User login
Guidance coming for mTOR inhibitors in infantile TSC
LOS ANGELES – Someday soon, physicians will probably have solid, evidence-based guidelines on how to use mTOR inhibitors in infants with tuberous sclerosis complex.
They couldn’t come soon enough. Everolimus (Afinitor) is being used off label more and more often in children under 2 years old for seizures and other manifestations of the genetic disorder, often with a decent response.
“I get emails all the time saying ‘how would you start treatment at this age?’ and we say there are no data,” he said at the American Academy of Neurology annual meeting.
Dr. Krueger presented a survey of 19 TSC centers to get the ball rolling. They reported on treating 45 children under 2 years old, at least one child at each center. The goal of the survey was to establish a baseline “to allow us to move forward with what proper studies would look like, and what needs to be done. Two-thirds of patients have seizures before their first birthday; we need to establish a safety [and efficacy] profile well before 2 years of age,” and evidence-based guidelines, he said.
On average, everolimus treatment started at 11.7 months, and continued for 27 months. Sirolimus treatment started at a mean of 16 months and continued for 16 months. Dosing ranged from once a week to daily. In contrast to case reports that skew heavily toward rhabdomyomas, most of the children were treated for epilepsy and subependymal giant cell astrocytoma (SEGA). Almost all the centers had participated in a clinical evaluation of everolimus for TSC epilepsy and SEGAs in older children, “so there was familiarity and a comfort level” for those indications, Dr. Krueger said.
Everolimus was used much more often than was sirolimus, which probably reflects ongoing clinical development of the drug. A handful of children switched between the two, probably because of side effects. Most centers reported a favorable response. The average initial daily dose of everolimus was 1.05 mg/m2; the average initial daily dose of sirolimus was 0.42 mg/m2.
Thirty-five children (78%) had an adverse event (AE), usually infections or lipid problems. Most were mild or moderate and didn’t affect treatment. Seven children (16%) had severe grade 3 events.
“The frequency of these AEs and the severity and type were not different from the earlier trials,” but almost 40% of the children discontinued treatment due to AEs, which was “much higher” than in past trials. “I think this represents the lack of real data with which to guide clinical judgment. There was a high tendency with any AE for discontinuation,” Dr. Krueger said.
There were more boys than girls among the 45 children. The majority were from the United States, and predominantly Cincinnati Children’s Hospital. Most had TSC2 mutations, which are associated with worse disease than TSC1 mutations.
Dr. Krueger reported research funding and personal compensation from Novartis, maker of everolimus. Almost all of his coinvestigators reported ties to the company. The work was supported by the Tuberous Sclerosis Alliance, which is funded in part by Novartis.
SOURCE: Krueger D et al. Neurology. 2018 Apr 90(15 Suppl.):S35.003.
LOS ANGELES – Someday soon, physicians will probably have solid, evidence-based guidelines on how to use mTOR inhibitors in infants with tuberous sclerosis complex.
They couldn’t come soon enough. Everolimus (Afinitor) is being used off label more and more often in children under 2 years old for seizures and other manifestations of the genetic disorder, often with a decent response.
“I get emails all the time saying ‘how would you start treatment at this age?’ and we say there are no data,” he said at the American Academy of Neurology annual meeting.
Dr. Krueger presented a survey of 19 TSC centers to get the ball rolling. They reported on treating 45 children under 2 years old, at least one child at each center. The goal of the survey was to establish a baseline “to allow us to move forward with what proper studies would look like, and what needs to be done. Two-thirds of patients have seizures before their first birthday; we need to establish a safety [and efficacy] profile well before 2 years of age,” and evidence-based guidelines, he said.
On average, everolimus treatment started at 11.7 months, and continued for 27 months. Sirolimus treatment started at a mean of 16 months and continued for 16 months. Dosing ranged from once a week to daily. In contrast to case reports that skew heavily toward rhabdomyomas, most of the children were treated for epilepsy and subependymal giant cell astrocytoma (SEGA). Almost all the centers had participated in a clinical evaluation of everolimus for TSC epilepsy and SEGAs in older children, “so there was familiarity and a comfort level” for those indications, Dr. Krueger said.
Everolimus was used much more often than was sirolimus, which probably reflects ongoing clinical development of the drug. A handful of children switched between the two, probably because of side effects. Most centers reported a favorable response. The average initial daily dose of everolimus was 1.05 mg/m2; the average initial daily dose of sirolimus was 0.42 mg/m2.
Thirty-five children (78%) had an adverse event (AE), usually infections or lipid problems. Most were mild or moderate and didn’t affect treatment. Seven children (16%) had severe grade 3 events.
“The frequency of these AEs and the severity and type were not different from the earlier trials,” but almost 40% of the children discontinued treatment due to AEs, which was “much higher” than in past trials. “I think this represents the lack of real data with which to guide clinical judgment. There was a high tendency with any AE for discontinuation,” Dr. Krueger said.
There were more boys than girls among the 45 children. The majority were from the United States, and predominantly Cincinnati Children’s Hospital. Most had TSC2 mutations, which are associated with worse disease than TSC1 mutations.
Dr. Krueger reported research funding and personal compensation from Novartis, maker of everolimus. Almost all of his coinvestigators reported ties to the company. The work was supported by the Tuberous Sclerosis Alliance, which is funded in part by Novartis.
SOURCE: Krueger D et al. Neurology. 2018 Apr 90(15 Suppl.):S35.003.
LOS ANGELES – Someday soon, physicians will probably have solid, evidence-based guidelines on how to use mTOR inhibitors in infants with tuberous sclerosis complex.
They couldn’t come soon enough. Everolimus (Afinitor) is being used off label more and more often in children under 2 years old for seizures and other manifestations of the genetic disorder, often with a decent response.
“I get emails all the time saying ‘how would you start treatment at this age?’ and we say there are no data,” he said at the American Academy of Neurology annual meeting.
Dr. Krueger presented a survey of 19 TSC centers to get the ball rolling. They reported on treating 45 children under 2 years old, at least one child at each center. The goal of the survey was to establish a baseline “to allow us to move forward with what proper studies would look like, and what needs to be done. Two-thirds of patients have seizures before their first birthday; we need to establish a safety [and efficacy] profile well before 2 years of age,” and evidence-based guidelines, he said.
On average, everolimus treatment started at 11.7 months, and continued for 27 months. Sirolimus treatment started at a mean of 16 months and continued for 16 months. Dosing ranged from once a week to daily. In contrast to case reports that skew heavily toward rhabdomyomas, most of the children were treated for epilepsy and subependymal giant cell astrocytoma (SEGA). Almost all the centers had participated in a clinical evaluation of everolimus for TSC epilepsy and SEGAs in older children, “so there was familiarity and a comfort level” for those indications, Dr. Krueger said.
Everolimus was used much more often than was sirolimus, which probably reflects ongoing clinical development of the drug. A handful of children switched between the two, probably because of side effects. Most centers reported a favorable response. The average initial daily dose of everolimus was 1.05 mg/m2; the average initial daily dose of sirolimus was 0.42 mg/m2.
Thirty-five children (78%) had an adverse event (AE), usually infections or lipid problems. Most were mild or moderate and didn’t affect treatment. Seven children (16%) had severe grade 3 events.
“The frequency of these AEs and the severity and type were not different from the earlier trials,” but almost 40% of the children discontinued treatment due to AEs, which was “much higher” than in past trials. “I think this represents the lack of real data with which to guide clinical judgment. There was a high tendency with any AE for discontinuation,” Dr. Krueger said.
There were more boys than girls among the 45 children. The majority were from the United States, and predominantly Cincinnati Children’s Hospital. Most had TSC2 mutations, which are associated with worse disease than TSC1 mutations.
Dr. Krueger reported research funding and personal compensation from Novartis, maker of everolimus. Almost all of his coinvestigators reported ties to the company. The work was supported by the Tuberous Sclerosis Alliance, which is funded in part by Novartis.
SOURCE: Krueger D et al. Neurology. 2018 Apr 90(15 Suppl.):S35.003.
REPORTING FROM AAN 2018
Key clinical point: A survey of treatment centers has begun to plug the evidence gap for using mTOR inhibitors in infants with tuberous sclerosis.
Major finding: Adverse events don’t seem more common than in trials of adults and older children, but the discontinuation rate is much higher, at about 40%.
Study details: Review of 45 children under 2 years old.
Disclosures: The lead investigator reported research funding and personal compensation from Novartis, maker of everolimus.
Source: Krueger D et al. Neurology. 2018 Apr 90(15 Suppl.):S35.003.
Long-acting bronchodilators increase CVD risk in certain COPD patients
Background: Long-acting inhaled bronchodilator use (LABA or LAMA) in patients with COPD is the mainstay of treatment. Prior studies have reported a possible interaction between LABA or LAMA use and increased rates of cardiovascular events; however, the results have been variable. The findings have been confounded by incomplete medical records, exclusion of patients with CVD in bronchodilator trials, and high patient drop out rates. This study aims to assess the association between LABA or LAMA use in patients with COPD and the risk of CVD.
Study design: Nested case control study.
Setting: Taiwanese national database.
