User login
Federal judge blocks merger between Anthem and Cigna
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
[email protected]
On Twitter @legal_med
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
[email protected]
On Twitter @legal_med
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
[email protected]
On Twitter @legal_med
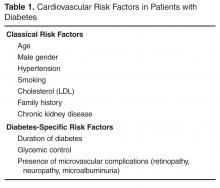
Cardiovascular Risk Reduction in Patients with Type 2 Diabetes
From the Division of Endocrinology, Department of Medicine, University of Toronto, Ontario, Canada.
Abstract
- Objective: To review the assessment of cardiovascular risk and prevention of vascular disease in patients with type 2 diabetes mellitus (T2DM).
- Methods: Literature and guidelines were reviewed and the evidence is presented around a clinical case.
- Results: T2DM has a high prevalence and confers significant lifetime risk for macrovascular disease, including stroke, heart disease, and peripheral arterial disease. There is strong evidence to support nonpharmacologic interventions, such as smoking cessation and weight loss, and pharmacologic interventions, such as statin therapy, in order to decrease lifetime risk. The effectiveness of an intervention as well as the strength of the evidence supporting an intervention differs depending on the stage of the disease.
- Conclusion: Once a patient is diagnosed with T2DM, it is important to recognize that their lifetime risk for vascular disease is high. Starting at this stage and continuing throughout the disease course, cardiovascular risk should be assessed in an ongoing manner and evidence-based interventions should be implemented whenever they are indicated. Using major guidelines as a framework, we provide an evidence-based approach to the reduction of vascular risk in these patients.
Key words: cardiovascular disease, diabetes, prevention, risk assessment, risk factors.
Type 2 diabetes (T2DM) is considered epidemic in the developed world, and is rapidly increasing in the developing world. Since 1980, there has been a near quadrupling of the number of adults with diabetes worldwide to an estimated 422 million in 2014 [1]. Because diabetes affects the whole body vascular system, there is a significant burden of vascular complications directly attributable to diabetes. Although the rates of diabetes-related complications are declining, the burden of disease remains high due to the increasing prevalence of diabetes [2]. The tremendous burden of diabetes and its complications on the population make it imperative that all health care practitioners understand the vascular effects of diabetes as well as evidence-based interventions that can mitigate them. In this review, we present an approach to the assessment, prevention, and treatment of cardiovascular disease in patients with T2DM.
Case Study
A 38-year-old male presents to his family physician’s office for a routine check-up. He is obese and a smoker, has no other health issues, and is taking no medications. He is sent for routine bloodwork and his A1c and fasting glucose are elevated and are diagnostic for diabetes. He returns to the clinic to discuss his results.
How are cardiovascular risk and risk factors assessed in a patient with diabetes?
There are many risk scores and risk calculators available for assessing cardiovascular risk. The Framingham Risk Score is the most commonly employed and takes into account the most common risk factors for cardiovascular risk, including cholesterol level, age, gender, and smoking status. Unfortunately, because a patient with diabetes may have a high lifetime risk but low or moderate short-term risk, these risk scores tend to underestimate overall risk in the population with diabetes [3,4]. Furthermore, since early intervention can decrease lifetime risk, it is important to recognize the limitations of these risk scores.
What interventions should be used for primary prevention at this stage?
A number of interventions can decrease lifetime risk for cardiovascular disease in persons with diabetes. First, smoking increases risk for all forms of vascular disease, including progression to end-stage renal disease, and is an independent predictor of mortality. Smoking cessation is one of the most effective interventions at decreasing these risks [6]. Second, lifestyle interventions such as diet and exercise are often recommended. The Look AHEAD trial studied the benefits of weight loss and exercise in the treatment of T2DM through a randomized control trial involving more than 5000 overweight patients with T2DM. Patients were randomly assigned to intensive lifestyle interventions targeting weight loss or a support and education group. Although the Action for Health in Diabetes (Look AHEAD) trial did not demonstrate clinical outcome benefit with this intensive intervention, there was improvement in weight, cholesterol level, blood pressure, and glycemic control, and clinical differences may have been related to study power or differences in cardioprotective medication use [7]. Furthermore, at least 1 large randomized trial of dietary intervention in high-risk cardiovascular patients, half of whom had diabetes (Prevención con Dieta Mediterránea [PREDIMED]), showed significant benefits in cardiovascular disease, reducing the incidence of major cardiovascular events [8]. According to most diabetes guidelines, diet and exercise continue to be stressed as initial management for all patients with diabetes [9–12].
In addition, although intensive glucose control decreases microvascular complication rates, it has been more difficult to demonstrate a reduction in cardiovascular disease with more intense glycemic control. However, long-term follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) cohort, a population that was earlier in their diabetes course, clearly demonstrated a reduction in cardiovascular events and mortality with better glycemic control over the long term [13,14]. For those who are later in their diabetes course, meta-analyses of glycemic control trials, along with follow-up studies, have also shown that better glycemic control can reduce cardiovascular events, but not mortality [15–17]. Therefore, glucose lowering should be pursued for cardiovascular risk reduction, in addition to its effects on microvascular complications.
It is well established that a multifactorial approach to cardiovascular risk reduction in patients with type 2 diabetes is effective. In the Steno-2 study, 160 patients with type 2 diabetes were randomly assigned to receive multidisciplinary, multifactorial intensive target-based lifestyle and pharmacologic intervention or standard of care. The intensive therapy group all received smoking cessation counseling, exercise and dietary advice, vitamin supplementation, and an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). Acetylsalicylic acid (ASA) was added for all patients with clinical macrovascular disease. Dyslipidemia, hypertension, and hyperglycemia were all treated in a protocolized way if lifestyle interventions did not achieve strict targets. During the mean 7.8 years of follow-up, the adjusted hazard ratio for a composite of cardiovascular death and macrovascular disease was 0.47 (95% confidence interval [CI] 0.22 to 0.74; P = 0.01) [18]. These patients were followed for an additional 5.5 years in an observational study with no further active intervention in both groups. Over the entire period, there was an absolute risk reduction of 20% for death from any cause, resulting in a number needed to treat of 5 for 13 years [19]. As a result of these compelling data, guidelines from around the world support a multifactorial approach, with the Canadian Diabetes Association (CDA) guidelines [20] promoting the use of the “ABCDES” of vascular protection:
A – A1C target
B – Blood pressure target
C – Cholesterol target
D – Drugs for vascular protection
E – Exercise/Eating
S – Smoking cessation
Is any particular dietary pattern recommended?
There is a large and ever-growing number of dietary patterns that are marketed to improve weight and cardiovascular health. Unfortunately, however, few of these interventions have been studied rigorously, and most dietary interventions are found to be unsustainable in the long term. In the case of motivated patients, there are some specific dietary patterns with high-quality evidence to recommend them. The simplest intervention is the implementation of a vegetarian or vegan diet. Over 18 months, this has been shown to improve fasting glucose and cholesterol profile, and promote weight loss [21]. In another study, a calorie-restricted vegetarian diet led to a reduction in diabetes medication in 46% of participants (versus 5% with conventional diet) [22].
A Mediterranean diet is comprised of large amounts of fruits, vegetables, legumes, nuts, and whole grains. In addition, it includes moderate consumption of olive oil, dairy, fish and poultry, with low consumption of red meat. This dietary pattern has been extensively studied, and in a meta-analysis has been shown to improve glycemic control, blood pressure, and lipid profile [23]. The PREDIMED study evaluated the efficacy of 2 versions of the Mediterranean diet, one supplemented with olive oil or mixed nuts, for reducing cardiovascular events. This multicenter randomized control trial of 7447 participants at high cardiovascular risk (48.5% of whom had diabetes) was stopped early due to benefit. Both versions of the diet reduced cardiovascular events by 30% over 5 years of follow-up [8].
The Dietary Approaches to Stop Hypertension (DASH) diet is similar to the Mediterranean diet in focusing on fruits, vegetables, low-fat dairy, whole grains, nuts, fish, and poultry, while avoiding red meat. In addition, it explicitly recommends avoiding sweets and sweetened beverages, as well as dietary fat. In a trial of patients with diabetes matched for moderate sodium intake, the DASH diet has been shown to decrease A1c, blood pressure, and weight and improve lipid profile within 8 weeks [24,25].
In addition to these specific dietary patterns, specific foods have been shown to improve glycemic control and cardiovascular risk profile, including mixed unsalted nuts, almonds, dietary pulses, and low-glycemic versus high-glycemic index carbohydrates [26–31].
In accordance with CDA, American Diabetes Association (ADA), and European Association for the Study of Diabetes (EASD) guidelines, we recognize that a variety of diets can improve the cardiovascular risk profile of a patient [12,32,33]. Therefore, we suggest a tailored approach to dietary changes for each individual patient. This should, whenever possible, be undertaken with a registered dietitian, with emphasis placed on the evidence for vascular protection, improved risk profile, patient preference, and likelihood of long-term sustainability.
Should therapy for weight loss be recommended for this patient?
There are currently a number of effective strategies for achieving weight loss, including lifestyle interventions, pharmacotherapy, and surgery. The evidence base for dietary interventions for diabetes is reviewed above. The Look AHEAD study randomized 5145 overweight or obese patients with T2DM to intensive lifestyle intervention for weight loss through promotion of decreased caloric intake and increased physical activity, or usual diabetes support and education. After a median follow-up of 9.6 years, the study was stopped early on the basis of a futility analysis despite greater weight loss in the intervention group throughout the study. However, other benefits were derived including reduced need for medications, reduced sleep apnea, and improved well-being [7].
Pharmacotherapy agents for weight loss have been approved by various regulatory agencies. None has as yet shown a reduction in cardiovascular events. Therefore, these cannot be recommended as therapies for vascular protection at this time.
Bariatric surgery is an effective option for weight loss in patients with diabetes, with marked and sustained improvements in clinically meaningful outcomes when compared with medical management. The longest study of bariatric surgery is the Swedish Obesity Study, a prospective case-control study of 2010 obese patients who underwent bariatric surgery and 2037 matched controls. After a median of 14.8 years of follow-up, there was a reduction in overall mortality (hazard ratio [HR] 0.71) and decreased incidence of diabetes (HR 0.17), myocardial infarction (HR 0.71), and stroke (HR 0.66). Diabetes remission, defined as normal A1c off of anti-hyperglycemic therapy, was increased at 2 years (odds ratio [OR] 13.3) and sustained at 15 years (OR 6.3) [34–36]. Randomized controlled trials of bariatric surgery have thus far been small and do show some decreases in cardiovascular risk factors [37–40]. However, these have not yet been of sufficient duration or size to demonstrate a decrease in cardiovascular event rate. Although local policies may vary in referral recommendation, the Obesity Society, ADA, and CDA recommend that patients with a body mass index greater than 40 kg/m2, or greater than 35 kg/m2 with an obesity-related comorbidity such as diabetes, should be referred to a center that specializes in bariatric surgery for evaluation [41–43].
Case Continued
After the initial diagnosis, the patient was seen by a registered dietitian and followed a Mediterranean diet for some time but has since stopped. He is seen regularly for follow-up of his diabetes at 3- to 6-month intervals. He initially lost some weight but has unfortunately regained the weight. He tells you proudly that he finally quit smoking. He was started on metformin about 6 months after diagnosis to address his glycemic control. He continues on the metformin now as his only medication.
The patient returns to clinic for his usual follow-up visit approximately 5 years after initial diagnosis. He is feeling well with no new medical issues. He has no clinically apparent retinopathy or macrovascular complications. On examination, his blood pressure is 140/90 mm Hg and the remainder of the exam is unremarkable. His bloodwork shows an A1c of 8% and a low-density lipoprotein cholesterol (LDL-C) level of 124 mg/dL. His albumin-to-creatinine ratio is normal.
How often should cardiovascular risk be reassessed?
When should initiating pharmacotherapy to reduce risk in primary prevention be considered?
In the population with diabetes, statins and renin-angiotensin-aldosterone inhibition are the mainstays of pharmacotherapy for cardiovascular risk reduction. In the presence of clinical macrovascular disease, the standard of care includes both of these therapies. However, there is also a great deal of data that supports the use of these therapies for primary prevention.
Statins
Major studies on the benefits of statin therapy in people with diabetes have consistently shown decreased cardiovascular disease and mortality. The Heart Protection Study included a subgroup of patients with diabetes in which patients over the age of 40 were randomly assigned to simvastatin or placebo. Consistently across all subgroups, there was a relative risk reduction of 22% to 33% for the primary outcome of first cardiovascular event over 5 years. This effect was maintained even in those who did not have elevated LDL-C at randomization [46]. Similarly, the Collaborative Atorvastatin Diabetes Study (CARDS) randomized patients with T2DM, over age 40, with at least 1 other vascular risk factor to atorvastatin 10 mg or placebo. They found a 37% risk reduction in time to first event over 4 years with atorvastatin, with consistent results across all subgroups [47].
Based on these studies, it is recommended that all patients with diabetes be placed on statin therapy to reduce vascular risk at age 40 years (CDA, ADA, American College of Cardiology/American Heart Association [ACC/AHA]) [20,45,48]. If under age 40 years, statin therapy should be considered in the presence of other risk factors (ADA, ACC/AHA) [45,48], or if diabetes duration is more than 15 years and age is greater than 30 years, or there are micro- or macrovascular complications (CDA) [20].
Renin-Angiotensin-Aldosterone Inhibition
Similar to research into statin therapy, a considerable amount of research has been dedicated to renin-angiotensin-aldosterone system (RAAS) blockade for the primary purpose of vascular risk reduction, even in the absence of hypertension, in those with diabetes. In a prespecified substudy of the Heart Outcomes Prevention Evaluation (HOPE) trial, known as MICRO HOPE, patients with diabetes who were older than 55 years of age, with at least 1 other cardiovascular risk factor, were randomized to receive ramipril 10 mg daily or placebo. In this study, ramipril reduced the risk for myocardial infarction (22%), stroke (33%), cardiovascular death (37%), and all-cause mortality (24%) over 4.5 years [49]. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), patients at high risk for cardiovascular disease were randomized to telmisartan 80 mg or ramipril 10 mg. In the diabetes subgroup, there were similar risk reductions and no statistical difference between the groups [50]. A 2012 meta-analysis assessed the benefits of RAAS blockade compared with placebo for primary prevention in high-risk individuals, or secondary prevention in those with established vascular disease. A reduction in cardiovascular death, all-cause mortality, fatal or nonfatal myocardial infarction, and stroke was seen across all subgroups, including those with and without diabetes or hypertension [51].
The CDA currently recommends that an ACE inhibitor or ARB be given to all patients with diabetes who are 55 years of age or older, or have macro- or microvascular disease, for the primary purpose of decreasing risk for vascular disease, even in the absence of hypertension. An agent and dose with proven vascular protective benefit should be chosen when selecting an ACE inhibitor or ARB [20].
Should this patient start ASA therapy?
Whether to start daily low-dose ASA for primary prevention of coronary artery disease has been a long-standing question in patients with diabetes. The benefits of ASA therapy with regards to coronary artery disease have long been known from a secondary prevention standpoint, and given the low risk and long experience, primary prevention seemed reasonable. However, no high-quality randomized controlled trials enrolling large numbers of patients with diabetes have been performed in the current era of medical therapy, specifically in the era of widespread statin use. The initial studies examining ASA use in primary prevention were analyzed in a meta-analysis in 1994 and showed a trend towards benefit for ASA in patients with diabetes [52]. Further trials increased the number of diabetes patient-years studied but did not change the initial result. Five meta-analyses have been conducted on the currently available trials, and all but one do not show a significant reduction in coronary artery disease or stroke in patients with diabetes [53–57]. In addition, ASA is known to cause a small absolute increase in the risk for gastrointestinal hemorrhage that is consistent across all studies, with a number needed to harm of approximately 100 over 2.5 years. Therefore, the possible small absolute benefit that was seen in ASA trials with regards to coronary artery disease in the era before statin therapy must be weighed against the known risk of bleeding. Because of this, the CDA and European Society of Cardiology have recommendations against the routine use of ASA for primary prevention in patients with diabetes [12,20].
A summary of pharmacotherapy for cardiovascular risk reduction is shown in the Figure.
Case Continued
The patient is started on a statin because of his elevated LDL-C level in the context of being over the age of 40 years with T2DM. He is also started on an ACE inhibitor to address the hypertension. In addition, a dipeptidyl peptidase-4 inhibitor is added to his metformin to address the elevated A1c. He continues to follow up every 3 to 6 months.
Six years later, he experiences an episode of retrosternal chest discomfort while exercising. He is brought to hospital and is found to have a non-ST elevation myocardial infarction. He is admitted to hospital, undergoes percutaneous revascularization of a single lesion, and is discharged to a rehabilitation center. He is discharged on aspirin, clopidogrel, an ACE inhibitor, a beta blocker, and a high-intensity statin. His blood pressure is well managed, and he has lost further weight since he was last seen. When he returns to clinic, he wonders if there is anything more he can do to prevent further events.
What secondary prevention of cardiovascular disease is recommended for patients with T2DM?
Optimal secondary prevention following a major vascular event includes a combination of pharmacologic and nonpharmacologic interventions. In the population without diabetes, evidence supports smoking cessation, exercise, cardiac-specific rehabilitation, antiplatelets, RAAS antag-onists, beta-blockade, and statins. Most of the trials that led to this standard suite of interventions had large diabetes subgroups. Therefore, there is no difference in the secondary prevention of cardiovascular disease in the population with diabetes with regard to these interventions.
Have any antihyperglycemic agents been shown to reduce cardiovascular events?
Metformin
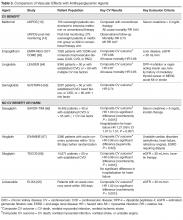
A summary of the vascular effects observed during trials of antihyperglycemic agents is shown in Table 3.
Empagliflozin
Many large randomized, controlled cardiovascular outcome trials have been completed or are ongoing looking at the cardiovascular safety of newer antihyperglycemic agents. The majority of the completed trials have shown a neutral effect, suggesting that the agents are safe. However, in September 2015, the first cardiovascular outcome trial of an antihyperglycemic agent with a positive result was published. The Empagliflozin Cardiovascular Outcome Event Trial (EMPA-REG OUTCOME) randomized 7020 patients with T2DM and cardiovascular disease (defined as nonacute myocardial infarction, multivessel coronary artery disease, unstable angina, nonacute stroke, or occlusive peripheral arterial disease) to placebo or 1 of 2 doses of empagliflozin. The primary outcome of cardiovascular mortality, nonfatal myocardial infarction, or stroke was reduced by 14% in the empagliflozin-treated group. Key secondary outcomes of all-cause mortality (HR 0.68) and heart failure hospitalization (HR 0.65) were also statistically different in favor of the empagliflozin arm [63].
On the basis of this trial’s results, empagliflozin should be considered for treatment of all patients with type 2 diabetes and known cardiovascular disease. It is as yet unknown whether this effect will translate to the other members of the sodium-glucose co-transporter 2 (SGLT-2) inhibitor class, although results of studies involving other SGLT-2 inhibitors are expected in the next 2 to 3 years.
Liraglutide
In 2016, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial reported results of its cardiovascular safety trial. In this trial, 9340 patients with either established vascular disease or risk factors for vascular disease were randomized to daily liraglutide or placebo injections. The primary composite outcome of cardiovascular death, nonfatal myocardial infarction, or stroke was reduced by 13%. A key secondary outcome of all-cause mortality also showed a significant reduction (HR 0.85). There was no reduction in hospitalization for heart failure [64].
Semaglutide
Most recently, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) was completed, assessing cardiovascular safety of a once-weekly injectable glucagon-like peptide-1 (GLP-1) analogue. This noninferiority trial studied 3297 patients with type 2 diabetes over the age of 50 years with established macrovascular disease, chronic heart failure, or chronic kidney disease (stage III or higher), or over the age of 60 years with at least 1 other cardiovascular risk factor. The patients were randomized to 1 of 2 doses of once-weekly semaglutide or placebo injection. A composite cardiovascular outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was decreased by 26% in the pooled semaglutide group. This was driven primarily by a reduction in nonfatal stroke, with no statistically significant reduction in nonfatal myocardial infarction or cardiovascular mortality. Significant secondary outcomes showed a reduction in new or worsening nephropathy (HR 0.64), and an unexpected increase in retinopathy (HR 1.76) [65].
All of these trials utilized their respective agents as add-on to existing antihyperglycemic therapy. Therefore, first-line antihyperglycemic therapy in a patient with T2DM remains metformin. For the patient with established vascular disease or who is at high risk for developing vascular disease, add-on therapy using an antihyperglycemic agent with proven cardiovascular benefits, such as empagliflozin or liraglutide, is suggested [9,11]. Semaglutide is not yet available for clinical use. The choice between these agents should be based on patient preference, cost, side effect profile, and absence of contraindications.
Currently, there are more studies underway with similar designs with different agents. As these studies are reported in the upcoming years, it is hoped that the options for reduction of cardiovascular risk will increase, and that we will have multiple antihyperglycemic agents that will provide not only glycemic benefit but also cardiovascular risk reduction.
Case Conclusion
The patient continues to abstain from smoking. He follows up with a dietitian and is enrolled in an exercise program. He remains on his cardiac medications. For glycemic control, he continues on his previous antihyperglycemic therapy and an antihyperglycemic agent with proven cardiovascular benefit is added. With these interventions, he understands that his risk is mitigated, but given his history and previous event, he remains at high risk for future vascular disease.
Conclusion
The care of a patient with diabetes requires a multifactorial approach. All patients are at risk for developing the vascular complications of diabetes, and it is these complications that ultimately result in the nearly doubled risk of mortality in patients with diabetes. Various trials have shown that targeted interventions can and do reduce the risk for cardiovascular disease in a measurable way. Above and beyond targeted interventions, we now know that strict multifactorial interventions can result in a clinically significant reduction in both mortality and cardiovascular disease. This multifactorial approach is supported by guidelines around the world [12,44,45]. A standardized approach to the assessment of risk and the application of interventions is critical. More recent data show that specific antihyperglycemic therapies can also reduce cardiovascular events above and beyond their glycemic effects. The rates of cardiovascular events in patients with diabetes have declined over time, and hopefully this trend will continue as further research supports additional interventions.
Corresponding author: Bikrampal S. Sidhu, MD, Toronto General Hospital, 200 Elizabeth St., 12 EN 242, Toronto, ON, M5G 2C4, [email protected].
Financial disclosures: Dr. Cheng has received fees for speaking and/or consulting from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck, Novo Nordisk, Sanofi, Servier, and Takeda.
1. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30.
2. Gregg EW, Li Y, Wang J, et al. Changes in Diabetes-Related Complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–23.
3. Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29–36.
4. Stevens RJ, Kothari V, Alder A, et al. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–9.
5. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
6. Solberg L, Desai JR, O’Connor PJ, et al. Diabetic patients who smoke: are they different? Diabetes Care 1999;22:1887–98.
7. Look Ahead Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54.
8. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.
9. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Pharmacologic management of type 2 diabetes: November 2016 Interim Update. Can J Diabetes 2016;40:484–6.
10. Harper W, Clement M, Goldenberg R, et al. Pharmacologic management of type 2 diabetes. Can J Diabetes 2013; 37:S61–S68.
11. American Diabetes Association. Pharmacologic approaches to glycemic management. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S64–74.
12. The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013;34:3035–87.
13. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65.
14. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
15. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–98.
16. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
17. ACCORD Study Group. Nine-year rffects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 2016;39:701–8.
18. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93.
19. Gaede P, Lund-Anderson H, Parving HH, Pederson O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91.
20. Stone JA, Fitchett D, Grover S, et al. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Vascular protection in people with diabetes. Can J Diabetes 2013;37 (suppl 1):S100–S104.
21. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588–96.
22. Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–59.
23. Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 2010;89:97–102.
24. Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–8.
25. Azadbakht L, Fard NR, Karimi M, et al. The Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care 2011;34:55–7.
26. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7.
27. Opperman AM, Venter CS, Oosthuizen W, et al. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004;92:367–81.
28. Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802.
29. Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes. Diabetologia 2009;52:1479–95.
30. Jenkins DJ, Kendall CW, Banach MS, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011;34:1706–11.
31. Li SC, Liu YH, Liu JF, et al. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9.
32. Dworatzek PD, Arcudi K, Gougeon R, et al. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: nutrition therapy. Can J Diabetes 2013;37(suppl 1):S45–55.
33. American Diabetes Association. Lifestyle Management. Sec. 4. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S33–43.
34. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med 2007;357:741–52.
35. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
36. Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–7.
37. Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–23.
38. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
39. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
40. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
41. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines and
The Obesity Society. Obesity (Silver Spring) 2014;22:S1–S410.
42. Wharton S, Sharma AM, Lau DCW. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Weight management in diabetes. Can J Diabetes 2013;37(suppl 1):S82–6.
43. American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes. Sec. 7. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S57–63.
44. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013;37(suppl 1):S1–S212.
45. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S1–S135.
46. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomized placebo controlled trial. Lancet 2003;361:2005–16.
47. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
48. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934.
49. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–9.
50. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59.
51. McAlister FA, Renin Angiotensin System Modulator Meta-Analysis Investigators. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are beneficial in normotensive atherosclerotic patients: a collaborative meta-analysis of randomized trials. Eur Heart J 2012;33:505–14.
52. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106.
53. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60.
54. Calvin AD, Aggarwal NR, Murad MH, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care 2009;32:2300–6.
55. De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009;339:b4531.
56. Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010;87:211–8.
57. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010;33:1395–402.
58. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomised clinical trial. JAMA 2014;312:2510–20.
59. ASCEND Trial Web site. Clinical Trials Service Unit, University of Oxford, and British Heart Foundation. https://ascend.medsci.ox.ac.uk/. Accessed September 20, 2016.
60. De Berardis G, Sacco M, Evangelista V, et al. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007;8:21.
61. Maruther NM, Tseng E, Hutfless SS, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
62. Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS Med 2012;9:e1001204.
63. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
64. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
65. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44
66. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
67. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
68. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
69. Pfeffer MA, Claggett B, Diaz R, Dickstein K, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57.
From the Division of Endocrinology, Department of Medicine, University of Toronto, Ontario, Canada.
Abstract
- Objective: To review the assessment of cardiovascular risk and prevention of vascular disease in patients with type 2 diabetes mellitus (T2DM).
- Methods: Literature and guidelines were reviewed and the evidence is presented around a clinical case.
- Results: T2DM has a high prevalence and confers significant lifetime risk for macrovascular disease, including stroke, heart disease, and peripheral arterial disease. There is strong evidence to support nonpharmacologic interventions, such as smoking cessation and weight loss, and pharmacologic interventions, such as statin therapy, in order to decrease lifetime risk. The effectiveness of an intervention as well as the strength of the evidence supporting an intervention differs depending on the stage of the disease.
- Conclusion: Once a patient is diagnosed with T2DM, it is important to recognize that their lifetime risk for vascular disease is high. Starting at this stage and continuing throughout the disease course, cardiovascular risk should be assessed in an ongoing manner and evidence-based interventions should be implemented whenever they are indicated. Using major guidelines as a framework, we provide an evidence-based approach to the reduction of vascular risk in these patients.
Key words: cardiovascular disease, diabetes, prevention, risk assessment, risk factors.
Type 2 diabetes (T2DM) is considered epidemic in the developed world, and is rapidly increasing in the developing world. Since 1980, there has been a near quadrupling of the number of adults with diabetes worldwide to an estimated 422 million in 2014 [1]. Because diabetes affects the whole body vascular system, there is a significant burden of vascular complications directly attributable to diabetes. Although the rates of diabetes-related complications are declining, the burden of disease remains high due to the increasing prevalence of diabetes [2]. The tremendous burden of diabetes and its complications on the population make it imperative that all health care practitioners understand the vascular effects of diabetes as well as evidence-based interventions that can mitigate them. In this review, we present an approach to the assessment, prevention, and treatment of cardiovascular disease in patients with T2DM.
Case Study
A 38-year-old male presents to his family physician’s office for a routine check-up. He is obese and a smoker, has no other health issues, and is taking no medications. He is sent for routine bloodwork and his A1c and fasting glucose are elevated and are diagnostic for diabetes. He returns to the clinic to discuss his results.
How are cardiovascular risk and risk factors assessed in a patient with diabetes?
There are many risk scores and risk calculators available for assessing cardiovascular risk. The Framingham Risk Score is the most commonly employed and takes into account the most common risk factors for cardiovascular risk, including cholesterol level, age, gender, and smoking status. Unfortunately, because a patient with diabetes may have a high lifetime risk but low or moderate short-term risk, these risk scores tend to underestimate overall risk in the population with diabetes [3,4]. Furthermore, since early intervention can decrease lifetime risk, it is important to recognize the limitations of these risk scores.
What interventions should be used for primary prevention at this stage?
A number of interventions can decrease lifetime risk for cardiovascular disease in persons with diabetes. First, smoking increases risk for all forms of vascular disease, including progression to end-stage renal disease, and is an independent predictor of mortality. Smoking cessation is one of the most effective interventions at decreasing these risks [6]. Second, lifestyle interventions such as diet and exercise are often recommended. The Look AHEAD trial studied the benefits of weight loss and exercise in the treatment of T2DM through a randomized control trial involving more than 5000 overweight patients with T2DM. Patients were randomly assigned to intensive lifestyle interventions targeting weight loss or a support and education group. Although the Action for Health in Diabetes (Look AHEAD) trial did not demonstrate clinical outcome benefit with this intensive intervention, there was improvement in weight, cholesterol level, blood pressure, and glycemic control, and clinical differences may have been related to study power or differences in cardioprotective medication use [7]. Furthermore, at least 1 large randomized trial of dietary intervention in high-risk cardiovascular patients, half of whom had diabetes (Prevención con Dieta Mediterránea [PREDIMED]), showed significant benefits in cardiovascular disease, reducing the incidence of major cardiovascular events [8]. According to most diabetes guidelines, diet and exercise continue to be stressed as initial management for all patients with diabetes [9–12].
In addition, although intensive glucose control decreases microvascular complication rates, it has been more difficult to demonstrate a reduction in cardiovascular disease with more intense glycemic control. However, long-term follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) cohort, a population that was earlier in their diabetes course, clearly demonstrated a reduction in cardiovascular events and mortality with better glycemic control over the long term [13,14]. For those who are later in their diabetes course, meta-analyses of glycemic control trials, along with follow-up studies, have also shown that better glycemic control can reduce cardiovascular events, but not mortality [15–17]. Therefore, glucose lowering should be pursued for cardiovascular risk reduction, in addition to its effects on microvascular complications.
It is well established that a multifactorial approach to cardiovascular risk reduction in patients with type 2 diabetes is effective. In the Steno-2 study, 160 patients with type 2 diabetes were randomly assigned to receive multidisciplinary, multifactorial intensive target-based lifestyle and pharmacologic intervention or standard of care. The intensive therapy group all received smoking cessation counseling, exercise and dietary advice, vitamin supplementation, and an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). Acetylsalicylic acid (ASA) was added for all patients with clinical macrovascular disease. Dyslipidemia, hypertension, and hyperglycemia were all treated in a protocolized way if lifestyle interventions did not achieve strict targets. During the mean 7.8 years of follow-up, the adjusted hazard ratio for a composite of cardiovascular death and macrovascular disease was 0.47 (95% confidence interval [CI] 0.22 to 0.74; P = 0.01) [18]. These patients were followed for an additional 5.5 years in an observational study with no further active intervention in both groups. Over the entire period, there was an absolute risk reduction of 20% for death from any cause, resulting in a number needed to treat of 5 for 13 years [19]. As a result of these compelling data, guidelines from around the world support a multifactorial approach, with the Canadian Diabetes Association (CDA) guidelines [20] promoting the use of the “ABCDES” of vascular protection:
A – A1C target
B – Blood pressure target
C – Cholesterol target
D – Drugs for vascular protection
E – Exercise/Eating
S – Smoking cessation
Is any particular dietary pattern recommended?
There is a large and ever-growing number of dietary patterns that are marketed to improve weight and cardiovascular health. Unfortunately, however, few of these interventions have been studied rigorously, and most dietary interventions are found to be unsustainable in the long term. In the case of motivated patients, there are some specific dietary patterns with high-quality evidence to recommend them. The simplest intervention is the implementation of a vegetarian or vegan diet. Over 18 months, this has been shown to improve fasting glucose and cholesterol profile, and promote weight loss [21]. In another study, a calorie-restricted vegetarian diet led to a reduction in diabetes medication in 46% of participants (versus 5% with conventional diet) [22].
A Mediterranean diet is comprised of large amounts of fruits, vegetables, legumes, nuts, and whole grains. In addition, it includes moderate consumption of olive oil, dairy, fish and poultry, with low consumption of red meat. This dietary pattern has been extensively studied, and in a meta-analysis has been shown to improve glycemic control, blood pressure, and lipid profile [23]. The PREDIMED study evaluated the efficacy of 2 versions of the Mediterranean diet, one supplemented with olive oil or mixed nuts, for reducing cardiovascular events. This multicenter randomized control trial of 7447 participants at high cardiovascular risk (48.5% of whom had diabetes) was stopped early due to benefit. Both versions of the diet reduced cardiovascular events by 30% over 5 years of follow-up [8].
The Dietary Approaches to Stop Hypertension (DASH) diet is similar to the Mediterranean diet in focusing on fruits, vegetables, low-fat dairy, whole grains, nuts, fish, and poultry, while avoiding red meat. In addition, it explicitly recommends avoiding sweets and sweetened beverages, as well as dietary fat. In a trial of patients with diabetes matched for moderate sodium intake, the DASH diet has been shown to decrease A1c, blood pressure, and weight and improve lipid profile within 8 weeks [24,25].
In addition to these specific dietary patterns, specific foods have been shown to improve glycemic control and cardiovascular risk profile, including mixed unsalted nuts, almonds, dietary pulses, and low-glycemic versus high-glycemic index carbohydrates [26–31].
In accordance with CDA, American Diabetes Association (ADA), and European Association for the Study of Diabetes (EASD) guidelines, we recognize that a variety of diets can improve the cardiovascular risk profile of a patient [12,32,33]. Therefore, we suggest a tailored approach to dietary changes for each individual patient. This should, whenever possible, be undertaken with a registered dietitian, with emphasis placed on the evidence for vascular protection, improved risk profile, patient preference, and likelihood of long-term sustainability.
Should therapy for weight loss be recommended for this patient?
There are currently a number of effective strategies for achieving weight loss, including lifestyle interventions, pharmacotherapy, and surgery. The evidence base for dietary interventions for diabetes is reviewed above. The Look AHEAD study randomized 5145 overweight or obese patients with T2DM to intensive lifestyle intervention for weight loss through promotion of decreased caloric intake and increased physical activity, or usual diabetes support and education. After a median follow-up of 9.6 years, the study was stopped early on the basis of a futility analysis despite greater weight loss in the intervention group throughout the study. However, other benefits were derived including reduced need for medications, reduced sleep apnea, and improved well-being [7].
Pharmacotherapy agents for weight loss have been approved by various regulatory agencies. None has as yet shown a reduction in cardiovascular events. Therefore, these cannot be recommended as therapies for vascular protection at this time.
Bariatric surgery is an effective option for weight loss in patients with diabetes, with marked and sustained improvements in clinically meaningful outcomes when compared with medical management. The longest study of bariatric surgery is the Swedish Obesity Study, a prospective case-control study of 2010 obese patients who underwent bariatric surgery and 2037 matched controls. After a median of 14.8 years of follow-up, there was a reduction in overall mortality (hazard ratio [HR] 0.71) and decreased incidence of diabetes (HR 0.17), myocardial infarction (HR 0.71), and stroke (HR 0.66). Diabetes remission, defined as normal A1c off of anti-hyperglycemic therapy, was increased at 2 years (odds ratio [OR] 13.3) and sustained at 15 years (OR 6.3) [34–36]. Randomized controlled trials of bariatric surgery have thus far been small and do show some decreases in cardiovascular risk factors [37–40]. However, these have not yet been of sufficient duration or size to demonstrate a decrease in cardiovascular event rate. Although local policies may vary in referral recommendation, the Obesity Society, ADA, and CDA recommend that patients with a body mass index greater than 40 kg/m2, or greater than 35 kg/m2 with an obesity-related comorbidity such as diabetes, should be referred to a center that specializes in bariatric surgery for evaluation [41–43].
Case Continued
After the initial diagnosis, the patient was seen by a registered dietitian and followed a Mediterranean diet for some time but has since stopped. He is seen regularly for follow-up of his diabetes at 3- to 6-month intervals. He initially lost some weight but has unfortunately regained the weight. He tells you proudly that he finally quit smoking. He was started on metformin about 6 months after diagnosis to address his glycemic control. He continues on the metformin now as his only medication.
The patient returns to clinic for his usual follow-up visit approximately 5 years after initial diagnosis. He is feeling well with no new medical issues. He has no clinically apparent retinopathy or macrovascular complications. On examination, his blood pressure is 140/90 mm Hg and the remainder of the exam is unremarkable. His bloodwork shows an A1c of 8% and a low-density lipoprotein cholesterol (LDL-C) level of 124 mg/dL. His albumin-to-creatinine ratio is normal.
How often should cardiovascular risk be reassessed?
When should initiating pharmacotherapy to reduce risk in primary prevention be considered?
In the population with diabetes, statins and renin-angiotensin-aldosterone inhibition are the mainstays of pharmacotherapy for cardiovascular risk reduction. In the presence of clinical macrovascular disease, the standard of care includes both of these therapies. However, there is also a great deal of data that supports the use of these therapies for primary prevention.
Statins
Major studies on the benefits of statin therapy in people with diabetes have consistently shown decreased cardiovascular disease and mortality. The Heart Protection Study included a subgroup of patients with diabetes in which patients over the age of 40 were randomly assigned to simvastatin or placebo. Consistently across all subgroups, there was a relative risk reduction of 22% to 33% for the primary outcome of first cardiovascular event over 5 years. This effect was maintained even in those who did not have elevated LDL-C at randomization [46]. Similarly, the Collaborative Atorvastatin Diabetes Study (CARDS) randomized patients with T2DM, over age 40, with at least 1 other vascular risk factor to atorvastatin 10 mg or placebo. They found a 37% risk reduction in time to first event over 4 years with atorvastatin, with consistent results across all subgroups [47].
Based on these studies, it is recommended that all patients with diabetes be placed on statin therapy to reduce vascular risk at age 40 years (CDA, ADA, American College of Cardiology/American Heart Association [ACC/AHA]) [20,45,48]. If under age 40 years, statin therapy should be considered in the presence of other risk factors (ADA, ACC/AHA) [45,48], or if diabetes duration is more than 15 years and age is greater than 30 years, or there are micro- or macrovascular complications (CDA) [20].
Renin-Angiotensin-Aldosterone Inhibition
Similar to research into statin therapy, a considerable amount of research has been dedicated to renin-angiotensin-aldosterone system (RAAS) blockade for the primary purpose of vascular risk reduction, even in the absence of hypertension, in those with diabetes. In a prespecified substudy of the Heart Outcomes Prevention Evaluation (HOPE) trial, known as MICRO HOPE, patients with diabetes who were older than 55 years of age, with at least 1 other cardiovascular risk factor, were randomized to receive ramipril 10 mg daily or placebo. In this study, ramipril reduced the risk for myocardial infarction (22%), stroke (33%), cardiovascular death (37%), and all-cause mortality (24%) over 4.5 years [49]. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), patients at high risk for cardiovascular disease were randomized to telmisartan 80 mg or ramipril 10 mg. In the diabetes subgroup, there were similar risk reductions and no statistical difference between the groups [50]. A 2012 meta-analysis assessed the benefits of RAAS blockade compared with placebo for primary prevention in high-risk individuals, or secondary prevention in those with established vascular disease. A reduction in cardiovascular death, all-cause mortality, fatal or nonfatal myocardial infarction, and stroke was seen across all subgroups, including those with and without diabetes or hypertension [51].
The CDA currently recommends that an ACE inhibitor or ARB be given to all patients with diabetes who are 55 years of age or older, or have macro- or microvascular disease, for the primary purpose of decreasing risk for vascular disease, even in the absence of hypertension. An agent and dose with proven vascular protective benefit should be chosen when selecting an ACE inhibitor or ARB [20].
Should this patient start ASA therapy?
Whether to start daily low-dose ASA for primary prevention of coronary artery disease has been a long-standing question in patients with diabetes. The benefits of ASA therapy with regards to coronary artery disease have long been known from a secondary prevention standpoint, and given the low risk and long experience, primary prevention seemed reasonable. However, no high-quality randomized controlled trials enrolling large numbers of patients with diabetes have been performed in the current era of medical therapy, specifically in the era of widespread statin use. The initial studies examining ASA use in primary prevention were analyzed in a meta-analysis in 1994 and showed a trend towards benefit for ASA in patients with diabetes [52]. Further trials increased the number of diabetes patient-years studied but did not change the initial result. Five meta-analyses have been conducted on the currently available trials, and all but one do not show a significant reduction in coronary artery disease or stroke in patients with diabetes [53–57]. In addition, ASA is known to cause a small absolute increase in the risk for gastrointestinal hemorrhage that is consistent across all studies, with a number needed to harm of approximately 100 over 2.5 years. Therefore, the possible small absolute benefit that was seen in ASA trials with regards to coronary artery disease in the era before statin therapy must be weighed against the known risk of bleeding. Because of this, the CDA and European Society of Cardiology have recommendations against the routine use of ASA for primary prevention in patients with diabetes [12,20].
A summary of pharmacotherapy for cardiovascular risk reduction is shown in the Figure.
Case Continued
The patient is started on a statin because of his elevated LDL-C level in the context of being over the age of 40 years with T2DM. He is also started on an ACE inhibitor to address the hypertension. In addition, a dipeptidyl peptidase-4 inhibitor is added to his metformin to address the elevated A1c. He continues to follow up every 3 to 6 months.
Six years later, he experiences an episode of retrosternal chest discomfort while exercising. He is brought to hospital and is found to have a non-ST elevation myocardial infarction. He is admitted to hospital, undergoes percutaneous revascularization of a single lesion, and is discharged to a rehabilitation center. He is discharged on aspirin, clopidogrel, an ACE inhibitor, a beta blocker, and a high-intensity statin. His blood pressure is well managed, and he has lost further weight since he was last seen. When he returns to clinic, he wonders if there is anything more he can do to prevent further events.
What secondary prevention of cardiovascular disease is recommended for patients with T2DM?
Optimal secondary prevention following a major vascular event includes a combination of pharmacologic and nonpharmacologic interventions. In the population without diabetes, evidence supports smoking cessation, exercise, cardiac-specific rehabilitation, antiplatelets, RAAS antag-onists, beta-blockade, and statins. Most of the trials that led to this standard suite of interventions had large diabetes subgroups. Therefore, there is no difference in the secondary prevention of cardiovascular disease in the population with diabetes with regard to these interventions.
Have any antihyperglycemic agents been shown to reduce cardiovascular events?
Metformin
A summary of the vascular effects observed during trials of antihyperglycemic agents is shown in Table 3.
Empagliflozin
Many large randomized, controlled cardiovascular outcome trials have been completed or are ongoing looking at the cardiovascular safety of newer antihyperglycemic agents. The majority of the completed trials have shown a neutral effect, suggesting that the agents are safe. However, in September 2015, the first cardiovascular outcome trial of an antihyperglycemic agent with a positive result was published. The Empagliflozin Cardiovascular Outcome Event Trial (EMPA-REG OUTCOME) randomized 7020 patients with T2DM and cardiovascular disease (defined as nonacute myocardial infarction, multivessel coronary artery disease, unstable angina, nonacute stroke, or occlusive peripheral arterial disease) to placebo or 1 of 2 doses of empagliflozin. The primary outcome of cardiovascular mortality, nonfatal myocardial infarction, or stroke was reduced by 14% in the empagliflozin-treated group. Key secondary outcomes of all-cause mortality (HR 0.68) and heart failure hospitalization (HR 0.65) were also statistically different in favor of the empagliflozin arm [63].
On the basis of this trial’s results, empagliflozin should be considered for treatment of all patients with type 2 diabetes and known cardiovascular disease. It is as yet unknown whether this effect will translate to the other members of the sodium-glucose co-transporter 2 (SGLT-2) inhibitor class, although results of studies involving other SGLT-2 inhibitors are expected in the next 2 to 3 years.
Liraglutide
In 2016, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial reported results of its cardiovascular safety trial. In this trial, 9340 patients with either established vascular disease or risk factors for vascular disease were randomized to daily liraglutide or placebo injections. The primary composite outcome of cardiovascular death, nonfatal myocardial infarction, or stroke was reduced by 13%. A key secondary outcome of all-cause mortality also showed a significant reduction (HR 0.85). There was no reduction in hospitalization for heart failure [64].
Semaglutide
Most recently, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) was completed, assessing cardiovascular safety of a once-weekly injectable glucagon-like peptide-1 (GLP-1) analogue. This noninferiority trial studied 3297 patients with type 2 diabetes over the age of 50 years with established macrovascular disease, chronic heart failure, or chronic kidney disease (stage III or higher), or over the age of 60 years with at least 1 other cardiovascular risk factor. The patients were randomized to 1 of 2 doses of once-weekly semaglutide or placebo injection. A composite cardiovascular outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was decreased by 26% in the pooled semaglutide group. This was driven primarily by a reduction in nonfatal stroke, with no statistically significant reduction in nonfatal myocardial infarction or cardiovascular mortality. Significant secondary outcomes showed a reduction in new or worsening nephropathy (HR 0.64), and an unexpected increase in retinopathy (HR 1.76) [65].
All of these trials utilized their respective agents as add-on to existing antihyperglycemic therapy. Therefore, first-line antihyperglycemic therapy in a patient with T2DM remains metformin. For the patient with established vascular disease or who is at high risk for developing vascular disease, add-on therapy using an antihyperglycemic agent with proven cardiovascular benefits, such as empagliflozin or liraglutide, is suggested [9,11]. Semaglutide is not yet available for clinical use. The choice between these agents should be based on patient preference, cost, side effect profile, and absence of contraindications.
Currently, there are more studies underway with similar designs with different agents. As these studies are reported in the upcoming years, it is hoped that the options for reduction of cardiovascular risk will increase, and that we will have multiple antihyperglycemic agents that will provide not only glycemic benefit but also cardiovascular risk reduction.
Case Conclusion
The patient continues to abstain from smoking. He follows up with a dietitian and is enrolled in an exercise program. He remains on his cardiac medications. For glycemic control, he continues on his previous antihyperglycemic therapy and an antihyperglycemic agent with proven cardiovascular benefit is added. With these interventions, he understands that his risk is mitigated, but given his history and previous event, he remains at high risk for future vascular disease.
Conclusion
The care of a patient with diabetes requires a multifactorial approach. All patients are at risk for developing the vascular complications of diabetes, and it is these complications that ultimately result in the nearly doubled risk of mortality in patients with diabetes. Various trials have shown that targeted interventions can and do reduce the risk for cardiovascular disease in a measurable way. Above and beyond targeted interventions, we now know that strict multifactorial interventions can result in a clinically significant reduction in both mortality and cardiovascular disease. This multifactorial approach is supported by guidelines around the world [12,44,45]. A standardized approach to the assessment of risk and the application of interventions is critical. More recent data show that specific antihyperglycemic therapies can also reduce cardiovascular events above and beyond their glycemic effects. The rates of cardiovascular events in patients with diabetes have declined over time, and hopefully this trend will continue as further research supports additional interventions.
Corresponding author: Bikrampal S. Sidhu, MD, Toronto General Hospital, 200 Elizabeth St., 12 EN 242, Toronto, ON, M5G 2C4, [email protected].
Financial disclosures: Dr. Cheng has received fees for speaking and/or consulting from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck, Novo Nordisk, Sanofi, Servier, and Takeda.
From the Division of Endocrinology, Department of Medicine, University of Toronto, Ontario, Canada.
Abstract
- Objective: To review the assessment of cardiovascular risk and prevention of vascular disease in patients with type 2 diabetes mellitus (T2DM).
- Methods: Literature and guidelines were reviewed and the evidence is presented around a clinical case.
- Results: T2DM has a high prevalence and confers significant lifetime risk for macrovascular disease, including stroke, heart disease, and peripheral arterial disease. There is strong evidence to support nonpharmacologic interventions, such as smoking cessation and weight loss, and pharmacologic interventions, such as statin therapy, in order to decrease lifetime risk. The effectiveness of an intervention as well as the strength of the evidence supporting an intervention differs depending on the stage of the disease.
- Conclusion: Once a patient is diagnosed with T2DM, it is important to recognize that their lifetime risk for vascular disease is high. Starting at this stage and continuing throughout the disease course, cardiovascular risk should be assessed in an ongoing manner and evidence-based interventions should be implemented whenever they are indicated. Using major guidelines as a framework, we provide an evidence-based approach to the reduction of vascular risk in these patients.
Key words: cardiovascular disease, diabetes, prevention, risk assessment, risk factors.
Type 2 diabetes (T2DM) is considered epidemic in the developed world, and is rapidly increasing in the developing world. Since 1980, there has been a near quadrupling of the number of adults with diabetes worldwide to an estimated 422 million in 2014 [1]. Because diabetes affects the whole body vascular system, there is a significant burden of vascular complications directly attributable to diabetes. Although the rates of diabetes-related complications are declining, the burden of disease remains high due to the increasing prevalence of diabetes [2]. The tremendous burden of diabetes and its complications on the population make it imperative that all health care practitioners understand the vascular effects of diabetes as well as evidence-based interventions that can mitigate them. In this review, we present an approach to the assessment, prevention, and treatment of cardiovascular disease in patients with T2DM.
