User login
Transgender care mandates endocrinologists share their expertise
BOSTON – Endocrinologists need to be familiar with new practice guidelines and changes in the landscape of transgender health care, Joshua D. Safer, MD, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York, said in a video interview at the annual meeting of the American Association of Clinical Endocrinologists.
“We endocrinologists ... need to be able to help (gender-dysphoric/gender-incongruent) individuals, even if it’s just an occasional patient, to do what is safe and to be expert (in transgender health care), just as we are with other hormone treatments,” he said in a discussion of aspects of the Endocrine Society clinical practice guideline on endocrine treatment of gender-dysphoric/gender-incongruent individuals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The new guidelines, published in November 2017, update 2009 guidance from the society. Among the big changes are the recognition that there may be “compelling reasons” to start cross-sex hormonal therapy prior to the old age cutoff of 16 years, which is “very late if you’re thinking about it from a biological perspective,” said Dr. Safer.
Another major change challenges the idea that a mental health professional is necessary to diagnose adults. Rather, any knowledgeable clinician could make the diagnosis, according to Dr. Safer.
The guidelines also recommend that endocrinologists provide education regarding onset and time course of physical changes induced by sex hormone treatments to transgender individuals undergoing treatment.
BOSTON – Endocrinologists need to be familiar with new practice guidelines and changes in the landscape of transgender health care, Joshua D. Safer, MD, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York, said in a video interview at the annual meeting of the American Association of Clinical Endocrinologists.
“We endocrinologists ... need to be able to help (gender-dysphoric/gender-incongruent) individuals, even if it’s just an occasional patient, to do what is safe and to be expert (in transgender health care), just as we are with other hormone treatments,” he said in a discussion of aspects of the Endocrine Society clinical practice guideline on endocrine treatment of gender-dysphoric/gender-incongruent individuals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The new guidelines, published in November 2017, update 2009 guidance from the society. Among the big changes are the recognition that there may be “compelling reasons” to start cross-sex hormonal therapy prior to the old age cutoff of 16 years, which is “very late if you’re thinking about it from a biological perspective,” said Dr. Safer.
Another major change challenges the idea that a mental health professional is necessary to diagnose adults. Rather, any knowledgeable clinician could make the diagnosis, according to Dr. Safer.
The guidelines also recommend that endocrinologists provide education regarding onset and time course of physical changes induced by sex hormone treatments to transgender individuals undergoing treatment.
BOSTON – Endocrinologists need to be familiar with new practice guidelines and changes in the landscape of transgender health care, Joshua D. Safer, MD, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York, said in a video interview at the annual meeting of the American Association of Clinical Endocrinologists.
“We endocrinologists ... need to be able to help (gender-dysphoric/gender-incongruent) individuals, even if it’s just an occasional patient, to do what is safe and to be expert (in transgender health care), just as we are with other hormone treatments,” he said in a discussion of aspects of the Endocrine Society clinical practice guideline on endocrine treatment of gender-dysphoric/gender-incongruent individuals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The new guidelines, published in November 2017, update 2009 guidance from the society. Among the big changes are the recognition that there may be “compelling reasons” to start cross-sex hormonal therapy prior to the old age cutoff of 16 years, which is “very late if you’re thinking about it from a biological perspective,” said Dr. Safer.
Another major change challenges the idea that a mental health professional is necessary to diagnose adults. Rather, any knowledgeable clinician could make the diagnosis, according to Dr. Safer.
The guidelines also recommend that endocrinologists provide education regarding onset and time course of physical changes induced by sex hormone treatments to transgender individuals undergoing treatment.
REPORTING FROM AACE 2018
Calcium, PTH predict permanent hypoparathyroidism
BOSTON – Low postoperative calcium serum and parathyroid hormone (PTH) levels may be strong predictors of permanent hypoparathyroidism in total thyroidectomy patients, according to results of a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
With incidence of transient hypoparathyroidism at 20-30%, being able to predict at-risk patients can significantly help clinicians with postoperative management, according to Steven Brown, DO, of the University of Arizona, Phoenix.
“It’s very important to draw the preoperative lab and postoperative lab in order to help those patients who are at risk,” said Dr. Brown in an oral abstract session.
To test the predictive accuracy of PTH and calcium levels, investigators conducted a single-center, retrospective study of 176 total thyroidectomy patients recorded during 1999-2013.
Patients with hypoparathyroidism had an average age of about 47 years, was almost entirely composed of females, was majority hispanic, and had mean postop calcium and PTH levels of 7.6 mg/dL and 8.0 pg/mL, respectively.
Those without hypoparathyroidism averaged about 51 years old, were equally hispanic and white, and had postop calcium and PTH levels of 8.08 mg/dL and 30.8 pg/dL, respectively.
Patients were split into four groups: Group 1 had low calcium and PTH levels (66), group 2 had low calcium and normal PTH levels (30), group 3 had normal calcium and low PTH levels (31), and group 4 had normal levels of both (49).
Over the study period, hypoparathyroidism developed in 30% of patients in group 1, 10% in group 2, 15% in group 3, and 2% in group 4.
Permanent hypothyroidism was defined as persistently low PTH (less than 12 pg/mL), low serum calcium (less than 8.0 mg/dL), and/or requiring calcitriol to maintain a normal calcium level for more than 6 months after total thyroidectomy.
Those with both low calcium and PTH levels were 4.3 times more likely to develop permanent hypoparathyroidism than those in the other groups, according to Dr. Brown.
Patients in the permanent hypoparathyroid group had a PTH drop of 70%, compared with 39% in the nonhypoparathyroid group. There was also a significant difference in respective drops of calcium levels (17.8% vs. 14.3%).
By comparing the levels before and after a thyroidectomy, physicians can act faster and more accurately when determining how best to treat patients to prevent hypoparathyroidism, a practice which, according to Dr. Brown, has already begun to be put into place.
“We’re starting to incorporate some of the practices into our patient care routine,” said Dr. Brown. “The next part of our project is going to be to actually do a prospective study at three different institution sites in order to evaluate this further.”
Dr. Brown reported no relevant financial disclosures.
SOURCE: Brown S et al. AACE 2018, Abstract 720.
