User login
FIGHT to remember PTSD
Certain clinical features of posttraumatic stress disorder (PTSD) appear in other psychiatric diagnoses and therefore can confound accurate diagnosis and treatment. PTSD is frequently comorbid with other classes of psychiatric disorders, including mood, personality, substance use, and psychotic disorders, which can further complicate diagnostic clarity. Comorbidity in PTSD is important to recognize because it has been associated with worse treatment outcomes.1
In DSM-5, the updated criteria for PTSD included Criterion D: “Negative alterations in cognitions and mood associated with the traumatic event(s) ….”2 In addition to inability to remember an important aspect of the trau
We created the mnemonic FIGHT to help remember the updated DSM-5 criteria for PTSD when considering the differential diagnosis.
Flight. Avoidant symptoms, including efforts to avoid distressing memories, thoughts, or feelings about the traumatic event, as well as avoidance of external reminders.
Intrusive symptoms, such as distressing dreams, intrusive memories, and physiological distress when exposed to cues.
Gloomy cognitions. Negative cognitions and mood associated with the traumatic event.
Hypervigilance. Alterations in arousal, such as irritability, angry outbursts, reckless behavior, and exaggerated startle response.
Trauma. Exposure to actual or threatened death, serious injury, or sexual violence.
A diagnosis of PTSD requires ≥1 month of symptoms that cause significant distress or impairment and are not attributable to the physiological effects of a substance or medical condition. Specifiers in DSM-5 include with depersonalization or derealization, as well as delayed expression.2
Vigilance in the assessment and treatment of PTSD will aid the clinician and patient in producing better care outcomes.
1. Angstman KB, Marcelin A, Gonzalez CA, et al. The impact of posttraumatic stress disorder on the 6-month outcomes in collaborative care management for depression. J Prim Care Community Health. 2016;7(3):159-164.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
Certain clinical features of posttraumatic stress disorder (PTSD) appear in other psychiatric diagnoses and therefore can confound accurate diagnosis and treatment. PTSD is frequently comorbid with other classes of psychiatric disorders, including mood, personality, substance use, and psychotic disorders, which can further complicate diagnostic clarity. Comorbidity in PTSD is important to recognize because it has been associated with worse treatment outcomes.1
In DSM-5, the updated criteria for PTSD included Criterion D: “Negative alterations in cognitions and mood associated with the traumatic event(s) ….”2 In addition to inability to remember an important aspect of the trau
We created the mnemonic FIGHT to help remember the updated DSM-5 criteria for PTSD when considering the differential diagnosis.
Flight. Avoidant symptoms, including efforts to avoid distressing memories, thoughts, or feelings about the traumatic event, as well as avoidance of external reminders.
Intrusive symptoms, such as distressing dreams, intrusive memories, and physiological distress when exposed to cues.
Gloomy cognitions. Negative cognitions and mood associated with the traumatic event.
Hypervigilance. Alterations in arousal, such as irritability, angry outbursts, reckless behavior, and exaggerated startle response.
Trauma. Exposure to actual or threatened death, serious injury, or sexual violence.
A diagnosis of PTSD requires ≥1 month of symptoms that cause significant distress or impairment and are not attributable to the physiological effects of a substance or medical condition. Specifiers in DSM-5 include with depersonalization or derealization, as well as delayed expression.2
Vigilance in the assessment and treatment of PTSD will aid the clinician and patient in producing better care outcomes.
Certain clinical features of posttraumatic stress disorder (PTSD) appear in other psychiatric diagnoses and therefore can confound accurate diagnosis and treatment. PTSD is frequently comorbid with other classes of psychiatric disorders, including mood, personality, substance use, and psychotic disorders, which can further complicate diagnostic clarity. Comorbidity in PTSD is important to recognize because it has been associated with worse treatment outcomes.1
In DSM-5, the updated criteria for PTSD included Criterion D: “Negative alterations in cognitions and mood associated with the traumatic event(s) ….”2 In addition to inability to remember an important aspect of the trau
We created the mnemonic FIGHT to help remember the updated DSM-5 criteria for PTSD when considering the differential diagnosis.
Flight. Avoidant symptoms, including efforts to avoid distressing memories, thoughts, or feelings about the traumatic event, as well as avoidance of external reminders.
Intrusive symptoms, such as distressing dreams, intrusive memories, and physiological distress when exposed to cues.
Gloomy cognitions. Negative cognitions and mood associated with the traumatic event.
Hypervigilance. Alterations in arousal, such as irritability, angry outbursts, reckless behavior, and exaggerated startle response.
Trauma. Exposure to actual or threatened death, serious injury, or sexual violence.
A diagnosis of PTSD requires ≥1 month of symptoms that cause significant distress or impairment and are not attributable to the physiological effects of a substance or medical condition. Specifiers in DSM-5 include with depersonalization or derealization, as well as delayed expression.2
Vigilance in the assessment and treatment of PTSD will aid the clinician and patient in producing better care outcomes.
1. Angstman KB, Marcelin A, Gonzalez CA, et al. The impact of posttraumatic stress disorder on the 6-month outcomes in collaborative care management for depression. J Prim Care Community Health. 2016;7(3):159-164.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
1. Angstman KB, Marcelin A, Gonzalez CA, et al. The impact of posttraumatic stress disorder on the 6-month outcomes in collaborative care management for depression. J Prim Care Community Health. 2016;7(3):159-164.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
‘Difficult’ patients: How to improve rapport
As psychiatrists, we all come across patients who press our buttons and engender negative feelings, such as anger, frustration, and inadequacy.1 These patients have been referred to as “hateful” or “difficult” because they disrupt the treatment alliance.1,2 We are quick to point our fingers at such patients for making our jobs harder, being noncompliant, resisting the therapeutic alliance, and in general, being “problem patients.”3 However, the physician–patient relationship is a 2-way street. Although our patients knowingly or unknowingly play a role in this dynamic, we could be overlooking our role in adversely affecting this relationship. The following factors influence the physician–patient bond.1,2
Countertransference. We may have negative feelings toward a patient based on our personalities and/or if the patient reminds us of someone we may not like, which could lead us to overprescribe or underprescribe medications, conduct unnecessary medical workups, distance ourselves from the patient, etc. Accepting our disdain for certain patients and understanding why we have these emotions will allow us to better understand them, ensure that we are not impeding the delivery of appropriate clinical care, and improve rapport.
Listening. It may seem obvious that not listening to our patients negatively impacts rapport. However, in today’s technological world, we may not be really listening to our patients even when we think we are. Answering a text message or reading the patient’s electronic medical record while they are talking to us may increase productivity, but doing so also can interfere with our ability to form a therapeutic alliance. Although we may hear what our patients are saying, such distractions can create a hurdle in listening to what they are telling us.
Empathy often is confused for sympathy. Sympathy entails expressing concern and compassion for one’s distress, whereas empathy includes recognizing and sharing the patient’s emotions. Identifying with and understanding our patients’ situations, drives, and feelings allows us to understand what they are experiencing, see why they are reacting in a negative manner, and protect them from unnecessary emotional distress. Empathy can lead us to know what needs to be said and what should be said. It also can demystify a patient’s suffering. Not providing empathy or substituting sympathy can disrupt the therapeutic alliance.
Projective identification. Patients can project intolerable and negative feelings onto us and coerce us into identifying with what has been projected, allowing them to indirectly take control of our emotions. Our subsequent reactions can unsettle the physician–patient relationship. We need to be attuned to this process and recognize what the patient is provoking within us. Once we understand the process, we can realize that this is how they deal with others under similarly stressful conditions, and then react in a more supportive and healthy manner, rather than reviling our patients and negatively impacting the therapeutic relationship.
1. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
3. Boland R. The ‘problem patient’: modest advice for frustrated clinicians. R I Med J (2013). 2014;97(6):29-32.
As psychiatrists, we all come across patients who press our buttons and engender negative feelings, such as anger, frustration, and inadequacy.1 These patients have been referred to as “hateful” or “difficult” because they disrupt the treatment alliance.1,2 We are quick to point our fingers at such patients for making our jobs harder, being noncompliant, resisting the therapeutic alliance, and in general, being “problem patients.”3 However, the physician–patient relationship is a 2-way street. Although our patients knowingly or unknowingly play a role in this dynamic, we could be overlooking our role in adversely affecting this relationship. The following factors influence the physician–patient bond.1,2
Countertransference. We may have negative feelings toward a patient based on our personalities and/or if the patient reminds us of someone we may not like, which could lead us to overprescribe or underprescribe medications, conduct unnecessary medical workups, distance ourselves from the patient, etc. Accepting our disdain for certain patients and understanding why we have these emotions will allow us to better understand them, ensure that we are not impeding the delivery of appropriate clinical care, and improve rapport.
Listening. It may seem obvious that not listening to our patients negatively impacts rapport. However, in today’s technological world, we may not be really listening to our patients even when we think we are. Answering a text message or reading the patient’s electronic medical record while they are talking to us may increase productivity, but doing so also can interfere with our ability to form a therapeutic alliance. Although we may hear what our patients are saying, such distractions can create a hurdle in listening to what they are telling us.
Empathy often is confused for sympathy. Sympathy entails expressing concern and compassion for one’s distress, whereas empathy includes recognizing and sharing the patient’s emotions. Identifying with and understanding our patients’ situations, drives, and feelings allows us to understand what they are experiencing, see why they are reacting in a negative manner, and protect them from unnecessary emotional distress. Empathy can lead us to know what needs to be said and what should be said. It also can demystify a patient’s suffering. Not providing empathy or substituting sympathy can disrupt the therapeutic alliance.
Projective identification. Patients can project intolerable and negative feelings onto us and coerce us into identifying with what has been projected, allowing them to indirectly take control of our emotions. Our subsequent reactions can unsettle the physician–patient relationship. We need to be attuned to this process and recognize what the patient is provoking within us. Once we understand the process, we can realize that this is how they deal with others under similarly stressful conditions, and then react in a more supportive and healthy manner, rather than reviling our patients and negatively impacting the therapeutic relationship.
As psychiatrists, we all come across patients who press our buttons and engender negative feelings, such as anger, frustration, and inadequacy.1 These patients have been referred to as “hateful” or “difficult” because they disrupt the treatment alliance.1,2 We are quick to point our fingers at such patients for making our jobs harder, being noncompliant, resisting the therapeutic alliance, and in general, being “problem patients.”3 However, the physician–patient relationship is a 2-way street. Although our patients knowingly or unknowingly play a role in this dynamic, we could be overlooking our role in adversely affecting this relationship. The following factors influence the physician–patient bond.1,2
Countertransference. We may have negative feelings toward a patient based on our personalities and/or if the patient reminds us of someone we may not like, which could lead us to overprescribe or underprescribe medications, conduct unnecessary medical workups, distance ourselves from the patient, etc. Accepting our disdain for certain patients and understanding why we have these emotions will allow us to better understand them, ensure that we are not impeding the delivery of appropriate clinical care, and improve rapport.
Listening. It may seem obvious that not listening to our patients negatively impacts rapport. However, in today’s technological world, we may not be really listening to our patients even when we think we are. Answering a text message or reading the patient’s electronic medical record while they are talking to us may increase productivity, but doing so also can interfere with our ability to form a therapeutic alliance. Although we may hear what our patients are saying, such distractions can create a hurdle in listening to what they are telling us.
Empathy often is confused for sympathy. Sympathy entails expressing concern and compassion for one’s distress, whereas empathy includes recognizing and sharing the patient’s emotions. Identifying with and understanding our patients’ situations, drives, and feelings allows us to understand what they are experiencing, see why they are reacting in a negative manner, and protect them from unnecessary emotional distress. Empathy can lead us to know what needs to be said and what should be said. It also can demystify a patient’s suffering. Not providing empathy or substituting sympathy can disrupt the therapeutic alliance.
Projective identification. Patients can project intolerable and negative feelings onto us and coerce us into identifying with what has been projected, allowing them to indirectly take control of our emotions. Our subsequent reactions can unsettle the physician–patient relationship. We need to be attuned to this process and recognize what the patient is provoking within us. Once we understand the process, we can realize that this is how they deal with others under similarly stressful conditions, and then react in a more supportive and healthy manner, rather than reviling our patients and negatively impacting the therapeutic relationship.
1. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
3. Boland R. The ‘problem patient’: modest advice for frustrated clinicians. R I Med J (2013). 2014;97(6):29-32.
1. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298(16):883-887.
3. Boland R. The ‘problem patient’: modest advice for frustrated clinicians. R I Med J (2013). 2014;97(6):29-32.
Understanding childhood cancer in sub-Saharan Africa
Researchers say they have published the most extensive data ever collected on childhood cancer in sub-Saharan Africa.
On the African continent, only South Africa operates a childhood cancer registry on the national level.
Researchers brought together data from 16 of the smaller, local registries, collecting this information for the first time and presenting it in an accessible format.
The data were published in ecancermedicalscience.
Examining the data in context allowed the researchers to notice trends in cancer incidence. For example, they found that, in Blantyre, Malawi’s second-largest city, the cumulative risk of a child developing Burkitt lymphoma is 2 in every thousand.
The researchers called this incidence “remarkable” and noted that the global research community is largely unaware of this.
“Everything starts with awareness,” said study author Cristina Stefan, global clinical leader of oncology for Roche Diagnostics International Ltd of Switzerland and director of the African Medical Research and Innovation Institute.
“It is highly necessary to publicize these data, which, at the moment, represent the best image of the malignant disease in children in the respective regions.”
The researchers also noted that factors such as the prevalence of malaria and the Epstein-Barr virus contribute to the unique epidemiology of childhood cancer in Africa.
“Our colleagues can learn that the patterns and distribution of cancers in Africa are totally different from Europe, and there is a need for further research into the roles of factors such as genetic predispositions and the influence of infections and other comorbidities in the evolution of cancer,” Dr Stefan said.
“We have learned many universal lessons about data collection as we prepared this work. Our hope is that the publication of this monograph will open the forums for future discussions and that the work will be referenced for the better understanding of cancer in children in Africa and used to improve outcomes for children affected there.” ![]()
Researchers say they have published the most extensive data ever collected on childhood cancer in sub-Saharan Africa.
On the African continent, only South Africa operates a childhood cancer registry on the national level.
Researchers brought together data from 16 of the smaller, local registries, collecting this information for the first time and presenting it in an accessible format.
The data were published in ecancermedicalscience.
Examining the data in context allowed the researchers to notice trends in cancer incidence. For example, they found that, in Blantyre, Malawi’s second-largest city, the cumulative risk of a child developing Burkitt lymphoma is 2 in every thousand.
The researchers called this incidence “remarkable” and noted that the global research community is largely unaware of this.
“Everything starts with awareness,” said study author Cristina Stefan, global clinical leader of oncology for Roche Diagnostics International Ltd of Switzerland and director of the African Medical Research and Innovation Institute.
“It is highly necessary to publicize these data, which, at the moment, represent the best image of the malignant disease in children in the respective regions.”
The researchers also noted that factors such as the prevalence of malaria and the Epstein-Barr virus contribute to the unique epidemiology of childhood cancer in Africa.
“Our colleagues can learn that the patterns and distribution of cancers in Africa are totally different from Europe, and there is a need for further research into the roles of factors such as genetic predispositions and the influence of infections and other comorbidities in the evolution of cancer,” Dr Stefan said.
“We have learned many universal lessons about data collection as we prepared this work. Our hope is that the publication of this monograph will open the forums for future discussions and that the work will be referenced for the better understanding of cancer in children in Africa and used to improve outcomes for children affected there.” ![]()
Researchers say they have published the most extensive data ever collected on childhood cancer in sub-Saharan Africa.
On the African continent, only South Africa operates a childhood cancer registry on the national level.
Researchers brought together data from 16 of the smaller, local registries, collecting this information for the first time and presenting it in an accessible format.
The data were published in ecancermedicalscience.
Examining the data in context allowed the researchers to notice trends in cancer incidence. For example, they found that, in Blantyre, Malawi’s second-largest city, the cumulative risk of a child developing Burkitt lymphoma is 2 in every thousand.
The researchers called this incidence “remarkable” and noted that the global research community is largely unaware of this.
“Everything starts with awareness,” said study author Cristina Stefan, global clinical leader of oncology for Roche Diagnostics International Ltd of Switzerland and director of the African Medical Research and Innovation Institute.
“It is highly necessary to publicize these data, which, at the moment, represent the best image of the malignant disease in children in the respective regions.”
The researchers also noted that factors such as the prevalence of malaria and the Epstein-Barr virus contribute to the unique epidemiology of childhood cancer in Africa.
“Our colleagues can learn that the patterns and distribution of cancers in Africa are totally different from Europe, and there is a need for further research into the roles of factors such as genetic predispositions and the influence of infections and other comorbidities in the evolution of cancer,” Dr Stefan said.
“We have learned many universal lessons about data collection as we prepared this work. Our hope is that the publication of this monograph will open the forums for future discussions and that the work will be referenced for the better understanding of cancer in children in Africa and used to improve outcomes for children affected there.” ![]()
Hand and arm pain: A pictorial guide to injections
Primary care physicians are frequently the first to evaluate hand, wrist, and forearm pain in patients, making knowledge of the symptoms, causes, and treatment of common diagnoses in the upper extremities imperative. Primary symptoms usually include pain and/or swelling. While most tendon disorders originating in the hand and wrist are idiopathic in nature, some patients occasionally report having recently performed unusual manual activity or having experienced trauma to the area days or weeks prior. A significant portion of patients are injured as a result of chronic repetitive activities at work.1
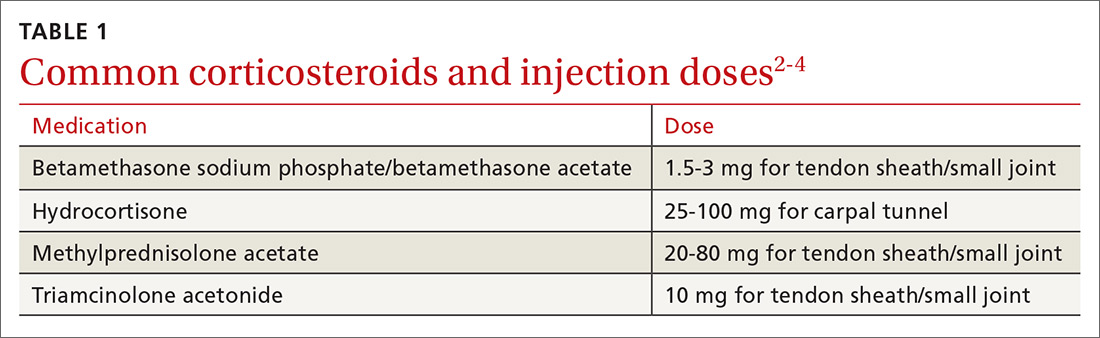
Most diagnoses can be made by pairing your knowledge of hand and forearm anatomy with an understanding of which tender points are indicative of which common conditions. (Care, of course, must be taken to ensure that there is no underlying infection.) Common conditions can often be treated nonsurgically with conservative treatments such as physical therapy, bracing/splinting, nonsteroidal anti-inflammatory drugs (NSAIDs), and injections of corticosteroids (eg, betamethasone, hydrocortisone, methylprednisolone, and triamcinolone) (TABLE 12-4) with or without the use of ultrasound. The benefits of corticosteroid injections for these conditions are well studied and documented in the literature, although physicians should always warn patients of the possible adverse effects prior to injection3,5 (TABLE 24).

To help you refine your skills, we review some of the more common hand and forearm conditions you are likely to encounter in the office and provide photos that reveal underlying anatomy so that you can administer injections without, in many cases, the need for ultrasound.
Trigger finger/thumb: New pathophysiologic findings?
Trigger finger most commonly occurs in the dominant hand. It is also more common in women, patients in their 50s, and in individuals with diabetes.6 Trigger finger/thumb is caused by inflammation and constriction of the flexor tendon sheath, which carries the flexor tendons through the palm and into the fingers and thumb. This, in turn, causes irritation of the tendons, sometimes via the formation of tendinous nodules, which may impinge upon the sheath’s “pulley system.”