Case Study
A 38-year-old male presents to his family physician’s office for a routine check-up. He is obese and a smoker, has no other health issues, and is taking no medications. He is sent for routine bloodwork and his A1c and fasting glucose are elevated and are diagnostic for diabetes. He returns to the clinic to discuss his results.
How are cardiovascular risk and risk factors assessed in a patient with diabetes?
There are many risk scores and risk calculators available for assessing cardiovascular risk. The Framingham Risk Score is the most commonly employed and takes into account the most common risk factors for cardiovascular risk, including cholesterol level, age, gender, and smoking status. Unfortunately, because a patient with diabetes may have a high lifetime risk but low or moderate short-term risk, these risk scores tend to underestimate overall risk in the population with diabetes [3,4]. Furthermore, since early intervention can decrease lifetime risk, it is important to recognize the limitations of these risk scores.
What interventions should be used for primary prevention at this stage?
A number of interventions can decrease lifetime risk for cardiovascular disease in persons with diabetes. First, smoking increases risk for all forms of vascular disease, including progression to end-stage renal disease, and is an independent predictor of mortality. Smoking cessation is one of the most effective interventions at decreasing these risks [6]. Second, lifestyle interventions such as diet and exercise are often recommended. The Look AHEAD trial studied the benefits of weight loss and exercise in the treatment of T2DM through a randomized control trial involving more than 5000 overweight patients with T2DM. Patients were randomly assigned to intensive lifestyle interventions targeting weight loss or a support and education group. Although the Action for Health in Diabetes (Look AHEAD) trial did not demonstrate clinical outcome benefit with this intensive intervention, there was improvement in weight, cholesterol level, blood pressure, and glycemic control, and clinical differences may have been related to study power or differences in cardioprotective medication use [7]. Furthermore, at least 1 large randomized trial of dietary intervention in high-risk cardiovascular patients, half of whom had diabetes (Prevención con Dieta Mediterránea [PREDIMED]), showed significant benefits in cardiovascular disease, reducing the incidence of major cardiovascular events [8]. According to most diabetes guidelines, diet and exercise continue to be stressed as initial management for all patients with diabetes [9–12].
In addition, although intensive glucose control decreases microvascular complication rates, it has been more difficult to demonstrate a reduction in cardiovascular disease with more intense glycemic control. However, long-term follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) cohort, a population that was earlier in their diabetes course, clearly demonstrated a reduction in cardiovascular events and mortality with better glycemic control over the long term [13,14]. For those who are later in their diabetes course, meta-analyses of glycemic control trials, along with follow-up studies, have also shown that better glycemic control can reduce cardiovascular events, but not mortality [15–17]. Therefore, glucose lowering should be pursued for cardiovascular risk reduction, in addition to its effects on microvascular complications.
It is well established that a multifactorial approach to cardiovascular risk reduction in patients with type 2 diabetes is effective. In the Steno-2 study, 160 patients with type 2 diabetes were randomly assigned to receive multidisciplinary, multifactorial intensive target-based lifestyle and pharmacologic intervention or standard of care. The intensive therapy group all received smoking cessation counseling, exercise and dietary advice, vitamin supplementation, and an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). Acetylsalicylic acid (ASA) was added for all patients with clinical macrovascular disease. Dyslipidemia, hypertension, and hyperglycemia were all treated in a protocolized way if lifestyle interventions did not achieve strict targets. During the mean 7.8 years of follow-up, the adjusted hazard ratio for a composite of cardiovascular death and macrovascular disease was 0.47 (95% confidence interval [CI] 0.22 to 0.74; P = 0.01) [18]. These patients were followed for an additional 5.5 years in an observational study with no further active intervention in both groups. Over the entire period, there was an absolute risk reduction of 20% for death from any cause, resulting in a number needed to treat of 5 for 13 years [19]. As a result of these compelling data, guidelines from around the world support a multifactorial approach, with the Canadian Diabetes Association (CDA) guidelines [20] promoting the use of the “ABCDES” of vascular protection:
A – A1C target
B – Blood pressure target
C – Cholesterol target
D – Drugs for vascular protection
E – Exercise/Eating
S – Smoking cessation
Is any particular dietary pattern recommended?
There is a large and ever-growing number of dietary patterns that are marketed to improve weight and cardiovascular health. Unfortunately, however, few of these interventions have been studied rigorously, and most dietary interventions are found to be unsustainable in the long term. In the case of motivated patients, there are some specific dietary patterns with high-quality evidence to recommend them. The simplest intervention is the implementation of a vegetarian or vegan diet. Over 18 months, this has been shown to improve fasting glucose and cholesterol profile, and promote weight loss [21]. In another study, a calorie-restricted vegetarian diet led to a reduction in diabetes medication in 46% of participants (versus 5% with conventional diet) [22].
A Mediterranean diet is comprised of large amounts of fruits, vegetables, legumes, nuts, and whole grains. In addition, it includes moderate consumption of olive oil, dairy, fish and poultry, with low consumption of red meat. This dietary pattern has been extensively studied, and in a meta-analysis has been shown to improve glycemic control, blood pressure, and lipid profile [23]. The PREDIMED study evaluated the efficacy of 2 versions of the Mediterranean diet, one supplemented with olive oil or mixed nuts, for reducing cardiovascular events. This multicenter randomized control trial of 7447 participants at high cardiovascular risk (48.5% of whom had diabetes) was stopped early due to benefit. Both versions of the diet reduced cardiovascular events by 30% over 5 years of follow-up [8].
The Dietary Approaches to Stop Hypertension (DASH) diet is similar to the Mediterranean diet in focusing on fruits, vegetables, low-fat dairy, whole grains, nuts, fish, and poultry, while avoiding red meat. In addition, it explicitly recommends avoiding sweets and sweetened beverages, as well as dietary fat. In a trial of patients with diabetes matched for moderate sodium intake, the DASH diet has been shown to decrease A1c, blood pressure, and weight and improve lipid profile within 8 weeks [24,25].
In addition to these specific dietary patterns, specific foods have been shown to improve glycemic control and cardiovascular risk profile, including mixed unsalted nuts, almonds, dietary pulses, and low-glycemic versus high-glycemic index carbohydrates [26–31].
In accordance with CDA, American Diabetes Association (ADA), and European Association for the Study of Diabetes (EASD) guidelines, we recognize that a variety of diets can improve the cardiovascular risk profile of a patient [12,32,33]. Therefore, we suggest a tailored approach to dietary changes for each individual patient. This should, whenever possible, be undertaken with a registered dietitian, with emphasis placed on the evidence for vascular protection, improved risk profile, patient preference, and likelihood of long-term sustainability.
Should therapy for weight loss be recommended for this patient?
There are currently a number of effective strategies for achieving weight loss, including lifestyle interventions, pharmacotherapy, and surgery. The evidence base for dietary interventions for diabetes is reviewed above. The Look AHEAD study randomized 5145 overweight or obese patients with T2DM to intensive lifestyle intervention for weight loss through promotion of decreased caloric intake and increased physical activity, or usual diabetes support and education. After a median follow-up of 9.6 years, the study was stopped early on the basis of a futility analysis despite greater weight loss in the intervention group throughout the study. However, other benefits were derived including reduced need for medications, reduced sleep apnea, and improved well-being [7].
Pharmacotherapy agents for weight loss have been approved by various regulatory agencies. None has as yet shown a reduction in cardiovascular events. Therefore, these cannot be recommended as therapies for vascular protection at this time.
Bariatric surgery is an effective option for weight loss in patients with diabetes, with marked and sustained improvements in clinically meaningful outcomes when compared with medical management. The longest study of bariatric surgery is the Swedish Obesity Study, a prospective case-control study of 2010 obese patients who underwent bariatric surgery and 2037 matched controls. After a median of 14.8 years of follow-up, there was a reduction in overall mortality (hazard ratio [HR] 0.71) and decreased incidence of diabetes (HR 0.17), myocardial infarction (HR 0.71), and stroke (HR 0.66). Diabetes remission, defined as normal A1c off of anti-hyperglycemic therapy, was increased at 2 years (odds ratio [OR] 13.3) and sustained at 15 years (OR 6.3) [34–36]. Randomized controlled trials of bariatric surgery have thus far been small and do show some decreases in cardiovascular risk factors [37–40]. However, these have not yet been of sufficient duration or size to demonstrate a decrease in cardiovascular event rate. Although local policies may vary in referral recommendation, the Obesity Society, ADA, and CDA recommend that patients with a body mass index greater than 40 kg/m2, or greater than 35 kg/m2 with an obesity-related comorbidity such as diabetes, should be referred to a center that specializes in bariatric surgery for evaluation [41–43].
Case Continued
After the initial diagnosis, the patient was seen by a registered dietitian and followed a Mediterranean diet for some time but has since stopped. He is seen regularly for follow-up of his diabetes at 3- to 6-month intervals. He initially lost some weight but has unfortunately regained the weight. He tells you proudly that he finally quit smoking. He was started on metformin about 6 months after diagnosis to address his glycemic control. He continues on the metformin now as his only medication.
The patient returns to clinic for his usual follow-up visit approximately 5 years after initial diagnosis. He is feeling well with no new medical issues. He has no clinically apparent retinopathy or macrovascular complications. On examination, his blood pressure is 140/90 mm Hg and the remainder of the exam is unremarkable. His bloodwork shows an A1c of 8% and a low-density lipoprotein cholesterol (LDL-C) level of 124 mg/dL. His albumin-to-creatinine ratio is normal.
How often should cardiovascular risk be reassessed?
When should initiating pharmacotherapy to reduce risk in primary prevention be considered?
In the population with diabetes, statins and renin-angiotensin-aldosterone inhibition are the mainstays of pharmacotherapy for cardiovascular risk reduction. In the presence of clinical macrovascular disease, the standard of care includes both of these therapies. However, there is also a great deal of data that supports the use of these therapies for primary prevention.
Statins
Major studies on the benefits of statin therapy in people with diabetes have consistently shown decreased cardiovascular disease and mortality. The Heart Protection Study included a subgroup of patients with diabetes in which patients over the age of 40 were randomly assigned to simvastatin or placebo. Consistently across all subgroups, there was a relative risk reduction of 22% to 33% for the primary outcome of first cardiovascular event over 5 years. This effect was maintained even in those who did not have elevated LDL-C at randomization [46]. Similarly, the Collaborative Atorvastatin Diabetes Study (CARDS) randomized patients with T2DM, over age 40, with at least 1 other vascular risk factor to atorvastatin 10 mg or placebo. They found a 37% risk reduction in time to first event over 4 years with atorvastatin, with consistent results across all subgroups [47].
Based on these studies, it is recommended that all patients with diabetes be placed on statin therapy to reduce vascular risk at age 40 years (CDA, ADA, American College of Cardiology/American Heart Association [ACC/AHA]) [20,45,48]. If under age 40 years, statin therapy should be considered in the presence of other risk factors (ADA, ACC/AHA) [45,48], or if diabetes duration is more than 15 years and age is greater than 30 years, or there are micro- or macrovascular complications (CDA) [20].
Renin-Angiotensin-Aldosterone Inhibition
Similar to research into statin therapy, a considerable amount of research has been dedicated to renin-angiotensin-aldosterone system (RAAS) blockade for the primary purpose of vascular risk reduction, even in the absence of hypertension, in those with diabetes. In a prespecified substudy of the Heart Outcomes Prevention Evaluation (HOPE) trial, known as MICRO HOPE, patients with diabetes who were older than 55 years of age, with at least 1 other cardiovascular risk factor, were randomized to receive ramipril 10 mg daily or placebo. In this study, ramipril reduced the risk for myocardial infarction (22%), stroke (33%), cardiovascular death (37%), and all-cause mortality (24%) over 4.5 years [49]. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), patients at high risk for cardiovascular disease were randomized to telmisartan 80 mg or ramipril 10 mg. In the diabetes subgroup, there were similar risk reductions and no statistical difference between the groups [50]. A 2012 meta-analysis assessed the benefits of RAAS blockade compared with placebo for primary prevention in high-risk individuals, or secondary prevention in those with established vascular disease. A reduction in cardiovascular death, all-cause mortality, fatal or nonfatal myocardial infarction, and stroke was seen across all subgroups, including those with and without diabetes or hypertension [51].
The CDA currently recommends that an ACE inhibitor or ARB be given to all patients with diabetes who are 55 years of age or older, or have macro- or microvascular disease, for the primary purpose of decreasing risk for vascular disease, even in the absence of hypertension. An agent and dose with proven vascular protective benefit should be chosen when selecting an ACE inhibitor or ARB [20].
Should this patient start ASA therapy?
Whether to start daily low-dose ASA for primary prevention of coronary artery disease has been a long-standing question in patients with diabetes. The benefits of ASA therapy with regards to coronary artery disease have long been known from a secondary prevention standpoint, and given the low risk and long experience, primary prevention seemed reasonable. However, no high-quality randomized controlled trials enrolling large numbers of patients with diabetes have been performed in the current era of medical therapy, specifically in the era of widespread statin use. The initial studies examining ASA use in primary prevention were analyzed in a meta-analysis in 1994 and showed a trend towards benefit for ASA in patients with diabetes [52]. Further trials increased the number of diabetes patient-years studied but did not change the initial result. Five meta-analyses have been conducted on the currently available trials, and all but one do not show a significant reduction in coronary artery disease or stroke in patients with diabetes [53–57]. In addition, ASA is known to cause a small absolute increase in the risk for gastrointestinal hemorrhage that is consistent across all studies, with a number needed to harm of approximately 100 over 2.5 years. Therefore, the possible small absolute benefit that was seen in ASA trials with regards to coronary artery disease in the era before statin therapy must be weighed against the known risk of bleeding. Because of this, the CDA and European Society of Cardiology have recommendations against the routine use of ASA for primary prevention in patients with diabetes [12,20].
A summary of pharmacotherapy for cardiovascular risk reduction is shown in the Figure.
Case Continued
The patient is started on a statin because of his elevated LDL-C level in the context of being over the age of 40 years with T2DM. He is also started on an ACE inhibitor to address the hypertension. In addition, a dipeptidyl peptidase-4 inhibitor is added to his metformin to address the elevated A1c. He continues to follow up every 3 to 6 months.
Six years later, he experiences an episode of retrosternal chest discomfort while exercising. He is brought to hospital and is found to have a non-ST elevation myocardial infarction. He is admitted to hospital, undergoes percutaneous revascularization of a single lesion, and is discharged to a rehabilitation center. He is discharged on aspirin, clopidogrel, an ACE inhibitor, a beta blocker, and a high-intensity statin. His blood pressure is well managed, and he has lost further weight since he was last seen. When he returns to clinic, he wonders if there is anything more he can do to prevent further events.
What secondary prevention of cardiovascular disease is recommended for patients with T2DM?
Optimal secondary prevention following a major vascular event includes a combination of pharmacologic and nonpharmacologic interventions. In the population without diabetes, evidence supports smoking cessation, exercise, cardiac-specific rehabilitation, antiplatelets, RAAS antag-onists, beta-blockade, and statins. Most of the trials that led to this standard suite of interventions had large diabetes subgroups. Therefore, there is no difference in the secondary prevention of cardiovascular disease in the population with diabetes with regard to these interventions.
Have any antihyperglycemic agents been shown to reduce cardiovascular events?
Metformin
A summary of the vascular effects observed during trials of antihyperglycemic agents is shown in Table 3.
Empagliflozin
Many large randomized, controlled cardiovascular outcome trials have been completed or are ongoing looking at the cardiovascular safety of newer antihyperglycemic agents. The majority of the completed trials have shown a neutral effect, suggesting that the agents are safe. However, in September 2015, the first cardiovascular outcome trial of an antihyperglycemic agent with a positive result was published. The Empagliflozin Cardiovascular Outcome Event Trial (EMPA-REG OUTCOME) randomized 7020 patients with T2DM and cardiovascular disease (defined as nonacute myocardial infarction, multivessel coronary artery disease, unstable angina, nonacute stroke, or occlusive peripheral arterial disease) to placebo or 1 of 2 doses of empagliflozin. The primary outcome of cardiovascular mortality, nonfatal myocardial infarction, or stroke was reduced by 14% in the empagliflozin-treated group. Key secondary outcomes of all-cause mortality (HR 0.68) and heart failure hospitalization (HR 0.65) were also statistically different in favor of the empagliflozin arm [63].
On the basis of this trial’s results, empagliflozin should be considered for treatment of all patients with type 2 diabetes and known cardiovascular disease. It is as yet unknown whether this effect will translate to the other members of the sodium-glucose co-transporter 2 (SGLT-2) inhibitor class, although results of studies involving other SGLT-2 inhibitors are expected in the next 2 to 3 years.
Liraglutide
In 2016, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial reported results of its cardiovascular safety trial. In this trial, 9340 patients with either established vascular disease or risk factors for vascular disease were randomized to daily liraglutide or placebo injections. The primary composite outcome of cardiovascular death, nonfatal myocardial infarction, or stroke was reduced by 13%. A key secondary outcome of all-cause mortality also showed a significant reduction (HR 0.85). There was no reduction in hospitalization for heart failure [64].
Semaglutide
Most recently, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) was completed, assessing cardiovascular safety of a once-weekly injectable glucagon-like peptide-1 (GLP-1) analogue. This noninferiority trial studied 3297 patients with type 2 diabetes over the age of 50 years with established macrovascular disease, chronic heart failure, or chronic kidney disease (stage III or higher), or over the age of 60 years with at least 1 other cardiovascular risk factor. The patients were randomized to 1 of 2 doses of once-weekly semaglutide or placebo injection. A composite cardiovascular outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was decreased by 26% in the pooled semaglutide group. This was driven primarily by a reduction in nonfatal stroke, with no statistically significant reduction in nonfatal myocardial infarction or cardiovascular mortality. Significant secondary outcomes showed a reduction in new or worsening nephropathy (HR 0.64), and an unexpected increase in retinopathy (HR 1.76) [65].
All of these trials utilized their respective agents as add-on to existing antihyperglycemic therapy. Therefore, first-line antihyperglycemic therapy in a patient with T2DM remains metformin. For the patient with established vascular disease or who is at high risk for developing vascular disease, add-on therapy using an antihyperglycemic agent with proven cardiovascular benefits, such as empagliflozin or liraglutide, is suggested [9,11]. Semaglutide is not yet available for clinical use. The choice between these agents should be based on patient preference, cost, side effect profile, and absence of contraindications.
Currently, there are more studies underway with similar designs with different agents. As these studies are reported in the upcoming years, it is hoped that the options for reduction of cardiovascular risk will increase, and that we will have multiple antihyperglycemic agents that will provide not only glycemic benefit but also cardiovascular risk reduction.
Case Conclusion
The patient continues to abstain from smoking. He follows up with a dietitian and is enrolled in an exercise program. He remains on his cardiac medications. For glycemic control, he continues on his previous antihyperglycemic therapy and an antihyperglycemic agent with proven cardiovascular benefit is added. With these interventions, he understands that his risk is mitigated, but given his history and previous event, he remains at high risk for future vascular disease.
Conclusion
The care of a patient with diabetes requires a multifactorial approach. All patients are at risk for developing the vascular complications of diabetes, and it is these complications that ultimately result in the nearly doubled risk of mortality in patients with diabetes. Various trials have shown that targeted interventions can and do reduce the risk for cardiovascular disease in a measurable way. Above and beyond targeted interventions, we now know that strict multifactorial interventions can result in a clinically significant reduction in both mortality and cardiovascular disease. This multifactorial approach is supported by guidelines around the world [12,44,45]. A standardized approach to the assessment of risk and the application of interventions is critical. More recent data show that specific antihyperglycemic therapies can also reduce cardiovascular events above and beyond their glycemic effects. The rates of cardiovascular events in patients with diabetes have declined over time, and hopefully this trend will continue as further research supports additional interventions.
Corresponding author: Bikrampal S. Sidhu, MD, Toronto General Hospital, 200 Elizabeth St., 12 EN 242, Toronto, ON, M5G 2C4, [email protected].
Financial disclosures: Dr. Cheng has received fees for speaking and/or consulting from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck, Novo Nordisk, Sanofi, Servier, and Takeda.
1. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30.
2. Gregg EW, Li Y, Wang J, et al. Changes in Diabetes-Related Complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–23.
3. Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29–36.
4. Stevens RJ, Kothari V, Alder A, et al. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–9.
5. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
6. Solberg L, Desai JR, O’Connor PJ, et al. Diabetic patients who smoke: are they different? Diabetes Care 1999;22:1887–98.
7. Look Ahead Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54.
8. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.
9. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Pharmacologic management of type 2 diabetes: November 2016 Interim Update. Can J Diabetes 2016;40:484–6.
10. Harper W, Clement M, Goldenberg R, et al. Pharmacologic management of type 2 diabetes. Can J Diabetes 2013; 37:S61–S68.
11. American Diabetes Association. Pharmacologic approaches to glycemic management. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S64–74.
12. The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013;34:3035–87.
13. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65.
14. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
15. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–98.
16. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
17. ACCORD Study Group. Nine-year rffects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 2016;39:701–8.
18. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93.
19. Gaede P, Lund-Anderson H, Parving HH, Pederson O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91.
20. Stone JA, Fitchett D, Grover S, et al. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Vascular protection in people with diabetes. Can J Diabetes 2013;37 (suppl 1):S100–S104.
21. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588–96.
22. Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–59.
23. Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 2010;89:97–102.
24. Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–8.
25. Azadbakht L, Fard NR, Karimi M, et al. The Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care 2011;34:55–7.
26. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7.
27. Opperman AM, Venter CS, Oosthuizen W, et al. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004;92:367–81.
28. Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802.
29. Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes. Diabetologia 2009;52:1479–95.
30. Jenkins DJ, Kendall CW, Banach MS, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011;34:1706–11.
31. Li SC, Liu YH, Liu JF, et al. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9.
32. Dworatzek PD, Arcudi K, Gougeon R, et al. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: nutrition therapy. Can J Diabetes 2013;37(suppl 1):S45–55.
33. American Diabetes Association. Lifestyle Management. Sec. 4. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S33–43.
34. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med 2007;357:741–52.
35. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
36. Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–7.
37. Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–23.
38. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
39. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
40. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
41. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines and
The Obesity Society. Obesity (Silver Spring) 2014;22:S1–S410.
42. Wharton S, Sharma AM, Lau DCW. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Weight management in diabetes. Can J Diabetes 2013;37(suppl 1):S82–6.
43. American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes. Sec. 7. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S57–63.
44. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013;37(suppl 1):S1–S212.
45. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S1–S135.
46. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomized placebo controlled trial. Lancet 2003;361:2005–16.
47. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
48. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934.
49. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–9.
50. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59.
51. McAlister FA, Renin Angiotensin System Modulator Meta-Analysis Investigators. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are beneficial in normotensive atherosclerotic patients: a collaborative meta-analysis of randomized trials. Eur Heart J 2012;33:505–14.
52. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106.
53. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60.
54. Calvin AD, Aggarwal NR, Murad MH, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care 2009;32:2300–6.
55. De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009;339:b4531.
56. Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010;87:211–8.
57. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010;33:1395–402.
58. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomised clinical trial. JAMA 2014;312:2510–20.
59. ASCEND Trial Web site. Clinical Trials Service Unit, University of Oxford, and British Heart Foundation. https://ascend.medsci.ox.ac.uk/. Accessed September 20, 2016.
60. De Berardis G, Sacco M, Evangelista V, et al. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007;8:21.
61. Maruther NM, Tseng E, Hutfless SS, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
62. Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS Med 2012;9:e1001204.
63. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
64. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
65. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44
66. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
67. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
68. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
69. Pfeffer MA, Claggett B, Diaz R, Dickstein K, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57.
1. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30.
2. Gregg EW, Li Y, Wang J, et al. Changes in Diabetes-Related Complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–23.
3. Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29–36.
4. Stevens RJ, Kothari V, Alder A, et al. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–9.
5. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
6. Solberg L, Desai JR, O’Connor PJ, et al. Diabetic patients who smoke: are they different? Diabetes Care 1999;22:1887–98.
7. Look Ahead Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54.
8. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.
9. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Pharmacologic management of type 2 diabetes: November 2016 Interim Update. Can J Diabetes 2016;40:484–6.
10. Harper W, Clement M, Goldenberg R, et al. Pharmacologic management of type 2 diabetes. Can J Diabetes 2013; 37:S61–S68.
11. American Diabetes Association. Pharmacologic approaches to glycemic management. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S64–74.
12. The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013;34:3035–87.
13. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65.
14. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
15. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–98.
16. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
17. ACCORD Study Group. Nine-year rffects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 2016;39:701–8.
18. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93.
19. Gaede P, Lund-Anderson H, Parving HH, Pederson O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91.
20. Stone JA, Fitchett D, Grover S, et al. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Vascular protection in people with diabetes. Can J Diabetes 2013;37 (suppl 1):S100–S104.
21. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588–96.
22. Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–59.
23. Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 2010;89:97–102.
24. Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–8.
25. Azadbakht L, Fard NR, Karimi M, et al. The Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care 2011;34:55–7.
26. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7.