Synopsis: This study included 284,200 LABA and LAMA naive patients who were aged 40 years or older and had COPD (mean age, 71.4 years); it retrieved health care claims data from 2007 through 2011 for these patients from the Taiwan National Health Insurance Research Database. During a mean follow-up of 2.0 years, 37,719 patients experienced a cardiovascular event, and 146,139 matched controls were identified. LABA or LAMA use was measured in the year preceding the cardiovascular event and stratified by duration since initiation of LABA or LAMA treatment. Logistical regression was performed to estimate the odds ratios of CVD from LABA and LAMA treatment. New LABA use was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) increased cardiovascular risk within 30 days of initiation, and new LAMA use was associated with a 1.52 fold (95% CI, 1.28-1.80; P less than .001) increased risk. In patients with prevalent LABA or LAMA use, the risk of CVD was absent or reduced.
Key limitations included the omission of contributors to cardiovascular disease, including smoking status and alcohol consumption, in the final analysis. Also, the contribution of worsening COPD to cardiovascular events was not accounted for.
Bottom line: Initiation of inhaled LABAs or LAMAs in patients with COPD is associated with a 1.5-fold increased risk of cardiovascular disease – including emergency or inpatient care for coronary artery disease, heart failure, ischemic stroke, or arrhythmia – in the first 30 days.
Citation: Wang MT et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease. JAMA Intern Med. 2018; 178(2):229-38.
Dr. Skinner is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Background: Long-acting inhaled bronchodilator use (LABA or LAMA) in patients with COPD is the mainstay of treatment. Prior studies have reported a possible interaction between LABA or LAMA use and increased rates of cardiovascular events; however, the results have been variable. The findings have been confounded by incomplete medical records, exclusion of patients with CVD in bronchodilator trials, and high patient drop out rates. This study aims to assess the association between LABA or LAMA use in patients with COPD and the risk of CVD.
Study design: Nested case control study.
Setting: Taiwanese national database.
Synopsis: This study included 284,200 LABA and LAMA naive patients who were aged 40 years or older and had COPD (mean age, 71.4 years); it retrieved health care claims data from 2007 through 2011 for these patients from the Taiwan National Health Insurance Research Database. During a mean follow-up of 2.0 years, 37,719 patients experienced a cardiovascular event, and 146,139 matched controls were identified. LABA or LAMA use was measured in the year preceding the cardiovascular event and stratified by duration since initiation of LABA or LAMA treatment. Logistical regression was performed to estimate the odds ratios of CVD from LABA and LAMA treatment. New LABA use was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) increased cardiovascular risk within 30 days of initiation, and new LAMA use was associated with a 1.52 fold (95% CI, 1.28-1.80; P less than .001) increased risk. In patients with prevalent LABA or LAMA use, the risk of CVD was absent or reduced.
Key limitations included the omission of contributors to cardiovascular disease, including smoking status and alcohol consumption, in the final analysis. Also, the contribution of worsening COPD to cardiovascular events was not accounted for.
Bottom line: Initiation of inhaled LABAs or LAMAs in patients with COPD is associated with a 1.5-fold increased risk of cardiovascular disease – including emergency or inpatient care for coronary artery disease, heart failure, ischemic stroke, or arrhythmia – in the first 30 days.
Citation: Wang MT et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease. JAMA Intern Med. 2018; 178(2):229-38.
Dr. Skinner is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Background: Long-acting inhaled bronchodilator use (LABA or LAMA) in patients with COPD is the mainstay of treatment. Prior studies have reported a possible interaction between LABA or LAMA use and increased rates of cardiovascular events; however, the results have been variable. The findings have been confounded by incomplete medical records, exclusion of patients with CVD in bronchodilator trials, and high patient drop out rates. This study aims to assess the association between LABA or LAMA use in patients with COPD and the risk of CVD.
Study design: Nested case control study.
Setting: Taiwanese national database.
Synopsis: This study included 284,200 LABA and LAMA naive patients who were aged 40 years or older and had COPD (mean age, 71.4 years); it retrieved health care claims data from 2007 through 2011 for these patients from the Taiwan National Health Insurance Research Database. During a mean follow-up of 2.0 years, 37,719 patients experienced a cardiovascular event, and 146,139 matched controls were identified. LABA or LAMA use was measured in the year preceding the cardiovascular event and stratified by duration since initiation of LABA or LAMA treatment. Logistical regression was performed to estimate the odds ratios of CVD from LABA and LAMA treatment. New LABA use was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) increased cardiovascular risk within 30 days of initiation, and new LAMA use was associated with a 1.52 fold (95% CI, 1.28-1.80; P less than .001) increased risk. In patients with prevalent LABA or LAMA use, the risk of CVD was absent or reduced.
Key limitations included the omission of contributors to cardiovascular disease, including smoking status and alcohol consumption, in the final analysis. Also, the contribution of worsening COPD to cardiovascular events was not accounted for.
Bottom line: Initiation of inhaled LABAs or LAMAs in patients with COPD is associated with a 1.5-fold increased risk of cardiovascular disease – including emergency or inpatient care for coronary artery disease, heart failure, ischemic stroke, or arrhythmia – in the first 30 days.
Citation: Wang MT et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease. JAMA Intern Med. 2018; 178(2):229-38.
Dr. Skinner is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
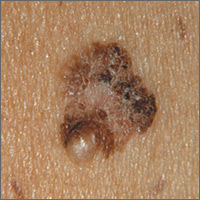
ESBL-B before colorectal surgery ups risk of surgical site infection
MADRID – Patients who are carriers of , despite a standard prophylactic antibiotic regimen.
Surgical site infections (SSIs) occurred in 23% of those who tested positive for the pathogens preoperatively, compared with 10.5% of ESBL-B–negative patients – a significant increased risk of 2.25, Yehuda Carmeli, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual congress.
ESBL-B was not the infective pathogen in most infection cases, but being a carrier increased the likelihood of an ESBL-B SSI. ESBL-B was the pathogen in 7.2% of the carriers and 1.6% of the noncarriers. However, investigators are still working to determine if the species present in the wound infection are the same as the ones present at baseline, said Dr. Carmeli of Tel Aviv Medical Center.
All of these results are emerging from the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in Gram-Negative Organisms: Studying Intervention Strategies” is a 12-million-euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multi-drug resistant Gram-negative bacteria. From 2012 to 2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures).
Several of the studies were reported at ECCMID 2018.
This portion of R-GNOSIS was intended to investigate the relationship between ESBL-B carriage and postoperative surgical site infections among colorectal surgery patients.
The study comprised 3,626 patients who were preoperatively screened for ESBL-B within 2 weeks of colorectal surgery. The ESBL-B carriage rate was 15.3% overall, but ranged from 12% to 20% by site. Of the carriers, 222 were included in this study sample. They were randomly matched with 444 noncarriers.
Anywhere from 2 weeks to 2 days before surgery, all of the patients received a standard prophylactic antibiotic. This was most often an infusion of 1.5 g cefuroxime plus 500 mg metronidazole. Other cephalosporins were allowed at the clinician’s discretion.
Patients were a mean of 62 years old. Nearly half (48%) had cardiovascular disease and about a third had undergone a prior colorectal surgical procedure. Cancer was the surgical indication in about 70%. Other indications were inflammatory bowel disease and diverticular disease.
A multivariate analysis controlled for age, cardiovascular disease, indication for surgery, and whether the procedure included a rectal resection, retention of drain at the surgical site, or stoma. The model also controlled for National Nosocomial Infection Surveillance score, a three-point scale that estimates surgical infection risk. Among this cohort, 48% were at low risk, 43% at moderate risk, and 10% at high risk.
Dr. Carmeli made no financial disclosures.
SOURCE: Carmeli et al, ECCMID 2018, Oral Abstract O1133.
MADRID – Patients who are carriers of , despite a standard prophylactic antibiotic regimen.
Surgical site infections (SSIs) occurred in 23% of those who tested positive for the pathogens preoperatively, compared with 10.5% of ESBL-B–negative patients – a significant increased risk of 2.25, Yehuda Carmeli, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual congress.
ESBL-B was not the infective pathogen in most infection cases, but being a carrier increased the likelihood of an ESBL-B SSI. ESBL-B was the pathogen in 7.2% of the carriers and 1.6% of the noncarriers. However, investigators are still working to determine if the species present in the wound infection are the same as the ones present at baseline, said Dr. Carmeli of Tel Aviv Medical Center.
All of these results are emerging from the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in Gram-Negative Organisms: Studying Intervention Strategies” is a 12-million-euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multi-drug resistant Gram-negative bacteria. From 2012 to 2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures).
Several of the studies were reported at ECCMID 2018.
This portion of R-GNOSIS was intended to investigate the relationship between ESBL-B carriage and postoperative surgical site infections among colorectal surgery patients.
The study comprised 3,626 patients who were preoperatively screened for ESBL-B within 2 weeks of colorectal surgery. The ESBL-B carriage rate was 15.3% overall, but ranged from 12% to 20% by site. Of the carriers, 222 were included in this study sample. They were randomly matched with 444 noncarriers.
Anywhere from 2 weeks to 2 days before surgery, all of the patients received a standard prophylactic antibiotic. This was most often an infusion of 1.5 g cefuroxime plus 500 mg metronidazole. Other cephalosporins were allowed at the clinician’s discretion.
