User login
Out to lunch
I’m sure there are folks here in town who wondered how I could keep up a professional pace that often included being on call 2 nights a week and working every third weekend. Even when I was in my early 50s, people asked me if I was getting ready to retire. I hope it wasn’t because I appeared unhappy or looked 15 years older than I was. I suspect that some parents who didn’t know me well predicted that my career would have ended far short of 40 years.
One of the secrets of what appeared to be my superhuman stamina was that almost every day at noon I was out to lunch. That doesn’t mean that I always took time to eat lunch. In fact, I must admit that more often than not my midday diet consisted of several handfuls of cashews or an energy bar eaten on the fly.
The feeling of invigoration and renewal that came in its wake fueled my commitment to my habit of lunchtime outdoor activity. Although to some people it may be counterintuitive, the physical activity energized me. The second half of my workday was no more fatiguing than the morning. However, if some thoughtless hospital or practice administrator scheduled a noon meeting, the rest of my day was a grump fest.
A recent study has demonstrated just how powerful lunchtime exercise can be in improving worker attitude and mood, even if the activity is just going for a walk. (“Changes in work affect in response to lunchtime walking in previously physically inactive employees: A randomized trial” (Scand J Med Sci Sports. 2015 Dec;25[6]:778-87). There have been other studies that have pointed to the value of an activity break, but these investigators collected real-time reports from subjects using their cell phones. “Lunchtime walks improved enthusiasm, relaxation, and nervousness at work,” the researchers noted.
The problem comes in getting employees to take that first step toward developing a lunchtime activity habit. A few, usually women, have discovered the value for themselves and enjoy the social interaction as much as they do the affect-improving aspects of the activity and change of scene. I have tried to encourage lunchtime walking in the workplace with several strategies, including small monetary rewards, prizes, and contests between groups of workers. One year we even bought umbrellas to encourage employees to walk even if it was raining. But without a vigorous and persistent support system, inertia wins, and only those who have discovered the benefits of lunchtime activity for themselves persist.
You may be asking yourself how I managed to find time in my schedule for that hour of lunchtime activity; actually it was usually an hour and half to include a shower. The answer is that I built my schedule around it, and that meant getting to the office earlier and working later. But in my mind that was a small price to pay for the benefits I received. The other secret to my apparent stamina was that I lived a 5-minute bike ride from both hospitals and my office. Don’t underestimate the toll your commute is taking on your life and happiness.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
I’m sure there are folks here in town who wondered how I could keep up a professional pace that often included being on call 2 nights a week and working every third weekend. Even when I was in my early 50s, people asked me if I was getting ready to retire. I hope it wasn’t because I appeared unhappy or looked 15 years older than I was. I suspect that some parents who didn’t know me well predicted that my career would have ended far short of 40 years.
One of the secrets of what appeared to be my superhuman stamina was that almost every day at noon I was out to lunch. That doesn’t mean that I always took time to eat lunch. In fact, I must admit that more often than not my midday diet consisted of several handfuls of cashews or an energy bar eaten on the fly.
The feeling of invigoration and renewal that came in its wake fueled my commitment to my habit of lunchtime outdoor activity. Although to some people it may be counterintuitive, the physical activity energized me. The second half of my workday was no more fatiguing than the morning. However, if some thoughtless hospital or practice administrator scheduled a noon meeting, the rest of my day was a grump fest.
A recent study has demonstrated just how powerful lunchtime exercise can be in improving worker attitude and mood, even if the activity is just going for a walk. (“Changes in work affect in response to lunchtime walking in previously physically inactive employees: A randomized trial” (Scand J Med Sci Sports. 2015 Dec;25[6]:778-87). There have been other studies that have pointed to the value of an activity break, but these investigators collected real-time reports from subjects using their cell phones. “Lunchtime walks improved enthusiasm, relaxation, and nervousness at work,” the researchers noted.
The problem comes in getting employees to take that first step toward developing a lunchtime activity habit. A few, usually women, have discovered the value for themselves and enjoy the social interaction as much as they do the affect-improving aspects of the activity and change of scene. I have tried to encourage lunchtime walking in the workplace with several strategies, including small monetary rewards, prizes, and contests between groups of workers. One year we even bought umbrellas to encourage employees to walk even if it was raining. But without a vigorous and persistent support system, inertia wins, and only those who have discovered the benefits of lunchtime activity for themselves persist.
You may be asking yourself how I managed to find time in my schedule for that hour of lunchtime activity; actually it was usually an hour and half to include a shower. The answer is that I built my schedule around it, and that meant getting to the office earlier and working later. But in my mind that was a small price to pay for the benefits I received. The other secret to my apparent stamina was that I lived a 5-minute bike ride from both hospitals and my office. Don’t underestimate the toll your commute is taking on your life and happiness.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
I’m sure there are folks here in town who wondered how I could keep up a professional pace that often included being on call 2 nights a week and working every third weekend. Even when I was in my early 50s, people asked me if I was getting ready to retire. I hope it wasn’t because I appeared unhappy or looked 15 years older than I was. I suspect that some parents who didn’t know me well predicted that my career would have ended far short of 40 years.
One of the secrets of what appeared to be my superhuman stamina was that almost every day at noon I was out to lunch. That doesn’t mean that I always took time to eat lunch. In fact, I must admit that more often than not my midday diet consisted of several handfuls of cashews or an energy bar eaten on the fly.
The feeling of invigoration and renewal that came in its wake fueled my commitment to my habit of lunchtime outdoor activity. Although to some people it may be counterintuitive, the physical activity energized me. The second half of my workday was no more fatiguing than the morning. However, if some thoughtless hospital or practice administrator scheduled a noon meeting, the rest of my day was a grump fest.
A recent study has demonstrated just how powerful lunchtime exercise can be in improving worker attitude and mood, even if the activity is just going for a walk. (“Changes in work affect in response to lunchtime walking in previously physically inactive employees: A randomized trial” (Scand J Med Sci Sports. 2015 Dec;25[6]:778-87). There have been other studies that have pointed to the value of an activity break, but these investigators collected real-time reports from subjects using their cell phones. “Lunchtime walks improved enthusiasm, relaxation, and nervousness at work,” the researchers noted.
The problem comes in getting employees to take that first step toward developing a lunchtime activity habit. A few, usually women, have discovered the value for themselves and enjoy the social interaction as much as they do the affect-improving aspects of the activity and change of scene. I have tried to encourage lunchtime walking in the workplace with several strategies, including small monetary rewards, prizes, and contests between groups of workers. One year we even bought umbrellas to encourage employees to walk even if it was raining. But without a vigorous and persistent support system, inertia wins, and only those who have discovered the benefits of lunchtime activity for themselves persist.
You may be asking yourself how I managed to find time in my schedule for that hour of lunchtime activity; actually it was usually an hour and half to include a shower. The answer is that I built my schedule around it, and that meant getting to the office earlier and working later. But in my mind that was a small price to pay for the benefits I received. The other secret to my apparent stamina was that I lived a 5-minute bike ride from both hospitals and my office. Don’t underestimate the toll your commute is taking on your life and happiness.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Ibrutinib, palbociclib yield durable complete responses in pretreated mantle cell lymphoma
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
AT ASH 2016
Key clinical point: Combination therapy with ibrutinib and palbociclib was generally well tolerated and induced complete responses in patients with pretreated mantle cell lymphoma.
Major finding: A total of 44% of patients had complete responses, and 67% remained alive and progression-free after a median of 11 months of follow-up. Severe rashes occurred at the highest dose studied (420 mg ibrutinib/100 mg palbociclib).
Data source: A phase I trial of 20 patients with previously treated mantle cell lymphoma.
Disclosures: The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
HHS pick Price made ‘brazen’ trades while committee was under scrutiny
Health and Human Services secretary nominee Tom Price showed little restraint in his personal stock trading during the 3 years that federal investigators were bearing down on a key House committee on which the Republican congressman served, a review of his financial disclosures shows.
Rep. Price (Ga.) made dozens of health industry stock trades during a 3-year investigation by the Securities and Exchange Commission that focused on the Ways and Means Committee, according to financial disclosure records he filed with the House of Representatives. The investigation was considered the first test of a law passed to ban members of Congress and their staffs from trading stock based on insider information.
Rep. Price, who is a retired orthopedic surgeon, was never a target of the federal investigation, which scrutinized a top Ways and Means staffer, and no charges were brought. But ethics experts say Rep. Price’s personal trading, even during the thick of federal pressure on his committee, shows he was unconcerned about financial investments that could create an appearance of impropriety.
“He should have known better,” Richard Painter, former White House chief ethics attorney under President George W. Bush and a professor at the University of Minnesota Law School said of Rep. Price’s conduct during the SEC inquiry.
As Rep. Price awaits a Senate vote on his confirmation, Senate Democrats and a number of watchdog groups have asked the SEC to investigate whether Rep. Price engaged in insider trading with some of his trades in health care companies. Rep. Price has said he abided by all ethics rules, although he acknowledged to the Senate Finance Committee that he did not consult the House Ethics Committee on trades that have now become controversial.
The SEC’s inquiry began in 2013, as it battled Ways and Means for documents to develop its case.
A few weeks ago, the day before President Donald Trump’s inauguration, the SEC quietly dropped its pursuit of committee documents without explanation, according to federal court records. No charges were brought against the staffer, Brian Sutter, who is now a health care lobbyist. Sutter’s lawyer declined to comment.
Craig Holman, government affairs lobbyist with Public Citizen, described Rep. Price’s volume of stock trades during the SEC inquiry as “brazen,” given the congressman’s access to nonpublic information affecting the companies’ fortunes.
“The public is seeing this and they really don’t like it,” said Holman, whose watchdog group recently filed complaints about Rep. Price’s stock trading with both the SEC and the Office of Congressional Ethics.
Trump administration officials and Rep. Price have dismissed questions that news reports and lawmakers have raised about stock trades coinciding with official actions to help certain companies, saying Rep. Price’s brokers chose the stocks independently and all of his conduct was transparent.
After acknowledging that he asked his broker to buy stock in an Australian drug company, he told the Senate Finance Committee that he did not direct his broker to make other trades.
“To the best of my knowledge, I have not undertaken such actions,” he wrote in response to finance committee questions. “I have abided by and adhered to all ethics and conflict of interest rules applicable to me.”
An analysis of Rep. Price’s trades shows that he bought health stocks in 2007, the first year Congress financial disclosures are posted online. In 2011, the first year Rep. Price sat on the health subcommittee, he traded no health-related stocks, according to his financial disclosures filed with Congress.
