User login
Resistant malaria parasite spreading through Asia

Image courtesy of
CDC/Mae Melvin
A lineage of multidrug-resistant malaria parasite has spread widely and is now established in parts of Thailand, Laos, and Cambodia, according to a study published in The Lancet Infectious Diseases.
The
researchers said their findings suggest an artemisinin-resistant
Plasmodium falciparum lineage probably arose in western Cambodia and
then spread to Thailand and Laos, outcompeting other parasites and
acquiring resistance to piperaquine.
“We now see this very successful, resistant parasite lineage emerging, outcompeting its peers, and spreading over a wide area,” said study author Arjen Dondorp, MD, of Mahidol University in Bangkok, Thailand, and the University of Oxford in Oxford, UK.
“It has also picked up resistance to the partner drug piperaquine, causing high failure rates of the widely used artemisinin combination therapy, DHA-piperaquine. We hope this evidence will be used to re-emphasize the urgency of malaria elimination in the Asia region before falciparum malaria becomes close to untreatable.”
For this study, Dr Dondorp and his colleagues examined blood spot samples from patients with uncomplicated, P falciparum malaria collected at sites in Cambodia, Laos, Thailand, and Myanmar.
The researchers found evidence to suggest that PfKelch13 C580Y, a single mutant parasite lineage, has spread across Cambodia, Laos, and Thailand, replacing parasites containing other, less resistant mutations.
Although the C580Y mutation does not confer a higher level of artemisinin resistance than many other PfKelch13 mutations, it appears to be fitter, more transmissible, and spreading more widely.
“It isn’t that the C580Y mutation itself makes the malaria parasites fitter, it is the other genetic changes that go along with it—hence, the critical emphasis on the term ‘lineage,’” said Nicholas White, also of Mahidol University and the University of Oxford.
“This is what makes superbugs—the evolution of multiple factors that make them fitter and more transmissible. The spread and emergence of drug-resistant malaria parasites across Asia into Africa has occurred before. Last time, it killed millions. We need to work with our policy, research, and funding partners to respond to this threat in Asia urgently to avoid history repeating itself.”
In particular, the researchers are calling for accelerated efforts in the Greater Mekong subregion and closer collaboration to monitor any further spread in neighboring regions.
“We are losing a dangerous race to eliminate artemisinin-resistant falciparum malaria before widespread resistance to the partner antimalarials makes that impossible,” White said. “The consequences of resistance spreading further into India and Africa could be grave if drug resistance is not tackled from a global public health emergency perspective.” ![]()

Image courtesy of
CDC/Mae Melvin
A lineage of multidrug-resistant malaria parasite has spread widely and is now established in parts of Thailand, Laos, and Cambodia, according to a study published in The Lancet Infectious Diseases.
The
researchers said their findings suggest an artemisinin-resistant
Plasmodium falciparum lineage probably arose in western Cambodia and
then spread to Thailand and Laos, outcompeting other parasites and
acquiring resistance to piperaquine.
“We now see this very successful, resistant parasite lineage emerging, outcompeting its peers, and spreading over a wide area,” said study author Arjen Dondorp, MD, of Mahidol University in Bangkok, Thailand, and the University of Oxford in Oxford, UK.
“It has also picked up resistance to the partner drug piperaquine, causing high failure rates of the widely used artemisinin combination therapy, DHA-piperaquine. We hope this evidence will be used to re-emphasize the urgency of malaria elimination in the Asia region before falciparum malaria becomes close to untreatable.”
For this study, Dr Dondorp and his colleagues examined blood spot samples from patients with uncomplicated, P falciparum malaria collected at sites in Cambodia, Laos, Thailand, and Myanmar.
The researchers found evidence to suggest that PfKelch13 C580Y, a single mutant parasite lineage, has spread across Cambodia, Laos, and Thailand, replacing parasites containing other, less resistant mutations.
Although the C580Y mutation does not confer a higher level of artemisinin resistance than many other PfKelch13 mutations, it appears to be fitter, more transmissible, and spreading more widely.
“It isn’t that the C580Y mutation itself makes the malaria parasites fitter, it is the other genetic changes that go along with it—hence, the critical emphasis on the term ‘lineage,’” said Nicholas White, also of Mahidol University and the University of Oxford.
“This is what makes superbugs—the evolution of multiple factors that make them fitter and more transmissible. The spread and emergence of drug-resistant malaria parasites across Asia into Africa has occurred before. Last time, it killed millions. We need to work with our policy, research, and funding partners to respond to this threat in Asia urgently to avoid history repeating itself.”
In particular, the researchers are calling for accelerated efforts in the Greater Mekong subregion and closer collaboration to monitor any further spread in neighboring regions.
“We are losing a dangerous race to eliminate artemisinin-resistant falciparum malaria before widespread resistance to the partner antimalarials makes that impossible,” White said. “The consequences of resistance spreading further into India and Africa could be grave if drug resistance is not tackled from a global public health emergency perspective.” ![]()

Image courtesy of
CDC/Mae Melvin
A lineage of multidrug-resistant malaria parasite has spread widely and is now established in parts of Thailand, Laos, and Cambodia, according to a study published in The Lancet Infectious Diseases.
The
researchers said their findings suggest an artemisinin-resistant
Plasmodium falciparum lineage probably arose in western Cambodia and
then spread to Thailand and Laos, outcompeting other parasites and
acquiring resistance to piperaquine.
“We now see this very successful, resistant parasite lineage emerging, outcompeting its peers, and spreading over a wide area,” said study author Arjen Dondorp, MD, of Mahidol University in Bangkok, Thailand, and the University of Oxford in Oxford, UK.
“It has also picked up resistance to the partner drug piperaquine, causing high failure rates of the widely used artemisinin combination therapy, DHA-piperaquine. We hope this evidence will be used to re-emphasize the urgency of malaria elimination in the Asia region before falciparum malaria becomes close to untreatable.”
For this study, Dr Dondorp and his colleagues examined blood spot samples from patients with uncomplicated, P falciparum malaria collected at sites in Cambodia, Laos, Thailand, and Myanmar.
The researchers found evidence to suggest that PfKelch13 C580Y, a single mutant parasite lineage, has spread across Cambodia, Laos, and Thailand, replacing parasites containing other, less resistant mutations.
Although the C580Y mutation does not confer a higher level of artemisinin resistance than many other PfKelch13 mutations, it appears to be fitter, more transmissible, and spreading more widely.
“It isn’t that the C580Y mutation itself makes the malaria parasites fitter, it is the other genetic changes that go along with it—hence, the critical emphasis on the term ‘lineage,’” said Nicholas White, also of Mahidol University and the University of Oxford.
“This is what makes superbugs—the evolution of multiple factors that make them fitter and more transmissible. The spread and emergence of drug-resistant malaria parasites across Asia into Africa has occurred before. Last time, it killed millions. We need to work with our policy, research, and funding partners to respond to this threat in Asia urgently to avoid history repeating itself.”
In particular, the researchers are calling for accelerated efforts in the Greater Mekong subregion and closer collaboration to monitor any further spread in neighboring regions.
“We are losing a dangerous race to eliminate artemisinin-resistant falciparum malaria before widespread resistance to the partner antimalarials makes that impossible,” White said. “The consequences of resistance spreading further into India and Africa could be grave if drug resistance is not tackled from a global public health emergency perspective.” ![]()
FDA approves IVIG product for PI and chronic ITP

Photo by Bill Branson
The US Food and Drug Administration (FDA) has approved an intravenous immunoglobulin (IVIG) product (Gammaplex® 10%) for the treatment of primary immunodeficiency (PI) and chronic immune thrombocytopenia (ITP) in adults.
PI includes, but is not limited to,
the humoral immune defect in common variable immunodeficiency, X-linked and congenital
agammaglobulinemia, Wiskott-Aldrich
syndrome, and severe combined immunodeficiencies.
Gammaplex 10% is manufactured by Bio Products Laboratory Limited (BPL).
Gammaplex 10% is made with the same process as BPL’s previously approved IVIG treatment, Gammaplex® 5% (immune globulin intravenous [human], 5% liquid).
Gammaplex 10% is more concentrated than Gammaplex 5%, with an immune globulin G (IgG) concentration of 100 g/L, and is stabilized with glycine.
The FDA’s approval of Gammaplex 10% was based on a 2-phase, crossover bioequivalence study comparing Gammaplex 10% and Gammaplex 5% in 33 adult patients with PI. This study is the first direct comparison of 10% and 5% IVIG products in the treatment of PI.
The primary endpoint of bioequivalence between the products was achieved, and trough levels of IgG were well maintained throughout the study.
Both Gammaplex 10% and Gammaplex 5% infusion rates were increased incrementally at 15-minute intervals if tolerated by the subject. The Gammaplex 10% infusion rate was increased to the maximum in 96% of infusions.
The mean infusion time for Gammaplex 10% was 1 hour and 51 minutes, which was 57 minutes faster than Gammaplex 5%.
There were no notable differences in the safety and tolerability of the 2 products.
The most common adverse events in patients receiving Gammaplex 10% were headache (12.5%), migraine (6.3%), and pyrexia (6.3%). There were no serious product-related adverse events.
The safety of Gammaplex 10% has not been established in adults with chronic ITP. The safety profile for Gammaplex 5% has been studied in a phase 3 trial of adults with chronic ITP, and it is anticipated that the safety profile for both formulations are comparable for ITP patients.
The most common adverse events in adults with chronic ITP receiving Gammaplex 5% were headache, vomiting, nausea, pyrexia, arthralgia, and dehydration. Serious adverse events were headache, vomiting, and dehydration.