When the “pulley” is compromised. The retinacular sheath is composed of 5 annular ligaments, or pulleys, that hold the tendons of the fingers close to the bone and allow the fingers to flex properly. The A1 pulley, at the level of the metacarpal head, is the first part of the sheath and is subject to the highest force; high forces may subsequently lead to the finger becoming locked in a flexed, or trigger, position.6 Patients may experience pain in the distal palm at the level of the A1 pulley and clicking of the finger.6
Additionally . . . recent studies show discrete histologic changes in trigger finger tendons, similar to findings with Achilles tendinosis and tendinopathy.7 In trigger finger tendons, collagen type 1A1 and 3A1, aggrecan, and biglycan are up-regulated, while metalloproteinase inhibitor 3 (TIMP-3) and matrix metallopeptidase3 (MMP-3) are down-regulated, a situation also described in Achilles tendinosis.7 This similarity in conditions provides new insight into the pathophysiology of the condition and may help provide future treatments.
Making the Dx: Look for swelling, check for carpal tunnel
During the examination, first look at both hands for swelling, arthropathy, or injury, and note the presence of any joint contractures. Next, examine all of the digits in flexion and extension while noting which ones are triggering, as the problem can occur in multiple digits on one hand. Then palpate the palms over the patient’s metacarpal heads, feeling for tender nodules.
Finally, examine the patient for carpal tunnel syndrome (CTS). A positive Tinel’s sign (shooting pain into the hand when the median nerve in the wrist is percussed), a positive Phalen maneuver (numbness or pain, usually within one minute of full wrist flexion), or thenar muscle wasting are highly indicative of CTS (compression of the median nerve at the transverse carpal ligament in the carpal tunnel). It is important to check for CTS when examining a patient for trigger finger because the 2 conditions frequently co-occur.6 (For more on CTS, see here.)
Treatment: Consider corticosteroids first
First-line treatment for patients with trigger finger or thumb is a corticosteroid injection into the subcutaneous tissue around the tendon sheath (FIGURES 1 and 2). (For this indication and for the others discussed throughout the article, there isn’t tremendous evidence for one particular type of corticosteroid over another; see TABLE 12-4 for choices.) Up to 57% of cases resolve with one injection, and 86% resolve with 2,8 but keep in mind that it may take up to 2 weeks to achieve the full clinical benefit.
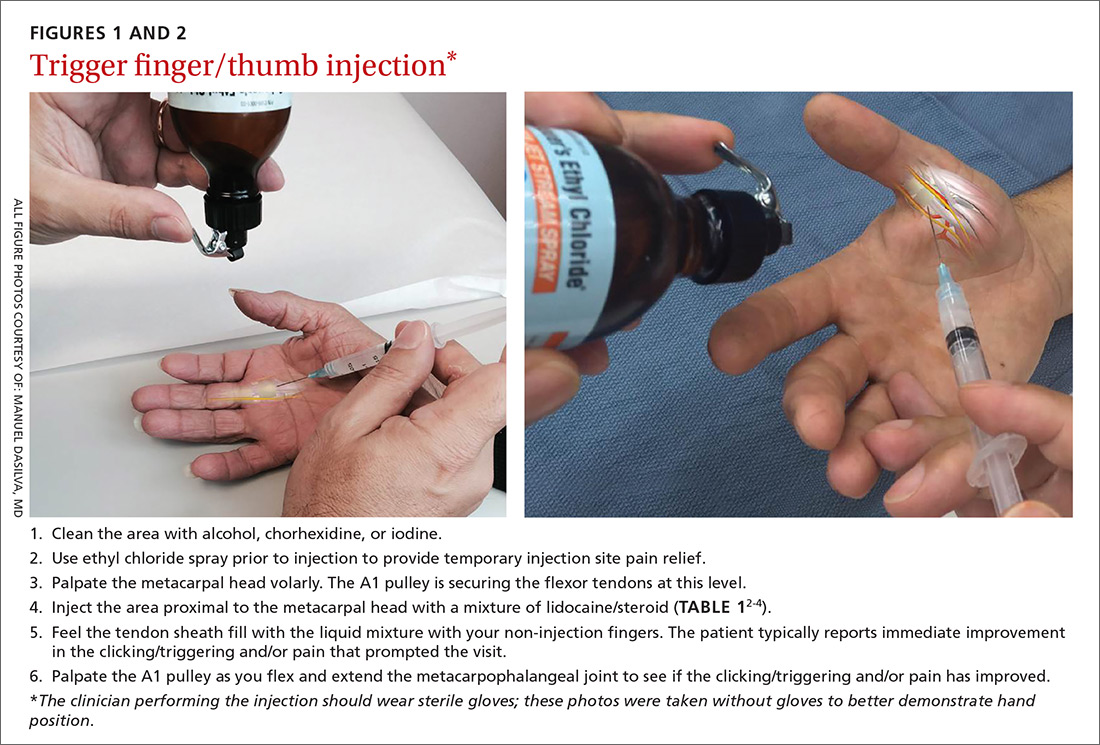
Patients with multiple trigger fingers can be treated with oral corticosteroids (eg, a methylprednisolone dose pack). Peters-Veluthamaningal et al performed a systematic review in 2009 and found 2 randomized controlled trials involving 63 patients (34 received injections of a corticosteroid [either methylprednisolone or betamethasone] and lidocaine and 29 received lidocaine only).2 The corticosteroid/lidocaine combination was more effective at 4 weeks (relative risk [RR]=3.15; 95% confidence interval [CI], 1.34 to 7.40).2
If 2 corticosteroid injections 6 weeks apart fail to provide benefit, or the finger is irreversibly locked in flexion, surgical release of the pulley is required and is performed through a palmar incision at the level of the A1 pulley. Complications from this surgery, including nerve damage, are exceedingly rare, but injury can occur, given the proximity of the digital nerves to the A1 pulley.
Patient is a child? Refer children with trigger finger or thumb to a hand surgeon for evaluation and management because the indications for nonoperative treatment in the pediatric population are unclear.9
Carpometacarpal arthritis: Common, with many causes
Osteoarthritis of the first carpometacarpal (CMC) joint is the most common site of arthritis in the hand/wrist region, affecting up to 11% of men and 33% of women in their 50s and 60s.10 Because the CMC joint lacks a bony restraint, it relies on a number of ligaments for stability—the strongest and most important of which is the palmar oblique “beak” ligament.11 A major cause of degenerative arthritis of this joint is attenuation and laxity of these ligaments, leading to abnormal and increased stress loads, which, in turn, can lead to loss of cartilage and bony impingement. While the exact mechanism of this process is not fully understood,10,12 acute or chronic trauma, advanced age, hormonal factors, and genetic factors seem to play a role.11
Many believe there is a relationship between a patient's occupation and the development of CMC arthritis, but studies are inconclusive.13 At risk are secretarial workers, tailors, domestic helpers/cleaners, and individuals whose jobs involve repetitive thumb use and/or insufficient rest of the joint throughout the day.
Making the Dx: Perform the Grind test
A detailed patient history (which is usually void of trauma to the hand) and physical examination are the keys to making the diagnosis of CMC arthritis. A history of pain at the base of the thumb during pinching and gripping tasks is often elucidated. Classically, patients describe pain upon turning keys, opening jars, and gripping doorknobs.11
It's important to focus on the dorsoradial aspect of the thumb during the physical exam and to rule out other causes of pain, such as de Quervain’s tenosynovitis, flexor carpi radialis tendinitis, CTS, and trigger thumb.11 Typical findings include pain with palpation directly over the dorsoradial aspect of the CMC joint and pain with axial loading and upon circumduction during a Grind test of the CMC joint. (The Grind test is performed by moving the metacarpal bone of the thumb in a circle and loading it with gentle axial forces. People with thumb joint arthritis generally experience sudden sharp pain at the CMC joint.)
Radiographic findings can be useful as a diagnostic adjunct, with staging of the disease, and in determining who can benefit from conservative management.11
Treatment: Start with NSAIDs and splinting
Depending on the degree of arthritis, management may include both conservative and surgical options.10 Patient education describing activity modification is useful during all stages of CMC arthritis. Research has shown that avoiding inciting activities, such as key turning, pinching, and grasping, helps to alleviate symptoms.14 Patients may also obtain relief from NSAIDs, especially when they are used in conjunction with activity modification and splinting. NSAIDs, however, do not halt or reverse the disease process; they only reduce inflammation, synovitis, and pain.11
Splinting. Studies have shown splinting of the thumb CMC joint to provide pain relief and to potentially slow disease progression.15 Because splints decrease motion and increase joint stability, they are especially useful for patients with joint hypermobility. The long opponens thumb spica splint is commonly used; it immobilizes the wrist and CMC, while leaving the thumb interphalangeal joint free. Short thumb spica and neoprene splints are also commercially available, and studies have shown that they provide good results.15 Splinting is most beneficial in patients with early-stage disease and may be used for either short-term flares or long-term treatment.11
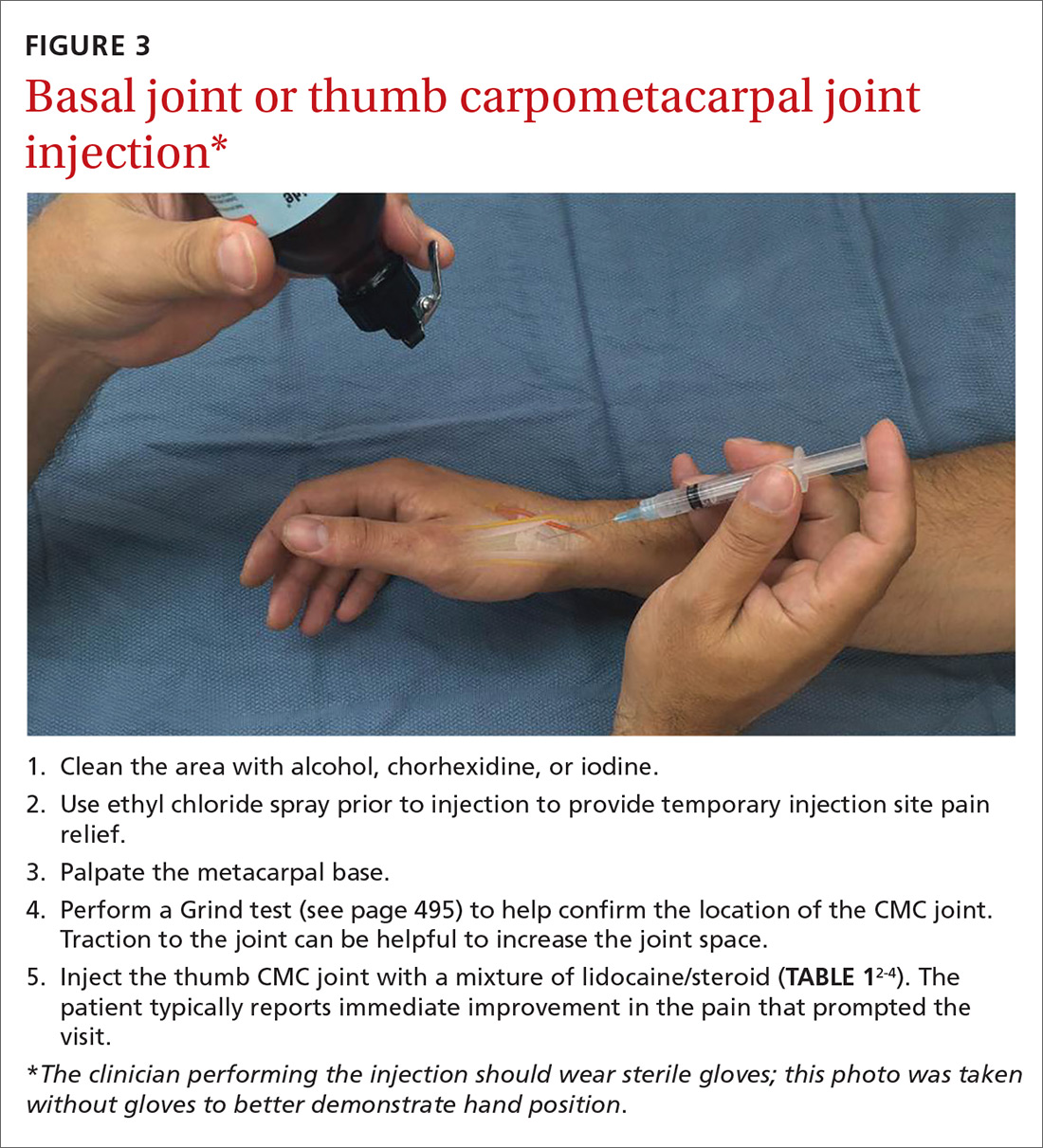
Cortisone injections. For those patients who do not respond to activity modification, NSAIDs, and/or splinting, consider cortisone injections (FIGURE 3). Intra-articular cortisone injections can decrease inflammation and provide good pain relief, especially in patients with early-stage disease. The effectiveness of cortisone injections in patients with more advanced disease is not clear; no benefit has been shown in studies to date.16 Equally unclear is the long-term benefit of injections.11 Patients who do not respond to conservative treatments will often require surgical care.
Carpal tunnel syndrome: Moving slower to surgery
CTS is one of the most common conditions of the upper extremities. Researchers estimate that 491 women per 100,000 person-years and 258 men per 100,000 person-years will develop CTS, with 109 per 100,000 person-years receiving carpal tunnel release surgery.17 Risk factors for the development of CTS include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, trauma, and occupations that involve repetitive tasks or long hours working at a computer.18
CTS is caused by compression of the median nerve as it passes through the carpal tunnel.19 The elevated pressure in the carpal tunnel restricts epineural blood flow and supply, causing the pain felt with CTS.20 Even after surgical decompression, recurrent or persistent CTS can be a problem.21
Making the Dx: Perform the Phalen maneuver, Durkan’s test
Patients typically present with complaints of weakness, pain, and/or numbness in at least 2 of 4 radial digits (thumb, index, middle, ring).19,22 The most common time of day for patients to have symptoms is at night.21
The diagnostic tools. Tinel’s sign is a useful diagnostic tool when you suspect carpal tunnel syndrome. Tinel’s sign is positive if percussion over the median nerve at the carpal tunnel elicits pain or paresthesia.18
When employing the Phalen maneuver, be certain to have the patient flex his/her wrist to 90 degrees and to document the number of seconds it takes for numbness to present in the fingers. Pain or paresthesia should occur in <60 seconds for the test to be positive.18
Median nerve compression over the carpal tunnel, also known as Durkan’s test, may also elicit symptoms. With Durkan’s test, you apply direct pressure over the transverse carpal ligament. If pain or paresthesia occurs in <30 seconds, the test is positive.18 Often clinicians will combine the Phalen maneuver and Durkan’s test to increase sensitivity and specificity.18 Nerve conduction studies are often performed to confirm the clinical diagnosis.
Is more than one condition at play? It is important to determine whether cervical spine disease and/or peripheral neuropathy is contributing to the patient’s symptoms, along with CTS; patients may have more than one condition contributing to their pain. We routinely check cervical spine motion, tenderness, and nerve compression as part of the exam on a patient with suspected CTS. In the office, a monofilament test or 2-point discrimination test can help make the clinical diagnosis by uncovering decreased sensation in the thumb, index, and/or middle fingers.23
The 5.07 monofilament test is performed with the clinician applying the monofilament to different dermatomal or sensory distributions while the patient has his/her eyes closed. The 2-point discrimination test is performed with a caliper device that measures the distance at which the patient can feel 2 separate stimuli. Often electromyography or nerve conduction studies are necessary.18
Treatment: Pursue nonoperative approaches
A survey of the membership of the American Society for Surgery of the Hand revealed that surgeons are utilizing nonoperative treatments for a longer duration of time and are employing narrowed surgical indications.24 Thus, clinicians are more likely to try splints and steroid injections before proceeding to operative release.24
Nonsurgical management. In our practice, we commonly recommend corticosteroid injections (TABLE 12-4) into the carpal tunnel (FIGURES 4 and 5) to patients who are poor candidates for surgery (ie, those who have too many medical comorbidities or wound healing concerns). This is one indication for which you may want to consider ultrasound-guided injections because the improved accuracy may provide symptom relief faster than “blind” or palpation-guided injections.25
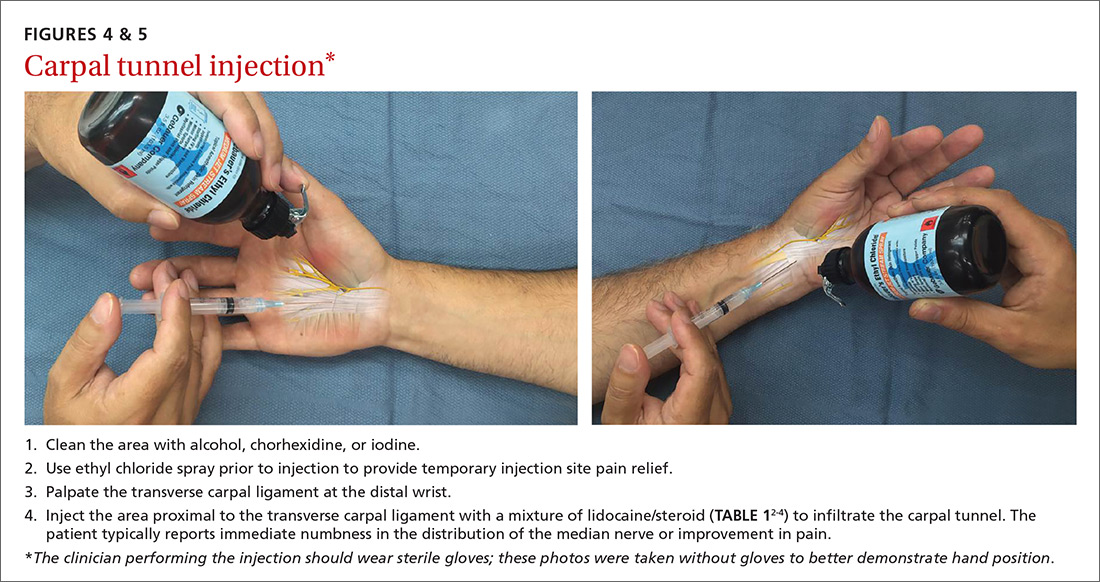
A recent randomized controlled trial from Sweden showed that injections of methylprednisolone relieved symptoms in patients with mild to moderate CTS at 10 weeks and reduced the rate of surgery one year after treatment; however, 3 out of 4 patients still went on to have surgery within a year.22 Patients in the study had failed a 2-month trial of splinting and were given either 80 mg or 40 mg of methylprednisolone or saline. There was no statistical difference between the doses of methylprednisolone in preventing surgery at one year. Compared to placebo, the 80-mg methylprednisolone group was less likely to have surgery with an odds ratio of 0.24 (P=.042).22
There is evidence that oral steroids, injected steroids, ultrasound, electromagnetic field therapy, nocturnal splinting, and use of ergonomic keyboards are effective nonoperative modalities in the short term, but evidence is sparse for mid- or long-term use.19 In addition, at least one randomized trial found traditional cupping therapy applied around the shoulder alleviated carpal tunnel symptoms in the short-term.26 Other nonoperative therapies include rest, NSAIDs, extracorporeal shock wave therapy, and activity modification.19,27
Surgical outcomes by either endoscopic, mini-open, or open surgical techniques are typically good.20,21 Surgical release involves cutting the transverse carpal ligament over the carpal tunnel to decompress the median nerve.24 You should inform patients of the risks and inconveniences associated with surgery, including the cost, absence from work, infection, and chronic pain. Patients who have recurrent or persistent symptoms after surgery may have had an incompletely released transverse carpal ligament or there may be no identifiable cause.21 Overall, surgical treatment, combined with physical therapy, seems to be more effective than splinting or NSAIDs for mid- and long-term treatment of CTS.28
De Quervain’s tenosynovitis: Common during pregnancy
De Quervain’s tenosynovitis (radial styloid tenosynovitis) involves painful inflammation of the 2 tendons in the first dorsal compartment of the wrist—the abductor pollicis longus (APL) and the extensor pollicis brevis (EPB). The tendons comprise the radial border of the anatomic snuffbox.
The APL abducts and extends the thumb at the CMC joint, while the EPB extends the thumb proximal phalanx at the metacarpophalangeal joint. These tendons are contained in a synovial sheath that is subject to inflammation and constriction and subsequent wear and damage.29 In addition, the extensor retinaculum in patients with de Quervain’s disease demonstrates increased vascularity and deposition of dense fibrous tissue resulting in thickening of the tendon up to 5 times its normal width.30
As a result, degeneration and thickening of the tendon sheath, as well as radial-sided wrist pain elicited at the first dorsal compartment, are common pathophysiologic and clinical findings.31 Pain is often accompanied by the build-up of protuberances and nodulations of the tendon sheath.
De Quervain’s disease commonly occurs during and after pregnancy.32 Other risk factors include racquet sports, golfing, wrist trauma, and other activities involving repetitive hand and wrist motions.33 Often, however, de Quervain’s is idiopathic.
Making the Dx: Perform a Finkelstein's test
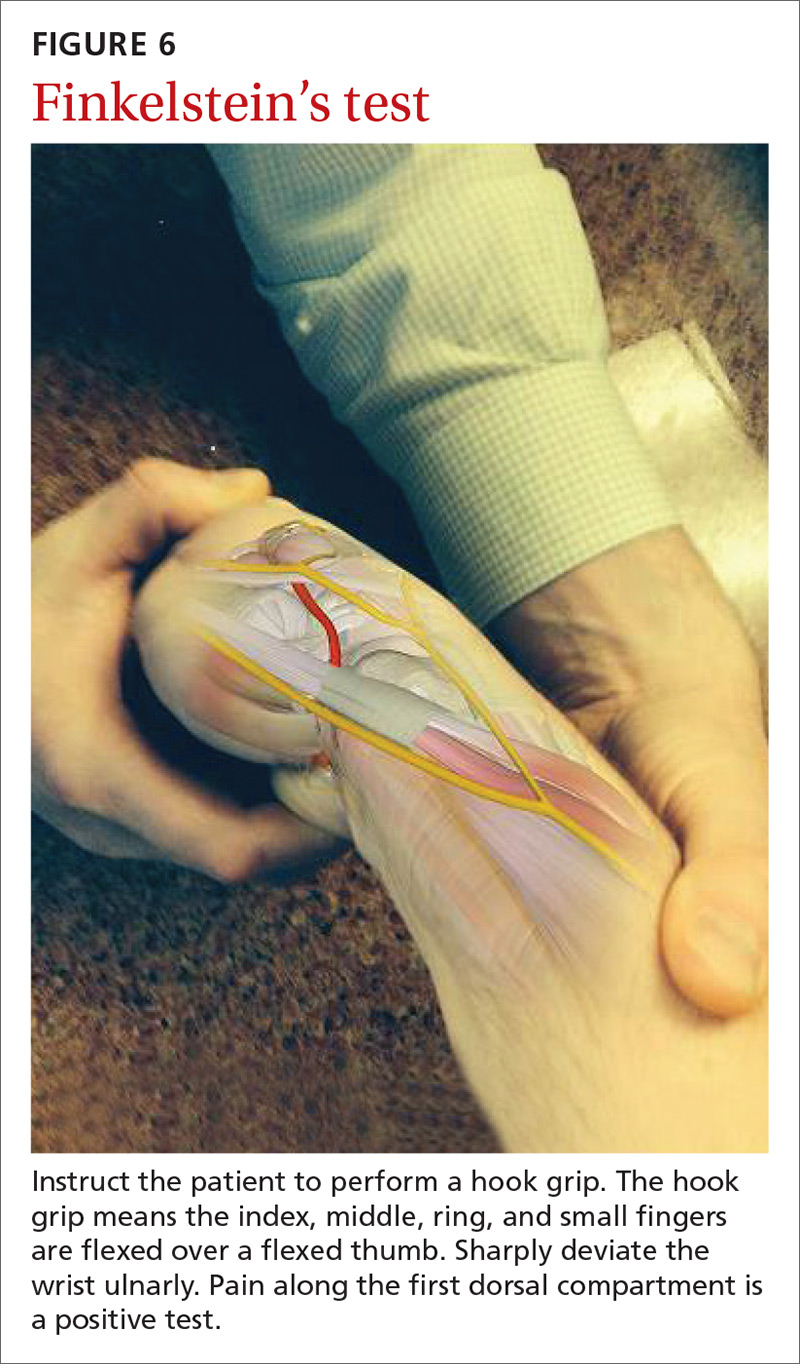
The major finding in patients with de Quervain’s tenosynovitis is a positive Finkelstein's test. To perform Finkelstein's test (FIGURE 6), ask the patient to oppose the thumb into the palm and flex the fingers of the same hand over the thumb. Holding the patient’s fingers around the thumb, ulnarly deviate the wrist. Finkelstein's test puts strain on the APL and EPB, causing pain along the radial border of the wrist and forearm in patients with de Quervain’s tenosynovitis. Since the maneuver can be uncomfortable, complete the exam on the unaffected side for comparison.
Stenosis of the tendon sheath may lead to crepitus over the first dorsal wrist compartment. This should be distinguished from intersection syndrome (tenosynovitis at the intersection of the first and second extensor compartments), which can also present with forearm and wrist crepitus. Patients usually have swelling of the wrist with marked discomfort upon palpation of the radial tendons. An x-ray can be useful to evaluate for CMC or radiocarpal arthritis, which may be an underlying cause.
Treatment: Select an approach based on symptom severity
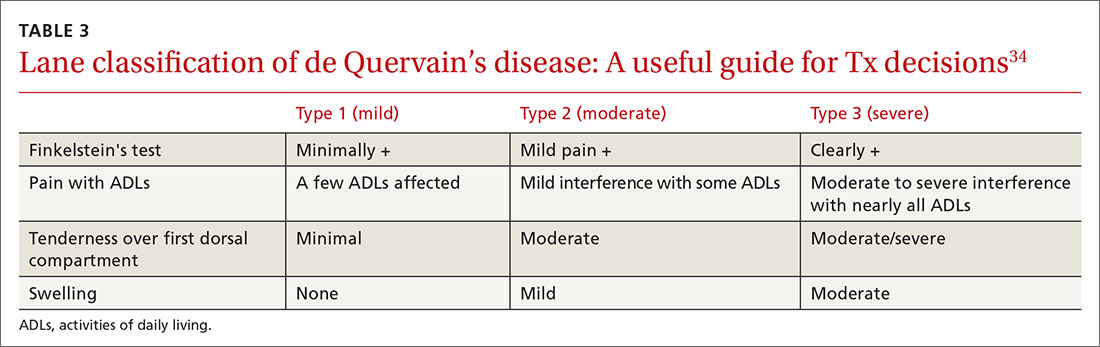
In a retrospective analysis, Lane et al concluded that classification of patients with de Quervain’s disease based on pretreatment symptoms may assist physicians in selecting the most efficacious treatment and in providing prognostic information to their patients (TABLE 334). Patients with mild to moderate (Types 1 and 2) de Quervain’s may benefit from immobilization in a thumb spica splint, rest, NSAIDs, and physical or occupational therapy. If work conditions played a role in causing the symptoms, they need to be addressed to improve outcomes. Types 2 and 3 can be initially treated with a corticosteroid injection, but may eventually require surgery.33
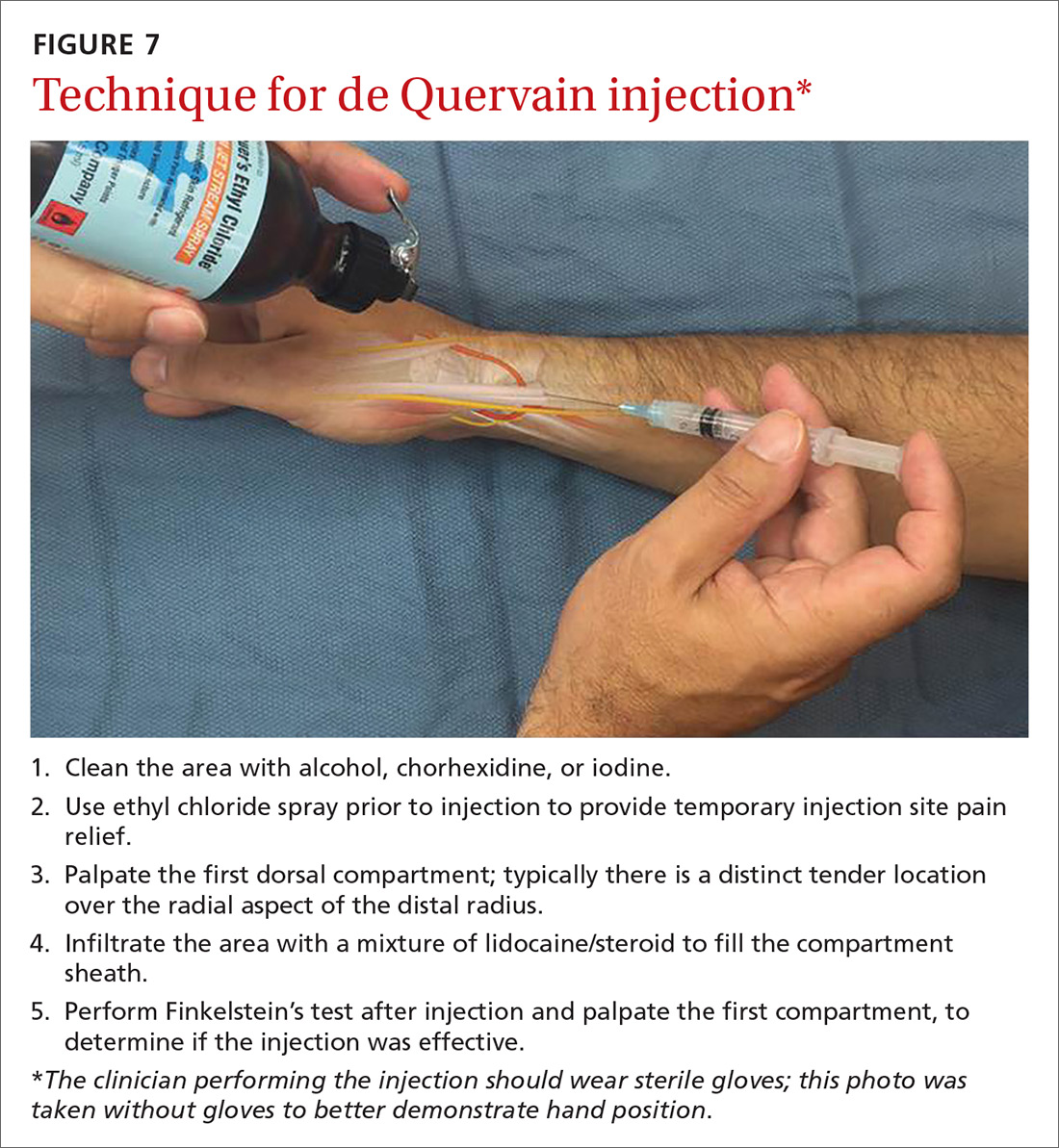
Treatment with NSAIDs or corticosteroid injections (see TABLE 12-4 for choices) in the first compartment of the extensor retinaculum (FIGURE 7) is usually adequate to provide relief. Peters-Veluthamaningal et al performed a systematic review in 2009 and found only one controlled trial of 18 participants (all pregnant or lactating women) who were either injected with corticosteroids or given a thumb spica splint.35 All 9 patients in the injection group had complete pain relief, whereas no one in the splint group had complete resolution of symptoms.35 Typical anatomic placement of corticosteroid injections is shown in FIGURE 7.
More complicated injection methods have been described, but injecting the first dorsal compartment is usually satisfactory. Patients will feel the tendon sheath filling with the injection material. The 2-point technique, implemented by Sawaizumi et al, which involves injecting corticosteroid into 2 points over the EPB and APL tendon in the area of maximum pain and soft tissue thickening, is more effective than the 1-point injection technique.36
Severe, recalcitrant cases. Professional and college athletes may be prone to recalcitrant de Quervain’s tenosynovitis. A 2010 study by Pagonis et al showed that recurrent symptomatic episodes commonly occur in athletes who engage in high-resistance, intense athletic training. In these severe cases, a 4-point injection technique offers better distribution of corticosteroid solution to the first extensor compartment than other methods.37 Consider referring severe cases to a hand surgeon.
Surgical release of the first dorsal compartmental sheath around the tendons serves as a final option for patients who fail conservative treatment. Care should be taken to release both tendons completely, as there may be at least 2 tendon slips of the APL or there may be a distinct EPB sheath dorsally.38
“Tennis elbow”— you don’t have to play tennis to have it
Lateral epicondylitis (tennis elbow) is a painful condition involving microtears within the extensor carpi radialis brevis muscle and the subsequent development of angiofibroblastic dysplasia.39 According to Regan et al who studied the histopathologic features of 11 patients with lateral epicondylitis, the underlying cause of recalcitrant lateral epicondylitis is, in fact, degenerative, rather than inflammatory.40
Although the condition has been nicknamed “tennis elbow,” only about 5% of tennis players have the condition.41 In tennis players, males are more often affected than females, whereas in the general population, incidence is approximately equal in men and women.41 Lateral epicondylitis occurs between 4 and 7 times more frequently than medial-sided elbow pain.42
Making the Dx: Look for localized pain, normal ROM
The diagnosis of lateral epicondylitis is based upon a history of pain over the lateral epicondyle and findings on physical examination, including local tenderness directly over the lateral epicondyle,43 pain aggravated by resisted wrist extension and radial deviation, pain with resisted middle finger extension, and decreased grip strength or pain aggravated by strong gripping. These findings typically occur in the presence of normal elbow range of motion.
Treatment: Choose from a range of options
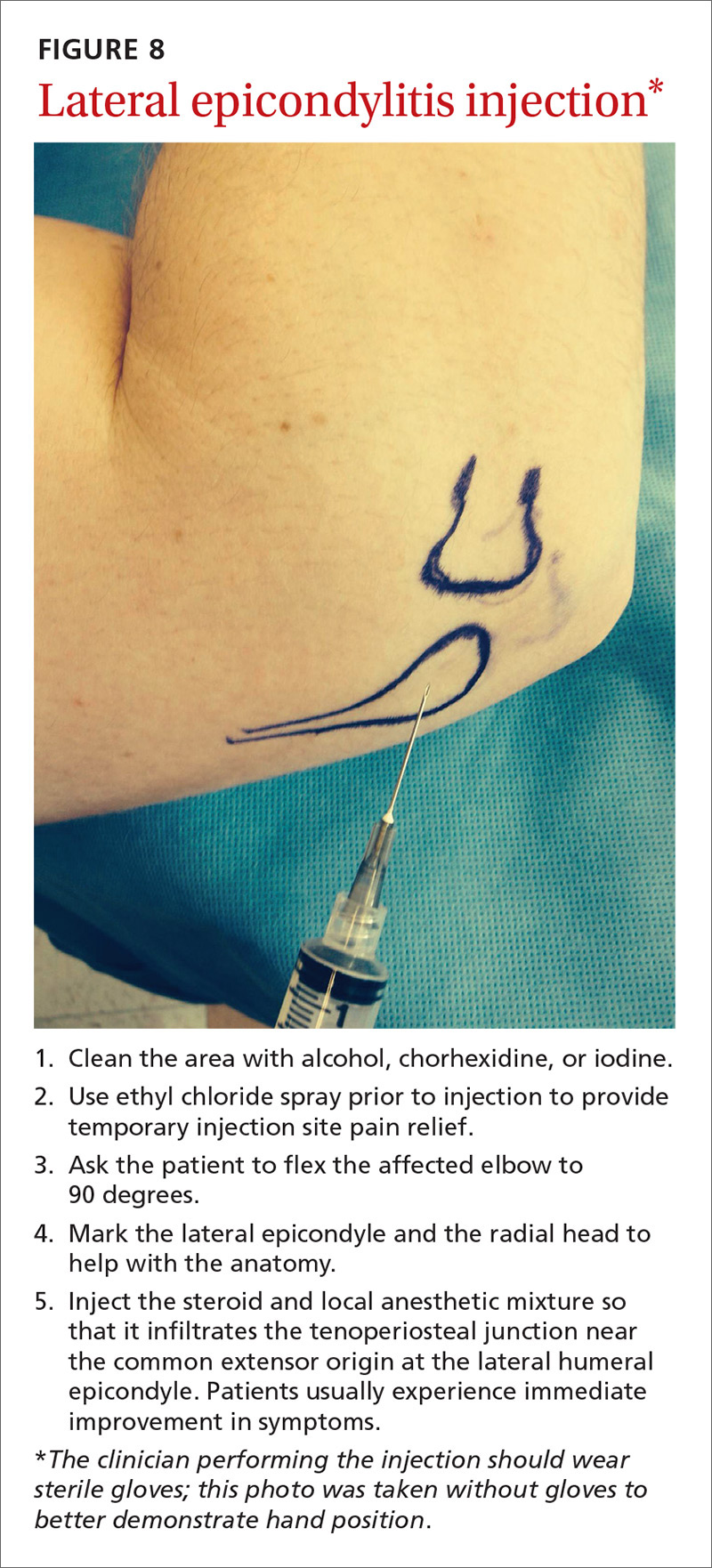
Since lateral epicondylitis was first described, researchers have proposed a wide variety of treatments as initial interventions including rest, activity, equipment modification, NSAIDs, wrist bracing/elbow straps, and physical therapy. If initial treatment does not produce the desired effect, second-line treatments include corticosteroid injections (FIGURE 8), prolotherapy (injection of an irritant, often dextrose; see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768), autologous blood injections, platelet-rich plasma injections (see “Is platelet-rich plasma right for your patient?” J Fam Pract. 2016;65:319-328), and needling of the extensor tendon origin. Refer patients who do not improve after one corticosteroid injection to an orthopedic surgeon for consideration of open or arthroscopic treatment.
CORRESPONDENCE
Gregory R. Waryasz, MD, Rhode Island Hospital, Department of Orthopaedic Surgery, 593 Eddy St., Providence, RI 02903; [email protected].
1. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775.
2. Peters-Veluthamaningal C, Van der Windt DA, Winters JC, et al. Corticosteroid injection for trigger finger in adults. Cochrane Database Syst Rev. 2009:CD005617.
3. Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11:141-147.
4. Waryasz GR, Tambone R, Borenstein TR, et al. A review of anatomical placement of corticosteroid injections for uncommon hand, wrist, and elbow pathologies. R I Med J. 2017;100:31-34.
5. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1:396-404.
6. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743.
7. Lundin AC, Aspenberg P, Eliasson P. Trigger finger, tendinosis, and intratendinous gene expression. Scand J Med Sci Sports. 2014;24:363-368.
8. Sato ES, Gomes Dos Santos JB, Belloti JC, et al. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology (Oxford). 2012;51:93-99.
9. Baek GH, Kim JH, Chug MS, et al. The natural history of pediatric trigger thumb. J Bone Joint Surg Am. 2008;90:980-985.
10. Gillis J, Calder K, Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19:134-138.
11. Yao J, Park MJ. Early treatment of degenerative arthritis of the thumb carpometacarpal joint. Hand Clin. 2008;24:251-261.
12. Ladd AL, Weiss AP, Crisco JJ, et al. The thumb carpometacarpal joint: anatomy, hormones, and biomechanics. Instr Course Lect. 2013;62:165-179.
13. Fontana L, Neel S, Claise JM, et al. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: a case-control study. J Hand Surg Am. 2007;32:459-465.
14. Stamm TA, Machold KP, Smolen JS, et al. Joint protection and home hand exercises improve hand function in patients with hand osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2002;47:44-49.
15. Weiss S, LaStayo P, Mills A, et al. Prospective analysis of splinting the first carpometacarpal joint: an objective, subjective, and radiographic assessment. J Hand Ther. 2000;13:218-226.
16. Day CS, Gelberman R, Patel AA, et al. Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J Hand Surg Am. 2004;29:247-251.
17. Gelfman R, Melton LJ 3rd, Yawn BP, et al. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33-41.
18. Wipperman J, Potter L. Carpal tunnel syndrome-try these diagnostic maneuvers. J Fam Pract. 2012;61:726-732.
19. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
20. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43:431-437.
21. Soltani AM, Allan BJ, Best MJ, et al. A systematic review of the literature on the outcomes of treatment for recurrent and persistent carpal tunnel syndrome. Plast Reconstr Surg. 2013;132:114-121.
22. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
23. Raji P, Ansari NN, Naghdi S, et al. Relationship between Semmes-Weinstein Monofilaments perception test and sensory nerve conduction studies in carpal tunnel syndrome. NeuroRehabilitation. 2014;35:543-552.
24. Leinberry CF, Rivlin M, Maltenfort M, et al. Treatment of carpal tunnel syndrome by members of the American Society for Surgery of the Hand: a 25-year perspective. J Hand Surg Am. 2012;37:1997-2003.e3.
25. Ustün N, Tok F, Yagz AE, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: a single-blind randomized prospective study. Am J Phys Med Rehabil. 2013;92:999-1004.
26. Michalsen A, Bock S, Lüdtke R, et al. Effects of traditional cupping therapy in patients with carpal tunnel syndrome: a randomized controlled trial. J Pain. 2009;10:601-608.
27. Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil. 2013;92:327-334.
28. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
29. Shehab R, Mirabelli MH. Evaluation and diagnosis of wrist pain: a case-based approach. Am Fam Physician. 2013;87:568-573.
30. Clarke MT, Lyall HA, Grant JW, et al. The histopathology of de Quervain’s disease. J Hand Surg Br. 1998;23:732-734.
31. Zychowicz MA. A closer look at hand and wrist complaints. Nurse Pract. 2013;38:46-53.
32. Avci S, Yilmaz C, Sayli U. Comparison of nonsurgical treatment measures for de Quervain’s disease of pregnancy and lactation. J Hand Surg Am. 2002;27:322-324.
33. Mani L, Gerr F. Work-related upper extremity musculoskeletal disorders. Prim Care. 2000;27:845-864.
34. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
35. Peters-Veluthamaningal C, Van der Windt JC, Winters JC, et al. Corticosteroid injection for de Quervain’s tenosynovitis. Cochrane Database Syst Rev. 2009;8:CD005616.
36. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
37. Pagonis T, Ditsios K, Toli P, et al. Improved corticosteroid treatment of recalcitrant de Quervain tenosynovitis with a novel 4-point injection technique. Am J Sports Med. 2011;39:398-403.
38. Scheller A, Schuh R, Hönle W, et al. Long-term results of surgical release of de Quervain’s stenosing tenosynovitis. Int Orthop. 2009;33:1301-1303.
39. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832-839.
40. Regan W, Wold LE, Coonrad R, et al. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20:746-749.
41. Van Hofwegen C, Baker CL 3rd, Baker CL Jr. Epicondylitis in the athlete’s elbow. Clin Sports Med. 2010;29:577-597.
42. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6:259-272.
43. Weerakul S, Galassi M. Randomized controlled trial local injection for treatment of lateral epicondylitis, 5 and 10 mg triamcinolone compared. J Med Assoc Thai. 2012;95 Supp 10:S184-188.
Primary care physicians are frequently the first to evaluate hand, wrist, and forearm pain in patients, making knowledge of the symptoms, causes, and treatment of common diagnoses in the upper extremities imperative. Primary symptoms usually include pain and/or swelling. While most tendon disorders originating in the hand and wrist are idiopathic in nature, some patients occasionally report having recently performed unusual manual activity or having experienced trauma to the area days or weeks prior. A significant portion of patients are injured as a result of chronic repetitive activities at work.1