27. Opperman AM, Venter CS, Oosthuizen W, et al. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004;92:367–81.
28. Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802.
29. Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes. Diabetologia 2009;52:1479–95.
30. Jenkins DJ, Kendall CW, Banach MS, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011;34:1706–11.
31. Li SC, Liu YH, Liu JF, et al. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9.
32. Dworatzek PD, Arcudi K, Gougeon R, et al. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: nutrition therapy. Can J Diabetes 2013;37(suppl 1):S45–55.
33. American Diabetes Association. Lifestyle Management. Sec. 4. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S33–43.
34. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med 2007;357:741–52.
35. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
36. Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–7.
37. Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–23.
38. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
39. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
40. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
41. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines and
The Obesity Society. Obesity (Silver Spring) 2014;22:S1–S410.
42. Wharton S, Sharma AM, Lau DCW. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Weight management in diabetes. Can J Diabetes 2013;37(suppl 1):S82–6.
43. American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes. Sec. 7. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S57–63.
44. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013;37(suppl 1):S1–S212.
45. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S1–S135.
46. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomized placebo controlled trial. Lancet 2003;361:2005–16.
47. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
48. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934.
49. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–9.
50. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59.
51. McAlister FA, Renin Angiotensin System Modulator Meta-Analysis Investigators. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are beneficial in normotensive atherosclerotic patients: a collaborative meta-analysis of randomized trials. Eur Heart J 2012;33:505–14.
52. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106.
53. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60.
54. Calvin AD, Aggarwal NR, Murad MH, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care 2009;32:2300–6.
55. De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009;339:b4531.
56. Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010;87:211–8.
57. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010;33:1395–402.
58. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomised clinical trial. JAMA 2014;312:2510–20.
59. ASCEND Trial Web site. Clinical Trials Service Unit, University of Oxford, and British Heart Foundation. https://ascend.medsci.ox.ac.uk/. Accessed September 20, 2016.
60. De Berardis G, Sacco M, Evangelista V, et al. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007;8:21.
61. Maruther NM, Tseng E, Hutfless SS, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
62. Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS Med 2012;9:e1001204.
63. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
64. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
65. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44
66. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
67. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
68. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
69. Pfeffer MA, Claggett B, Diaz R, Dickstein K, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57.
Effect of PCSK9 Inhibitors on Coronary Artery Disease Progression
Nicolls SJ, Puri S, Anderson, T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients. The GLAGOV randomized clinical trial. JAMA 2016;316:2372–84.
To Download a PDF of the Full Article:
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
References
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.
Nicolls SJ, Puri S, Anderson, T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients. The GLAGOV randomized clinical trial. JAMA 2016;316:2372–84.
To Download a PDF of the Full Article:
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
References
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.
Nicolls SJ, Puri S, Anderson, T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients. The GLAGOV randomized clinical trial. JAMA 2016;316:2372–84.
To Download a PDF of the Full Article:
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
References
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.
Increase in U.S. drug spending slowed in 2016
Prescription drug spending for those with employer-sponsored insurance increased by 3.8% in 2016, compared with a rise of 5.2% in 2015, according to pharmacy benefits manager Express Scripts.
Commercial plans managed by Express Scripts saw the cost of prescription drugs rise by 2.5% per person, while utilization was up by 1.3%, which adds up to the 3.8% overall increase. That represents a 27% drop from 2015, when drug use rose 2.0% and costs went up by 3.2%, Express Scripts said in its “2016 Drug Trend Report.”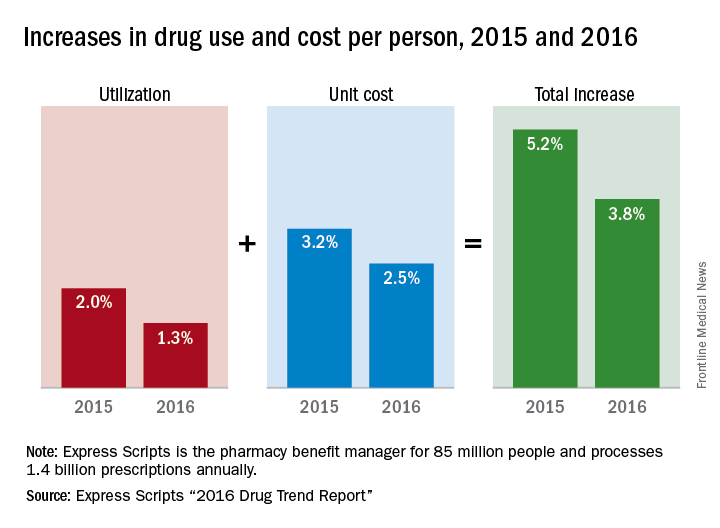
For a second consecutive year, patients of pharmacy plans managed by Express Scripts had a smaller out-of-pocket share of their total pharmacy costs, 14.6%, compared with 14.8% in 2015. That smaller share did represent an increase from $11.25 in 2015 to $11.34 in 2016, however, as the adjusted total cost per prescription rose from $75.85 to $77.84, Express Scripts said.
The company is the largest pharmacy benefits manager in the country, according to its corporate overview, processing 1.4 billion prescriptions annually for 85 million people.
Prescription drug spending for those with employer-sponsored insurance increased by 3.8% in 2016, compared with a rise of 5.2% in 2015, according to pharmacy benefits manager Express Scripts.
Commercial plans managed by Express Scripts saw the cost of prescription drugs rise by 2.5% per person, while utilization was up by 1.3%, which adds up to the 3.8% overall increase. That represents a 27% drop from 2015, when drug use rose 2.0% and costs went up by 3.2%, Express Scripts said in its “2016 Drug Trend Report.”
For a second consecutive year, patients of pharmacy plans managed by Express Scripts had a smaller out-of-pocket share of their total pharmacy costs, 14.6%, compared with 14.8% in 2015. That smaller share did represent an increase from $11.25 in 2015 to $11.34 in 2016, however, as the adjusted total cost per prescription rose from $75.85 to $77.84, Express Scripts said.
The company is the largest pharmacy benefits manager in the country, according to its corporate overview, processing 1.4 billion prescriptions annually for 85 million people.
Prescription drug spending for those with employer-sponsored insurance increased by 3.8% in 2016, compared with a rise of 5.2% in 2015, according to pharmacy benefits manager Express Scripts.
Commercial plans managed by Express Scripts saw the cost of prescription drugs rise by 2.5% per person, while utilization was up by 1.3%, which adds up to the 3.8% overall increase. That represents a 27% drop from 2015, when drug use rose 2.0% and costs went up by 3.2%, Express Scripts said in its “2016 Drug Trend Report.”
For a second consecutive year, patients of pharmacy plans managed by Express Scripts had a smaller out-of-pocket share of their total pharmacy costs, 14.6%, compared with 14.8% in 2015. That smaller share did represent an increase from $11.25 in 2015 to $11.34 in 2016, however, as the adjusted total cost per prescription rose from $75.85 to $77.84, Express Scripts said.
The company is the largest pharmacy benefits manager in the country, according to its corporate overview, processing 1.4 billion prescriptions annually for 85 million people.
Fractures in adult osteosarcoma patients presage worse survival
AMSTERDAM – Pathological fractures are prognostic of poor outcomes in adults with osteosarcoma, but not in children with osteosarcoma, investigators have found.
A retrospective review of data on consecutive patients treated over the course of 30 years showed that among patients of all ages, both 5-year and 10-year overall survival (OS) rates were worse among patients who had pathological fractures.
But in an analysis stratified by age, the survival difference attributable to fractures was limited entirely to patients who were 18 or older at the time of an osteosarcoma diagnosis, reported Lisa Kelley, a medical student at the Ludwig-Maximilians Universität in Munich.
“It’s important to understand what are the prognostic factors [in osteosarcoma], and while many of the factors have been thoroughly researched, on pathological fractures the data are still fairly inconclusive due to the results of studies contradicting each other, and also having a relatively small patient number due to the fact that osteosarcoma itself is rare, and the pathological fractures occur only in approximately 10% of cases,” she said at an annual congress sponsored by the European Cancer Organisation.
To get a better handle on possible correlations between pathological fractures and prognosis in patients with central high-grade osteosarcoma, Ms. Kelley and her coauthors collected data on consecutive patients treated for localized or metastatic osteosarcoma of the extremities from 1980 through 2010 at one of the member institutions of the Cooperative Osteosarcoma Study Group (COSS).
They identified 2,847 patients, of whom 2,193 (77%) were 18 or younger at the time of diagnosis. Of the entire cohort, 321 patients (11.3%) had a pathological fracture either at presentation or soon after diagnosis.
Comparing patients with and without pathological fractures, the investigators found that factors significantly associated with fracture risk included tumor location, especially the humerus (P less than .001), tumors occurring proximally and in a diaphysis (P less than .001), telangiectatic subtype (P less than .001), the presence of primary metastases (P = .025). and tumors comprising more than one-third of the affected bone (P less than .001).
There were no significant differences in the cohort as a whole between patients with or without fractures in either age in years, sex, body-mass index, history of pain or swelling symptoms, local surgical remission at the main tumor site, total surgical remission including metastases, response to chemotherapy, use of adjuvant chemotherapy rather than neoadjuvant, or type of surgery.
Among adult patients only, however, factors associated with pathological fractures included age (P less than .001). BMI (P = .021), tumor site (P less than .001), histologic subtype (P less than .001), primary metastases (P = .011), relative tumor size (P = .047), and total surgical remission (P = .015).
Among pediatric patients, factors associated with fracture risk were (P less than .001 for all unless otherwise specified) age, BMI (P = .018), history or symptoms (P = .001). tumor site, localization within bone, histologic subtype, and relative tumor size.
In univariate analysis, 5-year OS rates were 70.6% for patients without fractures compared with 63.0% for those with fractures, and respective 10-year OS rates were 64.9% vs. 58.1% (P = .007 for both comparisons).
Among pediatric patients, the Kaplan-Meier survival curves of patients with and without pathological fractures overlapped. But for adult patients, survival of those with fractures was significantly worse, with 5-year OS of 69.3% with no fractures vs. 45.9% with fractures, and respective 10-year OS rates of 62.1% vs. 36.8% (P less than .001 for both comparisons).
Also in univariate analysis, 5-year and 10-year event-free survival (EFS) rates were significantly lower for patients who experienced pathological fractures.
Finally, in multivariable analysis of overall survival by age group, the investigators found that among adults pathological fracture was associated with a nearly twofold risk for death (hazard ratio [HR] 1.893, P = .013). Other factors were primary metastases (HR 2.486, P = .001), response to chemotherapy (P less than .001) and total surgical remission (P less than .001).
As noted before, pathological fracture among pediatric patients was not associated with worse OS. Factors significantly associated with OS in younger patients were primary metastases (HR 2.187, P less than .001), relative tumor size (HR 1.239, P = .024), response to chemotherapy (HR 2.295, P less than .001), total surgical remission (HR 4.253, P less than .001), and type of surgery (HR 1.282, P = .008).
In contrast, pathological fracture was not associated with EFS among either adult or pediatric patients. Factors significantly associated with EFS in both children and adults were primary metastases and response to chemotherapy. Among pediatric patients only, tumor site and relative tumor size were also associated with EFS.
Ms. Kelley acknowledged that the study was limited by the retrospective design.
She said that exploration of the discrepancies between adult and pediatric patients in regard to the influence of pathological fracture on survival and of the role of pathologic fracture as a negative prognostic factor for OS but not EFS in adults is warranted.
The study was funded by the Wilhelm Sander-Stiftung Foundation for cancer research. Ms. Kelley reported having no conflicts of interest.
AMSTERDAM – Pathological fractures are prognostic of poor outcomes in adults with osteosarcoma, but not in children with osteosarcoma, investigators have found.
A retrospective review of data on consecutive patients treated over the course of 30 years showed that among patients of all ages, both 5-year and 10-year overall survival (OS) rates were worse among patients who had pathological fractures.
But in an analysis stratified by age, the survival difference attributable to fractures was limited entirely to patients who were 18 or older at the time of an osteosarcoma diagnosis, reported Lisa Kelley, a medical student at the Ludwig-Maximilians Universität in Munich.
“It’s important to understand what are the prognostic factors [in osteosarcoma], and while many of the factors have been thoroughly researched, on pathological fractures the data are still fairly inconclusive due to the results of studies contradicting each other, and also having a relatively small patient number due to the fact that osteosarcoma itself is rare, and the pathological fractures occur only in approximately 10% of cases,” she said at an annual congress sponsored by the European Cancer Organisation.
To get a better handle on possible correlations between pathological fractures and prognosis in patients with central high-grade osteosarcoma, Ms. Kelley and her coauthors collected data on consecutive patients treated for localized or metastatic osteosarcoma of the extremities from 1980 through 2010 at one of the member institutions of the Cooperative Osteosarcoma Study Group (COSS).
They identified 2,847 patients, of whom 2,193 (77%) were 18 or younger at the time of diagnosis. Of the entire cohort, 321 patients (11.3%) had a pathological fracture either at presentation or soon after diagnosis.
Comparing patients with and without pathological fractures, the investigators found that factors significantly associated with fracture risk included tumor location, especially the humerus (P less than .001), tumors occurring proximally and in a diaphysis (P less than .001), telangiectatic subtype (P less than .001), the presence of primary metastases (P = .025). and tumors comprising more than one-third of the affected bone (P less than .001).
There were no significant differences in the cohort as a whole between patients with or without fractures in either age in years, sex, body-mass index, history of pain or swelling symptoms, local surgical remission at the main tumor site, total surgical remission including metastases, response to chemotherapy, use of adjuvant chemotherapy rather than neoadjuvant, or type of surgery.
Among adult patients only, however, factors associated with pathological fractures included age (P less than .001). BMI (P = .021), tumor site (P less than .001), histologic subtype (P less than .001), primary metastases (P = .011), relative tumor size (P = .047), and total surgical remission (P = .015).
Among pediatric patients, factors associated with fracture risk were (P less than .001 for all unless otherwise specified) age, BMI (P = .018), history or symptoms (P = .001). tumor site, localization within bone, histologic subtype, and relative tumor size.
In univariate analysis, 5-year OS rates were 70.6% for patients without fractures compared with 63.0% for those with fractures, and respective 10-year OS rates were 64.9% vs. 58.1% (P = .007 for both comparisons).
Among pediatric patients, the Kaplan-Meier survival curves of patients with and without pathological fractures overlapped. But for adult patients, survival of those with fractures was significantly worse, with 5-year OS of 69.3% with no fractures vs. 45.9% with fractures, and respective 10-year OS rates of 62.1% vs. 36.8% (P less than .001 for both comparisons).
Also in univariate analysis, 5-year and 10-year event-free survival (EFS) rates were significantly lower for patients who experienced pathological fractures.
Finally, in multivariable analysis of overall survival by age group, the investigators found that among adults pathological fracture was associated with a nearly twofold risk for death (hazard ratio [HR] 1.893, P = .013). Other factors were primary metastases (HR 2.486, P = .001), response to chemotherapy (P less than .001) and total surgical remission (P less than .001).
As noted before, pathological fracture among pediatric patients was not associated with worse OS. Factors significantly associated with OS in younger patients were primary metastases (HR 2.187, P less than .001), relative tumor size (HR 1.239, P = .024), response to chemotherapy (HR 2.295, P less than .001), total surgical remission (HR 4.253, P less than .001), and type of surgery (HR 1.282, P = .008).
In contrast, pathological fracture was not associated with EFS among either adult or pediatric patients. Factors significantly associated with EFS in both children and adults were primary metastases and response to chemotherapy. Among pediatric patients only, tumor site and relative tumor size were also associated with EFS.
Ms. Kelley acknowledged that the study was limited by the retrospective design.
She said that exploration of the discrepancies between adult and pediatric patients in regard to the influence of pathological fracture on survival and of the role of pathologic fracture as a negative prognostic factor for OS but not EFS in adults is warranted.
The study was funded by the Wilhelm Sander-Stiftung Foundation for cancer research. Ms. Kelley reported having no conflicts of interest.
AMSTERDAM – Pathological fractures are prognostic of poor outcomes in adults with osteosarcoma, but not in children with osteosarcoma, investigators have found.
A retrospective review of data on consecutive patients treated over the course of 30 years showed that among patients of all ages, both 5-year and 10-year overall survival (OS) rates were worse among patients who had pathological fractures.
But in an analysis stratified by age, the survival difference attributable to fractures was limited entirely to patients who were 18 or older at the time of an osteosarcoma diagnosis, reported Lisa Kelley, a medical student at the Ludwig-Maximilians Universität in Munich.
“It’s important to understand what are the prognostic factors [in osteosarcoma], and while many of the factors have been thoroughly researched, on pathological fractures the data are still fairly inconclusive due to the results of studies contradicting each other, and also having a relatively small patient number due to the fact that osteosarcoma itself is rare, and the pathological fractures occur only in approximately 10% of cases,” she said at an annual congress sponsored by the European Cancer Organisation.
To get a better handle on possible correlations between pathological fractures and prognosis in patients with central high-grade osteosarcoma, Ms. Kelley and her coauthors collected data on consecutive patients treated for localized or metastatic osteosarcoma of the extremities from 1980 through 2010 at one of the member institutions of the Cooperative Osteosarcoma Study Group (COSS).
They identified 2,847 patients, of whom 2,193 (77%) were 18 or younger at the time of diagnosis. Of the entire cohort, 321 patients (11.3%) had a pathological fracture either at presentation or soon after diagnosis.
Comparing patients with and without pathological fractures, the investigators found that factors significantly associated with fracture risk included tumor location, especially the humerus (P less than .001), tumors occurring proximally and in a diaphysis (P less than .001), telangiectatic subtype (P less than .001), the presence of primary metastases (P = .025). and tumors comprising more than one-third of the affected bone (P less than .001).
There were no significant differences in the cohort as a whole between patients with or without fractures in either age in years, sex, body-mass index, history of pain or swelling symptoms, local surgical remission at the main tumor site, total surgical remission including metastases, response to chemotherapy, use of adjuvant chemotherapy rather than neoadjuvant, or type of surgery.
Among adult patients only, however, factors associated with pathological fractures included age (P less than .001). BMI (P = .021), tumor site (P less than .001), histologic subtype (P less than .001), primary metastases (P = .011), relative tumor size (P = .047), and total surgical remission (P = .015).
Among pediatric patients, factors associated with fracture risk were (P less than .001 for all unless otherwise specified) age, BMI (P = .018), history or symptoms (P = .001). tumor site, localization within bone, histologic subtype, and relative tumor size.
In univariate analysis, 5-year OS rates were 70.6% for patients without fractures compared with 63.0% for those with fractures, and respective 10-year OS rates were 64.9% vs. 58.1% (P = .007 for both comparisons).
Among pediatric patients, the Kaplan-Meier survival curves of patients with and without pathological fractures overlapped. But for adult patients, survival of those with fractures was significantly worse, with 5-year OS of 69.3% with no fractures vs. 45.9% with fractures, and respective 10-year OS rates of 62.1% vs. 36.8% (P less than .001 for both comparisons).
Also in univariate analysis, 5-year and 10-year event-free survival (EFS) rates were significantly lower for patients who experienced pathological fractures.
Finally, in multivariable analysis of overall survival by age group, the investigators found that among adults pathological fracture was associated with a nearly twofold risk for death (hazard ratio [HR] 1.893, P = .013). Other factors were primary metastases (HR 2.486, P = .001), response to chemotherapy (P less than .001) and total surgical remission (P less than .001).
As noted before, pathological fracture among pediatric patients was not associated with worse OS. Factors significantly associated with OS in younger patients were primary metastases (HR 2.187, P less than .001), relative tumor size (HR 1.239, P = .024), response to chemotherapy (HR 2.295, P less than .001), total surgical remission (HR 4.253, P less than .001), and type of surgery (HR 1.282, P = .008).
In contrast, pathological fracture was not associated with EFS among either adult or pediatric patients. Factors significantly associated with EFS in both children and adults were primary metastases and response to chemotherapy. Among pediatric patients only, tumor site and relative tumor size were also associated with EFS.
Ms. Kelley acknowledged that the study was limited by the retrospective design.
She said that exploration of the discrepancies between adult and pediatric patients in regard to the influence of pathological fracture on survival and of the role of pathologic fracture as a negative prognostic factor for OS but not EFS in adults is warranted.
The study was funded by the Wilhelm Sander-Stiftung Foundation for cancer research. Ms. Kelley reported having no conflicts of interest.
AT ECCO2017
Key clinical point: Pathological fractures are associated with worse survival of adults but not children with osteosarcoma of the extremities.
Major finding: Among adults pathological fracture was associated with a nearly twofold risk for death (hazard ratio, 1.893, P = .013).
Data source: Retrospective review of data on 2,847 consecutive patients with osteosarcoma.
Disclosures: The study was funded by the Wilhelm Sander-Stiftung Foundation for cancer research. Ms. Kelley reported having no conflicts of interest.
Rapid-Cycle Innovation Testing of Text-Based Monitoring for Management of Postpartum Hypertension
From the Maternal and Child Health Research Program, Department of Obstetrics and Gynecology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Dr. Hirshberg, Dr. Srinivas); Hospital of the University of Pennsylvania, Department of Nursing, Department of Obstetrics and Gynecology, Philadelphia, PA (Ms. Bittle); Penn Medicine Center for Health Care Innovation, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Mr. Vandertuyn, Ms. Mahraj, Dr. Asch, Mr. Rosin); and the Department of Family Medicine, University of Washington, Seattle, WA (Dr. Bennett).
Abstract
- Objective: To investigate engagement with a bidirectional text messaging system as an alternative to in-person follow-up for postpartum women with hypertensive disorders.
- Methods: We utilized rapid-cycle innovation processes to implement postpartum SMS text messaging follow-up in women with hypertensive disorders who delivered between September–December 2014. Patients were given electronic blood pressure cuffs and education before discharge. Standard texts reminded patients to send blood pressures daily on each of the 7 days post discharge. The study obstetrician sent text message responses based on a pre-specified management algorithm. Ability to meet ACOG guidelines was defined as receiving at least 1 reading on post-discharge days 1 or 2 and days 5, 6, or 7.
- Results: We enrolled 32 patients. Six (19%) returned for usual care office blood pressure checks. We received at least 1 blood pressure from 27 (84%) participants. Nearly 20 (65%) texted readings on 5 of the 7 days. 27 (84%) texted at least one reading on day 1 or 2, and 21 (66%) texted at least one pressure on day 5, 6, or 7 (P = 0.001 vs. usual care). Two patients required medications and none were readmitted for hypertension. Patients reported preference for home testing and text messaging over return visits.
- Conclusion: Remote blood pressure monitoring via text messaging is a patient-centered method for postpartum hypertension surveillance. Further testing is needed prior to widespread adoption within the broader obstetric community.
Key words: postpartum hypertension, remote monitoring, text-based intervention.
Hypertensive disease is a leading cause of maternal morbidity and mortality [1,2] and the leading cause of obstetric readmissions, accounting for 27% of obstetric readmissions in the United States in 2009 [3]. The majority of patients readmitted with hypertension have a diagnosis of hypertensive disorder of pregnancy on initial admission for delivery, indicating that these readmissions are the result of disease persistence or progression in contrast to new-onset disease. Peak blood pressure in these patients usually occurs 3 to 6 days postpartum [4–6] and is typically unaccompanied by warning symptoms. For these reasons, identifying patients who are at risk for persistent disease and being proactive in their postpartum care may decrease postpartum stroke and seizure. The recent Hypertension in Pregnancy guidelines provided by the American College of Obstetricians and Gynecologists (ACOG) recommend monitoring blood pressure for at least 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days after delivery in women in whom a hypertensive disease of pregnancy is diagnosed [6].
Although there is a clear need for effective and reliable blood pressure surveillance for high-risk women soon after delivery, significant obstacles exist. Our own high-risk blood pressure transition clinic, which occurred every other week and was staffed by maternal-fetal medicine specialists, had an average attendance of only 30% over a 2-year period. Moreover, all of the hypertension-related readmissions occurred in the first 7 days post discharge, which was before the scheduled clinic visit for approximately 50% of patients. Phone call reminders were also found to be an ineffective strategy, as the women did not answer or return voice messages left by the practice. In fact, a postpartum unit quality improvement project validated that follow-up phone calls after discharge from the postpartum unit were less effective than text messaging when reminding women of their blood pressure follow-up appointment at the clinic [7].
As an alternative to in-person visits or traditional voice telephonic communication, mobile phone “Short Message Service” (SMS) text messaging has been used successfully in health care for appointment reminders, result reporting, support of medication and treatment adherence, and dosage adjustment [8–13]. As of 2014, 90% of American adults own a cell phone and over 79% of those send and receive text messages [14]. Among a young population, which is at high risk for hypertensive disorders of pregnancy, data further reveals a preference for text messaging over live calls [15]. Among low-income women under age 30, the rates of cell phone use and text communication are very high [14,15], making text-based surveillance a promising and more patient-centered strategy for a broad population.