Patients were a mean of 62 years old. Nearly half (48%) had cardiovascular disease and about a third had undergone a prior colorectal surgical procedure. Cancer was the surgical indication in about 70%. Other indications were inflammatory bowel disease and diverticular disease.
A multivariate analysis controlled for age, cardiovascular disease, indication for surgery, and whether the procedure included a rectal resection, retention of drain at the surgical site, or stoma. The model also controlled for National Nosocomial Infection Surveillance score, a three-point scale that estimates surgical infection risk. Among this cohort, 48% were at low risk, 43% at moderate risk, and 10% at high risk.
Dr. Carmeli made no financial disclosures.
SOURCE: Carmeli et al, ECCMID 2018, Oral Abstract O1133.
MADRID – Patients who are carriers of , despite a standard prophylactic antibiotic regimen.
Surgical site infections (SSIs) occurred in 23% of those who tested positive for the pathogens preoperatively, compared with 10.5% of ESBL-B–negative patients – a significant increased risk of 2.25, Yehuda Carmeli, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual congress.
ESBL-B was not the infective pathogen in most infection cases, but being a carrier increased the likelihood of an ESBL-B SSI. ESBL-B was the pathogen in 7.2% of the carriers and 1.6% of the noncarriers. However, investigators are still working to determine if the species present in the wound infection are the same as the ones present at baseline, said Dr. Carmeli of Tel Aviv Medical Center.
All of these results are emerging from the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in Gram-Negative Organisms: Studying Intervention Strategies” is a 12-million-euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multi-drug resistant Gram-negative bacteria. From 2012 to 2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures).
Several of the studies were reported at ECCMID 2018.
This portion of R-GNOSIS was intended to investigate the relationship between ESBL-B carriage and postoperative surgical site infections among colorectal surgery patients.
The study comprised 3,626 patients who were preoperatively screened for ESBL-B within 2 weeks of colorectal surgery. The ESBL-B carriage rate was 15.3% overall, but ranged from 12% to 20% by site. Of the carriers, 222 were included in this study sample. They were randomly matched with 444 noncarriers.
Anywhere from 2 weeks to 2 days before surgery, all of the patients received a standard prophylactic antibiotic. This was most often an infusion of 1.5 g cefuroxime plus 500 mg metronidazole. Other cephalosporins were allowed at the clinician’s discretion.
Patients were a mean of 62 years old. Nearly half (48%) had cardiovascular disease and about a third had undergone a prior colorectal surgical procedure. Cancer was the surgical indication in about 70%. Other indications were inflammatory bowel disease and diverticular disease.
A multivariate analysis controlled for age, cardiovascular disease, indication for surgery, and whether the procedure included a rectal resection, retention of drain at the surgical site, or stoma. The model also controlled for National Nosocomial Infection Surveillance score, a three-point scale that estimates surgical infection risk. Among this cohort, 48% were at low risk, 43% at moderate risk, and 10% at high risk.
Dr. Carmeli made no financial disclosures.
SOURCE: Carmeli et al, ECCMID 2018, Oral Abstract O1133.
REPORTING FROM ECCMID 2018
Key clinical point: ESBL-B colonization increased the risk of surgical site infections after colorectal surgery, despite use of standard preoperative antibiotics.
Major finding: ESBL-B carriage more than doubled the risk of a colorectal surgical site infection by (OR 2.25).
Study details: The prospective study comprised 222 carriers and 444 noncarriers.
Disclosures: The study is part of the R-GNOSIS project, a 12-million-euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multi-drug resistant Gram-negative bacteria.
Source: Carmeli Y et al. ECCMID 2018, Oral Abstract O1130.
Simple QI intervention helped improve HPV vaccination rates
TORONTO – Teaching simple quality improvement principles to individual pediatric practices can improve adolescent human papillomavirus (HPV) vaccination rates, results from a multicenter study showed.
“We know that HPV vaccination rates are low, and there have been many efforts to improve the vaccination rates nationwide,” one of the study authors, Manika Suryadevara, MD, said in an interview at the Pediatric Academic Societies meeting. “One reason that vaccine rates are low in adolescents is missed opportunities. Adolescents don’t always show up for routine well-child visits where immunization records are reviewed, but they may show up with a cold, a sprained ankle, or a hospital follow-up. Providers do not routinely check immunizations at these visits, then don’t recommend vaccine to those who need it. These are the missed opportunities we need to act upon.”
Data were entered into the AAP Quality Improvement Data Aggregator and run charts were printed. Next, each practice held monthly team meetings for 5 months to discuss chart review data, run chart results, and to determine intervention change for the next cycle. “Most of the interventions included standing orders, optimizing nurse’s visits, using electronic medical reminders to review immunization records, and having immunization records pulled for all adolescents who show up in their practice,” Dr. Suryadevara said. “The goal of these systematic changes is to make the work flow seamless.”
Analysis of run chart data revealed that over the five monthly cycles, the HPV vaccine completion rate improved from 45% to 65%, while the overall HPV vaccine missed opportunities was reduced from 45% to 19%. Specifically, reductions in missed opportunities fell from 9% to 0% during well-child visits, from 80% to 61% during acute visits, from 25% to 0% during follow-up visits, and from 11% to 0% during nurse-only visits. “We did see missed opportunities for acute visits – those who come in sick, but even these missed opportunities decreased over the 6-month study period,” Dr. Suryadevara said.
During follow-up teleconference calls, practice representatives reported positive experiences about the QI process and outcome improvements. “Once the practices were able to pull everyone on board and develop practice changes, I wasn’t surprised that the interventions worked,” she said. “They were able to develop systematic interventions, change the work flow in their practice, and get the results we anticipated.”
The study was funded by the AAP Hub and Spoke Initiative. Dr. Suryadevara reported having no financial disclosures.
[email protected]
TORONTO – Teaching simple quality improvement principles to individual pediatric practices can improve adolescent human papillomavirus (HPV) vaccination rates, results from a multicenter study showed.
“We know that HPV vaccination rates are low, and there have been many efforts to improve the vaccination rates nationwide,” one of the study authors, Manika Suryadevara, MD, said in an interview at the Pediatric Academic Societies meeting. “One reason that vaccine rates are low in adolescents is missed opportunities. Adolescents don’t always show up for routine well-child visits where immunization records are reviewed, but they may show up with a cold, a sprained ankle, or a hospital follow-up. Providers do not routinely check immunizations at these visits, then don’t recommend vaccine to those who need it. These are the missed opportunities we need to act upon.”
Data were entered into the AAP Quality Improvement Data Aggregator and run charts were printed. Next, each practice held monthly team meetings for 5 months to discuss chart review data, run chart results, and to determine intervention change for the next cycle. “Most of the interventions included standing orders, optimizing nurse’s visits, using electronic medical reminders to review immunization records, and having immunization records pulled for all adolescents who show up in their practice,” Dr. Suryadevara said. “The goal of these systematic changes is to make the work flow seamless.”
Analysis of run chart data revealed that over the five monthly cycles, the HPV vaccine completion rate improved from 45% to 65%, while the overall HPV vaccine missed opportunities was reduced from 45% to 19%. Specifically, reductions in missed opportunities fell from 9% to 0% during well-child visits, from 80% to 61% during acute visits, from 25% to 0% during follow-up visits, and from 11% to 0% during nurse-only visits. “We did see missed opportunities for acute visits – those who come in sick, but even these missed opportunities decreased over the 6-month study period,” Dr. Suryadevara said.
During follow-up teleconference calls, practice representatives reported positive experiences about the QI process and outcome improvements. “Once the practices were able to pull everyone on board and develop practice changes, I wasn’t surprised that the interventions worked,” she said. “They were able to develop systematic interventions, change the work flow in their practice, and get the results we anticipated.”
The study was funded by the AAP Hub and Spoke Initiative. Dr. Suryadevara reported having no financial disclosures.
[email protected]
TORONTO – Teaching simple quality improvement principles to individual pediatric practices can improve adolescent human papillomavirus (HPV) vaccination rates, results from a multicenter study showed.
“We know that HPV vaccination rates are low, and there have been many efforts to improve the vaccination rates nationwide,” one of the study authors, Manika Suryadevara, MD, said in an interview at the Pediatric Academic Societies meeting. “One reason that vaccine rates are low in adolescents is missed opportunities. Adolescents don’t always show up for routine well-child visits where immunization records are reviewed, but they may show up with a cold, a sprained ankle, or a hospital follow-up. Providers do not routinely check immunizations at these visits, then don’t recommend vaccine to those who need it. These are the missed opportunities we need to act upon.”
Data were entered into the AAP Quality Improvement Data Aggregator and run charts were printed. Next, each practice held monthly team meetings for 5 months to discuss chart review data, run chart results, and to determine intervention change for the next cycle. “Most of the interventions included standing orders, optimizing nurse’s visits, using electronic medical reminders to review immunization records, and having immunization records pulled for all adolescents who show up in their practice,” Dr. Suryadevara said. “The goal of these systematic changes is to make the work flow seamless.”
Analysis of run chart data revealed that over the five monthly cycles, the HPV vaccine completion rate improved from 45% to 65%, while the overall HPV vaccine missed opportunities was reduced from 45% to 19%. Specifically, reductions in missed opportunities fell from 9% to 0% during well-child visits, from 80% to 61% during acute visits, from 25% to 0% during follow-up visits, and from 11% to 0% during nurse-only visits. “We did see missed opportunities for acute visits – those who come in sick, but even these missed opportunities decreased over the 6-month study period,” Dr. Suryadevara said.
