 44.3% (609/1374) of surgeons performed TSA in a minimum of 11 Medicare patients in all 3 years of the study period (consistent providers of TSA), providing a total of 55,538 TSA (77.2%; 55,538/71,973). When fellowship training was evaluated, 191 (31.4%; 191/609) of these surgeons were shoulder and elbow fellowship trained (21,444 TSA; 38.6%; Table 3). More than one-third (36.6%; 223/609) had completed a sports surgery fellowship (18,899 TSA; 34.0%). Surgeons trained in hand surgery (12.5%; 76/609) and adult reconstruction (5.3%; 32/610) also made contributions to meeting the TSA demand with 6971 (12.6%) and 2485 (4.5%) TSA, respectively. One-fifth of this cohort (18.1%; 110/609) had unknown fellowship training: they either reported no fellowship (13.6%; 83/609) or did not specify the type of training (4.4%; 27/609). Shoulder and elbow fellowship-trained surgeons performed more TSA (median: 89.0 TSA per surgeon between 2012 and 2014) than surgeons without shoulder and elbow fellowship training (median: 67.0 TSA per surgeon; P < 0.001). More than one-third (37%) of shoulder and elbow fellowship-trained surgeons’ surgical case volume was comprised of TSA, with an additional 35% from non-arthroplasty shoulder procedures (Figure 2). In order for the current supply of shoulder and elbow fellowship-trained surgeons to meet the Medicare TSA demand, each fellowship graduate would have to perform 140.6 TSA in Medicare patients annually. Shoulder and elbow fellowship-trained surgeons were more likely to practice in referral regions with an increased Medicare population (P < .001), an increased number of surgeons performing TSA (P < .001), and a higher proportion of Medicaid-eligible patients (P = .01; Table 4). Shoulder and elbow fellowship-trained surgeons (18.7 years post-medical school graduation) were also earlier in their careers than other consistent TSA surgeons (23.1 years post-graduation; P < .001).
Table 3. A Representation of Fellowships Among TSA Surgeons and Their Shoulder Arthroplasty Case Load*
| Fellowship | Surgeons (%) | 2012-2014 (no, %) | SA Medicare Cases (%) | Average Surgeon Annual SA Volume |
| Shoulder and elbow | 191 (31.4%) | 21,444 (38.6%) | 29.3% | 37.4 |
| Hand surgery | 76 (12.5%) | 6971 (12.6%) | 17.1% | 30.6 |
| Sports | 223 (36.6%) | 18,899 (34.0%) | 19.4% | 28.3 |
| Trauma | 14 (2.3%) | 1270 (2.3%) | 10.9% | 30.2 |
| Adult reconstruction | 32 (5.3%) | 2485 (4.5%) | 10.2% | 25.9 |
| Unknown/none | 110 (18.1%) | 8489 (15.3%) | 16.3% | 25.7 |
|
|
|
| |
| 1 Fellowship | 459 (75.3%) | 42,065 (75.7%) | 20.7% | 30.5 |
| ≥2 Fellowships | 67 (11.0%) | 7122 (12.8%) | 22.5% | 35.4 |
* Not all fellowships (eg, oncology) included due to small numbers. Also, many surgeons performed multiple fellowships.
Abbreviations: SA, shoulder arthroplasty; TSA, total shoulder arthroplasty.
Table 4. Breakdown of Geographic Characteristics of Orthopedic Surgeons Consistently
Performing TSA between 2012 and 2014 Stratified by Fellowship Training
Abbreviations: HRR, hospital referral region; TSA, total shoulder arthroplasty.
| Fellowship | Percentage in Non-Urban Area | Average No. of Other TSA Surgeons within HRR | Median Proportion of Patients Eligible for Medicaid within HRR | Average Proportion of Caucasian Patients within HRR | Average Population in Practicing Zip Code | Average No. of Medicare Beneficiaries in HRR | Average No. Years from Medical School Graduation |
| Shoulder and elbow | 7.3% | 10.5 | 12.6 | 84.7 | 26,620.1 | 224,868.3 | 18.7 |
| Other fellowships | 10.3% | 8.6 | 11.1 | 85.6 | 27,619.7 | 177,939.7 | 23.1 |
P value | 0.29 | <0.001 | 0.01 | 0.30 | 0.41 | <0.001 | <0.001 |
Hand surgery | 7.9% | 8.1 | 12.8 | 83.7 | 24,022.8 | 179,370.8 | 23.9 |
Sports | 11.2% | 8.9 | 11.9 | 85.6 | 28,588.9 | 185,902.4 | 21.2 |
Trauma | 21.4% | 7.7 | 13.8 | 85.5 | 20,065.9 | 170,807.6 | 25.6 |
Adult reconstruction | 6.3% | 8.7 | 12.8 | 86.9 | 26,601.5 | 173,280.1 | 22.4 |
None/unknown | 10.9% | 8.5 | 12.0 | 86.4 | 28,173.6 | 166,522.5 | 27.0 |
Continue to: DISCUSSION...
DISCUSSION
Utilization of TSA has continued to rise; however, access to this cost-effective procedure was recently demonstrated to be limited.11 In a separate analysis, we established the continued rise in use of TSA in the Medicare population, coupled with an increase in the number of surgeons routinely performing TSA.2 Multiple analyses have demonstrated the importance of high-volume surgeons and hospitals familiar with the intricacies of shoulder arthroplasty concepts in minimizing complications, improving the quality and decreasing the cost of TSA.6,10,17 Specifically, Singh and colleagues18 demonstrated from a multi-center registry that surgeons and hospitals with greater shoulder arthroplasty volumes had decreased intra-operative blood loss, operative time, and hospital length of stay. As the demand for TSA, both anatomic and reverse, continues to rise, it is imperative that the healthcare delivery system is optimized to provide the best possible care. Before we can determine whether specialized training in shoulder arthroplasty influences surgical outcomes, characteristics and training of surgeons performing TSA should be described.
