User login
New antibody shows potential as prognostic marker in early rheumatoid arthritis
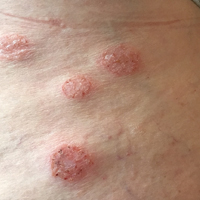
Individuals with rheumatoid arthritis who have antifibrillar collagen type II antibodies appear to have a unique disease phenotype characterized by acute but transient inflammation around the time of diagnosis, as well as a favorable prognosis, according to findings from a retrospective cohort study.
The antifibrillar collagen type II (anti-CII) phenotype also differed from the phenotype of individuals with anti–cyclic citrullinated peptide 2 (anti-CCP2) antibodies who typically exhibit inflammatory markers and higher disease activity later in the disease course, reported Vivek Anand Manivel of Uppsala (Sweden) University and his colleagues (Ann Rheum Dis. 2017 Mar 23. doi: 10.1136/annrheumdis-2016-210873).
Overall, the investigators detected anti-CII positivity in 6.6% and anti-CCP2 in 57.9%, including only anti-CII antibodies in 2.6%, only anti-CCP2 in 54%, double positivity in 3.9%, and double negativity in 39.4%. A control group of 960 patients from the Swedish general population who were matched for age, locality, and sex were positive for anti-CII in 1.6% and anti-CCP2 in 1.7%.
Significant differences were discovered between anti-CII positive and negative groups for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint count (SJC), and Disease Activity Score encompassing 28 joints (DAS28). Anti-CII seropositive patients had higher CRP at 0-6 months, higher ESR at 0-3 months, a higher SJC at 0-6 months, and a higher DAS28 at 0-3 months.
“Anti-CCP2 [seropositivity] on the other hand was associated with higher disease activity later during the 5-year follow-up, and with CRP and ESR during the full period,” the investigators wrote.
The same respective phenotypic patterns occurred when the comparisons were limited to patients with anti-CII positivity alone or anti-CCP2 positivity alone, the investigators noted. However, “patients in the anti-CII and anti-CCP2 double-positive group mainly followed the anti-CII pattern, with early but not late increased values for CRP, ESR, DAS28, and DAS28CRP, but with a mixed pattern for SJC.”
In comparisons where baseline clinical and laboratory measures were associated with anti-CII or anti-CCP2 positivity, anti-CII was more often associated with favorable late changes in CRP, ESR, SJC, TJC, DAS28, DAS28CRP, Health Assessment Questionnaire, and pain and global measurements on a visual analog scale when compared with antibody double-negative patients. There were fewer changes in clinical and laboratory measures associated with anti-CCP2 positivity. These significant changes associated with anti-CII positivity were always larger improvements than were seen for antibody double-negative patients, whereas the significant changes observed with anti-CCP2 patients were always smaller improvements than those for antibody double-negative patients.
Moderate or good European League Against Rheumatism responses were significantly associated with anti-CII positivity at 12, 24, 36, and 60 months, whereas they were negatively associated with anti-CCP2 positivity at 36 and 60 months.
Overall, the findings of the study show that since “anti-CII-positive patients with RA have an acute onset, but favorable prognosis” and anti-CCP2 is associated with poor prognosis, “the combined analysis of anti-CII and ACPA/anti-CCP2 may be a new two-dimensional tool for predicting the prognosis and choosing therapy in newly diagnosed patients with RA,” the authors suggested.
The study was funded by grants from the Swedish Research Council, Swedish Rheumatism Association, King Gustav Vth 80-Year Foundation, and Uppsala County Council. Dr. Manivel and his coauthors reported no relevant financial disclosures.
Individuals with rheumatoid arthritis who have antifibrillar collagen type II antibodies appear to have a unique disease phenotype characterized by acute but transient inflammation around the time of diagnosis, as well as a favorable prognosis, according to findings from a retrospective cohort study.
The antifibrillar collagen type II (anti-CII) phenotype also differed from the phenotype of individuals with anti–cyclic citrullinated peptide 2 (anti-CCP2) antibodies who typically exhibit inflammatory markers and higher disease activity later in the disease course, reported Vivek Anand Manivel of Uppsala (Sweden) University and his colleagues (Ann Rheum Dis. 2017 Mar 23. doi: 10.1136/annrheumdis-2016-210873).
Overall, the investigators detected anti-CII positivity in 6.6% and anti-CCP2 in 57.9%, including only anti-CII antibodies in 2.6%, only anti-CCP2 in 54%, double positivity in 3.9%, and double negativity in 39.4%. A control group of 960 patients from the Swedish general population who were matched for age, locality, and sex were positive for anti-CII in 1.6% and anti-CCP2 in 1.7%.
Significant differences were discovered between anti-CII positive and negative groups for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint count (SJC), and Disease Activity Score encompassing 28 joints (DAS28). Anti-CII seropositive patients had higher CRP at 0-6 months, higher ESR at 0-3 months, a higher SJC at 0-6 months, and a higher DAS28 at 0-3 months.
“Anti-CCP2 [seropositivity] on the other hand was associated with higher disease activity later during the 5-year follow-up, and with CRP and ESR during the full period,” the investigators wrote.
The same respective phenotypic patterns occurred when the comparisons were limited to patients with anti-CII positivity alone or anti-CCP2 positivity alone, the investigators noted. However, “patients in the anti-CII and anti-CCP2 double-positive group mainly followed the anti-CII pattern, with early but not late increased values for CRP, ESR, DAS28, and DAS28CRP, but with a mixed pattern for SJC.”
In comparisons where baseline clinical and laboratory measures were associated with anti-CII or anti-CCP2 positivity, anti-CII was more often associated with favorable late changes in CRP, ESR, SJC, TJC, DAS28, DAS28CRP, Health Assessment Questionnaire, and pain and global measurements on a visual analog scale when compared with antibody double-negative patients. There were fewer changes in clinical and laboratory measures associated with anti-CCP2 positivity. These significant changes associated with anti-CII positivity were always larger improvements than were seen for antibody double-negative patients, whereas the significant changes observed with anti-CCP2 patients were always smaller improvements than those for antibody double-negative patients.
Moderate or good European League Against Rheumatism responses were significantly associated with anti-CII positivity at 12, 24, 36, and 60 months, whereas they were negatively associated with anti-CCP2 positivity at 36 and 60 months.
Overall, the findings of the study show that since “anti-CII-positive patients with RA have an acute onset, but favorable prognosis” and anti-CCP2 is associated with poor prognosis, “the combined analysis of anti-CII and ACPA/anti-CCP2 may be a new two-dimensional tool for predicting the prognosis and choosing therapy in newly diagnosed patients with RA,” the authors suggested.
The study was funded by grants from the Swedish Research Council, Swedish Rheumatism Association, King Gustav Vth 80-Year Foundation, and Uppsala County Council. Dr. Manivel and his coauthors reported no relevant financial disclosures.
Individuals with rheumatoid arthritis who have antifibrillar collagen type II antibodies appear to have a unique disease phenotype characterized by acute but transient inflammation around the time of diagnosis, as well as a favorable prognosis, according to findings from a retrospective cohort study.
The antifibrillar collagen type II (anti-CII) phenotype also differed from the phenotype of individuals with anti–cyclic citrullinated peptide 2 (anti-CCP2) antibodies who typically exhibit inflammatory markers and higher disease activity later in the disease course, reported Vivek Anand Manivel of Uppsala (Sweden) University and his colleagues (Ann Rheum Dis. 2017 Mar 23. doi: 10.1136/annrheumdis-2016-210873).
Overall, the investigators detected anti-CII positivity in 6.6% and anti-CCP2 in 57.9%, including only anti-CII antibodies in 2.6%, only anti-CCP2 in 54%, double positivity in 3.9%, and double negativity in 39.4%. A control group of 960 patients from the Swedish general population who were matched for age, locality, and sex were positive for anti-CII in 1.6% and anti-CCP2 in 1.7%.
Significant differences were discovered between anti-CII positive and negative groups for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint count (SJC), and Disease Activity Score encompassing 28 joints (DAS28). Anti-CII seropositive patients had higher CRP at 0-6 months, higher ESR at 0-3 months, a higher SJC at 0-6 months, and a higher DAS28 at 0-3 months.
“Anti-CCP2 [seropositivity] on the other hand was associated with higher disease activity later during the 5-year follow-up, and with CRP and ESR during the full period,” the investigators wrote.
The same respective phenotypic patterns occurred when the comparisons were limited to patients with anti-CII positivity alone or anti-CCP2 positivity alone, the investigators noted. However, “patients in the anti-CII and anti-CCP2 double-positive group mainly followed the anti-CII pattern, with early but not late increased values for CRP, ESR, DAS28, and DAS28CRP, but with a mixed pattern for SJC.”
In comparisons where baseline clinical and laboratory measures were associated with anti-CII or anti-CCP2 positivity, anti-CII was more often associated with favorable late changes in CRP, ESR, SJC, TJC, DAS28, DAS28CRP, Health Assessment Questionnaire, and pain and global measurements on a visual analog scale when compared with antibody double-negative patients. There were fewer changes in clinical and laboratory measures associated with anti-CCP2 positivity. These significant changes associated with anti-CII positivity were always larger improvements than were seen for antibody double-negative patients, whereas the significant changes observed with anti-CCP2 patients were always smaller improvements than those for antibody double-negative patients.
Moderate or good European League Against Rheumatism responses were significantly associated with anti-CII positivity at 12, 24, 36, and 60 months, whereas they were negatively associated with anti-CCP2 positivity at 36 and 60 months.
Overall, the findings of the study show that since “anti-CII-positive patients with RA have an acute onset, but favorable prognosis” and anti-CCP2 is associated with poor prognosis, “the combined analysis of anti-CII and ACPA/anti-CCP2 may be a new two-dimensional tool for predicting the prognosis and choosing therapy in newly diagnosed patients with RA,” the authors suggested.
The study was funded by grants from the Swedish Research Council, Swedish Rheumatism Association, King Gustav Vth 80-Year Foundation, and Uppsala County Council. Dr. Manivel and his coauthors reported no relevant financial disclosures.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: RA patients with anti-CII seropositivity had significantly higher C-reactive protein levels at 0-6 months, higher ESR at 0-3 months, a higher SJC at 0-6 months, and a higher DAS28 at 0-3 months than did anti-CII seronegative patients.
Data source: Retrospective cohort study of EIRA study patients and 960 controls.
Disclosures: Study funded by Swedish Research Council, Swedish Rheumatism Association, King Gustav Vth 80-Year Foundation, and Uppsala County Council. The authors reported no relevant financial disclosures.
Suicide Risk in Older Adults: The Role and Responsibility of Primary Care
From the Primary Care Institute, Gainesville, FL.
Abstract
- Objective: To provide primary care practitioners with the knowledge required to identify and address older adult suicide risk in their practice.
- Methods: Review of the literature and good clinical practices.
- Results: Primary care practitioners play an important role in older adult suicide prevention and must have knowledge about older adult suicide risk, including risk factors and warning signs in this age-group. Practitioners also must appropriately screen for and manage suicide risk. Older adults, particularly older men, are at high risk for suicide, though they may be less likely to report suicide ideation. Additionally, older adults frequently see primary care practitioners within a month prior to death by suicide. A number of older adult–specific risk factors are reviewed, and appropriate screening and intervention for the primary care setting are discussed.
- Conclusion: Primary care practitioners are uniquely qualified to address a broad range of potential risk factors and should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide.
Key words: suicide; older adults; risk factors; screening; safety planning.
Primary care practitioners play an important role in older adult suicide prevention and have a responsibility to identify and address suicide risk among older adults. To do so, practitioners must understand the problem of older adult suicide, recognize risk factors for suicide in older adults, screen for suicide risk, and appropriately assess and manage suicide risk. Primary care practitioners may face challenges in completing these tasks; the goal of this article is to assist practitioners in addressing these challenges.
Suicide in Older Adults
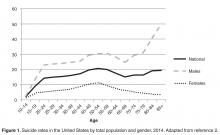
The United States has recently seen increases in suicide rates across the lifespan; from 1999 to 2014, the suicide rate rose by 24% across all ages [3]. Among both men and women aged 65 to 74, the suicide rate increased in this time period [3]. The high suicide rate among older adults is particularly important to address given the increasing numbers of older adults in the United States. By 2050, the older adult population in the United States is expected to reach 88.5 million, more than double the older adult population in 2010 [4]. Additionally, the generation that is currently aging into older adulthood has historically had higher rates of suicide across their lifespan [5]. Given that suicide rates also increase in older adulthood for men, the coming decades may evidence even higher rates of suicide among older adults than previously and it is critical that older adult suicide prevention becomes a public health priority.
It is also essential to discuss other suicide-related outcomes among older adults, including suicide attempts and suicide ideation. This is critical particularly because the ratio of suicide attempts to deaths by suicide in this age-group is 4 to 1 [1]. This is in contrast to the ratio of attempts to deaths across all ages, which is 25 suicide attempts per death by suicide [1]. This means that suicide prevention must occur before a first suicide attempt is made; suicide attempts cannot be used a marker of elevated suicide risk in older adults or an indication that intervention is needed. Intervention is required prior to suicide risk becoming elevated to the point of a suicide attempt.
It is also critical to recognize that despite the fact that suicide rates rise with age, reports of suicide ideation decrease with age [7,8]. Across all ages, 3.9% of Americans report past-year suicide ideation; however, only 2.7% of older adults report thoughts of suicide [9]. The discrepancy with the increasing rates of death by suicide with age suggest that suicide risk, and thereby opportunities for intervention, may be missed in this age-group [10].
However, older adults may be more willing to report death ideation, as research has found that over 15% of older adults endorse death ideation [11–13]. Death ideation is a desire for death without a specific desire to end one’s own life, and is an important suicide-related outcome, as older adults with death ideation appear the same as those with suicide ideation in terms of depression, hopelessness, and history of suicidal behavior [14]. Additionally, older adults with death ideation had more hospitalizations, more outpatient visits, and more medical issues than older adults with suicide ideation [15]. Therefore, death ideation should be taken as seriously as suicide ideation in older adults [14]. In sum, the high rates of death by suicide, the likelihood of death on a first or early suicide attempt, and the discrepancy between decreasing reports of suicide ideation and increasing rates of death by suicide among older adults indicate that older adult suicide is an important public health problem.
Suicide Prevention Strategies
Many suicide prevention strategies to date have focused on indicated prevention, which concentrates on individuals already identified at high risk (eg, those with suicide ideation or who have made a suicide attempt) [16]. However, because older adults may not report suicide ideation or survive a first suicide attempt, indicated prevention is likely not enough to be effective in older adult suicide prevention. A multilevel suicide prevention strategy [17] is required to prevent older adult suicide [18]. Older adult suicide prevention must include indicated prevention but must also include selective and universal prevention [16]. Selective prevention focuses on groups who may be at risk for suicide (eg, individuals with depression, older adults) and universal prevention focuses on the entire population (eg, interventions to reduce mental health stigma) [16]. To prevent older adult suicide, crisis intervention is critical, but suicide prevention efforts upstream of the development of a suicidal crisis are also essential.
The Importance of Primary Care
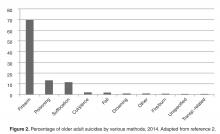
Research indicates that primary care is one of the best settings in which to engage in older adult suicide prevention [18]. Older adults are significantly less likely to receive specialty mental health care than younger adults, even when they have depressive symptoms [19]. Additionally, among older adults who died by suicide, 58% had contact with a primary care provider within a month of their deaths, compared to only 11% who had contact with a mental health specialist [20]. Among older adults who died by suicide, 67% saw any provider in the 4 weeks prior to their death [21]. Approximately 10% of older adults saw an outpatient mental health provider, 11% saw a primary care physician for a mental health issue, and 40% saw a primary care physician for a non-mental health issue [21]. Therefore, because older adults are less likely to receive specialty mental health treatment and so often seen a primary care practitioner prior to death by suicide, primary care may be the ideal place for older adult suicide risk to be detected and addressed, especially as many older adults visit primary care without a mental health presenting concern prior to their death by suicide.
Additionally, older adults may be more likely to disclose suicide ideation to primary care practitioners, with whom they are more familiar, than physicians in other settings (eg, emergency departments). Research has shown that familiarity with a primary care physician significantly increases the likelihood of patient disclosure of psychosocial issues to the physician [22]. Primary care providers also have a critical role as care coordinators; many older adults also see specialty physicians and use the emergency department. In fact, older adults are more likely to use the emergency department than younger adults, but emergency departments are not equipped to navigate the complex care needs of this population [23]. Primary care practitioners are important in ensuring that health issues of older adults are addressed by coordinating with specialists, hospitals (eg, inpatient stays, emergency department visits, surgery) and other health services (eg, home health care, physical therapy). Approximately 35% of older adults in the United States experience a lack of care coordination [24], which can negatively impact their health and leave issues such as suicide ideation unaddressed. Primary care practitioners may be critical in screening for mental health issues and suicide risk during even routine visits because of their familiarity with patients, and also play an important role in coordinating care for older adults to improve well-being and to ensure that critical issues, such as suicide ideation, are appropriately addressed.
Primary care practitioners can also be key in upstream prevention. Primary care practitioners are in a unique role to address risk factors for suicide prior to the development of a suicidal crisis. Because older adults frequently see primary care practitioners, such practitioners may have more opportunities to identify risk factors (eg, chronic pain, depression). Primary care practitioners are also trained to treat a broad range of conditions, providing the skills to address many different risk factors.
Finally, primary care is a setting in which screening for depression and suicide ideation among older adults is recommended. The US Preventive Services Task Force recommends screening for depression in all adults and older adults and provides recommended screening instruments, some of which include questions about self-harm or suicide risk [25]. However, this same group has concluded that there is insufficient evidence to support a recommendation for suicide risk screening [26]. Despite this, the Joint Commission recently released an alert that recommends screening for suicide risk in all settings, including primary care [27]. The Joint Commission requirement for ambulatory care that is relevant to suicide is PC.04.01.01: The organization has a process that addresses the patient’s need for continuing care, treatment, or services after discharge or transfer; behavioral health settings have additional suicide-specific requirements. The recommendations, though, go far beyond this requirement for primary care. The Joint Commission specifically notes that primary care clinicians play an important role in detecting suicide ideation and recommends that primary care practitioners review each patient’s history for suicide risk factors, screen all patients for suicide risk, review screenings before patients leave appointments, and take appropriate actions to address suicide risk when needed [27]. Further details are available in the Joint Commission’s Sentinel Event Alert titled, “Detecting and treating suicide ideation in all settings” [27]. Given these recommendations, primary care is an important setting in which to identify and address suicide risk.
Risk Factors for Older Adult Suicide
Numerous reviews exist that cover many risk factors for suicide in older adults [18,28]. This article will focus briefly on risk factors that are likely to be recognized and potentially addressed by primary care practitioners. Risk factors that apply across the lifespan can be recalled through a mnemonic: IS PATH WARM [29]. These risk factors include suicide Ideation, Substance abuse, Purposelessness, Anxiety (including agitation and poor sleep), feeling Trapped, Hopelessness, social Withdrawal, Anger or rage, Recklessness (ie, engaging in risky activities), and Mood changes. The National Suicide Prevention Lifeline also includes being in unbearable physical pain, perceiving one’s self as a burden to others, and seeking revenge on others as risk factors [30]. More specific to older adults, Conwell notes 5 categories or domains of risk factors with strong research support: psychiatric symptoms, somatic illness, functional impairment, social integration, and personality traits and coping [18,31].
Affective or mood disorders, particularly depression and depressive symptoms, are some of the most well-studied and strongest risk factors for older adult suicide [31]; 71% to 97% of all older adults who die by suicide have psychiatric illnesses [28]. Mood disorders, including major depressive episodes, are most consistently linked to older adult suicide risk; there is evidence as well for anxiety disorders and substance abuse disorders as risk factors, though it is somewhat mixed [28]. Therefore, screening for depression, anxiety, and substance abuse may be key to recognizing potential suicide risk. However, depression and anxiety do not present similarly in younger and older adults [32,33]. Depressive symptoms in older adults may be more somatic (eg, agitation, gastrointestinal symptoms) [32] and may reflect more anhedonia than mood changes [33]. Anxiety in older adults tends to be reported as stress or tension, whereas younger adults report feeling anxious or worried [33]. Additionally, substance abuse is often underrecognized, underdiagnosed, and undertreated in older adults [34]. Proactive screening for substance abuse is important as it may not interfere with work or other obligations in older adults, and therefore substance abuse may not be identified by older adults or others in their lives.
Physical illness may also be a risk factor for suicide [28,31]. Numerous diagnoses have been linked to suicide risk, including cancers, neurodegenerative diseases (eg, amyotrophic lateral sclerosis, Huntington disease), spinal cord injury, cardiovascular disease, and pulmonary disease [28,35]. However, overall illness burden (ie, number of chronic illnesses) [28] and self-perceived health [36] appear to be stronger risk factors than any specific illness. Additionally, authors have suggested that illness itself may not be a particularly strong risk factor, but the effect of illness on depressive symptoms [35], functioning, pain, or hopelessness due to the potential for decline over time [28] may increase suicide risk in older adults. Pain itself has been identified as a risk factor for suicide, as have perceptions of burden to others, hopelessness, and functional impairment [28].
In terms of functional impairment, research has shown that impairment in completing instrumental activities of daily living is associated with higher risk for death by suicide, and cognitive impairment may also be associated with elevated suicide risk [28]. However, there are some discrepant findings regarding the role of dementia in suicide risk, which may reflect medical and psychiatric comorbidities, as well as different stages of dementia or levels of cognitive impairment (eg, hopelessness about cognitive decline may increase suicide risk shortly after diagnosis, whereas lack of insight may decrease risk later in the course of the illness) [37]. Related to functional or cognitive impairment is perceived burdensomeness (ie, the perception that one is a liability or burden to others, to the point that others would be better off if one was gone) [38], which may also be associated with suicide risk in older adults [39,40]. Researchers have found that the interaction between perceived burdensomeness and thwarted belongingness (ie, a belief that one lacks reciprocal caring relationships and does not belong) identified older adults who were likely experiencing suicide ideation but did not report it [41]. These findings indicate that perceived burdensomeness and thwarted belongingness may be key in identifying older adults at risk for suicide.
Thwarted belongingness has also been linked to suicide ideation in older adults [41]. In fact, studies suggest that social integration is especially important for reducing suicide risk in this population [28,31,42]. A larger social network, living with others, and being active in the community are each protective against suicide [28]. Bereavement, which can reduce social connectedness and acts as a significant life stressor, is also an important risk factor [31]. Retirement may also reduce social connectedness, and employment changes have been identified as a suicide risk factor for older adults [28]. Retirement has been linked to risk for death by suicide in this population [43], and may not only serve to reduce social connectedness, but for some older adults may also be a significant role loss or loss of sense of purpose that can influence suicide risk.
Finally, rigid personality traits or coping styles are a risk factor for suicide among older adults [28,31]. As older adults face potential losses, health changes, and functional decline, effective positive coping strategies and flexibility are key to maintaining well-being. If older adults are unable to flexibly cope with these challenges, their risk for suicide increases [28].
In addition to risk factors, which confer suicide risk but do not necessarily suggest that an older adult is thinking about suicide, warning signs exist that indicate that suicide risk is imminent. These include suicidal communication (ie, talking or writing about suicide), seeking access to means, and making preparations for suicide (eg, ensuring a will is in place, giving away prized possessions). One important note is that discussing and preparing for death may be developmentally appropriate for older adults, particularly those with chronic illnesses; however, such appropriate preparation is critically different from talking about suicide or a desire for death.
Additionally, a lack of planning for the future may be a warning sign. For example, older adults who decline to schedule medical follow-up or do not wish to refill needed prescriptions may be exhibiting warning signs that should be addressed. Similarly, not following needed medical regimens (eg, an older adult with diabetes no longer taking insulin) is also a warning sign. Other, potentially more subtle warning signs may include significant changes in mood, sleep, or social interactions. Older adults may become agitated and sleep less when they are considering suicide, or may feel more at ease after they have made the decision to die by suicide and their sleep or mood may improve. Withdrawing from valued others may also be a warning sign. Finally, recent major changes (eg, loss of a spouse, moving to an assisted living facility) may be triggers for suicide risk and can serve as warning signs themselves.
Specific Screening Strategies
Given the numerous risk factors and warning signs for older adult suicide, as well as the time limitations that primary care practitioners face [44,45], it would be impractical to comprehensively assess each older adult who presents at a primary care practice. Therefore, more specific screening is necessary. Most importantly, every older adult should be screened for suicide ideation and death ideation at every visit. Screening at every visit is critical because suicide ideation may develop at any point. Previous research has included screening of over 29,000 older adults in 11 primary care settings for suicide ideation, risk of alcohol misuse, and mental health disorders [15], suggesting that suicide risk screening is feasible. Other studies have also successfully used widespread screening for depression and suicide ideation among older adults in primary care [46–48]. Additionally, in an emergency department setting, universal suicide risk screening has been associated with significantly improved risk detection [49], indicating that improved screening may be beneficial in identifying suicide risk. Importantly, asking about suicide does not cause thoughts of suicide [50]. Additionally, it is a myth that those who talk about suicide ideation will not act on these thoughts [51].
When primary care practitioners inquire about suicide ideation, they should also ask about death ideation; though some may believe that death ideation is not as significant in terms of suicide risk as suicide ideation, recall that research has not found differences in previous suicide attempts or current hopelessness among older adults with death ideation versus suicide ideation [14]. Therefore, screening for death ideation should be completed as part of every suicide risk screening.
Screening can take many forms. Screening may be oral; asking an older adult if he or she is having thoughts of suicide or is experiencing a desire to die is a brief, 2-question screening that may provide valuable information (eg, “Are you having thoughts about your own death or wanting to die?”, “Are you having thoughts of killing yourself or thinking about suicide?”). This screening may be conducted by medical assistants, nurses, care managers, or physicians, with the patient’s responses documented. Importantly, a standard procedure should be implemented to ensure older adults are consistently asked about suicide risk at each visit, but do not feel inundated by such questions from numerous staff.