During follow-up teleconference calls, practice representatives reported positive experiences about the QI process and outcome improvements. “Once the practices were able to pull everyone on board and develop practice changes, I wasn’t surprised that the interventions worked,” she said. “They were able to develop systematic interventions, change the work flow in their practice, and get the results we anticipated.”
The study was funded by the AAP Hub and Spoke Initiative. Dr. Suryadevara reported having no financial disclosures.
[email protected]
REPORTING FROM PAS 2018
Key clinical point: Teaching quality improvement to pediatric practices can improve adolescent human papillomavirus (HPV) vaccine completion rates.
Major finding:
Study details: A quality improvement project conducted at five large pediatric practices in New York state aimed at reducing missed vaccination opportunities in an effort to improve HPV vaccination completion rates.
Disclosures: The study was funded by the American Academy of Pediatrics Hub and Spoke Initiative. Dr. Suryadevara reported having no financial disclosures.
Upfront NGS testing of metastatic NSCLC saves money, time
Comprehensive testing of newly diagnosed metastatic non–small cell lung cancer (NSCLC) with next-generation sequencing (NGS) for known lung cancer–related genomic alterations is cost-saving relative to single-gene testing strategies and often faster, a new study finds.
“We know now that genomic testing for all patients with advanced NSCLC is the standard of care to help detect oncogenic drivers, to inform treatment decisions,” lead study author Nathan A. Pennell, MD, PhD, codirector of the Cleveland Clinic lung cancer program, said in a press briefing leading up to the ASCO annual meeting. But the optimal strategy for this testing is unclear.
He and his colleagues conducted a decision analytic modeling study among hypothetical insurance plans having 1 million enrollees. Outcomes were compared between NGS testing and three single-gene testing strategies.
Data indicated that compared with exclusionary, sequential, or hot-spot panel testing approaches, NGS testing simultaneously for eight genomic alterations having Food and Drug Administration–approved or investigational targeted therapies could save up to $2.1 million among Medicare beneficiaries and up to $250,842 among patients covered by commercial insurance. The costs to payers decreased as the percentage of patients receiving NGS testing increased. Moreover, the wait time for results was similar or roughly half as long with NGS.
“Our results showed that there were substantial cost savings associated with upfront NGS testing compared to all other strategies,” Dr. Pennell said. “In addition, NGS had a faster turnaround time than either sequential or exclusionary testing, which is critically important for sick lung cancer patients, to make sure they get their treatment as quickly as possible. Waiting a month or longer is simply no longer viable for patients because they get sick very quickly and these treatments work very well.”
Of note, the model indicated that some patients undergoing initial single-gene testing strategies never had their genomic alterations detected because tissue for testing ran out and they were too sick to undergo another biopsy.
“The bottom line is, ultimately, using the best single test upfront results in the fastest turnaround time, the highest percentage of patients with targetable alterations identified, and overall the lowest cost to payers,” he summarized.
A major challenge in this population is going back and retesting for known or new genomic alterations, agreed ASCO President Bruce E. Johnson, MD, FASCO. “At our upcoming meeting, we are going to hear about RET, which may end up as a target and may therefore need to be tested for.”
Recently, oncologists have a new attractive option of billing for NGS panels rather than for single gene tests, he noted.
“This study really shows that by doing all the testing at the same time, you can both get results back more quickly as well as get information,” said Dr. Johnson, professor of medicine at the Dana-Farber Cancer Institute and a leader of the Dana-Farber/Harvard Cancer Center Lung Cancer Program, Boston. “This study looked at an NGS panel of eight genes, but most of the NGS panels contain somewhere between 50 and 400 genes, so you get a lot more information with this at a cost that’s competitive or less. So this will be welcome news to people who are ordering these gene panels.”
Study details
For NSCLC, there are currently approved treatments that target alterations in EGFR, ALK, ROS1, and BRAF, and investigational treatments in clinical trials that target alterations in MET, HER2, RET, and NTRK1.
In the model Dr. Pennell and his colleagues developed, patients with newly diagnosed metastatic NSCLC received testing for programmed death ligand 1 (PD-L1) plus testing for the above known lung cancer–related genes using one of four strategies:
- NGS testing (testing of all eight genes plus KRAS simultaneously).
- Sequential testing (testing one gene at a time starting with EGFR).
- Exclusionary testing (testing for KRAS mutation, the most common genomic alteration, followed by sequential testing for changes in other genes only if KRAS was not mutated).
- Hot-spot panel testing (combined testing for EGFR, ALK, ROS1, and BRAF), followed by either single-gene or NGS testing for alterations in other genes.
Model results indicated that among 1 million hypothetical plan enrollees, 2,066 patients covered by the Centers for Medicare & Medicaid Services and 156 covered by U.S. commercial insurers would have newly diagnosed metastatic NSCLC and therefore be eligible for testing.
Estimated time to receive test results was 2 weeks for NGS testing and for panel testing, compared with 4.7 weeks for exclusionary testing and 4.8 weeks for sequential testing.
In the CMS population, NGS testing would save about $1.4 million compared with exclusionary testing, more than $1.5 million compared with sequential testing, and about $2.1 million compared with panel testing. In the commercial health plan cohort, NGS would save $3,809 compared with exclusionary testing, $127,402 compared with sequential testing, and $250,842 compared with panel testing.
Dr. Pennell disclosed that he has a consulting or advisory role with AstraZeneca, Lilly, and Regeneron, and that his institution receives research funding from Genentech, NewLink Genetics, Clovis Oncology, Astex Pharmaceuticals, Celgene, AstraZeneca, Pfizer, and Merck. The study received funding from Novartis.
SOURCE: Pennell et al., ASCO Annual Meeting Abstract 9031.
Comprehensive testing of newly diagnosed metastatic non–small cell lung cancer (NSCLC) with next-generation sequencing (NGS) for known lung cancer–related genomic alterations is cost-saving relative to single-gene testing strategies and often faster, a new study finds.
“We know now that genomic testing for all patients with advanced NSCLC is the standard of care to help detect oncogenic drivers, to inform treatment decisions,” lead study author Nathan A. Pennell, MD, PhD, codirector of the Cleveland Clinic lung cancer program, said in a press briefing leading up to the ASCO annual meeting. But the optimal strategy for this testing is unclear.
He and his colleagues conducted a decision analytic modeling study among hypothetical insurance plans having 1 million enrollees. Outcomes were compared between NGS testing and three single-gene testing strategies.
Data indicated that compared with exclusionary, sequential, or hot-spot panel testing approaches, NGS testing simultaneously for eight genomic alterations having Food and Drug Administration–approved or investigational targeted therapies could save up to $2.1 million among Medicare beneficiaries and up to $250,842 among patients covered by commercial insurance. The costs to payers decreased as the percentage of patients receiving NGS testing increased. Moreover, the wait time for results was similar or roughly half as long with NGS.
“Our results showed that there were substantial cost savings associated with upfront NGS testing compared to all other strategies,” Dr. Pennell said. “In addition, NGS had a faster turnaround time than either sequential or exclusionary testing, which is critically important for sick lung cancer patients, to make sure they get their treatment as quickly as possible. Waiting a month or longer is simply no longer viable for patients because they get sick very quickly and these treatments work very well.”
Of note, the model indicated that some patients undergoing initial single-gene testing strategies never had their genomic alterations detected because tissue for testing ran out and they were too sick to undergo another biopsy.
“The bottom line is, ultimately, using the best single test upfront results in the fastest turnaround time, the highest percentage of patients with targetable alterations identified, and overall the lowest cost to payers,” he summarized.
A major challenge in this population is going back and retesting for known or new genomic alterations, agreed ASCO President Bruce E. Johnson, MD, FASCO. “At our upcoming meeting, we are going to hear about RET, which may end up as a target and may therefore need to be tested for.”
Recently, oncologists have a new attractive option of billing for NGS panels rather than for single gene tests, he noted.
“This study really shows that by doing all the testing at the same time, you can both get results back more quickly as well as get information,” said Dr. Johnson, professor of medicine at the Dana-Farber Cancer Institute and a leader of the Dana-Farber/Harvard Cancer Center Lung Cancer Program, Boston. “This study looked at an NGS panel of eight genes, but most of the NGS panels contain somewhere between 50 and 400 genes, so you get a lot more information with this at a cost that’s competitive or less. So this will be welcome news to people who are ordering these gene panels.”
Study details
For NSCLC, there are currently approved treatments that target alterations in EGFR, ALK, ROS1, and BRAF, and investigational treatments in clinical trials that target alterations in MET, HER2, RET, and NTRK1.
In the model Dr. Pennell and his colleagues developed, patients with newly diagnosed metastatic NSCLC received testing for programmed death ligand 1 (PD-L1) plus testing for the above known lung cancer–related genes using one of four strategies:
- NGS testing (testing of all eight genes plus KRAS simultaneously).
- Sequential testing (testing one gene at a time starting with EGFR).
- Exclusionary testing (testing for KRAS mutation, the most common genomic alteration, followed by sequential testing for changes in other genes only if KRAS was not mutated).