That same year, members were facing public criticism because of a book detailing how they could use inside information and a “60 Minutes” investigation focused on how members and staff could legally use inside information to gain from their own stock trades.
In 2012, President Barack Obama signed the Stop Trading on Congressional Knowledge Act to rein in insider trading by members and require more disclosure. Public watchdog groups suggested at the time that the law would curb the practice.
That year, after his 1-year break in health care trades, Rep. Price resumed investing in health care companies.
Along with investments in technology, financial services, and retail stocks, he also bought and sold stock in companies that could be impacted by actions of his subcommittee, which has a role in determining rates the government pays under the Medicare program.
Health care firms spend heavily to influence members of Congress, lobbying on health matters, funding political campaigns, and seeking favor with Medicare officials who decide how much the program will pay for certain drugs and devices. The Food and Drug Administration holds similar power, approving or putting conditions on drug and device use.
Beyond his personal investments in health care companies, Rep. Price has also advocated their interests in letters to officials and proposed laws, government records show.
In 2012, disclosure records show Rep. Price sold stock in several drug firms, including more than $110,000 worth of Amgen stock. Amgen’s stock price had steadily climbed out of a recession-level slump, but Rep. Price’s sale came a few weeks before the company pleaded guilty to illegally marketing an anemia drug.
By 2013, the health subcommittee was at the center of a major conflict between Medicare, which sets Medicare Advantage rates, and the insurance industry. Medicare issued a notice early that year announcing its intention to reduce Medicare Advantage rates by 2.3 percent as part of a major cost-cutting initiative.
That prompted fierce lobbying by the health insurance industry. Members of Congress, including Rep. Price, wrote a letter to Marilyn Tavenner, then acting administrator for the Centers for Medicare & Medicaid Services, protesting the rate cut, saying the decrease would “disadvantage vulnerable beneficiaries with multiple chronic conditions.”
Ultimately, Medicare decided not to cut rates but instead, to increase them. Yet an hour before Medicare announced the change, a Height Securities analyst fired off a “flash” report to 200 clients that touched off a surge of trading.
The analyst’s report said a political deal was hatched on Capitol Hill to prevent the cuts as a condition for moving forward on Tavenner’s confirmation. Medicare officials increased rates by nearly 4 percent, a change that would positively impact the bottom lines of health insurance companies.
The SEC began looking for the leak’s source, and within weeks, FBI agents began interviewing staffers at the Ways and Means Committee, court records show.
They discovered communications between Sutter and a health care lobbyist. The HHS Inspector General also began a probe, and federal prosecutors briefly examined the matter as well.
As the case unfolded, Rep. Price bought more health care-related stocks, according to his financial disclosures. He has testified that his broker directed all of the trades, except for his investments in Innate Immunotherapeutics, an Australian company partly owned by Rep. Chris Collins (R-N.Y.), according to Collins’ disclosures. An HHS spokesman said Monday that Rep. Price held three broker-directed accounts.
Ethics experts have said that Rep. Price should have further distanced himself by placing his assets in a blind trust.
On April 30, 2013, Rep. Price bought $2,093 worth of stocks in Incyte, a company that develops cancer drugs; $2,076 in Onyx Pharmaceuticals, a drug maker that would soon merge with a larger drug firm; and $2,097 in Parexel International, a consultancy that helps drugs and devices win FDA approval, according to the financial disclosure records.
The same day, Rep. Price shed shares of Express Scripts, a drug management firm, and Danaher, which makes products hospitals and doctor’s offices using for testing and diagnostics. In August of that year, he bought a $2,429 stake in Jazz Pharmaceuticals, which makes sleep and cancer drugs.
On May 6, 2014, the SEC served its first subpoena for the Ways and Means Committee documents. The committee launched a vigorous fight, appealing a federal district judge’s ruling that it should comply with the SEC subpoena.
Rep. Price continued his health stock trades, including $1,000 to $15,000 in drug firms Amgen, Biogen, Bristol-Myers Squibb, Eli Lilly, and Pfizer. He also bought stock in Aetna, a major health insurer, and Athenahealth, which sells electronic medical record and medical billing software. In 2016, he also increased his investment in Innate Immunotherapeutics.
The purchase became controversial because both he and Collins bought stock in a private placement at a discounted price.
“You’re asking for trouble if you have access to nonpublic information about the health care industry and you’re buying and selling health care stocks,” Painter said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Health and Human Services secretary nominee Tom Price showed little restraint in his personal stock trading during the 3 years that federal investigators were bearing down on a key House committee on which the Republican congressman served, a review of his financial disclosures shows.
Rep. Price (Ga.) made dozens of health industry stock trades during a 3-year investigation by the Securities and Exchange Commission that focused on the Ways and Means Committee, according to financial disclosure records he filed with the House of Representatives. The investigation was considered the first test of a law passed to ban members of Congress and their staffs from trading stock based on insider information.
Rep. Price, who is a retired orthopedic surgeon, was never a target of the federal investigation, which scrutinized a top Ways and Means staffer, and no charges were brought. But ethics experts say Rep. Price’s personal trading, even during the thick of federal pressure on his committee, shows he was unconcerned about financial investments that could create an appearance of impropriety.
“He should have known better,” Richard Painter, former White House chief ethics attorney under President George W. Bush and a professor at the University of Minnesota Law School said of Rep. Price’s conduct during the SEC inquiry.
As Rep. Price awaits a Senate vote on his confirmation, Senate Democrats and a number of watchdog groups have asked the SEC to investigate whether Rep. Price engaged in insider trading with some of his trades in health care companies. Rep. Price has said he abided by all ethics rules, although he acknowledged to the Senate Finance Committee that he did not consult the House Ethics Committee on trades that have now become controversial.
The SEC’s inquiry began in 2013, as it battled Ways and Means for documents to develop its case.
A few weeks ago, the day before President Donald Trump’s inauguration, the SEC quietly dropped its pursuit of committee documents without explanation, according to federal court records. No charges were brought against the staffer, Brian Sutter, who is now a health care lobbyist. Sutter’s lawyer declined to comment.
Craig Holman, government affairs lobbyist with Public Citizen, described Rep. Price’s volume of stock trades during the SEC inquiry as “brazen,” given the congressman’s access to nonpublic information affecting the companies’ fortunes.
“The public is seeing this and they really don’t like it,” said Holman, whose watchdog group recently filed complaints about Rep. Price’s stock trading with both the SEC and the Office of Congressional Ethics.
Trump administration officials and Rep. Price have dismissed questions that news reports and lawmakers have raised about stock trades coinciding with official actions to help certain companies, saying Rep. Price’s brokers chose the stocks independently and all of his conduct was transparent.
After acknowledging that he asked his broker to buy stock in an Australian drug company, he told the Senate Finance Committee that he did not direct his broker to make other trades.
“To the best of my knowledge, I have not undertaken such actions,” he wrote in response to finance committee questions. “I have abided by and adhered to all ethics and conflict of interest rules applicable to me.”
An analysis of Rep. Price’s trades shows that he bought health stocks in 2007, the first year Congress financial disclosures are posted online. In 2011, the first year Rep. Price sat on the health subcommittee, he traded no health-related stocks, according to his financial disclosures filed with Congress.
That same year, members were facing public criticism because of a book detailing how they could use inside information and a “60 Minutes” investigation focused on how members and staff could legally use inside information to gain from their own stock trades.
In 2012, President Barack Obama signed the Stop Trading on Congressional Knowledge Act to rein in insider trading by members and require more disclosure. Public watchdog groups suggested at the time that the law would curb the practice.
That year, after his 1-year break in health care trades, Rep. Price resumed investing in health care companies.
Along with investments in technology, financial services, and retail stocks, he also bought and sold stock in companies that could be impacted by actions of his subcommittee, which has a role in determining rates the government pays under the Medicare program.
Health care firms spend heavily to influence members of Congress, lobbying on health matters, funding political campaigns, and seeking favor with Medicare officials who decide how much the program will pay for certain drugs and devices. The Food and Drug Administration holds similar power, approving or putting conditions on drug and device use.
Beyond his personal investments in health care companies, Rep. Price has also advocated their interests in letters to officials and proposed laws, government records show.
In 2012, disclosure records show Rep. Price sold stock in several drug firms, including more than $110,000 worth of Amgen stock. Amgen’s stock price had steadily climbed out of a recession-level slump, but Rep. Price’s sale came a few weeks before the company pleaded guilty to illegally marketing an anemia drug.
By 2013, the health subcommittee was at the center of a major conflict between Medicare, which sets Medicare Advantage rates, and the insurance industry. Medicare issued a notice early that year announcing its intention to reduce Medicare Advantage rates by 2.3 percent as part of a major cost-cutting initiative.
That prompted fierce lobbying by the health insurance industry. Members of Congress, including Rep. Price, wrote a letter to Marilyn Tavenner, then acting administrator for the Centers for Medicare & Medicaid Services, protesting the rate cut, saying the decrease would “disadvantage vulnerable beneficiaries with multiple chronic conditions.”
Ultimately, Medicare decided not to cut rates but instead, to increase them. Yet an hour before Medicare announced the change, a Height Securities analyst fired off a “flash” report to 200 clients that touched off a surge of trading.
The analyst’s report said a political deal was hatched on Capitol Hill to prevent the cuts as a condition for moving forward on Tavenner’s confirmation. Medicare officials increased rates by nearly 4 percent, a change that would positively impact the bottom lines of health insurance companies.
The SEC began looking for the leak’s source, and within weeks, FBI agents began interviewing staffers at the Ways and Means Committee, court records show.
They discovered communications between Sutter and a health care lobbyist. The HHS Inspector General also began a probe, and federal prosecutors briefly examined the matter as well.
As the case unfolded, Rep. Price bought more health care-related stocks, according to his financial disclosures. He has testified that his broker directed all of the trades, except for his investments in Innate Immunotherapeutics, an Australian company partly owned by Rep. Chris Collins (R-N.Y.), according to Collins’ disclosures. An HHS spokesman said Monday that Rep. Price held three broker-directed accounts.
Ethics experts have said that Rep. Price should have further distanced himself by placing his assets in a blind trust.
On April 30, 2013, Rep. Price bought $2,093 worth of stocks in Incyte, a company that develops cancer drugs; $2,076 in Onyx Pharmaceuticals, a drug maker that would soon merge with a larger drug firm; and $2,097 in Parexel International, a consultancy that helps drugs and devices win FDA approval, according to the financial disclosure records.