If verbal questions are asked, they must be asked appropriately. Euphemisms or indirect language should not be used during a screening; older adults should be directly asked about thoughts of death and suicide, not simply asked questions such as, “Have you ever had thoughts of harming or hurting yourself?” A question like this does not adequately assess current suicide risk, as it does not assess current thoughts, nor does it specifically inquire about suicide ideation (ie, killing one’s self). It is also important to phrase questions in a manner that invites honest responses and conveys an openness to listening. For example, asking, “You’re not thinking about suicide, are you?” suggests that the practitioner wants the older adult to say no and is not comfortable with the older adult endorsing suicide ideation. Open questions that allow endorsement or denial (eg, “Are you having thoughts of killing yourself?”) imply that the practitioner is receptive to either an endorsement or denial of suicide ideation.
Alternatively, a written screening can be used; older adults may complete a questionnaire prior to their appointment or while waiting to see their practitioner. Such an assessment may be a brief screening (eg, using similar yes/no questions to an oral screening), or may be a standardized measure. For example, the Geriatric Suicide Ideation Scale [52] is a 31-item self-report measure that provides scores for suicide ideation, death ideation, loss of personal and social worth, and perceived meaning in life. Though there are not standard cutoffs that suggest high versus low suicide risk, responses can be reviewed to identify whether older adults are reporting suicide ideation or death ideation, and can also be compared to norms (ie, average scores) from other older adults [52]. This measure also has the benefit of 2 subscales that do not specifically require reporting thoughts of suicide or death (ie, loss of personal and social worth, perceived meaning in life), which may give practitioners an indication of an older adult’s suicide risk even if the older adult is not comfortable disclosing suicide ideation, as has been shown in previous research [7,8].
Similarly, the Geriatric Depression Scale, which has a validated 15-item version [53], does not directly ask about suicide ideation but has a 5-item subscale that has been found to be highly correlated with reported suicide ideation [54]. When administered to older adult primary care patients, this subscale was an effective measure of suicide ideation; a score of ≥ 1 was the best cutoff for determining whether an older adult reported suicide ideation [55].
Additionally, as noted previously, the interaction between perceived burdensomeness and thwarted belongingness may identify older adults who are potentially experiencing, but not reporting, suicide ideation [41]. The Interpersonal Needs Questionnaire [56] is the validated assessment for both perceived burdensomeness and thwarted belongingness. Perceived burdensomeness is assessed via 6 self-report items, and thwarted belongingness is assessed via 9 self-report items on this measure [56]. There are not specific cutoffs that determine high versus low perceived burdensomeness or thwarted belongingness, but older adults’ responses can provide information about their experiences of these constructs. Administration of the Interpersonal Needs Questionnaire can provide information about potential risk for suicide among older adults who may otherwise deny thoughts of suicide or death.
If the screening for suicide ideation or death ideation is positive (ie, the older adult endorses thoughts of suicide or death), the treating primary care practitioner must then follow up with additional questions to determine current level of suicide risk. To make this determination, at a minimum, follow-up questions should focus on whether the older adult has any intent to die by suicide (eg, “Do you have any intent to act on your thoughts of suicide?”), as well as whether he or she has a plan to die by suicide (eg, “Have you begun formulating a plan to die by suicide?”). When asking about a plan, it is important to determine how specific the plan is. For example, an older adult with a specific method identified and date selected to implement the plan is at much higher risk than an older adult with a relatively vague idea. It is also critical to assess for the older adult’s access to means for suicide. If an older adult has a specific plan and has the capability to carry out the plan (eg, plans to overdose on prescription medication and has large quantities of medication or high-lethality medication at home), he or she is more likely to die by suicide than an older adult who does not have access to means (eg, only has small quantities of low-lethality medication available). A general assessment of risk factors and previous suicidal behavior (ie, any previous suicide attempts) also informs decisions about level of risk and interventions.
After a screening or assessment is completed, a risk determination must be made and documented. Acute suicide risk can be categorized as low, moderate, or high. It is not appropriate to say that there is “no” suicide risk present. Low risk occurs when there is no current suicide ideation, no plan to die by suicide, and no intent to act on suicidal thoughts, especially when the patient has no history of suicidal behavior and few risk factors [57]. Moderate risk is evident when there is current suicide ideation, but no specific plan to die by suicide or intent to act on suicidal thoughts. There are likely warning signs or risk factors, which may include previous suicidal behaviors, present in moderate suicide risk [57]. High risk is indicated by current suicide ideation with plan to die by suicide and suicidal intent. There are significant warning signs and risk factors present; there may also be a recent suicide attempt, though this is not a requirement for a high risk determination [57]. Undetermined suicide risk occurs when a practitioner cannot accurately assess risk, but concern regarding suicide is present; this is primarily used when a patient refuses to answer questions about suicide. Undetermined risk should be treated as at least moderate risk. Because research shows that death ideation has similar outcomes to suicide ideation in older adults [14], death ideation should also be factored into determinations of suicide risk; reports of death ideation may indicate low or moderate risk in older adults, dependent upon other risk factors, suicidal intent, and plan.
After a risk determination is made, it must be documented in the medical record. The level of risk and rationale for that determination must be included [58]. Stating only the level of risk without a rationale (ie, the older adult’s responses to questions) is not adequate, and documenting only the older adult’s responses without a determination of risk is also not sufficient. Finally, it is critical to document the intervention that occurred or steps taken after the level of risk was determined.
Critically, stating only that there was no indication of suicide risk is inadequate. For example, documenting “No evidence of suicide risk” is not appropriate. This documentation does not indicate that the older adult was specifically asked about suicide ideation, death ideation, suicidal intent, or plan to die by suicide. It also does not indicate a level of suicide risk. Examples of appropriate documentation include:
Patient was asked about suicide risk. She denied current suicide ideation but reported death ideation. She denied any current suicidal intent or plan. She also denied any previous suicide attempts. Therefore, acute suicide risk was deemed to be low. Provided patient with wallet card about the National Suicide Prevention Lifeline. Also called the Friendship Line while in the room with the patient to connect her with services. Finally, provided a brief list of local mental health professionals to patient; the patient reported she would like to see Dr. Smith. Called and left a message for Dr. Smith with referral information with patient during appointment.
Patient was asked about suicide risk. He reported both death ideation and suicide ideation. He also reported a nonspecific plan (ie, causing a single-vehicle motor vehicle accident, with no specific plan for the motor vehicle accident or timeframe) and denied any intent to act on his thoughts of suicide. He reported one previous suicide attempt, at age 22, by overdose on over-the-counter medication. He reported that this attempt did not require medical attention. Therefore, acute suicide risk was determined to be moderate. Patient was introduced to the behavioral health specialist, who met with the patient during the appointment to conduct further assessment and intervention.
Specific Intervention Strategies
Despite the fact that the pace of the primary care setting often does not allow for time-intensive intervention, there are ways to address suicide risk in this setting. Importantly, no-suicide contracts should not be used at any time [59,60]. No-suicide contracts are documents that patients who are experiencing suicide ideation are required to sign that state that they will not die by suicide while under the care of the practitioner. These contracts have no evidence of effectiveness, and some researchers argue that they may in fact damage the relationship with patients and serve the practitioner’s needs more than the patient’s needs [59].
One of the best options for older adults at low acute suicide risk is to provide resources and referrals. The National Suicide Prevention Lifeline can be reached at 1-800-273-TALK (8255); trained counselors are available to speak to patients at all times. Wallet cards with information about the National Suicide Prevention Lifeline are available at no charge from the US Substance Abuse and Mental Health Services Administration online store. The Friendship Line is another service available free to adults ages 60 and older, 24 hours per day, 7 days per week; this line can be reached at 1-800-971-0016. The Friendship Line, which is managed by the Institute on Aging, also provides outreach calls to older adults who may be isolated or lonely, increasing connectedness and potentially reducing suicide risk.
Having a ready list of local mental health professionals with expertise in geriatrics and suicide risk to provide to the patient is also beneficial. Recall, though, that older adults are less likely to seek out and receive mental health services [19]; therefore, connecting the patient with resources or referrals during the appointment is critical. If the practitioner does not have time to do this, having a medical assistant or other staff member that the patient knows engage in this step may be appropriate. For example, the patient can call the Friendship Line or National Suicide Prevention Lifeline while in the room with the practitioner, which may reduce anxiety or stigma about doing so and connect the patient with services. Similarly, calling a local mental health professional to make a referral during the appointment may increase the likelihood that the older adult will follow up on the referral.
The most ideal method of intervention for moderate or high acute suicide risk is a warm handoff to a behavioral or mental health specialist. As primary care and behavioral health become more integrated and financially viable as reimbursement through the Centers for Medicare and Medicaid Services improves [61], it is becoming increasingly likely that such a specialist will be on-site and available. Research has found that collaborative care in primary care reduces suicide risk in older adults [46–48,62]. Mental health specialists can conduct more comprehensive assessments and spend more time intervening to reduce suicide risk among older adults with death or suicide ideation. If an on-site behavioral health specialist is not available, older adults at high suicide risk may need to be referred to an emergency department for further evaluation and follow-up. Each state has its own laws and procedures regarding this process, which should be incorporated into a practice’s procedures for addressing high suicide risk. The procedure often involves ensuring that the older adult is accompanied at all times (ie, not left alone in a room), alerting emergency services (usually via phone call to an emergency line, such as 911), and completion of paperwork by a practitioner asserting that the patient is a danger to self. Police or other emergency personnel are then responsible for transporting the patient for further evaluation and determination of whether hospitalization is required.
If more time is available, either via the treating primary care practitioner or other patient care staff in the office, other brief interventions may be beneficial. First, means safety discussions are critical, particularly for older adults with plans for suicide or access to highly lethal means. In such discussions, patients are encouraged to restrict access to the methods that they may use to die by suicide. Plans for restricting access are developed, and when possible, a support person is enlisted to ensure that the plans are carried out. For example, if an older adult has access to firearms (eg, keeps a loaded weapon in his or her nightstand), he or she is encouraged to restrict his or her access to it. Ideally, this is through removing the weapon from the home, either permanently or until suicide risk reduces (eg, giving it to a friend, turning it over to police), but more safe storage may also be an option if the older adult is not willing to remove the weapon from the home. This may mean using a gun lock or storing the weapon in a gun safe, storing ammunition separately from an unloaded weapon, removing the firing pin, or otherwise disassembling the weapon. Means safety counseling has been shown to be effective in reducing suicide rates [63] and is acceptable to patients [64]. Studies indicate that over 90% of individuals who make a suicide attempt and survive do not go on to die by suicide [65]; therefore, reducing access to highly lethal means during a suicidal crisis may be key in reducing suicide rates. Though an in-depth review of means safety counseling is outside the scope of this article, readers are directed to Bryan, Stone, and Rudd’s article for a practical overview of means safety discussions [66].
Second, safety planning is a brief intervention that may be beneficial in the primary care setting [67,68]. The goal of a safety plan is to create an individualized plan to remain safe during a suicidal crisis. Means safety discussion is the last of 6 steps in the safety plan [68]. The first 5 steps include identifying warning signs, using internal coping strategies, social connectedness as distraction, social support for the crisis, and professionals that can be used as resources. When patients can identify specific, individualized warning signs that occur prior to a crisis, they can then use strategies to cope and prevent the crisis from worsening. Coping strategies that are encouraged are first internal (ie, those that can be done without relying on anyone else), such as exercise or journaling. If those do not improve the patient’s mood, then he or she is encouraged to use people or social settings as a distraction (eg, people watching at the mall, calling an acquaintance to chat), and if he or she is still feeling bad, encouraged to get social support for the crisis (eg, calling a family member to discuss the crisis and get support). Finally, if all of these steps are not effective, the older adult is encouraged to reach out to professional supports, such as a mental health provider, the National Suicide Prevention Lifeline, or 911 (or go to an emergency room). Readers are encouraged to review Stanley and Brown’s articles for comprehensive details about safety planning as an intervention [67,68]. Additionally, an article with specific adaptations for safety planning with older adults is forthcoming [69].
Conclusion
Older adults, particularly older men, are at high risk for suicide [1,2], and primary care practitioners are a critical component of older adult suicide prevention. Older adults frequently see primary care practitioners within a month prior to death by suicide [20,21]; primary care practitioners are uniquely qualified to address a broad range of potential risk factors, and may have more interactions and familiarity with older adults at risk for suicide than other medical professionals [20–22]. Primary care practitioners should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide. Screening can consist of standardized written questionnaires or oral questioning, and interventions may include providing resources and referrals, discussions about means safety, safety planning, and handoff to a mental health specialist. Interventions for suicide risk are likely feasible and acceptable in primary care [46–48]. Primary care practitioners have an important role to play in older adult suicide prevention, and must be prepared to interact with older adults who may be at risk for suicide.
Corresponding author: Danielle R. Jahn, PhD, Primary Care Institute, 605 NE 1st St, Gainesville, FL 32605, [email protected].
Financial disclosures: None reported.
1. American Association of Suicidology. U.S.A. suicide: 2014official final data. 2016. Accessed at www.suicidology.org/Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf.
2. Centers for Disease Control and Prevention. Leading causes of death reports, national and regional, 1999-2015. 2016. Accessed at https://webappa.cdc.gov/sasweb/ncipc/leadcaus10_us.html.
3. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief, No 241. Hyattsville, MD: National Center for Health Statistics; 2016.
4. US Census Bureau. The next four decades. The older population in the United States: 2010 to 2050. 2010. Accessed at www.census.gov/prod/2010pubs/p25-1138.pdf.
5. Phillips JA, Robin AV, Nugent CN, Idler EL. Understanding recent changes in suicide rates among the middle-aged: period or cohort effects? Public Health Rep 2010;125:680–8.
6. Substance Abuse and Mental Health Services Administration. Issue brief 4: preventing suicide in older adults. 2012. Accessed at https://aoa.acl.gov/AoA_Programs/HPW/Behavioral/docs2/Issue%20Brief%204%20Preventing%20Suicide.pdf.
7. Duberstein PR, Conwell Y, Seidlitz L, et al. Age and suicidal ideation in older depressed inpatients. Am J Geriatr Psychiatry 1999;7:289–96.
8. Lynch TR, Johnson CS, Mendelson T, et al. Correlates of suicidal ideation among an elderly depressed sample. J Affect Disord 1999;56:9–15.
9. Centers for Disease Control and Prevention. Suicide: facts at a glance 2015. 2015. Accessed at www.cdc.gov/violenceprevention/pdf/suicide-datasheet-a.pdf.
10. Cukrowicz KC, Duberstein PR, Vannoy SD, et al. What factors determine disclosure of suicide ideation in adults 60 and older to a treatment provider? Suicide Life Threat Behav 2014;44:331–7.
11. Kim YA, Bogner HR, Brown GK, Gallo JJ. Chronic medical conditions and wishes to die among older primary care patients. Int J Psychiatry Med 2006;36:183–98.
12. Scocco P, Fantoni G, Rapattoni M, et al. Death ideas, suicidal thoughts, and plans among nursing home residents. J Geriatr Psychiatry Neurol 2009;22:141–8.
13. Scocco P, Meneghel G, Caon F, et al. Death ideation and its correlates: survey of an over-65-year-old population. J Nerv Ment Dis 2001;189:210–8.
14. Szanto K, Reynolds III CF, Frank E, et al. Suicide in elderly patients: is active vs. passive suicidal ideation a clinically valid distinction? Am J Geriatr Psychiatry, 2002;4:197–207.
15. Bartels SJ, Coakley E, Oxman TE, et al. Suicidal and death ideation in older primary care patients with depression, anxiety, and at-risk alcohol use. Am J Geriatr Psychiatry 2002;10:417–27.
16. Yip PSF. A public health approach to suicide prevention. Hong Kong J Psychiatry 2005;15:29–31.
17. van der Feltz-Cornelis CM, Sarchiapone M, Postuvan V, et al. Best practice elements of multilevel suicide prevention strategies: a review of systematic reviews. Crisis 2011;32:319–33.
18. Conwell Y. Suicide and suicide prevention in later life. Focus 2013;11:39–47.
19. Crabb R, Hunsley J. Utilization of mental health services among older adults with depression. J Clin Psychol 2006;62:299–312.
20. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry 2002;159:909–16.
21. Ahmedani BK, Simon GE, Stewart C, et al. Health care contacts in the year before suicide death. J Gen Intern Med 2014;29:870–7.
22. Robinson JW, Roter DL. Psychosocial problem disclosure by primary care patients. Soc Sci Med 1999;48:1353–62.
23. Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002;39:238–47.
24. Osborn R, Moulds D, Squires D, et al. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff 2014;33:2247–55.
25. Siu AL, US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;315:380–7.
26. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:719–26.
27. The Joint Commission. Detecting and treating suicide risk in all settings. Sentinel Event Alert 2016;56:1–7.
28. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68.
29. American Association of Suicidology. Know the warning signs of suicide. 2016. Accessed at www.suicidology.org/resources/warning-signs.
30. National Suicide Prevention Lifeline. Suicide warning signs. 2011. Accessed at www.suicidepreventionlifeline.org/App_Files/Media/PDF/NSPL_WalletCard.pdf.
31. Conwell Y. Suicide later in life: challenges and priorities for prevention. Am J Prev Med 2014;47:S244–50.
32. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
33. Wuthrich VM, Johnco CJ, Wetherell JL. Differences in anxiety and depression symptoms: comparison between older and younger clinical samples. Int Psychogeriatr 2015;27:1523–32.
34. Substance Abuse and Mental Health Services Administration. Substance abuse among older adults. Treatment Improvement Protocol (TIP) Series, No. 26. HHS Publication No. (SMA) 12-3918. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1998.
35. Fiske A, O’Riley AA, Widoe RK. Physical health and suicide in late life: an evaluative review. Clin Gerontologist 2008;31:31–50.
36. Duberstein PR, Conwell Y, Conner KR, et al. Suicide at 50 years of age and older: perceived physical illness, family discord, and financial strain. Psychol Med 2004;34:137–46.
37. Draper B, Peisah C, Snowdon J, Brodaty H. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement 2010;6:75–82.
38. Joiner T. Why people die by suicide. Cambridge: Harvard University Press; 2005.
39. Jahn DR, Cukrowicz KC. The impact of the nature of relationships on perceived burdensomeness and suicide ideation in a community sample of older adults. Suicide Life Threat Behav 2011;41:635–49.
40. Jahn DR, Cukrowicz KC, Linton K, Prabhu F. The mediating effect of perceived burdensomeness on the relation between depressive symptoms and suicide ideation in a community sample of older adults. Aging Ment Health 2011;15:214–20.
41. Cukrowicz KC, Jahn DR, Graham RD, et al. Suicide risk in older adults: evaluating models of risk and predicting excess zeros in a primary care sample. J Abnorm Psychol 2013;122:1021–30.
42. Fassberg MM, Van Orden KA, Duberstein, P, et al. A systematic review of social factors and suicidal behavior in older adulthood. Int J Environ Res Public Health 2012;9:722–45.
43. Pompili M, Innamorati M, Masotti V, et al. Suicide in the elderly: a psychological autopsy study in a north Italy area (1994-2004). Am J Geriatr Psychiatry 2008;16:727–35.
44. Konrad TR, Link CL, Shackelton RJ, et al. It’s about time: physicians’ perceptions of time constraints in primary medical practice in three national healthcare systems. Med Care 2010;48:95–100.
45. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res 2006;42:1871–94.
46. Bruce ML, Ten Have TR, Reynolds III CF, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. J Am Med Assoc 2004;291:1081–91.
47. Alexopoulos GS, Reynolds CF III, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
48. Unutzer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc 2006;54:1550–6.
49. Boudreaux ED, Camargo Jr CA, Arias SA, et al. Improving suicide risk screening and detection in the emergency department. Am J Prev Med 2016;50:445–53.
50. Mathias CW, Furr RM, Sheftall AH, et al. What’s the harm in asking about suicidal ideation? Suicide Life Threat Behav 2012;42:341–51.
51. Joiner T. Myths about suicide. Cambridge: Harvard University Press; 2011.
52. Heisel MJ, Flett GL. The development and initial validation of the Geriatric Suicide Ideation Scale. Am J Geriatr Psychiatry 2006;14:742–51.
53. Sheikh JL, Yesavage JA. Geriatric Depression Scale: recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention. New York: Howarth Press; 1986: 165–73.
54. Heisel MJ, Flett GL, Duberstein PR, Lyness JM. Does the Geriatric Depression Scale (GDS) distinguish between older adults with high versus low levels of suicidal ideation? Am J Geriatr Psychiatry 2005;13:876–83.
55. Heisel MJ, Duberstein PR, Lyness JM, Feldman MD. Screening for suicide ideation among older primary care patients. J Am Board Fam Med 2010;23:260–9.
56. Van Orden KA, Cukrowicz KC, Witte TK, Joiner Jr TE. Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychol Assess 2012;24;197–215.
57. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. Accessed at www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf.
58. Freedenthal S. Documentation: do it well, for the client’s sake and yours. 2013. Accessed at www.speakingofsuicide.com/2013/05/25/documentation/.
59. McMyler C, Pryjmachuk S. Do ‘no-suicide’ contracts work? J Psychiatr Ment Hlt 2008;15:512–22.
60. Rudd MD, Mandrusiak M, Joiner Jr TE. The case against no-suicide contracts: the commitment to treatment statement as a practice alternative. J Clin Psychol 2006;62:243–51.
61. National Institute of Mental Health. Adding better mental health care to primary care: a new era of behavioral health integration. 2016. Accessed at www.nimh.nih.gov/news/science-news/2016/adding-better-mental-health-care-to-primary-care.shtml.
62. Lapierre S, Erlangsen A, Waern M, et al. A systematic review of elderly suicide prevention programs. Crisis 2011;32;88–98.
63. Hawton K. Restricting access to methods of suicide: rationale and evaluation of this approach to suicide prevention. Crisis 2007;28:4–9.
64. Walters H, Kulkarni M, Forman J, et al. Feasibility and acceptability of interventions to delay gun access in VA mental health settings. Gen Hosp Psychiatry 2012;34:692–8.
65. Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm: systematic review. Brit J Psychiatry 2002;181:193–9.
66. Bryan CJ, Stone SL, Rudd MD. A practical, evidence-based approach for means-restriction counseling with suicidal patients. Prof Psychol Res Pr 2011;42:339–46.
67. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. 2008. Accessed at www.mentalhealth.va.gov/docs/va_safety_planning_manual.pdf.
68. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract 2012;19:256–64.
69. Jahn DR, Conti EC, Simons KV, et al. Evidence and considerations for safety planning as a suicide prevention strategy for older adults. 2017. Manuscript in preparation.
70. Knox KL, Stanley B, Currier GW, et al. An emergency department-based brief intervention for veterans at risk for suicide (SAFE VET). Am J Public Health 2012;102:S33–7.
From the Primary Care Institute, Gainesville, FL.
Abstract
- Objective: To provide primary care practitioners with the knowledge required to identify and address older adult suicide risk in their practice.
- Methods: Review of the literature and good clinical practices.
- Results: Primary care practitioners play an important role in older adult suicide prevention and must have knowledge about older adult suicide risk, including risk factors and warning signs in this age-group. Practitioners also must appropriately screen for and manage suicide risk. Older adults, particularly older men, are at high risk for suicide, though they may be less likely to report suicide ideation. Additionally, older adults frequently see primary care practitioners within a month prior to death by suicide. A number of older adult–specific risk factors are reviewed, and appropriate screening and intervention for the primary care setting are discussed.
- Conclusion: Primary care practitioners are uniquely qualified to address a broad range of potential risk factors and should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide.
Key words: suicide; older adults; risk factors; screening; safety planning.
Primary care practitioners play an important role in older adult suicide prevention and have a responsibility to identify and address suicide risk among older adults. To do so, practitioners must understand the problem of older adult suicide, recognize risk factors for suicide in older adults, screen for suicide risk, and appropriately assess and manage suicide risk. Primary care practitioners may face challenges in completing these tasks; the goal of this article is to assist practitioners in addressing these challenges.
Suicide in Older Adults
The United States has recently seen increases in suicide rates across the lifespan; from 1999 to 2014, the suicide rate rose by 24% across all ages [3]. Among both men and women aged 65 to 74, the suicide rate increased in this time period [3]. The high suicide rate among older adults is particularly important to address given the increasing numbers of older adults in the United States. By 2050, the older adult population in the United States is expected to reach 88.5 million, more than double the older adult population in 2010 [4]. Additionally, the generation that is currently aging into older adulthood has historically had higher rates of suicide across their lifespan [5]. Given that suicide rates also increase in older adulthood for men, the coming decades may evidence even higher rates of suicide among older adults than previously and it is critical that older adult suicide prevention becomes a public health priority.
It is also essential to discuss other suicide-related outcomes among older adults, including suicide attempts and suicide ideation. This is critical particularly because the ratio of suicide attempts to deaths by suicide in this age-group is 4 to 1 [1]. This is in contrast to the ratio of attempts to deaths across all ages, which is 25 suicide attempts per death by suicide [1]. This means that suicide prevention must occur before a first suicide attempt is made; suicide attempts cannot be used a marker of elevated suicide risk in older adults or an indication that intervention is needed. Intervention is required prior to suicide risk becoming elevated to the point of a suicide attempt.
It is also critical to recognize that despite the fact that suicide rates rise with age, reports of suicide ideation decrease with age [7,8]. Across all ages, 3.9% of Americans report past-year suicide ideation; however, only 2.7% of older adults report thoughts of suicide [9]. The discrepancy with the increasing rates of death by suicide with age suggest that suicide risk, and thereby opportunities for intervention, may be missed in this age-group [10].
However, older adults may be more willing to report death ideation, as research has found that over 15% of older adults endorse death ideation [11–13]. Death ideation is a desire for death without a specific desire to end one’s own life, and is an important suicide-related outcome, as older adults with death ideation appear the same as those with suicide ideation in terms of depression, hopelessness, and history of suicidal behavior [14]. Additionally, older adults with death ideation had more hospitalizations, more outpatient visits, and more medical issues than older adults with suicide ideation [15]. Therefore, death ideation should be taken as seriously as suicide ideation in older adults [14]. In sum, the high rates of death by suicide, the likelihood of death on a first or early suicide attempt, and the discrepancy between decreasing reports of suicide ideation and increasing rates of death by suicide among older adults indicate that older adult suicide is an important public health problem.
