User login
Thermal ablation of duodenal tissue lowers transaminases
BOSTON – An endoscopic procedure that heats and ablates a few centimeters of the surface of the duodenum reduced hepatic transaminases in patients with type 2 diabetes. According to an abstract released in advance of the annual meeting of the American Association for the Study of Liver Diseases , a reduction in transaminases was also seen in a subset of patients who had ultrasonographic evidence of hepatic steatosis.
The meeting presentation will focus on the results of two studies of patients with type 2 diabetes who received duodenal mucosal resurfacing (DMR) to about 9 circumferential cm of their duodenum. The first study, which was a single-site study of 30 patients, was the first human study of DMR. The second study was a multicenter study of 22 patients.
“A single duodenal mucosal resurfacing procedure resulted in a decrease of liver transaminases sustained for 12 months,” Annieke van Baar, an MD-PhD candidate in gastroenterology and hepatology at the Academic University Center, Amsterdam, wrote in the study abstract.
At 6 months after the one-time procedure, the composite cohort of patients in both studies saw a 25% reduction in alanine transaminase, with an absolute reduction of a mean 9.9 plus or minus 22.3 IU/mL. Mean aspartate transaminase also decreased by a mean of 21%, with absolute reduction of a mean 6.1 plus or minus 14.8 IU/mL.
For the subset of 23 patients in the composite cohort who had ultrasound-identified steatosis, AST decreased by 14.4 plus or minus 23 IU/mL at 6 months post procedure; the reduction from baseline was 7.6 plus or minus 9.8 IU/mL at 9 months.
Duodenal mucosal resurfacing, performed endoscopically as a day procedure, has been shown to improve metabolic markers for type 2 diabetes. Though the exact mechanism for improvement is not known, reduction in incretin and other hormonal signaling from the duodenum is thought to increase insulin sensitization.
Patient characteristics were similar in both studies, with a mean age of 52.8 years in the first study and 56.8 in the second. Mean body mass index was 32 kg/m2 in both studies. In the first study, mean baseline hemoglobin A1c (HbA1c) was 9.7%; HbA1c was 8.4% in the second study.
Both study groups saw significant reductions in HbA1c at 6 months post DMR, with reductions of 1.2 plus or minus 1.8% and 0.8 plus or minus 0.9% (P less than .001). Homeostatic model assessment of insulin resistance (HOMA-IR) was reduced after DMR as well, with reductions of 0.9 plus or minus 4, and 2.4 plus or minus 6.8 in the two study groups, though the decrease was not statistically significant.
The transaminase reductions and improved glycemic markers were not accompanied by significant weight loss: Patients lost a mean 1.8 plus or minus 3.6 kg in the first study, and 2.4 plus or minus 3.8 kg in the second study.
“This unique endoscopic intervention deserves further study to ascertain its potential efficacy as a treatment for fatty liver disease,” wrote Ms. van Baar and her coauthors.
The studies were funded by Fractyl Laboratories, the manufacturer of the DMR device. Ms. van Baar had no financial disclosures, but two coauthors reported relationships with Fractyl Laboratories, and another author disclosed relationships with multiple other pharmaceutical companies.
[email protected]
On Twitter @karioakes
BOSTON – An endoscopic procedure that heats and ablates a few centimeters of the surface of the duodenum reduced hepatic transaminases in patients with type 2 diabetes. According to an abstract released in advance of the annual meeting of the American Association for the Study of Liver Diseases , a reduction in transaminases was also seen in a subset of patients who had ultrasonographic evidence of hepatic steatosis.
The meeting presentation will focus on the results of two studies of patients with type 2 diabetes who received duodenal mucosal resurfacing (DMR) to about 9 circumferential cm of their duodenum. The first study, which was a single-site study of 30 patients, was the first human study of DMR. The second study was a multicenter study of 22 patients.
“A single duodenal mucosal resurfacing procedure resulted in a decrease of liver transaminases sustained for 12 months,” Annieke van Baar, an MD-PhD candidate in gastroenterology and hepatology at the Academic University Center, Amsterdam, wrote in the study abstract.
At 6 months after the one-time procedure, the composite cohort of patients in both studies saw a 25% reduction in alanine transaminase, with an absolute reduction of a mean 9.9 plus or minus 22.3 IU/mL. Mean aspartate transaminase also decreased by a mean of 21%, with absolute reduction of a mean 6.1 plus or minus 14.8 IU/mL.
For the subset of 23 patients in the composite cohort who had ultrasound-identified steatosis, AST decreased by 14.4 plus or minus 23 IU/mL at 6 months post procedure; the reduction from baseline was 7.6 plus or minus 9.8 IU/mL at 9 months.
Duodenal mucosal resurfacing, performed endoscopically as a day procedure, has been shown to improve metabolic markers for type 2 diabetes. Though the exact mechanism for improvement is not known, reduction in incretin and other hormonal signaling from the duodenum is thought to increase insulin sensitization.
Patient characteristics were similar in both studies, with a mean age of 52.8 years in the first study and 56.8 in the second. Mean body mass index was 32 kg/m2 in both studies. In the first study, mean baseline hemoglobin A1c (HbA1c) was 9.7%; HbA1c was 8.4% in the second study.
Both study groups saw significant reductions in HbA1c at 6 months post DMR, with reductions of 1.2 plus or minus 1.8% and 0.8 plus or minus 0.9% (P less than .001). Homeostatic model assessment of insulin resistance (HOMA-IR) was reduced after DMR as well, with reductions of 0.9 plus or minus 4, and 2.4 plus or minus 6.8 in the two study groups, though the decrease was not statistically significant.
The transaminase reductions and improved glycemic markers were not accompanied by significant weight loss: Patients lost a mean 1.8 plus or minus 3.6 kg in the first study, and 2.4 plus or minus 3.8 kg in the second study.
“This unique endoscopic intervention deserves further study to ascertain its potential efficacy as a treatment for fatty liver disease,” wrote Ms. van Baar and her coauthors.
The studies were funded by Fractyl Laboratories, the manufacturer of the DMR device. Ms. van Baar had no financial disclosures, but two coauthors reported relationships with Fractyl Laboratories, and another author disclosed relationships with multiple other pharmaceutical companies.
[email protected]
On Twitter @karioakes
BOSTON – An endoscopic procedure that heats and ablates a few centimeters of the surface of the duodenum reduced hepatic transaminases in patients with type 2 diabetes. According to an abstract released in advance of the annual meeting of the American Association for the Study of Liver Diseases , a reduction in transaminases was also seen in a subset of patients who had ultrasonographic evidence of hepatic steatosis.
The meeting presentation will focus on the results of two studies of patients with type 2 diabetes who received duodenal mucosal resurfacing (DMR) to about 9 circumferential cm of their duodenum. The first study, which was a single-site study of 30 patients, was the first human study of DMR. The second study was a multicenter study of 22 patients.
“A single duodenal mucosal resurfacing procedure resulted in a decrease of liver transaminases sustained for 12 months,” Annieke van Baar, an MD-PhD candidate in gastroenterology and hepatology at the Academic University Center, Amsterdam, wrote in the study abstract.
At 6 months after the one-time procedure, the composite cohort of patients in both studies saw a 25% reduction in alanine transaminase, with an absolute reduction of a mean 9.9 plus or minus 22.3 IU/mL. Mean aspartate transaminase also decreased by a mean of 21%, with absolute reduction of a mean 6.1 plus or minus 14.8 IU/mL.
For the subset of 23 patients in the composite cohort who had ultrasound-identified steatosis, AST decreased by 14.4 plus or minus 23 IU/mL at 6 months post procedure; the reduction from baseline was 7.6 plus or minus 9.8 IU/mL at 9 months.
Duodenal mucosal resurfacing, performed endoscopically as a day procedure, has been shown to improve metabolic markers for type 2 diabetes. Though the exact mechanism for improvement is not known, reduction in incretin and other hormonal signaling from the duodenum is thought to increase insulin sensitization.
Patient characteristics were similar in both studies, with a mean age of 52.8 years in the first study and 56.8 in the second. Mean body mass index was 32 kg/m2 in both studies. In the first study, mean baseline hemoglobin A1c (HbA1c) was 9.7%; HbA1c was 8.4% in the second study.
Both study groups saw significant reductions in HbA1c at 6 months post DMR, with reductions of 1.2 plus or minus 1.8% and 0.8 plus or minus 0.9% (P less than .001). Homeostatic model assessment of insulin resistance (HOMA-IR) was reduced after DMR as well, with reductions of 0.9 plus or minus 4, and 2.4 plus or minus 6.8 in the two study groups, though the decrease was not statistically significant.
The transaminase reductions and improved glycemic markers were not accompanied by significant weight loss: Patients lost a mean 1.8 plus or minus 3.6 kg in the first study, and 2.4 plus or minus 3.8 kg in the second study.
“This unique endoscopic intervention deserves further study to ascertain its potential efficacy as a treatment for fatty liver disease,” wrote Ms. van Baar and her coauthors.
The studies were funded by Fractyl Laboratories, the manufacturer of the DMR device. Ms. van Baar had no financial disclosures, but two coauthors reported relationships with Fractyl Laboratories, and another author disclosed relationships with multiple other pharmaceutical companies.
[email protected]
On Twitter @karioakes
FROM THE LIVER MEETING 2016
Liver transplant waits shorter with DAAs
The rate of wait-listing for liver transplants for patients with hepatitis C and decompensated cirrhosis has decreased by over 30% since the entry of direct-acting antiviral (DAA) therapy and now equals the wait-list rate for nonalcoholic steatohepatitis, according to an abstract of a study that will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
Using the U.S. Scientific Registry of Transplant Recipients (SRTR), Jennifer Flemming, MD, and her coauthors developed a cohort of 47,591 adults who were wait-listed for liver transplant because of HCV or hepatitis B virus infection (HBV), or for nonalcoholic steatohepatitis (NASH).
Dr. Flemming, professor of gastroenterology at Queens University, Kingston, Ont., examined trends in liver transplant wait-listing between 2003 and 2015. The time period was divided into the “interferon era,” from 2003 to 2010, the “protease inhibitor era,” from 2011 to 2013, and the “DAA era,” from 2014 to 2015.
Examining annual standardized incidence rates of wait-listing, Dr. Flemming and her collaborators found that wait-listing for HCV patients with decompensated cirrhosis dropped by 5% during the protease inhibitor era, and by 32% in the DAA era (P = .004 and P less than .001, respectively).
Wait-listing for HBV also dropped, by 17% in the protease inhibitor era and by 24% in the DAA era (P = .002 and P less than .001, respectively). For NASH patients, conversely, wait-listing increased by 41% in the protease inhibitor era, and by 81% in the DAA era (P less than .001 for both). Hepatocellular carcinoma rates also rose during the protease inhibitor and DAA eras.
“Further reductions in [wait-listing] are anticipated with increased testing, linkage to care, and access to DAA therapy,” Dr. Flemming and her coauthors wrote in their study abstract.
Dr. Flemming reported no relevant financial disclosures. Several coauthors reported financial ties to pharmaceutical companies that market DAAs.
[email protected]
On Twitter @karioakes
The rate of wait-listing for liver transplants for patients with hepatitis C and decompensated cirrhosis has decreased by over 30% since the entry of direct-acting antiviral (DAA) therapy and now equals the wait-list rate for nonalcoholic steatohepatitis, according to an abstract of a study that will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
Using the U.S. Scientific Registry of Transplant Recipients (SRTR), Jennifer Flemming, MD, and her coauthors developed a cohort of 47,591 adults who were wait-listed for liver transplant because of HCV or hepatitis B virus infection (HBV), or for nonalcoholic steatohepatitis (NASH).
Dr. Flemming, professor of gastroenterology at Queens University, Kingston, Ont., examined trends in liver transplant wait-listing between 2003 and 2015. The time period was divided into the “interferon era,” from 2003 to 2010, the “protease inhibitor era,” from 2011 to 2013, and the “DAA era,” from 2014 to 2015.
Examining annual standardized incidence rates of wait-listing, Dr. Flemming and her collaborators found that wait-listing for HCV patients with decompensated cirrhosis dropped by 5% during the protease inhibitor era, and by 32% in the DAA era (P = .004 and P less than .001, respectively).
Wait-listing for HBV also dropped, by 17% in the protease inhibitor era and by 24% in the DAA era (P = .002 and P less than .001, respectively). For NASH patients, conversely, wait-listing increased by 41% in the protease inhibitor era, and by 81% in the DAA era (P less than .001 for both). Hepatocellular carcinoma rates also rose during the protease inhibitor and DAA eras.
“Further reductions in [wait-listing] are anticipated with increased testing, linkage to care, and access to DAA therapy,” Dr. Flemming and her coauthors wrote in their study abstract.
Dr. Flemming reported no relevant financial disclosures. Several coauthors reported financial ties to pharmaceutical companies that market DAAs.
[email protected]
On Twitter @karioakes
The rate of wait-listing for liver transplants for patients with hepatitis C and decompensated cirrhosis has decreased by over 30% since the entry of direct-acting antiviral (DAA) therapy and now equals the wait-list rate for nonalcoholic steatohepatitis, according to an abstract of a study that will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
Using the U.S. Scientific Registry of Transplant Recipients (SRTR), Jennifer Flemming, MD, and her coauthors developed a cohort of 47,591 adults who were wait-listed for liver transplant because of HCV or hepatitis B virus infection (HBV), or for nonalcoholic steatohepatitis (NASH).
Dr. Flemming, professor of gastroenterology at Queens University, Kingston, Ont., examined trends in liver transplant wait-listing between 2003 and 2015. The time period was divided into the “interferon era,” from 2003 to 2010, the “protease inhibitor era,” from 2011 to 2013, and the “DAA era,” from 2014 to 2015.
Examining annual standardized incidence rates of wait-listing, Dr. Flemming and her collaborators found that wait-listing for HCV patients with decompensated cirrhosis dropped by 5% during the protease inhibitor era, and by 32% in the DAA era (P = .004 and P less than .001, respectively).
Wait-listing for HBV also dropped, by 17% in the protease inhibitor era and by 24% in the DAA era (P = .002 and P less than .001, respectively). For NASH patients, conversely, wait-listing increased by 41% in the protease inhibitor era, and by 81% in the DAA era (P less than .001 for both). Hepatocellular carcinoma rates also rose during the protease inhibitor and DAA eras.
“Further reductions in [wait-listing] are anticipated with increased testing, linkage to care, and access to DAA therapy,” Dr. Flemming and her coauthors wrote in their study abstract.
Dr. Flemming reported no relevant financial disclosures. Several coauthors reported financial ties to pharmaceutical companies that market DAAs.
[email protected]
On Twitter @karioakes
FROM THE LIVER MEETING 2016
VIDEO: Recognizing the systemic impact of rosacea
LAS VEGAS – At its core, “we understand that rosacea is an inflammatory disease,” Linda Stein Gold, MD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
As with psoriasis, she added, “we understand that rosacea might have some systemic implications.”
In fact, data suggest that cardiovascular disease is independently associated with rosacea after controlling for multiple variables, she noted. “So we now believe it’s important to get the inflammation under control, not just for the skin, but for the body as a whole,” Dr. Stein Gold, director of dermatology research, Henry Ford Health System, Detroit, said in a video interview.
She disclosed receiving research support, serving as a consultant, serving on the speakers bureau, and/or serving on the scientific advisory board for multiple pharmaceutical companies.
SDEF and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – At its core, “we understand that rosacea is an inflammatory disease,” Linda Stein Gold, MD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
As with psoriasis, she added, “we understand that rosacea might have some systemic implications.”
In fact, data suggest that cardiovascular disease is independently associated with rosacea after controlling for multiple variables, she noted. “So we now believe it’s important to get the inflammation under control, not just for the skin, but for the body as a whole,” Dr. Stein Gold, director of dermatology research, Henry Ford Health System, Detroit, said in a video interview.
She disclosed receiving research support, serving as a consultant, serving on the speakers bureau, and/or serving on the scientific advisory board for multiple pharmaceutical companies.
SDEF and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – At its core, “we understand that rosacea is an inflammatory disease,” Linda Stein Gold, MD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
As with psoriasis, she added, “we understand that rosacea might have some systemic implications.”
In fact, data suggest that cardiovascular disease is independently associated with rosacea after controlling for multiple variables, she noted. “So we now believe it’s important to get the inflammation under control, not just for the skin, but for the body as a whole,” Dr. Stein Gold, director of dermatology research, Henry Ford Health System, Detroit, said in a video interview.
She disclosed receiving research support, serving as a consultant, serving on the speakers bureau, and/or serving on the scientific advisory board for multiple pharmaceutical companies.
SDEF and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Goals of Care Infrequently Discussed among Hospitalized Long-Term Care Residents
Clinical Question: How often are goals of care (GOC) discussed during hospitalization of long-term care residents, and what patient characteristics make this more likely to occur?
Background: GOC discussions during hospitalization have the potential to better align patient wishes with care received and to reduce unwanted care. Previous studies have examined barriers to GOC discussions, but less is known about factors associated with GOC discussions occurring and the outcomes of these discussions.
Study Design: Retrospective chart review.
Setting: Two academic hospitals in Toronto.
Synopsis: In the review, 665 hospitalized patients during a one-year period were identified as being >65 years old and from a long-term care facility. Of the 665 patients, a random sampling of 200 unique patients was reviewed. Of these, 37.5% had a documented GOC discussion. Lower Glasgow Coma Scale scores and higher respiratory rates were correlated with a higher incidence of GOC discussions. Patients with GOC discussions had higher rates of no resuscitation and comfort care orders; these patients also had higher odds of in-hospital death and one-year mortality. Of patients that had a change in their GOC, 74% did not have this change reflected in the discharge summary.
Although this study is a retrospective review and limited to two Canadian teaching hospitals, there is likely an opportunity for hospitalists to more frequently discuss and document GOC in hospitalized long-term care patients.
Bottom Line: In hospitalized long-term care patients, GOC are infrequently discussed and documented. Frequency of discussions is correlated with illness severity.
Citation: Wong HJ, Wang J, Grinman M, Wu RC. Goals of care discussions among hospitalized long-term care residents: predictors and associated outcomes of care [published online ahead of print July 21, 2016]. J Hosp Med.
Short Take
Sleep-Promoting Interventions Improve Sleep in Hospitalized Patients
A non-blinded, quasi-randomized pilot study of 112 patients demonstrated that sleep-promoting interventions, including education and environmental control to minimize sleep disruption, improved total nighttime sleep time as well as qualitative measures of sleep.
Citation: Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472.
Clinical Question: How often are goals of care (GOC) discussed during hospitalization of long-term care residents, and what patient characteristics make this more likely to occur?
Background: GOC discussions during hospitalization have the potential to better align patient wishes with care received and to reduce unwanted care. Previous studies have examined barriers to GOC discussions, but less is known about factors associated with GOC discussions occurring and the outcomes of these discussions.
Study Design: Retrospective chart review.
Setting: Two academic hospitals in Toronto.
Synopsis: In the review, 665 hospitalized patients during a one-year period were identified as being >65 years old and from a long-term care facility. Of the 665 patients, a random sampling of 200 unique patients was reviewed. Of these, 37.5% had a documented GOC discussion. Lower Glasgow Coma Scale scores and higher respiratory rates were correlated with a higher incidence of GOC discussions. Patients with GOC discussions had higher rates of no resuscitation and comfort care orders; these patients also had higher odds of in-hospital death and one-year mortality. Of patients that had a change in their GOC, 74% did not have this change reflected in the discharge summary.
Although this study is a retrospective review and limited to two Canadian teaching hospitals, there is likely an opportunity for hospitalists to more frequently discuss and document GOC in hospitalized long-term care patients.
Bottom Line: In hospitalized long-term care patients, GOC are infrequently discussed and documented. Frequency of discussions is correlated with illness severity.
Citation: Wong HJ, Wang J, Grinman M, Wu RC. Goals of care discussions among hospitalized long-term care residents: predictors and associated outcomes of care [published online ahead of print July 21, 2016]. J Hosp Med.
Short Take
Sleep-Promoting Interventions Improve Sleep in Hospitalized Patients
A non-blinded, quasi-randomized pilot study of 112 patients demonstrated that sleep-promoting interventions, including education and environmental control to minimize sleep disruption, improved total nighttime sleep time as well as qualitative measures of sleep.
Citation: Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472.
Clinical Question: How often are goals of care (GOC) discussed during hospitalization of long-term care residents, and what patient characteristics make this more likely to occur?
Background: GOC discussions during hospitalization have the potential to better align patient wishes with care received and to reduce unwanted care. Previous studies have examined barriers to GOC discussions, but less is known about factors associated with GOC discussions occurring and the outcomes of these discussions.
Study Design: Retrospective chart review.
Setting: Two academic hospitals in Toronto.
Synopsis: In the review, 665 hospitalized patients during a one-year period were identified as being >65 years old and from a long-term care facility. Of the 665 patients, a random sampling of 200 unique patients was reviewed. Of these, 37.5% had a documented GOC discussion. Lower Glasgow Coma Scale scores and higher respiratory rates were correlated with a higher incidence of GOC discussions. Patients with GOC discussions had higher rates of no resuscitation and comfort care orders; these patients also had higher odds of in-hospital death and one-year mortality. Of patients that had a change in their GOC, 74% did not have this change reflected in the discharge summary.
Although this study is a retrospective review and limited to two Canadian teaching hospitals, there is likely an opportunity for hospitalists to more frequently discuss and document GOC in hospitalized long-term care patients.
Bottom Line: In hospitalized long-term care patients, GOC are infrequently discussed and documented. Frequency of discussions is correlated with illness severity.
Citation: Wong HJ, Wang J, Grinman M, Wu RC. Goals of care discussions among hospitalized long-term care residents: predictors and associated outcomes of care [published online ahead of print July 21, 2016]. J Hosp Med.
Short Take
Sleep-Promoting Interventions Improve Sleep in Hospitalized Patients
A non-blinded, quasi-randomized pilot study of 112 patients demonstrated that sleep-promoting interventions, including education and environmental control to minimize sleep disruption, improved total nighttime sleep time as well as qualitative measures of sleep.
Citation: Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472.
Quality of End-of-Life Care Varies by Disease
Clinical Question: How do patterns of end-of-life care compare for patients with different diseases?
Background: Studies regarding quality of care at the end of life have focused primarily on patients dying from cancer. Few studies to date have looked at patients dying from other illnesses, and few have taken into account perspectives of family members.
Study Design: Retrospective cross-sectional.
Setting: 146 inpatient facilities in the Veterans Affairs (VA) health system.
Synopsis: The authors identified 57,753 patients who died while hospitalized at VA facilities during the study period, and 34,015 next of kin completed the Bereaved Family Survey. Overall, approximately half (43.7%–50.4%) of patients with end-stage renal disease (ESRD), frailty, or cardiopulmonary disease received palliative-care consultations compared with 73.5% and 61.4% of patients with cancer and dementia, respectively. Patients with cancer or dementia were less likely to die in the ICU compared to patients with other diagnoses (8.9%–13.4% compared to 32.3%–35.2%). Quality of care as perceived by the bereaved families was higher for patients with cancer or dementia (59.2%–59.3% compared to 53.7%–54.8%).
While large, this study was limited in applicability to different populations due to being conducted within the VA system. Overall, it showed significant differences in end-of-life care between patients who died from different diseases. This study suggests several practical steps that may improve disparities in end-of-life care, in particular, increasing discussion of goals of care and improving access to inpatient palliative-care consults for patients with ESRD, frailty, or cardiopulmonary disease.
Bottom Line: Quality and satisfaction indicators for end-of-life care for patients with ESRD, frailty, or cardiopulmonary disease were lower than for patients with dementia or cancer.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102.
Clinical Question: How do patterns of end-of-life care compare for patients with different diseases?
Background: Studies regarding quality of care at the end of life have focused primarily on patients dying from cancer. Few studies to date have looked at patients dying from other illnesses, and few have taken into account perspectives of family members.
Study Design: Retrospective cross-sectional.
Setting: 146 inpatient facilities in the Veterans Affairs (VA) health system.
Synopsis: The authors identified 57,753 patients who died while hospitalized at VA facilities during the study period, and 34,015 next of kin completed the Bereaved Family Survey. Overall, approximately half (43.7%–50.4%) of patients with end-stage renal disease (ESRD), frailty, or cardiopulmonary disease received palliative-care consultations compared with 73.5% and 61.4% of patients with cancer and dementia, respectively. Patients with cancer or dementia were less likely to die in the ICU compared to patients with other diagnoses (8.9%–13.4% compared to 32.3%–35.2%). Quality of care as perceived by the bereaved families was higher for patients with cancer or dementia (59.2%–59.3% compared to 53.7%–54.8%).
While large, this study was limited in applicability to different populations due to being conducted within the VA system. Overall, it showed significant differences in end-of-life care between patients who died from different diseases. This study suggests several practical steps that may improve disparities in end-of-life care, in particular, increasing discussion of goals of care and improving access to inpatient palliative-care consults for patients with ESRD, frailty, or cardiopulmonary disease.
Bottom Line: Quality and satisfaction indicators for end-of-life care for patients with ESRD, frailty, or cardiopulmonary disease were lower than for patients with dementia or cancer.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102.
Clinical Question: How do patterns of end-of-life care compare for patients with different diseases?
Background: Studies regarding quality of care at the end of life have focused primarily on patients dying from cancer. Few studies to date have looked at patients dying from other illnesses, and few have taken into account perspectives of family members.
Study Design: Retrospective cross-sectional.
Setting: 146 inpatient facilities in the Veterans Affairs (VA) health system.
Synopsis: The authors identified 57,753 patients who died while hospitalized at VA facilities during the study period, and 34,015 next of kin completed the Bereaved Family Survey. Overall, approximately half (43.7%–50.4%) of patients with end-stage renal disease (ESRD), frailty, or cardiopulmonary disease received palliative-care consultations compared with 73.5% and 61.4% of patients with cancer and dementia, respectively. Patients with cancer or dementia were less likely to die in the ICU compared to patients with other diagnoses (8.9%–13.4% compared to 32.3%–35.2%). Quality of care as perceived by the bereaved families was higher for patients with cancer or dementia (59.2%–59.3% compared to 53.7%–54.8%).
While large, this study was limited in applicability to different populations due to being conducted within the VA system. Overall, it showed significant differences in end-of-life care between patients who died from different diseases. This study suggests several practical steps that may improve disparities in end-of-life care, in particular, increasing discussion of goals of care and improving access to inpatient palliative-care consults for patients with ESRD, frailty, or cardiopulmonary disease.
Bottom Line: Quality and satisfaction indicators for end-of-life care for patients with ESRD, frailty, or cardiopulmonary disease were lower than for patients with dementia or cancer.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102.
Innovative Approaches to Substance Use Disorder Treatment at the VA
While much of private health care in the U.S. is only beginning to confront the burgeoning opioid epidemic, the VA has a long-standing commitment to providing treatment to veterans struggling with substance use disorders. To understand the scope of the challenge and the VA’s approach, Federal Practitioner Editor-in-Chief Cynthia M.A. Geppert, MD, talked with Karen Drexler, MD, national mental health program director, substance use disorders. Dr. Drexler served in the U.S. Air Force for 8 years before joining the Atlanta VAMC in Georgia, where she directed the substance abuse treatment program. She is an associate professor of psychiatry and behavioral sciences at Emory University. In 2014, Dr. Drexler was named deputy mental health program director, addictive disorders.
Editor-in-Chief Cynthia M.A. Geppert, MD. As national mental health program director for substance use disorders, what are the challenges facing VA substance use programs?
Karen Drexler, MD. The biggest challenge is the increasing demand for services. Veterans have been coming to the VA for mental health care in general and for substance use disorder treatment in particular. The most common substance use disorder that we treat in addition to tobacco is alcohol use disorder, and the demand for alcohol use disorder treatment continues to grow.
Also, with the opioid crisis, there’s increasing demand for opioid use disorder treatments, including what we recommend as first-line treatment, which is medication-assisted treatment using buprenorphine or methadone, or injectable naltrexone as a second-line treatment. Gearing up with medication-assisted treatment for both alcohol and opioid use disorders is probably our biggest challenge.
Dr. Geppert. What were the most important accomplishments of the VA substance abuse programs over the past 5 years?
Dr. Drexler. There have been a lot! First of all, one of the things I love about practicing within the VA is that we are an integrated health care system. When I talk with colleagues in the Emory system here in Atlanta and across the country, oftentimes mental health and substance use disorders are isolated. The funding streams through the public sector come in different ways. Third-party payers have carve-outs for behavioral health.
It’s really wonderful to be able to collaborate with my colleagues in primary care, medical specialty care, and general mental health care, to provide a holistic and team-based approach to our patients who have both medical and substance use disorder problems and often co-occurring mental illness. As we’ve moved from a hospital-based system to more outpatient [care], the VA continues to be able to collaborate through our electronic health records as well as just being able to pick up the phone or send a [Microsoft] Lync message or an Outlook encrypted e-mail to help facilitate that coordinated care.
Another accomplishment has been to [develop] policy. It’s one of our challenges, but it’s also an accomplishment even at this early stage. In 2008, we issued the Uniform Mental Health Services benefits package as part of VHA Handbook on Mental Health Services. From that very beginning, we have included medication-assisted therapy when indicated for veterans with substance use disorders.
Now, we also know there’s a lot of variability in the system, so not every facility is providing these indicated treatments yet; but at least it’s part of our policy, and it’s been a focus of ongoing quality improvement to make these treatments available for veterans when they need them.
Dr. Geppert. We’ve heard a lot lately about the CDC guidelines for prescribing opiates for chronic pain that were published earlier this year. How do you see these guidelines affecting the VA Opiate Safety Initiative? Do you see any important contradictions between current VA policy and these new guidelines?
Dr. Drexler. Yes—not necessarily with policy but with our VA/DoD Clinical Practice Guidelines from 2010 and the new CDC guidelines. There’s a shift in emphasis. The 2010 VA/DoD guidelines were maybe a little too optimistic about the safety of opioids for chronic, noncancer pain. As time goes on, more evidence is mounting of the potential harms, including from my perspective, the risk of developing an opioid use disorder when taking opioid analgesics as prescribed.
In the 1990s and the early 2000s, experts in the field reassured us. They told us all—patients and providers alike—that if opioid analgesics were taken as prescribed for legitimate pain by people who didn’t have a previous history of a substance use disorder, they could take them and would not be at risk for becoming addicted. We now know that’s simply not true. Patients who take these medicines as prescribed by their providers end up developing tolerance, developing hyperalgesia, needing more and more medication to get the desired effect, and crossing that line from just physiologic tolerance to developing an opioid use disorder.
We now have better guidelines about where that risk increases. We have some data from observational studies that patients who are maintained on opioid analgesics that are < 50 mg of daily morphine equivalent dose (MED) tend not to have as high a risk of overdose as those who are maintained on more, but the risk of developing an opioid use disorder actually is not insignificant even below 50 mg MED.
The key for developing an opioid use disorder is how long the patient is on the treatment. For patients who take opioid analgesics for < 90 days—again, these are observational studies—very few went on to develop opioid use disorder. However, those who took it for > 90 days, even those who were maintained on < 36 mg MED, had a significantly increased risk of developing an opioid use disorder. And for those who were on the higher dose, say, > 120 mg MED, the risk went up to 122-fold.
We just don’t see those kinds of odds ratios elsewhere in medicine. You know, we address risk factors when it increases the risk by 50%, but this is 122-fold.
Dr. Geppert. Pain as the fifth vital sign had a dark side there. Is there anything else you want to say to us about the Opiate Safety Initiative?
Dr. Drexler. I think it’s been incredibly successful; but like any change, there are unintended consequences and challenges with any change, even a good change. One of the things that has been a good change is raising awareness that opioids are not the answer for chronic pain. We have better evidence that self-care strategies (movement, exercise, physical therapy, and cognitive behavioral therapy for pain) help folks learn strategies for addressing automatic alarming thoughts about their pain—that it’s signaling something terrible when, really, it’s not. There are things that people can do to manage pain on their own. Those [strategies] are safer, and we have better evidence that they are effective than we have for opioids taken past 12 weeks.
Dr. Geppert. It’s really a paradigm shift in understanding. The Comprehensive Addiction and Recovery Act of 2016 has proposed to allow some nurse practitioners (NPs) and physician assistants (PAs) to obtain a waiver to prescribe buprenorphine. What role does the VA see for PAs and NPs in patient care for veterans with substance use disorders with medication-assisted therapy, and do you think that’ll involve prescribing buprenorphine?
Dr. Drexler. One of the things that I love about practicing within the VA is working within an interdisciplinary team and having that team-based approach. When we work together, our results far outshine anything that any one of us could do separately. I am very much looking forward to being able to provide or collaborate with other federal partners like the Substance Abuse and Mental Health Services Administration [SAMHSA] to provide the training for VA NPs and PAs who are able to prescribe controlled substances with their state licensure to provide buprenorphine for our patients.
Even without that prescriptive authority, NPs and PAs are already important partners and members of the interdisciplinary team who are managing patients and providing the evidence-based counseling, the monitoring, following up urine drug screen results, and doing a lot of the management, but not signing the prescription that’s being provided now by the Drug Addiction Treatment Act-waivered physicians. But SAMHSA has yet to define what the 24 hours of training will be that NPs and PAs will need to complete in order to apply for their U.S. Drug Enforcement Administration waiver, but we’re very much looking forward to it. And I am in contact with colleagues at SAMHSA, and they promise me they will keep me informed when they have the regulations set out for us to follow.
Dr. Geppert. The new VA/DoD Clinical Practice Guidelines for Management of Substance Use Disorders were published earlier this year. Could you give us an overview of the guidelines and what’s significant for most of our providers in the field?
Dr. Drexler. I’m very excited about the guidelines. One of the first things you should know is that they are very evidence based. If you’ve been practicing medicine as long as I have, you’ll remember, when the first clinical practice guidelines came out, they were expert opinion. They got some of the leading practitioners and researchers in the country together, put them in a room, and said, “What do you think people should do to treat this disorder?” Over time, clinical practice guidelines have become more evidence based; and the VA/DoD collaborative follows a highly respected methodology called the GRADE methodology.
Let me explain that just briefly. It means that our recommendations are based on systematic reviews of the literature. We all have our own screens and filters and our favorite studies. And so, if you get a bunch of experts together, they may not have as dispassionate a view of the entire literature as when you do a systematic review; and that’s what we started with.
We followed a specific methodology as well that took into account not only the strength of the evidence where the gold standard is a randomized, controlled clinical trial and, even better than that, a meta-analysis of multiple clinical trials. And for our recommendations, the strength of the evidence was, by far, supported mostly by clinical trials or meta-analyses. So, when you look at the VA/DoD Clinical Practice Guidelines, and I hope you will, you can feel confident that what we’re recommending is not just pulled out of the sky or some idiosyncratic favorite thing that Karen Drexler likes. It’s really based on good, strong clinical evidence.
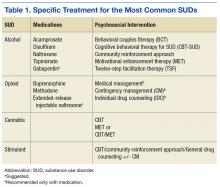
We went from the previous version that had over 160 recommendations. We distilled them down to 36, and those 36 recommendations cover everything from prevention in primary care settings to stabilization and withdrawal management for alcohol and sedatives and opioids, and then treatment or rehabilitation of the 4 most common substance use disorders and then some general principles (Table 1).
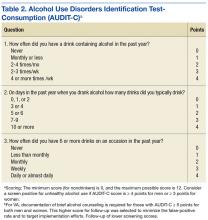
There’s good evidence that what we’re doing in VA with screening in primary care every year using the AUDIT-C [Alcohol Use Disorders Identification Test-Consumption] is making a difference (Table 2). That folks who screen positive for at-risk alcohol use are very likely to respond to a brief intervention by their health care provider and reduce their alcohol consumption to safe levels, where they’re not likely to go on to develop an addiction to the alcohol or to develop medical complications from heavy alcohol use.
After screening, then, if someone does develop a substance use disorder, we have evidence-based treatments, both medications for 2 of our most common disorders and psychosocial interventions for all 4 most common disorders after tobacco. And I don’t exclude tobacco because it’s not important; it’s incredibly important, but it gets its own clinical practice guidelines. So, with our limited budget, we just addressed the other 10 substances….
For alcohol we have great evidence for 5 different medications and for 6 different psychosocial approaches. It is the most common substance use disorder, but we also have a whole menu of different treatments that folks can choose from, everything from naltrexone—oral naltrexone is the most common medication that we prescribe—but also acamprosate; disulfiram; extended-release injectable naltrexone. Topiramate, interestingly, is not FDA approved for that indication, but we have found good evidence from clinical trials supporting topiramate, so we strongly recommend it. If any of those are not acceptable or not tolerated or ineffective, then we also have some evidence to support gabapentin as well. So we have a strong recommendation for the first ones and a suggestion for gabapentin if it’s clinically indicated.
In terms of psychosocial approaches, [we recommend] everything from motivation enhancement therapy or motivational interviewing; cognitive-behavioral therapy; 12-step facilitation, which is a structured way of getting folks to get more active in Alcoholics Anonymous; behavioral approaches like community reinforcement approach or contingency management.
For opioid use disorder, interestingly, we found good, strong evidence that medication-assisted treatments work but no evidence for any psychosocial intervention without medication. Let me say that again because, nationwide, the most common treatment that’s provided for opioid use disorder, for any substance use disorder, is a psychosocial intervention—talking therapy alone.
For all of the medicines for alcohol use disorder, the medicine improved upon talking therapy alone. For opioid use disorder, medication helps people stay sober; it retains them in treatment; it saves lives. But for talking therapy without medication, we have found insufficient evidence to recommend any particular treatment.
Dr. Geppert. Incredible. That leads to my next question: One of the most challenging issues facing primary care practitioners is how to manage patients who are on chronic opiate therapy but also use medical marijuana in the increasing number of states where it’s legal. Do you have some advice on how to approach these situations?
Dr. Drexler. It’s a great question, and it’s one I’m sure more and more VA practitioners are grappling with. By our policy, we do not exclude veterans who are taking medical marijuana from any VA services, and we encourage the veterans to speak up about it so that it can be considered as part of their treatment plan.
By policy, however, VA practitioners do not fill out forms for state-approved marijuana. Marijuana is still a Schedule I controlled substance by federal law, so it’s a very interesting legal situation where it’s illegal federally; but some states have passed laws making it legal by state law. It will be interesting to see how this plays out over the next few years.
But in that situation—and really, this is our policy, in general for substance use disorders—a patient having a substance use disorder should not be precluded or excluded from getting treatment for other medical conditions. Similarly, other medical conditions shouldn’t exclude patients from getting treatment for their substance use disorder. However, the best treatment comes when we look at the whole person and try to take both things into account.
In the same way that patients can become addicted to their opioid pain medicines, patients can become addicted to medical marijuana. So the marijuana use needs to be evaluated objectively; and whether or not they have a form, just like whether or not someone has a prescription for their opioid that may be causing them problems, that needs to be taken into account in the treatment plan; and the treatment needs to be individualized.
Dr. Geppert. What in VA substance use disorder programs and treatment do you think is especially innovative when compared to the community?
Dr. Drexler. One of the places where I think the VA is well ahead of our colleagues elsewhere in health care is…from SAIL [Strategic Analytics for Improvement and Learning] metrics. About 34% or 35% of veterans who are diagnosed clinically within our system with opioid use disorders are receiving medication-assisted therapy either with buprenorphine, methadone, or extended-release injectable naltrexone. When you look at the SAMHSA survey, the National Survey on Drug Use and Health, the percentage of patients in the community who meet criteria for a substance use disorder [who] actually receive treatment is more like 12%. We have no numbers from SAMHSA about what percentage are receiving medication-assisted treatment. They don’t ask. In that way, even though we have a long ways to go, we’re ahead of the curve when it comes to medication-assisted treatment. But the demand is growing, we’re going to have to keep working hard to keep up and to get ahead at this. When I think about it compared to other chronic illnesses, only 35% of our patients are getting the first-line recommended treatment. We still have a long ways to go.
Another place where we’re innovative is with contingency management. I mentioned psychosocial treatments. Behavioral treatment is very effective and yet is not often used in treatment of substance use disorders; but it works. And even better than that, it is fun.
Let me try to explain what this means. It’s based on the idea that the benefits of using drugs are immediate, and the benefits of staying sober take a long time to realize. And it is that difference that makes early recovery so challenging because, if someone has a craving for alcohol or another drug, they can satisfy that craving immediately and then have to pay the price, though, of health consequences or financial consequences or legal consequences from that alcohol or drug use.
On the other hand, staying sober is tough. But it takes a long time to put one’s life back together, to get one’s chronic health conditions that have been caused or made worse by the drug use back together. And the idea behind contingency management is to give immediate rewards early in recovery to keep folks engaged long enough to start realizing some of the longer-term benefits of recovery.
So the way this works, we use what we call the fishbowl model of contingency management. And we started with stimulant use disorders, which is one of the substance use disorders for which there’s no strong evidence for a medication that works; but this particular psychosocial intervention works very well. So patients come to the clinic twice a week, give a urine specimen for drug screen; and, based on those test results, if their test is negative and they haven’t used any stimulants, then they get a draw from the fishbowl, which is filled with slips of paper. Half of the slips of paper say, “Good job! Keep up the good work,” or something encouraging like that. And half of the slips of paper have a prize associated with them, and it could be a small prize like $1 or $5 for exchange at the VA Canteen store, or it could be a larger prize like $100 or $200. Of course, the less expensive prizes are more common than the more expensive prizes; but just that immediate reward can make such a difference.
With the first negative urine drug screen, you get 1 draw from the fishbowl. The second one in a row, you get 2; and so it goes that the longer you stay sober, the more chances you have for winning a prize. And this treatment can go on for as long as the veteran wants to continue for up to 12 weeks. Usually by 12 weeks, folks’ health and their lives are coming back together; and so there’s many more rewards for staying sober than just the draws from the fishbowl. And what we find is folks who get a good start with contingency management tend to stay on that trajectory towards recovery.
And this has been a wonderful partnership between the Center of Excellence for Substance Abuse Treatment and Education in Philadelphia and the VA Canteen Service, who has generously donated canteen coupons. So it doesn’t cost the medical center anything to offer these prizes, and we’ve had just some amazing results.
To listen to the unedited interview here
While much of private health care in the U.S. is only beginning to confront the burgeoning opioid epidemic, the VA has a long-standing commitment to providing treatment to veterans struggling with substance use disorders. To understand the scope of the challenge and the VA’s approach, Federal Practitioner Editor-in-Chief Cynthia M.A. Geppert, MD, talked with Karen Drexler, MD, national mental health program director, substance use disorders. Dr. Drexler served in the U.S. Air Force for 8 years before joining the Atlanta VAMC in Georgia, where she directed the substance abuse treatment program. She is an associate professor of psychiatry and behavioral sciences at Emory University. In 2014, Dr. Drexler was named deputy mental health program director, addictive disorders.
Editor-in-Chief Cynthia M.A. Geppert, MD. As national mental health program director for substance use disorders, what are the challenges facing VA substance use programs?
Karen Drexler, MD. The biggest challenge is the increasing demand for services. Veterans have been coming to the VA for mental health care in general and for substance use disorder treatment in particular. The most common substance use disorder that we treat in addition to tobacco is alcohol use disorder, and the demand for alcohol use disorder treatment continues to grow.
Also, with the opioid crisis, there’s increasing demand for opioid use disorder treatments, including what we recommend as first-line treatment, which is medication-assisted treatment using buprenorphine or methadone, or injectable naltrexone as a second-line treatment. Gearing up with medication-assisted treatment for both alcohol and opioid use disorders is probably our biggest challenge.
Dr. Geppert. What were the most important accomplishments of the VA substance abuse programs over the past 5 years?
Dr. Drexler. There have been a lot! First of all, one of the things I love about practicing within the VA is that we are an integrated health care system. When I talk with colleagues in the Emory system here in Atlanta and across the country, oftentimes mental health and substance use disorders are isolated. The funding streams through the public sector come in different ways. Third-party payers have carve-outs for behavioral health.
It’s really wonderful to be able to collaborate with my colleagues in primary care, medical specialty care, and general mental health care, to provide a holistic and team-based approach to our patients who have both medical and substance use disorder problems and often co-occurring mental illness. As we’ve moved from a hospital-based system to more outpatient [care], the VA continues to be able to collaborate through our electronic health records as well as just being able to pick up the phone or send a [Microsoft] Lync message or an Outlook encrypted e-mail to help facilitate that coordinated care.
Another accomplishment has been to [develop] policy. It’s one of our challenges, but it’s also an accomplishment even at this early stage. In 2008, we issued the Uniform Mental Health Services benefits package as part of VHA Handbook on Mental Health Services. From that very beginning, we have included medication-assisted therapy when indicated for veterans with substance use disorders.
Now, we also know there’s a lot of variability in the system, so not every facility is providing these indicated treatments yet; but at least it’s part of our policy, and it’s been a focus of ongoing quality improvement to make these treatments available for veterans when they need them.
Dr. Geppert. We’ve heard a lot lately about the CDC guidelines for prescribing opiates for chronic pain that were published earlier this year. How do you see these guidelines affecting the VA Opiate Safety Initiative? Do you see any important contradictions between current VA policy and these new guidelines?
Dr. Drexler. Yes—not necessarily with policy but with our VA/DoD Clinical Practice Guidelines from 2010 and the new CDC guidelines. There’s a shift in emphasis. The 2010 VA/DoD guidelines were maybe a little too optimistic about the safety of opioids for chronic, noncancer pain. As time goes on, more evidence is mounting of the potential harms, including from my perspective, the risk of developing an opioid use disorder when taking opioid analgesics as prescribed.
In the 1990s and the early 2000s, experts in the field reassured us. They told us all—patients and providers alike—that if opioid analgesics were taken as prescribed for legitimate pain by people who didn’t have a previous history of a substance use disorder, they could take them and would not be at risk for becoming addicted. We now know that’s simply not true. Patients who take these medicines as prescribed by their providers end up developing tolerance, developing hyperalgesia, needing more and more medication to get the desired effect, and crossing that line from just physiologic tolerance to developing an opioid use disorder.
We now have better guidelines about where that risk increases. We have some data from observational studies that patients who are maintained on opioid analgesics that are < 50 mg of daily morphine equivalent dose (MED) tend not to have as high a risk of overdose as those who are maintained on more, but the risk of developing an opioid use disorder actually is not insignificant even below 50 mg MED.
The key for developing an opioid use disorder is how long the patient is on the treatment. For patients who take opioid analgesics for < 90 days—again, these are observational studies—very few went on to develop opioid use disorder. However, those who took it for > 90 days, even those who were maintained on < 36 mg MED, had a significantly increased risk of developing an opioid use disorder. And for those who were on the higher dose, say, > 120 mg MED, the risk went up to 122-fold.
We just don’t see those kinds of odds ratios elsewhere in medicine. You know, we address risk factors when it increases the risk by 50%, but this is 122-fold.
Dr. Geppert. Pain as the fifth vital sign had a dark side there. Is there anything else you want to say to us about the Opiate Safety Initiative?
Dr. Drexler. I think it’s been incredibly successful; but like any change, there are unintended consequences and challenges with any change, even a good change. One of the things that has been a good change is raising awareness that opioids are not the answer for chronic pain. We have better evidence that self-care strategies (movement, exercise, physical therapy, and cognitive behavioral therapy for pain) help folks learn strategies for addressing automatic alarming thoughts about their pain—that it’s signaling something terrible when, really, it’s not. There are things that people can do to manage pain on their own. Those [strategies] are safer, and we have better evidence that they are effective than we have for opioids taken past 12 weeks.
Dr. Geppert. It’s really a paradigm shift in understanding. The Comprehensive Addiction and Recovery Act of 2016 has proposed to allow some nurse practitioners (NPs) and physician assistants (PAs) to obtain a waiver to prescribe buprenorphine. What role does the VA see for PAs and NPs in patient care for veterans with substance use disorders with medication-assisted therapy, and do you think that’ll involve prescribing buprenorphine?
Dr. Drexler. One of the things that I love about practicing within the VA is working within an interdisciplinary team and having that team-based approach. When we work together, our results far outshine anything that any one of us could do separately. I am very much looking forward to being able to provide or collaborate with other federal partners like the Substance Abuse and Mental Health Services Administration [SAMHSA] to provide the training for VA NPs and PAs who are able to prescribe controlled substances with their state licensure to provide buprenorphine for our patients.
Even without that prescriptive authority, NPs and PAs are already important partners and members of the interdisciplinary team who are managing patients and providing the evidence-based counseling, the monitoring, following up urine drug screen results, and doing a lot of the management, but not signing the prescription that’s being provided now by the Drug Addiction Treatment Act-waivered physicians. But SAMHSA has yet to define what the 24 hours of training will be that NPs and PAs will need to complete in order to apply for their U.S. Drug Enforcement Administration waiver, but we’re very much looking forward to it. And I am in contact with colleagues at SAMHSA, and they promise me they will keep me informed when they have the regulations set out for us to follow.
Dr. Geppert. The new VA/DoD Clinical Practice Guidelines for Management of Substance Use Disorders were published earlier this year. Could you give us an overview of the guidelines and what’s significant for most of our providers in the field?
Dr. Drexler. I’m very excited about the guidelines. One of the first things you should know is that they are very evidence based. If you’ve been practicing medicine as long as I have, you’ll remember, when the first clinical practice guidelines came out, they were expert opinion. They got some of the leading practitioners and researchers in the country together, put them in a room, and said, “What do you think people should do to treat this disorder?” Over time, clinical practice guidelines have become more evidence based; and the VA/DoD collaborative follows a highly respected methodology called the GRADE methodology.
Let me explain that just briefly. It means that our recommendations are based on systematic reviews of the literature. We all have our own screens and filters and our favorite studies. And so, if you get a bunch of experts together, they may not have as dispassionate a view of the entire literature as when you do a systematic review; and that’s what we started with.
We followed a specific methodology as well that took into account not only the strength of the evidence where the gold standard is a randomized, controlled clinical trial and, even better than that, a meta-analysis of multiple clinical trials. And for our recommendations, the strength of the evidence was, by far, supported mostly by clinical trials or meta-analyses. So, when you look at the VA/DoD Clinical Practice Guidelines, and I hope you will, you can feel confident that what we’re recommending is not just pulled out of the sky or some idiosyncratic favorite thing that Karen Drexler likes. It’s really based on good, strong clinical evidence.
We went from the previous version that had over 160 recommendations. We distilled them down to 36, and those 36 recommendations cover everything from prevention in primary care settings to stabilization and withdrawal management for alcohol and sedatives and opioids, and then treatment or rehabilitation of the 4 most common substance use disorders and then some general principles (Table 1).
There’s good evidence that what we’re doing in VA with screening in primary care every year using the AUDIT-C [Alcohol Use Disorders Identification Test-Consumption] is making a difference (Table 2). That folks who screen positive for at-risk alcohol use are very likely to respond to a brief intervention by their health care provider and reduce their alcohol consumption to safe levels, where they’re not likely to go on to develop an addiction to the alcohol or to develop medical complications from heavy alcohol use.
After screening, then, if someone does develop a substance use disorder, we have evidence-based treatments, both medications for 2 of our most common disorders and psychosocial interventions for all 4 most common disorders after tobacco. And I don’t exclude tobacco because it’s not important; it’s incredibly important, but it gets its own clinical practice guidelines. So, with our limited budget, we just addressed the other 10 substances….
For alcohol we have great evidence for 5 different medications and for 6 different psychosocial approaches. It is the most common substance use disorder, but we also have a whole menu of different treatments that folks can choose from, everything from naltrexone—oral naltrexone is the most common medication that we prescribe—but also acamprosate; disulfiram; extended-release injectable naltrexone. Topiramate, interestingly, is not FDA approved for that indication, but we have found good evidence from clinical trials supporting topiramate, so we strongly recommend it. If any of those are not acceptable or not tolerated or ineffective, then we also have some evidence to support gabapentin as well. So we have a strong recommendation for the first ones and a suggestion for gabapentin if it’s clinically indicated.
In terms of psychosocial approaches, [we recommend] everything from motivation enhancement therapy or motivational interviewing; cognitive-behavioral therapy; 12-step facilitation, which is a structured way of getting folks to get more active in Alcoholics Anonymous; behavioral approaches like community reinforcement approach or contingency management.
For opioid use disorder, interestingly, we found good, strong evidence that medication-assisted treatments work but no evidence for any psychosocial intervention without medication. Let me say that again because, nationwide, the most common treatment that’s provided for opioid use disorder, for any substance use disorder, is a psychosocial intervention—talking therapy alone.
For all of the medicines for alcohol use disorder, the medicine improved upon talking therapy alone. For opioid use disorder, medication helps people stay sober; it retains them in treatment; it saves lives. But for talking therapy without medication, we have found insufficient evidence to recommend any particular treatment.
Dr. Geppert. Incredible. That leads to my next question: One of the most challenging issues facing primary care practitioners is how to manage patients who are on chronic opiate therapy but also use medical marijuana in the increasing number of states where it’s legal. Do you have some advice on how to approach these situations?
Dr. Drexler. It’s a great question, and it’s one I’m sure more and more VA practitioners are grappling with. By our policy, we do not exclude veterans who are taking medical marijuana from any VA services, and we encourage the veterans to speak up about it so that it can be considered as part of their treatment plan.
By policy, however, VA practitioners do not fill out forms for state-approved marijuana. Marijuana is still a Schedule I controlled substance by federal law, so it’s a very interesting legal situation where it’s illegal federally; but some states have passed laws making it legal by state law. It will be interesting to see how this plays out over the next few years.
But in that situation—and really, this is our policy, in general for substance use disorders—a patient having a substance use disorder should not be precluded or excluded from getting treatment for other medical conditions. Similarly, other medical conditions shouldn’t exclude patients from getting treatment for their substance use disorder. However, the best treatment comes when we look at the whole person and try to take both things into account.
In the same way that patients can become addicted to their opioid pain medicines, patients can become addicted to medical marijuana. So the marijuana use needs to be evaluated objectively; and whether or not they have a form, just like whether or not someone has a prescription for their opioid that may be causing them problems, that needs to be taken into account in the treatment plan; and the treatment needs to be individualized.
Dr. Geppert. What in VA substance use disorder programs and treatment do you think is especially innovative when compared to the community?
Dr. Drexler. One of the places where I think the VA is well ahead of our colleagues elsewhere in health care is…from SAIL [Strategic Analytics for Improvement and Learning] metrics. About 34% or 35% of veterans who are diagnosed clinically within our system with opioid use disorders are receiving medication-assisted therapy either with buprenorphine, methadone, or extended-release injectable naltrexone. When you look at the SAMHSA survey, the National Survey on Drug Use and Health, the percentage of patients in the community who meet criteria for a substance use disorder [who] actually receive treatment is more like 12%. We have no numbers from SAMHSA about what percentage are receiving medication-assisted treatment. They don’t ask. In that way, even though we have a long ways to go, we’re ahead of the curve when it comes to medication-assisted treatment. But the demand is growing, we’re going to have to keep working hard to keep up and to get ahead at this. When I think about it compared to other chronic illnesses, only 35% of our patients are getting the first-line recommended treatment. We still have a long ways to go.
Another place where we’re innovative is with contingency management. I mentioned psychosocial treatments. Behavioral treatment is very effective and yet is not often used in treatment of substance use disorders; but it works. And even better than that, it is fun.
Let me try to explain what this means. It’s based on the idea that the benefits of using drugs are immediate, and the benefits of staying sober take a long time to realize. And it is that difference that makes early recovery so challenging because, if someone has a craving for alcohol or another drug, they can satisfy that craving immediately and then have to pay the price, though, of health consequences or financial consequences or legal consequences from that alcohol or drug use.
On the other hand, staying sober is tough. But it takes a long time to put one’s life back together, to get one’s chronic health conditions that have been caused or made worse by the drug use back together. And the idea behind contingency management is to give immediate rewards early in recovery to keep folks engaged long enough to start realizing some of the longer-term benefits of recovery.
So the way this works, we use what we call the fishbowl model of contingency management. And we started with stimulant use disorders, which is one of the substance use disorders for which there’s no strong evidence for a medication that works; but this particular psychosocial intervention works very well. So patients come to the clinic twice a week, give a urine specimen for drug screen; and, based on those test results, if their test is negative and they haven’t used any stimulants, then they get a draw from the fishbowl, which is filled with slips of paper. Half of the slips of paper say, “Good job! Keep up the good work,” or something encouraging like that. And half of the slips of paper have a prize associated with them, and it could be a small prize like $1 or $5 for exchange at the VA Canteen store, or it could be a larger prize like $100 or $200. Of course, the less expensive prizes are more common than the more expensive prizes; but just that immediate reward can make such a difference.
With the first negative urine drug screen, you get 1 draw from the fishbowl. The second one in a row, you get 2; and so it goes that the longer you stay sober, the more chances you have for winning a prize. And this treatment can go on for as long as the veteran wants to continue for up to 12 weeks. Usually by 12 weeks, folks’ health and their lives are coming back together; and so there’s many more rewards for staying sober than just the draws from the fishbowl. And what we find is folks who get a good start with contingency management tend to stay on that trajectory towards recovery.
And this has been a wonderful partnership between the Center of Excellence for Substance Abuse Treatment and Education in Philadelphia and the VA Canteen Service, who has generously donated canteen coupons. So it doesn’t cost the medical center anything to offer these prizes, and we’ve had just some amazing results.
To listen to the unedited interview here
While much of private health care in the U.S. is only beginning to confront the burgeoning opioid epidemic, the VA has a long-standing commitment to providing treatment to veterans struggling with substance use disorders. To understand the scope of the challenge and the VA’s approach, Federal Practitioner Editor-in-Chief Cynthia M.A. Geppert, MD, talked with Karen Drexler, MD, national mental health program director, substance use disorders. Dr. Drexler served in the U.S. Air Force for 8 years before joining the Atlanta VAMC in Georgia, where she directed the substance abuse treatment program. She is an associate professor of psychiatry and behavioral sciences at Emory University. In 2014, Dr. Drexler was named deputy mental health program director, addictive disorders.
Editor-in-Chief Cynthia M.A. Geppert, MD. As national mental health program director for substance use disorders, what are the challenges facing VA substance use programs?
Karen Drexler, MD. The biggest challenge is the increasing demand for services. Veterans have been coming to the VA for mental health care in general and for substance use disorder treatment in particular. The most common substance use disorder that we treat in addition to tobacco is alcohol use disorder, and the demand for alcohol use disorder treatment continues to grow.
Also, with the opioid crisis, there’s increasing demand for opioid use disorder treatments, including what we recommend as first-line treatment, which is medication-assisted treatment using buprenorphine or methadone, or injectable naltrexone as a second-line treatment. Gearing up with medication-assisted treatment for both alcohol and opioid use disorders is probably our biggest challenge.
Dr. Geppert. What were the most important accomplishments of the VA substance abuse programs over the past 5 years?
Dr. Drexler. There have been a lot! First of all, one of the things I love about practicing within the VA is that we are an integrated health care system. When I talk with colleagues in the Emory system here in Atlanta and across the country, oftentimes mental health and substance use disorders are isolated. The funding streams through the public sector come in different ways. Third-party payers have carve-outs for behavioral health.
It’s really wonderful to be able to collaborate with my colleagues in primary care, medical specialty care, and general mental health care, to provide a holistic and team-based approach to our patients who have both medical and substance use disorder problems and often co-occurring mental illness. As we’ve moved from a hospital-based system to more outpatient [care], the VA continues to be able to collaborate through our electronic health records as well as just being able to pick up the phone or send a [Microsoft] Lync message or an Outlook encrypted e-mail to help facilitate that coordinated care.
Another accomplishment has been to [develop] policy. It’s one of our challenges, but it’s also an accomplishment even at this early stage. In 2008, we issued the Uniform Mental Health Services benefits package as part of VHA Handbook on Mental Health Services. From that very beginning, we have included medication-assisted therapy when indicated for veterans with substance use disorders.
Now, we also know there’s a lot of variability in the system, so not every facility is providing these indicated treatments yet; but at least it’s part of our policy, and it’s been a focus of ongoing quality improvement to make these treatments available for veterans when they need them.
Dr. Geppert. We’ve heard a lot lately about the CDC guidelines for prescribing opiates for chronic pain that were published earlier this year. How do you see these guidelines affecting the VA Opiate Safety Initiative? Do you see any important contradictions between current VA policy and these new guidelines?
Dr. Drexler. Yes—not necessarily with policy but with our VA/DoD Clinical Practice Guidelines from 2010 and the new CDC guidelines. There’s a shift in emphasis. The 2010 VA/DoD guidelines were maybe a little too optimistic about the safety of opioids for chronic, noncancer pain. As time goes on, more evidence is mounting of the potential harms, including from my perspective, the risk of developing an opioid use disorder when taking opioid analgesics as prescribed.
In the 1990s and the early 2000s, experts in the field reassured us. They told us all—patients and providers alike—that if opioid analgesics were taken as prescribed for legitimate pain by people who didn’t have a previous history of a substance use disorder, they could take them and would not be at risk for becoming addicted. We now know that’s simply not true. Patients who take these medicines as prescribed by their providers end up developing tolerance, developing hyperalgesia, needing more and more medication to get the desired effect, and crossing that line from just physiologic tolerance to developing an opioid use disorder.
We now have better guidelines about where that risk increases. We have some data from observational studies that patients who are maintained on opioid analgesics that are < 50 mg of daily morphine equivalent dose (MED) tend not to have as high a risk of overdose as those who are maintained on more, but the risk of developing an opioid use disorder actually is not insignificant even below 50 mg MED.
The key for developing an opioid use disorder is how long the patient is on the treatment. For patients who take opioid analgesics for < 90 days—again, these are observational studies—very few went on to develop opioid use disorder. However, those who took it for > 90 days, even those who were maintained on < 36 mg MED, had a significantly increased risk of developing an opioid use disorder. And for those who were on the higher dose, say, > 120 mg MED, the risk went up to 122-fold.
We just don’t see those kinds of odds ratios elsewhere in medicine. You know, we address risk factors when it increases the risk by 50%, but this is 122-fold.
Dr. Geppert. Pain as the fifth vital sign had a dark side there. Is there anything else you want to say to us about the Opiate Safety Initiative?
Dr. Drexler. I think it’s been incredibly successful; but like any change, there are unintended consequences and challenges with any change, even a good change. One of the things that has been a good change is raising awareness that opioids are not the answer for chronic pain. We have better evidence that self-care strategies (movement, exercise, physical therapy, and cognitive behavioral therapy for pain) help folks learn strategies for addressing automatic alarming thoughts about their pain—that it’s signaling something terrible when, really, it’s not. There are things that people can do to manage pain on their own. Those [strategies] are safer, and we have better evidence that they are effective than we have for opioids taken past 12 weeks.
Dr. Geppert. It’s really a paradigm shift in understanding. The Comprehensive Addiction and Recovery Act of 2016 has proposed to allow some nurse practitioners (NPs) and physician assistants (PAs) to obtain a waiver to prescribe buprenorphine. What role does the VA see for PAs and NPs in patient care for veterans with substance use disorders with medication-assisted therapy, and do you think that’ll involve prescribing buprenorphine?
Dr. Drexler. One of the things that I love about practicing within the VA is working within an interdisciplinary team and having that team-based approach. When we work together, our results far outshine anything that any one of us could do separately. I am very much looking forward to being able to provide or collaborate with other federal partners like the Substance Abuse and Mental Health Services Administration [SAMHSA] to provide the training for VA NPs and PAs who are able to prescribe controlled substances with their state licensure to provide buprenorphine for our patients.
Even without that prescriptive authority, NPs and PAs are already important partners and members of the interdisciplinary team who are managing patients and providing the evidence-based counseling, the monitoring, following up urine drug screen results, and doing a lot of the management, but not signing the prescription that’s being provided now by the Drug Addiction Treatment Act-waivered physicians. But SAMHSA has yet to define what the 24 hours of training will be that NPs and PAs will need to complete in order to apply for their U.S. Drug Enforcement Administration waiver, but we’re very much looking forward to it. And I am in contact with colleagues at SAMHSA, and they promise me they will keep me informed when they have the regulations set out for us to follow.
Dr. Geppert. The new VA/DoD Clinical Practice Guidelines for Management of Substance Use Disorders were published earlier this year. Could you give us an overview of the guidelines and what’s significant for most of our providers in the field?
Dr. Drexler. I’m very excited about the guidelines. One of the first things you should know is that they are very evidence based. If you’ve been practicing medicine as long as I have, you’ll remember, when the first clinical practice guidelines came out, they were expert opinion. They got some of the leading practitioners and researchers in the country together, put them in a room, and said, “What do you think people should do to treat this disorder?” Over time, clinical practice guidelines have become more evidence based; and the VA/DoD collaborative follows a highly respected methodology called the GRADE methodology.
Let me explain that just briefly. It means that our recommendations are based on systematic reviews of the literature. We all have our own screens and filters and our favorite studies. And so, if you get a bunch of experts together, they may not have as dispassionate a view of the entire literature as when you do a systematic review; and that’s what we started with.
We followed a specific methodology as well that took into account not only the strength of the evidence where the gold standard is a randomized, controlled clinical trial and, even better than that, a meta-analysis of multiple clinical trials. And for our recommendations, the strength of the evidence was, by far, supported mostly by clinical trials or meta-analyses. So, when you look at the VA/DoD Clinical Practice Guidelines, and I hope you will, you can feel confident that what we’re recommending is not just pulled out of the sky or some idiosyncratic favorite thing that Karen Drexler likes. It’s really based on good, strong clinical evidence.
We went from the previous version that had over 160 recommendations. We distilled them down to 36, and those 36 recommendations cover everything from prevention in primary care settings to stabilization and withdrawal management for alcohol and sedatives and opioids, and then treatment or rehabilitation of the 4 most common substance use disorders and then some general principles (Table 1).
There’s good evidence that what we’re doing in VA with screening in primary care every year using the AUDIT-C [Alcohol Use Disorders Identification Test-Consumption] is making a difference (Table 2). That folks who screen positive for at-risk alcohol use are very likely to respond to a brief intervention by their health care provider and reduce their alcohol consumption to safe levels, where they’re not likely to go on to develop an addiction to the alcohol or to develop medical complications from heavy alcohol use.
After screening, then, if someone does develop a substance use disorder, we have evidence-based treatments, both medications for 2 of our most common disorders and psychosocial interventions for all 4 most common disorders after tobacco. And I don’t exclude tobacco because it’s not important; it’s incredibly important, but it gets its own clinical practice guidelines. So, with our limited budget, we just addressed the other 10 substances….
For alcohol we have great evidence for 5 different medications and for 6 different psychosocial approaches. It is the most common substance use disorder, but we also have a whole menu of different treatments that folks can choose from, everything from naltrexone—oral naltrexone is the most common medication that we prescribe—but also acamprosate; disulfiram; extended-release injectable naltrexone. Topiramate, interestingly, is not FDA approved for that indication, but we have found good evidence from clinical trials supporting topiramate, so we strongly recommend it. If any of those are not acceptable or not tolerated or ineffective, then we also have some evidence to support gabapentin as well. So we have a strong recommendation for the first ones and a suggestion for gabapentin if it’s clinically indicated.
In terms of psychosocial approaches, [we recommend] everything from motivation enhancement therapy or motivational interviewing; cognitive-behavioral therapy; 12-step facilitation, which is a structured way of getting folks to get more active in Alcoholics Anonymous; behavioral approaches like community reinforcement approach or contingency management.
For opioid use disorder, interestingly, we found good, strong evidence that medication-assisted treatments work but no evidence for any psychosocial intervention without medication. Let me say that again because, nationwide, the most common treatment that’s provided for opioid use disorder, for any substance use disorder, is a psychosocial intervention—talking therapy alone.
For all of the medicines for alcohol use disorder, the medicine improved upon talking therapy alone. For opioid use disorder, medication helps people stay sober; it retains them in treatment; it saves lives. But for talking therapy without medication, we have found insufficient evidence to recommend any particular treatment.
Dr. Geppert. Incredible. That leads to my next question: One of the most challenging issues facing primary care practitioners is how to manage patients who are on chronic opiate therapy but also use medical marijuana in the increasing number of states where it’s legal. Do you have some advice on how to approach these situations?
Dr. Drexler. It’s a great question, and it’s one I’m sure more and more VA practitioners are grappling with. By our policy, we do not exclude veterans who are taking medical marijuana from any VA services, and we encourage the veterans to speak up about it so that it can be considered as part of their treatment plan.
By policy, however, VA practitioners do not fill out forms for state-approved marijuana. Marijuana is still a Schedule I controlled substance by federal law, so it’s a very interesting legal situation where it’s illegal federally; but some states have passed laws making it legal by state law. It will be interesting to see how this plays out over the next few years.
But in that situation—and really, this is our policy, in general for substance use disorders—a patient having a substance use disorder should not be precluded or excluded from getting treatment for other medical conditions. Similarly, other medical conditions shouldn’t exclude patients from getting treatment for their substance use disorder. However, the best treatment comes when we look at the whole person and try to take both things into account.
In the same way that patients can become addicted to their opioid pain medicines, patients can become addicted to medical marijuana. So the marijuana use needs to be evaluated objectively; and whether or not they have a form, just like whether or not someone has a prescription for their opioid that may be causing them problems, that needs to be taken into account in the treatment plan; and the treatment needs to be individualized.
Dr. Geppert. What in VA substance use disorder programs and treatment do you think is especially innovative when compared to the community?
Dr. Drexler. One of the places where I think the VA is well ahead of our colleagues elsewhere in health care is…from SAIL [Strategic Analytics for Improvement and Learning] metrics. About 34% or 35% of veterans who are diagnosed clinically within our system with opioid use disorders are receiving medication-assisted therapy either with buprenorphine, methadone, or extended-release injectable naltrexone. When you look at the SAMHSA survey, the National Survey on Drug Use and Health, the percentage of patients in the community who meet criteria for a substance use disorder [who] actually receive treatment is more like 12%. We have no numbers from SAMHSA about what percentage are receiving medication-assisted treatment. They don’t ask. In that way, even though we have a long ways to go, we’re ahead of the curve when it comes to medication-assisted treatment. But the demand is growing, we’re going to have to keep working hard to keep up and to get ahead at this. When I think about it compared to other chronic illnesses, only 35% of our patients are getting the first-line recommended treatment. We still have a long ways to go.
Another place where we’re innovative is with contingency management. I mentioned psychosocial treatments. Behavioral treatment is very effective and yet is not often used in treatment of substance use disorders; but it works. And even better than that, it is fun.
Let me try to explain what this means. It’s based on the idea that the benefits of using drugs are immediate, and the benefits of staying sober take a long time to realize. And it is that difference that makes early recovery so challenging because, if someone has a craving for alcohol or another drug, they can satisfy that craving immediately and then have to pay the price, though, of health consequences or financial consequences or legal consequences from that alcohol or drug use.
On the other hand, staying sober is tough. But it takes a long time to put one’s life back together, to get one’s chronic health conditions that have been caused or made worse by the drug use back together. And the idea behind contingency management is to give immediate rewards early in recovery to keep folks engaged long enough to start realizing some of the longer-term benefits of recovery.
So the way this works, we use what we call the fishbowl model of contingency management. And we started with stimulant use disorders, which is one of the substance use disorders for which there’s no strong evidence for a medication that works; but this particular psychosocial intervention works very well. So patients come to the clinic twice a week, give a urine specimen for drug screen; and, based on those test results, if their test is negative and they haven’t used any stimulants, then they get a draw from the fishbowl, which is filled with slips of paper. Half of the slips of paper say, “Good job! Keep up the good work,” or something encouraging like that. And half of the slips of paper have a prize associated with them, and it could be a small prize like $1 or $5 for exchange at the VA Canteen store, or it could be a larger prize like $100 or $200. Of course, the less expensive prizes are more common than the more expensive prizes; but just that immediate reward can make such a difference.
With the first negative urine drug screen, you get 1 draw from the fishbowl. The second one in a row, you get 2; and so it goes that the longer you stay sober, the more chances you have for winning a prize. And this treatment can go on for as long as the veteran wants to continue for up to 12 weeks. Usually by 12 weeks, folks’ health and their lives are coming back together; and so there’s many more rewards for staying sober than just the draws from the fishbowl. And what we find is folks who get a good start with contingency management tend to stay on that trajectory towards recovery.
And this has been a wonderful partnership between the Center of Excellence for Substance Abuse Treatment and Education in Philadelphia and the VA Canteen Service, who has generously donated canteen coupons. So it doesn’t cost the medical center anything to offer these prizes, and we’ve had just some amazing results.
To listen to the unedited interview here
CHMP recommends expanding use of drug in CLL

Photo courtesy of GSK
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved indication for ofatumumab (Arzerra®).
The CHMP is recommending the drug be approved for use in combination with fludarabine and cyclophosphamide to treat adults with relapsed chronic lymphocytic leukemia (CLL).
The CHMP’s recommendation will be reviewed by the European Commission.
A final decision is expected in the coming months.
Ofatumumab is a monoclonal antibody designed to target CD20. The drug is marketed under a collaboration agreement between Genmab and Novartis.
The European Commission has already approved ofatumumab for the following indications:
- As a single-agent to treat CLL patients who are refractory to fludarabine and alemtuzumab
- For use in combination with chlorambucil or bendamustine in CLL patients who
have not received prior therapy and are not eligible for
fludarabine-based therapy.
COMPLEMENT 2 trial
The CHMP’s recommendation to approve ofatumumab in combination with fludarabine and cyclophosphamide was based on results from the phase 3 COMPLEMENT 2 study. Novartis reported top-line results from this study last April.
The trial enrolled 365 patients with relapsed CLL. The patients were randomized 1:1 to receive up to 6 cycles of ofatumumab in combination with fludarabine and cyclophosphamide or up to 6 cycles of fludarabine and cyclophosphamide alone.
The primary endpoint was progression-free survival, as assessed by an independent review committee.
The median progression-free survival was 28.9 months for patients receiving ofatumumab plus fludarabine and cyclophosphamide, compared to 18.8 months for patients receiving fludarabine and cyclophosphamide alone (hazard ratio=0.67, P=0.0032).
Novartis said the safety profile observed in this study was consistent with other trials of ofatumumab, and no new safety signals were observed. ![]()

Photo courtesy of GSK
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved indication for ofatumumab (Arzerra®).
The CHMP is recommending the drug be approved for use in combination with fludarabine and cyclophosphamide to treat adults with relapsed chronic lymphocytic leukemia (CLL).
The CHMP’s recommendation will be reviewed by the European Commission.
A final decision is expected in the coming months.
Ofatumumab is a monoclonal antibody designed to target CD20. The drug is marketed under a collaboration agreement between Genmab and Novartis.
The European Commission has already approved ofatumumab for the following indications:
- As a single-agent to treat CLL patients who are refractory to fludarabine and alemtuzumab
- For use in combination with chlorambucil or bendamustine in CLL patients who
have not received prior therapy and are not eligible for
fludarabine-based therapy.
COMPLEMENT 2 trial
The CHMP’s recommendation to approve ofatumumab in combination with fludarabine and cyclophosphamide was based on results from the phase 3 COMPLEMENT 2 study. Novartis reported top-line results from this study last April.
The trial enrolled 365 patients with relapsed CLL. The patients were randomized 1:1 to receive up to 6 cycles of ofatumumab in combination with fludarabine and cyclophosphamide or up to 6 cycles of fludarabine and cyclophosphamide alone.
The primary endpoint was progression-free survival, as assessed by an independent review committee.
The median progression-free survival was 28.9 months for patients receiving ofatumumab plus fludarabine and cyclophosphamide, compared to 18.8 months for patients receiving fludarabine and cyclophosphamide alone (hazard ratio=0.67, P=0.0032).
Novartis said the safety profile observed in this study was consistent with other trials of ofatumumab, and no new safety signals were observed. ![]()

Photo courtesy of GSK
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved indication for ofatumumab (Arzerra®).
The CHMP is recommending the drug be approved for use in combination with fludarabine and cyclophosphamide to treat adults with relapsed chronic lymphocytic leukemia (CLL).
The CHMP’s recommendation will be reviewed by the European Commission.
A final decision is expected in the coming months.
Ofatumumab is a monoclonal antibody designed to target CD20. The drug is marketed under a collaboration agreement between Genmab and Novartis.
The European Commission has already approved ofatumumab for the following indications:
- As a single-agent to treat CLL patients who are refractory to fludarabine and alemtuzumab
- For use in combination with chlorambucil or bendamustine in CLL patients who
have not received prior therapy and are not eligible for
fludarabine-based therapy.
COMPLEMENT 2 trial
The CHMP’s recommendation to approve ofatumumab in combination with fludarabine and cyclophosphamide was based on results from the phase 3 COMPLEMENT 2 study. Novartis reported top-line results from this study last April.
The trial enrolled 365 patients with relapsed CLL. The patients were randomized 1:1 to receive up to 6 cycles of ofatumumab in combination with fludarabine and cyclophosphamide or up to 6 cycles of fludarabine and cyclophosphamide alone.
The primary endpoint was progression-free survival, as assessed by an independent review committee.
The median progression-free survival was 28.9 months for patients receiving ofatumumab plus fludarabine and cyclophosphamide, compared to 18.8 months for patients receiving fludarabine and cyclophosphamide alone (hazard ratio=0.67, P=0.0032).
Novartis said the safety profile observed in this study was consistent with other trials of ofatumumab, and no new safety signals were observed. ![]()
NCCN issues challenge to ‘bag’ vincristine
Photo courtesy of ISMP
PHILADELPHIA—To ensure proper administration of vincristine, the National Comprehensive Cancer Network (NCCN) has issued a challenge to hospitals, medical centers, and oncology practices as part of its “Just Bag It!” campaign.
Vincristine—the “O” for Oncovin in the CHOP regimen—is widely used to treat patients with leukemia or lymphoma.
It is considered an important chemotherapeutic agent. However, if administered incorrectly, vincristine is uniformly fatal, usually within a week, according to the NCCN.
Vincristine is highly neurotoxic and should always be administered intravenously. If it is mistakenly given intrathecally along with other chemotherapy drugs, it causes ascending paralysis, neurological defects, and death.
Therefore, the NCCN recommends always diluting and administering vincristine in a mini IV-drip bag, never through a syringe.
This precaution decreases the chances of improper dosage and makes it impossible to accidentally administer vincristine into the spinal fluid.
The NCCN initiated the safe-handling campaign in response to the death 11 years ago of a 21-year-old patient who received vincristine incorrectly administered into his spinal fluid. He was referred to Robert W. Carlson, MD, NCCN’s chief executive officer, who, at the time, was at Stanford Hospital, not the hospital where the error occurred.
The patient, Christopher Wibeto, had a “likely curable” non-Hodgkin lymphoma and died 4 days later.
“When I first met Christopher, he was doing well,” Dr Carlson said. “He was a delightful young gentleman, very articulate. He was funny. Even in the ICU, he had me chuckling and laughing at what he was saying.”
“But we knew that the medical error would almost certainly lead to his death. Shortly thereafter, I met his parents, Debra and Robin, . . . and had to tell them what the consequences of that medical error were likely to be. And they joined me in Christopher’s room while we talked with him about what the consequences of that medical error were likely to be.”
Making the situation even more painful, Dr Carlson, at that time, was the father of a young son who is now almost the age Christopher was then.
Dr Carlson said he realized that “we needed to come up with systems to assure that this did not happen, not today or tomorrow or ever again.”
Motivated by the tragedy, Dr Carlson spearheaded a national effort to address this mistake when he joined NCCN as CEO, enlisting the help of NCCN’s Best Practices Committee.
The NCCN developed and issued guidelines, and all 27 member institutions have adopted policies in line with the guidelines.
The Institute for Safe Medication Practices (ISMP) has undertaken efforts over more than a decade to implement procedures for safe vincristine administration.
ISMP conducted surveys and follow-up self-assessments regarding use of IV bags for vincristine at oncology practice sites. They found that only about half the institutions surveyed dilute IV vincristine for administration in a small-volume bag.
Some practitioners associate the use of an IV bag with an increased risk of extravasation (when the chemotherapy agent leaks into the tissue surrounding the administration site). Research shows, however, that the risk of extravasation is extremely low (less than 0.5%), regardless of how vincristine is administered.
And cost is not an issue when implementing the mini-bag policy, according to the president of ISMP, Michael R. Cohen, RPh.
“It cost a few pennies more,” he said. “And I mean pennies. I think probably what is an issue is just the age-old habit of putting vincristine in a syringe and being able to change that habit.”
Since the introduction of vincristine use in the 1960s, 125 documented cases of accidental death in the US and abroad have been reported. While the error is relatively rare, it is preventable and unique in its level of mortality.
“It’s hard to understand why this idea of ‘Just Bag It’ hasn’t permeated healthcare at this point,” Cohen said. “Because it is a sure-fire way to prevent this type of error.”
The ISMP, the Joint Commission, the World Health Organization, and the Oncology Nursing Society also recommend the bag-it policy. ![]()

Photo courtesy of ISMP
PHILADELPHIA—To ensure proper administration of vincristine, the National Comprehensive Cancer Network (NCCN) has issued a challenge to hospitals, medical centers, and oncology practices as part of its “Just Bag It!” campaign.
Vincristine—the “O” for Oncovin in the CHOP regimen—is widely used to treat patients with leukemia or lymphoma.
It is considered an important chemotherapeutic agent. However, if administered incorrectly, vincristine is uniformly fatal, usually within a week, according to the NCCN.
Vincristine is highly neurotoxic and should always be administered intravenously. If it is mistakenly given intrathecally along with other chemotherapy drugs, it causes ascending paralysis, neurological defects, and death.
Therefore, the NCCN recommends always diluting and administering vincristine in a mini IV-drip bag, never through a syringe.
This precaution decreases the chances of improper dosage and makes it impossible to accidentally administer vincristine into the spinal fluid.
The NCCN initiated the safe-handling campaign in response to the death 11 years ago of a 21-year-old patient who received vincristine incorrectly administered into his spinal fluid. He was referred to Robert W. Carlson, MD, NCCN’s chief executive officer, who, at the time, was at Stanford Hospital, not the hospital where the error occurred.
The patient, Christopher Wibeto, had a “likely curable” non-Hodgkin lymphoma and died 4 days later.
“When I first met Christopher, he was doing well,” Dr Carlson said. “He was a delightful young gentleman, very articulate. He was funny. Even in the ICU, he had me chuckling and laughing at what he was saying.”
“But we knew that the medical error would almost certainly lead to his death. Shortly thereafter, I met his parents, Debra and Robin, . . . and had to tell them what the consequences of that medical error were likely to be. And they joined me in Christopher’s room while we talked with him about what the consequences of that medical error were likely to be.”
Making the situation even more painful, Dr Carlson, at that time, was the father of a young son who is now almost the age Christopher was then.
Dr Carlson said he realized that “we needed to come up with systems to assure that this did not happen, not today or tomorrow or ever again.”
Motivated by the tragedy, Dr Carlson spearheaded a national effort to address this mistake when he joined NCCN as CEO, enlisting the help of NCCN’s Best Practices Committee.
The NCCN developed and issued guidelines, and all 27 member institutions have adopted policies in line with the guidelines.
The Institute for Safe Medication Practices (ISMP) has undertaken efforts over more than a decade to implement procedures for safe vincristine administration.
ISMP conducted surveys and follow-up self-assessments regarding use of IV bags for vincristine at oncology practice sites. They found that only about half the institutions surveyed dilute IV vincristine for administration in a small-volume bag.
Some practitioners associate the use of an IV bag with an increased risk of extravasation (when the chemotherapy agent leaks into the tissue surrounding the administration site). Research shows, however, that the risk of extravasation is extremely low (less than 0.5%), regardless of how vincristine is administered.
And cost is not an issue when implementing the mini-bag policy, according to the president of ISMP, Michael R. Cohen, RPh.
“It cost a few pennies more,” he said. “And I mean pennies. I think probably what is an issue is just the age-old habit of putting vincristine in a syringe and being able to change that habit.”
Since the introduction of vincristine use in the 1960s, 125 documented cases of accidental death in the US and abroad have been reported. While the error is relatively rare, it is preventable and unique in its level of mortality.
“It’s hard to understand why this idea of ‘Just Bag It’ hasn’t permeated healthcare at this point,” Cohen said. “Because it is a sure-fire way to prevent this type of error.”
The ISMP, the Joint Commission, the World Health Organization, and the Oncology Nursing Society also recommend the bag-it policy. ![]()

Photo courtesy of ISMP
PHILADELPHIA—To ensure proper administration of vincristine, the National Comprehensive Cancer Network (NCCN) has issued a challenge to hospitals, medical centers, and oncology practices as part of its “Just Bag It!” campaign.
Vincristine—the “O” for Oncovin in the CHOP regimen—is widely used to treat patients with leukemia or lymphoma.
It is considered an important chemotherapeutic agent. However, if administered incorrectly, vincristine is uniformly fatal, usually within a week, according to the NCCN.
Vincristine is highly neurotoxic and should always be administered intravenously. If it is mistakenly given intrathecally along with other chemotherapy drugs, it causes ascending paralysis, neurological defects, and death.
Therefore, the NCCN recommends always diluting and administering vincristine in a mini IV-drip bag, never through a syringe.
This precaution decreases the chances of improper dosage and makes it impossible to accidentally administer vincristine into the spinal fluid.
The NCCN initiated the safe-handling campaign in response to the death 11 years ago of a 21-year-old patient who received vincristine incorrectly administered into his spinal fluid. He was referred to Robert W. Carlson, MD, NCCN’s chief executive officer, who, at the time, was at Stanford Hospital, not the hospital where the error occurred.
The patient, Christopher Wibeto, had a “likely curable” non-Hodgkin lymphoma and died 4 days later.
“When I first met Christopher, he was doing well,” Dr Carlson said. “He was a delightful young gentleman, very articulate. He was funny. Even in the ICU, he had me chuckling and laughing at what he was saying.”
“But we knew that the medical error would almost certainly lead to his death. Shortly thereafter, I met his parents, Debra and Robin, . . . and had to tell them what the consequences of that medical error were likely to be. And they joined me in Christopher’s room while we talked with him about what the consequences of that medical error were likely to be.”
Making the situation even more painful, Dr Carlson, at that time, was the father of a young son who is now almost the age Christopher was then.
Dr Carlson said he realized that “we needed to come up with systems to assure that this did not happen, not today or tomorrow or ever again.”
Motivated by the tragedy, Dr Carlson spearheaded a national effort to address this mistake when he joined NCCN as CEO, enlisting the help of NCCN’s Best Practices Committee.
The NCCN developed and issued guidelines, and all 27 member institutions have adopted policies in line with the guidelines.
The Institute for Safe Medication Practices (ISMP) has undertaken efforts over more than a decade to implement procedures for safe vincristine administration.
ISMP conducted surveys and follow-up self-assessments regarding use of IV bags for vincristine at oncology practice sites. They found that only about half the institutions surveyed dilute IV vincristine for administration in a small-volume bag.
Some practitioners associate the use of an IV bag with an increased risk of extravasation (when the chemotherapy agent leaks into the tissue surrounding the administration site). Research shows, however, that the risk of extravasation is extremely low (less than 0.5%), regardless of how vincristine is administered.
And cost is not an issue when implementing the mini-bag policy, according to the president of ISMP, Michael R. Cohen, RPh.
“It cost a few pennies more,” he said. “And I mean pennies. I think probably what is an issue is just the age-old habit of putting vincristine in a syringe and being able to change that habit.”
Since the introduction of vincristine use in the 1960s, 125 documented cases of accidental death in the US and abroad have been reported. While the error is relatively rare, it is preventable and unique in its level of mortality.
“It’s hard to understand why this idea of ‘Just Bag It’ hasn’t permeated healthcare at this point,” Cohen said. “Because it is a sure-fire way to prevent this type of error.”
The ISMP, the Joint Commission, the World Health Organization, and the Oncology Nursing Society also recommend the bag-it policy. ![]()
Health Canada approves drug for patients with VTE, NVAF

Image by Andre E.X. Brown
Health Canada has approved edoxaban (Lixiana®), an oral factor Xa inhibitor, for use in patients with venous thromboembolism (VTE) or nonvalvular atrial fibrillation (NVAF).
The drug can now be used to treat and prevent the recurrence of deep vein thrombosis (DVT) and pulmonary embolism (PE).
It can also be used to prevent stroke and systemic embolism in adults with NVAF in whom anticoagulation is appropriate.
Edoxaban was discovered and developed by Daiichi Sankyo Co., Ltd., but Servier Canada will market the drug in Canada.
Edoxaban has been approved in the US, European Union, Switzerland, Japan, South Korea, Taiwan, and Hong Kong. The drug is marketed as Savaysa® in the US and as Lixiana® elsewhere.
Health Canada’s approval of edoxaban is based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic VTE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()

Image by Andre E.X. Brown
Health Canada has approved edoxaban (Lixiana®), an oral factor Xa inhibitor, for use in patients with venous thromboembolism (VTE) or nonvalvular atrial fibrillation (NVAF).
The drug can now be used to treat and prevent the recurrence of deep vein thrombosis (DVT) and pulmonary embolism (PE).
It can also be used to prevent stroke and systemic embolism in adults with NVAF in whom anticoagulation is appropriate.
Edoxaban was discovered and developed by Daiichi Sankyo Co., Ltd., but Servier Canada will market the drug in Canada.
Edoxaban has been approved in the US, European Union, Switzerland, Japan, South Korea, Taiwan, and Hong Kong. The drug is marketed as Savaysa® in the US and as Lixiana® elsewhere.
Health Canada’s approval of edoxaban is based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic VTE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()

Image by Andre E.X. Brown
Health Canada has approved edoxaban (Lixiana®), an oral factor Xa inhibitor, for use in patients with venous thromboembolism (VTE) or nonvalvular atrial fibrillation (NVAF).
The drug can now be used to treat and prevent the recurrence of deep vein thrombosis (DVT) and pulmonary embolism (PE).
It can also be used to prevent stroke and systemic embolism in adults with NVAF in whom anticoagulation is appropriate.
Edoxaban was discovered and developed by Daiichi Sankyo Co., Ltd., but Servier Canada will market the drug in Canada.
Edoxaban has been approved in the US, European Union, Switzerland, Japan, South Korea, Taiwan, and Hong Kong. The drug is marketed as Savaysa® in the US and as Lixiana® elsewhere.
Health Canada’s approval of edoxaban is based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic VTE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()
Do Kidney Patients Know an App From a Nap?
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.