BOSTON – Low postoperative calcium serum and parathyroid hormone (PTH) levels may be strong predictors of permanent hypoparathyroidism in total thyroidectomy patients, according to results of a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
With incidence of transient hypoparathyroidism at 20-30%, being able to predict at-risk patients can significantly help clinicians with postoperative management, according to Steven Brown, DO, of the University of Arizona, Phoenix.
“It’s very important to draw the preoperative lab and postoperative lab in order to help those patients who are at risk,” said Dr. Brown in an oral abstract session.
To test the predictive accuracy of PTH and calcium levels, investigators conducted a single-center, retrospective study of 176 total thyroidectomy patients recorded during 1999-2013.
Patients with hypoparathyroidism had an average age of about 47 years, was almost entirely composed of females, was majority hispanic, and had mean postop calcium and PTH levels of 7.6 mg/dL and 8.0 pg/mL, respectively.
Those without hypoparathyroidism averaged about 51 years old, were equally hispanic and white, and had postop calcium and PTH levels of 8.08 mg/dL and 30.8 pg/dL, respectively.
Patients were split into four groups: Group 1 had low calcium and PTH levels (66), group 2 had low calcium and normal PTH levels (30), group 3 had normal calcium and low PTH levels (31), and group 4 had normal levels of both (49).
Over the study period, hypoparathyroidism developed in 30% of patients in group 1, 10% in group 2, 15% in group 3, and 2% in group 4.
Permanent hypothyroidism was defined as persistently low PTH (less than 12 pg/mL), low serum calcium (less than 8.0 mg/dL), and/or requiring calcitriol to maintain a normal calcium level for more than 6 months after total thyroidectomy.
Those with both low calcium and PTH levels were 4.3 times more likely to develop permanent hypoparathyroidism than those in the other groups, according to Dr. Brown.
Patients in the permanent hypoparathyroid group had a PTH drop of 70%, compared with 39% in the nonhypoparathyroid group. There was also a significant difference in respective drops of calcium levels (17.8% vs. 14.3%).
By comparing the levels before and after a thyroidectomy, physicians can act faster and more accurately when determining how best to treat patients to prevent hypoparathyroidism, a practice which, according to Dr. Brown, has already begun to be put into place.
“We’re starting to incorporate some of the practices into our patient care routine,” said Dr. Brown. “The next part of our project is going to be to actually do a prospective study at three different institution sites in order to evaluate this further.”
Dr. Brown reported no relevant financial disclosures.
SOURCE: Brown S et al. AACE 2018, Abstract 720.
BOSTON – Low postoperative calcium serum and parathyroid hormone (PTH) levels may be strong predictors of permanent hypoparathyroidism in total thyroidectomy patients, according to results of a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
With incidence of transient hypoparathyroidism at 20-30%, being able to predict at-risk patients can significantly help clinicians with postoperative management, according to Steven Brown, DO, of the University of Arizona, Phoenix.
“It’s very important to draw the preoperative lab and postoperative lab in order to help those patients who are at risk,” said Dr. Brown in an oral abstract session.
To test the predictive accuracy of PTH and calcium levels, investigators conducted a single-center, retrospective study of 176 total thyroidectomy patients recorded during 1999-2013.
Patients with hypoparathyroidism had an average age of about 47 years, was almost entirely composed of females, was majority hispanic, and had mean postop calcium and PTH levels of 7.6 mg/dL and 8.0 pg/mL, respectively.
Those without hypoparathyroidism averaged about 51 years old, were equally hispanic and white, and had postop calcium and PTH levels of 8.08 mg/dL and 30.8 pg/dL, respectively.
Patients were split into four groups: Group 1 had low calcium and PTH levels (66), group 2 had low calcium and normal PTH levels (30), group 3 had normal calcium and low PTH levels (31), and group 4 had normal levels of both (49).
Over the study period, hypoparathyroidism developed in 30% of patients in group 1, 10% in group 2, 15% in group 3, and 2% in group 4.
Permanent hypothyroidism was defined as persistently low PTH (less than 12 pg/mL), low serum calcium (less than 8.0 mg/dL), and/or requiring calcitriol to maintain a normal calcium level for more than 6 months after total thyroidectomy.
Those with both low calcium and PTH levels were 4.3 times more likely to develop permanent hypoparathyroidism than those in the other groups, according to Dr. Brown.
Patients in the permanent hypoparathyroid group had a PTH drop of 70%, compared with 39% in the nonhypoparathyroid group. There was also a significant difference in respective drops of calcium levels (17.8% vs. 14.3%).
By comparing the levels before and after a thyroidectomy, physicians can act faster and more accurately when determining how best to treat patients to prevent hypoparathyroidism, a practice which, according to Dr. Brown, has already begun to be put into place.
“We’re starting to incorporate some of the practices into our patient care routine,” said Dr. Brown. “The next part of our project is going to be to actually do a prospective study at three different institution sites in order to evaluate this further.”
Dr. Brown reported no relevant financial disclosures.
SOURCE: Brown S et al. AACE 2018, Abstract 720.
REPORTING FROM AACE 18
Key clinical point:
Major finding: Patients with low calcium and low PTH were 4.3 times as likely as those without to develop permanent hypoparathyroidism after total thyroidectomy.
Study details: Retrospective, single center study of 176 total thyroidectomy patients during 1999-2013.
Disclosures: The presenter reported no relevant financial disclosures.
Source: Brown S et al. AACE 2018, Abstract 720.
Multiple myeloma rates rising fastest in East Asia
The global incidence of multiple myeloma rose by 126% from 1990 to 2016, with the largest regional increases occurring in East Asia and tropical Latin America, according to data from the Global Burden of Disease 2016 study.
East Asia (China, North Korea, and Taiwan) saw incident cases of multiple myeloma jump by 262% – up to 1.0 per 100,000 population – from 1990 to 2016, which was the largest increase among any of the 21 global regions; tropical Latin America’s 256% rise took its age-standardized incidence rate to 1.8 per 100,000. Worldwide, incidence of multiple myeloma was 2.1 cases per 100,000 in 2016, Andrew J. Cowan, MD, and his associates reported in JAMA Oncology.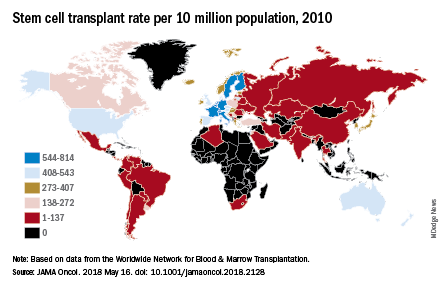
They also looked at treatment availability, with data on stem cell transplants for 2010 coming from the Worldwide Network for Blood & Marrow Transplantation (Lancet Haematol. 2015 Mar;2[3]:e91-100). The countries with the highest rates for all indications that year were Israel (814 per 10 million population), Italy (671), Germany (665), Sweden (625), and the Netherlands (614).