The number of surgeons performing >10 TSA in the Medicare population rose significantly between 2012 and 2014 (29.3%). However, the number of TSAs per surgeon over this time period remained consistent (approximately 25 per surgeon). Furthermore, the increase in the number of surgeons performing a reportable volume of TSA by 2014 was from the addition of already active surgeons (ie, the growth in TSA was not from the addition of newly trained arthroplasty surgeons but originated from the existing orthopedic surgeon workforce). In a recently published analysis, Somerson and colleagues, 11 using this same dataset, demonstrated persistent limitations in access to high-volume TSA surgeons. In a more recent analysis, we showed that while still lacking for some patients, access to a high-volume TSA surgeon has improved significantly over the past 3 years, with 96.9% of the United States population residing within 200 kilometers of a high-volume TSA surgeon (>20 Medicare cases).2 This analysis validates those findings, with the caveat that the average annual volume per surgeon is not increasing. What remains unknown, due to limitations of this dataset, is how many surgeons are not identified because they are performing ≤10 TSA each year or are performing TSA in non-Medicare patients.
With the specialization of healthcare delivery, specifically in orthopedics, it is imperative that mechanisms for providing specialty-focused care be established. However, the proportion of their practice that surgeons dedicate to TSA was unknown. This study demonstrates that this proportion is increasing. Including non-arthroplasty procedures, more than half (58%) of the procedures performed by this surgeon cohort were shoulder-specific. Furthermore, this analysis demonstrates that surgeons performing TSA have significant case diversity, including nearly half of the cohort performing TKA. Repeated evidence has demonstrated the effect of case volume on improved outcomes following orthopedic procedures.8,19–21 The pre-existing location-based model for delivering orthopedic care supports case diversity; however, this model continues to be challenged with high-volume centers of excellence and patient travel.22–24 Hip and knee arthroplasty experienced a similar surge in demand, with a subsequent shift in care to high-volume surgeons and centers.25 Shoulder and elbow fellowship-trained surgeons would need to nearly quadruple their current Medicare TSA volume to meet the entire current demand for TSA in the Medicare population (and this does not account for TSA performed by very low-volume surgeons not included in this cohort). With increased utilization of TSA, policymakers and the orthopedic community must determine the structure of delivery (centers of excellence or medium-volume disseminated throughout the country) that is optimal.
For those surgeons consistently performing TSA over the study period, fellowship training was diverse. While the current focus in orthopedics is on case volume, research in other specialties, namely general surgery, has provided repeated evidence that surgical specialization (more so than high case volume) provides improved outcomes.26–29Furthermore, Leopold and colleagues30 demonstrated an inverse relationship between competency in performing a procedure and confidence in one’s ability to do so. In their study, educational intervention provided improved competency in the procedure. Less than one-third (29.8%) of TSA in this cohort were performed by a shoulder and elbow fellowship-trained surgeon consistently performing this procedure. Approximately another quarter (26.2%) were performed by consistent TSA surgeons trained in sports surgery. Meanwhile, 34.6% of TSA in this study cohort were performed by a surgeon who did not consistently meet the minimum threshold in all study years (16,435 TSA; 22.8%) or by a surgeon performing TSA without fellowship training (8,489 TSA; 11.8%). There has been a trend toward orthopedic subspecialty training with an increased demand for fellowship-trained surgeons.31 Despite this and the complexities of TSA, many continue to be performed by surgeons with an inconsistent volume and those without arthroplasty-specific fellowship training. The available evidence supports a push toward the fellowship-trained, high-volume TSA surgeon in providing reproducible high-quality shoulder arthroplasty care. For now, that surgeon is more likely to be earlier in his/her career and reside in large, referral-based centers surrounded by other surgeons performing TSA.
These findings must be considered in the light of the study limitations. First, this is a large publicly available database. While this type of database provides a unique opportunity to assess the geographic distributions and characteristics of orthopedic surgeons, specifically those performing TSA, it completely prevents any assessment of the relationship between these findings and quality. As such, while the reader may generate hypotheses regarding the implications of our findings on the quality of TSA delivery, the true effects cannot be determined. In the same vein, for the purpose of privacy, surgeons performing ≤10 TSA were not included in this dataset. This limitation prevents the identification of low-volume TSA surgeons. Also, it is likely that the observed increase in surgeons over time is likely a reflection of small increases in volume for surgeons already performing TSA. Lastly, a web-based search was undertaken to identify surgeons’ self-reported fellowship training. The results of this web-based search could not be validated, and it is possible that fellowship training, or the lack thereof, was mischaracterized and simply not obtainable through a web-based search. Furthermore, it is not possible to fully assess the extent of high-quality TSA training in these various fellowships.
CONCLUSION
In just the past decade, the utilization of TSA in the Medicare population has increased significantly. However, this increase was not achieved by the addition of highly specialized, high-volume surgeons but by the addition of many surgeons performing lower numbers of TSA surgeries. Furthermore, for those performing this cost-effective procedure, TSA constitutes a relatively small proportion of the surgeries they perform. Shoulder and elbow fellowship-trained surgeons currently account for a low percentage of the overall number of surgeons performing TSA. The implications of these findings must be considered and investigated.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
2. Zmistowski B, Padegimas EM, Howley M, Abboud J, Williams G, Namdari S. Trends and Variability in the Use of Total Shoulder Arthroplasty for Medicare Patients. J Am Acad Orthop Surg. 2018;26(4):133-141. doi:10.5435/JAAOS-D-16-00720
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: http://www.jshoulderelbow.org/article/S1058-2746(10)00110-2/abstract.