The full prescribing information for Gammaplex 10%, which includes trial data, is available at http://www.gammaplex.com. ![]()

Photo by Bill Branson
The US Food and Drug Administration (FDA) has approved an intravenous immunoglobulin (IVIG) product (Gammaplex® 10%) for the treatment of primary immunodeficiency (PI) and chronic immune thrombocytopenia (ITP) in adults.
PI includes, but is not limited to,
the humoral immune defect in common variable immunodeficiency, X-linked and congenital
agammaglobulinemia, Wiskott-Aldrich
syndrome, and severe combined immunodeficiencies.
Gammaplex 10% is manufactured by Bio Products Laboratory Limited (BPL).
Gammaplex 10% is made with the same process as BPL’s previously approved IVIG treatment, Gammaplex® 5% (immune globulin intravenous [human], 5% liquid).
Gammaplex 10% is more concentrated than Gammaplex 5%, with an immune globulin G (IgG) concentration of 100 g/L, and is stabilized with glycine.
The FDA’s approval of Gammaplex 10% was based on a 2-phase, crossover bioequivalence study comparing Gammaplex 10% and Gammaplex 5% in 33 adult patients with PI. This study is the first direct comparison of 10% and 5% IVIG products in the treatment of PI.
The primary endpoint of bioequivalence between the products was achieved, and trough levels of IgG were well maintained throughout the study.
Both Gammaplex 10% and Gammaplex 5% infusion rates were increased incrementally at 15-minute intervals if tolerated by the subject. The Gammaplex 10% infusion rate was increased to the maximum in 96% of infusions.
The mean infusion time for Gammaplex 10% was 1 hour and 51 minutes, which was 57 minutes faster than Gammaplex 5%.
There were no notable differences in the safety and tolerability of the 2 products.
The most common adverse events in patients receiving Gammaplex 10% were headache (12.5%), migraine (6.3%), and pyrexia (6.3%). There were no serious product-related adverse events.
The safety of Gammaplex 10% has not been established in adults with chronic ITP. The safety profile for Gammaplex 5% has been studied in a phase 3 trial of adults with chronic ITP, and it is anticipated that the safety profile for both formulations are comparable for ITP patients.
The most common adverse events in adults with chronic ITP receiving Gammaplex 5% were headache, vomiting, nausea, pyrexia, arthralgia, and dehydration. Serious adverse events were headache, vomiting, and dehydration.
The full prescribing information for Gammaplex 10%, which includes trial data, is available at http://www.gammaplex.com. ![]()

Photo by Bill Branson
The US Food and Drug Administration (FDA) has approved an intravenous immunoglobulin (IVIG) product (Gammaplex® 10%) for the treatment of primary immunodeficiency (PI) and chronic immune thrombocytopenia (ITP) in adults.
PI includes, but is not limited to,
the humoral immune defect in common variable immunodeficiency, X-linked and congenital
agammaglobulinemia, Wiskott-Aldrich
syndrome, and severe combined immunodeficiencies.
Gammaplex 10% is manufactured by Bio Products Laboratory Limited (BPL).
Gammaplex 10% is made with the same process as BPL’s previously approved IVIG treatment, Gammaplex® 5% (immune globulin intravenous [human], 5% liquid).
Gammaplex 10% is more concentrated than Gammaplex 5%, with an immune globulin G (IgG) concentration of 100 g/L, and is stabilized with glycine.
The FDA’s approval of Gammaplex 10% was based on a 2-phase, crossover bioequivalence study comparing Gammaplex 10% and Gammaplex 5% in 33 adult patients with PI. This study is the first direct comparison of 10% and 5% IVIG products in the treatment of PI.
The primary endpoint of bioequivalence between the products was achieved, and trough levels of IgG were well maintained throughout the study.
Both Gammaplex 10% and Gammaplex 5% infusion rates were increased incrementally at 15-minute intervals if tolerated by the subject. The Gammaplex 10% infusion rate was increased to the maximum in 96% of infusions.
The mean infusion time for Gammaplex 10% was 1 hour and 51 minutes, which was 57 minutes faster than Gammaplex 5%.
There were no notable differences in the safety and tolerability of the 2 products.
The most common adverse events in patients receiving Gammaplex 10% were headache (12.5%), migraine (6.3%), and pyrexia (6.3%). There were no serious product-related adverse events.
The safety of Gammaplex 10% has not been established in adults with chronic ITP. The safety profile for Gammaplex 5% has been studied in a phase 3 trial of adults with chronic ITP, and it is anticipated that the safety profile for both formulations are comparable for ITP patients.
The most common adverse events in adults with chronic ITP receiving Gammaplex 5% were headache, vomiting, nausea, pyrexia, arthralgia, and dehydration. Serious adverse events were headache, vomiting, and dehydration.
The full prescribing information for Gammaplex 10%, which includes trial data, is available at http://www.gammaplex.com. ![]()
Investigators report new risk loci for CLL

Investigators say they have identified 9 new risk loci for chronic lymphocytic leukemia (CLL).
The team says the research, published in Nature Communications, provides additional evidence for genetic susceptibility to CLL and sheds new light on the biological basis of CLL development.
They also believe their findings could aid the development of new drugs for CLL or help in selecting existing therapies for CLL
patients.
“We knew people were more likely to develop chronic lymphocytic leukemia if someone in their family had suffered from the disease, but our new research takes a big step towards explaining the underlying genetics,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
“CLL is essentially a disease of the immune system, and it’s fascinating that so many of the new genetic variants we have uncovered seem to directly affect the behavior of white blood cells and their ability to fight disease. Understanding the genetics of CLL can point us towards new treatments for the disease and help us to use existing targeted drugs more effectively.”
For this study, Dr Houlston and his colleagues analyzed data from 8 studies involving a total of 6200 CLL patients and 17,598 controls.
From this, the team identified 9 CLL risk loci:
- 1p36.11 (rs34676223, P=5.04 × 10−13)
- 1q42.13 (rs41271473, P=1.06 × 10−10)
- 4q24 (rs71597109, P=1.37 × 10−10)
- 4q35.1 (rs57214277, P=3.69 × 10−8)
- 6p21.31 (rs3800461, P=1.97 × 10−8)
- 11q23.2 (rs61904987, P=2.64 × 10−11)
- 18q21.1 (rs1036935, P=3.27 × 10−8)
- 19p13.3 (rs7254272, P=4.67 × 10−8)
- 22q13.33 (rs140522, P=2.70 × 10−9).
The investigators noted that the 4q24 association marked by rs71597109 maps to intron 1 of the gene encoding BANK1 (B-cell scaffold protein with ankyrin repeats 1). BANK1 is only ever activated in B cells and is linked to the autoimmune disease lupus.
The team also pointed out that the 19p13.3 association marked by rs7254272 maps 2.5 kb 5′ to ZBTB7A (zinc finger and BTB domain-containing protein 7a), which is a master regulator of B versus T lymphoid fate. So errors in ZBTB7A could lead to too many B cells in the bloodstream and bone marrow.
And rs140522 maps to 22q13.33, which has been linked to the development of multiple sclerosis. The investigators noted that this region of linkage disequilibrium contains 4 genes. One of them, NCAPH2 (non-SMC condensin II complex subunit H2), is differentially expressed in CLL and normal B cells.
“This fascinating study makes a link between genetic variants in the immune system and the development of leukemia and implicates regions of DNA which are also involved in autoimmune diseases,” said Paul Workman, PhD, chief executive and president of The Institute of Cancer Research, who was not involved in this research.
“The findings could point us towards new ways of treating leukemia or better ways of using existing treatments—potentially including immunotherapies.” ![]()

Investigators say they have identified 9 new risk loci for chronic lymphocytic leukemia (CLL).
The team says the research, published in Nature Communications, provides additional evidence for genetic susceptibility to CLL and sheds new light on the biological basis of CLL development.
They also believe their findings could aid the development of new drugs for CLL or help in selecting existing therapies for CLL
patients.
“We knew people were more likely to develop chronic lymphocytic leukemia if someone in their family had suffered from the disease, but our new research takes a big step towards explaining the underlying genetics,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
“CLL is essentially a disease of the immune system, and it’s fascinating that so many of the new genetic variants we have uncovered seem to directly affect the behavior of white blood cells and their ability to fight disease. Understanding the genetics of CLL can point us towards new treatments for the disease and help us to use existing targeted drugs more effectively.”
For this study, Dr Houlston and his colleagues analyzed data from 8 studies involving a total of 6200 CLL patients and 17,598 controls.
From this, the team identified 9 CLL risk loci:
- 1p36.11 (rs34676223, P=5.04 × 10−13)
- 1q42.13 (rs41271473, P=1.06 × 10−10)
- 4q24 (rs71597109, P=1.37 × 10−10)
- 4q35.1 (rs57214277, P=3.69 × 10−8)
- 6p21.31 (rs3800461, P=1.97 × 10−8)
- 11q23.2 (rs61904987, P=2.64 × 10−11)
- 18q21.1 (rs1036935, P=3.27 × 10−8)
- 19p13.3 (rs7254272, P=4.67 × 10−8)
- 22q13.33 (rs140522, P=2.70 × 10−9).
The investigators noted that the 4q24 association marked by rs71597109 maps to intron 1 of the gene encoding BANK1 (B-cell scaffold protein with ankyrin repeats 1). BANK1 is only ever activated in B cells and is linked to the autoimmune disease lupus.
The team also pointed out that the 19p13.3 association marked by rs7254272 maps 2.5 kb 5′ to ZBTB7A (zinc finger and BTB domain-containing protein 7a), which is a master regulator of B versus T lymphoid fate. So errors in ZBTB7A could lead to too many B cells in the bloodstream and bone marrow.
