User login
Data Trends 2023: Pregnancy
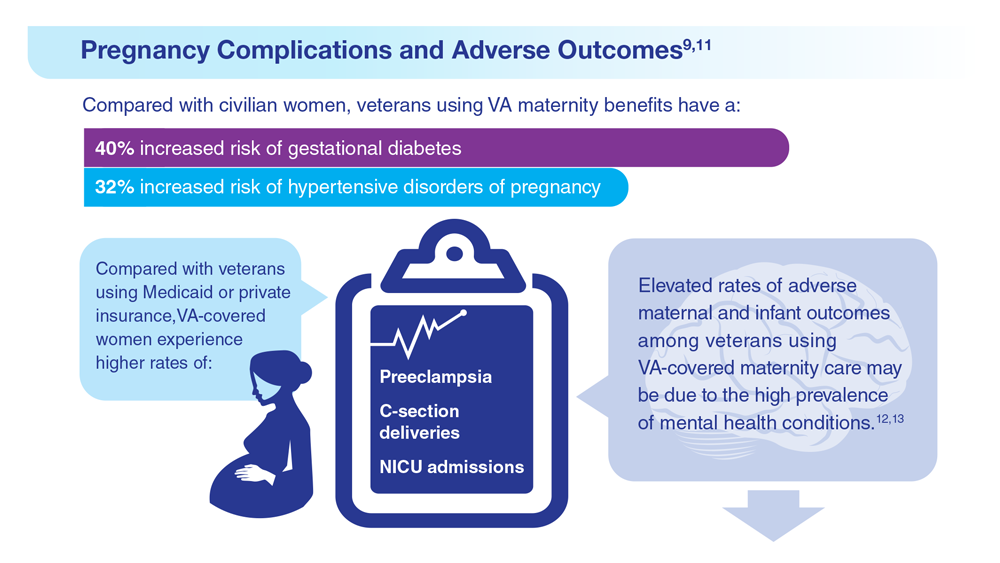
9. Frayne SM et al. Sourcebook: Women Veterans in the Veterans Health Administration. Volume 4: Longitudinal Trends in Sociodemographics, Utilization, Health Profile, and Geographic Distribution. Women’s Health Evaluation Initiative, Women’s Health Services, Veterans Health Administration. Published 2018. Accessed May 5, 2023. https://www.womenshealth.va.gov/WOMENSHEALTH/materials-and-resources/publications-and-reports.asp
10. Katon J et al. J Womens Health (Larchmt). 2014;23(10):792-800. doi:10.1089/jwh.2013.4681
11. Day MA et al. Am J Orthopsychiatry. 2023;93(1):41-49. doi:10.1037/ort0000654
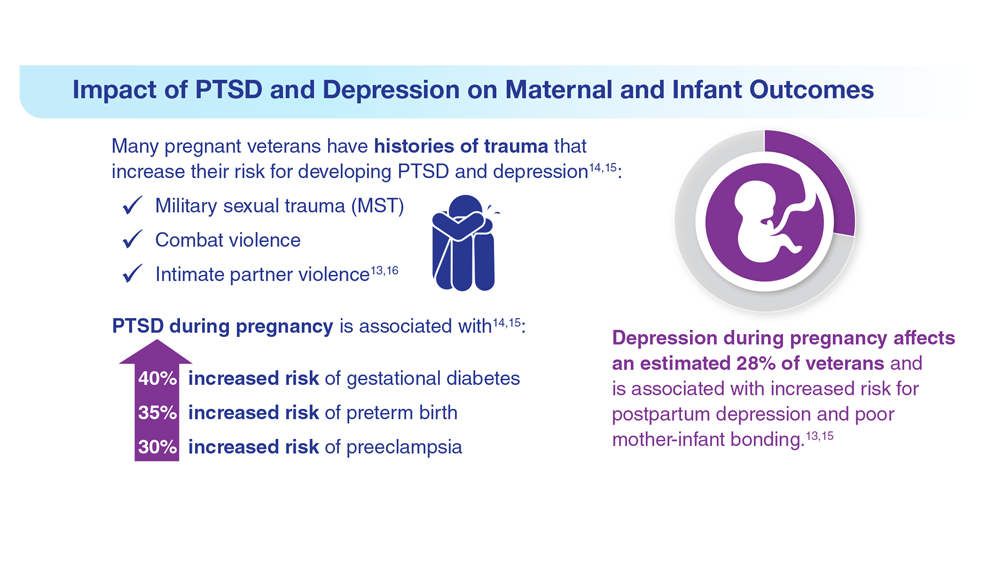
12. Shaw JG et al. Health Serv Res. 2018;53(suppl 3):5260-5284. doi:10.1111/1475-6773.13041
13. Shaw JG et al. Obstet Gynecol. 2014;124(6):1111-1119. doi:10.1097/AOG.0000000000000542
14. Shaw JG et al. Paediatr Perinat Epidemiol. 2017;31(3):185-194. doi:10.1111/ppe.12349
15. Kroll-Desrosiers A et al. J Gen Intern Med. 2022;37(suppl 3):762-769. doi:10.1007/s11606-022-07573-7
16. Creech SK et al. Depress Anxiety. 2022;39(3):201-210. doi:10.1002/da.23218
17. US Department of Defense. Department of Defense Releases Annual Demographics Report — Upward Trend in Number of Women Serving Continues. Published December 14, 2022. Accessed June 12, 2023. https://www.defense.gov/News/Releases/Release/Article/3246268/department-of-defense-releases-annual-demographics-report-upward-trend-in-numbe/
9. Frayne SM et al. Sourcebook: Women Veterans in the Veterans Health Administration. Volume 4: Longitudinal Trends in Sociodemographics, Utilization, Health Profile, and Geographic Distribution. Women’s Health Evaluation Initiative, Women’s Health Services, Veterans Health Administration. Published 2018. Accessed May 5, 2023. https://www.womenshealth.va.gov/WOMENSHEALTH/materials-and-resources/publications-and-reports.asp
10. Katon J et al. J Womens Health (Larchmt). 2014;23(10):792-800. doi:10.1089/jwh.2013.4681
11. Day MA et al. Am J Orthopsychiatry. 2023;93(1):41-49. doi:10.1037/ort0000654
12. Shaw JG et al. Health Serv Res. 2018;53(suppl 3):5260-5284. doi:10.1111/1475-6773.13041
13. Shaw JG et al. Obstet Gynecol. 2014;124(6):1111-1119. doi:10.1097/AOG.0000000000000542
14. Shaw JG et al. Paediatr Perinat Epidemiol. 2017;31(3):185-194. doi:10.1111/ppe.12349
15. Kroll-Desrosiers A et al. J Gen Intern Med. 2022;37(suppl 3):762-769. doi:10.1007/s11606-022-07573-7
16. Creech SK et al. Depress Anxiety. 2022;39(3):201-210. doi:10.1002/da.23218
17. US Department of Defense. Department of Defense Releases Annual Demographics Report — Upward Trend in Number of Women Serving Continues. Published December 14, 2022. Accessed June 12, 2023. https://www.defense.gov/News/Releases/Release/Article/3246268/department-of-defense-releases-annual-demographics-report-upward-trend-in-numbe/
9. Frayne SM et al. Sourcebook: Women Veterans in the Veterans Health Administration. Volume 4: Longitudinal Trends in Sociodemographics, Utilization, Health Profile, and Geographic Distribution. Women’s Health Evaluation Initiative, Women’s Health Services, Veterans Health Administration. Published 2018. Accessed May 5, 2023. https://www.womenshealth.va.gov/WOMENSHEALTH/materials-and-resources/publications-and-reports.asp