4. Day JS, Paxton ES, Lau E, Gordon VA, Abboud JA, Williams GR. Use of reverse total shoulder arthroplasty in the Medicare population. J Shoulder Elbow Surg. 2015;24(5):766-772. doi:10.1016/j.jse.2014.12.023.
5. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
6. Westermann RW, Pugely AJ, Martin CT, Gao Y, Wolf BR, Hettrich CM. Reverse shoulder arthroplasty in the United States: A comparison of national volume, patient demographics, complications, and surgical indications. Iowa Orthop J. 2015;35:1-7.
7. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367. doi: http://www.jshoulderelbow.org/article/S1058-2746(14)00036-6/abstract.
8. Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2003;85-A(12):2318-2324.
9. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop Relat Res. 2012;471(2):655-664. doi:10.1007/s11999-012-2481-6.
10. Lyman S, Jones EC, Bach PB, Peterson MGE, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;(432):132-137. doi:10.1097/01.blo.0000150571.51381.9a.
11. Somerson JS, Stein BA, Wirth MA. Distribution of high-volume shoulder arthroplasty surgeons in the United States: Data from the 2014 Medicare provider utilization and payment data release. J Bone Joint Surg Am. 2016;98(18):e77. doi:10.2106/JBJS.15.00776.
12. Fisher ES, Bell J-E, Tomek IM, Esty AR, Goodman DC. Trends and regional variation in hip, knee, and shoulder Replacement. Atlases and Reports. Dartmouth Atlas of Health Care. https://www.dartmouthatlas.org/atlases-and-reports/. Accessed December 14, 2018.
13. Daniels AH, DiGiovanni CW. Is subspecialty fellowship training emerging as a necessary component of contemporary orthopaedic surgery education? J Grad Med Educ. 2014;6(2):218-221. doi:10.4300/JGME-D-14-00120.1.
14. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Physician and other supplier Data 2012 CY 2012. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Tren.... Published October 5, 2015. Accessed July 25, 2016.
15. The Dartmouth Institute for Health Policy and Clinical Practice. Dartmouth Atlas of Health Care. Understanding the Efficiency and Effectiveness of the Health Care System. http://www.dartmouthatlas.org/. Accessed January 31, 2014.
16. United States Department of Commerce. United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed September 30, 2016.
17. Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469(9):2496-2504. doi:10.1007/s11999-011-1811-4.
18. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Bone Joint Surg Am. 2014;23(8):1187-1194. doi:10.1016/j.jse.2013.11.017.
19. Jain N, Pietrobon R, Hocker S, Guller U, Shankar A, Higgins LD. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(3):496-505.
20. Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital patient volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12(3):235-242. doi:10.1016/S0883-5403(97)90018-8.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Bone Joint Surg Am. 2012;21(11):1470-1477. doi:10.1016/j.jse.2011.11.010.
22. FitzGerald JD, Soohoo NF, Losina E, Katz JN. Potential impact on patient residence to hospital travel distance and access to care under a policy of preferential referral to high-volume knee replacement hospitals. Arthritis Care Res. 2012;64(6):890-897. doi:10.1002/acr.21611.
23. Maradit Kremers H, Salduz A, Schleck CD, Larson DR, Berry DJ, Lewallen DG. Referral bias in primary total knee arthroplasty: retrospective analysis of 22,614 surgeries in a tertiary referral center. J Arthroplasty. doi:10.1016/j.arth.2016.08.014.
24. Robinson JC, MacPherson K. Payers test reference pricing and centers of excellence to steer patients to low-price and high-quality providers. Health Affairs. 2012;31(9):2028-2036. doi: 10.1377/hlthaff.2011.1313
25. Laucis NC, Chowdhury M, Dasgupta A, Bhattacharyya T. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am. 2016;98(9):707-712. doi:10.2106/JBJS.15.00399.
26. Anwar S, Fraser S, Hill J. Surgical specialization and training–its relation to clinical outcome for colorectal cancer surgery. J Eval Clin Pract. 2012;18(1):5-11. doi:10.1111/j.1365-2753.2010.01525.x.
27. Snow BW, Catwright PC, Young MD. Does surgical subspecialization in pediatrics provide high-quality, cost-effective patient care? Pediatrics. 1996;97(1):14-17.
28. Smith J a. E, King PM, Lane RHS, Thompson MR. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90(5):583-592. doi:10.1002/bjs.4085.
29. Hall BL, Hsaio EY, Majercik S, Hirbe M, Hamilton BH. The impact of surgeon specialization on patient mortality: Examination of a continuous Herfindahl-Hirschman Index. Ann Surg. 2009;249(5):708-716. doi: 10.1097/SLA.0b013e3181a335f8.
30. Leopold SS, Morgan HD, Kadel NJ, Gardner GC, Schaad DC, Wolf FM. Impact of educational intervention on confidence and competence in the performance of a simple surgical task. J Bone Joint Surg Am. 2005;87(5):1031-1037. doi:10.2106/JBJS.D.02434.
31. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560. doi:10.3928/01477447-20120327-13.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
2. Zmistowski B, Padegimas EM, Howley M, Abboud J, Williams G, Namdari S. Trends and Variability in the Use of Total Shoulder Arthroplasty for Medicare Patients. J Am Acad Orthop Surg. 2018;26(4):133-141. doi:10.5435/JAAOS-D-16-00720
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi: http://www.jshoulderelbow.org/article/S1058-2746(10)00110-2/abstract.
4. Day JS, Paxton ES, Lau E, Gordon VA, Abboud JA, Williams GR. Use of reverse total shoulder arthroplasty in the Medicare population. J Shoulder Elbow Surg. 2015;24(5):766-772. doi:10.1016/j.jse.2014.12.023.
5. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97. doi:10.1016/j.jse.2014.08.026.
6. Westermann RW, Pugely AJ, Martin CT, Gao Y, Wolf BR, Hettrich CM. Reverse shoulder arthroplasty in the United States: A comparison of national volume, patient demographics, complications, and surgical indications. Iowa Orthop J. 2015;35:1-7.
7. Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363-1367. doi: http://www.jshoulderelbow.org/article/S1058-2746(14)00036-6/abstract.
8. Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2003;85-A(12):2318-2324.
9. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop Relat Res. 2012;471(2):655-664. doi:10.1007/s11999-012-2481-6.
10. Lyman S, Jones EC, Bach PB, Peterson MGE, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;(432):132-137. doi:10.1097/01.blo.0000150571.51381.9a.
11. Somerson JS, Stein BA, Wirth MA. Distribution of high-volume shoulder arthroplasty surgeons in the United States: Data from the 2014 Medicare provider utilization and payment data release. J Bone Joint Surg Am. 2016;98(18):e77. doi:10.2106/JBJS.15.00776.
12. Fisher ES, Bell J-E, Tomek IM, Esty AR, Goodman DC. Trends and regional variation in hip, knee, and shoulder Replacement. Atlases and Reports. Dartmouth Atlas of Health Care. https://www.dartmouthatlas.org/atlases-and-reports/. Accessed December 14, 2018.
13. Daniels AH, DiGiovanni CW. Is subspecialty fellowship training emerging as a necessary component of contemporary orthopaedic surgery education? J Grad Med Educ. 2014;6(2):218-221. doi:10.4300/JGME-D-14-00120.1.
14. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Physician and other supplier Data 2012 CY 2012. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Tren.... Published October 5, 2015. Accessed July 25, 2016.
15. The Dartmouth Institute for Health Policy and Clinical Practice. Dartmouth Atlas of Health Care. Understanding the Efficiency and Effectiveness of the Health Care System. http://www.dartmouthatlas.org/. Accessed January 31, 2014.
16. United States Department of Commerce. United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed September 30, 2016.
17. Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469(9):2496-2504. doi:10.1007/s11999-011-1811-4.
18. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Bone Joint Surg Am. 2014;23(8):1187-1194. doi:10.1016/j.jse.2013.11.017.
19. Jain N, Pietrobon R, Hocker S, Guller U, Shankar A, Higgins LD. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2004;86(3):496-505.
20. Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital patient volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12(3):235-242. doi:10.1016/S0883-5403(97)90018-8.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Bone Joint Surg Am. 2012;21(11):1470-1477. doi:10.1016/j.jse.2011.11.010.
22. FitzGerald JD, Soohoo NF, Losina E, Katz JN. Potential impact on patient residence to hospital travel distance and access to care under a policy of preferential referral to high-volume knee replacement hospitals. Arthritis Care Res. 2012;64(6):890-897. doi:10.1002/acr.21611.
23. Maradit Kremers H, Salduz A, Schleck CD, Larson DR, Berry DJ, Lewallen DG. Referral bias in primary total knee arthroplasty: retrospective analysis of 22,614 surgeries in a tertiary referral center. J Arthroplasty. doi:10.1016/j.arth.2016.08.014.
24. Robinson JC, MacPherson K. Payers test reference pricing and centers of excellence to steer patients to low-price and high-quality providers. Health Affairs. 2012;31(9):2028-2036. doi: 10.1377/hlthaff.2011.1313
25. Laucis NC, Chowdhury M, Dasgupta A, Bhattacharyya T. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am. 2016;98(9):707-712. doi:10.2106/JBJS.15.00399.
26. Anwar S, Fraser S, Hill J. Surgical specialization and training–its relation to clinical outcome for colorectal cancer surgery. J Eval Clin Pract. 2012;18(1):5-11. doi:10.1111/j.1365-2753.2010.01525.x.
27. Snow BW, Catwright PC, Young MD. Does surgical subspecialization in pediatrics provide high-quality, cost-effective patient care? Pediatrics. 1996;97(1):14-17.
28. Smith J a. E, King PM, Lane RHS, Thompson MR. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90(5):583-592. doi:10.1002/bjs.4085.
29. Hall BL, Hsaio EY, Majercik S, Hirbe M, Hamilton BH. The impact of surgeon specialization on patient mortality: Examination of a continuous Herfindahl-Hirschman Index. Ann Surg. 2009;249(5):708-716. doi: 10.1097/SLA.0b013e3181a335f8.
30. Leopold SS, Morgan HD, Kadel NJ, Gardner GC, Schaad DC, Wolf FM. Impact of educational intervention on confidence and competence in the performance of a simple surgical task. J Bone Joint Surg Am. 2005;87(5):1031-1037. doi:10.2106/JBJS.D.02434.
31. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560. doi:10.3928/01477447-20120327-13.
TAKE-HOME POINTS
- Between 2012 and 2014, 1,374 surgeons performed >10 total shoulder arthroplasties (TSA) in Medicare patients in at least one year.
- From 2012 to 2014, the number of surgeons performing at least 10 TSA in Medicare patients increased from 834 to 1,078, while the number of TSA increased from 21,137 (25.3 per surgeon) to 26,765 (24.9 per surgeon).
- Many of these surgeons had a diverse surgical practice, with nearly one-half performing total knee arthroplasty, one-third performing non-arthroplasty knee surgeries, and >80% performing non-arthroplasty shoulder procedures.
- Only one-third of these surgeons had formal fellowship training specific to shoulder and elbow.
- In order for the current supply of shoulder and elbow fellowship-trained surgeons to meet the Medicare TSA demand, each currently practicing fellowship graduate would have to perform 140.6 TSA in Medicare patients annually.