We report the results of rapid-cycle innovation and implementation of active, remote surveillance of hypertension with new text message communication strategies in the first 7 days post-discharge. We chose a Plan-Do-Study-Act cycle approach, in which small tests are performed and studied and changes made to accelerate improvement, in order to enhance our ability to acquire blood pressure data [16,17]. The goals of the work were to (1) assess patient engagement using a remote method of blood pressure monitoring, (2) increase ascertainment of postpartum blood pressure data and obtain at least once daily blood pressure readings on all patients on post discharge days 1–2 and 5–7, which is in accordance with the recommended guidelines [6] for blood pressure surveillance, and (3) address all “at risk” severe range blood pressure readings within a short time interval and prior to the need for readmission. We describe a program of remote blood pressure monitoring and communication via text message designed to increase patient engagement and participation, thereby having the potential to result in earlier interventions, reduce readmissions, and decrease overall morbidity.
Methods
We performed a series of 6 rapid-cycle innovation devel-opment and implementation interventions with a cohort of women with chronic hypertension (CHTN), gestational hypertension (GHTN), or preeclampsia (with and without severe features and superimposed) who delivered at our institution between 20 September 2014 and 14 December 2014. All patients were > 18 years old, able to speak and read English, had a hypertension diag-nosis listed above, and had access to a cell phone with unlimited text messaging capabilities. Patients received standard postpartum care and were continued or started on antihypertensive medications based on a standardized postpartum hypertension protocol previously developed at our institution (available on request). This project was undertaken as a quality improvement initiative and as such was exempt from formal review by our institutional review board. However, all patients signed a waiver acknowledging that SMS texting is not a secure communications technology. A single research telephone was used for physician-patient communication to further ensure privacy.
Patients who qualified for the intervention study were recruited on the postpartum unit following delivery. Those who agreed to participate were provided with electronic blood pressure monitors (CVS Pharmacy automatic blood pressure monitor and Omron 3 Series upper arm blood pressure monitor) prior to discharge and instructed on their use. Patients were told to expect their first text message reminder to send in their blood pressure the day after discharge; an example of a text reminder is “Good morning. Please send us a blood pressure reading by 12 pm.” Patients were enrolled for 7 days post discharge and were interviewed regarding their experience at the end of their 7-day enrollment. As this was primarily a feasibility and quality improvement study, patients were also instructed to continue to follow up with the standard of care at the hypertension clinic visit.
For each of the 7 days following discharge from the hospital, patients received a standard text message in the morning and afternoon reminding them to text their blood pressure to the research telephone by a specific time. Reported blood pressures were reviewed and a standard response was sent by the study obstetrician based on an algorithm consistent with the institution’s postpartum hypertension protocol. Patients were sent reminders at all time points whether or not they had texted any BPs.
The ACOG Hypertension in Pregnancy guidelines recommend monitoring blood pressure at 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days postpartum in women diagnosed with a hypertensive disorder of pregnancy [6]. We measured our ability to meet these guidelines by identifying how many patients texted blood pressures on post-discharge days 1 or 2 and post-discharge days 5, 6, or 7, as most patients were discharged home on postpartum day 2 or 3.
Strategies to enhance patient engagement were modified based on patient interviews and results from the immediately preceding cycle (for example, Cycle 1 interview information and results were used to make changes in Cycle 2), as well as studies on telemonitoring adherence in other populations [18]. The program ended after 6 cycles, as the study team felt there was sufficient promise to design an expanded platform suitable for a larger study.
Results
Overall
At the patient level, we received at least 1 blood pressure during the requested time frame from 27 of the 32 patients enrolled (84%). Nearly 65% of patients (20/32) texted at least 1 blood pressure reading on at least 5 out of the 7 days enrolled.
By Cycle
Cycle 1 - Basic
Cycle 1 tested our basic hypothesis that patients would take their blood pressure at home and transmit the results by text message: 5 of 7 patients responded to our reminders, each transmitting blood pressures on at least 5 of the 7 days requested.
Four severe-range blood pressures, defined as systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 [6], were sent to the physician responder, two times each in 2 patients. All four “at risk” severe blood pressures were addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 2 - Education
Patients in Cycle 1 reported during their follow-up interview that they became more aware of the possible morbidity associated with persistent postpartum hypertension as the cycle progressed. Therefore, Cycle 2 tested our hypothesis that focused education would improve patient engagement.
All five patients in this cohort sent in at least one blood pressure during the cycle period. All transmitted at least one blood pressure text on post-discharge day 1 or 2. Four of the five patients (80%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder.
Cycle 3 - Personalization
Patients in Cycle 2 reported during their interview that they felt the text message responses from the provider were too automated. Cycle 3 tested our hypothesis that added personalization, with patient and infant names included in the messages, would improve engagement.
Three of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (60%). Only one patient (20%) also sent in at least one blood pressure on day 5, 6, or 7.
One significantly elevated blood pressure was sent to the physician responder. This blood pressure was addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 4 - Response Timing
Patients in Cycle 3 had lower response rates than previous cycles and noted that they wanted more flexibility in the time to respond, as their schedules were unpredictable with a newborn at home. Although they enjoyed the personalized aspect, they did not feel it influenced their responses, which is evidenced by the low response rate on days 5, 6, or 7. Therefore, Cycle 4 tested our hypothesis that allowing patients to commit to a time of their choice for receiving the reminder texts would improve their response rate.
All five patients enrolled in this cohort sent in at least one blood pressure. We received at least one blood pressure text on post-discharge day 1 or 2 from all five patients in this cycle (100%). Three of the five patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
Five severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient had been discharged home on hydrochlorothiazide 12.5 mg for persistently elevated blood pressures while in the hospital after being diagnosed with severe preeclampsia. All five “at risk” blood pressures were addressed within 24 hours of the text message. On her fifth day of remote surveillance, 5 mg of amlodipine was added to her daily regimen for blood pressures ranging from 150–170/90–110 mm Hg. Her blood pressure at her 6-week postpartum visit was 120/60 mm Hg and she had seen her primary care doctor in the interim for further hypertension management.
Cycle 5 - Snooze and Countdown
Although most of the patients enrolled in Cycle 4 stated that they were very busy in the immediate postpartum period and not always able to respond in a timely fashion, allowing patients to receive the reminder text at their own designated convenient time did not increase engagement. Patients reported that while they always carried their cell phones, they did not always carry their blood pressure cuff, limiting their ability to send in a reading at the time of the reminder. Additionally, patients reported feeling less motivated to continue texting blood pressures towards the end of the cycle. Cycle 5 tested our hypothesis that patient engagement would improve if reminder text messages were sent closer to the morning or evening deadline. Patients were provided with the opportunity to request “snooze” response if they did have their cuff accessible. Additionally, standard responses were accompanied by a countdown message. For example, “Your blood pressure looks good. Four more days of checking your blood pressure to go.”
All five enrolled in this cohort sent in at least one blood pressure, and all (100%) transmitted at least one blood pressure text on post-discharge day 1 or 2 and on day 5, 6, or 7. Only two “snooze” requests were made over the course of the arm by a single patient, who responded both times after the additional reminder.
Four severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient was diagnosed with preeclampsia with severe features on delivery admission, and her blood pressures normalized prior to discharge. All four “at risk” blood pressures were addressed within 24 hours of the text message. Due to persistently elevated diastolic blood pressures ranging from 110–120 mm Hg, she was started on hydrochlorothiazide 12.5 mg on day 6 of the cycle and monitored for additional days following cycle completion with improved blood pressures.
Cycle 6 - Snooze and Support Person
The patients in Cycle 5 were overall satisfied with their experience and did not provide any suggestions for change. However, we sought to see if integrating support persons into the protocol would affect engagement. Cycle 6 tested our hypothesis that patients would be more engaged if they had a self-identified support person reminding them to text their blood pressures. Patients provided the name of a support person to contact if a morning blood pressure was not received. Additionally, patients received the same “snooze” option as in Cycle 5. A total of five patients were enrolled in this cohort; one patient enrolled in the trial but did not send in any blood pressures despite daily reminders to both her and her support buddy. Only 2 additional buddy notifications were required in patients who did not send in a morning blood pressure reading and both times a subsequent blood pressure was sent. Two “snooze” requests were made over the course of the cycle by a single patient, who responded both times after the reminder.
Four of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (80%). Three patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder and no medications were initiated.
Post-Cycle Interviews
Overall, patients reported satisfaction with the text messaging system in their post-cycle interviews. The convenience of the intervention was acknowledged by many, including one patient who commented that “this was a lot better than having to pay for the bus and waiting for hours in some waiting room.” One patient also reported that the increased awareness was important, stating that “when [she] got home and realized that [her blood pressure] was still high, [she] did her own research and learned more about hypertension and preeclampsia.” Others reported that they still checked their blood pressure after the cycle, and “would have went longer than a week if they had asked me to.”
Discussions
Our results suggest that remote blood pressure monitoring via text message communication engages patients and shows promise as a convenient and effective means of hypertension surveillance in the immediate postpartum period, in accordance to ACOG guidelines. Additionally, we were able to test this monitoring system using inexpensive, rapid-cycle validation techniques. Although these techniques are insufficiently controlled and of inadequate statistical power for definitive results, they were able to provide quick evidence toward a pragmatic and workable solution to an important clinical problem within the specific clinical context of our practice, though the results are likely to generalize to other settings. We found varied compliance based on the different engagement strategies, and although no single cycle proved superior, overall patient participation was good and provides a basis for different texting options in future work. Developing a method that both engages patients and is streamlined for providers is critical to our ability to translate this recommendation into practice. Although we did not specifically test how the system works from a provider’s point of view, the study obstetricians believe that this would help and can be fit within the existing workflows of the practices at most institutions.
This rapid-cycle intervention study provides several additional lessons, as we were able to rapidly implement this on our unit and test several hypotheses related to patient engagement. Most patients found the text messaging system to be a convenient way to communicate with their obstetrician. Even when patients had prenatal care at other institutions and delivered at our hospital without a prior patient/physician relationship (n = 5), we were able to engage them in text messaging. However, there was some evidence of patient drop out over the course of the week, as patients were more likely to text in blood pressure in the first few days of the cycle than the last few days (Figure 2).
Other telemedicine interventions have been studied in maternity care and have had inconsistent results. The Cochrane review on telephone support for women during pregnancy and up to 6 weeks after birth found that interventions were mainly aimed at smoking cessation, breastfeeding continuation, preterm birth, and postpartum depression [19]. To date, none of the randomized trials in pregnancy or the postpartum period have focused on postpartum hypertension. The results of our interventions are encouraging and support the use of text messaging in obstetrical care, particularly in the postpartum period. While text messaging cannot provide all the information that can be obtained in a doctor’s visit, such as physical exam, urine dipsticks, and review of symptoms, it can identify the minority of patients that may need to be seen in the office based on the severity of their blood pressures.
While some cases of postpartum preeclampsia occur in the absence of peripartum disease, most readmitted patients are diagnosed with preeclampsia prior to delivery and readmission is due to worsening or persistence of disease and therefore, potentially preventable. These patients are the primary target of our intervention, as remote hypertension surveillance provides an opportunity to start or adjust medications and minimize both patient inconvenience and hospital cost of a readmission.
However, our feasibility study has some limitations. Despite overall patient satisfaction, acceptability, and compliance with text message monitoring of hypertension, the small sample size and qualitative nature of our cycles merits further pursuit and follow-up studies prior to implementation. Overall, we had only a small number of elevated blood pressures requiring intervention; however, this underscores the need to identify patients most at risk for persistent or delayed hypertension and the importance of developing a method of follow-up that engages all patients. Additionally, as patients were asked to both text in blood pressure values and also present for office visits, and therefore acted as their own control, it is not surprising that more patients were compliant with the simple texting method than standard of care; however, even when comparing texting compliance to historical attendance in our clinic of only 30%, our results remain promising.
While our results are encouraging, we believe it is important to test text messaging surveillance and patient compliance in a larger trial prior to implementing within the broader community. This study provides critical data to support the development of a HIPAA-compliant, automated monitoring system that can provide timely responses to patient texts using a provider derived response to blood pressure values. Future work includes the development of an automated hypertension tool as well as a randomized controlled trial to more rigorously compare office blood pressure visits to remote text message surveillance. If effective, use of text messaging technology may allow for an improved patient partnership and more robust follow-up data, especially in patients with less than optimal compliance, as well as the ability to improve maternal care and decrease morbidity and mortality.
Corresponding author: Adi Hirshberg, MD, Dept. of Maternal-Fetal Medicine, 2 Silverstein, 3400 Spruce St., Philadelphia, PA 19104, [email protected].
Funding/support: Supported by a Penn Medicine Innovation Accelerator grant.
Financial disclosures. None reported.
1. Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol 2015;125:5–12.
2. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–S22.
3. Muri JH, Crawford N, Jellen BC. Reducing avoidable obstetrical and neonatal readmissions. American Hospital Association. Accessed 20 Sep 2016 at www.aha.org/content/11/PerinatalReadmissionscall1.pdf.
4. Walters BN, Walters T. Hypertension in the puerperium. Lancet 1987;2:330.
5. Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012;206:470–5.
6. Executive summary: Hypertension in pregnancy. American College of Obstetricians and Gynecologists. Obstet Gynecol 2013;122:1122–31.
7. Scalise LF, Stringer M. Follow-up text messages for patients at high risk for postpartum hypertension. J Obstet Gynecol Neonatal Nurs 2015;44:S6.
8. Using health text messages to improve consumer health knowledge, behaviors, and outcomes: an environmental scan. Rockville, MD: U.S. Department of Health and Human Services; 2014.
9. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, et al. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database Syst Rev 2013;5;12:CD007458.
10. Saffari M, Ghanizadeh G, Koenig HG. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. Prim Care Diabetes 2014;8:275–85.
11. Tran N, Coffma JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma 2014;51:536–43.
12. Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 2012;3:CD009756.
13. Kannisto KA, Koivunen MF, Valimaki MA. Use of mobile phone text message reminders in health care services: a narrative literature review. J Med Internet Res 2010;16:e222.
14. Pew Research Center. Mobile technology fact sheet. Accessed 17 Dec 2014 at www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/.
15. Duggan M. Cell phone activities 2013. Pew Research Center’s Internet and American Life Project. Available at www.pewinternet.org/Reports/2013/Cell-Activities.aspx.
16. Langley G, Nolan K, Nolan T, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
17. Brown P, Hare D. Rapid cycle improvement: controlling change. J Ark Med Soc 2003;99:320–1.
18. Aikens JE, Trivedi R, Aron DC, Piette JD. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med 2015;30:319–26.
19. Lavender T, Richens Y, Milan SJ, et al. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database Syst Rev 2013;7:CD009338.
From the Maternal and Child Health Research Program, Department of Obstetrics and Gynecology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Dr. Hirshberg, Dr. Srinivas); Hospital of the University of Pennsylvania, Department of Nursing, Department of Obstetrics and Gynecology, Philadelphia, PA (Ms. Bittle); Penn Medicine Center for Health Care Innovation, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Mr. Vandertuyn, Ms. Mahraj, Dr. Asch, Mr. Rosin); and the Department of Family Medicine, University of Washington, Seattle, WA (Dr. Bennett).
Abstract
- Objective: To investigate engagement with a bidirectional text messaging system as an alternative to in-person follow-up for postpartum women with hypertensive disorders.
- Methods: We utilized rapid-cycle innovation processes to implement postpartum SMS text messaging follow-up in women with hypertensive disorders who delivered between September–December 2014. Patients were given electronic blood pressure cuffs and education before discharge. Standard texts reminded patients to send blood pressures daily on each of the 7 days post discharge. The study obstetrician sent text message responses based on a pre-specified management algorithm. Ability to meet ACOG guidelines was defined as receiving at least 1 reading on post-discharge days 1 or 2 and days 5, 6, or 7.
- Results: We enrolled 32 patients. Six (19%) returned for usual care office blood pressure checks. We received at least 1 blood pressure from 27 (84%) participants. Nearly 20 (65%) texted readings on 5 of the 7 days. 27 (84%) texted at least one reading on day 1 or 2, and 21 (66%) texted at least one pressure on day 5, 6, or 7 (P = 0.001 vs. usual care). Two patients required medications and none were readmitted for hypertension. Patients reported preference for home testing and text messaging over return visits.
- Conclusion: Remote blood pressure monitoring via text messaging is a patient-centered method for postpartum hypertension surveillance. Further testing is needed prior to widespread adoption within the broader obstetric community.
Key words: postpartum hypertension, remote monitoring, text-based intervention.
Hypertensive disease is a leading cause of maternal morbidity and mortality [1,2] and the leading cause of obstetric readmissions, accounting for 27% of obstetric readmissions in the United States in 2009 [3]. The majority of patients readmitted with hypertension have a diagnosis of hypertensive disorder of pregnancy on initial admission for delivery, indicating that these readmissions are the result of disease persistence or progression in contrast to new-onset disease. Peak blood pressure in these patients usually occurs 3 to 6 days postpartum [4–6] and is typically unaccompanied by warning symptoms. For these reasons, identifying patients who are at risk for persistent disease and being proactive in their postpartum care may decrease postpartum stroke and seizure. The recent Hypertension in Pregnancy guidelines provided by the American College of Obstetricians and Gynecologists (ACOG) recommend monitoring blood pressure for at least 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days after delivery in women in whom a hypertensive disease of pregnancy is diagnosed [6].
Although there is a clear need for effective and reliable blood pressure surveillance for high-risk women soon after delivery, significant obstacles exist. Our own high-risk blood pressure transition clinic, which occurred every other week and was staffed by maternal-fetal medicine specialists, had an average attendance of only 30% over a 2-year period. Moreover, all of the hypertension-related readmissions occurred in the first 7 days post discharge, which was before the scheduled clinic visit for approximately 50% of patients. Phone call reminders were also found to be an ineffective strategy, as the women did not answer or return voice messages left by the practice. In fact, a postpartum unit quality improvement project validated that follow-up phone calls after discharge from the postpartum unit were less effective than text messaging when reminding women of their blood pressure follow-up appointment at the clinic [7].
As an alternative to in-person visits or traditional voice telephonic communication, mobile phone “Short Message Service” (SMS) text messaging has been used successfully in health care for appointment reminders, result reporting, support of medication and treatment adherence, and dosage adjustment [8–13]. As of 2014, 90% of American adults own a cell phone and over 79% of those send and receive text messages [14]. Among a young population, which is at high risk for hypertensive disorders of pregnancy, data further reveals a preference for text messaging over live calls [15]. Among low-income women under age 30, the rates of cell phone use and text communication are very high [14,15], making text-based surveillance a promising and more patient-centered strategy for a broad population.
We report the results of rapid-cycle innovation and implementation of active, remote surveillance of hypertension with new text message communication strategies in the first 7 days post-discharge. We chose a Plan-Do-Study-Act cycle approach, in which small tests are performed and studied and changes made to accelerate improvement, in order to enhance our ability to acquire blood pressure data [16,17]. The goals of the work were to (1) assess patient engagement using a remote method of blood pressure monitoring, (2) increase ascertainment of postpartum blood pressure data and obtain at least once daily blood pressure readings on all patients on post discharge days 1–2 and 5–7, which is in accordance with the recommended guidelines [6] for blood pressure surveillance, and (3) address all “at risk” severe range blood pressure readings within a short time interval and prior to the need for readmission. We describe a program of remote blood pressure monitoring and communication via text message designed to increase patient engagement and participation, thereby having the potential to result in earlier interventions, reduce readmissions, and decrease overall morbidity.
Methods
We performed a series of 6 rapid-cycle innovation devel-opment and implementation interventions with a cohort of women with chronic hypertension (CHTN), gestational hypertension (GHTN), or preeclampsia (with and without severe features and superimposed) who delivered at our institution between 20 September 2014 and 14 December 2014. All patients were > 18 years old, able to speak and read English, had a hypertension diag-nosis listed above, and had access to a cell phone with unlimited text messaging capabilities. Patients received standard postpartum care and were continued or started on antihypertensive medications based on a standardized postpartum hypertension protocol previously developed at our institution (available on request). This project was undertaken as a quality improvement initiative and as such was exempt from formal review by our institutional review board. However, all patients signed a waiver acknowledging that SMS texting is not a secure communications technology. A single research telephone was used for physician-patient communication to further ensure privacy.
Patients who qualified for the intervention study were recruited on the postpartum unit following delivery. Those who agreed to participate were provided with electronic blood pressure monitors (CVS Pharmacy automatic blood pressure monitor and Omron 3 Series upper arm blood pressure monitor) prior to discharge and instructed on their use. Patients were told to expect their first text message reminder to send in their blood pressure the day after discharge; an example of a text reminder is “Good morning. Please send us a blood pressure reading by 12 pm.” Patients were enrolled for 7 days post discharge and were interviewed regarding their experience at the end of their 7-day enrollment. As this was primarily a feasibility and quality improvement study, patients were also instructed to continue to follow up with the standard of care at the hypertension clinic visit.
For each of the 7 days following discharge from the hospital, patients received a standard text message in the morning and afternoon reminding them to text their blood pressure to the research telephone by a specific time. Reported blood pressures were reviewed and a standard response was sent by the study obstetrician based on an algorithm consistent with the institution’s postpartum hypertension protocol. Patients were sent reminders at all time points whether or not they had texted any BPs.
The ACOG Hypertension in Pregnancy guidelines recommend monitoring blood pressure at 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days postpartum in women diagnosed with a hypertensive disorder of pregnancy [6]. We measured our ability to meet these guidelines by identifying how many patients texted blood pressures on post-discharge days 1 or 2 and post-discharge days 5, 6, or 7, as most patients were discharged home on postpartum day 2 or 3.
Strategies to enhance patient engagement were modified based on patient interviews and results from the immediately preceding cycle (for example, Cycle 1 interview information and results were used to make changes in Cycle 2), as well as studies on telemonitoring adherence in other populations [18]. The program ended after 6 cycles, as the study team felt there was sufficient promise to design an expanded platform suitable for a larger study.
Results
Overall
At the patient level, we received at least 1 blood pressure during the requested time frame from 27 of the 32 patients enrolled (84%). Nearly 65% of patients (20/32) texted at least 1 blood pressure reading on at least 5 out of the 7 days enrolled.
By Cycle
Cycle 1 - Basic
Cycle 1 tested our basic hypothesis that patients would take their blood pressure at home and transmit the results by text message: 5 of 7 patients responded to our reminders, each transmitting blood pressures on at least 5 of the 7 days requested.
Four severe-range blood pressures, defined as systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 [6], were sent to the physician responder, two times each in 2 patients. All four “at risk” severe blood pressures were addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 2 - Education
Patients in Cycle 1 reported during their follow-up interview that they became more aware of the possible morbidity associated with persistent postpartum hypertension as the cycle progressed. Therefore, Cycle 2 tested our hypothesis that focused education would improve patient engagement.
All five patients in this cohort sent in at least one blood pressure during the cycle period. All transmitted at least one blood pressure text on post-discharge day 1 or 2. Four of the five patients (80%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder.
Cycle 3 - Personalization
Patients in Cycle 2 reported during their interview that they felt the text message responses from the provider were too automated. Cycle 3 tested our hypothesis that added personalization, with patient and infant names included in the messages, would improve engagement.
Three of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (60%). Only one patient (20%) also sent in at least one blood pressure on day 5, 6, or 7.
One significantly elevated blood pressure was sent to the physician responder. This blood pressure was addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 4 - Response Timing
Patients in Cycle 3 had lower response rates than previous cycles and noted that they wanted more flexibility in the time to respond, as their schedules were unpredictable with a newborn at home. Although they enjoyed the personalized aspect, they did not feel it influenced their responses, which is evidenced by the low response rate on days 5, 6, or 7. Therefore, Cycle 4 tested our hypothesis that allowing patients to commit to a time of their choice for receiving the reminder texts would improve their response rate.
All five patients enrolled in this cohort sent in at least one blood pressure. We received at least one blood pressure text on post-discharge day 1 or 2 from all five patients in this cycle (100%). Three of the five patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
Five severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient had been discharged home on hydrochlorothiazide 12.5 mg for persistently elevated blood pressures while in the hospital after being diagnosed with severe preeclampsia. All five “at risk” blood pressures were addressed within 24 hours of the text message. On her fifth day of remote surveillance, 5 mg of amlodipine was added to her daily regimen for blood pressures ranging from 150–170/90–110 mm Hg. Her blood pressure at her 6-week postpartum visit was 120/60 mm Hg and she had seen her primary care doctor in the interim for further hypertension management.
Cycle 5 - Snooze and Countdown
Although most of the patients enrolled in Cycle 4 stated that they were very busy in the immediate postpartum period and not always able to respond in a timely fashion, allowing patients to receive the reminder text at their own designated convenient time did not increase engagement. Patients reported that while they always carried their cell phones, they did not always carry their blood pressure cuff, limiting their ability to send in a reading at the time of the reminder. Additionally, patients reported feeling less motivated to continue texting blood pressures towards the end of the cycle. Cycle 5 tested our hypothesis that patient engagement would improve if reminder text messages were sent closer to the morning or evening deadline. Patients were provided with the opportunity to request “snooze” response if they did have their cuff accessible. Additionally, standard responses were accompanied by a countdown message. For example, “Your blood pressure looks good. Four more days of checking your blood pressure to go.”