Most diagnoses can be made by pairing your knowledge of hand and forearm anatomy with an understanding of which tender points are indicative of which common conditions. (Care, of course, must be taken to ensure that there is no underlying infection.) Common conditions can often be treated nonsurgically with conservative treatments such as physical therapy, bracing/splinting, nonsteroidal anti-inflammatory drugs (NSAIDs), and injections of corticosteroids (eg, betamethasone, hydrocortisone, methylprednisolone, and triamcinolone) (TABLE 12-4) with or without the use of ultrasound. The benefits of corticosteroid injections for these conditions are well studied and documented in the literature, although physicians should always warn patients of the possible adverse effects prior to injection3,5 (TABLE 24).

To help you refine your skills, we review some of the more common hand and forearm conditions you are likely to encounter in the office and provide photos that reveal underlying anatomy so that you can administer injections without, in many cases, the need for ultrasound.
Trigger finger/thumb: New pathophysiologic findings?
Trigger finger most commonly occurs in the dominant hand. It is also more common in women, patients in their 50s, and in individuals with diabetes.6 Trigger finger/thumb is caused by inflammation and constriction of the flexor tendon sheath, which carries the flexor tendons through the palm and into the fingers and thumb. This, in turn, causes irritation of the tendons, sometimes via the formation of tendinous nodules, which may impinge upon the sheath’s “pulley system.”

When the “pulley” is compromised. The retinacular sheath is composed of 5 annular ligaments, or pulleys, that hold the tendons of the fingers close to the bone and allow the fingers to flex properly. The A1 pulley, at the level of the metacarpal head, is the first part of the sheath and is subject to the highest force; high forces may subsequently lead to the finger becoming locked in a flexed, or trigger, position.6 Patients may experience pain in the distal palm at the level of the A1 pulley and clicking of the finger.6
Additionally . . . recent studies show discrete histologic changes in trigger finger tendons, similar to findings with Achilles tendinosis and tendinopathy.7 In trigger finger tendons, collagen type 1A1 and 3A1, aggrecan, and biglycan are up-regulated, while metalloproteinase inhibitor 3 (TIMP-3) and matrix metallopeptidase3 (MMP-3) are down-regulated, a situation also described in Achilles tendinosis.7 This similarity in conditions provides new insight into the pathophysiology of the condition and may help provide future treatments.
Making the Dx: Look for swelling, check for carpal tunnel
During the examination, first look at both hands for swelling, arthropathy, or injury, and note the presence of any joint contractures. Next, examine all of the digits in flexion and extension while noting which ones are triggering, as the problem can occur in multiple digits on one hand. Then palpate the palms over the patient’s metacarpal heads, feeling for tender nodules.
Finally, examine the patient for carpal tunnel syndrome (CTS). A positive Tinel’s sign (shooting pain into the hand when the median nerve in the wrist is percussed), a positive Phalen maneuver (numbness or pain, usually within one minute of full wrist flexion), or thenar muscle wasting are highly indicative of CTS (compression of the median nerve at the transverse carpal ligament in the carpal tunnel). It is important to check for CTS when examining a patient for trigger finger because the 2 conditions frequently co-occur.6 (For more on CTS, see here.)
Treatment: Consider corticosteroids first
First-line treatment for patients with trigger finger or thumb is a corticosteroid injection into the subcutaneous tissue around the tendon sheath (FIGURES 1 and 2). (For this indication and for the others discussed throughout the article, there isn’t tremendous evidence for one particular type of corticosteroid over another; see TABLE 12-4 for choices.) Up to 57% of cases resolve with one injection, and 86% resolve with 2,8 but keep in mind that it may take up to 2 weeks to achieve the full clinical benefit.

Patients with multiple trigger fingers can be treated with oral corticosteroids (eg, a methylprednisolone dose pack). Peters-Veluthamaningal et al performed a systematic review in 2009 and found 2 randomized controlled trials involving 63 patients (34 received injections of a corticosteroid [either methylprednisolone or betamethasone] and lidocaine and 29 received lidocaine only).2 The corticosteroid/lidocaine combination was more effective at 4 weeks (relative risk [RR]=3.15; 95% confidence interval [CI], 1.34 to 7.40).2
If 2 corticosteroid injections 6 weeks apart fail to provide benefit, or the finger is irreversibly locked in flexion, surgical release of the pulley is required and is performed through a palmar incision at the level of the A1 pulley. Complications from this surgery, including nerve damage, are exceedingly rare, but injury can occur, given the proximity of the digital nerves to the A1 pulley.
Patient is a child? Refer children with trigger finger or thumb to a hand surgeon for evaluation and management because the indications for nonoperative treatment in the pediatric population are unclear.9
Carpometacarpal arthritis: Common, with many causes
Osteoarthritis of the first carpometacarpal (CMC) joint is the most common site of arthritis in the hand/wrist region, affecting up to 11% of men and 33% of women in their 50s and 60s.10 Because the CMC joint lacks a bony restraint, it relies on a number of ligaments for stability—the strongest and most important of which is the palmar oblique “beak” ligament.11 A major cause of degenerative arthritis of this joint is attenuation and laxity of these ligaments, leading to abnormal and increased stress loads, which, in turn, can lead to loss of cartilage and bony impingement. While the exact mechanism of this process is not fully understood,10,12 acute or chronic trauma, advanced age, hormonal factors, and genetic factors seem to play a role.11
Many believe there is a relationship between a patient's occupation and the development of CMC arthritis, but studies are inconclusive.13 At risk are secretarial workers, tailors, domestic helpers/cleaners, and individuals whose jobs involve repetitive thumb use and/or insufficient rest of the joint throughout the day.
Making the Dx: Perform the Grind test
A detailed patient history (which is usually void of trauma to the hand) and physical examination are the keys to making the diagnosis of CMC arthritis. A history of pain at the base of the thumb during pinching and gripping tasks is often elucidated. Classically, patients describe pain upon turning keys, opening jars, and gripping doorknobs.11
It's important to focus on the dorsoradial aspect of the thumb during the physical exam and to rule out other causes of pain, such as de Quervain’s tenosynovitis, flexor carpi radialis tendinitis, CTS, and trigger thumb.11 Typical findings include pain with palpation directly over the dorsoradial aspect of the CMC joint and pain with axial loading and upon circumduction during a Grind test of the CMC joint. (The Grind test is performed by moving the metacarpal bone of the thumb in a circle and loading it with gentle axial forces. People with thumb joint arthritis generally experience sudden sharp pain at the CMC joint.)
Radiographic findings can be useful as a diagnostic adjunct, with staging of the disease, and in determining who can benefit from conservative management.11
Treatment: Start with NSAIDs and splinting
Depending on the degree of arthritis, management may include both conservative and surgical options.10 Patient education describing activity modification is useful during all stages of CMC arthritis. Research has shown that avoiding inciting activities, such as key turning, pinching, and grasping, helps to alleviate symptoms.14 Patients may also obtain relief from NSAIDs, especially when they are used in conjunction with activity modification and splinting. NSAIDs, however, do not halt or reverse the disease process; they only reduce inflammation, synovitis, and pain.11
Splinting. Studies have shown splinting of the thumb CMC joint to provide pain relief and to potentially slow disease progression.15 Because splints decrease motion and increase joint stability, they are especially useful for patients with joint hypermobility. The long opponens thumb spica splint is commonly used; it immobilizes the wrist and CMC, while leaving the thumb interphalangeal joint free. Short thumb spica and neoprene splints are also commercially available, and studies have shown that they provide good results.15 Splinting is most beneficial in patients with early-stage disease and may be used for either short-term flares or long-term treatment.11
Cortisone injections. For those patients who do not respond to activity modification, NSAIDs, and/or splinting, consider cortisone injections (FIGURE 3). Intra-articular cortisone injections can decrease inflammation and provide good pain relief, especially in patients with early-stage disease. The effectiveness of cortisone injections in patients with more advanced disease is not clear; no benefit has been shown in studies to date.16 Equally unclear is the long-term benefit of injections.11 Patients who do not respond to conservative treatments will often require surgical care.

Carpal tunnel syndrome: Moving slower to surgery
CTS is one of the most common conditions of the upper extremities. Researchers estimate that 491 women per 100,000 person-years and 258 men per 100,000 person-years will develop CTS, with 109 per 100,000 person-years receiving carpal tunnel release surgery.17 Risk factors for the development of CTS include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, trauma, and occupations that involve repetitive tasks or long hours working at a computer.18
CTS is caused by compression of the median nerve as it passes through the carpal tunnel.19 The elevated pressure in the carpal tunnel restricts epineural blood flow and supply, causing the pain felt with CTS.20 Even after surgical decompression, recurrent or persistent CTS can be a problem.21
Making the Dx: Perform the Phalen maneuver, Durkan’s test
Patients typically present with complaints of weakness, pain, and/or numbness in at least 2 of 4 radial digits (thumb, index, middle, ring).19,22 The most common time of day for patients to have symptoms is at night.21
The diagnostic tools. Tinel’s sign is a useful diagnostic tool when you suspect carpal tunnel syndrome. Tinel’s sign is positive if percussion over the median nerve at the carpal tunnel elicits pain or paresthesia.18
When employing the Phalen maneuver, be certain to have the patient flex his/her wrist to 90 degrees and to document the number of seconds it takes for numbness to present in the fingers. Pain or paresthesia should occur in <60 seconds for the test to be positive.18
Median nerve compression over the carpal tunnel, also known as Durkan’s test, may also elicit symptoms. With Durkan’s test, you apply direct pressure over the transverse carpal ligament. If pain or paresthesia occurs in <30 seconds, the test is positive.18 Often clinicians will combine the Phalen maneuver and Durkan’s test to increase sensitivity and specificity.18 Nerve conduction studies are often performed to confirm the clinical diagnosis.
Is more than one condition at play? It is important to determine whether cervical spine disease and/or peripheral neuropathy is contributing to the patient’s symptoms, along with CTS; patients may have more than one condition contributing to their pain. We routinely check cervical spine motion, tenderness, and nerve compression as part of the exam on a patient with suspected CTS. In the office, a monofilament test or 2-point discrimination test can help make the clinical diagnosis by uncovering decreased sensation in the thumb, index, and/or middle fingers.23
The 5.07 monofilament test is performed with the clinician applying the monofilament to different dermatomal or sensory distributions while the patient has his/her eyes closed. The 2-point discrimination test is performed with a caliper device that measures the distance at which the patient can feel 2 separate stimuli. Often electromyography or nerve conduction studies are necessary.18
Treatment: Pursue nonoperative approaches
A survey of the membership of the American Society for Surgery of the Hand revealed that surgeons are utilizing nonoperative treatments for a longer duration of time and are employing narrowed surgical indications.24 Thus, clinicians are more likely to try splints and steroid injections before proceeding to operative release.24
Nonsurgical management. In our practice, we commonly recommend corticosteroid injections (TABLE 12-4) into the carpal tunnel (FIGURES 4 and 5) to patients who are poor candidates for surgery (ie, those who have too many medical comorbidities or wound healing concerns). This is one indication for which you may want to consider ultrasound-guided injections because the improved accuracy may provide symptom relief faster than “blind” or palpation-guided injections.25

A recent randomized controlled trial from Sweden showed that injections of methylprednisolone relieved symptoms in patients with mild to moderate CTS at 10 weeks and reduced the rate of surgery one year after treatment; however, 3 out of 4 patients still went on to have surgery within a year.22 Patients in the study had failed a 2-month trial of splinting and were given either 80 mg or 40 mg of methylprednisolone or saline. There was no statistical difference between the doses of methylprednisolone in preventing surgery at one year. Compared to placebo, the 80-mg methylprednisolone group was less likely to have surgery with an odds ratio of 0.24 (P=.042).22
There is evidence that oral steroids, injected steroids, ultrasound, electromagnetic field therapy, nocturnal splinting, and use of ergonomic keyboards are effective nonoperative modalities in the short term, but evidence is sparse for mid- or long-term use.19 In addition, at least one randomized trial found traditional cupping therapy applied around the shoulder alleviated carpal tunnel symptoms in the short-term.26 Other nonoperative therapies include rest, NSAIDs, extracorporeal shock wave therapy, and activity modification.19,27
Surgical outcomes by either endoscopic, mini-open, or open surgical techniques are typically good.20,21 Surgical release involves cutting the transverse carpal ligament over the carpal tunnel to decompress the median nerve.24 You should inform patients of the risks and inconveniences associated with surgery, including the cost, absence from work, infection, and chronic pain. Patients who have recurrent or persistent symptoms after surgery may have had an incompletely released transverse carpal ligament or there may be no identifiable cause.21 Overall, surgical treatment, combined with physical therapy, seems to be more effective than splinting or NSAIDs for mid- and long-term treatment of CTS.28
De Quervain’s tenosynovitis: Common during pregnancy
De Quervain’s tenosynovitis (radial styloid tenosynovitis) involves painful inflammation of the 2 tendons in the first dorsal compartment of the wrist—the abductor pollicis longus (APL) and the extensor pollicis brevis (EPB). The tendons comprise the radial border of the anatomic snuffbox.
The APL abducts and extends the thumb at the CMC joint, while the EPB extends the thumb proximal phalanx at the metacarpophalangeal joint. These tendons are contained in a synovial sheath that is subject to inflammation and constriction and subsequent wear and damage.29 In addition, the extensor retinaculum in patients with de Quervain’s disease demonstrates increased vascularity and deposition of dense fibrous tissue resulting in thickening of the tendon up to 5 times its normal width.30
As a result, degeneration and thickening of the tendon sheath, as well as radial-sided wrist pain elicited at the first dorsal compartment, are common pathophysiologic and clinical findings.31 Pain is often accompanied by the build-up of protuberances and nodulations of the tendon sheath.
De Quervain’s disease commonly occurs during and after pregnancy.32 Other risk factors include racquet sports, golfing, wrist trauma, and other activities involving repetitive hand and wrist motions.33 Often, however, de Quervain’s is idiopathic.
Making the Dx: Perform a Finkelstein's test
The major finding in patients with de Quervain’s tenosynovitis is a positive Finkelstein's test. To perform Finkelstein's test (FIGURE 6), ask the patient to oppose the thumb into the palm and flex the fingers of the same hand over the thumb. Holding the patient’s fingers around the thumb, ulnarly deviate the wrist. Finkelstein's test puts strain on the APL and EPB, causing pain along the radial border of the wrist and forearm in patients with de Quervain’s tenosynovitis. Since the maneuver can be uncomfortable, complete the exam on the unaffected side for comparison.

Stenosis of the tendon sheath may lead to crepitus over the first dorsal wrist compartment. This should be distinguished from intersection syndrome (tenosynovitis at the intersection of the first and second extensor compartments), which can also present with forearm and wrist crepitus. Patients usually have swelling of the wrist with marked discomfort upon palpation of the radial tendons. An x-ray can be useful to evaluate for CMC or radiocarpal arthritis, which may be an underlying cause.
Treatment: Select an approach based on symptom severity
In a retrospective analysis, Lane et al concluded that classification of patients with de Quervain’s disease based on pretreatment symptoms may assist physicians in selecting the most efficacious treatment and in providing prognostic information to their patients (TABLE 334). Patients with mild to moderate (Types 1 and 2) de Quervain’s may benefit from immobilization in a thumb spica splint, rest, NSAIDs, and physical or occupational therapy. If work conditions played a role in causing the symptoms, they need to be addressed to improve outcomes. Types 2 and 3 can be initially treated with a corticosteroid injection, but may eventually require surgery.33

Treatment with NSAIDs or corticosteroid injections (see TABLE 12-4 for choices) in the first compartment of the extensor retinaculum (FIGURE 7) is usually adequate to provide relief. Peters-Veluthamaningal et al performed a systematic review in 2009 and found only one controlled trial of 18 participants (all pregnant or lactating women) who were either injected with corticosteroids or given a thumb spica splint.35 All 9 patients in the injection group had complete pain relief, whereas no one in the splint group had complete resolution of symptoms.35 Typical anatomic placement of corticosteroid injections is shown in FIGURE 7.

More complicated injection methods have been described, but injecting the first dorsal compartment is usually satisfactory. Patients will feel the tendon sheath filling with the injection material. The 2-point technique, implemented by Sawaizumi et al, which involves injecting corticosteroid into 2 points over the EPB and APL tendon in the area of maximum pain and soft tissue thickening, is more effective than the 1-point injection technique.36
Severe, recalcitrant cases. Professional and college athletes may be prone to recalcitrant de Quervain’s tenosynovitis. A 2010 study by Pagonis et al showed that recurrent symptomatic episodes commonly occur in athletes who engage in high-resistance, intense athletic training. In these severe cases, a 4-point injection technique offers better distribution of corticosteroid solution to the first extensor compartment than other methods.37 Consider referring severe cases to a hand surgeon.
Surgical release of the first dorsal compartmental sheath around the tendons serves as a final option for patients who fail conservative treatment. Care should be taken to release both tendons completely, as there may be at least 2 tendon slips of the APL or there may be a distinct EPB sheath dorsally.38
“Tennis elbow”— you don’t have to play tennis to have it
Lateral epicondylitis (tennis elbow) is a painful condition involving microtears within the extensor carpi radialis brevis muscle and the subsequent development of angiofibroblastic dysplasia.39 According to Regan et al who studied the histopathologic features of 11 patients with lateral epicondylitis, the underlying cause of recalcitrant lateral epicondylitis is, in fact, degenerative, rather than inflammatory.40
Although the condition has been nicknamed “tennis elbow,” only about 5% of tennis players have the condition.41 In tennis players, males are more often affected than females, whereas in the general population, incidence is approximately equal in men and women.41 Lateral epicondylitis occurs between 4 and 7 times more frequently than medial-sided elbow pain.42
Making the Dx: Look for localized pain, normal ROM
The diagnosis of lateral epicondylitis is based upon a history of pain over the lateral epicondyle and findings on physical examination, including local tenderness directly over the lateral epicondyle,43 pain aggravated by resisted wrist extension and radial deviation, pain with resisted middle finger extension, and decreased grip strength or pain aggravated by strong gripping. These findings typically occur in the presence of normal elbow range of motion.
Treatment: Choose from a range of options
Since lateral epicondylitis was first described, researchers have proposed a wide variety of treatments as initial interventions including rest, activity, equipment modification, NSAIDs, wrist bracing/elbow straps, and physical therapy. If initial treatment does not produce the desired effect, second-line treatments include corticosteroid injections (FIGURE 8), prolotherapy (injection of an irritant, often dextrose; see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768), autologous blood injections, platelet-rich plasma injections (see “Is platelet-rich plasma right for your patient?” J Fam Pract. 2016;65:319-328), and needling of the extensor tendon origin. Refer patients who do not improve after one corticosteroid injection to an orthopedic surgeon for consideration of open or arthroscopic treatment.

CORRESPONDENCE
Gregory R. Waryasz, MD, Rhode Island Hospital, Department of Orthopaedic Surgery, 593 Eddy St., Providence, RI 02903; [email protected].
Primary care physicians are frequently the first to evaluate hand, wrist, and forearm pain in patients, making knowledge of the symptoms, causes, and treatment of common diagnoses in the upper extremities imperative. Primary symptoms usually include pain and/or swelling. While most tendon disorders originating in the hand and wrist are idiopathic in nature, some patients occasionally report having recently performed unusual manual activity or having experienced trauma to the area days or weeks prior. A significant portion of patients are injured as a result of chronic repetitive activities at work.1

Most diagnoses can be made by pairing your knowledge of hand and forearm anatomy with an understanding of which tender points are indicative of which common conditions. (Care, of course, must be taken to ensure that there is no underlying infection.) Common conditions can often be treated nonsurgically with conservative treatments such as physical therapy, bracing/splinting, nonsteroidal anti-inflammatory drugs (NSAIDs), and injections of corticosteroids (eg, betamethasone, hydrocortisone, methylprednisolone, and triamcinolone) (TABLE 12-4) with or without the use of ultrasound. The benefits of corticosteroid injections for these conditions are well studied and documented in the literature, although physicians should always warn patients of the possible adverse effects prior to injection3,5 (TABLE 24).

To help you refine your skills, we review some of the more common hand and forearm conditions you are likely to encounter in the office and provide photos that reveal underlying anatomy so that you can administer injections without, in many cases, the need for ultrasound.
Trigger finger/thumb: New pathophysiologic findings?
Trigger finger most commonly occurs in the dominant hand. It is also more common in women, patients in their 50s, and in individuals with diabetes.6 Trigger finger/thumb is caused by inflammation and constriction of the flexor tendon sheath, which carries the flexor tendons through the palm and into the fingers and thumb. This, in turn, causes irritation of the tendons, sometimes via the formation of tendinous nodules, which may impinge upon the sheath’s “pulley system.”

When the “pulley” is compromised. The retinacular sheath is composed of 5 annular ligaments, or pulleys, that hold the tendons of the fingers close to the bone and allow the fingers to flex properly. The A1 pulley, at the level of the metacarpal head, is the first part of the sheath and is subject to the highest force; high forces may subsequently lead to the finger becoming locked in a flexed, or trigger, position.6 Patients may experience pain in the distal palm at the level of the A1 pulley and clicking of the finger.6
Additionally . . . recent studies show discrete histologic changes in trigger finger tendons, similar to findings with Achilles tendinosis and tendinopathy.7 In trigger finger tendons, collagen type 1A1 and 3A1, aggrecan, and biglycan are up-regulated, while metalloproteinase inhibitor 3 (TIMP-3) and matrix metallopeptidase3 (MMP-3) are down-regulated, a situation also described in Achilles tendinosis.7 This similarity in conditions provides new insight into the pathophysiology of the condition and may help provide future treatments.
Making the Dx: Look for swelling, check for carpal tunnel
During the examination, first look at both hands for swelling, arthropathy, or injury, and note the presence of any joint contractures. Next, examine all of the digits in flexion and extension while noting which ones are triggering, as the problem can occur in multiple digits on one hand. Then palpate the palms over the patient’s metacarpal heads, feeling for tender nodules.
Finally, examine the patient for carpal tunnel syndrome (CTS). A positive Tinel’s sign (shooting pain into the hand when the median nerve in the wrist is percussed), a positive Phalen maneuver (numbness or pain, usually within one minute of full wrist flexion), or thenar muscle wasting are highly indicative of CTS (compression of the median nerve at the transverse carpal ligament in the carpal tunnel). It is important to check for CTS when examining a patient for trigger finger because the 2 conditions frequently co-occur.6 (For more on CTS, see here.)
Treatment: Consider corticosteroids first
First-line treatment for patients with trigger finger or thumb is a corticosteroid injection into the subcutaneous tissue around the tendon sheath (FIGURES 1 and 2). (For this indication and for the others discussed throughout the article, there isn’t tremendous evidence for one particular type of corticosteroid over another; see TABLE 12-4 for choices.) Up to 57% of cases resolve with one injection, and 86% resolve with 2,8 but keep in mind that it may take up to 2 weeks to achieve the full clinical benefit.

Patients with multiple trigger fingers can be treated with oral corticosteroids (eg, a methylprednisolone dose pack). Peters-Veluthamaningal et al performed a systematic review in 2009 and found 2 randomized controlled trials involving 63 patients (34 received injections of a corticosteroid [either methylprednisolone or betamethasone] and lidocaine and 29 received lidocaine only).2 The corticosteroid/lidocaine combination was more effective at 4 weeks (relative risk [RR]=3.15; 95% confidence interval [CI], 1.34 to 7.40).2
If 2 corticosteroid injections 6 weeks apart fail to provide benefit, or the finger is irreversibly locked in flexion, surgical release of the pulley is required and is performed through a palmar incision at the level of the A1 pulley. Complications from this surgery, including nerve damage, are exceedingly rare, but injury can occur, given the proximity of the digital nerves to the A1 pulley.
Patient is a child? Refer children with trigger finger or thumb to a hand surgeon for evaluation and management because the indications for nonoperative treatment in the pediatric population are unclear.9
Carpometacarpal arthritis: Common, with many causes
Osteoarthritis of the first carpometacarpal (CMC) joint is the most common site of arthritis in the hand/wrist region, affecting up to 11% of men and 33% of women in their 50s and 60s.10 Because the CMC joint lacks a bony restraint, it relies on a number of ligaments for stability—the strongest and most important of which is the palmar oblique “beak” ligament.11 A major cause of degenerative arthritis of this joint is attenuation and laxity of these ligaments, leading to abnormal and increased stress loads, which, in turn, can lead to loss of cartilage and bony impingement. While the exact mechanism of this process is not fully understood,10,12 acute or chronic trauma, advanced age, hormonal factors, and genetic factors seem to play a role.11
Many believe there is a relationship between a patient's occupation and the development of CMC arthritis, but studies are inconclusive.13 At risk are secretarial workers, tailors, domestic helpers/cleaners, and individuals whose jobs involve repetitive thumb use and/or insufficient rest of the joint throughout the day.
Making the Dx: Perform the Grind test
A detailed patient history (which is usually void of trauma to the hand) and physical examination are the keys to making the diagnosis of CMC arthritis. A history of pain at the base of the thumb during pinching and gripping tasks is often elucidated. Classically, patients describe pain upon turning keys, opening jars, and gripping doorknobs.11
It's important to focus on the dorsoradial aspect of the thumb during the physical exam and to rule out other causes of pain, such as de Quervain’s tenosynovitis, flexor carpi radialis tendinitis, CTS, and trigger thumb.11 Typical findings include pain with palpation directly over the dorsoradial aspect of the CMC joint and pain with axial loading and upon circumduction during a Grind test of the CMC joint. (The Grind test is performed by moving the metacarpal bone of the thumb in a circle and loading it with gentle axial forces. People with thumb joint arthritis generally experience sudden sharp pain at the CMC joint.)
Radiographic findings can be useful as a diagnostic adjunct, with staging of the disease, and in determining who can benefit from conservative management.11
Treatment: Start with NSAIDs and splinting
Depending on the degree of arthritis, management may include both conservative and surgical options.10 Patient education describing activity modification is useful during all stages of CMC arthritis. Research has shown that avoiding inciting activities, such as key turning, pinching, and grasping, helps to alleviate symptoms.14 Patients may also obtain relief from NSAIDs, especially when they are used in conjunction with activity modification and splinting. NSAIDs, however, do not halt or reverse the disease process; they only reduce inflammation, synovitis, and pain.11
Splinting. Studies have shown splinting of the thumb CMC joint to provide pain relief and to potentially slow disease progression.15 Because splints decrease motion and increase joint stability, they are especially useful for patients with joint hypermobility. The long opponens thumb spica splint is commonly used; it immobilizes the wrist and CMC, while leaving the thumb interphalangeal joint free. Short thumb spica and neoprene splints are also commercially available, and studies have shown that they provide good results.15 Splinting is most beneficial in patients with early-stage disease and may be used for either short-term flares or long-term treatment.11
Cortisone injections. For those patients who do not respond to activity modification, NSAIDs, and/or splinting, consider cortisone injections (FIGURE 3). Intra-articular cortisone injections can decrease inflammation and provide good pain relief, especially in patients with early-stage disease. The effectiveness of cortisone injections in patients with more advanced disease is not clear; no benefit has been shown in studies to date.16 Equally unclear is the long-term benefit of injections.11 Patients who do not respond to conservative treatments will often require surgical care.

Carpal tunnel syndrome: Moving slower to surgery
CTS is one of the most common conditions of the upper extremities. Researchers estimate that 491 women per 100,000 person-years and 258 men per 100,000 person-years will develop CTS, with 109 per 100,000 person-years receiving carpal tunnel release surgery.17 Risk factors for the development of CTS include diabetes, hypothyroidism, rheumatoid arthritis, pregnancy, obesity, family history, trauma, and occupations that involve repetitive tasks or long hours working at a computer.18
CTS is caused by compression of the median nerve as it passes through the carpal tunnel.19 The elevated pressure in the carpal tunnel restricts epineural blood flow and supply, causing the pain felt with CTS.20 Even after surgical decompression, recurrent or persistent CTS can be a problem.21
Making the Dx: Perform the Phalen maneuver, Durkan’s test
Patients typically present with complaints of weakness, pain, and/or numbness in at least 2 of 4 radial digits (thumb, index, middle, ring).19,22 The most common time of day for patients to have symptoms is at night.21
The diagnostic tools. Tinel’s sign is a useful diagnostic tool when you suspect carpal tunnel syndrome. Tinel’s sign is positive if percussion over the median nerve at the carpal tunnel elicits pain or paresthesia.18
When employing the Phalen maneuver, be certain to have the patient flex his/her wrist to 90 degrees and to document the number of seconds it takes for numbness to present in the fingers. Pain or paresthesia should occur in <60 seconds for the test to be positive.18
Median nerve compression over the carpal tunnel, also known as Durkan’s test, may also elicit symptoms. With Durkan’s test, you apply direct pressure over the transverse carpal ligament. If pain or paresthesia occurs in <30 seconds, the test is positive.18 Often clinicians will combine the Phalen maneuver and Durkan’s test to increase sensitivity and specificity.18 Nerve conduction studies are often performed to confirm the clinical diagnosis.
Is more than one condition at play? It is important to determine whether cervical spine disease and/or peripheral neuropathy is contributing to the patient’s symptoms, along with CTS; patients may have more than one condition contributing to their pain. We routinely check cervical spine motion, tenderness, and nerve compression as part of the exam on a patient with suspected CTS. In the office, a monofilament test or 2-point discrimination test can help make the clinical diagnosis by uncovering decreased sensation in the thumb, index, and/or middle fingers.23
The 5.07 monofilament test is performed with the clinician applying the monofilament to different dermatomal or sensory distributions while the patient has his/her eyes closed. The 2-point discrimination test is performed with a caliper device that measures the distance at which the patient can feel 2 separate stimuli. Often electromyography or nerve conduction studies are necessary.18
Treatment: Pursue nonoperative approaches
A survey of the membership of the American Society for Surgery of the Hand revealed that surgeons are utilizing nonoperative treatments for a longer duration of time and are employing narrowed surgical indications.24 Thus, clinicians are more likely to try splints and steroid injections before proceeding to operative release.24
Nonsurgical management. In our practice, we commonly recommend corticosteroid injections (TABLE 12-4) into the carpal tunnel (FIGURES 4 and 5) to patients who are poor candidates for surgery (ie, those who have too many medical comorbidities or wound healing concerns). This is one indication for which you may want to consider ultrasound-guided injections because the improved accuracy may provide symptom relief faster than “blind” or palpation-guided injections.25

A recent randomized controlled trial from Sweden showed that injections of methylprednisolone relieved symptoms in patients with mild to moderate CTS at 10 weeks and reduced the rate of surgery one year after treatment; however, 3 out of 4 patients still went on to have surgery within a year.22 Patients in the study had failed a 2-month trial of splinting and were given either 80 mg or 40 mg of methylprednisolone or saline. There was no statistical difference between the doses of methylprednisolone in preventing surgery at one year. Compared to placebo, the 80-mg methylprednisolone group was less likely to have surgery with an odds ratio of 0.24 (P=.042).22
There is evidence that oral steroids, injected steroids, ultrasound, electromagnetic field therapy, nocturnal splinting, and use of ergonomic keyboards are effective nonoperative modalities in the short term, but evidence is sparse for mid- or long-term use.19 In addition, at least one randomized trial found traditional cupping therapy applied around the shoulder alleviated carpal tunnel symptoms in the short-term.26 Other nonoperative therapies include rest, NSAIDs, extracorporeal shock wave therapy, and activity modification.19,27
Surgical outcomes by either endoscopic, mini-open, or open surgical techniques are typically good.20,21 Surgical release involves cutting the transverse carpal ligament over the carpal tunnel to decompress the median nerve.24 You should inform patients of the risks and inconveniences associated with surgery, including the cost, absence from work, infection, and chronic pain. Patients who have recurrent or persistent symptoms after surgery may have had an incompletely released transverse carpal ligament or there may be no identifiable cause.21 Overall, surgical treatment, combined with physical therapy, seems to be more effective than splinting or NSAIDs for mid- and long-term treatment of CTS.28
De Quervain’s tenosynovitis: Common during pregnancy
De Quervain’s tenosynovitis (radial styloid tenosynovitis) involves painful inflammation of the 2 tendons in the first dorsal compartment of the wrist—the abductor pollicis longus (APL) and the extensor pollicis brevis (EPB). The tendons comprise the radial border of the anatomic snuffbox.
The APL abducts and extends the thumb at the CMC joint, while the EPB extends the thumb proximal phalanx at the metacarpophalangeal joint. These tendons are contained in a synovial sheath that is subject to inflammation and constriction and subsequent wear and damage.29 In addition, the extensor retinaculum in patients with de Quervain’s disease demonstrates increased vascularity and deposition of dense fibrous tissue resulting in thickening of the tendon up to 5 times its normal width.30
As a result, degeneration and thickening of the tendon sheath, as well as radial-sided wrist pain elicited at the first dorsal compartment, are common pathophysiologic and clinical findings.31 Pain is often accompanied by the build-up of protuberances and nodulations of the tendon sheath.
De Quervain’s disease commonly occurs during and after pregnancy.32 Other risk factors include racquet sports, golfing, wrist trauma, and other activities involving repetitive hand and wrist motions.33 Often, however, de Quervain’s is idiopathic.
Making the Dx: Perform a Finkelstein's test
The major finding in patients with de Quervain’s tenosynovitis is a positive Finkelstein's test. To perform Finkelstein's test (FIGURE 6), ask the patient to oppose the thumb into the palm and flex the fingers of the same hand over the thumb. Holding the patient’s fingers around the thumb, ulnarly deviate the wrist. Finkelstein's test puts strain on the APL and EPB, causing pain along the radial border of the wrist and forearm in patients with de Quervain’s tenosynovitis. Since the maneuver can be uncomfortable, complete the exam on the unaffected side for comparison.

Stenosis of the tendon sheath may lead to crepitus over the first dorsal wrist compartment. This should be distinguished from intersection syndrome (tenosynovitis at the intersection of the first and second extensor compartments), which can also present with forearm and wrist crepitus. Patients usually have swelling of the wrist with marked discomfort upon palpation of the radial tendons. An x-ray can be useful to evaluate for CMC or radiocarpal arthritis, which may be an underlying cause.
Treatment: Select an approach based on symptom severity
In a retrospective analysis, Lane et al concluded that classification of patients with de Quervain’s disease based on pretreatment symptoms may assist physicians in selecting the most efficacious treatment and in providing prognostic information to their patients (TABLE 334). Patients with mild to moderate (Types 1 and 2) de Quervain’s may benefit from immobilization in a thumb spica splint, rest, NSAIDs, and physical or occupational therapy. If work conditions played a role in causing the symptoms, they need to be addressed to improve outcomes. Types 2 and 3 can be initially treated with a corticosteroid injection, but may eventually require surgery.33

Treatment with NSAIDs or corticosteroid injections (see TABLE 12-4 for choices) in the first compartment of the extensor retinaculum (FIGURE 7) is usually adequate to provide relief. Peters-Veluthamaningal et al performed a systematic review in 2009 and found only one controlled trial of 18 participants (all pregnant or lactating women) who were either injected with corticosteroids or given a thumb spica splint.35 All 9 patients in the injection group had complete pain relief, whereas no one in the splint group had complete resolution of symptoms.35 Typical anatomic placement of corticosteroid injections is shown in FIGURE 7.

More complicated injection methods have been described, but injecting the first dorsal compartment is usually satisfactory. Patients will feel the tendon sheath filling with the injection material. The 2-point technique, implemented by Sawaizumi et al, which involves injecting corticosteroid into 2 points over the EPB and APL tendon in the area of maximum pain and soft tissue thickening, is more effective than the 1-point injection technique.36
Severe, recalcitrant cases. Professional and college athletes may be prone to recalcitrant de Quervain’s tenosynovitis. A 2010 study by Pagonis et al showed that recurrent symptomatic episodes commonly occur in athletes who engage in high-resistance, intense athletic training. In these severe cases, a 4-point injection technique offers better distribution of corticosteroid solution to the first extensor compartment than other methods.37 Consider referring severe cases to a hand surgeon.
Surgical release of the first dorsal compartmental sheath around the tendons serves as a final option for patients who fail conservative treatment. Care should be taken to release both tendons completely, as there may be at least 2 tendon slips of the APL or there may be a distinct EPB sheath dorsally.38
“Tennis elbow”— you don’t have to play tennis to have it
Lateral epicondylitis (tennis elbow) is a painful condition involving microtears within the extensor carpi radialis brevis muscle and the subsequent development of angiofibroblastic dysplasia.39 According to Regan et al who studied the histopathologic features of 11 patients with lateral epicondylitis, the underlying cause of recalcitrant lateral epicondylitis is, in fact, degenerative, rather than inflammatory.40
Although the condition has been nicknamed “tennis elbow,” only about 5% of tennis players have the condition.41 In tennis players, males are more often affected than females, whereas in the general population, incidence is approximately equal in men and women.41 Lateral epicondylitis occurs between 4 and 7 times more frequently than medial-sided elbow pain.42
Making the Dx: Look for localized pain, normal ROM
The diagnosis of lateral epicondylitis is based upon a history of pain over the lateral epicondyle and findings on physical examination, including local tenderness directly over the lateral epicondyle,43 pain aggravated by resisted wrist extension and radial deviation, pain with resisted middle finger extension, and decreased grip strength or pain aggravated by strong gripping. These findings typically occur in the presence of normal elbow range of motion.
Treatment: Choose from a range of options
Since lateral epicondylitis was first described, researchers have proposed a wide variety of treatments as initial interventions including rest, activity, equipment modification, NSAIDs, wrist bracing/elbow straps, and physical therapy. If initial treatment does not produce the desired effect, second-line treatments include corticosteroid injections (FIGURE 8), prolotherapy (injection of an irritant, often dextrose; see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768), autologous blood injections, platelet-rich plasma injections (see “Is platelet-rich plasma right for your patient?” J Fam Pract. 2016;65:319-328), and needling of the extensor tendon origin. Refer patients who do not improve after one corticosteroid injection to an orthopedic surgeon for consideration of open or arthroscopic treatment.

CORRESPONDENCE
Gregory R. Waryasz, MD, Rhode Island Hospital, Department of Orthopaedic Surgery, 593 Eddy St., Providence, RI 02903; [email protected].
1. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775.
2. Peters-Veluthamaningal C, Van der Windt DA, Winters JC, et al. Corticosteroid injection for trigger finger in adults. Cochrane Database Syst Rev. 2009:CD005617.
3. Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11:141-147.
4. Waryasz GR, Tambone R, Borenstein TR, et al. A review of anatomical placement of corticosteroid injections for uncommon hand, wrist, and elbow pathologies. R I Med J. 2017;100:31-34.
5. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1:396-404.
6. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743.
7. Lundin AC, Aspenberg P, Eliasson P. Trigger finger, tendinosis, and intratendinous gene expression. Scand J Med Sci Sports. 2014;24:363-368.
8. Sato ES, Gomes Dos Santos JB, Belloti JC, et al. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology (Oxford). 2012;51:93-99.
9. Baek GH, Kim JH, Chug MS, et al. The natural history of pediatric trigger thumb. J Bone Joint Surg Am. 2008;90:980-985.
10. Gillis J, Calder K, Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19:134-138.
11. Yao J, Park MJ. Early treatment of degenerative arthritis of the thumb carpometacarpal joint. Hand Clin. 2008;24:251-261.
12. Ladd AL, Weiss AP, Crisco JJ, et al. The thumb carpometacarpal joint: anatomy, hormones, and biomechanics. Instr Course Lect. 2013;62:165-179.
13. Fontana L, Neel S, Claise JM, et al. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: a case-control study. J Hand Surg Am. 2007;32:459-465.
14. Stamm TA, Machold KP, Smolen JS, et al. Joint protection and home hand exercises improve hand function in patients with hand osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2002;47:44-49.
15. Weiss S, LaStayo P, Mills A, et al. Prospective analysis of splinting the first carpometacarpal joint: an objective, subjective, and radiographic assessment. J Hand Ther. 2000;13:218-226.
16. Day CS, Gelberman R, Patel AA, et al. Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J Hand Surg Am. 2004;29:247-251.
17. Gelfman R, Melton LJ 3rd, Yawn BP, et al. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33-41.
18. Wipperman J, Potter L. Carpal tunnel syndrome-try these diagnostic maneuvers. J Fam Pract. 2012;61:726-732.
19. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
20. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43:431-437.
21. Soltani AM, Allan BJ, Best MJ, et al. A systematic review of the literature on the outcomes of treatment for recurrent and persistent carpal tunnel syndrome. Plast Reconstr Surg. 2013;132:114-121.
22. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
23. Raji P, Ansari NN, Naghdi S, et al. Relationship between Semmes-Weinstein Monofilaments perception test and sensory nerve conduction studies in carpal tunnel syndrome. NeuroRehabilitation. 2014;35:543-552.
24. Leinberry CF, Rivlin M, Maltenfort M, et al. Treatment of carpal tunnel syndrome by members of the American Society for Surgery of the Hand: a 25-year perspective. J Hand Surg Am. 2012;37:1997-2003.e3.
25. Ustün N, Tok F, Yagz AE, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: a single-blind randomized prospective study. Am J Phys Med Rehabil. 2013;92:999-1004.
26. Michalsen A, Bock S, Lüdtke R, et al. Effects of traditional cupping therapy in patients with carpal tunnel syndrome: a randomized controlled trial. J Pain. 2009;10:601-608.
27. Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil. 2013;92:327-334.
28. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
29. Shehab R, Mirabelli MH. Evaluation and diagnosis of wrist pain: a case-based approach. Am Fam Physician. 2013;87:568-573.
30. Clarke MT, Lyall HA, Grant JW, et al. The histopathology of de Quervain’s disease. J Hand Surg Br. 1998;23:732-734.
31. Zychowicz MA. A closer look at hand and wrist complaints. Nurse Pract. 2013;38:46-53.
32. Avci S, Yilmaz C, Sayli U. Comparison of nonsurgical treatment measures for de Quervain’s disease of pregnancy and lactation. J Hand Surg Am. 2002;27:322-324.
33. Mani L, Gerr F. Work-related upper extremity musculoskeletal disorders. Prim Care. 2000;27:845-864.
34. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
35. Peters-Veluthamaningal C, Van der Windt JC, Winters JC, et al. Corticosteroid injection for de Quervain’s tenosynovitis. Cochrane Database Syst Rev. 2009;8:CD005616.
36. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
37. Pagonis T, Ditsios K, Toli P, et al. Improved corticosteroid treatment of recalcitrant de Quervain tenosynovitis with a novel 4-point injection technique. Am J Sports Med. 2011;39:398-403.
38. Scheller A, Schuh R, Hönle W, et al. Long-term results of surgical release of de Quervain’s stenosing tenosynovitis. Int Orthop. 2009;33:1301-1303.
39. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832-839.
40. Regan W, Wold LE, Coonrad R, et al. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20:746-749.
41. Van Hofwegen C, Baker CL 3rd, Baker CL Jr. Epicondylitis in the athlete’s elbow. Clin Sports Med. 2010;29:577-597.
42. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6:259-272.
43. Weerakul S, Galassi M. Randomized controlled trial local injection for treatment of lateral epicondylitis, 5 and 10 mg triamcinolone compared. J Med Assoc Thai. 2012;95 Supp 10:S184-188.
1. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775.
2. Peters-Veluthamaningal C, Van der Windt DA, Winters JC, et al. Corticosteroid injection for trigger finger in adults. Cochrane Database Syst Rev. 2009:CD005617.
3. Cheng J, Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11:141-147.
4. Waryasz GR, Tambone R, Borenstein TR, et al. A review of anatomical placement of corticosteroid injections for uncommon hand, wrist, and elbow pathologies. R I Med J. 2017;100:31-34.
5. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1:396-404.
6. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743.
7. Lundin AC, Aspenberg P, Eliasson P. Trigger finger, tendinosis, and intratendinous gene expression. Scand J Med Sci Sports. 2014;24:363-368.
8. Sato ES, Gomes Dos Santos JB, Belloti JC, et al. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology (Oxford). 2012;51:93-99.
9. Baek GH, Kim JH, Chug MS, et al. The natural history of pediatric trigger thumb. J Bone Joint Surg Am. 2008;90:980-985.
10. Gillis J, Calder K, Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19:134-138.
11. Yao J, Park MJ. Early treatment of degenerative arthritis of the thumb carpometacarpal joint. Hand Clin. 2008;24:251-261.
12. Ladd AL, Weiss AP, Crisco JJ, et al. The thumb carpometacarpal joint: anatomy, hormones, and biomechanics. Instr Course Lect. 2013;62:165-179.
13. Fontana L, Neel S, Claise JM, et al. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: a case-control study. J Hand Surg Am. 2007;32:459-465.
14. Stamm TA, Machold KP, Smolen JS, et al. Joint protection and home hand exercises improve hand function in patients with hand osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2002;47:44-49.
15. Weiss S, LaStayo P, Mills A, et al. Prospective analysis of splinting the first carpometacarpal joint: an objective, subjective, and radiographic assessment. J Hand Ther. 2000;13:218-226.
16. Day CS, Gelberman R, Patel AA, et al. Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J Hand Surg Am. 2004;29:247-251.
17. Gelfman R, Melton LJ 3rd, Yawn BP, et al. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33-41.
18. Wipperman J, Potter L. Carpal tunnel syndrome-try these diagnostic maneuvers. J Fam Pract. 2012;61:726-732.
19. Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981-1004.
20. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43:431-437.
21. Soltani AM, Allan BJ, Best MJ, et al. A systematic review of the literature on the outcomes of treatment for recurrent and persistent carpal tunnel syndrome. Plast Reconstr Surg. 2013;132:114-121.
22. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
23. Raji P, Ansari NN, Naghdi S, et al. Relationship between Semmes-Weinstein Monofilaments perception test and sensory nerve conduction studies in carpal tunnel syndrome. NeuroRehabilitation. 2014;35:543-552.
24. Leinberry CF, Rivlin M, Maltenfort M, et al. Treatment of carpal tunnel syndrome by members of the American Society for Surgery of the Hand: a 25-year perspective. J Hand Surg Am. 2012;37:1997-2003.e3.
25. Ustün N, Tok F, Yagz AE, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: a single-blind randomized prospective study. Am J Phys Med Rehabil. 2013;92:999-1004.
26. Michalsen A, Bock S, Lüdtke R, et al. Effects of traditional cupping therapy in patients with carpal tunnel syndrome: a randomized controlled trial. J Pain. 2009;10:601-608.
27. Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil. 2013;92:327-334.
28. Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024.
29. Shehab R, Mirabelli MH. Evaluation and diagnosis of wrist pain: a case-based approach. Am Fam Physician. 2013;87:568-573.
30. Clarke MT, Lyall HA, Grant JW, et al. The histopathology of de Quervain’s disease. J Hand Surg Br. 1998;23:732-734.
31. Zychowicz MA. A closer look at hand and wrist complaints. Nurse Pract. 2013;38:46-53.
32. Avci S, Yilmaz C, Sayli U. Comparison of nonsurgical treatment measures for de Quervain’s disease of pregnancy and lactation. J Hand Surg Am. 2002;27:322-324.
33. Mani L, Gerr F. Work-related upper extremity musculoskeletal disorders. Prim Care. 2000;27:845-864.
34. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
35. Peters-Veluthamaningal C, Van der Windt JC, Winters JC, et al. Corticosteroid injection for de Quervain’s tenosynovitis. Cochrane Database Syst Rev. 2009;8:CD005616.
36. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
37. Pagonis T, Ditsios K, Toli P, et al. Improved corticosteroid treatment of recalcitrant de Quervain tenosynovitis with a novel 4-point injection technique. Am J Sports Med. 2011;39:398-403.
38. Scheller A, Schuh R, Hönle W, et al. Long-term results of surgical release of de Quervain’s stenosing tenosynovitis. Int Orthop. 2009;33:1301-1303.
39. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832-839.
40. Regan W, Wold LE, Coonrad R, et al. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20:746-749.
41. Van Hofwegen C, Baker CL 3rd, Baker CL Jr. Epicondylitis in the athlete’s elbow. Clin Sports Med. 2010;29:577-597.
42. Leach RE, Miller JK. Lateral and medial epicondylitis of the elbow. Clin Sports Med. 1987;6:259-272.
43. Weerakul S, Galassi M. Randomized controlled trial local injection for treatment of lateral epicondylitis, 5 and 10 mg triamcinolone compared. J Med Assoc Thai. 2012;95 Supp 10:S184-188.
PRACTICE RECOMMENDATIONS
› Diagnose common upper extremity conditions based on anatomic relationships. B
› Refer patients who do not respond to splinting, corticosteroid injections, or other conservative therapies to a surgeon for evaluation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Thalassemia case provides insight into history of malaria
The earliest documented case of β-thalassemia in Sardinia suggests malaria was widespread on the island long before the Middle Ages, according to researchers.
The team noted that Sardinia has one of the highest incidence rates of β-thalassemia in Europe due to its long history of endemic malaria.
However, it has been assumed that malaria was only endemic on the island since the Middle Ages (500-1500 CE).
New research, published in the American Journal of Physical Anthropology, suggests malaria was probably already endemic on Sardinia during the Roman period.
Since ancient DNA of malaria is difficult to extract, the researchers studied thalassemia and other genetic adaptations in its place.
The team studied a thalassemia allele called cod39 β-thalassemia, which is dominant on Sardinia. They were able to confirm the presence of the cod39 allele in the 2000-year-old (approximately 300 BCE to 100 CE) remains of a Roman man.
“This is the very first documented case of the genetic adaptation to malaria on Sardinia,” said study author Claudia Vigano, of the Institute for Evolutionary Medicine of the University of Zurich in Switzerland.
“We also discovered that the person was genetically a Sardinian in all probability and not an immigrant from another area.”
“Our study shows the importance of a multidisciplinary approach to history,” said Abigail Bouwman, also of the Institute for Evolutionary Medicine of the University of Zurich.
“We are researching the evolution of today’s diseases, such as malaria, to explain why the human body becomes sick at all and how adaptations occur.” ![]()
The earliest documented case of β-thalassemia in Sardinia suggests malaria was widespread on the island long before the Middle Ages, according to researchers.
The team noted that Sardinia has one of the highest incidence rates of β-thalassemia in Europe due to its long history of endemic malaria.
However, it has been assumed that malaria was only endemic on the island since the Middle Ages (500-1500 CE).
New research, published in the American Journal of Physical Anthropology, suggests malaria was probably already endemic on Sardinia during the Roman period.
Since ancient DNA of malaria is difficult to extract, the researchers studied thalassemia and other genetic adaptations in its place.
The team studied a thalassemia allele called cod39 β-thalassemia, which is dominant on Sardinia. They were able to confirm the presence of the cod39 allele in the 2000-year-old (approximately 300 BCE to 100 CE) remains of a Roman man.
“This is the very first documented case of the genetic adaptation to malaria on Sardinia,” said study author Claudia Vigano, of the Institute for Evolutionary Medicine of the University of Zurich in Switzerland.
“We also discovered that the person was genetically a Sardinian in all probability and not an immigrant from another area.”
“Our study shows the importance of a multidisciplinary approach to history,” said Abigail Bouwman, also of the Institute for Evolutionary Medicine of the University of Zurich.
“We are researching the evolution of today’s diseases, such as malaria, to explain why the human body becomes sick at all and how adaptations occur.” ![]()
The earliest documented case of β-thalassemia in Sardinia suggests malaria was widespread on the island long before the Middle Ages, according to researchers.
The team noted that Sardinia has one of the highest incidence rates of β-thalassemia in Europe due to its long history of endemic malaria.
However, it has been assumed that malaria was only endemic on the island since the Middle Ages (500-1500 CE).
New research, published in the American Journal of Physical Anthropology, suggests malaria was probably already endemic on Sardinia during the Roman period.
Since ancient DNA of malaria is difficult to extract, the researchers studied thalassemia and other genetic adaptations in its place.
The team studied a thalassemia allele called cod39 β-thalassemia, which is dominant on Sardinia. They were able to confirm the presence of the cod39 allele in the 2000-year-old (approximately 300 BCE to 100 CE) remains of a Roman man.
“This is the very first documented case of the genetic adaptation to malaria on Sardinia,” said study author Claudia Vigano, of the Institute for Evolutionary Medicine of the University of Zurich in Switzerland.
“We also discovered that the person was genetically a Sardinian in all probability and not an immigrant from another area.”
“Our study shows the importance of a multidisciplinary approach to history,” said Abigail Bouwman, also of the Institute for Evolutionary Medicine of the University of Zurich.
“We are researching the evolution of today’s diseases, such as malaria, to explain why the human body becomes sick at all and how adaptations occur.” ![]()
ASCO updates guidelines on antiemetic use in cancer patients
The American Society of Clinical Oncology (ASCO) has updated its clinical practice guidelines on the use of antiemetics in cancer patients.
The update, published in the Journal of Clinical Oncology, provides new evidence-based information on the appropriate use of olanzapine, NK1 receptor antagonists, and dexamethasone.
“The adverse impact of inadequately controlled nausea and vomiting on patients’ quality of life is well documented,” said Paul J. Hesketh, MD, co-chair of the ASCO expert panel that updated the guidelines.
“By following the ASCO antiemetics guideline, clinicians have the opportunity to improve patients’ quality of life by minimizing treatment-induced emesis.”
To update ASCO’s guidelines on antiemetics, the expert panel conducted a systematic review of the medical literature published between November 2009 and June 2016. The panel included members with expertise in medical oncology, radiation oncology, nursing, pharmacy, and health services research, as well as a patient representative.
“Tremendous progress has been realized over the last 25 years in the prevention of chemotherapy-induced nausea and vomiting with the introduction of new classes of antiemetic agents,” said Mark G. Kris, MD, co-chair of the expert panel that updated the guidelines.
“The full benefit of these treatment advances will only be realized, however, if evidence-based guidelines are fully implemented.”
Key recommendations in the updated guidelines include:
For adults receiving chemotherapy with a high risk for nausea and vomiting (eg, cisplatin or the combination of cyclophosphamide and an anthracycline), olanzapine should be added to standard antiemetic regimens (the combination of a 5-HT3 receptor antagonist, an NK1 receptor antagonist, and dexamethasone). Olanzapine also helps individuals who experience symptoms despite receiving medicines to prevent vomiting before chemotherapy is given.
For adults receiving carboplatin-based chemotherapy or high-dose chemotherapy and children receiving chemotherapy with a high risk for nausea and vomiting, an NK1 receptor antagonist should be added to the standard antiemetic regimen (the combination of 5-HT3 receptor antagonist and dexamethasone).
Dexamethasone treatment can be limited to the day of chemotherapy administration in patients receiving an anthracycline and cyclophosphamide.
Dronabinol and nabilone, cannabinoids approved by the US Food and Drug Administration, can be used to treat nausea and vomiting that is resistant to standard antiemetic therapies. Evidence remains insufficient to recommend medical marijuana for either prevention or treatment of nausea and vomiting in patients with cancer receiving chemotherapy or radiation therapy. ![]()
The American Society of Clinical Oncology (ASCO) has updated its clinical practice guidelines on the use of antiemetics in cancer patients.
The update, published in the Journal of Clinical Oncology, provides new evidence-based information on the appropriate use of olanzapine, NK1 receptor antagonists, and dexamethasone.
“The adverse impact of inadequately controlled nausea and vomiting on patients’ quality of life is well documented,” said Paul J. Hesketh, MD, co-chair of the ASCO expert panel that updated the guidelines.
“By following the ASCO antiemetics guideline, clinicians have the opportunity to improve patients’ quality of life by minimizing treatment-induced emesis.”
To update ASCO’s guidelines on antiemetics, the expert panel conducted a systematic review of the medical literature published between November 2009 and June 2016. The panel included members with expertise in medical oncology, radiation oncology, nursing, pharmacy, and health services research, as well as a patient representative.
“Tremendous progress has been realized over the last 25 years in the prevention of chemotherapy-induced nausea and vomiting with the introduction of new classes of antiemetic agents,” said Mark G. Kris, MD, co-chair of the expert panel that updated the guidelines.
“The full benefit of these treatment advances will only be realized, however, if evidence-based guidelines are fully implemented.”
Key recommendations in the updated guidelines include:
For adults receiving chemotherapy with a high risk for nausea and vomiting (eg, cisplatin or the combination of cyclophosphamide and an anthracycline), olanzapine should be added to standard antiemetic regimens (the combination of a 5-HT3 receptor antagonist, an NK1 receptor antagonist, and dexamethasone). Olanzapine also helps individuals who experience symptoms despite receiving medicines to prevent vomiting before chemotherapy is given.
For adults receiving carboplatin-based chemotherapy or high-dose chemotherapy and children receiving chemotherapy with a high risk for nausea and vomiting, an NK1 receptor antagonist should be added to the standard antiemetic regimen (the combination of 5-HT3 receptor antagonist and dexamethasone).
Dexamethasone treatment can be limited to the day of chemotherapy administration in patients receiving an anthracycline and cyclophosphamide.
Dronabinol and nabilone, cannabinoids approved by the US Food and Drug Administration, can be used to treat nausea and vomiting that is resistant to standard antiemetic therapies. Evidence remains insufficient to recommend medical marijuana for either prevention or treatment of nausea and vomiting in patients with cancer receiving chemotherapy or radiation therapy. ![]()
The American Society of Clinical Oncology (ASCO) has updated its clinical practice guidelines on the use of antiemetics in cancer patients.
The update, published in the Journal of Clinical Oncology, provides new evidence-based information on the appropriate use of olanzapine, NK1 receptor antagonists, and dexamethasone.
“The adverse impact of inadequately controlled nausea and vomiting on patients’ quality of life is well documented,” said Paul J. Hesketh, MD, co-chair of the ASCO expert panel that updated the guidelines.
“By following the ASCO antiemetics guideline, clinicians have the opportunity to improve patients’ quality of life by minimizing treatment-induced emesis.”
To update ASCO’s guidelines on antiemetics, the expert panel conducted a systematic review of the medical literature published between November 2009 and June 2016. The panel included members with expertise in medical oncology, radiation oncology, nursing, pharmacy, and health services research, as well as a patient representative.
“Tremendous progress has been realized over the last 25 years in the prevention of chemotherapy-induced nausea and vomiting with the introduction of new classes of antiemetic agents,” said Mark G. Kris, MD, co-chair of the expert panel that updated the guidelines.
“The full benefit of these treatment advances will only be realized, however, if evidence-based guidelines are fully implemented.”
Key recommendations in the updated guidelines include:
For adults receiving chemotherapy with a high risk for nausea and vomiting (eg, cisplatin or the combination of cyclophosphamide and an anthracycline), olanzapine should be added to standard antiemetic regimens (the combination of a 5-HT3 receptor antagonist, an NK1 receptor antagonist, and dexamethasone). Olanzapine also helps individuals who experience symptoms despite receiving medicines to prevent vomiting before chemotherapy is given.
For adults receiving carboplatin-based chemotherapy or high-dose chemotherapy and children receiving chemotherapy with a high risk for nausea and vomiting, an NK1 receptor antagonist should be added to the standard antiemetic regimen (the combination of 5-HT3 receptor antagonist and dexamethasone).
Dexamethasone treatment can be limited to the day of chemotherapy administration in patients receiving an anthracycline and cyclophosphamide.
Dronabinol and nabilone, cannabinoids approved by the US Food and Drug Administration, can be used to treat nausea and vomiting that is resistant to standard antiemetic therapies. Evidence remains insufficient to recommend medical marijuana for either prevention or treatment of nausea and vomiting in patients with cancer receiving chemotherapy or radiation therapy. ![]()
Children with noncomplex chronic diseases use one-third of annual Medicaid pediatric spending
, accounting for a third of Medicaid pediatric expenditures, according to a retrospective, cross-sectional analysis.
Generalizing to the 35 million children on Medicaid nationally, the NC-CD population accounts for $35 billion in annual Medicaid spending (Pediatrics. 2017. doi: 10.1542/peds.2017-0492).
“An improved understanding of children with NC-CDs and their associated health care expenditures is needed to improve health care delivery for this population and may provide opportunities for health policy interventions to reduce costs of care,” they said.
The per member per year (PMPY) expenditures for children with NC-CDs was significantly less than that of children with complex chronic disease (C-CDs), but the annual aggregate expenditure for the NC-CD group represents a substantial cost because of the high prevalence of these conditions. The annual expenditures for the entire group was $7,226,354,620 over the study period, or $3,037 PMPY. The total PMPY expenditure for children with NC-CDs ($2,801) was significantly greater than children without chronic disease ($1,151) and lower than children with C-CDs ($12,569).
Children with NC-CDs accounted for 36% of the study population and 33% of the annualized aggregate expenditure. Children without chronic disease accounted for 53% of the study population and 20% of annualized aggregate expenditure. Children with C-CDs accounted for 11% of the study population and 47% of the annualized aggregate expenditure.
, accounting for a third of Medicaid pediatric expenditures, according to a retrospective, cross-sectional analysis.
Generalizing to the 35 million children on Medicaid nationally, the NC-CD population accounts for $35 billion in annual Medicaid spending (Pediatrics. 2017. doi: 10.1542/peds.2017-0492).
“An improved understanding of children with NC-CDs and their associated health care expenditures is needed to improve health care delivery for this population and may provide opportunities for health policy interventions to reduce costs of care,” they said.
The per member per year (PMPY) expenditures for children with NC-CDs was significantly less than that of children with complex chronic disease (C-CDs), but the annual aggregate expenditure for the NC-CD group represents a substantial cost because of the high prevalence of these conditions. The annual expenditures for the entire group was $7,226,354,620 over the study period, or $3,037 PMPY. The total PMPY expenditure for children with NC-CDs ($2,801) was significantly greater than children without chronic disease ($1,151) and lower than children with C-CDs ($12,569).
Children with NC-CDs accounted for 36% of the study population and 33% of the annualized aggregate expenditure. Children without chronic disease accounted for 53% of the study population and 20% of annualized aggregate expenditure. Children with C-CDs accounted for 11% of the study population and 47% of the annualized aggregate expenditure.
, accounting for a third of Medicaid pediatric expenditures, according to a retrospective, cross-sectional analysis.
Generalizing to the 35 million children on Medicaid nationally, the NC-CD population accounts for $35 billion in annual Medicaid spending (Pediatrics. 2017. doi: 10.1542/peds.2017-0492).
“An improved understanding of children with NC-CDs and their associated health care expenditures is needed to improve health care delivery for this population and may provide opportunities for health policy interventions to reduce costs of care,” they said.
The per member per year (PMPY) expenditures for children with NC-CDs was significantly less than that of children with complex chronic disease (C-CDs), but the annual aggregate expenditure for the NC-CD group represents a substantial cost because of the high prevalence of these conditions. The annual expenditures for the entire group was $7,226,354,620 over the study period, or $3,037 PMPY. The total PMPY expenditure for children with NC-CDs ($2,801) was significantly greater than children without chronic disease ($1,151) and lower than children with C-CDs ($12,569).
Children with NC-CDs accounted for 36% of the study population and 33% of the annualized aggregate expenditure. Children without chronic disease accounted for 53% of the study population and 20% of annualized aggregate expenditure. Children with C-CDs accounted for 11% of the study population and 47% of the annualized aggregate expenditure.
FROM PEDIATRICS
Diagnosing and Classifying Anemia in Adult Primary Care
CE/CME No: CR-1708
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the importance of diagnosing the type of anemia in order to provide appropriate treatment.
• Describe how the complete blood count and its indices are used to initially determine if an anemia is microcytic, normocytic, or macrocytic.
• List the more common causes of microcytic, normocytic, and macrocytic anemia.
• Discuss addictional laboratory tests that may be used to further assess the cause of anemia.
FACULTY
Jean O’Neil is an Assistant Professor and Coordinator of the Adult Gerontology Acute Care Nurse Practitioner Program in the Patricia A. Chin School of Nursing at California State University, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2018.
Article begins on next page >>
Anemia affects more than 3 million people in the United States, making it a common problem in primary care practices. Once anemia is detected, clinicians must define the type and identify its underlying cause prior to initiating treatment. In most cases, the cause can be determined using information from the patient history, physical exam, and complete blood count.
Anemia is commonly identified during routine physical exams and laboratory testing.1-3 However, treating anemia can present a challenge for the primary care provider if the immediate cause is not apparent. Iron deficiency is a leading cause of anemia, but simply prescribing an iron supplement without determining the type or the cause of the anemia is not appropriate. Anemia that is misdiagnosed or goes untreated can be associated with a worse prognosis, as well as increased health care costs.4
Primary care providers often manage patients with common types of anemia and refer patients with severe or complex anemia to specialists for further testing and treatment. The most commonly used and cost-effective diagnostic tool for anemia is the complete blood count (CBC).2-6 The CBC provides details that can help the provider determine the type of anemia present, which in turn guides proper diagnostic testing and treatment.
EPIDEMIOLOGY
Anemia involves a reduction in the number of circulating red blood cells, the blood hemoglobin content, or the hematocrit, which leads to impaired delivery of oxygen to the body. Anemia affects more than 2 billion people worldwide, with iron deficiency the most common cause.7 Other leading nutritional causes of anemia include vitamin B12 and folate deficiency.4,7 Approximately 3 to 4 million Americans have anemia in some form, and it affects about 6.6% of men and 12.4% of women.5,8 The prevalence of anemia increases with age. Approximately 11% of men and 10% of women ages 65 or older have anemia, and in men ages 85 or older, prevalence of 20% to 44% has been reported.1,4 Anemia is present in about 3.5% of patients with chronic disease, but only 15% of them receive treatment.4

PATHOPHYSIOLOGY
Blood is composed of water-based plasma (54%), white blood cells and platelets (1%), and red blood cells (45%).5 Hemoglobin, the primary protein of the red blood cell, binds oxygen from the lungs and transports it to the rest of the body. Oxygen is then exchanged for carbon dioxide, which is carried back to the lungs to be exhaled.
Hemoglobin is made up of four globin chains, each containing an iron ion held in a porphyrin ring known as a heme group.5 When the body detects low tissue oxygen, the endothelial cells in the kidneys secrete the hormone erythropoietin (EPO), which stimulates the bone marrow to increase red cell production.5 This feedback loop can be interrupted by renal failure or chronic disease.4 In addition, bone marrow cannot produce enough red blood cells if there are insufficient levels of iron, amino acids, protein, carbohydrates, lipids, folate, and vitamin B12.5 Toxins (eg, lead), some types of cancer (eg, lymphoma), or even common infections (eg, pneumonia) can suppress the bone marrow, causing anemia. The more severe the anemia, the more likely oxygen transport will be compromised and organ failure will ensue.
Mutations affecting the genes that encode the globin chains within hemoglobin can cause one of the more than 600 known hemoglobinopathies (genetic defects of hemoglobin structure), such as sickle cell disease and thalassemias.5,9 While it is important to identify and treat patients with hemoglobinopathies, most anemias have other causes, such as iron deficiency, chronic disease, bone marrow defects, B12 deficiency, renal failure, medications, alcoholism, pregnancy, nutritional intake problems, gastrointestinal malabsorption, and active or recent history of blood loss.5,10
CLINICAL PRESENTATION
There are several signs and symptoms that should lead the primary care provider to suspect anemia (see Table 1).5,6 The severity of these symptoms can vary from mild to very serious. Severe anemia can lead to organ failure and death. However, most patients with anemia are asymptomatic, and anemia is typically detected incidentally during laboratory testing.1,2
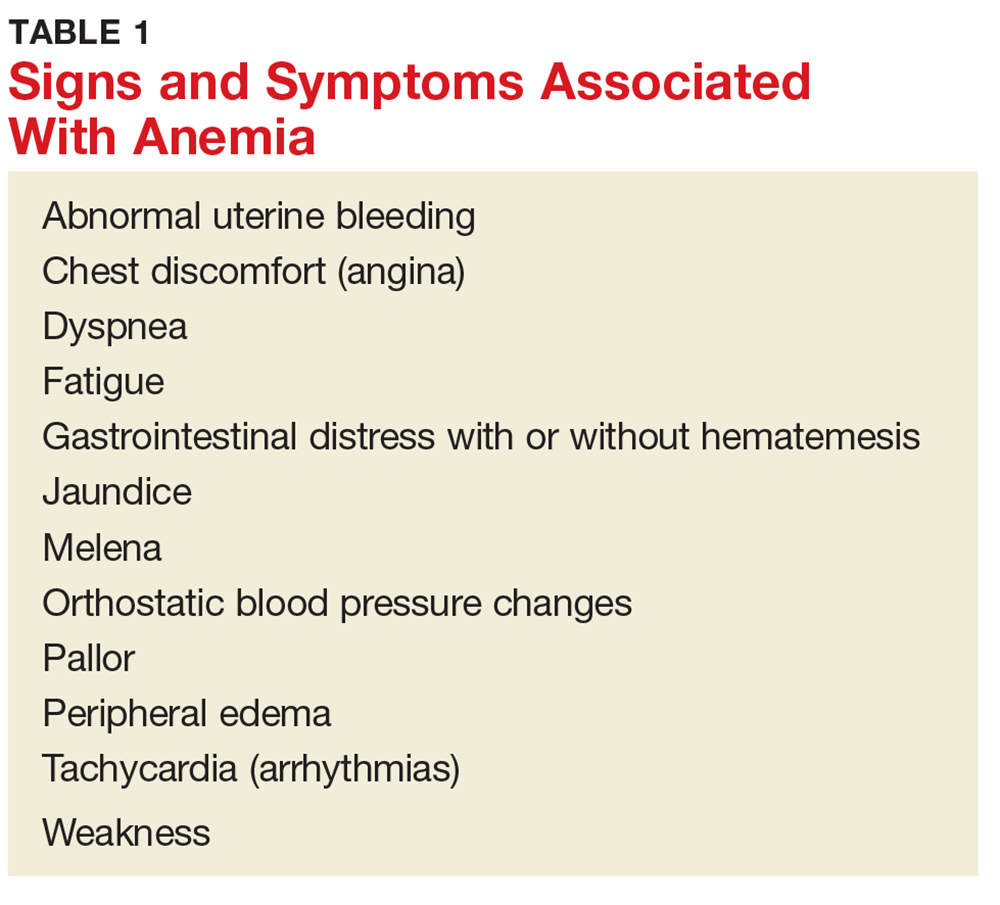
Once anemia is confirmed, the evaluation focuses on diagnosing its underlying cause. It should include a thorough patient history and review of systems to ascertain whether the patient has symptoms such as increased fatigue, palpitations, gastrointestinal distress, weakness, or dizziness.
If the provider has access to past CBC results, a comparison of the current and previous results will help determine whether the anemia is acute or chronic. Anemia caused by acute conditions, such as a suspicion of blood loss or bone marrow suppression, must be attended to immediately. A patient with chronic anemia should be carefully monitored and may need follow-up for ongoing treatment. While a provider has more time to work up a patient with chronic anemia, the causes may not be as straightforward.
DIAGNOSIS AND CLASSIFICATION
Anemia in adults is defined as hemoglobin less than 13 g/dL in males and 12 g/dL in females.6 The hemoglobin is part of the complete blood cell report, which also includes the white blood cell count (WBC), red blood cell count (RBC), hematocrit, platelet count, and indices.
When investigating the underlying cause of anemia, the most useful parts of the CBC are the hemoglobin and the mean corpuscular volume (MCV; see Table 2).6,10 The MCV is the average volume of red cells in a specimen. This parameter is used to classify the anemia as microcytic (MCV < 80 fL), normocytic (MCV 80-100 fL), or macrocytic (MCV > 100 fL), which helps to narrow the differential diagnosis and guide any further testing (see Figure).5,6,10
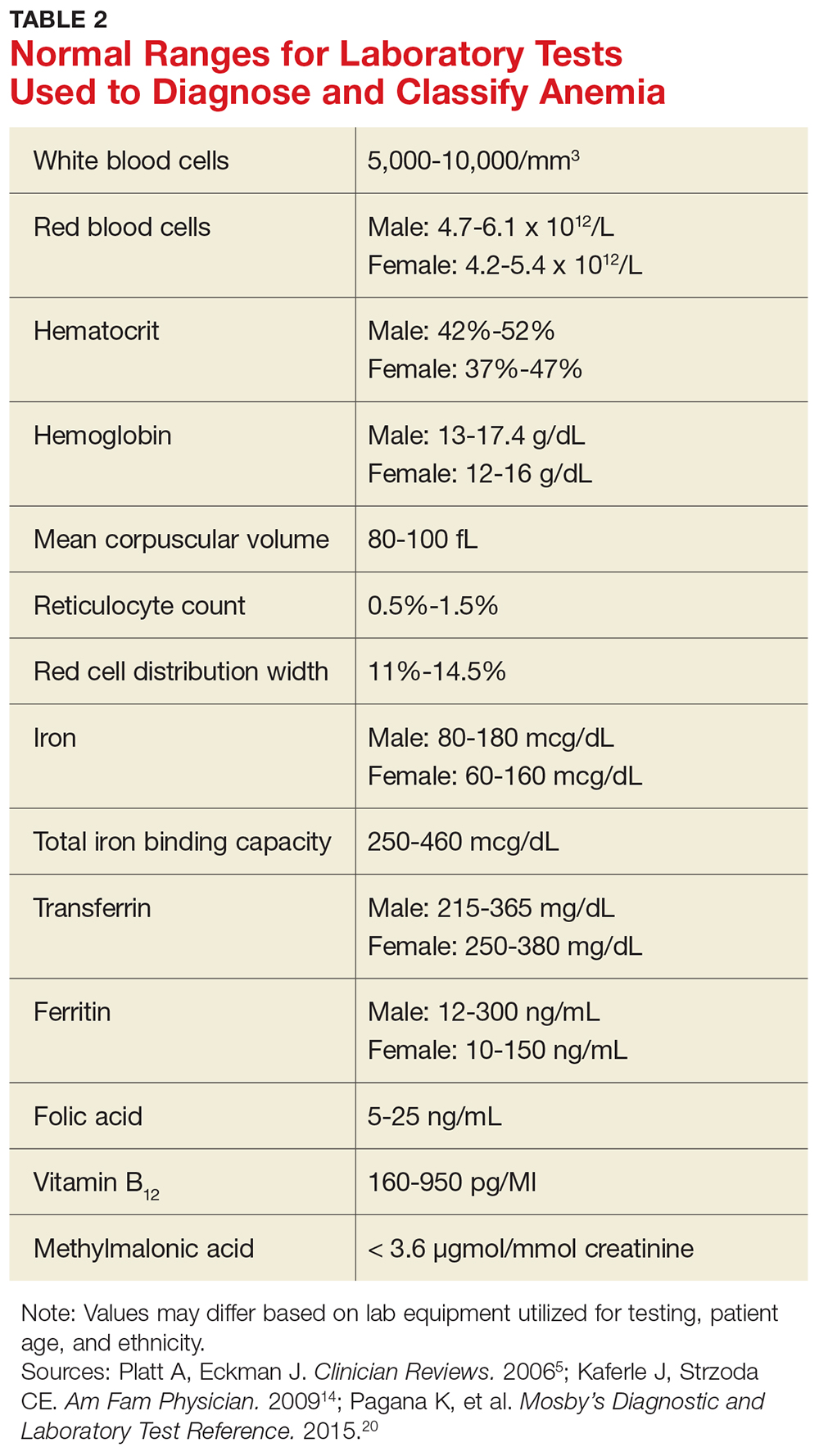
It is important to note that the normal ranges of the CBC parameters differ based on race, with persons of African ancestry having lower normal hemoglobin levels than persons of Caucasian ancestry.10 In addition, laboratories may have slightly different normal values for the CBC based on the equipment they utilize. Therefore, providers must follow their laboratory’s parameters, as well as adjust for the patient’s gender, age, and ethnicity.10
Microcytic Anemia
Iron deficiency
In microcytic anemia, the RBCs are smaller than average (MCV < 80 fL), as well as hypochromic due to lack of hemoglobin.9 Iron deficiency is the most common cause of microcytic anemia worldwide.11,12 Therefore, when a patient has microcytic anemia, a serum ferritin needs to be ordered. Further testing of total iron-binding capacity (TIBC), transferrin saturation, serum iron, and serum receptor levels may be helpful if the ferritin level is between 46-99 ng/mL and anemia due to iron deficiency is not confirmed (see Table 2).12
In iron deficiency anemia, serum ferritin and serum iron levels are low due to lack of iron, but serum TIBC is high.6 The elevated TIBC reflects increased synthesis of transferrin by the liver as it attempts to compensate for the patient’s low serum iron level.9 Since iron levels are controlled by absorption rather than excretion, iron is essentially only depleted from the body through blood loss.12 Therefore, an adult patient who is iron deficient has lost more iron through blood loss than was replaced through nutritional intake and gastrointestinal absorption. In children, increased growth-related iron requirements combined with poor nutritional intake of iron-rich foods is an additional mechanism for iron deficiency.11
Iron def
If the nutritional problem is corrected or the source of bleeding is controlled, treatment with oral or intravenous iron supplements should result in improved serum hemoglobin and reticulocyte counts.13 In the primary care setting, ferrous sulfate 325 mg, which provides 65 mg of elemental iron per tablet, orally three times daily is recommended for adults.13 This gives the patient the recommended dose of approximately 200 mg of elemental iron. Repeat hemoglobin and iron studies should be conducted again in three to six months.12,13
If the patient’s iron deficiency anemia does not improve after oral iron therapy, there may be a source of blood loss the provider missed or a problem with malabsorption of iron, which can be seen in those who have undergone gastric bypass surgery or who have inflammatory bowel disease.13 Such patients should be referred to a specialist, such as a gastroenterologist, for further evaluation.
Thalassemia
Microcytic anemia with normal or elevated serum iron and normal-to-increased serum ferritin can be seen in patients with a type of thalassemia (see Figure).2 Thalassemias are inherited blood disorders that reduce hemoglobin production, leading to microcytosis; they are more common in those of Mediterranean, African, and Southeast Asian descent.2 Red cells in patients with a form of thalassemia are usually very small (microcytic) and have normal or elevated red cell distribution width (RDW).10
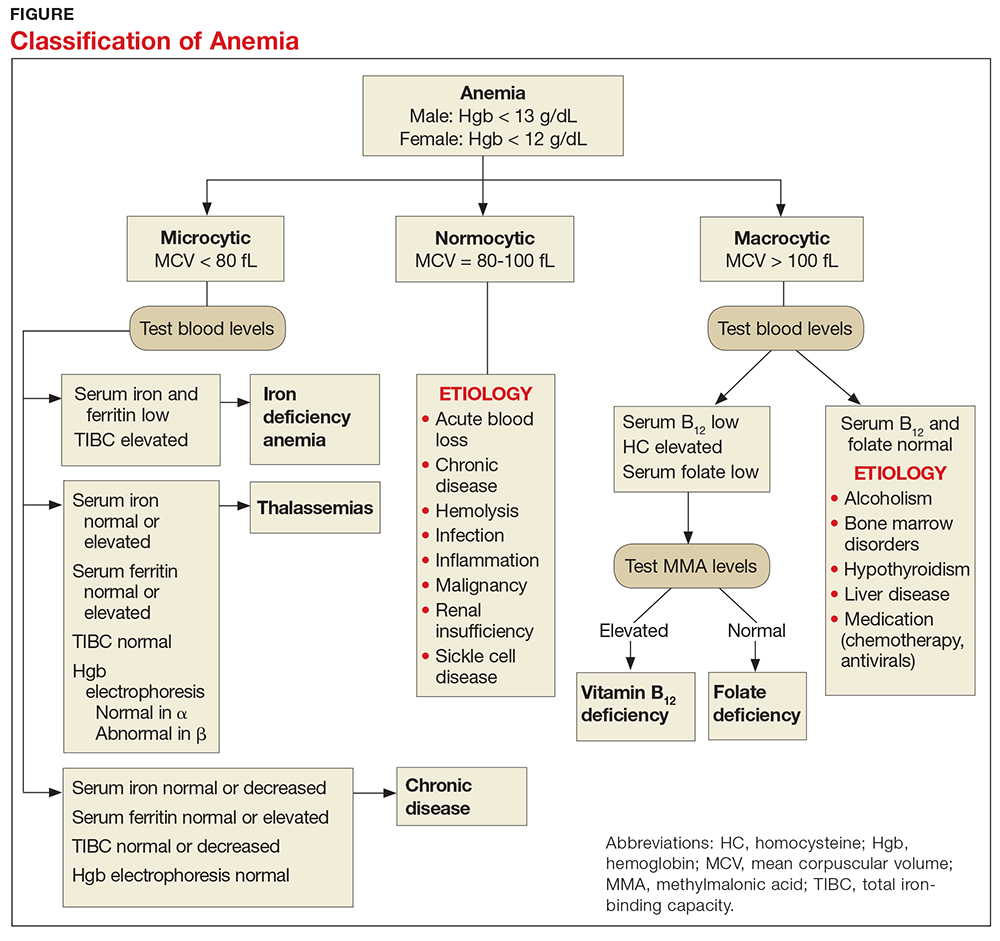
Moderate and severe forms of thalassemia can cause anemia. However, thalassemia syndromes that can cause severe (transfusion-dependent) anemia are usually diagnosed in childhood.9 Patients with one of the minor forms of thalassemia typically need minimal to no treatment.5 A patient with significant anemia suspicious for thalassemia should undergo hemoglobin electrophoresis testing to confirm the diagnosis and to determine the type of thalassemia.2 Typically, hemoglobin electrophoresis is normal in α thalassemia and is abnormal in ß thalassemia, as well as other forms of thalassemia. Referral to a hematologist for interpretation of these results and for further evaluation is appropriate.10
Chronic disease
If the patient has microcytic anemia and is not iron deficient or does not have thalassemia, then anemia related to a chronic disease should be considered.5 In such cases, the provider should order a reticulocyte count, which reveals how the bone marrow is responding to the anemia.5 Reticulocytes are immature red cells that have just been released from the bone marrow into the blood stream. The bone marrow increases the release of these cells in response to anemia.6
Any condition that stimulates reticulocyte production or prevents the bone marrow from producing reticulocytes will result in abnormal values (see Table 3). A normal reticulocyte count, expressed as the reticulocyte production index, is between 0.5% and 1.5%.5 The reticulocyte count is low in iron deficiency anemia and diseases that lead to decreased bone marrow production.5,6 Bone marrow suppression can occur in the context of chronic disease, infection, or inflammation. Malignancies are a less common cause for chronic disease microcytic anemia.6

If the cause of the decreased reticulocyte count is iron deficiency anemia, then treatment with iron supplementation should result in an increased reticulocyte count within one week.13 The primary care provider works in conjunction with the specialist to monitor the patient’s anemia when it is due to chronic disease or malignancy.
MACROCYTIC ANEMIA
In macrocytic anemia, the RBCs are larger than normal (MCV > 100 fL). This form of anemia is usually caused by vitamin B12 and folate deficiency, but it can also result from alcoholism, certain medications (eg, chemotherapy, antivirals), bone marrow disorders (eg, leukemia), and liver disease (eg, cirrhosis; see Figure).5,14 Common medications that can cause macrocytosis include the antiseizure drug phenytoin, the antibiotics trimethoprim/sulfamethoxazole and nitrofurantoin, the disease-modifying antirheumatic drug sulfasalazine, and immunosuppressants such as azathioprine.14,15 Antiviral agents, such as reverse transcriptase inhibitors (eg, zidovudine) used to treat HIV infection, can also cause macrocytosis with or without anemia.6,14
Macrocytic anemias caused by low serum levels of B12 and folate usually reflect problems with gastrointestinal malabsorption. For example, gastric bypass or Crohn disease can lead to malabsorption of vitamin B12 and increase a patient’s risk for macrocytic anemia.13
Vitamin B12 deficiency occurs in patients with pernicious anemia because they are missing intrinsic factor, which is necessary to facilitate B12 absorption in the ileum.10 Low vitamin B12 and folate levels also can result from inadequate dietary intake, although this is rare in the United States due to mandatory fortification of certain foods. A diet low in fresh vegetables is the leading cause of folate deficiency. While folate deficiency related to poor nutritional intake can be seen in all age groups, vitamin B12 deficiency more frequently affects the elderly or persons following a strict vegan diet.14
In addition to the fatigue and pallor associated with macrocytic anemia, patients with vitamin B12 deficiency may also have a smooth tongue, peripheral neuropathy, and edema.5,14 Severe vitamin B12 deficiency can lead to subacute combined degeneration of the spinal cord, with demyelination of the dorsal and lateral columns most often occurring in the cervical and thoracic regions.16,17 This spinal cord degeneration can cause paresthesia, muscle spasticity, and ataxia.16
When there is a macrocytic anemia, but the B12 or folate level is only borderline low, additional tests should be performed to help distinguish between B12 and folate deficiency. Both B12 and folate deficiencies can cause elevated homocysteine levels.13 Clinically significant B12 deficiency causes elevation of methylmalonic acid (MMA), whereas folate deficiency does not.13,14 Elevation of MMA can be very sensitive for B12 deficiency but lacks specificity in certain situations, such as pregnancy, renal insufficiency, and advanced age.13,14
Treatment of vitamin B12 and folate deficiencies with supplementation prevents progression of the disease, and has the potential to relieve most of the symptoms. Oral, sublingual, or parenteral vitamin B12 or oral folate supplements can be started in the primary care setting once the provider has identified whether the patient is B12 deficient, folate deficient, or both.
The vitamin B12 dose used for deficiency-induced macrocytic anemia depends on the cause—for example, a temporary condition such as pregnancy versus a lifelong disorder such as pernicious anemia.13 The usual oral dosing regimen is 2 mg/d; if intramuscular injections are used, 50 to 100 mcg are given daily for a week, followed by weekly injections for a month, and then monthly injections of 1 mg for life, if necessary.13 Bone marrow response to supplemental B12 is very rapid, with increased reticulocyte counts seen within four or five days.13
The usual dose for oral folic acid is 1 mg/d as needed.13 Folic acid can be given for folate deficiency only if the vitamin B12 level is normal. Giving folate to a patient with untreated vitamin B12 deficiency can potentially worsen subacute combined degeneration of the spinal cord.13,16
NORMOCYTIC ANEMIA
In normocytic anemia, the hemoglobin is low but the MCV is normal (see Figure).1 The history and physical exam should provide clues about whether the underlying cause of the anemia requires emergent (eg, active bleeding) or nonemergent (eg, anemia of chronic disease) management. Some of the causes of normocytic anemia are active bleeding, pregnancy, malnutrition, renal failure, chronic disease, hemolytic disorders, hypersplenism, congenital disorders, endocrine disorders, infection, and primary bone marrow disorders.1,5 Expanded plasma volume, as seen in pregnancy and overhydration, can also cause normocytic anemia.5 If gastrointestinal bleeding is suspected or the patient reports dark, tarry stools consistent with melena, fecal occult blood testing should be done. A positive result strongly supports gastrointestinal bleeding as the cause of the anemia.18
The reticulocyte count can also be helpful in identifying the cause of this type of anemia. A normocytic anemia with a normal reticulocyte and normal RDW count is usually related to chronic disease.1,10 For example, chronic kidney disease (CKD) is associated with decreased EPO production due to impaired renal function, which leads to reduced erythropoiesis. Decreased EPO prevents the bone marrow from making red blood cells, resulting in anemia. However, a normocytic anemia with an elevated reticulocyte count points to bleeding or hemolysis, as the reticulosis shows that the bone marrow is increasing red cell production to make up for the lost red cells.5
Additional diagnostic laboratory testing for patients with normocytic anemia may involve, for example, creatinine and blood urea nitrogen for patients with CKD, prothrombin time with an INR and liver function tests for patients with liver disease, and urine human chorionic gonadotropin if pregnancy is suspected.
For patients with an infection that is causing severe hemolysis (eg, sepsis due to a ß-hemolytic streptococcal infection), blood cultures should be drawn.5 If red blood cell destruction due to an artificial cardiac valve or an autoimmune disorder is suspected as the cause of the anemia, a hematology consult is needed.1 Anemia caused by disseminated intravascular coagulation or thrombotic thrombocytopenic purpura resulting in hemolysis are usually emergent conditions that require immediate intervention, including hospitalization and management by a hematologist.1
PATIENT EDUCATION
Patients and any accompanying family members should be educated about the signs and symptoms of anemia, the diagnostic testing and treatment regimens specific to their anemia, and medication compliance issues.
For instance, patients who abuse alcohol often have both vitamin B12 and folate deficiencies. If the macrocytosis is caused by alcohol intake, then the provider should educate the patient on the importance of alcohol abstention, as well as refer the patient for rehabilitation and psychologic counseling, as needed. These patients can sometimes recover from macrocytic anemia simply by stopping alcohol intake and improving their nutrition.19 Patients with microcytosis due to iron deficiency anemia should be advised about the importance of good nutrition and compliance with iron supplementation.
Repeat CBCs and a follow-up patient history and physical exam will help the provider assess whether the anemia is resolving. Individualized plans that target the specific type of anemia identified, as well as its underlying cause, are key to successful treatment.
CONCLUSION
When managing a patient with anemia, providers must define the type of anemia present and identify its underlying cause before starting treatment. Clues from the patient’s history, physical exam, and CBC can help isolate the cause of anemia. The MCV is the most helpful of the red blood cell indices because it allows the provider to classify the anemia as microcytic, macrocytic, or normocytic.
In cases in which the anemia is acute or severe—or in which the patient remains anemic even after being treated by the primary care provider—referral to a specialist is appropriate.
1. Brill JR, Baumgardner D. Normocytic anemia. Am Fam Physician. 2000;62(10):2255-2263.
2. Van Vranken M. Evaluation of microcytosis. Am Fam Physician. 2010;82(9):1117-1122.
3. National Institutes of Health/National Heart, Lung, and Blood Institute. How is anemia diagnosed? www.nhlbi.nih.gov/health/health-topics/topics/anemia/diagnosis. Accessed April 28, 2017.
4. Smith RE Jr. The clinical and economic burden of anemia. Am J Manag Care. 2010;16(3):S59-S66.
5. Platt A, Eckman J. Diagnosing anemia. Clinician Reviews. 2006;16(2):44-50.
6. Karnath B. Anemia in the adult patient. Hosp Physician. 2004;40(10):32-36.
7. World Health Organization. Micronutrient deficiencies. www.who.int/nutrition/topics/ida/en/#. Accessed April 29, 2017.
8. US Department of Health and Human Services, Office of Women’s Health. Iron-deficiency anemia. www.womenshealth.gov/publications/our-publications/fact-sheet/anemia.html#a. Accessed April 29, 2017.
9. DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14): 1324-1331.
10. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-936.
11. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015; 372(19):1832-1843.
12. Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671-678.
13. Rodgers GP, Young N. The Bethesda Handbook of Clinical Hematology. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 2013:1-21.
14. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
15. Hesdorffer CS, Longo DL. Drug-induced megaloblastic anemia. N Engl J Med. 2015;373(17):1649-1658.
16. Vasconcelos OM, Poehm EH, McCarter RJ, et al. Potential outcome factors in subacute combined degeneration. J Gen Intern Med. 2006;21(10):1063-1068.
17. Vide AT, Marques AM, Costa JD. MRI findings in subacute combined degeneration of the spinal cord in a patient with restricted diet. Neurol Sci. 2011;33(3):711-713.
18. Kessenich CR, Cronin K. Fecal occult blood testing in older adult patients with anemia. Nurse Pract. 2013;38(1):6-8.
19. Imashuku S, Kudo N, Kaneda S. Spontaneous resolution of macrocytic anemia: old disease revisited. J Blood Med. 2012;3:45-47.
20. Pagana K, Pagana T, Pagana T. Mosby’s Diagnostic and Laboratory Test Reference. 12th ed. St. Louis, MO: Elsevier Mosby; 2015: 497-501, 785-791, 805-806.
CE/CME No: CR-1708
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the importance of diagnosing the type of anemia in order to provide appropriate treatment.
• Describe how the complete blood count and its indices are used to initially determine if an anemia is microcytic, normocytic, or macrocytic.
• List the more common causes of microcytic, normocytic, and macrocytic anemia.
• Discuss addictional laboratory tests that may be used to further assess the cause of anemia.
FACULTY
Jean O’Neil is an Assistant Professor and Coordinator of the Adult Gerontology Acute Care Nurse Practitioner Program in the Patricia A. Chin School of Nursing at California State University, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2018.
Article begins on next page >>
Anemia affects more than 3 million people in the United States, making it a common problem in primary care practices. Once anemia is detected, clinicians must define the type and identify its underlying cause prior to initiating treatment. In most cases, the cause can be determined using information from the patient history, physical exam, and complete blood count.
Anemia is commonly identified during routine physical exams and laboratory testing.1-3 However, treating anemia can present a challenge for the primary care provider if the immediate cause is not apparent. Iron deficiency is a leading cause of anemia, but simply prescribing an iron supplement without determining the type or the cause of the anemia is not appropriate. Anemia that is misdiagnosed or goes untreated can be associated with a worse prognosis, as well as increased health care costs.4
Primary care providers often manage patients with common types of anemia and refer patients with severe or complex anemia to specialists for further testing and treatment. The most commonly used and cost-effective diagnostic tool for anemia is the complete blood count (CBC).2-6 The CBC provides details that can help the provider determine the type of anemia present, which in turn guides proper diagnostic testing and treatment.
EPIDEMIOLOGY
Anemia involves a reduction in the number of circulating red blood cells, the blood hemoglobin content, or the hematocrit, which leads to impaired delivery of oxygen to the body. Anemia affects more than 2 billion people worldwide, with iron deficiency the most common cause.7 Other leading nutritional causes of anemia include vitamin B12 and folate deficiency.4,7 Approximately 3 to 4 million Americans have anemia in some form, and it affects about 6.6% of men and 12.4% of women.5,8 The prevalence of anemia increases with age. Approximately 11% of men and 10% of women ages 65 or older have anemia, and in men ages 85 or older, prevalence of 20% to 44% has been reported.1,4 Anemia is present in about 3.5% of patients with chronic disease, but only 15% of them receive treatment.4

PATHOPHYSIOLOGY
Blood is composed of water-based plasma (54%), white blood cells and platelets (1%), and red blood cells (45%).5 Hemoglobin, the primary protein of the red blood cell, binds oxygen from the lungs and transports it to the rest of the body. Oxygen is then exchanged for carbon dioxide, which is carried back to the lungs to be exhaled.
Hemoglobin is made up of four globin chains, each containing an iron ion held in a porphyrin ring known as a heme group.5 When the body detects low tissue oxygen, the endothelial cells in the kidneys secrete the hormone erythropoietin (EPO), which stimulates the bone marrow to increase red cell production.5 This feedback loop can be interrupted by renal failure or chronic disease.4 In addition, bone marrow cannot produce enough red blood cells if there are insufficient levels of iron, amino acids, protein, carbohydrates, lipids, folate, and vitamin B12.5 Toxins (eg, lead), some types of cancer (eg, lymphoma), or even common infections (eg, pneumonia) can suppress the bone marrow, causing anemia. The more severe the anemia, the more likely oxygen transport will be compromised and organ failure will ensue.
Mutations affecting the genes that encode the globin chains within hemoglobin can cause one of the more than 600 known hemoglobinopathies (genetic defects of hemoglobin structure), such as sickle cell disease and thalassemias.5,9 While it is important to identify and treat patients with hemoglobinopathies, most anemias have other causes, such as iron deficiency, chronic disease, bone marrow defects, B12 deficiency, renal failure, medications, alcoholism, pregnancy, nutritional intake problems, gastrointestinal malabsorption, and active or recent history of blood loss.5,10
CLINICAL PRESENTATION
There are several signs and symptoms that should lead the primary care provider to suspect anemia (see Table 1).5,6 The severity of these symptoms can vary from mild to very serious. Severe anemia can lead to organ failure and death. However, most patients with anemia are asymptomatic, and anemia is typically detected incidentally during laboratory testing.1,2

Once anemia is confirmed, the evaluation focuses on diagnosing its underlying cause. It should include a thorough patient history and review of systems to ascertain whether the patient has symptoms such as increased fatigue, palpitations, gastrointestinal distress, weakness, or dizziness.
If the provider has access to past CBC results, a comparison of the current and previous results will help determine whether the anemia is acute or chronic. Anemia caused by acute conditions, such as a suspicion of blood loss or bone marrow suppression, must be attended to immediately. A patient with chronic anemia should be carefully monitored and may need follow-up for ongoing treatment. While a provider has more time to work up a patient with chronic anemia, the causes may not be as straightforward.
DIAGNOSIS AND CLASSIFICATION
Anemia in adults is defined as hemoglobin less than 13 g/dL in males and 12 g/dL in females.6 The hemoglobin is part of the complete blood cell report, which also includes the white blood cell count (WBC), red blood cell count (RBC), hematocrit, platelet count, and indices.
When investigating the underlying cause of anemia, the most useful parts of the CBC are the hemoglobin and the mean corpuscular volume (MCV; see Table 2).6,10 The MCV is the average volume of red cells in a specimen. This parameter is used to classify the anemia as microcytic (MCV < 80 fL), normocytic (MCV 80-100 fL), or macrocytic (MCV > 100 fL), which helps to narrow the differential diagnosis and guide any further testing (see Figure).5,6,10

It is important to note that the normal ranges of the CBC parameters differ based on race, with persons of African ancestry having lower normal hemoglobin levels than persons of Caucasian ancestry.10 In addition, laboratories may have slightly different normal values for the CBC based on the equipment they utilize. Therefore, providers must follow their laboratory’s parameters, as well as adjust for the patient’s gender, age, and ethnicity.10
Microcytic Anemia
Iron deficiency
In microcytic anemia, the RBCs are smaller than average (MCV < 80 fL), as well as hypochromic due to lack of hemoglobin.9 Iron deficiency is the most common cause of microcytic anemia worldwide.11,12 Therefore, when a patient has microcytic anemia, a serum ferritin needs to be ordered. Further testing of total iron-binding capacity (TIBC), transferrin saturation, serum iron, and serum receptor levels may be helpful if the ferritin level is between 46-99 ng/mL and anemia due to iron deficiency is not confirmed (see Table 2).12
In iron deficiency anemia, serum ferritin and serum iron levels are low due to lack of iron, but serum TIBC is high.6 The elevated TIBC reflects increased synthesis of transferrin by the liver as it attempts to compensate for the patient’s low serum iron level.9 Since iron levels are controlled by absorption rather than excretion, iron is essentially only depleted from the body through blood loss.12 Therefore, an adult patient who is iron deficient has lost more iron through blood loss than was replaced through nutritional intake and gastrointestinal absorption. In children, increased growth-related iron requirements combined with poor nutritional intake of iron-rich foods is an additional mechanism for iron deficiency.11
Iron def
If the nutritional problem is corrected or the source of bleeding is controlled, treatment with oral or intravenous iron supplements should result in improved serum hemoglobin and reticulocyte counts.13 In the primary care setting, ferrous sulfate 325 mg, which provides 65 mg of elemental iron per tablet, orally three times daily is recommended for adults.13 This gives the patient the recommended dose of approximately 200 mg of elemental iron. Repeat hemoglobin and iron studies should be conducted again in three to six months.12,13
If the patient’s iron deficiency anemia does not improve after oral iron therapy, there may be a source of blood loss the provider missed or a problem with malabsorption of iron, which can be seen in those who have undergone gastric bypass surgery or who have inflammatory bowel disease.13 Such patients should be referred to a specialist, such as a gastroenterologist, for further evaluation.
Thalassemia
Microcytic anemia with normal or elevated serum iron and normal-to-increased serum ferritin can be seen in patients with a type of thalassemia (see Figure).2 Thalassemias are inherited blood disorders that reduce hemoglobin production, leading to microcytosis; they are more common in those of Mediterranean, African, and Southeast Asian descent.2 Red cells in patients with a form of thalassemia are usually very small (microcytic) and have normal or elevated red cell distribution width (RDW).10

Moderate and severe forms of thalassemia can cause anemia. However, thalassemia syndromes that can cause severe (transfusion-dependent) anemia are usually diagnosed in childhood.9 Patients with one of the minor forms of thalassemia typically need minimal to no treatment.5 A patient with significant anemia suspicious for thalassemia should undergo hemoglobin electrophoresis testing to confirm the diagnosis and to determine the type of thalassemia.2 Typically, hemoglobin electrophoresis is normal in α thalassemia and is abnormal in ß thalassemia, as well as other forms of thalassemia. Referral to a hematologist for interpretation of these results and for further evaluation is appropriate.10
Chronic disease
If the patient has microcytic anemia and is not iron deficient or does not have thalassemia, then anemia related to a chronic disease should be considered.5 In such cases, the provider should order a reticulocyte count, which reveals how the bone marrow is responding to the anemia.5 Reticulocytes are immature red cells that have just been released from the bone marrow into the blood stream. The bone marrow increases the release of these cells in response to anemia.6
Any condition that stimulates reticulocyte production or prevents the bone marrow from producing reticulocytes will result in abnormal values (see Table 3). A normal reticulocyte count, expressed as the reticulocyte production index, is between 0.5% and 1.5%.5 The reticulocyte count is low in iron deficiency anemia and diseases that lead to decreased bone marrow production.5,6 Bone marrow suppression can occur in the context of chronic disease, infection, or inflammation. Malignancies are a less common cause for chronic disease microcytic anemia.6

If the cause of the decreased reticulocyte count is iron deficiency anemia, then treatment with iron supplementation should result in an increased reticulocyte count within one week.13 The primary care provider works in conjunction with the specialist to monitor the patient’s anemia when it is due to chronic disease or malignancy.
MACROCYTIC ANEMIA
In macrocytic anemia, the RBCs are larger than normal (MCV > 100 fL). This form of anemia is usually caused by vitamin B12 and folate deficiency, but it can also result from alcoholism, certain medications (eg, chemotherapy, antivirals), bone marrow disorders (eg, leukemia), and liver disease (eg, cirrhosis; see Figure).5,14 Common medications that can cause macrocytosis include the antiseizure drug phenytoin, the antibiotics trimethoprim/sulfamethoxazole and nitrofurantoin, the disease-modifying antirheumatic drug sulfasalazine, and immunosuppressants such as azathioprine.14,15 Antiviral agents, such as reverse transcriptase inhibitors (eg, zidovudine) used to treat HIV infection, can also cause macrocytosis with or without anemia.6,14
Macrocytic anemias caused by low serum levels of B12 and folate usually reflect problems with gastrointestinal malabsorption. For example, gastric bypass or Crohn disease can lead to malabsorption of vitamin B12 and increase a patient’s risk for macrocytic anemia.13
Vitamin B12 deficiency occurs in patients with pernicious anemia because they are missing intrinsic factor, which is necessary to facilitate B12 absorption in the ileum.10 Low vitamin B12 and folate levels also can result from inadequate dietary intake, although this is rare in the United States due to mandatory fortification of certain foods. A diet low in fresh vegetables is the leading cause of folate deficiency. While folate deficiency related to poor nutritional intake can be seen in all age groups, vitamin B12 deficiency more frequently affects the elderly or persons following a strict vegan diet.14
In addition to the fatigue and pallor associated with macrocytic anemia, patients with vitamin B12 deficiency may also have a smooth tongue, peripheral neuropathy, and edema.5,14 Severe vitamin B12 deficiency can lead to subacute combined degeneration of the spinal cord, with demyelination of the dorsal and lateral columns most often occurring in the cervical and thoracic regions.16,17 This spinal cord degeneration can cause paresthesia, muscle spasticity, and ataxia.16
When there is a macrocytic anemia, but the B12 or folate level is only borderline low, additional tests should be performed to help distinguish between B12 and folate deficiency. Both B12 and folate deficiencies can cause elevated homocysteine levels.13 Clinically significant B12 deficiency causes elevation of methylmalonic acid (MMA), whereas folate deficiency does not.13,14 Elevation of MMA can be very sensitive for B12 deficiency but lacks specificity in certain situations, such as pregnancy, renal insufficiency, and advanced age.13,14
Treatment of vitamin B12 and folate deficiencies with supplementation prevents progression of the disease, and has the potential to relieve most of the symptoms. Oral, sublingual, or parenteral vitamin B12 or oral folate supplements can be started in the primary care setting once the provider has identified whether the patient is B12 deficient, folate deficient, or both.
The vitamin B12 dose used for deficiency-induced macrocytic anemia depends on the cause—for example, a temporary condition such as pregnancy versus a lifelong disorder such as pernicious anemia.13 The usual oral dosing regimen is 2 mg/d; if intramuscular injections are used, 50 to 100 mcg are given daily for a week, followed by weekly injections for a month, and then monthly injections of 1 mg for life, if necessary.13 Bone marrow response to supplemental B12 is very rapid, with increased reticulocyte counts seen within four or five days.13
The usual dose for oral folic acid is 1 mg/d as needed.13 Folic acid can be given for folate deficiency only if the vitamin B12 level is normal. Giving folate to a patient with untreated vitamin B12 deficiency can potentially worsen subacute combined degeneration of the spinal cord.13,16
NORMOCYTIC ANEMIA
In normocytic anemia, the hemoglobin is low but the MCV is normal (see Figure).1 The history and physical exam should provide clues about whether the underlying cause of the anemia requires emergent (eg, active bleeding) or nonemergent (eg, anemia of chronic disease) management. Some of the causes of normocytic anemia are active bleeding, pregnancy, malnutrition, renal failure, chronic disease, hemolytic disorders, hypersplenism, congenital disorders, endocrine disorders, infection, and primary bone marrow disorders.1,5 Expanded plasma volume, as seen in pregnancy and overhydration, can also cause normocytic anemia.5 If gastrointestinal bleeding is suspected or the patient reports dark, tarry stools consistent with melena, fecal occult blood testing should be done. A positive result strongly supports gastrointestinal bleeding as the cause of the anemia.18
The reticulocyte count can also be helpful in identifying the cause of this type of anemia. A normocytic anemia with a normal reticulocyte and normal RDW count is usually related to chronic disease.1,10 For example, chronic kidney disease (CKD) is associated with decreased EPO production due to impaired renal function, which leads to reduced erythropoiesis. Decreased EPO prevents the bone marrow from making red blood cells, resulting in anemia. However, a normocytic anemia with an elevated reticulocyte count points to bleeding or hemolysis, as the reticulosis shows that the bone marrow is increasing red cell production to make up for the lost red cells.5
Additional diagnostic laboratory testing for patients with normocytic anemia may involve, for example, creatinine and blood urea nitrogen for patients with CKD, prothrombin time with an INR and liver function tests for patients with liver disease, and urine human chorionic gonadotropin if pregnancy is suspected.
For patients with an infection that is causing severe hemolysis (eg, sepsis due to a ß-hemolytic streptococcal infection), blood cultures should be drawn.5 If red blood cell destruction due to an artificial cardiac valve or an autoimmune disorder is suspected as the cause of the anemia, a hematology consult is needed.1 Anemia caused by disseminated intravascular coagulation or thrombotic thrombocytopenic purpura resulting in hemolysis are usually emergent conditions that require immediate intervention, including hospitalization and management by a hematologist.1
PATIENT EDUCATION
Patients and any accompanying family members should be educated about the signs and symptoms of anemia, the diagnostic testing and treatment regimens specific to their anemia, and medication compliance issues.
For instance, patients who abuse alcohol often have both vitamin B12 and folate deficiencies. If the macrocytosis is caused by alcohol intake, then the provider should educate the patient on the importance of alcohol abstention, as well as refer the patient for rehabilitation and psychologic counseling, as needed. These patients can sometimes recover from macrocytic anemia simply by stopping alcohol intake and improving their nutrition.19 Patients with microcytosis due to iron deficiency anemia should be advised about the importance of good nutrition and compliance with iron supplementation.
Repeat CBCs and a follow-up patient history and physical exam will help the provider assess whether the anemia is resolving. Individualized plans that target the specific type of anemia identified, as well as its underlying cause, are key to successful treatment.
CONCLUSION
When managing a patient with anemia, providers must define the type of anemia present and identify its underlying cause before starting treatment. Clues from the patient’s history, physical exam, and CBC can help isolate the cause of anemia. The MCV is the most helpful of the red blood cell indices because it allows the provider to classify the anemia as microcytic, macrocytic, or normocytic.
In cases in which the anemia is acute or severe—or in which the patient remains anemic even after being treated by the primary care provider—referral to a specialist is appropriate.
CE/CME No: CR-1708
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the importance of diagnosing the type of anemia in order to provide appropriate treatment.
• Describe how the complete blood count and its indices are used to initially determine if an anemia is microcytic, normocytic, or macrocytic.
• List the more common causes of microcytic, normocytic, and macrocytic anemia.
• Discuss addictional laboratory tests that may be used to further assess the cause of anemia.
FACULTY
Jean O’Neil is an Assistant Professor and Coordinator of the Adult Gerontology Acute Care Nurse Practitioner Program in the Patricia A. Chin School of Nursing at California State University, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2018.
Article begins on next page >>
Anemia affects more than 3 million people in the United States, making it a common problem in primary care practices. Once anemia is detected, clinicians must define the type and identify its underlying cause prior to initiating treatment. In most cases, the cause can be determined using information from the patient history, physical exam, and complete blood count.
Anemia is commonly identified during routine physical exams and laboratory testing.1-3 However, treating anemia can present a challenge for the primary care provider if the immediate cause is not apparent. Iron deficiency is a leading cause of anemia, but simply prescribing an iron supplement without determining the type or the cause of the anemia is not appropriate. Anemia that is misdiagnosed or goes untreated can be associated with a worse prognosis, as well as increased health care costs.4
Primary care providers often manage patients with common types of anemia and refer patients with severe or complex anemia to specialists for further testing and treatment. The most commonly used and cost-effective diagnostic tool for anemia is the complete blood count (CBC).2-6 The CBC provides details that can help the provider determine the type of anemia present, which in turn guides proper diagnostic testing and treatment.
EPIDEMIOLOGY
Anemia involves a reduction in the number of circulating red blood cells, the blood hemoglobin content, or the hematocrit, which leads to impaired delivery of oxygen to the body. Anemia affects more than 2 billion people worldwide, with iron deficiency the most common cause.7 Other leading nutritional causes of anemia include vitamin B12 and folate deficiency.4,7 Approximately 3 to 4 million Americans have anemia in some form, and it affects about 6.6% of men and 12.4% of women.5,8 The prevalence of anemia increases with age. Approximately 11% of men and 10% of women ages 65 or older have anemia, and in men ages 85 or older, prevalence of 20% to 44% has been reported.1,4 Anemia is present in about 3.5% of patients with chronic disease, but only 15% of them receive treatment.4

PATHOPHYSIOLOGY
Blood is composed of water-based plasma (54%), white blood cells and platelets (1%), and red blood cells (45%).5 Hemoglobin, the primary protein of the red blood cell, binds oxygen from the lungs and transports it to the rest of the body. Oxygen is then exchanged for carbon dioxide, which is carried back to the lungs to be exhaled.
Hemoglobin is made up of four globin chains, each containing an iron ion held in a porphyrin ring known as a heme group.5 When the body detects low tissue oxygen, the endothelial cells in the kidneys secrete the hormone erythropoietin (EPO), which stimulates the bone marrow to increase red cell production.5 This feedback loop can be interrupted by renal failure or chronic disease.4 In addition, bone marrow cannot produce enough red blood cells if there are insufficient levels of iron, amino acids, protein, carbohydrates, lipids, folate, and vitamin B12.5 Toxins (eg, lead), some types of cancer (eg, lymphoma), or even common infections (eg, pneumonia) can suppress the bone marrow, causing anemia. The more severe the anemia, the more likely oxygen transport will be compromised and organ failure will ensue.
Mutations affecting the genes that encode the globin chains within hemoglobin can cause one of the more than 600 known hemoglobinopathies (genetic defects of hemoglobin structure), such as sickle cell disease and thalassemias.5,9 While it is important to identify and treat patients with hemoglobinopathies, most anemias have other causes, such as iron deficiency, chronic disease, bone marrow defects, B12 deficiency, renal failure, medications, alcoholism, pregnancy, nutritional intake problems, gastrointestinal malabsorption, and active or recent history of blood loss.5,10
CLINICAL PRESENTATION
There are several signs and symptoms that should lead the primary care provider to suspect anemia (see Table 1).5,6 The severity of these symptoms can vary from mild to very serious. Severe anemia can lead to organ failure and death. However, most patients with anemia are asymptomatic, and anemia is typically detected incidentally during laboratory testing.1,2

Once anemia is confirmed, the evaluation focuses on diagnosing its underlying cause. It should include a thorough patient history and review of systems to ascertain whether the patient has symptoms such as increased fatigue, palpitations, gastrointestinal distress, weakness, or dizziness.
If the provider has access to past CBC results, a comparison of the current and previous results will help determine whether the anemia is acute or chronic. Anemia caused by acute conditions, such as a suspicion of blood loss or bone marrow suppression, must be attended to immediately. A patient with chronic anemia should be carefully monitored and may need follow-up for ongoing treatment. While a provider has more time to work up a patient with chronic anemia, the causes may not be as straightforward.
DIAGNOSIS AND CLASSIFICATION
Anemia in adults is defined as hemoglobin less than 13 g/dL in males and 12 g/dL in females.6 The hemoglobin is part of the complete blood cell report, which also includes the white blood cell count (WBC), red blood cell count (RBC), hematocrit, platelet count, and indices.
When investigating the underlying cause of anemia, the most useful parts of the CBC are the hemoglobin and the mean corpuscular volume (MCV; see Table 2).6,10 The MCV is the average volume of red cells in a specimen. This parameter is used to classify the anemia as microcytic (MCV < 80 fL), normocytic (MCV 80-100 fL), or macrocytic (MCV > 100 fL), which helps to narrow the differential diagnosis and guide any further testing (see Figure).5,6,10

It is important to note that the normal ranges of the CBC parameters differ based on race, with persons of African ancestry having lower normal hemoglobin levels than persons of Caucasian ancestry.10 In addition, laboratories may have slightly different normal values for the CBC based on the equipment they utilize. Therefore, providers must follow their laboratory’s parameters, as well as adjust for the patient’s gender, age, and ethnicity.10
Microcytic Anemia
Iron deficiency
In microcytic anemia, the RBCs are smaller than average (MCV < 80 fL), as well as hypochromic due to lack of hemoglobin.9 Iron deficiency is the most common cause of microcytic anemia worldwide.11,12 Therefore, when a patient has microcytic anemia, a serum ferritin needs to be ordered. Further testing of total iron-binding capacity (TIBC), transferrin saturation, serum iron, and serum receptor levels may be helpful if the ferritin level is between 46-99 ng/mL and anemia due to iron deficiency is not confirmed (see Table 2).12
In iron deficiency anemia, serum ferritin and serum iron levels are low due to lack of iron, but serum TIBC is high.6 The elevated TIBC reflects increased synthesis of transferrin by the liver as it attempts to compensate for the patient’s low serum iron level.9 Since iron levels are controlled by absorption rather than excretion, iron is essentially only depleted from the body through blood loss.12 Therefore, an adult patient who is iron deficient has lost more iron through blood loss than was replaced through nutritional intake and gastrointestinal absorption. In children, increased growth-related iron requirements combined with poor nutritional intake of iron-rich foods is an additional mechanism for iron deficiency.11
Iron def
If the nutritional problem is corrected or the source of bleeding is controlled, treatment with oral or intravenous iron supplements should result in improved serum hemoglobin and reticulocyte counts.13 In the primary care setting, ferrous sulfate 325 mg, which provides 65 mg of elemental iron per tablet, orally three times daily is recommended for adults.13 This gives the patient the recommended dose of approximately 200 mg of elemental iron. Repeat hemoglobin and iron studies should be conducted again in three to six months.12,13
If the patient’s iron deficiency anemia does not improve after oral iron therapy, there may be a source of blood loss the provider missed or a problem with malabsorption of iron, which can be seen in those who have undergone gastric bypass surgery or who have inflammatory bowel disease.13 Such patients should be referred to a specialist, such as a gastroenterologist, for further evaluation.
Thalassemia
Microcytic anemia with normal or elevated serum iron and normal-to-increased serum ferritin can be seen in patients with a type of thalassemia (see Figure).2 Thalassemias are inherited blood disorders that reduce hemoglobin production, leading to microcytosis; they are more common in those of Mediterranean, African, and Southeast Asian descent.2 Red cells in patients with a form of thalassemia are usually very small (microcytic) and have normal or elevated red cell distribution width (RDW).10

Moderate and severe forms of thalassemia can cause anemia. However, thalassemia syndromes that can cause severe (transfusion-dependent) anemia are usually diagnosed in childhood.9 Patients with one of the minor forms of thalassemia typically need minimal to no treatment.5 A patient with significant anemia suspicious for thalassemia should undergo hemoglobin electrophoresis testing to confirm the diagnosis and to determine the type of thalassemia.2 Typically, hemoglobin electrophoresis is normal in α thalassemia and is abnormal in ß thalassemia, as well as other forms of thalassemia. Referral to a hematologist for interpretation of these results and for further evaluation is appropriate.10
Chronic disease
If the patient has microcytic anemia and is not iron deficient or does not have thalassemia, then anemia related to a chronic disease should be considered.5 In such cases, the provider should order a reticulocyte count, which reveals how the bone marrow is responding to the anemia.5 Reticulocytes are immature red cells that have just been released from the bone marrow into the blood stream. The bone marrow increases the release of these cells in response to anemia.6
Any condition that stimulates reticulocyte production or prevents the bone marrow from producing reticulocytes will result in abnormal values (see Table 3). A normal reticulocyte count, expressed as the reticulocyte production index, is between 0.5% and 1.5%.5 The reticulocyte count is low in iron deficiency anemia and diseases that lead to decreased bone marrow production.5,6 Bone marrow suppression can occur in the context of chronic disease, infection, or inflammation. Malignancies are a less common cause for chronic disease microcytic anemia.6

If the cause of the decreased reticulocyte count is iron deficiency anemia, then treatment with iron supplementation should result in an increased reticulocyte count within one week.13 The primary care provider works in conjunction with the specialist to monitor the patient’s anemia when it is due to chronic disease or malignancy.
MACROCYTIC ANEMIA
In macrocytic anemia, the RBCs are larger than normal (MCV > 100 fL). This form of anemia is usually caused by vitamin B12 and folate deficiency, but it can also result from alcoholism, certain medications (eg, chemotherapy, antivirals), bone marrow disorders (eg, leukemia), and liver disease (eg, cirrhosis; see Figure).5,14 Common medications that can cause macrocytosis include the antiseizure drug phenytoin, the antibiotics trimethoprim/sulfamethoxazole and nitrofurantoin, the disease-modifying antirheumatic drug sulfasalazine, and immunosuppressants such as azathioprine.14,15 Antiviral agents, such as reverse transcriptase inhibitors (eg, zidovudine) used to treat HIV infection, can also cause macrocytosis with or without anemia.6,14
Macrocytic anemias caused by low serum levels of B12 and folate usually reflect problems with gastrointestinal malabsorption. For example, gastric bypass or Crohn disease can lead to malabsorption of vitamin B12 and increase a patient’s risk for macrocytic anemia.13
Vitamin B12 deficiency occurs in patients with pernicious anemia because they are missing intrinsic factor, which is necessary to facilitate B12 absorption in the ileum.10 Low vitamin B12 and folate levels also can result from inadequate dietary intake, although this is rare in the United States due to mandatory fortification of certain foods. A diet low in fresh vegetables is the leading cause of folate deficiency. While folate deficiency related to poor nutritional intake can be seen in all age groups, vitamin B12 deficiency more frequently affects the elderly or persons following a strict vegan diet.14
In addition to the fatigue and pallor associated with macrocytic anemia, patients with vitamin B12 deficiency may also have a smooth tongue, peripheral neuropathy, and edema.5,14 Severe vitamin B12 deficiency can lead to subacute combined degeneration of the spinal cord, with demyelination of the dorsal and lateral columns most often occurring in the cervical and thoracic regions.16,17 This spinal cord degeneration can cause paresthesia, muscle spasticity, and ataxia.16
When there is a macrocytic anemia, but the B12 or folate level is only borderline low, additional tests should be performed to help distinguish between B12 and folate deficiency. Both B12 and folate deficiencies can cause elevated homocysteine levels.13 Clinically significant B12 deficiency causes elevation of methylmalonic acid (MMA), whereas folate deficiency does not.13,14 Elevation of MMA can be very sensitive for B12 deficiency but lacks specificity in certain situations, such as pregnancy, renal insufficiency, and advanced age.13,14
Treatment of vitamin B12 and folate deficiencies with supplementation prevents progression of the disease, and has the potential to relieve most of the symptoms. Oral, sublingual, or parenteral vitamin B12 or oral folate supplements can be started in the primary care setting once the provider has identified whether the patient is B12 deficient, folate deficient, or both.
The vitamin B12 dose used for deficiency-induced macrocytic anemia depends on the cause—for example, a temporary condition such as pregnancy versus a lifelong disorder such as pernicious anemia.13 The usual oral dosing regimen is 2 mg/d; if intramuscular injections are used, 50 to 100 mcg are given daily for a week, followed by weekly injections for a month, and then monthly injections of 1 mg for life, if necessary.13 Bone marrow response to supplemental B12 is very rapid, with increased reticulocyte counts seen within four or five days.13
The usual dose for oral folic acid is 1 mg/d as needed.13 Folic acid can be given for folate deficiency only if the vitamin B12 level is normal. Giving folate to a patient with untreated vitamin B12 deficiency can potentially worsen subacute combined degeneration of the spinal cord.13,16
NORMOCYTIC ANEMIA
In normocytic anemia, the hemoglobin is low but the MCV is normal (see Figure).1 The history and physical exam should provide clues about whether the underlying cause of the anemia requires emergent (eg, active bleeding) or nonemergent (eg, anemia of chronic disease) management. Some of the causes of normocytic anemia are active bleeding, pregnancy, malnutrition, renal failure, chronic disease, hemolytic disorders, hypersplenism, congenital disorders, endocrine disorders, infection, and primary bone marrow disorders.1,5 Expanded plasma volume, as seen in pregnancy and overhydration, can also cause normocytic anemia.5 If gastrointestinal bleeding is suspected or the patient reports dark, tarry stools consistent with melena, fecal occult blood testing should be done. A positive result strongly supports gastrointestinal bleeding as the cause of the anemia.18
The reticulocyte count can also be helpful in identifying the cause of this type of anemia. A normocytic anemia with a normal reticulocyte and normal RDW count is usually related to chronic disease.1,10 For example, chronic kidney disease (CKD) is associated with decreased EPO production due to impaired renal function, which leads to reduced erythropoiesis. Decreased EPO prevents the bone marrow from making red blood cells, resulting in anemia. However, a normocytic anemia with an elevated reticulocyte count points to bleeding or hemolysis, as the reticulosis shows that the bone marrow is increasing red cell production to make up for the lost red cells.5
Additional diagnostic laboratory testing for patients with normocytic anemia may involve, for example, creatinine and blood urea nitrogen for patients with CKD, prothrombin time with an INR and liver function tests for patients with liver disease, and urine human chorionic gonadotropin if pregnancy is suspected.
For patients with an infection that is causing severe hemolysis (eg, sepsis due to a ß-hemolytic streptococcal infection), blood cultures should be drawn.5 If red blood cell destruction due to an artificial cardiac valve or an autoimmune disorder is suspected as the cause of the anemia, a hematology consult is needed.1 Anemia caused by disseminated intravascular coagulation or thrombotic thrombocytopenic purpura resulting in hemolysis are usually emergent conditions that require immediate intervention, including hospitalization and management by a hematologist.1
PATIENT EDUCATION
Patients and any accompanying family members should be educated about the signs and symptoms of anemia, the diagnostic testing and treatment regimens specific to their anemia, and medication compliance issues.
For instance, patients who abuse alcohol often have both vitamin B12 and folate deficiencies. If the macrocytosis is caused by alcohol intake, then the provider should educate the patient on the importance of alcohol abstention, as well as refer the patient for rehabilitation and psychologic counseling, as needed. These patients can sometimes recover from macrocytic anemia simply by stopping alcohol intake and improving their nutrition.19 Patients with microcytosis due to iron deficiency anemia should be advised about the importance of good nutrition and compliance with iron supplementation.
Repeat CBCs and a follow-up patient history and physical exam will help the provider assess whether the anemia is resolving. Individualized plans that target the specific type of anemia identified, as well as its underlying cause, are key to successful treatment.
CONCLUSION
When managing a patient with anemia, providers must define the type of anemia present and identify its underlying cause before starting treatment. Clues from the patient’s history, physical exam, and CBC can help isolate the cause of anemia. The MCV is the most helpful of the red blood cell indices because it allows the provider to classify the anemia as microcytic, macrocytic, or normocytic.
In cases in which the anemia is acute or severe—or in which the patient remains anemic even after being treated by the primary care provider—referral to a specialist is appropriate.
1. Brill JR, Baumgardner D. Normocytic anemia. Am Fam Physician. 2000;62(10):2255-2263.
2. Van Vranken M. Evaluation of microcytosis. Am Fam Physician. 2010;82(9):1117-1122.
3. National Institutes of Health/National Heart, Lung, and Blood Institute. How is anemia diagnosed? www.nhlbi.nih.gov/health/health-topics/topics/anemia/diagnosis. Accessed April 28, 2017.
4. Smith RE Jr. The clinical and economic burden of anemia. Am J Manag Care. 2010;16(3):S59-S66.
5. Platt A, Eckman J. Diagnosing anemia. Clinician Reviews. 2006;16(2):44-50.
6. Karnath B. Anemia in the adult patient. Hosp Physician. 2004;40(10):32-36.
7. World Health Organization. Micronutrient deficiencies. www.who.int/nutrition/topics/ida/en/#. Accessed April 29, 2017.
8. US Department of Health and Human Services, Office of Women’s Health. Iron-deficiency anemia. www.womenshealth.gov/publications/our-publications/fact-sheet/anemia.html#a. Accessed April 29, 2017.
9. DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14): 1324-1331.
10. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-936.
11. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015; 372(19):1832-1843.
12. Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671-678.
13. Rodgers GP, Young N. The Bethesda Handbook of Clinical Hematology. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 2013:1-21.
14. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
15. Hesdorffer CS, Longo DL. Drug-induced megaloblastic anemia. N Engl J Med. 2015;373(17):1649-1658.
16. Vasconcelos OM, Poehm EH, McCarter RJ, et al. Potential outcome factors in subacute combined degeneration. J Gen Intern Med. 2006;21(10):1063-1068.
17. Vide AT, Marques AM, Costa JD. MRI findings in subacute combined degeneration of the spinal cord in a patient with restricted diet. Neurol Sci. 2011;33(3):711-713.
18. Kessenich CR, Cronin K. Fecal occult blood testing in older adult patients with anemia. Nurse Pract. 2013;38(1):6-8.
19. Imashuku S, Kudo N, Kaneda S. Spontaneous resolution of macrocytic anemia: old disease revisited. J Blood Med. 2012;3:45-47.
20. Pagana K, Pagana T, Pagana T. Mosby’s Diagnostic and Laboratory Test Reference. 12th ed. St. Louis, MO: Elsevier Mosby; 2015: 497-501, 785-791, 805-806.
1. Brill JR, Baumgardner D. Normocytic anemia. Am Fam Physician. 2000;62(10):2255-2263.
2. Van Vranken M. Evaluation of microcytosis. Am Fam Physician. 2010;82(9):1117-1122.
3. National Institutes of Health/National Heart, Lung, and Blood Institute. How is anemia diagnosed? www.nhlbi.nih.gov/health/health-topics/topics/anemia/diagnosis. Accessed April 28, 2017.
4. Smith RE Jr. The clinical and economic burden of anemia. Am J Manag Care. 2010;16(3):S59-S66.
5. Platt A, Eckman J. Diagnosing anemia. Clinician Reviews. 2006;16(2):44-50.
6. Karnath B. Anemia in the adult patient. Hosp Physician. 2004;40(10):32-36.
7. World Health Organization. Micronutrient deficiencies. www.who.int/nutrition/topics/ida/en/#. Accessed April 29, 2017.
8. US Department of Health and Human Services, Office of Women’s Health. Iron-deficiency anemia. www.womenshealth.gov/publications/our-publications/fact-sheet/anemia.html#a. Accessed April 29, 2017.
9. DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14): 1324-1331.
10. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-936.
11. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015; 372(19):1832-1843.
12. Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671-678.
13. Rodgers GP, Young N. The Bethesda Handbook of Clinical Hematology. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 2013:1-21.
14. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
15. Hesdorffer CS, Longo DL. Drug-induced megaloblastic anemia. N Engl J Med. 2015;373(17):1649-1658.
16. Vasconcelos OM, Poehm EH, McCarter RJ, et al. Potential outcome factors in subacute combined degeneration. J Gen Intern Med. 2006;21(10):1063-1068.
17. Vide AT, Marques AM, Costa JD. MRI findings in subacute combined degeneration of the spinal cord in a patient with restricted diet. Neurol Sci. 2011;33(3):711-713.
18. Kessenich CR, Cronin K. Fecal occult blood testing in older adult patients with anemia. Nurse Pract. 2013;38(1):6-8.
19. Imashuku S, Kudo N, Kaneda S. Spontaneous resolution of macrocytic anemia: old disease revisited. J Blood Med. 2012;3:45-47.
20. Pagana K, Pagana T, Pagana T. Mosby’s Diagnostic and Laboratory Test Reference. 12th ed. St. Louis, MO: Elsevier Mosby; 2015: 497-501, 785-791, 805-806.
CDC: 4 conception strategies for HIV-discordant couples
What is the most effective treatment for scabies?
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Topical permethrin is the most effective treatment for classic scabies (strength of recommendation [SOR]: A, meta-analyses with consistent results).
Topical lindane and crotamiton are inferior to permethrin but appear equivalent to each other and benzyl benzoate, sulfur, and natural synergized pyrethrins (SOR: B, limited randomized trials).
Although not as effective as topical permethrin, oral ivermectin is an effective treatment compared with placebo (SOR: B, a single small randomized trial).
Oral ivermectin may reduce the prevalence of scabies at one year in populations with endemic disease more than topical permethrin (SOR: B, a single randomized trial).