Suicide Prevention Strategies
Many suicide prevention strategies to date have focused on indicated prevention, which concentrates on individuals already identified at high risk (eg, those with suicide ideation or who have made a suicide attempt) [16]. However, because older adults may not report suicide ideation or survive a first suicide attempt, indicated prevention is likely not enough to be effective in older adult suicide prevention. A multilevel suicide prevention strategy [17] is required to prevent older adult suicide [18]. Older adult suicide prevention must include indicated prevention but must also include selective and universal prevention [16]. Selective prevention focuses on groups who may be at risk for suicide (eg, individuals with depression, older adults) and universal prevention focuses on the entire population (eg, interventions to reduce mental health stigma) [16]. To prevent older adult suicide, crisis intervention is critical, but suicide prevention efforts upstream of the development of a suicidal crisis are also essential.
The Importance of Primary Care
Research indicates that primary care is one of the best settings in which to engage in older adult suicide prevention [18]. Older adults are significantly less likely to receive specialty mental health care than younger adults, even when they have depressive symptoms [19]. Additionally, among older adults who died by suicide, 58% had contact with a primary care provider within a month of their deaths, compared to only 11% who had contact with a mental health specialist [20]. Among older adults who died by suicide, 67% saw any provider in the 4 weeks prior to their death [21]. Approximately 10% of older adults saw an outpatient mental health provider, 11% saw a primary care physician for a mental health issue, and 40% saw a primary care physician for a non-mental health issue [21]. Therefore, because older adults are less likely to receive specialty mental health treatment and so often seen a primary care practitioner prior to death by suicide, primary care may be the ideal place for older adult suicide risk to be detected and addressed, especially as many older adults visit primary care without a mental health presenting concern prior to their death by suicide.
Additionally, older adults may be more likely to disclose suicide ideation to primary care practitioners, with whom they are more familiar, than physicians in other settings (eg, emergency departments). Research has shown that familiarity with a primary care physician significantly increases the likelihood of patient disclosure of psychosocial issues to the physician [22]. Primary care providers also have a critical role as care coordinators; many older adults also see specialty physicians and use the emergency department. In fact, older adults are more likely to use the emergency department than younger adults, but emergency departments are not equipped to navigate the complex care needs of this population [23]. Primary care practitioners are important in ensuring that health issues of older adults are addressed by coordinating with specialists, hospitals (eg, inpatient stays, emergency department visits, surgery) and other health services (eg, home health care, physical therapy). Approximately 35% of older adults in the United States experience a lack of care coordination [24], which can negatively impact their health and leave issues such as suicide ideation unaddressed. Primary care practitioners may be critical in screening for mental health issues and suicide risk during even routine visits because of their familiarity with patients, and also play an important role in coordinating care for older adults to improve well-being and to ensure that critical issues, such as suicide ideation, are appropriately addressed.
Primary care practitioners can also be key in upstream prevention. Primary care practitioners are in a unique role to address risk factors for suicide prior to the development of a suicidal crisis. Because older adults frequently see primary care practitioners, such practitioners may have more opportunities to identify risk factors (eg, chronic pain, depression). Primary care practitioners are also trained to treat a broad range of conditions, providing the skills to address many different risk factors.
Finally, primary care is a setting in which screening for depression and suicide ideation among older adults is recommended. The US Preventive Services Task Force recommends screening for depression in all adults and older adults and provides recommended screening instruments, some of which include questions about self-harm or suicide risk [25]. However, this same group has concluded that there is insufficient evidence to support a recommendation for suicide risk screening [26]. Despite this, the Joint Commission recently released an alert that recommends screening for suicide risk in all settings, including primary care [27]. The Joint Commission requirement for ambulatory care that is relevant to suicide is PC.04.01.01: The organization has a process that addresses the patient’s need for continuing care, treatment, or services after discharge or transfer; behavioral health settings have additional suicide-specific requirements. The recommendations, though, go far beyond this requirement for primary care. The Joint Commission specifically notes that primary care clinicians play an important role in detecting suicide ideation and recommends that primary care practitioners review each patient’s history for suicide risk factors, screen all patients for suicide risk, review screenings before patients leave appointments, and take appropriate actions to address suicide risk when needed [27]. Further details are available in the Joint Commission’s Sentinel Event Alert titled, “Detecting and treating suicide ideation in all settings” [27]. Given these recommendations, primary care is an important setting in which to identify and address suicide risk.
Risk Factors for Older Adult Suicide
Numerous reviews exist that cover many risk factors for suicide in older adults [18,28]. This article will focus briefly on risk factors that are likely to be recognized and potentially addressed by primary care practitioners. Risk factors that apply across the lifespan can be recalled through a mnemonic: IS PATH WARM [29]. These risk factors include suicide Ideation, Substance abuse, Purposelessness, Anxiety (including agitation and poor sleep), feeling Trapped, Hopelessness, social Withdrawal, Anger or rage, Recklessness (ie, engaging in risky activities), and Mood changes. The National Suicide Prevention Lifeline also includes being in unbearable physical pain, perceiving one’s self as a burden to others, and seeking revenge on others as risk factors [30]. More specific to older adults, Conwell notes 5 categories or domains of risk factors with strong research support: psychiatric symptoms, somatic illness, functional impairment, social integration, and personality traits and coping [18,31].
Affective or mood disorders, particularly depression and depressive symptoms, are some of the most well-studied and strongest risk factors for older adult suicide [31]; 71% to 97% of all older adults who die by suicide have psychiatric illnesses [28]. Mood disorders, including major depressive episodes, are most consistently linked to older adult suicide risk; there is evidence as well for anxiety disorders and substance abuse disorders as risk factors, though it is somewhat mixed [28]. Therefore, screening for depression, anxiety, and substance abuse may be key to recognizing potential suicide risk. However, depression and anxiety do not present similarly in younger and older adults [32,33]. Depressive symptoms in older adults may be more somatic (eg, agitation, gastrointestinal symptoms) [32] and may reflect more anhedonia than mood changes [33]. Anxiety in older adults tends to be reported as stress or tension, whereas younger adults report feeling anxious or worried [33]. Additionally, substance abuse is often underrecognized, underdiagnosed, and undertreated in older adults [34]. Proactive screening for substance abuse is important as it may not interfere with work or other obligations in older adults, and therefore substance abuse may not be identified by older adults or others in their lives.
Physical illness may also be a risk factor for suicide [28,31]. Numerous diagnoses have been linked to suicide risk, including cancers, neurodegenerative diseases (eg, amyotrophic lateral sclerosis, Huntington disease), spinal cord injury, cardiovascular disease, and pulmonary disease [28,35]. However, overall illness burden (ie, number of chronic illnesses) [28] and self-perceived health [36] appear to be stronger risk factors than any specific illness. Additionally, authors have suggested that illness itself may not be a particularly strong risk factor, but the effect of illness on depressive symptoms [35], functioning, pain, or hopelessness due to the potential for decline over time [28] may increase suicide risk in older adults. Pain itself has been identified as a risk factor for suicide, as have perceptions of burden to others, hopelessness, and functional impairment [28].
In terms of functional impairment, research has shown that impairment in completing instrumental activities of daily living is associated with higher risk for death by suicide, and cognitive impairment may also be associated with elevated suicide risk [28]. However, there are some discrepant findings regarding the role of dementia in suicide risk, which may reflect medical and psychiatric comorbidities, as well as different stages of dementia or levels of cognitive impairment (eg, hopelessness about cognitive decline may increase suicide risk shortly after diagnosis, whereas lack of insight may decrease risk later in the course of the illness) [37]. Related to functional or cognitive impairment is perceived burdensomeness (ie, the perception that one is a liability or burden to others, to the point that others would be better off if one was gone) [38], which may also be associated with suicide risk in older adults [39,40]. Researchers have found that the interaction between perceived burdensomeness and thwarted belongingness (ie, a belief that one lacks reciprocal caring relationships and does not belong) identified older adults who were likely experiencing suicide ideation but did not report it [41]. These findings indicate that perceived burdensomeness and thwarted belongingness may be key in identifying older adults at risk for suicide.
Thwarted belongingness has also been linked to suicide ideation in older adults [41]. In fact, studies suggest that social integration is especially important for reducing suicide risk in this population [28,31,42]. A larger social network, living with others, and being active in the community are each protective against suicide [28]. Bereavement, which can reduce social connectedness and acts as a significant life stressor, is also an important risk factor [31]. Retirement may also reduce social connectedness, and employment changes have been identified as a suicide risk factor for older adults [28]. Retirement has been linked to risk for death by suicide in this population [43], and may not only serve to reduce social connectedness, but for some older adults may also be a significant role loss or loss of sense of purpose that can influence suicide risk.
Finally, rigid personality traits or coping styles are a risk factor for suicide among older adults [28,31]. As older adults face potential losses, health changes, and functional decline, effective positive coping strategies and flexibility are key to maintaining well-being. If older adults are unable to flexibly cope with these challenges, their risk for suicide increases [28].
In addition to risk factors, which confer suicide risk but do not necessarily suggest that an older adult is thinking about suicide, warning signs exist that indicate that suicide risk is imminent. These include suicidal communication (ie, talking or writing about suicide), seeking access to means, and making preparations for suicide (eg, ensuring a will is in place, giving away prized possessions). One important note is that discussing and preparing for death may be developmentally appropriate for older adults, particularly those with chronic illnesses; however, such appropriate preparation is critically different from talking about suicide or a desire for death.
Additionally, a lack of planning for the future may be a warning sign. For example, older adults who decline to schedule medical follow-up or do not wish to refill needed prescriptions may be exhibiting warning signs that should be addressed. Similarly, not following needed medical regimens (eg, an older adult with diabetes no longer taking insulin) is also a warning sign. Other, potentially more subtle warning signs may include significant changes in mood, sleep, or social interactions. Older adults may become agitated and sleep less when they are considering suicide, or may feel more at ease after they have made the decision to die by suicide and their sleep or mood may improve. Withdrawing from valued others may also be a warning sign. Finally, recent major changes (eg, loss of a spouse, moving to an assisted living facility) may be triggers for suicide risk and can serve as warning signs themselves.
Specific Screening Strategies
Given the numerous risk factors and warning signs for older adult suicide, as well as the time limitations that primary care practitioners face [44,45], it would be impractical to comprehensively assess each older adult who presents at a primary care practice. Therefore, more specific screening is necessary. Most importantly, every older adult should be screened for suicide ideation and death ideation at every visit. Screening at every visit is critical because suicide ideation may develop at any point. Previous research has included screening of over 29,000 older adults in 11 primary care settings for suicide ideation, risk of alcohol misuse, and mental health disorders [15], suggesting that suicide risk screening is feasible. Other studies have also successfully used widespread screening for depression and suicide ideation among older adults in primary care [46–48]. Additionally, in an emergency department setting, universal suicide risk screening has been associated with significantly improved risk detection [49], indicating that improved screening may be beneficial in identifying suicide risk. Importantly, asking about suicide does not cause thoughts of suicide [50]. Additionally, it is a myth that those who talk about suicide ideation will not act on these thoughts [51].
When primary care practitioners inquire about suicide ideation, they should also ask about death ideation; though some may believe that death ideation is not as significant in terms of suicide risk as suicide ideation, recall that research has not found differences in previous suicide attempts or current hopelessness among older adults with death ideation versus suicide ideation [14]. Therefore, screening for death ideation should be completed as part of every suicide risk screening.
Screening can take many forms. Screening may be oral; asking an older adult if he or she is having thoughts of suicide or is experiencing a desire to die is a brief, 2-question screening that may provide valuable information (eg, “Are you having thoughts about your own death or wanting to die?”, “Are you having thoughts of killing yourself or thinking about suicide?”). This screening may be conducted by medical assistants, nurses, care managers, or physicians, with the patient’s responses documented. Importantly, a standard procedure should be implemented to ensure older adults are consistently asked about suicide risk at each visit, but do not feel inundated by such questions from numerous staff.
If verbal questions are asked, they must be asked appropriately. Euphemisms or indirect language should not be used during a screening; older adults should be directly asked about thoughts of death and suicide, not simply asked questions such as, “Have you ever had thoughts of harming or hurting yourself?” A question like this does not adequately assess current suicide risk, as it does not assess current thoughts, nor does it specifically inquire about suicide ideation (ie, killing one’s self). It is also important to phrase questions in a manner that invites honest responses and conveys an openness to listening. For example, asking, “You’re not thinking about suicide, are you?” suggests that the practitioner wants the older adult to say no and is not comfortable with the older adult endorsing suicide ideation. Open questions that allow endorsement or denial (eg, “Are you having thoughts of killing yourself?”) imply that the practitioner is receptive to either an endorsement or denial of suicide ideation.
Alternatively, a written screening can be used; older adults may complete a questionnaire prior to their appointment or while waiting to see their practitioner. Such an assessment may be a brief screening (eg, using similar yes/no questions to an oral screening), or may be a standardized measure. For example, the Geriatric Suicide Ideation Scale [52] is a 31-item self-report measure that provides scores for suicide ideation, death ideation, loss of personal and social worth, and perceived meaning in life. Though there are not standard cutoffs that suggest high versus low suicide risk, responses can be reviewed to identify whether older adults are reporting suicide ideation or death ideation, and can also be compared to norms (ie, average scores) from other older adults [52]. This measure also has the benefit of 2 subscales that do not specifically require reporting thoughts of suicide or death (ie, loss of personal and social worth, perceived meaning in life), which may give practitioners an indication of an older adult’s suicide risk even if the older adult is not comfortable disclosing suicide ideation, as has been shown in previous research [7,8].
Similarly, the Geriatric Depression Scale, which has a validated 15-item version [53], does not directly ask about suicide ideation but has a 5-item subscale that has been found to be highly correlated with reported suicide ideation [54]. When administered to older adult primary care patients, this subscale was an effective measure of suicide ideation; a score of ≥ 1 was the best cutoff for determining whether an older adult reported suicide ideation [55].
Additionally, as noted previously, the interaction between perceived burdensomeness and thwarted belongingness may identify older adults who are potentially experiencing, but not reporting, suicide ideation [41]. The Interpersonal Needs Questionnaire [56] is the validated assessment for both perceived burdensomeness and thwarted belongingness. Perceived burdensomeness is assessed via 6 self-report items, and thwarted belongingness is assessed via 9 self-report items on this measure [56]. There are not specific cutoffs that determine high versus low perceived burdensomeness or thwarted belongingness, but older adults’ responses can provide information about their experiences of these constructs. Administration of the Interpersonal Needs Questionnaire can provide information about potential risk for suicide among older adults who may otherwise deny thoughts of suicide or death.
If the screening for suicide ideation or death ideation is positive (ie, the older adult endorses thoughts of suicide or death), the treating primary care practitioner must then follow up with additional questions to determine current level of suicide risk. To make this determination, at a minimum, follow-up questions should focus on whether the older adult has any intent to die by suicide (eg, “Do you have any intent to act on your thoughts of suicide?”), as well as whether he or she has a plan to die by suicide (eg, “Have you begun formulating a plan to die by suicide?”). When asking about a plan, it is important to determine how specific the plan is. For example, an older adult with a specific method identified and date selected to implement the plan is at much higher risk than an older adult with a relatively vague idea. It is also critical to assess for the older adult’s access to means for suicide. If an older adult has a specific plan and has the capability to carry out the plan (eg, plans to overdose on prescription medication and has large quantities of medication or high-lethality medication at home), he or she is more likely to die by suicide than an older adult who does not have access to means (eg, only has small quantities of low-lethality medication available). A general assessment of risk factors and previous suicidal behavior (ie, any previous suicide attempts) also informs decisions about level of risk and interventions.
After a screening or assessment is completed, a risk determination must be made and documented. Acute suicide risk can be categorized as low, moderate, or high. It is not appropriate to say that there is “no” suicide risk present. Low risk occurs when there is no current suicide ideation, no plan to die by suicide, and no intent to act on suicidal thoughts, especially when the patient has no history of suicidal behavior and few risk factors [57]. Moderate risk is evident when there is current suicide ideation, but no specific plan to die by suicide or intent to act on suicidal thoughts. There are likely warning signs or risk factors, which may include previous suicidal behaviors, present in moderate suicide risk [57]. High risk is indicated by current suicide ideation with plan to die by suicide and suicidal intent. There are significant warning signs and risk factors present; there may also be a recent suicide attempt, though this is not a requirement for a high risk determination [57]. Undetermined suicide risk occurs when a practitioner cannot accurately assess risk, but concern regarding suicide is present; this is primarily used when a patient refuses to answer questions about suicide. Undetermined risk should be treated as at least moderate risk. Because research shows that death ideation has similar outcomes to suicide ideation in older adults [14], death ideation should also be factored into determinations of suicide risk; reports of death ideation may indicate low or moderate risk in older adults, dependent upon other risk factors, suicidal intent, and plan.
After a risk determination is made, it must be documented in the medical record. The level of risk and rationale for that determination must be included [58]. Stating only the level of risk without a rationale (ie, the older adult’s responses to questions) is not adequate, and documenting only the older adult’s responses without a determination of risk is also not sufficient. Finally, it is critical to document the intervention that occurred or steps taken after the level of risk was determined.
Critically, stating only that there was no indication of suicide risk is inadequate. For example, documenting “No evidence of suicide risk” is not appropriate. This documentation does not indicate that the older adult was specifically asked about suicide ideation, death ideation, suicidal intent, or plan to die by suicide. It also does not indicate a level of suicide risk. Examples of appropriate documentation include:
Patient was asked about suicide risk. She denied current suicide ideation but reported death ideation. She denied any current suicidal intent or plan. She also denied any previous suicide attempts. Therefore, acute suicide risk was deemed to be low. Provided patient with wallet card about the National Suicide Prevention Lifeline. Also called the Friendship Line while in the room with the patient to connect her with services. Finally, provided a brief list of local mental health professionals to patient; the patient reported she would like to see Dr. Smith. Called and left a message for Dr. Smith with referral information with patient during appointment.
Patient was asked about suicide risk. He reported both death ideation and suicide ideation. He also reported a nonspecific plan (ie, causing a single-vehicle motor vehicle accident, with no specific plan for the motor vehicle accident or timeframe) and denied any intent to act on his thoughts of suicide. He reported one previous suicide attempt, at age 22, by overdose on over-the-counter medication. He reported that this attempt did not require medical attention. Therefore, acute suicide risk was determined to be moderate. Patient was introduced to the behavioral health specialist, who met with the patient during the appointment to conduct further assessment and intervention.
Specific Intervention Strategies
Despite the fact that the pace of the primary care setting often does not allow for time-intensive intervention, there are ways to address suicide risk in this setting. Importantly, no-suicide contracts should not be used at any time [59,60]. No-suicide contracts are documents that patients who are experiencing suicide ideation are required to sign that state that they will not die by suicide while under the care of the practitioner. These contracts have no evidence of effectiveness, and some researchers argue that they may in fact damage the relationship with patients and serve the practitioner’s needs more than the patient’s needs [59].
One of the best options for older adults at low acute suicide risk is to provide resources and referrals. The National Suicide Prevention Lifeline can be reached at 1-800-273-TALK (8255); trained counselors are available to speak to patients at all times. Wallet cards with information about the National Suicide Prevention Lifeline are available at no charge from the US Substance Abuse and Mental Health Services Administration online store. The Friendship Line is another service available free to adults ages 60 and older, 24 hours per day, 7 days per week; this line can be reached at 1-800-971-0016. The Friendship Line, which is managed by the Institute on Aging, also provides outreach calls to older adults who may be isolated or lonely, increasing connectedness and potentially reducing suicide risk.
Having a ready list of local mental health professionals with expertise in geriatrics and suicide risk to provide to the patient is also beneficial. Recall, though, that older adults are less likely to seek out and receive mental health services [19]; therefore, connecting the patient with resources or referrals during the appointment is critical. If the practitioner does not have time to do this, having a medical assistant or other staff member that the patient knows engage in this step may be appropriate. For example, the patient can call the Friendship Line or National Suicide Prevention Lifeline while in the room with the practitioner, which may reduce anxiety or stigma about doing so and connect the patient with services. Similarly, calling a local mental health professional to make a referral during the appointment may increase the likelihood that the older adult will follow up on the referral.
The most ideal method of intervention for moderate or high acute suicide risk is a warm handoff to a behavioral or mental health specialist. As primary care and behavioral health become more integrated and financially viable as reimbursement through the Centers for Medicare and Medicaid Services improves [61], it is becoming increasingly likely that such a specialist will be on-site and available. Research has found that collaborative care in primary care reduces suicide risk in older adults [46–48,62]. Mental health specialists can conduct more comprehensive assessments and spend more time intervening to reduce suicide risk among older adults with death or suicide ideation. If an on-site behavioral health specialist is not available, older adults at high suicide risk may need to be referred to an emergency department for further evaluation and follow-up. Each state has its own laws and procedures regarding this process, which should be incorporated into a practice’s procedures for addressing high suicide risk. The procedure often involves ensuring that the older adult is accompanied at all times (ie, not left alone in a room), alerting emergency services (usually via phone call to an emergency line, such as 911), and completion of paperwork by a practitioner asserting that the patient is a danger to self. Police or other emergency personnel are then responsible for transporting the patient for further evaluation and determination of whether hospitalization is required.
If more time is available, either via the treating primary care practitioner or other patient care staff in the office, other brief interventions may be beneficial. First, means safety discussions are critical, particularly for older adults with plans for suicide or access to highly lethal means. In such discussions, patients are encouraged to restrict access to the methods that they may use to die by suicide. Plans for restricting access are developed, and when possible, a support person is enlisted to ensure that the plans are carried out. For example, if an older adult has access to firearms (eg, keeps a loaded weapon in his or her nightstand), he or she is encouraged to restrict his or her access to it. Ideally, this is through removing the weapon from the home, either permanently or until suicide risk reduces (eg, giving it to a friend, turning it over to police), but more safe storage may also be an option if the older adult is not willing to remove the weapon from the home. This may mean using a gun lock or storing the weapon in a gun safe, storing ammunition separately from an unloaded weapon, removing the firing pin, or otherwise disassembling the weapon. Means safety counseling has been shown to be effective in reducing suicide rates [63] and is acceptable to patients [64]. Studies indicate that over 90% of individuals who make a suicide attempt and survive do not go on to die by suicide [65]; therefore, reducing access to highly lethal means during a suicidal crisis may be key in reducing suicide rates. Though an in-depth review of means safety counseling is outside the scope of this article, readers are directed to Bryan, Stone, and Rudd’s article for a practical overview of means safety discussions [66].
Second, safety planning is a brief intervention that may be beneficial in the primary care setting [67,68]. The goal of a safety plan is to create an individualized plan to remain safe during a suicidal crisis. Means safety discussion is the last of 6 steps in the safety plan [68]. The first 5 steps include identifying warning signs, using internal coping strategies, social connectedness as distraction, social support for the crisis, and professionals that can be used as resources. When patients can identify specific, individualized warning signs that occur prior to a crisis, they can then use strategies to cope and prevent the crisis from worsening. Coping strategies that are encouraged are first internal (ie, those that can be done without relying on anyone else), such as exercise or journaling. If those do not improve the patient’s mood, then he or she is encouraged to use people or social settings as a distraction (eg, people watching at the mall, calling an acquaintance to chat), and if he or she is still feeling bad, encouraged to get social support for the crisis (eg, calling a family member to discuss the crisis and get support). Finally, if all of these steps are not effective, the older adult is encouraged to reach out to professional supports, such as a mental health provider, the National Suicide Prevention Lifeline, or 911 (or go to an emergency room). Readers are encouraged to review Stanley and Brown’s articles for comprehensive details about safety planning as an intervention [67,68]. Additionally, an article with specific adaptations for safety planning with older adults is forthcoming [69].
Conclusion
Older adults, particularly older men, are at high risk for suicide [1,2], and primary care practitioners are a critical component of older adult suicide prevention. Older adults frequently see primary care practitioners within a month prior to death by suicide [20,21]; primary care practitioners are uniquely qualified to address a broad range of potential risk factors, and may have more interactions and familiarity with older adults at risk for suicide than other medical professionals [20–22]. Primary care practitioners should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide. Screening can consist of standardized written questionnaires or oral questioning, and interventions may include providing resources and referrals, discussions about means safety, safety planning, and handoff to a mental health specialist. Interventions for suicide risk are likely feasible and acceptable in primary care [46–48]. Primary care practitioners have an important role to play in older adult suicide prevention, and must be prepared to interact with older adults who may be at risk for suicide.
Corresponding author: Danielle R. Jahn, PhD, Primary Care Institute, 605 NE 1st St, Gainesville, FL 32605, [email protected].
Financial disclosures: None reported.
From the Primary Care Institute, Gainesville, FL.
Abstract
- Objective: To provide primary care practitioners with the knowledge required to identify and address older adult suicide risk in their practice.
- Methods: Review of the literature and good clinical practices.
- Results: Primary care practitioners play an important role in older adult suicide prevention and must have knowledge about older adult suicide risk, including risk factors and warning signs in this age-group. Practitioners also must appropriately screen for and manage suicide risk. Older adults, particularly older men, are at high risk for suicide, though they may be less likely to report suicide ideation. Additionally, older adults frequently see primary care practitioners within a month prior to death by suicide. A number of older adult–specific risk factors are reviewed, and appropriate screening and intervention for the primary care setting are discussed.
- Conclusion: Primary care practitioners are uniquely qualified to address a broad range of potential risk factors and should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide.
Key words: suicide; older adults; risk factors; screening; safety planning.
Primary care practitioners play an important role in older adult suicide prevention and have a responsibility to identify and address suicide risk among older adults. To do so, practitioners must understand the problem of older adult suicide, recognize risk factors for suicide in older adults, screen for suicide risk, and appropriately assess and manage suicide risk. Primary care practitioners may face challenges in completing these tasks; the goal of this article is to assist practitioners in addressing these challenges.