The same day, Rep. Price shed shares of Express Scripts, a drug management firm, and Danaher, which makes products hospitals and doctor’s offices using for testing and diagnostics. In August of that year, he bought a $2,429 stake in Jazz Pharmaceuticals, which makes sleep and cancer drugs.
On May 6, 2014, the SEC served its first subpoena for the Ways and Means Committee documents. The committee launched a vigorous fight, appealing a federal district judge’s ruling that it should comply with the SEC subpoena.
Rep. Price continued his health stock trades, including $1,000 to $15,000 in drug firms Amgen, Biogen, Bristol-Myers Squibb, Eli Lilly, and Pfizer. He also bought stock in Aetna, a major health insurer, and Athenahealth, which sells electronic medical record and medical billing software. In 2016, he also increased his investment in Innate Immunotherapeutics.
The purchase became controversial because both he and Collins bought stock in a private placement at a discounted price.
“You’re asking for trouble if you have access to nonpublic information about the health care industry and you’re buying and selling health care stocks,” Painter said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Health and Human Services secretary nominee Tom Price showed little restraint in his personal stock trading during the 3 years that federal investigators were bearing down on a key House committee on which the Republican congressman served, a review of his financial disclosures shows.
Rep. Price (Ga.) made dozens of health industry stock trades during a 3-year investigation by the Securities and Exchange Commission that focused on the Ways and Means Committee, according to financial disclosure records he filed with the House of Representatives. The investigation was considered the first test of a law passed to ban members of Congress and their staffs from trading stock based on insider information.
Rep. Price, who is a retired orthopedic surgeon, was never a target of the federal investigation, which scrutinized a top Ways and Means staffer, and no charges were brought. But ethics experts say Rep. Price’s personal trading, even during the thick of federal pressure on his committee, shows he was unconcerned about financial investments that could create an appearance of impropriety.
“He should have known better,” Richard Painter, former White House chief ethics attorney under President George W. Bush and a professor at the University of Minnesota Law School said of Rep. Price’s conduct during the SEC inquiry.
As Rep. Price awaits a Senate vote on his confirmation, Senate Democrats and a number of watchdog groups have asked the SEC to investigate whether Rep. Price engaged in insider trading with some of his trades in health care companies. Rep. Price has said he abided by all ethics rules, although he acknowledged to the Senate Finance Committee that he did not consult the House Ethics Committee on trades that have now become controversial.
The SEC’s inquiry began in 2013, as it battled Ways and Means for documents to develop its case.
A few weeks ago, the day before President Donald Trump’s inauguration, the SEC quietly dropped its pursuit of committee documents without explanation, according to federal court records. No charges were brought against the staffer, Brian Sutter, who is now a health care lobbyist. Sutter’s lawyer declined to comment.
Craig Holman, government affairs lobbyist with Public Citizen, described Rep. Price’s volume of stock trades during the SEC inquiry as “brazen,” given the congressman’s access to nonpublic information affecting the companies’ fortunes.
“The public is seeing this and they really don’t like it,” said Holman, whose watchdog group recently filed complaints about Rep. Price’s stock trading with both the SEC and the Office of Congressional Ethics.
Trump administration officials and Rep. Price have dismissed questions that news reports and lawmakers have raised about stock trades coinciding with official actions to help certain companies, saying Rep. Price’s brokers chose the stocks independently and all of his conduct was transparent.
After acknowledging that he asked his broker to buy stock in an Australian drug company, he told the Senate Finance Committee that he did not direct his broker to make other trades.
“To the best of my knowledge, I have not undertaken such actions,” he wrote in response to finance committee questions. “I have abided by and adhered to all ethics and conflict of interest rules applicable to me.”
An analysis of Rep. Price’s trades shows that he bought health stocks in 2007, the first year Congress financial disclosures are posted online. In 2011, the first year Rep. Price sat on the health subcommittee, he traded no health-related stocks, according to his financial disclosures filed with Congress.
That same year, members were facing public criticism because of a book detailing how they could use inside information and a “60 Minutes” investigation focused on how members and staff could legally use inside information to gain from their own stock trades.
In 2012, President Barack Obama signed the Stop Trading on Congressional Knowledge Act to rein in insider trading by members and require more disclosure. Public watchdog groups suggested at the time that the law would curb the practice.
That year, after his 1-year break in health care trades, Rep. Price resumed investing in health care companies.
Along with investments in technology, financial services, and retail stocks, he also bought and sold stock in companies that could be impacted by actions of his subcommittee, which has a role in determining rates the government pays under the Medicare program.
Health care firms spend heavily to influence members of Congress, lobbying on health matters, funding political campaigns, and seeking favor with Medicare officials who decide how much the program will pay for certain drugs and devices. The Food and Drug Administration holds similar power, approving or putting conditions on drug and device use.
Beyond his personal investments in health care companies, Rep. Price has also advocated their interests in letters to officials and proposed laws, government records show.
In 2012, disclosure records show Rep. Price sold stock in several drug firms, including more than $110,000 worth of Amgen stock. Amgen’s stock price had steadily climbed out of a recession-level slump, but Rep. Price’s sale came a few weeks before the company pleaded guilty to illegally marketing an anemia drug.
By 2013, the health subcommittee was at the center of a major conflict between Medicare, which sets Medicare Advantage rates, and the insurance industry. Medicare issued a notice early that year announcing its intention to reduce Medicare Advantage rates by 2.3 percent as part of a major cost-cutting initiative.
That prompted fierce lobbying by the health insurance industry. Members of Congress, including Rep. Price, wrote a letter to Marilyn Tavenner, then acting administrator for the Centers for Medicare & Medicaid Services, protesting the rate cut, saying the decrease would “disadvantage vulnerable beneficiaries with multiple chronic conditions.”
Ultimately, Medicare decided not to cut rates but instead, to increase them. Yet an hour before Medicare announced the change, a Height Securities analyst fired off a “flash” report to 200 clients that touched off a surge of trading.
The analyst’s report said a political deal was hatched on Capitol Hill to prevent the cuts as a condition for moving forward on Tavenner’s confirmation. Medicare officials increased rates by nearly 4 percent, a change that would positively impact the bottom lines of health insurance companies.
The SEC began looking for the leak’s source, and within weeks, FBI agents began interviewing staffers at the Ways and Means Committee, court records show.
They discovered communications between Sutter and a health care lobbyist. The HHS Inspector General also began a probe, and federal prosecutors briefly examined the matter as well.
As the case unfolded, Rep. Price bought more health care-related stocks, according to his financial disclosures. He has testified that his broker directed all of the trades, except for his investments in Innate Immunotherapeutics, an Australian company partly owned by Rep. Chris Collins (R-N.Y.), according to Collins’ disclosures. An HHS spokesman said Monday that Rep. Price held three broker-directed accounts.
Ethics experts have said that Rep. Price should have further distanced himself by placing his assets in a blind trust.
On April 30, 2013, Rep. Price bought $2,093 worth of stocks in Incyte, a company that develops cancer drugs; $2,076 in Onyx Pharmaceuticals, a drug maker that would soon merge with a larger drug firm; and $2,097 in Parexel International, a consultancy that helps drugs and devices win FDA approval, according to the financial disclosure records.
The same day, Rep. Price shed shares of Express Scripts, a drug management firm, and Danaher, which makes products hospitals and doctor’s offices using for testing and diagnostics. In August of that year, he bought a $2,429 stake in Jazz Pharmaceuticals, which makes sleep and cancer drugs.
On May 6, 2014, the SEC served its first subpoena for the Ways and Means Committee documents. The committee launched a vigorous fight, appealing a federal district judge’s ruling that it should comply with the SEC subpoena.
Rep. Price continued his health stock trades, including $1,000 to $15,000 in drug firms Amgen, Biogen, Bristol-Myers Squibb, Eli Lilly, and Pfizer. He also bought stock in Aetna, a major health insurer, and Athenahealth, which sells electronic medical record and medical billing software. In 2016, he also increased his investment in Innate Immunotherapeutics.
The purchase became controversial because both he and Collins bought stock in a private placement at a discounted price.
“You’re asking for trouble if you have access to nonpublic information about the health care industry and you’re buying and selling health care stocks,” Painter said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Heart disease risk soars in young adults with coronary calcium
Younger adults who have any calcium deposited in their coronary arteries, even a small amount, are at increased risk for adverse coronary heart disease (CHD) outcomes and death, finds an analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
There’s no evidence, however, that treating such patients would make a difference in outcomes, John Jeffrey Carr, MD, reported in JAMA Cardiology on Feb. 8.
In the prospective, community-based, cohort study, 5,115 black and white adults underwent coronary computed tomographic (CT) imaging between the ages of 32 and 46 years, and had a mean follow-up of 12.5 years.
Compared with counterparts not having any coronary artery calcium (CAC), those having at least some had a 5.0-fold increased risk of CHD events and a 1.6-fold increased risk of death (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5493).
Estimates suggested that identification of individuals at elevated risk for developing CAC could inform a selective CT screening strategy whereby the number of younger adults screened could be reduced by half, and the number needing to be imaged to find one person with CAC could be reduced from 3.5 to 2.2.
“The finding that CAC present by ages 32-46 years is associated with increased risk of premature CHD and death emphasizes the need for reduction of risk factors and primordial prevention beginning in early life,” wrote Dr. Carr, professor radiology at Vanderbilt University in Nashville, Tenn.
“Whether any kind of general screening for CAC is warranted needs further study, although we suggest that a strategy in which all individuals aged 32 to 46 years are screened is not indicated. Rather, a more targeted approach based on measuring risk factors in early adult life to predict individuals at high risk for developing CAC in whom the CT scan would have the greatest value can be considered,” they propose.
Study details
Participants were recruited to CARDIA when aged 18-30 years, and they underwent CAC measurement at 15, 20, and 25 years after recruitment. Incident events were ascertained starting from the time of the year-15 scan.
At that year-15 scan, 10.2% of participants were found to have CAC. The geometric mean Agatston score was 21.6.
In adjusted analyses, participants with any CAC had sharply higher risks of CHD events (hazard ratio, 5.0), as well as cardiovascular disease events (HR, 3.0). The risk of CHD events increased with CAC score, with hazard ratios of 2.6, 5.8, and 9.8 for individuals with scores of 1-19, 20-99, and 100 or more, respectively.