And rs140522 maps to 22q13.33, which has been linked to the development of multiple sclerosis. The investigators noted that this region of linkage disequilibrium contains 4 genes. One of them, NCAPH2 (non-SMC condensin II complex subunit H2), is differentially expressed in CLL and normal B cells.
“This fascinating study makes a link between genetic variants in the immune system and the development of leukemia and implicates regions of DNA which are also involved in autoimmune diseases,” said Paul Workman, PhD, chief executive and president of The Institute of Cancer Research, who was not involved in this research.
“The findings could point us towards new ways of treating leukemia or better ways of using existing treatments—potentially including immunotherapies.” ![]()

Investigators say they have identified 9 new risk loci for chronic lymphocytic leukemia (CLL).
The team says the research, published in Nature Communications, provides additional evidence for genetic susceptibility to CLL and sheds new light on the biological basis of CLL development.
They also believe their findings could aid the development of new drugs for CLL or help in selecting existing therapies for CLL
patients.
“We knew people were more likely to develop chronic lymphocytic leukemia if someone in their family had suffered from the disease, but our new research takes a big step towards explaining the underlying genetics,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
“CLL is essentially a disease of the immune system, and it’s fascinating that so many of the new genetic variants we have uncovered seem to directly affect the behavior of white blood cells and their ability to fight disease. Understanding the genetics of CLL can point us towards new treatments for the disease and help us to use existing targeted drugs more effectively.”
For this study, Dr Houlston and his colleagues analyzed data from 8 studies involving a total of 6200 CLL patients and 17,598 controls.
From this, the team identified 9 CLL risk loci:
- 1p36.11 (rs34676223, P=5.04 × 10−13)
- 1q42.13 (rs41271473, P=1.06 × 10−10)
- 4q24 (rs71597109, P=1.37 × 10−10)
- 4q35.1 (rs57214277, P=3.69 × 10−8)
- 6p21.31 (rs3800461, P=1.97 × 10−8)
- 11q23.2 (rs61904987, P=2.64 × 10−11)
- 18q21.1 (rs1036935, P=3.27 × 10−8)
- 19p13.3 (rs7254272, P=4.67 × 10−8)
- 22q13.33 (rs140522, P=2.70 × 10−9).
The investigators noted that the 4q24 association marked by rs71597109 maps to intron 1 of the gene encoding BANK1 (B-cell scaffold protein with ankyrin repeats 1). BANK1 is only ever activated in B cells and is linked to the autoimmune disease lupus.
The team also pointed out that the 19p13.3 association marked by rs7254272 maps 2.5 kb 5′ to ZBTB7A (zinc finger and BTB domain-containing protein 7a), which is a master regulator of B versus T lymphoid fate. So errors in ZBTB7A could lead to too many B cells in the bloodstream and bone marrow.
And rs140522 maps to 22q13.33, which has been linked to the development of multiple sclerosis. The investigators noted that this region of linkage disequilibrium contains 4 genes. One of them, NCAPH2 (non-SMC condensin II complex subunit H2), is differentially expressed in CLL and normal B cells.
“This fascinating study makes a link between genetic variants in the immune system and the development of leukemia and implicates regions of DNA which are also involved in autoimmune diseases,” said Paul Workman, PhD, chief executive and president of The Institute of Cancer Research, who was not involved in this research.
“The findings could point us towards new ways of treating leukemia or better ways of using existing treatments—potentially including immunotherapies.” ![]()
Diabetes-Related Kidney Failure Drops Among Native Americans
Diabetes-related kidney failure has declined dramatically among Native Americans—54% between 1996 and 2013— largely thanks to team- and population-based approaches begun by the IHS in the mid-1980s.
In addition to lowering the prevalence of kidney failure, those approaches led to other improvements:
- Use of medicines to protect kidneys increased from 42% to 74% in 5 years
- Average blood pressure in people with hypertension is well controlled (133/76 mm Hg in 2015)
- Blood sugar control improved by 10% between 1996 and 2014
- Kidney testing in adults aged ≥ 65 years increased > 10% compared with the Medicare diabetes population
The CDC and IHS advise team-based care should include patient education; community outreach; care coordination; tracking of health outcomes; and access to health care providers, nutritionists, diabetes educators, pharmacists, community health workers, and behavioral health clinicians. For instance, care managers use clinical data to identify people who need to be linked to health care and call patients if they miss appointments. The care model also includes integrating kidney disease prevention and education into routine diabetes care.
Diabetes-related kidney failure has declined dramatically among Native Americans—54% between 1996 and 2013— largely thanks to team- and population-based approaches begun by the IHS in the mid-1980s.
In addition to lowering the prevalence of kidney failure, those approaches led to other improvements:
- Use of medicines to protect kidneys increased from 42% to 74% in 5 years
- Average blood pressure in people with hypertension is well controlled (133/76 mm Hg in 2015)
- Blood sugar control improved by 10% between 1996 and 2014
- Kidney testing in adults aged ≥ 65 years increased > 10% compared with the Medicare diabetes population
The CDC and IHS advise team-based care should include patient education; community outreach; care coordination; tracking of health outcomes; and access to health care providers, nutritionists, diabetes educators, pharmacists, community health workers, and behavioral health clinicians. For instance, care managers use clinical data to identify people who need to be linked to health care and call patients if they miss appointments. The care model also includes integrating kidney disease prevention and education into routine diabetes care.
Diabetes-related kidney failure has declined dramatically among Native Americans—54% between 1996 and 2013— largely thanks to team- and population-based approaches begun by the IHS in the mid-1980s.
In addition to lowering the prevalence of kidney failure, those approaches led to other improvements:
- Use of medicines to protect kidneys increased from 42% to 74% in 5 years
- Average blood pressure in people with hypertension is well controlled (133/76 mm Hg in 2015)
- Blood sugar control improved by 10% between 1996 and 2014
- Kidney testing in adults aged ≥ 65 years increased > 10% compared with the Medicare diabetes population
The CDC and IHS advise team-based care should include patient education; community outreach; care coordination; tracking of health outcomes; and access to health care providers, nutritionists, diabetes educators, pharmacists, community health workers, and behavioral health clinicians. For instance, care managers use clinical data to identify people who need to be linked to health care and call patients if they miss appointments. The care model also includes integrating kidney disease prevention and education into routine diabetes care.
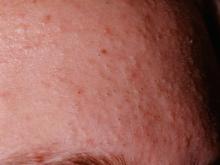
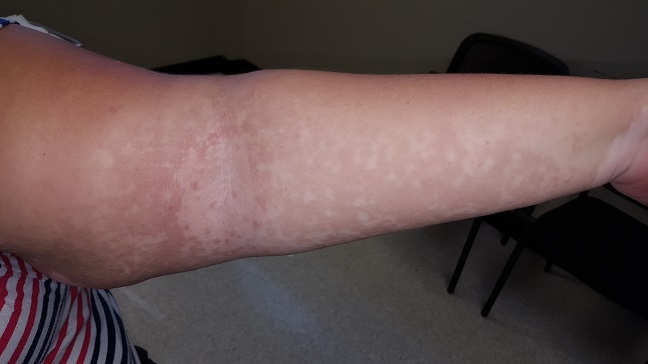
Lost Weight, Gained Rash?
A 25-year-old African-American woman presents for evaluation of an asymptomatic rash she has had for several months. It manifested shortly after she began an exercise program to help her lose weight. Her primary care provider made a presumptive diagnosis of tinea versicolor (TV), but the rash persists despite treatment attempts with topical selenium sulfide shampoo and a 10-day course of fluconazole (200 mg/d).
The patient denies having endocrine problems, such as diabetes. However, she states that given her weight and family history, she was warned about the possibility.
EXAMINATION
The patient is obese and has type V skin. Her rash is dark brown and feels slightly rough. It appears solid brown on the central back and chest, peripherally becoming sparser and more reticular (net-like). It extends to involve the flexural surfaces of both arms.
What is the diagnosis?
DISCUSSION
This is a fairly typical case of confluent and reticulated papillomatosis (CRP), also known as Gougerot-Carteaud syndrome. CRP is rare, mainly affecting young adults with darker skin after puberty. It can manifest in both genders. Originally believed to be a variant of acanthosis nigricans, CRP is now considered a distinct diagnostic entity.
At first glance, the appearance of CRP mimics that of TV. But the rough feel, reticular look, and dark color of CRP (which results from an increase in melanosomes) are totally missing in TV. Histologic studies of CRP show abnormal keratinocyte differentiation and maturation, a picture markedly at odds with that of TV.
TV, a result of the commensal yeast organism Malassezia furfur (M furfur) metabolizing normal sebum and leaving behind azelaic acid, causes color changes in the skin. But M furfur is not involved in CRP, and therefore the condition does not respond to oral or topical antiyeast medications.
The most effective treatment for CRP is minocycline (100 mg bid for 10 d). Long-term treatment includes weight loss and reduction of ambient heat.
TAKE-HOME LEARNING POINTS
- Confluent and reticulated papillomatosis (CRP) affects the trunk and extremities of obese patients with darker skin.
- In contrast with tinea versicolor (TV), CRP has a rough texture and reticulated look, especially on the periphery of the involved areas.
- Biopsy can help distinguish CRP from its lookalikes; it shows abnormal keratinocyte differentiation and maturation, as well as increased melanosomes.
- Oral minocycline is the best treatment, along with weight loss and reduction of ambient heat.
A 25-year-old African-American woman presents for evaluation of an asymptomatic rash she has had for several months. It manifested shortly after she began an exercise program to help her lose weight. Her primary care provider made a presumptive diagnosis of tinea versicolor (TV), but the rash persists despite treatment attempts with topical selenium sulfide shampoo and a 10-day course of fluconazole (200 mg/d).