10. Katon J et al. J Womens Health (Larchmt). 2014;23(10):792-800. doi:10.1089/jwh.2013.4681
11. Day MA et al. Am J Orthopsychiatry. 2023;93(1):41-49. doi:10.1037/ort0000654
12. Shaw JG et al. Health Serv Res. 2018;53(suppl 3):5260-5284. doi:10.1111/1475-6773.13041
13. Shaw JG et al. Obstet Gynecol. 2014;124(6):1111-1119. doi:10.1097/AOG.0000000000000542
14. Shaw JG et al. Paediatr Perinat Epidemiol. 2017;31(3):185-194. doi:10.1111/ppe.12349
15. Kroll-Desrosiers A et al. J Gen Intern Med. 2022;37(suppl 3):762-769. doi:10.1007/s11606-022-07573-7
16. Creech SK et al. Depress Anxiety. 2022;39(3):201-210. doi:10.1002/da.23218
17. US Department of Defense. Department of Defense Releases Annual Demographics Report — Upward Trend in Number of Women Serving Continues. Published December 14, 2022. Accessed June 12, 2023. https://www.defense.gov/News/Releases/Release/Article/3246268/department-of-defense-releases-annual-demographics-report-upward-trend-in-numbe/
Federal Health Care Data Trends 2023
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Data Trends 2023: Eating Disorders
29. Touma DA et al. Mil Med. 2022;usac180. doi:10.1093/milmed/usac180
30. Flatt RE et al. Eat Behav. 2021;43:101562. doi:10.1016/j.eatbeh.2021.101562
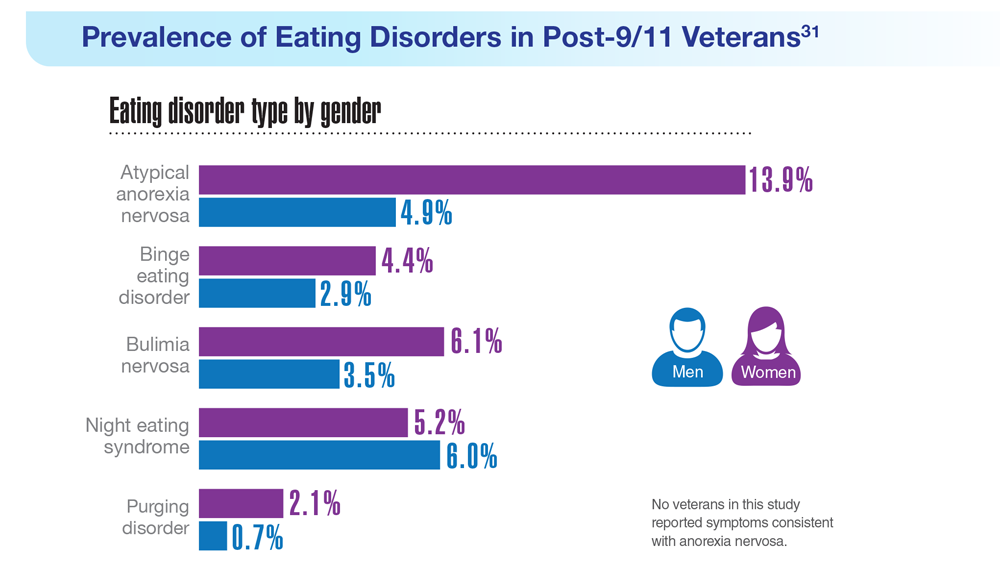
31. Masheb RM et al. Int J Eat Disord. 2021;54(7):1171-1180. doi:10.1002/eat.23501
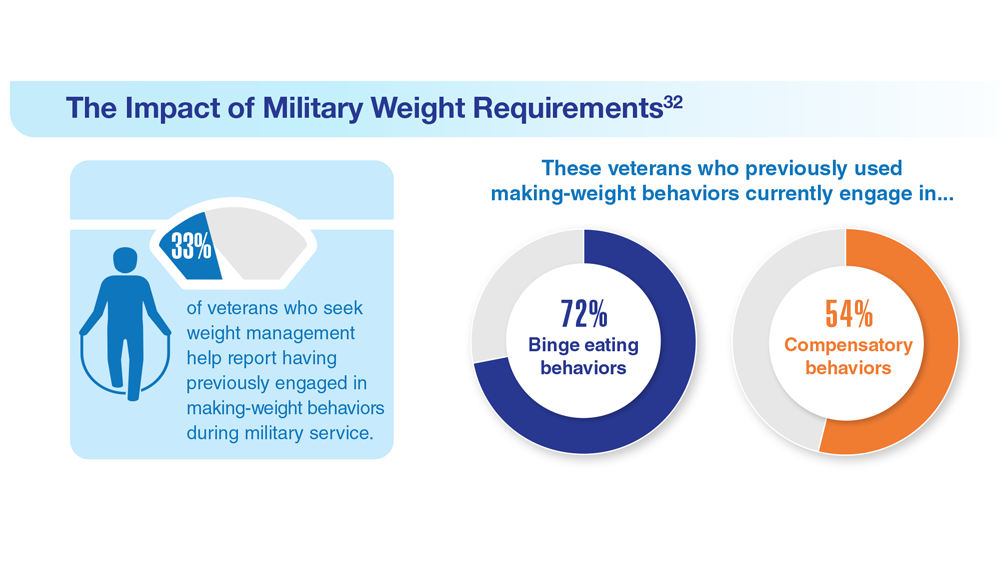
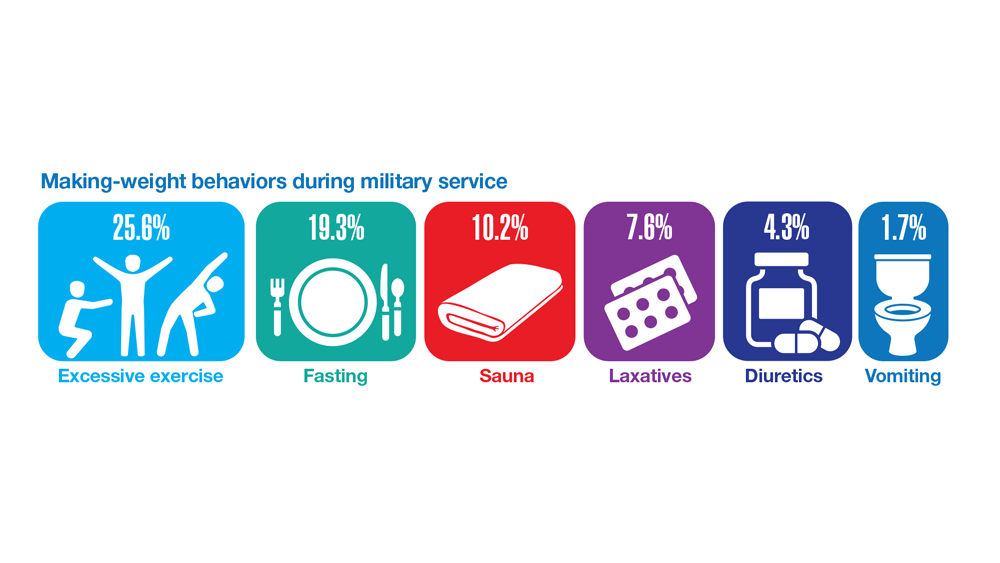
32. Masheb RM et al. Eat Weight Disord. 2019;24(6):1063-1070. doi:10.1007/s40519-019-00766-w
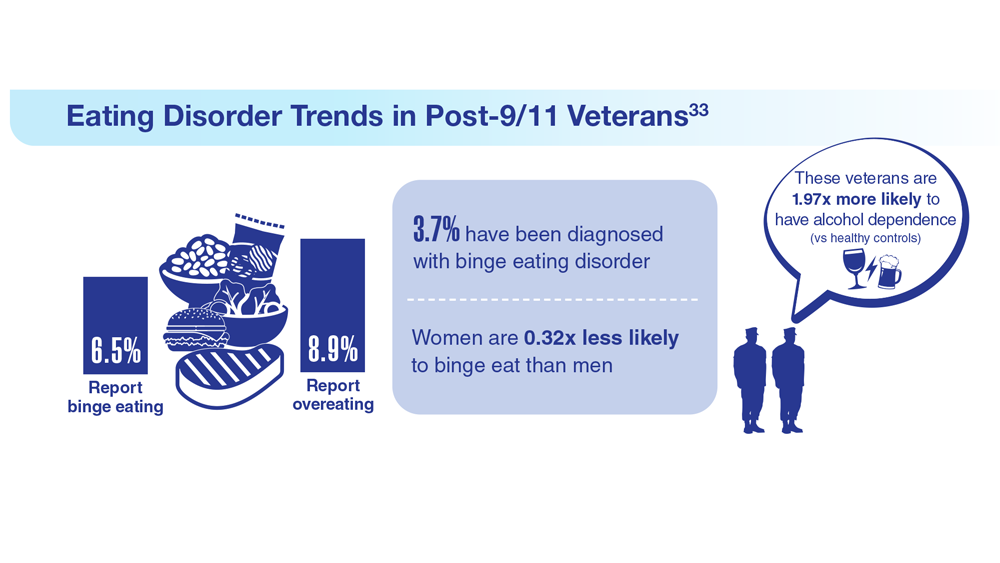
33. Etuk R et al. Mil Med. 2022;187(3-4):297-303. doi:10.1093/milmed/usab533
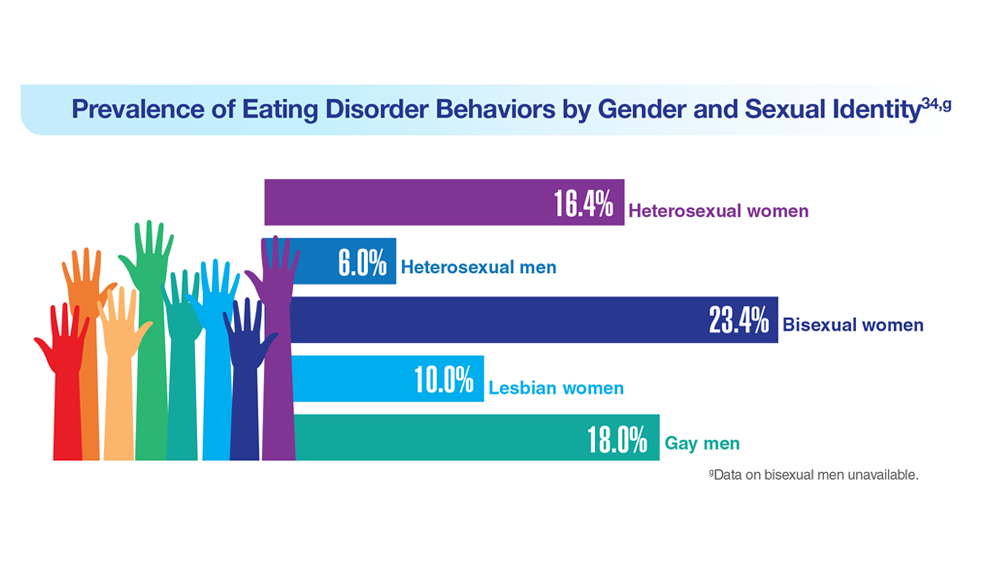
34. Serier KN et al. Int J Eat Disord. 2022;55(4):470-480. doi:10.1002/eat.23680
29. Touma DA et al. Mil Med. 2022;usac180. doi:10.1093/milmed/usac180
30. Flatt RE et al. Eat Behav. 2021;43:101562. doi:10.1016/j.eatbeh.2021.101562
31. Masheb RM et al. Int J Eat Disord. 2021;54(7):1171-1180. doi:10.1002/eat.23501
32. Masheb RM et al. Eat Weight Disord. 2019;24(6):1063-1070. doi:10.1007/s40519-019-00766-w
33. Etuk R et al. Mil Med. 2022;187(3-4):297-303. doi:10.1093/milmed/usab533
34. Serier KN et al. Int J Eat Disord. 2022;55(4):470-480. doi:10.1002/eat.23680
29. Touma DA et al. Mil Med. 2022;usac180. doi:10.1093/milmed/usac180
30. Flatt RE et al. Eat Behav. 2021;43:101562. doi:10.1016/j.eatbeh.2021.101562
31. Masheb RM et al. Int J Eat Disord. 2021;54(7):1171-1180. doi:10.1002/eat.23501
32. Masheb RM et al. Eat Weight Disord. 2019;24(6):1063-1070. doi:10.1007/s40519-019-00766-w
33. Etuk R et al. Mil Med. 2022;187(3-4):297-303. doi:10.1093/milmed/usab533
34. Serier KN et al. Int J Eat Disord. 2022;55(4):470-480. doi:10.1002/eat.23680
Metformin treatment shows benefit in gestational diabetes
HAMBURG –
Overall, the trial’s primary outcome, a composite of insulin initiation or a fasting glucose level ≥ 5.1 mmol/L (92 mg/dL) at gestation weeks 32 or 38, did not differ between women with gestational diabetes randomly assigned to either placebo or metformin. However, women taking metformin were significantly less likely to require insulin and had significantly lower fasting blood glucose levels at weeks 32 and 38.