Can robotics reduce hepatic surgery conversions?
The conversion rate from minimally invasive to open surgery for liver resection has been known to be high, but researchers from the University of Illinois, Chicago, have reported that conversion rates are considerably lower in robot-assisted liver resections, which may ultimately improve survival and complication rates.
Their study, published in the International Journal of Medical Robotics and Computer Assisted Surgery, found that the overall conversion rate of robot-assisted to open surgery for liver resection was 4.4%, considerably lower than that for the pure laparoscopic approach. “The robotic assist could potentially help in decreasing the conversion rate,” said Federico Gheza, MD, and his coauthors. They claimed that this is the first paper to focus on reasons for conversion from robot-assisted liver resection to open surgery.
The study findings are based on a systematic review of 29 series of 1,091 patients who had robot-assisted liver resection, including Dr. Gheza’s and his coauthors’ own series of 139 patients who had the operation from 2007 to 2017. The series were published from 2009 to 2017.
Dr. Gheza’s and his coauthors’ series had a conversion rate of 7.9%. When their results were included with those of the previously published studies, the conversion rate was 4.8%.
Dr. Gheza and his coauthors noted that the conversion rate for minimally invasive hepatic operations is one of the highest among all types of laparoscopic operations, with rates reported as high as 23% in published series (Ann Surg. 2016;263:761-77). “More importantly, preliminary database analysis and large series review suggested an association between conversion and higher morbidity and mortality,” the study noted.
The purpose of the systematic review was to better understand reasons for conversion from robotic to open in liver resections, Dr. Gheza and his coauthors said. They noted a study of 2,861 laparoscopic resections reported that more than one-third of patients were converted for bleeding, and almost one-fifth (18.9%) for peritoneal adhesions (Ann Surg. 2017;268:1051-7). “In the whole literature on hepatic robotic surgery, no conversion due to adhesion was reported,” Dr. Gheza and his coauthors said.
A subanalysis of nine series that included both robotic (360 patients) and laparoscopic (462 patients) surgeries found conversion rates of 2.5% vs. 8%, respectively (P less than .01).
Dr. Gheza and his coauthors called for a prospective trial to further define the impact of robotic surgery on conversions, but the clinical implications of conversions to open surgery in minimally invasive hepatic surgery remain unclear.
Dr. Gheza disclosed relationships with Medtronic. Coauthor P. C. Giulianotti, MD, reported financial relationships with Intuitive, Covidien/Medtronic and Ethicon/Johnson & Johnson. The other coauthors had no relationships to disclose.
SOURCE: Gheza F et al. Intl J Med Robotics Comp Assisted Surg. 2018; doi:10.1002/rcs.1976.
The conversion rate from minimally invasive to open surgery for liver resection has been known to be high, but researchers from the University of Illinois, Chicago, have reported that conversion rates are considerably lower in robot-assisted liver resections, which may ultimately improve survival and complication rates.
Their study, published in the International Journal of Medical Robotics and Computer Assisted Surgery, found that the overall conversion rate of robot-assisted to open surgery for liver resection was 4.4%, considerably lower than that for the pure laparoscopic approach. “The robotic assist could potentially help in decreasing the conversion rate,” said Federico Gheza, MD, and his coauthors. They claimed that this is the first paper to focus on reasons for conversion from robot-assisted liver resection to open surgery.
The study findings are based on a systematic review of 29 series of 1,091 patients who had robot-assisted liver resection, including Dr. Gheza’s and his coauthors’ own series of 139 patients who had the operation from 2007 to 2017. The series were published from 2009 to 2017.
Dr. Gheza’s and his coauthors’ series had a conversion rate of 7.9%. When their results were included with those of the previously published studies, the conversion rate was 4.8%.
Dr. Gheza and his coauthors noted that the conversion rate for minimally invasive hepatic operations is one of the highest among all types of laparoscopic operations, with rates reported as high as 23% in published series (Ann Surg. 2016;263:761-77). “More importantly, preliminary database analysis and large series review suggested an association between conversion and higher morbidity and mortality,” the study noted.
The purpose of the systematic review was to better understand reasons for conversion from robotic to open in liver resections, Dr. Gheza and his coauthors said. They noted a study of 2,861 laparoscopic resections reported that more than one-third of patients were converted for bleeding, and almost one-fifth (18.9%) for peritoneal adhesions (Ann Surg. 2017;268:1051-7). “In the whole literature on hepatic robotic surgery, no conversion due to adhesion was reported,” Dr. Gheza and his coauthors said.
A subanalysis of nine series that included both robotic (360 patients) and laparoscopic (462 patients) surgeries found conversion rates of 2.5% vs. 8%, respectively (P less than .01).
Dr. Gheza and his coauthors called for a prospective trial to further define the impact of robotic surgery on conversions, but the clinical implications of conversions to open surgery in minimally invasive hepatic surgery remain unclear.
Dr. Gheza disclosed relationships with Medtronic. Coauthor P. C. Giulianotti, MD, reported financial relationships with Intuitive, Covidien/Medtronic and Ethicon/Johnson & Johnson. The other coauthors had no relationships to disclose.
SOURCE: Gheza F et al. Intl J Med Robotics Comp Assisted Surg. 2018; doi:10.1002/rcs.1976.
The conversion rate from minimally invasive to open surgery for liver resection has been known to be high, but researchers from the University of Illinois, Chicago, have reported that conversion rates are considerably lower in robot-assisted liver resections, which may ultimately improve survival and complication rates.
Their study, published in the International Journal of Medical Robotics and Computer Assisted Surgery, found that the overall conversion rate of robot-assisted to open surgery for liver resection was 4.4%, considerably lower than that for the pure laparoscopic approach. “The robotic assist could potentially help in decreasing the conversion rate,” said Federico Gheza, MD, and his coauthors. They claimed that this is the first paper to focus on reasons for conversion from robot-assisted liver resection to open surgery.
The study findings are based on a systematic review of 29 series of 1,091 patients who had robot-assisted liver resection, including Dr. Gheza’s and his coauthors’ own series of 139 patients who had the operation from 2007 to 2017. The series were published from 2009 to 2017.
Dr. Gheza’s and his coauthors’ series had a conversion rate of 7.9%. When their results were included with those of the previously published studies, the conversion rate was 4.8%.
Dr. Gheza and his coauthors noted that the conversion rate for minimally invasive hepatic operations is one of the highest among all types of laparoscopic operations, with rates reported as high as 23% in published series (Ann Surg. 2016;263:761-77). “More importantly, preliminary database analysis and large series review suggested an association between conversion and higher morbidity and mortality,” the study noted.
The purpose of the systematic review was to better understand reasons for conversion from robotic to open in liver resections, Dr. Gheza and his coauthors said. They noted a study of 2,861 laparoscopic resections reported that more than one-third of patients were converted for bleeding, and almost one-fifth (18.9%) for peritoneal adhesions (Ann Surg. 2017;268:1051-7). “In the whole literature on hepatic robotic surgery, no conversion due to adhesion was reported,” Dr. Gheza and his coauthors said.
A subanalysis of nine series that included both robotic (360 patients) and laparoscopic (462 patients) surgeries found conversion rates of 2.5% vs. 8%, respectively (P less than .01).
Dr. Gheza and his coauthors called for a prospective trial to further define the impact of robotic surgery on conversions, but the clinical implications of conversions to open surgery in minimally invasive hepatic surgery remain unclear.
Dr. Gheza disclosed relationships with Medtronic. Coauthor P. C. Giulianotti, MD, reported financial relationships with Intuitive, Covidien/Medtronic and Ethicon/Johnson & Johnson. The other coauthors had no relationships to disclose.
SOURCE: Gheza F et al. Intl J Med Robotics Comp Assisted Surg. 2018; doi:10.1002/rcs.1976.
FROM THE INTERNATIONAL JOURNAL OF MEDICAL ROBOTICS AND COMPUTER ASSISTED SURGERY
Key clinical point: Robotic liver resection has the potential to lower conversion rates.
Major finding: The conversion rate in robot-assisted liver resection was 4.8%.
Study details: Systematic review of 29 series of 1,091 robot-assisted liver resections published from 2009 to 2017, including the authors’ own cohort of 139 consecutive patients from May 2007 to September 2017.
Disclosures: Dr. Gheza disclosed being a consultant for Medtronic. Coauthor P. C. Giulianotti, MD, reported financial relationships with Intuitive, Covidien/Medtronic and Ethicon/Johnson & Johnson. The other coauthors had no relationships to disclose.
Source: Gheza F et al. Intl J Med Robotics Comp Assisted Surg. 2018. doi: 10.1002/rcs.1976.
Positive results reported for ixekizumab versus adalimumab in PsA
Eli Lilly and Co. has announced positive results from the phase 3b/4, multicenter, randomized, open-label, parallel-group SPIRIT-H2H trial, which compared ixekizumab (Taltz) with adalimumab (Humira) in patients with psoriatic arthritis who had previously not taken a biologic disease-modifying antirheumatic drug.
The 52-week study included 566 patients with psoriatic arthritis. Patients received either ixekizumab at 80 mg every 4 weeks after a 160-mg loading dose or adalimumab at 40 mg every 2 weeks. The primary endpoint was the proportion of patients achieving at least a 50% reduction in American College of Rheumatology (ACR50) criteria at 24 weeks.
After 24 weeks, patients in the ixekizumab group were more likely to achieve ACR50, compared with those in the adalimumab group. In addition, patients receiving ixekizumab were more likely to achieve 100% skin clearance according to the Psoriasis Area and Severity Index. Ixekizumab also met all secondary trial endpoints.
“The positive results from the SPIRIT-H2H trial reinforce that Taltz effectively treats the debilitating joint signs and symptoms of active psoriatic arthritis, while also providing skin clearance. These results provide evidence that Taltz can be used as a first-line biologic treatment for patients with active psoriatic arthritis,” Lotus Mallbris, MD, PhD, vice president of immunology development at Lilly, said in the press release.
More detailed results will be presented at meetings and published in peer-reviewed journals in 2019, the company said.
Eli Lilly and Co. has announced positive results from the phase 3b/4, multicenter, randomized, open-label, parallel-group SPIRIT-H2H trial, which compared ixekizumab (Taltz) with adalimumab (Humira) in patients with psoriatic arthritis who had previously not taken a biologic disease-modifying antirheumatic drug.
The 52-week study included 566 patients with psoriatic arthritis. Patients received either ixekizumab at 80 mg every 4 weeks after a 160-mg loading dose or adalimumab at 40 mg every 2 weeks. The primary endpoint was the proportion of patients achieving at least a 50% reduction in American College of Rheumatology (ACR50) criteria at 24 weeks.
After 24 weeks, patients in the ixekizumab group were more likely to achieve ACR50, compared with those in the adalimumab group. In addition, patients receiving ixekizumab were more likely to achieve 100% skin clearance according to the Psoriasis Area and Severity Index. Ixekizumab also met all secondary trial endpoints.
“The positive results from the SPIRIT-H2H trial reinforce that Taltz effectively treats the debilitating joint signs and symptoms of active psoriatic arthritis, while also providing skin clearance. These results provide evidence that Taltz can be used as a first-line biologic treatment for patients with active psoriatic arthritis,” Lotus Mallbris, MD, PhD, vice president of immunology development at Lilly, said in the press release.
