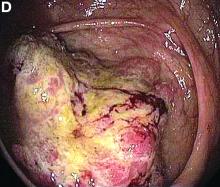
User login
Study reveals long-term survival in MM patients
A retrospective study suggests one in seven patients with newly diagnosed multiple myeloma (MM) who are eligible for transplant may live at least as long as similar individuals in the general population.
The study included more than 7,000 MM patients, and 14.3% of those patients were able to meet or exceed their expected survival based on data from matched subjects in the general population.
Researchers believe that figure may be even higher today, as more than 90% of patients in this study were treated in the era before novel therapies became available.
Saad Z. Usmani, MD, of the Levine Cancer Institute/Atrium Health in Charlotte, North Carolina, and his colleagues described this study in Blood Cancer Journal.
The researchers studied 7,291 patients with newly diagnosed MM who were up to 75 years old and eligible for treatment with high-dose melphalan and autologous stem cell transplant. The patients were treated on clinical trials in 10 countries.
Factors associated with survival
Patients who had achieved a complete response (CR) 1 year after diagnosis had better median progression-free survival (PFS) than patients who did not achieve a CR—3.3 years and 2.6 years, respectively (P<0.0001).
Patients with a CR also had better median overall survival (OS)—8.5 years and 6.3 years, respectively (P<0.0001).
The identification of early CR as a predictor of PFS and OS “underscores the importance of depth of response as we explore novel regimens for newly diagnosed MM along with MRD [minimal residual disease] endpoints,” Dr. Usmani and his colleagues wrote.
They did acknowledge, however, that the patients studied were a selected group eligible for transplant and treated on trials.
Dr. Usmani and his colleagues also performed multivariate analyses to assess clinical variables at diagnosis associated with 10-year survival as compared with 2-year death. The results indicated that patients were less likely to be alive at 10 years if they:
- Were older than 65 (odds ratio [OR]for death, 1.87, P=0.002)
- Had an IgA isotype (OR=1.53; P=0.004)
- Had an albumin level lower than 3.5 g/dL (OR=1.36; P=0.023)
- Had a beta-2 microglobulin level of at least 3.5 mg/dL (OR=1.86; P<0.001)
- Had a serum creatinine level of at least 2 mg/dL (OR=1.77; P=0.005)
- Had a hemoglobin level below 10 g/dL (OR=1.55; P=0.003)
- Had a platelet count below 150,000/μL (OR=2.26; P<0.001).
Cytogenetic abnormalities did not independently predict long-term survival, but these abnormalities were obtained only by conventional band karyotyping and were not available for some patients.
Comparison to general population
Overall, the MM patients had a relative survival of about 0.9 compared with the matched general population. Relative survival was the ratio of observed survival among MM patients to expected survival in a population with comparable characteristics, such as nationality, age, and sex.
With follow-up out to about 20 years, the cure fraction—or the proportion of patients achieving or exceeding expected survival compared with the matched general population—was 14.3%.
The researchers noted that recent therapeutic advances “have re-ignited the debate on possible functional curability of a subset of MM patients. [T]here are perhaps more effective drugs and drug classes in the clinician’s armamentarium than [were] available for MM patients being treated in the 1990s or even early 2000s.”
“This may mean that the depth of response after induction therapy may continue to improve over time, potentially further improving the PFS/OS of [the] biologic subset who previously achieved [a partial response] yet had good long-term survival.”
Dr. Usmani reported relationships with AbbVie, Amgen, BMS, Celgene, Janssen, Takeda, Sanofi, SkylineDx, Array Biopharma, and Pharmacyclics.
A retrospective study suggests one in seven patients with newly diagnosed multiple myeloma (MM) who are eligible for transplant may live at least as long as similar individuals in the general population.
The study included more than 7,000 MM patients, and 14.3% of those patients were able to meet or exceed their expected survival based on data from matched subjects in the general population.
Researchers believe that figure may be even higher today, as more than 90% of patients in this study were treated in the era before novel therapies became available.
Saad Z. Usmani, MD, of the Levine Cancer Institute/Atrium Health in Charlotte, North Carolina, and his colleagues described this study in Blood Cancer Journal.
The researchers studied 7,291 patients with newly diagnosed MM who were up to 75 years old and eligible for treatment with high-dose melphalan and autologous stem cell transplant. The patients were treated on clinical trials in 10 countries.
Factors associated with survival
Patients who had achieved a complete response (CR) 1 year after diagnosis had better median progression-free survival (PFS) than patients who did not achieve a CR—3.3 years and 2.6 years, respectively (P<0.0001).
Patients with a CR also had better median overall survival (OS)—8.5 years and 6.3 years, respectively (P<0.0001).
The identification of early CR as a predictor of PFS and OS “underscores the importance of depth of response as we explore novel regimens for newly diagnosed MM along with MRD [minimal residual disease] endpoints,” Dr. Usmani and his colleagues wrote.
They did acknowledge, however, that the patients studied were a selected group eligible for transplant and treated on trials.
Dr. Usmani and his colleagues also performed multivariate analyses to assess clinical variables at diagnosis associated with 10-year survival as compared with 2-year death. The results indicated that patients were less likely to be alive at 10 years if they:
- Were older than 65 (odds ratio [OR]for death, 1.87, P=0.002)
- Had an IgA isotype (OR=1.53; P=0.004)
- Had an albumin level lower than 3.5 g/dL (OR=1.36; P=0.023)
- Had a beta-2 microglobulin level of at least 3.5 mg/dL (OR=1.86; P<0.001)
- Had a serum creatinine level of at least 2 mg/dL (OR=1.77; P=0.005)
- Had a hemoglobin level below 10 g/dL (OR=1.55; P=0.003)
- Had a platelet count below 150,000/μL (OR=2.26; P<0.001).
Cytogenetic abnormalities did not independently predict long-term survival, but these abnormalities were obtained only by conventional band karyotyping and were not available for some patients.
Comparison to general population
Overall, the MM patients had a relative survival of about 0.9 compared with the matched general population. Relative survival was the ratio of observed survival among MM patients to expected survival in a population with comparable characteristics, such as nationality, age, and sex.
With follow-up out to about 20 years, the cure fraction—or the proportion of patients achieving or exceeding expected survival compared with the matched general population—was 14.3%.
The researchers noted that recent therapeutic advances “have re-ignited the debate on possible functional curability of a subset of MM patients. [T]here are perhaps more effective drugs and drug classes in the clinician’s armamentarium than [were] available for MM patients being treated in the 1990s or even early 2000s.”
“This may mean that the depth of response after induction therapy may continue to improve over time, potentially further improving the PFS/OS of [the] biologic subset who previously achieved [a partial response] yet had good long-term survival.”
Dr. Usmani reported relationships with AbbVie, Amgen, BMS, Celgene, Janssen, Takeda, Sanofi, SkylineDx, Array Biopharma, and Pharmacyclics.
A retrospective study suggests one in seven patients with newly diagnosed multiple myeloma (MM) who are eligible for transplant may live at least as long as similar individuals in the general population.
The study included more than 7,000 MM patients, and 14.3% of those patients were able to meet or exceed their expected survival based on data from matched subjects in the general population.
Researchers believe that figure may be even higher today, as more than 90% of patients in this study were treated in the era before novel therapies became available.
Saad Z. Usmani, MD, of the Levine Cancer Institute/Atrium Health in Charlotte, North Carolina, and his colleagues described this study in Blood Cancer Journal.
The researchers studied 7,291 patients with newly diagnosed MM who were up to 75 years old and eligible for treatment with high-dose melphalan and autologous stem cell transplant. The patients were treated on clinical trials in 10 countries.
Factors associated with survival
Patients who had achieved a complete response (CR) 1 year after diagnosis had better median progression-free survival (PFS) than patients who did not achieve a CR—3.3 years and 2.6 years, respectively (P<0.0001).
Patients with a CR also had better median overall survival (OS)—8.5 years and 6.3 years, respectively (P<0.0001).
The identification of early CR as a predictor of PFS and OS “underscores the importance of depth of response as we explore novel regimens for newly diagnosed MM along with MRD [minimal residual disease] endpoints,” Dr. Usmani and his colleagues wrote.
They did acknowledge, however, that the patients studied were a selected group eligible for transplant and treated on trials.
Dr. Usmani and his colleagues also performed multivariate analyses to assess clinical variables at diagnosis associated with 10-year survival as compared with 2-year death. The results indicated that patients were less likely to be alive at 10 years if they:
- Were older than 65 (odds ratio [OR]for death, 1.87, P=0.002)
- Had an IgA isotype (OR=1.53; P=0.004)
- Had an albumin level lower than 3.5 g/dL (OR=1.36; P=0.023)
- Had a beta-2 microglobulin level of at least 3.5 mg/dL (OR=1.86; P<0.001)
- Had a serum creatinine level of at least 2 mg/dL (OR=1.77; P=0.005)
- Had a hemoglobin level below 10 g/dL (OR=1.55; P=0.003)
- Had a platelet count below 150,000/μL (OR=2.26; P<0.001).
Cytogenetic abnormalities did not independently predict long-term survival, but these abnormalities were obtained only by conventional band karyotyping and were not available for some patients.
Comparison to general population
Overall, the MM patients had a relative survival of about 0.9 compared with the matched general population. Relative survival was the ratio of observed survival among MM patients to expected survival in a population with comparable characteristics, such as nationality, age, and sex.
With follow-up out to about 20 years, the cure fraction—or the proportion of patients achieving or exceeding expected survival compared with the matched general population—was 14.3%.
The researchers noted that recent therapeutic advances “have re-ignited the debate on possible functional curability of a subset of MM patients. [T]here are perhaps more effective drugs and drug classes in the clinician’s armamentarium than [were] available for MM patients being treated in the 1990s or even early 2000s.”