“With a composite outcome it’s more difficult to find a positive result ... So, although the primary composite outcome was not positive, the components of the primary outcome that are clinically meaningful were positive,” lead study author Fidelma Dunne, PhD, professor and endocrine consultant at the University of Galway, Ireland, said in an interview.
There were no differences in maternal or neonatal morbidities, but there was a nonsignificant increase in small for gestational age (SGA), a finding that has been seen in some but not all previous studies of metformin use in gestational diabetes.
Dr. Dunne presented the findings on Oct. 3 at the annual meeting of the European Association for the Study of Diabetes. The results were simultaneously published in JAMA.
Current recommendations from the United Kingdom’s National Institute for Health and Care Excellence say metformin is a suitable first-line therapy for gestational diabetes. However, both the American Diabetes Association and the Society of Maternal-Fetal Medicine do not, particularly for pregnancies with hypertension or preeclampsia or in those who are at risk for intrauterine growth restriction.
“Gestational diabetes is now reaching epidemic proportions. And of course, the vast majority of these women are in low- and middle-income countries where insulin might not be available, or the storage may not allow it to be used effectively. If you have a medication that in the majority of women is safe and effective it may actually help a lot of women in [those regions],” Dr. Dunne said.
Moreover, she noted, “women with gestational diabetes are testing their sugar with finger pricks four to seven times per day and we ask them to take insulin one to four times a day. So if you can relieve any of that pain related to treatment of their condition than that is a benefit for the women as well.”
Asked to comment, Katrien Benhalima, MD, PhD, of University Hospital Gasthuisberg, KU Leuven, Belgium, said, “I think it’s an interesting study because they investigated something novel, to initiate immediately metformin or placebo. Normally what we do with gestational diabetes is once we get the diagnosis, we treat them with lifestyle, and if that’s insufficient then we start with medical therapy. So this is a novel approach.”
She also agreed with Dr. Dunne that the lack of significance for the primary outcome “isn’t an issue of power but it is a composite outcome. If you look at the individual outcomes, as can be expected, the women taking metformin had less need for insulin treatment.”
But, Dr. Benhalima said, the study still leaves open the SGA issue. “It wasn’t significant, but it’s still something we are worried about in the sense that we feel we need more data, especially in the long-term for the offspring health ... You really need to follow them for 10 years or longer to see an effect.”
So for now, Dr. Benhalima said that she wouldn’t use metformin as a first-line treatment for gestational diabetes. “Normally if lifestyle isn’t enough we will still start insulin ... Another issue is why would you offer everybody medical treatment when pregnancy outcomes can be met with lifestyle alone?”
Then again, she added, “of course metformin is easier than an injection. Treatment satisfaction is improved, and the cost is less.”
Primary outcome didn’t differ, but study findings point toward metformin benefit
The double-blind, placebo-controlled trial was conducted at two sites in Ireland, with 510 individuals (535 gestational diabetes pregnancies) enrolled between June 2017 and September 2022. In addition to usual care, they were randomly assigned 1:1 to either placebo or metformin (maximum 2,500 mg) at the time of gestational diabetes diagnosis and continued until delivery.
The primary outcome, a composite of insulin initiation or a fasting glucose ≥ 5.1 mmol/L at gestation weeks 32 or 38, did not differ significantly between the two groups, with risk ratio 0.89 (P = 0.13).
Insulin initiation occurred in 38.4% of the metformin and 51.1% of the placebo groups (relative risk, 0.75, P = .004). The amount of insulin required at the last assessment prior to delivery did not differ between the two groups (P = .17).
Mean fasting glucose was significantly lower with metformin vs. placebo at gestational week 32 (4.9 vs. 5.0 mmol/L; P = .03) and at gestational week 38 (4.5 vs 4.7 mmol/L; P < .001).
On average, those in the metformin group gained less weight between randomization and delivery (0.8 kg vs. 2.0 kg; P = .003).
Gestational week at delivery didn’t differ between the groups, both 39.1 weeks, nor did preterm births prior to 37 weeks’ gestation (9.2% metformin vs. 6.5% placebo; P = .33) or any other pregnancy-related complications.
More participants in the metformin group said that they would choose the drug compared with placebo (76.2% vs. 67.1%, P = .04).
Mean birth weight was lower in the metformin group compared with placebo, 3,393 g vs. 3,506 g (P = .005), with fewer weighing > 4,000 g (7.6% vs. 14.8%; P = .02) or being large for gestational age, i.e., above the 90th percentile (6.5% vs. 14.9%; P = .003).
Proportions of offspring that were SGA (less than 10th percentile) were 5.7% in the metformin group vs. 2.7% with placebo (P = .13).
There were no other significant differences in neonatal variables.
Dr. Dunne told this news organization that her group has recently received funding for long-term follow-up of the SGA offspring. “As other papers have pointed out, if there’s any hint of SGA that’s really important to follow up. So we’re now beginning our longitudinal follow up of the mother and infants to see if the small number that were SGA will in fact turn out to have an increase in body mass index and weight in their childhood and adolescent years.”
The trial was funded by the Health Review Board (HRB) of Ireland, coordinated by the HRB-Clinical Research Facility Galway, and sponsored by the University of Galway, Ireland. Metformin and matched placebo were provided by Merck Healthcare KGaA, Darmstadt, Germany (operating as EMD Serono in the United States), and blood glucose monitoring strips were provided by Ascensia.
Dr. Dunne reported nonfinancial support from Merck and matched placebo and nonfinancial support from Ascensia during the conduct of the study. Dr. Benhalima receives research funds from Flemish Research Fund, study medication from Novo Nordisk, and devices and unrestricted grants from Medtronic and Dexcom.
A version of this article appeared on Medscape.com.
HAMBURG –
Overall, the trial’s primary outcome, a composite of insulin initiation or a fasting glucose level ≥ 5.1 mmol/L (92 mg/dL) at gestation weeks 32 or 38, did not differ between women with gestational diabetes randomly assigned to either placebo or metformin. However, women taking metformin were significantly less likely to require insulin and had significantly lower fasting blood glucose levels at weeks 32 and 38.
“With a composite outcome it’s more difficult to find a positive result ... So, although the primary composite outcome was not positive, the components of the primary outcome that are clinically meaningful were positive,” lead study author Fidelma Dunne, PhD, professor and endocrine consultant at the University of Galway, Ireland, said in an interview.
There were no differences in maternal or neonatal morbidities, but there was a nonsignificant increase in small for gestational age (SGA), a finding that has been seen in some but not all previous studies of metformin use in gestational diabetes.
Dr. Dunne presented the findings on Oct. 3 at the annual meeting of the European Association for the Study of Diabetes. The results were simultaneously published in JAMA.
Current recommendations from the United Kingdom’s National Institute for Health and Care Excellence say metformin is a suitable first-line therapy for gestational diabetes. However, both the American Diabetes Association and the Society of Maternal-Fetal Medicine do not, particularly for pregnancies with hypertension or preeclampsia or in those who are at risk for intrauterine growth restriction.
“Gestational diabetes is now reaching epidemic proportions. And of course, the vast majority of these women are in low- and middle-income countries where insulin might not be available, or the storage may not allow it to be used effectively. If you have a medication that in the majority of women is safe and effective it may actually help a lot of women in [those regions],” Dr. Dunne said.
Moreover, she noted, “women with gestational diabetes are testing their sugar with finger pricks four to seven times per day and we ask them to take insulin one to four times a day. So if you can relieve any of that pain related to treatment of their condition than that is a benefit for the women as well.”
Asked to comment, Katrien Benhalima, MD, PhD, of University Hospital Gasthuisberg, KU Leuven, Belgium, said, “I think it’s an interesting study because they investigated something novel, to initiate immediately metformin or placebo. Normally what we do with gestational diabetes is once we get the diagnosis, we treat them with lifestyle, and if that’s insufficient then we start with medical therapy. So this is a novel approach.”
She also agreed with Dr. Dunne that the lack of significance for the primary outcome “isn’t an issue of power but it is a composite outcome. If you look at the individual outcomes, as can be expected, the women taking metformin had less need for insulin treatment.”
But, Dr. Benhalima said, the study still leaves open the SGA issue. “It wasn’t significant, but it’s still something we are worried about in the sense that we feel we need more data, especially in the long-term for the offspring health ... You really need to follow them for 10 years or longer to see an effect.”
So for now, Dr. Benhalima said that she wouldn’t use metformin as a first-line treatment for gestational diabetes. “Normally if lifestyle isn’t enough we will still start insulin ... Another issue is why would you offer everybody medical treatment when pregnancy outcomes can be met with lifestyle alone?”
Then again, she added, “of course metformin is easier than an injection. Treatment satisfaction is improved, and the cost is less.”
Primary outcome didn’t differ, but study findings point toward metformin benefit
The double-blind, placebo-controlled trial was conducted at two sites in Ireland, with 510 individuals (535 gestational diabetes pregnancies) enrolled between June 2017 and September 2022. In addition to usual care, they were randomly assigned 1:1 to either placebo or metformin (maximum 2,500 mg) at the time of gestational diabetes diagnosis and continued until delivery.
The primary outcome, a composite of insulin initiation or a fasting glucose ≥ 5.1 mmol/L at gestation weeks 32 or 38, did not differ significantly between the two groups, with risk ratio 0.89 (P = 0.13).
Insulin initiation occurred in 38.4% of the metformin and 51.1% of the placebo groups (relative risk, 0.75, P = .004). The amount of insulin required at the last assessment prior to delivery did not differ between the two groups (P = .17).
Mean fasting glucose was significantly lower with metformin vs. placebo at gestational week 32 (4.9 vs. 5.0 mmol/L; P = .03) and at gestational week 38 (4.5 vs 4.7 mmol/L; P < .001).
On average, those in the metformin group gained less weight between randomization and delivery (0.8 kg vs. 2.0 kg; P = .003).
Gestational week at delivery didn’t differ between the groups, both 39.1 weeks, nor did preterm births prior to 37 weeks’ gestation (9.2% metformin vs. 6.5% placebo; P = .33) or any other pregnancy-related complications.
More participants in the metformin group said that they would choose the drug compared with placebo (76.2% vs. 67.1%, P = .04).
Mean birth weight was lower in the metformin group compared with placebo, 3,393 g vs. 3,506 g (P = .005), with fewer weighing > 4,000 g (7.6% vs. 14.8%; P = .02) or being large for gestational age, i.e., above the 90th percentile (6.5% vs. 14.9%; P = .003).