The patient denies having endocrine problems, such as diabetes. However, she states that given her weight and family history, she was warned about the possibility.
EXAMINATION
The patient is obese and has type V skin. Her rash is dark brown and feels slightly rough. It appears solid brown on the central back and chest, peripherally becoming sparser and more reticular (net-like). It extends to involve the flexural surfaces of both arms.

What is the diagnosis?
DISCUSSION
This is a fairly typical case of confluent and reticulated papillomatosis (CRP), also known as Gougerot-Carteaud syndrome. CRP is rare, mainly affecting young adults with darker skin after puberty. It can manifest in both genders. Originally believed to be a variant of acanthosis nigricans, CRP is now considered a distinct diagnostic entity.
At first glance, the appearance of CRP mimics that of TV. But the rough feel, reticular look, and dark color of CRP (which results from an increase in melanosomes) are totally missing in TV. Histologic studies of CRP show abnormal keratinocyte differentiation and maturation, a picture markedly at odds with that of TV.
TV, a result of the commensal yeast organism Malassezia furfur (M furfur) metabolizing normal sebum and leaving behind azelaic acid, causes color changes in the skin. But M furfur is not involved in CRP, and therefore the condition does not respond to oral or topical antiyeast medications.
The most effective treatment for CRP is minocycline (100 mg bid for 10 d). Long-term treatment includes weight loss and reduction of ambient heat.
TAKE-HOME LEARNING POINTS
- Confluent and reticulated papillomatosis (CRP) affects the trunk and extremities of obese patients with darker skin.
- In contrast with tinea versicolor (TV), CRP has a rough texture and reticulated look, especially on the periphery of the involved areas.
- Biopsy can help distinguish CRP from its lookalikes; it shows abnormal keratinocyte differentiation and maturation, as well as increased melanosomes.
- Oral minocycline is the best treatment, along with weight loss and reduction of ambient heat.
A 25-year-old African-American woman presents for evaluation of an asymptomatic rash she has had for several months. It manifested shortly after she began an exercise program to help her lose weight. Her primary care provider made a presumptive diagnosis of tinea versicolor (TV), but the rash persists despite treatment attempts with topical selenium sulfide shampoo and a 10-day course of fluconazole (200 mg/d).
The patient denies having endocrine problems, such as diabetes. However, she states that given her weight and family history, she was warned about the possibility.
EXAMINATION
The patient is obese and has type V skin. Her rash is dark brown and feels slightly rough. It appears solid brown on the central back and chest, peripherally becoming sparser and more reticular (net-like). It extends to involve the flexural surfaces of both arms.

What is the diagnosis?
DISCUSSION
This is a fairly typical case of confluent and reticulated papillomatosis (CRP), also known as Gougerot-Carteaud syndrome. CRP is rare, mainly affecting young adults with darker skin after puberty. It can manifest in both genders. Originally believed to be a variant of acanthosis nigricans, CRP is now considered a distinct diagnostic entity.
At first glance, the appearance of CRP mimics that of TV. But the rough feel, reticular look, and dark color of CRP (which results from an increase in melanosomes) are totally missing in TV. Histologic studies of CRP show abnormal keratinocyte differentiation and maturation, a picture markedly at odds with that of TV.
TV, a result of the commensal yeast organism Malassezia furfur (M furfur) metabolizing normal sebum and leaving behind azelaic acid, causes color changes in the skin. But M furfur is not involved in CRP, and therefore the condition does not respond to oral or topical antiyeast medications.
The most effective treatment for CRP is minocycline (100 mg bid for 10 d). Long-term treatment includes weight loss and reduction of ambient heat.
TAKE-HOME LEARNING POINTS
- Confluent and reticulated papillomatosis (CRP) affects the trunk and extremities of obese patients with darker skin.
- In contrast with tinea versicolor (TV), CRP has a rough texture and reticulated look, especially on the periphery of the involved areas.
- Biopsy can help distinguish CRP from its lookalikes; it shows abnormal keratinocyte differentiation and maturation, as well as increased melanosomes.
- Oral minocycline is the best treatment, along with weight loss and reduction of ambient heat.
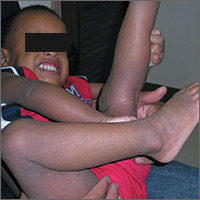
Severe itching in 2-year-old
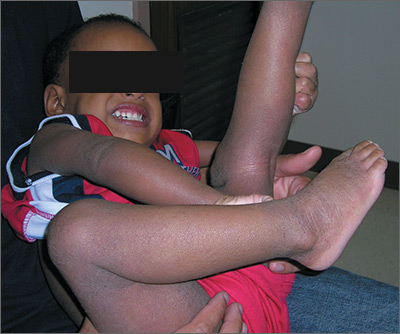
The FP recognized that the boy had a case of severe atopic dermatitis. There was considerable inflammation of the skin, but the erythema was less visible because of the boy’s darker skin color.
The father had the full atopic triad: asthma, allergic rhinitis, and atopic dermatitis. This explained the son’s inheritance of atopic dermatitis. The FP recommended aggressive treatment and the father was relieved, as it hurt him to see his son suffer.
The FP prescribed a tub (454 g) of 0.1% triamcinolone ointment to be used twice daily all over the involved skin, especially after bathing. The FP said that the child should be given a daily bath with mild soap. (There is no data to support the notion that bathing dries out the skin; bathing before the use of topical steroids actually helps the steroids absorb into the skin.)
Oral hydroxyzine was prescribed for use before bedtime, as this sedating antihistamine can improve sleep and diminish pruritus. Considering the severity of the condition, the FP decided to prescribe a short course of oral prednisolone for one week (1 mg/kg/d). There is some evidence that dilute bleach baths help atopic dermatitis, but the FP decided to discuss this at a future visit—after the flare had subsided. There was no evidence of a secondary infection, so antibiotics weren’t prescribed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP recognized that the boy had a case of severe atopic dermatitis. There was considerable inflammation of the skin, but the erythema was less visible because of the boy’s darker skin color.
The father had the full atopic triad: asthma, allergic rhinitis, and atopic dermatitis. This explained the son’s inheritance of atopic dermatitis. The FP recommended aggressive treatment and the father was relieved, as it hurt him to see his son suffer.
The FP prescribed a tub (454 g) of 0.1% triamcinolone ointment to be used twice daily all over the involved skin, especially after bathing. The FP said that the child should be given a daily bath with mild soap. (There is no data to support the notion that bathing dries out the skin; bathing before the use of topical steroids actually helps the steroids absorb into the skin.)
Oral hydroxyzine was prescribed for use before bedtime, as this sedating antihistamine can improve sleep and diminish pruritus. Considering the severity of the condition, the FP decided to prescribe a short course of oral prednisolone for one week (1 mg/kg/d). There is some evidence that dilute bleach baths help atopic dermatitis, but the FP decided to discuss this at a future visit—after the flare had subsided. There was no evidence of a secondary infection, so antibiotics weren’t prescribed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP recognized that the boy had a case of severe atopic dermatitis. There was considerable inflammation of the skin, but the erythema was less visible because of the boy’s darker skin color.
The father had the full atopic triad: asthma, allergic rhinitis, and atopic dermatitis. This explained the son’s inheritance of atopic dermatitis. The FP recommended aggressive treatment and the father was relieved, as it hurt him to see his son suffer.
The FP prescribed a tub (454 g) of 0.1% triamcinolone ointment to be used twice daily all over the involved skin, especially after bathing. The FP said that the child should be given a daily bath with mild soap. (There is no data to support the notion that bathing dries out the skin; bathing before the use of topical steroids actually helps the steroids absorb into the skin.)
Oral hydroxyzine was prescribed for use before bedtime, as this sedating antihistamine can improve sleep and diminish pruritus. Considering the severity of the condition, the FP decided to prescribe a short course of oral prednisolone for one week (1 mg/kg/d). There is some evidence that dilute bleach baths help atopic dermatitis, but the FP decided to discuss this at a future visit—after the flare had subsided. There was no evidence of a secondary infection, so antibiotics weren’t prescribed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Why CLL may go chemo free
NEW YORK – The approach to treating chronic lymphocytic leukemia (CLL) is evolving, and while chemoimmunotherapy remains a reasonable initial option in some cases, a “chemo-free” approach is also a very real possibility, according to Bruce D. Cheson, MD.
A number of studies showing survival benefits with targeted therapies vs. chemoimmunotherapy (CIT) regimens have been completed, including studies that look specifically at outcomes by mutation status and other factors. One example – a likely game changer – is the ALLIANCE trial, a randomized phase III study of bendamustine plus rituximab vs. ibrutinib plus rituximab vs. ibrutinib alone in untreated CLL patients aged 65 years or older, Dr. Cheson of Georgetown University Hospital, Washington, said at an international congress on hematologic malignancies.
“I think [the ALLIANCE] trial has the possibility of totally changing how we treat patients with CLL, even though it was done in older patients,” he said, noting that the study is completed, but final results are pending adequate follow-up.
Based on the available data, he suggests a treatment paradigm for untreated CLL patients who require therapy that begins with consideration of patient age, comorbidities, functional status, and fluorescence in situ hybridization (FISH).
While clinical trial enrollment is preferable, those who are CIT eligible based on age and comorbidities can be treated with bendamustine/rituximab (BR), fludarabine/cyclophosphamide/rituximab (FCR), or ibrutinib.
“I think BR and FCR are both reasonable options, although the latter primarily for young, IGHV-mutated patients, and certainly ibrutinib remains an option for this patient population,” he said.