Suicide in Older Adults
The United States has recently seen increases in suicide rates across the lifespan; from 1999 to 2014, the suicide rate rose by 24% across all ages [3]. Among both men and women aged 65 to 74, the suicide rate increased in this time period [3]. The high suicide rate among older adults is particularly important to address given the increasing numbers of older adults in the United States. By 2050, the older adult population in the United States is expected to reach 88.5 million, more than double the older adult population in 2010 [4]. Additionally, the generation that is currently aging into older adulthood has historically had higher rates of suicide across their lifespan [5]. Given that suicide rates also increase in older adulthood for men, the coming decades may evidence even higher rates of suicide among older adults than previously and it is critical that older adult suicide prevention becomes a public health priority.
It is also essential to discuss other suicide-related outcomes among older adults, including suicide attempts and suicide ideation. This is critical particularly because the ratio of suicide attempts to deaths by suicide in this age-group is 4 to 1 [1]. This is in contrast to the ratio of attempts to deaths across all ages, which is 25 suicide attempts per death by suicide [1]. This means that suicide prevention must occur before a first suicide attempt is made; suicide attempts cannot be used a marker of elevated suicide risk in older adults or an indication that intervention is needed. Intervention is required prior to suicide risk becoming elevated to the point of a suicide attempt.
It is also critical to recognize that despite the fact that suicide rates rise with age, reports of suicide ideation decrease with age [7,8]. Across all ages, 3.9% of Americans report past-year suicide ideation; however, only 2.7% of older adults report thoughts of suicide [9]. The discrepancy with the increasing rates of death by suicide with age suggest that suicide risk, and thereby opportunities for intervention, may be missed in this age-group [10].
However, older adults may be more willing to report death ideation, as research has found that over 15% of older adults endorse death ideation [11–13]. Death ideation is a desire for death without a specific desire to end one’s own life, and is an important suicide-related outcome, as older adults with death ideation appear the same as those with suicide ideation in terms of depression, hopelessness, and history of suicidal behavior [14]. Additionally, older adults with death ideation had more hospitalizations, more outpatient visits, and more medical issues than older adults with suicide ideation [15]. Therefore, death ideation should be taken as seriously as suicide ideation in older adults [14]. In sum, the high rates of death by suicide, the likelihood of death on a first or early suicide attempt, and the discrepancy between decreasing reports of suicide ideation and increasing rates of death by suicide among older adults indicate that older adult suicide is an important public health problem.
Suicide Prevention Strategies
Many suicide prevention strategies to date have focused on indicated prevention, which concentrates on individuals already identified at high risk (eg, those with suicide ideation or who have made a suicide attempt) [16]. However, because older adults may not report suicide ideation or survive a first suicide attempt, indicated prevention is likely not enough to be effective in older adult suicide prevention. A multilevel suicide prevention strategy [17] is required to prevent older adult suicide [18]. Older adult suicide prevention must include indicated prevention but must also include selective and universal prevention [16]. Selective prevention focuses on groups who may be at risk for suicide (eg, individuals with depression, older adults) and universal prevention focuses on the entire population (eg, interventions to reduce mental health stigma) [16]. To prevent older adult suicide, crisis intervention is critical, but suicide prevention efforts upstream of the development of a suicidal crisis are also essential.
The Importance of Primary Care
Research indicates that primary care is one of the best settings in which to engage in older adult suicide prevention [18]. Older adults are significantly less likely to receive specialty mental health care than younger adults, even when they have depressive symptoms [19]. Additionally, among older adults who died by suicide, 58% had contact with a primary care provider within a month of their deaths, compared to only 11% who had contact with a mental health specialist [20]. Among older adults who died by suicide, 67% saw any provider in the 4 weeks prior to their death [21]. Approximately 10% of older adults saw an outpatient mental health provider, 11% saw a primary care physician for a mental health issue, and 40% saw a primary care physician for a non-mental health issue [21]. Therefore, because older adults are less likely to receive specialty mental health treatment and so often seen a primary care practitioner prior to death by suicide, primary care may be the ideal place for older adult suicide risk to be detected and addressed, especially as many older adults visit primary care without a mental health presenting concern prior to their death by suicide.
Additionally, older adults may be more likely to disclose suicide ideation to primary care practitioners, with whom they are more familiar, than physicians in other settings (eg, emergency departments). Research has shown that familiarity with a primary care physician significantly increases the likelihood of patient disclosure of psychosocial issues to the physician [22]. Primary care providers also have a critical role as care coordinators; many older adults also see specialty physicians and use the emergency department. In fact, older adults are more likely to use the emergency department than younger adults, but emergency departments are not equipped to navigate the complex care needs of this population [23]. Primary care practitioners are important in ensuring that health issues of older adults are addressed by coordinating with specialists, hospitals (eg, inpatient stays, emergency department visits, surgery) and other health services (eg, home health care, physical therapy). Approximately 35% of older adults in the United States experience a lack of care coordination [24], which can negatively impact their health and leave issues such as suicide ideation unaddressed. Primary care practitioners may be critical in screening for mental health issues and suicide risk during even routine visits because of their familiarity with patients, and also play an important role in coordinating care for older adults to improve well-being and to ensure that critical issues, such as suicide ideation, are appropriately addressed.
Primary care practitioners can also be key in upstream prevention. Primary care practitioners are in a unique role to address risk factors for suicide prior to the development of a suicidal crisis. Because older adults frequently see primary care practitioners, such practitioners may have more opportunities to identify risk factors (eg, chronic pain, depression). Primary care practitioners are also trained to treat a broad range of conditions, providing the skills to address many different risk factors.
Finally, primary care is a setting in which screening for depression and suicide ideation among older adults is recommended. The US Preventive Services Task Force recommends screening for depression in all adults and older adults and provides recommended screening instruments, some of which include questions about self-harm or suicide risk [25]. However, this same group has concluded that there is insufficient evidence to support a recommendation for suicide risk screening [26]. Despite this, the Joint Commission recently released an alert that recommends screening for suicide risk in all settings, including primary care [27]. The Joint Commission requirement for ambulatory care that is relevant to suicide is PC.04.01.01: The organization has a process that addresses the patient’s need for continuing care, treatment, or services after discharge or transfer; behavioral health settings have additional suicide-specific requirements. The recommendations, though, go far beyond this requirement for primary care. The Joint Commission specifically notes that primary care clinicians play an important role in detecting suicide ideation and recommends that primary care practitioners review each patient’s history for suicide risk factors, screen all patients for suicide risk, review screenings before patients leave appointments, and take appropriate actions to address suicide risk when needed [27]. Further details are available in the Joint Commission’s Sentinel Event Alert titled, “Detecting and treating suicide ideation in all settings” [27]. Given these recommendations, primary care is an important setting in which to identify and address suicide risk.
Risk Factors for Older Adult Suicide
Numerous reviews exist that cover many risk factors for suicide in older adults [18,28]. This article will focus briefly on risk factors that are likely to be recognized and potentially addressed by primary care practitioners. Risk factors that apply across the lifespan can be recalled through a mnemonic: IS PATH WARM [29]. These risk factors include suicide Ideation, Substance abuse, Purposelessness, Anxiety (including agitation and poor sleep), feeling Trapped, Hopelessness, social Withdrawal, Anger or rage, Recklessness (ie, engaging in risky activities), and Mood changes. The National Suicide Prevention Lifeline also includes being in unbearable physical pain, perceiving one’s self as a burden to others, and seeking revenge on others as risk factors [30]. More specific to older adults, Conwell notes 5 categories or domains of risk factors with strong research support: psychiatric symptoms, somatic illness, functional impairment, social integration, and personality traits and coping [18,31].
Affective or mood disorders, particularly depression and depressive symptoms, are some of the most well-studied and strongest risk factors for older adult suicide [31]; 71% to 97% of all older adults who die by suicide have psychiatric illnesses [28]. Mood disorders, including major depressive episodes, are most consistently linked to older adult suicide risk; there is evidence as well for anxiety disorders and substance abuse disorders as risk factors, though it is somewhat mixed [28]. Therefore, screening for depression, anxiety, and substance abuse may be key to recognizing potential suicide risk. However, depression and anxiety do not present similarly in younger and older adults [32,33]. Depressive symptoms in older adults may be more somatic (eg, agitation, gastrointestinal symptoms) [32] and may reflect more anhedonia than mood changes [33]. Anxiety in older adults tends to be reported as stress or tension, whereas younger adults report feeling anxious or worried [33]. Additionally, substance abuse is often underrecognized, underdiagnosed, and undertreated in older adults [34]. Proactive screening for substance abuse is important as it may not interfere with work or other obligations in older adults, and therefore substance abuse may not be identified by older adults or others in their lives.
Physical illness may also be a risk factor for suicide [28,31]. Numerous diagnoses have been linked to suicide risk, including cancers, neurodegenerative diseases (eg, amyotrophic lateral sclerosis, Huntington disease), spinal cord injury, cardiovascular disease, and pulmonary disease [28,35]. However, overall illness burden (ie, number of chronic illnesses) [28] and self-perceived health [36] appear to be stronger risk factors than any specific illness. Additionally, authors have suggested that illness itself may not be a particularly strong risk factor, but the effect of illness on depressive symptoms [35], functioning, pain, or hopelessness due to the potential for decline over time [28] may increase suicide risk in older adults. Pain itself has been identified as a risk factor for suicide, as have perceptions of burden to others, hopelessness, and functional impairment [28].
In terms of functional impairment, research has shown that impairment in completing instrumental activities of daily living is associated with higher risk for death by suicide, and cognitive impairment may also be associated with elevated suicide risk [28]. However, there are some discrepant findings regarding the role of dementia in suicide risk, which may reflect medical and psychiatric comorbidities, as well as different stages of dementia or levels of cognitive impairment (eg, hopelessness about cognitive decline may increase suicide risk shortly after diagnosis, whereas lack of insight may decrease risk later in the course of the illness) [37]. Related to functional or cognitive impairment is perceived burdensomeness (ie, the perception that one is a liability or burden to others, to the point that others would be better off if one was gone) [38], which may also be associated with suicide risk in older adults [39,40]. Researchers have found that the interaction between perceived burdensomeness and thwarted belongingness (ie, a belief that one lacks reciprocal caring relationships and does not belong) identified older adults who were likely experiencing suicide ideation but did not report it [41]. These findings indicate that perceived burdensomeness and thwarted belongingness may be key in identifying older adults at risk for suicide.
Thwarted belongingness has also been linked to suicide ideation in older adults [41]. In fact, studies suggest that social integration is especially important for reducing suicide risk in this population [28,31,42]. A larger social network, living with others, and being active in the community are each protective against suicide [28]. Bereavement, which can reduce social connectedness and acts as a significant life stressor, is also an important risk factor [31]. Retirement may also reduce social connectedness, and employment changes have been identified as a suicide risk factor for older adults [28]. Retirement has been linked to risk for death by suicide in this population [43], and may not only serve to reduce social connectedness, but for some older adults may also be a significant role loss or loss of sense of purpose that can influence suicide risk.
Finally, rigid personality traits or coping styles are a risk factor for suicide among older adults [28,31]. As older adults face potential losses, health changes, and functional decline, effective positive coping strategies and flexibility are key to maintaining well-being. If older adults are unable to flexibly cope with these challenges, their risk for suicide increases [28].
In addition to risk factors, which confer suicide risk but do not necessarily suggest that an older adult is thinking about suicide, warning signs exist that indicate that suicide risk is imminent. These include suicidal communication (ie, talking or writing about suicide), seeking access to means, and making preparations for suicide (eg, ensuring a will is in place, giving away prized possessions). One important note is that discussing and preparing for death may be developmentally appropriate for older adults, particularly those with chronic illnesses; however, such appropriate preparation is critically different from talking about suicide or a desire for death.
Additionally, a lack of planning for the future may be a warning sign. For example, older adults who decline to schedule medical follow-up or do not wish to refill needed prescriptions may be exhibiting warning signs that should be addressed. Similarly, not following needed medical regimens (eg, an older adult with diabetes no longer taking insulin) is also a warning sign. Other, potentially more subtle warning signs may include significant changes in mood, sleep, or social interactions. Older adults may become agitated and sleep less when they are considering suicide, or may feel more at ease after they have made the decision to die by suicide and their sleep or mood may improve. Withdrawing from valued others may also be a warning sign. Finally, recent major changes (eg, loss of a spouse, moving to an assisted living facility) may be triggers for suicide risk and can serve as warning signs themselves.
Specific Screening Strategies
Given the numerous risk factors and warning signs for older adult suicide, as well as the time limitations that primary care practitioners face [44,45], it would be impractical to comprehensively assess each older adult who presents at a primary care practice. Therefore, more specific screening is necessary. Most importantly, every older adult should be screened for suicide ideation and death ideation at every visit. Screening at every visit is critical because suicide ideation may develop at any point. Previous research has included screening of over 29,000 older adults in 11 primary care settings for suicide ideation, risk of alcohol misuse, and mental health disorders [15], suggesting that suicide risk screening is feasible. Other studies have also successfully used widespread screening for depression and suicide ideation among older adults in primary care [46–48]. Additionally, in an emergency department setting, universal suicide risk screening has been associated with significantly improved risk detection [49], indicating that improved screening may be beneficial in identifying suicide risk. Importantly, asking about suicide does not cause thoughts of suicide [50]. Additionally, it is a myth that those who talk about suicide ideation will not act on these thoughts [51].
When primary care practitioners inquire about suicide ideation, they should also ask about death ideation; though some may believe that death ideation is not as significant in terms of suicide risk as suicide ideation, recall that research has not found differences in previous suicide attempts or current hopelessness among older adults with death ideation versus suicide ideation [14]. Therefore, screening for death ideation should be completed as part of every suicide risk screening.
Screening can take many forms. Screening may be oral; asking an older adult if he or she is having thoughts of suicide or is experiencing a desire to die is a brief, 2-question screening that may provide valuable information (eg, “Are you having thoughts about your own death or wanting to die?”, “Are you having thoughts of killing yourself or thinking about suicide?”). This screening may be conducted by medical assistants, nurses, care managers, or physicians, with the patient’s responses documented. Importantly, a standard procedure should be implemented to ensure older adults are consistently asked about suicide risk at each visit, but do not feel inundated by such questions from numerous staff.
If verbal questions are asked, they must be asked appropriately. Euphemisms or indirect language should not be used during a screening; older adults should be directly asked about thoughts of death and suicide, not simply asked questions such as, “Have you ever had thoughts of harming or hurting yourself?” A question like this does not adequately assess current suicide risk, as it does not assess current thoughts, nor does it specifically inquire about suicide ideation (ie, killing one’s self). It is also important to phrase questions in a manner that invites honest responses and conveys an openness to listening. For example, asking, “You’re not thinking about suicide, are you?” suggests that the practitioner wants the older adult to say no and is not comfortable with the older adult endorsing suicide ideation. Open questions that allow endorsement or denial (eg, “Are you having thoughts of killing yourself?”) imply that the practitioner is receptive to either an endorsement or denial of suicide ideation.
Alternatively, a written screening can be used; older adults may complete a questionnaire prior to their appointment or while waiting to see their practitioner. Such an assessment may be a brief screening (eg, using similar yes/no questions to an oral screening), or may be a standardized measure. For example, the Geriatric Suicide Ideation Scale [52] is a 31-item self-report measure that provides scores for suicide ideation, death ideation, loss of personal and social worth, and perceived meaning in life. Though there are not standard cutoffs that suggest high versus low suicide risk, responses can be reviewed to identify whether older adults are reporting suicide ideation or death ideation, and can also be compared to norms (ie, average scores) from other older adults [52]. This measure also has the benefit of 2 subscales that do not specifically require reporting thoughts of suicide or death (ie, loss of personal and social worth, perceived meaning in life), which may give practitioners an indication of an older adult’s suicide risk even if the older adult is not comfortable disclosing suicide ideation, as has been shown in previous research [7,8].
Similarly, the Geriatric Depression Scale, which has a validated 15-item version [53], does not directly ask about suicide ideation but has a 5-item subscale that has been found to be highly correlated with reported suicide ideation [54]. When administered to older adult primary care patients, this subscale was an effective measure of suicide ideation; a score of ≥ 1 was the best cutoff for determining whether an older adult reported suicide ideation [55].
Additionally, as noted previously, the interaction between perceived burdensomeness and thwarted belongingness may identify older adults who are potentially experiencing, but not reporting, suicide ideation [41]. The Interpersonal Needs Questionnaire [56] is the validated assessment for both perceived burdensomeness and thwarted belongingness. Perceived burdensomeness is assessed via 6 self-report items, and thwarted belongingness is assessed via 9 self-report items on this measure [56]. There are not specific cutoffs that determine high versus low perceived burdensomeness or thwarted belongingness, but older adults’ responses can provide information about their experiences of these constructs. Administration of the Interpersonal Needs Questionnaire can provide information about potential risk for suicide among older adults who may otherwise deny thoughts of suicide or death.
If the screening for suicide ideation or death ideation is positive (ie, the older adult endorses thoughts of suicide or death), the treating primary care practitioner must then follow up with additional questions to determine current level of suicide risk. To make this determination, at a minimum, follow-up questions should focus on whether the older adult has any intent to die by suicide (eg, “Do you have any intent to act on your thoughts of suicide?”), as well as whether he or she has a plan to die by suicide (eg, “Have you begun formulating a plan to die by suicide?”). When asking about a plan, it is important to determine how specific the plan is. For example, an older adult with a specific method identified and date selected to implement the plan is at much higher risk than an older adult with a relatively vague idea. It is also critical to assess for the older adult’s access to means for suicide. If an older adult has a specific plan and has the capability to carry out the plan (eg, plans to overdose on prescription medication and has large quantities of medication or high-lethality medication at home), he or she is more likely to die by suicide than an older adult who does not have access to means (eg, only has small quantities of low-lethality medication available). A general assessment of risk factors and previous suicidal behavior (ie, any previous suicide attempts) also informs decisions about level of risk and interventions.
After a screening or assessment is completed, a risk determination must be made and documented. Acute suicide risk can be categorized as low, moderate, or high. It is not appropriate to say that there is “no” suicide risk present. Low risk occurs when there is no current suicide ideation, no plan to die by suicide, and no intent to act on suicidal thoughts, especially when the patient has no history of suicidal behavior and few risk factors [57]. Moderate risk is evident when there is current suicide ideation, but no specific plan to die by suicide or intent to act on suicidal thoughts. There are likely warning signs or risk factors, which may include previous suicidal behaviors, present in moderate suicide risk [57]. High risk is indicated by current suicide ideation with plan to die by suicide and suicidal intent. There are significant warning signs and risk factors present; there may also be a recent suicide attempt, though this is not a requirement for a high risk determination [57]. Undetermined suicide risk occurs when a practitioner cannot accurately assess risk, but concern regarding suicide is present; this is primarily used when a patient refuses to answer questions about suicide. Undetermined risk should be treated as at least moderate risk. Because research shows that death ideation has similar outcomes to suicide ideation in older adults [14], death ideation should also be factored into determinations of suicide risk; reports of death ideation may indicate low or moderate risk in older adults, dependent upon other risk factors, suicidal intent, and plan.
After a risk determination is made, it must be documented in the medical record. The level of risk and rationale for that determination must be included [58]. Stating only the level of risk without a rationale (ie, the older adult’s responses to questions) is not adequate, and documenting only the older adult’s responses without a determination of risk is also not sufficient. Finally, it is critical to document the intervention that occurred or steps taken after the level of risk was determined.
Critically, stating only that there was no indication of suicide risk is inadequate. For example, documenting “No evidence of suicide risk” is not appropriate. This documentation does not indicate that the older adult was specifically asked about suicide ideation, death ideation, suicidal intent, or plan to die by suicide. It also does not indicate a level of suicide risk. Examples of appropriate documentation include:
Patient was asked about suicide risk. She denied current suicide ideation but reported death ideation. She denied any current suicidal intent or plan. She also denied any previous suicide attempts. Therefore, acute suicide risk was deemed to be low. Provided patient with wallet card about the National Suicide Prevention Lifeline. Also called the Friendship Line while in the room with the patient to connect her with services. Finally, provided a brief list of local mental health professionals to patient; the patient reported she would like to see Dr. Smith. Called and left a message for Dr. Smith with referral information with patient during appointment.
Patient was asked about suicide risk. He reported both death ideation and suicide ideation. He also reported a nonspecific plan (ie, causing a single-vehicle motor vehicle accident, with no specific plan for the motor vehicle accident or timeframe) and denied any intent to act on his thoughts of suicide. He reported one previous suicide attempt, at age 22, by overdose on over-the-counter medication. He reported that this attempt did not require medical attention. Therefore, acute suicide risk was determined to be moderate. Patient was introduced to the behavioral health specialist, who met with the patient during the appointment to conduct further assessment and intervention.
Specific Intervention Strategies
Despite the fact that the pace of the primary care setting often does not allow for time-intensive intervention, there are ways to address suicide risk in this setting. Importantly, no-suicide contracts should not be used at any time [59,60]. No-suicide contracts are documents that patients who are experiencing suicide ideation are required to sign that state that they will not die by suicide while under the care of the practitioner. These contracts have no evidence of effectiveness, and some researchers argue that they may in fact damage the relationship with patients and serve the practitioner’s needs more than the patient’s needs [59].
One of the best options for older adults at low acute suicide risk is to provide resources and referrals. The National Suicide Prevention Lifeline can be reached at 1-800-273-TALK (8255); trained counselors are available to speak to patients at all times. Wallet cards with information about the National Suicide Prevention Lifeline are available at no charge from the US Substance Abuse and Mental Health Services Administration online store. The Friendship Line is another service available free to adults ages 60 and older, 24 hours per day, 7 days per week; this line can be reached at 1-800-971-0016. The Friendship Line, which is managed by the Institute on Aging, also provides outreach calls to older adults who may be isolated or lonely, increasing connectedness and potentially reducing suicide risk.
Having a ready list of local mental health professionals with expertise in geriatrics and suicide risk to provide to the patient is also beneficial. Recall, though, that older adults are less likely to seek out and receive mental health services [19]; therefore, connecting the patient with resources or referrals during the appointment is critical. If the practitioner does not have time to do this, having a medical assistant or other staff member that the patient knows engage in this step may be appropriate. For example, the patient can call the Friendship Line or National Suicide Prevention Lifeline while in the room with the practitioner, which may reduce anxiety or stigma about doing so and connect the patient with services. Similarly, calling a local mental health professional to make a referral during the appointment may increase the likelihood that the older adult will follow up on the referral.
The most ideal method of intervention for moderate or high acute suicide risk is a warm handoff to a behavioral or mental health specialist. As primary care and behavioral health become more integrated and financially viable as reimbursement through the Centers for Medicare and Medicaid Services improves [61], it is becoming increasingly likely that such a specialist will be on-site and available. Research has found that collaborative care in primary care reduces suicide risk in older adults [46–48,62]. Mental health specialists can conduct more comprehensive assessments and spend more time intervening to reduce suicide risk among older adults with death or suicide ideation. If an on-site behavioral health specialist is not available, older adults at high suicide risk may need to be referred to an emergency department for further evaluation and follow-up. Each state has its own laws and procedures regarding this process, which should be incorporated into a practice’s procedures for addressing high suicide risk. The procedure often involves ensuring that the older adult is accompanied at all times (ie, not left alone in a room), alerting emergency services (usually via phone call to an emergency line, such as 911), and completion of paperwork by a practitioner asserting that the patient is a danger to self. Police or other emergency personnel are then responsible for transporting the patient for further evaluation and determination of whether hospitalization is required.
If more time is available, either via the treating primary care practitioner or other patient care staff in the office, other brief interventions may be beneficial. First, means safety discussions are critical, particularly for older adults with plans for suicide or access to highly lethal means. In such discussions, patients are encouraged to restrict access to the methods that they may use to die by suicide. Plans for restricting access are developed, and when possible, a support person is enlisted to ensure that the plans are carried out. For example, if an older adult has access to firearms (eg, keeps a loaded weapon in his or her nightstand), he or she is encouraged to restrict his or her access to it. Ideally, this is through removing the weapon from the home, either permanently or until suicide risk reduces (eg, giving it to a friend, turning it over to police), but more safe storage may also be an option if the older adult is not willing to remove the weapon from the home. This may mean using a gun lock or storing the weapon in a gun safe, storing ammunition separately from an unloaded weapon, removing the firing pin, or otherwise disassembling the weapon. Means safety counseling has been shown to be effective in reducing suicide rates [63] and is acceptable to patients [64]. Studies indicate that over 90% of individuals who make a suicide attempt and survive do not go on to die by suicide [65]; therefore, reducing access to highly lethal means during a suicidal crisis may be key in reducing suicide rates. Though an in-depth review of means safety counseling is outside the scope of this article, readers are directed to Bryan, Stone, and Rudd’s article for a practical overview of means safety discussions [66].
Second, safety planning is a brief intervention that may be beneficial in the primary care setting [67,68]. The goal of a safety plan is to create an individualized plan to remain safe during a suicidal crisis. Means safety discussion is the last of 6 steps in the safety plan [68]. The first 5 steps include identifying warning signs, using internal coping strategies, social connectedness as distraction, social support for the crisis, and professionals that can be used as resources. When patients can identify specific, individualized warning signs that occur prior to a crisis, they can then use strategies to cope and prevent the crisis from worsening. Coping strategies that are encouraged are first internal (ie, those that can be done without relying on anyone else), such as exercise or journaling. If those do not improve the patient’s mood, then he or she is encouraged to use people or social settings as a distraction (eg, people watching at the mall, calling an acquaintance to chat), and if he or she is still feeling bad, encouraged to get social support for the crisis (eg, calling a family member to discuss the crisis and get support). Finally, if all of these steps are not effective, the older adult is encouraged to reach out to professional supports, such as a mental health provider, the National Suicide Prevention Lifeline, or 911 (or go to an emergency room). Readers are encouraged to review Stanley and Brown’s articles for comprehensive details about safety planning as an intervention [67,68]. Additionally, an article with specific adaptations for safety planning with older adults is forthcoming [69].
Conclusion
Older adults, particularly older men, are at high risk for suicide [1,2], and primary care practitioners are a critical component of older adult suicide prevention. Older adults frequently see primary care practitioners within a month prior to death by suicide [20,21]; primary care practitioners are uniquely qualified to address a broad range of potential risk factors, and may have more interactions and familiarity with older adults at risk for suicide than other medical professionals [20–22]. Primary care practitioners should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide. Screening can consist of standardized written questionnaires or oral questioning, and interventions may include providing resources and referrals, discussions about means safety, safety planning, and handoff to a mental health specialist. Interventions for suicide risk are likely feasible and acceptable in primary care [46–48]. Primary care practitioners have an important role to play in older adult suicide prevention, and must be prepared to interact with older adults who may be at risk for suicide.
Corresponding author: Danielle R. Jahn, PhD, Primary Care Institute, 605 NE 1st St, Gainesville, FL 32605, [email protected].
Financial disclosures: None reported.
1. American Association of Suicidology. U.S.A. suicide: 2014official final data. 2016. Accessed at www.suicidology.org/Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf.
2. Centers for Disease Control and Prevention. Leading causes of death reports, national and regional, 1999-2015. 2016. Accessed at https://webappa.cdc.gov/sasweb/ncipc/leadcaus10_us.html.
3. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief, No 241. Hyattsville, MD: National Center for Health Statistics; 2016.
4. US Census Bureau. The next four decades. The older population in the United States: 2010 to 2050. 2010. Accessed at www.census.gov/prod/2010pubs/p25-1138.pdf.
5. Phillips JA, Robin AV, Nugent CN, Idler EL. Understanding recent changes in suicide rates among the middle-aged: period or cohort effects? Public Health Rep 2010;125:680–8.
6. Substance Abuse and Mental Health Services Administration. Issue brief 4: preventing suicide in older adults. 2012. Accessed at https://aoa.acl.gov/AoA_Programs/HPW/Behavioral/docs2/Issue%20Brief%204%20Preventing%20Suicide.pdf.
7. Duberstein PR, Conwell Y, Seidlitz L, et al. Age and suicidal ideation in older depressed inpatients. Am J Geriatr Psychiatry 1999;7:289–96.
8. Lynch TR, Johnson CS, Mendelson T, et al. Correlates of suicidal ideation among an elderly depressed sample. J Affect Disord 1999;56:9–15.
9. Centers for Disease Control and Prevention. Suicide: facts at a glance 2015. 2015. Accessed at www.cdc.gov/violenceprevention/pdf/suicide-datasheet-a.pdf.
10. Cukrowicz KC, Duberstein PR, Vannoy SD, et al. What factors determine disclosure of suicide ideation in adults 60 and older to a treatment provider? Suicide Life Threat Behav 2014;44:331–7.
11. Kim YA, Bogner HR, Brown GK, Gallo JJ. Chronic medical conditions and wishes to die among older primary care patients. Int J Psychiatry Med 2006;36:183–98.
12. Scocco P, Fantoni G, Rapattoni M, et al. Death ideas, suicidal thoughts, and plans among nursing home residents. J Geriatr Psychiatry Neurol 2009;22:141–8.
13. Scocco P, Meneghel G, Caon F, et al. Death ideation and its correlates: survey of an over-65-year-old population. J Nerv Ment Dis 2001;189:210–8.
14. Szanto K, Reynolds III CF, Frank E, et al. Suicide in elderly patients: is active vs. passive suicidal ideation a clinically valid distinction? Am J Geriatr Psychiatry, 2002;4:197–207.
15. Bartels SJ, Coakley E, Oxman TE, et al. Suicidal and death ideation in older primary care patients with depression, anxiety, and at-risk alcohol use. Am J Geriatr Psychiatry 2002;10:417–27.
16. Yip PSF. A public health approach to suicide prevention. Hong Kong J Psychiatry 2005;15:29–31.
17. van der Feltz-Cornelis CM, Sarchiapone M, Postuvan V, et al. Best practice elements of multilevel suicide prevention strategies: a review of systematic reviews. Crisis 2011;32:319–33.
18. Conwell Y. Suicide and suicide prevention in later life. Focus 2013;11:39–47.
19. Crabb R, Hunsley J. Utilization of mental health services among older adults with depression. J Clin Psychol 2006;62:299–312.
20. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry 2002;159:909–16.
21. Ahmedani BK, Simon GE, Stewart C, et al. Health care contacts in the year before suicide death. J Gen Intern Med 2014;29:870–7.
22. Robinson JW, Roter DL. Psychosocial problem disclosure by primary care patients. Soc Sci Med 1999;48:1353–62.
23. Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002;39:238–47.
24. Osborn R, Moulds D, Squires D, et al. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff 2014;33:2247–55.
25. Siu AL, US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;315:380–7.
26. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:719–26.
27. The Joint Commission. Detecting and treating suicide risk in all settings. Sentinel Event Alert 2016;56:1–7.
28. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68.
29. American Association of Suicidology. Know the warning signs of suicide. 2016. Accessed at www.suicidology.org/resources/warning-signs.
30. National Suicide Prevention Lifeline. Suicide warning signs. 2011. Accessed at www.suicidepreventionlifeline.org/App_Files/Media/PDF/NSPL_WalletCard.pdf.
31. Conwell Y. Suicide later in life: challenges and priorities for prevention. Am J Prev Med 2014;47:S244–50.
32. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
33. Wuthrich VM, Johnco CJ, Wetherell JL. Differences in anxiety and depression symptoms: comparison between older and younger clinical samples. Int Psychogeriatr 2015;27:1523–32.
34. Substance Abuse and Mental Health Services Administration. Substance abuse among older adults. Treatment Improvement Protocol (TIP) Series, No. 26. HHS Publication No. (SMA) 12-3918. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1998.
35. Fiske A, O’Riley AA, Widoe RK. Physical health and suicide in late life: an evaluative review. Clin Gerontologist 2008;31:31–50.
36. Duberstein PR, Conwell Y, Conner KR, et al. Suicide at 50 years of age and older: perceived physical illness, family discord, and financial strain. Psychol Med 2004;34:137–46.
37. Draper B, Peisah C, Snowdon J, Brodaty H. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement 2010;6:75–82.
38. Joiner T. Why people die by suicide. Cambridge: Harvard University Press; 2005.
39. Jahn DR, Cukrowicz KC. The impact of the nature of relationships on perceived burdensomeness and suicide ideation in a community sample of older adults. Suicide Life Threat Behav 2011;41:635–49.
40. Jahn DR, Cukrowicz KC, Linton K, Prabhu F. The mediating effect of perceived burdensomeness on the relation between depressive symptoms and suicide ideation in a community sample of older adults. Aging Ment Health 2011;15:214–20.
41. Cukrowicz KC, Jahn DR, Graham RD, et al. Suicide risk in older adults: evaluating models of risk and predicting excess zeros in a primary care sample. J Abnorm Psychol 2013;122:1021–30.
42. Fassberg MM, Van Orden KA, Duberstein, P, et al. A systematic review of social factors and suicidal behavior in older adulthood. Int J Environ Res Public Health 2012;9:722–45.
43. Pompili M, Innamorati M, Masotti V, et al. Suicide in the elderly: a psychological autopsy study in a north Italy area (1994-2004). Am J Geriatr Psychiatry 2008;16:727–35.
44. Konrad TR, Link CL, Shackelton RJ, et al. It’s about time: physicians’ perceptions of time constraints in primary medical practice in three national healthcare systems. Med Care 2010;48:95–100.
45. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res 2006;42:1871–94.
46. Bruce ML, Ten Have TR, Reynolds III CF, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. J Am Med Assoc 2004;291:1081–91.
47. Alexopoulos GS, Reynolds CF III, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
48. Unutzer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc 2006;54:1550–6.
49. Boudreaux ED, Camargo Jr CA, Arias SA, et al. Improving suicide risk screening and detection in the emergency department. Am J Prev Med 2016;50:445–53.
50. Mathias CW, Furr RM, Sheftall AH, et al. What’s the harm in asking about suicidal ideation? Suicide Life Threat Behav 2012;42:341–51.
51. Joiner T. Myths about suicide. Cambridge: Harvard University Press; 2011.
52. Heisel MJ, Flett GL. The development and initial validation of the Geriatric Suicide Ideation Scale. Am J Geriatr Psychiatry 2006;14:742–51.
53. Sheikh JL, Yesavage JA. Geriatric Depression Scale: recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention. New York: Howarth Press; 1986: 165–73.
54. Heisel MJ, Flett GL, Duberstein PR, Lyness JM. Does the Geriatric Depression Scale (GDS) distinguish between older adults with high versus low levels of suicidal ideation? Am J Geriatr Psychiatry 2005;13:876–83.
55. Heisel MJ, Duberstein PR, Lyness JM, Feldman MD. Screening for suicide ideation among older primary care patients. J Am Board Fam Med 2010;23:260–9.
56. Van Orden KA, Cukrowicz KC, Witte TK, Joiner Jr TE. Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychol Assess 2012;24;197–215.
57. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. Accessed at www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf.
58. Freedenthal S. Documentation: do it well, for the client’s sake and yours. 2013. Accessed at www.speakingofsuicide.com/2013/05/25/documentation/.
59. McMyler C, Pryjmachuk S. Do ‘no-suicide’ contracts work? J Psychiatr Ment Hlt 2008;15:512–22.
60. Rudd MD, Mandrusiak M, Joiner Jr TE. The case against no-suicide contracts: the commitment to treatment statement as a practice alternative. J Clin Psychol 2006;62:243–51.
61. National Institute of Mental Health. Adding better mental health care to primary care: a new era of behavioral health integration. 2016. Accessed at www.nimh.nih.gov/news/science-news/2016/adding-better-mental-health-care-to-primary-care.shtml.
62. Lapierre S, Erlangsen A, Waern M, et al. A systematic review of elderly suicide prevention programs. Crisis 2011;32;88–98.
63. Hawton K. Restricting access to methods of suicide: rationale and evaluation of this approach to suicide prevention. Crisis 2007;28:4–9.
64. Walters H, Kulkarni M, Forman J, et al. Feasibility and acceptability of interventions to delay gun access in VA mental health settings. Gen Hosp Psychiatry 2012;34:692–8.
65. Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm: systematic review. Brit J Psychiatry 2002;181:193–9.
66. Bryan CJ, Stone SL, Rudd MD. A practical, evidence-based approach for means-restriction counseling with suicidal patients. Prof Psychol Res Pr 2011;42:339–46.
67. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. 2008. Accessed at www.mentalhealth.va.gov/docs/va_safety_planning_manual.pdf.
68. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract 2012;19:256–64.
69. Jahn DR, Conti EC, Simons KV, et al. Evidence and considerations for safety planning as a suicide prevention strategy for older adults. 2017. Manuscript in preparation.
70. Knox KL, Stanley B, Currier GW, et al. An emergency department-based brief intervention for veterans at risk for suicide (SAFE VET). Am J Public Health 2012;102:S33–7.
1. American Association of Suicidology. U.S.A. suicide: 2014official final data. 2016. Accessed at www.suicidology.org/Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf.
2. Centers for Disease Control and Prevention. Leading causes of death reports, national and regional, 1999-2015. 2016. Accessed at https://webappa.cdc.gov/sasweb/ncipc/leadcaus10_us.html.
3. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief, No 241. Hyattsville, MD: National Center for Health Statistics; 2016.
4. US Census Bureau. The next four decades. The older population in the United States: 2010 to 2050. 2010. Accessed at www.census.gov/prod/2010pubs/p25-1138.pdf.
5. Phillips JA, Robin AV, Nugent CN, Idler EL. Understanding recent changes in suicide rates among the middle-aged: period or cohort effects? Public Health Rep 2010;125:680–8.
6. Substance Abuse and Mental Health Services Administration. Issue brief 4: preventing suicide in older adults. 2012. Accessed at https://aoa.acl.gov/AoA_Programs/HPW/Behavioral/docs2/Issue%20Brief%204%20Preventing%20Suicide.pdf.
7. Duberstein PR, Conwell Y, Seidlitz L, et al. Age and suicidal ideation in older depressed inpatients. Am J Geriatr Psychiatry 1999;7:289–96.
8. Lynch TR, Johnson CS, Mendelson T, et al. Correlates of suicidal ideation among an elderly depressed sample. J Affect Disord 1999;56:9–15.
9. Centers for Disease Control and Prevention. Suicide: facts at a glance 2015. 2015. Accessed at www.cdc.gov/violenceprevention/pdf/suicide-datasheet-a.pdf.
10. Cukrowicz KC, Duberstein PR, Vannoy SD, et al. What factors determine disclosure of suicide ideation in adults 60 and older to a treatment provider? Suicide Life Threat Behav 2014;44:331–7.
11. Kim YA, Bogner HR, Brown GK, Gallo JJ. Chronic medical conditions and wishes to die among older primary care patients. Int J Psychiatry Med 2006;36:183–98.
12. Scocco P, Fantoni G, Rapattoni M, et al. Death ideas, suicidal thoughts, and plans among nursing home residents. J Geriatr Psychiatry Neurol 2009;22:141–8.
13. Scocco P, Meneghel G, Caon F, et al. Death ideation and its correlates: survey of an over-65-year-old population. J Nerv Ment Dis 2001;189:210–8.
14. Szanto K, Reynolds III CF, Frank E, et al. Suicide in elderly patients: is active vs. passive suicidal ideation a clinically valid distinction? Am J Geriatr Psychiatry, 2002;4:197–207.
15. Bartels SJ, Coakley E, Oxman TE, et al. Suicidal and death ideation in older primary care patients with depression, anxiety, and at-risk alcohol use. Am J Geriatr Psychiatry 2002;10:417–27.
16. Yip PSF. A public health approach to suicide prevention. Hong Kong J Psychiatry 2005;15:29–31.
17. van der Feltz-Cornelis CM, Sarchiapone M, Postuvan V, et al. Best practice elements of multilevel suicide prevention strategies: a review of systematic reviews. Crisis 2011;32:319–33.
18. Conwell Y. Suicide and suicide prevention in later life. Focus 2013;11:39–47.
19. Crabb R, Hunsley J. Utilization of mental health services among older adults with depression. J Clin Psychol 2006;62:299–312.
20. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry 2002;159:909–16.
21. Ahmedani BK, Simon GE, Stewart C, et al. Health care contacts in the year before suicide death. J Gen Intern Med 2014;29:870–7.
22. Robinson JW, Roter DL. Psychosocial problem disclosure by primary care patients. Soc Sci Med 1999;48:1353–62.
23. Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002;39:238–47.
24. Osborn R, Moulds D, Squires D, et al. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff 2014;33:2247–55.
25. Siu AL, US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;315:380–7.
26. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:719–26.
27. The Joint Commission. Detecting and treating suicide risk in all settings. Sentinel Event Alert 2016;56:1–7.
28. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68.
29. American Association of Suicidology. Know the warning signs of suicide. 2016. Accessed at www.suicidology.org/resources/warning-signs.
30. National Suicide Prevention Lifeline. Suicide warning signs. 2011. Accessed at www.suicidepreventionlifeline.org/App_Files/Media/PDF/NSPL_WalletCard.pdf.
31. Conwell Y. Suicide later in life: challenges and priorities for prevention. Am J Prev Med 2014;47:S244–50.
32. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
33. Wuthrich VM, Johnco CJ, Wetherell JL. Differences in anxiety and depression symptoms: comparison between older and younger clinical samples. Int Psychogeriatr 2015;27:1523–32.
34. Substance Abuse and Mental Health Services Administration. Substance abuse among older adults. Treatment Improvement Protocol (TIP) Series, No. 26. HHS Publication No. (SMA) 12-3918. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1998.
35. Fiske A, O’Riley AA, Widoe RK. Physical health and suicide in late life: an evaluative review. Clin Gerontologist 2008;31:31–50.
36. Duberstein PR, Conwell Y, Conner KR, et al. Suicide at 50 years of age and older: perceived physical illness, family discord, and financial strain. Psychol Med 2004;34:137–46.
37. Draper B, Peisah C, Snowdon J, Brodaty H. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement 2010;6:75–82.
38. Joiner T. Why people die by suicide. Cambridge: Harvard University Press; 2005.
39. Jahn DR, Cukrowicz KC. The impact of the nature of relationships on perceived burdensomeness and suicide ideation in a community sample of older adults. Suicide Life Threat Behav 2011;41:635–49.
40. Jahn DR, Cukrowicz KC, Linton K, Prabhu F. The mediating effect of perceived burdensomeness on the relation between depressive symptoms and suicide ideation in a community sample of older adults. Aging Ment Health 2011;15:214–20.
41. Cukrowicz KC, Jahn DR, Graham RD, et al. Suicide risk in older adults: evaluating models of risk and predicting excess zeros in a primary care sample. J Abnorm Psychol 2013;122:1021–30.
42. Fassberg MM, Van Orden KA, Duberstein, P, et al. A systematic review of social factors and suicidal behavior in older adulthood. Int J Environ Res Public Health 2012;9:722–45.
43. Pompili M, Innamorati M, Masotti V, et al. Suicide in the elderly: a psychological autopsy study in a north Italy area (1994-2004). Am J Geriatr Psychiatry 2008;16:727–35.
44. Konrad TR, Link CL, Shackelton RJ, et al. It’s about time: physicians’ perceptions of time constraints in primary medical practice in three national healthcare systems. Med Care 2010;48:95–100.
45. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res 2006;42:1871–94.
46. Bruce ML, Ten Have TR, Reynolds III CF, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. J Am Med Assoc 2004;291:1081–91.
47. Alexopoulos GS, Reynolds CF III, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
48. Unutzer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc 2006;54:1550–6.
49. Boudreaux ED, Camargo Jr CA, Arias SA, et al. Improving suicide risk screening and detection in the emergency department. Am J Prev Med 2016;50:445–53.
50. Mathias CW, Furr RM, Sheftall AH, et al. What’s the harm in asking about suicidal ideation? Suicide Life Threat Behav 2012;42:341–51.
51. Joiner T. Myths about suicide. Cambridge: Harvard University Press; 2011.
52. Heisel MJ, Flett GL. The development and initial validation of the Geriatric Suicide Ideation Scale. Am J Geriatr Psychiatry 2006;14:742–51.
53. Sheikh JL, Yesavage JA. Geriatric Depression Scale: recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention. New York: Howarth Press; 1986: 165–73.
54. Heisel MJ, Flett GL, Duberstein PR, Lyness JM. Does the Geriatric Depression Scale (GDS) distinguish between older adults with high versus low levels of suicidal ideation? Am J Geriatr Psychiatry 2005;13:876–83.
55. Heisel MJ, Duberstein PR, Lyness JM, Feldman MD. Screening for suicide ideation among older primary care patients. J Am Board Fam Med 2010;23:260–9.
56. Van Orden KA, Cukrowicz KC, Witte TK, Joiner Jr TE. Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychol Assess 2012;24;197–215.
57. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. Accessed at www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf.
58. Freedenthal S. Documentation: do it well, for the client’s sake and yours. 2013. Accessed at www.speakingofsuicide.com/2013/05/25/documentation/.
59. McMyler C, Pryjmachuk S. Do ‘no-suicide’ contracts work? J Psychiatr Ment Hlt 2008;15:512–22.
60. Rudd MD, Mandrusiak M, Joiner Jr TE. The case against no-suicide contracts: the commitment to treatment statement as a practice alternative. J Clin Psychol 2006;62:243–51.
61. National Institute of Mental Health. Adding better mental health care to primary care: a new era of behavioral health integration. 2016. Accessed at www.nimh.nih.gov/news/science-news/2016/adding-better-mental-health-care-to-primary-care.shtml.
62. Lapierre S, Erlangsen A, Waern M, et al. A systematic review of elderly suicide prevention programs. Crisis 2011;32;88–98.
63. Hawton K. Restricting access to methods of suicide: rationale and evaluation of this approach to suicide prevention. Crisis 2007;28:4–9.
64. Walters H, Kulkarni M, Forman J, et al. Feasibility and acceptability of interventions to delay gun access in VA mental health settings. Gen Hosp Psychiatry 2012;34:692–8.
65. Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm: systematic review. Brit J Psychiatry 2002;181:193–9.
66. Bryan CJ, Stone SL, Rudd MD. A practical, evidence-based approach for means-restriction counseling with suicidal patients. Prof Psychol Res Pr 2011;42:339–46.
67. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. 2008. Accessed at www.mentalhealth.va.gov/docs/va_safety_planning_manual.pdf.
68. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract 2012;19:256–64.
69. Jahn DR, Conti EC, Simons KV, et al. Evidence and considerations for safety planning as a suicide prevention strategy for older adults. 2017. Manuscript in preparation.
70. Knox KL, Stanley B, Currier GW, et al. An emergency department-based brief intervention for veterans at risk for suicide (SAFE VET). Am J Public Health 2012;102:S33–7.
CMS recognizes Society of Hospital Medicine’s Center for Quality Improvement
PHILADELPHIA – The Society of Hospital Medicine (SHM)’s Center for Quality Improvement (QI) has been distinguished by the Centers for Medicare & Medicaid Services for maintaining an ongoing collaborative partnership with CMS to enhance patient safety.
The letter of recognition from Paul McGann, MD, Jean Moody-Williams, RN, MPP, and Dennis Wagner, MPA, of the CMS, to Jenna Goldstein, MA, director of SHM’s Center for QI, and Kevin Vuernick, MPA, senior project manager, noted: “Over the last several years, our team has been privileged to partner with you and the Society of Hospital Medicine on the work of quality improvement and patient safety. Without relationships like these, the results in the reduction of patient harm we have seen at a national scale, saving 87,000 lives and nearly $20 billion in cost savings, would never have been possible.”
In August 2016, CMS’ Hospital Improvement Innovation Networks contacted SHM to participate in their weekly Partnership for Patients (PfP) Pacing Event webinar to present strategies for reducing opioid use and preventing adverse drug events, including SHM’s Mentored Implementation pilot program on Reducing Adverse Drug Events Related to Opioids (RADEO). SHM’s contribution to this webinar was twofold: Thomas W. Frederickson, MD, the lead author of the RADEO guide and one of two program mentors, spoke about the development of the RADEO program and its importance in the acute care setting. Matthew Jared, MD, a hospitalist at St. Anthony Hospital in Oklahoma City, one of the five pilot RADEO sites, discussed his experience implementing specific RADEO interventions as well as the mentoring provided by Dr. Frederickson of the department of hospital medicine at CHI Health in Omaha, Neb.
As a result of this successful partnership, SHM was contacted in January to provide its perspective on best practices in managing inpatients receiving opioids and adverse drug event data collection. At that time, Mr. Vuernick discussed the lessons learned between RADEO’s pilot program and the second iteration of RADEO, which launched in November 2016.
For more information about SHM’s Center for QI, please visit www.hospitalmedicine.org/QI. For more information about SHM and hospital medicine, visit www.hospitalmedicine.org and follow SHM on Twitter at @SHMLive.
PHILADELPHIA – The Society of Hospital Medicine (SHM)’s Center for Quality Improvement (QI) has been distinguished by the Centers for Medicare & Medicaid Services for maintaining an ongoing collaborative partnership with CMS to enhance patient safety.
The letter of recognition from Paul McGann, MD, Jean Moody-Williams, RN, MPP, and Dennis Wagner, MPA, of the CMS, to Jenna Goldstein, MA, director of SHM’s Center for QI, and Kevin Vuernick, MPA, senior project manager, noted: “Over the last several years, our team has been privileged to partner with you and the Society of Hospital Medicine on the work of quality improvement and patient safety. Without relationships like these, the results in the reduction of patient harm we have seen at a national scale, saving 87,000 lives and nearly $20 billion in cost savings, would never have been possible.”
In August 2016, CMS’ Hospital Improvement Innovation Networks contacted SHM to participate in their weekly Partnership for Patients (PfP) Pacing Event webinar to present strategies for reducing opioid use and preventing adverse drug events, including SHM’s Mentored Implementation pilot program on Reducing Adverse Drug Events Related to Opioids (RADEO). SHM’s contribution to this webinar was twofold: Thomas W. Frederickson, MD, the lead author of the RADEO guide and one of two program mentors, spoke about the development of the RADEO program and its importance in the acute care setting. Matthew Jared, MD, a hospitalist at St. Anthony Hospital in Oklahoma City, one of the five pilot RADEO sites, discussed his experience implementing specific RADEO interventions as well as the mentoring provided by Dr. Frederickson of the department of hospital medicine at CHI Health in Omaha, Neb.
As a result of this successful partnership, SHM was contacted in January to provide its perspective on best practices in managing inpatients receiving opioids and adverse drug event data collection. At that time, Mr. Vuernick discussed the lessons learned between RADEO’s pilot program and the second iteration of RADEO, which launched in November 2016.
For more information about SHM’s Center for QI, please visit www.hospitalmedicine.org/QI. For more information about SHM and hospital medicine, visit www.hospitalmedicine.org and follow SHM on Twitter at @SHMLive.
PHILADELPHIA – The Society of Hospital Medicine (SHM)’s Center for Quality Improvement (QI) has been distinguished by the Centers for Medicare & Medicaid Services for maintaining an ongoing collaborative partnership with CMS to enhance patient safety.
The letter of recognition from Paul McGann, MD, Jean Moody-Williams, RN, MPP, and Dennis Wagner, MPA, of the CMS, to Jenna Goldstein, MA, director of SHM’s Center for QI, and Kevin Vuernick, MPA, senior project manager, noted: “Over the last several years, our team has been privileged to partner with you and the Society of Hospital Medicine on the work of quality improvement and patient safety. Without relationships like these, the results in the reduction of patient harm we have seen at a national scale, saving 87,000 lives and nearly $20 billion in cost savings, would never have been possible.”
In August 2016, CMS’ Hospital Improvement Innovation Networks contacted SHM to participate in their weekly Partnership for Patients (PfP) Pacing Event webinar to present strategies for reducing opioid use and preventing adverse drug events, including SHM’s Mentored Implementation pilot program on Reducing Adverse Drug Events Related to Opioids (RADEO). SHM’s contribution to this webinar was twofold: Thomas W. Frederickson, MD, the lead author of the RADEO guide and one of two program mentors, spoke about the development of the RADEO program and its importance in the acute care setting. Matthew Jared, MD, a hospitalist at St. Anthony Hospital in Oklahoma City, one of the five pilot RADEO sites, discussed his experience implementing specific RADEO interventions as well as the mentoring provided by Dr. Frederickson of the department of hospital medicine at CHI Health in Omaha, Neb.
As a result of this successful partnership, SHM was contacted in January to provide its perspective on best practices in managing inpatients receiving opioids and adverse drug event data collection. At that time, Mr. Vuernick discussed the lessons learned between RADEO’s pilot program and the second iteration of RADEO, which launched in November 2016.
For more information about SHM’s Center for QI, please visit www.hospitalmedicine.org/QI. For more information about SHM and hospital medicine, visit www.hospitalmedicine.org and follow SHM on Twitter at @SHMLive.
PSYCHIATRY UPDATE 2017
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 500 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, March 30 to April 1, 2017, at the Marriott Chicago Magnificent Mile in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us at Psychiatry Update Encore in Las Vegas, December 10 to 12, 2017 or next year in Chicago, March 22 to 24, 2018.
View summaries from the event on the following pages.
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Greg W. Mattingly, MD, Midwest Research Group and St. Charles Psychiatric Associates, St. Charles, Missouri.
In an industry-sponsored symposium, Dr. Mattingly reported that in the STAR-D study, approximately one-half of patients with major depressive disorder (MDD) did not experience adequate response to an initial selective serotonin reuptake inhibitor and 3 of 4 of those non-responding patients did not achieve full response with a second antidepressant, which prompts consideration of an adjunctive agent. Brexpiprazole (Rexulti) is a partial agonist for serotonin, dopamine, and noradrenergic systems. In pivotal trials as an adjunctive treatment in MDD, brexpiprazole, 2 mg/d, resulted in a statistically significant decrease in Montgomery-Åsburg Depression Rating Scale scores compared with placebo. Most common adverse reactions observed in ≥5% of patients and at least twice the rate of placebo included akathisia and weight increase.
Essentials of Malingering Assessment
Douglas Mossman, MD, University of Cincinnati
Malingering is intentional lying with an external incentive, such as avoiding work or obtaining drugs. Dr. Mossman gave 2 examples of malingered posttraumatic stress disorder and psychosis. Although lying cannot be detected by careful examination of facial expressions or gestures, a detailed evaluation can reveal malingering. An individual who is malingering psychosis may describe symptoms, such as “I talk to voices all the time,” but clinicians never observe such behavior. Signs of malingering include using “textbook” terms for symptoms; inconsistencies in their history or symptoms; sudden onset of delusions; exaggerating; and being unpleasant, dishonest, or demanding.
Beyond Efficacy and Effectiveness: Neurotoxicity vs Neuroprotection are the REAL Differences Between Typical and Atypical Antipsychotics
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Dr. Nasrallah discussed the difference between typical vs atypical antipsychotics—the former is neurotoxic, the latter is neuroprotective. Because patients with schizophrenia experience a loss of brain volume and cerebral grey matter and increased lateral ventricle volume, consider atypical antipsychotics for their neuroprotective properties.
In several studies typical antipsychotics, such as haloperidol, have been found to be neurotoxic, causing apoptosis and decreased cell viability. Atypical antipsychotics may be beneficial for patients with schizophrenia because they:
- stimulate production of new brain cells and increase neurotropic factors
- reverse PCP-induced changes in gene expression and loss of dendritic spines in the frontal cortex
- are neuroprotective against ischemic stroke damage
- prevent oligodendrocyte damage caused by interferon gamma-stimulated microglia.
Medicolegal Hazards in the Information Age: Malpractice and More
Douglas Mossman, MD, University of Cincinnati
Dr. Mossman began by answering the question, “What should I do if a patient ‘friended’ me on Facebook?” Such online relationships can blur boundaries or risk breaching confidentiality, therefore medical organizations recommend ignoring a friend request. Telemedicine via Skype is cost effective and enhances outreach to patients in rural areas or who cannot travel to the office, but online clinical encounters lack multidimensional aspects of the interpersonal encounter and might not be HIPAA compliant. E-mail carries some of the same concerns, such as confidentially of personal information, although the practice—when employed appropriately—is supported by some medical associations, including the American Psychiatric Association.
Treatment-Resistance and Suicidality in Schizophrenia: 2 Major Management Challenges
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Patients can seem treatment-resistant because of inadequate antipsychotic dosing, smoking, substance-induced relapse, nonadherence, or a general medical condition. Dr. Nasrallah discussed how to recognize true treatment-resistant schizophrenia and rule of spurious treatment resistance. If your patient is truly treatment-resistant, what do you do when everything else fails?
Risk factors for suicide include male sex, depressed mood, substance use, and social isolation. Clozapine, the only drug FDA-approved for refractory schizophrenia and suicidality, is underutilized for such patients. Dr. Nasrallah also presented evidence for the use of adjunctive modalities, such as lamotrigine, steroids, omega-3 fatty acids, NSAIDs, antidepressants, glutamatergic agents, and rTMS, as well as psychotherapy.
Luncheon Symposium
Depression, Its impact, and the Importance of Recognition and Treatment
Faculty: Jon Winston Draud, MS, MD, University of Tennessee Health Science Center
Major depressive disorder (MDD) is the most commonly diagnosed condition, second to cardiovascular disease. Dr. Draud recommended using a wellness screen such as the WHO-5 (World Health Organization, 5 item well-being index) in addition to a depression screening tool such as the PHQ-9. He emphasized that the goal of treatment should not be merely remission—but remission without residual symptoms. Cognitive impairment is the most common residual symptom. Patients with residual symptoms relapse earlier (5.5 times faster) and at a greater rate than patients without residual symptoms (76% vs 25%, respectively). Continue treatment and monitoring even after symptoms appear to subside. Recommended treatment is multi-modal and should include cognitive therapy and exercise.
New and Old Treatments for Opioid Abuse and Dependence
Mark S. Gold, MD, Washington University
Each day more than 1,000 people are treated in emergency departments for improper use of prescription opioids. But is naloxone saving lives or is overdose reversal nothing more than CPR? Dr. Gold spoke about the need for psychiatric assessment after a patient has been revived. Historically, treatment has stopped at abstinence or overdose treatment, but patients need ongoing treatment. Family therapy, vocational assistance, and psychotherapy are essential.
Dr. Gold reviewed established and newer treatments, including naloxone and naltrexone. Methadone and buprenorphine-naloxone can be effective for adherent patients who abuse only one drug. Naltrexone gives patients time to get their lives on track. Probuphine has comparable efficacy with buprenorphine-naloxone and methadone.
Impact of a Personality Disorder in Management of Comorbid Disorder
Donald W. Black, MD, University of Iowa
Personality disorder (PD) indicates patterns of long-term functioning and are not limited to episodes of illness. Abnormal personality traits are common among the general population, but are not considered a personality disorder unless they are inflexible, maladaptive, persisting, and cause distress either for the patient or the family. There are few cases of “pure” PDs without a comorbid psychiatric disorder. Personality disorders are not as stable as once understood; they wax and wane in response to stressors or depressed mood or anxiety. When a PD is comorbid with another disorder, patients are less likely to respond to medication and to experience remission from the comorbid psychiatric disorder.
Evaluation and Treatment of Patients Who Abuse Methamphetamine or Cocaine
Mark S. Gold, MD, Washington University
There are no FDA-approved medications or advancement in treatment for cocaine overdose—primary treatment is still ice baths. When assessing cocaine use, consider the route of ingestion and duration of use, which influence severity. Stimulants, whether methamphetamine or cocaine, cause changes in dopamine that are difficult to reverse. Substitute stimulants, such as modafinil, or vaccines have been proposed for cocaine abuse, but the evidence is not robust. Methamphetamine produces a schizophrenia-like illness, but antipsychotics are not effective. Naltrexone and bupropion showed some efficacy but was not statistically significant. There are no effective treatments for overdose or relapse prevention other than traditional group and residential treatment approaches.
Risks in Using Cannabis
Kevin Hill, MD, MHS, McLean Hospital
Although only 9% of Cannabis users become dependent, Dr. Hill recommended talking to all patients who use Cannabis about the risks, such as problems with work, school, and relationships. When treating patients with Cannabis use disorder, explore reasons that the individual would want to stop using Cannabis, take a careful history, and most importantly, build a good therapeutic alliance.
The most robust data for medical Cannabis is for chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis; however, there are more than 70 indications among the 28 states that allow its use. Dr. Hill suggests having a written policy, engage in conversation about why the patient wants medical Cannabis, be open to evaluating such a patient, and consider treating the patient’s symptoms with traditional modalities.
Marlene P. Freeman, MD, Massachusetts General Hospital
Dr. Freeman discussed the important role mental health providers play in helping women during pregnancy decrease medical and obstetrical risks, such as nutrition and maintaining a healthy weight. Because one-half of pregnancies in the United States are unplanned, consider medications that are compatible with pregnancy, and recommend omega-3 fatty acids and lifestyle changes such as diet.
To diagnose premenstrual dysphoric disorder, Dr. Freeman recommends asking your patient to document and rate daily moods using a mobile app or calendar. In perimenopause, the risk of depression increases because estrogen has antidepressant effects. Although, there are no guidelines for treating depression in women in perimenopause, consider serotonergic antidepressants, supplements such as omega-3 fatty acids, isoflavones, and black cohosh, and sleep aids for patients with insomnia—a common feature of menopause.
Jeffrey R. Strawn, MD, FAACP, University of Cincinnati
Attention-deficit/hyperactivity disorder (ADHD) and bipolar disorder (BD) may share an underlying biological etiology, Dr. Strawn explained. Shared risk factors include in utero events, dietary factors, and genetics. Differentiating ADHD from BD depends on the developmental stage of the patient. Symptoms overlap, which could lead to overdiagnosis of ADHD in youths with BD.
Dr. Strawn discussed how children with depression might display mood lability and irritability, rather than verbalizing feelings because they do not use language effectively until age 7. Children may have somatic symptoms early and irritability might decrease into adolescence. Anxiety disorder in children emerges early—usually as a phobia—around age 12 to 14, with an increase in onset of depressive disorders. Dr. Strawn reviewed screening tools to diagnose and track anxiety symptoms, as well as the pros and cons of pharmacological treatments.

George T. Grossberg, MD, Saint Louis University
Psychotic symptoms could be common in older adults; therefore it is important to evaluate whether these symptoms cause emotional suffering or impairment in daily function. Dr. Grossberg recommended that when treating psychotic disorders in geriatric patients to first evaluate and treat underlying medical problems and identify offending medications or environmental or psychosocial triggers, then consider psychosocial or environmental interventions. Consider antipsychotics for patients who are experiencing severe emotional distress or those who pose a high safety risk. If antipsychotics are necessary, pick an agent based on side effects, “start low, go slow,” and discuss the risks and benefits with the family.
Role of Psychiatrists in Long-term Care Facilities
In his presentation on the role of psychiatrists in long-term care facilities, Dr. Grossberg described common disorders including the behavioral and psychiatric symptoms of dementia, as well as risk for depression. Overprescribing is common in long-term care facilities; therefore when considering a patient’s medication regimen, often less is more. Dr. Grossberg also discussed common undertreated or undercorrected physical health problems, including hearing or vision deficits, obstructive sleep apnea, and malnutrition.
Alexander W. Thompson, MD, MBA, MPH, University of Iowa Carver College of Medicine
Somatizing patients experience symptoms all of the time, whether a headache or nausea, but most symptoms do not have an organic cause, and they might seek treatment for any or all symptoms. The goal of treating somatizing patients is to not harm them with unneeded workup and treatment. Dr. Thomspon recommends providing a letter to the patient’s primary care physician with your recommendations, which can reduce medical costs and improve physical function. Although there are no clear pharmacotherapies, cognitive-behavioral therapy focused on health and anxiety can help.
Fatigue
Fatigue experienced by patients with chronic fatigue syndrome is unrelenting, is not the result of ongoing exertion, and is unrelieved by rest. When approaching a patient with extreme fatigue, start with a thorough evaluation in collaboration with a primary care physician, Dr. Thompson said. Establish a rapport with the patient, limit iatrogenic harm, and treat chronic fatigue as you would any chronic condition. Rintatolimod and valganciclovir have showed some evidence of benefit, and graded exercise therapy has shown success.
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 500 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, March 30 to April 1, 2017, at the Marriott Chicago Magnificent Mile in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us at Psychiatry Update Encore in Las Vegas, December 10 to 12, 2017 or next year in Chicago, March 22 to 24, 2018.
View summaries from the event on the following pages.
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Greg W. Mattingly, MD, Midwest Research Group and St. Charles Psychiatric Associates, St. Charles, Missouri.
In an industry-sponsored symposium, Dr. Mattingly reported that in the STAR-D study, approximately one-half of patients with major depressive disorder (MDD) did not experience adequate response to an initial selective serotonin reuptake inhibitor and 3 of 4 of those non-responding patients did not achieve full response with a second antidepressant, which prompts consideration of an adjunctive agent. Brexpiprazole (Rexulti) is a partial agonist for serotonin, dopamine, and noradrenergic systems. In pivotal trials as an adjunctive treatment in MDD, brexpiprazole, 2 mg/d, resulted in a statistically significant decrease in Montgomery-Åsburg Depression Rating Scale scores compared with placebo. Most common adverse reactions observed in ≥5% of patients and at least twice the rate of placebo included akathisia and weight increase.
Essentials of Malingering Assessment
Douglas Mossman, MD, University of Cincinnati
Malingering is intentional lying with an external incentive, such as avoiding work or obtaining drugs. Dr. Mossman gave 2 examples of malingered posttraumatic stress disorder and psychosis. Although lying cannot be detected by careful examination of facial expressions or gestures, a detailed evaluation can reveal malingering. An individual who is malingering psychosis may describe symptoms, such as “I talk to voices all the time,” but clinicians never observe such behavior. Signs of malingering include using “textbook” terms for symptoms; inconsistencies in their history or symptoms; sudden onset of delusions; exaggerating; and being unpleasant, dishonest, or demanding.
Beyond Efficacy and Effectiveness: Neurotoxicity vs Neuroprotection are the REAL Differences Between Typical and Atypical Antipsychotics
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Dr. Nasrallah discussed the difference between typical vs atypical antipsychotics—the former is neurotoxic, the latter is neuroprotective. Because patients with schizophrenia experience a loss of brain volume and cerebral grey matter and increased lateral ventricle volume, consider atypical antipsychotics for their neuroprotective properties.
In several studies typical antipsychotics, such as haloperidol, have been found to be neurotoxic, causing apoptosis and decreased cell viability. Atypical antipsychotics may be beneficial for patients with schizophrenia because they:
- stimulate production of new brain cells and increase neurotropic factors
- reverse PCP-induced changes in gene expression and loss of dendritic spines in the frontal cortex
- are neuroprotective against ischemic stroke damage
- prevent oligodendrocyte damage caused by interferon gamma-stimulated microglia.
Medicolegal Hazards in the Information Age: Malpractice and More
Douglas Mossman, MD, University of Cincinnati
Dr. Mossman began by answering the question, “What should I do if a patient ‘friended’ me on Facebook?” Such online relationships can blur boundaries or risk breaching confidentiality, therefore medical organizations recommend ignoring a friend request. Telemedicine via Skype is cost effective and enhances outreach to patients in rural areas or who cannot travel to the office, but online clinical encounters lack multidimensional aspects of the interpersonal encounter and might not be HIPAA compliant. E-mail carries some of the same concerns, such as confidentially of personal information, although the practice—when employed appropriately—is supported by some medical associations, including the American Psychiatric Association.
Treatment-Resistance and Suicidality in Schizophrenia: 2 Major Management Challenges
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Patients can seem treatment-resistant because of inadequate antipsychotic dosing, smoking, substance-induced relapse, nonadherence, or a general medical condition. Dr. Nasrallah discussed how to recognize true treatment-resistant schizophrenia and rule of spurious treatment resistance. If your patient is truly treatment-resistant, what do you do when everything else fails?
Risk factors for suicide include male sex, depressed mood, substance use, and social isolation. Clozapine, the only drug FDA-approved for refractory schizophrenia and suicidality, is underutilized for such patients. Dr. Nasrallah also presented evidence for the use of adjunctive modalities, such as lamotrigine, steroids, omega-3 fatty acids, NSAIDs, antidepressants, glutamatergic agents, and rTMS, as well as psychotherapy.
Luncheon Symposium
Depression, Its impact, and the Importance of Recognition and Treatment
Faculty: Jon Winston Draud, MS, MD, University of Tennessee Health Science Center
Major depressive disorder (MDD) is the most commonly diagnosed condition, second to cardiovascular disease. Dr. Draud recommended using a wellness screen such as the WHO-5 (World Health Organization, 5 item well-being index) in addition to a depression screening tool such as the PHQ-9. He emphasized that the goal of treatment should not be merely remission—but remission without residual symptoms. Cognitive impairment is the most common residual symptom. Patients with residual symptoms relapse earlier (5.5 times faster) and at a greater rate than patients without residual symptoms (76% vs 25%, respectively). Continue treatment and monitoring even after symptoms appear to subside. Recommended treatment is multi-modal and should include cognitive therapy and exercise.
New and Old Treatments for Opioid Abuse and Dependence
Mark S. Gold, MD, Washington University
Each day more than 1,000 people are treated in emergency departments for improper use of prescription opioids. But is naloxone saving lives or is overdose reversal nothing more than CPR? Dr. Gold spoke about the need for psychiatric assessment after a patient has been revived. Historically, treatment has stopped at abstinence or overdose treatment, but patients need ongoing treatment. Family therapy, vocational assistance, and psychotherapy are essential.
Dr. Gold reviewed established and newer treatments, including naloxone and naltrexone. Methadone and buprenorphine-naloxone can be effective for adherent patients who abuse only one drug. Naltrexone gives patients time to get their lives on track. Probuphine has comparable efficacy with buprenorphine-naloxone and methadone.
Impact of a Personality Disorder in Management of Comorbid Disorder
Donald W. Black, MD, University of Iowa
Personality disorder (PD) indicates patterns of long-term functioning and are not limited to episodes of illness. Abnormal personality traits are common among the general population, but are not considered a personality disorder unless they are inflexible, maladaptive, persisting, and cause distress either for the patient or the family. There are few cases of “pure” PDs without a comorbid psychiatric disorder. Personality disorders are not as stable as once understood; they wax and wane in response to stressors or depressed mood or anxiety. When a PD is comorbid with another disorder, patients are less likely to respond to medication and to experience remission from the comorbid psychiatric disorder.
Evaluation and Treatment of Patients Who Abuse Methamphetamine or Cocaine
Mark S. Gold, MD, Washington University
There are no FDA-approved medications or advancement in treatment for cocaine overdose—primary treatment is still ice baths. When assessing cocaine use, consider the route of ingestion and duration of use, which influence severity. Stimulants, whether methamphetamine or cocaine, cause changes in dopamine that are difficult to reverse. Substitute stimulants, such as modafinil, or vaccines have been proposed for cocaine abuse, but the evidence is not robust. Methamphetamine produces a schizophrenia-like illness, but antipsychotics are not effective. Naltrexone and bupropion showed some efficacy but was not statistically significant. There are no effective treatments for overdose or relapse prevention other than traditional group and residential treatment approaches.
Risks in Using Cannabis
Kevin Hill, MD, MHS, McLean Hospital
Although only 9% of Cannabis users become dependent, Dr. Hill recommended talking to all patients who use Cannabis about the risks, such as problems with work, school, and relationships. When treating patients with Cannabis use disorder, explore reasons that the individual would want to stop using Cannabis, take a careful history, and most importantly, build a good therapeutic alliance.
The most robust data for medical Cannabis is for chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis; however, there are more than 70 indications among the 28 states that allow its use. Dr. Hill suggests having a written policy, engage in conversation about why the patient wants medical Cannabis, be open to evaluating such a patient, and consider treating the patient’s symptoms with traditional modalities.
Marlene P. Freeman, MD, Massachusetts General Hospital
Dr. Freeman discussed the important role mental health providers play in helping women during pregnancy decrease medical and obstetrical risks, such as nutrition and maintaining a healthy weight. Because one-half of pregnancies in the United States are unplanned, consider medications that are compatible with pregnancy, and recommend omega-3 fatty acids and lifestyle changes such as diet.
To diagnose premenstrual dysphoric disorder, Dr. Freeman recommends asking your patient to document and rate daily moods using a mobile app or calendar. In perimenopause, the risk of depression increases because estrogen has antidepressant effects. Although, there are no guidelines for treating depression in women in perimenopause, consider serotonergic antidepressants, supplements such as omega-3 fatty acids, isoflavones, and black cohosh, and sleep aids for patients with insomnia—a common feature of menopause.
Jeffrey R. Strawn, MD, FAACP, University of Cincinnati
Attention-deficit/hyperactivity disorder (ADHD) and bipolar disorder (BD) may share an underlying biological etiology, Dr. Strawn explained. Shared risk factors include in utero events, dietary factors, and genetics. Differentiating ADHD from BD depends on the developmental stage of the patient. Symptoms overlap, which could lead to overdiagnosis of ADHD in youths with BD.
Dr. Strawn discussed how children with depression might display mood lability and irritability, rather than verbalizing feelings because they do not use language effectively until age 7. Children may have somatic symptoms early and irritability might decrease into adolescence. Anxiety disorder in children emerges early—usually as a phobia—around age 12 to 14, with an increase in onset of depressive disorders. Dr. Strawn reviewed screening tools to diagnose and track anxiety symptoms, as well as the pros and cons of pharmacological treatments.

George T. Grossberg, MD, Saint Louis University
Psychotic symptoms could be common in older adults; therefore it is important to evaluate whether these symptoms cause emotional suffering or impairment in daily function. Dr. Grossberg recommended that when treating psychotic disorders in geriatric patients to first evaluate and treat underlying medical problems and identify offending medications or environmental or psychosocial triggers, then consider psychosocial or environmental interventions. Consider antipsychotics for patients who are experiencing severe emotional distress or those who pose a high safety risk. If antipsychotics are necessary, pick an agent based on side effects, “start low, go slow,” and discuss the risks and benefits with the family.
Role of Psychiatrists in Long-term Care Facilities
In his presentation on the role of psychiatrists in long-term care facilities, Dr. Grossberg described common disorders including the behavioral and psychiatric symptoms of dementia, as well as risk for depression. Overprescribing is common in long-term care facilities; therefore when considering a patient’s medication regimen, often less is more. Dr. Grossberg also discussed common undertreated or undercorrected physical health problems, including hearing or vision deficits, obstructive sleep apnea, and malnutrition.
Alexander W. Thompson, MD, MBA, MPH, University of Iowa Carver College of Medicine
Somatizing patients experience symptoms all of the time, whether a headache or nausea, but most symptoms do not have an organic cause, and they might seek treatment for any or all symptoms. The goal of treating somatizing patients is to not harm them with unneeded workup and treatment. Dr. Thomspon recommends providing a letter to the patient’s primary care physician with your recommendations, which can reduce medical costs and improve physical function. Although there are no clear pharmacotherapies, cognitive-behavioral therapy focused on health and anxiety can help.
Fatigue
Fatigue experienced by patients with chronic fatigue syndrome is unrelenting, is not the result of ongoing exertion, and is unrelieved by rest. When approaching a patient with extreme fatigue, start with a thorough evaluation in collaboration with a primary care physician, Dr. Thompson said. Establish a rapport with the patient, limit iatrogenic harm, and treat chronic fatigue as you would any chronic condition. Rintatolimod and valganciclovir have showed some evidence of benefit, and graded exercise therapy has shown success.
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 500 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, March 30 to April 1, 2017, at the Marriott Chicago Magnificent Mile in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us at Psychiatry Update Encore in Las Vegas, December 10 to 12, 2017 or next year in Chicago, March 22 to 24, 2018.
View summaries from the event on the following pages.
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Greg W. Mattingly, MD, Midwest Research Group and St. Charles Psychiatric Associates, St. Charles, Missouri.
In an industry-sponsored symposium, Dr. Mattingly reported that in the STAR-D study, approximately one-half of patients with major depressive disorder (MDD) did not experience adequate response to an initial selective serotonin reuptake inhibitor and 3 of 4 of those non-responding patients did not achieve full response with a second antidepressant, which prompts consideration of an adjunctive agent. Brexpiprazole (Rexulti) is a partial agonist for serotonin, dopamine, and noradrenergic systems. In pivotal trials as an adjunctive treatment in MDD, brexpiprazole, 2 mg/d, resulted in a statistically significant decrease in Montgomery-Åsburg Depression Rating Scale scores compared with placebo. Most common adverse reactions observed in ≥5% of patients and at least twice the rate of placebo included akathisia and weight increase.
Essentials of Malingering Assessment
Douglas Mossman, MD, University of Cincinnati
Malingering is intentional lying with an external incentive, such as avoiding work or obtaining drugs. Dr. Mossman gave 2 examples of malingered posttraumatic stress disorder and psychosis. Although lying cannot be detected by careful examination of facial expressions or gestures, a detailed evaluation can reveal malingering. An individual who is malingering psychosis may describe symptoms, such as “I talk to voices all the time,” but clinicians never observe such behavior. Signs of malingering include using “textbook” terms for symptoms; inconsistencies in their history or symptoms; sudden onset of delusions; exaggerating; and being unpleasant, dishonest, or demanding.
Beyond Efficacy and Effectiveness: Neurotoxicity vs Neuroprotection are the REAL Differences Between Typical and Atypical Antipsychotics
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Dr. Nasrallah discussed the difference between typical vs atypical antipsychotics—the former is neurotoxic, the latter is neuroprotective. Because patients with schizophrenia experience a loss of brain volume and cerebral grey matter and increased lateral ventricle volume, consider atypical antipsychotics for their neuroprotective properties.
In several studies typical antipsychotics, such as haloperidol, have been found to be neurotoxic, causing apoptosis and decreased cell viability. Atypical antipsychotics may be beneficial for patients with schizophrenia because they:
- stimulate production of new brain cells and increase neurotropic factors
- reverse PCP-induced changes in gene expression and loss of dendritic spines in the frontal cortex
- are neuroprotective against ischemic stroke damage
- prevent oligodendrocyte damage caused by interferon gamma-stimulated microglia.
Medicolegal Hazards in the Information Age: Malpractice and More
Douglas Mossman, MD, University of Cincinnati
Dr. Mossman began by answering the question, “What should I do if a patient ‘friended’ me on Facebook?” Such online relationships can blur boundaries or risk breaching confidentiality, therefore medical organizations recommend ignoring a friend request. Telemedicine via Skype is cost effective and enhances outreach to patients in rural areas or who cannot travel to the office, but online clinical encounters lack multidimensional aspects of the interpersonal encounter and might not be HIPAA compliant. E-mail carries some of the same concerns, such as confidentially of personal information, although the practice—when employed appropriately—is supported by some medical associations, including the American Psychiatric Association.
Treatment-Resistance and Suicidality in Schizophrenia: 2 Major Management Challenges
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Patients can seem treatment-resistant because of inadequate antipsychotic dosing, smoking, substance-induced relapse, nonadherence, or a general medical condition. Dr. Nasrallah discussed how to recognize true treatment-resistant schizophrenia and rule of spurious treatment resistance. If your patient is truly treatment-resistant, what do you do when everything else fails?
Risk factors for suicide include male sex, depressed mood, substance use, and social isolation. Clozapine, the only drug FDA-approved for refractory schizophrenia and suicidality, is underutilized for such patients. Dr. Nasrallah also presented evidence for the use of adjunctive modalities, such as lamotrigine, steroids, omega-3 fatty acids, NSAIDs, antidepressants, glutamatergic agents, and rTMS, as well as psychotherapy.
Luncheon Symposium
Depression, Its impact, and the Importance of Recognition and Treatment
Faculty: Jon Winston Draud, MS, MD, University of Tennessee Health Science Center
Major depressive disorder (MDD) is the most commonly diagnosed condition, second to cardiovascular disease. Dr. Draud recommended using a wellness screen such as the WHO-5 (World Health Organization, 5 item well-being index) in addition to a depression screening tool such as the PHQ-9. He emphasized that the goal of treatment should not be merely remission—but remission without residual symptoms. Cognitive impairment is the most common residual symptom. Patients with residual symptoms relapse earlier (5.5 times faster) and at a greater rate than patients without residual symptoms (76% vs 25%, respectively). Continue treatment and monitoring even after symptoms appear to subside. Recommended treatment is multi-modal and should include cognitive therapy and exercise.
New and Old Treatments for Opioid Abuse and Dependence
Mark S. Gold, MD, Washington University
Each day more than 1,000 people are treated in emergency departments for improper use of prescription opioids. But is naloxone saving lives or is overdose reversal nothing more than CPR? Dr. Gold spoke about the need for psychiatric assessment after a patient has been revived. Historically, treatment has stopped at abstinence or overdose treatment, but patients need ongoing treatment. Family therapy, vocational assistance, and psychotherapy are essential.
Dr. Gold reviewed established and newer treatments, including naloxone and naltrexone. Methadone and buprenorphine-naloxone can be effective for adherent patients who abuse only one drug. Naltrexone gives patients time to get their lives on track. Probuphine has comparable efficacy with buprenorphine-naloxone and methadone.
Impact of a Personality Disorder in Management of Comorbid Disorder
Donald W. Black, MD, University of Iowa
Personality disorder (PD) indicates patterns of long-term functioning and are not limited to episodes of illness. Abnormal personality traits are common among the general population, but are not considered a personality disorder unless they are inflexible, maladaptive, persisting, and cause distress either for the patient or the family. There are few cases of “pure” PDs without a comorbid psychiatric disorder. Personality disorders are not as stable as once understood; they wax and wane in response to stressors or depressed mood or anxiety. When a PD is comorbid with another disorder, patients are less likely to respond to medication and to experience remission from the comorbid psychiatric disorder.
Evaluation and Treatment of Patients Who Abuse Methamphetamine or Cocaine
Mark S. Gold, MD, Washington University
There are no FDA-approved medications or advancement in treatment for cocaine overdose—primary treatment is still ice baths. When assessing cocaine use, consider the route of ingestion and duration of use, which influence severity. Stimulants, whether methamphetamine or cocaine, cause changes in dopamine that are difficult to reverse. Substitute stimulants, such as modafinil, or vaccines have been proposed for cocaine abuse, but the evidence is not robust. Methamphetamine produces a schizophrenia-like illness, but antipsychotics are not effective. Naltrexone and bupropion showed some efficacy but was not statistically significant. There are no effective treatments for overdose or relapse prevention other than traditional group and residential treatment approaches.
Risks in Using Cannabis
Kevin Hill, MD, MHS, McLean Hospital
Although only 9% of Cannabis users become dependent, Dr. Hill recommended talking to all patients who use Cannabis about the risks, such as problems with work, school, and relationships. When treating patients with Cannabis use disorder, explore reasons that the individual would want to stop using Cannabis, take a careful history, and most importantly, build a good therapeutic alliance.
The most robust data for medical Cannabis is for chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis; however, there are more than 70 indications among the 28 states that allow its use. Dr. Hill suggests having a written policy, engage in conversation about why the patient wants medical Cannabis, be open to evaluating such a patient, and consider treating the patient’s symptoms with traditional modalities.
Marlene P. Freeman, MD, Massachusetts General Hospital
Dr. Freeman discussed the important role mental health providers play in helping women during pregnancy decrease medical and obstetrical risks, such as nutrition and maintaining a healthy weight. Because one-half of pregnancies in the United States are unplanned, consider medications that are compatible with pregnancy, and recommend omega-3 fatty acids and lifestyle changes such as diet.
To diagnose premenstrual dysphoric disorder, Dr. Freeman recommends asking your patient to document and rate daily moods using a mobile app or calendar. In perimenopause, the risk of depression increases because estrogen has antidepressant effects. Although, there are no guidelines for treating depression in women in perimenopause, consider serotonergic antidepressants, supplements such as omega-3 fatty acids, isoflavones, and black cohosh, and sleep aids for patients with insomnia—a common feature of menopause.
Jeffrey R. Strawn, MD, FAACP, University of Cincinnati
Attention-deficit/hyperactivity disorder (ADHD) and bipolar disorder (BD) may share an underlying biological etiology, Dr. Strawn explained. Shared risk factors include in utero events, dietary factors, and genetics. Differentiating ADHD from BD depends on the developmental stage of the patient. Symptoms overlap, which could lead to overdiagnosis of ADHD in youths with BD.
Dr. Strawn discussed how children with depression might display mood lability and irritability, rather than verbalizing feelings because they do not use language effectively until age 7. Children may have somatic symptoms early and irritability might decrease into adolescence. Anxiety disorder in children emerges early—usually as a phobia—around age 12 to 14, with an increase in onset of depressive disorders. Dr. Strawn reviewed screening tools to diagnose and track anxiety symptoms, as well as the pros and cons of pharmacological treatments.

George T. Grossberg, MD, Saint Louis University
Psychotic symptoms could be common in older adults; therefore it is important to evaluate whether these symptoms cause emotional suffering or impairment in daily function. Dr. Grossberg recommended that when treating psychotic disorders in geriatric patients to first evaluate and treat underlying medical problems and identify offending medications or environmental or psychosocial triggers, then consider psychosocial or environmental interventions. Consider antipsychotics for patients who are experiencing severe emotional distress or those who pose a high safety risk. If antipsychotics are necessary, pick an agent based on side effects, “start low, go slow,” and discuss the risks and benefits with the family.
Role of Psychiatrists in Long-term Care Facilities
In his presentation on the role of psychiatrists in long-term care facilities, Dr. Grossberg described common disorders including the behavioral and psychiatric symptoms of dementia, as well as risk for depression. Overprescribing is common in long-term care facilities; therefore when considering a patient’s medication regimen, often less is more. Dr. Grossberg also discussed common undertreated or undercorrected physical health problems, including hearing or vision deficits, obstructive sleep apnea, and malnutrition.
Alexander W. Thompson, MD, MBA, MPH, University of Iowa Carver College of Medicine
Somatizing patients experience symptoms all of the time, whether a headache or nausea, but most symptoms do not have an organic cause, and they might seek treatment for any or all symptoms. The goal of treating somatizing patients is to not harm them with unneeded workup and treatment. Dr. Thomspon recommends providing a letter to the patient’s primary care physician with your recommendations, which can reduce medical costs and improve physical function. Although there are no clear pharmacotherapies, cognitive-behavioral therapy focused on health and anxiety can help.
Fatigue
Fatigue experienced by patients with chronic fatigue syndrome is unrelenting, is not the result of ongoing exertion, and is unrelieved by rest. When approaching a patient with extreme fatigue, start with a thorough evaluation in collaboration with a primary care physician, Dr. Thompson said. Establish a rapport with the patient, limit iatrogenic harm, and treat chronic fatigue as you would any chronic condition. Rintatolimod and valganciclovir have showed some evidence of benefit, and graded exercise therapy has shown success.
Hormonal IUDs have higher expulsion rates immediately postpartum
Hormonal intrauterine devices inserted immediately postpartum had a nearly six times greater likelihood of expulsion compared with copper IUDs, but most women who requested any type of long-acting reversible contraception (LARC) postpartum were still using it half a year later, a recent study found.
“With more than eight out of ten women continuing use at 6 months, in-hospital placement of postpartum LARC devices is a worthwhile intervention,” reported Jennifer L. Eggebroten, MD, and her associates at the University of Utah. “More than half of women who experienced IUD expulsion without commencement of another highly effective contraceptive went on to become pregnant within 2 years, highlighting the need for appropriate counseling prior to device placement and backup contraception planning.”
Ninety percent of the patients were Hispanic, 87% had prior children, and 87% had an income below $24,000. Most (77%) had a vaginal delivery. Those who requested the copper IUD tended to be older and have more children compared with those who asked for the hormonal IUD or implant.
Among the 289 patients who completed the 6 months of follow-up, 17% of those with a hormonal IUD had an expulsion, compared with 4% of women with copper IUDs. That translated to a 5.8 times greater risk of expulsion for hormonal IUDs than for copper ones after the researchers accounted for age, mode of delivery, parity, and any breastfeeding. Expulsion rates were statistically similar between those who had vaginal deliveries and those who had cesarean deliveries.
Just 8% of the women requested removal of their device during the 6 months of follow-up. Most (67%) of the 21 women who had expulsions asked for a replacement. Cost of the device delayed or prevented replacement in some cases. Over the next 2 years, 6 of the 11 women who did not get replacement devices became pregnant.
Meanwhile, 81% of women with a hormonal IUD (88% including replacements), 83% with a copper IUD (86% including replacements), and 90% with an implant were still using that device 6 months later. A quarter of the women who completed the study follow-up reported that they did not return to their providers for their postpartum exams.
“For patients at high risk of rapid repeat pregnancy or who may not return for a postpartum visit, the benefit of placement of a highly effective method of contraception in the hospital prior to discharge may outweigh the increased risk of expulsion,” the researchers wrote. “In some states, by 8 weeks, public insurance coverage may expire and women face much more challenging obstacles to affordable, highly effective birth control options.”
The University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the research. The University of Utah receives research funding from LARC manufacturers and one of the coauthors reported financial relationships with LARC manufacturers.
Hormonal intrauterine devices inserted immediately postpartum had a nearly six times greater likelihood of expulsion compared with copper IUDs, but most women who requested any type of long-acting reversible contraception (LARC) postpartum were still using it half a year later, a recent study found.
“With more than eight out of ten women continuing use at 6 months, in-hospital placement of postpartum LARC devices is a worthwhile intervention,” reported Jennifer L. Eggebroten, MD, and her associates at the University of Utah. “More than half of women who experienced IUD expulsion without commencement of another highly effective contraceptive went on to become pregnant within 2 years, highlighting the need for appropriate counseling prior to device placement and backup contraception planning.”
Ninety percent of the patients were Hispanic, 87% had prior children, and 87% had an income below $24,000. Most (77%) had a vaginal delivery. Those who requested the copper IUD tended to be older and have more children compared with those who asked for the hormonal IUD or implant.
Among the 289 patients who completed the 6 months of follow-up, 17% of those with a hormonal IUD had an expulsion, compared with 4% of women with copper IUDs. That translated to a 5.8 times greater risk of expulsion for hormonal IUDs than for copper ones after the researchers accounted for age, mode of delivery, parity, and any breastfeeding. Expulsion rates were statistically similar between those who had vaginal deliveries and those who had cesarean deliveries.
Just 8% of the women requested removal of their device during the 6 months of follow-up. Most (67%) of the 21 women who had expulsions asked for a replacement. Cost of the device delayed or prevented replacement in some cases. Over the next 2 years, 6 of the 11 women who did not get replacement devices became pregnant.
Meanwhile, 81% of women with a hormonal IUD (88% including replacements), 83% with a copper IUD (86% including replacements), and 90% with an implant were still using that device 6 months later. A quarter of the women who completed the study follow-up reported that they did not return to their providers for their postpartum exams.
“For patients at high risk of rapid repeat pregnancy or who may not return for a postpartum visit, the benefit of placement of a highly effective method of contraception in the hospital prior to discharge may outweigh the increased risk of expulsion,” the researchers wrote. “In some states, by 8 weeks, public insurance coverage may expire and women face much more challenging obstacles to affordable, highly effective birth control options.”
The University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the research. The University of Utah receives research funding from LARC manufacturers and one of the coauthors reported financial relationships with LARC manufacturers.
Hormonal intrauterine devices inserted immediately postpartum had a nearly six times greater likelihood of expulsion compared with copper IUDs, but most women who requested any type of long-acting reversible contraception (LARC) postpartum were still using it half a year later, a recent study found.
“With more than eight out of ten women continuing use at 6 months, in-hospital placement of postpartum LARC devices is a worthwhile intervention,” reported Jennifer L. Eggebroten, MD, and her associates at the University of Utah. “More than half of women who experienced IUD expulsion without commencement of another highly effective contraceptive went on to become pregnant within 2 years, highlighting the need for appropriate counseling prior to device placement and backup contraception planning.”
Ninety percent of the patients were Hispanic, 87% had prior children, and 87% had an income below $24,000. Most (77%) had a vaginal delivery. Those who requested the copper IUD tended to be older and have more children compared with those who asked for the hormonal IUD or implant.
Among the 289 patients who completed the 6 months of follow-up, 17% of those with a hormonal IUD had an expulsion, compared with 4% of women with copper IUDs. That translated to a 5.8 times greater risk of expulsion for hormonal IUDs than for copper ones after the researchers accounted for age, mode of delivery, parity, and any breastfeeding. Expulsion rates were statistically similar between those who had vaginal deliveries and those who had cesarean deliveries.
Just 8% of the women requested removal of their device during the 6 months of follow-up. Most (67%) of the 21 women who had expulsions asked for a replacement. Cost of the device delayed or prevented replacement in some cases. Over the next 2 years, 6 of the 11 women who did not get replacement devices became pregnant.
Meanwhile, 81% of women with a hormonal IUD (88% including replacements), 83% with a copper IUD (86% including replacements), and 90% with an implant were still using that device 6 months later. A quarter of the women who completed the study follow-up reported that they did not return to their providers for their postpartum exams.
“For patients at high risk of rapid repeat pregnancy or who may not return for a postpartum visit, the benefit of placement of a highly effective method of contraception in the hospital prior to discharge may outweigh the increased risk of expulsion,” the researchers wrote. “In some states, by 8 weeks, public insurance coverage may expire and women face much more challenging obstacles to affordable, highly effective birth control options.”
The University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the research. The University of Utah receives research funding from LARC manufacturers and one of the coauthors reported financial relationships with LARC manufacturers.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Key clinical point:
Major finding: Hormonal IUDs were 5.8 times more likely to be expelled than were copper ones, but at least 80% of women who received a LARC still had it 6 months later.
Data source: A prospective cohort of 325 women who received a hormonal or copper IUD or a contraceptive implant immediately postpartum between October 2013 and February 2016.
Disclosures: The University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the research. The University of Utah receives research funding from LARC manufacturers and one of the coauthors reported financial relationships with LARC manufacturers.
Liver disease likely to become increasing indication for bariatric surgery
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
AGA Resource
The AGA Obesity Practice Guide provides tools for gastroenterologists to lead a multidisciplinary team of health-care professionals for the management of patients with obesity. Learn more at www.gastro.org/obesity.
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
AGA Resource
The AGA Obesity Practice Guide provides tools for gastroenterologists to lead a multidisciplinary team of health-care professionals for the management of patients with obesity. Learn more at www.gastro.org/obesity.
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
AGA Resource
The AGA Obesity Practice Guide provides tools for gastroenterologists to lead a multidisciplinary team of health-care professionals for the management of patients with obesity. Learn more at www.gastro.org/obesity.
AT DIGESTIVE DISEASES: NEW ADVANCES
Allergic Reaction to Vanadium Causes a Diffuse Eczematous Eruption and Titanium Alloy Orthopedic Implant Failure
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.
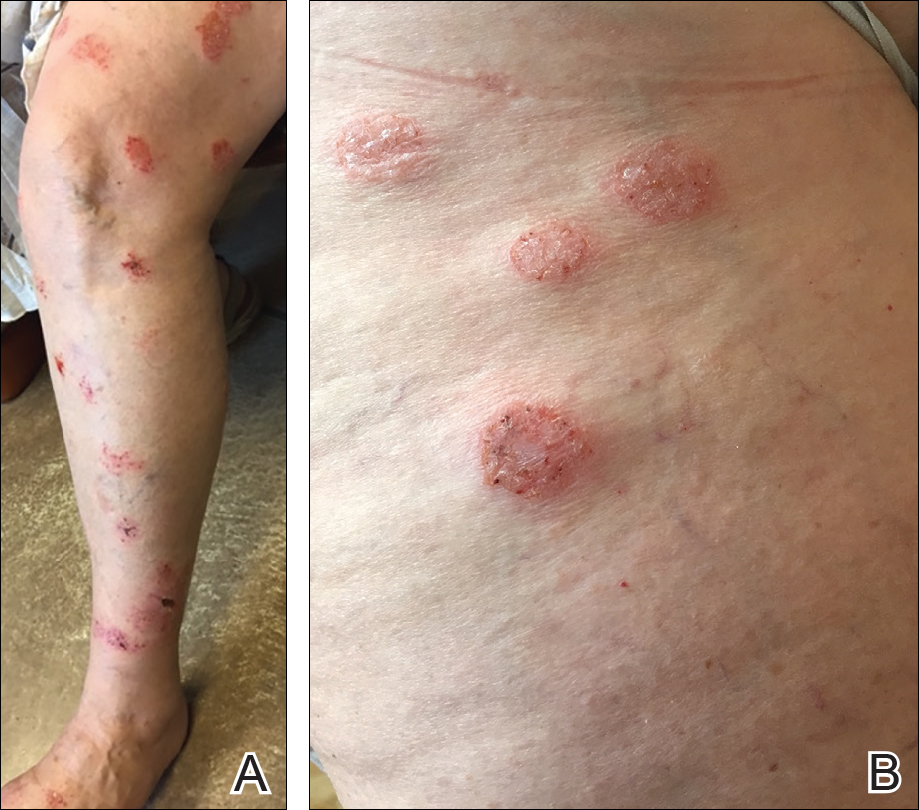
A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.
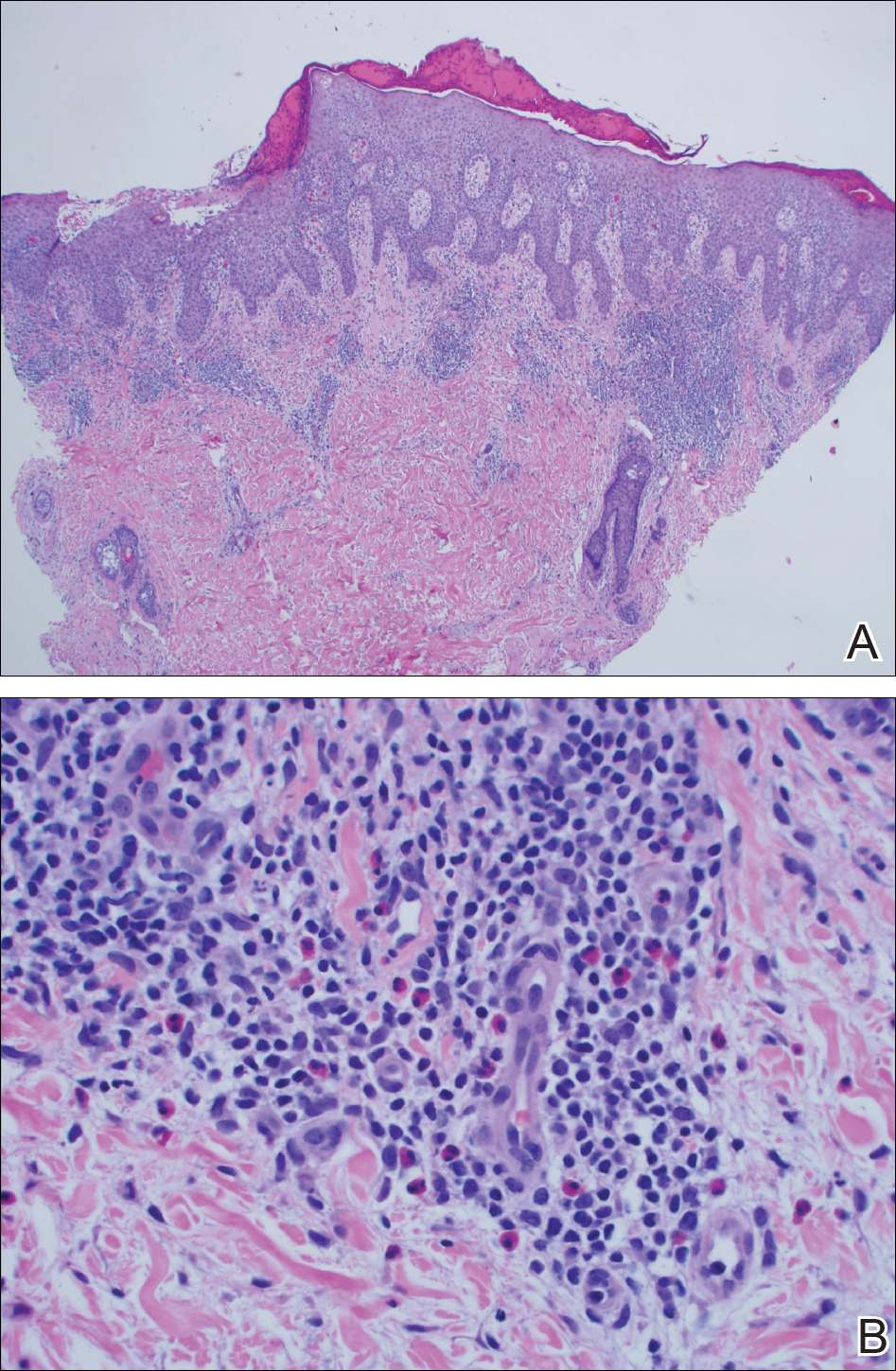
The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.

After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Practice Points
- Vanadium may be an underrecognized allergen in patients with metal implants.
- Consider vanadium allergy in those with surgical implants and signs of hypersensitivity reaction.
- Test for allergy with vanadium trichloride.
- Niobium is an alternative for implants in vanadium-allergic patients.
Refractory vasovagal syncope responds to DDD-CLS pacing
Washington – Help is finally at hand for the subgroup of patients who have severe refractory vasovagal syncope that recurs frequently and unpredictably, and is associated with a strong cardioinhibitory response on tilt table testing, Gonzalo Baron-Esquivias, MD, said at the annual meeting of the American College of Cardiology.
Results of the randomized, multicenter, double-blind, crossover, placebo-controlled SPAIN trial showed that implantation of a permanent pacemaker programmed to a closed loop stimulation (DDD-CLS) algorithm resulted in an 89% decrease in the risk of syncopal recurrences compared with sham DDI pacing, reported Dr. Baron-Esquivias, chief of clinical cardiology at Virgen del Rocio University Hospital in Seville, Spain.
Moreover, the number needed to treat with DDD-CLS pacing for 1 year to prevent a syncopal episode was a mere 2.7 patients in the SPAIN trial, he noted.
The study included 46 patients who met strict criteria for participation and received a permanent pacemaker for which they had no conventional indication. In half of the subjects, the device was programmed to DDD-CLS pacing, while the other half received sham DDI pacing, making them a placebo-treated control group. After 12 months, each group was switched over to the other form of pacing.
Dr. Baron-Esquivias emphasized that the tough SPAIN eligibility criteria enabled investigators to accurately define a specific CSL-responsive subgroup. This is not a form of therapy that’s appropriate for most patients who experience vasovagal syncope. In the right population, however, DDD-CLS pacing is life transforming. The SPAIN investigators documented severely impaired quality of life in the study population, with dramatic improvement on CLS pacing.
Vasovagal syncope is by far the most common cause of syncope. But roughly 70 out of every 100 patients who present to the emergency department with vasovagal syncope will never have a repeat episode. And of the 30 who do, perhaps only 10 will have frequent recurrences refractory to conventional measures and that impose a severe effect on quality of life. This is the subgroup for whom consideration of pacemaker therapy is appropriate.
To be eligible for the SPAIN trial, patients had to have at least five previous episodes of neuromediated vasovagal syncope, including at least two within the past year. They also had to be at least 40 years old, since CLS-responsive vasovagal syncope is skewed toward an older age group. In addition, all participants had to have normal results on a complete physical examination that included an orthostatic test, 12-lead echocardiogram, two-dimensional echocardiography, carotid sinus massage, and 24-hour Holter monitoring. Only then were they eligible for a tilt-table test. A positive head-up tilt test demonstrating a cardioinhibitory response required a heart rate drop to less than 40 bpm for at least 10 sec or a greater than 3-sec pause.
The average age of the 46 subjects who fulfilled all of these criteria was 56 years – several decades older than most patients who present with syncope. They averaged 12 prior syncopal episodes, including 4.5 during the prior 12 months. Three-quarters of the patients demonstrated asystole during the tilt test, with an average duration of 15 sec.
Four of the 46 patients experienced a syncopal recurrence during their 12 months on DDD-CLS pacing, while 21 of the same group of 46 had recurrent syncope during their year on DDI sham pacing. This translated into an absolute 37% reduction in syncopal episodes with active pacemaker therapy. Patients had an 8.8-fold greater risk of syncopal recurrence while on DDI sham pacing compared with DDD-CLS.
Dr. Baron-Esquivias explained that the DDD-CLS algorithm is designed to detect impending syncope at an early and actionable stage. The algorithm identifies the combination of increased myocardial contractility and reduced right ventricular intracardiac impedance, which heralds the first stage of vasovagal syncope. It then directs the pacemaker to activate high-rate atrioventricular sequential pacing to prevent arterial hypotension, bradycardia, and overt syncope.
Discussant Kenneth A. Ellenbogen, MD, applauded Dr. Baron-Esquivias and coinvestigators for “having the strength and conviction to attack this problem where so many prior studies have failed.”
For example, the earlier European SYNPACE trial showed no benefit for dual-chamber pacemaker therapy in DDD-RDR (rate-drop response) mode in patients with recurrent severe cardioinhibitory vasovagal syncope (Eur Heart J. 2004 Oct;25[19]:1741-8), probably due to a combination of a less robust early-syncope detection algorithm than that of DDD-CLS and less restrictive patient selection criteria, observed Dr. Ellenbogen, chairman of the division of cardiology and director of clinical cardiology electrophysiology and pacing at the Medical College of Virginia, Richmond.
The SPAIN trial was funded by the Spanish Society of Cardiology. Dr. Baron-Esquivias reported having no financial conflicts.
A considerably larger randomized trial known as BIOSync CLS, sponsored by Biotronic, which developed the CLS program, is ongoing.
Washington – Help is finally at hand for the subgroup of patients who have severe refractory vasovagal syncope that recurs frequently and unpredictably, and is associated with a strong cardioinhibitory response on tilt table testing, Gonzalo Baron-Esquivias, MD, said at the annual meeting of the American College of Cardiology.
Results of the randomized, multicenter, double-blind, crossover, placebo-controlled SPAIN trial showed that implantation of a permanent pacemaker programmed to a closed loop stimulation (DDD-CLS) algorithm resulted in an 89% decrease in the risk of syncopal recurrences compared with sham DDI pacing, reported Dr. Baron-Esquivias, chief of clinical cardiology at Virgen del Rocio University Hospital in Seville, Spain.
Moreover, the number needed to treat with DDD-CLS pacing for 1 year to prevent a syncopal episode was a mere 2.7 patients in the SPAIN trial, he noted.
The study included 46 patients who met strict criteria for participation and received a permanent pacemaker for which they had no conventional indication. In half of the subjects, the device was programmed to DDD-CLS pacing, while the other half received sham DDI pacing, making them a placebo-treated control group. After 12 months, each group was switched over to the other form of pacing.
Dr. Baron-Esquivias emphasized that the tough SPAIN eligibility criteria enabled investigators to accurately define a specific CSL-responsive subgroup. This is not a form of therapy that’s appropriate for most patients who experience vasovagal syncope. In the right population, however, DDD-CLS pacing is life transforming. The SPAIN investigators documented severely impaired quality of life in the study population, with dramatic improvement on CLS pacing.
Vasovagal syncope is by far the most common cause of syncope. But roughly 70 out of every 100 patients who present to the emergency department with vasovagal syncope will never have a repeat episode. And of the 30 who do, perhaps only 10 will have frequent recurrences refractory to conventional measures and that impose a severe effect on quality of life. This is the subgroup for whom consideration of pacemaker therapy is appropriate.
To be eligible for the SPAIN trial, patients had to have at least five previous episodes of neuromediated vasovagal syncope, including at least two within the past year. They also had to be at least 40 years old, since CLS-responsive vasovagal syncope is skewed toward an older age group. In addition, all participants had to have normal results on a complete physical examination that included an orthostatic test, 12-lead echocardiogram, two-dimensional echocardiography, carotid sinus massage, and 24-hour Holter monitoring. Only then were they eligible for a tilt-table test. A positive head-up tilt test demonstrating a cardioinhibitory response required a heart rate drop to less than 40 bpm for at least 10 sec or a greater than 3-sec pause.
The average age of the 46 subjects who fulfilled all of these criteria was 56 years – several decades older than most patients who present with syncope. They averaged 12 prior syncopal episodes, including 4.5 during the prior 12 months. Three-quarters of the patients demonstrated asystole during the tilt test, with an average duration of 15 sec.
Four of the 46 patients experienced a syncopal recurrence during their 12 months on DDD-CLS pacing, while 21 of the same group of 46 had recurrent syncope during their year on DDI sham pacing. This translated into an absolute 37% reduction in syncopal episodes with active pacemaker therapy. Patients had an 8.8-fold greater risk of syncopal recurrence while on DDI sham pacing compared with DDD-CLS.
Dr. Baron-Esquivias explained that the DDD-CLS algorithm is designed to detect impending syncope at an early and actionable stage. The algorithm identifies the combination of increased myocardial contractility and reduced right ventricular intracardiac impedance, which heralds the first stage of vasovagal syncope. It then directs the pacemaker to activate high-rate atrioventricular sequential pacing to prevent arterial hypotension, bradycardia, and overt syncope.
Discussant Kenneth A. Ellenbogen, MD, applauded Dr. Baron-Esquivias and coinvestigators for “having the strength and conviction to attack this problem where so many prior studies have failed.”
For example, the earlier European SYNPACE trial showed no benefit for dual-chamber pacemaker therapy in DDD-RDR (rate-drop response) mode in patients with recurrent severe cardioinhibitory vasovagal syncope (Eur Heart J. 2004 Oct;25[19]:1741-8), probably due to a combination of a less robust early-syncope detection algorithm than that of DDD-CLS and less restrictive patient selection criteria, observed Dr. Ellenbogen, chairman of the division of cardiology and director of clinical cardiology electrophysiology and pacing at the Medical College of Virginia, Richmond.
The SPAIN trial was funded by the Spanish Society of Cardiology. Dr. Baron-Esquivias reported having no financial conflicts.
A considerably larger randomized trial known as BIOSync CLS, sponsored by Biotronic, which developed the CLS program, is ongoing.
Washington – Help is finally at hand for the subgroup of patients who have severe refractory vasovagal syncope that recurs frequently and unpredictably, and is associated with a strong cardioinhibitory response on tilt table testing, Gonzalo Baron-Esquivias, MD, said at the annual meeting of the American College of Cardiology.
Results of the randomized, multicenter, double-blind, crossover, placebo-controlled SPAIN trial showed that implantation of a permanent pacemaker programmed to a closed loop stimulation (DDD-CLS) algorithm resulted in an 89% decrease in the risk of syncopal recurrences compared with sham DDI pacing, reported Dr. Baron-Esquivias, chief of clinical cardiology at Virgen del Rocio University Hospital in Seville, Spain.
Moreover, the number needed to treat with DDD-CLS pacing for 1 year to prevent a syncopal episode was a mere 2.7 patients in the SPAIN trial, he noted.
The study included 46 patients who met strict criteria for participation and received a permanent pacemaker for which they had no conventional indication. In half of the subjects, the device was programmed to DDD-CLS pacing, while the other half received sham DDI pacing, making them a placebo-treated control group. After 12 months, each group was switched over to the other form of pacing.
Dr. Baron-Esquivias emphasized that the tough SPAIN eligibility criteria enabled investigators to accurately define a specific CSL-responsive subgroup. This is not a form of therapy that’s appropriate for most patients who experience vasovagal syncope. In the right population, however, DDD-CLS pacing is life transforming. The SPAIN investigators documented severely impaired quality of life in the study population, with dramatic improvement on CLS pacing.
Vasovagal syncope is by far the most common cause of syncope. But roughly 70 out of every 100 patients who present to the emergency department with vasovagal syncope will never have a repeat episode. And of the 30 who do, perhaps only 10 will have frequent recurrences refractory to conventional measures and that impose a severe effect on quality of life. This is the subgroup for whom consideration of pacemaker therapy is appropriate.
To be eligible for the SPAIN trial, patients had to have at least five previous episodes of neuromediated vasovagal syncope, including at least two within the past year. They also had to be at least 40 years old, since CLS-responsive vasovagal syncope is skewed toward an older age group. In addition, all participants had to have normal results on a complete physical examination that included an orthostatic test, 12-lead echocardiogram, two-dimensional echocardiography, carotid sinus massage, and 24-hour Holter monitoring. Only then were they eligible for a tilt-table test. A positive head-up tilt test demonstrating a cardioinhibitory response required a heart rate drop to less than 40 bpm for at least 10 sec or a greater than 3-sec pause.
The average age of the 46 subjects who fulfilled all of these criteria was 56 years – several decades older than most patients who present with syncope. They averaged 12 prior syncopal episodes, including 4.5 during the prior 12 months. Three-quarters of the patients demonstrated asystole during the tilt test, with an average duration of 15 sec.
Four of the 46 patients experienced a syncopal recurrence during their 12 months on DDD-CLS pacing, while 21 of the same group of 46 had recurrent syncope during their year on DDI sham pacing. This translated into an absolute 37% reduction in syncopal episodes with active pacemaker therapy. Patients had an 8.8-fold greater risk of syncopal recurrence while on DDI sham pacing compared with DDD-CLS.
Dr. Baron-Esquivias explained that the DDD-CLS algorithm is designed to detect impending syncope at an early and actionable stage. The algorithm identifies the combination of increased myocardial contractility and reduced right ventricular intracardiac impedance, which heralds the first stage of vasovagal syncope. It then directs the pacemaker to activate high-rate atrioventricular sequential pacing to prevent arterial hypotension, bradycardia, and overt syncope.
Discussant Kenneth A. Ellenbogen, MD, applauded Dr. Baron-Esquivias and coinvestigators for “having the strength and conviction to attack this problem where so many prior studies have failed.”
For example, the earlier European SYNPACE trial showed no benefit for dual-chamber pacemaker therapy in DDD-RDR (rate-drop response) mode in patients with recurrent severe cardioinhibitory vasovagal syncope (Eur Heart J. 2004 Oct;25[19]:1741-8), probably due to a combination of a less robust early-syncope detection algorithm than that of DDD-CLS and less restrictive patient selection criteria, observed Dr. Ellenbogen, chairman of the division of cardiology and director of clinical cardiology electrophysiology and pacing at the Medical College of Virginia, Richmond.
The SPAIN trial was funded by the Spanish Society of Cardiology. Dr. Baron-Esquivias reported having no financial conflicts.
A considerably larger randomized trial known as BIOSync CLS, sponsored by Biotronic, which developed the CLS program, is ongoing.
At ACC 17
Key clinical point:
Major finding: The number of patients with severe recurrent cardioinhibitory reflex vasovagal syncope needed to be treated with DDD-CLS pacing for 1 year to prevent a recurrent episode is 2.7.
Data source: SPAIN, a 2-year randomized, multicenter, double-blind, prospective, placebo-controlled crossover trial of 46 patients with severe recurrent vasovagal syncope treated via pacemaker therapy.
Disclosures: The Spanish Society of Cardiology funded the study. The presenter reported having no financial conflicts.
Levothyroxine: No benefit for subclinical hypothyroidism in elderly
Levothyroxine provided no benefits in older patients with subclinical hypothyroidism in the first large randomized clinical trial of the treatment for this indication, which was presented at the annual meeting of the Endocrine Society.
An estimated 8%-18% of people aged 65 and older have subclinical hypothyroidism, which is defined as an elevated serum thyrotropin level and a serum free-thyroxine level within the reference range. The condition is considered to be a possible contributor to many problems affecting numerous physiologic systems including the vascular tree, the heart, the brain, skeletal muscle, and bone, said David J. Stott, MD, of the academic section of geriatric medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow.
The mean age of the study participants was 74 years, and they were followed for a median of 18 months after initiating treatment. The active intervention did boost thyroid function as expected, compared with placebo.
The two primary outcome measures were change between baseline and 1 year in hypothyroid symptoms and in tiredness scores on the 100-point ThyPRO (Thyroid-Related Quality of Life Patient-Reported Outcome) measure. There were no significant differences between the two study groups in changes in either of these scores. The mean 1-year score for hypothyroid symptoms was 16.6 for levothyroxine and 16.7 for placebo, and the mean 1-year score for tiredness was 28.7 for levothyroxine and 28.6 for placebo.
Secondary outcomes also did not differ significantly between the two study groups, including general health-related quality of life; hand-grip strength (reflecting possible effects on skeletal muscle); executive cognitive function (reflecting possible effects on the brain); blood pressure, weight, body mass index, and waist circumference (reflecting possible effects on cardiovascular health); or activities of daily living. In addition, both fatal and nonfatal cardiovascular events were similar between the two study groups at 1 year and at extended 3-year follow-up.
Further analyses did not identify any subgroup of adults who benefited from active treatment. The lack of benefit extended across all older age groups, both genders, and all serum thyrotropin levels at baseline. In addition, all the sensitivity analyses confirmed the results of the main analysis.
Adverse events also were not significantly different between the two study groups. This included four serious adverse events of special interest: new-onset atrial fibrillation, heart failure, fracture, or osteoporosis. There also was no increase in symptoms of hyperthyroidism in the active-treatment group.
Levothyroxine provided no benefits in older patients with subclinical hypothyroidism in the first large randomized clinical trial of the treatment for this indication, which was presented at the annual meeting of the Endocrine Society.
An estimated 8%-18% of people aged 65 and older have subclinical hypothyroidism, which is defined as an elevated serum thyrotropin level and a serum free-thyroxine level within the reference range. The condition is considered to be a possible contributor to many problems affecting numerous physiologic systems including the vascular tree, the heart, the brain, skeletal muscle, and bone, said David J. Stott, MD, of the academic section of geriatric medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow.
The mean age of the study participants was 74 years, and they were followed for a median of 18 months after initiating treatment. The active intervention did boost thyroid function as expected, compared with placebo.
The two primary outcome measures were change between baseline and 1 year in hypothyroid symptoms and in tiredness scores on the 100-point ThyPRO (Thyroid-Related Quality of Life Patient-Reported Outcome) measure. There were no significant differences between the two study groups in changes in either of these scores. The mean 1-year score for hypothyroid symptoms was 16.6 for levothyroxine and 16.7 for placebo, and the mean 1-year score for tiredness was 28.7 for levothyroxine and 28.6 for placebo.
Secondary outcomes also did not differ significantly between the two study groups, including general health-related quality of life; hand-grip strength (reflecting possible effects on skeletal muscle); executive cognitive function (reflecting possible effects on the brain); blood pressure, weight, body mass index, and waist circumference (reflecting possible effects on cardiovascular health); or activities of daily living. In addition, both fatal and nonfatal cardiovascular events were similar between the two study groups at 1 year and at extended 3-year follow-up.
Further analyses did not identify any subgroup of adults who benefited from active treatment. The lack of benefit extended across all older age groups, both genders, and all serum thyrotropin levels at baseline. In addition, all the sensitivity analyses confirmed the results of the main analysis.
Adverse events also were not significantly different between the two study groups. This included four serious adverse events of special interest: new-onset atrial fibrillation, heart failure, fracture, or osteoporosis. There also was no increase in symptoms of hyperthyroidism in the active-treatment group.
Levothyroxine provided no benefits in older patients with subclinical hypothyroidism in the first large randomized clinical trial of the treatment for this indication, which was presented at the annual meeting of the Endocrine Society.
An estimated 8%-18% of people aged 65 and older have subclinical hypothyroidism, which is defined as an elevated serum thyrotropin level and a serum free-thyroxine level within the reference range. The condition is considered to be a possible contributor to many problems affecting numerous physiologic systems including the vascular tree, the heart, the brain, skeletal muscle, and bone, said David J. Stott, MD, of the academic section of geriatric medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow.
The mean age of the study participants was 74 years, and they were followed for a median of 18 months after initiating treatment. The active intervention did boost thyroid function as expected, compared with placebo.
The two primary outcome measures were change between baseline and 1 year in hypothyroid symptoms and in tiredness scores on the 100-point ThyPRO (Thyroid-Related Quality of Life Patient-Reported Outcome) measure. There were no significant differences between the two study groups in changes in either of these scores. The mean 1-year score for hypothyroid symptoms was 16.6 for levothyroxine and 16.7 for placebo, and the mean 1-year score for tiredness was 28.7 for levothyroxine and 28.6 for placebo.
Secondary outcomes also did not differ significantly between the two study groups, including general health-related quality of life; hand-grip strength (reflecting possible effects on skeletal muscle); executive cognitive function (reflecting possible effects on the brain); blood pressure, weight, body mass index, and waist circumference (reflecting possible effects on cardiovascular health); or activities of daily living. In addition, both fatal and nonfatal cardiovascular events were similar between the two study groups at 1 year and at extended 3-year follow-up.
Further analyses did not identify any subgroup of adults who benefited from active treatment. The lack of benefit extended across all older age groups, both genders, and all serum thyrotropin levels at baseline. In addition, all the sensitivity analyses confirmed the results of the main analysis.
Adverse events also were not significantly different between the two study groups. This included four serious adverse events of special interest: new-onset atrial fibrillation, heart failure, fracture, or osteoporosis. There also was no increase in symptoms of hyperthyroidism in the active-treatment group.
FROM ENDO 2017
Key clinical point: Levothyroxine provided no benefits in older patients with subclinical hypothyroidism in the first large randomized clinical trial of the therapy.
Major finding: The mean score for hypothyroid symptoms was 16.6 for levothyroxine and 16.7 for placebo after 1 year of treatment, and the mean score for tiredness was 28.7 for levothyroxine and 28.6 for placebo.
Data source: An international randomized double-blind placebo-controlled clinical trial involving 737 older patients with subclinical hypothyroidism.
Disclosures: This study was supported by the European Union and several foundations. The levothyroxine and matching placebo were provided free of charge by Merck, which played no role in the design, analysis, or reporting of the study. Dr. Stott reported having no relevant financial disclosures; one of his associates reported ties to Bristol/Myers Squibb, Pfizer, and Bayer.
U.S. thyroid cancer incidence, mortality on the upswing
ORLANDO – with incidence-based mortality increasing by 1.1% annually from 1994 to 2013, according to a study conducted by the National Cancer Institute.
In a study of 77,726 patients diagnosed with thyroid cancer between 1974 and 2013, incidence rates increased from 4.56 per 100,000 person-years during 1974-1977 to 14.42 per 100,000 person-years during 2010-2013, according to Hyeyeun Lim, PhD, a postdoctoral fellow at National Cancer Institute, and colleagues (JAMA. 2017;317[13]:1338-48).
A majority of patients in the sample were female (75%) and white (82%); average age was 48 years.
A notable trend was the increase in papillary thyroid cancer (PTC). PTC was the most common thyroid cancer at 83.6% of diagnoses, followed by follicular, medullary, anaplastic, and other at 10.8%, 2.2%, 1.3%, and 2.1%, respectively. PTC was associated with the highest annual percent change (4.4%) and the only positive incidence-based mortality annual percent change (1.7%) among all histologic types, according to the researchers.
Regional and distant tumors accounted for 53.2% and 29% of deaths, respectively, compared to 13.5% for local tumors.
Dr. Lim and colleagues interpret the increase in incidence to contradict a common idea among researchers that attributes rising rates to new methods of detection such as ultrasound imaging and fine-needle aspiration biopsies.
“Such changes could account for the rapid increases in the incidence rates for localized and small PTCs that have been previously observed,” the researchers reported. “However, the significant, albeit less-rapid increase in advanced-stage and larger PTC incidence rates and increasing thyroid cancer mortality rates among patients diagnosed with advanced-stage PTC is not consistent with the notion that over-diagnosis is solely responsible for the changing trends in PTC incidence.”
While the researchers reported increased mortality rates among all PTC demographics, statistical significance was found solely in patients with distant disease (annual percentage changes, 2.9% [95% confidence interval, 1.1%-4.7%]), stage IV disease (APC, 12.9%[95%CI, 7.2%-19.0%]), or both, according to researchers.
Researchers speculate increased rates of obesity, childhood ionizing radiation exposure, and increased exposure to pesticides may be possible sources for increased rates of PTC, however Dr. Lim and peers assert further research must be conducted.
Based on these findings, researchers suggest “renewed focus on aggressive transdisciplinary management that includes surgery, adjuvant radioactive iodine, and, when indicated for the 5%-10% of patients who develop progressive disease, systemic therapy,” for patients with advanced-stage PTC.
Due to the nature of the study, researchers were limited to speculating potential reasons for increase in thyroid cancer incidence. Information of tumor size and stage was limited to the years when this information began to be recorded, after the initial years included in the study.
[email protected]
On Twitter @EAZTweets
ORLANDO – with incidence-based mortality increasing by 1.1% annually from 1994 to 2013, according to a study conducted by the National Cancer Institute.
In a study of 77,726 patients diagnosed with thyroid cancer between 1974 and 2013, incidence rates increased from 4.56 per 100,000 person-years during 1974-1977 to 14.42 per 100,000 person-years during 2010-2013, according to Hyeyeun Lim, PhD, a postdoctoral fellow at National Cancer Institute, and colleagues (JAMA. 2017;317[13]:1338-48).
A majority of patients in the sample were female (75%) and white (82%); average age was 48 years.
A notable trend was the increase in papillary thyroid cancer (PTC). PTC was the most common thyroid cancer at 83.6% of diagnoses, followed by follicular, medullary, anaplastic, and other at 10.8%, 2.2%, 1.3%, and 2.1%, respectively. PTC was associated with the highest annual percent change (4.4%) and the only positive incidence-based mortality annual percent change (1.7%) among all histologic types, according to the researchers.
Regional and distant tumors accounted for 53.2% and 29% of deaths, respectively, compared to 13.5% for local tumors.
Dr. Lim and colleagues interpret the increase in incidence to contradict a common idea among researchers that attributes rising rates to new methods of detection such as ultrasound imaging and fine-needle aspiration biopsies.
“Such changes could account for the rapid increases in the incidence rates for localized and small PTCs that have been previously observed,” the researchers reported. “However, the significant, albeit less-rapid increase in advanced-stage and larger PTC incidence rates and increasing thyroid cancer mortality rates among patients diagnosed with advanced-stage PTC is not consistent with the notion that over-diagnosis is solely responsible for the changing trends in PTC incidence.”
While the researchers reported increased mortality rates among all PTC demographics, statistical significance was found solely in patients with distant disease (annual percentage changes, 2.9% [95% confidence interval, 1.1%-4.7%]), stage IV disease (APC, 12.9%[95%CI, 7.2%-19.0%]), or both, according to researchers.
Researchers speculate increased rates of obesity, childhood ionizing radiation exposure, and increased exposure to pesticides may be possible sources for increased rates of PTC, however Dr. Lim and peers assert further research must be conducted.
Based on these findings, researchers suggest “renewed focus on aggressive transdisciplinary management that includes surgery, adjuvant radioactive iodine, and, when indicated for the 5%-10% of patients who develop progressive disease, systemic therapy,” for patients with advanced-stage PTC.
Due to the nature of the study, researchers were limited to speculating potential reasons for increase in thyroid cancer incidence. Information of tumor size and stage was limited to the years when this information began to be recorded, after the initial years included in the study.
[email protected]
On Twitter @EAZTweets
ORLANDO – with incidence-based mortality increasing by 1.1% annually from 1994 to 2013, according to a study conducted by the National Cancer Institute.
In a study of 77,726 patients diagnosed with thyroid cancer between 1974 and 2013, incidence rates increased from 4.56 per 100,000 person-years during 1974-1977 to 14.42 per 100,000 person-years during 2010-2013, according to Hyeyeun Lim, PhD, a postdoctoral fellow at National Cancer Institute, and colleagues (JAMA. 2017;317[13]:1338-48).
A majority of patients in the sample were female (75%) and white (82%); average age was 48 years.
A notable trend was the increase in papillary thyroid cancer (PTC). PTC was the most common thyroid cancer at 83.6% of diagnoses, followed by follicular, medullary, anaplastic, and other at 10.8%, 2.2%, 1.3%, and 2.1%, respectively. PTC was associated with the highest annual percent change (4.4%) and the only positive incidence-based mortality annual percent change (1.7%) among all histologic types, according to the researchers.
Regional and distant tumors accounted for 53.2% and 29% of deaths, respectively, compared to 13.5% for local tumors.
Dr. Lim and colleagues interpret the increase in incidence to contradict a common idea among researchers that attributes rising rates to new methods of detection such as ultrasound imaging and fine-needle aspiration biopsies.
“Such changes could account for the rapid increases in the incidence rates for localized and small PTCs that have been previously observed,” the researchers reported. “However, the significant, albeit less-rapid increase in advanced-stage and larger PTC incidence rates and increasing thyroid cancer mortality rates among patients diagnosed with advanced-stage PTC is not consistent with the notion that over-diagnosis is solely responsible for the changing trends in PTC incidence.”
While the researchers reported increased mortality rates among all PTC demographics, statistical significance was found solely in patients with distant disease (annual percentage changes, 2.9% [95% confidence interval, 1.1%-4.7%]), stage IV disease (APC, 12.9%[95%CI, 7.2%-19.0%]), or both, according to researchers.
Researchers speculate increased rates of obesity, childhood ionizing radiation exposure, and increased exposure to pesticides may be possible sources for increased rates of PTC, however Dr. Lim and peers assert further research must be conducted.
Based on these findings, researchers suggest “renewed focus on aggressive transdisciplinary management that includes surgery, adjuvant radioactive iodine, and, when indicated for the 5%-10% of patients who develop progressive disease, systemic therapy,” for patients with advanced-stage PTC.
Due to the nature of the study, researchers were limited to speculating potential reasons for increase in thyroid cancer incidence. Information of tumor size and stage was limited to the years when this information began to be recorded, after the initial years included in the study.
[email protected]
On Twitter @EAZTweets
AT ENDO 2017
Key clinical point:
Major finding: Thyroid cancer incidence increased 3.6% from 1974 to 2013 and incidence-based mortality increased by 1.1% per year from 1994 to 2013.
Data source: A retrospective study of 77,726 patient records attained from Surveillance, Epidemiology, and End Results–9 cancer registry database, analyzed via log-linear regression.
Disclosures: Dr. Julie Sosa reported being on the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry, which is sponsored by AstraZeneca, Eli Lilly, GlaxoSmithKline, and Novo Nordisk.