Proportions of offspring that were SGA (less than 10th percentile) were 5.7% in the metformin group vs. 2.7% with placebo (P = .13).
There were no other significant differences in neonatal variables.
Dr. Dunne told this news organization that her group has recently received funding for long-term follow-up of the SGA offspring. “As other papers have pointed out, if there’s any hint of SGA that’s really important to follow up. So we’re now beginning our longitudinal follow up of the mother and infants to see if the small number that were SGA will in fact turn out to have an increase in body mass index and weight in their childhood and adolescent years.”
The trial was funded by the Health Review Board (HRB) of Ireland, coordinated by the HRB-Clinical Research Facility Galway, and sponsored by the University of Galway, Ireland. Metformin and matched placebo were provided by Merck Healthcare KGaA, Darmstadt, Germany (operating as EMD Serono in the United States), and blood glucose monitoring strips were provided by Ascensia.
Dr. Dunne reported nonfinancial support from Merck and matched placebo and nonfinancial support from Ascensia during the conduct of the study. Dr. Benhalima receives research funds from Flemish Research Fund, study medication from Novo Nordisk, and devices and unrestricted grants from Medtronic and Dexcom.
A version of this article appeared on Medscape.com.
HAMBURG –
Overall, the trial’s primary outcome, a composite of insulin initiation or a fasting glucose level ≥ 5.1 mmol/L (92 mg/dL) at gestation weeks 32 or 38, did not differ between women with gestational diabetes randomly assigned to either placebo or metformin. However, women taking metformin were significantly less likely to require insulin and had significantly lower fasting blood glucose levels at weeks 32 and 38.
“With a composite outcome it’s more difficult to find a positive result ... So, although the primary composite outcome was not positive, the components of the primary outcome that are clinically meaningful were positive,” lead study author Fidelma Dunne, PhD, professor and endocrine consultant at the University of Galway, Ireland, said in an interview.
There were no differences in maternal or neonatal morbidities, but there was a nonsignificant increase in small for gestational age (SGA), a finding that has been seen in some but not all previous studies of metformin use in gestational diabetes.
Dr. Dunne presented the findings on Oct. 3 at the annual meeting of the European Association for the Study of Diabetes. The results were simultaneously published in JAMA.
Current recommendations from the United Kingdom’s National Institute for Health and Care Excellence say metformin is a suitable first-line therapy for gestational diabetes. However, both the American Diabetes Association and the Society of Maternal-Fetal Medicine do not, particularly for pregnancies with hypertension or preeclampsia or in those who are at risk for intrauterine growth restriction.
“Gestational diabetes is now reaching epidemic proportions. And of course, the vast majority of these women are in low- and middle-income countries where insulin might not be available, or the storage may not allow it to be used effectively. If you have a medication that in the majority of women is safe and effective it may actually help a lot of women in [those regions],” Dr. Dunne said.
Moreover, she noted, “women with gestational diabetes are testing their sugar with finger pricks four to seven times per day and we ask them to take insulin one to four times a day. So if you can relieve any of that pain related to treatment of their condition than that is a benefit for the women as well.”
Asked to comment, Katrien Benhalima, MD, PhD, of University Hospital Gasthuisberg, KU Leuven, Belgium, said, “I think it’s an interesting study because they investigated something novel, to initiate immediately metformin or placebo. Normally what we do with gestational diabetes is once we get the diagnosis, we treat them with lifestyle, and if that’s insufficient then we start with medical therapy. So this is a novel approach.”
She also agreed with Dr. Dunne that the lack of significance for the primary outcome “isn’t an issue of power but it is a composite outcome. If you look at the individual outcomes, as can be expected, the women taking metformin had less need for insulin treatment.”
But, Dr. Benhalima said, the study still leaves open the SGA issue. “It wasn’t significant, but it’s still something we are worried about in the sense that we feel we need more data, especially in the long-term for the offspring health ... You really need to follow them for 10 years or longer to see an effect.”
So for now, Dr. Benhalima said that she wouldn’t use metformin as a first-line treatment for gestational diabetes. “Normally if lifestyle isn’t enough we will still start insulin ... Another issue is why would you offer everybody medical treatment when pregnancy outcomes can be met with lifestyle alone?”
Then again, she added, “of course metformin is easier than an injection. Treatment satisfaction is improved, and the cost is less.”
Primary outcome didn’t differ, but study findings point toward metformin benefit
The double-blind, placebo-controlled trial was conducted at two sites in Ireland, with 510 individuals (535 gestational diabetes pregnancies) enrolled between June 2017 and September 2022. In addition to usual care, they were randomly assigned 1:1 to either placebo or metformin (maximum 2,500 mg) at the time of gestational diabetes diagnosis and continued until delivery.
The primary outcome, a composite of insulin initiation or a fasting glucose ≥ 5.1 mmol/L at gestation weeks 32 or 38, did not differ significantly between the two groups, with risk ratio 0.89 (P = 0.13).
Insulin initiation occurred in 38.4% of the metformin and 51.1% of the placebo groups (relative risk, 0.75, P = .004). The amount of insulin required at the last assessment prior to delivery did not differ between the two groups (P = .17).
Mean fasting glucose was significantly lower with metformin vs. placebo at gestational week 32 (4.9 vs. 5.0 mmol/L; P = .03) and at gestational week 38 (4.5 vs 4.7 mmol/L; P < .001).
On average, those in the metformin group gained less weight between randomization and delivery (0.8 kg vs. 2.0 kg; P = .003).
Gestational week at delivery didn’t differ between the groups, both 39.1 weeks, nor did preterm births prior to 37 weeks’ gestation (9.2% metformin vs. 6.5% placebo; P = .33) or any other pregnancy-related complications.
More participants in the metformin group said that they would choose the drug compared with placebo (76.2% vs. 67.1%, P = .04).
Mean birth weight was lower in the metformin group compared with placebo, 3,393 g vs. 3,506 g (P = .005), with fewer weighing > 4,000 g (7.6% vs. 14.8%; P = .02) or being large for gestational age, i.e., above the 90th percentile (6.5% vs. 14.9%; P = .003).
Proportions of offspring that were SGA (less than 10th percentile) were 5.7% in the metformin group vs. 2.7% with placebo (P = .13).
There were no other significant differences in neonatal variables.
Dr. Dunne told this news organization that her group has recently received funding for long-term follow-up of the SGA offspring. “As other papers have pointed out, if there’s any hint of SGA that’s really important to follow up. So we’re now beginning our longitudinal follow up of the mother and infants to see if the small number that were SGA will in fact turn out to have an increase in body mass index and weight in their childhood and adolescent years.”
The trial was funded by the Health Review Board (HRB) of Ireland, coordinated by the HRB-Clinical Research Facility Galway, and sponsored by the University of Galway, Ireland. Metformin and matched placebo were provided by Merck Healthcare KGaA, Darmstadt, Germany (operating as EMD Serono in the United States), and blood glucose monitoring strips were provided by Ascensia.
Dr. Dunne reported nonfinancial support from Merck and matched placebo and nonfinancial support from Ascensia during the conduct of the study. Dr. Benhalima receives research funds from Flemish Research Fund, study medication from Novo Nordisk, and devices and unrestricted grants from Medtronic and Dexcom.
A version of this article appeared on Medscape.com.
AT EASD 2023
Longer edoxaban may benefit cancer patients with distal DVT
Patients with active cancer and newly diagnosed isolated distal deep vein thrombosis (DVT) who received 12 months of edoxaban (Savaysa) had fewer thrombotic events at 1 year than those who received 3 months of treatment, without significantly increased bleeding, in the ONCO-DVT trial.
However, lead author Yugo Yamashita, MD, of Kyoto University noted that caution is needed when determining anticoagulation strategies in individual patients with distal DVT, especially those with high risk for bleeding.
Dr. Yamashita presented the results at the annual congress of the European Society of Cardiology, and the trial was simultaneously published in the journal Circulation.
“This is the first and only randomized trial to show the superiority of longer duration over shorter duration of anticoagulation therapy for reducing thrombotic events in cancer patients with isolated distal DVT,” he said in a press briefing.
The results provide support for 12 months of edoxaban in patients with active cancer and isolated distal DVD, he said in an email.
However, “considering the risk of bleeding associated with anticoagulation therapy, physicians should make the decision of anticoagulation strategies for these patients based on risk-benefit balance of anticoagulation therapy in individual patients,” he stressed.
The take-home message for clinicians is that, “if you find minor DVT in cancer patients, please be careful, because their thrombotic risk was not low” in this trial, Dr. Yamashita said.
The study was conducted in Japan, so whether or not the results are generalizable to other populations is not clear. “Subgroup analysis based on body weight did not show any signal of different effect,” he noted, which suggests that the main results could be applied to other populations, including the U.S. population. However, “generalizability of the current results should be carried out carefully.”
Caution needed when translating findings into clinical practice
The assigned discussant, Teresa Lopez-Fernandez, MD, from La Paz University Hospital, Madrid, who was co-chairperson of 2022 ESC guidelines on cardio-oncology, noted that the optimal anticoagulation therapy strategy is unclear in patients with cancer and isolated distal DVT.
“2022 ESC guidelines on cardio-oncology and [European Society for Medical Oncology] guidelines from this year,” she said, “are both in agreement that we need to prolong anticoagulation [therapy to prevent venous thromboembolism (VTE)] when active cancer exists, and particularly in patients with metastatic cancer. The problem is that none of this text refers specifically to distal DVT.”
The ONCO-DVT trial sheds light on this, but there are a few points to consider when interpreting the findings.
Major bleeding was slightly increased in the 12-month vs 3-month edoxaban groups, although this was not statistically significant, she noted. Moreover, 75% of the patients were treated with low-dose edoxaban, mainly due to their low weight. Also, bleeding risk probably differs in different cancer types.
“These are important things that we need to keep in mind when we try to transfer this data to [inform] our clinical practice,” Dr. Lopez-Fernandez said.
She drew attention to a recent study based on RIETE registry data that suggests that “isolated distal DVT is a big problem for patients with cancer in comparison with noncancer patients, where it seems it’s a low-risk problem.”
The main takeaways from ONCO-DVT, Dr. Lopez-Fernandez said, are that it confirms that cancer-associated isolated distal DVT is a marker of poor prognosis, and it supports the need for extended anticoagulation in patients with active, ongoing cancer and isolated distal DVT.