In addition, participants with any CAC had an elevated adjusted risk of all-cause mortality (HR, 1.6). This risk similarly rose with score but was significant for those having a score of 100 or greater only (hazard ratio, 3.7); the large majority of deaths in this group were deemed to be from CHD events.
The model that the investigators developed predicted the probability of CAC by ages 32-56 years based on risk factors assessed 7 years apart, between the ages of 18 and 38 years.
When stratified by this model, 4.2% of study participants falling into the lowest-risk decile had CAC, compared with 67.8% of those falling into the highest-risk decile.
Analyses suggested that if screening were restricted to those participants having an above-median risk score, fully 77.3% of all those with coronary calcium and 95.5% of all those with CHD events would be identified. Moreover, these yields would be obtained while reducing the number of individuals recommended to be screened by 50.0%.
Several challenges will need to be addressed before computed tomographic (CT) screening of younger adults for coronary artery calcium is ready for prime time.
First, such screenings must be efficient, and the investigator’s new model seems to be a step in this direction.
The model should be further validated in other populations as well as across younger populations (i.e., during the first CAC test, when the age of the cohort was aged 32-46 years) to help substantiate whether testing of younger individuals is efficient or if waiting to screen those who are older than 40-45 years may be preferable.
Second, even if coronary calcium is detected in young adults, individuals’ risk may not be sufficiently elevated to justify long-term statin therapy.
Finally, there are no data in this context to show that intervening with statins improves cardiovascular outcomes. The absence of such data, and consequently the fact that treatment is often not started until later in life, is owing to the economic and ethical considerations of performing a trial that would take decades to conduct.
In the meantime, the study’s findings have implications for care in younger adults who are found to have coronary calcium incidentally and underscore the importance of primordial prevention.
Future studies will be needed to refine our approaches to better select appropriate candidates for CAC testing, even more so in younger than in older individuals.
Ron Blankstein, MD, of Harvard University, Boston, and Philip Greenland, MD, of Northwestern University, Chicago, made these comments in an accompanying editorial (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5552). They reported having no relevant financial disclosures.
Several challenges will need to be addressed before computed tomographic (CT) screening of younger adults for coronary artery calcium is ready for prime time.
First, such screenings must be efficient, and the investigator’s new model seems to be a step in this direction.
The model should be further validated in other populations as well as across younger populations (i.e., during the first CAC test, when the age of the cohort was aged 32-46 years) to help substantiate whether testing of younger individuals is efficient or if waiting to screen those who are older than 40-45 years may be preferable.
Second, even if coronary calcium is detected in young adults, individuals’ risk may not be sufficiently elevated to justify long-term statin therapy.
Finally, there are no data in this context to show that intervening with statins improves cardiovascular outcomes. The absence of such data, and consequently the fact that treatment is often not started until later in life, is owing to the economic and ethical considerations of performing a trial that would take decades to conduct.
In the meantime, the study’s findings have implications for care in younger adults who are found to have coronary calcium incidentally and underscore the importance of primordial prevention.
Future studies will be needed to refine our approaches to better select appropriate candidates for CAC testing, even more so in younger than in older individuals.
Ron Blankstein, MD, of Harvard University, Boston, and Philip Greenland, MD, of Northwestern University, Chicago, made these comments in an accompanying editorial (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5552). They reported having no relevant financial disclosures.
Several challenges will need to be addressed before computed tomographic (CT) screening of younger adults for coronary artery calcium is ready for prime time.
First, such screenings must be efficient, and the investigator’s new model seems to be a step in this direction.
The model should be further validated in other populations as well as across younger populations (i.e., during the first CAC test, when the age of the cohort was aged 32-46 years) to help substantiate whether testing of younger individuals is efficient or if waiting to screen those who are older than 40-45 years may be preferable.
Second, even if coronary calcium is detected in young adults, individuals’ risk may not be sufficiently elevated to justify long-term statin therapy.
Finally, there are no data in this context to show that intervening with statins improves cardiovascular outcomes. The absence of such data, and consequently the fact that treatment is often not started until later in life, is owing to the economic and ethical considerations of performing a trial that would take decades to conduct.
In the meantime, the study’s findings have implications for care in younger adults who are found to have coronary calcium incidentally and underscore the importance of primordial prevention.
Future studies will be needed to refine our approaches to better select appropriate candidates for CAC testing, even more so in younger than in older individuals.
Ron Blankstein, MD, of Harvard University, Boston, and Philip Greenland, MD, of Northwestern University, Chicago, made these comments in an accompanying editorial (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5552). They reported having no relevant financial disclosures.
Younger adults who have any calcium deposited in their coronary arteries, even a small amount, are at increased risk for adverse coronary heart disease (CHD) outcomes and death, finds an analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
There’s no evidence, however, that treating such patients would make a difference in outcomes, John Jeffrey Carr, MD, reported in JAMA Cardiology on Feb. 8.
In the prospective, community-based, cohort study, 5,115 black and white adults underwent coronary computed tomographic (CT) imaging between the ages of 32 and 46 years, and had a mean follow-up of 12.5 years.
Compared with counterparts not having any coronary artery calcium (CAC), those having at least some had a 5.0-fold increased risk of CHD events and a 1.6-fold increased risk of death (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5493).
Estimates suggested that identification of individuals at elevated risk for developing CAC could inform a selective CT screening strategy whereby the number of younger adults screened could be reduced by half, and the number needing to be imaged to find one person with CAC could be reduced from 3.5 to 2.2.
“The finding that CAC present by ages 32-46 years is associated with increased risk of premature CHD and death emphasizes the need for reduction of risk factors and primordial prevention beginning in early life,” wrote Dr. Carr, professor radiology at Vanderbilt University in Nashville, Tenn.
“Whether any kind of general screening for CAC is warranted needs further study, although we suggest that a strategy in which all individuals aged 32 to 46 years are screened is not indicated. Rather, a more targeted approach based on measuring risk factors in early adult life to predict individuals at high risk for developing CAC in whom the CT scan would have the greatest value can be considered,” they propose.
Study details
Participants were recruited to CARDIA when aged 18-30 years, and they underwent CAC measurement at 15, 20, and 25 years after recruitment. Incident events were ascertained starting from the time of the year-15 scan.
At that year-15 scan, 10.2% of participants were found to have CAC. The geometric mean Agatston score was 21.6.
In adjusted analyses, participants with any CAC had sharply higher risks of CHD events (hazard ratio, 5.0), as well as cardiovascular disease events (HR, 3.0). The risk of CHD events increased with CAC score, with hazard ratios of 2.6, 5.8, and 9.8 for individuals with scores of 1-19, 20-99, and 100 or more, respectively.
In addition, participants with any CAC had an elevated adjusted risk of all-cause mortality (HR, 1.6). This risk similarly rose with score but was significant for those having a score of 100 or greater only (hazard ratio, 3.7); the large majority of deaths in this group were deemed to be from CHD events.
The model that the investigators developed predicted the probability of CAC by ages 32-56 years based on risk factors assessed 7 years apart, between the ages of 18 and 38 years.
When stratified by this model, 4.2% of study participants falling into the lowest-risk decile had CAC, compared with 67.8% of those falling into the highest-risk decile.
Analyses suggested that if screening were restricted to those participants having an above-median risk score, fully 77.3% of all those with coronary calcium and 95.5% of all those with CHD events would be identified. Moreover, these yields would be obtained while reducing the number of individuals recommended to be screened by 50.0%.
Younger adults who have any calcium deposited in their coronary arteries, even a small amount, are at increased risk for adverse coronary heart disease (CHD) outcomes and death, finds an analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
There’s no evidence, however, that treating such patients would make a difference in outcomes, John Jeffrey Carr, MD, reported in JAMA Cardiology on Feb. 8.
In the prospective, community-based, cohort study, 5,115 black and white adults underwent coronary computed tomographic (CT) imaging between the ages of 32 and 46 years, and had a mean follow-up of 12.5 years.
Compared with counterparts not having any coronary artery calcium (CAC), those having at least some had a 5.0-fold increased risk of CHD events and a 1.6-fold increased risk of death (JAMA Cardiol. 2017 Feb 8; doi: 10.1001/jamacardio.2016.5493).
Estimates suggested that identification of individuals at elevated risk for developing CAC could inform a selective CT screening strategy whereby the number of younger adults screened could be reduced by half, and the number needing to be imaged to find one person with CAC could be reduced from 3.5 to 2.2.
“The finding that CAC present by ages 32-46 years is associated with increased risk of premature CHD and death emphasizes the need for reduction of risk factors and primordial prevention beginning in early life,” wrote Dr. Carr, professor radiology at Vanderbilt University in Nashville, Tenn.
“Whether any kind of general screening for CAC is warranted needs further study, although we suggest that a strategy in which all individuals aged 32 to 46 years are screened is not indicated. Rather, a more targeted approach based on measuring risk factors in early adult life to predict individuals at high risk for developing CAC in whom the CT scan would have the greatest value can be considered,” they propose.
Study details
Participants were recruited to CARDIA when aged 18-30 years, and they underwent CAC measurement at 15, 20, and 25 years after recruitment. Incident events were ascertained starting from the time of the year-15 scan.
At that year-15 scan, 10.2% of participants were found to have CAC. The geometric mean Agatston score was 21.6.
In adjusted analyses, participants with any CAC had sharply higher risks of CHD events (hazard ratio, 5.0), as well as cardiovascular disease events (HR, 3.0). The risk of CHD events increased with CAC score, with hazard ratios of 2.6, 5.8, and 9.8 for individuals with scores of 1-19, 20-99, and 100 or more, respectively.
In addition, participants with any CAC had an elevated adjusted risk of all-cause mortality (HR, 1.6). This risk similarly rose with score but was significant for those having a score of 100 or greater only (hazard ratio, 3.7); the large majority of deaths in this group were deemed to be from CHD events.
The model that the investigators developed predicted the probability of CAC by ages 32-56 years based on risk factors assessed 7 years apart, between the ages of 18 and 38 years.
When stratified by this model, 4.2% of study participants falling into the lowest-risk decile had CAC, compared with 67.8% of those falling into the highest-risk decile.
Analyses suggested that if screening were restricted to those participants having an above-median risk score, fully 77.3% of all those with coronary calcium and 95.5% of all those with CHD events would be identified. Moreover, these yields would be obtained while reducing the number of individuals recommended to be screened by 50.0%.