“Some regions of the world lack access to stem cell transplantation entirely, particularly sub-Saharan Africa (with the exception of South Africa),” wrote Dr. Cowan of the University of Washington, Seattle, and his associates.
The approval status of lenalidomide (Revlimid) and bortezomib (Velcade) in 2016 was used as a surrogate for availability of drug treatment: Lenalidomide had been approved in 73 countries out of 195 countries and territories and bortezomib in 103 countries. “On a global level, there are marked discrepancies in the availability of effective therapies. In addition to ensuring universal access to health care … it is imperative to at least ensure access to highly effective medications,” they wrote.
Dr. Cowan reported that he has received research funding from Janssen and AbbVie.
SOURCE: Cowan AJ et al. JAMA Oncol. 2018 May 16. doi: 10.1001/jamaoncol.2018.2128.
The global incidence of multiple myeloma rose by 126% from 1990 to 2016, with the largest regional increases occurring in East Asia and tropical Latin America, according to data from the Global Burden of Disease 2016 study.
East Asia (China, North Korea, and Taiwan) saw incident cases of multiple myeloma jump by 262% – up to 1.0 per 100,000 population – from 1990 to 2016, which was the largest increase among any of the 21 global regions; tropical Latin America’s 256% rise took its age-standardized incidence rate to 1.8 per 100,000. Worldwide, incidence of multiple myeloma was 2.1 cases per 100,000 in 2016, Andrew J. Cowan, MD, and his associates reported in JAMA Oncology.
They also looked at treatment availability, with data on stem cell transplants for 2010 coming from the Worldwide Network for Blood & Marrow Transplantation (Lancet Haematol. 2015 Mar;2[3]:e91-100). The countries with the highest rates for all indications that year were Israel (814 per 10 million population), Italy (671), Germany (665), Sweden (625), and the Netherlands (614).
“Some regions of the world lack access to stem cell transplantation entirely, particularly sub-Saharan Africa (with the exception of South Africa),” wrote Dr. Cowan of the University of Washington, Seattle, and his associates.
The approval status of lenalidomide (Revlimid) and bortezomib (Velcade) in 2016 was used as a surrogate for availability of drug treatment: Lenalidomide had been approved in 73 countries out of 195 countries and territories and bortezomib in 103 countries. “On a global level, there are marked discrepancies in the availability of effective therapies. In addition to ensuring universal access to health care … it is imperative to at least ensure access to highly effective medications,” they wrote.
Dr. Cowan reported that he has received research funding from Janssen and AbbVie.
SOURCE: Cowan AJ et al. JAMA Oncol. 2018 May 16. doi: 10.1001/jamaoncol.2018.2128.
The global incidence of multiple myeloma rose by 126% from 1990 to 2016, with the largest regional increases occurring in East Asia and tropical Latin America, according to data from the Global Burden of Disease 2016 study.
East Asia (China, North Korea, and Taiwan) saw incident cases of multiple myeloma jump by 262% – up to 1.0 per 100,000 population – from 1990 to 2016, which was the largest increase among any of the 21 global regions; tropical Latin America’s 256% rise took its age-standardized incidence rate to 1.8 per 100,000. Worldwide, incidence of multiple myeloma was 2.1 cases per 100,000 in 2016, Andrew J. Cowan, MD, and his associates reported in JAMA Oncology.
They also looked at treatment availability, with data on stem cell transplants for 2010 coming from the Worldwide Network for Blood & Marrow Transplantation (Lancet Haematol. 2015 Mar;2[3]:e91-100). The countries with the highest rates for all indications that year were Israel (814 per 10 million population), Italy (671), Germany (665), Sweden (625), and the Netherlands (614).
“Some regions of the world lack access to stem cell transplantation entirely, particularly sub-Saharan Africa (with the exception of South Africa),” wrote Dr. Cowan of the University of Washington, Seattle, and his associates.
The approval status of lenalidomide (Revlimid) and bortezomib (Velcade) in 2016 was used as a surrogate for availability of drug treatment: Lenalidomide had been approved in 73 countries out of 195 countries and territories and bortezomib in 103 countries. “On a global level, there are marked discrepancies in the availability of effective therapies. In addition to ensuring universal access to health care … it is imperative to at least ensure access to highly effective medications,” they wrote.
Dr. Cowan reported that he has received research funding from Janssen and AbbVie.
SOURCE: Cowan AJ et al. JAMA Oncol. 2018 May 16. doi: 10.1001/jamaoncol.2018.2128.
FROM JAMA ONCOLOGY
Palliative care may reduce suicide among lung cancer patients
according to new research that will be presented at an international conference of the American Thoracic Society.
“Suicide is a significant national public health problem, especially among lung cancer patients and among veterans,” said lead author, Donald R. Sullivan, MD, of the division of pulmonary and critical care medicine at Oregon Health & Science University and a member of the OHSU Knight Cancer Institute, in a statement.
The investigators found that veterans who experienced at least one “palliative care encounter” after learning they had lung cancer were 82% less likely to die by suicide (odds ratio, 0.18; 95% confidence interval, 0.07-0.46; P less than .001), when compared with veterans who were diagnosed with lung cancer but did not receive palliative care.
The suicide rate for the advanced-stage lung cancer patients was 200/100,000 patient-years, which was more than five times higher than the suicide rate – adjusted for age, sex, and year – for all veterans using VA health care (37.5/100,000), according to the study abstract.
Of the 20,900 lung cancer patients analyzed, 30 committed suicide. Only six (20%) of the patients who died by suicide had received palliative care. Overall, most patients (18,192 or 87%) in the registry died of lung cancer. Other cancers, heart disease, and chronic obstructive pulmonary disease were some of the other common causes of death for the lung cancer patients, according to the abstract.
While several medical societies recommend palliative care for all patients with advanced-stage lung cancer, there is a gap between those recommendations and practice, noted Dr. Sullivan. “There are many barriers to palliative care, and unfortunately, some are related to clinician referrals. Not all doctors are aware of the benefits of palliative care,” he said in the statement.
Dr. Sullivan added that palliative care should be offered to all patients shortly after receiving a diagnosis of advanced-stage lung cancer.
More details on this study will be presented at the conference on Monday, May 21, at 2:30 p.m. in Room 14 A-B (Mezzanine Level) of the San Diego Convention Center.
according to new research that will be presented at an international conference of the American Thoracic Society.
“Suicide is a significant national public health problem, especially among lung cancer patients and among veterans,” said lead author, Donald R. Sullivan, MD, of the division of pulmonary and critical care medicine at Oregon Health & Science University and a member of the OHSU Knight Cancer Institute, in a statement.
The investigators found that veterans who experienced at least one “palliative care encounter” after learning they had lung cancer were 82% less likely to die by suicide (odds ratio, 0.18; 95% confidence interval, 0.07-0.46; P less than .001), when compared with veterans who were diagnosed with lung cancer but did not receive palliative care.
The suicide rate for the advanced-stage lung cancer patients was 200/100,000 patient-years, which was more than five times higher than the suicide rate – adjusted for age, sex, and year – for all veterans using VA health care (37.5/100,000), according to the study abstract.
Of the 20,900 lung cancer patients analyzed, 30 committed suicide. Only six (20%) of the patients who died by suicide had received palliative care. Overall, most patients (18,192 or 87%) in the registry died of lung cancer. Other cancers, heart disease, and chronic obstructive pulmonary disease were some of the other common causes of death for the lung cancer patients, according to the abstract.
While several medical societies recommend palliative care for all patients with advanced-stage lung cancer, there is a gap between those recommendations and practice, noted Dr. Sullivan. “There are many barriers to palliative care, and unfortunately, some are related to clinician referrals. Not all doctors are aware of the benefits of palliative care,” he said in the statement.
Dr. Sullivan added that palliative care should be offered to all patients shortly after receiving a diagnosis of advanced-stage lung cancer.
More details on this study will be presented at the conference on Monday, May 21, at 2:30 p.m. in Room 14 A-B (Mezzanine Level) of the San Diego Convention Center.
according to new research that will be presented at an international conference of the American Thoracic Society.
“Suicide is a significant national public health problem, especially among lung cancer patients and among veterans,” said lead author, Donald R. Sullivan, MD, of the division of pulmonary and critical care medicine at Oregon Health & Science University and a member of the OHSU Knight Cancer Institute, in a statement.
The investigators found that veterans who experienced at least one “palliative care encounter” after learning they had lung cancer were 82% less likely to die by suicide (odds ratio, 0.18; 95% confidence interval, 0.07-0.46; P less than .001), when compared with veterans who were diagnosed with lung cancer but did not receive palliative care.
The suicide rate for the advanced-stage lung cancer patients was 200/100,000 patient-years, which was more than five times higher than the suicide rate – adjusted for age, sex, and year – for all veterans using VA health care (37.5/100,000), according to the study abstract.
Of the 20,900 lung cancer patients analyzed, 30 committed suicide. Only six (20%) of the patients who died by suicide had received palliative care. Overall, most patients (18,192 or 87%) in the registry died of lung cancer. Other cancers, heart disease, and chronic obstructive pulmonary disease were some of the other common causes of death for the lung cancer patients, according to the abstract.
While several medical societies recommend palliative care for all patients with advanced-stage lung cancer, there is a gap between those recommendations and practice, noted Dr. Sullivan. “There are many barriers to palliative care, and unfortunately, some are related to clinician referrals. Not all doctors are aware of the benefits of palliative care,” he said in the statement.
Dr. Sullivan added that palliative care should be offered to all patients shortly after receiving a diagnosis of advanced-stage lung cancer.
More details on this study will be presented at the conference on Monday, May 21, at 2:30 p.m. in Room 14 A-B (Mezzanine Level) of the San Diego Convention Center.
FROM ATS 2018
Ocrelizumab safety update: Encouraging rates of serious infection, malignancy
LOS ANGELES – No new safety signals have emerged in multiple sclerosis patients treated with ocrelizumab, according to ongoing follow-up and postmarketing surveillance.
As of September 2017, patients with relapsing or primary progressive MS who were part of the pivotal OPERA I and II and ORATORIO trials – including phases 2 and 3 and open-label extensions – as well as an all-exposure population that included patients from prior studies, had nearly 9,500 patient-years of exposure to ocrelizumab (Ocrevus), a humanized anti-CD20 monoclonal antibody. The all-exposure population contributed about 1,500 of those patient-years, Stephen Hauser, MD, reported at the annual meeting of the American Academy of Neurology.
Postmarketing experience in approximately 37,000 treated patients with an additional 14,000 patient-years shows a fatality rate of 0.28/100 patient-years (49 fatalities) as of March 2018, he said.
“If one compares this to at least two reports of epidemiologic mortality estimates in MS, this is in line with – and in fact a little bit lower than – those estimates that range between 0.37 and 0.9, compared with 0.28,” he said. “So ... the updated safety profile in this all-exposure ocrelizumab population was generally consistent with what was seen during the controlled treatment period, and rates of serious infections fluctuated over time without any sustained increase.”
Only one serious opportunistic infection (Pasteurella) occurred in the controlled trials, and three more (two varicella zoster, one herpes simplex) occurred during open-marketing experience.
The rate of malignancies has been very encouraging, as well, as it appears to continue to align with population expectations, Dr. Hauser said.
Trial participants received 600-mg doses intravenously every 24 weeks in all three trials; in OPERA I/II, they received 96 weeks of treatment with the first dose given as two 300-mg infusions split by 14 days, and, in ORATORIO, they received at least 120 weeks of treatment with all doses split, Dr. Hauser said.
In the phase 2 study, they received split doses of 600-mg or 2,000-mg infusions through week 24; then through week 96, they received either 600-mg or 1,000-mg doses; those receiving 600 mg included those who started at that dose and those who received placebo or interferon beta-1a 30 mcg, and those receiving 1,000 mg were those who started on ocrelizumab at 2,000 mg.
The comparators were placebo in the ORATORIO and phase 2 trials, and interferon beta-1a given at a dose of 44 mcg subcutaneously three times weekly (OPERA I and II) or 30 mcg intramuscularly each week in the phase 2 trial.