However, “we need to be cautious to try to really understand what the bleeding risks of these patients are,” she said, “particularly because it is not always easy to transfer the results from an Asian population to other populations.”
There is also a need for further studies with other doses, with other novel oral anticoagulants, and in patients at high risk for bleeding, in clinical practice.
Dr. Yamashita said that the study suggests that there is a potential benefit of prolonged duration of anticoagulant therapy for some patients with isolated distal DVT, but not all patients should receive this dosing strategy, because some patients may be at high risk for bleeding or VTE recurrence. A subanalysis of data from ONCO-DVT study should shed further light on this.
“We need to individualize our risk stratification,” Dr. Lopez-Fernandez said, adding that notably, “a lot of patients in the 12-month group did not continue with the 12-month treatment,” which may have affected bleeding results. Dr. Yamashita agreed.
Study design and findings
From April 2019 to June 2022, the researchers enrolled and randomly assigned 604 patients with active cancer who had newly diagnosed isolated distal DVT, confirmed by ultrasonography, and were scheduled for DVT treatment with anticoagulation therapy, at 60 centers.
Active cancer was defined as a cancer diagnosis or cancer treatment (surgery, chemotherapy, radiotherapy, etc.) within 6 months of randomization, or current recurrence, local invasion, distant metastases, or hematopoietic malignancy without complete remission.
The most common reasons for ultrasonography were elevated D-dimer levels (62%) and suspected DVT because of symptoms (20%).
The patients had a mean age of 70.8 years and 28% were men. The most common cancer sites were ovaries (14%), uterus (13%), lung (11%), colon (9%), and pancreas (8%), followed by stomach, blood, and breast (each 5%).
The patients were randomly assigned 1:1 to receive 12 months or 3 months of oral edoxaban at a dose of 60 mg once daily or 30 mg once daily in patients with body weight of 60 kg or less, creatinine clearance of 30-50 mL/minute, or concomitant treatment with a potent P-glycoprotein inhibitor.
After excluding 3 patients who withdrew consent, 601 patients were included in the intention-to-treat population: 296 patients in the 12-month edoxaban group and 305 patients in the 3-month edoxaban group.
About 70% of patients had a body weight of 60 kg or less and about 22% had a creatinine clearance less than 50 mL/min. About three quarters received the lower dose of edoxaban.
In the 12-month edoxaban group, 223 patients completed the 1-year follow-up (66 patients had died and 7 were lost to follow-up). In the 3-month edoxaban group, 224 patients completed the 1-year follow-up (77 had died and 4 were lost to follow-up).
In the 12-month edoxaban group, 41% of the patients had discontinued treatment by 12 months. In the 3-month edoxaban group, 41% of patients had discontinued treatment by 3 months.
The primary endpoint – a symptomatic recurrent VTE event or VTE-related death – occurred in 3 of the 222 patients (1.2%) in the 12-month edoxaban group and in 22 of the 210 (8.5%) in the 3-month edoxaban group (odds ratio,0.13; 95% confidence interval, 0.03-0.44, P < .001). There were no VTE-related deaths.
The major secondary endpoint – major bleeding, according to International Society on Thrombosis and Hemostasis criteria – occurred in 28 of the 210 patients (10.2%) in the 12-month edoxaban group and in 22 of the 217 (7.6%) in the 3-month edoxaban group (OR, 1.34; 95% CI, 0.75-2.41, P = NS).
The researchers acknowledged that study limitations include an open-label design, a lower-than-expected primary endpoint rate, and less than high adherence to edoxaban, as well as the need for caution when generalizing the results to other populations.
The study was funded by Daiichi Sankyo. Dr. Yamashita disclosed receiving lecture fees from Bayer Healthcare, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo, and grant support from Bayer Healthcare and Daiichi Sankyo. Dr. Lopez-Fernandez disclosed receiving speaker fees from Phillips, Janssen, Daiichi Sankyo, Myocardial Solutions, AstraZeneca, Pfizer, Beigene, and Bayer not related to this study.
A version of this article appeared on Medscape.com.
Patients with active cancer and newly diagnosed isolated distal deep vein thrombosis (DVT) who received 12 months of edoxaban (Savaysa) had fewer thrombotic events at 1 year than those who received 3 months of treatment, without significantly increased bleeding, in the ONCO-DVT trial.
However, lead author Yugo Yamashita, MD, of Kyoto University noted that caution is needed when determining anticoagulation strategies in individual patients with distal DVT, especially those with high risk for bleeding.
Dr. Yamashita presented the results at the annual congress of the European Society of Cardiology, and the trial was simultaneously published in the journal Circulation.
“This is the first and only randomized trial to show the superiority of longer duration over shorter duration of anticoagulation therapy for reducing thrombotic events in cancer patients with isolated distal DVT,” he said in a press briefing.
The results provide support for 12 months of edoxaban in patients with active cancer and isolated distal DVD, he said in an email.
However, “considering the risk of bleeding associated with anticoagulation therapy, physicians should make the decision of anticoagulation strategies for these patients based on risk-benefit balance of anticoagulation therapy in individual patients,” he stressed.
The take-home message for clinicians is that, “if you find minor DVT in cancer patients, please be careful, because their thrombotic risk was not low” in this trial, Dr. Yamashita said.
The study was conducted in Japan, so whether or not the results are generalizable to other populations is not clear. “Subgroup analysis based on body weight did not show any signal of different effect,” he noted, which suggests that the main results could be applied to other populations, including the U.S. population. However, “generalizability of the current results should be carried out carefully.”
Caution needed when translating findings into clinical practice
The assigned discussant, Teresa Lopez-Fernandez, MD, from La Paz University Hospital, Madrid, who was co-chairperson of 2022 ESC guidelines on cardio-oncology, noted that the optimal anticoagulation therapy strategy is unclear in patients with cancer and isolated distal DVT.
“2022 ESC guidelines on cardio-oncology and [European Society for Medical Oncology] guidelines from this year,” she said, “are both in agreement that we need to prolong anticoagulation [therapy to prevent venous thromboembolism (VTE)] when active cancer exists, and particularly in patients with metastatic cancer. The problem is that none of this text refers specifically to distal DVT.”
The ONCO-DVT trial sheds light on this, but there are a few points to consider when interpreting the findings.
Major bleeding was slightly increased in the 12-month vs 3-month edoxaban groups, although this was not statistically significant, she noted. Moreover, 75% of the patients were treated with low-dose edoxaban, mainly due to their low weight. Also, bleeding risk probably differs in different cancer types.
“These are important things that we need to keep in mind when we try to transfer this data to [inform] our clinical practice,” Dr. Lopez-Fernandez said.
She drew attention to a recent study based on RIETE registry data that suggests that “isolated distal DVT is a big problem for patients with cancer in comparison with noncancer patients, where it seems it’s a low-risk problem.”
The main takeaways from ONCO-DVT, Dr. Lopez-Fernandez said, are that it confirms that cancer-associated isolated distal DVT is a marker of poor prognosis, and it supports the need for extended anticoagulation in patients with active, ongoing cancer and isolated distal DVT.
However, “we need to be cautious to try to really understand what the bleeding risks of these patients are,” she said, “particularly because it is not always easy to transfer the results from an Asian population to other populations.”
There is also a need for further studies with other doses, with other novel oral anticoagulants, and in patients at high risk for bleeding, in clinical practice.
Dr. Yamashita said that the study suggests that there is a potential benefit of prolonged duration of anticoagulant therapy for some patients with isolated distal DVT, but not all patients should receive this dosing strategy, because some patients may be at high risk for bleeding or VTE recurrence. A subanalysis of data from ONCO-DVT study should shed further light on this.
“We need to individualize our risk stratification,” Dr. Lopez-Fernandez said, adding that notably, “a lot of patients in the 12-month group did not continue with the 12-month treatment,” which may have affected bleeding results. Dr. Yamashita agreed.
Study design and findings
From April 2019 to June 2022, the researchers enrolled and randomly assigned 604 patients with active cancer who had newly diagnosed isolated distal DVT, confirmed by ultrasonography, and were scheduled for DVT treatment with anticoagulation therapy, at 60 centers.
Active cancer was defined as a cancer diagnosis or cancer treatment (surgery, chemotherapy, radiotherapy, etc.) within 6 months of randomization, or current recurrence, local invasion, distant metastases, or hematopoietic malignancy without complete remission.
The most common reasons for ultrasonography were elevated D-dimer levels (62%) and suspected DVT because of symptoms (20%).
The patients had a mean age of 70.8 years and 28% were men. The most common cancer sites were ovaries (14%), uterus (13%), lung (11%), colon (9%), and pancreas (8%), followed by stomach, blood, and breast (each 5%).
The patients were randomly assigned 1:1 to receive 12 months or 3 months of oral edoxaban at a dose of 60 mg once daily or 30 mg once daily in patients with body weight of 60 kg or less, creatinine clearance of 30-50 mL/minute, or concomitant treatment with a potent P-glycoprotein inhibitor.
After excluding 3 patients who withdrew consent, 601 patients were included in the intention-to-treat population: 296 patients in the 12-month edoxaban group and 305 patients in the 3-month edoxaban group.
About 70% of patients had a body weight of 60 kg or less and about 22% had a creatinine clearance less than 50 mL/min. About three quarters received the lower dose of edoxaban.
In the 12-month edoxaban group, 223 patients completed the 1-year follow-up (66 patients had died and 7 were lost to follow-up). In the 3-month edoxaban group, 224 patients completed the 1-year follow-up (77 had died and 4 were lost to follow-up).
In the 12-month edoxaban group, 41% of the patients had discontinued treatment by 12 months. In the 3-month edoxaban group, 41% of patients had discontinued treatment by 3 months.
The primary endpoint – a symptomatic recurrent VTE event or VTE-related death – occurred in 3 of the 222 patients (1.2%) in the 12-month edoxaban group and in 22 of the 210 (8.5%) in the 3-month edoxaban group (odds ratio,0.13; 95% confidence interval, 0.03-0.44, P < .001). There were no VTE-related deaths.
The major secondary endpoint – major bleeding, according to International Society on Thrombosis and Hemostasis criteria – occurred in 28 of the 210 patients (10.2%) in the 12-month edoxaban group and in 22 of the 217 (7.6%) in the 3-month edoxaban group (OR, 1.34; 95% CI, 0.75-2.41, P = NS).
The researchers acknowledged that study limitations include an open-label design, a lower-than-expected primary endpoint rate, and less than high adherence to edoxaban, as well as the need for caution when generalizing the results to other populations.
The study was funded by Daiichi Sankyo. Dr. Yamashita disclosed receiving lecture fees from Bayer Healthcare, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo, and grant support from Bayer Healthcare and Daiichi Sankyo. Dr. Lopez-Fernandez disclosed receiving speaker fees from Phillips, Janssen, Daiichi Sankyo, Myocardial Solutions, AstraZeneca, Pfizer, Beigene, and Bayer not related to this study.
A version of this article appeared on Medscape.com.
Patients with active cancer and newly diagnosed isolated distal deep vein thrombosis (DVT) who received 12 months of edoxaban (Savaysa) had fewer thrombotic events at 1 year than those who received 3 months of treatment, without significantly increased bleeding, in the ONCO-DVT trial.
However, lead author Yugo Yamashita, MD, of Kyoto University noted that caution is needed when determining anticoagulation strategies in individual patients with distal DVT, especially those with high risk for bleeding.
Dr. Yamashita presented the results at the annual congress of the European Society of Cardiology, and the trial was simultaneously published in the journal Circulation.
“This is the first and only randomized trial to show the superiority of longer duration over shorter duration of anticoagulation therapy for reducing thrombotic events in cancer patients with isolated distal DVT,” he said in a press briefing.
The results provide support for 12 months of edoxaban in patients with active cancer and isolated distal DVD, he said in an email.
However, “considering the risk of bleeding associated with anticoagulation therapy, physicians should make the decision of anticoagulation strategies for these patients based on risk-benefit balance of anticoagulation therapy in individual patients,” he stressed.
The take-home message for clinicians is that, “if you find minor DVT in cancer patients, please be careful, because their thrombotic risk was not low” in this trial, Dr. Yamashita said.
The study was conducted in Japan, so whether or not the results are generalizable to other populations is not clear. “Subgroup analysis based on body weight did not show any signal of different effect,” he noted, which suggests that the main results could be applied to other populations, including the U.S. population. However, “generalizability of the current results should be carried out carefully.”
Caution needed when translating findings into clinical practice
The assigned discussant, Teresa Lopez-Fernandez, MD, from La Paz University Hospital, Madrid, who was co-chairperson of 2022 ESC guidelines on cardio-oncology, noted that the optimal anticoagulation therapy strategy is unclear in patients with cancer and isolated distal DVT.
“2022 ESC guidelines on cardio-oncology and [European Society for Medical Oncology] guidelines from this year,” she said, “are both in agreement that we need to prolong anticoagulation [therapy to prevent venous thromboembolism (VTE)] when active cancer exists, and particularly in patients with metastatic cancer. The problem is that none of this text refers specifically to distal DVT.”
The ONCO-DVT trial sheds light on this, but there are a few points to consider when interpreting the findings.
Major bleeding was slightly increased in the 12-month vs 3-month edoxaban groups, although this was not statistically significant, she noted. Moreover, 75% of the patients were treated with low-dose edoxaban, mainly due to their low weight. Also, bleeding risk probably differs in different cancer types.
“These are important things that we need to keep in mind when we try to transfer this data to [inform] our clinical practice,” Dr. Lopez-Fernandez said.
She drew attention to a recent study based on RIETE registry data that suggests that “isolated distal DVT is a big problem for patients with cancer in comparison with noncancer patients, where it seems it’s a low-risk problem.”
The main takeaways from ONCO-DVT, Dr. Lopez-Fernandez said, are that it confirms that cancer-associated isolated distal DVT is a marker of poor prognosis, and it supports the need for extended anticoagulation in patients with active, ongoing cancer and isolated distal DVT.
However, “we need to be cautious to try to really understand what the bleeding risks of these patients are,” she said, “particularly because it is not always easy to transfer the results from an Asian population to other populations.”
There is also a need for further studies with other doses, with other novel oral anticoagulants, and in patients at high risk for bleeding, in clinical practice.
Dr. Yamashita said that the study suggests that there is a potential benefit of prolonged duration of anticoagulant therapy for some patients with isolated distal DVT, but not all patients should receive this dosing strategy, because some patients may be at high risk for bleeding or VTE recurrence. A subanalysis of data from ONCO-DVT study should shed further light on this.
“We need to individualize our risk stratification,” Dr. Lopez-Fernandez said, adding that notably, “a lot of patients in the 12-month group did not continue with the 12-month treatment,” which may have affected bleeding results. Dr. Yamashita agreed.
Study design and findings
From April 2019 to June 2022, the researchers enrolled and randomly assigned 604 patients with active cancer who had newly diagnosed isolated distal DVT, confirmed by ultrasonography, and were scheduled for DVT treatment with anticoagulation therapy, at 60 centers.
Active cancer was defined as a cancer diagnosis or cancer treatment (surgery, chemotherapy, radiotherapy, etc.) within 6 months of randomization, or current recurrence, local invasion, distant metastases, or hematopoietic malignancy without complete remission.
The most common reasons for ultrasonography were elevated D-dimer levels (62%) and suspected DVT because of symptoms (20%).
The patients had a mean age of 70.8 years and 28% were men. The most common cancer sites were ovaries (14%), uterus (13%), lung (11%), colon (9%), and pancreas (8%), followed by stomach, blood, and breast (each 5%).
The patients were randomly assigned 1:1 to receive 12 months or 3 months of oral edoxaban at a dose of 60 mg once daily or 30 mg once daily in patients with body weight of 60 kg or less, creatinine clearance of 30-50 mL/minute, or concomitant treatment with a potent P-glycoprotein inhibitor.
After excluding 3 patients who withdrew consent, 601 patients were included in the intention-to-treat population: 296 patients in the 12-month edoxaban group and 305 patients in the 3-month edoxaban group.
About 70% of patients had a body weight of 60 kg or less and about 22% had a creatinine clearance less than 50 mL/min. About three quarters received the lower dose of edoxaban.
In the 12-month edoxaban group, 223 patients completed the 1-year follow-up (66 patients had died and 7 were lost to follow-up). In the 3-month edoxaban group, 224 patients completed the 1-year follow-up (77 had died and 4 were lost to follow-up).
In the 12-month edoxaban group, 41% of the patients had discontinued treatment by 12 months. In the 3-month edoxaban group, 41% of patients had discontinued treatment by 3 months.
The primary endpoint – a symptomatic recurrent VTE event or VTE-related death – occurred in 3 of the 222 patients (1.2%) in the 12-month edoxaban group and in 22 of the 210 (8.5%) in the 3-month edoxaban group (odds ratio,0.13; 95% confidence interval, 0.03-0.44, P < .001). There were no VTE-related deaths.
The major secondary endpoint – major bleeding, according to International Society on Thrombosis and Hemostasis criteria – occurred in 28 of the 210 patients (10.2%) in the 12-month edoxaban group and in 22 of the 217 (7.6%) in the 3-month edoxaban group (OR, 1.34; 95% CI, 0.75-2.41, P = NS).
The researchers acknowledged that study limitations include an open-label design, a lower-than-expected primary endpoint rate, and less than high adherence to edoxaban, as well as the need for caution when generalizing the results to other populations.
The study was funded by Daiichi Sankyo. Dr. Yamashita disclosed receiving lecture fees from Bayer Healthcare, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo, and grant support from Bayer Healthcare and Daiichi Sankyo. Dr. Lopez-Fernandez disclosed receiving speaker fees from Phillips, Janssen, Daiichi Sankyo, Myocardial Solutions, AstraZeneca, Pfizer, Beigene, and Bayer not related to this study.