More detailed results will be presented at meetings and published in peer-reviewed journals in 2019, the company said.
Eli Lilly and Co. has announced positive results from the phase 3b/4, multicenter, randomized, open-label, parallel-group SPIRIT-H2H trial, which compared ixekizumab (Taltz) with adalimumab (Humira) in patients with psoriatic arthritis who had previously not taken a biologic disease-modifying antirheumatic drug.
The 52-week study included 566 patients with psoriatic arthritis. Patients received either ixekizumab at 80 mg every 4 weeks after a 160-mg loading dose or adalimumab at 40 mg every 2 weeks. The primary endpoint was the proportion of patients achieving at least a 50% reduction in American College of Rheumatology (ACR50) criteria at 24 weeks.
After 24 weeks, patients in the ixekizumab group were more likely to achieve ACR50, compared with those in the adalimumab group. In addition, patients receiving ixekizumab were more likely to achieve 100% skin clearance according to the Psoriasis Area and Severity Index. Ixekizumab also met all secondary trial endpoints.
“The positive results from the SPIRIT-H2H trial reinforce that Taltz effectively treats the debilitating joint signs and symptoms of active psoriatic arthritis, while also providing skin clearance. These results provide evidence that Taltz can be used as a first-line biologic treatment for patients with active psoriatic arthritis,” Lotus Mallbris, MD, PhD, vice president of immunology development at Lilly, said in the press release.
More detailed results will be presented at meetings and published in peer-reviewed journals in 2019, the company said.
Natural killer cells implicated in psoriatic arthritis
CHICAGO –
This natural killer cell interacts with the CD94/NKG2A receptor, part of a system believed to have been in place in humans for more than 90 million years.
“We believe there is a possible role for the innate immune system in the development of psoriatic arthritis and its distinction from psoriasis,” Vinod Chandran, MD, PhD, declared at the annual meeting of the American College of Rheumatology.
Dr. Chandran, of the University of Toronto, presented an analysis of a discovery cohort comprising 1,155 patients with dermatologist-diagnosed psoriasis of greater than 10 years duration, 664 rheumatologist-diagnosed psoriatic arthritis patients, and 3,118 controls, all participants in the International Psoriasis and Arthritis Research Team program. These findings were then independently confirmed in a separate University of Toronto replication cohort of 659 psoriasis patients, 1,177 psoriatic arthritis patients of European ancestry, and 1,096 controls.
By way of background, the rheumatologist explained that psoriasis and psoriatic arthritis are known to differ in terms of their genetic architecture, the biggest difference being in the HLA class I region, where HLA-C predominates in psoriasis and HLA-B in psoriatic arthritis. These structurally unrelated forms of HLA class I are known to educate natural killer cells and shape their function. Dr. Chandran and his coinvestigators were eager to shed new light on the mechanisms by which this leads to rheumatic disease.
Humans can be divided into three groups based upon whether they are HLA-B21 methionine/methionine (M/M), HLA-B21 M/threonine (T), or HLA-B21 T/T. The B21 M types educate CD94/NKG2A-positive natural killer cells by delivering functional peptides to the CD94/NKG2A receptor, while the B21 T/T version does not.
In the discovery cohort, individuals with psoriatic arthritis turned out to be 36% more likely to be HLA-B21 M/M or HLA-B21 M/T than were the psoriasis patients, while the psoriasis patients were 22% less likely to be B21 M–positive than controls. These relationships were confirmed in the replication cohort, where psoriatic arthritis patients were 40% more likely to be B21 M–positive than psoriasis patients, and psoriasis patients were 18% less likely to be B21 M–positive than controls, with all of these differences being statistically significant.
While this is translational science, Dr. Chandran explained that it has important clinical implications. He and his coinvestigators are developing a genetic marker panel to differentiate psoriatic arthritis from psoriasis, as are other research groups. And the Toronto investigators are now convinced that including HLA-B21 M/M and HLA-B21 M/T in their evolving genetic test is worthwhile in terms of boosting the test’s predictive power. The 36%-40% increased risk of psoriatic arthritis associated with B21 M–positivity isn’t sufficiently large for it to serve as a standalone test, but when the genetic test panel is finalized and the investigators can evaluate its positive and negative predictive value, it will be clear that the B21 M component will provide added value, he predicted.
Because psoriatic arthritis can take on a variety of disparate forms clinically, Dr. Chandran and his coworkers believe their genetic test will prove most useful for nonrheumatologists, especially dermatologists and primary care physicians.
He reported having no relevant financial relationships regarding this study, funded by the Canadian Institutes of Health Research, the Krembil Foundation, and the Arthritis Foundation.
SOURCE: Chandran V et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 2787.
CHICAGO –
This natural killer cell interacts with the CD94/NKG2A receptor, part of a system believed to have been in place in humans for more than 90 million years.
“We believe there is a possible role for the innate immune system in the development of psoriatic arthritis and its distinction from psoriasis,” Vinod Chandran, MD, PhD, declared at the annual meeting of the American College of Rheumatology.
Dr. Chandran, of the University of Toronto, presented an analysis of a discovery cohort comprising 1,155 patients with dermatologist-diagnosed psoriasis of greater than 10 years duration, 664 rheumatologist-diagnosed psoriatic arthritis patients, and 3,118 controls, all participants in the International Psoriasis and Arthritis Research Team program. These findings were then independently confirmed in a separate University of Toronto replication cohort of 659 psoriasis patients, 1,177 psoriatic arthritis patients of European ancestry, and 1,096 controls.
By way of background, the rheumatologist explained that psoriasis and psoriatic arthritis are known to differ in terms of their genetic architecture, the biggest difference being in the HLA class I region, where HLA-C predominates in psoriasis and HLA-B in psoriatic arthritis. These structurally unrelated forms of HLA class I are known to educate natural killer cells and shape their function. Dr. Chandran and his coinvestigators were eager to shed new light on the mechanisms by which this leads to rheumatic disease.