All five enrolled in this cohort sent in at least one blood pressure, and all (100%) transmitted at least one blood pressure text on post-discharge day 1 or 2 and on day 5, 6, or 7. Only two “snooze” requests were made over the course of the arm by a single patient, who responded both times after the additional reminder.
Four severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient was diagnosed with preeclampsia with severe features on delivery admission, and her blood pressures normalized prior to discharge. All four “at risk” blood pressures were addressed within 24 hours of the text message. Due to persistently elevated diastolic blood pressures ranging from 110–120 mm Hg, she was started on hydrochlorothiazide 12.5 mg on day 6 of the cycle and monitored for additional days following cycle completion with improved blood pressures.
Cycle 6 - Snooze and Support Person
The patients in Cycle 5 were overall satisfied with their experience and did not provide any suggestions for change. However, we sought to see if integrating support persons into the protocol would affect engagement. Cycle 6 tested our hypothesis that patients would be more engaged if they had a self-identified support person reminding them to text their blood pressures. Patients provided the name of a support person to contact if a morning blood pressure was not received. Additionally, patients received the same “snooze” option as in Cycle 5. A total of five patients were enrolled in this cohort; one patient enrolled in the trial but did not send in any blood pressures despite daily reminders to both her and her support buddy. Only 2 additional buddy notifications were required in patients who did not send in a morning blood pressure reading and both times a subsequent blood pressure was sent. Two “snooze” requests were made over the course of the cycle by a single patient, who responded both times after the reminder.
Four of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (80%). Three patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder and no medications were initiated.
Post-Cycle Interviews
Overall, patients reported satisfaction with the text messaging system in their post-cycle interviews. The convenience of the intervention was acknowledged by many, including one patient who commented that “this was a lot better than having to pay for the bus and waiting for hours in some waiting room.” One patient also reported that the increased awareness was important, stating that “when [she] got home and realized that [her blood pressure] was still high, [she] did her own research and learned more about hypertension and preeclampsia.” Others reported that they still checked their blood pressure after the cycle, and “would have went longer than a week if they had asked me to.”
Discussions
Our results suggest that remote blood pressure monitoring via text message communication engages patients and shows promise as a convenient and effective means of hypertension surveillance in the immediate postpartum period, in accordance to ACOG guidelines. Additionally, we were able to test this monitoring system using inexpensive, rapid-cycle validation techniques. Although these techniques are insufficiently controlled and of inadequate statistical power for definitive results, they were able to provide quick evidence toward a pragmatic and workable solution to an important clinical problem within the specific clinical context of our practice, though the results are likely to generalize to other settings. We found varied compliance based on the different engagement strategies, and although no single cycle proved superior, overall patient participation was good and provides a basis for different texting options in future work. Developing a method that both engages patients and is streamlined for providers is critical to our ability to translate this recommendation into practice. Although we did not specifically test how the system works from a provider’s point of view, the study obstetricians believe that this would help and can be fit within the existing workflows of the practices at most institutions.
This rapid-cycle intervention study provides several additional lessons, as we were able to rapidly implement this on our unit and test several hypotheses related to patient engagement. Most patients found the text messaging system to be a convenient way to communicate with their obstetrician. Even when patients had prenatal care at other institutions and delivered at our hospital without a prior patient/physician relationship (n = 5), we were able to engage them in text messaging. However, there was some evidence of patient drop out over the course of the week, as patients were more likely to text in blood pressure in the first few days of the cycle than the last few days (Figure 2).
Other telemedicine interventions have been studied in maternity care and have had inconsistent results. The Cochrane review on telephone support for women during pregnancy and up to 6 weeks after birth found that interventions were mainly aimed at smoking cessation, breastfeeding continuation, preterm birth, and postpartum depression [19]. To date, none of the randomized trials in pregnancy or the postpartum period have focused on postpartum hypertension. The results of our interventions are encouraging and support the use of text messaging in obstetrical care, particularly in the postpartum period. While text messaging cannot provide all the information that can be obtained in a doctor’s visit, such as physical exam, urine dipsticks, and review of symptoms, it can identify the minority of patients that may need to be seen in the office based on the severity of their blood pressures.
While some cases of postpartum preeclampsia occur in the absence of peripartum disease, most readmitted patients are diagnosed with preeclampsia prior to delivery and readmission is due to worsening or persistence of disease and therefore, potentially preventable. These patients are the primary target of our intervention, as remote hypertension surveillance provides an opportunity to start or adjust medications and minimize both patient inconvenience and hospital cost of a readmission.
However, our feasibility study has some limitations. Despite overall patient satisfaction, acceptability, and compliance with text message monitoring of hypertension, the small sample size and qualitative nature of our cycles merits further pursuit and follow-up studies prior to implementation. Overall, we had only a small number of elevated blood pressures requiring intervention; however, this underscores the need to identify patients most at risk for persistent or delayed hypertension and the importance of developing a method of follow-up that engages all patients. Additionally, as patients were asked to both text in blood pressure values and also present for office visits, and therefore acted as their own control, it is not surprising that more patients were compliant with the simple texting method than standard of care; however, even when comparing texting compliance to historical attendance in our clinic of only 30%, our results remain promising.
While our results are encouraging, we believe it is important to test text messaging surveillance and patient compliance in a larger trial prior to implementing within the broader community. This study provides critical data to support the development of a HIPAA-compliant, automated monitoring system that can provide timely responses to patient texts using a provider derived response to blood pressure values. Future work includes the development of an automated hypertension tool as well as a randomized controlled trial to more rigorously compare office blood pressure visits to remote text message surveillance. If effective, use of text messaging technology may allow for an improved patient partnership and more robust follow-up data, especially in patients with less than optimal compliance, as well as the ability to improve maternal care and decrease morbidity and mortality.
Corresponding author: Adi Hirshberg, MD, Dept. of Maternal-Fetal Medicine, 2 Silverstein, 3400 Spruce St., Philadelphia, PA 19104, [email protected].
Funding/support: Supported by a Penn Medicine Innovation Accelerator grant.
Financial disclosures. None reported.
From the Maternal and Child Health Research Program, Department of Obstetrics and Gynecology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Dr. Hirshberg, Dr. Srinivas); Hospital of the University of Pennsylvania, Department of Nursing, Department of Obstetrics and Gynecology, Philadelphia, PA (Ms. Bittle); Penn Medicine Center for Health Care Innovation, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Mr. Vandertuyn, Ms. Mahraj, Dr. Asch, Mr. Rosin); and the Department of Family Medicine, University of Washington, Seattle, WA (Dr. Bennett).
Abstract
- Objective: To investigate engagement with a bidirectional text messaging system as an alternative to in-person follow-up for postpartum women with hypertensive disorders.
- Methods: We utilized rapid-cycle innovation processes to implement postpartum SMS text messaging follow-up in women with hypertensive disorders who delivered between September–December 2014. Patients were given electronic blood pressure cuffs and education before discharge. Standard texts reminded patients to send blood pressures daily on each of the 7 days post discharge. The study obstetrician sent text message responses based on a pre-specified management algorithm. Ability to meet ACOG guidelines was defined as receiving at least 1 reading on post-discharge days 1 or 2 and days 5, 6, or 7.
- Results: We enrolled 32 patients. Six (19%) returned for usual care office blood pressure checks. We received at least 1 blood pressure from 27 (84%) participants. Nearly 20 (65%) texted readings on 5 of the 7 days. 27 (84%) texted at least one reading on day 1 or 2, and 21 (66%) texted at least one pressure on day 5, 6, or 7 (P = 0.001 vs. usual care). Two patients required medications and none were readmitted for hypertension. Patients reported preference for home testing and text messaging over return visits.
- Conclusion: Remote blood pressure monitoring via text messaging is a patient-centered method for postpartum hypertension surveillance. Further testing is needed prior to widespread adoption within the broader obstetric community.
Key words: postpartum hypertension, remote monitoring, text-based intervention.
Hypertensive disease is a leading cause of maternal morbidity and mortality [1,2] and the leading cause of obstetric readmissions, accounting for 27% of obstetric readmissions in the United States in 2009 [3]. The majority of patients readmitted with hypertension have a diagnosis of hypertensive disorder of pregnancy on initial admission for delivery, indicating that these readmissions are the result of disease persistence or progression in contrast to new-onset disease. Peak blood pressure in these patients usually occurs 3 to 6 days postpartum [4–6] and is typically unaccompanied by warning symptoms. For these reasons, identifying patients who are at risk for persistent disease and being proactive in their postpartum care may decrease postpartum stroke and seizure. The recent Hypertension in Pregnancy guidelines provided by the American College of Obstetricians and Gynecologists (ACOG) recommend monitoring blood pressure for at least 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days after delivery in women in whom a hypertensive disease of pregnancy is diagnosed [6].
Although there is a clear need for effective and reliable blood pressure surveillance for high-risk women soon after delivery, significant obstacles exist. Our own high-risk blood pressure transition clinic, which occurred every other week and was staffed by maternal-fetal medicine specialists, had an average attendance of only 30% over a 2-year period. Moreover, all of the hypertension-related readmissions occurred in the first 7 days post discharge, which was before the scheduled clinic visit for approximately 50% of patients. Phone call reminders were also found to be an ineffective strategy, as the women did not answer or return voice messages left by the practice. In fact, a postpartum unit quality improvement project validated that follow-up phone calls after discharge from the postpartum unit were less effective than text messaging when reminding women of their blood pressure follow-up appointment at the clinic [7].
As an alternative to in-person visits or traditional voice telephonic communication, mobile phone “Short Message Service” (SMS) text messaging has been used successfully in health care for appointment reminders, result reporting, support of medication and treatment adherence, and dosage adjustment [8–13]. As of 2014, 90% of American adults own a cell phone and over 79% of those send and receive text messages [14]. Among a young population, which is at high risk for hypertensive disorders of pregnancy, data further reveals a preference for text messaging over live calls [15]. Among low-income women under age 30, the rates of cell phone use and text communication are very high [14,15], making text-based surveillance a promising and more patient-centered strategy for a broad population.
We report the results of rapid-cycle innovation and implementation of active, remote surveillance of hypertension with new text message communication strategies in the first 7 days post-discharge. We chose a Plan-Do-Study-Act cycle approach, in which small tests are performed and studied and changes made to accelerate improvement, in order to enhance our ability to acquire blood pressure data [16,17]. The goals of the work were to (1) assess patient engagement using a remote method of blood pressure monitoring, (2) increase ascertainment of postpartum blood pressure data and obtain at least once daily blood pressure readings on all patients on post discharge days 1–2 and 5–7, which is in accordance with the recommended guidelines [6] for blood pressure surveillance, and (3) address all “at risk” severe range blood pressure readings within a short time interval and prior to the need for readmission. We describe a program of remote blood pressure monitoring and communication via text message designed to increase patient engagement and participation, thereby having the potential to result in earlier interventions, reduce readmissions, and decrease overall morbidity.
Methods
We performed a series of 6 rapid-cycle innovation devel-opment and implementation interventions with a cohort of women with chronic hypertension (CHTN), gestational hypertension (GHTN), or preeclampsia (with and without severe features and superimposed) who delivered at our institution between 20 September 2014 and 14 December 2014. All patients were > 18 years old, able to speak and read English, had a hypertension diag-nosis listed above, and had access to a cell phone with unlimited text messaging capabilities. Patients received standard postpartum care and were continued or started on antihypertensive medications based on a standardized postpartum hypertension protocol previously developed at our institution (available on request). This project was undertaken as a quality improvement initiative and as such was exempt from formal review by our institutional review board. However, all patients signed a waiver acknowledging that SMS texting is not a secure communications technology. A single research telephone was used for physician-patient communication to further ensure privacy.
Patients who qualified for the intervention study were recruited on the postpartum unit following delivery. Those who agreed to participate were provided with electronic blood pressure monitors (CVS Pharmacy automatic blood pressure monitor and Omron 3 Series upper arm blood pressure monitor) prior to discharge and instructed on their use. Patients were told to expect their first text message reminder to send in their blood pressure the day after discharge; an example of a text reminder is “Good morning. Please send us a blood pressure reading by 12 pm.” Patients were enrolled for 7 days post discharge and were interviewed regarding their experience at the end of their 7-day enrollment. As this was primarily a feasibility and quality improvement study, patients were also instructed to continue to follow up with the standard of care at the hypertension clinic visit.
For each of the 7 days following discharge from the hospital, patients received a standard text message in the morning and afternoon reminding them to text their blood pressure to the research telephone by a specific time. Reported blood pressures were reviewed and a standard response was sent by the study obstetrician based on an algorithm consistent with the institution’s postpartum hypertension protocol. Patients were sent reminders at all time points whether or not they had texted any BPs.
The ACOG Hypertension in Pregnancy guidelines recommend monitoring blood pressure at 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days postpartum in women diagnosed with a hypertensive disorder of pregnancy [6]. We measured our ability to meet these guidelines by identifying how many patients texted blood pressures on post-discharge days 1 or 2 and post-discharge days 5, 6, or 7, as most patients were discharged home on postpartum day 2 or 3.
Strategies to enhance patient engagement were modified based on patient interviews and results from the immediately preceding cycle (for example, Cycle 1 interview information and results were used to make changes in Cycle 2), as well as studies on telemonitoring adherence in other populations [18]. The program ended after 6 cycles, as the study team felt there was sufficient promise to design an expanded platform suitable for a larger study.
Results
Overall
At the patient level, we received at least 1 blood pressure during the requested time frame from 27 of the 32 patients enrolled (84%). Nearly 65% of patients (20/32) texted at least 1 blood pressure reading on at least 5 out of the 7 days enrolled.
By Cycle
Cycle 1 - Basic
Cycle 1 tested our basic hypothesis that patients would take their blood pressure at home and transmit the results by text message: 5 of 7 patients responded to our reminders, each transmitting blood pressures on at least 5 of the 7 days requested.
Four severe-range blood pressures, defined as systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 [6], were sent to the physician responder, two times each in 2 patients. All four “at risk” severe blood pressures were addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 2 - Education
Patients in Cycle 1 reported during their follow-up interview that they became more aware of the possible morbidity associated with persistent postpartum hypertension as the cycle progressed. Therefore, Cycle 2 tested our hypothesis that focused education would improve patient engagement.
All five patients in this cohort sent in at least one blood pressure during the cycle period. All transmitted at least one blood pressure text on post-discharge day 1 or 2. Four of the five patients (80%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder.
Cycle 3 - Personalization
Patients in Cycle 2 reported during their interview that they felt the text message responses from the provider were too automated. Cycle 3 tested our hypothesis that added personalization, with patient and infant names included in the messages, would improve engagement.
Three of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (60%). Only one patient (20%) also sent in at least one blood pressure on day 5, 6, or 7.
One significantly elevated blood pressure was sent to the physician responder. This blood pressure was addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 4 - Response Timing
Patients in Cycle 3 had lower response rates than previous cycles and noted that they wanted more flexibility in the time to respond, as their schedules were unpredictable with a newborn at home. Although they enjoyed the personalized aspect, they did not feel it influenced their responses, which is evidenced by the low response rate on days 5, 6, or 7. Therefore, Cycle 4 tested our hypothesis that allowing patients to commit to a time of their choice for receiving the reminder texts would improve their response rate.
All five patients enrolled in this cohort sent in at least one blood pressure. We received at least one blood pressure text on post-discharge day 1 or 2 from all five patients in this cycle (100%). Three of the five patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
Five severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient had been discharged home on hydrochlorothiazide 12.5 mg for persistently elevated blood pressures while in the hospital after being diagnosed with severe preeclampsia. All five “at risk” blood pressures were addressed within 24 hours of the text message. On her fifth day of remote surveillance, 5 mg of amlodipine was added to her daily regimen for blood pressures ranging from 150–170/90–110 mm Hg. Her blood pressure at her 6-week postpartum visit was 120/60 mm Hg and she had seen her primary care doctor in the interim for further hypertension management.
Cycle 5 - Snooze and Countdown
Although most of the patients enrolled in Cycle 4 stated that they were very busy in the immediate postpartum period and not always able to respond in a timely fashion, allowing patients to receive the reminder text at their own designated convenient time did not increase engagement. Patients reported that while they always carried their cell phones, they did not always carry their blood pressure cuff, limiting their ability to send in a reading at the time of the reminder. Additionally, patients reported feeling less motivated to continue texting blood pressures towards the end of the cycle. Cycle 5 tested our hypothesis that patient engagement would improve if reminder text messages were sent closer to the morning or evening deadline. Patients were provided with the opportunity to request “snooze” response if they did have their cuff accessible. Additionally, standard responses were accompanied by a countdown message. For example, “Your blood pressure looks good. Four more days of checking your blood pressure to go.”
All five enrolled in this cohort sent in at least one blood pressure, and all (100%) transmitted at least one blood pressure text on post-discharge day 1 or 2 and on day 5, 6, or 7. Only two “snooze” requests were made over the course of the arm by a single patient, who responded both times after the additional reminder.
Four severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient was diagnosed with preeclampsia with severe features on delivery admission, and her blood pressures normalized prior to discharge. All four “at risk” blood pressures were addressed within 24 hours of the text message. Due to persistently elevated diastolic blood pressures ranging from 110–120 mm Hg, she was started on hydrochlorothiazide 12.5 mg on day 6 of the cycle and monitored for additional days following cycle completion with improved blood pressures.
Cycle 6 - Snooze and Support Person
The patients in Cycle 5 were overall satisfied with their experience and did not provide any suggestions for change. However, we sought to see if integrating support persons into the protocol would affect engagement. Cycle 6 tested our hypothesis that patients would be more engaged if they had a self-identified support person reminding them to text their blood pressures. Patients provided the name of a support person to contact if a morning blood pressure was not received. Additionally, patients received the same “snooze” option as in Cycle 5. A total of five patients were enrolled in this cohort; one patient enrolled in the trial but did not send in any blood pressures despite daily reminders to both her and her support buddy. Only 2 additional buddy notifications were required in patients who did not send in a morning blood pressure reading and both times a subsequent blood pressure was sent. Two “snooze” requests were made over the course of the cycle by a single patient, who responded both times after the reminder.
Four of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (80%). Three patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder and no medications were initiated.
Post-Cycle Interviews
Overall, patients reported satisfaction with the text messaging system in their post-cycle interviews. The convenience of the intervention was acknowledged by many, including one patient who commented that “this was a lot better than having to pay for the bus and waiting for hours in some waiting room.” One patient also reported that the increased awareness was important, stating that “when [she] got home and realized that [her blood pressure] was still high, [she] did her own research and learned more about hypertension and preeclampsia.” Others reported that they still checked their blood pressure after the cycle, and “would have went longer than a week if they had asked me to.”
Discussions
Our results suggest that remote blood pressure monitoring via text message communication engages patients and shows promise as a convenient and effective means of hypertension surveillance in the immediate postpartum period, in accordance to ACOG guidelines. Additionally, we were able to test this monitoring system using inexpensive, rapid-cycle validation techniques. Although these techniques are insufficiently controlled and of inadequate statistical power for definitive results, they were able to provide quick evidence toward a pragmatic and workable solution to an important clinical problem within the specific clinical context of our practice, though the results are likely to generalize to other settings. We found varied compliance based on the different engagement strategies, and although no single cycle proved superior, overall patient participation was good and provides a basis for different texting options in future work. Developing a method that both engages patients and is streamlined for providers is critical to our ability to translate this recommendation into practice. Although we did not specifically test how the system works from a provider’s point of view, the study obstetricians believe that this would help and can be fit within the existing workflows of the practices at most institutions.
This rapid-cycle intervention study provides several additional lessons, as we were able to rapidly implement this on our unit and test several hypotheses related to patient engagement. Most patients found the text messaging system to be a convenient way to communicate with their obstetrician. Even when patients had prenatal care at other institutions and delivered at our hospital without a prior patient/physician relationship (n = 5), we were able to engage them in text messaging. However, there was some evidence of patient drop out over the course of the week, as patients were more likely to text in blood pressure in the first few days of the cycle than the last few days (Figure 2).
Other telemedicine interventions have been studied in maternity care and have had inconsistent results. The Cochrane review on telephone support for women during pregnancy and up to 6 weeks after birth found that interventions were mainly aimed at smoking cessation, breastfeeding continuation, preterm birth, and postpartum depression [19]. To date, none of the randomized trials in pregnancy or the postpartum period have focused on postpartum hypertension. The results of our interventions are encouraging and support the use of text messaging in obstetrical care, particularly in the postpartum period. While text messaging cannot provide all the information that can be obtained in a doctor’s visit, such as physical exam, urine dipsticks, and review of symptoms, it can identify the minority of patients that may need to be seen in the office based on the severity of their blood pressures.
While some cases of postpartum preeclampsia occur in the absence of peripartum disease, most readmitted patients are diagnosed with preeclampsia prior to delivery and readmission is due to worsening or persistence of disease and therefore, potentially preventable. These patients are the primary target of our intervention, as remote hypertension surveillance provides an opportunity to start or adjust medications and minimize both patient inconvenience and hospital cost of a readmission.
However, our feasibility study has some limitations. Despite overall patient satisfaction, acceptability, and compliance with text message monitoring of hypertension, the small sample size and qualitative nature of our cycles merits further pursuit and follow-up studies prior to implementation. Overall, we had only a small number of elevated blood pressures requiring intervention; however, this underscores the need to identify patients most at risk for persistent or delayed hypertension and the importance of developing a method of follow-up that engages all patients. Additionally, as patients were asked to both text in blood pressure values and also present for office visits, and therefore acted as their own control, it is not surprising that more patients were compliant with the simple texting method than standard of care; however, even when comparing texting compliance to historical attendance in our clinic of only 30%, our results remain promising.
While our results are encouraging, we believe it is important to test text messaging surveillance and patient compliance in a larger trial prior to implementing within the broader community. This study provides critical data to support the development of a HIPAA-compliant, automated monitoring system that can provide timely responses to patient texts using a provider derived response to blood pressure values. Future work includes the development of an automated hypertension tool as well as a randomized controlled trial to more rigorously compare office blood pressure visits to remote text message surveillance. If effective, use of text messaging technology may allow for an improved patient partnership and more robust follow-up data, especially in patients with less than optimal compliance, as well as the ability to improve maternal care and decrease morbidity and mortality.
Corresponding author: Adi Hirshberg, MD, Dept. of Maternal-Fetal Medicine, 2 Silverstein, 3400 Spruce St., Philadelphia, PA 19104, [email protected].
Funding/support: Supported by a Penn Medicine Innovation Accelerator grant.
Financial disclosures. None reported.
1. Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol 2015;125:5–12.
2. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–S22.
3. Muri JH, Crawford N, Jellen BC. Reducing avoidable obstetrical and neonatal readmissions. American Hospital Association. Accessed 20 Sep 2016 at www.aha.org/content/11/PerinatalReadmissionscall1.pdf.
4. Walters BN, Walters T. Hypertension in the puerperium. Lancet 1987;2:330.
5. Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012;206:470–5.
6. Executive summary: Hypertension in pregnancy. American College of Obstetricians and Gynecologists. Obstet Gynecol 2013;122:1122–31.
7. Scalise LF, Stringer M. Follow-up text messages for patients at high risk for postpartum hypertension. J Obstet Gynecol Neonatal Nurs 2015;44:S6.
8. Using health text messages to improve consumer health knowledge, behaviors, and outcomes: an environmental scan. Rockville, MD: U.S. Department of Health and Human Services; 2014.
9. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, et al. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database Syst Rev 2013;5;12:CD007458.
10. Saffari M, Ghanizadeh G, Koenig HG. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. Prim Care Diabetes 2014;8:275–85.
11. Tran N, Coffma JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma 2014;51:536–43.
12. Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 2012;3:CD009756.
13. Kannisto KA, Koivunen MF, Valimaki MA. Use of mobile phone text message reminders in health care services: a narrative literature review. J Med Internet Res 2010;16:e222.
14. Pew Research Center. Mobile technology fact sheet. Accessed 17 Dec 2014 at www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/.
15. Duggan M. Cell phone activities 2013. Pew Research Center’s Internet and American Life Project. Available at www.pewinternet.org/Reports/2013/Cell-Activities.aspx.
16. Langley G, Nolan K, Nolan T, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
17. Brown P, Hare D. Rapid cycle improvement: controlling change. J Ark Med Soc 2003;99:320–1.
18. Aikens JE, Trivedi R, Aron DC, Piette JD. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med 2015;30:319–26.
19. Lavender T, Richens Y, Milan SJ, et al. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database Syst Rev 2013;7:CD009338.
1. Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol 2015;125:5–12.
2. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–S22.
3. Muri JH, Crawford N, Jellen BC. Reducing avoidable obstetrical and neonatal readmissions. American Hospital Association. Accessed 20 Sep 2016 at www.aha.org/content/11/PerinatalReadmissionscall1.pdf.