A version of this article appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
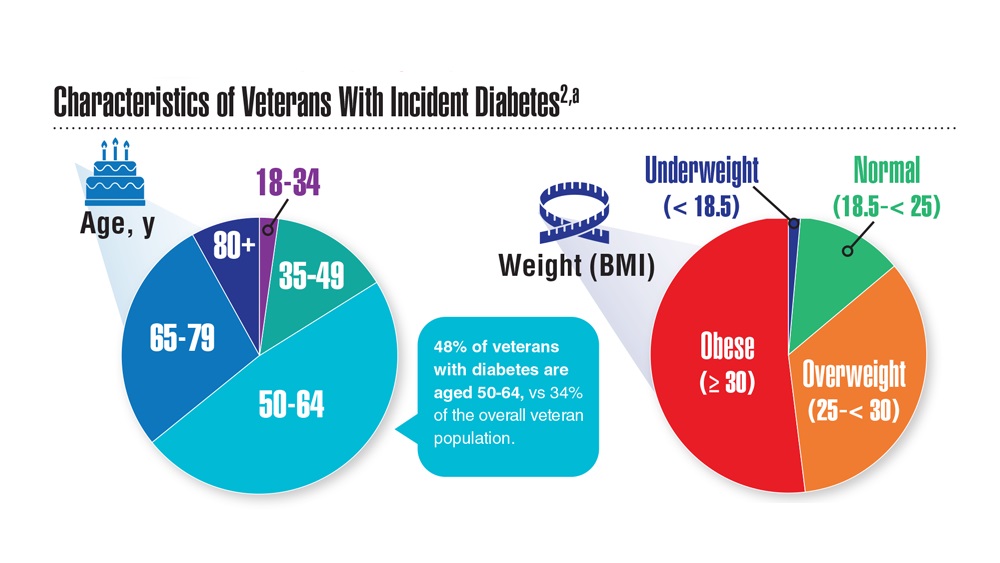
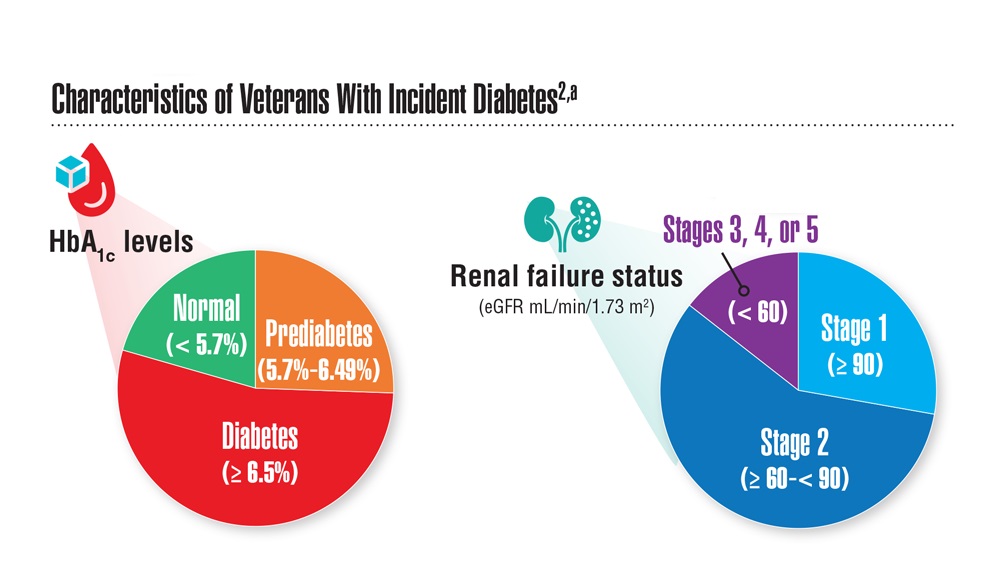
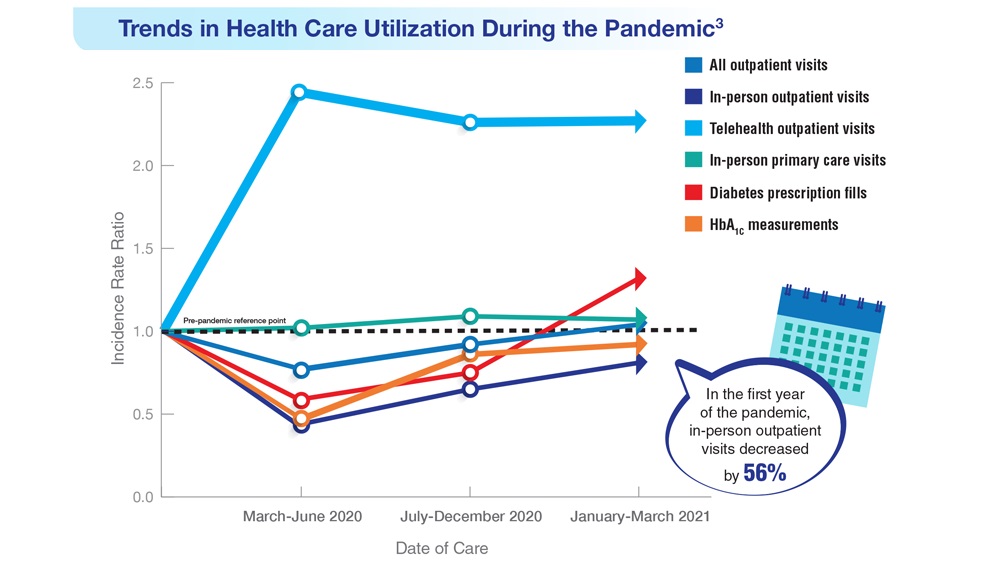
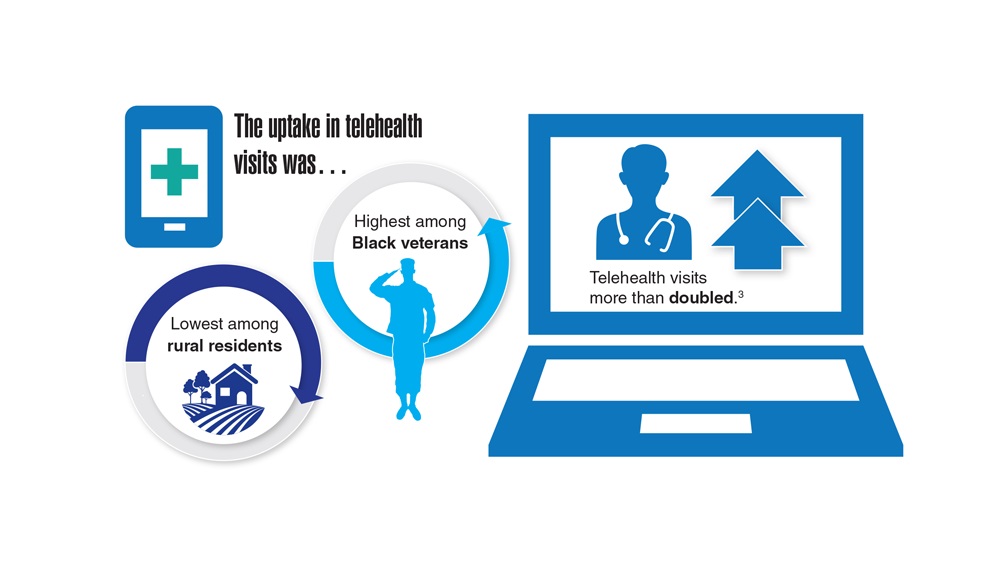
Data Trends 2023: Diabetes
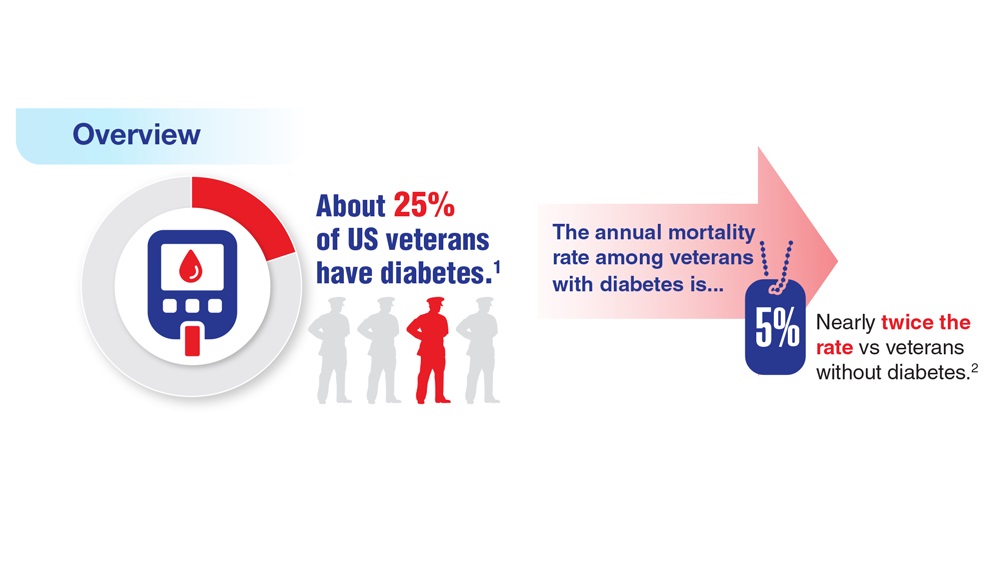
- US Department of Veterans Affairs. Nutrition and food services. Diabetes information. Updated December 1, 2022. Accessed April 14, 2023. https://www.nutrition.va.gov/Diabetes.asp
- Avramovic S et al. BMJ Open. 2020;10(12):e039489. doi:10.1136/bmjopen-2020-039489
- Adhikari S et al. BMC Health Serv Res. 2023;23(1):41. doi:10.1186/s12913-023-09057-8
- Zhou P et al. J Diabetes Metab Disord. 2022;21(1):759-768. doi:10.1007/s40200-022-01049-5
- Lamprea-Montealegre JA et al. JAMA. 2022;328(9):861-871. doi:10.1001/jama.2022.13885
- Fairman KA, Buckley K. Health Psychol. 2021;40(1):1-10. doi:10.1037/hea0000889
- US Department of Veterans Affairs. Nutrition and food services. Diabetes information. Updated December 1, 2022. Accessed April 14, 2023. https://www.nutrition.va.gov/Diabetes.asp
- Avramovic S et al. BMJ Open. 2020;10(12):e039489. doi:10.1136/bmjopen-2020-039489
- Adhikari S et al. BMC Health Serv Res. 2023;23(1):41. doi:10.1186/s12913-023-09057-8
- Zhou P et al. J Diabetes Metab Disord. 2022;21(1):759-768. doi:10.1007/s40200-022-01049-5
- Lamprea-Montealegre JA et al. JAMA. 2022;328(9):861-871. doi:10.1001/jama.2022.13885
- Fairman KA, Buckley K. Health Psychol. 2021;40(1):1-10. doi:10.1037/hea0000889
- US Department of Veterans Affairs. Nutrition and food services. Diabetes information. Updated December 1, 2022. Accessed April 14, 2023. https://www.nutrition.va.gov/Diabetes.asp
- Avramovic S et al. BMJ Open. 2020;10(12):e039489. doi:10.1136/bmjopen-2020-039489
- Adhikari S et al. BMC Health Serv Res. 2023;23(1):41. doi:10.1186/s12913-023-09057-8
- Zhou P et al. J Diabetes Metab Disord. 2022;21(1):759-768. doi:10.1007/s40200-022-01049-5
- Lamprea-Montealegre JA et al. JAMA. 2022;328(9):861-871. doi:10.1001/jama.2022.13885
- Fairman KA, Buckley K. Health Psychol. 2021;40(1):1-10. doi:10.1037/hea0000889
Advanced practice radiation therapists: Are they worth it?
An innovative care model involving in the radiation oncology department of Mount Sinai Health System in New York.
At a time when clinician burnout is rampant, a novel approach that brings value to both patients and health systems – and helps advance the careers of highly educated and skilled practitioners – represents a welcome step forward, according to Samantha Skubish, MS, RT, chief technical director of radiation oncology and Mount Sinai.
In the new care model, APRTs work alongside radiation oncologists and support “the care of resource-intensive patient populations,” according to the Association of Community Cancer Centers, which recently recognized the Mount Sinai Health System program as a 2023 ACCC Innovator Award winner.
The new and improved “model for continuity of care” with the APRT role has “helped improve the patient experience and create a more streamlined, efficient process while also alleviating some of the burden on our physicians,” Ms. Skubish said in the ACCC press release. She explained that APRTs possess the skills, knowledge, and judgment to provide an elevated level of care, as evidenced by decades of international research.
A 2022 systematic review of APRT-based care models outside the United States explored how the models have worked. Overall, the research shows that such models improve quality, efficiency, wellness, and administrative outcomes, according to investigators.
At Mount Sinai, the first health system to develop the APRT role in the United States, research to demonstrate the benefits of APRT model continues. In 2021, an APRT working group was established to “garner a network of individuals across the country focused on the work to prove the advanced practice radiation therapy model in the U.S.,” according to Danielle McDonagh, MS, RT, Mount Sinai’s clinical coordinator of radiation sciences education and research.
A paper published in May by Ms. McDonagh and colleagues underscored the potential for “positive change and impact” of the APRT care model in radiation oncology.
“We’re all in this current and longstanding crisis of clinician shortages,” Kimberly Smith, MPA, explained in a video introducing the Mount Sinai program.
“If you look at your therapists’ skill set and allow them to work at the top of their license, you can provide a cost-saving solution that lends itself to value-based care,” said Ms. Smith, vice president of radiation oncology services at Mount Sinai.
Indeed, Sheryl Green, MBBCh, professor and medical director of radiation oncology at Mount Sinai, noted that “the APRT has allowed us to really improve the quality of care that we deliver, primarily in the aspects of optimizing and personalizing the patient experience.”
Ms. Skubish and Ms. Smith will share details of the new care model at the ACCC’s upcoming National Oncology Conference.
An innovative care model involving in the radiation oncology department of Mount Sinai Health System in New York.
At a time when clinician burnout is rampant, a novel approach that brings value to both patients and health systems – and helps advance the careers of highly educated and skilled practitioners – represents a welcome step forward, according to Samantha Skubish, MS, RT, chief technical director of radiation oncology and Mount Sinai.
In the new care model, APRTs work alongside radiation oncologists and support “the care of resource-intensive patient populations,” according to the Association of Community Cancer Centers, which recently recognized the Mount Sinai Health System program as a 2023 ACCC Innovator Award winner.
The new and improved “model for continuity of care” with the APRT role has “helped improve the patient experience and create a more streamlined, efficient process while also alleviating some of the burden on our physicians,” Ms. Skubish said in the ACCC press release. She explained that APRTs possess the skills, knowledge, and judgment to provide an elevated level of care, as evidenced by decades of international research.
A 2022 systematic review of APRT-based care models outside the United States explored how the models have worked. Overall, the research shows that such models improve quality, efficiency, wellness, and administrative outcomes, according to investigators.
At Mount Sinai, the first health system to develop the APRT role in the United States, research to demonstrate the benefits of APRT model continues. In 2021, an APRT working group was established to “garner a network of individuals across the country focused on the work to prove the advanced practice radiation therapy model in the U.S.,” according to Danielle McDonagh, MS, RT, Mount Sinai’s clinical coordinator of radiation sciences education and research.
A paper published in May by Ms. McDonagh and colleagues underscored the potential for “positive change and impact” of the APRT care model in radiation oncology.
“We’re all in this current and longstanding crisis of clinician shortages,” Kimberly Smith, MPA, explained in a video introducing the Mount Sinai program.
“If you look at your therapists’ skill set and allow them to work at the top of their license, you can provide a cost-saving solution that lends itself to value-based care,” said Ms. Smith, vice president of radiation oncology services at Mount Sinai.
Indeed, Sheryl Green, MBBCh, professor and medical director of radiation oncology at Mount Sinai, noted that “the APRT has allowed us to really improve the quality of care that we deliver, primarily in the aspects of optimizing and personalizing the patient experience.”
Ms. Skubish and Ms. Smith will share details of the new care model at the ACCC’s upcoming National Oncology Conference.
An innovative care model involving in the radiation oncology department of Mount Sinai Health System in New York.
At a time when clinician burnout is rampant, a novel approach that brings value to both patients and health systems – and helps advance the careers of highly educated and skilled practitioners – represents a welcome step forward, according to Samantha Skubish, MS, RT, chief technical director of radiation oncology and Mount Sinai.
In the new care model, APRTs work alongside radiation oncologists and support “the care of resource-intensive patient populations,” according to the Association of Community Cancer Centers, which recently recognized the Mount Sinai Health System program as a 2023 ACCC Innovator Award winner.
The new and improved “model for continuity of care” with the APRT role has “helped improve the patient experience and create a more streamlined, efficient process while also alleviating some of the burden on our physicians,” Ms. Skubish said in the ACCC press release. She explained that APRTs possess the skills, knowledge, and judgment to provide an elevated level of care, as evidenced by decades of international research.
A 2022 systematic review of APRT-based care models outside the United States explored how the models have worked. Overall, the research shows that such models improve quality, efficiency, wellness, and administrative outcomes, according to investigators.
At Mount Sinai, the first health system to develop the APRT role in the United States, research to demonstrate the benefits of APRT model continues. In 2021, an APRT working group was established to “garner a network of individuals across the country focused on the work to prove the advanced practice radiation therapy model in the U.S.,” according to Danielle McDonagh, MS, RT, Mount Sinai’s clinical coordinator of radiation sciences education and research.
A paper published in May by Ms. McDonagh and colleagues underscored the potential for “positive change and impact” of the APRT care model in radiation oncology.
“We’re all in this current and longstanding crisis of clinician shortages,” Kimberly Smith, MPA, explained in a video introducing the Mount Sinai program.
“If you look at your therapists’ skill set and allow them to work at the top of their license, you can provide a cost-saving solution that lends itself to value-based care,” said Ms. Smith, vice president of radiation oncology services at Mount Sinai.
Indeed, Sheryl Green, MBBCh, professor and medical director of radiation oncology at Mount Sinai, noted that “the APRT has allowed us to really improve the quality of care that we deliver, primarily in the aspects of optimizing and personalizing the patient experience.”
Ms. Skubish and Ms. Smith will share details of the new care model at the ACCC’s upcoming National Oncology Conference.
Preparing for the viral trifecta: RSV, influenza, and COVID-19
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
New armamentaria available to fight an old disease.
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
Data Trends 2023: Traumatic Brain Injury
- Howard JT et al. JAMA Netw Open. 2022;5(2):e2148150. doi:10.1001/jamanetworkopen.2021.48150
- Cogan AM et al. PM R. 2020;12(3):301-314. doi:10.1002/pmrj.12237
- Stewart IJ et al. JAMA Neurol. 2022;79(11):1122-1129. doi:10.1001/jamaneurol.2022.2682
- Leng Y et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Winkler SL et al. Optom Vis Sci. 2022;99(1):3-8. doi:10.1097/OPX.0000000000001824
- Howard JT et al. JAMA Netw Open. 2022;5(2):e2148150. doi:10.1001/jamanetworkopen.2021.48150
- Cogan AM et al. PM R. 2020;12(3):301-314. doi:10.1002/pmrj.12237
- Stewart IJ et al. JAMA Neurol. 2022;79(11):1122-1129. doi:10.1001/jamaneurol.2022.2682
- Leng Y et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Winkler SL et al. Optom Vis Sci. 2022;99(1):3-8. doi:10.1097/OPX.0000000000001824
- Howard JT et al. JAMA Netw Open. 2022;5(2):e2148150. doi:10.1001/jamanetworkopen.2021.48150
- Cogan AM et al. PM R. 2020;12(3):301-314. doi:10.1002/pmrj.12237
- Stewart IJ et al. JAMA Neurol. 2022;79(11):1122-1129. doi:10.1001/jamaneurol.2022.2682
- Leng Y et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Winkler SL et al. Optom Vis Sci. 2022;99(1):3-8. doi:10.1097/OPX.0000000000001824
Data Trends 2023: Opioid Use Disorder
7. Bennett AS et al. Ann Med. 2022;54(1):1826-1838. doi:10.1080/07853890.2022.2092896
8. Finlay AK et al. Am J Prev Med. 2022;62(1):e29-e37. doi:10.1016/j.amepre.2021.06.014
9. Peltier MR et al. J Dual Diagn. 2021;17(2):124-134. doi:10.1080/15504263.2021.1904162
10. Beckman KL et al. Am J Prev Med. 2022;62(3):377-386. doi:10.1016/j.amepre.2021.08.020
11. Boyer TL et al. Am J Prev Med. 2022;63(2):168-177. doi:10.1016/j.amepre.2022.02.011
12. Jasuja GK et al. Med Care. 2021;59(suppl 2):S165-S169. doi:10.1097/MLR.0000000000001437
13. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA’s Rapid Naloxone Initiative recognized in fight against opioid overdose deaths. Published June 8, 2021. Accessed April 21, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5679
14. Chen EF, et. al. Fed Pract. 2022;39(3):136-141. doi:10.12788/fp.0236
7. Bennett AS et al. Ann Med. 2022;54(1):1826-1838. doi:10.1080/07853890.2022.2092896
8. Finlay AK et al. Am J Prev Med. 2022;62(1):e29-e37. doi:10.1016/j.amepre.2021.06.014
9. Peltier MR et al. J Dual Diagn. 2021;17(2):124-134. doi:10.1080/15504263.2021.1904162
10. Beckman KL et al. Am J Prev Med. 2022;62(3):377-386. doi:10.1016/j.amepre.2021.08.020
11. Boyer TL et al. Am J Prev Med. 2022;63(2):168-177. doi:10.1016/j.amepre.2022.02.011
12. Jasuja GK et al. Med Care. 2021;59(suppl 2):S165-S169. doi:10.1097/MLR.0000000000001437
13. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA’s Rapid Naloxone Initiative recognized in fight against opioid overdose deaths. Published June 8, 2021. Accessed April 21, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5679
14. Chen EF, et. al. Fed Pract. 2022;39(3):136-141. doi:10.12788/fp.0236
7. Bennett AS et al. Ann Med. 2022;54(1):1826-1838. doi:10.1080/07853890.2022.2092896
8. Finlay AK et al. Am J Prev Med. 2022;62(1):e29-e37. doi:10.1016/j.amepre.2021.06.014
9. Peltier MR et al. J Dual Diagn. 2021;17(2):124-134. doi:10.1080/15504263.2021.1904162
10. Beckman KL et al. Am J Prev Med. 2022;62(3):377-386. doi:10.1016/j.amepre.2021.08.020
11. Boyer TL et al. Am J Prev Med. 2022;63(2):168-177. doi:10.1016/j.amepre.2022.02.011
12. Jasuja GK et al. Med Care. 2021;59(suppl 2):S165-S169. doi:10.1097/MLR.0000000000001437
13. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA’s Rapid Naloxone Initiative recognized in fight against opioid overdose deaths. Published June 8, 2021. Accessed April 21, 2023. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5679
14. Chen EF, et. al. Fed Pract. 2022;39(3):136-141. doi:10.12788/fp.0236