For those not eligible for CIT, options include ibrutinib and chlorambucil/obinutuzumab. For those with deletion 17p, ibrutinib is the standard for frontline therapy. And for those who are frail, ibrutinib is a good option.
“Some might use an anti-CD20, but as a single agent, it’s not what I would prefer,” Dr. Cheson said, explaining that response rates with such agents are low and tend to lack durability.
CIT remains a reasonable initial option for those patients who are mutated, but with the prolonged progression-free survival seen with ibrutinib in several trials, he predicted that will change over time.
“The role of targeted approaches is a subject of discussion. It takes the most time in my clinic of any discussion I have. [Patients ask] ‘Should I get ibrutinib? Should I get chemoimmunotherapy?’ ” he said. “One needs to take into account patient age and comorbidities, the fact that with CIT you are six [treatments] and done vs. indefinite therapy [with ibrutinib]. There is cost and there is compliance that one needs to consider.”
As for relapsed/refractory CLL, the role of CIT is particularly diminished in the wake of trials such as HELIOS and RESONATE, showing survival benefits with ibrutinib, others showing survival benefits with rituximab/idelalisib (R-idelalisib), and trials showing better results with venetoclax non-CIT regimens than would be expected with CIT regimens. As with treatment-naive CLL patients, age, comorbidities, functional status, and FISH should be considered in those with previously treated CLL who require therapy, and if clinical trial enrollment is not possible, treatment options depend on certain patient characteristics.
“For patients who had a long first response to chemoimmunotherapy, I would still use ibrutinib, and in select patients, R-idelalisib,” he said.
If they had a long response with BR, or FCR, retreatment with those can be considered as well, he noted, adding, “But I don’t see the point when the results with kinase inhibitors are at least as good as, if not better than one would expect with CIT in this context.”
In patients who had short first response, ibrutinib and R-idelalisib are the best options. For those with deletion 17p, the best options are ibrutinib or venetoclax, and possibly R-idelalisib. For frail patients, options include ibrutinib, R-idelalisib, or anti-CD20, although, as with untreated patients, the latter is his least favorite option because of the increased risk of toxicity in this population, he said.
“I think the role of chemoimmunotherapy in CLL is vanishing, and a chemo-free world for CLL patients is a reality,” he said.
Dr. Cheson reported consulting for Acerta, Celgene Pharmacyclics, and Roche-Genentech.
NEW YORK – The approach to treating chronic lymphocytic leukemia (CLL) is evolving, and while chemoimmunotherapy remains a reasonable initial option in some cases, a “chemo-free” approach is also a very real possibility, according to Bruce D. Cheson, MD.
A number of studies showing survival benefits with targeted therapies vs. chemoimmunotherapy (CIT) regimens have been completed, including studies that look specifically at outcomes by mutation status and other factors. One example – a likely game changer – is the ALLIANCE trial, a randomized phase III study of bendamustine plus rituximab vs. ibrutinib plus rituximab vs. ibrutinib alone in untreated CLL patients aged 65 years or older, Dr. Cheson of Georgetown University Hospital, Washington, said at an international congress on hematologic malignancies.
“I think [the ALLIANCE] trial has the possibility of totally changing how we treat patients with CLL, even though it was done in older patients,” he said, noting that the study is completed, but final results are pending adequate follow-up.
Based on the available data, he suggests a treatment paradigm for untreated CLL patients who require therapy that begins with consideration of patient age, comorbidities, functional status, and fluorescence in situ hybridization (FISH).
While clinical trial enrollment is preferable, those who are CIT eligible based on age and comorbidities can be treated with bendamustine/rituximab (BR), fludarabine/cyclophosphamide/rituximab (FCR), or ibrutinib.
“I think BR and FCR are both reasonable options, although the latter primarily for young, IGHV-mutated patients, and certainly ibrutinib remains an option for this patient population,” he said.
For those not eligible for CIT, options include ibrutinib and chlorambucil/obinutuzumab. For those with deletion 17p, ibrutinib is the standard for frontline therapy. And for those who are frail, ibrutinib is a good option.
“Some might use an anti-CD20, but as a single agent, it’s not what I would prefer,” Dr. Cheson said, explaining that response rates with such agents are low and tend to lack durability.
CIT remains a reasonable initial option for those patients who are mutated, but with the prolonged progression-free survival seen with ibrutinib in several trials, he predicted that will change over time.
“The role of targeted approaches is a subject of discussion. It takes the most time in my clinic of any discussion I have. [Patients ask] ‘Should I get ibrutinib? Should I get chemoimmunotherapy?’ ” he said. “One needs to take into account patient age and comorbidities, the fact that with CIT you are six [treatments] and done vs. indefinite therapy [with ibrutinib]. There is cost and there is compliance that one needs to consider.”
As for relapsed/refractory CLL, the role of CIT is particularly diminished in the wake of trials such as HELIOS and RESONATE, showing survival benefits with ibrutinib, others showing survival benefits with rituximab/idelalisib (R-idelalisib), and trials showing better results with venetoclax non-CIT regimens than would be expected with CIT regimens. As with treatment-naive CLL patients, age, comorbidities, functional status, and FISH should be considered in those with previously treated CLL who require therapy, and if clinical trial enrollment is not possible, treatment options depend on certain patient characteristics.
“For patients who had a long first response to chemoimmunotherapy, I would still use ibrutinib, and in select patients, R-idelalisib,” he said.
If they had a long response with BR, or FCR, retreatment with those can be considered as well, he noted, adding, “But I don’t see the point when the results with kinase inhibitors are at least as good as, if not better than one would expect with CIT in this context.”
In patients who had short first response, ibrutinib and R-idelalisib are the best options. For those with deletion 17p, the best options are ibrutinib or venetoclax, and possibly R-idelalisib. For frail patients, options include ibrutinib, R-idelalisib, or anti-CD20, although, as with untreated patients, the latter is his least favorite option because of the increased risk of toxicity in this population, he said.
“I think the role of chemoimmunotherapy in CLL is vanishing, and a chemo-free world for CLL patients is a reality,” he said.
Dr. Cheson reported consulting for Acerta, Celgene Pharmacyclics, and Roche-Genentech.
NEW YORK – The approach to treating chronic lymphocytic leukemia (CLL) is evolving, and while chemoimmunotherapy remains a reasonable initial option in some cases, a “chemo-free” approach is also a very real possibility, according to Bruce D. Cheson, MD.
A number of studies showing survival benefits with targeted therapies vs. chemoimmunotherapy (CIT) regimens have been completed, including studies that look specifically at outcomes by mutation status and other factors. One example – a likely game changer – is the ALLIANCE trial, a randomized phase III study of bendamustine plus rituximab vs. ibrutinib plus rituximab vs. ibrutinib alone in untreated CLL patients aged 65 years or older, Dr. Cheson of Georgetown University Hospital, Washington, said at an international congress on hematologic malignancies.
“I think [the ALLIANCE] trial has the possibility of totally changing how we treat patients with CLL, even though it was done in older patients,” he said, noting that the study is completed, but final results are pending adequate follow-up.
Based on the available data, he suggests a treatment paradigm for untreated CLL patients who require therapy that begins with consideration of patient age, comorbidities, functional status, and fluorescence in situ hybridization (FISH).
While clinical trial enrollment is preferable, those who are CIT eligible based on age and comorbidities can be treated with bendamustine/rituximab (BR), fludarabine/cyclophosphamide/rituximab (FCR), or ibrutinib.
“I think BR and FCR are both reasonable options, although the latter primarily for young, IGHV-mutated patients, and certainly ibrutinib remains an option for this patient population,” he said.
For those not eligible for CIT, options include ibrutinib and chlorambucil/obinutuzumab. For those with deletion 17p, ibrutinib is the standard for frontline therapy. And for those who are frail, ibrutinib is a good option.
“Some might use an anti-CD20, but as a single agent, it’s not what I would prefer,” Dr. Cheson said, explaining that response rates with such agents are low and tend to lack durability.
CIT remains a reasonable initial option for those patients who are mutated, but with the prolonged progression-free survival seen with ibrutinib in several trials, he predicted that will change over time.
“The role of targeted approaches is a subject of discussion. It takes the most time in my clinic of any discussion I have. [Patients ask] ‘Should I get ibrutinib? Should I get chemoimmunotherapy?’ ” he said. “One needs to take into account patient age and comorbidities, the fact that with CIT you are six [treatments] and done vs. indefinite therapy [with ibrutinib]. There is cost and there is compliance that one needs to consider.”
As for relapsed/refractory CLL, the role of CIT is particularly diminished in the wake of trials such as HELIOS and RESONATE, showing survival benefits with ibrutinib, others showing survival benefits with rituximab/idelalisib (R-idelalisib), and trials showing better results with venetoclax non-CIT regimens than would be expected with CIT regimens. As with treatment-naive CLL patients, age, comorbidities, functional status, and FISH should be considered in those with previously treated CLL who require therapy, and if clinical trial enrollment is not possible, treatment options depend on certain patient characteristics.
“For patients who had a long first response to chemoimmunotherapy, I would still use ibrutinib, and in select patients, R-idelalisib,” he said.
If they had a long response with BR, or FCR, retreatment with those can be considered as well, he noted, adding, “But I don’t see the point when the results with kinase inhibitors are at least as good as, if not better than one would expect with CIT in this context.”
In patients who had short first response, ibrutinib and R-idelalisib are the best options. For those with deletion 17p, the best options are ibrutinib or venetoclax, and possibly R-idelalisib. For frail patients, options include ibrutinib, R-idelalisib, or anti-CD20, although, as with untreated patients, the latter is his least favorite option because of the increased risk of toxicity in this population, he said.
“I think the role of chemoimmunotherapy in CLL is vanishing, and a chemo-free world for CLL patients is a reality,” he said.
Dr. Cheson reported consulting for Acerta, Celgene Pharmacyclics, and Roche-Genentech.
Four factors signal complicated appendicitis
LAS VEGAS – Four clinical and imaging characteristics can preoperatively identify cases of complicated appendicitis, potentially saving many from long and unnecessary courses of antibiotics.
In a retrospective study, increasing age and days of pain, combined with the size of the appendix and the presence of an appendicolith on imaging were significantly associated with a histopathologic diagnosis of complicated appendicitis, Jonathan Imran, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
“A lot of patients get tagged as complicated but they really aren’t,” said Dr. Imran, a surgical resident at the University of Texas, Dallas. “This definition then guides clinical assessment and has a profound impact on postoperative antibiotics and length of stay. Despite its common use, intraoperative assessment of complicated appendicitis remains subjective.”
On the other hand, the standard of a histopathologic diagnosis isn’t available during surgery to guide postoperative management. Dr. Imran and his colleagues sought to create a risk assessment tool to identify patients at risk of complicated appendicitis on the basis of clinical and imaging findings.
They retrospectively examined 1,066 patients who underwent appendectomy at a single institution from 2011 to 2013. They compared the intraoperative designations of simple and complicated appendicitis with the histopathologic diagnosis.
Of the 827 patients designated as having simple appendicitis during surgery, 763 (93%) were confirmed by histopathology. The remainder had complicated appendicitis on histopathology.
Of the 239 patients designated as having complicated appendicitis during surgery, 143 (60%) were confirmed by histopathology. The remainder actually had simple appendicitis. Of these 96 patients, 60% went on to have prolonged courses of antibiotics that, by definition, were unnecessary.
The team then looked at 30 patient variables in an attempt to construct a prediction tool. Among the significant associations with a complicated presentation were older age, type 2 diabetes, longer duration of pain, less lower left quadrant pain, higher median temperature, higher serum creatinine, longer time from presentation of symptoms to surgery, larger appendix diameter, abscess, and the presence of an appendicolith.
Four of these factors remained significantly associated with complicated appendicitis in a multivariate regression analysis:
• Age (per 10 years) – odds ratio, 1.25.
• Duration of pain (per day) – OR, 1.21.
• Appendix diameter on imaging (per mm) – OR, 1.10.
• Presence of an appendicolith on imaging – OR, 1.65.
These findings are the basis of a preoperative risk assessment score the team is developing, which will be prospectively tested.
“We hope that these predictors, in combination with improved intraoperative grading, could be used to achieve a more timely and accurate diagnosis of complicated appendicitis,” Dr. Imran said.
He had no financial disclosures.
[email protected]
On Twitter @alz_gal
LAS VEGAS – Four clinical and imaging characteristics can preoperatively identify cases of complicated appendicitis, potentially saving many from long and unnecessary courses of antibiotics.
In a retrospective study, increasing age and days of pain, combined with the size of the appendix and the presence of an appendicolith on imaging were significantly associated with a histopathologic diagnosis of complicated appendicitis, Jonathan Imran, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
“A lot of patients get tagged as complicated but they really aren’t,” said Dr. Imran, a surgical resident at the University of Texas, Dallas. “This definition then guides clinical assessment and has a profound impact on postoperative antibiotics and length of stay. Despite its common use, intraoperative assessment of complicated appendicitis remains subjective.”
On the other hand, the standard of a histopathologic diagnosis isn’t available during surgery to guide postoperative management. Dr. Imran and his colleagues sought to create a risk assessment tool to identify patients at risk of complicated appendicitis on the basis of clinical and imaging findings.
They retrospectively examined 1,066 patients who underwent appendectomy at a single institution from 2011 to 2013. They compared the intraoperative designations of simple and complicated appendicitis with the histopathologic diagnosis.
Of the 827 patients designated as having simple appendicitis during surgery, 763 (93%) were confirmed by histopathology. The remainder had complicated appendicitis on histopathology.
Of the 239 patients designated as having complicated appendicitis during surgery, 143 (60%) were confirmed by histopathology. The remainder actually had simple appendicitis. Of these 96 patients, 60% went on to have prolonged courses of antibiotics that, by definition, were unnecessary.
The team then looked at 30 patient variables in an attempt to construct a prediction tool. Among the significant associations with a complicated presentation were older age, type 2 diabetes, longer duration of pain, less lower left quadrant pain, higher median temperature, higher serum creatinine, longer time from presentation of symptoms to surgery, larger appendix diameter, abscess, and the presence of an appendicolith.
Four of these factors remained significantly associated with complicated appendicitis in a multivariate regression analysis:
• Age (per 10 years) – odds ratio, 1.25.
• Duration of pain (per day) – OR, 1.21.
• Appendix diameter on imaging (per mm) – OR, 1.10.
• Presence of an appendicolith on imaging – OR, 1.65.
These findings are the basis of a preoperative risk assessment score the team is developing, which will be prospectively tested.
“We hope that these predictors, in combination with improved intraoperative grading, could be used to achieve a more timely and accurate diagnosis of complicated appendicitis,” Dr. Imran said.
He had no financial disclosures.
[email protected]
On Twitter @alz_gal
LAS VEGAS – Four clinical and imaging characteristics can preoperatively identify cases of complicated appendicitis, potentially saving many from long and unnecessary courses of antibiotics.
In a retrospective study, increasing age and days of pain, combined with the size of the appendix and the presence of an appendicolith on imaging were significantly associated with a histopathologic diagnosis of complicated appendicitis, Jonathan Imran, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
“A lot of patients get tagged as complicated but they really aren’t,” said Dr. Imran, a surgical resident at the University of Texas, Dallas. “This definition then guides clinical assessment and has a profound impact on postoperative antibiotics and length of stay. Despite its common use, intraoperative assessment of complicated appendicitis remains subjective.”
On the other hand, the standard of a histopathologic diagnosis isn’t available during surgery to guide postoperative management. Dr. Imran and his colleagues sought to create a risk assessment tool to identify patients at risk of complicated appendicitis on the basis of clinical and imaging findings.
They retrospectively examined 1,066 patients who underwent appendectomy at a single institution from 2011 to 2013. They compared the intraoperative designations of simple and complicated appendicitis with the histopathologic diagnosis.
Of the 827 patients designated as having simple appendicitis during surgery, 763 (93%) were confirmed by histopathology. The remainder had complicated appendicitis on histopathology.
Of the 239 patients designated as having complicated appendicitis during surgery, 143 (60%) were confirmed by histopathology. The remainder actually had simple appendicitis. Of these 96 patients, 60% went on to have prolonged courses of antibiotics that, by definition, were unnecessary.
The team then looked at 30 patient variables in an attempt to construct a prediction tool. Among the significant associations with a complicated presentation were older age, type 2 diabetes, longer duration of pain, less lower left quadrant pain, higher median temperature, higher serum creatinine, longer time from presentation of symptoms to surgery, larger appendix diameter, abscess, and the presence of an appendicolith.
Four of these factors remained significantly associated with complicated appendicitis in a multivariate regression analysis:
• Age (per 10 years) – odds ratio, 1.25.
• Duration of pain (per day) – OR, 1.21.
• Appendix diameter on imaging (per mm) – OR, 1.10.
• Presence of an appendicolith on imaging – OR, 1.65.
These findings are the basis of a preoperative risk assessment score the team is developing, which will be prospectively tested.
“We hope that these predictors, in combination with improved intraoperative grading, could be used to achieve a more timely and accurate diagnosis of complicated appendicitis,” Dr. Imran said.
He had no financial disclosures.
[email protected]
On Twitter @alz_gal
Key clinical point: Two clinical signs and two imaging findings can help identify patients with complicated appendicitis.
Major finding: Older age, days of pain, appendix diameter, and the presence of an appendicolith significantly predicted a complicated presentation.
Data source: The retrospective study comprised 1,066 patients.
Disclosures: Dr. Imran had no financial disclosures.
MDS gene mutations predict response to HSCT
Genetic mutations in blood samples may predict outcomes and guide treatment for patients of all ages who have myelodysplastic syndrome and are undergoing hematopoietic stem-cell transplantation, according to a report published online Feb. 8 in the New England Journal of Medicine.
Allogeneic hematopoietic stem-cell transplantation is the only potentially curative therapy currently available for myelodysplastic syndrome (MDS), but mortality due to relapse and to transplant-related complications is high. “Predicting which patients are most likely to benefit from transplantation is thus a central challenge,” and identifying patients most likely to relapse could help clinicians refine conditioning regimens and relapse-prevention strategies, said R. Coleman Lindsley, MD, PhD, of the division of hematological malignancies, Dana-Farber Cancer Institute, Boston, and his associates.
The analyses included targeted sequencing of 129 genes known or suspected to be involved in the pathogenesis of myeloid cancers or syndromes related to bone marrow failure. Approximately 80% of the study participants were found to have at least one such driver mutation, with a median of two mutations per patient.
Mutations in the TP53 gene turned out to be the single most powerful predictor of survival after transplantation, independent of factors such as patient age, performance status, and hematologic variables. Moreover, intensive (myeloablative) conditioning regimens did not attenuate this effect, “a finding that is consistent with clinical and experimental evidence showing TP53 mutation-mediated chemoresistance,” Dr. Lindsley and his associates said.
“Our data suggest that escalating the intensity of the conditioning regimen in order to improve outcomes in patients with TP53-mutated MDS will not be successful. ... These patients, who have an exceptionally high risk of relapse-related death after transplantation, should be considered for investigative approaches to conditioning or new relapse-prevention strategies after transplantation,” they added.
Among patients over age 40, mutations in the RAS pathway were associated with a significantly elevated risk of early relapse – an outcome that might be ameliorated by more intensive conditioning. “RAS-pathway mutations may thus reflect the presence of low-volume but biologically transformed disease that, without adequate cytoreduction before transplantation, outpaces the development of effective graft-versus-leukemia activity,” the investigators said.
However, this association between RAS mutations and relapse was not seen in patients younger than age 40 years, they noted.
Conversely, JAK2 mutations were associated with a higher rate of death without relapse but not a higher rate of relapse. And this association was not affected by conditioning intensity. Although the mechanism of such an effect is not yet known, early death without relapse may be driven by factors that are susceptible to targeting by JAK2 inhibitors. In addition, minimizing treatment toxicity should be the focus of treatment in patients who carry JAK2 mutations, since their poor survival rate is driven by deaths unrelated to relapse, Dr. Lindsley and his associates said.
Mutations in the PPM1D gene, especially when accompanied by TP53 mutations, were strongly associated with previous exposure to leukemogenic therapies. “PPM1D encodes a serine-threonine protein phosphatase that regulates the cellular response to environmental stress, in part by means of inhibition of TP53 activity, which suggests that TP53 and PPM1D mutations represent convergent mechanisms of clonal survival in the context of leukemogenic exposures,” the investigators said.
“Our results... provide strong genetic evidence of the role of PPM1D mutations in the pathogenesis of therapy-related myelodysplastic syndromes.”
Mutations in the SBDS gene, which has been linked to Shwachman-Diamond syndrome, were “unexpectedly common” in young-adult patients and were associated with a poor prognosis. (Shwachman-Diamond syndrome is a rare congenital syndrome of bone-marrow failure.) This finding suggests that early stem-cell transplantation should be considered for patients who have this disorder, since transplantation after full-blown MDS develops “may not offer long-term benefit.”
Genetic mutations in blood samples may predict outcomes and guide treatment for patients of all ages who have myelodysplastic syndrome and are undergoing hematopoietic stem-cell transplantation, according to a report published online Feb. 8 in the New England Journal of Medicine.
Allogeneic hematopoietic stem-cell transplantation is the only potentially curative therapy currently available for myelodysplastic syndrome (MDS), but mortality due to relapse and to transplant-related complications is high. “Predicting which patients are most likely to benefit from transplantation is thus a central challenge,” and identifying patients most likely to relapse could help clinicians refine conditioning regimens and relapse-prevention strategies, said R. Coleman Lindsley, MD, PhD, of the division of hematological malignancies, Dana-Farber Cancer Institute, Boston, and his associates.
The analyses included targeted sequencing of 129 genes known or suspected to be involved in the pathogenesis of myeloid cancers or syndromes related to bone marrow failure. Approximately 80% of the study participants were found to have at least one such driver mutation, with a median of two mutations per patient.
Mutations in the TP53 gene turned out to be the single most powerful predictor of survival after transplantation, independent of factors such as patient age, performance status, and hematologic variables. Moreover, intensive (myeloablative) conditioning regimens did not attenuate this effect, “a finding that is consistent with clinical and experimental evidence showing TP53 mutation-mediated chemoresistance,” Dr. Lindsley and his associates said.
“Our data suggest that escalating the intensity of the conditioning regimen in order to improve outcomes in patients with TP53-mutated MDS will not be successful. ... These patients, who have an exceptionally high risk of relapse-related death after transplantation, should be considered for investigative approaches to conditioning or new relapse-prevention strategies after transplantation,” they added.
Among patients over age 40, mutations in the RAS pathway were associated with a significantly elevated risk of early relapse – an outcome that might be ameliorated by more intensive conditioning. “RAS-pathway mutations may thus reflect the presence of low-volume but biologically transformed disease that, without adequate cytoreduction before transplantation, outpaces the development of effective graft-versus-leukemia activity,” the investigators said.
However, this association between RAS mutations and relapse was not seen in patients younger than age 40 years, they noted.
Conversely, JAK2 mutations were associated with a higher rate of death without relapse but not a higher rate of relapse. And this association was not affected by conditioning intensity. Although the mechanism of such an effect is not yet known, early death without relapse may be driven by factors that are susceptible to targeting by JAK2 inhibitors. In addition, minimizing treatment toxicity should be the focus of treatment in patients who carry JAK2 mutations, since their poor survival rate is driven by deaths unrelated to relapse, Dr. Lindsley and his associates said.
Mutations in the PPM1D gene, especially when accompanied by TP53 mutations, were strongly associated with previous exposure to leukemogenic therapies. “PPM1D encodes a serine-threonine protein phosphatase that regulates the cellular response to environmental stress, in part by means of inhibition of TP53 activity, which suggests that TP53 and PPM1D mutations represent convergent mechanisms of clonal survival in the context of leukemogenic exposures,” the investigators said.
“Our results... provide strong genetic evidence of the role of PPM1D mutations in the pathogenesis of therapy-related myelodysplastic syndromes.”
Mutations in the SBDS gene, which has been linked to Shwachman-Diamond syndrome, were “unexpectedly common” in young-adult patients and were associated with a poor prognosis. (Shwachman-Diamond syndrome is a rare congenital syndrome of bone-marrow failure.) This finding suggests that early stem-cell transplantation should be considered for patients who have this disorder, since transplantation after full-blown MDS develops “may not offer long-term benefit.”
Genetic mutations in blood samples may predict outcomes and guide treatment for patients of all ages who have myelodysplastic syndrome and are undergoing hematopoietic stem-cell transplantation, according to a report published online Feb. 8 in the New England Journal of Medicine.
Allogeneic hematopoietic stem-cell transplantation is the only potentially curative therapy currently available for myelodysplastic syndrome (MDS), but mortality due to relapse and to transplant-related complications is high. “Predicting which patients are most likely to benefit from transplantation is thus a central challenge,” and identifying patients most likely to relapse could help clinicians refine conditioning regimens and relapse-prevention strategies, said R. Coleman Lindsley, MD, PhD, of the division of hematological malignancies, Dana-Farber Cancer Institute, Boston, and his associates.
The analyses included targeted sequencing of 129 genes known or suspected to be involved in the pathogenesis of myeloid cancers or syndromes related to bone marrow failure. Approximately 80% of the study participants were found to have at least one such driver mutation, with a median of two mutations per patient.
Mutations in the TP53 gene turned out to be the single most powerful predictor of survival after transplantation, independent of factors such as patient age, performance status, and hematologic variables. Moreover, intensive (myeloablative) conditioning regimens did not attenuate this effect, “a finding that is consistent with clinical and experimental evidence showing TP53 mutation-mediated chemoresistance,” Dr. Lindsley and his associates said.
“Our data suggest that escalating the intensity of the conditioning regimen in order to improve outcomes in patients with TP53-mutated MDS will not be successful. ... These patients, who have an exceptionally high risk of relapse-related death after transplantation, should be considered for investigative approaches to conditioning or new relapse-prevention strategies after transplantation,” they added.
Among patients over age 40, mutations in the RAS pathway were associated with a significantly elevated risk of early relapse – an outcome that might be ameliorated by more intensive conditioning. “RAS-pathway mutations may thus reflect the presence of low-volume but biologically transformed disease that, without adequate cytoreduction before transplantation, outpaces the development of effective graft-versus-leukemia activity,” the investigators said.
However, this association between RAS mutations and relapse was not seen in patients younger than age 40 years, they noted.
Conversely, JAK2 mutations were associated with a higher rate of death without relapse but not a higher rate of relapse. And this association was not affected by conditioning intensity. Although the mechanism of such an effect is not yet known, early death without relapse may be driven by factors that are susceptible to targeting by JAK2 inhibitors. In addition, minimizing treatment toxicity should be the focus of treatment in patients who carry JAK2 mutations, since their poor survival rate is driven by deaths unrelated to relapse, Dr. Lindsley and his associates said.
Mutations in the PPM1D gene, especially when accompanied by TP53 mutations, were strongly associated with previous exposure to leukemogenic therapies. “PPM1D encodes a serine-threonine protein phosphatase that regulates the cellular response to environmental stress, in part by means of inhibition of TP53 activity, which suggests that TP53 and PPM1D mutations represent convergent mechanisms of clonal survival in the context of leukemogenic exposures,” the investigators said.
“Our results... provide strong genetic evidence of the role of PPM1D mutations in the pathogenesis of therapy-related myelodysplastic syndromes.”
Mutations in the SBDS gene, which has been linked to Shwachman-Diamond syndrome, were “unexpectedly common” in young-adult patients and were associated with a poor prognosis. (Shwachman-Diamond syndrome is a rare congenital syndrome of bone-marrow failure.) This finding suggests that early stem-cell transplantation should be considered for patients who have this disorder, since transplantation after full-blown MDS develops “may not offer long-term benefit.”
Key clinical point: Genetic mutations in blood samples may predict outcomes and guide treatment for patients of all ages who have myelodysplastic syndrome and are undergoing hematopoietic stem-cell transplantation.
Key numerical finding: Approximately 80% of the study participants were found to have at least one driver mutation, with a median of two such mutations per patient.
Data source: Targeted mutational analyses of banked blood samples from 1,514 patients treated at 130 transplantation centers in the U.S. and Germany.
Disclosures: This study was supported by the Edward P. Evans Foundation, the Harvard Catalyst Program, the National Marrow Donor Program, the National Institutes of Health, and the Leukemia and Lymphoma Society. Dr. Lindsley reported ties to Takeda, and one of his associates reported ties to Celgene, Genoptix, and H3 Biomedicine.