4. Walters BN, Walters T. Hypertension in the puerperium. Lancet 1987;2:330.
5. Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012;206:470–5.
6. Executive summary: Hypertension in pregnancy. American College of Obstetricians and Gynecologists. Obstet Gynecol 2013;122:1122–31.
7. Scalise LF, Stringer M. Follow-up text messages for patients at high risk for postpartum hypertension. J Obstet Gynecol Neonatal Nurs 2015;44:S6.
8. Using health text messages to improve consumer health knowledge, behaviors, and outcomes: an environmental scan. Rockville, MD: U.S. Department of Health and Human Services; 2014.
9. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, et al. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database Syst Rev 2013;5;12:CD007458.
10. Saffari M, Ghanizadeh G, Koenig HG. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. Prim Care Diabetes 2014;8:275–85.
11. Tran N, Coffma JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma 2014;51:536–43.
12. Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 2012;3:CD009756.
13. Kannisto KA, Koivunen MF, Valimaki MA. Use of mobile phone text message reminders in health care services: a narrative literature review. J Med Internet Res 2010;16:e222.
14. Pew Research Center. Mobile technology fact sheet. Accessed 17 Dec 2014 at www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/.
15. Duggan M. Cell phone activities 2013. Pew Research Center’s Internet and American Life Project. Available at www.pewinternet.org/Reports/2013/Cell-Activities.aspx.
16. Langley G, Nolan K, Nolan T, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
17. Brown P, Hare D. Rapid cycle improvement: controlling change. J Ark Med Soc 2003;99:320–1.
18. Aikens JE, Trivedi R, Aron DC, Piette JD. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med 2015;30:319–26.
19. Lavender T, Richens Y, Milan SJ, et al. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database Syst Rev 2013;7:CD009338.
Nurse Jackie and Patrick Kennedy
Nurse Jackie is an amazing woman: She’s a fantastically competent and compassionate ER nurse who will do anything for her patients, and she is far better than the caricature ER docs she works with who all care more about their designer shoes and designer egos then about their jobs. She’s a wife – at least in the early episodes – and mother to two adorable girls with a life that includes Mommy and Me tap dancing classes. She somehow juggles it all.
In her fictional life as the star of a Showtime series, “Jackie Peyton” – played by actress Edie Falco, formerly known for her role as Tony Soprano’s wife, Carmela – manages even more: She does it all while popping pain pills by the handful throughout the work day and snorting whatever there is to snort. The interesting thing about Jackie is that her drug habit never interferes with her ability to function. There are no episodes where she makes mistakes or falls asleep. She doesn’t get too wired and is rarely irritable, and she certainly doesn’t pass out from overdoses. She shows up at work on time and never falls off her tightrope of responsibilities in the busy All Saints Hospital ER of New York City.
Without the drugs, she gets ill, but with them, she just functions. And she doesn’t just hold her own with a busy life; she also juggles the secret life of what it takes to get her daily fix, including daily lunchtime sex with the hospital pharmacist, who provides her with pain medicines for her aching back and doesn’t initially know that she’s married with children.
By Season 6, her personal life has broken down, and her daughter Grace, now an angry teenager with her own difficulties and secret life, notes that mom Jackie is incredibly good at hiding her drug use, and that no one can tell when she’s using.
With each season, Jackie’s personal life unwinds a little more, but it’s not because of the effects constant pill-popping has on her behavior; it’s because of the difficulties she has obtaining the drugs. If she could go to the store and buy large amounts of oxycontin and whatever else she takes, she’d have no problem. Her cauldron, however, is filled with constant lies and deception and the secret lives she lives to obtain drugs and to avoid boiling in her own self-made addictive mess.
You wouldn’t expect a review of a dark comedy TV series together with a review of a very serious memoir written by former Rep. Patrick J. Kennedy, but somehow, the two fit together for me.
In “A Common Struggle,” Patrick Kennedy and author Stephen Fried march through Kennedy’s long history of mental illness and substance abuse. Kennedy talks about his life in psychotherapy starting in childhood, related to his parents’ separation. He talks about the treatment he received in college and during his time as a state legislator in Providence, R.I., and how he parked far from his psychiatrist’s office so no one would learn he was in treatment.
Unfortunately, while he found treatment to be very helpful, he stopped when his doctor, Peter Kramer, became a celebrity in his own right after the publication of “Listening to Prozac” (New York: Penguin Books, 1997), and Kennedy became “spooked.” He goes on to talk about the many treatments – including lists of medications – he received over the years and his diagnosis of bipolar disorder, type II. But he doesn’t document what symptoms or episodes led to this diagnosis, and I couldn’t help but wonder how much of his mood disorder was part and parcel of his substance abuse problem. I imagine his doctors may have had the same problem.
So while Kennedy is vague about his psychiatric illness, he is much more forthcoming with his substance abuse problem. Like Nurse Jackie, he has the constant flow of pills – prescribed opiates starting with the diagnosis of a spinal tumor, a handful of Adderall here, some benzodiazepines there, and an enormous issue with alcohol on top of all that. Unlike Nurse Jackie, Kennedy wears the results of his addictions openly. He sleeps through mornings, and his schedulers know to accommodate this. He says things he otherwise wouldn’t while intoxicated, and he embarrasses himself and has others on edge. His mentor in the House of Representatives was Dick Gephardt, and Gephardt’s chief of staff summoned Kennedy to ask him to become chairman of the Democratic Congressional Campaign Committee – a tremendous honor for a 31-year-old congressman.
“There was, however, one catch,” the authors wrote. “ ‘If you do this,’ he said, ‘you can’t drink.’ ” Kennedy notes that he didn’t have the insight to realize how odd this was, and in reflection calls it his first intervention.
While most of his difficulties remained relatively private, one night he mixed Ambien and Phenergan, and in a state of confusion, he went for a drive and crashed into a Capitol Hill concrete barrier – an event that led to media coverage, scandal, and, as his chief of staff put it, “ ‘Patrick,’ he said, ‘we have a problem.’ ” While his father, Sen. Ted Kennedy, dismissed this as “a little fendah bendah,” the son made a public statement and checked himself into a rehab unit at the Mayo Clinic. It was to be the second of many attempts at rehab, his first having been in high school.
What makes “A Common Struggle” (New York: Blue Rider Press, 2015) so special is not just that it is a strikingly candid memoir of addiction and illness in a successful congressman, nor that the congressman happens to be a Kennedy with all the glamour and tragedy that come with being born into that family. What makes it special is the way that Kennedy and Fried weave so much more into this riveting book. It’s the story of one man’s life, taken in the full context of a family bound by tragedies: the assassinations of two of his uncles before his birth, an aunt who’d had a lobotomy and inspired a whole advocacy organization for developmental disabilities, two siblings who struggled with cancer, and two parents with their own addictions and scandalous behaviors. His mother’s psychiatric illness was severe enough that her children obtained guardianship, and that’s just a smattering of all the Kennedy family events that are reported here.
The story unfolds against the backdrop of major news events in America, and combines itself with the complex story of parity legislation and the struggle to legitimize brain diseases as worthy of funding, awareness, and destigmatization. And if that’s not enough for one book to do, it all takes place within Patrick Kennedy’s poignant and powerful desire to gain his father’s approval. Ultimately, the son achieves an extended period of sobriety in the context of falling in love and starting a family, well into his 40s.
Nurse Jackie, despite her drug use and personal woes, is fictional and entertaining. “A Common Struggle” is a serious book and heartfelt look at how addiction and mental illness destroy lives; there is little doubt here that without the Kennedy machine for support and privilege, a 20-year-old compromised college student would not have been able to start or maintain a successful political career. Kudos to Mr. Kennedy for sharing his pain and for helping to break the stereotype that those with mental illness and substance abuse can only be found in jails or under bridges.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Care,” which was released last fall (Baltimore: Johns Hopkins University Press).
Nurse Jackie is an amazing woman: She’s a fantastically competent and compassionate ER nurse who will do anything for her patients, and she is far better than the caricature ER docs she works with who all care more about their designer shoes and designer egos then about their jobs. She’s a wife – at least in the early episodes – and mother to two adorable girls with a life that includes Mommy and Me tap dancing classes. She somehow juggles it all.
In her fictional life as the star of a Showtime series, “Jackie Peyton” – played by actress Edie Falco, formerly known for her role as Tony Soprano’s wife, Carmela – manages even more: She does it all while popping pain pills by the handful throughout the work day and snorting whatever there is to snort. The interesting thing about Jackie is that her drug habit never interferes with her ability to function. There are no episodes where she makes mistakes or falls asleep. She doesn’t get too wired and is rarely irritable, and she certainly doesn’t pass out from overdoses. She shows up at work on time and never falls off her tightrope of responsibilities in the busy All Saints Hospital ER of New York City.
Without the drugs, she gets ill, but with them, she just functions. And she doesn’t just hold her own with a busy life; she also juggles the secret life of what it takes to get her daily fix, including daily lunchtime sex with the hospital pharmacist, who provides her with pain medicines for her aching back and doesn’t initially know that she’s married with children.
By Season 6, her personal life has broken down, and her daughter Grace, now an angry teenager with her own difficulties and secret life, notes that mom Jackie is incredibly good at hiding her drug use, and that no one can tell when she’s using.
With each season, Jackie’s personal life unwinds a little more, but it’s not because of the effects constant pill-popping has on her behavior; it’s because of the difficulties she has obtaining the drugs. If she could go to the store and buy large amounts of oxycontin and whatever else she takes, she’d have no problem. Her cauldron, however, is filled with constant lies and deception and the secret lives she lives to obtain drugs and to avoid boiling in her own self-made addictive mess.
You wouldn’t expect a review of a dark comedy TV series together with a review of a very serious memoir written by former Rep. Patrick J. Kennedy, but somehow, the two fit together for me.
In “A Common Struggle,” Patrick Kennedy and author Stephen Fried march through Kennedy’s long history of mental illness and substance abuse. Kennedy talks about his life in psychotherapy starting in childhood, related to his parents’ separation. He talks about the treatment he received in college and during his time as a state legislator in Providence, R.I., and how he parked far from his psychiatrist’s office so no one would learn he was in treatment.
Unfortunately, while he found treatment to be very helpful, he stopped when his doctor, Peter Kramer, became a celebrity in his own right after the publication of “Listening to Prozac” (New York: Penguin Books, 1997), and Kennedy became “spooked.” He goes on to talk about the many treatments – including lists of medications – he received over the years and his diagnosis of bipolar disorder, type II. But he doesn’t document what symptoms or episodes led to this diagnosis, and I couldn’t help but wonder how much of his mood disorder was part and parcel of his substance abuse problem. I imagine his doctors may have had the same problem.
So while Kennedy is vague about his psychiatric illness, he is much more forthcoming with his substance abuse problem. Like Nurse Jackie, he has the constant flow of pills – prescribed opiates starting with the diagnosis of a spinal tumor, a handful of Adderall here, some benzodiazepines there, and an enormous issue with alcohol on top of all that. Unlike Nurse Jackie, Kennedy wears the results of his addictions openly. He sleeps through mornings, and his schedulers know to accommodate this. He says things he otherwise wouldn’t while intoxicated, and he embarrasses himself and has others on edge. His mentor in the House of Representatives was Dick Gephardt, and Gephardt’s chief of staff summoned Kennedy to ask him to become chairman of the Democratic Congressional Campaign Committee – a tremendous honor for a 31-year-old congressman.
“There was, however, one catch,” the authors wrote. “ ‘If you do this,’ he said, ‘you can’t drink.’ ” Kennedy notes that he didn’t have the insight to realize how odd this was, and in reflection calls it his first intervention.
While most of his difficulties remained relatively private, one night he mixed Ambien and Phenergan, and in a state of confusion, he went for a drive and crashed into a Capitol Hill concrete barrier – an event that led to media coverage, scandal, and, as his chief of staff put it, “ ‘Patrick,’ he said, ‘we have a problem.’ ” While his father, Sen. Ted Kennedy, dismissed this as “a little fendah bendah,” the son made a public statement and checked himself into a rehab unit at the Mayo Clinic. It was to be the second of many attempts at rehab, his first having been in high school.
What makes “A Common Struggle” (New York: Blue Rider Press, 2015) so special is not just that it is a strikingly candid memoir of addiction and illness in a successful congressman, nor that the congressman happens to be a Kennedy with all the glamour and tragedy that come with being born into that family. What makes it special is the way that Kennedy and Fried weave so much more into this riveting book. It’s the story of one man’s life, taken in the full context of a family bound by tragedies: the assassinations of two of his uncles before his birth, an aunt who’d had a lobotomy and inspired a whole advocacy organization for developmental disabilities, two siblings who struggled with cancer, and two parents with their own addictions and scandalous behaviors. His mother’s psychiatric illness was severe enough that her children obtained guardianship, and that’s just a smattering of all the Kennedy family events that are reported here.
The story unfolds against the backdrop of major news events in America, and combines itself with the complex story of parity legislation and the struggle to legitimize brain diseases as worthy of funding, awareness, and destigmatization. And if that’s not enough for one book to do, it all takes place within Patrick Kennedy’s poignant and powerful desire to gain his father’s approval. Ultimately, the son achieves an extended period of sobriety in the context of falling in love and starting a family, well into his 40s.
Nurse Jackie, despite her drug use and personal woes, is fictional and entertaining. “A Common Struggle” is a serious book and heartfelt look at how addiction and mental illness destroy lives; there is little doubt here that without the Kennedy machine for support and privilege, a 20-year-old compromised college student would not have been able to start or maintain a successful political career. Kudos to Mr. Kennedy for sharing his pain and for helping to break the stereotype that those with mental illness and substance abuse can only be found in jails or under bridges.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Care,” which was released last fall (Baltimore: Johns Hopkins University Press).
Nurse Jackie is an amazing woman: She’s a fantastically competent and compassionate ER nurse who will do anything for her patients, and she is far better than the caricature ER docs she works with who all care more about their designer shoes and designer egos then about their jobs. She’s a wife – at least in the early episodes – and mother to two adorable girls with a life that includes Mommy and Me tap dancing classes. She somehow juggles it all.
In her fictional life as the star of a Showtime series, “Jackie Peyton” – played by actress Edie Falco, formerly known for her role as Tony Soprano’s wife, Carmela – manages even more: She does it all while popping pain pills by the handful throughout the work day and snorting whatever there is to snort. The interesting thing about Jackie is that her drug habit never interferes with her ability to function. There are no episodes where she makes mistakes or falls asleep. She doesn’t get too wired and is rarely irritable, and she certainly doesn’t pass out from overdoses. She shows up at work on time and never falls off her tightrope of responsibilities in the busy All Saints Hospital ER of New York City.
Without the drugs, she gets ill, but with them, she just functions. And she doesn’t just hold her own with a busy life; she also juggles the secret life of what it takes to get her daily fix, including daily lunchtime sex with the hospital pharmacist, who provides her with pain medicines for her aching back and doesn’t initially know that she’s married with children.
By Season 6, her personal life has broken down, and her daughter Grace, now an angry teenager with her own difficulties and secret life, notes that mom Jackie is incredibly good at hiding her drug use, and that no one can tell when she’s using.
With each season, Jackie’s personal life unwinds a little more, but it’s not because of the effects constant pill-popping has on her behavior; it’s because of the difficulties she has obtaining the drugs. If she could go to the store and buy large amounts of oxycontin and whatever else she takes, she’d have no problem. Her cauldron, however, is filled with constant lies and deception and the secret lives she lives to obtain drugs and to avoid boiling in her own self-made addictive mess.
You wouldn’t expect a review of a dark comedy TV series together with a review of a very serious memoir written by former Rep. Patrick J. Kennedy, but somehow, the two fit together for me.
In “A Common Struggle,” Patrick Kennedy and author Stephen Fried march through Kennedy’s long history of mental illness and substance abuse. Kennedy talks about his life in psychotherapy starting in childhood, related to his parents’ separation. He talks about the treatment he received in college and during his time as a state legislator in Providence, R.I., and how he parked far from his psychiatrist’s office so no one would learn he was in treatment.
Unfortunately, while he found treatment to be very helpful, he stopped when his doctor, Peter Kramer, became a celebrity in his own right after the publication of “Listening to Prozac” (New York: Penguin Books, 1997), and Kennedy became “spooked.” He goes on to talk about the many treatments – including lists of medications – he received over the years and his diagnosis of bipolar disorder, type II. But he doesn’t document what symptoms or episodes led to this diagnosis, and I couldn’t help but wonder how much of his mood disorder was part and parcel of his substance abuse problem. I imagine his doctors may have had the same problem.
So while Kennedy is vague about his psychiatric illness, he is much more forthcoming with his substance abuse problem. Like Nurse Jackie, he has the constant flow of pills – prescribed opiates starting with the diagnosis of a spinal tumor, a handful of Adderall here, some benzodiazepines there, and an enormous issue with alcohol on top of all that. Unlike Nurse Jackie, Kennedy wears the results of his addictions openly. He sleeps through mornings, and his schedulers know to accommodate this. He says things he otherwise wouldn’t while intoxicated, and he embarrasses himself and has others on edge. His mentor in the House of Representatives was Dick Gephardt, and Gephardt’s chief of staff summoned Kennedy to ask him to become chairman of the Democratic Congressional Campaign Committee – a tremendous honor for a 31-year-old congressman.
“There was, however, one catch,” the authors wrote. “ ‘If you do this,’ he said, ‘you can’t drink.’ ” Kennedy notes that he didn’t have the insight to realize how odd this was, and in reflection calls it his first intervention.
While most of his difficulties remained relatively private, one night he mixed Ambien and Phenergan, and in a state of confusion, he went for a drive and crashed into a Capitol Hill concrete barrier – an event that led to media coverage, scandal, and, as his chief of staff put it, “ ‘Patrick,’ he said, ‘we have a problem.’ ” While his father, Sen. Ted Kennedy, dismissed this as “a little fendah bendah,” the son made a public statement and checked himself into a rehab unit at the Mayo Clinic. It was to be the second of many attempts at rehab, his first having been in high school.
What makes “A Common Struggle” (New York: Blue Rider Press, 2015) so special is not just that it is a strikingly candid memoir of addiction and illness in a successful congressman, nor that the congressman happens to be a Kennedy with all the glamour and tragedy that come with being born into that family. What makes it special is the way that Kennedy and Fried weave so much more into this riveting book. It’s the story of one man’s life, taken in the full context of a family bound by tragedies: the assassinations of two of his uncles before his birth, an aunt who’d had a lobotomy and inspired a whole advocacy organization for developmental disabilities, two siblings who struggled with cancer, and two parents with their own addictions and scandalous behaviors. His mother’s psychiatric illness was severe enough that her children obtained guardianship, and that’s just a smattering of all the Kennedy family events that are reported here.
The story unfolds against the backdrop of major news events in America, and combines itself with the complex story of parity legislation and the struggle to legitimize brain diseases as worthy of funding, awareness, and destigmatization. And if that’s not enough for one book to do, it all takes place within Patrick Kennedy’s poignant and powerful desire to gain his father’s approval. Ultimately, the son achieves an extended period of sobriety in the context of falling in love and starting a family, well into his 40s.
Nurse Jackie, despite her drug use and personal woes, is fictional and entertaining. “A Common Struggle” is a serious book and heartfelt look at how addiction and mental illness destroy lives; there is little doubt here that without the Kennedy machine for support and privilege, a 20-year-old compromised college student would not have been able to start or maintain a successful political career. Kudos to Mr. Kennedy for sharing his pain and for helping to break the stereotype that those with mental illness and substance abuse can only be found in jails or under bridges.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Care,” which was released last fall (Baltimore: Johns Hopkins University Press).
The last call
It’s 7:30 on a Tuesday evening, and you will be on call until 8 o’clock the next morning. You have already been in the office 9 hours. Usual start time is 8 a.m., but that extra hour at home is a perk you have earned by being on call tonight.
A quick glance at the schedule screen suggests that if nothing ugly crops up, you will finish seeing your last patient and be out the door and on your way home by 8:15 p.m. The phone has been quiet for the last half hour, but as you are making your quickstep transition between exam rooms, the nurse tells you that the receptionist has received a call from a very anxious mother who has just discovered that her 6-year-old has a fever of 103° F. The child didn’t eat any dinner and is now complaining that he has a sore throat. The mother is worried because the child had a couple of febrile seizures when he was a toddler, and she has heard of several cases of strep in his class at school.
On the other hand, you could ask the nurse to reassure the mother that a febrile seizure at age 6 is very unlikely and encourage the mother to call you if she continues to be concerned. The problem here hinges on the experience and skills of the nurse. Even if your office has a well-vetted portfolio of clinical algorithms, you may be relying on a nurse with whom you aren’t familiar. Or maybe your past experience makes you uncomfortable with this particular nurse. She or he may have missed some obvious red flags in the past or may be so unskillful at reassurance that it is very likely that you will be getting a 2 a.m. call from this worried parent.
Another option could be to suggest that after reassuring the mother, the nurse offer her a first of the morning appointment tomorrow. There are several problems with this strategy, and I have always discouraged our office staff from making these next morning appointments for sick children. The offer of the appointment seldom reassures the very anxious parents nor does it prevent the middle of the night calls. More importantly, our experience, and I suspect yours, is that half of those newly sick children with fevers will be better by the next morning or their parents ended up going to the emergency room. This will leave you with a wasted appointment slot that you would really like to have available when the phones heat up in the morning. A more efficient strategy is to promise parents that if the child is still sick in the morning, you can guarantee them a timely appointment.
Finally, there are two responses that worked best for me. The first is to have the nurse ask the parents how long it will take them to get to the office. Add 15 minutes to their estimate, and if you can accept that estimated time of arrival, have the nurse tell that family to hustle on in. Send the staff home unless they want the overtime, and see the patient yourself.
The second response is to get on the phone yourself and talk directly to the mother. You were probably going to end up speaking with her in the middle of the night anyway, so you might as well invest the time now in taking your own history. Even if your own version of reassurance fails to prevent a 2 a.m. call, at least you will have some frame of reference when you need to make one of those dangerous middle of the night clinical decisions. A quiet night may depend on how you manage that last call of the day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
It’s 7:30 on a Tuesday evening, and you will be on call until 8 o’clock the next morning. You have already been in the office 9 hours. Usual start time is 8 a.m., but that extra hour at home is a perk you have earned by being on call tonight.
A quick glance at the schedule screen suggests that if nothing ugly crops up, you will finish seeing your last patient and be out the door and on your way home by 8:15 p.m. The phone has been quiet for the last half hour, but as you are making your quickstep transition between exam rooms, the nurse tells you that the receptionist has received a call from a very anxious mother who has just discovered that her 6-year-old has a fever of 103° F. The child didn’t eat any dinner and is now complaining that he has a sore throat. The mother is worried because the child had a couple of febrile seizures when he was a toddler, and she has heard of several cases of strep in his class at school.
On the other hand, you could ask the nurse to reassure the mother that a febrile seizure at age 6 is very unlikely and encourage the mother to call you if she continues to be concerned. The problem here hinges on the experience and skills of the nurse. Even if your office has a well-vetted portfolio of clinical algorithms, you may be relying on a nurse with whom you aren’t familiar. Or maybe your past experience makes you uncomfortable with this particular nurse. She or he may have missed some obvious red flags in the past or may be so unskillful at reassurance that it is very likely that you will be getting a 2 a.m. call from this worried parent.
Another option could be to suggest that after reassuring the mother, the nurse offer her a first of the morning appointment tomorrow. There are several problems with this strategy, and I have always discouraged our office staff from making these next morning appointments for sick children. The offer of the appointment seldom reassures the very anxious parents nor does it prevent the middle of the night calls. More importantly, our experience, and I suspect yours, is that half of those newly sick children with fevers will be better by the next morning or their parents ended up going to the emergency room. This will leave you with a wasted appointment slot that you would really like to have available when the phones heat up in the morning. A more efficient strategy is to promise parents that if the child is still sick in the morning, you can guarantee them a timely appointment.
Finally, there are two responses that worked best for me. The first is to have the nurse ask the parents how long it will take them to get to the office. Add 15 minutes to their estimate, and if you can accept that estimated time of arrival, have the nurse tell that family to hustle on in. Send the staff home unless they want the overtime, and see the patient yourself.
The second response is to get on the phone yourself and talk directly to the mother. You were probably going to end up speaking with her in the middle of the night anyway, so you might as well invest the time now in taking your own history. Even if your own version of reassurance fails to prevent a 2 a.m. call, at least you will have some frame of reference when you need to make one of those dangerous middle of the night clinical decisions. A quiet night may depend on how you manage that last call of the day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
It’s 7:30 on a Tuesday evening, and you will be on call until 8 o’clock the next morning. You have already been in the office 9 hours. Usual start time is 8 a.m., but that extra hour at home is a perk you have earned by being on call tonight.