FROM JAMA CARDIOLOGY
Key clinical point:
Major finding: Individuals having any versus no coronary artery calcium when aged 32-46 years had elevated risks of CHD events (HR, 5.0) and death (HR, 1.6) by the age of 58 years.
Data source: A prospective community-based cohort study of 5,115 black and white adults (CARDIA Study).
Disclosures: Dr. Carr disclosed that he had no relevant conflicts of interest.
Tips for Living With Tardive Dyskinesia
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
Locum tenens physicians more popular than ever
Use of locum tenens physicians reached a new high in 2016, according to an annual survey by Staff Care, a health care staffing company.
Last year, 94% of hospitals, medical groups, and other health care facilities reported using temporary physicians, compared with 91% in 2014, which was the previous high, Staff Care reported in its “2017 Survey of Temporary Physician Staffing Trends.”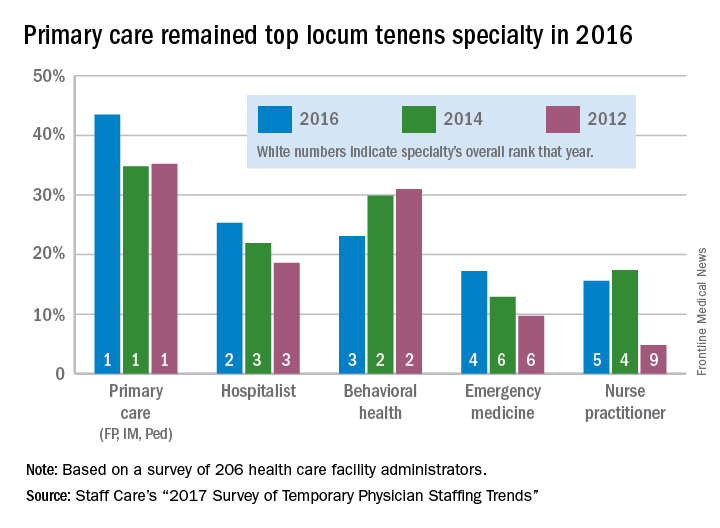
Primary care physicians (family physicians, internists, and pediatricians) were the leading locum tenens choice by specialty, with 43.5% of health care facilities reporting their use in 2016. Hospitalists were the next most popular specialists at 25%, followed by behavioral health professionals (23%), emergency physicians (17%), and nurse practitioners (16%), according to survey responses from 206 administrators of health care facilities.
Since about one-third of U.S. physicians practice primary care, “it is not particularly surprising that they are more utilized as locum tenens,” the report noted, but “only about 3.5% of all physicians are psychiatrists, [so] the fact that behavioral health professionals are the third most utilized type of locum tenens provider underlines the acute shortage of providers in this field.”
Use of locum tenens physicians reached a new high in 2016, according to an annual survey by Staff Care, a health care staffing company.
Last year, 94% of hospitals, medical groups, and other health care facilities reported using temporary physicians, compared with 91% in 2014, which was the previous high, Staff Care reported in its “2017 Survey of Temporary Physician Staffing Trends.”
Primary care physicians (family physicians, internists, and pediatricians) were the leading locum tenens choice by specialty, with 43.5% of health care facilities reporting their use in 2016. Hospitalists were the next most popular specialists at 25%, followed by behavioral health professionals (23%), emergency physicians (17%), and nurse practitioners (16%), according to survey responses from 206 administrators of health care facilities.
Since about one-third of U.S. physicians practice primary care, “it is not particularly surprising that they are more utilized as locum tenens,” the report noted, but “only about 3.5% of all physicians are psychiatrists, [so] the fact that behavioral health professionals are the third most utilized type of locum tenens provider underlines the acute shortage of providers in this field.”
Use of locum tenens physicians reached a new high in 2016, according to an annual survey by Staff Care, a health care staffing company.
Last year, 94% of hospitals, medical groups, and other health care facilities reported using temporary physicians, compared with 91% in 2014, which was the previous high, Staff Care reported in its “2017 Survey of Temporary Physician Staffing Trends.”
Primary care physicians (family physicians, internists, and pediatricians) were the leading locum tenens choice by specialty, with 43.5% of health care facilities reporting their use in 2016. Hospitalists were the next most popular specialists at 25%, followed by behavioral health professionals (23%), emergency physicians (17%), and nurse practitioners (16%), according to survey responses from 206 administrators of health care facilities.
Since about one-third of U.S. physicians practice primary care, “it is not particularly surprising that they are more utilized as locum tenens,” the report noted, but “only about 3.5% of all physicians are psychiatrists, [so] the fact that behavioral health professionals are the third most utilized type of locum tenens provider underlines the acute shortage of providers in this field.”
Subcutaneous high-dose methotrexate controls psoriasis
Subcutaneous high-dose methotrexate can be safely initiated in people with moderate to severe psoriasis, and produces a rapid and sustained response, researchers found.
Although methotrexate is a first-line agent in moderate to severe psoriasis, and is considerably cheaper than biological agents, much remains unknown about its ideal dosage and route of administration.
Authors of a 2016 systematic review noted that, despite the fact that methotrexate has been used for more than 50 years in psoriasis, high-quality trial evidence remains wanting (PLoS One 2016 May 11. doi: 10.1371/journal.pone.0153740). Recent, well-designed trials have compared methotrexate to biological drugs used in psoriasis rather than placebo. These studies also have used oral formulations of methotrexate, in a range of starting doses as low as 5 mg, rather than subcutaneous formulations.
In their 52-week, multicenter trial conducted across 13 study sites in Europe, Dr. Warren and his colleagues randomized 120 patients to subcutaneous methotrexate at a dose of 17.5 mg/week (n = 91) or sham injections (n = 29) for 16 weeks. Patients in the intervention arm who did not achieve at least 50% improvement on the baseline Psoriasis Area and Severity Index (PASI) score at 8 weeks were increased to 22.5 mg methotrexate per week; 31% received this dose increase.
The study’s primary endpoint was reduction of the PASI score by 75% or more at 16 weeks, which 41% of the intervention arm achieved, compared with 10% of patients in the placebo arm (relative risk 3.93, P = .0026). After 16 weeks, all patients in the cohort were converted to open-label methotrexate for the remainder of the trial, following the same dosing schedule of between 17.5 and 22.5 mg, depending on response at 8 weeks after initiation.
At week 52, PASI 75 response rates were 45% in the methotrexate-methotrexate group and 34% in the placebo-methotrexate group. This compared favorably, the researchers wrote, with a previous study in which the PASI 75 response rate at week 52 was 24% with oral methotrexate at doses of up to 25 mg per week.
No serious adverse events were associated with methotrexate, although gastrointestinal problems (mostly nausea) and elevated liver enzymes were more common in patients receiving the treatment.
“Our findings encourage the use of subcutaneous methotrexate for treatment of psoriasis, and suggest long-term clinical outcomes better than previously reported for oral administration, although final confirmation will be needed in a direct head-to-head trial of subcutaneous versus oral dosing. Our findings might also help to guide future recommendations for the optimum dosing of methotrexate,” the investigators wrote.
Medac Pharma funded the study. Dr. Warren and six of his coauthors disclosed financial relationships with multiple pharmaceutical firms, including the study sponsor, while three coauthors declared no financial conflicts of interest.
The results from this study compare favorably with those of a previous 52-week study of oral methotrexate in this population, suggesting that subcutaneous administration is superior to oral administration in the management of psoriasis. However, response rates for methotrexate are still lower than those reported with biological therapy, especially with infliximab, adalimumab, ustekinumab, and, more recently, the anti-interleukin-17 drugs secukinumab and ixekizumab.
The question that remains is whether methotrexate should remain the first-line systemic therapy for moderate to severe psoriasis. Because we now know that psoriasis is not just skin deep, and that many of the comorbidities – including psoriatic arthritis, metabolic syndrome, and cardiovascular events, in addition to premature death – are related to the extent of skin involvement, perhaps drugs that effectively control inflammation should be used initially. This approach could be addressed only via long-term observations of prospective studies of patients treated with methotrexate, compared with those treated with biological therapy, with collection of information not only about clinical improvement of skin disease, but also about comorbidities.
Dafna D. Gladman, MD, is director of the psoriatic arthritis program at the Centre for Prognosis Studies in The Rheumatic Diseases at Toronto Western Hospital. This comment was excerpted and modified from an editorial (Lancet. 2017;389[10068]:482-3). that accompanied the study by Warren et al. Dr. Gladman disclosed financial relationships, mostly grants and fees related to clinical trials, with several pharmaceutical manufacturers.
The results from this study compare favorably with those of a previous 52-week study of oral methotrexate in this population, suggesting that subcutaneous administration is superior to oral administration in the management of psoriasis. However, response rates for methotrexate are still lower than those reported with biological therapy, especially with infliximab, adalimumab, ustekinumab, and, more recently, the anti-interleukin-17 drugs secukinumab and ixekizumab.
The question that remains is whether methotrexate should remain the first-line systemic therapy for moderate to severe psoriasis. Because we now know that psoriasis is not just skin deep, and that many of the comorbidities – including psoriatic arthritis, metabolic syndrome, and cardiovascular events, in addition to premature death – are related to the extent of skin involvement, perhaps drugs that effectively control inflammation should be used initially. This approach could be addressed only via long-term observations of prospective studies of patients treated with methotrexate, compared with those treated with biological therapy, with collection of information not only about clinical improvement of skin disease, but also about comorbidities.
Dafna D. Gladman, MD, is director of the psoriatic arthritis program at the Centre for Prognosis Studies in The Rheumatic Diseases at Toronto Western Hospital. This comment was excerpted and modified from an editorial (Lancet. 2017;389[10068]:482-3). that accompanied the study by Warren et al. Dr. Gladman disclosed financial relationships, mostly grants and fees related to clinical trials, with several pharmaceutical manufacturers.
The results from this study compare favorably with those of a previous 52-week study of oral methotrexate in this population, suggesting that subcutaneous administration is superior to oral administration in the management of psoriasis. However, response rates for methotrexate are still lower than those reported with biological therapy, especially with infliximab, adalimumab, ustekinumab, and, more recently, the anti-interleukin-17 drugs secukinumab and ixekizumab.
The question that remains is whether methotrexate should remain the first-line systemic therapy for moderate to severe psoriasis. Because we now know that psoriasis is not just skin deep, and that many of the comorbidities – including psoriatic arthritis, metabolic syndrome, and cardiovascular events, in addition to premature death – are related to the extent of skin involvement, perhaps drugs that effectively control inflammation should be used initially. This approach could be addressed only via long-term observations of prospective studies of patients treated with methotrexate, compared with those treated with biological therapy, with collection of information not only about clinical improvement of skin disease, but also about comorbidities.