All patients were offered enrollment into open-label extension studies, and “there was rather massive interest in joining the open-label extension and continuing open-label extension in both trials,” he said.
“And earlier here at the AAN [meeting] we presented the clinical efficacy data from the open-label extension, now 2 years completed – so 4 years from onset of the study – in patients who received ocrelizumab continuously for [relapsing-remitting] MS during that time period, or who switched from three-times-weekly interferon beta-1a to ocrelizumab ... and the data continue to show the positive outcomes reported in the original trials,” he added.
Further, follow-up data on MRI outcomes from the open-label extensions demonstrate that the effects on focal disease activity and on progression persists and is durable with ongoing treatment, he noted.
“In conclusion, there is no pattern of serious infections or malignancies that has emerged thus far with increased exposure, but obviously long-term follow-up and postmarketing requirement studies are needed to monitor long-term patient safety and rare events that couldn’t be captured here,” he said.
This study was sponsored by F. Hoffmann-La Roche. Dr. Hauser has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Symbiotix, Annexon Biosciences, Bionure, Molecular Stethoscope, and for serving on the Board of Directors of Neurona Therapeutics.
SOURCE: Hauser S et al. Neurology. 2018 Apr 9;(15 Suppl.):S36.001.
LOS ANGELES – No new safety signals have emerged in multiple sclerosis patients treated with ocrelizumab, according to ongoing follow-up and postmarketing surveillance.
As of September 2017, patients with relapsing or primary progressive MS who were part of the pivotal OPERA I and II and ORATORIO trials – including phases 2 and 3 and open-label extensions – as well as an all-exposure population that included patients from prior studies, had nearly 9,500 patient-years of exposure to ocrelizumab (Ocrevus), a humanized anti-CD20 monoclonal antibody. The all-exposure population contributed about 1,500 of those patient-years, Stephen Hauser, MD, reported at the annual meeting of the American Academy of Neurology.
Postmarketing experience in approximately 37,000 treated patients with an additional 14,000 patient-years shows a fatality rate of 0.28/100 patient-years (49 fatalities) as of March 2018, he said.
“If one compares this to at least two reports of epidemiologic mortality estimates in MS, this is in line with – and in fact a little bit lower than – those estimates that range between 0.37 and 0.9, compared with 0.28,” he said. “So ... the updated safety profile in this all-exposure ocrelizumab population was generally consistent with what was seen during the controlled treatment period, and rates of serious infections fluctuated over time without any sustained increase.”
Only one serious opportunistic infection (Pasteurella) occurred in the controlled trials, and three more (two varicella zoster, one herpes simplex) occurred during open-marketing experience.
The rate of malignancies has been very encouraging, as well, as it appears to continue to align with population expectations, Dr. Hauser said.
Trial participants received 600-mg doses intravenously every 24 weeks in all three trials; in OPERA I/II, they received 96 weeks of treatment with the first dose given as two 300-mg infusions split by 14 days, and, in ORATORIO, they received at least 120 weeks of treatment with all doses split, Dr. Hauser said.
In the phase 2 study, they received split doses of 600-mg or 2,000-mg infusions through week 24; then through week 96, they received either 600-mg or 1,000-mg doses; those receiving 600 mg included those who started at that dose and those who received placebo or interferon beta-1a 30 mcg, and those receiving 1,000 mg were those who started on ocrelizumab at 2,000 mg.
The comparators were placebo in the ORATORIO and phase 2 trials, and interferon beta-1a given at a dose of 44 mcg subcutaneously three times weekly (OPERA I and II) or 30 mcg intramuscularly each week in the phase 2 trial.
All patients were offered enrollment into open-label extension studies, and “there was rather massive interest in joining the open-label extension and continuing open-label extension in both trials,” he said.
“And earlier here at the AAN [meeting] we presented the clinical efficacy data from the open-label extension, now 2 years completed – so 4 years from onset of the study – in patients who received ocrelizumab continuously for [relapsing-remitting] MS during that time period, or who switched from three-times-weekly interferon beta-1a to ocrelizumab ... and the data continue to show the positive outcomes reported in the original trials,” he added.
Further, follow-up data on MRI outcomes from the open-label extensions demonstrate that the effects on focal disease activity and on progression persists and is durable with ongoing treatment, he noted.
“In conclusion, there is no pattern of serious infections or malignancies that has emerged thus far with increased exposure, but obviously long-term follow-up and postmarketing requirement studies are needed to monitor long-term patient safety and rare events that couldn’t be captured here,” he said.
This study was sponsored by F. Hoffmann-La Roche. Dr. Hauser has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Symbiotix, Annexon Biosciences, Bionure, Molecular Stethoscope, and for serving on the Board of Directors of Neurona Therapeutics.
SOURCE: Hauser S et al. Neurology. 2018 Apr 9;(15 Suppl.):S36.001.
LOS ANGELES – No new safety signals have emerged in multiple sclerosis patients treated with ocrelizumab, according to ongoing follow-up and postmarketing surveillance.
As of September 2017, patients with relapsing or primary progressive MS who were part of the pivotal OPERA I and II and ORATORIO trials – including phases 2 and 3 and open-label extensions – as well as an all-exposure population that included patients from prior studies, had nearly 9,500 patient-years of exposure to ocrelizumab (Ocrevus), a humanized anti-CD20 monoclonal antibody. The all-exposure population contributed about 1,500 of those patient-years, Stephen Hauser, MD, reported at the annual meeting of the American Academy of Neurology.
Postmarketing experience in approximately 37,000 treated patients with an additional 14,000 patient-years shows a fatality rate of 0.28/100 patient-years (49 fatalities) as of March 2018, he said.
“If one compares this to at least two reports of epidemiologic mortality estimates in MS, this is in line with – and in fact a little bit lower than – those estimates that range between 0.37 and 0.9, compared with 0.28,” he said. “So ... the updated safety profile in this all-exposure ocrelizumab population was generally consistent with what was seen during the controlled treatment period, and rates of serious infections fluctuated over time without any sustained increase.”
Only one serious opportunistic infection (Pasteurella) occurred in the controlled trials, and three more (two varicella zoster, one herpes simplex) occurred during open-marketing experience.
The rate of malignancies has been very encouraging, as well, as it appears to continue to align with population expectations, Dr. Hauser said.
Trial participants received 600-mg doses intravenously every 24 weeks in all three trials; in OPERA I/II, they received 96 weeks of treatment with the first dose given as two 300-mg infusions split by 14 days, and, in ORATORIO, they received at least 120 weeks of treatment with all doses split, Dr. Hauser said.
In the phase 2 study, they received split doses of 600-mg or 2,000-mg infusions through week 24; then through week 96, they received either 600-mg or 1,000-mg doses; those receiving 600 mg included those who started at that dose and those who received placebo or interferon beta-1a 30 mcg, and those receiving 1,000 mg were those who started on ocrelizumab at 2,000 mg.
The comparators were placebo in the ORATORIO and phase 2 trials, and interferon beta-1a given at a dose of 44 mcg subcutaneously three times weekly (OPERA I and II) or 30 mcg intramuscularly each week in the phase 2 trial.
All patients were offered enrollment into open-label extension studies, and “there was rather massive interest in joining the open-label extension and continuing open-label extension in both trials,” he said.
“And earlier here at the AAN [meeting] we presented the clinical efficacy data from the open-label extension, now 2 years completed – so 4 years from onset of the study – in patients who received ocrelizumab continuously for [relapsing-remitting] MS during that time period, or who switched from three-times-weekly interferon beta-1a to ocrelizumab ... and the data continue to show the positive outcomes reported in the original trials,” he added.
Further, follow-up data on MRI outcomes from the open-label extensions demonstrate that the effects on focal disease activity and on progression persists and is durable with ongoing treatment, he noted.
“In conclusion, there is no pattern of serious infections or malignancies that has emerged thus far with increased exposure, but obviously long-term follow-up and postmarketing requirement studies are needed to monitor long-term patient safety and rare events that couldn’t be captured here,” he said.
This study was sponsored by F. Hoffmann-La Roche. Dr. Hauser has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Symbiotix, Annexon Biosciences, Bionure, Molecular Stethoscope, and for serving on the Board of Directors of Neurona Therapeutics.
SOURCE: Hauser S et al. Neurology. 2018 Apr 9;(15 Suppl.):S36.001.
REPORTING FROM AAN 2018
Key clinical point: Follow-up and postmarketing surveillance show no new safety signals with ocrelizumab.
Major finding: The fatality rate was 0.28/100 patient-years as of March 2018.
Study details: Follow-up of patients with nearly 9,500 patient-years of experience with ocrelizumab and postmarketing surveillance in patients with more than 14,000 patient-years of experience.
Disclosures: This study was sponsored by F. Hoffmann-La Roche. Dr. Hauser has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Symbiotix, Annexon Biosciences, Bionure, Molecular Stethoscope, and for serving on the board of directors of Neurona Therapeutics.
Source: Hauser S et al. Neurology. 2018 Apr 9;(15 Suppl.):S36.001.
Looking at Ourselves
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
ABIM, ASCO to build new pathway for maintenance of board certification
in how they maintain board certification.
The new 2-year option developed by the two groups will replace ABIM’s planned 2-year Knowledge Check-In in Medical Oncology that was scheduled to begin in 2020. ASCO membership is not required to follow the 2-year assessment pathway.
“The new assessment pathway is the result of more than 2 years of input and feedback from ASCO members, as well as our desire to collaborate with ABIM because of their recognized expertise in assessments,” ASCO CEO Clifford A. Hudis, MD, said in the statement. “Our dual goals for reshaping the recertification process are to ensure that assessment better reflects the realities of cancer care and recognizes oncologists for their knowledge gained through educational and quality improvement activities.”
in how they maintain board certification.
The new 2-year option developed by the two groups will replace ABIM’s planned 2-year Knowledge Check-In in Medical Oncology that was scheduled to begin in 2020. ASCO membership is not required to follow the 2-year assessment pathway.
“The new assessment pathway is the result of more than 2 years of input and feedback from ASCO members, as well as our desire to collaborate with ABIM because of their recognized expertise in assessments,” ASCO CEO Clifford A. Hudis, MD, said in the statement. “Our dual goals for reshaping the recertification process are to ensure that assessment better reflects the realities of cancer care and recognizes oncologists for their knowledge gained through educational and quality improvement activities.”
in how they maintain board certification.
The new 2-year option developed by the two groups will replace ABIM’s planned 2-year Knowledge Check-In in Medical Oncology that was scheduled to begin in 2020. ASCO membership is not required to follow the 2-year assessment pathway.
“The new assessment pathway is the result of more than 2 years of input and feedback from ASCO members, as well as our desire to collaborate with ABIM because of their recognized expertise in assessments,” ASCO CEO Clifford A. Hudis, MD, said in the statement. “Our dual goals for reshaping the recertification process are to ensure that assessment better reflects the realities of cancer care and recognizes oncologists for their knowledge gained through educational and quality improvement activities.”
Do “VILPs” signal a new era for viral endocrinology?
BOSTON – A new era for endocrinology could emerge based in part on the recent discovery of viral sequences that align with human peptide hormones, C. Ronald Kahn, MD, said in a plenary presentation at the annual meeting of the American Association of Clinical Endocrinologists.
Using a bioinformatics approach, including four very strongly aligned viral insulin/insulinlike growth factor–I peptides, or VILPs.
They also observed increased glucose uptake and activation of cell proliferation associated with VILPs, along with significantly lowered blood glucose when they were injected into mice.
Together, those results help define VILPs as the first viral hormones to be characterized, according to Dr. Kahn, head of the section on integrative physiology and metabolism at Joslin Diabetes Center, Harvard Medical School, Boston.
“We think that this is the tip of an iceberg, and could be the beginning of a new generation for endocrinologists – that of viral-hormone endocrinology,” Dr. Kahn said in his plenary presentation.
The positive outcomes seen in rodent and human cells suggest a potential role for using VILPs to treat human disease, according to Dr. Kahn.
Dr. Kahn credited Emrah Altindis, PhD, an instructor in medicine at Harvard and a research associate at Joslin, for finding the four VILPs, each encoded by a different member of the Iridoviridae family of viruses.
Iridoviridae are not known to infect humans, but they do infect fish, insects, and amphibians. In fact, iridovirus infection is the most common viral disease in saltwater fish, according to Dr. Kahn.
