User login
Screening for brain mets could improve quality of life for some with breast cancer
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
FROM JAMA ONCOLOGY
Key clinical point: Breast cancer patients presented with larger and more numerous brain metastases compared with non–small-cell lung cancer patients, but after brain-directed therapy, there were no differences in outcomes between groups.
Major finding: Median survival was 1.45 years for breast cancer patients and 1.09 for NSCLC patients.
Study details: A retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Disclosures: The authors reported no conflicts of interest.
Source: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
The ‘Other’ Risks of High Blood Pressure
Other cardiovascular disease outcomes, such as kidney disease and dementia, are less known than the “tradional” outcomes, such as heart attack, heart failure, and stroke. In a Centers for Disease Control and Prevention (CDC) study of 4,166 adults, only 38.5% of those with self-reported hypertension were aware of the risk of kidney disease, versus 24.8% of normotensive adults. Awareness of the risk of dementia was “markedly low”: 8.7% and 7.9%, respectively.
The researchers found “notable” socioeconomic and racial/ethnic differences in awareness of risks. For example, high-income respondents had greater awareness of the association between uncontrolled hypertension and kidney disease, stroke, and dementia, when compared with low-income respondents. Non-Hispanic whites had greater awareness of risk than non-Hispanic blacks (who have a higher prevalence of hypertension and uncontrolled hypertension).
Nearly 35 million people have uncontrolled hypertension, the researchers note. To educate more diverse patient populations about the risks, they recommend expanding current initiatives such as the “Mind Your Risks” program (https://mindyourrisks.nih.gov), promoted by the National Institute of Neurological Disorders and Stroke; and the “Measure Up/Pressure Down (www.measureuppressuredown.com) program, sponsored by the American Medical Group Association.
Other cardiovascular disease outcomes, such as kidney disease and dementia, are less known than the “tradional” outcomes, such as heart attack, heart failure, and stroke. In a Centers for Disease Control and Prevention (CDC) study of 4,166 adults, only 38.5% of those with self-reported hypertension were aware of the risk of kidney disease, versus 24.8% of normotensive adults. Awareness of the risk of dementia was “markedly low”: 8.7% and 7.9%, respectively.
The researchers found “notable” socioeconomic and racial/ethnic differences in awareness of risks. For example, high-income respondents had greater awareness of the association between uncontrolled hypertension and kidney disease, stroke, and dementia, when compared with low-income respondents. Non-Hispanic whites had greater awareness of risk than non-Hispanic blacks (who have a higher prevalence of hypertension and uncontrolled hypertension).
Nearly 35 million people have uncontrolled hypertension, the researchers note. To educate more diverse patient populations about the risks, they recommend expanding current initiatives such as the “Mind Your Risks” program (https://mindyourrisks.nih.gov), promoted by the National Institute of Neurological Disorders and Stroke; and the “Measure Up/Pressure Down (www.measureuppressuredown.com) program, sponsored by the American Medical Group Association.
Other cardiovascular disease outcomes, such as kidney disease and dementia, are less known than the “tradional” outcomes, such as heart attack, heart failure, and stroke. In a Centers for Disease Control and Prevention (CDC) study of 4,166 adults, only 38.5% of those with self-reported hypertension were aware of the risk of kidney disease, versus 24.8% of normotensive adults. Awareness of the risk of dementia was “markedly low”: 8.7% and 7.9%, respectively.
The researchers found “notable” socioeconomic and racial/ethnic differences in awareness of risks. For example, high-income respondents had greater awareness of the association between uncontrolled hypertension and kidney disease, stroke, and dementia, when compared with low-income respondents. Non-Hispanic whites had greater awareness of risk than non-Hispanic blacks (who have a higher prevalence of hypertension and uncontrolled hypertension).
Nearly 35 million people have uncontrolled hypertension, the researchers note. To educate more diverse patient populations about the risks, they recommend expanding current initiatives such as the “Mind Your Risks” program (https://mindyourrisks.nih.gov), promoted by the National Institute of Neurological Disorders and Stroke; and the “Measure Up/Pressure Down (www.measureuppressuredown.com) program, sponsored by the American Medical Group Association.
Perioperative rVWF alone sufficient for some VWD patients
GLASGOW—Recombinant von Willebrand factor (rVWF) alone can be sufficient as perioperative management for some patients with severe von Willebrand disease (VWD), according to researchers.
In a phase 3 study, 10 of 15 patients were able to achieve hemostatic efficacy ratings of “good” or “excellent” when receiving only rVWF before, during, and/or after surgery.
The remaining 5 patients also achieved favorable hemostatic efficacy ratings, but they received recombinant factor VIII (FVIII) as well.
These results were presented at the World Federation of Hemophilia (WFH) 2018 World Congress (abstract W-MP-63 [749]). The research was sponsored by Shire, the company marketing rVWF as Vonvendi.
“There is an unmet clinical need for those living with von Willebrand disease, as they face a heightened risk of bleeding during surgery,” said study investigator Flora Peyvandi, MD, PhD, of the University of Milan in Italy.
“People with von Willebrand disease lack proper function or quantity of von Willebrand factor, and some also have a secondary factor VIII deficiency. In this study, recombinant von Willebrand factor was administered to replace the insufficient or dysfunctional von Willebrand factor, allowing the body to naturally replenish FVIII in most patients. These study results demonstrate clinical promise as physicians were able to tailor treatment based on each patient’s individual need for one or both factor therapies.”
The study included 15 adults with severe VWD who were undergoing elective surgical procedures. Ten patients were undergoing major surgery, 4 minor, and 1 oral surgery.
The patients’ median age was 40 (range, 20-70), and 8 were female. Most (n=8) had type 3 VWD, 3 had type 1, 2 had type 2A, 1 had 2B, and 1 had 2M.
At baseline, the mean endogenous FVIII level (FVIII:C) was 16.4 IU/dL, and the mean VWF ristocetin cofactor (VWF:Rco) was 10.6 IU/dL.
The patients received rVWF at 40 to 60 IU/kg VWF:RCo intravenously 12 to 24 hours before surgery to allow FVIII:C levels to increase to at least 30 IU/dL for minor or oral surgery or to at least 60 IU/dL for major surgery, within 3 hours before surgery.
If the desired levels were achieved, rVWF could be given alone. If the levels were not achieved, patients would receive rFVIII as well, within 1 to 2 hours before surgery. Patients were monitored for 14 days after surgery.
Results
All 15 patients had overall/intraoperative hemostatic efficacy ratings of “excellent” (as good as or better than expected) or “good” (probably as good as expected).
The patients received a median of 6 (range, 2 to 15) rVWF infusions at a median dose of 55 IU/kg (range, 36.1 to 59.9). Most patients (n=11) did not receive rVWF every day. For some, infusions were separated by 2 to 9 days.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Most rVWF infusions (89.4%, 93/104) were given alone, and 70% (7/10) of the major surgeries were performed with rVWF alone.
The researchers said that, with rVWF alone, patients had hemostatically effective levels of FVIII:C as early as 6 hours after surgery, and this was sustained for 72 to 96 hours.
There were 5 patients who received rVWF with rFVIII. Of the 11 infusions these patients received, 9 were given when FVIII:C levels were above 60 IU/dL.
Three patients received rVWF with rFVIII 1 hour before major surgery—total hip replacement, molar extraction, and left ankle prosthesis. However, 2 of these patients had FVIII:C levels above 60 IU/dL.
The patient undergoing a molar extraction received rVWF with rFVIII 6 times after surgery. In 5 cases, the patient’s FVIII:C levels were 110 to 152 IU/dL. In the remaining case, the FVIII:C level was 23 IU/dL.
Two patients received rVWF with rFVIII for minor surgery. One patient undergoing a tooth extraction received rVWF with rFVIII intraoperatively when the FVIII:C level was 72 IU/dL.
The other patient received rVWF with rFVIII after radioisotope synovectomy when the FVIII:C level was 73 IU/dL. This patient received a postoperative dose of rVWF alone as well.
One patient tested positive for binding antibodies to VWF, and 1 patient developed deep vein thrombosis 3 days after total hip replacement while receiving rVWF.
GLASGOW—Recombinant von Willebrand factor (rVWF) alone can be sufficient as perioperative management for some patients with severe von Willebrand disease (VWD), according to researchers.
In a phase 3 study, 10 of 15 patients were able to achieve hemostatic efficacy ratings of “good” or “excellent” when receiving only rVWF before, during, and/or after surgery.
The remaining 5 patients also achieved favorable hemostatic efficacy ratings, but they received recombinant factor VIII (FVIII) as well.
These results were presented at the World Federation of Hemophilia (WFH) 2018 World Congress (abstract W-MP-63 [749]). The research was sponsored by Shire, the company marketing rVWF as Vonvendi.
“There is an unmet clinical need for those living with von Willebrand disease, as they face a heightened risk of bleeding during surgery,” said study investigator Flora Peyvandi, MD, PhD, of the University of Milan in Italy.
“People with von Willebrand disease lack proper function or quantity of von Willebrand factor, and some also have a secondary factor VIII deficiency. In this study, recombinant von Willebrand factor was administered to replace the insufficient or dysfunctional von Willebrand factor, allowing the body to naturally replenish FVIII in most patients. These study results demonstrate clinical promise as physicians were able to tailor treatment based on each patient’s individual need for one or both factor therapies.”
The study included 15 adults with severe VWD who were undergoing elective surgical procedures. Ten patients were undergoing major surgery, 4 minor, and 1 oral surgery.
The patients’ median age was 40 (range, 20-70), and 8 were female. Most (n=8) had type 3 VWD, 3 had type 1, 2 had type 2A, 1 had 2B, and 1 had 2M.
At baseline, the mean endogenous FVIII level (FVIII:C) was 16.4 IU/dL, and the mean VWF ristocetin cofactor (VWF:Rco) was 10.6 IU/dL.
The patients received rVWF at 40 to 60 IU/kg VWF:RCo intravenously 12 to 24 hours before surgery to allow FVIII:C levels to increase to at least 30 IU/dL for minor or oral surgery or to at least 60 IU/dL for major surgery, within 3 hours before surgery.
If the desired levels were achieved, rVWF could be given alone. If the levels were not achieved, patients would receive rFVIII as well, within 1 to 2 hours before surgery. Patients were monitored for 14 days after surgery.
Results
All 15 patients had overall/intraoperative hemostatic efficacy ratings of “excellent” (as good as or better than expected) or “good” (probably as good as expected).
The patients received a median of 6 (range, 2 to 15) rVWF infusions at a median dose of 55 IU/kg (range, 36.1 to 59.9). Most patients (n=11) did not receive rVWF every day. For some, infusions were separated by 2 to 9 days.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Most rVWF infusions (89.4%, 93/104) were given alone, and 70% (7/10) of the major surgeries were performed with rVWF alone.
The researchers said that, with rVWF alone, patients had hemostatically effective levels of FVIII:C as early as 6 hours after surgery, and this was sustained for 72 to 96 hours.
There were 5 patients who received rVWF with rFVIII. Of the 11 infusions these patients received, 9 were given when FVIII:C levels were above 60 IU/dL.
Three patients received rVWF with rFVIII 1 hour before major surgery—total hip replacement, molar extraction, and left ankle prosthesis. However, 2 of these patients had FVIII:C levels above 60 IU/dL.
The patient undergoing a molar extraction received rVWF with rFVIII 6 times after surgery. In 5 cases, the patient’s FVIII:C levels were 110 to 152 IU/dL. In the remaining case, the FVIII:C level was 23 IU/dL.
Two patients received rVWF with rFVIII for minor surgery. One patient undergoing a tooth extraction received rVWF with rFVIII intraoperatively when the FVIII:C level was 72 IU/dL.
The other patient received rVWF with rFVIII after radioisotope synovectomy when the FVIII:C level was 73 IU/dL. This patient received a postoperative dose of rVWF alone as well.
One patient tested positive for binding antibodies to VWF, and 1 patient developed deep vein thrombosis 3 days after total hip replacement while receiving rVWF.
GLASGOW—Recombinant von Willebrand factor (rVWF) alone can be sufficient as perioperative management for some patients with severe von Willebrand disease (VWD), according to researchers.
In a phase 3 study, 10 of 15 patients were able to achieve hemostatic efficacy ratings of “good” or “excellent” when receiving only rVWF before, during, and/or after surgery.
The remaining 5 patients also achieved favorable hemostatic efficacy ratings, but they received recombinant factor VIII (FVIII) as well.
These results were presented at the World Federation of Hemophilia (WFH) 2018 World Congress (abstract W-MP-63 [749]). The research was sponsored by Shire, the company marketing rVWF as Vonvendi.
“There is an unmet clinical need for those living with von Willebrand disease, as they face a heightened risk of bleeding during surgery,” said study investigator Flora Peyvandi, MD, PhD, of the University of Milan in Italy.
“People with von Willebrand disease lack proper function or quantity of von Willebrand factor, and some also have a secondary factor VIII deficiency. In this study, recombinant von Willebrand factor was administered to replace the insufficient or dysfunctional von Willebrand factor, allowing the body to naturally replenish FVIII in most patients. These study results demonstrate clinical promise as physicians were able to tailor treatment based on each patient’s individual need for one or both factor therapies.”
The study included 15 adults with severe VWD who were undergoing elective surgical procedures. Ten patients were undergoing major surgery, 4 minor, and 1 oral surgery.
The patients’ median age was 40 (range, 20-70), and 8 were female. Most (n=8) had type 3 VWD, 3 had type 1, 2 had type 2A, 1 had 2B, and 1 had 2M.
At baseline, the mean endogenous FVIII level (FVIII:C) was 16.4 IU/dL, and the mean VWF ristocetin cofactor (VWF:Rco) was 10.6 IU/dL.
The patients received rVWF at 40 to 60 IU/kg VWF:RCo intravenously 12 to 24 hours before surgery to allow FVIII:C levels to increase to at least 30 IU/dL for minor or oral surgery or to at least 60 IU/dL for major surgery, within 3 hours before surgery.
If the desired levels were achieved, rVWF could be given alone. If the levels were not achieved, patients would receive rFVIII as well, within 1 to 2 hours before surgery. Patients were monitored for 14 days after surgery.
Results
All 15 patients had overall/intraoperative hemostatic efficacy ratings of “excellent” (as good as or better than expected) or “good” (probably as good as expected).
The patients received a median of 6 (range, 2 to 15) rVWF infusions at a median dose of 55 IU/kg (range, 36.1 to 59.9). Most patients (n=11) did not receive rVWF every day. For some, infusions were separated by 2 to 9 days.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Most rVWF infusions (89.4%, 93/104) were given alone, and 70% (7/10) of the major surgeries were performed with rVWF alone.
The researchers said that, with rVWF alone, patients had hemostatically effective levels of FVIII:C as early as 6 hours after surgery, and this was sustained for 72 to 96 hours.
There were 5 patients who received rVWF with rFVIII. Of the 11 infusions these patients received, 9 were given when FVIII:C levels were above 60 IU/dL.
Three patients received rVWF with rFVIII 1 hour before major surgery—total hip replacement, molar extraction, and left ankle prosthesis. However, 2 of these patients had FVIII:C levels above 60 IU/dL.
The patient undergoing a molar extraction received rVWF with rFVIII 6 times after surgery. In 5 cases, the patient’s FVIII:C levels were 110 to 152 IU/dL. In the remaining case, the FVIII:C level was 23 IU/dL.
Two patients received rVWF with rFVIII for minor surgery. One patient undergoing a tooth extraction received rVWF with rFVIII intraoperatively when the FVIII:C level was 72 IU/dL.
The other patient received rVWF with rFVIII after radioisotope synovectomy when the FVIII:C level was 73 IU/dL. This patient received a postoperative dose of rVWF alone as well.
One patient tested positive for binding antibodies to VWF, and 1 patient developed deep vein thrombosis 3 days after total hip replacement while receiving rVWF.
FDA clears device for treatment of PE
The US Food and Drug Administration (FDA) has granted 510(k) clearance to the FlowTriever System for the treatment of pulmonary embolism (PE).
This makes the FlowTriever System the first and only thrombectomy device cleared by the FDA for the treatment of PE.
The 510(k) clearance was based on results from the FlowTriever Pulmonary Embolectomy (FLARE) study.
In this prospective, single-arm study, researchers evaluated the FlowTriever System in 106 patients with acute PE. Patients with proximal PE and right heart strain (RV/LV ratio ≥ 0.9) were eligible to participate.
Treatment with the FlowTriever System was used to non-surgically remove blood clots in the pulmonary arteries without the need for thrombolytic drugs.
The mean RV/LV ratio decreased from a baseline of 1.53 to 1.15 at 48 hours post-procedure, a difference of 0.39 (P<0.0001).
At 30 days, the rate of major adverse events was 3.8%. There were no device-related complications.
Patients had a median hospital stay of 3 days and a median stay in the intensive care unit of 1 day.
“The FlowTriever System is an exciting advancement in the treatment of acute pulmonary embolism patients,” said FLARE investigator Wissam Jaber, MD, of Emory University Hospital in Atlanta, Georgia.
“Until now, there has not been an approach to rapidly restore flow to reverse right heart strain without the use of thrombolytic drugs and their inherent risk of bleeding complications. The FlowTriever System represents a breakthrough in treatment options for this large patient population.”
The FlowTriever System is a product of Inari Medical, Inc.
The US Food and Drug Administration (FDA) has granted 510(k) clearance to the FlowTriever System for the treatment of pulmonary embolism (PE).
This makes the FlowTriever System the first and only thrombectomy device cleared by the FDA for the treatment of PE.
The 510(k) clearance was based on results from the FlowTriever Pulmonary Embolectomy (FLARE) study.
In this prospective, single-arm study, researchers evaluated the FlowTriever System in 106 patients with acute PE. Patients with proximal PE and right heart strain (RV/LV ratio ≥ 0.9) were eligible to participate.
Treatment with the FlowTriever System was used to non-surgically remove blood clots in the pulmonary arteries without the need for thrombolytic drugs.
The mean RV/LV ratio decreased from a baseline of 1.53 to 1.15 at 48 hours post-procedure, a difference of 0.39 (P<0.0001).
At 30 days, the rate of major adverse events was 3.8%. There were no device-related complications.
Patients had a median hospital stay of 3 days and a median stay in the intensive care unit of 1 day.
“The FlowTriever System is an exciting advancement in the treatment of acute pulmonary embolism patients,” said FLARE investigator Wissam Jaber, MD, of Emory University Hospital in Atlanta, Georgia.
“Until now, there has not been an approach to rapidly restore flow to reverse right heart strain without the use of thrombolytic drugs and their inherent risk of bleeding complications. The FlowTriever System represents a breakthrough in treatment options for this large patient population.”
The FlowTriever System is a product of Inari Medical, Inc.
The US Food and Drug Administration (FDA) has granted 510(k) clearance to the FlowTriever System for the treatment of pulmonary embolism (PE).
This makes the FlowTriever System the first and only thrombectomy device cleared by the FDA for the treatment of PE.
The 510(k) clearance was based on results from the FlowTriever Pulmonary Embolectomy (FLARE) study.
In this prospective, single-arm study, researchers evaluated the FlowTriever System in 106 patients with acute PE. Patients with proximal PE and right heart strain (RV/LV ratio ≥ 0.9) were eligible to participate.
Treatment with the FlowTriever System was used to non-surgically remove blood clots in the pulmonary arteries without the need for thrombolytic drugs.
The mean RV/LV ratio decreased from a baseline of 1.53 to 1.15 at 48 hours post-procedure, a difference of 0.39 (P<0.0001).
At 30 days, the rate of major adverse events was 3.8%. There were no device-related complications.
Patients had a median hospital stay of 3 days and a median stay in the intensive care unit of 1 day.
“The FlowTriever System is an exciting advancement in the treatment of acute pulmonary embolism patients,” said FLARE investigator Wissam Jaber, MD, of Emory University Hospital in Atlanta, Georgia.
“Until now, there has not been an approach to rapidly restore flow to reverse right heart strain without the use of thrombolytic drugs and their inherent risk of bleeding complications. The FlowTriever System represents a breakthrough in treatment options for this large patient population.”
The FlowTriever System is a product of Inari Medical, Inc.
Emicizumab controls bleeding regardless of inhibitors
GLASGOW—Emicizumab prophylaxis provides “clinically meaningful” control of bleeding whether or not patients have factor VIII inhibitors, according to researchers.
In the phase 3 HAVEN 4 study, researchers evaluated emicizumab prophylaxis, given every 4 weeks, in hemophilia A patients with or without factor VIII inhibitors.
At a median follow-up of about 26 weeks, patients had a median annualized bleeding rate (ABR) of 0.0 for treated bleeds and 2.1 for all bleeds.
About 30% of patients had 0 bleeds, and about 56% had 0 treated bleeds.
There were no serious adverse events (AEs) related to emicizumab. The most common AE was injection-site reaction.
Steve Pipe, MD, of Mott Children’s Hospital in Ann Arbor, Michigan, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The trial was sponsored by Hoffmann-La Roche.
HAVEN 4 included 48 patients, age 12 and older, who had hemophilia A with or without factor VIII inhibitors. Patients were previously treated with factor VIII or bypassing agents, on-demand or as prophylaxis.
The study was conducted in 2 parts: a pharmacokinetic (PK) run-in and an expansion cohort.
All patients in the PK run-in (n=7) were previously treated on-demand and received subcutaneous emicizumab at 6 mg/kg to fully characterize the PK profile after a single dose during 4 weeks. This was followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Patients in the expansion cohort (n=41) received subcutaneous emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Episodic treatment of breakthrough bleeds with factor VIII therapy or bypassing agents, depending on a patient’s factor VIII inhibitor status, was allowed per study protocol.
Results
The efficacy analysis included the 41 patients in the expansion cohort, 5 of whom had inhibitors at baseline.
The median efficacy period was 25.6 weeks. The median ABR was 2.1 for all bleeds and 0.0 for treated bleeds.
The percentage of patients with 0 bleeds was 29.3% for all bleeds, 56.1% for treated bleeds, 82.9% for treated spontaneous bleeds, 70.7% for treated joint bleeds, and 85.4% for treated target joint bleeds.
Most treated bleeds (74.5%, 38/51) were traumatic.
There were 148 AEs, and 73.2% of patients had at least 1 AE.
Injection-site reaction was the most common AE related to emicizumab, occurring in 22.0% of patients (n=9).
There were 2 serious AEs (grade ≥3)—hypertension and rhabdomyolysis. Both were considered unrelated to emicizumab.
There were no AEs leading to emicizumab discontinuation or withdrawal. There were no thrombotic events, cases of thrombotic microangiopathy, hypersensitivity reactions, or fatalities.
None of the patients developed de novo factor VIII inhibitors, and there were no anti-drug antibodies detected.
GLASGOW—Emicizumab prophylaxis provides “clinically meaningful” control of bleeding whether or not patients have factor VIII inhibitors, according to researchers.
In the phase 3 HAVEN 4 study, researchers evaluated emicizumab prophylaxis, given every 4 weeks, in hemophilia A patients with or without factor VIII inhibitors.
At a median follow-up of about 26 weeks, patients had a median annualized bleeding rate (ABR) of 0.0 for treated bleeds and 2.1 for all bleeds.
About 30% of patients had 0 bleeds, and about 56% had 0 treated bleeds.
There were no serious adverse events (AEs) related to emicizumab. The most common AE was injection-site reaction.
Steve Pipe, MD, of Mott Children’s Hospital in Ann Arbor, Michigan, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The trial was sponsored by Hoffmann-La Roche.
HAVEN 4 included 48 patients, age 12 and older, who had hemophilia A with or without factor VIII inhibitors. Patients were previously treated with factor VIII or bypassing agents, on-demand or as prophylaxis.
The study was conducted in 2 parts: a pharmacokinetic (PK) run-in and an expansion cohort.
All patients in the PK run-in (n=7) were previously treated on-demand and received subcutaneous emicizumab at 6 mg/kg to fully characterize the PK profile after a single dose during 4 weeks. This was followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Patients in the expansion cohort (n=41) received subcutaneous emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Episodic treatment of breakthrough bleeds with factor VIII therapy or bypassing agents, depending on a patient’s factor VIII inhibitor status, was allowed per study protocol.
Results
The efficacy analysis included the 41 patients in the expansion cohort, 5 of whom had inhibitors at baseline.
The median efficacy period was 25.6 weeks. The median ABR was 2.1 for all bleeds and 0.0 for treated bleeds.
The percentage of patients with 0 bleeds was 29.3% for all bleeds, 56.1% for treated bleeds, 82.9% for treated spontaneous bleeds, 70.7% for treated joint bleeds, and 85.4% for treated target joint bleeds.
Most treated bleeds (74.5%, 38/51) were traumatic.
There were 148 AEs, and 73.2% of patients had at least 1 AE.
Injection-site reaction was the most common AE related to emicizumab, occurring in 22.0% of patients (n=9).
There were 2 serious AEs (grade ≥3)—hypertension and rhabdomyolysis. Both were considered unrelated to emicizumab.
There were no AEs leading to emicizumab discontinuation or withdrawal. There were no thrombotic events, cases of thrombotic microangiopathy, hypersensitivity reactions, or fatalities.
None of the patients developed de novo factor VIII inhibitors, and there were no anti-drug antibodies detected.
GLASGOW—Emicizumab prophylaxis provides “clinically meaningful” control of bleeding whether or not patients have factor VIII inhibitors, according to researchers.
In the phase 3 HAVEN 4 study, researchers evaluated emicizumab prophylaxis, given every 4 weeks, in hemophilia A patients with or without factor VIII inhibitors.
At a median follow-up of about 26 weeks, patients had a median annualized bleeding rate (ABR) of 0.0 for treated bleeds and 2.1 for all bleeds.
About 30% of patients had 0 bleeds, and about 56% had 0 treated bleeds.
There were no serious adverse events (AEs) related to emicizumab. The most common AE was injection-site reaction.
Steve Pipe, MD, of Mott Children’s Hospital in Ann Arbor, Michigan, presented these results at the World Federation of Hemophilia (WFH) 2018 World Congress during the late-breaking abstract session on Monday.
The trial was sponsored by Hoffmann-La Roche.
HAVEN 4 included 48 patients, age 12 and older, who had hemophilia A with or without factor VIII inhibitors. Patients were previously treated with factor VIII or bypassing agents, on-demand or as prophylaxis.
The study was conducted in 2 parts: a pharmacokinetic (PK) run-in and an expansion cohort.
All patients in the PK run-in (n=7) were previously treated on-demand and received subcutaneous emicizumab at 6 mg/kg to fully characterize the PK profile after a single dose during 4 weeks. This was followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Patients in the expansion cohort (n=41) received subcutaneous emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 6 mg/kg every 4 weeks for at least 24 weeks.
Episodic treatment of breakthrough bleeds with factor VIII therapy or bypassing agents, depending on a patient’s factor VIII inhibitor status, was allowed per study protocol.
Results
The efficacy analysis included the 41 patients in the expansion cohort, 5 of whom had inhibitors at baseline.
The median efficacy period was 25.6 weeks. The median ABR was 2.1 for all bleeds and 0.0 for treated bleeds.
The percentage of patients with 0 bleeds was 29.3% for all bleeds, 56.1% for treated bleeds, 82.9% for treated spontaneous bleeds, 70.7% for treated joint bleeds, and 85.4% for treated target joint bleeds.
Most treated bleeds (74.5%, 38/51) were traumatic.
There were 148 AEs, and 73.2% of patients had at least 1 AE.
Injection-site reaction was the most common AE related to emicizumab, occurring in 22.0% of patients (n=9).
There were 2 serious AEs (grade ≥3)—hypertension and rhabdomyolysis. Both were considered unrelated to emicizumab.
There were no AEs leading to emicizumab discontinuation or withdrawal. There were no thrombotic events, cases of thrombotic microangiopathy, hypersensitivity reactions, or fatalities.
None of the patients developed de novo factor VIII inhibitors, and there were no anti-drug antibodies detected.
Large dark discoloration on the back
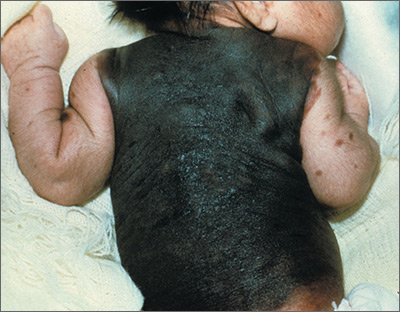
The FP recognized that this child had a large bathing trunk nevus with multiple small melanocytic satellite lesions on her arms.
He explained to the worried parents that their daughter had a bathing trunk nevus and that a local expert was needed. The FP consulted a local dermatologist, who subsequently explained to the parents that there was a significant risk of cutaneous melanoma if nothing was done about this large congenital nevus. The dermatologist indicated that while removal could decrease that risk, the process would require multiple large surgeries by a plastic surgeon. She also explained that a magnetic resonance imaging scan of the brain would be needed at about 6 months to look for neurocutaneous melanosis, which can cause seizures, hydrocephalus, and a central nervous system melanoma.
The parents were conflicted about whether to put their child through a series of massive surgeries or to accept the higher risk of melanoma and proceed with careful monitoring by the dermatologist. (No additional details on how this case resolved are available—Editor.)
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith, M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized that this child had a large bathing trunk nevus with multiple small melanocytic satellite lesions on her arms.
He explained to the worried parents that their daughter had a bathing trunk nevus and that a local expert was needed. The FP consulted a local dermatologist, who subsequently explained to the parents that there was a significant risk of cutaneous melanoma if nothing was done about this large congenital nevus. The dermatologist indicated that while removal could decrease that risk, the process would require multiple large surgeries by a plastic surgeon. She also explained that a magnetic resonance imaging scan of the brain would be needed at about 6 months to look for neurocutaneous melanosis, which can cause seizures, hydrocephalus, and a central nervous system melanoma.
The parents were conflicted about whether to put their child through a series of massive surgeries or to accept the higher risk of melanoma and proceed with careful monitoring by the dermatologist. (No additional details on how this case resolved are available—Editor.)
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith, M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized that this child had a large bathing trunk nevus with multiple small melanocytic satellite lesions on her arms.
He explained to the worried parents that their daughter had a bathing trunk nevus and that a local expert was needed. The FP consulted a local dermatologist, who subsequently explained to the parents that there was a significant risk of cutaneous melanoma if nothing was done about this large congenital nevus. The dermatologist indicated that while removal could decrease that risk, the process would require multiple large surgeries by a plastic surgeon. She also explained that a magnetic resonance imaging scan of the brain would be needed at about 6 months to look for neurocutaneous melanosis, which can cause seizures, hydrocephalus, and a central nervous system melanoma.
The parents were conflicted about whether to put their child through a series of massive surgeries or to accept the higher risk of melanoma and proceed with careful monitoring by the dermatologist. (No additional details on how this case resolved are available—Editor.)
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith, M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Atopic eczema linked to cardiovascular disease risk
according to a case-control study published online May 23 in BMJ.
In a population-based cohort study, researchers compared the electronic health records of 387,439 adults with eczema and 1,528,477 patients without eczema in the United Kingddom, matched according to age, sex, general practice, and calendar time, during 1998-2015. Patients were followed up for a median of 5.1 years
With the exception of cardiovascular death, atopic eczema was associated with all cardiovascular outcomes (MI, unstable angina, heart failure, atrial fibrillation, and stroke). The associations were stronger for severe atopic eczema, with significantly higher risks of MI, unstable angina, atrial fibrillation, stroke, cardiovascular death, and coronary revascularization among individuals with severe atopic eczema, compared with controls.
After adjustment for potential mediators such as body mass index, smoking, hyperlipidemia, diabetes, and severe alcohol use, individuals with severe eczema had a significant 37% increased risk of MI, 67% greater risk of heart failure, 35% greater risk of atrial fibrillation, 30% greater risk of cardiovascular death, and 36% greater risk of coronary revascularization, compared with controls with no eczema.
Increased cardiovascular risks also were seen in individuals whose atopic eczema was active more than half the time at follow-up. This group had a 37% greater risk of heart failure, 36% greater risk of unstable angina, and 19% greater risk of stroke, as well as significantly elevated risks of MI, atrial fibrillation, cardiovascular death, and coronary revascularization, compared with those without eczema.
Overall, atopic eczema contributed around 2.4% of the population-attributable risk for unstable angina, and 1.9% for heart failure (the highest population attributable risks). Ethnicity or high-dose corticosteroid use did not significantly impact outcomes.
Richard J. Silverwood, PhD, from the London School of Hygiene and Tropical Medicine, and his coauthors wrote that previous work examining the relationship between atopic eczema and cardiovascular disease had shown inconsistent outcomes, with some studies even pointing to a possible protective effect of mild atopic eczema.
“Mechanistic work suggests that atopic eczema may be associated with increased platelet activation and decreased fibrinolysis, which may increase the risk of clotting, though a recent study found no association with metabolite levels,” the authors wrote.
They noted that the strengths of their study were that it was largest to examine the association between atopic eczema and cardiovascular risk, and that they had access to data on body mass index, smoking, and severe alcohol use for most of the study population, which enabled them to adjust for these potential mediators.
“Consideration should be given to developing prevention strategies to reduce the risk of cardiovascular disease among patients with severe or predominantly active atopic eczema, including awareness of and screening for conventional cardiovascular risk factors by those providing clinical care,” they wrote.
The study was supported by the Wellcome Trust, and no relevant conflicts of interest were declared.
SOURCE: Silverwood R et al. BMJ 2018 May 23;361:k1786. doi: 10.1136/bmj.k1786.
The evidence in favor of a link between chronic inflammatory conditions, such as rheumatoid arthritis, and cardiovascular disease is growing. However, there are conflicting data and some of the uncertainty may be a result of a dose-response effect, where the increased cardiovascular risk is seen only in people with more severe disease.
This study and its finding of increased cardiovascular risk in patients with severe or more active eczema supports the case for targeted screening of this group for standard cardiovascular disease risk factors. It also could prompt incorporation of severe eczema as an independent cardiovascular disease risk factor in calculation of thresholds for primary prevention interventions.
The findings also may have implications for health care resources allocated to treatment of eczema, as prevention of cardiovascular disease could contribute to the argument in favor of the more expensive next-generation biologic treatments for eczema that are becoming available.
John R. Ingram, MD, is senior lecturer and consultant dermatologist, dermatology and academic wound healing in the division of infection and immunity at Cardiff (U.K.) University. These comments are taken from an accompanying editorial No conflicts of interest were declared. (BMJ. 2018 May 23. doi: 10.1136/bmj.k2064).
The evidence in favor of a link between chronic inflammatory conditions, such as rheumatoid arthritis, and cardiovascular disease is growing. However, there are conflicting data and some of the uncertainty may be a result of a dose-response effect, where the increased cardiovascular risk is seen only in people with more severe disease.
This study and its finding of increased cardiovascular risk in patients with severe or more active eczema supports the case for targeted screening of this group for standard cardiovascular disease risk factors. It also could prompt incorporation of severe eczema as an independent cardiovascular disease risk factor in calculation of thresholds for primary prevention interventions.
The findings also may have implications for health care resources allocated to treatment of eczema, as prevention of cardiovascular disease could contribute to the argument in favor of the more expensive next-generation biologic treatments for eczema that are becoming available.
John R. Ingram, MD, is senior lecturer and consultant dermatologist, dermatology and academic wound healing in the division of infection and immunity at Cardiff (U.K.) University. These comments are taken from an accompanying editorial No conflicts of interest were declared. (BMJ. 2018 May 23. doi: 10.1136/bmj.k2064).
The evidence in favor of a link between chronic inflammatory conditions, such as rheumatoid arthritis, and cardiovascular disease is growing. However, there are conflicting data and some of the uncertainty may be a result of a dose-response effect, where the increased cardiovascular risk is seen only in people with more severe disease.
This study and its finding of increased cardiovascular risk in patients with severe or more active eczema supports the case for targeted screening of this group for standard cardiovascular disease risk factors. It also could prompt incorporation of severe eczema as an independent cardiovascular disease risk factor in calculation of thresholds for primary prevention interventions.
The findings also may have implications for health care resources allocated to treatment of eczema, as prevention of cardiovascular disease could contribute to the argument in favor of the more expensive next-generation biologic treatments for eczema that are becoming available.
John R. Ingram, MD, is senior lecturer and consultant dermatologist, dermatology and academic wound healing in the division of infection and immunity at Cardiff (U.K.) University. These comments are taken from an accompanying editorial No conflicts of interest were declared. (BMJ. 2018 May 23. doi: 10.1136/bmj.k2064).
according to a case-control study published online May 23 in BMJ.
In a population-based cohort study, researchers compared the electronic health records of 387,439 adults with eczema and 1,528,477 patients without eczema in the United Kingddom, matched according to age, sex, general practice, and calendar time, during 1998-2015. Patients were followed up for a median of 5.1 years
With the exception of cardiovascular death, atopic eczema was associated with all cardiovascular outcomes (MI, unstable angina, heart failure, atrial fibrillation, and stroke). The associations were stronger for severe atopic eczema, with significantly higher risks of MI, unstable angina, atrial fibrillation, stroke, cardiovascular death, and coronary revascularization among individuals with severe atopic eczema, compared with controls.
After adjustment for potential mediators such as body mass index, smoking, hyperlipidemia, diabetes, and severe alcohol use, individuals with severe eczema had a significant 37% increased risk of MI, 67% greater risk of heart failure, 35% greater risk of atrial fibrillation, 30% greater risk of cardiovascular death, and 36% greater risk of coronary revascularization, compared with controls with no eczema.
Increased cardiovascular risks also were seen in individuals whose atopic eczema was active more than half the time at follow-up. This group had a 37% greater risk of heart failure, 36% greater risk of unstable angina, and 19% greater risk of stroke, as well as significantly elevated risks of MI, atrial fibrillation, cardiovascular death, and coronary revascularization, compared with those without eczema.
Overall, atopic eczema contributed around 2.4% of the population-attributable risk for unstable angina, and 1.9% for heart failure (the highest population attributable risks). Ethnicity or high-dose corticosteroid use did not significantly impact outcomes.
Richard J. Silverwood, PhD, from the London School of Hygiene and Tropical Medicine, and his coauthors wrote that previous work examining the relationship between atopic eczema and cardiovascular disease had shown inconsistent outcomes, with some studies even pointing to a possible protective effect of mild atopic eczema.
“Mechanistic work suggests that atopic eczema may be associated with increased platelet activation and decreased fibrinolysis, which may increase the risk of clotting, though a recent study found no association with metabolite levels,” the authors wrote.
They noted that the strengths of their study were that it was largest to examine the association between atopic eczema and cardiovascular risk, and that they had access to data on body mass index, smoking, and severe alcohol use for most of the study population, which enabled them to adjust for these potential mediators.
“Consideration should be given to developing prevention strategies to reduce the risk of cardiovascular disease among patients with severe or predominantly active atopic eczema, including awareness of and screening for conventional cardiovascular risk factors by those providing clinical care,” they wrote.
The study was supported by the Wellcome Trust, and no relevant conflicts of interest were declared.
SOURCE: Silverwood R et al. BMJ 2018 May 23;361:k1786. doi: 10.1136/bmj.k1786.
according to a case-control study published online May 23 in BMJ.
In a population-based cohort study, researchers compared the electronic health records of 387,439 adults with eczema and 1,528,477 patients without eczema in the United Kingddom, matched according to age, sex, general practice, and calendar time, during 1998-2015. Patients were followed up for a median of 5.1 years
With the exception of cardiovascular death, atopic eczema was associated with all cardiovascular outcomes (MI, unstable angina, heart failure, atrial fibrillation, and stroke). The associations were stronger for severe atopic eczema, with significantly higher risks of MI, unstable angina, atrial fibrillation, stroke, cardiovascular death, and coronary revascularization among individuals with severe atopic eczema, compared with controls.
After adjustment for potential mediators such as body mass index, smoking, hyperlipidemia, diabetes, and severe alcohol use, individuals with severe eczema had a significant 37% increased risk of MI, 67% greater risk of heart failure, 35% greater risk of atrial fibrillation, 30% greater risk of cardiovascular death, and 36% greater risk of coronary revascularization, compared with controls with no eczema.
Increased cardiovascular risks also were seen in individuals whose atopic eczema was active more than half the time at follow-up. This group had a 37% greater risk of heart failure, 36% greater risk of unstable angina, and 19% greater risk of stroke, as well as significantly elevated risks of MI, atrial fibrillation, cardiovascular death, and coronary revascularization, compared with those without eczema.
Overall, atopic eczema contributed around 2.4% of the population-attributable risk for unstable angina, and 1.9% for heart failure (the highest population attributable risks). Ethnicity or high-dose corticosteroid use did not significantly impact outcomes.
Richard J. Silverwood, PhD, from the London School of Hygiene and Tropical Medicine, and his coauthors wrote that previous work examining the relationship between atopic eczema and cardiovascular disease had shown inconsistent outcomes, with some studies even pointing to a possible protective effect of mild atopic eczema.
“Mechanistic work suggests that atopic eczema may be associated with increased platelet activation and decreased fibrinolysis, which may increase the risk of clotting, though a recent study found no association with metabolite levels,” the authors wrote.
They noted that the strengths of their study were that it was largest to examine the association between atopic eczema and cardiovascular risk, and that they had access to data on body mass index, smoking, and severe alcohol use for most of the study population, which enabled them to adjust for these potential mediators.
“Consideration should be given to developing prevention strategies to reduce the risk of cardiovascular disease among patients with severe or predominantly active atopic eczema, including awareness of and screening for conventional cardiovascular risk factors by those providing clinical care,” they wrote.
The study was supported by the Wellcome Trust, and no relevant conflicts of interest were declared.
SOURCE: Silverwood R et al. BMJ 2018 May 23;361:k1786. doi: 10.1136/bmj.k1786.
FROM THE BMJ
Key clinical point: Severe atopic eczema may be associated with a significantly increased risk of cardiovascular disease.
Major finding: Individuals with severe atopic eczema were at increased for cardiovascular disease, including a 67% greater risk of heart failure.
Study details: A population-based case-control cohort study in 387,439 patients with atopic eczema, compared with more than 1 million controls.
Disclosures: The study was supported by the Wellcome Trust, and no relevant conflicts of interest were declared.
Source: Silverwood R et al. BMJ. 2018 May 23;361:k1786.
Antidepressant use linked to increased weight gain
Taking antidepressants appears associated with weight gain in a recent study, though the researchers caution that the link might not be causal. Antidepressant use already was lowest in those with an initially normal BMI, and it increased with each category of greater BMI.
“Participants of normal weight showed an increased risk of transitioning to overweight or obesity, and overweight participants were more likely to become obese if they were treated with an antidepressant,” Rafael Gafoor, PhD, of King’s College London, and his associates wrote in BMJ.
“The risk of weight gain was substantially increased during the second and third years of treatment,” they reported. “The risk of weight gain remained increased during at least 6 years of follow-up.”
The researchers retrospectively analyzed EHRs from the U.K. Clinical Practice Research Datalink, representing about 7% of general practices in the United Kingdom, to compare the risk of weight gain among those who were and were not prescribed antidepressants. Of more than 2 million registered participants, a random sample of those with at least three measurements for body mass index (BMI), representing different weight classes, were included.
Out of 136,762 men and 157,957 women, 13% of the men and 22.4% of the women received prescriptions for antidepressants. The 10-year follow-up period included more than 1.8 million person-years. The proportion of people gaining at least 5% in BMI was 11.1% in those with antidepressants prescriptions, compared with 8.1% in those not prescribed antidepressants.
“Antidepressant use was greater in patients with comorbidity and coprescriptions, particularly with diagnoses of stroke and diabetes or coprescribing of antiepileptics or antipsychotics, than in those without,” the researchers wrote. “Antidepressant use was also associated with current smoking and higher deprivation category.”
The researchers adjusted the analysis to account for age, sex, initial BMI classification, smoking, prescriptions for antiepileptics or antipsychotics, year, region of residence, socioeconomic status, or having received advice on weight management or diet. They also accounted for diabetes, coronary heart disease, stroke, cancer, and depression diagnoses, and they eliminated the 280 days before a newborn delivery to account for pregnancy-related weight increases.
After adjustment for those potential confounders, people prescribed antidepressants were 21% more likely to experience substantial weight gain than those not prescribed them (adjusted rate ratio = 1.21; P less than .001). The researchers, cautioned, however, that residual confounding might have inflated the risk.
Those who had a normal weight when they began taking antidepressants had 29% greater risk of gaining enough weight to meet the criteria for overweight or obesity. Likewise, those already overweight when first prescribed antidepressants were 29% more likely to develop obesity.
For every 27 patients who took antidepressants for at least 1 year, 1 of them gained at least 5% in BMI during the subsequent year. Again, however, the researchers emphasized the absence of evidence for causality and the risk of bias from confounding.
Mirtazapine was the antidepressant associated with the highest incidence ratio of weight gain, but that drug was prescribed infrequently.
“From a clinical perspective, these observations reinforce the need for active body weight management to accompany widespread antidepressant treatment, although this might often be met with limited success,” the authors wrote.
The research was funded by the National Health Service Foundation Trust and by King’s College London. Aside from one author’s employment with the U.K. Clinical Practice Research Datalink data source, the authors had no disclosures.
SOURCE: Gafoor R et al. BMJ. 2018;361:k1951. doi: 10.1136/bmj.k1951.
Taking antidepressants appears associated with weight gain in a recent study, though the researchers caution that the link might not be causal. Antidepressant use already was lowest in those with an initially normal BMI, and it increased with each category of greater BMI.
“Participants of normal weight showed an increased risk of transitioning to overweight or obesity, and overweight participants were more likely to become obese if they were treated with an antidepressant,” Rafael Gafoor, PhD, of King’s College London, and his associates wrote in BMJ.
“The risk of weight gain was substantially increased during the second and third years of treatment,” they reported. “The risk of weight gain remained increased during at least 6 years of follow-up.”
The researchers retrospectively analyzed EHRs from the U.K. Clinical Practice Research Datalink, representing about 7% of general practices in the United Kingdom, to compare the risk of weight gain among those who were and were not prescribed antidepressants. Of more than 2 million registered participants, a random sample of those with at least three measurements for body mass index (BMI), representing different weight classes, were included.
Out of 136,762 men and 157,957 women, 13% of the men and 22.4% of the women received prescriptions for antidepressants. The 10-year follow-up period included more than 1.8 million person-years. The proportion of people gaining at least 5% in BMI was 11.1% in those with antidepressants prescriptions, compared with 8.1% in those not prescribed antidepressants.
“Antidepressant use was greater in patients with comorbidity and coprescriptions, particularly with diagnoses of stroke and diabetes or coprescribing of antiepileptics or antipsychotics, than in those without,” the researchers wrote. “Antidepressant use was also associated with current smoking and higher deprivation category.”
The researchers adjusted the analysis to account for age, sex, initial BMI classification, smoking, prescriptions for antiepileptics or antipsychotics, year, region of residence, socioeconomic status, or having received advice on weight management or diet. They also accounted for diabetes, coronary heart disease, stroke, cancer, and depression diagnoses, and they eliminated the 280 days before a newborn delivery to account for pregnancy-related weight increases.
After adjustment for those potential confounders, people prescribed antidepressants were 21% more likely to experience substantial weight gain than those not prescribed them (adjusted rate ratio = 1.21; P less than .001). The researchers, cautioned, however, that residual confounding might have inflated the risk.
Those who had a normal weight when they began taking antidepressants had 29% greater risk of gaining enough weight to meet the criteria for overweight or obesity. Likewise, those already overweight when first prescribed antidepressants were 29% more likely to develop obesity.
For every 27 patients who took antidepressants for at least 1 year, 1 of them gained at least 5% in BMI during the subsequent year. Again, however, the researchers emphasized the absence of evidence for causality and the risk of bias from confounding.
Mirtazapine was the antidepressant associated with the highest incidence ratio of weight gain, but that drug was prescribed infrequently.
“From a clinical perspective, these observations reinforce the need for active body weight management to accompany widespread antidepressant treatment, although this might often be met with limited success,” the authors wrote.
The research was funded by the National Health Service Foundation Trust and by King’s College London. Aside from one author’s employment with the U.K. Clinical Practice Research Datalink data source, the authors had no disclosures.
SOURCE: Gafoor R et al. BMJ. 2018;361:k1951. doi: 10.1136/bmj.k1951.
Taking antidepressants appears associated with weight gain in a recent study, though the researchers caution that the link might not be causal. Antidepressant use already was lowest in those with an initially normal BMI, and it increased with each category of greater BMI.
“Participants of normal weight showed an increased risk of transitioning to overweight or obesity, and overweight participants were more likely to become obese if they were treated with an antidepressant,” Rafael Gafoor, PhD, of King’s College London, and his associates wrote in BMJ.
“The risk of weight gain was substantially increased during the second and third years of treatment,” they reported. “The risk of weight gain remained increased during at least 6 years of follow-up.”
The researchers retrospectively analyzed EHRs from the U.K. Clinical Practice Research Datalink, representing about 7% of general practices in the United Kingdom, to compare the risk of weight gain among those who were and were not prescribed antidepressants. Of more than 2 million registered participants, a random sample of those with at least three measurements for body mass index (BMI), representing different weight classes, were included.
Out of 136,762 men and 157,957 women, 13% of the men and 22.4% of the women received prescriptions for antidepressants. The 10-year follow-up period included more than 1.8 million person-years. The proportion of people gaining at least 5% in BMI was 11.1% in those with antidepressants prescriptions, compared with 8.1% in those not prescribed antidepressants.
“Antidepressant use was greater in patients with comorbidity and coprescriptions, particularly with diagnoses of stroke and diabetes or coprescribing of antiepileptics or antipsychotics, than in those without,” the researchers wrote. “Antidepressant use was also associated with current smoking and higher deprivation category.”
The researchers adjusted the analysis to account for age, sex, initial BMI classification, smoking, prescriptions for antiepileptics or antipsychotics, year, region of residence, socioeconomic status, or having received advice on weight management or diet. They also accounted for diabetes, coronary heart disease, stroke, cancer, and depression diagnoses, and they eliminated the 280 days before a newborn delivery to account for pregnancy-related weight increases.
After adjustment for those potential confounders, people prescribed antidepressants were 21% more likely to experience substantial weight gain than those not prescribed them (adjusted rate ratio = 1.21; P less than .001). The researchers, cautioned, however, that residual confounding might have inflated the risk.
Those who had a normal weight when they began taking antidepressants had 29% greater risk of gaining enough weight to meet the criteria for overweight or obesity. Likewise, those already overweight when first prescribed antidepressants were 29% more likely to develop obesity.
For every 27 patients who took antidepressants for at least 1 year, 1 of them gained at least 5% in BMI during the subsequent year. Again, however, the researchers emphasized the absence of evidence for causality and the risk of bias from confounding.
Mirtazapine was the antidepressant associated with the highest incidence ratio of weight gain, but that drug was prescribed infrequently.
“From a clinical perspective, these observations reinforce the need for active body weight management to accompany widespread antidepressant treatment, although this might often be met with limited success,” the authors wrote.
The research was funded by the National Health Service Foundation Trust and by King’s College London. Aside from one author’s employment with the U.K. Clinical Practice Research Datalink data source, the authors had no disclosures.
SOURCE: Gafoor R et al. BMJ. 2018;361:k1951. doi: 10.1136/bmj.k1951.
FROM THE BMJ
Key clinical point: “These observations reinforce the need for active body weight management to accompany widespread antidepressant treatment.”
Major finding: People taking antidepressants had 21% higher risk of gaining at least 5% in body mass index and 29% greater risk of moving into a higher BMI category.
Study details: The findings are based on analysis of 10-year follow-up date from a British population-based cohort of 294,719 men and women, 18% of whom had been prescribed antidepressants.
Disclosures: The research was funded by the National Health Service Foundation Trust and by King’s College London. Aside from one author’s employment with the U.K. Clinical Practice Research Datalink data source, the authors had no disclosures.
Source: Gafoor R et al. BMJ. 2018;361:k1951. doi: 10.1136/bmj.k1951.
Hefty rewards pay off in smoking-cessation study
A new study finds it pays to pay people to stop lighting up: Smokers were more likely to quit if they had an opportunity to gain rewards worth $600 than if they simply received free cessation aids or free e-cigarettes.
The wide majority of the more than 6,000 smokers in the randomized study didn’t quit despite offers of various incentives. All the same, “programs that offered financial incentives tripled the rates of smoking cessation, reduced employers’ costs per successful quit, as compared with programs that offered cessation aids alone, and yielded total costs that compared favorably with the costs of employing smokers,” the study authors wrote.
The researchers reached out to employees and spouses at 54 companies that use wellness programs provided by the Vitality Institute, which supports research into health promotion. The institute provided grant support for the study.
Just over 6,000 employees and spouses who smoked were assigned to five groups. One group received usual care. The others received interventions: free smoking-cessation aids (nicotine replacement therapy, bupropion, or varenicline); free e-cigarettes; up to $600 worth of an unidentified “reward incentive” plus free cessation aids; and up to $600 via a redeemable deposit, plus free cessation aids.
Participants could only get the entire reward incentive or the full $600 redeemable deposit if they showed signs of sustained smoking cessation via blood or urine test at 1, 3, and 6 months.
The median age in the groups ranged from 43 to 45 years, and most were not college graduates. Just over half were women, and roughly 90% said they wanted to quit smoking.
Overall, 20% of the 6,006 participants logged onto the trial website, a sign that they were “engaged.” The number was highest in the free e-cigarette and reward groups (21%-23%) and lowest in the usual care group (16%).
The researchers focused on how many participants abstained from smoking – as confirmed by blood or urine test – for 6 months past the target quit date. The test data confirmed that just 1.3% of the total participants, 80 people, sustained cessation over 6 months.
Only 0.1% of the usual-care group sustained smoking cessation, and the number wasn’t much higher (0.5%) in the free cessation aids group.
One percent of those in the free e-cigarette group sustained cessation. However, the researchers noted there wasn’t a significant difference in the quit rates between the usual care, free cessation aid, and free e-cigarette groups.
“The observation of greater e-cigarette use in the free e-cigarette group than in the free cessation aids group, coupled with the absence of benefit of free e-cigarettes versus no intervention, supports the conclusion that offering free e-cigarettes does not promote smoking cessation,” they cautioned.
In the reward incentive and $600 redeemable-deposit groups, 2% and 2.9% of participants quit over a sustained period, respectively. Cessation rates in the $600 deposit group were superior to the free cessation aid group (P less than .001) and the free e-cigarette group (P = .008). Cessation rates in the incentive group were superior to those in the free cessation aid group (P = .006).
“Average costs per participant assigned to each intervention were lowest in the usual-care group ($0.82) and highest in the redeemable-deposit group ($100.96),” the researchers reported. “The overall cost of each program per participant who was abstinent for 6 months was lower in the rewards and redeemable-deposit groups than in the free e-cigarettes or free cessation aids groups.”
The study received grant support from the Vitality Institute. Most of the study authors reported no relevant disclosures. One author reported serving on the scientific advisory board of VAL Health, and another reported various grants and personal fees.
SOURCE: Halpern SD et al. N Engl J Med. 2018 May 23. doi: 10.1056/NEJMsa1715757.
A new study finds it pays to pay people to stop lighting up: Smokers were more likely to quit if they had an opportunity to gain rewards worth $600 than if they simply received free cessation aids or free e-cigarettes.
The wide majority of the more than 6,000 smokers in the randomized study didn’t quit despite offers of various incentives. All the same, “programs that offered financial incentives tripled the rates of smoking cessation, reduced employers’ costs per successful quit, as compared with programs that offered cessation aids alone, and yielded total costs that compared favorably with the costs of employing smokers,” the study authors wrote.
The researchers reached out to employees and spouses at 54 companies that use wellness programs provided by the Vitality Institute, which supports research into health promotion. The institute provided grant support for the study.
Just over 6,000 employees and spouses who smoked were assigned to five groups. One group received usual care. The others received interventions: free smoking-cessation aids (nicotine replacement therapy, bupropion, or varenicline); free e-cigarettes; up to $600 worth of an unidentified “reward incentive” plus free cessation aids; and up to $600 via a redeemable deposit, plus free cessation aids.
Participants could only get the entire reward incentive or the full $600 redeemable deposit if they showed signs of sustained smoking cessation via blood or urine test at 1, 3, and 6 months.
The median age in the groups ranged from 43 to 45 years, and most were not college graduates. Just over half were women, and roughly 90% said they wanted to quit smoking.
Overall, 20% of the 6,006 participants logged onto the trial website, a sign that they were “engaged.” The number was highest in the free e-cigarette and reward groups (21%-23%) and lowest in the usual care group (16%).
The researchers focused on how many participants abstained from smoking – as confirmed by blood or urine test – for 6 months past the target quit date. The test data confirmed that just 1.3% of the total participants, 80 people, sustained cessation over 6 months.
Only 0.1% of the usual-care group sustained smoking cessation, and the number wasn’t much higher (0.5%) in the free cessation aids group.
One percent of those in the free e-cigarette group sustained cessation. However, the researchers noted there wasn’t a significant difference in the quit rates between the usual care, free cessation aid, and free e-cigarette groups.
“The observation of greater e-cigarette use in the free e-cigarette group than in the free cessation aids group, coupled with the absence of benefit of free e-cigarettes versus no intervention, supports the conclusion that offering free e-cigarettes does not promote smoking cessation,” they cautioned.
In the reward incentive and $600 redeemable-deposit groups, 2% and 2.9% of participants quit over a sustained period, respectively. Cessation rates in the $600 deposit group were superior to the free cessation aid group (P less than .001) and the free e-cigarette group (P = .008). Cessation rates in the incentive group were superior to those in the free cessation aid group (P = .006).
“Average costs per participant assigned to each intervention were lowest in the usual-care group ($0.82) and highest in the redeemable-deposit group ($100.96),” the researchers reported. “The overall cost of each program per participant who was abstinent for 6 months was lower in the rewards and redeemable-deposit groups than in the free e-cigarettes or free cessation aids groups.”
The study received grant support from the Vitality Institute. Most of the study authors reported no relevant disclosures. One author reported serving on the scientific advisory board of VAL Health, and another reported various grants and personal fees.
SOURCE: Halpern SD et al. N Engl J Med. 2018 May 23. doi: 10.1056/NEJMsa1715757.
A new study finds it pays to pay people to stop lighting up: Smokers were more likely to quit if they had an opportunity to gain rewards worth $600 than if they simply received free cessation aids or free e-cigarettes.
The wide majority of the more than 6,000 smokers in the randomized study didn’t quit despite offers of various incentives. All the same, “programs that offered financial incentives tripled the rates of smoking cessation, reduced employers’ costs per successful quit, as compared with programs that offered cessation aids alone, and yielded total costs that compared favorably with the costs of employing smokers,” the study authors wrote.
The researchers reached out to employees and spouses at 54 companies that use wellness programs provided by the Vitality Institute, which supports research into health promotion. The institute provided grant support for the study.
Just over 6,000 employees and spouses who smoked were assigned to five groups. One group received usual care. The others received interventions: free smoking-cessation aids (nicotine replacement therapy, bupropion, or varenicline); free e-cigarettes; up to $600 worth of an unidentified “reward incentive” plus free cessation aids; and up to $600 via a redeemable deposit, plus free cessation aids.
Participants could only get the entire reward incentive or the full $600 redeemable deposit if they showed signs of sustained smoking cessation via blood or urine test at 1, 3, and 6 months.
The median age in the groups ranged from 43 to 45 years, and most were not college graduates. Just over half were women, and roughly 90% said they wanted to quit smoking.
Overall, 20% of the 6,006 participants logged onto the trial website, a sign that they were “engaged.” The number was highest in the free e-cigarette and reward groups (21%-23%) and lowest in the usual care group (16%).
The researchers focused on how many participants abstained from smoking – as confirmed by blood or urine test – for 6 months past the target quit date. The test data confirmed that just 1.3% of the total participants, 80 people, sustained cessation over 6 months.
Only 0.1% of the usual-care group sustained smoking cessation, and the number wasn’t much higher (0.5%) in the free cessation aids group.
One percent of those in the free e-cigarette group sustained cessation. However, the researchers noted there wasn’t a significant difference in the quit rates between the usual care, free cessation aid, and free e-cigarette groups.
“The observation of greater e-cigarette use in the free e-cigarette group than in the free cessation aids group, coupled with the absence of benefit of free e-cigarettes versus no intervention, supports the conclusion that offering free e-cigarettes does not promote smoking cessation,” they cautioned.
In the reward incentive and $600 redeemable-deposit groups, 2% and 2.9% of participants quit over a sustained period, respectively. Cessation rates in the $600 deposit group were superior to the free cessation aid group (P less than .001) and the free e-cigarette group (P = .008). Cessation rates in the incentive group were superior to those in the free cessation aid group (P = .006).
“Average costs per participant assigned to each intervention were lowest in the usual-care group ($0.82) and highest in the redeemable-deposit group ($100.96),” the researchers reported. “The overall cost of each program per participant who was abstinent for 6 months was lower in the rewards and redeemable-deposit groups than in the free e-cigarettes or free cessation aids groups.”
The study received grant support from the Vitality Institute. Most of the study authors reported no relevant disclosures. One author reported serving on the scientific advisory board of VAL Health, and another reported various grants and personal fees.
SOURCE: Halpern SD et al. N Engl J Med. 2018 May 23. doi: 10.1056/NEJMsa1715757.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Smokers are more likely to sustain cessation if they receive incentives worth $600.
Major finding: Cessation rates for 6 months past target quit date reached as high as 2.9% in smokers who had the opportunity to gain rewards, while they didn’t top 1% in those who received free cessation aids or free e-cigarettes.
Study details: A randomized, prospective study of 6,006 smokers (employees of 54 companies and spouses) who were assigned to usual care or one of four intervention groups.
Disclosures: The study received grant support from the Vitality Institute. Most of the study authors reported no relevant disclosures. One reported serving on the scientific advisory board of VAL Health, and another reported various grants and personal fees.
Source: Halpern SD et al. N Engl J Med. 2018 May 23. doi: 10.1056/NEJMsa1715757.
Doctors’ pay involves a lot of unseen work
“Doctors make a lot of money.” We hear that a lot, always from people who aren’t part of the profession.
Last month, I had to do my tax forms. Not my annual forms, but the quarterly withholding ones for the IRS, and for the state, along with the Arizona Department of Economic Security forms.
The whole thing takes me about 2 hours every 3 months. I suppose I could hire an accountant or office manager to deal with that stuff, but in solo practice, you do everything you can to keep the overhead low. So I do it myself.
Eight hours a year doesn’t sound too bad, but it got me thinking about all the other ways that work creeps into my home time.
I’m usually at the office around 5:00 a.m., when I start with reviewing charts, doing paperwork, and catching up on dictations until patients start at 8:00 a.m. From then on, they’re a steady stream until 4:00 p.m., when we close up and head home.
I get home and then have 1-2 hours of time paying bills, sorting mail, and catching up on phone calls and other unresolved issues.
On weekends, there’s always other stuff. Payroll for the coming weeks, office bills, and credit card statements I didn’t get to during the week, CME, forms, licensing paperwork, etc.
I’d guess about 15 hours/week goes into nonpatient-related stuff. Each year that’s more than 700 hours (or a little over a month) of extra time. Tack that on to the roughly 60 hours that I spend seeing patients between the office and hospital.
People say we make “a lot” (whatever that is), but they don’t see everything behind it. The 7-12 years of post-college training. The student loans of $200,000 and dating back to when I was 26 years old. The rising costs of overhead and dropping rates of reimbursement. The denied payments in disputes over claims. And, as mentioned above, the huge amount of time this job takes for stuff beyond just seeing patients.
We don’t get paid by the hour, but if we did, the rate would probably be a lot lower than what most would expect.
I suppose I could become employed, and let someone else worry about those things. But the financial impact doesn’t go away. Someone else still has to be doing those things, and since doctors are the ones who generate income in the majority of medical practices, the salaries for everyone else come out of ours. Plus, as I’ve previously written about, I’ve been employed before and got sick of the meetings and memos about cost-sharing, productivity numbers, and dollars earned per square foot.
But whenever I hear the refrain about our field being overpaid, I think about the actual hours the public doesn’t see (or care about). This isn’t a job for slackers, and
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“Doctors make a lot of money.” We hear that a lot, always from people who aren’t part of the profession.
Last month, I had to do my tax forms. Not my annual forms, but the quarterly withholding ones for the IRS, and for the state, along with the Arizona Department of Economic Security forms.
The whole thing takes me about 2 hours every 3 months. I suppose I could hire an accountant or office manager to deal with that stuff, but in solo practice, you do everything you can to keep the overhead low. So I do it myself.
Eight hours a year doesn’t sound too bad, but it got me thinking about all the other ways that work creeps into my home time.
I’m usually at the office around 5:00 a.m., when I start with reviewing charts, doing paperwork, and catching up on dictations until patients start at 8:00 a.m. From then on, they’re a steady stream until 4:00 p.m., when we close up and head home.
I get home and then have 1-2 hours of time paying bills, sorting mail, and catching up on phone calls and other unresolved issues.
On weekends, there’s always other stuff. Payroll for the coming weeks, office bills, and credit card statements I didn’t get to during the week, CME, forms, licensing paperwork, etc.
I’d guess about 15 hours/week goes into nonpatient-related stuff. Each year that’s more than 700 hours (or a little over a month) of extra time. Tack that on to the roughly 60 hours that I spend seeing patients between the office and hospital.
People say we make “a lot” (whatever that is), but they don’t see everything behind it. The 7-12 years of post-college training. The student loans of $200,000 and dating back to when I was 26 years old. The rising costs of overhead and dropping rates of reimbursement. The denied payments in disputes over claims. And, as mentioned above, the huge amount of time this job takes for stuff beyond just seeing patients.
We don’t get paid by the hour, but if we did, the rate would probably be a lot lower than what most would expect.
I suppose I could become employed, and let someone else worry about those things. But the financial impact doesn’t go away. Someone else still has to be doing those things, and since doctors are the ones who generate income in the majority of medical practices, the salaries for everyone else come out of ours. Plus, as I’ve previously written about, I’ve been employed before and got sick of the meetings and memos about cost-sharing, productivity numbers, and dollars earned per square foot.
But whenever I hear the refrain about our field being overpaid, I think about the actual hours the public doesn’t see (or care about). This isn’t a job for slackers, and
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“Doctors make a lot of money.” We hear that a lot, always from people who aren’t part of the profession.
Last month, I had to do my tax forms. Not my annual forms, but the quarterly withholding ones for the IRS, and for the state, along with the Arizona Department of Economic Security forms.
The whole thing takes me about 2 hours every 3 months. I suppose I could hire an accountant or office manager to deal with that stuff, but in solo practice, you do everything you can to keep the overhead low. So I do it myself.
Eight hours a year doesn’t sound too bad, but it got me thinking about all the other ways that work creeps into my home time.
I’m usually at the office around 5:00 a.m., when I start with reviewing charts, doing paperwork, and catching up on dictations until patients start at 8:00 a.m. From then on, they’re a steady stream until 4:00 p.m., when we close up and head home.
I get home and then have 1-2 hours of time paying bills, sorting mail, and catching up on phone calls and other unresolved issues.
On weekends, there’s always other stuff. Payroll for the coming weeks, office bills, and credit card statements I didn’t get to during the week, CME, forms, licensing paperwork, etc.
I’d guess about 15 hours/week goes into nonpatient-related stuff. Each year that’s more than 700 hours (or a little over a month) of extra time. Tack that on to the roughly 60 hours that I spend seeing patients between the office and hospital.
People say we make “a lot” (whatever that is), but they don’t see everything behind it. The 7-12 years of post-college training. The student loans of $200,000 and dating back to when I was 26 years old. The rising costs of overhead and dropping rates of reimbursement. The denied payments in disputes over claims. And, as mentioned above, the huge amount of time this job takes for stuff beyond just seeing patients.
We don’t get paid by the hour, but if we did, the rate would probably be a lot lower than what most would expect.
I suppose I could become employed, and let someone else worry about those things. But the financial impact doesn’t go away. Someone else still has to be doing those things, and since doctors are the ones who generate income in the majority of medical practices, the salaries for everyone else come out of ours. Plus, as I’ve previously written about, I’ve been employed before and got sick of the meetings and memos about cost-sharing, productivity numbers, and dollars earned per square foot.
But whenever I hear the refrain about our field being overpaid, I think about the actual hours the public doesn’t see (or care about). This isn’t a job for slackers, and
Dr. Block has a solo neurology practice in Scottsdale, Ariz.