“This may mean that the depth of response after induction therapy may continue to improve over time, potentially further improving the PFS/OS of [the] biologic subset who previously achieved [a partial response] yet had good long-term survival.”
Dr. Usmani reported relationships with AbbVie, Amgen, BMS, Celgene, Janssen, Takeda, Sanofi, SkylineDx, Array Biopharma, and Pharmacyclics.
The case for longer treatment in MM: Part 1
In Part 1 of this editorial, Katja Weisel, MD, of University Hospital Tubingen in Germany, describes the benefits of longer treatment in patients with multiple myeloma.
Despite recent progress in advancing the care of patients with multiple myeloma (MM), this cancer remains incurable.
Although novel combination regimens have driven major improvements in patient outcomes, most MM patients still experience multiple relapses, even those who respond to treatment initially.1
Historically, MM was treated for a fixed duration, followed by a treatment-free interval and additional treatment at relapse. However, evidence suggests that continuous therapy after an initial response may be a better approach.2,3
Pooled data from three large, phase 3 trials in newly diagnosed MM patients suggest that continuous therapy may lead to an increase in progression-free survival (PFS) and overall survival (OS).2
These results are supported by a meta-analysis, which showed favorable outcomes in PFS and OS with lenalidomide maintenance compared to placebo or observation in newly diagnosed MM patients who had received high-dose therapy and autologous stem cell transplant.3
Given these emerging findings and the availability of effective and tolerable therapies suitable for longer use, there is an opportunity to increase the adoption of this treatment strategy to improve outcomes for MM patients.
The concept of longer treatment for MM is not new. The first clinical trials in which researchers evaluated the efficacy and safety of this approach were conducted 40 years ago in patients initially treated with melphalan and prednisone. However, modest efficacy and substantial toxicity limited longer treatment with those agents.4-7
The intervening years saw the introduction of new agents with different mechanisms of action, such as proteasome inhibitors and immunomodulators. These therapies, commonly used as initial treatment, provided physicians with additional options for treating patients longer.
Research has shown that longer treatment with immunomodulatory agents and proteasome inhibitors can be clinically effective.8
Longer treatment—integrated in the first-line treatment strategy and before a patient relapses—may enhance conventional induction strategies, resulting in better PFS and OS.9,10
Continuous treatment, in which a patient receives treatment beyond a fixed induction period, has demonstrated extended PFS and OS as well.2,3
Data supporting the benefits of prolonged therapy with immunomodulatory drugs has been a key driver behind the shifting paradigm in favor of longer treatment as the standard of care.11,3
Additionally, continuing treatment with a proteasome inhibitor beyond induction therapy is associated with an improvement in the depth of response and prolonged OS.12
Longer treatment with proteasome inhibitors is also associated with deepening response rates and improved PFS following hematopoietic stem cell transplant.13-15
Recent research has also shown that patients may achieve deeper remission with longer treatment,16,17 overturning the long-held belief that longer duration of therapy can only extend a response rather than improve it.
Moreover, treating patients for longer may now be possible because of the favorable toxicity profile of some of the novel therapies currently available, which have fewer cumulative or late-onset toxicities.18
Dr. Weisel has received honoraria and/or consultancy fees from Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda. She has received research funding from Amgen, Celgene, Sanofi, and Janssen.
The W2O Group provided writing support for this editorial, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
1. Lonial S. Hematology Am Soc Hematol Educ Program. 2010; 2010:303-9. doi: 10.1182/asheducation-2010.1.303
2. Palumbo A et al. J Clin Oncol. 2015; 33(30):3459-66. doi: 10.1200/JCO.2014.60.2466
3. McCarthy PL et al. J Clin Oncol. 2017; 35(29):3279-3289. doi: 10.1200/JCO.2017.72.6679
4. Joks M et al. Eur J Haematol. 2015 ;94(2):109-14. doi: 10.1111/ejh.12412
5. Berenson JR et al. Blood. 2002; 99:3163-8. doi: http://www.bloodjournal.org/content/99/9/3163.long
6. Shustik C et al. Br J Haematol. 2007; 126:201-11. doi: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2141.2006.06405.x
7. Fritz E, Ludwig H. Ann Oncol. 2000 Nov;11(11):1427-36
8. Ludwig H et al. Blood. 2012; 119:3003-3015. doi: https://doi.org/10.1182/blood-2011-11-374249
9. Mateos MV et al. Am J Hematol. 2015; 90(4):314-9. doi: 10.1002/ajh.23933
10. Benboubker L et al. N Engl J Med. 2014; 371(10):906-17. doi: 10.1056/NEJMoa1402551
11. Holstein SA et al. Lancet Haematol. 2017; 4(9):e431-e442. doi: 10.1016/S2352-3026(17)30140-0
12. Mateos MV et al. Blood. 2014; 124:1887-1893. doi: https://doi.org/10.1182/blood-2014-05-573733
13. Sonneveld P et al. ASH Annual Meeting Abstracts. Blood. 2010;116. Abstract 40
14. Rosiñol L et al. Blood. 2012; 120(8):1589-96. doi: https://doi.org/10.1182/blood-2012-02-408922
15. Richardson PG et al. N Engl J Med. 2005; 352(24):2487-98. doi: 10.1056/NEJMoa043445
16. de Tute RM et al. ASH Annual Meeting Abstracts. Blood. 2017; 130: 904. Abstract 904
17. Dimopoulos M et al. J Hematol Oncol. 2018;11(1):49. doi: 10.1186/s13045-018-0583-7
18. Lipe B et al. Blood Cancer J. 2016; 6(10): e485. doi: 10.1038/bcj.2016.89
In Part 1 of this editorial, Katja Weisel, MD, of University Hospital Tubingen in Germany, describes the benefits of longer treatment in patients with multiple myeloma.
Despite recent progress in advancing the care of patients with multiple myeloma (MM), this cancer remains incurable.
Although novel combination regimens have driven major improvements in patient outcomes, most MM patients still experience multiple relapses, even those who respond to treatment initially.1
Historically, MM was treated for a fixed duration, followed by a treatment-free interval and additional treatment at relapse. However, evidence suggests that continuous therapy after an initial response may be a better approach.2,3
Pooled data from three large, phase 3 trials in newly diagnosed MM patients suggest that continuous therapy may lead to an increase in progression-free survival (PFS) and overall survival (OS).2
These results are supported by a meta-analysis, which showed favorable outcomes in PFS and OS with lenalidomide maintenance compared to placebo or observation in newly diagnosed MM patients who had received high-dose therapy and autologous stem cell transplant.3
Given these emerging findings and the availability of effective and tolerable therapies suitable for longer use, there is an opportunity to increase the adoption of this treatment strategy to improve outcomes for MM patients.
The concept of longer treatment for MM is not new. The first clinical trials in which researchers evaluated the efficacy and safety of this approach were conducted 40 years ago in patients initially treated with melphalan and prednisone. However, modest efficacy and substantial toxicity limited longer treatment with those agents.4-7
The intervening years saw the introduction of new agents with different mechanisms of action, such as proteasome inhibitors and immunomodulators. These therapies, commonly used as initial treatment, provided physicians with additional options for treating patients longer.
Research has shown that longer treatment with immunomodulatory agents and proteasome inhibitors can be clinically effective.8
Longer treatment—integrated in the first-line treatment strategy and before a patient relapses—may enhance conventional induction strategies, resulting in better PFS and OS.9,10
Continuous treatment, in which a patient receives treatment beyond a fixed induction period, has demonstrated extended PFS and OS as well.2,3
Data supporting the benefits of prolonged therapy with immunomodulatory drugs has been a key driver behind the shifting paradigm in favor of longer treatment as the standard of care.11,3
Additionally, continuing treatment with a proteasome inhibitor beyond induction therapy is associated with an improvement in the depth of response and prolonged OS.12
Longer treatment with proteasome inhibitors is also associated with deepening response rates and improved PFS following hematopoietic stem cell transplant.13-15
Recent research has also shown that patients may achieve deeper remission with longer treatment,16,17 overturning the long-held belief that longer duration of therapy can only extend a response rather than improve it.
Moreover, treating patients for longer may now be possible because of the favorable toxicity profile of some of the novel therapies currently available, which have fewer cumulative or late-onset toxicities.18
Dr. Weisel has received honoraria and/or consultancy fees from Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda. She has received research funding from Amgen, Celgene, Sanofi, and Janssen.
The W2O Group provided writing support for this editorial, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
1. Lonial S. Hematology Am Soc Hematol Educ Program. 2010; 2010:303-9. doi: 10.1182/asheducation-2010.1.303
2. Palumbo A et al. J Clin Oncol. 2015; 33(30):3459-66. doi: 10.1200/JCO.2014.60.2466
3. McCarthy PL et al. J Clin Oncol. 2017; 35(29):3279-3289. doi: 10.1200/JCO.2017.72.6679
4. Joks M et al. Eur J Haematol. 2015 ;94(2):109-14. doi: 10.1111/ejh.12412
5. Berenson JR et al. Blood. 2002; 99:3163-8. doi: http://www.bloodjournal.org/content/99/9/3163.long
6. Shustik C et al. Br J Haematol. 2007; 126:201-11. doi: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2141.2006.06405.x
7. Fritz E, Ludwig H. Ann Oncol. 2000 Nov;11(11):1427-36
8. Ludwig H et al. Blood. 2012; 119:3003-3015. doi: https://doi.org/10.1182/blood-2011-11-374249
9. Mateos MV et al. Am J Hematol. 2015; 90(4):314-9. doi: 10.1002/ajh.23933
10. Benboubker L et al. N Engl J Med. 2014; 371(10):906-17. doi: 10.1056/NEJMoa1402551
11. Holstein SA et al. Lancet Haematol. 2017; 4(9):e431-e442. doi: 10.1016/S2352-3026(17)30140-0
12. Mateos MV et al. Blood. 2014; 124:1887-1893. doi: https://doi.org/10.1182/blood-2014-05-573733
13. Sonneveld P et al. ASH Annual Meeting Abstracts. Blood. 2010;116. Abstract 40
14. Rosiñol L et al. Blood. 2012; 120(8):1589-96. doi: https://doi.org/10.1182/blood-2012-02-408922
15. Richardson PG et al. N Engl J Med. 2005; 352(24):2487-98. doi: 10.1056/NEJMoa043445
16. de Tute RM et al. ASH Annual Meeting Abstracts. Blood. 2017; 130: 904. Abstract 904
17. Dimopoulos M et al. J Hematol Oncol. 2018;11(1):49. doi: 10.1186/s13045-018-0583-7
18. Lipe B et al. Blood Cancer J. 2016; 6(10): e485. doi: 10.1038/bcj.2016.89
In Part 1 of this editorial, Katja Weisel, MD, of University Hospital Tubingen in Germany, describes the benefits of longer treatment in patients with multiple myeloma.
Despite recent progress in advancing the care of patients with multiple myeloma (MM), this cancer remains incurable.
Although novel combination regimens have driven major improvements in patient outcomes, most MM patients still experience multiple relapses, even those who respond to treatment initially.1
Historically, MM was treated for a fixed duration, followed by a treatment-free interval and additional treatment at relapse. However, evidence suggests that continuous therapy after an initial response may be a better approach.2,3
Pooled data from three large, phase 3 trials in newly diagnosed MM patients suggest that continuous therapy may lead to an increase in progression-free survival (PFS) and overall survival (OS).2
These results are supported by a meta-analysis, which showed favorable outcomes in PFS and OS with lenalidomide maintenance compared to placebo or observation in newly diagnosed MM patients who had received high-dose therapy and autologous stem cell transplant.3
Given these emerging findings and the availability of effective and tolerable therapies suitable for longer use, there is an opportunity to increase the adoption of this treatment strategy to improve outcomes for MM patients.
The concept of longer treatment for MM is not new. The first clinical trials in which researchers evaluated the efficacy and safety of this approach were conducted 40 years ago in patients initially treated with melphalan and prednisone. However, modest efficacy and substantial toxicity limited longer treatment with those agents.4-7
The intervening years saw the introduction of new agents with different mechanisms of action, such as proteasome inhibitors and immunomodulators. These therapies, commonly used as initial treatment, provided physicians with additional options for treating patients longer.
Research has shown that longer treatment with immunomodulatory agents and proteasome inhibitors can be clinically effective.8
Longer treatment—integrated in the first-line treatment strategy and before a patient relapses—may enhance conventional induction strategies, resulting in better PFS and OS.9,10
Continuous treatment, in which a patient receives treatment beyond a fixed induction period, has demonstrated extended PFS and OS as well.2,3
Data supporting the benefits of prolonged therapy with immunomodulatory drugs has been a key driver behind the shifting paradigm in favor of longer treatment as the standard of care.11,3
Additionally, continuing treatment with a proteasome inhibitor beyond induction therapy is associated with an improvement in the depth of response and prolonged OS.12
Longer treatment with proteasome inhibitors is also associated with deepening response rates and improved PFS following hematopoietic stem cell transplant.13-15
Recent research has also shown that patients may achieve deeper remission with longer treatment,16,17 overturning the long-held belief that longer duration of therapy can only extend a response rather than improve it.
Moreover, treating patients for longer may now be possible because of the favorable toxicity profile of some of the novel therapies currently available, which have fewer cumulative or late-onset toxicities.18
Dr. Weisel has received honoraria and/or consultancy fees from Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda. She has received research funding from Amgen, Celgene, Sanofi, and Janssen.
The W2O Group provided writing support for this editorial, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
1. Lonial S. Hematology Am Soc Hematol Educ Program. 2010; 2010:303-9. doi: 10.1182/asheducation-2010.1.303
2. Palumbo A et al. J Clin Oncol. 2015; 33(30):3459-66. doi: 10.1200/JCO.2014.60.2466
3. McCarthy PL et al. J Clin Oncol. 2017; 35(29):3279-3289. doi: 10.1200/JCO.2017.72.6679
4. Joks M et al. Eur J Haematol. 2015 ;94(2):109-14. doi: 10.1111/ejh.12412
5. Berenson JR et al. Blood. 2002; 99:3163-8. doi: http://www.bloodjournal.org/content/99/9/3163.long
6. Shustik C et al. Br J Haematol. 2007; 126:201-11. doi: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2141.2006.06405.x
7. Fritz E, Ludwig H. Ann Oncol. 2000 Nov;11(11):1427-36
8. Ludwig H et al. Blood. 2012; 119:3003-3015. doi: https://doi.org/10.1182/blood-2011-11-374249
9. Mateos MV et al. Am J Hematol. 2015; 90(4):314-9. doi: 10.1002/ajh.23933
10. Benboubker L et al. N Engl J Med. 2014; 371(10):906-17. doi: 10.1056/NEJMoa1402551
11. Holstein SA et al. Lancet Haematol. 2017; 4(9):e431-e442. doi: 10.1016/S2352-3026(17)30140-0
12. Mateos MV et al. Blood. 2014; 124:1887-1893. doi: https://doi.org/10.1182/blood-2014-05-573733
13. Sonneveld P et al. ASH Annual Meeting Abstracts. Blood. 2010;116. Abstract 40
14. Rosiñol L et al. Blood. 2012; 120(8):1589-96. doi: https://doi.org/10.1182/blood-2012-02-408922
15. Richardson PG et al. N Engl J Med. 2005; 352(24):2487-98. doi: 10.1056/NEJMoa043445
16. de Tute RM et al. ASH Annual Meeting Abstracts. Blood. 2017; 130: 904. Abstract 904
17. Dimopoulos M et al. J Hematol Oncol. 2018;11(1):49. doi: 10.1186/s13045-018-0583-7
18. Lipe B et al. Blood Cancer J. 2016; 6(10): e485. doi: 10.1038/bcj.2016.89
The case for longer treatment in MM: Part 2
In Part 2 of this editorial, Katja Weisel, MD, of University Hospital Tubingen in Germany, addresses the barriers to longer treatment in patients with multiple myeloma.
Attitudes regarding longer treatment can present barriers to widespread adoption of this approach in multiple myeloma (MM).
Indeed, some clinicians continue to follow a fixed-duration approach to treatment in MM, only considering further treatment once the patient has relapsed rather than treating the patient until disease progression.
In the MM community, some are reluctant to adopt a strategy of treating longer because of the modest efficacy gains observed with early research or concern over tolerability issues, including the risk of developing peripheral neuropathy or secondary malignancies.1
Others are uncertain about the optimal duration of therapy or the selection of an agent that will balance any potential gain in depth of response with the risk of late-onset or cumulative toxicities.
The potentially high cost of longer treatment for patients, their families, and/or the healthcare system overall also presents a challenge.
It is feasible that treating patients for longer may drive up healthcare utilization and take a toll on patients and caregivers, who may incur out-of-pocket costs because of the need to travel to a hospital or doctor’s office for intravenous therapies, requiring them to miss work.2
It is important to recognize, however, that more convenient all-oral treatment regimens are now available that do not require infusion at a hospital or clinic. Furthermore, results from recent studies suggest the majority of cancer patients prefer oral over intravenous therapies, which could reduce non-pharmacy healthcare costs.3,4
Healthcare providers might be more likely to accept and adopt a longer treatment approach for MM if they had access to data describing the optimal duration, dosage, schedule, toxicity, and quality of life standards.
Ongoing, randomized, phase 3 trials are evaluating the benefits of treating longer with an oral proteasome inhibitor in patients with newly diagnosed MM.5,6
Updated treatment guidelines and consensus statements will provide further guidance for clinicians on the benefits of maintenance therapy in both transplant-eligible and -ineligible patients with newly diagnosed MM.
The recently updated MM guidelines from the European Society for Medical Oncology (ESMO) recommend longer treatment or maintenance therapy in patients who have undergone hematopoietic stem cell transplant (HSCT).7
Based on evidence from studies such as FIRST and SWOG S0777, ESMO also recommends continuous treatment or treatment until progression with lenalidomide-dexamethasone and bortezomib-lenalidomide-dexamethasone in MM patients who are ineligible for HSCT.7-9
As there is no one-size-fits-all treatment approach in MM, a personalized treatment plan should be designed for each patient. This plan should take into account a number of factors, including age, disease characteristics, performance status, treatment history, and the patient’s goals of care and personal preferences.10
If the patient is a candidate for longer treatment, the clinician should carefully weigh the potential impact on disease-free and overall survival against the potential side effects, as well as assess the patient’s likelihood of adhering to the medication.
With the availability of newer, less-toxic medications that can be tolerated for a greater duration and are easy to administer, aiding in overall treatment compliance, sustained remissions are possible.11-13
Forty years ago, MM patients had very few treatment options, and the 5-year survival rate was 26%.14
Since then, novel therapies, including proteasome inhibitors and immunomodulatory drugs, have replaced conventional cytotoxic chemotherapy, leading to major improvements in survival.15,16
With emerging research that supports the value of longer treatment strategies for both patients and the healthcare system, clinicians will have a proven strategy to help their patients attain long-term disease control while maintaining quality of life.2, 17-19
Dr. Weisel has received honoraria and/or consultancy fees from Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda. She has received research funding from Amgen, Celgene, Sanofi, and Janssen.
The W2O Group provided writing support for this editorial, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
1. Lipe B et al. Blood Cancer J. 2016; 6(10): e485. doi: 10.1038/bcj.2016.89
2. Goodwin J et al. Cancer Nurs. 2013; 36(4):301-8. doi: 10.1097/NCC.0b013e3182693522
3. Eek D et al. Patient Prefer Adherence. 2016; 10:1609-21. doi: 10.2147/PPA.S106629
4. Bauer S et al. Value in Health. 2017; 20: A451. Abstract PCN217. doi: https://doi.org/10.1016/j.jval.2017.08.299
5. A Study of Oral Ixazomib Citrate (MLN9708) Maintenance Therapy in Participants With Multiple Myeloma Following Autologous Stem Cell Transplant. (2014). Retrieved from https://clinicaltrials.gov/ct2/show/NCT02181413 (Identification No. NCT02181413).
6. A Study of Oral Ixazomib Maintenance Therapy in Patients With Newly Diagnosed Multiple Myeloma Not Treated With Stem Cell Transplantation. (2014). Retrieved from https://clinicaltrials.gov/ct2/show/NCT02312258 (Identification No. NCT02312258).
7. Moreau P et al. Ann Oncol. 2017; 28: iv52-iv61. doi: https://org/10.1093/annonc/mdx096
8. Facon T et al. Blood. 2018131(3):301-310. doi: 10.1182/blood-2017-07-795047
9. Durie BG et al. Lancet. 2017; 389(10068):519-527. doi: 10.1016/S0140-6736(16)31594-X.
10. Laubach J et al. Leukemia. 2016; 30(5):1005-17. doi: 10.1038/leu.2015.356
11. Ludwig H et al. Blood. 2012; 119: 3003-3015. doi: https://doi.org/10.1182/blood-2011-11-374249
12. Lehners N et al. Cancer Med. 2018; 7(2): 307–316. doi: 10.1002/cam4.1283
13. Attal M et al. N Engl J Med. 2012; 366:1872-1791. doi: 10.1056/NEJMoa1114138
14. National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2014. National Cancer Institute. https://seer.cancer.gov/csr/1975_2014/. Accessed March 28, 2018.
15. Kumar SK et al. Blood. 2008 Mar 1;111(5):2516-20. doi: 10.1182/blood-2007-10-116129
16. Fonseca R et al. Leukemia. 2017 Sep;31(9):1915-1921. doi: 10.1038/leu.2016.380
17. Palumbo A, Niesvizky R. Leuk Res. 2012; 36 Suppl 1:S19-26. doi: 10.1016/S0145-2126(12)70005-X
18. Girnius S, Munshi NC. Leuk Suppl. 2013; 2(Suppl 1): S3–S9. doi: 10.1038/leusup.2013.2
19. Mateos M-V, San Miguel JF. Hematology Am Soc Hematol Educ Program. 2013; 2013:488-95. doi: 10.1182/asheducation-2013.1.488
In Part 2 of this editorial, Katja Weisel, MD, of University Hospital Tubingen in Germany, addresses the barriers to longer treatment in patients with multiple myeloma.
Attitudes regarding longer treatment can present barriers to widespread adoption of this approach in multiple myeloma (MM).
Indeed, some clinicians continue to follow a fixed-duration approach to treatment in MM, only considering further treatment once the patient has relapsed rather than treating the patient until disease progression.
In the MM community, some are reluctant to adopt a strategy of treating longer because of the modest efficacy gains observed with early research or concern over tolerability issues, including the risk of developing peripheral neuropathy or secondary malignancies.1
Others are uncertain about the optimal duration of therapy or the selection of an agent that will balance any potential gain in depth of response with the risk of late-onset or cumulative toxicities.
The potentially high cost of longer treatment for patients, their families, and/or the healthcare system overall also presents a challenge.
It is feasible that treating patients for longer may drive up healthcare utilization and take a toll on patients and caregivers, who may incur out-of-pocket costs because of the need to travel to a hospital or doctor’s office for intravenous therapies, requiring them to miss work.2
It is important to recognize, however, that more convenient all-oral treatment regimens are now available that do not require infusion at a hospital or clinic. Furthermore, results from recent studies suggest the majority of cancer patients prefer oral over intravenous therapies, which could reduce non-pharmacy healthcare costs.3,4
Healthcare providers might be more likely to accept and adopt a longer treatment approach for MM if they had access to data describing the optimal duration, dosage, schedule, toxicity, and quality of life standards.
Ongoing, randomized, phase 3 trials are evaluating the benefits of treating longer with an oral proteasome inhibitor in patients with newly diagnosed MM.5,6
Updated treatment guidelines and consensus statements will provide further guidance for clinicians on the benefits of maintenance therapy in both transplant-eligible and -ineligible patients with newly diagnosed MM.
The recently updated MM guidelines from the European Society for Medical Oncology (ESMO) recommend longer treatment or maintenance therapy in patients who have undergone hematopoietic stem cell transplant (HSCT).7
Based on evidence from studies such as FIRST and SWOG S0777, ESMO also recommends continuous treatment or treatment until progression with lenalidomide-dexamethasone and bortezomib-lenalidomide-dexamethasone in MM patients who are ineligible for HSCT.7-9
As there is no one-size-fits-all treatment approach in MM, a personalized treatment plan should be designed for each patient. This plan should take into account a number of factors, including age, disease characteristics, performance status, treatment history, and the patient’s goals of care and personal preferences.10
If the patient is a candidate for longer treatment, the clinician should carefully weigh the potential impact on disease-free and overall survival against the potential side effects, as well as assess the patient’s likelihood of adhering to the medication.
With the availability of newer, less-toxic medications that can be tolerated for a greater duration and are easy to administer, aiding in overall treatment compliance, sustained remissions are possible.11-13
Forty years ago, MM patients had very few treatment options, and the 5-year survival rate was 26%.14
Since then, novel therapies, including proteasome inhibitors and immunomodulatory drugs, have replaced conventional cytotoxic chemotherapy, leading to major improvements in survival.15,16
With emerging research that supports the value of longer treatment strategies for both patients and the healthcare system, clinicians will have a proven strategy to help their patients attain long-term disease control while maintaining quality of life.2, 17-19
Dr. Weisel has received honoraria and/or consultancy fees from Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda. She has received research funding from Amgen, Celgene, Sanofi, and Janssen.
The W2O Group provided writing support for this editorial, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
1. Lipe B et al. Blood Cancer J. 2016; 6(10): e485. doi: 10.1038/bcj.2016.89
2. Goodwin J et al. Cancer Nurs. 2013; 36(4):301-8. doi: 10.1097/NCC.0b013e3182693522
3. Eek D et al. Patient Prefer Adherence. 2016; 10:1609-21. doi: 10.2147/PPA.S106629
4. Bauer S et al. Value in Health. 2017; 20: A451. Abstract PCN217. doi: https://doi.org/10.1016/j.jval.2017.08.299
5. A Study of Oral Ixazomib Citrate (MLN9708) Maintenance Therapy in Participants With Multiple Myeloma Following Autologous Stem Cell Transplant. (2014). Retrieved from https://clinicaltrials.gov/ct2/show/NCT02181413 (Identification No. NCT02181413).
6. A Study of Oral Ixazomib Maintenance Therapy in Patients With Newly Diagnosed Multiple Myeloma Not Treated With Stem Cell Transplantation. (2014). Retrieved from https://clinicaltrials.gov/ct2/show/NCT02312258 (Identification No. NCT02312258).
7. Moreau P et al. Ann Oncol. 2017; 28: iv52-iv61. doi: https://org/10.1093/annonc/mdx096
8. Facon T et al. Blood. 2018131(3):301-310. doi: 10.1182/blood-2017-07-795047
9. Durie BG et al. Lancet. 2017; 389(10068):519-527. doi: 10.1016/S0140-6736(16)31594-X.
10. Laubach J et al. Leukemia. 2016; 30(5):1005-17. doi: 10.1038/leu.2015.356
11. Ludwig H et al. Blood. 2012; 119: 3003-3015. doi: https://doi.org/10.1182/blood-2011-11-374249
12. Lehners N et al. Cancer Med. 2018; 7(2): 307–316. doi: 10.1002/cam4.1283
13. Attal M et al. N Engl J Med. 2012; 366:1872-1791. doi: 10.1056/NEJMoa1114138
14. National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2014. National Cancer Institute. https://seer.cancer.gov/csr/1975_2014/. Accessed March 28, 2018.
15. Kumar SK et al. Blood. 2008 Mar 1;111(5):2516-20. doi: 10.1182/blood-2007-10-116129
16. Fonseca R et al. Leukemia. 2017 Sep;31(9):1915-1921. doi: 10.1038/leu.2016.380
17. Palumbo A, Niesvizky R. Leuk Res. 2012; 36 Suppl 1:S19-26. doi: 10.1016/S0145-2126(12)70005-X
18. Girnius S, Munshi NC. Leuk Suppl. 2013; 2(Suppl 1): S3–S9. doi: 10.1038/leusup.2013.2
19. Mateos M-V, San Miguel JF. Hematology Am Soc Hematol Educ Program. 2013; 2013:488-95. doi: 10.1182/asheducation-2013.1.488
In Part 2 of this editorial, Katja Weisel, MD, of University Hospital Tubingen in Germany, addresses the barriers to longer treatment in patients with multiple myeloma.
Attitudes regarding longer treatment can present barriers to widespread adoption of this approach in multiple myeloma (MM).
Indeed, some clinicians continue to follow a fixed-duration approach to treatment in MM, only considering further treatment once the patient has relapsed rather than treating the patient until disease progression.
In the MM community, some are reluctant to adopt a strategy of treating longer because of the modest efficacy gains observed with early research or concern over tolerability issues, including the risk of developing peripheral neuropathy or secondary malignancies.1
Others are uncertain about the optimal duration of therapy or the selection of an agent that will balance any potential gain in depth of response with the risk of late-onset or cumulative toxicities.
The potentially high cost of longer treatment for patients, their families, and/or the healthcare system overall also presents a challenge.
It is feasible that treating patients for longer may drive up healthcare utilization and take a toll on patients and caregivers, who may incur out-of-pocket costs because of the need to travel to a hospital or doctor’s office for intravenous therapies, requiring them to miss work.2
It is important to recognize, however, that more convenient all-oral treatment regimens are now available that do not require infusion at a hospital or clinic. Furthermore, results from recent studies suggest the majority of cancer patients prefer oral over intravenous therapies, which could reduce non-pharmacy healthcare costs.3,4
Healthcare providers might be more likely to accept and adopt a longer treatment approach for MM if they had access to data describing the optimal duration, dosage, schedule, toxicity, and quality of life standards.
Ongoing, randomized, phase 3 trials are evaluating the benefits of treating longer with an oral proteasome inhibitor in patients with newly diagnosed MM.5,6
Updated treatment guidelines and consensus statements will provide further guidance for clinicians on the benefits of maintenance therapy in both transplant-eligible and -ineligible patients with newly diagnosed MM.
The recently updated MM guidelines from the European Society for Medical Oncology (ESMO) recommend longer treatment or maintenance therapy in patients who have undergone hematopoietic stem cell transplant (HSCT).7
Based on evidence from studies such as FIRST and SWOG S0777, ESMO also recommends continuous treatment or treatment until progression with lenalidomide-dexamethasone and bortezomib-lenalidomide-dexamethasone in MM patients who are ineligible for HSCT.7-9
As there is no one-size-fits-all treatment approach in MM, a personalized treatment plan should be designed for each patient. This plan should take into account a number of factors, including age, disease characteristics, performance status, treatment history, and the patient’s goals of care and personal preferences.10
If the patient is a candidate for longer treatment, the clinician should carefully weigh the potential impact on disease-free and overall survival against the potential side effects, as well as assess the patient’s likelihood of adhering to the medication.
With the availability of newer, less-toxic medications that can be tolerated for a greater duration and are easy to administer, aiding in overall treatment compliance, sustained remissions are possible.11-13
Forty years ago, MM patients had very few treatment options, and the 5-year survival rate was 26%.14
Since then, novel therapies, including proteasome inhibitors and immunomodulatory drugs, have replaced conventional cytotoxic chemotherapy, leading to major improvements in survival.15,16
With emerging research that supports the value of longer treatment strategies for both patients and the healthcare system, clinicians will have a proven strategy to help their patients attain long-term disease control while maintaining quality of life.2, 17-19
Dr. Weisel has received honoraria and/or consultancy fees from Amgen, BMS, Celgene, Janssen, Juno, Sanofi, and Takeda. She has received research funding from Amgen, Celgene, Sanofi, and Janssen.
The W2O Group provided writing support for this editorial, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
1. Lipe B et al. Blood Cancer J. 2016; 6(10): e485. doi: 10.1038/bcj.2016.89
2. Goodwin J et al. Cancer Nurs. 2013; 36(4):301-8. doi: 10.1097/NCC.0b013e3182693522
3. Eek D et al. Patient Prefer Adherence. 2016; 10:1609-21. doi: 10.2147/PPA.S106629
4. Bauer S et al. Value in Health. 2017; 20: A451. Abstract PCN217. doi: https://doi.org/10.1016/j.jval.2017.08.299
5. A Study of Oral Ixazomib Citrate (MLN9708) Maintenance Therapy in Participants With Multiple Myeloma Following Autologous Stem Cell Transplant. (2014). Retrieved from https://clinicaltrials.gov/ct2/show/NCT02181413 (Identification No. NCT02181413).
6. A Study of Oral Ixazomib Maintenance Therapy in Patients With Newly Diagnosed Multiple Myeloma Not Treated With Stem Cell Transplantation. (2014). Retrieved from https://clinicaltrials.gov/ct2/show/NCT02312258 (Identification No. NCT02312258).
7. Moreau P et al. Ann Oncol. 2017; 28: iv52-iv61. doi: https://org/10.1093/annonc/mdx096
8. Facon T et al. Blood. 2018131(3):301-310. doi: 10.1182/blood-2017-07-795047
9. Durie BG et al. Lancet. 2017; 389(10068):519-527. doi: 10.1016/S0140-6736(16)31594-X.
10. Laubach J et al. Leukemia. 2016; 30(5):1005-17. doi: 10.1038/leu.2015.356
11. Ludwig H et al. Blood. 2012; 119: 3003-3015. doi: https://doi.org/10.1182/blood-2011-11-374249
12. Lehners N et al. Cancer Med. 2018; 7(2): 307–316. doi: 10.1002/cam4.1283
13. Attal M et al. N Engl J Med. 2012; 366:1872-1791. doi: 10.1056/NEJMoa1114138
14. National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2014. National Cancer Institute. https://seer.cancer.gov/csr/1975_2014/. Accessed March 28, 2018.
15. Kumar SK et al. Blood. 2008 Mar 1;111(5):2516-20. doi: 10.1182/blood-2007-10-116129
16. Fonseca R et al. Leukemia. 2017 Sep;31(9):1915-1921. doi: 10.1038/leu.2016.380
17. Palumbo A, Niesvizky R. Leuk Res. 2012; 36 Suppl 1:S19-26. doi: 10.1016/S0145-2126(12)70005-X
18. Girnius S, Munshi NC. Leuk Suppl. 2013; 2(Suppl 1): S3–S9. doi: 10.1038/leusup.2013.2
19. Mateos M-V, San Miguel JF. Hematology Am Soc Hematol Educ Program. 2013; 2013:488-95. doi: 10.1182/asheducation-2013.1.488
Premenstrual Dysphoric Disorder: Diagnosis and Management in Primary Care
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6
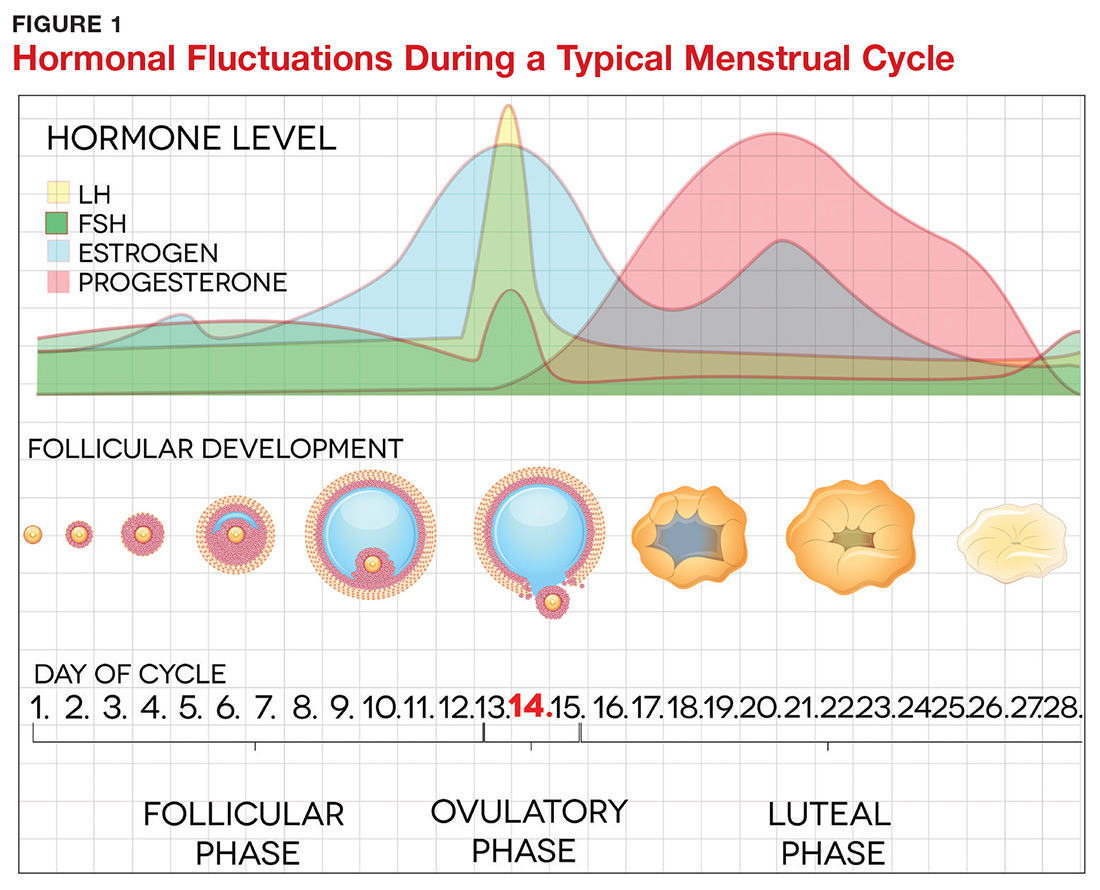
This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5
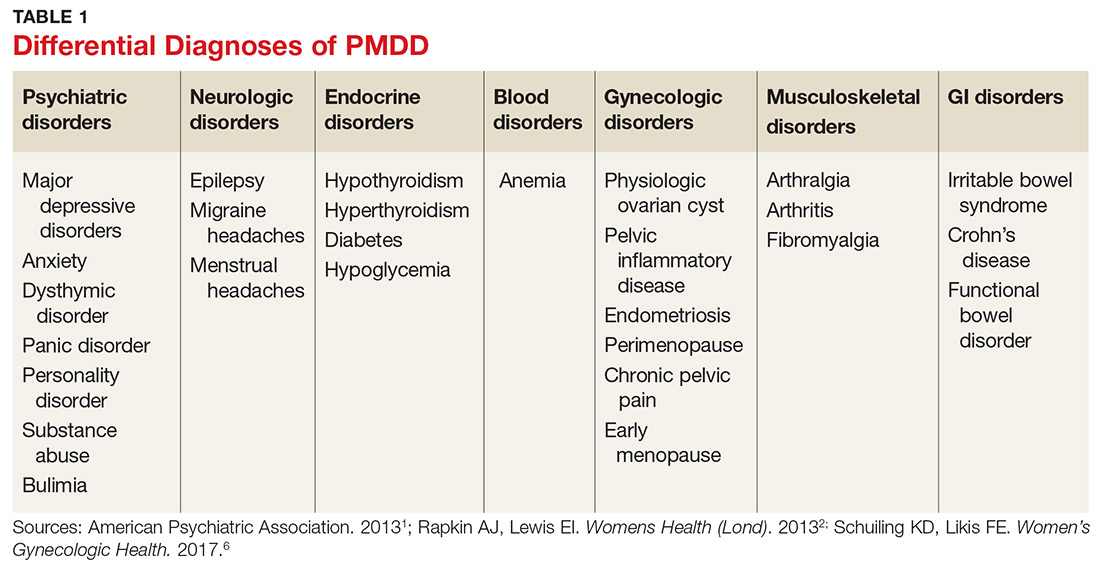
It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
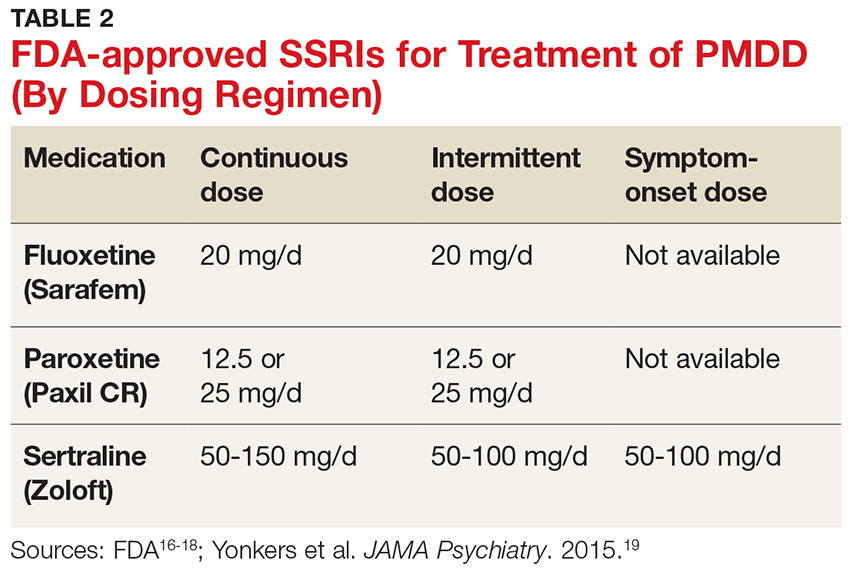
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19
Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6

This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5

It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19

Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6

This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5

It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19

Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
Stay tuned
Two events that will impact our practices occurred in November: 1) an election and 2) the Centers for Medicare & Medicaid Services final rule. The election returned us to a split government with Democrats controlling the U.S. House and Republicans controlling the Senate (without a filibuster-proof majority). This means that ACA repeal and dramatic alterations to Medicaid will be off the table. Pressures on ACA’s margins will remain in both the legislative and judicial arms of government. Federal and state governments will continue to try to stabilize the individual markets by using reinsurance and premium support. The number of states expanding Medicaid eligibility will continue to grow (now at 37). There will be further pressure on drug pricing, likely targeted to Part B and 340b drugs. This will affect academic centers and hospital margins substantially.
