User login
VA Establishes Presumption of Service Connection for Camp Lejeune
Veterans who were exposed to contaminated water at Camp Lejeune are now eligible for VA care and benefits if they have been diagnosed with any of 8 diseases: adult leukemia, aplastic anemia and other myelodysplastic syndromes, bladder cancer, kidney cancer, liver cancer, multiple myeloma, non-Hodgkin lymphoma, and Parkinson disease. The presumption of service connection regulations went into effect March 14.
The newly effective rule complements the health care already provided for 15 illnesses or conditions as part of the Honoring America’s Veterans and Caring for Camp Lejeune Families Act of 2012. The Camp Lejeune Act requires the VA to provide health care to veterans who served at Camp Lejeune and to reimburse family members or pay providers for medical expenses for those who lived there for ≥ 30 days between August 1, 1953, and December 31, 1987.
The act and new rule relate to 2 on-base water wells that were contaminated with trichloroethylene, perchloroethylene, benzene, vinyl chloride, and other compounds. The wells were shut down in 1985.
The presumption of service connection applies to active-duty, reserve, and National Guard members. The presumption also includes all of Camp Lejeune, Marine Corps Air Station New River, as well as satellite camps and housing areas.
Veterans who were exposed to contaminated water at Camp Lejeune are now eligible for VA care and benefits if they have been diagnosed with any of 8 diseases: adult leukemia, aplastic anemia and other myelodysplastic syndromes, bladder cancer, kidney cancer, liver cancer, multiple myeloma, non-Hodgkin lymphoma, and Parkinson disease. The presumption of service connection regulations went into effect March 14.
The newly effective rule complements the health care already provided for 15 illnesses or conditions as part of the Honoring America’s Veterans and Caring for Camp Lejeune Families Act of 2012. The Camp Lejeune Act requires the VA to provide health care to veterans who served at Camp Lejeune and to reimburse family members or pay providers for medical expenses for those who lived there for ≥ 30 days between August 1, 1953, and December 31, 1987.
The act and new rule relate to 2 on-base water wells that were contaminated with trichloroethylene, perchloroethylene, benzene, vinyl chloride, and other compounds. The wells were shut down in 1985.
The presumption of service connection applies to active-duty, reserve, and National Guard members. The presumption also includes all of Camp Lejeune, Marine Corps Air Station New River, as well as satellite camps and housing areas.
Veterans who were exposed to contaminated water at Camp Lejeune are now eligible for VA care and benefits if they have been diagnosed with any of 8 diseases: adult leukemia, aplastic anemia and other myelodysplastic syndromes, bladder cancer, kidney cancer, liver cancer, multiple myeloma, non-Hodgkin lymphoma, and Parkinson disease. The presumption of service connection regulations went into effect March 14.
The newly effective rule complements the health care already provided for 15 illnesses or conditions as part of the Honoring America’s Veterans and Caring for Camp Lejeune Families Act of 2012. The Camp Lejeune Act requires the VA to provide health care to veterans who served at Camp Lejeune and to reimburse family members or pay providers for medical expenses for those who lived there for ≥ 30 days between August 1, 1953, and December 31, 1987.
The act and new rule relate to 2 on-base water wells that were contaminated with trichloroethylene, perchloroethylene, benzene, vinyl chloride, and other compounds. The wells were shut down in 1985.
The presumption of service connection applies to active-duty, reserve, and National Guard members. The presumption also includes all of Camp Lejeune, Marine Corps Air Station New River, as well as satellite camps and housing areas.
Half of patients retain response to CAR T-cell therapy
WASHINGTON, DC—Roughly half of patients who responded to chimeric antigen receptor (CAR) T-cell therapy in the ZUMA-1 trial have retained that response at a median follow-up exceeding 8 months.
The CAR T-cell therapy, axicabtagene ciloleucel (formerly KTE-C19), initially produced an objective response rate (ORR) of 82% in this trial of patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL).
At a median follow-up of 8.7 months, 44% of all patients (53% of responders) are still in response, and 39% are in complete response (CR).
Thirteen percent of patients had grade 3 or higher cytokine release syndrome (CRS), and 28% had neurologic events.
There were 2 deaths related to axicabtagene ciloleucel.
Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa, Florida, presented these updated results from ZUMA-1 at the AACR Annual Meeting 2017 (abstract CT019).
ZUMA-1 is sponsored by Kite Pharma but is also funded, in part, by the Leukemia and Lymphoma Society Therapy Acceleration Program.
Patients and treatment
The trial enrolled 111 patients, 101 of whom were successfully treated with axicabtagene ciloleucel. Seven patients could not be treated due to serious adverse events, 1 due to unavailable product, and 2 due to non-measurable disease.
Seventy-seven of the patients had diffuse large B-cell lymphoma (DLBCL), and 24 had transformed follicular lymphoma (TFL) or primary mediastinal B-cell lymphoma (PMBCL). Eighty-five percent of patients had stage III/IV disease.
Seventy-nine percent were refractory to chemotherapy and did not have a prior autologous stem cell transplant (auto-SCT). Twenty-one percent did undergo auto-SCT and relapsed within 12 months of the procedure.
Sixty-nine percent of patients had received 3 or more lines of prior therapy, and 54% were refractory to 2 consecutive lines of prior therapy.
For this study, the patients received a conditioning regimen of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days.
Two days after the conditioning regimen was completed, patients received axicabtagene ciloleucel at a target dose of 2 × 106 CAR T cells/kg.
Efficacy
The following table shows overall response data, response data at 6 months, and ongoing responses at the primary analysis data cut-off.
| DLBCL (n=77) | TFL/PMBCL (n=24) | Combined (n=101) | ||||
| ORR (%) | CR (%) | ORR (%) | CR (%) | ORR (%) | CR (%) | |
| ORR | 82 | 49 | 83 | 71 | 82 | 54 |
| Month 6 | 36 | 31 | 54 | 50 | 41 | 36 |
| Ongoing | 36 | 31 | 67 | 63 | 44 | 39 |
The researchers said the ORR was generally consistent in key subgroups. The ORR was 83% in patients who were refractory to their second or greater line of therapy and 76% in patients who relapsed within 12 months of auto-SCT.
Overall, the median duration of response was 8.2 months. However, the median duration of response has not been reached for patients with a CR.
At a median follow-up of 8.7 months, the median overall survival has not been reached.
Safety
The most common grade 3 or higher adverse events included anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
The incidence of grade 3 or higher CRS was 13%, and the incidence of neurologic events was 28%. These represent decreases from the interim analysis of ZUMA-1, when the rate of grade 3+ CRS was 18%, and the rate of neurological events was 34%.
“We believe the rates of CRS and neurologic events decreased over the course of the study as clinicians gained experience in the management of adverse events,” said Jeff Wiezorek, MD, senior vice-president of clinical development at Kite Pharma.
There were 3 deaths throughout the course of the trial that were not a result of disease progression.
Two deaths were deemed related to axicabtagene ciloleucel. One was a case of hemophagocytic lymphohistiocytosis. The other was cardiac arrest in the setting of CRS.
The third death was the result of a pulmonary embolism and was considered unrelated to axicabtagene ciloleucel. ![]()
WASHINGTON, DC—Roughly half of patients who responded to chimeric antigen receptor (CAR) T-cell therapy in the ZUMA-1 trial have retained that response at a median follow-up exceeding 8 months.
The CAR T-cell therapy, axicabtagene ciloleucel (formerly KTE-C19), initially produced an objective response rate (ORR) of 82% in this trial of patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL).
At a median follow-up of 8.7 months, 44% of all patients (53% of responders) are still in response, and 39% are in complete response (CR).
Thirteen percent of patients had grade 3 or higher cytokine release syndrome (CRS), and 28% had neurologic events.
There were 2 deaths related to axicabtagene ciloleucel.
Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa, Florida, presented these updated results from ZUMA-1 at the AACR Annual Meeting 2017 (abstract CT019).
ZUMA-1 is sponsored by Kite Pharma but is also funded, in part, by the Leukemia and Lymphoma Society Therapy Acceleration Program.
Patients and treatment
The trial enrolled 111 patients, 101 of whom were successfully treated with axicabtagene ciloleucel. Seven patients could not be treated due to serious adverse events, 1 due to unavailable product, and 2 due to non-measurable disease.
Seventy-seven of the patients had diffuse large B-cell lymphoma (DLBCL), and 24 had transformed follicular lymphoma (TFL) or primary mediastinal B-cell lymphoma (PMBCL). Eighty-five percent of patients had stage III/IV disease.
Seventy-nine percent were refractory to chemotherapy and did not have a prior autologous stem cell transplant (auto-SCT). Twenty-one percent did undergo auto-SCT and relapsed within 12 months of the procedure.
Sixty-nine percent of patients had received 3 or more lines of prior therapy, and 54% were refractory to 2 consecutive lines of prior therapy.
For this study, the patients received a conditioning regimen of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days.
Two days after the conditioning regimen was completed, patients received axicabtagene ciloleucel at a target dose of 2 × 106 CAR T cells/kg.
Efficacy
The following table shows overall response data, response data at 6 months, and ongoing responses at the primary analysis data cut-off.
| DLBCL (n=77) | TFL/PMBCL (n=24) | Combined (n=101) | ||||
| ORR (%) | CR (%) | ORR (%) | CR (%) | ORR (%) | CR (%) | |
| ORR | 82 | 49 | 83 | 71 | 82 | 54 |
| Month 6 | 36 | 31 | 54 | 50 | 41 | 36 |
| Ongoing | 36 | 31 | 67 | 63 | 44 | 39 |
The researchers said the ORR was generally consistent in key subgroups. The ORR was 83% in patients who were refractory to their second or greater line of therapy and 76% in patients who relapsed within 12 months of auto-SCT.
Overall, the median duration of response was 8.2 months. However, the median duration of response has not been reached for patients with a CR.
At a median follow-up of 8.7 months, the median overall survival has not been reached.
Safety
The most common grade 3 or higher adverse events included anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
The incidence of grade 3 or higher CRS was 13%, and the incidence of neurologic events was 28%. These represent decreases from the interim analysis of ZUMA-1, when the rate of grade 3+ CRS was 18%, and the rate of neurological events was 34%.
“We believe the rates of CRS and neurologic events decreased over the course of the study as clinicians gained experience in the management of adverse events,” said Jeff Wiezorek, MD, senior vice-president of clinical development at Kite Pharma.
There were 3 deaths throughout the course of the trial that were not a result of disease progression.
Two deaths were deemed related to axicabtagene ciloleucel. One was a case of hemophagocytic lymphohistiocytosis. The other was cardiac arrest in the setting of CRS.
The third death was the result of a pulmonary embolism and was considered unrelated to axicabtagene ciloleucel. ![]()
WASHINGTON, DC—Roughly half of patients who responded to chimeric antigen receptor (CAR) T-cell therapy in the ZUMA-1 trial have retained that response at a median follow-up exceeding 8 months.
The CAR T-cell therapy, axicabtagene ciloleucel (formerly KTE-C19), initially produced an objective response rate (ORR) of 82% in this trial of patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL).
At a median follow-up of 8.7 months, 44% of all patients (53% of responders) are still in response, and 39% are in complete response (CR).
Thirteen percent of patients had grade 3 or higher cytokine release syndrome (CRS), and 28% had neurologic events.
There were 2 deaths related to axicabtagene ciloleucel.
Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa, Florida, presented these updated results from ZUMA-1 at the AACR Annual Meeting 2017 (abstract CT019).
ZUMA-1 is sponsored by Kite Pharma but is also funded, in part, by the Leukemia and Lymphoma Society Therapy Acceleration Program.
Patients and treatment
The trial enrolled 111 patients, 101 of whom were successfully treated with axicabtagene ciloleucel. Seven patients could not be treated due to serious adverse events, 1 due to unavailable product, and 2 due to non-measurable disease.
Seventy-seven of the patients had diffuse large B-cell lymphoma (DLBCL), and 24 had transformed follicular lymphoma (TFL) or primary mediastinal B-cell lymphoma (PMBCL). Eighty-five percent of patients had stage III/IV disease.
Seventy-nine percent were refractory to chemotherapy and did not have a prior autologous stem cell transplant (auto-SCT). Twenty-one percent did undergo auto-SCT and relapsed within 12 months of the procedure.
Sixty-nine percent of patients had received 3 or more lines of prior therapy, and 54% were refractory to 2 consecutive lines of prior therapy.
For this study, the patients received a conditioning regimen of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days.
Two days after the conditioning regimen was completed, patients received axicabtagene ciloleucel at a target dose of 2 × 106 CAR T cells/kg.
Efficacy
The following table shows overall response data, response data at 6 months, and ongoing responses at the primary analysis data cut-off.
| DLBCL (n=77) | TFL/PMBCL (n=24) | Combined (n=101) | ||||
| ORR (%) | CR (%) | ORR (%) | CR (%) | ORR (%) | CR (%) | |
| ORR | 82 | 49 | 83 | 71 | 82 | 54 |
| Month 6 | 36 | 31 | 54 | 50 | 41 | 36 |
| Ongoing | 36 | 31 | 67 | 63 | 44 | 39 |
The researchers said the ORR was generally consistent in key subgroups. The ORR was 83% in patients who were refractory to their second or greater line of therapy and 76% in patients who relapsed within 12 months of auto-SCT.
Overall, the median duration of response was 8.2 months. However, the median duration of response has not been reached for patients with a CR.
At a median follow-up of 8.7 months, the median overall survival has not been reached.
Safety
The most common grade 3 or higher adverse events included anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
The incidence of grade 3 or higher CRS was 13%, and the incidence of neurologic events was 28%. These represent decreases from the interim analysis of ZUMA-1, when the rate of grade 3+ CRS was 18%, and the rate of neurological events was 34%.
“We believe the rates of CRS and neurologic events decreased over the course of the study as clinicians gained experience in the management of adverse events,” said Jeff Wiezorek, MD, senior vice-president of clinical development at Kite Pharma.
There were 3 deaths throughout the course of the trial that were not a result of disease progression.
Two deaths were deemed related to axicabtagene ciloleucel. One was a case of hemophagocytic lymphohistiocytosis. The other was cardiac arrest in the setting of CRS.
The third death was the result of a pulmonary embolism and was considered unrelated to axicabtagene ciloleucel. ![]()
Study provides new insight into RBC resilience
Researchers say they have developed a new system for studying the resilience of red blood cells (RBCs).
The team’s microfluidic system allowed them to look at how RBCs spring back into shape after deforming to pass through a narrow channel.
The researchers believe their findings could aid the diagnosis and treatment of blood-related diseases such as septic shock and malaria.
Hiroaki Ito, PhD, of Osaka University in Suita, Japan, and his colleagues detailed these findings in Scientific Reports.
To study RBCs, the researchers built a “catch-load-launch” microfluidic platform.
The setup included a microchannel in which a single RBC could be held in place for any desired length of time. The RBC was ultimately launched into a wider section using a robotic pump, which simulates the transition from a capillary into a larger vessel.
“The cell was precisely localized in the microchannel by the combination of pressure control and real-time visual feedback,” explained study author Makoto Kaneko, of Osaka University.
“This let us ‘catch’ an erythrocyte in front of the constriction, ‘load’ it inside for a desired time, and quickly ‘launch’ it from the constriction to monitor the shape recovery over time.”
The researchers found that, as the time the RBC was held in the constricted region was increased—from 5 seconds all the way to 5 minutes—the time it took the cell to recover its normal shape also increased.
For very short constriction times, the cells bounced back within 1/10 of a second. But it took approximately 10 seconds for cells to recover if they were held in the narrow segment longer than about 3 minutes.
The researchers also used their “catch-load-launch” system to study septic shock. This condition can occur when bacteria invade the bloodstream and release endotoxins.
Patients with septic shock may suffer from reduced circulation inside the narrow blood vessels as RBCs become too stiff. The same problem can be caused by the malaria parasite Plasmodium falciparum.
The researchers exposed RBCs to endotoxin from the bacteria Salmonella minnesota and found the RBCs became stiffer and less resilient.
“There is a great deal of evidence that relates certain diseases, including sepsis and malaria, to a decrease in the deformability of red blood cells,” Dr Ito said.
“Such a stiffening can lead to a disturbance in microcirculation, and our ‘catch-load-launch’ platform has the potential to be applied to the mechanical diagnosis of these diseased blood cells.” ![]()
Researchers say they have developed a new system for studying the resilience of red blood cells (RBCs).
The team’s microfluidic system allowed them to look at how RBCs spring back into shape after deforming to pass through a narrow channel.
The researchers believe their findings could aid the diagnosis and treatment of blood-related diseases such as septic shock and malaria.
Hiroaki Ito, PhD, of Osaka University in Suita, Japan, and his colleagues detailed these findings in Scientific Reports.
To study RBCs, the researchers built a “catch-load-launch” microfluidic platform.
The setup included a microchannel in which a single RBC could be held in place for any desired length of time. The RBC was ultimately launched into a wider section using a robotic pump, which simulates the transition from a capillary into a larger vessel.
“The cell was precisely localized in the microchannel by the combination of pressure control and real-time visual feedback,” explained study author Makoto Kaneko, of Osaka University.
“This let us ‘catch’ an erythrocyte in front of the constriction, ‘load’ it inside for a desired time, and quickly ‘launch’ it from the constriction to monitor the shape recovery over time.”
The researchers found that, as the time the RBC was held in the constricted region was increased—from 5 seconds all the way to 5 minutes—the time it took the cell to recover its normal shape also increased.
For very short constriction times, the cells bounced back within 1/10 of a second. But it took approximately 10 seconds for cells to recover if they were held in the narrow segment longer than about 3 minutes.
The researchers also used their “catch-load-launch” system to study septic shock. This condition can occur when bacteria invade the bloodstream and release endotoxins.
Patients with septic shock may suffer from reduced circulation inside the narrow blood vessels as RBCs become too stiff. The same problem can be caused by the malaria parasite Plasmodium falciparum.
The researchers exposed RBCs to endotoxin from the bacteria Salmonella minnesota and found the RBCs became stiffer and less resilient.
“There is a great deal of evidence that relates certain diseases, including sepsis and malaria, to a decrease in the deformability of red blood cells,” Dr Ito said.
“Such a stiffening can lead to a disturbance in microcirculation, and our ‘catch-load-launch’ platform has the potential to be applied to the mechanical diagnosis of these diseased blood cells.” ![]()
Researchers say they have developed a new system for studying the resilience of red blood cells (RBCs).
The team’s microfluidic system allowed them to look at how RBCs spring back into shape after deforming to pass through a narrow channel.
The researchers believe their findings could aid the diagnosis and treatment of blood-related diseases such as septic shock and malaria.
Hiroaki Ito, PhD, of Osaka University in Suita, Japan, and his colleagues detailed these findings in Scientific Reports.
To study RBCs, the researchers built a “catch-load-launch” microfluidic platform.
The setup included a microchannel in which a single RBC could be held in place for any desired length of time. The RBC was ultimately launched into a wider section using a robotic pump, which simulates the transition from a capillary into a larger vessel.
“The cell was precisely localized in the microchannel by the combination of pressure control and real-time visual feedback,” explained study author Makoto Kaneko, of Osaka University.
“This let us ‘catch’ an erythrocyte in front of the constriction, ‘load’ it inside for a desired time, and quickly ‘launch’ it from the constriction to monitor the shape recovery over time.”
The researchers found that, as the time the RBC was held in the constricted region was increased—from 5 seconds all the way to 5 minutes—the time it took the cell to recover its normal shape also increased.
For very short constriction times, the cells bounced back within 1/10 of a second. But it took approximately 10 seconds for cells to recover if they were held in the narrow segment longer than about 3 minutes.
The researchers also used their “catch-load-launch” system to study septic shock. This condition can occur when bacteria invade the bloodstream and release endotoxins.
Patients with septic shock may suffer from reduced circulation inside the narrow blood vessels as RBCs become too stiff. The same problem can be caused by the malaria parasite Plasmodium falciparum.
The researchers exposed RBCs to endotoxin from the bacteria Salmonella minnesota and found the RBCs became stiffer and less resilient.
“There is a great deal of evidence that relates certain diseases, including sepsis and malaria, to a decrease in the deformability of red blood cells,” Dr Ito said.
“Such a stiffening can lead to a disturbance in microcirculation, and our ‘catch-load-launch’ platform has the potential to be applied to the mechanical diagnosis of these diseased blood cells.” ![]()
New BTK inhibitor may overcome resistance in CLL
WASHINGTON, DC—Preclinical research suggests a second-generation BTK inhibitor may overcome the acquired resistance observed with its predecessor in patients with chronic lymphocytic leukemia (CLL).
Investigators found the non-covalent BTK inhibitor SNS-062 was unaffected by the BTK C481S mutation, which confers resistance to the first-generation BTK inhibitor ibrutinib.
“[A] subset of patients acquire resistance to ibrutinib, the current standard-of-care BTK inhibitor,” said Amy Johnson, PhD, of The Ohio State University in Columbus.
“A key resistance mechanism to covalent BTK inhibitors is a point mutation in the BTK active site, converting cysteine 481 to serine, or C481S.”
“In this study, we demonstrate that SNS-062, which binds non-covalently to BTK, is a potent inhibitor of BTK unaffected by the presence of the C481S mutation. These findings support clinical investigation of SNS-062 to address acquired resistance to covalent BTK inhibitors in patients.”
Dr Johnson and her colleagues presented these findings at the AACR Annual Meeting 2017 (abstract 1207).
SNS-062 is being developed by Sunesis Pharmaceuticals, Inc., and company investigators were involved in this research. But the study was sponsored by The Ohio State University.
For this study, Dr Johnson and her colleagues tested SNS-062 in primary CLL cells and X-linked agammaglobulinemia human cell lines.
The investigators found that SNS-062 inhibited BTK, decreased the expression of B-cell activation markers, and reduced CLL cell viability in a dose-dependent manner. And these effects were comparable to those observed with ibrutinib.
SNS-062 and ibrutinib demonstrated comparable activity against wild-type BTK. However, ibrutinib and another BTK inhibitor, acalabrutinib, were hindered by the BTK C481S mutation, while SNS-062 was not.
The investigators said SNS-062 was 6 times more potent than ibrutinib against C481S BTK and more than 640 times more potent than acalabrutinib.
The team also noted that SNS-062 exhibited high specificity, affecting a limited number of kinases outside the TEC kinase family.
Finally, the investigators found that SNS-062 diminished stromal cell protection in CLL cells, suggesting the drug can hinder protection from the tumor microenvironment. ![]()
WASHINGTON, DC—Preclinical research suggests a second-generation BTK inhibitor may overcome the acquired resistance observed with its predecessor in patients with chronic lymphocytic leukemia (CLL).
Investigators found the non-covalent BTK inhibitor SNS-062 was unaffected by the BTK C481S mutation, which confers resistance to the first-generation BTK inhibitor ibrutinib.
“[A] subset of patients acquire resistance to ibrutinib, the current standard-of-care BTK inhibitor,” said Amy Johnson, PhD, of The Ohio State University in Columbus.
“A key resistance mechanism to covalent BTK inhibitors is a point mutation in the BTK active site, converting cysteine 481 to serine, or C481S.”
“In this study, we demonstrate that SNS-062, which binds non-covalently to BTK, is a potent inhibitor of BTK unaffected by the presence of the C481S mutation. These findings support clinical investigation of SNS-062 to address acquired resistance to covalent BTK inhibitors in patients.”
Dr Johnson and her colleagues presented these findings at the AACR Annual Meeting 2017 (abstract 1207).
SNS-062 is being developed by Sunesis Pharmaceuticals, Inc., and company investigators were involved in this research. But the study was sponsored by The Ohio State University.
For this study, Dr Johnson and her colleagues tested SNS-062 in primary CLL cells and X-linked agammaglobulinemia human cell lines.
The investigators found that SNS-062 inhibited BTK, decreased the expression of B-cell activation markers, and reduced CLL cell viability in a dose-dependent manner. And these effects were comparable to those observed with ibrutinib.
SNS-062 and ibrutinib demonstrated comparable activity against wild-type BTK. However, ibrutinib and another BTK inhibitor, acalabrutinib, were hindered by the BTK C481S mutation, while SNS-062 was not.
The investigators said SNS-062 was 6 times more potent than ibrutinib against C481S BTK and more than 640 times more potent than acalabrutinib.
The team also noted that SNS-062 exhibited high specificity, affecting a limited number of kinases outside the TEC kinase family.
Finally, the investigators found that SNS-062 diminished stromal cell protection in CLL cells, suggesting the drug can hinder protection from the tumor microenvironment. ![]()
WASHINGTON, DC—Preclinical research suggests a second-generation BTK inhibitor may overcome the acquired resistance observed with its predecessor in patients with chronic lymphocytic leukemia (CLL).
Investigators found the non-covalent BTK inhibitor SNS-062 was unaffected by the BTK C481S mutation, which confers resistance to the first-generation BTK inhibitor ibrutinib.
“[A] subset of patients acquire resistance to ibrutinib, the current standard-of-care BTK inhibitor,” said Amy Johnson, PhD, of The Ohio State University in Columbus.
“A key resistance mechanism to covalent BTK inhibitors is a point mutation in the BTK active site, converting cysteine 481 to serine, or C481S.”
“In this study, we demonstrate that SNS-062, which binds non-covalently to BTK, is a potent inhibitor of BTK unaffected by the presence of the C481S mutation. These findings support clinical investigation of SNS-062 to address acquired resistance to covalent BTK inhibitors in patients.”
Dr Johnson and her colleagues presented these findings at the AACR Annual Meeting 2017 (abstract 1207).
SNS-062 is being developed by Sunesis Pharmaceuticals, Inc., and company investigators were involved in this research. But the study was sponsored by The Ohio State University.
For this study, Dr Johnson and her colleagues tested SNS-062 in primary CLL cells and X-linked agammaglobulinemia human cell lines.
The investigators found that SNS-062 inhibited BTK, decreased the expression of B-cell activation markers, and reduced CLL cell viability in a dose-dependent manner. And these effects were comparable to those observed with ibrutinib.
SNS-062 and ibrutinib demonstrated comparable activity against wild-type BTK. However, ibrutinib and another BTK inhibitor, acalabrutinib, were hindered by the BTK C481S mutation, while SNS-062 was not.
The investigators said SNS-062 was 6 times more potent than ibrutinib against C481S BTK and more than 640 times more potent than acalabrutinib.
The team also noted that SNS-062 exhibited high specificity, affecting a limited number of kinases outside the TEC kinase family.
Finally, the investigators found that SNS-062 diminished stromal cell protection in CLL cells, suggesting the drug can hinder protection from the tumor microenvironment. ![]()
ASCO addresses needs of SGMs with cancer
The American Society of Clinical Oncology (ASCO) has issued recommendations addressing the needs of sexual and gender minority (SGM) populations with cancer.
The recommendations are designed to focus attention on the challenges facing the SGM community—including discrimination and greater risk of anxiety and depression, resulting in disparate care—and concrete steps that can help minimize health disparities among SGM individuals.
The recommendations were published in a policy statement in the Journal of Clinical Oncology.
“Sexual and gender minorities face unique challenges related to cancer risk, discrimination, and other psychosocial issues,” said ASCO President Daniel F. Hayes, MD.
“Compounding these challenges is the fact that providers may have a lack of knowledge and sensitivity about the health risks and health needs facing their SGM patients.”
SGMs include individuals who are lesbian, gay, bisexual, transgender, and intersex (also referred to as those with differences in sex development).
ASCO’s policy statement notes that SGM populations bear a disproportionate cancer burden stemming from several factors, including:
- Lower rates of cancer screening, in part due to lower rates of insurance coverage, exclusion from traditional screening campaigns, and previous experience of discrimination in the healthcare system
- A hesitancy on the part of SGM patients to disclose their sexual orientation to providers due to a fear of stigmatization, which can create additional barriers to care.
ASCO’s statement calls for a coordinated effort to address health disparities among SGM populations, including:
- Increased patient access to culturally competent support services
- Expanded cancer prevention education for SGM individuals
- Robust policies prohibiting discrimination
- Adequate insurance coverage to meet the needs of SGM individuals affected by cancer
- Inclusion of SGM status as a required data element in cancer registries and clinical trials
- Increased focus on SGM populations in cancer research.
“Our objective was to raise awareness among oncology providers, patients, policy makers, and other stakeholders about the cancer care needs of SGM populations and the barriers that SGM individuals face in getting the highest-quality care,” said Jennifer J. Griggs, MD, lead author of the policy statement and a professor at the University of Michigan in Ann Arbor.
“To address these barriers, a coordinated effort is needed to enhance education for patients and providers, to improve outreach and support, and to encourage productive policy and legislative action.” ![]()
The American Society of Clinical Oncology (ASCO) has issued recommendations addressing the needs of sexual and gender minority (SGM) populations with cancer.
The recommendations are designed to focus attention on the challenges facing the SGM community—including discrimination and greater risk of anxiety and depression, resulting in disparate care—and concrete steps that can help minimize health disparities among SGM individuals.
The recommendations were published in a policy statement in the Journal of Clinical Oncology.
“Sexual and gender minorities face unique challenges related to cancer risk, discrimination, and other psychosocial issues,” said ASCO President Daniel F. Hayes, MD.
“Compounding these challenges is the fact that providers may have a lack of knowledge and sensitivity about the health risks and health needs facing their SGM patients.”
SGMs include individuals who are lesbian, gay, bisexual, transgender, and intersex (also referred to as those with differences in sex development).
ASCO’s policy statement notes that SGM populations bear a disproportionate cancer burden stemming from several factors, including:
- Lower rates of cancer screening, in part due to lower rates of insurance coverage, exclusion from traditional screening campaigns, and previous experience of discrimination in the healthcare system
- A hesitancy on the part of SGM patients to disclose their sexual orientation to providers due to a fear of stigmatization, which can create additional barriers to care.
ASCO’s statement calls for a coordinated effort to address health disparities among SGM populations, including:
- Increased patient access to culturally competent support services
- Expanded cancer prevention education for SGM individuals
- Robust policies prohibiting discrimination
- Adequate insurance coverage to meet the needs of SGM individuals affected by cancer
- Inclusion of SGM status as a required data element in cancer registries and clinical trials
- Increased focus on SGM populations in cancer research.
“Our objective was to raise awareness among oncology providers, patients, policy makers, and other stakeholders about the cancer care needs of SGM populations and the barriers that SGM individuals face in getting the highest-quality care,” said Jennifer J. Griggs, MD, lead author of the policy statement and a professor at the University of Michigan in Ann Arbor.
“To address these barriers, a coordinated effort is needed to enhance education for patients and providers, to improve outreach and support, and to encourage productive policy and legislative action.” ![]()
The American Society of Clinical Oncology (ASCO) has issued recommendations addressing the needs of sexual and gender minority (SGM) populations with cancer.
The recommendations are designed to focus attention on the challenges facing the SGM community—including discrimination and greater risk of anxiety and depression, resulting in disparate care—and concrete steps that can help minimize health disparities among SGM individuals.
The recommendations were published in a policy statement in the Journal of Clinical Oncology.
“Sexual and gender minorities face unique challenges related to cancer risk, discrimination, and other psychosocial issues,” said ASCO President Daniel F. Hayes, MD.
“Compounding these challenges is the fact that providers may have a lack of knowledge and sensitivity about the health risks and health needs facing their SGM patients.”
SGMs include individuals who are lesbian, gay, bisexual, transgender, and intersex (also referred to as those with differences in sex development).
ASCO’s policy statement notes that SGM populations bear a disproportionate cancer burden stemming from several factors, including:
- Lower rates of cancer screening, in part due to lower rates of insurance coverage, exclusion from traditional screening campaigns, and previous experience of discrimination in the healthcare system
- A hesitancy on the part of SGM patients to disclose their sexual orientation to providers due to a fear of stigmatization, which can create additional barriers to care.
ASCO’s statement calls for a coordinated effort to address health disparities among SGM populations, including:
- Increased patient access to culturally competent support services
- Expanded cancer prevention education for SGM individuals
- Robust policies prohibiting discrimination
- Adequate insurance coverage to meet the needs of SGM individuals affected by cancer
- Inclusion of SGM status as a required data element in cancer registries and clinical trials
- Increased focus on SGM populations in cancer research.
“Our objective was to raise awareness among oncology providers, patients, policy makers, and other stakeholders about the cancer care needs of SGM populations and the barriers that SGM individuals face in getting the highest-quality care,” said Jennifer J. Griggs, MD, lead author of the policy statement and a professor at the University of Michigan in Ann Arbor.
“To address these barriers, a coordinated effort is needed to enhance education for patients and providers, to improve outreach and support, and to encourage productive policy and legislative action.” ![]()
Obese Man With Severe Pain and Swollen Hand
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
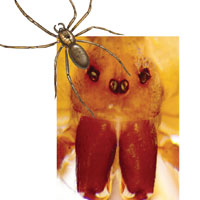
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).
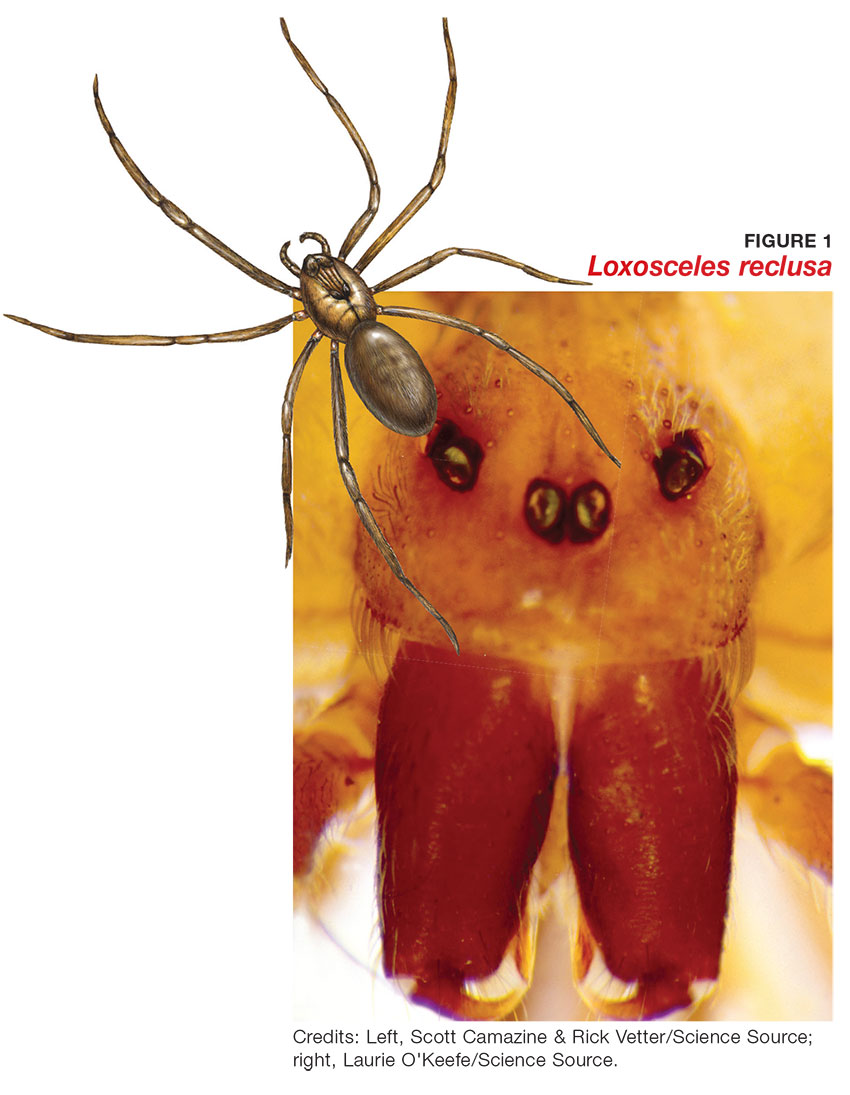
Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7
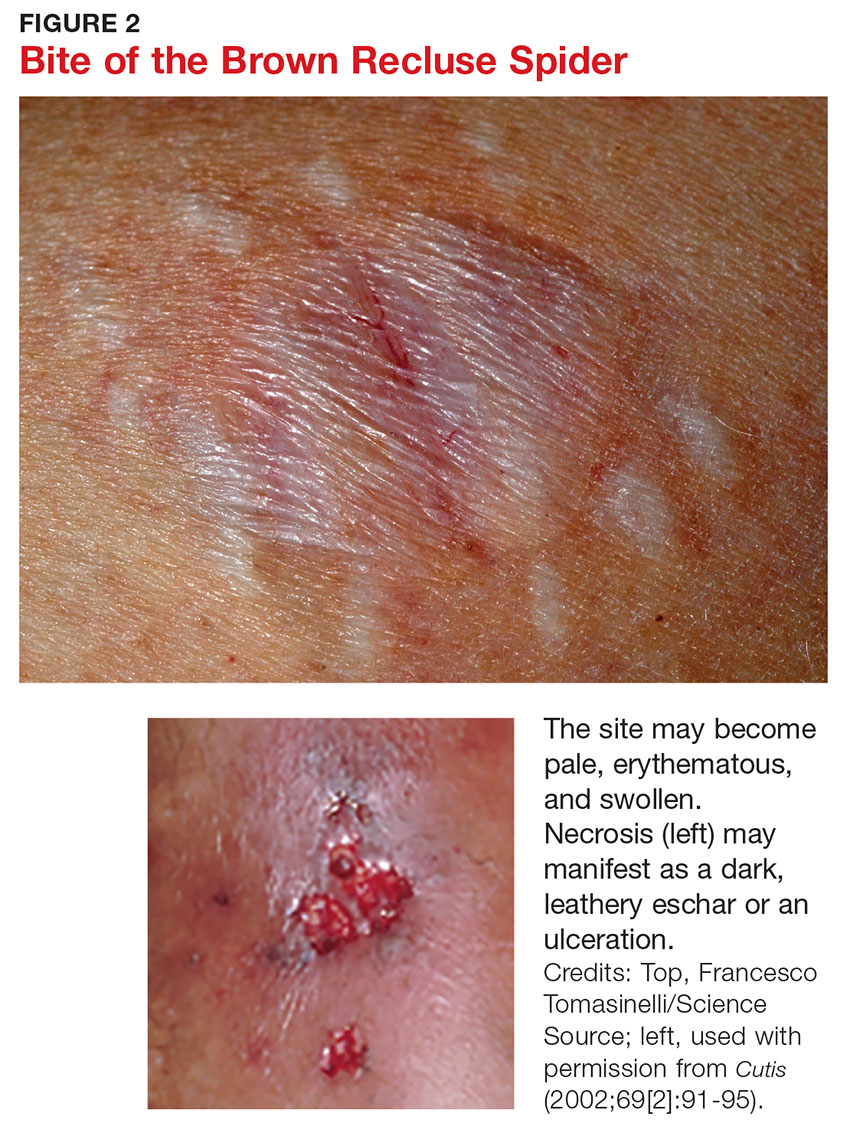
Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).

Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7

Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).

Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7

Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.
Prevention of Type 2 Diabetes: Evidence and Strategies
From the Maimonides Medical Center (Dr. Karam) and the SUNY Downstate Medical Center (Dr. Karam and Dr. McFarlane), Brooklyn, NY.
Abstract
- Objective. To discuss the epidemic of diabetes highlighting the natural history of the disease and the major clinical trials aimed at diabetes prevention in different prediabetic populations around the world.
- Results. Diabetes prevention studies have evaluated various interventions including lifestyle modifications, metformin, alpha-glucosidase inhibitors, thiazolidinediones, nateglinide, and xenical as well as the renin-angiotensin aldosterone system (RAS) inhibitors. Lifestyle modifications seem to be the safest, most effective, and most sustainable intervention to prevent diabetes. Except for metformin, the potential diabetes prevention benefits of the studied pharmacologic agents are limited by safety concerns or lack of durable efficacy or tolerability. RAS blockade and fibrates have a favorable glycemic effect, and, when indicated, are reasonable treatment options for hypertension and hyperlipidemia in prediabetic patients.
- Conclusion. As recommended by American Diabetes Association guidelines, patients with prediabetes should be referred to an intensive diet and physical activity behavioral counseling program; diet and activity goals include a loss of 7% of body weight and at least 150 minutes of moderate physical activity per week. Metformin therapy for diabetes prevention should be considered as well.
Key words: prediabetes; type 2 diabetes mellitus, diabetes prevention, lifestyle modifications.
Diabetes mellitus has reached pandemic proportions across the globe. The International Diabetes Federation (IDF) estimates that in 2015 around 415 million people, or 1 in 11 adults, had diabetes, compared to 285 million in 2010, with 5 million deaths, or 1 death every 6 seconds, occurring because of diabetes or diabetes complications [1]. In the United States, an estimated 29.1 million Americans, or 9.3% of the population, have diabetes, 27.8% of them undiagnosed [2]. The prevalence of diabetes increases significantly with age, affecting around 16.2% of American adults aged 45 to 64 years and 25.9% of adults aged 65 years or older [2]. The Centers for Disease Control and Prevention (CDC) estimates that, with current trends, as many as 1 in 3 American adults could have diabetes by 2050 [3].
Type 2 diabetes mellitus (T2DM) accounts for the majority of prevalent and newly diagnosed diabetes in the world, and is strongly linked to overweight and inactivity in adults [4]. T2DM is increasingly being diagnosed in pediatric patients, in whom type 1 diabetes has historically been predominant; it now accounts for approximately 30% of newly diagnosed diabetes in children aged 10 to 19 years, exceeding 50% in certain ethnicities such as non-Hispanic black and American Indian/Alaska Native children [2].
These alarming trends have spurred significant research and public efforts aimed at reducing the prevalence of diabetes by preventing T2DM. Indeed, insulin resistance and abnormal carbohydrate metabolism progress over many years prior to the diagnosis of diabetes and manifest with different clinical and biochemical features. Both the pathophysiology and the natural history of T2DM offer clinicians an opportunity to identify patients at risk for developing the disease and to implement prevention strategies. This article outlines the risk factors and diagnostic criteria for prediabetes, describes the studies that have explored diabetes prevention through lifestyle changes, pharmacotherapy, or surgery, and reviews recommendations for managing patients at risk.
Risk Factors and Screening for T2DM
The American Diabetes Association (ADA) recommends screening all adults for prediabetes by assessing for diabetes risk factors [8]. Glucose testing is recommended in individuals aged 45 years or older, and should be considered in adults of any age who are overweight or obese (body mass index [BMI] ≥ 25 kg/m2 or ≥ 23 kg/m2 in Asian Americans) and have 1 or more additional risk factors for diabetes. Testing also should be considered in children and adolescents who are overweight or obese and who have 2 or more additional risk factors. If tests are normal, repeat testing carried out at a minimum of 3-year intervals is suggested [8].
Prediabetes
Abnormalities in glucose metabolism progress along a continuum through various stages before T2DM develops. Years before the development of overt diabetes, and especially in the presence of excessive visceral fat, cellular sensitivity to insulin gradually decreases, leading to a compensatory increased insulin secretion [9]. With time, and under continuous increased demand, pancreatic beta cell function declines and ultimately fails to overcome insulin resistance and maintain a normal glucose metabolism, resulting in prediabetes followed by the development of diabetes. This early beta cell dysfunction was illustrated by the decreased beta cell volume observed on autopsy of obese patients with IFG or T2DM, when compared to obese individuals with normal glucose tolerance [10]. It is estimated that around 40% to 70% of beta cell function is already lost by the time diabetes is clinically diagnosed. This relatively slow pathophysiologic process allows the identification of at-risk patients well before their blood glucose levels reach the diabetic diagnostic thresholds, and therefore presents an opportunity for prevention.
Diagnostic Criteria
The ADA guidelines released in 2003 define prediabetes as IFG (fasting blood glucose [FBG] levels of 100–125 mg/dL), IGT (glucose levels of 140–199 mg/dL at 2 hours during an oral glucose tolerance test [OGTT] following an oral load of 75 g of dextrose), or both. Additionally, hemoglobin A1C (A1C) was introduced as a diagnostic tool for prediabetes in 2010, with values between 5.7% and 6.4% indicating prediabetes [8]. Most of these thresholds were chosen due to their association with increased rates of complications, notably retinopathy and cardiovascular disease.
A combined report from the World Health Organization (WHO) and the IDF published in 2006 defined intermediate hyperglycemia as IFG, but with a higher cutoff for FBG (110–125 mg/dL) than the ADA’s definition, and/or IGT (2-hour OGTT glucose level of 140–199 mg/dL) [11]. The rationale for a higher cut-point for IFG is the concern about the increased prevalence of IFG and its impact on individuals and health systems and the more favorable cardiovascular risk profile and decreased risk of progression to diabetes in the group of patients with FBG of 100 to 110 mg/dL when compared to the group with FBG of 110 to 125 mg/dL. The report does not recommend the use of A1C in the diagnosis of diabetes or intermediate hyperglycemia because of a lack of global consistency and the potential for other factors that can be prevalent in some developing countries, such as hemoglobinopathies and anemia, to interfere with the assay.
Prevalence and Progression to Diabetes
According to CDC data from 2014, up to 86 million American adults, more than 1 in 3, have prediabetes, and 9 out of 10 of these individuals are undiagnosed [2]. It is estimated that approximately 25% of people diagnosed with either IFG or IGT progress to diabetes mellitus over a 3- to 5-year period [12]. If observed for longer periods, most prediabetic persons will probably develop diabetes. The highest rate of progression to diabetes is observed in patients with both IFG and IGT, older age, overweight, or other diabetic risk factors.
Complications
In addition to increasing the risk for progression to diabetes, prediabetes is independently associated with microvascular and macrovascular complications and increased risk of death, prior to the actual onset of diabetes. The DECODE study demonstrated significantly increased mortality in 2766 individuals with IGT after 7 years of follow-up, when compared to normoglycemic patients; this effect was more prominent in participants with IGT than in participants with IFG [13]. In the Australian Diabetes, Obesity and Lifestyle Study, IFG was found to be an independent predictor for cardiovascular mortality after adjustment for age, sex, and other traditional cardiovascular risk factors [14].
Similarly, a recent meta-analysis demonstrated that the presence of IFG was significantly associated with future risk for coronary heart disease (CHD), with the risk increase starting when fasting plasma glucose was as low as 100 mg/dL; however, this finding may have been confounded by the presence of undetected IGT or other cardiovascular risk factors [15]. Another recent systematic review of 53 prospective cohort studies with 1,611,339 participants showed that prediabetes (IFG or IGT) was associated with an increased risk of composite cardiovascular disease, CHD, stroke, and all-cause mortality [16].
The association between retinopathy and prediabetes has been described in multiple reports and this association has helped guide authors on selected thresholds for diagnosis of prediabetes. For example, in 1 study, the incidence of retinopathy in individuals with IGT was 12% among Pima Indians [17]. Similarly, in a follow-up study of the Diabetes Prevention Program, 8% of prediabetic participants who remained nondiabetics had evidence of retinopathy [18].
Neuropathy also has been observed in prediabetes. A noninvasive neurologic evaluation of individuals with IGT revealed subclinical neural dysfunction suggestive of cardiovascular autonomic neuropathy [19]. At the clinical level, a study that evaluated 100 patients with chronic idiopathic axonal neuropathy of unknown etiology found IFG in 36 and IGT in 38 patients, underscoring the role of abnormal glucose metabolism in these patients [20].
Nephropathy may also be more prevalent in those with prediabetes. In a 1999–2006 National Health and Nutrition Examination Survey analysis, the adjusted prevalence of chronic kidney disease, defined by estimated glomerular filtration rate (eGFR) of 15 to 59 mL/min per 1.73 m2 or albumin-creatinine ratio ≥ 30 mg/g, was 17.1% in individuals with IFG, compared to 11.8% in individuals with normal fasting glucose [21].
Lifestyle Modifications
The alarming rapid increase in the prevalence of T2DM has been linked to a parallel rising epidemic of overweight, obesity, and lack of physical activity. Therefore, lifestyle changes aiming at weight reduction seemed to be a natural individual and public health strategy to prevent diabetes, and such strategies have been the focus of many randomized controlled trials around the world. As anticipated, weight loss, exercise, and diet have all been shown, separately or in combination, to be effective in decreasing the incidence of T2DM in high-risk patients [22–27]. Furthermore, and well beyond the benefit observed during the trials, follow-up studies revealed a sustained reduction of diabetes incidence in intervention groups several years after cessation of the intervention [28–32] (Table 2).
The Da Quing Diabetes Prevention Study (DQDPS), published in 1997, is one of the earliest prospective diabetes prevention trials [22]. This 6-year study conducted in 33 clinics in China from 1986 through 1992 included 577 participants with IGT who were randomly assigned to 1 of 4 groups: (1) diet (high vegetables, low sugar/alcohol) only, (2) exercise, (3) diet plus exercise, and (4) standard of care. At 6 years, diabetes incidence was significantly reduced by 46% in the exercise group, 31% in the diet group, and 42% in the diet plus exercise group compared to standard care. In 2006, 14 years after the end of the trial and 20 years after the initial enrollment, the cumulative incidence of diabetes was significantly lower in the intervention group at 80%, compared to 93% in the control group, and the annual incidence of diabetes was 7% and 11%, respectively, with a 43% lower incidence of diabetes over the 20-year period in the combination lifestyle changes group [28]. The preventive benefit of lifestyle changes persisted 2 decades after the initial randomization despite the standardization of treatment for all groups over the 14 years following the study, suggesting a strong and longitudinal preventive effect of the initial lifestyle modifications. In a follow-up study of the DQDPS conducted in 2009, at 23 years of follow-up, the cumulative incidences of cardiovascular mortality and all-cause mortality were significantly lower in the intervention group (11.9% versus 19.6%, and 28.1% versus 38.4%, respectively), highlighting the long-term clinical benefits of lifestyle intervention in patients with IGT [29].
Similarly, the Finnish Diabetes Prevention Study (FDPS), published in 2001, enrolled 522 middle-aged overweight participants with IGT [23]. The participants randomly assigned to the intervention group received individualized counseling designed to reduce weight, decrease total intake of fat and saturated fat, increase intake of fiber, and increase physical activity. The control group received standard therapy. At 4 years of follow-up, the cumulative incidence of diabetes was 11% in the intervention group and 23% in the control group, with a statistically significant 58% reduction in risk for progression to diabetes. A follow-up of the FDPS was published in 2006 [31]. Participants who did not progress to diabetes in the initial 4-year study were further followed for a median of 3 years. Interestingly, lifestyle changes were maintained by the intervention group participants despite the cessation of the individual counseling, leading to a 36% relative reduction in diabetes incidence during the post-intervention follow-up period alone (4.6 vs 7.2 per 100 person-years, P = 0.041) and a 43% cumulative diabetes incidence reduction over the 7-year follow-up, demonstrating, one more time, the sustained efficacy of lifestyle changes.
In the United States, the Diabetes Prevention Program (DPP) trial is a landmark NIH-sponsored multicenter randomized controlled trial published in 2002, and one of the largest diabetes prevention studies with lifestyle changes to date [24]. A total of 3234 participants with prediabetes, defined as an IFG or IGT, were randomly assigned to an intensive lifestyle modification program, metformin 850 mg twice daily, or matching placebo. Lifestyle changes included a low-fat (< 25% of caloric intake), 1200- to 1800-calorie diet and exercise for 150 minutes a week, with a 7% body weight reduction goal and a very well structured curriculum and professional support group. The study was discontinued early (at 3 years) as the data demonstrated the superiority of lifestyle changes, with a 58% reduction in diabetes incidence in the lifestyle intervention group and a 31% reduction in the metformin group when compared to placebo (cumulative incidence of diabetes at 3 years of 28.9%, 21.7 %, and 14.4% in the placebo, metformin, and lifestyle intervention groups, respectively). Lifestyle changes were significantly more effective than metformin and were consistently effective in men and women across age, BMI, and ethnic groups.
The DPPOS (DPP Outcome Study) was a 10-year follow-up of the DPP study published in 2009 where all participants were offered group-implemented lifestyle changes and were followed for an additional 5.7 years [32]. Unlike the Finnish follow-up study, diabetes incidence was similar in the 3 treatment groups in the follow-up period. However, the cumulative incidence of diabetes remained significantly the lowest in the original lifestyle group, with a 34% cumulative risk reduction in the lifestyle group and an 18% reduction in the metformin group at 10 years when compared to placebo. Interestingly, unlike most other studies of weight-reducing interventions, in the DPPOS, patients in the lifestyle changes and metformin groups maintained weight loss at 10 years’ follow-up.
In Japan, a diabetes prevention study assigned 458 male participants with IGT to a standard intervention group or an intensive intervention group receiving detailed lifestyle modification counseling every 3 to 4 months during hospital visits [25]. The cumulative 4-year incidence of diabetes was 9.3% in the control group versus 3.0% in the intervention group, and the reduction in diabetes risk was 67.4% (P < 0.001), with body weight reductions of 0.39 kg and 2.18 kg, respectively (P < 0.001). Of note, participants with higher FBG at baseline developed diabetes at a higher rate than those with lower values. This study suggested that lifestyle change counseling conducted in an outpatient clinic setting can be very effective in preventing diabetes.
Indian adults are thought to be more insulin resistant at a younger age and at a lower BMI than Caucasians. To assess whether the DPP findings can be replicated in an Indian population, the Indian Diabetes Prevention Program (IDPP) trial randomized a total of 531 participants with IGT to 4 groups: control, lifestyle modification, metformin, and lifestyle modifications with metformin [26]. The 3-year cumulative incidences of diabetes were 55.0%, 39.3%, 40.5%, and 39.5%, respectively, showing again a significant relative reduction in progression to diabetes of 28.5% with lifestyle changes, 26.4% with metformin, and 28.2% with both lifestyle changes and metformin, as compared with the control group.
In a Japanese unmasked, multicenter, randomized controlled trial published in 2011, 641 overweight adults with IFG were randomized to a frequent intervention group, receiving individual counseling and support for lifestyle modifications 9 times over 36 months, or a control group, receiving counseling 4 times over the same period. The 3-year cumulative incidence of T2DM was significantly lower in the frequent intervention group than in the control group (12.2% vs 16.6%) [27]. Interestingly, in a posthoc subgroup analysis, the protective effect was more prominent in patients with underlying associated IGT or elevated A1C, but was not observed in patients with isolated IFG, suggesting a possible prognostic value of an additional A1C or oral glucose tolerance test in individuals with IFG.
Diet
The diet followed in the major diabetes prevention trials discussed above has typically been a weight-reducing diet with decreased fat intake (eg, DPP, Finnish trial) and increased fiber intake (eg, Da Quing, DPP, Finnish trials). However, there has been more emphasis recently on the importance of the quality rather than the quantity of fats in preventing diabetes. For example, in a Spanish study, a non–calorie-restricted traditional Mediterranean diet, enriched with high-fat foods of vegetable origin (olive oil, nuts) decreased the incidence of diabetes by 52% in individuals at high cardiovascular risk after a median follow-up of 4.0 years, and in the absence of significant changes in body weight or physical activity among the groups [33]. These findings were reproduced by other studies. A recent meta-analysis examining the relation between intake of fruits and vegetables and the incidence of diabetes revealed that higher intake of fruit, especially berries, and green, leafy vegetables, yellow vegetables, cruciferous vegetables, or their fiber is associated with a lower risk of T2DM [34].
Exercise
Exercise is thought to improve insulin sensitivity and promote peripheral glucose uptake in normal individuals. Long-term moderate exercise, similar to the exercise recommended in DPP and FDPS, results in increased translocation of insulin-responsive glucose transporter (GLUT-4) from intracellular stores to the cell surface, facilitating glucose uptake [35]. A systematic review of 10 prospective cohort studies published in 2007 showed that, after adjustment for BMI, moderate-intensity physical activity was significantly associated with reduced diabetes incidence [36]. In the FDPS, participants who achieved at least 4 hours of exercise per week had a significant 80% decrease in incidence of diabetes, and this effect was observed even in the group that did not lose weight [23]. In the DQDPS, the greatest reduction in diabetes incidence was observed in the exercise group [22].
In a recent NIH-funded trial designed to examine the relative contribution of exercise alone to the overall beneficial effect of lifestyle changes in the DPP study, a total of 237 adults with IFG were randomly assigned to 4 different groups: low-amount moderate intensity exercise (similar to exercise followed in DPP), high-amount moderate intensity exercise, high-amount vigorous intensity exercise, and a combination of diet, weight loss, and low-amount moderate exercise. Only the diet and exercise group experienced a decrease in fasting glucose, whereas similar improvements in glucose tolerance were observed in both the diet and exercise group and the high-amount moderate-intensity exercise group, suggesting that such an exercise regimen may be as effective as a more intensive multicomponent approach involving diet, exercise, and weight loss for preventing diabetes [37].
Weight Loss
Weight reduction in prediabetic individuals has been consistently associated with reduced incidence of diabetes. Furthermore, the amount of weight loss needed to achieve this benefit seems to be relatively modest and a realistic goal to set for patients. Indeed, in the DPP trial, an average weight loss of only 5.6 kg was associated with a 58% lower incidence of diabetes [24]. Moreover, on further analysis of the DPP trial, and among weight, diet, and exercise, diabetes prevention correlated most strongly with weight loss, with an estimated 16% diabetes risk reduction for every single kilogram of weight reduction [38]. Similarly, within the same lifestyle intervention group in the FDPS, the participants who were able to achieve an initial body weight loss greater than 5% at 1 year had a nearly 70% relative risk reduction in progression to diabetes, when compared to their peers in the intervention group who had less or no weight loss [23].
In summary, numerous randomized controlled studies from various populations have proved that lifestyle modifications, including healthy diet, moderate weight loss, and moderate-intensity exercise, represent a very effective strategy to prevent diabetes in patients at risk, mostly patients with IGT, and this protective effect seems to be sustained over time.
Pharmacologic Interventions
Metformin
Metformin is an antidiabetic agent that works mostly at the liver site by suppressing hepatic glucose production and inhibiting production and oxidation of free fatty acids (FFA), thereby reducing FFA-induced insulin resistance and promoting peripheral glucose uptake [39]. This effect has the potential of preserving beta cell function by reducing the demand for insulin secretion.
In the DPP trial, metformin, although generally less effective than lifestyle changes, was associated with a significant 31% reduction in diabetes incidence (cumulative incidence of 22% in metformin group vs 29% in placebo group) and significant weight reduction (average of 2 kg) [24]. Further analysis of the DPP results showed that metformin efficacy, compared to placebo, was greater in patients who were younger, had higher BMI, and had higher FBG levels. In addition, a DPP substudy of 350 women with history of gestational diabetes and IGT revealed that this group of women, who had a higher risk of progression to diabetes (71% at 3 years) when compared to women with no history of gestational diabetes, despite similar baseline glucose levels, had similar diabetes risk reduction of 50% with both metformin and lifestyle changes [40].
In the IDPP study, both lifestyle changes and metformin reduced significantly and similarly the incidence of diabetes in adults with IGT, with no observed added benefit from combining both interventions [26]. It has not been clear, however, how much of this effect of metformin is a result of pharmacologic properties masking hyperglycemia or a true protective and preventive effect. In a washout study in which 1274 DPP participants who did not progress to diabetes underwent an OGTT after 1 to 2 weeks of discontinuing metformin or placebo, the incidence of diabetes was still reduced by 25% in the metformin group, after the washout period, compared to a 31% risk reduction in the primary DPP analysis, suggesting a partially sustained rather than temporary effect of metformin [41]. In the DPPOS long-term follow-up study, metformin (850 mg twice daily as tolerated) was continued in the group initially assigned to metformin in addition to lifestyle counseling [32]. Although the progression to diabetes was similar in all groups during the 5.7-year follow-up period, the cumulative incidence of diabetes at 10 years was still reduced in the metformin group by 18% when compared to control group. Furthermore, the weight loss associated with metformin was also interestingly sustained at 10 years. A meta-analysis echoed this beneficial effect of metformin observed in the DPP trial, reporting a relative risk reduction of new-onset diabetes of 40% with the use of metformin [42].
In summary, metformin has been shown to be effective in preventing diabetes in patients at risk, especially persons with younger age, higher BMI, and history of gestational diabetes and in native Asian Indians. The protective effect of metformin seems to be sustained over the long term in follow-up studies.
Thiazolidinediones
Thiazolidinediones (TZDs) are antidiabetic agents that have been evaluated in diabetes prevention trials. TZDs are peroxisome proliferator-activated gamma receptor (PPAR-γ) agonists that work by augmenting conversion of preadipocytes to adipocytes, which in turn increase adiponectin levels, promoting insulin sensitivity [43]. In addition to their antihyperglycemic properties, TZDs are thought to have a direct protective effect on beta cells, potentially translating into prevention and delay of diabetes [44].
The first study to demonstrate diabetes prevention with a TZD was the TRIPOD study (Troglitazone in Prevention of Diabetes), in which 266 Hispanic women with a history of gestational diabetes were randomly assigned to troglitazone or placebo [45]. Troglitazone use was significantly associated with reduction of progression to diabetes at 1.5-year follow-up when compared to placebo (relative risk reduction of 55%), with a decrease of endogenous insulin requirement at 3 months of therapy and sustained benefit after discontinuation of the TZD, suggesting an effect on beta cell preservation.
Moreover, troglitazone was an investigational drug in the DPP trial from 1996 to 1998, at which time it was discontinued because of associated fatal liver failure in a DPP participant. In the DPP trial, troglitazone was asso-ciated with a remarkable 75% decrease in progression to diabetes at 1 year. Troglitazone was withdrawn from the US market in 2000 because of its association with severe hepatotoxicity.
The international DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medications) trial randomly assigned more than 5000 participants with IFG and/or IGT to rosiglitazone, ramipril, or placebo in a 2 × 2 factorial design [46]. In participants receiving rosiglitazone, the risk for progression to diabetes was reduced by 60% and the likelihood of regression to normoglycemia was increased by 71% when compared to placebo. However, the use of rosiglitazone was associated with an increased risk of new-onset congestive heart failure and a mean weight gain of 2.2 kg, thought to reflect increased subcutaneous gluteal fat deposition, with an observed decreased waist-to-hip ratio.
Interestingly, in a passive follow-up of the DREAM study conducted a median 1.6 years after the end of the trial and 4.3 years after randomization, participants treated with rosiglitazone had a 39% lower incidence of diabetes compared to placebo participants, and 17% more of them regressed from prediabetes to normoglycemia [47]. Nonetheless, there was no difference between the 2 groups when the analysis was restricted to the passive follow-up period, suggesting a time-limited exposure to rosiglitazone reduces the longer-term incidence of diabetes by likely delaying but not reversing the underlying disease process.
The third large trial assessing the efficacy of a TZD in preventing diabetes was the Actos Now for the prevention of diabetes (ACT NOW) trial, which was a randomized, double-blinded study that assigned 602 patients with IGT to pioglitazone 45 mg daily or placebo [48]. Over a median follow-up of 2.6 years, pioglitazone was associated with a 72% lower annual rate of progression to diabetes (2.1% compared to 7.6 % in placebo group), and a higher rate of conversion to normal glucose tolerance (48%). In addition, pioglitazone had favorable effects on fasting and 2-hour blood glucose, A1C level, diastolic blood pressure, carotid intima thickness, and HDL cholesterol. As in the DREAM trial, an increased incidence of edema and weight gain was observed with pioglitazone.
Unlike the strong evidence supporting TZDs as an approach to diabetes prevention in the US trials, the Indian Diabetes Prevention Program-2 (IDPP-2) trial, which randomized 497 participants with IGT to lifestyle modifications with pioglitazone versus lifestyle modifications with placebo, did not demonstrate a significant reduction in diabetes at 3 years’ follow-up, suggesting a possible ethnicity-related variation in the effect of the medication [49]. In 2011, the French and German medications regulatory agency withdrew pioglitazone from the market because of a potential increase in incidence of bladder cancer with the cumulative use of more than 28 g of pioglitazone. In the United States, the Food and Drug Administration is performing an extensive review of data and advises against the use of pioglitazone in patients with a history of bladder cancer.
In summary, TZDs demonstrated significant efficacy in preventing diabetes in many patients at risk, but their safety concerns, particularly the associated new onset of congestive heart failure and potential increased risk of bladder cancer, might outweigh this benefit.
Combination Metformin and Thiazolidinediones
As metformin and rosiglitazone both have preventive benefits in diabetes, and rosiglitazone is associated with numerous side effects at a higher dose, a combination of metformin and low-dose rosiglitazone was evaluated in in the CAnadian Normoglycemia Outcomes Evaluation (CANOE) trial [50]. A total of 207 patients with IGT were randomly assigned to receive combination metformin (500 mg twice daily) and rosiglitazone (2 mg daily) versus placebo for a median of 3.9 years. The combination therapy was associated with a 66% relative risk reduction of progression to diabetes.
Alpha-glucosidase Inhibitors
Alpha-glucosidase inhibitors are antidiabetic agents that slow oral carbohydrate intestinal absorption, subsequently improving postprandial hyperglycemia, which can eventually reduce glucose toxicity of pancreatic beta cells. In addition, they have been shown to improve insulin sensitivity in individuals with IGT [51] and have been found to exert a favorable protective effect in a prediabetic population [52]. In a multicenter placebo-controlled randomized trial, the Study to Prevent Non-Insulin Dependent Diabetes Mellitus (STOP-NIDDM), 1429 participants with IGT were randomly assigned to receive acarbose 100 mg 3 times a day or placebo for 3 years [53]. As expected, diabetes incidence was significantly decreased by 25% in the acarbose group (relative risk of 32.4% vs 41.5% in acarbose and placebo group, respectively), and acarbose significantly increased reversion to normal glucose tolerance (P < 0.0001). Furthermore, the use of acarbose was associated with a statistically significant 49% decrease in the rate of any cardiovascular event, highlighting the cardiovascular protective effect of improving postprandial hyperglycemia with acarbose. This study had many limitations: a high percentage of participants discontinued treatment (31% in the acarbose group and 19% in the placebo group), most likely related to increased gastrointestinal adverse effects of acarbose. In addition, the diabetes prevention effect does not seem to be sustained: during a 3-month wash-out period where all patients received placebo, incidence of diabetes in the initial intervention group was higher than in the initial placebo group.
In a Japanese multicenter randomized double-blind trial, 1780 patients with IGT were randomly assigned to receive the alpha-glucosidase inhibitor voglibose or placebo [54]. An interim analysis at 48 weeks revealed a significantly lower risk of progression to diabetes in the voglibose group.
Combination Metformin and Acarbose
In a 6-year multicenter British study, the Early Diabetes Intervention Trial (EDIT), 631 participants with IFG were randomly assigned, in a factorial design, to double-blind treatment with acarbose or placebo and simultaneously to metformin or placebo [55]. At 3 years, there was a nonsignificant risk reduction of 8% and 37% in progression to 2 successive fasting plasma glucose values of 140 mg/dL or more in the acarbose and metformin groups, respectively, but a significantly lower 2-hour OGTT glucose in the acarbose group and significantly lower FBG in the metformin group. Interestingly, at 6 years of follow-up, there was no significant difference in relative risk of progression to diabetes with acarbose, metformin, or combination therapy [56]. However, unlike metformin or combination therapy, acarbose was associated with a significant relative risk reduction of diabetes (0.66, P = 0.046) in the subgroup of patients with IGT at baseline, suggesting a possible differential protective effect of certain agents in patients with IGT or IFG.
Nateglinide
Nateglinide is a short-acting insulin secretagogue that is mostly used in the treatment of postprandial hyperglycemia in diabetic patients. The protective effect of nateglinide in a prediabetic population was examined in the NAVIGATOR study (the NAteglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research), a large prospective multinational, randomized, double-blind, placebo-controlled trial. Nateglinide (30–60 mg 3 times daily) and valsartan (80–160 mg daily) versus placebo were used in a 2×2 factorial design in 9306 participants with IGT and increased risk of cardiovascular events [57]. At 5 years, nateglinide did not reduce the cumulative incidence of diabetes or cardiovascular outcomes, when compared to placebo, whereas risk of hypoglycemia was significantly increased in the intervention group.
Liraglutide
Liraglutide is an injectable glucagon-like peptide-1 (GLP-1) receptor agonist used to treat T2DM, and recently approved as a weight-reducing agent at the dose of 3 mg injected subcutaneously. GLP-1 receptor agonists work by stimulating insulin secretion in a glucose-dependent manner, suppressing glucagon secretion, inducing satiety, and slowing gastric emptying. In the international double-blind SCALE (Satiety and Clinical Adiposity-Liraglutide Evidence) trial, 3731 nondiabetic patients, among whom 61.2% had prediabetes, were randomly assigned to liraglutide 3 mg subcutaneous injection daily or placebo, in addition to diet and exercise [58]. Liraglutide was associated with lower glucose levels on OGTT and lower A1C values at the end of the study (56 weeks), with this decrease especially prominent in prediabetic patients. Significantly fewer participants in the liraglutide group (4/2219) compared to the placebo group (14/1225) developed diabetes at 56 weeks, nearly all of whom (except for 1 in the placebo group) had prediabetes at the beginning of the study. Of note, the liraglutide group had a mean 8.4-kg weight reduction by week 56, compared to 2.8 kg in the placebo group.
Insulin
Insulin has also been investigated as a possible diabetes prevention agent, given the assumed protective effect insulin could exert on beta cell reserve. In the landmark international Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial, 12,537 participants (mean age 63.5 years) with cardiovascular risk factors plus IFG, IGT, or type 2 diabetes were randomly assigned to receive insulin glargine (with a target FBG ≤ 95 mg/dL) or standard care and were monitored for cardiovascular outcomes and other secondary endpoints including incidence of diabetes [59]. After a median follow-up of 6.2 years, and 3 months after discontinuation of therapy, among the 1456 participants without baseline diabetes, new diabetes was diagnosed in 30% of participants receiving glargine versus 35% of those receiving standard therapy. However, rates of severe hypoglycemia and modest weight gain were higher in the insulin group, calling in to question the benefit/risk balance with the use of basal insulin for diabetes prevention.
ACE Inhibitors and ARBs
A possible diabetes preventive effect was observed with renin-angiotensin system (RAS) blockade agents in secondary analysis of several hypertension trials, such as with ramipril in the Heart Outcomes Prevention Evaluation study, captopril (compared to diuretics and beta blockers) in the CAptopril Prevention Project, lisinopril (compared to amlodipine and chlorthalidone) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, losartan (compared to atenolol) in the Losartan Intervention For Endpoint reduction in hypertension study), and multiple other randomized controlled trials [60–64]. Therefore, 2 major trials were designed to examine, as a primary outcome, the effect of RAS inhibition on diabetes prevention in a population at risk. The DREAM trial randomly assigned, in a 2 × 2 factorial design, 5269 relatively healthy participants with IGT and/or IFG to rosiglitazone, ramipril, or placebo [65]. Although the use of ramipril at a dose of 15 mg daily for 3.5 years did not prevent diabetes significantly, it was associated with a 9%, nonsignificant decrease in new-onset of diabetes and a 16%, significant increase in regression of IFG and IGT to normoglycemia, as well as a significant decrease in OGTT 2-hour glucose level (135.1 vs 140.5 mg/dL) with no improvement in FBG.
Similarly, in the NAVIGATOR trial that examined the effect of nateglinide and valsartan on the prevention of diabetes in 9306 participants with IGT and increased risk of cardiovascular events, valsartan significantly but slightly reduced the incidence of diabetes at 5 years, by 14%, when compared to placebo (33% versus 37%, respectively), with no significant reduction in cardiovascular outcome [66]. Unlike in the DREAM study, the patients enrolled in the NAVIGATOR trial had established cardiovascular disease or cardiovascular risk factors and assumable elevated RAS activation level. This baseline population difference might explain the more significant effect of RAS inhibition in the NAVIGATOR trial.
Given the positive glycemic effect of ACE inhibitors and ARBs, their use should be encouraged in prediabetic patients when indicated for treatment of high blood pressure or cardiovascular disease. Different mechanisms could explain this favorable glycemic impact: inhibition of the post-receptor insulin signaling abnormalities, increased blood flow to the skeletal muscle facilitating insulin action, enhanced differentiation of pre-adipocytes into mature adipocytes, and increased pancreatic islet blood perfusion leading to appropriate insulin release and possible partial PPAR-γ activity [67].
Xenical
Xenical is a gastrointestinal lipase inhibitor approved for use for weight reduction and maintenance. A possible diabetes prevention benefit of xenical was initially suggested by a retrospective analysis of xenical treatment effects on obese patients with IGT [68]. This finding was subsequently confirmed by a multicenter randomized placebo-controlled study, XENical in the prevention of Diabetes in Obese Subjects (XENDOS), where 3305 obese subjects, with normal glucose tolerance or IGT were randomly assigned to either xenical 120 mg 3 times a day or placebo, in addition to lifestyle changes for all participants [69]. In the group of patients with IGT (694 subjects), xenical treatment was associated with a 45% risk reduction of progression to diabetes at 4 years (18.8% versus 28.8% in placebo), whereas participants with baseline normal glucose tolerance had no significant change in incidence of diabetes. On the other hand, weight reduction at 4 years was significantly greater in all patients who received xenical (5.8 kg in intervention group vs 3 kg in control group). The beneficial effect of xenical in diabetes prevention seems to be additive to the benefit of weight loss. As in many weight reduction trials, this study was limited by the high discontinuation rate in both groups (48% in xenical group and 66% in control group), probably related to insufficient clinical response.
Fibric Acid Derivatives (Bezafibrate)
Bezafibrate, a nonselective ligand/activator for PPAR-α, was found to reduce not only triglycerides, but also FPG, fructosamine, and A1C levels significantly in T2DM patients with hyperlipidemia [70]. Different mechanisms of glucose lowering have been suggested with bezafibrate: nonselective activation of PPAR-γ, improving insulin sensitivity, and enhancing glucose disposal in adipose tissue and skeletal muscles [71]. Furthermore, bezafibrate treatment was associated with decreased incidence of diabetes in patients with IFG and in obese non-diabetic patients with normal glycemic levels [72,73]. In a posthoc analysis of the Bezafibrate Infarction Prevention study, 303 patients with IFG received either 400 mg of bezafibrate daily or placebo [73]. Over a mean follow-up of 6.2 years, development of diabetes was less prevalent (54.4% vs 42.3%, relative risk reduction of 22%) and delayed (mean 10 months) in the bezafibrate group compared to placebo. Multivariate analysis identified bezafibrate as an independent predictor of decreased risk of new diabetes development, regardless of BMI and lipid profile.
Surgery
Over the past decade, bariatric surgery has become one of the most effective interventions for inducing and sustaining weight reduction in severely obese patients, leading to a significant benefit in diabetes prevention or remission. The Swedish Obese Subject Study is a large ongoing prospective nonrandomized cohort study that between 1987 and 2001 enrolled 4047 nondiabetic obese participants who underwent gastric surgery or were matched obese control, with diabetes incidence measured at 2, 10 and 15 years [74–76]. At 15 years, analysis of the available cohort of the initial group showed that T2DM developed in 392 of 1658 control participants and in 110 of 1771 bariatric-surgery participants, corresponding to incidence rates of 28.4 and 6.8 cases per 1000 person-years, respectively (P < 0.001). The treatment effects on the incidence of T2DM were at least as strong after 2 years and 10 years of follow-up as after 15 years. This effect was most prominent among the 591 patients who had IFG at baseline, with a number needed to treat as low as 1.3. The surgery group maintained an average 20-kg weight loss at 15 years.
In another study of the effects of bariatric surgery, 150 of 152 obese participants with IGT who underwent gastric bypass achieved and maintained a normal glycemic profile at 14 years of follow-up [77]. Similarly, in a follow-up of 136 obese participants with IGT, 109 of whom underwent bariatric surgery, 1 participant in the surgical group developed diabetes, as compared with 6 out of 27 in the control group [78]. In a meta-analysis including studies involving 22,094 patients who underwent bariatric surgery, 76.8% had complete resolution of their diabetes [79]. The rapid improvement of glycemic profile after bariatric surgery is thought to be due to oral intake restriction as well as acute hormonal changes related to the exclusion of the upper gastrointestinal tract (eg, incretin and ghrelin levels variations) [80].
Conclusions and Recommendations
The natural history of T2DM allows identification of patients at risk for diabetes and implementation of prevention strategies, which seems to be a public health need given the alarming increase in diabetes incidence. Indeed, the onset of T2DM is typically preceded by many years of beta cell dysfunction translating into carbohydrate metabolism abnormalities such as IFG and IGT, providing an excellent window of opportunity to identify persons at risk and prevent progression to diabetes. Numerous randomized controlled trials established lifestyle modifications, including dietary changes, moderate weight loss, and moderate intensity physical activity, as safe and effective interventions to prevent diabetes. This protective effect has been consistently shown to be sustained for more than 10 years after the initial intervention. Pharmacologic agents such as metformin, thiazolidinediones, alpha-glucosidase inhibitors, xenical, liraglutide, and insulin have also been associated with diabetes prevention in patients at risk. However, except for metformin, safety concerns or lack of durable efficacy or tolerability seem to outweigh their potential diabetes prevention benefit.
Given their favorable glycemic effect, RAS blockade and fibrates should be considered, when indicated, as reasonable treatment options for hypertension and hyperlipidemia in prediabetic patients. Bariatric surgery has been associated with a dramatic reduction in diabetes incidence in obese prediabetic patients and can be considered an alternative prevention measure in patients with severe obesity and prediabetes.
The recently updated ADA guidelines recommend referring patients with prediabetes to an intensive diet and physical activity behavioral counseling program; diet and activity goals should adhere to the tenets of the DPP, with a loss of 7% of body weight and at least 150 minutes of moderate physical activity (eg, brisk walking) per week [8]. Metformin therapy for diabetes prevention should be considered in patients with prediabetes, especially in those with BMI greater than 35 kg/m2, those younger than 60 years of age, women with history of gestational diabetes, and/or those with rapidly rising A1C despite lifestyle modifications. Monitoring for development of diabetes, at least annually, and screening for and treatment of modifiable cardiovascular risk factors are suggested in patients with prediabetes [8].
Many lessons have been learned through the studies of diabetes prevention interventions. The challenge that remains is how to apply these interventions, especially the lifestyle modifications, in real world medical practice, at both the individual and public health level.
Corresponding author: Jocelyne Karam, MD, 4802 10th Avenue, Brooklyn, NY 11219, [email protected].
Financial disclosures: None reported.
1. International Diabetes Federation. Diabetes facts and figures. www.idf.org/about-diabetes/facts-figures. Accessed on January 29, 2017.
2. Centers for Disease Control and Prevention. National diabetes statistics report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed on January 29, 2017.
3. Centers for Disease Control and Prevention. Number of Americans with diabetes projected to double or triple by 2050. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed on January 29, 2017.
4. World Health Organization (WHO). Diabetes fact sheet. No. 312. November 2016. www.who.int/mediacentre/factsheets/fs312/en/. Accessed on January 29, 2017.
5. Karam JG, McFarlane SI. Update on the prevention of type 2 diabetes. Curr Diab Rep 2011;11:56–63.
6. Menke A, Rust KF, Fradkin J, et al. Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States: a series of cross-sectional studies. Ann Intern Med 2014;161:328–85.
7. Ford ES, Li C, Sattar N . Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008;31:1898–904.
8. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care 2017;40(Suppl. 1).
9. Kruszynska YT, Olefsky JM. Cellular and molecular mechanisms of non-insulin dependent diabetes mellitus. J Investig Med 1996;44:413–28..
10. Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–10.
11. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO and IDF Consultation. 2006. http://apps.who.int/iris/bitstream/10665/
43588/1/9241594934_eng.pdf. Accessed on February 1, 2017.
12. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. 2001 JAMA 2003;289:76–9.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999;354:617–21.
14. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–7.
15. Xu T, Liu W, Cai X, et al. Risk of coronary heart disease in different criterion of impaired fasting glucose: a meta-analysis. Medicine 2015;94:e1740.
16. Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953.
17. Nagi DK, Pettitt DJ, Bennett PH, et al. Diabetic retinopathy assessed by fundus photography in Pima Indians with impaired glucose tolerance and NIDDM. Diabet Med 1997;14:449 –56.
18. Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–44
19. Putz Z, Tabák AG, Tóth N, et al. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care 2009;32:181–3.
20. Hoffman-Snyder C, Smith BE, Ross MA, et al. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006;63:1075–9.
21. Plantinga LC, Crews DC, Coresh J, et al; CDC CKD Surveillance Team. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol 2010;5:673–82.
22. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–44.
23. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
24. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
25. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–62.
26. Ramachandran A, Snehalatha C, Mary S, et al; Indian Diabetes Prevention Programme (IDPP).The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97.
27. Saito T, Watanabe M, Nishida J, et al; Zensharen Study for Prevention of Lifestyle Diseases Group. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–60.
28. Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–9.
29. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–80.
30. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–80.
31. Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:673–9.
32. Diabetes Prevention Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–86.
33. Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14–19.
34. Wang P, Fang J, Gao Z, et al. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta‐analysis. J Diabetes Investig 2016;7:56–69.
35. Devlin JT. Effects of exercise on insulin sensitivity in humans. Diabetes Care 1992;15:1690–3.
36. Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30:744–52.
37. Slentz C, Bateman L, Willis L, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia 2016;59:2088–98.
38. Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–7.
39. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 2002;137:25–33.
40. Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–9.
41. Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003;26:977–80.
42. Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008;121:149–57.
43. El-Atat F, Nicasio J, Clarke L, et al. Beneficial cardiovascular effects of thiazolidinediones. Therapy 2005;2:113–19.
44. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803.
45. Azen SP, Peters RK, Berkowitz K. TRIPOD (Troglitazone In the Prevention Of Diabtes): a randomized placebo-controlled study of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials 1998;19:217–31.
46. Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105.
47. DREAM Investigators, Gerstein HC, Mohan V, Avezum A, et al. Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia 2011;54:487–95.
48. DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–15.
49. Ramachandran A, Snehalatha C, Mary S, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia 2009;52:1019–26.
50 Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010;376:103–11.
51. Chiasson JL, Josse RG, Leiter LA, et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care 1996;19:1190–3.
52. Van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev 2006(4):CD005061.
53. Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–7.
54. Kawamori R, Tajima N, Iwamoto Y, et al. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009;373:1607–14.
55. Holman RR, North BV, Tunbridge FK. Possible prevention of type 2 diabetes with acarbose or metformin. Diabetes 2000;49:Suppl 1:A111.
56. Holman RR, Blackwell L, Stratton IM et al. Six-year results from the Early Diabetes Intervention Trial. Diabet Med 2003;20(Suppl 2):15.
57. Holman RR, Haffner SM, McMurray JJ, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1463–76.
58. Pi-Sunyer X, Astrup A, Fujioka K et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22.
59. ORIGIN Trial Investigators, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–28.
60. Yusuf S, Gerstein H, Hoogwerf B, et al. Ramipril and the development of diabetes. JAMA 2001;286:1882–5.
61. Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999;353:611–6.
62. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–97.
63. Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:1004–10.
64. Gillespie EL, White CM, Kardas M, et al. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care 2005;28:2261–6.
65. Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–62.
66. McMurray JJ, Holman RR, Haffner SM, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1477–90.
67. McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol 2003;91(12A):30H–37H.
68. Heymsfield SB, Segal KR, Hauptman J, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Arch Intern Med 2000;160:1321–6.
69. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–61.
70. Rovellini A, Sommariva D, Branchi A, et al. Effects of slow release bezafibrate on the lipid pattern and on blood glucose of type 2 diabetic patients with hyperlipidaemia. Pharmacol Res 1992;25:237–45.
71. Tenenbaum A, Motro M, Fisman EZ. Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons. Cardiovasc Diabetol 2005;4:14.
72. Tenenbaum A, Motro M, Fisman EZ, et al. Effect of bezafibrate on incidence of type 2 diabetes mellitus in obese patients. Eur Heart J 2005;26:2032–8.
73. Tenenbaum A, Motro M, Fisman EZ, et al. Peroxisome proliferator-activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation 2004;109:2197–202.
74. Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93.
75. Sjostrom CD. Surgery as an intervention for obesity. Results from the Swedish obese subjects study. Growth Horm IGF Res 2003;13 Suppl A:S22–26.
76. Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704.
77. Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–50.
78. Long SD, O’Brien K, MacDonald KG Jr, et al. Weight loss in severely obese subjects prevents the progression of impaired glucose tolerance to type II diabetes. A longitudinal interventional study. Diabetes Care 1994;17:372–5.
79. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–37.
80. Tejirian T, Jensen C, Dutson E. Bariatric surgery and type 2 diabetes mellitus: surgically induced remission. J Diabetes Sci Technol 2008;2:685–91.
From the Maimonides Medical Center (Dr. Karam) and the SUNY Downstate Medical Center (Dr. Karam and Dr. McFarlane), Brooklyn, NY.
Abstract
- Objective. To discuss the epidemic of diabetes highlighting the natural history of the disease and the major clinical trials aimed at diabetes prevention in different prediabetic populations around the world.
- Results. Diabetes prevention studies have evaluated various interventions including lifestyle modifications, metformin, alpha-glucosidase inhibitors, thiazolidinediones, nateglinide, and xenical as well as the renin-angiotensin aldosterone system (RAS) inhibitors. Lifestyle modifications seem to be the safest, most effective, and most sustainable intervention to prevent diabetes. Except for metformin, the potential diabetes prevention benefits of the studied pharmacologic agents are limited by safety concerns or lack of durable efficacy or tolerability. RAS blockade and fibrates have a favorable glycemic effect, and, when indicated, are reasonable treatment options for hypertension and hyperlipidemia in prediabetic patients.
- Conclusion. As recommended by American Diabetes Association guidelines, patients with prediabetes should be referred to an intensive diet and physical activity behavioral counseling program; diet and activity goals include a loss of 7% of body weight and at least 150 minutes of moderate physical activity per week. Metformin therapy for diabetes prevention should be considered as well.
Key words: prediabetes; type 2 diabetes mellitus, diabetes prevention, lifestyle modifications.
Diabetes mellitus has reached pandemic proportions across the globe. The International Diabetes Federation (IDF) estimates that in 2015 around 415 million people, or 1 in 11 adults, had diabetes, compared to 285 million in 2010, with 5 million deaths, or 1 death every 6 seconds, occurring because of diabetes or diabetes complications [1]. In the United States, an estimated 29.1 million Americans, or 9.3% of the population, have diabetes, 27.8% of them undiagnosed [2]. The prevalence of diabetes increases significantly with age, affecting around 16.2% of American adults aged 45 to 64 years and 25.9% of adults aged 65 years or older [2]. The Centers for Disease Control and Prevention (CDC) estimates that, with current trends, as many as 1 in 3 American adults could have diabetes by 2050 [3].
Type 2 diabetes mellitus (T2DM) accounts for the majority of prevalent and newly diagnosed diabetes in the world, and is strongly linked to overweight and inactivity in adults [4]. T2DM is increasingly being diagnosed in pediatric patients, in whom type 1 diabetes has historically been predominant; it now accounts for approximately 30% of newly diagnosed diabetes in children aged 10 to 19 years, exceeding 50% in certain ethnicities such as non-Hispanic black and American Indian/Alaska Native children [2].
These alarming trends have spurred significant research and public efforts aimed at reducing the prevalence of diabetes by preventing T2DM. Indeed, insulin resistance and abnormal carbohydrate metabolism progress over many years prior to the diagnosis of diabetes and manifest with different clinical and biochemical features. Both the pathophysiology and the natural history of T2DM offer clinicians an opportunity to identify patients at risk for developing the disease and to implement prevention strategies. This article outlines the risk factors and diagnostic criteria for prediabetes, describes the studies that have explored diabetes prevention through lifestyle changes, pharmacotherapy, or surgery, and reviews recommendations for managing patients at risk.
Risk Factors and Screening for T2DM
The American Diabetes Association (ADA) recommends screening all adults for prediabetes by assessing for diabetes risk factors [8]. Glucose testing is recommended in individuals aged 45 years or older, and should be considered in adults of any age who are overweight or obese (body mass index [BMI] ≥ 25 kg/m2 or ≥ 23 kg/m2 in Asian Americans) and have 1 or more additional risk factors for diabetes. Testing also should be considered in children and adolescents who are overweight or obese and who have 2 or more additional risk factors. If tests are normal, repeat testing carried out at a minimum of 3-year intervals is suggested [8].
Prediabetes
Abnormalities in glucose metabolism progress along a continuum through various stages before T2DM develops. Years before the development of overt diabetes, and especially in the presence of excessive visceral fat, cellular sensitivity to insulin gradually decreases, leading to a compensatory increased insulin secretion [9]. With time, and under continuous increased demand, pancreatic beta cell function declines and ultimately fails to overcome insulin resistance and maintain a normal glucose metabolism, resulting in prediabetes followed by the development of diabetes. This early beta cell dysfunction was illustrated by the decreased beta cell volume observed on autopsy of obese patients with IFG or T2DM, when compared to obese individuals with normal glucose tolerance [10]. It is estimated that around 40% to 70% of beta cell function is already lost by the time diabetes is clinically diagnosed. This relatively slow pathophysiologic process allows the identification of at-risk patients well before their blood glucose levels reach the diabetic diagnostic thresholds, and therefore presents an opportunity for prevention.
Diagnostic Criteria
The ADA guidelines released in 2003 define prediabetes as IFG (fasting blood glucose [FBG] levels of 100–125 mg/dL), IGT (glucose levels of 140–199 mg/dL at 2 hours during an oral glucose tolerance test [OGTT] following an oral load of 75 g of dextrose), or both. Additionally, hemoglobin A1C (A1C) was introduced as a diagnostic tool for prediabetes in 2010, with values between 5.7% and 6.4% indicating prediabetes [8]. Most of these thresholds were chosen due to their association with increased rates of complications, notably retinopathy and cardiovascular disease.
A combined report from the World Health Organization (WHO) and the IDF published in 2006 defined intermediate hyperglycemia as IFG, but with a higher cutoff for FBG (110–125 mg/dL) than the ADA’s definition, and/or IGT (2-hour OGTT glucose level of 140–199 mg/dL) [11]. The rationale for a higher cut-point for IFG is the concern about the increased prevalence of IFG and its impact on individuals and health systems and the more favorable cardiovascular risk profile and decreased risk of progression to diabetes in the group of patients with FBG of 100 to 110 mg/dL when compared to the group with FBG of 110 to 125 mg/dL. The report does not recommend the use of A1C in the diagnosis of diabetes or intermediate hyperglycemia because of a lack of global consistency and the potential for other factors that can be prevalent in some developing countries, such as hemoglobinopathies and anemia, to interfere with the assay.
Prevalence and Progression to Diabetes
According to CDC data from 2014, up to 86 million American adults, more than 1 in 3, have prediabetes, and 9 out of 10 of these individuals are undiagnosed [2]. It is estimated that approximately 25% of people diagnosed with either IFG or IGT progress to diabetes mellitus over a 3- to 5-year period [12]. If observed for longer periods, most prediabetic persons will probably develop diabetes. The highest rate of progression to diabetes is observed in patients with both IFG and IGT, older age, overweight, or other diabetic risk factors.
Complications
In addition to increasing the risk for progression to diabetes, prediabetes is independently associated with microvascular and macrovascular complications and increased risk of death, prior to the actual onset of diabetes. The DECODE study demonstrated significantly increased mortality in 2766 individuals with IGT after 7 years of follow-up, when compared to normoglycemic patients; this effect was more prominent in participants with IGT than in participants with IFG [13]. In the Australian Diabetes, Obesity and Lifestyle Study, IFG was found to be an independent predictor for cardiovascular mortality after adjustment for age, sex, and other traditional cardiovascular risk factors [14].
Similarly, a recent meta-analysis demonstrated that the presence of IFG was significantly associated with future risk for coronary heart disease (CHD), with the risk increase starting when fasting plasma glucose was as low as 100 mg/dL; however, this finding may have been confounded by the presence of undetected IGT or other cardiovascular risk factors [15]. Another recent systematic review of 53 prospective cohort studies with 1,611,339 participants showed that prediabetes (IFG or IGT) was associated with an increased risk of composite cardiovascular disease, CHD, stroke, and all-cause mortality [16].
The association between retinopathy and prediabetes has been described in multiple reports and this association has helped guide authors on selected thresholds for diagnosis of prediabetes. For example, in 1 study, the incidence of retinopathy in individuals with IGT was 12% among Pima Indians [17]. Similarly, in a follow-up study of the Diabetes Prevention Program, 8% of prediabetic participants who remained nondiabetics had evidence of retinopathy [18].
Neuropathy also has been observed in prediabetes. A noninvasive neurologic evaluation of individuals with IGT revealed subclinical neural dysfunction suggestive of cardiovascular autonomic neuropathy [19]. At the clinical level, a study that evaluated 100 patients with chronic idiopathic axonal neuropathy of unknown etiology found IFG in 36 and IGT in 38 patients, underscoring the role of abnormal glucose metabolism in these patients [20].
Nephropathy may also be more prevalent in those with prediabetes. In a 1999–2006 National Health and Nutrition Examination Survey analysis, the adjusted prevalence of chronic kidney disease, defined by estimated glomerular filtration rate (eGFR) of 15 to 59 mL/min per 1.73 m2 or albumin-creatinine ratio ≥ 30 mg/g, was 17.1% in individuals with IFG, compared to 11.8% in individuals with normal fasting glucose [21].
Lifestyle Modifications
The alarming rapid increase in the prevalence of T2DM has been linked to a parallel rising epidemic of overweight, obesity, and lack of physical activity. Therefore, lifestyle changes aiming at weight reduction seemed to be a natural individual and public health strategy to prevent diabetes, and such strategies have been the focus of many randomized controlled trials around the world. As anticipated, weight loss, exercise, and diet have all been shown, separately or in combination, to be effective in decreasing the incidence of T2DM in high-risk patients [22–27]. Furthermore, and well beyond the benefit observed during the trials, follow-up studies revealed a sustained reduction of diabetes incidence in intervention groups several years after cessation of the intervention [28–32] (Table 2).
The Da Quing Diabetes Prevention Study (DQDPS), published in 1997, is one of the earliest prospective diabetes prevention trials [22]. This 6-year study conducted in 33 clinics in China from 1986 through 1992 included 577 participants with IGT who were randomly assigned to 1 of 4 groups: (1) diet (high vegetables, low sugar/alcohol) only, (2) exercise, (3) diet plus exercise, and (4) standard of care. At 6 years, diabetes incidence was significantly reduced by 46% in the exercise group, 31% in the diet group, and 42% in the diet plus exercise group compared to standard care. In 2006, 14 years after the end of the trial and 20 years after the initial enrollment, the cumulative incidence of diabetes was significantly lower in the intervention group at 80%, compared to 93% in the control group, and the annual incidence of diabetes was 7% and 11%, respectively, with a 43% lower incidence of diabetes over the 20-year period in the combination lifestyle changes group [28]. The preventive benefit of lifestyle changes persisted 2 decades after the initial randomization despite the standardization of treatment for all groups over the 14 years following the study, suggesting a strong and longitudinal preventive effect of the initial lifestyle modifications. In a follow-up study of the DQDPS conducted in 2009, at 23 years of follow-up, the cumulative incidences of cardiovascular mortality and all-cause mortality were significantly lower in the intervention group (11.9% versus 19.6%, and 28.1% versus 38.4%, respectively), highlighting the long-term clinical benefits of lifestyle intervention in patients with IGT [29].
Similarly, the Finnish Diabetes Prevention Study (FDPS), published in 2001, enrolled 522 middle-aged overweight participants with IGT [23]. The participants randomly assigned to the intervention group received individualized counseling designed to reduce weight, decrease total intake of fat and saturated fat, increase intake of fiber, and increase physical activity. The control group received standard therapy. At 4 years of follow-up, the cumulative incidence of diabetes was 11% in the intervention group and 23% in the control group, with a statistically significant 58% reduction in risk for progression to diabetes. A follow-up of the FDPS was published in 2006 [31]. Participants who did not progress to diabetes in the initial 4-year study were further followed for a median of 3 years. Interestingly, lifestyle changes were maintained by the intervention group participants despite the cessation of the individual counseling, leading to a 36% relative reduction in diabetes incidence during the post-intervention follow-up period alone (4.6 vs 7.2 per 100 person-years, P = 0.041) and a 43% cumulative diabetes incidence reduction over the 7-year follow-up, demonstrating, one more time, the sustained efficacy of lifestyle changes.
In the United States, the Diabetes Prevention Program (DPP) trial is a landmark NIH-sponsored multicenter randomized controlled trial published in 2002, and one of the largest diabetes prevention studies with lifestyle changes to date [24]. A total of 3234 participants with prediabetes, defined as an IFG or IGT, were randomly assigned to an intensive lifestyle modification program, metformin 850 mg twice daily, or matching placebo. Lifestyle changes included a low-fat (< 25% of caloric intake), 1200- to 1800-calorie diet and exercise for 150 minutes a week, with a 7% body weight reduction goal and a very well structured curriculum and professional support group. The study was discontinued early (at 3 years) as the data demonstrated the superiority of lifestyle changes, with a 58% reduction in diabetes incidence in the lifestyle intervention group and a 31% reduction in the metformin group when compared to placebo (cumulative incidence of diabetes at 3 years of 28.9%, 21.7 %, and 14.4% in the placebo, metformin, and lifestyle intervention groups, respectively). Lifestyle changes were significantly more effective than metformin and were consistently effective in men and women across age, BMI, and ethnic groups.
The DPPOS (DPP Outcome Study) was a 10-year follow-up of the DPP study published in 2009 where all participants were offered group-implemented lifestyle changes and were followed for an additional 5.7 years [32]. Unlike the Finnish follow-up study, diabetes incidence was similar in the 3 treatment groups in the follow-up period. However, the cumulative incidence of diabetes remained significantly the lowest in the original lifestyle group, with a 34% cumulative risk reduction in the lifestyle group and an 18% reduction in the metformin group at 10 years when compared to placebo. Interestingly, unlike most other studies of weight-reducing interventions, in the DPPOS, patients in the lifestyle changes and metformin groups maintained weight loss at 10 years’ follow-up.
In Japan, a diabetes prevention study assigned 458 male participants with IGT to a standard intervention group or an intensive intervention group receiving detailed lifestyle modification counseling every 3 to 4 months during hospital visits [25]. The cumulative 4-year incidence of diabetes was 9.3% in the control group versus 3.0% in the intervention group, and the reduction in diabetes risk was 67.4% (P < 0.001), with body weight reductions of 0.39 kg and 2.18 kg, respectively (P < 0.001). Of note, participants with higher FBG at baseline developed diabetes at a higher rate than those with lower values. This study suggested that lifestyle change counseling conducted in an outpatient clinic setting can be very effective in preventing diabetes.
Indian adults are thought to be more insulin resistant at a younger age and at a lower BMI than Caucasians. To assess whether the DPP findings can be replicated in an Indian population, the Indian Diabetes Prevention Program (IDPP) trial randomized a total of 531 participants with IGT to 4 groups: control, lifestyle modification, metformin, and lifestyle modifications with metformin [26]. The 3-year cumulative incidences of diabetes were 55.0%, 39.3%, 40.5%, and 39.5%, respectively, showing again a significant relative reduction in progression to diabetes of 28.5% with lifestyle changes, 26.4% with metformin, and 28.2% with both lifestyle changes and metformin, as compared with the control group.
In a Japanese unmasked, multicenter, randomized controlled trial published in 2011, 641 overweight adults with IFG were randomized to a frequent intervention group, receiving individual counseling and support for lifestyle modifications 9 times over 36 months, or a control group, receiving counseling 4 times over the same period. The 3-year cumulative incidence of T2DM was significantly lower in the frequent intervention group than in the control group (12.2% vs 16.6%) [27]. Interestingly, in a posthoc subgroup analysis, the protective effect was more prominent in patients with underlying associated IGT or elevated A1C, but was not observed in patients with isolated IFG, suggesting a possible prognostic value of an additional A1C or oral glucose tolerance test in individuals with IFG.
Diet
The diet followed in the major diabetes prevention trials discussed above has typically been a weight-reducing diet with decreased fat intake (eg, DPP, Finnish trial) and increased fiber intake (eg, Da Quing, DPP, Finnish trials). However, there has been more emphasis recently on the importance of the quality rather than the quantity of fats in preventing diabetes. For example, in a Spanish study, a non–calorie-restricted traditional Mediterranean diet, enriched with high-fat foods of vegetable origin (olive oil, nuts) decreased the incidence of diabetes by 52% in individuals at high cardiovascular risk after a median follow-up of 4.0 years, and in the absence of significant changes in body weight or physical activity among the groups [33]. These findings were reproduced by other studies. A recent meta-analysis examining the relation between intake of fruits and vegetables and the incidence of diabetes revealed that higher intake of fruit, especially berries, and green, leafy vegetables, yellow vegetables, cruciferous vegetables, or their fiber is associated with a lower risk of T2DM [34].
Exercise
Exercise is thought to improve insulin sensitivity and promote peripheral glucose uptake in normal individuals. Long-term moderate exercise, similar to the exercise recommended in DPP and FDPS, results in increased translocation of insulin-responsive glucose transporter (GLUT-4) from intracellular stores to the cell surface, facilitating glucose uptake [35]. A systematic review of 10 prospective cohort studies published in 2007 showed that, after adjustment for BMI, moderate-intensity physical activity was significantly associated with reduced diabetes incidence [36]. In the FDPS, participants who achieved at least 4 hours of exercise per week had a significant 80% decrease in incidence of diabetes, and this effect was observed even in the group that did not lose weight [23]. In the DQDPS, the greatest reduction in diabetes incidence was observed in the exercise group [22].
In a recent NIH-funded trial designed to examine the relative contribution of exercise alone to the overall beneficial effect of lifestyle changes in the DPP study, a total of 237 adults with IFG were randomly assigned to 4 different groups: low-amount moderate intensity exercise (similar to exercise followed in DPP), high-amount moderate intensity exercise, high-amount vigorous intensity exercise, and a combination of diet, weight loss, and low-amount moderate exercise. Only the diet and exercise group experienced a decrease in fasting glucose, whereas similar improvements in glucose tolerance were observed in both the diet and exercise group and the high-amount moderate-intensity exercise group, suggesting that such an exercise regimen may be as effective as a more intensive multicomponent approach involving diet, exercise, and weight loss for preventing diabetes [37].
Weight Loss
Weight reduction in prediabetic individuals has been consistently associated with reduced incidence of diabetes. Furthermore, the amount of weight loss needed to achieve this benefit seems to be relatively modest and a realistic goal to set for patients. Indeed, in the DPP trial, an average weight loss of only 5.6 kg was associated with a 58% lower incidence of diabetes [24]. Moreover, on further analysis of the DPP trial, and among weight, diet, and exercise, diabetes prevention correlated most strongly with weight loss, with an estimated 16% diabetes risk reduction for every single kilogram of weight reduction [38]. Similarly, within the same lifestyle intervention group in the FDPS, the participants who were able to achieve an initial body weight loss greater than 5% at 1 year had a nearly 70% relative risk reduction in progression to diabetes, when compared to their peers in the intervention group who had less or no weight loss [23].
In summary, numerous randomized controlled studies from various populations have proved that lifestyle modifications, including healthy diet, moderate weight loss, and moderate-intensity exercise, represent a very effective strategy to prevent diabetes in patients at risk, mostly patients with IGT, and this protective effect seems to be sustained over time.
Pharmacologic Interventions
Metformin
Metformin is an antidiabetic agent that works mostly at the liver site by suppressing hepatic glucose production and inhibiting production and oxidation of free fatty acids (FFA), thereby reducing FFA-induced insulin resistance and promoting peripheral glucose uptake [39]. This effect has the potential of preserving beta cell function by reducing the demand for insulin secretion.
In the DPP trial, metformin, although generally less effective than lifestyle changes, was associated with a significant 31% reduction in diabetes incidence (cumulative incidence of 22% in metformin group vs 29% in placebo group) and significant weight reduction (average of 2 kg) [24]. Further analysis of the DPP results showed that metformin efficacy, compared to placebo, was greater in patients who were younger, had higher BMI, and had higher FBG levels. In addition, a DPP substudy of 350 women with history of gestational diabetes and IGT revealed that this group of women, who had a higher risk of progression to diabetes (71% at 3 years) when compared to women with no history of gestational diabetes, despite similar baseline glucose levels, had similar diabetes risk reduction of 50% with both metformin and lifestyle changes [40].
In the IDPP study, both lifestyle changes and metformin reduced significantly and similarly the incidence of diabetes in adults with IGT, with no observed added benefit from combining both interventions [26]. It has not been clear, however, how much of this effect of metformin is a result of pharmacologic properties masking hyperglycemia or a true protective and preventive effect. In a washout study in which 1274 DPP participants who did not progress to diabetes underwent an OGTT after 1 to 2 weeks of discontinuing metformin or placebo, the incidence of diabetes was still reduced by 25% in the metformin group, after the washout period, compared to a 31% risk reduction in the primary DPP analysis, suggesting a partially sustained rather than temporary effect of metformin [41]. In the DPPOS long-term follow-up study, metformin (850 mg twice daily as tolerated) was continued in the group initially assigned to metformin in addition to lifestyle counseling [32]. Although the progression to diabetes was similar in all groups during the 5.7-year follow-up period, the cumulative incidence of diabetes at 10 years was still reduced in the metformin group by 18% when compared to control group. Furthermore, the weight loss associated with metformin was also interestingly sustained at 10 years. A meta-analysis echoed this beneficial effect of metformin observed in the DPP trial, reporting a relative risk reduction of new-onset diabetes of 40% with the use of metformin [42].
In summary, metformin has been shown to be effective in preventing diabetes in patients at risk, especially persons with younger age, higher BMI, and history of gestational diabetes and in native Asian Indians. The protective effect of metformin seems to be sustained over the long term in follow-up studies.
Thiazolidinediones
Thiazolidinediones (TZDs) are antidiabetic agents that have been evaluated in diabetes prevention trials. TZDs are peroxisome proliferator-activated gamma receptor (PPAR-γ) agonists that work by augmenting conversion of preadipocytes to adipocytes, which in turn increase adiponectin levels, promoting insulin sensitivity [43]. In addition to their antihyperglycemic properties, TZDs are thought to have a direct protective effect on beta cells, potentially translating into prevention and delay of diabetes [44].
The first study to demonstrate diabetes prevention with a TZD was the TRIPOD study (Troglitazone in Prevention of Diabetes), in which 266 Hispanic women with a history of gestational diabetes were randomly assigned to troglitazone or placebo [45]. Troglitazone use was significantly associated with reduction of progression to diabetes at 1.5-year follow-up when compared to placebo (relative risk reduction of 55%), with a decrease of endogenous insulin requirement at 3 months of therapy and sustained benefit after discontinuation of the TZD, suggesting an effect on beta cell preservation.
Moreover, troglitazone was an investigational drug in the DPP trial from 1996 to 1998, at which time it was discontinued because of associated fatal liver failure in a DPP participant. In the DPP trial, troglitazone was asso-ciated with a remarkable 75% decrease in progression to diabetes at 1 year. Troglitazone was withdrawn from the US market in 2000 because of its association with severe hepatotoxicity.
The international DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medications) trial randomly assigned more than 5000 participants with IFG and/or IGT to rosiglitazone, ramipril, or placebo in a 2 × 2 factorial design [46]. In participants receiving rosiglitazone, the risk for progression to diabetes was reduced by 60% and the likelihood of regression to normoglycemia was increased by 71% when compared to placebo. However, the use of rosiglitazone was associated with an increased risk of new-onset congestive heart failure and a mean weight gain of 2.2 kg, thought to reflect increased subcutaneous gluteal fat deposition, with an observed decreased waist-to-hip ratio.
Interestingly, in a passive follow-up of the DREAM study conducted a median 1.6 years after the end of the trial and 4.3 years after randomization, participants treated with rosiglitazone had a 39% lower incidence of diabetes compared to placebo participants, and 17% more of them regressed from prediabetes to normoglycemia [47]. Nonetheless, there was no difference between the 2 groups when the analysis was restricted to the passive follow-up period, suggesting a time-limited exposure to rosiglitazone reduces the longer-term incidence of diabetes by likely delaying but not reversing the underlying disease process.
The third large trial assessing the efficacy of a TZD in preventing diabetes was the Actos Now for the prevention of diabetes (ACT NOW) trial, which was a randomized, double-blinded study that assigned 602 patients with IGT to pioglitazone 45 mg daily or placebo [48]. Over a median follow-up of 2.6 years, pioglitazone was associated with a 72% lower annual rate of progression to diabetes (2.1% compared to 7.6 % in placebo group), and a higher rate of conversion to normal glucose tolerance (48%). In addition, pioglitazone had favorable effects on fasting and 2-hour blood glucose, A1C level, diastolic blood pressure, carotid intima thickness, and HDL cholesterol. As in the DREAM trial, an increased incidence of edema and weight gain was observed with pioglitazone.
Unlike the strong evidence supporting TZDs as an approach to diabetes prevention in the US trials, the Indian Diabetes Prevention Program-2 (IDPP-2) trial, which randomized 497 participants with IGT to lifestyle modifications with pioglitazone versus lifestyle modifications with placebo, did not demonstrate a significant reduction in diabetes at 3 years’ follow-up, suggesting a possible ethnicity-related variation in the effect of the medication [49]. In 2011, the French and German medications regulatory agency withdrew pioglitazone from the market because of a potential increase in incidence of bladder cancer with the cumulative use of more than 28 g of pioglitazone. In the United States, the Food and Drug Administration is performing an extensive review of data and advises against the use of pioglitazone in patients with a history of bladder cancer.
In summary, TZDs demonstrated significant efficacy in preventing diabetes in many patients at risk, but their safety concerns, particularly the associated new onset of congestive heart failure and potential increased risk of bladder cancer, might outweigh this benefit.
Combination Metformin and Thiazolidinediones
As metformin and rosiglitazone both have preventive benefits in diabetes, and rosiglitazone is associated with numerous side effects at a higher dose, a combination of metformin and low-dose rosiglitazone was evaluated in in the CAnadian Normoglycemia Outcomes Evaluation (CANOE) trial [50]. A total of 207 patients with IGT were randomly assigned to receive combination metformin (500 mg twice daily) and rosiglitazone (2 mg daily) versus placebo for a median of 3.9 years. The combination therapy was associated with a 66% relative risk reduction of progression to diabetes.
Alpha-glucosidase Inhibitors
Alpha-glucosidase inhibitors are antidiabetic agents that slow oral carbohydrate intestinal absorption, subsequently improving postprandial hyperglycemia, which can eventually reduce glucose toxicity of pancreatic beta cells. In addition, they have been shown to improve insulin sensitivity in individuals with IGT [51] and have been found to exert a favorable protective effect in a prediabetic population [52]. In a multicenter placebo-controlled randomized trial, the Study to Prevent Non-Insulin Dependent Diabetes Mellitus (STOP-NIDDM), 1429 participants with IGT were randomly assigned to receive acarbose 100 mg 3 times a day or placebo for 3 years [53]. As expected, diabetes incidence was significantly decreased by 25% in the acarbose group (relative risk of 32.4% vs 41.5% in acarbose and placebo group, respectively), and acarbose significantly increased reversion to normal glucose tolerance (P < 0.0001). Furthermore, the use of acarbose was associated with a statistically significant 49% decrease in the rate of any cardiovascular event, highlighting the cardiovascular protective effect of improving postprandial hyperglycemia with acarbose. This study had many limitations: a high percentage of participants discontinued treatment (31% in the acarbose group and 19% in the placebo group), most likely related to increased gastrointestinal adverse effects of acarbose. In addition, the diabetes prevention effect does not seem to be sustained: during a 3-month wash-out period where all patients received placebo, incidence of diabetes in the initial intervention group was higher than in the initial placebo group.
In a Japanese multicenter randomized double-blind trial, 1780 patients with IGT were randomly assigned to receive the alpha-glucosidase inhibitor voglibose or placebo [54]. An interim analysis at 48 weeks revealed a significantly lower risk of progression to diabetes in the voglibose group.
Combination Metformin and Acarbose
In a 6-year multicenter British study, the Early Diabetes Intervention Trial (EDIT), 631 participants with IFG were randomly assigned, in a factorial design, to double-blind treatment with acarbose or placebo and simultaneously to metformin or placebo [55]. At 3 years, there was a nonsignificant risk reduction of 8% and 37% in progression to 2 successive fasting plasma glucose values of 140 mg/dL or more in the acarbose and metformin groups, respectively, but a significantly lower 2-hour OGTT glucose in the acarbose group and significantly lower FBG in the metformin group. Interestingly, at 6 years of follow-up, there was no significant difference in relative risk of progression to diabetes with acarbose, metformin, or combination therapy [56]. However, unlike metformin or combination therapy, acarbose was associated with a significant relative risk reduction of diabetes (0.66, P = 0.046) in the subgroup of patients with IGT at baseline, suggesting a possible differential protective effect of certain agents in patients with IGT or IFG.
Nateglinide
Nateglinide is a short-acting insulin secretagogue that is mostly used in the treatment of postprandial hyperglycemia in diabetic patients. The protective effect of nateglinide in a prediabetic population was examined in the NAVIGATOR study (the NAteglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research), a large prospective multinational, randomized, double-blind, placebo-controlled trial. Nateglinide (30–60 mg 3 times daily) and valsartan (80–160 mg daily) versus placebo were used in a 2×2 factorial design in 9306 participants with IGT and increased risk of cardiovascular events [57]. At 5 years, nateglinide did not reduce the cumulative incidence of diabetes or cardiovascular outcomes, when compared to placebo, whereas risk of hypoglycemia was significantly increased in the intervention group.
Liraglutide
Liraglutide is an injectable glucagon-like peptide-1 (GLP-1) receptor agonist used to treat T2DM, and recently approved as a weight-reducing agent at the dose of 3 mg injected subcutaneously. GLP-1 receptor agonists work by stimulating insulin secretion in a glucose-dependent manner, suppressing glucagon secretion, inducing satiety, and slowing gastric emptying. In the international double-blind SCALE (Satiety and Clinical Adiposity-Liraglutide Evidence) trial, 3731 nondiabetic patients, among whom 61.2% had prediabetes, were randomly assigned to liraglutide 3 mg subcutaneous injection daily or placebo, in addition to diet and exercise [58]. Liraglutide was associated with lower glucose levels on OGTT and lower A1C values at the end of the study (56 weeks), with this decrease especially prominent in prediabetic patients. Significantly fewer participants in the liraglutide group (4/2219) compared to the placebo group (14/1225) developed diabetes at 56 weeks, nearly all of whom (except for 1 in the placebo group) had prediabetes at the beginning of the study. Of note, the liraglutide group had a mean 8.4-kg weight reduction by week 56, compared to 2.8 kg in the placebo group.
Insulin
Insulin has also been investigated as a possible diabetes prevention agent, given the assumed protective effect insulin could exert on beta cell reserve. In the landmark international Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial, 12,537 participants (mean age 63.5 years) with cardiovascular risk factors plus IFG, IGT, or type 2 diabetes were randomly assigned to receive insulin glargine (with a target FBG ≤ 95 mg/dL) or standard care and were monitored for cardiovascular outcomes and other secondary endpoints including incidence of diabetes [59]. After a median follow-up of 6.2 years, and 3 months after discontinuation of therapy, among the 1456 participants without baseline diabetes, new diabetes was diagnosed in 30% of participants receiving glargine versus 35% of those receiving standard therapy. However, rates of severe hypoglycemia and modest weight gain were higher in the insulin group, calling in to question the benefit/risk balance with the use of basal insulin for diabetes prevention.
ACE Inhibitors and ARBs
A possible diabetes preventive effect was observed with renin-angiotensin system (RAS) blockade agents in secondary analysis of several hypertension trials, such as with ramipril in the Heart Outcomes Prevention Evaluation study, captopril (compared to diuretics and beta blockers) in the CAptopril Prevention Project, lisinopril (compared to amlodipine and chlorthalidone) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, losartan (compared to atenolol) in the Losartan Intervention For Endpoint reduction in hypertension study), and multiple other randomized controlled trials [60–64]. Therefore, 2 major trials were designed to examine, as a primary outcome, the effect of RAS inhibition on diabetes prevention in a population at risk. The DREAM trial randomly assigned, in a 2 × 2 factorial design, 5269 relatively healthy participants with IGT and/or IFG to rosiglitazone, ramipril, or placebo [65]. Although the use of ramipril at a dose of 15 mg daily for 3.5 years did not prevent diabetes significantly, it was associated with a 9%, nonsignificant decrease in new-onset of diabetes and a 16%, significant increase in regression of IFG and IGT to normoglycemia, as well as a significant decrease in OGTT 2-hour glucose level (135.1 vs 140.5 mg/dL) with no improvement in FBG.
Similarly, in the NAVIGATOR trial that examined the effect of nateglinide and valsartan on the prevention of diabetes in 9306 participants with IGT and increased risk of cardiovascular events, valsartan significantly but slightly reduced the incidence of diabetes at 5 years, by 14%, when compared to placebo (33% versus 37%, respectively), with no significant reduction in cardiovascular outcome [66]. Unlike in the DREAM study, the patients enrolled in the NAVIGATOR trial had established cardiovascular disease or cardiovascular risk factors and assumable elevated RAS activation level. This baseline population difference might explain the more significant effect of RAS inhibition in the NAVIGATOR trial.
Given the positive glycemic effect of ACE inhibitors and ARBs, their use should be encouraged in prediabetic patients when indicated for treatment of high blood pressure or cardiovascular disease. Different mechanisms could explain this favorable glycemic impact: inhibition of the post-receptor insulin signaling abnormalities, increased blood flow to the skeletal muscle facilitating insulin action, enhanced differentiation of pre-adipocytes into mature adipocytes, and increased pancreatic islet blood perfusion leading to appropriate insulin release and possible partial PPAR-γ activity [67].
Xenical
Xenical is a gastrointestinal lipase inhibitor approved for use for weight reduction and maintenance. A possible diabetes prevention benefit of xenical was initially suggested by a retrospective analysis of xenical treatment effects on obese patients with IGT [68]. This finding was subsequently confirmed by a multicenter randomized placebo-controlled study, XENical in the prevention of Diabetes in Obese Subjects (XENDOS), where 3305 obese subjects, with normal glucose tolerance or IGT were randomly assigned to either xenical 120 mg 3 times a day or placebo, in addition to lifestyle changes for all participants [69]. In the group of patients with IGT (694 subjects), xenical treatment was associated with a 45% risk reduction of progression to diabetes at 4 years (18.8% versus 28.8% in placebo), whereas participants with baseline normal glucose tolerance had no significant change in incidence of diabetes. On the other hand, weight reduction at 4 years was significantly greater in all patients who received xenical (5.8 kg in intervention group vs 3 kg in control group). The beneficial effect of xenical in diabetes prevention seems to be additive to the benefit of weight loss. As in many weight reduction trials, this study was limited by the high discontinuation rate in both groups (48% in xenical group and 66% in control group), probably related to insufficient clinical response.
Fibric Acid Derivatives (Bezafibrate)
Bezafibrate, a nonselective ligand/activator for PPAR-α, was found to reduce not only triglycerides, but also FPG, fructosamine, and A1C levels significantly in T2DM patients with hyperlipidemia [70]. Different mechanisms of glucose lowering have been suggested with bezafibrate: nonselective activation of PPAR-γ, improving insulin sensitivity, and enhancing glucose disposal in adipose tissue and skeletal muscles [71]. Furthermore, bezafibrate treatment was associated with decreased incidence of diabetes in patients with IFG and in obese non-diabetic patients with normal glycemic levels [72,73]. In a posthoc analysis of the Bezafibrate Infarction Prevention study, 303 patients with IFG received either 400 mg of bezafibrate daily or placebo [73]. Over a mean follow-up of 6.2 years, development of diabetes was less prevalent (54.4% vs 42.3%, relative risk reduction of 22%) and delayed (mean 10 months) in the bezafibrate group compared to placebo. Multivariate analysis identified bezafibrate as an independent predictor of decreased risk of new diabetes development, regardless of BMI and lipid profile.
Surgery
Over the past decade, bariatric surgery has become one of the most effective interventions for inducing and sustaining weight reduction in severely obese patients, leading to a significant benefit in diabetes prevention or remission. The Swedish Obese Subject Study is a large ongoing prospective nonrandomized cohort study that between 1987 and 2001 enrolled 4047 nondiabetic obese participants who underwent gastric surgery or were matched obese control, with diabetes incidence measured at 2, 10 and 15 years [74–76]. At 15 years, analysis of the available cohort of the initial group showed that T2DM developed in 392 of 1658 control participants and in 110 of 1771 bariatric-surgery participants, corresponding to incidence rates of 28.4 and 6.8 cases per 1000 person-years, respectively (P < 0.001). The treatment effects on the incidence of T2DM were at least as strong after 2 years and 10 years of follow-up as after 15 years. This effect was most prominent among the 591 patients who had IFG at baseline, with a number needed to treat as low as 1.3. The surgery group maintained an average 20-kg weight loss at 15 years.
In another study of the effects of bariatric surgery, 150 of 152 obese participants with IGT who underwent gastric bypass achieved and maintained a normal glycemic profile at 14 years of follow-up [77]. Similarly, in a follow-up of 136 obese participants with IGT, 109 of whom underwent bariatric surgery, 1 participant in the surgical group developed diabetes, as compared with 6 out of 27 in the control group [78]. In a meta-analysis including studies involving 22,094 patients who underwent bariatric surgery, 76.8% had complete resolution of their diabetes [79]. The rapid improvement of glycemic profile after bariatric surgery is thought to be due to oral intake restriction as well as acute hormonal changes related to the exclusion of the upper gastrointestinal tract (eg, incretin and ghrelin levels variations) [80].
Conclusions and Recommendations
The natural history of T2DM allows identification of patients at risk for diabetes and implementation of prevention strategies, which seems to be a public health need given the alarming increase in diabetes incidence. Indeed, the onset of T2DM is typically preceded by many years of beta cell dysfunction translating into carbohydrate metabolism abnormalities such as IFG and IGT, providing an excellent window of opportunity to identify persons at risk and prevent progression to diabetes. Numerous randomized controlled trials established lifestyle modifications, including dietary changes, moderate weight loss, and moderate intensity physical activity, as safe and effective interventions to prevent diabetes. This protective effect has been consistently shown to be sustained for more than 10 years after the initial intervention. Pharmacologic agents such as metformin, thiazolidinediones, alpha-glucosidase inhibitors, xenical, liraglutide, and insulin have also been associated with diabetes prevention in patients at risk. However, except for metformin, safety concerns or lack of durable efficacy or tolerability seem to outweigh their potential diabetes prevention benefit.
Given their favorable glycemic effect, RAS blockade and fibrates should be considered, when indicated, as reasonable treatment options for hypertension and hyperlipidemia in prediabetic patients. Bariatric surgery has been associated with a dramatic reduction in diabetes incidence in obese prediabetic patients and can be considered an alternative prevention measure in patients with severe obesity and prediabetes.
The recently updated ADA guidelines recommend referring patients with prediabetes to an intensive diet and physical activity behavioral counseling program; diet and activity goals should adhere to the tenets of the DPP, with a loss of 7% of body weight and at least 150 minutes of moderate physical activity (eg, brisk walking) per week [8]. Metformin therapy for diabetes prevention should be considered in patients with prediabetes, especially in those with BMI greater than 35 kg/m2, those younger than 60 years of age, women with history of gestational diabetes, and/or those with rapidly rising A1C despite lifestyle modifications. Monitoring for development of diabetes, at least annually, and screening for and treatment of modifiable cardiovascular risk factors are suggested in patients with prediabetes [8].
Many lessons have been learned through the studies of diabetes prevention interventions. The challenge that remains is how to apply these interventions, especially the lifestyle modifications, in real world medical practice, at both the individual and public health level.
Corresponding author: Jocelyne Karam, MD, 4802 10th Avenue, Brooklyn, NY 11219, [email protected].
Financial disclosures: None reported.
From the Maimonides Medical Center (Dr. Karam) and the SUNY Downstate Medical Center (Dr. Karam and Dr. McFarlane), Brooklyn, NY.
Abstract
- Objective. To discuss the epidemic of diabetes highlighting the natural history of the disease and the major clinical trials aimed at diabetes prevention in different prediabetic populations around the world.
- Results. Diabetes prevention studies have evaluated various interventions including lifestyle modifications, metformin, alpha-glucosidase inhibitors, thiazolidinediones, nateglinide, and xenical as well as the renin-angiotensin aldosterone system (RAS) inhibitors. Lifestyle modifications seem to be the safest, most effective, and most sustainable intervention to prevent diabetes. Except for metformin, the potential diabetes prevention benefits of the studied pharmacologic agents are limited by safety concerns or lack of durable efficacy or tolerability. RAS blockade and fibrates have a favorable glycemic effect, and, when indicated, are reasonable treatment options for hypertension and hyperlipidemia in prediabetic patients.
- Conclusion. As recommended by American Diabetes Association guidelines, patients with prediabetes should be referred to an intensive diet and physical activity behavioral counseling program; diet and activity goals include a loss of 7% of body weight and at least 150 minutes of moderate physical activity per week. Metformin therapy for diabetes prevention should be considered as well.
Key words: prediabetes; type 2 diabetes mellitus, diabetes prevention, lifestyle modifications.
Diabetes mellitus has reached pandemic proportions across the globe. The International Diabetes Federation (IDF) estimates that in 2015 around 415 million people, or 1 in 11 adults, had diabetes, compared to 285 million in 2010, with 5 million deaths, or 1 death every 6 seconds, occurring because of diabetes or diabetes complications [1]. In the United States, an estimated 29.1 million Americans, or 9.3% of the population, have diabetes, 27.8% of them undiagnosed [2]. The prevalence of diabetes increases significantly with age, affecting around 16.2% of American adults aged 45 to 64 years and 25.9% of adults aged 65 years or older [2]. The Centers for Disease Control and Prevention (CDC) estimates that, with current trends, as many as 1 in 3 American adults could have diabetes by 2050 [3].
Type 2 diabetes mellitus (T2DM) accounts for the majority of prevalent and newly diagnosed diabetes in the world, and is strongly linked to overweight and inactivity in adults [4]. T2DM is increasingly being diagnosed in pediatric patients, in whom type 1 diabetes has historically been predominant; it now accounts for approximately 30% of newly diagnosed diabetes in children aged 10 to 19 years, exceeding 50% in certain ethnicities such as non-Hispanic black and American Indian/Alaska Native children [2].
These alarming trends have spurred significant research and public efforts aimed at reducing the prevalence of diabetes by preventing T2DM. Indeed, insulin resistance and abnormal carbohydrate metabolism progress over many years prior to the diagnosis of diabetes and manifest with different clinical and biochemical features. Both the pathophysiology and the natural history of T2DM offer clinicians an opportunity to identify patients at risk for developing the disease and to implement prevention strategies. This article outlines the risk factors and diagnostic criteria for prediabetes, describes the studies that have explored diabetes prevention through lifestyle changes, pharmacotherapy, or surgery, and reviews recommendations for managing patients at risk.
Risk Factors and Screening for T2DM
The American Diabetes Association (ADA) recommends screening all adults for prediabetes by assessing for diabetes risk factors [8]. Glucose testing is recommended in individuals aged 45 years or older, and should be considered in adults of any age who are overweight or obese (body mass index [BMI] ≥ 25 kg/m2 or ≥ 23 kg/m2 in Asian Americans) and have 1 or more additional risk factors for diabetes. Testing also should be considered in children and adolescents who are overweight or obese and who have 2 or more additional risk factors. If tests are normal, repeat testing carried out at a minimum of 3-year intervals is suggested [8].
Prediabetes
Abnormalities in glucose metabolism progress along a continuum through various stages before T2DM develops. Years before the development of overt diabetes, and especially in the presence of excessive visceral fat, cellular sensitivity to insulin gradually decreases, leading to a compensatory increased insulin secretion [9]. With time, and under continuous increased demand, pancreatic beta cell function declines and ultimately fails to overcome insulin resistance and maintain a normal glucose metabolism, resulting in prediabetes followed by the development of diabetes. This early beta cell dysfunction was illustrated by the decreased beta cell volume observed on autopsy of obese patients with IFG or T2DM, when compared to obese individuals with normal glucose tolerance [10]. It is estimated that around 40% to 70% of beta cell function is already lost by the time diabetes is clinically diagnosed. This relatively slow pathophysiologic process allows the identification of at-risk patients well before their blood glucose levels reach the diabetic diagnostic thresholds, and therefore presents an opportunity for prevention.
Diagnostic Criteria
The ADA guidelines released in 2003 define prediabetes as IFG (fasting blood glucose [FBG] levels of 100–125 mg/dL), IGT (glucose levels of 140–199 mg/dL at 2 hours during an oral glucose tolerance test [OGTT] following an oral load of 75 g of dextrose), or both. Additionally, hemoglobin A1C (A1C) was introduced as a diagnostic tool for prediabetes in 2010, with values between 5.7% and 6.4% indicating prediabetes [8]. Most of these thresholds were chosen due to their association with increased rates of complications, notably retinopathy and cardiovascular disease.
A combined report from the World Health Organization (WHO) and the IDF published in 2006 defined intermediate hyperglycemia as IFG, but with a higher cutoff for FBG (110–125 mg/dL) than the ADA’s definition, and/or IGT (2-hour OGTT glucose level of 140–199 mg/dL) [11]. The rationale for a higher cut-point for IFG is the concern about the increased prevalence of IFG and its impact on individuals and health systems and the more favorable cardiovascular risk profile and decreased risk of progression to diabetes in the group of patients with FBG of 100 to 110 mg/dL when compared to the group with FBG of 110 to 125 mg/dL. The report does not recommend the use of A1C in the diagnosis of diabetes or intermediate hyperglycemia because of a lack of global consistency and the potential for other factors that can be prevalent in some developing countries, such as hemoglobinopathies and anemia, to interfere with the assay.
Prevalence and Progression to Diabetes
According to CDC data from 2014, up to 86 million American adults, more than 1 in 3, have prediabetes, and 9 out of 10 of these individuals are undiagnosed [2]. It is estimated that approximately 25% of people diagnosed with either IFG or IGT progress to diabetes mellitus over a 3- to 5-year period [12]. If observed for longer periods, most prediabetic persons will probably develop diabetes. The highest rate of progression to diabetes is observed in patients with both IFG and IGT, older age, overweight, or other diabetic risk factors.
Complications
In addition to increasing the risk for progression to diabetes, prediabetes is independently associated with microvascular and macrovascular complications and increased risk of death, prior to the actual onset of diabetes. The DECODE study demonstrated significantly increased mortality in 2766 individuals with IGT after 7 years of follow-up, when compared to normoglycemic patients; this effect was more prominent in participants with IGT than in participants with IFG [13]. In the Australian Diabetes, Obesity and Lifestyle Study, IFG was found to be an independent predictor for cardiovascular mortality after adjustment for age, sex, and other traditional cardiovascular risk factors [14].
Similarly, a recent meta-analysis demonstrated that the presence of IFG was significantly associated with future risk for coronary heart disease (CHD), with the risk increase starting when fasting plasma glucose was as low as 100 mg/dL; however, this finding may have been confounded by the presence of undetected IGT or other cardiovascular risk factors [15]. Another recent systematic review of 53 prospective cohort studies with 1,611,339 participants showed that prediabetes (IFG or IGT) was associated with an increased risk of composite cardiovascular disease, CHD, stroke, and all-cause mortality [16].
The association between retinopathy and prediabetes has been described in multiple reports and this association has helped guide authors on selected thresholds for diagnosis of prediabetes. For example, in 1 study, the incidence of retinopathy in individuals with IGT was 12% among Pima Indians [17]. Similarly, in a follow-up study of the Diabetes Prevention Program, 8% of prediabetic participants who remained nondiabetics had evidence of retinopathy [18].
Neuropathy also has been observed in prediabetes. A noninvasive neurologic evaluation of individuals with IGT revealed subclinical neural dysfunction suggestive of cardiovascular autonomic neuropathy [19]. At the clinical level, a study that evaluated 100 patients with chronic idiopathic axonal neuropathy of unknown etiology found IFG in 36 and IGT in 38 patients, underscoring the role of abnormal glucose metabolism in these patients [20].
Nephropathy may also be more prevalent in those with prediabetes. In a 1999–2006 National Health and Nutrition Examination Survey analysis, the adjusted prevalence of chronic kidney disease, defined by estimated glomerular filtration rate (eGFR) of 15 to 59 mL/min per 1.73 m2 or albumin-creatinine ratio ≥ 30 mg/g, was 17.1% in individuals with IFG, compared to 11.8% in individuals with normal fasting glucose [21].
Lifestyle Modifications
The alarming rapid increase in the prevalence of T2DM has been linked to a parallel rising epidemic of overweight, obesity, and lack of physical activity. Therefore, lifestyle changes aiming at weight reduction seemed to be a natural individual and public health strategy to prevent diabetes, and such strategies have been the focus of many randomized controlled trials around the world. As anticipated, weight loss, exercise, and diet have all been shown, separately or in combination, to be effective in decreasing the incidence of T2DM in high-risk patients [22–27]. Furthermore, and well beyond the benefit observed during the trials, follow-up studies revealed a sustained reduction of diabetes incidence in intervention groups several years after cessation of the intervention [28–32] (Table 2).
The Da Quing Diabetes Prevention Study (DQDPS), published in 1997, is one of the earliest prospective diabetes prevention trials [22]. This 6-year study conducted in 33 clinics in China from 1986 through 1992 included 577 participants with IGT who were randomly assigned to 1 of 4 groups: (1) diet (high vegetables, low sugar/alcohol) only, (2) exercise, (3) diet plus exercise, and (4) standard of care. At 6 years, diabetes incidence was significantly reduced by 46% in the exercise group, 31% in the diet group, and 42% in the diet plus exercise group compared to standard care. In 2006, 14 years after the end of the trial and 20 years after the initial enrollment, the cumulative incidence of diabetes was significantly lower in the intervention group at 80%, compared to 93% in the control group, and the annual incidence of diabetes was 7% and 11%, respectively, with a 43% lower incidence of diabetes over the 20-year period in the combination lifestyle changes group [28]. The preventive benefit of lifestyle changes persisted 2 decades after the initial randomization despite the standardization of treatment for all groups over the 14 years following the study, suggesting a strong and longitudinal preventive effect of the initial lifestyle modifications. In a follow-up study of the DQDPS conducted in 2009, at 23 years of follow-up, the cumulative incidences of cardiovascular mortality and all-cause mortality were significantly lower in the intervention group (11.9% versus 19.6%, and 28.1% versus 38.4%, respectively), highlighting the long-term clinical benefits of lifestyle intervention in patients with IGT [29].
Similarly, the Finnish Diabetes Prevention Study (FDPS), published in 2001, enrolled 522 middle-aged overweight participants with IGT [23]. The participants randomly assigned to the intervention group received individualized counseling designed to reduce weight, decrease total intake of fat and saturated fat, increase intake of fiber, and increase physical activity. The control group received standard therapy. At 4 years of follow-up, the cumulative incidence of diabetes was 11% in the intervention group and 23% in the control group, with a statistically significant 58% reduction in risk for progression to diabetes. A follow-up of the FDPS was published in 2006 [31]. Participants who did not progress to diabetes in the initial 4-year study were further followed for a median of 3 years. Interestingly, lifestyle changes were maintained by the intervention group participants despite the cessation of the individual counseling, leading to a 36% relative reduction in diabetes incidence during the post-intervention follow-up period alone (4.6 vs 7.2 per 100 person-years, P = 0.041) and a 43% cumulative diabetes incidence reduction over the 7-year follow-up, demonstrating, one more time, the sustained efficacy of lifestyle changes.
In the United States, the Diabetes Prevention Program (DPP) trial is a landmark NIH-sponsored multicenter randomized controlled trial published in 2002, and one of the largest diabetes prevention studies with lifestyle changes to date [24]. A total of 3234 participants with prediabetes, defined as an IFG or IGT, were randomly assigned to an intensive lifestyle modification program, metformin 850 mg twice daily, or matching placebo. Lifestyle changes included a low-fat (< 25% of caloric intake), 1200- to 1800-calorie diet and exercise for 150 minutes a week, with a 7% body weight reduction goal and a very well structured curriculum and professional support group. The study was discontinued early (at 3 years) as the data demonstrated the superiority of lifestyle changes, with a 58% reduction in diabetes incidence in the lifestyle intervention group and a 31% reduction in the metformin group when compared to placebo (cumulative incidence of diabetes at 3 years of 28.9%, 21.7 %, and 14.4% in the placebo, metformin, and lifestyle intervention groups, respectively). Lifestyle changes were significantly more effective than metformin and were consistently effective in men and women across age, BMI, and ethnic groups.
The DPPOS (DPP Outcome Study) was a 10-year follow-up of the DPP study published in 2009 where all participants were offered group-implemented lifestyle changes and were followed for an additional 5.7 years [32]. Unlike the Finnish follow-up study, diabetes incidence was similar in the 3 treatment groups in the follow-up period. However, the cumulative incidence of diabetes remained significantly the lowest in the original lifestyle group, with a 34% cumulative risk reduction in the lifestyle group and an 18% reduction in the metformin group at 10 years when compared to placebo. Interestingly, unlike most other studies of weight-reducing interventions, in the DPPOS, patients in the lifestyle changes and metformin groups maintained weight loss at 10 years’ follow-up.
In Japan, a diabetes prevention study assigned 458 male participants with IGT to a standard intervention group or an intensive intervention group receiving detailed lifestyle modification counseling every 3 to 4 months during hospital visits [25]. The cumulative 4-year incidence of diabetes was 9.3% in the control group versus 3.0% in the intervention group, and the reduction in diabetes risk was 67.4% (P < 0.001), with body weight reductions of 0.39 kg and 2.18 kg, respectively (P < 0.001). Of note, participants with higher FBG at baseline developed diabetes at a higher rate than those with lower values. This study suggested that lifestyle change counseling conducted in an outpatient clinic setting can be very effective in preventing diabetes.
Indian adults are thought to be more insulin resistant at a younger age and at a lower BMI than Caucasians. To assess whether the DPP findings can be replicated in an Indian population, the Indian Diabetes Prevention Program (IDPP) trial randomized a total of 531 participants with IGT to 4 groups: control, lifestyle modification, metformin, and lifestyle modifications with metformin [26]. The 3-year cumulative incidences of diabetes were 55.0%, 39.3%, 40.5%, and 39.5%, respectively, showing again a significant relative reduction in progression to diabetes of 28.5% with lifestyle changes, 26.4% with metformin, and 28.2% with both lifestyle changes and metformin, as compared with the control group.
In a Japanese unmasked, multicenter, randomized controlled trial published in 2011, 641 overweight adults with IFG were randomized to a frequent intervention group, receiving individual counseling and support for lifestyle modifications 9 times over 36 months, or a control group, receiving counseling 4 times over the same period. The 3-year cumulative incidence of T2DM was significantly lower in the frequent intervention group than in the control group (12.2% vs 16.6%) [27]. Interestingly, in a posthoc subgroup analysis, the protective effect was more prominent in patients with underlying associated IGT or elevated A1C, but was not observed in patients with isolated IFG, suggesting a possible prognostic value of an additional A1C or oral glucose tolerance test in individuals with IFG.
Diet
The diet followed in the major diabetes prevention trials discussed above has typically been a weight-reducing diet with decreased fat intake (eg, DPP, Finnish trial) and increased fiber intake (eg, Da Quing, DPP, Finnish trials). However, there has been more emphasis recently on the importance of the quality rather than the quantity of fats in preventing diabetes. For example, in a Spanish study, a non–calorie-restricted traditional Mediterranean diet, enriched with high-fat foods of vegetable origin (olive oil, nuts) decreased the incidence of diabetes by 52% in individuals at high cardiovascular risk after a median follow-up of 4.0 years, and in the absence of significant changes in body weight or physical activity among the groups [33]. These findings were reproduced by other studies. A recent meta-analysis examining the relation between intake of fruits and vegetables and the incidence of diabetes revealed that higher intake of fruit, especially berries, and green, leafy vegetables, yellow vegetables, cruciferous vegetables, or their fiber is associated with a lower risk of T2DM [34].
Exercise
Exercise is thought to improve insulin sensitivity and promote peripheral glucose uptake in normal individuals. Long-term moderate exercise, similar to the exercise recommended in DPP and FDPS, results in increased translocation of insulin-responsive glucose transporter (GLUT-4) from intracellular stores to the cell surface, facilitating glucose uptake [35]. A systematic review of 10 prospective cohort studies published in 2007 showed that, after adjustment for BMI, moderate-intensity physical activity was significantly associated with reduced diabetes incidence [36]. In the FDPS, participants who achieved at least 4 hours of exercise per week had a significant 80% decrease in incidence of diabetes, and this effect was observed even in the group that did not lose weight [23]. In the DQDPS, the greatest reduction in diabetes incidence was observed in the exercise group [22].
In a recent NIH-funded trial designed to examine the relative contribution of exercise alone to the overall beneficial effect of lifestyle changes in the DPP study, a total of 237 adults with IFG were randomly assigned to 4 different groups: low-amount moderate intensity exercise (similar to exercise followed in DPP), high-amount moderate intensity exercise, high-amount vigorous intensity exercise, and a combination of diet, weight loss, and low-amount moderate exercise. Only the diet and exercise group experienced a decrease in fasting glucose, whereas similar improvements in glucose tolerance were observed in both the diet and exercise group and the high-amount moderate-intensity exercise group, suggesting that such an exercise regimen may be as effective as a more intensive multicomponent approach involving diet, exercise, and weight loss for preventing diabetes [37].
Weight Loss
Weight reduction in prediabetic individuals has been consistently associated with reduced incidence of diabetes. Furthermore, the amount of weight loss needed to achieve this benefit seems to be relatively modest and a realistic goal to set for patients. Indeed, in the DPP trial, an average weight loss of only 5.6 kg was associated with a 58% lower incidence of diabetes [24]. Moreover, on further analysis of the DPP trial, and among weight, diet, and exercise, diabetes prevention correlated most strongly with weight loss, with an estimated 16% diabetes risk reduction for every single kilogram of weight reduction [38]. Similarly, within the same lifestyle intervention group in the FDPS, the participants who were able to achieve an initial body weight loss greater than 5% at 1 year had a nearly 70% relative risk reduction in progression to diabetes, when compared to their peers in the intervention group who had less or no weight loss [23].
In summary, numerous randomized controlled studies from various populations have proved that lifestyle modifications, including healthy diet, moderate weight loss, and moderate-intensity exercise, represent a very effective strategy to prevent diabetes in patients at risk, mostly patients with IGT, and this protective effect seems to be sustained over time.
Pharmacologic Interventions
Metformin
Metformin is an antidiabetic agent that works mostly at the liver site by suppressing hepatic glucose production and inhibiting production and oxidation of free fatty acids (FFA), thereby reducing FFA-induced insulin resistance and promoting peripheral glucose uptake [39]. This effect has the potential of preserving beta cell function by reducing the demand for insulin secretion.
In the DPP trial, metformin, although generally less effective than lifestyle changes, was associated with a significant 31% reduction in diabetes incidence (cumulative incidence of 22% in metformin group vs 29% in placebo group) and significant weight reduction (average of 2 kg) [24]. Further analysis of the DPP results showed that metformin efficacy, compared to placebo, was greater in patients who were younger, had higher BMI, and had higher FBG levels. In addition, a DPP substudy of 350 women with history of gestational diabetes and IGT revealed that this group of women, who had a higher risk of progression to diabetes (71% at 3 years) when compared to women with no history of gestational diabetes, despite similar baseline glucose levels, had similar diabetes risk reduction of 50% with both metformin and lifestyle changes [40].
In the IDPP study, both lifestyle changes and metformin reduced significantly and similarly the incidence of diabetes in adults with IGT, with no observed added benefit from combining both interventions [26]. It has not been clear, however, how much of this effect of metformin is a result of pharmacologic properties masking hyperglycemia or a true protective and preventive effect. In a washout study in which 1274 DPP participants who did not progress to diabetes underwent an OGTT after 1 to 2 weeks of discontinuing metformin or placebo, the incidence of diabetes was still reduced by 25% in the metformin group, after the washout period, compared to a 31% risk reduction in the primary DPP analysis, suggesting a partially sustained rather than temporary effect of metformin [41]. In the DPPOS long-term follow-up study, metformin (850 mg twice daily as tolerated) was continued in the group initially assigned to metformin in addition to lifestyle counseling [32]. Although the progression to diabetes was similar in all groups during the 5.7-year follow-up period, the cumulative incidence of diabetes at 10 years was still reduced in the metformin group by 18% when compared to control group. Furthermore, the weight loss associated with metformin was also interestingly sustained at 10 years. A meta-analysis echoed this beneficial effect of metformin observed in the DPP trial, reporting a relative risk reduction of new-onset diabetes of 40% with the use of metformin [42].
In summary, metformin has been shown to be effective in preventing diabetes in patients at risk, especially persons with younger age, higher BMI, and history of gestational diabetes and in native Asian Indians. The protective effect of metformin seems to be sustained over the long term in follow-up studies.
Thiazolidinediones
Thiazolidinediones (TZDs) are antidiabetic agents that have been evaluated in diabetes prevention trials. TZDs are peroxisome proliferator-activated gamma receptor (PPAR-γ) agonists that work by augmenting conversion of preadipocytes to adipocytes, which in turn increase adiponectin levels, promoting insulin sensitivity [43]. In addition to their antihyperglycemic properties, TZDs are thought to have a direct protective effect on beta cells, potentially translating into prevention and delay of diabetes [44].
The first study to demonstrate diabetes prevention with a TZD was the TRIPOD study (Troglitazone in Prevention of Diabetes), in which 266 Hispanic women with a history of gestational diabetes were randomly assigned to troglitazone or placebo [45]. Troglitazone use was significantly associated with reduction of progression to diabetes at 1.5-year follow-up when compared to placebo (relative risk reduction of 55%), with a decrease of endogenous insulin requirement at 3 months of therapy and sustained benefit after discontinuation of the TZD, suggesting an effect on beta cell preservation.
Moreover, troglitazone was an investigational drug in the DPP trial from 1996 to 1998, at which time it was discontinued because of associated fatal liver failure in a DPP participant. In the DPP trial, troglitazone was asso-ciated with a remarkable 75% decrease in progression to diabetes at 1 year. Troglitazone was withdrawn from the US market in 2000 because of its association with severe hepatotoxicity.
The international DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medications) trial randomly assigned more than 5000 participants with IFG and/or IGT to rosiglitazone, ramipril, or placebo in a 2 × 2 factorial design [46]. In participants receiving rosiglitazone, the risk for progression to diabetes was reduced by 60% and the likelihood of regression to normoglycemia was increased by 71% when compared to placebo. However, the use of rosiglitazone was associated with an increased risk of new-onset congestive heart failure and a mean weight gain of 2.2 kg, thought to reflect increased subcutaneous gluteal fat deposition, with an observed decreased waist-to-hip ratio.
Interestingly, in a passive follow-up of the DREAM study conducted a median 1.6 years after the end of the trial and 4.3 years after randomization, participants treated with rosiglitazone had a 39% lower incidence of diabetes compared to placebo participants, and 17% more of them regressed from prediabetes to normoglycemia [47]. Nonetheless, there was no difference between the 2 groups when the analysis was restricted to the passive follow-up period, suggesting a time-limited exposure to rosiglitazone reduces the longer-term incidence of diabetes by likely delaying but not reversing the underlying disease process.
The third large trial assessing the efficacy of a TZD in preventing diabetes was the Actos Now for the prevention of diabetes (ACT NOW) trial, which was a randomized, double-blinded study that assigned 602 patients with IGT to pioglitazone 45 mg daily or placebo [48]. Over a median follow-up of 2.6 years, pioglitazone was associated with a 72% lower annual rate of progression to diabetes (2.1% compared to 7.6 % in placebo group), and a higher rate of conversion to normal glucose tolerance (48%). In addition, pioglitazone had favorable effects on fasting and 2-hour blood glucose, A1C level, diastolic blood pressure, carotid intima thickness, and HDL cholesterol. As in the DREAM trial, an increased incidence of edema and weight gain was observed with pioglitazone.
Unlike the strong evidence supporting TZDs as an approach to diabetes prevention in the US trials, the Indian Diabetes Prevention Program-2 (IDPP-2) trial, which randomized 497 participants with IGT to lifestyle modifications with pioglitazone versus lifestyle modifications with placebo, did not demonstrate a significant reduction in diabetes at 3 years’ follow-up, suggesting a possible ethnicity-related variation in the effect of the medication [49]. In 2011, the French and German medications regulatory agency withdrew pioglitazone from the market because of a potential increase in incidence of bladder cancer with the cumulative use of more than 28 g of pioglitazone. In the United States, the Food and Drug Administration is performing an extensive review of data and advises against the use of pioglitazone in patients with a history of bladder cancer.
In summary, TZDs demonstrated significant efficacy in preventing diabetes in many patients at risk, but their safety concerns, particularly the associated new onset of congestive heart failure and potential increased risk of bladder cancer, might outweigh this benefit.
Combination Metformin and Thiazolidinediones
As metformin and rosiglitazone both have preventive benefits in diabetes, and rosiglitazone is associated with numerous side effects at a higher dose, a combination of metformin and low-dose rosiglitazone was evaluated in in the CAnadian Normoglycemia Outcomes Evaluation (CANOE) trial [50]. A total of 207 patients with IGT were randomly assigned to receive combination metformin (500 mg twice daily) and rosiglitazone (2 mg daily) versus placebo for a median of 3.9 years. The combination therapy was associated with a 66% relative risk reduction of progression to diabetes.
Alpha-glucosidase Inhibitors
Alpha-glucosidase inhibitors are antidiabetic agents that slow oral carbohydrate intestinal absorption, subsequently improving postprandial hyperglycemia, which can eventually reduce glucose toxicity of pancreatic beta cells. In addition, they have been shown to improve insulin sensitivity in individuals with IGT [51] and have been found to exert a favorable protective effect in a prediabetic population [52]. In a multicenter placebo-controlled randomized trial, the Study to Prevent Non-Insulin Dependent Diabetes Mellitus (STOP-NIDDM), 1429 participants with IGT were randomly assigned to receive acarbose 100 mg 3 times a day or placebo for 3 years [53]. As expected, diabetes incidence was significantly decreased by 25% in the acarbose group (relative risk of 32.4% vs 41.5% in acarbose and placebo group, respectively), and acarbose significantly increased reversion to normal glucose tolerance (P < 0.0001). Furthermore, the use of acarbose was associated with a statistically significant 49% decrease in the rate of any cardiovascular event, highlighting the cardiovascular protective effect of improving postprandial hyperglycemia with acarbose. This study had many limitations: a high percentage of participants discontinued treatment (31% in the acarbose group and 19% in the placebo group), most likely related to increased gastrointestinal adverse effects of acarbose. In addition, the diabetes prevention effect does not seem to be sustained: during a 3-month wash-out period where all patients received placebo, incidence of diabetes in the initial intervention group was higher than in the initial placebo group.
In a Japanese multicenter randomized double-blind trial, 1780 patients with IGT were randomly assigned to receive the alpha-glucosidase inhibitor voglibose or placebo [54]. An interim analysis at 48 weeks revealed a significantly lower risk of progression to diabetes in the voglibose group.
Combination Metformin and Acarbose
In a 6-year multicenter British study, the Early Diabetes Intervention Trial (EDIT), 631 participants with IFG were randomly assigned, in a factorial design, to double-blind treatment with acarbose or placebo and simultaneously to metformin or placebo [55]. At 3 years, there was a nonsignificant risk reduction of 8% and 37% in progression to 2 successive fasting plasma glucose values of 140 mg/dL or more in the acarbose and metformin groups, respectively, but a significantly lower 2-hour OGTT glucose in the acarbose group and significantly lower FBG in the metformin group. Interestingly, at 6 years of follow-up, there was no significant difference in relative risk of progression to diabetes with acarbose, metformin, or combination therapy [56]. However, unlike metformin or combination therapy, acarbose was associated with a significant relative risk reduction of diabetes (0.66, P = 0.046) in the subgroup of patients with IGT at baseline, suggesting a possible differential protective effect of certain agents in patients with IGT or IFG.
Nateglinide
Nateglinide is a short-acting insulin secretagogue that is mostly used in the treatment of postprandial hyperglycemia in diabetic patients. The protective effect of nateglinide in a prediabetic population was examined in the NAVIGATOR study (the NAteglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research), a large prospective multinational, randomized, double-blind, placebo-controlled trial. Nateglinide (30–60 mg 3 times daily) and valsartan (80–160 mg daily) versus placebo were used in a 2×2 factorial design in 9306 participants with IGT and increased risk of cardiovascular events [57]. At 5 years, nateglinide did not reduce the cumulative incidence of diabetes or cardiovascular outcomes, when compared to placebo, whereas risk of hypoglycemia was significantly increased in the intervention group.
Liraglutide
Liraglutide is an injectable glucagon-like peptide-1 (GLP-1) receptor agonist used to treat T2DM, and recently approved as a weight-reducing agent at the dose of 3 mg injected subcutaneously. GLP-1 receptor agonists work by stimulating insulin secretion in a glucose-dependent manner, suppressing glucagon secretion, inducing satiety, and slowing gastric emptying. In the international double-blind SCALE (Satiety and Clinical Adiposity-Liraglutide Evidence) trial, 3731 nondiabetic patients, among whom 61.2% had prediabetes, were randomly assigned to liraglutide 3 mg subcutaneous injection daily or placebo, in addition to diet and exercise [58]. Liraglutide was associated with lower glucose levels on OGTT and lower A1C values at the end of the study (56 weeks), with this decrease especially prominent in prediabetic patients. Significantly fewer participants in the liraglutide group (4/2219) compared to the placebo group (14/1225) developed diabetes at 56 weeks, nearly all of whom (except for 1 in the placebo group) had prediabetes at the beginning of the study. Of note, the liraglutide group had a mean 8.4-kg weight reduction by week 56, compared to 2.8 kg in the placebo group.
Insulin
Insulin has also been investigated as a possible diabetes prevention agent, given the assumed protective effect insulin could exert on beta cell reserve. In the landmark international Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial, 12,537 participants (mean age 63.5 years) with cardiovascular risk factors plus IFG, IGT, or type 2 diabetes were randomly assigned to receive insulin glargine (with a target FBG ≤ 95 mg/dL) or standard care and were monitored for cardiovascular outcomes and other secondary endpoints including incidence of diabetes [59]. After a median follow-up of 6.2 years, and 3 months after discontinuation of therapy, among the 1456 participants without baseline diabetes, new diabetes was diagnosed in 30% of participants receiving glargine versus 35% of those receiving standard therapy. However, rates of severe hypoglycemia and modest weight gain were higher in the insulin group, calling in to question the benefit/risk balance with the use of basal insulin for diabetes prevention.
ACE Inhibitors and ARBs
A possible diabetes preventive effect was observed with renin-angiotensin system (RAS) blockade agents in secondary analysis of several hypertension trials, such as with ramipril in the Heart Outcomes Prevention Evaluation study, captopril (compared to diuretics and beta blockers) in the CAptopril Prevention Project, lisinopril (compared to amlodipine and chlorthalidone) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, losartan (compared to atenolol) in the Losartan Intervention For Endpoint reduction in hypertension study), and multiple other randomized controlled trials [60–64]. Therefore, 2 major trials were designed to examine, as a primary outcome, the effect of RAS inhibition on diabetes prevention in a population at risk. The DREAM trial randomly assigned, in a 2 × 2 factorial design, 5269 relatively healthy participants with IGT and/or IFG to rosiglitazone, ramipril, or placebo [65]. Although the use of ramipril at a dose of 15 mg daily for 3.5 years did not prevent diabetes significantly, it was associated with a 9%, nonsignificant decrease in new-onset of diabetes and a 16%, significant increase in regression of IFG and IGT to normoglycemia, as well as a significant decrease in OGTT 2-hour glucose level (135.1 vs 140.5 mg/dL) with no improvement in FBG.
Similarly, in the NAVIGATOR trial that examined the effect of nateglinide and valsartan on the prevention of diabetes in 9306 participants with IGT and increased risk of cardiovascular events, valsartan significantly but slightly reduced the incidence of diabetes at 5 years, by 14%, when compared to placebo (33% versus 37%, respectively), with no significant reduction in cardiovascular outcome [66]. Unlike in the DREAM study, the patients enrolled in the NAVIGATOR trial had established cardiovascular disease or cardiovascular risk factors and assumable elevated RAS activation level. This baseline population difference might explain the more significant effect of RAS inhibition in the NAVIGATOR trial.
Given the positive glycemic effect of ACE inhibitors and ARBs, their use should be encouraged in prediabetic patients when indicated for treatment of high blood pressure or cardiovascular disease. Different mechanisms could explain this favorable glycemic impact: inhibition of the post-receptor insulin signaling abnormalities, increased blood flow to the skeletal muscle facilitating insulin action, enhanced differentiation of pre-adipocytes into mature adipocytes, and increased pancreatic islet blood perfusion leading to appropriate insulin release and possible partial PPAR-γ activity [67].
Xenical
Xenical is a gastrointestinal lipase inhibitor approved for use for weight reduction and maintenance. A possible diabetes prevention benefit of xenical was initially suggested by a retrospective analysis of xenical treatment effects on obese patients with IGT [68]. This finding was subsequently confirmed by a multicenter randomized placebo-controlled study, XENical in the prevention of Diabetes in Obese Subjects (XENDOS), where 3305 obese subjects, with normal glucose tolerance or IGT were randomly assigned to either xenical 120 mg 3 times a day or placebo, in addition to lifestyle changes for all participants [69]. In the group of patients with IGT (694 subjects), xenical treatment was associated with a 45% risk reduction of progression to diabetes at 4 years (18.8% versus 28.8% in placebo), whereas participants with baseline normal glucose tolerance had no significant change in incidence of diabetes. On the other hand, weight reduction at 4 years was significantly greater in all patients who received xenical (5.8 kg in intervention group vs 3 kg in control group). The beneficial effect of xenical in diabetes prevention seems to be additive to the benefit of weight loss. As in many weight reduction trials, this study was limited by the high discontinuation rate in both groups (48% in xenical group and 66% in control group), probably related to insufficient clinical response.
Fibric Acid Derivatives (Bezafibrate)
Bezafibrate, a nonselective ligand/activator for PPAR-α, was found to reduce not only triglycerides, but also FPG, fructosamine, and A1C levels significantly in T2DM patients with hyperlipidemia [70]. Different mechanisms of glucose lowering have been suggested with bezafibrate: nonselective activation of PPAR-γ, improving insulin sensitivity, and enhancing glucose disposal in adipose tissue and skeletal muscles [71]. Furthermore, bezafibrate treatment was associated with decreased incidence of diabetes in patients with IFG and in obese non-diabetic patients with normal glycemic levels [72,73]. In a posthoc analysis of the Bezafibrate Infarction Prevention study, 303 patients with IFG received either 400 mg of bezafibrate daily or placebo [73]. Over a mean follow-up of 6.2 years, development of diabetes was less prevalent (54.4% vs 42.3%, relative risk reduction of 22%) and delayed (mean 10 months) in the bezafibrate group compared to placebo. Multivariate analysis identified bezafibrate as an independent predictor of decreased risk of new diabetes development, regardless of BMI and lipid profile.
Surgery
Over the past decade, bariatric surgery has become one of the most effective interventions for inducing and sustaining weight reduction in severely obese patients, leading to a significant benefit in diabetes prevention or remission. The Swedish Obese Subject Study is a large ongoing prospective nonrandomized cohort study that between 1987 and 2001 enrolled 4047 nondiabetic obese participants who underwent gastric surgery or were matched obese control, with diabetes incidence measured at 2, 10 and 15 years [74–76]. At 15 years, analysis of the available cohort of the initial group showed that T2DM developed in 392 of 1658 control participants and in 110 of 1771 bariatric-surgery participants, corresponding to incidence rates of 28.4 and 6.8 cases per 1000 person-years, respectively (P < 0.001). The treatment effects on the incidence of T2DM were at least as strong after 2 years and 10 years of follow-up as after 15 years. This effect was most prominent among the 591 patients who had IFG at baseline, with a number needed to treat as low as 1.3. The surgery group maintained an average 20-kg weight loss at 15 years.
In another study of the effects of bariatric surgery, 150 of 152 obese participants with IGT who underwent gastric bypass achieved and maintained a normal glycemic profile at 14 years of follow-up [77]. Similarly, in a follow-up of 136 obese participants with IGT, 109 of whom underwent bariatric surgery, 1 participant in the surgical group developed diabetes, as compared with 6 out of 27 in the control group [78]. In a meta-analysis including studies involving 22,094 patients who underwent bariatric surgery, 76.8% had complete resolution of their diabetes [79]. The rapid improvement of glycemic profile after bariatric surgery is thought to be due to oral intake restriction as well as acute hormonal changes related to the exclusion of the upper gastrointestinal tract (eg, incretin and ghrelin levels variations) [80].
Conclusions and Recommendations
The natural history of T2DM allows identification of patients at risk for diabetes and implementation of prevention strategies, which seems to be a public health need given the alarming increase in diabetes incidence. Indeed, the onset of T2DM is typically preceded by many years of beta cell dysfunction translating into carbohydrate metabolism abnormalities such as IFG and IGT, providing an excellent window of opportunity to identify persons at risk and prevent progression to diabetes. Numerous randomized controlled trials established lifestyle modifications, including dietary changes, moderate weight loss, and moderate intensity physical activity, as safe and effective interventions to prevent diabetes. This protective effect has been consistently shown to be sustained for more than 10 years after the initial intervention. Pharmacologic agents such as metformin, thiazolidinediones, alpha-glucosidase inhibitors, xenical, liraglutide, and insulin have also been associated with diabetes prevention in patients at risk. However, except for metformin, safety concerns or lack of durable efficacy or tolerability seem to outweigh their potential diabetes prevention benefit.
Given their favorable glycemic effect, RAS blockade and fibrates should be considered, when indicated, as reasonable treatment options for hypertension and hyperlipidemia in prediabetic patients. Bariatric surgery has been associated with a dramatic reduction in diabetes incidence in obese prediabetic patients and can be considered an alternative prevention measure in patients with severe obesity and prediabetes.
The recently updated ADA guidelines recommend referring patients with prediabetes to an intensive diet and physical activity behavioral counseling program; diet and activity goals should adhere to the tenets of the DPP, with a loss of 7% of body weight and at least 150 minutes of moderate physical activity (eg, brisk walking) per week [8]. Metformin therapy for diabetes prevention should be considered in patients with prediabetes, especially in those with BMI greater than 35 kg/m2, those younger than 60 years of age, women with history of gestational diabetes, and/or those with rapidly rising A1C despite lifestyle modifications. Monitoring for development of diabetes, at least annually, and screening for and treatment of modifiable cardiovascular risk factors are suggested in patients with prediabetes [8].
Many lessons have been learned through the studies of diabetes prevention interventions. The challenge that remains is how to apply these interventions, especially the lifestyle modifications, in real world medical practice, at both the individual and public health level.
Corresponding author: Jocelyne Karam, MD, 4802 10th Avenue, Brooklyn, NY 11219, [email protected].
Financial disclosures: None reported.
1. International Diabetes Federation. Diabetes facts and figures. www.idf.org/about-diabetes/facts-figures. Accessed on January 29, 2017.
2. Centers for Disease Control and Prevention. National diabetes statistics report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed on January 29, 2017.
3. Centers for Disease Control and Prevention. Number of Americans with diabetes projected to double or triple by 2050. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed on January 29, 2017.
4. World Health Organization (WHO). Diabetes fact sheet. No. 312. November 2016. www.who.int/mediacentre/factsheets/fs312/en/. Accessed on January 29, 2017.
5. Karam JG, McFarlane SI. Update on the prevention of type 2 diabetes. Curr Diab Rep 2011;11:56–63.
6. Menke A, Rust KF, Fradkin J, et al. Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States: a series of cross-sectional studies. Ann Intern Med 2014;161:328–85.
7. Ford ES, Li C, Sattar N . Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008;31:1898–904.
8. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care 2017;40(Suppl. 1).
9. Kruszynska YT, Olefsky JM. Cellular and molecular mechanisms of non-insulin dependent diabetes mellitus. J Investig Med 1996;44:413–28..
10. Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–10.
11. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO and IDF Consultation. 2006. http://apps.who.int/iris/bitstream/10665/
43588/1/9241594934_eng.pdf. Accessed on February 1, 2017.
12. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. 2001 JAMA 2003;289:76–9.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999;354:617–21.
14. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–7.
15. Xu T, Liu W, Cai X, et al. Risk of coronary heart disease in different criterion of impaired fasting glucose: a meta-analysis. Medicine 2015;94:e1740.
16. Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953.
17. Nagi DK, Pettitt DJ, Bennett PH, et al. Diabetic retinopathy assessed by fundus photography in Pima Indians with impaired glucose tolerance and NIDDM. Diabet Med 1997;14:449 –56.
18. Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–44
19. Putz Z, Tabák AG, Tóth N, et al. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care 2009;32:181–3.
20. Hoffman-Snyder C, Smith BE, Ross MA, et al. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006;63:1075–9.
21. Plantinga LC, Crews DC, Coresh J, et al; CDC CKD Surveillance Team. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol 2010;5:673–82.
22. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–44.
23. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
24. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
25. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–62.
26. Ramachandran A, Snehalatha C, Mary S, et al; Indian Diabetes Prevention Programme (IDPP).The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97.
27. Saito T, Watanabe M, Nishida J, et al; Zensharen Study for Prevention of Lifestyle Diseases Group. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–60.
28. Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–9.
29. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–80.
30. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–80.
31. Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:673–9.
32. Diabetes Prevention Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–86.
33. Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14–19.
34. Wang P, Fang J, Gao Z, et al. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta‐analysis. J Diabetes Investig 2016;7:56–69.
35. Devlin JT. Effects of exercise on insulin sensitivity in humans. Diabetes Care 1992;15:1690–3.
36. Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30:744–52.
37. Slentz C, Bateman L, Willis L, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia 2016;59:2088–98.
38. Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–7.
39. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 2002;137:25–33.
40. Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–9.
41. Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003;26:977–80.
42. Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008;121:149–57.
43. El-Atat F, Nicasio J, Clarke L, et al. Beneficial cardiovascular effects of thiazolidinediones. Therapy 2005;2:113–19.
44. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803.
45. Azen SP, Peters RK, Berkowitz K. TRIPOD (Troglitazone In the Prevention Of Diabtes): a randomized placebo-controlled study of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials 1998;19:217–31.
46. Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105.
47. DREAM Investigators, Gerstein HC, Mohan V, Avezum A, et al. Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia 2011;54:487–95.
48. DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–15.
49. Ramachandran A, Snehalatha C, Mary S, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia 2009;52:1019–26.
50 Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010;376:103–11.
51. Chiasson JL, Josse RG, Leiter LA, et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care 1996;19:1190–3.
52. Van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev 2006(4):CD005061.
53. Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–7.
54. Kawamori R, Tajima N, Iwamoto Y, et al. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009;373:1607–14.
55. Holman RR, North BV, Tunbridge FK. Possible prevention of type 2 diabetes with acarbose or metformin. Diabetes 2000;49:Suppl 1:A111.
56. Holman RR, Blackwell L, Stratton IM et al. Six-year results from the Early Diabetes Intervention Trial. Diabet Med 2003;20(Suppl 2):15.
57. Holman RR, Haffner SM, McMurray JJ, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1463–76.
58. Pi-Sunyer X, Astrup A, Fujioka K et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22.
59. ORIGIN Trial Investigators, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–28.
60. Yusuf S, Gerstein H, Hoogwerf B, et al. Ramipril and the development of diabetes. JAMA 2001;286:1882–5.
61. Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999;353:611–6.
62. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–97.
63. Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:1004–10.
64. Gillespie EL, White CM, Kardas M, et al. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care 2005;28:2261–6.
65. Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–62.
66. McMurray JJ, Holman RR, Haffner SM, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1477–90.
67. McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol 2003;91(12A):30H–37H.
68. Heymsfield SB, Segal KR, Hauptman J, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Arch Intern Med 2000;160:1321–6.
69. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–61.
70. Rovellini A, Sommariva D, Branchi A, et al. Effects of slow release bezafibrate on the lipid pattern and on blood glucose of type 2 diabetic patients with hyperlipidaemia. Pharmacol Res 1992;25:237–45.
71. Tenenbaum A, Motro M, Fisman EZ. Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons. Cardiovasc Diabetol 2005;4:14.
72. Tenenbaum A, Motro M, Fisman EZ, et al. Effect of bezafibrate on incidence of type 2 diabetes mellitus in obese patients. Eur Heart J 2005;26:2032–8.
73. Tenenbaum A, Motro M, Fisman EZ, et al. Peroxisome proliferator-activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation 2004;109:2197–202.
74. Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93.
75. Sjostrom CD. Surgery as an intervention for obesity. Results from the Swedish obese subjects study. Growth Horm IGF Res 2003;13 Suppl A:S22–26.
76. Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704.
77. Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–50.
78. Long SD, O’Brien K, MacDonald KG Jr, et al. Weight loss in severely obese subjects prevents the progression of impaired glucose tolerance to type II diabetes. A longitudinal interventional study. Diabetes Care 1994;17:372–5.
79. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–37.
80. Tejirian T, Jensen C, Dutson E. Bariatric surgery and type 2 diabetes mellitus: surgically induced remission. J Diabetes Sci Technol 2008;2:685–91.
1. International Diabetes Federation. Diabetes facts and figures. www.idf.org/about-diabetes/facts-figures. Accessed on January 29, 2017.
2. Centers for Disease Control and Prevention. National diabetes statistics report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed on January 29, 2017.
3. Centers for Disease Control and Prevention. Number of Americans with diabetes projected to double or triple by 2050. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed on January 29, 2017.
4. World Health Organization (WHO). Diabetes fact sheet. No. 312. November 2016. www.who.int/mediacentre/factsheets/fs312/en/. Accessed on January 29, 2017.
5. Karam JG, McFarlane SI. Update on the prevention of type 2 diabetes. Curr Diab Rep 2011;11:56–63.
6. Menke A, Rust KF, Fradkin J, et al. Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States: a series of cross-sectional studies. Ann Intern Med 2014;161:328–85.
7. Ford ES, Li C, Sattar N . Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008;31:1898–904.
8. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care 2017;40(Suppl. 1).
9. Kruszynska YT, Olefsky JM. Cellular and molecular mechanisms of non-insulin dependent diabetes mellitus. J Investig Med 1996;44:413–28..
10. Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–10.
11. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO and IDF Consultation. 2006. http://apps.who.int/iris/bitstream/10665/
43588/1/9241594934_eng.pdf. Accessed on February 1, 2017.
12. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. 2001 JAMA 2003;289:76–9.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999;354:617–21.
14. Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–7.
15. Xu T, Liu W, Cai X, et al. Risk of coronary heart disease in different criterion of impaired fasting glucose: a meta-analysis. Medicine 2015;94:e1740.
16. Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953.
17. Nagi DK, Pettitt DJ, Bennett PH, et al. Diabetic retinopathy assessed by fundus photography in Pima Indians with impaired glucose tolerance and NIDDM. Diabet Med 1997;14:449 –56.
18. Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–44
19. Putz Z, Tabák AG, Tóth N, et al. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care 2009;32:181–3.
20. Hoffman-Snyder C, Smith BE, Ross MA, et al. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006;63:1075–9.
21. Plantinga LC, Crews DC, Coresh J, et al; CDC CKD Surveillance Team. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol 2010;5:673–82.
22. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–44.
23. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
24. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
25. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–62.
26. Ramachandran A, Snehalatha C, Mary S, et al; Indian Diabetes Prevention Programme (IDPP).The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97.
27. Saito T, Watanabe M, Nishida J, et al; Zensharen Study for Prevention of Lifestyle Diseases Group. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–60.
28. Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–9.
29. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–80.
30. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–80.
31. Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:673–9.
32. Diabetes Prevention Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–86.
33. Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14–19.
34. Wang P, Fang J, Gao Z, et al. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta‐analysis. J Diabetes Investig 2016;7:56–69.
35. Devlin JT. Effects of exercise on insulin sensitivity in humans. Diabetes Care 1992;15:1690–3.
36. Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30:744–52.
37. Slentz C, Bateman L, Willis L, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia 2016;59:2088–98.
38. Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–7.
39. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 2002;137:25–33.
40. Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–9.
41. Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003;26:977–80.
42. Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008;121:149–57.
43. El-Atat F, Nicasio J, Clarke L, et al. Beneficial cardiovascular effects of thiazolidinediones. Therapy 2005;2:113–19.
44. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803.
45. Azen SP, Peters RK, Berkowitz K. TRIPOD (Troglitazone In the Prevention Of Diabtes): a randomized placebo-controlled study of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials 1998;19:217–31.
46. Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105.
47. DREAM Investigators, Gerstein HC, Mohan V, Avezum A, et al. Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia 2011;54:487–95.
48. DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–15.
49. Ramachandran A, Snehalatha C, Mary S, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia 2009;52:1019–26.
50 Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010;376:103–11.
51. Chiasson JL, Josse RG, Leiter LA, et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care 1996;19:1190–3.
52. Van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev 2006(4):CD005061.
53. Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–7.
54. Kawamori R, Tajima N, Iwamoto Y, et al. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009;373:1607–14.
55. Holman RR, North BV, Tunbridge FK. Possible prevention of type 2 diabetes with acarbose or metformin. Diabetes 2000;49:Suppl 1:A111.
56. Holman RR, Blackwell L, Stratton IM et al. Six-year results from the Early Diabetes Intervention Trial. Diabet Med 2003;20(Suppl 2):15.
57. Holman RR, Haffner SM, McMurray JJ, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1463–76.
58. Pi-Sunyer X, Astrup A, Fujioka K et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22.
59. ORIGIN Trial Investigators, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–28.
60. Yusuf S, Gerstein H, Hoogwerf B, et al. Ramipril and the development of diabetes. JAMA 2001;286:1882–5.
61. Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999;353:611–6.
62. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–97.
63. Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:1004–10.
64. Gillespie EL, White CM, Kardas M, et al. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care 2005;28:2261–6.
65. Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–62.
66. McMurray JJ, Holman RR, Haffner SM, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1477–90.
67. McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol 2003;91(12A):30H–37H.
68. Heymsfield SB, Segal KR, Hauptman J, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Arch Intern Med 2000;160:1321–6.
69. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–61.
70. Rovellini A, Sommariva D, Branchi A, et al. Effects of slow release bezafibrate on the lipid pattern and on blood glucose of type 2 diabetic patients with hyperlipidaemia. Pharmacol Res 1992;25:237–45.
71. Tenenbaum A, Motro M, Fisman EZ. Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons. Cardiovasc Diabetol 2005;4:14.
72. Tenenbaum A, Motro M, Fisman EZ, et al. Effect of bezafibrate on incidence of type 2 diabetes mellitus in obese patients. Eur Heart J 2005;26:2032–8.
73. Tenenbaum A, Motro M, Fisman EZ, et al. Peroxisome proliferator-activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation 2004;109:2197–202.
74. Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93.
75. Sjostrom CD. Surgery as an intervention for obesity. Results from the Swedish obese subjects study. Growth Horm IGF Res 2003;13 Suppl A:S22–26.
76. Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704.
77. Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–50.
78. Long SD, O’Brien K, MacDonald KG Jr, et al. Weight loss in severely obese subjects prevents the progression of impaired glucose tolerance to type II diabetes. A longitudinal interventional study. Diabetes Care 1994;17:372–5.
79. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–37.
80. Tejirian T, Jensen C, Dutson E. Bariatric surgery and type 2 diabetes mellitus: surgically induced remission. J Diabetes Sci Technol 2008;2:685–91.
Is MRI Safe in Patients with Implanted Cardiac Devices?
Study Overview
Objective. To assess the risks associated with magnetic resonance imaging (MRI) in patients with a pacemaker or implantable cardioverter-defibrillator (ICD) that is “non–MRI-conditional.”
Design. Prospective cohort study using the multicenter MagnaSafe Registry.
Setting and participants. Patients were included in the registry if they were 18 years of age or older and had a non–MRI-conditional pacemaker or ICD generator, from any manufacturer, that was implanted after 2001, with leads from any manufacturer, and if the patient’s physician determined that nonthoracic MRI at 1.5 tesla was clinically indicated. Exclusion criteria included an abandoned or inactive lead that could not be interrogated, an MRI-conditional pacemaker, a device implanted in a nonthoracic location, or a device with a battery that was near the end of its battery life. In addition, pacing-dependent patients with an ICD were also excluded.
Main outcome measures. The primary outcomes of the study were death, generator or lead failure requiring immediate replacement, loss of capture (for pacing-dependent patients with pacemakers), new-onset arrhythmia, and partial or full generator electrical reset. The secondary outcomes were changes in device settings including: a battery voltage decrease of 0.04V or more, a pacing lead threshold increase of 0.5V or more, a P-wave amplitude decrease of 50% or more, an R-wave amplitude decrease of 25% or more and of 50% or more, a pacing lead impedance change of 50 ohms or more, and a high-voltage (shock) lead impedance change of 3 ohms or more.
Main results. Between April 2009 and April 2014, clinically indicated nonthoracic MRI was performed in a total of 1000 pacemaker cases (818 patients) and 500 ICD cases (428 patients) across 19 centers in the United States. The majority (75%) of the MRI examinations were performed on the brain or the spine. The mean time patients spent within the magnetic field was 44 minutes. Four patients reported symptoms of generator-site discomfort; one patient with an ICD was removed from the scanner when a sensation of heating was described at the site of the generator implanted and did not complete the examination.
Regarding primary outcomes, no deaths, lead failures, losses of capture, or ventricular arrhythmias occurred during MRI. One ICD device was left in the active mode for anti-tachycardia therapy (a protocol violation) and the generator could not be interrogated after MRI and required immediate replacement. Four patients had atrial fibrillation and 2 patients had atrial flutter during or immediately after the MRI. All 6 patients returned to sinus rhythm within 49 hours after MRI. No ventricular arrhythmias were noted. There were also 6 cases of partial generator electrical reset with no clinical significance.
Regarding secondary outcomes, a decrease of 50% or more in P-wave amplitude was detected in 0.9% of pacemaker leads and in 0.3% of ICD leads; a decrease of 25% or more in R-wave amplitude was detected in 3.9% of pacemaker leads and in 1.5% of ICD leads, and a decrease of 50% or more in R-wave amplitude was detected in no pacemaker leads and in 0.2% of ICD leads. An increase in pacing lead threshold of 0.5 V or more was detected in 0.7% of pacemaker leads and in 0.8% of ICD leads. A pacing lead impedance change of 50 ohms or more was noted in 3.3% of pacemakers and in 4.2% of ICDs.
Conclusion. Device or lead failure did not occur in any patient with a non–MRI-conditional pacemaker or ICD who underwent clinically indicated nonthoracic MRI at 1.5 tesla when patients were appropriately screened and had the cardiac device reprogrammed in accordance with the protocol. Substantial changes in device settings were infrequent and did not result in clinical adverse events.
Commentary
It is estimated that 2 million people in the United States and an additional 6 million worldwide have an implanted non–MRI-conditional cardiac pacemaker or ICD [1]. At least half of patients with such devices are predicted to have a clinical indication for MRI during their lifetime after device implantation [2]. The use of MRI poses concerns due to the potential for magnetic field–induced cardiac lead heating, which could result in myocardial thermal injury and detrimental changes in pacing properties [3,4].
In this study, Russo and colleagues assessed the risks for patients with a non-MRI-conditional pacemaker or ICD receiving an MRI scan using a pre-scanning protocol. If the patient was asymptomatic and had an intrinsic heart rate of at least 40 beats per minute, the device was programmed to a no-pacing mode (ODO or OVO). Symptomatic patients or those with an intrinsic heart rate of less than 40 beats per minute were determined to be pacing-dependent, and the device was reprogrammed to an asynchronous pacing mode (DOO or VOO). All bradycardia and tachycardia therapies were inactivated before the MRI. Based on this standardized protocol, no major adverse outcomes occurred. All pacemaker or ICD device were reprogrammed in accordance with the pre-specified protocol except one case where the ICD device was left in the active mode for anti-tachycardia therapy (a protocol violation) and the generator could not be interrogated after MRI and required immediate replacement. In addition to patient safety, the authors also measure the functionality of the devices pre-MRI and post-MRI. One of these measurements were battery voltage changes, a small decrease was noted for both pacemakers and ICDs as expected. The radiofrequency energy generated during MRI scanning creates a temporary decrease in battery voltage, which had resolved in all pacemaker cases although some ICD voltage decreases of 0.04 V or more had not resolved by the end of the 6 month post-MRI follow-up.
Several limitations exist. The study registry included devices and leads from different manufacturers, but did not report outcomes by manufacturer. While overall it appears to be safe to conduct an MRI study for patients who have non–MRI-conditional devices, this study did not provide enough information for patients younger than 18 years of age, patients who required repeat MRI studies, MRI examinations of the thorax, or higher MRI field strengths—the newer 3 tesla high-resolution MRI machines.
Applications for Clinical Practice
This multicenter prospective cohort study provides strong evidence that patients with a non–MRI-conditional pacemaker or defibrillator can receive nonthoracic MRI studies at 1.5 tesla when a straight pre-scanning device interrogation is performed per the standardized protocol.
—Ka Ming Gordon Ngai, MD, MPH
1. Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med 2011;155:415–24.
2. Kalin R, Stanton MS. Current clnical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28:326–8.
3. Beinart R, Nazarian S. Effects of external electrical and magnetic fields on pacemakers and defibrillators: from engineering principles to clinical practice. Circulation 2013; 128:2799–809.
4. Luechinger R, Zeijlemaker VA, Pedersen EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J 2005;26:376–83.
Study Overview
Objective. To assess the risks associated with magnetic resonance imaging (MRI) in patients with a pacemaker or implantable cardioverter-defibrillator (ICD) that is “non–MRI-conditional.”
Design. Prospective cohort study using the multicenter MagnaSafe Registry.
Setting and participants. Patients were included in the registry if they were 18 years of age or older and had a non–MRI-conditional pacemaker or ICD generator, from any manufacturer, that was implanted after 2001, with leads from any manufacturer, and if the patient’s physician determined that nonthoracic MRI at 1.5 tesla was clinically indicated. Exclusion criteria included an abandoned or inactive lead that could not be interrogated, an MRI-conditional pacemaker, a device implanted in a nonthoracic location, or a device with a battery that was near the end of its battery life. In addition, pacing-dependent patients with an ICD were also excluded.
Main outcome measures. The primary outcomes of the study were death, generator or lead failure requiring immediate replacement, loss of capture (for pacing-dependent patients with pacemakers), new-onset arrhythmia, and partial or full generator electrical reset. The secondary outcomes were changes in device settings including: a battery voltage decrease of 0.04V or more, a pacing lead threshold increase of 0.5V or more, a P-wave amplitude decrease of 50% or more, an R-wave amplitude decrease of 25% or more and of 50% or more, a pacing lead impedance change of 50 ohms or more, and a high-voltage (shock) lead impedance change of 3 ohms or more.
Main results. Between April 2009 and April 2014, clinically indicated nonthoracic MRI was performed in a total of 1000 pacemaker cases (818 patients) and 500 ICD cases (428 patients) across 19 centers in the United States. The majority (75%) of the MRI examinations were performed on the brain or the spine. The mean time patients spent within the magnetic field was 44 minutes. Four patients reported symptoms of generator-site discomfort; one patient with an ICD was removed from the scanner when a sensation of heating was described at the site of the generator implanted and did not complete the examination.
Regarding primary outcomes, no deaths, lead failures, losses of capture, or ventricular arrhythmias occurred during MRI. One ICD device was left in the active mode for anti-tachycardia therapy (a protocol violation) and the generator could not be interrogated after MRI and required immediate replacement. Four patients had atrial fibrillation and 2 patients had atrial flutter during or immediately after the MRI. All 6 patients returned to sinus rhythm within 49 hours after MRI. No ventricular arrhythmias were noted. There were also 6 cases of partial generator electrical reset with no clinical significance.
Regarding secondary outcomes, a decrease of 50% or more in P-wave amplitude was detected in 0.9% of pacemaker leads and in 0.3% of ICD leads; a decrease of 25% or more in R-wave amplitude was detected in 3.9% of pacemaker leads and in 1.5% of ICD leads, and a decrease of 50% or more in R-wave amplitude was detected in no pacemaker leads and in 0.2% of ICD leads. An increase in pacing lead threshold of 0.5 V or more was detected in 0.7% of pacemaker leads and in 0.8% of ICD leads. A pacing lead impedance change of 50 ohms or more was noted in 3.3% of pacemakers and in 4.2% of ICDs.
Conclusion. Device or lead failure did not occur in any patient with a non–MRI-conditional pacemaker or ICD who underwent clinically indicated nonthoracic MRI at 1.5 tesla when patients were appropriately screened and had the cardiac device reprogrammed in accordance with the protocol. Substantial changes in device settings were infrequent and did not result in clinical adverse events.
Commentary
It is estimated that 2 million people in the United States and an additional 6 million worldwide have an implanted non–MRI-conditional cardiac pacemaker or ICD [1]. At least half of patients with such devices are predicted to have a clinical indication for MRI during their lifetime after device implantation [2]. The use of MRI poses concerns due to the potential for magnetic field–induced cardiac lead heating, which could result in myocardial thermal injury and detrimental changes in pacing properties [3,4].
In this study, Russo and colleagues assessed the risks for patients with a non-MRI-conditional pacemaker or ICD receiving an MRI scan using a pre-scanning protocol. If the patient was asymptomatic and had an intrinsic heart rate of at least 40 beats per minute, the device was programmed to a no-pacing mode (ODO or OVO). Symptomatic patients or those with an intrinsic heart rate of less than 40 beats per minute were determined to be pacing-dependent, and the device was reprogrammed to an asynchronous pacing mode (DOO or VOO). All bradycardia and tachycardia therapies were inactivated before the MRI. Based on this standardized protocol, no major adverse outcomes occurred. All pacemaker or ICD device were reprogrammed in accordance with the pre-specified protocol except one case where the ICD device was left in the active mode for anti-tachycardia therapy (a protocol violation) and the generator could not be interrogated after MRI and required immediate replacement. In addition to patient safety, the authors also measure the functionality of the devices pre-MRI and post-MRI. One of these measurements were battery voltage changes, a small decrease was noted for both pacemakers and ICDs as expected. The radiofrequency energy generated during MRI scanning creates a temporary decrease in battery voltage, which had resolved in all pacemaker cases although some ICD voltage decreases of 0.04 V or more had not resolved by the end of the 6 month post-MRI follow-up.
Several limitations exist. The study registry included devices and leads from different manufacturers, but did not report outcomes by manufacturer. While overall it appears to be safe to conduct an MRI study for patients who have non–MRI-conditional devices, this study did not provide enough information for patients younger than 18 years of age, patients who required repeat MRI studies, MRI examinations of the thorax, or higher MRI field strengths—the newer 3 tesla high-resolution MRI machines.
Applications for Clinical Practice
This multicenter prospective cohort study provides strong evidence that patients with a non–MRI-conditional pacemaker or defibrillator can receive nonthoracic MRI studies at 1.5 tesla when a straight pre-scanning device interrogation is performed per the standardized protocol.
—Ka Ming Gordon Ngai, MD, MPH
Study Overview
Objective. To assess the risks associated with magnetic resonance imaging (MRI) in patients with a pacemaker or implantable cardioverter-defibrillator (ICD) that is “non–MRI-conditional.”
Design. Prospective cohort study using the multicenter MagnaSafe Registry.
Setting and participants. Patients were included in the registry if they were 18 years of age or older and had a non–MRI-conditional pacemaker or ICD generator, from any manufacturer, that was implanted after 2001, with leads from any manufacturer, and if the patient’s physician determined that nonthoracic MRI at 1.5 tesla was clinically indicated. Exclusion criteria included an abandoned or inactive lead that could not be interrogated, an MRI-conditional pacemaker, a device implanted in a nonthoracic location, or a device with a battery that was near the end of its battery life. In addition, pacing-dependent patients with an ICD were also excluded.
Main outcome measures. The primary outcomes of the study were death, generator or lead failure requiring immediate replacement, loss of capture (for pacing-dependent patients with pacemakers), new-onset arrhythmia, and partial or full generator electrical reset. The secondary outcomes were changes in device settings including: a battery voltage decrease of 0.04V or more, a pacing lead threshold increase of 0.5V or more, a P-wave amplitude decrease of 50% or more, an R-wave amplitude decrease of 25% or more and of 50% or more, a pacing lead impedance change of 50 ohms or more, and a high-voltage (shock) lead impedance change of 3 ohms or more.
Main results. Between April 2009 and April 2014, clinically indicated nonthoracic MRI was performed in a total of 1000 pacemaker cases (818 patients) and 500 ICD cases (428 patients) across 19 centers in the United States. The majority (75%) of the MRI examinations were performed on the brain or the spine. The mean time patients spent within the magnetic field was 44 minutes. Four patients reported symptoms of generator-site discomfort; one patient with an ICD was removed from the scanner when a sensation of heating was described at the site of the generator implanted and did not complete the examination.
Regarding primary outcomes, no deaths, lead failures, losses of capture, or ventricular arrhythmias occurred during MRI. One ICD device was left in the active mode for anti-tachycardia therapy (a protocol violation) and the generator could not be interrogated after MRI and required immediate replacement. Four patients had atrial fibrillation and 2 patients had atrial flutter during or immediately after the MRI. All 6 patients returned to sinus rhythm within 49 hours after MRI. No ventricular arrhythmias were noted. There were also 6 cases of partial generator electrical reset with no clinical significance.
Regarding secondary outcomes, a decrease of 50% or more in P-wave amplitude was detected in 0.9% of pacemaker leads and in 0.3% of ICD leads; a decrease of 25% or more in R-wave amplitude was detected in 3.9% of pacemaker leads and in 1.5% of ICD leads, and a decrease of 50% or more in R-wave amplitude was detected in no pacemaker leads and in 0.2% of ICD leads. An increase in pacing lead threshold of 0.5 V or more was detected in 0.7% of pacemaker leads and in 0.8% of ICD leads. A pacing lead impedance change of 50 ohms or more was noted in 3.3% of pacemakers and in 4.2% of ICDs.
Conclusion. Device or lead failure did not occur in any patient with a non–MRI-conditional pacemaker or ICD who underwent clinically indicated nonthoracic MRI at 1.5 tesla when patients were appropriately screened and had the cardiac device reprogrammed in accordance with the protocol. Substantial changes in device settings were infrequent and did not result in clinical adverse events.
Commentary
It is estimated that 2 million people in the United States and an additional 6 million worldwide have an implanted non–MRI-conditional cardiac pacemaker or ICD [1]. At least half of patients with such devices are predicted to have a clinical indication for MRI during their lifetime after device implantation [2]. The use of MRI poses concerns due to the potential for magnetic field–induced cardiac lead heating, which could result in myocardial thermal injury and detrimental changes in pacing properties [3,4].
In this study, Russo and colleagues assessed the risks for patients with a non-MRI-conditional pacemaker or ICD receiving an MRI scan using a pre-scanning protocol. If the patient was asymptomatic and had an intrinsic heart rate of at least 40 beats per minute, the device was programmed to a no-pacing mode (ODO or OVO). Symptomatic patients or those with an intrinsic heart rate of less than 40 beats per minute were determined to be pacing-dependent, and the device was reprogrammed to an asynchronous pacing mode (DOO or VOO). All bradycardia and tachycardia therapies were inactivated before the MRI. Based on this standardized protocol, no major adverse outcomes occurred. All pacemaker or ICD device were reprogrammed in accordance with the pre-specified protocol except one case where the ICD device was left in the active mode for anti-tachycardia therapy (a protocol violation) and the generator could not be interrogated after MRI and required immediate replacement. In addition to patient safety, the authors also measure the functionality of the devices pre-MRI and post-MRI. One of these measurements were battery voltage changes, a small decrease was noted for both pacemakers and ICDs as expected. The radiofrequency energy generated during MRI scanning creates a temporary decrease in battery voltage, which had resolved in all pacemaker cases although some ICD voltage decreases of 0.04 V or more had not resolved by the end of the 6 month post-MRI follow-up.
Several limitations exist. The study registry included devices and leads from different manufacturers, but did not report outcomes by manufacturer. While overall it appears to be safe to conduct an MRI study for patients who have non–MRI-conditional devices, this study did not provide enough information for patients younger than 18 years of age, patients who required repeat MRI studies, MRI examinations of the thorax, or higher MRI field strengths—the newer 3 tesla high-resolution MRI machines.
Applications for Clinical Practice
This multicenter prospective cohort study provides strong evidence that patients with a non–MRI-conditional pacemaker or defibrillator can receive nonthoracic MRI studies at 1.5 tesla when a straight pre-scanning device interrogation is performed per the standardized protocol.
—Ka Ming Gordon Ngai, MD, MPH
1. Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med 2011;155:415–24.
2. Kalin R, Stanton MS. Current clnical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28:326–8.
3. Beinart R, Nazarian S. Effects of external electrical and magnetic fields on pacemakers and defibrillators: from engineering principles to clinical practice. Circulation 2013; 128:2799–809.
4. Luechinger R, Zeijlemaker VA, Pedersen EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J 2005;26:376–83.
1. Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med 2011;155:415–24.
2. Kalin R, Stanton MS. Current clnical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28:326–8.
3. Beinart R, Nazarian S. Effects of external electrical and magnetic fields on pacemakers and defibrillators: from engineering principles to clinical practice. Circulation 2013; 128:2799–809.
4. Luechinger R, Zeijlemaker VA, Pedersen EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J 2005;26:376–83.
Communicating Prognostic Information in Oncology
Study Overview
Objective. To assess the prevalence and determinants of patient–oncologist discordance in opinion of prognosis, and evaluate how often patients are aware of this discordance.
Design. Cross-sectional study.
Setting and participants. The study included 236 adult patients with advanced cancer and their 38 oncologists at academic and community oncology practices in Rochester, New York, and Sacramento, California. Inpatients and those already enrolled in hospice were excluded.
Main outcome measures. Patients and their oncologists independently reported their ratings of 2-year survival probability on a postindex visit multiple-choice questionnaire (response options included 100%, about 90%, about 75%, about 50%, about 25%, about 10%, and 0%). Prognostic discordance was defined as a more than 1 category difference between the patient and physician prognostic ratings. All patients were asked to report how they believed their oncologist would rate their 2-year survival probability. Those who correctly perceived their oncologist’s rating of their prognosis (within 1 category) were defined as knowing their oncologist’s opinion, and the rest were defined as not knowing their oncologist’s opinion. This distinction was used to categorize patients whose self-rating of their prognosis was discordant from their oncologist’s rating as either knowingly discordant or unknowingly discordant. Patient characteristics including age, sex, race/ethnicity, education, income, aggressiveness of cancer, self-efficacy with health care communication, recall of prognostic discussion with the oncologist, and end-of-life treatment preferences were evaluated as potential determinants of prognostic discordance.
Main results. 68% of patients rated their 2-year survival probability discordantly from their oncologists. Among these, 96% rated their prognosis more optimistically than their oncologists, and 89% were unaware that their opinions differed from that of their oncologists. Prognostic discordance was more common among nonwhite compared to white patients (95% versus 65%, P = 0.03). The prevalence of prognostic discordance did not significantly differ based on the other patient characteristics studied. Among patients whose prognostic ratings were discordant from their oncologist’s, 99% reported that they wanted to be involved in treatment decision making, and 70% were interested in involving palliative care when the end of life became near.
Conclusion. Patient–oncologist discordance about prognosis was common, particularly among nonwhite patients. In cases of prognostic discordance, patients rarely knew that their opinion differed from that of their oncologist, suggesting a lack of successful communication of prognostic information.
Commentary
Prior studies have noted that patients with advanced cancer perceive prognosis more optimistically than their physicians [1–3]. In a large national prospective observational study, the majority of patients receiving chemotherapy for metastatic (stage IV) lung or colorectal cancer inaccurately believed that chemotherapy was likely to be curative, potentially compromising their ability to make informed treatment decisions [4]. In the present study by Gramling et al, the authors confirm the observation that patients are more optimistic about prognosis than their oncologists, and furthermore demonstrate that most patients are unaware of the discrepancy, suggesting a failure of communication. As in prior studies, racial disparity in prognostic understanding was observed, with nonwhite patients being more likely to have overly optimistic views of their prognosis [4,5].
While the perceived 2-year survival probability is a somewhat arbitrary measure of prognostic opinion, it provides a useful representation of how one views the expected trajectory of disease. A high perceived likelihood of 2-year survival implies a view that long-term disease control can be achieved, whereas a low perceived likelihood of 2-year survival implies acknowledgement of terminal illness. This study effectively contrasts patient and physician opinions of 2-year survival probability, but it does not discriminate among clinically relevant differences in opinions within the 0–2 year prognostic range. For example, a patient whose oncologist believes his prognosis is < 6 months may be an appropriate candidate for hospice, but the patient may be unprepared to make the transition to hospice if he believes his prognosis is closer to a year or more. While the patient and oncologist may agree that 2-year survival is unlikely, they may have differing beliefs about the appropriateness of certain interventions based on their discrepant short-term prognostic views. Additional studies looking at perceived probabilities of short-term survival may be helpful in assessing patients’ readiness to transition to symptom-focused care when medically appropriate.
The authors designate 7 categories of 2-year survival probability (100%, about 90%, about 75%, about 50%, about 25%, about 10%, and 0%). The differences in percentage between prognostic categories are not evenly distributed, and therefore definition of discordance is non-uniform. The smaller percentage difference at the highest and lowest ends of the scale may result in overestimation of discordance at these extremes. For example, a patient rating her 2-year survival probability at 100% would be defined as having a discordant viewpoint from an oncologist rating her 2-year survival at 75% (as would be realistic for a diagnosis such as metastatic colon cancer). Given the imprecise nature of prognostication, the views of the patient and oncologist in this example are arguably similar, and perhaps should not be categorized as discordant.
As noted, patients already enrolled in hospice were excluded from the study, thus omitting a key group of patients whose prognostic views are more likely to be concordant with their physicians’ views. This group may be better captured in a prospective study of prognostic discordance among newly diagnosed advanced cancer patients after initial oncology consultation, allowing for inclusion of those who make an early transition to hospice.
Applications for Clinical Practice
Although clinicians tend to overestimate prognosis, their predictions correlate with outcomes in advanced cancer [6], and may therefore provide a useful framework for patients to understand the likely course of their disease. However, physicians often avoid explicit discussion of prognosis by shrouding prognostic information in discussions of radiographic findings, and quickly transitioning to discussion of treatment options [7]. Patients and families rarely inquire about prognosis, further limiting disclosure of prognostic information [7,8]. Even when prognostic information is explicitly stated, patients may misinterpret the information [4], potentially adversely affecting their ability to participate in shared decision making.
Some useful approaches for successfully communicating prognostic information may include asking patients what information they wish to hear before it is disclosed, providing prognostic data for patients with similar disease states while acknowledging individual variability, clearly defining the intent of proposed therapy (ie, curative versus noncurative), and asking the patient to restate information in order to assess understanding. Early involvement of palliative care specialists may help reinforce understanding about prognosis and goals of therapy, facilitate advance care planning, and reduce aggressive interventions at the end of life [2]. Ongoing research is directed at identifying effective interventions to improve communication between patients with advanced cancer and their oncologists [9].
—Irene M. Hutchins, MD
Scripps Cancer Center, La Jolla, CA
1. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998;279:1709–14.
2. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–26.
3. Pronzato P, Bertelli G, Losardo P, Landucci M. What do advanced cancer patients know of their disease? A report from Italy. Support Care Cancer 1994;2:242–4.
4. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616–25.
5. Ford D, Zapka J, Gebregziabher M, et al. Factors associated with illness perception among critically ill patients and surrogates. Chest 2010;138:59–67.
6. Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003;327:195–8.
7. Singh S, Cortez D, Maynard D, et al. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract 2017:JOP2016014621.
8. Leydon GM, Boulton M, Moynihan C, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ 2000;320:909–13.
9. Hoerger M, Epstein RM, Winters PC, et al. Values and options in cancer care (VOICE): study design and rationale for a patient-centered communication and decision-making intervention for physicians, patients with advanced cancer, and their caregivers. BMC Cancer 2013;13:188.
Study Overview
Objective. To assess the prevalence and determinants of patient–oncologist discordance in opinion of prognosis, and evaluate how often patients are aware of this discordance.
Design. Cross-sectional study.
Setting and participants. The study included 236 adult patients with advanced cancer and their 38 oncologists at academic and community oncology practices in Rochester, New York, and Sacramento, California. Inpatients and those already enrolled in hospice were excluded.
Main outcome measures. Patients and their oncologists independently reported their ratings of 2-year survival probability on a postindex visit multiple-choice questionnaire (response options included 100%, about 90%, about 75%, about 50%, about 25%, about 10%, and 0%). Prognostic discordance was defined as a more than 1 category difference between the patient and physician prognostic ratings. All patients were asked to report how they believed their oncologist would rate their 2-year survival probability. Those who correctly perceived their oncologist’s rating of their prognosis (within 1 category) were defined as knowing their oncologist’s opinion, and the rest were defined as not knowing their oncologist’s opinion. This distinction was used to categorize patients whose self-rating of their prognosis was discordant from their oncologist’s rating as either knowingly discordant or unknowingly discordant. Patient characteristics including age, sex, race/ethnicity, education, income, aggressiveness of cancer, self-efficacy with health care communication, recall of prognostic discussion with the oncologist, and end-of-life treatment preferences were evaluated as potential determinants of prognostic discordance.
Main results. 68% of patients rated their 2-year survival probability discordantly from their oncologists. Among these, 96% rated their prognosis more optimistically than their oncologists, and 89% were unaware that their opinions differed from that of their oncologists. Prognostic discordance was more common among nonwhite compared to white patients (95% versus 65%, P = 0.03). The prevalence of prognostic discordance did not significantly differ based on the other patient characteristics studied. Among patients whose prognostic ratings were discordant from their oncologist’s, 99% reported that they wanted to be involved in treatment decision making, and 70% were interested in involving palliative care when the end of life became near.
Conclusion. Patient–oncologist discordance about prognosis was common, particularly among nonwhite patients. In cases of prognostic discordance, patients rarely knew that their opinion differed from that of their oncologist, suggesting a lack of successful communication of prognostic information.
Commentary
Prior studies have noted that patients with advanced cancer perceive prognosis more optimistically than their physicians [1–3]. In a large national prospective observational study, the majority of patients receiving chemotherapy for metastatic (stage IV) lung or colorectal cancer inaccurately believed that chemotherapy was likely to be curative, potentially compromising their ability to make informed treatment decisions [4]. In the present study by Gramling et al, the authors confirm the observation that patients are more optimistic about prognosis than their oncologists, and furthermore demonstrate that most patients are unaware of the discrepancy, suggesting a failure of communication. As in prior studies, racial disparity in prognostic understanding was observed, with nonwhite patients being more likely to have overly optimistic views of their prognosis [4,5].
While the perceived 2-year survival probability is a somewhat arbitrary measure of prognostic opinion, it provides a useful representation of how one views the expected trajectory of disease. A high perceived likelihood of 2-year survival implies a view that long-term disease control can be achieved, whereas a low perceived likelihood of 2-year survival implies acknowledgement of terminal illness. This study effectively contrasts patient and physician opinions of 2-year survival probability, but it does not discriminate among clinically relevant differences in opinions within the 0–2 year prognostic range. For example, a patient whose oncologist believes his prognosis is < 6 months may be an appropriate candidate for hospice, but the patient may be unprepared to make the transition to hospice if he believes his prognosis is closer to a year or more. While the patient and oncologist may agree that 2-year survival is unlikely, they may have differing beliefs about the appropriateness of certain interventions based on their discrepant short-term prognostic views. Additional studies looking at perceived probabilities of short-term survival may be helpful in assessing patients’ readiness to transition to symptom-focused care when medically appropriate.
The authors designate 7 categories of 2-year survival probability (100%, about 90%, about 75%, about 50%, about 25%, about 10%, and 0%). The differences in percentage between prognostic categories are not evenly distributed, and therefore definition of discordance is non-uniform. The smaller percentage difference at the highest and lowest ends of the scale may result in overestimation of discordance at these extremes. For example, a patient rating her 2-year survival probability at 100% would be defined as having a discordant viewpoint from an oncologist rating her 2-year survival at 75% (as would be realistic for a diagnosis such as metastatic colon cancer). Given the imprecise nature of prognostication, the views of the patient and oncologist in this example are arguably similar, and perhaps should not be categorized as discordant.
As noted, patients already enrolled in hospice were excluded from the study, thus omitting a key group of patients whose prognostic views are more likely to be concordant with their physicians’ views. This group may be better captured in a prospective study of prognostic discordance among newly diagnosed advanced cancer patients after initial oncology consultation, allowing for inclusion of those who make an early transition to hospice.
Applications for Clinical Practice
Although clinicians tend to overestimate prognosis, their predictions correlate with outcomes in advanced cancer [6], and may therefore provide a useful framework for patients to understand the likely course of their disease. However, physicians often avoid explicit discussion of prognosis by shrouding prognostic information in discussions of radiographic findings, and quickly transitioning to discussion of treatment options [7]. Patients and families rarely inquire about prognosis, further limiting disclosure of prognostic information [7,8]. Even when prognostic information is explicitly stated, patients may misinterpret the information [4], potentially adversely affecting their ability to participate in shared decision making.
Some useful approaches for successfully communicating prognostic information may include asking patients what information they wish to hear before it is disclosed, providing prognostic data for patients with similar disease states while acknowledging individual variability, clearly defining the intent of proposed therapy (ie, curative versus noncurative), and asking the patient to restate information in order to assess understanding. Early involvement of palliative care specialists may help reinforce understanding about prognosis and goals of therapy, facilitate advance care planning, and reduce aggressive interventions at the end of life [2]. Ongoing research is directed at identifying effective interventions to improve communication between patients with advanced cancer and their oncologists [9].
—Irene M. Hutchins, MD
Scripps Cancer Center, La Jolla, CA
Study Overview
Objective. To assess the prevalence and determinants of patient–oncologist discordance in opinion of prognosis, and evaluate how often patients are aware of this discordance.
Design. Cross-sectional study.
Setting and participants. The study included 236 adult patients with advanced cancer and their 38 oncologists at academic and community oncology practices in Rochester, New York, and Sacramento, California. Inpatients and those already enrolled in hospice were excluded.
Main outcome measures. Patients and their oncologists independently reported their ratings of 2-year survival probability on a postindex visit multiple-choice questionnaire (response options included 100%, about 90%, about 75%, about 50%, about 25%, about 10%, and 0%). Prognostic discordance was defined as a more than 1 category difference between the patient and physician prognostic ratings. All patients were asked to report how they believed their oncologist would rate their 2-year survival probability. Those who correctly perceived their oncologist’s rating of their prognosis (within 1 category) were defined as knowing their oncologist’s opinion, and the rest were defined as not knowing their oncologist’s opinion. This distinction was used to categorize patients whose self-rating of their prognosis was discordant from their oncologist’s rating as either knowingly discordant or unknowingly discordant. Patient characteristics including age, sex, race/ethnicity, education, income, aggressiveness of cancer, self-efficacy with health care communication, recall of prognostic discussion with the oncologist, and end-of-life treatment preferences were evaluated as potential determinants of prognostic discordance.
Main results. 68% of patients rated their 2-year survival probability discordantly from their oncologists. Among these, 96% rated their prognosis more optimistically than their oncologists, and 89% were unaware that their opinions differed from that of their oncologists. Prognostic discordance was more common among nonwhite compared to white patients (95% versus 65%, P = 0.03). The prevalence of prognostic discordance did not significantly differ based on the other patient characteristics studied. Among patients whose prognostic ratings were discordant from their oncologist’s, 99% reported that they wanted to be involved in treatment decision making, and 70% were interested in involving palliative care when the end of life became near.
Conclusion. Patient–oncologist discordance about prognosis was common, particularly among nonwhite patients. In cases of prognostic discordance, patients rarely knew that their opinion differed from that of their oncologist, suggesting a lack of successful communication of prognostic information.
Commentary
Prior studies have noted that patients with advanced cancer perceive prognosis more optimistically than their physicians [1–3]. In a large national prospective observational study, the majority of patients receiving chemotherapy for metastatic (stage IV) lung or colorectal cancer inaccurately believed that chemotherapy was likely to be curative, potentially compromising their ability to make informed treatment decisions [4]. In the present study by Gramling et al, the authors confirm the observation that patients are more optimistic about prognosis than their oncologists, and furthermore demonstrate that most patients are unaware of the discrepancy, suggesting a failure of communication. As in prior studies, racial disparity in prognostic understanding was observed, with nonwhite patients being more likely to have overly optimistic views of their prognosis [4,5].
While the perceived 2-year survival probability is a somewhat arbitrary measure of prognostic opinion, it provides a useful representation of how one views the expected trajectory of disease. A high perceived likelihood of 2-year survival implies a view that long-term disease control can be achieved, whereas a low perceived likelihood of 2-year survival implies acknowledgement of terminal illness. This study effectively contrasts patient and physician opinions of 2-year survival probability, but it does not discriminate among clinically relevant differences in opinions within the 0–2 year prognostic range. For example, a patient whose oncologist believes his prognosis is < 6 months may be an appropriate candidate for hospice, but the patient may be unprepared to make the transition to hospice if he believes his prognosis is closer to a year or more. While the patient and oncologist may agree that 2-year survival is unlikely, they may have differing beliefs about the appropriateness of certain interventions based on their discrepant short-term prognostic views. Additional studies looking at perceived probabilities of short-term survival may be helpful in assessing patients’ readiness to transition to symptom-focused care when medically appropriate.
The authors designate 7 categories of 2-year survival probability (100%, about 90%, about 75%, about 50%, about 25%, about 10%, and 0%). The differences in percentage between prognostic categories are not evenly distributed, and therefore definition of discordance is non-uniform. The smaller percentage difference at the highest and lowest ends of the scale may result in overestimation of discordance at these extremes. For example, a patient rating her 2-year survival probability at 100% would be defined as having a discordant viewpoint from an oncologist rating her 2-year survival at 75% (as would be realistic for a diagnosis such as metastatic colon cancer). Given the imprecise nature of prognostication, the views of the patient and oncologist in this example are arguably similar, and perhaps should not be categorized as discordant.
As noted, patients already enrolled in hospice were excluded from the study, thus omitting a key group of patients whose prognostic views are more likely to be concordant with their physicians’ views. This group may be better captured in a prospective study of prognostic discordance among newly diagnosed advanced cancer patients after initial oncology consultation, allowing for inclusion of those who make an early transition to hospice.
Applications for Clinical Practice
Although clinicians tend to overestimate prognosis, their predictions correlate with outcomes in advanced cancer [6], and may therefore provide a useful framework for patients to understand the likely course of their disease. However, physicians often avoid explicit discussion of prognosis by shrouding prognostic information in discussions of radiographic findings, and quickly transitioning to discussion of treatment options [7]. Patients and families rarely inquire about prognosis, further limiting disclosure of prognostic information [7,8]. Even when prognostic information is explicitly stated, patients may misinterpret the information [4], potentially adversely affecting their ability to participate in shared decision making.
Some useful approaches for successfully communicating prognostic information may include asking patients what information they wish to hear before it is disclosed, providing prognostic data for patients with similar disease states while acknowledging individual variability, clearly defining the intent of proposed therapy (ie, curative versus noncurative), and asking the patient to restate information in order to assess understanding. Early involvement of palliative care specialists may help reinforce understanding about prognosis and goals of therapy, facilitate advance care planning, and reduce aggressive interventions at the end of life [2]. Ongoing research is directed at identifying effective interventions to improve communication between patients with advanced cancer and their oncologists [9].
—Irene M. Hutchins, MD
Scripps Cancer Center, La Jolla, CA
1. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998;279:1709–14.
2. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–26.
3. Pronzato P, Bertelli G, Losardo P, Landucci M. What do advanced cancer patients know of their disease? A report from Italy. Support Care Cancer 1994;2:242–4.
4. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616–25.
5. Ford D, Zapka J, Gebregziabher M, et al. Factors associated with illness perception among critically ill patients and surrogates. Chest 2010;138:59–67.
6. Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003;327:195–8.
7. Singh S, Cortez D, Maynard D, et al. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract 2017:JOP2016014621.
8. Leydon GM, Boulton M, Moynihan C, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ 2000;320:909–13.
9. Hoerger M, Epstein RM, Winters PC, et al. Values and options in cancer care (VOICE): study design and rationale for a patient-centered communication and decision-making intervention for physicians, patients with advanced cancer, and their caregivers. BMC Cancer 2013;13:188.
1. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998;279:1709–14.
2. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–26.
3. Pronzato P, Bertelli G, Losardo P, Landucci M. What do advanced cancer patients know of their disease? A report from Italy. Support Care Cancer 1994;2:242–4.
4. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616–25.
5. Ford D, Zapka J, Gebregziabher M, et al. Factors associated with illness perception among critically ill patients and surrogates. Chest 2010;138:59–67.
6. Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003;327:195–8.
7. Singh S, Cortez D, Maynard D, et al. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract 2017:JOP2016014621.
8. Leydon GM, Boulton M, Moynihan C, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ 2000;320:909–13.
9. Hoerger M, Epstein RM, Winters PC, et al. Values and options in cancer care (VOICE): study design and rationale for a patient-centered communication and decision-making intervention for physicians, patients with advanced cancer, and their caregivers. BMC Cancer 2013;13:188.
Senate committee moves Gorsuch nomination forward
Judge Neil Gorsuch has moved one step closer to becoming the next U.S. Supreme Court Justice.
The U.S. Senate Committee on the Judiciary approved Judge Gorsuch’s nomination by a 11-9 vote on April 3. The vote was a strict party line vote with 11 Republicans voting in favor of Judge Gorsuch and 9 Democrats voting against him.
“He’s a mainstream judge who’s earned the universal respect of his colleagues on the bench and in the bar,” Sen. Grassley said. “He applies the law as we in Congress write it – as the judicial oath says, ‘Without respect to persons.’ And he refuses to compromise his independence. This nominee ... is a judge’s judge. He’s a picture of the kind of justice we should have on the Supreme Court.”
Conversely, Sen. Dianne Feinstein (D-Calif.) expressed opposition to Judge Gorsuch, criticizing his past rulings and calling his answers during his nomination hearing vague and ambiguous.
“As I’ve said, our job is to assess whether the nominee will protect the legal and constitutional rights of all Americans and whether the nominee will recognize the humanity and justice required when evaluating the cases before him,” Sen. Feinstein said before the vote. “Unfortunately, based on the judge’s record at the Department of Justice, his tenure on the bench, his appearance before the Senate, and his written questions for the record, I cannot support his nomination.”
The full Senate is expected to vote on Judge Gorsuch’s nomination on April 7.
[email protected]
On Twitter @legal_med
Judge Neil Gorsuch has moved one step closer to becoming the next U.S. Supreme Court Justice.
The U.S. Senate Committee on the Judiciary approved Judge Gorsuch’s nomination by a 11-9 vote on April 3. The vote was a strict party line vote with 11 Republicans voting in favor of Judge Gorsuch and 9 Democrats voting against him.
“He’s a mainstream judge who’s earned the universal respect of his colleagues on the bench and in the bar,” Sen. Grassley said. “He applies the law as we in Congress write it – as the judicial oath says, ‘Without respect to persons.’ And he refuses to compromise his independence. This nominee ... is a judge’s judge. He’s a picture of the kind of justice we should have on the Supreme Court.”
Conversely, Sen. Dianne Feinstein (D-Calif.) expressed opposition to Judge Gorsuch, criticizing his past rulings and calling his answers during his nomination hearing vague and ambiguous.
“As I’ve said, our job is to assess whether the nominee will protect the legal and constitutional rights of all Americans and whether the nominee will recognize the humanity and justice required when evaluating the cases before him,” Sen. Feinstein said before the vote. “Unfortunately, based on the judge’s record at the Department of Justice, his tenure on the bench, his appearance before the Senate, and his written questions for the record, I cannot support his nomination.”
The full Senate is expected to vote on Judge Gorsuch’s nomination on April 7.
[email protected]
On Twitter @legal_med
Judge Neil Gorsuch has moved one step closer to becoming the next U.S. Supreme Court Justice.
The U.S. Senate Committee on the Judiciary approved Judge Gorsuch’s nomination by a 11-9 vote on April 3. The vote was a strict party line vote with 11 Republicans voting in favor of Judge Gorsuch and 9 Democrats voting against him.
“He’s a mainstream judge who’s earned the universal respect of his colleagues on the bench and in the bar,” Sen. Grassley said. “He applies the law as we in Congress write it – as the judicial oath says, ‘Without respect to persons.’ And he refuses to compromise his independence. This nominee ... is a judge’s judge. He’s a picture of the kind of justice we should have on the Supreme Court.”
Conversely, Sen. Dianne Feinstein (D-Calif.) expressed opposition to Judge Gorsuch, criticizing his past rulings and calling his answers during his nomination hearing vague and ambiguous.
“As I’ve said, our job is to assess whether the nominee will protect the legal and constitutional rights of all Americans and whether the nominee will recognize the humanity and justice required when evaluating the cases before him,” Sen. Feinstein said before the vote. “Unfortunately, based on the judge’s record at the Department of Justice, his tenure on the bench, his appearance before the Senate, and his written questions for the record, I cannot support his nomination.”
The full Senate is expected to vote on Judge Gorsuch’s nomination on April 7.
[email protected]
On Twitter @legal_med