New topical agents for acne rolling out
WAILEA, HAWAII – The Food and Drug Administration’s approval of adapalene gel 0.1% as an over-the-counter treatment for acne is a potential game changer that could lead to revision of guideline-recommended treatment algorithms, Lawrence F. Eichenfield, MD, predicted at the Hawaii Dermatology Seminar.
In addition to discussing the implications of the FDA’s unprecedented approval of a full prescription–strength topical retinoid for OTC use, he highlighted other developments in topical therapy for acne, including the 2016 approval of dapsone 7.5% gel, as well as several agents with novel mechanisms of action now wending their way through the developmental pipeline.
“This development could be very interesting from an access standpoint and in terms of how physicians write prescriptions for retinoids, in light of the copays for other agents,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego.
“We know that with other retinoids, access is an issue. In Southern California, for example, we have strong pharmacy benefits’ managers for the insurance companies, and they’re very restrictive. It seems like every 3 months, they change the tiering of the different retinoids. It’s something we have to work on to get our patients a fair price,” he explained.
Dapsone 7.5% gel, marketed as Aczone Gel, 7.5% by Allergan, is a once-daily reformulation of the older 5% product administered twice daily. It received FDA approval for use in patients aged 12 years and older based on two 12-week, double-blind, placebo-controlled, randomized trials totaling more than 4,300 acne patients. The studies showed the stronger once-daily product was extremely well tolerated, with application site dryness and itching rates similar to placebo. In terms of efficacy, a Global Acne Assessment Score of 0 or 1 with at least a 2-grade improvement was achieved in 30% of patients assigned to dapsone 7.5% gel, compared with 21% of vehicle-treated controls.
Dr. Eichenfield was lead investigator in a recently published positive phase IIb, randomized, vehicle-controlled study of a topical nitric oxide-releasing agent for acne known for now as SB204 (J Drugs Dermatol. 2016 Dec 1;15[12]:1496-502). The product has both antimicrobial and anti-inflammatory properties, bacteria don’t develop resistance to it, and there is no significant systemic absorption.
“I haven’t seen the data yet. We’ll have to wait and see whether this agent continues to go forward,” Dr. Eichenfield said at the meeting, sponsored by the Global Academy for Medical Education/Skin Disease Research Foundation.
In its press release, Novan stated that the company believes “its cash on hand is sufficient to fund operations at least through the end of 2017, of which the allocation of capital will be dependent upon further assessment of the SB204 phase III trial results.”
DRM01 is a novel topical inhibitor of acetyl coenzyme-A carboxylase, an enzyme involved in synthesis of the fatty acids that are an essential component of sebum. A phase IIb randomized trial in 420 adult acne patients yielded positive results, according to Dermira, which is developing DRM01. The company plans to begin a pivotal phase III trial in the first half of 2017.
Another investigational topical acne therapy to keep an eye on is cortexolone. This peripherally selective antiandrogenic agent is under development by Cassiopea* Pharmaceuticals.
Dr. Eichenfield’s financial disclosures included serving as an investigator for Novan, Regeneron, Galderma, and Astellas Pharma US; and as a consultant for Galderma, Genentech, Janssen, Lilly, Otsuka, and TopMD.
SDEF and this news organization are owned by the same parent company.
[email protected]
*An earlier version of this article misstated the company that developed the peripherally selective antiandrogenic agent.
WAILEA, HAWAII – The Food and Drug Administration’s approval of adapalene gel 0.1% as an over-the-counter treatment for acne is a potential game changer that could lead to revision of guideline-recommended treatment algorithms, Lawrence F. Eichenfield, MD, predicted at the Hawaii Dermatology Seminar.
In addition to discussing the implications of the FDA’s unprecedented approval of a full prescription–strength topical retinoid for OTC use, he highlighted other developments in topical therapy for acne, including the 2016 approval of dapsone 7.5% gel, as well as several agents with novel mechanisms of action now wending their way through the developmental pipeline.
“This development could be very interesting from an access standpoint and in terms of how physicians write prescriptions for retinoids, in light of the copays for other agents,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego.
“We know that with other retinoids, access is an issue. In Southern California, for example, we have strong pharmacy benefits’ managers for the insurance companies, and they’re very restrictive. It seems like every 3 months, they change the tiering of the different retinoids. It’s something we have to work on to get our patients a fair price,” he explained.
Dapsone 7.5% gel, marketed as Aczone Gel, 7.5% by Allergan, is a once-daily reformulation of the older 5% product administered twice daily. It received FDA approval for use in patients aged 12 years and older based on two 12-week, double-blind, placebo-controlled, randomized trials totaling more than 4,300 acne patients. The studies showed the stronger once-daily product was extremely well tolerated, with application site dryness and itching rates similar to placebo. In terms of efficacy, a Global Acne Assessment Score of 0 or 1 with at least a 2-grade improvement was achieved in 30% of patients assigned to dapsone 7.5% gel, compared with 21% of vehicle-treated controls.
Dr. Eichenfield was lead investigator in a recently published positive phase IIb, randomized, vehicle-controlled study of a topical nitric oxide-releasing agent for acne known for now as SB204 (J Drugs Dermatol. 2016 Dec 1;15[12]:1496-502). The product has both antimicrobial and anti-inflammatory properties, bacteria don’t develop resistance to it, and there is no significant systemic absorption.
“I haven’t seen the data yet. We’ll have to wait and see whether this agent continues to go forward,” Dr. Eichenfield said at the meeting, sponsored by the Global Academy for Medical Education/Skin Disease Research Foundation.
In its press release, Novan stated that the company believes “its cash on hand is sufficient to fund operations at least through the end of 2017, of which the allocation of capital will be dependent upon further assessment of the SB204 phase III trial results.”
DRM01 is a novel topical inhibitor of acetyl coenzyme-A carboxylase, an enzyme involved in synthesis of the fatty acids that are an essential component of sebum. A phase IIb randomized trial in 420 adult acne patients yielded positive results, according to Dermira, which is developing DRM01. The company plans to begin a pivotal phase III trial in the first half of 2017.
Another investigational topical acne therapy to keep an eye on is cortexolone. This peripherally selective antiandrogenic agent is under development by Cassiopea* Pharmaceuticals.
Dr. Eichenfield’s financial disclosures included serving as an investigator for Novan, Regeneron, Galderma, and Astellas Pharma US; and as a consultant for Galderma, Genentech, Janssen, Lilly, Otsuka, and TopMD.
SDEF and this news organization are owned by the same parent company.
[email protected]
*An earlier version of this article misstated the company that developed the peripherally selective antiandrogenic agent.
WAILEA, HAWAII – The Food and Drug Administration’s approval of adapalene gel 0.1% as an over-the-counter treatment for acne is a potential game changer that could lead to revision of guideline-recommended treatment algorithms, Lawrence F. Eichenfield, MD, predicted at the Hawaii Dermatology Seminar.
In addition to discussing the implications of the FDA’s unprecedented approval of a full prescription–strength topical retinoid for OTC use, he highlighted other developments in topical therapy for acne, including the 2016 approval of dapsone 7.5% gel, as well as several agents with novel mechanisms of action now wending their way through the developmental pipeline.
“This development could be very interesting from an access standpoint and in terms of how physicians write prescriptions for retinoids, in light of the copays for other agents,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego.
“We know that with other retinoids, access is an issue. In Southern California, for example, we have strong pharmacy benefits’ managers for the insurance companies, and they’re very restrictive. It seems like every 3 months, they change the tiering of the different retinoids. It’s something we have to work on to get our patients a fair price,” he explained.
Dapsone 7.5% gel, marketed as Aczone Gel, 7.5% by Allergan, is a once-daily reformulation of the older 5% product administered twice daily. It received FDA approval for use in patients aged 12 years and older based on two 12-week, double-blind, placebo-controlled, randomized trials totaling more than 4,300 acne patients. The studies showed the stronger once-daily product was extremely well tolerated, with application site dryness and itching rates similar to placebo. In terms of efficacy, a Global Acne Assessment Score of 0 or 1 with at least a 2-grade improvement was achieved in 30% of patients assigned to dapsone 7.5% gel, compared with 21% of vehicle-treated controls.
Dr. Eichenfield was lead investigator in a recently published positive phase IIb, randomized, vehicle-controlled study of a topical nitric oxide-releasing agent for acne known for now as SB204 (J Drugs Dermatol. 2016 Dec 1;15[12]:1496-502). The product has both antimicrobial and anti-inflammatory properties, bacteria don’t develop resistance to it, and there is no significant systemic absorption.
“I haven’t seen the data yet. We’ll have to wait and see whether this agent continues to go forward,” Dr. Eichenfield said at the meeting, sponsored by the Global Academy for Medical Education/Skin Disease Research Foundation.
In its press release, Novan stated that the company believes “its cash on hand is sufficient to fund operations at least through the end of 2017, of which the allocation of capital will be dependent upon further assessment of the SB204 phase III trial results.”
DRM01 is a novel topical inhibitor of acetyl coenzyme-A carboxylase, an enzyme involved in synthesis of the fatty acids that are an essential component of sebum. A phase IIb randomized trial in 420 adult acne patients yielded positive results, according to Dermira, which is developing DRM01. The company plans to begin a pivotal phase III trial in the first half of 2017.
Another investigational topical acne therapy to keep an eye on is cortexolone. This peripherally selective antiandrogenic agent is under development by Cassiopea* Pharmaceuticals.
Dr. Eichenfield’s financial disclosures included serving as an investigator for Novan, Regeneron, Galderma, and Astellas Pharma US; and as a consultant for Galderma, Genentech, Janssen, Lilly, Otsuka, and TopMD.
SDEF and this news organization are owned by the same parent company.
[email protected]
*An earlier version of this article misstated the company that developed the peripherally selective antiandrogenic agent.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR