User login
FDA Efforts to Advance Development of Gene Therapies
FDA Commissioner Scott Gottlieb, MD, releases a statement on the agency’s efforts to advance development of gene therapies.
Once just a theory, gene therapies are now a therapeutic reality for some patients. These platforms may have the potential to treat and cure some of our most intractable and vexing diseases. The policy framework we construct for how these products should be developed, reviewed by regulators, and reimbursed, will help set the stage for the continued advancement of this new market. Last year, we announced our comprehensive policy framework for regenerative medicine, including a draft guidance that describes the expedited programs, such as the breakthrough therapy designation, and the regenerative medicine advanced therapy (RMAT) designation, that may be available to sponsors of these therapies. Today, we’re unveiling a complementary framework for the development, review and approval of gene therapies.
In the past 12 months, we’ve seen three separate gene therapy products approved by the FDA. This reflects the rapid advancements in this field. An inflection point was reached with the development of vectors that could reliably deliver gene cassettes in vivo, into cells and human tissue. In the future, we expect this field to continue to expand, with the potential approval of new treatments for many debilitating diseases. These therapies hold great promise. Our new steps are aimed at fostering developments in this innovative field.
Gene therapies are being studied in many areas, including genetic disorders, autoimmune diseases, heart disease, cancer and HIV/AIDS. We look forward to working with the academic and research communities to make safe and effective products a reality for more patients. But we know that we still have much to learn about how these products work, how to administer them safely, and whether they will continue to work properly in the body without causing adverse side effects over long periods of time. In contrast to traditional drug review, some of the more challenging questions when it comes to gene therapy relate to product manufacturing and quality, or questions about the durability of response, which often can’t be fully answered in any reasonably sized pre-market trial. For some of these products, we may need to accept some level of uncertainty around these questions at the time of approval. For example, in some cases the long-term durability of the effect won’t be fully understood at the time of approval. Effective tools for reliable post-market follow up, such as required post-market clinical trials, are going to be one key to advancing this field and helping to ensure that our approach fosters safe and innovative treatments.
Even when there may be uncertainty about some questions, we need to make certain we assure patient safety and adequately characterize the potential risks and demonstrated benefits of these products. In part because of the added questions that often surround a new technology like gene therapy, these products are initially being aimed at devastating diseases, many of which lack available therapies, including some diseases that are fatal. In such cases of devastating diseases without available therapies, we’ve traditionally been willing to accept more uncertainty to facilitate timely access to promising therapies. In such cases, drug sponsors are generally required to conduct post-marketing clinical trials, known as phase 4 confirmatory trials, to confirm clinical benefit of the drug. This is the direction Congress gave the FDA by creating vehicles like the accelerated approval pathway.
When it comes to novel technologies like gene therapy, the FDA is steadfastly committed to a regulatory path that maintains the agency’s gold standard for assuring safety and efficacy. As we develop this evidence-based framework, we’re going to have to modernize how we approach certain aspects of these products in order to make sure our approach is tailored to the unique challenges created by these new platforms.
Today, we’re taking a step toward shaping this modern structure for the regulation of gene therapy. The agency is issuing a suite of six scientific guidance documents intended to serve as the building blocks of a modern, comprehensive framework for how we’ll help advance the field of gene therapy while making sure new products meet the FDA’s gold standard for safety and effectiveness.
These policies are part of our efforts to communicate the steps we’re taking to provide clear recommendations to sponsors and researchers, so that we can better support innovation. The documents are being issued in draft form so that we can solicit public input on these new policies. As with all draft guidances, all of the comments we receive will be carefully considered prior to finalizing these documents. We’re committed to working with stakeholders to bring novel treatments to the market while ensuring the safety of patients.
Disease-Specific Gene Therapy Guidances
The FDA has issuing three new draft guidance documents on the development of gene therapy products for specific disease categories. These are the first three disease-specific guidances that the agency is issuing for gene therapy products. Our new commitment to develop disease-specific guidance documents reflects the increasing activity in this field, and its growing importance to advancing public health.
Human Gene Therapy for Hemophilia: Gene therapy products for hemophilia are now being developed as single-dose treatments that may enable long-term production of the missing or abnormal coagulation factor in patients. This may reduce or eliminate the need for coagulation factor replacement. To define the proper development pathway for such products, we’re issuing a new draft guidance on gene therapy products that are targeted to the treatment of hemophilia. Once finalized, this new guidance will provide recommendations on the FDA’s current thinking on clinical trial design and preclinical considerations to support the development of these gene therapy products. Among other elements, the draft guidance provides recommendations regarding surrogate endpoints that could be used by sponsors pursuing accelerated approval of gene therapy products that are intended for treatment of hemophilia.
Human Gene Therapy for Retinal Disorders: Another area of fast-paced activity is gene therapy products targeted to the treatment of retinal disorders. The Human Gene Therapy for Retinal Disorders guidance, once finalized, will assist those developing gene therapy products for a wide variety of retinal disorders affecting both adult and pediatric patients. Gene therapy products currently undergoing clinical trials in the United States for retinal disorders are commonly delivered by intravitreal injections (into the fluid portion of the eye), or by subretinal injections (beneath the retina). In some cases, the gene therapy products are encapsulated in a device to be implanted within the eye. This new guidance document will focus on issues that are specific to gene therapies for retinal disorders. The document provides recommendations related to product development, preclinical testing, and clinical trial design for such products.
Human Gene Therapy for Rare Diseases: Rare diseases are those that affect fewer than 200,000 people in the United States. The National Institutes of Health reports that nearly 7,000 rare diseases affect more than 25 million Americans. About 80 percent of rare diseases are caused by a single-gene defect, and about half of all rare diseases affect children. Since most rare diseases have no approved therapies, there is a significant unmet need. The Human Gene Therapy for Rare Diseases guidance, once finalized, will provide recommendations on preclinical, manufacturing and clinical trial design for all phases of the clinical development program for these types of gene therapies. The information is intended to assist sponsors in the design of clinical development programs, where there may be limited study population size, potential feasibility and safety issues, as well as issues relating to the interpretation of effectiveness.
Guidances on Manufacturing Gene Therapies
The FDA is also providing new and comprehensive updates to three existing guidances that address manufacturing issues related to gene therapy. These updates reflect input from many stakeholders. We encourage additional feedback on these documents during the comment period.
The first draft guidance, Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs), provides sponsors with recommendations on how to provide sufficient CMC information to assure safety, identity, quality, purity and strength/potency of investigational gene therapy products. The guidance applies to human gene therapies and to combination products that contain a human gene therapy in combination with a drug or device. In addition, this guidance is organized to follow the structure of the FDA guidance on the Common Technical Document.
The second draft guidance, Testing of Retroviral Vector-Based Gene Therapy Products for Replication Competent Retrovirus (RCR) during Product Manufacture and Patient Follow-up, provides additional recommendations regarding the proper testing for RCR during the manufacture of retroviral vector-based gene therapy products, as well as during the follow-up monitoring of patients who’ve received retroviral vector-based gene therapy products. Specifically, the draft guidance recommends the identification and amount of material to be tested. The guidance also provides advice on general testing methods.
The third draft guidance, Long Term Follow-Up After Administration of Human Gene Therapy Products, provides recommendations regarding the design of long-term follow-up (LTFU) observational studies for the collection of data on delayed adverse events following administration of a gene therapy product. Because of some of the additional uncertainty intrinsic to a novel platform like gene therapy -- including questions related to the durability of the treatment effects as well as the theoretical potential for off-target effects if the genes do not insert correctly -- there’s an increased need for robust long-term follow-up of patients in the post-market period. This guidance describes product characteristics, patient-related factors, and the preclinical and clinical data that should be considered when assessing the need for LTFU observations and describes the features related to effective post-market follow up.
Once finalized, these draft guidances will replace previous guidances issued by the FDA in April 2008 (CMC) and November 2006 (RCR and LTFU).
The field of gene therapy has progressed rapidly since these guidances were first issued. Therefore, the FDA is updating these guidances to provide sponsors with the agency’s most up-to-date thinking.
Our goal is to help promote safe and effective product development in this field. We’ll continue to work with the product sponsors to help make the development and approval of these innovative gene therapies more efficient, while putting in place the regulatory controls needed to ensure that the resulting therapies are both safe and effective. We’ll also make full use of our expedited programs such as breakthrough therapy designation and regenerative medicine advanced therapy designation whenever possible.
Gene therapy represents one of the most promising opportunities for developing highly effective and even curative treatments for many vexing disorders. Some of these products are almost certainly going to change the contours of medical practice, and the destiny of patients with some debilitating diseases.
The FDA’s goal is to help these innovations advance in a framework that assures the safety and effectiveness of these resulting treatments, and continues to build peoples’ confidence in this novel area of medicine.
–Scott Gottlieb, MD
FDA Commissioner Scott Gottlieb, MD, releases a statement on the agency’s efforts to advance development of gene therapies.
FDA Commissioner Scott Gottlieb, MD, releases a statement on the agency’s efforts to advance development of gene therapies.
Once just a theory, gene therapies are now a therapeutic reality for some patients. These platforms may have the potential to treat and cure some of our most intractable and vexing diseases. The policy framework we construct for how these products should be developed, reviewed by regulators, and reimbursed, will help set the stage for the continued advancement of this new market. Last year, we announced our comprehensive policy framework for regenerative medicine, including a draft guidance that describes the expedited programs, such as the breakthrough therapy designation, and the regenerative medicine advanced therapy (RMAT) designation, that may be available to sponsors of these therapies. Today, we’re unveiling a complementary framework for the development, review and approval of gene therapies.
In the past 12 months, we’ve seen three separate gene therapy products approved by the FDA. This reflects the rapid advancements in this field. An inflection point was reached with the development of vectors that could reliably deliver gene cassettes in vivo, into cells and human tissue. In the future, we expect this field to continue to expand, with the potential approval of new treatments for many debilitating diseases. These therapies hold great promise. Our new steps are aimed at fostering developments in this innovative field.
Gene therapies are being studied in many areas, including genetic disorders, autoimmune diseases, heart disease, cancer and HIV/AIDS. We look forward to working with the academic and research communities to make safe and effective products a reality for more patients. But we know that we still have much to learn about how these products work, how to administer them safely, and whether they will continue to work properly in the body without causing adverse side effects over long periods of time. In contrast to traditional drug review, some of the more challenging questions when it comes to gene therapy relate to product manufacturing and quality, or questions about the durability of response, which often can’t be fully answered in any reasonably sized pre-market trial. For some of these products, we may need to accept some level of uncertainty around these questions at the time of approval. For example, in some cases the long-term durability of the effect won’t be fully understood at the time of approval. Effective tools for reliable post-market follow up, such as required post-market clinical trials, are going to be one key to advancing this field and helping to ensure that our approach fosters safe and innovative treatments.
Even when there may be uncertainty about some questions, we need to make certain we assure patient safety and adequately characterize the potential risks and demonstrated benefits of these products. In part because of the added questions that often surround a new technology like gene therapy, these products are initially being aimed at devastating diseases, many of which lack available therapies, including some diseases that are fatal. In such cases of devastating diseases without available therapies, we’ve traditionally been willing to accept more uncertainty to facilitate timely access to promising therapies. In such cases, drug sponsors are generally required to conduct post-marketing clinical trials, known as phase 4 confirmatory trials, to confirm clinical benefit of the drug. This is the direction Congress gave the FDA by creating vehicles like the accelerated approval pathway.
When it comes to novel technologies like gene therapy, the FDA is steadfastly committed to a regulatory path that maintains the agency’s gold standard for assuring safety and efficacy. As we develop this evidence-based framework, we’re going to have to modernize how we approach certain aspects of these products in order to make sure our approach is tailored to the unique challenges created by these new platforms.
Today, we’re taking a step toward shaping this modern structure for the regulation of gene therapy. The agency is issuing a suite of six scientific guidance documents intended to serve as the building blocks of a modern, comprehensive framework for how we’ll help advance the field of gene therapy while making sure new products meet the FDA’s gold standard for safety and effectiveness.
These policies are part of our efforts to communicate the steps we’re taking to provide clear recommendations to sponsors and researchers, so that we can better support innovation. The documents are being issued in draft form so that we can solicit public input on these new policies. As with all draft guidances, all of the comments we receive will be carefully considered prior to finalizing these documents. We’re committed to working with stakeholders to bring novel treatments to the market while ensuring the safety of patients.
Disease-Specific Gene Therapy Guidances
The FDA has issuing three new draft guidance documents on the development of gene therapy products for specific disease categories. These are the first three disease-specific guidances that the agency is issuing for gene therapy products. Our new commitment to develop disease-specific guidance documents reflects the increasing activity in this field, and its growing importance to advancing public health.
Human Gene Therapy for Hemophilia: Gene therapy products for hemophilia are now being developed as single-dose treatments that may enable long-term production of the missing or abnormal coagulation factor in patients. This may reduce or eliminate the need for coagulation factor replacement. To define the proper development pathway for such products, we’re issuing a new draft guidance on gene therapy products that are targeted to the treatment of hemophilia. Once finalized, this new guidance will provide recommendations on the FDA’s current thinking on clinical trial design and preclinical considerations to support the development of these gene therapy products. Among other elements, the draft guidance provides recommendations regarding surrogate endpoints that could be used by sponsors pursuing accelerated approval of gene therapy products that are intended for treatment of hemophilia.
Human Gene Therapy for Retinal Disorders: Another area of fast-paced activity is gene therapy products targeted to the treatment of retinal disorders. The Human Gene Therapy for Retinal Disorders guidance, once finalized, will assist those developing gene therapy products for a wide variety of retinal disorders affecting both adult and pediatric patients. Gene therapy products currently undergoing clinical trials in the United States for retinal disorders are commonly delivered by intravitreal injections (into the fluid portion of the eye), or by subretinal injections (beneath the retina). In some cases, the gene therapy products are encapsulated in a device to be implanted within the eye. This new guidance document will focus on issues that are specific to gene therapies for retinal disorders. The document provides recommendations related to product development, preclinical testing, and clinical trial design for such products.
Human Gene Therapy for Rare Diseases: Rare diseases are those that affect fewer than 200,000 people in the United States. The National Institutes of Health reports that nearly 7,000 rare diseases affect more than 25 million Americans. About 80 percent of rare diseases are caused by a single-gene defect, and about half of all rare diseases affect children. Since most rare diseases have no approved therapies, there is a significant unmet need. The Human Gene Therapy for Rare Diseases guidance, once finalized, will provide recommendations on preclinical, manufacturing and clinical trial design for all phases of the clinical development program for these types of gene therapies. The information is intended to assist sponsors in the design of clinical development programs, where there may be limited study population size, potential feasibility and safety issues, as well as issues relating to the interpretation of effectiveness.
Guidances on Manufacturing Gene Therapies
The FDA is also providing new and comprehensive updates to three existing guidances that address manufacturing issues related to gene therapy. These updates reflect input from many stakeholders. We encourage additional feedback on these documents during the comment period.
The first draft guidance, Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs), provides sponsors with recommendations on how to provide sufficient CMC information to assure safety, identity, quality, purity and strength/potency of investigational gene therapy products. The guidance applies to human gene therapies and to combination products that contain a human gene therapy in combination with a drug or device. In addition, this guidance is organized to follow the structure of the FDA guidance on the Common Technical Document.
The second draft guidance, Testing of Retroviral Vector-Based Gene Therapy Products for Replication Competent Retrovirus (RCR) during Product Manufacture and Patient Follow-up, provides additional recommendations regarding the proper testing for RCR during the manufacture of retroviral vector-based gene therapy products, as well as during the follow-up monitoring of patients who’ve received retroviral vector-based gene therapy products. Specifically, the draft guidance recommends the identification and amount of material to be tested. The guidance also provides advice on general testing methods.
The third draft guidance, Long Term Follow-Up After Administration of Human Gene Therapy Products, provides recommendations regarding the design of long-term follow-up (LTFU) observational studies for the collection of data on delayed adverse events following administration of a gene therapy product. Because of some of the additional uncertainty intrinsic to a novel platform like gene therapy -- including questions related to the durability of the treatment effects as well as the theoretical potential for off-target effects if the genes do not insert correctly -- there’s an increased need for robust long-term follow-up of patients in the post-market period. This guidance describes product characteristics, patient-related factors, and the preclinical and clinical data that should be considered when assessing the need for LTFU observations and describes the features related to effective post-market follow up.
Once finalized, these draft guidances will replace previous guidances issued by the FDA in April 2008 (CMC) and November 2006 (RCR and LTFU).
The field of gene therapy has progressed rapidly since these guidances were first issued. Therefore, the FDA is updating these guidances to provide sponsors with the agency’s most up-to-date thinking.
Our goal is to help promote safe and effective product development in this field. We’ll continue to work with the product sponsors to help make the development and approval of these innovative gene therapies more efficient, while putting in place the regulatory controls needed to ensure that the resulting therapies are both safe and effective. We’ll also make full use of our expedited programs such as breakthrough therapy designation and regenerative medicine advanced therapy designation whenever possible.
Gene therapy represents one of the most promising opportunities for developing highly effective and even curative treatments for many vexing disorders. Some of these products are almost certainly going to change the contours of medical practice, and the destiny of patients with some debilitating diseases.
The FDA’s goal is to help these innovations advance in a framework that assures the safety and effectiveness of these resulting treatments, and continues to build peoples’ confidence in this novel area of medicine.
–Scott Gottlieb, MD
Once just a theory, gene therapies are now a therapeutic reality for some patients. These platforms may have the potential to treat and cure some of our most intractable and vexing diseases. The policy framework we construct for how these products should be developed, reviewed by regulators, and reimbursed, will help set the stage for the continued advancement of this new market. Last year, we announced our comprehensive policy framework for regenerative medicine, including a draft guidance that describes the expedited programs, such as the breakthrough therapy designation, and the regenerative medicine advanced therapy (RMAT) designation, that may be available to sponsors of these therapies. Today, we’re unveiling a complementary framework for the development, review and approval of gene therapies.
In the past 12 months, we’ve seen three separate gene therapy products approved by the FDA. This reflects the rapid advancements in this field. An inflection point was reached with the development of vectors that could reliably deliver gene cassettes in vivo, into cells and human tissue. In the future, we expect this field to continue to expand, with the potential approval of new treatments for many debilitating diseases. These therapies hold great promise. Our new steps are aimed at fostering developments in this innovative field.
Gene therapies are being studied in many areas, including genetic disorders, autoimmune diseases, heart disease, cancer and HIV/AIDS. We look forward to working with the academic and research communities to make safe and effective products a reality for more patients. But we know that we still have much to learn about how these products work, how to administer them safely, and whether they will continue to work properly in the body without causing adverse side effects over long periods of time. In contrast to traditional drug review, some of the more challenging questions when it comes to gene therapy relate to product manufacturing and quality, or questions about the durability of response, which often can’t be fully answered in any reasonably sized pre-market trial. For some of these products, we may need to accept some level of uncertainty around these questions at the time of approval. For example, in some cases the long-term durability of the effect won’t be fully understood at the time of approval. Effective tools for reliable post-market follow up, such as required post-market clinical trials, are going to be one key to advancing this field and helping to ensure that our approach fosters safe and innovative treatments.
Even when there may be uncertainty about some questions, we need to make certain we assure patient safety and adequately characterize the potential risks and demonstrated benefits of these products. In part because of the added questions that often surround a new technology like gene therapy, these products are initially being aimed at devastating diseases, many of which lack available therapies, including some diseases that are fatal. In such cases of devastating diseases without available therapies, we’ve traditionally been willing to accept more uncertainty to facilitate timely access to promising therapies. In such cases, drug sponsors are generally required to conduct post-marketing clinical trials, known as phase 4 confirmatory trials, to confirm clinical benefit of the drug. This is the direction Congress gave the FDA by creating vehicles like the accelerated approval pathway.
When it comes to novel technologies like gene therapy, the FDA is steadfastly committed to a regulatory path that maintains the agency’s gold standard for assuring safety and efficacy. As we develop this evidence-based framework, we’re going to have to modernize how we approach certain aspects of these products in order to make sure our approach is tailored to the unique challenges created by these new platforms.
Today, we’re taking a step toward shaping this modern structure for the regulation of gene therapy. The agency is issuing a suite of six scientific guidance documents intended to serve as the building blocks of a modern, comprehensive framework for how we’ll help advance the field of gene therapy while making sure new products meet the FDA’s gold standard for safety and effectiveness.
These policies are part of our efforts to communicate the steps we’re taking to provide clear recommendations to sponsors and researchers, so that we can better support innovation. The documents are being issued in draft form so that we can solicit public input on these new policies. As with all draft guidances, all of the comments we receive will be carefully considered prior to finalizing these documents. We’re committed to working with stakeholders to bring novel treatments to the market while ensuring the safety of patients.
Disease-Specific Gene Therapy Guidances
The FDA has issuing three new draft guidance documents on the development of gene therapy products for specific disease categories. These are the first three disease-specific guidances that the agency is issuing for gene therapy products. Our new commitment to develop disease-specific guidance documents reflects the increasing activity in this field, and its growing importance to advancing public health.
Human Gene Therapy for Hemophilia: Gene therapy products for hemophilia are now being developed as single-dose treatments that may enable long-term production of the missing or abnormal coagulation factor in patients. This may reduce or eliminate the need for coagulation factor replacement. To define the proper development pathway for such products, we’re issuing a new draft guidance on gene therapy products that are targeted to the treatment of hemophilia. Once finalized, this new guidance will provide recommendations on the FDA’s current thinking on clinical trial design and preclinical considerations to support the development of these gene therapy products. Among other elements, the draft guidance provides recommendations regarding surrogate endpoints that could be used by sponsors pursuing accelerated approval of gene therapy products that are intended for treatment of hemophilia.
Human Gene Therapy for Retinal Disorders: Another area of fast-paced activity is gene therapy products targeted to the treatment of retinal disorders. The Human Gene Therapy for Retinal Disorders guidance, once finalized, will assist those developing gene therapy products for a wide variety of retinal disorders affecting both adult and pediatric patients. Gene therapy products currently undergoing clinical trials in the United States for retinal disorders are commonly delivered by intravitreal injections (into the fluid portion of the eye), or by subretinal injections (beneath the retina). In some cases, the gene therapy products are encapsulated in a device to be implanted within the eye. This new guidance document will focus on issues that are specific to gene therapies for retinal disorders. The document provides recommendations related to product development, preclinical testing, and clinical trial design for such products.
Human Gene Therapy for Rare Diseases: Rare diseases are those that affect fewer than 200,000 people in the United States. The National Institutes of Health reports that nearly 7,000 rare diseases affect more than 25 million Americans. About 80 percent of rare diseases are caused by a single-gene defect, and about half of all rare diseases affect children. Since most rare diseases have no approved therapies, there is a significant unmet need. The Human Gene Therapy for Rare Diseases guidance, once finalized, will provide recommendations on preclinical, manufacturing and clinical trial design for all phases of the clinical development program for these types of gene therapies. The information is intended to assist sponsors in the design of clinical development programs, where there may be limited study population size, potential feasibility and safety issues, as well as issues relating to the interpretation of effectiveness.
Guidances on Manufacturing Gene Therapies
The FDA is also providing new and comprehensive updates to three existing guidances that address manufacturing issues related to gene therapy. These updates reflect input from many stakeholders. We encourage additional feedback on these documents during the comment period.
The first draft guidance, Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs), provides sponsors with recommendations on how to provide sufficient CMC information to assure safety, identity, quality, purity and strength/potency of investigational gene therapy products. The guidance applies to human gene therapies and to combination products that contain a human gene therapy in combination with a drug or device. In addition, this guidance is organized to follow the structure of the FDA guidance on the Common Technical Document.
The second draft guidance, Testing of Retroviral Vector-Based Gene Therapy Products for Replication Competent Retrovirus (RCR) during Product Manufacture and Patient Follow-up, provides additional recommendations regarding the proper testing for RCR during the manufacture of retroviral vector-based gene therapy products, as well as during the follow-up monitoring of patients who’ve received retroviral vector-based gene therapy products. Specifically, the draft guidance recommends the identification and amount of material to be tested. The guidance also provides advice on general testing methods.
The third draft guidance, Long Term Follow-Up After Administration of Human Gene Therapy Products, provides recommendations regarding the design of long-term follow-up (LTFU) observational studies for the collection of data on delayed adverse events following administration of a gene therapy product. Because of some of the additional uncertainty intrinsic to a novel platform like gene therapy -- including questions related to the durability of the treatment effects as well as the theoretical potential for off-target effects if the genes do not insert correctly -- there’s an increased need for robust long-term follow-up of patients in the post-market period. This guidance describes product characteristics, patient-related factors, and the preclinical and clinical data that should be considered when assessing the need for LTFU observations and describes the features related to effective post-market follow up.
Once finalized, these draft guidances will replace previous guidances issued by the FDA in April 2008 (CMC) and November 2006 (RCR and LTFU).
The field of gene therapy has progressed rapidly since these guidances were first issued. Therefore, the FDA is updating these guidances to provide sponsors with the agency’s most up-to-date thinking.
Our goal is to help promote safe and effective product development in this field. We’ll continue to work with the product sponsors to help make the development and approval of these innovative gene therapies more efficient, while putting in place the regulatory controls needed to ensure that the resulting therapies are both safe and effective. We’ll also make full use of our expedited programs such as breakthrough therapy designation and regenerative medicine advanced therapy designation whenever possible.
Gene therapy represents one of the most promising opportunities for developing highly effective and even curative treatments for many vexing disorders. Some of these products are almost certainly going to change the contours of medical practice, and the destiny of patients with some debilitating diseases.
The FDA’s goal is to help these innovations advance in a framework that assures the safety and effectiveness of these resulting treatments, and continues to build peoples’ confidence in this novel area of medicine.
–Scott Gottlieb, MD
Antibodies to HCV core protein can be expressed in E. coli and silkworms
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
FROM PROTEIN EXPRESSION AND PURIFICATION
Antegrade Femoral Nail Distal Interlocking Screw Causing Rupture of the Medial Patellofemoral Ligament and Patellar Instability
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
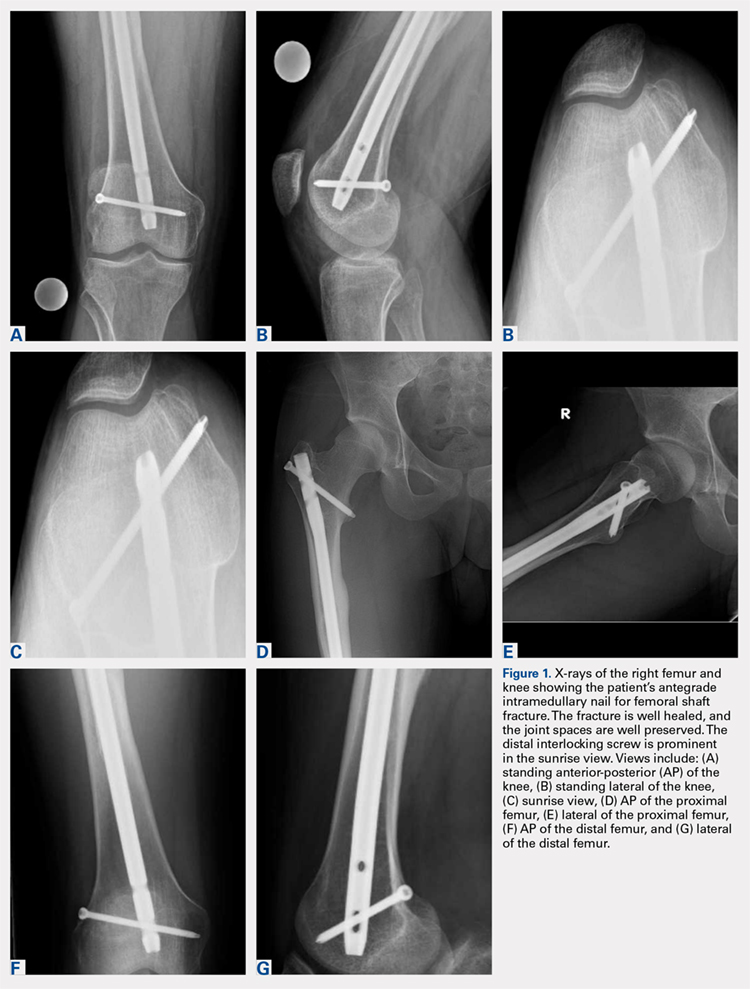
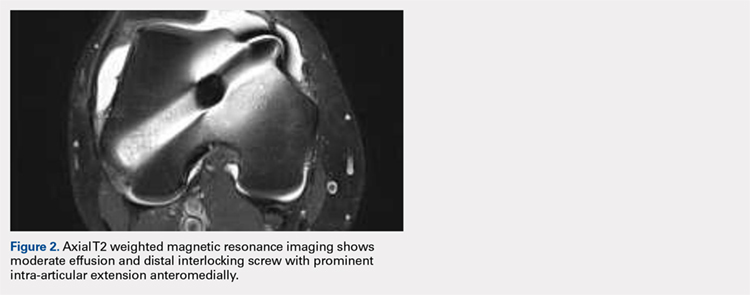
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.
Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).
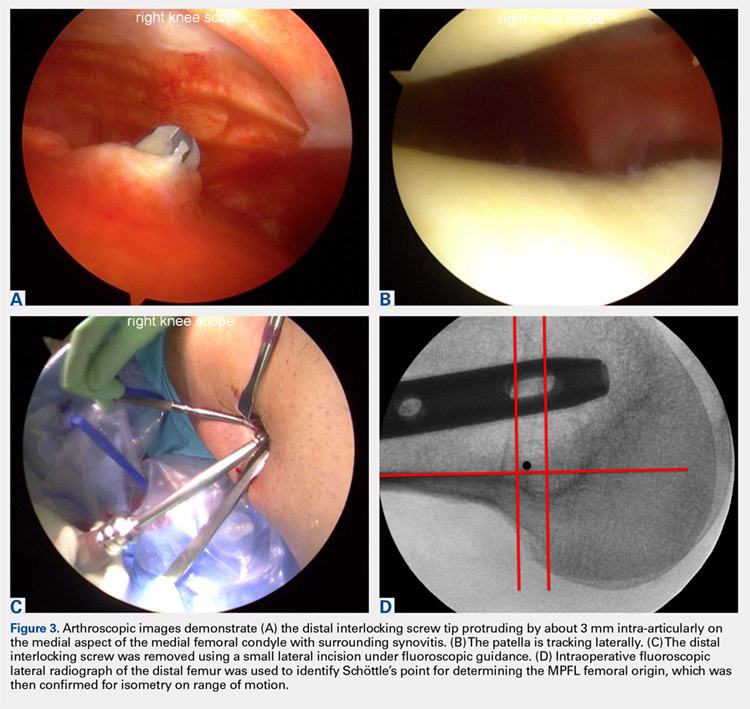
Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
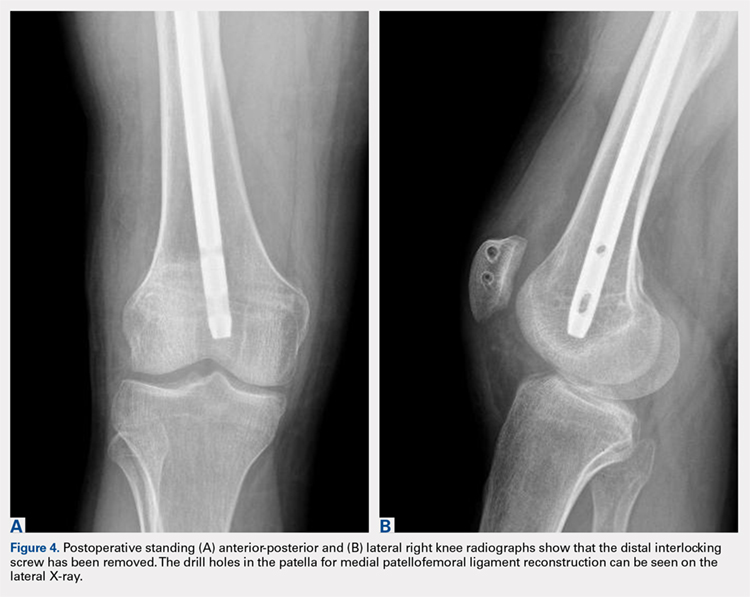
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).
DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
TAKE-HOME POINTS
- Anterograde intramedullary nailing is the gold standard for fixation of diaphyseal femur fractures.
- Damage to the MPFL can be caused by the distal interlocking screw of an anterograde intramedullary nail.
- The trajectory of the distal interlocking screw from posterolateral to anteromedial, and a prominent screw tip, likely contributed to the injury to the MPFL observed in this case.
- Surgeons treating these conditions should pursue advanced imaging if patients present with effusion and patellar instability after femoral intramedullary nail placement.
- Distal interlocking screw removal and arthroscopic MPFL reconstruction can result in successful return of function and normal activities.
Facial and Orbital Asymmetry in Oculofacial Surgery Patients
Facial symmetry plays a role in attractiveness, but a small degree of asymmetry is normal and more common than symmetry. Mild asymmetry has been noted in the general population, even in the absence of pathology such as trauma or craniosynostosis.1,2 Asymmetry may be static or dynamic and is thought to arise from a multitude of developmental factors, including skeletal, neurologic, and soft tissue changes, as well as photoaging.3-5 Cosmetic and reconstructive surgical procedures strive to achieve facial symmetry. Patients often are unaware of their preexisting facial asymmetry.6 Anecdotally, we have found patients tend to be more cognizant of preexisting facial asymmetry following a notable change in facial appearance (eg, surgery). In counseling patients who are considering reconstructive or cosmetic surgery, it is beneficial to identify any preexisting facial asymmetries and discuss if they are within normal limits. The current literature, however, lacks thresholds for what is considered normal in many cases. In this study, we reviewed 100 faces without unilateral or orbital pathology or diplopia to describe the occurrence of facial asymmetries, including larger hemiface and hemiface with greater excursion of motion upon smiling (interpreted to signify stronger seventh cranial nerve), hemiface with more rhytides at rest, higher globe, higher earlobe, and higher lip.
Methods
One hundred oculofacial surgery patients without unilateral or orbital pathology or diplopia were included in this retrospective evaluation of static and dynamic facial asymmetry via facial photography (100 participants). Three graders were provided standard frontal and frontal smiling photographs with overlying facial grids to aid in assessing larger hemiface and hemiface with stronger seventh cranial nerve, which was judged in smiling photographs by assessing the excursion and the vector of motion; more rhytides at rest; higher globe; higher earlobe; and higher lip. Difference in globe height was measured relative to interpupillary distance (IPD) and recorded as the ratio of difference in globe height to IPD. The data were analyzed to see if there were any correlations among the 6 variables. This study was approved by the Duke University Health System (Durham, North Carolina) institutional review board.
Results
One hundred photographs were analyzed including 82 women aged 42 to 85 years and 18 men aged 22 to 88 years (overall average age, 61.64 years). The average difference in globe height was 1.2% of IPD; the maximum was 4.4% of IPD. The difference in globe height was verified by 3 graders via 2 different methods. Fifty-four patients were found to have a larger right hemiface, 36 had a larger left hemiface, and 10 had symmetrically sized hemifaces. Nearly half of patients were judged to have greater seventh cranial nerve action on the left (n=47), approximately one-quarter had greater action on the right (n=28), and another quarter were judged to have equal action (n=25). Most patients had static facial asymmetry; 72 had rhytides more pronounced on one hemiface compared to the other, 79 with a difference in globe height, and 68 with a difference in lip height. In approximately 40% of photographs, the graders were unable to judge earlobe height difference; therefore, this data was not analyzed. There was no correlation among the 6 variables.
Discussion
Facial asymmetry has long been a topic of interest in the plastic and reconstructive surgery fields. Ercan et al7 used statistical shape analysis to study facial asymmetry in young healthy subjects and found the left hemiface to be larger than the right hemiface in both sexes. Smith4 evaluated facial asymmetry in healthy college students and found the left hemiface to be larger in males and the right hemiface to be larger in females. Our group was predominantly female, but we found the right hemiface to be larger in both females and males, similar to the findings of Lepich et al.8
We also found that most patients had static and dynamic facial asymmetry despite no known unilateral pathology. The present literature lacks normative values to help determine what degree of asymmetry should be considered pathologic. Vertical orbital dystopia is defined as an inequality in the horizontal levels of the whole orbits.9 It has been hypothesized that most vertical dystopia is caused by congenital malformations, but no threshold has been set for the difference in height that qualifies as dystopia.10 Regarding the difference we found in globe height relative to IPD, if one takes the mean IPD of 63.36 mm (based on a study of 3976 American adults aged 17–51 years)11 and makes the assumption that our patients have this IPD, then one can extrapolate that on average there was a difference of 0.76 mm between the 2 globe heights. Likewise, nearly all patients (n=96) had less than a 2-mm difference (21 had symmetric globe heights, 46 had a difference in globe height of <1 mm, and 29 had a difference of >1 mm and <2 mm). Four patients had a difference greater than 2 mm, with the largest difference being 2.75 mm. A limitation of this retrospective study is the need to extrapolate these distances, as our patients were not photographed with rulers.
Hafezi et al12 looked at the facial asymmetry in patients without history of trauma or nasal fracture who were seeking rhinoplasty. They noted vertical orbital dystopia in this patient population, but the degree of dystopia was not quantified.12 We believe our data highlight the importance of counseling patients about preexisting facial asymmetry with normative values in mind. Patients may be dissatisfied by new or preexisting asymmetry following surgery, even if such asymmetries are less objectively apparent than in the patient’s preoperative appearance. Even when patients are already acutely aware of their facial asymmetries, they should learn that facial asymmetries, to varying degrees, are natural and not necessarily unattractive. In fact, a 2006
- Wang TT, Wessels L, Hussain G, et al. Discriminative thresholds in facial asymmetry: a review of the literature. Aesthet Surg J. 2017;37:375-385.
- Zaidel DW, Cohen JA. The face, beauty, and symmetry: perceiving asymmetry in beautiful faces. Int J Neurosci. 2005;115:1165-1173.
- Rossi M, Ribeiro E, Smith R. Craniofacial asymmetry in development: an anatomical study. Angle Orthod. 2003;73:381-385.
- Smith WM. Hemispheric and facial asymmetry: gender differences. Laterality. 2000;5:251-258.
- Gordon JR, Brieva JC. Images in clinical medicine. unilateral dermatoheliosis. N Engl J Med. 2012;366:e25.
- Macdonald KI, Mendez AI, Hart RD, et al. Eyelid and brow asymmetry in patients evaluated for upper lid blepharoplasty. J Otolaryngol Head Neck Surg. 2014;43:36.
- Ercan I, Ozdemir ST, Etoz A, et al. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J Anat. 2008;213:663-669.
- Lepich T, Dabek J, Witkowska M, et al. Female and male orbit asymmetry: digital analysis. Adv Clin Exp Med. 2017;26:69-76.
- Tan ST, Ashworth G, Czypionka S, et al. Vertical orbital dystopia. Plast Reconstr Surg. 1996;97:1349-1361.
- De Ponte FS, Fadda T, Rinna C, et al. Early and late surgical treatment of orbital dystopia in craniofacial malformation. J Craniofac Surg. 1997;8:17-22.
- Dodgson NA. Variation and extrema of human interpupillary distance. Proc Int Soc Opt Eng. 2004;5291:36-46.
- Hafezi F, Naghibzadeh B, Nouhi A, et al. Asymmetric facial growth and deviated nose: a new concept. Ann Plast Surg. 2010;64:47-51.
- Ing E, Safarpour A, Ing T, et al. Ocular adnexal asymmetry in models: a magazine photograph analysis. Can J Ophthalmol. 2006;41:175-182.
Facial symmetry plays a role in attractiveness, but a small degree of asymmetry is normal and more common than symmetry. Mild asymmetry has been noted in the general population, even in the absence of pathology such as trauma or craniosynostosis.1,2 Asymmetry may be static or dynamic and is thought to arise from a multitude of developmental factors, including skeletal, neurologic, and soft tissue changes, as well as photoaging.3-5 Cosmetic and reconstructive surgical procedures strive to achieve facial symmetry. Patients often are unaware of their preexisting facial asymmetry.6 Anecdotally, we have found patients tend to be more cognizant of preexisting facial asymmetry following a notable change in facial appearance (eg, surgery). In counseling patients who are considering reconstructive or cosmetic surgery, it is beneficial to identify any preexisting facial asymmetries and discuss if they are within normal limits. The current literature, however, lacks thresholds for what is considered normal in many cases. In this study, we reviewed 100 faces without unilateral or orbital pathology or diplopia to describe the occurrence of facial asymmetries, including larger hemiface and hemiface with greater excursion of motion upon smiling (interpreted to signify stronger seventh cranial nerve), hemiface with more rhytides at rest, higher globe, higher earlobe, and higher lip.
Methods
One hundred oculofacial surgery patients without unilateral or orbital pathology or diplopia were included in this retrospective evaluation of static and dynamic facial asymmetry via facial photography (100 participants). Three graders were provided standard frontal and frontal smiling photographs with overlying facial grids to aid in assessing larger hemiface and hemiface with stronger seventh cranial nerve, which was judged in smiling photographs by assessing the excursion and the vector of motion; more rhytides at rest; higher globe; higher earlobe; and higher lip. Difference in globe height was measured relative to interpupillary distance (IPD) and recorded as the ratio of difference in globe height to IPD. The data were analyzed to see if there were any correlations among the 6 variables. This study was approved by the Duke University Health System (Durham, North Carolina) institutional review board.
Results
One hundred photographs were analyzed including 82 women aged 42 to 85 years and 18 men aged 22 to 88 years (overall average age, 61.64 years). The average difference in globe height was 1.2% of IPD; the maximum was 4.4% of IPD. The difference in globe height was verified by 3 graders via 2 different methods. Fifty-four patients were found to have a larger right hemiface, 36 had a larger left hemiface, and 10 had symmetrically sized hemifaces. Nearly half of patients were judged to have greater seventh cranial nerve action on the left (n=47), approximately one-quarter had greater action on the right (n=28), and another quarter were judged to have equal action (n=25). Most patients had static facial asymmetry; 72 had rhytides more pronounced on one hemiface compared to the other, 79 with a difference in globe height, and 68 with a difference in lip height. In approximately 40% of photographs, the graders were unable to judge earlobe height difference; therefore, this data was not analyzed. There was no correlation among the 6 variables.
Discussion
Facial asymmetry has long been a topic of interest in the plastic and reconstructive surgery fields. Ercan et al7 used statistical shape analysis to study facial asymmetry in young healthy subjects and found the left hemiface to be larger than the right hemiface in both sexes. Smith4 evaluated facial asymmetry in healthy college students and found the left hemiface to be larger in males and the right hemiface to be larger in females. Our group was predominantly female, but we found the right hemiface to be larger in both females and males, similar to the findings of Lepich et al.8
We also found that most patients had static and dynamic facial asymmetry despite no known unilateral pathology. The present literature lacks normative values to help determine what degree of asymmetry should be considered pathologic. Vertical orbital dystopia is defined as an inequality in the horizontal levels of the whole orbits.9 It has been hypothesized that most vertical dystopia is caused by congenital malformations, but no threshold has been set for the difference in height that qualifies as dystopia.10 Regarding the difference we found in globe height relative to IPD, if one takes the mean IPD of 63.36 mm (based on a study of 3976 American adults aged 17–51 years)11 and makes the assumption that our patients have this IPD, then one can extrapolate that on average there was a difference of 0.76 mm between the 2 globe heights. Likewise, nearly all patients (n=96) had less than a 2-mm difference (21 had symmetric globe heights, 46 had a difference in globe height of <1 mm, and 29 had a difference of >1 mm and <2 mm). Four patients had a difference greater than 2 mm, with the largest difference being 2.75 mm. A limitation of this retrospective study is the need to extrapolate these distances, as our patients were not photographed with rulers.
Hafezi et al12 looked at the facial asymmetry in patients without history of trauma or nasal fracture who were seeking rhinoplasty. They noted vertical orbital dystopia in this patient population, but the degree of dystopia was not quantified.12 We believe our data highlight the importance of counseling patients about preexisting facial asymmetry with normative values in mind. Patients may be dissatisfied by new or preexisting asymmetry following surgery, even if such asymmetries are less objectively apparent than in the patient’s preoperative appearance. Even when patients are already acutely aware of their facial asymmetries, they should learn that facial asymmetries, to varying degrees, are natural and not necessarily unattractive. In fact, a 2006
Facial symmetry plays a role in attractiveness, but a small degree of asymmetry is normal and more common than symmetry. Mild asymmetry has been noted in the general population, even in the absence of pathology such as trauma or craniosynostosis.1,2 Asymmetry may be static or dynamic and is thought to arise from a multitude of developmental factors, including skeletal, neurologic, and soft tissue changes, as well as photoaging.3-5 Cosmetic and reconstructive surgical procedures strive to achieve facial symmetry. Patients often are unaware of their preexisting facial asymmetry.6 Anecdotally, we have found patients tend to be more cognizant of preexisting facial asymmetry following a notable change in facial appearance (eg, surgery). In counseling patients who are considering reconstructive or cosmetic surgery, it is beneficial to identify any preexisting facial asymmetries and discuss if they are within normal limits. The current literature, however, lacks thresholds for what is considered normal in many cases. In this study, we reviewed 100 faces without unilateral or orbital pathology or diplopia to describe the occurrence of facial asymmetries, including larger hemiface and hemiface with greater excursion of motion upon smiling (interpreted to signify stronger seventh cranial nerve), hemiface with more rhytides at rest, higher globe, higher earlobe, and higher lip.
Methods
One hundred oculofacial surgery patients without unilateral or orbital pathology or diplopia were included in this retrospective evaluation of static and dynamic facial asymmetry via facial photography (100 participants). Three graders were provided standard frontal and frontal smiling photographs with overlying facial grids to aid in assessing larger hemiface and hemiface with stronger seventh cranial nerve, which was judged in smiling photographs by assessing the excursion and the vector of motion; more rhytides at rest; higher globe; higher earlobe; and higher lip. Difference in globe height was measured relative to interpupillary distance (IPD) and recorded as the ratio of difference in globe height to IPD. The data were analyzed to see if there were any correlations among the 6 variables. This study was approved by the Duke University Health System (Durham, North Carolina) institutional review board.
Results
One hundred photographs were analyzed including 82 women aged 42 to 85 years and 18 men aged 22 to 88 years (overall average age, 61.64 years). The average difference in globe height was 1.2% of IPD; the maximum was 4.4% of IPD. The difference in globe height was verified by 3 graders via 2 different methods. Fifty-four patients were found to have a larger right hemiface, 36 had a larger left hemiface, and 10 had symmetrically sized hemifaces. Nearly half of patients were judged to have greater seventh cranial nerve action on the left (n=47), approximately one-quarter had greater action on the right (n=28), and another quarter were judged to have equal action (n=25). Most patients had static facial asymmetry; 72 had rhytides more pronounced on one hemiface compared to the other, 79 with a difference in globe height, and 68 with a difference in lip height. In approximately 40% of photographs, the graders were unable to judge earlobe height difference; therefore, this data was not analyzed. There was no correlation among the 6 variables.
Discussion
Facial asymmetry has long been a topic of interest in the plastic and reconstructive surgery fields. Ercan et al7 used statistical shape analysis to study facial asymmetry in young healthy subjects and found the left hemiface to be larger than the right hemiface in both sexes. Smith4 evaluated facial asymmetry in healthy college students and found the left hemiface to be larger in males and the right hemiface to be larger in females. Our group was predominantly female, but we found the right hemiface to be larger in both females and males, similar to the findings of Lepich et al.8
We also found that most patients had static and dynamic facial asymmetry despite no known unilateral pathology. The present literature lacks normative values to help determine what degree of asymmetry should be considered pathologic. Vertical orbital dystopia is defined as an inequality in the horizontal levels of the whole orbits.9 It has been hypothesized that most vertical dystopia is caused by congenital malformations, but no threshold has been set for the difference in height that qualifies as dystopia.10 Regarding the difference we found in globe height relative to IPD, if one takes the mean IPD of 63.36 mm (based on a study of 3976 American adults aged 17–51 years)11 and makes the assumption that our patients have this IPD, then one can extrapolate that on average there was a difference of 0.76 mm between the 2 globe heights. Likewise, nearly all patients (n=96) had less than a 2-mm difference (21 had symmetric globe heights, 46 had a difference in globe height of <1 mm, and 29 had a difference of >1 mm and <2 mm). Four patients had a difference greater than 2 mm, with the largest difference being 2.75 mm. A limitation of this retrospective study is the need to extrapolate these distances, as our patients were not photographed with rulers.
Hafezi et al12 looked at the facial asymmetry in patients without history of trauma or nasal fracture who were seeking rhinoplasty. They noted vertical orbital dystopia in this patient population, but the degree of dystopia was not quantified.12 We believe our data highlight the importance of counseling patients about preexisting facial asymmetry with normative values in mind. Patients may be dissatisfied by new or preexisting asymmetry following surgery, even if such asymmetries are less objectively apparent than in the patient’s preoperative appearance. Even when patients are already acutely aware of their facial asymmetries, they should learn that facial asymmetries, to varying degrees, are natural and not necessarily unattractive. In fact, a 2006
- Wang TT, Wessels L, Hussain G, et al. Discriminative thresholds in facial asymmetry: a review of the literature. Aesthet Surg J. 2017;37:375-385.
- Zaidel DW, Cohen JA. The face, beauty, and symmetry: perceiving asymmetry in beautiful faces. Int J Neurosci. 2005;115:1165-1173.
- Rossi M, Ribeiro E, Smith R. Craniofacial asymmetry in development: an anatomical study. Angle Orthod. 2003;73:381-385.
- Smith WM. Hemispheric and facial asymmetry: gender differences. Laterality. 2000;5:251-258.
- Gordon JR, Brieva JC. Images in clinical medicine. unilateral dermatoheliosis. N Engl J Med. 2012;366:e25.
- Macdonald KI, Mendez AI, Hart RD, et al. Eyelid and brow asymmetry in patients evaluated for upper lid blepharoplasty. J Otolaryngol Head Neck Surg. 2014;43:36.
- Ercan I, Ozdemir ST, Etoz A, et al. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J Anat. 2008;213:663-669.
- Lepich T, Dabek J, Witkowska M, et al. Female and male orbit asymmetry: digital analysis. Adv Clin Exp Med. 2017;26:69-76.
- Tan ST, Ashworth G, Czypionka S, et al. Vertical orbital dystopia. Plast Reconstr Surg. 1996;97:1349-1361.
- De Ponte FS, Fadda T, Rinna C, et al. Early and late surgical treatment of orbital dystopia in craniofacial malformation. J Craniofac Surg. 1997;8:17-22.
- Dodgson NA. Variation and extrema of human interpupillary distance. Proc Int Soc Opt Eng. 2004;5291:36-46.
- Hafezi F, Naghibzadeh B, Nouhi A, et al. Asymmetric facial growth and deviated nose: a new concept. Ann Plast Surg. 2010;64:47-51.
- Ing E, Safarpour A, Ing T, et al. Ocular adnexal asymmetry in models: a magazine photograph analysis. Can J Ophthalmol. 2006;41:175-182.
- Wang TT, Wessels L, Hussain G, et al. Discriminative thresholds in facial asymmetry: a review of the literature. Aesthet Surg J. 2017;37:375-385.
- Zaidel DW, Cohen JA. The face, beauty, and symmetry: perceiving asymmetry in beautiful faces. Int J Neurosci. 2005;115:1165-1173.
- Rossi M, Ribeiro E, Smith R. Craniofacial asymmetry in development: an anatomical study. Angle Orthod. 2003;73:381-385.
- Smith WM. Hemispheric and facial asymmetry: gender differences. Laterality. 2000;5:251-258.
- Gordon JR, Brieva JC. Images in clinical medicine. unilateral dermatoheliosis. N Engl J Med. 2012;366:e25.
- Macdonald KI, Mendez AI, Hart RD, et al. Eyelid and brow asymmetry in patients evaluated for upper lid blepharoplasty. J Otolaryngol Head Neck Surg. 2014;43:36.
- Ercan I, Ozdemir ST, Etoz A, et al. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J Anat. 2008;213:663-669.
- Lepich T, Dabek J, Witkowska M, et al. Female and male orbit asymmetry: digital analysis. Adv Clin Exp Med. 2017;26:69-76.
- Tan ST, Ashworth G, Czypionka S, et al. Vertical orbital dystopia. Plast Reconstr Surg. 1996;97:1349-1361.
- De Ponte FS, Fadda T, Rinna C, et al. Early and late surgical treatment of orbital dystopia in craniofacial malformation. J Craniofac Surg. 1997;8:17-22.
- Dodgson NA. Variation and extrema of human interpupillary distance. Proc Int Soc Opt Eng. 2004;5291:36-46.
- Hafezi F, Naghibzadeh B, Nouhi A, et al. Asymmetric facial growth and deviated nose: a new concept. Ann Plast Surg. 2010;64:47-51.
- Ing E, Safarpour A, Ing T, et al. Ocular adnexal asymmetry in models: a magazine photograph analysis. Can J Ophthalmol. 2006;41:175-182.
Resident Pearl
- A small degree of asymmetry is normal and more common than perfect symmetry.
RISE: Insulin glargine, metformin offer no beta cell function benefit in youth
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
EXPERT ANALYSIS AT ADA 2018
Treatment of Grade III Acromioclavicular Separations in Professional Baseball Pitchers: A Survey of Major League Baseball Team Physicians
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
TAKE-HOME POINTS
- There was no difference in return to previous level of play between professional pitchers treated nonoperatively and operatively for grade III AC separation.
- MLB team physicians prefer nonoperative management for acute grade III AC joint separation in professional pitchers.
- The majority of MLB physicians do not use injections for nonoperative treatment of grade III AC separations; however, use of orthobiologics (eg, PRP) is becoming more commonplace.
- Persistent functional limitations and pain are the most common surgical indications for treatment of grade III AC separation in high level throwing athletes.
- If operative intervention is indicated for grade III AC separation, open coracoclavicular reconstruction and adjunct distal clavicle excision are preferred by most MLB team physicians.
Ibrutinib/venetoclax shows early promise in relapsed CLL
STOCKHOLM – A chemotherapy-free regimen of ibrutinib plus venetoclax was generally safe and showed promising early efficacy in patients with relapsed or refractory chronic lymphocytic leukemia, investigators reported.
A planned interim analysis performed after the first 15 patients who had received two cycles of ibrutinib plus one of ibrutinib and venetoclax showed no treatment-related deaths or treatment interruptions, and all patients had clinical responses, including 8 with complete clinical remission (CR), reported Carsten U. Niemann, MD, PhD, from Rigshospitalet in Copenhagen, and his colleagues.
The goal of the ongoing VISION/HOVEN 141 study is to evaluate whether minimal residual disease (MRD)–guided therapy with the Bruton tyrosine kinase inhibitor ibrutinib and the BCL2 inhibitor venetoclax could lead to MRD negativity and allow select patients to stop treatment, Dr. Niemann said in an interview at the annual congress of the European Hematology Association.
“It’s a 100% clinical response rate and 53% CR. Obviously these are clinical responses, so we don’t have the CT scans, we don’t have the bone marrow biopsies, but we’re very happy to see even in the relapsed/refractory setting such good response rates,” he said.
The investigators are enrolling patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic leukemia requiring treatment and starting all patients on ibrutinib 420 mg daily for the first 2 cycles, with venetoclax added in a 5-week ramp-up from 20 mg beginning with cycle 3 to a final dose of 400 mg daily for 15 total treatment cycles.
At the end of the induction phase, patients who are determined to be MRD-negative by flow cytometry at cycles 12 and 15, and by bone marrow at cycle 15, are randomized on a 1:2 basis to ibrutinib maintenance until disease progression or intolerable toxicity, or to observation until progression or loss of MRD negativity, at which time they start maintenance with ibrutinib until progression or toxicity, plus 12 months of venetoclax.
All 15 patients who were followed for 3 months had clinical responses, including 8 CRs (53%), 6 partial remissions (40%), and 1 partial remission with lymphocytosis (7%).
Three patients had ibrutinib dose reductions and two had venetoclax dose reductions, but no patients stopped treatment. Three patients had grade 2 adverse events (AEs), three had grade 3 AEs, and two had grade 4 AEs. There were no grade 5 AEs.
Two patients had serious AEs during the first two cycles with ibrutinib alone, one of which was a case of febrile neutropenia and one which was an adenocarcinoma of the lung. There were no serious AEs reported during venetoclax ramp-up. To date, there have been no cases of tumor lysis syndrome, atrial fibrillation, or bleeding events reported.
The results suggest that treatment with ibrutinib and venetoclax ramp-up is manageable in this patient population, and the study is ongoing, with further results expected to be reported at either the 2018 annual meeting of the American Society of Hematology or the 2019 annual meeting of the American Society of Clinical Oncology, Dr. Niemann said.
The study is supported by AbbVie and Janssen, which supplied the drugs and had the right to comment on the presentation. Dr. Niemann has previously disclosed consultancy fees from those companies and others.
SOURCE: Niemann CU et al. EHA Congress, Abstract PF346.
STOCKHOLM – A chemotherapy-free regimen of ibrutinib plus venetoclax was generally safe and showed promising early efficacy in patients with relapsed or refractory chronic lymphocytic leukemia, investigators reported.
A planned interim analysis performed after the first 15 patients who had received two cycles of ibrutinib plus one of ibrutinib and venetoclax showed no treatment-related deaths or treatment interruptions, and all patients had clinical responses, including 8 with complete clinical remission (CR), reported Carsten U. Niemann, MD, PhD, from Rigshospitalet in Copenhagen, and his colleagues.
The goal of the ongoing VISION/HOVEN 141 study is to evaluate whether minimal residual disease (MRD)–guided therapy with the Bruton tyrosine kinase inhibitor ibrutinib and the BCL2 inhibitor venetoclax could lead to MRD negativity and allow select patients to stop treatment, Dr. Niemann said in an interview at the annual congress of the European Hematology Association.
“It’s a 100% clinical response rate and 53% CR. Obviously these are clinical responses, so we don’t have the CT scans, we don’t have the bone marrow biopsies, but we’re very happy to see even in the relapsed/refractory setting such good response rates,” he said.
The investigators are enrolling patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic leukemia requiring treatment and starting all patients on ibrutinib 420 mg daily for the first 2 cycles, with venetoclax added in a 5-week ramp-up from 20 mg beginning with cycle 3 to a final dose of 400 mg daily for 15 total treatment cycles.
At the end of the induction phase, patients who are determined to be MRD-negative by flow cytometry at cycles 12 and 15, and by bone marrow at cycle 15, are randomized on a 1:2 basis to ibrutinib maintenance until disease progression or intolerable toxicity, or to observation until progression or loss of MRD negativity, at which time they start maintenance with ibrutinib until progression or toxicity, plus 12 months of venetoclax.
All 15 patients who were followed for 3 months had clinical responses, including 8 CRs (53%), 6 partial remissions (40%), and 1 partial remission with lymphocytosis (7%).
Three patients had ibrutinib dose reductions and two had venetoclax dose reductions, but no patients stopped treatment. Three patients had grade 2 adverse events (AEs), three had grade 3 AEs, and two had grade 4 AEs. There were no grade 5 AEs.
Two patients had serious AEs during the first two cycles with ibrutinib alone, one of which was a case of febrile neutropenia and one which was an adenocarcinoma of the lung. There were no serious AEs reported during venetoclax ramp-up. To date, there have been no cases of tumor lysis syndrome, atrial fibrillation, or bleeding events reported.
The results suggest that treatment with ibrutinib and venetoclax ramp-up is manageable in this patient population, and the study is ongoing, with further results expected to be reported at either the 2018 annual meeting of the American Society of Hematology or the 2019 annual meeting of the American Society of Clinical Oncology, Dr. Niemann said.
The study is supported by AbbVie and Janssen, which supplied the drugs and had the right to comment on the presentation. Dr. Niemann has previously disclosed consultancy fees from those companies and others.
SOURCE: Niemann CU et al. EHA Congress, Abstract PF346.
STOCKHOLM – A chemotherapy-free regimen of ibrutinib plus venetoclax was generally safe and showed promising early efficacy in patients with relapsed or refractory chronic lymphocytic leukemia, investigators reported.
A planned interim analysis performed after the first 15 patients who had received two cycles of ibrutinib plus one of ibrutinib and venetoclax showed no treatment-related deaths or treatment interruptions, and all patients had clinical responses, including 8 with complete clinical remission (CR), reported Carsten U. Niemann, MD, PhD, from Rigshospitalet in Copenhagen, and his colleagues.
The goal of the ongoing VISION/HOVEN 141 study is to evaluate whether minimal residual disease (MRD)–guided therapy with the Bruton tyrosine kinase inhibitor ibrutinib and the BCL2 inhibitor venetoclax could lead to MRD negativity and allow select patients to stop treatment, Dr. Niemann said in an interview at the annual congress of the European Hematology Association.
“It’s a 100% clinical response rate and 53% CR. Obviously these are clinical responses, so we don’t have the CT scans, we don’t have the bone marrow biopsies, but we’re very happy to see even in the relapsed/refractory setting such good response rates,” he said.
The investigators are enrolling patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic leukemia requiring treatment and starting all patients on ibrutinib 420 mg daily for the first 2 cycles, with venetoclax added in a 5-week ramp-up from 20 mg beginning with cycle 3 to a final dose of 400 mg daily for 15 total treatment cycles.
At the end of the induction phase, patients who are determined to be MRD-negative by flow cytometry at cycles 12 and 15, and by bone marrow at cycle 15, are randomized on a 1:2 basis to ibrutinib maintenance until disease progression or intolerable toxicity, or to observation until progression or loss of MRD negativity, at which time they start maintenance with ibrutinib until progression or toxicity, plus 12 months of venetoclax.
All 15 patients who were followed for 3 months had clinical responses, including 8 CRs (53%), 6 partial remissions (40%), and 1 partial remission with lymphocytosis (7%).
Three patients had ibrutinib dose reductions and two had venetoclax dose reductions, but no patients stopped treatment. Three patients had grade 2 adverse events (AEs), three had grade 3 AEs, and two had grade 4 AEs. There were no grade 5 AEs.
Two patients had serious AEs during the first two cycles with ibrutinib alone, one of which was a case of febrile neutropenia and one which was an adenocarcinoma of the lung. There were no serious AEs reported during venetoclax ramp-up. To date, there have been no cases of tumor lysis syndrome, atrial fibrillation, or bleeding events reported.
The results suggest that treatment with ibrutinib and venetoclax ramp-up is manageable in this patient population, and the study is ongoing, with further results expected to be reported at either the 2018 annual meeting of the American Society of Hematology or the 2019 annual meeting of the American Society of Clinical Oncology, Dr. Niemann said.
The study is supported by AbbVie and Janssen, which supplied the drugs and had the right to comment on the presentation. Dr. Niemann has previously disclosed consultancy fees from those companies and others.
SOURCE: Niemann CU et al. EHA Congress, Abstract PF346.
REPORTING FROM THE EHA CONGRESS
Key clinical point:
Major finding: All of the 15 patients analyzed to date had clinical responses to the combination, including 8 complete clinical remissions.
Study details: An ongoing, open-label, phase 2, randomized trial in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic leukemia.
Disclosures: The study is supported by AbbVie and Janssen, which supplied the drugs and had the right to comment on the presentation. Dr. Niemann has previously disclosed consultancy fees from those companies and others.
Source: Niemann CU et al. EHA Congress, Abstract PF346
Identifying patients with a high risk of AML
Researchers say they have found ways to identify patients with a high risk of developing acute myeloid leukemia (AML).
The researchers used basic clinical and laboratory data to identify patients 6 to 12 months before AML presentation.
Using genetic information, the researchers were able to identify high-risk patients several years before AML presentation.
“This long time window gives us the first opportunity to think about how to prevent AML,” said John Dick, PhD, of Princess Margaret Cancer Centre in Toronto, Ontario, Canada.
However, Dr Dick and his colleagues noted that neither the genetic method nor the clinical data method were entirely accurate in identifying patients who would develop AML.
The researchers described both methods in Nature.
The team’s work began with the goal of distinguishing patients who will develop AML from patients who simply develop age-related clonal hematopoiesis.
“We wanted to know if there was any difference between these 2 groups in the genetics of their ‘normal’ blood samples taken at enrollment,” Dr Dick said. “To find out, we developed a gene-sequencing tool that captured the most common genes that get altered in AML and sequenced all the 500 blood samples.”
The researchers analyzed samples from 95 patients who ultimately developed AML and 414 age- and gender-matched controls. The samples from AML patients were obtained an average of 6.3 years before AML diagnosis.
The researchers found that pre-AML cases had more mutations per sample, higher variant allele frequencies, and a higher frequency of certain mutations than controls. Specifically, pre-AML cases had a significantly greater frequency of DNMT3A, TET2, SRSF2, ASXL1, TP53, U2AF1, JAK2, RUNX1, and IDH2 mutations.
The researchers used these findings to develop a model for predicting AML. They tested the model in a validation cohort of 29 pre-AML cases and 262 controls, as well as in a cohort combining the validation and discovery cohorts. The model predicted AML development with 41.9% sensitivity and 95.7% specificity.
However, the researchers said widespread use of this model would not be practical because AML is so rare. The team said millions of people would need to undergo screening to identify a few pre-AML cases, and there would be “many” false-positives.
Therefore, the researchers developed a model using clinical information from electronic health records—particularly blood counts. This model was able to predict AML development 6 to 12 months before diagnosis with a sensitivity of 25.7% and specificity of 98.2%.
The researchers said these results suggest clinical data can be used to identify patients with a high risk of AML who may benefit from targeted genetic screening. And combining clinical and genetic information in a single model could improve predictive accuracy.
“Our study provides, for the first time, evidence that we can identify people at risk of developing AML many years before they actually develop this life-threatening disease,” said study author George Vassiliou, PhD, of the Wellcome Trust Sanger Institute in Hinxton, UK.
“We hope to build on these findings to develop robust screening tests for identifying those at risk and drive research into how to prevent or stall progression towards AML. Our aspiration is that, one day, AML prevention would provide a compelling alternative to treatment.”
Researchers say they have found ways to identify patients with a high risk of developing acute myeloid leukemia (AML).
The researchers used basic clinical and laboratory data to identify patients 6 to 12 months before AML presentation.
Using genetic information, the researchers were able to identify high-risk patients several years before AML presentation.
“This long time window gives us the first opportunity to think about how to prevent AML,” said John Dick, PhD, of Princess Margaret Cancer Centre in Toronto, Ontario, Canada.
However, Dr Dick and his colleagues noted that neither the genetic method nor the clinical data method were entirely accurate in identifying patients who would develop AML.
The researchers described both methods in Nature.
The team’s work began with the goal of distinguishing patients who will develop AML from patients who simply develop age-related clonal hematopoiesis.
“We wanted to know if there was any difference between these 2 groups in the genetics of their ‘normal’ blood samples taken at enrollment,” Dr Dick said. “To find out, we developed a gene-sequencing tool that captured the most common genes that get altered in AML and sequenced all the 500 blood samples.”
The researchers analyzed samples from 95 patients who ultimately developed AML and 414 age- and gender-matched controls. The samples from AML patients were obtained an average of 6.3 years before AML diagnosis.
The researchers found that pre-AML cases had more mutations per sample, higher variant allele frequencies, and a higher frequency of certain mutations than controls. Specifically, pre-AML cases had a significantly greater frequency of DNMT3A, TET2, SRSF2, ASXL1, TP53, U2AF1, JAK2, RUNX1, and IDH2 mutations.
The researchers used these findings to develop a model for predicting AML. They tested the model in a validation cohort of 29 pre-AML cases and 262 controls, as well as in a cohort combining the validation and discovery cohorts. The model predicted AML development with 41.9% sensitivity and 95.7% specificity.
However, the researchers said widespread use of this model would not be practical because AML is so rare. The team said millions of people would need to undergo screening to identify a few pre-AML cases, and there would be “many” false-positives.
Therefore, the researchers developed a model using clinical information from electronic health records—particularly blood counts. This model was able to predict AML development 6 to 12 months before diagnosis with a sensitivity of 25.7% and specificity of 98.2%.
The researchers said these results suggest clinical data can be used to identify patients with a high risk of AML who may benefit from targeted genetic screening. And combining clinical and genetic information in a single model could improve predictive accuracy.
“Our study provides, for the first time, evidence that we can identify people at risk of developing AML many years before they actually develop this life-threatening disease,” said study author George Vassiliou, PhD, of the Wellcome Trust Sanger Institute in Hinxton, UK.
“We hope to build on these findings to develop robust screening tests for identifying those at risk and drive research into how to prevent or stall progression towards AML. Our aspiration is that, one day, AML prevention would provide a compelling alternative to treatment.”
Researchers say they have found ways to identify patients with a high risk of developing acute myeloid leukemia (AML).
The researchers used basic clinical and laboratory data to identify patients 6 to 12 months before AML presentation.
Using genetic information, the researchers were able to identify high-risk patients several years before AML presentation.
“This long time window gives us the first opportunity to think about how to prevent AML,” said John Dick, PhD, of Princess Margaret Cancer Centre in Toronto, Ontario, Canada.
However, Dr Dick and his colleagues noted that neither the genetic method nor the clinical data method were entirely accurate in identifying patients who would develop AML.
The researchers described both methods in Nature.
The team’s work began with the goal of distinguishing patients who will develop AML from patients who simply develop age-related clonal hematopoiesis.
“We wanted to know if there was any difference between these 2 groups in the genetics of their ‘normal’ blood samples taken at enrollment,” Dr Dick said. “To find out, we developed a gene-sequencing tool that captured the most common genes that get altered in AML and sequenced all the 500 blood samples.”
The researchers analyzed samples from 95 patients who ultimately developed AML and 414 age- and gender-matched controls. The samples from AML patients were obtained an average of 6.3 years before AML diagnosis.
The researchers found that pre-AML cases had more mutations per sample, higher variant allele frequencies, and a higher frequency of certain mutations than controls. Specifically, pre-AML cases had a significantly greater frequency of DNMT3A, TET2, SRSF2, ASXL1, TP53, U2AF1, JAK2, RUNX1, and IDH2 mutations.
The researchers used these findings to develop a model for predicting AML. They tested the model in a validation cohort of 29 pre-AML cases and 262 controls, as well as in a cohort combining the validation and discovery cohorts. The model predicted AML development with 41.9% sensitivity and 95.7% specificity.
However, the researchers said widespread use of this model would not be practical because AML is so rare. The team said millions of people would need to undergo screening to identify a few pre-AML cases, and there would be “many” false-positives.
Therefore, the researchers developed a model using clinical information from electronic health records—particularly blood counts. This model was able to predict AML development 6 to 12 months before diagnosis with a sensitivity of 25.7% and specificity of 98.2%.
The researchers said these results suggest clinical data can be used to identify patients with a high risk of AML who may benefit from targeted genetic screening. And combining clinical and genetic information in a single model could improve predictive accuracy.
“Our study provides, for the first time, evidence that we can identify people at risk of developing AML many years before they actually develop this life-threatening disease,” said study author George Vassiliou, PhD, of the Wellcome Trust Sanger Institute in Hinxton, UK.
“We hope to build on these findings to develop robust screening tests for identifying those at risk and drive research into how to prevent or stall progression towards AML. Our aspiration is that, one day, AML prevention would provide a compelling alternative to treatment.”
FDA grants EUA for freeze-dried plasma product
The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for a freeze-dried plasma product to be used by the military.
The product is Pathogen-Reduced Leukocyte-Depleted Freeze-Dried Plasma manufactured by the Centre de Transfusion Sanguine des Armées, referred to in the EUA as “French FDP.”
Under the EUA, French FDP is authorized for the treatment of hemorrhage or coagulopathy in US military personnel during an emergency involving agents of military combat (eg, firearms, projectiles, and explosive devices) when plasma is not available or when the use of plasma is not practical.
The use of plasma in combat settings is severely limited by logistical and operational challenges, such as the need for refrigeration and, in the case of frozen plasma, a long thawing period.
French FDP is a powdered, freeze-dried product that can be used following reconstitution in settings where refrigeration is not available, thus enabling the rapid availability of plasma for use at the point of injury.
The FDA issued the EUA for French FDP in response to a request from the US Department of Defense (DoD) and after receiving the required determination by DoD and a declaration by the Secretary of the Department of Health and Human Services.
This action is the result of a collaboration between the FDA and the DoD to prioritize development of medical products intended to help save the lives of American military personnel. The FDA outlined its approach to advancing the development of such products in a work plan developed with DoD.
“Earlier this year, we reaffirmed our commitment to the Department of Defense and to the dedicated men and women protecting our country by expediting the development and availability of safe and effective priority medical products that are essential to the health of our military service members,” said FDA Commissioner Scott Gottlieb, MD.
“Through our collaborative program with the DoD, they’ve made clear the importance of access to freeze-dried plasma in initial efforts to control hemorrhage from battlefield trauma. Granting this authorization will support access to this important product in the event it’s needed. The FDA remains deeply committed to implementing an enduring pathway to ensure that these potentially life-saving medical products are made available in the most expeditious, safe, and effective manner possible.”
The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for a freeze-dried plasma product to be used by the military.