A quick glance at the schedule screen suggests that if nothing ugly crops up, you will finish seeing your last patient and be out the door and on your way home by 8:15 p.m. The phone has been quiet for the last half hour, but as you are making your quickstep transition between exam rooms, the nurse tells you that the receptionist has received a call from a very anxious mother who has just discovered that her 6-year-old has a fever of 103° F. The child didn’t eat any dinner and is now complaining that he has a sore throat. The mother is worried because the child had a couple of febrile seizures when he was a toddler, and she has heard of several cases of strep in his class at school.
On the other hand, you could ask the nurse to reassure the mother that a febrile seizure at age 6 is very unlikely and encourage the mother to call you if she continues to be concerned. The problem here hinges on the experience and skills of the nurse. Even if your office has a well-vetted portfolio of clinical algorithms, you may be relying on a nurse with whom you aren’t familiar. Or maybe your past experience makes you uncomfortable with this particular nurse. She or he may have missed some obvious red flags in the past or may be so unskillful at reassurance that it is very likely that you will be getting a 2 a.m. call from this worried parent.
Another option could be to suggest that after reassuring the mother, the nurse offer her a first of the morning appointment tomorrow. There are several problems with this strategy, and I have always discouraged our office staff from making these next morning appointments for sick children. The offer of the appointment seldom reassures the very anxious parents nor does it prevent the middle of the night calls. More importantly, our experience, and I suspect yours, is that half of those newly sick children with fevers will be better by the next morning or their parents ended up going to the emergency room. This will leave you with a wasted appointment slot that you would really like to have available when the phones heat up in the morning. A more efficient strategy is to promise parents that if the child is still sick in the morning, you can guarantee them a timely appointment.
Finally, there are two responses that worked best for me. The first is to have the nurse ask the parents how long it will take them to get to the office. Add 15 minutes to their estimate, and if you can accept that estimated time of arrival, have the nurse tell that family to hustle on in. Send the staff home unless they want the overtime, and see the patient yourself.
The second response is to get on the phone yourself and talk directly to the mother. You were probably going to end up speaking with her in the middle of the night anyway, so you might as well invest the time now in taking your own history. Even if your own version of reassurance fails to prevent a 2 a.m. call, at least you will have some frame of reference when you need to make one of those dangerous middle of the night clinical decisions. A quiet night may depend on how you manage that last call of the day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
Complicated appendicitis outcomes worse with delayed surgery
LAS VEGAS – Contrary to what some recent studies suggest, patients with complicated appendicitis may benefit from immediate surgery, with shorter hospital stays and fewer postoperative complications.
According to findings from a large database review, delaying the surgery for a complicated case is likely to result in worse patient outcomes, Matthew Symer, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
There are no firm guidelines about the timing of surgery for complicated appendicitis, he said. “There is in fact some controversy about the timing of surgery,” with studies coming to conflicting conclusions about the benefits and risks of both immediate and delayed treatment. “We theorized that the potential morbidity of operating at the height of the inflammatory process would be less than the risk of complications associated with delay,” he said.
To investigate the question, Dr. Symer and his colleagues queried the New York Statewide Planning and Research Cooperative Database, which contains information on all hospital admissions with an ICD-9 code on any patient covered by any payer in the state. Each patient has a unique identifier that allows tracking over time and across facilities.
From 2000 to 2013, the investigators identified 38,840 patients who presented with complicated appendicitis, defined as a perforation. Of these, 31,167 had an appendectomy within 1 year of the index admission. These patients were separated into two groups: those who had surgery within 48 hours of the index admission (28,015) and those who had later surgery (3,152).
The delayed surgery group was further parsed into three: those who had surgery during the index admission, but at least 48 hours after admission (51%); those who had an appendectomy at a subsequent urgent admission (23%); and those who had an elective interval appendectomy sometime within that year (26%).
In comparing the early vs. late surgery groups overall, Dr. Symer noticed some significant initial differences. Patients in the early surgery group were significantly younger (48 vs. 53 years), more likely to be male (55% vs. 47%), white (70% vs. 64%), and to have private insurance (53% vs. 45%).
Comorbidities were more common among the delayed surgery group. These included chronic obstructive pulmonary disease, renal failure, coronary artery disease, hypertension, diabetes, and congestive heart failure. Delayed-surgery patients were more likely to be treated at high-volume hospitals (45% vs. 34%).
Abscess was more common among the delayed surgery group (72% vs. 51%). Their median length of stay was significantly longer (9 vs. 5 days).
Delayed-surgery patients experienced significantly more iatrogenic complications (4% vs. 2%), and more urinary and wound complications. Overall, two or more complications occurred in 23% of the delayed surgery group and 14% of the early surgery group. The readmission rate was higher (28% vs. 18%). Significantly more in the delayed group reached the 75th percentile in hospital charges (62% vs. 26%).
In a multivariate regression analysis, patients with delayed surgery were more likely to experience a prolonged length of stay (odds ratio, 6); high hospital charges (OR, 4.8), iatrogenic complications (OR, 1.9), any complications (OR, 1.5) and readmission (OR, 1.5).
These findings were largely recapitulated when Dr. Symer broke the delayed group down into the three subgroups: patients who had surgery late in the index admission, patients who had an urgent later appendectomy, and patients who had a later elective procedure.
“All of these relationships held up, with patients who delayed surgery having worse overall complications, whether iatrogenic or any complications, more readmissions, and a longer stay in the hospital,” Dr. Symer said.
He had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
LAS VEGAS – Contrary to what some recent studies suggest, patients with complicated appendicitis may benefit from immediate surgery, with shorter hospital stays and fewer postoperative complications.
According to findings from a large database review, delaying the surgery for a complicated case is likely to result in worse patient outcomes, Matthew Symer, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
There are no firm guidelines about the timing of surgery for complicated appendicitis, he said. “There is in fact some controversy about the timing of surgery,” with studies coming to conflicting conclusions about the benefits and risks of both immediate and delayed treatment. “We theorized that the potential morbidity of operating at the height of the inflammatory process would be less than the risk of complications associated with delay,” he said.
To investigate the question, Dr. Symer and his colleagues queried the New York Statewide Planning and Research Cooperative Database, which contains information on all hospital admissions with an ICD-9 code on any patient covered by any payer in the state. Each patient has a unique identifier that allows tracking over time and across facilities.
From 2000 to 2013, the investigators identified 38,840 patients who presented with complicated appendicitis, defined as a perforation. Of these, 31,167 had an appendectomy within 1 year of the index admission. These patients were separated into two groups: those who had surgery within 48 hours of the index admission (28,015) and those who had later surgery (3,152).
The delayed surgery group was further parsed into three: those who had surgery during the index admission, but at least 48 hours after admission (51%); those who had an appendectomy at a subsequent urgent admission (23%); and those who had an elective interval appendectomy sometime within that year (26%).
In comparing the early vs. late surgery groups overall, Dr. Symer noticed some significant initial differences. Patients in the early surgery group were significantly younger (48 vs. 53 years), more likely to be male (55% vs. 47%), white (70% vs. 64%), and to have private insurance (53% vs. 45%).
Comorbidities were more common among the delayed surgery group. These included chronic obstructive pulmonary disease, renal failure, coronary artery disease, hypertension, diabetes, and congestive heart failure. Delayed-surgery patients were more likely to be treated at high-volume hospitals (45% vs. 34%).
Abscess was more common among the delayed surgery group (72% vs. 51%). Their median length of stay was significantly longer (9 vs. 5 days).
Delayed-surgery patients experienced significantly more iatrogenic complications (4% vs. 2%), and more urinary and wound complications. Overall, two or more complications occurred in 23% of the delayed surgery group and 14% of the early surgery group. The readmission rate was higher (28% vs. 18%). Significantly more in the delayed group reached the 75th percentile in hospital charges (62% vs. 26%).
In a multivariate regression analysis, patients with delayed surgery were more likely to experience a prolonged length of stay (odds ratio, 6); high hospital charges (OR, 4.8), iatrogenic complications (OR, 1.9), any complications (OR, 1.5) and readmission (OR, 1.5).
These findings were largely recapitulated when Dr. Symer broke the delayed group down into the three subgroups: patients who had surgery late in the index admission, patients who had an urgent later appendectomy, and patients who had a later elective procedure.
“All of these relationships held up, with patients who delayed surgery having worse overall complications, whether iatrogenic or any complications, more readmissions, and a longer stay in the hospital,” Dr. Symer said.
He had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
LAS VEGAS – Contrary to what some recent studies suggest, patients with complicated appendicitis may benefit from immediate surgery, with shorter hospital stays and fewer postoperative complications.
According to findings from a large database review, delaying the surgery for a complicated case is likely to result in worse patient outcomes, Matthew Symer, MD, said at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
There are no firm guidelines about the timing of surgery for complicated appendicitis, he said. “There is in fact some controversy about the timing of surgery,” with studies coming to conflicting conclusions about the benefits and risks of both immediate and delayed treatment. “We theorized that the potential morbidity of operating at the height of the inflammatory process would be less than the risk of complications associated with delay,” he said.
To investigate the question, Dr. Symer and his colleagues queried the New York Statewide Planning and Research Cooperative Database, which contains information on all hospital admissions with an ICD-9 code on any patient covered by any payer in the state. Each patient has a unique identifier that allows tracking over time and across facilities.
From 2000 to 2013, the investigators identified 38,840 patients who presented with complicated appendicitis, defined as a perforation. Of these, 31,167 had an appendectomy within 1 year of the index admission. These patients were separated into two groups: those who had surgery within 48 hours of the index admission (28,015) and those who had later surgery (3,152).
The delayed surgery group was further parsed into three: those who had surgery during the index admission, but at least 48 hours after admission (51%); those who had an appendectomy at a subsequent urgent admission (23%); and those who had an elective interval appendectomy sometime within that year (26%).
In comparing the early vs. late surgery groups overall, Dr. Symer noticed some significant initial differences. Patients in the early surgery group were significantly younger (48 vs. 53 years), more likely to be male (55% vs. 47%), white (70% vs. 64%), and to have private insurance (53% vs. 45%).
Comorbidities were more common among the delayed surgery group. These included chronic obstructive pulmonary disease, renal failure, coronary artery disease, hypertension, diabetes, and congestive heart failure. Delayed-surgery patients were more likely to be treated at high-volume hospitals (45% vs. 34%).
Abscess was more common among the delayed surgery group (72% vs. 51%). Their median length of stay was significantly longer (9 vs. 5 days).
Delayed-surgery patients experienced significantly more iatrogenic complications (4% vs. 2%), and more urinary and wound complications. Overall, two or more complications occurred in 23% of the delayed surgery group and 14% of the early surgery group. The readmission rate was higher (28% vs. 18%). Significantly more in the delayed group reached the 75th percentile in hospital charges (62% vs. 26%).
In a multivariate regression analysis, patients with delayed surgery were more likely to experience a prolonged length of stay (odds ratio, 6); high hospital charges (OR, 4.8), iatrogenic complications (OR, 1.9), any complications (OR, 1.5) and readmission (OR, 1.5).
These findings were largely recapitulated when Dr. Symer broke the delayed group down into the three subgroups: patients who had surgery late in the index admission, patients who had an urgent later appendectomy, and patients who had a later elective procedure.
“All of these relationships held up, with patients who delayed surgery having worse overall complications, whether iatrogenic or any complications, more readmissions, and a longer stay in the hospital,” Dr. Symer said.
He had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT THE ACADEMIC SURGICAL CONGRESS
Key clinical point: Delayed surgery for complicated appendicitis was associated with worse patient outcomes than immediate surgery.
Major finding: Patients with delayed surgery were more likely to experience a prolonged length of stay (odds ratio, 6); high hospital charges (OR, 4.8), iatrogenic complications (OR, 1.9), any complications (OR, 1.5) and readmission (OR, 1.5).
Data source: The database review comprised almost 39,000 patients.
Disclosures: Dr. Symer had no financial disclosures.
Trials refine device therapy options for heart failure
SNOWMASS, COLO. – The indication for primary prophylactic implantable cardioverter-defibrillator therapy in patients with nonischemic heart failure is likely to be downgraded in the next iteration of the ACC/AHA heart failure guidelines as a consequence of the negative results of the DANISH trial, William T. Abraham, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
In addition to outlining where the guideline recommendations for implantable cardioverter-defibrillator (ICD) therapy stand today, and how they’re likely to change in response to the DANISH findings, he highlighted the latest patient selection criteria for cardiac resynchronization therapy (CRT), which have grown considerably more complicated over time.
“Following the success of neurohormonal inhibitors and antagonists, our only other breakthroughs for the management of heart failure have been CRT and ICDs,” he noted.
The two device therapies are complementary, and indeed are often employed in combination.
“CRT makes patients feel better and saves lives, while ICDs prolong survival without an effect on improving heart failure per se,” the cardiologist explained.
To put the quality of life benefits of CRT into perspective, studies show that the device therapy results in a placebo-subtracted improvement on the Minnesota Living With Heart Failure Questionnaire of 9-10 points.
“This is a large and clinically meaningful improvement in quality of life. Our best drugs for heart failure – beta blockers and ACE inhibitors – improve this same measure by 4 or 5 points,” Dr. Abraham said.
Current American College of Cardiology/American Heart Association heart failure guidelines give a class I, level of evidence: A, recommendation for prophylactic ICD therapy in patients with an left ventricular ejection fraction (LVEF) of 35% or less and New York Heart Association functional class II or III symptoms despite optimal medical therapy, regardless of whether their heart failure is attributable to ischemic heart disease or nonischemic dilated cardiomyopathy.
The DANISH trial investigators looked at the evidence base for primary prevention ICDs in nonischemic heart failure and concluded it needed shoring up. The recommendation relied mainly on subgroup analyses of larger landmark trials done about 15 years ago, before major improvements in medical therapy had occurred. These reservations were the impetus for the DANISH trial, in which more than 1,100 patients with symptomatic systolic heart failure were randomized to an ICD or usual care.
The primary outcome in the DANISH trial – all-cause mortality – occurred in 21.6% of patients in the ICD group and 23.4% of controls during a median follow-up of 68 months, a nonsignificant difference (N Engl J Med. 2016 Sep 29;375[13]:1221-30).
Turning to the CRT guidelines, Dr. Abraham noted that the simple, broad, class I recommendation for this form of device therapy in patients with cardiac dyssynchrony as defined by a QRS duration greater than 120 msec contained in the 2005 ACC/AHA heart failure guidelines has been whittled down over time as new evidence has unfolded. The only class I recommendation in the current guidelines is in patients with an LVEF of 35% or less, sinus rhythm, left bundle branch block with a QRS duration of 150 msec or longer, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy (Circulation. 2012 Oct 1;126:1784-800). “That’s the money group right there. That’s the group for whom we have the greatest confidence of producing the greatest benefit with the application of cardiac resynchronization therapy,” he explained.
Studies examining the use of CRT in heart failure patients with a non–left bundle branch morphology and a QRS duration of less than 150 msec have yielded negative findings. So have attempts to utilize echocardiographic evidence of mechanical dyssynchrony rather than ECG measurement of QRS duration to guide patient selection for CRT.
“In our practice, any patient with a left bundle branch block gets a CRT device. Our confidence in its efficacy is greater in patients with a QRS of at least 150 msec, but the studies demonstrate clear benefit for patients with left bundle branch block and a QRS of 120-149 msec as well,” according to the cardiologist.
Studies also show that patients who are dependent upon ventricular pacing benefit from CRT.
“If you have a patient who requires at least 40% or more ventricular pacing and also has reduced ejection fraction heart failure, that patient should have a CRT device rather than a dual chamber ICD or standard right-sided right ventricular pacemaker,” he said.
All of this presupposes that first and foremost the patient is already on optimized guideline-directed medical therapy.
“With optimal medical therapy, some of these patients may improve their left ventricular ejection fraction above 35%, or they may become asymptomatic and no longer have an indication for CRT,” Dr. Abraham added.
The rationale for utilizing CRT in combination with an ICD is a bit shaky, resting on a single older landmark study, the COMPANION trial (N Engl J Med. 2004; 350:2140-50).
“That study wasn’t powered to answer the question of whether CRT-D [a combined CRT/ICD device] is better than CRT. Really, this remains somewhat of an unanswered question. So where are we today? Essentially, if a patient has an indication for CRT and an indication for an ICD, we implant a combined device,” he said.
Dr. Abraham reported serving as a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – The indication for primary prophylactic implantable cardioverter-defibrillator therapy in patients with nonischemic heart failure is likely to be downgraded in the next iteration of the ACC/AHA heart failure guidelines as a consequence of the negative results of the DANISH trial, William T. Abraham, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
In addition to outlining where the guideline recommendations for implantable cardioverter-defibrillator (ICD) therapy stand today, and how they’re likely to change in response to the DANISH findings, he highlighted the latest patient selection criteria for cardiac resynchronization therapy (CRT), which have grown considerably more complicated over time.
“Following the success of neurohormonal inhibitors and antagonists, our only other breakthroughs for the management of heart failure have been CRT and ICDs,” he noted.
The two device therapies are complementary, and indeed are often employed in combination.
“CRT makes patients feel better and saves lives, while ICDs prolong survival without an effect on improving heart failure per se,” the cardiologist explained.
To put the quality of life benefits of CRT into perspective, studies show that the device therapy results in a placebo-subtracted improvement on the Minnesota Living With Heart Failure Questionnaire of 9-10 points.
“This is a large and clinically meaningful improvement in quality of life. Our best drugs for heart failure – beta blockers and ACE inhibitors – improve this same measure by 4 or 5 points,” Dr. Abraham said.
Current American College of Cardiology/American Heart Association heart failure guidelines give a class I, level of evidence: A, recommendation for prophylactic ICD therapy in patients with an left ventricular ejection fraction (LVEF) of 35% or less and New York Heart Association functional class II or III symptoms despite optimal medical therapy, regardless of whether their heart failure is attributable to ischemic heart disease or nonischemic dilated cardiomyopathy.
The DANISH trial investigators looked at the evidence base for primary prevention ICDs in nonischemic heart failure and concluded it needed shoring up. The recommendation relied mainly on subgroup analyses of larger landmark trials done about 15 years ago, before major improvements in medical therapy had occurred. These reservations were the impetus for the DANISH trial, in which more than 1,100 patients with symptomatic systolic heart failure were randomized to an ICD or usual care.
The primary outcome in the DANISH trial – all-cause mortality – occurred in 21.6% of patients in the ICD group and 23.4% of controls during a median follow-up of 68 months, a nonsignificant difference (N Engl J Med. 2016 Sep 29;375[13]:1221-30).
Turning to the CRT guidelines, Dr. Abraham noted that the simple, broad, class I recommendation for this form of device therapy in patients with cardiac dyssynchrony as defined by a QRS duration greater than 120 msec contained in the 2005 ACC/AHA heart failure guidelines has been whittled down over time as new evidence has unfolded. The only class I recommendation in the current guidelines is in patients with an LVEF of 35% or less, sinus rhythm, left bundle branch block with a QRS duration of 150 msec or longer, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy (Circulation. 2012 Oct 1;126:1784-800). “That’s the money group right there. That’s the group for whom we have the greatest confidence of producing the greatest benefit with the application of cardiac resynchronization therapy,” he explained.
Studies examining the use of CRT in heart failure patients with a non–left bundle branch morphology and a QRS duration of less than 150 msec have yielded negative findings. So have attempts to utilize echocardiographic evidence of mechanical dyssynchrony rather than ECG measurement of QRS duration to guide patient selection for CRT.
“In our practice, any patient with a left bundle branch block gets a CRT device. Our confidence in its efficacy is greater in patients with a QRS of at least 150 msec, but the studies demonstrate clear benefit for patients with left bundle branch block and a QRS of 120-149 msec as well,” according to the cardiologist.
Studies also show that patients who are dependent upon ventricular pacing benefit from CRT.
“If you have a patient who requires at least 40% or more ventricular pacing and also has reduced ejection fraction heart failure, that patient should have a CRT device rather than a dual chamber ICD or standard right-sided right ventricular pacemaker,” he said.
All of this presupposes that first and foremost the patient is already on optimized guideline-directed medical therapy.
“With optimal medical therapy, some of these patients may improve their left ventricular ejection fraction above 35%, or they may become asymptomatic and no longer have an indication for CRT,” Dr. Abraham added.
The rationale for utilizing CRT in combination with an ICD is a bit shaky, resting on a single older landmark study, the COMPANION trial (N Engl J Med. 2004; 350:2140-50).
“That study wasn’t powered to answer the question of whether CRT-D [a combined CRT/ICD device] is better than CRT. Really, this remains somewhat of an unanswered question. So where are we today? Essentially, if a patient has an indication for CRT and an indication for an ICD, we implant a combined device,” he said.
Dr. Abraham reported serving as a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – The indication for primary prophylactic implantable cardioverter-defibrillator therapy in patients with nonischemic heart failure is likely to be downgraded in the next iteration of the ACC/AHA heart failure guidelines as a consequence of the negative results of the DANISH trial, William T. Abraham, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
In addition to outlining where the guideline recommendations for implantable cardioverter-defibrillator (ICD) therapy stand today, and how they’re likely to change in response to the DANISH findings, he highlighted the latest patient selection criteria for cardiac resynchronization therapy (CRT), which have grown considerably more complicated over time.
“Following the success of neurohormonal inhibitors and antagonists, our only other breakthroughs for the management of heart failure have been CRT and ICDs,” he noted.
The two device therapies are complementary, and indeed are often employed in combination.
“CRT makes patients feel better and saves lives, while ICDs prolong survival without an effect on improving heart failure per se,” the cardiologist explained.
To put the quality of life benefits of CRT into perspective, studies show that the device therapy results in a placebo-subtracted improvement on the Minnesota Living With Heart Failure Questionnaire of 9-10 points.
“This is a large and clinically meaningful improvement in quality of life. Our best drugs for heart failure – beta blockers and ACE inhibitors – improve this same measure by 4 or 5 points,” Dr. Abraham said.
Current American College of Cardiology/American Heart Association heart failure guidelines give a class I, level of evidence: A, recommendation for prophylactic ICD therapy in patients with an left ventricular ejection fraction (LVEF) of 35% or less and New York Heart Association functional class II or III symptoms despite optimal medical therapy, regardless of whether their heart failure is attributable to ischemic heart disease or nonischemic dilated cardiomyopathy.
The DANISH trial investigators looked at the evidence base for primary prevention ICDs in nonischemic heart failure and concluded it needed shoring up. The recommendation relied mainly on subgroup analyses of larger landmark trials done about 15 years ago, before major improvements in medical therapy had occurred. These reservations were the impetus for the DANISH trial, in which more than 1,100 patients with symptomatic systolic heart failure were randomized to an ICD or usual care.
The primary outcome in the DANISH trial – all-cause mortality – occurred in 21.6% of patients in the ICD group and 23.4% of controls during a median follow-up of 68 months, a nonsignificant difference (N Engl J Med. 2016 Sep 29;375[13]:1221-30).
Turning to the CRT guidelines, Dr. Abraham noted that the simple, broad, class I recommendation for this form of device therapy in patients with cardiac dyssynchrony as defined by a QRS duration greater than 120 msec contained in the 2005 ACC/AHA heart failure guidelines has been whittled down over time as new evidence has unfolded. The only class I recommendation in the current guidelines is in patients with an LVEF of 35% or less, sinus rhythm, left bundle branch block with a QRS duration of 150 msec or longer, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy (Circulation. 2012 Oct 1;126:1784-800). “That’s the money group right there. That’s the group for whom we have the greatest confidence of producing the greatest benefit with the application of cardiac resynchronization therapy,” he explained.
Studies examining the use of CRT in heart failure patients with a non–left bundle branch morphology and a QRS duration of less than 150 msec have yielded negative findings. So have attempts to utilize echocardiographic evidence of mechanical dyssynchrony rather than ECG measurement of QRS duration to guide patient selection for CRT.
“In our practice, any patient with a left bundle branch block gets a CRT device. Our confidence in its efficacy is greater in patients with a QRS of at least 150 msec, but the studies demonstrate clear benefit for patients with left bundle branch block and a QRS of 120-149 msec as well,” according to the cardiologist.
Studies also show that patients who are dependent upon ventricular pacing benefit from CRT.
“If you have a patient who requires at least 40% or more ventricular pacing and also has reduced ejection fraction heart failure, that patient should have a CRT device rather than a dual chamber ICD or standard right-sided right ventricular pacemaker,” he said.
All of this presupposes that first and foremost the patient is already on optimized guideline-directed medical therapy.
“With optimal medical therapy, some of these patients may improve their left ventricular ejection fraction above 35%, or they may become asymptomatic and no longer have an indication for CRT,” Dr. Abraham added.
The rationale for utilizing CRT in combination with an ICD is a bit shaky, resting on a single older landmark study, the COMPANION trial (N Engl J Med. 2004; 350:2140-50).
“That study wasn’t powered to answer the question of whether CRT-D [a combined CRT/ICD device] is better than CRT. Really, this remains somewhat of an unanswered question. So where are we today? Essentially, if a patient has an indication for CRT and an indication for an ICD, we implant a combined device,” he said.
Dr. Abraham reported serving as a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS