User login
Dropping the A-bomb
Your first patient of the day is a 2½-year-old who has a runny nose and a cough. His mother has brought him in because his cough is more frequent and persistent than she is accustomed to hearing. He is happy and playful, and has a low-grade fever. You notice that he is slightly tachypneic, and you hear fine wheezes scattered throughout his lung fields. You also recall that at age 6 months, he was diagnosed with bronchiolitis but was never hospitalized.
Will you give him antibiotics and send him home with a nebulizer? Just the nebulizer? Just the antibiotics? Neither? We can debate those answers for hours, and you can plead for more information before you commit to an answer. But let’s skip over the question about what you are going to do and focus on what you are going to say. I want to know what diagnosis you are going to share with this mother.
Or are you going to try a pseudoscientific smoke screen and tell her that her that her son has “reactive airway disease”? You could soften it even further by reassuring her that his diagnosis is so common that it has an abbreviation: “We usually just call it RAD.”
You may not have trouble telling a parent that her child has asthma, but most clinicians struggle with dropping the A-bomb. Why? It may be that we don’t want the family to freak out. You could end up spending the rest of the morning coaxing them back off the ledge because you have diagnosed their child with a chronic illness that could kill him. This kind of exaggerated reaction is far less of a problem now than it was 30 or 40 years ago. Almost every parent knows at least one family with an asthmatic child who seems to be doing just fine. In my opinion, this apparent increase in prevalence of asthma is primarily the result of an improved awareness and a relabeling phenomenon.
Your own experience probably reflects the national statistics that less than a third of preschoolers with recurrent wheezing still have asthma by the time they finish kindergarten. And you may be hesitant to use the asthma diagnosis because you don’t want to be labeled as a clinician who cries wolf.
It may be that subconsciously you are afraid that by raising the asthma red flag you will be committing yourself to the time gobbling task of managing another patient with a chronic disease. You could gamble that he will only have one or two more episodes of wheezing, and you will be able to treat his illnesses simply as a short series of unconnected events.
Is there any harm in dancing around the asthma diagnosis? The authors of a Perspectives article in the January 2017 issue of Pediatrics argue persuasively that vague descriptive and nondiagnostic terms such as “reactive airways disease” are confusing and should be abandoned (“RAD: Reactive Airway Disease or Really Asthma Disease?” Pediatrics. 2017 Jan. doi: 10.1542/peds.2016-0625). They question why we would treat a condition with asthma medications and not call it asthma just because a child will probably out grow it later.
It’s more than just about sloppy language. Jose A. Castro-Rodriguez, MD, a physician who has pioneered one of the tools than can be used to predict persistent asthma in young children, observes that by failing to signal to parents that the child has a chronic condition, we run the risk that the child will be less adherent to the medication and management program we recommend. (“The Asthma Predictive Index,” Curr Opin Allergy Clin Immunol. 2011;11[3]:157-61).
If we are going to tighten up our language and drop the vague substitute terms like RAD, and if we are hesitant to drop the A-bomb because it sounds too much like a lifelong disease when the truth is that most young children will outgrow asthma, what should we tell all those parents of wheezing preschoolers? The authors of the article in Pediatrics have several suggestions. Their favorite and the one that appeals most to me is toddler asthma. As they observe, the term “toddler asthma” implies an endpoint and the need for reevaluation to determine if the child is one of the minority who has “real” asthma.
Although it’s almost always about the money. When it's not about the money, it's usually about the labels we use.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
Your first patient of the day is a 2½-year-old who has a runny nose and a cough. His mother has brought him in because his cough is more frequent and persistent than she is accustomed to hearing. He is happy and playful, and has a low-grade fever. You notice that he is slightly tachypneic, and you hear fine wheezes scattered throughout his lung fields. You also recall that at age 6 months, he was diagnosed with bronchiolitis but was never hospitalized.
Will you give him antibiotics and send him home with a nebulizer? Just the nebulizer? Just the antibiotics? Neither? We can debate those answers for hours, and you can plead for more information before you commit to an answer. But let’s skip over the question about what you are going to do and focus on what you are going to say. I want to know what diagnosis you are going to share with this mother.
Or are you going to try a pseudoscientific smoke screen and tell her that her that her son has “reactive airway disease”? You could soften it even further by reassuring her that his diagnosis is so common that it has an abbreviation: “We usually just call it RAD.”
You may not have trouble telling a parent that her child has asthma, but most clinicians struggle with dropping the A-bomb. Why? It may be that we don’t want the family to freak out. You could end up spending the rest of the morning coaxing them back off the ledge because you have diagnosed their child with a chronic illness that could kill him. This kind of exaggerated reaction is far less of a problem now than it was 30 or 40 years ago. Almost every parent knows at least one family with an asthmatic child who seems to be doing just fine. In my opinion, this apparent increase in prevalence of asthma is primarily the result of an improved awareness and a relabeling phenomenon.
Your own experience probably reflects the national statistics that less than a third of preschoolers with recurrent wheezing still have asthma by the time they finish kindergarten. And you may be hesitant to use the asthma diagnosis because you don’t want to be labeled as a clinician who cries wolf.
It may be that subconsciously you are afraid that by raising the asthma red flag you will be committing yourself to the time gobbling task of managing another patient with a chronic disease. You could gamble that he will only have one or two more episodes of wheezing, and you will be able to treat his illnesses simply as a short series of unconnected events.
Is there any harm in dancing around the asthma diagnosis? The authors of a Perspectives article in the January 2017 issue of Pediatrics argue persuasively that vague descriptive and nondiagnostic terms such as “reactive airways disease” are confusing and should be abandoned (“RAD: Reactive Airway Disease or Really Asthma Disease?” Pediatrics. 2017 Jan. doi: 10.1542/peds.2016-0625). They question why we would treat a condition with asthma medications and not call it asthma just because a child will probably out grow it later.
It’s more than just about sloppy language. Jose A. Castro-Rodriguez, MD, a physician who has pioneered one of the tools than can be used to predict persistent asthma in young children, observes that by failing to signal to parents that the child has a chronic condition, we run the risk that the child will be less adherent to the medication and management program we recommend. (“The Asthma Predictive Index,” Curr Opin Allergy Clin Immunol. 2011;11[3]:157-61).
If we are going to tighten up our language and drop the vague substitute terms like RAD, and if we are hesitant to drop the A-bomb because it sounds too much like a lifelong disease when the truth is that most young children will outgrow asthma, what should we tell all those parents of wheezing preschoolers? The authors of the article in Pediatrics have several suggestions. Their favorite and the one that appeals most to me is toddler asthma. As they observe, the term “toddler asthma” implies an endpoint and the need for reevaluation to determine if the child is one of the minority who has “real” asthma.
Although it’s almost always about the money. When it's not about the money, it's usually about the labels we use.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
Your first patient of the day is a 2½-year-old who has a runny nose and a cough. His mother has brought him in because his cough is more frequent and persistent than she is accustomed to hearing. He is happy and playful, and has a low-grade fever. You notice that he is slightly tachypneic, and you hear fine wheezes scattered throughout his lung fields. You also recall that at age 6 months, he was diagnosed with bronchiolitis but was never hospitalized.
Will you give him antibiotics and send him home with a nebulizer? Just the nebulizer? Just the antibiotics? Neither? We can debate those answers for hours, and you can plead for more information before you commit to an answer. But let’s skip over the question about what you are going to do and focus on what you are going to say. I want to know what diagnosis you are going to share with this mother.
Or are you going to try a pseudoscientific smoke screen and tell her that her that her son has “reactive airway disease”? You could soften it even further by reassuring her that his diagnosis is so common that it has an abbreviation: “We usually just call it RAD.”
You may not have trouble telling a parent that her child has asthma, but most clinicians struggle with dropping the A-bomb. Why? It may be that we don’t want the family to freak out. You could end up spending the rest of the morning coaxing them back off the ledge because you have diagnosed their child with a chronic illness that could kill him. This kind of exaggerated reaction is far less of a problem now than it was 30 or 40 years ago. Almost every parent knows at least one family with an asthmatic child who seems to be doing just fine. In my opinion, this apparent increase in prevalence of asthma is primarily the result of an improved awareness and a relabeling phenomenon.
Your own experience probably reflects the national statistics that less than a third of preschoolers with recurrent wheezing still have asthma by the time they finish kindergarten. And you may be hesitant to use the asthma diagnosis because you don’t want to be labeled as a clinician who cries wolf.
It may be that subconsciously you are afraid that by raising the asthma red flag you will be committing yourself to the time gobbling task of managing another patient with a chronic disease. You could gamble that he will only have one or two more episodes of wheezing, and you will be able to treat his illnesses simply as a short series of unconnected events.
Is there any harm in dancing around the asthma diagnosis? The authors of a Perspectives article in the January 2017 issue of Pediatrics argue persuasively that vague descriptive and nondiagnostic terms such as “reactive airways disease” are confusing and should be abandoned (“RAD: Reactive Airway Disease or Really Asthma Disease?” Pediatrics. 2017 Jan. doi: 10.1542/peds.2016-0625). They question why we would treat a condition with asthma medications and not call it asthma just because a child will probably out grow it later.
It’s more than just about sloppy language. Jose A. Castro-Rodriguez, MD, a physician who has pioneered one of the tools than can be used to predict persistent asthma in young children, observes that by failing to signal to parents that the child has a chronic condition, we run the risk that the child will be less adherent to the medication and management program we recommend. (“The Asthma Predictive Index,” Curr Opin Allergy Clin Immunol. 2011;11[3]:157-61).
If we are going to tighten up our language and drop the vague substitute terms like RAD, and if we are hesitant to drop the A-bomb because it sounds too much like a lifelong disease when the truth is that most young children will outgrow asthma, what should we tell all those parents of wheezing preschoolers? The authors of the article in Pediatrics have several suggestions. Their favorite and the one that appeals most to me is toddler asthma. As they observe, the term “toddler asthma” implies an endpoint and the need for reevaluation to determine if the child is one of the minority who has “real” asthma.
Although it’s almost always about the money. When it's not about the money, it's usually about the labels we use.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
Essential tips to diagnose and intervene early in hair loss
MIAMI – and watch for a new trend – man bun traction alopecia.
These and other clinical pearls on hair loss come courtesy of Wendy E. Roberts, MD.
“Hair loss can be scary,” but if physicians diagnose the underlying cause and treat early, a number of existing and upcoming treatments can be effective, said Dr. Roberts, a dermatologist in private practice in Rancho Mirage, Calif. “Timing is very critical.”
A dermatology full body examination is a perfect opportunity to ask patients about their hair because “you’re checking them from head to toe,” Dr. Roberts said. She recommends asking: “How is your hair doing?” This advice prompted a bit of uproar from the ODAC audience, suggesting this may be too sensitive a subject for some to broach with patients. Dr. Roberts responded, “No, not at all. Your patients will be glad you asked.
“That simple question will open up doors of opportunity” for dermatologists, she said. The condition remains very common: About 80 million people in the United States are affected by its No. 1 cause, hereditary hair loss. An Internet search for “hair loss,” in fact, reveals an overwhelming amount of consumer interest in hair loss treatment and management, yielding approximately 35 million results.
Paying attention to hair loss is not just question of appearance or aesthetics; hair loss can also indicate declining health, Dr. Roberts said at the Orlando Dermatology Aesthetic and Clinical Conference.
Suggest supplements backed by science
Given the millions of online searches, it’s apparent that people continue to look for the latest solutions to treat or prevent further hair loss. But as with many “treatments” and “cures” touted online, caution is warranted – not every claim is backed by solid research, she said. “Go with supplements that do have peer review literature.”
For this reason, Dr. Roberts suggests considering the following three supplements for patients with hair loss:
• Nutrafol – “What I like about this supplement is it has ashwagandha, an Indian herb that reduces stress,” she said.
• Viviscal – This supplement has the marine extract AminoMar, which includes shark cartilage and oyster extract powder.
• Vitalize Hair – Its active ingredient, Redensyl, contains two molecules, Dr. Roberts said, one to boost metabolism of the hair follicle and the other to increase hair volume over time.
Application of platelet rich plasma is another option showing promise for hair loss. It works by activating hair bulb cells. “It kind of whispers to the hair: ‘Be young again.’ Many times it comes in at the original hair color – tell your patients to be prepared to see their hair color from childhood.” Dr. Roberts said.
Man bun alert
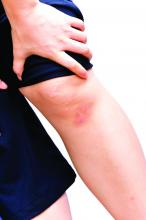
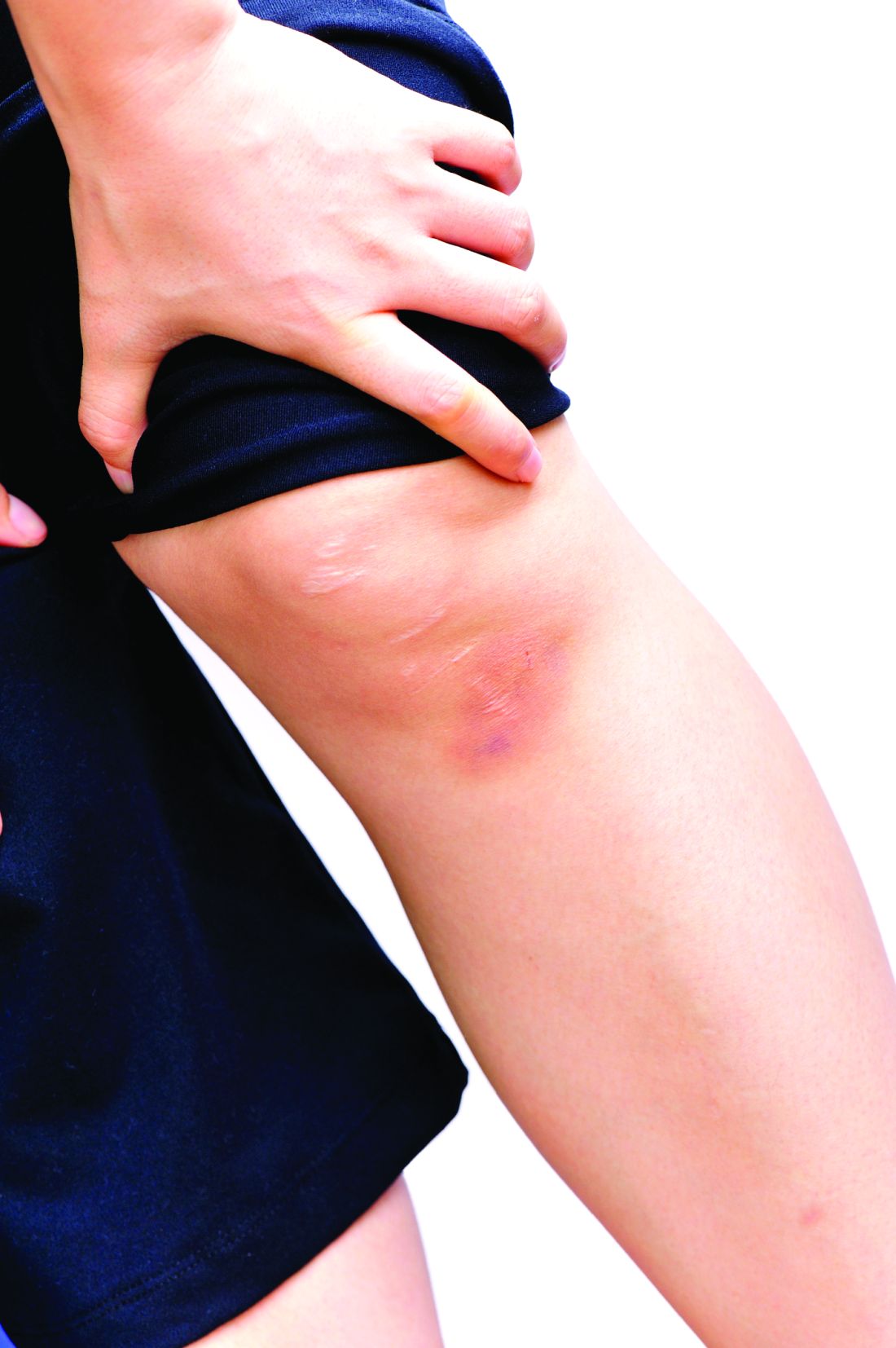
There is a new consideration in male hair loss based on changing hairstyles. “One thing that is trending: man bun traction alopecia. We see a lot of this in Southern California where I practice,” Dr. Roberts said. She showed meeting attendees the photo of a man with a tight hair bun whose hairline was starting to recede.
Basics to remember: differential diagnosis
When a patient presents with hair loss, things to consider in a differential diagnosis include chemical hair treatments, hairstyles that can cause traction alopecia, and behaviors such as compulsive hair pulling. Trichotillomania is on the increase. It affects approximately 2% of the population, but 90% of those are women, Dr. Roberts said. To diagnose, “look at the scalp and lower area near the nape of neck, especially in younger patients.”
She suggested that dermatologists show male patients the Norwood Scale illustrations of hair loss. The illustrations can help them understand when their hair loss is progressing over time, she added. For female patients with hair loss, the Ludwig Scale for hair loss in women can be very illustrative.
In the work-up of the patient, conduct a physical exam and consider overall health and nutritional status. Ask about family history as well, because relatives are the best window for insight into hair loss caused by genetics and aging, Dr. Roberts said.
Review patient medications. Older patients, in particular, often take multiple medications, which increases the likelihood for interactions or side effects leading to hair loss.
In addition, she had a few specific recommendations. “When you have male patient with hair loss taking Propecia [finasteride], talk to them about the dangers of exposure to a female partner and pregnancy in particular.” Also ask patients to bring in any supplements they are taking. Watch out for keratin supplements in patients with renal disease, she added, because elevated levels can be associated with kidney problems.
In terms of laboratory testing, consider checking for thyroid function, hormonal imbalance, and anemia. “The most overlooked is hemoglobin levels,” Dr. Roberts said, although anemia can cause hair loss in some patients.
Dr. Roberts is a speaker, consultant, investigator for and/or receives honoraria from Allergan, Colorescience, Galderma, Lytera, MDRejuvena, Restorsea, SkinMedica, Theraplex, Top MD Skincare, Valeant Pharmaceutical International, and Viviscal.
MIAMI – and watch for a new trend – man bun traction alopecia.
These and other clinical pearls on hair loss come courtesy of Wendy E. Roberts, MD.
“Hair loss can be scary,” but if physicians diagnose the underlying cause and treat early, a number of existing and upcoming treatments can be effective, said Dr. Roberts, a dermatologist in private practice in Rancho Mirage, Calif. “Timing is very critical.”
A dermatology full body examination is a perfect opportunity to ask patients about their hair because “you’re checking them from head to toe,” Dr. Roberts said. She recommends asking: “How is your hair doing?” This advice prompted a bit of uproar from the ODAC audience, suggesting this may be too sensitive a subject for some to broach with patients. Dr. Roberts responded, “No, not at all. Your patients will be glad you asked.
“That simple question will open up doors of opportunity” for dermatologists, she said. The condition remains very common: About 80 million people in the United States are affected by its No. 1 cause, hereditary hair loss. An Internet search for “hair loss,” in fact, reveals an overwhelming amount of consumer interest in hair loss treatment and management, yielding approximately 35 million results.
Paying attention to hair loss is not just question of appearance or aesthetics; hair loss can also indicate declining health, Dr. Roberts said at the Orlando Dermatology Aesthetic and Clinical Conference.
Suggest supplements backed by science
Given the millions of online searches, it’s apparent that people continue to look for the latest solutions to treat or prevent further hair loss. But as with many “treatments” and “cures” touted online, caution is warranted – not every claim is backed by solid research, she said. “Go with supplements that do have peer review literature.”
For this reason, Dr. Roberts suggests considering the following three supplements for patients with hair loss:
• Nutrafol – “What I like about this supplement is it has ashwagandha, an Indian herb that reduces stress,” she said.
• Viviscal – This supplement has the marine extract AminoMar, which includes shark cartilage and oyster extract powder.
• Vitalize Hair – Its active ingredient, Redensyl, contains two molecules, Dr. Roberts said, one to boost metabolism of the hair follicle and the other to increase hair volume over time.
Application of platelet rich plasma is another option showing promise for hair loss. It works by activating hair bulb cells. “It kind of whispers to the hair: ‘Be young again.’ Many times it comes in at the original hair color – tell your patients to be prepared to see their hair color from childhood.” Dr. Roberts said.
Man bun alert
There is a new consideration in male hair loss based on changing hairstyles. “One thing that is trending: man bun traction alopecia. We see a lot of this in Southern California where I practice,” Dr. Roberts said. She showed meeting attendees the photo of a man with a tight hair bun whose hairline was starting to recede.
Basics to remember: differential diagnosis
When a patient presents with hair loss, things to consider in a differential diagnosis include chemical hair treatments, hairstyles that can cause traction alopecia, and behaviors such as compulsive hair pulling. Trichotillomania is on the increase. It affects approximately 2% of the population, but 90% of those are women, Dr. Roberts said. To diagnose, “look at the scalp and lower area near the nape of neck, especially in younger patients.”
She suggested that dermatologists show male patients the Norwood Scale illustrations of hair loss. The illustrations can help them understand when their hair loss is progressing over time, she added. For female patients with hair loss, the Ludwig Scale for hair loss in women can be very illustrative.
In the work-up of the patient, conduct a physical exam and consider overall health and nutritional status. Ask about family history as well, because relatives are the best window for insight into hair loss caused by genetics and aging, Dr. Roberts said.
Review patient medications. Older patients, in particular, often take multiple medications, which increases the likelihood for interactions or side effects leading to hair loss.
In addition, she had a few specific recommendations. “When you have male patient with hair loss taking Propecia [finasteride], talk to them about the dangers of exposure to a female partner and pregnancy in particular.” Also ask patients to bring in any supplements they are taking. Watch out for keratin supplements in patients with renal disease, she added, because elevated levels can be associated with kidney problems.
In terms of laboratory testing, consider checking for thyroid function, hormonal imbalance, and anemia. “The most overlooked is hemoglobin levels,” Dr. Roberts said, although anemia can cause hair loss in some patients.
Dr. Roberts is a speaker, consultant, investigator for and/or receives honoraria from Allergan, Colorescience, Galderma, Lytera, MDRejuvena, Restorsea, SkinMedica, Theraplex, Top MD Skincare, Valeant Pharmaceutical International, and Viviscal.
MIAMI – and watch for a new trend – man bun traction alopecia.
These and other clinical pearls on hair loss come courtesy of Wendy E. Roberts, MD.
“Hair loss can be scary,” but if physicians diagnose the underlying cause and treat early, a number of existing and upcoming treatments can be effective, said Dr. Roberts, a dermatologist in private practice in Rancho Mirage, Calif. “Timing is very critical.”
A dermatology full body examination is a perfect opportunity to ask patients about their hair because “you’re checking them from head to toe,” Dr. Roberts said. She recommends asking: “How is your hair doing?” This advice prompted a bit of uproar from the ODAC audience, suggesting this may be too sensitive a subject for some to broach with patients. Dr. Roberts responded, “No, not at all. Your patients will be glad you asked.
“That simple question will open up doors of opportunity” for dermatologists, she said. The condition remains very common: About 80 million people in the United States are affected by its No. 1 cause, hereditary hair loss. An Internet search for “hair loss,” in fact, reveals an overwhelming amount of consumer interest in hair loss treatment and management, yielding approximately 35 million results.
Paying attention to hair loss is not just question of appearance or aesthetics; hair loss can also indicate declining health, Dr. Roberts said at the Orlando Dermatology Aesthetic and Clinical Conference.
Suggest supplements backed by science
Given the millions of online searches, it’s apparent that people continue to look for the latest solutions to treat or prevent further hair loss. But as with many “treatments” and “cures” touted online, caution is warranted – not every claim is backed by solid research, she said. “Go with supplements that do have peer review literature.”
For this reason, Dr. Roberts suggests considering the following three supplements for patients with hair loss:
• Nutrafol – “What I like about this supplement is it has ashwagandha, an Indian herb that reduces stress,” she said.
• Viviscal – This supplement has the marine extract AminoMar, which includes shark cartilage and oyster extract powder.
• Vitalize Hair – Its active ingredient, Redensyl, contains two molecules, Dr. Roberts said, one to boost metabolism of the hair follicle and the other to increase hair volume over time.
Application of platelet rich plasma is another option showing promise for hair loss. It works by activating hair bulb cells. “It kind of whispers to the hair: ‘Be young again.’ Many times it comes in at the original hair color – tell your patients to be prepared to see their hair color from childhood.” Dr. Roberts said.
Man bun alert
There is a new consideration in male hair loss based on changing hairstyles. “One thing that is trending: man bun traction alopecia. We see a lot of this in Southern California where I practice,” Dr. Roberts said. She showed meeting attendees the photo of a man with a tight hair bun whose hairline was starting to recede.
Basics to remember: differential diagnosis
When a patient presents with hair loss, things to consider in a differential diagnosis include chemical hair treatments, hairstyles that can cause traction alopecia, and behaviors such as compulsive hair pulling. Trichotillomania is on the increase. It affects approximately 2% of the population, but 90% of those are women, Dr. Roberts said. To diagnose, “look at the scalp and lower area near the nape of neck, especially in younger patients.”
She suggested that dermatologists show male patients the Norwood Scale illustrations of hair loss. The illustrations can help them understand when their hair loss is progressing over time, she added. For female patients with hair loss, the Ludwig Scale for hair loss in women can be very illustrative.
In the work-up of the patient, conduct a physical exam and consider overall health and nutritional status. Ask about family history as well, because relatives are the best window for insight into hair loss caused by genetics and aging, Dr. Roberts said.
Review patient medications. Older patients, in particular, often take multiple medications, which increases the likelihood for interactions or side effects leading to hair loss.
In addition, she had a few specific recommendations. “When you have male patient with hair loss taking Propecia [finasteride], talk to them about the dangers of exposure to a female partner and pregnancy in particular.” Also ask patients to bring in any supplements they are taking. Watch out for keratin supplements in patients with renal disease, she added, because elevated levels can be associated with kidney problems.
In terms of laboratory testing, consider checking for thyroid function, hormonal imbalance, and anemia. “The most overlooked is hemoglobin levels,” Dr. Roberts said, although anemia can cause hair loss in some patients.
Dr. Roberts is a speaker, consultant, investigator for and/or receives honoraria from Allergan, Colorescience, Galderma, Lytera, MDRejuvena, Restorsea, SkinMedica, Theraplex, Top MD Skincare, Valeant Pharmaceutical International, and Viviscal.
EXPERT ANALYSIS FROM ODAC 2017
Dual fractional laser offers advantages for facial rejuvenation
MIAMI – A device that combines nonablative and ablative laser energies can promote mild to moderate facial photo rejuvenation and improve the appearance of fine lines and wrinkles, according to Jason Pozner, MD.
Clinicians can tailor the depth for the 1470 nm nonablative diode and the 2940 nm Er:YAG lasers for each individual patient, Dr. Pozner said at the Orlando Dermatology Aesthetic and Clinical Conference. Advantages of resurfacing with the device, the HALO laser, include a cost-effective disposable tip and the ability to combine treatment with other therapies, he noted.
Before treatment begins, clinicians use the device to take facial measurements. Many patients find this precision reassuring, Dr. Pozner said during a live patient demonstration. Also, the device uses the information to help clinicians deliver the appropriate duration of therapy.
At this stage, it is a simple procedure, said Dr. Pozner, a plastic surgeon in a group practice in Boca Raton, Fla. Suction is turned on and the probe is then slowly advanced back and forth until the zone is finished, and “the laser beeps at you and you know you’re done,” he explained.
The HALO laser is useful for rejuvenation with little downtime. Most women treated with the device can wear makeup the same day, although more aggressively treated patients generally wait 1 additional day, Dr. Pozner said.
“I’ve never seen anything in our practice that gives this good a clinical result with this little downtime,” he added. He initially expected results to fall in between those associated with typical nonablative and ablative fractional laser treatments. But “in our experience, we get better results than ablative fractional [laser therapy], a story of one plus one equals three,” he said. “No matter what laser setting you use, patients are better by 5 days.”
When combined with intense pulsed light (IPL) treatment you can get a “double whammy effect,” Dr. Pozner said.
A meeting attendee asked about the appropriate order of IPL and HALO treatments. “When you combine the BBL (IPL) and HALO, yes, you do the IPL first,” Joel L. Cohen, MD, a private practice aesthetic dermatologist and Mohs surgeon in Denver who moderated the session at the meeting and also gave his own lecture on resurfacing options for the face.
Aside from the laser itself, the HALO system contains two tubes integrated into the handpiece, one of which is a Zimmer to deliver cooling during the procedure and the other is an air evacuator, he explained. “By having all of this integrated into the handpiece itself, it makes it much easier for the nurse who is circulating in the room to assist.”
Patients may feel warm for about 90 minutes post procedure, Dr. Cohen said. Make sure patients’ hands are clean and that the circulating nurse has given them an ice pack to minimize discomfort. “Even though I practice in Denver, where it is freezing cold right now, I’ve had patients drive home with the air conditioning on – just to try to cool down in the hour or so immediately following the laser treatment.”
Dr. Pozner said that a decrease in pore counts was an unexpected effect of HALO treatment, and he estimated that patients end up with about 20% fewer pores in treated areas, which can be advantage because “nothing else works on pores.” In his experience, most of the pore reduction persists over time.
A HALO disposal tip costs approximately $50, which he said was inexpensive, compared with other devices.
Dr. Cohen said that in his practice, using HALO, “We can give patients a significant improvement in overall photodamage and mild improvement in wrinkles with only about 5 days of redness and swelling, and on the last few days, some coffee-ground appearance.” The nonablative component can promote coagulation, so there is less bleeding when you turn up the erbium component, “offering synergistic results for the patient,” he added.
Dr. Pozner has received equipment, consulting fees, and honoraria from Halo manufacturer Sciton and is a member of the company’s advisory board and speakers bureau. Dr. Cohen is a consultant for Sciton.
MIAMI – A device that combines nonablative and ablative laser energies can promote mild to moderate facial photo rejuvenation and improve the appearance of fine lines and wrinkles, according to Jason Pozner, MD.
Clinicians can tailor the depth for the 1470 nm nonablative diode and the 2940 nm Er:YAG lasers for each individual patient, Dr. Pozner said at the Orlando Dermatology Aesthetic and Clinical Conference. Advantages of resurfacing with the device, the HALO laser, include a cost-effective disposable tip and the ability to combine treatment with other therapies, he noted.
Before treatment begins, clinicians use the device to take facial measurements. Many patients find this precision reassuring, Dr. Pozner said during a live patient demonstration. Also, the device uses the information to help clinicians deliver the appropriate duration of therapy.
At this stage, it is a simple procedure, said Dr. Pozner, a plastic surgeon in a group practice in Boca Raton, Fla. Suction is turned on and the probe is then slowly advanced back and forth until the zone is finished, and “the laser beeps at you and you know you’re done,” he explained.
The HALO laser is useful for rejuvenation with little downtime. Most women treated with the device can wear makeup the same day, although more aggressively treated patients generally wait 1 additional day, Dr. Pozner said.
“I’ve never seen anything in our practice that gives this good a clinical result with this little downtime,” he added. He initially expected results to fall in between those associated with typical nonablative and ablative fractional laser treatments. But “in our experience, we get better results than ablative fractional [laser therapy], a story of one plus one equals three,” he said. “No matter what laser setting you use, patients are better by 5 days.”
When combined with intense pulsed light (IPL) treatment you can get a “double whammy effect,” Dr. Pozner said.
A meeting attendee asked about the appropriate order of IPL and HALO treatments. “When you combine the BBL (IPL) and HALO, yes, you do the IPL first,” Joel L. Cohen, MD, a private practice aesthetic dermatologist and Mohs surgeon in Denver who moderated the session at the meeting and also gave his own lecture on resurfacing options for the face.
Aside from the laser itself, the HALO system contains two tubes integrated into the handpiece, one of which is a Zimmer to deliver cooling during the procedure and the other is an air evacuator, he explained. “By having all of this integrated into the handpiece itself, it makes it much easier for the nurse who is circulating in the room to assist.”
Patients may feel warm for about 90 minutes post procedure, Dr. Cohen said. Make sure patients’ hands are clean and that the circulating nurse has given them an ice pack to minimize discomfort. “Even though I practice in Denver, where it is freezing cold right now, I’ve had patients drive home with the air conditioning on – just to try to cool down in the hour or so immediately following the laser treatment.”
Dr. Pozner said that a decrease in pore counts was an unexpected effect of HALO treatment, and he estimated that patients end up with about 20% fewer pores in treated areas, which can be advantage because “nothing else works on pores.” In his experience, most of the pore reduction persists over time.
A HALO disposal tip costs approximately $50, which he said was inexpensive, compared with other devices.
Dr. Cohen said that in his practice, using HALO, “We can give patients a significant improvement in overall photodamage and mild improvement in wrinkles with only about 5 days of redness and swelling, and on the last few days, some coffee-ground appearance.” The nonablative component can promote coagulation, so there is less bleeding when you turn up the erbium component, “offering synergistic results for the patient,” he added.
Dr. Pozner has received equipment, consulting fees, and honoraria from Halo manufacturer Sciton and is a member of the company’s advisory board and speakers bureau. Dr. Cohen is a consultant for Sciton.
MIAMI – A device that combines nonablative and ablative laser energies can promote mild to moderate facial photo rejuvenation and improve the appearance of fine lines and wrinkles, according to Jason Pozner, MD.
Clinicians can tailor the depth for the 1470 nm nonablative diode and the 2940 nm Er:YAG lasers for each individual patient, Dr. Pozner said at the Orlando Dermatology Aesthetic and Clinical Conference. Advantages of resurfacing with the device, the HALO laser, include a cost-effective disposable tip and the ability to combine treatment with other therapies, he noted.
Before treatment begins, clinicians use the device to take facial measurements. Many patients find this precision reassuring, Dr. Pozner said during a live patient demonstration. Also, the device uses the information to help clinicians deliver the appropriate duration of therapy.
At this stage, it is a simple procedure, said Dr. Pozner, a plastic surgeon in a group practice in Boca Raton, Fla. Suction is turned on and the probe is then slowly advanced back and forth until the zone is finished, and “the laser beeps at you and you know you’re done,” he explained.
The HALO laser is useful for rejuvenation with little downtime. Most women treated with the device can wear makeup the same day, although more aggressively treated patients generally wait 1 additional day, Dr. Pozner said.
“I’ve never seen anything in our practice that gives this good a clinical result with this little downtime,” he added. He initially expected results to fall in between those associated with typical nonablative and ablative fractional laser treatments. But “in our experience, we get better results than ablative fractional [laser therapy], a story of one plus one equals three,” he said. “No matter what laser setting you use, patients are better by 5 days.”
When combined with intense pulsed light (IPL) treatment you can get a “double whammy effect,” Dr. Pozner said.
A meeting attendee asked about the appropriate order of IPL and HALO treatments. “When you combine the BBL (IPL) and HALO, yes, you do the IPL first,” Joel L. Cohen, MD, a private practice aesthetic dermatologist and Mohs surgeon in Denver who moderated the session at the meeting and also gave his own lecture on resurfacing options for the face.
Aside from the laser itself, the HALO system contains two tubes integrated into the handpiece, one of which is a Zimmer to deliver cooling during the procedure and the other is an air evacuator, he explained. “By having all of this integrated into the handpiece itself, it makes it much easier for the nurse who is circulating in the room to assist.”
Patients may feel warm for about 90 minutes post procedure, Dr. Cohen said. Make sure patients’ hands are clean and that the circulating nurse has given them an ice pack to minimize discomfort. “Even though I practice in Denver, where it is freezing cold right now, I’ve had patients drive home with the air conditioning on – just to try to cool down in the hour or so immediately following the laser treatment.”
Dr. Pozner said that a decrease in pore counts was an unexpected effect of HALO treatment, and he estimated that patients end up with about 20% fewer pores in treated areas, which can be advantage because “nothing else works on pores.” In his experience, most of the pore reduction persists over time.
A HALO disposal tip costs approximately $50, which he said was inexpensive, compared with other devices.
Dr. Cohen said that in his practice, using HALO, “We can give patients a significant improvement in overall photodamage and mild improvement in wrinkles with only about 5 days of redness and swelling, and on the last few days, some coffee-ground appearance.” The nonablative component can promote coagulation, so there is less bleeding when you turn up the erbium component, “offering synergistic results for the patient,” he added.
Dr. Pozner has received equipment, consulting fees, and honoraria from Halo manufacturer Sciton and is a member of the company’s advisory board and speakers bureau. Dr. Cohen is a consultant for Sciton.
AT THE ODAC CONFERENCE
In active CLL with deletion 17p, consider trial enrollment
NEW YORK – Outside of clinical trials, therapy for early stage chronic lymphocytic leukemia in patients with deletion of the short arm of chromosome 17 (del[17]p) and/or mutation of the tumor suppressor gene TP53 requires the presence of active disease, according to Neil E. Kay, MD.
“Right now, we would propose that patients with del[17]p should have additional prognostic work-up. It’s very important to know if they are unmutated or mutated for the IgVH gene,” he said, adding that stimulated karyotype is also important to perform in those with del[17]p.
“The median overall survivorship in many phase II and phase III trials appears to be around 2 to 3 years,” he said.
Those with a chromosome 17 (del[17]p) and/or mutation of the tumor suppressor gene TP53 who receive chemoimmunotherapy very rarely achieve a complete response, or if they do they have a short duration of response, he added.
In treated patients, 17p deletion and p53 mutation are the most common abnormalities acquired during the course of the disease.
“Unfortunately there appears to be a selection pressure, and [in treated patients] the incidence of 17p and the p53 mutation has been reported up to 23%-44%. No one understands completely the biology of this, but it may be that subclones are present and expand, or that new mutations occur due to selection pressure of [chemoimmunotherapy] and the overgrowth of these subclones,” he said.
Importantly, not all patients with del[17]p or p53 mutation have bad outcomes; there are patients with indolent disease, he noted, adding that various criteria have been shown to help identify which patients are at risk for poor outcomes and to classify them according to risk. In general, lower-risk patients have mutated immunoglobulin heavy chain variable gene status, early stage disease, younger age, good performance status, and normal serum lactate dehydrogenase. These criteria could be used to identify low-risk patients who can be followed, he said.
Fluorescence in situ hybridization (FISH) evaluation of patients is a useful tool when there is no access to sequencing and other tests, Dr. Kay said, describing a recent multinational CLL Research Consortium study of nearly 1,600 patients (Br J Haematol. 2016 Apr;173[1]:105-13).
In that study, he and his colleagues found that patients with less than 50% 17p did not have such poor outcomes, but at 50%-plus they did much worse in terms of time to first treatment.
Based on the available data, Dr. Kay said that treatment is unnecessary in asymptomatic patients, except, perhaps, in high-risk patients identified using recently published risk models, for whom clinical trial enrollment may be considered.
“We do advocate having a discussion about allogeneic stem cell transplant since this may still be the only curative approach,” he added.
In patients with del[17]p and/or p53 mutation who have progressive disease, Dr. Kay said his take on the available data is that patients should first be categorized by age, then by whether they are fit or frail, and finally by whether or not they have del[17]p. Those under age 70 years without del[17]p and who have a mutated IgVH status should be considered for a clinical trial, and are also good candidates for chemoimmunotherapy. If they do have del[17]p or p53 mutation, consider clinical trial enrollment or treatment with ibrutinib, he said.
Fit patients in a complete response can be referred for transplant evaluation, but while the other treatments can be considered in frail patients or those aged 70 years or older, transplant is not advised, he added.
For relapsed or refractory patients, FISH testing should be performed or repeated, because such patients are at high risk of progression to develop del[17]p or mutation, he noted.
Those who are asymptomatic can be observed or enrolled in a clinical trial, and those who are symptomatic can be enrolled in a clinical trial or treated with various novel agents, including ibrutinib, idelalisib/rituximab, venetoclax, or combination therapies with methylpred–anti-CD20, or alemtuzumab with or without rituximab. Referral for transplant may be warranted in these patients if they are fit.
“Progressive CLL patients with 17p deletion/p53 mutations are much less likely to do well with chemoimmunotherapy, and novel inhibitors are effective, but we still need to enhance complete response rates and minimal residual disease-negative status for these high-risk patients,” he said.
Dr. Kay reported consulting for or receiving grant/research support from Acerta, Celgene, Gilead, Infinity, MorphoSys, Pharmacyclics, and Tolero.
NEW YORK – Outside of clinical trials, therapy for early stage chronic lymphocytic leukemia in patients with deletion of the short arm of chromosome 17 (del[17]p) and/or mutation of the tumor suppressor gene TP53 requires the presence of active disease, according to Neil E. Kay, MD.
“Right now, we would propose that patients with del[17]p should have additional prognostic work-up. It’s very important to know if they are unmutated or mutated for the IgVH gene,” he said, adding that stimulated karyotype is also important to perform in those with del[17]p.
“The median overall survivorship in many phase II and phase III trials appears to be around 2 to 3 years,” he said.
Those with a chromosome 17 (del[17]p) and/or mutation of the tumor suppressor gene TP53 who receive chemoimmunotherapy very rarely achieve a complete response, or if they do they have a short duration of response, he added.
In treated patients, 17p deletion and p53 mutation are the most common abnormalities acquired during the course of the disease.
“Unfortunately there appears to be a selection pressure, and [in treated patients] the incidence of 17p and the p53 mutation has been reported up to 23%-44%. No one understands completely the biology of this, but it may be that subclones are present and expand, or that new mutations occur due to selection pressure of [chemoimmunotherapy] and the overgrowth of these subclones,” he said.
Importantly, not all patients with del[17]p or p53 mutation have bad outcomes; there are patients with indolent disease, he noted, adding that various criteria have been shown to help identify which patients are at risk for poor outcomes and to classify them according to risk. In general, lower-risk patients have mutated immunoglobulin heavy chain variable gene status, early stage disease, younger age, good performance status, and normal serum lactate dehydrogenase. These criteria could be used to identify low-risk patients who can be followed, he said.
Fluorescence in situ hybridization (FISH) evaluation of patients is a useful tool when there is no access to sequencing and other tests, Dr. Kay said, describing a recent multinational CLL Research Consortium study of nearly 1,600 patients (Br J Haematol. 2016 Apr;173[1]:105-13).
In that study, he and his colleagues found that patients with less than 50% 17p did not have such poor outcomes, but at 50%-plus they did much worse in terms of time to first treatment.
Based on the available data, Dr. Kay said that treatment is unnecessary in asymptomatic patients, except, perhaps, in high-risk patients identified using recently published risk models, for whom clinical trial enrollment may be considered.
“We do advocate having a discussion about allogeneic stem cell transplant since this may still be the only curative approach,” he added.
In patients with del[17]p and/or p53 mutation who have progressive disease, Dr. Kay said his take on the available data is that patients should first be categorized by age, then by whether they are fit or frail, and finally by whether or not they have del[17]p. Those under age 70 years without del[17]p and who have a mutated IgVH status should be considered for a clinical trial, and are also good candidates for chemoimmunotherapy. If they do have del[17]p or p53 mutation, consider clinical trial enrollment or treatment with ibrutinib, he said.
Fit patients in a complete response can be referred for transplant evaluation, but while the other treatments can be considered in frail patients or those aged 70 years or older, transplant is not advised, he added.
For relapsed or refractory patients, FISH testing should be performed or repeated, because such patients are at high risk of progression to develop del[17]p or mutation, he noted.
Those who are asymptomatic can be observed or enrolled in a clinical trial, and those who are symptomatic can be enrolled in a clinical trial or treated with various novel agents, including ibrutinib, idelalisib/rituximab, venetoclax, or combination therapies with methylpred–anti-CD20, or alemtuzumab with or without rituximab. Referral for transplant may be warranted in these patients if they are fit.
“Progressive CLL patients with 17p deletion/p53 mutations are much less likely to do well with chemoimmunotherapy, and novel inhibitors are effective, but we still need to enhance complete response rates and minimal residual disease-negative status for these high-risk patients,” he said.
Dr. Kay reported consulting for or receiving grant/research support from Acerta, Celgene, Gilead, Infinity, MorphoSys, Pharmacyclics, and Tolero.
NEW YORK – Outside of clinical trials, therapy for early stage chronic lymphocytic leukemia in patients with deletion of the short arm of chromosome 17 (del[17]p) and/or mutation of the tumor suppressor gene TP53 requires the presence of active disease, according to Neil E. Kay, MD.
“Right now, we would propose that patients with del[17]p should have additional prognostic work-up. It’s very important to know if they are unmutated or mutated for the IgVH gene,” he said, adding that stimulated karyotype is also important to perform in those with del[17]p.
“The median overall survivorship in many phase II and phase III trials appears to be around 2 to 3 years,” he said.
Those with a chromosome 17 (del[17]p) and/or mutation of the tumor suppressor gene TP53 who receive chemoimmunotherapy very rarely achieve a complete response, or if they do they have a short duration of response, he added.
In treated patients, 17p deletion and p53 mutation are the most common abnormalities acquired during the course of the disease.
“Unfortunately there appears to be a selection pressure, and [in treated patients] the incidence of 17p and the p53 mutation has been reported up to 23%-44%. No one understands completely the biology of this, but it may be that subclones are present and expand, or that new mutations occur due to selection pressure of [chemoimmunotherapy] and the overgrowth of these subclones,” he said.
Importantly, not all patients with del[17]p or p53 mutation have bad outcomes; there are patients with indolent disease, he noted, adding that various criteria have been shown to help identify which patients are at risk for poor outcomes and to classify them according to risk. In general, lower-risk patients have mutated immunoglobulin heavy chain variable gene status, early stage disease, younger age, good performance status, and normal serum lactate dehydrogenase. These criteria could be used to identify low-risk patients who can be followed, he said.
Fluorescence in situ hybridization (FISH) evaluation of patients is a useful tool when there is no access to sequencing and other tests, Dr. Kay said, describing a recent multinational CLL Research Consortium study of nearly 1,600 patients (Br J Haematol. 2016 Apr;173[1]:105-13).
In that study, he and his colleagues found that patients with less than 50% 17p did not have such poor outcomes, but at 50%-plus they did much worse in terms of time to first treatment.
Based on the available data, Dr. Kay said that treatment is unnecessary in asymptomatic patients, except, perhaps, in high-risk patients identified using recently published risk models, for whom clinical trial enrollment may be considered.
“We do advocate having a discussion about allogeneic stem cell transplant since this may still be the only curative approach,” he added.
In patients with del[17]p and/or p53 mutation who have progressive disease, Dr. Kay said his take on the available data is that patients should first be categorized by age, then by whether they are fit or frail, and finally by whether or not they have del[17]p. Those under age 70 years without del[17]p and who have a mutated IgVH status should be considered for a clinical trial, and are also good candidates for chemoimmunotherapy. If they do have del[17]p or p53 mutation, consider clinical trial enrollment or treatment with ibrutinib, he said.
Fit patients in a complete response can be referred for transplant evaluation, but while the other treatments can be considered in frail patients or those aged 70 years or older, transplant is not advised, he added.
For relapsed or refractory patients, FISH testing should be performed or repeated, because such patients are at high risk of progression to develop del[17]p or mutation, he noted.
Those who are asymptomatic can be observed or enrolled in a clinical trial, and those who are symptomatic can be enrolled in a clinical trial or treated with various novel agents, including ibrutinib, idelalisib/rituximab, venetoclax, or combination therapies with methylpred–anti-CD20, or alemtuzumab with or without rituximab. Referral for transplant may be warranted in these patients if they are fit.
“Progressive CLL patients with 17p deletion/p53 mutations are much less likely to do well with chemoimmunotherapy, and novel inhibitors are effective, but we still need to enhance complete response rates and minimal residual disease-negative status for these high-risk patients,” he said.
Dr. Kay reported consulting for or receiving grant/research support from Acerta, Celgene, Gilead, Infinity, MorphoSys, Pharmacyclics, and Tolero.
EXPERT ANALYSIS FROM LYMPHOMA & MYELOMA
Antibiotics have a role in PANS even with no infection
SAN FRANCISCO – Antibiotics might help in pediatric acute-onset neuropsychiatric syndrome (PANS) even if there’s no apparent infection, according to Kiki Chang, MD, director of PANS research at Stanford (Calif.) University.
The first step at Stanford is to look for an active infection, and knock it out with antibiotics. Dr. Chang has seen remarkable turnarounds in some of those cases, but even if there’s no infection, “we still do use antibiotics.” There are positive data, “although not a lot,” indicating that they can help. Some kids even seem to need to be on long-term antibiotics, and flair if taken off long after infections should have been cleared.
“We don’t know what’s going on. We try to stop antibiotics if we can; if patients relapse, we think the benefit [of ongoing treatment] outweighs the risks. Some kids just have to be on antibiotics for a long time, and that’s an issue.” Perhaps it has something to do with the anti-inflammatory properties of antibiotics like azithromycin and amoxicillin, or there might be a lingering infection, he said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
PANS is a recently coined term for the sudden onset of obsessive compulsive disorder (OCD) within a few days of an infection, metabolic disturbance, or other inflammatory insult. Anxiety, mood problems, and tics are common. There might be severe food restriction – only eating white foods, for instance – that are not related to body image.
PANS broadened the concept of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), which was first described in 1998, although it’s been known for generations that acute streptococcus infections can lead to abrupt psychiatric symptoms.
PANS is the topic of ongoing investigation, and Dr. Chang and many others are working to define the syndrome and its treatment, and trying especially to determine how PANS differs from typical OCD and other problems with more insidious onset. The idea is that inflammation in the patient’s brain, whatever the source, triggers an OCD mechanism in susceptible patients. As a concept, “we believe it’s true,” he said.
For now, it’s best to refer suspected cases to one of several academic PANS programs in the United States, as diagnosis and treatment isn’t ready for general practice, he said.
If more than antibiotics are needed, Stanford considers targeting inflammation. Some children respond to easy options such as ibuprofen. Dr. Chang has seen some helped with prednisone, but treatment is tricky. There might be an occult infection, and PANS can present with psychiatric issues that prednisone can make worse, including depression and mania. Intravenous immunoglobulin is another of the many options, “but we really need about four treatments” to see if it helps.
Cognitive behavioral therapy and family support also helps. As for psychotropic medication, “we often use them, but they rarely take away the acute symptoms,” and PANS children seem especially sensitive to side effects. “I’ve seen many of them become manic on SSRIs. I’ve seen some of them have very strong [extrapyramidal symptoms] with atypical antipsychotics. You have to be very careful; we don’t have any good studies” of psychiatric drugs in this population, he said.
At the moment, PANS seems to be more common in boys than girls, and most patients have a relapsing/remitting course and a family history of autoimmune disease. Suicidal and homicidal ideation can be part of the condition.
Dr. Chang believes PANS could be part of the overall increase in autoimmune disease and psychiatric disorders in children over the past few decades.
“We have more kids who have special needs than ever before,” large, objective increases in bipolar, autism, and other psychiatric problems, as well as increases in psoriasis, nut allergies, and other autoimmune issues. “What causes it is harder to say, but there has been a change for sure in kids and their immune system development that does affect the brain, and has probably led to more neuropsychiatric disturbances,” he said.
“No one talks about it. Everyone thinks that it’s some sort of pharmaceutical industry conspiracy” to sell more drugs by increasing scrutiny of children. “I think it’s caused by something in the environment interacting with genetics,” whether it’s infections, toxins, or something else. “We don’t know. Any kind of inflammation can be a trigger” and “we know inflammation” is key to “many psychiatric symptoms. I do think there’s something going on with kids over the last 30 years,” he said.
Dr. Chang is a consultant for and/or has received research support from Bristol-Myers Squibb, Lilly, Merck, GlaxoSmithKline, and other companies.
SAN FRANCISCO – Antibiotics might help in pediatric acute-onset neuropsychiatric syndrome (PANS) even if there’s no apparent infection, according to Kiki Chang, MD, director of PANS research at Stanford (Calif.) University.
The first step at Stanford is to look for an active infection, and knock it out with antibiotics. Dr. Chang has seen remarkable turnarounds in some of those cases, but even if there’s no infection, “we still do use antibiotics.” There are positive data, “although not a lot,” indicating that they can help. Some kids even seem to need to be on long-term antibiotics, and flair if taken off long after infections should have been cleared.
“We don’t know what’s going on. We try to stop antibiotics if we can; if patients relapse, we think the benefit [of ongoing treatment] outweighs the risks. Some kids just have to be on antibiotics for a long time, and that’s an issue.” Perhaps it has something to do with the anti-inflammatory properties of antibiotics like azithromycin and amoxicillin, or there might be a lingering infection, he said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
PANS is a recently coined term for the sudden onset of obsessive compulsive disorder (OCD) within a few days of an infection, metabolic disturbance, or other inflammatory insult. Anxiety, mood problems, and tics are common. There might be severe food restriction – only eating white foods, for instance – that are not related to body image.
PANS broadened the concept of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), which was first described in 1998, although it’s been known for generations that acute streptococcus infections can lead to abrupt psychiatric symptoms.
PANS is the topic of ongoing investigation, and Dr. Chang and many others are working to define the syndrome and its treatment, and trying especially to determine how PANS differs from typical OCD and other problems with more insidious onset. The idea is that inflammation in the patient’s brain, whatever the source, triggers an OCD mechanism in susceptible patients. As a concept, “we believe it’s true,” he said.
For now, it’s best to refer suspected cases to one of several academic PANS programs in the United States, as diagnosis and treatment isn’t ready for general practice, he said.
If more than antibiotics are needed, Stanford considers targeting inflammation. Some children respond to easy options such as ibuprofen. Dr. Chang has seen some helped with prednisone, but treatment is tricky. There might be an occult infection, and PANS can present with psychiatric issues that prednisone can make worse, including depression and mania. Intravenous immunoglobulin is another of the many options, “but we really need about four treatments” to see if it helps.
Cognitive behavioral therapy and family support also helps. As for psychotropic medication, “we often use them, but they rarely take away the acute symptoms,” and PANS children seem especially sensitive to side effects. “I’ve seen many of them become manic on SSRIs. I’ve seen some of them have very strong [extrapyramidal symptoms] with atypical antipsychotics. You have to be very careful; we don’t have any good studies” of psychiatric drugs in this population, he said.
At the moment, PANS seems to be more common in boys than girls, and most patients have a relapsing/remitting course and a family history of autoimmune disease. Suicidal and homicidal ideation can be part of the condition.
Dr. Chang believes PANS could be part of the overall increase in autoimmune disease and psychiatric disorders in children over the past few decades.
“We have more kids who have special needs than ever before,” large, objective increases in bipolar, autism, and other psychiatric problems, as well as increases in psoriasis, nut allergies, and other autoimmune issues. “What causes it is harder to say, but there has been a change for sure in kids and their immune system development that does affect the brain, and has probably led to more neuropsychiatric disturbances,” he said.
“No one talks about it. Everyone thinks that it’s some sort of pharmaceutical industry conspiracy” to sell more drugs by increasing scrutiny of children. “I think it’s caused by something in the environment interacting with genetics,” whether it’s infections, toxins, or something else. “We don’t know. Any kind of inflammation can be a trigger” and “we know inflammation” is key to “many psychiatric symptoms. I do think there’s something going on with kids over the last 30 years,” he said.
Dr. Chang is a consultant for and/or has received research support from Bristol-Myers Squibb, Lilly, Merck, GlaxoSmithKline, and other companies.
SAN FRANCISCO – Antibiotics might help in pediatric acute-onset neuropsychiatric syndrome (PANS) even if there’s no apparent infection, according to Kiki Chang, MD, director of PANS research at Stanford (Calif.) University.
The first step at Stanford is to look for an active infection, and knock it out with antibiotics. Dr. Chang has seen remarkable turnarounds in some of those cases, but even if there’s no infection, “we still do use antibiotics.” There are positive data, “although not a lot,” indicating that they can help. Some kids even seem to need to be on long-term antibiotics, and flair if taken off long after infections should have been cleared.
“We don’t know what’s going on. We try to stop antibiotics if we can; if patients relapse, we think the benefit [of ongoing treatment] outweighs the risks. Some kids just have to be on antibiotics for a long time, and that’s an issue.” Perhaps it has something to do with the anti-inflammatory properties of antibiotics like azithromycin and amoxicillin, or there might be a lingering infection, he said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
PANS is a recently coined term for the sudden onset of obsessive compulsive disorder (OCD) within a few days of an infection, metabolic disturbance, or other inflammatory insult. Anxiety, mood problems, and tics are common. There might be severe food restriction – only eating white foods, for instance – that are not related to body image.
PANS broadened the concept of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), which was first described in 1998, although it’s been known for generations that acute streptococcus infections can lead to abrupt psychiatric symptoms.
PANS is the topic of ongoing investigation, and Dr. Chang and many others are working to define the syndrome and its treatment, and trying especially to determine how PANS differs from typical OCD and other problems with more insidious onset. The idea is that inflammation in the patient’s brain, whatever the source, triggers an OCD mechanism in susceptible patients. As a concept, “we believe it’s true,” he said.
For now, it’s best to refer suspected cases to one of several academic PANS programs in the United States, as diagnosis and treatment isn’t ready for general practice, he said.
If more than antibiotics are needed, Stanford considers targeting inflammation. Some children respond to easy options such as ibuprofen. Dr. Chang has seen some helped with prednisone, but treatment is tricky. There might be an occult infection, and PANS can present with psychiatric issues that prednisone can make worse, including depression and mania. Intravenous immunoglobulin is another of the many options, “but we really need about four treatments” to see if it helps.
Cognitive behavioral therapy and family support also helps. As for psychotropic medication, “we often use them, but they rarely take away the acute symptoms,” and PANS children seem especially sensitive to side effects. “I’ve seen many of them become manic on SSRIs. I’ve seen some of them have very strong [extrapyramidal symptoms] with atypical antipsychotics. You have to be very careful; we don’t have any good studies” of psychiatric drugs in this population, he said.
At the moment, PANS seems to be more common in boys than girls, and most patients have a relapsing/remitting course and a family history of autoimmune disease. Suicidal and homicidal ideation can be part of the condition.
Dr. Chang believes PANS could be part of the overall increase in autoimmune disease and psychiatric disorders in children over the past few decades.
“We have more kids who have special needs than ever before,” large, objective increases in bipolar, autism, and other psychiatric problems, as well as increases in psoriasis, nut allergies, and other autoimmune issues. “What causes it is harder to say, but there has been a change for sure in kids and their immune system development that does affect the brain, and has probably led to more neuropsychiatric disturbances,” he said.
“No one talks about it. Everyone thinks that it’s some sort of pharmaceutical industry conspiracy” to sell more drugs by increasing scrutiny of children. “I think it’s caused by something in the environment interacting with genetics,” whether it’s infections, toxins, or something else. “We don’t know. Any kind of inflammation can be a trigger” and “we know inflammation” is key to “many psychiatric symptoms. I do think there’s something going on with kids over the last 30 years,” he said.
Dr. Chang is a consultant for and/or has received research support from Bristol-Myers Squibb, Lilly, Merck, GlaxoSmithKline, and other companies.
EXPERT ANALYSIS FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE
Thigh muscle weakness predicts knee osteoarthritis in women only
Weakness in the lower thigh muscles is a stronger risk factor for knee osteoarthritis in women than in men, possibly because of the greater influence that high body mass index has on thigh muscle strength in women, according to new case-control study findings.
The findings, published online Feb. 8 in Arthritis Care & Research (doi: 10.1002/acr.23182), come from a study enrolling 161 patients with radiographic knee osteoarthritis and 186 controls without arthritis at baseline who were observed over a 4-year period in the ongoing multicenter Osteoarthritis Initiative cohort study. About 60% of patients and controls were women.
They found that lower muscle-specific strength in these areas significantly increased the risk of incident knee osteoarthritis in women, with odds ratios of 1.47 (95% confidence interval, 1.10-1.96) for knee flexors and 1.41 (95% CI, 1.06-1.89) for extensors. This relationship was not significant in men, and in women, the relationship lost statistical significance after adjustment for high body mass index (BMI). Lower specific strength was associated with higher BMI in women (r = –0.29, P less than .001), but not in men.
“The lower muscle-specific strength in the presence of higher BMI in women (possibly driven by greater intramuscular adiposity), but not in men, may provide a possible explanation” for the differences in knee osteoarthritis incidence in men and women with muscle strength deficits, Dr. Culvenor and colleagues wrote in their analysis.
“The response of thigh muscle to variations in BMI differed between men and women, with apparently more contractile tissue (and strength) being present in men with greater BMI, and apparently more noncontractile (adipose) tissue in women with greater BMI,” the researchers concluded.
The Osteoarthritis Initiative is cofunded by the National Institutes of Health and a consortium of pharmacological manufacturers. The work for this analysis also received funding from Paracelsus Medical University and the European Union Seventh Framework Programme. Three of the study’s seven coauthors disclosed extensive financial relationships, including employment, with Chondrometrics GmbH, a company that provides MRI analysis services, among other firms, while four disclosed no commercial conflicts.
Weakness in the lower thigh muscles is a stronger risk factor for knee osteoarthritis in women than in men, possibly because of the greater influence that high body mass index has on thigh muscle strength in women, according to new case-control study findings.
The findings, published online Feb. 8 in Arthritis Care & Research (doi: 10.1002/acr.23182), come from a study enrolling 161 patients with radiographic knee osteoarthritis and 186 controls without arthritis at baseline who were observed over a 4-year period in the ongoing multicenter Osteoarthritis Initiative cohort study. About 60% of patients and controls were women.
They found that lower muscle-specific strength in these areas significantly increased the risk of incident knee osteoarthritis in women, with odds ratios of 1.47 (95% confidence interval, 1.10-1.96) for knee flexors and 1.41 (95% CI, 1.06-1.89) for extensors. This relationship was not significant in men, and in women, the relationship lost statistical significance after adjustment for high body mass index (BMI). Lower specific strength was associated with higher BMI in women (r = –0.29, P less than .001), but not in men.
“The lower muscle-specific strength in the presence of higher BMI in women (possibly driven by greater intramuscular adiposity), but not in men, may provide a possible explanation” for the differences in knee osteoarthritis incidence in men and women with muscle strength deficits, Dr. Culvenor and colleagues wrote in their analysis.
“The response of thigh muscle to variations in BMI differed between men and women, with apparently more contractile tissue (and strength) being present in men with greater BMI, and apparently more noncontractile (adipose) tissue in women with greater BMI,” the researchers concluded.
The Osteoarthritis Initiative is cofunded by the National Institutes of Health and a consortium of pharmacological manufacturers. The work for this analysis also received funding from Paracelsus Medical University and the European Union Seventh Framework Programme. Three of the study’s seven coauthors disclosed extensive financial relationships, including employment, with Chondrometrics GmbH, a company that provides MRI analysis services, among other firms, while four disclosed no commercial conflicts.
Weakness in the lower thigh muscles is a stronger risk factor for knee osteoarthritis in women than in men, possibly because of the greater influence that high body mass index has on thigh muscle strength in women, according to new case-control study findings.
The findings, published online Feb. 8 in Arthritis Care & Research (doi: 10.1002/acr.23182), come from a study enrolling 161 patients with radiographic knee osteoarthritis and 186 controls without arthritis at baseline who were observed over a 4-year period in the ongoing multicenter Osteoarthritis Initiative cohort study. About 60% of patients and controls were women.
They found that lower muscle-specific strength in these areas significantly increased the risk of incident knee osteoarthritis in women, with odds ratios of 1.47 (95% confidence interval, 1.10-1.96) for knee flexors and 1.41 (95% CI, 1.06-1.89) for extensors. This relationship was not significant in men, and in women, the relationship lost statistical significance after adjustment for high body mass index (BMI). Lower specific strength was associated with higher BMI in women (r = –0.29, P less than .001), but not in men.
“The lower muscle-specific strength in the presence of higher BMI in women (possibly driven by greater intramuscular adiposity), but not in men, may provide a possible explanation” for the differences in knee osteoarthritis incidence in men and women with muscle strength deficits, Dr. Culvenor and colleagues wrote in their analysis.
“The response of thigh muscle to variations in BMI differed between men and women, with apparently more contractile tissue (and strength) being present in men with greater BMI, and apparently more noncontractile (adipose) tissue in women with greater BMI,” the researchers concluded.
The Osteoarthritis Initiative is cofunded by the National Institutes of Health and a consortium of pharmacological manufacturers. The work for this analysis also received funding from Paracelsus Medical University and the European Union Seventh Framework Programme. Three of the study’s seven coauthors disclosed extensive financial relationships, including employment, with Chondrometrics GmbH, a company that provides MRI analysis services, among other firms, while four disclosed no commercial conflicts.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Weaker thigh muscle significantly increased the risk of incident knee osteoarthritis in women, but after adjusting for BMI the relationship lost significance.
Data source: Men and women (n = 161) with knee osteoarthritis and matched controls (n = 186) without osteoarthritis at baseline who were recruited from a multisite longitudinal cohort.
Disclosures: The Osteoarthritis Initiative is cofunded by the National Institutes of Health and a consortium of pharmacological manufacturers. The work for this analysis also received funding from Paracelsus Medical University and the European Union Seventh Framework Programme. Three of the study’s seven coauthors disclosed extensive financial relationships, including employment, with Chondrometrics GmbH, a company that provides MRI analysis services, among other firms, while four disclosed no commercial conflicts.
Nicardipine okay to use after pediatric cardiac surgery
HOUSTON – The use of nicardipine following cardiac surgery in children appears to be safe and effective, results from a single-center study suggest.
“There has been a traditional hesitation to use calcium channel blockers, particularly in infants, due to underdevelopment of their calcium channels,” study investigator Matthew L. Stone, MD, PhD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
“Further, these agents have commonly lacked selectivity to the vascular smooth muscles affecting both the blood vessels and the heart. Nicardipine offers a unique advantage over other calcium channel blockers in that it has more direct effects on vascular smooth muscles than it does on the actual myocardium.”
In their study, Dr. Stone, a first-year fellow in the division of cardiothoracic surgery at the University of Virginia Health System, Charlottesville, and his associates noted that nicardipine offers a favorable pharmacokinetic profile with both rapid onset and short half-life. The purpose of the study was to evaluate the use of nicardipine as a first-line agent for treatment of postoperative hypertension and to compare outcomes between children younger than 6 months of age and those older than 6 months. The researchers retrospectively reviewed the medical records of 68 children who received nicardipine for postoperative hypertension after undergoing cardiac surgery at the University of Virginia during 2010-2015. They compared the incidence of adverse postoperative events between 33 children who were younger than 6 months (group 1) and 35 who were older than 6 months (group 2). Major events including stroke or cardiogenic shock were considered failure of therapy.
Dr. Stone and his associates found that all children received nicardipine within a median of 90 minutes following cardiac surgery; 22 (33%) were started on the drug prior to leaving the operating room and most required dosing for less than 24 hours. Clinically significant hypertension that required dose titration or cessation of therapy occurred in 13% of patients, but there were no significant differences between age groups (17% in group 1 vs. 9% in group 2; P = 0.47). “While the incidence of hypotension following nicardipine administration did not reach statistical significance, it’s important to note that going forward, a lower starting dose in infants less than 6 months of age may be most appropriate. This would certainly be an important focus for future prospective study in the development of postoperative blood pressure control protocols,” said Dr. Stone.
No significant adverse events including stroke or cardiogenic shock occurred in either group. In addition, no operative or postoperative factors reviewed were associated with the development of complications during administration of nicardipine. This included cardiopulmonary bypass time, cross-clamp time, ventilator time, nicardipine duration, ICU length of stay, and hospital length of stay.
“Our traditional hesitation to use this class of agents in infants should be reevaluated,” Dr. Stone concluded. “As we move toward standardization and optimization of perioperative care, our study supports the use and prospective clinical study of nicardipine. Additionally, further pharmacologic study of dose-specific responses within myocardial and vascular smooth muscle cells may further optimize this treatment strategy and provide a more reliable standard with which to control blood pressure.
“Our traditional agents such as beta-blockers and nitroprusside have side effects that need to be considered, the most significant of which being myocardial depression and cyanide toxicity. In a limited number of very-high-risk children, we’ve shown that nicardipine may provide an option with less deleterious side effects. It’s a foundation for future study.”
Dr. Stone reported having no financial disclosures.
HOUSTON – The use of nicardipine following cardiac surgery in children appears to be safe and effective, results from a single-center study suggest.
“There has been a traditional hesitation to use calcium channel blockers, particularly in infants, due to underdevelopment of their calcium channels,” study investigator Matthew L. Stone, MD, PhD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
“Further, these agents have commonly lacked selectivity to the vascular smooth muscles affecting both the blood vessels and the heart. Nicardipine offers a unique advantage over other calcium channel blockers in that it has more direct effects on vascular smooth muscles than it does on the actual myocardium.”
In their study, Dr. Stone, a first-year fellow in the division of cardiothoracic surgery at the University of Virginia Health System, Charlottesville, and his associates noted that nicardipine offers a favorable pharmacokinetic profile with both rapid onset and short half-life. The purpose of the study was to evaluate the use of nicardipine as a first-line agent for treatment of postoperative hypertension and to compare outcomes between children younger than 6 months of age and those older than 6 months. The researchers retrospectively reviewed the medical records of 68 children who received nicardipine for postoperative hypertension after undergoing cardiac surgery at the University of Virginia during 2010-2015. They compared the incidence of adverse postoperative events between 33 children who were younger than 6 months (group 1) and 35 who were older than 6 months (group 2). Major events including stroke or cardiogenic shock were considered failure of therapy.
Dr. Stone and his associates found that all children received nicardipine within a median of 90 minutes following cardiac surgery; 22 (33%) were started on the drug prior to leaving the operating room and most required dosing for less than 24 hours. Clinically significant hypertension that required dose titration or cessation of therapy occurred in 13% of patients, but there were no significant differences between age groups (17% in group 1 vs. 9% in group 2; P = 0.47). “While the incidence of hypotension following nicardipine administration did not reach statistical significance, it’s important to note that going forward, a lower starting dose in infants less than 6 months of age may be most appropriate. This would certainly be an important focus for future prospective study in the development of postoperative blood pressure control protocols,” said Dr. Stone.
No significant adverse events including stroke or cardiogenic shock occurred in either group. In addition, no operative or postoperative factors reviewed were associated with the development of complications during administration of nicardipine. This included cardiopulmonary bypass time, cross-clamp time, ventilator time, nicardipine duration, ICU length of stay, and hospital length of stay.
“Our traditional hesitation to use this class of agents in infants should be reevaluated,” Dr. Stone concluded. “As we move toward standardization and optimization of perioperative care, our study supports the use and prospective clinical study of nicardipine. Additionally, further pharmacologic study of dose-specific responses within myocardial and vascular smooth muscle cells may further optimize this treatment strategy and provide a more reliable standard with which to control blood pressure.
“Our traditional agents such as beta-blockers and nitroprusside have side effects that need to be considered, the most significant of which being myocardial depression and cyanide toxicity. In a limited number of very-high-risk children, we’ve shown that nicardipine may provide an option with less deleterious side effects. It’s a foundation for future study.”
Dr. Stone reported having no financial disclosures.
HOUSTON – The use of nicardipine following cardiac surgery in children appears to be safe and effective, results from a single-center study suggest.
“There has been a traditional hesitation to use calcium channel blockers, particularly in infants, due to underdevelopment of their calcium channels,” study investigator Matthew L. Stone, MD, PhD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
“Further, these agents have commonly lacked selectivity to the vascular smooth muscles affecting both the blood vessels and the heart. Nicardipine offers a unique advantage over other calcium channel blockers in that it has more direct effects on vascular smooth muscles than it does on the actual myocardium.”
In their study, Dr. Stone, a first-year fellow in the division of cardiothoracic surgery at the University of Virginia Health System, Charlottesville, and his associates noted that nicardipine offers a favorable pharmacokinetic profile with both rapid onset and short half-life. The purpose of the study was to evaluate the use of nicardipine as a first-line agent for treatment of postoperative hypertension and to compare outcomes between children younger than 6 months of age and those older than 6 months. The researchers retrospectively reviewed the medical records of 68 children who received nicardipine for postoperative hypertension after undergoing cardiac surgery at the University of Virginia during 2010-2015. They compared the incidence of adverse postoperative events between 33 children who were younger than 6 months (group 1) and 35 who were older than 6 months (group 2). Major events including stroke or cardiogenic shock were considered failure of therapy.
Dr. Stone and his associates found that all children received nicardipine within a median of 90 minutes following cardiac surgery; 22 (33%) were started on the drug prior to leaving the operating room and most required dosing for less than 24 hours. Clinically significant hypertension that required dose titration or cessation of therapy occurred in 13% of patients, but there were no significant differences between age groups (17% in group 1 vs. 9% in group 2; P = 0.47). “While the incidence of hypotension following nicardipine administration did not reach statistical significance, it’s important to note that going forward, a lower starting dose in infants less than 6 months of age may be most appropriate. This would certainly be an important focus for future prospective study in the development of postoperative blood pressure control protocols,” said Dr. Stone.
No significant adverse events including stroke or cardiogenic shock occurred in either group. In addition, no operative or postoperative factors reviewed were associated with the development of complications during administration of nicardipine. This included cardiopulmonary bypass time, cross-clamp time, ventilator time, nicardipine duration, ICU length of stay, and hospital length of stay.
“Our traditional hesitation to use this class of agents in infants should be reevaluated,” Dr. Stone concluded. “As we move toward standardization and optimization of perioperative care, our study supports the use and prospective clinical study of nicardipine. Additionally, further pharmacologic study of dose-specific responses within myocardial and vascular smooth muscle cells may further optimize this treatment strategy and provide a more reliable standard with which to control blood pressure.
“Our traditional agents such as beta-blockers and nitroprusside have side effects that need to be considered, the most significant of which being myocardial depression and cyanide toxicity. In a limited number of very-high-risk children, we’ve shown that nicardipine may provide an option with less deleterious side effects. It’s a foundation for future study.”
Dr. Stone reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point:
Major finding: The incidence of adverse postoperative events was similar between children who were younger than 6 months and those who were older than 6 months (17% vs. 9%, respectively), but no significant adverse events, including stroke and cardiogenic shock, occurred in either group.
Data source: A retrospective review of 68 children who received nicardipine for postoperative hypertension after undergoing cardiac surgery during 2010-2015.
Disclosures: Dr. Stone reported having no financial disclosures.
In high-risk myeloma, look beyond VRD for induction
NEW YORK – There’s more to induction therapy for transplant-eligible multiple myeloma patients than the go-to combination of bortezomib, lenalidomide, and dexamethasone, commonly known as VRD, according to Joseph R. Mikhael, MD.
“Outstanding evidence” has emerged that demonstrates the value of combining multiple mechanisms of action, but there are numerous options available – there is a trend toward increasing use of carfilzomib, for example – and VRD isn’t necessarily the best option for all patients, nor is it available in all cases, Dr. Mikhael of Mayo Clinic Arizona, Scottsdale, said at an international congress on hematologic malignancies.
Further, choosing the treatment method shown to give the most powerful or deepest response as a default isn’t necessarily the best option, either, he said, noting data showing that the regimen most often associated with at least very good partial response (VGPR) after four cycles in newly diagnosed multiple myeloma (carfilzomib, lenalidomide, dexamethasone, or KRD, about a 90% response rate) costs 2.5 times more than does the regimen (lenalidomide, dexamethasone, or RD) that provided about a 50% response rate ($250,000 vs. $100,000 per year).“But it’s also the toxicity cost ... do we need that many [drugs]?” he said, adding that “just because a clinical trial shows that three drugs work better than two, it doesn’t mean that every time I have a patient who is eligible I’m going to go with all three.”
It may be that a patient can start on two, with a third added if needed, he added.
“That being said, we want to maximize the synergy of those three, especially in high-risk patients,” he said.
Prior to 2016, popular regimens included CyBorD (cyclophosphamide, bortezomib, dexamethasone) and VTD (bortezomib, thalidomide, dexamethasone), which were commonly used worldwide. CTD (cyclophosphamide, thalidomide, dexamethasone) was used in Brazil and other countries where it was difficult to obtain bortezomib; VRD was “king of the hill” in the United States, and transplant was routinely recommended, Dr. Mikhael said.
A number of recent studies are changing that paradigm, he said.
One such study, the prospective randomized Intergroupe Francophone du Myélome (IFM) 2013-04 trial, showed that VTD is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) for induction in patients with de novo multiple myeloma. VGPR and PR rates were significantly superior in the VTD arm.
Of note, hematologic toxicity increased in the VCD arm, and the peripheral neuropathy rate was higher in the VTD arm. However, the study was conducted at a time when bortezomib was generally used twice weekly; now it is standard to use it once weekly and subcutaneously, which significantly reduces the risk of peripheral neuropathy.
The authors advocated the preferential use of VTD over VCD for induction, based on the synergistic effect of a proteasome inhibitor and immunomodulatory drug, Dr. Mikhael said.
“Meanwhile, back in the United States, many trials were starting to be built with VRD,” he said, noting that “really extraordinary” responses were seen in phase I and phase II studies.
Perhaps the VRD study (called RVD in this case) that is most cited is the phase III IFM 2009 study comparing RVD with and without autologous stem cell transplant in newly diagnosed younger multiple myeloma patients. Although final results are still pending, interim results reported at the American Society of Hematology 2015 meeting (abstract 391) have been very helpful, Dr. Mikhael said.
The authors reported a 31% reduction in the risk of progression or death with autologous stem cell transplant versus RVD alone in patients with newly diagnosed multiple myeloma, as well as improved rates of thrombotic thrombocytopenic purpura (TTP) and minimal residual disease negativity (MRD). Mortality rates were similar.
The findings show that transplantation is feasible (93%), and associated with acceptable mortality (1.4%), an increased rate of negative MRD versus with RVD alone (80% vs. 65%), an improved 4-year progression-free survival (47% vs. 35%), and improved 4-year TTP (49% vs. 35%).
“Here the question was not so much is RVD the standard ... but is RVD plus transplant superior to RVD alone,” he said.
Surprising to many, especially the “dissenters in the crowd” who thought perhaps RVD would be the death knell to autologous stem cell transplant, transplant actually significantly improved the depth of response, he added.
Median progression-free survival at 10 months was 43% with versus 34% without transplantation.
“So there was definitely an argument here that transplant remains the standard of care,” he said, noting that transplant should be considered as decisions are made about up-front therapy.
As for the trend of bringing carfilzomib in earlier on in treatment, a number of studies, including the phase II CYKLONE study by Dr. Mikhael and his colleagues, suggest it improves the rates of deep response; some show that is especially true when used with transplant.
One study looking at KRD showed that the regimen provides high rates of deep and durable responses with acceptable tolerability – a finding that needs confirmation in a randomized setting.
Other studies are comparing KRD and VRD, and KRD and KCyd (carfilzomib, cyclophosphamide), as well as adding monoclonal antibodies such as daratumumab to treatment regimens, and results are very much anticipated, Dr. Mikhael said.
Currently, however, VRD remains the standard of care in standard risk and intermediate risk patients.
“But consideration in the highest-risk patients should be given to something like KRD,” he said. “We still recommend transplant across the board, we still recommend some form of maintenance therapy, be it with lenalidomide for most patients, but potentially bortezomib combined with carfilzomib in the highest risk patients.”
Dr. Mikhael reported consulting for Abbvie, Celgene, Onyx, and Sanofi.
NEW YORK – There’s more to induction therapy for transplant-eligible multiple myeloma patients than the go-to combination of bortezomib, lenalidomide, and dexamethasone, commonly known as VRD, according to Joseph R. Mikhael, MD.
“Outstanding evidence” has emerged that demonstrates the value of combining multiple mechanisms of action, but there are numerous options available – there is a trend toward increasing use of carfilzomib, for example – and VRD isn’t necessarily the best option for all patients, nor is it available in all cases, Dr. Mikhael of Mayo Clinic Arizona, Scottsdale, said at an international congress on hematologic malignancies.
Further, choosing the treatment method shown to give the most powerful or deepest response as a default isn’t necessarily the best option, either, he said, noting data showing that the regimen most often associated with at least very good partial response (VGPR) after four cycles in newly diagnosed multiple myeloma (carfilzomib, lenalidomide, dexamethasone, or KRD, about a 90% response rate) costs 2.5 times more than does the regimen (lenalidomide, dexamethasone, or RD) that provided about a 50% response rate ($250,000 vs. $100,000 per year).“But it’s also the toxicity cost ... do we need that many [drugs]?” he said, adding that “just because a clinical trial shows that three drugs work better than two, it doesn’t mean that every time I have a patient who is eligible I’m going to go with all three.”
It may be that a patient can start on two, with a third added if needed, he added.
“That being said, we want to maximize the synergy of those three, especially in high-risk patients,” he said.
Prior to 2016, popular regimens included CyBorD (cyclophosphamide, bortezomib, dexamethasone) and VTD (bortezomib, thalidomide, dexamethasone), which were commonly used worldwide. CTD (cyclophosphamide, thalidomide, dexamethasone) was used in Brazil and other countries where it was difficult to obtain bortezomib; VRD was “king of the hill” in the United States, and transplant was routinely recommended, Dr. Mikhael said.
A number of recent studies are changing that paradigm, he said.
One such study, the prospective randomized Intergroupe Francophone du Myélome (IFM) 2013-04 trial, showed that VTD is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) for induction in patients with de novo multiple myeloma. VGPR and PR rates were significantly superior in the VTD arm.
Of note, hematologic toxicity increased in the VCD arm, and the peripheral neuropathy rate was higher in the VTD arm. However, the study was conducted at a time when bortezomib was generally used twice weekly; now it is standard to use it once weekly and subcutaneously, which significantly reduces the risk of peripheral neuropathy.
The authors advocated the preferential use of VTD over VCD for induction, based on the synergistic effect of a proteasome inhibitor and immunomodulatory drug, Dr. Mikhael said.
“Meanwhile, back in the United States, many trials were starting to be built with VRD,” he said, noting that “really extraordinary” responses were seen in phase I and phase II studies.
Perhaps the VRD study (called RVD in this case) that is most cited is the phase III IFM 2009 study comparing RVD with and without autologous stem cell transplant in newly diagnosed younger multiple myeloma patients. Although final results are still pending, interim results reported at the American Society of Hematology 2015 meeting (abstract 391) have been very helpful, Dr. Mikhael said.
The authors reported a 31% reduction in the risk of progression or death with autologous stem cell transplant versus RVD alone in patients with newly diagnosed multiple myeloma, as well as improved rates of thrombotic thrombocytopenic purpura (TTP) and minimal residual disease negativity (MRD). Mortality rates were similar.
The findings show that transplantation is feasible (93%), and associated with acceptable mortality (1.4%), an increased rate of negative MRD versus with RVD alone (80% vs. 65%), an improved 4-year progression-free survival (47% vs. 35%), and improved 4-year TTP (49% vs. 35%).
“Here the question was not so much is RVD the standard ... but is RVD plus transplant superior to RVD alone,” he said.
Surprising to many, especially the “dissenters in the crowd” who thought perhaps RVD would be the death knell to autologous stem cell transplant, transplant actually significantly improved the depth of response, he added.
Median progression-free survival at 10 months was 43% with versus 34% without transplantation.
“So there was definitely an argument here that transplant remains the standard of care,” he said, noting that transplant should be considered as decisions are made about up-front therapy.
As for the trend of bringing carfilzomib in earlier on in treatment, a number of studies, including the phase II CYKLONE study by Dr. Mikhael and his colleagues, suggest it improves the rates of deep response; some show that is especially true when used with transplant.
One study looking at KRD showed that the regimen provides high rates of deep and durable responses with acceptable tolerability – a finding that needs confirmation in a randomized setting.
Other studies are comparing KRD and VRD, and KRD and KCyd (carfilzomib, cyclophosphamide), as well as adding monoclonal antibodies such as daratumumab to treatment regimens, and results are very much anticipated, Dr. Mikhael said.
Currently, however, VRD remains the standard of care in standard risk and intermediate risk patients.
“But consideration in the highest-risk patients should be given to something like KRD,” he said. “We still recommend transplant across the board, we still recommend some form of maintenance therapy, be it with lenalidomide for most patients, but potentially bortezomib combined with carfilzomib in the highest risk patients.”
Dr. Mikhael reported consulting for Abbvie, Celgene, Onyx, and Sanofi.
NEW YORK – There’s more to induction therapy for transplant-eligible multiple myeloma patients than the go-to combination of bortezomib, lenalidomide, and dexamethasone, commonly known as VRD, according to Joseph R. Mikhael, MD.
“Outstanding evidence” has emerged that demonstrates the value of combining multiple mechanisms of action, but there are numerous options available – there is a trend toward increasing use of carfilzomib, for example – and VRD isn’t necessarily the best option for all patients, nor is it available in all cases, Dr. Mikhael of Mayo Clinic Arizona, Scottsdale, said at an international congress on hematologic malignancies.
Further, choosing the treatment method shown to give the most powerful or deepest response as a default isn’t necessarily the best option, either, he said, noting data showing that the regimen most often associated with at least very good partial response (VGPR) after four cycles in newly diagnosed multiple myeloma (carfilzomib, lenalidomide, dexamethasone, or KRD, about a 90% response rate) costs 2.5 times more than does the regimen (lenalidomide, dexamethasone, or RD) that provided about a 50% response rate ($250,000 vs. $100,000 per year).“But it’s also the toxicity cost ... do we need that many [drugs]?” he said, adding that “just because a clinical trial shows that three drugs work better than two, it doesn’t mean that every time I have a patient who is eligible I’m going to go with all three.”
It may be that a patient can start on two, with a third added if needed, he added.
“That being said, we want to maximize the synergy of those three, especially in high-risk patients,” he said.
Prior to 2016, popular regimens included CyBorD (cyclophosphamide, bortezomib, dexamethasone) and VTD (bortezomib, thalidomide, dexamethasone), which were commonly used worldwide. CTD (cyclophosphamide, thalidomide, dexamethasone) was used in Brazil and other countries where it was difficult to obtain bortezomib; VRD was “king of the hill” in the United States, and transplant was routinely recommended, Dr. Mikhael said.
A number of recent studies are changing that paradigm, he said.
One such study, the prospective randomized Intergroupe Francophone du Myélome (IFM) 2013-04 trial, showed that VTD is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) for induction in patients with de novo multiple myeloma. VGPR and PR rates were significantly superior in the VTD arm.
Of note, hematologic toxicity increased in the VCD arm, and the peripheral neuropathy rate was higher in the VTD arm. However, the study was conducted at a time when bortezomib was generally used twice weekly; now it is standard to use it once weekly and subcutaneously, which significantly reduces the risk of peripheral neuropathy.
The authors advocated the preferential use of VTD over VCD for induction, based on the synergistic effect of a proteasome inhibitor and immunomodulatory drug, Dr. Mikhael said.
“Meanwhile, back in the United States, many trials were starting to be built with VRD,” he said, noting that “really extraordinary” responses were seen in phase I and phase II studies.
Perhaps the VRD study (called RVD in this case) that is most cited is the phase III IFM 2009 study comparing RVD with and without autologous stem cell transplant in newly diagnosed younger multiple myeloma patients. Although final results are still pending, interim results reported at the American Society of Hematology 2015 meeting (abstract 391) have been very helpful, Dr. Mikhael said.
The authors reported a 31% reduction in the risk of progression or death with autologous stem cell transplant versus RVD alone in patients with newly diagnosed multiple myeloma, as well as improved rates of thrombotic thrombocytopenic purpura (TTP) and minimal residual disease negativity (MRD). Mortality rates were similar.
The findings show that transplantation is feasible (93%), and associated with acceptable mortality (1.4%), an increased rate of negative MRD versus with RVD alone (80% vs. 65%), an improved 4-year progression-free survival (47% vs. 35%), and improved 4-year TTP (49% vs. 35%).
“Here the question was not so much is RVD the standard ... but is RVD plus transplant superior to RVD alone,” he said.
Surprising to many, especially the “dissenters in the crowd” who thought perhaps RVD would be the death knell to autologous stem cell transplant, transplant actually significantly improved the depth of response, he added.
Median progression-free survival at 10 months was 43% with versus 34% without transplantation.
“So there was definitely an argument here that transplant remains the standard of care,” he said, noting that transplant should be considered as decisions are made about up-front therapy.
As for the trend of bringing carfilzomib in earlier on in treatment, a number of studies, including the phase II CYKLONE study by Dr. Mikhael and his colleagues, suggest it improves the rates of deep response; some show that is especially true when used with transplant.
One study looking at KRD showed that the regimen provides high rates of deep and durable responses with acceptable tolerability – a finding that needs confirmation in a randomized setting.
Other studies are comparing KRD and VRD, and KRD and KCyd (carfilzomib, cyclophosphamide), as well as adding monoclonal antibodies such as daratumumab to treatment regimens, and results are very much anticipated, Dr. Mikhael said.
Currently, however, VRD remains the standard of care in standard risk and intermediate risk patients.
“But consideration in the highest-risk patients should be given to something like KRD,” he said. “We still recommend transplant across the board, we still recommend some form of maintenance therapy, be it with lenalidomide for most patients, but potentially bortezomib combined with carfilzomib in the highest risk patients.”
Dr. Mikhael reported consulting for Abbvie, Celgene, Onyx, and Sanofi.
EXPERT ANALYSIS FROM LYMPHOMA & MYELOMA
Maternal mental health: New consensus on optimal care
A new consensus bundle of recommendations calls on ob.gyns. to integrate mental health care into their care of pregnant and postpartum women.
The interdisciplinary Council on Patient Safety in Women’s Health Care issued a consensus document summarizing existing recommendations on maternal mental health and pairing them with appropriate screening and other resources. The bundle of recommendations was jointly issued by the American College of Obstetricians and Gynecologists, the National Association of Nurse Practitioners in Women’s Health, and other groups (Obstet Gynecol. 2017 Mar;129(3):422-430)*.
While no novel information is included in the bundle, the goal is to provide a streamlined document that pairs previous recommendations with resources and tools.
“Embedding a mental health professional with the women’s health care provider would seem to be the ideal, [but] in reality, this may not be available in every setting or geographic location,” Susan Kendig, JD, MSN, WHNP-BC, FAANP, policy director for the National Association of Nurse Practitioners in Women’s Health, and one of the authors of the document, said in an interview. “The purpose of the consensus bundle is to provide a framework for women’s health care providers to use in developing a system of care that includes standardized risk assessment for existing or emerging mental health issues, strategies for brief interventions, identification of community resources available to the woman, and/or resources to support the provider in addressing the woman’s needs and facilitating appropriate referrals, and addressing emergencies to prevent harm.”
The framework is grouped in four domains: readiness, recognition and prevention, response, and reporting and systems learning.
Readiness is focused largely on setting up clinical and administrative protocols, including mental health screening that is seamlessly integrated into the patient visit, established algorithms that are triggered according to screening results, and designated staff to both educate colleagues on the protocols and to drive their implementation.
The goal for physicians in all settings, according to the authors, is to balance cost, availability of tools, ease of use, and the validity of the tools against the need to capture often unrecognized signs and symptoms of mood and anxiety disorders, including changes in sleep patterns, appetite, or anxiety levels that often are attributed to the physiologic and neuroendocrinologic changes inherent in childbirth. More than 80% of women know something is “off” but do not bring up their symptoms to their clinician, according to the recommendation document.
“Coordination and team cohesiveness is not necessarily dependent on co-location,” Ms. Kendig said. “Virtual teams, where care is coordinated among providers at different locations, can also achieve positive outcomes.”
The follow-on to this – recognition and prevention – views every patient visit as an opportunity to draw a thorough picture of mental and physical health by taking a complete family and patient history, maintaining routine screening, and offering psychoeducation to patients and their families or others on whom they rely for emotional support.
When a woman does screen positive for perinatal mood or anxiety disorders, the next bundle offers a rough outline of a stage-based response for intervention and follow-up.
“Screening alone does not appear to improve pregnancy or maternal-child outcomes,” the authors wrote.
Several possible algorithms are included in the document, including for emergent mental health concerns such as suicidal or homicidal ideation.
The final domain relies on data capture, taking a systems approach to not only delivering care, but also improving it. This includes scheduling regular staff debriefings after severe maternal mental health crises, troubleshooting why some patients become lost to follow-up, and examining data to see patterns.
On Twitter @whitneymcknight
CORRECTION, 3/20/17: An earlier version of this article misstated the citation.
A new consensus bundle of recommendations calls on ob.gyns. to integrate mental health care into their care of pregnant and postpartum women.
The interdisciplinary Council on Patient Safety in Women’s Health Care issued a consensus document summarizing existing recommendations on maternal mental health and pairing them with appropriate screening and other resources. The bundle of recommendations was jointly issued by the American College of Obstetricians and Gynecologists, the National Association of Nurse Practitioners in Women’s Health, and other groups (Obstet Gynecol. 2017 Mar;129(3):422-430)*.
While no novel information is included in the bundle, the goal is to provide a streamlined document that pairs previous recommendations with resources and tools.
“Embedding a mental health professional with the women’s health care provider would seem to be the ideal, [but] in reality, this may not be available in every setting or geographic location,” Susan Kendig, JD, MSN, WHNP-BC, FAANP, policy director for the National Association of Nurse Practitioners in Women’s Health, and one of the authors of the document, said in an interview. “The purpose of the consensus bundle is to provide a framework for women’s health care providers to use in developing a system of care that includes standardized risk assessment for existing or emerging mental health issues, strategies for brief interventions, identification of community resources available to the woman, and/or resources to support the provider in addressing the woman’s needs and facilitating appropriate referrals, and addressing emergencies to prevent harm.”
The framework is grouped in four domains: readiness, recognition and prevention, response, and reporting and systems learning.
Readiness is focused largely on setting up clinical and administrative protocols, including mental health screening that is seamlessly integrated into the patient visit, established algorithms that are triggered according to screening results, and designated staff to both educate colleagues on the protocols and to drive their implementation.
The goal for physicians in all settings, according to the authors, is to balance cost, availability of tools, ease of use, and the validity of the tools against the need to capture often unrecognized signs and symptoms of mood and anxiety disorders, including changes in sleep patterns, appetite, or anxiety levels that often are attributed to the physiologic and neuroendocrinologic changes inherent in childbirth. More than 80% of women know something is “off” but do not bring up their symptoms to their clinician, according to the recommendation document.
“Coordination and team cohesiveness is not necessarily dependent on co-location,” Ms. Kendig said. “Virtual teams, where care is coordinated among providers at different locations, can also achieve positive outcomes.”
The follow-on to this – recognition and prevention – views every patient visit as an opportunity to draw a thorough picture of mental and physical health by taking a complete family and patient history, maintaining routine screening, and offering psychoeducation to patients and their families or others on whom they rely for emotional support.
When a woman does screen positive for perinatal mood or anxiety disorders, the next bundle offers a rough outline of a stage-based response for intervention and follow-up.
“Screening alone does not appear to improve pregnancy or maternal-child outcomes,” the authors wrote.
Several possible algorithms are included in the document, including for emergent mental health concerns such as suicidal or homicidal ideation.
The final domain relies on data capture, taking a systems approach to not only delivering care, but also improving it. This includes scheduling regular staff debriefings after severe maternal mental health crises, troubleshooting why some patients become lost to follow-up, and examining data to see patterns.
On Twitter @whitneymcknight
CORRECTION, 3/20/17: An earlier version of this article misstated the citation.
A new consensus bundle of recommendations calls on ob.gyns. to integrate mental health care into their care of pregnant and postpartum women.
The interdisciplinary Council on Patient Safety in Women’s Health Care issued a consensus document summarizing existing recommendations on maternal mental health and pairing them with appropriate screening and other resources. The bundle of recommendations was jointly issued by the American College of Obstetricians and Gynecologists, the National Association of Nurse Practitioners in Women’s Health, and other groups (Obstet Gynecol. 2017 Mar;129(3):422-430)*.
While no novel information is included in the bundle, the goal is to provide a streamlined document that pairs previous recommendations with resources and tools.
“Embedding a mental health professional with the women’s health care provider would seem to be the ideal, [but] in reality, this may not be available in every setting or geographic location,” Susan Kendig, JD, MSN, WHNP-BC, FAANP, policy director for the National Association of Nurse Practitioners in Women’s Health, and one of the authors of the document, said in an interview. “The purpose of the consensus bundle is to provide a framework for women’s health care providers to use in developing a system of care that includes standardized risk assessment for existing or emerging mental health issues, strategies for brief interventions, identification of community resources available to the woman, and/or resources to support the provider in addressing the woman’s needs and facilitating appropriate referrals, and addressing emergencies to prevent harm.”
The framework is grouped in four domains: readiness, recognition and prevention, response, and reporting and systems learning.
Readiness is focused largely on setting up clinical and administrative protocols, including mental health screening that is seamlessly integrated into the patient visit, established algorithms that are triggered according to screening results, and designated staff to both educate colleagues on the protocols and to drive their implementation.
The goal for physicians in all settings, according to the authors, is to balance cost, availability of tools, ease of use, and the validity of the tools against the need to capture often unrecognized signs and symptoms of mood and anxiety disorders, including changes in sleep patterns, appetite, or anxiety levels that often are attributed to the physiologic and neuroendocrinologic changes inherent in childbirth. More than 80% of women know something is “off” but do not bring up their symptoms to their clinician, according to the recommendation document.
“Coordination and team cohesiveness is not necessarily dependent on co-location,” Ms. Kendig said. “Virtual teams, where care is coordinated among providers at different locations, can also achieve positive outcomes.”
The follow-on to this – recognition and prevention – views every patient visit as an opportunity to draw a thorough picture of mental and physical health by taking a complete family and patient history, maintaining routine screening, and offering psychoeducation to patients and their families or others on whom they rely for emotional support.
When a woman does screen positive for perinatal mood or anxiety disorders, the next bundle offers a rough outline of a stage-based response for intervention and follow-up.
“Screening alone does not appear to improve pregnancy or maternal-child outcomes,” the authors wrote.
Several possible algorithms are included in the document, including for emergent mental health concerns such as suicidal or homicidal ideation.
The final domain relies on data capture, taking a systems approach to not only delivering care, but also improving it. This includes scheduling regular staff debriefings after severe maternal mental health crises, troubleshooting why some patients become lost to follow-up, and examining data to see patterns.
On Twitter @whitneymcknight
CORRECTION, 3/20/17: An earlier version of this article misstated the citation.
FROM OBSTETRICS & GYNECOLOGY
iPSCs used to identify potential treatment for DBA
from cells from a DBA patient
Image courtesy of
Boston Children’s Hospital
Researchers have used induced pluripotent stem cells (iPSCs) to identify a compound that could treat Diamond Blackfan anemia (DBA).
The team used iPSCs to generate expandable hematopoietic progenitor cells (HPCs) that recapitulate the defects in erythroid differentiation observed in patients with DBA.
The researchers then used the HPCs to screen chemical compounds that might be used to treat DBA.
One of these compounds, SMER28, enhanced erythropoiesis in models of DBA.
“It is very satisfying as physician-scientists to find new potential treatments for rare blood diseases such as Diamond Blackfan anemia,” said study author Leonard Zon, MD, of Boston Children’s Hospital and Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Zon and his colleagues described this work in Science Translational Medicine.
The researchers first took fibroblasts from 2 patients with DBA and reprogrammed them into iPSCs. From the iPSCs, the team generated HPCs, which they loaded into a high-throughput drug screening system.
Testing a library of 1440 chemicals, the researchers found several that showed promise for treating DBA in vitro. One compound, SMER28, was able to induce red blood cell (RBC) production in mice and zebrafish.
The researchers believe this study marks an important advance in the stem cell field.
“[iPSCs] have been hard to instruct when it comes to making blood,” said study author Sergei Doulatov, PhD, of the University of Washington in Seattle.
“This is the first time [iPSCs] have been used to identify a drug to treat a blood disorder.”
Making RBCs
As in DBA itself, the patient-derived HPCs, studied in vitro, failed to generate erythroid cells. The same was true when the cells were transplanted into mice.
However, the chemical screen got several “hits.” In wells loaded with certain chemicals, erythroid cells began appearing. Because of its especially strong effect, SMER28 was put through additional testing.
When SMER28 was used to treat the marrow in zebrafish and mouse models of DBA, the animals made erythroid progenitor cells that, in turn, made RBCs, reversing or stabilizing anemia. The same was true in cells from DBA patients transplanted into mice.
The higher the dose of SMER28, the more RBCs were produced, and no ill effects were found. However, formal toxicity studies have not been conducted.
SMER28 has been tested preclinically for some neurodegenerative diseases. It activates an autophagy pathway that recycles damaged cellular components.
In DBA, SMER28 appears to turn on autophagy in erythroid progenitors. Dr Doulatov plans to further explore how this interferes with RBC production. ![]()

from cells from a DBA patient
Image courtesy of
Boston Children’s Hospital
Researchers have used induced pluripotent stem cells (iPSCs) to identify a compound that could treat Diamond Blackfan anemia (DBA).
The team used iPSCs to generate expandable hematopoietic progenitor cells (HPCs) that recapitulate the defects in erythroid differentiation observed in patients with DBA.
The researchers then used the HPCs to screen chemical compounds that might be used to treat DBA.
One of these compounds, SMER28, enhanced erythropoiesis in models of DBA.
“It is very satisfying as physician-scientists to find new potential treatments for rare blood diseases such as Diamond Blackfan anemia,” said study author Leonard Zon, MD, of Boston Children’s Hospital and Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Zon and his colleagues described this work in Science Translational Medicine.
The researchers first took fibroblasts from 2 patients with DBA and reprogrammed them into iPSCs. From the iPSCs, the team generated HPCs, which they loaded into a high-throughput drug screening system.
Testing a library of 1440 chemicals, the researchers found several that showed promise for treating DBA in vitro. One compound, SMER28, was able to induce red blood cell (RBC) production in mice and zebrafish.
The researchers believe this study marks an important advance in the stem cell field.
“[iPSCs] have been hard to instruct when it comes to making blood,” said study author Sergei Doulatov, PhD, of the University of Washington in Seattle.
“This is the first time [iPSCs] have been used to identify a drug to treat a blood disorder.”
Making RBCs
As in DBA itself, the patient-derived HPCs, studied in vitro, failed to generate erythroid cells. The same was true when the cells were transplanted into mice.
However, the chemical screen got several “hits.” In wells loaded with certain chemicals, erythroid cells began appearing. Because of its especially strong effect, SMER28 was put through additional testing.
When SMER28 was used to treat the marrow in zebrafish and mouse models of DBA, the animals made erythroid progenitor cells that, in turn, made RBCs, reversing or stabilizing anemia. The same was true in cells from DBA patients transplanted into mice.
The higher the dose of SMER28, the more RBCs were produced, and no ill effects were found. However, formal toxicity studies have not been conducted.
SMER28 has been tested preclinically for some neurodegenerative diseases. It activates an autophagy pathway that recycles damaged cellular components.
In DBA, SMER28 appears to turn on autophagy in erythroid progenitors. Dr Doulatov plans to further explore how this interferes with RBC production. ![]()

from cells from a DBA patient
Image courtesy of
Boston Children’s Hospital
Researchers have used induced pluripotent stem cells (iPSCs) to identify a compound that could treat Diamond Blackfan anemia (DBA).
The team used iPSCs to generate expandable hematopoietic progenitor cells (HPCs) that recapitulate the defects in erythroid differentiation observed in patients with DBA.
The researchers then used the HPCs to screen chemical compounds that might be used to treat DBA.
One of these compounds, SMER28, enhanced erythropoiesis in models of DBA.
“It is very satisfying as physician-scientists to find new potential treatments for rare blood diseases such as Diamond Blackfan anemia,” said study author Leonard Zon, MD, of Boston Children’s Hospital and Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Zon and his colleagues described this work in Science Translational Medicine.
The researchers first took fibroblasts from 2 patients with DBA and reprogrammed them into iPSCs. From the iPSCs, the team generated HPCs, which they loaded into a high-throughput drug screening system.
Testing a library of 1440 chemicals, the researchers found several that showed promise for treating DBA in vitro. One compound, SMER28, was able to induce red blood cell (RBC) production in mice and zebrafish.
The researchers believe this study marks an important advance in the stem cell field.
“[iPSCs] have been hard to instruct when it comes to making blood,” said study author Sergei Doulatov, PhD, of the University of Washington in Seattle.
“This is the first time [iPSCs] have been used to identify a drug to treat a blood disorder.”
Making RBCs
As in DBA itself, the patient-derived HPCs, studied in vitro, failed to generate erythroid cells. The same was true when the cells were transplanted into mice.
However, the chemical screen got several “hits.” In wells loaded with certain chemicals, erythroid cells began appearing. Because of its especially strong effect, SMER28 was put through additional testing.
When SMER28 was used to treat the marrow in zebrafish and mouse models of DBA, the animals made erythroid progenitor cells that, in turn, made RBCs, reversing or stabilizing anemia. The same was true in cells from DBA patients transplanted into mice.
The higher the dose of SMER28, the more RBCs were produced, and no ill effects were found. However, formal toxicity studies have not been conducted.
SMER28 has been tested preclinically for some neurodegenerative diseases. It activates an autophagy pathway that recycles damaged cellular components.
In DBA, SMER28 appears to turn on autophagy in erythroid progenitors. Dr Doulatov plans to further explore how this interferes with RBC production. ![]()