The product is Pathogen-Reduced Leukocyte-Depleted Freeze-Dried Plasma manufactured by the Centre de Transfusion Sanguine des Armées, referred to in the EUA as “French FDP.”
Under the EUA, French FDP is authorized for the treatment of hemorrhage or coagulopathy in US military personnel during an emergency involving agents of military combat (eg, firearms, projectiles, and explosive devices) when plasma is not available or when the use of plasma is not practical.
The use of plasma in combat settings is severely limited by logistical and operational challenges, such as the need for refrigeration and, in the case of frozen plasma, a long thawing period.
French FDP is a powdered, freeze-dried product that can be used following reconstitution in settings where refrigeration is not available, thus enabling the rapid availability of plasma for use at the point of injury.
The FDA issued the EUA for French FDP in response to a request from the US Department of Defense (DoD) and after receiving the required determination by DoD and a declaration by the Secretary of the Department of Health and Human Services.
This action is the result of a collaboration between the FDA and the DoD to prioritize development of medical products intended to help save the lives of American military personnel. The FDA outlined its approach to advancing the development of such products in a work plan developed with DoD.
“Earlier this year, we reaffirmed our commitment to the Department of Defense and to the dedicated men and women protecting our country by expediting the development and availability of safe and effective priority medical products that are essential to the health of our military service members,” said FDA Commissioner Scott Gottlieb, MD.
“Through our collaborative program with the DoD, they’ve made clear the importance of access to freeze-dried plasma in initial efforts to control hemorrhage from battlefield trauma. Granting this authorization will support access to this important product in the event it’s needed. The FDA remains deeply committed to implementing an enduring pathway to ensure that these potentially life-saving medical products are made available in the most expeditious, safe, and effective manner possible.”
The US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for a freeze-dried plasma product to be used by the military.
The product is Pathogen-Reduced Leukocyte-Depleted Freeze-Dried Plasma manufactured by the Centre de Transfusion Sanguine des Armées, referred to in the EUA as “French FDP.”
Under the EUA, French FDP is authorized for the treatment of hemorrhage or coagulopathy in US military personnel during an emergency involving agents of military combat (eg, firearms, projectiles, and explosive devices) when plasma is not available or when the use of plasma is not practical.
The use of plasma in combat settings is severely limited by logistical and operational challenges, such as the need for refrigeration and, in the case of frozen plasma, a long thawing period.
French FDP is a powdered, freeze-dried product that can be used following reconstitution in settings where refrigeration is not available, thus enabling the rapid availability of plasma for use at the point of injury.
The FDA issued the EUA for French FDP in response to a request from the US Department of Defense (DoD) and after receiving the required determination by DoD and a declaration by the Secretary of the Department of Health and Human Services.
This action is the result of a collaboration between the FDA and the DoD to prioritize development of medical products intended to help save the lives of American military personnel. The FDA outlined its approach to advancing the development of such products in a work plan developed with DoD.
“Earlier this year, we reaffirmed our commitment to the Department of Defense and to the dedicated men and women protecting our country by expediting the development and availability of safe and effective priority medical products that are essential to the health of our military service members,” said FDA Commissioner Scott Gottlieb, MD.
“Through our collaborative program with the DoD, they’ve made clear the importance of access to freeze-dried plasma in initial efforts to control hemorrhage from battlefield trauma. Granting this authorization will support access to this important product in the event it’s needed. The FDA remains deeply committed to implementing an enduring pathway to ensure that these potentially life-saving medical products are made available in the most expeditious, safe, and effective manner possible.”
New approach to AE reporting needed, group says
A group of experts has called for improvements in reporting adverse events (AEs) that occur in patients with hematologic malignancies.
The group highlighted deficiencies in capturing chronic, cumulative, and late AEs; collecting patient-reported outcomes (PROs); reporting AEs associated with hematopoietic stem cell transplant (HSCT); assessing long-term toxicity in survivors; reporting AEs to regulatory agencies; and tracking AEs that occur in routine clinical practice.
The experts discussed these problems and made recommendations for fixing them in The Lancet Haematology.
“The success in outcomes and survival in many hematological malignancies is historically unparalleled and fueled by scientific discovery and implementation,” said author Gita Thanarajasingam, MD, of the Mayo Clinic in Rochester, Minnesota.
“Patients are now living with the challenge of managing not just their hematological malignancy but also managing chronic therapy for their illness, with new types of acute, chronic, cumulative, and late toxicities. Measures to address the broad facets of toxicity assessment must be prioritized and further developed to ultimately enhance accurate, comprehensive, patient-centered toxicity reporting that will meaningfully inform the care of patients with blood cancers.”
Dr Thanarajasingam and a group of clinicians, investigators, regulators, biostatisticians, and patient advocates analyzed the evidence on AEs and proposed recommendations to policy makers, researchers, industry, and regulators.
First, the group noted that chronic, delayed, and cumulative AEs may go unreported in patients with hematologic malignancies. Therefore, longitudinal methods for AE analysis are needed, and phase 1 trials should have longer periods for evaluating dose-limiting toxicity.
The experts also said PROs are often overlooked, but it should be standard to assess PROs in clinical trials of patients with hematologic malignancies.
Another of the group’s concerns is the “cumbersome” reporting of AEs associated with HSCT acting as a barrier to clinical trials. The experts recommended using registry data to develop a consensus on expected AEs after HSCT.
The group also highlighted deficiencies in the “description and management” of cumulative and late toxicities in survivors of hematologic malignancies. Potential solutions to this problem include developing infrastructure to collect data for adult survivors and standardizing the use and content of survivorship care plans.
The experts said “meaningful” AEs are often underreported to regulatory agencies, so better systems are needed for collecting and analyzing AE data, and the electronic submission of AEs should be simplified.
Finally, the group said AEs occurring in routine clinical practice are difficult to capture and analyze on a large scale. This suggests a need to optimize the systematic, objective collection of toxicity data at multiple points in real-world databases, according to the experts.
Additional details and recommendations are available in the full report.
A group of experts has called for improvements in reporting adverse events (AEs) that occur in patients with hematologic malignancies.
The group highlighted deficiencies in capturing chronic, cumulative, and late AEs; collecting patient-reported outcomes (PROs); reporting AEs associated with hematopoietic stem cell transplant (HSCT); assessing long-term toxicity in survivors; reporting AEs to regulatory agencies; and tracking AEs that occur in routine clinical practice.
The experts discussed these problems and made recommendations for fixing them in The Lancet Haematology.
“The success in outcomes and survival in many hematological malignancies is historically unparalleled and fueled by scientific discovery and implementation,” said author Gita Thanarajasingam, MD, of the Mayo Clinic in Rochester, Minnesota.
“Patients are now living with the challenge of managing not just their hematological malignancy but also managing chronic therapy for their illness, with new types of acute, chronic, cumulative, and late toxicities. Measures to address the broad facets of toxicity assessment must be prioritized and further developed to ultimately enhance accurate, comprehensive, patient-centered toxicity reporting that will meaningfully inform the care of patients with blood cancers.”
Dr Thanarajasingam and a group of clinicians, investigators, regulators, biostatisticians, and patient advocates analyzed the evidence on AEs and proposed recommendations to policy makers, researchers, industry, and regulators.
First, the group noted that chronic, delayed, and cumulative AEs may go unreported in patients with hematologic malignancies. Therefore, longitudinal methods for AE analysis are needed, and phase 1 trials should have longer periods for evaluating dose-limiting toxicity.
The experts also said PROs are often overlooked, but it should be standard to assess PROs in clinical trials of patients with hematologic malignancies.
Another of the group’s concerns is the “cumbersome” reporting of AEs associated with HSCT acting as a barrier to clinical trials. The experts recommended using registry data to develop a consensus on expected AEs after HSCT.
The group also highlighted deficiencies in the “description and management” of cumulative and late toxicities in survivors of hematologic malignancies. Potential solutions to this problem include developing infrastructure to collect data for adult survivors and standardizing the use and content of survivorship care plans.
The experts said “meaningful” AEs are often underreported to regulatory agencies, so better systems are needed for collecting and analyzing AE data, and the electronic submission of AEs should be simplified.
Finally, the group said AEs occurring in routine clinical practice are difficult to capture and analyze on a large scale. This suggests a need to optimize the systematic, objective collection of toxicity data at multiple points in real-world databases, according to the experts.
Additional details and recommendations are available in the full report.
A group of experts has called for improvements in reporting adverse events (AEs) that occur in patients with hematologic malignancies.
The group highlighted deficiencies in capturing chronic, cumulative, and late AEs; collecting patient-reported outcomes (PROs); reporting AEs associated with hematopoietic stem cell transplant (HSCT); assessing long-term toxicity in survivors; reporting AEs to regulatory agencies; and tracking AEs that occur in routine clinical practice.
The experts discussed these problems and made recommendations for fixing them in The Lancet Haematology.
“The success in outcomes and survival in many hematological malignancies is historically unparalleled and fueled by scientific discovery and implementation,” said author Gita Thanarajasingam, MD, of the Mayo Clinic in Rochester, Minnesota.
“Patients are now living with the challenge of managing not just their hematological malignancy but also managing chronic therapy for their illness, with new types of acute, chronic, cumulative, and late toxicities. Measures to address the broad facets of toxicity assessment must be prioritized and further developed to ultimately enhance accurate, comprehensive, patient-centered toxicity reporting that will meaningfully inform the care of patients with blood cancers.”
Dr Thanarajasingam and a group of clinicians, investigators, regulators, biostatisticians, and patient advocates analyzed the evidence on AEs and proposed recommendations to policy makers, researchers, industry, and regulators.
First, the group noted that chronic, delayed, and cumulative AEs may go unreported in patients with hematologic malignancies. Therefore, longitudinal methods for AE analysis are needed, and phase 1 trials should have longer periods for evaluating dose-limiting toxicity.
The experts also said PROs are often overlooked, but it should be standard to assess PROs in clinical trials of patients with hematologic malignancies.
Another of the group’s concerns is the “cumbersome” reporting of AEs associated with HSCT acting as a barrier to clinical trials. The experts recommended using registry data to develop a consensus on expected AEs after HSCT.
The group also highlighted deficiencies in the “description and management” of cumulative and late toxicities in survivors of hematologic malignancies. Potential solutions to this problem include developing infrastructure to collect data for adult survivors and standardizing the use and content of survivorship care plans.
The experts said “meaningful” AEs are often underreported to regulatory agencies, so better systems are needed for collecting and analyzing AE data, and the electronic submission of AEs should be simplified.
Finally, the group said AEs occurring in routine clinical practice are difficult to capture and analyze on a large scale. This suggests a need to optimize the systematic, objective collection of toxicity data at multiple points in real-world databases, according to the experts.
Additional details and recommendations are available in the full report.