- Hot-spot panel testing (combined testing for EGFR, ALK, ROS1, and BRAF), followed by either single-gene or NGS testing for alterations in other genes.
Model results indicated that among 1 million hypothetical plan enrollees, 2,066 patients covered by the Centers for Medicare & Medicaid Services and 156 covered by U.S. commercial insurers would have newly diagnosed metastatic NSCLC and therefore be eligible for testing.
Estimated time to receive test results was 2 weeks for NGS testing and for panel testing, compared with 4.7 weeks for exclusionary testing and 4.8 weeks for sequential testing.
In the CMS population, NGS testing would save about $1.4 million compared with exclusionary testing, more than $1.5 million compared with sequential testing, and about $2.1 million compared with panel testing. In the commercial health plan cohort, NGS would save $3,809 compared with exclusionary testing, $127,402 compared with sequential testing, and $250,842 compared with panel testing.
Dr. Pennell disclosed that he has a consulting or advisory role with AstraZeneca, Lilly, and Regeneron, and that his institution receives research funding from Genentech, NewLink Genetics, Clovis Oncology, Astex Pharmaceuticals, Celgene, AstraZeneca, Pfizer, and Merck. The study received funding from Novartis.
SOURCE: Pennell et al., ASCO Annual Meeting Abstract 9031.
Comprehensive testing of newly diagnosed metastatic non–small cell lung cancer (NSCLC) with next-generation sequencing (NGS) for known lung cancer–related genomic alterations is cost-saving relative to single-gene testing strategies and often faster, a new study finds.
“We know now that genomic testing for all patients with advanced NSCLC is the standard of care to help detect oncogenic drivers, to inform treatment decisions,” lead study author Nathan A. Pennell, MD, PhD, codirector of the Cleveland Clinic lung cancer program, said in a press briefing leading up to the ASCO annual meeting. But the optimal strategy for this testing is unclear.
He and his colleagues conducted a decision analytic modeling study among hypothetical insurance plans having 1 million enrollees. Outcomes were compared between NGS testing and three single-gene testing strategies.
Data indicated that compared with exclusionary, sequential, or hot-spot panel testing approaches, NGS testing simultaneously for eight genomic alterations having Food and Drug Administration–approved or investigational targeted therapies could save up to $2.1 million among Medicare beneficiaries and up to $250,842 among patients covered by commercial insurance. The costs to payers decreased as the percentage of patients receiving NGS testing increased. Moreover, the wait time for results was similar or roughly half as long with NGS.
“Our results showed that there were substantial cost savings associated with upfront NGS testing compared to all other strategies,” Dr. Pennell said. “In addition, NGS had a faster turnaround time than either sequential or exclusionary testing, which is critically important for sick lung cancer patients, to make sure they get their treatment as quickly as possible. Waiting a month or longer is simply no longer viable for patients because they get sick very quickly and these treatments work very well.”
Of note, the model indicated that some patients undergoing initial single-gene testing strategies never had their genomic alterations detected because tissue for testing ran out and they were too sick to undergo another biopsy.
“The bottom line is, ultimately, using the best single test upfront results in the fastest turnaround time, the highest percentage of patients with targetable alterations identified, and overall the lowest cost to payers,” he summarized.
A major challenge in this population is going back and retesting for known or new genomic alterations, agreed ASCO President Bruce E. Johnson, MD, FASCO. “At our upcoming meeting, we are going to hear about RET, which may end up as a target and may therefore need to be tested for.”
Recently, oncologists have a new attractive option of billing for NGS panels rather than for single gene tests, he noted.
“This study really shows that by doing all the testing at the same time, you can both get results back more quickly as well as get information,” said Dr. Johnson, professor of medicine at the Dana-Farber Cancer Institute and a leader of the Dana-Farber/Harvard Cancer Center Lung Cancer Program, Boston. “This study looked at an NGS panel of eight genes, but most of the NGS panels contain somewhere between 50 and 400 genes, so you get a lot more information with this at a cost that’s competitive or less. So this will be welcome news to people who are ordering these gene panels.”
Study details
For NSCLC, there are currently approved treatments that target alterations in EGFR, ALK, ROS1, and BRAF, and investigational treatments in clinical trials that target alterations in MET, HER2, RET, and NTRK1.
In the model Dr. Pennell and his colleagues developed, patients with newly diagnosed metastatic NSCLC received testing for programmed death ligand 1 (PD-L1) plus testing for the above known lung cancer–related genes using one of four strategies:
- NGS testing (testing of all eight genes plus KRAS simultaneously).
- Sequential testing (testing one gene at a time starting with EGFR).
- Exclusionary testing (testing for KRAS mutation, the most common genomic alteration, followed by sequential testing for changes in other genes only if KRAS was not mutated).
- Hot-spot panel testing (combined testing for EGFR, ALK, ROS1, and BRAF), followed by either single-gene or NGS testing for alterations in other genes.
Model results indicated that among 1 million hypothetical plan enrollees, 2,066 patients covered by the Centers for Medicare & Medicaid Services and 156 covered by U.S. commercial insurers would have newly diagnosed metastatic NSCLC and therefore be eligible for testing.
Estimated time to receive test results was 2 weeks for NGS testing and for panel testing, compared with 4.7 weeks for exclusionary testing and 4.8 weeks for sequential testing.
In the CMS population, NGS testing would save about $1.4 million compared with exclusionary testing, more than $1.5 million compared with sequential testing, and about $2.1 million compared with panel testing. In the commercial health plan cohort, NGS would save $3,809 compared with exclusionary testing, $127,402 compared with sequential testing, and $250,842 compared with panel testing.
Dr. Pennell disclosed that he has a consulting or advisory role with AstraZeneca, Lilly, and Regeneron, and that his institution receives research funding from Genentech, NewLink Genetics, Clovis Oncology, Astex Pharmaceuticals, Celgene, AstraZeneca, Pfizer, and Merck. The study received funding from Novartis.
SOURCE: Pennell et al., ASCO Annual Meeting Abstract 9031.
REPORTING FROM THE ASCO ANNUAL MEETING
Key clinical point:
Major finding: Relative to exclusionary, sequential, or panel testing, NGS testing could save up to $2.1 million among CMS beneficiaries and up to $250,842 among patients covered by commercial insurance.
Study details: A decision analytic modeling study in hypothetical cohorts of 1 million insurance plan enrollees.
Disclosures: Dr. Pennell disclosed that he has a consulting or advisory role with AstraZeneca, Lilly, and Regeneron, and that his institution receives research funding from Genentech, NewLink Genetics, Clovis Oncology, Astex Pharmaceuticals, Celgene, AstraZeneca, Pfizer, and Merck. The study received funding from Novartis.
Source: Pennell et al. ASCO Annual Meeting, Abstract 9031.
FDA’s Gottlieb floats ideas on Medicare drug coverage
When the current Part B drug reimbursement scheme – average sales price plus an additional 6% to cover administration and storage – was developed, there was little competition in most of the covered therapeutic categories, Scott Gottlieb, MD, Food and Drug Administration commissioner, said May 15 at an event hosted by the Alliance for Health Policy.
The situation is different now. “Not only are these product categories multisource and in some cases quite crowded, but there is also a lot of therapeutic equivalence,” Dr. Gottlieb said. “There are a lot of other types of drugs [physicians] might use to try to address the same clinical condition.”
He specifically noted that autoimmune and inflammatory conditions have a wealth of products that address them in different ways, with the opportunity for therapeutic substitutions. He also mentioned drugs that are delivered through durable medical equipment as another area open to being moved into a more price-competitive space.
“I don’t think anyone envisioned how competitive these categories would become,” he said.
Switching drug coverage from Medicare Part B to Part D is just one of more than 50 proposals contained in a broad package introduced by the White House on May 11 to address the rising prices of prescription drugs.
“In situations where you have a lot of therapeutic variety ... you have [insurance] plans negotiating pricing using formularies and using things like step therapy, putting drugs on preferred tiers relative to the price concessions they are able to extract,” Dr. Gottlieb said.
In contrast, the current Part B [program], “looks like the small molecule world with respect to how much competition we see within some of these categories, but you don’t have the same structure. Times have changed, and I think that is why you see Secretary [Alex Azar of the Health & Human Services department] rethinking how we bid out those Part B drugs into a competitive scheme.”
Dr. Gottlieb suggested that more competition could come from the moving coverage to Medicare Part D or possibly through a reinvigorated competitive acquisition program for Part B.
When the current Part B drug reimbursement scheme – average sales price plus an additional 6% to cover administration and storage – was developed, there was little competition in most of the covered therapeutic categories, Scott Gottlieb, MD, Food and Drug Administration commissioner, said May 15 at an event hosted by the Alliance for Health Policy.
The situation is different now. “Not only are these product categories multisource and in some cases quite crowded, but there is also a lot of therapeutic equivalence,” Dr. Gottlieb said. “There are a lot of other types of drugs [physicians] might use to try to address the same clinical condition.”
He specifically noted that autoimmune and inflammatory conditions have a wealth of products that address them in different ways, with the opportunity for therapeutic substitutions. He also mentioned drugs that are delivered through durable medical equipment as another area open to being moved into a more price-competitive space.
“I don’t think anyone envisioned how competitive these categories would become,” he said.
Switching drug coverage from Medicare Part B to Part D is just one of more than 50 proposals contained in a broad package introduced by the White House on May 11 to address the rising prices of prescription drugs.
“In situations where you have a lot of therapeutic variety ... you have [insurance] plans negotiating pricing using formularies and using things like step therapy, putting drugs on preferred tiers relative to the price concessions they are able to extract,” Dr. Gottlieb said.
In contrast, the current Part B [program], “looks like the small molecule world with respect to how much competition we see within some of these categories, but you don’t have the same structure. Times have changed, and I think that is why you see Secretary [Alex Azar of the Health & Human Services department] rethinking how we bid out those Part B drugs into a competitive scheme.”
Dr. Gottlieb suggested that more competition could come from the moving coverage to Medicare Part D or possibly through a reinvigorated competitive acquisition program for Part B.
When the current Part B drug reimbursement scheme – average sales price plus an additional 6% to cover administration and storage – was developed, there was little competition in most of the covered therapeutic categories, Scott Gottlieb, MD, Food and Drug Administration commissioner, said May 15 at an event hosted by the Alliance for Health Policy.
The situation is different now. “Not only are these product categories multisource and in some cases quite crowded, but there is also a lot of therapeutic equivalence,” Dr. Gottlieb said. “There are a lot of other types of drugs [physicians] might use to try to address the same clinical condition.”
He specifically noted that autoimmune and inflammatory conditions have a wealth of products that address them in different ways, with the opportunity for therapeutic substitutions. He also mentioned drugs that are delivered through durable medical equipment as another area open to being moved into a more price-competitive space.
“I don’t think anyone envisioned how competitive these categories would become,” he said.
Switching drug coverage from Medicare Part B to Part D is just one of more than 50 proposals contained in a broad package introduced by the White House on May 11 to address the rising prices of prescription drugs.
“In situations where you have a lot of therapeutic variety ... you have [insurance] plans negotiating pricing using formularies and using things like step therapy, putting drugs on preferred tiers relative to the price concessions they are able to extract,” Dr. Gottlieb said.
In contrast, the current Part B [program], “looks like the small molecule world with respect to how much competition we see within some of these categories, but you don’t have the same structure. Times have changed, and I think that is why you see Secretary [Alex Azar of the Health & Human Services department] rethinking how we bid out those Part B drugs into a competitive scheme.”
Dr. Gottlieb suggested that more competition could come from the moving coverage to Medicare Part D or possibly through a reinvigorated competitive acquisition program for Part B.
Diabetic Ketoacidosis Takes a Turn for the Worse
Diabetic ketoacidosis (DKA) is life threatening, but preventable. It was good news that between 2000-2009, rates of DKA began to decline, but researchers from the Centers for Disease Control and Prevention (CDC) say it is “concerning” to find that those rates began to reverse between 2009-2014 , increasing 54.9%. All age groups saw an increase of ≥ 6% annually. The highest rates were among people aged < 45 years: approximately 27 times higher than among people aged > 65 years.
The causes for the reversal are not clear, the researchers say, but they offer several possibilities, including changes in case definition, new medications that might raise the risk of DKA, and higher admission rates for people with less serious disease.
Because DKA rates were highest among people aged > 45 years, the researchers say information from studies in that age group might help determine whether prevention strategies should focus on factors such as symptom recognition, adherence to treatment, self-management skills, access to care, or cost of treatment.
Diabetic ketoacidosis (DKA) is life threatening, but preventable. It was good news that between 2000-2009, rates of DKA began to decline, but researchers from the Centers for Disease Control and Prevention (CDC) say it is “concerning” to find that those rates began to reverse between 2009-2014 , increasing 54.9%. All age groups saw an increase of ≥ 6% annually. The highest rates were among people aged < 45 years: approximately 27 times higher than among people aged > 65 years.
The causes for the reversal are not clear, the researchers say, but they offer several possibilities, including changes in case definition, new medications that might raise the risk of DKA, and higher admission rates for people with less serious disease.
Because DKA rates were highest among people aged > 45 years, the researchers say information from studies in that age group might help determine whether prevention strategies should focus on factors such as symptom recognition, adherence to treatment, self-management skills, access to care, or cost of treatment.
Diabetic ketoacidosis (DKA) is life threatening, but preventable. It was good news that between 2000-2009, rates of DKA began to decline, but researchers from the Centers for Disease Control and Prevention (CDC) say it is “concerning” to find that those rates began to reverse between 2009-2014 , increasing 54.9%. All age groups saw an increase of ≥ 6% annually. The highest rates were among people aged < 45 years: approximately 27 times higher than among people aged > 65 years.
The causes for the reversal are not clear, the researchers say, but they offer several possibilities, including changes in case definition, new medications that might raise the risk of DKA, and higher admission rates for people with less serious disease.
Because DKA rates were highest among people aged > 45 years, the researchers say information from studies in that age group might help determine whether prevention strategies should focus on factors such as symptom recognition, adherence to treatment, self-management skills, access to care, or cost of treatment.
Regimen can improve DFS in newly diagnosed T-ALL
The addition of nelarabine can improve treatment outcomes for certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to a phase 3 trial.
Patients with newly diagnosed, intermediate- or high-risk T-ALL had a significant improvement in 4-year disease-free survival (DFS) if they received nelarabine in addition to chemotherapy and cranial irradiation.
The DFS benefit with nelarabine was significant for patients who received high-dose methotrexate but not for those who received escalating-dose methotrexate.
This study also included patients with T-cell lymphoblastic lymphoma (T-LL), and they did not experience an improvement in DFS with the addition of nelarabine.
Kimberly Dunsmore, MD, of Virginia Tech Carilion School of Medicine in Roanoke, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting. Additional results are scheduled to be presented at the meeting as abstract 10500.
This research was supported by the National Cancer Institute/National Institutes of Health and St. Baldrick’s Foundation. The researchers’ disclosures are listed with the abstract.
Patients and treatment
The trial enrolled 1895 patients, ages 1 to 30, who were newly diagnosed with T-ALL (94%) or T-LL (6%).
Patients received standard 4-drug induction chemotherapy, and 1307 of these patients were then randomized to 1 of 4 treatment arms.
Regardless of which arm they were randomized to, patients received an 11-drug chemotherapy regimen—the augmented Berlin-Frankfurt-Munster regimen. Intermediate- and high-risk patients in all 4 arms also received cranial irradiation.
In the first treatment arm, T-LL (n=58) and T-ALL (n=372) patients received escalating-dose methotrexate without leucovorin rescue and pegaspargase (C-MTX).
In the second treatment arm, patients with intermediate- and high-risk T-ALL (n=147) and T-LL (n=60) received C-MTX plus nelarabine (six 5-day courses at 650 mg/m2/day).
In the third arm, T-ALL patients (n=451) received high-dose methotrexate with leucovorin rescue (HD-MTX). T-LL patients were not eligible for this arm or the fourth treatment arm.
In the fourth arm, intermediate- and high-risk T-ALL patients (n=219) received HD-MTX and nelarabine (same schedule as above). This included 43 T-ALL patients who had induction failure and were assigned to this arm non-randomly.
Results
For T-ALL patients, the 4-year disease-free survival (DFS) rate was 84%, and the 4-year overall survival rate was 90%.
There was a significant improvement in DFS for T-ALL patients who received nelarabine compared to those who did not—89% and 83%, respectively (P=0.0332).
“Historically, about 80% of people [with T-ALL] live at least 4 years after being treated for their disease, but we felt we could and must do better,” Dr Dunsmore said. “Our trial shows that we could further increase survival rates by about 10%, which is very encouraging.”
Dr Dunsmore also noted that patients who received nelarabine had fewer central nervous system relapses.
Among T-ALL patients who received C-MTX, there was no significant difference in DFS for those who received nelarabine and those who did not—92% and 90%, respectively (P=0.3825).
However, for patients who received HD-MTX, the difference in DFS was significant. The DFS rate was 86% in patients who received nelarabine and 78% in those who did not (P=0.024).
For the T-ALL patients who failed induction and were assigned to HD-MTX and nelarabine, the 4-year DFS rate was 55%.
Patients with T-LL did not benefit from the addition of nelarabine. The 4-year DFS rate was 85% in the nelarabine recipients and 89% in non-recipients (P=0.2788).
There were no significant differences in overall toxicity or peripheral neurotoxicity between the treatment arms.
Dr Dunsmore said the next step with this research will be to examine the implications and potential benefits of using nelarabine in treatment protocols that do not include cranial radiation.
The addition of nelarabine can improve treatment outcomes for certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to a phase 3 trial.
Patients with newly diagnosed, intermediate- or high-risk T-ALL had a significant improvement in 4-year disease-free survival (DFS) if they received nelarabine in addition to chemotherapy and cranial irradiation.
The DFS benefit with nelarabine was significant for patients who received high-dose methotrexate but not for those who received escalating-dose methotrexate.
This study also included patients with T-cell lymphoblastic lymphoma (T-LL), and they did not experience an improvement in DFS with the addition of nelarabine.
Kimberly Dunsmore, MD, of Virginia Tech Carilion School of Medicine in Roanoke, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting. Additional results are scheduled to be presented at the meeting as abstract 10500.