Dafna D. Gladman, MD, is director of the psoriatic arthritis program at the Centre for Prognosis Studies in The Rheumatic Diseases at Toronto Western Hospital. This comment was excerpted and modified from an editorial (Lancet. 2017;389[10068]:482-3). that accompanied the study by Warren et al. Dr. Gladman disclosed financial relationships, mostly grants and fees related to clinical trials, with several pharmaceutical manufacturers.
Subcutaneous high-dose methotrexate can be safely initiated in people with moderate to severe psoriasis, and produces a rapid and sustained response, researchers found.
Although methotrexate is a first-line agent in moderate to severe psoriasis, and is considerably cheaper than biological agents, much remains unknown about its ideal dosage and route of administration.
Authors of a 2016 systematic review noted that, despite the fact that methotrexate has been used for more than 50 years in psoriasis, high-quality trial evidence remains wanting (PLoS One 2016 May 11. doi: 10.1371/journal.pone.0153740). Recent, well-designed trials have compared methotrexate to biological drugs used in psoriasis rather than placebo. These studies also have used oral formulations of methotrexate, in a range of starting doses as low as 5 mg, rather than subcutaneous formulations.
In their 52-week, multicenter trial conducted across 13 study sites in Europe, Dr. Warren and his colleagues randomized 120 patients to subcutaneous methotrexate at a dose of 17.5 mg/week (n = 91) or sham injections (n = 29) for 16 weeks. Patients in the intervention arm who did not achieve at least 50% improvement on the baseline Psoriasis Area and Severity Index (PASI) score at 8 weeks were increased to 22.5 mg methotrexate per week; 31% received this dose increase.
The study’s primary endpoint was reduction of the PASI score by 75% or more at 16 weeks, which 41% of the intervention arm achieved, compared with 10% of patients in the placebo arm (relative risk 3.93, P = .0026). After 16 weeks, all patients in the cohort were converted to open-label methotrexate for the remainder of the trial, following the same dosing schedule of between 17.5 and 22.5 mg, depending on response at 8 weeks after initiation.
At week 52, PASI 75 response rates were 45% in the methotrexate-methotrexate group and 34% in the placebo-methotrexate group. This compared favorably, the researchers wrote, with a previous study in which the PASI 75 response rate at week 52 was 24% with oral methotrexate at doses of up to 25 mg per week.
No serious adverse events were associated with methotrexate, although gastrointestinal problems (mostly nausea) and elevated liver enzymes were more common in patients receiving the treatment.
“Our findings encourage the use of subcutaneous methotrexate for treatment of psoriasis, and suggest long-term clinical outcomes better than previously reported for oral administration, although final confirmation will be needed in a direct head-to-head trial of subcutaneous versus oral dosing. Our findings might also help to guide future recommendations for the optimum dosing of methotrexate,” the investigators wrote.
Medac Pharma funded the study. Dr. Warren and six of his coauthors disclosed financial relationships with multiple pharmaceutical firms, including the study sponsor, while three coauthors declared no financial conflicts of interest.
Subcutaneous high-dose methotrexate can be safely initiated in people with moderate to severe psoriasis, and produces a rapid and sustained response, researchers found.
Although methotrexate is a first-line agent in moderate to severe psoriasis, and is considerably cheaper than biological agents, much remains unknown about its ideal dosage and route of administration.
Authors of a 2016 systematic review noted that, despite the fact that methotrexate has been used for more than 50 years in psoriasis, high-quality trial evidence remains wanting (PLoS One 2016 May 11. doi: 10.1371/journal.pone.0153740). Recent, well-designed trials have compared methotrexate to biological drugs used in psoriasis rather than placebo. These studies also have used oral formulations of methotrexate, in a range of starting doses as low as 5 mg, rather than subcutaneous formulations.
In their 52-week, multicenter trial conducted across 13 study sites in Europe, Dr. Warren and his colleagues randomized 120 patients to subcutaneous methotrexate at a dose of 17.5 mg/week (n = 91) or sham injections (n = 29) for 16 weeks. Patients in the intervention arm who did not achieve at least 50% improvement on the baseline Psoriasis Area and Severity Index (PASI) score at 8 weeks were increased to 22.5 mg methotrexate per week; 31% received this dose increase.
The study’s primary endpoint was reduction of the PASI score by 75% or more at 16 weeks, which 41% of the intervention arm achieved, compared with 10% of patients in the placebo arm (relative risk 3.93, P = .0026). After 16 weeks, all patients in the cohort were converted to open-label methotrexate for the remainder of the trial, following the same dosing schedule of between 17.5 and 22.5 mg, depending on response at 8 weeks after initiation.
At week 52, PASI 75 response rates were 45% in the methotrexate-methotrexate group and 34% in the placebo-methotrexate group. This compared favorably, the researchers wrote, with a previous study in which the PASI 75 response rate at week 52 was 24% with oral methotrexate at doses of up to 25 mg per week.
No serious adverse events were associated with methotrexate, although gastrointestinal problems (mostly nausea) and elevated liver enzymes were more common in patients receiving the treatment.
“Our findings encourage the use of subcutaneous methotrexate for treatment of psoriasis, and suggest long-term clinical outcomes better than previously reported for oral administration, although final confirmation will be needed in a direct head-to-head trial of subcutaneous versus oral dosing. Our findings might also help to guide future recommendations for the optimum dosing of methotrexate,” the investigators wrote.
Medac Pharma funded the study. Dr. Warren and six of his coauthors disclosed financial relationships with multiple pharmaceutical firms, including the study sponsor, while three coauthors declared no financial conflicts of interest.
FROM THE LANCET
Key clinical point:
Major finding: At 16 weeks, 41% of patients started on methotrexate achieved a 75% reduction in psoriasis severity scores, compared with 10% of patients in the placebo arm. At 1 year of treatment, 45% of patients saw this level of response.
Data source: A multisite, placebo-controlled trial randomizing 120 patients to subcutaneous methotrexate or placebo for 16 weeks, then converting all patients to open-label subcutaneous methotrexate through week 52.
Disclosures: Medac Pharma funded the study. Dr. Warren and six of his coauthors disclosed financial relationships with multiple pharmaceutical firms, including the study sponsor, while three coauthors declared no financial conflicts of interest.
HIV research update: Late January 2017
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Researchers demonstrated the safety and activity of the HIV-neutralizing monoclonal antibody 10-1074 in humans and said their study supports the idea that antibodies targeting the V3 glycan supersite might be useful for the treatment and prevention of HIV-1 infection.
Efforts to support HIV disclosure (such as counseling) may be needed for TB-HIV coinfected patients, particularly for women and those unaware of their partners’ status, a study found.
Elevated levels of procoagulants may exert a particularly detrimental effect on neurocognitive functioning among older HIV-infected persons, according to a study in the journal AIDS.
A recent study found that a combination of the anti-HIV drugs tenofovir, lopinavir, and ritonavir in a lipid-stabilized nanosuspension enhanced and sustained intracellular drug levels and exposures in lymph node and blood cells above those in plasma.
Hepatitis B virus infection continues to be acquired in adulthood among HIV-positive Ugandans, but HBV incidence is dramatically reduced with HBV-active antiretroviral therapy, a study found.
In antiretroviral therapy-naive Ugandan children, insulin resistance changed significantly after 48 weeks of ART and correlated with monocyte activation, according to a study in the Pediatric Infectious Disease Journal.
Oxidative stress is associated with a higher risk of serious non-AIDS events in HIV patients, including non-AIDS deaths, according to a study in JAIDS. The authors said the effect is independent and additive to biomarkers of inflammation, monocyte activation and coagulation.
A study at a California teaching hospital found the yield of opt-out HIV rapid antibody screening in inpatients was comparable to the national HIV prevalence average. However, uptake of screening was markedly limited where opt-out screening was delivered by physicians during routine care, with limited time resources being the major barrier.
Variations within fat mass and the obesity-associated (FTO) gene may be predictors of fatty liver disease in HIV-infected patients independently of metabolic factors, according to a study in HIV Medicine.
After adjustment for sex, years of HIV experience, and patient caseload, providing primary care to HIV-infected patients remained associated with delivering comprehensive reproductive health counseling, according to a study in AIDS Care.
A recent study found that treatment dose nevirapine-based combination antiretroviral therapy was generally well tolerated by HIV-infected neonates, and associated with normalization of trough levels over time in most cases without dose adjustment.
Immune function may be impacted by various forms of psychological stress in HIV-positive women, a study found, and investigators said interventions that target stress reduction may be useful in improving immune parameters and quality of life.
Annual HIV incidence among men who have sex with men attending STI clinics in England is high, a study in HIV Medicine revealed, and previous STIs were predictors of HIV acquisition but accounted only for one in five infections.
A study in Public Health Reports found that the prevalence of HIV testing provision at organizations serving young people in Baltimore was low, and few organizations offered linkages to HIV testing.
A study in Hepatology found that eradication of hepatitis C virus infection in HIV/HCV coinfected patients is associated not only with a reduction in the frequency of death, HIV progression, and liver-related events, but also with a reduced hazard of diabetes mellitus and possibly of chronic renal failure.
A recent study found modest but significant improvements in self-assessed quality of life among HIV patients initiating antiretroviral therapy immediately compared to deferring treatment, supporting patient-perceived health benefits of initiating ART as soon as possible after an HIV diagnosis.
Distinct mechanisms may underlie cortical and subcortical injury in people with HIV, a study in JAIDS found, suggesting the potential importance of early initiation of HAART to protect long-term brain health.
A three-hospital study found that an EMR prompt for hospitalized patients was associated with a large increase in HIV testing, a diversification of patients tested, and an increase in diagnoses made by screening.
[email protected]
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Researchers demonstrated the safety and activity of the HIV-neutralizing monoclonal antibody 10-1074 in humans and said their study supports the idea that antibodies targeting the V3 glycan supersite might be useful for the treatment and prevention of HIV-1 infection.
Efforts to support HIV disclosure (such as counseling) may be needed for TB-HIV coinfected patients, particularly for women and those unaware of their partners’ status, a study found.
Elevated levels of procoagulants may exert a particularly detrimental effect on neurocognitive functioning among older HIV-infected persons, according to a study in the journal AIDS.
A recent study found that a combination of the anti-HIV drugs tenofovir, lopinavir, and ritonavir in a lipid-stabilized nanosuspension enhanced and sustained intracellular drug levels and exposures in lymph node and blood cells above those in plasma.
Hepatitis B virus infection continues to be acquired in adulthood among HIV-positive Ugandans, but HBV incidence is dramatically reduced with HBV-active antiretroviral therapy, a study found.
In antiretroviral therapy-naive Ugandan children, insulin resistance changed significantly after 48 weeks of ART and correlated with monocyte activation, according to a study in the Pediatric Infectious Disease Journal.
Oxidative stress is associated with a higher risk of serious non-AIDS events in HIV patients, including non-AIDS deaths, according to a study in JAIDS. The authors said the effect is independent and additive to biomarkers of inflammation, monocyte activation and coagulation.
A study at a California teaching hospital found the yield of opt-out HIV rapid antibody screening in inpatients was comparable to the national HIV prevalence average. However, uptake of screening was markedly limited where opt-out screening was delivered by physicians during routine care, with limited time resources being the major barrier.
Variations within fat mass and the obesity-associated (FTO) gene may be predictors of fatty liver disease in HIV-infected patients independently of metabolic factors, according to a study in HIV Medicine.
After adjustment for sex, years of HIV experience, and patient caseload, providing primary care to HIV-infected patients remained associated with delivering comprehensive reproductive health counseling, according to a study in AIDS Care.
A recent study found that treatment dose nevirapine-based combination antiretroviral therapy was generally well tolerated by HIV-infected neonates, and associated with normalization of trough levels over time in most cases without dose adjustment.
Immune function may be impacted by various forms of psychological stress in HIV-positive women, a study found, and investigators said interventions that target stress reduction may be useful in improving immune parameters and quality of life.
Annual HIV incidence among men who have sex with men attending STI clinics in England is high, a study in HIV Medicine revealed, and previous STIs were predictors of HIV acquisition but accounted only for one in five infections.
A study in Public Health Reports found that the prevalence of HIV testing provision at organizations serving young people in Baltimore was low, and few organizations offered linkages to HIV testing.
A study in Hepatology found that eradication of hepatitis C virus infection in HIV/HCV coinfected patients is associated not only with a reduction in the frequency of death, HIV progression, and liver-related events, but also with a reduced hazard of diabetes mellitus and possibly of chronic renal failure.
A recent study found modest but significant improvements in self-assessed quality of life among HIV patients initiating antiretroviral therapy immediately compared to deferring treatment, supporting patient-perceived health benefits of initiating ART as soon as possible after an HIV diagnosis.
Distinct mechanisms may underlie cortical and subcortical injury in people with HIV, a study in JAIDS found, suggesting the potential importance of early initiation of HAART to protect long-term brain health.
A three-hospital study found that an EMR prompt for hospitalized patients was associated with a large increase in HIV testing, a diversification of patients tested, and an increase in diagnoses made by screening.
[email protected]
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Researchers demonstrated the safety and activity of the HIV-neutralizing monoclonal antibody 10-1074 in humans and said their study supports the idea that antibodies targeting the V3 glycan supersite might be useful for the treatment and prevention of HIV-1 infection.
Efforts to support HIV disclosure (such as counseling) may be needed for TB-HIV coinfected patients, particularly for women and those unaware of their partners’ status, a study found.
Elevated levels of procoagulants may exert a particularly detrimental effect on neurocognitive functioning among older HIV-infected persons, according to a study in the journal AIDS.
A recent study found that a combination of the anti-HIV drugs tenofovir, lopinavir, and ritonavir in a lipid-stabilized nanosuspension enhanced and sustained intracellular drug levels and exposures in lymph node and blood cells above those in plasma.
Hepatitis B virus infection continues to be acquired in adulthood among HIV-positive Ugandans, but HBV incidence is dramatically reduced with HBV-active antiretroviral therapy, a study found.
In antiretroviral therapy-naive Ugandan children, insulin resistance changed significantly after 48 weeks of ART and correlated with monocyte activation, according to a study in the Pediatric Infectious Disease Journal.
Oxidative stress is associated with a higher risk of serious non-AIDS events in HIV patients, including non-AIDS deaths, according to a study in JAIDS. The authors said the effect is independent and additive to biomarkers of inflammation, monocyte activation and coagulation.
A study at a California teaching hospital found the yield of opt-out HIV rapid antibody screening in inpatients was comparable to the national HIV prevalence average. However, uptake of screening was markedly limited where opt-out screening was delivered by physicians during routine care, with limited time resources being the major barrier.
Variations within fat mass and the obesity-associated (FTO) gene may be predictors of fatty liver disease in HIV-infected patients independently of metabolic factors, according to a study in HIV Medicine.
After adjustment for sex, years of HIV experience, and patient caseload, providing primary care to HIV-infected patients remained associated with delivering comprehensive reproductive health counseling, according to a study in AIDS Care.
A recent study found that treatment dose nevirapine-based combination antiretroviral therapy was generally well tolerated by HIV-infected neonates, and associated with normalization of trough levels over time in most cases without dose adjustment.
Immune function may be impacted by various forms of psychological stress in HIV-positive women, a study found, and investigators said interventions that target stress reduction may be useful in improving immune parameters and quality of life.
Annual HIV incidence among men who have sex with men attending STI clinics in England is high, a study in HIV Medicine revealed, and previous STIs were predictors of HIV acquisition but accounted only for one in five infections.
A study in Public Health Reports found that the prevalence of HIV testing provision at organizations serving young people in Baltimore was low, and few organizations offered linkages to HIV testing.
A study in Hepatology found that eradication of hepatitis C virus infection in HIV/HCV coinfected patients is associated not only with a reduction in the frequency of death, HIV progression, and liver-related events, but also with a reduced hazard of diabetes mellitus and possibly of chronic renal failure.
A recent study found modest but significant improvements in self-assessed quality of life among HIV patients initiating antiretroviral therapy immediately compared to deferring treatment, supporting patient-perceived health benefits of initiating ART as soon as possible after an HIV diagnosis.
Distinct mechanisms may underlie cortical and subcortical injury in people with HIV, a study in JAIDS found, suggesting the potential importance of early initiation of HAART to protect long-term brain health.
A three-hospital study found that an EMR prompt for hospitalized patients was associated with a large increase in HIV testing, a diversification of patients tested, and an increase in diagnoses made by screening.
[email protected]
On Twitter @richpizzi
Hypertensive disease of pregnancy linked to earlier mortality
LAS VEGAS – Women who develop any form of hypertensive disease during pregnancy have a significantly increased mortality rate until they reach age 50, compared with women without the condition.
The findings, presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine, are based on a review of more than 2 million mothers who delivered babies in Utah during 1939-2012.
The relatively increased mortality rate linked with hypertensive disease of pregnancy (HDP) reached its peak during the first decade immediately following the index delivery and was dramatically higher in women for whom the index HDP was preceded by at least one earlier HDP. Increased mortality was especially elevated for certain types of deaths including stroke, diabetes, circulatory disease, and ischemic heart disease, said Lauren Theilen, MD, a maternal-fetal medicine researcher at the University of Utah in Salt Lake City.
She calculated that during the decade following an index case of HDP, life expectancy among mothers who had two or more HDP-affected pregnancies was about 49 years, life expectancy among women with a history of one HDP was 52 years, and postpartum women without HDP had a life expectancy of 55 years.
The data came from 2,083,331 singleton pregnancies delivered in Utah during 1939-2012 where the mother remained in Utah for at least 1 year following delivery. From this group, Dr. Theilen and her associates identified 67,384 women (3%) with HDP, including 49,598 women without a prior history of HDP and 7,786 with at least one prior HDP pregnancy. They included four different diagnoses as HDP: gestational hypertension, preeclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count), or eclampsia.
The analysis excluded women with chronic hypertension, antiphospholipid syndrome, pregestational diabetes, or chronic kidney disease. It also excluded women who died within a year of the index delivery. For each of the included 67,384 cases with HDP, the researchers selected two controls with a delivery unaffected by HDP and matched them to a case by age, year of childbirth, and parity.
The women in the study were 26 years old on average. Gestational age at delivery among the HDP cases averaged 1 week less than among the controls, 37.8 weeks compared with 38.9 weeks, a statistically significant difference. Average birth weight also differed by a significant amount, 3,079 grams in the HDP neonates and 3,319 grams in the control newborns. During follow-up, 8% of the HDP women died, compared with 6% of the control women.
Analysis showed that relative mortality rates in HDP women were especially elevated for certain types of death: endocrine and metabolic, circulatory, genitourinary, infectious disease, and digestive. In most types of death, the increased risk linked with HDP was markedly higher in the women with recurrent HDP.
Dr. Theilen reported the relative risks of death for certain specific mortality causes (Am J Obstet Gynecol. 2017 Jan;216[1][suppl]:S32-3). In women with a single index case of HDP, the mortality rate compared to women with no HDP was threefold higher for diabetes, twofold higher for ischemic heart disease, and 85% higher for stroke. In women with recurrent HDP, the relative mortality risks were fourfold higher for diabetes, threefold higher for ischemic heart disease, and fivefold higher for stroke.
For all causes of death except stroke, the increased relative risk from HDP existed only for women younger than 51 years. Once HDP women passed the age of 50, their excess mortality risk was substantially muted, even in women with recurrent HDP. But for stroke mortality, the added risk persisted among older women with a history of at least two HDPs. In this subgroup, stroke deaths were nearly fourfold higher than in the matched controls.
Dr. Theilen reported having no financial disclosures.
[email protected]
On Twitter @mitchelzoler
The findings Dr. Theilen and her associates made in this study confirm results reported several decades ago that first established a link between hypertensive disease of pregnancy (HDP) and significantly reduced life expectancy. We have known for some time that HDP identifies women with a long-term mortality risk. The real question is: Can we ameliorate the risk linked with HDP?
We know that HDP requires acute management, but we are not sure how to best manage these women once they have delivered. To address that, we need prospective studies.
Not all women who experience HDP receive appropriate follow-up after delivery. We need to ensure a better handoff of women who developed HDP from the physicians who cared for these women during pregnancy to the physicians who care for these women postpartum and during the balance of their life. Ideally, a primary care physician should closely follow and manage blood pressure and other risk factors of HDP women once they are no longer pregnant.
Mary E. D’Alton, MD , is professor and chair of ob.gyn. at Columbia University Medical Center in New York City. She is on the advisory board of Merck for Mothers. She made these comments in an interview.
The findings Dr. Theilen and her associates made in this study confirm results reported several decades ago that first established a link between hypertensive disease of pregnancy (HDP) and significantly reduced life expectancy. We have known for some time that HDP identifies women with a long-term mortality risk. The real question is: Can we ameliorate the risk linked with HDP?
We know that HDP requires acute management, but we are not sure how to best manage these women once they have delivered. To address that, we need prospective studies.
Not all women who experience HDP receive appropriate follow-up after delivery. We need to ensure a better handoff of women who developed HDP from the physicians who cared for these women during pregnancy to the physicians who care for these women postpartum and during the balance of their life. Ideally, a primary care physician should closely follow and manage blood pressure and other risk factors of HDP women once they are no longer pregnant.
Mary E. D’Alton, MD , is professor and chair of ob.gyn. at Columbia University Medical Center in New York City. She is on the advisory board of Merck for Mothers. She made these comments in an interview.
The findings Dr. Theilen and her associates made in this study confirm results reported several decades ago that first established a link between hypertensive disease of pregnancy (HDP) and significantly reduced life expectancy. We have known for some time that HDP identifies women with a long-term mortality risk. The real question is: Can we ameliorate the risk linked with HDP?
We know that HDP requires acute management, but we are not sure how to best manage these women once they have delivered. To address that, we need prospective studies.
Not all women who experience HDP receive appropriate follow-up after delivery. We need to ensure a better handoff of women who developed HDP from the physicians who cared for these women during pregnancy to the physicians who care for these women postpartum and during the balance of their life. Ideally, a primary care physician should closely follow and manage blood pressure and other risk factors of HDP women once they are no longer pregnant.
Mary E. D’Alton, MD , is professor and chair of ob.gyn. at Columbia University Medical Center in New York City. She is on the advisory board of Merck for Mothers. She made these comments in an interview.
LAS VEGAS – Women who develop any form of hypertensive disease during pregnancy have a significantly increased mortality rate until they reach age 50, compared with women without the condition.
The findings, presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine, are based on a review of more than 2 million mothers who delivered babies in Utah during 1939-2012.
The relatively increased mortality rate linked with hypertensive disease of pregnancy (HDP) reached its peak during the first decade immediately following the index delivery and was dramatically higher in women for whom the index HDP was preceded by at least one earlier HDP. Increased mortality was especially elevated for certain types of deaths including stroke, diabetes, circulatory disease, and ischemic heart disease, said Lauren Theilen, MD, a maternal-fetal medicine researcher at the University of Utah in Salt Lake City.
She calculated that during the decade following an index case of HDP, life expectancy among mothers who had two or more HDP-affected pregnancies was about 49 years, life expectancy among women with a history of one HDP was 52 years, and postpartum women without HDP had a life expectancy of 55 years.
The data came from 2,083,331 singleton pregnancies delivered in Utah during 1939-2012 where the mother remained in Utah for at least 1 year following delivery. From this group, Dr. Theilen and her associates identified 67,384 women (3%) with HDP, including 49,598 women without a prior history of HDP and 7,786 with at least one prior HDP pregnancy. They included four different diagnoses as HDP: gestational hypertension, preeclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count), or eclampsia.
The analysis excluded women with chronic hypertension, antiphospholipid syndrome, pregestational diabetes, or chronic kidney disease. It also excluded women who died within a year of the index delivery. For each of the included 67,384 cases with HDP, the researchers selected two controls with a delivery unaffected by HDP and matched them to a case by age, year of childbirth, and parity.
The women in the study were 26 years old on average. Gestational age at delivery among the HDP cases averaged 1 week less than among the controls, 37.8 weeks compared with 38.9 weeks, a statistically significant difference. Average birth weight also differed by a significant amount, 3,079 grams in the HDP neonates and 3,319 grams in the control newborns. During follow-up, 8% of the HDP women died, compared with 6% of the control women.
Analysis showed that relative mortality rates in HDP women were especially elevated for certain types of death: endocrine and metabolic, circulatory, genitourinary, infectious disease, and digestive. In most types of death, the increased risk linked with HDP was markedly higher in the women with recurrent HDP.
Dr. Theilen reported the relative risks of death for certain specific mortality causes (Am J Obstet Gynecol. 2017 Jan;216[1][suppl]:S32-3). In women with a single index case of HDP, the mortality rate compared to women with no HDP was threefold higher for diabetes, twofold higher for ischemic heart disease, and 85% higher for stroke. In women with recurrent HDP, the relative mortality risks were fourfold higher for diabetes, threefold higher for ischemic heart disease, and fivefold higher for stroke.
For all causes of death except stroke, the increased relative risk from HDP existed only for women younger than 51 years. Once HDP women passed the age of 50, their excess mortality risk was substantially muted, even in women with recurrent HDP. But for stroke mortality, the added risk persisted among older women with a history of at least two HDPs. In this subgroup, stroke deaths were nearly fourfold higher than in the matched controls.
Dr. Theilen reported having no financial disclosures.
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Women who develop any form of hypertensive disease during pregnancy have a significantly increased mortality rate until they reach age 50, compared with women without the condition.
The findings, presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine, are based on a review of more than 2 million mothers who delivered babies in Utah during 1939-2012.
The relatively increased mortality rate linked with hypertensive disease of pregnancy (HDP) reached its peak during the first decade immediately following the index delivery and was dramatically higher in women for whom the index HDP was preceded by at least one earlier HDP. Increased mortality was especially elevated for certain types of deaths including stroke, diabetes, circulatory disease, and ischemic heart disease, said Lauren Theilen, MD, a maternal-fetal medicine researcher at the University of Utah in Salt Lake City.
She calculated that during the decade following an index case of HDP, life expectancy among mothers who had two or more HDP-affected pregnancies was about 49 years, life expectancy among women with a history of one HDP was 52 years, and postpartum women without HDP had a life expectancy of 55 years.
The data came from 2,083,331 singleton pregnancies delivered in Utah during 1939-2012 where the mother remained in Utah for at least 1 year following delivery. From this group, Dr. Theilen and her associates identified 67,384 women (3%) with HDP, including 49,598 women without a prior history of HDP and 7,786 with at least one prior HDP pregnancy. They included four different diagnoses as HDP: gestational hypertension, preeclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count), or eclampsia.
The analysis excluded women with chronic hypertension, antiphospholipid syndrome, pregestational diabetes, or chronic kidney disease. It also excluded women who died within a year of the index delivery. For each of the included 67,384 cases with HDP, the researchers selected two controls with a delivery unaffected by HDP and matched them to a case by age, year of childbirth, and parity.
The women in the study were 26 years old on average. Gestational age at delivery among the HDP cases averaged 1 week less than among the controls, 37.8 weeks compared with 38.9 weeks, a statistically significant difference. Average birth weight also differed by a significant amount, 3,079 grams in the HDP neonates and 3,319 grams in the control newborns. During follow-up, 8% of the HDP women died, compared with 6% of the control women.
Analysis showed that relative mortality rates in HDP women were especially elevated for certain types of death: endocrine and metabolic, circulatory, genitourinary, infectious disease, and digestive. In most types of death, the increased risk linked with HDP was markedly higher in the women with recurrent HDP.
Dr. Theilen reported the relative risks of death for certain specific mortality causes (Am J Obstet Gynecol. 2017 Jan;216[1][suppl]:S32-3). In women with a single index case of HDP, the mortality rate compared to women with no HDP was threefold higher for diabetes, twofold higher for ischemic heart disease, and 85% higher for stroke. In women with recurrent HDP, the relative mortality risks were fourfold higher for diabetes, threefold higher for ischemic heart disease, and fivefold higher for stroke.
For all causes of death except stroke, the increased relative risk from HDP existed only for women younger than 51 years. Once HDP women passed the age of 50, their excess mortality risk was substantially muted, even in women with recurrent HDP. But for stroke mortality, the added risk persisted among older women with a history of at least two HDPs. In this subgroup, stroke deaths were nearly fourfold higher than in the matched controls.
Dr. Theilen reported having no financial disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Postpartum life expectancy was 49 years in women with two or more hypertensive pregnancies and 55 years in women without hypertension.
Data source: Retrospective, case-control study of 2,083,331 singleton pregnancies delivered in Utah during 1939-2012.
Disclosures: Dr. Theilen reported having no financial disclosures.
Obinutuzumab approved to treat FL in Canada

Health Canada has approved the use of obinutuzumab (Gazyva®), an anti-CD20 monoclonal antibody, in patients with follicular lymphoma (FL).
The approval means obinutuzumab can be given, first in combination with bendamustine and then alone as maintenance therapy, to FL patients who relapsed after, or are refractory to, a rituximab-containing regimen.
Obinutuzumab is also approved in Canada for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
Obinutuzumab is a product of Roche.
Health Canada’s approval of obinutuzumab in FL is based on results from the phase 3 GADOLIN trial.
The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by the IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% (18.7% CR, 56% PR) for those receiving bendamustine alone, as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
At last follow-up, the median overall survival had not been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()

Health Canada has approved the use of obinutuzumab (Gazyva®), an anti-CD20 monoclonal antibody, in patients with follicular lymphoma (FL).
The approval means obinutuzumab can be given, first in combination with bendamustine and then alone as maintenance therapy, to FL patients who relapsed after, or are refractory to, a rituximab-containing regimen.
Obinutuzumab is also approved in Canada for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
Obinutuzumab is a product of Roche.
Health Canada’s approval of obinutuzumab in FL is based on results from the phase 3 GADOLIN trial.
The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by the IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% (18.7% CR, 56% PR) for those receiving bendamustine alone, as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
At last follow-up, the median overall survival had not been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()

Health Canada has approved the use of obinutuzumab (Gazyva®), an anti-CD20 monoclonal antibody, in patients with follicular lymphoma (FL).
The approval means obinutuzumab can be given, first in combination with bendamustine and then alone as maintenance therapy, to FL patients who relapsed after, or are refractory to, a rituximab-containing regimen.
Obinutuzumab is also approved in Canada for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
Obinutuzumab is a product of Roche.
Health Canada’s approval of obinutuzumab in FL is based on results from the phase 3 GADOLIN trial.
The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by the IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% (18.7% CR, 56% PR) for those receiving bendamustine alone, as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
At last follow-up, the median overall survival had not been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()