“What’s interesting, of course, is that we eat fish, and we can potentially eat fish that are contaminated with these viruses,” Dr. Kahn said in his presentation.
“So, could these VILPs play a role in human disease? This is really an important area of challenge,” he added.
There are many possibilities that need to be researched. If the viruses can infect human cells, then maybe they could mimic insulin signaling and cause hypoglycemia, or on the other hand, maybe they could act as competitive antagonists and impair insulin signaling, Dr. Kahn said.
The four VILPs found to date represent the tip of a potential iceberg because only about 2% of viruses have been sequenced. That means there could be 50 times as many other viral hormones out there that could influence human health and disease, Dr. Kahn said in his presentation.
Dr. Kahn reported disclosures related to CohBar, ERX Therapeutics, Kaleido Biosciences, and Merck.
BOSTON – A new era for endocrinology could emerge based in part on the recent discovery of viral sequences that align with human peptide hormones, C. Ronald Kahn, MD, said in a plenary presentation at the annual meeting of the American Association of Clinical Endocrinologists.
Using a bioinformatics approach, including four very strongly aligned viral insulin/insulinlike growth factor–I peptides, or VILPs.
They also observed increased glucose uptake and activation of cell proliferation associated with VILPs, along with significantly lowered blood glucose when they were injected into mice.
Together, those results help define VILPs as the first viral hormones to be characterized, according to Dr. Kahn, head of the section on integrative physiology and metabolism at Joslin Diabetes Center, Harvard Medical School, Boston.
“We think that this is the tip of an iceberg, and could be the beginning of a new generation for endocrinologists – that of viral-hormone endocrinology,” Dr. Kahn said in his plenary presentation.
The positive outcomes seen in rodent and human cells suggest a potential role for using VILPs to treat human disease, according to Dr. Kahn.
Dr. Kahn credited Emrah Altindis, PhD, an instructor in medicine at Harvard and a research associate at Joslin, for finding the four VILPs, each encoded by a different member of the Iridoviridae family of viruses.
Iridoviridae are not known to infect humans, but they do infect fish, insects, and amphibians. In fact, iridovirus infection is the most common viral disease in saltwater fish, according to Dr. Kahn.
“What’s interesting, of course, is that we eat fish, and we can potentially eat fish that are contaminated with these viruses,” Dr. Kahn said in his presentation.
“So, could these VILPs play a role in human disease? This is really an important area of challenge,” he added.
There are many possibilities that need to be researched. If the viruses can infect human cells, then maybe they could mimic insulin signaling and cause hypoglycemia, or on the other hand, maybe they could act as competitive antagonists and impair insulin signaling, Dr. Kahn said.
The four VILPs found to date represent the tip of a potential iceberg because only about 2% of viruses have been sequenced. That means there could be 50 times as many other viral hormones out there that could influence human health and disease, Dr. Kahn said in his presentation.
Dr. Kahn reported disclosures related to CohBar, ERX Therapeutics, Kaleido Biosciences, and Merck.
BOSTON – A new era for endocrinology could emerge based in part on the recent discovery of viral sequences that align with human peptide hormones, C. Ronald Kahn, MD, said in a plenary presentation at the annual meeting of the American Association of Clinical Endocrinologists.
Using a bioinformatics approach, including four very strongly aligned viral insulin/insulinlike growth factor–I peptides, or VILPs.
They also observed increased glucose uptake and activation of cell proliferation associated with VILPs, along with significantly lowered blood glucose when they were injected into mice.
Together, those results help define VILPs as the first viral hormones to be characterized, according to Dr. Kahn, head of the section on integrative physiology and metabolism at Joslin Diabetes Center, Harvard Medical School, Boston.
“We think that this is the tip of an iceberg, and could be the beginning of a new generation for endocrinologists – that of viral-hormone endocrinology,” Dr. Kahn said in his plenary presentation.
The positive outcomes seen in rodent and human cells suggest a potential role for using VILPs to treat human disease, according to Dr. Kahn.
Dr. Kahn credited Emrah Altindis, PhD, an instructor in medicine at Harvard and a research associate at Joslin, for finding the four VILPs, each encoded by a different member of the Iridoviridae family of viruses.
Iridoviridae are not known to infect humans, but they do infect fish, insects, and amphibians. In fact, iridovirus infection is the most common viral disease in saltwater fish, according to Dr. Kahn.
“What’s interesting, of course, is that we eat fish, and we can potentially eat fish that are contaminated with these viruses,” Dr. Kahn said in his presentation.
“So, could these VILPs play a role in human disease? This is really an important area of challenge,” he added.
There are many possibilities that need to be researched. If the viruses can infect human cells, then maybe they could mimic insulin signaling and cause hypoglycemia, or on the other hand, maybe they could act as competitive antagonists and impair insulin signaling, Dr. Kahn said.
The four VILPs found to date represent the tip of a potential iceberg because only about 2% of viruses have been sequenced. That means there could be 50 times as many other viral hormones out there that could influence human health and disease, Dr. Kahn said in his presentation.
Dr. Kahn reported disclosures related to CohBar, ERX Therapeutics, Kaleido Biosciences, and Merck.
EXPERT ANALYSIS FROM AACE 2018
New ILD diagnostic test is available
IPF can be difficult to distinguish from other ILDs, S. Samuel Weigt, MD, of the University of California, Los Angeles, and director of UCLA Health’s Interstitial Lung Disease Center, said in a statement from Veracyte, the company marketing the test.
In fact, more than half of patients with ILDs were misdiagnosed at least once, according to a study published by the Pulmonary Fibrosis Foundation.
The new test, known as the Envisia Genomic Classifier, combines RNA sequencing and machine learning to help physicians differentiate IPF from ILDs in samples obtained through transbronchial biopsy. Its specificity and sensitivity for detecting the genomic pattern of usual interstitial pneumonia, are 88% and 70%, respectively, according to the Veracyte statement.
“Multiple studies have demonstrated that the Envisia Genomic Classifier supports more confident IPF diagnosis and optimal patient management,” Bonnie Anderson, chairman and CEO of Veracyte, said in the statement.
A benefit of the new test is that its use does not require patients to undergo risky, expensive surgery, which may not even be possible for some patients, noted Dr. Weigt. “We are pleased to be one of the few medical facilities in the country to have access to this breakthrough technology.”
To obtain more information about the Envisia Genomic Classifier and how to use the early-access program, contact Veracyte at 844-464-5864 or [email protected].
IPF can be difficult to distinguish from other ILDs, S. Samuel Weigt, MD, of the University of California, Los Angeles, and director of UCLA Health’s Interstitial Lung Disease Center, said in a statement from Veracyte, the company marketing the test.
In fact, more than half of patients with ILDs were misdiagnosed at least once, according to a study published by the Pulmonary Fibrosis Foundation.
The new test, known as the Envisia Genomic Classifier, combines RNA sequencing and machine learning to help physicians differentiate IPF from ILDs in samples obtained through transbronchial biopsy. Its specificity and sensitivity for detecting the genomic pattern of usual interstitial pneumonia, are 88% and 70%, respectively, according to the Veracyte statement.
“Multiple studies have demonstrated that the Envisia Genomic Classifier supports more confident IPF diagnosis and optimal patient management,” Bonnie Anderson, chairman and CEO of Veracyte, said in the statement.
A benefit of the new test is that its use does not require patients to undergo risky, expensive surgery, which may not even be possible for some patients, noted Dr. Weigt. “We are pleased to be one of the few medical facilities in the country to have access to this breakthrough technology.”
To obtain more information about the Envisia Genomic Classifier and how to use the early-access program, contact Veracyte at 844-464-5864 or [email protected].
IPF can be difficult to distinguish from other ILDs, S. Samuel Weigt, MD, of the University of California, Los Angeles, and director of UCLA Health’s Interstitial Lung Disease Center, said in a statement from Veracyte, the company marketing the test.
In fact, more than half of patients with ILDs were misdiagnosed at least once, according to a study published by the Pulmonary Fibrosis Foundation.
The new test, known as the Envisia Genomic Classifier, combines RNA sequencing and machine learning to help physicians differentiate IPF from ILDs in samples obtained through transbronchial biopsy. Its specificity and sensitivity for detecting the genomic pattern of usual interstitial pneumonia, are 88% and 70%, respectively, according to the Veracyte statement.
“Multiple studies have demonstrated that the Envisia Genomic Classifier supports more confident IPF diagnosis and optimal patient management,” Bonnie Anderson, chairman and CEO of Veracyte, said in the statement.
A benefit of the new test is that its use does not require patients to undergo risky, expensive surgery, which may not even be possible for some patients, noted Dr. Weigt. “We are pleased to be one of the few medical facilities in the country to have access to this breakthrough technology.”
To obtain more information about the Envisia Genomic Classifier and how to use the early-access program, contact Veracyte at 844-464-5864 or [email protected].
Trends in teen consumption of sports drinks are up and down
Although daily consumption of sports drinks decreased from 2010 to 2015 among teenagers, sugar-sweetened sports drinks still are popular, with numerous high school students drinking them at least weekly, said Kyla Cordery of the Steven and Alexandra Cohen Children’s Medical Center of New York, Lake Success, N.Y., and her associates.
Yet sports drink consumption in the previous week increased from 58% in 2010 to 60% in 2015 (P = .0002). And daily consumption of sports drinks also increased among teenagers watching television for more than 2 hours per day and among obese teens.
Boys were more than twice as likely as girls to drink one of more sports drinks daily (19% vs. 9%), as were more athletic/active children than those weren’t very athletic/active (18% vs. 10%).
“ Like many sugar-sweetened beverages, the excessive consumption of sports drinks is associated with weight gain, dental erosion, obesity, poor nutrition, and diabetes,” Ms. Cordery and her associates wrote. “The America Academy of Pediatrics’ Committee on Nutrition and Council on Sports Medicine and Fitness stated that the level of physical activity of the average child does not require the electrolyte replenishment offered by sports drinks.” Rehydration with water should be encouraged for most sports-related activities.
SOURCE: Cordery K et al. doi: 10.1542/peds.2017-2784.
Although daily consumption of sports drinks decreased from 2010 to 2015 among teenagers, sugar-sweetened sports drinks still are popular, with numerous high school students drinking them at least weekly, said Kyla Cordery of the Steven and Alexandra Cohen Children’s Medical Center of New York, Lake Success, N.Y., and her associates.
Yet sports drink consumption in the previous week increased from 58% in 2010 to 60% in 2015 (P = .0002). And daily consumption of sports drinks also increased among teenagers watching television for more than 2 hours per day and among obese teens.
Boys were more than twice as likely as girls to drink one of more sports drinks daily (19% vs. 9%), as were more athletic/active children than those weren’t very athletic/active (18% vs. 10%).
“ Like many sugar-sweetened beverages, the excessive consumption of sports drinks is associated with weight gain, dental erosion, obesity, poor nutrition, and diabetes,” Ms. Cordery and her associates wrote. “The America Academy of Pediatrics’ Committee on Nutrition and Council on Sports Medicine and Fitness stated that the level of physical activity of the average child does not require the electrolyte replenishment offered by sports drinks.” Rehydration with water should be encouraged for most sports-related activities.
SOURCE: Cordery K et al. doi: 10.1542/peds.2017-2784.
Although daily consumption of sports drinks decreased from 2010 to 2015 among teenagers, sugar-sweetened sports drinks still are popular, with numerous high school students drinking them at least weekly, said Kyla Cordery of the Steven and Alexandra Cohen Children’s Medical Center of New York, Lake Success, N.Y., and her associates.
Yet sports drink consumption in the previous week increased from 58% in 2010 to 60% in 2015 (P = .0002). And daily consumption of sports drinks also increased among teenagers watching television for more than 2 hours per day and among obese teens.
Boys were more than twice as likely as girls to drink one of more sports drinks daily (19% vs. 9%), as were more athletic/active children than those weren’t very athletic/active (18% vs. 10%).
“ Like many sugar-sweetened beverages, the excessive consumption of sports drinks is associated with weight gain, dental erosion, obesity, poor nutrition, and diabetes,” Ms. Cordery and her associates wrote. “The America Academy of Pediatrics’ Committee on Nutrition and Council on Sports Medicine and Fitness stated that the level of physical activity of the average child does not require the electrolyte replenishment offered by sports drinks.” Rehydration with water should be encouraged for most sports-related activities.
SOURCE: Cordery K et al. doi: 10.1542/peds.2017-2784.
FROM PEDIATRICS