This research was supported by the National Cancer Institute/National Institutes of Health and St. Baldrick’s Foundation. The researchers’ disclosures are listed with the abstract.
Patients and treatment
The trial enrolled 1895 patients, ages 1 to 30, who were newly diagnosed with T-ALL (94%) or T-LL (6%).
Patients received standard 4-drug induction chemotherapy, and 1307 of these patients were then randomized to 1 of 4 treatment arms.
Regardless of which arm they were randomized to, patients received an 11-drug chemotherapy regimen—the augmented Berlin-Frankfurt-Munster regimen. Intermediate- and high-risk patients in all 4 arms also received cranial irradiation.
In the first treatment arm, T-LL (n=58) and T-ALL (n=372) patients received escalating-dose methotrexate without leucovorin rescue and pegaspargase (C-MTX).
In the second treatment arm, patients with intermediate- and high-risk T-ALL (n=147) and T-LL (n=60) received C-MTX plus nelarabine (six 5-day courses at 650 mg/m2/day).
In the third arm, T-ALL patients (n=451) received high-dose methotrexate with leucovorin rescue (HD-MTX). T-LL patients were not eligible for this arm or the fourth treatment arm.
In the fourth arm, intermediate- and high-risk T-ALL patients (n=219) received HD-MTX and nelarabine (same schedule as above). This included 43 T-ALL patients who had induction failure and were assigned to this arm non-randomly.
Results
For T-ALL patients, the 4-year disease-free survival (DFS) rate was 84%, and the 4-year overall survival rate was 90%.
There was a significant improvement in DFS for T-ALL patients who received nelarabine compared to those who did not—89% and 83%, respectively (P=0.0332).
“Historically, about 80% of people [with T-ALL] live at least 4 years after being treated for their disease, but we felt we could and must do better,” Dr Dunsmore said. “Our trial shows that we could further increase survival rates by about 10%, which is very encouraging.”
Dr Dunsmore also noted that patients who received nelarabine had fewer central nervous system relapses.
Among T-ALL patients who received C-MTX, there was no significant difference in DFS for those who received nelarabine and those who did not—92% and 90%, respectively (P=0.3825).
However, for patients who received HD-MTX, the difference in DFS was significant. The DFS rate was 86% in patients who received nelarabine and 78% in those who did not (P=0.024).
For the T-ALL patients who failed induction and were assigned to HD-MTX and nelarabine, the 4-year DFS rate was 55%.
Patients with T-LL did not benefit from the addition of nelarabine. The 4-year DFS rate was 85% in the nelarabine recipients and 89% in non-recipients (P=0.2788).
There were no significant differences in overall toxicity or peripheral neurotoxicity between the treatment arms.
Dr Dunsmore said the next step with this research will be to examine the implications and potential benefits of using nelarabine in treatment protocols that do not include cranial radiation.
The addition of nelarabine can improve treatment outcomes for certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to a phase 3 trial.
Patients with newly diagnosed, intermediate- or high-risk T-ALL had a significant improvement in 4-year disease-free survival (DFS) if they received nelarabine in addition to chemotherapy and cranial irradiation.
The DFS benefit with nelarabine was significant for patients who received high-dose methotrexate but not for those who received escalating-dose methotrexate.
This study also included patients with T-cell lymphoblastic lymphoma (T-LL), and they did not experience an improvement in DFS with the addition of nelarabine.
Kimberly Dunsmore, MD, of Virginia Tech Carilion School of Medicine in Roanoke, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting. Additional results are scheduled to be presented at the meeting as abstract 10500.
This research was supported by the National Cancer Institute/National Institutes of Health and St. Baldrick’s Foundation. The researchers’ disclosures are listed with the abstract.
Patients and treatment
The trial enrolled 1895 patients, ages 1 to 30, who were newly diagnosed with T-ALL (94%) or T-LL (6%).
Patients received standard 4-drug induction chemotherapy, and 1307 of these patients were then randomized to 1 of 4 treatment arms.
Regardless of which arm they were randomized to, patients received an 11-drug chemotherapy regimen—the augmented Berlin-Frankfurt-Munster regimen. Intermediate- and high-risk patients in all 4 arms also received cranial irradiation.
In the first treatment arm, T-LL (n=58) and T-ALL (n=372) patients received escalating-dose methotrexate without leucovorin rescue and pegaspargase (C-MTX).
In the second treatment arm, patients with intermediate- and high-risk T-ALL (n=147) and T-LL (n=60) received C-MTX plus nelarabine (six 5-day courses at 650 mg/m2/day).
In the third arm, T-ALL patients (n=451) received high-dose methotrexate with leucovorin rescue (HD-MTX). T-LL patients were not eligible for this arm or the fourth treatment arm.
In the fourth arm, intermediate- and high-risk T-ALL patients (n=219) received HD-MTX and nelarabine (same schedule as above). This included 43 T-ALL patients who had induction failure and were assigned to this arm non-randomly.
Results
For T-ALL patients, the 4-year disease-free survival (DFS) rate was 84%, and the 4-year overall survival rate was 90%.
There was a significant improvement in DFS for T-ALL patients who received nelarabine compared to those who did not—89% and 83%, respectively (P=0.0332).
“Historically, about 80% of people [with T-ALL] live at least 4 years after being treated for their disease, but we felt we could and must do better,” Dr Dunsmore said. “Our trial shows that we could further increase survival rates by about 10%, which is very encouraging.”
Dr Dunsmore also noted that patients who received nelarabine had fewer central nervous system relapses.
Among T-ALL patients who received C-MTX, there was no significant difference in DFS for those who received nelarabine and those who did not—92% and 90%, respectively (P=0.3825).
However, for patients who received HD-MTX, the difference in DFS was significant. The DFS rate was 86% in patients who received nelarabine and 78% in those who did not (P=0.024).
For the T-ALL patients who failed induction and were assigned to HD-MTX and nelarabine, the 4-year DFS rate was 55%.
Patients with T-LL did not benefit from the addition of nelarabine. The 4-year DFS rate was 85% in the nelarabine recipients and 89% in non-recipients (P=0.2788).
There were no significant differences in overall toxicity or peripheral neurotoxicity between the treatment arms.
Dr Dunsmore said the next step with this research will be to examine the implications and potential benefits of using nelarabine in treatment protocols that do not include cranial radiation.
Treating insomnia in cancer survivors
Treatment with acupuncture or cognitive behavioral therapy (CBT) can decrease the severity of insomnia among cancer survivors, according to new research.
Overall, improvements in insomnia were greatest in patients treated with CBT, and patients with mild insomnia at baseline had a significantly greater improvement with CBT than with acupuncture.
However, among patients who had moderate to severe insomnia at baseline, there was no significant difference in results with CBT and acupuncture.
Jun J. Mao, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting.
Additional results are scheduled to be presented at the meeting as abstract 10001.
“Up to 60% of cancer survivors have some form of insomnia, but it is often underdiagnosed and undertreated,” Dr Mao said. “Our trial showed that both CBT-I [CBT for insomnia] and acupuncture were effective in treating moderate to severe insomnia, although CBT-I was more effective for those with mild symptoms of insomnia. Now, patients have more choices to manage their insomnia.”
Dr Mao and his colleagues studied 160 individuals who had completed cancer treatment. Their mean time since cancer diagnosis was about 6 years.
The subjects had received treatment for hematologic malignancies (8%) as well as breast (31%), prostate (23%), head and neck (7%), colorectal (6%), gynecological (4%), and other cancers (14%). Six percent of subjects had been treated for more than 1 type of cancer.
All subjects had been clinically diagnosed with insomnia. Seventy-nine percent had moderate (n=94) to severe (n=33) insomnia, and 21% had mild insomnia (n=33).
Interventions
Subjects were randomized to receive CBT or acupuncture for 8 weeks. Those who received CBT worked with a therapist to re-establish a restorative sleep schedule by:
- Reducing the amount of time spent in bed
- Limiting activities performed in bed to sleep and sexual activity
- Modifying unhelpful beliefs about sleep
- Promoting good sleep hygiene (setting a regular sleep schedule and avoiding activities that include light from tablets and cellphones, eating too late, and performing vigorous activities).
The study’s primary outcome was reduction in insomnia severity, as measured by the Insomnia Severity Index (ISI), from study entry to the end of treatment at week 8. Subjects were also reassessed at 20 weeks.
The ISI is a questionnaire that asks people to rate the severity of insomnia problems, such as difficulty falling asleep and staying asleep, and the impact of insomnia on their daily functioning and quality of life.
ISI scoring ranges from 0 to 28. Scores of 0 to 7 denote no clinically significant insomnia, 8 to 14 denote mild insomnia, 15 to 21 denote moderate insomnia, and 22 to 28 denote severe insomnia.
Results
At 8 weeks, the mean ISI score fell 10.9 points (from 18.5 to 7.5) for subjects who received CBT and 8.3 points (from 17.55 to 9.23) for those who received acupuncture (P=0.0007).
Among subjects with mild insomnia at baseline, far more responded to CBT than to acupuncture—85% and 18%, respectively (P<0.0001).
However, response rates were similar in subjects who had moderate to severe insomnia at baseline. Seventy-five percent of those who received CBT achieved a response, as did 66% of those who received acupuncture (P=0.26).
Both treatment groups had few mild adverse events, and all subjects maintained their improvements in insomnia at the 20-week assessment.
This study was funded by the Patient-Centered Outcomes Research Institute. The researchers’ disclosures are listed with the abstract.
Treatment with acupuncture or cognitive behavioral therapy (CBT) can decrease the severity of insomnia among cancer survivors, according to new research.
Overall, improvements in insomnia were greatest in patients treated with CBT, and patients with mild insomnia at baseline had a significantly greater improvement with CBT than with acupuncture.
However, among patients who had moderate to severe insomnia at baseline, there was no significant difference in results with CBT and acupuncture.
Jun J. Mao, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting.
Additional results are scheduled to be presented at the meeting as abstract 10001.
“Up to 60% of cancer survivors have some form of insomnia, but it is often underdiagnosed and undertreated,” Dr Mao said. “Our trial showed that both CBT-I [CBT for insomnia] and acupuncture were effective in treating moderate to severe insomnia, although CBT-I was more effective for those with mild symptoms of insomnia. Now, patients have more choices to manage their insomnia.”
Dr Mao and his colleagues studied 160 individuals who had completed cancer treatment. Their mean time since cancer diagnosis was about 6 years.
The subjects had received treatment for hematologic malignancies (8%) as well as breast (31%), prostate (23%), head and neck (7%), colorectal (6%), gynecological (4%), and other cancers (14%). Six percent of subjects had been treated for more than 1 type of cancer.
All subjects had been clinically diagnosed with insomnia. Seventy-nine percent had moderate (n=94) to severe (n=33) insomnia, and 21% had mild insomnia (n=33).
Interventions
Subjects were randomized to receive CBT or acupuncture for 8 weeks. Those who received CBT worked with a therapist to re-establish a restorative sleep schedule by:
- Reducing the amount of time spent in bed
- Limiting activities performed in bed to sleep and sexual activity
- Modifying unhelpful beliefs about sleep
- Promoting good sleep hygiene (setting a regular sleep schedule and avoiding activities that include light from tablets and cellphones, eating too late, and performing vigorous activities).
The study’s primary outcome was reduction in insomnia severity, as measured by the Insomnia Severity Index (ISI), from study entry to the end of treatment at week 8. Subjects were also reassessed at 20 weeks.
The ISI is a questionnaire that asks people to rate the severity of insomnia problems, such as difficulty falling asleep and staying asleep, and the impact of insomnia on their daily functioning and quality of life.
ISI scoring ranges from 0 to 28. Scores of 0 to 7 denote no clinically significant insomnia, 8 to 14 denote mild insomnia, 15 to 21 denote moderate insomnia, and 22 to 28 denote severe insomnia.
Results
At 8 weeks, the mean ISI score fell 10.9 points (from 18.5 to 7.5) for subjects who received CBT and 8.3 points (from 17.55 to 9.23) for those who received acupuncture (P=0.0007).
Among subjects with mild insomnia at baseline, far more responded to CBT than to acupuncture—85% and 18%, respectively (P<0.0001).
However, response rates were similar in subjects who had moderate to severe insomnia at baseline. Seventy-five percent of those who received CBT achieved a response, as did 66% of those who received acupuncture (P=0.26).
Both treatment groups had few mild adverse events, and all subjects maintained their improvements in insomnia at the 20-week assessment.
This study was funded by the Patient-Centered Outcomes Research Institute. The researchers’ disclosures are listed with the abstract.
Treatment with acupuncture or cognitive behavioral therapy (CBT) can decrease the severity of insomnia among cancer survivors, according to new research.
Overall, improvements in insomnia were greatest in patients treated with CBT, and patients with mild insomnia at baseline had a significantly greater improvement with CBT than with acupuncture.
However, among patients who had moderate to severe insomnia at baseline, there was no significant difference in results with CBT and acupuncture.
Jun J. Mao, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, presented these results in a press briefing in advance of the 2018 ASCO Annual Meeting.
Additional results are scheduled to be presented at the meeting as abstract 10001.
“Up to 60% of cancer survivors have some form of insomnia, but it is often underdiagnosed and undertreated,” Dr Mao said. “Our trial showed that both CBT-I [CBT for insomnia] and acupuncture were effective in treating moderate to severe insomnia, although CBT-I was more effective for those with mild symptoms of insomnia. Now, patients have more choices to manage their insomnia.”
Dr Mao and his colleagues studied 160 individuals who had completed cancer treatment. Their mean time since cancer diagnosis was about 6 years.
The subjects had received treatment for hematologic malignancies (8%) as well as breast (31%), prostate (23%), head and neck (7%), colorectal (6%), gynecological (4%), and other cancers (14%). Six percent of subjects had been treated for more than 1 type of cancer.
All subjects had been clinically diagnosed with insomnia. Seventy-nine percent had moderate (n=94) to severe (n=33) insomnia, and 21% had mild insomnia (n=33).
Interventions
Subjects were randomized to receive CBT or acupuncture for 8 weeks. Those who received CBT worked with a therapist to re-establish a restorative sleep schedule by:
- Reducing the amount of time spent in bed
- Limiting activities performed in bed to sleep and sexual activity
- Modifying unhelpful beliefs about sleep
- Promoting good sleep hygiene (setting a regular sleep schedule and avoiding activities that include light from tablets and cellphones, eating too late, and performing vigorous activities).
The study’s primary outcome was reduction in insomnia severity, as measured by the Insomnia Severity Index (ISI), from study entry to the end of treatment at week 8. Subjects were also reassessed at 20 weeks.
The ISI is a questionnaire that asks people to rate the severity of insomnia problems, such as difficulty falling asleep and staying asleep, and the impact of insomnia on their daily functioning and quality of life.
ISI scoring ranges from 0 to 28. Scores of 0 to 7 denote no clinically significant insomnia, 8 to 14 denote mild insomnia, 15 to 21 denote moderate insomnia, and 22 to 28 denote severe insomnia.
Results
At 8 weeks, the mean ISI score fell 10.9 points (from 18.5 to 7.5) for subjects who received CBT and 8.3 points (from 17.55 to 9.23) for those who received acupuncture (P=0.0007).
Among subjects with mild insomnia at baseline, far more responded to CBT than to acupuncture—85% and 18%, respectively (P<0.0001).
However, response rates were similar in subjects who had moderate to severe insomnia at baseline. Seventy-five percent of those who received CBT achieved a response, as did 66% of those who received acupuncture (P=0.26).
Both treatment groups had few mild adverse events, and all subjects maintained their improvements in insomnia at the 20-week assessment.
This study was funded by the Patient-Centered Outcomes Research Institute. The researchers’ disclosures are listed with the abstract.
Changing mole on arm
The FP suspected that this was a melanoma.
He thought through the ABCDEs of melanoma and saw that it was Asymmetric, had an irregular Border, had varied Colors, the Diameter was larger than 6 mm, and it was Evolving. The FP also noted that one area was elevated and another area (the center) seemed to be regressing. Using his dermatoscope, he noted strong evidence of regression in the center and an “atypical network.” He told the patient that this was very suspicious for melanoma and recommended a skin biopsy without delay. The patient agreed and after local anesthesia with lidocaine with epinephrine, a saucerization biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The FP easily removed all of the visible tumor with the saucerization (deep shave). The bleeding was stopped with topical aluminum chloride, and the specimen was sent in formalin to the pathologist. The pathology report came back as a melanoma of 2.1 mm depth arising in a pre-existing nevus.
The patient was referred to a surgical oncologist for sentinel lymph node biopsy and excision with wide margins.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP suspected that this was a melanoma.
He thought through the ABCDEs of melanoma and saw that it was Asymmetric, had an irregular Border, had varied Colors, the Diameter was larger than 6 mm, and it was Evolving. The FP also noted that one area was elevated and another area (the center) seemed to be regressing. Using his dermatoscope, he noted strong evidence of regression in the center and an “atypical network.” He told the patient that this was very suspicious for melanoma and recommended a skin biopsy without delay. The patient agreed and after local anesthesia with lidocaine with epinephrine, a saucerization biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The FP easily removed all of the visible tumor with the saucerization (deep shave). The bleeding was stopped with topical aluminum chloride, and the specimen was sent in formalin to the pathologist. The pathology report came back as a melanoma of 2.1 mm depth arising in a pre-existing nevus.
The patient was referred to a surgical oncologist for sentinel lymph node biopsy and excision with wide margins.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP suspected that this was a melanoma.
He thought through the ABCDEs of melanoma and saw that it was Asymmetric, had an irregular Border, had varied Colors, the Diameter was larger than 6 mm, and it was Evolving. The FP also noted that one area was elevated and another area (the center) seemed to be regressing. Using his dermatoscope, he noted strong evidence of regression in the center and an “atypical network.” He told the patient that this was very suspicious for melanoma and recommended a skin biopsy without delay. The patient agreed and after local anesthesia with lidocaine with epinephrine, a saucerization biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The FP easily removed all of the visible tumor with the saucerization (deep shave). The bleeding was stopped with topical aluminum chloride, and the specimen was sent in formalin to the pathologist. The pathology report came back as a melanoma of 2.1 mm depth arising in a pre-existing nevus.
The patient was referred to a surgical oncologist for sentinel lymph node biopsy and excision with wide margins.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.