CMS issued its final rule for the Physician Fee Schedule. AGA and the other GI societies have published a detailed member alert that can be found here. Key points involve simplified documentation for evaluation and management visits, site-neutrality reimbursement for clinic visits, identification of colonoscopy and EGD codes for CMS review, and changes in calculating practice expense, among others. MACRA rules are evolving with further pressure on practices and health systems to evolve into alternative payment models. Commercial insurers are finally near a tipping point in pressing for two-sided risk contracts. Practices should be alert for local and regional pressures around price transparency and narrow networks. Health systems (including academic centers) must plan for margin reductions due to changes in pharmacy reimbursement, network price tiering, a continued shift toward government payers, and other pressures that could drive large systems into the red.
For the first time since 1996, discretionary programs including NIH, CDC, AHRQ, and VA research all have been included in a budget (as opposed to a Continuing Resolution) that was passed by Congress and signed into law. This gives us some stability and predictability; however, the looming (and increasing) budget deficit will prompt Congress to increase fiscal pressure on domestic programs such as Social Security, Medicare, and Medicaid. Stay tuned and stay involved.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Two events that will impact our practices occurred in November: 1) an election and 2) the Centers for Medicare & Medicaid Services final rule. The election returned us to a split government with Democrats controlling the U.S. House and Republicans controlling the Senate (without a filibuster-proof majority). This means that ACA repeal and dramatic alterations to Medicaid will be off the table. Pressures on ACA’s margins will remain in both the legislative and judicial arms of government. Federal and state governments will continue to try to stabilize the individual markets by using reinsurance and premium support. The number of states expanding Medicaid eligibility will continue to grow (now at 37). There will be further pressure on drug pricing, likely targeted to Part B and 340b drugs. This will affect academic centers and hospital margins substantially.
CMS issued its final rule for the Physician Fee Schedule. AGA and the other GI societies have published a detailed member alert that can be found here. Key points involve simplified documentation for evaluation and management visits, site-neutrality reimbursement for clinic visits, identification of colonoscopy and EGD codes for CMS review, and changes in calculating practice expense, among others. MACRA rules are evolving with further pressure on practices and health systems to evolve into alternative payment models. Commercial insurers are finally near a tipping point in pressing for two-sided risk contracts. Practices should be alert for local and regional pressures around price transparency and narrow networks. Health systems (including academic centers) must plan for margin reductions due to changes in pharmacy reimbursement, network price tiering, a continued shift toward government payers, and other pressures that could drive large systems into the red.
For the first time since 1996, discretionary programs including NIH, CDC, AHRQ, and VA research all have been included in a budget (as opposed to a Continuing Resolution) that was passed by Congress and signed into law. This gives us some stability and predictability; however, the looming (and increasing) budget deficit will prompt Congress to increase fiscal pressure on domestic programs such as Social Security, Medicare, and Medicaid. Stay tuned and stay involved.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Two events that will impact our practices occurred in November: 1) an election and 2) the Centers for Medicare & Medicaid Services final rule. The election returned us to a split government with Democrats controlling the U.S. House and Republicans controlling the Senate (without a filibuster-proof majority). This means that ACA repeal and dramatic alterations to Medicaid will be off the table. Pressures on ACA’s margins will remain in both the legislative and judicial arms of government. Federal and state governments will continue to try to stabilize the individual markets by using reinsurance and premium support. The number of states expanding Medicaid eligibility will continue to grow (now at 37). There will be further pressure on drug pricing, likely targeted to Part B and 340b drugs. This will affect academic centers and hospital margins substantially.
CMS issued its final rule for the Physician Fee Schedule. AGA and the other GI societies have published a detailed member alert that can be found here. Key points involve simplified documentation for evaluation and management visits, site-neutrality reimbursement for clinic visits, identification of colonoscopy and EGD codes for CMS review, and changes in calculating practice expense, among others. MACRA rules are evolving with further pressure on practices and health systems to evolve into alternative payment models. Commercial insurers are finally near a tipping point in pressing for two-sided risk contracts. Practices should be alert for local and regional pressures around price transparency and narrow networks. Health systems (including academic centers) must plan for margin reductions due to changes in pharmacy reimbursement, network price tiering, a continued shift toward government payers, and other pressures that could drive large systems into the red.
For the first time since 1996, discretionary programs including NIH, CDC, AHRQ, and VA research all have been included in a budget (as opposed to a Continuing Resolution) that was passed by Congress and signed into law. This gives us some stability and predictability; however, the looming (and increasing) budget deficit will prompt Congress to increase fiscal pressure on domestic programs such as Social Security, Medicare, and Medicaid. Stay tuned and stay involved.
John I. Allen, MD, MBA, AGAF
Editor in Chief
FDA approves congenital CMV diagnostic test
, a new test to be used as an aid in the diagnosis of congenital cytomegalovirus (CMV) in newborns less than 21 days of age.
The Alethia CMV Assay Test System detects CMV DNA from a saliva swab. Results from the test should be used only in conjunction with the results of other diagnostic tests and clinical information, according to an FDA statement.
“This test for detecting the virus, when used in conjunction with the results of other diagnostic tests, may help health care providers more quickly identify the virus in newborns,” said Timothy Stenzel, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
In a prospective clinical study, 1,472 saliva samples out of 1,475 samples collected from newborns were correctly identified by the device as negative for the presence of CMV DNA. Three samples were incorrectly identified as positive when they were negative. Five collected saliva specimens were correctly identified as positive for the presence of CMV DNA.
In a testing of 34 samples of archived specimens from babies known to be infected with CMV, all of the archived specimens were correctly identified by the device as positive for the presence of CMV DNA.
The FDA reviewed the Alethia CMV Assay Test System through a regulatory pathway established for novel, low- to moderate-risk devices. Along with this authorization, the FDA is establishing criteria, called special controls, which determine the requirements for demonstrating accuracy, reliability, and effectiveness of tests intended to be used as an aid in the diagnosis of congenital CMV infection.
With this new regulatory classification, subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
The FDA granted marketing authorization of the Alethia CMV Assay Test System to Meridian Bioscience.
, a new test to be used as an aid in the diagnosis of congenital cytomegalovirus (CMV) in newborns less than 21 days of age.
The Alethia CMV Assay Test System detects CMV DNA from a saliva swab. Results from the test should be used only in conjunction with the results of other diagnostic tests and clinical information, according to an FDA statement.
“This test for detecting the virus, when used in conjunction with the results of other diagnostic tests, may help health care providers more quickly identify the virus in newborns,” said Timothy Stenzel, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
In a prospective clinical study, 1,472 saliva samples out of 1,475 samples collected from newborns were correctly identified by the device as negative for the presence of CMV DNA. Three samples were incorrectly identified as positive when they were negative. Five collected saliva specimens were correctly identified as positive for the presence of CMV DNA.
In a testing of 34 samples of archived specimens from babies known to be infected with CMV, all of the archived specimens were correctly identified by the device as positive for the presence of CMV DNA.
The FDA reviewed the Alethia CMV Assay Test System through a regulatory pathway established for novel, low- to moderate-risk devices. Along with this authorization, the FDA is establishing criteria, called special controls, which determine the requirements for demonstrating accuracy, reliability, and effectiveness of tests intended to be used as an aid in the diagnosis of congenital CMV infection.
With this new regulatory classification, subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
The FDA granted marketing authorization of the Alethia CMV Assay Test System to Meridian Bioscience.
, a new test to be used as an aid in the diagnosis of congenital cytomegalovirus (CMV) in newborns less than 21 days of age.
The Alethia CMV Assay Test System detects CMV DNA from a saliva swab. Results from the test should be used only in conjunction with the results of other diagnostic tests and clinical information, according to an FDA statement.
“This test for detecting the virus, when used in conjunction with the results of other diagnostic tests, may help health care providers more quickly identify the virus in newborns,” said Timothy Stenzel, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
In a prospective clinical study, 1,472 saliva samples out of 1,475 samples collected from newborns were correctly identified by the device as negative for the presence of CMV DNA. Three samples were incorrectly identified as positive when they were negative. Five collected saliva specimens were correctly identified as positive for the presence of CMV DNA.
In a testing of 34 samples of archived specimens from babies known to be infected with CMV, all of the archived specimens were correctly identified by the device as positive for the presence of CMV DNA.
The FDA reviewed the Alethia CMV Assay Test System through a regulatory pathway established for novel, low- to moderate-risk devices. Along with this authorization, the FDA is establishing criteria, called special controls, which determine the requirements for demonstrating accuracy, reliability, and effectiveness of tests intended to be used as an aid in the diagnosis of congenital CMV infection.
With this new regulatory classification, subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
The FDA granted marketing authorization of the Alethia CMV Assay Test System to Meridian Bioscience.
What is your diagnosis? - December 2018
Cecal carcinoma–associated paraneoplastic Sweet’s syndrome
Based on the tomographic appearance of an “apple core”–like lesion in the right lower quadrant, the patient was referred for colonoscopy, which revealed a malignant-appearing cecal mass (Figure D), with biopsies confirming adenocarcinoma; despite these findings, no bowel-related symptoms were reported. The patient underwent laparoscopic right hemicolectomy, after which the skin lesions began to resolve, and corticosteroids were successfully tapered. The overall presentation was consistent with Sweet’s syndrome, with a paraneoplastic etiology being favored given the clinical scenario, including absence of alternative etiologies and dependence on corticosteroids for control of skin disease until resection of the underlying malignancy was performed.
Sweet’s syndrome was first described in a case series of eight patients published in 1964 by the English dermatologist Dr. Robert Douglas Sweet.1,2 Sweet’s syndrome is characterized by fever, neutrophilia, and sterile erythematous plaques or nodules, which most commonly involve the upper extremities and face and respond to corticosteroid therapy. It may be malignancy associated, drug induced, autoimmune disease related, or idiopathic.2,3 The pathogenesis of Sweet’s syndrome is unclear, but T-lymphocyte, neutrophil chemotaxis, and cytokine (e.g., interleukin-6 and granulocyte colony–stimulating factor) abnormalities have been suggested.2 Diagnosis is based on the clinical presentation and context together with typical dermatopathologic findings, including a dense neutrophilic infiltrate. Skin lesions may be phasic, but persist typically until appropriate therapy (e.g., corticosteroids, chemotherapy) is administered or the offending drug removed. Malignancy-associated (i.e., paraneoplastic) Sweet’s syndrome accounts for approximately 20% of all cases; these primarily involve hematologic malignancies, most commonly leukemia, but adenocarcinomata have also been implicated.3 Recurrence of Sweet’s syndrome can occur and often heralds relapse of the underlying disease.
References
1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-56.
2. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-56.
3. Cohen PR. Sweet’s syndrome–a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
Cecal carcinoma–associated paraneoplastic Sweet’s syndrome
Based on the tomographic appearance of an “apple core”–like lesion in the right lower quadrant, the patient was referred for colonoscopy, which revealed a malignant-appearing cecal mass (Figure D), with biopsies confirming adenocarcinoma; despite these findings, no bowel-related symptoms were reported. The patient underwent laparoscopic right hemicolectomy, after which the skin lesions began to resolve, and corticosteroids were successfully tapered. The overall presentation was consistent with Sweet’s syndrome, with a paraneoplastic etiology being favored given the clinical scenario, including absence of alternative etiologies and dependence on corticosteroids for control of skin disease until resection of the underlying malignancy was performed.
Sweet’s syndrome was first described in a case series of eight patients published in 1964 by the English dermatologist Dr. Robert Douglas Sweet.1,2 Sweet’s syndrome is characterized by fever, neutrophilia, and sterile erythematous plaques or nodules, which most commonly involve the upper extremities and face and respond to corticosteroid therapy. It may be malignancy associated, drug induced, autoimmune disease related, or idiopathic.2,3 The pathogenesis of Sweet’s syndrome is unclear, but T-lymphocyte, neutrophil chemotaxis, and cytokine (e.g., interleukin-6 and granulocyte colony–stimulating factor) abnormalities have been suggested.2 Diagnosis is based on the clinical presentation and context together with typical dermatopathologic findings, including a dense neutrophilic infiltrate. Skin lesions may be phasic, but persist typically until appropriate therapy (e.g., corticosteroids, chemotherapy) is administered or the offending drug removed. Malignancy-associated (i.e., paraneoplastic) Sweet’s syndrome accounts for approximately 20% of all cases; these primarily involve hematologic malignancies, most commonly leukemia, but adenocarcinomata have also been implicated.3 Recurrence of Sweet’s syndrome can occur and often heralds relapse of the underlying disease.
References
1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-56.
2. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-56.
3. Cohen PR. Sweet’s syndrome–a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
Cecal carcinoma–associated paraneoplastic Sweet’s syndrome
Based on the tomographic appearance of an “apple core”–like lesion in the right lower quadrant, the patient was referred for colonoscopy, which revealed a malignant-appearing cecal mass (Figure D), with biopsies confirming adenocarcinoma; despite these findings, no bowel-related symptoms were reported. The patient underwent laparoscopic right hemicolectomy, after which the skin lesions began to resolve, and corticosteroids were successfully tapered. The overall presentation was consistent with Sweet’s syndrome, with a paraneoplastic etiology being favored given the clinical scenario, including absence of alternative etiologies and dependence on corticosteroids for control of skin disease until resection of the underlying malignancy was performed.
Sweet’s syndrome was first described in a case series of eight patients published in 1964 by the English dermatologist Dr. Robert Douglas Sweet.1,2 Sweet’s syndrome is characterized by fever, neutrophilia, and sterile erythematous plaques or nodules, which most commonly involve the upper extremities and face and respond to corticosteroid therapy. It may be malignancy associated, drug induced, autoimmune disease related, or idiopathic.2,3 The pathogenesis of Sweet’s syndrome is unclear, but T-lymphocyte, neutrophil chemotaxis, and cytokine (e.g., interleukin-6 and granulocyte colony–stimulating factor) abnormalities have been suggested.2 Diagnosis is based on the clinical presentation and context together with typical dermatopathologic findings, including a dense neutrophilic infiltrate. Skin lesions may be phasic, but persist typically until appropriate therapy (e.g., corticosteroids, chemotherapy) is administered or the offending drug removed. Malignancy-associated (i.e., paraneoplastic) Sweet’s syndrome accounts for approximately 20% of all cases; these primarily involve hematologic malignancies, most commonly leukemia, but adenocarcinomata have also been implicated.3 Recurrence of Sweet’s syndrome can occur and often heralds relapse of the underlying disease.
References
1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-56.
2. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-56.
3. Cohen PR. Sweet’s syndrome–a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
What is the diagnosis?
Firdapse approved: First treatment for rare autoimmune disorder
The Food and Drug Administration has approved amifampridine (Firdapse) as the first treatment for the rare autoimmune disorder known as Lambert-Eaton myasthenic syndrome, which causes the immune system to attack the neuromuscular junction and thereby disrupts the nerves’ ability to send signals to muscle cells. This causes fatigue and weakness in those affected, so they can experience difficulties with activities of daily living as a result.
The most common side effects included prickling sensation, upper respiratory tract infection, abdominal pain, and muscle spasms.
More information can be found in the FDA’s press announcement.
The Food and Drug Administration has approved amifampridine (Firdapse) as the first treatment for the rare autoimmune disorder known as Lambert-Eaton myasthenic syndrome, which causes the immune system to attack the neuromuscular junction and thereby disrupts the nerves’ ability to send signals to muscle cells. This causes fatigue and weakness in those affected, so they can experience difficulties with activities of daily living as a result.
The most common side effects included prickling sensation, upper respiratory tract infection, abdominal pain, and muscle spasms.
More information can be found in the FDA’s press announcement.
The Food and Drug Administration has approved amifampridine (Firdapse) as the first treatment for the rare autoimmune disorder known as Lambert-Eaton myasthenic syndrome, which causes the immune system to attack the neuromuscular junction and thereby disrupts the nerves’ ability to send signals to muscle cells. This causes fatigue and weakness in those affected, so they can experience difficulties with activities of daily living as a result.
The most common side effects included prickling sensation, upper respiratory tract infection, abdominal pain, and muscle spasms.
More information can be found in the FDA’s press announcement.
DMARDs improve vascular abnormalities in early RA
, Maya H. Buch, PhD, said at the annual meeting of the American College of Rheumatology.
She presented a study of 71 treatment-naive patients with recently diagnosed RA and no history of cardiovascular disease. Patients were randomized to either etanercept plus methotrexate or methotrexate with treat-to-target escalation to triple nonbiologic disease-modifying antirheumatic drug therapy by week 12. Patients in the latter group were switched to etanercept plus methotrexate at week 24 if at that point they had a Disease Activity Score 28 of 2.6 or more.
All participants underwent cardiovascular MRI at baseline and again after 1 and 2 years in order to measure change in aortic stiffness, the primary study endpoint chosen because it is clinically meaningful and can reliably and reproducibly be measured by MRI.
The study was designed to shed light on possible mechanisms for the epidemiologic observation that effective treatment with methotrexate and/or tumor necrosis factor (TNF) inhibitors curbs the excess risk of cardiovascular events that accompanies RA. The thinking is that abnormal aortic distensibility represents an early harbinger of the increased cardiovascular risk associated with this form of inflammatory arthritis, explained Dr. Buch, professor of rheumatology at the University of Leeds (England) and section head of clinical and translational rheumatology at the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The mean aortic distensibility at baseline, when the study population was treatment naive, was 2.99 x 10–3 mm Hg–1. This value is abnormally low when compared with values seen in healthy age- and sex-matched controls. After 1 year of treatment, however, mean aortic distensibility improved significantly to 3.59 x 10–3 mm Hg–1. This improvement was maintained at year 2, when the mean value was 3.55.
Interestingly, neither treatment response status or disease activity was associated with the improvement in aortic stiffness. However, in a prespecified exploratory comparison between the 20 responders to etanercept plus methotrexate and the 10 responders to first-line methotrexate who never received etanercept, the group on biologic therapy had a 16% greater improvement in aortic distensibility at 1 year.
“It’s not a statistically significant difference. The study wasn’t powered for that,” she said in an interview. “But these data suggest that an anti-TNF treatment strategy may confer additional benefit. Of course, that will need to be confirmed in a larger trial. And if it is confirmed, it would suggest a role for etanercept plus methotrexate in personalizing tailored therapy in early RA patients at particularly high cardiovascular risk.”
Dr. Buch reported receiving funding from Pfizer, Roche, UCB, AbbVie, Sanofi, Eli Lilly, and Sandoz, but reported having no financial conflicts of interest regarding this study, which was sponsored by the U.K. National Institute for Healthcare Research.
SOURCE: Buch MH et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L05.
, Maya H. Buch, PhD, said at the annual meeting of the American College of Rheumatology.
She presented a study of 71 treatment-naive patients with recently diagnosed RA and no history of cardiovascular disease. Patients were randomized to either etanercept plus methotrexate or methotrexate with treat-to-target escalation to triple nonbiologic disease-modifying antirheumatic drug therapy by week 12. Patients in the latter group were switched to etanercept plus methotrexate at week 24 if at that point they had a Disease Activity Score 28 of 2.6 or more.
All participants underwent cardiovascular MRI at baseline and again after 1 and 2 years in order to measure change in aortic stiffness, the primary study endpoint chosen because it is clinically meaningful and can reliably and reproducibly be measured by MRI.
The study was designed to shed light on possible mechanisms for the epidemiologic observation that effective treatment with methotrexate and/or tumor necrosis factor (TNF) inhibitors curbs the excess risk of cardiovascular events that accompanies RA. The thinking is that abnormal aortic distensibility represents an early harbinger of the increased cardiovascular risk associated with this form of inflammatory arthritis, explained Dr. Buch, professor of rheumatology at the University of Leeds (England) and section head of clinical and translational rheumatology at the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The mean aortic distensibility at baseline, when the study population was treatment naive, was 2.99 x 10–3 mm Hg–1. This value is abnormally low when compared with values seen in healthy age- and sex-matched controls. After 1 year of treatment, however, mean aortic distensibility improved significantly to 3.59 x 10–3 mm Hg–1. This improvement was maintained at year 2, when the mean value was 3.55.
Interestingly, neither treatment response status or disease activity was associated with the improvement in aortic stiffness. However, in a prespecified exploratory comparison between the 20 responders to etanercept plus methotrexate and the 10 responders to first-line methotrexate who never received etanercept, the group on biologic therapy had a 16% greater improvement in aortic distensibility at 1 year.
“It’s not a statistically significant difference. The study wasn’t powered for that,” she said in an interview. “But these data suggest that an anti-TNF treatment strategy may confer additional benefit. Of course, that will need to be confirmed in a larger trial. And if it is confirmed, it would suggest a role for etanercept plus methotrexate in personalizing tailored therapy in early RA patients at particularly high cardiovascular risk.”
Dr. Buch reported receiving funding from Pfizer, Roche, UCB, AbbVie, Sanofi, Eli Lilly, and Sandoz, but reported having no financial conflicts of interest regarding this study, which was sponsored by the U.K. National Institute for Healthcare Research.
SOURCE: Buch MH et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L05.
, Maya H. Buch, PhD, said at the annual meeting of the American College of Rheumatology.
She presented a study of 71 treatment-naive patients with recently diagnosed RA and no history of cardiovascular disease. Patients were randomized to either etanercept plus methotrexate or methotrexate with treat-to-target escalation to triple nonbiologic disease-modifying antirheumatic drug therapy by week 12. Patients in the latter group were switched to etanercept plus methotrexate at week 24 if at that point they had a Disease Activity Score 28 of 2.6 or more.
All participants underwent cardiovascular MRI at baseline and again after 1 and 2 years in order to measure change in aortic stiffness, the primary study endpoint chosen because it is clinically meaningful and can reliably and reproducibly be measured by MRI.
The study was designed to shed light on possible mechanisms for the epidemiologic observation that effective treatment with methotrexate and/or tumor necrosis factor (TNF) inhibitors curbs the excess risk of cardiovascular events that accompanies RA. The thinking is that abnormal aortic distensibility represents an early harbinger of the increased cardiovascular risk associated with this form of inflammatory arthritis, explained Dr. Buch, professor of rheumatology at the University of Leeds (England) and section head of clinical and translational rheumatology at the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The mean aortic distensibility at baseline, when the study population was treatment naive, was 2.99 x 10–3 mm Hg–1. This value is abnormally low when compared with values seen in healthy age- and sex-matched controls. After 1 year of treatment, however, mean aortic distensibility improved significantly to 3.59 x 10–3 mm Hg–1. This improvement was maintained at year 2, when the mean value was 3.55.
Interestingly, neither treatment response status or disease activity was associated with the improvement in aortic stiffness. However, in a prespecified exploratory comparison between the 20 responders to etanercept plus methotrexate and the 10 responders to first-line methotrexate who never received etanercept, the group on biologic therapy had a 16% greater improvement in aortic distensibility at 1 year.
“It’s not a statistically significant difference. The study wasn’t powered for that,” she said in an interview. “But these data suggest that an anti-TNF treatment strategy may confer additional benefit. Of course, that will need to be confirmed in a larger trial. And if it is confirmed, it would suggest a role for etanercept plus methotrexate in personalizing tailored therapy in early RA patients at particularly high cardiovascular risk.”
Dr. Buch reported receiving funding from Pfizer, Roche, UCB, AbbVie, Sanofi, Eli Lilly, and Sandoz, but reported having no financial conflicts of interest regarding this study, which was sponsored by the U.K. National Institute for Healthcare Research.
SOURCE: Buch MH et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L05.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Abnormal aortic stiffness is present in patients with newly diagnosed RA but improves with disease-modifying antirheumatic drug therapy.
Major finding: Mean aortic distensibility in patients with early RA improved from an abnormally low 2.99 x 10–3 mm Hg–1 prior to treatment to a more robust 3.59 x 10–3 mm Hg–1 after 1 year of disease-modifying antirheumatic drug therapy.
Study details: This study included 71 patients with newly diagnosed RA whose aortic stiffness was measured serially via cardiovascular MRI over a 2-year period.
Disclosures: The presenter reported receiving funding from Pfizer, Roche, UCB, AbbVie, Sanofi, Eli Lilly, and Sandoz, but reported having no financial conflicts of interest regarding this study, which was sponsored by the U.K. National Institute for Healthcare Research.
Source: Buch MH et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L05.
PCOS linked to increased cancer risk in premenopausal women
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
FROM JAMA ONCOLOGY
Key clinical point: Polycystic ovarian syndrome may be associated with increased cancer risks among younger women.
Major finding: Among premenopausal women, there was a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and a more than doubling in the risk of ovarian and pancreatic cancers
Study details: The study examined risks in 3.5 million women with up to 24 years of follow-up.
Disclosures: The study authors had no financial disclosures.
Source: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.