Humans can be divided into three groups based upon whether they are HLA-B21 methionine/methionine (M/M), HLA-B21 M/threonine (T), or HLA-B21 T/T. The B21 M types educate CD94/NKG2A-positive natural killer cells by delivering functional peptides to the CD94/NKG2A receptor, while the B21 T/T version does not.
In the discovery cohort, individuals with psoriatic arthritis turned out to be 36% more likely to be HLA-B21 M/M or HLA-B21 M/T than were the psoriasis patients, while the psoriasis patients were 22% less likely to be B21 M–positive than controls. These relationships were confirmed in the replication cohort, where psoriatic arthritis patients were 40% more likely to be B21 M–positive than psoriasis patients, and psoriasis patients were 18% less likely to be B21 M–positive than controls, with all of these differences being statistically significant.
While this is translational science, Dr. Chandran explained that it has important clinical implications. He and his coinvestigators are developing a genetic marker panel to differentiate psoriatic arthritis from psoriasis, as are other research groups. And the Toronto investigators are now convinced that including HLA-B21 M/M and HLA-B21 M/T in their evolving genetic test is worthwhile in terms of boosting the test’s predictive power. The 36%-40% increased risk of psoriatic arthritis associated with B21 M–positivity isn’t sufficiently large for it to serve as a standalone test, but when the genetic test panel is finalized and the investigators can evaluate its positive and negative predictive value, it will be clear that the B21 M component will provide added value, he predicted.
Because psoriatic arthritis can take on a variety of disparate forms clinically, Dr. Chandran and his coworkers believe their genetic test will prove most useful for nonrheumatologists, especially dermatologists and primary care physicians.
He reported having no relevant financial relationships regarding this study, funded by the Canadian Institutes of Health Research, the Krembil Foundation, and the Arthritis Foundation.
SOURCE: Chandran V et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 2787.
CHICAGO –
This natural killer cell interacts with the CD94/NKG2A receptor, part of a system believed to have been in place in humans for more than 90 million years.
“We believe there is a possible role for the innate immune system in the development of psoriatic arthritis and its distinction from psoriasis,” Vinod Chandran, MD, PhD, declared at the annual meeting of the American College of Rheumatology.
Dr. Chandran, of the University of Toronto, presented an analysis of a discovery cohort comprising 1,155 patients with dermatologist-diagnosed psoriasis of greater than 10 years duration, 664 rheumatologist-diagnosed psoriatic arthritis patients, and 3,118 controls, all participants in the International Psoriasis and Arthritis Research Team program. These findings were then independently confirmed in a separate University of Toronto replication cohort of 659 psoriasis patients, 1,177 psoriatic arthritis patients of European ancestry, and 1,096 controls.
By way of background, the rheumatologist explained that psoriasis and psoriatic arthritis are known to differ in terms of their genetic architecture, the biggest difference being in the HLA class I region, where HLA-C predominates in psoriasis and HLA-B in psoriatic arthritis. These structurally unrelated forms of HLA class I are known to educate natural killer cells and shape their function. Dr. Chandran and his coinvestigators were eager to shed new light on the mechanisms by which this leads to rheumatic disease.
Humans can be divided into three groups based upon whether they are HLA-B21 methionine/methionine (M/M), HLA-B21 M/threonine (T), or HLA-B21 T/T. The B21 M types educate CD94/NKG2A-positive natural killer cells by delivering functional peptides to the CD94/NKG2A receptor, while the B21 T/T version does not.
In the discovery cohort, individuals with psoriatic arthritis turned out to be 36% more likely to be HLA-B21 M/M or HLA-B21 M/T than were the psoriasis patients, while the psoriasis patients were 22% less likely to be B21 M–positive than controls. These relationships were confirmed in the replication cohort, where psoriatic arthritis patients were 40% more likely to be B21 M–positive than psoriasis patients, and psoriasis patients were 18% less likely to be B21 M–positive than controls, with all of these differences being statistically significant.
While this is translational science, Dr. Chandran explained that it has important clinical implications. He and his coinvestigators are developing a genetic marker panel to differentiate psoriatic arthritis from psoriasis, as are other research groups. And the Toronto investigators are now convinced that including HLA-B21 M/M and HLA-B21 M/T in their evolving genetic test is worthwhile in terms of boosting the test’s predictive power. The 36%-40% increased risk of psoriatic arthritis associated with B21 M–positivity isn’t sufficiently large for it to serve as a standalone test, but when the genetic test panel is finalized and the investigators can evaluate its positive and negative predictive value, it will be clear that the B21 M component will provide added value, he predicted.
Because psoriatic arthritis can take on a variety of disparate forms clinically, Dr. Chandran and his coworkers believe their genetic test will prove most useful for nonrheumatologists, especially dermatologists and primary care physicians.
He reported having no relevant financial relationships regarding this study, funded by the Canadian Institutes of Health Research, the Krembil Foundation, and the Arthritis Foundation.
SOURCE: Chandran V et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 2787.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: A genetic panel designed to differentiate psoriatic arthritis from psoriasis is drawing closer to fruition.
Major finding: The prevalence of HLA-B21 methionine is increased by roughly 40% in patients with psoriatic arthritis, compared with psoriasis patients.
Study details: This translational study included two independent cohorts totaling 1,814 psoriasis patients, 1,841 with psoriatic arthritis, and 4,214 controls.
Disclosures: The presenter reported having no relevant financial relationships regarding this study, which was funded by the Canadian Institutes of Health Research, the Krembil Foundation, and the Arthritis Foundation.
Source: Chandran V et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 2787.